Screening Assessment Report
Phenol, 2-(2H-benzotriazol-2-yl)-4,6-bis(1,1-dimethylpropyl)- (BDTP)
Chemical Abstracts Service Registry Number
25973-55-1
Environment and Climate Change Canada
Health Canada
May 2016
Table of Contents
- Synopsis
- 1. Introduction
- 2. Substance Identity
- 3. Physical and Chemical Properties
- 4. Sources
- 5. Uses
- 6. Releases to the Environment
- 7. Environmental Fate
- 8. Potential to Cause Ecological Harm
- 9. Potential to Cause Harm to Human Health
- 10. Conclusion
- References
- Appendix A: Critical Body Burden (CBB) approach for BDTP
- Appendix B: Environmental exposure monitoring for BDTP and other phenolic benzotriazole UV stabilizers in other countries
- Appendix C: Upper-bounding estimates of daily intakes of BDTP for various age groups
- Appendix D: Summary of health effects information for BDTP (CAS RN 25973-55-1) and analogues BBMPP (CAS RN 70321-86-7) and BMP (CAS RN 2440-22-4)
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Ministers of the Environment and Climate Change and of Health have conducted a screening assessment on Phenol, 2-(2H-benzotriazol-2-yl)-4,6-bis(1,1-dimethylpropyl)-, hereinafter referred to as BDTP, Chemical Abstracts Service Registry Number (CAS RN) 25973-55-1. This substance was identified as a priority for screening assessments because it met the categorization criteria under subsection 73(1) of CEPA.
BDTP does not occur naturally in the environment. The substance is not manufactured in Canada; results from a survey conducted under the authority of section 71 of CEPA indicate that, in the year 2000, between 100 000 and 1 000 000 kg of the substance was imported into Canada for use as an ultraviolet light absorber in automotive and industrial coatings, paints and plastics. Based on more recent information provided by stakeholders on a voluntary basis, Canadian import and use quantities of BDTP were in the range of 10 000 and 100 000 kg in 2012 and 2013.
BDTP has low solubility in water, a high octanol-water partition coefficient and a low vapour pressure. It is not expected to be significantly present in air and is not subject to long-range atmospheric transport. If released to water, the substance is likely to largely partition to particles and organic matter because of its hydrophobic nature, consequently ending up in sediment. If released to soil, it remains in that medium.
Experimental data indicate that BDTP does not degrade rapidly in water, soil or sediment. Empirical data and model predictions also suggest that the substance has the potential to bioconcentrate and bioaccumulate in aquatic organisms and may biomagnify in trophic food webs.
In Canada, BDTP is expected to be primarily released from industrial uses to surface water and ultimately partition in sediment. In a recent wastewater monitoring project, the substance was found in very low concentrations in influents and effluents of the wastewater treatment systems, biosolids, surface water and sediment in Canada. It has also been found in soil and biota in other countries.
Ecological assessment
To evaluate potential exposure to BDTP in the Canadian aquatic environment, predicted environmental concentrations (PECs) were conservatively estimated for two industrial sites based on the highest use quantities identified for each site, aiming to characterize the industrial releases of this substance to surface waters from the manufacture of plastics and from the manufacture of paints and coating products. To deal with uncertainty associated with the industrial uses of BDTP and potential non-representativeness of the selected sites, a number of generic scenarios were also developed. Both the short-term concentration near the BDTP discharge location and the longer-term exposure to aquatic organisms in the receiving water body were estimated.
To assess the exposure of fish and wildlife, the aquatic PECs were used to estimate the tissue residue of BDTP in mid-trophic level fish, which was further applied to calculate the total daily intakes (TDI) for fish-consuming terrestrial organisms (mink and river otters) as indicators of exposure. Exposure of BDTP in sediment was also estimated; however, due to a lack of sediment effects data, a risk quotient analysis was not conducted for this compartment.
The only empirical toxicity data available for BDTP are from acute toxicity studies on aquatic organisms reporting no effect at the water saturation level. Given the poor bioavailability of the test substance in water, especially during a short-term exposure, the uptake rate of the substance from water alone may not be adequate to reach the internal effect concentration in test organisms. Therefore, experimental data reported from acute aquatic toxicity studies are considered insufficient to characterize the toxicity of this substance.
A critical body burden (CBB) approach and a wildlife exposure assessment were used to characterize the effect of BDTP on aquatic and wildlife organisms. In the CBB approach, the acute and chronic external effect concentrations were estimated for fish, based on the bioaccumulation potential of this substance and the internal effect thresholds for hindered phenols. In the wildlife exposure assessment, the effect on terrestrial wildlife was characterized by the chronic toxicity reference values (TRVs) determined for mink and river otters, which were developed based on data from a repeated dose toxicity study in rats.
In the two industrial site specific scenarios, both the short-term concentrations near the discharge point and the longer-term exposure concentrations in the receiving water were found to be below the corresponding external effect concentrations determined for fish in the CBB approach. This suggests that the risk to aquatic organisms in the surrounding Canadian environment is low. For wildlife, TDIs for mink and river otters were below their respective chronic toxicity thresholds, indicating that the risk to terrestrial wildlife associated with a long-term consumption of BDTP-contaminated fish is not significant for the sites selected to represent the plastics manufacture and the paints and coatings sector.
In the generic scenarios, high tissue residue concentrations could be found in fish if the total release of BDTP from a plastics manufacturing company was assumed to enter a small river. Assuming the wildlife receptor spends 100% of its time in the contaminated area and eats contaminated fish, long-term TDIs of BDTP by terrestrial wildlife are close to or slightly higher than the chronic TRVs. Considering that a potential risk was identified only in the generic scenarios when very conservative assumptions were made, more weight of evidence is given to the outcomes from the site specific scenarios.
Based on the overall results of the ecological assessment, it is concluded that BDTP does not meet the criteria under paragraph 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity, or that constitute or may constitute a danger to the environment on which life depend.
Human health assessment
The health effects database for BDTP is limited, but chronic toxicity studies for selected analogues indicated no evidence of carcinogenicity in experimental animals, and the available data do not indicate genotoxic potential. Based on the collective information on BDTP and selected analogues, the primary health effect associated with exposure to BDTP is liver toxicity. However, exposure of the general population of Canada to BDTP through environmental media is expected to be minimal, and exposure from use of consumer products is not expected. Based on this, the risk to human health is considered to be low. It is concluded that BDTP does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Overall Conclusion
Based on the information available, it is concluded that BDTP does not meet any criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to sections 68 and 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and Climate Change and the Minister of Health conduct screening assessments of substances to determine whether these substances present or may present a risk to the environment or to human health.
A screening assessment was undertaken on phenol, 2-(2H-benzotriazol-2-yl)-4,6-bis(1,1-dimethylpropyl)-, Chemical Abstracts Service Registration Number (CAS RN) 25973-55-1, hereinafter referred as BDTP. BDTP was identified as a priority for assessment because it met the criteria for bioaccumulation and inherent toxicity to non-human organisms during the categorization of the Domestic Substances List (DSL).
Screening assessments focus on information critical to determining whether a substance meets criteria set out in section 64 of CEPA. Screening assessments examine scientific information and develop conclusions by incorporating a weight-of-evidence approach and precautionFootnote[1].
This screening assessment includes consideration of information on chemical properties, hazards, uses and exposure to BDTP. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents, stakeholder research reports, voluntary submissions, and the environmental monitoring project up to August 2014 for ecological sections and March 2013 for human health sections of the document. When available and relevant, information presented in hazard assessments from other jurisdictions was considered.
An industry survey was conducted in 2001 through a Canada Gazette Notice issued under authority of section 71 of CEPA (Canada 2001). This survey collected data on the Canadian manufacture and import on a subset of DSL substances (Environment Canada 2001b). Key studies and submissions from industry were critically evaluated; modelling results were used where necessary to reach conclusions. More recent information on the industrial uses and the import quantity of BDTP in Canada was obtained via stakeholder consultation in 2011-2012 and voluntary surveys in 2014.
Evaluation of risk to human health involves consideration of data relevant to estimation of exposure of the general population, as well as information on health hazards. Decisions for human health are based on the nature of the critical effect and/or margins between conservative effect levels and estimates of exposure, taking into account confidence in the completeness of the identified databases on both exposure and effects, within a screening context.
The screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents a summary of the critical information upon which the conclusion is based.
This screening assessment was prepared by staff in the Existing Substances programs at Health Canada and Environment and Climate Change Canada. The ecological portion of this assessment has undergone external written peer review and consultation. dditionally, the draft of this screening assessment was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening risk assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
The critical information and considerations upon which the assessment is based are summarized below.
2. Substance Identity
For the purpose of this assessment phenol, 2-(2H-benzotriazol-2-yl)-4,6-bis(1,1-dimethylpropyl)- is referred to as BDTP, an acronym derived from the DSL inventory name. Information relevant to the identity of BDTP is presented in Table 2-1. BDTP has other inventory names and chemical names, which can be found in National Chemical Inventories (NCI 2014).
| CAS Registry Number | 25973-55-1 |
|---|---|
| DSL Inventory name | Phenol, 2-(2H-benzotriazol-2-yl)-4,6-bis(1,1-dimethylpropyl)- |
| Chemical group | Organic |
| Chemical sub-group | Benzotriazole, Phenol |
| Chemical formula | C22H29N3O |
| Chemical structure |  |
| SMILESFootnote Table 2-1[a] string | Oc(c(cc(c1)C(CC)(C)C)C(CC)(C)C)c1n(nc(c2ccc3)c3)n2 |
BDTP is a phenolic benzotriazole compound. The representative chemical structure of phenolic benzotriazoles is illustrated in Figure 1 below. Substituents (R1 and R2) on the phenolic group vary. A few other phenolic benzotriazole compounds are included in this assessment as structural analogues for BDTP (see Table 2-2), most of which have either R1 or R2 or both R1 and R2 as a tertiary carbon group. These structurally analogues have also been used as the UV stabilizers. Available experimental data for these analogues are considered in the assessment.

Figure 1. The basic structure of phenolic benzotriazole compounds
| CAS RN | Molecular Mass (g/mol) |
Chemical Structure |
|---|---|---|
| 25973-55-1 (BDTP) |
351 | 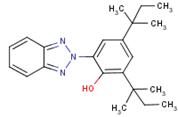 |
| 36437-37-3 | 323 | 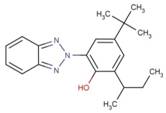 |
| 3846-71-7 | 323 | 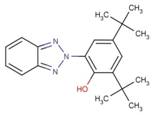 |
| 3896-11-5 | 316 | 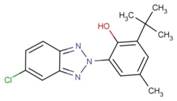 |
| 70321-86-7 | 448 | 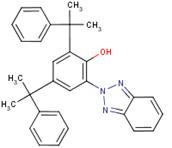 |
| 3147-75-9 | 323 | 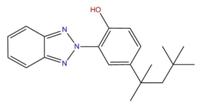 |
| 3864-99-1 | 358 | 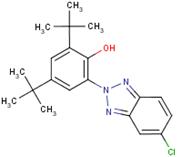 |
| 2440-22-4 | 225 | 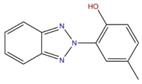 |
3. Physical and Chemical Properties
Data relevant to the physical and chemical properties of BDTP and its structural analogues are presented in Table 3-1, and are used for predicting the environmental fate and effects of BDTP in this assessment.
| Property | Substance or analogue CAS RN |
Type | Value | ConditionsFootnote Table 3-1[a] | Reference |
|---|---|---|---|---|---|
| Physical state | BDTP | Experimental | Solid (yellow powder) | 20°C, 101kPa | US EPA 2009 |
| Melting point (°C) | BDTP | Experimental | 80-83 | - | US EPA 2009 |
| Melting point (°C) | Experimental | 81.2 | - | ECHA 2013 | |
| Boiling point (°C) | BDTP | Experimental | greater than 180 | - | ECHA 2013 |
| DensityFootnote Table 3-1[b] (kg/m3) |
BDTP | Experimental | 1.17 × 103 | 20 °C | ECHA 2013 |
| Vapour pressure (Pa) |
BDTP | Experimental | 4.7 × 10-6 | 20 °C | ECHA 2013 |
| Water solubility (mg/L) |
BDTP | Modelled | 0.015 | - | WSKOWWIN (EPI Suite 4.0) |
| Water solubility (mg/L) |
BDTP | Experimental | less than 0.001 | 20 °C, pH=6.32-6.43 | ECHA 2013 |
| Water solubility (mg/L) |
2440-22-4 | Experimental | 0.173 | - | US EPA 2009 |
| Water solubility (mg/L) |
70321-86-7 | Experimental | 0.04 | - | US EPA 2009 |
| Henry's Law constant (Pa·m3/mol) | BDTP | Calculated | 1.65 | - | Based on HLC=VP/WS |
| Octanol/water partition coefficient (log Kow) | BDTP | Modelled | 7.25 | - | EPI Suite 4.0 |
| Octanol/water partition coefficient (log Kow) | BDTP | Experimental | greater than 6.5 | 23 °C, pH=6.4 | ECHA 2013 |
| Octanol/water partition coefficient (log Kow) | 2440-22-4 | Experimental | 4.2 | - | US EPA 2009 |
| Octanol/water partition coefficient (log Kow) | 2440-22-4 | Experimental | 4.3 | - | Hansch et al. 1995 |
| Octanol/water partition coefficient (log Kow) | 70321-86-7 | Experimental | 6.5 | - | US EPA 2009 |
| Cross-section diameter (nm) | BDTP | Calculated | 1.45-1.60 | - | CPOPs 2008 |
BDTP is a light yellow powder at the room temperature and has a low vapour pressure (measured as 4.7 × 10-6 Pa) and a moderate-low Henry's Law constant (calculated as 1.65 Pa·m3/mol). Partitioning into the atmosphere is anticipated to be minor for this substance. Similar to its analogues in the phenolic benzotriazoles group, BDTP has low water solubility (estimated as 0.015 mg/L and measured as less than 0.001 mg/L) and high octanol-water partition coefficient (log Kow estimated as 7.25 and measured as greater than 6.5). The substance has a density greater than that of water (measured as ~1.17 × 103 kg/m3).
4. Sources
BDTP is an anthropogenic substance and as such does not naturally occur in the environment.
A survey was conducted under section 71 of CEPA to collect data on the manufacture, import and uses of BDTP in Canada for the year 2000 (Canada 2001). According to results, no company manufactured this substance in Canada in 2000 above the reporting threshold of 100 kg/year (Environment Canada 2001b). Several companies reported importing a total of between 100 000 and 1 000 000 kg of BDTP into Canada (Environment Canada 2001b). Such information was considered out-of-date and not representative of the current use of this substance in the country.
In 2011, stakeholders provided new information relevant to the current use of this substance in Canada. No manufacture of BDTP in Canada was reported, but 10 000 to 100 000 kg of BDTP are estimated to have been imported into Canada in 2010, to be used at more than 10 industrial facilities across the country (emails between stakeholders and Chemicals Sector Directorate, Environment Canada, 2011-2012, unreferenced). A few additional industrial users were identified via responses to voluntary surveys; the total import quantity identified remained in the same range of 10 000 to 100 000 kg for the year 2013 (Environment Canada 2014).
The quantities reported above did not include quantities present in imported finished articles. No information is available on the total volume of BDTP in finished articles that has been imported into Canada in any recent year.
BDTP has been identified as a High Production Volume (HPV) substance in the United States with an annual production volume between 1 to 10 million pounds (approximately 455 000 to 4 555 000 kg) from 1986 to 2006 in the United States (US EPA 2011). In 2012, the national production volume for this substance is 2 246 476 pounds (approximately 1 019 000 kg) (US EPA 2014).
The United Kingdom Environment Agency published a report on prioritization outcomes of approximately 8000 substances in commerce in the European market in the range of 10 tonnes/year to 1000 tonnes/year (UK Environment Agency 2010). BDTP and a few other phenolic benzotriazole chemicals listed in Table 2-2 (CAS RN 3147-75-9, CAS RN 36437-37-3, CAS RN 3846-71-7, and CAS RN 3864-99-1) were identified in a list of approximately 100 substances with a high priority for further investigation, based on criteria of persistence and bioaccumulation potentials.
BDTP has been reported to be used in Nordic countries since 1999 (SPIN 2012). Reported use quantities ranged from 13 to 45 tonnes/year during 1999 to 2007; however, a decrease was noted in the most recent years recorded (8.1 tonnes in 2008, 6 tonnes in 2009 and 2010, and 2.3 tonnes in 2011, as originally reported, SPIN 2012).
5. Uses
Globally, BDTP is used in a variety of products including automotive and industrial coatings and paints, as well as plastic additives. BDTP reduces or prevents the absorption of ultraviolet (UV) light by chromophores, which in an excited state can form radicals that may have damaging effects on materials or alter their properties (PBA 2001).
Data compiled for the Domestic Substances List, which included production and use data for Canada in 1986, indicated that BDTP was used for industrial purposes only, with usage of 63% being from the plastics sector and 37% for paint and coatings in the automotive industry (Environment Canada 1986). According to results from section 71 survey, uses in the year 2000 were primarily as UV absorbers for paint and coatings in the automotive industry and for polymer additives in the plastics sector (Environment Canada 2001b). BDTP is identified to be used as an additive in the non-food contact layer in food packaging materials, which are used for frozen or refrigerated products. Therefore, BDTP would not be expected to be present in food. This substance has not been identified to be used/present in formulations of incidental additives (Food Directorate, Health Canada, unreferenced).
Industrial uses of BDTP were also identified as a result of a section 71 notice under CEPA for the year 2000 (Environment Canada 2001b). Fewer than four companies reported the use of BDTP in automotive paint at concentrations of 0 - 2.0% by weight (Environment Canada 2001b). Fewer than four companies also reported that BDTP is used as a sealant in the manufacture of automobiles.
According to recent information obtained from stakeholder consultation and voluntary surveys, the use of BDTP in Canada remains the same (emails between stakeholders and Chemicals Sector Directorate, Environment Canada, 2011-2012, unreferenced; Environment Canada 2014).
6. Releases to the Environment
The National Pollutant Release Inventory (NPRI 1994-2013) provides information on releases and transfers of key pollutants in Canada; however, BDTP is not a reportable substance.
According to the outcomes from a section 71 survey (Environment Canada 2001b) and follow-ups with stakeholders (emails between stakeholders and Chemicals Sector Directorate, Environment Canada, 2011-2012, unreferenced), BDTP was not reported as being manufactured in Canada; however, the substance was imported into the country and used as a UV absorber in the manufacture of plastics and coatings materials. According to the industrial uses of BDTP, the substance is expected to be released to surface waters. It may also potentially enter soil from wastewater biosolids which are commonly used for soil enrichment as well as from the disposal of products that degrade and release the substance.
Dispersive use of this substance is not anticipated, and the ultimate disposal of the BDTP-containing end-use products (e.g., paints and adhesives) is not addressed in the assessment, mainly because BDTP is expected to be contained in the polymer matrix like a plastic article or a coating product and its release is unlikely. For recycling unwanted or end-of-life vehicles, most of the ferrous parts are expected to be reused or subject under materials recycling; the plastic parts, if not recycled, are landfilled. Therefore, releases from these sources are expected to be negligible and not considered in the assessment. Details are presented in the section of Ecological Exposure Assessment.
7. Environmental Fate and Behaviour
7.1 Environmental Distribution
Based on its physical and chemical properties (Table 3-1), the environmental fate of BDTP was predicted using Level III fugacity modelling (EQC 2003).
Three assumptions for mode-of-entry were selected to explore the fate and transport of BDTP in air, water, soil and sediment. The results from EQC are presented in Table 7-1 below.
| Substance released to | Partitioning in air (%) | Partitioning in water (%) | Partitioning in soil (%) | Partitioning in sediment (%) |
|---|---|---|---|---|
| Air (100%) | 0.0 | 0.4 | 78.5 | 21.1 |
| Water (100%) | 0.0 | 1.9 | 0.0 | 98.1 |
| Soil (100%) | 0.0 | 0.0 | 99.9 | 0.1 |
When it is assumed that 100% of BDTP is released to air, BDTP is expected to partition mainly to soil and sediment, with trace amounts residing in water but none partitioning to air. This is supported by BDTP's density (~1.17 g/cm3), low vapour pressure (4.7 × 10-6 Pa) and moderate-low Henry's Law constant (calculated 1.65 Pa·m3/mole).
When it is assumed that 100% of BDTP is released to water, it is expected to adsorb to suspended solids in sediments, due to the high log Kow value (estimated 7.25). Results of the Level III simulation for release to water show that the majority of BDTP will reside in the solid phase (suspended sediment and bed sediments), while a small amount will reside in the aqueous phase (water column). Volatilization from surface water is expected to be negligible, based upon the low vapour pressure and the Henry's Law constant.
When it is assumed that 100% of BDTP is released to soil through, for example, applications of wastewater sludge to moist agricultural soils, most of the mass fraction will partition in the same medium associated with solids for the same reasons described for sediment (high log Kow).
7.2 Environmental Persistence
For assessing the environmental persistence and bioaccumulation of BDTP, relevant experimental information has been obtained from a literature search on the substance and its analogues. QSAR models were also used to estimate potentials for biodegradation and bioaccumulation.
Considering the chemical structure, the experimental data, the biodegradation model results, BDTP is expected to degrade very slowly in the environment.
7.2.1 Experimental Studies on Environmental Persistence
In the atmosphere, BDTP may react with photochemically produced hydroxyl radicals at an estimated reaction rate of 1.58 × 10-11 cm3/molecule-sec (AOPWIN 2008), which translates to an estimated half life in the atmosphere of 0.679 days, assuming a hydroxyl radical concentration of 1.5 × 106 OH/cm3, a 12 hour day, and a first order reaction. Based its low vapour pressure and short estimated half-life, the substance is not expected to be subject to long range transport in the atmosphere.
Experimental data for the degradation of BDTP and its analogues in water and/or activated sludge are presented in Table 7-2.
| Substance or analogue CAS RN | Concentration (mg/L) |
Media | Degradation Endpoint and Value | Reference |
|---|---|---|---|---|
| BDTP | 10 | Activated sludge | 28-day Biodegradation = 8% | PBA 2001 |
| BDTP | 20 | Activated sludge | 28-dayBiodegradation = 2% | PBA 2001 |
| 3846-71-7 | 100 | Activated sludge | 28-day Biodegradation = 0 | CHRIP c2008 |
| 3864-99-1 | 100 | Activated | 14-day Biodegradation = 0 | CHRIP c2008 |
Based on its chemical structure, BDTP is not expected to degrade rapidly. Indeed, in water, biodegradation does not appear to be a significant removal mechanism for BDTP. The substance has shown limited biodegradation in the OECD CO2 Evolution test (301B) (PBA 2001). At concentrations of 10 mg/L and 20 mg/L, BDTP was observed to have 8 and 2% degradation, respectively, after 28 days. The test results indicate that the substance does not biodegrade rapidly in water.
Experimental biodegradation data of the other phenolic benzotriazoles have been found in the Chemical Risk Information Platform (CHRIP) database of National Institute of Technology and Evaluation (NITE) in Japan. Japanese ministries (Ministry of Health, Labour and Welfare (MHLW), Ministry of Economy, Trade and Industry (METI), and Ministry of the Environment (MOE)) concluded BDTP to be non-degradable, according to the Chemical Substances Control Law in Japan, although its biodegradation data are not publicly available (CHRIP c2008). Two analogues, CAS RN 3846-71-7 and CAS RN 3864-99-1, both showed 0% degradation in a 28- and a 14-day biodegradation study respectively (see Table 7-2). The ministries also concluded another structural analogue (CAS RN 36437-37-3) to be persistent (CHRIP c2008); however details of the biodegradation studies are not published.
BDTP does not contain functional groups that are expected to undergo hydrolysis in aerobic environments. This applies primarily to the portion of a substance that is present in the environment in the dissolved form, recognizing that a significant proportion would also likely exist in dispersed form as solid particles, which is expected to have reduced potential hydrolysis.
Environmental monitoring data in other jurisdictions have provided additional information relevant to the degradation of BDTP. The substance was detected in samples of sediment cores collected from Narragansett Bay, in Rhode Island, United States of America (USA) (Reddy et al. 2000; Hartmann et al. 2005). This location was near an industrial plant that had manufactured BDTP; however the production had been stopped 12 years prior to the sampling year. These findings suggest slow degradation of BDTP in anaerobic sediments.
7.2.2 Model Predictions for Environmental Persistence
The environmental persistence of BDTP was also examined using predictive QSAR models, produced by Syracuse Research Corporation's BIOWIN Biodegradation Probability Program (BIOWIN 2008) and CATABOL (c2004-2008), for estimating aerobic biodegradation in water. The model prediction is based on the chemical structure of the subject chemical. BIOWIN and CATABOL outcomes for BDTP are presented in Table 7-3 below.
| Fate Process | Model | Model Result and Prediction |
|---|---|---|
| Primary biodegradation (aerobic) | BIOWIN 2008 Sub-model 4: Expert Survey (Qualitative Results) |
3.07Footnote Table 7-3[a].1 "biodegrades fast" |
| Ultimate biodegradation (aerobic) | BIOWIN 2008 Sub-model 3: Expert Survey (Qualitative Results) |
2.05[a] "biodegrades slowly" |
| Ultimate biodegradation (aerobic) | BIOWIN 2008 Sub-model 5 (MITI Linear Model Probability) |
0.02Footnote Table 7-3[b].1 "biodegrades slowly" |
| Ultimate biodegradation (aerobic) | BIOWIN 2008 Sub-model 6 (MITI Non-Linear Model Probability) |
0.01[b] "biodegrades slowly" |
| Ultimate biodegradation (aerobic) | CATABOL c2004-2008 % 28-day BOD (biological oxygen demand) |
28-day BOD 0.02 "biodegrades slowly" |
It is noted that the outcome from a primary biodegradation model (BIOWIN Sub-model 4) suggests a rapid biodegradation for BDTP; however results from all ultimate biodegradation models indicate a slow biodegradation for the substance. The probability models (BIOWIN sub-models 5 and 6) suggest that BDTP does not biodegrade rapidly (Table 6b). All probability results are less than 0.3, which is the cut-off value suggested by Aronson et al. (2006) to identify a substance as having a half-life greater than 180 days. Further, the ultimate degradation models (BIOWIN sub-model 3 and CATABOL) also predict that this substance does not biodegrade rapidly in water.
When the results of the probability and all degradation models are considered, there is model consensus suggesting the ultimate biodegradation half-life in water is greater than 182 days. This finding is consistent with what would be expected for this chemical's structure (i.e., few degradable functional groups, solid sparingly insoluble particle).
Specific degradation information for BDTP in soils and sediments was not available. To extrapolate a half-life in water to half-lives in soil and sediment, Boethling's factors (t1/2 water : t1/2 soil : t1/2 sediment = 1:1:4) from Boethling et al. 1995 can be used. Based on the above empirically derived water half-life of greater than or equal to 182 days and the extrapolation factor of one, half-life in soil is expected to be greater than or equal to 182 days. For sediment, half-life is expected to be four times higher (i.e. greater than or equal to 365 days).
Considering the chemical structure, the experimental data, the biodegradation model results, BDTP is expected to be persistent in water, soil and sediment. Therefore, it is proposed that BDTP meets the regulatory criteria for the persistence in water, soil and sediment as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
7.3 Potential for Bioaccumulation
Based on the measured BCF, modelled BAFs, high lipophilicity, and a low metabolic transformation rate, BDTP is considered to possess a high bioaccumulation potential in organisms, suggesting that the exposure to this substance is expected largely via the food intake. There is a need to assess effects due to a secondary poisoning in environmental organisms at a higher trophic level, including wildlife. Coupled with a high potential for persistence, greater exposure is also very likely in the near field.
7.3.1 Empirical Bioconcentration Factor (BCF)
Empirical BCFs for BDTP and its structural analogues have been reported from studies using carp (CHRIP c2008; ECHA 2013). Testing concentrations of substances used in these studies and the outcomes within the testing periods are summarized in Table 7-4.
| Substance or Analogue CAS RN | Treatment Concentration (mg/L) | Bioconcentration Factor (BCF) (L/kg) | Testing Period (days) |
Reference |
|---|---|---|---|---|
| BDTP | 1 × 10-4 | 940 | 60 | CHRIP c2008 |
| BDTP | 1 × 10-5 | 1800 | 60 | CHRIP c2008 |
| BDTP | 1 × 10-5 | 2400 | 60 | CHRIP c2008 |
| BDTP | 1 × 10-3 | 1405 | 28 | ECHA 2013 |
| BDTP | 1 × 10-3 | 2230 | 42 | ECHA 2013 |
| BDTP | 1 × 10-3 | 2230 | 56 | ECHA 2013 |
| BDTP | 1 × 10-4 | 3635 | 28 | ECHA 2013 |
| BDTP | 1 × 10-4 | 4990 | 42 | ECHA 2013 |
| BDTP | 1 × 10-4 | 4590 | 56 | ECHA 2013 |
| 3864-99-1 | 1 × 10-3 | 900 | 70 | CHRIP c2008 |
| 3864-99-1 | 1 × 10-4 | 4700 | 70 | CHRIP c2008 |
| 3864-99-1 | 1 × 10-4 | 7600 | 63 | CHRIP c2008 |
| 3864-99-1 | 1 × 10-5 | 6500 | 63 | CHRIP c2008 |
| 3846-71-7 | 1 × 10-2 | 365-2250 | 98 | CHRIP c2008 |
| 3846-71-7 | 1 × 10-3 | 1380-8180 | 98 | CHRIP c2008 |
| 3846-71-7 | 1 × 10-4 | 2960-10,000 | 98 | CHRIP c2008 |
Data provided from NITE (CHRIP c2008) reported a 60-day bioconcentration factor (BCF) of between 940 and 2400 for BDTP at a test concentration between 1 × 10-4 to 1 × 10-5 mg/L. The structural analogues however showed much higher bioconcentration potential than BDTP. For example, CAS RN 3864-99-1 presented a BCF of 7600 in a 60-day study, and CAS RN 3846-71-1 possessed a BCF of up to 10,000 (L/kg) in a 14-week study (98 days).
In another bioconcentration study using carp (ECHA 2013), there were two concentrations of BDTP used in the test medium, at 1 × 10-3 and 1 × 10-4 mg/L respectively. The average BCF determined after 6 and 8 weeks were 4990 and 4590 L/kg respectively for the concentration group at 1 × 10-4 mg/L, which was higher than the average BCF determined at the higher concentration level (1 × 10-3 mg/L) for each determining time point (see Table 7-4). A BCF of 4767 L/kg was determined for BDTP as the geometric average of the reported values for the 1 × 10-4 mg/L concentration group at 6 and 8 weeks.
7.3.2 Modelled Bioaccumulation Factor (BAF) and BCF
It is noted that the BCF reported from the laboratory experiments may not adequately account for the bioaccumulation potential of substances via the diet, which can be a predominant aspect for substances with log Kow greater than ~4.0 (Arnot and Gobas 2003). Arnot and Gobas (2006) collected approximately 2900 BCF and BAF observations from the scientific literature on more than 450 chemicals on the DSL. The authors concluded that, for chemicals with log Kow greater than 4, the BAF is substantially higher than the BCF. They also provided examples where poorly metabolized substances had measured BAFs that were 1 to 2 orders of magnitude higher than the corresponding BCFs (Arnot and Gobas 2006).
For BDTP with a log Kow of 7.25, the BCFs measured from laboratory experiments have only accounted for the exposure to the substance from water alone and does not consider the uptake from food. A BAF with the metabolism correction in test organisms is considered more appropriate for characterizing the bioaccumulation potential for this substance. Given the lack of experimental BAF data for BDTP or its analogues, a kinetic mass-balance model called AQUAWEB was used to generate an estimate of this endpoint and fill the data gap.
AQUAWEB model (v1.3, Arnot and Gobas 2004) is a modified version of a previous food web bioaccumulation model (Gobas 1993). The model is presented in rate constant format for assessing the bioaccumulation of non-ionic hydrophobic organic chemicals (i.e. log Kow 1~9) at steady-state. Metabolic biotransformation rate data can also be taken into account as a mechanism of chemical elimination from the environmental organism.
Like the previous version of this food web model, AQUAWEB (v1.3) is parameterized to Western Lake Erie environmental conditions and contains empirical data for PCBs from the Lake food web for model evaluation (Morrison et al. 1996, 1997). BDTP remains in a neutral form under the environmental conditions and possesses a slow metabolism rate same as PCBs. The passive diffusion is considered to be most important uptake mechanism for PCBs and BDTP. Therefore, the AQUAWEB model is considered applicable to BDTP.
By considering the calculated concentrations of this substance in water and the sediment (see the section of Ecological Exposure section below), AQUAWEB can calculate BAF and whole body tissue residue concentration of the substance in approximately 20 aquatic organisms. From these, black crappie, white perch, and yellow perch were selected to represent the mid trophic level fish in the Canadian aquatic ecosystem, since they are near shore species likely to be consumed by piscivores.
AQUAWEB also considers the metabolic rate of a chemical, at which a parent compound can be eliminated via metabolic transformation from an organism. The metabolic rate represents the ability of organisms in the food web to metabolize absorbed parents compounds (Arnot and Gobas 2003). If metabolic transformation is significant, it can counteract the effects of biomagnification in the food web and actually cause the chemical concentration to decrease with increasing trophic level.
In order to estimate the metabolic rate constant (kM) for BDTP, the approach outlined in Arnot et al. (2008a), when the experimental BCF is known, was used. The purpose of this procedure is to fit the kinetic model to agree with the observed BCF data, thus providing reasonable estimations of elimination rate constants. The kM was then body weight normalized to the weight of a middle trophic level fish (184g) at a temperature of 10 °C as outlined in Arnot et al. (2008b). The metabolism rate of BDTP in a 184g fish was calculated as 0.011/day, which is considered to be low among estimated kM for various organic chemicals (Arnot et al. 2008a), indicating that the metabolic transformation of BDTP in an aquatic organism is not significant. The substance is expected to remain at a steady-state concentration in an organism; when such organism is consumed by a higher trophic level predator, biomagnification is likely to occur.
Using CPOPs to characterize the metabolism in fish, sulfate conjugation and arene hydroxylation are suggested as the possible biotransformation for BDTP, based on the chemical structure. However, the probability of such metabolism is close to zero (see Figure 1 below). The outcomes from CPOPs also suggest a slow biotransformation of BDTP in aquatic organisms, indicating a likely high bioaccumulation of this substance in the food web that the substance concentration increases with increasing trophic level.
Figure 2. Predicted degradation pathway and metabolites of BDTP (CPOPs 2008)

Long description of the figure 2
The degradation pathway of BDTP in fish and possible metabolites, which are predicted by Canadian Persistent organic pollutants (POPs) model. Sulfate conjugation and arene hydroxylation are suggested as the possible biotransformation for this substance, based on the chemical structure. However, the probability of such metabolism is close to zero.
Estimates of BCFs and BAFs for mid trophic fish are summarized in Table 7-5 below, considering the metabolic rate for the 10 g fish (kM-10g = kM-184g × 2 = 0.022 /day).
| Species | BCF (L/kg) | BAF (L/kg) |
|---|---|---|
| Black crappie | 3240 | 101710 |
| White perch | 3610 | 77350 |
| Yellow perch | 4040 | 79300 |
As summarized in Table 7-5 above, the estimated BCF values in AQUAWEB range from 3240 to 4040 L/kg for mid trophic level fish, which are at the same magnitude as the experimental data of this endpoint (ECHA 2013). Coupled with the potential for biotransformation, the BAF is estimated at approximately 86653 L/kg (the geometric average of BAFs for three fishes) for BDTP in mid trophic level fish, indicating that bioaccumulation potential is expected to be significant in aquatic organisms if considering the uptake of BDTP from dietary source.
8. Potential to Cause Ecological Harm
8.1 Ecological Effects Assessment
8.1.1 Aquatic Toxicity Data
It is noted that BDTP, as most phenolic benzotriazole UV stabilizers, is poorly soluble in water and difficult to test in this medium, as these substances do not dissolve naturally. Often, auxiliary agents are used in experiments to facilitate dissolution and support stable dispersions. By doing so, the exposure to the test chemicals tends to occur above their water solubility. Such high concentrations of the test substances are not likely realistic in the Canadian environment. An ECETOC (1996) report states that the use of any auxiliary at a low concentration should not add additional toxic effects on the test organisms. If toxic effects are apparent, these should be identified and eliminated from the study by using the solvent control group.
The reported findings from acute aquatic toxicity studies on BDTP are summarized in Table 8-1.
| Organism | Species | Ecotoxicity EndpointFootnote Table 8-1[a].2 and Value (mg/L) | Note | Reference |
|---|---|---|---|---|
| Fish | Brachydanio rerio (Danio rerio) | 96h EC0 greater than 100 96h EC0 greater than 100 |
|
ECHA 2013 |
| Fish | Oryzias latipes | 96h EC0 greater than 0.078 |
|
CHRIP c2008 ECHA 2013 |
| Crustacean | Daphnia pulex | 48h EC0 greater than 10 48h EC0 greater than 10 |
|
Kim et al. 2011a |
| Crustacean | Daphnia magna | (Immobilization) 24h EC0 = 5.8 |
|
ECHA 2013 |
| Crustacean | Daphnia magna | (Immobilization) 48h EC0 greater than 0.083 |
|
CHRIP c2008 ECHA 2013 |
| Alga | Scenedesmus subspicatus (Desmodesmus subspicatus) |
72h EC50 greater than 10 72h NOEC less than 0.1 |
|
ECHA 2013 |
| Alga | Pseudokirchneriella subcapitata | (growth) 72h NOEC = 0.016 |
|
ECHA 2013 |
| Bacteria | Activated sludge | 3h IC20 greater than 100 | ECHA 2013 |
Experimental acute toxicity data of BDTP were available for four major taxa of aquatic organisms. Different exposure concentrations of BDTP (all nominal) were used in the laboratory experiments; the reported endpoint values ranged from 10-2 to 102 mg/L for the test organisms. Most aquatic toxicity studies were somewhat preliminary experiments, in which only a single concentration of BDTP was used on the test organisms. No mortality or toxic effect was observed in fish and crustacean when the exposure to BDTP was at 10 mg/L. In algae, there was some effect observed at the lowest concentration in a 72-hour toxicity study (0.1 mg/L); however the EC50 was expected to be higher than 10 mg/L. As well, bacteria do not seem to be highly sensitive to this substance with a 3h IC20 being greater than 100 mg/L. It should be noted that all of reported endpoint values exceed the water solubility of the test substance ( less than 0.001 mg/L, ECHA 2013). Given that such exposure is not likely to realistically occur in the environment, no acute aquatic toxic effects are expected at saturation.
In an algae (Pseudokirchneriella subcapitata) growth inhibition study (ECHA 2013), a nominal concentration at 0.1 mg/L was applied in a range finding experiment, using an auxiliary agent to facilitate dissolution. No effect in algae was observed after a 72-hour exposure. Concentrations were measured at 0 and 72 hours of the experiment; however, the exact values were not reported. The concentration at the end of the experiment was below the quantitative limit and was characterized by half value of the quantitative limit. A 72h NOEC of 0.016 mg/L was calculated, based on the geometric average of the measured concentrations at 0 and 72 hours.
In another algae (Scenedesmus subspicatus) growth inhibition study (ECHA 2013), three exposure concentrations were used in the experiment. At 72 hours, algal cell counts for the 0.1, 1.0, and 10 mg/L (nominal) test levels were 61, 80, and 74% of the pooled control mean population, respectively. A 72h NOEC of less than 0.1 mg/L was reported. Considering the use of an auxiliary agent in the experiment, the lowest test concentration is much higher than the water solubility of BDTP; therefore no effect is anticipated at saturation of this substance in water.
These studies are considered reliable for characterizing the aquatic toxicity of BDTP. Findings suggest a low acute toxicity of this substance on aquatic organisms. However, no reported value of any toxicity endpoint is considered acceptable to calculate a predicted no effect concentration (PNEC) for risk quotient analysis of the aquatic compartment.
Chronic ecotoxicity data have not been identified for phenolic benzotriazoles. The predictive QSAR-based ECOSAR model (2008) was used to estimate the chronic effects of BDTP on aquatic organisms. The modelled chronic toxicity data suggest a possible toxic effect on fish after long-term exposure at a very low concentration (see Table 8-2 below).
| Organism | Chronic Toxicity Value (mg/L) |
|---|---|
| Fish | 0.000823 |
| Daphnid | 0.002Footnote Table 8-2[a].3 |
| Green Algae | 0.037[a] |
8.1.2 Estimated Critical Body Burden (CBB) for BDTP in Fish
According to its physical and chemical properties, BDTP has low water solubility and very limited bioavailability to test organisms during short term exposure in water. This could explain why the substance has demonstrated low acute toxicity to aquatic organisms in ecotoxicity studies.
As a persistent and bioaccumulative substance, BDTP is expected to remain in the environment causing long-term exposure. If bioaccumulation happens in an organism in a food chain, there is likely a tendency to concentrate as it may move from one trophic level to a higher one.
To deal with the uncertainty associated with the empirical acute toxicity data and to fill the data gap in the chronic toxicity, a critical body burden (CBB) approach, also referred to as an internal critical concentration (ICC) approach, was applied as a "check mechanism." In this approach, an external effect concentration of BDTP that would cause the mortality of organism is calculated, extrapolated from the internal effect concentration. Details of the CBB approach are described in Appendix A. In summary, the internal effect concentration for BDTP was determined based on the mode of action for the class of hindered phenols. The external effect concentrations were calculated accordingly, using BCF to account of exposure water during the short exposure and BAF to account for the long-term exposure via both water and food (see Table 8-3).
| Exposure duration | Internal effect concentration (mg/kg) | Bioaccumulation potential | External Effect Concentration (LC50) (mg/L) |
|---|---|---|---|
| Short-term | 703 (deriving from 2 mmol/kg) |
BCFL = 6583 L/kg | 1.04×10-1 |
| Long-term | Chronic: 70.30 (derived from 0.2 mmol/L) |
BAFL = 119 664 L/kg | 5.87×10-4 |
These calculated data indicate that, to reach the CBB threshold level and cause 50% mortality in the aquatic organisms, the short-term exposure to BDTP must be equal to or more than 1.04×10-1 mg/L and the long-term exposure must be equal or more than 5.87×10-4 mg/L. These external effect concentrations help to characterize potential risks associated with the exposure to BDTP in the aquatic environment (see Ecological Risk Characterization section).
8.1.3 Sediments and Soil
According to the industrial uses of BDTP, the substance is expected to primarily enter surface water and end up in sediment. It may also potentially enter soil from wastewater biosolids which are commonly used for soil enrichment as well as from the disposal of products that degrade and release the substance. There is potential exposure for both soil-dwelling organisms and sediment-dwelling species to the substance. It would be desirable to have toxicity data for sediment and soil organisms. However, no suitable ecological effects studies were found for this substance in media other than water, where no effect has been reported at saturation.
8.1.4 Wildlife
Given the environmental persistence and bioaccumulation potential for BDTP, other routes of exposure (e.g., the secondary route of exposure through food chain of a keystone receptor) should be accounted for in the assessment.
To characterize the toxicity of BDTP on wildlife, the toxicity reference value (TRV) for the terrestrial organism is determined based on a toxicological study on experimental rodents exposed to BDTP and its analogues (see Health Effects Assessment). According to findings from short-term and subchronic toxicity studies on rats (Til et al. 1968; IBT 1969b; Leuschner et al. 1970; IIBF 1970), the lowest observed adverse effect level (LOAEL) for repeated dose oral exposure to BDTP was determined to be 15 mg/kg-bw/day. Using an assessment factor of 10, a no observed adverse effect level (NOAEL) was derived to be 1.5 mg/kg-bw/day in rat.
Applying the rat NOAEL and LOAEL in a wildlife exposure model, the chronic toxicological reference values (TRVs) were estimated for mink and river otters with consideration of species-specific parameters for these fish-consuming terrestrial organisms (Table 8-4).
| Wildlife Organism | Chronic TRV (mg/kg-bw/day) |
|---|---|
| Mink | 3.86 |
| River otters | 2.34 |
8.2 Ecological Exposure Assessment
8.2.1 Measured Environmental Concentrations
8.2.1.1 Environmental Monitoring Data for Canada
Measured concentrations of a few phenolic benzotriazole UV stabilizers (including BDTP) in Canada were reported from a recent wastewater monitoring project (De Silva et al. 2014). Samples of influents and effluents of wastewater treatment systems (WWTS), biosolids, surface water and sediment were collected and analyzed. Measured concentrations of BDTP are summarized in Table 8-5.
| Sample Period | Subject Media | Number of Locations (number of detections) | Concentration |
|---|---|---|---|
| January 2014 to July 2014 | WWTS influents | 9 (9) | 8.3 to 107 ng/L |
| January 2014 to July 2014 | WWTS effluents | 9 (9) | 0.52 to 4.0 ng/L |
| July 2013 to April 2014 | Biosolids | 12 (12) | 39 to 278 ng/g dw |
| July 2012 to November 2012 | Surface water | 32 (12Footnote Table 8-5[a].4) | 0.05 to 1.5 ng/L |
| July 2012 to November 2012 | Sediment | 19 (19) | 0.26 to 16 ng/g dw |
A sediment core was also collected from Lake Ontario in June 2013 aiming to analyze phenolic benzotriazole UV stabilizers (De Silva et al. 2014). The 16-cm-long sediment core was considered to represent the past 110-year history of the lake. The top 8 cm was sliced into 0.5 cm segments and analyzed for phenolic benzotriazole UV stabilizers (De Silva et al. 2014). BDTP was found in all 16 portions corresponding to years between 1975 and 2013 and concentrations range from 36 to 77 ng/g dw.
It is noted that sampling sites under the wastewater monitoring project (De Silva et al. 2014) were not specifically determined based on the industrial uses of BDTP identified in Canada.
No information of this substance in other environmental compartments in Canada has been identified.
8.2.1.2 Environmental monitoring data for other jurisdictions
BDTP has been found in water, soil, sediments and aquatic organisms in other countries (see Appendix B).
The substance was reported in the environment as early as 1978, in the Pawtuxet River in Rhode Island, USA. A chemical plant that manufactured BDTP and other UV stabilizers was located on the Pawtuxet River, which flows into the Providence River section of Narragansett Bay (Lopaz-Avila and Hites 1980). Measured concentrations range from 5×10-4 and 1×10-2 mg/L. In studies for Narragansett Bay, BDTP was still detected in samples collected in 1997 in sediment cores, even though BDTP production at the nearby industrial plant had been stopped 12 years prior to the sampling year (Reddy et al. 2000; Hartmann et al. 2005). Such findings indicate a slow degradation of BDTP in the anaerobic environment. In a more recent study, the substance was found in surface water and storm water in Sweden, ranging from 1.9×10-7 and 1×10-5 mg/L (Brorström-Lundén et al. 2011).
BDTP and a few phenolic benzotriazole UV stabilizers were also reported existing in a variety of aquatic organisms in the Manila Bay (Philippines) and Ariake Sea (Japan) (Tables 8-6 and 8-7).
| Substance or Analogue CAS RN | Detection Frequency (% of 58 samples) | Species detected with the highest concentration | Highest concentration detected (ng/g lipid weight) |
|---|---|---|---|
| BDTP | 88 | Bumpnose trevally | 207 |
| 2440-22-4 | 86 | Coral grouper (adult) | 160 |
| 3846-71-7 | 79 | Common ponyfish | 22.5 |
| 70321-86-7 | 55 | Yellowstriped goatfish | 62.9 |
In the fish samples from Manila Bay in Philippinethe Philippines, BDTP was reported with the highest frequency among 8 phenolic benzotriazole UV stabilizers (Kim et al. 2011b). The highest average concentration of BDTP was found in the bumpnose trevally at 207 ng/g lipid weight (see Table 8e8-6). According to the study, this contamination by phenolic benzotriazole UV stabilizers in Manila Bay would be caused by the release of untreated wastewater to the coastal waters. The study findings also suggest there is an accumulation of these chemicals and/or lower metabolic capacity in fish to eliminate them (Kim et al. 2011b).
| Biota | Sampling Period | Number of Samples | Average or Range of Concentrations (ng/L or ng/g wet weight) |
Reference |
|---|---|---|---|---|
| Aquatic organisms (tidal flat organisms) |
2006-2007 | 9 | 0.69-14 (soft tissue) | Nakata et al. 2009 |
| Aquatic organisms (tidal flat organisms) |
2006-2007 | 10 | 0.35-1.2 (whole) | Nakata et al. 2009 |
| Aquatic organisms (shallow water organisms) |
2004-2007 | 9 | 0.19-0.29 (whole) | Nakata et al. 2009 |
| Aquatic organisms (shallow water organisms) |
2004-2007 | 18 | 0.15-101 (liver) | Nakata et al. 2009 |
| Aquatic organisms (shallow water organisms) |
2004-2007 | 7 | 0.3-13.6 (whole except liver) | Nakata et al. 2009 |
| Aquatic organisms (shallow water organisms) |
2004-2007 | 2 | 0.79 (Hepatopancreas) | Nakata et al. 2009 |
| Finless porpoises (blubbers) | 1999 | 2 | 20-64 (female) | Nakata et al. 2010 |
| Finless porpoises (blubbers) | 2008 | 1 | 11 (male) | Nakata et al. 2010 |
| Finless porpoises (blubbers) | 2009 | 2 | 34 (female) 16 (male) |
Nakata et al. 2010 |
Nakata et al. (2009) sampled marine organisms and sediments for BDTP and other phenolic benzotriazoles from Japan. Fifty-five samples, including tidal flat organisms, fishes, shallow water species, teleost fish, cartilaginous fish, and coastal birds were collected from the Ariake Sea during 2004 and 2007. The whole body, soft tissue, hepatopancreas, and liver samples were analyzed depending on the species. Sixteen coastal and river sediments were also sampled around the Ariake Sea during 2006-2007. Concentrations of BDTP in biota were variable and species-specific. Results indicate that environmental releases of BDTP and other phenolic benzotriazole UV stabilizer in Japan and significant bioaccumulation of this class of chemicals through the marine food-webs are occurring.
In another publication, Nakata et al. (2009b) reported the geographical distribution of BDTP and other UV stabilizers in Asian coastal regions. The reported concentrations of these chemicals were high in mussels from Korea, Japan, and Hong Kong, but low in samples from India and Vietnam, which suggested different usage volumes of UV stabilizers among countries and regions in Asia.
The concentrations of phenolic benzotriazole UV stabilizers in marine mammals in Japan have been found to be increasing since the 1990s, which strongly suggest a continuous input of these chemicals into the marine environment (Shinohara et al 2009). These chemicals were found in effluents from wastewater treatment plants that were further released in the aquatic environment (Nakata and Shinohara 2010).
It is noted that due to differences in the aquatic ecosystems in different countries, the monitoring data for BDTP in biota in other jurisdictions may not represent the situation in Canada.
8.2.2 Exposure Scenarios and Predicted Environmental Concentrations (PECs)
As discussed in the Releases to the Environment section, anthropogenic releases of BDTP to the environment depend upon various losses that occur during the industrial uses of the substance. The majority of BDTP released from any industrial site is expected to be caught in the sludge of the local WWTS. There may be some release to the environment via the application of biosolids to agricultural land or disposal in landfill. Releases of this substance would enter surface water via the effluents from the WWTS and may ultimately partition in sediment.
8.2.2.1 Estimate for PECs in Aquatic Compartment
Aquatic exposure was estimated for BDTP released from industrial use activities to an off-site wastewater system that discharges its effluent to a receiving surface water body. Concentrations in the receiving water near the discharge point of the wastewater treatment system were used as the predicted environmental concentration (PEC) for short-term exposure. Based on its persistence, it is assumed that the substance remains in the receiving compartment for a long period of time. The long-term exposure concentrations in the receiving water were therefore calculated by averaging the total annual release over 365 days. Both short-term and long-term exposures were further considered in characterizing the aquatic risk of this substance.
The estimated aquatic concentration due to releases of BDTP from industrial activities to a wastewater system that discharges its effluent to a receiving surface water body was calculated using the equation as follows.
Cw,A = [1000 × Q × L × (1 - R)] / (N × F)
where
- C w,A:
- aquatic concentration resulting from industrial releases, averaged over the whole year and considering full dilution capacity, mg/L
- Q:
- total substance quantity used annually at an industrial site, kg/yr
- L:
- loss to wastewater of an industrial facility in percentage of the total quantity used at such facility, %
- R:
- wastewater system removal rate, fraction
- N:
- number of days per year (d/yr)
- F:
- average flow of the waterbody m 3/d
Based on the information obtained from the stakeholder consultations (emails between stakeholders and Chemicals Sector Directorate, Environment Canada, 2011-2012, unreferenced) and voluntary submissions (Environment Canada 2014), the greatest use of BDTP was identified for industrial companies manufacturing plastics and coating products. A site-specific scenario for the largest user was developed for each sector to estimate the resulting concentrations of BDTP in the environment. Additionally, generic scenarios were also developed to estimate the environmental releases associated with potential uses of BDTP at other theoretical sites.
Based on the available information, releases of BDTP from the industrial sites are assumed to be periodical. The total number of days per year that such release occurs (N) at the industrial site was assumed as 10 days, based on the European Chemicals Agency guidance document (ECHA 2012). This number was used to estimate the predicted environmental concentration (PEC) for the short-term exposure in the receiving water near the discharge point of the wastewater treatment system after each release. Considering the persistence of BDTP, long-term exposure concentrations of this substance in the receiving water were characterized by the total release per year divided by 365 days (N). Both short-term and long-term exposure concentrations in surface water were further considered in characterizing the aquatic risk of substances.
Estimation of the aquatic PECs from the site-specific use and in the generic scenarios in the plastics manufacture and the paints and coatings industry are discussed in detail below and input values of parameters used in the calculations are summarized accordingly.
8.2.2.1.1 Releases of BDTP from the plastics manufacture
For use as an additive in plastics manufacture, the greatest quantity of BDTP used by an industrial company was identified as up to 25 000 kg in a recent year (Environment Canada 2014). There are some processes in the plastics manufacture during which releases of BDTP may occur. Emission factors for these processes were estimated based on the OECD Emission Scenario Document on Plastic Additives (OECD 2009b) and information provided by stakeholders. Considering industrial operations in Canada, emission factors were determined assuming an average particle size of greater than 40 μm, a processing temperature of approximately 200 °C, and activities at a medium or large sized processing plant.
It is noted that the site-specific scenario was developed based on the information identified from stakeholder consultations and voluntary surveys. To capture the potential use of BDTP for manufacturing plastics, a generic scenario was developed for the plastics sector, in which the environmental release was calculated accordingly. The largest use quantity (25 000 kg) identified at an industrial site was used to represent the annual use quantity of a medium to large facility; meanwhile, the average flow rates of a small and a medium-sized river were considered to account for dilution of the substance in the receiving water.
Input values of key parameters for the site-specific and generic scenarios are summarized in Table 8-8.
| Parameter | Input Value for the site-specific scenario | Input Value for the generic scenario |
|---|---|---|
| Quantity used per site (kg)Footnote Table 8-8 [a] | 25 000 | 25 000 |
| Site activities assumed | Raw material handling and compounding | Raw material handling, compounding and conversion |
| Loss to wastewater (%)Footnote Table 8-8 [b] | 0.211 | 0.231 |
| On-site wastewater system removal efficiency (%)Footnote Table 8-8 [c] | 0 | 0 |
| Off-site wastewater system removal efficiency (%)Footnote Table 8-8 [d] | 82.6 | 82.6 |
| Number of daysFootnote Table 8-8 [e] | 10 or 365 | 10 or 365 |
| Wastewater system effluent flow (L/day) | 3.64×108 | Not applicable |
| Dilution in the receiving waterFootnote Table 8-8 [f] | Dilution factor - 10 | Average flow rate in the receiving water - 1.1×108 and 7.8×109 L/day |
Considering the above information, concentrations of BDTP in surface water are calculated to represent the short-term peak concentrations after each release near the discharge site and the long-term exposure concentration to aquatic organisms in the receiving water. It is noted that the PECs obtained for the generic scenario are conservative, considering the average dilution capacity for a small and a medium river, and are therefore much higher than the PECs calculated for the site-specific scenario.
| Exposure duration | Scenario | Aquatic PEC (mg/L) |
|---|---|---|
| Short-term concentration after per release near the discharge point | Site-specific | 2.52×10-4 |
| Short-term concentration after per release near the discharge point | GenericFootnote Table 8-9 [a] | 1.28×10-4 – 8.81×10-3 |
| Long-term exposure concentration in the receiving water | Site-specific | 6.90×10-6 |
| Long-term exposure concentration in the receiving water | Generic | 3.52×10-6 – 2.41×10-4 |
8.2.2.1.2 Releases from the paints and coatings industry
Considerations similar to the ones used in the plastics manufacture scenarios were applied to estimate releases from industrial uses in the paints and coatings industry. A site-specific scenario was developed, based on the identification of an industrial company with the greatest annual use of BDTP. Generic scenarios were also used to capture other potential uses of BDTP in this sector.
To better estimate releases of BDTP from industrial uses in the coating industry, the generic scenarios included both the solvent-based coating and aqueous-based coating operations. A different use quantity was applied to each coating operation, based on the information obtained from voluntary submissions (Environment Canada 2014).
Input values of all parameters are summarized in Table 8-10 below.
| Parameter | Input value for the site-specific use | Input value for the generic scenario (solvent-based coating) | Input value for the generic scenario (aqueous-based coating) |
|---|---|---|---|
| Quantity used per site (kg)Footnote Table 8-10 [a] | 12 000 | 12 000 | 1000 |
| Dusting loss from raw material handling (%)Footnote Table 8-10 [b] | 0.2 | 0.2 | 0.2 |
| Allowance for removal of dust by ventilation system (%) | 95 | 95 | 95 |
| Potential losses to waste water from vessel cleaning (%) | 0 | 0 | 0.5 |
| On-site wastewater system removal efficiency (%)Footnote Table 8-10 [c] | 0 | 0 | 0 |
| Off-site wastewater system removal efficiency (%)Footnote Table 8-10 [d] | 82.6 | 82.6 | 82.6 |
| Number of days (days)Footnote Table 8-10 [e] | 10 or 365 | 10 or 365 | 10 or 365 |
| Wastewater system effluent flow (L/day) | 4.24×107 | Not applicable | Not applicable |
| Dilution in the receiving waterFootnote Table 8-10 [f] | Dilution factor - 10 | Average flow rate in the receiving water - 1.14 × 108 and 7.83×109 L/day | Average flow rate in the receiving water - 1.14 × 108 and 7.83 × 109 L/day |
Considering the above information, concentrations of BDTP in surface water were calculated to characterize the short-term concentration after each release near the discharge point and the long-term exposure concentration in the receiving water to the aquatic organisms. Similarly to the plastics manufacturing scenarios, the PECs calculated for the generic scenarios are higher than those for the site-specific uses.
| Exposure duration | Scenario | Aquatic PEC (mg/L) |
|---|---|---|
| Short-term concentration after per release near the discharge point | Site-specific | 4.92 × 10-5 |
| Short-term concentration after per release near the discharge point | GenericFootnote Table 8-11 [a] | 2.67 × 10-6 to 7.78×10-4 |
| Long-term exposure concentration in the receiving water | Site-specific | 1.35 × 10-6 |
| Long-term exposure concentration in the receiving water | Generic | 7.31 × 10-8 to 2.13×10-5 |
Measured concentrations of BDTP in the environment have been identified in Canada. The highest measured concentration of BDTP in surface water was reported as 1.5 × 10-6 mg/L, which is at the same magnitude as the long-term PECs for the site-specific scenarios in the plastics manufacturing sector (6.90 × 10-6 mg/L) and the paints and coatings sector (1.35 × 10-6 mg/L). The highest measure concentration of this substance in surface water is also lower than the upper bonds of long-term PECs for the generic scenarios (2.41×10-4 mg/L for the plastics manufacturing sector and 2.13×10-5 mg/L for the paints and coatings sector).
8.2.2.2 Estimate for PECs in Sediment Compartment
An equilibrium sediment-water partition approach was used to estimate the concentration of BDTP in bottom sediment. This approach is based on a partitioning principle described by the European Chemicals Agency (ECHA 2012) and incorporates two additional calculation methods. The first method is to estimate the substance’s concentration in the aqueous phase (truly dissolved) of the overlying water from its total concentration, according to studies by Gobas (2007 and 2010). The second method is to estimate a substance’s concentration in bottom sediment from its concentration in the aqueous phase of the overlying water based on an equilibrium partitioning assumption between bottom sediment and overlying water as described by the USEPA’s National Center for Environmental Assessment (USEPA 2003). At equilibrium, the PEC in bottom sediment can linearly correlate with the concentration in the aqueous phase of the overlying water. Sediment exposure scenarios were developed as an extension of the industrial aquatic release scenarios described above to determine equilibrium sediment exposure concentrations, standardized to 3% organic carbon (a typical organic carbon content in bottom sediment for rivers and lakes).
Considering the long-term exposure of BDTP in surface water, the sediment PECs were calculated as follows (Table 8-12).
| Sector | Scenario | PEC in sediment (mg/kg dw) |
|---|---|---|
| Plastics | Site specific | 0.19 |
| Plastics | GenericFootnote Table 8-12 [a] | 6.80 |
| Paints and coatings | Site specific | 0.038 |
| Paints and coatings | Generica (solvent-based coating) | 0.14 |
| Paints and coatings | Generica (aqueous-based coating) | 0.60 |
It is noted that all calculated sediment PECs are greater than the highest measured concentration in this compartment of 0.016 mg/kg dw (originally reported as 16 ng/g dw, De Silva et al. 2014).
8.2.2.3 Estimate for PECs in Soil Compartment
Based on the environmental fate anticipated for BDTP and the environmental monitoring data (Table 8-5), the majority of BDTP is expected to be removed via the wastewater treatment and caught in the biosolids.
To estimate releases of BDTP in soil, an approach described by the European Chemicals Agency (ECHA 2010) was used to quantify this substance sorbed to biosolids and further estimate predicted environmental concentrations in soil (soil PECs) resulting from the land application of biosolids. This approach employed the quantity of biosolids accumulated within the top 20-cm layer (ploughing depth) of soil over 10 consecutive years as the basis for soil PECs. One underlying assumption of the approach was that substances were subject to no loss due to degradation, volatilization, leaching and soil run-off upon their entry into soil. This assumption, therefore, yields conservative soil PECs. Soil exposure scenarios were developed as an extension of the aquatic release scenarios described above, using sludge concentrations and production rates based on the standard wastewater treatment system and biosolids application in Canada.
Standard assumptions/considerations are applied as follows:
- Removal from WWTS – According to ASTreat 1.0 model, a 82.6% removal rate for the wastewater treatment was considered at all off-site treatment plants.
- Biosolids application rate is 8.3 tonne/ha-yr.
- Biosolids application period is 10 consecutive years.
- Soil depth and density - 0.2 m and 1200 kg/m3.
Same use quantities of BDTP for the site specific scenarios and generic scenarios were applied. To calculate the daily biosolids production, the population of two specific sites were used; while in the generic scenarios, a 10,000 population representative of a small city was used, which is conservative.
Estimated concentrations of BDTP in biosolids and soil PECs are summarized in Table 8-13 below.
| Sector | Scenario | Quantity (kg/yr) | Population | Concentration in biosolids (mg/kg dw) | PEC in soil (mg/kg dw) |
|---|---|---|---|---|---|
| Plastics | Site specific | 25 000 | 1 200 000 | 18.62 | 0.64 |
| Plastics | GenericFootnote Table 8-13 [a] | 25,000 | 10,000 | 2446.23 | 84.60 |
| Paints and coatings | Site specific | 12 000 | 110 000 | 92.42 | 3.20 |
| Paints and coatings | Generica (solvent based coating) | 12,000 | 10,000 | 1016.62 | 35.16 |
| Paints and coatings | Generica (aqueous based coating) | 1000 | 10,000 | 84.72 | 2.93 |
It is recognized that the predicted biosolids concentrations are significantly different from the measured data (see section 8.2.1.1). However, it should be noted that the two sets of concentrations do not represent a similar situation. The measured data likely represents background concentrations associated with consumer usage, as the sampling time and location was not linked specifically with industrial activity associated with BDTP.
On the opposite, the calculated concentrations have estimated the anticipated spike in biosolids concentration resulting from the specific use and release of BDTP from an industrial facility discharging to the wastewater treatment system over a short period of time. Therefore, the calculated biosolids concentrations that are presented in the assessment are high end concentrations. They were calculated assuming that high concentrations in wastewater resulting from the releases of BDTD over a short period of time would remain over a longer period of time, resulting in elevated biosolids concentrations. In reality, there would be further mixing and dilution of the biosolids at the wastewater treatment site during the processing and storage of these biosolids, leading to a decrease in the concentration of BDTP in the biosolids potentially being applied to land. Additionally, in the calculation of soil concentrations, it is currently assumed that these biosolids with an elevated concentration of BDTP would be applied to the same field in successive years, leading to exaggerated buildup in soil.
8.2.2.4 Exposure Estimates for Wildlife
For the purpose of characterizing the exposure of BDTP to the terrestrial organisms, the food web bioaccumulation of BDTP in aquatic organisms was first estimated; outcomes were further used to calculate the corresponding exposure in the wildlife.
1. Tissue residue concentrations in fish
Tissue residue concentrations in fish were estimated based on the exposure of BDTP in surface water with consideration of its bioaccumulation. The exposure of BDTP to fish was characterized by the aquatic PECs obtained from both site-specific and generic scenarios. The modelled BAF was used to represent the bioaccumulation potential in fish, to account for intake from both water and food. For mid-trophic level fishes, the tissue residue concentrations of BDTP were estimated accordingly as follows (Table 8-14).
| Sector | Scenario | Tissue residue in fish (mg/kg) |
|---|---|---|
| Plastics | Site specific | 0.83 |
| Plastics | GenericFootnote Table 8-14 [a] | 28.84 |
| Paints and coatings | Site specific | 0.16 |
| Paints and coatings | Generica (solvent-based coating) | 0.60 |
| Paints and coatings | Generica (aqueous-based coating) | 0.25 |
2. Total Daily Intake for the wildlife
Applying the estimated residues in fishes to a bio-energetic wildlife model, the exposure of BDTP to selected wildlife receptors (i.e., mink and river otters) was further calculated. Both mink and river otters are selected to represent fish-consuming terrestrial organisms in the Canadian environment. The exposure in wildlife is presented in a form of the total daily intake (TDI), as illustrated in the formula below.
TDI = [FMR × (N, i=1)Σ [(Ci × Pi) / (GEi × AEi)] + (Cs × IRs) + (Cw × IRw)] × Ed × Pt
where:
- TDI =
- total daily intake (mg/kg bw/day)
- FMR =
- normalized free metabolic rate of wildlife receptor of interest (kcal/kg bw/day)
- Ci =
- concentration of contaminant in the ith prey species (mg/kg)
- Pi =
- proportion of the ith prey species in the diet (%)
- GEi =
- gross energy of the ith prey species (kcal/kg prey)
- AEi =
- assimilation efficiency of the ith prey species by the wildlife receptor of interest
- Cs =
- concentration of contaminant in soil or sediments (mg/kg dw)
- IRs =
- intake rate of soil or sediments (assumed to be zero) (kg dw/kg bw/day)
- Cw =
- concentration of contaminant in water (mg/L)
- IRw =
- intake rate of water (L/kg bw/day)
- ED =
- the dietary assimilation efficiency of the contaminant by the predator (assumed to be 100%) (%)
- Pt =
- proportion of the time the receptor spends in the contaminated area (assumed to be 100%) (%)
- N =
- the number of prey species
Among contributing factors listed above, it is difficult at the bench scale of wildlife risk assessment to specify the proportion of time (Pt) that a focal species may spend foraging in a contaminated area. A worst case of 100% for this parameter was used in the calculation.
The resulting TDIs for mink and river otters are summarized as follows (Table 8-15). Difference in TDIs is largely due to a higher proportion of fish (Pi) in the diet for river otters (84%) compared to mink (61%).
| Sector | Scenario | TDIs (mg/kg-bw/day) |
|---|---|---|
| Plastics | Site specific | Mink: 0.083 River otter: 0.088 |
| Plastics | Generic | Mink: 3.75 River otter: 3.94 |
| Paints and coatings | Site specific | Mink: 0.021 River otter: 0.022 |
| Paints and coatings | Generic (solvent-based coating) | Mink: 0.083 River otter: 0.088 |
| Paints and coatings | Generic (aqueous-based coating) | Mink: 0.033 River otter: 0.035 |
8.3 Ecological Risk Characterization
The approach taken in this ecological screening assessment was to examine various supporting information and propose a conclusion using a weight-of-evidence approach and precaution as required under CEPA. Lines of evidence considered include available information relevant to BDTP on its physical-chemical properties, sources, uses, environmental fate, persistence, bioaccumulation potential, exposure and effects, including risk quotient analyses for aquatic and wildlife organisms.
Predicted environmental concentrations (PECs) were developed to characterize aquatic exposure to this substance resulting from two industrial release scenarios: during the manufacture of plastics and during the manufacture of coating materials (see Ecological Exposure Assessment section). Each scenario considered the quantity of BDTP used at the industrial site, the emission factor to wastewater, the removal rate and effluent flow rate of the wastewater treatment system, and the receiving water body dilution factor.
A few generic scenarios were additionally developed to address uncertainty associated with the industrial uses and quantities of BDTP. The average flow rates of small and medium rivers were used in the calculations.
As a persistent and bioaccumulative substance, BDTP can remain in the environment for a long time and lead to long-term exposure. Having a slow metabolic biotransformation, the substance has a tendency to build up in the organisms and may move from one trophic level to the next in the food chain. The tissue residue concentrations of BDTP in the mid-trophic level fish were therefore calculated based on the aquatic PECs and BAF; these tissue residue concentrations were further used to estimate the TDIs in wildlife organisms.
To deal with the uncertainty associated with the empirical acute toxicity data and to account for the long-term exposure as well as exposure via the dietary intake, a CBB approach was applied; the external effect concentrations were calculated, derived from the internal effect concentrations (for the class of hindered phenols) and BAF. These external effect concentrations were used as PNECs for BDTP. To characterize the effects in wildlife with regards the long-term consumption of contaminated fish, the chronic TRVs were calculated for mink and river otters.
8.3.1 Risk Quotient Analysis Based on Critical Body Burden in Fish
Applying the CBB approach for the aquatic organisms, the risk quotient analysis was conducted, by comparing 1) the short-term concentration of BDTP near the discharge location after each release to the acute external concentration; and 2) the long-term exposure concentration in the receiving water to the chronic external effect concentration. Outcomes are summarized in Tables 8-15 and 8-16 below.
| Sector | Exposure duration | Aquatic PEC (mg/L) | External effect concentration (mg/L) | RQFootnote Table 8-16 [a] |
|---|---|---|---|---|
| Plastics | Short-term exposure after each release | 2.52×10-4 | 1.04×10-1 | 0.0024 |
| Plastics | Long-term exposure | 6.90×10-6 | 5.87×10-4 | 0.018 |
| Paints and coatings | Short-term exposure after each release | 4.92×10-5 | 1.04×10-1 | 0.00047 |
| Paints and coatings | Long-term exposure | 1.35×10-6 | 5.87×10-4 | 0.0023 |
In the site-specific scenarios, both of the short-term exposure and the long-term exposure in water are below the external effect concentrations. This suggests that, considering the current uses of BDTP in Canada, there is a low potential for the substance to pose a risk to organisms in the aquatic environment.
| Sector | Exposure duration | Aquatic PEC (mg/L) | External effect concentration (mg/L) | RQFootnote Table 8-17 [a] |
|---|---|---|---|---|
| Plastics | Short-term exposure after each release | 8.81×10-3 | 1.04×10-1 | 0.085 |
| Plastics | Long-term exposure | 2.41×10-4 | 5.87×10-4 | 0.41 |
| Paints and coatings (solvent-based coating) | Short-term exposure after each release | 1.83×10-4 | 1.04×10-1 | 0.0016 |
| Paints and coatings (solvent-based coating) | Long-term exposure | 5.02×10-6 | 5.87×10-4 | 0.0086 |
| Paints and coatings (aqueous-based coating) | Short-term exposure after each release | 7.78×10-4 | 1.04×10-1 | 0.0075 |
| Paints and coatings (aqueous-based coating) | Long-term exposure | 2.13×10-5 | 5.87×10-4 | 0.036 |
In both the site specific and the generic scenarios, both the short-term exposure and the long-term exposure in water are below the external effect concentrations and outcomes from the RQ analysis are all below 1. This suggests that, considering a general industrial use of BDTP in Canada, the risk to organisms in the aquatic environment is low.
8.3.2 Risk Quotient Analysis for Wildlife
A wildlife exposure assessment was also conducted to account for the substance’s persistence and potential for accumulation in organisms and possible build up in the food chain. The water and sediment PECs for the industrial sites in both the plastics sector and the paints and coatings sector were applied in a wildlife exposure model to estimate the tissue residue concentrations of BDTP in mid-trophic level fish. Consequently, the TDIs of the substance by fish-consuming wildlife mammals (mink and otters) were further calculated with consideration of the metabolism of these organisms. The resulting TDI was estimated as 1.50 mg/kg bw/day for mink and 1.58 mg/kg bw/day for otters, based on the different proportions of fish in the mammals’ diet (see Ecological Exposure Assessment section).
The chronic toxicity reference values (TRV) for wildlife was determined based on the mammalian toxicity data for BDTP and the analogues. The NOAEL and the LOAEL were determined to be 1.5 mg/kg-bw/day and 15 mg/kg-bw/day for rats (see Ecological Effects Assessment section). These two values were further used calculate chronic TRVs as 3.86 and 2.34 mg/kg-bw/day for the mink and river otter, respectively.
The risk quotient analysis was conducted by comparing the TDIs to the chronic TRVs. Outcomes are summarized in Table 8-17 below.
| Sector and scenario | TDI (mg/kg-bw/day) |
RQ=TDI/Chronic TRVFootnote Table 8-18 [a] |
|---|---|---|
| Plastics - site specific | Mink: 0.107 River otter: 0.113 |
Mink: 0.028 River otter: 0.048 |
| Plastics - generic | Mink: 3.75 River otter: 3.94 |
Mink:0.97 River otter: 1.68 |
| Paints and coatings - site specific | Mink: 0.021 River otter: 0.022 |
Mink: 0.0054 River otter: 0.0094 |
| Paints and coatings - generic (solvent-based coating) |
Mink: 0.083 River otter: 0.088 |
Mink:0.020 River otter: 0.035 |
| Paints and coatings - generic (aqueous-based coating) |
Mink: 0.033 River otter: 0.035 |
Mink: 0.0086 River otter: 0.015 |
For the site-specific scenarios in both of the plastics and paintings and coatings sectors, the resulting RQs for wildlife organisms (mink and river otters) are below 1. Outcomes from risk quotient analysis indicate, based on the information identified for the current industrial use and quantity of BDTP in Canada, that there is a low risk for terrestrial organisms associated with the long-term consumption of fish contaminated with BDTP.
In the generic scenarios, there is an RQ greater than 1 for the plastics sector only when the greatest quantity of BDTP is assumed to be used at an industrial site and releases of this substance enter a small river. A very low dilution capacity in a small river may cause a high-exposure concentration in surface water, resulting in a high tissue residue of this substance in exposed fish, which in turn could pose a risk for terrestrial organisms that consume contaminated fish over a long period of time. These conditions are considered very conservative and unlikely.
8.3.3 Soil and Sediment
Release of BDTP to soil may happen if biosolids are applied on land. For the substance primarily released to water, it would ultimately end up in sediment. Given that there is insufficient toxicity data available to derive PNEC for these compartments, a risk quotient analysis is therefore not conducted for these two compartments for BDTP.
8.4 Consideration of Lines of Evidence and Conclusion of Ecological Risk Characterization
Based on activities identified in Canada, BDTP has been currently used in a moderate quantity in Canada, ranging from 10 000 to 100 000 kg per year. The substance is expected to be released to the environment during its use in the manufacture of plastics and coating materials. These releases would first enter into the water compartment (via wastewater treatment plant effluent) and may ultimately end up in sediments. Releases are also expected to soil via the application of biosolids to agricultural land. Water, soil and sediment are therefore the media of focus for this ecological risk characterization.
BDTP has very low water solubility (measured less than 0.001 mg/L) and high log Kow (modelled 7.25). Given the substance's low vapour pressure (measured 10-6 to 10-4 Pa), the substance is not expected to be volatile nor transported over long distances.
Empirical data have been identified and were used, coupled with model predictions, to assess the potentials for persistence and bioaccumulation of BDTP. BDTP is concluded to be persistent in water, sediments and soil and bioaccumulative in organisms. It may also biomagnify in trophic food webs.
Recent monitoring data reported the presence of BDTP in the Canadian environment. BDTP was detected in very low concentrations in influents and effluents of WWTS, biosolids, surface water and sediment. The highest measured concentration in surface water is below any aquatic PECs in the site specific scenarios, which were considered in the CBB approach and further used to calculate the tissue residue concentrations in fish. So there is moderate confidence with the aquatic PECs.
BDTP was also found in soil, sediment and biota in other countries. It degrades slowly and remains in the environment, particularly in anaerobic environment (sediment) where the substance has been detected 12 years after the cessation of nearby production. Accumulation of BDTP in biota was also reported in a variety of aquatic organisms in other jurisdictions.
As discussed in the section of Ecological Effects, findings from aquatic toxicity studies suggest a low acute toxicity of BDTP to aquatic organisms. It is noted that in the standard toxicity tests in water, BDTP is not bioavailable to the test organisms during short-term exposure. Uptake of the substance from water alone may be insufficient to achieve the maximum internal concentration. The reported toxicity value may have underestimated effect thresholds and are therefore not acceptable for deriving a PNEC.
Empirical toxicity data for BDTP are only available for the aquatic compartment, and there is a lack of soil and sediment toxicity data. Given that findings from acute aquatic studies may have underestimated the toxicity of BDTP, additional lines of evidence were taken into account. Exposure to the aquatic and wildlife organisms were calculated for the selected industrial sites. Outcomes from the CBB approach and the wildlife exposure assessment indicated that the substance is expected to pose low risk to both fish and wildlife following long-term exposure via water and food.
To account for uncertainty associated with potential use of BDTP in Canada, a few generic scenarios were developed to account for the potential non-representativeness of the selected sites. The potential for risk was identified from the use of BDTP in plastics manufacturing where the substance is released to a small river; such very conservative conditions are considered unlikely. Therefore, greater weight of evidence is given to outcomes from the site-specific scenarios.
Based on all lines of evidence presented in the ecological risk characterization above, it is concluded that BDTP does not meet the criteria under paragraph 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity.
8.5 Uncertainty in Ecological Risk Assessment
A major uncertainty is associated with a lack of information on the current sources and industrial uses of BDTP in Canada. Data collected in response to the section 71 notice in 2001 were out-of-date. Information obtained from the stakeholder consultations and voluntary surveys helped to update the current use of this substance. Two industrial sites were selected for characterizing the risk associated with the current industrial operations and use of BDTP in Canada. However, new information strongly suggested that the use quantity identified was not truly equivalent to import volume. Given that, generic scenarios in the Ecological Exposure Assessment were used to address the potential uses of BDTP at any non-representative site.
There is an uncertainty with respect to measured concentrations of this substance in Canada. It is noted that this substance was analyzed in influents and effluents of WWTS, biosolids, and surface water in a very recent wastewater monitoring project; however, sampling was conducted at a limited number of locations and there was no information to determine whether there is any source of release upstream or nearby. Given that, PECs in water, sediment and soil were calculated for two industrial sites where the highest use quantities were reported; a few generic scenarios were also developed in the exposure assessment to estimate the potential releases at any non-representative site.
Potential exposure in surface water was estimated considering the batch releases from the industrial uses and their impact on the long-term exposure in water, sediment, soil and fish. Having no information for Canada, the number of days of release was determined based on the OECD guidance and used to estimate the PECs near the discharge site after each release. Furthermore, to characterize the long-term exposure in the environment, the concentrations of BDTP in surface water was estimated by considering the total releases to the environment per year divided by 365 days and used to calculate the sediment concentrations and tissue residue concentrations in fish.
Uncertainty is also associated with the fact that experimental ecotoxicity data were limited for BDTP. For the aquatic compartment, due to the low water solubility of phenolic benzotriazoles, acute toxicity studies were conducted using saturated solutions. Most of the reported endpoints of LC50s and EC50s were much higher than the measured and estimated maximum water solubilities; therefore, they cannot be used in directly predicting the ecotoxicity of BDTP. It is also noted that, due to their low water solubility, bioavailability of the phenolic benzotriazoles is poor to the test organisms. The uptake of these chemicals from water may not be adequate to reach any internal effect concentration, which may explain the lack of effect observed in the acute studies. The findings of standard aquatic toxicity studies may also have underestimated to toxicity of this substance since they do not take the chronic effects or the dietary factor into consideration. Therefore, external effect concentrations in fish with consideration of BAF and the chronic toxicity reference values for wildlife were calculated to fill the data gap and used to further characterize the risk in aquatic and wildlife organisms.
It is noted that a few other phenolic benzotriazoles have been used as the UV stabilizers in Canada. These substances are structurally similar to BDTP and are expected to possess similar environmental fates and modes of action. Effects associated with the cumulative exposure of this class of substances cannot be well represented by the assessment of BDTP or any single phenolic benzotriazole substances. Consequently, it is proposed that BDTP and other phenolic benzotriazole substances contributing to cumulative exposure in the environment be considered in a future cumulative assessment.
9. Potential to Cause Harm to Human Health
9.1 Exposure Assessment
9.1.1 Environmental media
Empirical data on concentrations of BDTP in environmental media in Canada were not identified, however, environmental monitoring data is available from other countries. As mentioned in the environmental fate section, BDTP is expected to be most prevalent in soil and sediment when released to the environment. It is not expected to remain in the atmosphere based on its low vapour pressure. When released to water, it is expected to partition in sediments based on its low water solubility and high log Kow, limiting the likelihood of finding BDTP in water.
In a study from Spain, BDTP was detected in indoor dust collected from private homes with a maximum concentration of 149 ng/g (mean of 91 ng/g) (Carpinteiro et al 2010a). No residential soil studies were identified for BDTP, and this indoor dust study is considered a suitable surrogate for estimating exposure from soil ingestion. There are several studies in which BDTP was detected in surface water from the United States, Japan, and Europe. Most of the sampling was done around industrial areas from effluents or discharge to wastewater with high monitoring levels in the United States ranging from 0.0005 and 4.7 mg/L and much lower levels of 34 ng/L in Japan and 19 ng/L in Spain (Appendix B). BDTP was also measured in surface water away from point sources in Sweden, with concentrations ranging from 1.7 - 4.1 ng/L (Brorström-Lundén et al. 2011). No other environmental monitoring studies were found.
Conservative daily intake estimates of BDTP for the general population in Canada were derived based on the Brorström-Lundén (2011) study for surface water and the Carpinteiro (2010a) study for soil/dust, resulting in an upper-bounding estimate of exposure in the order of nanograms (10-9 g) per kg-bw (kilogram of body weight) per day (see Appendix C). Given the very low vapour pressure of BDTP and no manufacturing in Canada above 100 kg/year, presence in the air is not expected.
9.1.2 Consumer products
BDTP is mainly used as a UV stabilizer in plastics, adhesives, and industrial coatings not intended for use by the general population (Environment Canada 2001b). It is also an ingredient in automotive paint and finishes for professional auto repair shops with a concentration up to 2% by weight (Environment Canada 2004). The section 71 submissions do not report use in consumer products.
In the United States, BDTP is identified in Material Safety Data Sheets (MSDS) for the consumer use of automotive clearcoat finish and topcoat glaze for boats with concentrations ranging up to 10% (Advantage Refinish 2009, Akzo Nobel Coatings 2008). BDTP is also an ingredient in a two-part primer paint for glass surfaces, containing between 0.5 - 1.5% w/w BDTP (Glassprimer 2008). However, these products are not sold to consumers in Canada and exposure of the general population of Canada through use of these products is not expected.
9.2 Health Effects Assessment
No classifications or in-depth reviews of the health effects of BDTP by national or international regulatory agencies were identified. The US EPA conducted a screening-level hazard characterization of BDTP and three other substances in the phenolic benzotriazoles category for the High Production Volume Challenge Program (US EPA 2009).
9.2.1 Analogue justification
In this Screening Assessment Report, mammalian toxicological information of 2-(2H-Benzotriazol-2-yl)-4,6-bis(1-methyl-1-phenylethyl)phenol (BBMPP) (CAS RN 70321-86-7) and 2-(2H-Benzotriazol-2-yl)-4-methylphenol (BMP) (CAS RN 2440-22-4) were extrapolated to BDTP as they are associated with a larger health effects database and were considered to have a similar structural backbone and similar chemical, physical and toxicological properties as BDTP.The elements considered for the analogue justification are discussed below.
9.2.1.1 Structural similarity
The analogues and BDTP have the identical molecular base structure of a benzotriazole group. They also have in common a phenolic group attached to the benzotriazole structure at the same location but the alkyl substituents on the phenolic group vary.
9.2.1.2 Similarity in physical-chemical properties
As a result of structural similarity, the analogues and BDTP exhibit similar physical-chemical properties. They are solids with low water solubility, low to negligible vapour pressure and relatively high octanol-water partition coefficient (log Kow). The rate of hydrolysis of these phenolic benzotriazoles cannot be measured due to low water solubility; however the chemical structure of these compounds suggests that hydrolysis is likely to be negligible under environmental conditions (US EPA 2009).
9.2.1.3 Similarity in mammalian toxicokinetics and toxicity
No toxicokinetics data were available for BDTP and the analogues identified. In the absence of empirical data, the ACD [Advanced Chemistry Development Inc.] Percepta model was used to generate predictions for oral absorption for each substance (ACD/Percepta c1997-2012). Based on the model results, all three substances are expected to be nonionized in the small intestine and anticipated to be absorbed in the gastro-intestinal tract to a certain extent after oral administration. BMP is predicted to have better oral bioavailability than the other two compounds, which correlates with its lower molecular weight, lower log Kow and higher water solubility.
BDTP and the analogues share common toxicological properties. The acute mammalian toxicity of these compounds is low via oral route. They did not induce gene mutations in bacterial tests in vitro. Some toxicological effects were observed after repeated oral exposure to these substances and the liver was the principal target organ for all of them.
Based upon the similarities of the analogues to BDTP in structures, physical-chemical properties and mammalian toxicity, they are suitable to be used for read-across in order to fill the data gaps in the database. The analogue approach is justifiable according to guidance provided by the Organisation for Economic Co-operation and Development (OECD 2007). Note that these two analogues are also members of the phenolic benzotriazoles category that was reviewed by the US EPA in their screening-level hazard characterization (US EPA 2009).
The available studies for BDTP and analogues that have been incorporated in this assessment are summarized below and are presented in more detail in Appendix D.
9.2.2 Acute toxicity
The results from acute oral toxicity studies indicated that the LD50 values in rats and mice for BDTP were all greater than 2000 mg/kg-bw following a single exposure (CIBA-GEIGY Corporation 1989; PBA 2001). Measured acute inhalation LC50 values in rats were greater than 400-4050 mg/m3 (CIBA-GEIGY Corporation 1989). Measured acute dermal LD50 values in rabbits were greater than 1100-3000 mg/kg-bw (CIBA-GEIGY Corporation 1989).
Acute oral toxicity studies indicated that the LD50 values in rats or mice for all of the analogues were greater than 2000 mg/kg-bw (PBA 2001). Acute inhalation LC50 value reported in rats was greater than 1420 mg/m3 for BMP (PBA 2001). The measured acute dermal LD50 value for BBMPP was greater than 2000 mg/kg-bw in rats (PBA 2001).
9.2.3 Short-term and subchronic toxicity
In short-term and subchronic studies with oral exposure to BDTP in experimental animals, the primary effect was liver toxicity. In a 49-day study in which albino rats were orally exposed to BDTP in the feed at 0 or 100 mg/kg-bw per day, reduced body weights were reported in males at 100 mg/kg-bw per day compared to controls. At this dose, increased relative liver weights with accompanying pathological changes in the liver (enlargement and discolouration of the liver, necrosis of hepatocytes, etc.) in male and female rats were also reported. There was an increase in relative kidney weight in both sexes in the BDTP-treated animals but no histological changes in the kidney were found (Til et al. 1968). In a subchronic oral feeding study, Wistar derived albino rats were exposed to BDTP at 0 to 80 mg/kg-bw per day for 90 days. Hemoglobin content and packed cell volume showed a dose-related decrease at 10 mg/kg-bw per day and above. Glucose-6-phosphatase (G6Pase) activity in the livers was increased at all levels (5-80 mg/kg-bw per day). Relative weights of the livers, kidneys, thyroids and testes were increased at the three highest dose levels (20, 40 and 80 mg/kg-bw per day), and relative liver weight was also significantly increased at 5 and 10 mg/kg-bw per day. Gross pathologic examination revealed enlargement and discolouration of livers at all dose levels in males. Livers of females and kidneys of males and females showed distinct enlargement and discolouration only at higher feeding levels (40 and 80 mg/kg-bw per day). Microscopically, hepatic lesions (enlarged and discoloured hepatocytes) were observed at all levels in males and females (Til et al. 1968). In two other 90-day oral studies in which rats were fed BDTP up to 50 mg/kg-bw per day (IBT 1969b; Leuschner et al. 1970), elevated serum liver enzymes were detected and increased liver weights were observed. However, there were no histopathological correlates to these changes. Similarly to that reported in rats, when Beagle dogs were exposed to BDTP in the diet at 0 to 240 mg/kg-bw per day for 3 months, certain liver effects were observed in the lowest dose group (15 mg/kg-bw per day), which included increased liver enzyme levels, elevated serum bilirubin levels and fatty changes in the Kupffer cells. Other effects observed in this study included fatty changes in the renal glomeruli (30 mg/kg-bw per day and above), abnormal spermiogenesis and atrophy of tubules in testes (60 mg/kg-bw per day and above), atrophy of the prostate (30 mg/kg-bw per day and above) and atrophy of the uterus (60 mg/kg-bw per day and above) (IIBF 1970).
No short-term repeated-dose toxicity studies were identified for the analogues, however in longer duration studies, similar to BDTP, the primary effect seen in subchronic studies (90 days exposure) with oral exposure to the analogues was liver toxicity. The lowest oral LOAELs for subchronic exposures to the analogues were 15 mg/kg-bw per day for BBMPP and 317.5 mg/kg-bw per day for BMP. The critical effect level for subchronic toxicity of BBMPP is based on increased absolute and relative liver weights with accompanying histopathological changes in the liver of female rats (PBA 2001), while the critical effect level for BMP is based on decreased food consumption and body weight gain and elevated liver enzymes (alanine aminotransferase (ALT) and gamma-glutamyl transpeptidase (GGT)) in dogs (PBA 2001).
9.2.4 Chronic toxicity and carcinogenicity
No chronic toxicity/carcinogenicity studies were identified for BDTP.
For the analogues, in a two-year (104 weeks) chronic toxicity/carcinogenicity study in rats orally exposed to BMP, decreased body weight gain in males and reduced food consumption in females were observed at the highest dose level, 142 mg/kg-bw per day for males or 169 mg/kg-bw per day for females (3000 ppm in food), during the last 52 weeks. No significant differences were observed between treated groups and controls with respect to tumour incidence. The lowest oral LOAEL for non-cancer effects was 142 or 169 mg/kg-bw per day for male or female rats, respectively, based on decreased body weight gain in males and reduced food intake in females (Hunter et al. 1975). In a lifetime carcinogenicity study in mice exposed to BMP in the diet for 24 months, there were no exposure related effects on incidence or type of neoplasms and on mortality, body weight, food consumption or clinical signs. No systemic toxicity was observed (CIBA-GEIGY Limited 1981).
No repeated-dose inhalation or dermal toxicity studies were available for BDTP and the analogues, however as noted earlier these compounds are sparingly soluble in water and are relatively non-volatile. BDTP has very low water solubility (measured less than 0.001 mg/L) and high log Kow (modelled 7.25). In light of the substance's low vapour pressure (measured 10-6 to 10-4 Pa), the substance is not expected to be volatile nor transported over long distances. When released to the environment, the majority of the substance is expected to reside in soil and sediment compartments.
Based on consideration of the available data on BDTP and the analogues, low effect levels in studies range upward from 5 mg/kg-bw per day. The Lowest Observed Adverse Effect Level (LOAEL) for repeated dose oral exposure is determined to be 15 mg/kg-bw per day.
9.2.5 Genotoxicity
In genotoxicity studies for BDTP, Ames assays were conducted using Salmonella typhimurium strains (TA97, TA98, TA100, TA102, TA1535 and TA 1537) (Hachiya and Takizawa 1994; PBA 2001) and Escherichia coli WP2 (PKM101) (Hachiya and Takizawa 1994). BDTP did not induce gene mutations in these bacterial tests either with and without metabolic activation.There were no other in vitro studies and no in vivo genotoxicity data identified.
Available genotoxicity data showed that the analogues were not mutagenicin the Ames test with Salmonella typhimurium or Escherichia coli (PBA 2001). Additionally, no induction of DNA damage in rat hepatocytes was observed when tested with BBMPP (PBA 2001). However, although BMP was not mutagenic in bacterial mutation assays using Salmonella typhimurium, it was positive in a mouse lymphoma cell mutation assay with metabolic activation (EG&G Mason Research Institute 1981). It was also positive for an unscheduled DNA synthesis assay in rat hepatocytes (Miami Valley Laboratories 1982). The mutagenicity results in bacterial cells for the analogues were similar to those for BDTP. In vivo genotoxicity data were identified for BBMPP and BMP. No evidence of genotoxicity was demonstrated in in vivo assays conducted in hamsters or mice, including the dominant lethal assay, micronucleus test, sister chromatid exchange test and chromosomal aberration assay (PBA 2001).
9.2.6 Reproductive and developmental toxicity
No reproductive toxicity studies were available for BDTP and the analogues. The effects of analogues on reproductive organs were examined in repeated-dose toxicity studies. No obvious exposure related effects on reproductive organs were reported in rats, mice and dogs orally exposed to the analogues subchronically or chronically (US EPA 2009).
No developmental toxicity studies were identified for BDTP. For one of the analogues, the developmental LOAEL is determined to be 1000 mg/kg-bw per day based on decreased body weights and increased delay of skeletal maturation of fetuses at this dose level after pregnant rat dams were exposed to BBMPP at 0-3000 mg/kg-bw per day for 10 days (during days 6-15 of gestation) (PBA 2001). The external examination of fetuses revealed an omphalocele in one fetus in the high dose group (3000 mg/kg-bw per day). Due to the lack of information on the number of dams and fetuses in each group, there was no indication whether a sufficient number of dams and fetuses were evaluated. No developmental effects were reported in rats and mice orally exposed to BMP (PBA 2001). No evidence of maternal toxicity was found in any of the developmental studies.
9.2.7 Confidence in Health Effects Database
The confidence in the health effects database of BDTP by itself is low as limited empirical data were identified, however with the incorporation of the toxicological information from phenolic benzotriazole analogues, the overall confidence is considered to be moderate.
Although for BDTP, there was a lack of in vivo genotoxicity, chronic toxicity/carcinogenicity and reproductive/developmental toxicity data by all routes of exposure, information on these endpoints was available for the two other phenolic benzotriazoles and was used to fill the data gaps (Appendix D).
9.3 Characterization of Risk to Human Health
As only limited empirical data were available with respect to the toxicity of BDTP, relevant health effects information on BBMPP and BMP, two analogues of BDTP and members of the phenolic benzotriazole group that were also reviewed by the US EPA in their screening-level hazard characterization (US EPA 2009), was considered in the characterization of potential human health effects of BDTP in this Screening Assessment Report.
No chronic toxicity/carcinogenicity studies were identified for BDTP. A chronic study conducted on another member of the phenolic benzotriazole class, i.e., BMP, indicated no evidence of carcinogenicity in experimental animals. Limited in vitro genotoxicity data for BDTP indicated no evidence of mutagenicity; however, no in vivo genotoxicity studies were identified for BDTP. The results of in vitro genotoxicity tests (including bacterial mutation assays and DNA repair test) for BBMPP and BMP were mostly negative. No evidence of genotoxicity was found in a few in vivo assays with the analogues, including the dominant lethal assay, micronucleus test, sister chromatid exchange test and chromosomal aberration assay. Consideration of the available information for BDTP and analogues on genotoxicity indicates that BDTP is not likely to be genotoxic.
The critical effects observed in short-term and subchronic toxicity studies for BDTP, BBMPP and BMP were liver effects in experimental animals with exposure upward of 5 mg/kg-bw per day. The lowest observed adverse effect level (LOAEL) identified is 15 mg/kg-bw per day based on consideration of the toxicological information available for both BDTP and its analogues.
General population exposure to BDTP is considered to be negligible as exposure via environmental media was estimated to be in the order of nanograms (10-9 g) per kg-bw (kilogram of body weight) per day across all age groups. This takes into consideration BDTP's physical-chemical properties and that exposure via water or air is not expected.
As exposure of the general population through environmental media in Canada is expected to be negligible, the risk to human health is considered to be low. General population exposure to BDTP from use of consumer products is not expected.
9.4 Uncertainties in Evaluation of Risk to Human Health
This screening assessment does not include a full analysis of the mode of induction of effects associated with exposure to BDTP nor does it take into account possible differences between humans and experimental species with respect to effects induced by this substance. Uncertainty regarding the hazard potential of BDTP is high due to limited empirical health effects data for BDTP by itself. However the similarities in the structures and hazard profiles of phenolic benzotriazoles (BBMPP, BMP and BDTP) provide confidence in the overall conclusion. The combined information available for the phenolic benzotriazoles (BBMPP, BMP, and BDTP) shows low potential for mammalian toxicity including genotoxicity and no evidence of carcinogenicity.
Confidence in the estimates of exposure to BDTP of the general Canadian population from environmental media is considered to be low. Because there were no data for BDTP levels in Canadian environmental media, environmental monitoring studies for dust and surface water from Europe were used as surrogates in order to estimate potential population exposures in Canada.
The commercial uses identified in the section 71 submissions are not expected to result in general population exposure, as they are not used in consumer products. There remains some uncertainty with regards to indirect exposure from contact with treated products, such as automotive or boat surfaces that have used coatings containing BDTP. However, this exposure would be incidental and is expected to be negligible because of the physiochemical properties of BDTP.
10. Conclusion
Based on the overall results of the ecological assessment, it is concluded that BDTP does not meet the criteria under paragraph 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity, and it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger to the environment on which life depends.
Based on the currently available information on its potential to cause harm to human health, it is concluded that BDTP does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that BDTP does not meet any criteria set out in section 64 of CEPA.
References
ACD/Percepta [Prediction Module]. c1997-2012. Toronto (ON): Advanced Chemistry Development. [cited 2013 July 31]. Available from: www.acdlabs.com/products/percepta/
Advantage Refinish Products; MSDS AD-54501-G; Autobody Brands International; Scottsdale, AZ, April 4, 2009. [cited March 2, 2011]. Available from: http://www.advantagerefinishproducts.com/advantage-refinish-products/msds/ad-545-01.pdf
[AIEPS] Artificial Intelligence Expert Predictive System. 2003-2007. Version 2.05. Ottawa (ON): Environment Canada. Model developed by Stephen Niculescu. Available from: Environment Canada, Ecological Assessment Division, New Substances Evaluation Section
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2008. Version 1.92a. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited July 2009]. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Arnot JA and Gobas FAPC. 2003. A Generic QSAR for Assessing the Bioaccumulation Potential of Organic Chemicals in Aquatic Food Webs. QSAR & Combinatorial Science. 22(3): 337-345
Arnot JA and Gobas FAPC. 2004. A food web bioaccumulation model for organic chemicals in aquatic ecosystem. Environmental Technology and Chemistry. 23(10): 2343-2355.
Arnot JA and Gobas FAPC. 2006. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environmental Reviews. 14: 257-297.
Arnot JA and Gobas FAPC, Mackay D, Bonnell M. 2006. A tiered method to assess the bioaccumulation of organic chemicals in aquatic systems. Presentation at SETAC North America, 27th Annual Meeting, Montreal, QC, November 5-9, 2006
Arnot JA, Mackay D, Bonnell M. 2008a. Estimating metabolic biotransformation rates in fish from laboratory data. Environ Toxicol Chem. 27(2): 341-351
Arnot JA, Mackay D, Parkerton T, Bonnell M. 2008b. A database of fish biotransformation rate constants for organic chemicals. Environ Toxicol Chem. 27(11): 2263-2270
Aronson D, Boethling B, Howard P, Stiteler W. 2006. Estimating biodegradation half-lives for use in chemical screening. Chemosphere. 63: 1953-1960
AWLCRAFT 2000 Clear; MSDS OF 3029-3; Akzo Nobel Coatings; Union, NJ, September 17, 2008. Available from: http://www.kelloggmarine.com/msds/AWL-Awlgrip%20Paint/AWL_F3029_MSDS.pdf [cited: March 10, 2011]
[BCFBAF] Bioaccumulation Program for Windows [Estimation Model]. 2008. Version 3.00. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited in July 2009]. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Beyer A, Mackay D, Matthies M, Wania F, Webster E. 2000. Assessing Long-Range Transport Potential of Persistent Organic Pollutants. Environmental Science and Technology: 34 (4): 699-703
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2008. Version 4.10. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited July 2009]. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere. 30(4): 741-752
Brorström-Lundén E, Remberger M, Kaj L, Hansson K, Andersson H, Haglund P, Andersson R, Liljelind P, Grabic R. 2011. Screening of benzothiazoles, benzenediamines, dicyclohexylamine and benzotriazoles 2009. 1-64. Cited in Germany 2013
Canada. 2000. Canadian Environmental Protection Act, 1999: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March, 2000, SOR/2000-107. Ottawa: Queen's Printer. Available from: http://canadagazette.gc.ca/partII/2000/20000329/pdf/g2-13407.pdf
Canada, Dept. of the Environment. 2001. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List (DSL). Canada Gazette, Part I, vol. 145, no. 45, p. 4194-4210. Ottawa: Queen's Printer. Available from: http://www.gazette.gc.ca/archives/p1/2001/2001-11-17/pdf/g1-13546.pdf
Carpinteiro I, Abuin B, Rodriguez I, Ramil M, Cela R. 2010a. Pressurized solvent extraction followed by gas chromatography tandem mass spectrometry for the determination of benzotriazole light stabilizers in indoor dust. Journal of Chromatography A. 1217: 3729-3735
Carpinteiro I, Abuin B, Rogriguiez I, Cela R, Ramil M. 2010b. Headspace solid-phase microextraction followed by gas chromatography tandem mass spectrometry for the sensitive determination of benzotriazole UV stabilizers in water samples. Analytical and Bioanalytical Chemistry. 397: 829-839
CATABOL. [Computer Model]. c2004−2008. Version 5.10.2. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. [cited July 2009]. Available from: http://oasis-lmc.org/?section=software&swid=1
Choi BH, Yamashita T, and Lee HS. 2010. Regional ocean tide simulator: application to adjacent seas of Korea and Japan. Journal of International Development and Cooperation. 16(2): 21-28
[CHRIP] Chemical Risk Information Platform [database on the Internet]. c2008. Tokyo (JP): National Institute of Technology and Evaluation, Chemical Management Centre (CMC). [cited in July 2009]. Available from: http://www.safe.nite.go.jp/english/db.html
Ciba. 2004a. Material Safety Data Sheet. Tinuvin 328. 25973-55-1. Ciba Specialty Chemicals Corporation.
Ciba. 2004b. Ciba Specialty Chemicals Corporation. Revised Robust Summaries and Test Plan for Phenolic Benzotriazole Category. CAS 2440-22-4. Available from: http://www.epa.gov/HPV/pubs/summaries/phenbenz/c13266rr.pdf
CIBA-GEIGY Corporation. 1989. Letter from Ciba Geigy Corp. to US EPA regarding study results for Tinuvin 328. TSCA OTS0516611-1, Document ID: 89-890000077
CIBA-GEIGY Limited. 1981. Final report - TK 10047 - Lifetime carcinogenicity study in mice. Project No. 784334; TSCA OTS0000572-0, Document ID: FYI-OTS-0887-0572
[CPOPs] Canadian POPs Model. 2008. Gatineau (QC): Environment Canada, Ecological Assessment Division; Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. [Model developed based on Mekenyan et al. 2005]. Available upon request
De Silva A, Muir D, Smyth SA. 2014. Unpublished monitoring data submitted to Ecological Assessment Division of Environment Canada, Gatineau QC.
ECETOC. 1996 Monograph No. 26 Aquatic Toxicity Testing of Sparingly Soluble, Volatile and Unstable Substances. European Centre for Ecotoxicology and Toxicology of Chemicals. Monograph No. 16
ECHA. 2012. Guidance on information requirements and chemical safety assessment, Chapter R.16: Environmental Exposure Estimation- Version 2.1. European Chemicals Agency. October, 2012
[ECHA] European Chemicals Agency. 2013. Registered Substances database. Helsinki (FI): ECHA. [cited March 2013]. Available from: http://echa.europa.eu/web/guest/information-on-chemicals/registered-substances
[ECOSAR] Ecological Structural Activity Relationships [Internet]. 2008. Version 1.00. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited July 2009]. Available from:
http://www.epa.gov/oppt/exposure/pubs/episuite.htm
EG&G Mason Research Institute. 1981. Test for chemical induction of mutation in mammalian cells in culture - the L5178Y TK+/- mouse lymphoma assay - test material: T1015.01. Study report 003-471-679-7, conducted for The Procter & Gamble Company; TSCA OTS 0543787, Document ID: 88-920006782.
Environment Canada. 1986. Domestic Substances List. CAS RN 25973-55-1.
Environment Canada. 2001a. Canadian Environmental Protection Act, 1999. Notice with Respect to Certain Substances on the Domestic Substances List (DSL). Canada Gazette. Vol. 135, No. 46. Available from: http://canadagazette.gc.ca/archives/p1/2001/2001-11-17/html/notice-avis-eng.html#i1
Environment Canada. 2001b. Data collected pursuant to Section 71 (CEPA, 1999) and in accordance with the published notice "Notice with Respect to Certain Substances on the Domestic Substances List (DSL)", Canada Gazette, Vol. 135, No. 46
Environment Canada. 2004. Preliminary Report of Section 71 (CEPA, 1999) "Notice with Respect to Certain Substances on the Domestic Substances List (DSL)", Canada Gazette, Vol. 135, No. 46.
Environment Canada. 2006. Approach to ecological screening assessments under Paragraph 64(a) of CEPA for existing substances that are both persistent and bioaccumulative. In: CEPA DSL Categorization: Overview and Results [CD-ROM], released in September 2006. Environment Canada, Existing Substances Division, Gatineau (QC), K1A 0H3. Available upon request.
Environment Canada. 2008. Guidance for conducting ecological assessments under CEPA, 1999: science resource technical series, technical guidance module: Mass Flow Tool. Preliminary draft working document. Gatineau (QC): Environment Canada, Ecological Assessment Division
Environment Canada. 2013. Internal database of Canadian surface water sediments. Ecological Assessment Division, Environment Canada.
Environment Canada. 2014. Voluntary data submissions in response to the data gap described in the draft Risk Management Scope for BDTP (CAS RN 25973-55-14).
[EPISUITE] Estimation Programs Interface Suite for Microsoft Windows [Estimation Model]. 2008. Version 4.0. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited July 2009]. Available from: http://www.epa.gov/oppt/exposure/pubs/episuitedl.htm
[EQC] Equilibrium Criterion Model. 2003. Version 2.02. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre. [cited in July 2009]. Available from: http://www.trentu.ca/academic/aminss/envmodel/models/EQC2.html
Feron VJ, Til HP, de Groot AP. 1966. Sub-chronic feeding tests as to the toxicity of Tinuvin P in rats. Central Institute for Nutrition and Food Research TNO. Study report 2205, conducted for J.R. Geigy A.G., Basle, Switzerland; TSCA OTS0539880, Document ID: 88-920002920.
Germany. 2013. Proposal for identification of a substance as a CMR 1a or 1b, PBT, vPvB or a substance of an equivalent level of concern. CAS RN 25973-55-1. Available from: http://echa.europa.eu/proposals-to-identify-substances-of-very-high-concern-previous-consultations.
Gobas FAPC. 2007. Development and review of a generic water–sediment modelling framework for organic chemicals. Report prepared for Environment Canada. Burnaby (BC): Simon Fraser University, Faculty of Environment. March 26, 2007.
Gobas FAPC. 2010. Comments on approach to sediment exposure approach. Report prepared for Environment Canada. Burnaby (BC): Simon Fraser University, Faculty of Environment. March 25, 2010.
Hachiya N, Takizawa Y. 1994. Mutagenicity of plastic additives. Hen'Igensei Shiken 3(3):147-154. As cited in the Chemical Carcinogenesis Research Information System (CCRIS) for CAS 25973-55-1 accessed through ChemIDplus [database on the Internet]. Available from: http://chem.sis.nlm.nih.gov/chemidplus/
Hansch C, Leo A, Hoekman D. 1995. Exploring QSAR: Hydrophobic, Electronic, and Steric Constants. American Chemical Society, Washington, DC, USA.
Hartmann PC, Quinn JG, Cairns RW, King JW. 2005. Depositional history of organic contaminants in Narragansett Bay, Rhode Island, USA. Mar Pollut Bull. 50(4): 388-395.
Health Canada. 1994. Human health risk assessment for priority substances. Ottawa (ON): Health Canada, Environmental Health Directorate. [cited March 4, 2006]. Available from: http://www.hc-sc.gc.ca/ewh-semt/pubs/contaminants/approach/index_e.html
[HENRYWIN] Henry's Law Constant Program for Microsoft Windows [Estimation Model]. 2008. Version 3.20. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited July 2009]. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Hunter B, Graham C, Street AE, Heywood R, Cherry CP. 1975. Long-term feeding of TK 10047 to rats (final report 0 - 104 weeks). Huntingdon Research Centre. TSCA OTS0000572-0, Document ID: FYI-OTS-0887-0572.
[IBT] Industrial Bio-Test Laboratories, Inc. 1969a. Ninty-day subacute oral toxicity of Tinuvin P in beagle dogs. Study report C7008, conducted for Geigy Chemical Corporation; TSCA OTS0000572-0, Document ID: FYI-OTS-0887-0572.
[IBT] Industrial Bio-Test Laboratories, Inc. 1969b. Ninety-day subacute oral toxicity of TU-1102, albino rats. Study report B6680, conducted for Geigy Chemical Corporation; TSCA OTS0516611, Document ID: 88-880000056.
[IIBF] Institut für Industrielle und Biologische Forschung. 1970. Three-months toxicity study, Tinuvin 328, dietary administration - beagle dogs. Study report A 0176/049, conducted for J.R. Geigy A.G., Basle, Switzerland; TSCA OTS0516611, Doc. No. 88-880000056.
Jungclaus G, Lopez-Avila V, Hites RA. 1978. Organic compounds in an industrial wastewater: A case study of their environmental impact. Environmental Science and Technology. 12: 88- 96.
Kim J-W, Chang K-H, Isobe T, Tanabe S. 2011a. Acute toxicity of benzotriazole ultraviolet stabilizers on freshwater crustacean (Daphnis pulex). The Journal of Toxicological Sciences. 36(2): 247-251.
Kim J-W, Isobe T, Ramaswamy BR, Chang K-H, Amano A, Miller TM, Siringan FP, Tanabe S. 2011b. Contamination and bioaccumulation of benzotriazole ultraviolet stabilizers in fish from Manila Bay, the Philippines using an altra-fast liquid chromatography-tandem basis mass spectrometry. Chemosphere. In press.
Klasmeier, J, Matthies M, MacLeod M, Fenner K, Scheringer M, Stroebe M, Le Gall AC, McKone T, van de Meent D, and Wania F. 2006. Application of Multimedia Models for Screening Assessment of Long-Range Transport Potential and Overall Persistence. Environmental Science and Technology. 40(1): 53-60.
Klimisch HJ, Andreae M, Tillmann U. 1997. A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regulatory Toxicology and Pharmacology. 25:1-5.
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2008. Version 1.67. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited July 2009]. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Latimer JS and Quinn JG. 1996. Historical trends and current inputs of hydrophobic organic compounds in an urban estuary: the sedimentary record. Environmental Science and Technology. 30: 623-633.
Leuschner F, Leuschner A, Schwerdtfeger W, Dontenwill W. 1970. Investigation of the 13-week toxicity of six substances administered orally to Sprague-Dawley rats. Laboratory for Pharmacology and Toxicology. TSCA OTS0516611, Document ID: 88-880000056.
Lopez-Avila V and Hites RA. 1980. Organic compounds in an industrial wastewater: Their transport into sediments. Environmental Science and Technology. 14: 1382- 1390.
Mackay D. 2006. The OECD Persistence and Long Range Transport Potential Screening Tool, Paper prepared for distribution at an OECD workshop held in Ottawa, Canada, May 31 to June 2, 2006.
Mackey D, Hughes DM, Romano ML, Bonnell M. 2014. The role of persistence in chemical evaluations. Integrated Environmental Assessment and Management. In press.
Mayer CM and Wahl DH. 1997. The relationship between prey selectivity and growth and survival in a larval fish. Canadian Journal of Fisheries and Aquatic Sciences. 54(7): 1504-1512
Mayzo. 2003. Product Data Sheet for BLS 5411. CAS RN 3147-75-9. Published by Mayzo Inc.
McCarty LS. 1986. The relationship between aquatic toxicity QSARs and bioconcentration for some organic chemicals. Environ Toxicol Chem. 5:1071-1080
McCarty LS. 1987a. Relationship between toxicity and bioconcentration for some organic chemicals: I Examination of the relationship. In: QSAR in Environmental Toxicology-II, KLE Kaiser (ed). D Reidel Publishing Co, Dordecht, The Netherlands. Pp. 207-220.
McCarty LS. 1987b. Relationship between toxicity and bioconcentration for some organic chemicals: II Application of the relationship. In: QSAR in Environmental Toxicology-II, KLE Kaiser (ed). D Reidel Publishing Co, Dordecht, The Netherlands . Pp. 221-229.
McCarty LS. 1990. A kinetics-based analysis of quantitative structure-activity relationships in aquatic toxicity and bioconcentration bioassays with organic chemicals. Ph.D. thesis. University of Waterloo. Waterloo, Ontario, Canada.
McCarty LS, Hodson PV, Craig GR, Kaiser KLE. 1985. On the use of quantitative structure-activity relationships to predict the acute and chronic toxicity of chemicals to fish. Environ Toxicol Chem. 4:595-606.
McCarty LS, Mackay D, Smith AD, Ozburn GW, Dixon DG. 1991. Interpreting aquatic toxicity QSARs: the significance of toxicant body residues at the pharmacologic endpoint. Science of the Total Environment, Special Issue: QSAR in Environmental Toxicology 109:515-525.
McCarty LS, Mackay D. 1993. Enhancing ecotoxicological modeling and assessment: critical body residues and modes of toxic action. Environ Sci Technol 27:1719-1728.
Mekenyan G, Dimitrov SD, Pavlov TS, Veith GD. 2005. POPs: a QSAR system for creating PBT profiles of chemicals and their metabolites. SAR QSAR Environmental Research. 16(1-2): 103-133
MHLW. 2007. Revision of the Cabinet Order of the Law Concerning the Evaluation of Chemical Substances and Regulation of their Manufacture, etc. CAS RN 3846-71-7. Ministry of Health, Labour and Welfare, Ministry of Economy, Trade and Industry, and Ministry of the Environment. Japan.
Miami Valley Laboratories. 1982. Unscheduled DNA Synthesis Assay in primary cultures of rat hepatocytes with TSIN T1015.01. Study report M0013, conducted for The Procter & Gamble Company; TSCA OTS0543815, Document ID: 88-920006810.
Nakata H, Murata S, Filatreau J. 2009a. Occurrence and concentrations of benzotriazole UV stabilizers in marine organisms and sediments from the Ariake Sea, Japan. Environmental Science and Technology. 43: 6920- 6926.
Nakata H, Murata S, Shinohara R, Filatreau J, Isobe T, Takahashi S, Tanane S. 2009b. Occurrence and concentrations of persistent personal care products, organic uv filters, in the marine environment. Interdisiplinary Studies on Environmental Chemistry - Environmental Research in Asia, Eds. Y. Obayashi, T. Isobe, A. Subramanian, S. Suzuki, and S. Tanabe, pp. 239-246.
Nakata H, Shinohara R. 2010. Concentrations of benzotriazole UV stabilizers and polycyclic musks in wastewater treatment plant samples in Japan. Interdisciplinary Studies on Environmental Chemistry - Environmental Specimen Bank. Edited by Tomohiko Isobe, Kei Nomiyama, Annamalai Subramanian and Shinsuke Tanabe. Pp 51-59. TERRAPUB, 2010.
[NCI] National Chemical Inventories. 2014. Columbus (OH): American Chemical Society. Available from: http://www.cas.org/products/cd/nci/index.html
[NPRI] National Pollutant Release Inventory. 1994-2013. Available from: http://www.ec.gc.ca/pdb/npri/
[OECD] Organisation for Economic Co-operation and Development. 2004a. OECD Series On Emission Scenario Documents, Number 3, Emission Scenario Document on Plastic Additives. ENV/JM/MONO(2004)8. Paris, France
[OECD] Organisation for Economic Co-operation and Development. 2004b. OECD Series On Emission Scenario Documents, Number 11, Emission Scenario Document on Coating Application Via Spray-Painting In The Automotive Refinishing Industry. ENV/JM/MONO(2004)22. Paris, France
[OECD] Organisation for Economic Co-operation and Development. 2007. Guidance on Grouping of Chemicals. Paris (FR): OECD, Environment Directorate. (Series on Testing and Assessment No.80). Report No.:ENV/JM/MONO(2007)28, JT03232745. [cited July 31, 2013]. Paris (FR): OECD. Available from: http://search.oecd.org/officialdocuments/displaydocumentpdf/?cote=env/jm/mono%282007%2928&doclanguage=en
[OECD] Organisation for Economic Co-operation and Development. 2009. Manual for the Assessment of Chemicals. Annex 1: Guidance for Completing a SIDS Dossier. [Internet]. Paris (FR): OECD, Environment Directorate. [cited July, 2011]. Available from: http://www.oecd.org/dataoecd/13/17/36045066.pdf
[PBA] The Phenolic Benzotriazoles Association. 2001. High Production Volume (HPV) Challenge Program. Data summary and Test Plan for Phenolic Benzotriazoles. Available from: http://www.epa.gov/oppt/chemrtk/pubs/summaries/phenbenz/c13266.pdf
Reddy CM, Quinn J, King JW. 2000. Free and Bound Benzotriazoles in Marine and Freshwater Sediments. Environmental Science and Technology. 34:973-979
Rodríguez Pereiro I, Casado Agrelo J. 2012. Benzotriazole UV Stabilizers in Soil and Suspended Particulate Matter Samples. Cited in Germany 2013
Scheringer M, MacLeod M, Wegmann F. 2006. The OECD POV and LRTP Screening Tool. Version 2.0
Shinohara R, Nakata H, Murata S, Isobe T, Takahashi S, Tanabe S. 2009. Temporal trends and seasonal variation of organic UV filters in coastal waters of Japan. Abstract of 18th Symposium on Environmental Chemistry. Pp 354-355. Cited in Nakata and Shinohara 2010.
Sijm DTHM, Hermens JLM JLM. 2000. Internal effect concentration: link between bioaccumulation and ecotoxicity for organic chemicals. In: The Handbook of Environmental Chemistry. Vol. 2, Part J. Bioaccumulation (ed. by B. Beek). Springer-Verlag Berlin Heidelberg, 2000. Pp. 167-199.
[SPIN] Substances in Preparations in Nordic Countries [database on the Internet]. 2012. Copenhagen (DK): Nordic Council of Ministers. [cited 2012]. Available from: http://195.215.251.229/Dotnetnuke/Home/tabid/58/Default.aspx
[TaPL3] Long Range Transport and Persistence Level III model [Internet]. 2000. Version 2.10. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre. Available from: http://www.trentu.ca/academic/aminss/envmodel/models/TaPL3.html
Til HP, van der Meulen HC, Huismans JW, de Groot AP. 1968. Short-term (49-day) and sub-chronic (90-day) toxicity studies with "RY 1137" in rats. Central Institute for Nutrition and Food Research TNO. Study report 2640, conducted for J.R. Geigy A.G., Basle, Switzerland; TSCA OTS0516611, Document ID: 88-880000056
UK Environmental Agency. 2010. Environmental prioritisation of low product volume substances under REACH: PBT screening. United Kingdom Environmental Agency
[US EPA] US Environmental Protection Agency. 1993. Wildlife exposure factors handbook. Office of Health and Environmental Assessment, U.S. Environmental Protection Agency, Washington, D.C. (EPA/600/R-93/187). Available from: http://www.epa.gov/ncea/pdfs/wild2.pdf
[US EPA] US Environmental Protection Agency. 2001. High Production Volume (HPV) Challenge Program. Data Summary and Test Plan for Phenolic Benzotriazoles. Prepared by the Phenolic Benzotriazoles Association. Available from: http://www.epa.gov/chemrtk/pubs/summaries/phenbenz/c13266tc.htm
[US EPA] US Environmental Protection Agency. 2003. Exposure and human health reassessment of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds. Part I: Estimating exposure to dioxin-like compounds. Volume 3: Site-specific assessment procedures. Chapter 4: Estimating exposure media concentrations. EPA/600/P-00/001Cb. U.S. Environmental Protection Agency, National Center for Environmental Assessment, Washington DC, December 2003.
[US EPA] US Environmental Protection Agency. 2004. High Production Volume Challenge Program. Data Summary and Test Plan for Phenolic Benzotriazoles. Revised by the Phenolic Benzotriazoles Association. Available from: http://www.epa.gov/chemrtk/pubs/summaries/phenbenz/c13266rr.htm
[US EPA] United States Environmental Protection Agency. 2007. Screening Level Hazard Characterization of High Production Volume Chemical. Phenolic Benzotriazoles. (CAS No. 2440-22-4, CAS No. 3147-75-9, CAS No. 25973-55-1, CAS No. 70321-86-7). Prepared by High Production Volume Chemicals Branch, Risk Assessment Division, US EPA. August 2007
[US EPA] United States Environmental Protection Agency. 2009. U.S. Environmental Protection Agency Hazard Characterization Document. Screening-level hazard characterization. Sponsored chemicals. Phenolic Benzotriazoles Category. Washington (DC): US EPA, Office of Pollution Prevention and toxics. Available from: http://www.epa.gov/chemrtk/hpvis/hazchar/Category_Phenolic%20Benzotriazoles_Sept2009.pdf
[US EPA] United States Environmental Protection Agency. 2011. US EPA Inventory Update Reporting. [cited January 2011]. Available from: http://www.epa.gov/oppt/iur/tools/data/index.html
[US EPA] US Environmental Protection Agency. 2013. Benchmark Dose Software (BMDS). [Internet]. Washington (DC): US Environmental Protection Agency; [cited July 3, 2013]. Available from: http://www.epa.gov/ncea/bmds/index.html
[US EPA] US Environmental Protection Agency. 2014. Chemical Data Reporting. Updated June 2014. [Internet]. [cited July 2013]. Available from: http://java.epa.gov/oppt_chemical_search/
Van Hoogen G, Opperhuizen A. 1988. Toxicokinetics of chlorobenzenes in fish. Environ. Toxicol. Chem. 7:213-219.
Velasquez, IB., Jacinto, G.S., Valera, F.S., 2002. The speciation of dissolved copper, cadmium and zinc in Manila Bay, Philippines. Marine Pollution Bulletin. 45: 210-217
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [Estimation Model]. 2008. Version 1.41. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Zhang Z, Ren N, Li Y-F, Kunisue T, Gao D, Kannan K. 2011. Determination of Benzotriazole and Benzophenone UV Filters in Sediment and Sewage Sludge. Environmental Science and Technology. 45: 3909-3916
Appendix A: Critical Body Burden (CBB) approach for BDTP
In terms of aquatic toxicity, the critical body burden (CBB) concept shows that an aquatic organism, which takes up a chemical from water, may accumulate this chemical until a certain critical body burden has been attained, which then causes the mortality of the organism. McCarty (1986, 1987a, 1987b, 1990), McCarty and Mackay (1993), McCarty et al. (1985, 1991), and Van Hoogen and Opperhuizen (1988) have indeed shown that internal concentrations of halogenated organic chemicals in fish causing death are fairly constant: about 2-8 mmol/kg.
Sijm and Hermens (2000) indicate that McCarty (1987a, 1987b) and McCarty et al. (1991) have provided a mathematical explanation: . The fairly constant internal effect concentration or critical body burden (CBB) is the result of the bioconcentration factor (BCF), which increases with Kow, and the external effect concentration (LC50), which decreases with Kow:
CBB = LC50 × BCF, and therefore:
log CBB ≈ log (LC50) + log (BCF) ≈ (-log Kow + b1) + (log Kow + b2) ≈ b1 + b2 ≈ constant (where b1 and b2 are constants).
Having analyzed the literature data, Sijm and Hermens (2000) emphasized that for narcotic (e.g., polychlorinated benzenes and biphenyls) and polar-narcotic compounds (e.g., chlorinated phenols and anilines), sufficient information is available to study this assumption. The authors conclude that for different organisms, the lethal body burdens for polar narcotics vary approximately by two orders of magnitude, and thus again show a significant reduction in the variation of the ecotoxicological effect concentrations compared to the more than five orders of magnitude differences that are found in external effect concentrations for this type of mechanism of action.
While applying a CBB approach for BDTP, the following assumptions have been made: 1) the substance is not a reactive or specifically acting reactive chemical, i.e., it provokes toxicity only through non-specific mechanisms (i.e., narcotic mode of action); 2) there are no interactions between the substance and other ingredients in its formulation; 3) the purity of the substance is very high; 4) for lethal effects, once aquatic organism has reached the critical body burden associated with a lethal effect, it dies; 5) the CBB threshold is similar to those for phenols.
Critical body burden (CBB) and external effect concentration (EEC) calculations
As indicated above that CBB = LC50 × BCF, the external effect concentration (LC50) can be back-calculated as:
LC50 (mmol/L) = CBB (mmol/kg) / BCF (L/kg).
The experimental whole-body wet-weight BCF (BCFwb-ww) for BDTP is ~4767 L/kg (ECHA 2013), calculated by averaging the measured values at 6 and 8 weeks. It is noted that the lipid content (Lf) has been reported as 4.2% in fish in the bioconcentration study (ECHA 2013); meanwhile the average lipid content for the mid-trophic level (MTL) fish is assumed as 5.8%. Therefore the BCFwb-ww shall be normalized, based on the average lipid content in the MTL fish accordingly, resulting the BCFL as follows.
BAFL = BAFwb-ww/Lf × 5.8% = 82220 (L/kg) / 0.042 × 0.058 = 113,542 L/kg
Given that the substance possesses a log Kow as 7.25 (estimated), BAF is considered to better account for the bioaccumulation potential of the substance via the diet. A bioaccumulation model (AQUAWEB v1.3, Arnot and Gobas 2004) is used to estimate bioaccumulation of BDTP via the aquatic food webs. Considering BCFL (6583 L/kg), the metabolic rate (kM as 0.022/day) for a 10 g fish, and the concentrations of BDTP in water and sediments, a BAFL (the whole-body BAF on a wet weight basis normalized based on the average lipid content in the MTL fish) is estimated at 119 664 L/kg.
BDTP is basically a benzotriazole-hindered phenol. This class of substances demonstrate toxicity via the polar narcosis as mode of action. The CBB threshold for this type of mode of action (acute 2-7 mmol/kg; chronic 0.2 – 0.7 mmol/kg) is considered in calculating the external effect concentration. BCFL and BAFL are used to account for the intake mainly via water during the short-term exposure and the intake via both water and food during the long-term exposure.
Using the profiling mechanism of the OECD Toolbox, there are a few structural alerts generated for BDTP and other phenolic benzotriazoles, suggesting that this class of chemicals could be very reactive in organisms. If entering an organism, they can cause toxic effects above the baseline of narcosis. Therefore, the lower bound of the CBB threshold for the polar narcosis (acute 2 mmol/kg; chronic 0.2 mmol/kg) is used in the calculation, as follows:
Acute LC50 = CBB (mmol/kg) / BCFL = 2 (mmol/kg) / 6,583 (L/kg) = 3.04 × 10-4 mmol/L.
Chronic LC50 = CBB (mmol/kg) / BAFL = 0.2 (mmol/kg) / 113,542 (L/kg) = 1.76 × 10-6 mmol/L
Using the molecular weight of BDTP (~351.49 g/mol or 351.49 mg/mmol) and assuming that the bulk density of water is ~1 g/cm3 (i.e., 1 mmol/L water ~ 1 mmol/kg water), the external effect concentration for this substance, expressed in mg/L, is:
Acute 1.67 × 10-5 mmol/L × 351.49 mg/mmol = 1.04×10-1 mg/L.
Chronic 1.67 × 10-6 mmol/L × 351.49 mg/mmol = 5.87×10-4 mg/L
Appendix B: Environmental Exposure Monitoring exposure monitoring for BDTP and other phenolic benzotriazole UV stabilizers in other countries
No environmental monitoring data for BDTP in Canada have been identified. However the substance has been detected in water, sediments and aquatic organisms in other countries. Information is summarized in tables below.
The detection of BDTP in the environment was reported as early as 1978 in US (see Table B1) by Jungclaus et al. (1978) who conducted a study at the Pawtuxet River in Rhode Island. The Pawtuxet River flowed by a plant that manufactured BDTP and other UV stabilizers. The plant wastewater was discharged after some treatment into the river, and entered the brackish Providence River through the Pawtuxet Cove. BDTP was detected in wastewater at an average concentration higher than that in the river water. There was an accumulation of this substance in Pawtuxet River sediment up to 100 ppm (equivalent to 100 mg/kg). Lopez-Avila and Hites (1980) also determined that the BDTP concentrations in water nearest the plant were highest in the Pawtuxet River, and followed the rules of simple dilution in the Pawtuxet River and Cove, and in the Providence River. The substance was found in wastewater (0.55-4.7 ppm, equivalent to 0.55-4.7 mg/L), river water (0.007-0.085 ppm, equivalent to 0.007-0.085 mg/L) and sediment (1-100 ppm, equivalent to 1-100 mg/kg).
There are also a few studies on the contamination of BDTP and other organic compounds at Pawtuxet River in Rhode Island. Lopez-Avila and Hites (1980) examined the transport of 120 organic compounds in an industrial wastewater. BDTP was not only detected at a concentration of 40 ppb (equivalent to 0.040 mg/L), but also determined with a sedimentation rate at 3 cm/year. Latimer and Quinn (1996) studied the sedimentary record of hydrophobic organic compounds at Narragansett Bay, and concluded a range of sedimentation rate from 0.23 to 5.5 cm/year among 4 sampling sites. Reddy et al. (2000) and Hartmann et al. (2005) reported their studies on free and bound benzotriazoles in marine and freshwater sediments from the Pawtuxet River and Narragansett Bay in Rhode Island.
In a recent publication (Zhang et al. 2011), BDTP and three other phenolic benzotriazole UV stabilizers (CAS RN 3896-11-5; CAS RN 3864-99-1; CAS RN 3147-76-0) were detected in Saginaw and Detroit Rivers, Michigan, USA. BDTP was detected in a range of 0.72-224 ng/g dry weight in the sediments at 5 out of 6 sites at these two rivers (see Table B1). The reported concentrations and the number of sites detecting BDTP were higher than for the other three phenolic benzotriazole compounds in the study.
| Location | Subject Media | Number of Samples or Locations | Average or Range of Concentrations (mg/L or mg/kg dry weight) |
Reference |
|---|---|---|---|---|
| Pawtuxet River, Rhode Island | Wastewater | n/a | 0.55-4.7 | Jungclaus et al. 1978 |
| Pawtuxet River, Rhode Island | River water | n/a | 0.007-0.085 | Jungclaus et al. 1978 |
| Pawtuxet River, Rhode Island | Sediment | n/a | 1-100 | Jungclaus et al. 1978 |
| Pawtuxet River, Rhode Island | Water | n/a | 0.01-0.04 | Lopez-Avila and Hites 1980 |
| Pawtuxet Cove, Rhode Island | Water | n/a | 0.008-0.009 | Lopez-Avila and Hites 1980 |
| Providence River, Rhode Island | Water | n/a | 0.0005-0.002 | Lopez-Avila and Hites 1980 |
| Pawtuxet River, Rhode Island | Sediments | 8 | 70-300 | Lopez-Avila and Hites 1980 |
| Pawtuxet Cove, Rhode Island | Sediments | 8 | 100 | Lopez-Avila and Hites 1980 |
| Providence River, Rhode Island | Sediments | 8 | 0.6-10 | Lopez-Avila and Hites 1980 |
| Pawtuxet River, Rhode Island | Sediments (collected in 1989) |
n/a | 1 | Reddy et al. 2000 |
| Narragansett Bay, Rhode Island | Sediments (collected in 1997) |
n/a | 25 | Reddy et al. 2000 |
| Narragansett Bay, Rhode Island | Sediments (collected in 1997) |
3 | 1.2 | Hartmann et al. 2005 |
| Saginaw and Detroit Rivers, Michigan | Sediments | 6 (5)Footnote Table B1[a] | 0.72-224 (ng/g dw) | Zhang et al. 2011 |
Nakata et al. (2009a) sampled marine organisms and sediments for BDTP and other phenolic benzotriazoles from Japan. Fifty-five samples, including tidal flat organisms, fishes, shallow water species, teleost fish, cartilaginous fish, and coastal birds were collected from the Ariake Sea during 2004 and 2007. The whole body, soft tissue, hepatopancreas, and liver samples were analyzed, depending on the species. Sixteen coastal and river sediments were also collected around the Ariake Sea during 2006-2007. Concentrations of BDTP in biota were variable and species-specific. The highest concentration was found in tidal flat gastropod at 460 µg/g (lipid wt), followed by mullet (120 µg/g in whole body and 250 µg/g in liver) and hammerhead shark (130 µg/g in liver) from shallow waters. The oysters and clams in tidal flat contained high concentrations of BDTP, at greater than 100 µg/g. Among all subject chemicals analyzed in sediments samples, BDTP was found at the highest concentrations (6.3 ± 4.0 µg/g). Results indicated a use of phenolic benzotriazole UV stabilizer in Japan and suggest the significant bioaccumulation of BDTP and other phenolic benzotriazole UV stabilizers through the marine food-webs.
In another publication, Nakata et al. (2009b) reported the geographical distribution of BDTP and other UV stabilizers in Asian coastal regions. The reported concentrations of these chemicals were high in mussels from Korea, Japan, and Hong Kong, but low in samples from India and Vietnam, which suggested different usage volumes of UV stabilizers among countries and regions in Asia.
The concentrations of phenolic benzotriazole UV stabilizers in marine mammals in Japan have been found to be increasing since the 1990s, which strongly suggest a continuous input of these chemicals into the marine environment (Shinohara et al 2009). These chemicals were found in effluents from wastewater treatment plants that were further released to the aquatic environment.
To identify the source of these chemicals into the marine ecosystems, Nakata and Shinohara analyzed samples from wastewater treatment plants surrounding a city in Japan (Nakata and Shinohara 2010). BDTP was reported with an average concentration of 510 ng/g dry weight in the wastewater biosolids samples from the wastewater treatment plants. The average removal rate of BDTP during the wastewater treatment was calculated as 91±3.2% in the study; at the same time, BDTP was detected at an average concentration as 2.6±0.32 ng/L in the effluent released from the wastewater treatment plant.
| Subject Media | Sampling Period | Number of Samples | Average or Range of Concentrations (ng/L or ng/g wet weight) |
Reference |
|---|---|---|---|---|
| Aquatic organisms (tidal flat organisms) |
2006-2007 | 9 | 0.69-14 (soft tissue) | Nakata et al. 2009 |
| Aquatic organisms (tidal flat organisms) |
2006-2007 | 10 | 0.35-1.2 (whole) | Nakata et al. 2009 |
| Aquatic organisms (shallow water organisms) |
2004-2007 | 9 | 0.19-0.29 (whole) | Nakata et al. 2009 |
| Aquatic organisms (shallow water organisms) |
2004-2007 | 18 | 0.15-101 (liver) | Nakata et al. 2009 |
| Aquatic organisms (shallow water organisms) |
2004-2007 | 7 | 0.3-13.6 (whole except liver) | Nakata et al. 2009 |
| Aquatic organisms (shallow water organisms) |
2004-2007 | 2 | 0.79 (Hepatopancreas) | Nakata et al. 2009 |
| Coastal sediments | 2006-2007 | 16 | 2.6-320 | Nakata et al. 2009 |
| Finless porpoises (blubbers) | 1999 | 2 | 20-64 (female) | Nakata et al. 2010 |
| Finless porpoises (blubbers) | 2008 | 1 | 11 (male) | Nakata et al. 2010 |
| Finless porpoises (blubbers) | 2009 | 2 | 34 (female) 16 (male) |
Nakata et al. 2010 |
| Wastewater treatment plants (influent) |
May to October, 2009 | 9 | 34±15 | Nakata et al. 2010 |
| Wastewater treatment plants (effluent) |
May to October, 2009 | 5 | 2.6±0.32 | Nakata et al. 2010 |
| Wastewater treatment plants (biosolids) |
May to October, 2009 | 10 | 510±67 (dry weight) | Nakata et al. 2010 |
Zhang et al. (2011) reported detection of phenolic benzotriazoles UV stabilizers in north eastern China. Samples were collected from sediments at the Songhua River and wastewater biosolids at the wastewater treatment plants serving five large cities located along the river. BDTP was reported with highest concentrations in both of sediment samples from the river and biosolids samples from the wastewater treatment plants (see Table B3). In this study, the concentration of one phenolic benzotriazole UV stabilizer (CAS RN 3147-76-0) was below the detection limits for the sediment sample of the river, but the substance was detected in the biosolids samples from the wastewater systems. Such findings may be associated with the physical and chemical properties of these compounds that CAS RN 3147-76-0 has considerable higher water solubility and lower log Kow than any of the other three phenolic benzotriazole UV stabilizers. Therefore, if released into the river, CAS RN 3147-76-0 mainly dissolves and remains in aquatic medium while the other phenolic benzotriazole chemicals condense and partition into sediments of the river.
| Substance or Analogue CAS RN | Log KowFootnote Table B3[a] | Location | Subject Media | Number of Sites (Number of Sites that the Subject Chemical was Detected) | Average and Range of Concentrations (ng/g dry weight) |
|---|---|---|---|---|---|
| BDTP | 7.22 | Songhua River | Sediments | 6 (6) | 3.81 2.06-7.12 |
| BDTP | 7.22 | Wastewater system | Biosolids | 5 (5) | 1300 40.6-5920 |
| 3896-11-5 | 5.52 | Songhua River | Sediments | 6 (2) | 1.86 1.71-2.01 |
| 3896-11-5 | 5.52 | Wastewater system | Biosolids | 5 (5) | 77.4 23.3-136 |
| 3864-99-1 | 6.75 | Songhua River | Sediments | 6 (1) | 0.310 0.310 |
| 3864-99-1 | 6.75 | Wastewater system | Biosolids | 5 (4) | 3.68 1.80-8.40 |
| 3147-76-0 | 3.24 | Songhua River | Sediments | NDFootnote Table B3[b] | -- |
| 3147-76-0 | 3.24 | Wastewater system | Biosolids | 5 (2) | 0.955 0.730-1.18 |
Carpinteiro et al. (2010a) in Spain established a laboratory analysis method for determination of benzotriazole UV stabilizers in indoor dust. The use of efficient insulation in private houses and administrative buildings, together with the low impact of light-induced degradation reaction in indoor areas, leads to low removal rates of compounds associated with dust particles. Among five benzotriazole chemicals in the study, BDTP was reported with an average concentration of 91 ng/g in dust samples collected in five private houses, one public building, and three vehicle interiors (see Table 4d). Findings from this study indicate that benzotriazole UV stabilizers as additives were slowly released from host materials, and eventually were settled in dust.
Carpinteiro et al. (2010b) analyzed benzotriazole UV stabilizers in water samples. BDTP was reported in raw wastewater samples ranged from 1.0±0.1 to 19±2 ng/L (see Table B4). The results from the study indicated that BDTP and other phenolic benzotriazole UV stabilizer existed at a very low concentration in environmental media somewhat close to or even below detection limits.
| Chemical or CAS RN | Subject Media | Average Concentrations (ng/g) | Range of Concentrations (ng/g or ng/L) | Reference |
|---|---|---|---|---|
| BDTP | Indoor dust | 91 | 46±3 - 149±4 | Carpinteiro et al. 2010a |
| 2440-22-4 | Indoor dust | 160 | 65±6 - 657±27 | Carpinteiro et al. 2010a |
| 3896-11-5 | Indoor dust | 80 | 42±4 - 4883±150 | Carpinteiro et al. 2010a |
| 3864-99-1 | Indoor dust | 71 | 22±1 - 131±7 | Carpinteiro et al. 2010a |
| BDTP | Water | Not available | 1.0±0.1 - 19±2 | Carpinteiro et al. 2010b |
| 2440-22-4 | Water | Not available | 5±2 - 16±1 | Carpinteiro et al. 2010b |
| 3896-11-5 | Water | Not available | 3.5±0.5 | Carpinteiro et al. 2010b |
| 3864-99-1 | Water | Not available | NDFootnote Table b4[a] | Carpinteiro et al. 2010b |
Kim et al. (2011b) analyzed concentrations of some phenolic benzotriazole UV stabilizers in the fish samples from Manila Bay in Philippine. BDTP was reported with the highest frequency among 8 phenolic benzotriazole UV stabilizers and the highest average concentration of BDTP was found in bumpnose trevally at 207 ng/g lipid weight (see Table B5). According to the study (Kim et al. 2011b), the release of untreated wastewater to the coastal waters caused the contamination of phenolic benzotriazole UV stabilizers in Manila Bay. At the same time, the study findings also suggest the active uptake and/or lower metabolic capacity to eliminate these chemicals (Kim et al. 2011b).
| Chemical or Analogue CAS RN | Detection Frequency (% of 58 samples) | Species detected with the highest concentration | Highest concentration detected (ng/g lipid weight) |
|---|---|---|---|
| BDTP | 88 | Bumpnose trevally | 207 |
| 2440-22-4 | 86 | Coral grouper (adult) | 160 |
| 3846-71-7 | 79 | Common ponyfish | 22.5 |
| 70321-86-7 | 55 | Yellowstriped goatfish | 62.9 |
BDTP was included in a Swedish monitoring study on benzotriazoles (Brorström-Lundén et al. 2011). The substance was not found in samples of air or air deposition or fish samples, but was detected in samples of soil, surface water, sediments, WWTP effluents and sludges, landfill effluents, and storm water. Findings reported by Brorström-Lundén et al. (2011) are summarized in Table B6 below, as cited in an ECHA report for identifying substances of very high concern (Germany 2013). However the original reference is not accessible.
| Subject Media | Concentrations (ng/L or µg/g dw) | # of Detection in All Samples |
|---|---|---|
| Soil | 0.74 µg/g dw | 1/4 |
| Surface water | 1.3-10 ng/L | 6/6 |
| Sediment | 0.65 - 1.3 µg/g dw | 4/6 |
| WWTP effluents | 6.8-15 ng/L | 5/5 |
| WWTP effluent particles | Not detectable (the detection limit = 110 µg/g dw) |
1/1 |
| WWTP sludge | 2.8-37 µg/g dw | 4/8 |
| Landfill effluents | 7-91 ng/L | 3/3 |
| Landfill effluent particles | 3.1 µg/g dw | 1/1 |
| Storm water | 0.19 - 1.3 ng/L | 3/4 |
In the ECHA report (Germany 2013), BDTP was also reported with environmental data in Germany (Rodríguez Pereiro and Casado Agrelo 2012). Soil from high anthropogenic influence and background sites was analyzed; however, BDTP was not detected in any sample collected. The substance was detected in one of five samples of suspended particulate matter from water in the River Rhine (see Table B7 below). It was noted that the sampling site at the River Rhine downstream of Basel, Switzerland, is influenced by the Swiss chemical industry.
| Subject Media | Concentrations (ng/g dw) | # of Detection in All Samples |
|---|---|---|
| Suspended solids (from river water) |
26 | 1/5 |
Appendix C: Upper-bounding estimates of daily intakes of BDTP for various age groups.
| Route of exposure | 0-0.5 yearsFootnote Appendix C[a], Footnote Appendix C[b], Footnote Appendix C[c] (breast milk fed) | 0-0.5 yearsa, b, c (formula fed) | 0-0.5 yearsa, b, c (not formula fed) | 0.5-4 yearsFootnote Appendix C[d] | 5-11 yearsFootnote Appendix C[e] | 12-19 yearsFootnote Appendix C[f] | 20-59 yearsFootnote Appendix C[g] | 60+ yearsFootnote Appendix C[h] |
|---|---|---|---|---|---|---|---|---|
| Drinking waterFootnote Appendix C[i] | N/A | 4.37 × 10-4 | 1.64 × 10-4 | 1.85 × 10-4 | 1.45 × 10-4 | 8.28 × 10-5 | 8.67 × 10-5 | 9.11 × 10-5 |
| Soil/DustFootnote Appendix C[j] | 5.96 × 10-4 | 5.96 × 10-4 | 5.96 × 10-4 | 9.61 × 10-4 | 3.12 × 10-4 | 7.53 × 10-5 | 6.30 × 10-5 | 6.21 × 10-5 |
| Total intake | 5.96 × 10-4 | 1.03 × 10-3 | 7.1 × 10-4 | 1.2 × 10-3 | 4.6 × 10-4 | 1.6 × 10-4 | 1.5 × 10-4 | 1.5 × 10-4 |
Appendix D: Summary of health effects information for BDTP (CAS RN 25973-55-1) and analogues BBMPP (CAS RN 70321-86-7) and BMP (CAS RN 2440-22-4)
| Compound Endpoints |
Phenol, 2-(2H-benzotriazol-2-yl)-4,6-bis(1,1-dimethylpropyl)- (BDTP) (CAS RN 25973-55-1) | 2-(2H-Benzotriazol-2-yl)-4,6-bis(1-methyl-1-phenylethyl)phenol (BBMPP) (CAS RN 70321-86-7) | 2-(2H-Benzotriazol-2-yl)-4-methylphenol (BMP) (CAS RN 2440-22-4) |
|---|---|---|---|
| Acute toxicity | Oral LD50s (rat or mouse) greater than 2325-10 000 mg/kg-bw (CIBA-GEIGY Corporation 1989; PBA 2001) Inhalation LC50s (rat, 4h) greater than 400-4050 mg/m3 (CIBA-GEIGY Corporation 1989) Dermal LD50s (rabbit) greater than 1100-3000 mg/kg-bw (CIBA-GEIGY Corporation 1989) |
Oral LD50 (rat) greater than 7750 mg/kg-bw (PBA 2001) Dermal LD50 (rat) greater than 2000 mg/kg-bw (PBA 2001) No inhalation studies were identified. |
Oral LD50 (rat) greater than 10 000 mg/kg-bw (PBA 2001) Inhalation LC50 (rat, 4h) greater than 1420 mg/m3 (PBA 2001) No dermal studies were identified. |
| Short-term repeated-dose toxicity | (Lowest) oral LOAEL = 100 mg/kg-bw per day (2000 ppm in food), based on decreased growth rate (statistically significant only in males) and increased relative liver weight with accompanying pathological changes in the liver (gross pathology: enlarged livers and greenish-drab in colour; histopathology: enlarged parenchymal cells with homogeneous, eosinophilic cytoplasm and nuclei varying greatly in size, shape and quantity of chromatin, increased number of mitotic figures and binucleated hepatocytes, eosinophilic droplets and yellowish-green pigment granules occasionally found within the cytoplasm of parenchymal cells, necrosis of individual hepatocytes and a slight proliferation of bile duct epithelium in some livers) in male and female Wistar derived albino rats (15/sex/group) exposed to BDTP in the diet at 0 or 2000 ppm (equivalent to 0 or 100 mg/kg-bw per day based on Health Canada 1994) for 49 days (Til et al. 1968). No inhalation or dermal studies were identified. |
No studies were identified. | No studies were identified. |
| Subchronic toxicity | Lowest oral LOEL = 5 mg/kg-bw per day (100 ppm in food), based on increased G6Pase activity in the liver, increased relative liver weights with accompanying histopathological abnormalities in the liver (slightly enlarged parenchymal cells with somewhat homogeneous, eosinophilic cytoplasm and high incidence of "dark" cells in some instances) in male and female albino rats (10/sex/group) exposed to BDTP in the diet at 0, 100, 200, 400, 800 or 1600 ppm (equivalent to 0, 5, 10, 20, 40 or 80 mg/kg-bw per day based on Health Canada 1994) for 90 days (Til et al. 1968). Other oral LOEL = 15 mg/kg-bw per day, based on elevated bilirubin concentration and increased activities of glutamic pyruvic transaminase (GPT), glutamic oxaloacetic transaminase (GOT) and alkaline phosphatase (ALP) in serum in males, increased serum ALP activity in females and histopathological changes in the liver (fatty changes in Kupffer cells, protein globules in cytoplasm, yellow pigmentation in Kupffer cells and Kupffer cell hyperplasia) in both sexes in a dog study (male and female Beagle dogs, 3/sex/group in treatment groups and 5/sex in control group, were exposed to BDTP in the diet at 0, 15, 30, 60, 120 or 240 mg/kg-bw per day for 3 months). In this study, decreases in body weight and food consumption were evident in higher dose groups. Anemia was noted in animals at dose levels of 120 and 240 mg/kg-bw per day. Liver damage such as icterus (i.e. jaundice) was observed upon gross and histopathological examination in a few dogs of the two highest dose groups. In addition, fatty changes in the renal glomeruli (30 mg/kg-bw per day and above), abnormal spermiogenesis and atrophy of tubules in testes (60 mg/kg-bw per day and above), atrophy of the prostate (30 mg/kg-bw per day and above) and atrophy of the uterus (60 mg/kg-bw per day and above) were also observed (IIBF 1970). Other oral studies: In another 90-day oral feeding study in which rats received BDTP up to 50 mg/kg-bw per day, the serum ALP values were elevated at the highest dietary level; increased absolute and relative weights of the livers and kidneys were observed; however, there were no histopathological correlates to these organ weight changes (IBT 1969bFootnote Table D1[d]). When rats were exposed to BDTP in the diet at a dose of 50 mg/kg-bw per day in another 13-week study, increased activities of GPT, GOT and ALP in serum were detected. Distinct enlargement of the heart, liver, kidney and gonads was observed although no pathologic organ changes were found to be attributable to BDTP exposure (Leuschner et al. 1970). No inhalation or dermal studies were identified. |
Lowest oral LOAEL = 15 mg/kg-bw per day (300 ppm in food), based on increased absolute and relative liver weights (liver to body and/or liver to brain weight ratios) with accompanying hypertrophy and/or cytoplasmic vacuolization of hepatocytes in female rats exposed for 92-94 days (male and female Tif:RAIF (SPF) albino rats, 10 per sex per group, were exposed to BBMPP in the diet at 0, 50, 300, 2000 or 10 000 ppm, equivalent to 0, 2.5, 15, 100 or 500 mg/kg-bw per day based on Health Canada 1994) (PBA 2001). No inhalation or dermal studies were identified. |
Lowest oral LOAEL = 317.5 mg/kg-bw per day and 346 mg/kg-bw per day for males and females, respectively (10 000 ppm in food), based on decreased food consumption and body weight gain and elevated liver enzymes (ALT and GGT) in dogs in a 13-week study (male and female Beagle dogs, 6 per sex per group, were exposed to BMP in the diet at 0, 1000, 3000 or 10 000 ppm, equal to 0, 31.75, 95.25 or 317.5 mg/kg-bw per day for males and 0, 34.6, 103.8 or 346 mg/kg-bw per day for females) (PBA 2001). Other oral LOAEL = 500 mg/kg-bw per day (1% in food), based on increased relative liver weights in both sexes, decreased relative testes weights and histolopathological changes in the kidney (a distinct nephropathy) in males in a 90-day rat study (male and female Wistar derived albino rats, 10 per sex per group, were exposed to BMP in the diet at 0, 0.2, 1 or 5%, i.e., 0, 2000, 10 000 or 50 000 ppm which is equivalent to 0, 100, 500 or 2500 mg/kg-bw per day based on Health Canada 1994) (Feron et al. 1966). Other oral study: IBT 1969a[d]. No inhalation or dermal studies were identified. |
| Chronic toxicity/carcinogenicity | No studies were identified. | No studies were identified. | Oral study in rats: Groups of 50 CFY rats per sex were exposed to BMP in the diet at 0, 100, 300, 1000 or 3000 ppm (equal to 0, 4, 14, 47 or 142 mg/kg-bw per day and 0, 6, 17, 58 or 169 mg/kg-bw per day for males and females, respectively) daily for 104 weeks. There were no exposure-related effects on haematology, clinical chemistry and gross or histopathology of organs and tissues examined. Although not statistically significant, marginally lower survival rate was observed during the last 26 weeks in males at 3000 ppm. Neoplastic changes were seen in lymphoreticular system, liver, kidney, reproductive system, endocrine glands and subcutaneous tissues in both control and treated rats. There was slightly increased incidence of tumours in females at 300 or 1000 ppm, although the differences were not statistically significant. There was no evidence of exposure-related effect on tumour occurrence. (Lowest) oral LOAEL for non-neoplastic effects = 142 mg/kg-bw per day for males or 169 mg/kg-bw per day for females, based on slightly decreased body weight gain in males and slightly reduced food consumption in females during the last 52 weeks of the study (Hunter et al. 1975). Oral study in mice: Groups of 50 Tif:MAGf (SPF) mice per sex were exposed to BMP in the diet at 0, 5, 50 or 500 ppm (equal to 0, 0.8, 6.5 or 64 mg/kg-bw per day and 0, 0.8, 6.7 or 62 mg/kg-bw per day for males and females, respectively) daily for 24 months. There were no exposure-related effects on mortality, body weight, food consumption and clinical signs. No systemic toxicity was observed. Neoplastic changes were seen in both control and treated mice in different organs and tissues such as testis, ovary, uterus, mammary gland, spleen, lung, liver, urinary bladder, skin, subcutaneous tissue, haematopoietic tissue, lymphoreticular tissue and spleen etc., but the frequency and type of the neoplasms were not treatment related. Also other gross or histopathological lesions described as developmental, degenerative or inflammatory in origin were not attributed to the exposure to BMP. Oral NOAEL for chronic toxicity and carcinogenicity = 64 mg/kg-bw per day for males and 62 mg/kg-bw per day for females (the highest dose tested) (CIBA-GEIGY Limited 1981). No inhalation or dermal studies were identified. |
| Genotoxicity and related endpoints: in vivo | No studies were identified. | Micronucleus test Negative: bone marrow cells, Chinese hamsters, oral (0, 1250, 2500 or 5000 mg/kg-bw per day by gavage for 2 days) (PBA 2001). Sister chromatid exchange test Negative: bone marrow cells, Chinese hamsters, oral (single exposure to 0, 1250, 2500 or 5000 mg/kg-bw by gavage) (PBA 2001). |
Dominant lethal assay Negative: male albino mice (NMRI derived), oral (single exposure to 0, 1000 or 3000 mg/kg-bw by gavage) (PBA 2001). Micronucleus test Negative: bone marrow cells, Chinese hamsters, oral (0, 500, 1000 or 2000 mg/kg-bw per day by gavage for 2 days) (PBA 2001). Chromosomal aberrations Negative: bone marrow, Chinese hamsters, oral (0, 500, 1000 or 2000 mg/kg-bw per day by gavage for 2 days) (PBA 2001). |
| Genotoxicity and related endpoints: in vitro | Mutagenicity in bacteria Negative: Salmonella typhimurium strains TA97, TA98, TA100, TA102, TA1535 and TA1537 with and without metabolic activation (Hachiya and Takizawa 1994; PBA 2001). Negative: Escherichia coli WP2 (PKM101) with and without S9 (Hachiya and Takizawa 1994). Negative: bacterial strains not specified (CIBA-GEIGY Corporation 1989). |
Mutagenicity in bacteria Negative: Salmonella typhimurium strains TA98, TA100, TA 1535and TA 1537 with and without metabolic activation (PBA 2001). DNA repair test Negative: Male rat hepatocytes (PBA 2001). |
Mutagenicity in bacteria Negative: Salmonella typhimurium strains TA98, TA100, TA 1535 and TA 1537 with and without metabolic activation (PBA 2001). Mammalian cell mutation assay Positive: Mouse lymphoma L5178Y TK+/- with S9 (EG&G Mason Research Institute 1981) Unscheduled DNA Synthesis Assay Positive: Rat hepatocytes (Miami Valley Laboratories 1982) |
| Developmental toxicity | No studies were identified. | Lowest oral LOAEL for developmental toxicity = 1000 mg/kg-bw per day, based on decreased body weights and increased delay of skeletal maturation of fetuses in a study where pregnant female Tif:RAIF (SPF) albino rats (number not specified) were exposed at 0, 300, 1000 or 3000 mg/kg-bw per day by gavage during days 6-15 of gestation. One fetus in the high dose group (3000 mg/kg-bw per day) showed an omphalocele (failure of ventral closure during late stages of embryonic development). Oral NOAEL for maternal toxicity = 3000 mg/kg-bw per day (the highest dose tested). There were no exposure-related effects on maternal body weight, food consumption and clinical signs (PBA 2001). |
Oral NOAELs for developmental and maternal toxicity = 1000 mg/kg-bw per day (the highest dose tested) in two studies where female Sprague-Dawley rats and NMRI derived albino mice (number of animals not specified for either studies) were exposed by gavage at 0, 150, 500 or 1000 mg/kg-bw per day during days 6-15 of gestation. No maternal toxicity and no teratogenic effects were noted (developmental endpoints not specified for either studies) (PBA 2001). |
| Sensitization | Skin sensitization (guinea pig): no animals sensitized in a maximization test (no study details provided) (CIBA-GEIGY Corporation 1989). | No studies were identified. | Skin sensitization (guinea pig): 80-90% of the animals tested showed skin reactions in a maximization test. "BMP was classified as extreme sensitizer in albino guinea pig" (US EPA 2009). |
| Irritation | Skin irritation (rabbit): Minimally irritating, Draize score 0.125/8.0 (no study details provided) (CIBA-GEIGY Corporation 1989). Eye irritation (rabbit): Non-irritant or minimally irritating in two studies, Draize score 0/120 or 5.0/110.0, respectively (no study details provided) (CIBA-GEIGY Corporation 1989). |
No studies were identified. | Skin irritation (rat or mouse): No irritation was observed in rats or mice (strain and sex not specified) exposed to BMP in 5% suspension in gum arabic on a clipped spot on the back at 0.1 cm3 or 0.4 cm3, respectively, for 5 days (US EPA 2009). Eye irritation (rabbit): Minimal irritation to eye mucosa was noted in 2 of the 6 rabbits (strain and sex not specified) exposed to 100 mg of BMP that was instilled in the eyes (US EPA 2009). |
| Compound Endpoint |
Phenol, 2-(2H-benzotriazol-2-yl)-4,6-bis(1,1-dimethylpropyl) (BDTP) (CAS RN 25973-55-1) |
2-(2H-Benzotriazol-2-yl)-4,6-bis(1-methyl-1-phenylethyl)phenol (BBMPP) (CAS RN 70321-86-7) |
2-(2H-Benzotriazol-2-yl)-4-methylphenol (BMP) (CAS RN 2440-22-4) |
|---|---|---|---|
| Sensitization / Irritation | No studies were identified. | No studies were identified. | Patch testing of 59 human subjects (12 men, 47 women) with 0.5 mL of 5% BMP in dimethyl phthalate produced no irritation following initial application. No evidence of irritation or sensitization was noted when the test was repeated (three times weekly for three weeks followed by a similar challenge exposure in the sixth week) (US EPA 2009). |

