Screening assessment phenol, 4-chloro-3-methyl- (Chlorocresol)
Official title: Screening Assessment - Phenol, 4-chloro-3-methyl- (Chlorocresol)
Chemical Abstracts Service Registry Number
59-50-7
Environment and Climate Change Canada
Health Canada
May 2021
Cat. No.: En84-220/2021E-PDF
ISBN: 978-0-660-37511-3
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of phenol, 4-chloro-3-methyl-, hereinafter referred to as chlorocresol. The Chemical Abstracts Service Registry Number (CAS RNFootnote 1 ) for chlorocresol is 59-50-7. This substance was identified as a priority for assessment as it met categorization criteria under subsection 73(1) of CEPA.
Chlorocresol was included in a survey issued pursuant to Section 71 of CEPA. There were no reports of manufacture of chlorocresol in Canada above the reporting threshold of 100 kg in 2011. Chlorocresol was reported as being imported into Canada with a total volume in the range of 100 to 1 000 kg for commercial uses as a component in an admixture to concrete. Other uses in Canada include as a component in certain body moisturizer creams/lotions at concentrations up to 0.2%. Chlorocresol was also identified as a non-medicinal ingredient in licensed natural health product creams at concentrations up to 0.2%, as a non-medicinal ingredient in a limited number of pharmaceuticals at concentrations up to 0.1%, and as an active ingredient in one registered pest control product in Canada, which is formulated into concrete admixtures. The sodium salt form of chlorocresol is also registered in two pest control products.
The ecological risk of chlorocresol was characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, chlorocresol is considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from chlorocresol. It is concluded that chlorocresol does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the low volumes of chlorocresol reported in commerce in Canada, and the low frequency of detection of chlorocresol in Canadian drinking water, wastewater treatment system sludge and indoor air, exposure to the general population to chlorocresol from environmental media is expected to be minimal. Accordingly, risk to human health is considered to be low.
Consumer exposure is not expected from small quantities of chlorocresol used for commercial purposes in certain building or construction materials as a concrete admixture.
In Canada, exposure to chlorocresol may occur through the use of certain body moisturizer creams/lotions, or topical licensed natural health products or pharmaceuticals, in which it is present at concentrations up to 0.2%. The highest exposures were estimated for the use of moisturizers when applied to infants (birth to 6 months old).
The critical health effect for chlorocresol was identified as decreased adrenal gland weights in a chronic exposure study. A comparison of estimated exposure to chlorocresol from its use in body lotions, to the critical health effect level resulted in margins of exposure (MOEs) which were considered inadequate to address uncertainties in the health effects and exposure databases.
With respect to short-term dermal exposure to chlorocresol from the use of topical licensed natural health products or pharmaceuticals, a comparison of the estimated exposure to the critical effect level resulted in MOEs that are considered adequate to address uncertainties in the health effects and exposure databases.
Considering all the information presented in this screening assessment, it is concluded that chlorocresol meets the criteria under paragraph 64(c) of CEPA as it is entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that chlorocresol meets one or more of the criteria set out in section 64 of CEPA.
It has also been determined that chlorocresol does not meet the persistence or bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
1. Introduction
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of phenol, 4-chloro-3-methyl-, hereinafter referred to by its common name chlorocresol, to determine whether this substance presents or may present a risk to the environment or to human health. The substance was identified as a priority for assessment as it met categorization criteria under subsection 73(1) of CEPA (ECCC, HC [modified 2017]).
The ecological risk of chlorocresol was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to January 2020. Empirical data from key studies as well as results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. Additionally, the draft of this screening assessment published July 27, 2019, was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether the substance meets the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precaution.Footnote 2 This screening assessment presents the critical information and considerations on which the conclusions are based.
2. Substance identity
The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 3 ), Domestic Substances List (DSL) name and common name for phenol, 4-chloro-3-methyl- are presented in Table 2‑1.
| CAS RN | DSL name(common name) | Chemical structure and molecular formulaa | Molecular weight (g/mol)a |
|---|---|---|---|
| 59-50-7 | phenol, 4-chloro-3-methyl- (chlorocresol) | 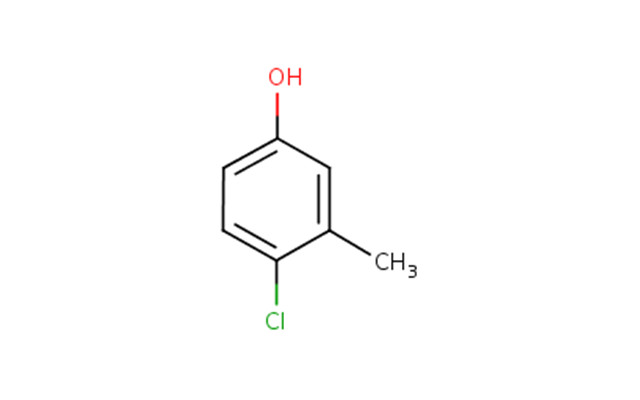 C7H7ClO
C7H7ClO | 142.58 |
a ChemIDPlus 1993- ; US EPA 1997
3. Physical and chemical properties
A summary of physical and chemical properties of chlorocresol are presented in Table 3‑1. Additional physical chemical properties are reported in ECCC (2016b).
| Property | Value (or range) | Key reference(s) |
|---|---|---|
| Physical state | White or pink crystals or crystalline powder | ChemSpider 2018; IPCS 1997; O’Neil 2006 |
| Melting point (°C) | 63–68 | ChemIDplus 2018; ChemSpider 2018; IPCS 1997 |
| Boiling point (°C) | 234–236 | ChemIDPlus 2018; ChemSpider 2018; IPCS 1997 |
| Vapour pressure (Pa) | 6.67 | ChemIDPlus 2018 |
| Henry’s law constant (Pa·m3/mol) | 0.248 | ChemIDPlus 2018 |
| Water solubility (mg/L) | 3 830 | ChemIDPlus 2018 |
| Other solubilities | Soluble in alkalies, organic solvents, fats and oils | O’Neil 2006 |
| Skin permeability constant (cm/hr) | 2.85 × 10−2 | RAIS 2018 |
| Log Kow (dimensionless) | 3.1 | ChemIDPlus 2018; IPCS 1997 |
| Log Koc (dimensionless) | 2.69 | RAIS 2018 |
| pKa (dimensionless) | 9.55 | ChemIDPlus 2018 |
Abbreviations: Kow, octanol-water partition coefficient; Koc, organic carbon-water partition coefficient; pKa, acid dissociation constant.
4. Sources and uses
Chlorocresol was included in a survey issued pursuant to Section 71 of CEPA (Canada 2012). In Canada, chlorocresol was not reported to be manufactured above the reporting threshold of 100 kg during the 2011 calendar year, while total import quantities during that same period were reported in a range of 100 to 1000 kgFootnote 4 , for commercial uses as a component in an admixture to concrete (Environment Canada 2013). Additional uses of chlorocresol in Canada are listed in Table 4‑1.
| Use | Details |
|---|---|
| Incidental additivea,b | A component in lubricants used in food processing facilities with the potential for incidental food contact. |
| Medicinal or non-medicinal ingredient in final pharmaceutical, disinfectant or veterinary drug productsc | Medicinal ingredient in a veterinary drug and non-medicinal ingredient in topical creams to treat temporary skin irritations. |
| Medicinal or non-medicinal ingredient in licensed natural health products in Canadad | Non-medicinal ingredient in topical creams used to treat temporary skin irritations. |
| Present in cosmetics, according to notifications submitted under the Cosmetic Regulationse | Notified as present in certain body moisturizer creams/lotions. |
| Active ingredient in registered pest control productsf | Active ingredient in certain material preservatives (e.g., in concrete admixtures and lubricants). |
a Email from Foods Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated January 9, 2018; unreferenced.
b While not defined under the Food and Drugs Act, incidental additives may be regarded, for administrative purposes, as those substances which are used in food processing plants and which may potentially become adventitious residues in foods (e.g. cleaners, sanitizers).
c Email from Therapeutic Products Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated January 2, 2018; unreferenced.
d LNHPD [modified 2018].
e Email from Consumer and Hazardous Products Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated February 9, 2018; unreferenced.
f Email from Pest Management Regulatory Agency, Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated July 13, 2016; unreferenced.
5. Environmental fate and behaviour
5.1 Environmental persistence
According to models used in ERC (ECCC 2016a), chlorocresol is not expected to persist in water, air, sediment or soil.
5.2 Potential for bioaccumulation
Given its low Kow and low bioconcentration factors (ECCC 2016b), chlorocresol is not expected to significantly bioaccumulate in organisms.
6. Potential to cause ecological harm
6.1 Characterization of ecological risk
The ecological risk of chlorocresol was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox 2014), and responses to surveys issued pursuant to section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over and under classification of hazard, exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models. However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for chlorocresol, and the hazard, exposure and risk classification results, are presented in ECCC (2016b).
On the basis of low hazard and low exposure potential according to information considered under ERC, chlorocresol was classified as having a low potential for ecological risk. It is unlikely that this substance is resulting in concerns for the environment in Canada.
7. Potential to cause harm to human health
7.1 Exposure assessment
Chlorocresol was not detected (detection limit 1.0 µg/L) in treated drinking water samples collected province-wide from water treatment plants in Alberta in 2013 (SEAWA 2013). A City of Toronto annual water quality report indicated chlorocresol was not detected (detection limit, 0.5 µg/L) in 19 drinking water samples collected quarterly (Toronto 2003). Liquid sludge samples obtained from 12 wastewater treatment systems (WWTSs) across Canada between September 1993 and February 1994 were analyzed for chlorocresol. Chlorocresol was detected at a single WWTS location, at a maximum concentration of 0.1 mg/kg dry weight. The remaining samples were all reported at concentrations below method detection limits (Webber and Nichols 1995).
According to data from the Canadian Health Measures Survey (CHMS) Cycle 2 (2012), indoor air concentrations of chlorocresol were not detected above the stated detection limit of 0.18 µg/m3 (Zhu et al. 2013).
Given the low volumes of chlorocresol reported in commerce in Canada, and the low frequency of detection of chlorocresol in Canadian drinking water, WWTS sludge and indoor air, exposure of the general population to chlorocresol from environmental media is expected to be minimal.
Chlorocresol is reported to be used in small quantities for commercial purposes in certain building or construction materials as a preservative in concrete admixture (Environment Canada 2013; Lanxess 2020). Consumer exposure to chlorocresol from this use is not expected.
Chlorocresol may be used as a component in the manufacture of lubricants that are used in food processing facilities which may have the potential for incidental food contact. The potential exposure from this use is considered negligible (personal communication, email from Foods Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated January 9, 2018; unreferenced).
Exposure to chlorocresol may occur through the use of certain body moisturizer creams/lotions in which it is present at concentrations up to 0.2% (personal communication, email from Consumer and Hazardous Products Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated June 6, 2018; unreferenced). Chlorocresol was identified in licensed natural health products (LNHPs) as a non-medicinal ingredient in eight anti-itch creams at concentrations up to 0.2% (LNHPD [modified 2018]). When present as a non-medicinal ingredient in pharmaceuticals used to treat skin conditions, such as fungi or eczema, chlorocresol was reported at concentrations up to 0.1% (personal communication, email from Therapeutic Products Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated January 2, 2018; unreferenced). Compared to body moisturizers, which may be used daily, LNHPs and pharmaceuticals used to treat skin conditions have shorter recommended use durations (typically one to four weeks).
In studies with guinea pigs, 0.2% to 1.6% of applied doses remained as free chlorocresol at the exposed (patch) site and 75% of chlorocresol in aqueous suspension permeated the skin (Andersen et al. 1985). A dermal absorption value of 75% was also applied in a risk assessment for chlorocresol completed under Regulation (EU) No 528/2012, which was adopted from the European Food Safety Authority (EFSA) guidance (2012). However, it is considered that up to 100% dermal absorption would be possible when the products are being applied to abraded or broken skin (e.g., for treatment of fungi or eczema) (Brown et al. 2006).
Table 7-1 summarizes exposure scenarios for products available to consumers containing chlorocresol. A dermal absorption value of 75% was applied for scenarios estimating exposure to body lotion, assuming the skin to be in a healthy state.
To estimate exposures to the LNHPs and pharmaceuticals that contain chlorocresol, a scenario for use of an anti-itch cream was selected. LNHPs and pharmaceuticals containing chlorocresol are considered to be applied similarly when used as directed. For this reason, the highest concentration reported in these products (0.2% in an LNHP) was used for estimating exposures. For these scenarios, an adjustment for dermal absorption was not applied as they are being compared with a dermal health effect endpoint.
| Products available to consumers scenario | Maximum concentrationa (%) | Estimated systemic exposure (mg/kg bw/day)b |
|---|---|---|
| Body lotion (infant) | 0.2 | 0.40 |
| Body lotion (toddler) | 0.2 | 0.32 |
| Body lotion (child) | 0.2 | 0.19 |
| Body lotion (adolescent) | 0.2 | 0.18 |
| Body lotion (adult) | 0.2 | 0.21 |
| Anti-itch cream to treat eczema (infant) | 0.2 | 0.80 |
| Anti-itch cream to treat eczema (adult) | 0.2 | 0.20 |
a Body lotion: email from Consumer and Hazardous Products Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated June 6, 2018, unreferenced; Anti-itch cream: LNHPD [modified 2018]. Chlorocresol-containing LNHPs and pharmaceuticals are considered to be applied similarly when used as directed; however, the maximum chlorocresol concentration identified in LNHPs was chosen as it is greater than the maximum chlorocresol concentration identified in pharmaceuticals.
b For comparison with an oral endpoint the estimated systemic exposure for the body lotion scenarios was adjusted using the dermal absorption factor. A dermal absorption value of 75% was applied for body lotion use scenarios, assuming the skin to be in a healthy state. Scenarios for anti-itch cream to treat eczema were not adjusted for dermal absorption as they are being compared with a dermal endpoint.
7.2 Health effects assessment
7.2.1 Toxicokinetics
Cresols in general may be absorbed through the skin, respiratory tract and digestive tract. Following absorption, cresols are metabolized by the liver and then excreted primarily via the kidney or in smaller amounts through the lungs (Andersen 2006).
The absorption, distribution, metabolism and excretion of chlorocresol has been characterized in some laboratory studies. The dermal absorption of chlorocresol was evaluated in a study where four groups of female albino guinea pigs were exposed to various concentrations of chlorocresol solutions via occlusive patches for 24 hours. Exposure solutions consisted of either 0.2 mL of 5% chlorocresol aqueous suspension with Carbomer 941, a saturated aqueous solution of 0.38% chlorocresol, 5% chlorocresol in an oil/acetone (4/1) solution, or a solution of 5% chlorocresol in propylene glycol. The guinea pigs were sacrificed after 96 hours and the skin at the exposure (patch) site was removed for analysis along with the patches. The results of the study indicated that 25% and 46% of the aqueous chlorocresol and saturated aqueous chlorocresol solutions remained on the patches, respectively. Only 0.2% and 0.5% of the aqueous chlorocresol and saturated aqueous chlorocresol solutions remained in the skin at the patch site. In comparison, 65% and 66% of the chlorocresol in propylene glycol and olive oil/acetone solutions, respectively, were found in the patch, with 0.7% and 1.6%, respectively, remaining in the skin at the patch site (Andersen et al. 1985).
Dermal absorption of chlorocresol was investigated in an in vitro study using abdominal skin from SKH-hr-1 mice. Whole skin and skin repeatedly stripped (to remove the stratum corneum) from the abdomen were mounted in a two-compartment diffusion cell, with two half-cells being filled with normal saline. Absorption was measured at 245 nm using a spectrophotometer and permeability coefficients were assessed. The apparent permeability coefficient of chlorocresol for the whole skin and stripped skin were 119 ± 1.8 x 10-3 cm/hour and 241 ± 22 x 10-3 cm/hour, respectively. Estimated permeability coefficients for chlorocresol for viable tissue and stratum corneum were 302 x 10-3 and 235 x10-3 cm/hour, respectively (Huq et al. 1986).
In rats exposed to 300 mg/kg chlorocresol via oral dosing, chlorocresol was reported to be rapidly eliminated through the kidneys. A corresponding study showed no accumulation of chlorocresol in fatty and hepatic tissues of rats that were exposed orally for 13 weeks to feed containing 150 to 1500 ppm chlorocresol (Paulus and Genth 1983).
ECHA (2016) reported that chlorocresol is extensively metabolized in rats and that five metabolic fractions have been observed in urine; however, specific details on the metabolites or metabolic processes were not reported.
7.2.2 Repeated-dose toxicity
In a short-term dermal toxicity study, male and female New Zealand white rabbits (10 per sex per dose) were exposed to 0, 10, 40 or 160 mg/kg bw/day of technical grade 99.9% chlorocresol via cutaneous application five days per week for three weeks (Mobay Chemical Corporation 1980). US EPA (1997) indicated that information on the use of a vehicle was not complete, but that it appeared that chlorocresol was applied without a vehicle. Given this study, US EPA (1997) reported that dermal irritation was observed in all treated groups ranging from slight erythema and very slight edema in the 10 mg/kg bw/day treatment group to severe erythema and slight edema in the 160 mg/kg bw/day treatment group. US EPA (1997) also reported that no chlorocresol-related systemic effects were observed in the 10 and 40 mg/kg bw/day treatment groups; however, a compound-related enhancing effect on nonsuppurative pericholangitis (males and females) and bile duct proliferation (females only) in the liver was observed in the 160 mg/kg bw/day treatment group. US EPA (1997) reported a systemic no observed effect level (NOEL) of 40 mg/kg bw/day and a lowest observed effect level (LOEL) of 160 mg/kg bw/day based on enhanced liver pathology in males and females. Health Canada (PMRA 2013) reported a NOEL of 160 mg/kg bw/day based on the lack of adverse systemic effects at that dose (highest dose tested).
In a sub-chronic oral toxicity study by Madsen et al. (1986), 20 Wistar SPF rats (ten per sex per group) were exposed to 0, 50, 200, or 400 mg/kg bw/day chlorocresol in food-grade soybean oil administered by gavage for 28 days. After 21 days of dosing, blood samples were taken from eight male and eight female rats to examine hematological and clinical chemistry. Organs were also weighed and examined upon necropsy at the termination of the study. A statistically significant decrease in body weight gains was observed in male and female rats exposed to 400 mg/kg bw/day chlorocresol (32% and 41% lower body weight gain for male and female rats, respectively, compared to the controls). No other treatment related effects were reported to be significant (Madsen et al. 1986). Andersen (2006) reported the NOEL to be 200 mg/kg/day. US EPA (2009) reported the no observed adverse effect level (NOAEL) to be 200 mg/kg bw/day and the lowest observed adverse effect level (LOAEL) to be 400 mg/kg bw/day based on the decreases in body weight gain in the exposed rats. The authors considered the decreased body weight gain to be toxicologically significant (US EPA 2009).
In another sub-chronic oral toxicity study by Bayer AG (1992), male and female Wistar rats (20 per sex per group) were exposed to 0, 150, 500 or 1500 ppm (equivalent to approximately 0, 12, 41 or 120 mg/kg bw/day for males and 0, 17, 54 or 167 mg/kg bw/day for females) chlorocresol in the diet for 13 weeks. A decrease in body weight gain (5% to 6%) compared to controls was reported for male rats in the 500 and 1500 ppm treatment groups. No other treatment-related effects were observed. US EPA (2009) reported the NOAEL as the highest dose tested (i.e., 167 mg/kg bw/day) since the toxicological significance of the decreased body weight gain in adult rats is unknown and the study protocol was not described.
In an oral chronic exposure study by Bayer AG (1993), male and female Wistar rats (60 animals per sex per group) were administered chlorocresol in their diet at concentrations of 0, 400, 2000 or 10 000 ppm (equivalent to approximately 0, 21, 103.1 or 558.9 mg/kg bw/day for males and 0, 27.7, 134.3 or 743.5 mg/kg bw/day for females) over two years. Rats were observed daily for clinical signs following exposure to chlorocresol. All major tissues and organs of the euthanized rats, and on rats that were dead or moribund prior to study termination, were examined (Bayer AG 1993). Other than general poor condition in high dose females, no treatment-related clinical signs of toxicity were noted in any of the groups. Body weight for high dose males was significantly lower (up to 8%) compared to controls, throughout the study. For all treated females, body weight was significantly lower throughout the study compared to controls, but, this was only considered to be adverse in the high-dose females since the average body weight decrease was greater than 10%.
While statistically significant decreases in body weight were observed in females for all treated groups, in males, the decreases in body weight were only significant at the highest dose (8%) (Bayer AG 1993). In males, decreases in adrenal gland weights were observed at the mid-dose, where statistically significant decreases in body weight were not observed. The decreases in absolute gland weights were significant at both mid (26%) and high dose (30%) compared to controls. (Bayer AG 1993). Given the significance of the decrease in the adrenal gland weights at the mid dose, in the absence of a significant decrease in body weights, the changes are considered to be toxicologically relevant. The NOAEL for this study was determined to be 21 mg/kg bw/day (400 ppm), with a LOAEL of 103.1 mg/kg bw/day (2000 ppm), based on significant decreases in absolute adrenal gland weights compared to controls.
In male rats in the 2000 and 10 000 ppm treatment groups, a statistically significant increase in the incidence of unilateral and combined unilateral and bilateral degeneration of seminiferous tubules was observed compared to the control groups (Bayer 1993). Male rats within these two dose groups also showed a statistically significant decrease in unilateral and combined unilateral and bilateral spermatozoa in the epididymides in comparison to the controls. Inadequate information was available from this study to characterize the relevance of this effect on reproduction.
7.2.3 Developmental and reproductive toxicity
In a study by Miles Inc. (1992), groups of 25 pregnant Wistar rats were exposed via gavage to 0, 30, 100, or 300 mg/kg bw/day of Preventol CMK (chlorocresol) in 0.5% aqueous methyl cellulose. The rats were dosed once per day on days six through 15 of gestation and gross pathological examinations were performed on gestation day (GD) 20. In the 300 mg/kg bw/day treatment group, clinical signs of toxicity included prostration and convulsions, laboured breathing and bloody nasal exudates. At this dose, six dams died. Pathological findings in these dams included gas-filled intestines and vaginal bleeding in three animals. Decreased food and water intake, and a decrease in mean body weight gain were all observed in dams exposed to 300 mg/kg bw/day chlorocresol at various stages throughout GD 6 to 20 (US EPA 2009). US EPA (1997) also indicated that two dams in the 300 mg/kg bw/day group totally resorbed their litters. During the treatment period, decreased food intake and significantly decreased body weight (25%) compared to controls were noted in dams exposed to 100 mg/kg bw/day chlorocresol. Laboured breathing was noted in two dams in the 100 mg/kg bw/day group following treatment. No clinical or pathological signs of maternal toxicity were observed in the low-dose group (30 mg/kg bw/day). In all groups, no significant treatment-related effects were reported for number of corpora lutea, implantations, live fetuses and live fetuses per sex. Signs of fetotoxicity were observed only in the high-dose group (300 mg/kg bw/day) which included a significant increase in early resorptions as well as a significant decrease in mean fetal weight compared to controls. In the 100 mg/kg bw/day treatment group, a significant skewing of the normal sex ratio from 55.6% males to 45.6% males was observed. No treatment-related fetal malformations were observed at any dose level (US EPA 2009). US EPA (2009) indicated that the maternal NOAEL was 30 mg/kg bw/day and the maternal LOAEL was 100 mg/kg bw/day based on laboured breathing and decreased body weight. US EPA (2009) also indicated the developmental NOAEL was 30 mg/kg bw/day and the developmental LOAEL was 100 mg/kg bw/day based on changes in the sex ratio, which may be indicative of an estrogenic/anti-androgenic effect of the chemical which was supported by the male reproductive endpoints described above from the Bayer AG (1993) study.
The European Union (ECHA 2016), in an evaluation of chlorocresol in biocidal products, also reported on a two-generation reproduction study in Wistar rats exposed to chlorocresol. The dosing regimen of the study was not specified; however, the author noted three separate NOAELs based on the study. A NOAEL of 47 mg/kg bw/day was noted for offspring toxicity based on an unspecified effect on pup weights. A parental NOAEL of 90 mg/kg bw/day was noted which was reportedly based on a significant decrease in body weight gain and on liver and kidney effects observed in the 365 mg/kg/day treatment group. Lastly, a NOAEL for toxicity on fertility of 288 mg/kg bw/day was reported based on increased seminal vesicle weights, which occurred in the 12 000 ppm treatment group. ECHA (2016) also noted that ovarian atrophy, increased metoestrus, decreased dioestrus and atrophy of the vaginal epithelium were observed in first and second generation females in the 12 000 ppm treatment groups. ECHA (2016) also noted that other reports and articles discussed the potential endocrine disruption activity of chlorocresol, most notably in vitro, and that the results support a conclusion that chlorocresol possesses “a slight endocrine disruption potential in vitro”. However, ECHA (2016) noted that based on the sub-chronic studies, teratogenicity studies and chronic/carcinogenic studies, no changes in endocrine function were observed and the two-generation study in rats also showed no indication of endocrine related activity of chlorocresol. Additional details were not available (ECHA 2016).
7.2.4 Genotoxicity and carcinogenicity
Various genotoxicity studies of chlorocresol in Salmonella typhimurium have been completed and summarized in the literature, with all studies reporting negative results (Ames et al. 1975; Bayer AG 1980; Herbold 1991a; Herbold and Lorke 1980; Madsen et al. 1986; Rapson et al. 1980; Zeiger et al. 1992). In a single mutation assay, chlorocresol tested positive in S. typhimurium strain TA97 with metabolic activation, but negative in chromosomal aberration and unscheduled DNA synthesis assays, suggesting chlorocresol is not genotoxic (Durham et al. 2004). The genotoxicity of chlorocresol was also tested in Escherichia coli strain PQ37 and SOS-DNA repair synthesis was induced without metabolic activation (Malaveille et al. 1991).
In vitro and in vivo genotoxicity studies completed for chlorocresol in other mammalian systems, including hamsters, rats and mice, have also produced generally negative results (Cifone 1988; Herbold 1990, 1991b; Lehn 1989; Van Goethem 1991).
According to US EPA (1997, 2009), there is inadequate data to assess the carcinogenic potential of chlorocresol. In the US EPA (1997, 2009) cancer classification, the oral chronic exposure study by Bayer AG (1993) conducted on male and female Wistar rats (60 animals per sex per group) discussed above was the only study referenced. No statistically significant treatment-related effects on the incidence of neoplastic changes were reported. A statistically significant increase in the incidence of pituitary adenomas was noted in females in the mid-dose group, but not the high-dose group, failing to provide a significant dose-related trend. In males, there was a statistically significant decreasing trend for incidence of pituitary adenomas (significant increase noted in the low-dose males but not in mid- or high-dose males), and the incidence was within the historical control range (US EPA 1997, 2009). US EPA (1997) also notes that analogues of chlorocresol tested in NTP bioassays produced negative results.
7.3 Characterization of risk to human health
Chlorocresol is not a naturally occurring substance and is not detected in environmental media in Canada, such as drinking water, indoor air or WWTS sludge, at levels that would result in significant exposure to the general population.
Exposure of the general population to chlorocresol is expected as a result of the substance being present in cosmetics, licensed natural health products, and pharmaceutical products.
Compared to the natural health products or pharmaceuticals identified as including chlorocresol, a greater potential for exposure to chlorocresol is expected to result from its use in certain body moisturizer creams/lotions, as they are typically applied in greater quantities per application, and are used daily (as opposed to intermittent use for treatment of a skin condition).
To calculate margins of exposure (MOEs) for chlorocresol through use of body lotions, the most appropriate toxicological endpoint was considered to be the NOAEL of 21 mg/kg bw/day from the Bayer AG (1993) oral chronic exposure study. The critical effect in this study was a significant decrease in absolute adrenal gland weights, in the absence of significant decreases in body weights, in males.
To calculate MOEs for chlorocresol resulting from the as-directed short-term use of anti-itch creams for the treatment of eczema the most appropriate toxicological endpoint was considered to be the dermal NOEL of 160 mg/kg bw/day from the Mobay Chemical Corporation (1980) short-term dermal toxicity study. This NOEL is also consistent with the point of departure used by Health Canada in the evaluation of the pesticidal uses of chlorocresol (PMRA 2013). If these creams were used on a longer term basis, these exposures would be compared to the chronic oral NOAEL of 21 mg/kg bw/day and MOEs would be similar to those calculated for the use of body lotion.
Table 7‑2 compares the estimated systemic exposure (from Table 7‑1) to the selected critical health effect levels to calculate the MOEs for chlorocresol present in body lotion, and anti-itch cream.
| Exposure scenario | Systemic exposure(mg/kg bw/day)a | Critical effect level(mg/kg bw/day) | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Body lotion(infant) | 0.40 | Oral NOAEL of 21 | decrease in absolute adrenal gland weights | 53 |
| Body lotion(toddler) | 0.32 | Oral NOAEL of 21 | decrease in absolute adrenal gland weights | 66 |
| Body lotion(child) | 0.19 | Oral NOAEL of 21 | decrease in absolute adrenal gland weights | 111 |
| Body lotion(adolescent) | 0.17 | Oral NOAEL of 21 | decrease in absolute adrenal gland weights | 124 |
| Body lotion(adult) | 0.21 | Oral NOAEL of 21 | decrease in absolute adrenal gland weights | 100 |
| Anti-itch cream to treat eczema (infant) | 0.80 | Dermal NOEL of 114b | no evidence of systemic effects | 142 |
| Anti-itch cream to treat eczema (adult) | 0.20 | Dermal NOEL of 114b | no evidence of systemic effects | 570 |
a The calculation of systemic exposure includes an adjustment for dermal absorption for the body lotion use scenarios. A dermal absorption value of 75% was applied for body lotion use scenarios, assuming the skin to be in a healthy state. No dermal absorption value was applied to for the anti-itch cream to treat eczema use scenarios as the treatment scenario is not a chronic exposure and is being compared to a dermal endpoint.
b Dermal NOAEL of 160 mg/kg bw/day was based on a 5 day/week exposure. This value was amortized to adjust for continuous exposure.
The calculated MOEs for use of chlorocresol in body lotion are considered inadequate to account for uncertainties in the health effects and exposure databases. Exposures could be higher than those estimated if the product is being used more frequently due to dry or itchy skin, or if it is being used on abraded or broken skin.
The calculated MOEs for the short-term topical use of licensed natural health products or pharmaceuticals are considered adequate to address uncertainties in the health effects and exposure database for all age groups.
7.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in Table 7‑3.
| Key source of Uncertainty | Impact |
|---|---|
| Dermal absorption was assumed to be 75% for topical products; however, this may be closer to 100%, if the skin is abraded (e.g., for treatment of fungi or eczema). | - |
| There are no chronic animal studies for dermal exposure. | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over or under estimation of risk.
8. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from chlorocresol. It is concluded that chlorocresol does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Considering all the information presented in this screening assessment, it is concluded that chlorocresol meets the criteria under paragraph 64(c) of CEPA as it is entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that chlorocresol meets one or more of the criteria set out in section 64 of CEPA.
It has also been determined that chlorocresol does not meet the persistence or bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
References
Ames BN, McCann J, Yamasaki E. 1975. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian microsome mutagenicity test. Mutat. Res. 31: 347-364. [as cited in CIR 1997].
Andersen KE, Carlsen L, Egsgaard H, Larsen E. 1985. Contact sensitivity and bioavailability of chlorocresol. Contact Dermatitis, 13: 246-251. [as cited in Andersen 2006].
Andersen KE. 2006. Final report on the safety assessment of sodium p-chloro-m-cresol, p-chloro-m-cresol, chlorothymol, mixed cresols, m-cresol, o-cresol, p-cresol, isopropyl cresols, thymol, o-cymen-5-ol, and carcacro. International Journal of Toxicology. 25:29-127.
Bayer AG. 1980. Salmonella/microsome test with PCMC. Unpublished data (Report No. 9122) submitted by COLIPA (1 page). [as cited in CIR 1997 and Andersen 2006].
Bayer AG. 1992. Chronic toxicity and carcinogenicity study in Wistar rats (administration in feed for 104 weeks – interim report) with cover letter dated 03/27/92. NTIS Report No. OTS0535962. [as cited in CIR 1997, Andersen 2006, US EPA 1997, US EPA 2009].
Bayer AG. 1993. Preventol CMK: chronic toxicity and carcinogenicity study in Wistar rats: lab project number: T9030673: 22168. Unpublished study prepared by Bayer AG Institute of Industrial Toxicology. MRID 42784801.
Brown MB, Martin GP, Jones SA, Akomeah FK. 2006. Dermal and transdermal drug delivery systems: Current and future prospects. Drug Delivery, 13 (3): 175-187.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
ChemIDPlus [database]. 1993- . Search results for CAS RN 59-50-7. Bethesda (MD): US National Library of Medicine. [accessed 2018 February 14].
ChemSpider [database]. 2018. Royal Society of Chemistry. [accessed 2018 February 13].
Cifone M, 1988. Parachlorometacresol (Preventol CMK) in the rat primary hepatocyte unscheduled dna synthesis assay: HLA study No.: 10285-0-447: Sponsor Study No.: T3027707. Unpublished study prepared by Hazelton Laboratories America, Inc. 30 p. [cited in US EPA 1997 and US EPA 2009].
[CIR] Cosmetic Ingredient Review. 1997. Final report on the safety assessment of p-chloro-m-cresol. Int J Toxicol. 16:235-268.
Durham J, Bhat VS, Ball GL, Mclellan CJ. 2004. Human health risk assessment for p-chloro-m-cresol to determine drinking water action levels. The Toxicologist. Society of Toxicology 43rd Annual Meeting, Baltimore, Maryland.
[ECCC] Environment and Climate Change Canada. 2016a. Science Approach Document: Ecological Risk Classification of Organic Substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2017 August 9].
[ECHA] European Chemicals Agency. 2015. Committee for Risk Assessment RAC, Annex 1, background document to the opinion proposing harmonised classification and labelling at EU level of chlorocresol; 4-chloro-m-cresol; 4-chloro-3-methylphenol [PDF]. Helsinki (FI): ECHA.
[ECHA] European Chemicals Agency. 2016. Regulation (EU) No 528/2012 concerning the making available on the market and use of biocidal products. Evaluation of active substances, Assessment Report: Chlorocresol (CMK). Product-type PT 2 (Private area and public health area disinfectants and other biocidal products) [PDF]. Helsinki (FI): ECHA.
[EFSA] European Food Safety Authority. 2012. Guidance on Dermal Absorption. EFSA Journal. 10(4): 2665.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Ficheux AS, Wesolek N, Chevillotte G, Roudot AC, 2015. Consumption of cosmetic products by the French population. First part: Frequency data. Food Chem Toxicol. 78:159-169.
[HSDB] Hazardous Substances Data Bank [database]. 1983- . Bethesda (MD): US National Library of Medicine. [updated 2012 February 14; accessed 2018 February 21].
Health Canada. 1995. Investigating human exposure to contaminants in the environment: A handbook for exposure calculations [PDF] . Ottawa (ON): Health Canada, Great Lakes Health Effects Program.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Herbold B, Lorke, D. 1980. Preventol CMK: salmonella/microsome test for detection of point mutagenic effects: Report No. 9122. A translation of: Preventol CMK: salmonella/mikrosomen-test zur utersuchung auf punktmutagen wirkung. (Unpublished study, including German text, received Jul 1, 1981 under 39967-1; prepared by Bayer, AG, West Germany, submitted by Mobay Chemical Corp., Pittsburgh, Pa.; CDL:245551-C). [cited in US EPA 1997].
Herbold B. 1990. Preventol CMK: micronucleus test on the mouse: Lab Project Number: T 3033061. Unpublished study prepared by Bayer AG. 45 p. [cited in US EPA 1997].
Herbold B. 1991a. Preventol CMK: salmonella/microsome test: Lab Project Number: T 4038030. Unpublished study prepared by Bayer AG. 45 p. [cited in US EPA 1997].
Herbold B. 1991b. Preventol CMK: micronucleus test on the mouse: supplement to: Lab Project Number: 18686A: T 3033061. Unpublished study prepared by Bayer Ag. 12 p. [cited in US EPA 1997].Huq AS, Ho NFH, Husari N, Flynn GL, Jetzer WE, Condie L. Permeation of Water Contaminative Phenols Through Hairless Mouse Skin. Arch Environ Contam Toxicol. 15(5):557-566.
[InformedHealth] InformedHealth.org [Internet]. 2006- . Eczema: Steroids and other topical medications. Cologne (DE): Institute for Quality and Efficiency in Health Care (IQWiG). [updated 2017 February 23; accessed 2020 January 21]
[IPCS] International Programme on Chemical Safety. 1997. ICSC: 0131, 4-chloro-m-cresol. Geneva (CH): United Nations Environment Programme; International Labour Organization; World Health Organization. [accessed: 2018 February 18].
Lanxess. 2020. Concrete admixtures [internet]. Leverkusen (DE): Lanxess AG. [accessed 2020 Aug 27]. Product portfolio.
Lehn, H. 1989. Parachlorometacresol (Preventol CMK): mutagenicity study for the detection of induced forward mutations in the CHO-HGPRT assay in vitro: Lab Report No.: T1029785; 17755. Unpublished study prepared by Bayer AG, Dept. of Toxicology. 36 p. [as cited in US EPA 1997].
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2018 Feb 6]. Ottawa (ON): Government of Canada. [accessed 2019 Oct 11].
Madsen C, Andersen PH, Meyer O, Würtzen G. 1986. 4-Chloro-3-methylphenol: Salmonella/mammalian microsome mutagenicity test and subacute toxicity test in rats. Bull Environ Contam Toxicol. 37:651-654. [cited in Andersen 2006; CIR 1997; US EPA 2009].
Malaveille C, Brun G, Bartsch H. 1991. Genotoxicity of ochratoxin A and structurally related compounds in Escherichia coli strains: studies on their mode of action. IARC Monographs. 115:261-266. [cited in Andersen 2006; CIR 1997; US EPA 2009].
Miles Inc. 1992. Initial Submission from Miles Inc to US EPA submitting an enclosed embryotoxicity report on p-chloro-m-cresol with attachments. TSCA Section 8E Submission. US EPA Doc. No. 88-920000850. Fiche. No. OTS0533951. [cited in US EPA 1997, 2009].
Mobay Chemical Corporation. 1980. Subchronic dermal study in rabbits. Unpublished data submitted by COLIPA. Pittsburgh (PA): Mobay Chemical Corporation. [cited in Andersen 2006; CIR 1997; US EPA 1997].
O'Neil MJ. 2006. The Merck Index - an encyclopedia of chemicals, drugs, and biologicals. Readington Township (NJ): Merck and Co., Inc. [cited in HSDB 1983- ].
OECD QSAR Toolbox [Read-across tool]. 2014. Version 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Paulus W, Genth H. 1983. Microbiocidal phenolic compounds - a critical examination. In: Biodeterioration, Vol. 5, ed. Oxley TA, Barry S. New York: John Wiley & Sons. [cited in Andersen 2006: CIR 1997].
[PMRA]. Pest Management Regulatory Agency. 2013. Proposed Re-evaluation Decision: p-Choro-m-cresol and Sodium p-chloro-m-cresolate [PDF]. PRVD2013-03. Ottawa (ON): PMRA.
Rapson WH, Nazar MA, Butsky VV. 1980. Mutagenicity produced by aqueous chlorination of organic compounds [PDF]. Bull Environ Contam Toxicol. 24:590-596. [cited in Andersen 2006: CIR 1997].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2006. Cosmetic fact sheet to assess the risk for the consumer [PDF]. Updated Version for ConsExpo 4. Bilthoven (NL): RIVM. RIVM Report No. 320104001/2006. [accessed 2018 February 14].
[RAIS] Risk Assessment Information System [database]. 2018. Search results for CAS RN 59-50-7. Washington (DC): US Department of Energy. [accessed 2018 February 21].
Rutter et al. 1979. Acute dermal administration study in male and female rabbits. Preventol CMK. Hazleton Laboratories America, Inc., Virginia, USA Project No. 339-108. [as cited in ECHA 2015].
[SEAWA] South East Alberta Watershed Alliance. 2013. Environmental analytical report [final report; 9 Aug 2013. PDF]. Calgary (AB).
[Toronto]. 2003. City of Toronto. Water Quality Quarterly Report. Toronto Works and Emergency Services, Waste and Wastewater Services.
[US EPA] United States Environmental Protection Agency. 1997. Reregistration Eligibility Decision (RED) [PDF]. P-chloro-m-cresol. Prevention, Pesticides and Toxic Substances (7508W). Washington (DC): US EPA. EPA-738-R-96-008.
[US EPA] United States Environmental Protection Agency. 2009. Provisional peer-reviewed toxicity values for 4-chloro-3-methylphenol (p-chloro-m-cresol) (CAS RN 59-50-7) [PDF]. Washington (DC): US EPA.
Van Goethem D. 1991. Mutagenicity test on Preventol CMK in the rat primary hepatocyte unscheduled DNA synthesis assay: Lab Project Number: 10285-0-447: T3027707. Unpublished study prepared by Hazleton Labs America, Inc. 6p. [cited in US EPA 1997].
Webber MD, Nichols JA. 1995. Organic and metal contaminants in Canadian municipal sludges and a sludge compost. Burlington (ON): Wastewater Technology Centre, Rockcliffe Research Management Inc. 168 p.
Wu X, Bennett DH, Ritz B, Cassady DL, Lee K, Hertz-Picciotto I. 2010. Usage pattern of personal care products in California households. Food Chem Toxicol. 48:3109-3119.
Zeiger E, Andersen B, Haworth S, Lawlor T, Mortelmans K. 1992. Salmonella mutagenicity tests: V. Results from the testing of 311 chemicals. Environ Mol Mutagen. 19:2-141. [cited in Andersen 2006; CIR 1997].
Zhu J, Wong SL, Cakmak S. 2013. Nationally representative levels of selected volatile organic compounds in Canadian residential indoor air: population based survey. Environ. Sci. Technol. 47(23): 13276-13283.
Appendices
Appendix A - Exposure parameters for estimating human exposure to chlorocresol
Dermal exposure to products containing chlorocresol was estimated using the following equation,
EAD = (C × Q × F × A ) ÷ BW
EAD: Estimated absorbed dose (mg/kg bw/day)
C: Concentration of chlorocresol in product (%)
Q: Quantity of product applied per event (mg per event)
F: Frequency of events per day (events per day)
A: Dermal absorption (%) (when applicable)
BW: Body weight (kg)
Values selected for the parameters included in this equation were obtained from published literature and described in Table A-1.
Exposure scenarios for anti-itch creams consider directions for use, as printed on product labels and packaging. Consumers are directed to discontinue use of these types of products after several weeks (details described in Table A-1).
Unless specified otherwise, the parameter values are taken from relevant ConsExpo Fact Sheets (RIVM 2006) for the scenario presented.
| Exposure scenario | Model input parameter |
|---|---|
| Body lotion (infant) | Frequency of Use: 0.8 applications per day (Ficheux et al. 2015) Product amount: 2.5 g/application (Ficheux et al. 2015). Includes adjustment by a factor of 0.637 to account for skin surface area difference between infant and toddler (Health Canada 1995). Dermal absorption value: 75% (Andersen et al. 1985, EFSA 2012) Body weight: 7.5 kg (Health Canada 1998) |
| Body lotion (toddler) | Frequency of Use: 0.8 applications per day (Ficheux et al. 2015) Product amount: 4.1 g/application (Ficheux et al. 2015) Dermal absorption value: 75% (Andersen et al. 1985, EFSA 2012) Body weight: 15.5 kg (Health Canada 1998) |
| Body lotion(child) | Frequency of Use: 0.8 applications per day (Wu et al. 2010) Product amount: 5.0 g/application (Ficheux et al. 2015). Includes adjustment by a factor of 0.531 to account for skin surface area difference between child and adult (Health Canada 1995). Dermal absorption value: 75% (Andersen et al. 1985, EFSA 2012) Body weight: 31 kg (Health Canada 1998) |
| Body lotion(adolescent) | Frequency of Use: 0.8 applications per day (Wu et al. 2010) Product amount: 8.7 g/application (Ficheux et al. 2015). Includes adjustment by a factor of 0.890 to account for skin surface area difference between adolescent and adult (Health Canada 1995). Dermal absorption value: 75% (Andersen et al. 1985, EFSA 2012) Body weight: 59.4 kg (Health Canada 1998) |
| Body lotion(adult) | Frequency of Use: 1.0 application per day (Ficheux et al. 2015; Wu et al. 2010) Product amount: 10 g/application (Ficheux et al. 2015) Dermal absorption value: 75% (Andersen et al. 1985, EFSA 2012) Body weight: 70.9 kg (Health Canada 1998) |
| Anti-itch cream to treat eczema (infants) | Frequency of Use: 2 applications per day (InformedHealth 2006- ). Product amount: 1.5 g per application (InformedHealth 2006- ). Chlorocresol concentration: 0.2% (LNHPD [modified 2018]). Body weight: 7.5 kg (infants, Health Canada 1998) |
| Anti-itch cream to treat eczema (adults) | Frequency of Use: 2 applications per day (InformedHealth 2006- ). Product amount: 3.5 g per application (InformedHealth 2006- ). Chlorocresol concentration: 0.2% (LNHPD [modified 2018]). Body weight: 70.9 kg (adults, Health Canada 1998) |
