Archived Annual Report on Compliance and Enforcement Activities (Tobacco Control) 2016-2017
Download the entire report
(PDF format, 331 KB, 17 pages)
Table of Contents
What We Do
Mission and Vision
Health Canada is the federal department responsible for helping the people of Canada maintain and improve their health.
Health Canada is committed to improving the lives of all of Canada's people and to making this country's population among the healthiest in the world as measured by longevity, lifestyle, and effective use of the public health care system.
Tobacco Control in Canada
Tobacco use is the leading preventable cause of disease and premature death in Canada, killing one in two long-term users. It causes many serious chronic illnesses, including cancer, respiratory ailments, and heart disease. Each year, about 37,000 Canadians die from tobacco use.
Since 2001, the Government of Canada has been addressing this national public health problem through the Federal Tobacco Control Strategy (FTCS). Built on the principles of prevention, protection, cessation, and product regulation, the FTCS aims to reduce tobacco use prevalence and the resulting disease and deaths.
Provinces and territories also have tobacco control strategies, a situation which reflects the shared responsibility in tobacco control among levels of government.
Canada's Tobacco Act
The Tobacco Act is a key component of the FTCS. The Act regulates the manufacture, sale, labelling, and promotion of tobacco products. The Act recognizes the inherent harmful nature of tobacco products; even when tobacco manufacturers comply with all the provisions of the Tobacco Act, their products remain harmful.
Within Health Canada, the Tobacco Act and its regulations are administered by the Tobacco Control Directorate of the Healthy Environments and Consumer Safety Branch, while the related inspection and investigation activities are carried out by the Regulatory Operations and Regions Branch.
Canada and the World Health Organization Framework Convention on Tobacco Control
Canada, together with 179 other jurisdictions, is a Party to the World Health Organization Framework Convention on Tobacco Control (FCTC). The FCTC is the first international treaty negotiated under the auspices of the World Health Organization.
The FCTC, which came into force in 2005, was developed in response to the global tobacco epidemic. It reaffirms the right of all people to the highest standard of health. Based on scientific evidence, it sets out minimum requirements for action; Parties to the treaty are invited to exceed those requirements.
Executive Summary
The purposes of this report are to inform interested parties about Health Canada's level of effort in monitoring compliance with the Tobacco Act and its regulations, and to identify areas where the tobacco industry has not complied with the applicable legislation.
Tobacco control initiatives in Canada have led to a significant reduction in tobacco use prevalence. However, despite decades of effort, there are still 4.6 million tobacco users in Canada, including 3.9 million current smokers. In 2015, 115,000 Canadians became daily smokers.Footnote 1
Canada's current tobacco control efforts are grounded in the Federal Tobacco Control Strategy (FTCS). Actions taken pursuant to the Tobacco Act contribute to preventing people--in particular youth--from taking up tobacco use, and to helping tobacco users quit.
Health Canada actively monitors tobacco manufacturers and importers, retailers, and others to identify cases of non-compliance with the Act and regulations. Health Canada takes enforcement actions where warranted.
Key Statistics for 2016-2017
Through its compliance monitoring and enforcement activities, between April 2016 and March 2017 (i.e. fiscal year 2016-2017), Health Canada:
- Collected and analyzed approximately 700 samples from the tobacco manufacturing and importing sector. Among the subsets of samples analyzed, 14% were found to be non-compliant with respect to the prohibition on the promotion of additives on packaging, 6% were non-compliant with respect to the Tobacco Products Labelling Regulations (Cigarettes and Little Cigars) and 1% were found to be non-compliant with the ban on certain additives in tobacco products.
- Completed 6792 inspections of tobacco retailers. The most frequently observed infractions were the labelling of cigarettes and little cigars that did not meet the Tobacco Products Labelling Regulations (Cigarettes and Little Cigars), the presence of self-service tobacco displays (where consumers were able to handle tobacco products before purchase), and the promotion of tobacco products not permitted under the Tobacco Act.
- Reviewed 1037 reports (reports that are required under the Tobacco Reporting Regulations of the Tobacco Act) from tobacco manufacturers and importers. During the year, 68 letters of deficiency were issued due to incomplete or missing reports. These letters indicate to the manufacturer or importer which missing information is to be submitted to Health Canada, along with the time-frame for doing so.
It is important to note that tobacco manufacturers, importers, and retailers who comply with the different provisions of the Tobacco Act still manufacture or sell a harmful product: every year, 37,000 people die in Canada from tobacco use, which is about one of every six deaths.
Compliance and Enforcement Activities
Scope of Tobacco Control Efforts
Current tobacco control legislation supports the FTCS in helping prevent people from taking up tobacco use, and helping users quit. Specifically, the Tobacco Act is intended to protect young people and others from inducements to use tobacco products, restrict youth access to these products, and enhance public awareness of the health hazards of using tobacco products. The Act does not directly address the harmful effects of tobacco products.
Since its coming into force in 1997, the Tobacco Act has been amended twice, and a number of regulations have been adopted.
Key Measures
- Protecting youth from inducements to use tobacco products by banning additives, including flavours, which contribute to making these products appealing: With the Cracking Down on Tobacco Marketing Aimed at Youth Act, which amended the Tobacco Act in 2009, Canada became the first country in the world to ban the use of certain additives in cigarettes, little cigars, and blunt wraps. The banned additives include flavours like chocolate and bubble gum that were making tobacco products more appealing to youth. This ban was extended in 2015 to include cigars with a wrapper that is not fitted in spiral form and cigars with tipping paper. Cigars that have a wrapper fitted in spiral form and that weigh more than 1.4 g but not more than 6 g, excluding the weight of any mouthpiece or tip, were also included in this ban, but are allowed to contain additives that impart a flavour that is generally attributed to port, wine, rum or whisky.
- Restricting advertising for tobacco products: The promotion of tobacco products by means of "information advertising" or "brand-preference advertising" is limited to publications delivered by mail to a named adult and on signs in places where young persons are not permitted by law.
- Labelling requirements, such as health warnings: Canada was the first country to require pictorial health warnings on tobacco packages in 2000. In June 2011, the Tobacco Products Labelling Regulations (Cigarettes and Little Cigars) were adopted to renew and strengthen these messages. These regulations increased the size of the health warnings from 50% to 75% of the main panels of cigarette and little cigar packages.
- Disclosure requirements for the tobacco industry: Under the Tobacco Reporting Regulations, tobacco manufacturers and importers must submit regular reports that include sales data, manufacturing information, information on the ingredients used in their products, constituents and emissions information, as well as information on their research and promotional activities.
Three Pillars of Compliance and Enforcement Activities
Compliance and enforcement activities are based on three pillars, as described below:
- Compliance Promotion
- Compliance Monitoring
- Enforcement
1. Compliance Promotion
To promote industry's compliance with the Tobacco Act and its regulations, Health Canada carries out a range of activities, including publishing public notices and conducting presentations with businesses. By sharing information on the applicable restrictions and requirements, Health Canada expects that manufacturers, importers and retailers will make well-informed decisions regarding how they manufacture, sell, label and promote their products.
Compliance promotion initiatives may be stand-alone activities, such as information provided in trade publications or at trade shows, direct e-mail communication to affected industry members, or information sessions, or they may be part of a planned inspection.
During on-site inspections at both manufacturer/importer and retail locations, inspectors provide, as needed, information on the provisions being monitored. They also answer questions and listen to concerns that company representatives raise.
When new provisions are adopted, Health Canada informs those sectors of the industry that will be affected by these new measures.
2. Compliance Monitoring
Through its compliance monitoring work, Health Canada oversees regulated businesses to verify that their activities are carried out in accordance with the Tobacco Act and its regulations and to identify and assess cases of non-compliance. Both inspectors and reviewers play a role in the Department's tobacco compliance monitoring activities:
- Inspectors conduct inspections at manufacturer/importer facilities and retailer locations across Canada. During an inspection, inspectors may perform audits, collect samples, and carry out analysis of tobacco product packaging and of promotional materials.
- Reviewers examine reports received from tobacco companies to determine if they comply with the Tobacco Reporting Regulations.
3. Enforcement
Health Canada uses a progressive enforcement regime, in which activities range from promoting compliance up to and including prosecution and court orders.Footnote 2 If a business is found to be non-compliant with a specific section of the Tobacco Act or its regulations, Health Canada considers a range of actions to induce, encourage, or compel the business to correct the observed non-compliance. The Department considers many factors in determining the appropriate enforcement action. Among these are: how severe the alleged non-compliance is; how likely it is to reoccur; how likely it is that the business will cooperate and become compliant; and, the expected impact of a possible enforcement action.
The main enforcement actions taken when Health Canada observes non-compliance include negotiating compliance with the regulated business, issuing warning letters including those that outline deficiencies in industry reports, and seizing products.
Compliance Promotion, Monitoring, and Enforcement Activities involving the Manufacturing / Importing Sector
In Canada, about 60 manufacturers and importers are actively involved in the sale of tobacco products. The majority are located in Ontario and Québec. The main measures Health Canada monitors compliance with are:
- The cigarette ignition propensity standard, as set out in the Cigarette Ignition Propensity (Consumer Products) Regulations ("CIPR") under the Canada Consumer Product Safety Act;
- The prohibition on the use of certain additives in cigarettes, most cigars and blunt wraps, as per sections 5.1 and 5.2 of the Tobacco Act ("Prohibited additives");
- The prohibition on promoting, by means of cigarette, most cigar and blunt wrap packaging, the presence of additives that cannot be in used in said products, as per section 23.1 of the Tobacco Act ("Prohibition of Promotion of Banned Additives on Packaging");
- The labelling requirements (specifically, health warnings, toxic emissions statements, and health information messages) as set out in the Tobacco Products Labelling Regulations (Cigarettes and Little Cigars) ("TPLR").
Inspections in fiscal year 2016-2017, and trends over time
Tobacco inspections in the manufacturing/importing sector consist of sampling tobacco products, which are then analyzed for compliance with the applicable provisions of the Tobacco Act and its regulations. Once the analyses are completed, the determination of an appropriate enforcement action is made for all cases of observed non-compliance.
Non-compliance rates based on samples from the manufacturing/importing sector analyzed against specific provisions are shown in the table below. The non-compliance rate reflects the number of samples found to be non-compliant with the provisions in question.
| Provisions | Number of Samples Analyzed | Non-Compliance Rate (%) |
|---|---|---|
| Cigarette Ignition Propensity (Consumer Products) Regulations (CIPR) | 16 | 0% |
| Prohibited Additives | 203 | 1% |
| Prohibition of Promotion of Banned Additives on Packaging | 91 | 14% |
| Tobacco Products Labelling Regulations (Cigarettes and Little Cigars) (TPLR)Footnote * | 360 | 6% |
|
||
No tobacco product samples analyzed against section 3 of the CIPR were found to be non-compliant. Among the tobacco products analyzed for "Prohibited additives", 1% were found to contain a prohibited additive. Additionally, 14% of the tobacco products analyzed against the "Prohibition of Promotion of Banned Additives on Packaging" did not respect the prohibition on the promotion of prohibited additives on the packaging of tobacco products. Finally, 6% of the tobacco products analyzed against the labelling provisions did not meet the requirements set forth in the TPLR, including minimum packaging requirements set out in section 10.1 of the Tobacco Act.
The number of warning letters issued and the number of seizures conducted by Health Canada in the manufacturing/importing sector in fiscal year 2016-2017 are shown in the table below.
| Enforcement Action | Health Canada Region | Number of Actions | ||||
|---|---|---|---|---|---|---|
| AtlanticFootnote 3 | Québec | Ontario | PrairiesFootnote 4 | British Columbia | ||
| Number of seizures conducted | 0 | 0 | 3 | 0 | 0 | 3 |
| Number of warning letters issued to manufacturersFootnote * | 0 | 3Footnote ** | 15Footnote ** | 0 | 0 | 18 |
|
||||||
Non-compliance rates based on samples from the manufacturing/importing sector analyzed over the last three fiscal years are set out in the following table. It shows trends over time for non-compliance with specific regulatory provisions. The non-compliance rate for each provision was obtained by dividing the number of non-compliant samples identified (not shown) by the number of samples analyzed (shown).
| Provisions | 2014-2015 | 2015-2016 | 2016-2017 | |||
|---|---|---|---|---|---|---|
| Number of Samples Analyzed (1357) |
Non-Compliance Rate (%) | Number of Samples Analyzed (795) |
Non-Compliance Rate (%) | Number of Samples Analyzed (670) |
Non-Compliance Rate (%) | |
| CIPR | 20 | 0% | 18 | 0% | 16 | 0% |
| Prohibited Additives | 197 | 0% | 191 | 1% | 203 | 1% |
| Prohibition of Promotion of Banned Additives on Packaging | 436 | 0% | 110 | 11% | 91 | 14% |
| Minimum Packaging | 247 | 0% | 79 | 0% | - | - |
| TPLR | 457 | 9% | 397 | 3% | 360Footnote * | 6% |
|
||||||
The non-compliance rates (from the above table) are presented in graph form below to show trends for fiscal years 2014-2015 to 2016-2017. The percentages represent the non-compliance rate for samples analyzed for each provision.
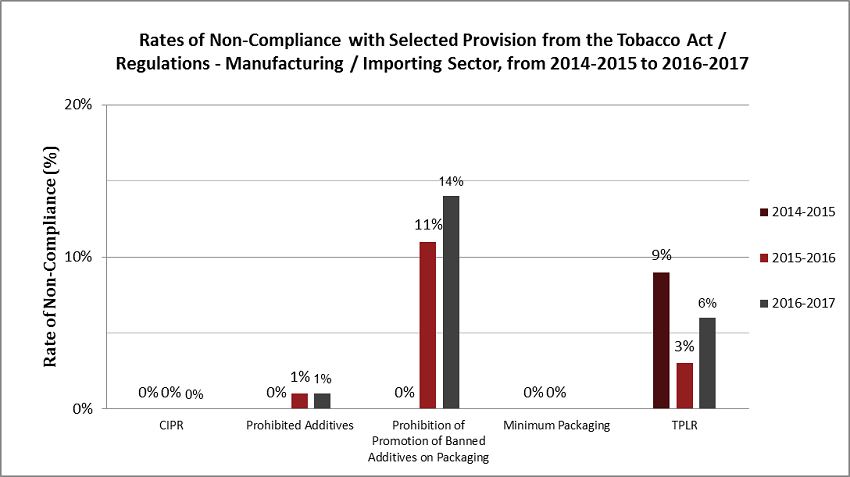
Description - Rates of Non-Compliance with Selected Provisions of the Tobacco Act/Regulations - Manufacturing/Importing Sector, 2014-2015 to 2016-2017.
Bar chart showing rates of non-compliance for selected provisions of the Tobacco Act and Regulations for fiscal years 2014-2015, 2015-2016 and 2016-2017. CIPR = 0% non-compliance for 2014-2015, 0% non-compliance for 2015-2016 and 0% non-compliance for 2016-2017; Prohibited Additives = 0% non-compliance for 2014-2015, 1% non-compliance for 2015-2016 and 1% non-compliance for 2016-2017; Prohibition of Promotion of Banned Additives on Packaging = 0% non-compliance for 2014-2015, 11% non-compliance for 2015-2016 and 14% non-compliance for 2016-2017; Minimum Packaging = 0% non-compliance for 2014-2015, 0% non-compliance for 2015-2016, not assessed independently for 2016-2017; TPLR = 9% non-compliance for 2014-2015, 3% non-compliance for 2015-2016 and 6% non-compliance for 2016-2017.
Compliance Promotion, Monitoring, and Enforcement Activities involving the Retail Sector
There are about 30,000 to 35,000 points of sale for tobacco products across Canada. Health Canada monitors their compliance with the following key measures:
- Minimum packaging requirements
- Prohibition of promotion of banned additives on packaging
- Labelling requirements
- Prohibited promotional activities.
Inspections in fiscal year 2016-2017, and trends over time
Health Canada conducts inspections of tobacco retailers in order to promote and assess retailers' compliance with the Tobacco Act and its regulations. A retailer is identified as non-compliant if at least one case of non-compliance that relates to the above-mentioned key measures is observed during the inspection.
The retail environment is where tobacco users have access to tobacco products. Taking timely action on observed non-compliance in the retail sector prevents the sale and promotion of tobacco products that are appealing to youth. It also helps prevent the sale of tobacco products that do not display the required health warnings.
The number of inspections and the non-compliance rate based on samples from the retail sector analyzed in fiscal year 2016-2017 are shown in the table below. The non-compliance rate is based on the percentage of retailers inspected where at least one case of non-compliance was observed.
| Provisions | Number of Inspections | Non-Compliance Rate (%) |
|---|---|---|
|
6792 | 11% |
In fiscal year 2016-2017, Health Canada completed 6792 retail inspections across Canada, with an overall non-compliance rate of 11% with respect to the provisions listed in the above table.
The number of enforcement activities (both seizures at retail and warning letters issued) in each region is shown in the table below.
| Enforcement Action | Atlantic | Québec | Ontario | Prairies | British Columbia | Total Number of Actions |
|---|---|---|---|---|---|---|
| Number of seizures at retail | 42 | 231 | 117 | 58 | 14 | 462 |
| Number of warning letters issued to retailersFootnote * | 0 | 0 | 0 | 1 | 0 | 1 |
|
||||||
The Order Amending the Schedule to the Tobacco Act came into force in December 2015, banning the manufacture, packaging and sale of certain cigars that contain or promote, through their packaging, a prohibited additive. The increased number of seizures observed across the country (462), compared with 271 in the previous fiscal year, is a result of enforcement actions to get these products off the market.
The number of inspections conducted in the retail sector over the last three fiscal years is shown in the table below. The non-compliance rate for each year is expressed as the percentage of retailers inspected where at least one case of non-compliance was observed.
| Fiscal Year | Number of InspectionsFootnote * | Non-Compliance Rate |
|---|---|---|
| 2014-2015 | 6774 | 12% |
| 2015-2016 | 6719 | 13% |
| 2016-2017 | 6792 | 11% |
|
||
The number of inspections conducted at retail has remained relatively constant in fiscal years 2014-2015, 2015-2016 and 2016-2017, as have the non-compliance rates.
Compliance Promotion, Monitoring and Enforcement Activities concerning Industry Reporting
As noted earlier, the Tobacco Reporting Regulations require tobacco manufacturers and importers to regularly submit reports to the Minister of Health (see page 5 for section on Disclosure requirements for the tobacco industry for a list of the information to be submitted). Health Canada assesses the reports to verify that the required information is provided. When a report is assessed as missing required information, Health Canada issues a letter outlining the deficiency to the company who submitted the incomplete report.
As the information contained in the industry reports is used to inform policies and support the development of tobacco regulations, it is critical that the information received be complete and accurate.
In fiscal year 2016-2017, Health Canada reviewed 1037 reports from manufacturers and importers. Of the reports reviewed, 119 (11%) were determined to be incomplete, and a total of 68 letters of deficiency were issued.Footnote 5
The number of reports reviewed, the number of these deemed incomplete and the number of letters of deficiency issued over the last three fiscal years are displayed in the table below to show the trend over time.
| Fiscal Year | Number of Reports Reviewed | Number (and Percentage) of Reports Deemed Incomplete | Number of Letters of Deficiency Issued |
|---|---|---|---|
| 2014-2015 | 1607 | 172 (11%) | 81 |
| 2015-2016 | 1485 | 131 (9%) | 58 |
| 2016-2017 | 1037 | 119 (11%) | 68 |
Prosecution
In 2006, Health Canada investigated Compagnie de tabac Dynasty Inc. for alleged failure to comply with the requirements set out in the Tobacco Reporting Regulations. In April 2007, 35 charges were filed before the Court of Québec against the company and its majority shareholder. On May 19, 2016, the company and its majority shareholder, Mr. Ahmad Said Trad, pleaded guilty to all 35 counts. The court fined the company $305,000 and the shareholder, $68,000.
Litigation
Canadian tobacco control legislation has been challenged by members of the tobacco industry for most of the period since 1988. The most recent case involves the Tobacco Products Labelling Regulations (Cigarettes and Little Cigars), which were challenged by Imperial Tobacco Canada and JTI-Macdonald in April 2012. The case is still before the courts.
In another case filed in April 2016, GVA Distribution Inc. (GVA) applied for a judicial review, seeking a declaratory judgment in Federal Court on the interpretation of the exception to the prohibition of certain additives (listed in the Schedule to the Tobacco Act), which allows the use of additives that "impart a flavour that is generally attributed to port, wine, rum or whisky" in some cigars. On February 21, 2017, the Court granted GVA's application in part. On March 23, 2017, the Attorney General of Canada filed an appeal of the judgment with the Federal Court of Appeal. The case is still before the courts.
Conclusion
Despite decades of effort, there are still 4.6 million tobacco users in Canada (including 3.9 million current smokers).
Canada's Tobacco Act is a key component of the FTCS. Health Canada actively monitors compliance with the Tobacco Act and its regulations. Where a business is found to be non-compliant, Health Canada considers a range of actions to induce, encourage, or compel the business to correct the observed non-compliance.
In fiscal year 2016-2017, a non-compliance rate of 11% was observed at retail. Retail establishments are identified as non-compliant when at least one instance of non-compliance is observed for that establishment.
With respect to the manufacturing and importing sector, non-compliance was mainly the result of the promotion of banned additives on the packaging of tobacco products (14% of analyzed samples deemed non-compliant) and a lack of proper disclosure of information (11% of reports deemed incomplete).
The Tobacco Act recognizes the inherent harmful nature of tobacco products; even when tobacco manufacturers comply with all the provisions of the Tobacco Act, their products remain harmful.
Key Priorities for fiscal year 2017-2018
Health Canada's key priorities with respect to compliance and enforcement activities in relation to the Tobacco Act for fiscal year 2017-2018 are:
- To continue to promote compliance with the Tobacco Act and its regulations and actively monitor the market.
- To promote compliance with the Order Amending the Schedule to the Tobacco Act (Menthol), which prohibits the manufacture and sale of cigarettes, blunt wraps and most cigars that contain menthol, and the promotion of menthol on the packaging of these tobacco products. The Order takes effect on October 2, 2017.
Footnotes
- Footnote 1
-
Health Canada. "Canadian Tobacco, Alcohol and Drugs Survey (CTADS): 2015 Summary." Ottawa, ON, (Corrections posted in 2017) https://www.canada.ca/en/health-canada/services/canadian-tobacco-alcohol-drugs-survey/2015-summary.html
- Footnote 2
-
Court orders can be sought to prevent a person from contravening the Tobacco Act or its regulations.
- Footnote 3
-
The Atlantic region includes Newfoundland and Labrador, Prince Edward Island, Nova Scotia and New Brunswick.
- Footnote 4
-
The Prairies region includes Manitoba, Saskatchewan and Alberta, as well as Nunavut, Northwest Territories and the Yukon.
- Footnote 5
-
In a number of cases, one letter referred to more than one deficiency.