Screening Assessment
5,12-Naphthacenedione, 10-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, (8S-cis)-
(Doxorubicin)
Chemical Abstracts Service Registry Number
23214-92-8
Environment Canada
Health Canada
February 2015
Table of Contents
- Synopsis
- 1. Introduction
- 2. Substance Identity
- 3. Physical and Chemical Properties
- 4. Sources and Uses
- 5. Releases to the Environment
- 6. Measured Environmental Concentrations
- 7. Environmental Fate
- 8. Potential to Cause Ecological Harm
- 9. Potential to Cause Harm to Human Health
- 10. Proposed Conclusion
- 11. References
Tables
- Table 1-1: Substance identity: doxorubicin
- Table 1-2: Substance identity: doxorubicinol, the major metabolite of doxorubicin..
- Table 2-1: A summary of the physical and chemical properties of the neutral form of doxorubicin
- Table 2-2: A summary of the physical and chemical properties of the metabolite, doxorubicinol
- Table 7-1: Summary of the Level III fugacity modelling (EQC 2003) indicating the percentage of doxorubicin partitioning into each compartment
- Table 7-2: Summary of modelled data for degradation of doxorubicin
- Table 7-3: Summary of modelled data for degradation of doxorubicinol, the major metabolite of doxorubicin
- Table 7-4: Summary of modelled bioaccumulation data for the neutral form of doxorubicin
- Table 7-5: Summary of modelled bioaccumulation data for doxorubicinol, the major metabolite of doxorubicin
- Table 8-1: Summary of empirical aquatic toxicity data from key studies for doxorubicin
- Table 8-2: Summary of modelled data for aquatic toxicity for the metabolite, doxorubicinol
- Table 8-3: Summary of empirical in vitro cytotoxicity data for doxorubicin
- Table 8-4: Summary of input values used for estimating aquatic concentrations resulting from industrial releases of doxorubicin
- Table 8-5: Summary of input values used for estimating aquatic concentrations resulting from use of doxorubicin
Synopsis
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a screening assessment of the substance 5,12-naphthacenedione, 10-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, (8S-cis)-,Chemical Abstracts Service Registry NumberFootnote[1] 23214-92-8. This substance will be referred to by its common name, doxorubicin. Doxorubicin was prioritized for assessment because it had been identified as posing a potential high hazard to human health based on classifications by other national or international agencies for carcinogenicity.
Drugs containing doxorubicin as an ingredient are assessed under the Food and Drugs Act (F&DA) with respect to their safety, effectiveness and quality. This screening assessment focused on uses and exposures that were not covered as part of the F&DA assessment, specifically the risks posed by the residues resulting from manufacture, formulation and disposal after use.
Doxorubicin is an organic substance that occurs naturally in the environment. It is produced by mutating a strain of Streptomyces using N-nitroso-N-methyl urethane into a new strain (S. peucetius var. caesius) that produces the red-coloured compound called doxorubicin. Doxorubicin is registered for use in Canada primarily for cancer therapy.
Information available for this substance indicates that its uses are limited to pharmaceuticals and research. There are several pharmaceutical companies licensed to market doxorubicin in Canada for human consumption or research. Chemical-grade doxorubicin can be purchased from chemical manufacturers. No information was found regarding alternative uses or releases of this substance in Canada. Data were available to estimate that 31 kg, 4.5 kg and 4.3 kg of the substance were sold to hospitals and pharmacies across Canada in 2007, 2011 and 2012, respectively. Although doxorubicin was included in a survey conducted under section 71 of CEPA 1999 to collect information relevant to its manufacture and import in 2009, no responses were received from the Canadian public or industry, which indicates there was no manufacture or import of the substance in that year above the reporting threshold of 100 kg.
Based on its physical and chemical properties (high water solubility, low volatility), doxorubicin is expected to reside predominantly in water, sediment and soil, depending on the compartment of release. Doxorubicin can make its way into surface waters through release from manufacturing or formulation sites and/or releases of the substance in feces or urine from consumers directly using this substance. The main source of ecological exposure to doxorubicin is through surface water. However, no information was available regarding actual releases of this substance in Canada. Based on modelled data, it is concluded that doxorubicin meets the persistence criteria in water, soil and sediment, but does not meet the criterion for air, as set out in the Persistence and Bioaccumulation Regulations of CEPA 1999.
Doxorubicin has low bioaccumulation potential given its physical and chemical properties (i.e., high molecular weight, low octanol–water partition coefficient [log Kow]) and the ability for some aquatic organisms to reduce cellular accumulation of doxorubicin. It is therefore concluded that doxorubicin does not meet the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations. Additionally, doxorubicin has the potential to harm aquatic organisms at moderately low concentrations.
For the ecological assessment, realistic conservative exposure scenarios were selected for the aquatic environment based on expected releases for a site-specific industrial operation and for down-the-drain releases of the substance. The predicted environmental concentrations in water were below the predicted no-effect concentrations calculated for fish, daphnids and algae.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to organisms and the broader integrity of the environment from doxorubicin. It is concluded that doxorubicin does not meet the criteria under paragraph 64(a) or 64(b) of CEPA 1999, as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
In terms of general population exposure, the principal potential source of exposure is drinking water containing the pharmaceutical. The exposure to doxorubicin present in drinking water is significantly smaller than the exposure to doxorubicin through the use of pharmaceuticals.
For this assessment, conservative assumptions were used when estimating the potential indirect exposure of the general population to doxorubicin. Doxorubicin was not detected in wastewater treatment plant influent or effluent at six plants across Canada. In regards to potential general population exposure, upper-bounding estimated intakes from environmental media are low. Based on these low exposures, risks posed by this substance are not expected. To further support this risk characterization, the upper-bounding estimated indirect exposures of the general population were compared with the lowest therapeutic dose identified for the substance. The margins of exposure are large (greater than 100 000).
Based on a comparison of conservative exposure estimates with the lowest therapeutic dose identified for oral use of doxorubicin, the calculated margins of exposure are considered to be adequate to address uncertainties in the database and to be protective of human health.
Based on the adequacy of the margins of exposure, it is concluded that doxorubicin does not meet the criteria under paragraph 64(c) of CEPA 1999, as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Conclusion
It is concluded that doxorubicin does not meet any of the criteria set out in section 64 of CEPA 1999.
1. Introduction
Pursuant to sections 68 and 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999), the Minister of the Environment and the Minister of Health conduct screening assessments of substances to determine whether these substances present or may present a risk to the environment or to human health.
A screening assessment was undertaken on the substance 5,12-naphthacenedione, 10-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, (8S-cis)-, Chemical Abstracts Service Registry Number (CAS RN) 23214-92-8, as it was identified during the categorization of substances on the Domestic Substances List (DSL) as posing a potential high hazard to human health based on classifications by other national or international agencies for carcinogenicity. This substance did not meet the ecological categorization criteria for persistence or bioaccumulation potential, but it met the criteria for inherent toxicity to aquatic organisms.
Screening assessments focus on information critical to determining whether a substance meets the criteria as set out in section 64 of the Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999). Screening assessments examine scientific information and develop conclusions by incorporating a weight of evidence approach and using precaution.Footnote[2]
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposure, including additional information submitted by stakeholders. Relevant data were identified up to March 2013. Empirical data from key studies, as well as some results from models were used to reach conclusions. When available and relevant, information presented in risk and hazard assessments from other jurisdictions was considered. The screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents the most critical studies and lines of evidence pertinent to the conclusion.
Drugs containing doxorubicin as an ingredient were assessed under the Food and Drugs Act (F&DA) (Canada 1985) with respect to their safety, effectiveness and quality. This assessment focused on uses and exposures that were not covered as part of the FDA assessment, specifically the risks posed by the residues resulting from manufacture, formulation and disposal after use.
The screening assessment was prepared by staff in the Existing Substances programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. The ecological and human health portions of this assessment have undergone external written peer review and/or consultation. Comments on the technical portions relevant to the environment were received from Chris Metcalfe, Trent University and Vance Trudeau, University of Ottawa. Comments on the approach used to assess the substance with respect to human health were received from Warren Foster, McMaster University, Sam Kacew, McLaughlin Centre for Population Health Risk Assessment, and Beate Escher, University of Queensland.
Additionally, the draft of this screening assessment was subject to a 60-day public comment period. While comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment Canada.
The critical information and considerations upon which the assessment is based are summarized below.
2. Substance Identity
For the purposes of this assessment, the substance 5,12-naphthacenedione, 10-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, (8S-cis)-, will be referred to by its common name, doxorubicin.
Doxorubicin can be manufactured as chemical-grade doxorubicin (CAS RN 23214-92-8) or as pharmaceutical-grade doxorubicin hydrochloride (CAS RN 25316-40-9). Doxorubicin hydrochloride is available as a pharmaceutical for human use. The presence of hydrochloride is omitted from this assessment, given that its function is predominantly pharmacokinetic and that it is not expected to contribute to the toxicity of or exposure to doxorubicin itself. Both the chemical and pharmaceutical grades of doxorubicin have been used in a variety of human and ecological toxicity studies. For the purpose of this screening assessment, the pharmaceutical and chemical grades of doxorubicin are treated equally and interchangeably.
Doxorubicin is part of the anthracycline class of chemotherapy drugs that are also antibiotics. Anthracyclines have a four-ring nucleus (tetrahydrotetrahydrotetracenedione) that is lipophilic; however, the saturated end of the ring system contains hydroxyl groups adjacent to the amino sugar, producing a hydrophilic centre (see Table 1a). The molecule is amphoteric, containing acidic functions in the ring phenolic group and a basic function in the amino sugar (glycoside) group.
Approximately 3.5–5.7% of doxorubicin is excreted unmetabolized via urine in humans (Mahnik et al. 2006, 2007). The urinary excretion of the major metabolite of doxorubicin, doxorubicinol, is 0.7–4.3% (Mahnik et al. 2006). Although not clearly established, doxorubicinol may be ten times more cytotoxic than doxorubicin (Mahnik et al. 2006). Therefore, this metabolite is evaluated concurrently with doxorubicin in the ecological screening assessment. Substance identity information for doxorubicin and its metabolite is presented in Tables 1-1 and 1-2, respectively.
| CAS RN | 23214-92-8 |
|---|---|
| DSL name | 5,12-Naphthacenedione, 10-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, (8S-cis)- |
| NCI names | Doxorubicine (French) (DSL, EINECS); Doxorubicin (English, German) (EINECS); D5,12-Naphthacenedione, 10-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-, (8S,10S)- (ASIA-PAC, NZIoC) |
| Other namesFootnote Table 1-1[a] | (1S,3S)-3-Glycoloyl-1,2,3,4,6,11-hexahydro-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-1-naphthacenyl-(3-amino-2,3,6-tridesoxy-α-L-lyxo-hexopyranosid); (8S,10S)-10-((3-Amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy)-8-glycoloyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione; 1,2,3,4,6,11-Hexahydro-4β,5,12-trihydroxy-4-(hydroxyacetyl)-10-methoxy-6,11-dioxonaphthacen-1β-yl-3-amino-2,3,6-trideoxy-α-L-lyxohexopyranoside; 10-((3-Amino-2,3,6-trideoxy-D-lyxohexopyranosyl)oxy)-8-glycolcyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione; 14-Hydroxydaunomycin; 14-Hydroxydaunorubicine; ;ADM; Adriablastin; Adriamycin semiquinone; Adriblastin; Adriblastina; CCRIS 739; Doxil; |
| Major chemical class | Anthracycline glycoside antibiotic |
| Chemical formula | C27H29NO11 |
| Chemical structure | 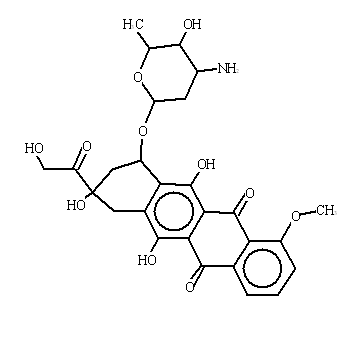 |
| SMILES | COc4cccc5C(=O)c3c(O)c2CC(O)(CC(OC1CC (N)C(O)C(C)O1)c2c(O)c3C(=O)c45)C(=O)CO |
| Molecular mass | 543.53 g/mol |
| CAS RN | 54193-28-1 |
|---|---|
| Chemical structure |  |
| Molecular mass (g/mol) | 545.55 |
| Chemical formula | C27N31NO11 |
| SMILES | c3c(c5c(cc3)C(=O)c4c(C5(=O))c(O)c2c (c4O)CC(C(O)CO)(O)CC2OC1CC(C (C(O1)C)O)N)OC |
Structural analogues having relevant empirical data may be used to help assess those substances that lack empirical data. For this assessment, the Organization for Economic Co-operation and Development (OECD) Quantitative Structure–Activity Relationship (QSAR) Toolbox (OECD QSAR Toolbox 2012) was employed to determine whether potential analogues with measured data for physical and chemical properties, persistence, bioaccumulation and toxicity were available. Some analogues were identified through literature review (e.g., epirubicin and pirarubicin); however, no useful corresponding experimental data were available for read-across purposes.
3. Physical and Chemical Properties
Tables 2-1 and 2-2 provide a summary of experimental and modelled physical and chemical properties of doxorubicin and doxorubicinol, respectively, that are relevant to their environmental fate and ecotoxicity.
The QSAR models used are mainly based on fragment addition methods; that is, they sum the contributions of sub-structural fragments of a molecule to make predictions for a property or endpoint. Most of these models rely on the neutral form of a chemical as input (in simplified molecular input line entry system [SMILES] form). Consequently, except where noted, the modelled values shown in Tables 2-1 and 2-2 are for the neutral forms of doxorubicin and doxorubicinol, respectively.
Doxorubicin is amphoteric, containing acidic functions in the ring phenolic group and a basic function in the amino sugar (glycoside) group. The estimated acid dissociation constants (pKa values) indicate that this substance will also exist in the cationic form at pH 5–9 in the environment. Approximately 50% of the substance is ionized at pH 8.3; at pH 7, about 44% of the substance is ionized (ACD/Percepta ©1997–2012). Therefore, the model predictions for the physical and chemical properties of doxorubicin do not fully represent the properties and environmental behaviour of this substance; however, the neutral form is also relevant based on the pKa values identified in Table 2-1.
| Property | Type | ValueFootnote Table 2-1[a].1 | Temperature (°C) | Reference |
|---|---|---|---|---|
| Physical form | NA | Red-orange crystalline solid | NA | Pfizer Canada Inc. 2010 |
| Melting point (°C) | Modelled | 344.5 | NA | MPBPVPWIN 2008 |
| Melting point (°C) | Experimental | 204–205 | NA | Pfizer Canada Inc. 2010 |
| Melting point (°C) | Experimental | 230 | NA | Lide 2007 |
| Boiling point (°C) | Modelled | 782.4 | NA | MPBPVPWIN 2008 |
| Density (kg/m3) | Experimental | NA | NA | NA |
| Vapour pressure (Pa) | Modelled | 3.37 × 10−21* (2.53 × 10−23 mmHg)Footnote Table 2-1[b] |
25 | MPBPVPWIN 2008 |
| Vapour pressure (Pa) | Modelled | 8.99 × 10−25 (6.74 × 10−27 mmHg)[b] |
25 | Rowney et al. 2009 |
Henry’s Law constant (Pa·m3/mol) |
Modelled (Bond estimate only, as Group estimate incomplete) |
2.26 × 10−18 (2.23 × 10−23 atm·m3/mol)[b] |
NA | HENRYWIN 2008 |
| Log Dow (for pH 5–9) | Modelled | −2.74 to −2.28 | NA | ACD/pKaDB 2005 |
Log Kow (dimensionless) |
Modelled | 1.85 | NA | KOWWIN 2008 |
Log Kow (dimensionless) |
Modelled | 1.27* | NA | Hansch et al. 1995 |
| Log Koc (dimensionless) | Modelled | 1.89 (estimate using log Kow) 3.78 (estimate using MCI) |
NA | KOCWIN 2008 |
| Log Koa (dimensionless) | Modelled | 22.31 | NA | KOAWIN 2008 |
| Water solubility (mg/L) | Modelled | 92.8* | 25 | WSKOWWIN 2008 |
Water solubility (mg/L) |
Modelled | 65.1 | NA | AIEPS 2003-2007 |
| pKa (dimensionless) | Modelled | pKa1 = 7.34 (phenol); pKa2 = 8.46 (amine); pKa3 = 9.46 (estimated) |
NA | SPARC 2008 |
| pKa (dimensionless) | Experimental | 8.22 in N/20 sodium hydroxide solution | NA | Sopherion Therapeutics, Inc. 2006 |
| pKa (dimensionless) | Modelled | 13.81 | NA | ACD/pKaDB 2005 |
| pKa (dimensionless) | Modelled | pKa1 = 9.28–11.21 (phenol) pKa2 = 8.57 (amine) pKa3 = 14.15–15.38 (estimated) |
NA | PALLAS 1994–1995 |
| Property | Type | ValueFootnote Table 2-2[a].2 | Temperature (°C) | Reference |
|---|---|---|---|---|
| Melting point (°C) | Modelled | 349.2 | NA | MPBPVPWIN 2008 |
| Boiling point (°C) | Modelled | 792.5 | NA | MPBPVPWIN 2008 |
| Density (kg/m3) | Modelled | NA | NA | |
| Vapour pressure (Pa) | Modelled | 4.58 × 10−23 | 25 | MPBPVPWIN 2008 |
Henry’s Law constant (Pa·m3/mol) |
Modelled (Bond estimate only, as Group estimate incomplete) |
1.19 × 10−23 (1.17 × 10−28 atm·m3/mol)[a] |
NA | HENRYWIN 2008 |
Log Kow (dimensionless) |
Modelled | 0.91 | NA | KOWWIN 2008 |
Log Koc (dimensionless) |
Modelled | 1.69 (estimate using log Kow) 3.78 (estimate using MCI) |
NA | KOCWIN 2008 |
| Log Koa (dimensionless) | Modelled | 27.2 | NA | KOAWIN 2008 |
Water solubility (mg/L) |
Modelled | 183.4 | 25 | WSKOWWIN 2008 |
4. Sources and Uses
Doxorubicin is an organic substance that occurs naturally in the environment. It is produced by mutating a strain of Streptomyces using N-nitroso-N-methyl urethane into a new strain (S. peucetius var. caesius) that produces the red-coloured compound called doxorubicin (Weiss 1992). Doxorubicin is registered for use in Canada primarily for cancer therapy (DPD 2010).
Although doxorubicin was included in a survey conducted under section 71 of CEPA 1999 to collect information relevant to its manufacture and import for the year 2009, no responses were received above the reporting threshold of 100 kg.
Entry characterization for doxorubicin in Canada consisted of searching for information on sources and releases of the substance in relevant databases to identify the potential for exposure of the general population from all sources, including pharmaceutical use (Canada [1978]; HSDB 1983– ; Household Products Database 1993– ; LNHPD 2008; DPD 2010; EAFUS 2011; NHPID 2011). Based on notifications submitted under the Cosmetic Regulations to Health Canada, doxorubicin is not used in cosmetic products in Canada (2012 email from the Consumer Product Safety Directorate, Health Canada, to the Existing Substance Risk Assessment Bureau, Health Canada; unreferenced). Information available for this substance indicates that its uses are limited to pharmaceuticals and research. Searches for this substance were conducted up to March 2013, and no information was found regarding alternative uses or releases of this substance in Canada. Data were available to estimate that 31 kg of the substance was sold to hospitals and pharmacies across Canada for prescription for the year 2007 (McLaughlin and Belknap 2008). Data were also available to estimate that 4.5 kg and 4.3 kg of doxorubicin were sold to hospitals and pharmacies across Canada for the years 2011 and 2012, respectively (IMS 2013). Doxorubicin may also be used for additional off-label or veterinary uses that are not considered in this assessment. The quantity of the substance being used for these purposes is unknown.
5. Releases to the Environment
Pharmaceuticals can make their way into surface waters through release from manufacturing or formulation sites and release of the un-metabolized substances or their metabolites in feces or urine from consumers directly using these substances. For example, in humans, urinary excretion of doxorubicin is minimal with only 5 % excreted during the first 5 days. After an injection of 1.5 mg/kg of tritium-labelled doxorubicin, approximately 50% of the substance was detected in the feces in 7 days, while the fecal excretion accounted for only 20% in patients with impaired liver function. Doxorubicin is metabolized predominantly by the liver to adriamycinol and several aglycone derivatives. Approximately 50% of the drug excreted in the bile was unchanged and 30 % were metabolites (Janssen Inc. 2011; Novopharm Limited 2008; Hospira Healthcare Corporation 2008; Sopherion Therapeutics, Inc. 2006; Pfizer Canada Inc., 2010). Undetectable or low plasma concentrations (i.e., 0.8-26.2 ng/ml) of the metabolite, doxorubicinol, have been reported following intravenous administration of a single 10- to 50-mg/sq m dose of doxorubicin hydrochloride as a polymer poly-(ethylene glycol (PEG)-stabilized liposomal injection. Additionally, doxorubicin hydrochloride encapsulated in liposomes that have not been PEG-stabilized is metabolized to doxorubicinol (HSDB c1993-2008). With non-encapsulated doxorubicin, more than 20% of the total drug in plasma is present as metabolites 5 minutes after a dose, 70% in 30 minutes, 75% in 4 hours, and 90% in 24 hours (HSDB c1993-2008). Therefore, the use of pharmaceutical products containing doxorubicin may result in the release of doxorubicin and its major metabolite, doxorubicinol, to the environment through various waste streams. The potential for exposure to doxorubicin from direct sources (i.e., releases during manufacture or formulation) is also assessed (see section on “Ecological Exposure Assessment”); however, it should be noted that no information was available regarding actual releases of doxorubicin from manufacturing or formulation of the pharmaceutical.
No data on doxorubicin concentrations in Canadian biosolids or soil were found. Mahnik et al. (2007) assessed the elimination of doxorubicin by activated sludge and found that doxorubicin was 90% eliminated from the liquid phase. Over time, the recovery of doxorubicin ranged between 20% and 40% in the sludge, but only between 6% and 12% in the liquid phase, with the elimination caused by adsorption (Rowney et al. 2009). The ring structure of doxorubicin remains stable when it is degraded (i.e., only primary biodegradation). The application of biosolids containing doxorubicin to agricultural land is therefore a possibility, but it cannot be quantified at the present time.
6. Measured Environmental Concentrations
In Canada, samples collected at six municipal wastewater treatment plants representing typical Canadian treatment systems and geographic variations were analyzed, but no measurable concentrations of doxorubicin were detected (Smyth and Teslic 2013). The Canadian municipal wastewater treatment plant influents/effluents, treated biosolids and landfill leachate samples were obtained during the summer period (July–September 2012). The reporting limit was 37.9–303 ng/L for liquids and 163–1790 ng/g for solids (Smyth and Teslic 2013).
Yin et al. (2010) investigated the concentrations of cytostatic drugs, including doxorubicin and its major metabolite, doxorubicinol, in the wastewater effluent of 21 hospitals in Beijing, China. Yin et al. (2010) chose cytostatic drugs based on their frequent use in hospitals and ease of analysis. However, neither doxorubicin nor its major metabolite was detectable (limit of detection 10 ng/L). The authors attributed the lack of detection either to the low usage of these drugs in the hospitals investigated or the fact that the wastewater from these hospitals was disinfected with chlorine before discharge, resulting in drugs that may be partially or completely transformed and hence concentrations below the limits of detection.
Elsewhere, doxorubicin was measured in the wastewater of the oncologic in-patient treatment ward of a hospital in Vienna, Austria, where concentrations ranged from the detection limit, 0.26 µg/L, to 1.35 µg/L (Lenz et al. 2007; Mahnik et al. 2007). Doxorubicin was not detected (limit of detection 0.1 ng/L) in samples taken from a wastewater treatment plant in Catalonia, Spain (Negreira et al. 2013).
7. Environmental Fate
Level III fugacity modelling (EQC 2003) simulates the distribution of a substance in a hypothetical, evaluative environment known as the “unit world.” The EQC model simulates the environmental distribution of a chemical at a regional scale (i.e., 100 000 km2) and outputs the fraction of the total mass in each compartment from an emission into the unit world and the resulting concentration in each compartment. Environment Canada uses only the mass fraction distribution results for general information on the environmental fate of a substance and generally does not use the compartmental concentration results for the predicted environmental concentration (PEC) in a substance assessment. Some exceptions to this may occur, such as when a wide dispersive release of a substance suggests that regional-scale concentrations are appropriate for the PEC(s).
The mass fraction distribution for the neutral form of doxorubicin is given in Table 3-1 using individual steady-state emissions to air, water and soil. The level III EQC model assumes non-equilibrium conditions between environmental compartments, but equilibrium within compartments. The level III EQC model cannot address the potential for doxorubicin to ionize in the aquatic environment as a salt, which will likely be more soluble than the free acid form (i.e., non-salt form). Nor can the model address the potential for binding to soil components via electrostatic interactions (cation exchange) or binding to clays that are negatively surface charged. Therefore, the model cannot fully simulate the fate distribution of doxorubicin in the environment.
The results in Table 7-1 represent the net effect of chemical partitioning, inter-media transport and loss by both advection (out of the modelled region) and degradation or transformation processes. It should be noted that the modelled vapour pressure for doxorubicin, 3.37 × 10−21 Pa, is not accurately estimated, but the value does suggest that it is negligible. The Level III fugacity model’s reasonable limits for vapour pressure are 10+1 to 10−11 Pa. Therefore, an input value of 3.37 × 10−11 Pa was used to run the model, given the negligible difference between 10−11 and 10−21 Pa (i.e., vapour pressure is not a sensitive model input at less than 10−11 Pa).
| Substance released to: | Air (%) | Water (%) | Soil (%) | Sediment (%) |
|---|---|---|---|---|
| Air (100%) | 0.85 | 29 | 70 | 0.07 |
| Water (100%) | Negligible | 99.8 | Negligible | 0.24 |
| Soil (100%) | Negligible | 27 | 73 | 0.06 |
If released to air, given its vapour pressure of 3.37 × 10−11 Pa, doxorubicin is expected to exist solely in the particulate phase in the ambient atmosphere. Particulate-phase doxorubicin may be removed from the air by wet or dry deposition. However, it is not likely that doxorubicin would be released to air.
If released into water, the neutral form of doxorubicin is expected to be highly soluble in water (estimated water solubility is 92.8 mg/L), but the cationic form may undergo binding to negatively charged substrates (i.e., suspended solids) via electrostatic interactions, which can be as strong as covalent bonds. Doxorubicin will likely exist in the cationic form at pH values of 5–9, given its estimated pKa values; therefore, volatilization from water is not expected to be an important fate process.
If released to soil, doxorubicin is expected to have minimal mobility, based on an estimated organic carbon–water partition coefficient (log Koc) of 1.89–3.78. Estimated pKa values indicate that this compound will exist in the cationic form at pH 5–9 in the environment. Cations generally adsorb more strongly to soils containing organic carbon and clay compared with their neutral counterparts (HSDB c1993-2008). Doxorubicin is not expected to volatilize from dry soil surfaces based on an estimated vapour pressure of 3.37 × 10−21 Pa.
The results of Level III fugacity modelling) suggest that the neutral form of doxorubicin will reside predominantly in water (i.e., neutral form is highly water soluble; see Table 2-1), but that the cationic form of doxorubicin will likely undergo binding to suspended particles in water. Doxorubicin may also reside in soil (as the cationic form at pH 5–9) should the substance be released to that compartment. Doxorubicin is expected to occur in surface waters through the release from manufacturing or formulation sites and/or through the release of the un-metabolized substance or its metabolite in feces or urine from consumers directly using this substance. Given these potential releases, this assessment examined water as the main source of exposure in the ecological environment. The application of biosolids containing doxorubicin to agricultural land is a possibility, but the exposure potential cannot be quantified in the absence of toxicity data and data on concentrations of doxorubicin in soil/biosolids in Canada.
7.1 Environmental Persistence
In order to provide the best possible weight of evidence for determination of the persistence of doxorubicin and doxorubicinol in the environment, both empirical and modelled data were considered. Model estimates for doxorubicin are strictly structure based and not expected to be influenced by chemical speciation. Chemical speciation, however, may affect bioavailability for biodegradation. This is not accounted for in model estimates of biodegradation.
7.1.1 Empirical Data
Biological degradation has not been reported for doxorubicin; however, doxorubicin absorbs light at wavelengths greater than 290 nm and thus may be susceptible to direct photolysis by sunlight (IARC 1987; Mahnik et al. 2006). Nawara et al. (2012) showed that nitrogen-purged 40 µM doxorubicin exhibits photoreactivity in laboratory aqueous solutions at 320 and 420 nm. One photoreactive pathway leads to the formation of 3-methoxysalicyclic acid, a stable degradation product. The other possible pathway is a photoreduction of doxorubicin to form dihydroquinone, which undergoes spontaneous oxidation mediated by dissolved oxygen to recover doxorubicin with the formation of hydrogen peroxide.
Castegnaro et al. (1997) reported the complete degradation of doxorubicin after a 1-hour treatment with sodium hypochlorite (5.25%) and Fenton reagent (30%). Treatment of doxorubicin with hydrogen peroxide ( less than 30%) was inefficient, with 32% of the parent compound remaining after 48 hours.
7.1.2 Modelling Data
Since few environmentally relevant experimental data on the degradation of doxorubicin or doxorubicinol are available, a QSAR-based weight of evidence approach (Environment Canada 2007) was applied using the degradation models shown in Tables 7-2 and 7-3.
Given the ecological importance of the water compartment and the fact that doxorubicin and its major metabolite are expected to be released to this compartment, biodegradation in water was primarily examined. However, some of the models in Tables 7-2 and 7-3 (i.e., AOPWIN for ozone reaction and HYDROWIN) could not provide an estimate for doxorubicin, as chemicals with comparable structures are not contained in their training sets. Thus, the results for these specific models were not considered in the weight of evidence approach.
| Fate process | Model and model basis | Model result and prediction | Extrapolated half-life (days) |
|---|---|---|---|
| Atmospheric oxidation | AOPWIN 2008Footnote Table 7-2[a] | t½ = 0.074 day | less than 2 |
| Ozone reaction | AOPWIN 2008[a] | NAFootnote Table 7-2[b] | NA |
| Hydrolysis | HYDROWIN 2008[a] | NA[b] | NA |
| Primary biodegradation (aerobic) | BIOWIN 2008[a] Sub-model 4: Expert Survey (qualitative results) |
“biodegrades fast” |
less than 182 |
Primary biodegradation (aerobic) |
CATALOGIC 2009 | 1.69 daysFootnote Table 7-2[d] | less than 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2008[c] Sub-model 3: Expert Survey (qualitative results) |
“biodegrades slowly” |
greater than or equal to 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2008[c] Sub-model 5: MITI linear probability |
0.30[e] “biodegrades slowly” |
greater than or equal to 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2008[c] Sub-model 6: MITI non-linear probability |
0.004[e] “biodegrades very slowly” |
greater than or equal to 182 |
| Ultimate biodegradation (aerobic) | TOPKAT 2004 Probability |
0[e] “biodegrades very slowly” |
greater than or equal to 182 |
Ultimate biodegradation (aerobic) |
CATALOGIC 2009 | 6 months 27 daysd | greater than or equal to 182 |
| Fate process | Model and model basis | Model result and prediction | Extrapolated half-life (days) |
|---|---|---|---|
| Atmospheric oxidation | AOPWIN 2008Footnote Table 7-3[a] | t½ = 0.060 day | less than 2 |
| Ozone reaction | AOPWIN 2008[a] | NAFootnote Table 7-3[b] | NA |
| Hydrolysis | HYDROWIN 2008[a] | NA[b] | NA |
| Primary biodegradation (aerobic) | BIOWIN 2008[a] Sub-model 4: Expert Survey (qualitative results) |
“biodegrades fast” |
less than 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2008[c] Sub-model 3: Expert Survey (qualitative results) |
“biodegrades slowly” |
greater than or equal to 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2008[c] Sub-model 5: MITI linear probability |
0.28[d] “biodegrades slowly” |
greater than or equal to 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2008[c] Sub-model 6: MITI non-linear probability |
0.004[d] “biodegrades very slowly” |
greater than or equal to 182 |
For both doxorubicin and doxorubicinol, the ultimate biodegradation models suggest that biodegradation is very slow and that the half-life in water would be greater than or equal to 182 days, whereas the result of the BIOWIN Sub-model 4 (primary survey model) would suggest that these substances have a primary half-life of less than 182 days. The results from the BIOWIN Sub-models 3, 5 and 6 (for both doxorubicin and doxorubicinol) and TOPKAT (for doxorubicin) indicate that these substances may not undergo fast biodegradation. Considering all model results as well as the structural features of doxorubicin, there is a weight of evidence to suggest that the biodegradation half-life of doxorubicin and doxorubicinol is greater than or equal to 182 days in water.Footnote[3]
In air, predicted atmospheric oxidation half-life values of 0.074 day for doxorubicin and 0.060 day for doxorubicinol (see Tables 4a and 4b) demonstrate that these substances are likely to be rapidly oxidized. Doxorubicin is susceptible to direct photolysis in sunlight (Mahnik et al. 2006). With a half-life of 0.074 day via reactions with hydroxyl radicals, doxorubicin is not considered persistent in air. Also, with a half-life of 0.060 day via reactions with hydroxyl radicals, doxorubicinol is not considered persistent in air. Due to the very low proportion of doxorubicin expected to partition to air and the short half-life of the substance in air (0.074 day), it is considered unlikely that doxorubicin would be transported through the atmosphere. Its long-range atmospheric transport potential is considered negligible.
Doxorubicin and doxorubicinol do not contain functional groups expected to undergo hydrolysis in water. However, under acidic conditions, doxorubicin breaks into adriamycione and daunosamine (Mahnik et al. 2006; O’Neil 2006). The only hydrolyzable function detected was amides; however, with the exception of a few halogenated acetamides, most amides hydrolyze to acids extremely slowly at 25°C and at the environmentally relevant pH 7, with half-lives measured in centuries (HSDB c1993-2008). Electronegative groups on carbon or nitrogen greatly accelerate base-catalyzed hydrolysis, but alkyl groups on nitrogen retard both acid- and base-catalyzed processes. The ring structure of doxorubicin also remains stable when it is degraded (i.e., only primary biodegradation), as found in the study of the removal of doxorubicin by activated sludge (Mahnik et al. 2007). Therefore, no neutral hydrolysis is evident.
Using an extrapolation ratio of 1:1:4 for water:soil:sediment biodegradation half-lives (Boethling et al. 1995), the half-life in soil is also greater than or equal to 182 days, and the half-life in sediments is greater than or equal to 365 days. This indicates that doxorubicin and doxorubicinol are expected to be persistent in water, soil and sediment.
Based on empirical and modelled data ,both doxorubicin and doxorubicinol is persistent in water, soil and sediment (half-lives in water and soil greater than or equal to 182 days and half-life in sediment greater than or equal to 365 days), but not in air (half-life greater than or equal to 2 days),
7.2 Potential for Bioaccumulation
Modelled octanol–water partition coefficient (log Kow) values (1.27–1.85) for doxorubicin and modelled pH-dependent octanol–water distribution ratio (log Dow ) values (−2.74 to −2.28) for its ionized fraction show that both the neutral and ionized forms of the substance have a low potential to bioaccumulate in biota (see Tables 2-1 and 2-2). The combination of a log Kow of 1.27 and an octanol–air partition coefficient (log Koa) of 22.3 indicates that doxorubicin will not have the potential to biomagnify in terrestrial food webs, as suggested by Gobas et al. (2003) and Kelly et al. (2007). However, Environment Canada does not consider the use of these partition coefficients in isolation as sufficient evidence to determine bioaccumulation potential, as they cannot account for physiological parameters such as metabolism.
No empirical bioaccumulation values were available for doxorubicin, its analogues or its major metabolite (doxorubicinol). In order to provide the best possible weight of evidence analysis of the bioaccumulation potential of doxorubicin, the physical and chemical properties (e.g., log Kow, solubility), modelled data and empirical metabolism and excretion data were considered to arrive at an overall conclusion.
7.2.1 Metabolism and Excretion
In some aquatic organisms, the uptake of many cytostatic drugs, including doxorubicin, is reduced by the expression of a P-glycoprotein-like activity that provides multi-xenobiotic resistance (MXR). The MXR mechanism is of most concern in organs that have an excretion (liver, kidneys), absorption (intestine) or blood–brain barrier function (Bard and Gadbois 2007). The MXR mechanism provides resistance through over-expression of P-glycoprotein transporters, encoded by the highly conserved MDR gene family. This gene family is found in a broad range of taxa, including bacteria, protozoa, nematodes, insects, fish and humans (Hildebrand et al. 2009). The protective role of the MXR mechanism and the presence of the P-glycoprotein family of transporters have been demonstrated in more than 40 aquatic species, such as the killifish (Fundulus heteroclitus), rainbow trout (Oncorhynchus mykiss), flounder (Pleuronectes americanus), turbot (Scophthalmus maximus), zebrafish (Danio rerio) and other marine and freshwater bivalves (Bard 2000; Zaja et al. 2008).
Through the maintenance and regulation of defence mechanisms, it is likely that there are energetic costs imposed on organisms under chronic exposure to xenobiotics. In addition, the exposure of cells to the selection pressures of cytotoxic substances can lead to the induction and over-expression of these energy-consuming efflux transporters (Hildebrand et al. 2009). The effect of energy consumption by cellular defence mechanisms is not well understood, but it is suggested that these systems have significant energetic or metabolic costs and may be disadvantageous to other processes, such as growth and reproduction, through decreases in energy allocations. Hildebrand et al. (2009) used an in vitro rainbow trout hepatocyte cell line to investigate the energetic costs associated with xenobiotic efflux pumps for doxorubicin. Energetic costs were measured through changes in cellular adenylate nucleotide and inorganic phosphate concentrations. In the Hildebrand et al. (2009) study, for all doxorubicin concentrations (5–125 μM), cell accumulation was linear for 10–15 minutes, after which it levelled off, and steady state was assumed. Cells were then placed in a doxorubicin-free medium; initial efflux was characterized by a rapid decline in intracellular concentrations, followed by a slower elimination phase. The rates of doxorubicin efflux were calculated as 3.57– 39.17 μg/min per 106 cells for a 5 μM treatment concentration. The results from the Hildebrand et al. (2009) study suggest that there may be significant energetic costs associated with the active transport activity of P-glycoprotein. However, it is unknown whether these costs are substantial in terms of the overall energy budget of the organism. The metabolic competency of an organism can be related to body weight and temperature. The lipid content of fish differs from the lipid content of humans, and the temperature of Canadian waters is on average lower than normal room temperature. Although phase I and phase II metabolic activity may be significantly lower in fish than in humans, elimination processes in humans are rapid enough to suggest that the bioaccumulation potential in aquatic species would be low. Albertus and Laine (2001) observed that the uptake of doxorubicin (50 µg/mL) in killifish hepatocytes was limited, with no significant increase in doxorubicin accumulation over 20–80 minutes. In the presence of 10 μmol/L verapamil (a competitive substrate or inhibitor of the MXR mechanism ) doxorubicin was time-dependently accumulated in killifish hepatocytes.
Doxorubicin hydrochloride was administered to the rotifer, Philodina acuticornis odiosa Milne, at a single concentration of 100 µM (54.3 mg/L) for a period of 24 hours (Poeggeler et al. 2005). At this concentration, doxorubicin accumulated 100-fold in the rotifer mitochondria, yielding organelle concentrations of 10 mM (5.4 g/L).
7.2.2 Estimating bioconcentration factor (BCF) and bioconcentration factor (BAF)
Since few experimental data on bioaccumulation factors (BAFs) or bioconcentration factors (BCFs) for either doxorubicin or doxorubicinol were available, a predictive approach was applied using available BAF and BCF models, as shown in Tables 7-4 and 7-5.
Measures of BAF are the preferred metric for assessing bioaccumulation potential of substances. This is because BCF may not adequately account for the bioaccumulation potential of substances via the diet, which predominates for substances with log Kow greater than ~4.0 (Arnot and Gobas 2003). Kinetic mass balance modelling is in principle considered to be the most reliable prediction method for determining bioaccumulation potential because it allows for metabolism correction as long as the log Kow of the substance is within the log Kow domain of the model.
BCF and BAF estimates, corrected for potential biotransformation, were generated using the BCFBAF model (EPIsuite 2008). Metabolic rate constants (kM) were derived using structure–activity relationships described further in Arnot et al. (2008a, b, 2009). The middle trophic level fish was used to represent overall model output and is most representative of the fish weight likely to be consumed by an avian or terrestrial piscivore. The median kM is greater than 25.0/day. The results of the BCF and BAF modelling are given in Tables 7-4 and 7-5.
| kM (/day) | Test organism | Model and model basis | Endpoint | Value (L/kg wet weight) | Reference |
|---|---|---|---|---|---|
| 100Footnote Table 7-4[a] | Fish | BCFBAF Sub-model 1 (linear regression) |
BCF | 0.462 | BCFBAF 2008 |
| 100[a] | Fish | BCFBAF Sub-model 2 (mass balance) |
BCFFootnote Table 7-4[b] | 1.36 | BCFBAF 2008 |
| 0.17 | Fish | BCFmax with mitigating factors | BCFFootnote Table 7-4[c] | 4.26Footnote Table 7-4[d] | CPOPs 2008 |
| 100[a] | Fish | BCFBAF Sub-model 3 (Gobas mass balance) |
BAF[b] | 1.36 | BCFBAF 2008 |
| kM (/day) | Test organism | Model and model basis | Endpoint | Value (L/kg wet weight) | Reference |
|---|---|---|---|---|---|
| 125Footnote Table 7-5[a] | Fish | BCFBAF Sub-model 1 (linear regression) |
BCF | 1.1 | BCFBAF 2008 |
| 125[a] | Fish | BCFBAF Sub-model 2 (mass balance) |
BCFFootnote Table 7-5[b] | 3.16 | BCFBAF 2008 |
| 125[a] | Fish | BCFBAF Sub-model 3 (Gobas mass balance) |
BAF[b] | 1.1 | BCFBAF 2008 |
The BCFBAF (2010) model flagged that the predicted metabolic rate constant (i.e., 100–125/day for a 10 g fish) exceeds the theoretical whole-body maximum value, suggesting that doxorubicin and doxorubicinol may be readily metabolized in fish. In addition, at a log Kow of 1.27, the BAF value suggests insignificant dietary uptake. Metabolism is also less important, as the main loss process is gill exchange (i.e., BAF = BCF). The model predictions for bioaccumulation were considered acceptable as an indication of fast metabolism, although there is some uncertainty (i.e., error would suggest that the kM will not be slow, with potential for a false negative).
Based on three-dimensional analysis of conformers calculated using the BCFmax model with mitigating factors (Dimitrov et al. 2005), the maximum diameters (Dmax) of doxorubicin range from 1.51 to 1.89 nm. This suggests that doxorubicin may likely experience restricted uptake from steric effects at the gill surface. Information regarding molecular size and cross-sectional diameters is useful to consider and is commonly used by international jurisdictions such as the European Union (ECHA 2008) as weight of evidence for bioaccumulation potential. Recent investigations relating fish BCF data to molecular size parameters (Dimitrov et al. 2002, 2005) suggest that the probability of a molecule crossing cell membranes as a result of passive diffusion declines significantly with increasing Dmax. The probability of passive diffusion decreases appreciably when the Dmax is greater than ~1.5 nm and much more so for molecules having a Dmax of greater than 1.7 nm. Sakuratani et al. (2008) also investigated the effect of cross-sectional diameter on passive diffusion in a BCF test set of about 1200 new and existing chemicals. They observed that substances that do not have a very high bioconcentration potential (BCF less than 5000) often have a Dmax of greater than 2.0 nm and an effective diameter (Deff) of greater than 1.1 nm. However, as Arnot et al. (2010) noted, there are uncertainties associated with the thresholds proposed by Dimitrov et al. (2002, 2005) and Sakuratani et al. (2008), since the BCF studies used to derive them were not critically evaluated. Arnot et al. (2010) pointed out that molecular size influences solubility and diffusivity in water and organic phases (membranes), and larger molecules may have slower uptake rates. However, these same kinetic constraints apply to diffusive routes of chemical elimination (i.e., slow in = slow out). Thus, significant bioaccumulation potential may remain for substances that are subject to slow absorption processes, if they are slowly biotransformed or slowly eliminated by other processes. However, if the rate of gill uptake is sufficiently mitigated by steric hindrance to the point where the rate of elimination exceeds uptake, bioconcentration will be lowered.
The available evidence indicates that doxorubicin and doxorubicinol are expected to have low bioaccumulation potential due to their physical and chemical properties (i.e., high molecular weight, low log Kow and log Dow ), relatively large cross-sectional diameter, resulting in restricted uptake from steric effects at the gill surface, and the presence of the MXR efflux mechanism, by which aquatic organisms can prevent the entry and accumulation of doxorubicin within the cells. Metabolism-corrected BCF and BAF values are also less than 5000, depending on the rate of metabolism. Therefore, based on the available data and kinetic-based modelled values corrected for metabolism, doxorubicin and doxorubicinol are not bioaccumulative
8. Potential to Cause Ecological Harm
8.1 Ecological Effects Assessment
Anthracycline substances, including doxorubicin, are “difficult to model”; the physical and chemical properties of many of the structural classes of anthracyclines are not amenable to model prediction because they are considered “out of the model domain of applicability” (e.g., structural and water solubility domains) for ecotoxicity models. Therefore, in order to provide the best possible weight of evidence for assessing the ecological effects of doxorubicin, only empirical data were considered. In the absence of empirical data for the metabolite doxorubicinol, modelling data were considered.
8.1.1 Mode of Action
Doxorubicin has the potential to cause cell death in non-human organisms, as it interferes with the function of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) and can act directly on the cell membrane. The cytotoxic effect of doxorubicin may result from a complex system of multiple modes of action related to free radical formation secondary to metabolic activation of the doxorubicin by electron reduction, intercalation of doxorubicin into DNA, induction of DNA breaks and chromosomal aberrations, and alterations in cell membranes (Bonnet et al. 2003). Doxorubicin may inhibit protein synthesis and may be active throughout the cell cycle, including the interphase (HSDB c1993-2008). Doxorubicin can also undergo enzymatic one- and two-electron reduction to the corresponding semiquinone and dihydroquinone. 7-Deoxyaglycones are formed enzymatically by one-electron reduction, and the resulting semiquinone free radical reacts with oxygen to produce the hydroxyl radical in a cascade of reactions. This radical may lead to cell death by reacting with DNA, RNA, cell membranes and proteins (HSDB c1993-2008).
8.1.2 Empirical Aquatic Toxicity Data
Zounková et al. (2007) investigated the toxicity of doxorubicin to green algae, bacteria, yeast and Daphnia magna. A 96-hour algal (Pseudokirchneriella subcapitata) growth inhibition test resulted in a no-observed-effect concentration (NOEC) of 1 mg/L, a lowest-observed-effect concentration (LOEC) of 10 mg/L and a median effective concentration (EC50) of 13 mg/L. A 16-hour Pseudomonas putida growth inhibition test resulted in a NOEC of 1 mg/L, a LOEC of 10 mg/L and an EC50 greater than 1000 mg/L. A 48-hour Daphnia magna acute immobilization test resulted in a NOEC of 0.01 mg/L, a LOEC of 0.1 mg/L and an EC50 of 2.0 mg/L.
Poeggeler et al. (2005) administered doxorubicin hydrochloride at a single concentration of 100 µM (54.3 mg/L) for a period of 24 hours to the rotifer, Philodina acuticornis odiosa Milne. The concentration of 100 µM was lethal, with only 1.0 ± 0.5% survival.
Bonnet et al. (2003) assessed the toxicity of doxorubicin to Tetrahymena pyriformis, a freshwater ciliated protozoan. This ciliate is characterized by a short generation time--3 hours--enabling effects to be studied over several generations. Bonnet et al. (2003) assessed population growth impairment and non-specific esterase activities.Footnote[4] The median inhibitory concentration (IC50) for population growth was 44.8 mg/L, with an average IC50 of 43.3 mg/L over a period corresponding to approximately three generations of the control population. The IC50 for the effect of doxorubicin on non-specific esterase activities was 25.9 mg/L.
Belyaeva et al. (2009) described toxicity to zebrafish (Danio rerio) embryos exposed to doxorubicin. The authors compared normally developing zebrafish embryos with zebrafish embryos exposed to concentrations of doxorubicin ranging from 0.08 to 2.0 mg/L. The concentration of 0.08 mg/L caused tail flexure; 0.11 mg/L caused tail flexure, head and cardiac edema; and 0.2 mg/L caused tail flexure, strong cardiac and yolk sac edema, and impaired locomotor activity. The authors also noted that the increase in doxorubicin concentration was accompanied by an increase in the number of abnormalities in the developing zebrafish embryos.
Krysanov and Demidova (2012) determined that a doxorubicin concentration of 10 µg/L had no significant effect on the rate of embryo hatching for zebrafish (Danio rerio). The authors noted that there was no increase in the percentage of embryos with abnormal development compared with the controls.The aquatic toxicity studies on doxorubicin are summarized in Table8-1.
| Test organism | Type of test | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|
| Pseudokirchneriella subcapitata (green alga) | Acute (96 h) | EC50 | 13 (12–17) |
Zounková et al. 2007 |
| Pseudokirchneriella subcapitata (green alga) | Acute (96 h) | NOEC | 1 | Zounková et al. 2007 |
| Pseudokirchneriella subcapitata (green alga) | Acute (96 h) | LOEC | 10 | Zounková et al. 2007 |
| Daphnia magna (water flea) | Acute (48 h) | EC50 | (0.52–4.8) |
Zounková et al. 2007 |
| Daphnia magna (water flea) | Acute | NOEC | 0.01 | Zounková et al. 2007 |
| Daphnia magna (water flea) | Acute (48 h) | LOEC | 0.1 | Zounková et al. 2007 |
| Pseudomonas putida (soil bacterium) | Acute (16 h) | EC50 | greater than 1000 | Zounková et al. 2007 |
| Pseudomonas putida (soil bacteria) | Acute | NOEC | 1 | Zounková et al. 2007 |
| Pseudomonas putida (soil bacterium) | Acute (16 h) | LOEC | 10 | Zounková et al. 2007 |
| Philodina acuticornis odiosa Milne (rotifer, zooplankton) | Acute (24 h) | 99% lethality (single concentration) | 54.3 (100 μM) |
Poeggeler et al. 2005 |
| Danio rerio (zebrafish) | Acute (96 h) | Embryotoxicity LC50 | less than 2 | Belyaeva et al. 2009 |
| Tetrahymena pyriformis (ciliated protozoan) | Chronic (24 h – 3 generations) | IC50 (population growth) | 44.8 | Bonnet et al. 2003 |
| Tetrahymena pyriformis (ciliated protozoan) | Chronic (24 hours – 3 generations) | IC50 (non-specific esterase activities) | 25.9 | Bonnet et al. 2003 |
The modelled data for doxorubicinol (see Table 8-2) suggest that doxorubicinol is not expected to cause acute harm to aquatic organisms at low concentrations (acute LC50s are greater than or equal to 1.0 mg/L).
| Test organism | Type of test | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|
| Alga | Acute (96 h) | EC50 | 10.5 | EPISuite 2008 |
| Daphnia magna (water flea) | Acute (48 h) | EC50 | 35 | EPISuite 2008 |
| Fish | Acute (96 h) | LC50 | 434.6Footnote Table 8-2[a] | EPISuite 2008 |
8.1.3 Other Ecological Effects: Cytotoxicity and Genotoxicity
Fish cell lines from different species and different organs may serve as models for correlation between in vitro and in vivo tests. Caminada et al. (2006) investigated the cytotoxicity of doxorubicin by applying two assays widely used for investigations of cytotoxicity in many fish cell lines. The 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay is based on the uptake of thiazolyl blue tetrazolium bromide and its following reduction in the mitochondria of living cells to MTT formazan, while dead cells are completely negative in this cleavage activity. The neutral red (NR) assay is based on the uptake and accumulation of neutral red in the lysosomes of living cells. Damaged cells have altered uptakes, and dead cells are not able to retain the dye. In this study, the fish cell lines used were the fish hepatoma cell line (PLHC-1) and the rainbow trout gonadal cell line (RTG-2). Cytotoxicity for doxorubicin was observed at an EC50 of 1.14 mg/L (0.002 60 mM) in the fish hepatoma cell line for the MTT. In the rainbow trout gonadal cell line, cytotoxicity was observed at an EC50 of 2.56 mg/L (0.004 70 mM) for the MTT assay. The results of this study are summarized in Table 8-3.
Lehmann et al. (2003) studied the genotoxic effects of doxorubicin using the wing somatic mutation and recombination assay (SMART) in Drosophila melanogaster. The SMART assay using D. melanogaster was developed to detect the loss of heterozygosity of suitable gene markers that have detectable phenotypes expressed on the wings and can quantitatively determine the recombinogenic and mutagenic potential of chemical and physical agents. Using the standard version of the SMART assay, the authors estimated the quantitative and qualitative genotoxic effects by comparing the wing spot frequencies in marker and balancer heterozygous recombination, which is the major event responsible for genetic toxicity. The flies were allowed to lay eggs during an 8-hour period; 72 hours after the end of the egg-laying stage, larvae were collected and distributed into containers with 5 mL of test solutions at four different doxorubicin concentrations: 0 mM, 0.25 mM (135.9 mg/L), 0.5 mM (271.8 mg/L), 1.0 mM (543.5 mg/L) and 2.5 mM (1.36 g/L). The larvae fed on this medium until the end of their development (chronic feeding). The hatching flies were collected and stored in 70% ethanol. In the mwhflr3 genotype, doxorubicin showed significant increases (95% recombination) in all spot categories analyzed, indicating that doxorubicin is capable of damaging the DNA of D. melanogaster. De Rezende et al. (2011) showed that recombination was the major effect of doxorubicin on D. melanogaster. Doxorubicin at 0.2 mM (108.7 mg/L) statistically increased all categories of spots compared with the control with the standard cross and the high bioactivation cross of D. melanogaster.
| Test organism | Type of test | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|
| Fish (Poeciliopsis lucida) hepatoma cell line PLHC-1 | MTT assay (24 h) | EC50 (cell death) |
1.14 | Caminada et al. (2006) |
| Fish (Poeciliopsis lucida) hepatoma cell line PLHC-1 | NR assay (24 h) | EC50 (cell death) |
1.18 (0.002 17 mM) |
Caminada et al. (2006) |
| Fish (Oncorhynchus mykiss) hepatoma cell line PLHC-1 | MTT assay (24 h) | EC50 (cell death) |
2.56 (0.004 70 mM) |
Caminada et al. (2006) |
Fick et al. (2010) calculated the critical environmental concentration (CEC)--that is, the surface water concentration expected to cause a pharmacological effect in fish. The CEC is based on literature data on human potencies together with a predicted BCF in fish based on lipophilicity. Fick et al. (2010) proposed that CECs could be used as preliminary indicators of a drug’s potential to cause adverse pharmacological effects at specific concentrations in water. In Fick et al. (2010), the estimated log Kow was based on the neutral form of the substance. These values were also used for substances that would be dissociated at physiological pH valeus. The lowest value for human therapeutic plasma concentrations (HTPC) were used to get a conservative estimate of the risk. Theoretical plasma bioconcentration factors (Pblood:water) were estimated for each substance from the following equation: log Pblood:water = 0.73 × log Kow − 0.88, and the CECs were calculated from the equation: CEC = HTPC/(CR × Pblood:water) for each pharmaceutical using a concentration ratio of 1. This approach takes both the estimated potency (as described by the HTPC) and the physical and chemical properties (as described by the log Kow) of each substance into account. The calculated CEC for doxorubicin (i.e., the surface water concentration expected to cause a pharmacological effect in fish) was 2031 ng/L (0.002 031 mg/L).
8.1.4 Derivation of the PNEC
A conservative predicted no-effect concentration (PNEC) was derived from the critical toxicity value (CTV) as described by the acute EC50 toxicity value of 2 mg/L (as the most valid experimental value from a concentration–response curve) for Daphnia magna. The CTV (2 mg/L) was divided by an assessment factor of 500 to account for uncertainties and possible long-term effects resulting from exposure to reactive compounds, as follows: a factor of 100 was applied to account for uncertainty related to interspecies and intraspecies variability in sensitivity, extrapolation from acute to chronic effects and extrapolation from laboratory conditions to the field. A supplementary factor of 5 was applied to account for the possible effects related to cytotoxicity. These effects would not be recorded using standard short-term laboratory tests because these tests are not designed to observe cellular or gene-level interactions. Possible carcinogenic, mutagenic or hormonal effects from reactive substances may not be observed in the lifetime of the organism, but “molecular initiating events” may commence quite rapidly upon permeation of the cell by a reactive compound (i.e., a cancer treatment drug), disrupting cellular processes nonetheless. Therefore, additional precaution is warranted to account for this non-quantifiable source of uncertainty, which could result in non-predictable excess toxicity in other species.
Accounting for these uncertainties, the PNEC was calculated to be 0.004 mg/L
8.2 Ecological Exposure Assessment
No data on the concentrations of doxorubicin in water in Canada have been identified. PECs have been estimated from available information, including estimated substance quantities, estimated release rates and characteristics of the receiving environment. PECs have been estimated for an industrial release scenario and a down-the-drain release scenario, as described in the following subsections.
8.2.1 Industrial Release
Exposure to doxorubicin is expected if the substance is released during industrial manufacturing and processing to a wastewater system that discharges its effluent to a receiving surface water body. The concentration of the substance in the receiving water near the discharge point of the wastewater system is used as the PEC in evaluating the aquatic risk of the substance. It can be calculated using the equation:
PECaq = (1000 × Q × L) × (1 − R) / (N × F × D)
where:
- PEC aq:
- Aquatic concentration resulting from industrial releases (
- Q:
- Total substance quantity produced annually at an industrial site (kg/year)
- L:
- Loss to wastewater (fraction)
- R:
- Wastewater treatment plant removal rate (fraction)
- N:
- Number of annual release days (days/year)
- F:
- Wastewater treatment plant effluent flow (m 3/day)
- D:
- Receiving water dilution factor (dimensionless)
As doxorubicin may be manufactured and/or processed by industrial facilities in Canada (CAREX Canada 2010; DPD 2010) and subsequently may be released to Canadian surface waters, an aquatic site-specific industrial release scenario was developed with realistic conservative assumptions. Table 8-4 presents the inputs used to estimate resulting aquatic concentrations close to a generic industrial point of discharge, which is assumed to be located in Mississauga, an area known to have numerous pharmaceutical manufacturing plants. Based on these assumptions, this scenario yields a PEC of 0.000 002 2 mg/L (Environment Canada 2011a). This PEC value represents the level of exposure in the receiving water near the point of discharge from the wastewater treatment system at the site.
| Input | Value | Justification and reference |
|---|---|---|
| Q: Quantity (kg/year) | 31 | McLaughlin and Belknap 2008; IMS 2013 Estimated quantity as prescribed at hospitals and pharmacies across Canada for the year 2007 as the most conservative quantity in comparison with the years 2011 and 2012 |
| L: Loss to wastewater (%) | 0.5 | Health Canada (pers. comm.)Footnote[a] |
| R: Wastewater system removal efficiency (%) | 1.9 | EPI Suite 2008 |
| N: Number of annual release days (days/year) | 21 | Assumed to be manufactured or processed in small batches over 1 month, due to the assumption of the low substance quantity manufactured or processed per industrial site |
| F: Wastewater system effluent flow (m3/day) | 332 624 | Effluent flow of a large wastewater treatment plant located in Mississauga (a typical Canadian pharmaceuticals manufacturing site, assumed to be located in Mississauga) |
| D: Receiving water dilution factor (dimensionless) | 10 | Environment Canada’s default assumption for large lakes, the WWTP in the scenario discharges to Lake Ontario |
8.2.2 Down-the-Drain Releases from Pharmaceutical Use
As doxorubicin is used in pharmaceutical products and can be released to water, an aquatic exposure scenario resulting from down-the-drain releases from pharmaceutical use was developed. The scenario estimates the concentration of doxorubicin in multiple water bodies receiving wastewater treatment system effluents where pharmaceutical products that contain doxorubicin may have been released (Environment Canada 2009). This scenario provides estimates for approximately 1000 release sites across Canada.
Table 8-5 presents a summary of the inputs used to estimate aquatic concentrations resulting from the use of pharmaceutical products containing doxorubicin. The approach and equations used to calculate the PECs are described in Environment Canada (2011b).
The total mass of doxorubicin used in Canada was assumed to be evenly distributed across the country. Releases may result from the excretion of un-metabolized or unchanged doxorubicin by patients in feces and urine. Mahnik et al. (2006, 2007) measured doxorubicin in the Vienna University Hospital (Austria) wastewater effluent at concentrations ranging from 0.26 to 5 µg/L. Although, Lenz et al. (2007) calculated that 0.1–0.2% of the administered amount of doxorubicin would be in the wastewater, the authorsdid not find doxorubicin in a Vienna (Austria) hospital wastewater effluent above the detection limit of 0.05 µg/L. The authors suggest that as the presence of suspended solids in the storage tanks and biomass production could not be inhibited, elimination of doxorubicin during the storage period may be reason why the substance was not found above the detection limit. Rowney et al. (2009) suggested that doxorubicin is excreted, unchanged, in urine at 6–45%. Therefore, the loss to wastewater of unmetabolized or unchanged doxorubicin was assumed to range between 3.5% and 45% (Mahnik et al. 2007, Rowney et al. (2009) (see Table 8-5). In addition, in light of the uncertainty relating to the environmental stability of the major metabolite of doxorubicin, a conservative environmental concentration value was also obtained by considering metabolism in the derivation of the PECs.
The number of annual release days was assumed to be 365 to account for the variable use of the drug throughout the year as well as the variability between locations (i.e., hospitals where the drug is administered).
The PECs of doxorubicin in receiving water bodies were estimated to range between 0.000 001 6 and 0.000 046 mg/L. These PEC values are maximum estimates for 1000 sites and are based on 10% flows for all watercourses covered in the scenario.
| Input | Value(s) | Justification and reference |
|---|---|---|
| Quantity (kg/year) | 31 | McLaughlin and Belknap 2008. IMS 2013 Estimated quantity as sold to hospitals and pharmacies across Canada for the year 2007 as the most conservative quantity in comparison with the years 2011 and 2012 |
| Loss to wastewater (%) | 1) 3.5–45% | 1. Assumes some uptake or metabolism of the substance within human body (Mahnik et al. 2007; Rowney et al. 2009) |
| Loss to wastewater (%) | 2) 100% (assumes no metabolism) | 2. Assumes no metabolism in light of the uncertainty relating to the environmental stability of doxorubicinol, the major metabolite of doxorubicin |
| Variability factorFootnote Table 8-5[a] | 2 | Default |
| Wastewater system removal efficiency (%) | 1.9 | EPIsuite 2008 |
| Number of annual release days (days/year) | 365 | Number of annual release days |
| Receiving water dilution factor (dimensionless) | 1–10 | Environment Canada Existing Substances default assumption |
8.3 Characterization of Ecological Risk
The approach taken in this ecological screening assessment was to examine various supporting information and develop conclusions based on a weight of evidence approach and using precaution, as required under CEPA 1999. Lines of evidence considered include results from risk quotient calculations as well as information on persistence, bioaccumulation, inherent or ecological toxicity, sources and fate of the substance, and presence and distribution in the environment.
Doxorubicin can make its way into surface waters through release from manufacturing or formulation sites and/or release of the un-metabolized substance or its metabolites in feces or urine from consumers directly using doxorubicin.
The loss to wastewater of unmetabolizedun-metabolized/unchanged doxorubicin was assumed to range between 3.5% and 45% (Mahnik et al. 2007; Rowney et al. 2009). Therefore, the use of pharmaceutical products containing doxorubicin may result in the release of doxorubicin and its major metabolite, doxorubicinol, to the environment through various waste streams. The available information on the use of doxorubicin as a pharmaceutical product in Canada indicates a potential for a dispersive release into the Canadian environment. Doxorubicin is expected to be persistent in water, soil and sediment, but is expected to have a low bioaccumulation potential. Once released into the environment, doxorubicin may be found mainly in water and soil. The application of sewage sludgebiosolids containing doxorubicin to agricultural land is a possibility, but it cannot be quantified in the absence of toxicity data and data on concentrations of doxorubicin in soil/sewage sludgebiosolids in Canada. Therefore, given the potential releases through prescribed use and industrial manufacture/formulation, this assessment examined water as the main source of exposure in the ecological environment.
Empirical evidence indicates that doxorubicin can cause harm to aquatic organisms at moderately low concentrations. Some of the evidence of potential ecological harm caused by doxorubicin relates to endpoints such as cytotoxicity (i.e., fish cell lines) and genotoxicity (i.e., fruit flies). These effects are part of the weight of evidence indicating that doxorubicin has the potential to be hazardous to organisms. There is uncertainty in how these effects translate to long-term effects on whole organisms and wildlife populations in the environment. These effects are part of the weight of evidence considered in the development of the PNEC, reflected in the increase of the assessment factor to 500. Modelling results indicate that doxorubicinol, the major metabolite, may not cause harm to aquatic organisms (acute LC50s are greater than or equal to 1.0 mg/L).
A risk quotient analysis, integrating a realistic conservative exposure with toxicity information, was performed for the aquatic medium to determine whether there is potential for ecological harm in Canada. The site-specific industrial scenario (considering the actual receiving water body) presented above yielded a PEC of 0.000 002 2 mg/L (Environment Canada 2011a). A PNEC was derived from the EC50 of 2.0 mg/L (as the most sensitive valid experimental value) for the aquatic invertebrate Daphnia magna, by dividing this value by an assessment factor of 500 (to account for interspecies and intraspecies variability in sensitivity, to estimate a long-term no-effects concentration from a short-term EC50 and to account for its inherent genotoxicity and cytotoxicity), to give a value of 0.004 mg/L. The resulting risk quotient (PEC/PNEC) is 0.0006. Therefore, harm to aquatic organisms as a result of industrial releases of the substance is unlikely.
The PECs (0.000 001 6 – 0.000 046 mg/L) will not exceed the PNEC (0.004 mg/L) at any site across Canada for exposures resulting from down-the-drain releases through the consumption of pharmaceutical products that contain doxorubicin (Environment Canada 2011b). Based on the estimated number of receiving water bodies that will not be negatively affected by the use of the substance, coupled with the magnitude of the risk quotient and the more realistic scenario run, doxorubicin is not expected to cause harm to aquatic organisms due to down-the-drain releases.
Based on the information available, there is low risk of harm to organisms or the broader integrity of the environment from this substance. It is therefore concluded that doxorubicin does not meet the criteria under paragraph 64(a) or 64(b) of CEPA 1999, as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
8.4 Uncertainties in Evaluation of Ecological Risk
There is a lack of information on the sources of doxorubicin in the Canadian environment. Uncertainties are also present due to the lack of information on manufacturing and use in Canada and the quantity of this substance imported into Canada. The proportion of doxorubicin manufactured by and released from each individual industrial facility is unknown. These uncertainties were addressed by making conservative assumptions using best model estimates. Additionally, the locations of the release sites are unknown. As such, the quantitative results provide only a general indication of the magnitude of the potential risk to aquatic organisms. Uncertainties are also associated with the fraction of the substance that is released during use and with the fraction that is removed in wastewater treatment plants. Therefore, it was conservatively assumed that all doxorubicin used in Canada was manufactured at a single location. Similarly, as the distribution of the pharmaceutical across Canada is unknown, a variability factor of 2 was applied to every location in Mega Flush to account for uneven distribution.
The assessment of bioaccumulation potential is limited by the absence of empirical bioaccumulation data. Modelled BAFs and BCFs were derived, and, although all predictions using models have some degree of error (e.g., low log Kow and high water solubility) bioaccumulation model results could be interpreted as a false negative), the metabolism-corrected model outputs also support the expectation that doxorubicin, given its structural characteristics, has low bioaccumulation potential.
Regarding ecotoxicity, based on the predicted partitioning behaviour of doxorubicin, the significance of soil and sediment as important media of exposure is not well addressed by the available effects data. The application of sewage sludgebiosolids containing doxorubicin to agricultural land is a possibility, but it cannot be quantified in the absence of toxicity data and data on concentrations of doxorubicin in soil/sewage sludgebiosolids in Canada. In addition, it should be noted that the partitioning model cannot address the potential for doxorubicin to ionize in the aquatic environment or the potential for binding to soil components via electrostatic interactions (cation exchange) or binding to clays that are negatively surface charged. Thus, the model cannot fully account for the fate distribution of doxorubicin in the environment.
Regarding the ecotoxicity of doxorubicin, there is uncertainty associated with the consideration of modelled results in the effects characterization of the substance, as doxorubicin may not be included in the training sets of the QSAR models. Anthracycline substances, including doxorubicin, are “difficult to model,” as the physical and chemical properties of many of the structural classes of anthracyclines are not amenable to toxicity model prediction. They are considered “out of the model domain of applicability” (e.g., structural and water solubility domains). In addition to acute toxicity, some of the evidence of harm for doxorubicin relates to endpoints such as cytotoxicity (i.e., fish cell lines) and genotoxicity (i.e., fruit flies). There is uncertainty in how these effects translate to long-term effects on whole organisms and wildlife populations in the environment. These effects are part of the weight of evidence that has been considered in the development of the PNEC, reflected in the increase of the application factor to 500.
9. Potential to Cause Harm to Human Health
Doxorubicin has been classified as probably carcinogenic to humans (Group 2A) by the International Agency for Research on Cancer (IARC 1987) and as reasonably anticipated to be a human carcinogen by the National Toxicology Program in the United States (NTP 2011).
Drugs containing doxorubicin as an ingredient are assessed under the F&DA with respect to their safety, effectiveness and quality. This assessment focused on uses and exposures that were not covered as part of the F&DA assessment, specifically the risks posed by the residues resulting from manufacture, formulation and disposal after use.
As discussed in section 8.2.1, doxorubicin may be manufactured and/or processed by industrial facilities in Canada and subsequently may be released to Canadian surface waters. An aquatic site-specific industrial release scenario was developed with realistic conservative assumptions, which yields a PEC of 0.000 002 2 mg/L (2.2 ng/L)(Environment Canada 2011a). This PEC value represents the level of exposure in the receiving water near the point of discharge from the wastewater treatment system at the site.
When patients use pharmaceuticals, some of the drugs may not be absorbed or metabolized, and even drugs that are metabolized may have active metabolites or may revert to the parent form in environmental media. This may lead to excretion of active drug residues into the wastewater system and release of the wastewater effluent containing these residues into surface water (i.e., lakes, rivers), and this surface water has the potential to be used as drinking water. Additionally, the drug may be released to wastewater during the manufacturing process or via incorrect disposal of the excess pharmaceutical. Therefore, a focus of this assessment is on the potential for indirect exposure of humans to these pharmaceuticals through drinking water.
Only a portion of the pharmaceutical used in Canada would be released into the wastewater system. Metabolism results in a smaller portion of the pharmaceutical being excreted by the patient in the urine and/or feces. This amount can be further reduced as a result of wastewater treatment, environmental biodegradation and/or drinking water treatment prior to consumption. The concentration in the water source is also significantly reduced via dilution as the waste is released into waterways.
For this assessment, conservative assumptions were used when estimating the potential indirect exposure of the general population to doxorubicin. Releases to surface water were modelled using a down-the-drain release from pharmaceutical use scenario, as described above. For the purposes of modelling, it was assumed that 100% of the pharmaceutical that was prescribed was excreted and released into wastewater. It was also assumed that only 1.9% of the doxorubicin was removed during wastewater treatment.
This scenario estimates concentrations in approximately 1000 waterways across Canada. The highest values estimated by this scenario are typically in small waterways with low dilution capacity, which are unlikely to be sources of drinking water. As a result, this scenario would be expected to highly overestimate actual concentrations in drinking water. The maximum PEC estimated was 0.000 046 mg/L (46 ng/L).
Limited measured concentration data for doxorubicin were identified. Smyth and Teslic (2013) analyzed influent and effluent samples from six wastewater treatment plants across Canada for doxorubicin. Doxorubicin was not detected in any of the samples of influent or effluent, with detection limits ranging from 37.9 to 303 ng/L. As this substance was not detected even at the lowest reporting limit, this value (37.9 ng/L) is considered to be a conservative proxy for actual concentrations. It is recognized that this concentration would not be expected to be found in drinking water, as it would be further reduced via dilution after the effluent was released to surface water and possibly reduced during the drinking water treatment process prior to consumption. However, this value can be used as an upper-bounding estimate of exposure of Canadians.
The estimated intakes of doxorubicin by humans can be represented by formula-fed infants 0–6 months of age, which is estimated to be the most highly exposed age class, on a body weight basis, of those examined. The equation for deriving the estimated intake is given below:
Intake = (PEC × IR) / bw
where:
- Intake:
- Estimated intake of the substance from drinking water (mg/kg bw per day)
- PEC:
- Predicted environmental concentration in receiving water from modelled or measured data (mg/L)
- IR:
- Ingestion rate of drinking water for formula-fed infants: 0.8 L/day (Health Canada 1998)
- bw:
- Default body weight for infants 0–6 months of age: 7.5 kg (Health Canada 1998)
The maximum estimated intake for doxorubicin based on a modelled concentration of 46 ng/L is 5 ng/kg bw per day. The maximum intake based on samples of wastewater influent and effluent in which doxorubicin was not detected based on a detection limit of 37.9 ng/L is 4 ng/kg bw per day. It is expected that these are conservative upper-bounding estimates of possible exposure and that actual exposures would be significantly lower. Given the low levels of estimated exposure, the potential risk associated with indirect exposure to doxorubicin is expected to be low.
To further characterize potential risks associated with the intake of doxorubicin via drinking water, the lowest therapeutic dose (LTD) for doxorubicin was identified, and a margin of exposure (MOE) was calculated to determine the ratio between the upper-bounding estimate of intake by the general population and the dose that would be expected to produce a pharmacological effect. This approach is consistent with methodology described elsewhere (Webb et al. 2003; Schwab et al. 2005; Watts et al. 2007; Bull et al. 2011; WHO 2011). The LTD is the lowest concentration that evokes a desired therapeutic effect among target populations and is equivalent to the lowest dose prescribed or recommended, taking into account the number of doses per day (WHO 2011). These values are derived from an assessment of the balance between safety and efficacy.
The products registered for use in Canada are only for intravenous administration (DPD 2010). Dosage information indicates a recommended dose of 20–75 mg/m2 per day (Hospira Healthcare Corporation 2008; Novopharm Limited 2008; Pfizer Canada Inc. 2010; Janssen Inc. 2011). Using an adult body weight of 70.9 kg (Health Canada 1998) and a body surface area of 1.82 m2 for an adult (Health Canada 1995), the LTD of 20 mg/m2 is equivalent to a dose of 0.5 mg/kg bw per day.
MOEs were derived using the equation below:
MOE = LTD/Intake
where:
- MOE:
- Margin of exposure (dimensionless)
- LTD:
- Lowest therapeutic dose (mg/kg bw per day)
- Intake:
- Maximum estimated intake for drinking water derived from modelled or measured concentrations (mg/kg bw per day)
For doxorubicin, using the intake based on the reporting limit of samples of wastewater influent and effluent in which doxorubicin was not detected results in an MOE of 125 000. The MOE using the maximum modelled PEC would be 100 000. Given the very highly conservative nature of the exposure inputs and the use of human data to derive a point of departure for risk characterization, these MOEs support the determination that risks from indirect exposure to doxorubicin are low.
Based on the available information, it is concluded that doxorubicin does not meet the criteria set out in paragraph 64(c) of CEPA 1999, as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health
9.1 Uncertainties in Evaluation of Risk to Human Health
There is uncertainty regarding the estimation of exposure due to the lack of representative Canadian surface water or drinking water data and the use of models for estimating risk to human health. However, confidence is high that actual exposures would be lower than the ones used from both the models and the concentrations in influent and effluent. This is supported by the data available from other countries and the highly conservative default assumptions used. The uncertainty in the human risk estimates could be reduced significantly by the use of measured doxorubicin concentrations in Canadian surface water and/or drinking water.
Potential exposures to doxorubicin could occur via other sources, such as ingestion of fish or swimming in waters where the pharmaceutical is present, but these exposures are expected to be much less than the exposure through drinking water and so are not considered in this assessment.
Doxorubicin may also be used for additional off-label or veterinary uses that are not considered in this assessment. The quantity of the substance being used for these purposes is unknown, and so estimation of releases is not possible at this time. These potential releases may be accounted for in the measured concentrations.
It is recognized that the LTD represents an exposure level at which a desired pharmacological response is achieved and further that at this exposure level, adverse effects, in addition to intended effects, may occur in some patients. For certain indications and certain classes of drugs, the nature of these unintended effects may be significant. However, the LTD is developed for patients who require treatment for a particular illness and therefore are likely to be more susceptible to potential effects than a healthy individual. Although the use of the LTD provides a tier 1 type of assessment that does not utilize all the toxicity data that may be available for each substance, the highly conservative exposure defaults that have been used lead to significant margins between the LTD and the estimated intakes. The LTD also allows for derivation of an MOE based on a human dose as the point of departure, which is preferable to using a point of departure developed using experimental animals.
10. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to organisms and the broader integrity of the environment from doxorubicin. It is concluded that doxorubicin does not meet the criteria under paragraph 64(a) or 64(b) of CEPA 1999, as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Based on the information presented in this screening assessment, it is concluded that doxorubicin does not meet the criteria set out in paragraph 64(c) of CEPA 1999, as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is concluded that this substance does not meet any of the criteria set out in section 64 of CEPA 1999.
11. References
ACD/pKaDB [Prediction Module]. 2005. Version 9.04. Toronto (ON): Advanced Chemistry Development. [cited 2011 Nov -24]. Available from: http://www.acdlabs.com/products/phys_chem_lab/pka/. [restricted access].
[AIEPS] Artificial Intelligence Expert Predictive System. 2003–2007. Version 2.05. Ottawa (ON): Environment Canada. Model developed by Stephen Niculescu. Available from: Environment Canada, Ecological Assessment Division, New Chemicals Evaluation Section.
Albertus JA, Laine RO. 2001. Enhanced xenobiotic transporter expression in normal teleost hepatocytes: response to environmental and chemotherapeutic toxins. J Exp Biol 204:217–227.
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2010. Version 1.92a. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Arnot JA, Gobas FAPC. 2003. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs [Internet]. QSAR Comb Sci 22(3):337–345. Available from: onlinelibrary.wiley.com/doi/10.1002/qsar.v22:3/issuetoc [restricted access]
Arnot JA, Mackay D, Bonnell M. 2008a. Estimating metabolic biotransformation rates in fish from laboratory data. Environ Toxicol Chem 27(2):341–351.
Arnot JA, Mackay D, Parkerton TF, Bonnell M. 2008b. A database of fish biotransformation rates for organic chemicals. Environ Toxicol Chem 27(11):2263–2270.Arnot JA, Meylan W, Tunkel J, Howard PH, Mackay D, Bonnell M, Boethling RS. 2009. A quantitative structure–activity relationship for predicting metabolic biotransformation rates for organic chemicals in fish. Environ Toxicol Chem 28(6):1168–1177.
Arnot JA, Arnot MI, Mackay D, Couillard Y, MacDonald D, Bonnell M, Doyle P. 2010. Molecular size cutoff criteria for screening bioaccumulation potential: fact or fiction? Integr Environ Assess Manag 6(2):210–224.
Bard SM. 2000. Multixenobiotic resistance as a cellular defence mechanism in aquatic organisms. Aquat Toxicol 48:357–389.
Bard SM, Gadbois S. 2007. Assessing neuroprotective P-glycoprotein activity at the blood–brain barrier in killifish (Fundulus heteroclitus) using behavioural profiles. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Belyaeva NF, Kashirtseva VN, Medvedeva NV, Khudoklinova YY, Ipatova OM, Archakov AI. 2009. Zebrafish as a model system for biomedical studies. Biochem (Mosc) Suppl Ser B Biomed Chem 3(4):343–350.
[BCFBAF] Bioaccumulation Program for Windows [Estimation Model]. [2008]. Version [3.00]. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2010. Version 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4):741–752.
Bonnet J-L, Dusser M, Bohaiter J, Laffosse J. 2003. Cytotoxicity assessment of three therapeutic agents, cyclosporin A, cisplatin and doxorubicin, with the ciliated protozoan Tetrahymena pyriformis. Res Microbiol 154:375–385.
Bull RJ, Crook J, Whittaker M, Cotruvo JA. 2011. Therapeutic dose as the point of departure in assessing potential health hazards from drugs in drinking water and recycled municipal wastewater. Regul Toxicol Pharmacol 60:1–19.
Caminada D, Escher C, Fent K. 2006. Cytotoxicity of pharmaceuticals found in aquatic systems: comparison of PLHC-1 and RTG-2 fish cell lines. Aquat Toxicol 79:114–123.
Canada. [1978]. Food and Drug Regulations, C.R.C., c. 870. Available from: www.canlii.org/en/ca/laws/regu/crc-c-870/latest/crc-c-870.html
Canada. 1985. Food and Drugs Act, R.S.C. 1985, c. F-27. Available from: www.canlii.org/ca/sta/f-27/whole.html
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33. Canada Gazette, Part III, vol. 22, no. 3. Available from: publications.gc.ca/gazette/archives/p3/1999/g3-02203.pdf
Canada. 2000. Canadian Environmental Protection Act, 1999: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March 2000, SOR/2000-107, Canada Gazette, Part II, vol. 134, no. 7, p. 607−612. Available from: http://laws-lois.justice.gc.ca/eng/regulations/SOR-2000-107/page-1.html
CAREX Canada. 2010. Substance fact sheet: Adriamycin®. Vancouver (BC): University of British Columbia, School of Environmental Health.
Castegnaro M, De Meo M, Laget M, Michelon J, Garren L, Sportouch MH, Hansel S. 1997. Chemical degradation of wastes of antineoplastic agents. 2: Six anthracyclines: idarubicin, doxorubicin, epirubicin, pirarubicin, aclarubicin, and daunorubicin. Int Arch Occup Environ Health 70:378–384.
[CATALOGIC]A Computer System for Predicting Biodegradability Metabolic Pathways and Toxicity of Stable Biodegradation Products [Estimation Model]. 2009. [version 5.10.8]. Laboratory of Mathematical Chemistry; University "Prof. As. Zlatarov", Bourgas, Bulgaria.
ChemIDplus [Internet chemicals search system]. 1993-- . Bethesda (MD): National Library of Medicine (US). [cited 2014 01 02]. Available from: www.chem.sis.nlm.nih.gov/chemidplus/
[CHRIP] Chemical Risk Information Platform [database on the Internet]. ©2008. Tokyo (JP): National Institute of Technology and Evaluation, Chemical Management Centre (CMC). [cited 2012]. Available from: www.safe.nite.go.jp/english/db.html
[CPOPs] Canadian Persistent Organic Pollutants Profiler Model. 2008. Gatineau (QC): Environment Canada, Ecological Assessment Division; Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. [Model developed based on Mekenyan et al. 2005]. Available from: Environment Canada, Ecological Assessment Division.
de Rezende AAA, e Silva MLA, Tavares DC, Cunha WR, Rezende KCS, Bastos JK, Lehmann M, de Andrade HHR, Guterres ZR, Silva LP, Spanó MA. 2011. The effect of the dibenzylbutyrolactolic lignan (−)-cubebin on doxorubicin mutagenicity and recombinogenicity in wing somatic cells of Drosophila melanogaster. Food Chem Toxicol 49:1235–1241.
Dimitrov S, Dimitrova N, Walker J, Veith G, Mekenyan O. 2002. Predicting bioconcentration potential of highly hydrophobic chemicals. Effect of molecular size. Pure Appl Chem 74(10):823–1830.
Dimitrov S, Dimitrova N, Parkerton T, Comber M, Bonnell M, Mekenyan O. 2005. Base-line model for identifying the bioaccumulation potential of chemicals. SAR QSAR Environ Res 16(6):531–554.
Doroshow JH. 1983. Anthracycline antibiotic-stimulated superoxide, hydrogen peroxide, and hydroxyl radical production by NADH dehydrogenase. Cancer Res 43 (2)460.
[EAFUS] Everything Added to Food in the United States [database on the Internet]. 2011. Silver Spring (MD): US Food and Drug Administration. [cited 2013 Mar]. Available from: www.fda.gov/Food/IngredientsPackagingLabeling/FoodAdditivesIngredients/ucm115326.htm
[ECHA] European Chemicals Agency. 2008. Guidance on information requirements and chemical safety assessment. Chapter R.11: PBT assessment. May 2008. Guidance for the implementation of REACH. Helsinki (FI): European Chemicals Agency.
Environment Canada. 2007. Guidance for conducting ecological assessments under CEPA, 1999--Science Resource Technical Series. Technical guidance module: QSARs. Reviewed draft working document. Gatineau (QC): Environment Canada, Ecological Assessment Division.
Environment Canada. 2009. Guidance for conducting ecological assessments under CEPA, 1999--Science Resource Technical Series. Technical guidance module: Mega Flush consumer release scenario. Working document. Gatineau (QC): Environment Canada, Ecological Assessment Division.
Environment Canada. 2011a. Site specific analysis report: CAS RN 23214-92-8 [2011-11-14]. Unpublished report. Gatineau (QC): Environment Canada, Ecological Assessment Division.
Environment Canada. 2011b. Mega Flush file: CAS RN 23214-92-8 [2011 Nov 14]. Version 2.11. Unpublished report. Gatineau (QC): Environment Canada, Ecological Assessment Division.
[EPI Suite] Estimation Programs Interface Suite for Microsoft Windows [Estimation Model]. [2008]. Version 4.00]. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
[EQC] Equilibrium Criterion Model. 2003. Version 2.02. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre. Available from: http://www.trentu.ca/academic/aminss/envmodel/models/EQC2.html
Fick J, Lindberg RH, Tysklind M, Larsson DGJ. 2010. Predicted critical environmental concentrations for 500 pharmaceuticals. Regul Toxicol Pharmacol 58:516–523.
Gobas FAPC, Kelly BC, Arnot JA. 2003. Quantitative structure activity relationships for predicting the bioaccumulation of POPs in terrestrial food-webs. QSAR Comb Sci 22:329–336.
Gribaldo L, Casati S, Figliuzzi L, Marafante E. 1998. In vitro myelotoxicity of environmental contaminants. Environmental Toxicology and Pharmacology 6:135-141
Hamscher G, Mohring SAI, Knobloch A, Eberle N, Nau H, Nolte I, Simon D. 2010. Determination of drug residues in urine of dogs receiving anti-cancer chemotherapy by liquid chromatography-electrospray ionization-tandem mass spectrometry: is there an environmental or occupational risk? Journal of Analytical Toxicology 34: 142- 148.
Hansch C, Leo A, Hoekman D. 1995. Exploring QSAR. Vol. 2. Hydrophobic, electronic, and steric constants. Washington (DC): American Chemical Society; p. 187.
Hartmann A, Alder AC, Koller T, Widmer RM. 1998. Environ Toxicol Chem 17(3): 377-382.
Health Canada. 1995. Investigating human exposure to contaminants in the environment: a handbook for exposure calculations. Ottawa (ON): Health Canada, Health Protection Branch, Great Lakes Health Effects Program.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
[HENRYWIN] Henry’s Law Constant Program for Microsoft Windows [Estimation Model]. 2008. Version 3.20. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Hildebrand JL, Bains OS, Lee DSH, Kennedy CJ. 2009. Functional and energetic characterization of P-gp-mediated transport in rainbow trout (Oncorhynchus mykiss) hepatocytes. Comp Biochem Physiol C 149:65–72.
Hospira Healthcare Corporation. 2008. Product monograph for Doxorubicin hydrochloride for injection, USP. [revised 2008 Feb 18]. [cited in DPD 2010].
Household Products Database [database on the Internet]. 1993– . Bethesda (MD): National Library of Medicine (US). [updated 2013 Jan; cited 2013 Mar]. Available from: www.householdproducts.nlm.nih.gov/
[HSDB] Hazardous Substances Data Bank [database on the internet]. c1993-2008. United States National Library of Medicine, National Library of Medicine (US). [revised 2006 Dec 20; cited 2013 Mar]. Available from: www.toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB
[HYDROWIN] Hydrolysis Rates Program for Microsoft Windows [Estimation Model]. 2008. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
[IMS] Intercontinental Marketing Services. 2013. Health Canada Sales Database 2011 & 2012 [MIDAS database on CD]. IMS Brogan,Toronto (ON), IMS Brogan
Janssen Inc. 2011. Product monograph for CAELYX. Date of preparation: January 1, 2011. Available from DPD 2013.
Kelly BC, Ikonomou MG, Blair JD, Morin AE, Gobas FAPC. 2007. Food web-specific biomagnification of persistent organic pollutants. Science 317:236–239.
[KOAWIN] Octanol Air Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2008. Version 1.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
[KOCWIN] The Soil Adsorption Coefficient Program [Estimation Model]. 2008. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2008. Version 1.67. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Krysanov EY, Demidova TB. 2012. The effect of low concentrations on nanocrystalline cerium dioxide on the embryotoxicity of doxorubicin for fish. Dokl Biol Sci 443:117–119.
Lehmann M, Franco A, Vilar KSP, Reguly ML, de Andrade HHR. 2003. Doxorubicin and two of its analogues are preferential inducers of homologous recombination compared with mutational events in somatic cells of Drosophila melanogaster. Mutat Res 539:167–175.
Lenz K, Mahnik SN, Weissenbacher N, Mader RM, Krenn P, Hann S, Koellensperger G, Uhl M, Knasmüller S, Ferk F, Bursch W, Fuerhacker M. 2007. Monitoring, removal, and risk assessment of cytostatic drugs in hospital wastewater. Water Sci Technol 15(12):141–149.
Lide DR, editor. 2007. CRC handbook of chemistry and physics. 88th ed. 2007–2008. Boca Raton (FL): CRC Press.
[LNHPD] Licensed Natural Health Products Database [database on the Internet]. 2008.
Version 1.0. Ottawa (ON): Health Canada. [cited 2013 Mar]. Available from: http://www.hc-sc.gc.ca/dhp-mps/prodnatur/applications/licen-prod/lnhpd-bdpsnh-eng.phpwebprod3.hc-sc.gc.ca/lnhpd-bdpsnh/index-eng.jsp
Mahnik SN, Rizovski B, Fuerhacker M, Mader RM. 2006. Development of an analytical method for the determination of anthracyclines in hospital effluent. Chemosphere 65:1419-1425
Mahnik SN, Lenz K, Weissenbacher N, Mader RM, Fuerhacker M. 2007. Fate of 5-fluorouracil, doxorubicin, epirubicin, and daunorubicin in hospital wastewater and their elimination by activated sludge and treatment in a membrane-bio-reactor system. Chemosphere 66:30–37.
McLaughlin A, Belknap A. 2008. Annual kg quantity of medicinal ingredients distributed and dispensed in Canada: analysis of intercontinental medical statistics (IMS) data for 2007. [Excel format data summary]. Ottawa (ON): Health Canada, Health Products and Food Branch, Environmental Impact Initiative.
Mekenyan G, Dimitrov SD, Pavlov TS, Veith GD. 2005. POPs: a QSAR system for creating PBT profiles of chemicals and their metabolites. SAR QSAR Environ Res 16(1–2):103−133.
Momparler RL, Karon M, Siegal SE. 1976. Effect of Adriamycin on DNA, RNA, and protein synthesis in cell-free systems and intact cells. Cancer Res. 36: 2891-2895.
[MPBPWIN] Melting Point Boiling Point Program for Microsoft Windows [Estimation Model]. 2008]. Version 1.43. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Nawara K, Krysinski P, Blanchard GJ. 2012. Photoinduced reactivity of doxorubicin: catalysis and degradation. J Phys Chem A 116:4330–4337.
Negreira N, López de Alda M, Barceló D. 2013. On-line solid phase extraction–liquid chromatography–tandem mass spectrometry for the determination of 17 cytostatics and metabolites in waste, surface and groundwater samples. J Chromatogr A 1280:64–74.
[NHPID] Natural Health Products Ingredients Database [database on the Internet]. 2011. Version 2.1. Ottawa (ON): Health Canada. [cited 2013 Mar]. Available from:.hc-sc.gc.ca/nhpid-bdipsn/search-rechercheReq.do
Nichols JW, Fitzsimmons PN, Burkhard LP. 2007. In vitro – in vivo extrapolation of quantitative hepatic biotransformation data for fish. II. Modeled effects on chemical bioaccumulation. Environ Toxicol Chem 26:1304−1319.
Novopharm Limited. 2008. Product monograph for Doxorubicin hydrochloride injection, USP. [revised 2008 Jul 18]. [cited in DPD 2010].
[NTP] National Toxicology Program (US). 2011. Doxorubicin. In: Report on carcinogens. 12th ed. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program. Available from: ntp.niehs.nih.gov/go/roc12
O’Neil MJ. 2006. Merck index. 14th ed. Whitehouse Station (NJ): Merck and Co., Inc.; p. 582.
Pallas [Prediction module]. 1994–1995. Version 4.0. Bal Harbour (FL): CompuDrug Chemistry Ltd.
Pfizer Canada Inc. 2010. Product monograph for Adriamycin PFS. [revised 2010 Jan 8]. [cited in DPD 2010].
Poeggeler B, Durand G, Polidori A, Pappolla MA, Vega-Naredo I, Coto-Mones A, Böker J, Hardeland R, Pucci B. 2005. Mitochondrial medicine: neuroprotection and life extension by the amphiphilic nitrone LPBNAH acting as a highly potent antioxidant agent. J Neurochem 95:962–973.
Rowney NC, Johnson AC, Williams RJ. 2009. Cytotoxic drugs in drinking water: a prediction and risk assessment exercise for the Thames catchment in the United Kingdom. Environ Toxicol Chem 28:2733–2743.
Sakuratani Y, Noguchi Y, Kobayashi K, Yamada J, Nishihara T. 2008. Molecular size as a limiting characteristic for bioconcentration in fish. J Environ Biol 29(1):89–92.
Schwab BW, Hayes EP, Fiori JM, Mastrocco FJ, Roden NM, Cragin D, Meyerhoff RD, D’Aco VJ, Anderson PD. 2005. Human pharmaceuticals in US surface waters: a human health risk assessment. Regul Toxicol Pharmacol 42:296–312.
SPARC; pKa/property server. Ver 4.2 Mar, 2008. Available from, as of Mar 8, 2010: http://ibmlc2.chem.uga.edu/sparc/
Takayama S, Thorgeirsson UP, Adamson RH. 2008. Chemical carcinogenesis studies in nonhuman primates. Proc Jpn Acad, Ser B, 84: 176- 188.
Smyth SA, Teslic S. 2013. Occurrence and fate of pharmaceuticals and personal care products in municipal wastewater treatment systems. Unpublished year-end report, March 22, 2013. Ottawa (ON): Health Canada, New Substances Assessment and Control Bureau; 14 p.
[TOPKAT] TOxicity Prediction by Komputer Assisted Technology [Internet]. 2004. Version 6.2. San Diego (CA): Accelrys Software Inc. Available from: http://www.accelrys.com/products/topkat/index.html
Takayama S, Thorgeirsson UP, Adamson RH. 2008. Chemical carcinogenesis studies in nonhuman primates. Proc Jpn Acad, Ser B, 84: 176- 188.
[US NIH] US National Institute of Health. 2009a. DailyMed: current medication information for doxorubicin hydrochloride (doxorubicin hydrochloride) injection, powder, lyophilized, for solution (October 2006). [Accessed 2011 Sept 11]. Available from: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=2256
[US NIH] US National Institute of Health. 2009b. DailyMed: current medication information for Doxil (doxorubicin hydrochloride) injectable, liposomal for intravenous use (January 2008) [accessed 2011 Sept 11] Available from: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6429
Watts C, Maycock D, Crane M, Fawell J, Goslan E. 2007. Desk based review of current knowledge on pharmaceuticals in drinking water and estimation of potential levels. Final report prepared by Watts and Crane Associates for Drinking Water Inspectorate, Department for Food, Environment and Rural Affairs (Defra Project Code: CSA 7184/WT02046/DWI70/2/213). Available from: dwi.defra.gov.uk/research/completed-research/reports/dwi70-2-213.pdf
Webb S, Ternes T, Gibert M, Olejniczak K. 2003. Indirect human exposure to pharmaceuticals via drinking water. Toxicol Lett 142:157–167.
Weiss RB. 1992. The anthracyclines: will we ever find a better doxorubicin? Semin Oncol 19(6):670–686.
[WHO] World Health Organization. 2011. Pharmaceuticals in drinking-water. Geneva (CH): World Health Organization, Public Health and Environment, Water, Sanitation, Hygiene and Health. Report No.: WHO/HSE/WSH/11.05.
Williams JH. 1999. Regulations on additions of sludge-borne metals to soil and their adaptation to local conditions in treatment and use of sewage sludge and liquid agricultural wastes. edited by L’Hermite P. Commission of the European Communities, Elsevier Applied Science, London, England p.243-250.
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [Estimation Model]. 2008. Version 1.41. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Yin J, Shao B, Zhang J, Li K. 2010. A preliminary study on the occurrence of cytostatic drugs in hospital effluents in Beijing, China. Bull Environ Contam Toxicol 84:39–45.
Zaja R, Munic V, Klobucar RS, Ambriovic-Ristov A, Smital T. 2008. Cloning and molecular characterization of apical efflux transporters (ABCB1, ABCB11, ABCC2) in rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquat Toxicol 90:322–332.
Zounková R, Odráška P, Dolezalová L, Hilscherová K, Maršálek B, Bláha L. 2007. Ecotoxicity and genotoxicity assessment of cytostatic pharmaceuticals. Environ Toxicol Chem 26:2208–2214.