Screening Assessment
Aromatic Azo and Benzidine-based Substance Grouping
Certain Azo Acid Dyes
Environment and Climate Change Canada
Health Canada
June 2016
Table of Contents
List of Tables
- Table 2-1. Description and representative chemical structure of the 20 monoazo acid dyes
- Table 2-2. Identity of the 20 monoazo acid dyes
- Table 2-3. Description and representative chemical structure of the 24 disazo acid dyes
- Table 2-4. Identity of the 24 disazo acid dyes
- Table 2-5. Description and representative chemical structure of the eight polyazo acid dyes
- Table 2-6. Identity of the eight polyazo acid dyes
- Table 2-7. Identities of analogues for monoazo acid dyes and parameters used to inform the physical and chemical properties, environmental fate, potential to cause ecological harm and potential to cause harm to human health
- Table 2-8. Identities of analogues for disazo acid dyes and parameters used to inform the physical and chemical properties, environmental fate, potential to cause ecological harm and potential to cause harm to human health
- Table 2-9. Identities of analogues for polyazo acid dyes and parameters used to inform the physical and chemical properties, environmental fate and potential to cause ecological harm
- Table 3-1. A summary of experimental physical and chemical properties (at standard temperature of ~25°C) for the monoazo acid dyes and their analogues (except where noted)
- Table 3-2. A summary of experimental physical and chemical properties (at standard temperature of ~25°C) for disazo acid dyes and polyazo acid dyes and their analogues (except where noted)
- Table 4-1. Annual import quantity ranges and major uses of certain Azo Acid Dyes in Canada based on Consumer and Commercial Codes (indicated in parentheses) identified in the section 71 survey for the year 2010
- Table 5-1. Select empirical data for biodegradation of Azo Acid Dyes under aerobic conditions
- Table 5-2. Select empirical data for biodegradation of Azo Acid Dyes under anaerobic conditions
- Table 5-3. Empirical data for bioconcentration of Azo Acid Dyes (Tartrazine, CAS RN 1934-21-0)
- Table 6-1. Empirical aquatic toxicity data for fish exposed to representative substances in the monoazo acid dyes subgroup
- Table 6-2. Empirical aquatic toxicity data for fish and invertebrates exposed to representative substances in the disazo acid dyes subgroup
- Table 6-3. Empirical aquatic toxicity data for fish exposed to representative substances in the polyazo acid dyes subgroup
- Table 6-4. Algae toxicity data for Azo Acid Dyes
- Table 7-1. Estimates of dietary exposure to Amaranth and Tartrazine.
- Table 7-2. Summary of highest estimated oral and dermala exposures to Amaranth from use of cosmetic products
- Table 7-3. Summary of highest estimated dermala exposures to New Coccine from use of cosmetic products
- Table 7-4. Summary of highest estimated oral and dermala exposures to Orange II from use of cosmetic products
- Table 7-5. Summary of highest estimated oral and dermala exposures to Tartrazine from use of cosmetics and non-prescription drugs at the cosmetic-drug interface
- Table 7-6. Summary of exposure estimates from use of textilea products for Acid Black 24, Acid Black 26, Acid Blue 113, Acid Orange 33, Acid Red 6, Acid Red 138, CAS RN 70210-05-8, CAS RN 70210-06-9, CAS RN 71873-51-3 and CAS RN 84962-50-5
- Table 7-7. Summary of exposure estimates from use of leathera products for Acid Black 24, Acid Blue 113, CAS RN 68155-63-5,CAS RN 70210-05-8, CAS RN 70210-06-9, CAS RN 70210-25-2 and CAS RN 70210-34-3
- Table 7-8. Evidence considered for azo bond reductive cleavage for 20 monoazo acid dyes
- Table 7-9. Evidence considered for azo bond reductive cleavage for 24 disazo acid dyes
- Table 7-10. Evidence considered for azo bond reductive cleavage for eight polyazo acid dyes
- Table 7-11. Available genotoxicity data on Azo Acid Dyes and unsulfonated aromatic amine metabolites
- Table 7-12. Margins of exposure for Amaranth (cosmetics)
- Table 7-13. Margins of exposure for New Coccine (cosmetic products)
- Table 7-14. Margins of exposure for Orange II (cosmetic products)
- Table 7-15. Margins of exposure for Tartrazine (cosmetics, non-prescription drugs at the cosmetic-drug interface)
- Table 7-16. Margins of exposure for Acid Red 138, CAS RN 71873-51-3,CAS RN 84962-50-5, Acid Orange 33, Acid Red 6, Acid Black 26, CAS RN 70210-05-8, Acid Blue 113, Acid Black 24 and CAS RN 70210-06-9
Synopsis
Pursuant to sections 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment on 52 Azo Acid Dyes. These substances constitute a subgroup of the Aromatic Azo and Benzidine-based Substance Grouping being assessed as part of the Substance Groupings Initiative of the Government of Canada’s Chemicals Management Plan (CMP) based on similar chemical structures and application. Substances in this grouping were identified as priorities for action as they met categorization criteria under subsection 73(1) of CEPA and/or were considered as priority substances under the CMP based on other human health concerns.
The Chemical Abstracts Service Registry Number (CAS RN)Footnote1, Domestic Substances List (DSL) name and Colour Index (C.I.) generic name or common name of the 52 Azo Acid Dyes are presented in the following table.
| CAS RN | DSL name | C.I. name or common name |
|---|---|---|
| 587-98-4 | Benzenesulfonic acid, 3-[[4-(phenylamino)phenyl]azo]-, monosodium salt | Metanil Yellow |
| 633-96-5[a] | Benzenesulfonic acid, 4-[(2-hydroxy-1-naphthalenyl)azo]-, monosodium salt | Orange II |
| 915-67-3a | 2,7-Naphthalenedisulfonic acid, 3-hydroxy-4-[(4-sulfo-1-naphthalenyl)azo]-, trisodium salt | Amaranth |
| 1934-21-0a | 1H-Pyrazole-3-carboxylic acid, 4,5-dihydro-5-oxo-1-(4-sulfophenyl)-4-[(4-sulfophenyl)azo]-, trisodium salt | Tartrazine |
| 2611-82-7a | 1,3-Naphthalenedisulfonic acid, 7-hydroxy-8-[(4-sulfo-1-naphthalenyl)azo]-, trisodium salt | New Coccine |
| 3071-73-6 | 1-Naphthalenesulfonic acid, 8-(phenylamino)-5-[[4-[(5-sulfo-1-naphthalenyl)azo]-1-naphthalenyl]azo]-, disodium salt | Acid Black 24 |
| 3351-05-1 | 1-Naphthalenesulfonic acid, 8-(phenylamino)-5-[[4-[(3-sulfophenyl)azo]-1-naphthalenyl]azo]-, disodium salt | Acid Blue 113 |
| 3761-53-3 | 2,7-Naphthalenedisulfonic acid, 4-[(2,4-dimethylphenyl)azo]-3-hydroxy-, disodium salt | Ponceau MX |
| 6262-07-3 | 2-Naphthalenesulfonic acid, 6-hydroxy-5-[[4-[[4-(phenylamino)-3-sulfophenyl]azo]-1-naphthalenyl]azo]-, disodium salt | Acid Black 26 |
| 6507-77-3 | 1,3-Naphthalenedisulfonic acid, 7-hydroxy-8-[[4-[1-[4-[(4-hydroxyphenyl)azo]phenyl]cyclohexyl]phenyl]azo]-, disodium salt | Acid Orange 33 |
| 15792-43-5 | 2,7-Naphthalenedisulfonic acid, 5-(acetylamino)-3-[(4-dodecylphenyl)azo]-4-hydroxy-, disodium salt | Acid Red 138 |
| 25317-22-0 | 1-Naphthalenesulfonic acid, 3-[[4-(benzoylethylamino)-2-methylphenyl]azo]-4-hydroxy- | Acid Red 6 |
| 29706-48-7 | Benzenesulfonic acid, 3-[[[4-(2-benzothiazolylazo)-3-methylphenyl]ethylamino]methyl]- | NA |
| 35342-16-6 | 7-Benzothiazolesulfonic acid, 2-[4-[(hexahydro-2,4,6-trioxo-5-pyrimidinyl)azo]phenyl]-6-methyl-, monolithium salt | NA |
| 51988-24-0 | Benzenesulfonic acid, 3-[[4-[(4-hydroxy-3-methylphenyl)azo]-3-methoxyphenyl]azo]-, monolithium salt | NA |
| 52236-73-4a | Benzenesulfonic acid, 4-[(5-amino-3-methyl-1-phenyl-1H-pyrazol-4-yl)azo]-2,5-dichloro-, monolithium salt | NA |
| 62133-79-3 | 2-Naphthalenesulfonic acid, 5-[[4-[ethyl[(3-sulfophenyl)methyl]amino]phenyl]azo]-8-(phenylazo)-, disodium salt | NA |
| 62133-80-6 | 2-Naphthalenesulfonic acid, 8-[[4-[ethyl[(3-sulfophenyl)methyl]amino]phenyl]azo]-5-(phenylazo)-, disodium salt | NA |
| 67892-55-1 | 1-Naphthalenesulfonic acid, 5-[[4-[(2-chlorophenyl)azo]-6(or 7)-sulfo-1-naphthalenyl]azo]-8-(phenylamino)-, disodium salt | NA |
| 68155-63-5 | 2,7-Naphthalenedisulfonic acid, 5-[[2,4-dihydroxy-5-[(4-nitrophenyl)azo]phenyl]azo]-4-hydroxy-3-[(2-hydroxy-3,5-dinitrophenyl)azo]-, disodium salt | NA |
| 68555-86-2 | Benzenesulfonic acid, 4-[[5-methoxy-4-[(4-methoxyphenyl)azo]-2-methylphenyl]azo]-, sodium salt | Acid Orange 156 |
| 70210-05-8 | 2,7-Naphthalenedisulfonic acid, 3-[[2,4-bis(2-methylphenoxy)phenyl]azo]-4-hydroxy-5-[[(4-methylphenyl)sulfonyl]amino]-, disodium salt | NA |
| 70210-06-9 | Benzenesulfonic acid, 3-[[ethyl[4-[[4-[(3-sulfophenyl)azo]-1-naphthalenyl]azo]phenyl]amino]methyl]-, disodium salt | NA |
| 70210-25-2 | 2,7-Naphthalenedisulfonic acid, 5-[[2,4-dihydroxy-5-[(2-hydroxy-3,5-dinitrophenyl)azo]phenyl]azo]-4-hydroxy-3-[(4-nitrophenyl)azo]-, disodium salt | NA |
| 70210-34-3 | 2,7-Naphthalenedisulfonic acid, 5-[[2,4-dihydroxy-5-[[4-[(4-nitro-2-sulfophenyl)amino]phenyl]azo]phenyl]azo]-4-hydroxy-3-[[4-[(4-nitro-2-sulfophenyl)amino]phenyl]azo]-, tetrasodium salt | NA |
| 71720-89-3 | 2-Naphthalenesulfonic acid, 5-[[4-(4-cyclohexylphenoxy)-2-sulfophenyl]azo]-6-[(2,6-dimethylphenyl)amino]-4-hydroxy-, disodium salt | NA |
| 71873-51-3 | Benzenesulfonic acid, 2,5-dichloro-4-[4-[[5-[[(dodecyloxy)carbonyl]amino]-2-sulfophenyl]azo]-4,5-dihydro-3-methyl-5-oxo-1H-pyrazol-1-yl]-, disodium salt | NA |
| 72496-92-5 | Naphthalenesulfonic acid, 5-[[2,4-dihydroxy-5-[[4-[(4-nitro-2-sulfophenyl)amino]phenyl]azo]phenyl]azo]-8-[[4-[(4-nitro-2-sulfophenyl)amino]phenyl]azo]-, trisodium salt | NA |
| 72828-67-2 | 1,3-Naphthalenedisulfonic acid, 7-hydroxy-8-[[4-[1-[4-[(4-hydroxyphenyl)azo]phenyl]cyclohexyl]phenyl]azo]-, potassium sodium salt | NA |
| 72828-83-2 | 2,7-Naphthalenedisulfonic acid, 5-(benzoylamino)-3-[[2-(2-cyclohexylphenoxy)phenyl]azo]-4-hydroxy-, disodium salt | NA |
| 72968-80-0 | 2-Naphthalenesulfonic acid, 5-[[4-[[(4-methylphenyl)sulfonyl]oxy]phenyl]azo]-8-[[4-[(4-nitro-2-sulfophenyl)amino]phenyl]azo]-, disodium salt | NA |
| 72968-81-1 | 2-Naphthalenesulfonic acid, 8-[[4-[[(4-methylphenyl)sulfonyl]oxy]phenyl]azo]-5-[[4-[(4-nitro-2-sulfophenyl)amino]phenyl]azo]-, disodium salt | NA |
| 72986-60-8 | 2-Naphthalenesulfonic acid, 5-[[4-[(4-nitro-2-sulfophenyl)amino]phenyl]azo]-8-[[4-[(phenylsulfonyl)oxy]phenyl]azo]-, disodium salt | NA |
| 72986-61-9 | 2-Naphthalenesulfonic acid, 8-[[4-[(4-nitro-2-sulfophenyl)amino]phenyl]azo]-5-[[4-[(phenylsulfonyl)oxy]phenyl]azo]-, disodium salt | NA |
| 75949-73-4 | Benzenesulfonic acid, 4,4′-[methylenebis[4,1-phenyleneazo(4,5-dihydro-3-methyl-5-oxo-1H-pyrazole-4,1-diyl)]]bis[3-methyl-, disodium salt | NA |
| 79234-36-9 | 2,7-Naphthalenedisulfonic acid, 5-(benzoylamino)-3-[[2-(4-cyclohexylphenoxy)phenyl]azo]-4-hydroxy-, disodium salt | NA |
| 83006-48-8 | Benzenesulfonic acid, 4-[4-[[3-[(ethylphenylamino)sulfonyl]-4-methylphenyl]azo]-4,5-dihydro-3-methyl-5-oxo-1H-pyrazol-1-yl]- | NA |
| 83006-74-0 | 1-Naphthalenesulfonic acid, 8-(phenylamino)-5-[[4-[(5-sulfo-1-naphthalenyl)azo]-1-naphthalenyl]azo]-, ammonium sodium salt | NA |
| 83006-77-3 | 1-Naphthalenesulfonic acid, 8-(phenylamino)-5-[[4-[(3-sulfophenyl)azo]-1-naphthalenyl]azo]-, ammonium sodium salt | NA |
| 83027-51-4 | 1,7-Naphthalenedisulfonic acid, 6-[[2-(4-cyclohexylphenoxy)phenyl]azo]-4-[[(2,4-dichlorophenoxy)acetyl]amino]-5-hydroxy-, disodium salt | NA |
| 83027-52-5 | 1,7-Naphthalenedisulfonic acid, 6-[[2-(2-cyclohexylphenoxy)phenyl]azo]-4-[[(2,4-dichlorophenoxy)acetyl]amino]-5-hydroxy-, disodium salt | NA |
| 83221-60-7 | 1,6-Naphthalenedisulfonic acid, 4-[[4-[[1-hydroxy-6-(phenylamino)-3-sulfo-2-naphthalenyl]azo]-1-naphthalenyl]azo]-, ammonium sodium salt | NA |
| 84559-92-2 | 2,7-Naphthalenedisulfonic acid, 3,3′-[azoxybis[(2-methoxy-4,1-phenylene)azo]]bis[4,5-dihydroxy-, tetralithium salt | NA |
| 84962-50-5 | Benzenesulfonic acid, 2,5-dichloro-4-[[2-(dibutylamino)-4-methyl-6-[[2-(4-sulfophenyl)ethyl]amino]-5-pyrimidinyl]azo]-, sodium salt | NA |
| 85030-31-5 | 2,7-Naphthalenedisulfonic acid, 3-hydroxy-4-[[4-[[4-[(2-hydroxy-6-sulfo-1-naphthalenyl)azo]-2-methylphenyl]methyl]-3-methylphenyl]azo]-, sodium salt | NA |
| 85136-25-0 | 2,7-Naphthalenedisulfonic acid, 3,3′-[azoxybis[(2-methoxy-4,1-phenylene)azo]]bis[4,5-dihydroxy-, lithium sodium salt | NA |
| 85223-35-4 102616-51-3[b] |
Benzoic acid, 3,3′-methylenebis[6-[[2,4-dihydroxy-5-[(4-sulfophenyl)azo]phenyl]azo]-, sodium salt | NA |
| 90218-20-5 | Benzenesulfonic acid, 5-amino-2,4-dimethyl-, diazotized, coupled with diazotized 2,4-, 2,5- and 2,6-xylidine and 4-[(2,4-dihydroxyphenyl)azo]benzenesulfonic acid, sodium salts | NA |
| 90432-08-9 | 2,7-Naphthalenedisulfonic acid, 4-amino-5-hydroxy-, diazotized, coupled with diazotized 4-nitro-1,3-benzenediamine and resorcinol, potassium sodium salts | NA |
| 90459-02-2 | 2,7-Naphthalenedisulfonic acid, 5-amino-4-hydroxy-3-[[6-sulfo-4-[(4-sulfo-1-naphthalenyl)azo]-1-naphthalenyl]azo]-, diazotized, coupled with diazotized 4-nitrobenzenamine and resorcinol, potassium sodium salts | NA |
| 106028-58-4 | 2,7-Naphthalenedisulfonic acid, 6-amino-4-hydroxy-3-[[7-sulfo-4-[(4-sulfophenyl)azo]-1-naphthalenyl]azo]-, tetralithium salt | NA |
| 114910-04-2 | 1-Naphthalenediazonium, 4-[[4-[(4-nitro-2-sulfophenyl)amino]phenyl]azo]-6-sulfo-, chloride, reaction products with formaldehyde and salicylic acid, ammonium sodium salts | NA |
Table Notes
Abbreviations: NA: not available
- [a]
- This substance was not identified under subsection 73(1) of CEPA but was included in this assessment as it was considered as a priority based on other human health concerns.
- [b]
- Two CAS RNs represent the same substance.
Azo Acid Dyes are not expected to occur naturally in the environment. No manufacture of any substance above the 100 kg/year reporting threshold has been reported in response to any recent surveys under section 71 of CEPA. Ten substances have been reported as having an import quantity above the 100 kg/year survey reporting threshold (during 2010).
Environment
All Azo Acid Dyes are soluble in water, generally with solubilities well above 1 g/L. Given the import and use of ten Azo Acid Dyes in Canada above reporting thresholds, potential releases to the aquatic environment have been estimated. Considering the physical and chemical properties of these substances, when released to water, it is expected that the Azo Acid Dyes may remain in the water column for relatively long periods of time due to their hydrophilicity, but will ultimately partition to suspended solids, sediments or soil particles via electrostatic interactions. Available experimental and modelled data regarding the abiotic and biotic degradation of the Azo Acid Dyes indicate that these substances are likely to persist in water, sediment and soil. In anaerobic environments (i.e., anoxic layers of sediments), there is the potential for these substances to degrade to aromatic amines as a result of cleavage of the azo bond under anaerobic or reducing conditions.
Although there are limited experimental data available, information on the log octanol-water partition coefficients and fish bioconcentration factors indicates that these substances are not likely to bioconcentrate or bioaccumulate in aquatic organisms.
While all Azo Acid Dyes are structurally related and are expected to have a common mode of action and environmental fate profile, review of physical-chemical and ecotoxicity data allowed them to be divided into subsets of monoazo, disazo and polyazo acid dyes for which aquatic toxicity levels were variable. Disazo acid dyes presented the highest levels of toxicity (effects at concentrations below 10 mg/L) while monoazo acid dyes showed lower toxicity (effects at concentrations below 100 mg/L). Polyazo substances were the least toxic to aquatic organisms (no effects below 100 mg/L). Soil toxicity data were limited, while sediment toxicity data were not available for these substances.
Risk quotient analyses were focused on exposure scenarios representing potential major environmental releases due to industrial activities involving Azo Acid Dyes that may result in high levels of exposure of aquatic organisms. Predicted environmental concentrations were calculated for the aquatic environment for those substances identified in industrial formulation activities. Predicted environmental concentrations were not found to exceed the predicted no-effect concentrations for water.
Considering all available lines of evidence presented in this Screening Assessment, there is low risk of harm to organisms and the broader integrity of the environment from the Azo Acid Dyes evaluated in this assessment. It is concluded that these 52 Azo Acid Dyes do not meet the criteria under paragraph 64(a) or 64(b) of CEPA, as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Human Health
Environmental media are not considered to be a significant source of exposure to Azo Acid Dyes for the general population in Canada. Seventeen Azo Acid Dyes (i.e., Acid Black 24, Acid Black 26, Acid Blue 113, Acid Orange 33, Acid Red 6, Acid Red 138, Amaranth, New Coccine, Orange II, Tartrazine, CAS RN 68155-63-5, CAS RN 70210-05-8, CAS RN 70210-06-9, CAS RN 70210-25-2, CAS RN 70210-34-3, CAS RN 71873-51-3 and CAS RN 84962-50-5) were indicated to be present in products in the Canadian marketplace that may lead to exposure of the general population to these substances. Exposure to Amaranth and Tartrazine occurs predominantly through food intake and use of cosmetics, as well as through use of certain non-prescription drugs (including natural health products, regulated under the Food and Drug Regulations and Natural Health Products Regulations) at the cosmetic-drug interface for Tartrazine. Exposure to New Coccine and Orange II is predominantly through use of cosmetics. Exposure to the other 13 Azo Acid Dyes is through contact with textile and/or leather products.
Margins between the upper-bounding estimates of oral exposure to Amaranth from food and cosmetics and the critical health effect level from a chronic dietary study in rats are considered adequate to address uncertainties in the health effects and exposure databases.
Margins between the upper-bounding estimates of exposure to New Coccine in certain cosmetics and the critical health effect levels are considered adequate to address uncertainties in the health effects and exposure databases.
Margins between the upper-bounding estimates of oral exposure to Orange II in lipstick and the critical health effect levels determined in oral studies in laboratory animals are considered adequate to address uncertainties in the health effects and exposure databases. Similarly, margins between the upper-bounding estimates of dermal exposure to Orange II in certain cosmetics and the no-observed-effect level from the dermal study in mice are considered adequate to address uncertainties in the health effects and exposure databases.
Margins between the upper-bounding estimates of exposure to Tartrazine in food, cosmetics and non-prescription drugs at the cosmetic-drug interface, and the critical health effect levels are considered adequate to address uncertainties in the health effects and exposure databases.
A range of critical effect levels from oral repeated-dose studies on Azo Acid Dyes in this subgroup and relevant analogues was identified. However, no effects were observed in a chronic study in which mice received weekly applications to the skin of several Azo Acid Dyes in this subgroup. These data were the basis for the risk characterization of 10 Azo Acid Dyes lacking empirical data (i.e., Acid Red 138, CAS RN 71873-51-3, CAS RN 84962-50-5, Acid Orange 33, Acid Red 6, Acid Black 26, CAS RN 70210-05-8, Acid Blue 113, Acid Black 24 and CAS RN 70210-06-9). Margins between upper-bounding estimates of oral exposure via mouthing of textile objects by infants and the range of oral critical effect levels were considered adequate to address uncertainties in the health effects and exposure databases. Margins between upper-bounding estimates of dermal exposure from direct and prolonged contact with textiles containing these dyes and the no-observed-effect level from the dermal study were considered adequate to address uncertainties in the health effects and exposure databases.
For three Azo Acid Dyes (i.e., CAS RN 68155-63-5, CAS RN 70210-25-2 and CAS RN 70210-34-3), the only potential exposure of the general population identified was from use of leather products. As exposures to leather products are considered short term and intermittent and as available information does not indicate that Azo Acid Dyes demonstrate high acute toxicity, the risk to human health for the general population is expected to be low.
For the remaining 35 Azo Acid Dyes, available information did not identify potential for direct and prolonged exposure of the general population. Accordingly, risk for the general population to these substances is not expected.
Some of the Azo Acid Dyes addressed in this assessment have effects of concern based on potential carcinogenicity. Whereas available information does not indicate a risk to human health for Canadians at current exposure levels to these substances, there may be a concern if exposures were to increase.
Based on the information presented in this Screening Assessment, it is concluded that the 52 Azo Acid Dyes evaluated in this assessment do not meet the criteria for human health effects under paragraph 64(c) of CEPA as they are not currently entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Overall Conclusion
It is concluded that the 52 Azo Acid Dyes evaluated in this assessment do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to sections 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and Climate Change and the Minister of Health conduct screening assessments of substances to determine whether these substances present or may present a risk to the environment or to human health.
The Substance Groupings Initiative is a key element of the Government of Canada’s Chemicals Management Plan (CMP). The Aromatic Azo and Benzidine-based Substance Grouping consists of 358 substances that were identified as priorities for assessment, as they met the categorization criteria under section 73 of CEPA and/or were considered as a priority based on human health concerns (Environment Canada and Health Canada 2007). Some substances within this Substance Grouping have been identified by other jurisdictions as a concern due to the potential cleavage of the azo bonds, which can lead to the release of aromatic amines that are known or likely to be carcinogenic.
While many of these substances have common structural features and similar functional uses as dyes or pigments in multiple sectors, diversity within the substance group has been taken into account through the establishment of subgroups. Subgrouping based on structural similarities, physical and chemical properties, and common functional uses and applications accounts for variability within this Substance Grouping and allows for subgroup-specific approaches in the conduct of screening assessments. This Screening Assessment considers substances that belong to the Azo Acid Dyes subgroup. Consideration of potential azo bond cleavage products (aromatic amines) is a key element of human health assessment in each subgroup. Some aromatic amines, commonly referred to as EU22 aromatic aminesFootnote3, as well as associated azo dyes, are restricted in other countries (EU 2006). Information on the CMP subgrouping approach for the Aromatic Azo and Benzidine-based Substance Grouping, as well as additional background information and regulatory context, is provided in a previously published document (Environment Canada and Health Canada 2013).
Screening assessments focus on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA, by examining scientific information to develop conclusions based on a weight of evidence approach and using precautionFootnote4.
This Screening Assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposure, including additional information submitted by stakeholders. Relevant data were identified up to April 2013. Empirical data from key studies as well as some results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
The Screening Assessment does not represent an exhaustive or critical review of all available data. Rather, it presents the most critical studies and lines of evidence pertinent to the conclusion.
The Screening Assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological and human health portions of this assessment have undergone external peer review and/or consultation. Comments on the technical portions relevant to the environment were received from Dr. Harold Freeman (North Carolina State University, USA) and Dr. Gisela Umbuzeiro (University of Campinas, Brazil). Comments on the technical portions relevant to human health were received from Dr. Harold Freeman (North Carolina State University, USA), Dr. David Josephy (University of Guelph, Canada), Dr. Michael Bird (University of Ottawa, Canada) and Dr. Kannan Krishnan (Université de Montréal, Canada). Additionally, the draft of this Screening Assessment was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the Screening Assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
The critical information and considerations upon which the Screening Assessment is based are given below.
2. Identity of Substances
This Screening Assessment focuses on 52 substances in the subgroup of Azo Acid Dyes that is part of the Aromatic Azo and Benzidine-based Substance Grouping. This subgroup is based on structural similarity and similar applications. One substance, CAS RN 106028-58-4, classified as a food dye in the Subgrouping and Background document (Environment Canada and Health Canada 2013) has similar uses and a chemical structure analogous to others in this subgroup, therefore it will be considered an Azo Acid Dye for the purpose of this Screening Assessment.
The Azo Acid Dyes subgroup includes three subsets based on the number of azo functional groups: monoazo (one azo functional group), disazo (two azo functional groups) and polyazo (three or four azo functional groups, known as tris- and tetrakis- azo, respectively). For ecological purposes, monoazo, disazo and polyazo acid dyes are discussed separately when sufficient data are available, as there is some variation in the molecular weights, certain physical-chemical properties and ecotoxicities of the three subsets.
The subsets and identities of the individual Azo Acid Dyes in each subset are presented in Tables 2-1 to 2-6. When available, a Colour Index (C.I.) or recognized common name is used to identify the substance. If these terms are unavailable, the Chemical Abstracts Service Registry Number (CAS RN) is used as the primary identifier. A list of additional chemical names (e.g., trade names) is available from the National Chemical Inventories (NCI 2012).
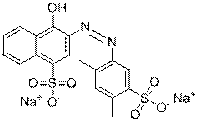
| Description of critical functional groups | Azo group (1), benzene rings (2-4), sulfonic groups (1-3), naphthalene groups (1-2), carboxyl group (0-1), pyrazole ring (1), sodium or lithium |
|---|---|
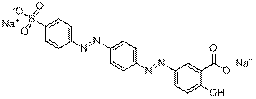
| Representative chemical structure |  C16H8N4O9S2Na4(CAS RN 1934-21-0) |
| CAS RN | DSL name | C.I. name and/or common name | Molecular weight (g/mol) |
|---|---|---|---|
| 587-98-4 | Benzenesulfonic acid, 3-[[4-(phenylamino)phenyl]azo]-, monosodium salt | Metanil Yellow (also known as Acid Yellow 36) | 375 |
| 633-96-5 | Benzenesulfonic acid, 4-[(2-hydroxy-1-naphthalenyl)azo]-, monosodium salt | Orange II (also known as Acid Orange 7) | 351 |
| 915-67-3 | 2,7-Naphthalenedisulfonic acid, 3-hydroxy-4-[(4-sulfo-1-naphthalenyl)azo]-, trisodium salt | Amaranth (also known as C.I. Food Red 9, Acid Red 27) | 604 |
| 1934-21-0 | 1H-Pyrazole-3-carboxylic acid, 4,5-dihydro-5-oxo-1-(4-sulfophenyl)-4-[(4-sulfophenyl)azo]-, trisodium salt | Tartrazine (also known as Acid Yellow 23, C.I. Food Yellow 4) | 534 |
| 2611-82-7 | 1,3-Naphthalenedisulfonic acid, 7-hydroxy-8-[(4-sulfo-1-naphthalenyl)azo]-, trisodium salt | New Coccine (also known as C.I. Food Red 7, Acid Red 18, Ponceau 4R) | 604 |
| 3761-53-3 | 2,7-Naphthalenedisulfonic acid, 4-[(2,4-dimethylphenyl)azo]-3-hydroxy-, disodium salt | Ponceau MX (also known as Acid Red 26, C.I. Food Red 5) | 480 |
| 15792-43-5 | 2,7-Naphthalenedisulfonic acid, 5-(acetylamino)-3-[(4-dodecylphenyl)azo]-4-hydroxy-, disodium salt | Acid Red 138 | 678 |
| 25317-22-0 | 1-Naphthalenesulfonic acid, 3-[[4-(benzoylethylamino)-2-methylphenyl]azo]-4-hydroxy- | Acid Red 6 | 490 |
| 29706-48-7 | Benzenesulfonic acid, 3-[[[4-(2-benzothiazolylazo)-3-methylphenyl]ethylamino]methyl]- | NA | 467 |
| 35342-16-6 | 7-Benzothiazolesulfonic acid, 2-[4-[(hexahydro-2,4,6-trioxo-5-pyrimidinyl)azo]phenyl]-6-methyl-, monolithium salt | NA | 465 |
| 52236-73-4 | Benzenesulfonic acid, 4-[(5-amino-3-methyl-1-phenyl-1H-pyrazol-4-yl)azo]-2,5-dichloro-, monolithium salt | NA | 432 |
| 70210-05-8 | 2,7-Naphthalenedisulfonic acid, 3-[[2,4-bis(2-methylphenoxy)phenyl]azo]-4-hydroxy-5-[[(4-methylphenyl)sulfonyl]amino]-, disodium salt | NA | 834 |
| 71720-89-3 | 2-Naphthalenesulfonic acid, 5-[[4-(4-cyclohexylphenoxy)-2-sulfophenyl]azo]-6-[(2,6-dimethylphenyl)amino]-4-hydroxy-, disodium salt | NA | 746 |
| 71873-51-3 | Benzenesulfonic acid, 2,5-dichloro-4-[4-[[5-[[(dodecyloxy)carbonyl]amino]-2-sulfophenyl]azo]-4,5-dihydro-3-methyl-5-oxo-1H-pyrazol-1-yl]-, disodium salt | NA | 779 |
| 72828-83-2 | 2,7-Naphthalenedisulfonic acid, 5-(benzoylamino)-3-[[2-(2-cyclohexylphenoxy)phenyl]azo]-4-hydroxy-, disodium salt | NA | 746 |
| 79234-36-9 | 2,7-Naphthalenedisulfonic acid, 5-(benzoylamino)-3-[[2-(4-cyclohexylphenoxy)phenyl]azo]-4-hydroxy-, disodium salt | NA | 746 |
| 83006-48-8 | Benzenesulfonic acid, 4-[4-[[3-[(ethylphenylamino)sulfonyl]-4-methylphenyl]azo]-4,5-dihydro-3-methyl-5-oxo-1H-pyrazol-1-yl]- | NA | 556 |
| 83027-51-4 | 1,7-Naphthalenedisulfonic acid, 6-[[2-(4-cyclohexylphenoxy)phenyl]azo]-4-[[(2,4-dichlorophenoxy)acetyl]amino]-5-hydroxy-, disodium salt | NA | 845 |
| 83027-52-5 | 1,7-Naphthalenedisulfonic acid, 6-[[2-(2-cyclohexylphenoxy)phenyl]azo]-4-[[(2,4-dichlorophenoxy)acetyl]amino]-5-hydroxy-, disodium salt | NA | 845 |
| 84962-50-5 | Benzenesulfonic acid, 2,5-dichloro-4-[[2-(dibutylamino)-4-methyl-6-[[2-(4-sulfophenyl)ethyl]amino]-5-pyrimidinyl]azo]-, sodium salt | NA | 718 |
Abbreviation: NA: not available.
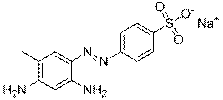
| Group description with critical functional groups | Azo groups (2), benzene rings (1-4), sulfonic acid groups (1-4), naphthalene groups (2-3) |
|---|---|
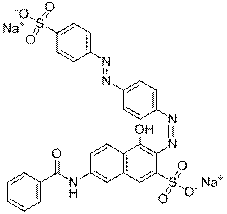
| Representative chemical structure | 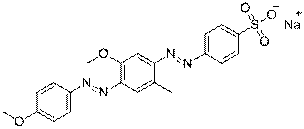 C21H19N4O5S1Na (CAS RN 68555-86-2) |
| CAS RN | DSL name | C.I. name and/or common name | Molecular weight (g/mol) |
|---|---|---|---|
| 3071-73-6 | 1-Naphthalenesulfonic acid, 8-(phenylamino)-5-[[4-[(5-sulfo-1-naphthalenyl)azo]-1-naphthalenyl]azo]-, disodium salt | Acid Black 24 | 732 |
| 3351-05-1 | 1-Naphthalenesulfonic acid, 8-(phenylamino)-5-[[4-[(3-sulfophenyl)azo]-1-naphthalenyl]azo]-, disodium salt | Acid Blue 113 | 682 |
| 6262-07-3 | 2-Naphthalenesulfonic acid, 6-hydroxy-5-[[4-[[4-(phenylamino)-3-sulfophenyl]azo]-1-naphthalenyl]azo]-, disodium salt | Acid Black 26 | 698 |
| 6507-77-3 | 1,3-Naphthalenedisulfonic acid, 7-hydroxy-8-[[4-[1-[4-[(4-hydroxyphenyl)azo]phenyl]cyclohexyl]phenyl]azo]-, disodium salt | Acid Orange 33 | 731 |
| 51988-24-0 | Benzenesulfonic acid, 3-[[4-[(4-hydroxy-3-methylphenyl)azo]-3-methoxyphenyl]azo]-, monolithium salt | NA | 432 |
| 62133-79-3 | 2-Naphthalenesulfonic acid, 5-[[4-[ethyl[(3-sulfophenyl)methyl]amino]phenyl]azo]-8-(phenylazo)-, disodium salt | NA | 674 |
| 62133-80-6 | 2-Naphthalenesulfonic acid, 8-[[4-[ethyl[(3-sulfophenyl)methyl]amino]phenyl]azo]-5-(phenylazo)-, disodium salt | NA | 674 |
| 67892-55-1 | 1-Naphthalenesulfonic acid, 5-[[4-[(2-chlorophenyl)azo]-6(or 7)-sulfo-1-naphthalenyl]azo]-8-(phenylamino)-, disodium salt | NA | 717 |
| 68555-86-2 | Benzenesulfonic acid, 4-[[5-methoxy-4-[(4-methoxyphenyl)azo]-2-methylphenyl]azo]-, sodium salt | Acid Orange 156 | 462 |
| 70210-06-9 | Benzenesulfonic acid, 3-[[ethyl[4-[[4-[(3-sulfophenyl)azo]-1-naphthalenyl]azo]phenyl]amino]methyl]-, disodium salt | NA | 674 |
| 72828-67-2 | 1,3-Naphthalenedisulfonic acid, 7-hydroxy-8-[[4-[1-[4-[(4-hydroxyphenyl)azo]phenyl]cyclohexyl]phenyl]azo]-, potassium sodium salt | NA | 747 |
| 72968-80-0 | 2-Naphthalenesulfonic acid, 5-[[4-[[(4-methylphenyl)sulfonyl]oxy]phenyl]azo]-8-[[4-[(4-nitro-2-sulfophenyl)amino]phenyl]azo]-, disodium salt | NA | 847 |
| 72968-81-1 | 2-Naphthalenesulfonic acid, 8-[[4-[[(4-methylphenyl)sulfonyl]oxy]phenyl]azo]-5-[[4-[(4-nitro-2-sulfophenyl)amino]phenyl]azo]-, disodium salt | NA | 845 |
| 72986-60-8 | 2-Naphthalenesulfonic acid, 5-[[4-[(4-nitro-2-sulfophenyl)amino]phenyl]azo]-8-[[4-[(phenylsulfonyl)oxy]phenyl]azo]-, disodium salt | NA | 833 |
| 72986-61-9 | 2-Naphthalenesulfonic acid, 8-[[4-[(4-nitro-2-sulfophenyl)amino]phenyl]azo]-5-[[4-[(phenylsulfonyl)oxy]phenyl]azo]-, disodium salt | NA | 833 |
| 75949-73-4 | Benzenesulfonic acid, 4,4′-[methylenebis[4,1-phenyleneazo(4,5-dihydro-3-methyl-5-oxo-1H-pyrazole-4,1-diyl)]]bis[3-methyl-, disodium salt | NA | 803 |
| 83006-74-0 | 1-Naphthalenesulfonic acid, 8-(phenylamino)-5-[[4-[(5-sulfo-1-naphthalenyl)azo]-1-naphthalenyl]azo]-, ammonium sodium salt | NA | 767 |
| 83006-77-3 | 1-Naphthalenesulfonic acid, 8-(phenylamino)-5-[[4-[(3-sulfophenyl)azo]-1-naphthalenyl]azo]-, ammonium sodium salt | NA | 677 |
| 83221-60-7 | 1,6-Naphthalenedisulfonic acid, 4-[[4-[[1-hydroxy-6-(phenylamino)-3-sulfo-2-naphthalenyl]azo]-1-naphthalenyl]azo]-, ammonium sodium salt | NA | 1085 |
| 85030-31-5 | 2,7-Naphthalenedisulfonic acid, 3-hydroxy-4-[[4-[[4-[(2-hydroxy-6-sulfo-1-naphthalenyl)azo]-2-methylphenyl]methyl]-3-methylphenyl]azo]-, sodium salt | NA | 843 |
| 90218-20-5[a] | Benzenesulfonic acid, 5-amino-2,4-dimethyl-, diazotized, coupled with diazotized 2,4-, 2,5- and 2,6-xylidine and 4-[(2,4-dihydroxyphenyl)azo]benzenesulfonic acid, sodium salts | NA | 550 |
| 90432-08-9a | 2,7-Naphthalenedisulfonic acid, 4-amino-5-hydroxy-, diazotized, coupled with diazotized 4-nitro-1,3-benzenediamine and resorcinol, potassium sodium salts | NA | 664 |
| 106028-58-4 | 2,7-Naphthalenedisulfonic acid, 6-amino-4-hydroxy-3-[2-[7-sulfo-4-[2-(4-sulfophenyl)diazenyl]-1-naphthalenyl]diazenyl]-, lithium salt | NA | 766 |
| 114910-04-2a | 1-Naphthalenediazonium, 4-[[4-[(4-nitro-2-sulfophenyl)amino]phenyl]azo]-6-sulfo-, chloride, reaction products with formaldehyde and salicylic acid, ammonium sodium salts | NA | 732 |
Table 2-4 Notes
Abbreviation: NA, not available.
- [a]
- UVCB, unknown or variable composition, complex reaction products, or biological materials. Note that molecular weight is provided for a representative structure.
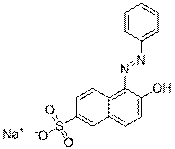
| Group description with critical functional groups | Azo groups(3-4), benzene rings (2-6), sulfonic acid groups (2-4), naphthalene groups (1-2) |
|---|---|
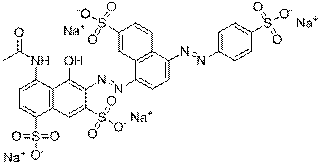
| Representative chemical structure | 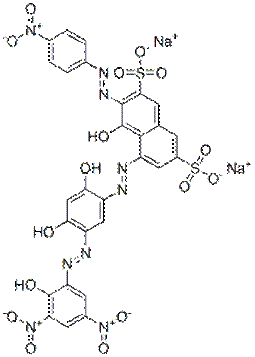 C28H15N9O16S2Na2(CAS RN 70210-25-2) |
| CAS RN | DSL name | C.I. name and/or common name | Molecular weight (g/mol) |
|---|---|---|---|
| 68155-63-5 | 2,7-Naphthalenedisulfonic acid, 5-[[2,4-dihydroxy-5-[(4-nitrophenyl)azo]phenyl]azo]-4-hydroxy-3-[(2-hydroxy-3,5-dinitrophenyl)azo]-, disodium salt | NA | 844 |
| 70210-25-2 | 2,7-Naphthalenedisulfonic acid, 5-[[2,4-dihydroxy-5-[(2-hydroxy-3,5-dinitrophenyl)azo]phenyl]azo]-4-hydroxy-3-[(4-nitrophenyl)azo]-, disodium salt | NA | 844 |
| 70210-34-3 | 2,7-Naphthalenedisulfonic acid, 5-[[2,4-dihydroxy-5-[[4-[(4-nitro-2-sulfophenyl)amino]phenyl]azo]phenyl]azo]-4-hydroxy-3-[[4-[(4-nitro-2-sulfophenyl)amino]phenyl]azo]-, tetrasodium salt | NA | 1169 |
| 72496-92-5 | Naphthalenesulfonic acid, 5-[[2,4-dihydroxy-5-[[4-[(4-nitro-2-sulfophenyl)amino]phenyl]azo]phenyl]azo]-8-[[4-[(4-nitro-2-sulfophenyl)amino]phenyl]azo]-, trisodium salt | NA | 1051 |
| 84559-92-2 | 2,7-Naphthalenedisulfonic acid, 3,3′-[azoxybis[(2-methoxy-4,1-phenylene)azo]]bis[4,5-dihydroxy-, tetralithium salt | NA | 975 |
| 85136-25-0 | 2,7-Naphthalenedisulfonic acid, 3,3′-[azoxybis[(2-methoxy-4,1-phenylene)azo]]bis[4,5-dihydroxy-, lithium sodium salt | NA | 1006 |
| 85223-35-4 (102616-51-3[a]) | Benzoic acid, 3,3'-methylenebis[6-[[2,4-dihydroxy-5-[(4-sulfophenyl)azo]phenyl]azo]-, sodium salt | NA | 980 |
| 90459-02-2[b] | 2,7-Naphthalenedisulfonic acid, 5-amino-4-hydroxy-3-[[6-sulfo-4-[(4-sulfo-1-naphthalenyl)azo]-1-naphthalenyl]azo]-, diazotized, coupled with diazotized 4-nitrobenzenamine and resorcinol, potassium sodium salts | NA | 788 |
Table 2-6 Notes
Abbreviation: NA, not available
- [a]
- CAS RN 102616-51-3 has been removed from the CAS registry, as it is the same as CAS RN 85223-35-4.
- [b]
- UVCB, unknown or variable composition, complex reaction products, or biological materials. Note that molecular weight is provided for a representative structure.
2.1 Selection of Analogues and Use of (Q)SAR Models
Guidance on the use of read-across approaches has been prepared by various organizations, such as the Organisation for Economic Co-operation and Development (OECD 2014). It has been applied in various regulatory programs, including the European Union’s (EU) Existing Substances Programme. The general method for analogue selection and the use of (quantitative) structure-activity relationship ((Q)SAR) models for the Azo Acid Dyes is reported in Environment Canada and Health Canada (2013).
Analogues used to inform the ecological assessment were selected based on structural similarity and the availability of relevant empirical data pertaining to physical-chemical properties, persistence, bioaccumulation and ecotoxicity. Such data were used as read-across data for those Azo Acid Dyes that lacked empirical data, where appropriate, or to support the weight of evidence of existing empirical information. Although analogue data are used preferentially to fill data gaps for the substances in this assessment, the applicability of (Q)SAR models to the Azo Acid Dyes is determined on a case-by-case basis.
A list of the various analogues used to inform this assessment is presented in Tables 2-7 to 2-9, along with an indication of the potential read-across data available for different parameters. All of these substances are azo compounds, most of them being azo acid, direct or food dyes. More detailed information regarding the identity of these substances can be found in Appendix A (Tables A-6 and A-7). In the cases where analogues for one azo group are used for multiple subsets, this is indicated in the text.
| C.I. name and/or common name (CAS RN) | Chemical structure and formula | Molecular weight (g/mol) | Parameters to be used in the read-across approach |
|---|---|---|---|
| Acid Yellow 17 (6359-98-4) |
 C16H10Cl2N4O7S2Na2 |
551 | Water solubility, pKa, ecotoxicity |
| C.I. Food Red 3 (3567-69-9) |
 C20H12N2O7S2Na2 |
502 | Melting or decomposition point, water solubility health effects |
| NA (22915-90-8) |
 C20H14N2O7S2 |
458 | Water solubility |
| Acid Orange 10 (1936-15-8) |
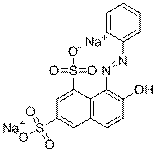 C16H10N2O7S2Na2 |
452 | Melting or decomposition point, water solubility, pKa, volatility, health effects |
| C.I. Food Yellow 3 (2783-94-0) |
 C16H12N2O7S2Na2 |
454 | Melting or decomposition point, water solubility, log Kow, pKa, volatility, ecotoxicity, health effects |
| Ponceau 3R (3564-09-8) |
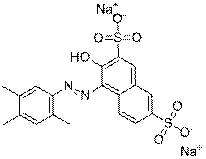 C19H16N2O7S2Na2 |
494 | Health effects |
| C.I. Food Red 1, Ponceau SX (4548-53-2) |
 C18H14N2O7S2Na2 |
480 | Health effects |
| Acid Red 1 (3734-67-6) |
 C18H13N3O8S2Na2 |
509 | Health effects |
| Brown FK (6300-61-4) |
 C13H13N4O3SNa |
328 | Health effects |
| Orange RN (1934-20-9) |
 C16H11N2O4SNa |
350 | Health effects |
Abbreviations: Kow: octanol-water partition coefficient; NA: not available; pKa: acid dissociation constant
| C.I. name and/or common name (CAS RN) | Chemical structure and formula | Molecular weight (g/mol) | Parameters to be used in the read-across approach |
|---|---|---|---|
| Acid Black 1 (1064-48-8) | 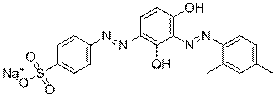 C22H14N6O9S2Na2 |
Water solubility | |
| NA (3564-27-0) |
 C19H12N4O6SNa2 |
470 | Water solubility |
| Direct Red 81 (2610-11-9) |
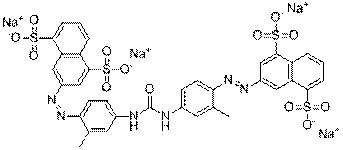
 C29H19N5O8S2Na2 |
676 | Melting or decomposition point, ecotoxicity |
| C.I. Food Black 1[a] (2519-30-4) |
 C28H17N5O14S4Na4 |
868 | Water solubility, volatility, health effects |
| Direct Yellow 50a (3214-47-9) |
 C35H24N6O13S4Na4 |
957 | Water solubility, volatility, ecotoxicity |
| NA (72245-50-2) |
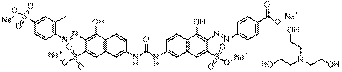 C41H37N7O17S3Na4 |
1088 | Ecotoxicity |
| NA (4553-89-3) |
 C27H18N4O9S2Na2 |
653 | Water solubility, health effects |
Table 2-8 Note
Abbreviations: Kow, octanol-water partition coefficient
- [a]
- CAS RNs 2519-30-4 and 3214-47-9 are also used as analogues for polyazo acid dyes
| C.I. name and/or common name (CAS RN) | Chemical structure and formula | Molecular weight (g/mol) | Parameters to be used in the read-across approach |
|---|---|---|---|
| Acid Orange 24 (1320-07-6) |
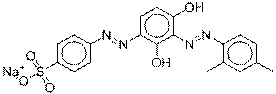 C20H17N4OSNa4 |
448 | Ecotoxicity |
| Acid Black 1 (1064-48-8) |
 C22H14N6O9S2Na2 |
616 | Melting or decomposition point, water solubility, log Kow, ecotoxicity |
| NA (90432-06-7) |
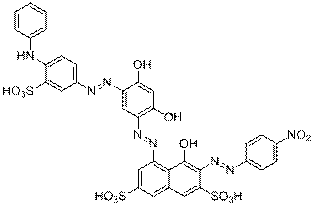 C10H9NO7S2 |
865 | Ecotoxicity |
| NA (70210-31-0) |
 C43H26N8O14S4Na4 |
1099 | Ecotoxicity |
| NA (72829-12-0) |
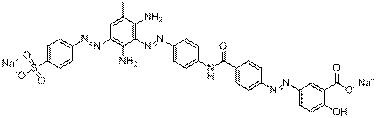 C33H25N9O7SNa2 |
738 | Ecotoxicity |
| NA (108936-08-9) |
 C34H24N9O11S3Li3 |
852 | Ecotoxicity |
3. Physical and Chemical Properties
Physical and chemical properties play a critical role in the overall characteristics of a substance and are used to determine the suitability of different substances for different applications. Such properties also play a critical role in determining the environmental fate of substances (including their potential for long-range transport), as well as their toxicity to humans and non-human organisms.
Several physical and chemical properties of Azo Acid Dyes--namely, melting point, water solubility, size, log octanol-water partition coefficient (log Kow)are important in terms of ecological and human health assessment. A summary of the experimental physical and chemical properties relevant to the environmental fate and ecotoxicity of monoazo as well as disazo and polyazo acid dyes and their analogues is presented in Tables 3-1 and 3-2 Pivotal values, including either single mean data points (e.g., melting point and decomposition) or a range of values, have been chosen to represent the properties of each subset. Detailed substance-specific information for these properties can be found in Tables A4-A7 in Appendix A of this report.
The Azo Acid Dyes are substances with molar weights distributed over a wide range (i.e., 319-1684 g/mol). Most Azo Acid Dyes are complex anionic molecules that tend to dissociate at environmentally relevant pH levels and are highly soluble in water (generally with water solubilities greater than 1 g/L) due to the presence of solubilizing functional groups (Hunger 2003; Tables 3-1 and 3-2). Azo Acid Dyes are primarily sodium and lithium salts that contain functional groups such as sulfonic and carboxylic acids, which increase solubility. Given their hydrophilicity and ionic character, as demonstrated by low pKa values (an indicator of acid dissociation), Azo Acid Dyes tend to have very low (even negative) experimental log Kow values (Tables 3-1 and 3-2).
| Property | Value(s) or range (for more than three data points) | Pivotal value(s) for this risk assessment (basis for selection) |
|---|---|---|
| Molar weight (g/mol)[a] | 351-845 | 351-845 (range was used) |
| Melting point and decomposition (°C) | 141-390 (n = 10) | 141-390 (range was used) |
| Water solubility (g/L) | 2-300 (n = 11) | 2-300 (range was used) |
| Log Kow (dimensionless) | -1.28 to 0.7 (n = 6) | -0.59 (mean) |
| Dmin (nm)a | 0.820-1.28 (n = 20) | Range was used and discussed in text |
| Dmax (nm)a | 0.919-1.70 (n = 20) | Range was used and discussed in text |
| pKa (dimensionless) | 5.5-11.5 (n = 6) | 5.5-11.5 (range was used) |
Table 3-1 Note
Abbreviations: Dmax: effective maximum cross-sectional diameter; Dmin: effective minimum cross-sectional diameter; Kow: octanol-water partition coefficient; pKa: acid dissociation constant.
- [a]
- Molar weight and cross-sectional diameter ranges are shown here only for the specific 20 monoazo acid dyes included in this assessment (i.e., analogue data are not included).
| Property | Value(s) or range (for more than three data points) | Pivotal value(s) for this risk assessment (basis for selection) |
|---|---|---|
| Molar weight (g/mol)[a] | 319-1684 | 319-1684 (range was used) |
| Melting point and decomposition (°C) | 240 - greater than 350 (n = 2) | 240 - greater than 350 (range was used) |
| Water solubility (g/L) | 1.8-180 (n = 4) | 1.8-180 (range was used) |
| Log Kow (dimensionless) | -4.5, 1.2 (n = 2) | -1.65 (mean) |
| Dmin (nm)a | 0.857-1.47 (n = 26) | Range was used and discussed in text |
| Dmax (nm)a | 1.19-1.99 (n = 26) | Range was used and discussed in text |
| pKa (dimensionless) | NA | NA |
Table 3-2 Notes
Abbreviations: Dmax: effective maximum cross-sectional diameter; Dmin: effective minimum cross-sectional diameter; Kow: octanol-water partition coefficient; pKa: acid dissociation constant.
- [a]
- Molar weight and cross-sectional diameter ranges are shown here only for the specific 24 disazo acid dyes and 8 polyazo acid dyesincluded in this assessment (i.e., analogue data are not included).
Physical and chemical data are more prevalent for monazo acid dyes than for disazo and polyazo acid dyes, which are data poor. However, while there are some differences in the ranges demonstrated among the two groups, the general trend of highly anionic substances with high water solubility and low log Kow is observed. Disazo and polyazo acid dyes tend to have higher molar weights and cross-sectional diameters and lower water solubilities. Results pertaining to cross-sectional diameter are discussed further in the section "Potential for Bioaccumulation."
Similar to other azo colourants, Azo Acid Dyes may undergo tautomerization between the azo and hydrazone forms. This tautomerization is well known for the Azo Acid Dyes, where a hydroxyl group and the azo bond are present in the ortho- or para- position. Tautomerization is important commercially, since the tautomeric forms may differ in colour, performance properties, toxicological profile and tinctorial strength (Environment Canada and Health Canada 2013). However, the degree to which this affects the fate and behaviour of these substances in the environment or their toxicological properties is not well understood.
4. Sources and Uses
4.1 Sources
All of the Azo Acid Dyes are anthropogenically produced and are not expected to occur naturally in the environment.
In recent years (i.e., 2005 to present), all 52 substances considered in this Screening Assessment have been included in surveys issued pursuant to section 71 of CEPA. These surveys aimed to collect information on manufacturing and import activities in Canada, with a reporting threshold of 100 kg/year. One survey, which focused on the commercial activity of the Aromatic Azo and Benzidine-based Substance Grouping for the year 2010 (Canada 2011), yielded submissions above the reporting threshold.
Based on the information received from the survey conducted for the year 2010 (Canada 2011), no manufacturing activity in Canada was reported for these Azo Acid Dyes. Importation data for 10 substances found to be used in commerce above the survey threshold are summarized in Table 4-1.
| C.I. name or CAS RN | 2010 annual import quantity ranges (kg) | Food and beverage (C562) | Fabric, textile and leather articles (C104) | Drugs (C563) |
|---|---|---|---|---|
| Tartrazine[b] | 10 000 - 100 000 | X | ||
| Amaranthb | 10 000 - 100 000 | X | ||
| New Coccine[c] | 1 000 - 10 000 | X | X | |
| 70210-34-3 | 1 000 - 10 000 | X | ||
| Metanil Yellow | 100 - 1 000 | |||
| Acid Orange 156 | 100 - 1 000 | |||
| Acid Blue 113 | 100 - 1 000 | X | ||
| 68155-63-5 | 100 - 1 000 | X | ||
| 70210-05-8 | 100 - 1 000 | |||
| 71873-51-3 | 100 - 1 000 | X |
Table 4-1 Notes
- [a]
- Other Consumer and Commercial Codes were also submitted for Acid Orange 156, Metanil Yellow and CAS RN 70210-05-8 but are not indicated in this table due to confidentiality.
- [b]
- Additional information regarding uses of Amaranth and Tartrazine that was submitted in response to a section 71 survey is not included in this table due to confidentiality.
- [c]
- New Coccine is a non-permitted food additive--it is not listed in the List of Permitted Colouring Agents (Health Canada 2012). Follow-up with the submitter has been initiated regarding its use of this substance.
Nine of the ten substances in Table 4-1 (all except for CAS RN 68155-63-5), as well as ten additional substances (i.e., Orange II, Acid Black 24, Acid Black 26, Acid Orange 33, Acid Red 138, Acid Red 6, CAS RN 70210-06-9, CAS RN 70210-25-2, CAS RN 72828-67-2 and CAS RN 84962-50-5), were identified as being used in Canada in 2010, based on information submitted by the Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers (ETAD) (personal communication, email from ETAD to Environment Canada, dated August 2010; unreferenced).
4.2 Uses
In general, Azo Acid Dyes are used principally for colouring application to nylon, wool, silk and leather (Kirk-Othmer 2010; CII 2011; personal communication, email from ETAD to Existing Substances Risk Assessment Bureau, Health Canada, dated May 2013; unreferenced). They are also used to colour cosmetics, drugs, soaps and cleaning compounds, paper and inks (Freeman and Peters 2000; Kirk-Othmer 2010; CII 2011). Some substances in this subgroup, namely Amaranth, New Coccine, Ponceau MX and Tartrazine, have C.I. classification as food dyes, and all but Ponceau MX are permitted colouring agents in food in some jurisdictions.
Colouring Agents in Food
In Canada, food colouring agents are regulated as food additives under the Food and Drug Regulations. Colouring agents that are permitted for use in food are listed in the List of Permitted Colouring Agents incorporated by reference in the Marketing Authorization for Food Additives that May be Used As Colouring Agents, issued under the authority of the Food and Drugs Act. Of all substances in this Screening Assessment, only Amaranth and Tartrazine are listed on the List of Permitted Colouring Agents as permitted food colouring agents in Canada.
Food Packaging
None of the Azo Acid Dyes was identified as being used in food packaging applications in Canada (personal communications, emails from Food Directorate, Health Canada to Risk Management Bureau, Health Canada, dated 2011; unreferenced).
Colouring Agents in Drugs and Natural Health Products
Colouring agents used in drugs that are permitted in Canada are regulated under Part C, Division 1 of the Food and Drug Regulations (Canada [1978]). Under the Food and Drugs Act, drugs also include biologics, natural health products and veterinary drugs. Amaranth, New Coccine and Tartrazine are listed in the Food and Drug Regulations as colouring agents permitted in drugs for internal and external use, while Orange II is only permitted for external use. Health Canada’s Therapeutic Products Directorate’s internal Non-Medicinal Ingredients Database (NMID) identified Amaranth as a non-medicinal ingredient in human drugs and disinfectants, New Coccine as a non-medicinal ingredient in human drugs, Orange II as a non-medicinal ingredient in disinfectants and Tartrazine as a non-medicinal ingredient in drugs (human and veterinary) and disinfectants (personal communication, email from Therapeutic Products Directorate, Health Canada to Risk Management Bureau, Health Canada, dated August 2011; unreferenced).
Amaranth and New Coccine (as Ponceau 4R) are listed in the Natural Health Products Ingredients Database (NHPID) with a non-medicinal role for internal and external use as colour additives in natural health products (NHPID 2015). These substances are listed in the Licensed Natural Health Products Database (LNHPD) as being present as non-medicinal ingredients in a limited number of currently licensed oral natural health products (LNHPD 2015). Tartrazine is listed in the NHPID with a non-medicinal role for internal and external use as a colour additive in natural health products (NHPID 2015). Tartrazine is listed in the LNHPD to be present as a non-medicinal ingredient in currently licensed natural health products (LNHPD 2015). Orange II and Metanil Yellow are listed in the NHPID with a non-medicinal role for topical use as colour additives in natural health products (NHPID 2015).
Some of the drugsand natural health products regulated in Canada containing Tartrazine are also considered to be at the cosmetic-drug interface (Health Canada 2008. Under the Food and Drugs Act, natural health products are considered to be a subset of “drugs”; hence in this report, the term “drug” includes “natural health products” unless differentiated for specific reasons.
None of the 52 substances in this Screening Assessment have been identified to be present in biologics in Canada (June 2011 email from the Biologics and Genetic Therapies Directorate, Health Canada, to the Risk Management Bureau, Health Canada; unreferenced).
Cosmetics
Amaranth, Orange II, New Coccine, and Tartrazine were identified as ingredients in cosmetic products according to notifications submitted under the Cosmetic Regulations to Health Canada. Cosmetic products containing these substances include bath products, cleansers, creams, deodorants, douches, eye and face makeup, face paint, fragrances, genitalia products, hair dyes, hair grooming products, hair removal and shaving products, massage oils, moisturizers, nail care products and soaps (2011 and 2013 emails from Consumer Product Safety Directorate, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). Based on notifications submitted under the Cosmetic Regulations to Health Canada, Amaranth and Tartrazine are used in cosmetic makeup tattoo inks in Canada (2011 and 2013 emails from Consumer Product Safety Directorate, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). Similarly, New Coccine was identified as an ingredient in semi-permanent cosmetic makeup tattoo and body tattoo inks; however, these products containing New Coccine are not on the Canadian market (2013 email from Consumer Product Safety Directorate, Health Canada to Existing Substances Risk Assessment Bureau [Health Canada], dated 2013; unreferenced).
Metanil Yellow, a substance prohibited for use in cosmetics, is listed under the name C.I. 13065 on Health Canada’s Cosmetic Ingredient Hotlist. The Hotlist is an administrative tool that Health Canada uses to communicate to manufacturers and others that certain substances, when present in a cosmetic, may contravene (a) the general prohibition found in section 16 of the Food and Drugs Act or (b) a provision of the Cosmetic Regulations (Health Canada 2014).
Formulants in Pest Control Products
Amaranth, Metanil Yellow, Orange II and Tartrazine were identified as being present in pest control products registered in Canada (June 2011 email from Pest Management Regulatory Agency, Health Canada to Risk Management Bureau, Health Canada; unreferenced; July 2013 email from Pest Management Regulatory Agency, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). As the Pest Control Products Act is a CEPA equivalent legislation, the use of these substances as formulants in pest control products registered in Canada are not further considered in this assessment.
5. Environmental Fate and Behaviour
The environmental fate of chemicals describes the processes by which chemicals become distributed and are transformed in the environment. In this section, some general characteristics of the substances considered in this screening assessment will be discussed with respect to their environmental fate in different compartments in an effort to understand how organisms come into contact with the substances in a particular medium, the persistence of the substances in environmental compartments, and their degradation, distribution among media, migration in groundwater, removal from effluents by standard wastewater treatment methods and bioaccumulation in organisms.
As explained in Environment Canada and Health Canada (2013), mass balance fate models, such as the Equilibrium Criterion model or EQC (New EQC 2011), are not applicable for strongly ionic acid dyes, and their use with these substances would not conform to good modelling practice for multimedia models (Buser et al. 2012). Therefore, the environmental fate and compartmentalization of these substances will be discussed qualitatively using information on physical and chemical properties.
5.1 Water and Sediment
Azo Acid Dyes are highly water soluble and have an inherently high affinity for substrates, which results in high fixation rates (ETAD 1995; Environment Canada and Health Canada 2013). Given a continuous steady-state emission to water, a large proportion of Azo Acid Dyes will reside in the water column due to their very high water solubility. Over time, negatively charged (anionic) Azo Acid Dyes are expected to bind to suspended organic matter, specifically positively charged particulates, due to electrostatic interactions and eventually settle out to bed sediments or wastewater sludge (ETAD 1995). Some ionic dyes can also bind to organic material via hydrogen bonds and van der Waals forces (Oster 1955).
Other factors, such as increasing molecular size, hardness of the water and salinity, as well as decreasing pH, are thought to favour some sorption of azo dyes to suspended solids (HSDB 1983- ; Øllgaard et al. 1998). It has been stated generally that due to the recalcitrant nature of azo dyes in aerobic environments, they eventually end up in anaerobic sediments, shallow aquifers and groundwater (Razo-Flores et al. 1997). After partitioning to sediment or wastewater sludge, some azo dyes may bind reversibly and become resuspended, while others will bind irreversibly and remain buried.
5.2 Soil
There are two major routes for the release of azo dyes to soil: directly via the use or application of a dye in the environment and indirectly via the application of wastewater sludge to agricultural land or deposition in landfills. Acid dyes have an inherently high affinity for substrates, with fixation levels ranging from 85% to 98% for acid dyes with more than one sulfonic acid group (ETAD 1995).
5.3 Air
Acid-based dyes are not expected to be released to air and are not expected to partition to this compartment due to very low vapour pressures, high water solubilities and low Henry’s Law constants (HSDB 1983- ; Øllgaard et al. 1998). Water-soluble dyes such as Azo Acid Dyes are intended for use in water-based treatments, which also limits their release, as they are hydrophilic. While premixed dyes in their solid states may have some limited capacity for dispersal into the air as large particles, air is not considered to be a carrying medium for dyes, as these substances exhibit low or negligible volatility (ETAD 1995; Øllgaard et al. 1998).
Given their low levels of volatility and physicochemical preference for partitioning to other media, it is also not expected that Azo Acid Dyes will be subject to long-range atmospheric transport.
5.4 Environmental Persistence
To characterize the environmental persistence of Azo Acid Dyes under both aerobic and anaerobic conditions, empirical and modelled data for these substances were considered.
5.4.1 Empirical Data for Persistence
Empirical biodegradation data related to the persistence of Azo Acid Dyes are limited. While a standard biodegradation test was not used, Shaul et al. (1990) demonstrated that 10 azo acid dyes, including two substances of focus in this assessment (Tartrazine and New Coccine), were not biodegradable under aerobic conditions, as the concentration of these dyes remained essentially constant in the influent, primary effluent and activated sludge of a wastewater treatment plant. Two other substances addressed in this assessment, Orange II and Acid Blue 113, had different results in the same study. The results indicated that Acid Blue 113 adsorbed on the activated sludge but did not degrade, while Orange II may have biodegraded to some degree, as concentrations decreased throughout the treatment process.
Select empirical tests (Table 5-1), with more quantitative results under aerobic conditions, showed little to no degradation of Tartrazine (Tarimci et al. 1987). Despite some limitations, additional degradation data from Brown and Hamburger (1987) for two Azo Acid Dyes also show relatively low levels of aerobic degradation following initial anaerobic decolourization experiments of up to 56 days. However, since sludge was acclimated to the dyes, longer test periods were used, which makes the values difficult to compare with the results of standard OECD Test Guideline 301C tests. Also, it is unclear whether commercial formulations may have been used in some of these tests instead of high-purity dyes. Brown and Hamburger (1987) noted that the percentage of dye may be as low as 80%. If this was the case, the degradation value may not be representative of the pure substance.
| Substance (CAS RN) | Method | Degradation value (%, except where noted)[a] | Degradation endpoint | Test duration (days) | Reference |
|---|---|---|---|---|---|
| Tartrazine (1934-21-0) | N/A | 300 days | Half-life | N/A | Tarimci et al. 1987 |
| Orange II (633-96-5) |
Modified OECD 301E screening test | less than 25 | DOC degradation | 15 | Brown and Hamburger 1987 |
| Acid Black 24 (3071-73-6) | Modified OECD 301E screening Test | less than 25 | DOC degradation | 49 | Brown and Hamburger 1987 |
Table 5-1 Note
Abbreviations: DOC: dissolved organic carbon; N/A: not applicable; OECD: Organisation for Economic Co-operation and Development
- [a]
- Percent biodegradation at a given concentration of the test substance.
5.4.2 Anaerobic Biodegradation of Azo Acid Dyes
Under anaerobic or reducing conditions, biotic degradation of dyes may take place relatively rapidly (Yen et al. 1991; Baughman and Weber 1994; ETAD 1995; Øllgaard et al. 1998; Isik and Sponza 2004). Dyes have a high tendency to cleave at the azo bond, with the formation of aromatic amines (Øllgaard et al. 1998; Hunger 2005). Select empirical tests (Table 5-2) under anaerobic conditions showed significant degradation.
| Substance (CAS RN) | Method | Degradation value (%)[a] | Degradation endpoint | Test duration (days) | Reference |
|---|---|---|---|---|---|
| Orange II (633-96-5) | Brown and Laboureur 1983 | 97 | Colour removal | 28 | Brown and Hamburger 1987 |
| Metanil Yellow (587-98-4) | Brown and Laboureur 1983 | 97 | Colour removal | 7 | Brown and Hamburger 1987 |
| Acid Black 24 (3071-73-6) | Brown and Laboureur 1983 | 100 | Colour removal | 7 | Brown and Hamburger 1987 |
Table 5-2 Note
- [a]
- Percent biodegradation at a given concentration of the test substance.
5.4.3 Modelling of Persistence
In addition to the experimental data, a (Q)SAR-based weight of evidence approach (Environment Canada 2007) was applied using biodegradation models. These models are considered acceptable for use, as they are based on chemical structure, and the azo structure is represented in the training sets of all of the BIOWIN models used (BIOWIN 2010), thereby increasing the reliability of the predictions. Given the ecological relevance of the water compartment, the fact that most of the available models apply to water and the fact that Azo Acid Dyes are expected to be released to this compartment, primarily aerobic biodegradation in water was examined.
Aquatic degradation models used in this analysis were HYDROWIN (2010), BIOWIN Submodels 3-6 (BIOWIN 2010), DS TOPKAT (©2005-2009) and CATALOGIC (2012).
All of the model outputs for Azo Acid Dyes (other than for a few substances using BIOWIN Submodels 3 and 4) consistently predicted that these substances would biodegrade slowly in water under aerobic conditions. These results are consistent with information included in Environment Canada and Health Canada (2013), which outlines the general persistence of azo dyes in aerobic environments. Two substances (Amaranth and New Coccine) appear to be more persistent in air than the other Azo Acid Dyes in the report, according to AOPWIN (2010).
5.4.4 Hydrolysis
The majority of the Azo Acid Dyes do not contain functional groups expected to undergo hydrolysis. This is consistent with published studies that note hydrolysis as being an insignificant factor in the cleavage of azo compounds (Baughman and Perenich 1988). However, eight substances (CAS RN 79234-36-9, CAS RN 72828-83-2, CAS RN 83027-51-4, CAS RN 83027-52-5, Acid Red 138, CAS RN 71873-51-3, CAS RN 83006-48-8, CAS RN 75949-73-4) contain amide functional groups that were flagged by EPI Suite (2012) as having the potential to undergo some degree of hydrolysis. One substance (CAS RN 35342-16-6) was also flagged as having a carbonyl urea functional group that potentially could undergo some degree of hydrolysis.
5.4.5 Summary of Persistence
Due to the persistence of Azo Acid Dyes in aerobic environments in combination with their high water solubility, it is expected that these substances will have relatively long residence times in water. As these substances are predicted to stay in the water for long periods of time, they may disperse widely. Eventually, due to electrostatic interactions with particulate matter, they may also disperse widely in sediment. In sediment and soil, biodegradation is also expected to be slow under aerobic conditions and fast under anaerobic conditions. Short residence times in air are expected to result in low potential for long-range atmospheric transport.
5.5 Potential for Bioaccumulation
In this assessment, a variety of lines of evidence have been used to determine the bioaccumulation potential of Azo Acid Dyes. Experimental data for traditional bioaccumulation metrics, such as bioconcentration factor (BCF), are minimal and restricted to the water compartment for these substances. In addition, the use of (Q)SAR bioaccumulation modelling was not pursued for Azo Acid Dyes, since these substances were outside the model domains of applicability.
5.5.1 Octanol-Water Partition Coefficient
As indicated in Tables 3-1 and 3-2, Azo Acid Dyes as a whole subgroup have relatively high water solubilities (1.8-300 g/L), and the limited number of experimental data for these dyes suggest very low log Kow values (-4.5 to 1.2), which would also suggest a very low bioaccumulation potential, according to equilibrium partitioning theory. This is consistent with the general view from other sources that note the very low bioaccumulation potential of ionic dyes (ETAD 1995).
5.5.2 Bioconcentration Factor (BCF)
Estimated and experimental log Kow values were compared with experimental BCFs for fish for a number of dyes (Anliker et al. 1981; ETAD 1995; Øllgaard et al. 1998). With respect to the data for six acid dyes, reported BCFs were less than 10, indicating that these dyes are not likely to bioconcentrate in aquatic organisms. Data available for Tartrazine (Table 5-3) illustrate low BCF values ( less than 0.29 and less than 3 L/kg) for carp exposed to two different concentrations.
| Test organism | Experimental concentration | Endpoint (BCF, L/kg) | Reference |
|---|---|---|---|
| Common carp (Cyprinus carpio) |
600 µg/L | less than 0.29 | MITI 1992 |
| Common carp (Cyprinus carpio) |
60 µg/L | less than 3 | MITI 1992 |
5.5.3 Other Factors for Assessing Bioaccumulation Potential
As outlined in the “Potential for Bioaccumulation” section of Environment Canada and Health Canada (2013), due to the lack of empirical bioaccumulation data available for Azo Acid Dyes, available data on water solubility, molecular weight and cross-sectional diameter are considered in order to determine bioaccumulation potential. Given their relatively high water solubility, ionic nature and high degree of dissociation under typical environmental conditions, the lipid partitioning tendency of these substances is expected to be limited. Also, bioaccumulation data resulting from exposures of organisms to these substances in soil and sediment are minimal and limited, in large part due to the high water solubility of these substances (Environment Canada and Health Canada 2013).
In general, Azo Acid Dyes are relatively hydrophilic, large molecules with high molecular weight (most above 400 g/mol). The minimum (Dmin) and maximum cross-sectional diameters (Dmax) for Azo Acid Dyes range from 0.820 (Dmin) to 1.99 nm (Dmax) (Tables 3-1 and 3-2). These characteristics suggest that molecular dimensions of substances may also restrict their rate of uptake when crossing cell membranes in fish from water, thereby reducing the bioaccumulation potential for these substances.
5.5.4 Summary of Bioaccumulation Potential
Azo Acid Dyes are expected to have a low bioaccumulation potential due to low observed bioconcentration in empirical tests. This is supported by and consistent with their physical and chemical properties (i.e., low log Kow, ionized at relevant environmental pH, high molecular weight, large cross-sectional diameters, high water solubility) and likely high degree of biotransformation by organisms. The low potential for bioaccumulation of these substances suggests that there may also be low potential for internal concentrations in organisms to reach levels that could cause adverse effects. Potential for adverse effects is discussed further in the following section.
6. Potential to Cause Ecological Harm
6.1 Ecological Effects Assessment
Only empirical data for specific substances within the subgroups and analogues were considered for assessment of the potential of Azo Acid Dyes to cause ecological harm, given the high level of uncertainty associated with modelling the ecotoxicity of these substances.
6.1.1 Empirical Studies for the Aquatic Compartment
Limited empirical ecotoxicity studies were available for Azo Acid Dyes (Tables 6-1 to 6-3). In particular, all studies were of short-term duration and deemed to be lethality tests. Most substances had few to no empirical data, whereas other substances, such as Metanil Yellow 36, had multiple studies in the aquatic compartment. Sufficient acute toxicity data were available to consider the three subsets (monoazo, disazo and polyazo) of Azo Acid Dyes separately, but not to separate out structural subsets of higher similarity any further.
Several key data points originated from an empirical study on the acute toxicity of 46 dyes to fathead minnow (Pimephales promelas), which is described first in Little and Lamb (1973) and then summarized in Little et al. (1974). While this bioassay is not recent, it was carried out according to published standard methods, including pertinent information on the test organisms, dilution water and the chemical and physical parameters of the test water. The experiment was designed to estimate the threshold concentration at which 50% of the experimental animals survived after 96 hours. This was originally reported in the two studies as a median tolerable limit (TL50), but may also be interpreted as a median lethal concentration (LC50).
Empirical toxicity studies on two different species of fish using five different monoazo acid dyes were found in the literature (Table 6-1). Acute toxicity values ranged widely from 68 to greater than 1000 mg/L for Oryzias latipes (Japanese rice fish) exposed to Metanil Yellow and Tartrazine, respectively (Table 6-1). The 48-hour LC50 value of 68 mg/L (Tonogai et al. 1982) is of significance because it is the most sensitive value obtained in a test with a fish species that is relevant to the Canadian environment and for which enough information is available to interpret its reliability. This study was deemed acceptable in part because it was performed using a standard method, fish were acclimated and measurements of key test properties were evaluated throughout.
While toxicity tests were available for a paramecium (Paramecium caudatum), a protozoan (Tetrahymena pyriformis)and brine shrimp(Artemia salina), these were not included in the assessment, as they are not standard laboratory species for toxicity tests, and insufficient data were available to assess the results. In addition, some data were available for the tropical fish Channa punctata (96-hour LC50s of 1.5 and 155 mg/L for Metanil Yellow in Rathore et al., 1989); however, given that this fish is native to East Asia and requires water temperatures higher than those generally found in the Canadian environment, it was not considered as a critical toxicity value (CTV). In addition, the reported values from Rathore et al. (1989) ranged over 2 orders of magnitude for the same substance and test organism, making the results uncertain. Tests by MacPhee and Ruelle (1969) focused on the exposure of two fish species (Ptychocheilus oregonensis and Oncorhynchus kisutch) to the analogue CAS RN 2150-33-6. While a value of 10 mg/L was reported, the study is dated, and key information regarding the tests is not available (e.g. duration, endpoint, conditions) to properly evaluate the methods; hence, it was also not considered as a key value in this assessment.
| Test organism | Type of test (duration) | Endpoint | Value (mg/L) (C.I. name or CAS RN) |
Reference |
|---|---|---|---|---|
| Oryzias latipes | Acute (48 h) | LC50 | 68 (Metanil Yellow) |
Tonogai et al. 1982 |
| Oryzias latipes | Acute (24 h) | LC50 | 90 (Metanil Yellow) |
Tonogai et al. 1982 |
| Pimephales promelas | Acute (96 h) | LC50 | 165 (Orange II) |
Little and Lamb 1973; Little et al. 1974 |
| Oryzias latipes | Acute (48 and 96 h) | LC50 | greater than 180 (Acid Yellow 17) |
Little and Lamb 1973; Little et al. 1974 |
| Oryzias latipes | Acute (24 and 48 h) | LC50 | 1000 (Orange II) |
Tonogai et al. 1982 |
| Oryzias latipes | Acute (48 h) | LC50 | greater than 1000 (Tartrazine) |
MITI 1992 |
Abbreviations: LC50: the concentration of a substance that is estimated to be lethal to 50% of the test organisms
Toxicity tests on disazo acid dyes and analogues are available for two fish species, Pimephales promelas and Danio rerio, as well as the invertebrate Daphnia magna. The test results also varied widely over 3 orders of magnitude. An acute toxicity test result for Pimephales promelas exposed to Acid Blue 113 (96-hour LC50 of 4.4 mg/L) represents the lowest LC50 value for the disazo acid dyes (Table 9b). While this is an older toxicity value, it was deemed acceptable in part because it was performed with a standard method and because the key physical and chemical parameters of the test water were well characterized throughout the test (Little and Lamb 1973; Little et al. 1974).
| Test organism | Type of test (duration) | Endpoint | Value (mg/L) (C.I. name) |
Reference |
|---|---|---|---|---|
| Pimephales promelas | Acute (96 h) | LC50 | 4.4 (Acid Blue 113) |
Little and Lamb 1973; Little et al. 1974 |
| Pimephales promelas | Acute (24, 48 and 96 h) | LC50 | greater than 180 (Direct Red 81) |
Little and Lamb 1973; Little et al. 1974 |
| Danio rerio | Acute (96 h) | LC50 | 220 (Acid Orange 156) |
ETAD 1992 |
| Daphnia magna | Acute (24 h) | EC50 | 24 (Acid Orange 156) |
ETAD 1992 |
Abbreviations: EC50: the concentration of a substance that is estimated to cause some effect on 50% of the test organisms; LC50: the concentration of a substance that is estimated to be lethal to 50% of the test organisms
Ecotoxicity data for the polyazo acid dyes and analogues are limited but illustrate lower levels of acute aquatic toxicity. While only one endpoint was identified for polyazo acid dyes. The most sensitive value is a 96-hour LC50 for Pimephales promelas of 130 mg/L from the same studies discussed above (Little and Lamb 1973; Little et al. 1974), which were considered reliable.
| Test organism | Type of test (duration) | Endpoint | Value (mg/L) (C.I. name) |
Reference |
|---|---|---|---|---|
| Pimephales promelas | Acute (96 h) | LC50 | 130 (Acid Orange 24) |
Little and Lamb 1973; Little et al. 1974 |
Abbreviations: LC50: the concentration of a substance that is estimated to be lethal to 50% of the test organisms.
Algae appear to be somewhat sensitive to Azo Acid Dyes with respect to short-term studies in the aquatic environment (Table 6-4). The lowest toxicity value for algae obtained in a study with sufficient information (e.g., effect endpoint specified) is a 72-hour EC50 of 20.8 mg/L for Acid Orange 156 (Greene and Baughman 1996). Regarding ecotoxicity studies using algae, the algal growth inhibition test is one of the most common tests for determining aquatic toxicity, by measuring changes of the growth rate in response to exposure to test chemicals in water. Particularly when testing coloured substances, it has been noted that these substances are capable of attenuating light penetration into the test medium, by light absorption and reflection. Using solvents or emulsifiers to create a homogeneous dispersion in water, attenuation of light is likely to be proportional to the amount of substance added. The inhibition of algal growth due to light attenuation can result in reduced algal population growth in relation to the amount of substance added to the test medium. However, such inhibition is not considered a true ecotoxicological effect of the test chemicals (Rufli et al. 1998; Cleuvers and Weyers 2003).
There have been recommendations to deal with light attenuation in algal tests with coloured substances. It was suggested to put algae back into a test substance-free medium at the end of the exposure period, in order to discriminate between an algistatic and an algicidal effect (Whitehouse and Mallett 1993). A reduced light path through the test solution was also proposed, so that the algal growth rate is not affected (Comber et al. 1995). However, in studies identified to assess the ecotoxicological effects of Azo Acid Dyes and analogues in algae, there is no report of any light attenuation and hence no indication of whether light attenuation has been impacted. Therefore, it is suspected that the reported ecotoxicity in the algae studies may not represent the “true” effects of the test dyes on the organisms.
| Test organism | Type of test (duration) | Endpoint | Value (mg/L) (C.I. name) |
Reference |
|---|---|---|---|---|
| Alga(Scenedesmus subspicatus) | Chronic (72 h) | Population biomass, EC50 | 20.8 (Acid Orange 156) |
Greene and Baughman 1996 |
| Alga(Scenedesmus subspicatus) | Chronic (72 h) | Population biomass, EC50 | 28 (Acid Orange 156) |
ETAD 1992 |
| Alga(Scenedesmus subspicatus) | Chronic (72 h) | Population growth, EC50 | 37 (Acid Orange 156) |
ETAD 1992 |
| Alga (Pseudokirchneriella subcapitata) | Chronic (1 and 14 days) | Population biomass (no effect concentration listed) |
10 mg/L (Acid Black 1) |
Ericson 1977 |
| Alga (Pseudokirchneriella subcapitata) | Chronic (1 and 14 days) | Population biomass (no effect concentration listed) | 10 mg/L (Acid Orange 24) |
Ericson 1977 |
6.1.2 Empirical Studies for Other Environmental Compartments
Toxicity data for sediment-dwelling organisms are unavailable, and toxicity data for terrestrial species exposed to Azo Acid Dyes are limited. A single plant study was found in which three species (sorghum, sunflower and soy) were exposed to the monoazo acid dye C.I. 13155 (Brown and Anliker 1988). There were no effects on seed germination at any concentration up to 1000 mg/L. Growth rate was also not affected up to 100 mg/L. However, at 21 days, there was variable growth at 1000 mg/L, and monoazo acid dye C.I. 13155 could be detected in the plant foliage at a soil concentration of 1000 mg/kg. While this is not sufficient to generate a reliable terrestrial endpoint value, it does illustrate that, based on these limited data, effects on soil organisms are not expected at moderate to low concentrations.
6.1.3 Derivation of the Aquatic and Terrestrial PNECs
A grouping approach was also used in the development of the predicted no-effect concentrations (PNECs) for the Azo Acid Dyes subgroup. CTVs in the aquatic medium were chosen to represent each subset, as data were available. There were insufficient data to calculate a CTV for soil, and no data were available to calculate a CTV for sediment.
The aquatic CTV selected for monoazo acid dyes was the acute 48-hour LC50 of 68 mg/L for Oryzias latipes(Tonogai et al. 1982), as this was the most sensitive valid experimental value. The aquatic PNEC was then derived by dividing this value by an assessment factor of 100 (to account for interspecies and intraspecies variability and the estimation of a long-term no-effects concentration from a short-term LC50). Therefore, a PNEC of 0.68 mg/L was calculated for the monoazo acid dyes.
The aquatic CTV selected for disazo acid dyes was the acute 96-hour LC50 of 4.4 mg/L for Pimephales promelas (Little et al. 1974), as this was the most sensitive valid experimental value (updated from the study on the same substance published by Little and Lamb in 1973). The aquatic PNEC was then derived by dividing this value by an assessment factor of 100 (to account for interspecies and intraspecies variability and the estimation of a long-term no-effects concentration from a short-term LC50). Therefore, a PNEC of 0.044 mg/L was calculated for the disazo acid dyes.
The aquatic CTV selected for polyazo acid dyes was the acute 96-hour LC50 of 130 mg/L, also for Pimephales promelas (Little et al. 1974), as this was the most sensitive valid experimental value (also updated from the study on the same substance published by Little and Lamb in 1973). The aquatic PNEC was then derived by dividing this value by an assessment factor of 100 (to account for interspecies and intraspecies variability and the estimation of a long-term no-effects concentration from a short-term LC50). Therefore, a PNEC of 1.3 mg/L was calculated for the polyazo acid dyes.
A terrestrial CTV was not calculated for Azo Acid Dyes, given the limited data available. However, based on the existing plant study, effects on soil organisms are not expected at moderate to low concentrations.
6.1.4 Ecological Effects Summary
Based on lines of evidence involving empirical and read-across aquatic ecotoxicity data, it may be concluded that some monoazo and disazo acid dyes may cause harm to aquatic organisms at moderate concentrations (i.e., LC50s are less than 100 mg/L). It may be concluded that polyazo acid dyes are not expected to cause harm to aquatic organisms at moderate or low concentrations (i.e., LC50s are greater than 100 mg/L). Azo Acid Dyes are not expected to cause harm to soil organisms at moderate to low concentrations (no effects at concentrations greater than 100 mg/L, and detected in foliage at a concentration of 1000 mg/kg).
6.2 Ecological Exposure Assessment
6.2.1 Releases to the Environment
No measured concentrations of Azo Acid Dyes in the Canadian environment have been identified. Environmental concentrations have therefore been estimated from available information.
Anthropogenic releases of a substance to the environment depend upon various losses that occur during the manufacture, industrial use, consumer/commercialFootnote5 use and disposal of a substance. In order to estimate releases to the environment occurring at different stages of the life cycle of the acid dyes, Environment and Climate Change Canada compiles information on the relevant sectors and product lines, as well as emission factorsFootnote6 to raw industrial wastewater or wastewater collected by publicly-owned wastewater systems, land and air at different life cycle stages in order to identify the life cycle stages that are likely significant contributors to overall environmental concentrations. Recycling activities and transfer to waste disposal sites (landfill, incineration) are also considered. However, releases to the environment from disposal were not quantitatively accounted for unless reliable specific information on the rate (or potential) for release from landfills and incinerators was available.
This information is used to further develop exposure characterization scenarios to estimate resulting environmental concentrations.
Factors relevant to key life cycle stages of these substances have been considered, uncertainties have been recognized and assumptions have been made related to different stages, subject to the availability of information. Exposure scenarios for the uses/media of concern have been developed, including the determination of applicable predicted environmental concentrations (PECs).
6.2.2 Identification of Important Exposure Scenarios
Exposure characterization is focused on important release scenarios. These scenarios represent major environmental releases and relatively high concentrations. In general, the magnitude of releases is a direct function of the quantity of a substance manufactured or used and its applicable emission factors. In cases where industrial releases are similar in quantity to consumer and/or commercial releases, the former normally results in higher environmental concentrations. This is in part because industrial releases are concentrated at a limited number of sites, while consumer and/or commercial releases are dispersed across the country.
The Azo Acid Dyes were not manufactured in Canada according to the section 71 survey on Aromatic Azo and Benzidine-based Substances (Canada 2011). They were imported and used in the production of the following seven product categories:
- Food and beverage
- Cleaning and care products
- Paintballs
- Agricultural, lawn and garden products
- Personal care and cosmetics
- Pharmaceuticals
- Textile and leather
The Azo Acid Dyes were used in the following two types of industrial operation:
- Formulation to produce concentrates (e.g., flavouring concentrates for food and beverage, chemical concentrates for textile and leather processing);
- Industrial use of imported Azo Acid Dyes or formulated concentrates (as stated in point 1) to produce the products in the above-mentioned seven categories (e.g., use of imported Azo Acid Dyes in the production of cleaning compounds, use of chemical concentrates in textile dyeing).
Exposure scenarios resulting from these two types of operation have been developed for this assessment.
6.2.3 Estimates of Predicted Environmental Concentrations
The PECs of the Azo Acid Dyes were estimated for the formulation and industrial use scenarios. These concentrations are based on available information on the quantities of the Azo Acid Dyes, emission factors, the characteristics of the industrial or publicly-owned wastewater treatment systems involved and the characteristics of the receiving environment.
The PECs estimated were focused on the aquatic compartment. This is because the Azo Acid Dyes are expected to be released to the aquatic compartment through wastewater treatment systems and to remain in the water column after release. The Azo Acid Dyes are grouped into three subsets: monoazo, disoazo and polyazo. These subsets are all highly water soluble, with solubilities in the range of 1.8-300 g/L. While it is expected that some Azo Acid Dyes may partition to sludge during wastewater treatment or to sediment after they are released to receiving water, the water column is nonetheless expected to be the major compartment of their environmental presence.
The environmental concentrations of the Azo Acid Dyes in sediment and biosolids-amended soil could not be calculated, as ionic substances are outside the domain of applicability for equilibrium partitioning models. Although these substances are ionizable in water and can sorb to sediment or biosolids via electrostatic bonding, the amounts going to sediment or soil are expected to be less than those present in water at any given location due to their high water solubilities. While some Azo Acid Dyes may reach and persist in the more aerobic top layers of sediment, this accumulation should be dispersive due to their persistence in the water column. Also, experimental biodegradation data indicate that Azo Acid Dyes can degrade under anaerobic conditions, which are likely to occur as they move to deeper layers of sediment. Thus, the PECs in sediment and biosolids-amended soil are predicted to be low, and quantitative calculations are not pursued.
To estimate the aquatic PECs for the formulation and industrial use scenarios, 72 facilities were found to be the major users of the Azo Acid Dyes from the survey. These facilities each used 100 kg/year or more of the Azo Acid Dyes in their operations. They represented 99% of the Azo Acid Dyes found in Canadian commerce (10 000-100 000 kg/year). Two other sites were also identified, but insufficient information was available in order to complete a quantitative exposure scenario at those locations.
The 72 facilities were located in 40 municipalities in British Columbia, Manitoba, New Brunswick, Nova Scotia, Ontario, Quebec and Saskatchewan. These municipalities were referred to as sites in this assessment, as they represent a common point of entry to the environment via the wastewater treatment system. The number of facilities at a site varied from 1 to 10.
Where the survey information was not sufficient to determine which facilities were involved with the Azo Acid Dyes (e.g., when a survey respondent was found to operate multiple facilities), this information was identified based on the respondent’s website and Environment and Climate Change Canada’s National Pollutant Release Inventory database (NPRI 2006). When a survey respondent reported the total Azo Acid Dyes used from multiple facilities, each of these facilities was conservatively assumed to use the Azo Acid Dyes at a quantity equal to the total quantity reported by the respondent. In some cases, this may be a very conservative assumption.
The aquatic PEC from each individual facility was estimated based on its annual use quantity from the survey, an applicable emission factor for emissions to raw industrial wastewater, municipal wastewater influent or effluent flow and receiving water dilution. The PECs ranged between 0.06 and 607.3 µg/L at sites where monoazo acid dyes are used, between 3.1 and 40.0 µg/L where disazo acid dyes are used and between 33.6 and 81.7 µg/L where polyazo acid dyes were used. The calculations, as well as assumptions used, are explained in Appendix B. These results were conservative, since the emission factors used were relatively high and the removal by either on-site industrial wastewater treatment or off-site municipal wastewater treatment was assumed to be zero.
6.3 Characterization of Ecological Risk
The approach taken in this ecological screening assessment was to examine various supporting information and develop proposed conclusions based on a weight of evidence approach and using precaution as required under CEPA. Lines of evidence considered include information on physical and chemical properties, environmental fate, ecotoxicity and sources of the substances, as well as results from conservative risk quotient analyses, which are outlined below.
6.3.1 Aquatic Risk Quotient Analysis
Risk quotient analyses compare the PECs with the appropriate PNEC values in order to evaluate potential risks. A risk quotient analysis, integrating conservative estimates of exposure with toxicity information, was performed for the aquatic medium to determine whether there is potential for ecological harm in Canada.
The PECs outlined in the previous section (see “Estimates of Predicted Environmental Concentrations”) for sites using monoazo, disazo and/or polyazo acid dyes for industrial uses or in the formulation of concentrates were compared with the PNECs of 680, 44 and 1300 µg/L derived for monoazo, disazo and polyazo acid dyes, respectively (see “Derivation of the Aquatic and Terrestrial PNECs”). The resulting risk quotients for each of the subsets were less than 0.001 to 0.89 for monoazo acid dyes, 0.070 to 0.91 for disazo acid dyes and 0.026 to 0.063 for polyazo acid dyes, respectively.
The comparison between PEC and PNEC values indicates that none of the 40 sites exceeds a risk quotient of one, with a majority (38) of sites having maximum risk quotients of less than 0.35. However, two of the 40 locations show potential concern, with relatively high risk quotients approaching one. These higher risk quotients can be explained by one site having low wastewater flow, which does not provide as much dilution, and the second site using more generic and conservative parameters due to a lack of more refined site-specific data. In addition, given that at least a small percentage of these substances are expected to be removed in wastewater treatment systems or to eventually deposit to sediment due to electrostatic interactions, these results show low concern in the aquatic compartment.
Therefore, harm to aquatic organisms is unlikely at these sites, due in part to the large dilution capacities at many of the locations.
6.3.2 Soil Risk Quotient Analysis
A PNEC in soil was not calculated, as sufficient data were not available. However, Azo Acid Dyes are not expected to cause harm to soil organisms at moderate to low concentrations based on a terrestrial plant toxicity study. Also, no PEC is available, as no monitoring data exist, and these substances, being reactive, are not within the domain of applicability of the exposure model for equilibrium partitioning.
6.3.3 Characterization of Ecological Risk
The approach taken in this ecological screening assessment was to examine various supporting information and develop conclusions based on a weight of evidence approach and using precaution as required under CEPA. Lines of evidence considered include results from conservative risk quotient calculations, as well as information on persistence, bioaccumulation, ecological effects, sources, fate of the substances and presece and distribution in the environment. Various lines of evidence for each ecological subset are summarized below, along with relevant uncertainties, leading to overall conclusions.
Azo Acid Dyes are anthropogenically produced and not expected to occur naturally in the environment. No data concerning concentrations of these substances in the Canadian environment have been identified. Azo Acid Dyes are complex anionic molecules that have relatively high water solubility ( greater than 1 g/L) and are expected to dissociate at environmentally relevant pH levels. Since there is a relative paucity of data, disazo and polyazo substances were examined as a group with respect to their physical and chemical properties. All Azo Acid Dyes were grouped together with respect to environmental fate due to their similar physical and chemical properties as well as relatively similar chemical structures (e.g., sharing common functional groups, but varying in number). Due to their high water solubility and affinity for oppositely charged organic particles, Azo Acid Dyes are expected to be found in water, sediment and soil. Given their very low expected vapour pressures and low Henry’s Law constants, they are unlikely to stay in air if released to this compartment. Therefore, long-range atmospheric transport is not anticipated to be of concern. Given their hydrophilicity and charged character, Azo Acid Dyes have low experimental log Kow values ( less than 2). Estimated and experimental log Kow values were compared with experimental BCFs for fish for a number of dyes (Anliker et al. 1981; Øllgaard et al. 1998; ETAD 1995). The reported BCF values for Azo Acid Dyes were low, indicating that these dyes are not likely to bioconcentrate in aquatic organisms. Furthermore, Azo Acid Dyes are not expected to bioconcentrate due to their high molecular weights ( greater than 400 g/mol) and relatively large Dmin and Dmax, which suggest slow uptake potential. Bioaccumulation resulting from exposures of organisms to these substances in soil and sediment is not well understood due to minimal and limited data, in large part due to the high water solubility of these substances. According to empirical and modelled data, Azo Acid Dyes are expected to biodegrade very slowly in aerobic environments and are therefore considered to be persistent in water, sediment and soil. However, Azo Acid Dyes may degrade and transform to certain aromatic amines if they reach anaerobic environments.
Based on lines of evidence involving empirical aquatic and terrestrial ecotoxicity data for specific Azo Acid Dyes and analogues, it may be concluded that monoazo and disazo acid dyes may be expected to cause harm to aquatic organisms at moderate concentrations (68 and 4.4 mg/L, respectively), while aquatic organisms are less sensitive to polyazo acid dyes. Toxicity data are limited for the terrestrial environment and unavailable for sediment-dwelling organisms. A conservative exposure analysis of the food and beverage sector and certain industrial operations involved in formulation to produce various products was done, because those sectors were anticipated to present the highest potential ecological risk related to industrial releases to the environment for these substances. Risk quotients calculated from PECs for Azo Acid Dyes released from 40 sites and 72 facilities and PNECs in the aquatic environment show that at conservatively predicted levels of release, Azo Acid Dyes are not likely to result in significant aquatic exposure at the majority of sites.
Considering all available lines of evidence with respect to the persistence, potential for bioaccumulation, ecotoxicity, industrial uses and potential releases of the substances, it is concluded that the 52 Azo Acid Dyes assessed in the ecological portion of this assessment have a low potential to cause ecological harm in Canada.
6.3.4 Uncertainties in Ecological Assessment
Many specific substances, especially disazo and polyazo acid dyes, addressed in this report had limited data available. As a result, a read-across approach using data from selected analogues was the best alternative to estimating physical and chemical properties. This introduces some uncertainties, as there is still some degree of structural variation between the substances assessed and the analogues.
Long-term (chronic) toxicity data would be beneficial in evaluating these substances due to the fact that they are predicted to be persistent in the environment, but the availability of such data is minimal. The use of an assessment factor in determining a PNEC is intended to address this uncertainty. While water was found to be the key medium of interest, soil and sediment also hold some importance due to potential adsorption and electrostatic interactions. Therefore, the minimal available effects data for Azo Acid Dyes in soil and sediment are a source of uncertainty.
The lack of measured environmental concentrations of these substances (e.g., monitoring data) in Canada resulted in the need to evaluate risk based on predicted concentrations in water near industrial point sources. Conservative assumptions were made when using models to estimate concentrations in receiving water bodies.
Given the use of some of these substances in other countries, it is possible that they may enter the Canadian market as components of manufactured items and/or products available to consumers. However, it is anticipated that the proportions of these substances released to the various environmental media would not be significantly different from those estimated here, given the conservative assumptions used in the exposure analyses.
7. Potential to Cause Harm to Human Health
The human health assessment of Azo Acid Dyes focuses on 17 substances that are in commerce above the section 71 reporting threshold or for which there is other information to indicate that they are in commerce in Canada. These substances are 17 Azo Acid Dyes (Acid Black 24, Acid Black 26, Acid Blue 113, Acid Orange 33, Acid Red 6, Acid Red 138, Amaranth, New Coccine, Orange II, Tartrazine, CAS RN 68155-63-5, CAS RN 70210-05-8, CAS RN 70210-06-9, CAS RN 70210-25-2, CAS RN 70210-34-3, CAS RN 71873-51-3 and CAS RN 84962-50-5). Metanil Yellow is also in commerce above the section 71 reporting threshold, but reported uses of this substance do not result in significant exposure for the general population in Canada.
7.1 Exposure Assessment
7.1.1 Environmental Media and Food
Environmental Media
Empirical data on concentrations of Azo Acid Dyes in environmental media in Canada or elsewhere were not identified. As described in the “Sources” section, only 10 Azo Acid Dyes were identified to be imported into Canada, most of them at quantities equal to or below 1000 kg/year. Due to the very low volatility and limited commercial quantities of Azo Acid Dyes in Canada, environmental media are not considered to be a significant source of exposure to these substances for the general population.
Food
The Canadian Food Inspection Agency (CFIA) has tested for the presence of certain dyes in foods, including 9 of the Azo Acid Dyes in this assessment: Acid Black 24, Acid Blue 113, Amaranth, Metanil Yellow, New Coccine, Orange II, Ponceau MX, Tartrazine and CAS RN 106028-58-4 (sodium salt of Food Black 2). In the 2009-2010 and 2010-2011 targeted food surveys, the CFIA analyzed domestic and imported food, including spices, selected for their high likelihood of containing food colouring agents (CFIA 2010, 2011). Of the 9 Azo Acid Dyes listed above, four substances were found at levels above the analytical method limit of detection: New Coccine, Orange II, Amaranth and Tartrazine.
Neither New Coccine nor Orange II are permitted food colouring agents in Canada, and the CFIA’s compliance activities aim to prevent the sale of foods in Canada that are non-compliant with Canadian regulations. Therefore, food is not expected to be a significant source of exposure to these two dyes. This conclusion is supported by the results of the CFIA’s targeted food surveys which detected New Coccine and Orange II in only 0.4% and 0.1%, respectively, of the 1646 samples across both surveys; follow-up risk management actions were taken for each violative sample as appropriate.
Amaranth and Tartrazine are permitted food colouring agents in Canada, and were found in food samples at 3% and 18%, respectively, across both targeted food surveys. As such, exposures of the general population in Canada to Amaranth and Tartrazine from food were estimated for this Screening Assessment.
Dietary exposures to Amaranth and Tartrazine were estimated using consumption data from the 24 hour dietary recall component of the Canadian Community Health Survey (CCHS Cycle 2.2). A tiered approach was used, whereby exposure estimates were first calculated using a conservative single-day intake approach which may overestimate long-term or chronic exposure. As the 90th percentile of dietary exposure to Amaranth calculated on this tier was close to the Acceptable Daily Intake (ADI) for some age groups, intakes were further refined using a “usual intake” approach. This approach adjusts for the frequency of food consumption using a second day of food recall data, thereby reducing the impact of occasional high consumption of a single food item on the exposure estimate (e.g., a given respondent may have reported consuming three beverages containing Amaranth on the first day of the survey, but may not necessarily consume this quantity every day). While this approach requires more complicated computations, it provides a more accurate estimate of long-term dietary exposure, particularly in the upper percentiles of the distribution. For Tartrazine, such additional refinements were not required, as even the more conservative initial tier approach did not indicate exceedance of the ADI; therefore, single-day intakes were retained for Tartrazine. Toddlers and children had the highest estimated exposure to Amaranth and Tartrazine due to their high food consumption relative to body weight (Table 7-1 below). See Appendix C for more details.
| Amaranth | Tartrazine | |
|---|---|---|
| Estimated exposure based on single-day intakes or usual intakes | Usual intakes | Single-day intakes |
| Mean estimate of dietary exposures | 0.01 mg/kg-bw per day (seniors) to 0.21 mg/kg-bw per day (toddlers and children) | 0.2 mg/kg-bw per day (seniors) to 1.0 mg/kg-bw per day (toddlers and children) |
| 90th percentile estimate of dietary exposure | 0.04 mg/kg-bw per day (seniors) to 0.54 mg/kg-bw per day (toddlers) | 0.5 mg/kg-bw per day (seniors) to 2.0 mg/kg-bw per day (toddlers and children) |
Abbreviations: kg-bw, kilogram of body weight.
Seniors considered to be 60+ years of age. Toddlers considered to be 6 months to 4 years of age. Children considered to be 4 to 11 years of age.
None of the 52 Azo Acid Dyes in this screening assessment was identified to be used in food packaging applications; therefore, exposure from food packaging is expected to be negligible.
7.1.2 Products
A variety of exposure scenarios for products available to consumers are considered to be relevant to general population exposure, including use of cosmetics, drugs, textiles, leather, cleaning products and tattoo inks. Where substance-specific information was available, exposure estimates were derived for each substance. Otherwise, generic default parameters were applied (refer to Appendix D for details). Exposure estimates that result in the highest levels are presented below. Details of the exposure characterization and estimates for all identified uses are available in Appendix D. Limited data on dermal absorption were identified for most of the Azo Acid Dyes. As a tier 1 approach, dermal absorption was assumed to be 100% for estimating exposure via the dermal route. This is considered to be conservative as, in reality, dermal absorption is expected to be less than 100%.
7.1.2.1 Amaranth, New Coccine, Orange II and Tartrazine
Amaranth, New Coccine and Orange II are used in cosmetic products in Canada (2013 emails from Consumer Product Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). Tartrazine is used in cosmetics and certain non-prescription drugs at the cosmetic-drug interface in Canada (LNHPD 2015; NMID 2011; Environment Canada 2012; 2011 and 2013 emails from Consumer Product Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). The highest estimated dermal and oral exposures are summarized in Tables 7-1 to 7-4; also see Appendix D. Inhalation exposure to vapours is expected to be negligible due to the very low vapour pressure of these substances. Exposure via inhalation to droplets of spray products is considered to be low relative to exposure by other routes.
Although these substances are used in other products available to consumers (as outlined in the ‘Exposure from Other Products’ section of Appendix D), the predominant source of non-dietary exposure of the general population is considered to be the use of the products listed in Tables 7-1 to 7-4.
| Exposure scenario | Age group | Route | Concentration (% w/w)[b] |
Estimated daily exposure (mg/kg-bw per day) |
|---|---|---|---|---|
| Toothpaste | Adults | Oral | less than or equal to 0.1 | less than or equal to 0.0023 |
| Lipstick | Adults | Oral | less than or equal to 3 | less than or equal to 0.01 |
| Lip balm | Toddlers | Oral | less than or equal to 0.3 | less than or equal to 0.001 |
| Body moisturizer | Adults | Dermal | less than or equal to 0.1 | less than or equal to 0.068 |
Table 7-2 Notes
Abbreviations: kg-bw: kilogram of body weight; w/w: weight per weight
- [a]
- Dermal absorption was conservatively assumed to be 100%.
- [b]
- Based on notifications submitted under the Cosmetic Regulations to Health Canada (2011 and 2013 emails from Consumer Product Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
| Cosmetic product exposure scenario | Age group | Concentration (% w/w)[b] |
Estimated daily exposure (mg/kg-bw per day) |
|---|---|---|---|
| Body moisturizer | Adults | less than or equal to 1 | less than or equal to 0.68 |
| Leave-in hair conditioner | Adults | 1-3 | 0.02-0.61 |
Table 7-3 Notes
Abbreviations: kg-bw: kilogram of body weight; w/w: weight per weight
- [a]
- Dermal absorption was conservatively assumed to be 100%.
- [b]
- Based on notifications submitted under the Cosmetic Regulations to Health Canada (2011 and 2013 emails from Consumer Product Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
| Cosmetic product exposure scenario | Age group | Route | Concentration (% w/w)[b] |
Estimated daily exposure (mg/kg-bw per day) |
|---|---|---|---|---|
| Lipstick | Adults | Oral | less than or equal to 0.1 | less than or equal to 0.0003 |
| Body moisturizer | Adults | Dermal | less than or equal to 0.1 | less than or equal to 0.068 |
| Hair shampoo | Infants | Dermal | less than or equal to 0.1 | less than or equal to 9.3 × 10-5 |
Table 7-4 Notes
Abbreviations: kg-bw: kilogram of body weight; w/w: weight per weight
- [a]
- Dermal absorption was conservatively assumed to be 100%.
- [b]
- Based on notifications submitted under the Cosmetic Regulations to Health Canada (2011 and 2013 emails from Consumer Product Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
| Cosmetic and personal care drug product exposure scenario | Age group | Route | Concentration (% w/w)[b] |
Estimated daily exposure (mg/kg-bw per day) |
|---|---|---|---|---|
| Lip balm | Toddlers | Oral | less than or equal to 30 | less than or equal to 0.11 |
| Lipstick | Adults | Oral | less than or equal to 30 | less than or equal to 0.10 |
| Toothpaste | Toddlers | Oral | less than or equal to 0.1 | less than or equal to 0.68 |
| Toothpaste | Adults | Oral | less than or equal to 0.1 | less than or equal to 0.0011 |
| Facial makeup | Adult | Dermal | less than or equal to 10 | less than or equal to 0.94 |
| Body moisturizer | Adult | Dermal | less than or equal to 1 | less than or equal to 0.68 |
| Baby cream | Infant | Dermal | less than or equal to 0.1 | less than or equal to 0.32 |
Table 7-5 Notes
Abbreviations: kg-bw: kilogram of body weight; w/w: weight per weight
- [a]
- Dermal absorption was conservatively assumed to be 100%.
- [b]
- LNHPD 2008; NMID 2011; and based on notifications submitted under the Cosmetic Regulations to Health Canada (2011 and 2013 emails from Consumer Product Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
7.1.2.2 Acid Black 24, Acid Black 26, Acid Blue 113, CAS RN 70210-25-2, Acid Orange 33, Acid Red 6, Acid Red 138, CAS RN 68155-63-5, CAS RN 70210-05-8, CAS RN 70210-06-9, CAS RN 70210-34-3, CAS RN 71873-51-3 and CAS RN 84962-50-5
Upper-bounding estimates of exposure of the general population to 10 substances (i.e., Acid Black 24, Acid Black 26, Acid Blue 113, Acid Orange 33, Acid Red 6, Acid Red 138, CAS RN 70210-05-8, CAS RN 70210-06-9, CAS RN 71873-51-3 and CAS RN 84962-50-5) used as dyes in textile products were estimated via the dermal route from direct skin contact (refer to Table 7-5). Assumptions and default parameters for these scenarios are outlined in Appendix D. Upper-bounding oral exposures due to mouthing of textiles by infants were also estimated.
| Exposure scenario | Age group | Route | Upper-bounding daily exposure (mg/kg-bw per day) |
|---|---|---|---|
| Textiles: Personal apparel | Adults | Dermal[b] | 0.026 |
| Textiles: Baby sleeper | Infants | Dermalb | 0.040 |
| Mouthing of textile objects | Infants | Oral | 0.000 27 |
Table 7-6 Notes
Abbreviations: kg-bw: kilogram of body weight;
- [a]
- Concentration of dye assumed to be 1% (BfR 2007).
- [b]
- Dermal absorption was conservatively assumed to be 100%.
Exposure to dyes from contact with leather products was also considered. Seven Azo Acid Dyes-- Acid Black 24, Acid Blue 113, CAS RN 68155-63-5, CAS RN 70210-05-8, CAS RN 70210-06-9, CAS RN 70210-25-2 and CAS RN 70210-34-3--are commonly used as dyes for leather, based on available information (Environment Canada 2012) and according to the Colour Index International database that is published jointly by the Society of Dyers and Colourists and the American Association of Textile Chemists and Colorists (CII 2013). Estimated exposures from direct skin contact with leather products are included in Table 7-6 (refer to Appendix D for more details).
| Exposure scenario | Age group | Route | Per event exposure (mg/kg-bw) |
|---|---|---|---|
| Leather products | Adults | Dermal[b] | 0.0021-0.077 |
| Toys of leather material | Infants | Dermalb | 0.040 |
Table 7-7 Notes
Abbreviations: kg-bw: kilogram of body weight;
- [a]
- Concentration of dye assumed to be 2% (Øllgaard et al. 1998).
- [b]
- Dermal absorption was conservatively assumed to be 100%.
7.1.3 Uncertainties in Human Exposure Assessment
Estimated exposures to Tartrazine and Amaranth from consumption of foods are considered to be conservative for several reasons. Where possible, they were based on the highest levels of use reported by the Canadian food industry (2013-4 personal communications from various food industry stakeholders to Food Directorate, Health Canada; unreferenced) and results measured by the CFIA from targeted food surveys. In the absence of such data, they were based either on data submitted to Health Canada’s Food Directorate Bureau of Chemical Safety in response to a call for data issued in July 1976 or on the maximum levels of use permitted in the List of Permitted Colouring Agents; as food additive provisions are enabling, not all food producers necessarily use Amaranth and Tartrazine at these maximum levels. In addition, all foods in a given category were assumed to contain the food dye in question. As mentioned previously, estimated dietary exposures to Amaranth were based on usual intakes derived from two 24-hour dietary recall surveys (CCHS cycle 2.2); while for Tartrazine, estimates are based on one 24-hour dietary recall survey, which may overestimate long-term usual exposure. More details regarding underlying assumptions are summarized in Appendix C.
Estimated exposures to Amaranth, New Coccine, Orange II and Tartrazine from dermal contact with certain products are also based on conservative assumptions (see Appendix D). Only limited dermal absorption studies were identified for two of these four substances. Dermal absorption of Tartrazine was reported to be low (less than 1%) in an in vitro study on the outer skin of porcine ears (unpublished report referenced in SCCNFP 2004a). Similarly, dermal absorption of Orange II was reported to be low (less than 5%) in an in vitro study on pig skin (Honarvar et al. 2005). Both studies were conducted with a 30-minute exposure duration; limited details on these studies were available. Another in vitro study in which Orange II was applied to human skin in four different hair dye formulations indicated that 0.1% to 13.8% of the applied dose was absorbed; percent absorbed includes Orange II found in the stratum corneum, epidermis, dermis, receptor fluid, receptor rinse and receptor chamber rinse. However, the duration of exposure was again only 30 minutes, and the potential for azo bond cleavage was not accounted for; therefore, the study was considered inadequate for refinement of the dermal uptake fraction (Charles River Laboratories 2013). In the absence of adequate dermal absorption data and skin metabolism data, the dermal uptake fraction of these substances was conservatively assumed to be 1. This is considered to be conservative as, in reality, dermal absorption is expected to be significantly less than 100%.
There is uncertainty with respect to exposure estimates for textiles and leather. The estimates are based on generic assumptions for dye content in textiles or leather not specific to Azo Acid Dyes and are likely overestimates.
7.2 Health Effects Assessment
Carcinogenicity and genotoxicity are the critical health effects of potential concern for Aromatic Azo and Benzidine-based Substances. The mechanism by which Azo Acid Dyes exert their toxicity involves the reductive cleavage of the azo bonds and the subsequent release of the free aromatic amines. These aromatic amines are, in turn, converted to reactive electrophilic intermediates through metabolic oxidation (Environment Canada and Health Canada 2013). The health effects of the 52 Azo Acid Dyes were assessed by examining their ability to undergo reductive cleavage and their hazard potential. This analysis, based on consideration of the available information, is presented in the next two subsections. The focus of the health effects assessment was on those Azo Acid Dyes for which exposure of the general population of Canada is expected (see “Exposure Assessment”). For substances for which exposure of the general population of Canada is not expected, the health effects assessment focused on carcinogenicity and genotoxicity.
For six Azo Acid Dyes (i.e., Amaranth, Metanil Yellow, New Coccine, Orange II, Ponceau MX and Tartrazine; all monoazo acid dyes), some data were available on carcinogenicity and in vivo and in vitro genotoxicity. Limited in vitro genotoxicity data were identified for an additional 12 Azo Acid Dyes (i.e., Acid Black 24, Acid Blue 113, Acid Black 26, Acid Orange 33, Acid Red 138, CAS RN 29706-48-7, CAS RN 52236-73-4, CAS RN 68155-63-5, Acid Orange 156, CAS RN 70210-06-9, CAS RN 72828-83-2 and CAS RN 79234-36-9). For the remaining Azo Acid Dyes, no data on carcinogenicity or genotoxicity were identified. For four Azo Acid Dyes for which exposure to Canadians is expected (i.e., Amaranth, New Coccine, Orange II and Tartrazine), repeated-dose toxicity data were also available on non-cancer endpoints, including reproductive and developmental endpoints. No relevant health effects data were identified for the other Azo Acid Dyes for which exposure is expected. Due to the limited data for many of the substances in this assessment, information on analogues was used to inform the health effects assessment, as appropriate.
As the hazard potential of azo dyes can be attributed to the effects of the azo bond cleavage products (i.e., aromatic amines), carcinogenicity and genotoxicity data on the corresponding aromatic amines were considered in order to inform the health effects assessment of the Azo Acid Dyes. All of the substances in this subgroup have sulfonic acid substituents (SO3-) at one or more positions, and therefore many of the aromatic amines released are sulfonated. Available data pertaining to sulfonated aromatic amines indicate that these substances generally have no or very low genotoxic effects. Jung et al. (1992) demonstrated that mutagenicity seen in several aromatic amines is absent in their corresponding sulfonated analogues. The mitigating effect of sulfonation on the mutagenic potential of aromatic amines, including increased electronegativity and water solubility, is recognized (Marchisio et al. 1976; Lin and Solodar 1988; OECD QSAR Toolbox 2013). The effect of sulfonation also appears to contribute to the overall lower systemic toxicity observed for sulfonated aniline and sulfonated naphthylamine derivatives. These generally have effect levels at doses higher than those of their unsulfonated counterparts and do not have strong hemolytic effects that are typical of aniline and other un-sulfonated aromatic amines (Sakuratani et al. 2008; see also Sections 7.2.2 and 7.2.3 of Environment and Climate Change Canada, Health Canada 2015). With respect to the effect of sulfonation status on the toxicity of aromatic amine metabolites, those Azo Acid Dyes which are fully sulfonated (i.e., one or more sulfonate groups on all potential aromatic amine metabolites) and those theoretically generating at least one un-sulfonated aromatic amine are indicated in the footnotes of Tables 7-7, 7-8, and 7-9.
The potential link between food colouring agents (including certain azo dyes) and behavioural effects, including hyperactivity, in children has been investigated by several national and international organizations, including Health Canada, the US Food and Drug Administration (US FDA) and the European Food Safety Authority (EFSA). To date, no causal link has been established between particular food colouring agents and behavioural effects; however, it has been acknowledged that there may be individuals who are particularly sensitive to some food colouring agents (EFSA 2008; Health Canada 2010; US FDA 2010, 2011a, b). These reviews included Tartrazine, which is an approved food colour in Canada, and New Coccine, which is not on the list of approved food colours in Canada.
7.2.1 Azo Bond Cleavage Potential
The azo bond cleavage potential of the substances considered in this assessment was determined based on several lines of evidence that have been previously discussed (Environment Canada and Health Canada 2013). The types of evidence considered in this assessment range from in vivo assays to general read-across.
In vivo ADME (absorption, metabolism, distribution, elimination) studies provide the most direct evidence for reductive cleavage, and this type of study was identified for 6 of 20 monoazo acid dyes (Table 7-7). In all instances, one or more of the released aromatic amines were identified in the urine and feces of one or more mammalian species that were orally exposed to the dye, thus indicating that these six monoazo acid dyes are capable of undergoing in vivo cleavage of the azo bond and of releasing their corresponding aromatic amines (Daniel 1962; Radomski and Mellinger 1962; Jones et al. 1964; Hall et al. 1966; Urakubo 1967; Pritchard et al. 1976; Ruddick et al. 1977; Willes et al. 1980; Raza et al. 1981; Phillips et al. 1982, 1987; Singh 1989; Singh et al. 1991; Poul et al. 2009). Similar findings were observed in metabolism studies in vivo for other fully sulfonated monoazo acid dyes not evaluated in this assessment including Food Red 17 (CAS RN 25956-17-6), Food Yellow 3 (CAS RN 2783-94-0) and Food Red 3 (CAS RN3567-69-9) (EFSA 2008) supporting that azo cleavage readily occurs by the oral route for the fully sulfonated azo acid dyes. While no ADME studies were identified for the disazo and polyazo acid dyes in this assessment, all of which are at least partially sulfonated, azo cleavage by the oral route was also observed for related disazo benzidine-based acid and direct dyes, including Acid Red 114 (CAS RN 6459-94-5), Direct Blue 14 (CAS RN 72-57-1) and Direct Red 28 (CAS RN 573-58-0) and for related polyazo benzidine-based direct dyes, Direct Black 38 (CAS RN 1937-37-7) and Direct Brown 95 (CAS RN 16071-86-6), which are all similarly partially sulfonated (Environment Canada, Health Canada 2014a).
In vitro metabolism studies were identified for seven monoazo, five disazo and one polyazo acid dye (Tables 7-7 to 7-9). The aromatic amines generated from the reductive cleavage of the azo bond were identified following incubation of the dye with intestinal contents (bacteria) from various species, human feces or species of bacteria commonly found on the surface of human skin (Roxon et al. 1967; Larsen et al. 1976; Chung et al. 1978; Watabe et al. 1980; Combes and Haveland-Smith 1982; Phillips et al. 1982; Ghosh et al. 1988; Singh et al. 1995, 1997; Bragger et al. 1997; Chen et al. 2004; Stingley et al. 2010; Pan et al. 2011; BRI 2012, 2013a, b). In all these studies, the potential for reductive cleavage by intestinal or skin microflora was demonstrated.
The results obtained from the Ames assay under reductive conditions could be considered to infer reductive cleavage potential if the Ames test yielded positive results only after such conditions were employed (Environment Canada and Health Canada 2013). For the monoazo acid dyes, only Metanil Yellow had positive results when the azo dye protocol was used (Muzzall and Cook 1979; Rastogi and Levin 1996). In addition, four disazo acid dyes tested positive under reductive conditions in Ames tests using one or more strains of Salmonella typhimurium (ILS 2011, 2012).
In the absence of empirical data, the reductive cleavage potential of an Azo Acid Dye can be inferred based on read-across among closely related substances (Environment Canada and Health Canada 2013). Read-across from substances with empirical data for azo bond reductive cleavage to those with no data was conducted for the Azo Acid Dyes subgroup.
As all of the substances in this subgroup are expected to have the potential to release aromatic amines, the hazard potential of the released aromatic amines will therefore be considered in the health effects assessment of the Azo Acid Dyes. The structures of the potential metabolites released for each Azo Acid Dye were proposed based on theoretical cleavage of all azo bonds. The resulting structures were used to identify CAS RNs associated with each metabolite, when possible.
| Azo Acid Dye (name or CAS RN) |
ADME data | In vitro metabolism data | Ames assay (positive only with reductive conditions) | Read-across |
|---|---|---|---|---|
| Metanil Yellow | X | X | X | |
| Orange II | X | X | ||
| Amaranth[b] | X | X | ||
| Tartrazineb | X | X | ||
| New Coccine | X | X | ||
| Ponceau MX | X | |||
| Acid Red 138 | X | |||
| Acid Red 6 | X | |||
| 29706-48-7 | X | |||
| 35342-16-6 | X | |||
| 52236-73-4 | X | |||
| 70210-05-8 | X | |||
| 71720-89-3b | X | |||
| 71873-51-3b | X | |||
| 72828-83-2 | X | |||
| 79234-36-9 | X | |||
| 83006-48-8 | X | |||
| 83027-51-4 | X | |||
| 83027-52-5 | X | |||
| 84962-50-5b | X |
Table 7-8 Notes
Abbreviations: ADME: absorption, distribution, metabolism, elimination;
- [a]
- An "X" is placed where empirical data are available or to indicate that read-across was used to determine azo bond reductive cleavage.
- [b]
- All the aromatic amine metabolites are sulfonated.
| Azo Acid Dye (name or CAS RN) |
ADME data | In vitro metabolism data | Ames assay (positive only with reductive conditions) | Read-across |
|---|---|---|---|---|
| Acid Black 24 | X | |||
| Acid Blue 113 | X | X | ||
| Acid Black 26 | X | |||
| Acid Orange 33 | X | X | ||
| 51988-24-0 | X | |||
| 62133-79-3 | X | |||
| 62133-80-6 | X | |||
| 67892-55-1 | X | |||
| Acid Orange 156 | X | X | ||
| 70210-06-9 | X | X | ||
| 72828-67-2 | X | |||
| 72968-80-0 | X | |||
| 72968-81-1 | X | |||
| 72986-60-8 | X | |||
| 72986-61-9 | X | |||
| 75949-73-4 | X | |||
| 83006-77-3 | X | |||
| 83006-74-0 | X | |||
| 83221-60-7 | X | |||
| 85030-31-5 | X | |||
| 90218-20-5 | X | |||
| 90432-08-9 | X | |||
| 106028-58-4[b] | X | |||
| 114910-04-2 | X |
Table 7-9 Notes
Abbreviations: ADME: absorption, distribution, metabolism, elimination;
- [a]
- An "X" is placed where empirical data are available or to indicate that read-across was used to determine azo bond reductive cleavage.
- [b]
- All the aromatic amine metabolites are sulfonated.
| Azo Acid Dye (CAS RN) |
ADME data | In vitro metabolism data | Ames assay (positive only with reductive conditions) | Read-across |
|---|---|---|---|---|
| 68155-63-5 | X | |||
| 70210-25-2 | X | |||
| 70210-34-3 | X | |||
| 72496-92-5 | X | |||
| 84559-92-2 | X | |||
| 85136-25-0 | X | |||
| 85223-35-4[b] 102616-51-3 |
X | |||
| 90459-02-2 | X |
Table 7-10 Notes
Abbreviations: ADME: absorption, distribution, metabolism, elimination;
- [a]
- An "X" is placed where empirical data are available or to indicate that read-across was used to determine azo bond reductive cleavage.
- [b]
- CAS RNs 85223-35-4 and 102616-51-3 correspond to the same substance.
7.2.2 Health Effects
7.2.2.1 Amaranth (CAS RN 915-67-3)
The health effects of Amaranth have been previously reviewed by the EU SCF (1983), JECFA (1984) and EFSA (2010). In addition, Amaranth has been reviewed and classified as group 3 ("not classifiable as to its carcinogenicity") by IARC (1975b, 1987c). ADI values have been set by the EU SCF (0-0.8 mg/kg-bw; SCF 1983), JECFA (0-0.5 mg/kg-bw; JECFA 1984) and EFSA (0.15 mg/kg-bw per day; EFSA 2010). Health Canada has established an ADI of 0.5 mg/kg-bw per day, consistent with that derived by JECFA (2014 memorandum from Chemical Health Hazard Assessment Division, Health Canada to Bureau of Chemical Safety, Health Canada; unreferenced).
By the oral route, Amaranth is reduced, and the aromatic amines released are absorbed into the systemic circulation. In vitro data have shown that liver enzymes, gut bacteria and at least one species of skin bacteria can mediate the azo cleavage of the dye (EFSA 2010; BRI 2013b).
Carcinogenicity and Genotoxicity
The carcinogenic potential of Amaranth has been investigated in a number of studies in rats and mice in which animals were exposed to the dye orally through the diet or subcutaneously (Nelson and Hagan 1953; Willheim and Ivy 1953; DFG 1957; Mannell et al. 1958; US FDA 1964; JECFA 1966; Baigusheva 1968; Andrianova 1970). The majority of these studies were reviewed by JECFA (1984) and IARC (1975b) and found to be limited or confounded by insufficient information about dye purity, by very low purity of the dye or by deficiencies in study design and execution (EFSA 2010).
The concerns regarding the possible carcinogenic potential of Amaranth were addressed in a long-term study with an in utero phase in which Wistar-derived rats (54 of each sex per treated group; 90 of each sex for controls) were exposed to 0, 50, 250 or 1250 mg/kg-bw per day of the dye through their diet for 2 years after weaning. The parental generation rats were exposed to the respective dose levels for 60 days before mating, and dams were dosed during gestation and lactation as well. No treatment-related increase in the incidence of neoplasms was observed in males. While there was a statistically significant increase in the incidence of uterine polyps and vaginal fibromas in the high-dose females when compared with the controls, they were within expected or historical control ranges and were therefore not considered to be treatment related (Clode et al. 1987).
A dermal carcinogenicity study was also identified for Amaranth. Swiss Webster mice (50 of each sex) received weekly uncovered skin applications of about 33 mg/kg-bw of dye for 19.5 months in the dorsal area (approximately 5 mg/kg-bw per day). No treatment-related differences in the incidence of any lesions, either neoplastic or non-neoplastic, were reported (including incidence of lymphomas, which are common in this strain) (Carson 1984a).
Amaranth was not mutagenic in the Ames assay with or without metabolic activation or in the presence of reductive conditions (Stanford Research Institute 1972; Baker et al. 1974; Auletta et al. 1977; Garner and Nutman 1977; Brown et al. 1978; Lecointe and Lesca 1978; Bonin and Baker 1980; Haveland-Smith and Combes 1980; Chung et al. 1981; Ishidate et al. 1981, 1984; Prival et al. 1988; Izbirak et al. 1990; Sweeney et al. 1994). Chromosomal aberrations were detected following incubation of the dye with Chinese hamster fibroblasts and diploid human embryonic lung cells, but not with human lymphocytes (Stanford Research Institute 1972; Zhurkov 1975; Ishidate et al. 1981, 1984). Mixed results were obtained in in vitro genotoxicity assays for deoxyribonucleic acid (DNA) damage and/or repair (Kada et al. 1972; Stanford Research Institute 1972; Sankaranarayanan and Murthy 1979; Haveland-Smith and Combes 1980; Kornbrust and Barfknecht 1985; von der Hude et al. 1988; Sweeney et al. 1994; Mpountoukas et al. 2010).
In vivo genotoxicity assays were generally negative (Stanford Research Institute 1972; Arnold et al. 1976; Münzner 1979; Tripathy et al. 1995). Comet assays following oral gavage, however, showed that Amaranth was able to selectively damage DNA in the colon, stomach and bladder of two strains of mice exposed to up to 2000 mg/kg-bw of the dye; positive results were also obtained in the stomach of rats orally exposed to 10 mg/kg-bw of the dye, but this result was considered sporadic by the authors (Tsuda et al. 2001; Sasaki et al. 2002; Shimada et al. 2010). Given the lack of genotoxicity and increase in mitotic cells observed in the gut micronucleus assay in mice orally exposed to up to 1000 mg/kg-bw twice at 24-hour intervals, as well as the significant presence of Amaranth and its main aromatic amine metabolites among colonic cells, the authors concluded that the increase in comet tail length observed in the comet assays represented transient DNA damage rather than stable genotoxic lesions and may be partly explained by local cytotoxicity of the dye (Poul et al. 2009).
The expected metabolites following cleavage of the azo bond in Amaranth are naphthionic acid (CAS RN 84-86-6) and 1-amino-2-naphthol-3,6-disulfonic acid (CAS RN 42579-07-7). The available data on the azo cleavage metabolites of Amaranth suggest that neither of the aromatic amines released is mutagenic (Garner and Nutman 1977; Chung et al. 1981; Jung et al. 1992; Poul et al. 2009).
Based on the available data reviewed above, Amaranth does not appear to have carcinogenic potential and is not expected to be genotoxic. This is consistent with previous evaluations by several international bodies (IARC 1975b, 1987c; SCF 1983; JECFA 1984; EFSA 2010).
Other Health Effects
In the long-term study with an in utero phase (described above), Wistar rats were exposed to 0, 50, 250 or 1250 mg/kg-bw per day of the dye through their diet for 2 years after weaning. In females, there was an increased incidence of renal calcification and pelvic epithelial hyperplasia with degenerative changes noted in all treated groups. The authors noted that the hyperplasia was focal and also associated with mineralization, suggesting an irritant effect. The lowest dose was identified as a lowest-observed-adverse-effect level (LOAEL) for this study, and no no-observed-adverse-effect level (NOAEL) was identified in the publication (Clode et al. 1987). A second, unpublished evaluation of the data from this study in which tissue histological assessment was randomized indicated that the changes were not statistically significant until 250 mg/kg-bw per day (Butler and Conning 1983). JECFA (1984) derived an ADI for Amaranth based on selection of 50 mg/kg-bw per day as a NOAEL in the chronic study . Health Canada has established an ADI of 0.5 mg/ kg-bw per day, consistent with that derived by JECFA (2014 memorandum from Chemical Health Hazard Assessment Division, Health Canada to Bureau of Chemical Safety, Health Canada; unreferenced).
In a follow-up study focusing only on kidney effects, there was a positive dose-related trend in the incidence of renal pelvic calcification and pelvic hyperplasia in male rats treated with Amaranth for 90 days, which was significant at the highest dose (1250 mg/kg-bw per day). The increased calcification, however, was seen only in rats with kidneys already compromised by senile nephrosis. The highest dose (1250 mg/kg-bw per day) was identified as the LOAEL, and the next lower dose of 80 mg/kg-bw per day was the NOAEL for this study (Clode et al. 1987). The EU SCF (1983) derived an ADI for Amaranth based on the NOAEL of 80 mg/kg-bw per day in the 90-day study.
In the only dermal repeated-dose study identified, mice received weekly uncovered skin applications of about 33 mg/kg-bw of dye for 19.5 months (approximately 5 mg/kg-bw per day) (Carson 1984a) (see above section; no treatment-related effect in the incidence of any lesions). There were no significant differences between treatment and control groups in body weight, behaviour or survival, and there was no indication of treatment-related pathology.
Several reproductive and developmental toxicity studies were identified in the rat. However, only one study was identified from which both a NOAEL and a LOAEL could be determined (Collins and McLaughlin 1972). Female Osborne-Mendel rats were given Amaranth (purity 94%) by gavage from gestation days 0 to 19 at a dose level of 7.5, 15, 30, 100 or 200 mg/kg-bw per day. There were no treatment-related changes in average fetal weight, skeletal or soft tissue abnormalities, or number of implantations per dam; however, the percentage of viable implantations (and number of live fetuses) per litter decreased with increasing dosage. The number of litters with one or more resorptions, the number of litters with two or more resorptions and the number of resorptions per litter increased with increasing dose; these increases were significant from 30 mg/kg-bw per day and above. The authors identified the study LOAEL as 15 mg/kg-bw per day, based on a non-statistically significant increase in resorptions. An EFSA Panel selected 30 mg/kg-bw per day as the LOAEL, based on the dose at which the responses became statistically significant (EFSA 2010).
In a follow-up collaborative study, Osborne-Mendel and Charles River rats were treated with Amaranth by gavage or in drinking water during gestation (days 0-19, 6-15 or 7-9 ) at 0 or 200 mg/kg-bw per day. There was no increase in resorptions in Osborne-Mendel rats during the same gestational period and at a dose level the same as the highest dose in the Collins and McLaughlin (1972) study. However, in Charles River rats gavaged on gestation days 0-19, significant increases in litters with two or more resorptions and in the percentage of resorptions per litter were observed. The authors indicated that they did not consider these effects were biologically significant because of the significant variability in the incidence of resorptions, the absence of effects on the number of viable pups, fetal weight or abnormalities and the lack of reproducibility of the effect in Osborne-Mendel rats (Collins et al. 1976a, b; Holson et al. 1976; Keplinger et al. 1976).
No effects on fertility or developmental parameters were observed in a two-generation feeding study in Wistar rats (up to 1250 mg/kg-bw per day) (Clode et al. 1987). The only effects in a three-generation feeding study in Osborne-Mendel rats were a decrease in weanling weights at the high dose (~2400 mg/kg-bw per day) in some litters and decreased survival index at 14 days (one litter at the high dose) or 21 days (one litter at a mid-dose), with no dose-response relationship (Collins et al. 1975b). Some of the litters from the three-generation feeding study in Osborne-Mendel rats were used for a teratology study (Collins et al. 1975a). Some instances of increased resorptions were observed, but only in some dose groups. There were no consistent dose-dependent adverse effects observed in the animals of any generation.
Several other feeding and gavage studies in different rat strains were identified in which no effects on development were identified at up to the highest dose tested (150-2000 mg/kg-bw per day) (FDRL 1972; Burnett et al. 1974; Keplinger et al. 1974; Khera et al. 1974; Willes et al. 1980).
Amaranth has also been studied in developmental toxicity tests in multiple other species, including mice, hamsters, rabbits, cats and dogs. No LOAELs were identified from any study. Although effects were observed in some cases, none was consistent and dose related. Based on the highest dose tested, NOAELs for developmental toxicity were identified for mouse (100 mg/kg-bw per day), rabbit (15 mg/kg-bw per day) and dog (90 mg/kg-bw per day) (Keplinger et al. 1974; Larsson 1975; Mastalski et al. 1975).
EFSA (2010) determined an ADI for Amaranth based on the developmental toxicity NOAELs of 15-100 mg/kg-bw per day, as well as a LOAEL of 50 mg/kg-bw per day in the chronic dietary study.
In two of publications in which mice were given Amaranth in food or drinking water before and during gestation, as well as for 9 weeks after birth, at doses of 39-450 mg/kg-bw per day, no consistent, significant dose-related effects were observed. No NOAELs could be derived from these studies (Tanaka 1992, 1993).
7.2.2.2 Metanil Yellow (CAS RN 587-98-4)
Carcinogenicity and Genotoxicity
One limited carcinogenicity study was identified for Metanil Yellow, in which female mice developed tumours following administration of Metanil Yellow at a high dose in the diet for 1 year. The publication lacked key study details (e.g., limited reporting of methodology and results; purity of the test substance was not reported) (Prasad and Rastogi 1982a). A number of studies that investigated the tumour-promoting effects of Metanil Yellow on the development of hepatic preneoplastic lesions induced by diethylnitrosamine (DEN) in male rats were also identified (Fernandes and Rao 1994; Sundarrajan et al. 2000, 2001; Gupta et al. 2003). In all instances, liver carcinogenesis was enhanced by Metanil Yellow, and this tumour-promoting effect involved increases in the expression of proliferating cell nuclear antigen (PCNA), a marker of cell proliferation, and of G1/S cyclins, as well as an increase in DNA synthesis.
In vitro, Metanil Yellow was mutagenic only in the presence of reductive metabolism and metabolic activation in the Ames assay and exerted mutagenicity in human lymphoblast cells in all three treatment regimens used (Muzzall and Cook 1979; Rastogi et al. 1991; Rastogi and Levin 1996). A dose-dependent increase in chromosomal aberrations was also observed in human leukocytes in vitro (Vaidya and Godbole 1978). In vivo, Metanil Yellow was positive in chromosomal aberration and sister chromatid exchange assays in mice and in the micronucleus assay in rats (Giri and Banerjee 1986; Giri et al. 1986a, b; Pino et al. 1996). It was also positive in a dominant lethal study in mice; however, a small number of animals and a single dose were tested (Prasad 1986).
7.2.2.3 New Coccine (CAS RN 2611-82-7)
The health effects of New Coccine (Ponceau 4R) were previously reviewed by JECFA (1983, 2011) and EFSA (2009a). ADI values have been set by JECFA (0-4 mg/kg-bw; JECFA 1983, 2011) and EFSA (0.7 mg/kg-bw per day; EFSA 2009a). No carcinogenicity classifications were identified.
By the oral route, New Coccine is reduced, and the aromatic amines released are absorbed into the systemic circulation. In vitro data have shown that both liver enzymes and gut bacteria can mediate the azo cleavage of the dye (EFSA 2009a). No data were available on New Coccine regarding potential for azo reduction on the skin.
Carcinogenicity and Genotoxicity
No evidence of carcinogenicity was demonstrated in eight long-term carcinogenicity studies in rats and mice exposed to New Coccine in their diet, in their drinking water or subcutaneously (Allmark et al. 1957; DFG 1957; Mason et al. 1974; Brantom et al. 1987a). Although some of these studies are limited, no carcinogenic effect was observed across these eight studies up to dose levels of approximately 1463 mg/kg-bw per day in rats and 1625 mg/kg-bw per day in mice in feeding studies.
In vitro mutagenicity was not observed in the Ames assay with and without metabolic activation using various protocols, including under reductive conditions, or in mouse lymphoma cells (Viola and Nosotti 1978; Haveland-Smith and Combes 1980; Ishidate et al. 1984; Cameron et al. 1987; Izbirak et al. 1990; Ozaki et al. 1998). Genotoxicity was also not observed in vitro in several DNA damage and/or repair assays, and limited mixed results were seen in chromosomal aberration assays (Kada et al. 1972; Ishidate et al. 1978, 1984; Sankaranarayanan and Murthy 1979; Tonogai et al. 1979; Haveland-Smith and Combes 1980; Kornbrust and Barfknecht 1985).
In vivo, negative results were obtained when New Coccine (purity of 91%) was assayed in a micronucleus assay (Hayashi et al. 1988), but a dose-related increase in chromosomal aberrations was observed in mice exposed to 0, 4, 10 or 20 mg/kg-bw of the dye (purity not reported) (Agarwal et al. 1993). While no DNA damage was observed in vivo in rats (Kornbrust and Barfknecht 1985; Shimada et al. 2010), comet assays following oral gavage showed that New Coccine caused DNA damage in the colon, stomach and bladder of two strains of mice exposed to up to 2000 mg/kg-bw of the dye (Tsuda et al. 2001; Sasaki et al. 2002; Shimada et al. 2010).
The postulated azo bond cleavage products of New Coccine are naphthionic acid (CAS RN 84-86-6) and 1-amino-2-naphthol-6,8-disulfonic acid (n° CAS RN identified). The available data on these substances suggest that neither of them is mutagenic (Garner and Nutman 1977; Chung et al. 1981; Jung et al. 1992; Poul et al. 2009).
Despite the DNA damage observed in mice following oral gavage dosing with New Coccine, no carcinogenicity was observed in a number of long-term rat and mouse studies. The majority of in vitro and in vivo studies also tested negative for genotoxicity, and neither of the aromatic amines released is expected to be mutagenic. Overall, neither carcinogenicity nor genotoxicity is an endpoint of toxicological concern for New Coccine.
Other Health Effects
Key studies focusing on relevant routes of exposure are presented below.
When rats (16 of each sex per group) were exposed to 0%, 0.5%, 1.0% or 2.0% New Coccine (purity greater than or equal to 82%; equivalent to 0, 220, 560 and 1140 mg/kg-bw per day for males and 0, 280, 630 and 1250 mg/kg-bw per day for females, respectively) in their diet for 90 days, the mid-dose was identified as the NOAEL and the high dose as the LOAEL, based on a statistically significant increase in alanine and aspartate aminotransferases in both sexes and a significant decrease in hemoglobin concentrations and liver weight in females at the highest dose tested (Gaunt et al. 1967). In another subchronic study, pigs (three of each sex per group) were exposed to the dye (purity greater than or equal to 82%) in their feed at a concentration of 0, 100, 300 or 900 mg/kg-bw per day for 90 days. Although a slight transient decrease in the number of erythrocytes was observed at the highest dose, 900 mg/kg-bw per day was considered the NOAEL of the study (Gaunt et al. 1969).
NOAEL and LOAEL values were also identified in three chronic toxicity studies in which mice or rats were exposed to New Coccine through their feed for 64-118 weeks (Allmark et al. 1957; Mason et al. 1974; Brantom et al. 1987a).
When male and female mice were exposed to 0%, 0.01%, 0.05%, 0.25% or 1.25% of the dye (equivalent to 0, 13, 65, 325 and 1625 mg/kg-bw per day) for 82 weeks, a NOAEL of 65 mg/kg bw per day and a LOAEL of 325 mg/kg-bw per day were determined based on an increased incidence of glomerulonephrosis. Mild anemia during the first 6 months (which became significant at the next higher dose) was also observed at the LOAEL (Mason et al. 1974). JECFA used the dose level of 0.25% (which was converted to 375 mg/kg-bw per day) as a NOAEL in the derivation of the ADI (JECFA 1983, 2011), whereas EFSA selected 0.05% (which was converted to 70 mg/kg-bw per day) as a NOAEL for the ADI (EFSA 2009a).
In another study, rats were given diets containing 0%, 0.03%, 0.3% or 3% (equivalent to 0, 16, 153 and 1462 mg/kg-bw per day for males and 0, 16, 199 and 1772 mg/kg-bw per day for females, respectively, based on week 64 body weight and food consumption data provided in the study) of the dye for 64 weeks. At the highest dose, females had lower food consumption and decreased body weight throughout the experiment, as well as increased relative weights of heart, liver and kidneys. No other adverse effects were reported (Allmark et al. 1957).
In a chronic toxicity study that also included an in utero phase, rats were fed diets containing New Coccine (purity 81%) at a concentration of 0, 50, 500 or 1250 mg/kg-bw per day. The F0 generation was treated for 60 days prior to mating as well as during gestation, and the F1 generation was treated for 114-118 weeks. A NOAEL of 500 mg/kg-bw per day and a LOAEL of 1250 mg/kg-bw per day were established based on lower body weight gain without reduction in food intake and increased protein in the urine of female rats in the F1 generation with histopathological signs of renal damage at the highest dose tested. It cannot, however, be determined if the signs of renal damage are due to the developmental effects from in utero exposure or to chronic postnatal exposure. No treatment-related effects were observed, including effects on fertility and pup rearing, for the parental generation following their 9-week exposure (Brantom et al. 1987a).
No reproductive or developmental toxicity was observed when mice or rats were orally exposed to the dye (purity 70-82%), by gavage or diet, at different time points during gestation or in a three-generation study at the highest dose tested (NOAELs of 100-4000 mg/kg-bw per day) (Larsson 1975; Meyer and Hansen 1975; Kihara et al. 1977; Momma et al. 1981; Brantom et al. 1987b; Tanaka 2006a).
Neurobehavioural toxicity, however, was observed in a study in which mice were exposed to 0%, 0.12%, 0.24% or 0.48% New Coccine (purity greater than 85%) in their feed from 5 weeks of age in the F0 generation to 9 weeks of age in the F1 generation (equivalent to 0, 212, 423 and 819 mg/kg-bw per day for the F1 generation; Tanaka 2006a). Males, but not females, exhibited dose-related impaired performance on the water T-maze test (a test of learning ability) at the mid- and high-doses at 7 weeks and impaired performance in surface righting (indicative of coordinated movement) at the high dose on postnatal day (PND) 4. A NOAEL of 212 mg/kg-bw per day and a LOAEL of 423 mg/kg-bw per day for neurobehavioural toxicity were therefore identified in this study. The authors noted that, in their opinion, the absence of significant adverse effects in females was due to the poor score of a few female controls.
7.2.2.4 Orange II (CAS RN 633-96-5)
Orange II has previously been reviewed by the EU SCCS (2011b, 2014) and the EU SCCNFP (2004b).
In a metabolism study in rabbits administered Orange II by gavage, most of the Orange II administered was excreted in urine as conjugates of the 1-amino-2-naphthol moiety, indicating reductive cleavage, presumably by gut bacteria, and absorption of the resulting amine (Daniel 1962). In vitro assays indicate that reduction of Orange II can be mediated by intestinal bacteria (Chung et al. 1978; Singh et al. 1995; Bragger et al. 1997; Chen et al. 2004), and several species of skin bacteria cultured in vitro were shown to be able to completely reduce Orange II within 24 hours (Stingley et al. 2010).
Carcinogenicity and Genotoxicity
Two carcinogenicity studies identified in mice were both considered limited. The first study, pre-dating OECD Guidelines, was limited to examining the liver of mice exposed to 15-20 mg per week of Orange II in their diet, 5 days/week, for 538 days (equivalent to 71-95 mg/kg-bw per day). Even though neither cholangioma nor hepatoma was observed in these mice, only the livers of 6 mice of the 30 treated were microscopically examined (Cook et al. 1940). In the second study, mice (50 of each sex) received weekly uncovered skin applications of about 33 mg/kg-bw of dye for 18 months in the dorsal region (Carson 1984a). No treatment-related differences in the incidence of any lesions, either neoplastic or non-neoplastic, were reported (including incidence of lymphomas, which are common in this strain).
Orange II was not mutagenic in several in vitro aerobic Ames assays with or without metabolic activation (Garner and Nutman 1977; Chung et al. 1981; Miyagoshi et al. 1983; Yamada et al. 1995; Wollny 1999a), but mixed results were obtained in the presence of reductive conditions (Zhou et al. 1987; Rastogi and Levin 1996; Rafii et al. 1997). Although the dye induced mutations at the HPRT locus in a metabolically competent human lymphoblast cell line, it did not induce mutations at the TK locus in mouse lymphoma cells with and without metabolic activation (Rastogi et al. 1991; Wollny 1999b). In vitro assays for DNA damage and/or repair were generally negative, except in one study following incubation under reductive conditions (Mamber et al. 1983, 1984; Gottlieb et al. 2003). In vivo, Orange II was not mutagenic in Ames assays using samples of bile, urine or feces from male rats treated with 1000 mg/kg-bw by oral gavage (Wever et al. 1989). Orange II was also negative in the micronucleus assay in mouse bone marrow, in a study conducted according to OECD Guidelines (Honarvar 2003), and in a non-guideline study on micronuclei, chromosomal aberrations and sister chromatid exchanges in Chinese hamsters and three strains of mice (Wever et al. 1989). Positive results for chromosomal aberrations were observed in two studies in mice; however, the purity of the test substance was not provided in either study (Prasad and Rastogi 1982b; El-Sherbeny and Ibrahim 1999).
The postulated azo bond cleavage products of Orange II are sulfanilic acid (CAS RN 121-57-3) and 1-amino-2-naphthol (CAS RN 2834-92-6). The available data on these substances indicate that neither of them is mutagenic (Garner and Nutman 1977; Chung et al. 1981; Freeman et al. 1987; Zeiger et al. 1988; Dillon et al. 1994; Mansour et al. 2009).
Overall, the available information indicates that Orange II is not genotoxic in vivo.
Other Health Effects
A number of studies have been identified for various endpoints, but only the critical studies focusing on relevant routes of exposure are presented.
No gross or histopathological lesions were observed in a skin painting study in which mice received weekly uncovered skin applications of about 33 mg/kg-bw of dye for 18 months (approximately 5 mg/kg-bw per day) (Carson 1984a; see above).
In oral gavage and dietary repeated-dose studies on Orange II, spleen and blood effects were consistently observed, characteristic of aromatic amine-induced anemia.
In a range-finding study, extramedullary hematopoiesis was observed in male and female rats orally dosed with Orange II (purity 90%) by gavage for 14 days at the lowest dose tested (10 mg/kg-bw per day); significant increases in absolute and relative spleen weights were seen at the next dose level of 60 mg/kg-bw per day. Hematology parameters were not analyzed in this study (Rosner 1999a). When the 14-day gavage study was repeated with lower doses in order to check the hematology parameters associated with the spleen pathology, a small but statistically significant increase in methemoglobin levels was observed at 10 but not 5 mg/kg-bw per day, clearly indicating a gavage dosing threshold in rats. No histopathology was performed in the second study (Rosner 1999b).
When male and female rats were dosed by gavage at 0, 2.5, 5 or 10 mg/kg-bw per day of Orange II (purity 90%) in a 90-day study, changes in hematology parameters, including increased methemoglobin as well as extramedullary hematopoiesis in the spleen, were observed at the mid- and high- doses, and Heinz bodies were observed in one female at the high dose. The relative spleen weights in males were significantly increased at 10 mg/kg-bw per day (Hamann et al. 2000). Although Hamann et al. (2000) considered the low dose of 2.5 mg/kg-bw per day to be the NOAEL, based on an insignificant increase in methemoglobin levels and reticulocyte counts in males without concurrent effects on the spleen, SCCS (2011b, 2014) noted that slight early hematological effects were already observed at this dose and could be test article related. At the mid-dose of 5 mg/kg-bw per day, the methemoglobin levels (1.5% and 1.6% in males and females, respectively) were still within historical control levels, despite being statistically different from controls (0.9% and 0.8% in males and females, respectively). Therefore, Health Canada considers 10 mg/kg-bw per day to be the lowest-observed-effect level (LOEL) for this study, based on small but statistically significant increases in methemoglobin (2.6% and 2.1% in males and females, respectively), increased relative spleen weight in males and an increase in the severity and incidence of extramedullary hematopoiesis in the spleen.
Hematological effects, including the presence of Heinz bodies, and spleen effects were also observed in several feeding studies at the lowest dose tested (50-420 mg/kg-bw per day) when male rats were exposed to Orange II in their diet for 28-90 days (Rofe 1957; Singh and Khanna 1979; Singh et al. 1987).
Relative spleen weights were increased significantly at all doses in a 90-day rat feeding study; the LOAEL was the lowest tested dose, 128 mg/kg-bw per day, but decreased red blood cell hemoglobin content was evident only at the high dose of 3770 mg/kg-bw per day. Splenomegaly and proliferation of myeloid cells were also observed at the high dose (Singh et al. 1987).
When male rats were exposed to 0%, 0.5% or 1% Orange II (equivalent to 0, 250 and 500 mg/kg-bw per day) in their feed for 300 days, significant alterations in hematological parameters suggestive of normocytic hypochromic anemia (23% decrease in red blood cells and 42% decrease in hemoglobin) were observed at the low dose (Singh and Khanna 1979). In the same study, the formation of Heinz bodies was observed over 60 days of exposure and was shown to be related to dose and exposure duration. Heinz body formation (representing hemoglobin structural changes) was observed at the lowest dose of 250 mg/kg-bw per day, starting on the 8th day of exposure. Patchy degeneration of the testes, including extensive desquamation of almost all seminiferous epithelium and vacuolar degeneration and sloughing off of gametogenic layers, was also observed in the chronic portion of the study at both dose levels. The study authors noted that the effects were not localized to the testicular hormone-producing cells. These testicular effects were not observed in the short-term and subchronic gavage studies on Orange II (Rosner et al. 1999a, b; Hamann et al. 2000).
Increases in chromosomal abnormalities were, however, reported in spermatogonial metaphase cells when ICR/Swiss mice were orally exposed to the dye for 180 days at a very high dose (3000 mg/kg-bw per day, which is above the OECD Guidelines limit dose). Enlargement of the spleen and liver and lethargy were also reported at the LOAEL. A NOAEL of 500 mg/kg-bw per day was identified (Prasad and Rastogi 1982b).
In 2000, Becker and Biedermann reported a maternal toxicity NOAEL of 5 mg/kg-bw per day and a LOAEL of 40 mg/kg-bw per day for Orange II (90% purity) in a rat developmental toxicity study, based on an increase in maternal spleen weight following gavage dosing at 0, 5, 40 or 320 mg/kg-bw per day during gestational days 6-17. The high dose of 320 mg/kg-bw per day was identified as the NOAEL for fetotoxicity (Becker and Biedermann 2000).
Overall, Orange II or its metabolite alters hemoglobin, and repeated exposure over an extended time would be expected to lead to decreased hemoglobin, extramedullary hematopoiesis and subsequent anemia.
7.2.2.5 Ponceau MX (CAS RN 3761-53-3)
Carcinogenicity and Genotoxicity
Carcinogenicity studies were identified in rats and mice, many of which had been previously reviewed by IARC (1975a). IARC has classified Ponceau MX as a Group 2B carcinogen (“possibly carcinogenic to humans”) (IARC 1975a, 1987b); however, carcinogenicity occurred only at high doses and was secondary to liver toxicity, thus suggesting a non-genotoxic mode of action. This is further supported by the overall lack of mutagenicity and genotoxicity observed in the genotoxicity assays.
7.2.2.6 Tartrazine (CAS RN 1934-21-0)
The health effects of Tartrazine have been previously reviewed by Health Canada (1986 memorandum from Toxicology Evaluation Section, Health Canada to Food Additives and Contaminants Section, Health Canada; unreferenced), JECFA (1966), the EU SCF (1983) and EFSA (2009b). ADI values have been set by Health Canada (8.5 mg/kg-bw; 1986 memorandum from Toxicology Evaluation Section, Health Canada to Food Additives and Contaminants Section, Health Canada; unreferenced), JECFA (0-7.5 mg/kg-bw; JECFA 1966) and EFSA (7.5 mg/kg-bw per day; EFSA 2009b). No carcinogenicity classifications were identified.
By the oral route, Tartrazine is reduced, and the aromatic amines released are absorbed into the systemic circulation. In vitro data have shown that gut bacteria can mediate the azo cleavage of the dye (EFSA 2009b). No data were available on Tartrazine regarding potential for azo reduction on the skin.
Carcinogenicity and Genotoxicity
Numerous carcinogenicity studies have been identified for Tartrazine, many of which were previously reviewed by JECFA (1966). Although no increase in the incidence of tumours was observed in mice, rats and dogs exposed to the dye in their diet, all the studies were conducted before OECD Guidelines were established. More recent studies were identified and are briefly described below.
There was no increase in the incidence of neoplasias or time of tumour appearance or any significant differences in their primary location or histological characteristics between control and treated animals when mice (60 of each sex per group) were exposed to 0%, 0.5%, 1.5% or 5% Tartrazine (90% purity; equivalent to 0, 714, 2173 and 8103 mg/kg-bw per day for males and 0, 870, 2662 and 9735 mg/kg-bw per day for females, respectively) in their diet for 104 weeks (Borzelleca and Hallagan 1988a).
Similarly, when rats were exposed to the dye (90% purity) at levels of 0%, 0.1%, 1% or 2% (equivalent to 0, 48, 491 and 984 mg/kg-bw per day for males and 0, 58, 589 and 1225 mg/kg-bw per day for females, respectively) or at 0% or 5% (equivalent to 0 and 2641 mg/kg-bw per day for males and 0 and 3348 mg/kg-bw per day for females, respectively) in their diet during an in uterophase followed by a chronic exposure of 113-125 weeks, there was no difference in the incidence of lesions, including neoplasms, and those identified were of types commonly found in aging rats (Borzelleca and Hallagan 1988b).
In another study, rats were exposed to Tartrazine (93.4% purity) through their drinking water at concentrations of 0%, 1% or 2% (equivalent to 0, 1400 and 2800 mg/kg-bw per day) for 104 weeks followed by an 8-week recovery period (Maekawa et al. 1987). The only significant increase in tumours observed was an increase in mesotheliomas in males and endometrial stromal polyps in females at the low dose only. No positive trend was noted, however, in the occurrence of these two tumours using an age-adjusted statistical analysis, and no significant differences were observed between the control and treated groups in hyperplastic or preneoplastic changes in the mesothelium or endometrium. The authors as well as the EFSA Panel (EFSA 2009b) therefore concluded that these two tumour types were not attributable to Tartrazine administration.
No carcinogenic changes in any gastric area of the esophagus-gastroduodenal segment were observed in a study in which male rats were exposed to 0 or 7.5 mg/kg-bw per day of the dye in drinking water for 46 weeks (Moutinho et al. 2007).
Carcinogenicity studies were also identified for other routes of exposure, including dermal and subcutaneous, but no significant treatment-related increase in neoplasms was observed (Price et al. 1978; Carson 1984b; Hazleton Laboratories 1992).
In vitro mutagenicity was not observed in the Ames assay with or without metabolic activation using various protocols, including under reductive conditions, or in mouse lymphoma cells (Brown et al. 1978; Viola and Nosotti 1978; Muzzall and Cook 1979; Bonin and Baker 1980; Haveland-Smith and Combes 1980; Chung et al. 1981; Ishidate et al. 1981, 1984; Brown and Dietrich 1983; De France et al. 1986; Henschler and Wild 1986; Cameron et al. 1987; Münzner and Wever 1987; Prival et al. 1988; Izbirak et al. 1990; Pollastrini et al. 1990; Rafii et al. 1997). Mixed results were observed in in vitro chromosomal aberration assays, and results were negative overall for DNA damage and/or repair (Kada et al. 1972; Zhurkov 1975; Ishidate et al. 1978, 1981, 1984; Au and Hsu 1979; Sankaranarayanan and Murthy 1979; Haveland-Smith and Combes 1980; Kawachi et al. 1980; Patterson and Butler 1982; Kornbrust and Barfknecht 1985; Mpountoukas et al. 2010).
In vivo genotoxicity assays for Tartrazine gave mixed results. Mainly negative results were obtained when urine and fecal extracts from rats exposed to the dye by gavage were incubated with Salmonella typhimurium strains TA98 and TA100, respectively, in the presence of metabolic activation (Henschler and Wild 1985; Münzner and Wever 1987). Chromosomal aberrations were detected in bone marrow cells when male rats were exposed to Tartrazine (unknown purity) at doses of 5-50 mg/kg-bw per day in their feed for 3, 6 or 9 months (Giri et al. 1990), but not when Chinese hamsters were administered 200 mg/kg-bw of the dye by oral gavage in an acute study (Renner 1984). Sister chromatid exchange assays done on bone marrow cells were negative when Chinese hamsters were administered 50 mg/kg-bw of Tartrazine by oral gavage (Renner 1984), but positive when mice were exposed to up to 200 mg/kg-bw of the dye (purity unknown) by intraperitoneal injection (Giri et al. 1990). Tartrazine did not induce any DNA repair in rat hepatocytes at a dose of 500 mg/kg-bw by oral gavage (Kornbrust and Barfknecht 1985), but induced a dose-dependent increase in DNA damage in the colon of male mice exposed to 10, 100 or 2000 mg/kg-bw of the dye, using the comet assay (Sasaki et al. 2002). Given the lack of genotoxicity and increase in mitotic cells observed in the gut micronucleus assay in mice orally exposed to up to 1000 mg/kg-bw twice at 24-hour intervals, as well as the significant presence of Tartrazine and its main aromatic amine metabolite, sulfanilic acid, among colonic cells, the authors concluded that the increase in comet tail length observed in the comet assays represented transient DNA damage that was unable to be converted into stable genotoxic lesions and may be partly explained by local cytotoxicity of the dye (Poul et al. 2009).
The expected metabolite following cleavage of the azo bond in Tartrazine is sulfanilic acid (CAS RN 121-57-3). The available data on sulfanilic acid indicate that it is not mutagenic in vitro (Chung et al. 1981; Zeiger et al. 1988; Mansour et al. 2009).
Based on the available information from the carcinogenicity and genotoxicity studies examined, Tartrazine does not induce either benign or malignant neoplasias in long-term carcinogenicity studies; Tartrazine and its primary metabolite are not mutagenic. Consistent with the most recent evaluation by an international agency (EFSA 2009b), Tartrazine is not expected to be carcinogenic.
Other Health Effects
Several short-term, subchronic and chronic studies were reviewed by JECFA (1966) and, more recently, by EFSA (2009b). Key studies as well as more recent studies are briefly summarized below.
When rats (15 of each sex per group) were exposed to Tartrazine at a concentration of 0%, 0.03%, 0.3% or 1.5% (equivalent to 0, 15, 150 or 750 mg/kg-bw per day) in their diet for 64 weeks, no treatment-related effect was observed at the highest dose tested (Mannell et al. 1958). JECFA used the high dose in this study as a NOAEL to set its ADI (JECFA 1966).
In studies conducted according to OECD guidelines, mice and rats were exposed to Tartrazine (purity 90%) for approximately 2 years through their diet. In the mouse study, where the animals were exposed to 0%, 0.5%, 1.5% or 5% of the dye for 104 weeks, the highest dose tested (equivalent to 8103 and 9735 mg/kg-bw per day for males and females, respectively) was identified as the NOAEL (Borzelleca and Hallagan 1988a). The rat study also included an in utero phase that was followed by a chronic exposure of 113-125 weeks. The rats were exposed to the dye at a level of 0%, 0.1%, 1% or 2% or at 0% or 5%. A no-observed-effect level (NOEL) of 2% and a LOEL of 5% were identified based on a significant decrease in body weight, markedly elevated food consumption and increased incidence of two non-neoplastic lesions (mineralization of renal tissue and pancreatic arteritis) in both sexes, as well as an increase in thyroid weights in females, at the interim sacrifice. According to the study authors, based on combined data from the in utero and long-term phases of the study, these doses in food are equivalent to 984 and 2641 mg/kg-bw per day for males and 1225 or 3348 mg/kg-bw per day for females (Borzelleca and Hallagan 1988b). The Health Canada ADI was derived based on the NOAEL of 2% Tartrazine in the diet (converted to 850 mg/kg-bw per day, based on data from only the long-term phase of the study, which was later published as Borzelleca and Hallagan 1988b) (1986 memorandum from Toxicology Evaluation Section, Health Canada to Food Additives and Contaminants Section, Health Canada; unreferenced).
In a more recent study, significant increases in the number of eosinophils and lymphocytes in the gastric antrum mucosa were observed in treated animals when male rats were exposed to the dye through their drinking water at a concentration of 0 or 7.5 mg/kg-bw per day for 46 weeks (Moutinho et al. 2007). These effects, however, were not considered systemic, and observations were confined to the esophagus-gastroduodenal segment of the animals.
In a range-finding study in which rats were given Tartrazine in the drinking water for 13 weeks, liver weights were significantly decreased at 3500 mg/kg-bw per day, but not at 1750 mg/kg-bw per day (Maekawa et al. 1987).
In a subchronic oral toxicity study and two short-term oral studies, rats were exposed to one of three doses of the dye, ranging from 5 to 500 mg/kg-bw per day. Although changes in body weight, biochemical markers and histopathology were described, the pattern of effects was not consistent and does not suggest biologically significant treatment-related effects. In addition, all three studies were found to be inadequate for use in risk assessment due to poor study design and limited detail on methods, including purity of the test substance (Amin et al. 2010; Himri et al. 2011; Abd El-Wahab and Moram 2013).
No sign of treatment-related systemic toxicity was seen in mice dermally exposed to Tartrazine (purity 92%), twice a week, for 18 months at an equivalent daily dose of 0 or 8.1 mg/kg-bw (Carson 1984b; Hazleton Laboratories 1992).
With the exception of one study, no reproductive toxicity was observed in rats and mice orally exposed to the dye during gestation or up to 2 months prior to mating, with NOAEL values ranging from 608 to 3348 mg/kg-bw per day (Borzelleca and Hallagan 1988b; Collins et al. 1991, 1992; Tanaka 2006b; Tanaka et al. 2008). In one study, a LOAEL of 5541 mg/kg-bw per day was identified, based on effects on testes and sperm when male mice were given 0%, 0.1%, 1% or 2.5% (equivalent to 0, 174, 1768 and 5541 mg/kg-bw per day) of the dye (purity 87%) through drinking water for 13 weeks. However, the number of animals in the treatment and control groups (n = 6) was too low to accurately detect an effect on fertility (Mehedi et al. 2009).
No effects on developmental parameters were observed in several studies. When rats were orally exposed to Tartrazine (purity 93%) by gavage at a dose level of 0, 60, 100, 200, 400, 600 or 1000 mg/kg-bw per day or in drinking water at a level of 0%, 0.05%, 0.1%, 0.2%, 0.4% or 0.7% (equivalent to 0, 67, 132, 292, 568 and 1064 mg/kg-bw per day) throughout gestation, no dose-related effects on the number and type of implantations or on fetal viability, size, or skeletal and visceral development were observed (Collins et al. 1990, 1991, 1992).
In a two-generation study, mice (10 of each sex per group) were exposed to 0%, 0.05%, 0.15% or 0.45% (equivalent to 0, 83, 259 and 773 mg/kg-bw per day) of Tartrazine (purity 85%) in their diet from 5 weeks of age in the F0 generation to 9 weeks of age in the F1 generation (Tanaka 2006b). Several behavioural parameters were affected, including a dose-related acceleration of surface righting on PND 4, but not PND 7, in F1 males, a dose-dependent reduction in the number of exploratory movements of F1 males at 21 days relative to controls, and delayed negative geotaxis at PND 4 in F1 females. These changes, however, were seen in only one sex and were not consistent over time.
Some neurobehavioural parameters were also affected in a follow-up three-generation study in which mice were exposed to the same doses of Tartrazine in their diet from 5 weeks of age in the F0 generation to 9 weeks of age in the F2 generation. In the F1 generation, swimming direction (indicating coordinated movement) was accelerated in males and surface righting was delayed in females on PND 7; both effects were significantly dose related in a trend test. In the F2 generation, swimming direction in males and surface righting in females were accelerated on PND 7 (direction in females opposite to that seen in F1 generation), and olfactory orientation in males was accelerated on PND 14. The effects in males were significantly dose related in a trend test on both PND 7 and PND 14. In addition, exploratory activity on PND 21 was reduced in males of both the F1 and F2 generations; in both generations, the effect was significantly dose related in a trend test (Tanaka et al. 2008).
Although the Tanaka (2006b) and Tanaka et al. (2008) studies were well conducted, the EFSA Panel did not agree with the authors that any neurobehavioural development was affected, based on the absence of a dose-response relationship for many of the effects and the fact that the effects were observed at only one time point, in only one of the two studies or only in one of two generations (EFSA 2009b). The Panel also noted that the litters were not culled to a uniform size on the day of birth and that the authors did not specify in their statistical analyses whether litters or individual pups were used as the independent variable; both factors are likely to have a significant impact on the results and in the interpretation of the results. The method for testing exploratory activity did not take into consideration the possibility of habituation over time within each test period or the biphasic pattern of normal locomotor development in the first 3 weeks of life. In addition, some of the statistically significant findings from both studies are indicative of faster neurological development, some possibly related to the higher pup body weight, and should therefore not be considered adverse. On balance, Health Canada shares EFSA’s reservations concerning the neurobehavioural effects described by the authors.
There is a substantial body of literature, including clinical trials, volunteer studies and individual case reports, in which adverse responses to Tartrazine in medications and food have been observed. It is recognized that in a small proportion of the general population, dietary exposure to Tartrazine may be associated with individual intolerance (EFSA 2009b; US FDA 2013).
7.2.2.7 Forty-six Remaining Substances
Carcinogenicity and Genotoxicity
For the remaining 14 monoazo acid dyes as well as all the 24 disazo and 8 polyazo acid dyes in this assessment, no carcinogenicity or in vivo genotoxicity data were identified. Limited in vitro genotoxicity data, including Ames tests under reductive conditions, were identified for 12 substances. As shown in Table 7-11, six substances were found to be non-mutagenic in the Ames assay in the presence or absence of reductive conditions, four substances were mutagenic in one or both of the test strains (TA98 and TA100) only under reductive conditions and two were mutagenic in the presence or absence of reductive conditions (ILS 2011, 2012; BioReliance 2012). Four substances were negative for DNA damage and/or repair in the SOS/umu test (Kosaka and Nakamura 1990; Nakamura et al. 1993).
A literature search was conducted to identify empirical data on carcinogenicity and genotoxicity for the postulated aromatic amine metabolites for which a CAS RN was available. The empirical data available on specific sulfonated aromatic amine metabolites are consistent with a general lack of genotoxic effects for sulfonated aromatic amines. For unsulfonated aromatic amine metabolites, limited carcinogenicity and genotoxicity data were identified.
Following azo bond cleavage, one disazo acid dye (CAS RN 75949-73-4) may release the EU22 aromatic amine 4,4′-methylenedianiline (MDA) (CAS RN 101-77-9), which is considered to be mutagenic and carcinogenic (OECD 2002).
Two disazo acid dyes were identified that could release aniline (CAS RN 62-53-5). Aniline was previously evaluated by Health Canada (2011b). The genotoxicity of aniline in various in vitroor in vivo assays was mixed, but was generally observed only at high doses. In carcinogenicity studies, aniline induced a rare spectrum of tumours in the spleen of male Fischer 344 rats at very high doses at which massive effects on the blood and non-neoplastic splenotoxicity as a consequence of profound methemoglobinemia were also observed. Although the mode of action of potential carcinogenicity of aniline is not fully understood, the underlying mechanism of aniline-related splenic tumours supports a non-genotoxic mode of action (Health Canada 2011).
Other than these two amines, no other aromatic amine metabolites of the 52 Azo Acid Dyes had a cancer classification by a national or international agency or empirical data indicating a carcinogenic effect in experimental animals.
Additional information is provided below on the azo bond cleavage products (aromatic amines) of 13 Azo Acid Dyes to which exposure of the general population of Canada is expected, and which are lacking health effects data (5 monoazo, 5 disazo, and 3 polyazo). This information is also shown in Table 7-11.
| Azo Acid Dye (name or CAS RN) |
In vitro genotoxicity data on Azo Acid Dyes | Relevant genotoxicity data on unsulfonated aromatic amines |
|---|---|---|
| Acid Black 24 | Positive in standard and reductive Ames (ILS 2012) | CAS RN 2243-61-0 Some positive Ames (Mortelmans et al. 1986; Zeiger et al. 1992) |
| Acid Blue 113[b] | Positive in reductive Ames only (Tamaro and Banfi 1976; ETAD 1988; ILS 2011) Negative for DNA damage in bacteria (Kosaka and Nakamura 1990; Nakamura et al. 1993) |
CAS RN 2243-61-0 Some positive Ames (Mortelmans et al. 1986; Zeiger et al. 1992) |
| Acid Black 26b | Negative in standard and reductive Ames (Venturini and Tamaro 1979; Bioreliance 2012) Negative for DNA damage in bacteria (Kosaka and Nakamura 1990; Nakamura et al. 1993) |
CAS RN 2243-61-0 Some positive Ames (Mortelmans et al. 1986; Zeiger et al. 1992) |
| Acid Orange 33b | Positive in reductive Ames only (Venturini and Tamaro 1979; ILS 2012) | CAS RN 123-30-8 Clastogenic, not mutagenic (SCCP 2005; OECD 2010) |
| Acid Red 138b | Negative in standard and reductive Ames (ILS 2012) Negative for DNA damage in bacteria (Kosaka and Nakamura 1990; Reifferscheid and Heil 1996) |
CAS RN 104-42-7 No data |
| Acid Red 6b | No data | CAS RN 5856-00-8 No data |
| 29706-48-7 | Negative in standard and reductive Ames (BioReliance 2012) | N/A[c] |
| 52236-73-4 | Negative in standard and reductive Ames (ILS 2011) | N/Ac |
| 68155-63-5b | Positive in standard and reductive Ames (ILS 2012) | CAS RN 100-01-6 Probably non-genotoxic (US EPA 2009) CAS RN 96-91-3 Positive in Ames but negative in other in vitro and in vivo genotoxicity assays (Becker et al. 2009) CAS RN 16523-31-2 No data |
| Acid Orange 156 | Positive in reductive Ames only (ILS 2012) | N/Ac |
| 70210-05-8b | No data | CAS RN 73637-04-4 No data |
| 70210-06-9b | Positive in reductive Ames only (ILS 2011) Negative for DNA damage in bacteria (Kosaka and Nakamura 1990; Nakamura et al. 1993) |
CAS RN 2243-61-0 Some positive Ames (Mortelmans et al. 1986; Zeiger et al. 1992) |
| 70210-25-2b | No data | CAS RN 100-01-6 Probably non-genotoxic (US EPA 2009) CAS RN 96-91-3 Positive in Ames but negative in other in vitro and in vivo genotoxicity assays (Becker et al. 2009 CAS RN 16523-31-2 No data |
| 70210-34-3b | No data | CAS RN 16523-31-2 No data |
| 71873-51-3b | No data | All sulfonated |
| 72828-83-2 | Negative in standard and reductive Ames (BioReliance 2012) | N/Ac |
| 79234-36-9 | Negative in standard and reductive Ames (ILS 2011) | N/Ac |
| 84962-50-5b | No data | All sulfonated |
Table 7-11 Notes
Abbreviations: NA: not available; N/A: not applicable
- [a]
- Only those Azo Acid Dyes with empirical data or for which exposure of the general population is expected are shown.
- [b]
- Substances with exposure of the general population of Canada.
- [c]
- Data on unsulfonated aromatic amines are shown only for substances with exposure.
The monoazo acid dyes with CAS RNs 71873-51-3 and 84962-50-5 release only sulfonated aromatic amines, and therefore they are not likely to be mutagenic. Although no data were identified for the unsulfonated aromatic amines of Acid Red 138, the dye itself was non-mutagenic in reductive and standard Ames assays. The monoazo acid dyes Acid Red 6 and CAS RN 70210-05-8 each release one unsulfonated aromatic amine with no data, and therefore there is less confidence in the prediction of non-mutagenicity for these substances.
Four disazo acid dyes share a common unsulfonated aromatic amine, 1,4-naphthalenediamine (CAS RN 2243-61-0), which is mutagenic in bacteria. One of the dyes containing the 1,4-naphthalenediamine structure (CAS RN 70210-06-9) was mutagenic in an Ames assay only under reductive conditions, suggesting that this metabolite could drive the mutagenicity of the parent dye. However, two of the dyes (Acid Black 24 and Acid Blue 113) were Ames positive under standard and reductive conditions, and one dye (Acid Black 26) was negative in both the presence and the absence of reductive conditions. Therefore, it is not clear whether the mutagenicity of some of these dyes in Ames tests is really due to the release of 1,4-naphthalenediamine or some other pathway. Data on endpoints other than bacterial mutagenicity are not available for 1,4-naphthalenediamine.
One disazo acid dye (Acid Orange 33) could release two unsulfonated aromatic amines, p-aminophenol (CAS RN 123-30-8) and an amine with n° CAS RN. Acid Orange 33 was mutagenic in an Ames assay only under reductive conditions, which may be due to the release of one of these amines. However, p-aminophenol is generally negative in bacterial mutagenicity tests in strains TA98 and TA100, the strains tested for Acid Orange 33, and is considered clastogenic but not mutagenic (SCCP 2005; OECD 2010). It is possible that the mutagenicity observed for Acid Orange 33 under reductive conditions is due to the release of the unknown metabolite.
Three polyazo acid dyes share one unsulfonated aromatic amine metabolite (1,3-benzenediol, 4,6-diamino-,dihydrochloride; CAS RN 16523-31-2). No data are available on this metabolite. Two of the three polyazo acid dyes also share two other unsulfonated aromatic amine metabolites: p-nitroaniline (CAS RN 100-01-6) and picramic acid (CAS RN 96-91-3), both of which are not likely to be genotoxic in vivo (Becker et al. 2009; US EPA 2009). One of the polyazo acid dyes (CAS RN 68155-63-5) is positive in both standard and reductive Ames, so it is not clear whether the mutagenicity is driven by the release of a mutagenic aromatic amine metabolite or some other pathway.
Overall, although some of these 13 Azo Acid Dyes have the potential to be mutagenic in vitro, available information does not indicate that they would be mutagenic in vivo or subsequently carcinogenic.
Other Health Effects
No data on relevant endpoints were identified for any other Azo Acid Dyes for which exposure of the general population of Canada is expected.
Read-across Considerations
Due to the limited data available for these 46 substances, the OECD QSAR Toolbox (2013) was used as an application of a (Q)SAR approach to search for related substances with data on repeated-dose toxicity, including carcinogenicity, to supplement the health effects database. As this subgroup contains only substances that are non-benzidine Azo Acid Dyes, benzidine-based substances were excluded. The functional group searching used the sulfonic acid group (with proper metal salt to eliminate pigments) and the azo group. In addition to the 6 substances discussed previously in this section of the assessment, 11 related substances were identified. Nine of the 11 substances are monoazo and 2 are disazo acid dyes; all are composed of simple substituted aniline or naphthalene rings. Five of the 11 substances are sulfonated on all of the component aromatic amines; 6 are composed of both sulfonated and unsulfonated amines. The structures of the analogues are shown in Tables 2-7 and 2-8, and some of their physical and chemical properties are shown in Appendix A.
Seven of the 11 substances identified have data in the Carcinogenic Potency Database (CPDB 2011) or the “Carcinogenicity & Mutagenicity ISSCAN” database (ISSCAN 2012). Of these, five substances (CAS RNs 1936-15-8, 2519-30-4, 2783-94-0, 3567-69-9 and 4553-89-3) were identified as having negative results in oral cancer bioassays. Estimates of carcinogenic potency were identified for two substances: CAS RN 4548-53-2 (Ponceau SX) and CAS RN 3564-09-8 (Ponceau 3R) (CPDB 2011). However, an IARC review of Ponceau SX describes it as negative for carcinogenicity in rat, mouse and dog (IARC 1975d, 1987a), and the potency estimate for Ponceau 3R (LTD10, of 47 mg/kg-bw per day in rat) was based on a study that IARC considered inadequate for evaluation (IARC 1975c, 1987d). Overall, there is no strong evidence to suggest that carcinogenicity is a critical effect for any of the seven substances identified.
As described previously in this section of the assessment, carcinogenicity data were available for four monoazo acid dyes in this subgroup. The available data on three Azo Acid Dyes (Amaranth, Tartrazine and New Coccine; all monoazo acid dyes) clearly indicate that carcinogenicity and genotoxicity are not endpoints of concern for these substances, whereas Ponceau MX is a possible carcinogen with a non-genotoxic mode of action. For Orange II and Metanil Yellow, although no adequate carcinogenicity data were identified, the substances are not expected to be mutagenic in vivo.
All of the substances for which carcinogenicity data were identified (including six substances in this subgroup and seven others) share similar structures (one or more azo linkage, and one or more sulfonate group) and physical-chemical properties (soluble in water, very low log Kow and high pKas). However, there are other parameters that vary widely, most notably the presence and position of various functional groups. In general, the lack of carcinogenicity potential for the majority of the non-benzidine-based sulfonated azo dyes that have been tested in animal bioassays lends support to the idea that the substances in this subgroup would not be expected to be genotoxic carcinogens.
Oral non-cancer effect levels were identified in the OECD QSAR Toolbox (2013) for eight substances. The lowest effect level identified was for CAS RN 3734-67-6 (Acid Red 1). The LOEL of 46 mg/kg-bw per day was based on spleen effects in rats given Acid Red 1 in the diet for 2 years (Munro et al. 1996). Effect levels for the other seven substances ranged from 64 to 3700 mg/kg-bw per day, and the effects were listed as “nonspecific effects,” organ weight changes and body weight changes.
Repeated-dose toxicity data for four monoazo acid dyes in this subgroup are described in the previous sections. The lowest oral effect level is a LOEL of 10 mg/kg-bw per day, based on spleen and blood effects in a subchronic oral gavage study on Orange II, for which one of the expected aromatic amine metabolites is unsulfonated. In contrast, much lower toxicity is observed for the other three monoazo acid dyes, Amaranth, New Coccine, and Tartrazine, which all contain one or more sulfonate groups on either side of the azo bond. The critical effect levels for Amaranth and New Coccine were based on kidney effects at 250 and 325 mg/kg-bw per day, respectively, and the critical effect level for Tartrazine was much higher, 2600 mg/kg-bw per day. In dermal dosing studies, no neoplastic or non-neoplastic effects were observed in mice when Amaranth or Orange II was applied weekly or when Tartrazine was applied twice weekly, at approximately 5-8 mg/kg-bw per day.
The most sensitive effects identified for these azo acid dyes were hematological, including increased methemoglobin, evidence of hemolysis, and subsequent effects on the spleen and is expected to be due to one or more of the non-sulfonated aromatic amine metabolites released following reduction of the azo bond. This is a well-studied mechanism of toxicity for aniline and other non-sulfonated aromatic amines (Neumann 2005, 2010; IARC 2010; Health Canada 2011). For Orange II specifically, the observed hematotoxicity is assumed to be due to the release of 1-amino-2-naphthol, and not the other expected metabolite sulfanilic acid. This is considered a reasonable assumption since hematology findings of this type were not observed in toxicity studies of tartrazine (see Section 7.2.2.6 Tartrazine) where near complete in vivo metabolism to sulfanilic acid is expected to occur following oral exposure (EFSA 2009b). In addition, similar toxicity on red blood cells and related responses in the spleen are observed following oral exposure to the structurally related β-naphthol pigment lakes where release of the 1-amino-2-naphthol metabolite is also considered responsible for these effects (Environment Canada, Health Canada 2015). Based on the assumption that Orange II hematotoxicity is due to the release of the 1-amino-2-naphthol metabolite, the effect level of the amine itself would be in the range of 5 mg/kg-bw per day by gavage (based on a molecular weight of approximately half, and assuming 100% cleavage).
In comparison, the critical LOAEL for the tolerable daily intake (TDI) derivation for aniline was 7.5 mg/kg-bw per day (Health Canada 2011). One of the postulated aromatic amine metabolites of Acid Red 1 is aniline, and the observation of spleen effects is consistent with an aniline-like mode of action for toxicity. As aniline is approximately 20% of the molecular weight of Acid Red 1, the LOAEL of 46 mg/kg-bw per day for the parent dye would be comparable to approximately 8.5 mg/kg-bw per day of aniline (assuming complete cleavage). The lowest LOEL identified in the OECD QSAR Toolbox (2013) for aromatic amines was 6 mg/kg-bw per day.
Based on the available toxicity data on azo azo dyes, as well as considering the potential effects of any non-sulfonated aromatic amines released (if present), no substances in this subgroup would be expected to have an effect level lower than the LOEL of 10 mg/kg-bw per day identified for Orange II in a 90-day rat oral gavage study. It should be noted that this worst-case effect level would not be expected to directly apply to those azo acid dyes that have solubilizing group substitution on all sides of the azo bond(s), since the released sulfonated aromatic amines would be unlikely to produce the hematotoxicity typical of non-sulfonated aromatic amines.
7.2.3 Uncertainties in Human Health Effects Assessment
The information available to determine azo bond reductive cleavage for this subgroup is considered moderate to strong. The reductive cleavage potential for many substances and structurally related groups is based on several types of evidence. All of the Azo Acid Dyes evaluated in this assessment are expected to release aromatic amines upon reductive cleavage. Confidence is highest in the monoazo acid dyes, as in vivo data, including metabolite identification, were available for several substances; the generation of individual aromatic amines from disazo and polyazo acid dyes is less certain, as only in vitro data were available for some substances, and the relative cleavage rates of multiple azo bonds are unknown. In general, confidence in the read-across approach for substances without empirical data is high, as they have been grouped together based on their similar uses, properties and structures.
Health effects data are available only for a limited number of substances. For the majority of the substances in this subgroup, read-across from related substances is used to characterize the potential health effects. Confidence in the read-across to individual substances varies depending on the differences between properties, including molecular size, and the presence and position of functional groups. However, in the absence of other data, critical effect levels for substances in the Azo Acid Dyes subgroup are considered to be within the range identified for other sulfonated azo dyes with data on non-cancer endpoints.
Dye purity was often low or not reported in toxicology studies. Impurities in the test substance, including aromatic amines, may contribrute to the health effects noted in some studies. There may be significant variation in the amount and types of impurities present in the toxicology studies, which could lead to variable responses.
7.3 Risk Characterization
Environmental media are not considered to be a significant source of exposure to Azo Acid Dyes for the general population in Canada. Exposure to 17 Azo Acid Dyes for the general population of Canada via food or use of products available to consumers was identified. Food is considered the primary source of exposure to Tartrazine and Amaranth. Non-dietary exposure via cosmetics and non-prescription drugs at the cosmetic-drug interface was also identified for Tartrazine, and via cosmetic products for Amaranth, New Coccine and Orange II. For Acid Black 24, Acid Black 26, Acid Blue 113, CAS RN 70210-25-2, Acid Orange 33, Acid Red 6, Acid Red 138, CAS RN 68155-63-5, CAS RN 70210-05-8, CAS RN 70210-06-9, CAS RN 70210-34-3, CAS RN 71873-51-3 and CAS RN 84962-50-5, the primary source of exposure is from the use of textiles and/or leather products.
Carcinogenicity is not considered to be the key endpoint of concern for Amaranth, Tartrazine and New Coccine, based on the available empirical data on health effects. In addition, although no adequate carcinogenicity data were identified for Orange II, it is not expected to be genotoxic in vivo. For these four Azo Acid Dyes, the toxicology database is sufficient to derive individual margins of exposure (MOEs) for non-cancer endpoints.
7.3.1 Amaranth (CAS RN 915-67-3)
Exposure of the general population in Canada to Amaranth occurs predominantly through food intake and use of cosmetics. The mean usual intake of the general population from food was estimated to range from 0. 01 mg/kg-bw per day (seniors) to 0. 21 mg/kg-bw per day (toddlers and children), while 90th percentile estimates ranged from 0.04 mg/kg-bw per day (seniors) to 0.54 mg/kg-bw per day (toddlers). Estimates of risk associated with upper-bounding cosmetic scenarios (i.e., toothpaste, lip balm, lipstick and body moisturizer) for Amaranth are presented in Table 7-12.
The critical effect for Amaranth was determined to be renal calcification and hyperplasia. The LOAEL in a chronic dietary study in rats was 250 mg/kg-bw per day; the NOAEL was 50 mg/kg-bw per day (Butler and Conning 1983; Clode et al. 1987). JECFA and Health Canada each derived an ADI of 0.5 mg/kg bw per day based on this NOAEL and an uncertainty factor of 100 (JECFA 1984; 2014 memorandum from Chemical Health Hazard Assessment Division, Health Canada to Bureau of Chemical Safety, Health Canada; unreferenced).
In a subchronic dietary study in rats designed to further investigate the kidney effects, a NOAEL of 80 mg/kg-bw per day and a LOAEL of 1250 mg/kg-bw per day were identified (Clode et al. 1987). The authors noted that renal calcification was present in rats whose kidneys were compromised by senile change and that hyperplasia was focal and associated with mineralization, suggesting an irritant effect. Female rats were also found to be more susceptible than males due to estrogen-induced mineralization (Clode et al. 1987).
In a developmental toxicity study in rats, a LOAEL of 30 mg/kg-bw per day and a NOAEL of 15 mg/kg-bw per day were identified based on a significant increase in resorptions (Collins and McLaughlin 1972). However, this effect was not observed in several other developmental toxicity studies in rats, in which NOAELs ranged from 150 to 2000 mg/kg-bw per day (generally the highest doses tested); no other effects on reproductive or developmental toxicity were observed in a number of studies, including a two-generation and a three-generation study in rats (FDRL 1972; Burnett et al. 1974; Keplinger et al. 1974; Khera et al. 1974; Collins et al. 1975a, b, 1976a, b; Holson et al. 1976; Keplinger et al. 1976; Willes et al. 1980; Clode et al. 1987). NOAELs for developmental toxicity based on the highest dose tested were also identified for mouse (100 mg/kg-bw per day), rabbit (15 mg/kg-bw per day) and dog (90 mg/kg-bw per day) (Keplinger et al. 1974; Larsson 1975; Mastalski et al. 1975). Considering all of the evidence from the toxicological database including the absence of adverse effects in all other developmental and reproductive studies in rats, at doses much higher than the LOAEL identified by Collins and McLaughlin (1972), and the absence of adverse effects in developmental studies in mice, rabbits, and dogs, the NOAEL in the Collins and McLaughlin (1972) study was not used as a critical effect level for risk characterization.
The 90th percentile dietary exposure estimate from food for toddlers (6 months to 4 years) is marginally higher (0.54 mg/kg-bw per day) than the ADI (0.5 mg/kg-bw per day). This estimated intake in toddlers, however, does not raise health concerns given the conservative nature of the exposure estimate, the very minor exceedance of the ADI, and the relatively short period in a human lifetime during which this conservatively identified exceedance could occur. In addition, the toxicological endpoint selected (renal calcification) occurred only in older rats following life time exposure to amaranth and is not a condition known to develop in toddlers.
Comparison of estimated mean usual intakes to Amaranth from food , modelled based on currently identified maximum levels of use, with the critical effect level (chronic NOAEL of 50 mg/kg-bw per day) results in MOEs ranging from 240 (toddlers and children) to 5000 (seniors). This range of MOEs is considered adequate to address uncertainties in the health effectsand exposure databases.
Comparison of upper-bounding estimates for oral and dermal exposure to Amaranth from use of cosmetics with the critical effect level results in MOEs ranging from greater than or equal to 740 to greater than or equal 50 000, which are considered adequate to address uncertainties in the health effects and exposure databases.
| Exposure duration and route | Exposure Scenario | Daily exposure (mg/kg-bw per day) |
Critical effect levels (mg/kg-bw per day) | MOEs |
|---|---|---|---|---|
| Daily oral | Toothpaste (adults) | less than or equal to 0.0023 | Chronic oral feeding NOAEL = 50 |
greater than or equal to 20 000 |
| Daily oral | Lipstick (adults) | less than or equal to 0.01 | Chronic oral feeding NOAEL = 50 |
greater than or equal to 5000 |
| Daily oral | Lip balm (toddlers) | less than or equal to 0.001 | Chronic oral feeding NOAEL = 50 |
greater than or equal to 50 000 |
| Daily dermal | Body moisturizer (adults) | less than or equal to 0.068 | Chronic oral feeding NOAEL = 50 | greater than or equal to 740 |
Abbreviations: MOE: margin of exposure; NOAEL: no-observed-adverse-effect level; NOEL: no-observed-effect level
7.3.2 New Coccine (CAS RN 2611-82-7)
Exposure of the general population in Canada to New Coccine occurs predominantly through use of cosmetics. Estimates of risk associated with upper-bounding cosmetic product exposure scenarios (i.e., body moisturizer and leave-in hair conditioner) for New Coccine are presented in Table 7-13.
The critical effect level for New Coccine is a NOAEL of 65 mg/kg-bw per day (0.05% in the diet), based on glomerulonephrosis and mild anemia at the LOAEL of 325 mg/kg-bw per day (0.25% in the diet) in an 82-week dietary study in mice (Mason et al. 1974). EFSA (2009a) selected 70 mg/kg-bw per day (based on 0.05% in the diet) as a NOAEL for its ADI, whereas JECFA (1983, 2011) used a dose level of 375 mg/kg-bw per day (based on 0.25% in the diet) as a NOAEL in its derivation of the ADI.
Comparison of upper-bounding estimates of dermal exposure to New Coccine from use of body moisturizer and leave-in hair conditioner with the critical effect level (chronic oral NOAEL of 65 mg/kg-bw per day) results in MOEs of greater than or equal to 100-3250, which are considered adequate to address uncertainties in the health effects and exposure databases. Although dermal acute exposure scenarios were identified for New Coccine (e.g., hair dye and hair dye spray for children), the available information does not indicate that New Coccine demonstrates high acute toxicity. Consequently, the risk for the general population is considered low.
| Exposure duration and route | Cosmetic products | Daily exposure (mg/kg-bw per day) | Critical effect levels (mg/kg-bw per day) | MOEs |
|---|---|---|---|---|
| Daily dermal | Body moisturizer (adults) | less than or equal to 0.68 | Chronic oral feeding NOAEL = 65 |
greater than or equal to 100 |
| Daily dermal | Leave-in hair conditioner (adults) | 0.02-0.61 | Chronic oral feeding NOAEL = 65 |
110-3250 |
7.3.3 Orange II (CAS RN 633-96-5)
Exposure of the general population in Canada to Orange II occurs predominantly through use of cosmetic products. Estimates of risk associated with upper-bounding cosmetic product exposure scenarios (i.e., lipstick, body moisturizer and hair shampoo for infants) for Orange II are presented in Table 7-14.
In oral gavage and dietary repeated-dose toxicity studies on Orange II, spleen and blood effects, characteristic of aromatic amine-induced anemia, were observed consistently. No NOAEL was identified for any study in which exposure was by the dietary route, as effects were observed at the lowest dose tested in each study. LO(A)ELs ranged from 50 to 250 mg/kg-bw per day in feeding studies of 60-300 days’ duration in rats, based on decreased red blood cells and hemoglobin, Heinz body formation and increased spleen weights, as well as testicular seminiferous tubule histopathology (Singh and Khanna 1979; Singh et al. 1987).
In the short-term and subchronic gavage studies identified, similar effects in blood and spleen were observed at lower doses, likely due to the higher peak concentrations obtained with a bolus dose compared with continuous exposure in food. LOELs of 10-40 mg/kg-bw per day were identified in 90-day, 14-day and developmental toxicity gavage studies, based on increased severity and incidence of extramedullary hematopoiesis in the spleen, increased methemoglobin and increased spleen weights (Rosner 1999a, b; Becker and Biedermann 2000; Hamann et al. 2000).
Comparison of the upper-bounding estimate of oral exposure to Orange II from use of lipstick with a range of critical effect levels--from 10-40 mg/kg-bw per day (subchronic oral LOEL via gavage) to 50-250 mg/kg-bw per day (oral LOAELs from 60- to 300-day feeding studies)--results in MOEs of greater than 33 000. This range of MOEs is considered adequate to address uncertainties in the exposure and health effects databases.
No effects were observed in a study in which mice received weekly uncovered skin applications of dye of about 33 mg/kg-bw for 18 months (approximately 5 mg/kg-bw per day) (Carson 1984a). Comparison of the upper-bounding estimate of dermal exposure to Orange II from use of baby shampoo for infants with the critical effect level (chronic dermal NOEL of 5 mg/kg-bw per day) results in an MOE of greater than 50 000. Comparison of the upper-bounding estimate of dermal exposure to Orange II from use of body moisturizer by adults with the same critical effect level results in an MOE of greater than or equal to 74. Both MOEs are considered adequate to address uncertainties in the exposure and health effects databases, taking into account that based on the chronic oral NOAEL (50 mg/kg-bw per day) and assuming 100% dermal absorption would result in MOEs of greater than 500 000 and greater than 700 for baby shampoo for infants and body moisturizer for adults, respectively.
Dermal acute exposure to Orange II via use of hair dye has also been identified. However, the available information does not indicate that Orange II demonstrates high acute toxicity. Consequently, the risk for the general population is considered low.
| Exposure duration and route | Exposure Scenario | Daily exposure (mg/kg-bw per day) | Critical effect levels (mg/kg-bw per day) | MOEs |
|---|---|---|---|---|
| Daily oral | Lipstick (adults) | less than or equal to 0.0003 | Subchronic and chronic oral (gavage and feeding) LO(A)ELs = 10-250 | greater than or equal to 33 300 - 830 000 |
| Daily dermal | Body moisturizer (adults) | less than or equal to 0.068 | Chronic dermal NOEL = 5 | greater than or equal to 74 |
| Daily dermal | Hair shampoo (infants) | less than or equal to 9.3 × 10-5 | Chronic dermal NOEL = 5 | greater than or equal to 53 800 |
7.3.4 Tartrazine (CAS RN 1934-21-0)
Exposure of the general population in Canada to Tartrazine occurs predominantly through food intake and use of cosmetics and certain non-prescription drugs at the cosmetic-drug interface. The mean dietary exposure of the general population to Tartrazine was estimated to range from 0.2 mg/kg-bw per day (seniors) to 1.0 mg/kg-bw per day (children). Estimates of risk associated with upper-bounding exposure scenarios for cosmetics and non-prescription drugs at the cosmetic-drug interface for Tartrazine are presented in Table 7-15.
The critical effect level for Tartrazine is a chronic oral LOAEL of 2600 mg/kg-bw per day in a rat dietary study, based on decreased body weight, non-neoplastic lesions in kidney and pancreas and increased thyroid weight. The NOAEL was 984 mg/kg-bw per day (2% in the diet) (Borzelleca and Hallegan 1988b). The study authors used combined data from the entire study to convert the dietary doses. The Health Canada ADI of 8.5 mg/kg-bw was derived from a NOAEL of 850 mg/kg-bw per day (2% in the diet, converted using only the long-term exposure portion of the study later published by Borzelleca and Hallegan 1988b) and an uncertainty factor of 100 (1986 memorandum from Toxicology Evaluation Section, Health Canada to Food Additives and Contaminants Section, Health Canada; unreferenced). The JECFA ADI of 0-7.5 mg/kg-bw was established based on a lack of effects at the highest dose tested (750 mg/kg-bw per day) in an earlier 64-week study in rats and using an uncertainty factor of 100 (JECFA 1966).
Comparison of estimated mean dietary exposures to Tartrazine from food intake with the critical effect level (chronic NOAEL of 984 mg/kg-bw per day) results in MOEs ranging from 980 (children) to 4920 (infants). This range of MOEs is considered adequate to address uncertainties in the health effects and exposure databases. Exposures are also below the ADI of 8.5 mg/kg-bw established by Health Canada and the upper bound of the ADI of 0-7.5 mg/kg-bw established by JECFA, respectively.
Comparison of upper-bounding estimates of oral exposure to Tartrazine via use of lip balm, lipstick or toothpaste with the critical effect level (chronic NOAEL of 984 mg/kg-bw per day) results in MOEs of greater than 8000, which are also considered adequate to address uncertainties in the health effects and exposure databases.
No effects were observed in the only available dermal study in which mice received twice weekly uncovered skin applications of about 8.1 mg/kg-bw per day for 18 months (Carson 1984b). As this dose level is more than 100-fold lower than the oral no-effect level, the oral NOAEL of 984 mg/kg-bw per day was used as the critical effect level. Comparison of upper-bounding estimates for dermal exposure to Tartrazine from use of facial makeup, baby cream or body moisturizer with this critical effect level results in MOEs of greater than or equal to 1000, which are considered adequate to address uncertainties in the health effects and exposure databases.
Dermal acute exposure to Tartrazine via use of other products (e.g., face paint and genitalia product) has also been identified. However, the available information does not indicate that Tartrazine demonstrates high acute toxicity. Consequently, the risk for the general population is considered low.
| Exposure duration and route | Exposure Scenario | Daily exposure (mg/kg-bw per day) | Critical effect levels (mg/kg-bw per day) | MOEs |
|---|---|---|---|---|
| Daily oral | Lip balm (toddlers) | less than or equal to 0.11 | Oral feeding chronic NOAEL = 984 |
greater than or equal to 8950 |
| Daily oral | Lipstick (adults) | less than or equal to 0.10 | Oral feeding chronic NOAEL = 984 |
greater than or equal to 9840 |
| Daily oral | Toothpaste (toddlers) | less than or equal to 0.068 | Oral feeding chronic NOAEL = 984 |
greater than or equal to 14 470 |
| Daily dermal | Facial makeup (adults) | less than or equal to 0.94 | Oral feeding chronic NOAEL = 984 |
greater than or equal to 1050 |
| Daily dermal | Body moisturizer (adults) | less than or equal to 0.68 | Oral feeding chronic NOAEL = 984 |
greater than or equal to 1450 |
| Daily dermal | Baby cream (infants) | less than or equal to 0.32 | Oral feeding chronic NOAEL = 984 |
greater than or equal to 3080 |
7.3.5 Thirteen Azo Acid Dyes (Exposure via Textile and Leather Products)
Exposure of the general population of Canada to the following 13 Azo Acid Dyes is expected via use of textile and/or leather products: Acid Red 138, CAS RN 71873-51-3, CAS RN 84962-50-5, Acid Orange 33, Acid Red 6 and Acid Black 26 as textile dyes; CAS RN 68155-63-5, CAS RN 70210-34-3 and CAS RN 70210-25-2 as leather dyes; and CAS RN 70210-05-8, Acid Blue 113, Acid Black 24 and CAS RN 70210-06-9. Upper-bounding daily exposures of the general population to dyes in textile and leather products were estimated via the dermal route from direct and prolonged skin contact with such products. Upper-bounding oral exposures due to mouthing of textiles by infants were also estimated. Estimates of risk associated with the upper-bounding exposure scenarios for these 13 Azo Acid Dyes are presented in Table 26.
Health effects data on the other 13 Azo Acid Dyes with exposure are limited; however, based on available data on related substances, carcinogenicity and genotoxicity are not expected to be endpoints of concern.
The lowest critical oral non-cancer effect level was identified for Orange II (subchronic oral LOEL of 10 mg/kg-bw per day) among all Azo Acid Dyes in this subgroup and relevant analogues. Effect levels identified for oral repeated-dose toxicity data on other Azo Acid Dyes ranged from 46 to 3700 mg/kg-bw per day. Comparison of the upper-bounding estimate of exposure to Azo Acid Dyes via mouthing of textile objects by infants with the range of oral critical effect levels (subchronic/chronic LO(A)ELs of 10-3700 mg/kg-bw per day) results in a MOE of greater than 37 000, which is considered adequate to address uncertainties in the exposure and health effects databases.
By the dermal route, no cancer or non-cancer effects were observed in mice at approximately 5-8 mg/kg-bw per day when Amaranth or Orange II was applied weekly or Tartrazine was applied twice per week. Comparison of upper-bounding estimates of dermal exposure to 10 Azo Acid Dyes (Acid Red 138, CAS RN 71873-51-3, CAS RN 84962-50-5, Acid Orange 33, Acid Red 6, Acid Black 26, CAS RN 70210-05-8, Acid Blue 113, Acid Black 24 and CAS RN 70210-06-9) via textile clothing with this range of critical effect levels results in a range of MOEs from 125 to 310, which are considered adequate to address uncertainties in the exposure and health effects databases. For the following seven substances identified in leather products, CAS RN 68155-63-5, CAS RN 70210-34-3, CAS RN 70210-25-2, CAS RN 70210-05-8, Acid Blue 113, Acid Black 24 and CAS RN 70210-06-9, dermal acute exposure to these substances is not considered to result in risk for the general population of Canada, as the available information does not indicate that Azo Acid Dyes demonstrate high acute toxicity.
| Exposure duration and route | Consumer products | Daily exposure (mg/kg-bw per day) | Critical effect levels (mg/kg-bw per day) | MOEs |
|---|---|---|---|---|
| Daily oral | Textile objects (infants) | 2.7×10-5 | Subchronic/chronic oral (gavage and feeding) LO(A)ELs = 10-3700 | greater than or equal to 370 000-1.3x108 |
| Daily dermal | Textiles (personal apparel: adult) | 0.0026 | Chronic dermal NOELs = 5-8 | 1900-3100 |
| Daily dermal | Textiles (baby sleeper: infant) | 0.0040 | Chronic dermal NOELs = 5-8 | 1250-2000 |
7.3.6 The Remaining 35 Azo Acid Dyes
No exposure of the general population is expected for the remaining 35 Azo Acid Dyes; therefore, the risk to human health is not expected.
7.3.7 Uncertainties in Characterization of Risk to Human Health
A read-across approach was used to infer the azo cleavage of many substances in this subgroup. Although all of the available data suggest that Azo Acid Dyes have the potential to undergo reductive cleavage, there is uncertainty as to the rate and degree of cleavage that would occur in vivo for some substances.
Read-across was used for 13 Azo Acid Dyes with limited health effects data. Data on multiple related substances as well as component aromatic amines were considered, and the use of the lowest effect levels as a point of departure for the Azo Acid Dyes lacking data is considered a conservative approach.
As no adequate dermal toxicity studies were identified, oral toxicity data were used to derive MOEs for dermal exposures in some cases. By the oral route, Azo Acid Dyes are reduced by gut bacteria, and the aromatic amines released are absorbed into the systemic circulation. Reductive cleavage may also occur on the skin. This has been demonstrated in vitro by some species of skin bacteria for Orange II, Acid Red 138 and Acid Orange 33. The aromatic amine metabolites may be better absorbed than the parent dyes by the oral and dermal routes (Environment Canada and Health Canada 2013). In the absence of empirical data, the toxicity of the dyes due to the release and absorption of the corresponding aromatic amines is assumed to be of similar potency by the oral and dermal routes.
Uncertainty is recognized in impurities in the dyes tested in toxicological studies, which may be partly responsible for some observed health effects. In addition, commercial dyes to which Canadians are exposed may contain different amounts and types of impurities from those studies.
7.3.8 Azo Acid Dyes with Effects of Concern
Overall, human health risk from the substances in this assessment is low based on the current levels of exposure. However, as indicated above, some of the Azo Acid Dyes in this assessment have health effects of concern based on potential carcinogenicity. A list of these substances is shown in Appendix E.
8. Conclusion
Considering all available lines of evidence presented in this Screening Assessment, there is low risk of harm to organisms and the broader integrity of the environment from Azo Acid Dyes. It is concluded that the 52 Azo Acid Dyes do not meet the criteria under paragraphs 64(a) or 64(b) of CEPA, as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Based on the information presented in this Screening Assessment, it is concluded that the 52 Azo Acid Dyes evaluated in this assessment for human health do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is concluded that the 52 Azo Acid Dyes evaluated in this assessment do not meet any of the criteria set out in section 64 of CEPA.
References
Abd El-Wahab HMF, Moram GS. 2013. Toxic effects of some synthetic food colorants and/or flavor additives on male rats. Toxicol Ind Health 29(2):224-232.
Acros Organics. 2008a Material Safety Data Sheet: Metanil Yellow (587-98-4) [Internet]. Fair Lawn (NJ): MSDSonline. [cited 2013 Jul]. [restricted access].
Acros Organics. 2008b Material Safety Data Sheet: Xylidine Ponceau 2R (3761-53-3) [Internet]. Fair Lawn (NJ): MSDSonline. [cited 2013 Jul]. [restricted access].
Acros Organics. 2009. Material Safety Data Sheet: Orange(II) (633-96-5) [Internet]. Fair Lawn (NJ): MSDSonline. [cited 2013 Jul]. [restricted access].
Agarwal K, Mukherjee A, Sharma A. 1993. In vivocytogenetic studies on male mice exposed to Ponceau 4R and beta-carotene. Cytobios 74(296):23-28.
Allmark MG, Mannell WA, Grice HC. 1957. Chronic toxicity studies on food colours. III. Observations on the toxicity of malachite green, new coccine and nigrosine in rats. J Pharm Pharmacol 9(9):622-628.
Amin KA, Abdel Hameid H II, Abd Elsttar AH. 2010. Effect of food azo dyes tartrazine and carmoisine on biochemical parameters related to renal, hepatic function and oxidative stress biomarkers in young male rats. Food Chem Toxicol 48(10):2994-2999.
Andrianova MM. 1970. Carcinogenous properties of red food pigments--amaranth, SX purple and 4R purple. Vop Pitan 29:61-65. [cited in IARC 1975b; CPDB 2011].
Anliker R, Clarke EA, Moser P. 1981. Use of the partition coefficient as an indicator of bioaccumulation tendency of dyestuffs in fish. Chemosphere 10(3):263-274.
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2010. Version 1.92a. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Arnold DW, Kennedy GL Jr, Keplinger ML, Calandra JC. 1976. Failure of FD & C Red No. 2 to produce dominant lethal effects in the mouse. Food Cosmet Toxicol 14(3):163-165.
Ashraf MA, Hussain M, Mahmood K, Wajid A, Yusof M, Alias Y, Yusoff I. 2013. Removal of acid yellow-17 dye from aqueous solution using eco-friendly biosorbent. Desalin Water Treat 51:4530-4545.
ASTreat Model [sewage treatment plant removal model]. 2006. Version 1.0. Cincinnati (US): Procter & Gamble Company. [cited July 2013]. Available from Procter & Gamble Company, P.O. Box 538707, Cincinnati, OH 45253-8707, U.S.
Au W, Hsu TC. 1979. Studies on the clastogenic effects of biologic stains and dyes. Environ Mutagen 1(1):27-35.
Auletta AE, Kuzava JM, Parmar AS. 1977. Lack of mutagenic activity of a series of food dyes for Salmonella typhimurium. Mutat Res 56(2):203-206.
Baigusheva MM. 1968. Carcinogenic properties of the amaranth paste. Vop Pitan 27:46-50. [cited in IARC 1975b].
Baker CH, Johnston MR, Barber WW. 1974. Cherry plum pigment as a natural red food colorant: sensory evaluation and mutagenicity testing. Food Prod Dev 8(4):65-70.
Baughman GL, Perenich TA. 1988. Investigating the fate of dyes in the environment. Am Dyest Rep 7(2):19-20, 22, 47-48. [cited in Øllgaard et al. 1998].
Baughman GL, Weber EJ. 1994. Transformation of dyes and related compounds in anoxic sediment: kinetics and products. Environ Sci Technol 28(2):267-276.
Becker H, Biedermann K. 2000. D&C Orange 4 (C.I.15510). Study for effects on embryo-fetal development in the rat. Itingen (CH): RCC Ltd. RCC Report No.: 733961.
Becker LC, Bergfeld WF, Belsito DV, Klaassen CD, Marks JG Jr, Shank RC, Slaga TJ, Snyder PW, Alan Andersen F. 2009. Amended safety assessment of sodium picramate and picramic acid. Int J Toxicol 28(6 Suppl 2):205S-216S.
[BfR] Federal Institute for Risk Assessment. 2007. Introduction to the problems surrounding garment textiles. BfR Information No. 018/2007, 1 June 2007. Available upon request.
BioReliance. 2012. Bacterial reverse mutation assay of selected azo dyes. Unpublished report prepared for Health Canada. Rockville (MD): BioReliance. Study Report No.: AD46RB-RL.502.BTL.
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2010. Version 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Bonin AM, Baker RU. 1980. Mutagenicity testing of some approved food additives with the Salmonella/microsome assay. Food Technol Aust 32:608-611.
Borzelleca JF, Hallagan JB. 1988a. A chronic toxicity/carcinogenicity study of FD & C yellow no. 5 (tartrazine) in mice. Food Chem Toxicol 26(3):189-194.
Borzelleca JF, Hallagan JB. 1988b. Chronic toxicity/carcinogenicity studies of FD & C yellow no. 5 (tartrazine) in rats. Food Chem Toxicol 26(3):179-187.
Bragger JL, Lloyd AW, Soozandehfar SH, Bloomfield SF, Marriott C, Martin GP. 1997. Investigations into the azo reducing activity of a common colonic microorganism. Int J Pharm 157(1):61-71.
Brantom PG, Stevenson BI, Wright MG. 1987a. Long-term toxicity study of Ponceau 4R in rats using animals exposed in utero. Food Chem Toxicol 25(12):955-962.
Brantom PG, Stevenson BI, Ingram AJ. 1987b. A three-generation reproduction study of Ponceau 4R in the rat. Food Chem Toxicol 25:963-968.
[BRI] Biopharmaceutical Research Inc. 2012. Testing the metabolism potential of selected aromatic azo and benzidine dyes in vitro in two representative human skin bacterial cultures under aerobic conditions. Unpublished report prepared for Health Canada. Vancouver (BC): BRI. Study Report No.: RPT-HEA-2011-002.
[BRI] Biopharmaceutical Research Inc. 2013a. Determination of the metabolic reduction potential of selected aromatic azo- and benzidine-based substances: in vitro anaerobic fecal bacterial cultures of the human gastrointestinal tract. Unpublished report prepared for Health Canada. Vancouver (BC): BRI. Study Report Nos.: HEA-2012-001; HEA-2012-002.
[BRI] Biopharmaceutical Research Inc. 2013b. Identification of azo reductive cleavage products in metabolic reaction of azo dyes by Staphylococcus epidermidis and Micrococcus luteus under aerobic condition. Unpublished report prepared for Health Canada. Vancouver (BC): BRI. Study Report No.: RPT-HEA-2013-001.
Brown D, Anliker R. 1988. Dyestuffs and the environment--A risk assessment. In: Richardson M, editor. Risk assessment of chemicals in the environment. London (GB): The Royal Society of Chemistry; pp. 398-413. [cited in Øllgaard et al. 1998].
Brown D, Hamburger B. 1987. The degradation of dyestuffs: Part III--Investigations of their ultimate degradability. Chemosphere 16(7):1539-1553.
Brown D, Laboureur P. 1983. The degradation of dyestuffs: Part I--Primary biodegradation under anaerobic conditions. Chemosphere 12(3):397-404.
Brown JP, Dietrich PS. 1983. Mutagenicity of selected sulfonated azodyes in the Salmonella/microsome assay: use of aerobic and anaerobic activation procedures. Mutat Res 116(3-4):305-315.
Brown JP, Roehm GW, Brown RJ. 1978. Mutagenicity testing of certified food colors and related azo, xanthene and triphenylmethane dyes with the Salmonella/microsome system. Mutat Res 56(3):249-272.
Burnett CM, Agersborg HPK, Borzelleca JF, Eagle E, Ebert AG, Pierce EC, Kirschman JC, Scala RA. 1974. Teratogenic studies with certified colors in rats. Toxicol Appl Pharmacol 29:121-128. [cited in EFSA 2010].
Buser AM, MacLeod M, Scheringer M, Mackay D, Bonnell M, Russell MH, DePinto JV, Hungerbühler K. 2012. Good modeling practice guidelines for applying multimedia models in chemical assessments. Integr Environ Assess Manag 8(4):703-708.
Butler WH, Conning DM. 1983. Further investigation of the pathology of tissues from rats treated with Amaranth. Unpublished report provided by Sensient Food Colors, UK. BIBRA Report No.: 452/1/82. [cited in EFSA 2010].
Cai Y, Pailthorpe MT, David CK. 1999. A new method for improving the dyeability of cotton with reactive dyes. Text Res J 69:440-446.
Cameo Chemicals [database on the Internet]. 2013. Version 2.4.1. National Oceanic and Atmospheric Administration. Available from: http://cameochemicals.noaa.gov/chemical/20944
Cameron TP, Hughes TJ, Kirby PE, Fung VA, Dunkel VC. 1987. Mutagenic activity of 27 dyes and related chemicals in the Salmonella/microsome and mouse lymphoma TK+/- assays. Mutat Res 189(3):223-261.
Canada. [1978]. Food and Drug Regulations, C.R.C., c. 870. Available from: www.canlii.org/en/ca/laws/regu/crc-c-870/latest/crc-c-870.html
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33. Canada Gazette, Part III, vol. 22, no. 3. Available from: http://publications.gc.ca/gazette/archives/p3/1999/g3-02203.pdf
Canada. 2000. Canadian Environmental Protection Act, 1999: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 29 March 2000, SOR/2000-107. Available from: http://publications.gc.ca/gazette/archives/p2/2000/2000-03-29/pdf/g2-13407.pdf
Canada, Dept. of the Environment. 2011. Canadian Environmental Protection Act, 1999: Notice with respect to certain aromatic amines and aromatic azo- and benzidine-based substances. Canada Gazette, Part I, vol. 145, no. 51, Supplement to the Canada Gazette. Available from: www.gazette.gc.ca/rp-pr/p1/2011/index-eng.html
Carson S. 1984a. Skin painting studies in mice on 11 FD&C and D&C colors: FD&C Green No. 3, Red No. 2, Red No. 4, Yellow No. 6, and external D&C No. 7, D&C Orange No. 4, Violet No. 2, Red No. 17, Red No. 34, and Yellow No. 7. J Toxicol Cutan Ocul Toxicol 3(3):309-331.
Carson S. 1984b. Skin painting studies in mice with 14 FD&C and D&C colors: FD&C Blue No. 1, Red No. 3, and Yellow No. 5, D&C Red No.7, Red No. 9, Red No. 10, Red No. 19, Red No. 21, Red No. 27, Red No. 31, Red No. 36, Orange No. 5, Orange No. 10, and Orange No. 17. J Toxicol Cutan Ocul Toxicol 3(4):357-370.
CATALOGIC [Computer Model]. 2012. Version 5.11.6. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. Available from: www.oasis-lmc.org/?section=software&swid=1
[CCRIS] Chemical Carcinogenesis Research Information System [database on the Internet]. 2012. Bethesda (MD): National Library of Medicine (US). [cited 2013 May]. Available from: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?CCRIS
[CFIA] Canadian Food Inspection Agency. 2010. Food Safety Action Plan report. 2009-2010 targeted surveys chemistry. Food colours used in the production of manufactured foods. Ottawa (ON): CFIA. Report No.: TS-CHEM-09/10-05.
[CFIA] Canadian Food Inspection Agency. 2011. Food Safety Action Plan report. 2010-2011 targeted surveys chemistry. Food colours used in the production of manufactured foods. Ottawa (ON): CFIA. Report No.: TS-CHEM-10/11.
Charles River Laboratories. 2013. The in vitropercutaneous absorption of radiolabelled Acid Orange 7(C015) in two formulations under oxidative conditions and two formulations under non-oxidative conditions through human skin. Edinburgh (GB): Charles River Test Facility. Study No.: 793145. Report No.: 33937. Sponsored by The Proctor & Gamble Company.
ChemicalBook [database on the Internet]. 2008. Acid Orange 10 (1936-15-8) basic information. [cited 2013 Jul]. Available from: http://www.chemicalbook.com/ProductChemicalPropertiesCB5777321_EN.htm
ChemicalLand21 [database on the Internet]. 2010a. C.I. Acid Red 1. [cited 2012]. Available from: http://chemicalland21.com/specialtychem/finechem/RED%202G.htm
ChemicalLand21 [database on the Internet]. 2010b. Carmoisine/C.I. Food Red 3. [cited 2012]. Available from: http://www.chemicalland21.com/lifescience/foco/CARMOISINE.htm
Chen H, Wang RF, Cerniglia CE. 2004. Molecular cloning, overexpression, purification, and characterization of an aerobic FMN-dependent azoreductase from Enterococcus faecalis. Protein Expres Purif 34:302-310.
[CHRIP] Chemical Risk Information Platform [database on the Internet]. ©2008. Tokyo (JP): National Institute of Technology and Evaluation, Chemical Management Centre. [cited 2011 Jan]. Available from: www.safe.nite.go.jp/english/db.html
Chung KT, Fulk GE, Egan M. 1978. Reduction of azo dyes by intestinal anaerobes. Appl Environ Microbiol 35(3):558-562.
Chung KT, Fulk GE, Andrews AW. 1981. Mutagenicity testing of some commonly used dyes. Appl Environ Microbiol 42(4):641-648.
[CII] Colour Index International [database on the Internet]. 2011. 4th ed. online [Internet]. Research Triangle Park (NC): Society of Dyers and Colourists and American Association of Textile Chemists and Colorists. Available from: www.colour-index.com
Cleuvers M, Weyers A. 2003. Algal growth inhibition test: does shading of coloured substances really matter? Water Res 37(11):2718-2722.
Clode SA, Hooson J, Grant D, Butler WH. 1987. Long-term toxicity study of amaranth in rats using animals exposed in utero. Food Chem Toxicol 25(12):937-946.
Collins TF, McLaughlin J. 1972. Teratology studies on food colourings. Part 1. Embryotoxicity of amaranth (FD&C red no. 2) in rats. Food Cosmet Toxicol 10:619-624.
Collins TF, Keeler HV, Black TN, Ruggles DI. 1975a. Long-term effects of dietary amaranth in rats. I. Effects on reproduction. Toxicology 3(1):115-128.
Collins TF, Black TN, Ruggles DI. 1975b. Long-term effects of dietary amaranth in rats. II. Effects on fetal development. Toxicology 3:129-140.
Collins TF, Ruggles DI, Holson JF Jr, Schumacher H, Gaylor DW, Kennedy GL Jr. 1976a. Teratological evaluation of FD&C red no. 2--a collaborative government-industry study. I. introduction, experimental materials, and procedures. J Toxicol Environ Health 1(5):851-856.
Collins TF, Black TN, Ruggles DI, Gray GC. 1976b. Teratological evaluation of FD&C red no. 2--a collaborative government-industry study. II. FDA’s study. J Toxicol Environ Health 1(5):857-862.
Collins TF, Black TN, Brown LH, Bulhack P. 1990. Study of the teratogenic potential of FD & C yellow no. 5 when given by gavage to rats. Food Chem Toxicol 28(12):821-827.
Collins TF, Black TN, O’Donnell MJ, Bulhack P. 1991. Teratogenic potential of FD&C yellow no. 5 when administered in drinking water to Osborne-Mendel rats. Teratology 43(5):456 [abstract].
Collins TF, Black TN, O’Donnell MW Jr, Bulhack P. 1992. Study of the teratogenic potential of FD & C yellow no. 5 when given in drinking-water. Food Chem Toxicol 30(4):263-268.
Comber MHI, Smyth DV, Thompson RS. 1995. Assessment of the toxicity to algae of coloured substances. Bull Environ Contam Toxicol 55:922-928. [cited in Rufli et al. 1998; Cleuvers and Weyers 2003].
Combes RD, Haveland-Smith RB. 1982. A review of the genotoxicity of food, drug and cosmetic colours and other azo, triphenylmethane and xanthene dyes. Mutat Res 98(2):101-248.
[ConsExpo] Consumer Exposure Model [Internet]. 2006. Version 4.1. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. Available from: www.rivm.nl/en/healthanddisease/productsafety/ConsExpo.jsp#tcm:13-42840
Cook JW, Hewett CL, Kennaway EL, Kennaway NM. 1940. Effects produced in the livers of mice by azonaphthalenes and related compounds. Am J Cancer 40:62-77.
[CPDB] Carcinogenic Potency Database [database on the Internet]. 2011. Berkeley (CA): University of California. [cited 2013 May]. Available from: http://potency.berkeley.edu/
[CTFA] Cosmetic, Toiletry and Fragrance Association. 1983. Summary for the results of surveys of the amount and frequency of use of cosmetic products by women. Report prepared by Environ Corporation, Washington, DC. Washington (DC): CTFA.
Daniel J. 1962. The excretion and metabolism of edible food colors. Toxicol Appl Pharmacol 4:572-594.
[Danish EPA] Danish Environmental Protection Agency. 2012. Annex XV report: Proposal for a restriction. Substance name(s): Chromium (VI) compounds [Internet]. Copenhagen (DK): Danish EPA. Available from: http://echa.europa.eu/documents/10162/4d88d444-4b8b-48ab-9c11-6e74819e047c
De France BF, Carter MH, Josephy PD. 1986. Comparative metabolism and mutagenicity of azo and hydrazone dyes in the Ames test. Food Chem Toxicol 24(2):165-169.
[DFG] Deutsche Forschungsgemeinschaft. 1957. Farbstoff Kommission, Mitteilung 6, 2, Auflage Toxikologische Daten von Farbstoffen und ihre zullassung fur Lebensmittel in verschiedenen Landern. Wiesbaden (DE): Franz Steiner Verlag GmbH; p. 38. [cited in IARC 1975b; JECFA 1983].
Dharma Trading Co. 2009. Material Safety Data Sheet: Acid Blue 113 [Internet]. Petaluma (CA): Dharma Trading Co. Available from: http://www.google.ca/url?q=http://www.clearsynth.com/docs/MSD-CS-AC-21782.pdf&sa=U&ei=nn1hUuaFBKjE4AO02IEw&ved=0CCcQFjAD&usg=
AFQjCNG0C1izw2HmUdpUlUWlgObAiYKkxA
Dillon D, Combes R, Zeiger E. 1994. Activation by caecal reduction of the azo dye D & C red no. 9 to a bacterial mutagen. Mutagenesis 9(4):295-299.
[DS TOPKAT] Discovery Studio TOxicity Prediction by Komputer Assisted Technology [Prediction Module]. ©2005-2009. Version 2.5.0.9164. San Diego (CA): Accelrys Software Inc. Available from: www.accelrys.com/products/
[EC] European Commission. 2001. Integrated Pollution Prevention and Control (IPPC) - Best Available Techniques in the Pulp and Paper Industry, European Commission, December 2001, p.238).
[EC] European Commission. 2003a. Technical guidance document on risk assessment. Part I. Ispra (IT): European Commission, European Chemicals Bureau, Institute for Health and Consumer Protection. Available from: http://ihcp.jrc.ec.europa.eu/our_activities/public-health/risk_assessment_of_Biocides/doc/tgd/tgdpart1_2ed.pdf
[EC] European Commission. 2003b. Technical guidance document on risk assessment. Part II. Ispra (IT): European Commission, European Chemicals Bureau, Institute for Health and Consumer Protection. Available from: http://www.google.ca/url?q=http://ihcp.jrc.ec.europa.eu/our_activities/public-health/risk_assessment_of_Biocides/doc/tgd/tgdpart2_2ed.pdf&sa=U&ei=
uHlhUunWDrT_4APd24DwCA&ved=0CBoQFjAA&usg=
AFQjCNElhih6cIzUrE4tZEu8aK6Ptt5aAA
[ECHA] European Chemicals Agency. ©2007-2013. Registered Substances database. Search results for various CAS RNs. Helsinki (FI): ECHA. [updated 2012 Nov 27; cited 2012 Jan-Nov]. Available from: www.echa.europa.eu/information-on-chemicals/registered-substances
[EFSA] European Food Safety Authority. 2008. Assessment of the results of the study by McCann et al. (2007) on the effect of some colours and sodium benzoate on children’s behaviour. Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Food Contact Materials (AFC). EFSA J 660[54p.]. Available from: www.efsa.europa.eu/en/efsajournal/pub/660.htm
[EFSA] European Food Safety Authority. 2009a. Scientific opinion on the re-evaluation of Ponceau 4R (E 124) as a food additive on request from the European Commission. EFSA J 7(11):1328 [39 p.]. Available from: www.efsa.europa.eu/en/efsajournal/pub/1328.htm
[EFSA] European Food Safety Authority. 2009b. Scientific opinion on the re-evaluation of tartrazine (E 102) on request from the European Commission. EFSA J 7(11):1331[52 p.]. Available from: www.efsa.europa.eu/en/efsajournal/pub/1331.htm
[EFSA] European Food Safety Authority. 2010. Scientific opinion on the re-evaluation of amaranth (E 123) as a food additive on request from the European Commission. EFSA J 8(7):1649 [41 p.]. Available from: www.efsa.europa.eu/en/efsajournal/pub/1649.htm
El-Sherbeny KM, Ibrahim AAE. 1999. Induction of chromosome aberrations in bone-marrow and germ cells and sperm head abnormalities in mice by orange II and the protective effect of royal jelly. Bull Natl Res Cent (Egypt) 24(4):469-479.
Engel E, Vasold R, Santarelli F, Maisch T, Gopee NV, Howard PC, Landthaler M, Baumler W. 2009. Tattooing of skin results in transportation and light-induced decomposition of tattoo pigments--a first quantification in vivo using a mouse model. Exp Dermatol 19:54-60.
Environment Canada. 2007. Overview of the ecological assessment of substances under the Canadian Environmental Protection Act, 1999. Gatineau (QC): Environment and Climate Change Canada, Existing Substances Division and New Substances Division. Available from:
www.ec.gc.ca/lcpe-cepa/documents/substances/eas_overview-eng.pdf
Environment Canada. 2012. Data for certain aromatic amines and aromatic azo- and benzidine-based substances collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain aromatic amines and aromatic azo- and benzidine-based substances. Data prepared by: Environment and Climate Change Canada, Program Development and Engagement Division.
Environment Canada, Health Canada. 2007. Chemical substances: Categorization [Internet]. Ottawa (ON): Government of Canada. [updated 2007 April 20; cited 2014 June 10]. Available from: http://www.chemicalsubstanceschimiques.gc.ca/approach-approche/categor-eng.php
Environment Canada, Health Canada. 2013. The Chemicals Management Plan Substance Grouping Initiative. Subgrouping Approach and Background Information for the Screening Assessment of Aromatic Azo and Benzidine-based Substances. May 2013. Environment and Climate Change Canada, Health Canada. Available upon request.
Environment Canada, Health Canada. 2014a. Screening Assessment. Aromatic Azo and Benzidine-based Substance Grouping. Certain Benzidine-based Dyes and Related Substances. Environment and Climate Change Canada, Health Canada. Available from: http://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=6A3D4735-1
Environment Canada, Health Canada. 2014b. Rapid Screening of Substances from Phase One of the Domestic Substances List Inventory Update. March 2014. Environment and Climate Change Canada, Health Canada. Available from: http://www.ec.gc.ca/ese-ees/7340E1B7-1809-4564-8C49-F05875D511CB/FSAR_RSII_EN.pdf
Environment and Climate Change Canada, Health Canada. 2015. Screening Assessment. Aromatic Azo and Benzidine-based Substance Grouping. Certain Monoazo Pigments. Environment and Climate Change Canada, Health Canada.
[EPI Suite] Estimation Programs Interface Suite for Microsoft Windows [Estimation Model]. 2012. Version 4.1. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Ericson JW. 1977. Applicability of ATP measurements for the determination of dye toxicity in short term algal assays. In: Proceedings of the Bi-Annual ATP Methodology Symposium. San Diego (CA): SAI Technology Co.; p. 415-439.
[ETAD] The Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 1983. Final report on extractability of dyestuffs from textiles. Basel (CH): ETAD. Project No. A 4007.
[ETAD] The Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 1988. Executive summary on the ETAD Project T 2015--Toxicological testing of major colorants, with attachments and cover letter dated 06/03/88. Basel (CH): ETAD. Report No.: TSCA OTS0000621, Document No. FYI-AX-0688-0621.
[ETAD] The Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 1992. ETAD project E3020--data summary (disperse dyes). Unpublished results summary. Submitted to Environment and Climate Change Canada, Existing Substances Division, by ETAD, Basel (CH).
[ETAD] The Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 1995. Health and environmental information on dyes used in Canada. An overview to assist in the implementation of the New Substances Notification Regulation under the Canadian Environmental Protection Act. Basel (CH): ETAD.
[EU] European Union. 2006. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemical Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC [Internet]. Off J Eur Union L 396:1-849. Available from: http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=oj:l:2006:396:0001:0849:en:pdf
[FDRL] Food and Drug Research Laboratories. 1972. Teratologic evaluation of FDA 71-23
(Amaranth FD/C Red No. 2). Springfield (VA): National Technical Information Service; p. 49. Report No.: PB-221 771. [cited in EFSA 2010].
Fernandes C, Rao KV. 1994. Dose related promoter effect of metanil yellow on the development of hepatic pre-neoplastic lesions induced by N-nitrosodiethylamine in rats. Indian J Med Res 100:140-149.
Fisher. 2012. Material Safety Data Sheet: Acid Black 1 [Internet]. Fair Lawn (NJ): Fisher Scientific. Available from: http://www.fishersci.ca/viewmsds.do?catNo=BP12410
Freeman HS, Peters AT. 2000. Colorants for non-textile applications. Amsterdam (NL): Elsevier B.V.
Freeman HS, Esancy JF, Esancy MK, Mills KP, Whaley WM, Dabney BJ. 1987. An approach to the design of non-mutagenic azo dyes. 1. The identification of non-mutagenic precursors and potential metabolites. Dyes Pigm 8(6):417-430.
Fujita S, Peisach J. 1978. Liver microsomal cytochromes P-450 and azoreductase activity. J Biol Chem 253(13):4512-4513.
Garner RC, Nutman CA. 1977. Testing of some azo dyes and their reduction products for mutagenicity using Salmonella typhimurium TA1538. Mutat Res 44:9-19.
Gaunt IF, Farmer M, Grasso P, Gangolli SD. 1967. Acute (mouse and rat) and short-term (rat) toxicity studies on Ponceau 4R. Food Cosmet Toxicol 5:187-194.
Gaunt IF, Grasso P, Creasey M, Gangolli SD. 1969. Short-term toxicity study on Ponceau 4R in the pig. Food Cosmet Toxicol 7(5):443-449.
Ghosh DK, Chaudhuri J, Mandal A. 1988. Degradation of seven food dyes by some pathogenic and non-pathogenic intestinal microflora. Microbios Lett 39(154):81-86.
Giri AK, Banerjee TS. 1986. Antagonistic activity of herbal drug (Phyllanthus emblica) on cytological effects of environmental chemicals on mammalian cells. Cytologia 51(2):375-380.
Giri AK, Talukder G, Sharma A. 1986a. Sister chromatid exchange induced by metanil yellow and nitrite singly and in combination in vivo on mice. Cancer Lett 31(3):299-303.
Giri AK, Banerjee TS, Talukder G, Sharma A. 1986b. Effects of dyes (indigo carmine, metanil yellow, fast green FCF) and nitrite in vivo on bone marrow chromosomes of mice. Cancer Lett 30(3):315-320.
Giri AK, Das SK, Talukder G, Sharma A. 1990. Sister chromatid exchange and chromosome aberrations induced by curcumin and tartrazine on mammalian cells in vivo. Cytobios 62(249):111-117.
Gottlieb A, Shaw C, Smith A, Wheatley A, Forsythe S. 2003. The toxicity of textile reactive azo dyes after hydrolysis and decolourisation. J Biotechnol 101:49-56.
Green FJ. 1990. The Sigma-Aldrich handbook for dyes, stains and indicators. Milwaukee (WI): Aldrich Chemical Co.; p. 513. [cited in EPI Suite 2012].
Greene JC, Baughman GL. 1996. Effects of 46 dyes on population growth of freshwater green alga Selenastrum capricornutum. Text Chem Color 28(4): 23-30.
Gupta S, Sundarrajan M, Rao KV. 2003. Tumor promotion by metanil yellow and malachite green during rat hepatocarcinogenesis is associated with dysregulated expression of cell cycle regulatory proteins. Teratog Carcinog Mutagen Suppl 1:301-312.
Haag WR, Mill T. 1987. Direct and indirect photolysis of water soluble azodyes: kinetic measurements and structure-activity relationships. Environ Toxicol Chem 6:359-369. [cited in EPI Suite 2012].
Hall DE, Lee FS, Fairweather FA. 1966. Acute (mouse and rat) and short-term (rat) toxicity studies on Ponceau MX. Food Cosmet Toxicol 4(4):375-382.
Hall B, Tozer S, Safford B, Coroama M, Steiling W, Leneveu-Duchemin MC, McNamara C, Gibney M. 2007. European consumer exposure to cosmetic products, a framework for conducting population exposure assessments. Food Chem Toxicol 45: 2097-2108.
Hamann HJ, Richard D, Robert N, Knuppe C. 2000. 13-week oral toxicity (gavage) study
in rat with D&C Orange 4 (C.I. 15510). Itingen (CH): RCC Ltd. RCC Project No. 736312, Test Report .
Hamchem. 2013. Material Safety Data Sheet: C.I. Food Red 17 [Internet]. Hangzhou (CN): Hamchem. Available from: http://www.vinayakcorporation.com/alluramsdc1.htm
Haveland-Smith RB, Combes RD. 1980. Screening of food dyes for genotoxic activity. Food Cosmet Toxicol 18:215-221.
Hayashi M, Kishi M, Sofuni T, Ishidate MJR. 1988. Micronucleus tests in mice on 39 food additives and eight miscellaneous chemicals. Food Chem Toxicol 26(6):487-500.
Hazleton Laboratories. 1992. Initial submission: Repeated dermal applications in mice of FD&C blue 1, FD&C red 3, FD&C yellow 5, D&C red 19 with cover letter dated 08/26/92. Doc ID: 88-920008654. Microfiche No.: OTS0555152. Available from: www.ntis.gov
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Health Canada. 2008. Classification of products at the cosmetic-drug interface. Ottawa (ON): Health Canada, Health Products and Food Branch, and Healthy Environments and Consumer Safety Branch. [cited 2014 Dec 12]. Available from: www.hc-sc.gc.ca/cps-spc/alt_formats/hecs-sesc/pdf/pubs/indust/cosmet_drug_guide-drogue_ref/cosmet_drug_guide-drogue_ref-eng.pdf
Health Canada. 2010. Health Canada proposal to improve food colour labelling requirements--February 2010. Ottawa (ON): Health Canada, Bureau of Chemical Safety, Food Directorate, Health Products and Food Branch. [cited 2013 Mar 21]. Available from: www.hc-sc.gc.ca/fn-an/consult/_feb2010-food-aliments-col/draft-ebauche-eng.php
Health Canada. 2011b. Canadian Environmental Protection Act, 1999. Follow-up report on a PSL substance for aniline. Chemical Abstract Registry Number 62-53-3 [Internet]. [cited 2013 Oct]. Available from: www.ec.gc.ca/ese-ees/default.asp?lang=En&n=CCDA73CC-1
Health Canada. 2012. List of Permitted Colouring Agents (Lists of Permitted Food Additives) [Internet]. Ottawa (ON): Health Canada. [cited 2012 Oct]. Available from: www.hc-sc.gc.ca/fn-an/securit/addit/list/3-colour-color-eng.php
Henschler D, Wild D. 1985. Mutagenic activity in rat urine after feeding with the azo dye tartrazine. Arch Toxicol 57:214-215.
Health Canada. 2014. The Cosmetic Ingredient Hotlist March 2011 [Internet]. Ottawa (ON): Health Canada, Consumer Product Safety. [cited 2012 Sep]. Available from: http://www.hc-sc.gc.ca/cps-spc/alt_formats/pdf/cosmet-person/hot-list-critique/hotlist-liste-eng.pdf
Henschler D, Wild D. 1986. Mutagenicity of tartrazine revisited. Arch Toxicol 59:69-70.
Himri I, Bellahcen S, Souna F, Belmekki F, Aziz M, Bnouham M, Zoheir J, Berkia Z, Mekhfi H, Saalaoui E. 2011. A 90-day oral toxicity study of tartrazine, a synthetic food dye, in Wistar rats. Int J Pharm Pharm Sci 3(Suppl 3):159-169.
Holson JF Jr, Gaylor DW, Schumacher HJ, Collins TF, Ruggles DI, Keplinger ML, Kennedy GL Jr. 1976. Teratological evaluation of FD&C red no. 2--a collaborative government-industry study. V. Combined findings and discussion. J Toxicol Environ Health 1(5):875-885.
Honarvar N. 2003. Micronucleus assay in bone marrow cells of the mouse with Orange 4 (C.I. 15510). Rossdorf (DE): RCC Ltd. RCC-CCR Test Report No.: 741302.
Honarvar N et al. 2005. In vitro dermal absorption through porcine ear skin with SC Clear + 0.5% D&C Orange 4 (Lot AJ 3559). Rossdorf (DE): RCC Ltd. RCC-CCR Study No. 860802. [cited in SCCS 2011b].
[HSDB] Hazardous Substances Data Bank [database on the Internet]. 1983- . Bethesda (MD): National Library of Medicine (US). [cited 2012]. Available from: www.toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB
Hunger K, editor. 2003. Industrial dyes: chemistry, properties, applications. Weinheim (DE): Wiley-VCH Verlag GmbH & Co. KGaA.
Hunger K. 2005. Toxicology and toxicological testing of colorants. Rev Prog Color 35:76-89.
[HYDROWIN] Hydrolysis Rates Program for Microsoft Windows [Estimation Model]. 2010. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1975a. Ponceau MX. In: Some aromatic azo compounds. IARC Monogr Eval Carcinog Risk Hum 8:189-198.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1975b. Amaranth. In: Some aromatic azo compounds. IARC Monogr Eval Carcinog Risk Hum 8:41-51.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1975c. Ponceau 3R. In: Some aromatic azo compounds. IARC Monogr Eval Carcinog Risk Hum 8:199.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1975d. Ponceau SX. In: Some aromatic azo compounds. IARC Monogr Eval Carcinog Risk Hum 8:207.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1987a. Ponceau SX (group 3). In: Overall evaluations of carcinogenicity: an updating of IARC monographs volumes 1-42. IARC Monogr Eval Carcinog Risk Chem Hum Suppl 7: 70.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1987b. Ponceau MX (group 2B). In: Overall evaluations of carcinogenicity: an updating of IARC monographs volumes 1-42. IARC Monogr Eval Carcinog Risk Chem Hum Suppl 7:70.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1987c. Amaranth (group 3). In: Overall evaluations of carcinogenicity: an updating of IARC monographs volumes 1-42. IARC Monogr Eval Carcinog Risk Chem Hum Suppl 7:56.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1987d. Ponceau 3R (group 2B). In: Overall evaluations of carcinogenicity: an updating of IARC monographs volumes 1-42. IARC Monogr Eval Carcinog Risk Chem Hum Suppl 7:70.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 2010. Some aromatic amines, organic dyes, and related exposures. IARC Monogr Eval Carcinog Risks Hum 99. Available from: http://monographs.iarc.fr/ENG/Monographs/vol99/mono99.pdf
[ILS] Integrated Laboratory Systems, Inc. 2011. Final report: Assessment of mutagenicity of twenty aromatic azo substances in the Ames mutagenicity assay using the Prival modification with and without FMN. Unpublished report prepared for Health Canada. Durham (NC): ILS. Study Report No.: C191-006.
[ILS] Integrated Laboratory Systems, Inc. 2012. Final report: Assessment of mutagenicity of fifteen aromatic azo substances in the Ames mutagenicity assay using the Prival modification with and without FMN. Unpublished report prepared for Health Canada. Durham (NC): ILS. Study Report No.: C191-009.
Ishidate MJR, Hayashi M, Sawada M, Matsuoka A, Yoshikawa K, Ono M, Nakadate M. 1978. Cytotoxicity test on medical drugs. chromosome aberration tests with Chinese hamster cells in vitro. Eisei Shikenjo Hokoku 96(55):61.
Ishidate MJR, Sofuni T, Yoshikawa K. 1981. Chromosomal aberration tests in vitro as a primary screening tool for environmental mutagens and/or carcinogens. Gann Monogr Cancer Res 27:95-108.
Ishidate MJR, Sofuni T, Yoshikawa K, Hayashi M, Nohmi T, Sawada M, Matsuoka A. 1984. Primary mutagenicity screening of food additives currently used in Japan. Food Chem Toxicol 22:623-636.
Isik M, Sponza DT. 2004. Monitoring of toxicity and intermediates of C.I. Direct Black 38 azo dye through decolourization in an anaerobic/aerobic sequential reactor system. J Hazard Mater B114:29-39.
[ISSCAN] Istituto Superiore di Sanità Cancer. 2012 Chemical carcinogens: structures and experimental data [database on the Internet]. [cited in OECD QSAR Toolbox 2013]. Description at: www.epa.gov/ncct/dsstox/sdf_isscan_external.html; available from: www.iss.it/index.php?lang=1&anno=2013&tipo=39
Izbirak A, Sumer S, Diril N. 1990. [Mutagenicity testing of some azo dyes used as food additives]. Mikrobiyol Bul 24(1):48-56. [in Turkish].
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 1966. Specifications for identity and purity and toxicological evaluation of food colours. Eighth report of the Joint FAO/WHO Expert Committee on Food Additives. Geneva (CH): World Health Organization; p. 88-92. Report No.: WHO/Food Add/66.25.
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 1983. Ponceau 4R. In: Toxicological evaluation of certain food additives and contaminants. Geneva (CH): World Health Organization. WHO Food Additive Series, No. 18. Available from: www.inchem.org/documents/jecfa/jecmono/v18je12.htm
[JECFA] Joint FAO/WHO Expert Committee on Food Additives.1984. Amaranth. In: Toxicological evaluation of certain food additives and contaminants. Geneva (CH): World Health Organization. WHO Food Additive Series, No. 19. Available from: www.inchem.org/documents/jecfa/jecmono/v19je02.htm
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2011. Evaluation of certain food additives and contaminants. Seventy-fourth report of the Joint FAO/WHO Expert Committee on Food Additives. Geneva (CH): World Health Organization. WHO Technical Report Series, No. 966. Available from: http://whqlibdoc.who.int/trs/WHO_TRS_966_eng.pdf
Jones R, Ryan AJ, Wright SE. 1964. The metabolism and excretion of tartrazine in the rat, rabbit and man. Food Cosmet Toxicol 2:447-452.
Jung R, Steinle D, Anliker R. 1992. A compilation of genotoxicity and carcinogenicity data on aromatic aminosulphonic acids. Food Chem Toxicol 30(7):635-660.
Kada T, Tutikawa K, Sadaie Y. 1972. In vitro and host-mediated “rec-assay” procedures for screening chemical mutagens; and phloxine, a mutagenic red dye detected. Mutat Res 16(2):165-174.
Karpinski K. and Nargundkar M. 1992. Nova Scotia Nutrition Survey Methodology Report. Technical Document #E451311-001, Bureau of Biostatistics and Computer Applications, Food Directorate, Health Canada.
Kawachi T, Yahagi T, Kada T, Tazima Y, Ishidate M, Sasaki M, Sugiyama T. 1980. Cooperative program on short-term assays for carcinogenicity in Japan. IARC Sci Publ 27:323-330.
Keplinger ML, Wright PL, Plank JB, Calandra JC. 1974. Teratologic studies with FD & C red no. 2 in rats and rabbits. Toxicol Appl Pharmacol 28:209-215.
Keplinger ML, Kinoshita FK, Smith SH, Kennedy GL Jr. 1976. Teratological evaluation of FD&C red no. 2--a collaborative government-industry study. III. IBT’s study. J Toxicol Environ Health 1(5):863-866.
Khera KS, Przybylski W, McKinley WP. 1974. Implantation and embryonic survival in rats treated with amaranth during gestation. Food Cosmet Toxicol 12(4):507-510.
Kihara T, Yasuda Y, Tanimura T. 1977. Effects on pre- and post-natal offspring of pregnant rats fed food red no. 102. Teratology 16:111.
[Kirk-Othmer] Kirk-Othmer Encyclopedia of Chemical Technology. 2010. John Wiley & Sons, Inc.
Kornbrust D, Barfknecht T. 1985. Testing of 24 food, drug, cosmetic, and fabric dyes in the in vitro and the in vivo/in vitro rat hepatocytes primary culture/DNA repair assay. Environ Mutagen 7:101-120.
Kosaka H, Nakamura S. 1990. [Genotoxicity of synthetic dyes in umu test using Salmonella typhimurium TA1535/pSK1002 (1). Results of examination for acid dyes, direct dyes, disperse dyes and reactive dyes]. Sangyo Igaku 32(2):89-104. [in Japanese].
Larsen JC, Meyer T, Scheline RR. 1976. Reduction of sulphonated water-soluble azo dyes by caecal microorganisms from the rat. Acta Pharmacol Toxicol 38:353-357. [cited in EFSA 2010].
Larsson KS. 1975. Teratologic study with the dyes amaranth and Ponceau 4R in mice. Toxicology 4:75-82.
Lea PJ, Pawlowski A. 1987. Human tattoo: electronic microscopic assessment of epidermis, epidermal-dermal junction, and dermis. Int J Dermatol 26(7):453-458.
Lecointe P, Lesca P. 1978. Absence of activity of amaranth (FD & C red no. 2) in the Salmonella/microsome mutagenicity test. Food Cosmet Toxicol 16(1):89-90.
Lewis RJ Sr. 2007. Hawley’s condensed chemical dictionary. 15th ed. New York (NY): John Wiley & Sons, Inc.
Lin GHY, Solodar WE. 1988. Structure-activity relationship studies on the mutagenicity of some azo dyes in the Salmonella/microsome assay. Mutagenesis 3(4):311-315.
Little LW, Lamb JC III. 1973. Acute toxicity of 46 selected dyes to the fathead minnow, Pimephales promelas. In: Dyes and the environment--Reports on selected dyes and their effects. Vol. 1. American Dye Manufacturers Institute, Inc.; 130 p.
Little LW, Lamb JC III, Chillingworth MA, Durkin WB. 1974. Acute toxicity of selected commercial dyes to the fathead minnow and evaluation of biological treatment for reduction of toxicity. In: Proceedings of the 29th Industrial Waste Conference, Purdue University, West Lafayette (IN); p. 524-534.
[LNHPD] Licensed Natural Health Products Database [database on the Internet]. Created 2008. Searched Feb 2015. Version 1.0. Ottawa (ON): Health Canada. [cited 2011]. Available from: webprod3.hc-sc.gc.ca/lnhpd-bdpsnh/index-eng.jsp
Loretz LG, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, Rothenstein A, Scrafford CG, Sewall C. 2005. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol 43:279-291.
Loretz L, Api AM, Barraj L, Burdick J, Davis DA, Dressler W, Gilberti E, Jarrett G, Mann S, Pan YHL, Re T, Renskers K, Scrafford C, Vater S. 2006. Exposure data for personal care products: hairspray, spray perfume, liquid foundation, shampoo, body wash, and solid antiperspirant. Food Chem Toxicol 44:2008-2018.
Loretz LG, Api AM, Babcock L, Barraj LM, Burdick J, Cater KC, Jarrett G, Mann S, Pan YHL, Re TA, Renskers KJ, Scrafford CG. 2008. Exposure data for cosmetic products: facial cleanser, hair conditioner, and eye shadow. Food Chem Toxicol 46:1516-1524.
MacPhee C, Ruelle R. 1969. Lethal effects of 1888 chemicals upon four species of fish from western North America. Moscow (RU): Forest, Wildlife, and Range Experiment Station.
Maekawa A, Matsuoka C, Onodera H, Tanigawa H, Furuta K, Kanno J, Jang JJ, Hayashi Y, Ogiu T. 1987. Lack of carcinogenicity of tartrazine (FD & C yellow no. 5) in the F344 rat. Food Chem Toxicol 25(12):891-896.
Mamber SW, Bryson V, Katz SE. 1983. The Escherichia coli WP2/WP100 rec assay for detection of potential chemical carcinogens. Mutat Res 119(2):135-144.
Mamber SW, Bryson V, Katz SE. 1984. Evaluation of the Escherichia coli K12 inductest for detection of potential chemical carcinogens. Mutat Res 130(3):141-151.
Mannell WA, Grice HC, Lu FC, Allmark MG. 1958. Chronic toxicity studies on food colours. IV. Observations on the toxicity of tartrazine, amaranth and sunset yellow in rats. J Pharm Pharmacol 10(10):625-634.
Mansour HB, Barillier D, Corroler D, Ghedira K, Chekir-Ghedira L, Mosrati R. 2009. In vitro study of DNA damage induced by acid orange 52 and its biodegradation derivatives. Environ Toxicol Chem 28:489-495.
Marchisio MA, Dubini M, Serra G, Mennini T, Manara L. 1976. Excretion of 35S-tobias acid (2-naphthylamino, 1-sulfonic acid) by the rat after oral and intravenous administration. Br J Ind Med 33(4):269-271.
Marmion DM. 1991. Handbook of U.S. colorants: foods, drugs, and cosmetics and medical devices. New York (NY): John Wiley & Sons, Inc.
Martin CN, Kennelly JC. 1981. Rat liver microsomal azoreductase activity on four azo dyes derived from benzidine, 3,3′-dimethylbenzidine or 3,3′-dimethoxybenzidine. Carcinogenesis 2(4):307-312.
Mason PL et al. (1974) Long-term toxicity study of Ponceau 4R in mice. Unpublished report no. 1/1974. Submitted to WHO by the British Industrial Biological Research Association. [cited in JECFA 1983; EFSA 2009a].
Masson P. 2002. Exposure to cosmetic products: General consideration--Calculation of exposure according to the SCCNFP Notes of Guidance. Bordeaux (FR): EVIC France.
Mastalski K, Jenkins DH, Plank JB, Kinoshita FK, Keplinger ML, Calandra JC. 1975. Teratologic study in dogs with FD&C red no. 2. Toxicol Appl Pharmacol 33:122-123.
Mehedi N, Ainad-Tabet S, Mokrane N, Addou S, Zaoui C, Kheroua O, Saidi D. 2009. Reproductive toxicology of tartrazine (FD and C yellow no. 5) in Swiss albino mice. Am J Pharmacol Toxicol 4(4):130-135.
Meyer O, Hansen EV. 1975. Study of the embryotoxicity of the food color Ponceau 4 R in rats. Toxicology 5:201-207.
[MITI] Ministry of International Trade and Industry (JP), Basic Industries Bureau, Chemical Products Safety Division. 1992. Biodegradation and bioaccumulation data of existing chemicals based on the CSCL Japan. Tokyo (JP): Japan Chemical Industry Ecology-Toxicology & Information Center.
Miyagoshi M, Hayakawa Y, Nagayama T. 1983. Studies on the mutagenicity of cosmetic azo-dyes. Eisei Kagaku 29:212-220ey
Momma J, Kawamata K, Takada K, Horiuchi S, Tobe M. 1981. A study on teratogenicity of new coccine, food red no. 102, in mice. Eisei Shikenjo Hokoku 99:73-78.
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, Zeiger E. 1986. Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals. Environ Mutagen 8(Suppl 7):1-119.
Moutinho IL, Bertges LC, Assis RV. 2007. Prolonged use of the food dye tartrazine (FD&C yellow no 5) and its effects on the gastric mucosa of Wistar rats. Braz J Biol 67(1):141-145.
Mpountoukas P, Pantazaki A, Kostareli E, Christodoulou P, Kareli D, Poliliou S, Mourelatos C, Lambropoulou V, Lialiaris T. 2010. Cytogenetic evaluation and DNA interaction studies of the food colorants amaranth, erythrosine and tartrazine. Food Chem Toxicol 48(10):2934-2944.
Munro IC, Ford RA, Kennepohl E, Sprenger JG. 1996. Correlation of structural class with no-observed-effect levels: a proposal for establishing a threshold of concern. Food Chem Toxicol 34(9):829-867.
Münzner R. 1979. Mutagenicity testing of the urine of rats treated with amaranth. Food Cosmet Toxicol 17(5):563.
Münzner R, Wever J. 1987. Mutagenic activity of the feces of rats following oral administration of tartrazine. Arch Toxicol 60:328-330.
Muzzall JM, Cook WL. 1979. Mutagenicity test of dyes used in cosmetics with the Salmonella/mammalian-microsome test. Mutat Res 67(1):1-8.
Nakamura S, Kosaka H, Ugawa M. 1993. [Genotoxicity of chemical synthetic dyes. Results of umu test using Salmonella typhimurium TA1535/pSK1002]. Hen’igensei Shiken 2(3):162-174. [in Japanese].
[NCI] National Chemical Inventories [database on a CD-ROM]. 2012. Issue 2. Columbus (OH): American Chemical Society. [cited 2013 Oct 13]. Available from: www.cas.org/products/other-cas-products/nci-on-cd
Nelson AA, Hagan EC. 1953. Production of fibrosarcomas in rats at site of SQ infection of various food dyes. Fed Proc 12:397-398. [cited in IARC 1975b].
Nesnow S, Bergman H, Bryant B-J, Helton S, Richard A. 1988. A CASE-SAR study of mammalian hepatic azoreduction. J Toxicol Environ Health 24(4):499-514.
Neumann HG. 2005. Monocyclic aromatic amino and nitro compounds: toxicity, genotoxicity and carcinogenicity, classification in a carcinogen category. In: The MAK-Collection Part I: MAK Value Documentations, Vol. 21. Weinheim (DE): Deutsche Forschungsgemeinschaft (DFG).
Neumann HG. 2010. Aromatic amines: mechanisms of carcinogenesis and implications for risk assessment. Frontiers Biosci 15:1119-1130.
[New EQC] New Equilibrium Criterion Model. 2011. Version 1.00 (Beta). Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry. Available from: www.trentu.ca/academic/aminss/envmodel/models/NewEQCv100.html
[NHPID] Natural Health Products Ingredients Database [database on the Internet]. 2015. Version 2.1. Ottawa (ON): Health Canada. [cited 2015]. Available from: webprod.hc-sc.gc.ca/nhpid-bdipsn/search-rechercheReq.do
[NMID] Non-Medicinal Ingredients Database (internal use only). 2011. Ottawa (ON): Health Canada, Therapeutic Products Directorate. [searched 2011 Aug].
[NPRI] National Pollutant Release Inventory [database on the Internet]. 2006. Gatineau (QC): Environment Canada. [cited 2013 May]. Available from: www.ec.gc.ca/inrp-npri
[NTP] National Toxicology Program. 1992. Chemical Repository Database. Research Triangle Park (NC): National Institutes of Health, Institute of Environmental Health Sciences, NTP. [cited in Cameo Chemicals 2013].
[OECD] Organisation for Economic Co-operation and Development. 2002. SIDS summary risk assessment report for: 4,4′-Methylenedianiline (MDA). CAS no. 101-77-9. EINECS no. 202-974-4. Available from: www.inchem.org/documents/sids/sids/101779.pdf
[OECD] Organisation for Economic Co-operation and Development. 2004a. Emission scenario document on textile finishing industry [Internet]. Paris (FR): OECD, Environment Directorate. Report No.: ENV/JM/MONO(2004)12, JT00166691. Available from: www.oecd.org/dataoecd/2/47/34003719.pdf
[OECD] Organisation for Economic Co-operation and Development. 2004b. Chemicals Screening Information Dataset (SIDS) for Cooperative Chemicals Assessment Programme. Available from: http://webnet.oecd.org/hpv/ui/Publications.aspx
[OECD] Organisation for Economic Co-operation and Development. 2014. Guidance on Grouping of Chemicals. Second Edition. Series on Testing & Assessment No. 194. Available at: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2014)4&doclanguage=en
OECD QSAR Toolbox [Read-across tool]. 2013. Version 3.1. Paris (FR): Organisation for Economic Co-operation and Development, Environment Directorate. [cited 2013]. Available from: www.oecd.org/document/23/0,3343,en_2649_34379_33957015_1_1_1_1,00.html
Øllgaard H, Frost L, Galster J, Hansen OC. 1998. Survey of azo-colorants in Denmark: consumption, use, health and environmental aspects. Copenhagen (DK): Danish Ministry of Environment and Energy, Environmental Protection Agency. Available from: www2.mst.dk/udgiv/publications/1999/87-7909-548-8/pdf/87-7909-546-1.pdf
Oster G. 1955. Dye binding to high polymers. J Polym Sci 16:235-244.
Ozaki A, Kitano M, Itoh N, Kuroda K, Furusawa N, Masuda T, Yamaguchi H. 1998. Mutagenicity and DNA-damaging activity of decomposed products of food colours under UV irradation. Food Chem Toxicol 36(9-10):811-817.
Pan H, Feng J, Cerniglia CE, Chen H. 2011. Effects of orange II and sudan III azo dyes and their metabolites on Staphylococcus aureus. J Ind Microbiol Biotechnol 38:1729-1738.
Patterson RM, Butler JS. 1982. Tartrazine-induced chromosomal aberrations in mammalian cells. Food Chem Toxicol 20(4):461-465.
Pérez-Urquiza M, Beltrán JL. 2001. Determination of the dissociation constants of sulfonated azo dyes by capillary zone electrophoresis and spectrophotometry methods. J Chromatogr A 917:331-336.
Peterson FJ, Mason RP, Holtzman JL. 1977. Microsomal azo reduction--role of cytochrome-P-450 and NADPH cytochrome-c reductase. Pharmacologist 19:210. [cited in EFSA 2010].
Phillips JC, Bex CS, Gaunt IF. 1982. The metabolic disposition of 14C-labelled Ponceau 4R in the rat, mouse and guinea-pig. Food Chem Toxicol 20(5):499-505.
Phillips JC, Bex CS, Mendis D, Walters DG, Gaunt IF. 1987. Metabolic disposition of 14C-labelled amaranth in the rat, mouse and guinea-pig. Food Chem Toxicol 25(12):947-954. [cited in EFSA 2010].
Pino A, Maura A, Gardella A. 1996. Induction of micronuclei in bone marrow of male and female rats following oral administration of metanil yellow. Toxic Subst Mech 15(1):43-47.
Pollastrini MT, Barea M, Salas J. 1990. [Genotoxic study of commercial dyes with tartrazine base in S. typhimuriumhis- and E. coli trp-]. Rev Sanid Hig Publica (Madr) 64(3-4):203-209. [in Spanish].
Poul M, Jarry G, Elhkim MO, Poul JM. 2009. Lack of genotoxic effect of food dyes amaranth, sunset yellow and tartrazine and their metabolites in the gut micronucleus assay in mice. Food Chem Toxicol 47(2):443-448.
Prasad O. 1986. Induction of dominant lethals by two nonpermitted food colors metanil yellow and orange II in albino mice. Proc Indian Natl Sci Acad B56(4):343-345.
Prasad O, Rastogi PB. 1982a. Carcinogenic effect of common food colour metanil yellow on albino mice. Nat Acad Sci Lett 5:205-208.
Prasad O, Rastogi PB. 1982b. Orange II induced cytogenetical changes in albino mice. Experientia 38:1240-1242.
Price PJ, Suk WA, Freeman AE, Lane WT, Peters RL, Vernon ML, Huebner RJ. 1978. In vitro and in vivoindications of the carcinogenicity and toxicity of food dyes. Int J Cancer 21(3):361-367.
Pritchard AB, Holmes PA, Kirschman JC. 1976. The fate of FD&C red no. 2 and its metabolite, naphthionic acid, after different routes of administration in the rat. Toxicol Appl Pharmacol 35(1):1-10.
Prival MJ, Davis VM, Peiperl MD, Bell SJ. 1988. Evaluation of azo food dyes for mutagenicity and inhibition of mutagenicity by methods using Salmonella typhimurium. Mutat Res 206(2):247-259.
Radomski JL, Mellinger TJ. 1962. The absorption, fate and excretion in rats of the water-soluble azo dyes, FD&C Red No. 2, FD&C Red No. 4, and FD&C Yellow No. 6. J Pharmacol Exp Ther 136:259-266. [cited in EFSA 2010].
Rafii F, Hall JD, Cerniglia CE. 1997. Mutagenicity of azo dyes used in foods, drugs and cosmetics before and after reduction by Clostridium species from the human intestinal tract. Food Chem Toxicol 35(9):897-901.
Rastogi PB, Levin RE. 1996. Metabolic activation and inactivation of metanil yellow and orange II in the Salmonella typhimurium his- reversion assay. J Food Prot 59(3):244-248.
Rastogi PB, Thilly WG, Shirname-More L. 1991. Long-term low-dose mutation studies in human cells: metanil yellow and orange II. Mutat Res 249(1):265-273.
Rathore VS, Saxena R, John PJ, . 1989. Acute toxicity of textile dyes to Channa punctatus. J Nat Conserv 1(2):155-157.
Raza H, Singh GB, Khanna SK. 1981. Mode of bio degradation of metanil yellow in rats. Annual Meeting and 2nd Congress of the Federation of Asian and Oceanian Biochemists, Bangalore (IN), December 14-18, 1980. Indian J Biochem Biophys 18(4):151.
Razo-Flores E, Luijten M, Donlon B, Lettinga G, Field J. 1997. Biodegradation of selected azo dyes under methanogenic conditions. Water Sci Technol 36(6-7):65-72.
Reckitt Benckiser. 2006. Produt safety data sheet: Vanish Versatile Oxi Action. Available from: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&ved=0CEAQFjAB&url=http%3A%2F%2Foneeuro.hu%2Findex.php%3Foption%3Dcom_rokdownloads%26view%3Dfile%26task%3Ddownload%26id%3D1142%253Apsds-vanishmultigel-2005-10-13-reckitt-benckiser%26Itemid%3D124&ei=2v1eUqzyFPTF4AOzlYCoDw&usg=AFQjCNGSyo6wE1jL5SmGrP8eQKsvTEAk0g&sig2=oMqQjNKhellq5ei6KjFu-Q&bvm=bv.54176721,d.dmg
Reifferscheid G, Heil J. 1996. Validation of the SOS/umu test using test results of 486 chemicals and comparison with the Ames test and carcinogenicity data. Mutat Res 369(3-4):129-145.
Renner HW. 1984. Tartrazine--a reinvestigation by in vivo cytogenetic methods. [letter to journal editor]. Food Chem Toxicol 22(4):327.
Ricca Chemical Company. 2004. Material Safety Data Sheet: Metanil Yellow Stain [Internet]. Arlington (TX): Ricca Chemical Company LLC. Available from: http://www.riccachemical.com/Technical-Support/MSDS/R4810000
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu. 2002. Children's Toys Fact Sheet: To assess the risks for the consumer. RIVM report 612810012/2002. The Netherlands: The National Institute for Public Health and the Environment (Rijksinstituut voor Volksgezondheid en Milieu). Available from: http://www.rivm.nl/bibliotheek/rapporten/612810012.pdf
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment (NL)]. 2006a. Cosmetics fact sheet: To assess the risks for the consumer. Updated version for ConsExpo 4 [Internet]. Bilthoven (NL): RIVM (National Institute for Public Health and the Environment). Report No.: 320104001/2006. Available from: www.rivm.nl/bibliotheek/rapporten/320104001.pdf
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment (NL)]. 2006b. Cleaning products fact sheet: to assess the risks for the consumer [Internet]. Bilthoven (NL): RIVM (National Institute for Public Health and the Environment). Report No: 320104003/2006. Available from: www.rivm.nl/bibliotheek/rapporten/320104003.pdf
Rofe P. 1957. Azo dyes and Heinz bodies. Br J Ind Med 14:275-280.
Rosner E. 1999a. 14-day oral toxicity (gavage) study in the rat. Itingen (CH): RCC Ltd. RCC Report No.: 736288.
Rosner E. 1999b. 14-day oral toxicity (gavage) study in the rat. Itingen (CH): RCC Ltd. RCC Report No.: 746460.
Roxon JJ, Ryan AJ, Welling PG, Wright SE. 1967. Origin of urinary sulfanilic acid from tartrazine. Food Cosmet Toxicol 5(3):447-449. [cited in EFSA 2010].
Ruddick JA, Willes RF, Stavric B, Munro I. 1977. Teratology 15: 31A. [cited in EFSA 2010].
Rufli H,Fisk PR, Girling AE, King JMH, Länge R, Lejeune EX, Stelter N, Stevens C,Suteau P, Tapp J, Thus J, Versteeg DJ, Niessen HJ. 1998. Aquatic toxicity testing of sparingly soluble, volatile, and unstable substances and interpretation and use of data. Ecotoxicol Environ Saf 39:72-77.
Sakuratani Y, Sato S, Nishikawa S, Yamada J, Maekawa A, and Hayashi M. 2008. Category analysis of the substituted anilines studied in a 28-day repeat-dose toxicity test conducted on rats: correlation between toxicity and chemical structure. SAR QSAR Environ Res. 19(7-8):681-96.
Sankaranarayanan N, Murthy MS. 1979. Testing of some permitted food colors for the induction of gene conversion in diploid yeast. Mutat Res 67:309-314.
Santa Cruz. 2009. Material Safety Data Sheet: Brilliant Black BN [Internet]. Santa Cruz (CA): Santa Cruz Biotechnology, Inc. Available from: http://datasheets.scbt.com/sc-214622.pdf
Santa Cruz. 2010. Material Safety Data Sheet: Direct Yellow 50 [Internet]. Santa Cruz (CA): Santa Cruz Biotechnology, Inc. Available from: http://datasheets.scbt.com/sc-214914.pdf
Sasaki YF, Kawaguchi S, Kamaya A, Ohshita M, Kabasawa K, Iwama K, Taniguchi K, Tsuda S. 2002. The comet assay with 8 mouse organs: results with 39 currently used food additives. Mutat Res 519(1-2):103-119.
[SCCNFP] Scientific Committee on Cosmetic Products and Non-Food Products. 2004a. Opinion of the Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers concerning Acid Yellow 23. Colipa no. C29. Report No.: SCCNFP/0786/04. Available from: http://ec.europa.eu/health/archive/ph_risk/committees/sccp/documents/out260_en.pdf
[SCCNFP] Scientific Committee on Cosmetic Products and Non-Food Products. 2004b. Opinion of the Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers concerning Acid Orange 7. Colipa no. C15. Report No.: SCCNFP/0785/04. Available from: http://ec.europa.eu/health/archive/ph_risk/committees/sccp/documents/out259_en.pdf
[SCCP] Scientific Committee on Consumer Products. 2005. Opinion on para-aminophenol. Colipa no. A16. Report No.: SCCP/0867/05. Available from: http://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_00e.pdf
[SCCP] Scientific Committee on Consumer Products. 2006. The SCCP’s Notes of Guidance for the Testing of Cosmetic Ingredients and their Safety Evaluation, 6th Revision.
[SCCS] Scientific Committee on Consumer Safety. 2010. Opinion on Acid Black 1. Colipa no. B15. Report No.: SCCS/1226/09. Available from: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_018.pdf
[SCCS] Scientific Committee on Consumer Safety. 2011a. The SCCS’s notes of guidance for the testing of cosmetic ingredients and their safety evaluation. 7th Revision. [Internet]. Scientific Committee on Consumer Safety. [cited 27 Jul 2012]. Available from: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_s_004.pdf
[SCCS] Scientific Committee on Consumer Safety. 2011b. Opinion on Acid Orange 7. Colipa no. C15. Report No.: SCCS/1382/10. Available from: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_057.pdf
SCCS] Scientific Committee on Consumer Safety. 2014. Revision of the opinion on Acid Orange 7. Colipa no. C15. Report No.: SCCS/1536/14. Available from: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_158.pdf
[SCF] Scientific Committee for Food. 1983. Report of the Scientific Committee for Food on colouring matters authorized for use in foodstuffs intended for human consumption. In: Reports of the Scientific Committee for Food (fourteenth series). Report No.: EUR 8752 EN. Available from: http://ec.europa.eu/food/fs/sc/scf/reports/scf_reports_14.pdf
ScienceLab. 2013. Material Safety Data Sheet: Metanil Yellow [Internet]. Houston (TX): Sciencelab.com, Inc. Available from: http://www.google.ca/url?sa=t&rct=j&q=&esrc=s&frm=1&source=web&cd=1&cad=rja&ved=0CCkQFjAA&url=http%3A%2F%2Fwww.sciencelab.com%2Fmsds.php%3FmsdsId%3D9924629&ei=fltkUo2HL6ea2AWD5oDgAw&usg=AFQjCNGAz7DNPzf9ZBf4GkEVszuETAgHhw&bvm=bv.54934254,d.aWc
Scott B, Moore L. 2000. Assessment of the risks to human health posed by azo colourants in toys, writing inks and paper products, and analysis of the advantages and drawbacks of restrictions on their marketing and use. Final Report. Laboratory of the Government Chemist.
[SDA] Soap and Detergent Association. 2010. Appendix II-A-1: Dermal exposure parameters to estimate screening exposures to consumer products--North America. In: Consumer product ingredient safety: exposure and risk screening methods for consumer product ingredients. 2nd ed. Washington (DC): SDA.
Shaul GM, Holdsworth TJ, Dempsey CR, Dostal KA. 1990. Fate of water soluble azo dyes in the activated sludge process. Chemosphere 22(1-2):1117-1119.
Shimada C, Kano K, Sasaki YF, Sato I, Tsudua S. 2010. Differential colon DNA damage induced by azo food additives between rats and mice. J Toxicol Sci 35(4):547-554.
Singh GB, Khanna SK. 1979. Toxicity studies in rats fed orange II. Indian J Exp Biol 17(10):1100-1102.
Singh RL. 1989. Metabolic disposition of [14C]-metanil yellow in rats. Biochem Int 19(5):1109-1116.
Singh RL, Khanna SK, Singh GB. 1987. Acute and short-term toxicity studies in orange II. Vet Hum Toxicol 29(4):300-304.
Singh RL, Khana SK, Singh GB. 1991. Metabolic disposition of carbon-14-labelled metanil yellow in guinea-pigs. Vet Hum Toxicol 33(3):220-223.
Singh S, Das M, Khanna SK. 1995. Bio-metabolic disposition of metanil yellow, orange II and their blend by caecal microflora of rats. Indian J Exp Biol 33(7):543-544.
Singh S, Das M, Khanna SK. 1997. Comparative azo reductase activity of red azo dyes through caecal and hepatic microsomal fraction in rats. Indian J Exp Biol 35(9):1016-1018.
Smyth SA. 2012. Wastewater 101: for CMP monitoring. Presentation to Science Risk Assement Division, Gatineau (QC), October 16.
Sperry K. 1992. Tattoos and tattooing: Part II: Gross pathology, histopathology, medical complications, and applications. Am J Forensic Med Pathol 13(1):7-17.
Srivastava LP, Khanna SK, Singh GB, Murti CR. 1982. In vitro studies on the biotransformation of metanil yellow. Environ Res 27:185-189.
Stanford Research Institute. 1972. Study of mutagenic effects of FD and C red no. 2 (FDA 71-23). Springfield (VA): National Technical Information Service; 132 p. Report No.: FDABF-GRAS-080. Document No.: PB-221814.
Statistics Canada. 2004. Canadian Community Health Survey - Nutrition (CCHS). Detailed information for 2004 (Cycle 2.2) [Internet]. Ottawa (ON): Statistics Canada. Available from: www.statcan.gc.ca/cgi-bin/imdb/p2SV.pl?Function=getSurvey&SDDS=5049&lang=en&db=imdb&adm=8&dis=2
Statistics Canada. 2012. Canadian Health Measures Survey (CHMS) - Cycle 1. Ottawa (ON): Statistics Canada. Available upon request.
Stingley RL, Zou W, Heinze TM, Chen H, Cerniglia CE. 2010. Metabolism of azo dyes by human skin microbiota. J Med Microbiol 59:108-114.
Sundarrajan M, Fernandis AZ, Subrahmanyam G, Prabhudesai S, Krishnamurthy SC, Rao KVK. 2000. Overexpression of G1/S cyclins and PCNA and their relationship to tyrosine phosphorylation and dephosphorylation during tumor promotion by metanil yellow and malachite green. Toxicol Lett 116(1-2):119-130.
Sundarrajan M, Prabhudesai S, Krishnamurthy SC, Rao KVK. 2001. Effect of metanil yellow and malachite green on DNA synthesis in N-nitrosodiethylamine induced preneoplastic rat livers. Indian J Exp Biol 39(9):845-852.
Sweeney EA, Chipman JK, Forsythe SJ. 1994. Evidence for direct-acting oxidative genotoxicity by reduction products of azo dyes. Environ Health Perspect 102(Suppl 6):119-122.
Tamaro M, Banfi E. 1976. Comparative mutagenicity test of dyes on E. coli Wp2 and S. typhimurium as indicator organisms. Boll Ist Sieroter Milan 55:191-194.
Tanaka T. 1992. Effects of amaranth on F1 generation mice. Toxicol Lett 60(3):315-324.
Tanaka T. 1993. Reproductive and neurobehavioral effects of amaranth administered to mice in drinking water. Toxicol Ind Health 9(6):1027-1035.
Tanaka T. 2006a. Reproductive and neurobehavioural toxicity study of Ponceau 4R administered to mice in the diet. Food Chem Toxicol 44(10):1651-1658.
Tanaka T. 2006b. Reproductive and neurobehavioural toxicity study of tartrazine administered to mice in the diet. Food Chem Toxicol 44(2):179-187.
Tanaka T, Takahashi O, Oishi S, Ogata A. 2008. Effects of tartrazine on exploratory behavior in a three-generation toxicity study in mice. Reprod Toxicol 26(2):156-163.
Tarimci N, Agabeyoglu I, Gönül N, Baykara T. 1987. Investigation of stability of the pharmaceutical colorants exposed to sunlight. FABAD (J Pharm Sci) 12: 289-295. [cited in HSDB 1983- ].
[Therapeutic Guidelines] Therapeutic Guidelines Limited. 2008. Lund and Browder chart for calculating the percentage of total body surface area burnt (Figure 14.19). Published in eTG complete, March 2008. Available upon request.
Tonogai Y, Ito Y, Iwaida M, Tati M, Ose Y, Sato T. 1979. Studies on the toxicity of coal tar dyes. 2. Examination of the biological reaction of coal tar dyes to vital body. J Toxicol Sci 4:211-220.
Tonogai Y, Ogawa S, Ito Y, Iwaida M. 1982. Actual survey on TLm (median tolerance limit) values of environmental pollutants, especially on amines, nitriles, aromatic nitrogen compounds and artificial dyes. J Toxicol Sci 7:193-203.
Tripathy NK, Nabi MJ, Sahu GP, Kumar AA. 1995. Genotoxicity testing of two red dyes in the somatic and germ line cells of Drosophila. Food Chem Toxicol 33(11):923-927.
Tsuda S, Murakami M, Matsusaka N, Kano K, Taniguchi K, Sasaki YF. 2001. DNA damage induced by red food dyes orally administered to pregnant and male mice. Toxicol Sci 61:92-99.
Urakubo G. 1967. Distribution (in experimental animals) and excretion of sulfur-35-labelled Ponceau MX. Shokuhin Eiseigaku Zasshi 8:489-493. [cited in IARC 1975a].
[US EPA] US Environmental Protection Agency. 1994. Generic scenario--fabric finishing. Washington (DC): US EPA, Chemical Engineering Branch.
[US EPA] US Environmental Protection Agency. 1997. Exposure factors handbook. Washington (DC): US EPA, Office of Research and Development, National Center for Environmental Assessment.
[US EPA] US Environmental Protection Agency. 2009. Screening-level hazard characterization--mononitroanilines category: 2-nitrobenzenamine (CASRN 88-74-4), 4-nitrobenzenamine (CASRN 100-01-6) [Internet]. Washington (DC): US Environmental Protection Agency. Available from: www.epa.gov/hpvis/hazchar/Category_Mononitroanilines_Sept2009.pdf
[US EPA] US Environmental Protection Agency. 2012. Standard operating procedures for residential pesticide exposure assessment. Washington (DC): US EPA, Office of Pesticide Programs, Health Effects Division. Available from: www.epa.gov/pesticides/science/EPA-OPP-HED_Residential%20SOPS_Feb2012.pdf
[US FDA] US Food and Drug Administration. 1964. Unpublished report submitted to the World Health Organization by the US Food and Drug Administration. [cited in EFSA 2010].
[US FDA] US Food and Drug Administration. 2010. Food Advisory Committee meeting materials--Interim toxicology review memorandum (certified color additives). [cited 2013 Mar 25]. Available from: www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/FoodAdvisoryCommittee/ucm248008.htm
[US FDA] US Food and Drug Administration. 2011a. Background document for the Food Advisory Committee: certified color additives in food and possible association with attention deficit hyperactivity disorder in children. [cited 2013 Mar 21]. Available from: www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/FoodAdvisoryCommittee/UCM248549.pdf
[US FDA] US Food and Drug Administration. 2011b. Quick minutes: Food Advisory Committee meeting March 30-31, 2011. [cited 2013 Mar 21]. Available from: www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/FoodAdvisoryCommittee/ucm250901.htm
[US FDA] US Food and Drug Administration. 2013. Overview of food ingredients, additives & colors. [cited 2013 Mar 21]. Available from: www.fda.gov/Food/IngredientsPackagingLabeling/FoodAdditivesIngredients/ucm094211.htm
[US NRC] US National Research Council. 1986. Nutrient Adequacy. National Academy Press, pp 16-25.
Vaidya VG, Godbole NN. 1978. Effects of metanil yellow on human leukocyte chromosomes in vitro. Indian J Exp Biol 16:820-821.
Venturini S, Tamaro M. 1979. Mutagenicity of anthraquinone and azo dyes in Ames’ Salmonella typhimurium test. Mutat Res 68(4):307-312.
Vinayak. 2013. Material Safety Data Sheet: Chocolate Brown HT [Internet]. Monmouth (NJ): Vinayak Corporation. Available from: http://www.vinayakcorporation.com/lbrimsdc.htm
Viola M, Nosotti A. 1978. Application of the Ames test on some dyes. Boll Chim Farm 117(7):402-415.
von der Hude W, Behm C, Gürtler R, Basler A. 1988. Evaluation of the SOS chromotest. Mutat Res 203(2):81-94.
Watabe T, Ozawa N, Kobayashi F, Kurata H. 1980. Reduction of sulphonated water-soluble azo dyes by micro-organisms from human faeces. Food Cosmet Toxicol 18(4):349-352.
Weerdesteijn MCH, Bremner HJ, Zeilmaker MJ, van Veen MP. 1999. Hygienic cleaning products used in the kitchen. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute of Public Health and the Environment]. RIVM Report No.: 612810008. Available from: http://rivm.openrepository.com/rivm/bitstream/10029/10194/1/612810008.pdf
Wever J, Muenzner R, Renner HW. 1989. Testing of sunset yellow and orange II for genotoxicity in different laboratory animal species. Environ Mol Mutagen 13(3):271-276.
Whitehouse P, Mallett M. (1993). Aquatic toxicity testing for notification of new substances. An advisory document on dealing with difficult substances. Report to the Chemical Notification Unit, Department of the Environment, WRc plc., Medmenham, Marlow, Buckinghamshire, UK. [cited in Rufli et al. 1998; Cleuvers and Weyers 2003].
Willes RF, Stavric B, Craig JC, Ruddick JA. 1980. Circulating naphthionic acid in nonpregnant and pregnant rats after feeding amaranth. Toxicol Appl Pharmacol 54:276-284.
Willheim R, Ivy AC. 1953. A preliminary study concerning the possibility of dietary carcinogenesis. Gastroenterology 23:1-19. [cited in IARC 1975b].
Wollny HE. 1999a. Salmonella typhimurium and Escherichia coli reverse mutation assay for azo dyes with D&C Orange 4. Rossdorf (DE): RCC Ltd. RCC-CCR Project No.: 636401.
Wollny HE. 1999b. Cell mutatation assay at the thymidine kinase locus (TK +/-) in mouse lymphoma L5178Y cells with D&C Orange 4 (C.I. 15510). Rossdorf (DE): RCC Ltd. RCC-CCR Project No.: 636402.
Wormuth M, Scheringer M, Hungerbühler K. 2005. Linking the use of scented consumer products to consumer exposure to polycyclic musk fragrances. J Ind Ecol 9(1-2):237-258.
Wormuth M, Scheringer M, Vollenweider M, Hungerbühler K. 2006. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Analysis. 26(3):803-824.
Wu X, Bennett DH, Ritz B, Cassady DL, Lee K, Hertz-Picciotto I. 2010. Usage pattern of personal care products in California households. Food Chem Toxicol 48:3109-3119.
Yamada M, Kato Y, Nakamura M, Ito Y. 1995. Mutagenicity study of Sunset Yellow Fcf and its impurity. Foods Food Ingredients J Jpn 164:93-99. [cited in CCRIS 2012].
Yen CC, Perenich TA, Baughman GL. 1991. Fate of commercial disperse dyes in sediments. Environ Toxicol Chem 10:1009-1017.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K. 1988. Salmonella mutagenicity tests: IV. Results from the testing of 300 chemicals. Environ Mol Mutagen 11(Suppl 12):1-157.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K. 1992. Salmonella mutagenicity tests: V. Results from the testing of 311 chemicals. Environ Mol Mutagen 19(Suppl 21):2-141.
Zeilmaker MJ, Kroese ED, van Haperen P, van Veen MP, Bremmer HJ, van Kranen HJ, Wouters MFA, Janus JA. 1999. Cancer risk assessment of azo dyes and aromatic amines from garment and footwear. [Internet]. Bilthoven (NL): Rijkinstituut voor Volkgezondheid en Milieu [National Institute of Public Health and the Environment]. RIVM Report No.: 601503 014. Available from: www.rivm.nl/bibliotheek/rapporten/601503014.html
Zeilmaker MJ, van Kranen HJ, van Veen MP, Janus JA. 2000. Cancer risk assessment of azo dyes and aromatic amines from tattoo bands, folders of paper, toys, bed clothes, watch straps and ink. RIVM report 601503 019. Published: Feb 2000. [Internet]. Rijksinstituut voor Volksgezondheid en Milieu(National Institute of Public Health and the Environment). 45 p. Available from: http://www.rivm.nl/bibliotheek/rapporten/601503019.html
Zhou Y, You X, Ye X. 1987. Mutagenicity of benzidines and their congener derivative dyes. Huanjing Kexue 8(2):31-34.
Zhurkov VS. 1975. Investigation of the mutagenic activity of drug preparations and food additives in a culture of human lymphocytes. Sov Genet 11:528-530.