Screening Assessment Report
Ethylbenzene
Chemical Abstracts Service Registry Number:
100-41-4
Environment and Climate Change Canada
Health Canada
April 2016
Table of Contents
- List of Tables
- Synopsis
- 1. Introduction
- 2. Substance Identity
- 3. Physical and Chemical Properties
- 4. Sources
- 5. Uses
- 6. Releases to the Environment
- 7. Environmental Fate
- 8. Persistence and Bioaccumulation Potential
- 9. Potential to Cause Ecological Harm
- 10. Potential to Cause Harm to Human Health
- 11. Conclusion
- References
- Appendices
List of Tables
- Table 2-1: Substance identity for ethylbenzene
- Table 3-1: Physical and chemical properties of ethylbenzene
- Table 4-1: Canadian import and export quantities of ethylbenzene from the years 2000 to 2013 (CIMT 2010, 2014)
- Table 6-1: NPRI release and disposal data (tonnes) for ethylbenzene from 2004 to 2013 (Environment Canada 2013b)
- Table 7-1: Results of the Level III fugacity modelling (EQC 2003)
- Table 8-1: Environmental half-lives and removal processes of ethylbenzene
- Table 9-1: Concentrations (µg/L) of ethylbenzene in surface waters and effluents in Canada
- Table 9-2: Concentrations (µg/L) of ethylbenzene in groundwater in Canada
- Table 9-3: Concentrations (µg/kg) of ethylbenzene in soil in Canada
- Table 9-4: Concentrations (µg/kg wet weight) of ethylbenzene in biota in Canada
- Table 9-5: Empirical data for toxicity of ethylbenzene to aquatic organisms
- Table 9-6: Empirical data for toxicity of ethylbenzene to soil organisms
- Table 9-7: Values used to calculate risk quotients (RQs) for all media
- Table 10-1: Range of ethylbenzene emission factors of selected materials (µg/m2/h) at 24 hours (Won et al. 2005)
- Table 10-2: Weighted average residual ethylbenzene concentrations for polystyrene packaging and disposable items (PSWG 1997, cited in VCCEP 2007)
- Table 10-3: Summary of inhalation and dermal ethylbenzene exposure from use of consumer prducts by adults, estimated using ConsExpo v. 4.1
- Table 10-4: Summary of the endpoints selected for risk characterization of ethylbenzene
- Table 10-5: Margins of exposure from use of consumer products containing ethylbenzene for acute and short-term durations - Inhalation
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Ministers of the Environment and Climate Change and of Health have conducted a screening assessment of benzene, ethyl- also known as ethylbenzene (Chemical Abstracts Service Registry Number 100-41-4). Ethylbenzene was identified as a priority for assessment on the basis of greatest potential for human exposure and also because it was classified by other agencies on the basis of carcinogenicity.
Ethylbenzene occurs naturally in the environment in crude oil and some natural gas streams and as a result of incomplete combustion of natural materials, making it a component of forest fire smoke. Ethylbenzene is a component of vehicle and aviation fuels as well as a component of mixed xylenes, which are used as solvents in various applications including in paints, stains, and automotive cleaners. Ethylbenzene is also synthetically produced and mainly used in the manufacture of styrene. Styrene is then used to manufacture various types of polymers such as polystyrene. Ethylbenzene is used in the oil and gas industry in a number of oilfield applications such as a non-emulsifier, as an acid additive and as a surfactant in hydraulic fracturing fluids. Minor applications of the synthetically produced ethylbenzene include use as a solvent and in the production of other chemicals such as diethylbenzene.
The most recent available information on ethylbenzene production in Canada is from 2003, during which a total of 906 000 tonnes of ethylbenzene was produced. Approximately 545 tonnes of ethylbenzene was imported into Canada in 2009, and approximately 51.6 tonnes were exported the same year. According to the results from a section 71 Notice with Respect to Certain Substances on the Domestic Substances List (DSL) conducted for the year 2000, approximately 1 700 000 tonnes of ethylbenzene at a concentration greater than 1% were manufactured in and imported into Canada during that year, mainly by companies in the petrochemical sector. Ethylbenzene has been internationally identified as a high production volume (HPV) chemical.
Ethylbenzene is included in the National Pollutant Release Inventory (NPRI), to which facilities manufacturing, processing, or otherwise using more than 10 tonnes per year of the substance must report their releases. In 2013, facilities across Canada reported to the NPRI on-site environmental releases totalling approximately 326 tonnes, transfers for disposal totalling 1346 tonnes, and transfers for off-site recycling totalling 3482 tonnes.
Ethylbenzene has been detected in ambient and indoor air, drinking water, surface water, groundwater, soil, and biota but not in sediment in Canada. Ethylbenzene has also been detected in various food items in the United States. Ethylbenzene has been identified in numerous consumer products such as liquid and aerosol coatings, caulking, lacquers, stains and varnishes, and building materials. Ethylbenzene has also been measured in the blood of individuals living in the United States.
Based on its physical and chemical properties and half-lives in surface water, groundwater, wastewater treatment systems, soil, and sediments, ethylbenzene is expected to degrade relatively rapidly in water, soil, and sediment under aerobic conditions, but degradation under anaerobic conditions is slower. Ethylbenzene will degrade in air with an estimated half-life of about 2 days. Ethylbenzene has a low potential to accumulate in organisms or biomagnify in trophic food chains.
Short-term effects to aquatic and terrestrial organisms range from 1.8 to 9.6 mg/L and 112 to 259 mg/kg dry weight, respectively. Predicted environmental concentrations (PECs) in air, surface water, sediment, and soil do not exceed concentrations associated with effects. While there is some uncertainty respecting the extent of risk in groundwater due to the fact that the concentration data is not recent and to the consideration of surrogate organisms, concern to the environment is not identified.
Based on the information available, there is low risk of harm to organisms or the broader integrity of the environment from ethylbenzene. It is therefore concluded that ethylbenzene does not meet the criteria under paragraph 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
The critical health effects associated with exposure to ethylbenzene are considered to be tumour induction and non-cancer systemic effects, primarily on the auditory system and on the liver, kidney and pituitary glands.
The general population of Canada is exposed to ethylbenzene from environmental media, food, and the use of consumer products. The margins between levels associated with effects in experimental animals and upper-bounding estimates of exposure from environmental media (including vehicle interior air), food, and from scenarios such as pumping gasoline or living near service stations are considered to be adequate to account for uncertainties in the health effects and exposure for both cancer and non-cancer effects. The margins between upper-bounding estimates of exposure from use of consumer products and critical effect levels are also considered adequate to account for uncertainties in the health effects and exposure databases.
Based on the information available, it is concluded that ethylbenzene does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Based on the information available, it is concluded that ethylbenzene does not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
The Canadian Environmental Protection Act, 1999(CEPA) (Canada 1999) requires the Minister of the Environment and Climate Change and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or to human health.
Ethylbenzene, CAS RN (Chemical Abstracts Service Registry Number) 100-41-4 was identified as a priority for assessment because it met the criteria for greatest potential for human exposure and had been classified by other agencies on the basis of carcinogenicity and it met the criteria for persistence, but did not meet the criteria for bioaccumulation potential or inherent toxicity to non-human organisms.
The 2006 version of the State of the Science Report for a Screening Health Assessment of ethylbenzene was posted on the Health Canada website on January 30th, 2006 (Health Canada 2006). The State of the Science Report for a Screening Health Assessment was externally reviewed by staff of Toxicology Advice and Consulting Limited and by V.C. Armstrong (consultant) for adequacy of data coverage and defensibility of the conclusions. The external comments were taken into consideration in drafting the State of the Science Report. The health screening assessment included here is an update of the State of the Science Report and supersedes that report.
Screening assessments focus on information critical to determining whether a substance presents, or may present, a risk to the environment or to human health, according to the criteria set out in section 64 of CEPA. Screening assessments examine scientific information and develop conclusions by incorporating a weight-of-evidence approach and precaution.
This screening assessment includes consideration of information on chemical properties, hazards, uses, and exposure. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents and stakeholder research reports and from recent literature searches, up to June 2014 for ecological sections of the document and August 2014 for human health sections of the document. In addition, an industry survey was conducted in 2001 through a Canada Gazette notice issued under the authority of section 71 of CEPA (Canada 2001). This survey collected data on the Canadian manufacture and import of substances selected for the Domestic Substances List (DSL) screening assessment pilot project (Environment Canada 2001). Key studies were critically evaluated; modelling results were used to reach conclusions. When available and relevant, information presented in hazard assessments from other jurisdictions was considered. This screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents the most critical studies and lines of evidence pertinent to the conclusion.
Evaluation of risk to human health involves consideration of data relevant to estimation of exposure (non-occupational) of the general population, as well as information on health hazards (based principally on the weight-of-evidence assessments of other agencies that were used for prioritization of the substance). Decisions for risks to human health are based on the nature of the critical effect and/or margins between conservative effect levels and estimates of exposure, taking into account confidence in the completeness of the identified databases on both exposure and effects, within a screening context.
This screening assessment was prepared by staff in the Existing Substances programs at Health Canada and Environment and Climate Change Canada. As mentioned above, the State of the Science Report was also previously externally reviewed. The ecological component of this assessment has undergone external written scientific peer review/consultation and comments received were considered in the production of this report. Comments on the technical portions relevant to human health in the draft screening assessment were received from scientific experts, including Cathy Petito Boyce, Leslie Beyer and Chris Long from Gradient. Additionally, the draft of this screening assement was subject to a 60-day public comment period. Although external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
The critical information and considerations upon which this assessment is based are summarized below.
2. Substance Identity
Substance Name
Information relevant to the identity of ethylbenzene is presented in Table 1.
| Chemical Abstracts Service Registry Number (CAS RN) | 100-41-4 |
|---|---|
| DSL name | Benzene, ethyl- |
| National Chemical Inventories (NCI) namesFootnote Table 2-1[a] | Benzene, ethyl (TSCA, AICS, SWISS, PICCS, ASIA-PAC, NZIoC) Ethylbenzene (EINECS, ENCS, ECL, PICCS) |
| Other names | α-Methyltoluene; EB; Ethyl benzene; Ethylbenzol; NSC 406903; Phenylethane; UN 1175; UN 1175 (DOT) Aethylbenzol; Ethylbenzeen; Etilbenzene; Etylobenzen |
| Chemical group (DSL Stream) |
Discrete organics |
| Major chemical class or use | Cyclic organic |
| Major chemical subclass | Monoaromatic hydrocarbon |
| Chemical formula | C8H10 |
| Chemical structure | 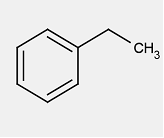 |
| SMILESFootnote Table 2-1[b] | CCc1ccccc1 |
| Molecular mass | 106.17 g/mol |
3. Physical and Chemical Properties
The experimental and modeled physical and chemical properties of ethylbenzene relevant to its environmental fate are summarized in Table 2.
| Property | Type | ValueFootnote Table 3-1[a].1 | Temperature (°C) |
Reference |
|---|---|---|---|---|
| Physical characteristics | colourless, flammable liquid | 20 | O'Neil et al. 2006 | |
| Melting point (°C) | Experimental | -94.9 to -95 | - | Mabey et al. 1982; O'Neil et al. 2006; Lide and Haynes 2010 |
| Melting point (°C) | Modelled | -46.94 | - | MPBPWIN 2008 |
| Boiling point (°C) | Experimental | 136.2 | - | Mabey et al. 1982; O'Neil et al. 2006; Lide and Haynes 2010 |
| Boiling point (°C) | Modelled | 148.30 | - | MPBPWIN 2008 |
| Density (kg/m3) | Experimental | 866 | 20 | O'Neil et al. 2006; |
| Density (kg/m3) | Experimental | 863 (0.8626 g/mL) |
25 | Lide and Haynes 2010 |
| Vapour pressure (Pa) | Experimental | 930 (7 torr) |
20 | Mabey et al. 1982; O'Neil et al. 2006 |
| Vapour pressure (Pa) | Experimental | 1280 (9.6 mm Hg) |
25 | Daubert and Danner 1985 |
| Vapour pressure (Pa) | Experimental | 1270 | 25 | ATSDR 2010 |
| Vapour pressure (Pa) | Modelled | 1010 (7.596 mm Hg) |
25 | MPBPWIN 2008 |
| Henry's law constant (Pa·m3/mol) | Experimental | 675 (0.0066 atm·m3/mol) |
20 | Mabey et al. 1982 |
| Henry's law constant (Pa·m3/mol) | Experimental | 854 (0.00843 atm·m3/mol) |
25 | Mackay et al. 1979 |
| Henry's law constant (Pa·m3/mol) | Experimental | 798 (0.00788 atm·m3/mol) |
25 | Sanemesa et al. 1982 |
| Henry's law constant (Pa·m3/mol) | Modelled | 800Footnote Table 3-1[b].1 | 25 | HENRYWIN 2008 |
| Log Kow (octanol-water partition coefficient) (dimensionless) |
Experimental | 3.13-3.15 | 25 | Tewari et al. 1982; Kamlet et al. 1988 |
| Log Kow (octanol-water partition coefficient) (dimensionless) |
Modelled | 3.03 | - | KOWWIN 2008 |
| Log Koc (organic carbon-water partition coefficient) (dimensionless) |
Experimental | 2.21Footnote Table 3-1[c] (soil OMFootnote Table 3-1[d]) |
- | Chiou et al. 1983; OECD 2005 |
| Log Koc (organic carbon-water partition coefficient) (dimensionless) |
Experimental | 3.04 (sediment OM) |
- | Mabey et al. 1982 |
| Log Koc (organic carbon-water partition coefficient) (dimensionless) |
Modelled | 2.65-2.73 | - | KOCWIN 2008 |
| Water solubility (mg/L) | Experimental | 140 | 15 | Verschueren 1983 |
| Water solubility (mg/L) | Experimental | 152 | 20 | Verschueren 1983 |
| Water solubility (mg/L) | Experimental | 111 (seawater) | 25 | Verschueren 1983 |
| Water solubility (mg/L) | Experimental | 169 | 25 | Verschueren 1983 |
| Water solubility (mg/L) | Modelled | 52.19 | 25 | WSKOWWIN 2008 |
4. Sources
Ethylbenzene is naturally present in crude oil and some natural gas streams and is a by-product of petroleum and coal refining (IPCS 1996; CAPP 2006; EURAR 2007; VCCEP 2007). It is also produced by incomplete combustion of natural materials, which makes it a component of forest fire or wood burning smoke (IPCS 1996; IARC 2000). Anthropogenic sources of ethylbenzene to the environment include releases from petrochemical plants, coal power plants, landfills, contaminated sites, and gasoline (e.g., evaporative emissions from vehicles and service stations; CONCAWE 1997). As a natural constituent of petroleum substances, ethylbenzene is often found in emissions from industrial activities related to the upstream oil and gas sector (glycol dehydrators, oil sands upgrading, and fugitive equipment leaks; Picard et al. 2002), the petroleum refining sector (manufacture, processing, use, storage, and disposal), and the combustion of vehicle and aviation fuels and coal (IPCS 1996; German Chemical Society 1997). Environmental tobacco smoke (ETS) has also been shown to be a source of ethylbenzene (Nelson et al 1998; Daisey et al. 1994; Xie et al. 2003).
Globally, the majority of manufactured ethylbenzene is produced by alkylating benzene with ethylene in the liquid phase with an aluminum chloride catalyst or in the vapour phase with a synthetic zeolite or Lewis acid catalyst (IARC 2000; Berthiaume and Ring 2006). Other methods of producing ethylbenzene include preparation from acetophenone, benzene, chlorobenzene, ethylenebenzene, naphthenes, and xylene (VCCEP 2007; ATSDR 2010). Ethylbenzene is also produced from the mixed xylenes stream in the petroleum refining industry (Fishbein 1985; Coty et al. 1987).
According to Camford Information Services (2004), two companies were manufacturing ethylbenzene in 2003 and a total of 906 kt of ethylbenzene was produced in the same year. Camford Information Services (2004) estimated that quantities of ethylbenzene manufactured in Canada have remained stable at 985 kt/year since 1999. More recent information on the manufacturing of ethylbenzene in Canada was not identified; approximately 650 002 kg of ethylbenzene were imported into Canada in 2013, and approximately 257 880 kg were exported the same year (CIMT 2014). Import and export quantities for the years 2000-2013 (CIMT 2010, 2014) are listed in Table 4-1. Import and export quantities have been variable over the years. Ethylbenzene has been internationally identified as a high production volume (HPV) chemical (OECD 2005).
| Year | Import quantities (kg) | Export quantities (kg) |
|---|---|---|
| 2013 | 650 002 | 257 880 |
| 2012 | 61 487 | 20 744 035 |
| 2011 | 126 358 | 480 517 |
| 2010 | 137 524 | 420 674 |
| 2009 | 545 147 | 90 290 |
| 2008 | 162 767 | 63 070 |
| 2007 | 133 315 | No data |
| 2006 | 116 588 | No data |
| 2005 | 161 656 | 35 474 963 |
| 2004 | 170 239 | 8 524 901 |
| 2003 | 130 640 | 18 167 873 |
| 2002 | 284 954 | 40 540 |
| 2001 | 164 154 | 30 289 662 |
| 2000 | 212 089 | 319 749 |
Based on information received in response to a notice issued under the authority of section 71 survey of CEPA (Canada 2001), approximately 1700 kilotonnes (kt) of ethylbenzene at a concentration higher than 1% were manufactured in or imported into Canada during the year 2000, mainly by companies in the petrochemical sector (Environment Canada 2004). In addition, several companies reported either importing or manufacturing ethylbenzene at a concentration lower than 1% and in a quantity meeting the reporting threshold of 10 000 kg (Environment Canada 2004). Both upstream petroleum producing facilities and downstream petroleum industries (refinery/petrochemical) responded as manufacturers of ethylbenzene. Because refineries are supplied by the extractors, it is possible that double counting had occurred; however, it was not possible to determine to what extent (Environment Canada 2004).
5. Uses
Globally, almost all ( greater than 99%) of the ethylbenzene commercially produced is used as an intermediate in the manufacture of styrene (IARC 2000; Berthiaume and Ring 2006). Styrene is subsequently used to produce various polymers including polystyrene, acrylonitrile-butadiene styrene, styrene-acrylonitrile, styrene-butadiene latexes, styrene-butadiene rubber, and unsaturated polyester resins (Berthiaume and Ring 2006; VCCEP 2007). These styrenic polymers are used in a variety of applications such as for food packaging, appliances, and sporting goods, in the automotive and electronic industry, and in building materials (VCCEP 2007). The remaining synthetically produced ethylbenzene is used as a solvent or occasionally in the production of diethylbenzene, acetophenone, ethyl anthraquinone, cellulose acetate, ethylbenzene sulfonic acids, propylene oxide, and a-methylbenzyl alcohol (Berthiaume and Ring 2006; ATSDR 2010).
The ethylbenzene that is naturally occurring in crude oil is a component of automotive and aviation fuels including gasoline (VCCEP 2007; Dow 2009). Levels of ethylbenzene in gasoline range from less than 1 to 5.4% (IARC 2000; FLL 2008). It is also a constituent of refined products including mixed xylenes at a concentration of 15 to 20%. Mixed xylenes are used as a solvent in various applications including spray paints, primers, paint removers and thinners, wood stains, and varnishes, as well as household and automotive cleaners (IPCS 1996; VCCEP 2007; Dow 2009). Ethylbenzene may also be a component of asphalt and naphtha (VCCEP 2007).
Ethylbenzene has been reported to be used as a component in a number of hydraulic fracturing fluids used in the United States (US) for developing and unlocking natural gas supplies in shale and other unconventional oil and gas formations across the country (US House of Representatives 2011).
In Canada, the results of a notice issued under the authority of section 71 of CEPA for the year 2000 reported the use of ethylbenzene as a feedstock for petrochemicals and other organic chemicals, as a solvent in paints and coatings, and in other solvent applications (Environment Canada 2004). According to Camford Information Services (2004), the majority of ethylbenzene in Canada is manufactured for its use in the production of styrene monomer with small amounts being used as a solvent. Ethylbenzene is also used in the oil and gas industry in a number of oilfield applications such as a non-emulsifier, an acid additive and as a surfactant in hydraulic fracturing fluids (FracFocus 2013).
Ethylbenzene is not an active ingredient in pest control products registered for use in Canada but is a formulant and is currently present in approximately 130 pest control products with concentrations ranging from close to zero to 3.2% (e-mail from Pest Management and Regulatory Agency, Health Canada to Risk Management Bureau, Health Canada, 2014; unreferenced). Ethylbenzene was identified in manicure preparation products in Canada (CNS 2010). Ethylbenzene is not currently listed on Health Canada's List of Prohibited and Restricted Cosmetic Ingredients (or The Cosmetic Ingredient Hotlist), an administrative tool that Health Canada uses to communicate to manufacturers and others that certain substances, when present in a cosmetic, may contravene (a) the general prohibition found in section 16 of the Food and Drugs Act or (b) a provision of the Cosmetic Regulations (Health Canada 2011).
The use of ethylbenzene in insecticides, printing inks, glues, perfumes, and pharmaceuticals has also been reported (IARC 2000; EURAR 2007; VCCEP 2007). Reported uses in other jurisdictions fall into the categories of manufacture, solvents, fuels, and coatings (HSDB 2009; ATSDR 2007). The Danish Environmental Protection Agency (EPA) also detected ethylbenzene in candles (Eggert et al. 2002), incense (Eggert and Hansen 2004), adult toys (Nilsson et al. 2006), products made of exotic wood (Witterseh 2004), printed material (Hansen and Eggert 2003), Christmas decorations (Danish EPA 2003), and waders and dive gloves made with chloroprene (Nilsson and Pedersen 2004).
6. Releases to the Environment
Ethylbenzene is released from facilities that manufacture the substance or use it as a solvent or as an intermediate in the production of other chemicals. It is also a component of vehicle exhaust after combustion (Health Canada 2004). Recent ethylbenzene releases reported to the National Pollutant Release Inventory (NPRI) (Environment Canada 2013b) by Canadian industries are presented in Table 4. Most releases occur to air, with smaller releases to water and land. The NPRI database indicates the following methods are used for the disposal of ethylbenzene: incineration, underground injection, physical treatment, containment landfill, and biological treatment (Environment Canada 2009).
The number of companies reporting to the NPRI for the years 1994 to 2011 has increased from 68 in 1994 to 233 in 2011. In 2011, facilities from across Canada reported to the NPRI on-site environmental releases totalling approximately 325 tonnes, transfers for disposal totalling 1800 tonnes, and transfers for recycling totalling 541 tonnes. The majority of the ethylbenzene released from glycol dehydrators is not reported to the NPRI due to reporting thresholds for the conventional oil and gas extraction.
| Year | On-site releases to air |
On-site releases to water |
On-site releases to land |
Disposal on-site |
Disposal off-site |
Off-site recycling |
|---|---|---|---|---|---|---|
| 2013 | 322 | 4.1 | 0.003 | 900 | 446 | 3482 |
| 2012 | 345 | 2.0 | 2.5 | 1241 | 434 | 1268 |
| 2011 | 328 | 2.4 | 0.011 | 1213 | 65 | 1215 |
| 2010 | 900 | 2.8 | 0.626 | 983 | 102 | 541 |
| 2009 | 838 | 4.1 | 0.125 | 1059 | 81 | 427 |
| 2008 | 940 | 3.8 | 0.069 | 966 | 121 | 1041 |
| 2007 | 599 | 3.0 | 0.343 | 914 | 112 | 977 |
| 2006 | 778 | 4.4 | 0.121 | 879 | 378 | 1328 |
| 2005 | 815 | 4.7 | 0.386 | 26 | 388 | 1242 |
| 2004 | 911 | 2.6 | 0.837 | 15 | 146 | 1021 |
As a component of benzene, toluene, ethylbenzene, and xylenes (BTEX) emissions, ethylbenzene is also released from glycol dehydrators used to remove water from natural gas prior to entering the pipeline (Health Canada 2004). In 2007, the estimated population of glycol dehydrators was 5,195 units. These units emit approximately 1470 tonnes of benzene per year (CAPP 2009). In a study of BTEX wet gas out of the Western Sedimentary Basin, ethylbenzene concentrations from glycol dehydrators were found to be approximately 8.4% of the benzene values, resulting in estimated ethylbenzene emissions from glycol dehydrators of approximately 123.5 tonnes per year (Murray 2010). Based on 2007 NPRI data for Oil and Gas extraction release, of the 137 tonnes released to air, 8.5 tonnes were from conventional upstream oil and gas extraction and 128.5 tonnes originated from oil sands extraction. Therefore, a minimum of 115 tonnes of additional ethylbenzene are released from glycol dehydrators.
Ethylbenzene may be released to the aquatic compartment through industrial or household waste effluents contaminated with ethylbenzene-containing products. Releases of ethylbenzene to soil may result from landfilling of industrial or household waste (Health Canada 2004).
According to the U.S. Toxics Release Inventory, total on- and off-site disposal and industrial releases of approximately 2 kt and 1.5 kt were reported in 2008 and 2009, respectively (US EPA 2009).
Industrial and non-industrial emissions to air were estimated for ethylbenzene in the Great Lakes region of the United States and Ontario. In 2001, 42.5 kt of ethylbenzene were released according to the Inventory of Toxic Air Emissions (Great Lakes Commission 2004). These emissions were attributed as follows: 28% from light-duty gasoline vehicles, 12% from light-duty gasoline trucks ( less than 2.7 tons [2.4 tonnes] gross vehicle weight), 12% from recreational vehicles, 8% from lawn and garden equipment, 7% from light-duty trucks (2.7-3.9 tons [2.4-3.5 tonnes] gross vehicle weight), 5% from pleasure craft, 5% from architectural surface coating, and 23% from other sources, where individual sectors contributed less than 5% of the total emissions. In 2002, the estimated ethylbenzene emissions to air were reported to be 32.4 kt (Great Lakes Commission 2006). The Ontario-specific emissions from all sources were estimated to be 3.7 kt and 3.8 kt in 2001 and 2002, respectively (Great Lakes Commission 2004, 2006).
Ethylbenzene concentrations have also been measured in individual releases to Canadian air, surface water, and groundwater from major anthropogenic sources, including from the upstream oil and gas sector, the petroleum refining sector, coal power plants, landfills, deep injection wells, and former gasworks sites; however, no total annual release quantities have been calculated. No release data were located for soil and sediment in Canada, but contamination of these media from petroleum-related activities and disposal sites is likely.
7. Environmental Fate
Environmental fate analysis combines information on the chemical behaviour of the substance with the properties of the receiving environment. The objective of fate analysis is to determine the multimedia distribution of the substance after its release into the environment. This includes consideration of the persistence and bioaccumulation of the substance in the environment.
The results of Level III fugacity modelling (Table 7-1) (EQC 2003) show that ethylbenzene is expected to remain mostly in the medium to which it is released: if emitted only to air, 99.3% of the ethylbenzene remains in the air; if released only to water or soil, 91.2% and 92.1% remain in these media, respectively.
| Substance released to: | Air % | Water % | Soil % | Sediment % |
|---|---|---|---|---|
| Air (100%) | 99.3 | 0.3 | 0.4 | 0.0 |
| Water (100%) | 7.6 | 91.2 | 0.0 | 1.2 |
| Soil (100%) | 7.6 | 0.3 | 92.1 | 0.0 |
8. Persistence and Bioaccumulation Potential
8.1 Environmental Persistence
Ethylbenzene is expected to persist in air but not in water, soil, or sediment, based on degradation half-lives (Table 8-1). Ethylbenzene mobility in soil is relatively low (Swann et al. 1983). It can however leach to groundwater, based on its moderate log organic carbon-water partition coefficient (Koc) of 2.21-3.04. Removal can also be by advection which is not affected by anaerobic conditions. Degradation in groundwater may be slower than in surface water owing to anaerobic conditions (Wilson et al. 1986, 1988).
| Medium | Fate process | Degradation value | Degradation endpoint / units | Reference |
|---|---|---|---|---|
| Air | Photodegradation | 7.0 × 10−12 | Rate coefficient / cm3·molecule-1·second-1 | Calvert et al. 2002 |
| Air | Photodegradation | 0.5-2.7 | Half-life / days | Singh et al. 1981; Ohta and Ohyama 1985; Atkinson 1989; Howard 1989 |
| Surface water | Biodegradation | 2 | Half-life / days | Bouwer and McCarty 1984 |
| Surface water | Volatilization | 13 (winter) 20 (spring) 2.1 (summer) |
Half-life / days | Wakeham et al. 1983 |
| Groundwater | Biodegradation | 4-4 (aerobic) |
Aronson et al. 1999 | |
| Groundwater | Biodegradation | 8-46 (anaerobic) |
Half-life / days | Kappeler and Wuhrmann 1978; Aronson and Howard 1997 |
| Wastewater treatment systems | Biodegradation | 71-96 | Activated sludge system, facultative lagoon and aerated lagoon, trickling filter | Hannah et al. 1986 |
| Landfill aquifer material | Biodegradation | 74 | Anaerobic biodegradation / % after 40 weeks | Wilson et al. 1986, 1988 |
| Soil | Biodegradation | 3-10 | Half-life / days | Howard 1991 |
| River sediments | Mineralization | 19 | Half-life / days | Ludzak and Ettinger 1963 |
Ethylbenzene is probably subject to long-range transport, based on an intermediate characteristic travel distance of 700-2000 km, estimated by TaPL3 fugacity modelling (Beyer et al. 2000; TaPL3 2000). According to the model, up to 5% of the mass fraction of the substance can travel farther than three times this distance. This is supported by the detection of ethylbenzene in the tissues of fish in remote areas (Lockhart et al. 1989, 1992) and in Antarctic snow (Desideri et al. 1994).
8.2 Potential for Bioaccumulation
Experimental and modelled log Kow values for ethylbenzene indicate that this chemical has low potential to bioaccumulate (see Table 3-1).
Ethylbenzene is not expected to bioaccumulate significantly in aquatic organisms, based on its highest reported bioaccumulation factor (log BAF) of 1.78 (BAF of 60), which is a calculated value (Park and Lee 1993), and its highest reported bioconcentration factor (log BCF) of 1.19 (BCF of 15.5), which was determined experimentally in goldfish, Carassius auratus (Ogata et al. 1984).
Based on the above, it is proposed that ethylbenzene does not meet the criteria for bioaccumulation (BAF or BCF greater than 5000) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
9. Potential to Cause Ecological Harm
9.1 Ecological Exposure Assessment
Ethylbenzene is expected to be found throughout Canada, given its persistence in air, its potential for long-range transport, and its numerous sources (including natural ones). Ethylbenzene concentrations measured in surface water, groundwater, soil, and biota in Canada and other relevant locations are summarized in Tables 7-10.
9.1.1 Air
The data and studies on ethylbenzene in ambient air in Canada are summarized in Appendix A. This appendix also contains data from five Canadian studies (Windsor, Regina, Halifax, Edmonton, and Ottawa) that measured ethylbenzene in outdoor air just outside of residencesp.
The National Air Pollution Surveillance (NAPS) program maintains an extensive database of ambient air concentrations monitored across Canada, including volatile organic compounds such as ethylbenzene. The NAPS program has been in existence since 1969 and currently has 368 monitoring sites in 255 communities located in every province and territory in Canada (Environment Canada 2009). Measured concentrations of ethylbenzene in air were compiled from 42 NAPS monitoring stations dating from 2005 to 2009 with a minimum of 90 samples per station for a total of 13 462 samples across Canada. However, only stations that measured ethylbenzene for all 5 years and therefore containing many samples were included in the compilation (31 stations). Mean 24-hour concentrations ranged from 0.103 to 1.28 μg/m3 with corresponding 95thpercentile 24-hour concentrations ranging from 0.206 to 4.40 μg/m3. The maximum ethylbenzene concentration measured across all NAPS monitoring stations from 2005 to 2009 occurred within the Burnaby area of the Greater Vancouver Regional District, British Colombia with a value of 35.84 μg/m3 (with a mean and 95% percentile value of 0.71 ± 3.07 and 1.06 μg/m3, respectively, for that monitoring station between 2005 and 2009) (Environment Canada 2011a). An analysis of the NAPS data from 2010 to 2012 showed ethylbenzene concentrations fell within the same range as those reported from 2005-2009.
Published data on ethylbenzene in air are also available from a number of sites in Alberta (Alberta Environment 2005, 2010; FAP 2010). Fort Air Partnership (FAP), a multi-stakeholder group with members from industry, government, and the public, monitored eight permanent continuous ambient air quality monitoring stations in an area northeast of Edmonton in 2009. Five sites are in the immediate vicinity of petrochemical and oil and gas facilities, one is not in close proximity to an industry site, one is located in the city of Fort Saskatchewan, and one station is located in Elk Island National Park.
Ethylbenzene was measured on a semi-continuous (four samples per hour) basis at the Scotford 2 Air Quality Monitoring station which monitors local industrial emissions on air quality. Industries monitored at the station include: Shell Canada Energy Scotford Upgrader, Shell Canada Products Scotford Oil Refinery, Shell Chemicals Canada Ltd. Styrene and MEG Plant, and BA Energy Heatland Bitumen Upgrader. Ethylbenzene concentrations were measured below the limit of detection (detection limit of 0.08 µg/m3) more than 87% of the time and a maximum value of 87.7 µg/m3 was measured over the year.
Alberta Environment investigated odour complaints from February to May 2010 from the Three Creeks area, Alberta (Alberta Environment 2010). Air samples were collected at the sites by drawing air into 6 liter evacuated stainless steel canisters. Two types of sample collection methods were used: i) the sample was drawn in at a constant rate for a period of time or ii) the canister was used to grab instantaneous samples. The first method resulted in an integrated sample and the concentrations quantified using this method was an average for the sample time. Air was sampled for 1 hour at seven sites and a 10-minute sampling interval was used at the eighth site. A fifteen minute sampling interval was used at the eighth site. One-hour average concentrations ranging from 0.29 to 4.03 µg/m3 were reported.
Ethylbenzene was detected in air in a study of volatile organic compounds sampled every day for 24 hours at two sites commencing 12 September 2004 to 30 March 2006 in an area with more than 30 major industrial facilities in Fort Saskatchewan, Alberta (Mintz and McWhinney 2008). Maximum concentrations ranged from 2.14 to 6.49 μg/m3. You et al. (2008) observed that oil and gas facilities contributed to airborne concentrations of ethylbenzene (maximum of 6.21 μg/m3) in rural western Canada.
Atari and Luginaah (2009) monitored ethylbenzene using 37 samplers in Sarnia, Ontario, where more than 40% of Canadian chemicals are manufactured. A mean of 0.46 μg/m3 and maximum of 1.06 μg/m3 ethylbenzene in air was measured over 2 weeks in October 2005. Miller et al. (2009) carried out a similar study using the same sampling sites as Atari and Luginaah (2009) but focused on the spatial variability of ethylbenzene in Sarnia during October 2005. Results indicated that spatial variability is significant in Sarnia with high pollution occurring where there is a cluster of industrial and chemical facilities or in areas that are a short distance downwind of these facilities.
Ethylbenzene was monitored as part of an ambient air monitoring program at six stations over approximately 2 years (1 June 2003 to 31 March 2005) in the Clarkson Airshed (Oakville and Mississauga, Ontario) (OMOE 2006). The highest annual average concentration was 1.46 μg/m3, and a maximum 24-hour value of 9.63 μg/m3 was reported for the substance. Badjagbo et al. (2009) presented results for three urban locations in Canada (general mechanics garage, storm drain of an industrial waste landfill site, two-lane street in an industrial area) and found mean concentrations ranging from less than 8 μg/m3 to 13 μg/m3.
In 2007, 60% of the biggest landfills (permitted to receive 40 000 tonnes of waste per year) captured their landfill gas and 95% captured their leachate. Only 5% used no treatment, only natural attenuation, to treat their leachate (Conestoga Rovers and Associates 2009). Ethylbenzene is expected to be present in landfill gas, but assumed it will be destroyed at a 99% rate by combustion.
9.1.2 Surface Water
Canadian surface water data for ethylbenzene are limited to measurements made as part of the Municipal/Industrial Strategy for Abatement, a provincial monitoring program of the Ontario petroleum refining sector conducted from 1 December 1988 to 30 November 1989 (OMOE 1990, 1992). Concentrations of ethylbenzene were measured in industrial process effluent streams, cooling and intake water, farmland leachate, and storm-water effluent (see Table 9-1).
The Sarnia-Lambton Environmental Association (SLEA), a voluntary environmental association co-operative of 20 industrial facilities in Lambton County, Ontario, has been monitoring air and water quality along the St. Clair River since 1988. Levels of ethylbenzene in the river have ranged from a maximum value of 285 µg/L in 1990 down to 1 µg/L in 1995 (SLEA 2007-2008). Maximum concentrations of ethylbenzene in the St. Clair River decreased in 2007 and 2008 with results of 0.15 and 0.09 µg/L, respectively.
| Media | Details | Mean concentrationFootnote Table 9-1[a].2 (µg/L) | Maximum concentration (µg/L) | Reference |
|---|---|---|---|---|
| Surface water | 150 samples of intake water from an Esso Sarnia plant in Ontario | 0.71* | 31.2 | OMOE 1992 |
| Surface water | St. Clair River | 0.09-0.15 | SLEA 2007-2008 | |
| Process effluent stream water | 1095 samples of process effluent stream water at 7 refineries in Ontario | 0.347 | 0.060-24.300 | OMOE 1992 |
| Once-through cooling water effluent stream | 143 samples of once-through cooling water effluent stream at 4 refineries in Ontario | 1.101 | 0.180-43.390 | OMOE 1992 |
| Landfarm leachate | 25 samples of landfarm leachate at 2 refineries in Ontario | 0.234 | 0.060-0.36 | OMOE 1992 |
| Storm-water effluent stream | 150 samples of storm-water effluent stream at 3 refineries in Ontario | 0.443 | 0.060-13.810 | OMOE 1992 |
9.1.3 Groundwater
Groundwater concentrations of ethylbenzene, often measured with other BTEX (benzene, toluene, ethylbenzene, xylenes) chemicals, are available for several contaminated sites in Ontario (Reinhard et al. 1984; Jackson et al. 1985; Cherry 1987; Barker 1988; Barker et al. 1989; Lesage et al. 1990a, 1990b, 1991, 1993, 1997; MacRitchie et al. 1994; OMOE 2005), as well as for natural background levels at other locations in Canada (Reinhard et al. 1984; Cherry 1987) (see Table 9-2).
| Details | ConcentrationFootnote Table 8[a] (µg/L) | Reference |
|---|---|---|
| Background concentration, Canada | 0.1 | Reinhard et al. 1984 |
| Background concentration, North Bay, Ontario | 0.1 | Cherry 1987 |
| 5 sites in the Niagara Falls area, Ontario | 1-3 | Lesage et al. 1997 |
| From gasoline stations (on-site): Scarborough, Ontario Aurora, Ontario Flamborough County, Ontario (total of 56 groundwater samples) |
111 (median) 541 (median) 1 (median) |
Lesage et al. 1997 |
| Concentrations measured in contaminant plume in groundwater at the landfill in North Bay, Ontario | 0.03-14 | Cherry 1987 |
| Westbay multilevel monitoring well (8 different depths from surface in 1988) installed close to industrial waste disposal wells that had been used for deep injection of liquid waste in order to compare these BTEXFootnote Table 8[b] concentrations with those at other sampling wells in shallow aquifers | 5-133 | Lesage et al. 1991 |
| Leachate concentrations at: Landfill, Guelph, Ontario (1988 and 1989) and Landfill, Muskoka, Ontario (1989) |
35-83 | Lesage et al. 1993 |
| At 5 of 6 southern Ontario landfills (Old Borden, North Bay, New Borden, Upper Ottawa Street, Woolwich, Tricil) (note: this is a summary report; values from this report are also presented below) | Maximum range 1-3320 | Barker 1988 |
| Landfill, North Bay, Ontario, measured 460 m off-site | 58* | Barker et al. 1989 |
| Landfills: North Bay, Old Borden, New Borden, Upper Ottawa Street, Woolwich, and Tricil, Ontario | less than 1-3320 | Barker et al. 1989; MacRitchie et al. 1994 |
| Landfill, Elmira, Ontario (monitoring wells installed beside or into former waste disposal lagoons) |
2000-120 000 | Lesage et al. 1990b |
| Concentrations measured at different wells within the landfill in Woolwich, Ontario (1981), and the landfill in North Bay, Ontario (1981) | 0.08-480 | Reinhard et al. 1984 |
| Concentrations measured at different wells within the landfill in Gloucester, Ontario (1982) | 0.6-38 | Jackson et al. 1985 |
| Landfill, Gloucester, Ontario (1988) In outwash aquifer: 3% frequency of identification of ethylbenzene in 37 samples collected |
2 | Lesage et al. 1990a |
| Detected in 1 of 5 monitoring wells | 3 | Lesage et al. 1990a |
| Various brownfield sites in Ottawa and Toronto, Ontario | 1.09-1.5 | OMOE 2005 |
9.1.4 Soil
No data were found for concentrations of ethylbenzene in Canadian sediment. Soil data were found for three parkland sites in Ontario (OMEE 1993), and more recent data are available for Ontario sites in the Brownfields Environmental Site Registry (OMOE 2005) (see Table 9). However, both of these data sources pertain to contaminated sites and do not provide details on soil sampling sites or methodology.
| Details | ConcentrationFootnote Table 9-3[a].4 (µg/kg) | Reference |
|---|---|---|
| Rural parkland in Ontario | 0.46* | OMEE 1993 |
| Old urban parkland in Ontario | 0.40 | |
| Various brownfield sites in Ottawa and Toronto, Ontario | 40-50 | OMOE 2005 |
9.1.5 Biota
Data available for levels of ethylbenzene in biota are for fish and are presented in Table 9-4. Concentrations of ethylbenzene in Burbot and Whitefish muscle tissue were higher than in the Burbot liver tissue from the Northwest Territories, Canada. Mean levels ranged from 2.45 to 104 μg/kg in muscle tissue compared to 1.81 to 46.3 in Burbot liver tissue. (Lockhart et al 1992).
| Details | Mean Concentration (µg/kg) | Maximum Concentration (µg/kg) |
Reference |
|---|---|---|---|
| Burbot, Lota lota, muscle tissue from Mackenzie River, Northwest Territories | 2.45-49.6 | 115 | Lockhart et al. 1992 |
| Burbot liver tissue from Mackenzie River, Northwest Territories | 1.81-46.3 | 84 | Lockhart et al. 1992 |
| Whitefish, Coregonus clupeaformis, muscle tissue from Mackenzie River, Northwest Territories | 7.46-104 | 273 | Lockhart et al. 1992 |
9.2 Ecological Effects Assessment
Key toxicity studies for aquatic and soil organisms are presented in Tables 11 and 12. Acute and chronic endpoint values for fish, aquatic invertebrates, and algae fall in the range of 1-10 mg/L (Table 11), indicating that ethylbenzene is moderately toxic to aquatic species. Among the more sensitive species are the freshwater water flea, Daphnia magna, with the lowest 48-hour EC50 of 1.8 mg/L (Vigano 1993) and the estuarine mysid shrimp, Mysidopsis bahia, with a 96-hour LC50 of 2.6 mg/L (Masten et al. 1994). In addition, Niederlehner et al. (1998) reported a 7-day No-Observed-Effect Concentration (NOEC) and Lowest-Observed-Effect Concentration (LOEC) of 1.0 and 1.7 mg/L, respectively, for significantly reduced reproduction in the freshwater water flea, Ceriodaphnia dubia, while Tsai and Chen (2007) used a novel closed-system testing technique to determine a 48-hour EC50 of 1.34 mg/L for significantly inhibited growth in the freshwater green alga, Pseudokirchneriella subcapitata.
| Classification | Test organism | Endpoint | Value (mg/L)Footnote Table 9-5[a].5 | Reference |
|---|---|---|---|---|
| Vertebrate | Atlantic silverside, Menidia menidia |
96-hour LC50Footnote Table 9-5[b] (mortality) |
5.1 | Masten et al. 1994 |
| Vertebrate | Fathead minnow, Pimephales promelas |
96-hour LC50 (mortality) | 9.1 | Brooke 1987 |
| Vertebrate | Rainbow trout, Oncorhynchus mykissFootnote Table 9-5[c].1 |
96-hour LC50 (mortality) | 4.2 | Galassi et al. 1988 |
| Vertebrate | Guppy, Poecilia reticulata |
96-hour LC50 (mortality) |
9.6 | Galassi et al. 1988 |
| Invertebrate | Water flea, Ceriodaphnia dubia |
2-day LC50 (mortality) |
3.2 (30 µM) |
Niederlehner et al. 1998 |
| Invertebrate | Water flea, Ceriodaphnia dubia |
7-day LC50 (mortality) |
3.6 (34 µM) |
Niederlehner et al. 1998 |
| Invertebrate | Water flea, Ceriodaphnia dubia |
7-day NOECFootnote Table 9-5[d](reproduction) | 1.0 (9 µM) |
Niederlehner et al. 1998 |
| Invertebrate | Water flea, Ceriodaphnia dubia |
7-day LOECFootnote Table 9-5[e] (reproduction) |
1.7 (16 µM) |
Niederlehner et al. 1998 |
| Invertebrate | Water flea, Ceriodaphnia dubia |
7-day IC50Footnote Table 9-5[f] (reproduction) |
3.3 (31 µM) |
Niederlehner et al. 1998 |
| Invertebrate | Water flea, Daphnia magna |
24-hour IC50 (immobilization) |
2.2 | Galassi et al. 1988 |
| Invertebrate | Water flea, Daphnia magna |
48-hour EC50Footnote Table 9-5[g] (immobilization) |
2.9 | MacLean and Doe 1989 |
| Invertebrate | Water flea, Daphnia magna |
48-hour EC50 (immobilization) |
1.8-2.4 | Vigano 1993 |
| Invertebrate | Brine shrimp, Artemia salina |
48-hour EC50 (immobilization) |
9.2 | MacLean and Doe 1989 |
| Invertebrate | Mysid shrimp, Mysidopsis bahia |
96-hour LC50 (mortality) | 2.6 | Masten et al. 1994 |
| Plant | Diatom, Skeletonema costatum |
96-hour EC50 (growth inhibition) |
7.7 | Masten et al. 1994 |
| Plant | Green alga, Pseudokirchneriella subcapitata |
48-hour EC50 (growth inhibition) |
1.3 | Tsai and Chen 2007 |
| Plant | Green alga, Selenastrum capricornutumFootnote Table 9-5[h] |
72-hour EC50 (growth inhibition) |
4.6 | Galassi et al. 1988 |
| Plant | Green alga, Selenastrum capricornutumh |
96-hour EC50 (growth inhibition) |
3.6 | Masten et al. 1994 |
Toxicity data for soil organisms are limited to two studies. One study by Neuhauser et al. (1985), which determined an LC50 of 47 µg/cm2 for the earthworm, Eisenia fetida, used filter paper rather than actual soil as the substrate. A more recent study by ESG International, Inc. (2002), with soil concentrations recalculated by Komex International Ltd. (2002), reported acute toxicity values (14-day LC25) for a soil invertebrate, the hexapod collembolan, Onychiurus folsomi, of 576 mg/kg dry weight (dw) for coarse sandy loam soil and 259 mg/kg dw for fine clay loam soil. ESG International, Inc. (2002) also reported a 14-day NOEC for the earthworm, Eisenia andrei, of 16 mg/kg dw and a 14-day LOEC of 112 mg/kg dw in coarse sandy loam soil. Komex International Ltd. (2002) recalculated the values from ESG International, Inc. (2002) and reported a NOEC of 16 mg/kg dw and a LOEC of 112 mg/kg dw for a 14-day exposure of the earthworm in fine clay loam soil (Table 9-6).
| Organism | Endpoint | Concentration (mg/kg dwFootnote Table 9-6[a].6) | Reference |
|---|---|---|---|
| Collembola (springtail), Onychiurus folsomi |
14-day LC25Footnote Table 9-6[b].4 (mortality) |
576 (coarse sandy loam) |
ESG International, Inc. 2002 |
| Earthworm, Eisenia andrei |
14-day NOECFootnote Table 9-6[c].2 | 16 (coarse sandy loam, and fine clay loam) |
ESG International, Inc. 2002; Komex International Ltd. 2002 |
| Collembola (springtail), Onychiurus folsomi |
14-day NOECc | 259 (fine clay loam) |
Komex International Ltd. 2002 |
| Earthworm, Eisenia andrei |
14-day LOECFootnote Table 9-6[d].2 | 112 (coarse sandy loam, and fine clay loam) |
ESG International, Inc. 2002; Komex International Ltd. 2002 |
| Northern wheatgrass, Agropyron dasystachyum |
14-day IC25Footnote Table 9-6[e].1 (reduction of root wet mass) |
3 (coarse sandy loam) |
ESG International, Inc. 2002 |
| Northern wheatgrass, Agropyron dasystachyum |
14-day IC25e (reduction of root wet mass) |
218 (fine clay loam) |
Komex International Ltd. 2002 |
| Alfalfa, Medicago sativa |
14-day IC25 (reduction of root length) |
462 (coarse sandy loam) |
Komex International Ltd. 2002 |
| Alfalfa, Medicago sativa |
14-day IC25 (reduction of root length) |
316 (fine clay loam) |
Komex International Ltd. 2002 |
In terms of soil toxicity to plants, the ESG and Komex studies reported the most sensitive endpoint for northern wheatgrass, Agropyron dasystachyum, to be significantly reduced root wet mass, with 14-day IC25 values of 3 mg/kg dw for coarse sandy loam soil and 218 mg/kg dw for fine clay loam soil (ESG International, Inc. 2002; Komex International Ltd. 2002). The most sensitive endpoint for alfalfa, Medicago sativa, was a significant reduction in root length, with 14-day IC25values of 462 and 316 mg/kg dw for coarse sandy loam and fine clay loam, respectively (Table 9-6).
No toxicity data were found for terrestrial wildlife; however, laboratory studies using rodents and other mammals have been conducted to evaluate the potential for impacts on human health, and relevant data from these studies are considered here in the context of terrestrial wildlife species. The results indicate that chronic inhalation exposure to ethylbenzene may be associated with organ damage, reproductive and developmental effects, and possible carcinogenicity in mammals (see Human Health Effects section). The study endpoint value considered most relevant to potential effects in terrestrial wildlife is a LOEC of 326 mg/m3 (75 ppm) reported for increased severity of nephropathy in female rats exposed to ethylbenzene for 104 weeks (6 hours/day, 5 days/week) (NTP 1999).
9.3 Characterization of Ecological Risk
The approach taken in this ecological screening assessment is to examine various supporting information and develop conclusions based on a weight of evidence approach as required under CEPA. Particular consideration has been given to risk quotient analyses, as well as persistence, bioaccumulation, and trends in ambient concentrations.
Risk Quotient Analysis
Risk quotient (RQ) analyses, integrating known or potential exposures with known or potential adverse ecological effects, were performed for each relevant compartment. This involved first selecting a Critical Toxicity Value (CTV) based on the most sensitive species of the compartment. A Predicted No-Effect Concentration (PNEC) was then derived from the CTV by applying an assessment factor (AF) to account for the following sources of uncertainty: (1) inter- and intraspecies variations in sensitivity, (2) extrapolation of results from laboratory to field, and (3) the use of short-term studies to model long-term exposure. For each medium, a Predicted Exposure Concentration (PEC) was selected for conservative exposure scenarios based on reasonable worst-case situations. PECs, CTVs, AFs, PNECs, and resulting RQs for each medium are presented in Table 9-7. A RQ value of greater than 1 suggests the possibility of adverse effects.
| Medium/ exposure scenario | Organism | Endpoint | CTV | Reference | AF | PNEC | PECFootnote Table 9-7[a].7 | RQ |
|---|---|---|---|---|---|---|---|---|
| Air | Rat | 104-week LOEC | 326 mg/m3 | NTP 1999 | 100 | 3.26 mg/m3 | 4.40 µg/m3 | 0.001 |
| Surface water | C. dubia | 7-d LOEC | 1.7 mg/L | Niederlehner et al. 1998 | 10 | 0.17 mg/L |
0.000 71 mg/L | 0.004 |
| Ground- water |
C. dubia | 7-d LOEC | 1.7 mg/L | Niederlehner et al. 1998 | 100 | 0.017 mg/L | 0.058 mg/L | 3.4 |
| Sediment (pore water) |
C. dubia | 7-d LOEC | 1.7 mg/L | Niederlehner et al. 1998 | 100 | 0.017 mg/L | 0.0058 mg/L | 0.34 |
| Soil (pore water) |
C. dubia | 7-d LOEC | 1.7 mg/L | Niederlehner et al. 1998 | 100 | 0.017 mg/L | 0.000 14 mg/L | 0.008 |
| Soil | Northern wheatgrass | 14-day IC25 | 3 mg/kg dw | ESG International, Inc. 2002; Komex International Ltd. 2002 | 100 | 0.03 mg/kg | 0.000 46 mg/kg | 0.02 |
The first scenario was developed for exposure of terrestrial wildlife to ethylbenzene in air. Given the lack of toxicity data for terrestrial wildlife, data for laboratory mammals were considered in choosing the CTV. The study endpoint value considered most relevant to potential effects in terrestrial wildlife is a LOEC of 326 mg/m3 (75 ppm) reported for increased severity of nephropathy in female rats exposed for 104 weeks (6 hours/day, 5 days/week) (NTP 1999). A conservative AF of 100 was applied to this chronic endpoint to account for species variability and the extrapolation from laboratory to field conditions. The resulting PNECair is 3.26 mg/m3.
A 95th percentile ambient air concentration of 4.40 µg/m3 was selected as the worst-case predicted environmental concentration (PEC) (Environment Canada 2011a, Appendix A). This site is located in Montreal, Québec. Therefore, the conservative RQ for exposure of terrestrial wildlife to ethylbenzene in air is
RQAir1 = PEC / PNEC = 4.40 µg/m3 / 3260 μg/m3 = 0.001.
For the aquatic compartment, exposure scenarios were analyzed for both surface water and groundwater. The lowest chronic effect value, a 7-day LOEC of 1.7 mg/L for significantly reduced reproduction in Ceriodaphnia dubia (Niederlehner et al. 1998), was selected as the CTV for both surface water and groundwater assuming comparative sensitivity between surface water and groundwater invertebrates to ethylbenzene. Given the comparatively rich empirical database for toxicity to surface water species (Table 9-5), an AF of 10 was applied to the CTV to yield a PNEC value of 0.17 mg/L. In the absence of empirical data for groundwater organisms, a larger AF of 100 was used and the resulting PNEC for groundwater is therefore 0.017 mg/L.
For the surface water scenario, Canadian data are mostly limited to measurements made as part of a provincial monitoring program of the Ontario petroleum refining sector in 1988-1989 (OMOE 1992). The maximum ethylbenzene concentration found in intake water (which would correspond to surface water before use in any chemical process) is reported to be 31.2 µg/L for a station outside the Esso Sarnia plant. The PEC was selected as this site's average concentration (0.71 µg/L) based on 150 measurements (see Table 9-1). The RQ for surface water can be calculated as follows:
RQSurface Water = PEC / PNEC = 0.000 71 mg/L / 0.17 mg/L = 0.004.
For the groundwater exposure scenario, the PEC was selected as 58 µg/L (0.058 mg/L), the highest dissolved concentration of ethylbenzene measured in groundwater near a landfill in North Bay, Ontario (Barker et al. 1989). Higher concentrations have been reported (Table 9-2), but this study was chosen for its good quality in terms of experimental design of monitoring and sampling. As opposed to other monitoring reports, Barker et al. (1989) clearly outlined the geological and hydrological situation of the site and the different wells and described the sampling site selection for measured concentrations of ethylbenzene in groundwater. The selected value, 58 µg/L, corresponds to the maximum concentration found in an off-site sampling well at a distance of approximately 460 m downstream from the landfill (Barker et al. 1989). Although higher ethylbenzene concentrations were identified closer to the landfill, Barker et al. (1989) demonstrated clearly that with increasing distance from the main source, the concentrations decrease rapidly. At a distance of 620 m downstream from the groundwater flow, ethylbenzene could not be detected at one well and was measured at 2.1 µg/L at another well. The authors assumed that this large decrease in concentrations was due to microbial degradation. According to provincial regulations (e.g., the Hazardous Waste Regulation of the British Columbia Environmental Management Act), a distance of 300 m between hazardous waste sites and landfill sites and the closest surface water body must be respected (Government of British Columbia 1988). Assuming that the sensitivities of groundwater invertebrates are similar to those inhabiting surface waters, the RQ for groundwater can be calculated as follows:
RQGroundwater = PEC / PNEC = 0.058 mg/L / 0.017 mg/L = 3.4.
For sediment exposure (to determine a PEC for sediment pore water concentrations), a scenario was developed in which ethylbenzene in groundwater discharges into a surface water body, such as a river or creek, or into a wetland. Given its properties (moderate log Koc of 2.65-3.04 and water solubility of 111-169 mg/L), ethylbenzene would not adsorb significantly to soil particles but instead would partition into the pore water of river sediments and possibly present a risk to benthic organisms. For this scenario, the concentration of ethylbenzene in sediment pore water is estimated to be comparable with an ethylbenzene concentration found in groundwater at a distance of 460 m from the main cell of the landfill in North Bay (Barker et al. 1989). At the North Bay landfill, the groundwater flow actually crosses a sandy aquifer containing springs and wetlands before discharging first into the Chippewa Creek about 800 m from the landfill site and then into other rivers downstream (Barker et al. 1989). Therefore, the PECpore water for sediments was derived using the groundwater concentration (0.058 mg/L, Barker et al. 1989) and an application factor (AF)Footnote[2] of 10 to account for the uncertainty in estimating the PECpore water based on a measured groundwater concentration.
In the absence of suitable sediment toxicity data, the CTV selected for sediments is the same as the CTV for the aquatic scenario (i.e., 7-d LOEC of 1.7 mg/L for Ceriodaphnia dubia), when C. dubia is used as a surrogate for benthic organisms. A PNEC of 0.017 mg/L is then obtained by applying an AF of 100. Thus,the RQ for sediment is
RQ Sediment = PECpore water / PNECbenthic invertebrate
= 0.0058 mg/L / 0.017 mg/L = 0.34,
where PECpore water = PECgroundwater / AF.
For the soil compartment, two different scenarios were analyzed because of the limited exposure data available. The highest soil concentration published for a Canadian site other than a brownfield or other urban site (see Table 9-3) is 0.00046 mg/kg dw, from rural parkland in Ontario (OMEE 1993). No details of the sampling and analytical methods could be located. In the absence of more reliable or more recent data for non-urban soil concentrations, for the first exposure scenario, a soil PEC was developed from this bulk soil PEC, based on the equilibrium partitioning of pore water and soil carbon (DiToro et al. 1991). More specifically, the PECpore water for soil was calculated with the following equation, which is based on an equation originally developed for sediment (DiToro et al. 1991):
PECsoil pore water (mg/L) = PECbulk soil(mg/L) / [Koc-soil (L/kg) × foc (no units)],
where
PECbulk soil = 0.00046 mg/kg dw soil in rural parkland (OMEE 1993)
Koc-soil = partition coefficient for soil (L/kg) = 163 (Chiou et al. 1983; OECD 2005)
foc = organic carbon content (default value for soil is 2% = 0.02)
Therefore:
PECsoil pore water = 0.00046 mg/kg / (163 L/kg × 0.02) = 0.000 14 mg/L.
For this scenario, the CTV for aquatic invertebrates (7-d LOEC of 1.7 mg/L for C. dubia) was selected as representative of a CTV for soil invertebrates exposed to pore water. Dividing the CTV by an AF of 100 results in a PNECsoil invertebratesof 0.017 mg/L. The RQ is calculated as follows:
RQsoil pore water = PECsoil pore water / PNECsoil invertebrates
= 0.00014 mg/L / 0.017 mg/L = 0.008.
For comparison, in a second scenario, a RQsoil was derived directly from the PECbulk soil of 0.00046 mg/kg dw from soil in rural parkland (OMEE 1993) and a CTV based on the northern wheatgrass, Agropyron dasystachyum, 14-day IC25 value of 3 mg/kg dw for coarse sandy loam soil (ESG International, Inc. 2002; Komex International Ltd. 2002). Applying an AF of 100 to the CTV results in a PNEC of 0.03 mg/kg dw.
RQsoil = PECbulk soil / PNECplant = 0.00046 mg/kg / 0.03 mg/kg = 0.02.
9.3.1 Consideration of Lines of Evidence and Conclusion
The approach taken in this ecological screening assessment was to examine various supporting information and develop conclusions based on a weight-of-evidence approach, using precaution as required under CEPA. Lines of evidence considered include results from a risk quotient analysis, as well as information on persistence, bioaccumulation, ecotoxicity, sources, and fate of the substance.
Ethylbenzene meets the Persistence and Bioaccumulation Regulations (Canada 2000) criteria for persistence in air but does not meet the criteria for water, sediment, and soil. Ethylbenzene does not meet the bioaccumulation criteria as specified in the Persistence and Bioaccumulation Regulations. The available toxicity data indicate that ethylbenzene is moderately toxic to aquatic and terrestrial species.
Ethylbenzene has many possible sources of release throughout Canada, mainly to air but also to other compartments, especially near disposal sites, and it is expected to be found in all media. Calculated RQs for air, surface water, sediment, and soil indicate that ethylbenzene concentrations in these compartments probably do not exceed concentrations associated with effects, even when conservative scenarios and assumptions are used. The RQ obtained for groundwater, however, exceeded 1 (a value of 3.4), which suggests some potential risk to organisms living in groundwater near landfills. However, there is high uncertainty relating to the lack of data for effects on groundwater organisms, which required use of an additional application factor.
The manufacture of ethylbenzene in Canada has remained relatively stable since 1999. Ethylbenzene is a high production volume (HPV) chemical. Reported industrial releases of ethylbenzene appear to have slightly increased in recent years with the number of reporting companies (mainly from the petrochemical industry). Other releases of ethylbenzene, especially as a product of fuel combustion, may be increasing as well, with increasing population and demand for energy. However, several regulations made under CEPA directly or indirectly limit hydrocarbon emissions from- on-road and off-road vehicles as well as from the refuelling of on-road vehicles. These include, but are not limited to the Gasoline and Gasoline Blend Dispensing Flow Rate Regulations, the Heavy-duty Vehicle and Engine Greenhouse Gas Emission Regulations, the On-Road Vehicle and Engine Emission Regulationsand the Passenger Automobile and Light Truck Greenhouse Gas Emission Regulations. Actual quantification of Canadian releases and exposure concentrations for this substance at this time is limited.
Based on the information available, there is low risk of harm to organisms or the broader integrity of the environment from this substance. It is therefore concluded that ethylbenzene does not meet the criteria under paragraph 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
9.3.2 Uncertainties in Evaluation of Ecological Risk
Uncertainties associated with the ecological screening assessment of ethylbenzene are discussed below.
A range of experimental values was available for certain physical/chemical properties. Although experimental data were used as input to models, some uncertainties are introduced in the resulting model output.
There were limited data available for ethylbenzene exposure concentrations in surface water, soil, and sediments. PECs were therefore based on older or extrapolated data, usually from contaminated sites or related sites representing worst-case exposure scenarios in air, water, sediment, and soil.
Many uncertainties are associated with the PNEC determinations. Toxicity data for aquatic organisms were readily available; however, for effects on soil organisms and terrestrial plants exposed to ethylbenzene in soil, only one recent study was found to be acceptable. No acceptable data were found for sediment or groundwater organisms exposed to ethylbenzene.
10. Potential to Cause Harm to Human Health
10.1 Exposure Assessment
Exposure to ethylbenzene through various environmental media has been well documented (HSDB 1983; VCCEP 2007; ATSDR 2010). According to results from Level III fugacity modelling (Table 7-1), ethylbenzene is expected to remain mostly in the medium to which it is released. Given that air is the predominant medium of release based on results from NPRI (Table 6-1), inhalation is expected to be the predominant route of human exposure. Data pertaining to concentrations of ethylbenzene in ambient air, indoor air, drinking water, food, and consumer products, identified for Canada and elsewhere, are presented in this section. Although numerous studies were identified, only those deemed most relevant for assessing ethylbenzene exposure for the general population of Canada are summarized.
10.1.1 Ambient Air
Appendix A lists the various Canadian studies that have measured ethylbenzene in outdoor air.
The National Air Pollution Surveillance (NAPS) program referred to in the Ecological Exposure Assessment Section was used to estimate human exposures to ethylbenzene in ambient air. Measured concentrations of ethylbenzene in air are available for 14 commercial, 13 residential, 2 industrial and 2 undeveloped rural NAPS monitoring stations in Canada dating from 2005 to 2009. For this screening assessment, commercial, residential and industrial monitoring stations are considered urban areas and undeveloped rural areas are considered rural. Among the rural and urban monitoring locations, for years 2005 to 2009, the mean 24-hour concentrations of ethylbenzene ranged from 0.199 to 1.08 μg/m3 and from 0.103 to 1.28 μg/m3, respectively. The 95th percentile 24-hour concentrations ranged from 0.586 to 2.54 μg/m3 for rural locations and from 0.206 to 4.40 μg/m3 for urban locations. The maximum ethylbenzene 24-hour concentration measured across all NAPS monitoring stations from 2005 to 2009 occurred within the Burnaby area of the Greater Vancouver Regional District, British Columbia; with a value of 35.84 μg/m3. During this same time period, the mean± standard deviation and 95thpercentile values were 0.71 ± 3.07 μg/m3 and 1.06 μg/m3, respectively (Environment Canada 2011a). An analysis of the NAPS data from 2010 to 2012 showed ethylbenzene concentrations fell within the same range as those reported from 2005-2009.
Five Canadian studies measured ethylbenzene in outdoor air in the immediate area of residential homes (Zhu 2005; Health Canada 2010a,b; Health Canada 2012; Health Canada 2013) and the results are presented in Appendix B (Table B1). Measurements took place in Windsor, Ontario (Health Canada 2010a), Regina, Saskatchewan (Health Canada 2010b), Halifax, Nova Scotia (Health Canada 2012), Edomonton, Alberta (Health Canada 2013), and Ottawa, Ontario (Zhu 2005). The maximum concentration identified from all five studies was 146.5 μg/m3 in Edmonton (Health Canada 2013), with mean concentrations across studies ranging from 0.13 to 1.14 μg/m3, and 95th percentile values across studies ranging from 0.3 to 2.0μg/m3.
Individuals living near the vicinity of an oil and gas facility or a manufacturing facility that releases ethylbenzene into the air may be exposed to higher concentrations of ethylbenzene in outdoor air. In the Ecological Exposure Assessment Section on air, the results of several studies that measured ethylbenzene in the air near petrochemical and oil and gas facilities, and industrial sites in Canada were described. Mean concentrations of ethylbenzene in the air near these industrial sites ranged from 0.054-13 μg/m3 and maximum ethylbenzene concentrations ranged from 0.93 to 87.7 μg/m3 (Badjagbo et al. 2009; FAP 2010; Alberta Environment 2010; Mintz and McWhinney 2008; You et al. 2008; Atari and Luginaah 2009; Miller et al. 2009). The maximum concentration of 87.7 μg/m3 was measured in Alberta however 87% of the samples measured were below the limit of detection of 0.08 μg/m3 (FAP 2010); this measurement was not considered representative of a typical high end concentration and was not used to estimate exposure. The University of British Columbia (UBC) conducted a human health impact assessment on air emissions from the Chevron North Burnaby Refinery as a result of concerns from residents living near the refinery (Kennedy et al. 2002). The maximum concentration of ethylbenzene measured in the area adjacent to the tank farm was 5.5 µg/m3, and the mean value was 1.82 µg/m3. This maximum concentration is lower than the maximum concentration detected in the NAPS data and is similar to the highest 95th percentile value measured in the same data set.
The highest 95th percentile value of 4.40 μg/m3 from the NAPS 2005-2009 data is considered upper-bounding representing long-term inhalation exposure for both rural and urban populations as well as populations living near a point source; this value was used to estimate the upper-bounding daily intake of ethylbenzene by the general population from outdoor air (Appendix D, Table D1).
10.1.2 Indoor Air
Empirical data on ethylbenzene levels in indoor air were identified in the literature and are presented in this section. The presence of ethylbenzene in indoor air may be attributed to various sources including smoking, vehicle exhaust and fuel vapour intrusion from attached garages, use of building materials, and consumer products (Wallace et al. 1987; Batterman et al. 2007; Héroux et al. 2008). Although data on emissions of ethylbenzene from attached garages, smoking, building materials and electronic products are presented in this section, emissions from these uses are considered to be captured by the empirical indoor air measurements of ethylbenzene. Information on exposures while using certain consumer products is presented in the Consumer Products section.
Canadian data on concentrations of ethylbenzene in residential indoor air have been identified and reviewed. Results and technical details from the various studies are presented in Appendix B.
In a Canadian indoor air survey conducted in 1991, volatile organic compounds (VOCs) were measured in 754 randomly selected homes from across Canada (Fellin et al. 1992). For each residence, the indoor air concentration of ethylbenzene was measured over a 24-hour period. The maximum indoor air concentration of ethylbenzene measured within a residence was 539 μg/m3(mean, 8.2 μg/m3; detection limit, 0.66 μg/m3).
A more recent Canadian indoor air survey was conducted from 2009 to 2011 as part of the second cycle of the Canadian Health Measures Survey (CHMS), an on going national survey that collects important health information from individuals aged 3 to 79 years old living in private households (Statistics Canada 2012; Wheeler et al. 2013). Eighty-four VOCs, including ethylbenzene, were measured by survey participants who deployed the samplers in their homes for 7 consecutive days. A total of 3857 valid indoor air samplers, from various dwellings including houses, apartments, mobile homes, and hotels, and including both smoking and non-smoking occupants, were analysed from 18 sites across Canada (personal communication from Environmental Health Science and Research Bureau December 2012). The mean concentration of ethylbenzene in indoor air was 4.09 μg/m3 and the 95th percentile value was 15.07 μg/m3 (weighted data at the person level) (Wheeler et al. 2013). The mean and 95th percentile indoor air concentrations weighted at the household level were 4.22 μg/m3 and 13.63 μg/m3, respectively (Zhu et al. 2013). The five Canadian surveys mentioned in the outdoor air section also monitored indoor air for ethylbenzene (Health Canada 2010a,b; Health Canada 2012; Health Canada 2013a; Zhu 2005). In the Windsor survey, 46-47 non-smoking participant homes were monitored between January 2005 and August 2006 with samples collected every 24 hours for 5 consecutive days (reported as an average of the 5 individual 24-hour samples). In the Regina survey, 146 homes, of which 34 homes had at least one smoking participant, were monitored in 2007 with samples collected over a single 24 hour period. In the Halifax study, 50 homes were monitored in both the winter and the summer of 2009 with samples collected every 24 hours for 7 consecutive days. In the Edmonton study, 50 homes were monitored in both the winter and the summer of 2010 with samples collected every 24 hours for 7 consecutive days. All four studies deployed active air samplers concurrently inside and outside the home. The Ottawa survey is an earlier study sponsored by Health Canada in which ethylbenzene was measured in 75 homes between November 2002 and March 2003. Each home was sampled once and indoor and outdoor active samplers were deployed with 10 L of air collected over 100 minutes (Zhu 2005). Health Canada also conducted an indoor air study of 96 homes in Québec City, Québec, during the winter and spring of 2005 with samples collected continuously over 7 consecutive days using passive samplers (Héroux et al. 2008).
The mean residential indoor concentrations of ethylbenzene recorded across all six studies ranged from 1.8 to 15.3 μg/m3, and 95th percentile concentrations ranged from 5.0 to 54.3 μg/m3. The highest indoor air concentration of ethylbenzene reported across the Canadian studies occurred in a residence in Windsor, which had a value of 1199 μg/m3. Several other high maximum values were noted in the various studies and are presented in Appendix B (Table B1).
A number of adult participants in the 2005 Windsor study also wore backpacks equipped with sampling apparatus, over 24-hour periods for five consecutive days, to measure personal exposure to ethylbenzene in air. Participants were asked to wear the sampling equipment during the normal course of a day. The highest concentration reported among Windsor participants wearing a personal backpack during the winter was 565 μg/m3, while the corresponding mean and 95th percentile values were 8.3 and 9.8 μg/m3, respectively. The maximum concentration of ethylbenzene reported during the summer sampling was 392 μg/m3, while the corresponding mean and 95th percentile values were 10.6 and 27.3 μg/m3, respectively (Health Canada 2010a).
10.1.2.1 Attached Garages
A study conducted in Québec City, Québec, compared housing characteristics and indoor air concentrations of VOCs including ethylbenzene. Higher indoor air concentrations of ethylbenzene were associated with homes that had attached garages (n,18; geometric mean, 5.15 µg/m3) compared to those without (n,78; geometric mean, 2.31 µg/m3) (p = 0.0006) (Héroux et al. 2008). Graham et al. 2004 studied the contribution of vehicle emissions from attached garages to indoor air in 16 residential homes in Ottawa, Ontario. Indoor air and garage air samples were collected for various compounds including ethylbenzene, before and during hot-soak and cold-start operation of a light-duty vehicle (the same vehicle was used in all homes). Results from the study showed that there was a positive net change in the concentration of hydrocarbons including ethylbenzene between the background sample in the houses before the tests commenced (referred to as pre-test) and samples collected while the car was operating in the garage (referred to as during-test). Cold-start and hot-soak pre-test results for ethylbenzene ranged from 1.17 to 15.2 µg/m3 while during-test results for ethylbenzene ranged from 2.51 to 59.3 µg/m3. Levels of ethylbenzene in the air of the garage during cold-start and hot-soak tests ranged from 31.5 to 675 µg/m3 (Graham et al. 2004). Wheeler et al. (2013) used univariate regressions to determine that having an attached garage was one of the significant predictors of ethylbenzene in indoor air based on data from the recent CHMS indoor air survey.
Batterman et al. (2007) examined the migration of pollutants from attached garages into 15 houses in southeast Michigan in the United States. The mean concentration of ethylbenzene in attached garages was 28.0 µg/m3 while the corresponding mean indoor air concentration in the same homes was 2.3 µg/m3 and the mean outdoor concentration was 0.2 µg/m3. Dodson et al. (2008) reported similar results in a study of 55 homes in Boston, Massachusetts.
The presence of ethylbenzene and other compounds in garages are likely attributed to emissions related to vehicles, other gasoline-powered equipment and gasoline storage containers. Ethylbenzene levels measured in the Graham et al. (2004) and Batterman et al. (2007) study were higher in garages than either indoors or outdoors, suggesting that evaporative emissions from attached garages represent a source of indoor ethylbenzene.
10.1.2.2 Tobacco Smoke
Environmental tobacco smoke (ETS) has been shown to be a source of ethylbenzene (Nelson et al.1998). Daisey et al. (1994) measured volatile organic compounds from various cigarette brands over a 4-hour period after 24- and 27-minute sessions of smoking, using a smoking machine, in a room sized (20m3) environmental chamber. The concentrations of ethylbenzene in ETS range from 10.1 to 21.1 µg/m3 (detection limit not stated) (mean concentrations from different cigarette brands range from 11.5 to 19.3 µg/m3). Xie et al. (2003) reported ethylbenzene concentrations of 1.02 to 16.4 µg/m3 (mean of 9.38 µg/m3) from environmental tobacco smoke in a vacant office. Bi et al. (2005) conducted a similar study and reported the level of ethylbenzene in environmental tobacco smoke from 3 brands of cigarettes to range between 69.2 to 84.2 µg/cigarette. Polzin et al. (2007) measured ethylbenzene in mainstream smoke following ISO 3308:2000 standard where an automated smoking machine simulated smoking conditions (35 mL puff of 2-second duration every 60 seconds). Levels of ethylbenzene in mainstream cigarette smoke from various brands of cigarettes ranged from 0.8 to 7.8 µg/cigarette (Polzin et al. 2007). Wallace and Pellizzari (1986) measured the concentration of ethylbenzene in the breath of 198 smokers and 322 non-smokers. The ethylbenzene breath concentration in smokers (2.6 µg/m3) was significantly higher (P less than 0.001) than in non-smokers (0.8 µg/m3) (Wallace and Pellizzari 1986).
Data from the air study conducted in Regina indicated that cigarette smoking in the home did not result in a large concentration increase of ethylbenzene in air when compared with homes of non-smokers (Health Canada 2010b). Indoor air concentrations of ethylbenzene ranged from 0.27 to 13.5 µg/m3 and from 0.10 to 33.6 µg/m3 in homes with at least one smoker and without any smokers, respectively. The mean concentrations for these homes ranged from 1.8 to 2.4 µg/m3 and from 1.9 to 3.8 µg/m3, respectively (see Appendix B, Table B1) (Health Canada 2010b). This suggests that although cigarette smoke may contribute to the concentration of ethylbenzene in the home, it is unlikely a significant source. Kim et al.(2001) reported among home studies, certain VOCs including ethylbenzene were higher in the homes of non-smokers compared with homes of smokers. The authors suggest that non-cigarette sources of VOCs including infiltration of vehicle exhaust, cooking, and use of solvent-based products contribute to indoor air levels of ethylbenzene. Furthermore, in a tobacco smoking simulation experiment conducted in a vacant office, ethylbenzene concentrations in air did not correlate well with ETS markers produced during cigarette smoking. The authors propose that the concentration of ethylbenzene in indoor air is mainly attributed to non-smoking sources (Xie et al.2003). Ethylbenzene exposure estimates for individuals who smoke cigarettes are presented in the Consumer Products section.
10.1.2.3 Building Materials
Ethylbenzene has been identified in various building materials such as flooring and furniture. National Research Council Canada has a database containing information on emissions of VOCs from various building materials created through a series of projects entitled "Consortium of Material Emissions and Indoor Air Quality Modeling". The emission testing was conducted using a flow-through chamber system for 69 different materials including carpet, plywood and adhesive (Won et al. 2005). A list of some of the materials in which ethylbenzene was detected and the corresponding emission factors can be found in Table 14.
| Material Type | Specific Material | Minimum | Maximum |
|---|---|---|---|
| Solid & Engineered Wood Materials | Oriented Strand Board (OSB) | 0.13 | 1.3 |
| Solid & Engineered Wood Materials | Plywood | 0.05 | 0.09 |
| Solid & Engineered Wood Materials | Solid Wood | 0.03 | 0.28 |
| Solid & Engineered Wood Materials | Medium Density Fibreboard (MDF) | 0.94 | 0.94 |
| Flooring | Carpet/Assembly | 0.03 | 291 |
| Flooring | Underpad | 0.21 | 0.23 |
| Flooring | Laminate/Assembly | 0.01 | 0.20 |
| Flooring | Linoleum/Vinyl Flooring | 0.04 | 0.11 |
| Installation Materials | Adhesive | 5 | 5 |
| Installation Materials | Caulking | 151 | 4 457 281 |
Park et al. (1996) conducted chamber studies (chamber volume of 0.006 m3) on the flooring, wall, and ceiling materials similar to those that would be installed in a newly constructed residence. Initial emission rates of ethylbenzene from flooring, wall, and ceiling material were 67.1 µg/m2/h, 64.6 µg/m2/h, and not detected (detection limit not stated), respectively (Park et al. 1996). Salthammer (1996) reported levels of ethylbenzene emitted from five wood cabinets treated with different coatings. The concentration of ethylbenzene in the air surrounding and inside the cabinet ranged from not detected (detection limit not stated) to 962 µg/m3 after 24 hours. Concentrations of ethylbenzene in the air surrounding and inside of the cabinets were lower when measured after 400 hours (Salthammer 1996). Qin et al. (1999) conducted a study using two experimental rooms to determine the air concentration of various volatile organic compounds after installing plastic flooring, after using floor wax, and after using wall paint. Ethylbenzene concentrations in indoor air after installing plastic flooring was 557 µg/m3 after 5 hours but dropped to 17 µg/m3 after 9 days. Ethylbenzene concentrations in indoor air after using wall paint were 283 µg/m3 after 2 hours but dropped to levels not detected after 10 days (Qin et al. 1999). Wallace et al. (1987) detected ethylbenzene in the air above glued carpet (6.4 µg/m3) in a chamber study and estimated an emission rate of 77 ng/m2 per minute. Ethylbenzene was one of the main VOCs emitted from laminate flooring in a chamber study conducted in Korea, however, only values for total VOCs were reported (An et al. 2011).
10.1.2.4 Electronic Products
Ethylbenzene is emitted from various electronic products such as television sets and video monitors. Malmgren-Hansen et al. (2003) used test chambers to measure emissions from television sets (2 µg/unit per hour at 7 hours and 3 µg/unit per hour at 9 hours or 0.23 and 0.34 µg/m3), from monitors (33 and 14 µg/unit per hour at 7 hours and 9 hours, respectively, or 3.8 and 1.6 µg/m3), and from voltage converters (139 and 74 µg/unit per hour at 7 hours and 9 hours, respectively, or 16.0 and 8.5 µg/m3 ).
Ethylbenzene has been detected in various types of office equipment such as printers and photocopiers. Lee et al. (2001) conducted a chamber study to determine emissions from laser printers, ink-jet printers, and an all-in-one machine. Ethylbenzene was detected in both laser printers, one of two ink-jet printers, and the all-in-one machine. Average levels of ethylbenzene ranged from 1.26 to 3.00 ppb (5.5 to 13 µg/m3) for machines in operation and from 1.2 to 2.07 ppb (5.2 to 9 µg/m3) for the same machines not in operation (idle) (Lee et al. 2001).
Levoic et al. (1996) conducted chamber studies to estimate emission rates of various compounds from dry-process photocopiers used in an office environmentboth in idle mode and during operation. The emission rates for ethylbenzene ranged from less than 10 to 180 µg/hour per copier for the photocopiers in idle mode and from less than 50 to 28 000 µg/hour per copier for the photocopiers in operation mode (Levoic et al. 1996). Levoic et al. (1998) conducted a similar study using various laboratories to test their method. Emission rates for ethylbenzene, while photocopiers were in operation, ranged from 23 000 to 29 000 µg/hour per copier used in an office environment. Headspace analysis of the toner used in the photocopiers was also conducted with ethylbenzene headspace concentrations in toner cartridges ranging from 260 to 620 ng/mL headspace (Levoic et al. 1998). Brown (1999) conducted a chamber study to determine various types of chemicals that are emitted from dry-process photocopiers. The average ethylbenzene air concentrations while the copier was in operation ranged from 552 to 608 µg/m3, and the average ethylbenzene air concentration was 4.1 µg/m3 while the copier was in idle-mode (Brown 1999).
10.1.2.5 Summary
Ethylbenzene has been identified in indoor air in Canada and may be attributed to various sources. The highest 95thpercentile value of 54.3 µg/m3 reported in the Windsor study over the summer of 2006 (Health Canada 2010a) is considered to be an upper-bounding ethylbenzene concentration representative of long-term inhalation for the general population from indoor air and accounts for daily exposures to ethylbenzene from attached garages, building materials, and electronic products.
10.1.3 Vehicle Interior Air
Ethylbenzene has been identified in new car interiors in Spain, Australia, and Taiwan (Grabbs et al. 1999; Brown and Cheng 2000; Chien 2007; Esteve-Turrillas et al. 2007). Chien (2007) measured interior air concentrations (sampling time of 45 minutes) of various VOCs, including ethylbenzene, in new domestic and imported cars (20 cars in total) in Taiwan in order to examine inter-brand, intra-brand, and intra-model variations. In total, 20 cars were included in the study ranging in age from a few weeks to 4 months after manufacture. Ethylbenzene concentrations ranged from not detected (method detection limit of 5.5 µg/m3) to 240 µg/m3. The concentrations of all the analyzed VOCs including ethylbenzene varied between brands and models most likely as a result of the different types of materials used in the different cars such as upholstery, adhesives, and lubricant (Chien 2007).
According to Brown and Cheng (2000), levels of ethylbenzene in car interiors decrease over time. Various VOCs were measured in three new cars (two in Australia and one imported from Korea). Only the two cars made in Australia had detectable levels of ethylbenzene. One car had an ethylbenzene air concentration of 140 µg/m3 after 10 weeks and only 0.9 µg/m3after 115 weeks. The other car had ethylbenzene levels of 880 µg/m3 after 3 weeks, 56 µg/m3 after 9 weeks, and 7.5 µg/m3 after 95 weeks (Brown and Cheng 2000).
Yoshida and Matsunaga (2006) measured interior air concentrations in one car over a 3-year period in Japan. The concentration of ethylbenzene the day after delivery, approximately 2 weeks after manufacture, was 361 µg/m3 (average over a 24-hour period). The concentration of ethylbenzene, as well as for other aromatic hydrocarbons, decreased rapidly for the first 6 months with concentrations ranging from one-hundreth to one-tenth of the original concentration (3.6 to 36 µg/m3). During the first summer, concentrations increased slightly with the rise in outdoor temperatures; however, by the second year, little difference was noticed during the winter and summer months (Yoshida and Matsunaga 2006).
Ethylbenzene may also be present in vehicles while in transit. Novamann International (1994a,b) examined driver exposures to various substances while commuting during morning and evening rush hour in winter and summer in Toronto, Ontario. Ethylbenzene concentrations ranged from below method detection limit (BMDL) to 109.8 µg/m3 (average of 14.1 µg/m3) and from BMDL to 15.2 µg/m3 (average of 3.5 µg/m3) in winter and summer, respectively. The concentration of ethylbenzene as well as other compounds were usually higher in winter than in the summer most likely as a result of windows being closed during the winter months (Novamann International 1994a,b).
Karmen and Graham (2002) examined the concentration of various compounds in ambient air on a busy street in Ottawa, Ontario as well as in vehicles on long commuting trips. Sampling took place in January/February and July/August of the year 2000. Mean (±standard deviation) concentrations of ethylbenzene on the roadside were 2.49(2.62) µg/m3 and 1.36(0.78) µg/m3 in winter and summer, respectively. Mean (±standard deviation) concentrations of ethylbenzene in cars were 3.09(3.24) µg/m3 and 2.90(1.30) µg/m3, and in buses were 2.58(1.23) µg/m3 and 3.57(1.77) µg/m3in winter and summer, respectively (Karmen and Graham 2002).
In-vehicle monitoring of ethylbenzene was also performed on public buses in northern Spain; concentrations of ethylbenzene ranged from 0.20 to 4.89 µg/m3 (mean concentrations ranged from 1.05 to 1.30 µg/m3) (Parra et al. 2008). Ethylbenzene was also detected on 22 public buses in Changsha, China; concentrations of ethylbenzene ranged from 19.6 to 95.9 µg/m3. The authors reported that levels of BTEX increased when in-vehicle temperatures or relative humidity increased and levels decreased with age of the vehicle or if the travel distance increased (Chen et al. 2011). Shiohara et al. (2005) reported median in-vehicle concentrations of ethylbenzene of 36.8 µg/m3 in cars, 25.6 µg/m3 in microbuses, 17.8 µg/m3 in buses, and 11.3 µg/m3 in the metro in Mexico City during commutes along defined routes. The authors suggested that the VOCs they measured (benzene, toluene, ethylbenzene, m/p-xylenes) in vehicles were probably from gasoline vapors or exhaust fume penetration from the vehicle itself or from the surrounding vehicles (Shiohara et al. 2005). Novamann International (1994a) stated that the major sources of substances in a vehicle are: exhaust from the vehicle itself; exhaust from the surrounding vehicles; substances in ambient air while in transit; and, compounds being emitted from inside the vehicle itself.
Exposures to ethylbenzene from vehicle interior air varies greatly depending on the age and brand of the vehicle, ventilation within the vehicle, the location, and season, as well as on the frequency and duration of exposures. The maximum value of 240 µg/m3 measured in the Chien (2007) study was chosen to represent an upper-bound inhalation exposure level from interior air in vehicles. Higher concentrations were measured in other studies; however, these had smaller sample sizes and were therefore not as representative of potential exposures.
10.1.4 Drinking Water
Canadian data relating to the concentration of ethylbenzene in drinking water has been identified and reviewed. Ethylbenzene is listed in the Guidelines for Canadian Drinking Water Quality published by Health Canada (Health Canada 2014a). A Maximum Acceptable Concentration or MAC has been established at 140 μg/L, based on health effects considerations and an Aesthetic Objective or AO has been established at 1.6 ug/L based on considerations such as taste and odour. The World Health Organization (WHO) has published guidelines for chemicals in drinking water based on human health concerns. The WHO recommends that the concentration of ethylbenzene in drinking water not exceed 300 μg/L (WHO 1996).
Ethylbenzene has been detected in drinking water in several surveys in Canada. Otson et al. (1982) tested the raw and effluent water from 29 municipalities across Canada in which concentrations as high as 10 μg/L (detection limit, 1 μg/L) were observed in a treated sample; however, the mean concentration did not exceed 1 μg/L. In a similar survey of nine municipalities along the Great Lakes from 1982 to 1983, ethylbenzene was not identified above the detection limit (detection limit, 0.1-0.4 μg/L) in 12 of 24 raw water samples and 14 of 42 treated water samples (Otson 1987). A survey of municipal drinking water sources in the Atlantic region determined mean concentrations of ethylbenzene to be 0.2 μg/L and 0.5 μg/L (detection limits not stated) in 1987 and 1988, respectively (Environment Canada 1989). More recent data were available through the Ontario Ministry of the Environment's Drinking Water Surveillance Program (MOE 2009). During the 2007 reporting year, the concentration of ethylbenzene was measured across 120 water systems throughout Ontario. The highest reported ethylbenzene concentrations among samples of raw water, treated water, and samples from the distribution system did not exceed trace levels (0.1-0.2 μg/L; detection limit, 0.05 μg/L).
Approximately 30% of Canadians use groundwater in their homes (Environment Canada 2011b). Background levels of ethylbenzene in wells in North Bay were identified at 0.1 µg/L (Reinhard et al. 1984; Cherry 1987). Goss et al. (1998) sampled 160 wells in rural Ontario and were unable to detect ethylbenzene in any of the samples (detection limit, 1.17 μg/L). Several other studies, listed in Table 9-2 of the Ecological Exposure Assessment section showing levels of ethylbenzene in Canadian groundwater located near landfills, waste sites, leaking underground fuel tanks, or other contaminated sites, which most likely would not be used as a source of drinking water. The U.S. Geological Survey (USGS) conducted a study on 55 VOCs in groundwater from large aquifers, some of which are used for drinking water. Levels of ethylbenzene in domestic and public wells collected from 1985 to 2001 ranged from 0.003 to 5.4 µg/L with the majority of samples containing less than 0.03 µg/L (Zogorski et al. 2006). More recent data from a similar study conducted by the USGS identified ethylbenzene concentrations ranging from 0.013 to 0.52 µg/L from 2002 to 2005 (Carter et al. 2007).
Drinking water is not expected to be a significant route of general population exposure to ethylbenzene in Canada. In all but one study examined, ethylbenzene concentrations fell below the Health Canada Aesthetic Objective of 1.6 μg/L and all sources examined fell far below the Health Canada MAC or the WHO drinking water guideline. The maximum value found by Otson et al. (1982) does not reflect the findings found in more current available databases and was published before the availability of the Health Canada Guideline. Studies identified on the presence of ethylbenzene in Canadian municipal and well water are limited, and therefore the Canadian Drinking Water Aesthetic Objective of 1.6 µg/L will be used in derivation of the upper-bounding estimate of daily intake of ethylbenzene from drinking water. It is recognized, however, that this is a conservative assumption since ethylbenzene concentrations at this level and above are expected to alter the taste and smell of the water and would likely result in complaints and action to reduce levels in the drinking water.
10.1.5 Food and Beverages
Data on levels of ethylbenzene in food in Canada are limited, and available measurements of ethylbenzene in food from other countries are presented in this section.
Ethylbenzene does not likely occur naturally in plants (Tang et al. 2000); however, it has been identified in various unpackaged or fresh food items. According to VCCEP (2007), ethylbenzene may accumulate in foods as a result of its presence in the atmosphere. The empirical data on levels of ethylbenzene in unpackaged food items is presented below.
Enviro-Test Laboratories (1991, 1992, 1993) conducted a study of 34 to 36 food groups in grocery stores located in Alberta, Ontario, and Québec from 1991 to 1993. Ethylbenzene was below the detection limit (50 μg/kg in solids for the Alberta study and 5 µg/kg in solids for the Ontario and Québec studies, 1 μg/kg in liquids for all studies) in all of the food groups tested. In the Northwest Territories and northern Manitoba, in 1985 and 1986, ethylbenzene was detected in the muscle tissue and liver tissue of burbot, Lota lota, with concentrations ranging from not detectable to 115.0 μg/kg (weighted mean of 10. 6 µg/kg, detection limit not stated) and from not detectable to 84.0 µg/kg (weighted mean of 26.7 µg/kg, detection limit not stated), respectively. Ethylbenzene was also detected in the muscle tissue of whitefish, Coregonus clupeaformis, with concentrations ranging from not detectable to 273 µg/kg (weighted mean: 19.8 µg/kg, detection limit not stated) (see Table 9-4) (Lockhart et al. 1992). These concentrations of ethylbenzene are in fish tissue near industrial sources in northern areas and, therefore, are not considered to be representative of typical levels to which most of the Canadian population would be exposed. Segments of the population, however, who consume fish and live in northern areas, may be exposed to these higher levels of ethylbenzene.
In 1986, ethylbenzene was detected in 43 out of 138 fish samples in Japan with concentrations ranging from 1.0 to 9.8 µg/kg wet weight (detection limit of 1 µg/kg wet weight) (Government of Japan 2004; IPCS 1996; IARC 2000). Ethylbenzene was detected in various Korean salt-fermented fish and shrimp pastes with mean concentrations of 76.6 µg/kg for anchovy, 38.3 µg/kg for hairtail, and 72.5 µg/kg for shrimp (Cha and Cadwallader 1995). Ethylbenzene was identified in the neutral fraction of roast beef flavour isolate; however, the actual concentrations were not reported (Min et al. 1979).
Ethylbenzene has been detected in various fruits, vegetables, and legumes. It was detected in parsley at 256.7 µg/kg and in orange peel at 23.6 µg/kg (detection limit not stated) in a European study that examined the peel, pulp, and roots of 14 different vegetables and 10 different fruits (Górna-Binkul et al. 1996). Lovegren et al. (1979) reported the presence of ethylbenzene in various dry legumes including beans (concentrations ranging from 0 to 11 µg/kg), split peas (13 µg/kg), and lentils (5 µg/kg). Ethylbenzene was also detected in chickpea seed (Rembold et al. 1989). Ethylbenzene was identified in tomatoes and tomato products, apples (Golden Delicious), strawberries, and kiwis, but no concentrations were reported (Dirinck et al. 1977; Chung et al. 1983; Takeoka et al. 1986).
Ethylbenzene was detected in various forms of olive oil, including extra-virgin olive oil, virgin olive oil, olive oil, and refined olive oil, contained in different types of packaging (glass, plastic, or metal) at concentrations ranging from not detected (limit of detection of 0.25 ng/mL) to 34.3 ng/mL in a study conducted in Spain (Carrillo-Carrión et al. 2007). Vichi et al. (2007) also detected ethylbenzene in 54 samples of virgin olive oils from three different crops with concentrations ranging from 14 to 201 µg/kg (mean of 45 µg/kg, limit of detection of 0.6 µg/kg) in Spain. The presence of ethylbenzene, as well as other aromatic hydrocarbons, in olive oil is thought to arise as a result of its presence in the atmosphere from spills, combustion, and evaporation of fuel oil, vehicular and industrial emissions, and geochemical processes. The aromatic hydrocarbons are lipophilic in nature and tend to contaminate oils and fats (Vichi et al. 2007).
Ethylbenzene may also be present in foods as a result of migration from food packaging and containers made from styrenic polymers (VCCEP 2007). Polystyrene, including general purpose polystyrene (GPPS), high impact polystyrene (HIPS), and foam, is used in a variety of food packaging and food contact materials such as cutlery, drink cups, meat trays, egg cartons, dinnerware, fast-food packaging, cookie and cake trays, dairy containers, soda fountain cups, and lids (Shariq and Funada 2008). The European Union's specific migration limit (SML) for ethylbenzene is 600 µg/kg (0.6 mg/kg) (Nerín et al. 2002).
Tang et al. (2000) reported that certain polymer food packaging materials, mainly polystyrene, may contain ethylbenzene as a residual. Ethylbenzene was reported to range from 8 to 473 ppm (median of 50 ppm) in 41 out of 44 samples of polystyrene products (Hempel and Rüdt, in Tang et al. 2000). The same study reported ethylbenzene in all 12 samples of styrene graft and copolymer products with concentrations ranging from 61 to 202 ppm (median of 84 ppm) (Hempel and Rüdt, in Tang et al. 2000). The Polystyrene Work Group (PSWG) of the Society of the Plastics Industry's Food, Drug, and Cosmetic Packaging Materials Committee conducted a study to determine the potential dietary exposure to ethylbenzene from food-contact items made of polystyrene. An industrial survey was carried out to collect data on the residual levels of ethylbenzene present in various polystyrene food packaging and disposable food-contact items. The weighted average residual ethylbenzene concentrations collected during the survey (PSWG 1997, cited in VCCEP 2007) are shown in Table 10-2. The concentration of ethylbenzene in commercial polystyrene resins will depend on the technical process used (Durst and Laperle 1990, in Tang et al. 2000), and the eventual residual content of ethylbenzene in foods is therefore variable and difficult to predict (2010 Dec 6 conversation and e-mail from Food Packaging/Incidental Additives Section to Existing Substances Risk Assessment Bureau; unreferenced).
| Material Type | Polymer/applicationsFootnote Table 10-2[a].8 | Residual ethylbenzene (ppm ) |
|---|---|---|
| Packaging | GPPS | 18 |
| Packaging | HIPS | 29 |
| Packaging | Polystyrene foam | 66 |
| Disposables | GPPS | 42 |
| Disposables | HIPS | 108 |
| Disposables | Polystyrene foam | 37 |
| Disposables | Expandable polystyrene foam | 37 |
Several studies have been identified on the presence of ethylbenzene in food as a result of its migration from packaging. Chiesa et al. (2008) reported levels of ethylbenzene in various types of cheese mainly packaged in plastic and stored at 4ºC. Concentrations in the cheese ranged from 0.52 to 76.1 µg/kg (Chiesa et al. 2008). Ethylbenzene was reported to migrate from various types of plastic containers, intended for high temperature use, into powdered whole and skimmed milk (López et al. 2008). Four types of plastic (polypropylene random, polypropylene copolymer, polycarbonate, and styrene-acrylonitirile copolymer) were heated at various temperatures (75, 100, and 121°C) and exposure times (30, 60, and 120 minutes) with the powdered milk. Ethylbenzene was detected in all samples of the powdered skimmed and whole milk with concentrations ranging from 0.03 to 0.09 µg/kg and 0.02 to 11 µg/kg, respectively. Concentrations of ethylbenzene in the powdered whole milk were greater than those of the powdered skimmed milk most likely because of the higher fat content in the powdered whole milk (López et al. 2008). In addition, concentrations of ethylbenzene increased with increasing temperature for the powdered whole milk.
Nerín et al. (2002) detected ethylbenzene in various plastics (polycarbonate, polypropylene-copolymer, polypropylene-20% talcum, polypropylene random, and styrene-acrylonitrile) used in high-temperature food containers designed for heating food in microwave ovens. Concentrations of ethylbenzene released as vapour from the packaging containers at 100ºC ranged from 0.147 to 0.360 µg/kg resulting in potential migration of ethylbenzene to food from the vapour phase ranging from 0.0165 to 0.0273 µg/kg (Nerín et al. 2002).
Gramshaw and Vandenburg (1995) reported that the migration of ethylbenzene into pork belly, cooked at 175°C for 1.5 hours in thermoset polyesters dishes (containing between 6 and 25 mg/kg ethylbenzene), ranged from less than 6 to 34 µg/kg (detection limit, 6 µg/kg). Ethylbenzene was detected in low fat yoghurts and chocolate desserts packaged in polystyrene with concentrations ranging from not detected (detection limit not specified) to 4 µg/kg (Ehret-Henry et al. 1994). Tan and Okada (1978) examined the migration of styrene and ethylbenzene from polystyrene cups. Ethylbenzene was detected in the following food items: in sour milk beverage at less than 0.0025 to 0.006 ppm; in noodle soup at 0.015 to 0.021 ppm; in noodle curry at 0.089 to 0.153 ppm; and in instant wonton soup at 0.009 to 0.028 ppm. The concentration of ethylbenzene in the various polystyrene cups ranged from 108 to 424 ppm (Tan and Okada 1978).
In the U.S. Food and Drug Administration (US FDA) Total Diet Study (US FDA 2006), which encompasses data from 1991 to 2004, ethylbenzene was detected in approximately 80 packaged and unpackaged food items (detection limit not stated), as summarized in Appendix C, with the greatest concentrations detected in muffins (plain or fruit) at 224 µg/kg (mean concentration of 10 µg/kg, detection limit not stated) and in popcorn (microwave, butter-flavour) at 129 µg/kg (mean concentration of 0.043 µg/kg, detection limit not stated). The data from the US FDA Total Diet Study (presented in Appendix C) were considered to be the most representative of potential levels in food in Canada and were used to estimate upper-bound dietary intakes of ethylbenzene to the general population of Canada (see Appendix D, Table D1). Ethylbenzene concentrations in fish from the Lockhart et al. (1992) study were used in estimating ethylbenzene exposure of individuals living in northern parts of the country (presented in Appendix D, Table D2).
Ethylbenzene has also been detected in human breast milk. Blount et al. (2010) developed and validated a method for collecting, storing and analyzing 36 VOCs, including ethylbenzene, in breast milk. Breast milk was collected from 12 healthy women at least 30 days post-partum, in Baltimore, Maryland. Concentrations of ethylbenzene ranged from 0.053-0.58 ng/mL with a mean value of 0.232 ng/mL and a median value of 0.149 ng/mL (Blount et al. 2010). The maximum value of 0.58 ng/mL was used to estimate upper-bound exposures to infants (see Appendix 5).
10.1.6 Soil and Sediment
Limited data on levels of ethylbenzene in soil were identified. Table 9-3 in in the Ecological Exposure Assessment Section on soil, shows the limited Canadian soil data available. In a study of Ontario parkland, the upper 97.5th percentile concentration of ethylbenzene in soil samples was calculated to be 0.40 ng/g (detection limit, 2.0 ng/g) while the concentration of ethylbenzene in soil in rural parkland was 0.46 ng/g (OMEE 1993). The detection limit was higher than the highest detected level, and therefore there is low confidence in these data. Data from the Ontario Brownfields Environmental Site Registry ranged from 40-50 ng/g (OMOE 2005). Both of these data sources pertain to contaminated sites and do not provide details on soil sampling sites or methodology and were therefore not used to derive human exposure estimates.
The Canadian Soil Quality Guidelines for agricultural, residential, commercial, and industrial land uses, intended to be protective of environmental and human health, have been established by the Canadian Council of Ministers of the Environment (CCME) for ethylbenzene (CCME 2004). The values for coarse and fine soil are 0.082 and 0.018 mg/kg, respectively, and are identical across all land uses. In the absence of quality Canadian data, the guidelines proposed by CCME (2004) were used as a conservative surrogate value for the calculation of the upper-bound estimate of daily intake of ethylbenzene from soil ingestion.
10.1.7 Consumer Products
The survey conducted pursuant to section 71 of CEPA reported the use of ethylbenzene in numerous consumer products in the year 2000, representing several consumer product types. Ethylbenzene was reported to be used in coating products with concentrations ranging from 0.002 to 40%, and as a component of fuels, including gasoline, with concentrations ranging from 0.42 to 8.0%. However, some survey respondants had interpreted consumer to mean customer, and products and concentrations of ethylbenzene specifically available to the general population could not be determined; therefore, alternative sources of information on levels of ethylbenzene in consumer products were used to derive exposure estimates, as presented below.
10.1.7.1 Household Products
The presence of ethylbenzene in consumer products is primarily as a result of its presence in mixed xylenes which are used as solvents (ECHA 2008). Available data on concentrations of ethylbenzene in Canadian products was limited; therefore information was collected on the presence of ethylbenzene in products from the United States as a first step. The Household Products Database (HPD 2011) listed over 300 products containing ethylbenzene including various spray paints and paint products, automotive cleaners, arts and craft supplies, sealants, wood stains and varnish, pesticide products, and an adhesive. The concentration of ethylbenzene in these products ranged from 0.01 to 25%. Other sources consulted included the Source Ranking Database (SRD) and the public literature. A summary of the information on types of products and ethylbenzene concentrations identified from various US sources is provided in Appendix E.
Not all of the products and ethylbenzene concentrations identified from the U.S. Household Products Database and the SRD are available in Canada. Therefore, follow-up research was conducted to confirm concentrations in products in Canada including searches of Canadian retailer websites and Canadian material safety data sheets as well as contacting the Canadian industry. The types of consumer products that were reported to contain ethylbenzene included both liquid and aerosol forms of interior and exterior coatings, wood finishes, stains and varnishes. The concentration in aerosol paint-products ranged from 0.01 to 3.79% and those in liquid paint-products were usually less than 1% except for certain specialty products used outdoors which could be as high as 10-14%.
In addition, Health Canada's Product Safety Laboratory measured the concentration of ethylbenzene in over 100 consumer products including various paints, coatings, stains, finishes, cleaners, and fuel treatments (Health Canada, unpublished (compositional analyses conducted in 2013-2015). The ethylbenzene concentrations in the majority of the products tested were below 1% with a few products ranging in concentration from 1 to 4.5% (Health Canada, unpublished (compositional analyses conducted in 2013-2015). Two concrete/ stone sealer products had ethylbenzene concentrations between 16 and 18% (Health Canada 2014b); however, after communicating with the manufacturers, one of these products has been discontinued and should no longer be available to consumers and the other is available for exterior use only (2014 email(s) from Risk Management Bureau, Health Canada to Existing Substances Risk Assessment Bureau; unreferenced).
Based on this information, Table 10-3 shows the product types and corresponding ethylbenzene concentrations for which exposure estimates were derived. Only exposures to products that are likely to be used indoors or in garages were estimated since these would result in the highest exposure estimates. More details on the concentration ranges selected for each scenario are provided in Appendix F.
The ConsExpo model, version 4.1 (ConsExpo 2006), was used to estimate inhalation and dermal exposures to ethylbenzene from use of spray and liquid paints, paint remover, lacquer/stain/varnish, and joint sealant (or caulking). ConsExpo is a multi-tiered predictive model used to derive estimates of exposure to substances in consumer products. It contains exposure factors for various products and uses and it is a well-established model. The US EPA's Wall Paint Exposure Model was also used to derive estimates of exposure for the liquid paint scenario. The results from this model were similar to the outputs from ConsExpo; therefore, to be consistent across all product scenarios, and to estimate dermal exposures as well as inhalation exposures, the ConsExpo model was used in this report. As illustrated in Appendix F and Table 10-3, concentrations of ethylbenzene in consumer products may vary substantially. Accordingly, a range of concentrations of ethylbenzene in each product type were used to derive estimates of exposure (see Appendix F for details). A summary of the lower- and upper-bound estimates of inhalation and dermal exposures to ethylbenzene resulting from use of certain consumer products is provided in Table 10-3. Information on the parameters used for each scenario is detailed in Appendix F. Direct use of these products by children was not considered likely and exposure estimates were derived for adults only.
As shown in Table 10-3, exposure estimates vary as a result of differences in the concentration of ethylbenzene in the products. Mean air concentrations over the day of the event ranged from 0.006 mg/m3 for adults using spray paint to 13 mg/m3 for adults using liquid paint. Dermal exposure ranged from 0.002 mg/kg-body weight per event for adults using spray paint to 1.1 mg/kg-body weight (bw) per event for adults using caulking.
| Product | Concentration of ethylbenzene in Canadian products | Mean air concentration on day of event (mg/m3) |
Dermal exposure (mg/kg-bw per event) |
|---|---|---|---|
| Spray paint | 0.01 to 5 % | 0.006 to 3 | 0.002 to 1.1 |
| Liquid paint | 0.1 to 1 % | 1.3 to 13 | 0.051 to 0.51 |
| Paint remover | 4 % | 2.8 | 0.28 |
| Lacquer/Stain/varnish | 0.1 to 2 % | 0.5 to 9.4 | 0.051 to 1.0 |
| Caulking (or sealant) | 0.1 to 5 % | 0.1 to 5.2 | 0.021 to 1.1 |
Data on concentrations of ethylbenzene in air from use of products have been identified in the literature. In a study by Nielsen et al. (2003), five products were analyzed. Measured concentrations of ethylbenzene around a simulated user spraying the product for 60 seconds were determined to range from 0.006 to 17 mg/m3 (concentration in the 5 products ranged from not present to 1.83%) (Nielsen et al. 2003). The mean air concentration over the day of the event could not be determined from the study. Ethylbenzene was detected in proofing sprays (2 out of 16 products; 0.027 and 0.97 mg/g) (Feilberg et al. 2008). Chang et al. (2007) evaluated inhalation and dermal exposures to solvents (including ethylbenzene) in 15 male shipyard spray painters. The personal 8-hour time-weighted average (±SD) exposure concentration of ethylbenzene outside the workers mask was 59.2 ± 10.4 ppm (257 mg/m3) and 2.60 ± 0.49 ppm (11 mg/m3) inside the workers mask. Dermal exposures of ethylbenzene inside and outside block units of assembled ships ranged from 281.8 to 342.4 mg/9 cm2 tape and 42.5 to 70.7 mg/9 cm2 tape, respectively (Chang et al. 2007). Whitehead et al. (1984) conducted a study on occupational exposures to solvents in paints and glues that are applied by spraying, which mainly takes place in spray booths. The average time-weighted averages (TWAs) for workers spraying high- and low-aromatic paint ranged from 0.4 to 13.2 ppm (1.7 to 57 mg/m3), and the highest TWAs ranged from 3.4 to 52 ppm (15 to 226 mg/m3). The average TWAs for workers spraying aromatic- and chlorinated-hydrocarbon-dominated glues ranged from 0.3 to 37.5 ppm (1.3 to 163 mg/m3), and the highest TWAs ranged from 1.4 to 123 ppm (6.1 to 534 mg/m3) (Whitehead et al. 1984). These measured concentrations do not exceed occupational standards based on eye, skin and upper-respiratory irritation set out by various agencies such as OSHA, NIOSH and ACGIH (ATSDR 2010; OSHA 2011).
Ethylbenzene was detected in 16 out of 26 air freshener products during a headspace analysis conducted by Jo et al. (2008) in Korea. The same study measured mean concentrations of ethylbenzene in the air within gasoline- and diesel-fueled cars with and without air fresheners. The cars with air fresheners had only slightly higher levels of ethylbenzene than the cars without the air fresheners indicating that the level of ethylbenzene in the vehicles was likely a result of ambient air within the transportation corridor than from the air fresheners (Jo et al. 2008). Lim et al. (2011) analyzed for BTEX in 207 consumer products obtained from a supermarket in Korea. High concentrations of ethylbenzene were detected in shoe polish (not detected (nd) - 277,928 ppm), leather cleaner (nd - 42,223 ppm), whiteout (nd - 2770 ppm), permanent pen (nd - 345,065 ppm) and glue (nd - 792 ppm) (Lim et al. 2011 - abstract only). Ethylbenzene was detected in all 5 newly produced household furniture products tested (desk chair, bedside table, dining table, sofa and cabinet) in a 5 m3 chamber over 14 days in Korea. Mean concentrations of ethylbenzene ranged from 1.16 µg/m3 (for desk chair) to 563 µg/m3(dining table) (Ho et al. 2011).
Based on notifications submitted under the Cosmetic Regulations to Health Canada, ethylbenzene is used in certain cosmetic products in Canada such as a few manicure preparation products (2013 email(s) from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). Exposure from use of manicure products that may contain small quantities of ethylbenzene was estimated to be low compared with exposure from use of other household products.
10.1.7.2 Products Intended for use by Children
The Danish EPA has identified ethylbenzene in several children's products. Glensvig and Ports (2006) conducted emission tests (collection on solid adsorbents and analysis by GC/MS) of various children's toys and identified ethylbenzene in two out of seven children's toys that contain perfume; a rubber figurine emitting ethylbenzene at a concentration of 1100 µg/m3(equivalent to 1.9 µg/m3 of ethylbenzene in a room), and a soft cube emitting 540 µg/m3 (equivalent to 0.94 µg/m3 of ethybenzene in a room). Hansen et al. (2004) reported the presence of xylenes/ethylbenzene in all six of the children's tents and tunnels that were sampled with levels of xylenes/ethylbenzene ranging from 2 to 40 µg/m3. Svendson et al. (2005) measured ethylbenzene in all 14 slimy-type toys that were analyzed. Migration analyses that used artificial sweat and saliva were conducted by the Danish EPA to determine the potential release of certain substances when in contact with skin (via sweating) or saliva. The migration of ethylbenzene from slimy-type toys was determined to be less than 0.05 to 0.56 µg/g into artificial sweat (i.e., potential dermal exposure) and less than 0.05 to 0.64 µg/g into artificial saliva (i.e., potential oral exposure) (Svendson et al. 2005). Ethylbenzene was also detected in children's marker pens (no concentration given) (Hansen et al. 2008).
The Danish EPA has also identified ethylbenzene in various hobby products that can be used by adults and children. Mikkelsen et al. (2005) detected ethylbenzene in glass, window, or porcelain colourants (3 out of 10 products; not detected to 82 mg/kg). Egmose and Pors (2005) measured ethylbenzene in textile colourants such as fabric dyes (4 out of 15 products; not detected to 19 mg/kg). Ethylbenzene has also been detected in ironing beads (4 out of 6 products; 11 to 950 µg/kg) (Pors and Fuhlendorff 2002).
Styrene-containing polymers such as polystyrene and acrylonitrile-butadiene-styrene, are used to make a variety of consumer products including children's toys (Ormonde and Yokose 2008; Shariq and Funada 2008). There is a potential for young children to mouth toys made of styrenic polymers, which most likely contain residual levels of ethylbenzene. VCCEP (2007) estimated exposure to ethylbenzene by young children mouthing toys using conservative assumptions and by predicting the daily migration rate of ethylbenzene drawing on information from the PWSG (1997) study on ethylbenzene residuals in food-contact materials (PWSG 1997, cited in VCCEP 2007). VCCEP (2007) made use of the residual concentrations of ethylbenzene in polystyrene food packaging and disposable food-contact items shown in Table 10-2 as well as some assumptions on ethylbenzene's structural similarity to styrene to estimate the potential daily migration rate of ethylbenzene from children's toys. Using the weighted-average residual concentration of ethylbenzene in non-disposable HIPS (29 ppm or 29 mg/kg), the estimated daily migration rate was determined to be 0.0002 µg/cm2-day, which was used to predict oral intakes for young children (aged 2 to 36 months old) mouthing toys. These predicted oral intakes ranged from 6.8 × 10−10 to 1.4 × 10−7 mg/kg-bw per day, and it was concluded that this potential source of ethylbenzene exposure was unlikely to be significant (VCCEP 2007).
To characterize potential oral exposures from other types of toys identified by the Danish EPA, the predicted oral intakes determined with the VCCEP method were re-calculated with the highest weighted-average residual concentration of ethylbenzene of 108 ppm (108 mg/kg) for disposable HIPS (higher concentration than those identified by the Danish EPA). This resulted in an estimated daily migration rate of 0.00075 µg/cm2-day. The predicted oral intakes ranged from 2.5 × 10−9 to 5.2 × 10−7 mg/kg-bw per day for children aged from 2 to 36 months old (presented in more detail in Appendix F).
10.1.7.3 Gasoline
Ethylbenzene is naturally present in crude oil and is therefore present in gasoline. Levels of ethylbenzene in gasoline range from less than 1 to 5.4% (IARC 2000; FLL 2008). In Ontario, typical ethylbenzene concentrations were reported to be 1.4% in regular unleaded gasoline and 1.7% in premium unleaded gasoline (CCME 2004). Evaporative losses of gasoline and therefore ethylbenzene may occur during refuelling and from gasoline storage tanks.
A national survey was conducted by the Petroleum Association for Conservation of the Canadian Environment (PACE 1987, 1989) in 1985 on the ambient concentration of ethylbenzene around gas stations in Halifax, Montreal, Toronto, Calgary, and Vancouver during summer and winter. Eight-hour air samples were collected near the gas stations; 160 samples were taken during the summer study and 156 samples during the winter for a total of 316 samples. Mean concentrations of ethylbenzene in 8-hour air samples ranged from 30 to 46 mg/m3, with 95th percentile concentrations ranging from 83 to 184 µg/m3 (maximum concentrations ranged from 816 to 1163 µg/m3). A total of 233 (114 during the summer and 119 during the winter) 10- to 15-minute samples measured during fill-ups at full-serve stations (representing three to five fill-ups) were taken with a battery-operated portable pump attached close to the breathing zone of volunteers (pump operators). Mean concentrations of ethylbenzene from all gas types (regular leaded, regular unleaded, and super unleaded) in the breathing zone ranged from 142 to 389 µg/m3, with 95th percentile concentrations ranging from 263 to 1461 µg/m3 (maximum concentrations ranged from 733 to 2275 µg/m3). More recent Canadian data were not available; however, Esteve-Turrillas et al. (2007) reported levels of ethylbenzene in the air near gas stations in Spain to range from 46 to 99 µg/m3 (three samples) and from 32 to 2280 µg/m3 (six samples) near the breathing zone of individuals refueling their vehicles. Backer et al. (1997) measured air in the personal breathing zone of 30 individuals pumping gasoline during winter in 1995. The average concentrations of ethylbenzene from the high- and low-volume sampling pumps did not exceed 200 ppb (880 µg/m3).
The highest 95th percentile concentration (1461 µg/m3) identified in the PACE studies was used to estimate upper-bound inhalation exposures to ethylbenzene while refuelling a vehicle. Dermal exposures to ethylbenzene while refuelling a vehicle at a service station may occur periodically and were estimated with a range of ethylbenzene concentrations (1 to 5.4%). The resulting dermal doses that used the thin-film approach and assumed 100% dermal absorption ranged from 0.01 to 0.07 mg/kg-bw per event for adults (see Appendix G). Canadians may also be exposed to gasoline when using it at home to operate lawn mowers, emergency power generators, motor chain saws, and similar equipment. No data are currently available to estimate these types of exposures.
The highest 95th percentile concentration for an 8-hour air sample (184 µg/m3) was used to estimate upper-bound inhalation exposures to ethylbenzene for individuals living near service stations that could be exposed to higher concentrations of ethylbenzene compared with those who do not live near a service station.
10.1.8 Confidence in Exposure Database
Confidence in the database on exposure to ethylbenzene through environmental media is considered moderate to high, as representative Canadian data were available for ambient and indoor air, the most relevant sources of exposure via the environment. Confidence is moderate for exposures to ethylbenzene while inside a vehicle. Some Canadian data was available on levels while in traffic but no data on levels in new vehicles in Canada were available. Some Canadian data were available on levels of ethylbenzene in drinking water, but were limited for soil. Confidence in the exposure to ethylbenzene from food is considered moderate as levels in various food items were identified in the United States, but no recent data were available on levels in Canada. Confidence in the exposure estimates from use of consumer products is considered to be moderate as there was some Canadian-specific information available from in-house product testing, information submitted by industry stakeholders and from a more in-depth review of Canadian retailers and material safety data sheets on the types of products found in the country but there was limited information on some of the parameters used in the model including the amount of ethylbenzene dermally absorbed; however, confidence is high that the estimated exposures to ethylbenzene from all pathways are conservative.
10.2 Health Effects Assessment
An assessment by the International Agency for Research on Cancer (IARC 2000) concluded that ethylbenzene was possibly carcinogenic to humans (Group 2B), based on sufficient evidence in experimental animals and inadequate evidence in humans. Although, the US EPA has classified ethylbenzene as a Group D substance, not classifiable as to its human carcinogenicity (US EPA 1991), this assessment was conducted before completion of a 2-year inhalation carcinogenicity bioassay conducted by the US National Toxicology Program (NTP) in 1999. The available health effects information for ethylbenzene is summarized in Appendix H.
10.2.1 Carcinogenicity and Genotoxicity
The carcinogenicity of ethylbenzene was evidenced in experimental animals via inhalation and oral exposure routes. In the NTP inhalation carcinogenicity bioassay, male and female B6C3F1 mice and F344/N rats were exposed to 0, 75, 250, or 750 ppm (0, 326, 1090, or 3260 mg/m3) ethylbenzene vapour for 103 and 104 weeks, respectively (Chan et al. 1998; NTP 1999). A significant and concentration-related increase in incidence of both alveolar/bronchiolar adenomas and combined alveolar/bronchiolar adenomas and carcinomas of the lung, as well as a significant increase in alveolar epithelium metaplasia, were observed in male mice at 3260 mg/m3 (750 ppm) compared with concurrent controls, but were within the NTP historical control ranges (10-42%) at this dose. In the exposed female mice, there were concentration-related increases in the incidence of both hepatocellular adenomas and combined adenomas and carcinomas, which were significant at 3260 mg/m3 compared with concurrent controls, but remained in the NTP historical control ranges (3-54%). The incidence of eosinophilic foci in the liver was significantly greater in the female mice at 3260 mg/m3and was considered a precursor to hepatocellular neoplasia. In the exposed rats, a concentration-dependent increase in incidence of combined renal tubular adenomas and carcinomas, significant at 3260 mg/m3, was observed in the males. Significant increases in incidence of renal tubular adenomas in the females and testicular adenomas in the males were also observed at 3260 mg/m3. It should be noted that testicular adenomas are present in nearly all aged rats of this strain and were found in 80-88% of the males at 3260 mg/m3 which is within the NTP historical control range (54-83%). In both sexes, there was a significant increase in the incidence of focal renal tubular hyperplasia at 3260 mg/m3, which was considered to be a precursor stage of adenoma development by the study's authors. Dose-dependent increases in the severity of chronic progressive nephropathy were observed in females at all exposure levels and in males at the highest concentration (Chan et al. 1998; NTP 1999). In an oral carcinogenicity bioassay, significantly increased incidences in total malignant tumours were observed in Sprague-Dawley rats exposed to 500 mg/kg-bw per day via gavage for 104 weeks (Maltoni et al. 1985). Increased incidences in nasal cavity tumours, neuroesthesioepitheliomas, and oral cavity tumours (statistical analysis was not provided) were also observed in rats exposed to 800 mg/kg-bw per day ethylbenzene by gavage for 2 years (Miltonic et al. 1997).
Ethylbenzene has not demonstrated mutagenic or clastogenic activity in in vivo assays, and negative results have been shown for chromosome aberrations in rat bone marrow (ethylbenzene was administered in a mixture with xylene, Donner et al. 1980) and mouse micronuclei assays (Mohtashamipur et al. 1985; NTP 1992, 1999). Results were also negative in gene mutation assays in vitro in bacteria, with and without metabolic activation, and yeasts, and in insects (Nestmann and Lee 1983; Dean et al. 1985; NTP 1992, 1999). However, there were some positive results from in vitro assays in mammalian cells, including cell transformation after prolonged exposure periods (7 days) and micronuclei in Syrian hamster embryo cells at all dose levels tested (25 to 200 µg/mol). In addition, there was a positive response at the highest non-lethal dose (80 µg/mol; the reported lethal dose was 100 µg/mol) in the mouse lymphoma assay in the presence of cytoxicity. Exposure to ethylbenzene at concentrations of 100-200 µM (10-20 µg/mL) also induced single DNA strand breaks in human blood lymphocytes, whereas exposure to 50 µM ethylbenzene did not elicit this effect (Chen et al. 2008). At a very high dose level (10 mM), ethylbenzene was able to induce marginal sister chromatid exchange in human lymphocytes (Norppa and Vainio 1983). Furthermore, sunlight-irradiated ethylbenzene and ethylbenzene metabolites, ethylhydroquinone and 4-ethylcatechol, in the presence of Cu(II) were able to induce oxidative DNA damage and DNA adduct formation in a dose-dependent manner (Toda et al.2003; Midorikawa et al.2004). Overall, the weight of evidence suggests that ethylbenzene is not likely to be directly genotoxic.
10.2.1.1 Mode of Action for Carcinogenicity
The mode of action for ethylbenzene carcinogenicity has not been fully elucidated. Midorikawa et al. (2004) reported that the ethylbenzene metabolites ethylhydroquinone and 4-ethylcatechol have the ability to induce oxidative DNA damage in vitro. It should be noted the study used calf thymus DNA and oxidative damage was only observed in the presence of copper catalyst. The level of copper used may be higher than physiological level. The Agency for Toxic Substances and Disease Registry (ATSDR) toxicological profile for ethylbenzene concluded that the carcinogenic activity of ethylbenzene may be, at least in part, attributed to the parent compound and/or reactive oxidative metabolites (ATSDR 2010). The California EPA, Office of Environmental Health Hazard Assessment (OEHHA 2007) also stated that although cytotoxicity or exacerbation of existing degenerative processes may be involved in tumourigenicity of ethylbenzene, which might be considered as a non-genotoxic mode of action, the current data do not conclusively establish any particular mode of action for ethylbenzene carcinogenesis.
In contrast, the Voluntary Children's Chemical Evaluation Program (VCCEP 2007), an industry led initiative, examined genotoxicity and non-genotoxicity mediated modes of action for ethylbenzene carcinogenicity based on Hill's criteria (VCCEP 2007). The VCCEP (2007) review, which was subjected to a peer-review (TERA 2007), concluded that all in vivo studies have been negative for genotoxicity and the in vitro studies have been predominantly negative for genotoxicity, and direct genotoxicity does not seem to be a relevant mode of action for ethylbenzene induced species-, sex- , and tissue-specific (kidney, liver, Leydig cell, or lung) tumours. Notably, unpublished genotoxicity test results that were included in the VCCEP data set (Seidel et al. 2006) repeated the gene mutation assay in mouse lymphoma cells and did not find mutagenic response with concentrations up to 120 mg/L. A genotoxicity review article of ethylbenzene similarly concluded a non-genotoxic mechanism based on available data from the standard battery of genotoxicity assays (Henderson et al. 2007).
The VCCEP assessment proposed various non-genotoxic modes of action for ethylbenzene carcinogenicity including a mode of action for kidney tumours (secondary to chronic progressive nephropathy [CPN] caused by a primary ethylbenzene metabolite, 1-phenylethanol, may involve α2u-globulin accumulation), a mode of action for lung tumours (chronic cell proliferation, secondary to metabolism of ethylbenzene to cytotoxic metabolites by CYP2F2, which is expressed at relatively higher levels in mouse lung (Cruzan et al. 2009; Saghir et al. 2009, 2010a); however, it is not clear whether reactive metabolites formed in the liver could also distribute through blood to the lungs (Huff et al. 2010; Saghir et al. 2010b), a mode of action for liver tumours (secondary to a phenobarbital-like enzyme induction and cell proliferation), and a mode of action for Leydig cell tumours (Leydig cell hyperplasia, secondary to ethylbenzene-induced hepatic expression of different cytochrome P450 isozymes, resulting increased hydroxylation and clearance of testosterone). The CPN-mediated renal tubule tumours, the phenobarbital-type liver responses, and the perturbation of serum testosterone-induced Leydig cell tumours were considered qualitatively irrelevant to humans by VCCEP (2007). Even though work by Seely et al. (2002) showed that the association between CPN and renal tubule cell neoplasm is marginal (but statistically significant), more recent analyses have concluded that chemically-induced exacerbation of CPN in rats should not be acknowledged as an indicator of hazard in humans and furthermore, the renal tumours described in this case are CPN-related and their increased incidence should not be considered relevant to humans (Hard 2002; Lock and Hard 2004; Hard and Seely 2005; Hard et al. 2009, 2012, 2013). Others have argued that advanced CPN observed after ethylbenzene exposure was not sufficient to account for the increased kidney tumours in rats (Melnick et al. 2012, 2013).
Although data for ethylbenzene in rodents indicate that it is carcinogenic at high doses, available information on the potential modes of action by which ethylbenzene induces different organ tumours (lung, Leydig cell, liver and kidney) indicates there is a threshold below which tumour formation would not be expected.
10.2.2 Reproductive/Developmental Effects
There were no indications of reproductive toxicity in either sex in rats exposed to ethylbenzene vapour up to 500 ppm (2174 mg/m3) over two generations. No significant exposure-related changes were observed with respect to oestrous cycle length, pre-coital intervals, male and female mating and fertility indices, gestation length, spermatogenic endpoints, reproductive organ weights, ovarian follicle counts, or F1 and F2 litter parameters, including pup sex ratios, live litter sizes, number of dead pups, viability indices, pup body weights, and the general physical condition of the pups. The oestrous cycle length was significantly reduced in the F0 but not in the F1 generation, and the authors considered it was not an ethylbenzene exposure-related effect. A no-observed-adverse-effect concentration (NOAEC) for reproductive toxicity was considered by the investigators to be 2174 mg/m3 (Stump 2004a; Faber et al. 2006). In addition, no significant concentration-related adverse effects on female fertility were observed in Wistar rats that inhaled ethylbenzene vapour at concentrations up to 4348 mg/m3 (1000 ppm) for 3 weeks before breeding in a developmental toxicity study (Hardin et al. 1981; NIOSH 1981). A similar absence of adverse effects on reproductive organs was observed in rats, mice, and rabbits exposed to ethylbenzene vapour up to 3400 mg/m3 (rodents, 782 ppm) or 7000 mg/m3 (rabbits, 1610 ppm) for 4 weeks (Cragg et al. 1989). No treatment-related effects were observed on sperm counts or motility, testicular morphology, length of oestrous cycle, or caudal or epididymal weights in rats or mice exposed to ethylbenzene vapour up to 4348 mg/m3 (1000 ppm) for 13 weeks (NTP 1992). However, decreased peripheral hormone levels during the dioestrus stage were observed in rats that were orally administered ethylbenzene at dose levels of 500 mg/kg and above (Ungváry 1986).
Minor developmental effects were observed in the offspring of rodents and rabbits exposed to ethylbenzene during gestation. A significant increase in the incidence of foetuses with extra ribs was observed in rats exposed to ethylbenzene at 435 mg/m3 (100 ppm, the lowest inhalation lowest-observed-effect concentration [LOEC] for developmental effects) during gestation (Hardin et al. 1981; NIOSH 1981). In the same study where rats were exposed during pregestation and gestation, increase in the incidences of fetuses with extra ribs was only observed at a higher dose level of 4350 mg/m3(1000 ppm), but not in rats that were exposed to a lower concentration. Hence, the authors considered the dose-response relationship for this effect at 435 mg/mg3 was not consistent. Maternal toxicity, such as significantly increased relative and absolute liver, kidney, and spleen weights, was observed at 4350 mg/m3 (1000 ppm) in rats in this study. Other studies have also noted minor developmental effects following in utero exposure to ethylbenzene during gestation, including increased number of foetuses with skeletal retardation in rats exposed to 600 mg/m3 (138 ppm), increased incidence of foetal uropoietic apparatus malformation in mice, and reduced mean foetal body weights in rabbits observed at exposure concentrations of 500 mg/m3 (115 ppm) (Ungváry and Tatrai 1985). Mice were treated for only one dose level of 500 mg/m3 (115 ppm) and anomalies of the uropoietic apparatus was not observed in other recent developmental studies (Faber et al. 2006, 2007; Saillenfait et al. 2003, 2006, 2007). Moderate and dose-dependent maternal toxicity was observed in rats in the latter study (no further details were provided) and spontaneous abortion was observed in rabbits at 1000 mg/m3. ATSDR (2010) noted that the Ungváry and Tatrai (1985) study did not include sufficient details regarding the adverse effects, dictating caution in the interpretation of the study findings. In the two-generation study in rats, as described in the reproductive effects section, no adverse developmental or neurodevelopmental effects or maternal effects were observed in F1 and F2 rats exposed to ethylbenzene up to 2174 mg/m3(500 ppm) (Faber et al.2007). In more recent development toxicity studies, significant increases in the incidence of foetal skeletal variations per litter were observed in the offspring of rats exposed to 8696 mg/m3 (2000 ppm) and significant reductions in foetal body weights were observed in the offspring of rats exposed to greater than or equal to 4348 mg/m3(1000 ppm) ethylbenzene during gestation in the presence of maternal toxicity (Saillenfait et al.2003, 2006, 2007.
In 4-week and 13-week repeated-dose toxicity studies where rats were orally administrated ethylbenzene for up to 750 mg/kg-bw/day, there were no pathological changes or weight changes to reproductive organs in male and female rats (Mellert et al. 2007).
10.2.3 Ototoxicity and Central Nervous System Effects
Ototoxicity (i.e., hearing loss featured by increased auditory thresholds and the outer hair cell losses) was consistently observed in experimental rats following repeated inhalation and oral exposure to ethylbenzene when the auditory threshold changes were measured by electrocochleography. A short-term inhalation no-observed-adverse-effect concentration (NOAEC) was identified at 1305 mg/m3 (300 ppm) based on ototoxicity (increased auditory thresholds and outer hair cell loss) observed at 1740 mg/m3 (400 ppm) (Cappaert et al. 2000). A subchronic inhalation lowest-observed-adverse-effect concentration (LOAEC) of 870 mg/m3 (200 ppm) was identified based on outer hair cell loss (no NOAEC were identified in the study, Gagnaire et al. 2007), and an oral lowest-observed-adverse-effect level (LOAEL) of 900 mg/kg-bw per day (Gagnaire and Langlais 2005) was identified, also based on ototoxicity. Such auditory system effects were not detected when tested by acoustic startle in offsprings of rats (postnatal days (PND) 20 and 60) that were exposed to ethylbenzene by inhalation to doses as high as 2174 mg/m3 (500 ppm) in a two generation study (Faber et al. 2007) and in rats administered doses of ethylbenzene up to 500 mg/kg-bw per day for 90 days via the oral route (Li et al. 2010). In addition, guinea pigs were not susceptible to ethylbenzene-induced ototoxicity after exposure to 10 879 mg/m3 (2500 ppm) ethylbenzene for 5 days (Cappaert et al. 2002).
In an effort to develop a weight of evidence approach to determining ototoxicity from exposure to industrial chemicals, Vyskosil et al (2011) created a structured database examining the potential ototoxicity of industrial chemicals alone or in combination with noise exposure. According to this exercise, given the current evidence from animal studies, ethylbenzene appeared to affect auditory function mainly in the cochlear mid-frequency range and could be considered a possible ototoxic agent.
Other nervous system effects induced by ethylbenzene were observed. Depletion of striatal and tubero-infundibular dopamine was observed in rabbits at a concentration of 3261 mg/m3(750 ppm) and above (Romanelli et al.1986; Mutti et al.1988) and ethylbenzene-induced moderate activation in motor behaviour was observed in rats following an acute 4 hour inhalation exposure, with a LOAEC of 1740 mg/m3 (400 ppm; the lowest dose tested) (Molnar et al. 1986), and following subchronic oral exposure, with a NOAEL of 75 mg/kg-bw per day (Mellert et al. 2007). Acute exposure to ethylbenzene also caused non-specific depression of the central nervous system in humans and animals at higher concentrations (Yant et al. 1930; Bardodej and Bardodejova 1970).
No developmental neurotoxicity effects were observed in rats (Faber et al. 2007; Li et al. 2010), which was also described in Section 10.2.2.
There were a number of epidemiological investigations or human reports on health effects, such as altered neuronal behaviour and short-term memory capacity, and ototoxicity, associated with occupational exposure to hydrocarbon mixtures (e.g., paints and gasoline) that contain ethylbenzene. In these studies, there is uncertainty with respect to the relative contribution of ethylbenzene exposure in the case of workers who experienced ototoxicity.
A cross-sectional study was conducted from workers in petrochemical plants (Zhang et al. 2013). The workers had relatively specific exposures to ethylbenzene, since the levels of other volatile aromatic hydrocarbons (styrene, bezene, toluene and xylene) were below the limit of detection. The prevalence of hearing loss for the ethylbenzene-exposed workers was higher when compared to two reference groups (unexposed office personel in these plants and workers in a power station exposed to similar noise level), with age, cigarette smoking and alcohol drinking adjusted. Neurobehavioural function alternation was observed in these exposed workers.
10.2.4 Other Systemic Effects
Species-specific kidney and liver toxicity was consistently observed in rodents following repeated inhalation and oral exposure to ethylbenzene. Liver and/or kidney weight changes were observed in several studies (Wolf et al.1956; Elovaara et al.1985; Cragg et al.1989; NTP 1992; Stump 2004b; Mellert et al. 2007; Li et al. 2010), and clear pathological changes in the mouse liver and rat kidneys were observed at higher concentrations following chronic inhalation exposure (Chan et al. 1998; NTP 1999). The NTP 2-year chronic inhalation study examined effects in both rats and mice. Increased severity of chronic progressive nephropathy (CPN) was observed in female rats at the lowest dose tested (326 mg/m3) and in male rats at the highest dose tested (Chan et al. 1998; NTP 1999). CPN is a spontaneously occurring disease in laboratory rats that occurs with age and its progression and severity is dependant on the strain of rat used and the diet consumed during the study (Hard et al 2009). In some strains, like the strain described above (Fisher 344), CPN starts to develop at a relatively young age and has been observed to occur in 100% of animals with first detectable histological lesions at 4-5 months of age in control males regardless of diet used. This disease does develop in females, but is less severe (Hard et al 2009). On the basis of differences in physiology and pathology, Hard et al (2009) concluded that there is no clear human counterpart for CPN and recommended that chemically-induced exacerbation of CPN alone should not be used as a reliable indicator of hazard for humans. This was supported by the observation that CPN was not seen in mice exposed to ethylbenzene at similar or higher dose levels and therefore is considered a species-specific effect. The LOAEC for exposed mice in this study was set at 1090 mg/m3 (250 ppm), based on significantly increased incidences of hyperplasia of the pituitary gland pars distalis in exposed female mice and significantly increased incidences of syncytial alteration of hepatocytes in exposed males (Chan et al. 1998; NTP 1999). The NOAEC for this study was established at 326 mg/m3 (75 ppm).
The highest oral repeat dose NOAEL was identified to be 75 mg/kg-bw per day, based on significantly increased liver and kidney weights with corresponding liver enzyme changes, cellular effects in the kidney, and haematological parameters in subchronically (13 weeks) exposed rats at the next higher dose of 250 mg/kg-bw per day (Mellert et al.2007). Other systemic effects, such as pathological changes in lung, thyroid, prostate gland, bone marrow, and testes in rats, were also observed in the repeated inhalation studies at higher dose levels and after prolonged exposure (Chan et al. 1998; NTP 1999).
Haematological effects (significantly increased platelets in male rats and increased leukocyte counts in female rats) were observed in a 4-week inhalation study at 3401 mg/m3 (782 ppm, Cragg et al. 1989); however, these adverse effects were not observed in a 13-week inhalation study with rats exposed to doses up to 4350 mg/m3 (1000 ppm, NTP 1992). Some haematological effects (increased mean corpuscular volume in both sexes of rats and decreased platelets in female rats) were also observed following subchronic oral exposure (Mellert et al. 2007). Rats exposed to up to 2174 mg/m3 (500 ppm) ethylbenzene for 28 days did not exhibit alterations in their immune response (Stump 2004b; Li et al. 2010).
In addition, ethylbenzene is irritating to the mucous membranes (eye and respiratory tract); such effects were observed both in humans and animals (Yant et al. 1930; Wolf et al. 1956; Smyth et al. 1962; Gerarde 1963; Bardodej and Bardodejova 1970; Moscato et al. 1987; Lewis 1992; Cometto-Muñiz and Cain 1995).
There were a number of epidemiological investigations or human reports on other health effects not previously mentioned, such as changed blood cell counts, reduced semen counts, and genotoxicity, associated with occupational exposure to hydrocarbon mixtures (e.g., paints and gasoline) that contain ethylbenzene. There was no evidence that exposure to ethylbenzene was associated with increased cancer risk in these workers. These data were not used to assess ethylbenzene effects in humans due to the co-exposure to other chemicals, such as benzene, xylene, or toluene (Nicholson et al. 1978; Angerer and Wulf 1985; Bardodej and Círek 1988; Triebig et al. 1988; Lu and Zhen 1989; Holz et al. 1995; De Celis et al. 2000; Sliwinska-Kowalska et al. 2001; Sram et al. 2004; Chang et al 2011).
VOCs have been associated with effects on the respiratory system (e.g., asthma, reduced lung function, rhinitis), but no epidemiological study has determined these effects were directly linked to ethylbenzene alone (Rumchev et al 2004; Arif and Shah 2007; Hulin et al 2010; Billionnet et al 2011; Hwang et al 2011; Martins et al 2012).
10.2.5 Toxicokinetics
There were a considerable number of studies that investigated the absorption, distribution, metabolism, and excretion of ethylbenzene in humans and animals (VCCEP 2007; ATSDR 2010). Ethylbenzene is well absorbed from the skin (ethylbenzene liquid, but not ethylbenzene vapour), lungs, and gastrointestinal tract and is rapidly distributed throughout the body. Data pertaining to oral absorption of ethylbenzene from rabbits and rats following exposure to single oral doses of ethylbenzene suggests rapid and effective absorption by this route with 72 and 92% of the administered dose recovered in rabbits and 84% in rats, respectively (Climie et al. 1983; El Masry et al. 1956). More recently, Faber et al. (2006) reported that ethylbenzene was detected at 0.49, 3.51, and 18.28 mg/L in maternal blood of pregnant rats 1 hour after the last administration of 0, 8.67, 30, and 114 mg/kg ethylbenzene by gavage for 4 days, respectively. Further, ethylbenzene was not detected in blood of weanlings from the same dams. When applied dermally, Morgan et al. (1991) reported that the peak blood level of ethylbenzene (5.6 µg/ml) was reached within 2 hours of topical application of neat ethylbenzene to approximately 1% of the body surface in rats and slowly declined after 24-hrs. The total amount absorbed was reduced when ethylbenzene was administered in aqueous solutions.
Ethylbenzene can be rapidly metabolized and then eliminated from the body, primarily as urinary metabolites and conjugates. The half-life of ethylbenzene in blood was measured in the range of 3.3 minutes at 326 mg/m3 (75ppm) to 63 minutes at 4348 mg/m3 (1000 ppm) in mice following a 4-hour exposure (Charest-Tardif et al. 2006). In addition, saturation kinetics of ethylbenzene was observed in this study at exposure concentrations above 2174 mg/m3 (500 ppm), while it was linear at lower concentrations.
The metabolism of ethylbenzene is mediated by cytochrome P450 enzymes (e.g., CYP2E1, 1A2, and 2B6 in human liver; CYP2B1, 1E1, 2E1, and 1A1 in rat liver; CYP 1A1, 1E1, and 2B1 in mouse liver), with the ethyl moiety (side-chain) oxidation as the major metabolic pathway and the ring oxidation as a minor one, followed by conjugation reactions. The metabolism of ethylbenzene, in terms of major metabolites and the percentages of the metabolites, varies with species, sex, and nutrition status. No significant qualitative metabolic differences between oral and inhalation routes were reported (ATSDR 2010). However, metabolic differences between inhalation and dermal exposure routes were observed in humans. The major metabolites of ethylbenzene in humans after inhalation exposure are mandelic acid (64-71%), phenylglyoxylic acid (19-25%), and 1-phenylethanol (5%), whereas excretion of mandelic acid was only 4.6% of a dermally absorbed dose. In rats, after exposure to ethylbenzene orally or via inhalation, the major metabolites were identified as hippuric and benzoic acids (38%), 1-phenylethanol (25%), mandelic acid (15-23%), phenylglyoxylic acid (10%), and more recently measured mercapturic acids (0.3%; Cossec et al 2010). In rabbits, the most important metabolite is hippuric acid, which is probably formed by oxidative decarboxylation of phenylglyoxylic acid. Ring oxidation products include para- and meta-hydroxyacetophenone, 2-ethylphenol, and 4-ethylphenol. Metabolism of ethylbenzene has not been studied in children or immature animals. However, some enzymes (e.g., uridine 5'-diphospho-glucuronosyltransferase and sulfotransferases) involved in conjugation of phase I ethylbenzene metabolites are known to be developmentally regulated (VCCEP 2007; ATSDR 2010). Species and organ differences in the metoblism of ethylbenzene were also observed in in vitro assays (Saghir et al. 2009, 2010a). Overall, the rate of ethylbenzene metabolism by mouse liver microsomes was higher than that in rats and humans, while the latter two were similar. Both rat and mouse lung microsomes were more active in metabolizing ethylbenzene than were liver microsomes, while human lung microsomes did not metabolize ethylbenzene to any metabolites above the detection limit. Both CYP 2E1 and 2F2 were involved in the ring-oxylation of ethylbenzene to generate reactive metabolites, while CYP 2F2 activity in mouse lung was higher than that in rat lung and much higher than that in human lung.
Several physiologically based pharmacokinetic (PBPK) models have been developed that simulate the kinetics of inhaled ethylbenzene in animals and humans (Tardif et al. 1997; Dennison et al. 2003; Nong et al. 2007). A model of dermal absorption of ethylbenzene in humans has also been reported predicting that, based on its lipophilicity, exposure to ethylbenzene via the dermal route would be almost an order of magnitude greater than that of VOCs with lower Kow values (Shatkin and Brown 1991).
The confidence in the toxicological database is high as data on acute toxicity, carcinogenicity, repeated-dose toxicity, genotoxicity, reproductive and developmental toxicity, neurotoxicity, immunotoxicity, and toxicological kinetics and dynamics are available, although data on health effects associated with dermal exposure are limited.
10.3 Characterization of Risk to Human Health
On the basis of the available health effects information, mainly obtained from the studies in experimental animals and the assessments conducted by other international agencies, the critical health effects associated with exposure to ethylbenzene are considered to be tumour induction and non-cancer systemic effects, primarily on the auditory system and on the liver, kidney and pituitary glands. Minor developmental effects, haematological effects, effects on the endocrine glands (thyroid hyperplasia), and on the central nervous system were also observed at high dose levels and following prolonged exposure periods.
Ethylbenzene is not mutagenic or clastogenic in vivo. It did not induce gene mutations in bacteria and yeasts and only induced gene mutations in mouse lymphoma cells at high dose levels in the presence of cytotoxicity. Although ethylbenzene elicited weak clastogenicity and DNA damage in some in vitroassays, overall the available information indicates that ethylbenzene is not likely to be directly genotoxic. In addition, saturated toxicokinetics of ethylbenzene was observed in mice at dose levels below the concentration where increased tumour incidence became significant, indicating the existence of a threshold exposure level for ethylbenzene-induced tumourigenesis. A summary of the critical endpoints selected for risk characterization for both cancer and non-cancer effects from exposure to ethylbenzene is presented below in Table 10-4.
| Duration and route | Critical effect | Critical effect level |
|---|---|---|
| Acute and short term inhalation | Inhalation NOAEC based on significant hearing loss in rats exposed to ethylbenzene for 5 days (Cappaert et al 2000). | 1305 mg/ m3 (300 ppm) |
| Subchronic inhalation | Inhalation LOAEC based on hearing loss in rats in a 90-day study, a NOAEC was not identified from the study (Gagnaire et al 2007). | 870 mg/m3 (200 ppm) |
| Chronic inhalation | Inhalation NOAEC based on increased incidences of pituitary gland hyperplasia in female mice and syncytial alterations in livers of male mice at the next dose of 1090 mg/m3 (250 ppm) in a chronic study (NTP 1999) (increased severity of CPN in exposed female rats was observed at 326 mg/m3, however, this effect is not considered to be relevant to humans). Further, this level is protective of effects shown in subchronic inhalation studies, including ototoxicity at 870 mg/m3. Significant increase in tumour incidences in various organs in both rodent species were observed in this study at 3260 mg/m3 (750 ppm) (NTP 1999). | 326 mg/m3 (75 ppm) |
| Chronic oral | Oral NOAEL based on increased kidney and liver weights with corresponding liver enzyme changes, cellular effects in the kidney, and haematological parameters in rats in a 13-week study (Mellert et al 2007; Li et al. 2010). | 75 mg/kg-bw per day |
The general population of Canada can be exposed to ethylbenzene through environmental media (i.e., ambient air, indoor air, drinking water, and soil), food, and during the intermittent use of consumer products containing the substance. Critical health effects observed in experimental animals were used to characterize the potential human health risk associated with ethylbenzene exposure.
The chronic inhalation NOAEC of 326 mg/m3 observed in a 2-year chronic study in mice (NTP 1999) was used to characterize the human health risk associated with inhalation exposure from environmental media. Comparing this effect concentration with the highest 95th percentile concentrations measured in indoor air (54 µg/m3), or with the highest 95th percentile concentration measured in personal air (27.3 µg/m3), results in a margins of exposure (MOE) of 6000 and 12 000, respectively. Significantly increased tumour incidences were observed in a 2-year study at 3260 mg/m3, and thus MOEs of 60 000 and 120 000, based on indoor and personal air respectively, were derived for tumour occurrence. These MOEs are considered adequate to account for uncertainties in the health effects and exposure databases for cancer and non-cancer effects for inhalation exposures from environmental media.
The presence of ethylbenzene in vehicle interior air is also a source of inhalation exposure. The available data indicate that the peak concentrations of ethylbenzene in the interior air of new vehicles decreased rapidly over a few months and then reached levels that were comparable to the highest 95thpercentile of indoor air concentration (i.e., 54 µg/m3). The upper-bound value of 240 µg/m3identified in interior air of new vehicles (Chien 2007) is considered to represent a conservative exposure from this source. Comparing this value with the lowest subchronic LO(A)EC of 870 mg/m3(200 ppm), based on outer hair cell loss (hearing loss) in rats exposed to ethylbenzene for 90 days (Gagnaire et al. 2007), results in an MOE of approximately 3600. This MOE is considered adequate to account for uncertainties in the health effects and exposure databases.
Oral intake from environmental media (i.e., water and soil) and food represents a chronic oral exposure scenario. However, the available oral chronic toxicity studies (Maltoni et al. 1985, 1997) did not provide sufficient information on non-cancer effects in the exposed rats and tested high doses only. The highest subchronic oral NOAEL of 75 mg/kg-bw per day, based on a significant increase in liver and kidney weights with corresponding liver enzyme changes, cellular effects in the kidney, and haematological parameters in rats exposed to ethylbenzene for 13 weeks (Mellert et al. 2007), was used to characterize the human health risk of non-cancer effects from potential chronic oral exposure. Comparing this effect level with the highest oral exposure from food, water, and soil (3 µg/kg-bw per day estimated in infants aged 0-6 months, not formula fed) results in an MOE of 25 000. Following a 2-year oral dosing, a significant increase in tumour incidences was reported in rats at 500 mg/kg-bw per day and above (Maltoni et al. 1985, 1997), which is seven times the subchronic oral NOAEL. Thus, MOEs are considered adequate to account for uncertainties in the health effects and exposure databases for cancer and non-cancer effects for oral exposure from environmental media. The highest oral exposure from food, water, and soil for individuals living in northern parts of the country that may consume fish with higher concentrations of ethylbenzene (3.3 µg/kg-bw per day estimated in individuals from 6 months to 4 years old) was not dissimilar from estimates for the general population of Canada and, therefore, results in similar MOEs.
Ethylbenzene has been reported to be present in several types of consumer products that may be used indoors or in a garage. These consumer products would be used on an intermittent and sporadic basis and are likely to result in both inhalation and dermal exposure of the user. Some of the products, such as caulking, or spray paints, are only used occasionally, a few times a year or less, and the applications are normally completed within a day. Other consumer products might be used on consecutive days, such as paint remover, liquid paint, or stain. In both cases, ethylbenzene could be released into indoor air over several days, e.g., during application as well as after paint is applied. Therefore, potential inhalation exposure of the general population from use of products such as paint or wood stains is considered to be short term rather than acute in duration.
The lowest short-term NOAEC available in the database is 1305 mg/m3 (300 ppm) based on significant hearing loss in rats exposed to ethylbenzene for 5 days (Cappaert et al. 2000). MOEs were derived by comparing the NOAEC with the estimated mean concentrations on the day of the event derived from ConsExpo, and are shown in Table 10-5. Resulting margins of exposure are considered adequate to account for uncertainties in the health effects and exposure databases at the concentrations listed in Table 10-5.
| Type of products | Concentrations of ethylbenzene in Canadian products | Mean concentration on day of event (mg/m3) | Margins of exposureFootnote Table 10-5[a].9 |
|---|---|---|---|
| Spray paint | 0.01 to 5% | 0.006 to 3 | 217 500-435 |
| Liquid paint | 0.1 to 1% | 1.3 to 13 | 1003-100 |
| Paint remover | 4% | 2.8 | 466 |
| Lacquer/stain/ varnish | 0.1 to 2% | 0.5 to 9.4 | 2610-139 |
| Caulking (sealant) | 0.1 to 5% | 0.1 to 5.2 | 13 050-251 |
Use of these products is expected to be associated with dermal exposure to ethylbenzene. The toxicological database was inadequate to derive a critical effect level via the dermal route. Although dermal exposure would be expected to contribute to the overall exposure during use of consumer products, the primary route is considered to be inhalation. Part of the ethylbenzene deposited on skin will be volatilized, and only a portion on the non-volatilized substance will be systemically absorbed. Accordingly, the increase in exposure resulting from dermal contact is not considered to be significant enough to result in inadequate margins of exposure for those scenarios for which margins of exposure from the inhalation route are considered adequate.
Consumers are also potentially exposed to ethylbenzene while refuelling personal vehicles. A comparison of the lowest short-term NOAEC of 1305 mg/m3 (300 ppm), based on significant hearing loss in rats exposed to ethylbenzene for 5 days (Cappaert et al. 2000), with the 95th percentile concentration of ethylbenzene measured while pumping gasoline (1461 µg/m3) results in a MOE of 893. This MOE is considered adequate to account for uncertainties in the health effects and exposure databases. The increase in overall exposure from dermal contact with ethylbenzene while refuelling personal vehicles is not considered to result in potentially inadequate margins of exposure.
Individuals living near service stations may be exposed to higher levels of ethylbenzene every day; the highest 95th percentile 8-hour air concentration of 184 µg/m3 measured near gas stations was compared with the lowest inhalation NOAEC of 326 mg/m3, based on liver and pituitary gland effects in mice at the higher dose level of 1090 mg/m3 following chronic exposure (NTP 1999), resulting in an MOE of 1770. This MOE is considered adequately protective of non-neoplastic effects. This exposure was also compared with the effect level associated with increased tumour incidences (3260 mg/m3), resulting in a MOE of 17 700, which is considered adequately protective of neoplastic effects.
Mainstream cigarette smoke is a source of exposure for ethylbenzene and would contribute to exposures of ethylbenzene.
Although ethylbenzene was also detected in some young children's toys that may be mouthed, the conservative oral exposure estimates from use of these products (i.e., 2.5 ´ 10-9 to 5.2 ´ 10-7 mg/kg-bw per day) indicate that the contribution of this source of exposure is minimal.
10.3.1 Biomonitoring Data
No Canadian biomonitoring data were identified; however, concentrations of ethylbenzene in blood for the general population of the United States are available. The National Health and Nutrition Examination Survey (NHANES) conducted by the U.S. Center for Disease Control and Prevention (CDC) is a series of surveys that collect data on levels of chemicals found in blood, serum, and urine, as well as other information related to the health and nutritional status of the U.S. population (CDC 2009, 2014). The report provides data on levels of ethylbenzene in the blood of adults aged 20 to 59 years old for the years 2001 to 2006, for adults 60 years and older and for adolescents aged 12 to 19 years old for the year 2005-2006 (CDC 2014). The geometric mean blood concentrations for adults were 0.034 ng/mL, 0.035 ng/mL and 0.040 ng/mL for the years 2001-2002, 2003-2004, and 2005-2006 respectively. The 95th percentile values were 0.140 ng/mL, 0.110 ng/mL, and 0.150 ng/mL for the years 2001-2002, 2003-2004 and 2005-2006, respectively (CDC 2014). Similar ethylbenzene levels in blood have also been measured through the NHANES surveys in 1988-1994 and in 1999-2000 (CDC 2009). For adults 60 years and older, the geometric mean blood concentration was 0.037 ng/mL with a 95th percentile blood concentration of 0.130 ng/mL (CDC 2014). For adolescents aged 12 to 19 years old, the geometric mean blood concentration was 0.032 ng/mL with a 95th percentile blood concentration of 0.096 ng/mL (CDC 2014). U.S. data on the concentration of ethylbenzene in the blood of children were also identified. In a study conducted in the years 2000 and 2001, a stratified random sample of 152 children aged 6-10 years was selected across two elementary schools in Minneapolis, Minnesota (Sexton et al. 2005). The mean concentration of ethylbenzene measured in the blood of 134 children was 0.04 ng/mL and the 95th percentile value was reported to be 0.07 ng/mL. According to CDC (2009), the presence of ethylbenzene in blood indicates recent exposure.
Aylward et al. (2010) derived equations to convert external exposure concentrations of ethylbenzene into human steady-state venous blood concentrations, using metabolic parameters provided by a human PBPK model (Haddad et al. 2001; Alyward et al. 2010). Conversion of the chronic oral NOAEL (75 mg/kg-bw per day, Mellert et al. 2007) and inhalation NOAEC (326 mg/m3, NTP 1999), identified as critical effects for risk characterization of ethylbenzene, results in steady-state venous blood concentrations that are two orders of magnitude above the highest blood concentrations identified from the NHANES surveys (0.150 ng/L). These results are consistent with the risk characterization conclusion that margins of exposure between chronic exposure to ethylbenzene and critical effect levels are adequate taking into consideration uncertainties in the exposure and health effects databases.
Based on the available health effects and exposure information, it is concluded that ethylbenzene does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
10.4 Uncertainties in Evaluation of Risk to Human Health
Although a wide range of toxicological studies are available, adequate epidemiological data for characterizing the human health risks associated with ethylbenzene exposure were not identified.
In light of the species differences in ethylbenzene toxicokinetics and dynamics, there might be some quantitative and qualitative differences between human responses to ethylbenzene exposure and those observed in experimental animals. There have been efforts made to reduce the uncertainty in interspecies extrapolation using PBPK models (Tardif et al. 1997; Dennison et al. 2003; Nong et al. 2007). Nevertheless, as a conservative approach in the absence of reliable human health effects data, effects observed in experimental animals and the exposure levels associated with those effects were used to characterize the human health risks.
For characterizing the risks associated with chronic inhalation exposure from environmental media and from pumping gasoline, the critical health effects chosen for risk characterization were non-neoplastic liver and pituitary gland effects in 2-year inhalation-exposed mice. Although the human relevance of ethylbenzene-induced tumours from both the rat and mouse chronic inhalation studies is uncertain, risk characterization for both non-neoplastic and neoplastic effects from chronic exposure was conducted and the resulting MOEs were adequate to address uncertainties in the health effects and exposure databases. This approach is considered conservative.
The available health effects data were inadequate to identify a chronic oral endpoint and a subchronic LO(A)EL based on increased liver and kidney weights observed in rats exposed for 13 weeks was used for characterizing the risk associated with chronic oral intake from food, water, and soil. There is uncertainty in using a subchronic LOAEL since effects might occur at exposure levels higher than in a chronic study. However, margins of exposure were considered large enough to address this uncertainty.
Ototoxicity (hearing loss) was observed in experimental animals and in workers occupationally exposed to solvents including ethylbenzene. There is some uncertainty with respect to the relative contribution of ethylbenzene exposure of workers observed to suffer from ototoxicity.
In addition, no dermal effect level was identified in the data set to be suitable for risk characterization.
There is some uncertainty in how much ethylbenzene is present in vehicles (new and old) and while they are in traffic as only a few studies on this topic were identified. More information on characterizing the sources of ethylbenzene concentration in indoor air would be useful. There is some uncertainty related to the estimation of daily intakes from food and beverages, as Canada-specific data were limited; however, confidence is high that the estimated exposures are conservative and most likely overestimate potential exposures to ethylbenzene from foods. There is some uncertainty in the estimation of exposure to individuals living in northern areas that may consume fish with higher concentrations of ethylbenzene. Only one document was available that measured concentrations of ethylbenzene in fish in Canada. The maximum concentration of ethylbenzene in fish was used as a conservative approach to estimating exposures to these populations. There is also uncertainty in the estimated intakes of ethylbenzene from soil as no relevant Canadian studies were available.
Exposure estimates could not be derived for all potential consumer products identified to contain ethylbenzene, such as automotive and arts and craft products, owing to a lack of data specific to each of these products; however, the upper-bounding estimates of exposure from use of paint, caulking and other coating products derived with the ConsExpo model are considered to account for these other scenarios. There is some uncertainty in the estimates of exposure to ethylbenzene from use of certain consumer products because of the lack of information on specific parameters used in the model (e.g., amount of product used for certain scenarios). There is also some uncertainty associated with exposures from use of consumer products to other age groups such as infants, toddlers, and teenagers. The use of upper-bound Canadian-specific ethylbenzene concentrations for each product scenario and the ConsExpo model which contains conservative assumptions ensures that upper-bound exposures are estimated.
There is also some uncertainty related to the estimates of both inhalation and dermal exposures to ethylbenzene while refueling a vehicle and for those living near service stations as measured concentrations may not be representative of current levels of ethylbenzene in gasoline. However, conservative assumptions were used to estimate these exposures to ethylbenzene such as the length of time spent at a gas station, and the use of the highest 95th percentile concentrations measured both at the pump and near the gas stations. There is also uncertainty regarding exposures to gasoline when refuelling motor-operated equipment used at home, such as lawn mowers; however, exposures associated with these uses are considered limited and intermittent and are considered to be covered by the automobile refuelling scenario.
11. Proposed Conclusion
On the basis of the information presented in this screening assessment, it is concluded that ethylbenzene does not meet the criteria under paragraph 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Based on the information presented in this screening assessment, it is concluded that ethylbenzene does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is concluded that ethylbenzene does not meet any of the criteria set out in section 64 of CEPA.
References
Alberta Environment. 2005. Alberta ambient air quality objective. Ethylbenzene. Effective 1 May 2005.
Alberta Environment. 2010. Three Creeks odour issue: a report on air quality monitoring conducted between February and May 2010. ISBN No. 978-0-7785-8994-5 (online edition). 2 July 2010.
Andersson K, Fuxe K, Nilsen OG, Toftgård R, Eneroth P, Gustafsson JÅ. 1981. Production of discrete changes in dopamine and noradrenaline levels and turnover in various parts of the rat brain following exposure to xylene, ortho-, meta-, and para-xylene, and ethylbenzene.Toxicol Appl Pharmacol 60:535-548. [cited in IPCS 1996; IARC 2000].
Angerer J, Wolf H. 1985. Occupational chronic exposure to organic solvents. XI. Alkylbenzene exposure of varnish workers: effects on hematopoietic system. Int Arch Occup Environ Health 56:307-321.
Arif AA, Shah SM. 2007. Association between personal exposure to volatile organic compounds and asthma among US adult population. Int Arch Occup Environ Health 80:711-719.
Aronson D, Howard PH. 1997. Anaerobic biodegradation of organic chemicals in groundwater: a summary of field and laboratory studies. North Syracuse (NY): Environmental Science Centre, Syracuse Research Corporation. Available from: http://www.syrres.com/pdfs/ratecon.pdf http://www.syrres.com/pdfs/ratecon.pdf
Aronson D, Citra M, Shuler K, Printup H, Howard PH. 1999. Aerobic biodegradation of organic chemicals in environmental media: a summary of field and laboratory studies. North Syracuse (NY): Environmental Science Centre, Syracuse Research Corporation. Available from: http://www.syrres.com/pdfs/AERBIO.pdf (SRC TR 99-002).
Atari DO, Luginaah IN. 2009. Assessing the distribution of volatile organic compounds using land use regression in Sarnia, "Chemical Valley", Ontario, Canada. Environ Health 8(16):1-14.
Atkinson R. 1989. Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds. J Phys Chem Ref Data Monogr 1:1-246.
[ATSDR] Agency for Toxic Substances and Disease Registry. 2007. Toxicological profile for ethylbenzene (update). Washington (DC): US Department of Health and Human Services, Public Health Service. [cited 2009 Dec]. Available from: http://www.atsdr.cdc.gov/toxprofiles/tp110.html
[ATSDR] Agency for Toxic Substances and Disease Registry. 2010. Toxicological profile for ethylbenzene (update). Washington (DC): US Department of Health and Human Services, Public Health Service. [cited 2010 Dec]. Available from: http://www.atsdr.cdc.gov/ToxProfiles/tp110.pdf
Aylward LL, Kirman CR, Blount BC, Hays SM. 2010. Chemical-specific screening criteria for interpretation of biomonitoring data for volatile organic compounds (VOCs) - Application of steady-state PBPK model solutions. Regul Toxicol Pharmacol 58:33-44.
Backer LC, Egeland GM, Ashley DL, Lawryk NJ, Weisel CP, White MC, Bundy T, Shortt E, Middaugh JP. 1997. Exposure to regular gasoline and ethanol oxyfuel during refuelling in Alaska. Environ Health Perspect 105(8): 850-855.
Badjagbo K, Picard P, Moore S, Sauve S. 2009. Direct atmospheric pressure chemical ionization-tandem mass spectrometry for the continuous real-time trace analysis of benzene, toluene, ethylbenzene, and xylenes in ambient air. J Am Soc Mass Spectrom 20:829-836.
Bardodej Z, Bardodejova E. 1970. Biotransformation of ethylbenzene, styrene, and alphamethylstyrene in man. Am Ind Hyg Assoc J 31:206-209.
Bardodej Z, Círek A. 1988. Long-term study on workers occupationally exposed to ethylbenzene. J Hyg Epidemiol Microbiol Immunol 32:1-5. [cited in VCCEP 2007].
Barker JF. 1988. Organic contaminants affected by landfill leachates. In: Workshop on research needs in toxicology. AECL-9718. Waterloo (ON): Atomic Energy of Canada Ltd. p. 200-206.
Barker JF, Cherry JA, Reinhard M, Pankow JF, Zapico M. 1989. The occurrence and mobility of hazardous organic chemicals in groundwater at several Ontario landfills. Final Report. R.A.C. Project No. 118 PL. Toronto (ON): Queen's Printer for Ontario.
Barnett JF Jr. 2006. Oral (gavage) subchronic neurotoxicity study of ethylbenzene in rats with recovery group. Laboratory Project ID MHV00001. Horsham (PA): Charles River DDS Argus Division Laboratory. Sponsored by the Ethylbenzene Panel, American Chemistry Council, Arlington, VA. [cited in VCCEP 2007].
Batterman S, Jia C, Hatzivasilis G. 2007. Migration of volatile organic compounds from attached garages to residences: a major exposure source. Environ Res 104:224-240.
Berthiaume S, Ring KL. 2006. CEH marketing research report: ethylbenzene. In: Chemical economics handbook [Internet]. Menlo Park (CA): SRI Consulting. [cited 2010 Jan]. Available from: http://www.sriconsulting.com/CEH/Public/Reports/645.3000/ [restricted access].
Beyer A, Mackay D, Matthies M, Wania F, Webster E. 2000. Assessing long-range transport potential of persistent organic pollutants. Environ Sci Technol 34:699-703.
Bi X, Sheng G, Feng Y, Fu J, Xie J. 2005. Gas- and particulate-phase specific tracer and toxic organic compounds in environmental tobacco smoke. Chemosphere 61:1512-1522.
Billionnet C, Gay E, Kirchner S, Leynaert B, Annesi-Maesano I. 2011. Quantitative assessments of indoor air pollution and respiratory health in a population-based sample of French dwellings. Environ Res 111:425-434.
Blount BC, McElprang DO, Chambers DM, Waterhouse MG, Squibb KS, LaKind JS. 2010. Methodology for collecting, storing, and analyzing human milk for volatile organic compounds. J Environ Monit. 12:1265-1273.
Bouwer EJ, McCarty PL. 1984. Modeling of trace organics biotransformation in the subsurface. Ground Water 22(4):433-440.
Brooke L. 1987. Report of the flow-through and static acute test comparisons with fathead minnows and acute tests with an amphipod and a cladoceran. Superior (WI): Center for Lake Superior Environmental Studies, University of Wisconsin-Superior [cited in ECOTOX 2006].
Brown SK. 1999. Assessment of pollutant emissions from dry-process photocopiers. Indoor Air 9:259-267.
Brown SK, Cheng M. 2000. Volatile organic compounds (VOCs) in new car interiors. In: Proceedings of 15th International Clean Air and Environment Conference, 26-30 Nov2000. Sydney (AU): Clean Air Society, Australia and New Zealand. p. 464-468.
Calvert JG, Atkinson R, Becker KH, Kamens RM, Seinfeld JH, Wallington TJ, Yarwood G. 2002. The mechanisms of atmospheric oxidation of aromatic hydrocarbons. New York (NY): Oxford University Press. [cited in Jenkin et al. 2003].
Camford Information Services. 2004. CPI product profiles: ethylbenzene (phenylethane). Toronto (ON): Camford Information Services, Inc.
[CAPP] Canadian Association of Petroleum Producers. 2009. Benzene Emissions from Glycol Dehydrators, 2007 Operating Year (Status Report). Calgary (AB). www.capp.ca/getdoc.aspx?DocId=105760&DT=PDF
CAPP, 2006. Control of Benzene emissions from Glycol Dehydrators P.19. Available from: www.capp.ca/getdoc.aspx?DocId=105760&DT=PDF
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33, Canada Gazette. Part III, vol. 22, no. 3. Available from: http://www.gazette.gc.ca/archives/p3/1999/g3-02203.pdf
Canada. 2000. Canadian Environmental Protection Act, 1999: Persistence and Bioaccumulation Regulations,P.C. 2000-348, 23 March 2000, SOR/2000-107, Canada Gazette, Part II, vol. 134, no. 7, p. 607-612. Available from: http://www.gazette.gc.ca/archives/p2/2000/2000-03-29/pdf/g2-13407.pdf
Canada, Dept. of the Environment. 2001. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List (DSL).Canada Gazette, Part I, vol. 135, no. 46, p. 4194-4211. Available from: http://www.gazette.gc.ca/archives/p1/2001/2001-11-17/pdf/g1-13546.pdf
Cappaert NLM, Klis SFL, Muijser H, de Groot JCMJ, Kulig BM, Smoorenburg GF. 1999. The ototoxic effects of ethylbenzene in rats. Hear Res 137(1-2):91-102.
Cappaert NLM, Klis SFL, Baretta AB, Muijser H, Smoorenburg GF. 2000. Ethyl benzene-induced ototoxicity in rats: a dose-dependent mid-frequency hearing loss. J Assoc Res Otolaryngol 1:292-299.
Cappaert NLM, Klis SFL, Muijser H, Kulig BM, Smoorenburg GF. 2001. Simultaneous exposure to ethyl benzene and noise synergistic effects on outer hair cells. Hear Res 162:67-79.
Cappaert NLM, Klis SFL, Muijser H, Kulig BM, Ravensbery LC, Smoorenburg GF. 2002. Differential susceptibility of rats and guinea pigs to the ototoxic effects of ethyl benzene. Neurotoxicol Teratol 24:503-510.
Carrillo-Carrión C, Lucena R, Cárdenas S, Valcárcel. 2007. Liquid-liquid extraxtion/headspace/gas chromatographic/mass spectrometric determination of benzene, toluene, ethylbenzene, (o-, m- and p-)xylene and styrene in olive oil using surfactant-coated carbon nanotubes as extractant. J Chromatogr A 1171:1-7.
Carter JM, Delzer GC, Kingsbury JA, Hopple JA. 2007. Concentration data for anthropogenic organic compounds in ground water, surface water, and finished water of selected community water systems in the United States, 2002-05. Data Series 268. [Internet]. Reston (VA): U.S. Geological Survey. [cited Mar 2011]. Available from: http://pubs.usgs.gov/ds/2007/268/downloads/ds268webV1-4.pdf
Casto BC, Hatch GG. 1977. Progress Report NIH-NCI-N01-CP-45615. p. 1-24.
[CCME] Canadian Council of Ministers of the Environment. 2004. Canadian soil quality guidelines for the protection of environmental and human health: ethylbenzene (2004). In: Canadian environmental quality guidelines. Winnipeg (MB): CCME. [cited 2009 Dec]. Available from: http://ceqg-rcqe.ccme.ca/
[CDC] Centres for Disease Control and Prevention (US). 2009. Fourth national report on human exposure to environmental chemicals [Internet]. Atlanta (GA): CDC, National Center for Health Statistics. [cited 2010 Oct]. Available from: http://www.cdc.gov/exposurereport/pdf/FourthReport.pdf
[CDC] Centres for Disease Control and Prevention (US). 2014. Fourth national report on human exposure to environmental chemicals, updated tables, August, 2014 [Internet]. Atlanta (GA): CDC, National Center for Health Statistics. [cited 2014 Sept]. Available from: http://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Aug2014.pdf
CH2M Hill Engineering Ltd. 1989. CMHC Kitchener townhouse study of soil gas ventilation as a remedial measure for methane entry into basements. Waterloo (ON): CH2M Hill.
Cha YJ, Cadwallader KR. 1995. Volatile components in salt-fermented fish and shrimp pastes. J Food Sci 60(1):19-24.
Chan PC, Hasemani JK, Mahleri J, Aranyi C. 1998. Tumor induction in F344/N rats and B6C3F1 mice following inhalation exposure to ethylbenzene. Toxicol Lett 99(1):23-32.
Chang FK, Chen ML, Cheng SF, Shih TS, Mao IF. 2007. Dermal absorption of solvents as a major source of exposure among shipyard spray painters. J Occup Environ Med 49(4):430-436.
Chang FK, Mao IF, Chen ML, Cheng SF. 2011. Urinary 8-hyrdrodeoxyguanosine as a biomarker of oxidative DNA damage in workers exposed to ethylbenzene. Ann Occup Hyg 55(5):519-525.
Charest-Tardif G, Tardif R, Krishnan K. 2006. Inhalation pharmacokinetics of ethylbenzene in B6C3F1 mice. Toxicol Appl Pharmacol 210:63-69.
Chen CS, Hseu YC, Liang SH, Kuo JY, Chen SC.2008. Assessment of genotoxicity of methyl-tert-butyl ether, benzene, toluene, ethylbenzene and xylene to human lymphocytes using comet assay. J Hazard Mater 153:351-356.
Chen X, Zhang G, Zhang Q, Chen H. 2011. Mass concentrations of BTEX inside air environment of buses in Changsha, China. Building and Environment. 46: 421-427.
Cherry JA. 1987. Groundwater occurrence and contamination in Canada. Can Bull Fish Aquat Sci 215:387-426.
Chien YC. 2007. Variations in amounts and potential sources of volatile organic chemical in new cars. Sci Total Environ 382: 228-239.
Chiesa LM, Soncin S, Panseri S, Cantoni C. 2008. Release of ethylbenzene and styrene from plastic cheese containers. Vet Res Commun 32(Suppl 1):S319-S321.
Chiou CT, Porter PE, Schmedding DW. 1983. Partition equilibria of non-ionic organic compounds between soil organic matter and water. Environ Sci Technol 17(4):227-231.
Chung TY, Hayase F, Kato H. 1983. Volatile components of ripe tomatoes and their juices, purées and pastes. Agric Biol Chem 47(2):343-351.
[CIMT] Canadian International Merchandise Trade [database on the Internet]. 2010. Ottawa (ON): Statistics Canada. [cited 2010 Jan]. Available from: http://www.statcan.gc.ca/trade/scripts/trade_search.cgi
[CIMT] Canadian International Merchandise Trade [database on the Internet]. 2014. Ottawa (ON): Statistics Canada. [cited 2014 Aug]. Available from: http://www5.statcan.gc.ca/cimt-cicm/home-accueil?lang=eng
Clay P. 2001. Ethylbenzene: in vivo mouse liver unscheduled DNA synthesis assay. Central Toxicology Laboratory Report. CTL/SM0998/REG/REPT. Sponsored by the Styrenics Steering Committee, CEFIC, Brussels, Belgium. [cited in VCCEP 2007].
Climie IJG, Hutson DH, Stoydin G. 1983. The metabolism of ethylbenzene hydroperoxide in the rat. Xenobiotica 13:611-618.
Cometto-Muñiz JE, Cain WS. 1995. Relative sensitivity of the ocular trigeminal, nasal trigeminal and olfactory systems to airborne chemicals. Chem Senses 20:191-198.
CONCAWE. 1997. Exposure profile: gasoline [Internet]. Brussels (BE): CONCAWE. [cited 2011 Mar]. Available from: http://www.concawe.be/DocShareNoFrame/docs/1/PNPLNMMBEDPMNCIEDKCMHPJEVEVC7391P3PDB69DWGTD/CEnet/docs/DLS/Rpt_97-52-2003-01970-01-E.pdf
Conestoga Rovers & Associates. 2009. ,Baseline information on major municipal solid waste landfill in Canada. Prepared for Environment Canada.
[ConsExpo] Consumer Exposure Model [Internet]. 2006. Version 4.1. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu (National Institute for Public Health and the Environment). Available from: http://www.rivm.nl/en/healthanddisease/productsafety/ConsExpo.jsp#tcm:13-42840
Cossec B, Cosnier F, Burgart M, Nunge H, Grossmann S. 2010. Glutathione pathway in ethylbenzene metabolism: novel biomarkers of exposure in the rat. Chemosphere 81:1334-1341.
Coty RR, Welch VA, Ram S, Singh J. 1987. Ethylbenzene. In: Ullmann's encyclopaedia of industrial chemistry. Vol. A 10. 5th ed. Weinheim (DE): Verlag Chemie. p. 36-43.
Cragg ST, Clarke EA, Daly IW, Miller RR, Terrill JB, Ouellette RE. 1989. Subchronic inhalation toxicity of ethylbenzene in mice, rats, and rabbits. Fundam Appl Toxicol 13(3):399-408.
Cruzan G, Bus J, Banton M, Gingell R, Carlson G. 2009. Mouse specific lung tumors from CYP2F2-mediated cytotoxic metabolism: an endpoint/toxic response where data from multiple chemicals converge to support a mode of action. Regul Toxicol Pharmacol 55(2)205-18.
[CTUMS] Canadian Tobacco Use Monitoring Survey. 2008. Supplementary tables, CTMUS Annual 2008 [Internet]. [cited 2011 Jan]. Available from: http://www.hc-sc.gc.ca/hc-ps/tobac-tabac/research-recherche/stat/_ctums-esutc_2008/ann-table1-eng.php
Daisey JM, Mahanama KRR, Hodgson AT. 1994. Toxic volatile organic compounds in environmental tobacco smoke: emission factors for modeling exposures of California populations. Final Report. Sacramento (CA): California Air Resources Board, Research Division. Report No. A133-186.
[Danish EPA] Danish Environmental Protection Agency. 2003. Mapping and exposure of chemical substances in Christmas decorations. Survey of Chemical Substances in Consumer Products, No. 37. Copenhagen (DK): Danish Environmental Protection Agency (performed by Eurofins Intecon Consultancy A/S).
Daubert TE, Danner RP. 1985. Data compilation tables of properties of pure compounds. New York (NY): American Institute of Chemical Engineers. p. 450.
Dean BJ, Brooks TM, Hodson-Walker G, Hutson DH. 1985. Genetic toxicology testing of 41 industrial chemicals. Mutat Res 153:57-77. [cited in IPCS 1996].
De Celis R, Feria-Velasco A, Gonzalez-Unzaga M, Torres-Calleja J, Pedron-Nuevo N. 2000. Semen quality of workers occupationally exposed to hydrocarbons. Fertil Steril 73:221-228. [cited in VCCEP 2007].
Dennison JE, Andersen ME, Yang RSH. 2003. Characterization of the pharmacokinetics of gasoline using PBPK modeling with a complex mixtures chemical lumping approach. Inhal Toxicol 15:961-986. [cited in ATSDR 2010].
Desideri P, Lepri G, Checcini L, Santianni D. 1994. Organic compounds in surface and deep Antarctic snow. Int J Environ Anal Chem 55:33-46.
Diamond Vogel. 2010. Material safety data sheet: high solids miracle glaze urethane [Internet]. Minneapolis (MN): Diamond Vogel Paint. [updated 18 May 2010; cited 2010 Oct]. Available from: http://www.diamondvogel.com/prod_msds/LG-0414.pdf
Dirinck P, Schreyen L, Schamp N. 1977. Aroma quality evaluation of tomatoes, apples, and strawberries. J Agric Food Chem 25(4):759-763.
DiToro DM, Zarba CS, Hansen DJ, Berry WJ, Swartz RC, Cowan CE, Pavlou SP, Allen HE, Thomas NA, Paquin PR. 1991. Technical basis for establishing sediment quality criteria for nonionic organic chemicals using equilibrium partitioning. Environ Toxicol Chem 10:1541-1583.
Dodson RE, Levy JI, Spengler JD, Shine JP, Bennett DH. 2008. Influence of basements, garages, and common hallways on indoor residential volatile organic compound concentrations. Atmos Environ 42:1569-1581.
Donner M, Maki-Paakkanen J, Norppa H, Sorsa M, Vainio H. 1980. Genetic toxicology of xylenes. Mutat Res 74:171-172. [cited in IPCS 1996].
[Dow] The Dow Chemical Company. 2009. Product safety assessment: Dow™ ethylbenzene [Internet]. Form No. 233-00283-MM-0409. [cited 2010 Jan]. Available from: http://www.dow.com/PublishedLiterature/dh_0272/0901b80380272cb0.pdf?filepath=productsafety/pdfs/noreg/233-00283.pdf&fromPage=GetDoc
Durst GL, Laperle EA. 1990. Styrene monomer migration as monitored by purge and trap gas chromatography and sensory analysis for polystyrene containers. J Food Sci 55:522-524. [cited in Tang et al. 2000].
Dynamac Corporation. 1986. Information review ethylbenzene. Rockville, Maryland (IR-478).
[ECETOC] European Chemical Industry Ecology and Toxicology Centre. 1986. Joint assessment of commodity chemicals no. 7 ethylbenzene CAS: 100-41-4. Brussels (BE): European Chemical Industry Ecology and Toxicology Centre. 61 pp.
[ECHA] European Chemicals Agency. 2008. Guidance on information requirements and chemical safety assessment Part D: Exposure scenario building May 2008 (version 1.1) Guidance on the implementation of REACH [Internet]. [cited July 2011]. Available from: http://guidance.echa.europa.eu/docs/guidance_document/information_requirements_part_d_en.pdf
[ECOTOX] ECOTOXicology database [database on the Internet]. 2006. Version 4. Washington (DC): US Environmental Protection Agency, Office ofResearch and Development, National Health and Environmental Effects Research Laboratory, Mid-Continent Ecology Division. Available from: http://cfpub.epa.gov/ecotox
Eggert T, Bødker J, Hansen OC. 2002. Chemical ingredients in candles sold in Danish retail shops. Survey of Chemical Substances in Consumer Products, No. 6 [Internet]. Copenhagen (DK): Danish Environmental Protection Agency. [updated 2010 Apr 14; cited 2010 Jan]. Available from: http://www.mst.dk/NR/rdonlyres/4DBF68B7-77DD-4D13-8911-B42581EBACCC/0/lys_uk.pdf
Eggert T, Hansen OC. 2004. Survey and emissions of chemical substances from incense. Survey of Chemical Substances in Consumer Products, No. 39 [Internet]. Copenhagen (DK): Danish Environmental Protection Agency. [updated 2010 Apr 14; cited 2010 Jan]. Available from: http://www.mst.dk/NR/rdonlyres/1DBB5B30-34F9-4F9D-BD0E-D8EA4A88118D/0/39.pdf
Egmose K, Pors J. 2005. Survey of chemical substances in textile colorants. Survey of Chemical Substances in Consumer Products, No. 58 [Internet]. Copenhagen (DK): Danish Environmental Protection Agency. [updated 2010 Apr 14; cited 2010 Jan]. Available from: http://www2.mst.dk/Udgiv/publications/2005/87-7614-677-4/pdf/87-7614-678-2.pdf
EHD (Environmental Health Directorate). 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada.Draft internal report. Ottawa (ON): Bureau of Chemical Hazards, Health Canada.
Ehret-Henry J, Ducruet V, Luciani A, Feigenbaum A. 1994. Styrene and ethylbenzene migration from polystyrene into dairy products by dynamic purge-and-trap gas chromatography. J Food Sci 59(5):990-992
El Masry AM, Smith JN, Williams RT. 1956. The metabolism of alkylbenzenes: n-Propylbenzene and n-butylbenzene with further observations on ethylbenzene. Biochem J 64:50-56.
Elovaara E, Engstrom K, Nickels J, Aitio A, Vainio H. 1985. Biochemical and morphological effects of long-term inhalation exposure of rats to ethylbenzene. Xenobiotica 15:299-308. [cited in IPCS 1996].
Engelhardt G. 2006. In vivo micronucleus test in mice with 1-phenylethanol. Arch Toxicol 80:868-872.
[ENS] Environment News Service. 2011. Toxic Diesel Fuel Used Without Permits in Fracking Operations. Sept 26, 2011. Available from: http://www.ens-newswire.com/ens/feb2011/2011-02-04-092.html
Environment Canada. 1988. Atlantic Region Federal-Provincial toxic chemical survey of municipal drinking water sources 1985-1988.
Environment Canada. 1989. Atlantic Region Federal-Provincial toxic chemical survey of municipal drinking water sources. Data summary report, Province of New Brunswick, 1985-1988. Inland Waters Directorate, Water Quality Branch. IWD-AR-WQB-89-155.
Environment Canada. 2001. Data for selected substances collected under the Canadian Environmental Protection Act, 1999. Section 71: Notice with Respect to Certain Substances on the Domestic Substances List (DSL). Data prepared by: Environment Canada, Existing Substances Program.
Environment Canada. 2004. Benzene, ethyl- (ethyl benzene) (CAS RN 100-41-4). Report of Section 71 (CEPA, 1999) Notice with Respect to Certain Substances on the Domestic Substances List (DSL). Existing Substances Branch, Environment Canada. November 2004 (Version 2.0).
Environment Canada 2009. The National Air Pollution Surveillence program: Forty years of air quality monitoring and assessment [Internet]. Gatineau (QC): Environment Canada [cited 2011 Jun 10]. Available from: http://www.ec.gc.ca/Publications/default.asp?lang=En&xml=BABE2D5A-D182-4B9C-85E0-940F5C85187C
Environment Canada. 2011a. NAPS Annual raw data [Internet]. Gatineau (QC): Environment Canada, Environmental Technology Centre [cited 2011 Jun]. Available from: http://www.etc-cte.ec.gc.ca/NapsAnnualRawData/Default.aspx?ReturnUrl=%2fNapsAnnualRawData%2fMain.aspx (protected)
Environment Canada. 2011b. Groundwater [Internet]. Gatineau (QC): Environment Canada. [created 2007 Jan 9; last updated 2011 Feb 15; cited 2011 Mar 14]. Available from: http://www.ec.gc.ca/eau-water/default.asp?lang=En&n=300688DC-1&printfullpage=true&nodash=1
Environment Canada. 2013. National pollutant release inventory [database on the Internet]. Gatineau (QC): Environment Canada. [cited 2013 May 6]. Available from: http://www.ec.gc.ca/inrp-npri/default.asp?lang=en&n=BF14CADF-1
Environment Canada. 2014. NAPS Annual raw data [Internet]. Gatineau (QC): Environment Canada, Environmental Technology Centre [cited 2014 Aug]. Available from: http://maps-cartes.ec.gc.ca/rnspa-naps/data.aspx?lang=en
Enviro-Test Laboratories. 1991. Cayley background study: analysis of food products for target organic and inorganic parameters. Series: 91-E1208. Prepared under contract to Hazardous Waste Section, Environmental Health Directorate, Health and Welfare Canada, Ottawa, Ontario.
Enviro-Test Laboratories. 1992. Windsor area background study: analysis of food products for target organic and inorganic parameters. Prepared under contract to Hazardous Waste Section, Environmental Health Directorate, Health and Welfare Canada, Ottawa, Ontario.
Enviro-Test Laboratories. 1993. Ville-Mercier background study: analysis of food products for target organic and inorganic parameters. Prepared under contract to Hazardous Waste Section, Environmental Health Directorate, Health and Welfare Canada, Ottawa, Ontario.
[EPA] Ethylbenzene Producers Association. 1986a. A four day inhalation study of ethylbenzene in the rat, mouse, and rabbit. Ethylbenzene Producers Association. Submitted to the U.S. Environmental Protection Agency under TSCA Section 8D. OTS0513170.
[EPA] Ethylbenzene Producers Association. 1986b. A four week inhalation study of ethylbenzene in the rat, mouse, and rabbit. Ethylbenzene Producers Association. Submitted to the U.S. Environmental Protection Agency under TSCA Section 8D. OTS0513173.
[EQC] Equilibrium Criterion Model. 2003. Version 2.02. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre. Available from: http://www.trentu.ca/academic/aminss/envmodel/models/EQC2.html
ESG International, Inc. 2002. Quantification of the exposure concentrations and toxicity of BTEX compounds in soil. Report No. G1603. June 2002. Report prepared for the Soil Quality Guidelines Task Group, Canadian Council of Ministers of the Environment. Guelph (ON): ESG International, Inc. [cited in CME 2004].
Esteve-Turillas FA, Pastor A, de la Guardia M. 2007. Assessing air quality inside vehicles and at filling stations by monitoring benzene, toluene, ethylbenzene and xylenes with the use of semipermeable devices. Anal Chim Acta 593:108-116.
[EURAR] European Union Risk Assessment Report: CAS: 100-41-4: Ethylbenzene: environmental part only [Internet]. 2007. Luxembourg (LU): Office for Official Publications of the European Communities. Report No.: R057_0704_ENV.DOC. [cited 2010 Jan]. 106 pp. (On the cover, European Commission Joint Research Centre). Available from: http://esis.jrc.ec.europa.eu/doc/existing-chemicals/risk_assessment/REPORT/ethylbenzenereport057.pdf
Faber WD, Roberts LSG, Stump DG, Tardif R, Krishnan K, Tort M, Dimond S, Dutton D, Moran E, Lawrence W. 2006. Two generation reproduction study of ethylbenzene by inhalation in Crl-CD rats. Birth Defects Res B Dev Reprod Toxicol 77(1):10-21.
Faber WD, Roberts LSG, Stump DG, Beck M, Kirkpatrick D, Regan KS, Tort M, Moran E, Banton M. 2007. Inhalation developmental neurotoxicity study of ethylbenzene in Crl-CD rats. Birth Defects Res B Dev Reprod Toxicol 80:34-48.
[FAP] Fort Air Partnership. 2010. Fort Air Partnership ambient air monitoring network 2009. Annual technical report: network and data summary. Fort Saskatchewan (AB): Fort Air Partnership.
Feilberg A, Tønning K, Jacobsen E, Hemmersam AG, Søborg I, Cohr KH. 2008. Survey and health assessment of possible health hazardous compounds in proofing sprays. Survey of Chemical Substances in Consumer Products, No. 98 [Internet]. Copenhagen (DK): Danish Environmental Protection Agency. [updated 2010 Apr 14; cited 2010 Jan]. Available from: http://www2.mst.dk/udgiv/publications/2008/978-87-7052-837-5/pdf/978-87-7052-838-2.pdf
Fellin P, Barnett SE, Tran QA. 1992. Results of a national pilot survey of airborne volatile organic compounds in Canadian residences. Unpublished report. Downsview (ON): Concord Environmental. Prepared for Health and Welfare Canada.
Fishbein L. 1985. An overview of environmental and toxicological aspects of hydrocarbons. IV. Ethylbenzene. Sci Total Environ 44:269-287.
[FLL] Fuels and Lubricants Laboratory. 2008. Gasoline and diesel compositional analysis. Edmonton (AB): Fuels and Lubricants Laboratory, Alberta Research Council. Prepared for Environment Canada, Edmonton.
FracFocus. 2013. Chemical Disclosure Registry. Available at: http://fracfocus.ca/
Frantik E, Hornychova M, Horvath M.1994. Relative acute neurotoxicity of solvents: isoeffective air concentrations of 48 compounds evaluated in rats and mice. Environ Res 66:173-185.
Gagnaire F, Langlais C. 2005. Relative ototoxicity of 21 aromatic solvents. Arch Toxicol 79(6):346-354.
Gagnaire F, Langlais C, Grossmann S, Grossmann S, Wild P. 2007. Ototoxicity in rats exposed to ethylbenzene and to two technical xylene vapours for 13 weeks. Arch Toxicol 81(2):127-143.
Galassi S, Mingazzini M, Vigano L, Cesareo D, Tosato ML. 1988. Approaches to modeling toxic responses of aquatic organisms to aromatic hydrocarbons. Ecotoxicol Environ Saf 16(2):158-169.
Gerarde HW. 1960. In: Browning E, editor. Toxicology and biochemistry of aromatic hydrocarbons. Elsevier monographs on toxic agents. New York (NY): Elsevier. [cited in VCCEP 2007].
Gerarde HW. 1963. The aromatic hydrocarbons. Chapter XXX. In: Patty FA, editor. Industrial hygiene and toxicology. Vol II. 2nd rev. ed. New York (NY): Interscience Publishers. P12191240.
German Chemical Society. 1997. Ethylbenzene. BUA Report 178. Weinheim (DE): GDCh-Advisory Committee on Existing Chemicals of Environmental Relevance (BUA). S. Hirzel Wissenschaftliche Verlagsgesellschaft.
Gibson DP, Brauninger R, Shaffu HS, Kerckaert GA, LeBoeuf RA, Isfort RJ, Aardema MJ. 1997. Induction of micronuclei in Syrian hamster embryo cell: comparison to results in SHE cell transformation assay for National Toxicology Program test chemicals. Mutat Res 392(1-2):61-70.
Glensvig D, Ports J. 2006. Mapping of perfume in toys and children's articles. Survey of Chemical Substances in Consumer Products, No. 68 [Internet]. Copenhagen (DK): Danish Environmental Protection Agency [updated 2010 Apr 14; cited 2010 Jan]. Available from: http://www2.mst.dk/udgiv/Publications/2006/87-7052-018-6/pdf/87-7052-019-4.pdf
Goss MJ, Barry DAJ, Rudolph DL. 1998. Contamination in Ontario farmstead domestic wells and its association with agriculture: 1. Results from drinking water wells. J Contam Hydrol 32(3-4):267-293.
Gòrna-Binkul A, Keymeulen R, Van Langenhove H, Buszewski B. 1996. Determination of monocyclic aromatic hydrocarbons in fruit and vegetables by gas chromatography-mass spectrometry. J Chromatogr A 734:297-302.
Government of British Columbia. 1988. Environmental Management Act. Hazardous Waste Regulation.Division 6 - Secure landfills. Section 25. Siting requirements. [accessed 2005 Jun 15]. Available from: http://www.qp.gov.bc.ca/statreg/reg/e/envmgmt/envmgmt63%5F88/63_88part1-4.htm#part4_division6
Government of Japan. 2004. Surveyed chemical substances and their detected levels in the environment (a cumulative list for fiscal year 1974 - 2003) [Internet]. Tokyo (JP): Environmental Health and Safety Division, Ministry of Environment. [cited 2010 Feb]. Available from: http://www.env.go.jp/chemi/kurohon/en/http2004e/03-cie/summary2004.pdf
Grabbs JS, Corsi RL, Torres VM. 1999. A screening assessment of volatile organic compounds in the interior of new automobiles. Presented at: First NSF International Conference on Indoor Air Health, 1999 May 3-5, Denver, Colorado. p. 119-128.
Graham LA, Noseworthy L, Fugler D, O'Leary K, Karman D, Grande C. 2004. Contribution of vehicle emissions from an attached garage to residential indoor air pollution levels. Air & Waste Manage Assoc 54:563-584.
Gramshaw JW, Vandenburg HJ. 1995. Compositional analysis of samples of thermoset polyester and migration of ethylbenzene and styrene from thermoset polyester into pork during cooking. Food Addit Contam 12(2):223-234.
Great Lakes Commission. 2004. 2001 Inventory of toxic air emissions: point, area, and mobile sources. Report submitted to the U.S. Environmental Protection Agency. Ann Arbor (MI): Great Lakes Commission.
Great Lakes Commission. 2006. 2002 Inventory of toxic air emissions for the Great Lakes Region [Internet]. Report submitted to the U.S. Environmental Protection Agency. Ann Arbor (MI): Great Lakes Commission. [cited 2011 Apr]. Available from: http://www.glc.org/air/inventory/2002/2002report_Full.pdf
Haddad S, Be´liveau M, Tardif R, Krishnan K. 2001. A PBPK modeling-based approach to account for interactions in the health risk assessment of chemical mixtures. Toxicol Sci 63:125-131.
Hannah SA, Austern BM, Eralp AE, Wise RH. 1986. Comparative removal of toxic pollutants by six wastewater treatment processes. J Water Pollut Control Fed 58:27-34.
Hansen J, Hansen OC, Pommer K. 2004. Release of chemical substances from tents and tunnels for children. Mapping of Chemical Substances in Consumer Products, No. 46 [Internet]. Copenhagen (DK): Danish Environmental Protection Agency [updated 2010 Apr 14; cited 2010 Jan]. Available from: http://www.mst.dk/NR/rdonlyres/26E770C9-2FFB-4059-BD1B-139D6D373C85/0/46.pdf
Hansen OC, Eggert T. 2003. Survey, emission and evaluation of volatile organic chemicals in printed matter. Survey of Chemical Compounds in Consumer Products, No. 36 [Internet]. Copenhagen (DK): Danish Environmental Protection Agency. [updated 2010 Apr 14; cited 2010 Jan]. Available from: http://www.mst.dk/NR/rdonlyres/C2C6FC89-9584-4757-A286-B6D6DFCB571A/0/36.pdf
Hansen PL, Tønning K, Malmgren-Hansen B, Jacobsen E. 2008. Survey and health assessment of chemical substances in hobby products for children. Survey of Chemical Substances in Consumer Products, No. 93 [Internet]. Copenhagen (DK): Danish Environmental Protection Agency. [updated 2010 Apr 14; cited 2010 Jan]. Available from: http://www2.mst.dk/udgiv/publications/2008/978-87-7052-763-7/pdf/978-87-7052-764-4.pdf
Hard GC. 2002. Significance of the renal effects of ethyl benzene in rodents for assessing human carcinogenic risk. Toxicol Sci 69:30-41.
Hard GC, Seely JC. 2005. Recommendations for the interpretation of renal tubule proliferative lesions occurring in rat kidneys with advanced chronic progressive nephropathy (CPN). Toxicol Pathol 33:641-649.
Hard GC, Johnson KJ, Cohen SM. 2009. A comparison of rat chronic progressive nephropathy with human renal disease - implications for human risk assessment. Crit Rev Toxicol 39(4):332-346.
Hard GC, Betz LJ, Seely JC. 2012. Association of advanced chronic nephropathy (CPN) with renal tubule tumors and precursor hyperplasia in control F344 rats from two-year carcinogenicity studies. Toxicol Pathol. DOI:10.1177/0192623311431948. Available at: http://tpx.sagepub.com/content/early/2012/01/30/0192623311431948
Hard GC, Banton MI, Bretzlaff RS, Dekant W, Fowles JR, Mallett AK, McGregor DB, Roberts KM, Sielken RL Jr, Valdez-Flores C, Cohen SM. 2013. Consideration of rat chronic progressive nephropathy in regulatory evaluations for carcinogenicity. Toxicol Sci 132(2):268-75.
Hardin BD, Bond GP, Sikov MR, Andrew FD, Beliles RP, Niemeier RW. 1981. Testing of selected workplace chemicals for teratogenic potential. Scand J Work Environ Health 7(Suppl 4):66-75. [cited in IPCS 1996].
Harton EE, Rawl RR. 1976. Toxicological and skin corrosion testing of selected hazardous chemicals. Prepared for the Office of Hazardous Materials Operation, U.S. Department of Transportation, Washington, D.C. (NTIS Report No. PB264-975). [cited in Dynamac Corporation 1986].
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate
Health Canada. 2004. State of the science report for a screening health assessment. Supporting working document. Ethylbenzene. August 2004. Ottawa (ON): Health Canada, Existing Substances Division.
Health Canada. 2006. State of the science report for ethylbenzene. Ottawa (ON): Health Canada. 19 pp.
Health Canada. 2010a. Windsor exposure assessment study (2005-2006): Data summary for volatile organic compound sampling. Ottawa (ON): Health Canada. 85 pp.
Health Canada. 2010b. Regina indoor air quality study (2007): Data summary for volatile organic compound sampling. Ottawa (ON): Health Canada. 164 pp.
Health Canada. 2011a. The cosmetic ingredient hotlist-March 2011 [Internet]. Ottawa (ON): Health Canada, Consumer Product Safety. [cited 2011 Dec]. Available from: www.hc-sc.gc.ca/cps-spc/cosmet-person/indust/hotlist-critique/index-eng.php
Health Canada. 2012. Halifax indoor air quality study 2009: VOC sampling data summary (Draft). Ottawa (ON): Health Canada. 37 pp.
Health Canada. 2013. Edmonton indoor air quality study 2020: Volatile Organic Compounds (VOC) data summary. Ottawa (ON): Health Canada. 50 pp.
Health Canada. 2014a. Guidelines for Canadian Drinking Water Quality: Guideline Technical Document - Toluene, Ethylbenzene and Xylenes. Ottawa (ON): Health Canada. 87 pp.
Health Canada. 2014b. Determination of Ethylbenzene in Consumer Chemicals: Survey 2014-15. Ottawa (ON): Product Safety Laboratory, Health Canada.
Hempel G, Rüdt U. 1988. Bestimmung von monomeren flüchtigen Anteilen in Polystyrol und Styrol-Misch- und Pfropfpolymerisaten. Dtsch Levensm Rdsch 84:239-242. [cited in Tang et al. 2000].
Henderson L, Brusick D, Ratpan F, Veenstra G. 2007. A review of the genotoxicity of ethylbenzene. Mut Res 635: 81-89.
Henkel. 2008. Material safety data sheet: Quad advanced formula sealant [Internet]. Mentor (OH): Henkel Corporation [cited 2010 Oct]. Available from: http://www.osipro.com/msds/quad_clear_msds.pdf
Henkel. 2009. Technical data sheet: QUAD® advanced formula sealant [Internet]. Avon (OH): Henkel Corporation [cited 2010 Oct]. Available from: http://www.osipro.com/tds/quad-tds.pdf
[HENRYWIN] Henry's Law Constant Program for Microsoft Windows [Estimation Model]. 2008. Version 3.20. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Héroux MÈ, Gauvin D, Gilbert NL, Guay M, Dupuis G, Legris M, Lévesque B. 2008. Housing characteristics and indoor concentrations of selected volatile organic compounds (VOCs) in Quebec City, Canada. Indoor Built Environ 17(2):128-137.
Ho DX, Kim KH, Sohn JR, Oh YH, Ahn JW. 2011. Emission rates of volatile organic compounds released from newly produced household furniture products using a large-scale chamber testing method. The Scientific World Journal. 11: 1597-1622.
Howard PH. 1989. Handbook of environmental fate and exposure data for organic chemicals. Vol. 1. Large production and priority pollutants. Chelsea (MI): Lewis Publishers. p. 325-334.
Howard PH. 1991. Handbook of environmental degradation rates. Chelsea (MI): Lewis Publishers. p. 340-341.
Holz O, Scherer G, Brodtmeier S, Koops F, Wamcke K, Krause T, Austen A, Angerer J, Tricker AR, AdIkofer F, Rudiger HW. 1995. Determination of low level exposure to volatile aromatic hydrocarbons and genotoxic effects in workers at a styrene plant. Occup Environ Med 52:420-428.
Home Hardware. 2013. Material Safety Data Sheet: Beauti-tone Enamel Forest Green Aerosol [Internet]. Burford (ON): Home Hardware Stores Limited. [cited 2013 Feb]. Available from: http://msds.homehardware.ca/OpenRepositoryFile.asp?s=139441&v=4
[HPD] Household Products Database [database on the Internet]. 2011. Ethylbenzene (CAS No. 100-41-4). Bethesda (MD): National Library of Medicine (US). [revised 2011 Oct; cited 2012 Dec 6]. Available from: http://householdproducts.nlm.nih.gov/cgi-bin/household/brands?tbl=chem&id=271&query=100-41-4&searchas=TblChemicals
[HSDB] Hazardous Substances Data Bank [database on the Internet]. 1983 - . Ethylbenzene CAS No. 100-41-4. Bethesda (MD): National Library of Medicine (US). [revised 2005 Jul 7; cited 2009 Dec]. Available from: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB
Huff J, Chan P, Melnick R. 2010. Clarifying carcinogenicity of ethylbenzene. Regul Toxicol Pharmacol 58:167-169.
Hulin M, Caillaud D, Annesi-Maesano I. 2010. Indoor air pollution and childhood asthma: variations between urban and rural areas. Indoor Air 20:502-514.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 2000. Some industrial chemicals. Monographs on ethylbenzene. IARC Monogr Eval Carcinog Risks Hum 77:227-241.
ICI Paints. 2010. Material Safety Data Sheet: 200 Xpert Jammer Prim [Internet].Concord (ON): ICI Paints (Canada) and Akzo Nobel Paints LLC. [cited 2013 Feb]. Available from: http://www.duspec.com/DuSpec2/document/DocumentDisplayController.htm?documentId=11153
[IPCS] International Programme on Chemical Safety. 1996. Ethylbenzene. Geneva (CH): World Health Organization. (Environmental Health Criteria 186). Jointly sponsered by the United Nations Environment Programme, the International Labour Organization, and the World Health Organization.
Ivanov SV. 1962. Toxicity of ethylbenzene. Tr Voronezh Gos Med Inst 47: 80. [cited via International Uniform Chemical Information Database (IUCLID) in Dynamac Corporation 1986].
Jackson RE, Patterson RJ, Graham BW, Bahr J, Belanger D, Lockwood J, Priddle M. 1985. Contaminant hydrogeology of toxic organic chemicals at a disposal site, Gloucester, Ontario. 1. Chemical concepts and site assessment. NHRI Paper No. 23. IWD Scientific Series No. 141. Ottawa (ON): Environment Canada, Inland Waters Directorate, National Hydrology Research Institute.
Jenkin ME, Saunders SM, Wagner V, Pilling MJ. 2003. Protocol for the development of the master chemical mechanism, MCM v3 (Part B): tropospheric degradation of non-aromatic volatile organic compounds. Atmos Chem Phys 3:1861-1893.
Jo WK, Lee JH, Kim MK. 2008. Head-space, small-chamber and in-vehicle tests for volatile organic compounds (VOCs) emitted from air freshners for the Korean market. Chemosphere 70:1827-1834.
Kamlet MJ, Doherty RM, Abraham MH, Marcus Y, Taft RW. 1988. Linear solvation energy relationship. 46. An improved equation for correlation and prediction of octanol/water partition coefficients of organic nonelectrolytes (including strong hydrogen bond donor solutes). J Phys Chem 92(18):5244-5255.
Kappeler T, Wuhrmann K. 1978. Microbial degradation of the water-soluble fraction of gas oil - 1. Water Res 12:327-333.
Karmen D, Graham L. 2002. Measurement and modelling of motor vehicle related air toxics along urban streets. Health Canada Toxic Substances Research Initiative Project #55, Final Report May 2001, Revised November 2002. Ottawa (ON): Carleton University and Environment Canada. 38 pp.
Kennedy SM, Copes R, Henderson S, Na S, MacKay C. 2002. Air emissions from the Chevron North Burnaby Refinery, human health impact assessment. Final Report [Internet]. Vancouver (BC): UBC School of Occupational and Environmental Hygiene. [cited 2010 Oct]. Available from: http://www.cher.ubc.ca/PDFs/burnaby2.pdf
Kerckaert GA, Brauninger R, LeBoeuf RA, Isfort RJ. 1996. Use of the Syrian hamster embryo cell transformation assay for carcinogenicity prediction of chemicals currently being tested by the National Toxicology Program in rodent bioassays. Environ Health Perspect 104(Suppl 5):1075-1084.
Kim YM, Harrad S, Harrison RM. 2001. Concentrations and sources of VOCs in urban domestic and public microenvironments. Environ Sci Technol 35:997-1004.
Kligman AM. 1974. Report to RIFM (1974). Cited in Opdyke D LJ. 1975. Ethylbenzene. Food Cosmet Toxicol 13:803-804. [cited in ECETOC 1986].
[KOCWIN] Organic Carbon-Water Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2008. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Komex International Ltd. 2002. Derivation of revised benzene, toluene, ethylbenzene, and xylenes soil guidelines. Prepared by Komex International Ltd. for the Soil Quality Guidelines Task Group of the Canadian Council of Ministers of the Environment. Calgary (AB): Komex International Ltd. [cited in CCME 2004].
Korzite. 2012. Material Safety Data Sheet: SR/CCCSC [Internet]. Guelph (ON): Korzite Coatings Inc. [cited 2013 June]. Available from: http://www.stonesaver.ca/documents/pdf/msds_concrete_sealer1.pdf
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2008. Version 1.67. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Leddy MB, Phipps DW, Ridgway HF. 1995. Catabolite-mediated mutations in alternate toluene degradative pathways in Pseudomonas putida.J Bacteriol 177(16):4713-4720.
Lee SC, Lam S, Fai HK. 2001. Characterization of VOCs, ozone, and PM10 emissions from office equipment in an environmental chamber. Building Environ 36:837-842.
Lesage S, Jackson RE, Priddle MW, Riemann PR. 1990a. Occurrence and fate of organic solvent residues in anoxic groundwater at the Gloucester landfill, Canada. Environ Sci Technol 24:559-566.
Lesage S, Ritch JK, Treciokas EJ. 1990b. Characterisation of groundwater contaminants at Elmira, Ontario, by thermal desorption, solvent extraction GC-MS and HPLC. Water Pollut Res J Can 25(3):275-292.
Lesage S, Jackson RE, Priddle M, Beck P, Raven KG. 1991. Investigation of possible contamination of shallow ground water by deeply injected liquid industrial wastes. Ground Water Monit Rev 11:151-159.
Lesage S, McBride RA, Cureton PM, Brown S. 1993. Fate of organic solvents in landfill leachates under simulated field conditions and in anaerobic microcosms. Waste Manag Res 11:215-226.
Lesage S, Xu H, Novakowski KS. 1997. Distinguishing natural hydrocarbons from anthropogenic contamination in ground water. Ground Water 35(1):149-160.
Levoic KW, Sheldon LS, Whitaker DA, Hetes RG, Cacagni JA, Baskir JN. 1996. Measurement of indoor air emissions from dry-process photocopy machines. J Air Waste Manag Assoc 46:821-829.
Levoic K, Whitaker D, Northeim C, Sheldon L. 1998. Evaluation of a test method for measuring indoor air emissions from dry-process photocopiers. J Air Waste Manag Assoc 48:915-923.
Lewis RJ Sr, editor. 1992. Sax's dangerous properties of industrial materials. 8th ed. New York (NY): Van Nostrand Reinhold. p. 1579. [cited in NTP 1999].
Li AA, Maurissen JPJ, Barnett JF Jr, Foss J, Freshwater L, Garman RH, Peachee VL, Hong SJ, Stump DG, Bus JS. 2010. Oral gavage subchronic neurotoxicity and inhalation subchronic immunotoxicity studies of ethylbenzene in the rat. Neurotoxicology 31:247-258.
Lide DR, Haynes WM, editors. 2010. CRC handbook of chemistry and physics. 90th ed. [Internet version 2010]. Physical constants of organic compounds [Internet]. Section 3: 238. [cited 2009 Dec]. Available from: http://www.hbcpnetbase.com/
Lim SK, Lee BM, Han EY, Bae JY, Ahn IY, Kwon MJ, Kim SM, Cho MC. 2011. Analysis of benzene, toluene, ethylbenzene, and xylene (BTEX) by GC/MS in consumer products in Korea. Abstracts/Toxicology Letters. 205S: S180-S300.
Lock EA and Hard GC. 2004. Chemically induce renal tubule tumors in the laboratory rat and mouse: review of the NCI/NTP database and categorization of renal carcinogens based on mechanistic information. Crit Rev Toxicol 34(3):211-299.
Lockhart WL, Metner DA, Murray DAJ, Danell RW, Billeck BN, Baron CL, Muir DCG, Chang-Kue K.1989. Studies to determine whether the condition of fish from the lower Mackenzie River is related to hydrocarbon exposure. Environmental Studies 61. Ottawa (ON): Department of Indian Affairs and Northern Development.
Lockhart WL, Wagemann R, Tracey B, Sutherland D, Thomas DJ. 1992. Presence and implications of chemical contaminants in the freshwater of the Canadian Arctic. Sci Total Environ 122(1/2):165-243.
López P, Batlle R, Salafranca J, Nerín C. 2008. Efficiency of whole and skimmed powdered milk for trapping volatile compounds released from plastic containers in high-temperature applications. J Food Prot 71(9):1889-1897.
Lovegren NV, Fisher GS, Legendre MG, Schuller WH. 1979. Volatile constituents of dried legumes. J Agric Food Chem 27(4):851-853.
Lu BQ, Zhen ZH. 1989. Health standards for ethylbenzene in the air of workplaces. Beijing (CN): Chinese Academy of Preventive Medicine, Institute of Occupational Medicine (in Chinese). [cited in VCEEP 2007].
Ludzak FJ, Ettinger MB. 1963. Biodegradation of organic chemicals isolated from rivers. Engl Bull Ext Service No. 115. West Lafayette (IN): Purdue University. p. 278-282. [cited in Aronson et al. 1999].
Mabey WR, Smith JH, Podoll RT, Johnson HL, Mill T, Chou TW, Gates J, Waight Partridge I, Jaber H, Vandenberg D. 1982. Aquatic fate process data for organic priority pollutants. EPA 440/4-81-014. Washington (DC): US Environmental Protection Agency.
Mackay D, Shiu WY, Sutherland RP. 1979. Determination of air-water Henry's law constants for hydrophobic pollutants. Environ Sci Technol 13(3):333-337.
MacLean MM, Doe KG. 1989. The comparative toxicity of crude and refined oils to Daphnia magna and Artemia. Unpublished Environment Canada Report EE-111. Ottawa (ON): Environmental Protection Directorate, River Road Environmental Technology Centre.
MacRitchie SM, Pupp C, Grove G, Howard KWF, Lapcevic P. 1994. Groundwater in Ontario; hydrogeology, quality concerns, management. NHRI Contribution No. CS-94011. Burlington (ON): Environment Canada, National Hydrology Research Institute.
Malmgren-Hansen B, Olesen S, Pommer K, Funch LW, Pedersen E, Willum O, Olsen S. 2003. Emission and evaluation of chemical substances from selected electrical and electronic products. Survey of Chemical Substances in Consumer Products, No. 32 [Internet]. Copenhagen (DK): Danish Environmental Protection Agency [updated 2010 Apr 14; cited 2010 Jan]. Available from: http://www.mst.dk/NR/rdonlyres/F6F58F52-2D13-48DC-B7B2-71A58387AC57/0/32.pdf
Maltoni C, Conti B, Cotti G, Belpoggi F. 1985. Experimental studies on benzene carcinogenicity at the Bologna Institute of Oncology: current results and ongoing research. Am J Ind Med 7:415-446. [cited in IPCS 1996].
Maltoni C, Ciliberti A, Pinto C, Soffritti M, Belpoggi F, Menarini L. 1997. Results of long-term experimental carcinogenicity studies of the effects of gasoline, correlated fuels, and major gasoline aromatics on rats. Ann NY Acad Sci 837:15-52. [cited in OEHHA 2007].
Masten LW, Boeri RL, Walker JD. 1994. Strategies employed to determine the acute aquatic toxicity of ethyl benzene, a highly volatile, poorly water-soluble chemical. Ecotoxicol Environ Saf 27:335-348.
McGregor DB, Brown A, Cattanach P, Edwards I, McBride D, Riach C, Caspary WJ. 1988. Responses of the L5178Y tk+/tk− mouse lymphoma cell forward mutation assay: III. 72 coded chemicals. Environ Mol Mutagen 12:85-154. [cited in IPCS 1996].
Mellert W, Deckhardt K, Kaufmann W, van Ravenzwaay B. 2007. Ethylbenzene: 4- and 13-week rat oral toxicity. Arch Toxicol 81:361-370.
Melnick, R. L., Burns, K. M., Ward, J. M. and Huff, J. 2012. Chemically exacerbated chronic progressive nephropathy not associated with renal tubular tumor induction in rats: An evaluation based on 60 carcinogenicity studies by the national toxicology program. Toxicol. Sci. 128 (2): 346-356.
Melnick RL, Ward JM, Huff J. 2013. War on carcinogens: industry disputes human relevance of chemicals causing cancer in laboratory animals based on uproven hypotheses, using kidney tumors as an example. Int J Occup Environ Health 19(4):255-60.
Meyer-Monath M, Beaumont J, Morel I, Rouget F, Tack K, Lestremau F. 2014. Analysis of BTEX and chlorinated solvents in meconium by headspace-solid-phase microextraction gas chromatography coupled with mass spectrometry. Anal Bioanal Chem. 406:4481-4490.
Midorikawa K, Uchida T, Okamoto Y, Todab C, Sakaib Y, Uedab K, Hirakua Y, Murataa M, Kawanishia S, Kojimab N. 2004. Metabolic activation of carcinogenic ethylbenzene leads to oxidative DNA damage. Chem Biol Interact 150:271-281.
Mikkelsen SH, Havelund S, Mogensen AS, Stuer-Lauridsen F. 2005. Survey and assessments of chemical substances in glass and porcelain colours. Survey of Chemical Substances in Consumer Products, No. 59 [Internet]. Copenhagen (DK): Danish Environmental Protection Agency [updates 2010 Apr 14; cited 2010 Jan]. Available from: http://www2.mst.dk/Udgiv/publications/2005/87-7614-716-9/pdf/87-7614-717-7.pdf
Miller L, Xiaohuong X, Luginaah I. 2009. Spatial variability of volatile organic compound concentrations in Sarnia, Ontario, Canada. J Toxicol Environ Health A 72:610-624.
Min DBS, Ina K, Peterson RJ, Chang SS. 1979. Preliminary identification of volatile flavor compounds in the neutral fraction of roast beef. J Food Sci 44:639-642.
Mintz R, McWhinney RD. 2008. Characterization of volatile organic compound emission sources in Fort Saskatchewan, Alberta using principal component analysis. J Atmos Chem 60:83-101.
[MOE] Ministry of the Environment. 2009. Drinking water surveillance program: 2007 data. Toronto (ON): Minstry of the Environment. [updated 2009 Mar 23]. Available from: http://www.ene.gov.on.ca/en/publications/dataproducts/2Water/DWSP_by_year/index4.php
Morgan DL, Cooper SW, Carlock DL, Sykora JJ, Sutton B, Mattie DR, and McDougal JN. 1991. Dermal absorption of neat and aqueous volatile organic chemicals in the Fischer 344 rat. Environ Res 55:51-63.
Mohtashamipur E, Norpoth K, Woelke U, Huber P. 1985. Effects of ethylbenzene, toluene, and xylene on the induction of micronuclei in bone marrow polychromatic erythrocytes of mice. Arch Toxicol 58(2):106-109.
Molnar J, Katalin A, Naray M. 1986. Changes in the rat's motor behaviour during 4-hr inhalation exposure to prenarcotic concentrations of benzene and its derivatives. Acta Physiol Hung 67(3):349-354. [cited in VCCEP 2007].
Moscato G, Biscaldi G, Cottica D, Pugliese F, Candura S, Candura F. 1987. Occupational asthma due to styrene: two case reports. J Occup Med 29:957-960.
[MPBPWIN] Melting Point Boiling Point Program for Microsoft Windows [Estimation Model]. 2008. Version 1.43. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Murray R. 2010. Acceptability of Methods for Predicting Benzene Emissions from Glycol Dehydrators, Suncor Energy. Calgary (AB).
Mutti A, Falzoi M, Romanelli A, Bocchi MC, Ferroni C, Franchini I. 1988. Brain dopamine as a target for solvent toxicity: effects of some monocyclic aromatic hydrocarbons. Toxicology 49:77-82.
[NCI] National Chemical Inventories [database on CD-ROM]. 2007. Issue 1. Columbus (OH): American Chemical Society. Available from: http://www.cas.org/products/cd/nci/index.html
Nelson PR, Conrad FW, Kelly SP, Reynolds RJ. 1998. Comparison of environmental tobacco smoke to aged and diluted sidestream smoke. J Aerosol Sci 29:281-282.
Nerín C, Acosta D, Rubio C. 2002. Potential migration release of volatile compounds from plastic containers destined for food use in microwave ovens. Food Addit Contam 19(6):594-601.
Nestmann ER, Lee EG. 1983. Mutagenicity of constituents of pulp and paper mill effluent in growing cells of Saccharomyces cerevisiae. Mutat Res 119:273-280.
Neuhauser EF, Loehr RC, Malecki MR. 1985. Contact and artificial soil tests using earthworms to evaluate the impact of wastes in soil. In: Petros JK Jr, Lacy WJ, Conway RA, editors. Hazardous and industrial solid waste testing: 4th symposium. ASTM STP 886. Philadelphia (PA): American Society for Testing and Materials. p. 192-203.
New Brunswick Department of Environment. 2009. New Brunswick air quality monitoring results for the year 2007. Technical Report T-2009-2. Sciences and Reporting Branch, New Brunswick Deaprtment of Environment, Frederiction, New Brunswick.
NHW (Department of National Health and Welfare). 1990. Present patterns and trends in infant feeding in Canada. Ottawa (ON): National Health and Welfare. 9 pp. (Catalogue No. H39-199/1990E). [cited in EHD 1998].
Nicholson WJ, Selikoff IJ, Sedman H. 1978. Mortality experience of styrene-polystyrene polymerization workers. Initial findings. Scand J Work Environ Health 3(Suppl 2):247-252.
Niederlehner BR, Cairns J Jr, Smith EP. 1998. Modeling acute and chronic toxicity to non-polar narcotic chemicals and mixtures to Ceriodaphnia dubia. Ecotoxicol Environ Saf 39:136-146.
Nielsen J, Niemann AL, Mikkelsen J, Hansen MK. 2003. Chemical substances in spray paint. Surveys on Chemical Substances in Consumer Products, No. 45. Copenhagen (DK): Danish Environmental Protection Agency.
Nilsson NH and Pedersen V. 2004. Mapping and release of chemical substances from products made of chloroprene. Survey of Chemical Substances in Consumer Products, No. 51 [Internet]. Copenhagen (DK): Danish Environmental Protection Agency. [updated 2010 Apr 14; cited 2010 Jan]. Available from: http://www2.mst.dk/Udgiv/publications/2004/87-7614-767-3/pdf/87-7614-768-1.pdf
Nilsson NH, Malmgren-Hansen B, Bernth N, Pedersen E, Pommer K. 2006. Survey and health assessment of chemical substances in sex toys. Survey of Chemical Substances in Consumer Products, No. 77 [Internet]. Copenhagen (DK): Danish Environmental Protection Agency. [updated 2010 Apr 14; cited 2010 Jan]. Available from: http://www2.mst.dk/udgiv/Publications/2006/87-7052-227-8/pdf/87-7052-228-6.pdf
NIOSH. 1981. Teratologic assessment of ethylbenzene and 2-ethoxyethanol. Cincinnati (OH): National Institute for Occupational Safety and Health. PB83208074.
Nong A, Charest-Tardif G, Tardif R, et al. 2007. Physiologically based modeling of the inhalation pharmacokinetics of ethylbenzene in B6C3F1 mice. J Toxicol Environ Health A 70(21):1838-1848.
Norppa H, Vainio H. 1983. Induction of sister-chromatid exchanges by styrene analogues in cultured human lymphocytes.Mutat Res 116:379-387. [cited in IPCS 1996].
Novamann International. 1994a. Commuter exposure to vehicle related air pollutants. Report presented to Health Canada May 1994. Mississauga (ON): Novamann International. 52 pp.
Novamann International. 1994b. Commuter exposure to vehicle related air pollutants. Report presented to Health Canada September 1994. Mississauga (ON): Novamann International. 44 pp.
[NTP] National Toxicology Program (US). 1986. Toxicology and carcinogenesis studies of xylenes (mixed) (60% m-xylene, 14% p-xylene, 9% o-xylene, and 17% ethylbenzene) (CAS No. 1330-20-7) in F344/N rats and B6C3F1 mice (gavage studies). Research Triangle Park (NC): National Institutes of Health, U.S. Department of Health and Human Services, National Toxicology Program. Technical Report Series No. 327; NIH Publication. No. 87-2583.
[NTP] National Toxicology Program (US). 1992. Toxicity studies of ethylbenzene in F344/N rats and B6C3F1 mice (inhalation studies). Research Triangle Park (NC): National Institutes of Health, U.S. Department of Health and Human Services, National Toxicology Program. NIH Publication No. 92-3129.
[NTP] National Toxicology Program (US). 1999. Toxicology and carcinogenesis studies of ethylbenzene (CAS No. 100-41-4) in F344/N rats and B6C3F1 mice (inhalation study). Research Triangle Park (NC): U.S. Department of Health and Human Services, National Toxicology Program. NTP TR 466; NIH Publication No. 99-3956.
[OECD] Organisation for Economic Co-operation and Development. 2005. Ethylbenzene. CAS No. 100-41-4. SIDS Initial Assessment Report for SIAM 14, 26-28March 2002. Paris (FR): OECD. Available from: http://www.chem.unep.ch/irptc/sids/OECDSIDS/100414.pdf.
[OEHHA] Office of Environmental Health Hazard Assessment. 2007. Notice of adoption of unit risk value for ethylbenzene. Attachment: Long-term health effects of exposure to ethylbenzene. Sacramento (CA): Air Toxicology and Epidemiology Branch, OEHHA. Available from: http://oehha.ca.gov/air/hot_spots/ebenz111407.html
[OEHHA] Office of Environmental Health Hazard Assessment. 2008. No significant risk levels (NSRLs) for the proposition 65 carcinogen ethylbenzene. Sacramento (CA): Air Toxicology and Epidemiology Branch, OEHHA. Available at: http://www.oehha.org/prop65/law/pdf_zip/EthylbenzeneNSRL032808.pdf
Ogata M, Fujisaw K, Ogino Y, Mano E. 1984. Partition coefficient as a measure of bioconcentration potential of crude oil compounds in fish and shellfish. Bull Environ Contam Toxicol 33:561-567.
Ohta T, Ohyama T. 1985. A set of rate constants for the reactions of OH radicals with aromatic-hydrocarbons. Bull Chem Soc Jpn 58:3029-3030.
[OMEE] Ontario Ministry of Environment and Energy. 1993. Ontario typical range of chemical parameters in soil, vegetation, moss bags and snow. Toronto (ON): Queen's Printer for Ontario. [cited 2009 Dec]. Available from: http://www.ene.gov.on.ca/envision/sudbury/ontario_typical_range/index.htm
[OMOE] Ontario Ministry of the Environment. 1990. Municipal/industrial strategy for abatement MISA program of the petroleum refining sector. Toronto (ON): Queen's Printer for Ontario.
[OMOE] Ontario Ministry of the Environment. 1992. Background document on the development of the draft petroleum refining sector effluent limits regulation. Toronto (ON): Queen's Printer for Ontario.
[OMOE] Ontario Ministry of the Environment. 2005. Brownfields environmental site registry. [accessed 2005 Jan 5]. Available from: http://www.ene.gov.on.ca/environet/BESR/search.htm
[OMOE] Ontario Ministry of the Environment. 2006. Clarkson airshed study. A scientific approach to improving air quality. Part II - the ambient air monitoringprogram. November 2006. Available from: http://www.ene.gov.on.ca/envision/techdocs/6031e.pdf
O'Neil MJ, Heckelman PE, Koch CB, Roman KJ, editors. 2006. The Merck index. An encyclopedia of chemicals, drugs, and biologicals. 14th ed. Whitehouse Station (NJ): Merck & Co., Inc.
Ormonde EV, Yokose K. 2008. CEH marketing research report: acrylonitrile-butadiene-styrene (ABS) resins. In: Chemical economics handbook [Internet]. Menlo Park (CA): SRI Consulting. [cited 2010 Mar]. Available from: http://www.sriconsulting.com/CEH/Public/Reports/580.0180/ [restricted access].
[OSHA] Occupational Safety & Health Administration. 2011. Occupational safety and health guideline for ethyl benzene [Internet]. Washington (DC): United States Department of Labor. [cited 2011 Dec]. Available from: http://www.osha.gov/SLTC/healthguidelines/ethylbenzene/recognition.html
Otson R. 1987. Purgeable organics in Great Lakes raw and treated water. Int J Environ Anal Chem 31:41-53.
Otson R, Williams DT, Bothwell PD. 1982. Volatile organic compounds in water at thirty Canadian potable water treatment facilities. J Assoc Off Anal Chem 65(6):1370-1374.
[PACE] Petroleum Association for Conservation of the Canadian Environment. 1987. A study of exposure to motor gasoline hydrocarbon vapours at service stations. Phase II - Summer study (PACE Report No. 87-5).
[PACE] Petroleum Association for Conservation of the Canadian Environment. 1989. A study of exposure to motor gasoline hydrocarbon vapours at service stations. Phase III - Winter study (PACEReport No. 89-3).
Park JH, Lee HJ. 1993. Estimation of bioconcentration factor in fish, adsorption coefficient for soil and sediments and interfacial tension with water for organic nonelectrolytes based on the linear energy relationships. Chemosphere 26(10):1905-1916.
Park JS, Fujii S, Yuasa K, Kagi N, Toyzumi A, Tamura H. 1996. Characteristics of volatile organic compounds in residence. In: Indoor Air '96: Proceedings of the 7th International Conference on Indoor Air Quality and Climate, Vol. 3. 1996 Jul 21-26. Nagoya (JP): Organizing Committee of the Conference. p. 579-584.
Parra MA, Elustondo D, Bermejo R, Santamaría JM. 2008. Exposure to volatile organic compounds (VOC) in public buses of Pamplona, Northern Spain. Sci Total Environ 404:18-25.
Picard D, Ross B, Palibrk G. 2002. A summary of NPRI emissions from the upstream oil and gas inventory. Calgary (AB): Clearstone Engineering Ltd. Report prepared for T. Mah, Common Air Contaminants, Pollution Prevention Control Branch, Environment Canada.
Performance Coatings. 2013. Material Safety Data Sheet: Semitransparent Stain [Internet]. Ukiah (CA): Performance Coatings Inc. [cited 2013 June]. Available from: http://www.penofin.com/pdf/msds/MSDS-blue-gold-label550.pdf
Phatrabuddha N, Maharatchpong N, Keadtongtawee S, Saowakhontha S. 2013. Comparison of personal BTEX exposure and pregnancy outcomes amont pregnant women residing in and near petrochemical industrial area. Environment Asia. 6(2):34-41.
Polzin GM, Kosa-Maines RW, Ashley DL, Watson CH. 2007. Analysis of volatile organic compounds in mainstream cigarette smoke. Environ Sci Technol 41:1297-1302.
Pors J, Fuhlendorff R. 2002. Mapping of compound discharge when ironing of beads. Survey of Chemical Substances in Consumer Products, No. 7 [Internet]. Copenhagen (DK): Danish Environmental Protection Agency [updated 2010 Apr 14; cited 2010 Jan]. Available from: http://www.mst.dk/English/Chemicals/Consumer_Products/Survey_no_7_2002.htm
[PSWG] Polystyrene Working Group. 1997. Potential exposure to ethylbenzene from food-contact use of polystyrene resins. Washington (DC): Food, Drug, and Cosmetic Packaging Materials Committee, Society of the Plastics Industry, Inc. [cited in VCCEP 2007 Appendix L].
Qin Y, Han K, Jing H, Kong J. 1999. Indoor air pollution from building materials. In: Indoor Air '99: Proceedings of the 8th International Conference on Indoor Air Quality and Climate, Vol. 1. 1999 Aug 8-13, Edinburgh (UK): Construction Research Communications Ltd. p. 426-429.
Reinhard M, Goodman NL, Barker JF. 1984. Occurrence and distribution of organic chemicals in two landfill leachate plumes. Environ Sci Technol 18:953-961.
Rembold H, Wallner P, Nitz S, Kollmannsberger H, Drawert F. 1989. Volatile components of chickpea (Cicer arietinum L.) seed. J Agric Food Chem 37:659-662.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu. 2007a. Paint products fact sheet: to assess the risks for the consumer. Updated version for ConsExpo 4 [Internet]. Bilthoven (NL): RIVM (National Institute for Public Health and the Environment). Report No: 320104008/2007. [cited 2010 Oct]. Available from: http://www.rivm.nl/bibliotheek/rapporten/320104008.pdf
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu. 2007b. Do-it-yourself products fact sheet: to assess the risks for the consumer [Internet]. Bilthoven (NL): RIVM (National Institute for Public Health and the Environment). Report No.: 320104007/2007. [cited 2010 Oct]. Available from: http://www.rivm.nl/bibliotheek/rapporten/320104007.pdf
Romanelli A, Falzoi M, Mutti A, Bergamaschi E, Franchini I. 1986. Effects of some monocyclic aromatic solvents and their metabolites on brain dopamine in rabbits. J Appl Toxicol 6(6):431-438.
Rumchev K, Spickett J, Bulsara M, Phillips M, Stick S. 2004. Association of domestic exposure to volatile organic compounds with asthma in young children. Thorax 59:746-751.
Rust-Oleum. 2011. Material Safety Data Sheet: WDCARE 1-GL 2 PK Watco Lacqr Fin Satin [Internet]. Vernon Hills (IL): Rust-Oleum Corporation. [cited 2013 Feb]. Available from: http://www.rustoleum.com/MSDS/ENGLISH/63231.PDF
Rust-Oleum. 2013a. Material Safety Data Sheet: PTouch 6X340GM Gloss Key Lime [Internet]. Vernon Hills (IL): Rust-Oleum Corporation. [cited 2013 Feb]. Available from: http://www.rustoleum.ca/CBGResourceCenter.asp
Rust-Oleum. 2013b. Material Safety Data Sheet: TRMCLD 2 X 3.78 LT Rust Paint Fire Red [Internet]. Concord (ON): Rust-Oleum Consumer Brands Canada. [cited 2013 Feb]. Available from: http://www.rustoleum.ca/CBGResourceCenter.asp
Sack TM, Steele DH, Hammerstrom K, Remmers J. 1992. A survey of household products for volatile organic compounds. Atmos Environ 26A(6):1063-1070.
Saghir SA, Rick DL, McClymont EL, Zhang F, Bartels MJ, Bus JS. 2009. Mechanism of ethylbenzene-induced mouse-specific lung tumor: metabolism of ethylbenzene by rat, mouse, and human liver and lung microsomes. Toxicol Sci 107:352-366.
Saghir SA, Zhang F, Rick DL, Kan L, Bus JS, Bartels MJ. 2010. In vitro metabolism and covalent binding of ethylbenzene to microsomal protein as a possible mechanism of ethylbenzene-induced mouse lung tumorigenesis. Regul Toxicol Pharmacol 57:129-135.
Saghir SA, Zhang F, Rick DL, Kan L, Bus JS, Bartels MJ. 2010b. Authors' response to Huff et al., "Clarifying carcinogenicity of ethylbenzene." Regul Toxicol Pharmaco 58:170-172.
Saillenfait AM, Gallissot F, More G, Bonnet P. 2003. Developmental toxicities of ethylbenzene, ortho-, meta-, para-xylene and technical xylene in rats following inhalation exposure. Food Chem Toxicol 41(3):415-429.
Saillenfait AM, Gallissot F, Sabaté JP, Bourges-Abella N, Cadot R, Morel G, Lambert AM. 2006. Developmental toxicities of combined ethylbenzene and methylethylketone administered by inhalation to rats. Food Chem Toxicol 44(8):1287-1298.
Saillenfait AM, Gallissot F, Sabat JP, Bourges-Abella N, Muller S. 2007. Developmental toxic effects of ethylbenzene to toluene alone and in combination with butyl acetate in rats after inhalation exposure. J Appl Toxicol 27(1):32-42.
Salthammer T. 1996. VOC emissions from cabinet furnitures: concentrations in the test chamber and in the cabinet. In: Indoor Air '96: Proceedings of the 7th International Conference on Indoor Air Quality and Climate, Vol. 3. 1996 Jul 21-26. Nagoya (JP): Organizing Committee of the Conference. p. 567-572.
Sanemesa I, Araki M, Deguchi T et al. 1982. Solubility measurements of benzene and alkylbenzenes in water by making use of solute vapour. Bull Chem Soc Jpn 55:1054-1082. [cited in HSDB 2009].
Seely JC, Hasman JK, Nyska A, Wolf DC, Everitt JI, Hailey JR. 2002. The effect of chronic progressive nephropathy on the incidence of renal tubule cell neoplasms in control male F344 rats. Toxicol Pathol 30:681-686.
Seidel SD, Schisler MR, Kleinert KM. 2006. Evaluation of ethylbenzene in the mouse lymphoma (L5178YTK+/-) forward mutation assay. Midland (MI): Toxicology and Environmental Research and Consulting, The Dow Chemical Company. Study ID: 051157. [cited in VCCEP 2007].
Sexton K, Adgate JL, Church TR, Ashley DL, Needham LL, Ramachandran G, Fredrickson AL, Ryan AD. 2005. Children's exposure to volatile organic compounds as determined by longitudinal measurements in blood. Environ Health Perspect 113(3):342-349.
Shariq K and Funada C. 2008. CEH marketing research report: polystyrene. In: Chemical economics handbook [Internet]. Menlo Park (CA): SRI Consulting. [cited 2010 Mar]. Available from: http://www.sriconsulting.com/CEH/Public/Reports/580.1500/ [restricted access].
Shatkin JA and Brown HS. 1991. Pharmacokinetics of the dermal route of exposure to volatile organic chemicals in water: a computer simulation model. Environ Res 56(1):90-108. [cited in ATSDR 2010].
Sherwin-Williams. 2008. Material safety data sheet: Steampede™-1 polyurethane sealant [Internet]. Cleveland (OH): The Sherwin-Williams Company. [cited 2010 Oct]. Available from: http://www.sherlink.com/sher-link/ImgServ?id=1637495.pdf&basePath=/ecomm_apps/ecommerce/temp/sher-link/msds/
Sherwin-Williams. 2010. Material safety data sheet: Wood Classics® Fast Dry oil varnish, satin [Internet]. Cleveland (OH): The Sherwin-Williams Company. [cited 2010 Oct]. Available from: http://www.sherlink.com/sher-link/ImgServ?id=640315933.pdf&basePath=/ecomm_apps/ecommerce/temp/sher-link/msds/
Shiohara N, Fernández-Bremauntz AA, Jiménez SB, Yanagisawa Y. 2005. The commuters' exposure to volatile chemicals and carcinogenic risk in Mexico City. Atmos Environ 39:3481-3489.
Singh HB, Salas LJ, Smith AJ, Shigeishi H. 1981. Measurements of some potentially hazardous organic chemicals in urban environments. Atmos Environ 15:601-612.
[SLEA] Sarnia-Lambton Environmental Association. 2007-2008. Progress review technical summary. Sarnia, Ontario.
Sliwinska-Kowalska M, Zamyslowska-Szmytke E, Szymczak W, Kotylo P, Fiszer M, Dudarewicz A, Wesolowski W, Pawlaczyk-Luszczynska M, Stolarek R. 2001. Hearing loss among workers exposed to moderate concentrations of solvents. Scand J Work Environ Health 27(5):335-342. [cited in ATSDR 2010].
Smyth HF, Carpenter CP, Weil CS, Pozzani UC, Streigel JA. 1962. Range finding toxicity data: list IV. Am Ind Hyg J 23:95-97.
Sram RJ, Beskid O, Binkova B, Rossner P, Smerhovsky Z. 2004. Cytogenetic analysis using fluorescence in situ hybridization (FISH) to evaluate occupational exposure to carcinogens. Toxicol Lett 149:335-344.
[SRD] Source Ranking Database [database on the Internet]. 2004. Version 4.0. Washington (DC): US Environmental Protection Agency. [updated 2004 Apr; cited 2010 Mar]. Available from: http://www.epa.gov/oppt/exposure/pubs/srd.htm
Statistics Canada. 2012. Canadian Health Measures Survey [Internet]. Ottawa (ON): Statistics Canada. [cited 2013 April]. Available from: http://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=5071&Item_Id=129548&lang=en
Stott WT, Johnson KA, Bahnemann R, Day SJ, McGuirk RJ. 2003. Evaluation of potential modes of action of inhaled ethylbenzene in rats and mice. Toxicol Sci 71(1):53-66.
Stump DG. 2004a. A pilot inhalation study for a reproductive toxicity study of ethylbenzene in rats. Study number - WIL-186028. Ashland (OH): WIL Research Laboratories, Inc. Sponsored by the Ethylbenzene Panel, American Chemistry Council, Arlington, Virginia. [cited in VCCEP 2007].
Stump DG. 2004b. A 28-day inhalation splenic antibody formation study of ethylbenzene in rats. Study number - WIL-186028. Ashland (OH): WIL Research Laboratories, Inc. Sponsored by the Ethylbenzene Panel, American Chemistry Council, Arlington, Virginia. [cited in VCCEP 2007].
Svendsen N, Pedersen SF, Hansen OC, Pedersen E, Bernth N. 2005. Survey and release of chemical substances in "slimy toys". Survey of Chemical Substances in Consumer Products, No. 67 [Internet]. Copenhagen (DK): Danish Environmental Protection Agency [updated 2010 Apr 14; cited 2010 Jan]. Available from: http://www2.mst.dk/udgiv/Publications/2006/87-7052-013-5/pdf/87-7052-014-3.pdf
Swann RL, Laskowski DA, McCall PJ, VanderKuty K, Dishburger HJ. 1983. A rapid method for the estimation of the environmental parameters octanol/water partition coefficient, soil sorption constant,water to air ratio, and water solubility. Residue Rev Pestic 85:17-28.
Takeoka GR, Güntert M, Flath RA, Wurz RE, Jennings W. 1986. Volatile constituents of kiwi fruit (Actinidia chinensisPlanch.). J Agric Food Chem 34:576-578.
Tan S, Okada T. 1978. Hygienic studies on plastic containers and packages. (II) Analysis of styrene monomer in food content. Shokuhin Eiseigaku Zasshi (J Food Hyg Soc Jpn) 19(2):172-177.
Tang W, Hemm I, Eisenbrand G. 2000. Estimation of human exposure to styrene and ethylbenzene. Toxicology 144:39-50.
[TaPL3] Long Range Transport and Persistence Level III model [Internet]. 2000. Version 2.10. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre. Available from: http://www.trentu.ca/academic/aminss/envmodel/models/TaPL3.html
Tardif R, Charest-Tardif G, Brodeur J, Krishnan K. 1997. Physiologically based pharmacokinetic modeling of a ternary mixture of alkyl benzenes in rats and humans. Toxicol Appl Pharmacol 144:120-134. [cited in ATSDR 2010].
Tegeris JS, Balster RL. 1994. A comparison of the acute behavioral effects of alkylbenzenes using a functional observational battery in mice. Fundam Appl Toxicol 22:240-250.
[TERA] Toxicology Excellence for Risk Assessment. 2007. Report of the peer consultation meeting on ethylbenzene. Submission by American Chemistry Council Ethylbenzene Panel for the Voluntary Children's Chemical Evaluation Program (VCCEP). May 31, 2007. [cited 22 August 2014]. Available at http://www.tera.org/Peer/VCCEP/Ethylbenzene/EBWelcome.html
Tewari YB, Miller MM, Wasik SP, Maritre DE. 1982. Aqueous solubility and octanol/water partition coefficient of organic compounds at 25.0°C. J Chem Eng Data 27:451-454.
Triebig G, Claus D, Csuzdal I, Druschky KF, Holler P, Kinze W, Lehrl S, Reichwein S, Weidenhammer W, Weitbrecht WU, Weltle D, Schaller KH, Valentin H. 1988. Cross-sectional epidemiological study on neurotoxicity of solvents in paints and lacquers. Int Arch Occup Environ Health 60:233-241.
Toda C, Uchida T, Midorikawa K, Murata M, Hiraku Y, Okamoto Y, Ueda K, Kojima N, Kawanishib S. 2003. DNA damage by ethylbenzenehydroperoxide formed from carcinogenic ethylbenzene by sunlight irradiation. Biochem Biophys Res Comm 304:638-642.
Toftgård R, Nilsen OG. 1982. Effects of xylene and xylene isomers on cytochrome P-450 and in vitro enzymatic activities in rat liver, kidney and lung. Toxicology 23: 197-212.
Triebig G, Claus D, Csuzda I, Druschky KF, Holler P, Kinzel W, Lehrl S, Reichwein P, Weidenhammer W, Weitbrecht WU, Weltle D, Schaller KH, Valentin H. 1988. Cross-sectional epidemiological study on neurotoxicity of solvents in paints and lacquers. Int Arch Occup Environ Health 60:233-241. [cited in VCCEP 2007].
Tsai KP, Chen CY. 2007. An algal toxicity database of organic toxicants derived by a closed-system technique. Environ Toxicol Chem 26(9):1931-1939.
Ungváry G. 1986. Solvent effects on reproduction: experimental toxicity. Prog Clin Biol Res 220:169-177.
Ungváry G, Tatrai E. 1985. On the embryotoxic effects of benzene and its alkyl derivatives in mice, rats and rabbits. Arch Toxicol 8(Suppl):425-430. [cited in IPCS 1996].
[US EPA] US Environmental Protection Agency. 1991. Integrated risk information system for ethylbenzene, carcinogenicity assessment. [cited 2010 Feb]. Available from: http://www.epa.gov/ncea/iris/subst/0051.htm
[US EPA] US Environmental Protection Agency. 1992. Dermal exposure assessment: principles and applications. Washington (DC): US Environmental Protection Agency.
[US EPA] US Environmental Protection Agency. 1997. Exposure factors handbook. Chapter 16: Consumer products. Washington (DC): US EPA, National Centre for Environmental Assessment, Office of Research and Development.
[US EPA] US Environmental Protection Agency. 2009. Toxics release inventory (TRI) program [database on the Internet]. Washington (DC): US EPA, Office of Information Analysis and Access, TRI Program Division. Available from: http://www.epa.gov/triexplorer/chemical.htm
[US EPA] US Environmental Protection Agency. 2011. Exposure factors handbook: 2011 edition. Chapter 7: Dermal exposure factors [Internet]. Washington (DC): US EPA, National Centre for Environmental Assessment, Office of Research and Development. [cited October 2011]. Available from: http://www.epa.gov/ncea/efh/report.html
[US FDA] US Food and Drug Administration. 2006. Total diet study market baskets 1991-3 through to 2003-4 [Internet]. [cited 2010 Jan]. Available from: http://www.fda.gov/downloads/Food/FoodSafety/FoodContaminantsAdulteration/TotalDietStudy/UCM184304.pdf
US House of Representatives. 2011. Chemicals used in hydraulic fracturing [Internet]. Washington (DC): United States House of Representatives, Committee on Energy and Commerce, Minority Staff [updated 2011 April; cited 2012] Available from: http://democrats.energycommerce.house.gov/sites/default/files/documents/Hydraulic%20Fracturing%20Report%204.18.11.pdf
[VCCEP] Voluntary Children's Chemical Evaluation Program. 2007. Tier 1 pilot submission for ethylbenzene (CAS No. 100-41-4). Washington (DC): US Environmental Protection Agency. [cited 2010 Feb]. Available from: http://www.epa.gov/oppt/vccep/pubs/chem8.html
Versar, Inc. 1986. Standard scenarios for estimating exposure to chemical substances during use of consumer products. Vol. I. Consumer use of household paints. Springfield (VA): Versar, Inc. Prepared for US Environmental Protection Agency.
Verschueren K. 1983. Handbook of environmental data on organic chemicals. 2nd ed. New York (NY): Van Nostrand Reinhold Co. p. 628-630.
Vichi S, Pizzale L, Conte LS, Buxaderas S, López-Tamames E. 2007. The occurrence of volatile and semi-volatile aromatic hydrocarbons in virgin olive oils from north-eastern Italy. Food Control 18:1204-1210.
Vigano L. 1993. Reproductive strategy of Daphnia magnaand toxicity of organic compounds. Water Res 27(5):903-909.
Vyskocil A.Truchon,G. Leroux T. Lemay F. Gendron M. Gagnon F. Majidi N.E. Boudjerida A. Lim S. Emond C. Viau C. 2012. A weight of evidence approach for the assessment of the ototoxic potential of industrial chemicals. Toxicol.Ind.Health. 28(9):796-819.
Wakeham SG, Davis AC, Karas JL. 1983. Mesocosm experiments to determine the fate and persistence of volatile organic compounds in coastal seawater. Environ Sci Technol 17:611-617.
Wallace LA, Pellizzari ED. 1986. Personal air exposures and breath concentrations of benzene and other volatile hydrocarbons for smokers and nonsmokers. Toxicol Lett 35: 113-116.
Wallace LA, Pellizzari E, Leaderer B, Zelon H, Sheldon L. 1987. Emissions of volatile organic compounds from building materials and consumer products. Atmos Environ 21(2):385-393.
Wheeler A, Wong SL, Khoury C, Zhu J. 2013. Predictors of indoor BTEX concentrations in Canadian residences. Health Reports. 24(5): 11-17.
Whitehead LW, Ball GL, Fine LJ, Langolf GD. 1984. Solvent vapour exposures in booth spray painting and spray glueing, and associated operations. Am Ind Hyg Assoc J 45(11):767-772.
[WHO] World Health Organization. 1996. Guidelines for drinking-water quality. Vol. 2. 2nd ed. [Internet]: Ethylbenzene. Geneva (CH): World Health Organization. [cited 2009 Dec]. Available from: http://www.who.int/water_sanitation_health/dwq/chemicals/ethylbenzene.pdf
Wilson BH, Smith GB, Rees JF. 1986. Biotransformation of selected alkylbenzene and halogenated aliphatic hydrocarbons in methanogenic aquifer material: a microcosm study. Environ Sci Technol 20(10):997-1002.
Wilson JT, Henson JM, Piwoni MD, Wilson BH, Banerjee P. 1988. Biodegradation and sorption of organic solvents and hydrocarbon fuel constituents in subsurface environments. Tyndall Air Force Base (FL): Air Force Engineering and Services Centre, Engineering and Services Laboratory.
Witterseh T. 2004. Emission of chemical substances from products made of exotic wood. Mapping of Chemical Substances in Consumer Products, No. 49 [Internet]. Copenhagen (DK): Danish Environmental Protection Agency. [updated 2010 Apr 14; cited 2010 Jan]. Available from: http://www.mst.dk/NR/rdonlyres/AA1FFDA1-D5E0-4BF8-B4DF-B69A23E5471F/0/49.pdf
WM Barr. 2012. Material safety data sheet: Goof Off Professional Strength VOC Compliant. Memphis (TN): W.M. Barr. [cited 2013 Jan]. Available from: http://www.goofoffstainremover.com/sites/default/files/msds/go_professional_voc_2410.3_msds_91312_2.pdf
Wolf MA, Rowe VK, McCollister DD, Hollingsworth RL, Oyen F. 1956. Toxicological studies of certain alkylated benzenes and benzene. Arch Ind Health 14:387-398. [cited in IPCS 1996].
Won D, Magee RJ, Yang W, Lusztyk E, Nong G, Shaw CY. 2005. A material emission database for 90 target VOCs NRCC-48314 [Internet]. Ottawa (ON): Institute for Reaseach in Construction, National Research Council Canada. [cited Jul 2011]. Available from: http://www.nrc-cnrc.gc.ca/obj/irc/doc/pubs/nrcc48314/nrcc48314.pdf
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [Estimation Model]. 2008. Version 1.41. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Xie J, Wang X, Sheng G, Bi X, Fu J. 2003. Determination of tobacco smoking influence on volatile organic compounds constituent by indoor tobacco smoking simulation experiment. Atmos Environ 37:3365-3374.
Yant WP, Schrenk HH, Waite CP, Patty FA. 1930. Acute response of guinea pigs to vapors of some new commercial organic compounds. II. Ethylbenzene. Public Health Rep 45:1241-1250.
Yoshida T, Matsunaga I. 2006. A case study on identification of airborne organic compounds and time courses of their concentrations in the cabin of a new car for private use. Environ Int 32:58-79.
You X(I), Senthilselvan A, Cherry NM, Kim HM, Burstyn I. 2008. Determinants of airborne concentrations of volatile organic compounds in rural areas of Western Canada. J Exposure Sci Environ Epidemiol 18:117-128.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K. 1992. Salmonella mutagenicity tests: V. Results from the testing of 311 chemicals. Environ Mol Mutagen 19(Suppl 21):2-141.
Zhang M, Wang Y, Wang Q, Yang J, Yang D, Liu J, Li J. 2010. Involvement of mitochondria-mediated apoptosis in ethylbenzene-induced renal toxicity in rat. Toxicol Sci 115(1):295-303.
Zhang M, Wang Y, Wang Q, Yang D, Zhang J, Wang F, Gu Q. 2013. Ethylbenzene-induced hearing loss, neurobehavioral function, and neurotransmitter alterations in petrochemical workers. J Occup Environ Med 55(9):1001-6.
Zhu J, Newhook R, Marro L, Chan C. 2005. Selected volatile organic compounds in residential air in the city of Ottawa, Canada. Environ Sci Technol 39:3964-3971.
Zhu J, Wong SL, Cakmak S. 2013. Nationally representative levels of selected volatile organic compounds in Canadian residential indoor air: Population-based survey. Environ. Sci. Technol. 47:13276-13283.
Zogorski JS, Carter JM, Ivahnenko T, Lapham WW, Moran MJ, Rowe BL, Squillace PJ, Toccalino PL. 2006. The quality of our nation's waters: Volatile organic compounds in the nation's ground water and drinking-water supply wells. National Water-Quality Assessment Program Circular 1292. [Internet]. Reston (VA): U.S. Geological Survey. [cited Mar 2011]. Available from: http://pubs.usgs.gov/circ/circ1292/
Appendices
- Appendix A: Summary of Canadian Indoor Air Studies
- Appendix B: Summary of Ethylbenzene Concentrations in Various Food Items (US FDA 2006)
- Appendix C: Ethylbenzene in Various Food Items
- Appendix D: Estimates of Daily Intake of Ethylbenzene by Canadians
- Appendix E: Consumer Product Information
- Appendix F: Estimates of Exposure to Ethylbenzene
- Appendix G: Estimates of Potential Exposure to Ethylbenzene from Gasoline
- Appendix H: Summary of Health Effects