Final Report of the National Pharmacare Committee of Experts
Download in PDF format
(1.78 MB, 82 pages)
2025
Note: The views expressed in this publication are those of the National Pharmacare Committee of Experts and do not necessarily reflect the views of Health Canada.
On this page
- Message from the Chair
- Executive summary
- Introduction
- Recommendations and explanations
- Scope, context and approach of the committee of experts
- Acknowledgements
- Appendix 1: Biographies
- Appendix 2: Estimating the cost of pharmacare
- Appendix 3: List of medicines
- Endnotes
Message from the Chair
The cost-of-living crisis is dramatically illustrated by unaffordable life-saving medicines. People die because medicines are still not included in Canada’s publicly funded health care system.Footnote 1Footnote 2 In addition to saving lives, pharmacare will save billions of dollars.Footnote 3Footnote 4 Pharmacare’s savings provoke opposition from corporations that profit off the unfair status quo while exploiting government subsidies and tax loopholes.
The right to essential medicines should be recognized by swiftly implementing pharmacare. The principles of the Canada Health Act should apply to medicines: public administration, comprehensiveness, universality, portability and accessibility. The federal government should fully fund access to essential medicines for everyone living in Canada. Governmental reports from 2018 and 2019 clearly made the case for bringing medicines into our publicly funded system.Footnote 5Footnote 6 Progress on pharmacare is reported to the United Nations as part of Canada’s efforts to progressively realize the right to health.Footnote 7Footnote 8
Pharmacare will help make good on promises made by the Crown to Indigenous Peoples dating back to at least to 1876 with the Medicine Chest clause of Treaty 6.Footnote 9Footnote 10 The government has committed to reconciliation through the United Nations Declaration on the Rights of Indigenous Peoples Act.
There are 2 options: take charge and build pharmacare in Canada now or continue our over reliance on American-style private insurance schemes. These schemes transfer wealth outside of Canada and import inherent problems:
- discriminatory access exhibiting sexism, racism and ableism
- poor health outcomes
- high costs
The current approach will never provide good value, and we cannot save money in a system geared for making money.
Total spending on prescribed drugs in Canada jumped by $10 billion in 5 years – from $34 billion in 2019 to $44 billion in 2024Footnote 11 – with no measurable improvements in health or access to medicines.
Private insurance plans incentivize high drug prices because insurers take a percentage of claims. Per-pill drug costs are higher in Canada than in comparable countries. Some medicines produced in Canada are sold at lower prices overseas than they are here. People in Canada already have health cards that provide access to publicly funded necessary health services, so the costs of administering private plans and profit-taking by plan owners represent waste.
Private health insurance is generally tied to higher-income employment, which means that some households have no members with private insurance. Others have one member with a plan that covers all household members, while some households have two members with duplicative insurance plans that both cover all household members. In these double insurance situations, where 2 household members have separate and overlapping insurance plans, insurance premiums are paid as though full coverage will be provided but the costs are shared between the 2 plans. This makes little sense and does not happen when every resident has public insurance through a health card.
Private insurance companies have always enjoyed more governmental support than pharmacare. Manulife was created by an act of Parliament in 1887, and its first president was John A. Macdonald, the sitting prime minister. Large insurers demutualized in the 1990s to become publicly traded companies, bound to yield profits for shareholders. Today Manulife and Sun Life are among the largest insurance companies in Canada with a combined value of assets over $1 trillion. Privately administered plans, available to only some, are supported with a public subsidy (non-taxation of employer contributions to private insurance plans) that costs us all $5 billion each year.Footnote 12
Before asking how we can afford pharmacare, ask how every year we afford a $5 billion public subsidy for private insurance plans available to only some? Ask who is ultimately paying $50 billion for medicines right now? We can choose to pay less and get much more.
Pharmacare is what most would choose. Support from more than 80% of the public and multiple previous governmental recommendations demonstrate a rare and clear consensus that medicines should be included in our publicly funded system.Footnote 13
What’s needed now is the political will to shrug off industry lobbying and take these long-promised steps.
Today, we can choose to build a Canadian institution that serves us for generations to come. Pharmacare means including medicines in our publicly funded system, which already funds medically necessary services from seeing a family doctor about high blood pressure to having a heart transplant. The same rationale for publicly funding necessary health care services applies to essential medicines. Pharmacare should be built around a rigorously developed and maintained essential medicines list that is at the centre of a national strategy.
Action on pharmacare will address a multitude of connected issues.
Medicine access is connected with care. Provinces and territories are taking action to decrease the number of people without a family physician. Federal funding for pharmacare will free up money that can be reinvested in ensuring everyone has access to a primary care provider who can appropriately prescribe medicines. Indigenous, provincial and territorial governments will continue to provide and administer health care with federal funding in relationships built on mutual respect.
“If the United States no longer wants to lead, Canada will.” This was a strong statement from Canada’s new Prime Minister in April 2025 regarding Canada’s global role. One international goal is to achieve the United Nations HIV 95-95-95 target (95 % diagnosed, 95 % treated, 95 % virally suppressed) by the end of 2025.Footnote 14 Some jurisdictions within Canada are on target to meet this goal, but HIV transmits across borders, so it must be addressed everywhere at once. HIV is a clear example of the need for federal leadership.
Investments in care and dispensing medicines are particularly needed in remote communities with inequitable healthcare access. Sovereign Indigenous health systems should flourish and culturally appropriate care for Indigenous Peoples should be available everywhere.
Private medicine infusion clinics currently provide medically necessary care for patients who need medicines like infliximab for conditions such as Crohn’s disease or rheumatoid arthritis. Multinational pharmaceutical companies exploit this gap in our publicly funded medicines to market more expensive products in a biologic and specialty medicines drug category that costs more than $5 billion per year, according to the Canadian Institute of Health Information (CIHI). Medicines and the services needed to receive them should be both be included in Canada’s publicly funded health care system.
Many in Canada’s health system, including Health Canada, purchase drug dispensing data from a company headquartered in Durham, North Carolina. IQVIA sells personal health information collected at pharmacies to third parties. Canada should not be reliant on an American company to improve prescribing practices. We need to take back control of our data and ensure it is used to improve care. Investments in our data infrastructure and new ways of collaborating between jurisdictions are critical.
While improving access to medicines, we must also reduce the harms from their inappropriate use. The opioid crisis was caused by illegal marketing of drugs to physicians who wrote deadly prescriptions. The thousands of deaths per year in the still burning opioid crisis are a reminder that we cannot repeat past mistakes. A national strategy that addresses appropriate prescribing and medicine use is vital.
Virtual care might help to expand access to care and medicines, but some services skirt regulations and practice standards to rapidly churn out prescriptions. Pharmacare must be implemented in ways that maintain standards while broadening access.
Canada’s attempts to bring down the prices of patented drugs have failed since 1987. In 2017, the Minister of Health announced enhancements of the Patented Medicines Prices Review Board (PMPRB) that were supposed to lower drug prices and pave the way to pharmacare. Since then the PMPRB has been hobbled and drug prices have taken off.Footnote 15 The correct sequence is crystal clear: implement pharmacare today to reduce drug prices and spending tomorrow. Over decades, the United States has pressured Canada to adopt a weak stance that benefits American companies that evergreen patents and Canada continues to be pulled by the ear and pay through the nose.
Pharmacare represents an opportunity to ensure the environmental impact of medicines as part of a one health approach.Footnote 16 Medicine selection decisions can take into account the fact that some, but not other, medicines have byproducts that remain in the water and air for centuries. While ensuring medicines are easily available to all from coast to coast to coast, we must protect the water, land and air which support all life.
Most of the best medicines are old. These include anti-infectives, treatments for high blood pressure, treatments for depression and schizophrenia as well as other life saving treatments that were discovered decades ago. While difficult decisions may lay ahead regarding new and expensive medicines, we can immediately provide free access to many effective ones at a relatively low cost and within a set budget. In the future, the list of covered medicines can expand using international best practices and precedents that balance limited public resources and the right to health.Footnote 17Footnote 18Footnote 19
Although pharmacare will reduce drug spending by billions in direct savings on medicines and indirectly through improved health, its rationale transcends the material. Pharmacare is about life, health and fairness. Basic human rights.Footnote 18Footnote 19
The best route forward is uphill. Bold decisiveness is needed now for pharmacare, made for and in Canada, that is resilient to real challenges. The easier option is to shuffle around in the status quo in submission to fear and misinformation.
Canada will continue to be a big spender on medicines, and we can decide to invest in Canadian innovation and productive capacity. Canada gave the world insulin, first through its discovery and then through exports from Connaught Laboratories in Toronto. However, today people living in Canada struggle to afford insulin products shipped to us from those more interested in our money than our health. Specific policy gaps have held Canada back, and we can decide to move toward a position of strength and leadership. Strong and free.
Executive summary
Millions of people in Canada do not take essential medicines because they cannot afford them. Specifically, this affects outpatients -- people who receive medical treatments but do not need to be in a hospital. Access to medicines depends on private insurance or the ability to pay out of pocket, making it inequitable or unfair.
The absence of a national and coordinated approach for access to medicines is unacceptable. Change is overdue.
This report presents a comprehensive framework for universal, single-payer, publicly administered pharmacare in Canada. It is grounded in the recognition of access to essential medicines as a human right and will:
- close gaps in medication access
- reduce health disparities
- improve health outcomes
- save billions of dollars
Pharmacare legislation, by the end of 2023, was part of the Delivering for Canadians Now agreement announced by the Prime Minister on March 22, 2022.Footnote 5 On October 10, 2024, An Act Respecting Pharmacare came into force.Footnote 6 The act laid the legal and policy groundwork for a universal pharmacare system, starting with contraceptives and diabetes medications and working towards a more comprehensive formulary. The act also created a committee of experts to “make recommendations respecting options for the operation and financing of national, universal, single payer pharmacare.”Footnote 6
The committee has taken an open-minded approach to its mandate. Committee members looked at a wide range of options, considering their context and history. They wanted their work to build on and complement existing work, including:
- a 2018 parliamentary committee report titled “Pharmacare Now”Footnote 1
- a 2019 National Advisory Council report on the implementation of National PharmacareFootnote 21
The committee consulted with people who have different perspectives on the operation and financing of drug programs. They reviewed large amounts of data, including summaries of government data not usually available. Committee members completed a robust review of domestic and international sources and considered the history of health policy in this context. They considered different approaches for drug coverage in Canada as well as internationally and heeded international guidance.
The committee found that the current approach to access medicines in Canada undermines the right to health as well as Canada's identity as a nation committed to equity and universal care. The committee rejects the idea of a limited "fill-the-gaps" model and instead advocates for universal, single-payer, publicly administered pharmacare based on human rights.
The committee concluded that a national pharmacare system will improve health outcomes and reduce disparities and optimize existing public investments in medicines. It will streamline access, harmonize data collection, enable efficacy and cost evaluations, stabilize supply chains, and could support domestic manufacturing. With anticipated system-wide savings, governments could gain flexibility to reinvest in services like primary health care and community-based supports - in particular, services prioritized by Indigenous Peoples and underserved populations.
These 8 recommendations offer a detailed roadmap to guide the federal government in operating and financing pharmacare that is rights-based, evidence-informed, and aligned with Canada’s legal and treaty obligations.
The committee urges federal leadership to act now.
Summary of recommendations
Recommendation 1
The federal government should quickly advance new legislation explicitly recognizing the right to essential medicines - building upon the Canada Health Act of 1984, the United Nations Declaration on the Rights of Indigenous Peoples Act of 2021 and the 2024 Act Respecting Pharmacare – defining exactly how the policy provides universal, first-dollar coverage through a single-payer and publicly administered plan that is equitable and fair.
The government should enact federal legislation that formally recognizes access to essential medicines as a human right, grounded in Canada’s constitutional obligations and international commitments. New legislative provisions should:
- establish universal, single-payer, publicly administered pharmacare with equitable first-dollar coverage
- include specific implementation criteria tied to federal funding
- advance reconciliation through the integration of Indigenous rights and treaty obligations
- ensure consistent access to essential medicines across all jurisdictions in Canada
Recommendation 2
The federal government should fully fund a list of essential medicines, ensuring free access for all people living in Canada through existing processes, such as provincial and territorial health cards.
The federal government should commit to fully funding a core list of essential medicines to ensure free and equal access across all provinces and territories, using existing public health infrastructure. This single-payer approach avoids the drawbacks of bilateral agreements, enshrines universal access and strengthens the ability to negotiate drug prices with drug companies.
Pharmacare should be delivered through existing provincial, territorial, and federal drug plans using current health card systems, allowing seamless access with no user fees. Pharmacare should serve as the first payer for essential medicines, while respecting Indigenous health sovereignty and enabling portability between jurisdictions in Canada. Private insurers may offer complementary coverage, as is done in other international jurisdictions.
Recommendation 3
The federal government should use international best practices to establish an independent body that maintains the list of essential medicines to be publicly funded for everyone in Canada. This independent body should be free from financial conflict of interests.
The federal government should use international best practices to establish an independent body that maintains the list of essential medicines to be publicly funded for everyone in Canada. This independent body should be free from financial conflict of interests.
An independent, conflict-free body should be created to evaluate and maintain the essential medicines list based on public health needs. This body should:
- be above and free from political influence
- reflect Canada’s diversity
- prioritize primary health care perspectives
- adopt a transparent, evidence-based process aligned with international standards like those from the World Health Organization
Recommendation 4
The Federal government should develop a national essential medicines strategy that ensures affordability and accessibility. By implementing competitive procurement and strategic financing agreements, Canada can strengthen its healthcare system, safeguard supply chains, and promote domestic pharmaceutical production.
The government should adopt a multi-dimensional strategy to optimize access, cost-efficiency, and patient safety for essential medicines. Components should include:
- a competitive procurement strategy, including tendering to lower prices
- cost oversight and cost containment tools for patented drugs
- sustainable drug distribution, pharmacy compensation and rural access
- safeguards against medicine shortages
- evidence-based prescribing
- data monitoring
This integrated approach will stabilize supply chains, address inequities and deliver better health outcomes at lower cost.
Recommendation 5
The federal government should fully fund the initial list of essential medicines through various revenue-generating measures that are fair, neutral and efficient.
Pharmacare, like other national initiatives, can be fully funded through general federal revenues. If needed, equitable options include revisiting tax exemptions for private drug plans or adjusting insurance sector taxation. These measures promote fairness without introducing regressive taxes.
Recommendation 6
Indigenous Peoples must be at the forefront of a monitoring and evaluation plan to assess the impact of pharmacare on access to medicines. First Nations and Inuit representatives should decide how saving from the Non-Insured Health Benefits program will be reinvested into Indigenous health priorities.
Pharmacare will reduce spending through the Non-Insured Health Benefits program. This presents an opportunity for Indigenous determination to redirect the budget to ensure equitable, culturally appropriate health care for Indigenous Peoples. Redirecting funding in this way will support Indigenous-led systems and address the harms of colonial health policies without risking treaty rights.
Recommendation 7
The federal government should immediately meet with provincial and territorial governments to agree on specific plans for improving primary health care and pharmacy services. They should focus on services that ensure access and appropriate use of medicines that will be supported using provincial and territorial savings from pharmacare.
Provinces and territories should reinvest pharmacare-generated savings into primary health care expansion, rural pharmacy services, equitable distribution systems, and public infusion clinics. Without access to prescribers and pharmacy support, the full potential of pharmacare cannot be realized, especially for underserved populations.
Recommendation 8
Data on health outcomes (including mortality, morbidity and disparities) and prescribing patterns should be continuously and rapidly acted upon by health system partners and practitioners to improve care. Annual reporting to the United Nations Committee on Economic, Social and Cultural Rights will demonstrate Canada’s commitment to advancing the right to health.
Pharmacare’s impact on health outcomes, disparities, affordability, prescribing patterns, and systemic efficiency should be systematically tracked by CIHI annually. Patients, Indigenous communities, prescribers and pharmacy stakeholders should be engaged in ongoing evaluation. Transparent reporting (domestically and to the UN) will demonstrate Canada’s commitment to the right to health.
Introduction
Today, millions of people in Canada do not take essential medicines because they cannot afford them and this problem is much less common in comparable countries.Footnote 22Footnote 23Footnote 24
Health inequities create avoidable differences in health outcomes, and in Canada there are inequities in access to life saving medicines. Older adults, those with a low income, women, Indigenous Peoples and racialized people are more likely to not take a medication due to the cost.Footnote 25Footnote 26Footnote 27Footnote 28
Health issues become more common as people age, so the ability to access medically necessary and appropriate medications becomes increasingly important. The majority of people in Canada 65 years and over are currently living with at least one chronic disease, while a growing number are living with multiple diseases. In fact, a recent report found that 25% of older adults in Canada in 2016 were prescribed medications belonging to 10 or more medication classes. Older adults typically receive some level of provincial or territorial support for access to prescription medications. However, provincial and territorial drug plans for older adults vary across Canada. In most cases, co-pays and deductibles are still in place, which can reduce access.Footnote 29
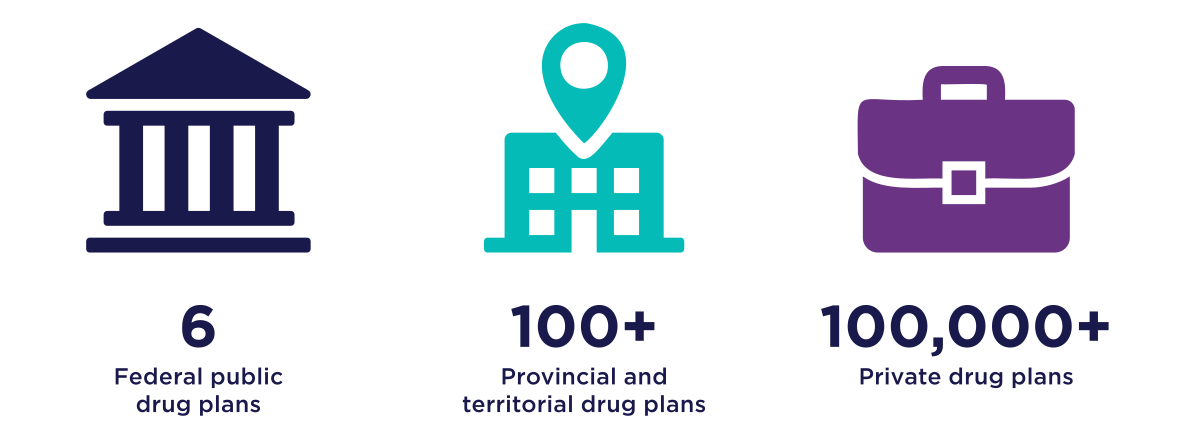
Figure 1: Text description
A series of three graphics that illustrates the number and type of existing drug plans in Canada.
- The first graphic shows there are 6 federal public drug plans.
- The second graphic shows there are over 100 provincial and territorial drug plans.
- The last graphic shows there are over 100,000 private drug plans.
Other factors that should not affect access to essential medicines effectively determine access in Canada. Being a newcomer (versus being born in Canada) and being separated or divorced (versus married) are factors associated with lower access to health insurance and essential medicines.Footnote 26 Racialized people are less likely to have private insurance, and women are less likely to be able to afford medicines whether or not they have private insurance.Footnote 26
Medicine access policy is also rooted in colonial processes that unfold in unfair access for Indigenous Peoples. Due to historical and contemporary segregation, underfunding, and jurisdictional gaps in the Canadian health care system, Indigenous people have among the lowest rates of access to medicines and access to care.Footnote 26Footnote 27Footnote 28
Inequities are caused both by a lack of universal public pharmacare and a reliance on privately administered drug plans to mitigate coverage needs. These plans are inequitably available and often tied to employment. These inequities are related to discrimination in hiring, and promotion and employment practices.Footnote 29
Discrimination in employment is often based on gender, racialization and having a disability.Footnote 30 Women get paid less than men, and women have worse access to medicines.Footnote 26Footnote 27Footnote 28Footnote 29Footnote 30Footnote 31 Racialized people are less likely to be promoted, and they are more likely to report not being able to afford medicines.Footnote 32 An intersectional lens shows that Indigenous and racialized women are the most disadvantaged.Footnote 31
None of this should be allowed to happen. Access to health care is meant be a right for all people living in Canada. The absence of a national and harmonized approach for access to medicines can no longer be an accepted standard.
Over 150 countries have an essential medicines list, but Canada does not.Footnote 33 The federal government has a responsibility to ensure that people are not harmed due to poor access to essential medicines. Government currently spends billions of dollars each year supporting systems that do not adequately provide equitable access to necessary medicines. Since every person has rights, every person should have access to essential medicines. Essential medicines meet the priority health needs of the population and should always be available in a functioning health system.Footnote 33
In Canada, the right to health reflects a vital factor in our collective identity. Through this report and its predecessors, the inequity of access to medicines weakens and threatens this right in its intention and thus weakens the core identity of what it means to be Canadian.
A rights-based approach has crucial implications for national pharmacare. Enshrining federally led national pharmacare improves the health rights of people living in Canada, adding robustness, sustainability and strength to meet our nation’s future health care needs.
Some have argued that there is no need for Canada to establish a universal, single-payer, publicly administered prescription drug program. They note that most people living in Canada have access to some level of drug coverage and that insurance gaps are typically small and geographically concentrated. They advocate for a “fill-the-gaps” pharmacare model that would take care of the uninsured and under-insured and will purportedly cost less.
The committee of experts recognized and respected this feedback as they deliberated on recommendations for operating and financing national pharmacare. However, the committee’s consensus was that all people living in Canada should have access to essential medicines, no matter their identity or employment circumstances. As such, the committee has firmly anchored its recommendations on the right of every person in Canada to have equal and equitable access to essential medicines.
The committee further investigated the financial risks associated with the current fragmented approach to drug coverage. They determined that a national strategy would be transparent in managing the high expenditures already being invested in providing access to medicines. They saw fundamental inefficiencies that threaten the sustainability of the existing model. It is critical for federal leadership to participate in partnerships, negotiations and planning to optimize investment.
The committee viewed implementing universal, single-payer, publicly administered pharmacare as an investment in risk management for government. It allows government to monitor, evaluate and continuously improve upon access to medicines. This will allow for:
- streamlined and standardized access to essential medicines
- centralized and harmonized prescribing and dispensing data
- independent evaluation of effectiveness and cost of effectiveness of essential medicines and the pharmacare itself
- opportunities for provincial and territorial governments, and NIHB end users, to reinvest direct and indirect savings to improve existing federal, provincial and territorial drug plans so that they can better meet the distinct needs of the patient populations
- a sustainable supply chain that will:
- strengthen domestic distribution
- meet the needs of urban, remote and rural communities
- be responsive to communities that may be impacted by climate disasters or other emergencies
- opportunities to work with other countries on a national basis to address challenges within the pharmaceutical ecosystem
- an ability to renegotiate with manufacturers and suppliers in the event of distribution or manufacturing challenges, including navigating geopolitical or climate barriers to medications access for people in Canada
The committee anticipates that national pharmacare will improve health outcomes and result in savings for all drug insurance plans, including existing federal, provincial, territorial and private drug plans. These savings will free up significant resources that can be repurposed to support improved access to primary health services and health interventions to better meet the needs of all people living in Canada. These include mental health, care for seniors (including community home and long-term care) and palliative care. To this end, the committee encourages the federal government to work with Indigenous Peoples and provincial and territorial governments to create priorities and strategies to optimize this reinvestment opportunity for the health system.
Recommendations and explanations
The committee’s 8 interconnected recommendations apply a rights-based approach to including medicines in Canada’s publicly funded and administered health care system.
Recommendation 1
The federal government should quickly advance new legislation explicitly recognizing the right to essential medicines - building upon the Canada Health Act of 1984, the United Nations Declaration on the Rights of Indigenous Peoples Act of 2021 and the 2024 Act Respecting Pharmacare – defining exactly how the policy provides universal, first-dollar coverage through a single-payer and publicly administered plan that is equitable and fair.
Nations must protect rights and take steps toward their realization with an urgency that reflects the fundamental nature of human rights.Footnote 10
People who need access to health services should not be refused care due to an inability to pay. Similarly, access to essential medicines should be guaranteed and viewed as a human right.Footnote 34 This right is recognized in Canada as it is around the world.Footnote 21Footnote 34
Canada is a party to the International Covenant on Economic, Social and Cultural Rights (ICESCR) that recognizes essential medicine provision as a core obligation under the right to health.Footnote 8 In September of 2024, the Government of Canada responded to questions about the right to health from the United Nations Committee on Economic, Social and Cultural Rights by outlining progress being made toward national pharmacare.Footnote 9 In April of 2025, Canada supported a motion at the United Nations Human rights council that repeatedly affirmed the need to realize the right to health.Footnote 18
The 2024 Act Respecting Pharmacare recognizes that “quality health care, including access to prescription drugs and related products, is critical to protecting the health and well-being of Canadians”. It also recognizes that multiple government reports have recommended establishing “universal, single-payer, public pharmacare in Canada”.Footnote 6
Governments in Canada currently spend billions of dollars supporting various public and privately administered drug plans that leave some with poor access to essential medicines. This goes to the core of the responsibility of government under the Charter of Rights and Freedoms.
One might argue that government interventions that support access to medicine only for some are discriminatory under the Charter.Footnote 35Footnote 36 Such supports include substantial direct and indirect federal funding through tax exemptions: for example, for those with privately administered plans. The Government of Canada makes substantial investments in protecting human rights internationally, including with respect to reproductive health access.Footnote 37Likewise, similar political will should be applied to essential medicine access in Canada where people have the same human rights.
With respect to Indigenous Peoples, in 1876 the Government of Canada both promised a Medicine Chest in Treaty 6. At the same time, they also investing heavily in colonial projects by passing the Indian Act in a transparent attempt to strip Indigenous Peoples of their rights.Footnote 10Footnote 38 Treaty 6 applies within and beyond Treaty 6 territory, crossing multiple jurisdictions. The Medicine Chest was also promised as parts of multiple Treaties including 7, 8, 10 and 11 as well as others.Footnote 39
The importance of these Treaties was enshrined in Section 25 of the Charter of Rights and Freedoms of 1982 that references the 1763 Royal Proclamation.Footnote 40 The United Nations Declaration on the Rights of Indigenous Peoples Act (UNDRIP Act) of 2021 affirms the UNDRIP as an international human rights instrument applicable in Canada. This makes it abundantly clear that the right to health (that is explicitly mentioned in UNDRIP) applies here and now for Indigenous Peoples.Footnote 41 This includes access to medicines.
Legislation
Pharmacare could be implemented within the existing legislative framework without new or amended legislation. However, committee members agreed that the preferred approach is for government to quickly enact more robust legislation that clearly and explicitly recognizes the right to medicine and the relevance of the UNDRIP Act.
The new legislative provisions should:
- articulate the federal government’s overall commitment to supporting universal, single-payer pharmacare
- integrate clear criteria on how it will be publicly administered and by whom
- charge the federal Minister of Health with enforcing those criteria so that provinces and territories receive federal funding for essential medicines
New pharmacare legislation should enable the federal government to transfer funds to provincial and territorial governments that provide access to a list of essential medicines. This would be similar to Canada Health Transfers made according to the Canada Health Act (a federal law that requires provinces and territories to meet certain requirements to access federal funding). This would assure provincial and territorial governments that if they meet certain requirements, they will receive adequate funding. This funding would allow all jurisdictions to consistently and sustainably administer access to essential medicines.
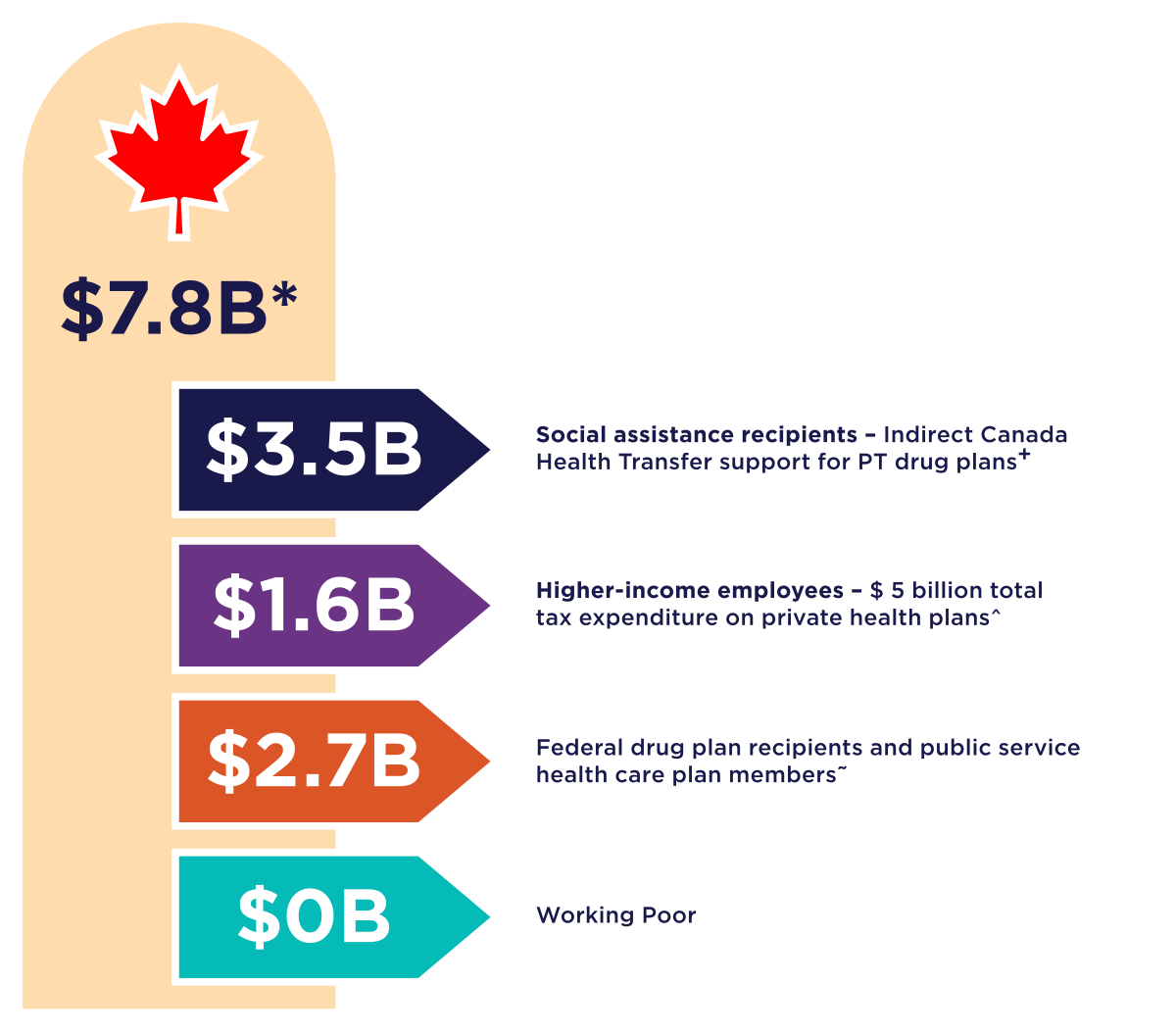
Figure 2: Text description
A vertical graphic showing the amount of federal funds that support medicine access.
- The first graphic indicates total funds of 7.8 Billion (all numbers are approximate).
- Underneath is a black arrow indicating 3.5 Billion for social assistance recipients – the committee’s estimate of indirect Canada Health Transfer funds that could support PT drug program services.
- Next is a purple arrow indicating 1.6 Billion for higher income employees – Estimated 2025 costs for non-taxation of benefits from private health and dental plans is $5B. In 2023, 32% of private plan pay-outs were for drug claims.
- Next is a red arrow indicating 2.7 Billion for Federal drug plan members, including Non-Insured Health Benefits, Veterans, RCMP, Correctional Services Canada, Interim Federal Health Program, Canadian Armed Force and public service health care plan members.
- Lastly a green arrow indicating 0 Billion for the working poor.
These new provisions would underpin the extension of Canada’s publicly funded health care system, supported by the pillars of the Canada Health Act principles. They would also provide an opportunity for government to further implement UNDRIP by applying a distinction-based approach to demonstrate the value of respecting Indigenous knowledge systems in major policy and legislative changes. This would ultimately serve to improve health for everyone, including Indigenous people living in urban settings who currently face the most obstacles to obtaining essential medicines. This is because pharmacare designed to support Indigenous people without status will by its nature support others with poor access.
Recommendation 2
The federal government should fully fund a list of essential medicines, ensuring free access for all people living in Canada through existing processes, such as provincial and territorial health cards.
Federal funding
Pharmacare should be built upon a rigorously developed and updated list of essential medicines. The list would define the minimum medicine coverage to be eligible for federal funding.
Providing a list of medicines for free is based on international guidance and has been shown in a Canadian study to:Footnote 2Footnote 4Footnote 42Footnote 43Footnote 44Footnote 45Footnote 46
- improve health outcomes
- make it easier to afford necessities like food
- reduce overall health care costs
- be acceptable to patients, clinicians and decision makers
The total cost of publicly funding a list of essential medicines will likely be between $6 to $10 billion dollars (see Appendix 2).
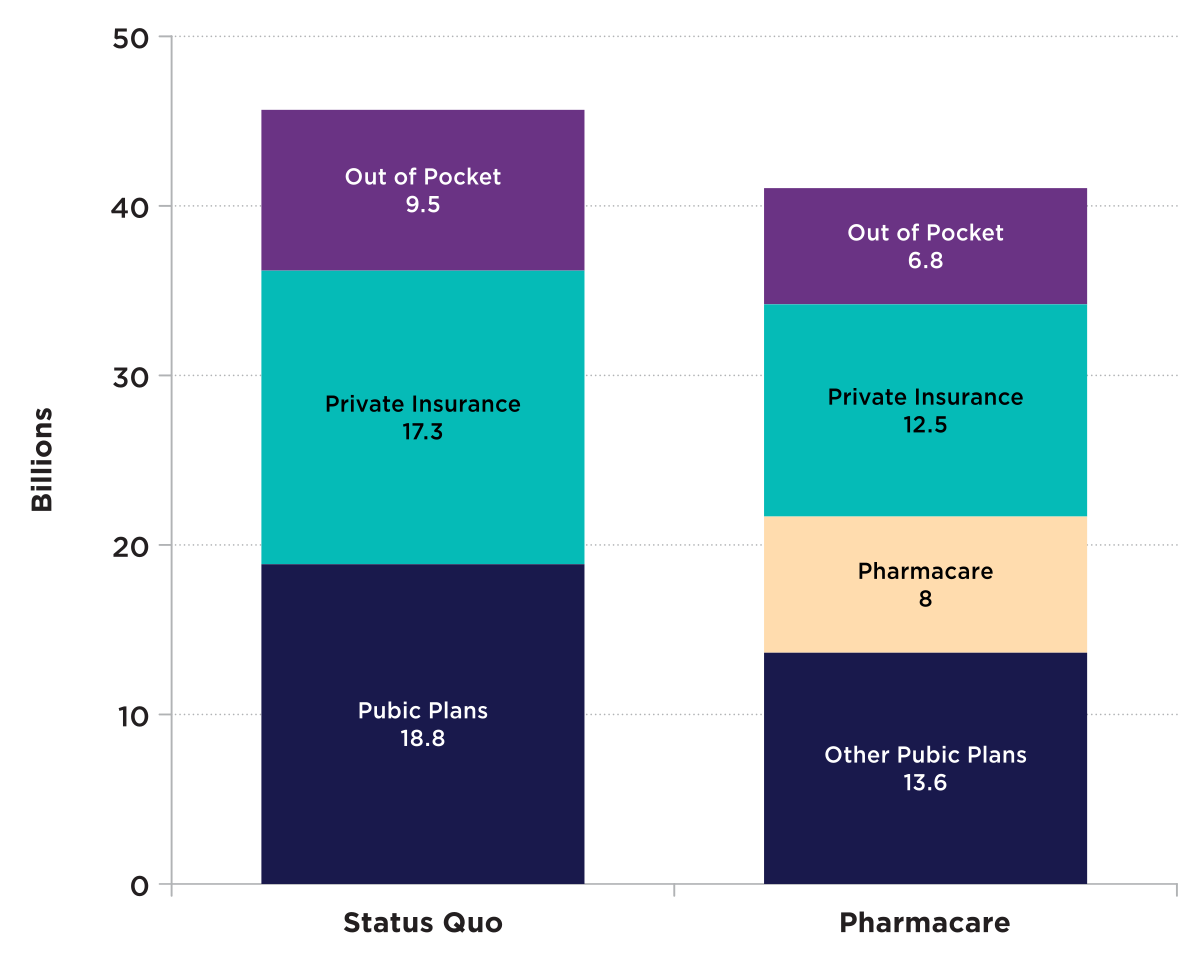
Figure 3: Text description
| Status Quo | Pharmacare | |
|---|---|---|
| Out of pocket | 9.5 | 6.8 |
| Private Insurance | 17.3 | 12.5 |
| Public plans | 18.8 | 13.6 |
| Pharmacare | 0 | 8 |
Source : Source : Committee estimations using data from IQVIA Solutions Canada, the Canadian Institute for Health Information and reports from the Parliamentary Budget Officer
The committee recommends that the federal government be entirely responsible for paying for the list of essential medicines. Full federal funding will ensure that the right to essential medicines is protected for everyone in Canada, regardless of the province or territory in which they live.
The committee’s position is that the federal government’s responsibility is to initially protect the right to a minimal standard of essential medicine. This responsibility should not be devolved to provincial and territorial governments. Similarly, the outcome of bilateral negotiations should not in any way prevent the federal government from meeting their responsibility to provide a minimum standard of access to essential medicines. Once essential medicines are funded, it will fall to the provinces and territories to administer pharmacare within their respective jurisdictions.
The federal government’s role in funding access to medicines is clear in law, in policy, and in reality.
The supreme court summarized the overlapping roles of different levels of government in health:
“In sum ‘health’ is not a matter which is subject to specific constitutional assignment but instead is an amorphous topic which can be addressed by valid federal or provincial legislation, depending in the circumstances of each case on the nature or scope of the health problem in question.” (Schneider vs British Columbia, 1982).
The 2024 Act Respecting Pharmacare clarifies that the federal government has a role in funding medicines. It also speaks to the importance of cooperation with provincial and territorial governments.Footnote 6 Cooperation between different levels of government in providing access to medicines has been recommended in multiple reports,Footnote 6Footnote 45 mostly in the form of shared funding.Footnote 21Footnote 47 To date those recommendations have not resulted in national pharmacare. In contrast, initiatives fully funded by the federal government that provide dental care, childcare and other services administered by provincial and territorial governments have all been successfully implemented within short time frames.
The federal government should commit to fully fund a list of essential medicines, rather topping up provincial and territorial funding for increased drug plan coverage for universal, single-payer, first-dollar coverage.
This would ensure the Canada Health Act’s principles of public administration, comprehensiveness, universality, portability and accessibility are upheld by providing sustainable funding for a consistent list of medicines across jurisdictions. It would also immediately reduce current provincial and territorial expenditures for those same medicines. The provinces and territories could then be asked to commit to reinvesting these direct savings to enhance related primary health care services to meet the needs of their residents (see Recommendations 3 and 4).
Before making this recommendation, the committee considered other funding options, such as:
- providing provinces and territories with a proportion of the funding based upon their current share of drug expenditures for covered medicines
- contributing half of the total cost of covered medicines
These options would require the type of bilateral funding agreements which have been proven to be challenging and time consuming, as demonstrated by implementation of the 2024 Act Respecting Pharmacare.
At the time of writing, only four of the 13 Canadian jurisdictions – BC, Manitoba, PEI and Yukon – have reached agreements with the federal government to provide access to just two classes of medicines, contraceptives and diabetes treatments, and even those agreements have inconsistencies in what medicines are included and how they are covered. In those agreements, on average the federal contribution is approximately 70 percent of total expenditures on included medicines.Footnote 48
Using a bilateral approach to implement universal, single-payer, first-dollar coverage for a list of essential medicines would undoubtedly prove to be much more challenging. It would also likely further erode the ability of pharmacare to address universality and portability inequities. If bilateral funding negotiations were to proceed, respective governments would be joint funders. As such, they would be entitled to take part in selection and procurement activities related to the medicines to be included on the essential medicines list. A lack of consensus on these matters could lead to further disparities in coverage across jurisdictions.
Disparities in coverage and its timing created via bilateral agreements can also have an impact on drug prices, as demonstrated by the existing bilateral approach. Drug companies contend that they are able to provide larger drug price rebates to public drug plans because they can command higher prices from private payers. If private insurers decide to delist medicines that are identified in one or only a few bilateral agreements, the rebates offered to those jurisdictions may be reduced. Building pharmacare around a single federal list of essentials medicines allows pharmacare to capitalize on economies of scale. Of course, provincial and territorial governments are free to publicly fund additional medicines.
Eliminating the complex and political nature of negotiations required for shared funding models in the early stages of pharmacare implementation will increase the likelihood that the foundational principles will be acceptable to all governments. These principles concern the right to medicine, universality, accessibility and portability of coverage. Providing full funding for all essential medicines on the list will also avoid penalizing provinces and territories that have already enhanced access to some essential medicines.
The funding model for pharmacare should be straightforward. It should be based upon federal reimbursement for essential medicines dispensed within the province or territory. It should also be supported by an appropriate medicines and prescribing strategy that ensures optimal care.
Progressive realization
The committee’s recommendation is to fully implement pharmacare now. The right to essential medicines must be progressively realized and always focused on medicines commonly prescribed in primary care. Progressive realization means that the federal government is obliged to use the maximum available resources to develop and implement rights-based legislation and needed policies to implement pharmacare.Footnote 49
The concept of progressive realization recognizes resource constraints, and a rights-based approach means constant and inexorable progress. It does not allow for finding excuses or pretexts for further delays in implementing pharmacare despite past recommendations and promises.Footnote 5Footnote 6Footnote 10Footnote 1Footnote 21 Progress toward realizing the right to health should be reported annually to the United Nations.Footnote 7 The report prepared by the first anniversary of these recommendations being tabled in parliament should describe the provision of a list of essential medicines to all residents of Canada for free through a single-payer and publicly administered plan.
Future expansions of national pharmacare
Lists of publicly funded medicines tend to grow over time. Adding medicines to the list of medicines included in national pharmacare will help to ensure people have access to needed treatments.
The committee’s recommendation for the federal government to fully fund medicines included in pharmacare would not necessarily apply to all future expansions of the list of covered medicines. The federal government should guarantee permanent and ongoing full funding for medicines included initially and those medicines added during the first 2 years.
As pharmacare is evaluated, a longer list of medicines may be considered for inclusion and funding agreements may have to be established based on:
- the actual direct and indirect savings realized by provincial and territorial governments
- factors such as the rubric of listing decisions and price negotiations that have been achieved
- this is further elaborated upon in Recommendation 3
Such cost sharing arrangements could be triggered based on:
- time (e.g. for medicines added more than 2 years after pharmacare is implemented)
- federal spending (e.g. when federal spending on pharmacare crosses a threshold)
- the share of federal spending on medicines
Administration of pharmacare
The current patchwork of drug insurance coverage in Canada complicates access for patients and administration for providers. Many providers and patients alike demand modernization of Canada’s publicly funded health systems and simplified access to essential health needs. The current system includes overlapping programs and plans, which leads to duplicative drug coverage. This results in challenges in coordinating coverage across different plans, and subsequent idle investment which could be redistributed to those with little to no coverage.
Federally funded coverage for a list of essential medicines should be administered through existing public drug programs to simplify coverage.
Federal, provincial and territorial (FPT) drug plan managers have indicated that existing program infrastructure can already accommodate free access to certain medicines. For example, all FPT governments provide first-dollar coverage for the abortion pill mifegymiso. Some, like BC and Manitoba, also provide first-dollar coverage for certain contraceptives. Additionally, provinces that have implemented bilateral agreements for diabetes medicines and contraceptives have not had challenges administering free access using their existing systems and provincial health cards. On the front line, pharmacists have indicated that they inevitably experience reduced administrative burden with the application of first-dollar, zero copay plans.Footnote 50
With pharmacare, provincial governments would use existing administrative processes for drug-specific first-dollar coverage. This shifts all brand name and generic products on the essential medicines list into a category or plan where there is no charge. Pharmacare would then be the first payer for those medicines, providing 100% of drug and dispensing cost for all residents with zero deductibles or co-payments.
Beneficiaries of other drug plans will continue to access medicines not covered by pharmacare, as per the existing plan rules and eligibility requirements of those plans. Examples of these plans include federal plans like the Non Insured Health Benefits (NIHB) program, and provincial and territorial drug plans.
With respect to beneficiaries of private drug plans, the proposed pharmacare legislation would be perfectly compatible with private insurance, unlike the Canada Health Act, which:
- ensures that medically necessary hospital and physician services are funded only publicly
- bans private payments for medically necessary health services
Private insurance companies and drug plan sponsors could continue to offer coverage for essential medicines in addition to other medicines and health benefits if they so choose.
This is similar to other countries with universal first dollar public drug plans where private insurance typically plays a supplementary role. Many employers in these countries continue to offer private drug plans as part of a competitive compensation packages that bundle drug coverage with other health benefits like dental, vision care and other paramedical services. These plans may include drugs listed on the public formulary as well as non-formulary drugs, and often offer quicker access to new medicines or cover brand-name drugs when the public plan only covers generics.Footnote 51
In the past, Canada’s insurance industry has expressed support for the concept of government implementing a pharmacare formulary based on essential medicines: “There have been some interesting discussions around a national formulary based on the WHO definition of essential medicines…It can be done quickly, and we can all get behind it.”Footnote 52
Effecting first dollar coverage within existing public drug programs will be relatively simple. However, the committee recognizes that drug plan managers may want to assess the impact that moving to the essential medicine formulary may have in terms of beneficiaries reaching deductibles, co-payments and annual family maximums.
The committee is not aware of any scenario where pharmacare would result in negative consequences for existing beneficiaries. We recommend that a robust monitoring, evaluation and engagement strategy be put in place to rapidly identify and mitigate any unintended consequences.
The committee also recognized that there may be costs incurred by provinces and territories in adapting existing drug plan administrative infrastructure to accommodate pharmacare. Public drug plans should be provided with an opportunity to submit funding requests to use drug plan savings incurred by pharmacare to “operationalize” pharmacare. This may include support for human resources, IT and communication needs.
Pharmacare should be simple and universal. Simplicity means that pharmacare is easy for members of the public to understand and use. Every resident of Canada will be eligible for the pharmacare by using provincial or territorial health card numbers or other public identifying numbers, such as NIHB or refugee immigration card numbers. This approach seems natural since pharmacare will bring medicines for outpatients into the existing publicly funded health care system. This approach uses existing administrative structures and thus would be the easiest to implement, especially in the short term.
Considerations related to free access to essential medicines apply to Indigenous Peoples on the same terms as others. However, there are several special considerations which must recognize the inherent, international and Treaty Rights of Indigenous Peoples, including the rights to self-determination in health. Many non-Status First Nations, non-registered Inuit, and Métis do not have any access to medicines via the NIHB program. Pharmacare will represent a substantial improvement in access for Indigenous people who do not currently have access to a drug plan.
Pharmacare, as the first payer for essential medicines for all people living in Canada, will improve access for all people without coverage, including non-status Indigenous people and Métis. However, the committee is aware through its consultations that Indigenous Peoples expect the federal government to fund Indigenous-led health services. Indigenous Peoples may also perceive the administration of federally funded pharmacare by provincial governments as a dereliction of this relationship. Further consultation will be required to ensure concerns raised by Indigenous Peoples in this regard are addressed.
Additionally, national pharmacare should provide access to medicines for residents who frequently spend extended periods outside of their home province or territory. Pharmacare should be available across Canada and its portability is important for people who spend time living in more than one province or territory.
Patients should provide prescriptions for medicines covered by pharmacare at pharmacy counters, along with a valid health card from another province or territory, or an NIHB registration number. They should then be provided with the essential medicine at no cost. Optimally an IT system would be in place to allow the pharmacy to quickly verify health card numbers at the point of care and make a claim to the relevant provincial or territorial drug plan. Portability should be phased in, as ensuring portability will require upgrades to information technology and changes to provincial legislation. Initially out-of-province claims could be compiled by out-of-province pharmacies and submitted to the province or territory at defined intervals for reconciliation.
Simplicity helps to build upon the principles of the Canada Health Act and to modernize the healthcare system. Pharmacare covers medicines for every resident. There will no longer be a distinction in coverage because the medicine is needed as a result of a workplace incident, or because a person is a Canadian Forces veteran, for example.
The committee considered other eligibility validation options, including issuing new federal cards specifically for pharmacare. The main benefit of this approach would be the ability to access medicines regardless of location in Canada. It would also allow the federal government to directly track utilization rather than relying on data provided by provincial and territorial governments that use different administrative systems.
The committee is not recommending this option, as issuing new federal cards to every person in Canada would be a substantial undertaking. It would also likely effectively exclude many people who have trouble accessing medicines now.
Recommendation 3
The federal government should use international best practices to establish an independent body that maintains the list of essential medicines to be publicly funded for everyone in Canada. This independent body should be free from financial conflict of interests.
An independent body, free of financial conflict of interests, should be established without further delay to evaluate and maintain the list of essential medicines based upon public health needs.Footnote 53Footnote 54 This independent body should be led by an executive director who considers recommendations by the committee and ultimately makes decisions on whether a medicine is listed on the pharmacare formulary. The executive director should not be allowed to communicate with elected officials regarding specific drug files. This would prevent lobbyists and others from approaching elected officials to influence decisions.
The essential medicines list can be an adaptation of the list created by Canada’s Drug Agency (CDA). However, there should be a rigorous process for adding and removing medicines from the list, based primarily on the effectiveness of a medicine and its need. The list should be a positive list, where listed medicines are available for free with no restrictions or conditions.
Anyone should be allowed to suggest changes to the list of essential medicines, but primary care providers should play a key role in deciding which medicines are listed. This is because they prescribe the most medicines in Canada, and they have expertise seeking input from specialists where needed. Those involved in listing decisions should also reflect the diversity of Canada and represent the unique health and access challenges facing the population. For example, emerging health crises, and the health impacts of colonization on Indigenous Peoples.
The list should be reviewed and updated on a regular schedule. The World Health Organization updates its Model List of Essential Medicines every 2 years and can serve as a guide for pharmacare. Ad hoc updates could be made under special circumstances, such as when COVID vaccines were made available at no cost during the pandemic.
Recommendation 4
The federal government should develop a national essential medicines strategy that ensures affordability and accessibility. By implementing competitive procurement and strategic financing agreements, Canada can strengthen its healthcare system, safeguard supply chains, and promote domestic pharmaceutical production.
National essential medicines strategy
A single, national strategy focused on essential medicines will help to coordinate efforts in a complex sector that involves multiple governmental and non-governmental institutions playing different roles. The federal government should lead the development of the strategy, working with:
- Indigenous Peoples, in partnership with Indigenous Services Canada
- provincial and territorial governments
- health care professionals, organizations and patients
The strategy should include essential medicines formulary management, procurement approaches, evaluations of effectiveness and appropriateness of prescribing, drug distribution and optimal use of medicines.
Providing a list of medicines for free is based on international guidance and has been shown in a Canadian study to:Footnote 2Footnote 4Footnote 42Footnote 43Footnote 44Footnote 45Footnote 46
- improve health outcomes
- make it easier to afford necessities like food
- reduce overall health care costs
- be acceptable to patients, clinicians and decision makers
Within 6 months of receiving this report a list of essential medicines should be publicly funded, and efforts to ensure appropriate use of these medicines should be undertaken with coordination by the CDA.
Once access is provided for the essential medicines, it is critical that a procurement strategy is pursued as soon as possible to achieve greater value for these drugs.
Reducing the cost of essential medicines
A procurement strategy should focus on reducing the costs of medicines through internationally proven approaches, such as tendering. It should use a rubric of diverse criteria to ensure value for investment and be directed by principles that serve the distinct health needs of people in Canada. In addition to price, the agility to navigate disruptions in supply chains and to support investments in domestic production should be considered by the pan-Canadian Pharmaceutical Alliance (pCPA) or an alternative entity responsible for drug price negotiations.
Drug spending per person is higher in Canada than in comparable countries such as Australia, New Zealand, Ireland, the United Kingdom, and Mexico.Footnote 53 Only a handful of countries spend more than Canada per person, such as the United States, Germany and Switzerland.Footnote 55
The approximate $45 billion spent on prescription medicines (from all payers) in 2024 in Canada is similar to the amount spent on post-secondary education and more than the total budgets of Indigenous Services Canada, Health Canada, Veterans Affairs, the Department of Industry and the Mortgage and Housing Corporation combined.Footnote 56
Total spending on medicines is equivalent to around 11% of the federal budget.Footnote 56
Spending on medicines in Canada is growing even faster than other costs with recent increases in public spending ranging from 6.4% to 7.4%.Footnote 57 The health benefits of medicines are not correspondingly increasing at this high rate. There are no reports of life-expectancy, health or satisfaction with health care increasing at anywhere near this rate.Footnote 58
Alarmingly, life expectancy in Canada has dropped in recent years.Footnote 58 This is in part due to the opioid crisis that was fuelled by investments in opioid therapies. The harms of these therapies were not anticipated due to the lack of a national medicine strategy and robust expert oversight.Footnote 59Footnote 60
The value of medicine spending is decreasing, yet we spend more year after year. A waning return on investment which threatens the sustainability of access to essential health products cannot continue. Drug spending is increasing much faster than government revenues through taxation and other sources.
There are several related reasons for overspending on medicines in Canada. The pricing structure for both patented and generic medicines often result in an estimate of billions of dollars being left unused each year.Footnote 61Footnote 62 These funds could be reinvested into evidence-based, effective, and sustainable access to medications and primary health care services.
Escalating drug spending is mainly caused by rising drug prices, rather than differences in the medicines being prescribed or the way they are used. Newer expensive medicine makes up a substantial proportion of expenditures, and their market share continues to grow. Meanwhile, older commonly prescribed medicines that meet most medical needs represent a relatively small portion of total drug spending. The prices of these older medicines are fair and predictable.Footnote 57
Existing business models perpetuate a preference for costly pharmaceuticals over more economical, established alternatives. For instance, some private drug plan providers benefit from a commission based on the monetary value of each claim submitted, derived from the drug’s price. This creates a financial incentive for these insurers to promote higher-priced medications instead of generic substitutes or lower-cost options.
To reduce drug prices, competitive value approaches like tendering should be used.Footnote 63 Tendering can be used wherever multiple manufacturers are likely to submit bids. This seems likely for most essential medicines. For other products, such as infrequently used and relatively inexpensive single-source medicines, tendering may not reduce drug prices substantially or at all. Procurement approaches like current ones being used in Canada should continue to obtain best value.
FPT governments currently collaborate through the pan-Canadian Pharmaceutical Alliance (pCPA) to negotiate prices for brand and generic drugs for public drug plans.Footnote 64
In addition to negotiating the price of brand-named drug products, the pCPA’s current approach is to tie the price of generic products to a percentage of the list price of the brand product. This approach recognizes that generic drug manufacturers do not fund innovative medicines research or marketing, even if they are invested in the development of generic molecules.
The pCPA uses price thresholds for commonly prescribed generic medicines for which there are several manufacturers (generally 25% of the brand price).Footnote 65
Generic drug prices in Canada indirectly include the cost of supplemental pharmacy operations and services in the form of professional allowances. These allowances are allocated by manufacturers to drive market share in pharmacies. They incentivize pharmacy chains, groups or independent owners to choose to stock one generic company’s product over others. As well as inflating prices, this business practice causes inequities in the funding of pharmacy services from one dispensing site to the next. This is because funding is negotiated with pharmacy distributors on a case-by-case basis. Investment of these professional allowances by pharmacies is neither monitored nor regulated and does not necessarily result in investments in pharmacy services. Professional allowances also shift in response to external pricing and other industry pressures, adding further uncertainty to the funding of essential pharmacy services.Footnote 66Footnote 67
Some countries that see much lower drug spending use competitive tendering processes to achieve lower drug prices. New Zealand is an example of a country that has implemented tendering despite prior concerns about shortages and industry exiting a relatively small “market” that is an ocean away from some manufacturers.Footnote 68Footnote 69 New Zealand is just one example; many countries use tendering to lower drug prices.Footnote 70
Since 2018 in Canada, the pCPA has reached agreements with generic manufacturers to lower prices in deals reported to save billions of dollars. In exchange, the pCPA has not implemented tendering processes to achieve lower prices. These savings, however, are presumably less than the savings that would be achieved through tendering. The agreement to avoid competitive tendering processes expires in 2026.Footnote 65
For the relatively small number of medicines on the essential medicines list protected by a patent, a variety of measures may be used to reduce drug prices.
Brand name drugs are theoretically priced to allow a pharmaceutical company to recover the investment in the research and development required to:
- bring the product to market
- promote the drug through marketing strategies
The price is intended to allow the manufacturer to make a reasonable profit during the time in which the patent is protected.
There are some measures in place to protect the public from overpriced brand medicines. The Patented Medicine Prices Review Board (PMPRB) was established to ensure that patented drug prices in Canada are not excessive. It reviews the pricing information provided by pharmaceutical companies and sets limits on the prices they can charge for patented medicines. It does not, however, ensure drugs are priced to be cost-effective and recent attempts to do so have failed.
In 2017, the Government of Canada announced changes to the way patented medicine prices were to be regulated with the intention of making them more affordable.Footnote 71 This was announced as action that would pave the way for pharmacare. First drug prices would come down, and then medicines would be included in our publicly funded health care system.Footnote 72 After 8 years and a court challenge of the announced reforms, little progress has been made in bringing down patented drug prices.Footnote 73Footnote 74
Historically, the PMPRB has assessed the price of patented drugs in part on industry commitments to reinvest a percentage of revenue into domestic research and development, so they bring innovative therapies to Canadians. However, PMPRB’s reporting has consistently shown that these R&D targets (typically set at 10% of sales revenue) are rarely met. It would seem, based on recent events – including legal challenges, internal leadership resignations and stalled guideline updates -- that this approach is not a singular solution to ensuring cost-efficacy and sustainable access for innovative medicines.Footnote 62
The pCPA currently enters negotiations with pharmaceutical companies to reduce the list price of a medicine being considered for public funding. Public drug plans receive confidential rebates that are non-transparent to the public based on volumes of sales and other factors. However, going forward, if it is determined that some essential medicines exceed a willingness-to-pay threshold in attempts to negotiate, the federal government could consider using other cost containment tools such as compulsory licensing. In compulsory licensing, a regulator determines that a price of a necessary medicine is beyond acceptable limits. They then offer the patent holder a reduced price while reserving the ability to grant other manufacturers the ability to produce the needed medicine, while paying the patent holder a reasonable licensing fee.Footnote 75
The Federal Minister of Health has requested that the CDA develop a national bulk purchasing strategy for prescription drugs and related products. That work may further inform the procurement strategy for the list of essential medicines that will make up the essential medicine’s formulary.
As the national essential medicines strategy is developed, a procurement agency, whether pCPA or otherwise, should be tasked with engaging with the manufacturers of the drugs selected for an essential medicines list to negotiate value. This may mean, if feasible, reopening existing agreements with drug companies in recognition of the economy of scale that a single, universal payer would bring to the table.
In addition to achieving lower prices for specific medicines, the committee heard from stakeholders, particularly drug plan administrators, that the strategy should consider value beyond price.
Product listing agreements should include assurances that:
- prevent supply chain disruptions
- minimize drug shortages
- support domestic production
- prevent long-term market dominance
- consider factors such as environmental impact
Once product listing agreements for the essential medicines have been established with manufacturers, pharmacies should be given time to adjust inventory before the new prices take effect.
Other guidance
One consequence of reducing the price of drugs is the impact it may have on the pharmaceutical distribution model that is currently fully or partially funded through markups on the price of drugs.
Pharmacy distributors serve as wholesalers and operate regional warehouses and deliver products to pharmacies located across the country. The ability to adequately distribute drugs to rural and remote locations is of particular concern if revenues from mark-ups tied to drug prices significantly decrease. For example, access to products requiring specific transportation standards, such controlled narcotics, temperature-controls, or those deemed to be hazardous materials may be negatively impacted by price reductions.
The committee recommends that the federal government provides predictable funding for distributors of essential medicines to maintain distribution sustainability and improve access in remote areas.
This could be achieved by offering:
- financial incentives for enhanced services to remote and rural areas
- investments in regional warehouse infrastructure
- other co-designed initiatives to offset the reduction in price related markup operating revenue
Distributors should be included in the monitoring and evaluation strategy of pharmacare’s impact to address any consequences on the supply and distribution chain and equitable access.
Pharmacy owners and operators generally rely on revenue from dispensing fees and mark-ups to compensate them for the pharmacy services they provide. Dispensing fees negotiated by public drug plan administrators are considerably lower than those reimbursed by private insurers or those charged to uninsured individuals. Pharmacy stakeholders have expressed concern that transitioning essential medicines to a universal, single-public payer system may result in a significant reduction in dispensing fee revenue. This potential decline could adversely affect the scope of services provided by pharmacies.
Pharmacare administrators should engage with pharmacy operators to determine an appropriate compensation model for essential medicines to sustain and improve pharmacy services to support primary care.
A higher dispensing fee should be applied to all prescriptions being dispensed to northern or isolated communities to enhance services to underserved populations. Freight charges for air shipments to remote communities should be reimbursed.
A northern or isolated community is defined by the Organisation for Economic Co-operation and Development (OECD) as a community where “at least 50% of its population needs to drive 60 minutes or more to reach a populated centre with more than 50,000 inhabitants.” For the purposes of considering access to medicines, the definition should describe a community with access as being one which is within 1 hour to a populated centre which has a fully operational pharmacy. A fully operational pharmacy is one that is eligible to receive daily orders from distributors and operates a minimum of 40 hours per week.
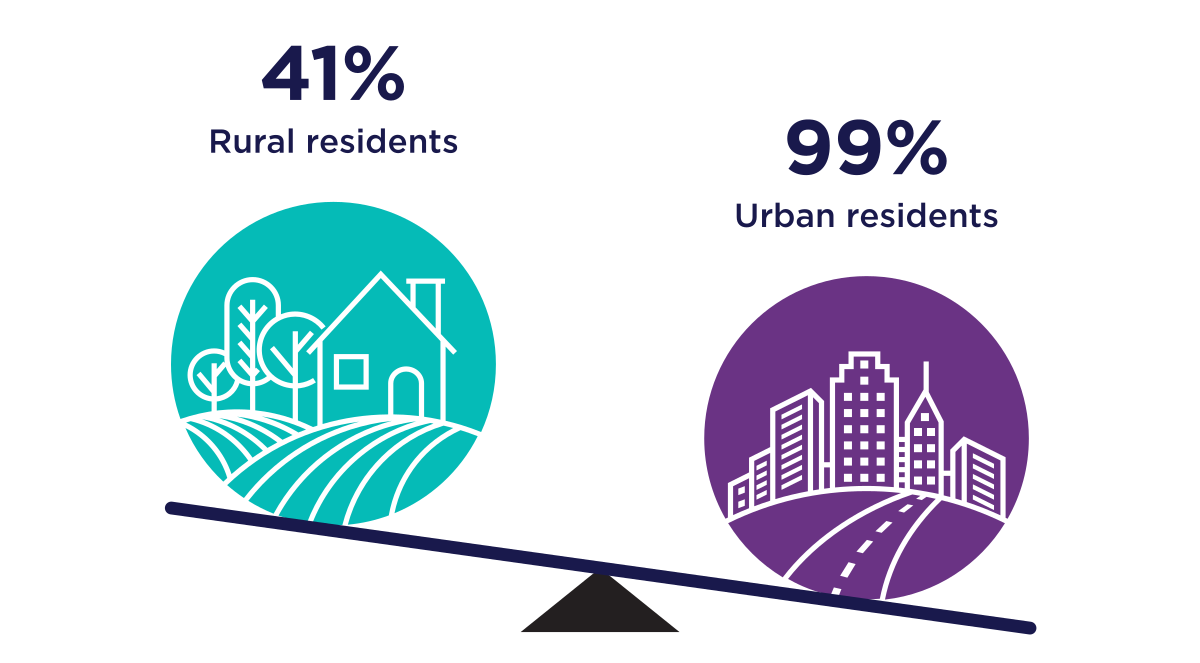
Figure 4: Text description
A series of two graphics that illustrate the percentage Ontario residents living within 5km of a pharmacy.
- The first graphic shows 41% of rural residents live within 5km of a pharmacy
- The second graphic shows 99% of urban residents live within 5km of pharmacy
Source : Timony, P, Houle SKD et al (2022) Geographic distribution of Ontario pharmacists: A focus on rural and northern communities
Any changes to distribution, dispensing or other funding structures for dispensing which are implemented for essential medicines within pharmacare should be mirrored in the NIHB program. This would ensure equitable access and prevent preferential provision of service to beneficiaries who have more robust reimbursement. This is just one step in addressing anti-Indigenous systemic racism experienced by patients within segregated drug benefit plans.
Protecting the supply of medicines and avoiding shortages
The essential medicines strategy should include plans to avoid and mitigate the effects of drug shortages. These should include effective strategies for the early identification of shortage risks, and responsive approval processes that allow alternative products to be rapidly and temporarily covered during shortages (while ensuring quality). In the longer term, there should be substantial engagement with manufacturers to strategize and invest in domestic drug production.
Rare disease treatments
The National Strategy for Drugs for Rare Diseases should advance alongside pharmacare. Medicines can be essential even if they are not used by most. Different standards will be needed for adding medicines for infrequently encountered conditions (or “rare diseases”) because there is often a lack of evidence of efficacy from multiple clinical trials. This is not an issue unique to these medicines and thus an essential medicines formulary could include drugs for rare diseases to further stabilize the access to medicines across provincial jurisdictions.
Appropriate use
Medicines can lead to health harms and even deaths when not prescribed and used properly. Inappropriate prescribing and use of medicines is common, harmful and costly.Footnote 76Footnote 77Footnote 78 Medicines prescribed or used inappropriately represent the worst value and estimates of direct drug costs related to inappropriately prescribed medicines are around $1 billion annually.Footnote 76
There are several reasons for inappropriate prescribing and use of medicines, ranging from lack of clinician knowledge to structural issues in the health care system.Footnote 79Footnote 80
One of the many causes of inappropriate prescribing is related to marketing used by pharmaceutical companies.Footnote 81Footnote 82Footnote 83 Some medicines that are commonly prescribed inappropriately were marketed misleadingly. Examples include gabapentinoids, antidepressants, antipsychotics and opioids.Footnote 84
Canada's health systems are plagued by the contemporary harms of inappropriate prescribing and utilization of medications. The opioid crisis has killed well over 100,000 people in Canada, resulting largely from misleading marketing of products like OxyContin. The death rate from OxyContin is still over 7,000 per year and counting.Footnote 85Footnote 86Footnote 87Footnote 88 All efforts must be made to prevent these circumstances from occurring again. As pharmacare is implemented steps must be taken to ensure medicines are prescribed and used appropriately. Pharmacare represents a necessary investment in safe and sustainable prescribing, and vital access to resources, including preventative measures and treatments to mitigate the current opioid crisis.
Pharmacare will improve access to medicines through a strategy that will ensure that its investment realizes the inherent benefits while carefully navigating and mitigating potential harms. This will require coordinated efforts involving prescribers, pharmacists, professional associations, academics, and the people who take medicines.
Improving appropriate medicine use relies on accurate data being collected and acted upon.Footnote 87Footnote 88 Collecting and accessing data on prescribing in Canada is fragmented across Canada. This includes data that private companies sell to pharmaceutical companies for marketing purposes and to others for research.Footnote 91 This reliance on a third party may limit the use of data to improve care and creates privacy concerns.Footnote 92 Pharmacare may represent an opportunity for the federal government to support efforts to ensure prescribing data is used to promote the appropriate prescribing and use of medicines among prescribers.
Some provincial and territorial governments, and other institutions, have developed approaches to leveraging data to improve care. The Canadian Institute for Health Information also tracks costs for public drug plans and some prescribing trends (such as opioids and Beers list prescribing for seniors). Prescribing data should be used to rapidly intervene to address regionally variation in prescribing that may represent
Prescribing and use of medicines should also be monitored to identify:Footnote 89Footnote 90Footnote 93
- regional variation that may be a result of specific population needs (for example single doses versus infusion medicines)
- other signals that may be actionable with respect to promoting the appropriate use of medicines (such as academic detailing and audit and feedback)
This component of the recommendation may link with ongoing work by the CDA committees. The federal government has published a pan-Canadian strategy regarding the appropriate use of prescription drugs and related products on the Department of Health website. Within 3 years, the government will have the CDA report on the progress made in advancing that strategy.
Recommendation 5
The federal government should fully fund the initial list of essential medicines through various revenue-generating measures that are fair, neutral and efficient.
Revenue to the federal government is used to support federal spending in general, without dollars being marked for specific purposes. Major initiatives with budgetary implications are routinely implemented without identifying the source of each dollar needed.
Federal funding for important priorities such as health care services, dental care, and childcare is derived from the federal government’s revenues. The right to essential medicines is no less important than other federally funded priorities. It carries with it the opportunity to build cost-efficiency and targeted values into this already substantial investment.
Current federal spending on medicines, like most other federal spending, is from general revenues totalling $448 billion annuallyFootnote 94, derived from:
- personal income tax ($208 billion)
- corporate tax ($94 billion)
- goods and services tax ($46 billion)
- other sources
The federal government does not levy specific taxes to fund medicines or health care in the way that some provincial or territorial governments levy payroll taxes to partially fund public health care.
In 1977, the federal government “transferred” some of its taxation of personal and corporate income tax (13.5% and 1% respectively) to provinces and territories at the same time as it reduced its cash transfers.Footnote 95 This was primarily to support health and post-secondary education. Over decades, the federal government subsequently reduced, and then slightly increased, its cash contributions to provinces and territories. Contributions currently sits at around 21% of provincial and territorial health spending.Footnote 95
The federal government has recently announced several new policies that advance national priorities related to equity including a dental care program and a childcare program (with annual costs estimated at $4.4 billion and $7.7 billion, respectively).Footnote 96Footnote 97Footnote 98 In both instances no specific revenue source was identified, thus leaving them to be paid for using general revenues. Pharmacare could be funded based on general revenues, just like other government priorities.
It would be unusual to tie pharmacare funding to a specific source of revenue. However, some revenue sources could be considered if federal support for essential medicine access needs to be justified from a fiscal perspective.
If the government decides to implement specific revenue generating mechanisms for pharmacare, the committee recommends that the approach should be fair, neutral and efficient in accordance with generally accepted standards for public financing.
Additional sources of revenue above current general revenues relevant to the pharmaceutical sector include:
- end the federal income tax exemption for employer contributions to privately administered extended health benefits
- federal insurance premium tax
- federal capital tax on large insurance companies
- excise taxes such as an excise tax on long-acting opioid products that contribute to the opioid crisis
All these approaches have drawbacks and limitations, and these are just some examples of measures that could be implemented. These measures vary in how well they meet the criteria of being fair, neutral and efficient as well as in their expected revenue.
Insurance firm taxation was low before 1969 when some of the Royal Commission on Taxation (Carter report) recommendations were implemented and corporate taxes were applied to insurance companies.Footnote 99
The main findings of the Carter report were that the tax system should move away from loopholes and exemptions that disproportionately favor wealthy individuals and toward a more fair and simple system.Footnote 99
Many recommendations were never implemented, although the idea of making the tax system fair, neutral and efficient is widely cited and accepted.
Taxation of insurance has evolved since the 1980s, and the current approach is summarized in the following table.
| Tax | Application | Rate |
|---|---|---|
Premium |
Insurance premiums |
Federal: not applicableFootnote 1 |
Corporate |
Corporate income, standard and not specific to industry |
Federal: 15 % |
Additional on Life Insurers & Banks |
Corporate income above $100 million |
Federal: 1.5 % |
Capital |
Assets over $1 billion |
Federal: 0% |
Minimum (Capital) |
Eliminated or reduced based on corporate income tax payments but alternatively applicable to capital over $1 billion |
Federal: 1.5% |
Sales |
Certain insurance-related transactions other than premiums |
Federal: 5 % |
One-Time Federal (2022) |
Corporate income above $1 billion as a temporary measure in 2022 |
Federal: 15% |
|
||
The Canadian Life and Health Insurance Association reports that private health insurance that includes coverage for medicines, dental care, physiotherapy and mental health supports represented $61 billion in collected premiums in 2023.Footnote 100 These companies paid out $48 billion in claims, with medicines accounting for $15 billion (or 32%). There are 4 large insurance companies in Canada: Manulife (assets $849 billion), Canada Life ($701 billion), Desjardins ($407 billion) and Sun Life ($331 billion).Footnote 100
Reductions in tax revenue due to tax code exemptions relevant to medicine access include the exemption of employer contributions to privately administered insurance plans from income tax ($5 billion annually) and the exemption of prescription medicines from sales tax for consumers ($1.1 billion annually). The $5 billion public subsidy represents a tax advantage for those with privately administered plans unavailable to many.Footnote 12
Normally taxing this income like other would represent a fair approach that could fund pharmacare.Footnote 99 This approach would treat employer contributions to private plans the same as employer contributions to public health plans (that are currently taxed regularly as income). Unlike other potential revenue sources, insurance premiums have been increasing at a rate similar to drug spending, and this will likely continue. As a result, this could represent a sustainable source of funding for pharmacare.
Pharmacare might be expected to decrease premiums in privately administered plans. However, prior expansions of public drug plans have not resulted in decreases in insurance premiums or the number of people with privately administered plans.Footnote 102
The benefits of the current tax exemptions are elusive. These longstanding tax exemptions are not known to have increased the number of people who have access to medicines in the way pharmacare will.Footnote 102
Although the committee mentions specific revenue generating measures above, none are needed to implement pharmacare. The committee understands that prior reports did not delve into this aspect of pharmacare, and understood that part of its mandate was to outline options for revenue generating options.
The committee respects the fact that the federal government decides how to generate revenue based on several considerations beyond the scope of pharmacare or health care. The committee notes that much larger investments in health have been announced and implemented without identifying any specific source of funding.Footnote 101
Recommendation 6
Indigenous Peoples must be at the forefront of a monitoring and evaluation plan to assess the impact of pharmacare on access to medicines. First Nations and Inuit representatives should decide how saving from the Non-Insured Health Benefits program will be reinvested into Indigenous health priorities.
Over the years, multiple reports have detailed the need to ensure culturally appropriate care is offered to Indigenous PeoplesFootnote 103Footnote 104Footnote 105Footnote 106Footnote 107 to address the legacy of historical and contemporary harms associated with colonialization. This can be achieved by facilitating sovereign Indigenous health systems that are independently operated by and for Indigenous Peoples.Footnote 108Footnote 109Footnote 110Footnote 111Footnote 112Footnote 113
Indigenous Peoples’ participation in pharmacare could be a vital step toward universal access, and away from colonial and discriminatory health system processes which have historically caused harm.
One benefit of pharmacare is that it will save money and provide opportunities to have Indigenous Peoples direct investment in other areas of the health care system.
Pharmacare could eventually become the first payor for essential medicines for all Indigenous Peoples, including those currently eligible for NIHB. However, it is especially important to ensure that NIHB program funding is not reduced due to existing budgeting policies that base funding on utilization. A decrease in NIHB claims could over time, inadvertently erode budgeting for the NIHB program. This must be avoided.
Any resulting savings to the NIHB program must be carefully documented and evaluated and not used to justify a reduced budget for NIHB program, but instead, be used as a metric to engage with status First Nations and Inuit program recipients to determine how reduced drug expenditures can be reinvested in primary health services, including enhanced primary health care access and access to pharmacy services. For example, health related travel, dental care, vision care and mental health supports could all be enhanced. The priorities for reinvestment should ultimately be determined by Indigenous people eligible for NIHB.
Pharmacare implementation should be accompanied by efforts to achieve 100% access to primary health care. This should include culturally appropriate care for Indigenous Peoples within sovereign health care systems. Access to prescription medicines requires access to primary care. As such, the value of the investment in pharmacare will not be fully realized until all people have access to primary health care.
Based on its consultations, the committee believes that the engagement process with Indigenous Peoples regarding existing NIHB benefits should be reviewed. Necessary improvements must be addressed to ensure it is effective and meets the needs of its beneficiaries.
An Indigenous-led strategy should include engagement on several metrics for monitoring and evaluation, such as:
- unmet health priorities through existing drug coverage
- principles of sovereignty in the delivery of care
- enshrining treaty rights in the application and expansion of pharmacare
Such a strategy will serve as a step further by the federal government in meeting the commitment to UNDRIP, and Indigenous self-determination and sovereignty, in the application of Indigenous-specific health funding decision making.
Indigenous Peoples should lead the careful monitoring and evaluation of the impact of pharmacare on medication access for Indigenous patients and communities, with the support of the federal government. The process should include thorough measurement of impacts on remote and rural access and effective primary health services.
Recommendation 7
The federal government should immediately meet with provincial and territorial governments to agree on specific plans for improving primary health care and pharmacy services. They should focus on services that ensure access and appropriate use of medicines that will be supported using provincial and territorial savings from pharmacare.
Full federal funding of pharmacare will result in substantial direct and indirect savings for provincial and territorial governments.
Pharmacare would cover medicines currently funded by PT governments for their eligible beneficiaries, for example people with disabilities, those with low incomes, and older adults. First dollar coverage for certain classes of beneficiaries varies from jurisdiction to jurisdiction, but there will be direct savings of up to $18 billion to all public drug plans.Footnote 11
Pharmacare is also expected to make people healthier and to reduce hospitalizations related to medication nonadherence, resulting in indirect savings estimated to be larger than $1,000 per person per year for those currently unable to afford medicines.Footnote 2
The direct and indirect savings to provincial and territorial governments can be used to support services needed to ensure appropriate and equitable access to medicines. This is consistent with the priorities of the FPT governments and the Canada Health Act. These savings could be used to:
- improve access to primary health care, mental health and seniors care
- enhance pharmacy services
- standardize infusion services
- improve the distribution of medicines to remote communities
There is little point in providing free medicines to people if those people are unable to receive a prescription because they do not have access to health care. Right now, millions of people in Canada do not have a primary health care provider to prescribe life-saving treatments or access to essential pharmacy services.Footnote 114
People are affected differently by the lack of primary health care access. Those with asymptomatic conditions that require medical treatment may defer treatment if it’s not readily accessible. An example of such a condition is high blood pressure, which can lead to serious complications including heart attacks, stroke and death. People living in remote communities and those with a lower income have the most trouble getting care. Indigenous people both have lower levels of access and face discrimination in many health care settings.
Pharmacare will help only some unless it is also accompanied by improved access to primary health care. Savings to provincial and territorial governments should be reinvested in improving access to this care.
There should be separate public funding for essential pharmacy services including dispensing and transportation of medicines to remote communities. Examples from other countries such as Australia support the use of dedicated funding to enhance medicine distribution to people living in remote communities.Footnote 115
The number of pharmacies per capita is high in Canada compared with comparable countries. However, their distribution across the country leads to inequities and challenges for people living in rural communities.Footnote 116Footnote 117
In urban settings pharmacists play a vital role in ensuring medicines are prescribed and used appropriately.Footnote 118 Yet many in rural and remote communities people effectively have no access to pharmacy services. They often simply receive packages with medicines, but not the usual associated services like reviews and counselling by a pharmacist. The current approach to dispensing in remote communities is less than optimal. Funding, implementing, and evaluating pharmacare must address access to pharmacy services, drug distribution and sustainability of related services in remote and rural locations.
Medication access for some products includes additional requirements for administration, which have now become linked to the complexities of funding and distribution. Certain medicines must be administered via intravenous infusion. These include expensive branded medicines for autoimmune conditions such as rheumatoid arthritis.Footnote 119
Since intravenous infusion of these medicines is medically necessary, one might expect these services to be included in Canada’s publicly funded health care system. However, many receive these medicines as services funded by the pharmaceutical companies selling the products (with drug costs ultimately paid in large part from public funds).Footnote 120
These services provide wrap around “concierge” type care that includes close connections between patients and nurses which patients come to rely upon.Footnote 120 Patients then become resistant to moving from brand-name medicines to biosimilar products. These products are proven to have the same clinical effects as branded products at a lower cost.
Privately paid infusion services are only present in certain locations. There are no incentives or regulations that require the establishment of these infusion clinics in rural communities although they are needed. Right now, these privately funded services fill a gap in publicly funded services. Savings from pharmacare could be reinvested in publicly funding infusion services.
Recommendation 8
Data on health outcomes (including mortality, morbidity and disparities) and prescribing patterns should be continuously and rapidly acted upon by health system partners and practitioners to improve care. Annual reporting to the United Nations Committee on Economic, Social and Cultural Rights will demonstrate Canada’s commitment to advancing the right to health.
Pharmacare should provide an opportunity for government to monitor and evaluate its investment in implementing a universal, first dollar single-payer drug plan in order to improve care and health outcomes.
The ultimate purpose of pharmacare is to improve health and to address inequities through realizing the right to essential medicines. Today, there are wide disparities in health outcomes based on social factors. This is unacceptable. The continuous development, monitoring, and evaluation of pharmacare must be data-driven against the impact on health outcomes, including mortality and morbidity rates and disparities in health outcomes. There should be a focus on common medicine-amenable conditions, including certain infectious diseases (including HIV and Hepatitis C), cardiovascular disease, diabetes, hematological cancers, asthma and pain management.
The rates of inappropriate prescribing and use should be carefully tracked and addressed through mitigating strategies such as guidelines, training and professional and institutional standards.
In addition to overall health equity and outcomes, other dimensions of value should be monitored and evaluated, including improvements to:
- affordability through reductions in drug prices
- the appropriate use of medicines
- the ability of manufacturers and distributors to sustainably and equitably supply medicines
- the distribution and dispensing of essential medicines
Prices paid for medicines included in pharmacare and other public drug plans should be contrasted with prices paid by hospitals and public drug plans in other countries in regular public reports, similar to the 2017 Ontario Auditor General’s report.Footnote 63
Health systems-wide cost savings resulting from pharmacare should be monitored and evaluated continually. Impacted stakeholders should be invited to provide regular data and feedback on several metrics. For example, pharmacy stakeholders will report on factors like:
- drug shortages
- the distribution ecosystem
- supply chain complexities
- impact on rural and remote communities
- impact on equity-deserving patient populations
Representative patient or community stakeholders should provide their perspectives on the impact of pharmacare on medication access, health outcomes and equity. An engagement strategy for Indigenous peoples should be co-designed and implemented with Indigenous peoples.
Progress on realizing the right to essential medicines should continue to be reported to the United Nations Committee on Economic, Social and Cultural Rights. These reports will demonstrate how Canada is progressively realizing the right to health along with concrete plans to address any identified inequities.
Scope, context and approach of the committee of experts
Pharmacare legislation by the end of 2023 was part of the Delivering for Canadians Now agreement announced by the prime minister on 22 March 2022.Footnote 5 This committee of experts was created through An Act Respecting Pharmacare, legislation that was enacted on 10 October 2024, to “make recommendations respecting options for the operation and financing of national, universal, single-payer pharmacare”.Footnote 6
In undertaking its work, the committee took a broad and open-minded approach to its mandate. It considered a wide range of options, their rationale and their provenance and history.
Committee members thought carefully about how their work could build upon and complement work that had already been done. Previous reports had recommended including medicines in Canada’s publicly funded health care system. These reports included a 2018 parliamentary committee report titled “Pharmacare Now” and a 2019 National Advisory Council report on the implementation of National Pharmacare.Footnote 1Footnote 21
The committee consulted widely with people with different perspectives on the operation and financing of drug plans. It reviewed large swaths of data, including summaries of government data not usually available. Committee members completed a robust review of reports, references, and policy documents from domestic and international stakeholders and considered the history of health policy in this context. They considered different approaches used in Canada and internationally and heeded international guidance.
The committee has made 8 interrelated recommendations that should be acted upon in concert. The rationale for the recommendations and details that can be used during implementation are included in this report. The committee’s overall advice is to fully implement pharmacare now. The right to essential medicines must be progressively realized and always focused on medicines commonly prescribed in primary care.
Acknowledgements
The committee is very grateful to all of the individuals and organizations that provided written submissions and attended meetings. The committee was particularly appreciative of the opportunity to meet on Treaty 6 land with representatives of Indigenous Peoples in Canada.
The committee is grateful to the Minister of Health for the opportunity to provide advice on such an important issue. The committee thanks Mitch Moneo (Executive Secretary) and Kaireen Patton (Secretariat) and the staff and leadership of Health Canada who supported this work.
List of individuals and organizations who provided input
Individuals
Senator Yvonne Boyer
Douglas Clark
Marc-Andre Gagnon, Carleton University
Quinn Grundy, University of Toronto
Matthew Herder, Dalhousie University
Dr. Eric Hoskins
Josée G. Lavoie, University of Manitoba
Michael Law, University of Calgary
Dr. Wendy V. Norman, University of British Columbia
Senator Kim Pate
Indigenous engagement
Lindsay Batt, Atlantic Policy Congress of First Nations
Tessy Big Plume, Stoney Nakoda Tsuut’ina Tribal Council
Jim Devoe, Congress of Aboriginal Peoples
Tanya Davoren, Métis Nation British Columbia
Dallas Fiddler, Federation of Sovereign Indigenous Nations
Zachariah General, Chiefs of Ontario
Kohkum Rita LaPlante
Charlene Lavallee, Association of Métis, Non and Status Indians of Saskatchewan
John Mah, First Nations Health Authority
Chief Brendan Moore, Congress of Aboriginal Peoples
Garret Munch, Manitoba Métis Federation
Rachelle Neault, Manitoba Métis Federation
Bobbi Paul-Alook, Otipemisiwak Métis Government
Chief David Pratt, Federation of Sovereign Indigenous Nations
Tanya Pruden, Métis Nation Saskatchewan
Sandra Romain, Inuit Tapiriit Kanatami
Tim Saunders, MEDOcare Pharmacy
Tina Yellowdirt-Mitsuing, Confederacy of Treaty 6
Organizations
Access BC
Better Pharmacare Coalition
Biosimilars Canada
Canada’s Drug Agency
- Essential Prescription Drugs and Related Products Advisory Panel
- National Bulk Purchasing Advisory Panel
- Patient and Community Advisory Committee
Canadian Association for Pharmaceutical Distribution Management
Canadian Generic Pharmaceutical Association
Canadian Labour Congress
Canadian Life and Health Insurance Association
Canadian Medical Association
Canadian Paediatric Society
Canadian Pharmacists Association
Competition Bureau of Canada
Federal Provincial Territorial Pharmaceutical Executive Group
Heart & Stroke
Innovative Medicines Canada
Neighbourhood Pharmacy Association
Pan Canadian Pharmaceutical Alliance
Patented Medicine Prices Review Board
Appendix 1: Biographies
Nav Persaud, Chair

Dr. Nav Persaud is the Canada Research Chair in Health Justice and Professor at the University of Toronto.
He is a Staff Physician in the Department of Family and Community Medicine at St. Michael's Hospital in Unity Health Toronto, and a scientist with MAP Centre for Urban Health Solutions at St. Michael’s Hospital.
He trained at the University of Toronto and the University of Oxford.
His research focuses on health equity, especially as it relates to medicine access. He also compares national essential medicines lists in collaboration with the World Health Organization.
Amy Lamb

Amy Lamb is the Executive Director of Indigenous Pharmacy Professionals of Canada and advocates for pharmacy practice, Indigenous and holistic health, women's health, and leadership development.
As a Métis woman and member of the Métis Nation-Saskatchewan, Amy advocates for vulnerable and systemically harmed community members, access, and other systemic barriers in pharmacy practice and Canadian healthcare systems.
Amy has a Bachelor of Science in Pharmacy from the University of Saskatchewan and has worked as a front-line community pharmacist for 10 years, specializing in women's and holistic health. She has in-depth experience navigating the health access and equity needs of diverse populations, with experience serving urban, remote, and fly-in Indigenous communities in Saskatchewan.
She gives back to her community by empowering the structural determinants of health fulfilled by local non-profits, including as Chair of the YWCA Prince Albert for the past 5 years. She adds to diverse perspectives and guidance work as a member of the Canadian Medical Association's Indigenous Guiding Circle, the Canadian Pharmacists Association Workplace Wellness Task Force, and the Canadian Medication Appropriateness and Deprescribing Network's Indigenization Working Group.
Linda Silas

Linda Silas has been the President of the 250,000-strong Canadian Federation of Nurses Unions (CFNU) since 2003. As the dynamic and charismatic leader of Canada's largest nurses' organization, Linda is recognized as the foremost advocate on behalf of nurses in Canada.
Linda has earned a reputation for being a caring listener who is focused and solution-oriented in everything she does. A proud New Brunswicker, Linda credits her home province for both her impressive work ethic and her well-known zest for life and adventure.
Linda has fine-tuned her skills as a union leader at the local, provincial, national and international levels over the course of two decades. She is a passionate speaker whose straight-talking in both official languages inspires nurses and earns the respect of policy-makers and stakeholders.
Linda was previously the President of the New Brunswick Nurses Union (NBNU) for 10 years. Linda is a graduate of l'Université de Moncton, where she earned a Bachelor of Science in Nursing, and has practiced in the ICU, emergency, and labour and delivery.
Appendix 2: Estimating the cost of pharmacare
We set out to estimate the direct cost to the federal government of implementing pharmacare during the 2025 to 2026 fiscal year.
We assume that the federal government will cover the entire cost of covered medicines, and that the contribution from provincial and territorial governments will be zero. This is unlike the bilateral agreements for diabetes treatments and contraceptives, where there is cost sharing and the federal government covers only the anticipated incremental cost. Our estimates of the cost of pharmacare include the full cost of contraceptives and treatments for diabetes as well as other medicines that might be addressed by bilateral agreements. Our estimates disregard these bilateral agreements that exist for a minority of jurisdictions.
We consider only amounts paid for drugs and dispensing fees. We do not consider any savings related to improved health, such as avoided hospitalizations or increased productivity.
We estimated a reasonable range of the cost to the federal government, such that the actual cost will likely be within the estimated range. The lower bound is intended to provide a realistic estimate of the cost to the federal government in a scenario with savings through several mechanisms. The upper bound is intended to represent a fiscally conservative estimate of the most pharmacare would cost the federal government based on parameters that result in higher costs. As each estimate details, we used past published estimates of the cost of implementing pharmacare and dispensing data tracked at community pharmacies.
The approach explained below yielded a range in costs for pharmacare from $6.0 to $9.8 billion in 2025 to 26.
Upper estimate
We used the 2023 report from the Parliamentary Budget Office to estimate the upper bound of the cost of pharmacare.Footnote 3Footnote 121 The estimated total cost of publicly funding a longer list of medicines (the RAMQ list from Quebec) was $35 billion in 2025 to 26. The incremental cost to the public sector $11.9 billion. For a shorter list of essential medicines, the estimated cost in 2025 to 26 was $9.8 billion.
We take $9.8 billion to be a reasonable estimate of the cost of implementing pharmacare in 2025-26. Some have suggested that this may represent an overestimate based on some of the assumptions. However, this estimate accords with the committee’s estimates of the cost of funding essential medicines lists longer than the one used in the PBO estimate. This estimate is a reasonable estimate of the upper bound because it does not account for tendering or reductions in administrative costs. Including additional medicines would increase the cost.
Lower estimate
We used the list of 185 medicines included in the essential prescription drugs list prepared by the Canada’s Drug Agency committee to estimate the lower bound of the cost of pharmacare. The pan-Canadian Pharmaceutical Alliance identified that these medicines may be suitable for competitive pricing through tendering (as all have multiple sources and none are protected by a patent). We used 2024 dispensing and spending data from IQVIA Compuscript: The CompuScript dataset is derived from a sample of electronic dispensing records representing around 82 % of retail pharmacies. The spending data includes dispensing fees and mark ups. In 2024, total spending on these 185 medicines was $12.6 billion.
For the lower bound estimate, we used reasonable estimates that reflect a confluence of changes that result in savings. We adjusted the estimate upward to account for 3 factors:
Jurisdictions represented (2%): We did not have dispensing data for 5 jurisdictions (Newfoundland and Labrador, Prince Edward Island, Yukon, Nunavut and Northwest Territories) representing around 2% of the population of Canada. As such, we adjusted the estimates upward by 2%.
Year over year increase in use (4%): There was a 4% increase in the volume of prescriptions between 2023 and 2024, and we assume a similar increase between 2024 and the 2025 to 2026 financial year.
Increased use due to pharmacare (5%): The expected increase in drug use cannot be known in advance. However, it can be estimated based on the prevalence of cost-related nonadherence, and prior expansions of public drug plans that were more limited in scope than pharmacare, such as expansions. Some studies have found no increases in use with public coverage, while others have found increases above 10%.Footnote 122Footnote 123 We selected 5% for our lower bound estimate, while the upper bound estimate from the Parliamentary Budget Office assumes an increase of 13.5%, which is larger than estimates in the literature.
We adjusted the estimate downward to account for these 3 factors:
Higher prices for branded products (14%): We used pricing for generic products to address reasons for higher reported spending on brand name products. Based on the available dispensing cost data, we determined savings if all dispensations of a medicine (defined by the molecule and route of administration) were priced as a generic product. Based on the 2024 dispensing data, this would reduce overall spending by approximately 14% (14.0% based on price per unit or 14.5% based on the price per prescription). Note that the rate of 14% represents an aggregate effect of higher prices for branded products. For some medicines there is no effect and for others the effect is larger.
Lower mark-ups and dispensing fees for publicly funded medicines (17%): Based on available information, public drug plans generally allow a lower mark-up rate compared with privately administered insurance plans, which differ between jurisdictions. One estimate from Quebec indicates that generic medicines are 27.8% less expensive in public versus private plans.Footnote 124 This differential applies only to private spending, which represents around 60% of drug spending.
Lower prices due to competitive pricing (40%): In exchange for not instituting competitive pricing processes such as tendering, drug manufacturers have offered in 2018 to reduce prices paid by public drug plans by a reported 25% to 40%.Footnote 125 This implies that tendering would reduce prices even more. Prices of medicines in jurisdictions that use tendering are reported to be several times lower than in Canada, where generic prices are pegged to the price of brand products.Footnote 69
All of these adjustments yield a lower limit for the cost of pharmacare to be $6.0 billion annually in 2025 to 26. Annual increases of around 4% might be expected.Footnote 11 Cost would increase as more medicines are included.
Comparison with other estimates and data points
The lower bound of the estimate represents a substantial reduction in spending compared with current dispensing data that should be checked against available information regarding spending on medicines. Overall per capita drug spending in Canada is substantially higher than comparable countries:Footnote 55
- Ireland (41% higher in Canada)
- United Kingdom (70%)
- Iceland (40%)
- France (8%)
- Australia (23%)
This overall drug spending includes spending that would not be affected by pharmacare.
Some countries, such as New Zealand, employ tendering processes for medicines not protected by a patent, such as those included in the list of 185 medicines used for the lower bound. These countries see prices that are substantially lower. For some medicines, the price may be 10 times higher in Canada.Footnote 69 One estimate indicated that overall drug prices for essential medicines are 84% lower in New Zealand.Footnote 4 A 2017 report from the Auditor General of Ontario found that prices paid the Ontario Public Drugs Program were 70% higher than prices in New Zealand, and also 85% higher than prices paid by hospitals in Ontario.Footnote 63 Drug prices in Canada may have declined since these comparisons were made, and repeating such comparisons now would help to quantify potential savings from tendering.
Strengths and limitations of estimate approach
The upper bound estimate from the Parliamentary Budget Office is based on a previously published estimate that is intended to be fiscally conservative or cautious. The lower bound estimate is based on fairly complete and quite recent dispensing data.
The limitations include uncertainty about increases in medicine use, including switching within a class after pharmacare is implemented. The dispensing cost data include ingredient costs, dispensing fees mark ups and confidential rebates, which can only be estimated based on the available information. There is a high degree of uncertainty in some estimates, such as the effect of competitive pricing or tendering. Lower costs seen in other countries may not be realized in Canada, especially not immediately as tendering processes develop.
Although public plans generally have lower dispensing fees than private payers, we did not adjust for this as it was difficult to disentangle dispensing fees from other costs.
The estimates are for the direct cost to the federal government of assuming 100% of the cost of medicines included for all residents of Canada, and current spending is disregarded. The estimated cost does not account for:
- current public spending on included medicines, such as through provincial and territorial drug plans (which may account for more than 30% of the cost)
- current private spending on these medicines
All prior estimates indicate that pharmacare will reduce overall spending and reduce private spending. The estimates also do not address savings to the federal government (or provincial and territorial governments) related to decreased spending on current federal drug plans including privately administered plans for civil servants. The estimates also do not include reductions in federal tax expenditures related to non-taxation of employer contributions to privately administered health insurance plans that might result from lowered premiums.
The estimates are restricted to direct medicine costs. Pharmacare would likely result in indirect savings through improved health and reduced healthcare use.Footnote 4 Some administrative costs associated with privately administered plans might be avoided. The Canadian Life and Health Insurance Association reported 9% operating costs and 3% profit taking on $60.8 billion in health premiums in 2024.Footnote 100Footnote 126
Conclusion
The cost of pharmacare in 2025 to 26 is projected to be between $6.0 and $9.8 billion. The actual cost could be substantially lower if fewer medicines are included, or if prices are brought down more than projected. Indeed the total cost of pharmacare could be fit with a defined budget by including only a subset of medicines initially. The cost could be substantially higher if a longer list of medicines is used, or if current prices prevail.
Acknowledgements for cost estimation
The committee appreciates expert advice from Professor Michael Law.
Disclaimer
The statements, findings, conclusions, views, and opinions expressed in this report are based in part on data obtained under license from IQVIA Solutions Canada Inc. concerning the following information service(s): CompuScript, from: January 1st, 2024 to December 31st, 2024. All Rights Reserved. The statements, findings, conclusions, views, and opinions expressed herein are not necessarily those of IQVIA Solutions Canada Inc. or any of its affiliated or subsidiary entities.
Appendix 3: List of medicines
Both selected by Canada’s Drug Agency Essential Prescription Drugs and Related Products Advisory Panel and identified as being candidates for competitive pricing
Abiraterone
Acetaminophen
Acetaminophen-oxycodone
Acetylsalicylic acid
Acyclovir
Alendronate
Allopurinol
Amikacin
Amiodarone
Amitriptyline
Amlodipine
Amoxicillin
Amoxicillin-clavulanic acid
Amphetamine
Anastrazole
Apixaban
Aripiprazole
Atazanavir
Atenolol
Atomoxetine
Atorvastatin
Azithromycin
Baclofen
Bicalutamide
Bisacodyl
Bisoprolol
Calcitriol
Candesartan
Candesartan-hydrochlorothiazide
Capecitabine
Carbidopa-levodopa
Carvedilol
Cefazolin
Ceftriaxone
Cefuroxime
Celecoxib
Cephalexin
Cetirizine
Ciprofloxacin
Citalopram
Clarithromycin
Clindamycin
Clonazepam
Clonidine
Clopidogrel
Cyanocobalamin
Cyclobenzaprine
Darunavir
Dasatinib
Deferasirox
Desvenlafaxine
Dexamethasone
Dextroamphetamine-amphetamine
Diclofenac
Diltiazem
Dimethyl fumarate
Docusate
Domperidone
Donepezil
Dorzolamide-timolol
Doxycycline
Doxylamine-pyridoxine
Duloxetine
Dutasteride
Efavirenz
Efavirenz-emtricitabine-tenofovir
Eletriptan
Emtricitabine-tenofovir
Enalapril
Entacapone
Entecavir
Erlotinib
Escitalopram
Esomeprazole
Everolimus
Ezetimibe
Famotidine
Febuxostat
Fentanyl
Finasteride
Fingolimod
Flecainide
Fluconazole
Fluoxetine
Fosinopril
Fulvestrant
Furosemide
Gabapentin
Gefitinib
Glycopyrrolate
Hydralazine
Hydrochlorothiazide
Hydrocortisone-urea
Hydromorphone
Hydroxychloroquine
Ibuprofen
Imatinib
Irbesartan
Irbesartan-hydrochlorothiazide
Lactulose
Lamivudine-zidovudine
Lamotrigine
Lansoprazole
Latanoprost
Latanoprost-timolol
Leflunomide
Lenalidomide
Letrozole
Leucovorin
Levetiracetam
Levofloxacin
Levonorgestrel-ethinyl estradiol
Linezolid
Lisdexamfetamine
Lisinopril
Losartan
Losartan-hydrochlorothiazide
Lurasidone
Meropenem
Metformin
Methadone
Methotrexate
Metoclopramide
Metoprolol
Metronidazole
Mirtazapine
Modafinil
Mometasone
Morphine
Moxifloxacin
Mycophenolate
Mycophenolic
Nifedipine
Nystatin
Olanzapine
Olmesartan
Olmesartan-hydrochlorothiazide
Olopatadine
Omeprazole
Ondansetron
Oseltamivir
Pantoprazole
Paroxetine
Perindopril
Perindopril-indapamide
Piperacillin-tazobactam
Pomalidomide
Potassium chloride
Pramipexole
Pravastatin
Pregabalin
Progesterone
Quetiapine
Rabeprazole
Raloxifene
Ramipril
Ranitidine
Risedronate
Risperidone
Rivaroxaban
Rivastigmine
Rosuvastatin
Sertraline
Simvastatin
Sitagliptin
Sitagliptin-metformin
Spironolactone
Sumatriptan
Sunitinib
Tadalafil
Tamsulosin
Telmisartan
Telmisartan-hydrochlorothiazide
Tenofovir
Terbinafine
Teriflunomide
Ticagrelor
Timolol
Tobramycin
Topiramate
Trandolapril
Tranexamic acid
Trazodone
Valacyclovir
Valproic acid
Valsartan
Valsartan-hydrochlorothiazide
Vancomycin
Varenicline
Venlafaxine
Voriconazole
Zopiclone
Endnotes
- Footnote 1
-
Casey B. Pharmacare now prescription medicine coverage for all Canadians. Ottawa: House of Commons, Canada; 2018. (Report of the Standing Committee on Health; 14th report, 42nd Parliament, 1st session).
- Footnote 2
-
Persaud N, Bedard M, Boozary A, Glazier RH, Gomes T, Hwang SW, et al. Effect of Free Medicine Distribution on Health Care Costs in Canada Over 3 Years: A Secondary Analysis of the CLEAN Meds Randomized Clinical Trial. JAMA Health Forum. 2023 May 5;4(5):e231127.
- Footnote 3
-
PBO-DPB [Internet]. [cited 2021 Mar 3]. Federal Cost of a National Pharmacare Program. Available from: https://www.pbo-dpb.gc.ca/en/blog/news/Pharmacare
- Footnote 4
-
Morgan SG, Li W, Yau B, Persaud N. Estimated effects of adding universal public coverage of an essential medicines list to existing public drug plans in Canada. CMAJ. 2017 Feb 27;189(8):E295–302.
- Footnote 5
-
Prime Minister of Canada [Internet]. 2022 [cited 2022 Mar 31]. Delivering for Canadians Now. Available from: https://pm.gc.ca/en/news/news-releases/2022/03/22/delivering-canadians-now
- Footnote 6
-
Government Bill (House of Commons) C-64 (44-1) - Royal Assent - Pharmacare Act - Parliament of Canada [Internet]. [cited 2024 Oct 24]. Available from: https://www.parl.ca/documentviewer/en/44-1/bill/C-64/royal-assent
- Footnote 7
-
OHCHR [Internet]. [cited 2025 July 30]. Canada. Available from: https://www.ohchr.org/en/countries/canada
- Footnote 8
-
International Covenant on Economic, Social and Cultural Rights | OHCHR [Internet]. [cited 2025 Feb 18]. Available from: https://www.ohchr.org/en/instruments-mechanisms/instruments/international-covenant-economic-social-and-cultural-rights
- Footnote 9
-
Canada G of CCIR and NA. Treaty Texts: Treaty No. 6 [Internet]. 2008 [cited 2025 Feb 19]. Available from: https://www.rcaanc-cirnac.gc.ca/eng/1100100028710/1581292569426
- Footnote 10
-
Treaty Right to Health | Federation of Sovereign Indigenous Nations [Internet]. [cited 2025 Feb 18]. Available from: https://www.fsin.ca/treaty-right-to-health/
- Footnote 11
-
National health expenditure trends | CIHI [Internet]. [cited 2025 July 29]. Available from: https://www.cihi.ca/en/national-health-expenditure-trends
- Footnote 12
-
Canada D of F. Report on Federal Tax Expenditures - Concepts, Estimates and Evaluations 2025: Table of Contents [Internet]. 2025 [cited 2025 Sept 5]. Available from: https://www.canada.ca/en/department-finance/services/publications/federal-tax-expenditures/2025.html
- Footnote 13
-
There’s universal support for pharmacare, finds new poll – Canadian Health Coalition [Internet]. [cited 2025 Sept 5]. Available from: https://www.healthcoalition.ca/theres-universal-support-for-pharmacare-finds-new-poll/
- Footnote 14
-
UNAIDS calls for greater focus on ending inequalities to end AIDS with new 95-95-95 Target [Internet]. [cited 2025 Sept 5]. Available from: https://bccfe.ca/bccfe-documents/unaids-calls-for-greater-focus-on-ending-inequalities-to-end-aids-with-new-95-95-95-target/
- Footnote 15
-
Committee Report No. 17 - HESA (44-1) - House of Commons of Canada [Internet]. [cited 2025 Sept 5]. Available from: https://www.ourcommons.ca/documentviewer/en/44-1/HESA/report-17/page-69#13
- Footnote 16
-
One health [Internet]. [cited 2025 Aug 6]. Available from: https://www.who.int/health-topics/one-health
- Footnote 17
-
Soobramoney v Minister of Health (Kwazulu-Natal) (CCT32/97) [1997] ZACC 17; 1998 (1) SA 765 (CC); 1997 (12) BCLR 1696 (27 November 1997) [Internet]. [cited 2025 Aug 1]. Available from: https://www.saflii.org/za/cases/ZACC/1997/17.html
- Footnote 18
-
Question of the realization in all countries of economic, social and cultural rights: resolution [Internet]. Geneva: UN; 4 [cited 2025 Sept 22]. Available from: https://digitallibrary.un.org/record/4081128
- Footnote 19
-
Neve A. Universal: renewing human rights in a fractured world. Toronto: Anansi; 2025.
- Footnote 20
-
Lipscombe LL, Austin PC, Manuel DG, Shah BR, Hux JE, Booth GL. Income-related differences in mortality among people with diabetes mellitus. CMAJ. 2010 Jan 12;182(1):E1–17.
- Footnote 21
-
A prescription for Canada: achieving pharmacare for all : final report of the Advisory Council on the Implementation of National Pharmacare. Ottawa, Ontario: Health Canada = Santé Canada; 2019
- Footnote 22
-
Holbrook AM, Wang M, Lee M, Chen Z, Garcia M, Nguyen L, et al. Cost-related medication nonadherence in Canada: a systematic review of prevalence, predictors, and clinical impact. Systematic Reviews. 2021 Jan 6;10(1):11.
- Footnote 23
-
Rebić N, Cheng L, Law MR, Cragg JJ, Brotto LA, De Vera MA. Predictors of cost-related medication nonadherence in Canada: a repeated cross-sectional analysis of the Canadian Community Health Survey. CMAJ. 2024 Nov 24;196(40):E1331–40.
- Footnote 24
-
Morgan SG, Lee A. Cost-related non-adherence to prescribed medicines among older adults: a cross-sectional analysis of a survey in 11 developed countries. BMJ Open. 2017 Jan 1;7(1):e014287.
- Footnote 25
-
Angus Reid Institute. As Canadians age, struggles over access to health care extend to prescription drugs [Internet]. 2019. Available from: https://angusreid.org/wp-content/uploads/2019/08/2019.08.09_Senior-Health-II.pdf
- Footnote 26
-
Government of Canada SC. Exploring gaps in prescription drug insurance coverage among men and women in Canada using an intersectional lens [Internet]. 2024 [cited 2025 Feb 19]. Available from: https://www150.statcan.gc.ca/n1/pub/75-006-x/2024001/article/00001-eng.htm
- Footnote 27
-
Cortes K, Smith L. Pharmaceutical access and use during the pandemic. Insights on Canadian Society [Internet]. 2022 Nov 2; Available from: https://www150.statcan.gc.ca/n1/pub/75-006-x/2022001/article/00011-eng.htm
- Footnote 28
-
Government of Canada SC. Access to prescription insurance and medication use during the COVID-19 pandemic among First Nations people living off reserve, Métis and Inuit in the provinces [Internet]. 2024 [cited 2025 Feb 19]. Available from: https://www150.statcan.gc.ca/n1/pub/41-20-0002/412000022024003-eng.htm
- Footnote 29
-
National Institute of Ageing. National Seniors Strategy for Canada [Internet]. 2020. Available from: https://nationalseniorsstrategy.ca/wp-content/uploads/2020/09/NSS_2020_Third_Edition.pdf
- Footnote 30
-
Taking the pulse: People’s opinions on human rights in Ontario | Ontario Human Rights Commission [Internet]. [cited 2025 Feb 19]. Available from: https://www.ohrc.on.ca/en/taking-pulse-peoples-opinions-human-rights-ontario
- Footnote 31
-
Government of Canada SC. The Daily — Intersectional Gender Wage Gap in Canada, 2007 to 2022 [Internet]. 2023 [cited 2025 Feb 19]. Available from: https://www150.statcan.gc.ca/n1/daily-quotidien/230921/dq230921b-eng.htm
- Footnote 32
-
Statistics Canada. Racialized Canadians are less likely to find as good jobs as their non-racialized and non-Indigenous counterparts early in their careers [Internet]. 2023. Available from: https://www150.statcan.gc.ca/n1/daily-quotidien/230118/dq230118b-eng.htm
- Footnote 33
-
World Health Organization. Essential medicines [Internet]. 2024 [cited 2025 Apr 9]. Available from: https://www.who.int/news-room/fact-sheets/detail/essential-medicines#:~:text=Essential%20medicines%20should%20always%20be,individuals%20and%20the%20health%20system.
- Footnote 34
-
Perehudoff K, Persaud N, Forman L. The human right to essential medicines applies to Canadians. Canadian family physician. 2021 June 14;67(6):400–2.
- Footnote 35
-
CJCCL 2015, Volume 1 | The Canadian Journal of Comparative and Contemporary Law [Internet]. [cited 2025 Feb 19]. Available from: https://www.cjccl.ca/posts/health-law/
- Footnote 36
-
Da Silva M. Positive Charter Rights: When Can We Open the ‘Door?’ [Internet]. Rochester, NY: Social Science Research Network; 2021 [cited 2025 Feb 19]. Available from: https://papers.ssrn.com/abstract=3853161
- Footnote 37
-
Canada GA. GAC. 2017 [cited 2025 Feb 19]. Sexual and reproductive health and rights. Available from: https://www.international.gc.ca/world-monde/issues_development-enjeux_developpement/global_health-sante_mondiale/reproductive-reproductifs.aspx?lang=eng
- Footnote 38
-
Health (NCCIH) TNCC for I. NCCIH. 2022 [cited 2025 Feb 18]. NCCIH - National Collaborating Centre for Indigenous Health > Home > NCCIH PUBLICATIONS. Available from: http://www.nccah-ccnsa.ca/en/
- Footnote 39
-
Aboriginal health: a constitutional rights analysis. Native Law Centre; 2003.
- Footnote 40
-
Government of Canada D of J. The Canadian Charter of Rights and Freedoms [Internet]. 2021 [cited 2025 Feb 18]. Available from: https://www.justice.gc.ca/eng/csj-sjc/rfc-dlc/ccrf-ccdl/
- Footnote 41
-
Branch LS. Consolidated federal laws of Canada, United Nations Declaration on the Rights of Indigenous Peoples Act [Internet]. 2024 [cited 2025 Feb 18]. Available from: https://www.laws-lois.justice.gc.ca/eng/acts/u-2.2/page-1.html
- Footnote 42
-
WHO. Global Essential Medicines [Internet]. Available from: https://global.essentialmeds.org/about#about
- Footnote 43
-
Persaud N, Bedard M, Boozary A, Glazier RH, Gomes T, Hwang SW, et al. Adherence at 2 years with distribution of essential medicines at no charge: The CLEAN Meds randomized clinical trial. PLOS Medicine. 2021 May 21;18(5):e1003590.
- Footnote 44
-
Persaud N, Bedard M, Boozary AS, Glazier RH, Gomes T, Hwang SW, et al. Effect on Treatment Adherence of Distributing Essential Medicines at No Charge: The CLEAN Meds Randomized Clinical Trial. JAMA Intern Med. 2020 Jan 1;180(1):27.
- Footnote 45
-
Ally MZ, Woods H, Adekoya I, Bali A, Persaud N. Acceptability of a short list of essential medicines to patients and prescribers: Multimethod study. Can Fam Physician. 2022 July;68(7):e204–14.
- Footnote 46
-
Jarvis JD, Murphy A, Perel P, Persaud N. Acceptability and feasibility of a national essential medicines list in Canada: a qualitative study of perceptions of decision-makers and policy stakeholders. CMAJ. 2019 Oct 7;191(40):E1093–9.
- Footnote 47
-
Canada H. Standing Senate Committee on Social Affairs, Science and Technology on the State of the Health Care System in Canada (Kirby Committee) - Health Canada [Internet]. 2005 [cited 2025 Feb 20]. Available from: https://www.canada.ca/en/health-canada/services/health-care-system/commissions-inquiries/federal-commissions-health-care/standing-senate-committee-social-affairs-science-technology-state-health-care-system-canada.html
- Footnote 48
-
Canada H. National pharmacare bilateral agreements [Internet]. 2025 [cited 2025 Sept 5]. Available from: https://www.canada.ca/en/health-canada/corporate/transparency/health-agreements/national-pharmacare-bilateral-agreements.html
- Footnote 49
-
WHO. Human rights [Internet]. 2025. Available from: https://www.who.int/news-room/fact-sheets/detail/human-rights-and-health
- Footnote 50
-
Dangerfield K. Canada’s pharmacare bill is now law. What this means for you [Internet]. 2024. Available from: https://globalnews.ca/news/10807237/canada-pharmacare-plan-what-to-know/
- Footnote 51
-
Brandt J, Shearer B, Morgan SG. Prescription drug coverage in Canada: a review of the economic, policy and political considerations for universal pharmacare. Journal of pharmaceutical policy and practice. 2018;11(1):28–28.
- Footnote 52
-
House of Commons of Canada. Standing Committee on Health. 42nd Parliament, 1st Session, Meeting 74 [Internet]. Available from: https://www.ourcommons.ca/DocumentViewer/en/42-1/hesa/meeting-74/evidence
- Footnote 53
-
World Health Organization. How to Develop and Implement a National Drug Policy (Second Edition) [Internet]. Geneva: World Health Organization; 2001. Available from: https://www.who.int/publications/i/item/924154547X
- Footnote 54
-
How to create and update an essential medicines list for Canada [Internet]. MAP Centre for Urban Health Solutions. [cited 2024 Mar 5]. Available from: https://maphealth.ca/canada-eml-process/
- Footnote 55
-
OECD [Internet]. [cited 2025 Feb 20]. Pharmaceutical spending. Available from: https://www.oecd.org/en/data/indicators/pharmaceutical-spending.html
- Footnote 56
-
Secretariat TB of C. 2024–25 Estimates [Internet]. 2021 [cited 2025 Feb 20]. Available from: https://www.canada.ca/en/treasury-board-secretariat/services/planned-government-spending/government-expenditure-plan-main-estimates/2024-25-estimates.html
- Footnote 57
-
Prescribed drug spending in Canada, 2023 | CIHI [Internet]. [cited 2025 Feb 20]. Available from: https://www.cihi.ca/en/prescribed-drug-spending-in-canada-2023
- Footnote 58
-
Canada - Place Explorer - Data Commons [Internet]. [cited 2025 Feb 25]. Available from: https://datacommons.org/place/country/CAN?utm_medium=explore&mprop=lifeExpectancy&popt=Person&hl=en
- Footnote 59
-
Government of Canada SC. The Daily — Key findings from the Health of Canadians report, 2024 [Internet]. 2025 [cited 2025 Sept 18]. Available from: https://www150.statcan.gc.ca/n1/daily-quotidien/250305/dq250305a-eng.htm
- Footnote 60
-
Ledlie S, Leece P, Yang J, Iacono A, Kolla G, Boyd R, et al. Trends, characteristics, and circumstances surrounding stimulant toxicity deaths in Ontario, Canada from 2018 to 2021. J Subst Use Addict Treat. 2024 Dec 21;209614.
- Footnote 61
-
Government of Canada. Competition Bureau Submission to the OECD on Excessive Pricing in Pharmaceuticals [Internet]. 2018. Available from: https://competition-bureau.canada.ca/en/how-we-foster-competition/promotion-and-advocacy/regulatory-adviceinterventions-competition-bureau/competition-bureau-submission-oecd-excessive-pricing-pharmaceuticals
- Footnote 62
-
HESA - Patented Medicine Prices Review Board [Internet]. [cited 2025 Feb 21]. Available from: https://www.ourcommons.ca/committees/en/HESA/StudyActivity?studyActivityId=12106120
- Footnote 63
-
Office of the Auditor General of Ontario [Internet]. [cited 2025 Aug 6]. Available from: https://auditor.on.ca/en/content/annualreports/arbyyear/ar2017.html
- Footnote 64
-
pan-Canadian Pharmaceutical Alliance. About pCPA [Internet]. 2025. Available from: https://www.pcpacanada.ca/about
- Footnote 65
-
pan-Canadian Pharmaceutical Alliance. Generic drugs [Internet]. 2025. Available from: https://www.pcpacanada.ca/generic-drug-framework
- Footnote 66
-
CBC News. “Professional allowances” and the price of generic drugs [Internet]. 2010 Apr. Available from: https://www.cbc.ca/news/science/professional-allowances-and-the-price-of-generic-drugs-1.874458
- Footnote 67
-
Government of Canada I. Generic Drug Sector Study [Internet]. Innovation, Science and Economic Development Canada; 2007 [cited 2025 Feb 26]. Available from: https://competition-bureau.canada.ca/en/generic-drug-sector-study
- Footnote 68
-
Kelley LT, Tenbensel T, Johnson A. Ontario and New Zealand Pharmaceuticals: Cost and Coverage. Healthc Policy. 2018 May;13(4):23–34.
- Footnote 69
-
Morgan SG, Persaud N. New generic pricing scheme maintains high prices and risks of shortages. CMAJ. 2018 Apr 9;190(14):E410–1.
- Footnote 70
-
From Regulated Prices to Prices Set in Tenders [Internet]. [cited 2025 Feb 21]. Available from: https://www.iqvia.com/library/white-papers/from-regulated-prices-to-prices-set-in-tenders
- Footnote 71
-
Canada H. Backgrounder: Regulation of patented drug prices in Canada [Internet]. 2017 [cited 2025 Feb 21]. Available from: https://www.canada.ca/en/health-canada/news/2017/05/backgrounder_regulationofpatenteddrugpricesincanada.html
- Footnote 72
-
Bureau ABOB Raisa Patel Ottawa. Toronto Star. 2023 [cited 2025 Feb 21]. Justin Trudeau’s former health minister slams his government for delay in lowering drug prices. Available from: https://www.thestar.com/politics/federal/justin-trudeau-s-former-health-minister-slams-his-government-for-delay-in-lowering-drug-prices/article_be4cc987-ec4d-5b3a-8d33-5082b656230b.html
- Footnote 73
-
Crowe K. CBC News. 2022 [cited 2022 Aug 29]. After a 5-year fight to lower drug prices, Ottawa’s pledge quietly falls apart. Available from: https://www.cbc.ca/news/health/drug-prices-canada-regulations-1.6449265
- Footnote 74
-
Crowe K. Canada has found the key to lowering drug prices, but it won't be used any time soon. CBC News [Internet]. 2018 Nov 24 [cited 2025 Feb 21]; Available from: https://www.cbc.ca/news/health/canada-drug-price-patented-medicine-pharmaceutical-industry-pmprb-1.4919200
- Footnote 75
-
Government of Canada. Compulsory licences under Canada’s Access to Medicines Regime [Internet]. 2009. Available from: https://www.canada.ca/en/health-canada/services/canada-access-medicines-regime/countries/eligibility/compulsory-licences.html
- Footnote 76
-
Huon JF, Sanyal C, Gagnon CL, Turner JP, Khuong NB, Bortolussi-Courval É, et al. The cost of potentially inappropriate medications for older adults in Canada: A comparative cross-sectional study. J Am Geriatr Soc. 2024 Sept 5;
- Footnote 77
-
Squires JE, Cho-Young D, Aloisio LD, Bell R, Bornstein S, Brien SE, et al. Inappropriate use of clinical practices in Canada: a systematic review. CMAJ. 2022 Feb 28;194(8):E279–96.
- Footnote 78
-
Reason B, Terner M, Moses McKeag A, Tipper B, Webster G. The impact of polypharmacy on the health of Canadian seniors. Fam Pract. 2012 Aug;29(4):427–32.
- Footnote 79
-
Ailabouni NJ, Rebecca Weir K, Reeve E, Turner JT, Wilson Norton J, Gray SL. Barriers and enablers of older adults initiating a deprescribing conversation. Patient Educ Couns. 2022 Mar;105(3):615–24.
- Footnote 80
-
Doherty AJ, Boland P, Reed J, Clegg AJ, Stephani AM, Williams NH, et al. Barriers and facilitators to deprescribing in primary care: a systematic review. BJGP Open. 2020 Aug;4(3):bjgpopen20X101096.
- Footnote 81
-
DeFrank JT, Berkman ND, Kahwati L, Cullen K, Aikin KJ, Sullivan HW. Direct-to-Consumer Advertising of Prescription Drugs and the Patient-Prescriber Encounter: A Systematic Review. Health Commun. 2020 May;35(6):739–46.
- Footnote 82
-
Every-Palmer S, Duggal R, Menkes DB. Direct-to-consumer advertising of prescription medication in New Zealand. N Z Med J. 2014 Aug 29;127(1401):102–10.
- Footnote 83
-
Spurling GK, Mansfield PR, Montgomery BD, Lexchin J, Doust J, Othman N, et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med. 2010 Oct 19;7(10):e1000352.
- Footnote 84
-
Tamblyn R, Huang A, Perreault R, Jacques A, Roy D, Hanley J, et al. The medical office of the 21st century (MOXXI): effectiveness of computerized decision-making support in reducing inappropriate prescribing in primary care. Cmaj. 2003;169(6):549–56.
- Footnote 85
-
Canada PHA of. Key findings: Opioid- and Stimulant-related Harms in Canada — Canada.ca [Internet]. 2019 [cited 2025 Feb 20]. Available from: https://health-infobase.canada.ca/substance-related-harms/opioids-stimulants/
- Footnote 86
-
Hutchinson M, Lavigne É, Patterson Z. Opioid use in the era of COVID-19: a multifaceted study of the opioid epidemic in Canada. Front Pharmacol. 2023;14:1122441.
- Footnote 87
-
Belzak L, Halverson J. The opioid crisis in Canada: a national perspective. Health Promot Chronic Dis Prev Can. 2018 June;38(6):224–33.
- Footnote 88
-
Degenhardt L, Grebely J, Stone J, Hickman M, Vickerman P, Marshall BDL, et al. Global patterns of opioid use and dependence: harms to populations, interventions, and future action. Lancet. 2019 Oct 26;394(10208):1560–79.
- Footnote 89
-
Soresi J, Bertilone C, Banks E, Marshall T, Murray K, Preen DB. Features and effectiveness of electronic audit and feedback for patient safety and quality of care in hospitals: A systematic review. Health Informatics J. 2025;31(1):14604582251315414.
- Footnote 90
-
Xu AXT, Brown K, Schwartz KL, Aghlmandi S, Alderson S, Brehaut JC, et al. Audit and Feedback Interventions for Antibiotic Prescribing in Primary Care: A Systematic Review and Meta-analysis. Clin Infect Dis. 2025 Feb 24;80(2):253–62.
- Footnote 91
-
Connected Intelligence Copy [Internet]. [cited 2025 Feb 25]. Available from: http://view.ceros.com/quintiles-ims/core-2-0-module-2-3-5
- Footnote 92
-
Privacy commissioner to investigate sale of health data [Internet]. [cited 2025 Feb 25]. Available from: https://www.thestar.com/news/investigations/privacy-commissioner-to-investigate-sale-of-health-data/article_cf43dab3-bc4b-5307-8074-51787701b5ac.html
- Footnote 93
-
Rome BN, Dancel E, Chaitoff A, Trombetta D, Roy S, Fanikos P, et al. Academic Detailing Interventions and Evidence-Based Prescribing: A Systematic Review. JAMA Netw Open. 2025 Jan 2;8(1):e2453684.
- Footnote 94
-
Fun With Data. How much money does the federal government collect? [Internet]. 2025. Available from: https://www.funwithdata.ca/canada-facts/economy/how-much-money-does-the-federal-government-collect
- Footnote 95
-
Canada D of F. History of Health and Social Transfers [Internet]. 2000 [cited 2025 Mar 7]. Available from: https://www.canada.ca/en/department-finance/programs/federal-transfers/history-health-social-transfers.html
- Footnote 96
-
Canada E and SD. Canada-British Columbia Canada-Wide Early Learning and Child Care Agreement – 2021 to 2026 [Internet]. 2021 [cited 2025 Sept 5]. Available from: https://www.canada.ca/en/early-learning-child-care-agreement/agreements-provinces-territories/british-columbia-canada-wide-2021.html
- Footnote 97
-
Canada H. The Canadian Dental Care Plan [Internet]. 2023 [cited 2025 Sept 5]. Available from: https://www.canada.ca/en/health-canada/news/2023/12/the-canadian-dental-care-plan.html
- Footnote 98
-
Sourang D. New Canadian Dental Care Plan [Internet]. Office of the Parliamentary Budget Officer. Office of the Parliamentary Budget Officer; 2023 June [cited 2025 Sept 5]. Available from: https://www.pbo-dpb.ca/en/publications/LEG-2324-009-S--new-canadian-dental-care-plan--nouveau-regime-canadien-soins-dentaires
- Footnote 99
-
Government of Canada PS and PC. Report of the Royal Commission on Taxation. Vol. 1 : Introduction, acknowledgments and minority reports / Kenneth LeM. Carter, chairman. : Z1-1962/1-1E-PDF - Government of Canada Publications - Canada.ca [Internet]. 2002 [cited 2025 Sept 18]. Available from: https://publications.gc.ca/site/eng/471998/publication.html
- Footnote 100
-
CLHIA - Canadian Life and Health Insurance Facts, 2024 Edition [Internet]. [cited 2025 July 29]. Available from: https://www.clhia.ca/facts
- Footnote 101
-
Dhalla IA, Guyatt GH, Stabile M, Bayoumi AM. Broadening the base of publicly funded health care. CMAJ. 2011 Mar 22;183(5):E296–305.
- Footnote 102
-
Gagnon MA. Pharmacare and Access to Medicines in Canada: Is Bill C-64 a Step in the Right Direction? [Internet]. 2024. Available from: https://perspectivesjournal.ca/pharmacare-bill-c64/
- Footnote 103
-
Honouring the Truth, Reconciling for the Future - Summary of the Final Report of the Truth and Reconciliation Commission of Canada [Internet]. Truth and Reconciliation Commission of Canada; 2015. Available from: www.trc.ca
- Footnote 104
-
Well Living House. Justice for Joyce: Public Reports and Evidence Briefs [Internet]. 2020 Oct [cited 2021 Sept 28]. Available from: http://www.welllivinghouse.com/justice-for-joyce-public-reports-and-evidence-briefs/
- Footnote 105
-
Report of the Special Rapporteur on the rights of indigenous peoples, James Anaya [Internet]. United Nations General Assembly Human Rights Council; 2014 July [cited 2021 Mar 3]. Available from: https://www.ohchr.org/en/issues/ipeoples/srindigenouspeoples/pages/sripeoplesindex.aspx
- Footnote 106
-
LibGuides, University of Manitoba. Indigenous Health: For Brian Sinclair [Internet]. 2025. Available from: https://libguides.lib.umanitoba.ca/indigenoushealth/ForBrian
- Footnote 107
-
Addressing Racism in BC Health Care [Internet]. 2020 [cited 2025 Feb 24]. Available from: https://engage.gov.bc.ca/addressingracism/
- Footnote 108
-
Barbo G, Alam S. Indigenous people’s experiences of primary health care in Canada: a qualitative systematic review. Health Promot Chronic Dis Prev Can. 2024 Apr;44(4):131–51.
- Footnote 109
-
Canuto K, Brown A, Wittert G, Harfield S. Understanding the utilization of primary health care services by Indigenous men: a systematic review. BMC Public Health. 2018 Oct 23;18(1):1198.
- Footnote 110
-
Davy C, Harfield S, McArthur A, Munn Z, Brown A. Access to primary health care services for Indigenous peoples: A framework synthesis. Int J Equity Health. 2016 Sept 30;15(1):163.
- Footnote 111
-
Harfield SG, Davy C, McArthur A, Munn Z, Brown A, Brown N. Characteristics of Indigenous primary health care service delivery models: a systematic scoping review. Global Health. 2018 Jan 25;14(1):12.
- Footnote 112
-
Gomersall JS, Gibson O, Dwyer J, O’Donnell K, Stephenson M, Carter D, et al. What Indigenous Australian clients value about primary health care: a systematic review of qualitative evidence. Aust N Z J Public Health. 2017 Aug;41(4):417–23.
- Footnote 113
-
Shrivastava R, Couturier Y, Girard F, Papineau L, Emami E. Two-eyed seeing of the integration of oral health in primary health care in Indigenous populations: a scoping review. Int J Equity Health. 2020 June 30;19(1):107.
- Footnote 114
-
OurCare [Internet]. [cited 2023 Apr 20]. OurCare. Available from: https://www.ourcare.ca
- Footnote 115
-
Niesche C. How the latest medicines get into the remotest communities [Internet]. 2024. Available from: https://www.themandarin.com.au/242833-community-service-obligation-medicine-remote-locations/
- Footnote 116
-
Grootendorst P. Pharmacy location and medical need: regional evidence from Canada. BMC Health Services Research. 2022 Nov 3;22(1):1309.
- Footnote 117
-
Wang L, Ramroop S. Geographic disparities in accessing community pharmacies among vulnerable populations in the Greater Toronto Area. Can J Public Health. 2018 Dec;109(5–6):821–32.
- Footnote 118
-
Canadian Pharmacists Association. CPhA POSITION STATEMENT Pharmacist Prescribing [Internet]. 2011. Available from: https://www.pharmacists.ca/cpha-ca/assets/File/cpha-on-the-issues/Pharmacists%20Prescribing%20in%20Canada.pdf
- Footnote 119
-
The Globe and Mail [Internet]. 2018 [cited 2025 Feb 24]. How a blockbuster drug tells the story of why Canada’s spending on prescriptions is sky high. Available from: https://www.theglobeandmail.com/canada/article-how-a-blockbuster-drug-tells-the-story-of-why-canadas-spending-on/
- Footnote 120
-
Grundy Q, Hart D, Elkhalifa S, Lexchin J, Gagnon MA, Persaud N, et al. Mapping the Landscape of Infusion Care for People Prescribed IV Medicines in Canada. Canadian Journal of Health Technologies [Internet]. 2025 Jan 30 [cited 2025 Feb 24];5(1). Available from: https://canjhealthtechnol.ca/index.php/cjht/article/view/JA0003
- Footnote 121
-
Barkova L, Malanik-Busby C, Barkova L, Malanik-Busby C. Cost Estimate of a Single-payer Universal Drug Plan [Internet]. Office of the Parliamentary Budget Officer. Office of the Parliamentary Budget Officer; 2023 Oct [cited 2025 July 9]. Available from: https://www.pbo-dpb.ca/en/publications/RP-2324-016-S--cost-estimate-single-payer-universal-drug-plan--estimation-couts-un-regime-assurance-medicaments-universel-payeur-unique
- Footnote 122
-
Kitchen SA, Gomes T, Tadrous M, Pajer K, Gardner W, Lunsky Y, et al. Association between a publicly funded universal drug program and antipsychotic and antidepressant medication dispensing to children. BMC Pediatr. 2025 Feb 8;25:105.
- Footnote 123
-
Antoniou T, McCormack D, Kitchen S, Pajer K, Gardner W, Lunsky Y, et al. Impact of a publicly-funded pharmacare program policy on benzodiazepine dispensing among children and youth: a population-based natural experiment. BMC Pediatr. 2023 Oct 19;23:519.
- Footnote 124
-
M. C, A. F, I. C, M. S, L. B. Difference in drug cost between private and public drug plans in Quebec, Canada. BMC Health Services Research. 2022 Feb 14;22(1):200.
- Footnote 125
-
Gonzalez-Sirois G. A Joint Statement from the pan-Canadian Pharmaceutical Alliance and the Canadian Generic Pharmaceutical Association [Internet]. CGPA – Canadian Generic Pharmaceutical Association. 2018 [cited 2025 July 9]. Available from: https://canadiangenerics.ca/a-joint-statement-from-the-pan-canadian-pharmaceutical-alliance-and-the-canadian-generic-pharmaceutical-association/
- Footnote 126
-
Law MR, Kratzer J, Dhalla IA. The increasing inefficiency of private health insurance in Canada. CMAJ. 2014 Sept 2;186(12):E470-474.
