Consultation summary: proposals for the regulation of vaping products

Download the alternative format
(PDF format, 2.2 MB, 19 pages)
Organization: Health Canada
Published: April 2018
Cat.: H149-8/2018E-PDF
ISBN: 978-0-660-24512-6
Pub.: 170393
Table of contents
- Executive Summary
- 1. Background
- 2. Overview of Comments on Proposals to Regulate Vaping Products
- 3. Summary of Comments on Each Proposed Measure
- 4. Additional Comments on Other Applicable Legislation
- 5. Next Steps
Executive Summary
Health Canada published a consultation document on August 25, 2017, setting out proposals to regulate vaping products in Canada. The document Proposals for the Regulation of Vaping Products was open for comments from the public and interested stakeholders for a 60-day period ending October 27, 2017. In total, 105 comments were received from across nine groups: academics; the general public; other levels of government (municipal, provincial and territorial); the health products industry; the vaping industry; the tobacco industry; non-governmental organizations (NGOs); public health groups; and retailers (including vape shops).
Comments on the proposed regulations were generally supportive, with some clear but expected differences of opinion and specific concerns identified by some groups. For example, some public health groups and NGOs expressed concern about the negative health consequences of vaping, and advocated for additional restrictions than those being proposed. The vaping industry and retailers cited vaping products as an important harm reduction tool that, in order to achieve its potential health benefits, should not be over-regulated.
This report provides a summary of the feedback received during the consultation period on each of the 10 proposed regulatory measures.
The consultation was not intended to capture comments on the federal government's broader legislative approach to vaping products (as set out in Bill S-5, An Act to amend the Tobacco Act and the Non-smokers' Health Act and to make consequential amendments to other Acts) being currently examined by Parliament. However, many comments received spoke to Bill S-5 and a high level summary of comments on this issue is included in this report.
1. Background
In March 2015, the House of Commons Standing Committee on Health issued a report entitled "Vaping: Towards a regulatory framework for e-cigarettes."Footnote 1 Following eight meetings with 33 witnesses, the Committee put forward 14 recommendations, one of which asked the Government of Canada to "work with all affected stakeholders to establish a new legislative framework (under the Tobacco Act, new legislation, or other relevant statutes) for regulating electronic cigarettes and related devices."
In response, the Government of Canada introduced in Parliament Bill S-5, an Act to amend the Tobacco Act and the Non-smokers' Health Act and to make consequential amendments to other Acts, in November 2016.Footnote 2 The Bill's framework for vaping products is based on the following principles:
- Protecting youth and non-users of tobacco products from nicotine addiction and inducements to tobacco use;
- Allowing adults-in particular, adult smokers-to access vaping products as a less harmful alternative to tobacco;
- Providing a mechanism through the Canada Consumer Product Safety Act (CCPSA) to address potential health and safety risks from nicotine-containing vaping products without therapeutic claims;
- Preserving the current regulatory process through the Food and Drugs Act (FDA) for vaping products marketed for a therapeutic use, such as smoking cessation.
Bill S-5 contains provisions that would amend the Tobacco Act, including changing its title to the "Tobacco and Vaping Products Act" (TVPA). The proposed TVPA would regulate the manufacture, sale, labelling and promotion of vaping products as a set of products separate from tobacco products.
Under the framework proposed by Bill S-5, vaping products would also be required to meet the applicable provisions of either the FDA or the CCPSA, depending on whether the product is marketed with or without therapeutic claims. Information on the applicability of the FDA and the CCPSA to vaping products is included at the end of this document, under Additional Comments on Other Applicable Legislation.
On August 25, 2017, Health Canada proposed 10 measures for the regulation of vaping products to be made under the proposed TVPA. The Department invited all interested organizations and individuals to review the measures being considered and to provide feedback that would be used to inform the development of regulations.
2. Overview of Comments on Proposals to Regulate Vaping Products
In total, Health Canada received 105 submissions during the 60-day consultation period (August 25 to October 27, 2017), spread across nine groups, as set out in Figure 1, below. Of the 105 comments received, only 40% of respondents commented specifically on the regulatory proposals. Most respondents took the opportunity to provide comments about the government's legislative approach proposed in Bill S-5. Comments received on the regulatory proposals were mostly supportive, however, commenters raised various concerns about their implementation.
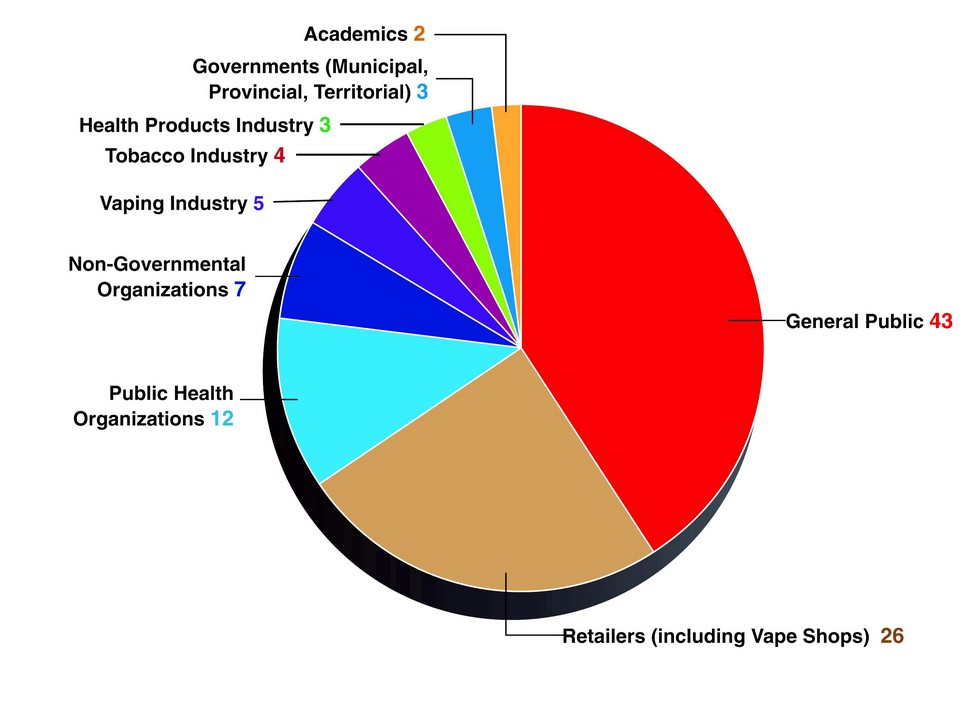
Figure 1 - Text description
| Stakeholder Group | Number of submissions |
|---|---|
| General Public | 43 |
| Retailers (Including vape shops) | 26 |
| Public Health Organizations | 12 |
| Non-Governmental Organizations | 7 |
| Vaping Industry | 5 |
| Tobacco Industry | 4 |
| Health Products Industry | 3 |
| Governments (Municipal, Territorial, Provincial) | 3 |
| Academics | 3 |
Most submissions were made by members of the general public (43) and retailers, including vape shops (26), with most identifying themselves as vapers and former smokers. Of those who identified themselves as vapers, most framed vaping products as lifesaving, describing their past difficulties quitting smoking and how they used vaping products to finally stop smoking.
Public health groups that made submissions (12 in total) included medical practitioners and public health advocates. For this group, as well as those in the NGO group (7 commenters), views were divergent. Some identified vaping products as a harm reduction tool, while others characterized them as a risk to public health. Of those who expressed concern about the negative impact of vaping product use, most were worried about their availability to youth and inducements that would promote vaping product use. The public health groups generally advocated for additional restrictions on advertising, as well as more stringent requirements for labelling and industry reporting beyond what was being proposed in the consultation document.
Submissions from industry were provided by three sub-groups based on the products they market:
- The health products industry (three submissions from marketers of medical devices and therapeutic products) generally expressed concern that there was overlap between the FDA and the proposed TVPA, which will regulate vaping products.
- The tobacco industry (four submissions) generally supported the proposed regulations. They expressed concern, however, about the proposals related to the disclosure of confidential business information and the potential public dissemination of that information (Proposal 5). These commenters also advocated that additional authority be added to Bill S-5 to allow them to promote certain tobacco products as less harmful products, similar to proposed regulatory measures for vaping products.
- The vaping industry (five commenters representing vaping product manufacturers and importers) expressed concern that the proposed regulations were too restrictive. They argued that vaping products are an important harm reduction tool and, in order to achieve their potential health benefits, should not be overregulated. They said the regulations would be too burdensome for smaller vaping industry players, forcing them underground. This same group did indicate support for many of the other proposals, as described later in this report.
Submissions from municipal, provincial and territorial governments were generally supportive of the proposals and, at the same time, expressed concern that vaping product use and promotion would renormalize smoking behaviours and be detrimental to public health. Of the two submissions from academics, one did not support the proposed regulation of vaping products at all, citing vaping as a vastly important harm reduction tool in the fight against smoking that should not be regulated; the other was generally supportive of the proposals.
Although Bill S-5 was not the subject of this consultation, many commenters stated that they did not support the inclusion of vaping products in the same legislation as tobacco products, in turn subjecting them to similar restrictions. Concerns over the restrictions on the promotion of flavours were also expressed as this may be detrimental to efforts to entice smokers to switch to vaping products. As well, concerns were expressed over the possible future taxation of vaping products (which is not part of Bill S-5).
3. Summary of Comments on Each Proposed Measure
Labelling: Proposals 1 and 2
Labelling-Nicotine content in mg/ml
Proposal 1: Health Canada proposes that all vaping products which contain nicotine display their nicotine concentration in milligrams/millilitre (mg/ml).
There was strong support for Proposal 1.
Almost all (approximately 85%) that commented on this proposal were in support. One commenter suggested that the percent (%) of nicotine concentration should also be considered for display on labels.
Only two commenters were opposed to this proposal. One commenter stated that vaping products-as a harm reduction tool-should not be required to display any labelling requirements. Another commenter was concerned about the legislative overlap for labelling requirements under the FDA and those being proposed under the TVPA.
Labelling-Products that contain more than 0.1mg/ml of nicotine
Proposal 2: To prevent consumers from being misled about the presence or absence of nicotine, Health Canada proposes that any vaping product be considered to contain nicotine if nicotine is present at a concentration of 0.1 mg/ml or higher.
There was strong support for Proposal 2.
Almost all of those that commented on this proposal were in support (approximately 95%) of establishing a level of nicotine to require labelling. However, some public health groups and municipal/ provincial/territorial governments that commented stated that a vaping product should be considered to contain nicotine at any detectable level, as opposed to the proposed 0.1 mg/ml. Approximately the same number of retailers and manufacturers of vaping products noted that a higher level, such as 0.5mg/ml, would be more appropriate. According to them, current methods are only capable of detecting the presence of nicotine above 0.5mg/ml. Lastly, there was a comment to allow the nicotine content to be expressed as a range of +/-10% of the actual nicotine content.
Only two commenters were opposed to this proposal. Their concerns are the same as those raised for Proposal 1.
Warning Statements: Proposals 3 and 4
Warning statement
Proposal 3: Health Canada proposes to require that vaping products that contain nicotine display a warning such as "WARNING: This product contains nicotine. Nicotine is an addictive substance. Use of nicotine during pregnancy may harm the fetus."
There was strong support for Proposal 3.
Many of those that responded to this proposal (approximately 93%) supported the display of a warning although several suggested different warning statements. Several commenters also mentioned the label size as a limiting factor, with concern that the information would be illegible due to tiny print size.
Some commenters felt that the warning statement needed to be stronger to truly warn of harm, while others felt that the proposed warning might discourage smokers, especially pregnant women who smoke, from switching to a less harmful alternative (i.e. vaping). Of the few who were opposed to this proposal, one opposed all labelling regulations due to vaping's harm reduction potential. Another (a retailer) expressed concern that the limited space on the label of a small 30ml bottle of e-liquid would prevent compliance with all of the proposed requirements. Rather, it was suggested that the warning could be displayed in the retail store, instead of on the bottle.
A requirement for plain and standardized packaging (packages without any distinctive or attractive features, that are similar in appearance and shape, and of the same ordinary colour), was suggested as a potential requirement for vaping products by a few public health groups and NGOs, similar to those proposed for tobacco products. There were other suggestions, including the use of a rotating set of graphic warnings (akin to current cigarette warnings) to be placed on the vaping product label, covering 70% of the package.
List of ingredients
Proposal 4: Health Canada proposes to require that products that contain a vaping liquid display a complete list of ingredients in descending order by weight.
There was strong support for Proposal 4.
There was strong support for the proposal, however several of those in support raised concerns over how flavourings should be listed. One frequent suggestion (mainly from retailers and members of the vaping industry) was that only "flavouring" or "natural and artificial flavourings" should be listed in order to keep manufacturers' flavouring ingredients proprietary, and to ensure that there is enough room on the label for other health-related information.
Among the few who opposed this proposal, one commenter stated that the proposed measure is burdensome and far too cumbersome, given the harm reduction potential of vaping products. Another supported having a list of ingredients for vaping products, but was also concerned about overlap with the FDA, which requires that medicinal and non-medicinal ingredients be listed on the label or package insert.
Information Reporting: Proposals 5 to 8
Information reporting-information to be reported to Minister of Health
Proposal 5: Health Canada proposes that manufacturers be required to report the information set out belowFootnote 3 at the frequency specified.
Feedback about Proposal 5 was mixed.
Approximately half of respondents who commented on this proposal were in support while the other half expressed concerns. Among the supporters were mainly public health groups and NGOs, as well as retailers and other members of the vaping industry. A number of them suggested additional items to be reported, and some suggested a change to the timing of reporting. A few NGOs specified that they would like to see emissions testing for vaping products included in reports to the Minister of Health. Other calls were for collected data to be made available to the provinces or to the public, and that regulations for vaping products should be similar to the Tobacco Reporting Regulations. Some of those in favour of this proposal wanted clarification and additional detail on the reporting elements.
Some retailers and both the tobacco and vaping industries expressed concern about releasing proprietary information to Health Canada. Some were concerned that regulations would be too burdensome, which would drive the industry underground, creating an industry of "Do-It-Yourselfers." Other commenters said they worried that their custom e-juice manufacturing business would not be able to meet the reporting requirements. Some claimed that the proposal would freeze innovation by requiring the industry to hand over confidential business information. Industry commenters noted that there are provisions in Bill S-5 to make some information public, and they expressed concern that their confidential business information would be made public under the Bill S-5 authority.
Requests for supplemental information
Proposal 6: Health Canada proposes that manufacturers of vaping products be required to provide supplementary information in a form, manner and within the time frame specified, once notified by the Minister. The form, manner and time frame allowed for manufacturers to provide the supplementary information would be specified in the request and could vary according to the nature of the information requested.
Feedback on Proposal 6 was mixed, and consistent with Proposal 5.
Many NGOs, public health groups, retailers and industry members supported this proposal. Those critical of Proposal 5 were also critical of this proposal. Reasons include the broader "overreach" of the reporting requirement. At least one commenter questioned what might constitute "supplemental information", requesting that it not be overly burdensome and that clarity be provided about what information would be required.
Measures to enhance compliance with reporting requirements
Proposal 7: Health Canada proposes that manufacturers of vaping products be given a period of no more than 30 calendar days to address any deficiency in the reporting of information prescribed by the regulations, once they are notified of the deficiency by Health Canada. Should the manufacturer fail to address the deficiency, or should the information provided continue to be deficient, the sale of the product in question would be suspended until the missing information is submitted to Health Canada, and the manufacturer would be informed accordingly.
There was mixed support for Proposal 7.
As is the case for Proposals 5 and 6, both support for and opposition to this proposal were expressed. Concerns included that the proposal constitutes government overreach, and that it would drive the industry underground. There was also concern about how this proposal would be enforced. While some supported the idea of having measures to enhance compliance, some retailers and industry groups stated that 30 days may not be enough time to comply and that provisions should be in place to extend the timeline as necessary.
Other suggestions included that the timeline could be negotiated for more complex situations. One commenter pointed out that halting the sale of a product can have a major detrimental effect on the industry. For this reason, the suggestion was made that the decision to halt sale should be made at a higher level within government (e.g. at the Director or Director General level), and that there should also be a mechanism that would allow companies to appeal decisions if they believe that they have complied with the regulations.
There was support for this proposal among the municipal/provincial/territorial governments, public health groups, the vaping industry and NGOs. Some said that 30 days is too lenient, and that sales should be halted as soon as the deficiency is noted.
Record-keeping practices by manufacturers
Proposal 8: Health Canada proposes that manufacturers of vaping products be required to maintain all records and documents used to prepare their information reports for a period of six (6) years after the end of the year to which the document relates. This documentation would have to be kept in a form and manner prescribed by the regulations, so that it could be readily accessed and viewed in Canada during audits.
There was general support for Proposal 8.
This record-keeping proposal had broad support (approximately 95%) from almost all groups, including public health groups, NGOs, industry and retailers. One frequent suggestion from public health groups and NGOs was to significantly lengthen the time that industry must keep their records (to 25 or 30 years, much longer than the six years proposed in the regulations). As was the case with some of the other proposals, some expressed concern about overlap (being regulated by both the FDA and the proposed TVPA). Also in line with some other proposals, the vaping industry expressed concern about the burdensome requirements of this proposal.
Relative Risk Statements: Proposal 9
Relative risk statements
Proposal 9: Health Canada proposes to establish regulations that would specify the conditions upon which manufacturers, retailers and others could use authorized relative risk statements in vaping product promotions. The regulations would incorporate by reference a selection of authorized statements regarding the relative health risks of using vaping products or comparing the potential health effects arising from the use of a vaping product relative to that of a tobacco product. As the authorized statements may need to be amended from time to time to keep up with scientific knowledge, these regulations would also set out the requirement for public consultations on such amendments.
There was general support for Proposal 9.
While there was broad support (approximately 90%) expressed across commenter groups for this proposal to allow "relative risk" statements, there were different views about how to move forward with the regulation of such statements, and concern about who would be responsible for developing acceptable statements.
Public health groups, NGOs and those not affiliated with the vaping industry tended to support relative risk claims, however many had concerns about the implementation of this proposal. Among those with concerns, widely divergent views were offered, from complete opposition, to preventing any form of relative risk statements for vaping products, to support for a precautionary approach that would allow such statements only when there is unequivocal evidence of their reduced harm. A few offered full support for relative risk statements as a means to educate consumers about their reduced harm, compared to that of cigarettes.
Some from the health products industries expressed concern that relative risk statements may drift into therapeutic claims (such as claims that vaping products aid in smoking cessation), which would be better regulated under the FDA. One commenter noted their concern that smoking cessation products that are authorized under the FDA might not be allowed to have relative risk statements, yet under this proposed new regime, vaping products that do not undergo a pre-market review under the FDA prior to their sale would be able to make a relative risk statement.
Some NGOs offered that relative risk statements should be accessible only to smokers and never to youth or non-smokers, and to achieve this should only be placed in cigarette and little cigar packages as part of mandated Health Information Messages, to persuade smokers to switch to vaping product use.
Commenters across all nine groups expressed interest in being involved in the decision-making process, to help ensure that approved relative risk statements are scientifically sound and appropriately address reduced risk. Some commenters said that any industry should not be involved in the generation of reduced risk statements. However, the vaping industry indicated strong support for relative risk statements and advocated for their involvement in the creation of such statements. The tobacco industry expressed support for relative risk statements if they could also be allowed to make similar statements for different tobacco products (such as "heated" tobacco and smokeless tobacco). The tobacco industry commenters also advocated for product-specific reduced risk claims that are supported by science. Both the tobacco and vaping industries also stated they prefer general guidelines for the development of relative risk statements (rather than prescribed statements).
Vaping retailers as well as the general public said they want clear guidance on what can and cannot be said about the health benefits of vaping products. At the same time, some commenters from both these groups said that this is an example of government overreach.
One academic expert was opposed to any restriction on the use of relative risk statements due to the much lower risk associated with vaping products compared to cigarettes.
Advertising Restrictions: Proposal 10
Advertising restrictions
Proposal 10: Health Canada proposes to establish regulations to help limit youth exposure to information and brand-preference advertising of vaping products. These regulations would include restrictions on the type, medium and content of advertising of vaping products. In line with the objectives of the proposed TVPA, the restrictions would be based on limiting advertising that has a high likelihood of being viewed by youth, while still allowing vaping product manufacturers to advertise their products and brands to adult smokers. Restrictions would therefore seek to limit advertising in or near locations that are attended predominantly by youth, such as schools, parks, recreational and sporting facilities. Restrictions would also be placed on advertising in certain media-for example, by either prohibiting advertisements on television and radio or restricting the times of the day when such ads may appear or be heard to limit youth exposure to them.
There was strong support for Proposal 10.
Similar to feedback on Proposal 9, there was strong support (approximately 95%) for this proposal; however, commenters were divided about the need to further restrict advertising. In general, public health groups and NGOs were strong advocates for additional advertising restrictions, with many suggesting that vaping advertising restrictions should align with those for tobacco products. Other suggestions included plain packaging of vaping products, banning of all promotion on television, radio, social media, billboards, point-of-sale and the use of promotional e-mails and giveaways. They also advocated for a ban on all lifestyle advertising, including in age-restricted areas.
Those in the vaping industry (manufacturers and retailers) expressed support for advertising restrictions in order to protect youth, while still allowing marketing of a reduced risk product. Some advocated that social media be allowed as an advertising medium, noting that they already have age-restricted access to their social media feeds, to adults over the age of 19. Others suggested time-of-day bans on vaping advertising on television and radio. One suggested banning all promotional materials near schools.
The vaping industry and retail group raised concern about their ability to comply with these promotional regulations, and called for clear guidance on what would/would not be considered legal. Some expressed confusion about how online user reviews of products would be treated, as well as about the difference between factual and promotional information. Some retailers noted that proposed reduced risk statements (Proposal 9) would benefit the industry as a promotional tool.
4. Additional Comments on Other Applicable Legislation
Canada Consumer Product Safety Act-Child-Resistant Containers
Comments on the proposal to require child-resistant containers (CRCs) for vaping products were received from 27 stakeholders from various groups, including from the general public, retailers, NGOs, public health organizations, the vaping industry and the tobacco industry.
There was strong support for CRCs to be required on vaping substances (liquids) and strong opposition to requiring CRCs on tanks (devices). In many cases, the same commenters expressed support for CRCs on containers of vaping substances (liquids) and strong opposition to CRC on tanks (devices). Sixteen commenters specifically expressed broad support from many different groups for CRCs on bottles of vaping substances, and/or indicated that the bottles they sell already have CRCs. No commenters expressed opposition to the proposal to require CRCs on bottles of vaping substances.
Nineteen of the 27 stakeholders (from the general public, NGOs, retailers, the vaping industry and the tobacco industry) expressed strong opposition to requiring CRCs on the tanks of vaping devices. Most stated that virtually all vaping devices are imported from China, that there are very few vaping device models available with CRCs on the tank, and that manufacturers would be unwilling to develop compliant models for the small Canadian market. Not one commenter expressed support specifically for CRCs on tanks. Several commenters offered additional remarks: that the risk of harmful effects from swallowing the liquid from the tank would be low, because most tanks hold a volume of only 1 to 5 ml. Several indicated that requiring CRCs on tanks would have a devastating effect on the Canadian vaping industry.
Five commenters indicated that among vapers, many are older adults, and that CRCs on tanks would be very difficult for older adults to open and close, and/or that many older customers have difficulty with the closures on current vaping products.
Canada Consumer Product Safety Act - Labelling Requirements
Comments on the proposed labelling requirements under the CCPSA and the Consumer Chemicals and Containers Regulations, 2001 (CCCR, 2001) were received from 13 stakeholders, including from the general public, retailers, NGOs and the vaping industry. Commenters were all supportive of toxicity labelling on vaping substances, although some retailers and one representative from the vaping industry expressed general concern that limited label space may make compliance with the CCPSA and other government labelling requirements problematic. One NGO and one retailer expressed concerns that toxicity warnings on containers of e-liquids with low nicotine concentrations or on tanks may give consumers an exaggerated impression of nicotine toxicity and of the relative risks of vaping compared to smoking.
Canada Consumer Product Safety Act - Other Issues
Other Health and Safety Risks
- Five commenters (including from the public, NGOs, municipal, provincial, territorial governments and the vaping industry) stated that requirements should be introduced to address safety issues related to batteries and other mechanical hazards.
- Two retailers said that requirements to address chemical hazards other than nicotine should be introduced.
- Five commenters (from the general public and the vaping industry) said, more generally, that minimum safety and quality standards for the manufacture of vaping product should be introduced.
Assessment of Nicotine Toxicity under CCCR, 2001, Criteria
- Two commenters, one academic and one from the vaping industry stated that they believed Health Canada's application of CCCR, 2001, toxicity classification criteria to nicotine-containing vaping substances, is incorrect and will result in an overly restrictive regulatory regime.
Manufacturing Processes
- Five commenters (from the public, one academic and one vaping industry) said that the vaping industry needs clarity on requirements for the manufacture of vaping substances. Some of these commenters also indicated that the proposed maximum concentration of 66 mg/ml nicotine for vaping substances sold as consumer products is not appropriate or workable for nicotine solutions used as manufacturing inputs.
Regulatory Pathway
- One retailer stated that the CCCR, 2001, is not an appropriate instrument for regulating CRC and labelling of vaping products. This commenter and a commenter from the health products industry said that the Government of Canada should introduce specific regulations to address health and safety risks related to vaping products.
Food and Drugs Act
Comments on provisions that may affect the FDA came mainly from those in the health products industry. Commenters expressed concern that products authorized for sale under the FDA may also have to meet requirements under the proposed TVPA and its supporting regulations, thus possibly creating confusion on how to comply with both the FDA and TVPA.
An additional concern identified was that the proposed TVPA has fewer restrictions than the FDA. For example, an authorized smoking cessation product that has had a full scientific review may not be allowed to have certain relative risk claims under the FDA, while a vaping product that has had no pre-market review may be allowed to make such claims under the proposed TVPA.
5. Next Steps
Health Canada will take the comments received as part of this consultation into consideration while developing regulations for vaping products under Bill S-5.
Should Bill S-5 receive royal assent, and regulations under it be formally put forward, any proposed regulations and their accompanying Regulatory Impact Assessment Statements would be pre-published in the Canada Gazette, Part I, to give Canadians and interested parties the opportunity to review and provide comments before they are finalized.
Footnotes
- Footnote 1
-
House of Commons Standing Committee on Health. Vaping: Towards a regulatory framework for e-cigarettes, report of the Standing Committee on Health. 2015 Mar [cited 2017 Jun 5]. Available from: http://www.ourcommons.ca/DocumentViewer/en/41-2/HESA/report-9.
- Footnote 2
-
An overview of the Bill is available from: https://www.canada.ca/en/health-canada/programs/consultation-regulation-vaping-products/s5-overview-regulate-vaping-products.html.
- Footnote 3
-
Proposed information and frequency are listed on page 5, "Proposals for the Regulation of Vaping Products. Document for Consultation, August 2017" (Health Canada).