State of the Science Report
Phthalates Substance Grouping
Long-chain Phthalate Esters
1,2-Benzenedicarboxylic acid, diisodecyl ester
(diisodecyl phthalate; DIDP)
and
1,2-Benzenedicarboxylic acid, diundecyl ester
(diundecyl phthalate; DUP)
Chemical Abstracts Service Registry Numbers
26761-40-0, 68515-49-1;
3648-20-2
Environment Canada
Health Canada
August 2015
Table of Contents
- Tables and Figures
- Synopsis
- 1. Introduction
- 2. Identity of Substances
- 3. Physical and Chemical Properties
- 4. Sources
- 5. Uses
- 6. Releases to the Environment
- 7. Environmental Fate and Behaviour
- 8. Potential to Cause Ecological Harm
- 9. Potential to Cause Harm to Human Health
- References
- Appendices
Tables and Figures
- Figure 1. General structure of ortho-phthalates
- Table 2-1. Substance identities for long-chain phthalate esters in the Phthalates Substance Grouping
- Table 2-2. Read-across data used to inform various parameters evaluated in this assessment
- Table 2-3. Information on identity, chemical structure, and branching of analogues used for human health assessment of DUP
- Table 3-1. Range of experimental and predicted physical and chemical properties (at standard conditions) for long-chain phthalate esters in the Phthalates Substance Grouping
- Table 4-1. Section 71 reporting for DIDP and DUP in 2012 (kg)
- Table 4-2. Inventory Update Reporting national aggregated production volumes in the United States (kg)
- Table 4-3. ECHA REACH Registered Substances quantities manufactured and imported (kg)
- Table 5-1. Summary of the major uses of DIDP and DUP identified internationally
- Table 7-1. Summary of Level III fugacity modelling (EQC 2011) for long-chain phthalate esters in the Phthalate Substance Grouping, showing percent partitioning into each medium for three release scenarios
- Table 7-2. Summary of key abiotic degradation data for long-chain phthalate esters in the Phthalates Substance Grouping
- Table 7-3. Summary of key primary biodegradation data under aerobic conditions for long-chain phthalate esters in the Phthalates Substance Grouping
- Table 7-4. Summary of key ultimate biodegradation data for long-chain phthalate esters in the Phthalates Substance Grouping
- Table 7-5. Summary of empirical bioconcentration (BCF) data for DIDP
- Table 7-6. Summary of modelled fish bioaccumulation data for long-chain phthalate esters in the Phthalates Substance Grouping
- Table 8-1. Key aquatic toxicity studies for the long-chain phthalates
- Table 8-2. Key sediment toxicity studies for DIDP
- Table 8-3. Key soil toxicity studies for DIDP
- Table 9-1. DIDP concentrations in dust
- Table 9-2. Parameters used in recent evaluations of dermal exposure to DIDP from plastic articles by other jurisdictions
- Table 9-3. Migration rates of DEHP into simulated sweat from various articles
- Table 9-4. Dermal exposure estimates to DIDP for infants (0 to 18 months) and adults (20+)
- Table 9-5. Urine excretion fractions (FUE's) for metabolites of DIDP in risk assessment
- Table 9-6. Metabolites used for intake calculations in NHANES and P4 analyses
- Table 9-7. 2009 to 2010 NHANES daily intakes (µg/kg/day), males (using creatinine correction)
- Table 9-8. 2009 to 2010 NHANES daily intakes (µg/kg/day), females (using creatinine correction)
- Table 9-9. P4 Pregnant women and MIREC-CD Plus (preliminary data) infants (intakes (µg/kg/day)
- Table 9-10. DUP concentrations in dust
- Table 9-11. Dermal exposure estimates to DUP for infants (0 to 18 months) and adults (20+)
- Table 9-12. Summary of the rates of oral absorption for DIDP and DnOP
- Table 9-13. Summary of metabolites of DIDP and DnOp found in urine after oral administration in rats and human in vivo
- Table 9-14. Effects from gestational exposure to DIDP in male offspring (mg/kg bw/day)
- Table 9-15. Effects from exposure to DIDP in prepubertal-pubertal males (mg/kg bw/day)
- Table 9-16. Effects from exposure to DIDP in adult males (mg/kg bw/day)
- Table 9-17. Short-term and subchronic studies in rodents
- Table 9-18. Carcinogenicity studies in rodents
- Table 9-19. Effects from gestational exposure to DUP, D911P and DnOP in male offspring (mg/kg bw/day)
- Table 9-20. Effects from exposure to DUP and DnOP in prepubertal-pubertal males (mg/kg bw/day)
- Table 9-21. Effects from exposure to DUP, D911P and DnOP in adult males (mg/kg bw/day)
- Table 9-22. Summary of critical systemic effects after oral exposure to DIDP
- Table 9-23. Summary of margins of exposure to DIDP for subpopulations with highest exposure
- Table 9-24. Summary of critical systemic effects associated with oral exposure to DUP
- Table 9-25. Summary of margins of exposure to DUP for subpopulations with highest exposure
Synopsis
The Minister of the Environment and the Minister of Health have prepared a state of the science report on two long-chain phthalate esters, 1,2-Benzenedicarboxylic acid, diisodecyl ester (DIDP) and 1,2-Benzenedicarboxylic acid, diundecyl ester (DUP). The purpose of this report is to review the currently available science on these substances so that the public has an opportunity to review, comment, and/or provide additional information for consideration prior to proposing conclusions through the publication of a draft screening assessment. A proposed approach for considering the cumulative risk of phthalates has also been prepared for public review and comment, and will be used in the development of the draft screening assessment.
DIDP and DUP are two of 14 phthalate esters (or phthalates) identified for screening assessment under the Chemicals Management Plan (CMP) Substance Grouping Initiative. Key selection considerations for this group were based on similar potential health effects of concern; potential ecological effects of concern for some phthalates; potential exposure of consumers and children; potential to leverage/align with international activity; and potential risk assessment and risk management efficiencies and effectiveness.
While many phthalate substances have common structural features and similar functional uses, differences in the potential health hazard, as well as environmental fate and behaviour, have been taken into account through the establishment of subgroups. The primary basis for the subgroups from a health hazard perspective is a structure activity relationship (SAR) analysis using studies related to important events in the mode of action for phthalate-induced androgen insufficiency during male reproductive development in the rat. The effects of phthalate esters for these important events appear to be structure dependent, and highly related to the length and nature of their alkyl chain. Further information on the approach by which the substances in the Phthalate Substance Grouping were divided into three subgroupings from a health hazard perspective is provided in Health Canada (2015a). From an ecological perspective, subgrouping was based primarily on differences in log Kow and water solubility, and their resulting effects on bioaccumulation and ecotoxicity. Further information on the ecological rationale for the subgroups is provided in an appendix to the draft approach for considering the cumulative risk of phthalates (Environment Canada and Health Canada 2015a). DIDP and DUP belong to the long-chain Phthalate Esters subgroup.
The Chemical Abstracts Service Registry Numbers (CAS RNsFootnote[1]), Domestic Substances List (DSL) names and common names and acronyms for DIDP and DUP are listed in the table below.
| CAS RN | Domestic Substances List name | Common name |
|---|---|---|
| 26761-40-0; 68515-49-1 |
1,2-Benzenedicarboxylic acid, diisodecyl ester | Diisodecyl phthalate (DIDP) |
| 3648-20-2 | 1,2-Benzenedicarboxylic acid, diundecyl ester | Diundecyl phthalate (DUP) |
DIDP and DUP are organic substances that are primarily used as plasticizers in a wide variety of consumer, commercial and industrial products. Neither substance is naturally occurring in the environment. Information obtained for the year 2012 determined that DIDP and DUP were both manufactured in Canada and imported into the country in that year, with a combined production and import quantity in the range of 20 000 000 kg/y. Both are found in wiring and cable to make the insulating sheath more flexible. DIDP is associated with a greater variety of uses than DUP in Canada with applications in building and construction materials, paper products, toys, sporting equipment and rubber materials. Based on high use quantities and potential use in a variety of manufactured items, DIDP and DUP are considered to have high potential to be released into the Canadian environment.
Air and water are expected to be the primary receiving media for DIDP and DUP in the environment. Based on properties of low water solubility and vapour pressure, and high partitioning potential into organic carbon, DIDP and DUP released into water will distribute into sediment and the suspended particulate fraction of surface waters. When released into air, both substances are expected to distribute primarily into soil and sediments through wet and dry deposition processes. DIDP and DUP released into soil are predicted to remain within this environmental compartment and are not expected to leach through soil into groundwater.
DIDP and DUP are expected to degrade rapidly in aerobic environments, but may take longer to break down under low oxygen conditions such as those occurring in sub-surface sediments and soil. However, neither substance is expected to persist in the environment. DIDP has been detected in air, water and sediments, while DUP was present in a small number of sediment samples, indicating that sources of the substances into the environment result in detectable concentrations reflecting the balance of emission inputs and degradation losses.
Based on high partition coefficients and low water solubilities, exposure of DIDP and DUP to organisms will occur primarily through the diet. Empirical and modelled data suggest that both substances have low bioaccumulation and biomagnification potential. However, DIDP has been measured in a variety of aquatic species and this confirms that the substance is bioavailable. No reliable biota monitoring data were found for DUP.
Results from standard laboratory tests suggest that DIDP and DUP have low hazard potential in aquatic and terrestrial species, with no adverse effects on survival, growth, development or reproduction seen in acute and chronic testing at concentrations up to and exceeding solubility and saturation limits. Results from an analysis of critical body residues (CBRs) conducted for aquatic organisms determined that maximum tissue concentrations of DIDP and DUP based on solubility limits will be much lower than levels associated with adverse acute or chronic lethality effects due to neutral narcosis. An analysis conducted for DIDP in sediment and soil organisms indicated that maximum tissue concentrations calculated from the saturation limit of DIDP in a 4% organic carbon (OC) sediment or soil do not exceed minimum concentrations estimated to cause narcotic effects. A similar result was determined for DIDP measured directly in the tissues of Canadian aquatic biota. Equivalent CBR analyses could not be conducted for DUP due to a lack of data. However, based on the similarity in chemical properties, results obtained for DIDP are considered applicable to DUP. Therefore, based on the analyses of CBRs, it is considered unlikely that internal body concentrations of DIDP and DUP in exposed organisms will reach levels causing adverse narcotic effects. It should be noted that the CBR analysis does not consider the potential for adverse effects resulting from modes of action other than baseline narcosis.
With regard to human health, the principal source of exposure to DIDP and DUP for the general population is expected to be house dust (oral ingestion) as well as food and beverages for DIDP (oral ingestion). Expsoure scenarios were identified to characterize dermal exposure for adults and children for both long-chain phthalates. Finally, concentrations of DIDP metabolites in urine were also used to estimate exposure of DIDP for the Canadian general population.
The health effects database for long-chain phthalates shows that the critical effect for risk characterization is effects on the liver. An examination of the potential developmental and reproductive toxicity of long-chain phthalates indicated that this group of phthalates has limited effects on the developing male.
Comparisons of estimates for exposure to DIDP and DUP from various sources such as environmental media, food and contact with plastic articles as well as from biomonitoring levels, as available, with critical effect levels results in margins that are considered adequate to address uncertainties in the exposure and health effects databases and are protective of potential limited developmental and reproductive effects of DIDP and DUP toxicity not only in males, but also in females as well as other systemic effects. Further, these margins are also considered adequate as they address potential carcinogenicity of DIDP. Although the MOEs are currently considered adequate on an individual basis, this does not address potential risk of exposure to long-chain phthalates in a cumulative context when considered with other phthalates exhibiting a similar mode of action.
Accordingly, a proposed cumulative risk assessment approach for certain phthalates is provided in a separate report (Environment Canada and Health Canada 2015a).
1. Introduction
Pursuant to sections 68 and 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999), the Minister of the Environment and the Minister of Health conduct evaluations of substances to determine whether these substances present or may present a risk to the environment or to human health.
The Substance Groupings Initiative is a key element of the Government of Canada's Chemicals Management Plan (CMP). The Phthalate Substance Grouping consists of 14 substances that were identified as priorities for assessment, as they met the categorization criteria under section 73 of CEPA 1999 and/or were considered as a priority based on human health concerns (Environment Canada, Health Canada 2007). Certain substances within this Substance Grouping have been identified by other jurisdictions as a concern due to potential reproductive and developmental effects in humans. There are also potential ecological effects of concern for some phthalates. A survey conducted for phase 1 of the Domestic Substances List (DSL) Inventory Update identified that a subset of phthalates have a wide range of consumer applications that could result in exposure to humans, including children (Environment Canada 2012). Addressing these substances as a group allows for consideration of cumulative risk, where warranted.
This state of the science (SOS) report provides a summary and evaluation of the current available science intended to form the basis for a draft screening assessment scheduled for publication in 2016. The Government of Canada developed a series of SOS reports for the Phthalate Substance Grouping to proide an opportunity for early public comment on a proposed cumulative assessment approach for certain phthalates (Environment Canada and Health Canada 2015a), prior to that approach being used to propose conclusions on the substances in Phthalate Substance Grouping through publication of a draft screening assessment report.
This SOS report focuses on 1,2-Benzenedicarboxylic acid, diisodecyl ester or DIDP (CAS RNs 26761-40-0 or 68515-49-1) and 1,2-Benzenedicarboxylic acid, diundecyl ester or DUP (CAS RN 3648-20-2). These substances were identified in the categorization of the DSL under subsection 73(1) of CEPA 1999 as priority for assessment. These substances also met the categorization criteria for inherent toxicity to non-human organisms, but not for persistence or bioaccumulation.
While phthalates have common structural features and similar functional uses, differences in their potential health hazard, environmental fate and behaviour have been taken into account through the establishment of subgroups. The primary basis for the subgroups from a health hazard perspective is a structure activity relationship (SAR) analysis using studies related to important mechanistic events for phthalate-induced androgen insufficiency during male reproductive development in the rat. The effects of phthalate esters for these important events appear to be structure dependent, and highly related to the length and nature of their alkyl chain (Health Canada 2015a). From an ecological perspective, subgrouping was based primarily on differences in log Kow and water solubility, and their resulting effects on bioaccumulation and ecotoxicity (Environment Canada and Health Canada 2015a).
This SOS report includes consideration of information on chemical properties, environmental fate, hazards, uses and exposure, including additional information submitted by stakeholders. Relevant data were identified up to October 2014 for the ecological portion and up to August 2014 for the health portion of the assessment. Empirical data from key studies as well as some results from models were used. When available and relevant, information presented in assessments from other jurisdictions was considered.
The SOS report does not represent an exhaustive or critical review of all available data. Rather, it presents the most critical and reliable studies and lines of evidence pertinent to develop a screening assessment in the future.
This SOS report was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. The ecological and human health portions of this report have undergone external written peer review and/or consultation. Comments on the technical portions relevant to the environment were received from Dr. Frank Gobas (Frank Gobas Environmental Consulting), Dr. Chris Metcalfe (Ambient Environmental Consulting, Inc.), Dr. Thomas Parkerton (ExxonMobil Biomedical Sciences, Inc.), and Dr. Charles Staples (Assessment Technologies, Inc.). Comments on the technical portions relevant to human health were received from Dr. Jack Dempsey (EnRisks), Dr. Michael Jayjock (The Lifeline Group) and Dr. Bernard Gadagbui (Toxicology Excellence for Risk Assessment). While external comments were taken into consideration, the final content and outcome of the report remain the responsibility of Health Canada and Environment Canada.
2. Identity of Substances
Phthalate esters are synthesized through the esterification of phthalic anhydride (1,2-benzenedicarboxylic acid anhydride; CAS RN 85-44-9) with various alcohols (ACC 2001). The resulting phthalate esters are diesters of benzenedicarboxylic acid comprised of a benzene ring with two side chain ester groups. Phthalates have the general structure outlined in Figure 1, where R1 and R2 represent ester side chains that can vary in length and structure (ACC 2001). The ester side groups can be the same or different and the nature of the side groups determines both the identity of the particular phthalate and its physical and toxicological properties. All substances in the Phthalate Grouping are ortho-phthalates (o-phthalates), with their ester side chains situated adjacent to each other at the 1 and 2 positions of the benzene ring (refer to Figure 1; US EPA 2012).
The structural formula for phthalate esters is derived from the isomeric composition of the alcohol used in their manufacture (Parkerton and Winkelmann 2004). Dialkyl phthalates have ester groups of linear or branched alkyl chains containing from one to thirteen carbons, while benzyl phthalates generally contain a phenylmethyl group and an alkyl chain as ester side groups and cyclohexyl phthalates contain a saturated benzene ring as an ester group (Parkerton and Winkelmann 2004).
Figure 1. General structure of ortho-phthalates

Long description for figure 1
A two-dimensional representation of the general molecular structure for the phthalates of interest.
The depiction has two elements:
1) Starting on the left is the general molecular structure for the phthalates of interest. The general molecular structure consists of a benzene ring with ester substitutions at the 1 and 2 positions. The chains on the ester linkage are represented by “R1” and “R2”.
2) On the right of the figure are the definitions of the R groups. R1 and R2 may be saturated linear or branched alkyl chains. R1 and R2 may also be a phenyl group or a cyclohexyl ring.
Diisodecyl phthalate (DIDP) and diundecyl phthalate (DUP) are two of the 14 phthalate esters in the Phthalate Substance Grouping. Information on the chemical structure and identity of DIDP and DUP is given in Table 2-1 , with further details provided in Appendix A and Environment Canada (2015). Together, DIDP and DUP comprise the long-chain phthalate esters subgroup of the Phthalates Substance Grouping.
DIDP is a complex isomeric mixture containing mainly C10-branched isomers on its side chains (ECJRC 2003). While two different CAS RNs have been assigned to DIDP, the European Council for Plasticisers and Intermediates (ECPI) has indicated that the products represented by these two CAS RNs are prepared from the same feed, through an identical olefin oligomerisation process, and through similar manufacturing and phthalate esterification processes (ECPI 1996). For this reason, the two CAS RNs 26761-40-0 and 68515-49-1 are considered fully interchangeable (ECJRC 2003) and will be treated as one product in this SOS report. DIDP is prepared from propylene and butenes that are chemically processed to form the alcohol mixture designated Alcohols, C9-11 branched and linear, C10 rich (CAS RN 93821-11-5). This alcohol product is then reacted with phthalic anhydride to form DIDP (ECJRC 2003). It should be noted that because DIDP is an isomeric mixture, the chemical structures provided in Table 2-1 are considered to be representative structures for the substance. For example, the structure provided for CAS RN 26761-40-0 indicates quaternary carbons occur at the end of the alkyl chain. However, the branching pattern is variable across the alkyl chain, consistent with the way in which the alcohols are synthesized from polymerization of low molecular weight olefins (personal communication, correspondence from ExxonMobil Biomedical Sciences, Inc., Houston, TX to Ecological Assessment Division, Environment Canada dated November 2014; unreferenced).
By contrast, DUP is a single constituent, discrete chemical comprised of two linear ester side chains each with a backbone of 11 carbons (NICNAS 2008b). DUP is produced primarily from C11 alcohols such as 1-undecanol (CAS RN 112-42-5) (European Commission 2000; SciFinder 2013).
| CAS RN acronym |
DSL name and common name | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 26761-40-0 DIDP |
1,2-Benzenedicarboxylic acid, diisodecyl ester Diisodecyl phthalate |
 C28H46O4 |
446.68 (average) |
| 68515-49-1 DIDP |
1,2-Benzenedicarboxylic acid, di-C9-11-branched alkyl esters, C10-rich Diisodecyl phthalate |
 C28H46O4 |
446.68 (average) |
| 3648-20-2 DUP |
1,2-Benzenedicarboxylic acid, diundecyl ester Diundecyl phthalate |
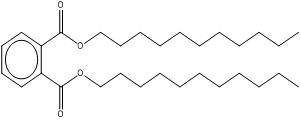 C30H50O4 |
474.73 |
2.1 Selection of Analogues and Use of (Q)SAR Models
Guidance on the use of a read-across approach and Quantitative Structure-Activity Relationships or (Q)SAR models for filling data gaps has been prepared by various organizations such as the Organisation for Economic Co-operation and Development (OECD). These methods have been applied in various regulatory programs including the European Union's (EU) Existing Substances Programme. In this assessment, a read-across approach using data from analogues and the results of (Q)SAR models, where appropriate, has been used to inform the ecological and human health assessments. Analogues were selected that were structurally similar and/or functionally similar to substances within this subgroup (e.g., based on physical-chemical properties, toxicokinetics) and that had relevant empirical data that could be used to read-across to substances that were data poor. The applicability of (Q)SAR models was determined on a case-by-case basis.
2.1.1 Selection of Analogues for Ecological Assessment
Analogues used to inform the ecological component of this SOS report are presented in Table 2-2, along with an indication of the read-across data used for different parameters. Further information relating to the analogue substances is provided in Appendix Table A-1.
| CAS RN for Analogue | Common name | Type of data used |
|---|---|---|
| 28553-12-0 68515-48-0 |
Diisononyl phthalate (DINP) | Anaerobic biodegradation; biodegradation and aquatic toxicity of primary degradation product |
| 85507-79-5 | Diisoundecyl phthalate (DIUP) | Biomagnification factor |
DINP was selected as a source of read-across data for the anaerobic biodegradation of the long-chain phthalates, as well as the aerobic biodegradation and ecotoxicity of their primary degradation products, while DIUP was used for read-across to evaluate the biomagnification potential of DUP. Both analogue substances have structural comparability of greater than 84% with DIDP and greater than 81% with DUP as determined by the OECD QSAR Toolbox software (2012; see Appendix Table A-1). This indicates that all four substances (DIDP, DUP, DINP and DIUP) can be expected to biodegrade in a similar manner and with similar biodegradation products, making DINP suitable as a source of read-across biodegradation data for DIDP and DUP. In addition, comparability in their molecular dimensions (maximum diameter range 27 to 35 nm, effective diameter range 19 to 22 nm; Table A-1) and chemical properties (water solubility less than 0.0001 mg/L, log Kow greater than 8 and log Koc in the range of 5.5 to 7) suggests that all four substances may have similar uptake and bioaccumulation potential, making DIUP acceptable as a source of read-across data for evaluating the biomagnification potential of DUP.
As well, some data for DIDP were considered to be representative of DUP (i.e., for the evaluation of bioaccumulation potential and potential toxicity to sediment and soil organisms).
2.1.2 Selection of Analogues for Human Health Assessment
As there were no specific gaps in the toxicological database for DIDP related to the characterization of risk to human health from exposure to DIDP, no analogues were necessary. Table 2-3 presents information on the analogues selected to support the characterization of risk from exposure to DUP (Health Canada 2015a).
| CAS RN | DSL name | Common name (acronym) | Chemical structure and molecular formula | Branching (Number of carbons in longest backbone) |
|---|---|---|---|---|
| 68515-43-5 | 1,2-Benzenedicarboxylic acid, di-C9-11-branched and linear alkyl esters | di-C9-11-alkyl phthalate (D911P) |
n-nonyl ester groups n-decyl ester groups 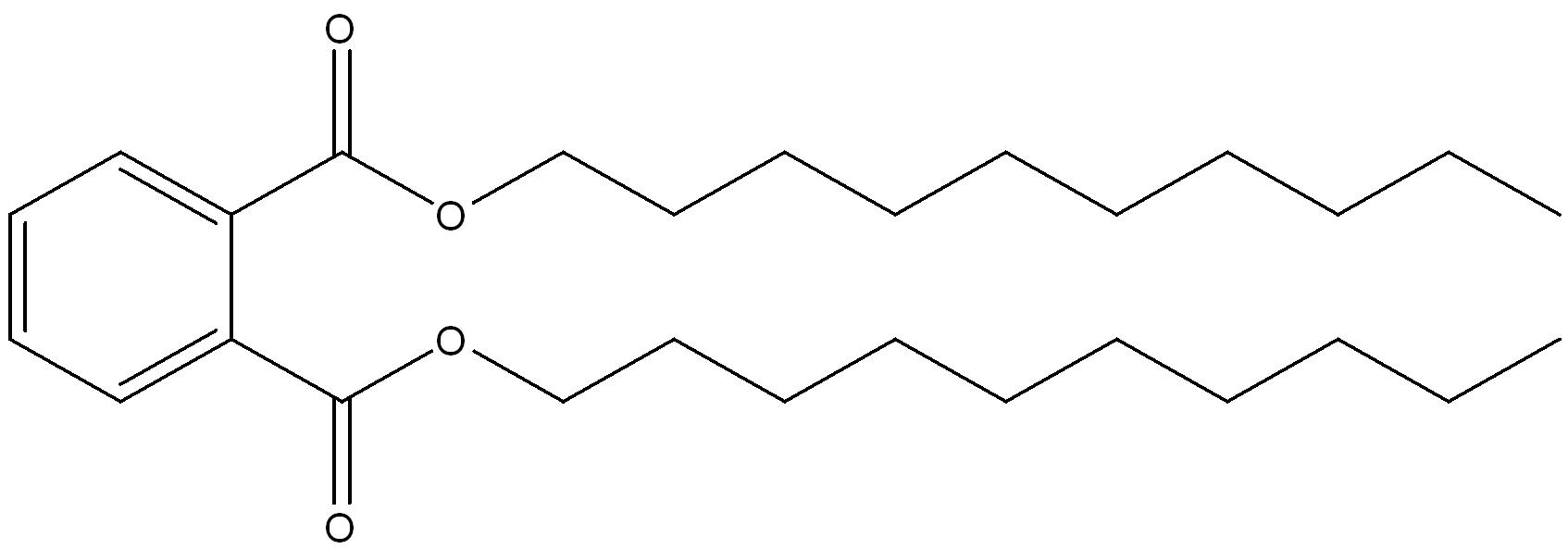 n-undecyl ester groups 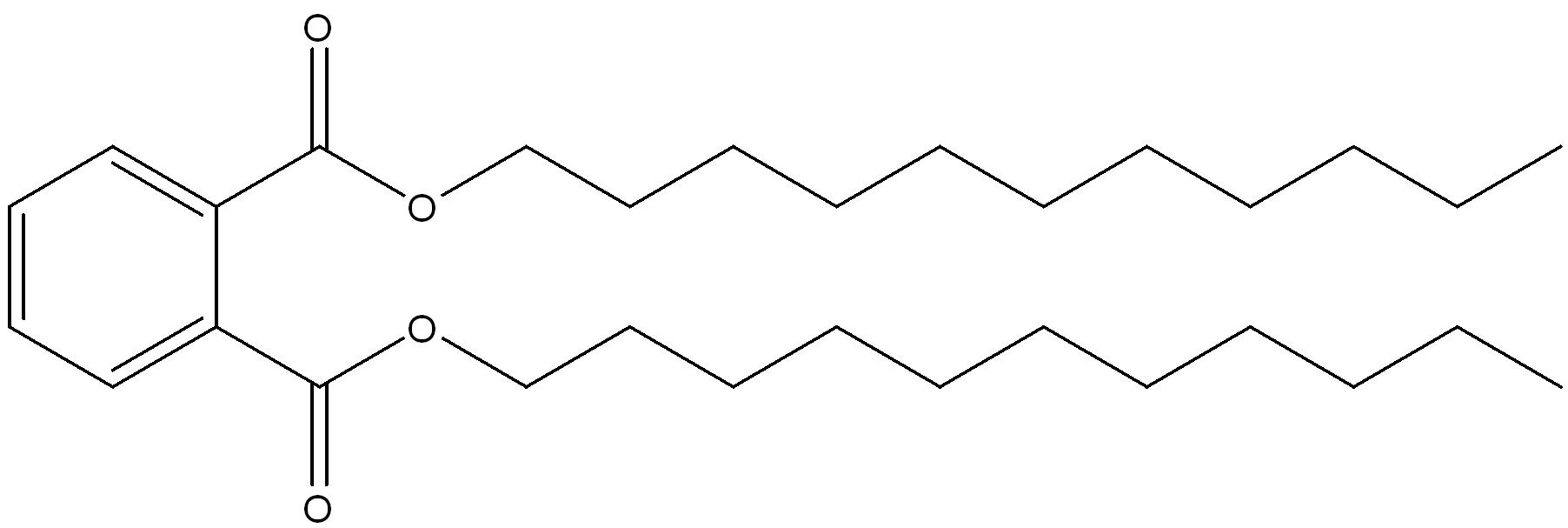 |
Mostly Linear (greater than 80%) Branched (20%) (9-11) |
| 117-84-0 | 1,2-Benzenedicarboxylic acid, 1,2-dioctyl ester | di-n-octyl phthalate (DnOP) |
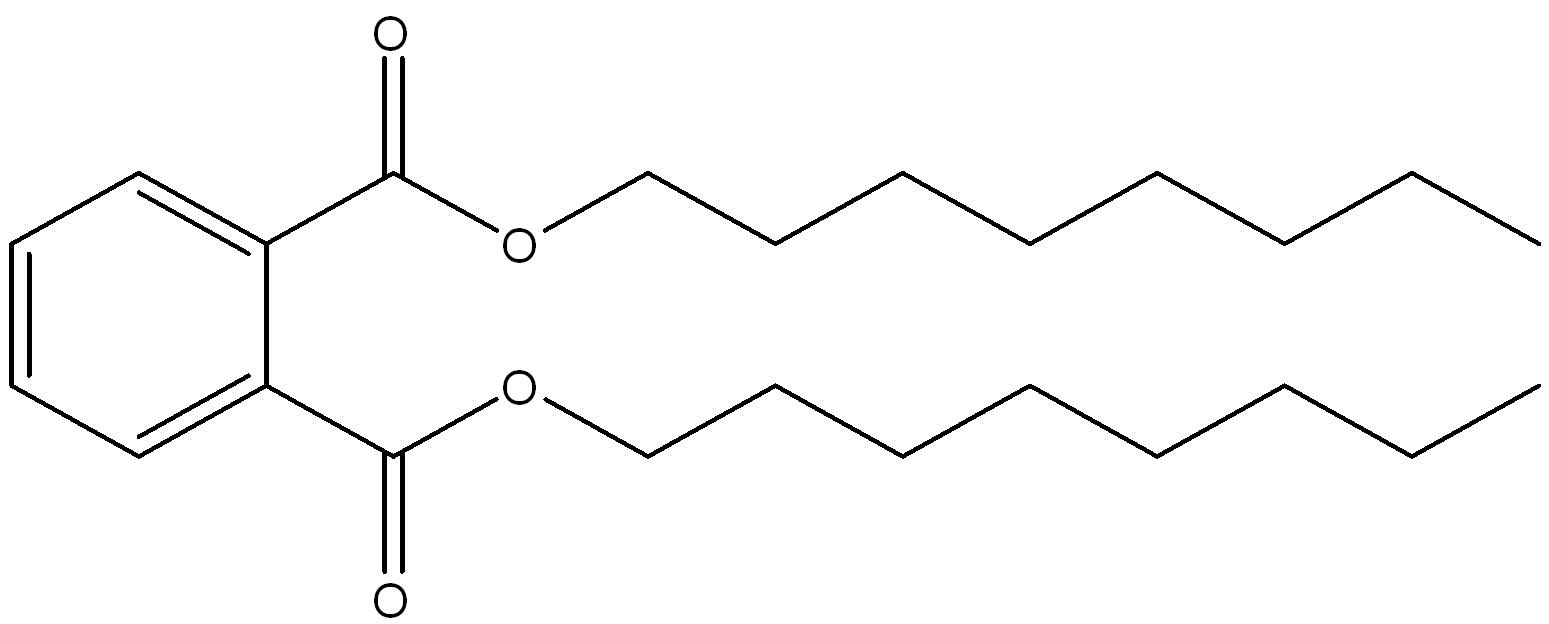 |
Linear (8) |
3. Physical and Chemical Properties
Physical and chemical properties determine the overall characteristics of a substance and are used to determine the suitability of different substances for different types of applications. Such properties also play a critical role in determining the environmental fate of substances (including their potential for long-range transport), as well as their toxicity to humans and non-human organisms.
A summary of physical and chemical properties for DIDP and DUP is presented in Table 3-1. More detailed information for the individual substances is available in Appendix B. Property values designated for use in modelling are identified in Appendix Tables B-1 and B-2.
| Property | Value or rangeFootnote Table 3-1[a] | Type of data | Key references |
|---|---|---|---|
| Physical state | Liquid | Experimental | European Commission 2000 |
| Melting point (°C) | -40 to 35.5 | Experimental | European Commission 2000; Mackay et al. 2006; ECHA 2014 |
| Melting point (°C) | 106 to 156 | Modelled | MPBPVPWIN 2010 |
| Boiling point (°C) | 336 to 463 | Experimental | Mackay et al. 2006; ECHA 2014 |
| Boiling point (°C) | 454 to 500 | Modelled | MPBPVPWIN 2010 |
| Density (kg/m3) | 954 to 970 | Experimental | European Commission 2000; ECHA 2014 |
| Vapour pressure (Pa) | 4.97 × 10-7 to 7.0 × 10-5 | Experimental, Calculated | Yaws 1994; Cousins and Mackay 2000 |
| Vapour pressure (Pa) | 6.55 × 10-5 to 3.77 × 10-2 | Modelled | MPBPVPWIN 2010 |
| Water solubility (mg/L) | 4.41 × 10-6 to 1.2Footnote Table 3-1[b] | Experimental, Calculated | Howard et al. 1985; Cousins and Mackay 2000 |
| Water solubility (mg/L) | 7.1 × 10-7 to 0.078 | Modelled | VCCLab 2005; WSKOWWIN 2010 |
| Henry's Law constant (Pa·m3/mol) | 21.6 to 50.5 | Calculated | Cousins and Mackay 2000 |
| Henry's Law constant (Pa·m3/mol) | 3.7 to 6.5 | Modelled | HENRYWIN 2011 Bond and Group estimates |
| Henry's Law constant (Pa·m3/mol) | 1.75 × 102 to 4.4 × 104 | Modelled | HENRYWIN 2011 VP/WS estimateFootnote Table 3-1[c] |
| Log Kow (dimensionless) | greater than 8 to 10.33 | Experimental, Calculated | Staples et al. 1997; Cousins and Mackay 2000 |
| Log Kow (dimensionless) | 9.12 to 12.13 | Modelled | VCCLab 2005; ACD/Percepta c1997-2012 |
| Log Koc (dimensionless) | 5.5 | Experimental | Williams et al. 1995 |
| Log Koc (dimensionless) | 5.8 to 7.1 | Modelled | KOCWIN 2010 |
| Log Koa (dimensionless) | 11.5 to 12.0 | Calculated | Cousins and Mackay 2000 |
| Log Koa (dimensionless) | 13.1 to 14.7 | Modelled | KOAWIN 2010 |
Models based on quantitative structure-activity relationships (QSARs) were used to generate data for some of the physical and chemical properties of DIDP and DUP. These models are mainly based on fragment addition methods, i.e., they sum the contributions of sub-structural fragments of a molecule to make predictions for a property or endpoint. Most of these models rely on the neutral form of a chemical as input; this is appropriate for DIDP and DUP as they occur as neutral (non-ionized) substances in the environment.
DIDP and DUP are oily liquids at room temperature. Based on experimental and modelled physicochemical property values, both DIDP and DUP have very low to low solubility in water, very low to low vapour pressure, and high to very high partition coefficients (Kow, octanol-water partition coefficient; Koc, organic carbon-water partition coefficient; Koa, octanol-air partition coefficient).
4. Sources
DIDP and DUP do not occur naturally in the environment.
An industry survey, issued pursuant to section 71 of CEPA 1999, was conducted in 2013 to obtain information on quantities in commerce for substances in the Phthalate Substance Grouping in Canada (Canada 2013). Quantities of DIDP and DUP reported for import, export and manufacturing in Canada in the year 2012 are summarized in Table 4-1 (Environment Canada 2014a). Due to the targeted nature of the survey, reported use quantities may not fully reflect all uses in Canada.
| Phthalate | CAS RN | Manufactured | Imported | Exported |
|---|---|---|---|---|
| DIDP | 26761-40-0 68515-49-1 | 10 000 to 100 000 | 1 million to 10 million | 100 000 to 1 million |
| DUP | 3648-20-2 | greater than 10 000 000 | 100 000 to 1 million | 1 million to 10 million |
In the United States, national aggregated production volumes of DIDP and DUP were reported through Inventory Update Reporting (IUR) between 1986 and 2002 and in 2006 (US EPA 2014a,b). The reported production volumes are summarized in Table 4-2.
| Phthalate | CAS RN | 2002 | 2006 |
|---|---|---|---|
| DIDP | 26761-40-0 68515-49-1 |
greater than 4.54 × 105 to 2.27× 108 | greater than 4.54 × 105 to less than 2.27× 108 |
| DUP | 3648-20-2 | greater than 4.54 × 106 to 2.27× 107 | 4.54 × 106 to less than 2.27×107 |
Production and use volumes of DIDP and DUP per annum reported by registrants under the European Union's REACH Registered Substances are provided in Table 4-3 (ECHA 2014).
| Phthalate | CAS RN | Reported quantity range |
|---|---|---|
| DIDP | 68515-49-1 | 1.0 × 108 to 1.0 × 109 |
| DIDP | 26761-40-0 | Not reported |
| DUP | 3648-20-2 | 1.0 × 107 to 1.0 × 108 |
DIDP and DUP are identified as high production volume chemicals in Europe as CAS RNs 68515-49-1 and 3648-20-2, respectively (ESIS 2014).
5. Uses
Information on uses of DIDP and DUP in Canada was submitted in response to a notice issued pursuant to section 71 of CEPA 1999 (Canada 2013). The data from the survey showed that both substances are used primarily as plasticizers. They are also used in adhesives and sealants, chemical manufacturing and have a role in the manufacturing of automobiles and automotive and transportation products. Both are found in electrical and electronic products (e.g., wiring and cable) to make the insulating sheath more flexible. DIDP is associated with a greater variety of uses than DUP in Canada with applications in building and construction materials, lubricants and greases, paints and coatings, fabric coatings, rubber materials and other manufactured items (Environment Canada 2014a).
DIDP and DUP have been identified to be used as plasticizers in food contact materials (September 2014 emails from the Food Directorate, Health Canada to the Risk Management Bureau, Health Canada; unreferenced). Specifically, DIDP is a plasticizer in polyvinylchloride (PVC) liners used to package aqueous, acidic and low alcohol food products and DUP is a plasticizer in PVC hose liners.
DIDP and DUP are not listed in the Drug Products Database, the Therapeutic Product Directorate's internal Non-Medicinal Ingredients Database, the Natural Health Products Ingredients Database or the Licensed Natural Health Products Database as medicinal or non-medicinal ingredients present in final pharmaceutical products, veterinary drugs or natural health products in Canada (DPD 2014; NHPID 2014; LNHPD 2014; September 2014 email from the Therapeutic Products Directorate, Health Canada to the Risk Management Bureau, Health Canada).
DIDP and DUP are not included on the List of Prohibited and Restricted Cosmetic Ingredients (more commonly referred to as the Cosmetic Ingredient Hotlist or simply the Hotlist), an administrative tool that Health Canada uses to communicate to manufacturers and others that certain substances, when present in a cosmetic, may contravene the general prohibition found in section 16 of the Food and Drugs Act or a provision of the Cosmetic Regulations (Health Canada 2011).
DUP was not identified as being present in pest control products registered in Canada (April 2012 email from the Pest Management Regulatory Agency (PMRA), Health Canada to the Risk Management Bureau, Health Canada; unreferenced). DIDP is registered as a formulant in Canada (January 2015 email from PMRA, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
A search of international uses was also conducted to provide information on potential manufactured items containing these substances that could be imported into Canada as these may not have been captured by the section 71 survey. Table 5-1 provides a summary of the general use of DIDP and DUP internationally.
| Uses | DIDP | DUP | References |
|---|---|---|---|
| Plastic and rubber sheeting | X | X | Ash and Ash 2003; ExxonMobil Chemical 2014a,b |
| Thermoplastics and flame-retardant plastics | X | X | NICNAS 2008a,b; Versar and SRC 2011 |
| Automotive applications | X | X | COWI, IOM and AMEC 2012; NICNAS 2008a,b; Cheminfo 2013a; ECHA 2014 |
| Electronics and appliances | X | X | US EPA 2014b; ECHA 2014 |
| Surfactant | X | NICNAS 2008a | |
| Wires and cables | X | X | BASF 2009; Versar and SRC 2011; ExxonMobil Chemical 2014a,b |
| Lubricating oils | X | Ash and Ash 2003; NICNAS 2008b; ECHA 2014 | |
| Printing Ink | X | X | NICNAS 2008a,b |
| Children and baby products | X | CSPA Reports 2014 | |
| Construction materials | X | HPD 2014 | |
| Food and pharmaceutical packaging | X | Ash and Ash 2003 | |
| Textiles | X | X | HSDB 2010; COWI, IOM and AMEC 2012; US EPA 2014a,b |
| Petrochemical manufacturing | X | US EPA 2014a,b | |
| Adhesives, sealants, paints, and coatings | X | X | Ash and Ash 2003; NICNAS 2008a,b; COWI, IOM and AMEC 2012; ExxonMobil Chemical 2014a,b; HPD 2014 |
| Paper products | X | X | NICNAS 2008a,b |
| Cosmetics and personal care productsFootnote Table 5-1[a] | X | X | ECHA 2014; SCCP 2007; HSDB 2010 |
| Manufacturing moulding | X | COWI, IOM and AMEC 2012 |
6. Releases to the Environment
There are no known natural sources of DIDP and DUP, and potential releases to the environment are restricted to those associated with anthropogenic activities.
Releases of DIDP and DUP to the Canadian environment could occur during their manufacture and processing, including the transportation and storage of materials, as well as during the production, use and disposal of products containing them. Releases from processing include losses during the manufacture of DIDP and DUP, the compounding of plasticizers and PVC resins to make flexible PVC, the fabrication of flexible PVC into products, and the production of construction materials, plastisols, coatings, and other products containing the PVC product (Leah 1977). Losses could also occur during transportation activities, such as during the cleaning of holding containers and truck tanks. Releases of DIDP and DUP from use and disposal activities include losses from products during service life, as well as during the final disposal of products in landfills and by incineration (Leah 1977). DIDP and DUP contained in products and manufactured items that are disposed of in landfills may migrate out of the products and items and could end up in landfill leachate. In 94% of large landfill sites in Canada (permitted to receive 40 000 tonnes of municipal solid waste annually), leachate is collected and treated on-site and/or off-site (sent to nearby wastewater treatment systemsFootnote[2]) prior to being released to receiving water. However, leachate is most likely not treated in smaller landfills (Conestoga-Rovers and Associates 2009). At these sites, DIDP and DUP may potentially be released to ground or surface water via leachate. Based on this, both non-dispersive and dispersive releases of DIDP and DUP to the environment are possible.
Releases are expected to occur primarily to air and to water. As DIDP and DUP are not chemically bound into polymer matrices during processing activities (Hakkarainen 2008), they can migrate to the surface of polymer products over time and potentially enter air through vapourization and water through leaching or abrasion. The rate of this migration is expected to be slow, however, and counteracted by chemical and physical attractive forces which work to hold the phthalates within polymers (personal communication, correspondence from Assessment Technologies, Inc., Keswick, VA to Ecological Assessment Division, Environment Canada dated October 2014; unreferenced). While both substances have low vapour pressures (4.97 × 10-7 to 3.77 × 10-2 Pa at 25°C; see Table 3-1), higher temperatures associated with some processing activities and environmental conditions could enhance their volatility and result in increased release into air.
Results from a section 71 survey conducted for the year 2012 (Canada 2013) indicate that manufacturing and processing activities for DIDP and DUP during that year were restricted to the industrial areas of Quebec and southern Ontario and, for this reason, potential releases during these activities are likely to be in these regions (Environment Canada 2014a). In all parts of Canada, releases are expected to primarily result from the use and disposal of products which contain the substances.
DIDP and DUP are not reportable substances under Environment Canada's National Pollutant Release Inventory (NPRI) program (Environment Canada 2014b).
7. Environmental Fate and Behaviour
7.1 Environmental Distribution
A summary of the steady-state mass distribution for the long-chain phthalates based on three emission scenarios to either air, water or soil is given in Table 7-1 below. Results for the individual CAS RNs are provided in Environment Canada (2015). The results in Table 7-1 represent the net effect of chemical partitioning, inter-media transport, and loss by both advection (out of the modelled region) and degradation/transformation processes. The results of Level III fugacity modelling indicate that the long-chain phthalates can be expected to distribute primarily into soil or sediment, depending upon the compartment of release, with smaller proportions distributing into air and water.
| Substances released to: | Air (%) | Water (%) | Soil (%) | Sediment (%) |
|---|---|---|---|---|
| Air (100%) | 2.1 - 7.8 | 1.1 - 2.2 | 71 - 78 | 19 - 20 |
| Water (100%) | 0 | 5.5 - 10 | 0 - 0.2 | 90 - 94 |
| Soil (100%) | 0 | 0 | 100 | 0 |
When released into air, these substances are predicted to distribute primarily into soil (71 to 78%; Table 7-1). High solid phase partition coefficients (log Kow greater than 8 to 12, log Koc 5.5 to 7.1; see Table 3-1) indicate that DIDP and DUP entering water from air can be expected to mainly distribute into sediment (19 to 20%), with only a small proportion (1.1 to 2.2%) remaining in the water column. A small proportion (2.1 to 7.8%) of the amount released into air is predicted to remain within this medium. High calculated and predicted log Koa values of 11.5 to 14.7 (Table 3-1) suggest that DIDP and DUP present in the atmosphere will be mainly sorbed to particulates in the air (Cousins et al. 2003). EQC (2011) predicts that 60 to 100% of DIDP and DUP released directly into air will distribute to the aerosol (particulate) fraction. These particulates may subsequently be deposited to soil and vegetation through wet or dry deposition processes, thereby limiting the potential for transport of DIDP and DUP in air. As well, there is potential for DIDP and DUP sorbed to air particulates to be transported some distance from the site of release; however, the rapid photolytic degradation of these substances (see Abiotic degradation section below) indicates that long-range atmospheric transport of DIDP and DUP is unlikely to occur.
DIDP and DUP released into water are predicted to distribute primarily into sediment (90 to 94%), with a smaller proportion (5.5 to 10%) remaining in the water. The very low vapour pressure (4.97 × 10-7 to 3.77 × 10-2 Pa at 25°C; see Table 3-1), indicates that DIDP and DUP released into water will have little tendency to distribute into air. While moderate to high calculated and modelled Henry's Law constant values (3.7 to 4.4 × 104 Pa·m3/mol at 25°C; Table 3-1) suggest that DIDP and DUP may potentially volatilize from water, this effect will likely be mitigated by strong sorption of the substances to suspended material in the water column (Cousins et al. 2003).
Level III fugacity modelling predicts that DIDP and DUP released into soil will remain within this compartment (100%). High solid phase partition coefficients indicate that these substances will sorb strongly to organic matter in soil and, together with the low water solubility (7.1 × 10-7 to 1.2 mg/L at 20 to 25°C; Table 3-1); this suggests that they will have low mobility and are unlikely to leach through soil into groundwater.
7.2 Environmental Persistence
Biodegradation is the primary removal mechanism for DIDP and DUP in aquatic and terrestrial environments, while indirect photolysis (reaction with atmospheric hydroxyl radicals) will predominate in air. Both substances biodegrade rapidly under aerobic conditions, with complete removal (mineralization) occurring in the order of weeks to months. No information was found on the biodegradation potential of DIDP and DUP under anaerobic conditions. However, data for a structurally similar substance, DINP, indicate that anaerobic biodegradation will proceed more slowly and may result in substance half-lives exceeding one year. Based on this, DIDP and DUP may have the potential to remain for longer periods in low-oxygen media, such as sub-surface sediments and soils. However, evidence for relatively rapid degradation of the primary transformation product, even under low-oxygen conditions, indicates that while DIDP and DUP may remain resident in the environment for longer periods than would be predicted based on laboratory testing, neither substance is expected to persist in the environment.
No soil degradation data were found for DIDP and DUP. Similar to sediment, it is likely that residence times in this medium may be longer due to sorption to soil particulates. However, neither substance is expected to persist in soil.
7.2.1 Abiotic Degradation
As with all phthalates, DIDP and DUP can mineralize completely through a degradation pathway that occurs abiotically or through biological mechanisms and involves sequential hydrolysis of the ester linkages on the molecule (Liang et al. 2008; Otton et al. 2008). The first hydrolytic step results in the formation of the mono-alkyl phthalate ester (MPE). The MPE can then undergo further ester hydrolysis to form phthalic acid, which degrades to benzoic acid and ultimately to carbon dioxide (Otton et al. 2008). As hydrolysis reactions are important in the breakdown of phthalates, the fairly slow rates of DIDP and DUP hydrolytic degradation in water (Table 7-2) are likely to be influenced by the very low water solubility of these substances.
Table 7-2 presents key abiotic degradation data for DIDP and DUP. No empirical data were found and model estimates were used to evaluate the potential for degradation through abiotic processes.
| Common name | Fate process | Degradation endpoint or prediction | Extrapolated half-life (t1/2 = days) |
Reference |
|---|---|---|---|---|
| DIDP | Atmospheric oxidation | Half-life | 0.20 - 0.28 | AOPWIN 2010 |
| DIDP | Ozone reaction | N/A | N/A | AOPWIN 2010 |
| DIDP | Hydrolysis | Half-life (pH = 7) |
1251 | HYDROWIN 2010 |
| DIDP | Hydrolysis | Half-life (pH = 8) |
125 | HYDROWIN 2010 |
| DUP | Atmospheric oxidation | Half-life | 0.18 | AOPWIN 2010 |
| DUP | Ozone reaction | N/A | N/A | AOPWIN 2010 |
| DUP | Hydrolysis | Half-life (pH = 7) |
2808 | HYDROWIN 2010 |
| DUP | Hydrolysis | Half-life (pH = 8) |
281 | HYDROWIN 2010 |
AOPWIN (2010) predicts that DIDP and DUP will undergo photolytic degradation through reaction with atmospheric hydroxyl radicals, with estimated half-lives of less than one day (see Table 7-2). In addition, these substances contain chromophores that will absorb light at wavelengths of greater than 290 nm and this indicates that they may be susceptible to direct photolysis by sunlight (Lyman et al. 1990). Therefore, DIDP and DUP are unlikely to remain for long periods of time in air.
DIDP and DUP are predicted to hydrolyze slowly at 25°C, with half-lives of 125 and 281 days, respectively, at a water pH of 8 and much longer half-lives of 1251 and 2808 days (3.4 and 7.7 years), respectively, at neutral pH (pH 7; HYDROWIN 2010).
7.2.2 Biodegradation
Table 7-3 and Table 7-4 summarize key primary and ultimate biodegradation data for DIDP and DUP.
DIDP is rapidly biodegraded to intermediate products (primary biodegradation) in aerobic aqueous environments, with 68% removal of the parent substance reported to occur within 1 day (O'Grady et al. 1985) and 90 to 100% removal of the parent in 10 to 28 days using acclimated (Sugatt et al. 1984; O'Grady et al. 1985) and non-acclimated (O'Grady et al. 1985) microorganisms (see Table 7-3). Primary biodegradation of DUP proceeds more slowly, with 29 to 45% removal of the parent substance occurring in one day (Saeger and Tucker 1976) and 76 to 100% removal in one to five weeks (Saeger and Tucker 1976; Sugatt et al. 1984; Furtmann 1993). BIOWIN (2010) predicts that DIDP and DUP will undergo primary biodegradation over a period of days or weeks (Table 7-3).
| Common name | Fate process | Degradation endpoint or prediction | Extrapolated half-life (t1/2 = days) |
Reference |
|---|---|---|---|---|
| DIDP | Aerobic | 68% at 1 dFootnote Table 7-3[a] | N/A | O'Grady et al. 1985 |
| DIDP | Aerobic | 10 d to achieve greater than or equal to 90% biodegradationFootnote Table 7-3[b] |
N/A | O'Grady et al. 1985 |
| DIDP | Aerobic | greater than 99% at 28 dFootnote Table 7-3[c] | N/A | Sugatt et al. 1984 |
| DIDP | Aerobic | 3.3 - 3.7Footnote Table 7-3[d],Footnote Table 7-3[e] "biodegrades fast" |
Days to weeks | BIOWIN 2010 |
| DUP | Aerobic | 29, 45% at 1 da | N/A | Saeger and Tucker 1976 |
| DUP | Aerobic | 76 - 100% in 7 da | N/A | Furtmann 1993 |
| DUP | Aerobic | greater than 99% at 28 dFootnote Table 7-3[f] | N/A | Sugatt et al. 1984 |
| DUP | Aerobic | less than 20% remaining at 5 weeksa,Footnote Table 7-3[g] | N/A | Saeger and Tucker 1976 |
| DUP | Aerobic | 4.1d,e "biodegrades fast" |
Days | BIOWIN 2010 |
Rates of ultimate biodegradation (mineralization) are similar for the two substances, with 28-day removal rates of 56 to 74% and 57 to 76% for DIDP and DUP, respectively (Table 7-4). Calculated half-lives based on standard biodegradation testing are also similar, with values of 9.6 and 6.2 days reported for DIDP and DUP, respectively (Sugatt et al. 1984). Both BIOWIN (2010) and CATALOGIC (2012) predict that ultimate biodegradation of DIDP and DUP will be rapid, in the range of weeks to months.
| Common name | Degradation endpoint or prediction | Test method or model basis | Extrapolated half-life (t1/2 = days) |
Reference |
|---|---|---|---|---|
| DIDP | 42% at 21 dFootnote Table 7-4[a],Footnote Table 7-4[b] | BOD | N/A | CHRIP 2014 |
| DIDP | 56% at 28 dFootnote Table 7-4[c] | CO2 evolution | 9.6 | Sugatt et al. 1984 |
| DIDP | 74% at 28 da | O2 consumption | N/A | ExxonMobil Biomedical Sciences, Inc. 2010 |
| DIDP | 74% at 47 da | ThOD | N/A | Exxon Biomedical Sciences, Inc. 1998 |
| DIDP | 2.1 - 2.5Footnote Table 7-4[d] "biodegrades fast" |
Sub-model 3: Expert Survey (qualitative) | Weeks to months | BIOWIN 2010 |
| DIDP | 0.7 - 0.8Footnote Table 7-4[e] "biodegrades fast" |
Sub-model 5: MITI linear probability | Readily biodegradable | BIOWIN 2010 |
| DIDP | 0.7e "biodegrades fast" |
Sub-model 6: MITI non-linear probability | Readily biodegradable | BIOWIN 2010 |
| DIDP | 53 - 84 "biodegrades fast" |
% BOD | 10.5-25.6Footnote Table 7-4[f] | CATALOGIC 2012 |
| DUP | 57% at 28 da | O2 consumption | N/A | Exxon Biomedical Sciences, Inc. 1995 |
| DUP | 76% at 28 dFootnote Table 7-4[g] | CO2 evolution | 6.17 | Sugatt et al. 1984 |
| DUP | 3.0d "biodegrades fast" |
Sub-model 3: Expert Survey (qualitative) | Weeks | BIOWIN 2010 |
| DUP | 1.0e "biodegrades fast" |
Sub-model 5: MITI linear probability | Readily biodegradable | BIOWIN 2010 |
| DUP | 0.9e "biodegrades fast" |
Sub-model 6: MITI non-linear probability | Readily biodegradable | BIOWIN 2010 |
| DUP | 84 "biodegrades fast" |
% BOD | 10.6Footnote Table 7-4[h] | CATALOGIC 2012 |
No information was found on the potential for anaerobic biodegradation of DIDP and DUP. Data derived for a structurally similar substance, DINP (CAS RNs 28553-12-0 and 68515-48-0), determined anaerobic biodegradation half-lives of about one to two years for DINP (Lertsirisopon et al. 2006), indicating much slower biodegradation under anaerobic conditions. The slower removal rate suggests there is potential for the substance to remain longer in low-oxygen media, such as sub-surface sediments and soils.
In sediment toxicity testing with DIDP and the midge, Chironomus riparius, Brown et al. (1996) reported that DIDP remained essentially unchanged in the test sediment, with little or no degradation occurring over the 28-day period of the study. This study is described in more detail in the Potential for Bioaccumulation (section 7.3) and Ecological Effects (section 8.1) sections of this SOS report.
Kickham et al. (2012) investigated the relationship between biodegradation rates, hydrophobicity and sorption potential of phthalates in sediment and determined that while phthalates have the inherent capacity to be rapidly degraded by sediment microbes, the rate of biodegradation in natural sediments is influenced by the sorption potential of the phthalate to sediment. Phthalates with high sorption potential will have slower biodegradation rates, mainly due to a reduced fraction of bioavailable, freely dissolved chemical concentration in the interstitial water (Kickham et al. 2012). While DIDP and DUP were not included in the study, examination was given to the structurally-similar DINP. DINP has high hydrophobicity (log Kow 8.4 to 10, log Koc 5.5 to 5.7; Environment Canada and Health Canada 2015b) and therefore high sorption potential, and this is reflected in the long sediment biodegradation half-life of 12 000 days (about 33 years) calculated from the study. The study concluded that inherently biodegradable substances that are subject to a high degree of sorption, such as DINP and the long-chain phthalates, can be expected to exhibit long half-lives in natural sediments. The reduced bioavailability to microbial attack due to sorption also implies that the substances will be less bioavailable for uptake by benthic organisms.
Primary degradation products of phthalates, the monoesters or MPEs, appear to biodegrade rapidly even under conditions of low oxygen and this rapid removal is expected to impact the overall stability of long-chain phthalates in the environment. Otton et al. (2008) measured a mean biodegradation half-life of about one day for monoisodecyl phthalate (MIDP), the MPE of DIDP, in field-collected marine sediments tested at 22°C. Biodegradation was preceded by a lag phase of 22 to 30 hours. DUP was not examined in the study. Scholz (2003) reported 89% removal, after a two-day lag phase, of the MPE of DINP, monoisononyl phthalate (MINP), as determined by standard 28-day OECD ready biodegradation testing (OECD 1992). DIDP and DUP were not examined in the study. The results of these studies suggest that the initial conversion of the phthalate to its MPE may act as the rate-limiting step in the degradation process and, once accomplished, ultimate biodegradation of the MPE proceeds more rapidly.
A number of other factors may contribute to the stability and biodegradation potential of DIDP and DUP in the environment. Johnson et al. (1984) observed slower rates of primary biodegradation for phthalates having longer and/or more complex alkyl chain configurations, as well as for all phthalates at lower chemical concentrations and lower test temperatures. Decreased biodegradability at low chemical concentrations was reported by Boethling and Alexander (1979), who hypothesized that the energy obtained from oxidizing chemicals at low concentrations may be insufficient to meet the energy demands of the microorganisms. This, in turn, limits the proliferation of the organisms to levels needed to cause appreciable loss of the chemical (Boethling and Alexander 1979). Considered together with evidence for relatively rapid biodegradation of the primary MPE transformation products, the information suggests that DIDP and DUP may have the potential to remain resident in the environment for longer periods than would be indicated by laboratory-based biodegradation testing; however, it is unlikely that either substance will persist in the environment.
No soil degradation data were found for DIDP and DUP. Similar to their presence in sediment, it is likely that the high sorptive potential of these substances will result in longer soil residence times due to sorption to soil particulates. However, the evidence for biodegradation of the parent substances, as well as for the primary MPE degradation products, suggests that neither substance will persist in soil.
7.3 Potential for Bioaccumulation
Empirical bioconcentration factors (BCFs) of less than 14 and 147 L/kg wet weight (ww), and biota-soil/sediment accumulation factors (BSAFs) of 0.015 and 0.16, suggest that DIDP has low potential to bioaccumulate in aquatic and terrestrial organisms. Higher BCF values of about 3000 to 4000 L/kg ww were reported for DIDP in blue mussels; however, these values are considered to be not reliable. DIDP has been measured in a number of Canadian aquatic species (Mackintosh et al. 2004; McConnell 2007; Blair et al. 2009) and this confirms that the substance is bioavailable. A field-based food web magnification factor (FWMF), equivalent to a trophic magnification factor (TMF), of 0.44 indicates that the substance was not biomagnifying across trophic levels of the studied food web but was instead undergoing trophic dilution. No empirical bioconcentration or bioaccumulation data were found for DUP; however, based on structural and chemical similarities with DIDP, this substance is also expected to not bioaccumulate to any great extent in organisms. Modelled BCFs and bioaccumulation factors (BAFs) for DIDP and DUP range from 1.0 to 186 L/kg ww and 1.4 to 40 L/kg ww, respectively, providing further evidence for low bioaccumulation potential.
7.3.1 Bioconcentration Factor (BCF) and Bioaccumulation Factor (BAF)
Experimentally-derived BCF values for DIDP are presented in Table 7-5. Some laboratory-based soil and sediment bioaccumulation study data are also available for earthworms and midges. No experimental bioconcentration or bioaccumulation data were found for DUP. The accurate determination of a water-based BCF for these substances is likely to be complicated by their very low water solubility and high octanol-water partition coefficients. For example, the water solubility and log Kow of DIDP are 9.97 × 10-6 to 1.2 mg/L and greater than 8 to 9.78, respectively (see Appendix Table B-2).
BCF values of less than 3.6 L/kg ww and less than 14 L/kg ww were reported for carp, Cyprinus carpio, exposed to water concentrations of 0.10 to 1.0 mg/L DIDP for 56 days (CHRIP 2014; Table 7-5). Water flea, Daphnia magna, exposed to 0.003 to 0.10 mg/L for 21 days bioconcentrated DIDP by factors of 90 to 147 L/kg ww (mean BCF value 116 L/kg ww; Brown and Thompson 1982a). Much higher BCFs of 2998 to 3977 L/kg ww were measured in mussels, Mytilus edulis, exposed through water-only testing to concentrations of 0.004 to 0.04 mg/L for 28 days (Brown and Thompson 1982b). DIDP residues were rapidly lost from the tissue following cessation of exposure, with calculated 14-day depuration half-lives of 3.5 to 3.8 days (Brown and Thompson 1982b).
While fish BCF values were determined through direct analysis of tissue and water concentrations, Daphnia and mussel BCFs were derived using radiolabelling of carbon in the ring structure of the DIDP molecule (see Table 2-1). Therefore, these BCF values reflect not only the presence of the parent DIDP but also any metabolites containing the labelled carbonyl carbon (Brown and Thompson 1982a,b). The 14C label position in the aromatic ring of DIDP was selected as it was considered likely to give the most significant information on possibly stable metabolites (Brown and Thompson 1982b).
Additionally, mussels in the Brown and Thompson (1982b) study were fed continuously throughout the exposure phase, while Daphnia were fed daily by direct addition (Brown and Thompson 1982a). Given the high partition coefficients of DIDP (log Kow greater than 8 to 9.78; log Koc 5.5 to 6.5; see Table B-2), sorption to food particles with subsequent ingestion by the test organisms is therefore likely to have contributed to the calculated BCFs. Both studies also included a carrier substance (acetone) to enhance the low water solubility of DIDP and this will have influenced the bioavailability of DIDP in the test solutions. No information was found on the feeding protocol or possible carrier used in the 56-day fish study cited in CHRIP (2014).
A BCF of 1 L/kg ww was calculated for DIDP using an empirically-derived elimination rate constant of 0.83 day-1 determined in a feeding study with rainbow trout, Oncorhynchus mykiss (ECHA 2014). A mean measured dietary concentration of 1145 mg DIDP/kg feed was administered to the fish over a 14-day exposure phase, which was followed by an 8-day depuration period during which the fish were fed untreated food. The study was assigned a reliability of 2 (reliable with restrictions) by the European Chemicals Agency (ECHA), based on the rationale that although the data were developed using a non-standard test procedure rather than an established BCF study protocol, the information was well documented and testing followed accepted scientific procedures.
| Test organism | Experimental concentration (duration) |
BCF (L/kg ww) |
Reference |
|---|---|---|---|
| Common carp (Cyprinus carpio) |
0.10 - 1.0 mg/L (56 days) |
less than 3.6 - less than 14.4 |
CHRIP 2014 |
| Water flea (Daphnia magna) |
0.003 - 0.10 mg/L (21 days) |
90 - 147 (mean 116) |
Brown and Thompson 1982a |
| Blue mussel (Mytilus edulis) |
0.004 - 0.04 mg/L (28 days) |
2998 - 3977 (mean 3488) |
Brown and Thompson 1982b |
| Rainbow trout (Oncorhynchus mykiss) |
182 mg/kg feed (14 days) |
1Footnote Table 7-5[a] | ECHA 2014 |
ECHA (2014) describes an unpublished 14-d acute toxicity study using earthworm, Eisenia fetida, and DIDP at a mean measured concentration of 7829 mg/kg dw soil. The test was conducted according to OECD Test Guideline 207 (Earthworm, acute toxicity; OECD 1984a) and was assigned a reliability of 1 (reliable without restriction) by ECHA when evaluating the study for data quality. Although not a specified endpoint under the test guideline, a biota-soil/sediment accumulation factor (BSAF) of 0.015 was calculated based on DIDP concentrations determined in the worm tissues and test soil. The results suggested that DIDP did not bioaccumulate in earthworms under the conditions of the study.
An earlier study by Brown et al. (1996) reported a BSAF of 0.6 for midge (Chironomus riparius) larvae exposed for 28 days to nominal DIDP concentrations of 100, 1000, and 10 000 mg/kg dw in a natural river sediment. Acetone was used as a carrier solvent and concentrations of DIDP in the sediment, as determined by total radioactive 14C-count, remained within 100 to 120% of nominal values throughout the course of the test. As well, at the start of the test, 89 to 93% of the measured radioactivity in the sediment could be attributed to the parent DIDP while, at the end of the test, this range was 89 to 92%. These results indicate that the percentage of parent DIDP in the sediment remained essentially unchanged, with little or no degradation occurring over the 28-day study period. The results also confirm that only a small proportion of the DIDP was taken up by the larval midges. Tissue concentrations measured in the emerging midge adults increased with increasing exposure concentrations and, as radiolabelling was used to determine measured values, the resulting BSAFs represent the presence of both DIDP and its metabolites.
No empirical BAF values were found for DIDP and DUP. In order to provide an additional line of evidence for bioaccumulation potential, modelled BCF and BAF estimates were derived using the BCFBAF (2010) model in EPI Suite (2000-2008) and the Baseline Bioaccumulation Model with Mitigating Factors (BBM 2008). BCFBAF (2010) sub-model 1 estimates BCF values of 76 to 83 L/kg ww for DIDP and 21 L/kg ww for DUP, using a regression-based approach that does not include consideration of metabolism (Table 7-6). Sub-model 2 of BCFBAF incorporates metabolism and yields lower BCF estimates of 1.0 to 1.4 and 1.0 L/kg ww for DIDP and DUP, respectively. BBM (2008) estimates BCFs of 166 to 186 and 31.6 L/kg ww for DIDP and DUP, respectively, citing mitigating factors of metabolism, large molecular size and low water solubility. BAF values of 1.4 to 40 and 16 L/kg ww were derived for DIDP and DUP, respectively, using the Arnot-Gobas mass balance approach of BCFBAF sub-model 3, which also incorporates consideration of whole body primary biotransformation but does not consider gut metabolism, a process that has been recognized to be of importance for phthalates in fish (Webster 2003). Therefore, the estimates derived using BCFBAF sub-models 2 and 3 are conservative values. Predicted BCFs and BAFs for DUP are lower than those for DIDP. There is good agreement between modelled bioaccumulation estimates and those derived empirically, with both indicating low bioaccumulation potential for the long-chain phthalates.
| Common name | Model and model basis | Endpoint | Value (L/kg ww) |
Reference |
|---|---|---|---|---|
| DIDP | BCFBAF Sub-model 1: linear regression |
BCF | 76, 83 | BCFBAF 2010 |
| DIDP | BCFBAF Sub-model 2: mass balance |
BCF | 1.4Footnote Table 7-6[a], 1.0Footnote Table 7-6[b] | BCFBAF 2010 |
| DIDP | BCFmax with mitigating factors | BCF | 166, 186Footnote Table 7-6[c] | BBM with Mitigating Factors 2008 |
| DIDP | BCFBAF Sub-model 3: Arnot-Gobas mass balance |
BAF | 40a, 1.4b | BCFBAF 2010 |
| DUP | BCFBAF Sub-model 1: linear regression |
BCF | 21 | BCFBAF 2010 |
| DUP | BCFBAF Sub-model 2: mass balance |
BCF | 1.0Footnote Table 7-6[d] | BCFBAF 2010 |
| DUP | BCFmax with mitigating factors | BCF | 31.6c | BBM with Mitigating Factors 2008 |
| DUP | BCFBAF Sub-model 3: Arnot-Gobas mass balance |
BAF | 16d | BCFBAF 2010 |
The results of both models emphasize the importance of metabolism in determining the bioaccumulation potential of DIDP and DUP. While no empirical metabolism data were available specific to these substances, a number of aquatic and terrestrial species have demonstrated the capacity to metabolize phthalates, including long-chain phthalates (e.g., Barron et al. 1995; Bradlee and Thomas 2003; Gobas et al. 2003), and it is expected that DIDP and DUP will also be effectively metabolized. Further evidence for the metabolic potential is provided by the results of ready biodegradation testing which confirm that microorganisms are able to readily break down both substances (see Biodegradation section 7.2.2).
The low water solubility of these substances, as well as their tendency to form stable emulsions in water (Bradlee and Thomas 2003), is expected to limit exposure to aquatic organisms, thereby limiting the potential for uptake and accumulation. Active metabolism will further reduce the potential for bioaccumulation.
7.3.2 Biomagnification Factor (BMF) and Trophic Magnification Factor (TMF)
BMF values describe the process by which the concentration of a chemical in an organism reaches a level that is higher than that in the organism's diet, due to dietary absorption (Gobas and Morrison 2000). ECHA (2014) reported a lipid-normalized BMF of less than 0.1 and a tissue elimination half-life of less than one day for DIDP, based on an empirically-determined elimination rate constant of 0.83 day-1 in rainbow trout, Oncorhynchus mykiss, fed a mean measured dietary concentration of 1145 mg/kg feed over 14 days. A BMF below 1 indicates that biomagnification is not likely to be occurring.
A lipid-normalized BMF of 0.0045 was calculated for rainbow trout, O. mykiss, fed a mean measured concentration of 988 µg/g feed DIUP (diisoundecyl phthalate; CAS RN 85507-79-5) for nine days, followed by a three-day depuration period (ExxonMobil Biomedical Sciences, Inc. 2005). DIUP is a long-chain phthalate that is structurally and chemically similar to DUP. An assimilation efficiency of 11% and growth-corrected whole body half-life of 0.19 day were also determined from the study.
Mackintosh et al. (2004) evaluated the distribution of DIDP and 12 other phthalates in a Canadian marine aquatic food web. DUP was not examined in the study. Concentrations of the target substances were measured in 18 marine species, representing approximately four trophic levels, and a food web magnification factor (FWMF) was calculated for each phthalate. The FWMF provided a measure of the degree of biomagnification occurring in the food web, and was determined from the average increase in lipid-equivalent chemical concentration for each unit increase in trophic position. Based on this description, the FWMF can be considered equivalent to a TMF. The FWMF for DIDP was calculated as 0.44, indicating that DIDP was not likely to be biomagnifying in this aquatic food web. Rather, the substance was undergoing trophic dilution, consistent with substances that are predominantly absorbed via the diet and depurated at a rate greater than the passive elimination rate via fecal egestion and respiratory ventilation, due to metabolism (Mackintosh et al. 2004).
7.4 Summary of Environmental Fate
DIDP and DUP may be released during industrial activities and through consumer use, with releases occurring primarily to air and to water. As these substances are not chemically bound into polymer matrices, they can slowly migrate to the surface of polymer products over time and potentially enter air through vapourization and water through leaching or abrasion. The rate of this migration is expected to be slow, however, and counteracted by chemical and physical attractive forces which work to hold the phthalates within polymers (see Releases to the Environment section). DIDP and DUP entering air will distribute into soil and to a lesser extent into water and then sediment. DIDP and DUP released into water will distribute into sediment and the suspended particulate fraction of surface waters. Both substances degrade rapidly through abiotic and biological means and are not expected to persist in the environment. However, degradation may be slower under low oxygen conditions and this may promote slower removal and higher relative concentrations of the substances in the environment. As well, high use quantities suggest that releases to the environment, and therefore organism exposure, may be continuous. Based on information relating to releases and the predicted distribution in the environment, organisms residing in soil and in the aquatic environment (water column and sediment species) will have the highest exposure potential to DIDP and DUP. The relatively rapid biodegradation rates of both substances suggests that exposure will be greatest for organisms inhabiting areas close to release sites, as concentrations are expected to decrease with increasing distance from points of discharge into the environment. The very low water solubility and high hydrophobicity of both substances suggest that exposure will be primarily through the diet rather than via the surrounding medium. Empirical and modelled evidence indicate that DIDP and DUP have low bioaccumulation and biomagnification potential, likely as a result of reduced potential for uptake and high biotransformation capacity.
8. Potential to Cause Ecological Harm
8.1 Ecological Effects
All phthalates, including DIDP and DUP, are considered to exert adverse effects through a non-specific, narcotic mode of toxic action. Parkerton and Konkel (2000) estimated critical body residues (CBRs) for parent phthalates and their metabolites to be in the range for nonpolar narcotics, suggesting that these substances exert adverse effects through baseline toxicity. Some phthalates and phthalate metabolites may also operate as polar narcotics. A more detailed discussion on the possible mode of action for substances in the Phthalates Substances Grouping is provided in the Approach document for considering cumulative risk (Environment Canada and Health Canada 2015a).
Parkerton and Konkel (2000) proposed that phthalates with high hydrophobicity (i.e., log Kow greater than 5.5), which includes DIDP and DUP, do not cause acute or chronic toxicity in aquatic organisms because the combined effects of low water solubility and limited bioconcentration potential prevent concentrations of the substance in the tissues of organisms from reaching levels sufficient to cause adverse effects.
Results from standard laboratory toxicity studies conducted using aquatic, sediment and terrestrial species indicate no adverse effects up to the water solubility or saturation limit of DIDP and DUP. One Daphnia study reported a definitive endpoint value for DIDP; however, the observed daphnid mortality was attributed to physical effects resulting from the presence of undissolved DIDP in the test system rather than to direct chemical toxicity. It is important to note that standard toxicity tests were conducted using test concentrations that are well above those expected to occur in the environment and do not therefore represent realistic exposure conditions.
In vitro testing using porcine ovarian cells suggests that DIDP may have the ability to influence normal endocrine activity in mammals. However, no evidence of endocrine effects was seen in two in vivo fish studies.
8.1.1 Water
Table 8-1 summarizes the key aquatic toxicity studies for DIDP and DUP. Acute median lethality or effects data (L/EC50or acute lowest- and no-effect levels) are available for fish, invertebrates and bacteria, while endpoint values for chronic testing (EC50, lowest- and no-effect levels) were found for algae and Daphnia. In all studies except the chronic Daphnia testing of Rhodes et al. (1995), the selected endpoint value exceeded the highest test concentration used in the study. As studies were conducted using test concentrations that approached or exceeded maximum water solubility limits under the conditions of the particular study (Brown and Thompson 1982a; Adams et al. 1995; Rhodes et al. 1995; ECHA 2014), the results indicate that adverse effects are not expected to occur up to the maximum water solubility limit of the substance.
| Common name | Test organism | Endpoint | Value (mg/L)Footnote Table 8-1[a] | Reference |
|---|---|---|---|---|
| DIDP | Rainbow trout, Oncorhynchus mykiss |
96 h LC50 mortality |
less than 0.62 | Adams et al. 1995 |
| DIDP | Fathead minnow, Pimephales promelas |
96 h LC50 mortality |
less than 0.47, less than 1.00 |
Adams et al. 1995 |
| DIDP | Bluegill sunfish, Lepomis macrochirus |
96 h LC50 mortality |
less than 0.37 | Adams et al. 1995 |
| DIDP | Sheepshead minnow, Cyprinodon variegatus |
96 h LC50 mortality |
less than 0.47 | Adams et al. 1995 |
| DIDP | Midge, Paratanytarsus parthenogenetica | 96 h LC50 mortality |
less than 0.64 | Adams et al. 1995 |
| DIDP | Mysid shrimp, Mysidopsis bahiaFootnote Table 8-1[b] |
96 h LC50 mortality |
less than 0.08 | Adams et al. 1995 |
| DIDP | Water flea, Daphnia magna |
48 h EC50 immobilization |
less than 0.02 | Adams et al. 1995 |
| DIDP | Water flea, Daphnia magna |
48 h EC50 immobilization |
less than 0.32Footnote Table 8-1[c] | Brown and Thompson 1982a |
| DIDP | Water flea, Daphnia magna |
21 d NOEC 21 d LOEC survival, reproduction |
0.030 0.060Footnote Table 8-1[d] |
Rhodes et al. 1995 |
| DIDP | Water flea, Daphnia magna |
21 d NOEC 21 d LOEC survival, reproduction |
0.10 less than 0.10 |
Brown and Thompson 1982a |
| DIDP | Water flea, Daphnia magna |
21 d NOEC 21 d LOEC survival, reproduction, growth |
1.0 less than 1.0Footnote Table 8-1[e] |
Brown et al. 1998 |
| DIDP | Green algae, Selenastrum capricornutumf |
96 h EC50 growth |
less than 0.80 | Adams et al. 1995 |
| DIDP | Marine bacterium, Photobacterium phosphoreum |
15 minute NOEC 15 minute LOEC photo-luminescence inhibition |
83 less than 83 |
ECHA 2014 |
| DIDP | Activated sludge microorganisms | 30 minute EC50 respiration inhibition |
less than 83.9 | ECHA 2014 |
| DUP | Rainbow trout, Oncorhynchus mykiss |
96 h LC50 Mortality |
less than 1.40 | Adams et al. 1995 |
| DUP | Rainbow trout, Oncorhynchus mykiss |
120 d NOEC 120 d LOEC survival, growth |
0.30 less than 0.30 |
Rhodes et al. 1995 |
| DUP | Fathead minnow, Pimephales promelas |
96 h LC50 mortality |
less than 0.74, less than 1.30 |
Adams et al. 1995 |
| DUP | Bluegill sunfish, Lepomis macrochirus |
96 h LC50 mortality |
less than 0.73 | Adams et al. 1995 |
| DUP | Sheepshead minnow, Cyprinodon variegatus |
96 h LC50 mortality |
less than 0.22 | Adams et al. 1995 |
| DUP | Midge, Paratanytarsus parthenogenetica | 96 h LC50 mortality |
less than 0.39 | Adams et al. 1995 |
| DUP | Mysid shrimp, Mysidopsis bahiab |
96 h LC50 mortality |
less than 0.29 | Adams et al. 1995 |
| DUP | Water flea, Daphnia magna |
48 h EC50 immobilization |
less than 0.02 | Adams et al. 1995 |
| DUP | Water flea, Daphnia magna |
21 d NOEC 21 d LOEC survival, reproduction |
0.059 less than 0.059 |
Rhodes et al. 1995 |
| DUP | Water flea, Daphnia magna |
21 d NOEC 21 d LOEC survival, reproduction, growth |
1.0 less than 1.0e |
Brown et al. 1998 |
| DUP | Green algae, Selenastrum capricornutumFootnote Table 8-1[f] |
96 h EC50 growth |
less than 2.10 | Adams et al. 1995 |
Rhodes et al. (1995) reported reduced survival in Daphnia magna exposed for 21 days to concentrations of 0.06 and 0.14 mg/L DIDP. The observed mortality was attributed to physical effects associated with surface entrapment of the daphnids, rather than toxicity from exposure of the animals to dissolved aqueous-phase chemical (Rhodes et al. 1995). This entrapment may have resulted from the presence of undissolved DIDP in micro-droplet form or as a surface layer on the water (Knowles et al. 1987; Rhodes et al. 1995). No adverse effects were observed in Daphnia exposed for 21 days to a maximum concentration of 0.059 mg/L DUP, although test organisms were also observed to be floating on the surface of the test solutions.
In 48-hour acute testing with DIDP and Daphnia, Brown and Thompson (1982a) noted that all Daphnia at the highest test concentration of 0.32 mg/L (nominal) were floating in the surface layer, while those at lower test concentrations (0.056 to 0.18 mg/L nominal) were not floating. The researchers hypothesized that DIDP at concentrations of 0.18 mg/L or less gave stable solutions, whereas loss from the bulk solution occurred at concentrations above this level. Therefore, the observed daphnid flotation behaviour may have resulted from the presence of DIDP above its solubility level precipitating onto the surfaces of the Daphnia (Brown and Thompson 1982a).
Brown et al. (1998) conducted 21-day D. magna testing using a single concentration of DIDP or DUP (1 mg/L nominal; measured range was 1.0 to 1.0 and 0.91 to 0.86 mg/L, respectively ) solubilized in the dispersant, "Marlowet R40" (castor oil-40-ethoxylate) at a proportion of 1:10 phthalate:dispersant. The testing was conducted according to OECD Guideline 202 (OECD 1984b), modified by individually separating the Daphniainto single test vessels. Endpoints examined were survival of the parent generation, number of young produced, and mean body length of the surviving parent daphnids. A dispersant was used to enhance the solubility of the test substances in order to more clearly delineate between adverse effects associated with physical entrapment and those relating to direct chemical toxicity. No adverse effects on Daphnia survival, reproduction or growth were observed with either test substance under the conditions of the study. Based on these results, the researchers concluded that any toxic effects observed in laboratory toxicity testing with long-chain phthalates were due to surface entrapment and not to inherent toxicity (Brown et al. 1998).
No ecotoxicity information was found on the primary monoester degradation products of DIDP and DUP; however, some data are available for the monoester of the closely-related phthalate, DINP. Scholz (2003) conducted standard toxicity testing on the primary metabolic degradation product of DINP, the monoester MINP. The 96-hour LC50 for MINP in carp, Cyprinus carpio, was 40 mg/L, while a 48-hour EC50 of 29 mg/L was determined for the water flea, Daphnia magna. The 72-hour EC50 for the green alga, Desmodesmus subspicatus, was greater than the highest test concentration of 51 mg/L. The water solubility limit for MINP is reported to be 56 mg/L (Scholz 2003), therefore all endpoint values fall below the solubility limit of the substance. Acute median lethal effect values of 29 to greater than 51 mg/L indicate that MINP does not have high toxicity to the species tested and this suggests that the primary monoester degradation products of DIDP and DUP will also not cause adverse effects in these species.
Patyna et al. (2006) conducted a multigenerational feeding study using Japanese medaka, Oryzias latipes, and DIDP at a nominal concentration of 20 µg/g of food (mean measured concentration was 19.3 µg/g). A feeding study is of particular relevance as the high log Kow of DIDP (greater than 8 to 9.78; see Appendix Table B-2) indicates that dietary exposure will be the primary exposure route for this substance. The study evaluated the potential for DIDP to cause reproductive and developmental effects in three generations of medaka, and examined a number of endpoints relating to biochemical, individual and population parameters. No significant effects were seen on survival, development, growth, and egg production of the DIDP-treated fish relative to the controls (negative and acetone). In addition, there were no significant effects to parameters associated with evidence of endocrine modulation, such as sex ratios and gonadal-somatic index, and no effects on 7-ethoxyresorufin-o-deethylase (EROD) activity. The metabolism of testosterone was elevated in female DIDP-treated fish as compared with that seen in the negative control fish. However, the effect was not statistically different from that of fish in the acetone control. The significance of this observation is unclear, as there appeared to be no adverse impacts to the individual fish in the DIDP treatment, nor were development and fecundity affected. Testosterone metabolism was slightly elevated in male DIDP-treated fish relative to that in the controls, but the effect was not statistically significant. Based on the results of the study, the researchers concluded that chronic dietary exposure to DIDP did not adversely impact Japanese medaka at the biochemical, individual, or population level (Patyna et al. 2006).
Chen et al. (2014) examined the potential for DIDP to exert acute toxicity or estrogenic activity in two species of fish. Embryos of zebrafish, Danio rerio, were exposed to 0.01 to 500 mg/L DIDP (nominal) in methanol carrier for 72 hours. While some toxicity occurred at high concentrations, greater than 50% lethality was not observed at any exposure level and the 72-hour LC50 was therefore greater than 500 mg/L. Twenty-four hour testing with estrogen-responsive transgenic medaka, Oryzias melastigma, was then conducted to determine the potential for estrogenic activity. The transgenic medaka contained an estrogen-dependent liver-specific gene which exhibited fluorescence when stimulated by estrogenic activity. Eleutheroembryos, the stage of hatched fish that rely on yolk and have not started external feeding, were used in the study. DIDP showed no estrogenic activity, both on its own and when tested together with a known estrogen-active compound, estradiol (E2). By comparison, fish exposed to increasing concentrations of E2 alone exhibited a dose-dependent increase in fluorescence in the estrogen-dependent marker gene, indicating enhanced estrogenic activity was occurring.
A dose of 10 mg DIDP/kg body weight was administered intraperitoneally to goldfish, Carassius auratus, for 10 days to investigate effects on the levels of three antioxidant enzymes (superoxide dismutase, catalase and glutathione peroxidase) in the liver of the fish (Zheng et al. 2013). The activity of all three enzymes decreased significantly in the treated fish relative to that seen in the controls, an indicator of reduced enzyme function and potential oxidative stress. It should be noted, however, that intraperitoneal injection of the test compound would circumvent metabolic processes occurring in the gut or elsewhere in the fish. For this reason, this exposure route is not realistic for environmental conditions.
Modelled aquatic toxicity estimates were also considered in a weight-of-evidence approach to evaluating the potential for adverse effects in organisms (Environment Canada 2007). Modelled ecotoxicity values derived using the ECOSAR program (ECOSAR 2009) in EPI Suite (2000-2008) were deemed to be not reliable because the model domain of applicability for log Kow was exceeded in both the ester and neutral organic (baseline toxicity) SARs.
8.1.1.1 Derivation of a predicted no-effect concentration
No evidence of chemical toxicity was seen in standard aquatic toxicity testing with DIDP and DUP up to their respective water solubility limits, although physical effects were sometimes observed.
As noted, the very low water solubility and high hydrophobicity of these substances suggest that dietary exposure will be the major route of exposure for organisms, rather than from the surrounding medium. For this reason, endpoint values derived from water concentrations may not fully describe the potential for effects. The toxic potential of substances that are taken up primarily through the diet is better captured by examining whole-body residues (internal concentrations) of the substance in an organism. Critical body residues (CBRs) can then be calculated in order to estimate the potential for the substance to reach internal concentrations that are sufficiently high to cause effects through baseline neutral narcosis (McCarty and Mackay 1993; McCarty et al. 2013). Baseline narcosis refers to a mechanism of toxic action which results from the disruption of cellular membranes due to the physical presence of the substance in tissues, rather than from chemical changes caused by exposure to a substance (Schultz 1989; McCarty et al. 2013).
CBRs were calculated for DIDP and DUP using the McCarty and Mackay (1993) equation:
CBR = BAF × WS / MW
where:
- CBR =
- the critical body residue (mmol/kg)
- BAF =
- fish bioaccumulation factor (L/kg); normalized to 5% body lipid
- WS =
- water solubility of the substance (mg/L)
- MW =
- molecular weight of the substance (g/mol)
Input values for DIDP were
- BAF 40 L/kg (highest Arnot-Gobas mass balance prediction; Table 7-6);
- Water solubility 1.7 × 10-4 mg/L (Letinski et al. 2002; Table B-2);
- Molecular weight 446.68 g/mol (Table 2-1).
Input values for DUP were
- BAF 16 L/kg (Arnot-Gobas mass balance prediction; Table 7-6);
- Water solubility 1.73 × 10-6 mg/L (EVA-adjusted WATERNT value; Table B-2);
- Molecular weight 474.73 g/mol (Table 2-1).
The input parameters selected for use in the calculation of CBR represent a conservative but realistic scenario.
Based on the above input values, the calculated CBR for DIDP was 1.5 × 10-5 mmol/kg while that of DUP was 5.8 × 10-8 mmol/kg.
McCarty and Mackay (1993) determined that CBRs associated with acutely lethal baseline neutral narcosis in small aquatic organisms typically range from about 2 to 8 mmol/kg, while those for chronic exposures range from 0.2 to 0.8 mmol/kg. CBR values calculated for DIDP and DUP are much lower than these, indicating that internal concentrations are unlikely to reach levels sufficient to elicit acute or chronic effects through a neutral narcosis mode of toxic action.
8.1.2 Sediment
Table 8-2 summarizes the key sediment toxicity studies for DIDP. No adverse effects were observed in sediment testing up to the highest concentrations of DIDP tested. No sediment toxicity data were found for DUP.
| Test organism | Endpoint | Value (mg/kg dw)Footnote Table 8-2[a] |
Reference |
|---|---|---|---|
| Chironomid, Chironomus tentans |
10 d NOEC 10 d LOEC survival, growth |
2630 greater than 2630 |
Call et al. 2001 |
| Amphipod, Hyalella azteca |
10 d NOEC 10 d LOEC survival, growth |
2090 greater than 2090 |
Call et al. 2001 |
| Chironomid, Chironomus tentans |
28 d NOEC 28 d LOEC emergence, sex ratio |
10 000Footnote Table 8-2[b] greater than 10 000 |
Brown et al. 1996 |
| Moor frog, Rana arvalis |
14 d NOEC 14 d LOEC egg hatching |
657 greater than 657 |
Wennberg et al. 1997 |
| Moor frog, Rana arvalis |
29 d NOEC 29 d LOEC tadpole survival, growth |
657 greater than 657 |
Wennberg et al. 1997 |
The maximum saturation of DIDP in sediment can be determined using the relationship:
Cs = Cw × Koc × foc
where:
- C s =
- maximum saturation of DIDP in sediment (mg/kg dw)
- C w =
- water solubility of DIDP (mg/L)
- K oc =
- organic carbon-water partition coefficient of DIDP (L/kg OC)
- f oc =
- fraction of organic carbon (OC) in the sediment (kg OC/kg)
Maximum saturation reflects the theoretical maximum amount of a substance that can dissolve into a given medium at equilibrium. It cannot be exceeded according to thermodynamic principles. In surface waters, the presence of co-solvents or surfactants can create conditions that allow for an "apparent solubility" which is slightly greater than the maximum solubility value. In solid phases, such as sediments and soils, maximum saturation is a direct function of the amount of organic carbon present in the matrix if it is assumed that only hydrophobic interactions with organic matter occur. Sediment organic carbon content can vary from location to location and often average carbon contents are used for calculating maximum saturation in sediments. The apparent solubility in water, and saturation in sediment or soil, can increase or decrease the bioavailability of a compound.
Selecting best values for water solubility and Koc of 1.7 × 10-4 mg/L and 9.12 × 105 (mean log Koc of 5.92 based on all experimental and modelled estimates), respectively (see Appendix Table B-2), and an foc value of 0.04 (default value for average Canadian sediment OC content), the maximum saturation of DIDP is calculated as 6.2 mg/kg dw of sediment. This value is much lower than the highest test concentrations used in the Call et al. (2001) study, suggesting that free DIDP was present in the test system. However, while the saturation limit was exceeded under the conditions of the study, no adverse effects were observed in either test species (see Table 8-2).
Similarly, Brown et al. (1996) used test sediment containing 3.6% OC and the saturation limit of DIDP under the conditions of the study was therefore 5.6 mg/kg dw. This value is much lower than the highest test concentration, suggesting free DIDP was present in the test system. Parkerton and Staples (2003) estimated an OC content of 9.0% for the sediment used in the study by Wennberg et al. (1997). Applying the above equation, the maximum saturation of DIDP in this sediment was 13.9 mg/kg dw, again much lower than the test concentrations used. Therefore, while the maximum saturation limits of DIDP were exceeded in all sediment toxicity testing, no adverse effects were seen in the test organisms.
8.1.2.1 Derivation of a predicted no-effect concentration
As with water column testing, no adverse effects were seen with sediment testing up to and above the saturation limit of DIDP. No sediment toxicity data were found for DUP.
A biota-sediment accumulation factor (BSAF) of 0.6 was reported for DIDP in the midge, Chironomus riparius (Brown et al. 1996; see section 7.3.1) and a CBR analysis was conducted to determine the potential for adverse effects.
Applying the CBR relationship described in section 8.1.1 above to DIDP in sediment,
CBR = BSAF × Cs / MW
where:
- CBR =
- the critical body residue (mmol/kg)
- BSAF =
- biota-sediment accumulation factor (kg/kg); normalized to 5% body lipid
- Cs =
- saturation limit of the substance in sediment (mg/kg)
- MW =
- molecular weight of the substance (g/mol)
Input values to the equation were
- BSAF 0.6 kg/kg (Brown et al. 1996 for midge, C. tentans; see section 7.3.1);
- Saturation limit of DIDP in sediment 6.2 mg/kg (assuming 4% OC content for average Canadian sediment);
- Molecular weight 446.68 g/mol (Table 2-1).
Using the maximum saturation in the calculation of CBR represents a conservative but realistic scenario.
Based on these input values, the calculated CBR is 0.008 mmol/kg. This value is less than the minimum effect threshold ranges of 2 to 8 mmol/kg and 0.2 to 0.8 mmol/kg proposed by McCarty and Mackay (1993) for acute and chronic narcotic effects, respectively. This suggests that while tissue levels of DIDP for sediment-dwelling organisms may reach higher concentrations than those in water column species, the levels will still remain below those predicted to result in acute or chronic effects due to baseline narcosis.
No empirical BSAF data were found for DUP; therefore, a CBR cannot be calculated for this substance in sediment species. As the log Kow of DUP is higher than that of DIDP (maximum 12.13 for DUP vs. maximum 9.78 for DIDP; see Appendix Table B-2), the uptake potential of DUP is expected to be similar to or less than that of DIDP. Therefore, tissue concentrations of DUP in sediment organisms are not likely to reach levels predicted to result in acute or chronic effects due to baseline narcosis.
8.1.3 Terrestrial
Terrestrial toxicity data for DIDP are presented in Table 8.3. No data were found for DUP.
| Test organism | Endpoint | Value (mg/kg dw)Footnote Table 8-3[a] |
Reference |
|---|---|---|---|
| Earthworm, Eisenia fetida |
14 d NOEC 14 d LOEC survival |
8808, 7829Footnote Table 8-3[b] greater than 8808, greater than 7829 |
Exxon Biomedical Sciences, Inc. 1996a |
| Lettuce, Lactuca sativa |
5 d NOEC 5 d LOEC seed germination |
8551, 8630b greater than 8551, greater than 8630 |
Exxon Biomedical Sciences, Inc. 1996b |
| Rye grass, Lolium sp. |
5 d NOEC 5 d LOEC seed germination |
8551, 8630b greater than 8551, greater than 8630 |
Exxon Biomedical Sciences, Inc. 1996b |
No adverse effects were observed in acute toxicity testing conducted with lettuce and rye grass and in chronic testing with earthworm.
Using the procedure described in section 8.1.2 above and reported organic carbon (OC) contents in the test soils of 1.7 and 4.0% (Parkerton and Staples 2003), the maximum saturation of DIDP in the three test systems described in Table 8-3 (i.e., Exxon Biomedical Sciences, Inc. 1996a,b) can be calculated as:
Csoil = Cw × Koc × foc
where:
- C soil =
- maximum saturation of DIDP in soil (mg/kg dw)
- C w =
- water solubility of DIDP (mg/L) = 1.7 × 10 -4mg/L
- K oc =
- organic carbon-water partition coefficient of DIDP = 9.12 × 10 5 L/kg OC
- f oc =
- fraction of OC in the soil = 0.017 and 0.040 kgOC/kg
The maximum saturation limit of DIDP was calculated as 2.6 and 6.2 mg/kg dw of soil for the 1.7 and 4.0% OC soils, respectively. These values are well below the highest test concentrations used in the studies, suggesting that free DIDP may have been present in the test systems. However, while the saturation limit was exceeded, no adverse effects were observed in any of the species tested (see Table 8-3).
No data were found on the potential for adverse effects to terrestrial plants through atmospheric exposure to DIDP and DUP. While these substances are expected to be released into air (see Releases to the Environment section), the estimated short atmospheric half-life of 0.20 to 0.28 day (AOPWIN 2010; Environmental Persistence section) and low levels measured in this environmental medium (Environment Canada 2015) suggest that organism exposure to DIDP and DUP via ambient air is likely to be limited.
No information was found on potential effects of DIDP and DUP in wildlife species. Laboratory studies using rodents and other mammalian species, considered as surrogates for mammals such as piscivores, have been conducted in order to evaluate the potential for impacts to human health. Relevant data from these studies are presented in the Human Health Effects section of this SOS report.
Results from in vitro testing provide preliminary indications that DIDP may have the potential to influence normal endocrine activity in mammalian species. Cultured porcine granulosa cells exposed simultaneously to 10-4 to 10-8 M DIDP and follicle-stimulating hormone (FSH) over a 72-hour incubation period exhibited enhanced levels of progesterone production and decreased estradiol production relative to FSH-only controls (Mlynarčíková et al. 2007). No significant change occurred in the production of either hormone when cells were exposed to DIDP in the absence of FSH. The results indicate that DIDP can alter the production of steroid hormones in the presence of an endocrine-active substance and under laboratory conditions.
8.1.3.1 Derivation of a predicted no-effect concentration
No adverse effects were observed in soil toxicity testing with DIDP and no soil toxicity data are available for DUP.
A biota-soil accumulation factor (BSAF) of 0.015 was reported in ECHA (2014) for earthworm, Eisenia fetida, exposed to DIDP (see section 7.3.1), and a CBR analysis was conducted to determine the potential for adverse effects.
Applying the CBR relationship described in sections 8.1.1 and 8.1.2 to DIDP in soil,
CBR = BSAF × Csoil / MW
where:
- CBR =
- the critical body residue (mmol/kg)
- BSAF =
- biota-soil accumulation factor (kg/kg); normalized to 5% body lipid
- C soil =
- saturation limit of the substance in soil (mg/kg)
- MW =
- molecular weight of the substance (g/mol)
Input values for the equation were
- BSAF 0.015 kg/kg (ECHA 2014; see section 7.3.1);
- Saturation limit of DIDP in 4% OC soil 6.2 mg/kg (determined above);
- Molecular weight 446.68 g/mol (Table 2-1).
Using the maximum saturation in the calculation of CBR represents a conservative but realistic scenario.
Based on these input values, the calculated CBR is 2.1 × 10-4 mmol/kg. This suggests that internal concentrations of DIDP in the earthworms did not reach levels sufficient to elicit acute or chronic effects through a neutral narcosis mode of toxic action (i.e., 2 to 8 mmol/kg or 0.2 to 0.8 mmol/kg, respectively; McCarty and Mackay 1993).
The mean measured test concentration of 7829 mg/kg dw reported by ECHA (2014) is equivalent to that of Exxon Biomedical Sciences, Inc. (1996a), in which no adverse effects were observed in 14-day earthworm toxicity testing (see Table 8-3). The lack of observed toxicity in this testing aligns well with the result obtained in the CBR analysis.
As with the sediment compartment, an empirical BSAF for soil organisms was not found for DUP. Based on the higher log Kow and therefore lower potential for uptake, the CBR of DUP is expected to be lower than that of DIDP. Therefore, it is unlikely that tissue concentrations of DUP will reach levels predicted to result in acute or chronic effects in soil organisms.
The results of rodent toxicity studies conducted for the evaluation of potential human health effects indicate that lowest observed adverse effect levels (LOAELs) from DIDP dietary studies are much higher than levels measured in the environment. For example, a LOAEL of 400 ppm (400 mg DIDP/kg food, corresponding to 22 mg/kg body weight per day) was reported for histologic changes in the livers of male rats exposed for two years to DIDP in the diet (Cho et al. 2008; see Human Health Effects section). Levels measured in the Canadian environment are low (see Measured Environmental Concentrations section below), which suggests that wildlife is unlikely to be exposed to concentrations approaching this LOAEL value. No chronic toxicity studies were found for DUP and few short-term oral toxicity studies were found. A lowest dietary LOAEL of 1145 mg/kg body weight per day, based on decreased body weight gain, increased absolute and relative liver weights and liver lesions in rats, was reported for DUP (Barber et al. 1987; see Human Health Effects section). Assuming an average body weight for rats of 0.35 kg (Sample et al. 1996), this corresponds to an approximate exposure concentration of 400 mg DUP per day for similarly-sized wildlife. No soil monitoring data were found for DUP and levels measured in sludge and sediments are low. Therefore, it seems likely that environmental concentrations will be lower than the reported LOAEL.
While the in vitro testing of Mlynarčíková et al. (2007) provides preliminary evidence to suggest that DIDP may have the capacity to influence normal mammalian hormonal function in the presence of an endocrine-active substance and under laboratory conditions, further supporting information is needed in order for the study results to be deemed acceptable for use in a quantitative analysis.
8.2 Ecological Exposure
A number of factors can contribute to variability and uncertainty in environmental monitoring data for DIDP and DUP. The widespread use of these substances can lead to issues with contamination during sample collection and especially during sample preparation and analysis, where phthalates can be present in low background levels in analytical equipment, laboratory air and reagents (Lin et al. 2003; McConnell 2007). This is especially problematic when measuring environmental occurrence at concentrations approaching the method detection limit. As well, incomplete separation of commercial mixtures can complicate the accurate determination of specific phthalates (Lin et al. 2003). There are also analytical issues associated with the chemical properties of these substances; for example, the very low water solubility and high hydrophobicity can result in emulsion formation in water samples and/or sorption to glassware, both of which will influence the accuracy of analytical determinations (Staples et al. 1997; Cousins et al. 2003).
8.2.1 Measured Concentrations in Environmental Media and Wastewater
Data concerning the measured presence of DIDP and DUP in the environment are presented in Environment Canada (2015). Canadian monitoring data are available for DIDP in surface waters, sediments and biota. Canadian data are also available for wastewater systems and their effluents.No Canadian monitoring data are available for DIDP in air and soil. No Canadian monitoring data were found for DUP.
A mean measured concentration of 67 ng/L DIDP was determined for 10 surface seawater samples collected in 1999 from False Creek, in Vancouver, British Columbia (Mackintosh et al. 2004). Blair et al. (2009) reported a mean value of 70 ng/L (estimated from graphical data) in 10 samples collected from the same inlet over the period 2004 to 2006. False Creek is an urbanized marine inlet in Vancouver and has a history of contamination from various industrial and urban sources.
Mackintosh et al. (2006) analyzed for DIDP in surface water and sediment samples collected in False Creek. Concentrations in total seawater samples ranged from 45 to 129 ng/L; however, much higher concentrations of 23 300 to 79 900 ng/g dry weight (dw) were determined in the suspended particulate fraction of water samples as compared with the dissolved fraction, where concentrations ranged from 15 to 44 ng/L. The results illustrate that, based on its physicochemical properties of low water solubility and high partition coefficients (Table 3-1), DIDP in water will associate to a high degree with suspended organic material present in the water column rather than occurring in the dissolved fraction. Interestingly, sediment samples collected from the same area contained lesser quantities than those measured in suspended particulates, ranging from 252 to 589 ng/g dw. The researchers proposed that the observed decline in concentration between suspended and bottom sediments suggested that desorption and biodegradation of DIDP were occurring at rates which exceeded the rate of decline in organic carbon content between suspended particulates (40% OC) and bed sediment (2.8% OC) (Mackintosh et al. 2006). A mean concentration of about 200 ng/g dw has also been reported for False Creek sediments (McConnell 2007; Blair et al. 2009).
Concentrations of 22 to 1296 ng DIDP/L were measured in effluents collected in 2002 and 2003 from eight wastewater treatment systems in Alberta (Sosiak and Hebben 2005). DIDP was also detected in surface water samples collected upstream (43 ng/L) and downstream (27 to 128 ng/L) of the plants.
Mackintosh et al. (2004) reported concentrations of 6 to 13 804 ng/g lipid weight (lw) in marine aquatic biota collected in 1999 from False Creek, Vancouver. The sampling program analyzed for DIDP in 18 marine species representing approximately four trophic levels of the marine aquatic food web and was designed to examine the potential for biomagnification of DIDP within the food web. In general, highest concentrations were associated with lower trophic level species, including plankton, algae (Enteromorpha intestinalis), geoduck clams (Panope abrupta) and Manila clams (Tapes philippinarum). However, high concentrations were also measured in striped seaperch (Embiotoca lateralis) and surf scoter (Melanitta perspicillata) (see Environment Canada 2015). Lower levels were found in most fish species (shiner perch, Cymatogaster aggregata; Pacific staghorn sculpin, Leptocottus armatus; English sole, Pleuronectes ventulis; white-spotted greenling, Hexogrammos stelleri; spiny dogfish (Squalus acanthias). DIDP was below the detection limit of 1.6 ng/g lw in samples of Pacific herring (Clupea harengus pallasi) and pile perch (Rhacochilus vacca). Further details on this study are provided in the Potential for Bioaccumulation section of this report.
McConnell (2007) and Blair et al. (2009) also reported the presence of DIDP in aquatic biota collected from False Creek, including green algae (Prasiola meridionlis; 6500 ng/g lw, 91 ng/g wet weight (ww) mean), blue mussel (M. edulis; 1300 ng/g lw, 6 to 7 ng/g ww mean), soft-shell clam (Mya arenaria; 4100 ng/g lw, 14 ng/g ww mean), Dungeness crab (Cancer magister; 270 to 720 ng/g lw, 1.8 to 21 ng/g ww mean), shiner perch (C. aggregata; 3000 ng/g lw, 57 ng/g ww mean) and spiny dogfish (S. acanthius; 1600 ng/g lw, 150 ng/g ww maximum). The substance was not detected (detection limits 2 to 37 ng/g ww) in white-spotted greenling (Hexogrammus stelleri). McConnell (2007) noted, but could not account for, the differences observed between the two datasets which reported DIDP concentrations for the same or similar species collected from the same sampling location but in two different years (1999 for Mackintosh et al. and 2005 for McConnell).
DIDP has been measured in Swedish air samples, as well as in surface waters, precipitation, sediments, and biota collected at a number of European locations. It has also been detected in European urban stormwaters and wastewater treatment system sludges. DUP was present in a small number of sediment and wastewater treatment system sludge samples collected in several Nordic countries in 2010 and 2011 (Remberger et al. 2013). Additional environmental monitoring data for DIDP and DUP are provided in Environment Canada (2015).
8.3 Characterization of Ecological Risk
8.3.1 Consideration of Lines of Evidence
This state of the science report presents information relating to the potential for the long-chain phthalates, DIDP and DUP, to cause harm to the Canadian environment and/or to human health. Lines of evidence considered in the report include those pertaining to use patterns, environmental release and distribution, potential for environmental persistence, bioaccumulation potential, toxicity and hazard potential, and the results of environmental monitoring studies. The database of information for DUP is considerably smaller than that of DIDP and, for this reason, data for DIDP were also considered to be representative of DUP, as in the evaluation of bioaccumulation potential and potential toxicity to sediment and soil organisms. This approach was deemed reasonable in light of similarities in the chemical structure and properties of the two substances.
DIDP and DUP are plasticizers and are used in a wide variety of consumer, commercial and industrial products. These substances are not chemically bound into the polymer matrices of products containing them and can slowly migrate to the surface of polymer products over time, potentially entering environmental media such as air or water. The rate of this migration is expected to be slow and counteracted by chemical and physical attractive forces which work to hold the phthalates within polymers. Based on high use quantities and widespread distribution in products, DIDP and DUP are considered to have high potential to be released into the Canadian environment.
Air and water are the primary receiving media for DIDP and DUP in the environment. DIDP and DUP released into the environment are predicted to distribute primarily into soil and sediment. They will also associate with suspended particulates in air and water. DIDP and DUP adsorbed to air particulates will distribute into soil and surface waters through wet and dry deposition processes. Therefore, sediments, soil and suspended particulates in surface waters are the primary exposure routes for organisms to DIDP and DUP in the environment.
DIDP and DUP degrade rapidly under aerobic conditions. No data were found on the potential for anaerobic degradation, although a sediment toxicity test with DIDP reported only minimal loss of the test substance over the 28-day study period and this implies a degree of stability in sediment. As sediments are generally lower in oxygen content than surface waters, this would indicate that DIDP degrades more slowly under lower oxygen conditions. As well, data derived for a suitable analogue substance, DINP, also suggest that DIDP and DUP will biodegrade more slowly under anaerobic conditions. However, neither substance is expected to persist in the environment. A lack of persistence indicates that the substance will eventually be removed through degradation and continued releases will dictate the resulting environmental concentrations reported in field monitoring programs. Therefore, organisms in the environment will not be exposed to increasing quantities of the substance over time, provided that future emissions remain unchanged or decline.
Limited monitoring data are available for DIDP and DUP. DIDP has been detected in air and water, while both substances have been reported in sediment samples, indicating that ongoing sources of these substances into the environment result in detectable concentrations that reflect the balance of emission inputs and degradation losses. Given the evidence for degradation potential, it is unlikely that DIDP and DUP will be transported long distances from the point of release and highest organism exposures are therefore expected to primarily occur near discharge sites. The predicted limited distribution into air, along with short atmospheric half-lives, suggests that DIDP and DUP will have little potential for long-range atmospheric transport.
High partition coefficients and low water solubility indicate that uptake of DIDP and DUP into organisms will occur primarily via the diet. Based on empirical and modelled data, DIDP has low bioaccumulation and biomagnification potential. Despite this, DIDP has been measured in a variety of aquatic species and this confirms that the substance is able to be taken up by organisms. Highest concentrations are usually associated with lower trophic level species, possibly due to feeding strategies which may include exposure through the ingestion of planktonic organisms and detritus having DIDP adsorbed to their external surfaces and filter feeding that can include co-uptake of DIDP adsorbed to particulates. As well, lower trophic organisms commonly have reduced metabolic capacities when compared with higher trophic species such as fish. However, DIDP has also been detected in some higher trophic species. Given the higher metabolic capabilities of these species, the presence of DIDP in these organisms is suggestive of high exposure, possibly from local sources into the environment. No empirical bioaccumulation data were found for DUP. Based on modelled BCF and BAF data for DUP, as well as an empirical BMF for a suitable analogue substance, DIUP, DUP is considered to have low potential to bioaccumulate within organisms and through food webs. This suggests that DUP will have little tendency to accumulate in tissues to levels that are high enough to cause adverse effects in the organism, nor will it be likely to transfer between organisms in predator-prey interactions in high enough amounts to cause toxicity.
DIDP and DUP demonstrate low hazard potential in standard laboratory testing conducted with aquatic and terrestrial species. In most cases, no adverse effects were observed at concentrations up to and exceeding the water solubility for water column testing with DIDP and DUP, and saturation limits for DIDP in sediment and soil toxicity tests. No sediment or soil ecotoxicity data are available for DUP; however, the potential for adverse effects in these media is considered to be comparable with that of DIDP and therefore low. No evidence for potential hormonal effects was found in in vivo laboratory testing with DIDP and medaka, although results from an in vitro study using porcine granulosa cells suggest that DIDP is capable of altering the production of steroid hormones under laboratory conditions. The potential for DIDP to influence normal hormonal function in organisms when in the presence of an endocrine-active substance in the environment has not been established. In light of monitoring data which indicate the potential for continuous exposure in the environment, the possible role of DIDP as an endocrine-active substance requires further exploration.
Results from an analysis of critical body residues (CBRs) derived using the water solubility limit of each substance indicate that maximum tissue concentrations of DIDP and DUP based on solubility limits will be much lower than levels associated with adverse acute or chronic effects in organisms due to neutral narcosis. Similar analyses conducted for DIDP in sediment and soil organisms indicated that maximum tissue concentrations calculated from the saturation limit of DIDP in a 4% OC sediment or soil do not exceed minimum concentrations estimated to cause narcotic effects. Therefore, while DIDP has been measured in Canadian surface waters and sediment (no soil monitoring data are available), it is unlikely that internal body concentrations in exposed organisms will reach levels that are sufficiently high to cause adverse effects. For example, a maximum freshwater concentration of 128 ng/L was reported for DIDP downstream of a wastewater treatment system (Sosiak and Hebben 2005; see Measured Environmental Concentrations section) and this corresponds to a CBR in aquatic organisms of 1.1 × 10-5 mmol/kg (see CBR calculation in Ecological Effects Assessment section). As this value falls below the threshold ranges of 2 to 8 mmol/kg and 0.2 to 0.8 mmol/kg for acute and chronic effects, respectively, aquatic organisms exposed to this concentration in the environment are unlikely to exhibit adverse effects resulting from baseline narcosis. Similarly, Mackintosh et al. (2006) reported a highest sediment concentration of 589 ng/g dw for DIDP in an estuarine sediment. The resulting CBR in sediment organisms exposed to this concentration is 8.0 × 10-4 mmol/kg, indicating that adverse effects due to neutral narcosis are unlikely to occur.
Several studies report the presence of DIDP in Canadian aquatic species. A mean concentration in fish of 57 ng/g ww was measured in shiner perch, Cymatogaster aggregata (McConnell 2007), and this value was converted to CBR units in order to investigate whether tissue levels in the fish were high enough to potentially result in adverse effects attributable to baseline narcosis. The CBR for this tissue concentration is 1.3 × 10-4 mmol/kg (0.057 mg/kg / MW 446.68 g/mol). This value is below the ranges of 2 to 8 and 0.2 to 0.8 mmol/kg attributed to acute and chronic narcotic effects, respectively, suggesting that the perch in the study are not likely to be experiencing adverse narcotic effects due to the presence of DIDP in their tissues. While Mackintosh et al. (2004) reported a higher concentration of 13 804 ng/g lw in striped seaperch, Embiotoca lateralis, insufficient information are available from the study report to allow the conversion to CBR units (i.e., tissue wet weight concentrations are required).
It should be noted that the CBR analysis does not consider the potential for adverse effects resulting from modes of action other than baseline narcosis. The analysis does not therefore provide a measure of potential for effects from modes of action such as peroxisome proliferation or disruption to normal hormonal function. The structure and chemical properties of DIDP and DUP suggest that baseline narcosis will be the primary mode of action for both substances.
Estimated total daily intake (TDI) values for DIDP in two fish-eating mammalian wildlife species, mink and river otter, were calculated using Canadian monitoring data in order to compare the potential daily ingestion rates for these two species with the lowest LOAEL of 22 mg/kg bw/d reported for the rat (Cho et al. 2008; see Human Health Effects Assessment). The procedure was based on the methods of Sample et al. (1996) and considered average body weights for mink and otter (1.08 and 7.98 kg, respectively), as well as the fish BAF of 40 (BCFBAF 2010; see Potential for Bioaccumulation section) and a highest Canadian surface water concentration of 128 ng/L (Sosiak and Hebben 2005). Estimated TDIs were 9.4 × 10-4 and 7.8 × 10-4 mg/kg bw/d for mink and otter, respectively, indicating that daily intake rates of DIDP would be very low and much lower than the lowest reported LOAEL. Together with the low bioaccumulation potential, this suggests that effects are unlikely to occur in either wildlife species. No Canadian surface water concentrations were found for DUP; however, the low bioaccumulation potential of the substance suggests that TDI values would also be low.
8.3.2 Uncertainties in Evaluation of Ecological Risk
Empirical and modelled data indicate that DIDP and DUP have low bioaccumulation and biomagnification potential. However, DIDP has been measured in a variety of aquatic species, confirming that the substance can be taken up by organisms. Since dietary exposure is the main exposure route for these substances, the measured presence in organisms implies that some degree of trophic transfer is possible. Further research is needed in order to clarify the nature and extent of uptake, as well as the relative roles of environmental exposure and metabolism in determining the ultimate fate of these substances in organisms.
While DIDP and DUP are considered to present low hazard potential in terms of baseline narcosis based on empirical and modelled data and CBR analyses, there is uncertainty regarding the potential for effects relating to other modes of toxic action. Preliminary data suggest that DIDP may be capable of influencing the activity of endocrine-active substances. Further chronic toxicity information, particularly for species with lower metabolic capabilities than fish and therefore potentially higher internal concentrations, would help to more fully address chronic hazard potential.
Recent Canadian monitoring data are lacking for DIDP and, in particular, for DUP as no Canadian environmental concentrations data were found for this substance. Both substances have substantial use in Canada and information on measured environmental presence would help to inform the risk assessment on potential for exposure to organisms in the Canadian environment.
Some physical and chemical properties of DIDP and DUP are not easily measured by standard laboratory methods, and this introduces uncertainty into some of the property values reported in the literature. Special measurement procedures are needed to account for the characteristics of very low water solubility and vapour pressure and high partition coefficients displayed by these substances. For this reason, chemical property values obtained using techniques specifically designed for hydrophobic substances, such as the slow-stir method for determining water solubility, have received greater weight in the assessment than those derived from procedures which may not account as well for the behaviour of these substances.
9. Potential to Cause Harm to Human Health
9.1 Exposure
9.1.1 DIDP
Environment media and food
Ambient air, drinking water and soil
No Canadian data were identified for DIDP in ambient air. Elsewhere, DIDP was not detected in background air and was detected in all samples measured from the vicinity of industrial and urban waste water sites in Sweden during the years of 2006 to 2007 (Cousins et al. 2007).
No Canadian data were identified for DIDP in drinking water. DIDP has been detected in surface seawater (suspended and dissolved fractions) in Vancouver (Mackintosh et al. 2004, 2006; Blair et al. 2009). It was also found to be present downstream, upstream, and in the effluent of urban wastewater treatment systems in Alberta (Sosiak and Hebben 2005). Furthermore, DIDP has been detected in sludge from a wastewater treatment system in Sweden (Cousins et al. 2007; Remberger et al. 2013). Finally, it has also been observed in river water in the UK (Fawell et al. 2001), in precipitation and storm water in the Netherlands and Sweden (Peters et al. 2008; Björklund et al. 2009), and in road runoff water in Austria (Clara et al. 2010).
No Canadian data were identified for DIDP in soil. Limited data are available indicating the presence of DIDP in sediments in Vancouver, Canada (Blair et al. 2009; McConnell 2007; Mackintosh et al. 2006) and in Sweden (Cousins et al. 2007). A summary of environmental monitoring data for DIDP and DUP is provided in Environment Canada (2015).
Due to the absence of Canadian and North American air, drinking water and soil data, daily intakes from these sources were not estimated.
Indoor air and dust
Phthalates are semi-volatile compounds and are generally present in the indoor environment, presumably due to their ubiquitous presence in plastic products (Weschler and Nazaroff 2010; Fromme et al. 2004; Bergh et al. 2011a,b; Rudel et al. 2010; Bornehag et al. 2005). Long-chain phthalates tend to partition to settled dust and surfaces,whereas short chain and low molecular weight medium chain phthalates may actually partition in greater proportions to the gaseous or particle phases of the indoor air (Weschler and Nazaroff 2010; Fromme et al. 2004; and Bergh et al. 2011a,b). No data were identified for DIDP in indoor air in Canada or elsewhere.
DIDP, a long-chain phthalate, has been monitored primarily in settled house dust (Kubwabo et al. 2013; Kang et al. 2012; Abb et al. 2009). No monitoring as to its presence in gaseous of particle phases was identified. Concentrations of DIDP in house dust are presented in Table 9-1.
DIDP has applications as a plasticizer in the manufacturing of automobiles and automobile parts (ECHA 2013; Environment Canada 2014a). For the general population, indirect exposure (e.g., off-gassing) is considered a relevant source, but no data on this exposure source has been identified, which is currently an uncertainty in the assessment.
| Location | Detection frequency | Concentration (µg/g) | Reference |
|---|---|---|---|
| Canada | 100% of 126 homes | Median: 110.7 95th: 433.9 Range: 5.3 - 1428.5 |
Kubwabo et al. 2013 |
| Germany | 100% of 30 homes | Median: 33.6 | Abb et al. 2009 |
| China | Not Reported | Median: 2.9 Range: 0.16 - 63.3 |
Kang et al. 2012 |
The results from the Kubwabo et al. (2013) study are considered to be representative of Canadian dust levels and were used to estimate Canadian general population daily intakes of DIDP from dust; the highest estimates of exposure were for infants 0 to 6 months old) at 0.562 and 2.20 µg/kg/day, based on the median (111 µg/g) and 95th percentile concentrations (433.9 µg/g), respectively (See Appendix C-1, Table C-1a).
Food and beveragres
Some phthalates are present in various PVC food packaging and processing articles such as PVC tubing, gloves, food packaging films, PVC gaskets for glass jars, and printing inks in food packaging (Fasano et al. 2012), and have been known to migrate into food and beverages (Alin and Hakkarainen 2011; Barros et al. 2011; Bradley et al. 2013; Gartner et al. 2009; Page and Lacroix 1992; Fierens et al. 2012; Petersen and Jensen 2010; Xu et al. 2010; Xue et al. 2010).
In Canada, DIDP presence in food was monitored as part of the Canadian Food Inspection Agency's (CFIA) 2013-2014 Food Safety Action Plan (FSAP) (Personal communication Food Directorate to Existing Substances Risk Assessment Bureau, April 2014). DIDP was detected in 10% of 677 (Limit of detection (LOD): 0.1 µg/g) packaged and processed foods sampled. The highest frequency products were oils and fats (44% of 73 samples), and frozen meals (14% of 91 samples). The highest concentrations were obtained in oils and fats (mean: 55 µg/g, 95th percentile: 245 µg/g detected in 32 of 73 samples) and frozen desserts (3.79 ug/g detected in 1 of 5 samples).
No data were identified monitoring the presence of DIDP in breast milk in Canada or elsewhere. The CFIA surveillance data detected DIDP in 5% of 20 samples of infant foods and in 6% of 32 samples of infant formula. It was not detected in any of 7 samples of infant cereal.
Limited international studies monitoring the presence of DIDP in foods were identified. Sørensen (2006) did not detect (LOD of 5 µg/kg) DIDP in milk and milk products, including infant formula (powdered and liquid) in Europe. Fankhauser-Noti and Grob (2006) reported DIDP in oily foods in jars at a concentration range of 55 to 705 mg/kg in Switzerland, which are similar to those reported in Canada in the CFIA surveillance study mentioned above.
Using CFIA surveillance data, and US and UK data to supplement data gaps, probabilistic estimates of dietary intakes for DIDP were derived for the Canadian general population and results are outlined in Appendix C-1, Table C-1b (the methodology for estimating probabilistic intakes is provided in Appendix C-3).
The highest estimate of dietary intake of DIDP in Canada is for the 1- to 3-year-old group with median and 90th percentile intakes of 0.128 and 1.327 µg/kg/day, respectively (See Appendix C-1, Table C-1b). Coefficients of variations for infant intake estimates are not sufficiently low to allow for reporting the intake (See Appendix C-1, Table C-1b).
Similarly, ECHA (2013) recently estimated intakes of DIDP from food. The highest intakes they reported were 0.97 and 6.3 µg/kg/day, as typical and reasonable worst case estimates, for children aged 12 to 18 months, respectively (ECHA 2013).
ECHA (2013) estimated intakes of DIDP for infants aged 0 to 6 months and infants aged 6 to 12 months ranged from 1 to 5.4 µg/kg/day, including typical and reasonable worst cases.
Due to the limited Canadian data for infant food and the absence of data for breast milk, exposure from food was not quantified for infants less than 1 year, which represents an uncertainty in this assessment. However, based on the available dietary intakes for infants reported in ECHA (2013), 1- to 3-year-old children may be expected to be associated with the highest daily intake of DIDP from food and beverages.
Products used by consumers
Manufactured items, children's articles, children's toys, textiles
DIDP may also be present in manufactured items, children's articles, children's toys and textiles (see Table 5-2). In Canada, DIDP use was also reported for various manufactured items (Environment Canada 2014a).
Oral Exposure
Some studies have reported the presence of DIDP in children's toys (ECHA 2013, Biedermann-Brem 2008; Rastogi 1998; Johnson et al. 2011).
Health Canada has surveyed toys over a number of years for multiple phthalates, including DIDP, and it was not detected in any samples in any survey (Health Canada 2007, 2009, 2012, 2014). DIDP was also monitored in toys purchased in Canada, but made elsewhere; it was not detected in any samples (Stringer et al. 2000).
Currently, Canada (along with the US and EU) has regulations in place limiting the amount of certain phthalates (including DIDP) in toys and childcare articles. The European Union's RAPEX database also shows very low reporting of toys tested in violation of the regulation for DIDP (RAPEX 2012), and the US CPSC recently stated that less that 10% of exposures to DIDP for infants and children would result from mouthing of toys and childcare articles (US CPSC CHAP 2014).
Due to the absence of DIDP in toys and childcare articles reported in Canadian and international monitoring studies, exposure is expected to be negligible and oral intakes from mouthing toys and/or childcare articles were not estimated.
Dermal exposure Globally, DIDP is known to have applications in the production of articles and textiles that may come in contact with skin. Examples of these are artificial leather, upholstery, general and athletic footwear (See a summary of the major uses of DIDP identified internationally in Table 5-1). These potential uses were confirmed in Canada, as DIDP was reported to be used in fabric coatings (e.g., upholstery, artificial leather) and in manufactured items (Environment Canada 2014a).
Two recent assessments by ECHA and the CHAP include an evaluation of dermal exposure to DIDP in plastic (PVC, polyurethane, polyester, etc.) articles and parameters used in these assessments are provided in Table 9-2 (ECHA 2013; US CPSC CHAP 2014).
| Jurisdiction/reference | Population assessed | Exposure parameters | Scenario outline |
|---|---|---|---|
| ECHA 2013 | Infants (0 - 18 months) |
|
|
| ECHA 2013 | Adults |
|
Typical scenario: Wearing gloves all day + holding a steering wheel
|
| US CPSC CHAP 2014 | Infants (0 to 18 months) | Change pad
|
Migration rate of DEHP (Deisinger et al. 1998). |
| US CPSC CHAP 2014 |
Infants (0 to 18 months) | Play pen
|
Migration rate of DEHP (Deisinger et al. 1998). |
| US CPSC CHAP 2014 |
Adults (women) | Gloves
|
Migration rate of DEHP (Deisinger et al. 1998). |
| US CPSC CHAP 2014 |
Adults (women) | Sitting on a couch
|
Migration rate of DEHP (Deisinger et al. 1998). |
No studies are available regarding migration rates of DIDP in simulated sweat. DEHP has been evaluated for migration into simulated sweat from various articles (see Table 9-3).
| Method | Type of Article | Migration (µg/cm2) | Concentration in article (% content) | Reference |
|---|---|---|---|---|
| In vitro, staticFootnote Table 9-3[a] | Sandals | NDFootnote Table 9-3[d] - 1.7 | ND - 46 | Danish EPA 2010a |
| In vitro, staticFootnote Table 9-3[b] | Balance balls, articles | ND - 0.38 | ND - 47 | Danish EPA 2010b |
| In vitro, staticFootnote Table 9-3[c] | Pencil cases | 0.039 | NSd | Danish EPA 2007 |
| In vitro, staticc | School bags, toy bags | 0.0098 - 0.011 | NS | Danish EPA 2007 |
A conservative dermal exposure assessment was conducted, using the DEHP migration rates as a surrogate. Use of DEHP migration rates for DIDP is similar to the approach used by ECHA, which used DEHP dermal flux from a plastic article as a surrogate for DIDP exposure to various types of plastic articles (see Table 9-2).
Two scenarios were developed to model exposure of infants in contact with various plastic objects for 1 hour/day with 25% of their body surface area (representative of multiple diaper changes per day on a change pad) and for 4 h/day with 50% of their body surface area (representative of holding a plastic article and being changed on a plastic change pad multiple times a day and playing on a plastic mat).
Representative scenarios to model exposure of adults in contact with various plastic articles were also assessed. The first for 3hours/day with 16% of their body surface area (analogous to sitting on a couch and wearing plastic gloves); the second for 3 hours/day with 50% of their body surface area (representative of various contacts with plastic articles including wearing gloves, holding a plastic steering wheel, sitting on a couchand wearing PVC articles). Estimated intakes are outlined in Table 9-4.
| Migration rate (µg/cm2/h) | Infant exposure µg/kg/dayFootnote Table 9-4[a]p |
Adult exposure µg/kg/daya |
|---|---|---|
| 0.22Footnote Table 9-4[b] | 0.27 (SAFootnote Table 9-4[c]=922 cm2; TFootnote Table 9-4[d]=1h) 2.16 (SA=1840 cm2; T=4h) |
0.27(SA=2912 cm2; T=3h) 0.85(SA=9100 cm2; T=3h) |
The average migration rate of 0.22 µg/cm2/h was derived without adjusting for experiment duration (e.g., the highest migration rate of 1.7 µg/cm2/h was not averaged over 16 hours). Evaluation of migration rate data show that a majority of phthalates leach out in the first 1 to 3 hours; therefore, dividing the migration rate by 16 hours would lead to underestimation of exposure. Note that this scenario does not account for plasticizer depletion; another conservatism. A dermal absorption factor of 1% was used to estimate the systemic exposure (see section 9.2.1.3 for approach to characterizing dermal absorption to DIDP).
Intakes for infants (0 to 18 months) were estimated to be 0.27 and 2.16 µg/kg/day for estimated lower end and upper bound, respectively. However, intakes from adults were estimated to be 0.27 and 0.85 µg/kg/day for estimated lower end and upper bound, respectively. These intakes are comparable to the internal dermal estimates derived by ECHA (infants 0 to 18 months: 1.0 to 2.2 µg/kg/day, adults: 1 to 2.0 µg/kg/day) and CHAP (infants 0 to 18 months: 0.24 to 0.99 µg/kg/day, adults: 0 to 2.9 µg/kg/day).
Adult toys
No Canadian use of DIDP in adult toys was reported under the Section 71 industry survey (Environment Canada 2014a). Based on global use patterns, there is potential for use of DIDP in adult toys. DIDP was reported in 8 of 71 articles (range 14 to 55 %, VWA 2009 in ECHA 2013). Another study reporting the presence of phthalates in adult toys in the EU reported DIDP in 2 of 7 toys tested (48% and 49%, BSMEPH 2012 in ECHA 2013). Recently, ECHA 2013 assessed exposure to DIDP and DINP from these products and derived a combined intake of 4.8 to 63 µg/kg/day for typical and worst-case exposure estimates (using merged migration rates for DIDP and DINP derived from Danish EPA 2006).
Biomonitoring
Data on DIDP metabolism is limited. The fractional urinary excretion (FUE) of a substance is defined as the mole ratio of the amount of metabolites excreted in urine (at 24 hrs) to that of total parent compound ingested. Due to their structural similarities, the FUEs for DINP have been used as a surrogate for DIDP (Anderson et al. 2011; Kransler et al. 2012). The selected fractionary urinary excretions, for selected metabolites, are outlined below (Table 9-5).
| Metabolite | Molecular weight | FUEFootnote Table 9-5[a] | Reference |
|---|---|---|---|
| Mono-(2-propyl-6-oxo-heptyl) phthalate (MOiDP) | 320 | 0.114 | Anderson et al. 2011; Kransler et al. 2012 |
| Mono-(hydroxy-isodecyl) phthalate (MHiDP) | 322 | 0.063 | Anderson et al. 2011; Kransler et al. 2012 |
| Mono-(7-carboxy-2,7-dimethylheptyl) phthalate (MCiNP) | 336 | 0.099 | Anderson et al. 2011; Kransler et al. 2012 |
MCiNP, a secondary metabolite of DIDP, was measured in NHANES in the USA (CDC 2013).
All of the metabolites of DIDP listed in Table 9-5 above have been measured by Health Canada in two cohort surveys: Plastics and Personal-Care Product Use in Pregnancy survey (P4, n = 31 women, 542 individual spot samples, women provided multiple urine samples over two visits) and Maternal-Infant Research on Environmental Chemicals - Child development plus study (MIREC-CD Plus, 193 children, 2-3 years old, 1 spot sample per individual). MHiDP and MOiDP were detected in 38 and 92% of urine samples in P4 and 75 and 80% of urine samples in MIREC-CD Plus, respectively (Table 9-8; personal communication from Environmental Health Science and Radiation Directorate [EHSRD] to Existing Substances Risk Assessment Bureau [ESRAB], October 2013, 2014).
Peaks that may be attributed to MCiNP were detected in every sample, but the retention times did not match the analytical standard (personal communication from Environmental Health Science and Radiation Directorate [EHSRD] to Existing Substances Risk Assessment Bureau [ESRAB], October 2014). Therefore, MCiNP could not be reliably quantified and was not included in the intake calculation for pregnant women. However, it is important to note that since DIDP is a mixture, it is expected that metabolites will be mixtures; thus an inability to quantify does not necessarily mean non-detection of MCINP in samples. Metabolites of DIDP have also been measured elsewhere (Fromme et al. 2013; Frederiksen et al. 2013; Enke et al. 2013; Koch et al. 2013; Koch and Calafat 2009; Casas et al. 2011; Colacino et al. 2011; Kasper-Sonnenberg et al. 2012; Koch et al. 2011).
Results from the study conducted by NHANES (CDC 2014) and from the P4 datasets were used to generate intake estimates for the Canadian general population. Table 9-6 shows which metabolites were used for intake calculations. Results are presented in Table 9-7 (NHANES) and 9-8 (P4) below (see Appendix D for further information on the methodology). Intakes were corrected for urine dilution using the creatinine correction method, which is a commonly used method for phthalate biomonitoring assessment (Fromme et al. 2007; Christensen et al. 2014, US CPSC CHAP 2014, Frederiksen et al. 2014). Daily creatinine excretion rates, for participants, were estimated using the Mage equation and biomonitoring intakes are presented in Tables 9-7, through to 9-9 below (see Appendix D for further information on the methodology).
| Survey used for Intake AnalysisFootnote Table 9-6[a] | Metabolite | Total FUE |
|---|---|---|
| NHANES | MCiNP | 0.099 |
| P4, MIREC-CD Plus | MOiDP, MHiDP, MCiNPFootnote Table 9-6[b] | 0.177 |
| Age group (years) | n | Geometric mean | 50th | 75th | 95th |
|---|---|---|---|---|---|
| 6-11 | 209 | 1.4 | 1.4 | 2.3 | 4.4 |
| 12-19 | 225 | 0.75 | 0.67 | 1.1 | 4Footnote Table 9-7[a] |
| 20+ | 949 | 0.83 | 0.76 | 1.5 | 4.4 |
| Age group (years) | n | Geometric mean | 50th | 75th | 95th |
|---|---|---|---|---|---|
| 6-11 | 204 | 1.2 | 1.1 | 2 | 4.3 |
| 12-19 | 189 | 0.74 | 0.71 | 1.3 | 3.7 |
| 20+ | 948 | 0.73 | 0.65 | 1.3 | 4.9 |
| Age Group (years) | Arithmetic mean | 50th | 75th | 95th |
|---|---|---|---|---|
| 2-3 | 0.36 | 0.13 | 0.24 | 0.76 |
| 19+ | 0.11Footnote Table 9-9[a] | 0.05 | 0.10 | 0.3 |
The highest intakes from biomonitoring data were from NHANES in the USA, including children (ages 6-11, males) with calculated intakes of 1.4 (median) and 4.4 (95th) µg/kg-bw/day. As well as adults (20+) with calculated intakes of 0.76 (median), 4.4 (95th) and 0.65 (median), 4.9 (95th) µg/kg-bw/day for males and females, respectively (Table 9-7, Appendix D). These intakes were considered a conservative representation of the Canadian population. The P4 and MIREC-CD Plus calculated intakes for younger children (2-3 years) and pregnant women (19+) were lower and these were actually based on Canadian data.
9.1.2 DUP
Environment media and food
Ambient air, drinking water and soil
No studies monitoring DUP in ambient air, drinking water or soil were identified in Canada or elsewhere. DUP has been reported in sludge and sediments in Nordic countries (Remberger et al. 2013).
Indoor air and dust
Phthalates are semi-volatile compounds and are generally present in the indoor environment, presumably due to their ubiquitous presence in plastic products (Wechler and Nazaroff 2010; Fromme et al. 2004; Bergh et al. 2011a,b; Rudel et al. 2010; Bornehag et al. 2005). Long-chain phthalates tend to partition more to settled dust and surfaces, whereas short-chain and low molecular weight, medium-chain phthalates may actually partition in greater proportions to the gaseous or particle phases of the indoor air (Wechler and Nazaroff 2010; Fromme et al. 2004; and Bergh et al. 2011a,b). DUP is considered to be a long-chain phthalate. No monitoring as to its presence in gaseous of particle phases was identified in Canada or elsewhere. Only one study reported the presence of DUP in household dust (see Table 9-10).
DUP has applications as a plasticizer in the manufacturing of automobiles and automobile parts (OECD 2004; HSDB 2010; Environment Canada 2014a). For the general population, indirect exposure (e.g., off-gassing) is considered a relevant source, but no data on this exposure source has been identified, which is currently an uncertainty in the assessment.
| Location | Detection Frequency | Concentration (µg/g) | Reference |
|---|---|---|---|
| Canada | 99% of 126 homes | Median: 3.9 95th: 68.8 Range: ND-259.3 |
Kubwabo et al. 2013 |
The results from the Kubwabo et al. (2013) were used to estimate Canadian general population daily intakes of DUP from dust. The highest estimates of exposure (infants 0 to 6 months old) are 0.0198 and 0.349 µg/kg/day, based on the median (3.9 µg/g) and 95th percentile (68.8 µg/g) concentrations, respectively (See Appendix C-2, Table C-2a).
Food and beverages
No Canadian data on DUP in foods were identified. DUP is used in food packaging applications in Canada (September 2014 email from the Food Directorate, Health Canada to the Risk Management Bureau, Health Canada; unreferenced). In the USA, it is on the list of indirect additives used in food contact substances (FDA 2014). Food may be a potential route of exposure for DUP, and based on the similar molecular weight, phys-chem properties and given that DUP is also used in food contact materials, exposure is expected to be similar to DIDP. However, due to the absence of data from this source, exposure was not quantified and is currently an uncertainty in this assessment.
Products used by consumers
DUP may also be used as a plasticizer in the coating of textiles and other manufactured items (OECD 2004). Given the high production volumes of this substance (see section 4) and its global use pattern, potential exposure to the general population to DUP from its use as a plasticizer in manufactured items (e.g., PVC, polyurethane, polyester) was characterized.
A conservative exposure estimate was conducted to estimate exposure to DUP from dermal contact with the various manufactured items (see table 9-11). Two scenarios were developed to model exposure of infants in contact with various plastic objects for 1 hour/day with 25% of their body surface area (representative of multiple diaper changes per day on a change pad) and for 4 hours/day with 50% of their body surface area (representative of holding a plastic article and being changed on a plastic change pad multiple times a day and playing on a plastic mat).
Two scenarios to model exposure of adults in contact with various plastic articles were also assessed. The first for 3hours/day with 16% of their body surface area (representative of sitting on a couch and wearing plastic gloves), the second for 3 hours/day with 50% of their body surface area (representative of various daily contacts with plastic articles including wearing gloves or holding a plastic steering wheel, sitting on a couch and wearing plastic clothing). Estimated intakes are outlined in Table 9-11.
Migration studies have shown that various phthalates can migrate from articles (sandals, children's articles, toys, etc.) into saliva and sweat (Danish EPA 2010a,b; RIVM 1998; Babich et al. 2004). DUP migration rates have not been reported in the literature. Therefore, DEHP which has been evaluated for migration into simulated sweat from various articles has been used as a surrogate (Table 9-3 above in DIDP exposure assessment). A dermal absorption factor of 10% was used to estimate the internal dose (see section 9.2.1.3).
| Migration rate (µg/cm2/h) |
Infant (0 - 18 months) intake µg/kg/dayFootnote Table 9-11[a] |
Adult 20 + Intake µg/kg/daya |
|---|---|---|
| 0.22Footnote Table 9-11[b] | 2.7 (SAFootnote Table 9-11[c]=922 cm2; TFootnote Table 9-11[d]=1h) 21.6 (SA=1840 cm2; T=4h) |
2.7 (SA=2912 cm2; T=3h) 8.5 (SA=9100 cm2; T=3h) |
Conservative estimates of dermal exposure from contact with plastic articles, depending on the scenario, were 2.7 and 21.6 µg/kg/day for infants. For adults, conservative estimates of dermal exposure, depending on the scenario, were 2.7 and 8.5 µg/kg/day.
Finally, DUP was reported to be used in adhesives, sealants and coatings (Environment Canada 2014a); but a do-it-yourself use (e.g., applying a coating to a hard surface) is also feasible. Given that phthalates are metabolized quickly, do not bioaccumulate in the body and are known to have low acute toxicity, acute dermal exposure from incidental use of these types of products is not anticipated to contribute significantly to the overall exposure of the general population in Canada. Therefore, estimates were not generated and the focus will be on sub-chronic and chronic exposure assessments (see Risk Characterization section for additional information).
9.2 Health Effects
9.2.1 Toxicokinetics
Among the phthalates in this subgroup, oral, inhalation and dermal toxicokinetics data are available for DIDP. Since no information is available for DUP, DnOP was identified as the most appropriate analogue (see section 2.3.2; Health Canada 2015a). Oral data are available for DnOP.
9.2.1.1 Oral route
In a gavage study, absorption of DIDP was reported to decrease in rats with increasing exposure to this phthalate, suggesting that metabolic pathways may saturate at high doses. The reported absorption rates were 56% and 46% in rats exposed to a single dose of 0.1 and 11.2 mg/kg-bw, respectively, over 72 h. Absorption was 17% at the highest dose of 1000 mg/kg-bw over the same time period. Almost all of the administered dose (99%) was excreted through urine and feces with only 1% remaining in the tissues. The highest level of absorbed radioactivity was seen in the GI tract, liver and kidneys (General Motors Research Laboratories 1983; US CPSC 2010a).The absorption rate of DnOP in rats was reported to be 31% over 48 h when animals were exposed twice by gavage to 0.2 ml at 24 h interval (Albro and Moore 1974). See Table 9-12 below.
| Substance | Species | Dose | Basis | Absorption rates (% of dose) | Reference |
|---|---|---|---|---|---|
| DIDP | Rat | 0.1 mg/kg 11.2 mg/kg 1000 mg/kg |
Urine+bile | 55.6% over 72 h 45.9% over 72 h 17.3% over 72 h |
General Motors Research Laboratories (1983) |
| DnOP | Rat | Twice 0.2 ml (85% radioactive; at 24 h interval) | Urine | 31% over 48 h | Albro and Moore (1974) |
It has been shown that the oxidative metabolic pathways are predominant in the metabolism of DnOP in the rat (Albro and Moore 1974; Silvaet al. 2005; Calafatet al.2006). Silvaet al.(2005) have observed high urinary levels of mono-(3-carboxypropyl) phthalate (MCPP) and mono-(7-carboxy-n-heptyl) phthalate (MCHpP), suggesting that ω-oxidation followed by β-oxidation(s) is the major metabolic pathway for DnOP metabolism in rats. MCPP has been estimated to be over 560-fold higher than the monoester MnOP in 24-hour urine samples of exposed rats (Silvaet al.2005; Calafatet al.2006). The presence of multiple peaks for mono-hydroxy-n-octyl phthalate (MHOP) and mono-oxo-n-octyl phthalate (MOOP) metabolites suggested active (ω-1) to (ω-n) pathways (Silvaet al.2005).
In a study in which rats were exposed orally to a single dose of DIDP14 (300 mg/kg-bw), it was shown that DIDP is first metabolized to MIDP and then to oxidative metabolites that are excreted in the urine. DIDP had a rapid clearance and the half-life of all metabolites was estimated to be around 14 hours. The major metabolite in urine was MCINP, while MIDP was only present in minor quantity. The authors of this study identified MCINP, MHIDP, and MOIDP as suitable biomarkers for biomonitoring studies (Kato et al. 2007). In humans, it has also been shown that DIDP metabolism results in the formation of oxidative metabolites. Similar to what has been observed in rats, the monoester of DIDP (MIDP) was not detected whereas MCINP, MHIDP, and MOIDP were identified (Silva et al.2007).
See Table 9-13 for a summary of long-chain phthalate diesters and their metabolites found in urine after oral administration in rats and humans.
Table 9-13. Summary of metabolites of DIDP and DnOp found in urine after oral administration in rats and human in vivo
| Metabolite found in urine after oral administration | Abb. | Reference (species) |
|---|---|---|
| Monoisodecyl phthalate | MIDP | Calafat et al. 2006 (rat) Not detected in human samples (Silva et al. 2007) |
| Mono(carboxyisodecyl) phthalate | CO2-MIDP (MCIDP) |
Koch et al. 2012 (human)Footnote Table 9-13[a] Kato et al. 2007 (rat) |
| Mono(hydroxyisodecyl) phthalate | OH-MIDP (MHIDP) | Silva et al. 2007 (human) Koch et al. 2012 (human)a Kato et al. 2007 (rat) |
| Mono(hydroxyisononyl) phthalate | OH-MINP (MHINP) |
Kato et al. 2007 (rat) |
| Mono(oxyisodecyl) phthalate | Oxo-MIDP (MOIDP) |
Silva et al. 2007 (human) Koch et al. 2012 (human)a Kato et al. 2007 (rat) |
| Mono(carboxyisononyl) phthalate | MCINP | Silva et al. 2007 (human) Kato et al. 2007 (rat) |
| Mono-n-octyl phthalate | MnOP | Calafat et al. 2006 (rat) |
| Monoisononyl phthalates | MINP | Calafat et al. 2006 (rat) |
| Mono-(3-carboxypropyl) phthalate | MCPP | Calafat et al. 2006 (rat) |
| Phthalic Acid | PA | General Motors Research Laboratories 1983 (rat) |
| Metabolite found in urine after oral administration | Abb. | Reference (species) |
|---|---|---|
| Mono-n-octyl phthalate | MnOP | Albro and Moore 1974 (rat) Calafat et al. 2006 (rat) Silva et al. 2005 (rat) |
| Mono(3-carboxypropyl) phthalate | MCPP | Calafat et al. 2006 (rat) Silva et al. 2005 (rat) |
| Mono-hydroxy-n-octyl phthalate | MHOP | Silva et al. 2005 (rat) |
| Mono-oxo-n-octyl phthalate | MOOP | Silva et al. 2005 (rat) |
| Mono-(7-carboxy-n-heptyl) phthalate | MCHpP | Silva et al. 2005 (rat) |
| Mono-(5-carboxy-n-pentyl) phthalate | MCPepP | Silva et al. 2005 (rat) |
| Mono-carboxymethyl phthalate | MCMP | Silva et al. 2005 (rat) |
| Phthalic Acid | PA | Albro and Moore 1974 (rat) Silva et al. 2005 (rat) |
| Other acids/esters/alcohols | - | Albro and Moore 1974 (rat) |
9.2.1.2 Inhalation route
There is limited information on long-chain phthalate absorption via inhalation. The only experimental study found was an unpublished report on DIDP by General Motors Research Laboratories (1981) using rats. Male rats were exposed (head-only) to 14C-DIDP aerosol (MMAD: 0.98 μm) atmosphere (100 mg/m3) for 6 hours. The absorption of DIDP was estimated to be at least 54%, based on the sum of radioactivity recovered over 72 h from the urine (45.3%) and carcass and tissues (9.4%) of exposed rats (General Motors Research Laboratories 1981). Other unpublished reports provided only qualitative data indicating that some absorption through the lungs does in fact occur in rats (Warf Institute 1976; Pegg 1979).
The distribution of DIDP after inhalation was also studied by General Motors Research Laboratories (1981) immediately after cessation of exposure and 72 h later (n=3). The highest concentration of radioactivity was measured immediately after exposure in the lungs (0.663 μmol equivalents), followed by the GI tract (0.095), the liver (0.015) and the kidneys (0.006). The remaining tissues (brain, thymus, heart, spleen, testes, fat) contained far lesser amounts. Radioactivity decreased after 72 hrs by 3.6-fold in the lungs, 8.5-fold in the GI tract and the liver, 10-fold in the kidneys, and 2.5-fold in the thymus and was below detection limits in the brain, spleen and testes. In contrast, the radioactivity did not decrease in fat (same value at both time points) (General Motors Research Laboratories 1981).
9.2.1.3 Dermal route
It has been suggested by Elsisiet al. (1989) that dermal absorption of DIDP is very low and that generally phthalates with alkyl chain lengths of more than four carbons have lower rates of absorption through the skin than phthalates with shorter chains lengths of C2 and C4. It was suggested that the extent of dermal absorption of the phthalates diesters may depend on several competing factors such as lipophilicity, molecular size, and metabolism. In this study, several [14C] phthalate diesters including DIDP were applied topically to the dorsal side of rats (5-8 mg/cm2; skin not washed after exposure, site of application covered with a perforated cap). A large part of the dose remained at the site of application (i.e., retained in the skin) 7 days after application for all phthalates. For DIDP, about 75% and 6% of the dose remained at the skin area of application and the cap, respectively (Elsisiet al. 1989).
Among the phthalates examined, DIDP was the most slowly excreted in urine and feces. Only 0.04 and 0.5% of the applied dose was excreted after 24 h and 7 days, respectively. For most diesters, distribution in tissues after 7 days was generally low. For DIDP, ranking for distribution in tissues was muscle (0.33%) greater than fat (0.14%) greater than skin (0.10%), and less than 0.5 % was recovered in total in other tissues collected (including brain, lung, liver, spleen, small intestine, kidney, testis, spinal cord, and blood) (Elsisi et al. 1989). The dermal absorption rate for DIDP in rat is estimated to be about 1% over 7 days based on the percentage of initial dose recovered in urine, feces and tissues. While no in vivo data in humans are available for DIDP, it has been shown in in vitro experiments conducted with rat and human epidermis that human skin is less permeable than rat skin to phthalate diesters (Scott et al. 1987; Barber et al. 1992; Mint and Hotchkiss 1993). Based on this, it is considered appropriate to assume that dermal absorption of DIDP in humans is not likely to exceed 1%. Since DIDP is the shorter of the two long-chain phthalates examined in this document, and has a lower molecular weight than DUP, it would be reasonable to also assume a dermal absorption of 1% for DUP. However, considering the lack of substance specific data, dermal absorption is conservatively assumed to be 10% in humans. The assignment of 10% as a default value for the human dermal absorption of DUP is based on the study by Janjua et al. (2007, 2008) which showed a maximal dermal absorption of DEP, a short-chain phthalate expected to be more dermally available than DUP in humans (see SOS Reports on Short and Medium Chain Phthalate Esters; Environment Canada and Health Canada 2015c,d). This default of 10% is also supported by assignment of a dermal absorption of 10%, for other smaller chain phthalates such as DIBP, DBP, DMP, and DEP, by other international agencies (see MCP and SCP document). Finally, the assignment of a 10% default for DUP is further supported by the European Commission which recommends the use of 10% (EC 2004), as a default dermal absorption, when the molecular weight of the substance is 500 g/mol or greater and the Log Kow is greater than 4 (DUP has a molecular weight of 475 g/mol and a Log Kow of 8.7).
At doses not associated with metabolic saturation, fecal excretion is generally less important than urinary excretion for phthalates. However, for DIDP, fecal excretion is the major elimination route. Elsisi et al.(1989) reported a relationship between the side-chain length and the fecal excretion where 24 hours after dermal exposure to phthalate diesters (alkyl chains from C1 to C10), the fraction of fecal excretion increased as a function of the side-chain length. The authors indicated that fecal excretion was less than 16% of total excretion for phthalate diesters with chain length of C6 or less while fecal excretion was 100% for DIDP. As suggested, this agrees well with the molecular weight of this phthalate which supports higher biliary excretion.
9.2.2 DIDP
9.2.2.1 Reproductive and developmental effects in males
In this section, the first three segments focus on reproductive and developmental effects of the male gender in three different life stages (gestational exposure [GD0-21], (pre)pubertal-pubertal [PND1-55], and adult [PND55+]), with particular focus on the male gender. Adverse effects observed subsequent to gestational exposure are further organized and presentedas follows: 1) changes in hormone levels (serum or testicular); 2) feminizationeffects; 3) reproductive tract malformations and/or effects on fertility; and 4) other developmental effects.Footnote[3]Descriptions of effects within each life stage are structured such that effects occurring at the lowest doses are summarized first. The potential reproductive developmental effects of DIDP in female animals are presented next in a similar manner in considering life stage and species sensitivity. The last segments focus on endocrine studies and reproductive and developmental effects observed in humans.
9.2.2.1.1 Early development: in utero exposure
A literature search identified seven developmental studies for DIDP. Five of those studies examined the effects of DIDP when administered during gestation in pregnant rats during the foetal masculinization programming window (gestational days [GD] 15-17). A limited study in mice using only one high-dose exposure to DIDP was also identified (Hardin et al. 1987). Summaries of the studies are described in Table 9-14 below.
In two multi-generational animal studies reported in Hushka et al. (2001, study A and B), DIDP was associated with toxicity in the parental (F0) and both filial generations (F1 and F2) in a dose-responsive manner.
In the first study (study A), Sprague Dawley rats were given 0, 0.2, 0.4, or 0.8% DIDP in their diet for ten weeks prior to and during mating. Females continued to receive DIDP throughout gestation and lactation. Treatment of the P2 generation was as described for the P1 generation. Estimated dose levels for the F0 and F1 generation males were approximately 0, 131-152, 262-297 and 524-611 mg/kg bw/day (ECJRC 2003). Developmental effects were observed in F1 and F2 male pups. There was a significant decrease in pup survival at birth and on postnatal day (PND)1 in the 0.8% treatment group for the F1 generation and at PND 1 and 4 in all treatment groups in the F2 generation. Reduced survival was also observed on lactation day 7 and at weaning at 0.8% in the F2 generation. Offspring body weights were reduced in the 0.8% dose group in both generations. By PND 35, the offspring body weights were similar to control values. Brain and liver weights were above control values in the 0.8% group, but there were no differences in any of the sexual organs. Dose-related enlargement of hepatocytes with cytoplasmic eosinophilia was seen in the livers of offspring of both sexes of animals treated with 0.4% and 0.8%. No treatment-related microscopic findings were noted in kidneys or other organs. No clinical signs of toxicity or gross post-mortem observations were noted in the offspring.
In the second two-generation study (Study B), rats were treated similarly as in study A but with lower doses, i.e., DIDP was given by gavage at doses of 0, 0.02, 0.06, 0.2 or 0.4%, equivalent to approximately 0, 13-15, 39-44, 127-150 and 254-295 for F0 and 0, 13-15, 38-44, 134-150, 256-284 mg/kg bw/day for F1 parental animals, respectively (ECJRC 2003). Some parameters related to RPS (e.g., altered feminization parameters such as anogenital distance (AGD), nipple retention (NR), hypospadias (HYP), and testicular pathological changes) were not evaluated in earlier studies, but were included in this study. In the F1 and F2 pups, there were no effects on body weight gain, organ weight, AGD, NR, or PPS. In the F2 pups, there was significantly decreased pup survival on PND 1 and 4 at 0.2 and 0.4% DIDP. No difference in survival was observed for the F1 generation. Age of preputial separation (PPS) was increased by 1.2 days in high dose F2, but not F1 pups, but was not considered adverse by the authors. There were no histological lesions or weight changes in the productive organs of either sex in both generations.
Two satellite experiments were included in study A in an attempt to differentiate between potential effects on offspring body weights from in utero vs postnatal exposure. In the first of these (cross fostering protocol), ten litters from the F1 generation (0.8% group) were switched with an equal number of control litters. The second experiment used a switched diet protocol to determine whether the effects following in utero exposure were reversible, and, if so, how quickly. Results show that control pups switched to a 0.8% DIDP fed dam had significantly lower body weight on PND 14 and 21 which could be attributed to lactational exposure to DIDP or poor palatability. Pups exposed to DIDP in utero but nursed by a control dam did not show body weight changes. In the switched diet study, pups exposed to DIDP in utero and while nursing recovered body weight after receiving control diets after weaning (Hushka et al. 2001).
Developmental studies
In a study in which pregnant rats were exposed by gavage to 0, 40, 200, and 1000 mg DIDP/kg bw/day during GD 6 to 15, DIDP did not affect the incidence of malformations although there was some evidence of increased foetal variations. The main variations observed were hydroureter, dilated renal pelves, increased rudimentary cervical ribs and accessory 14th ribs at 1000 mg/kg bw/day on a per litter basis (statistically significant). The types of skeletal variations at 200 mg/kg bw/day were not reported and not considered treatment-related. Maternal toxicity was observed at 1000 mg/kg bw/day and included increased liver weights and vaginal hemorrhage. The developmental LOAEL in the study was 1000 mg/kg bw/day based on increased skeletal variations (Hellwig et al. 1997).
In Waterman et al. (1999), pregnant rats were administered DIDP by gavage at 0, 100, 500 or 1000 mg/kg bw/day from GD 6 to 15. There was no incidence of malformations at any dose although there was evidence of increased foetal variations. The main variations observed were dose-related increases in rudimentary lumbar ribs and supernumerary cervical ribs at both 500 and 1000 mg/kg bw/day dose levels. No maternal toxicity was observed.
A more recent study presenting the potential for DIDP and other phthalates to perturb foetal testosterone production (ex vivo) in pregnant SD rats showed that this phthalate did not disturb testicular testosterone production during gestation at doses as high as 1500 mg/kg bw/day (Furr et al. 2014). However, contrary to other studies, foetal viability did not appear to be affected, although limited description was provided.
| Strain and species; Dose (mg/kg bw/day); Route; Duration (reference) |
Testosterone levelsFootnote Table 9-14[a] (T, S) |
Feminization parametersFootnote Table 9-14[b] | Reproductive tract malformations and/or fertilityFootnote Table 9-14[c] | Other developmental parametersFootnote Table 9-14[d] | Maternal effects |
|---|---|---|---|---|---|
| Crl:CDBR Rats; 0, 0.2, 0.4, 0.8%; est. F0 Female intake during gestation: 0, 131-149, 262-287, 524-551; diet; 10 wks before mating-PND21 (Hushka et al. 2001, study A) |
NM | NM | NM (CRY) NM (HYP) NE (TP) NM (FER) |
524-511 (BW) NE (ROW) NM (EMB) 524-551 (FV) NM (ESV) |
LOEL= 131-149 (↑ liver wt) |
| Crl:CDBR Rats; 0, 0.2, 0.4, 0.8%; est. F1 Female intake during gestation: 0, 135-152, 262-297, 574-611; diet; 10 wks before mating-PND21 (Hushka et al. 2001, study A) |
NM | NM | NM (CRY) NM (HYP) NE (TP) NM (FER) |
574-611 (BW) NM (ROW) NM (EMB) 135-152NDR and above(FV) NM (ESV) |
LOEL= 135-152 (↑ liver, kidney wt) |
| Crl:CDBR Rats; 0, 0.02, 0.06, 0.2, 0.4%; est. F0 Female intake during gestation: 0, 13-15, 39-43, 127-147, 254-295; diet; 10 wks before mating-PND21 (Hushka et al. 2001, study B) |
NM | NE | NM (CRY) NM (HYP) NE (TP) NM (FER) |
NE (BW) NE (ROW) NM (EMB) NM (ESV) |
LOEL= 254-295 (↑ liver wt) |
| Crl:CDBR Rats; 0, 0.02, 0.06, 0.2, 0.4%; est. F1 Female intake during gestation: 0, 13-15, 38-44, 134-150, 256-284; diet; 10 wks before mating-PND21 (Hushka et al. 2001, study B) |
NM | NE (AGD) NE (NR) 256-284* (PPS) |
NM (CRY) NM (HYP) NE (TP) NM (FER) |
NE (BW) NE (ROW) NM (EMB) 134-150 (FV) NM (ESV) |
LOEL= 134-150 (↑ liver and kidney wt) |
| Crl:CDBR Rats; 0, 0.25, 0.5, 0.75, 1%; est. M: 0, 32-264, 262-521, 414-776, 542-1014; F: 0, 165- 479, 314-897, 500-1334, 631- 1571; diet;10 wks before mating-PND21 (Exxon Biomedical Sciences 1997 (in EC 2003); Huska et al. 2001) |
NR | NR | NM (CRY) NM (HYP) NE (TP) NP (FER) |
262-521 (BW) NE (ROW) NM (EMB) NP (FV) NM (ESV) |
LOEL = 414-776 (bodywt, food consump.) |
| SD Rats; 0, 100, 500, 1000; gavage; GD:6-15 (Waterman et al. 1999) |
NM | NM | NM | NE (BW) NM (ROW) NE (EMB) NE (FV) 5) 500 (ESV) |
LOEL= 1000 (body wt, food consump.) |
| Outbred Wistar Rats; 0, 40, 200, 1000; gavage; GD:6-15 (Hellwig et al. 1997) |
NM | NM | NM | NE (BW) NM (ROW) NE (FV) NE (EMB) 200 (ESV) |
LOEL = 1000 (↑ liver wt and vaginal hemorrhage) |
| Harlan SD Rats; 0, 500, 750, 1000, 1500; GD14-18 (Furr et al. 2014) |
NE (T) NM (S) |
NM | NM | NM (BW) NM (ROW) NE (FV) NM (EMB) NM (ESV) |
NE |
| CD-1 Mice; 0, 9650; gavage; GD:6-14 (Hardin et al. 1987) |
NM |
NM | NM | NE (BW) NM (ROW) NM (EMB) NE (FV) NE (ESV) |
NE |
Overall, based on the findings in the two 2-generation studies, the NOAEL and lowest LOAEL for reproductive and developmental toxicity were 0.06% and 0.2%, respectively (approximately 38-44 mg/kg bw/day and 134-150 mg/kg bw/day as calculated by Hushka et al. 2001) based on significant reduction in offspring survival. The same effect level from this study was set by other international agencies in recent years (ECJRC 2003; EFSA 2005; SCCP 2007; NICNAS 2008a; ECHA 2013). Parental toxicity was observed in developmental and reproductive toxicity studies but was limited mostly to changes in organ weights, with the lowest LOEL for maternal toxicity at 131-149 mg/kg bw/day based on increase in liver weights. The lowest overall LOEL from these studies was in adult parental males where there were increases in liver and kidney weights at 103 mg/kg bw/day (Study A, P0) and decreases in body weight gain and food consumption at higher doses (Hushka et al. 2001).
In summary, although there were some statistically significant effects on some developmental landmarks, these differences were small, not consistent at all levels, and within the range of normal biologic variability. Results of these studies, along with previous reports that DIDP is inactive in screening tests for endocrine modulation (Harris et al. 1997 and Zacharewski et al. 1998; Furr et al. 2014) provided no evidence that DIDP treatment affects male reproductive development in laboratory animals.
9.2.2.1.2 Exposure at prepubertal-pubertal life stages
Anti-androgenicity of DIDP was examined in a Hershberger assay. Castrated prepubertal rats were treated with DIDP at dose levels of 20, 100, or 500 mg/kg bw/day in combination with 0.4 mg/kg bw/day of testosterone (Lee and Koo 2007) for 10 days. Seminal vesicle weight and ventral prostrate weight were significantly decreased in animals treated with DIDP at dose level of 500 mg/kg bw/day compared to the testosterone positive control. Significantly increased liver weight was also observed in animals of that group. Significant differences in luteinizing hormone levels and serum testosterone were also observed at mid and low doses, respectively.
In a limited comparative study using a single dose of 500 mg/kg bw/day, DIDP had no significant effect on sperm count after a 4-week exposure of juvenile rats (oral gavage; Kwack et al. 2009). However, DIDP reduced sperm motility, straight-line velocity, curvilinear velocity, straightness, and linearity of the epididymal sperm motion. Liver weight was significantly increased at this dose level but testis weight was unchanged (slight decrease but no statistical significance). From the hematological and clinical chemistry parameters, only the platelet count and serum alkaline phosphatase (ALP) were significantly increased.
In another study where rats were fed with DIDP for 21 continuous days, no effects were observed on the testes up to doses of 2200 mg/kg bw/day (Lington et al. 1993). Summaries of the studies are described in Table 9-15 below.
| Strain and species; Dose (mg/kg bw/day); Route; Duration (reference) |
Life stage at the start of study (age) | Hormone levelsFootnote Table 9-15[a] (T, S, LH) |
FertilityFootnote Table 9-15[b] | Reproductive tract pathologyFootnote Table 9-15[c] | Other effectsFootnote Table 9-15[d] |
|---|---|---|---|---|---|
| SD Rats; 0, 500; gavage; 28 daysFootnote Table 9-15[e] (Kwack et al. 2009) |
Prepubertal (PND 35) |
NM | 500Footnote Table 9-15[f] (motility) | NM | NE (BW) NE (ROW) 500* (ST- ↑ relative liver wt) |
| SD RatsFootnote Table 9-15[g]; 0, 20, 100, 500; gavage; 10 days (Lee and Koo 2007) (CAS not defined) |
Pubertal (PND 42) |
NM (T) 20* (↓ S) 100 (↑ LH) |
NM | NM | NE (BW) 500 (ROW) 500 (ST- ↑ relative liver wt) |
| F344 Rats; 0, 0.3, 0.6, 1.2, 2.5%; est. 0, 600, 1200, 2200; diet; 21 days (Lington et al. 1993) (CAS not defined) |
Not specified "young" |
NM | NM | NE | NE (BW) NE (ROW) 2200 (ST- ↓ food consumption) |
Overall, the only LOEL for reproductive toxicity identified for this life stage was 500 mg/kg-bw per day based on a significant decrease in seminal vesicle and ventral prostrate weights (no NOAEL; Lee and Koo 2007). However, it is difficult to evaluate what would be the adverse effects from changes in weight of accessory sex organs, as well as hormones variation, since they are most likely due to liver metabolism. DIDP also significantly increased liver weight at that dose. No studies were identified on any other species via any route of exposure at this life stage.
9.2.2.1.3 Oral exposure at the mature male adult stage
Information on effects of DIDP on the adult male (PND55+) was available from the two-generational toxicity study by Hushka et al. (2001), described in section 9.2.2.1. In the first study (study A), male body weights in the 0.8% dose groups were significantly below control values during the pre-mating period. Relative testes, epidydimis and seminal vesicle weight were significantly increased versus the control weights in P1 treated males; however, there were no pathologic changes in sexual organs. There was a small but significant decrease in normal sperm in all treated groups. There were no effects on mating, fertility, or gestational indices (mean length of gestation and mean litter size).
The results from the second study (study B) were similar to those from the first study. In the P1 generation, there were no treatment-related effects on parental survival, no clinical effects, and no differences in body weights or food consumption. There were no differences in the various reproductive indices, live birth indices, or male/female sex ratios. There were no significant differences in fertility indices, litter size, or sex ratio.
Two other repeated-dose studies in which animals were treated with DIDP showed limited effect on testis in male rats, with only increase in relative testes weights observed at high dose (BIBRA 1986; Cho et al. 2011). Common effects of DIDP were mostly a decrease in body weight and liver and kidney toxicity (see Table 9-16 and section 9.2.2.1 for detailed information).
| Strain and species; Dose (mg/kg bw/day); Route; Duration (reference) |
Life stage at the start of study (age) | Hormone levelsFootnote Table 9-16[a] (T, S, LH) | FertilityFootnote Table 9-16[b] | Reproductive tract pathologyFootnote Table 9-16[c] | Other effectsFootnote Table 9-16[d] |
|---|---|---|---|---|---|
| Crl:CDBR Rats; 0, 0.2, 0.4, 0.8%; est. F0 Adult males: 0, 103-198, 211-405, 427-787; diet; 10 wks before mating-PND21 (Hushka et al. 2001A) |
Not specified | NM | NE | NE | 427-787 (BW) 211-405 (ROW) 103-198 (ST- ↑ kidney wt) |
| Crl:CDBR Rats; 0, 0.02, 0.06, 0.2, 0.4%; est. F0 Adult males: 0, 12-23, 33-68, 114-225, 233-453; diet; 10 wks before mating-PND21 (Hushka et al. 2001B) |
Not specified | NM | NE | NE | NE (BW) NM (ROW) 233-453 (ST- ↑ liver, kidney wt) |
| Crl:CDBR Rats; 0, 0.25, 0.5, 0.75, 1%; est. M: 0, 32-264, 262-521, 414-776, 542-1014; diet;10 wks before mating-PND21 (Exxon Biomedical Sciences 1997; Huska et al. 2001) |
Not specified | NM | NE | NM | NE (BW) NR (ROW) 414-776 (ST- ↓body wt, food consump.) |
| F344 Rats; 0, 0.3, 1.2, 2.5%; est. 0, 300, 1000, 2000; diet; 21 days (BIBRA 1986) |
Not specified | NP | NP | NE (only tested at highest dose) | NE (BW) 2000 (ROW- ↓ absolute wt, ↑ relative testes wt) 300Footnote Table 9-16[f] (ST- ↑ liver, kidney wt) |
| F344 Rats; 0, 0.02, 0.05, 0.1, 0.3, 1%; est. 0, 25, 57, 116, 353, 1287; diet; 28 days (Lake et al. 1991) |
PND 42 | NP | NP | NE (only tested at highest dose) | NE (BW) NP (ROW) 116 (ST- ↑ relative liver wt) |
| CB6F1-Tg rasH2 mice; 0, 0.1, 0.33, 1%; est. 0, 130, 429, 1300 (based on Health Canada 1994); diet; 26 wks (Cho et al. 2011) |
PND 49 | NM | NM | NP | 1300 (BW) 1300 (ROW- ↑ relative testes wt) 130 (ST- histologic changes in liver) |
| CB6F1-Tg rasH2 wildtypemice; 0, 1%; est. 0, 1300; diet; 26 wks (Cho et al. 2011) |
PND 49 | NM | NM | NP | 1300 (BW) 1300 (ROW- ↑ relative testes wt) 1300 (ST- histologic changes in liver) |
These studies demonstrated little toxicity in adult reproductive organs. Effects were primarily decreases in body weight and increases in kidney and liver weight with corresponding histologic changes. No overt signs of reproductive toxicity or effect on fertility parameters were observed up to the highest dose tested. The NOAEL for fertility and reproductive developmental effects was 542 to 1014 mg/kg bw/day based on no effects observed in the one generation study identified (Huska et al. 2001).
9.2.2.2 Oral exposure in females
Eight oral studies on the reproductive and developmental effects of DIDP in females were identified. They include two two-generation studies.
The review identified four studies that examined the developmental/reproductive effects of DIDP in both sexes. In two, DIDP did not induce reproductive toxicity in males and females. In the two remaining studies (two-generation studies in rat, Hushka et al. 2001), gender-related differences were observed (small differences in time to vaginal patency) with no clear consistency.
Overall, data indicate that DIDP is a developmental and reproductive toxicant at similar doses as described above in section 9.2.2.1.1 (estimated doses of 103 mg/kg bw/day and above; Hushka et al. 2001). It induced postnatal lethality, growth alterations and mild teratogenicity in female offspring as well as alterations of reproductive-relative organ weights and vaginal haemorrhage in female parents. Developmental toxicity occurred at doses lower or similar to maternally toxic doses. Gender-related differences were observed in the reviewed studies but no consistent trend was observed.
9.2.2.3 Endocrine studies
In vitro studies have been done to test the potential effects of DIDP on steroidogenesis in mammalian systems. Many of these steroidogenesis assays are related to reproductive hormones, while others focus on glucocorticoid effects. Additionally, there are some miscellaneous effects assays.
In Hannas et al. (2012), DIDP was administered by gavage at dose levels of 0, 100, 300, 600 or 900 mg/kg bw/day to pregnant rats on GD 14-18 to examine its effect and define its potency relative to other phthalates on several genomic biomarkers of male reproductive developmental toxicity. Measurements were conducted to test effects of DIDP treatment on gene expression levels in foetal testes (e.g., Cyp11b1, Scarb1, StAR, Cyp11a1, Cyp17a1, Insl3, and Hsd3b). DIDP significantly reduced expression of only one gene, Wnt7a, at the highest dose tested. In addition to gene expressions referred to above, DIDP did not affect PPAR-related genes in foetal testes. Also, DIDP had no effect on ex vivo foetal testicular testosterone (T) production. Based on these results, DIDP does not appear to have anti-androgenic activity.
In in vitro studies, DIDP was involved in progesterone release in granulosa cells, was not oestrogenic and showed contradictory results for anti-oestrogenicity (Mlynarcikova et al. 2007; Akahori et al. 2008; Takeuchi et al. 2005; Ghisari and Bonefeld-Jorgensen 2009). DIDP did not affect androgen receptors (AR), but had a weak agonistic AhR activity (Kruger et al. 2008; Takeuchi et al. 2005).
DIDP may affect the sulphate supply pathway leading to increase in the availability of free hormones and decreased capacity for detoxification via sulphate conjugation (Harris et al. 2007; Turan et al. 2005). In addition, DIDP enhanced iodide uptake in thyroid cell line and had thyroid hormone-like effects in pituitary cells (Wenzel et al. 2005; Breous et al. 2005; Ghisari and Bonefeld-Jorgensen 2009).
9.2.2.4 Reproductive and developmental toxicity: evidence in humans
Available information on the potential effects of phthalates on humans was evaluated. The published literature was searched and human studies with an epidemiological focus were identified for further consideration. The evaluation included cross-sectional, case-control and cohort studies that encompassed 14 phthalate parent compounds and their metabolites. Given the large number of studies available in humans and the diverse outcomes identified for this substance grouping, all studies collected were scored for quality using a consistent evaluation metricFootnote[4] (Downs and Black 1988).This allowed for a reliable, objective assessment tool that captured the dimensions of study quality across various study designs. Statistically significant exposure-response associations were evaluated for each health outcome. A conclusion as to the level of evidence of association of a phthalate and each health outcome was based on the strength and consistency of the relationship as well as the quality of the epidemiology studies, as determined by the Downs and Black scores. Based on the overall score obtained from the evaluation approach, the level of evidence for association was designated as sufficient, limited, inadequate, or evidence suggesting no association. Studies that were rated in the lowest quartile (Quartile 1) based on the evaluation were not included in this report. This evaluation did not consider the biological plausibility of the relationship, meaning that no causal inference was established. More detail is provided in Health Canada (2015b) available upon request.
Limited human epidemiological data were available measuring DIDP or its metabolites with reproductive-developmental health outcomes. The available studies showed no associations for maternal urinary metabolite monocarboxyisononyl phthalate with any birth measures (birth weight, birth length and head circumference) (Philippat et al. 2012), or with the risk of preterm birth (Meeker et al. 2009).
More recent studies have found associations between metabolites of DIDP and various reproductive and developmenta endpoints, but these have not yet been assessed using the Downs and Black evaluation approach. More recent studies have reported some associations with MCNP and testosterone (Meeker and Ferguson 2014) and no significant associations between higher levels of MCNP and IGF2/H19 expression in placenta (LaRocca et al. 2014).
9.2.2.5 Other systemic effectsFootnote[5]
9.2.2.5.1 Considerations
It is well documented that phthalates can induce peroxisome proliferation in the liver as well as increase liver weight in rats and mice. In some cases, liver cancer was also observed following longer-term oral administration of high doses of phthalates. It is well established that the peroxisome proliferator-activated receptor (PPAR) α plays a role in peroxisome proliferation-induced liver effects (Corton and Lapinskas 2005). However, the relevance of the hepatotoxic effects of phthalates observed in rodents is difficult to establish due to the species-specific differences in the peroxisomal proliferation response (rodents being significantly more sensitive than humans to PPARα-mediated induction of peroxisome proliferation) (ECB 2008, NICNAS 2010, US CPSC 2010b). Several recent studies have suggested that the mechanisms of liver toxicity of peroxisome proliferators have not been entirely elucidated and that multiple pathways may exist, some of those likely PPARα-independent (Ito et al. 2007, Yang et al. 2007, Eveillard et al. 2009, Ren et al. 2010, IARC 2012). Based on this, liver effects cannot be precluded as an effect potentially relevant to humans and should be included in the characterization of health effects of phthalates (see Health Canada 2015c for more detailed information on the mode of action of liver carcinogenicity in rodents with peroxisome proliferators).
9.2.2.5.2 Repeated-dose studies
In repeated-dose studies identified in the literature for DIDP, the main effects observed following oral exposure were increased liver weights with correlating histological changes, effects that are considered to be related to peroxisome proliferation. Changes in kidney weights were reported as well in a few studies. No effects were reported in an inhalation study and no dermal studies were identified. The available studies are summarized below. NOAELs and LOAELs identified from these studies are presented in Table 9-17. In an inhalation study in which rats were exposed to 505 mg/m3 DIDP vapour for six hours per day, five days per week for two weeks, no signs of systemic toxicity were observed (General Motors Research Laboratories 1981).
In a 10-day gavage study in castrated male rats, an increase in liver weights was the only effect noted in animals treated with 0.4 mg/kg bw/day testosterone propionateand 500 mg/kg bw/day DIDP, the highest dose tested (Lee and Koo 2007, as described in section 9.2.2.1.2). In a 21-day study in which rats were fed 0, 0.3, 1.2 or 2.5% DIDP (0, 300, 1000 or 2000 mg/kg bw/day), elevated liver and kidney weights were observed in all exposed male rats. Increase in absolute and relative liver weights and increase in relative kidney weight was also observed in females at 1.2 and 2.5%. Electron microscopy of the liver revealed increased number and size of hepatic peroxisomes in both sexes at 2.5%. Histopathology revealed reduced hepatocyte basophilia at 1.2 and 2.5%, and increased eosinophilia and 2.5%. Non-dose related decreases in serum triglycerides and cholesterol were noted in males from 1.2%; as well, cyanide insensitive palmitoylcoA oxidation was increased in both sexes from 1.2%. 11- and 12-hydroxylation of lauric acid was increased in treated males; in females, high-dose females had increased 12-hydroxylase activity. Histology revealed no changes in the testes of treated males. Absolute testes weight was lower at highest dose, but higher when expressed relative to body weight. A significant reduction in body weight gain was noted at the highest dose in both sexes and food consumption was significantly reduced in males (BIBRA 1986). In this study, a NOAEL of 300 mg/kg bw/day was identified for females and a LOAEL of 300 mg/kg bw/day was identified for males based on liver effects observed at all doses tested.
In a 28-day feeding study, male rats were exposed to 0, 0.02, 0.05, 0.1, 0.3 and 1% DIDP in feed (approximately 0, 25, 57, 116, 353, 1287 mg/kg bw/day). A NOEL of 57 mg/kg bw/day and a LOEL of 116 mg/kg bw/day was identified based on a significant and dose-related increase in relative liver weight and in palmitol-CoA oxidation activity from 0.1%. Absolute liver weight was significantly increased from 0.3%. No testicular atrophy was noted (Lake et al. 1991). In other 28-day feeding studies in male and/or female rats and mice, increase in liver weight was noted at higher dose than in the study by Lake et al. (500 mg/kg-bw of DIDP per day and above) (BASF AG 1969a; Smith et al. 2000; Kwack et al. 2009).
When rats were exposed for 90 days to 0, 800, 1600, 3200, 6400 ppm DIDP (approximately 0, 55, 100, 200, 400 mg/kg bw/day for males and 0, 60, 120, 250, 500 mg/kg bw/day for females) through the diet, a NOEL of 60 mg/kg bw/day and a LOEL of 120 mg/kg bw/day were identified based on dose-dependent increase in relative liver weight in females. Female absolute liver weights were increased significantly at doses of 250 and 500 mg/kg bw/day. In males, absolute liver weight was significantly increased at the highest dose tested. Relative liver weight was also increased, although no dose-response relationship could be established. Female relative kidney weights were significantly elevated at 120 and 250 mg/kg bw/day, but not at 500 mg/kg bw/day. Relative kidney weights were significantly increased in all treated males, but also without dose-concordance. No histopathological findings were noted in any organ in both sexes (BASF AG 1969b). In another 90-day feeding study in rats, increases in absolute and relative liver weights were noted only in males and females exposed to the highest dose of DIDP (586 to 686 mg/kg bw/day). No kidney changes were reported (Hazleton Laboratories 1968a).
In a 13-week diet study in dogs (3 per sex per group) exposed to 0, 0.3, 0.5, or 1% DIDP (equivalent to approximately 0, 15, 75, and 300 mg/kg bw/day), hepatic effects were also reported. A dose-related increase in mean absolute liver weight was observed (not statistically significant due to small numbers of animal used and variability). A slight increase in relative liver weight was also noted at the highest dose. In mid- and high-dose animals, there was slight to moderate swelling and vacuolation of hepatocytes. Clinical markers of hepatic injury were similar to control (ALT, AST, and BSP clearance) (Hazleton Laboratories 1968b). While there is some limitations in this study (inadequate statistical analysis due to small study size), a NOAEL of 15 mg/kg bw/day and a LOAEL of 75 mg/kg bw/day were identified based on increase in liver weight accompanied with histological changes.
| Strain and species; Dose (mg/kg bw/day); Route; Duration (reference) | NOAEL (mg/kg bw/day) | LOAEL (mg/kg bw/day); (Effects) |
|---|---|---|
| SD RatsFootnote Table 9-17[a] (males); 0, 20, 100, 500; gavage; 10 days (Lee and Koo, 2007) (CAS not defined) |
NOEL: 100 | LOEL: 500 (significant increase in liver weights) |
| Fischer 344 rats; 0, 0.3, 1.2, 2.5%; est. 0, 300, 1000, 2000; diet; 21 days (BIBRA 1986) |
- (males) 300 (females) |
300 (significant increase in absolute and relative liver weights accompanied with histopathological changes in the liver at the highest dose) (males) 1000 (significant increase in absolute and relative liver weights accompanied with histopathological changes in the liver at the highest dose) (females) |
| Fisher 344 rats (males); 0, 1000 or 12 000 ppm; est. 0, 50, 600 (based on Health Canada 1994); diet; 2 or 4 weeks (Smith et al. 2000) |
NOEL: 50 | LOEL: 600 (significant increase in relative liver weights, periportal DNA synthesis and PBOX activity) |
| Fischer 344 rats (males); 0, 0.02, 0.05, 0.1, 0.3 and 1%; est. 0, 25, 57, 116, 353, 1287; diet; 28 days (Lake et al. 1991) |
NOEL: 57 | LOEL: 116 (significant and dose-related increase in relative liver weights and increase in palmitol-CoA oxidation activity) |
| Rats; 0, 5000 or 10 000 ppm; est. 0, 600 and 1250 (males); 0, 1100 and 2100 (females); diet; 28 days (BASF AG 1969a) |
NOEL: 600 - 1100 (males/females) | LOEL: 1250-2100 (significant and dose-related increase in absolute and relative liver weights) (males/females) |
| SD Rats (males); 0, 500; gavage; 28 days (Kwack et al. 2009) |
- | LOEL: 500 (significant increase in relative liver weights) |
| Rats;0, 800, 1600, 3200, 6400 ppm; est. 0, 55, 100, 200, 400 (males); 0, 60, 120, 250, 500 (females); diet; 90 days (BASF AG 1969b) |
NOEL: 200 (males) 60 (females) |
LOEL: 400 (significant increase in absolute liver weights) (males) LOEL: 120 (significant increase in relative liver weights) (females) |
| Rats; 0, 0.05, 0.3, 1% in feed; est. 0, 28, 170, 586 (males); 0, 35, 211, 686 (females); diet; 90 days (Hazleton Laboratories 1968a) |
NOEL: 170 - 211 (males/females) | LOEL: 586-686 (Significant increase in absolute and relative liver weights and minimal increase in thyroid activity) (males/females) |
| B6C3F1 mice (males); 0, 500 or 6000 ppm; est. 0, 65, 780 (based on Health Canada 1994); diet; 2 or 4 weeks (Smith et al. 2000) |
NOEL: 65 | LOEL: 780 (significant increase in relative liver weights after 2 weeks but not after 4 weeks, increase in periportal DNA synthesis and PBOX activity) |
| Dogs; 0, 0.3, 0.5, 1% in feed; est. 0, 15, 75, 300 (based on NICNAS 2008a); diet; 13 weeks (Hazleton Laboratories 1968b) |
15 (males/females) | 75 (dose-related increase in absolute liver weights and histological changes) (males/females) |
| Rats (males);0, 505 mg/m3; inhalation; 6h per day, 5 days per week, for 2 weeks (General Motors Research Laboratories 1981) |
505 mg/m3 | No systemic effects reported. |
Overall, the lowest LOAEL for short-term oral exposure identified for DIDP was 300 mg/kg bw/day based on increase in absolute and relative liver weights accompanied with histopathological changes in the liver at the highest dose in all treated male rats in a 21-day study (BIBRA 1986). As mentioned earlier in this section, no effects were noted in rats exposed to DIDP in one inhalation study (2-week duration) and a NOAEC for short-term inhalation exposure was identified at 505 mg/m3 (General Motors Research Laboratories 1981). In a subchronic oral study in dogs, which is considered a more relevant species to humans with respect to peroxisome proliferation than rodents, a NOAEL of 15 mg/kg bw/day and a LOAEL of 75 mg/kg bw/day were identified based on increase in liver weight accompanied with histological changes (Hazleton Laboratories 1968b). However, it is important to note that some limitations were observed in this study (e.g., inadequate statistical analysis due to small study size).
9.2.2.5.3 Carcinogenicity
DIDP has not been classified for its potential carcinogenicity by other international agencies.
In a 2-year oral carcinogenicity study in which F344 rats were fed diets containing 0, 400, 2000, and 8000 ppm DIDP (equivalent to 0, 22, 110, 479 mg/kg bw/day for males and 0, 23, 128, 620 mg/kg bw/day for females), an increased incidence of mononuclear cell leukemia (MNCL) was observed in both sexes at the highest dose. However, the incidences in the different dose groups were not dose-related and according to the author, were within the NTP historical ranges in control F344 rats (historical data from the breeder used by the authors in their study was not provided) (see Table 9-18 for incidence rates). No other treatment-related neoplastic lesions were noted in other organs, including the liver. Non-neoplastic effects observed in males and females included histopathological changes in the liver, such as an increase in microgranuloma and a statistically significant increase in incidence of spongiosis hepatis in males exposed at all doses and a significant increase in liver necrosis in both sexes at the highest dose. Histopathological changes in the kidney (mineralization and interstitial nephritis) were also noted in males exposed at 479 mg/kg bw/day. A significant decrease in survival and body weight and a significant increase in relative liver and kidney weights were also observed at that dose level (Cho et al. 2008). In this study, the LOAEL for non-neoplastic effect was 22 mg/kg bw/day based on spongiosishepatis and other signs of hepatotoxicity in the liver of all treated males.
Cho et al. (2008) also assessed DIDP potential to induce peroxisome proliferation by measuring the levels of the H2O2-degrading enzyme catalase contained within the peroxisomes, which is a marker of peroxisome proliferating activity. A group of 50 rats were fed diets containing up to 8000 ppm DIDP and 12 000 ppm di(2-ethylhexyl)phthalate (DEHP) as a positive control, for 12 or 32 weeks. After 12 weeks of treatment, levels of catalase in the liver of rats treated at the highest dose were significantly increased compared to the vehicle control. However, after 32 weeks of treatment, no significant differences were found in the level and activity of catalase protein among the DIDP-treated liver tissues while an increase was noted in the liver of rats administered DEHP. Likewise, DIDP treatment caused no significant changes in the expression of catalase at the end of the study (104 weeks). It was proposed that long-term exposure to DIDP results in limited peroxisomal proliferating activity and thus explains the non-carcinogenicity of this phthalate in rats (Cho et al. 2008).
In a recent carcinogenicity study, transgenic rasH2 mice of both sexes were exposed through the diet to 0, 0.1, 0.33, and 1% DIDP (equivalent to 0, 130, 429, 1300 mg/kg bw/day, based on a dose conversion by Health Canada 1994) for 26 weeks. Wild-type mice were also exposed to 0 and 1% DIDP (0 and 1300 mg/kg bw/day) for the duration of the study. A statistically significant increase in hepatocellular adenomas was observed in male transgenic mice administered DIDP at the highest dose, but not in the wild-type mice. No hepatocellular adenomas were observed in both transgenic and wild-type female mice and no MNCL was observed in the study.
Non-neoplastic effects observed included histologic changes in the liver (parenchymal inflammation, diffuse hypertrophy with eosinophilic granules, focal necrosis, pigmented hepatocytes/Kupffer cells or prominent Kupffer cells) seen in males and/or females transgenic and wild-type mice starting from the lowest dose tested or at higher dose. Among the other effects observed, a significant increase in relative liver and kidney weights was noted in male and female transgenic mice at the highest dose. Liver weights were also significantly increased in transgenic male mice exposed to 0.33% DIDP. Significant increase in relative liver weight was observed in both sexes in exposed wild-type mice, but increase in relative kidney weight was noted only in exposed females. At 1% DIDP, a higher incidence of tubular basophilia and tubular hyperplasia was observed in the kidneys of transgenic and wild-type male mice. A significant decrease in body weights was also noted in both transgenic and wild-type mice (male and female) exposed at that dose level (Cho et al. 2011). A LOAEL for non-neoplastic effect of 130 mg/kg bw/day was identified in this study based on histologic changes in the liver in mice of both sexes.
The transgenic rasH2 mice are reported to be sensitive to genotoxic and non-genotoxic carcinogens, but have a relatively low susceptibility for liver carcinogenicity. It has been suggested by Cho et al. that peroxisome proliferation could be the underlying mode of action for development of liver tumours in mice in this study (Cho et al. 2011; ECHA 2013). However, neoplastic lesions in the liver were not observed in rats exposed for 2 years, and it has been shown that DIDP has a limited peroxisomal proliferating activity in that species in comparison with other phthalates such as DEHP.
The mode of action (MOA) for the liver tumours in transgenic male mice has not been fully elucidated and the carcinogenic potential of DIDP in humans remains unclear. More detailed information on the MOA of liver carcinogenicity in rodents with peroxisome proliferators is available in Health Canada (2015c).
| Strain and species; Dose (mg/kg bw/day); Route; Duration (reference) | Result |
|---|---|
| Fischer 344 rats; 0, 400, 2000, and 8000 ppm; est. 0, 22, 110, 479 (males); 0, 23, 128, 620 (females); diet; 2 years (Cho et al. 2008) |
No treatment related neoplastic lesions observed. Incidence of MNCL lesions (males: 20%, 32%, 28%, 46% at 0, 22, 110, 479 mg/kg bw/day, respectively; females: 23%, 14%, 22%, 45% at 0, 23, 128, 620 mg/kg bw/day, respectively). Incidence of MNCL lesions in NTP historical control (males: 32-74%; females: 14-52%) LOAEL (non-neoplastic): 22 mg/kg bw/day (histopathological changes in the liver) (males) |
| Fischer 344 rats; 0, 400, 2000, and 8000 ppm; est. 0, 22, 110, 479 (males); 0, 23, 128, 620 (females); diet; 12 and 32 weeks (Cho et al. 2008) |
Increase of catalase activity after 12 weeks but not after 32 weeks. |
| CB6F1-Tg rasH2 mice; 0, 0.1, 0.33, and 1% DIDP; est. 0, 130, 429, 1300 (based on Health Canada 1994) Wild-type mice; 0, 1% DIDP; est. 0, 1300; diet; 26 weeks (Cho et al. 2011) |
Significant increase in incidence of hepatocellular adenomas in male rasH2 mice receiving 1% DIDP (males: 0%, 7%, 7%, 33%; females: 0%, 0%, 0%, 0% at 0, 130, 429, 1300 mg/kg bw/day, respectively) No increase in hepatocellular adenomas in wild-type mice (males: 0%, 7%; females: 0%, 0% at 0, 1300 mg/kg bw/day, respectively) LOAEL (non-neoplastic): 130 mg/kg bw/day (histologic changes in the liver) (males/females) |
Overall, the lowest oral LOAEL for chronic non-cancer effects was 22 mg/kg bw/day, based on spongiosis hepatis and other histopathological changes in the liver of male rats exposed to DIDP in a two-year carcinogenicity study (Cho et al. 2008).
9.2.2.5.4 Genotoxicity
In in vitro assays, negative results were observed with DIDP in the Ames test using Salmonella typhimurium strains TA 98, TA 100, TA 1535, and TA 1537, and in an 8-azaguanine assay, with and without metabolic activation (Seed 1982; Zeiger 1985). In mouse lymphoma cell mutation assays, negative results were also reported, with and without metabolic activation (Litton Bionetics 1985a; Hazleton Biotechnologies 1986; Barber 2000). Positive results were seen in one of two in vitro transformation assays. In the first assay, DIDP was tested on Balb/c-3T3 mouse cells at concentrations up to 20 µl/ml. The cells were exposed for 72 hours and then incubated from four weeks. There were no significant increases in transforming activity (Litton Bionetics 1985b). In the second assay, Balb/3T3 Clone A31 mouse embryo cells were treated with DIDP for 20-24 hours then incubated from four to six weeks. DIDP led to an increase in transforming frequencies at one µl/ml but not at 0.01 or 0.1 µl/ml (Microbiological Associates 1981). Since positive results were seen only in one of the assay, it is impossible to conclude on DIDP potential to induce cell transformation.
In an in vivo mouse micronucleus assay, negative results were observed when CD-1 mice were orally administered single dose of DIDP at up to 5000 mg/kg-bw (Hazleton Washington 1994; Mckee et al. 2000).
Based on the collective evidence on genotoxicity, which include negative results from in vitro bacterial mutation assays, in vitro mouse lymphoma assays and in an in vivo mousemicronucleus assay, DIDP is likely non-genotoxic.
9.2.2.5.5 Evidence of systemic toxicity in humans
Available information on the potential effects of DIDP on humans was evaluated using the Downs and Black approach (Appendix E). As previously mentioned, this evaluation did not consider the biological plausibility. More detail is provided in Health Canada (2015b) available upon request.
Based on this evaluation, there were no associations between metabolites of DIDP and cardiovascular function (Trasande et al. 2014), and allergy/asthma related symptoms (Hoppin et al. 2013). There was inadequate evidence of associations for MCNP and biomarkers of oxidative stress and inflammation (Ferguson et al. 2011, 2012).
More recent studies have found associations between DIDP and various endpoints, but these have not yet been assessed using the Downs and Black evaluation approach. An additional study by Ferguson et al. (2014) reported associations between MCNP and biomarkers of oxidative stress and inflammation. Buser et al. (2014) reported associations with MCNP and obesity. No significant associations were reported between MCNP and blood pressure considering both genders together (Shiue 2014a,b; Shiue and Hristova 2014), and considering men only (Shiue and Hristova 2014). However, significant association between MCNP and blood pressure in women was reported (Shiue and Hristova 2014). No significant associations were found between higher levels of MCNP and osteoporosis (Min and Min 2014).
9.2.3 DUP
9.2.3.1 Reproductive and developmental effects in males
In this section, the first three segments focus on reproductive and developmental effects of the male gender in three different life stages (gestational exposure [GD0-21], (pre)pubertal-pubertal [PND1-55], and adult [PND55+]). Adverse effects observed after gestational exposure are further organized and presented in the following subcategories: 1) changes in hormone levels (serum or testicular); 2) feminization parameters; 3) reproductive tract malformations and/or effects on fertility; and 4) other developmental parameters.Footnote[6]Descriptions of effects within each life stage are structured such that effects occurring at the lowest doses are summarized first. The potential reproductive developmental effects of DUP in female animals are presented next in a similar manner in considering life stage and species sensitivity. When no studies were available for DUP for a particular life stage or exposure period, an analysis of health effects for its analogue DnOP was conducted (Health Canada 2015a). The last segments focus on endocrine studies and reproductive and developmental effects observed in humans
9.2.3.1.1 Early development: in utero exposure
A literature search identified one study examining the potential effects of DUP during gestation in rats. A summary of the study is described in Table 9-19 below.
In a developmental study, Saillenfait et al. (2013) administered DUP to pregnant Sprague-Dawley rats at doses of 0, 250, 500 or 1000 mg/kg bw/day, by gavage (in olive oil), on GD 6-20. Male anogenital distance (AGD) was observed to be slightly reduced at the mid and high dose (500 and 1000 mg/kg bw/day, 3 and 4% change with concurrent control, respectively) in foetuses of dams exposed to DUP. Statistical significance was only obtained in the 500 mg/kg bw/day male group when adjustment with the cubic root of foetal weight or when foetal body weight was used as covariate (with litter based analysis or mixed-effects model). No effects on testicular descent (i.e., cryptorchidism) were observed. An increase in the occurrence of lumbar ribs was also observed in foetuses from the 500 and 1000 mg/kg bw/day dose groups, compared to control. According to the authors, although this variation did not occur in a clear dose-related manner, a relationship to treatment cannot be ruled out. No significant differences were observed in the number of corpora lutea or incidence of pre-implantation loss. No effects were observed on post-implantation loss, resorptions, live foetuses, on foetal sex ratio (percent male foetuses per litter), or on foetal body weights. Also, no significant maternal toxicity was noted. The LOAEL for this study was 500 mg/kg bw/day based on increase occurrence of supernumerary lumbar ribs.
It should be noted that most reproductive parameters directly pertaining to the male reproductive system as it relates to RPS were not measured in mice or any other species; therefore, no conclusions can be made regarding the particular potential of DUP to induce this syndrome in animals.
No other developmental studies were identified examining gestational exposure to DUP using other species.
Effects observed following exposure to phthalates with similar chain length/size:
Since only one study examining effects from exposure during the masculinization programming window (GD15-17) was identified for DUP, an analysis of similar phthalates that could be used as potential analogues for read-across to inform the characterization of reproductive and developmental effects of DUP was performed. It identified di-C9-11-alkyl phthalate [D911P] (1,2-Benzenedicarboxylic acid, di-C9-11-branched and linear alkyl esters: CAS # 68515-43-5) and di-n-octyl phthalate [DnOP] (1,2-Benzenedicarboxylic acid, 1,2-dioctyl ester: CAS # 117-84-0) as the "closest analogue" phthalates to DUP within the subcategory based on consideration of similarities in the length and nature of the ester chains (section 2.3.2; Health Canada 2015a). Summaries of the studies are presented in Table 9-19 below.
D911P was tested in a two-generation reproductive toxicity study by Willoughby et al. (2000). D911P was administered daily to Sprague-Dawley rats for 10 weeks prior to mating in the F0 generation at dietary levels of 0, 0.1, 0.5, and 1.0% (Table 9-19). Treatments of the F1 generation were as described for the F0 generation. Estimated dose levels for the F0 and F1 generation males were approximately 0, 71-81, 361-432 and 756-854 mg/kg bw/day. A significant dose-related decrease in mean pup weights was observed at 756-854 mg/kg bw/day in F1 and F2 male and female pups at postnatal days 14 to 25. Although this effect was also observed at 432 mg/kg bw/day in F2 female pups at PND 14, it was not seen on other PND in this group. Authors reported that D911P treatment did not affect oestrous cyclicity, number mated, sex ratio, pup weight at birth, pup gross abnormalities, or pup sexual maturation in either generation of rats. A dose-related and significant reduction in gestation length (0.5 days) was observed in the F0 (when treated with 361 mg/kg bw/day and above) and F1 generation rats (at a dose of 854 mg/kg bw/day). Dose-related decrements were reported for the number of rats pregnant, the number of live litters, the number of implantation sites, and the litter size in the F1 generation. However, these effects were probably related to maternal toxicity, since final female body weights were decreased in a dose-related fashion in both the F0 and F1generation. Pup sexual maturation (preputial separation) was delayed (by one day) in the F1 pups treated with the highest dose of D911P (854 mg/kg bw/day), but according to the authors, the difference was not statistically significant or outside the range of historical controls. Differences in maturation may have been related to observed body weight effects. In summary, exposure of male and female Sprague-Dawley rats to D911P at up to 854 mg/kg bw/day in the diet for two generations did not affect sexual behaviour, fertility, or fecundity in the absence of maternal toxicity (Willoughby et al. 2000). The NOAEL for effects on development was 361 to 432 mg/kg bw/day and the LOAEL was 756 to 854 mg/kg bw/day based on decreased pup weight during weaning.
In a developmental toxicity study by Fulcher et al. (2001), D911P was administered daily by oral gavage to mated female Sprague-Dawley rats at doses of 0, 250, 500, and 1000 mg/kg bw/day from GD1 to 19. There were no statistically significant differences in body weight, fertility, reproductive organs, litter size, placental weights or foetal survival observed between treated animals and control groups at any dose at any time during gestation. Visceral and skeletal examinations of foetuses revealed an increased incidence of dilated renal pelvis in pups at 1000 mg/kg bw/day. Pups of the mid- and high-dose groups (500 mg/kg bw/day and above) also showed increased incidences of supernumerary lumbar ribs. It was reported that D911P did not induce maternal toxicity.
Several studies examined the potential developmental effects of DnOP in rodents. Two studies examined effects in rats when DnOP is administered during gestation during the masculinization programming window, and are described here.
In a developmental study by Saillenfait et al. (2011), DnOP was administered daily by oral gavage to mated female Sprague-Dawley rats at doses of 0, 250, 500, and 1000 mg/kg bw/day from GD6 to 20. No effects on AGD were observed. The incidence of supernumerary lumbar ribs in foetuses or litters was reported to be significantly higher than controls in all treated groups. The number of implants and live foetuses, and the incidence of post-implantation loss, resorptions and foetal deaths were similar across all groups including control. No significant maternal toxicity was noted. The LOAEL for this study was determined to be 250 mg/kg bw/day based on increase occurrence of supernumerary lumbar ribs.
DnOP had no effect on fertility or reproductive performance in Swiss albino CD-1 mice at doses up to 7500 mg/kg bw/day in the diet for 7 days before and throughout a 98-day continuous breeding period (Heindel et al. 1989; Lamb et al. 1997). No significant differences in the fertility and reproductive performance of the parental generation were observed compared to controls. Reproductive effects were also examined in the offsprings from high-dose treated parents (second generation, F1). There was a decrease in the weight of the seminal vesicles in the absence of any changes in the weight of the testes, prostate, and epididymis, or effects on the sperm of the F1. There were no significant differences in the fertility and reproductive performance at any parental generations compared to controls, though the liver weight in both sexes and the kidney weights in females were increased in the F1. The LOEL for maternal toxicity was 8640 mg/kg bw/day based on increases in organ weights observed at this dose.
Other developmental studies showed no effects on male reproductive organs.
| Strain and species; Dose (mg/kg bw/day); Route; Duration (reference) |
Testosterone levelsFootnote Table 9-19[a] (T, S) |
Feminization parametersFootnote Table 9-19[b] | Reproductive tract malformations and/or fertilityFootnote Table 9-19[c] | Other developmental parametersFootnote Table 9-19[d] | Maternal effects |
|---|---|---|---|---|---|
| DUP SD Rats; 0, 250, 500, 1000; gavage; GD:6-20 (Saillenfait et al. 2013) |
NM | 500Footnote Table 9-19[e], NDR (AGD) NM (NR) NM (PPS) |
NE (CRY) NM (HYP) NM (TP NM (FER) |
NE (BW) NM (ROW) NE (FV) NE (EMB) 500 (ESV) |
NE |
| D911P SD Rats; 0, 0.1, 0.5, 2/1%f; est. F0 Female intake during gestation: 0, 71, 361, 756 6 weeks prior to mating -PND21 (Willoughby et al. 2000) |
NM | NM (AGD) NM (NR) 756NS (PPS) |
NM (CRY) NM (HYP) NE (TP- adult) NE (FER- adult) |
NE (BW) 756 (ROW) NE (FV) NM (EMB) NM (ESV) |
1389 (↑ liver lesions), ↓ BW in 1st week of treatment) |
| D911P SD Rats; 0, 0.1, 0.5, 2/1%Footnote Table 9-19[f]; est. F1 Female intake during gestation: 0, 81, 432, 854 (In utero exposed- PND21) (Willoughby et al. 2000) |
NM | NM | NM (CRY) NM (HYP) NP (TP) NP (FER) |
854 (BW) NP (ROW) NE (FV) NM (EMB) NM (ESV) |
1264 (↑ liver wt, ↓ BW) |
| DnOP SD Rats; 0, 250, 500, 1000; gavage; GD:6-20 (Saillenfait et al. 2011) |
NM | NE (AGD) NM (NR) NM (PPS) |
NM | 250Footnote Table 9-19[g] (BW) NM (ROW) NE (FV) NE (EMB) 250g (ESV) |
NE |
| DnOP CD-1 Mice; 0, 0.5, 1.25, 2.5, 5.0%; est. F1: 0, 1800, 3600, 7500 (diet) 7 days prior to mate- PND98 (Heindel et al. 1989; Lamb et al. 1997) |
NM | NM | NM (CRY) NM (HYP) NP (TP) NM (FER) |
NE (BW) NM (ROW) NE (FV) NM (EMB) NM (ESV) |
8640 (↑ liver and kidney wt) |
| DnOP CD-1 Mice; 0, 9780; gavage; GD:6-13 (Hardin et al. 1987) |
NM | NM | NM | 9780g (BW) NM (ROW) 9780g (FV) NM (EMB) NE (ESV) |
NE |
Overall, the highest no observed adverse effect level (NOAEL) for developmental effects of DUP following in uteroexposure was identified to be 250 mg/kg bw/day based on an increase occurrence of lumbar ribs in foetuses at the next highest dose of 500 mg/kg bw/day (LOAEL; Saillenfait et al. 2013). The effect on AGD in this study was not considered significant at the highest dose tested and the magnitude of change was within historical controls. An increase in occurrence of lumbar ribs in foetuses was also observed in a study conducted with DnOP at a dose of 250 mg/kg bw/day. No effect on AGD following gestational exposure was observed (Saillenfait et al. 2011). The highest NOAEL for systemic toxicity for these studies was between 88 to 117 mg/kg bw/day based on increased liver lesions in the parental male rats (Willoughby et al. 2000). No indication of adverse effects on dams was observed.
9.2.31.2 Exposure at prepubertal-pubertal life stage
In a limited comparative 4-week toxicity study, Sprague-Dawley male rats were administered DUP by gavage at a single dose of 500 mg/kg per day. Sperm count and motility were significantly reduced at this dose (28% and 63%). Specific motility parameters significantly reduced were curvilinear velocity (17%), straightness (19%), and linearity (19%). No significant changes in body weight, food consumption or organ weight (e.g., liver, testis, epididymis) were observed. However, total protein, glutamate oxaloacetate transferase (GOT), and alkaline phosphatase activities were increased (Kwack et al. 2009).
In another study where rats were fed DUP in their diet for 21 continuous days, DUP did not reduce testis weights or induce testicular atrophy in rats up to doses of 2200 mg/kg bw/day (Lington et al. 1993).
No other studies were identified examining prepubertal-pubertal exposure to DUP using other species.
Effects observed following exposure to DnOP:
Results appear to be similar in studies examining effects of di-n-octyl phthalate (DnOP; CAS 117-84-0). DnOP was also observed to cause decreases in sperm count and motility at 500 mg/kg bw/day in prepubertal rats after short-term oral exposure in a limited one-dose study (Kwack et al. 2009).
There were also observations of subtle effects in the testes (alterations in the smooth and rough endoplasmic reticulum in Leydig cells) in pubertal rats at very high doses (2000 mg/kg bw/day; Jones et al. 1993). No effects in males following exposure to doses as high as 2800 mg/kg bw/day were observed (Mann et al. 1985; Foster et al. 1980; Oishi and Hiraga 1980). Summaries of the studies are described in Table 9-20 below.
| Strain and species; Dose (mg/kg bw/day); Route; Duration (reference) |
Life stage at the start of study (age) | Hormone levelsFootnote Table 9-20[a] (T, S, LH) |
FertilityFootnote Table 9-20[b] | Reproductive tract pathologyFootnote Table 9-20[c] | Other effectsFootnote Table 9-20[d] |
|---|---|---|---|---|---|
| DUP SD Rats; 0, 500; gavage; 28 days* (Kwack et al. 2009) |
Prepubertal (PND35) |
NM | 500Footnote Table 9-20[e] (sperm count, motility) | NM | NE |
| DUP F344 Rats; 0, 0.3, 0.6, 1.2, 2.5%; est. 0, 600, 1200, 2200; diet; 21 days (Lington et al. 1993) (CAS not defined) |
Not specified "young" |
NM | NM | NE | NE (BW) NE (ROW) NM (ST) |
| DnOP SD Rats; 0, 500; gavage; 28 daysFootnote Table 9-20[f] (Kwack et al. 2009) |
Prepubertal (PND 35) |
NM | 500e (sperm count, motility) | NM | NE |
| DnOP SD Rats; 0, 2800; gavage; 4 days (Foster et al. 1980) (CAS not defined) |
Prepubertal (weight based) |
NM | NM | NE | NE (BW) NE (ROW) NM (ST) |
| DnOP Wistar Rats; 0, 2000; gavage; 2 days (Jones et al. 1993) (CAS not defined) |
Pubertal (PND42-56) |
NM | NM | 2000* | NM |
| DnOP Wistar Albino Rats; 0, 2%; est. 1000 (based on Health Canada 1994); diet; 21 days (Mann et al. 1985) |
Pubertal-Adult (PND35) |
NM | NM | NE | NE (BW) NE (ROW) 1000e (ST- ↑relative liver wt) |
| DnOP Wistar Rats; 0, 2%; est. 0, 1000 (US CPSC 2010); diet; 7 days (Oishi and Hiraga 1980) |
Prepubertal (PND 35) |
NE (T) NE (S) NM (LH) |
NE | NM | NE (BW) NE (ROW) 1000e (ST- ↑ liver wt) |
Overall, the only observed effect level for reproductive toxicity of DUP identified for this life stage was 500 mg/kg bw/day based on a significant decrease in sperm count and motility (Kwack et al. 2009). However, this is a limited study where only one dose was used. Also, the length of the study did not allow a full cycle of spermatogenesis to occur and therefore, it is difficult to interpret the adverse outcomes of a decrease in sperm count and motility. Doses below 500 mg/kg bw/day were not tested for the analogue DnOP. Liver weight was decrease at 1000 mg/kg bw/day in studies using DnOP (Mann et al. 1985; Oishi and Hiraga 1980).
9.2.3.1.3 Oral exposure at the mature male adult stage
Only one study was identified in which reproductive parameters were measured after male adult rodents were treated with DUP. In a 21-day study, male rats were fed DUP in their diet at doses of 0, 282, 1145 and 2305 mg/kg bw/day (BIBRA 1985; Barber et al. 1987). Dose-related increases in relative, but not absolute, testes weight were observed in males treated at the mid and high doses (1145 mg/kg bw/day and above); however, the relative weights were within historical control range. According to the authors, the increase in relative testis weight reflected the decrease in body weight observed in these animals rather than a specific effect on the testis. There were no treatment-related histological abnormalities in the testis.
Effects observed following exposure to DnOP:
In a one-generation reproductive toxicity study where mice were given in the feed 1800, 3600 or 7500 mg/kg bw/day DnOP before mating, at mating and through lactation (Heindel et al. 1989; Morrissey et al. 1989), statistically significantly lower seminal vesicle weight compared with controls was observed in F1 males treated with 7500 mg/kg bw/day (males in the F1 generation treated at the same dose as their parent; only the highest dose was tested in the F1 generation), but no other male reproductive differences from controls in reproductive organ weights, sperm concentrations, percentage morphologically abnormal sperm, or percentage motile sperm were observed in the study. Liver weights were also increased compared with controls in the F1 male group.
Using the two-generational toxicity study described in section 9.2.3.1.1 (Willoughby et al. 2000), information was extracted to determine the effects of D911P on the adult male (PND55+). Histopathological evaluations of reproductive organs and tissues from F0 males revealed no evidence of treatment-related effects. Relative (not absolute) testes weight was increased and testicular sperm count was increased in the F0 generation in the group receiving a dose of 1%. Relative epididymal weights were decreased in the 1.0% group for F0 (absolute epididymal weights were reduced for both generations), although sperm concentration, motility, and morphology were not affected. There was no impairment of fertility, fecundity, or development in either generation. The changes in reproductive organ weights in the F0 males may reflect treatment-related effects on body weight. Histological findings included a dose-related increase in the number of small epididymides and small, dark, flaccid testes in theF0 generation. The NOAEL for effects on reproduction was established at 1238 to 1510 mg/kg bw/day, the highest dose tested. Systemic toxicity was observed at 1238 to 1510 mg/kg bw per day based on liver effects. Summaries of the studies are presented in Table 9-21 below.
| Strain and species; Dose (mg/kg bw/day); Route; Duration (reference) |
Age at the start of dosing | Hormone levelsFootnote Table 9-21[a] (T, S, LH) |
FertilityFootnote Table 9-21[b] | Reproductive tract pathologyFootnote Table 9-21[c] | Other effectsFootnote Table 9-21[d] |
|---|---|---|---|---|---|
| DUP Rat (Not specified); 0, 0.3, 1.2, 2.5%; est. 0, 282, 1145, 2305 (based on HPVIS); diet; 21 days (Barber et al. 1987, BIBRA 1985) |
Not specified | NM | NM | NE | 1145 (BW) 1145 (↑ ROW) 1145 (ST- ↑ relative kidney and liver wt, liver lesions) |
| D911P SD Rats; 0, 0.1, 0.5, 2/1%; est. F0 Adult males; 0, 88, 444, 1510; 6 weeks prior to mating -PND21 (Willoughby et al. 2000) |
6-7 wks | NM | NE | NE | 1510 (BW) 1510 (↑ testes wt; ↓ epididymal wt) 444 (liver lesions) |
| DnOP CD-1 Mice; 0, 1.25, 2.5, 5.0%; est. 0, 1800, 3600, 7500; diet; 7 days prior to mate- PND98 (Heindel et al. 1989; Morrissey et al. 1989Footnote Table 9-21[e]) |
11 wks | NM | NE | NP | NEe (BW) 7500e (ROW- Seminal vesicle) 7500e(ST- ↑ relative liver wt) |
| DnOP SD Rats; 0, 5, 50, 500, 5000 ppm; est. 0, 0.4, 3.5, 37, 350; diet; 13 wks (Poon et al. 1997) |
Not specified "young" |
NM | NM | NE | NM (BW) NM (ROW) 350 (ST- histologic effects in the liver and thyroid) |
The lowest LOEL for reproductive toxicity identified for DUP was 1145 mg/kg bw/day based on an increase in relative testes weight observed in males treated with doses of 1145 (8%) and 2305 (15%) mg/kg bw/day (NOEL of 282 mg/kg bw/day; Barber et al. 1987). The increase in relative testis weight likely reflects the decrease in body weight observed in these animals rather than a specific effect on the testis. The lowest LOAEL for systemic toxicity for these studies was 1145 mg/kg bw/day in male based on decrease in body weight gain, increase in relative liver and kidney weights, and liver lesions (NOAEL of 282 mg/kg bw/day; BIBRA 1985; Barber et al. 1987).
9.2.3.2 Oral exposure in females
Two studies related to the reproductive and developmental effects of DUP in females have been published. Data indicate no adverse effects on reproductive parameters of females and mild teratogenicity (variations) after exposure to DUP at high doses (NOAEL of 500 mg/kg bw/day) during gestation (Saillenfait et al. 2013).
9.2.3.3 Reproductive developmental toxicity: evidence in humans
No information is currently available on the potential reproductive-developmental effects of DUP in humans.
9.2.3.4 Other systemic effectsFootnote[7]
9.2.3.4.1 Considerations
It is well documented that phthalates can induce peroxisome proliferation in the liver as well as increase liver weight in rats and mice. In some cases, liver cancer was also observed following longer-term oral administration of high doses of phthalates. It is well established that the peroxisome proliferator-activated receptor (PPAR) α plays a role in peroxisome proliferation-induced liver effects. However, the relevance of the hepatotoxic effects of phthalates observed in rodents is difficult to establish due to the species-specific differences in the peroxisomal proliferation response (rodents being significantly more sensitive than humans to PPARα-mediated induction of peroxisome proliferation). Several recent studies have suggested that the mechanisms of liver toxicity of peroxisome proliferators have not been entirely elucidated and that multiple pathways may exist, some of which are likely PPARα-independent. Based on this, liver effects cannot be precluded as an effect potentially relevant to humans and should be included in the characterization of health effects of phthalates (See Health Canada 2015c for more detailed information on the mode of action of liver carcinogenicity in rodents with peroxisome proliferators).
9.2.3.4.2 Repeated-dose studies
The database for repeated-dose toxicity studies using DUP includes a few short-term oral rat studies identified in the public literature.
In a 21-day repeated-dose study, male and female Fischer 344 rats received DUP through the diet at approximately 0, 282, 1145, or 2305 mg/kg bw/day (BIBRA 1985; Barber et al. 1987). Statistically significant decreases in body weight gain were observed at the mid- and high doses for both males and females, with no significant changes in food intake observed in either sex. At the mid- and high doses, liver and kidney weights were also increased. The liver weight increases were found to be dose related in both sexes. In addition, an increase in liver enzymes and palmitoyl-CoA (PCoA) oxidation was seen, which is an indicator of peroxisome proliferation. The incidences of liver lesions (slight necrosis, slight/moderate vacuolation) were significantly increased in males treated with the two highest doses. A NOAEL of 282 mg/kg bw/day and a LOAEL of 1145 mg/kg bw/day was established for the study, based on decreased body weight gain and increased absolute and relative liver weights in both sexes accompanied with increased incidence of liver lesions in males.
In a more recent study, groups of male Sprague-Dawley rats were administered 0 or 500 mg DUP/kg bw/day by gavage in corn oil for 4 weeks (Kwack et al. 2009, also described in 9.2.3.1.2). There was no mortality and no clinical signs other than salivation immediately after dosing. Neither food consumption nor body weight was significantly altered by treatment with DUP. Most relative organ weights and hematological parameters were also not affected by dosing with DUP. Relative liver weight was increased 18%, but this difference was not found to be statistically significant. Serum chemistry analyses showed significant increases in total protein (9.1% over control), aspartate aminotransferase (AST; 50% over control), and alkaline phosphatise (ALP; 81% over control). Results of the urinalyses were unremarkable.
The LOAEL for short-term oral exposure was 1145 mg/kg bw/day based on decrease in body weight gain and increase in liver and kidney weights. Changes in liver enzymes and liver lesions were also observed.
No subchronic studies were identified for DUP.
For DnOP, the only subchronic study identified in which a wide range of endpoints was examined was a dietary study in rats conducted by Poon et al. (1997). Groups of 10 male and female Sprague-Dawley rats were administered 0, 5, 50, 500, 5000 ppm DnOP (est. 0, 0.4, 3.5, 37, 350 mg/kg bw/day in males; 0, 0.4, 4.1, 41, 403 mg/kg bw/day in females) for 13 weeks. In both sexes, effects were observed at the highest dose only (350 to 403 mg/kg bw/day for male and female, respectively). These included a significant increase in hepatic ethoxyresorufin-O-deethylase (EROD) activity, a biomarker of Ah receptor binding. Other effects such as mild histological changes in the thyroid consisting of reduced follicle size and colloid density, and in the liver consisting of endothelial nuclear prominence, nuclear hyperchromicity and anisokaryosis, were observed. Accentuation of zonation of the hepatic lobules and increased perivenous cytoplasmic vacuolation in DnOP-treated rats was also observed at the highest dose. In this study, a NOAEL of 37 mg/kg bw/day and a LOAEL of 350 to 403 mg/kg bw/day were identified based on histologic effects in the liver and the thyroid at the highest dose tested.
9.2.3.4.3 Carcinogenicity
DUP has not been classified for its potential carcinogenicity by other international agencies. No chronic toxicity/carcinogenicity studies were available for this phthalate or its analogue, DnOP.
DnOP has been reported to induce small increases in hepatic peroxisomes in some studies (Mann et al. 1985; Hinton et al. 1986) but not in others (Lake et al. 1984; Poon et al. 1997). Increases in enzymatic markers associated with peroxisome proliferation (i.e., carnitine acetyltransferase, cyanide-insensitive palmitoyl-CoA oxidase, and enoyl-CoA hydratase activity), have also been described in vivo in rats (Lake et al. 1984) and in vitro with MnOP (Lake et al. 1986).
Partially hepatectomized male Sprague-Dawley rats initiated with a single subcarcinogenic intraperitoneal dose of diethylnitrosamine (DENA) and then subsequently promoted with 500 mg/kg-bw DnOP mixed in feed for 10 weeks showed a significant increase in their number of gamma-glutamyltranspeptidase (GGT) positive liver foci when compared with controls (DeAngelo et al. 1986). A similar change in the GGT liver enzyme activity was also observed in those rats. No evidence of liver enlargement was noted and the average body weight did not differ between groups of rats. Similar results were reported by Carter et al. (1992) when using partially hepatectomized and DENA initiated male F344 rats. In this case, 26 weeks of 250 and 500 mg/kg-bw DnOP treatment significantly increased the expression of liver enzyme (GGT and gluthatione-S-transferase (GST-P)). The number of GST-P+ nodules, but not GST+altered foci, was also higher in 500 mg/kg-bw DnOP treatments. Results from these studies suggest that DnOP may act as a promoter of precancer hepatic lesions in rats.
Overall, only a limited number of studies (of short-term and subchronic duration) have evaluated the potential of DnOP to induce carcinogenicity in animals. Even though there are some data suggesting that DnOP may promote prencancer lesions in the rat liver, there are no data showing that DnOP may cause cancer in animals or humans.
9.2.3.4.4 Genotoxicity
In in vitro assays, DUP was not mutagenic in the Ames test using Salmonella typhimurium strains TA98, TA100, TA1535 and TA1537, in the presence and absence of S-9 metabolic activation (Zeiger et al. 1985). DUP has also shown no mutagenic activity in an in vitro mouse lymphoma assay with or without metabolic activation (Barber et al. 2000). In the same study, DUP did not induce transformation of BALB/3T3 cells; no metabolic activation was used in this assay. No in vivostudies have been identified in the literature.
9.2.3.4.5 Evidence of systemic toxicity in humans
No information is currently available on the potential systemic effects of DUP in humans.
9.3 Characterization of Risk to Human Health
The health effects data for long-chain phthalates shows that the critical effect for risk characterization is effects on the liver. Systemic toxicity has been associated with the lowest levels of exposure to this subcategory of phthalates examined to date in animal experiments. An examination of the potential developmental and reproductive toxicity of LCP revealed that this group of phthalates has limited effects on the developing male. Below are different aspects taken into consideration for the characterization of risk to human health.
9.3.1 Risk Characterization of DIDP
Based principally on the weight of evidence from the available information, critical effects associated with oral exposure to DIDP are carcinogenicity and liver effects.
DIDP has not been classified for its carcinogenicity by other international agencies. In a chronic study, an increased incidence of mononuclear cell leukemia (MNCL) was observed in F344 rats of both sexes at the highest dose tested (479-620 mg/kg bw/day, in males and females, respectively). The incidences in the different dose groups were not dose related. While the tumour incidences in the different dose groups were within NTP historical ranges in control F344 rats, it could be argued that historical control data coming from a different testing laboratory has limited relevance, and incidences of tumours could be potentially treatment-related. The historical data from the breeder used by Cho et al. (2008) would have been more appropriate for comparison but this data has not been found in the literature. However, increased incidence of MNCL is not likely relevant to humans since this lesion is a common neoplasm in aging F344 rats, and increased incidence of MNCL in F344 rats exposed to phthalates is likely to be a species- and strain-specific effect (see Health Canada 2015c for additional information on the MOA).
No other treatment-related tumours were observed in the above study, including the liver. However, DIDP has been shown to induce liver adenomas in a 26-week study in transgenic male mice exposed to 1300 mg/kg bw/day. The author has suggested that the increased incidence of liver neoplasms in mice is related to peroxisome proliferation. The mode of action of liver carcinogenicity in rodents with peroxisome proliferators has not been fully elucidated and the carcinogenic potential of DIDP in humans remains unclear (Health Canada 2015c).
Consideration of the available information on genotoxicity indicates that DIDP is not likely to be genotoxic.
An examination of the potential systemic toxicity of DIDP revealed that this phthalate induces effects mainly in the liver. No systemic effect was reported in rats following short-term exposure to DIDP through inhalation (only one study available). However, several repeated-dose studies have shown that the liver is the primary target organ in rats and dogs following repeated exposure to this phthalate through the oral route (see Table 9-22). The main effects observed include increase in liver weight, increase in peroxisomal enzyme levels and histological changes. Increases in kidney weight were also observed in a few studies. Histopathological changes were noted in this organ in male rats and mice in two carcinogenicity studies, at higher doses than the one at which histopathological changes in the liver were observed. These effects could be related to alpha 2 u-globulin nephropathy (rat specific effect in male) and be of limited relevance to human health risk assessment.
| Endpoint | Species | Effect | LOAEL (mg/kg bw/day) |
NOAEL (mg/kg bw/day) |
Reference |
|---|---|---|---|---|---|
| Short term | Rat | Increase in liver weight in males accompanied with histological changes at the highest dose | 300 | 300 (females) |
BIBRA 1986 |
| Subchronic | Dog | Increase in liver weight accompanied with histological changes. | 75 | 15 | Hazleton Laboratories 1968b |
| Chronic | Rat | Histopathological changes in the liver in males. | 22 | - | Cho et al. 2008 |
An examination of the potential developmental and reproductive toxicity of DIDP showed that this phthalate reduced pup survival in multiple generations at doses higher than those at which liver effects are observed in subchronic and chronic studies (LOAELs of 134 to 150 mg/kg bw/day; Hushka et al. 2001). Adverse effects in developing males were limited to changes in organ weights.
The principal source of exposure to DIDP for the general population is expected to be house dust (oral ingestion) for infants and children aged 0 to 11 years of age and food and beverages (oral ingestion) for individuals aged 12 years and above. Dermal contact with plastic articles (furniture, gloves, etc.) was also evaluated for infants (0-18 months) and adults. Finally, concentrations of DIDP metabolites (MOiDP, MHiDP, and MCiNP) in urine samples were converted to daily intake estimates of DIDP for the Canadian general population using reverse dosimetry; these estimates represent an internal dose from all sources.
Comparisons of estimates for exposure to DIDP from various sources with the LOAEL of 22 mg/kg bw/day of DIDP based on histopathological effects in the liver of male rats after chronic oral exposure (Cho et al. 2008) are presented in Table 9-23.
| Age Group and Exposure Scenario | Central tendency (Upper bounding) Estimate of exposure (µg/kg per day) | Margin of Exposure (MOE) Footnote Table 9-23[c] based on a oral LOAEL of 22 mg/kg bw/day from Cho et al. 2008 |
|---|---|---|
| Children (males) 6 - 11 years: Biomonitoring, mean (95th percentile), NHANES | 1.4 (4.4) | 15 714 (5000) |
| Infants (0 - 18 months)Footnote Table 9-23[a]: Exposure to plastic articles, dermal |
0.27Footnote Table 9-23[b] (2.16) | 81 481 (10 185) |
| Children 0.5 - 4 years: food and dust, oral | 0.514 (2.87) | 42 802 (7666) |
| Adolescents 12 - 19 years: food and dust, oral | 0.075 (0.726) | 293 333 (30 303) |
| Adults (males) 20+ years: Biomonitoring, mean (95thpercentile), NHANES | 0.76 (4.4) | 28 947 (5000) |
| Adults (females) 20+ years: Biomonitoring, mean (95th percentile), NHANES | 0.65 (4.9) | 33 846 (4490) |
| Adults 20 - 59 years of age: food and dust, oral | 0.068 (0.715) | 323 529 (30769) |
| Adults 20+a years: Exposure to plastic articles, dermal | 0.27b (0.85) | 81 481 (25 581) |
The above MOEs are considered adequate to account for uncertainties in the exposure and health effects databases and further, protective of potential developmental and reproductive effects of DIDP toxicity not only in males, but also in females as well as effects in other organ systems. These MOEs are also considered adequate as they address potential carcinogenicity of DIDP that could occur at higher doses.
9.3.2 Risk Characterization of DUP
DUP has not been classified for its potential carcinogenicity by other international agencies and no chronic toxicity/carcinogenicity studies were available for this phthalate. Even though there are some data from short-term and suchronic studies suggesting that its analogue DnOP may promote precancer lesions in the rat liver at high doses (500 mg/kg bw/day), there are no data showing that DnOP may cause cancer in animals and humans. Consideration of the available information on genotoxicity indicates that DUP is not likely to be genotoxic.
An examination of the potential systemic toxicity of DUP revealed that this phthalate induces effects mainly in the liver reported as liver weight changes accompanied by histopathological effects (Table 9-24). The lowest LOAEL for short-term exposure was 1145 mg/kg bw/day based on decreased body weight gain and increased absolute and relative liver weights in both sexes accompanied with increased incidence of liver lesions in males (BIBRA 1985; Barber et al. 1987). The lowest LOAEL for subchronic oral exposure was 350-403 mg/kg bw/day based on increased in liver enzyme activities and histological effects in the liver and thyroid in DnOP-treated rats (Poon et al. 1997).
| Endpoint | Species | Effect | LOAEL (mg/kg bw/day) | NOAEL (mg/kg bw/day) | Reference |
|---|---|---|---|---|---|
| Short term | Rat | Decreased body weight gain and increased liver and kidney weights accompanied with liver lesions | 1145 | 282 | Barber et al. 1987 |
| Subchronic | Rat (DnOP) | Increases in liver enzyme activities and histological effects in the liver and thyroid | less than or equal to 350-403 | 37 | Poon et al. 1997 |
An examination of the potential developmental and reproductive effects of DUP showed that this phthalate had a limited effect on the reproductive system of males. Male AGD was slightly reduced in foetuses of dams exposed to DUP during gestation (GD6-20), and a statistically significant difference was only noted at the mid-dose. An increase in occurrence of supernumerary lumbar ribs was observed in foetuses from the 500 and 1000 mg/kg bw/day dose groups, compared to controls (NOAEL of 250 mg/kg bw/day; Saillenfait et al. 2013). Decreases in sperm count and motility were observed in pubertal rats, but no other adverse effects in the testes were found at relatively high doses (2200 mg/kg bw/day; Kwack et al. 2009). There were also no severe reproductive effects in adult males following exposure to D911P or DnOP, two substances considered analogous to DUP. Based on these results, the potential of DUP to induce effects related to RPS in animals is unlikely.
The principal source of exposure to DUP for the general population is expected to be house dust (oral ingestion) for children and adults aged 0 to 19 years of age. Dermal contact with plastic articles (furniture, gloves, etc.) was also evaluated for infants (0 to 18 months) and adults.
Comparisons of estimates for exposure to DUP through oral ingestion of house dust and through dermal exposure from plastic articles with the NOAEL of 37 mg/kg bw/day of DnOP based on increases in liver enzyme activities and histological effects in the liver and thyroid in rats after subchronic oral exposure (Poon et al. 1997) are presented in Table 9-25.
| Age Group and Exposure Scenario | Central tendency (Upper bounding) Estimate of exposure (µg/kg per day) | Margin of Exposure (MOE) Footnote Table 9-25[c] based on a oral LOAEL of 22 mg/kg bw/day from Cho et al. 2008 |
|---|---|---|
| Infants 0 - 0.5 years: dust, oral | 0.0198 (0.349) | Over 1 million (106 000) |
| Infants (0 - 18 months)Footnote Table 9-25[a]: Exposure to plastic articles, dermal | 2.7Footnote Table 9-25[b] (21.6) | 13 704 (1712) |
| Adolescents/Adults 12 - 19 years: dust, oral | 0.000234 (0.004) | Over 1 million |
| Adults (20+)a: Exposure to plastic articles, dermal | 2.7b (8.5) | 13 704 (4353) |
The above MOEs are considered adequate to account for uncertainties in the exposure and health effects databases and further, protective of potential developmental and reproductive effects of DUP toxicity not only in males, but also in females as well as effects in other organ systems.
9.3.3 Considerations
The most significant source of phthalate exposure to the general population of Canada is from environmental media, dust and food/food packaging. Other sources include, but are not limited to, wearing textiles containing phthalates and use of other products used by consumers (do-it-yourself products, personal care products, etc.).
With respect to the use of adhesive, sealants, and coatings which contain long-chain phthalates, exposure would not be considered to be of concern for human health based on the following:
Dermal absorption of long-chain phthalates is rats is low (1-4%), and evidence shows that human skin is less permeable than rat skin to phthalate diesters. Also, retention in skin is 3 to 6 fold higher in rat compared to human (Mint and Hotchkiss 1993; Mint et al. 1994). Distribution in tissues of rats is generally low showing no accumulation, and excretion is rapid, within hours to days.
Exposure from use of these products would be of very short duration (acute) via the dermal route.
Phthalates in general are not considered acute toxicants, with LD50 levels from dermal exposure being at minimum 2 to 5 fold higher than oral values (Draize et al. 1948; Eastman Kodak 1978; David et al. 2001; Monsanto Company 1970 cited in US EPA 2006, 2010).
Acute dermal toxicokinetic information indicates that reproductive organs are not a target organ, and that presence and residence time in other tissues (adipose and muscle) is extremely low after 7 days (0.14 to 0.33% of applied dose; Elsisi et al. 1989).
This is consistent with the assessments of other jurisdictions who have focussed their assessment on repeated exposures (ECHA 2013a; US CPSC CHAP 2014).
9.4 Uncertainties in Evaluation of Risk to Human Health
There is some uncertainty associated with the use of analogues such as DnOP and D911P to characterize human health effects of DUP.
There are limited to no studies by any route of administration on neurodevelopmental toxicity of DIDP or DUP. Further, there is no 2-generation study available for DUP.
The majority of the studies in the health effects database for DIDP and DUP related to reproductive-developmental toxicity are limited to one species (rat) and mostly in males. There is some uncertainty associated not only with the potential biological significance of effects, but also in sensitivity of effects after exposure to this substance group in both female and male humans, but current information does not allow for conclusions to state otherwise.
There is a lack of repeated-dose studies of short to long-term duration for DUP and no carcinogenicity studies were available for this phthalate. There is uncertainty associated with the mode of induction of tumours for DIDP. Postulated modes of action have been identified but have not been fully elucidated. Thus, the carcinogenic potential of DIDP in humans remains unclear and is an uncertainty.
Inhalation studies are limited to acute studies for both DIDP and DUP. No dermal studies were identified for either substance.
Although a rigorous evaluation approach was conducted with the available human epidemiological data, uncertainty still exists in the relevance of these studies implicating the potential hazard of certain phthalates to humans. Thoroughly conducted epidemiologic studies showing robust and consistent associations between an exposure factor and an outcome may provide strong implication for causal inference. However, observational studies in diverse populations pose challenges in both the measure of exposure and the measure of the outcome, and inherently have biases and confounding factors (Lucas and McMichael 2005). The majority of epidemiological studies examined were cross-sectional in which a temporal sequence whereby exposure precedes the outcome cannot be established. In addition, several outcomes associated with phthalate exposure in human epidemiological studies have long latencies (such as cancer, diabetes, obesity, cardiovascular disease) and multifactorial etiologies (geographical location, socioeconomic status, diet, lifestyle factors, genetic propensity, nonchemical stressors) and are chronic in nature, whereas phthalates have short biological half-lives and their measurement therefore reflects a snapshot of recent exposure. Moreover, biomonitoring data showed that exposure to certain phthalates is ubiquitous and therefore cannot be dichotomized as present or absent but is instead a continuous variable, often with a limited range.
While it has been argued that even in the absence of consistent methods, a robust association should yield consistent findings (La Kind et al., 2012), poor reproducibility continues to feature prominently in epidemiological studies involving phthalates. Adding to the lack of clarity is the fact that humans are simultaneously exposed to multiple phthalates from multiple sources via multiple routes, as well as other environmental agents that may share coinciding effect domains, including bisphenol A, certain metals and organochlorine compounds, such as PCBs, dioxins and various persistent organic pesticides. In its final report in 2014, the US Chronic Hazard Advisory Panel (CHAP) on Phthalates concluded that although there is a growing body of studies reporting associations between phthalate exposure and human health, and many of the reported health effects are consistent with testicular dysgenesis syndrome in humans, there are acknowledged limitations of these studies similar to those described above. These were therefore not used in risk characterization (US CPSC CHAP 2014). Another recent review also found that epidemiological evidence for associations with reproductive and developmental effects from phthalates is minimal to weak in most cases (Kay et al. 2014).
There are uncertainties associated with estimating intakes of DIDP and DUP from environmental media due to very limited monitoring data available for these phthalates in air, drinking water and soil. Confidence is moderate to high that derived intake estimates from household dust are representative of the potential exposure of the general Canadian population, since the data are based on the results from a Canadian house dust monitoring study.
Confidence is moderate to high that the derived dietary exposure estimate for DIDP is representative of the general population of Canada, as recent Canadian monitoring data were available. However, uncertainty exists regarding methodology issues of measuring mixtures in various food matrices. For phthalate presence in food, there is uncertainty in the literature regarding presentation of LODs and LOQs as some publications present the LOD of the instrument rather than incorporating the background level of phthalate contamination. There is also uncertainty associated with potential intake from food of DUP since this substance is present on international databases indicating potential food contact exposure; however, no monitoring data as to its presence in food were identified. There is also uncertainty associated with intake from breast milk for DIDP and DUP.
Generally, for both phthalates assessed in this document, there is uncertainty in estimating dermal exposure from contact with products used by consumers, based on limited substance specific information with regards to the presence and migration over time of phthalates from these products. Therefore, it is unclear how much phthalate is available for transfer to skin from contact. Additional uncertainty is associated with parameters used (e.g., surface area, exposure duration, and migration from plastic articles onto skin), leading to increased variability and uncertainty in deriving dermal exposure estimates. Additionally, the methods used to derive scenarios and migration rates (especially relevant for these substances since migration rates for DEHP into sweat were used) use professional judgement, thus further reinforcing the uncertainty in derived exposure estimates; however, there is confidence that the assumptions used were conservative.
A number of assumptions are made to derive intake estimates from biomonitoring data which represent a source of uncertainty; i.e., assumptions that spot urine samples are representative of steady-state concentrations; assumptions around creatinine correction; use of read across of fractional urinary excretion factors based on structural similarities for DIDP. However, there is confidence that the assumptions used in deriving estimates of intakes are appropriate and conservative.
Additionally, there is uncertainty related to the use of NHANES derived DIDP intakes as a surrogate for the Canadian population and around the quantification of mixtures of metabolites in urine samples (observed multiple peaks). However, confidence in the biomonitoring database for DIDP is high, as it represents a substantially large number of data points collected recently in North American individuals spanning a wide age spectrum, and including Canadian subpopulations such as pregnant woman.
Due to the lack of or limited health effects data for all relevant routes and durations of exposure, route-to-route extrapolation was required and/or use of effect levels from studies with a longer or shorter duration of exposure than the exposure scenarios was applied. In the case of inconsistencies in duration scenarios, provided that the daily exposure is being compared with health effect levels from animal studies of longer duration, confidence is high that the derived MOEs are conservative.
Uncertainty is recognized in the potential oral bioavailability of long-chain phthalates, in particular the estimated systemic exposure at which effects were observed in animal studies after administration. Information exists that absorption of these phthalates could be, based on their size, lower than 100% (31 to 56%; see section 9.2.1), although studies are limited for this subgrouping. Absorption of a substance is influenced by rates of metabolism and excretion of an organism and by different routes at any given time of measurement. These limitations do not allow for accurate adjustments in characterization of risk for each phthalate; however, estimated MOEs are considered adequate to account for this uncertainty.
References
Abb M, Heinrich T, Sorkau E, Lorenz W. 2009. Phthalates in house dust. Environ Int 35:965-970.
[ACC] American Chemistry Council. 2001. High production volume (HPV) chemical challenge program test plan for the phthalate esters category. December 10, 2001. Prepared by ExxonMobil Biomedical Sciences, Inc. for the Phthalate Esters Panel HPV Testing Group of the American Chemistry Council. Washington (DC): American Chemistry Council.
ACD/Percepta [Prediction Module]. c1997-2012. Toronto (ON): Advanced Chemistry Development [cited 2014 October 23]. Available from: www.acdlabs.com/products/percepta/
Adams WJ, Biddinger GR, Robillard KA, Gorsuch JW. 1995. A summary of the acute toxicity of 14 phthalate esters to representative aquatic organisms. Environ Toxicol Chem 14(9):1569-1574.
Akahori Y, Nakai M, Yamasaki K, Takatsuki M, Shimohigashi Y, Ohtaki M. 2008. Relationship between the results of in vitro receptor binding assay to human estrogen receptor and in vivo uterotrophic assay: comparative study with 65 selected chemicals. Toxicol in Vitro 22:225-231.
Albro PW, Moore B. 1974. Identification of the metabolites of simple phthalate diesters in rat urine. J Chromatogr 94(0):209-18.
Albro PW, Lavenhar SR. 1989. Metabolism of di(2-ethylhexyl)phthalate. Drug Metab Rev 21(1):13-34.
Alin J, Hakkarainen M. 2011. Microwave heating causes rapid degradation of antioxidants in polypropylene packaging, leading to greatly increased specific migration to food simulants as shown by ESI-MS and GC-MS. J Agr Food Chem 59:5418-5427.
Anderson WA, Castle L, Hird S, Jeffery J, Scotter MJ. 2011. A twenty-volunteer study using deuterium labelling to determine the kinetics and fractional excretion of primary and secondary urinary metabolites of di-2-ethylhexylphthalate and di-iso-nonylphthalate. Food Chem Toxicol. 49:2022-2029. doi: 10.1016/j.fct.2011.05.013.
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2010. Version 1.92a. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Ash M, Ash I. 2003. Handbook of paint and coating raw materials. Vol. 2, 2nd ed. Endicott (NY): Synapse Information Resources Inc.
Babich MA, Chen SB, Greene MA, Kiss CT, Porter WK, Smith TP, Wind ML, Zamula WW. 2004. Risk assessment of oral exposure to diisononyl phthalate from children's products. Regul Toxicol Pharmacol 40:151-167.
Barber ED, Astill BD, Moran EJ, Schneider BF, Gray TJ, Lake BG, Evans JG. 1987. Peroxisome induction studies on seven phthalate esters. Toxicol and Indust Health 3(2):7-24.
Barber ED, Teetsel NM, Kolberg KF, Guest D. 1992. A comparative study of the rates of in vitro percutaneous absorption of eight chemicals using rat and human skin. Fundam Appl Toxicol 19(4):493-7.
Barber ED, Cifone M, Rundell J, Przygoda R, Astill BD, Moran E, Mulholland A, Robinson E, Schneider B. 2000. Results of the L5178Y mouse lymphoma assay and the Balb/3T3 cell in vitro transformation assay for eight phthalate esters. J Appl Toxicol 20:69-80 [cited in US CPSC 2010a].
Barron MG, Albro PW, Hayton WL. 1995. Biotransformation of di(2-ethylhexyl)phthalate by rainbow trout. Environ Toxicol Chem 14(5):873-876.
Barros HD, Pereira da Silva Zamith H, Bazilio FS, Jaeger de Carvalho L, de Mello Pereira Abrantes S. 2011. Identification of fatty foods with contamination possibilities by plasticizers when stored in PVC film packaging. Ciênc Technol Aliment Campinas 31:547-552.
BASF AG. 1969a. Bericht über den 28-Tage-Ratten-Fütterungsversuch mit PALATINOL Z [cited in ECJRC 2003].
BASF AG. 1969b. Bericht über den 90-Tage-Ratten-Fütterungsversuch mit PALATINOL Z [cited in ECJRC 2003, NICNAS 2008a, US CPSC 2010a].
BASF. 2009. Palatinol® 111P-I Di-Undecyl Phthalate (stabilized with 0.1% Topanol CA). Available from: http://www2.basf.us/plasticizers/pdfs/Palatinol111P-I(01TCA)TDSFebruary2009.pdf [cited 2014 June 23].
[BBM with Mitigating Factors] Baseline Bioaccumulation Model with Mitigating Factors. 2008. Gatineau (QC): Environment Canada, Existing Substances Division [Model based on Dimitrov et al. 2005].
[BCFBAF] Bioaccumulation Program for Windows [Estimation Model]. 2010. Version 3.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2014 Jun 4]. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Bergh C, Torgrip R, Emenius G, Ostman C. 2011a. Organophosphate and phthalate esters in air and settled dust - a multi-location indoor study. Indoor Air 21:67-76.
Bergh C, Aberg K, Svartengren M, Emenius G, Ostman C. 2011b. Organophosphate and phthalate esters in indoor air: a comparison between multi-storey buildings with high and low prevalence of sick building symptoms. J Environ Monit 13:2001-2009.
Bertelsen, RJ, Carlsen KC, Calafat AM, Hoppin JA, Haland G, Mowinckel P, Carlsen KH, Løvik M. 2013. Urinary biomarkers for phthalates associated with asthma in Norwegian children. Environ Health Perspect 121(2): 251-256.
Bi X, Pan X, Yuan S, Wang Q. 2013. Plasticizer contamination in edible vegetable oil in a U.S. retail market. J Agr Food Chem 61:9502-9509.
[BIBRA] British Industrial Biological Research Association. 1985. A 21-day feeding study of diundecyl phthalate to rats: effects on the liver and liver lipids. Report no 0495/4/84 [cited in HPVIS 2002].
[BIBRA] British Industrial Biological Research Association. 1986. A 21-day feeding study of di-isodecyl phthalate to rats: effects on the liver and liver lipids. Report no 0495/5/85 [cited in ECJRC 2003].
Biedermann-Brem S, Biedermann M, Pfenninger S, Bauer M, Altkofer W, Rieger K, Hauri U, Droz C, Grob K. 2008. Plasticizers in PVC toys and childcare products: what succeeds the phthalates? Market survey 2007. Chromatographia 68:227-234.
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2010. Version 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [cited 2014-05-30]. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Björklund K, Cousins AP, Strömvall A-M, Malmqvist P-A. 2009. Phthalates and nonylphenols in urban runoff: Occurrence, distribution and area emission factors. Sci Total Environ 407:4665-4672.
Blair JD, Ikonomou MG, Kelly BC, Surridge B, Gobas FAPC. 2009. Ultra-trace determination of phthalate ester metabolites in seawater, sediments, and biota from an urbanized marine inlet by LC/ESI-MS/MS. Environ Sci Technol 43(16):6262-6268.
Boethling RS, Alexander M. 1979. Effect of concentration of organic chemicals on their biodegradation by natural microbial communities. Appl Environ Microbiol 37(6):1211-1216.
Bornehag C, Lundgren B, Weschler C J, Sigsgaard T, Hagerhed-Engman L, Sundell J. 2005. Phthalates in indoor dust and their association with building characteristics. Environ Health Perspect 113(10):1399-1404.
Bradlee CA, Thomas P. 2003. Aquatic toxicity of phthalate esters. In: Hutzinger O, ed. The handbook of environmental chemistry. Volume 3. Anthropogenic compounds. Part Q. Berlin (DE): Springer-Verlag. pp. 263-298.
Bradley EL, Burden RA, Bentayeb K, Driffield M, Harmer N, Mortimer DN, Speck DR, Ticha J, Castle L. 2013. Exposure to phthalic acid, phthalate diesters and phthalate monoesters from food stuff: UK total diet study results. Food Addit Contam A 30(4):735-742.
Breous E, Wenzel A, Loos U. 2005. The promoter of the human sodium/iodide symporter responds to certain phthalate plasticisers. Mol Cell Endocrinol 244(1):75-78.
Brown D, Thompson RS. 1982a. Phthalates and the aquatic environment: Part 1. The effect of di-2-ethylhexyl phthalate (DEHP) and di-isodecyl phthalate (DIDP) on the reproduction of Daphnia magna and observations on their bioconcentration. Chemosphere 11(4):417-426.
Brown D, Thompson RS. 1982b. Phthalates and the aquatic environment: Part II. The bioconcentration and depuration of di-2-ethylhexyl phthalate (DEHP) and di-isodecyl phthalate (DIDP) in mussels (Mytilus edulis). Chemosphere 11(4):427-435.
Brown D, Thompson RS, Stewart KM, Croudace CP, Gillings E. 1996. The effect of phthalate ester plasticisers on the emergence of the midge (Chironomus riparius) from treated sediments. Chemosphere 32(11):2177-2187.
Brown D, Croudace CP, Williams NJ, Shearing JM, Johnson PA. 1998. The effect of phthalate ester plasticisers tested as surfactant stabilised dispersions on the reproduction of the Daphnia magna. Chemosphere 36(6):1367-1379.
[BSMEPH] Bavarian State Ministry of the Environment and Public Health (2012). Submission of information via the public consultation on ECHA's draft review report by the Bavarian State Ministry of the Environment and Public Health (Bayerisches Staatsministerium für Umwelt und Gesundheit). Comment reference number 19. Public comments are available on http://echa.europa.eu/web/guest/addressing-chemicals-ofconcern/restriction/consultations-draft-review-report [cited in ECHA 2013].
Buser MC, Murray HE, Scinicariello F. 2014. Age and sex differences in childhood and adulthood obesity association with phthalates: Analyses of NHANES 2007-2010. Int J Hygiene and Env Health 217:687-694.
Calafat AM, Silva MJ, Reidy JA, Earl Gray L, Samandar E, Preau JL, Herbert AR, Needham LL. 2006. Mono-(3-carboxypropyl) phthalate, a metabolite of di-n-octyl phthalate. J Toxicol Environ Health 69(3-4):215-27.
Call DJ, Cox DA, Geiger DL, Genisot KI, Markee TP, Brooke LT, Polkinghorne CN, VandeVenter FA, Gorsuch JW, Robillard KA, Parkerton TF, Reiley MC, Ankley GT, Mount DR. 2001. An assessment of the toxicity of phthalate esters to freshwater benthos. 2. Sediment exposures. Environ Toxicol Chem 20(8):1805-1815.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33, Canada Gazette. Part III, vol. 22, no. 3. Available from: http://www.gazette.gc.ca/archives/p3/1999/g3-02203.pdf
Canada. 2013. Canadian Environmental Protection Act, 1999: Notice with respect to certain phthalate substances. Canada Gazette Part I, vol. 147, no. 28, p. 1801-1823. Available from: http://gazette.gc.ca/rp-pr/p1/2013/2013-07-13/html/notice-avis-eng.html#phthalates
Carter JH, Richmond RE, Carter HW, Potter CL, Daniel FB, DeAngelo AB. 1992. Quantitative image cytometry of hepatocytes expressing gamma-glutamyl transpeptidase and glutathione S-transferase in diethylnitrosamine-initiated rats treated with phenobarbital and/or phthalate esters. J Histochem Cytochem 40(8):1105-1115.
Casas L, Fernandez MF, Llop S, Guzens M, Ballester F, Olea N, Irurzun MB, Rodriguez LSM, Riano I, Tardon A, Vrijheid M, Calafat AM, Sunyer J. 2011. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ Int 37:858-866.
CATALOGIC [Computer Model]. 2012. Version 5.11.6. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. Available from: www.oasis-lmc.org/?section=software&swid=1
[CDC] Centres for Disease Control and Prevention. 2013. Fourth National Report on Human Exposure to Environmental Chemicals. Updated Tables, March, 2013 www.cdc.gov/exposeuereport/pdf/FourthReport_UpdatedTables_Mar2013.pdf
Cheminfo Services Inc. 2013a. Chemical Management Plan 2 (CMP2) Scoping Project for Substance Information. Draft Final Report on Phthalates. Markham (ON): Cheminfo Services Inc. Submitted to Environment Canada, Gatineau QC.
Cheminfo Services Inc. 2013b. Plastic product study (Review of the potential for releases of CMP II substances and organotins from plastic products). Final report. March 28, 2013. Submitted to Environment Canada, Gatineau QC.
Chen X, Xu S, Tan T, Lee ST, Cheng SH, Lee FWF, Xu SJL, Ho KC. 2014. Toxicity and estrogenic endocrine disrupting activity of phthalates and their mixtures. Int J Environ Res Public Health 11:3156-3168.
Cho W-S, Han BS, Ahn B, Nam KT, Choi M, Oh SY, Kim SH, Jeong J, Jang DD. 2008. Peroxisome proliferator di-isodecyl phthalate has no carcinogenic potential in Fischer 344 rats. Toxicol Letters 178:110-116.
Cho WS, Jeong J, Choi M, Park SN, Han BS, Son WC. 2011. 26-Week carcinogenicity study of di-isodecyl phthalate by dietary administration to CB6F1-rasH2 transgenic mice. Arch Toxicol 85:59-66.
[CHRIP] Chemical Risk Information Platform [database on the Internet]. 2014. Tokyo (JP): National Institute of Technology and Evaluation, Chemical Management Centre (CMC) [cited 2014 May 30]. Available from: www.safe.nite.go.jp/english/db.html
Christensen KLY, Makris SL, Lorber M. 2014. Generation of hazard indices for cumulative exposure to phthalates for use in cumulative risk assessment. Regul Toxicol Pharm 69(3):380-389.
Clara M, Windhofer G, Hartl W, Braun K, Simon M, Gans O, Scheffknecht C, Chovanec A. 2010. Occurrence of phthalates in surface runoff, untreated and treated wastewater and fate during wastewater treatment. Chemosphere 78:1078-1084.
Colacino JA, Soliman AS, Calafat AM, Nahar MS, Van Zomeren-Dohm A, Hablas A, Seifeldin IA, Rozek LS, Dolinoy DC. 2011. Exposure to phthalates among premenstrual girls from rural and urban Gharbiah, Egypt: A pilot exposure assessment study. Environmental Health 10:40.
Conestoga-Rovers and Associates. 2009. Baseline information on major municipal solid waste landfills in Canada. Unpublished report prepared for Environment Canada. June 2009. Gatineau (QC): Environment Canada.
Corton JC and Lapinskas PJ. 2005. Peroxisome proliferator-activated receptors: mediators of phthalate ester-induced effects in the male reproductive tract? Toxicol Sci 83(1):4-17.
Cousins AP, Remberger M, Kaj L, Ekheden Y, Dusan B, Brorström-Lundén E. 2007. Results from the Swedish National Screening Programme 2006. Subreport 1: Phthalates. IVL Report B1750. June 2007. Stockholm (SE): Swedish Environmental Research Institute.
Cousins I, Mackay D. 2000. Correlating the physical-chemical properties of phthalate esters using the 'three solubility' approach. Chemosphere 41:1389-1399.
Cousins IT, Mackay D, Parkerton TF. 2003. Physical-chemical properties and evaluative fate modelling of phthalate esters. In: Hutzinger O, ed. The handbook of environmental chemistry. Volume 3. Anthropogenic compounds. Part Q. Berlin (DE): Springer-Verlag. pp. 263-298.
[COWI, IOM and AMEC] COWI, IOM Consulting and AMEC. 2012. Evaluation of new scientific evidence concerning the restrictions on DINP and DIDP contained in entry 52 of Annex xvii to Regulation (EC) n° 1907/2006 (REACH). Final report, volumes of DINP and DIDP. Available from: http://echa.europa.eu/documents/10162/a35fa99b-ed8f-4451-a4d5-f012e9ba69c7
[CPOPs] Canadian Persistent Organic Pollutants Profiler Model. 2012. Version 1.1.18. Gatineau (QC): Environment Canada, Existing Substances Division; Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. [Model developed based on Mekenyan et al. 2005].
CSPA Reports. 2014. Children's Safe Product Actreported data. Lacey (WA): Washington State Department of Ecology. Available from: https://fortress.wa.gov/ecy/cspareporting/ [cited 2014 July 3].
[Danish EPA] Danish Environmental Protection Agency. 2006. Survey and Health Assessment of Chemicals substances in sex toys. Survey of chemical substances in consumer products. No 77. Available from: http://mst.dk/service/publikationer/publikationsarkiv/2006/sep/survey-and-health-assessment-of-chemicals-substances-in-sex-toys/
[Danish EPA] Danish Ministry of the Environment. Environmental Protection Agency. 2007. Survey as well as health assessment of chemical substances in school bags, toy bags, pencil cases and erasers. Survey of Chemical Substances in Consumer Products, No. 84. Available from: http://mst.dk/service/publikationer/publikationsarkiv/2007/aug/survey-as-well-as-health-assessment-of-chemical-substances-in-school-bags,-toy-bags,-pencil-cases-and-erasers/
[Danish EPA] Danish Ministry of the Environment. Environmental Protection Agency. 2010a. Phthalates in products that children are in direct contact with. Survey of Chemical Substances in Consumer Products, No. 109. Available from: http://mst.dk/service/publikationer/publikationsarkiv/2010/dec/phthalates-in-products-that-children-are-in-direct-contact-with/
[Danish EPA] Danish Ministry of the Environment. Environmental Protection Agency. 2010b. Phthalates in plastic sandals. Survey of Chemical Substances in Consumer Products, No. 107. Available from: http://mst.dk/service/publikationer/publikationsarkiv/2010/dec/phthalates-in-plastic-sandals/
David RM. 2000. Exposure to phthalate esters. Environ Health Perspect 108:A440.
DeAngelo AB, Garrett CT, Manolukas LA, Yario T. 1986. Di-n-octyl phthalate (DOP), a relatively ineffective peroxisome inducing straight chain isomer of the environmental contaminant di (2-ethylhexyl) phthalate (DEHP), enhances the development of putative preneoplastic lesions in rat liver. Toxicology 41(3):279-288.
Deisinger PJ, Perry LG, Guest D. 1998. In vivo percutaneous absorption of [14C]DEHP from [14C]DEHP-plasticized polyvinyl chloride film in male Fischer 344 rats. Food Chem Toxicol 36:521-527.
Dimitrov S, Dimitrova N, Parkerton T, Comber M, Bonnell M, Mekenyan O. 2005. Base-line model for identifying the bioaccumulation potential of chemicals. SAR QSAR Environ Res 16(6):531-554.
Downs SH, Black N. 1998. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health52:377-84.
[DPD] Drug Product Database [database on the Internet]. 2014. Ottawa (ON): Health Canada. Available from: http://webprod5.hc-sc.gc.ca/dpd-bdpp/index-eng.jsp
Draize JH, Alvarez E, Whitesell MF, Woodward G, Conway Hagan E, Nelson AA. 1948. Toxicological investigations of compounds proposed for use as insect repellants; A. Local and systemic effects following topical skin application; B. Acute oral toxicity; C. Pathological examination. J Pharmacol Exp Ther 93:26-39.
Eastman Kodak. 1978. Toxicity and Health Hazard Summary. Submitted under TSCA Section 8D. Document No 878214402; OTS 0206525.
[EC] European Commission. 2004. Guidance document on dermal absorption. 19 March 2004. Brussels (BE): European Commission, Health & Consumer Protection Directorate-General. Available from: http://ec.europa.eu/food/plant/protection/evaluation/guidance/wrkdoc20_rev_en.pdf
[ECB] European Chemicals Bureau. 2008. European Union risk assessment report; CAS No: 117-81-7; EINECS No: 204-211-0; bis(2-ethylhexyl)phthalate (DEHP). 2nd Priority List, Volume 80. Institute for Health and Consumer Protection. Report no. EUR 23384 EN. pp. 588. http://publications.jrc.ec.europa.eu/repository/bitstream/111111111/5648/1/dehpreport042.pdf
[ECHA] European Chemicals Agency. 2013. Evaluation of new scientific evidence concerning DINP and DIDP. Final Review Report. Available from: http://echa.europa.eu/documents/10162/31b4067e-de40-4044-93e8-9c9ff1960715
[ECHA] European Chemicals Agency. 2014. Registered Substances database. Search results for CAS RN [3648-20-2]. Helsinki (FI): ECHA [accessed 28 February 2014]. Available from: www.echa.europa.eu/information-on-chemicals/registered-substances
[ECJRC] European Commission Joint Research Centre. 2003. European Union risk assessment report: CAS: 68515-49-1, 26761-40-0: 1,2-benzenedicarboxylic acid, di-C9-11-branched alkyl esters, C10-rich and di-"isodecyl" phthalate (DIDP) [Internet]. Report No.: EUR 20785 EN. Luxembourg: Office for Official Publications of the European Communities. Available from: http://publications.jrc.ec.europa.eu/repository/bitstream/111111111/5459/1/EUR 20785
[ECPI] European Council for Plasticisers and Intermediates. 1996. Personal communication [cited in ECJRC 2003]
[ECOSAR] Ecological Structure Activity Relationships Class Program [Estimation Model]. 2009. Version 1.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
[EFSA] European Food Safety Authority. 2005. Statement of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a request from the Commission on the possibility of allocating a group-TDI for Butylbenzylphthalate (BBP), di-Butylphthalate (DBP), Bis(2-ethylhexyl) phthalate (DEHP), di-Isononylphthalate (DINP) and di-Isodecylphthalate (DIDP). Minutes' statement expressed on 28 June 2005 at its 12th Plenary meeting, corresponding to the item 10 of the agenda. Available from: http://www.efsa.europa.eu/en/home/doc/phthalategroup_minutes_statement.pdf
Elsisi AE, Carter DE, Sipes IG. 1989. Dermal absorption of phthalate diesters in rats. Fundam Appl Toxicol 12(1):70-7.
Enke U, Schleussner E, Palmke C, Seyfarth L, Koch HM. 2013. Phthalate exposure in pregnant women and newborns - The urinary metabolite excretion pattern differs distinctly. Int J Hyg Envir Heal. DOI: http://dx.doi.org/10.1016/j.ijheh.2013.01.006
Environment Canada. 2007. Guidance for Conducting Ecological Assessments under CEPA 1999, Science Resource Technical Series, Technical Guidance Module: QSARs. Reviewed draft working document. Gatineau (QC): Environment Canada, Ecological Assessment Division.
Environment Canada. 2012. Final Results from Phase One of the Domestic Substances List Inventory Update Rapid Screening Assessment of Substances of Lower Ecological Concern - Detailed Spreadsheet. Gatineau (QC): Ecological Assessment Division, Environment Canada.
Environment Canada. 2014a. Data for phthalates collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain phthalate substances. Data prepared by: Environment Canada, Health Canada, Existing Substances Program.
Environment Canada. 2014b. National Pollutant Release Inventory. Gatineau (QC): Environment Canada. Available from: http://www.ec.gc.ca/inrp-npri/default.asp?lang=En&n=4A577BB9-1
Environment Canada. 2015. Supporting documentation: Phthalates Grouping. Information in support of the State of the Science reports for phthalates: Short-, Medium- and Long-Chain, and DINP. Gatineau (QC): Environment Canada. Available on request from: substances@ec.gc.ca
Environment Canada, Health Canada. 2007. Chemical substances: Categorization [Internet]. Ottawa (ON): Government of Canada [updated 2007 April 20; cited 2014 June 10]. Available from: http://www.chemicalsubstanceschimiques.gc.ca/approach-approche/categor-eng.php
Environment Canada and Health Canada. 2015a. Draft approach for considering cumulative risk of certain phthalates under the Chemicals Management Plan. Gatineau (QC): Environment Canada, Health Canada: Existing Substances Program. Available on request from: substances@ec.gc.ca
Environment Canada and Health Canada. 2015b. State of the Science Report: the Phthalate Substance Grouping: 1,2-Benzenedicarboxylic acid, diisononyl ester; 1,2-Benzenedicarboxylic acid, di-C8-10-branched alkyl esters, C9-rich (DINP). Chemical Abstracts Service Registry Numbers: 28553-12-0, 68515-48-0. Gatineau (QC): Environment Canada, Health Canada: Existing Substances Program. Available on request from: substances@ec.gc.ca
Environment Canada and Health Canada. 2015c. State of the Science Report: the Phthalate Substance Grouping: Short-Chain Phthalate Ester: 1,2-Benzenedicarboxylic acid, dimethyl ester (DMP). Chemical Abstracts Service Registry Number: 131-11-3. Gatineau (QC): Environment Canada, Health Canada: Existing Substances Program. Available on request from: substances@ec.gc.ca
Environment Canada and Health Canada. 2015d. State of the Science Report: the Phthalate Substance Grouping: Medium-Chain Phthalate Esters. Chemical Abstracts Service Registry Numbers: 84-61-7; 84-64-0; 84-69-5; 523-31-9; 5334-09-8;16883-83-3; 27215-22-1; 27987-25-3; 68515-40-2; 71888-89-6. Gatineau (QC): Environment Canada, Health Canada: Existing Substances Program. Available on request from: substances@ec.gc.ca
[EPI Suite] Estimation Programs Interface Suite for Microsoft Windows [Estimation Model]. 2000-2008. Version 4.1. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuitedl.htm
[EQC] Equilibrium Criterion Model. 2011. Version 2.02. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre. Available from: http://www.trentu.ca/academic/aminss/envmodel/models/EQC2.html
[ESIS] European Chemical Substances Information System [database on the Internet]. 2014. Ispra, ITA: European Chemical Substances Information System, Institute for Health and Consumer Protection, Joint Research Centre, European Commission. Available from: http://esis.jrc.ec.europa.eu/ [cited 2014 June 24].
European Commission. 2000. IUCLID Dataset. Ispra (IT): European Commission, Joint Research Centre, Institute for Health and Consumer Protection, European Chemicals Bureau. Available from: http://esis.jrc.ec.europa.eu/
Eveillard A, Mselli-Lakhal L, Mogha A, Lasserre F, Polizzi A, Pascussi JM, Guillou H, Martin PG, Pineau T. 2009. Di-(2-ethylhexyl)-phthalate (DEHP) activates the constitutive androstane receptor (CAR): a novel signalling pathway sensitive to phthalates. Biochem Pharmacol 77:1735-1746.
Exxon Biomedical Sciences. 1997. Reproduction toxicity study in rats with di-isodecyl phtalate (DIDP; MRD- 94-775). Report no 177533 [cited in ECJRC 2003].
Exxon Biomedical Sciences, Inc. 1995. Ready biodegradability manometric respirometry test. Final report 95 MRL 77 [cited in Staples et al. 1997].
Exxon Biomedical Sciences, Inc. 1996a. Earthworm limit test. Project number: 199692. July 9, 1996. Submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division.
Exxon Biomedical Sciences, Inc. 1996b. Seed germination limit test with rye grass and lettuce. Unpublished report No. 199674. Submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division.
Exxon Biomedical Sciences, Inc. 1998. Ready biodegradability: OECD 301F manometric respirometry test. Final report. Project number: 118594A(3). 27 October, 1998. Submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division.
ExxonMobil Biomedical Sciences, Inc. 2005. Fish, dietary bioaccumulation study. Final report. Study number: 176447. 10 October 2005. Submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division.
ExxonMobil Biomedical Sciences, Inc. 2010. Ready biodegradability: OECD 301F manometric respirometry tests. Study number: 0946479. 21 October 2010. Submitted to Environment Canada under the Chemicals Management Plan initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division.
ExxonMobil Chemical. 2014a. Jayflex™ DIDP plasticizers. Available from: http://www.exxonmobilchemical.com/Chem-English/brands/jayflex-didp.aspx?ln=productsservices [cited 2014 July 4].
ExxonMobil Chemical. 2014b. Jayflex™ linear plasticizers. Available from: http://www.exxonmobilchemical.com/Chem-English/brands/jayflex-linear-plasticizers.aspx?ln=productsservices [cited 2014 June 23].
Fankhauser-Noti A, Grob K. 2006. Migration of plasticizers from PVC gaskets of lids for glass jars into oily foods: amount of gasket material in food contact, proportion of plasticizer migrating into food and compliance testing by simulation. Trends Food Sci Technol 17:105-12.
Fasano E, Bono-Blay F, Cirillo T, Montuori P, Lacorte S. 2012. Migration of phthalates, alkylphenols, bisphenol A and di(2-ethylhexyl)adipate from food packaging. Food Control 27:123-128.
Fawell JK, Sheahan D, James HA, Hurst M, Scott S. 2001. Oestrogens and oestrogenic activity in raw and treated water in Severn Trent water. Water Res 35(5):1240-1244.
[FDA] U.S. Food and Drug Administration. 2014. List of indirect additives used in food contact substances. Available from: http://www.fda.gov/Food/IngredientsPackagingLabeling/PackagingFCS/IndirectAdditives/default.htm [cited 2014]
Ferguson KK, Loch-Caruso R, Meeker JD. 2011. Urinary phthalate metabolites in relation to biomarkers of inflammation and oxidative stress: NHANES 1999-2006. Environ Res 111:718-726.
Ferguson KK, Loch-Caruso R, Meeker JD. 2012. Exploration of oxidative stress and inflammatory markers in relation to urinary phthalate metabolites: NHANES 1999-2006. Environ Sci Technol 46:477-485.
Ferguson KK, McElrath TF, Chen YH, Mukherjee B, Meeker JD. 2014. Urinary phthalate metabolites and biomarkers of oxidative stress in pregnant women: a repeated measures analysis. Environ Health Perspect 123(3): 210-6.
Fierens T, Servaes K, Van Holderbeke M, Geerts L, De Henauw S, Sioen I,Vanerman G. 2012. Analysis of phthalates in food products and packaging materials sold on the Belgian market. Food Chem Toxicol 50:2575-2583.
Foster P, Thomas LV, Cook MW, Gangolli SD. 1980. Study of the testicular effects and changes in zinc excretion produced by some n-alkyl phthalates in the rat. Toxicol Appl Pharm 54(3):392-398.
Frederiksen H, Nielsen JKS, Morck TA, Hansen PW, Jensen JF, Nielsen O, Andersson A-M, Knudsen LE. 2013. Urinary excretion of phthalate metabolites, phenols and parabens in rural and urban Danish mother-child pairs. Int J Hyg Envir Heal 216:772-783.
Frederiksen H, Hanninen TK, Main KM, Dunkel L, Sankilampi. 2014. A longitudinal study of urinary phthalate excretion in 58 full-term and 67 preterm infants from birth through 14 months. Environ Health Persp 122(9):998-1005.
Fromme H, Lahrz T, Piloty M, Gebhart H, Oddoy A, Rüden H. 2004. Occurrence of phthalates and musk fragrances in indoor air and dust from apartments and kindergartens in Berlin (Germany). Indoor Air 14(3):188-195.
Fromme H, Gruber L, Schlummer M, Wolz G, Böhmer S, Angerer J, Mayer R, Liebl B, Bolte G. 2007. Intake of phthalates and di (2-ethylhexyl) adipate: Results of the Integrated Exposure Assessment Survey based on duplicate diet samples and biomonitoring data. Environ Int 33(8):1012-1020.
Fromme H, Gruber L, Schuster R, Schlummer M, Kiranoglu M, Bolte G, Völkel W. 2013. Phthalate and di-(2-ethylhexyl) adipate (DEHA) intake by German infants based on the results of a duplicate diet study and biomonitoring data (INES 2). Food Chem Toxicol 53:272-280.
Fulcher SM, Willoughby CR, Heath JA, Veenstra GE, Moore NP. 2001. Developmental toxicity of di-(C(7)-C(9) alkyl) phthalate and di-(C(9)-C(11) alkyl) phthalate in the rat. Reprod Toxicol 15(1):95-102.
Furr J, Lambright C, Wilson V, Foster P, Gray L Jr. 2014. A short-term in vivo screen using fetal testosterone production, a key event in the phthalate adverse outcome pathway, to predict disruption of sexual differentiation. Toxicol Sci 140(2):403-432.
Furtmann K. 1993. Phthalates in the aquatic environment. PhD dissertation, Regional Water and Wastewater Authority, Nordrhein-Westfalen [cited in Staples et al. 1997].
Gartner S, Balski M, Koch M, Nehls I. 2009. Analysis and migration of phthalates in infant food packed in recycled paperboard. J Agric Food Chem 57:10675-10681.
General Motors Research Laboratories. 1981. Toxicity and fate of diisodecyl phthalate following inhalation exposure in rats. EPA Document No 878210881, OTW206189 [cited in ECJRC 2003; US CPSC 2010a].
General Motors Research Laboratories. 1983. Effect of dose on di-isodecyl phthalate disposition in rats. EPA document No 878213821, OTS 206315 [cited in NTP 2000; ECJRC 2003; European Chemicals Bureau 2004].
Ghisari M, Bonefeld-Jorgensen EC. 2009. Effects of plasticizers and their mixtures on estrogen receptor and thyroid hormone functions. Toxicol Lett 189(1):67-77.
Gobas FAPC, Morrison HA. 2000. Bioconcentration and biomagnification in the aquatic environment. In: Boethling RS, Mackay D, editors. Handbook of property estimation methods for chemicals, environmental and health sciences. Boca Raton (FL): CRC Press. pp. 189-231.
Gobas FAPC, Mackintosh CE, Webster G, Ikonomou M, Parkerton TF, Robillard K. 2003. Bioaccumulation of phthalate esters in aquatic food-webs. In: Hutzinger O, ed. The handbook of environmental chemistry. Volume 3. Anthropogenic compounds. Part Q. Berlin (DE): Springer-Verlag. pp. 201-225.
Hakkarainen M. 2008. Migration of monomeric and polymeric PVC plasticizers. Adv Polym Sci 211:159-185 [cited in Cheminfo Services Inc. 2013b].
Hannas BR, Lambright CS, Furr J, Evans N, Foster PMD, Gray EL, Wilson VS. 2012. Genomic biomarkers of phthalate-induced male reproductive developmental toxicity: a targeted RT-PCR array approach for defining relative potency. Toxicol Sci 125(2):544-557.
Hardin BD, Schuler RL, Burg JR, Booth GM, Hazleden KP, Mackenzie KM, Piccirillo VJ, Smith KN. 1987. Evaluaton of 60 chemicals in a preliminary developmental toxicity test. Teratogen Carcin Mut 7:29-48.
Harris CA, Henttu P, Parker MG, Sumpter JP. 1997. The oestrogenic activity of phthalate esters in vitro. Envion Health Perspect 105:802-811 [cited in NICNAS 2008a].
Harris KR, Bair S. 2007. Temperature and pressure dependence of the viscosity of diisodecyl phthalate at temperatures between (0 and 100) C and at pressures to 1 GPa. J Chemical Eng Data 52(1):272-278.
Hart R, Doherty DA, Frederiksen H, Keelan JA, Hickey M, Sloboda D, Pennell CE, Newnham JP, Skakkebaek NE, Main KM. 2014. The influence of antenatal exposure to phthalates on subsequent female reproductive development in adolescence: a pilot study. Reproduction. 147: 379-390.
Haynes WM, Lide DR. 2010. CRC handbook of chemistry and physics. 91st edition. 2010-2011. Boca Raton (FL): CRC Press, Taylor & Francis Group.
Hazleton Biotechnologies Company. 1986. Mutagenicity of 1 L in a mouse lymphoma mutation assay. Report no 20989 [cited in ECJRC 2003].
Hazleton Laboratories. 1968a. Three-month dietary administration - albino rats DIDP - FDA grade (plasticiser) [cited in ECJRC 2003; NICNAS 2008a; US CPSC 2010a].
Hazleton Laboratories. 1968b. 13-week dietary administration - dogs plasticiser (DIDP) [cited in ECJRC 2003; NICNAS 2008a; US CPSC 2010a].
Hazleton Washington. 1994. Mutagenicity test on Jayflex DIDP in an in vivo mouse micronucleus assay. Project No 20996 [as cited in NICNAS 2008a].
Health Canada. 1994. Human health risk assessment for priority substances. Ottawa (ON): Health Canada, Environmental Health Directorate. Available from: http://www.hc-sc.gc.ca/ewh-semt/pubs/contaminants/approach/index_e.html
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Health Canada. 2007. Market Evaluation: Analysis of phthalate content in children's toys. Consumer Product Safety Bureau. Project #850950.
Health Canada. 2009. Survey-Determination of phthalate in various children's toys. Consumer Product Safety Bureau. Project #2008-1090.
Health Canada. 2011. The cosmetic ingredient hotlist - September 2011 [Internet]. Ottawa (ON): Health Canada, Consumer Project Safety. Available from: http://www.hc-sc.gc.ca/cps-spc/cosmet-person/indust/hot-list-critique/index-eng.php
Health Canada. 2012. Phthalates in Toys: Cyclical Enforcement 2011-2012. Consumer Product Safety Bureau. Project #2011-1387.
Health Canada. 2014. Survey 2014-15: Determination of a Series of 34 Phthalates in Plastic Consumer Products . Consumer Product Safety Bureau. Project # 2014-2047
Health Canada. 2015a. Technical Document: Approach for using chemical categories and read-across to address data gaps for effects on the developing male reproductive system: Phthalate Substance Grouping. Ottawa (ON): Health Canada. Available from: http://www.chemicalsubstanceschimiques.gc.ca/group/phthalate/index-eng.php
Health Canada. 2015b. Supporting documentation: Evaluation of epidemiologic studies on phthalate compounds and their metabolites. Ottawa (ON): Health Canada. Available on request from: substances@ec.gc.ca
Health Canada. 2015c. Supporting documentation: Carcinogenicity of phthalates - Common MOA by tumor types. Ottawa (ON): Health Canada. Available on request from: substances@ec.gc.ca
Heindel JJ, Gulati DK, Mounce RC, Russell SR, Lamb JC IV. 1989. Reproductive toxicity of three phthalic acid esters in a continuous breeding protocol. Fundam Appl Toxicol 12(3):508-518.
Hellwig J, Freudenberger H, Jackh R. 1997. Differential prenatal toxicity of branched phthalate esters in rats. Food Chemical Toxicol 35:501-512.
[HENRYWIN] Henry's Law Constant Program for Microsoft Windows [Estimation Model]. 2011. Version 3.20. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Hinton RH, Mitchell FE, Mann A, Chescoe D, Price SC, Nunn A, Grasso P, Bridges JW. 1986. Effects of phthalic acid esters on the liver and thyroid. Environ Health Perspect 70:195-210.
Hoppin JA, Jaramillo R, London SJ, Bertelsen RJ, Salo RM, Sandler DP, Zeldin DC. 2013. Phthalate exposure and allergy in the U.S. population: results from NHANES 2005-2006. Environ Health Perspect 121:1129-1134.
Howard PH, Banerjee S, Robillard KH. 1985. Measurement of water solubilities, octanol/water partition coefficients and vapor pressures of commercial phthalate esters. Environ Toxicol Chem 4:653-661.
[HPD] Household Products Database [database on the Internet]. 2014. Bethesda (MD): U.S. National Library of Medicine. Available from: http://householdproducts.nlm.nih.gov/. [cited 2014 July 3].
[HPVIS] High Production Volume Information System. 2002. Detailed chemical results on 1,2 Benzenedicarboxylic acid, diundecyl ester. CAS Number 3648-20-2. US EPA. Available from: http://ofmpub.epa.gov/oppthpv/quicksearch.display?pChem=100979
[HSDB] Hazardous Substances Data Bank [database on the Internet]. 2010. Bethesda (MD): U.S. National Library of Medicine [cited 2014 Jun 2]. Available from: http://toxnet.nlm.nih.gov/cgi-bin/sis/search2
Huber DR, Blount BC, Mage DT, Letkiewicz FJ, Kumar A, Allen RH. 2010. Estimating perchlorate exposure from food and tap water based on US biomonitoring and occurrence data. J Expo Sci Environ Epidemiol 21:395-407.
Hushka LJ, Waterman SJ, Keller LH, Trimmer GW, Freeman JJ, Ambroso JL, Nicolich M, McKee RH. 2001 study A. Two-generation reproduction studies in rats fed di-isodecyl phthalate. Reprod Toxicol 15:153-169.
Hushka LJ, Waterman SJ, Keller LH, Trimmer GW, Freeman JJ, Ambroso JL, Nicolich M, Mckee RH. 2001 study B. Two-generation reproduction studies in rats fed di-isodecyl phthalate. Reprod Toxicol 15:153-169.
[HYDROWIN] Hydrolysis Rates Program for Microsoft Windows [Estimation Model]. 2010. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
[IARC] International Agency for Research on Cancer. 2012. Di(2-ethylhexyl) phthalate. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 101 - Some Chemicals Present in Industrial and Consumer Products, Food and Drinking-water. Pp. 149-284. Available from: http://monographs.iarc.fr/ENG/Monographs/vol101/mono101-006.pdf.
Ito Y, Yamanoshita O, Asaeda N, Tagawa Y, Lee CH, Aoyama T, Ichihara G, Furuhashi K, Kamijima M, Gonzalez FJ, Nakajima T. 2007. Di(2-ethylhexyl)phthalate induces hepatic tumorigenesis through a peroxisome proliferator-activated receptor alpha-independent pathway. J Occup Health 49:172-182.
Janjua NR, Mortensen GK, Andersson AM, Kongshoj B, Skakkebaek NE, Wulf HC. 2007. Systemic uptake of diethyl phthalate, dibutyl phthalate, and butyl paraben following whole-body topical application and reproductive and thyroid hormone levels in humans. Environ Sci Technol 41(15):5564-5570.
Janjua NR, Frederiksen H, Skakkebaek NE, Wulf HC, Andersson AM. 2008. Urinary excretion of phthalates and paraben after repeated whole-body topical application in humans. Int J Androl 31(2):118-30.
Johnson S, Saikia N, Sahu R. 2011. Phthalates in toys available in Indian Market. 2011. Bull Environ Contam Toxicol 86:621-626.
Johnson TB, Heitkamp MA, Jones JR. 1984. Environmental and chemical factors influencing the biodegradation of phthalic acid esters in freshwater sediments. Environ Pollut B 8(2):101-118.
Jones HB, Garside DA, Liu R, Roberts JC. 1993. The influence of phthalate esters on Leydig cell structure and function in vitro and in vivo. Exp Mol Pathol 58(3):179-193.
Kang Y, Man YB, Cheung KC, Wong MH. 2012. Risk assessment of human exposure to bioaccessible phthalate esters via indoor dust around the Pearl River Delta. Environ Sci Technol 46(15):8422-8430. DOI: 10.1021/es300379v.
Kasper-Sonnenberg M, Koch HM, Wittsiepe J, Wilhelm M. 2012. Levels of phthalate metabolites in urine among mother-child-pairs - Results from the Duisburg birth cohort study, Germany. Int J Hyg Envir Heal 215:373-382.
Kato K, Silva MJ, Wolf C, Gray LE, Needham LL, Calafat AM. 2007. Urinary metabolites of diisodecyl phthalate in rats. Toxicology 236(1-2):114-22.
Kickham P, Otton SV, Moore MM, Ikonomou MG, Gobas FAPC. 2012. Relationship between biodegradation and sorption of phthalate esters and their metabolites in natural sediments. Environ Toxicol Chem 31(8):1730-1737.
Kim M, Yun SJ, Chung G-S. 2009. Determination of phthalates in raw bovine milk by gas chromatography/time-of-flight mass spectrometry (GC/TOF-MS) and dietary intakes. Food Addit Contam A 26(1):134-138.
Knowles CO, McKee MJ, Palawski DU. 1987. Chronic effects of di-2-ethylhexyl phthalate on biochemical composition and reproduction of Daphnia magna. Environ Toxicol Chem 6(3):201-208.
[KOAWIN] Octanol Air Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2010. Version 1.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Koch HM, Becker K, Wittassek M, Seiwert M, Angerer J, Kolossa-Gehring M. 2007 Di-n-butylphthalate and butylbenzylphthalate--urinary metabolite levels and estimated daily intakes: pilot study for the German Environmental Survey on children. Journal of Exposure Science and Environmental Epidemiology 17:378-387.
Koch HM, Calafat AM. 2009. Human body burdens of chemicals used in plastic manufacture. Phil Trans R Soc B 364:2063-2078.
Koch HM, Wittassek M, Burning T, Angerer J, Heudorf U. 2011. Exposure to phthalates in 5-6 years old primary school starters in Germany - A human biomonitoring study and cumulative risk assessment. Int J Hyg Envir Heal 214:188-195.
Koch HM, Haller A, Weiss T, Käfferlein HU, Stork J, Brüning T. 2012. Phthalate exposure during cold plastisol application-a human biomonitoring study. Toxicol Lett 213(1):100-106.
Koch HM, Lorber M, Christensen KLY, Palmke C, Koslitz S, Bruning T. 2013. Identifying sources of phthalate exposure with human biomonitoring: Results of a 48h fasting study with urine collection and personal activity patterns. Int J Hyg Envir Heal. DOI: http://dx.doi.org/10.1016/j.ijheh.2012.12.002
[KOCWIN] The Soil Adsorption Coefficient Program [Estimation Model]. 2010. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2010. Version 1.68. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Kransler KM, Bachman AN McKee RH. 2012. A comprehensive review of intake estimates of di-isononyl phthalate (DINP) based on indirect exposure models and urinary biomonitoring data. Regul Toxicol Pharm 62:248-256.
Krüger T, Long M, Bonefeld-Jorgensen EC. 2008. Plastic components affect the activation of the aryl hydrocarbon and the androgen receptor. Toxicology 246:112-123.
Kubwabo C, Rasmussen PE, Fan X, Kosarac I, Wu F, Zidek A, Kuchta SL. 2013. Analysis of selected phthalates in Canadian indoor dust collected using household vacuum and standardized sampling techniques. Indoor Air 23(6):506-514. doi:10.1111/ina.12048.
Kwack SJ, Kim KB, Kim HS, Lee BM. 2009. Comparative toxicological evaluation of phthalate diesters and metabolites in Sprague-Dawley male rats for risk assessment. J Toxicol Env Health Part A 72:1446-1454.
Lake BG, Rijcken W, Gray TJ, Foster JR, Gangolli SD. 1984. Comparative studies of the hepatic effects of di-and mono-n-octyl phthalates, di-(2-ethylhexyl) phthalate and clofibrate in the rat. Acta Pharmacol Tox 54(3):167-176.
Lake BG, Gray TJ, Gangolli SD. 1986. Hepatic effects of phthalate esters and related compounds-in vivo and in vitro correlations. Environ Health Perspect 67:283-90.
Lake BG, Cook WM, Worrell NR, Cunningham ME, Evans JG, Price RJ, Young PJ, Carpanini F. 1991. Dose-response relationships for induction of hepatic peroxisome proliferation and testicular atrophy by phthalate esters in the rat. Human Exp Toxicol 10:67-68 [cited in ECJRC 2003, NICNAS 2008a, US CPSC 2010a].
Lamb JC, Gulati DK, Chambers R, Shaver S, Sabharwal PS. 1997. Di-n-octylphthalate. Environ Health Perspect 105(1):253-254.
LaRocca J, Binder AM, McElrath TF, Michels KB. 2014. The impact of first trimester phthalate and phenol exposure on IGF2/H19 genomic imprinting and birth outcomes. Env Res 133:396-406.
Leah TD. 1977. Environmental contaminants inventory study no. 4. The production, use and distribution of phthalic acid esters in Canada. Report Series No. 47. Burlington (ON): Fisheries and Environment Canada, Inland Waters Directorate, Ontario Region, Water Planning and Management Branch.
Lee BM, Koo HJ. 2007. Hershberger assay for antiandrogenic effects of phthalates. J Toxicol Env Health Part A 70:1365-1370.
Leitz J, Kuballa T, Rehm J, Lachenmeier DW. 2009. Chemical analysis and risk assessment of diethyl phthalate in alcoholic beverages with special regard to unrecorded alcohol. PLoS ONE 4(12):e8127. doi:10.1371/journal.pone.0008127.
Lertsirisopon R, Soda S, Sei K, Ike M, Fujita M. 2006. Biodegradability of four phthalic acid esters under anaerobic condition assessed using natural sediment. J Environ Sci 18(4):793-796.
Letinski DG, Connelly MJ Jr., Peterson DR, Parkerton TF. 2002. Slow-stir water solubility measurements of selected alcohols and diesters. Chemosphere 48:257-265.
Li Z, Xue F, Xu L, Chifang P, Kuang H, Ding T, Xu C, Sheng C, Gong Y, Wang L. 2011. Simultaneous determination of nine types of phthalate residues in commercial milk products using HPLC-ESI-MS-MS. J Chromatogr Sci 49:338-343.
Liang D-W, Zhang T, Fang HHP, He J. 2008. Phthalates biodegradation in the environment. Appl Microbiol Biotechnol 80:183-198.
Lin Z-P, Ikonomou MG, Jing H, Mackintosh C, Gobas FAPC. 2003. Determination of phthalate ester congeners and mixtures by LC/ESI-MS in sediments and biota of an urbanized marine inlet. Environ Sci Technol 37:2100-2108.
Lington AW, Gray TJB, Evans J, Lake B, Moran B. 1993. Short-term feeding studies assessing the testicular effects of nine plasticizers in the F344 rat. Acta Pharmacol Toxicol 73(Suppl 11):132.
Litton Bionetics. 1985a. Evaluation of 1L in the mouse lymphoma toxicity assay final report. Assay No 7160. EPA/OTS Doc 40-8526196. US EPA TSCA 8(d) files [cited in European Commission 2000].
Litton Bionetics. 1985b. Evaluation of 1 L in the in vitro transformation of BALB/C-3T3 cells assay. Report no. 20992 [cited in ECJRC 2003].
[LNHPD] Licensed Natural Health Products Database [database on the internet]. 2014. Ottawa (ON): Health Canada. Available from: http://www.hc-sc.gc.ca/dhp-mps/prodnatur/applications/licen-prod/lnhpd-bdpsnh-eng.php
Lyman WJ, Rosenblatt DH, Reehl WJ, eds. 1990. Handbook of chemical property estimation methods. Washington (DC): American Chemical Society [cited in HSDB 2010].
Ma Y, Hashi Y, Ji F, Lin J-M. 2010. Determination of phthalates in fruit jelles by dispersive SPE coupled with HPLC-MS. J Sep Sci 33:251-257.
Mackay D, Shiu WY, Ma K-C, Lee SC. 2006. Handbook of physical-chemical properties and environmental fate for organic chemicals. 2nd edition. Volume III. Oxygen containing compounds. Boca Raton (FL): CRC Press, Taylor & Francis Group. Available from: http://files.rushim.ru/books/spravochniki/mackay1.pdf
Mackintosh CE, Maldonado J, Hongwu J, Hoover N, Chong A, Ikonomou MG, Gobas FAPC. 2004. Distribution of phthalate esters in a marine aquatic food web: Comparison to polychlorinated biphenyls. Environ Sci Technol 38:2011-2020.
Mackintosh CE, Maldonado JA, Ikonomou MG, Gobas FAPC. 2006. Sorption of phthalate esters and PCBs in a marine ecosystem. Environ Sci Technol 40(11):3481-3488.
Mann AH, Price SC, Mitchell FE, Grasso P, Hinton RH, Bridges JW. 1985. Comparison of the short-term effects of di (2-ethylhexyl) phthalate, di (n-hexyl) phthalate, and di (n-octyl) phthalate in rats. Toxicol Appl Pharm 77(1):116-132.
McCarty LS, Mackay D. 1993. Enhancing ecotoxicological modeling and assessment: body residues and modes of toxic action. Environ Sci Technol 27(9):1719-1728.
McCarty LS, Arnot JA, Mackay D. 2013. Evaluation of critical body residue data for acute narcosis in aquatic organisms. Environ Toxicol Chem 32(10):2301-2314.
McConnell ML. 2007. Distribution of phthalate monoesters in an aquatic food web. School of Resource and Environmental Management Master of Resource Management Thesis Project Report No. 426. Spring 2007. Burnaby (BC): Simon Fraser University.
Mckee RH, Przygoda RT, Chirdon MA, Engelhardt G, Stanley M. 2000. Di(isononyl) phthalate (DINP) and di(isodecyl) phthalate (DIDP) are not mutagenic. J Appl Toxicol 20(6):491-497 [cited in US CPSC 2010a].
Mekenyan G, Dimitrov SD, Pavlov TS, Veith GD. 2005. POPs: a QSAR system for creating PBT profiles of chemicals and their metabolites. SAR QSAR Environ Res 16(1−2):103-133.
Microbiological Associates. 1981. Activity of T1678 in the in vitro mammalian cell transformation assay in the absence of exogenous metabolic activation. EPA Document No 878210225, Fiche No OTS206260 [cited in ECJRC 2003].
Meeker JD, Hu H, Cantonwine DE. 2009. Urinary phthalate metabolites in relation to preterm birth in Mexico City. Environ Health Perspect 117(10):1587-92.
Meeker JD, Ferguson KK. 2014. Urinary phthalate metabolites are associated with decreased serum testosterone in men, women, and children from NHANES 2011 -2012 .. J Clin Endocrinol Metab 99(11): 4346-4352.
Min K, Min J. 2014. Urinary phthalate metabolites and the risk of low bone mineral density and osteoporosis in older women. JCEM 99(10):E1997-E2003.
Mint A, Hotchkiss SAM. 1993. Percutaneous absorption of dimethyl phthalate and di-n-butyl phthalate through rat and human skin in vitro. In: Prediction of percutaneous penetration. Brain KR, JV, Hadgraft J, Walters KA, eds. 3B pp 646-657 [cited in ECJRC 2003].
Mint A, Hotchkiss SA, Caldwell J. 1994. Percutaneous absorption of diethyl phthalate through rat and human skin in vitro. Toxicol in Vitro 8(2):251-256.
Mlynarčíková A, Ficková M, Scsuková S. 2007. The effects of selected phenol and phthalate derivatives on steroid hormone production by cultured porcine granulosa cells. ATLA 35:71-77.
Morrissey RE, Lamb JC IV, Morris RW, Chapin RE, Gulati DK, Heindel JJ. 1989. Results of evaluations of 48 continuous breeding reproduction studies conducted in mice. Fundam Appl Toxicol 13:747-777 [cited in NTP 2003].
[MPBPVPWIN] Melting Point Boiling Point Vapour Pressure Program for Microsoft Windows [Estimation Model]. 2010. Version 1.43. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Nanni N, Fiselier K, Grob K, Di Pasquale M, Fabrizi L, Aureli P,Coni E. 2011. Contamination of vegetable oils marketed in Italy by phthalic acid esters. Food Control 22:209-214.
[NHPID] Natural Health Products Ingredients Database [database on the Internet]. 2014. Ottawa (ON): Health Canada. Available from: http://webprod.hc-sc.gc.ca/nhpid-bdipsn/search-rechercheReq.do
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme. 2008a. Existing chemical hazard assessment report. Diisodecyl phthalate. June 2008. Sydney (AU): Australian Government, Department of Health and Ageing.
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme. 2008b. Existing chemical hazard assessment report. Diundecyl phthalate. June 2008. Sydney (AU): Australian Government, Department of Health and Ageing.
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme. 2010. Diethylhexyl Phthalate. Priority Existing Chemical Assessment Report No.32. Sydney, NSW. Australian Government. Department of Health and Ageing. Available from: http://www.nicnas.gov.au/chemical-information/pec-assessments
[NTP] National Toxicology Program- CERHR. 2003. Monograph on the Potential Human Reproductive and Developmental Effects of Di-n -Octyl Phthalate (DnOP). NIH Publication No. 03-4488. Available from: http://ntp.niehs.nih.gov/ntp/ohat/phthalates/dnop/dnop_monograph_final.pdf
[OECD] Organisation for Economic Co-operation and Development. 1984a. OECD guideline for testing of chemicals. TG 301: Earthworm, acute toxicity tests. Adopted 9 April 1984. Paris (FR): OECD.
[OECD] Organisation for Economic Co-operation and Development. 1984b. OECD guideline for testing of chemicals. TG 202: Daphnia sp., acute immobilisation test and reproduction test. Adopted 4 April 1984. Paris (FR): OECD.
[OECD] Organisation for Economic Co-operation and Development. 1992. OECD guideline for testing of chemicals. TG 207: Ready biodegradability. Adopted 17 July 1992. Paris (FR): OECD.
[OECD] Organisation for Economic Cooperation and Development. 2004. SIDS Initial Assessment Profile, SIAM 19, 19-22 October 2014. Available from: http://webnet.oecd.org/hpv/UI/handler.axd?id=3744a3ff-ef6d-4a04-ba90-f311d99e62d0 [cited 2014].
OECD QSAR Toolbox [Read across tool]. 2012. Version 3.0. Paris (FR): Organisation for Economic Co-oporation and Development, Environment Directorate. [cited 2014 Aug 21]. Available from: www.oecd.org/document/23/0,3343,en_2649_34379_33957015_1_1_1_1,00.html
O'Grady DP, Howard PH, Werner AF. 1985. Activated sludge biodegradation of 12 commercial phthalate esters. Appl Environ Microbiol 49(2):443-445.
Oishi S, Hiraga K. 1980. Testicular atrophy induced by phthalic acid esters: effect on testosterone and zinc concentrations. Toxicol Appl Pharm 53(1):35-41.
O'Reilly JT. 1989. Personal communication from James T. O'Reilly, the Procter & Gamble Company, Cincinnati, OH to Andrew Ulsamer, U.S. Consumer Product Safety Commission, Washington, DC [cited in US CPSC CHAP 2014].
Otton VS, Sura S, Blair J, Ikonomou MG, Gobas FAPC. 2008. Biodegradation of mono-alkyl phthalate esters in natural sediments. Chemosphere 71:2011-2016.
Page BD, Lacroix GM. 1992. Studies into the transfer and migration of phthalate esters from aluminium foil-paper laminates to butter and margarine. Food Addit Contam 9(3):197-212.
Parkerton TF, Konkel WJ. 2000. Application of quantitative structure-activity relationships for assessing the aquatic toxicity of phthalate esters. Ecotox Environ Saf 45:61-78.
Parkerton TF, Staples CA. 2003. As assessment of the potential environmental risks posed by phthalates in soil and sediment. In: Hutzinger O, ed. The handbook of environmental chemistry. Volume 3. Anthropogenic compounds. Part Q. Berlin (DE): Springer-Verlag. pp. 317-349.
Parkerton T, Winkelmann D. 2004. An assessment of the persistence, bioaccumulation, and inherent toxicity of selected phthalates, trimellitates, adipates, and related monoesters on the Canadian Domestic Substance List (DSL). Prepared for the Phthalate Esters Panel of the American Chemistry Council. August 9, 2004.
Patyna PJ, Brown RP, Davi RA, Letinski DJ, Thomas PE, Cooper KR, Parkerton TF. 2006. Hazard evaluation of diisononyl phthalate and diisodecyl phthalate in a Japanese medaka multigenerational assay. Ecotox Environ Saf 65(1):36-47.
Philippat C, Mortamais M, Chevrier C. 2012. Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environ Health Perspect 120(3):464-70.
Pegg DG. 1979. Research Report No. 81-135, unpublished work [cited in Albro and Lavenhar 1989].
Peters RJB, Beeltje H, van Delft RJ. 2008. Xeno-estrogenic compounds in precipitation. J Environ Monit 10:760-769.
Petersen JH, Jensen LK. 2010. Phthalates and food-contact materials: enforcing the 2008 European Union plastics legislation. Food Addit Contam A 27(11):1608-1616.
Poon R, Lecavalier P, Mueller R, Valli VE, Procter BG, Chu I. 1997. Subchronic oral toxicity of di-n-octyl phthalate and di (2-ethylhexyl) phthalate in the rat. Food Chem Toxicol 35(2):225-239.
[RAPEX] Rapid Alert System for non-food dangerous products, 'RAPid EXchange'. 2012. Weekly Notification reports. http://ec.europa.eu/consumers/dyna/rapex/rapex_archives_en.cfm
Rastogi SC. 1998. Gas chromatographic analysis of phthalate esters in plastic toys. Chromatographia 47:724-726.
Remberger M, Kaj L, Hansson K, Andersson H, Brorström-Lundén, Lunder H, Schlabach M. 2013. Selected plasticisers and additional sweeteners in the Nordic environment. TemaNord 2013:505. Copenhagen (DK): Norden, Nordic Council of Ministers.
Ren H, Aleksunes LM, Wood C, Vallanat B, George MH, Klaassen CD, Corton JC. 2010. Characterization of peroxisome proliferator-activated receptor alpha-independent effects of PPARalpha activators in the rodent liver: di- (2-ethylhexyl) phthalate also activates the constitutive-activated receptor. Toxicol Sci 113:45-59.
Rhodes JE, Adams WJ, Biddinger GR, Robillard KA, Gorsuch JW. 1995. Chronic toxicity of 14 phthalate esters to Daphnia magna and rainbow trout (Oncorhynchusmykiss). Environ Toxicol Chem 14(11):1967-1976.
[RIVM] National Institute of Public Health and the Environment. 1998. Phthalate release from soft PVC baby toys. Report from the Dutch Consensus Group. Könemann WH, ed. RIVM Report 613320 002. September 1998. Bilthoven, NL: RIVM. Available from: http://www.rivm.nl/bibliotheek/rapporten/613320002.pdf
Rudel RA, Dodson RE, Perovich LJ, Morello-Frosch R, Camann DE, Zuniga MM, Yau AY, Just AC, Brody JG. 2010. Semivolatile endocrine-disrupting compounds in paired indoor and outdoor air in two Northern California communities. Environ Sci Technol 44(17):6583-6590.
Saeger VW, Tucker ES. 1976. Biodegradation of phthalic acid esters in river water and activated sludge. Appl Environ Microbiol 31(1):29-34.
Saillenfait AM, Roudot AC, Gallissot F, Sabaté JP. 2011. Prenatal developmental toxicity studies on di- n-heptyl and di-n-octyl phthalates in Sprague-Dawley rats. Reprod Toxicol 32(3):268-276.
Saillenfait AM, Gallissot F, Sabaté JP, Remy A. 2013. Prenatal developmental toxicity studies on diundecyl and ditridecyl phthalates in Sprague-Dawley rats. Reprod Toxicol 37:49-55.
Sample BE, Opresko DM, Suter II GW. 1996. Toxicological benchmarks for wildlife: 1996 revision. ES/ER/TM-86/R3. June 1996. Oakridge (TN): United States Department of Energy, Risk Assessment Program, Health Sciences Research Division.
Sannino A. 2009. Survey of phthalate levels in Italian oily foods contained in glass jars with PVC gaskets. Food Addit Contam B 2(2):166-170.
[SCCP] Scientific Committee on Consumer Products. 2007. Opinion on phthalates in cosmetic products. Brussels (BE): European Commission, Health & Consumer Protection Directorate. Available from: http://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_106.pdf
Schecter A, Lorber M, Guo Y, Wu Q, Hun Yun S, Kannan K, Hommel M, Imran N, Hynan LS, Cheng D, Colacino JA, Birnbaum L. 2013. Phthalate concentrations and dietary exposure from food purchased in New York State. Environ Health Perspect 121(4):473-479.
Scholz N. 2003. Ecotoxicity and biodegradation of phthalate monoesters. Chemosphere 53:921-926.
Schultz TW. 1989. Nonpolar narcosis: A review of the mechanism of action for baseline aquatic toxicity. In: Aquatic toxicology and hazard assessment, 12th volume. Cowgill UM, Williams LR, eds. ASTM STP 1027. Philadelphia (PA): American Society for Testing and Materials.
SciFinder [database on the Internet]. 2013. Columbus (OH): American Chemical Society [cited 2014 May 01]. Restricted access. Available from: https://scifinder.cas.org/scifinder/
Scott RC, Dugard PH, Ramsey JD, Rhodes C. 1987. In vitro absorption of some o-phthalate diesters through human and rat skin. Environ Health Perspect 74:223-7.
Seed JL. 1982. Mutagenic activity of phthalate esters in bacterial liquid suspension assays. Environ Health Perspect 45:111-114 [cited in European Commission 2000].
Shiue I. 2014a. Higher urinary heavy metal, arsenic, and phthalate concentrations in people with high blood pressure: US NHANES, 2009-2010. Blood Press 23(6):363-9.
Shiue I. 2014b. Higher urinary heavy metal, phthalate, and arsenic but not parabens concentrations in people with high blood pressure , U.S. NHANES, 2011-2012. Int J Environ Res Public Health 11(6): 5989-99.
Shiue I, Hristova K. 2014. Higher urinary heavy metal, phthalate and arsenic concentrations accounted for 3-19% of the population attributable risk for high blood pressure: US NHANES, 2009-2012. Hypertens Res 37(12):1075-81.
Silva MJ, Kato K, Gray EL, Wolf C, Needham LL, Calafat AM. 2005. Urinary metabolites of di-n-octyl phthalate in rats. Toxicol 210(2-3):123-33.
Silva MJ, Reidy JA, Kato K, Preau JL Jr, Needham LL, Calafat AM. 2007. Assessment of human exposure to di-isodecyl phthalate using oxidative metabolites as biomarkers. Biomarkers 12(2):133-44.
Smith JH, Isenberg JS, Pugh G, Kamendulis LM, Ackley D, Lington AW, Klaunig JE. 2000. Comparative in vivo hepatic effects of di-isononyl phthalate (DINP) and related C7-C11 di-alkyl phthalates on gap junctional intercellular communication (GJIC), peroxisomal beta-oxidation (PBOX), and DNA synthesis in rat and mouse liver. Toxicol Sci 54:312-321.
Sørensen LK. 2006. Determination of phthalates in milk and milk products by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 20:1135-1143.
Sosiak A, Hebben T. 2005. A preliminary survey of pharmaceuticals and endocrine disrupting compounds in treated municipal wastewaters and receiving rivers of Alberta. September 2005. Edmonton (AB): Environmental Monitoring and Evaluation Branch, Alberta Environment.
Staples CA, Peterson DR, Parkerton TF, Adams WJ. 1997. The environmental fate of phthalate esters: A literature review. Chemosphere 35(4):667-749.
Statistics Canada. 2004. Canadian Community Health Survey - Nutrition (CCHS). Detailed information for 2004 (Cycle 2.2). Ottawa (ON): Statistics Canada. Available from: http://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=5049&lang=en&db=imdb&adm=8&dis=2
Stringer R, Labunska I, Santillo D, Johnston P, Siddorn J, Stephenson A. 2000. Concentrations of phthalate esters and identification of other additives in PVC children's toys. Environ Sci Pollut Res 7(1):27-36.
Sugatt RH, O'Grady DP, Banerjee S, Howard PH, Gledhill WE. 1984. Shake flask biodegradation of 14 commercial phthalate esters. Appl Environ Microbiol 47(4):601-606.
Sun Q, Li L, Jiang Z, Xin S, Wu S, Yu L. 2013. Characterization of phthalate plasticizer from bottled beverages by GC-MS. Appl Mech Mater 401-403:590-593.
Takeuchi S, Iida M, Kobayashi S, Jin K, Matsuda T, Kojima H. 2005. Differential effects of phthalate esters on transcriptional activities via human estrogen receptors and, and androgen receptor. Toxicol 210:223-233.
Trasande L, Sathyanarayana S, Trachtman H. 2014. Dietary phthalates and low-grade albuminuria in US children and adolescents. Clin J Am Soc Nephrol 9(1):100-109.
[US CPSC] United States Consumer Product Safety Commission. 2010a. Toxicity review for Di(isodecyl) phthalate. Bethesda (MD). Available from: http://www.cpsc.gov/PageFiles/126534/toxicityDIDP.pdf
[US CPSC] United States Consumer Product Safety Commission. 2010b. Toxicity Review of Di(2-ethylhexyl) Phthalate (DEHP). Bethesda (MD). Available from: http://www.cpsc.gov//PageFiles/126533/toxicityDEHP.pdf
[US CPSC CHAP] United States Consumer Product Safety Commission Chronic Hazard Advisory Panel. 2014. Chronic Hazard Advisory Panel on Phthalates and Phthalate Alternatives Final Report. Available from: http://www.cpsc.gov/PageFiles/169902/CHAP-REPORT-With-Appendices.pdf
[US EPA] United States Environmental Protection Agency. 2011. Exposure Factors Handbook: 2011 Edition. U.S. Environmental Protection Agency, Office of Research and Development, Washington, DC 20460. EPA/600/R-090/052F. September 2011. http://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=236252
[US EPA] United States Environmental Protection Agency. 2012. Phthalates action plan. Revised 03/14/2012. Washington (DC): United States Environmental Protection Agency
[US EPA] United States Environmental Protection Agency. 2014a. Non-confidential IUR 2002 production volume information. Washington (DC): Environmental Protection Agency. Available from: http://www.epa.gov/oppt/cdr/tools/data/2002-vol.html
[US EPA] United States Environmental Protection Agency. 2014b. Non-confidential IUR 2006 production volume information. Washington (DC): Environmental Protection Agency. Available from: http://www.epa.gov/oppt/cdr/tools/data/2006-vol.html
[VCCLab] Virtual Computational Chemistry Laboratory. 2005. ALOGPS 2.1 non-Java interface [cited 2014 October 23]. Available from: http://www.vcclab.org/lab/alogps/
Versar Inc. and SRC Inc. 2011. Final toxicity review for diundecyl phthalate (DUP, CASRN 3648-20-2). Springfield (VA): Versar Inc. North Syracuse (NY): SRC, Inc. Available from: https://www.cpsc.gov/PageFiles/125795/dup.pdf
VWA. 2009. Consumentenproducten in de eroticabranche. Fact sheet. Voedsel en Waren Autoriteit (VWA), Afdeling Signalering en Ontwikkeling, Regio Noord, Juni 2009 [cited in ECHA 2013].
Warf Institute. 1976. Acute inhalation LCSO sample LL-I 132, unpublished work [cited in Albro and Lavenhar 1989].
Waterman SJ, Ambroso JL, Keller LH, Trimmer GW, Nikiforov AI, Harris SB. 1999. Developmental toxicity of di-idodecyl and di-isononyl phthalates in rats. Reprod Toxicol 13(2):131-136.
[WATERNT] Water Solubility Program [Estimation Model]. 2010. Version 1.01. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Webster GM. 2003. Dietary uptake and biotransformation of phthalate esters in staghorn sculpin. School of Resource and Environmental Management Master of Resource Management Thesis Project Report No. 335. August 2003. Burnaby (BC): Simon Fraser University.
Wennberg L, Parkman H, Remberger M, Viktor T, Williams C. 1997. The influence of sediment-associated phthalate esters (DEHP and DIDP) on hatching and survival of the moorfrog, Rana arvalis. IVL Swedish Environmental Institute Report B1260. Stockholm (SE): IVL [cited in Parkerton and Staples 2003 and ECJRC 2003].
Wenzel A, Franz C, Breous E, Loos U. 2005. Modulation of iodide uptake by dialkyl phthalate plasticisers in FRTL-5 rat thyroid follicular cells. Mol Cell Endocrinol 244:63-71.
Weschler CJ, Nazaroff WW. 2010. SVOC partitioning between the gas phase and settled dust indoors. Atmos Environ 44:3609-3620.
Williams MD, Adams WJ, Parkerton TF, Biddinger GR, Robillard KA. 1995. Sediment sorption coefficient measurements for four phthalate esters: Experimental results and model theory. Environ Toxicol Chem 14(9):1477-1486.
Willoughby CR, Fulcher SM, Creasy DM, Health JA, Priston RA, Moore NP. 2000. Two-generation reproduction toxicity studies of di-(C(7)-C(9 )alkyl) phthalate and di-(C(9)-C(11) alkyl) phthalate in the rat. Reprod Toxicol 14(5):427-450.
Wilson R, Jones-Otazo H, Petrovic S, Mitchell I, Bonvalot Y, Williams D, Richardson GM. 2013. Revisiting dust and soil ingestion rates based on hand-to-mouth transfer. Hum Ecol Risk Assess 19(1):158-188.
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [Estimation Model]. 2010. Version 1.42. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Xing Y, Wu J. 2014. Determination of 15 PAEs in fatty food by SPE-GC/MS. Appl Mech Mater 469:458-463.
Xu Q, Yin X, Wang M, Wang H, Zhang N, Shen Y, Xu S, Zhang L, Zhongze G. 2010. Analysis of phthalate migration from plastic containers to packaged cooking oil and mineral water. J Agric Food Chem 58:11311-11317.
Xue M-G, Wang S-F, Huang C-X, Xia N-N. 2010. The analysis of organic contaminants in printing paper food packaging materials. Proceedings of the 17th IAPRI World Conference on Packaging. Tianjin, China. October 12 to 15 2010. International Association of Packaging Research Institutes.
Yang Q, Nagano T, Shah Y, Cheung C, Ito S, Gonzalez FJ. 2007. The PPARα-Humanized Mouse: A Model to Investigate Species Differences in Liver Toxicity Mediated by PPARα. Toxicol Sci 101(1):132-139.
Yaws CL. 1994. Handbook of vapor pressure. Volume 3: C8 to C28 Compounds. Houston (TX): Gulf Publishing Co. [cited in HSDB 2010].
Zacharewski TR, Meek MD, Clemons JH, Wu ZF, Fielden MR, Matthews JB. 1998. Examination of the in vivo and in vitro estrogenic activities of eight commercial phthalate esters. Toxicol Sci 46(2):282-293.
Zeiger E, Haworth S, Mortelmans K, Speck W. 1985. Mutagenicity testing of di(2- ethylhexy1) phthalate and related chemicals in Salmonella. Environ Mutagen 7:213-232 [cited in European Commission 2000].
Zheng Q, Feng M, Dai Y. 2013. Comparative antioxidant responses in liver of Carassius auratus exposed to phthalates: An integrated biomarker approach. Environ Toxicol Pharmacol 36:741-749.
Appendices
- Appendix A: Information on analogues used for substances in the Long-Chain Phthalates Grouping
- Appendix B: Physical and chemical properties for substances in the Long-Chain Phthalates Grouping
- Appendix C: Estimates of daily intake of DIDP and DUP
- Appendix D: Methodology for biomonitoring intake calculations
- Appendix E: Description and Application of the Downs and Black Scoring and Guidance for Level of Evidence of An Association