Screening Assessment
Internationally Classified Substance Grouping
Ethanol, 2-[(2-aminoethyl)amino]-
(AEEA)
Chemical Abstracts Service Registry Number
111-41-1
Environment and Climate Change Canada
Health Canada
May 2016
Table of Contents
- Synopsis
- Introduction
- Substance Identity
- Physical and Chemical Properties
- Sources
- Uses
- Releases to the Environment
- Measured Environmental Concentrations
- Environmental Fate
- Persistence and Bioaccumulation Potential
- Potential to Cause Ecological Harm
- Ecological Effects Assessment
- Ecological Exposure Assessment
- Characterization of Ecological Risk
- Uncertainties in Evaluation of Ecological Risk
- Potential to Cause Harm to Human Health
- Exposure Assessment
- Health Effects Assessment
- Characterization of Risk to Human Health
- Uncertainties in Evaluation of Risk to Human Health
- Conclusion
- References
Synopsis
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA), the Ministers of the Environment and Climate Change and of Health have conducted a screening assessment of Ethanol, 2-[(2-aminoethyl)amino]-, hereinafter referred to as AEEA. The Chemical Abstracts Service Registry Number (CAS RN) for AEEA is 111-41-1. This substance is part of the Internationally Classified Substance Grouping, which includes substances that were prioritised for screening assessment because they were classified by certain international agencies as potentially of concern for human health.
AEEA does not occur naturally in the environment. In Canada, AEEA is imported both as a pure substance and as a component of products. In 2008, a lesser quantity than the reporting threshold of 100 kg of AEEA was manufactured in Canada, and more than 500 000 kg of AEEA were imported into Canada. In 2011, AEEA was not manufactured in Canada, and between 100 000 and 500 000 kg of AEEA was imported into Canada in the same year. AEEA can be used as a chemical intermediate, a component of adhesives and sealants used in asphalt paving or patching, a curing agent for epoxy resins, in building products with mainly commercial applications, a component in super glue, and a component of corrosion inhibitors and lubricant additives. As a chemical intermediate, AEEA is used to manufacture surfactants which in turn have applications as industrial detergents and in consumer products such as cosmetics. AEEA is used as a component in food-packaging adhesives and inks with no direct contact with food, and as a component of an agent used in the paper manufacturing process. AEEA is also used as a component in additives for closed recirculating cooling systems where the water treated will not come into direct contact with food.
AEEA is characterized by a moderate vapour pressure, a very low Henry’s Law constant, and very low log Koc and log Kow values. AEEA is miscible in water. Monitoring data on AEEA in the Canadian environment have not been identified.
AEEA has a short half-life in air, and it is not expected to be present in the atmosphere. AEEA is readily biodegradable in water and it is not expected to remain in soil or sediments for prolonged periods of time. Based on the available empirical and modelled evidence, AEEA is expected to have a limited persistence in air, water, soil and sediments.
AEEA has a low bioaccumulation potential. This was evidenced by very low empirical and modelled bioconcentration and bioaccumulation values for fish.
Several studies have been conducted for AEEA to identify ecotoxicological effects of the substance on aquatic organisms, including micro-organisms, crustaceans and fish. Results of these studies indicated that AEEA has a low to moderate potential to cause acute toxic effects in exposed organisms. Longer-term ecotoxicological studies were not available for AEEA. It was noted that at higher exposure concentrations, AEEA increased the alkalinity of the aqueous test solutions, and this may have contributed to additional toxic effects to exposed organisms. Effects of AEEA on soil and sediment organisms have not been studied; however, such effects are not expected to be greater than those determined in aquatic species.
AEEA is imported as a minor component in products or mixtures that are used in industrial , commercial or consumer applications. Many of these products undergo curing. The potential for AEEA releases into the environment from these cured products as well as from AEEA applications in asphalt cement is not expected to be significant. AEEA can also be a minor constituent of imported solid products in building materials, but with very limited potential for releases. The main source of release of AEEA is expected to occur from industrial uses of the pure substance as a chemical intermediate. AEEA is assumed to be chemically converted during industrial processes, and it is expected that it no longer exists in its parent form. The only quantitative scenario considered was based on disposal of unreacted residual AEEA from the cleaning of empty transport and processing containers. Based on this scenario, and using conservative assumptions, exposure to organisms in the environment would be below levels expected to cause harm.
Considering all available lines of evidence presented in this Screening Assessment, there is low risk of harm to organisms and the broader integrity of the environment from AEEA. It is concluded that AEEA does not meet the criteria under paragraph 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity, or that constitute or may constitute a danger to the environment on which life depends.
Critical effects for characterization of risk to human health for AEEA are developmental and reproductive effects in animal studies.
There were no reports of AEEA in environmental media identified for Canada. Exposure of the general population to AEEA from environmental media is not expected, given that it is not manufactured in Canada and its use is limited to a few industrial applications. Canadians are also not expected to be exposed to AEEA through food consumption or the use of consumer products. Accordingly, the risk to human health is considered to be low.
Based on the information presented in this Screening Assessment, it is concluded that AEEA does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Conclusion
It is concluded that AEEA does not meet any of the criteria set out in section 64 of CEPA.
Introduction
Pursuant to sections 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999) the Minister of the Environment and Climate Change Canada and the Minister of Health conduct Screening Assessments of substances to determine whether these substances present or may present a risk to the environment or to human health.
The Substance Groupings Initiative is a key element of the Government of Canada’s Chemicals Management Plan (CMP). The Internationally Classified Substance Grouping consists of six substances that were identified as priorities for action, because they met the categorization criteria under section 73 of CEPA and/or were considered as priority substances under the CMP based on human health concerns (Environment Canada, Health Canada 2013). Substances in this substance grouping have been identified by other jurisdictions as a concern for human health due to high hazard potential as recognized by international agencies.
The Internationally Classified Substance Grouping includes four cresols (phenol, methyl- substances), as well as two other substances, Ethanol, 2-[(2-aminoethyl)amino]- (CAS RN 111-41-1) and Carbamic acid, ethyl ester (CAS RN 51-79-6). These substances are not necessarily similar in terms of chemical structure, physical and chemical properties, uses, or other assessment parameters. For this reason, three separate Screening Assessments have been conducted within the Internationally Classified Substance Grouping, with one screening assessment for the sub-grouping of the four cresols, and individual assessments for Ethanol, 2-[(2-aminoethyl)amino]- and Carbamic acid, ethyl ester.
Screening assessments focus on information critical to determining whether substances within a grouping meet the criteria as set out in section 64 of CEPA, by examining scientific information to develop conclusions by using a weight-of-evidence approach and precaution.Footnote[1]
This Screening Assessment includes consideration of information on physical and chemical properties, hazards, uses and exposure, including additional information submitted by stakeholders. Relevant data were identified up to July 2013. Empirical data from key studies as well as some results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
The Screening Assessment does not represent an exhaustive or critical review of all available data. Rather, it presents the most critical studies and lines of evidence pertinent to the proposed conclusion.
The Screening Assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. The ecological and human health portions of this assessment have undergone external written peer review and/or consultation. Comments on the technical portions relevant to the environment were received from Tim Fletcher (Ontario Ministry of the Environment) and Dr. Pamela Welbourn (Queen’s University). Comments on the technical portions relevant to human health were received from scientific experts selected and directed by Toxicology Excellence for Risk Assessment (TERA), including Dr. Sue Ross (TERA), Dr. Barry Ryan (Emory University), Dr. Pamela Williams (E Risk Sciences and University of Colorado) and Dr. Calvin Willhite (McLaughlin Centre for Population Health Risk Assessment and Risk Sciences International). Additionally, the draft of this Screening Assessment was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
The critical information and considerations upon which the Screening Assessment is based are summarized below.
Substance Identity
The substance Ethanol, 2-[(2-aminoethyl)amino]-, (CAS RN 111-41-1), hereinafter referred to by its acronym AEEA, is a simple organic chemical that belongs to the class of organic substances known as ethanolamines. Information regarding substance identity of AEEA is summarized in Table 1.
| CAS RN | Chemical structure | Molecular weight (g/mol) | Chemical formula | SMILESFootnote Table 1 [a] |
|---|---|---|---|---|
| 111-41-1 | 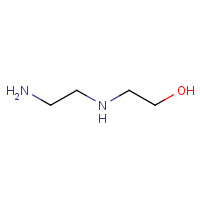 |
104.15 | C4H12N2O | OCCNCCN |
Physical and Chemical Properties
A summary of experimental and modelled values of physical and chemical properties of AEEA that are relevant to its environmental fate and ecotoxicity is provided in Table 2.
Models based on quantitative structure-activity relationships [(Q)SAR] were used to generate data for some of the physical and chemical properties of AEEA. These models are mainly based on fragment addition methods, i.e., they sum the contributions of sub-structural fragments of a molecule to make predictions for a property or endpoint. Most of these models rely on the neutral form of AEEA as input (see Table 1 for SMILES sequence). Consequently, except where noted, the modelled values shown in Table 2 are for the neutral form of AEEA. It is noted that AEEA ionizes at environmentally relevant pH (ECHA c2007-2014b; ACD/Percepta c1997-2012; see Table 2), and consequently some of the substance could be present in the +1 and/or +2 ionized form, available to undergo electrostatic interactions in environmental media. Nonetheless, the modelled physical and chemical properties are considered relevant and predictive of its fate in the environment. Some AEEA is expected to be present in its neutral form at environmentally relevant pH. In addition, when ionized, AEEA behaves as a base. In aquatic toxicity studies, rapid increases in pH to as high as pH 10 were noted when AEEA was applied at high concentrations of approximately greater than or equal to 250 mg/L (ECHA c2007-2014b; European Commission 2000). AEEA exposure concentrations of less than or equal to 250 mg/L had no effect on pH levels of aqueous test solutions.
At room temperature, AEEA is a colourless to pale yellow liquid, and it has a mild ammonia odour (HSDB 1983– ; ECHA c2007-2014b). Generally, a very good correlation was found between the available empirical physical and chemical property values and the modelled values. AEEA has a moderate vapour pressure (less than or equal to 1 to 1.3 Pa at room temperature) (ECHA c2007-2014b; Hawley’s 2007; Sax’s 2012) and a very low modelled Henry’s Law constant (HLC) of 10-10 to 10-8 Pa·m3/mol (EPI Suite 2012). The empirical and modelled log Kow values for AEEA were determined to be negligible, at -2.13 to -1.37 (European Commission 2000; EPI Suite 2012; ACD/Percepta c1997-2012). The modelled log D values varied with pH and were found to be in the range of -4.46 to -3.26 for pH 6.5–8.0, respectively. The modelled log Koc values were very low, at 0.42 (based on the MCI method) and -0.359 (based on the Kow method) (EPI Suite 2012). AEEA is completely miscible in water (ECHA c2007-2014b; EPI Suite 2012; ACD/Percepta c1997-2012; Lide 2012). Lastly, the modelled pKa of the ammonium ion suggests that AEEA is an ionizing substance that is likely to exist primarily in either the 1+ or 2+ ionized form at a pH lower than approximately 9 (ECHA c2007-2014b; ACD/Percepta c1997-2012). Additional physical and chemical properties such as the melting and boiling points, density, and other partition coefficients are provided in Table 2 below.
| Property | Type | ValueFootnote Table 2 [b] | Temperature (°C) | Reference |
|---|---|---|---|---|
| Melting point (ºC) |
Experimental | -18Footnote Table 2 [a] | NA | European Commission 2000 |
| Melting point (ºC) |
Experimental | -38 (measured as pour point) |
NA | ECHA c2007-2014b |
| Melting point (ºC) |
Modelled | 9–58 | NA | EPI Suite 2012 |
| Boiling point (ºC) |
Experimental | 243.1; 243.7 | NA | ECHA c2007-2014ba; Sax’s 2012 |
| Boiling point (ºC) |
Experimental | 237–243; 140 (at 44 hPa) |
NA | European Commission 2000 |
| Boiling point (ºC) |
Experimental | 238–240[*] | NA | HSDB 1983– |
| Water solubilityFootnote Table 2 [c] (mg/L) |
Experimental | 1 × 106[*] (1000 g/L) |
20 | ECHA c2007-2014b |
| Water solubility[c] (mg/L) |
Modelled | 1 × 106 | 25 | EpiSuite 2012; ACD/Percepta c1997-2012 |
| Density (kg/m³) | Experimental | 1030 | 20 | European Commission 2000 |
| Density (kg/m³) | Experimental | 1024 | 25 | ECHA c2007-2014b |
| Vapour pressure (Pa) |
Experimental | 1.2; (0.012 hPa) 1.3[*]; (0.01 mm Hg) less than 1 (less than 0.01 hPa ) |
20 | ECHA c2007-2014b; Hawley’s 2007; Sax’s 2012 |
| Vapour pressure (Pa) |
Experimental | 1.09 (0.00819 mm Hg) |
25 | ECHA c2007-2014b |
| Vapour pressure (Pa) |
Experimental | 100 (1 hPa) |
83.8 | ECHA c2007-2014b |
| Vapour pressure (Pa) |
Modelled | 0.824 | 25 | EPI Suite 2012 |
| Henry’s Law constant (Pa·m3/mol) |
Modelled | 1.1 × 10−8[*]; (bond method) 6.2 × 10−10 (group method) |
25 | EPI Suite 2012 |
| Log Kow (dimensionless) |
Experimental | -1.46[*] | NA | European Commission 2000 |
| Log Kow (dimensionless) |
Experimental | -1.37 | 25 | European Commission 2000 |
| Log Kow (dimensionless) |
Modelled | -2.13; -1.4 |
NA | EPI Suite 2012; ACD/Percepta c1997-2012 |
| Log D (dimensionless) |
Modelled | -4.46 (at pH 6.5) -3.81 (at pH 7.4) -3.26 (at pH 8.0) |
NA | ACD/Percepta c1997-2012 |
| Log Koc (dimensionless) |
Modelled | 0.42 (MCI method) -0.359[*] (Kow method) |
NA | EPI Suite 2012 |
| Log Koa (dimensionless) | Modelled | 9.89 | 25 | EPI Suite 2012 |
| pKa (dimensionless) |
Experimental | pKa1 =6.49 pKa2 = 9.52 |
25 | ECHA c2007-2014b |
| pKa (dimensionless) |
Modelled | pKa1 = 6.1 (secondary amine) pKa2 = 9.6 (primary amine) pKa3 = 14.9 (primary hydroxyl) |
NA | ACD/Percepta c1997-2012 |
Sources
No natural sources of AEEA have been identified. Sources of exposure to AEEA are anthropogenic, primarily resulting from industrial manufacturing and processing activities.
Results from the DSL IU conducted under section 71 of CEPA (Canada 2009) indicate that for the year 2008, a quantity lower than the reporting threshold of 100 kg of AEEA was manufactured in Canada, and more than 500 000 kg of AEEA was imported into Canada (Environment Canada 2010). Based on the more recent voluntary information submitted to Environment Canada and Health Canada in 2012–2014 as a follow up to the DSL IU (Environment Canada, Health Canada 2012-2014), AEEA was not manufactured in Canada in 2011, while between 100 000 and 500 000 kg of AEEA was imported into Canada in the same year. Moreover, approximately one quarter of the total AEEA imported was in the form of a pure substance, with the rest imported as a component of products.
Globally in 2003, the production capacity for ethyleneamines, including AEEA, was estimated at 295 million kg (295 000 tonnes). In Europe, AEEA is identified as a high-production volume chemical in the Organisation for Economic Co-operation and Development (OECD) Cooperative Chemicals Assessment Programme. The production capacity was highest for Europe, followed by the United States and Japan; however, individual capacity data on AEEA were not available at that time (OECD 2006). In the United States, the national production volume of AEEA was listed as approximately 14.5 million kg/year (31.7 million lb/year) for the 2012 reporting year (CDAT 2013).
Uses
Globally, ethanolamines are used as chemical intermediates, corrosion inhibitors and cement additives. They can also be used in gas purification to capture carbon dioxide. As chemical intermediates, ethanolamines are widely used to manufacture surfactants with applications in lubricating fluids, detergents and fabric softeners (Frauenkron et al. 2001; Dow Chemical Company 2010; BASF AG 2013). AEEA is also used in the synthesis of adhesion additive for latex paints (BASF AG 2013).
Other specific uses of AEEA have been reported for the United States (Geosyntec 2012a), Europe and Japan (OECD 2006). In the United States, AEEA has also been used as a reactant in the production of an organic flotation agent used to process ground marble ore (Geosyntec 2012a). In Europe, AEEA is used in the production of polyurethane and hardeners for epoxy resins. In Switzerland, AEEA is reported as a constituent of soldering flux. In Japan, AEEA is primarily used as a chemical intermediate to produce surfactants and waxes for consumer uses (OECD 2006). Additional uses of AEEA identified by other jurisdictions include its application as an additive in textiles, fuel, 2,4-D-based herbicide salts, oils used in metal cutting, gas processing chemicals, resins, rubber products, insecticides and certain medicinal soaps (NJ Health 2008; HSDB 1983– ).
Most of the AEEA imported into Canada is used as a chemical intermediate (Environment Canada and Health Canada 2012-2014), a curing agent for epoxy resins (Henkel 2010, 2012a–c), used in commercial building products (Henkel 2013, Mapei 2013), and as a component of adhesives and sealants used in asphalt paving or patching (Environment Canada and Health Canada 2012-2014). AEEA may also be present in some epoxy adhesives, or super glues, used for small scale repairs or hobbies (Henkel Canada 2012b). AEEA can also be found as a component of corrosion inhibitors, lubricant additives, as a component of pigments used in fibres (e.g., carpets) (Environment Canada, Health Canada 2012-2014. AEEA can also be a minor constituent of solid products in building materials, with very limited potential for release (Environment Canada 2010).
Furthermore, in Canada, AEEA is used as a component in food-packaging adhesives and inks with no direct contact with food, and as a component of an agent used in the paper manufacturing process. AEEA is also used as a component in additives for closed recirculating cooling systems where the treated water will not come into direct contact with food (personal communication, 2011 email from the Food Directorate to the Risk Management Bureau; unreferenced).Based on notifications submitted under the Cosmetic Regulations to Health Canada, AEEA is not used in cosmetic products in Canada (2013 emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). AEEA is not listed in the Drug Product Database (DPD 2013), the Therapeutic Products Directorate’s internal Non-Medicinal Ingredients Database, the Natural Health Products Ingredients Database (NHPID 2013), or the Licensed Natural Health Products Database (LNHPD 2008) as a medicinal or non-medicinal ingredient present in final pharmaceutical products, natural health products, or veterinary drugs (personal communication, 2011 emails from Therapeutic Products Directorate, Natural Health Products Directorate and Veterinary Drugs Directorate to the Risk Management Bureau; unreferenced).
Lastly, AEEA is a component of formulants that are used in four herbicides classified as commercial-use products by the Pest Management Regulatory Agency (personal communication; email from Pest Management Regulatory Agency, Health Canada to Ecological Assessment Division, Environment Canada, dated 18 September, 2013; unreferenced).
Releases to the Environment
Anthropogenic releases of a substance to the environment depend upon various losses that occur during the manufacture, industrial use, consumer or commercialFootnote[2] use, and disposal of a substance. In order to estimate releases to the environment occurring at different stages of the life cycle of AEEA, information on the relevant sectors and product lines, as well as emission factorsFootnote[3] to wastewater, land and air at different life cycle stages is compiled. This is done in order to identify the life cycle stages that are likely to be significant contributors to overall environmental concentrations. Recycling activities and transfer to waste disposal sites (landfill, incineration) are also considered. However, releases to the environment from disposal are not quantitatively accounted for because reliable specific information on the rate of (or potential for) release from landfills and incinerators is not available.
AEEA is used as a chemical intermediate and can also be a component of imported additives that are mainly used as a chemical intermediate (Environment Canada and Health Canada 2012-2014). The substance is also used as a curing agent for epoxy resins (Henkel 2010, 2012a–c), and as an anti-stripping agent to enhance adhesion properties in asphalt cement (Environment Canada and Health Canada 2012-2014).
AEEA has a limited potential for release to the environment. Expected environmental releases for AEEA stem mainly from its uses as a chemical intermediate. Potential releases may occur during handling of AEEA when it is added, as a pure substance, in industrial processes to prepare other chemicals. These releases are expected to result mainly from the cleaning operations of the transport and processing containers and could yield emission factors as high as 3% (OECD 2006).
Releases of AEEA from life cycle stages subsequent to its use as a chemical intermediate are not expected. Quantitative estimates of residual AEEA were not identified in published literature. However, given that AEEA is a reactive chemical (contains primary amine and primary alcohol functional groups), it can be expected that the majority of the substance would be fully chemically transformed (i.e., no longer existing in its original chemical form), and covalently bound, when used as a chemical intermediate. Under such conditions, releases of AEEA would not be expected.
Similarly, the potential for AEEA releases from cured products and its applications in asphalt cement is not expected to be significant, since AEEA is expected to undergo irreversible chemical reactions in these applications that would result in its full chemical transformation.
Other known uses of AEEA in Canada (e.g., minor constituent of solid products in building materials) (Environment Canada 2010) are estimated to have very limited potential of releases.
Measured Environmental Concentrations
AEEA is an anthropogenic chemical, and it is not found naturally in the environment. No data on the concentrations of AEEA in the Canadian environment have been identified.
In the United States, levels of AEEA in surface water and groundwater were reported in the industrial zone of Florence, Vermont, where ground calcium carbonate is produced from marble ore (Geosyntec 2012a, 2012b). It is noted that since 2010, tailings management practices at this industrial site have been improved, resulting in a decrease in the detection of AEEA in the water samples (Geosyntec 2012a). Based on the most recent 2012 monitoring data, AEEA was not detected above the detection limit of 0.002 mg/L in any on-site or off-site surface water samples (Geosyntec 2012a). In the past, on-site surface water concentrations of AEEA were measured to be as high as 0.3 mg/L in 2006, and the highest off-site concentrations were up to 0.009 mg/L in 2007 (Geosyntec 2012b). AEEA was detected in groundwater in one of ten monitoring wells at an estimated concentration of 0.016 mg/L (based on a measured concentration of 0.004 mg/L adjusted for 25% recovery), and at a concentration of less than 0.01 mg/L in the remaining nine wells. Historical groundwater concentrations of AEEA, adjusted for incomplete recovery, ranged from below the detection limit of less than 0.002 mg/L to 0.07 mg/L, and a decrease in concentrations has been observed over time (Geosyntec 2012a).
Environmental Fate
Fugacity modelling
Level III fugacity modelling (New EQC 2011) simulates the distribution of a substance in a hypothetical, evaluative environment known as the “unit world”. The EQC model simulates the environmental distribution of a chemical at a regional scale and outputs the fraction of the total mass in each compartment from an emission into the unit world and the resulting concentration in each compartment. The mass-fraction distribution results are used for general information on the environmental fate of a substance rather than the compartmental concentration results for the predicted environmental concentration (PEC) in a substance assessment. Some exceptions to this may occur, such as when a wide dispersive release of a substance suggests that regional-scale concentrations are appropriate for the PEC(s).
The mass-fraction distribution of AEEA is given in Table 3 below using individual steady-state emissions to air, water and soil. The Level III EQC model (New EQC 2011) assumes non-equilibrium conditions between environmental compartments, but equilibrium within compartments. The results in Table 4 represent the net effect of chemical partitioning, inter-media transport, and loss by both advection (out of the modelled region) and degradation/transformation processes.
| Substance released to | Air | Water | Soil | Sediment |
|---|---|---|---|---|
| Air (100%) | negligible | 19 | 81 | negligible |
| Water (100%) | negligible | 100 | negligible | negligible |
| Soil (100%) | negligible | 11 | 89 | negligible |
Model inputs: half-lives in air, water, soil and sediment were 1.1 h, 120 h, 120 h, and 480 h, respectively; log Koc = -0.359; log Kow= -1.46; water solubility = 1 × 106 g/L; vapour pressure = 1.3 Pa.
The results of Level III fugacity modelling (summarized in Table 3) indicate that AEEA is not likely to reside in air because of a very short predicted half-life, moderate vapour pressure and very high water solubility. When released to air, AEEA is expected to deposit to soil, and to a lesser extent to water, based on wet deposition from the atmosphere. Level III fugacity modelling indicates that if released to water, AEEA would remain in water. Similarly, when released to soil, AEEA is expected to remain in soil, and also, given its high solubility in water, run off soil to water. Partitioning to sediments was predicted to be negligible in all scenarios. However, given its high water solubility, AEEA could potentially be found in pore water, and thereby be found in sediment samples. It is also noted that AEEA ionizes at environmentally relevant pH (ECHA c2007-2014b; ACD/Percepta c1997-2012) (see Table 2). Therefore, AEEA may undergo electrostatic interactions in sediment as well as in soil that are not accounted for by the EQC modelling.
In summary, based on the results of Level III fugacity modelling, water and soil are the main receiving compartments of AEEA, depending on the compartment of release. Therefore, considering the physical and chemical properties and the associated predicted environmental fate of AEEA, aquatic and soil organisms could potentially be exposed to this substance. Exposure to sediment-dwelling organisms is not expected. Exposure to terrestrial organisms through inhalation is not expected, given that AEEA is not likely to remain in and/or partition to air.
Persistence and Bioaccumulation Potential
Environmental persistence
Relevant media and data sources
Based on the known uses, potential releases, and expected partitioning of AEEA, the environmental media of primary interest are water and soil. In order to provide the best possible weight of evidence for persistence of AEEA, empirical and modelled data for AEEA were considered. Available information is organized and presented based on the environmental compartment (i.e., air, water, soil and sediment). Available data are summarized in Tables 4a–c.
Data for persistence
Air – Modelled data
Empirical data for the degradation potential of AEEA in air were not available. Modelled results, based on the available (Q)SAR model, indicated a short half-life of 1.1 hours in air (AOPWIN 2010). It is therefore expected that the substance will be rapidly degraded by reaction with hydroxyl radicals in the atmosphere. In contrast, the ozone reaction half-life could not be modelled since chemicals of structural comparability to AEEA are not contained in the training set of the AOPWIN (2010) model. Nonetheless, based on the short half-life in air, AEEA is considered readily degradable and not persistent in this compartment. Modelled data in air are summarized in Table 4a.
| Fate process | Model and model basis |
Model result and prediction | Extrapolated half-life (days) |
|---|---|---|---|
| Atmospheric oxidation | AOPWIN 2010 | t 1/2 = 1.1 hoursFootnote Table 4a [a] | less than or equal to 2 days |
| Ozone reaction | AOPWIN 2010 | N/A | N/A |
Water – Empirical studies
Several ready and inherent biodegradation studies and one field study have been conducted for AEEA using activated sludge. Original unpublished studies were not available for review. However, study summaries prepared for the European Union Regulation concerning the Registration Evaluation Authorization and Restriction of Chemical Substances (REACH), available from ECHA (c2007-2014a) were considered. The summary of the more recent ready biodegradation study (performed according to OECD Guideline 301F) conducted in 2005 was comprehensive. The three other ready or inherent biodegradation studies are briefly summarized in the European Commission’s IUCLID Dataset for AEEA (European Commission 2000). However, given that no experimental details and methodologies were provided, little can be concluded based on the available study results. Finally, a field study was performed by Emtiazi and Knapp (1994) characterizing biodegradation of AEEA in water samples taken from the River Aire (located in Leeds, United Kingdom).
Overall, available biodegradation studies using activated sludge indicate that AEEA undergoes relatively rapid biodegradation in water. In addition, it is noted that more severe toxic effects on micro-organisms, including those in activated sludge, were observed at AEEA exposure concentration greater than 100 mg/L (ECHA c2007-2014b). The large extent of inhibition of sludge micro-organisms, which could affect the degradation of AEEA in studies using sludge, is not expected at substance concentration of less than 100 mg/L. OECD ready or inherent biodegradation study protocols specify that substance concentrations not exceeding 100 mg/L are applied (Environment Canada 2009). Biodegradation study summaries are provided below, and study endpoints are listed in Table 4b.
In general, ready biodegradability tests include the modified OECD screening tests, CO2 evolution test, manometric respirometry test, dissolved organic carbon (DOC) die-away test, closed bottle test, and the MITI(I) test, and measure mineralization over a 28-day period using a low concentration of a sewage or activated sludge as an inoculum and a high concentration of the test compound (2–100 mg/L). Inherent biodegradability tests are comprised of the Zahn-Wellens test, SCAS test and MITI(II) test, and are typically run with high microbial population densities, also using a sewage or activated sludge inoculum. In general, a substance is considered to undergo ready, ultimate biodegradation if at least 60% biodegradation has occurred in 28 days, and inherent, ultimate biodegradation if at least 70% has occurred in 28 days in an inherent test (Aronson and Howard 1999). Biodegradation above 20% but lower than 60–70% may be regarded as evidence of inherent, primary biodegradability (Environment Canada 2009).
AEEA was tested in 2005 using OECD Guideline 301F (Ready biodegradability, Manonmetric respirometry test) (ECHA c2007-2014b) at concentrations of 19 and 64 mg/L over 28 days. In this study, biodegradation was measured based on oxygen consumption, mineralization and DOC removal. Results from oxygen consumption tests indicated mean biological oxygen demand (BOD) values ranging from 66.3 to 109.6% of the theoretical oxygen demand (ThOD) after 28 days. Similar results, i.e., biodegradation values of greater than 60%, were obtained from measurements of CO2 evolution and DOC removal, thereby indicating mineralization of AEEA under these study conditions (ECHA c2007-2014b).
Other unpublished ready and inherent biodegradation studies are briefly summarized in the European Commission’s IUCLID Dataset for AEEA (European Commission 2000). Too few experimental details were provided to infer conditions of these studies and thereby to allow for interpretation of the test results. In one study performed according to OECD Guideline 301C (Ready biodegradability, Modified MITI Test I), 0% biodegradation in 14 days was reported for AEEA, tested at a concentration of 100 mg/L. In another study (according to a BSB-test method), less than 1% aerobic biodegradation was observed after 5 days, but sample concentrations were not provided. Finally, results from the 1977 inherent Zahn-Wellens study indicated 30–50% biodegradation (based on DOC removal) after 37 days, using a high concentration of AEEA of 400 mg/L (European Commission 2000). In addition, this study reported a 1% biodegradation of AEEA after 3 hours. Given that study duration was less than 28 days in two of these studies, it is difficult to extrapolate whether more complete biodegradation would occur if the studies were run longer. Results of the 1977 Zahn-Wellens (European Commission 2000) study suggested that AEEA biodegrades under inherent test conditions but did not pass the 70% threshold. In addition, it is possible that if applied at high test concentrations, AEEA may have caused toxic effects to bacteria found in the sludge, and consequently affected the biodegradation test results. Inhibitory effects in micro-organisms have been observed at concentrations of less than 400mg/L (ECHA c2007-2014b) (see the Potential to Cause Ecological Harm section).
A study using water samples from the River Aire located in central Leeds in the United Kingdom reported complete degradation of AEEA in 30 days based on a die-away test method (Emtiazi and Knapp 1994). It was noted that at the time of sampling, the river was recovering from the impact of treated domestic and industrial effluents emitted from the surrounding cities. Environmental samples containing activated sludge as well as soil suspensions were added to a sterile water solution containing AEEA, for a final concentration of approximately 100 mg/L (1 mM). It was observed that AEEA remained in aqueous solution and did not adsorb or partition into solids. Complete degradation of AEEA was observed in 8–30 days, based on 4 or 5 experimental determinations. The observed lag time was reported in the range of 3–12 days. Mean degradation value was also calculated by Emtiazi and Knapp (1994) based on individual results, and was reported as 20 days for complete degradation with a lag time of 6.5 days. The results of this study provide additional evidence that AEEA can undergo complete mineralization in a relatively short amount of time (less than or equal to 30 days). Available empirical biodegradation results are summarized in Table 4b.
| Fate process | Degradation endpoint / units |
Degradation value | Reference |
|---|---|---|---|
| Biodegradation (aerobic) | % BOD, % CO2 evolution, % DOC removal (28 days) |
greater than 60%Footnote Table 4b [a] | ECHA c2007-2014b |
| Biodegradation (aerobic) | die-away test (20 days) |
100 % | Emtiazi and Knapp 1994 |
| Biodegradation (aerobic) | % BOD NR (14 days) |
0% | European Commission 2000 |
| Biodegradation (aerobic) | BSB test NR (5 days) |
less than 1% | European Commission 2000 |
| Biodegradation (aerobic) | % DOCNR(37 days) % DOCNR (3 hours) |
30–50% 1% |
European Commission 2000 |
In summary, the available experimental data suggest that AEEA biodegrades relatively quickly in water. Two studies, the 2005 OECD 301F ready biodegradation study (ECHA c2007-2014b) and the field study by Emtiazi and Knapp (1994) indicate that AEEA undergoes complete mineralization in as little as 8 days and up to 30 days. The results of the 1977 Zahn-Wellens study suggest that AEEA has the potential for inherent biodegradation under favourable conditions (European Commission 2000). Available results from two other ready biodegradation studies indicate limited biodegradation of AEEA up to 14 days (European Commission 2000). However, given the lack of experimental details provided for these studies from the available source (European Commission 2000), the fact they were conducted for a period of time too short to evaluate biodegradation potential (i.e., less than 28 days), and may have used concentrations of AEEA high enough to cause toxic effects in sludge micro-organisms, little weight is placed on these results.
Overall, there exists compelling empirical evidence to support that AEEA is not persistent in water.
Water – Modelled data
In addition to the available empirical data for the degradation of AEEA in water, a (Q)SAR-based weight-of-evidence approach was also applied using the degradation models shown in Table 4c.
| Fate process | Model and model basis |
Model result and prediction | Extrapolated half-life (days) |
|---|---|---|---|
| Hydrolysis | HYDROWIN 2010Footnote Table 4c [a] | N/A | N/A |
| Primary biodegradation (aerobic) | BIOWIN 2010 Sub-model 4: Expert Survey (qualitative results) |
3.91Footnote Table 4c [b] “biodegrades fast” |
less than or equal to 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2010 Sub-model 3: Expert Survey (qualitative results) |
3.18[b] “biodegrades fast” |
less than or equal to 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2010 Sub-model 5: MITI linear probability |
0.83Footnote Table 4c [c] “biodegrades fast” |
less than or equal to 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2010 Sub-model 6: MITI non-linear probability |
0.84[c] “biodegrades fast” |
less than or equal to 182 |
| Ultimate biodegradation (aerobic) | DS TOPKAT c2005-2009 Probability |
N/A[c] | N/A |
| Ultimate biodegradation (aerobic) | CPOPs 2012 % BOD (biological oxygen demand) |
% BOD = 0 “biodegrades slowly” |
greater than or equal to 182 |
Modelled hydrolysis
Hydrolysis reaction half-life using the available HYDROWIN (2010) model was not calculable for AEEA, as chemicals of structural comparability are not contained in the training set of the model. AEEA is not expected to undergo hydrolysis, as the substance contains functional groups that typically do not hydrolyze (i.e., primary amine and hydroxyl functional groups) (Boethling and Mackay 2000). However, AEEA is expected to ionize, and the pka1 of 6.1–6.49 (see Table 2) suggests a potential for ionization at environmentally relevant pH levels.
Modelled primary and ultimate biodegradation
Results from the primary biodegradation (BIOWIN Sub-model 4), and most of the ultimate biodegradation models (BIOWIN sub-models 3, 5 and 6) (EPI Suite 2012) suggest that biodegradation is rapid and that the expected half-life in water would be less than or equal to 182 days. In contrast, results from the ultimate model CPOPs (2012) suggest that this substance does not biodegrade at all (0% BOD). However, the CPOPs model output also indicates that AEEA has a suspected BOD inhibition effect. It is noted that toxic effects to micro-organisms in sludge stemming from AEEA exposure have been observed at substance concentrations exceeding 100 mg/L. AEEA also contains structural features associated with chemicals that are easily biodegraded (e.g., primary amine and hydroxyl functional groups). Therefore, results from CPOPs (2012) are considered unreliable for AEEA. Lastly, results for AEEA were not available from DS TOPKAT (c2005-2009), as the model does not provide biodegradation estimates for this type of structure.
In summary, based on the reliable primary and ultimate modelled biodegradation results as well as the structural features of AEEA, there is sufficient evidence to indicate that AEEA undergoes mineralization in water and that its expected half-life would beless than or equal to 182 days in this compartment. Model results for AEEA support the available empirical biodegradation data (ECHAc2007-2014b; Emtiazi and Knapp 1994; European Commission 2000) described in the preceding section.
Soil and sediment
No experimental studies were found for the biodegradation of AEEA in soil or sediments. Limited modelling is available for these compartments. Therefore, an extrapolation ratio of 1:1:4 for water:soil:sediment biodegradation half-life based on Boethling et al. (1995) was applied. Consequently, given that the half-life of AEEA in water is less than182 days, it follows that the half-life in soil is also less than182 days and the half-life in sediments is expected to be less than 365 days. This indicates that AEEA is not expected to be persistent in either soil or sediment.
Conclusion
AEEA is not considered to be persistent in air, based on a model prediction (AOPWIN 2008). Available boidegradation studies as well as modelled data suggest that the substance boidegardes rapidly in water. AEEA is also expected to undergo rapid boidegradation in soil and sediment, based on the extrapolation criteria from the half-life in water (Boethling et al. 1995). Therefore, based on the empirical and modelled data (Tables 4a-c), AEEA is expected to have a limited persistence in environmental media.
Potential for Bioaccumulation
In order to provide the best possible weight of evidence for the bioaccumulation potential of AEEA, its physical and chemical properties as well as empirical and modelled data for AEEA were considered.
Physical and chemical properties of AEEA relevant to bioaccumulation potential
AEEA is completely miscible in water, indicating that the substance can be readily bioavailable for uptake in water. Experimental and modelled log Kow values (-1.37 to -2.13) for AEEA suggest that this chemical has a low potential to bioaccumulate in biota (see Table 2). In addition, a combination of log Kow of -2.13 and log Koa of 9.89 indicates that, given a terrestrial dietary exposure, AEEA is unlikely to have the potential to biomagnify in terrestrial food webs, as suggested by Gobas et al. (2003) and Kelly et al. (2007).
Information regarding molecular size and cross-sectional diameters is useful to consider as weight of evidence for bioaccumulation potential. Studies relating fish BCF data and molecular size parameters (Dimitrov et al. 2002, 2005) and the effects of cross-sectional diameter on passive diffusion (Sakuratani et al. 2008) suggest that the probability of a molecule crossing cell membranes as a result of passive diffusion declines significantly with increasing maximum and effective diameters (Dmax and Deff). It was observed that the probability of passive diffusion decreases appreciably for molecules with maximum diameters greater than 1.5 nm (Dimitrov et al. 2002, 2005), and that substances that do not have a very high bioconcentration potential (BCF less than 5000) often have a Dmax of greater than 2.0 nm and an effective diameter (Deff) greater than 1.1 nm. However, significant bioaccumulation potential may remain for substances that are subject to slow absorption processes, if they are slowly biotransformed or slowly eliminated by other processes (Arnot et al. 2010). Based on 3D analysis of AEEA conformers calculated using the BCFmax Model with Mitigating Factors (Dimitrov et al. 2005), the maximum and effective diameters of AEEA are 1.0 and 0.8 nm, respectively, suggesting that the probability that a molecule will cross cell membranes as a result of passive diffusion is high. This indicates that AEEA is not likely to experience restricted uptake from steric effects at the gill surface.
Physical and chemical properties of AEEA relevant to its bioaccumulation potential are listed in Table 5a.
| Molecular mass (g) | Water solubilityFootnote Table 5a [a] (mg/L) | Log Kow | Log Koa | Molecular diameter (nm) |
|---|---|---|---|---|
| 104.15 | 1 000 000 | -1.46 | 9.89 | 0.961 (Dmax), 0.792 (Deff) |
Empirically determined bioaccumulation
Bioconcentration factor (BCF)
One empirical bioconcentration study in fish was identified for AEEA. The summary of this unpublished study was available from ECHA (c2007-2014b). This study was performed in 1992 according to OECD Guildeline 305C (Bioaccumulation: test for the degree of bioconcentration in fish) in flow-through conditions. Carp (Cyprinus carpio) of about 30 g and 10 cm in length were used in the study, and fish lipid content was calculated at 4.8%. AEEA was tested at two concentrations, 0.1 and 1.0 mg/L, for 42 days. Bioconcentration factors (BCFs) were determined at both test concentrations as 2.1 to less than 3.7 at 0.1 mg/L and less than 0.2 at 1 mg/L. These results indicated that AEEA has a very low potential to bioconcentrate in fish. No data were provided regarding the elimination of AEEA by carp. However, as the substance is likely to undergo passive diffusion across the gills based on its small molecular size, cross-sectional diameters and unhindered structure, it is expected that the depuration rates in fish would likely be high. Study results are summarized in Table 5b.
| Test organism | Kinetic and/or steady-state value (L/kg)Footnote Table 5b [a] | Reference |
|---|---|---|
| Carp (Cyprinus carpio) |
2.1 to less than 3.7 (0.1) | ECHA c2007-2014b |
| Carp (Cyprinus carpio) |
less than 0.2 (1.0) | ECHA c2007-2014b |
Bioaccumulation factor (BAF)
Bioaccumulation factors are measured under field conditions as the ratio of the whole body burden of chemical taken up from all exposures to that of the ambient water concentrations. Measures of BAF are the preferred metric for assessing the bioaccumulation potential of substances because they incorporate all chemical exposures including the diet, which predominates for substances with log Kowgreater than ~4.0 (Arnot and Gobas 2003).
No empirical BAF values were available for AEEA at the time of this analysis, and therefore metabolism-corrected kinetic mass-balance modelling was used to fill this data gap. For chemical substances characterized by log Kow values of less than 3.5, BCF values tend to be equivalent to BAF values for chemical substances, due to lack of dietary uptake (Arnot and Gobas 2003, 2006).
Modelling of BCF and BAF
Since few empirical BCF and no BAF data were available, the BCF and BAF of AEEA were estimated using both structure-based models and a three trophic level kinetic mass-balance model. All estimates of BCF and BAF, [except sub-model 1 of the BCFBAF (2010) model in EPI Suite (2012)], were corrected for metabolism using the metabolism rate constant (kM) derived based on the fragment addition (Q)SAR within the BCFBAF model, a structure-based QSAR method. The (Q)SAR kM for a 10 g fish at 15oC reported in the BCFBAF (2010) model was17.58 days–1.
The results of the BCF and BAF modelling are given in Table 5c below. These results are reported for a middle-trophic-level fish representative of Canadian waters, based on a modification of the mass-balance model from Arnot and Gobas (2003).
| Log Kow | kM (days–1) |
Model and model basis |
Endpoint | Value wet weight (L/kg) |
Reference |
|---|---|---|---|---|---|
| -1.46 | 17.58 | BCFBAF Sub-model 1 (linear regression) |
BCF | 3.16 | BCFBAF 2010 |
| -1.46 | 17.58 | BCFBAF Sub-model 2 (mass balance) |
BCFFootnote Table 5c [a] | 0.93 | BCFBAF 2010 |
| -1.46 | 17.58 | BCFBAF Sub-model 3 (Gobas-mass balance) |
BAF[a] | 0.93 | BCFBAF 2010 |
| -1.46 | N/A | BCFmax with mitigating factors | BCFFootnote Table 5c [b] | 0.37 | Dimitrov et al. 2005 |
Modelled BCF values for AEEA ranged from 0.37 to 3.16 depending on the model used and application of the metabolic rate constant. These values are similar to the empirically derived BCF values of less than 0.2 to less than 3.7 in carp (see Table 5b), and point to a very low potential for bioaccumulation of AEEA. The modelled BAF value of 0.93 was also in agreement with modelled and empirical BCF values.
Conclusion
AEEA is water-miscible, has a very low log Kow and has a relatively small molecular diameter (see Table 5a). These properties suggest that it may easily diffuse across biological membranes such as gills. Empirical and modelled data indicate that AEEA does not bioaccumulate in organisms. Available empirical evidence in fish from exposure to AEEA over 42 days indicated very low BCF values (see Table 5b). Similarly, modelled BCF and BAF results, corrected for metabolism, also indicated a very low potential for bioaccumulation (see Table 5c). Overall, based on the available evidence it is considered that AEEA has a low bioaccumulation potential.
Potential to Cause Ecological Harm
Ecological effects assessment
Data sources
Ecological effects of AEEA in the aquatic compartment have been well characterized through empirical studies. In addition, modelled data for AEEA were considered for aquatic species. Studies addressing ecological effects in soil and sediment were not found, and suitable models are also not available. However, it is expected, based on the physical and chemical properties, use and release patterns, and results from fugacity modelling, that the majority of exposure to AEEA would occur in the aquatic compartment.
Empirical studies – in the aquatic compartment
Empirical studies describing the ecotoxicological effects of AEEA on aquatic organisms were available from published literature as well as unpublished sources. The majority of the available data were from unpublished aquatic toxicity studies performed according to internationally accepted standard protocols (such as OECD testing guidelines) and described effects of AEEA on micro-organisms (freshwater bacteria and activated sludge), algae (Scenedesmus subspicatus), invertebrates (Daphnia magna) and fish (three species). Effects studies on invertebrates and fish were limited to short-term AEEA exposures.
None of the unpublished aquatic toxicity studies was available for review; however, study summaries were found from sources including the European Chemicals Agency website (ECHA c2007-2014a) and a IUCLID Dataset for AEEA prepared by the European Commission (European Commission 2000). It is noted that study summaries provided in the IUCLID Dataset (European Commission 2000) are limited to study endpoints and provide minimal details; they therefore do not provide sufficient basis for scientific evaluation. Nonetheless, available endpoints are included for comparison with other available empirical data. A summary of the toxicological endpoints in aquatic organisms, such as lethal and effect concentrations, is provided in Table 6a. However, only those endpoints where sufficient experimental details were available were included in this table. Additionally, experimental observations including percent mortality are summarized in the text.
Effects of AEEA were studied in two species of freshwater Gram-negative bacteria, Pseudomonas putida and Pseudomonas aeruginosa, and in micro-organisms from industrial activated sludge (ECHA c2007-2014b; European Commission 2000; Emtiazi and Knapp 1994). The effects on P. putida were evaluated in 1988 in a study according to the Bringmann-Kuehn Standards (similar to the German Industrial Standard DIN 38412, part 8) (ECHA c2007-2014b; European Commission 2000). In the study, P. putida were exposed to AEEA at nominal concentrations ranging from 8 mg/L to 1000 mg/L. The 10% effects concentration (EC10), median effects concentration (EC50) and 90% effects concentration (EC90) values for growth inhibition were determined as 82.2, 134.8 and 231.3 mg/L, respectively, following 17 hours of exposure.
Growth inhibition of AEEA to P. putida and Pseudomonas aeruginosa was also observed by Emtiazi and Knapp (1994). Specific growth rate and growth inhibition were measured at AEEA concentrations of 1000 mg/L (10 mM) and 10 000 mg/L (100 mM). Following 8 hours of AEEA exposure, it was observed that the maximum growth of both P. putida and P. aeruginosa was reduced by 30% at 1000 mg/L, and by nearly 60% for P. putida and 40% for P. aeruginosa at 10 000 mg/L. Similar observations for specific growth rate were also made after 1-hour exposure to AEEA. Specific growth rate of P. putida was reduced by about 25% and 45% at 1000 mg/L and 10 000 mg/L exposure concentrations, respectively. For P. aeruginosa, the specific growth rate was reduced to a lesser extent, by 6% at 1000 mg/L, and by 11% at 10 000 mg/L. These results are not presented in Table 6a.
The effects of AEEA exposure on respiration rate were studied in industrial activated sludge in 1986, according to the ISO 8192 test protocol (Test for Inhibition of Oxygen Consumption by Activated Sludge) (European Commission 2000). AEEA was tested at the nominal test concentration of 1003 mg/L. It is noted that typically studies with activated sludge are performed using domestic sewage sludge rather than industrial activated sludge (OECD 2005). Following 30 minutes of exposure, no inhibition of respiration rate was observed; instead, observations of increased respiration activity of the industrial activated sludge when compared to the study control were made. These results are also not presented in Table 6a.
In algae (Scenedesmus subspicatus), AEEA was tested in two studies, dated 1989 and 1988 (ECHA c2007-2014b; European Commission 2000). The 1989 study was performed according to German Industrial Standard DIN 38412, part 9 with AEEA at nominal concentrations ranging from 8 mg/L to 500 mg/L for 72 hours(ECHA c2007-2014b). The addition of AEEA at 500 mg/L increased the pH up to pH ~9, and a subset of the test samples was pH neutralized to pH 7.8–8.2. Endpoints were reported based on measurements of the growth rate (growth inhibition) and biomass reduction.For biomass reduction, the EC10 and EC50 values were determined as 100, and 204 mg/L, respectively. For growth inhibition, EC10 was determined as 156 mg/L, and EC50 values were determined as 358 mg/L, and >500 mg/L from the pH-neutralized samples. Adverse effects were not observed in the neutralized, 500 mg/L-AEEA treatments. respectively.
The effects on S. subspicatus were also evaluated in a growth inhibition study in 1988 (European Commission 2000). This study was performed according to German Industrial Standard DIN 38412, part 8. EC20, EC50 and EC90 values for growth inhibition were determined as 130, 210 and 490 mg/L, following 72 hours of exposure to AEEA.
The ecotoxicological effects of AEEA were determined for the water flea (Daphnia magna) in short-term studies. In a 1993 study, acute ecotoxicological effects of AEEA to D. magna were determined according to OECD Guideline 202 (Daphnia sp. Acute Immobilisation Test) (ECHA c2007-2014b). AEEA was tested at nominal concentrations ranging from 1 to 100 mg/L for 48 hours. NOEC and EC50 values for mobility were determined as 10 and 22 mg/L, respectively. It was observed that at the three highest concentrations tested (32, 56 and 100 mg/L), the pH levels in test solutions increased to above the recommended pH maximum of 9, subsequently decreasing to pH below 9 during the 48-hour course of the test.. Nevertheless, as was noted by the study authors, the high alkalinity at these test concentrations may have exerted adverse effects on daphnids and contributed to the observed toxicity.
In another acute toxicity study performed in 1989 (according to Directive 79/831/EEC, Annex V, Part C), daphnids were exposed to somewhat higher nominal AEEA concentrations of up to 500 mg/L for 24 and 48 hours. At 24 hours, the NOEC, EC50, and EC100 values for mobility were determined as 125, 225 and 500 mg/L, respectively. At 48 hours, the NOEC, EC50, and EC100 values were determined as 125, 190 and 500 mg/L, respectively. Increases in alkalinity were also observed at AEEA concentrations greater than 250 mg/L, where pH values of test solutions increased to approximately 10, and subsequently normalized to about 8.5 after 48 hours. It was noted that this marked increase in alkalinity may have produced additional toxic effects in daphnids; however, observations detailing such effects were not provided.
In fish, the short-term toxicity of AEEA was established through a series of unpublished studies conducted between 1978 and 1994.These studies were short term, i.e., 48- and 96-hour studies, and the tested fish species included the rice fish (Oryzias latipes), rainbow trout (Oncorhynchus mykiss), and fathead minnow (Pimephales promelas). In the 1978 study, the effects of AEEA in the fathead minnow (P. promelas) were performed according to an industry protocol comparable to the accepted international standards (ECHA c2007-2014b). In this study, fish were exposed to AEEA at nominal concentrations ranging from 155 to 1150 mg/L for 96 hours. The NOEC (mortality), 10% lethal concentration (LC10), median lethal concentration (LC50), and 100% lethal concentration (LC100) were determined as 490, 514, 640 and 1000 mg/L, respectively. It was observed that at the four highest concentrations tested (i.e., 650–1150 mg/L), the pH of the test solutions increased, to values up 10.2, and increased the mortality of fish, ranging from 70 to 100%. It was noted by the authors that adverse effects due to alkaline conditions may have contributed to the observed mortality at these exposure concentrations. However, it was also pointed out that at the highest concentration of AEEA that caused no mortality (i.e., 490 mg/L), the pH of the test solution was similarly high, at 9.8. Overall, these results suggest that the combination of high and critical AEEA concentration (greater than or equal to 650 mg/L) together with the increased pH levels of water had the most detrimental effects on fish. It is noted that such high exposure concentrations of AEEA are not representative realistic environmental exposures.
Acute toxic effects of AEEA were also determined in the Japanese rice fish (Oryzias latipes), in a 1992 study (ECHA c2007-2014b). The study was performed according to Japanese Industrial Standard JIS K 0102-1986-71, in semi-static test conditions. Additional details of methodology and observations were not available. The LC50 value was determined as greater than 1000 mg/L following 48 hours of AEEA exposure.
Two other acute fish studies were also briefly summarized in the IUCLID Dataset prepared by the European Commission (European Commission 2000). In one study using fathead minnows, the 96-hour LC10, LC50 and LC100 values were determined as 617, 728 and 859 mg/L, respectively. Another study using rainbow trout listed the NOEC and LC50 values of greater than or equal to 100 and greater than 100mg/L, respectively (exposure time was not provided) (European Commission 2000). These endpoints are not listed in Table 6a.
| Test organism | Type of test | Endpoint | Value (mg/L) | ReferenceNAR |
|---|---|---|---|---|
| Bacteria (Pseudomonas putida) | Chronic (17 hours) |
EC10; EC50; EC90 (growth inhibition) |
82.2; 134.8; 231.3 | ECHA c2007-2014b; European Commission 2000 |
| Algae (Scenedesmus subspicatus) | Acute (72 hours) |
EC10; EC50 |
156; 358 greater than 500 (pH neutralized); |
ECHA c2007-2014b |
| Algae (Scenedesmus subspicatus) | Acute (72 hours) |
EC10; EC50 (biomass increase) |
100;204 | ECHA c2007-2014b |
| Algae (Scenedesmus subspicatus) | Acute (72 hours) |
EC20; EC50; EC90 (biomass increase) |
130; 210; 490 |
European Commission 2000 |
| Water flea (Daphnia magna) |
Acute (24 hours) |
NOEC; EC50; EC100 (immobilization) |
125; 225; 500 |
ECHA c2007-2014b |
| Water flea (Daphnia magna) |
Acute (48 hours) |
NOEC; EC50; (immobilization) |
10; 22 | ECHA c2007-2014b |
| Water flea (Daphnia magna) |
Acute (48 hours) |
NOEC; EC50; EC100 (immobilization) |
125; 190; 500 |
ECHA c2007-2014b |
| Japanese rice fish (Oryzias latipes) |
Acute (48 hours) |
LC50 | greater than 1000 | ECHA c2007-2014b |
| Fathead minnow (Pimephales promelas) |
Acute (96 hours) |
NOEC; LC10; LC50; LC100 | 490; 514; 640; 1000 |
ECHA c2007-2014b |
In summary, a number of ecotoxicological studies determining the effects of AEEA exposure were available for different aquatic organisms including micro-organisms, crustaceans and fish. Overall, based on the endpoints studied, AEEA has moderate to low toxicity to aquatic organisms based on short-term exposure. The lowest observed EC50 value was 22 mg/L for immobilization of daphnia, based on 48 hours of exposure to AEEA. A notable effect of AEEA at the highest test concentrations was the marked increase in alkalinity, to pH levels as high as 10. Increased fish mortality was noted at those high pH levels, but only when combined with the high AEEA concentrations of greater than or equal to 650 mg/L. Exposure concentration of 450 mg/L combined with a high alkalinity of the test solution (pH 9.8) did not induce mortality. In a study with D. magna, a neutralized AEEA test solution indicated approximately two-fold lower toxicity (for growth rate, an EC50 of greater than 500mg/L for a neutralized test was observed, compared to about 350 mg/L in the non-neutralized test). These results point to additional toxic effects induced by increased pH levels at high exposure concentrations of AEEA. However, these effects, and notably mortality, transpire in test conditions where high alkalinity occurs with highest AEEA exposure concentrations. Such high AEEA exposure concentrations are not representative of realistic environmental exposures.
Long-term studies characterizing the ecotoxicological effects of AEEA in aquatic invertebrates and vertebrates were not located in the published literature or in any unpublished materials.
Modelled results – in the aquatic compartment
In addition to the available empirical data for aquatic species, modelled data were also considered. The structural classes of ethanol amines are amenable to most model predictions.
Short-term aquatic toxicity values were obtained from the ECOSAR model in EPI Suite (2012) (see Table 6b). Predicted EC50 or LC50 values for fish, daphnids and algae estimated for short-term exposures fall in the range of 26–5555 mg/L. Model results for chronic exposure to AEEA were also generated; however, AEEA or similar substances were not part of the aliphatic substances training sets of ECOSAR (2012). As such, these chronic modelled results are not considered reliable for AEEA.
In general, model results generated for algae, daphinds and fish agreed with the available empirical results for these species, and pointed to a low toxicity potential of AEEA. Therefore, based on the model results, AEEA is not expected to cause acute harm to aquatic organisms at low concentrations [acute median lethal concentrations (LC50s) are greater than or equal to1 mg/L]. Modelled results are summarized in Table 6b.
| Test organism | Type of test |
Endpoint | Value (mg/L) |
Reference |
|---|---|---|---|---|
| Fish | Acute (96 hours) |
LC50 | 5555 | ECOSAR 2012 |
| Water flea (Daphnia magna) |
Acute (48 hours) |
EC50 | 238 | ECOSAR 2012 |
| Algae | Acute (96 hours) |
EC50 | 26.8 | ECOSAR 2012 |
Empirical studies – in other environmental compartments
AEEA effects studies were not identified in other environmental compartments, including soil and sediments. Suitable (Q)SAR models were not available for characterization of ecological effects in soil and sediment organisms.
Derivation of the PNEC
A predicted no-effect concentration (PNEC) was derived from the acute toxicity value of 22 mg/L, which was the most sensitive valid experimental value, determined for daphnids. This value was divided by an assessment factor of 100, to account for interspecies and intraspecies variability in sensitivity, and to extrapolate from acute to chronic exposure duration, to give a value of 0.22 mg/L.
Conclusion
Based on various lines of evidence, AEEA is unlikely to cause harm to aquatic organisms at low concentrations. Given that AEEA has low bioaccumulation potential characterized by factors such as BCF values of approximately 1 L/kg, and a negative log Kow, it has limited potential to be incorporated into organisms and thereby cause toxic effects. The available empirical and modelled data are in good agreement for all trophic levels (i.e., fish, daphnids and algae) in the aquatic compartment.
Ecological Exposure Assessment
No data on measured environmental concentrations (in water, soils or sediments) of AEEA in Canada have been identified. Therefore, environmental concentrations have been estimated from available information, including substance quantities, estimated release rates and characteristics of the receiving environment.
Identification of important exposure scenarios
Exposure characterization is focused on scenarios which represent the highest potential for environmental releases and exposure. In general, the magnitude of releases is a direct function of the quantity of a substance manufactured or used and its applicable emission factors. In cases where industrial releases are similar in quantities to consumer and/or commercial releases, the former typically result in higher levels of environmental exposure than the latter. This is because industrial releases are concentrated at a limited number of sites while consumer and/or commercial releases are dispersed across the country.
Asphalt
AEEA is used in asphalt as a component of an anti-stripping agent (additive). The role of this anti-stripping agent is to enhance asphalt-aggregate adhesion and reduce moisture damage on asphalt by improving the bond between the asphalt cement and the aggregates (Harnish 2010). The recommended amount of total anti-stripping agent in asphalt cement is 0.25% to 0.75% (based on the weight of the liquid asphalt cement). According to an industry product information sheet about anti-stripping products, one half pound of product per ton of asphalt can increase the lifespan of asphalt pavements (Unique Paving Materials c2013). The concentration of AEEA in asphalt should be well below the recommended concentration of the anti-stripping agent in the asphalt cement, because AEEA is only a minor component of this anti-stripping agent.
During the preparation of the asphalt, aggregates are dried to remove moisture, and the anti-stripping agent is added to asphalt cement through a pipe system. Asphalt cement is then transferred to an aggregates vessel and mixed for a short period of time. During this operation, volatile organic compounds (VOCs) can be released from organic compounds. The US EPA (2000) has estimated that releases of total semi-volatile compounds from mixing asphalt, unloading mixer and asphalt storage can be less than 2 lbs per 100 000 tons of asphalt made. This results in an emission factor of 1 × 10−8 (US EPA 2000). As AEEA has a moderate vapour pressure, it is possible that AEEA can be a minor component of these VOCs. Water is not used in this process, and therefore releases of wastewater containing AEEA are not expected.
Reclaimed asphalt pavement may be added into a new batch mix (US EPA 2000). Residues in mixing equipment are assumed to be cleaned and then are expected to be re-inserted in future batches.
In general, releases of AEEA from the process of asphalt paving are considered to be minimal. Releases of any remaining unreacted AEEA to air or adjacent soil are expected to be negligible. As wastewater is typically not produced during asphalt paving, releases of AEEA in water to the environment are also considered negligible from this operation (Lutes et al. 1994). In contrast, releases of AEEA from asphalt leaching are possible, as suggested by Lindgren (2011). Lindgren (2011) has studied leaching of emulsifiers from new asphalt wash down. The function of the emulsifier in asphalt is to increase bonding between asphalt cement and aggregates. This function is similar to an anti-strip agent as described by Harnish (2010); therefore, Lindgrens’ findings can be used for comparison. Lindgren (2011) found that retention of emulsifiers in asphalt was almost 100% with a fraction release of less than 0.005% that occurs primarily with the first rain events after application. Additionally, leachate will be highly diluted by water runoff (Lindgren 2011).
In summary, the potential for AEEA releases from the preparation of asphalt cement is considered to be negligible based on characteristics of the typical process in place (US EPA 2000). Release of AEEA from asphalt pavement is expected to be negligible based on the function of the additive to promote asphalt adhesion to aggregates and due to the known limited leaching potential of an asphalt product with a similar function as that of AEEA (Harnish 2010; Lindgren 2011).
Epoxy resins (component of imported additive)
In Canada, AEEA is also a component of imported additives, mainly used as a curing agent or chemical intermediate for epoxy resins (Henkel 2010; 2012a–c). These activities are discussed together because both imply that AEEA undergoes irreversible chemical reaction, with the subsequent chemical conversion of AEEA into another substance.
In some cases, AEEA is imported in products for commercial uses where AEEA is a minor component (Environment Canada and Health Canada 2012-2014). The function of AEEA in these products is not clearly understood but is often presented as a curing agent (Henkel 2010, 2012a–c). Therefore, in such applications, AEEA is assumed to be cured within the product. It is expected that any residual AEEA should be captured in the matrix of the solid material formed after the epoxy is cured. Under optimal curing conditions, which are known and specified by product use instructions, AEEA would be largely consumed by the reversible chemical reactions that characterize the curing processes. Therefore, the potential for releases from fully cured products is considered negligible.
Imported as chemical intermediate
Some companies reported imports of AEEA as a pure substance, to formulate as an additive for industrial uses, or to prepare other substances. In these cases, the function of AEEA was reported as a chemical intermediate (Environment Canada and Health Canada 2012-2014). When used as a chemical intermediate, AEEA is considered to be chemically converted during the process, and it is expected that AEEA no longer exists in its original chemical form. Therefore, the only potential significant releases for unreacted AEEA stem from the cleaning of empty transport and processing containers. This scenario is further developed to determine quantitative estimates for aquatic concentrations resulting from this activity.
Estimates for predicted environmental concentrations
The exposure to AEEA was estimated in the form of predicted environmental concentrations (PECs) for the cleaning of equipment scenario (when pure AEEA is used as chemical intermediate). These concentrations are based on available information on quantities of AEEA, sector-specific emission factors, the characteristics of wastewater treatment systems and the receiving environment.
The PECs estimated were focused on the aquatic compartment. This is because AEEA is primarily released to the aquatic compartment through wastewater treatment systems.
Given its high water solubility and low Kow, AEEA is not expected to reach the soil medium through the application of biosolids (resulting from wastewater treatment operations) to land.
Industrial releases to aquatic medium
As AEEA is used by industrial facilities and can be released to water, an aquatic industrial release scenario was developed. This scenario considered the release of AEEA at an industrial facility where pure AEEA is used as a chemical intermediate. AEEA is assumed to be fully chemically converted during the industrial processes. Therefore, only a scenario for release of residual AEEA resulting from cleaning of empty transport and processing containers was considered.
Industrial uses of pure AEEA in Canada have been reported (Environment Canada and Health Canada 2012-2014). To estimate the potential of AEEA releases to water resulting from its industrial use as a chemical intermediate (i.e., the applications of AEEA in its pure chemical form), conservative but realistic scenarios at several industrial facilities were developed. These scenarios were refined by applying the exact substance quantities reported for use in 2011 (Environment Canada and Health Canada 2012-2014), as well as considering the characteristics of the receiving water bodies, wastewater treatment, and industrial operations at each site. In the conservative scenario presented below, the exact quantity of AEEA between 1000 kg and 10 000 kg reported for 2011 (Environment Canada and Health Canada 2012-2014) for an industrial facility that discharges into a receiving environment with low dilution was considered.
Aquatic exposure to AEEA could occur if this substance is released from industrial activity to a wastewater system that discharges its effluent to a receiving surface water body. The concentration of the substance in the receiving water near the discharge point of the wastewater system is used as the predicted environmental concentration (PEC) in evaluating the aquatic risk of the substance. It can be calculated using the equation:
Cwater-ind = [1000 × Q × L × (1 – R)] / [N × F × D]
where
- C water-ind:
- aquatic concentration resulting from industrial releases, mg/L
- Q:
- total substance quantity used annually at an industrial site, kg/yr
- L:
- loss to wastewater, fraction
- R:
- wastewater system removal rate, fraction
- N:
- number of annual release days, d/yr
- F:
- wastewater system effluent flow, m 3/d
- D:
- receiving water dilution factor, dimensionless
Table 7 presents the inputs used to estimate resulting aquatic concentrations close to the industrial point of discharge. It is noted that the assumptions considered are conservative and provide a basis for a conservative release scenario.
| Input | Value | Justification and reference |
|---|---|---|
| Quantity (kg) | 1000–10 000 | Quantity of AEEA reported at one site in Canada for 2011 (Environment Canada and Health Canada 2012-2014) |
| Loss to wastewater (%) | 3% | Maximum residual in container that can be washed and released to wastewater (OECD 2011 |
| Wastewater system removal efficiency (%) | 60% | Model estimates of efficiency of a typical secondary wastewater plant; removal of AEEA will be between 60 and 80%. Models used are ASTreat 1.0 (2006), SimpleTreat 3.0 (1997), STP Model 2.1 (2006) and STP-EX (2011). Value of 60% is obtained from model ASTreat 1.0 (2006). |
| Number of annual release days (days) | 100 | Value based on estimated intermittent cleaning (2 days per week for 50 weeks) |
Based on these conservative realistic assumptions, this scenario yielded a predicted environmental concentration (PEC) of 0.11 mg/L. This PEC value represents the level of exposure in the receiving water near the point of the discharge of a small sized wastewater treatment system with secondary treatment characteristics.
Characterization of Ecological Risk
The approach taken in this ecological screening assessment was to examine various supporting information and to develop conclusions based on a weight-of-evidence approach and using precaution as required under CEPA. Lines of evidence considered in the assessment of AEEA include results from a conservative risk quotient calculation, as well as information on persistence, bioaccumulation, ecological toxicity, sources, and fate of the substance and its presence and distribution in the environment.
AEEA is not expected to be persistent in air, water, soil or sediment. It is also expected to have a low bioaccumulation potential and has been shown to have a low to moderate potential for toxicity to aquatic organisms. Information on AEEA uses in Canada indicates that its potential for release into the Canadian environment is minimal. However, releases of AEEA into water as a result of cleaning transport and processing containers of AEEA following its use as a chemical intermediate were considered.
A risk quotient analysis that integrated conservative but realistic estimates of exposure with toxicity information was performed for the aquatic medium. The conservative scenario presented above (which also considers secondary-level treatment) yielded a predicted environmental concentration (PEC) of 0.11 mg/L. A predicted no-effect concentration (PNEC) of 0.22 mg/L was derived from the acute toxicity value of 22 mg/L for daphnids (as the most sensitive, valid experimental endpoint), and by dividing this value by an assessment factor of 100. The resulting risk quotient (PEC/PNEC) is 0.49. Table 8 provides a summary of this information.
| Media | Scenario | PNEC (mg/L) | PEC (mg/L) | RQ |
|---|---|---|---|---|
| Water | Industrial release | 0.22 | 0.11 | 0.49 |
Consideration of the factors presented above indicates that AEEA does not have the potential to cause ecological harm in Canada.
Uncertainties in Evaluation of Ecological Risk
Adverse effects of AEEA in aquatic organisms were characterized based on acute exposure duration. Chronic effects studies were not found for AEEA, and model results were considered unreliable. The lack of chronic effects characterization for AEEA is considered to be a data gap. AEEA is noted to be a narcotic chemical based on the ECOSAR (2012) aquatic toxicity classification. Generally, for narcotic chemicals an equilibrium tends to be reached quickly in exposed organisms, resulting in the short-term and longer-term effects being similar. However, specific mode(s) of action underlie some of the effects observed in mammals. To derive the predicted no effect concentration (PNEC) for aquatic organisms, an assessment factor of 100 was applied to consider extrapolation of acute to chronic exposure and inter- and intra-species variability.
There is uncertainty related to toxic effects stemming from increased alkalinity of water at high exposure concentrations of AEEA. Increased mortality of fish was observed in tests at high concentrations of AEEA. It is uncertain, based on the available study details, whether increased water alkalinity alone caused these effects, or in combination with the high AEEA concentrations. However, high exposure concentrations of AEEA characteristic of the available aquatic studies are not considered to be representative of realistic environmental exposures.
No ecotoxicity data were available for soil and sediment organisms as a result of exposure to AEEA. Based on the predicted partitioning behavior, if released to soil, AEEA would tend to remain in this compartment. Due to lack of relevant data, ecological effects in soil and sediment are not well defined. Nonetheless, given the low potential for toxicity in the aquatic species determined through a number of empirical studies, it is not expected that AEEA would exhibit a markedly higher toxicity to soil and sediment organisms.
There is also some uncertainly related to the fate and behavior of the ionized forms of AEEA at pH 6-9. The available models, including the fugacity model, use the neutral form of chemicals as model inputs. Therefore, ionizing substances are not easily amenable to modelling, and as a result, some uncertainty exists in the interpretation of modelling data for this substance. However, ionization characteristics would have been taken into account in most empirical data generated at environmentally relevant pH.
The potential for environmental releases from the uses of AEEA in asphalt cement or as a curing agent are assumed to be negligible. However, is noted that no measurements or quantitative estimates characterizing the extent of conversion of chemical intermediates in cured products are currently available. It is also assumed that the potential for releases from the cured products made with AEEA as a chemical intermediate is negligible. Studies or reliable quantitative estimates addressing this potential release of chemical intermediates such as AEEA from cured products have not been conducted. Therefore, there is potential for underestimation of AEEA exposure to soil organisms. However, the ecotoxicological effects and impacts of AEEA exposure are not expected to be substantial.
Potential to Cause Harm to Human Health
Exposure assessment
Environmental media
AEEA is an anthropogenic chemical that is not naturally found in the environment. No reports of environmental monitoring of AEEA in air, soil, sediment, or dust in Canada were identified. One water monitoring study conducted outside of Canada was identified in which AEEA was measured in surface water and groundwater from Florence, Vermont, United States (Geosyntec 2012a) near an industrial zone that produces ground calcium carbonate from marble ore using AEEA as a component of ore flotation agents. Given this use was not identified for Canada, and given that AEEA is not naturally found in the environment, these measured levels in the vicinity of the quarrying facility are not considered representative of potential levels that would be found in the Canadian environment.
Level III fugacity modelling (New EQC 2011) indicated that AEEA is not likely to reside in air. When released to air or soil, AEEA would primarily partition to soil and would remain in water when released to water (see Table 3).
According to information submitted to Environment Canada and Health Canada (Environment Canada, Health Canada 2012-2014), AEEA was not manufactured in Canada in 2011. Between 100 000 and 500 000 kg of AEEA was imported into Canada in the same year. As indicated in the Releases to the Environment section, AEEA is expected to be fully converted during its use as a chemical intermediate, and the only scenario that could result in potential release of residual AEEA into the environment is the cleaning of empty transport and processing containers. The portion of AEEA that could be released into wastewater is expected to be small (a maximum of 3%) (OECD 2011), and is expected to be further treated before it is released into surface water, resulting in a negligible amount of residual AEEA in drinking water. An analysis of the submitted information also showed that the potential for dispersive releases of AEEA during use of imported products is minimal (see the Ecological Exposure Assessment section for details).
Based on these considerations, the potential for exposure of the Canadian population to AEEA in environmental media is not expected.
Food
No reports of AEEA in food in Canada or elsewhere were identified. In Canada, AEEA is used as a component in adhesives and inks with no direct contact with food, as well as an ingredient in an agent used in the paper manufacturing process. AEEA is also used as a component in additives for closed re-circulating cooling systems where the treated water will not come into direct contact with food. The potential for exposure from food as a result of these uses is expected to be minimal (personal communication, 2011 email from the Food Directorate to the Risk Management Bureau; unreferenced).
Consumer Products
Results of an OECD initial assessment on AEEA indicated that un-reacted AEEA may be found at very low concentrations in final consumer products (OECD 2009); AEEA was present at 3 parts per million (ppm) in surfactant and waxes, which was in the range of the detection limit (Degaussa 2005, as cited in OECD 2009). AEEA was not detected in a study of European cosmetics containing amphoteric surfactants; the detection limit for that study was 10 ppm (TEGEWA 2005, as cited in OECD 2009).
In Canada, AEEA was reported to be used as a chemical intermediate in the manufacture of surfactants, which can then be used in consumer products such as cosmetics. However, information provided by manufacturers of the surfactant used in consumer products indicates that AEEA is not present in the surfactant (Environment Canada, Health Canada 2012-2014) Based on notifications submitted under the Cosmetic Regulations to Health Canada. AEEA is not used in cosmetic products in Canada (2013 emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
AEEA may also be present in some epoxy adhesives, or super glues, used for small scale repairs or hobbies (Henkel 2012b). Given that these products are typically used in small amounts and given the moderate volatility of AEEA, negligible inhalation exposure is expected. Skin contact during application of super glues is typically avoided to protect the dermal layer from physical harm of tearing. In the event of incidental contact with super glue, a fingertip may be exposed to 5% of the glue used, based on this the amount of AEEA that could potentially reach the skin is considered negligible.
Overall, exposure of the Canadian population to AEEA through the use of consumer products is not expected.
Health Effects AssessmentFootnote[4]
The European Commission classified AEEA as Category 2 (causes developmental toxicity in humans) Risk phrase R61 (may cause harm to the unborn child) for developmental toxicity (European Commission 2004). This classification was based on damage in blood vessels in fetuses and pups in studies in which rats were exposed to AEEA orally. The European Commission also classified AEEA as Category 3 (causes concern for human fertility) Risk phrase R62 (possible risk of impaired fertility) for reproductive toxicity, based on decreased fertility in rats treated by the oral route (European Commission 2003, 2004). Subsequent changes to the classification schemes for the hazard class within the European Union’s Classification, Labelling and Packaging (CLP) regulations (EC No 1272/2008) resulted in a change in the status of AEEA to Category 1B - reproductive toxicant (presumed human reproductive toxicant).
In a combined reproduction/developmental toxicity screening test in which rats were given 0, 0.2, 1, 5, or 50 mg AEEA per kilogram of body weight (kg-bw) per day by gavage for 38 or 54 days (males exposed for 14 days prior to mating and during mating period; females treated from 14 days prior to mating until day 4 of lactation), aneurysms and focal hemorrhages of pericardial blood vessels (aorta, pulmonary trunk, ductus arteriosus, innominate arteria) were observed in fetuses at all doses tested. While the increase in focal hemorrhages was not dose-related and not statistically significant at any dose level tested, it was higher than the historical controls for hemorrhagic lesions (0.2%). The increase in aneurysms was not dose-related at the lowest doses (0.2-5 mg/kg-bw per day) but was statistically significant at 50 mg/kg-bw per day. The no-observed-adverse-effect level (NOAEL) for maternal toxicity in this study was 50 mg/kg-bw per day based on no treatment-related effects observed in dams at the highest dose tested (BASF AG 2008;Treumann et al. 2011; Schneider et al. 2012). In another combined reproduction/developmental toxicity study in rats using higher dose levels (50-1000 mg AEEA/kg-bw per day given by gavage for 32 or 65 days [ (males treated 5 weeks pre-mating, 2 weeks during mating period and 1 week post-mating; females treated from 2 weeks prior to mating until day 4 post-delivery]), aneurysms of the aorta and pulmonary trunk and dilatations of carotid and descending aorta were observed in pups at all dose levels. Maternal toxicity was noted at 1000 mg/kg-bw per day based on decreased body weight gain, salivation and impairment of care of fur (EPSDG 2002; BASF AG 2003b, 2003c; Schneider et al. 2012). When pregnant rats were administered AEEA by gavage during gestational day (GD) 6 to day 4 post partum (pp), aneurysms and focal hyperplasia were observed in or near the aorta in pups at 50 mg/kg-bw per day, the highest dose tested, in absence of maternal toxicity. When the pups were treated by gavage with the same dose level on days 14 to 28 pp or days 14 to 60 pp, other changes of the large blood vessels were observed such as dilated aorta and focal scar in the region of the aortic arch (BASF 2005). In a more recent study in which female rats were administered 250 mg AEEA/kg-bw per day by gavage from GD 6 to GD 19 (group 1 and 2; animals euthanized and examined on GD 21 in group 1 or postnatal day 4 in group 2) or from GD 6 to postnatal day 3 (group 3; animals euthanized and examined on postnatal day 4), heart malformations were observed with an incidence of 91.1% in group 1 versus100% in groups 2 and 3, and aneurysms were more prevalent in group 3 compared to group 2. The findings of this study indicate that exposure to AEEA during gestation alone was sufficient to induce malformations of the great vessels and aneurysms, but that the critical period of susceptibility to AEEA-induced aneurysms in the rat extends beyond gestation into the early postnatal period (Moore et al. 2012b). However, in another study where rats were exposed to AEEA by gavage during GD 6 to 19 at doses of 0, 0.5, 2, 10, or 50 mg/kg-bw per day, no treatment-related signs of developmental toxicity or teratogenicity (in particular, no effect on the fetal cardiovascular system) were observed. No maternal toxicity was noted (BASF 2003a).
In the majority of these studies, increase in aneurysms and other adverse effects to blood vessels was found to be dose-related and statistically significant in animals administered 50 mg/kg-bw per day. In one study (BASF AG 2008), increase in aneurysms and focal hemorrhages of pericardial blood vessels was observed at lower doses. However, this increase was not dose-related and not statistically significant at any dose level tested. Based on this, the lowest oral lowest-observed-adverse-effect level (LOAEL) for developmental toxicity was considered to be 50 mg/kg-bw per day in fetuses of pregnant rats exposed to AEEA by gavage, based on aneurysms of pericardial blood vessels. The lowest LOAEL for maternal toxicity in rats was 1000 mg/kg-bw per day based on decreased body weight gain, salivation, and impairment of care of fur in dams.
No data on the potential developmental toxicity via other routes of exposure were identified for AEEA.
Treatment-related reproductive effects were also observed in rats exposed to AEEA in the reproduction/developmental toxicity study described earlier (BASF AG 2008; Schneider et al. 2012). Effects observed included a significantly lower viability index and increased number of stillborn pups at 250 mg/kg-bw per day and above, a reduction in fertility index, reduced implantations per dam and decreased absolute weight of ovaries or testes at the highest dose (BASF AG 2008; Schneider et al. 2012). The lowest oral LOAEL for reproductive toxicity was identified at 250 mg/kg-bw per day. A parental LOAEL of 1000 mg/kg-bw per day was identified based on decreased body weight gain, salivation, and impairment of care of fur in both males and females. No reproductive toxicity studies with exposure to AEEA via other routes of exposure have been found in the literature.
Although no long-term studies have been identified, the toxicity of AEEA has been investigated in a limited number of shorter-term studies. The primary effect of AEEA is irritancy after oral and dermal exposure. In a 28-day gavage study in rats, the lowest LOAEL for repeated-dose oral exposure was 250 mg/kg-bw per day based on histopathological changes in both sexes. In males, the histopathological changes observed were deposition of amphophilic bodies and swelling in the renal proximal tubules and mucosal thickening in the stomach. In females, mucosal thickening in the stomach was noted (Okazaki et al. 1996). With respect to repeated-dose dermal exposure, the lowest LOAEL was 1000 mg/kg-bw per day based on skin irritation, ulcers, inflammation of the dermis and epidermis, and increase in epidermal hyperplasia in male and female rats exposed for 4 weeks (Dow Chemical Company 1994).
No chronic toxicity/carcinogenicity studies were available for AEEA.
Although no carcinogenic bioassays have been conducted with AEEA, the substance has been tested in a range of genotoxicity tests. The collective evidence indicates that AEEA is not genotoxic. In in vitro assays, AEEA was not mutagenic in the majority of bacterial mutation assays using Salmonella typhimurium, with or without activation (Zeiger et al. 1987; BASF AG 1991; Morton International 1994; Prival and Zeiger 1998). Similarly, assays for chromosomal aberration in Chinese hamster V79 and lung cells, for gene mutation at the HGPRT locus in Chinese hamster ovary and V79 cells, and for sister chromatid exchange in Chinese hamster ovary cells showed negative responses, all in the presence and absence of metabolic activation (Leung 1994; Morton International 1994; Tanaka et al. 1996; Kusakabe et al. 2002). A negative result was also reported for induction of unscheduled deoxyribonucleic acid (DNA) synthesis in rat hepatocytes (Leung 1994). In vivo, negative results were obtained in a micronuclei assay in bone marrow cells of mice dosed orally (Shibuya et al. 1996). Negative results were also observed in male Drosophila melanogaster using the sex-linked recessive mutation assay in feeding and injection experiments (Foureman et al. 1994).
In acute studies, AEEA administered at high doses causes mainly effects related to severe local irritation following oral and dermal administration to experimental animals (Sidorov et al. 1968; BASF AG 1979, 1980). AEEA is classified as Category 1B - skin corrosive and as Category 1 - skin sensitizer according to the European Union’s Classification, Labelling and Packaging (CLP) regulations (EC No 1272/2008). Several irritation studies have shown that AEEA is corrosive to the skin (BASF AG 1979; Dow Chemical Company 1980, 1992; Bushy Run Research Center 1990; Myers and Ballantyne 1997) and the eyes (BASF AG 1970; Dow Chemical Company 1980, 1992) in rabbits. Sensitization tests performed in guinea pigs or mice indicate sensitization in animals subsequent to exposure to the diluted substance (Dow Chemical Company 1980, 1992; Bio/dynamics Inc. 1990; Leung and Auletta 1997; Dearman and Kimber 2001).
Toxicokinetic studies on radiolabelled AEEA in non-pregnant and pregnant rats have been identified in the literature (Dow Chemical Company 2004; Moore et al. 2012a). These studies show that AEEA is rapidly absorbed following oral administration of a single dose (0.5 or 50 mg/kg-bw). Absorption was more than 85% within 48 hours in all doses groups, based on the recovered radioactivity in urine, tissue, cage wash, and expired air. Excretion was rapid and occurred mainly via urine, with approximately 85-98% of the oral dose recovered in the first 48 hours, 5.2-11.5% in feces, and 0.02-0.03% in expired volatiles and as 14CO2. In tissues, 2.3 to 3.0% of the administered radioactivity was recovered. The overall recovery ranged between 99% and 107%. Bioavailability of AEEA was fairly linear with dose. At termination, the radioactivity in tissues was low, and there was no statistically significant difference between dose groups, or between pregnant and non-pregnant animals. Tissues that retained AEEA were carcass, skin, liver, and kidneys, in decreasing order of concentration. Three metabolites and unmetabolized AEEA were observed in urine from all oral dose groups. No significant differences in metabolic profile were observed between dose level or pregnancy status. Following repeated oral administration during GD 17 to 19, AEEA readily partitioned into the fetus and the maternal compartments, but cleared approximately twofold slower from the fetal blood and tissues than the maternal blood and chorioallantoic placenta. Although AEEA appeared to be distributed preferentially from the blood into tissues of the fetal compartment, it did not appear to specifically concentrate in the great vessels. When administered to lactating dams during lactation days 1 to 12, AEEA preferentially partitioned into the milk; levels reached there were 1.6- to 2.5-fold higher than the levels in maternal blood. However, the concentration of AEEA in milk fell by almost 40% between lactation days 4 and 12, probably due to an increase in milk production over the same period. Transfer via the milk in nursing offspring resulted in at least 10-fold lower exposure to AEEA than in the dams. Pup exposure to radiolabelled AEEA equivalents was estimated to be 9.6, 8.8, and 4.5% of the dose administered to the dams in lactation days 4, 8, and 12, respectively (Dow Chemical Company 2004; Moore et al. 2012a). In a toxicokinetic study in which 14C-AEEA was administered through dermal application, the substance was rapidly absorbed and excreted by female rats (Dow Chemical Company 2004). Quantifiable amounts of radioactivity were found in urine, but not in plasma. Based on radioactivity in cage wash, excreta and tissues, the estimated dermal absorption was 7.73±1.56%. The organ distribution was low and comparable in all experiments. Excretion was rapid and occurred mainly via urine within 8 hours post-application. Excretion in feces was low in the 0- to 8-hour interval (0.18%) and the 8- to 24-hour interval (0.33%) (Dow Chemical Company 2004).
The confidence in the health effects database is considered to be low to moderate based mainly on the absence of oral or dermal chronic carcinogenicity studies for AEEA. There is adequate information to identify critical effects following short-term oral exposure (developmental toxicity, reproductive toxicity, repeated-dose toxicity, genetic toxicity and acute toxicity). However, dermal exposure studies are limited.
Characterization of Risk to Human Health
Based on consideration of the weight-of-evidence-based classification of AEEA by the European Commission as Category 2 for developmental toxicity (European Commission 2004) and consideration of relevant data for the substance, a critical effect for characterization of risk to human health for AEEA is developmental toxicity due to damage in blood vessels in rat fetuses and pups. The European Commission has also classified AEEA as Category 3 for reproductive toxicity (European Commission 2003, 2004) based on decreased fertility but these effects were seen at higher doses. Acute and repeated-dose studies in experimental animals showed effects mainly related to irritancy after oral and dermal exposure (effects on the skin, stomach and kidney) at higher doses as well. The lowest LOAEL for developmental toxicity identified for AEEA was 50 mg/kg-bw per day in fetuses of pregnant rats exposed orally, based on increased incidence of aneurysms of pericardial blood vessels.
There were no reports of AEEA in environmental media identified for Canada. Based on data submitted to Environment Canada and Health Canada for 2011 (Environment Canada, Health Canada 2012-2014), AEEA is not manufactured in Canada and the potential release of AEEA from its use in industrial processes or during use of imported products containing AEEA is expected to be minimal. It is used in food-packaging applications with no direct contact with food (personal communication, 2011 email from the Food Directorate to the Risk Management Bureau; unreferenced), and exposure of the Canadian population to AEEA through the use of consumer products is not expected. Based on this, exposure to the general population is not expected and the risk to human health is considered to be low.
Human health risk from this substance in this assessment is low based on the current levels of exposure. However, AEEA has effects of concern based on reproductive and developmental toxicity.
Uncertainties in Evaluation of Risk to Human Health
This Screening Assessment does not include a full analysis of the mode of induction of effects associated with exposure to AEEA, nor does it take into account all possible intra-species and inter-species variation. There is uncertainty surrounding the potential carcinogenicity and chronic toxicity of AEEA due to the lack of long-term studies. In addition, dermal exposure studies are limited.
Confidence in the exposure database is low. No data on the presence of AEEA in environmental media were identified for Canada or elsewhere. Based on information submitted to Environment Canada and known international use patterns, exposure to the general population is not expected.
Canadians are not expected to be exposed to this substance through the use of consumer products. However, levels of AEEA (3 ppm) have been detected in surfactants and waxes in other countries. The lack of data on residual AEEA in similar Canadian products is an uncertainty.
Conclusion
Considering all available lines of evidence presented in this Screening Assessment, there is low risk of harm to organisms and the broader integrity of the environment from AEEA. It is concluded that AEEA does not meet the criteria under paragraph 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Based on the information presented in this Screening Assessment, it is concluded that AEEA does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is concluded that AEEA does not meet any of the criteria set out in section 64 of CEPA.
References
ACD/Percepta [Prediction Module]. c1997-2012. Toronto (ON): Advanced Chemistry Development. [cited 2013 May 10]. Available from: http://www.acdlabs.com/products/percepta/
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2010. Version 1.92a. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2013 May]. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Arnot JA, Gobas FAPC. 2003. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci 22(3):337–345.
Arnot JA, Gobas FAPC. 2006. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ Rev 14:257–297.
Arnot JA, Arnot MI, Mackay D, Couillard Y, MacDonald D, Bonnell M, Doyle P. 2010. Molecular size cutoff criteria for screening bioaccumulation potential: fact or fiction? Integr Environ Assess Manage 6(2):210–224.
Aronson D, Howard PH. 1999. Evaluating potential POP/PBT compounds for environmental persistence. North Syracuse (NY): Syracuse Research Corp., Environmental Science Centre. Report No.: SRC-TR-99-020.
ASTreat Model [sewage treatment plant removal model]. 2006. Version 1.0. Cincinnati (US): Procter & Gamble Company. Available from Procter & Gamble Company, PO Box 538707, Cincinnati, OH 45253-8707, USA.
BASF AG. 1970. Toxikologie, unveroeffentlichte Untersuchungen, ZST Nr. XX/174, 16.09.1970 [cited in European Commission 2000; OECD 2008, 2009].
BASF AG. 1979. Toxikologie, unveroeffentlichte Untersuchungen, ZST Nr. 77/715, 23.08.1979 [cited in European Commission 2000; OECD 2008, 2009].
BASF AG. 1980. Department of Toxicology. Prüfung der akutendermalenToxizität an der Rückenhautweißer Kaninchen (77/213), unpublished data, 5 May 1980 [cited in European Commission 2000; OECD 2008, 2009].
BASF AG. 1991. Toxikologie, unveroeffentlichte Untersuchungen, ZST Nr. 89/233, 18.04.1991 [cited in European Commission 2000; OECD 2008, 2009].
BASF AG. 2003a. Report on the study of AEEA in the prenatal developmental toxicity study in Wistar rats. Unpublished study No. 30R0019/01105. Department of Toxicology [cited in OECD 2008; 2009].
BASF AG. 2003b. AEEA-Reproduction/developmental toxicity screening test (SIDS) in Wistar rats. Unpublished report No. R0019/01075. Department of Experimental Toxicology and Ecology [cited in OECD 2008; 2009].
BASF AG. 2003c. Additional histopathological examination of pups from an OECD 421 screening study. Unpublished report to project No. 90R0019/01075. Department of Experimental Toxicology and Ecology [cited in OECD 2008, 2009].
BASF AG. 2005. N-(2-aminoethyl)ethanolamine - Mechanistic toxicity screening test in Wistar rats. Oral application (gavage). Unpublished report No. 06R0019/01136. Sponsored by the Ethyleneamines Product Stewardship Discussion Group (EPSDG) and CEFIC Ethyleneamines Group. Department of Experimental Toxicology and Ecology [cited in OECD 2008, 2009].
BASF AG. 2008. N-(2-aminoethyl)ethanolamine. Enhanced reproduction/developmental toxicity screening test in Wistar rats.Oral administration (gavage). Unpublished study report No. 90R0019/01195 [cited in OECD 2008, 2009].
BASF AG. 2013. N-(2-Aminoethyl)ethanolamin (AEEA) [Internet]. [cited 2013 Sept]. Available from: http://www.basf.com/group/corporate/en/brand/N_2_AMINOETHYL_ETHANOLAMINE
[BCFBAF] Bioaccumulation Program for Windows [Estimation Model]. 2010. Version 3.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Bio/dynamics Inc. 1990. Guinea pig maximization test - Report 5502-89. Sponsored by Union Carbide Corporation. NTIS/OTS 0537564.
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2010. Version 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermediate extrapolations in biodegradability assessment. Chemosphere 30(4):741−752.
Boethling RS, Mackay D. 2000. Handbook of property estimation methods for chemicals. Chapter 13. Hydrolysis. New York (NY): Lewis Publishers.
Bushy Run Research Center. 1990. Aminoethylethanolamine: primary skin irritancy studies in the rabbit. Prepared for Union Carbide Chemicals and Plastics Company Inc. Report No. 53-63. Sponsored by Union Carbide Corporation. NTIS/OTS0541035 [cited in OECD 2008, 2009].
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33, Canada Gazette. Part III, vol. 22, no. 3. Available from: http://publications.gc.ca/gazette/archives/p3/1999/g3-02203.pdf
Canada, Dept. of the Environment. 2009. Canadian Environmental Protection Act, 1999: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List. Canada Gazette, Part I, vol. 143, no. 40, p. 2945-2967. Available from: http://www.gazette.gc.ca/rp-pr/p1/2009/index-eng.html
[CDAT] Chemical Data Access Tool. 2013. Non-confidential 2012 Chemical Data Reporting Information: search results for CAS RN 111-41-1. Washington (DC): US Environmental Protection Agency. [updated 2013 May 3; cited 2013 May 29]. Available from: http://java.epa.gov/oppt_chemical_search/
ChemCAN [Level III fugacity model of 24 regions of Canada]. 2003. Version 6.00. Peterborough (ON): Trent University, Centre for Environmental Modelling and Chemistry. Available from: http://www.trentu.ca/academic/aminss/envmodel/models/CC600.html
Clark B, Henry JG, Mackay D. 1995.Fugacity analysis and model of organic-chemical fate in a sewage-treatment plant. Environ Sci Technol 29:1488–1494.
[CPOPs] Canadian Persistent Organic Pollutants Profiler Model. 2012. Version 1.1.18. Gatineau (QC): Environment Canada, Existing Substances Division; Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. [Model developed based on Mekenyan et al. 2005].
Dearman RJ, Kimber I. 2001. Assessment of the allergenic and respiratory sensitizing potential of ethyleneamine and ethanolamine. Central Toxicology Laboratory Alderley Park Macclesfield (sponsored by the Dow Chemical Co.). Report No. CTL/L/8918 (unpublished) [cited in OECD 2008,2009].
Degaussa. 2005. Residual content of AEEA in amphoteric tenside. Unpublished report AI 05077937, 25 Nov 2005 [cited in OECD 2009].
Dimitrov S, Dimitrova N, Walker J, Veith G, Mekenyan O. 2002. Predicting bioconcentration potential of highly hydrophobic chemicals. Effect of molecular size. Pure and Appl Chem 74(10):1823–1830.
Dimitrov S, Dimitrova N, Parkerton T, Comber M, Bonnell M, Mekenyan O. 2005. Base-line model for identifying the bioaccumulation potential of chemicals. SAR QSAR Environ Res 16(6):531–554.
Dow Chemical Company. 1980. Acute toxicological properties and skin sensitization potential of aminoethylethanolamine. R+D report, TXT: K-4409-(3) [cited in European Commission 2000; OECD 2008, 2009].
Dow Chemical Company. 1992. Initial submission: Acute toxicological properties and skin sensitization potential of aminoethylethanolamine (final report) with cover letter dated 032792. NTIS/OTS 0537184 [cited in European Commission 2000; OECD 2008, 2009].
Dow Chemical Company. 1994. Unpublished report (1991): zitiertim HEDSET von DOW, 21.05.1994 [cited in European Commission 2000; OECD 2008, 2009].
Dow Chemical Company. 2004. AEEA: Dermal and oral absorption and limited pharmacokinetic study in Wistar rats. Unpublished study report (study ID 031053). Department of Toxicology [cited in OECD 2008].
Dow Chemical Company. 2010. Product safety assessment. Aminoethylethanolamine. Available from: http://msdssearch.dow.com/PublishedLiteratureDOWCOM/dh_0436/0901b80380436aae.pdf?filepath=productsafety/pdfs/noreg/233-00317.pdf&fromPage=GetDoc
[DPD] Drug Product Database [database on the Internet]. 2013. Ottawa (ON): Health Canada. [cited 2013 April]. Available from: http://www.hc-sc.gc.ca/dhp-mps/prodpharma/databasdon/index-eng.php
[DS TOPKAT] Discovery Studio TOxicity Prediction by Komputer Assisted Technology [Prediction Module]. c2005-2009. Version 2.5.0.9164. San Diego (CA): Accelrys Software Inc. Available from: http://www.accelrys.com/products/
[ECHA] European Chemicals Agency. c2007-2014a. Registered Substances database. Helsinki (FI): ECHA. [updated 2014 Sept 24; cited 2014 Sept 26]. Available from: http://www.echa.europa.eu/information-on-chemicals/registered-substances
[ECHA] European Chemicals Agency. c2007-2014b. Registered Substances database. Search results for CAS RN [111-41-1]. Helsinki (FI): ECHA. [updated 2014 Sept 24; cited 2014 Sept 26]. Available from: http://apps.echa.europa.eu/registered/data/dossiers/DISS-9d96d548-8651-68ae-e044-00144f67d249/DISS-9d96d548-8651-68ae-e044-00144f67d249_DISS-9d96d548-8651-68ae-e044-00144f67d249.html
[ECOSAR] Ecological Structure Activity Relationships Class Program [Estimation Model]. 2012. Version 1.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2013 May]. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Emtiazi G, Knapp JS. 1994. The biodegradation of piperazine and structurally-related linear and cyclic amines. Biodegradation 5:83–92.
Environment Canada. 2009. Guidance for Conducting Ecological Assessments under CEPA, 1999. Science Resource Technical Series, Mini Guidance Module: Determining the persistence of a chemical from biodegradation data. Gatineau (QC): Environment Canada, Ecological Assessment Division.
Environment Canada. 2010. Data for AEEA collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada, Existing Substances Program.
Environment Canada, Health Canada. 2012-2014. Voluntary submission of follow-up data for AEEA in the Internationally Classified Substance Grouping. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada and Health Canada. 2013. Chemical substances: Categorization [Internet]. Ottawa (ON): Government of Canada. [updated 252007 April 20; cited 2014 Sept 25]. Available from: http://www.chemicalsubstanceschimiques.gc.ca/approach-approche/categor-eng.php
[EPSDG] Ethyleneamines Product Stewardship Discussion Group. 2002. Initial Submission: letter from EPSDG to USEPA w/summary of interim results for reproduction/developmental study with 2-(2-aminoethylamino)ethanol, dated 070302. NTIS/OTS 0574400.
European Commission. 2000. IUCLID Dataset, 2-(2-aminoethylamino)ethanol, CAS No. 111-41-1 [Internet]. Year 2000 CD-ROM edition. European Chemicals Agency, European Commission. 33 p. [created 2000 Feb 18; cited 2013 May]. Available from: http://www.esis.jrc.ec.europa.eu/index.php?PGM=dat
European Commission. 2003. Summary record: Meeting of the Commission Working Group on the Classification and Labelling of Dangerous Substances. ECB Ispra, 17-19 November 2003. European Commission, Directorate General JRC, Joint Research Centre, Institute for Health and Consumer Protection, European Chemicals Bureau. ECBI/65/04 – Rev.2. Available from: http://apps.kemi.se/hclass/DocumentDownload.aspx?DocId=925126
European Commission. 2004. Summary record: Meeting of the Technical Committee C & L on the Classification and Labelling of Dangerous Substances. ECB Riga, 12-14 May 2004. European Commission, Directorate General JRC, Joint Research Centre, Institute for Health and Consumer Protection, European Chemicals Bureau. ECBI/147/04 – Rev.3. Available from: http://apps.kemi.se/hclass/DocumentDownload.aspx?DocId=922689
[EPI Suite] Estimation Programs Interface Suite for Microsoft Windows [Estimation Model]. 2012. Version 4.1. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2013 May 27]. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Foureman P, Mason JM, Valencia R, Zimmering S. 1994. Chemical mutagenesis testing in Drosophilia. IX. Results of 50 coded compounds tested for the National Toxicology Program. Environ Mol Mut 23:51-63 [cited in OECD 2008, 2009].
Frauenkron M, Melder J-P, Ruider G, Rossbacher R, Höke H. 2001. Ethanolamines and propanolamines. In: Elvers, B, editor-in-chief. Ullmann’s encyclopedia of industrial chemistry, Vol. 13. 7th ed. New York (NY): Wiley. p. 405-431.
[Geosyntec] Geosyntec Consultants. 2012a. October 2012 Monitoring Report: Omya Verpol Facility, Florence, Vermont. Prepared for: Omya Inc. (Florence, VT). Prepared by: Geosyntec Consultants (Acton, MA). Project Number: BR0139. December 2012. [cited 2013 Sept 3]. Available from: http://www.omyainvermont.com/C125786200464C7C/vwLookupDownloads/Oct_2012_Omya_Monitoring_Report.pdf/$FILE/Oct_2012_Omya_Monitoring_Report.pdf
[Geosyntec] Geosyntec Consultants. 2012b. October 2012 Omya Tables. [cited 2013 Sept 4]. Available from: http://www.omyainvermont.com/C125786200464C7C/vwLookupDownloads/Oct_2012_Omya_Tables.pdf/$FILE/Oct_2012_Omya_Tables.pdf
Gobas FAPC, Kelly BC, Arnot JA. 2003. Quantitative structure activity relationships for predicting the bioaccumulation of POPs in terrestrial food-webs. QSAR Comb Sci 22:329–336.
Harnish IC. 2010. Liquid anti-strip additives in asphalt & anionic emulsion. Presentation to AEMA Asphalt emulsion technologies workshop, Niagara Falls, Ontario, Canada 2-3 November, 2010. ArrMAz Custom Chemicals. 35 p. [cited 2013 May 27]. Available from:http://www.aema.org/index.php?option=com_docman&task=doc_view&gid=389&Itemid=132
[Hawley’s] Hawley’s Condensed Chemical Dictionary [Internet]. 2007. Wiley Online Library. Hydroxyethylethylenediamine. [cited 2013 May]. Available from: http://onlinelibrary.wiley.com/doi/10.1002/9780470114735.hawley08710/full
Health Canada. 2013. Supporting document: Summary of health effects information for AEEA. Ottawa (ON): Health Canada. Available on request from: substances@ec.gc.ca
Henkel. 2010. Material Safety Data Sheet: Loctite(R) Nordbak(R) Locking Compound [Internet]. Mississauga (ON): Henkel Corporation. [cited 2013 July 3]. Available from: http://hybris.cms.henkel.com/henkel/msdspdf?matnr=702270&country=CA&language=EN.
Henkel. 2012a. Material Safety Data Sheet: Loctite(R) Nordbak(R) Backing [Internet]. Mississauga (ON): Henkel Corporation. [cited 2013 July 3]. Available from: http://hybris.cms.henkel.com/henkel/msdspdf?matnr=702292&country=CA&language=EN.
Henkel. 2012b. Material Safety Data Sheet: LEPAGE 5 MINUTE EPOXY - HARDENER [Internet]. Mississauga (ON): Henkel Corporation. [cited 2013 July 3]. Available from: http://hybris.cms.henkel.com/henkel/msdspdf?matnr=901649&country=CA&language=EN.
Henkel. 2012c. Material Safety Data Sheet: Fixmaster Fast Set Grout [Internet]. Mississauga (ON): Henkel Corporation. [cited 2013 July 3]. Available from: http://hybris.cms.henkel.com/henkel/msdspdf?matnr=702082&country=CA&language=EN.
Henkel. 2013. Material Safety Data Sheet: Fixmaster Deep Pour Grout [Internet]. Revision No.: 004.0. Mississauga (ON): Henkel Corporation. [cited 2014 Dec 3]. Available from: http://hybris.cms.henkel.com/henkel/msdspdf?matnr=702290&country=CA&language=EN.
[HSDB] Hazardous Substances Data Bank [database on the Internet]. 1983 – . Search results for CAS RN 111-41-1. Bethesda (MD): National Library of Medicine (US). [cited 2013 May]. Available from: http://www.toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB
[HYDROWIN] Hydrolysis Rates Program for Microsoft Windows [Estimation Model]. 2010. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Kelly BC, Ikonomou MG, Blair JD, Morin AE, Gobas FAPC. 2007. Food web-specific biomagnification of persistent organic pollutants. Science 317:236–239.
Kusakabe H, Yamakage K, Wakuri S, Sasaki K, Nakagawa Y, Watanabe M, Hayashi M, Sofuni T, Ono H, Tanaka N. 2002. Relevance of chemical structure and cytotoxicity to the induction of chromosome aberrations based on the testing results of 98 high production volume industrial chemicals. Mut Res 517:187-198. [cited in OECD 2008, 2009].
Leung HW. 1994. Evaluation of the genotoxic potential of alkyleneamines. Mut Res 1(2):31-43 [cited in OECD 2008, 2009].
Leung HW, Auletta CS. 1997. Evaluation of skin sensitization and cross-reaction of nine alkyleneamines in the Guinea pig maximization test. J Toxicol Cut and Ocular Toxicol 16:189-195 [cited in OECD 2008, 2009].
[LNHPD] Licensed Natural Health Products Database [database on the Internet]. 2008. Version 1.0. Ottawa (ON): Health Canada. [cited 2013]. Available from: http://webprod3.hc-sc.gc.ca/lnhpd-bdpsnh/index-eng.jsp
Lide DR, editor. 2012. CRC Handbook of Chemistry and Physics. 93rd ed. Boca Raton (FL): CRC Press. Available from: http://www.hbcpnetbase.com/
Lindgren A-L. 2011. Leachability and toxicity of cationic emulsifiers used in asphalt application. University of Gothenburg. June 2011 [cited 2013 May 27]. Available from: http://www.bioenv.gu.se/digitalAssets/1338/1338271_anna-lena-lindgren.pdf
Lutes CC, Thomas RJ, Burnette R (Acurex Environmental Corporation, Research Triangle Park, NC). 1994. Evaluation of emissions from paving asphalts. Report EPA-600/R-94-135. Washington (DC): United States Environmental Protection Agency, Office of Research and Development. [cited 2013 June13]. Available from: http://cfpub.epa.gov/si/si_public_record_Report.cfm?dirEntryId=126520&CFID=116889056&CFTOKEN=43638243&jsessionid=863040eeeb954a0807001812736264f18206
Mapei. 2013. Material Safety Data Sheet: Primer E – Part A [Internet]. Revision 1.0001. Deerfield Beach (FL): Mapei. [cited 2014 Dec 3]. Available from: http://whatsinproducts.com/files/brands_pdf/10739_13038020%20MSDS%20Mapei%20Primer%20E.pdf
Mekenyan G, Dimitrov SD, Pavlov TS, Veith GD. 2005. POPs: a QSAR system for creating PBT profiles of chemicals and their metabolites. SAR QSAR Environ Res 16(1−2):103−133.
Moore NP, Saghir SA, Clark AJ, Hansen SC, Carney EW, Marshall VA, Rasoulpour R, Bartels MJ. 2012a. Toxicokinetic profile of N-(2-aminoethyl)ethanolamine in the female Wistar rat and distribution into the late gestation fetus and milk. Birth Defects Res (Part B) 95:107-115.
Moore NP, Tornesi B, Yano BL, Nitschke KD, Carney EW. 2012b. Developmental sensitivity to the induction of great vessel malformations by N-(2-aminoethyl)ethanolamine. Birth Defects Res (Part B) 95:116-122.
Morton International. 1994. Unpublished report: Germany (1992) zitiertim HEDSET von DOW, 21.05.1994 [cited in European Commission 2000; OECD 2008].
Myers RC, Ballantyne B. 1997.Comparative acute toxicity and primary irritancy of various classes of amines. In: Taylor and Francis, editors. Toxic Substance Mechanisms. p. 151–193 [cited in OECD 2008, 2009].
[New EQC] New Equilibrium Criterion Model. 2011. Version 1.0 (Beta). Peterborough (ON): Trent University, Canadian Environmental Modelling Centre. [cited 2013 June 6]. Available from: http://www.trentu.ca/academic/aminss/envmodel/models/NewEQCv100.html
[NJ Health] New Jersey Department of Health. 2008. Right to Know: Hazardous Substance Fact Sheet: aminoethylethanolamine. Trenton (NJ): New Jersey Department of Health and Senior Services. Available from: http://www.nj.gov/health/eoh/rtkweb/documents/fs/0074.pdf
[NHPID] Natural Health Products Ingredients Database [database on the Internet]. 2013. Ottawa (ON): Health Canada. [cited 2013 April 11]. Available from: http://webprod.hc-sc.gc.ca/nhpid-bdipsn/search-rechercheReq.do
[OECD] Organisation for Economic Co-operation and Development. 2005. OECD guideline for testing of chemicals. Annex1. ENV/JM/TG(2005)5/REV1. Available from: www.oecd.org/chemicalsafety/testing/34898616.pdf
[OECD] Organisation for Economic Co-operation and Development. 2006. SIDS Initial Assessment Report for: 2-(2-Aminoethylamino)ethanol; CAS RN 111-41-1. SIDS Initial Assessment Meeting 23; March 2006. Available from: http://webnet.oecd.org/Hpv/UI/handler.axd?id=7c999614-f9fe-4046-a4ad-a7949b751ec3
[OECD] Organisation for Economic Co-operation and Development. 2008. 2-(2-aminoethylamino)ethanol. Screening Information Data Set for High Production (SIDS). SIDS Dossier. Report. 207 p.
[OECD] Organisation for Economic Co-operation and Development. 2009. 2-(2-aminoethylamino)ethanol. Screening Information Data Set for High Production (SIDS). SIDS Initial Assessment Report for SIAM 23. Report. 32 p.
[OECD] Organisation for Economic Co-operation and Development. 2011. Emission scenario document on the use of metalworking fluids. Paris (FR): OECD, Environment Directorate. Series on Emission Scenario Documents No. 28. Report No. ENV/JM/MONO(2011)18, JT03304938.
Okazaki S, Enami T, Nakamura H, Hatayama K, Tamura K, Numata H, Katsumata T. 1996. Twenty-eight-day repeat dose oral toxicity test of N-(aminoethyl)ethanolamine in rats. Bozo Research Center IncInc., Japan. In: Toxicity testing reports of environmental chemicals (Ministry of Health and Welfare, Japan). p. 351-365 [cited in OECD 2008; 2009].
Prival MJ, Zeiger E. 1998. Chemicals mutagenic in Salmonella typhimurium strain TA1535 but not in TA100. Mut Res 412(3):251–260 [cited in OECD 2008, 2009].
Sakuratani Y, Noguchi Y, Kobayashi K, Yamada J, Nishihara T. 2008. Molecular size as a limiting characteristic for bioconcentration in fish. J Environ Biol 29(1):89–92.
[Sax’s] Sax’s dangerous properties of chemical substances [Internet]. 2012. Wiley Online Library. N-Aminoethylethanolamine 111-41-1. [cited 2013 May]. Available from: http://onlinelibrary.wiley.com/doi/10.1002/0471701343.sdp27176/full
Schneider S, Treumann S, Moore NP. 2012. Malformations of the great vessels in the neonatal rat induced by N-(2-aminoethyl)ethanolamine. Birth Defects Res (Part B) 95:95–106.
Seth R, Webster E, Mackay D. 2008. Continued development of a mass balance model of chemical fate in a sewage treatment plant. Water Res 42:595–604.
Shibuya T, Horiya N, Katoh M, Takumi H, Sekino S, Matsuki Y, Lida S, Nakagomi M. 1996. Micronucleus test of N-(aminoethyl)ethanolamine on mice. Hatano Research Institute, Food and Drug Safety Center, Japan. In: Toxicity testing reports of environmental chemicals (Ministry of Health and Welfare, Japan). p. 371-376 [cited in OECD 2008, 2009].
Sidorov KK, Gorban GM, Tikhonova GP. 1968. Comparative toxicological characteristics of some regenerable absorbers of carbon dioxide. Environ Space Sci 2:289–292 [cited in OECD 2008, 2009]
SimpleTreat [sewage treatment plant removal model]. 1997. Version 3.0. Bilthoven (NL): National Institute for Public Health and the Environment (RIVM). Available from: National Institute for Public Health and the Environment (RIVM), Laboratory for Ecological Risk Assessment, PO Box 1, 3720 BA Bilthoven, the Netherlands.
[STP-EX] Sewage Treatment Plant Expanded Model. 2008. Windsor (ON): University of Windsor, Dept. of Civil and Environmental Engineering [model described in Seth et al. 2008].
[STP Model] Fugacity-based Sewage Treatment Plant Model. 2006. Version 2.11. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre [model based on Clark et al. 1995].
Tanaka N, Yamakage K, Wakuri S, Hashimoto K, Nagao T, Ohta R. 1996. In vitro chromosomal aberration test of N-(aminoethyl)ethanolamine on cultured Chinese hamster cells. Hatano Research Institute, Food and Drug Safety Center, Japan. In: Toxicity testing reports of environmental chemicals (Ministry of Health and Welfare, Japan). p. 367–370 [cited in OECD 2008, 2009].
Treumann S, Schneider S, Gröters S, Moore NP, Boor PJ. 2011. Spontaneous occurrence of dissecting aneurysms in the region of the ductusarteriosus in four-day-old Wistar rat pup. Toxicol Pathol 39:969–974.
Unique Paving Materials. c2013. Pavegrip hot mix adhesion promoter: Performance and longevity to improve hot mix (product information sheet). [cited 2013 June 15]. Available from: http://uniquepavingmaterials.com
[US EPA] United States Environmental Protection Agency. 2000. Hot mix asphalt plant, emission assessment report. EPA-454/R-00-019.United States Offices of Air Quality. Environmental Protection Planning and Standards United States Environmental Protection Agency. Agency research Triangle Park, NC27711. December 2000. [cited 2013 June13]. Available from: http://www.epa.gov/ttnchie1/ap42/ch11/related/ea-report.pdf
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K,Speck W. 1987. Salmonella mutagenicity tests III. Results from the testing of 255 chemicals. Environ Mutagen Suppl 9 (SUPPL. 9):1-110 [cited in OECD 2008, 2009].