Guidelines for Canadian Drinking Water Quality: Guideline Technical Document – Antimony
Table of Contents
- Guideline
- 1.0 Exposure Considerations
- 2.0 Health Considerations
- 3.0 Derivation of the health-based value (HBV)
- 4.0 Analytical and Treatment Considerations
- 5.0 Management Strategies
- 6.0 International considerations
- 7.0 Rationale for maximum acceptable concentration
- 8.0 References
- Appendix A: List of abbreviations
- Appendix B: Canadian water quality data
Guideline
A maximum acceptable concentration (MAC) of 0.006 mg/L (6 μg/L) is established for total antimony in drinking water.
Executive summary
This guideline technical document was prepared in collaboration with the Federal-Provincial-Territorial Committee on Drinking Water.
Exposure
Antimony naturally occurs in the environment in the form of organic and inorganic compounds. Antimony enters the environment from natural sources and human activities, with coal combustion, mining and smelting being the most important sources of release from human activities.
Canadians can be exposed to antimony via food, drinking water, air and consumer products. Exposure to antimony through environmental media, food and water is considered as low. Antimony may enter drinking water from plumbing solders in drinking water distribution systems. Food (including breast milk for infants), beverages and, to a lesser extent, drinking water are identified as the main contributors for exposure to the general population.
Canadian data indicate that antimony is not commonly found in drinking water. The detection frequency for antimony in drinking water is very low and reported levels are largely below detection limits.
Health effects
Oral exposure to antimony may induce adverse effects mainly on the gastrointestinal tract and the liver. Kidney, cardiovascular, metabolic, and developmental adverse effects have also been reported in the literature. The health-based value (HBV) of 0.003 mg/L (3 µg/L) was derived based on histopathological changes in the liver and changes in serum biochemistry observed in animal studies. These effects are indicative of impacts on the liver.
The overall weight of scientific evidence indicates that antimony and related compounds are not considered carcinogenic via the oral route of exposure.
Analytical and treatment considerations
The development of a drinking water guideline takes into consideration the ability to both measure the contaminant and remove it from drinking water supplies. Several analytical methods are available for measuring antimony concentrations in water well below the MAC. Measurements should be for total antimony, which includes both the dissolved and particulate forms of antimony in a water sample.
At the municipal level, treatment technologies that are available to achieve antimony drinking water concentrations below the MAC include coagulation, adsorption, reverse osmosis and coagulation followed by ultrafiltration. The performance of these technologies depends on factors such as antimony species, pH, coagulant type, coagulant dose and type of adsorbent.
At the residential scale, there are no treatment units currently certified for the removal of antimony from drinking water Technology that is expected to be effective is reverse osmosis, and distillation may also be effective. When using such treatment units, it is important to send samples of water entering and leaving the treatment unit to an accredited laboratory for analysis to ensure that adequate antimony removal is occurring. Routine operation and maintenance of treatment units, including replacement of the filter components, should be conducted according to manufacturer specifications.
It is recommended that water utilities develop a distribution system management plan to minimize the accumulation and release of co-occurring contaminants, including antimony. This typically involves minimizing the antimony concentration entering the distribution system and implementing best practices to maintain stable chemical and biological water quality conditions throughout the system, as well as to minimize any physical hydraulic disturbances.
Application of the guidelines
Specific guidance related to the implementation of drinking water guidelines should be obtained from the appropriate drinking water authority.
All water utilities should implement a comprehensive, up-to-date risk management water safety plan. A source-to-tap approach should be taken that ensures water safety is maintained. This approach requires a system assessment to characterize the source water; describe the treatment barriers that prevent or reduce contamination; identify the conditions that can result in contamination; and implement control measures. Operational monitoring is then established and operational/management protocols are instituted (for example, standard operating procedures, corrective actions and incident responses). Compliance monitoring is determined and other protocols to validate the water safety plan are implemented (for example, record keeping and consumer satisfaction). Operator training is also required to ensure the effectiveness of the water safety plan at all times.
The guidelines are protective against health effects from exposure to antimony in drinking water over a lifetime. Any exceedance of the MAC should be investigated and followed by the appropriate corrective actions, if required. For exceedances in source water where there is no treatment in place, additional monitoring to confirm the exceedance should be conducted. If it is confirmed that antimony concentrations in the water source are above the MAC, then an investigation to determine the most appropriate way to reduce exposure to antimony should be conducted. This may include use of an alternate water supply or installation of an antimony treatment system. Where treatment is already in place and an exceedance occurs, an investigation should be conducted to verify treatment and to determine whether adjustments are needed to lower the treated water concentration below the MAC.
Discolouration (coloured water) episodes are likely to be accompanied by the release of accumulated contaminants, including antimony, because dissolved antimony can adsorb onto deposits in the distribution and plumbing systems. Therefore, discoloured water events should not be considered only an aesthetic issue; they should trigger sampling for metals and possibly distribution system maintenance.
1.0 Exposure Considerations
1.1 Sources, uses and identity
Elemental antimony (Sb) is a group 15 metalloid, which has two stable isotopes (121Sb and 123Sb) and two allotropic forms: the stable metallic form and the amorphous black form. Metallic antimony is an insoluble, silvery white, brittle crystalline solid with poor electrical and heat conductivity properties (Reimann et al., 2010; Anderson, 2012; Tylenda et al., 2015; Multani et al., 2016; Hammond and Lide, 2019).
Elemental antimony rarely occurs free in the environment but rather occurs in the form of either organic or inorganic compounds. Over 200 inorganic compounds of antimony exist in the environment, with stibnite being the most abundant followed by the oxides of antimony and the antimonides of heavy metals, with arsenic (As - another group 15 element) being predominant (Andrewes and Cullen, 2003; McCallum, 2005; Reimann et al., 2010; Tylenda et al., 2015).
Antimony occurs in four oxidation states (-3, 0, +3, and +5) with the trivalent [Sb(III)] and the pentavalent [Sb(V)] forms being the most environmentally prevalent and toxicologically relevant species (DFG, 2007; Filella et al., 2009). The physical/chemical properties of select antimony compounds are presented in Table 1.
| Property | Antimony (elemental) | Antimony trioxide | Antimony pentoxide | Antimony potassium tartrate | Sodium hexahydroxy-antimonate |
|---|---|---|---|---|---|
| CAS# | 7440-36-0 | 1309-64-4 | 1314-60-9 | 28300-74-5 | 33908-66-6 |
| Molecular formula | Sb | Sb2 O3 | Sb2O5 | C8H4K2O12Sb2∙3H2O | NaSb(OH)6 |
| Molecular weight (g/mol) | 121.75 | 291.50 | 323.5 (anhydrous) | 333.93 | 246.79 |
| Water solubility (mg/L) | Insoluble | Slightly soluble | Very slightly soluble | 8.3 x 104 (highly soluble) | 594 (moderately soluble) |
| Vapour pressure (mm Hg) | 1 at 886 °C | 1 at 574 °C | N/A | N/A | N/A |
| N/A – Not available. | |||||
1.2 Environmental fate
Antimony enters the environment from natural sources such as windblown dust, weathering of mineral rocks (predominantly sulphides and sulphosalts) and volcanic ash. It can also enter the environment through anthropogenic activities, with coal combustion, mining and smelting being the most important. Antimony is also emitted in areas of high motor vehicle traffic (for example, abrasion of tires and brake linings). Other anthropogenic sources include fire retardants, shooting ranges (in military sites), pharmaceuticals and pesticides (Andrewes and Cullen, 2003; Filella et al., 2009; Environment Canada and Health Canada, 2010; Belzile et al., 2011; Multani et al., 2016; Herath et al., 2017). Antimony may enter drinking water from plumbing solders in drinking water distribution systems; however, this is not a significant source (WHO, 2003).
In general, the emission of inorganic antimony compounds, more specifically antimony trioxide (also known as diantimony trioxide [ATO]; CAS RN 1309-64-4), represents the major source of environmental antimony in industrial regions (Oorts et al., 2008; Filella et al., 2009). According to the Canadian National Pollutant Release Inventory (NPRI), in 2017, antimony compounds released to the environment totalled approximately 5.4 tonnes (NPRI, 2017).
Once in the environment, antimony undergoes redox transformations, with both the Sb(III) and the Sb(V) forms interconverting between one another to subsequently form a variety of dissolved antimony species. Both Sb(III) and Sb(V) ions readily hydrolyze, forming dissolved hydroxides in the Sb(III) and Sb(V) states, such as antimonite [Sb(OH)3] and the antimonate anion [Sb(OH)6- oxyanion], respectively (Oorts et al., 2008; Okkenhaug et al., 2012; Ilgen et al., 2014; Hockmann et al., 2015).
Antimony in the particulate form is mobile and easily transported in the air, favouring wet deposition (Belzile et al., 2011). Once in the soil and water, the fate of antimony is driven by precipitation and adsorption to metal oxyhydroxides. Antimony can be immobilized in soil and water by complexation with alkaline (for example, calcium and magnesium), alkali (for example, sodium and potassium), and heavy (iron and manganese being the most important) metals, forming highly stable secondary minerals such as calcium antimonates. Natural/synthetic amorphous iron and manganese (oxyhydr)oxides are known for enhancing the oxidation of Sb(III) to Sb(V) (the most stable species) (Ettler et al., 2007; Oorts et al., 2008; Filella et al., 2009; Reimann et al., 2010; Okkenhaug et al., 2012; Ilgen et al., 2014; Cai et al., 2015; Hockmann et al., 2015; Herath et al., 2017). Fate studies have shown that, due to its highest sorption capacity, antimonite predominates in the soil matrix, specifically the topsoil.
Most of the dissolved antimony (pentavalent) that might be discharged to natural waters would rapidly precipitate as antimony trioxide or antimony pentoxide and be removed by sedimentation (McKee and Wolf, 1963). In natural water sources, the antimonate anion is more mobile and is the most prevalent form of antimony under aerobic conditions (ATSDR, 2019). In drinking water, the prevalence of Sb(V) can be explained by the oxidizing nature of the treatment processes generally applied (for example, chlorination or ozonation) which oxidize Sb(III) to Sb(V), and by the types of plumbing solder and pipes in the distribution systems. Despite all of the above, some evidence supports that both species can coexist in the same oxygen-dependent environment as they interconvert between one another (Andrewes and Cullen, 2003; Leuz et al., 2006; Ettler et al., 2007; Oorts et al., 2008; Filella et al., 2009; Reimann et al., 2010; Belzile et al., 2011; Okkenhaug et al., 2012; Skeaff et al., 2013; Ilgen et al., 2014; Cai et al., 2015; Hockmann et al., 2015; Herath et al., 2017).
Elemental antimony is mainly used in the manufacture of alloys and certain types of semi-conductors such as infrared detectors and diodes (Multani et al., 2016; Hammond and Lide, 2019). Antimony alloys and many inorganic antimony compounds are widely used in the manufacture of lead-acid batteries, electrical equipment, anti-friction materials, flame retardants, paints, type metal in printing presses, art glass and ceramics, plastics and pottery, ammunition and fireworks, plumbing solder and pipes, transportation vehicles, and lubricants (Hjortenkrans et al., 2007; Tylenda et al., 2015; Multani et al., 2016; Hammond and Lide, 2019). Antimony organic compounds are widely used as therapeutics for some parasitic diseases including visceral, mucosal, and cutaneous leishmaniasis, schistosomiasis, trypanosomiasis and ulcerative granuloma (Health Canada, 1997; DFG, 2007; Tylenda et al., 2015; Multani et al., 2016; NTP, 2018; ECCC and Health Canada, 2020). Despite its past uses, in Canada, antimony is prohibited in cosmetics and is not used as an active ingredient in pesticides (ECCC and Health Canada, 2020).
Canadian production of antimony is minimal, significantly decreasing over time from a 2013 estimate of 148 tonnes (about 0.1% of the global production) to 1 tonne in 2015, with no production anticipated after 2016 (ECCC and Health Canada, 2020). Estimated global production of the metalloid in 2020 was 153 000 tonnes, down from 162 000 tonnes in 2019, with China being the largest producer (U.S. GS, 2020).
Antimony trioxide is the most significant commercial antimony compound accounting for over 80% of global antimony use (2005 production estimate was 120 000 tonnes) (Environment Canada and Health Canada, 2010; ECCC and Health Canada, 2020). One to 10 million kg of the compound was manufactured in Canada in 2006 with importation above 1.8 million kg and average use around 3 million reported by Canadian companies the same year (Environment Canada and Health Canada, 2010). In Canada, antimony trioxide is primarily used in combination with other compounds to provide flame retardant properties. Globally, flame-retardants are expected to remain the main consumption product of antimony (U.S. GS, 2016).
Antimony compounds are not allowed as food additives in Canada. Antimony oxide is used in the manufacture of polyethylene terephthalate (PET) which is used in various food packaging applications (Environment Canada and Health Canada, 2010; CFIA, 2016; ECCC and Health Canada, 2020).
1.3 Exposure
Canadians can be exposed to antimony via food, drinking water, air and consumer products. Exposure to antimony trioxide and antimony containing substances (11 inorganic compounds) has been assessed previously (Environment Canada and Health Canada, 2010; ECCC and Health Canada, 2020); this section builds on those exposure assessments. Exposure to antimony through environmental media, food and water is expected to be low, with average daily intakes of total antimony estimated at 0.019–0.057 µg/kg body weight (bw) per day and the highest intake (i.e., 0.27 µg/kg bw per day) estimated in infants aged up to 6 months. Food (including breast milk and beverages; range 68%–80%) and, to a lesser extent, drinking water (range 17%–29%) have been identified as the main contributors for exposure (ECCC and Health Canada, 2020). Based on these estimated daily intakes, a source allocation factor of 30% is considered appropriate for drinking water.
Water: Water monitoring data from the provinces (municipal and non-municipal supplies) were obtained and included data for raw water, treated water and water from distribution systems. Where indicated, data were separated into groundwater and surface water sources. When the source type could not be discerned, it was classified as ground and/or surface water. Samples were divided into raw, treated and distribution water, and when not indicated or not possible to determine, samples were classified as unspecified. Total antimony concentrations were also obtained from the First Nations and Inuit Health Branch (FNIHB) (Indigenous Services Canada, 2019) and the National Drinking Water Survey (Health Canada, 2017). The exposure data provided reflect different detection limits of accredited laboratories used within and amongst the jurisdictions, as well as their respective monitoring programs. As a result, the statistical analysis of exposure data provides only a limited picture.
Overall, within all three datasets, the detection frequency was very low, indicating that a large number of the samples had antimony concentrations below the detection limit. For this reason, the mean, median and lower percentiles were not calculated. The range of detection limits, number of detects, number of samples, 90th percentile antimony concentration and maximum antimony concentration are presented in Table 2 for the provincial and FNIHB data and in Table 3 for the National Drinking Water Survey. When the per cent detection is less than 10%, the 90th percentile is presented as below the detection limit (DL). Ambient antimony datasets were obtained from Environment and Climate Change Canada's (ECCC) surface water monitoring (ECCC, 2017) and select groundwater monitoring studies supplied by some provinces (Appendix C). Overall, for total antimony, these datasets show that:
- Most of the maximum antimony concentrations from provincial drinking water data were low. In cases with higher maximum values, the 90th percentile is either below the detection limit or below 1.5 μg/L.
- The National Drinking Water Survey of Canada had maximum antimony concentrations below 1.0 μg/L during the summer months but had some higher concentrations in the raw and treated lake water during the winter months. There were negligible differences between raw, treated and distribution system waters.
- The ECCC surface water monitoring dataset had low 90th percentiles (≤ 0.5μg/L) for each basin.
- Groundwater monitoring studies, which are ambient studies and not representative of sources for drinking water, showed higher antimony levels that reflect the respective groundwater system.
| Jurisdiction |
System type |
Water Type |
# Detects /samples |
% Detect |
Total antimony (μg/L) |
|
|---|---|---|---|---|---|---|
| 90th percentile |
Maximum |
|||||
| Atlantic – FNIHB (0.1–1.0) [2013–2018]Table 2 Footnote 1 |
Public and semi-public | Ground - Raw | 2/41 | 4.9 | < DL | 0.5 |
| Ground - Treated | 0/58 | 0 | < DL | < DL | ||
| Ground - Distribution | 4/185 | 2.2 | < DL | 1.2 | ||
| Surface - Raw | 0/9 | 0 | NC | < DL | ||
| Surface - Treated | 0/19 | 0 | < DL | < DL | ||
| Surface - Distribution | 0/27 | 0 | < DL | < DL | ||
| Private wells and systems | Ground - Raw | 0/1 | 0 | NC | < DL | |
| Ground - Distribution | 10/95 | 10.5 | 0.5 | 1.9 | ||
| British ColumbiaTable 2 Footnote 2 (0.1–1) [2014–2019] |
Municipal | Ground - Raw | 87/280 | 31.1 | 1.00 | 15.0 |
| Ground - Treated | 2/21 | 9.5 | < DL | 0.50 | ||
| Ground - Distribution | 54/257 | 21.0 | 1.00 | 2.00 | ||
| Ground - Unspecified | 99/256 | 38.7 | 0.05 | 1.28 | ||
| Surface - Raw | 10/56 | 17.9 | 0.65 | 3.00 | ||
| Surface - Treated | 2/2 | 100 | NC | 0.05 | ||
| Surface - Distribution | 11/30 | 36.7 | 1.00 | 2.00 | ||
| Surface - Unspecified | 1/24 | 4.2 | < DL | 0.25 | ||
| Ground &/or Surface-Raw | 17/39 | 43.6 | 1.00 | 1.46 | ||
| Ground &/or Surface-Treated | 6/9 | 66.7 | 1.23 | 2.50 | ||
| Ground &/or Surface-Distribution | 95/240 | 39.6 | 0.50 | 11.30 | ||
| Ground &/or Surface - Unspecified | 40/134 | 29.9 | 0.50 | 1.95 | ||
| ManitobaTable 2 Footnote 3 (0.2–2) [2009–2018] |
Municipal | Ground - Raw | 58/775 | 7.5 | 0.20 | 0.99 |
| Ground -Treated | 65/1 141 | 5.7 | < DL | 1.08 | ||
| Ground -Distribution | 6/88 | 6.8 | < DL | 0.92 | ||
| Surface - Raw | 131/578 | 22.7 | 0.36 | 1.65 | ||
| Surface - Treated | 94/618 | 15.2 | 0.27 | 1.69 | ||
| Surface - Distribution | 22/74 | 29.7 | 0.40 | 0.58 | ||
| Ground &/or Surface-Raw | 30/174 | 17.2 | 0.25 | 0.5 | ||
| Ground &/or Surface-Treated | 27/205 | 13.2 | 0.23 | 0.58 | ||
| Ground &/or Surface-Distribution | 6/29 | 20.7 | 0.25 | 0.42 | ||
| Manitoba – FNIHBTable 2 Footnote 1 (0.1–1.0) [2013–2018] |
Public and semi-public | Ground - Raw | 26/164 | 15.9 | 0.5 | 1.5 |
| Ground - Treated | 19/155 | 12.3 | 0.5 | 0.9 | ||
| Ground - Distribution | 2/29 | 6.9 | < DL | 0.2 | ||
| Surface - Raw | 31/239 | 13.0 | 0.5 | 1.7 | ||
| Surface - Treated | 20/241 | 8.3 | < DL | 0.7 | ||
| Surface - Distribution | 0/4 | 0 | NC | < DL | ||
| Private wells and systems | Ground - Raw | 1/12 | 8.3 | < DL | 0.2 | |
| Ground - Distribution | 0/13 | 0 | < DL | < DL | ||
| Surface - Raw | 4/7 | 57.1 | NC | 0.2 | ||
| Surface - Treated | 3/7 | 42.9 | NC | 0.3 | ||
| New BrunswickTable 2 Footnote 4 (0.1–2) [2013–2018] |
Municipal | Ground - Raw | 72/1 053 | 6.8 | < DL | 6.3 |
| Ground - Treated | 5/74 | 6.8 | < DL | 0.5 | ||
| Ground - Distribution | 10/504 | 2.0 | < DL | 0.5 | ||
| Surface - Raw | 3/99 | 3.0 | < DL | 0.1 | ||
| Surface - Distribution | 9/298 | 3.0 | < DL | 0.2 | ||
| Ground &/or Surface-Raw | 6/91 | 6.6 | < DL | 0.3 | ||
| Ground &/or Surface-Treated | 25/268 | 9.3 | < DL | 4.9 | ||
| Ground &/or Surface-Distribution | 7/188 | 3.7 | < DL | 0.3 | ||
| NewfoundlandTable 2 Footnote 5 (0.5–1) [2015–2017] |
Municipal | Ground - Raw | 0/99 | 0 | < DL | < DL |
| Ground - Distribution | 37/1 216 | 3.0 | < DL | 4.5 | ||
| Surface - Raw | 0/627 | 0 | < DL | < DL | ||
| Surface - Distribution | 1/3 225 | 0.03 | < DL | 0.7 | ||
| Nova ScotiaTable 2 Footnote 6 (1–2) [2014–2019] |
Municipal | Ground - Raw | 0/388Table 2 Footnote a | 0 | < DL | < DL |
| Ground - Treated | 2/388Table 2 Footnote a | 0.5 | < DL | 2.6 | ||
| Surface - Raw | 0/400Table 2 Footnote b | 0 | < DL | < DL | ||
| Surface - Treated | 1/400Table 2 Footnote b | 0.3 | < DL | 5.0 | ||
| OntarioTable 2 Footnote 7 (0.08) [2014–2018] |
Municipal | Ground &/or Surface - Raw | 1 613/1 613 | 100 | 0.80 | 4.0 |
| Ground &/or Surface -Treated | 1 305/1 305 | 100 | 0.80 | 1.1 | ||
| Ground &/or Surface -Distribution | 1 367/1 367 | 100 | 0.80 | 2.2 | ||
| Ontario – FNIHBTable 2 Footnote 1 (0.1–0.6) [2013–2018] |
Public and semi-public | Ground Raw | 0/22 | 0 | < DL | < DL |
| Ground Treated | 1/236 | 0.4 | < DL | 0.5 | ||
| Ground - Distribution | 13/111 | 11.7 | 0.3 | 2.3 | ||
| Surface - Raw | 0/60 | 0 | < DL | < DL | ||
| Surface - Treated | 2/377 | 0.5 | < DL | 0.6 | ||
| Surface - Distribution | 0/34 | 0 | < DL | < DL | ||
| Private wells and systems | Ground - Raw | 0/1 | 0 | NC | < DL | |
| Ground - Treated | 0/4 | 0 | NC | < DL | ||
| Ground - Distribution | 0/53 | 0 | < DL | < DL | ||
| Surface - Treated | 0/5 | 0 | NC | < DL | ||
| Prince Edward IslandTable 2 Footnote 8 (1.00) |
Non-municipal | Ground - Raw | 0/sample size not given | 0 | < DL | < DL |
| QuebecTable 2 Footnote 9 (0.02-6) [2013–2019] |
Municipal | Ground - Distribution | 310/6 400 | 5 | < DL | 7 |
| Surface - Distribution | 109/2 223 | 5 | < DL | 6 | ||
| SaskatchewanTable 2 Footnote 10 (0.1–1) [2014–2018] |
Municipal | Ground - Raw | 3/50 | 6.0 | < DL | 2.6 |
| Surface - Raw | 6/61 | 9.8 | < DL | 0.8 | ||
| Ground &/or Surface -Treated | 10/50 | 20 | 0.5 | 0.7 | ||
| Ground &/or Surface -Distribution | 55/607 | 9.0 | < DL | 1.1 | ||
DL – detection limit; < DL – below detection limit (for maximum with 0% detects; for 90th percentile with < 10% detects); FNIHB – First Nations and Inuit Health Branch; NC – not calculated due to insufficient sample size; Unspecified – not specified whether raw, treated or distribution water.
|
||||||
| Water Type | Summer (μg/L)Table 3 Footnote a | Winter (μg/L)Table 3 Footnote a | ||||
|---|---|---|---|---|---|---|
| Detects/ Samples | %Detect | Max | Detects/ Samples | %Detect | Max | |
| Well – raw | 1/18 | 5.6 | 0.90 | 1/17 | 5.9 | 0.60 |
| Well – treated | 1/17 | 5.9 | 0.50 | 0/16 | 0 | < DL |
| Well – distribution | 1/18 | 5.6 | 0.80 | 1/9 | 11.1 | 0.50 |
| Lake – raw | 3/21 | 14 | 0.50 | 4/20 | 20 | 9.40 |
| Lake – treated | 1/21 | 4.8 | 0.50 | 3/20 | 15 | 9.00 |
| Lake – distribution | 1/21 | 4.8 | 0.80 | 0/10 | 0 | < DL |
| River – raw | 1/26 | 3.8 | 0.80 | 2/22 | 9.1 | 0.80 |
| River – treated | 2/26 | 7.7 | 0.60 | 1/22 | 4.5 | 0.60 |
| River – distribution | 1/26 | 3.8 | 0.50 | 1/12 | 8.3 | 0.60 |
DL – detection limit; < DL – below detection limit (for maximum with 0% detects; for 90th percentile with < 10% detects). Source: Health Canada, 2017
|
||||||
A report for Quebec distribution system sampling showed two systems with antimony concentrations exceeding 6 μg/L between 2013 and 2017. The maximum antimony concentration reported was 7 μg/L (Ministère de l’Environnement et de la Lutte contre les changements climatiques, 2020).
Additionally, US data were examined and a sampling campaign of 1 172 private wells in North Carolina showed antimony concentrations below 0.1 µg/L in 74.5% of first draw samples and 91.4% of 5-minute flush samples. The 90th percentiles were 0.3 µg/L and 0.1 µg/L in first draw samples and 5-minute flush samples, respectively (Pieper, 2021).
Food: Antimony is absorbed by the roots of vegetables and other crops grown on antimony-containing soils (WHO, 2003). Estimates of dietary exposure to total antimony for the general Canadian population were generated by Health Canada’s Food Directorate and are based on over 40 000 analytical results from 19 surveys conducted by the Canadian Food Inspection Agency (CFIA). Total antimony has been measured in a variety of food items (including cereals, dairy products, fruits and vegetables, meat and seafood, and beverages) with most (87%) of the results exhibiting levels below the limits of detection (i.e., 0.0001 to 0.01 µg/g) (CFIA, 2016; ECCC and Health Canada, 2020). Similar average levels were observed (0.001–0.002 µg/g) for total antimony in foods and beverages in the 2016–2018 Canadian Total Diet Study (Health Canada, 2020). A level of 0.002 µg/g has been reported for total antimony in breast milk, representing an arithmetic mean of concentrations from the scientific literature in the absence of data for human milk in Canada (ECCC and Health Canada, 2020).
Dietary exposure to total antimony is expected to be low, with average daily intakes of total antimony estimated at 0.013 to 0.130 µg/kg bw per day and the highest intake (i.e., 0.26 µg/kg per day) estimated in infants aged up to 6 months (95th percentile exposure 0.023 to 0.27 µg/kg bw per day). Median and 95th percentile exposure estimates to antimony for exclusively breast-fed infants under 6 months were 0.259 and 0.306 µg/kg bw per day, respectively, as determined from scientific literature in the absence of Canadian occurrence data (ECCC and Health Canada, 2020). Orange juice, milk, and breakfast cereals were the main contributors to total dietary exposure for total antimony in adults aged 19 or above, accounting for approximately 16%, 12%, and 9%, respectively. Total dietary exposure for total antimony in children aged 1 to 3 years was influenced by consumption of milk (26%), apple juice (19%), and orange juice (14%) among foods in the diet (ECCC and Health Canada, 2020).
Other than environmental sources, PET food packaging materials, such as trays and bottles, may also contribute to antimony in food and in bottled water because antimony-related catalysts are used in the manufacture of PET resins (Filella et al., 2009; Filella, 2020). Low parts per billion levels (ppb) of total antimony were reported in water packaged in PET bottles with levels rarely exceeding drinking water regulatory levels (Shotyk et al., 2006; Westerhoff et al., 2008; Carneado et al., 2015; Filella, 2020). None of the packaged food (i.e., domestic and imported beverages, nut and seed butters, condiments, frozen/shelf-stable heat-and-serve meals, and processed fruits and vegetable products) samples from the 2012–2014 CFIA survey had detectable levels of antimony (CFIA, 2016; ECCC and Health Canada, 2020). In Canada, the contribution of food packaging to the overall dietary exposure to antimony is considered negligible.
Consumer products: Canadians can potentially be exposed to antimony (specifically ATO) from its use in consumer products either as a polymerization catalyst, a pigment or flame retardant. The concentration of antimony compounds in a given product depends on the polymer and the intended use of the finished product. Antimony is usually in the range of 2% to 5% in polymers (Environment Canada and Health Canada, 2010; ECCC and Health Canada, 2020). Investigations have shown that children are expected to have the greatest exposure from direct skin contact (for example, from carpets while crawling), mouthing of toys and other products, and potential inhalation of dusts containing antimony (NTP, 2018; ECCC and Health Canada, 2020).
Air: Canadians can be exposed to antimony through the air via fine particulate matter (PM2.5), which can penetrate deep into the lungs. Air antimony levels are generally higher in urban areas. Little is known about the chemical form(s) of antimony in air (ECCC and Health Canada, 2020). Rural atmospheric aerosol levels of antimony ranging from 0.04 ng/m3 in Quebec to 2.17 ng/m3 in Nova Scotia have been reported (Hopper and Barrie, 1988). Furthermore, outdoor exposure is higher than indoor exposure from household products (for example, fabrics, carpets and paints). In Windsor, Ontario, a concentration of 1.9 ng/m3 (n = 447) was estimated for the 95th percentile of antimony in PM2.5 in Canadian outdoor air (Rasmussen, 2016), increasing from a 95th percentile of 0.7 ng/m3 (n = 910) previously reported by the National Air Pollution Surveillance in 2011. A lower 95th percentile of 0.7 ng/m3 was estimated for PM2.5 in Canadian indoor air during the same period (Rasmussen, 2016). A median level up to 8.5 mg/kg was reported in dust (95th percentile of 32 mg/kg) from a Canadian house dust study in 2010, and levels even higher, up to 63 mg/kg, were reported in locations close to smelters in 2016 (ECCC and Health Canada, 2020).
Soil: Environmental exposure to antimony from the soil varies as a reflection of the mineralogy of the bedrock and proximity to human sources. Total antimony levels ranging from 0 to 8 mg/kg were measured in soils from some Canadian provinces (i.e., Ontario, Alberta and British Columbia) (ECCC and Health Canada, 2020).
Canadian biomonitoring data: Total antimony was measured in the urine of Canadians aged 6–79 years and 3–79 years in cycle 1 (2007–2009) and 2 (2009–2011), respectively, in the Canadian Health Measures Survey. Urinary median levels up to 0.045 µg/L (95th percentile up to 0.19 µg/L) and 0.048 µg/L (95th percentile up to 0.22 µg/L) were reported for cycle 1 and 2, respectively. In general, the measured antimony levels were slightly higher in teenagers (12–19 years old) and tended to be slightly higher in men as compared to women (Health Canada, 2013).
2.0 Health Considerations
2.1 Kinetics
Absorption: Gastrointestinal (GI) absorption of antimony has been shown in humans and several animal species (Environment Canada and Health Canada, 2010; Borborema et al., 2013). The current scientific literature indicates GI absorption of antimony is low and dependant on the solubility and chemical form (oxidation state) (WHO, 2003; OEHHA 2016; ATSDR, 2019). GI absorption of the relatively insoluble ATO in humans is reported as approximately 1% (EU, 2008). From acute intoxication (poisoning) data for four individuals exposed to antimony potassium tartrate (APT), a highly water-soluble form of antimony, 5% absorption was reported (Iffland and Bösche, 1987; Lauwers et al., 1990). Quantitative information on the absorption of antimony is not available for all forms of antimony. The International Commission on Radiological Protection recommends the use of a 10% absorption factor for the dietary intake of antimony and, due to variability in the absorption data available, an absorption of 5% is recommended for situations where specific information is not available (ICRP, 1981, 1995, 2017). Data on the dermal absorption of antimony are limited. The low water/lipid solubilities of antimony and its compounds suggest that dermal exposure is not a significant route of exposure (OEHHA, 2016). In a study by Roper and Stupart (2006), skin samples from the abdomen (1 sample) and breast (5 samples) of women were exposed in vitro to 100 μg/cm2 and 300 μg/cm2 of diantimony trioxide resulting in total estimated dermal absorptions of 0.26% and 0.14%, respectively, after a 24-hour exposure period.
Distribution: Ingested antimony, once absorbed, is distributed mainly to the liver, spleen and bone and, to a lesser extent, the gall bladder, kidneys, nails, ovaries, testes, thyroid, and hair (DFG, 2007; Tylenda et al., 2015; Kip et al., 2017; Sztajnkrycer, 2017). Studies in humans (Gerhardsson et al., 1982, 1988; Kip et al., 2017), rhesus monkeys (Friedrich et al., 2012), and rats (Poon et al., 1998; Coelho et al., 2014b) using radioactively labelled (Sb124) sodium antimony mercapto-succinate, meglumine antimoniate and APT, respectively, indicate that accumulation is dose-dependent. A study by Sunagawa (1981) in rats found that exposure to metallic antimony resulted in similar antimony concentrations in the liver and blood; however, exposure to antimony trioxide resulted in a 10-fold higher antimony concentration in the blood compared to the liver. The distribution of the different oxidation states (for example, +3, +5) following oral exposure to antimony is not known (ECCC and Health Canada, 2020). In the blood, pentavalent antimony is primarily found in the serum (Felicetti et al., 1974; Edel et al., 1983; Ribeiro et al., 2010) and trivalent antimony is primarily found in the hemoglobin fraction of red blood cells (Lippincott et al., 1947; Edel et al., 1983; Newton et al., 1994; Poon et al., 1998; Kobayashi and Ogra, 2009). However, both trivalent and pentavalent antimony have been shown to enter red blood cells (Quiroz et al., 2013; Lopez et al., 2015; Barrera et al., 2016). In vitro studies have found that pentavalent antimony can enter erythrocytes via protein channels (Quiroz et al., 2013; Barrera et al., 2016).
Trans-placental and mammary gland (via maternal milk) transfers of antimony have been reported in humans and animals (Miranda et al., 2006; Coelho et al., 2014b; NTP, 2018; Li et al., 2019).
Metabolism: In vitro evidence of the metabolism of ingested antimony in mammals shows intracellular interconversion between both the Sb(III) and Sb(V) valence states (NTP, 2018). For ingested antimony, there is a reduction of Sb(V) into Sb(III) which is dose-dependent and promoted by acidic pH and elevated temperature (25°C–37°C) (Frezard et al., 2001; DFG, 2007; NTP, 2018). This reduction is followed by the conjugation of Sb(III) with reduced glutathione (GSH), and the subsequent enterohepatic recycling of the Sb(III)-GSH complex (Bailly et al., 1991; DFG, 2007).
There is no convincing evidence for methylation of antimony in mammals, although methylated forms of antimony have been reported in the environment (Filella and Williams, 2010; Herath et al., 2017; Sztajnkrycer, 2017).
Elimination: Ingested antimony is primarily excreted through the feces and, to a lesser extent, the urine (Environment Canada and Health Canada, 2010; Borborema et al., 2013; OEHHA, 2016). Sb(V) is preferentially excreted in the urine, whereas Sb(III) is excreted in the feces (Friedrich et al., 2012; Sztajnkrycer, 2017). Evidence from human pharmacokinetic studies indicates that the pharmacokinetics of antimony is age-dependent with young children eliminating more of the chemical than adults (Cruz et al., 2007). In patients treated with meglumine antimoniate (5 mg Sb/kg bw per day by intramuscular injection) for 30 days, half-lives for elimination were reported to range from 24–72 hours for the rapid excretion phase and a half-life of > 50 days for the slow elimination phase (Miekeley et al., 2002).
2.2 Health effects
The information on the toxicity of ingested antimony and its compounds has been described elsewhere in more detail (OEHHA, 2016; NTP, 2018; ATSDR, 2019). This assessment focuses on oral exposure data which are most relevant for exposure from drinking water. According to the available data, oral exposure to antimony may induce adverse effects mainly on the GI tract (for example, abdominal pain, nausea, vomiting, and diarrhea) and the liver. Kidney, cardiovascular, metabolic (for example, decreased serum glucose levels), and developmental adverse effects have also been reported (Lauwers et al., 1990; Hepburn et al., 1993; WHO, 2003; Alvarez et al., 2005; OEHHA, 2016; Scinicariello and Buser, 2016; Sztajnkrycer, 2017; NTP, 2018; ATSDR, 2019).
2.2.1 Health effects in humans
There are limited data on the toxicological effects of antimony in humans. The majority of the human data in the literature come from the reported side effects observed during therapeutic applications of antimony-based drugs (antimonials). Side effects observed following therapeutic-level doses include GI tract distress, cardiotoxicity, pancreatitis, hepatotoxicity, and nephrotoxicity (Hepburn et al., 1994; Oliveira et al., 2009, 2011; Mlika et al., 2012; Wise et al., 2012). Although these studies provide useful insight into the potential effects following antimony exposure, the relevance of these reported effects following environmental exposures is uncertain due to the poor absorption of antimony compounds.
Hepatotoxicity: In humans exposed to antimony for the treatment of leishmaniasis (a parasitic disease), hepatocellular damage and impaired liver metabolism has been demonstrated (OEHHA, 2016). Patients treated for cutaneous leishmaniasis have been shown to have alterations in liver enzymes such as alanine aminotransferase and glutathione S-transferase B1, which indicate potential liver damage and impairment of liver metabolism (Hepburn et al., 1993, 1994; Andersen et al., 2005; Oliveira et al., 2011). In the treatment of visceral leishmaniasis, impaired peroxisomal function, hepatitis, and hepatic failure were observed in patients (Gupta et al., 2009; Oliveira et al., 2009). Patients treated for mucosal leishmaniasis also showed increased liver enzymes (Franke et al., 1990; Saenz et al., 1991).
Gastrointestinal effects: Antimony has long been known for its emetic properties (ATSDR, 2019). Although rarely reported, some cases of poisoning have occurred after accidental ingestion of beverages or food contaminated with antimony. The most frequently reported effects from ingested antimony poisoning include GI disturbances (for example, abdominal pain, nausea, vomiting, and diarrhea). Exposure to levels between 0.4 mg and 0.9 mg Sb/kg bw has been reported to induce vomiting in adults (Lauwers et al., 1990; Health Canada, 1997; Cooper and Harrison, 2009; Sundar and Chakravarty, 2010; Tylenda et al., 2015; Sztajnkrycer, 2017; NTP, 2018).
Reproductive and developmental effects: Data from retrospective and prospective studies in pregnant women treated for visceral leishmaniasis with therapeutic doses of antimony (i.e., 20 mg/kg sodium stibogluconate, intramuscular route, once daily for 30 days) suggest an association between antimony and developmental toxicity (i.e., spontaneous abortions) (Mueller et al., 2006; Adam et al., 2009). Moreover, this effect appears to be specific to the first (Mueller et al., 2006; Adam et al., 2009; Forns et al., 2014) and possibly second trimesters of pregnancy (Mueller et al., 2006). As previously mentioned, given the poor absorption of antimony compounds, the relevance of such effects following environmental exposures is uncertain. A recent study investigated the impact of a mixture of metals on fetal size during mid-pregnancy in a largely Hispanic cohort in Los Angeles (Howe et al., 2021). The authors found an association between urinary antimony (as a component of a mixture of urinary metals including total arsenic, barium, cadmium, mercury, molybdenum, tin, cobalt, nickel and thallium) and reduced fetal weight. It was concluded that the analysis identified antimony as a potential element of concern due to its inverse association with fetal size and that more investigation of antimony exposure within this specific study population is required. A later study by Howe et al. (2022) conducted an environmental mixture analysis of metal impacts on fetal growth. This study pooled data from three geographically and demographically diverse cohorts (the Maternal and Developmental Risks from Environmental and Social Stressors, the New Hampshire Birth Cohort Study and the Puerto Rico Test site for Exploring Contamination Threats) participating in the Environmental Influences on Child Health Outcomes program in the U.S. Seven metals (including antimony) were measured in maternal urine samples of 1 002 participants collected during pregnancy (median: 16.0 weeks gestation). An inverse relationship between increasing urinary antimony and birth weight for gestational age was observed for the pooled analysis and across the three individual cohorts in both males and females. The authors indicated that, among other study limitations, several additional metals which may also impact fetal growth (for example, arsenic, manganese and lead) were excluded from the analysis because urine is not a suitable matrix or because the metal was not measured in all three cohorts. These metals could have influenced the observed associations.
Liu et al. (2022) evaluated the association between exposure during pregnancy to multiple metals (including antimony) and neurodevelopment in children aged two to three years of age. Serum antimony levels in pregnant women were 2.27 μg/L (50th percentile). The authors reported that antimony was found to be negatively correlated with the language and social behaviour developmental quotient for infants. According to the authors, important confounding factors not considered in the study include education and genetic factors, which may have biased the results. Another important limitation of the study is that only single measurements of metals were used to evaluate impacts on child neurodevelopment, which can lead to exposure misclassification.
To assess the relationship between prenatal blood levels of metals and spontaneous abortion (SA) risk, Vigeh et al. (2021) compared blood concentrations of some heavy metals in samples taken from apparently healthy mothers recruited in the Tehran Environment and Neurodevelopmental Defects (TEND) study who subsequently experienced SA with those from mothers whose pregnancy ended in live births. During early gestation, 206 women were enrolled and followed until fetal abortion or successful deliveries occurred. The mean blood levels of lead, antimony, and nickel were higher in SA mothers than mothers with ongoing pregnancy; however, the difference was not statistically significant. When adjusted for covariates, a significant association between maternal age and the risk of SA in all regression models was observed. Only antimony had a noticeable positive relation with the risk of SA (odds ratio: 1.65, 95% confidence interval: 1.08–2.52, P value: 0.02) compared to the other metals. Pearson's correlation coefficient showed significant (P < 0.05) positive correlations among prenatal blood metals levels, except for nickel. The authors concluded that although the study did not provide strong evidence for metal-induced effects on the occurrence of SA at relatively low-levels, these metals should be avoided in women who plan pregnancy and/or during the early stages of gestation to prevent the potential for adverse effects.
Other endpoints: Data from human antimonials therapy and chronic inhalation of antimony-containing dusts in the workplace indicate that antimony may also induce nephrotoxicity, cardiotoxicity and effects on the musculoskeletal system, pancreas and nervous system (Hepburn et al., 1993; Hepburn et al., 1994; Health Canada, 1997; WHO, 2003; OEHHA, 2016; ATSDR, 2019).
2.2.2 Health effects in experimental animals
Antimony is acutely toxic to experimental animals, as indicated by the oral median lethal dose (LD50) values reported in the literature including 115 mg/kg bw and 600 mg/kg bw for APT in rabbits/rats and mice, respectively (Omura et al., 2002; WHO, 2003). Oral LD50 values higher than 2 000 mg/kg bw have been reported for sodium hexahydroxoantimonate (ECHA, 2014) and above 20 000 mg/kg bw for ATO (WHO, 2003).
Similar to humans, data on the toxic effects of antimony following oral exposure in experimental animals are limited but indicate that exposure may result in a number of adverse health effects. Acute oral exposure to Sb(III) and Sb(V) has been shown to affect the GI tract (NTP, 1992; Tylenda et al., 2015). Subchronic and chronic oral exposures (mostly to ATO, APT, and antimony trichloride) have been shown to impact the liver, thyroid and kidneys (Sunagawa, 1981; NTP, 1992; Poon et al., 1998; Hext et al., 1999; NTP, 2018), as well as potentially induce adverse developmental effects (Imai and Nakamura, 2006; Chen et al., 2010; ECHA, 2014, Khosravi et al., 2018). These effects have also been demonstrated in animal injection studies (Paumgartten and Chahoud, 2001; Omura et al., 2002; Grimaldi et al., 2010; Coelho et al., 2014a; Kato et al., 2014); however, given this route of exposure is not applicable to the drinking water exposure context, these studies will not be discussed further in this risk assessment. Other reported effects include altered blood glucose (Schroeder et al., 1970; Poon et al., 1998) and lipid levels (Schroeder et al., 1970; Poon et al., 1998; Hext et al., 1999) following subchronic and chronic exposures of rats to Sb(III) (i.e., APT or ATO) via drinking water or food. The results from antimonial studies also suggest the potential for cardiotoxicity and nephrotoxicity of antimony (NTP, 1992; Tirmenstein et al., 1995; Poon et al., 1998; Tylenda et al., 2015) as well as its oestrogenic potential (Choe et al., 2003; Darbre, 2006). Table 4 provides a summary of the relevant animal toxicity studies available for antimony.
| Species, number | Exposure duration | Compound and dose(s) (as mg Sb/kg bw per day) |
POD (mg Sb/kg bw per day) |
Critical effects | Ref. |
|---|---|---|---|---|---|
| B6C3F1 mice (10/sex/dose) | 14 days (ad libitum) | APT in drinking water: 0, 21, 36, 63, 99, 150 | NOAEL = 99 | Forestomach lesions in the high dose group. Dose-related increases in relative liver weight; lesions in the liver of most mice in the high dose group. | NTP (1992) |
| F344/N rats (10/sex/dose) | 14 days (ad libitum) | APT in drinking water: 0, 5.8, 10, 21, 34, 61 | NOAEL = 61 | Increase in relative liver weight in the high dose group. | NTP (1992) |
| Sprague-Dawley rats (15/sex/dose) | 13 weeks (and 4-week recovery for high dose animals) | APT in drinking water: males: 0.06, 0.56, 5.58, and 42.17; females: 0.06, 0.64, 6.13 and 45.69 | NOAEL = 0.06 | Dose-related increases in liver anisokaryosis reaching moderate severity in the high dose group. Dose-dependent decreased serum glucose levels in females reaching statistical significance in the three highest dose groups. Dose-related accumulation of antimony in red blood cells and the spleen with marked accumulation beginning in the second lowest dose group and persistence of antimony in the spleen beyond recovery. | Poon et al. (1998) |
| Wistar rats (Alpk:APSD strain; 12/sex/dose) | 90 days | ATO by diet: males: 0, 70, 353, 1 408; females: 0, 81, 413, 1 570 | NOAEL = 1 408 | Small increase in liver weight, small decrease in plasma alkaline phosphatase activity and small increase in plasma aspartate and alanine aminotransferase levels in the high dose group. No histological effects on liver. | Hext et al. (1999) |
| Wistar rats (male; 5/dose) | 24 weeks | ATO by diet: 0, 418, 836 | LOEL = 418 | Liver histopathological changes and increased aspartate transaminase (AST) activity. | Sunagawa (1981) |
| SD rats, female (20/dose) | GD 6 to 19 | Sodium hexahydroxoantimonate via gavage: 0, 49, 148, 493 | NOAEL = 49 | Increased (non-significant) incidence in delayed skeletal development in the mid and high dose groups. Most values were only slightly above historical control data. When considering skeletal malformations overall, incidence was observed in 99.3% to 100% of fetuses and 100% of litters including controls. No reproductive toxicity, embryotoxicity or fetotoxicity. | ECHA (2014) |
| Long-Evans rats (50–60/sex/dose) | Lifetime | APT: 0 and 0.43 in drinking water; dose estimated by the U.S. EPA (1992) | LOAEL = 0.43 | Reduced survival rate in males and females; at the median life spans, survival was reduced by 106 and 107 days for males and females, respectively, compared to controls. Non-fasting serum glucose levels were reduced by 28%–30% in the dosed animals. | Schroeder et al. (1970) |
| APT – antimony potassium tartrate; ATO – antimony trioxide; GD – gestational day; LOAEL – lowest observed adverse effect level; LOEL – lowest observed effect level; NOAEL – no observed adverse effect level; POD – point of departure; SD – Sprague-Dawley. | |||||
2.2.3 Genotoxicity and carcinogenicity
Both the genotoxicity and the carcinogenicity of antimony and its compounds have been previously reviewed (WHO, 2003; Porquet and Filella, 2007; NTP, 2018; ATSDR, 2019). For genotoxicity, overall in vivo studies for antimony trioxide were negative for clastogenicity and bone marrow aberrations, and chromosomal aberrations and micronuclei formation were negative for in vivo assays. Occupational studies were also negative for micronuclei formation and sister chromatid exchange. In vitro assays were generally negative for gene mutations. However, some positive responses for antimony trichloride and pentachloride (highly soluble antimony substances) in chromosomal aberration and micronuclei formation assays were observed. Overall, there is low concern for genotoxicity for the antimony substances in the group (ECCC and Health Canada, 2020).
The International Agency for Research on Cancer (IARC) has classified trivalent antimony as a group 2A carcinogen (IARC, 2023) by the inhalation route. The European Commission classified antimony trioxide as a Category 2 carcinogen (suspected human carcinogen) under the regulation on classification, labelling and packaging (CLP-Regulation (CE) No 1272/2008) (EU, 2008a). According to a European Union risk assessment report, antimony trioxide is classified as a Category 3 carcinogen (Annex 1, Directive 67/548/EEC) based on limited evidence of a carcinogenic effect (EU, 2008). The EU (2008) further indicated that there is no evidence of tumours following oral exposure to antimony. A critical review by Lynch et al. (1999) concluded the available chronic studies contained many flaws in design and experimental methodology, making them unsuitable for making any definitive conclusions about carcinogenicity. ATSDR (2019) notes that cancer incidence was not increased in chronic studies where mice and rats were orally exposed to APT.
2.3 Mode of action
The mode of action for antimony-induced toxicity in mammals has not been fully elucidated. However, the current evidence indicates that treatment-related hepatotoxicity likely involves oxidative stress (NTP, 2018) which is preceded by the reduction of Sb(V) (the form most prevalent in drinking water) to its Sb(III) form. Both the in vitro reduction of Sb(V) to Sb(III) (which is dose-dependent, and favoured at acidic pH and high temperature) and the involvement of Sb(III) in hepatotoxicity have been demonstrated (Frezard et al., 2001; DFG, 2007; Kato et al., 2014). Thiol homeostasis imbalance has also been demonstrated through depletion of intracellular glutathione and inhibition of thiol-containing enzyme systems by Sb(III) (for example, APT) (Lauwers et al., 1990; DFG, 2007; Kato et al., 2014). These processes result in increased production of reactive oxygen species (ROS), oxidative stress, and the induction of peroxidase activity and apoptosis (Lecureur et al., 2002b; Kato et al., 2014).
Hashemzaei et al. (2015) observed that the antimony-induced lysis of isolated rat hepatocytes was mediated by ROS formation, lipid peroxidation and a decline in mitochondrial membrane potential. Increased oxidative stress was also observed in the liver of mice and rats treated with Sb(V) antimonials (for example, meglumine antimoniate) (Dzamitika et al., 2006; Frezard et al., 2009; Bento et al., 2013). The acute treatment of mice with meglumine antimoniate also induced oxidative stress as evidenced by increased lipoperoxidation and superoxide dismutase (SOD) activity in the liver. An imbalance between SOD and catalase activities in heart, liver, spleen and brain tissue was also reported (Bento et al., 2013).
Finally, the cytotoxicity of APT, as evidenced by APT induced apoptosis, was observed in various lymphoid cell lines, including the HL60 acute myeloid leukemia cell lines. There was also an association between the underlying apoptosis and increased cellular production of ROS, as well as loss of mitochondrial membrane potential (Lecureur et al., 2002a, 2002b). Increased levels of ROS and deleterious effects on mitochondria by ATO were also reported (NTP, 2018).
Wan et al. (2021) investigated the nephrotoxicity induced by arsenic and/or antimony exposure via the induction of autophagy and pyroptosis in vivo and in vitro. In vivo, mice were dosed with 4 mg/kg arsenic trioxide or/and 15 mg/kg antimony trichloride by intragastric intubation for 60 days. In vitro, renal tubular epithelial (TCMK-1) cells were treated with arsenic trioxide (12.5 μM) and/or antimony trichloride (25 μM) for 24 hours. The in vivo results showed the potential for arsenic and/or antimony exposure to induce histopathological changes in the kidneys as indicated by elevated levels of creatinine and carbamine (which serve as indicators of nephrotoxicity). Additionally, arsenic and/or antimony exposure induced oxidative stress activating autophagy and pyroptosis processes (two types of programmed cell death) via increasing/decreasing anti-autophagy/pyroptosis gene expression. In vitro, arsenic and/or antimony increased reactive oxygen species generation and decreased mitochondrial membrane potential in TCMK cells.
2.4 Selected key study
The adverse health effects of antimony have been evaluated in several subchronic studies with rats and mice. The available data from humans are not suitable for deriving a health-based value (HBV) due to study weaknesses including the route of administration of antimony (i.e. intravenous and intramuscular injection) as well as exposure to high doses via poisoning events or therapeutic applications of antimony-based drugs; thus, animal data are considered the most appropriate for risk assessment.
For the derivation of an HBV for drinking water, the study by Poon et al. (1998) is chosen as the critical study because it used an adequate number of animals, administered antimony by drinking water over multiple doses, assessed numerous health outcomes, and reports the lowest NOAEL in the animal toxicity database. Sprague-Dawley rats (15/sex/dose) were given 0, 0.5, 5, 50 and 500 ppm APT (0.06, 0.56, 5.58, 42.17 mg Sb/kg bw per day for males; 0.06, 0.64, 6.13, 45.69 mg Sb/kg bw per day for females) in drinking water for 13 weeks. Ten additional animals per sex were included in each of the control and the highest dose groups and were given tap water for a further 4-week recovery period.
The authors observed no mortalities or clinical signs of toxicity and several of the observed histological changes in the internal organs assessed were considered as adaptive. Histological changes observed in the liver were anisokaryosis (i.e., variation in size and shape) and hyperchromicity (increased optical density) of the liver nuclei, as well as increased portal density and perivenous homogeneity in the cytoplasm of hepatocytes. Anisokaryosis occurred with a dose-related increased incidence and severity in both sexes with persistence observed through the recovery period in the high dose animals, indicating that these effects were not readily reversible. According to the authors, all of the high dose group animals had a moderate severity of anisokaryosis and most animals in the lower dose groups showed low to minimal severity of anisokaryosis. Hyperchromicity was observed in the high dose males which also persisted through the recovery period. Increased hepatocyte portal density and perivenous homogeneity was observed in all treated rats (which persisted through recovery) but were considered adaptive and were less prominent in females. Other reported effects include: mild histological changes in the thyroid observed in all treated animals progressing in severity with increasing dose; dose-related decreases in serum glucose in females starting in the second lowest dose group with statistical significance reached in the highest dose group; decreased red blood cell and platelet counts with increased mean corpuscular volume in the high dose males; dose-related accumulation of antimony in red blood cells starting in the second lowest dose group animals; and dose-related increased accumulation of antimony in the spleen starting in the lowest dose group with persistence in the high dose group animals through recovery. Poon and colleagues identified a NOAEL of 0.06 mg/kg bw per day based on histological changes in the liver and thyroid, biochemical changes (namely decreased serum glucose levels in females), and accumulation of antimony in red blood cells and the spleen.
In a review of the Poon et al. (1998) study by Lynch et al. (1999), Lynch and colleagues conclude that the observed histological effects in the liver were not necessarily indicative of overt toxicity and thus proposed an alternative NOAEL of 6 mg Sb/kg bw per day. Poon and colleagues, however, responded in a later publication indicating that while the liver histological changes were considered as adaptive, these effects should be considered along with the changes in serum biochemistry, which together indicate a change in liver function (Valli et al., 2000). In conclusion, Poon and colleagues maintain that the identified NOAEL of 0.06 mg Sb/kg bw per day is appropriate. Based on the consideration of liver histological effects and serum chemistry together as indicating a change in liver function (Valli et al, 2000), the NOAEL of 0.06 mg Sb/kg bw per day was selected as the most appropriate point of departure (POD) from the Poon et al. (1998) study.
Other recent assessments by OEHHA (2016) and ATSDR (2019) have also used data from Poon et al. (1998) for developing their health advice on antimony. In developing the public health goal for antimony in drinking water, the OEHHA (2016) identified liver anisokaryosis in males as the key health endpoint for risk assessment and derived a POD of 0.14 mg Sb/kg bw per day (10% benchmark dose level, BMDL10) for the basis of the public health goal. OEHHA indicates that the choice of key health endpoint is supported by evidence of liver damage in humans exposed to antimony for the treatment of leishmaniasis and in animals in repeated dose studies, and that liver anisokaryosis has been documented as a toxic response following exposure to other xenobiotic compounds such as hydroquinone and toxaphene. In developing its intermediate minimal risk level for antimony, ATSDR (2019) also used the NOAEL of 0.06 mg/kg bw per day as identified by Poon et al. (1998). However the NOAEL was chosen based on changes in serum glucose levels which ATSDR (2019) identifies as one of the most sensitive health endpoints in the animal toxicity database.
Under the Chemicals Management Plan, the risks to human health and the environment posed by a group of 11 antimony-containing substances have been previously evaluated (ECCC and Health Canada, 2020). Using a screening assessment approach, a POD of 49 mg Sb/kg bw per day for fetal skeletal effects reported by ECHA (2014) was used for characterizing the risks associated with exposure to antimony-containing substances from environmental media, food, drinking water and consumer products. The ECHA (2014) study administered a less soluble form of antimony (sodium hexahydroxoantimonate) via gavage, yielding a higher POD than that identified by Poon et al. (1988) which administered APT via drinking water. ECCC and Health Canada (2020) did not consider studies administering APT or antimony trichloride for risk characterization since it was deemed that exposure to these forms of antimony from environmental media, food, drinking water and consumer products is not anticipated.
For the derivation of an HBV for antimony in drinking water, a POD of 0.06 mg Sb/kg bw per day from Poon et al. (1998) for changes in liver histology (anisokaryosis) along with the changes in serum biochemistry (which together are indicative of a change in liver function) is chosen. The choice of liver impacts as the key health endpoint is supported by evidence of liver damage in humans exposed to antimony during the treatment of leishmaniasis and in animal repeated dose studies. Since the form of antimony used in the Poon et al. (1998) study (APT) is more soluble than other forms of antimony that may be present in drinking water, the HBV is expected to be protective of all forms of antimony in drinking water.
3.0 Derivation of the health-based value (HBV)
A NOAEL of 0.06 mg Sb/kg bw per day based on histopathological changes (anisokaryosis) in the liver and changes in serum biochemistry that are indicative of liver effects, as reported by Poon et al. (1998), is chosen as the POD for deriving the HBV for antimony in drinking water.
Using the NOAEL of 0.06 mg Sb/kg bw per day, a tolerable daily intake (TDI) for total antimony is calculated as follows:
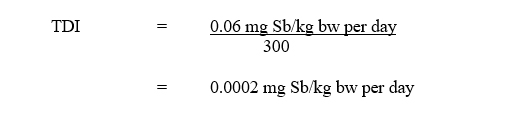
Text description
The tolerable daily intake is equal to 0.06 milligrams of antimony per kilogram of body weight per day divided by 300. This is equivalent to 0.0002 milligrams of elemental antimony per kilogram of body weight per day.
where:
- 0.06 mg Sb/kg bw per day is the NOAEL identified from Poon et al. (1998), based on histopathological changes (anisokaryosis) in the liver and changes in serum biochemistry indicative of liver effects; and
- 300 is the uncertainty factor, accounting for interspecies variation (×10), intraspecies variation (×10), and the use of a subchronic study (×3).
Using this TDI, the HBV for total antimony in drinking water is derived as follows:
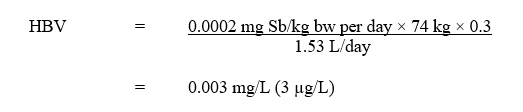
Text description
The health-based value is equal to 0.0002 milligrams of antimony per kilogram of body weight per day multiplied by 74 kilograms multiplied by 0.3. The product is then divided by 1.53 litres per day. This is equivalent to 0.003 milligrams per litre, or 3 micrograms per litre.
where:
- 0.0002 mg Sb/kg bw per day is the TDI derived above;
- 74 kg is the average body weight for an adult (Health Canada, 2021);
- 0.3 is the drinking water allocation factor based on the upper bound of the estimated intake for drinking water (see section 1.3);
- 1.53 L/day is the drinking water intake rate for a Canadian adult (Health Canada, 2021). Due to its low volatility and low dermal absorption (OEHHA, 2016), exposure to antimony from showering or bathing is unlikely to be significant; consequently, a multi-route exposure assessment, as outlined by Krishnan and Carrier (2008), was not performed.
4.0 Analytical and Treatment Considerations
4.1 Analytical methods to detect antimony
4.1.1 Standardized methods
Standardized analytical methods available for the analysis of total antimony in drinking water and their respective method detection limits (MDLs) are summarized in Table 5. MDLs are dependent on the sample matrix, instrumentation, and selected operating conditions and will vary between individual laboratories. These methods are subject to a variety of interferences, which are outlined in the respective references. The total concentration of antimony is determined from these methods and not the antimony species. A number of accredited laboratories in Canada were contacted to determine the MDLs and the method reporting limits (MRLs) for total antimony analysis and the MDLs were in the range of those reported in Table 5. The MRL were between 0.5 μg/L and 1 μg/L by methods based on inductively coupled plasma – Mass Spectrometry (ICP-MS) (AGAT Laboratories, 2019a, b, c; Paracel Laboratories Ltd., 2019).
The MDLs or MRLs from provincial data are in the range of 0.1 μg/L to 2 μg/L (see section 1.3).
Drinking water utilities should discuss sampling requirements with the accredited laboratory conducting the analysis to ensure that quality control procedures are met and that MRLs are low enough to ensure accurate monitoring at concentrations below the MAC.
| Method (Reference) |
Methodology | MDL (µg/L) |
Interferences/Comments |
|---|---|---|---|
| U.S. EPA Methods | |||
EPA 200.5 Rev. 4.2 (U.S. EPA, 2003) |
Axially viewed inductively coupled plasma-atomic emission spectrometry (AVICP-AES) |
0.9 |
Spectral, physical, chemical and memory interferences. Matrix interferences: Ca, Mg and Na > 125 mg/L and Si > 250 mg/L. |
EPA 200.8, Rev. 5.4 (U.S. EPA, 1994a) |
ICP-MS |
Isobaric elemental and polyatomic ion and physical interferences. Matrix interferences: TDS > 0.2 % (w/v). |
|
EPA 200.9, Rev 2.2 (U.S. EPA, 1994b) |
Stabilized temperature graphite furnace atomic absorption |
0.8 |
Spectral, matrix and memory interferences. The HCl present from the digestion procedure can influence the sensitivity. Interference by K2SO4 can be reduced by using H/Ar in char step. |
EPA 6020B (U.S. EPA, 2014) |
ICP-MS |
0.1 (IDL) |
Isobaric elemental and molecular and memory interferences. Matrix interferences: TDS > 0.2 % Sb concentrations of 50–500μg/L require 1% (v/v) HCl for stability. |
| APHA Standard Methods (SM) | |||
SM 3113B (APHA et al., 2017) |
Electrothermal atomic absorption spectrometry |
0.8 (Estimated detection level) |
Sb not recovered unless HCl used in digestion. Molecular absorption, chemical and matrix interferences. |
SM 3125 (APHA et al., 2017) |
ICP-MS |
0.07 (IDL) |
Samples should not contain more than 0.5 % dissolved solids. Isobaric, abundance sensitivity, polyatomic ion, physical, memory and ionization interferences. |
| ASTM Methods | |||
D5673-16 (ASTM, 2016) |
ICP-MS |
0.08 (IDL) |
Abundance sensitivity and isobaric elemental, isobaric polyatomic ion, physical and memory interferences. |
ICP-MS – inductively coupled plasma mass spectrometry; IDL – instrument detection level; MDL – method detection limit; TDS – total dissolved solids.
|
|||
4.1.2 Sample preparation
Total antimony includes both the dissolved and particulate (suspended) fractions of antimony in a water sample and is analyzed using methods for total recoverable antimony. Analysis of total antimony is needed for comparison to the MAC.
Sample processing considerations for the analysis of antimony in drinking water (i.e., sample preservation, storage, digestion, etc.) can be found in the references listed in Table 5. Accurate quantification of dissolved, particulate and total antimony in samples is dependent on proper sample preservation and processing steps. SM 3030B and SM 3030D provide guidance on filtration, preservation (acidification) and digestion procedures for the determination of dissolved or particulate metals (APHA et al., 2017). SM 3030D provides guidance for metal digestion, including antimony and the necessity of using hydrochloric acid along with nitric acid for proper digestion (APHA et al., 2017). In order to determine dissolved antimony concentrations, samples should be filtered at the time of collection (not at the laboratory) and the filtrate should be acidified to pH < 2 with concentrated nitric acid.
Currently, United States Environmental Protection Agency (U.S. EPA) methods 200.5, 200.8, 200.9 and SM 3113B do not require hot acid digestion for total recoverable metals, unless turbidity of the sample is greater than 1 nephelometric turbidity unit (NTU). However, research conducted on other metals (for example, lead, chromium and cadmium) has found that this may not accurately quantify the total metal concentration in a drinking water sample for all metals. This approach may underestimate total antimony in drinking water when the particulate form of antimony is present and hot acid digestion may be necessary. Hot acid digestion is described in U.S. EPA methods 200.5, 200.8 and 200.9 (U.S. EPA, 2003, 1994a, b). Microwave-assisted digestion, outlined in method SM 3030 K (APHA et al., 2017), can also be used for analysis of total recoverable metals for methods that are based on ICP-MS.
4.2 Treatment considerations
Treatment technologies that are available to decrease antimony concentrations in drinking water include: ferric-based conventional coagulation, with best removal achieved at low pH (95% removal at pH 5.1); adsorption using titanium-based adsorbents (100 000–170 000 bed volumes to breakthrough of 6 μg/L at pH 6.5); reverse osmosis (RO) (46%–99%); and a combination of coagulation/flocculation and ultrafiltration (16%–98%). The effectiveness of these technologies varies depending on water quality parameters such as the species of antimony, the pH and presence of competing ions. At the residential scale, certified treatment devices relying on RO and distillation are expected to be effective for removal of antimony (U.S. EPA, 1998). Operational complexity (for example, pre- and post-treatment pH adjustment and alkalinity adjustment) may need to be considered in the selection of treatment options, particularly for small systems.
The antimony species present in water entering a treatment plant is an important factor in determining the effectiveness of treatment. Smaller, neutral species are generally more difficult to remove than larger, charged species. The two main species of antimony present in natural waters are antimonite (Sb(III)), in the form of Sb(OH)3, and antimonate (Sb(V)), an anion in the form of Sb(OH)6- (U.S. EPA, 2006; Deng et al., 2017). Typically, Sb(V) is the form present in oxic surface water and Sb(III) is the form in anoxic groundwater (He et al., 2015).
The redox chemistry of antimony is important in the treatment and removal of antimony from drinking water. The treatment type for antimony determines whether Sb(III) or Sb(V) is better removed. Conventional treatment bench-scale studies indicate that Sb(III) is better removed than Sb(V) whereas removal through RO exhibited better removal of Sb(V).
4.2.1 Municipal-scale
The selection of an appropriate treatment process will depend on many factors, including the raw water source and its characteristics, the species of antimony present in the water, the operational conditions of the selected treatment method and the water utility’s treatment goals. Treatment goals may require that pH be adjusted post-treatment to address corrosion issues in the distribution system (Health Canada, 2015). Pilot- and bench-scale testing is critical to ensure the source water can be successfully treated and to optimize operating conditions.
4.2.1.1 Conventional coagulation
Conventional coagulation was evaluated for antimony removal through numerous bench-scale studies, with some using natural waters and others deionized waters (see Tables 6, 7 and 8). The first study listed in Table 6 evaluated two source waters, collected in and distributed through two historic mine tunnels (Spiro and Judge tunnels) in Park City, Utah, with high total antimony levels as well as other co-occurring contaminants (CH2M, 2016; Najm et al., 2017). Ferric chloride (FC) was used in jar tests to determine the impact of coagulant dose and pH (see Table 6). Overall, the results showed that the removal of total antimony was:
- only partially achieved with FC (Najm et al., 2017),
- most effective at highest dose of FC and lowest pH (Najm et al., 2017), and
- not as effective using high pH coagulation (CH2M, 2016; Najm et al., 2017).
| Water | Influent Sb (μg/L) | pH | Sb effluent (μg/L)Table 6 Footnote b | Water Quality | Co-occurring contaminants | ||
|---|---|---|---|---|---|---|---|
| FeCl3 Dose (mg/L) | |||||||
| 5 | 20 | 40 | |||||
Spiro Tunnel |
9.3 |
5.5 |
3 |
4 |
2.9 |
|
|
6.5 |
6.8 |
5.8 |
4.7 |
||||
7.5 |
7.6 |
7 |
5.3 |
||||
Judge Tunnel |
6.1 |
5.5 |
4.5 |
3 |
1.6 |
|
|
6.5 |
5.1 |
4.5 |
3.1 |
||||
7.5 |
5.1 |
4.7 |
3.4 |
||||
As – arsenic; Cd – cadmium; Fe – iron; Tl: thallium; Zn – zinc.
|
|||||||
Other bench-scale studies also evaluated Sb(V) and Sb(III) removal using coagulation (see Tables 7 and 8) and these showed that:
- Ferric-based coagulants performed better than aluminum-based (Kang et al., 2003; Guo et al., 2009);
- Sb(III) was better removed than Sb(V) (Kang et al., 2003; Guo et al., 2018);
- Optimal pH for Sb(V) removal with FC was between 4.5 and 5.5 (Kang et al., 2003; Guo et al., 2009, 2018);
- Sb(V) removal declined with increasing pH (Guo et al., 2009, 2018);
- Sb(III) removal was less impacted by pH (4.0–10.0) (Guo et al., 2009, 2018); and
- Generally, Sb(III) and Sb(V) removal increased with ferric-based coagulant dose (Kang et al., 2003; Wu et al., 2010; Guo et al., 2018; Inam et al., 2018).
| Influent (μg/L) |
% RemovalTable 7 Footnote b |
Coagulant Type |
Coagulant Dose |
pH |
Water |
References |
|---|---|---|---|---|---|---|
| Aluminum-based coagulants | ||||||
Sb(V) = 6 |
10% |
Polyaluminum chloride |
5.4 mg/L |
5.1 |
|
Kang et al. (2003) |
Sb(III) = 6 (Sb2O3) |
40% |
5.3 mg/L |
5.1 |
|
||
Sb(III) = 4 (SbCl3) |
20% |
|||||
Sb(V) = 50 |
< 20% |
Aluminum sulphate |
1 - 3 x 10-4 mol/L |
3.5–9.8 |
|
Guo et al. (2009) |
Sb(III) = 50 |
< 25% |
1 - 3 x 10-4 mol/L |
||||
| Iron-based coagulants | ||||||
Sb(V) = 6 |
65% |
Ferric chloride |
10.3 mg/L |
5 |
|
Kang et al. (2003) |
90% |
20.0 mg/L |
|||||
Sb(III) = 6 (Sb2O3) |
90% |
10.3 - 20.0 mg/L |
5.1 |
|
||
Sb(III) = 4 (SbCl3) |
> 95% |
|||||
Sb(V) = 250 |
> 99% |
Polymeric ferric sulfate |
8 x 10-4 mol/L |
4 |
Deionized water with 4.0x10-3 mol/L NaHCO3 added |
Guo et al. (2018) |
78% |
6 |
|||||
< 55% |
> 8.5 |
|||||
95% |
4 x 10-4 mol/L |
5 |
||||
50% |
7 |
|||||
< 45% |
> 8 |
|||||
Sb(III) = 100 |
85% |
4 x 10-5 mol/L |
4 |
|||
> 95% |
> 5.5 |
|||||
70% |
2 x 10-5 mol/L |
4.5 |
||||
80% |
6 |
|||||
> 90% |
> 8 |
|||||
DOC – dissolved organic carbon.
|
||||||
| FC Dose (mol/L) | Initial Sb(V) (μg/L) | Initial Sb(III) (μg/L) | ||||
|---|---|---|---|---|---|---|
| Sb(V) = 49.2 | Sb(V) = 98.4 | Sb(V) = 492 | Sb(III) = 50.6 | Sb(III) = 101 | Sb(III) = 506 | |
| Treated Sb(V) concentration (μg/L) | Treated Sb(III) concentration (μg/L) | |||||
| pH 6.0 ± 0.2 | ||||||
| 2 x 10-4 | 22.1 | 47.7 | 241 | 6.6 | 13.8 | 35.2 |
| 6 x 10-4 | 0.7 | 10.8 | 50.5 | 1.5 | 4.6 | 13.2 |
| 10 x 10-4 | Undetectable | 2.9 | 8.2 | 0.7 | 3.5 | 6.4 |
| pH 7.8 ± 0.2 | ||||||
| 2 x 10-4 | 38.3 | 73.2 | 341 | 11.3 | 13.5 | 36.1 |
| 6 x 10-4 | 20.8 | 31.8 | 106 | 3.9 | 9.8 | 21.5 |
| 10 x 10-4 | 4.7 | 25.1 | 60.0 | 3.5 | 7.1 | 6.5 |
FC – ferric chloride.
|
||||||
A bench-scale study used to evaluate ferric-based coagulants found that the addition of Fe(III) had better Sb(III) removal than that using Fe(II), in artificially contaminated tap water (Mitrakas et al., 2018).
Sb(III) was shown to be better removed than Sb(V) when using FC-based coagulants, and pre-oxidation may have a negative impact on overall removal since this oxidation step converts the Sb(III) to the Sb(V) form. The impact of pre-chlorination (residual free chlorine of 0.5 mg/L at 10 minutes) was examined in a bench-scale study on antimony removal using FC (dose = 10.3 mg as Fe/L) (Kang et al., 2003). When Sb(III) was present, pre-chlorination resulted in declined removal over the entire range of pH values (pH 5–10).
Kang et al. (2003) discussed antimony removals compared to those of arsenic. For arsenic, As(V) is better removed than As(III), which differs from antimony, in which Sb(III) is better removed than Sb(V). The authors stated that for Sb(V) removal, the required FC dose at a pH of 5 is about nine times higher than that for removal of As(V).
The presence of competing ions had variable effects on removal and was a function of the antimony species, pH, coagulant dose and the concentration of competing ion. A bench-scale study evaluated effects of silicate, bicarbonate (HCO3-), sulfate (SO42-), phosphate (PO43-) and humic acid (HA) on both Sb(V) and Sb(III) removal (Wu et al., 2010). The jar tests were conducted at various FC doses, competing ion concentrations and pH for each of Sb(III) and Sb(V). Silicate was found to have minimal effect on antimony removal. HCO3-, SO42-, HA and PO43- presence had a negative impact on Sb(V) removal. The effect of HA and PO43- on Sb(III) removal was variable depending on pH and coagulant dose. The authors also conducted experiments using a synthetic water containing various anions and cations and indicated that Sb(V) removal was impacted to a larger extent than Sb(III). Other bench-scale studies were conducted that showed similar impacts as a result of competing ions (Guo et al., 2009, 2018).
Inam et al. (2019) investigated the impact of various natural organic matter (NOM) concentrations on antimony removal through jar tests. Different NOM were investigated to determine the impact on the optimal FC dose in removing Sb(III) and Sb(V). The NOM types that were investigated included hydrophilic salicylic acid and L-cysteine and hydrophobic HA. The tests included rapid mixing, slow mixing and settling stages and were conducted with either Sb(III) or Sb(V) at 1 mg/L in deionized water, with NOM at a concentration of 10 mg/L. The optimal FC dose was found in the presence of each type of NOM for both Sb(III) and Sb(V), and in all cases was higher for Sb(V) than for Sb(III) with greater than 90% removed. For both Sb(III) and Sb(V), the presence of HA resulted in the highest FC optimal dose over the other types of NOM. The authors stated that the hydrophobic molecules in HA suppressed the antimony adsorption onto the FC.
4.2.1.2 Adsorption
Adsorption can be used to remove contaminants and effectiveness of this treatment technology depends on the contaminant, the sorbent material, water quality parameters and presence of competing ions (U.S. EPA, 1998). Other ions present in the water may compete with antimony for adsorption sites and impact the number of bed volumes (BVs) to breakthrough and the regeneration or replacement frequency.
The town of Alta in Utah uses the Bay-City Tunnel (an abandoned mine tunnel) to collect and store percolating groundwater which is then used as the source for drinking water (Najm et al., 2010). The tunnel water contains total antimony concentrations between 1.2 μg/L to 29 μg/L (average of 13 μg/L), along with many other co-occurring contaminants such as arsenic and cadmium. Pilot-scale testing was conducted using a titanium-based media, operating for 11 hrs/day. At a natural pH of 7.3, antimony reached breakthrough concentration of 6 μg/L between 50 000 BVs and 75 000 BVs. When the pH was lowered to 6.5, the performance improved to approximately 100 000 BVs to reach breakthrough. The full-scale system for this site consisted of two columns in a lead-lag configuration, with effluent remaining below 1 μg/L during the evaluation period of 30 000 BVs for the lead column and 15 000 BVs for both columns. Comparison of the pilot- and full-scale results during the first 30 000 BVs show better performance at the full-scale with < 1 μg/L of total antimony in the treated water compared to the pilot-scale with approximately 2 μg/L. Finally, fully treated water was compared against a blend of treated and untreated water (at a ratio of 2:1). The results showed that the treated water antimony concentration remained close to 0 μg/L for > 390 days of operation and that the blended water concentrations were between 3 μg/L and 6 μg/L over this same time frame.
The Spiro and Judge Tunnel waters in Park City Utah presented in section 4.2.1.1 were also evaluated for removal of antimony, using various adsorbents, through bench- and pilot-scale studies (CH2M, 2016; Najm et al., 2017; Swaim et al., 2017). Antimony and other co-occurring contaminants had to be removed from the mine tunnel waters. The bench-scale study evaluated a titanium dioxide (TiO2)-based adsorbent at pH 8, achieving antimony removal to less than 1 μg/L at a dose of 60 mg/L (Najm et al., 2017). The pilot-scale study was carried out in advance of full-scale implementation to ensure adequate removals of antimony, arsenic, cadmium (Cd), iron (Fe), manganese (Mn), thallium (Tl), zinc (Zn), selenium (Se) and lead (Pb). The treatment train consisted of pre-oxidation, high pH coagulation/settling, MnO2 filtration, adsorption, disinfection and conditioning (CH2M, 2016; Swaim et al., 2017). All of the co-occurring contaminants were removed to satisfactory levels after the MnO2 filtration stage, with the exception of antimony. The removal of antimony was examined through evaluation of three different adsorption media including TiO2, ferric oxide and ferric hydroxide. It was estimated that ferric oxide and ferric hydroxide at pH 6.5 would need to be changed every 12 000 BVs to 18 000 BVs and 29 000 BVs to 50 000 BVs, respectively. For the TiO2 adsorbent, it was expected to have approximately 170 000 BVs between media changes at pH 6.5 and 66 000 BVs to 91 000 BVs at pH 7.6. The TiO2 column running at a pH of 7.6 was lowered to 6.5, resulting in an increased antimony removal. When the pH was returned to 7.6, the antimony concentrations returned to previous values. Overall, this pilot-scale study showed that:
- Antimony was removed by all media at an empty bed contact time of 2.5 minutes;
- TiO2 exhibited the best antimony removal; and
- Lowering to pH 6.5 improved performance (CH2M, 2016).
A full-scale study carried out as part of the U.S. EPA’s Arsenic Treatment Technology Demonstration program included investigation of antimony. The study included three parallel columns that evaluated adsorption of arsenic and antimony using commercially available granular ferric hydroxide (β-FeOOH + Fe(OH)3) (Cumming et al., 2009). The study ran over approximately five and a half months and treated 15 753 000 gallons of groundwater. Breakthrough of antimony at 6 μg/L occurred at 3 000 BVs, which the authors stated was unexpectedly short and may have been due to presence of silica and phosphorus in the source water.
Other pilot- and bench-scale studies provided a range of adsorption capacities for various iron-based adsorbents to remove antimony (see Table 9). Overall, these studies showed that:
- Sb(V) removal is better under acidic conditions (Guo et al., 2014; Miao et al., 2014);
- Sb(III) removal is relatively unaffected by pH (Guo et al., 2014);
- Antimony removal is not impacted by the presence of arsenic (Sazakli et al., 2015), NO3- or SO43- (Qi and Pichler, 2017);
- Phosphate has negative impact on antimony removal (Qi and Pichler, 2017);
- Freshly prepared ferric hydroxide (in-situ FeOxHy) exhibited better adsorption, as freshly prepared adsorbents better maintain the adsorption sites (He et al., 2015); and
- The surface of the iron-based adsorbent may catalyze the oxidation of Sb(III) that is adsorbed, leading to partial desorption of Sb(V) into treated water (Leuz et al., 2006; Qi and Pichler, 2017).
| Adsorbent | Antimony species | Adsorption capacity (mg/g) | References |
|---|---|---|---|
| Iron-based | Not specified | 0.42–1.63 | Ilavský et al. (2014, 2015); Sazakli et al. (2015); Barloková et al. (2017) |
| Sb(III) | 19.4–53.5 | Xi et al. (2013); Guo et al. (2014); Qi and Pichler (2016) | |
| Sb(V) | 6.5–62.5 | Guo et al. (2014); Miao et al. (2014); Qi and Pichler (2016) | |
| In-situ FeOxHy | Sb(III) | 12.77 mmol/g(1 555 mg/g) | He et al. (2015) |
| Sb(V) | 10.21 mmol/g(1 243 mg/g) | ||
| In-situ FeOxHy – freshly prepared ferric hydroxide. | |||
Three pilot-scale studies evaluated the removal of antimony from spring water and the use of commercially available iron-based adsorbents by determining the number of BVs to reach 5 μg/L (see Table 10) (Ilavský et al., 2014, 2015; Barloková et al., 2017). Barloková et al. (2017) determined that the number of BVs increased with increased column height. Ilavský et al. (2014, 2015) indicated that CeO2·nH2O was the better adsorbent over 90.1% α-FeOOH, FeOOH and β-FeOOH + Fe(OH)3. In these three studies, the authors stated iron-based adsorbents could possibly decrease antimony to drinking water levels.
| Adsorbent type | Influent (μg/L) | EBCT (min) | BV | HeightTable 10 Footnote a (cm) | Filtration rate (m/h) | Description | References |
|---|---|---|---|---|---|---|---|
| β-FeOOH + Fe(OH)3 | 90.3 (ave) | 5.5 | 1 537 | 50 | 5.3–5.4 (Average) | Spring water (high antimony due to mining) | Barloková et al., (2017) |
| 7.7 | 3 736 | 70 | |||||
| 10.1 | 4 659 | 100 | |||||
| 27.7 ± 3.41 | 5.29 | 3 236 | 48–49 | 5.55–5.58 (Average) | Spring water pH 8.2; TDS 190 mg/L | Ilavský et al., (2015) | |
| CeO2·nH2O | 5.16 | 3 967 | |||||
| β-FeOOH + Fe(OH)3 | 58.3 (ave) | 6.0 | 1 700 | 50–52 | 4.7–5.3 | Spring water | Ilavský et al., (2014) |
| 90.1% α-FeOOH | 6.4 | 715 | 4.3–4.9 | ||||
| FeOOH | 6.3 | 790 | 4.3–5.1 | ||||
BV – bed volumes; EBCT – empty bed contact time; TDS -- total dissolved solids.
|
|||||||
He et al. (2015) examined the removal of antimony in deionized water with potassium nitrate solute at 0.01 M using in-situ FeOxHy at the bench-scale. The maximum adsorption capacity of in-situ FeOxHy was shown to be 6.68 mmol/g and 5.34 mmol/g for Sb(III) and Sb(V), respectively. The authors then conducted a pilot-scale study to examine the removal of Sb(V) over 716 hours from two columns in series. The influent concentration ranged between 20 and 30 μg/L with the pH gradually being decreased in a step-wise manner from 7.6 to 5.2. The effluent Sb(V) concentration from each column was found to increase slowly over time and then decrease corresponding to the step-wise decline in pH. The authors stated that the pH adjustment was valuable to increase the adsorption capacity and decrease the regeneration frequency.
4.2.1.3 Reverse osmosis
Studies investigating antimony removal by RO illustrate good antimony removal (see Table 11). As part of the U.S. EPA’s Arsenic Treatment Technology Demonstration program, a point-of-entry RO system was evaluated at a school (Wang et al., 2011). Antimony removal was examined in addition to arsenic removal and had 99% removal over the eight-month study period.
A bench-scale study examined the removal of Sb(III) and Sb(V) by RO and showed that Sb(V) was better removed than Sb(III) and was less impacted by pH (Kang et al., 2000). The removals of Sb(III) and Sb(V) are almost constant over the pH range of 3 to 10 with both the polyamide and polyvinyl alcohol membranes. The exception was for the removal of Sb(III) with the polyvinyl alcohol membrane, where it decreased sharply from 60.2% to 45.7% when pH changed from 7 to 10.
| Influent (μg/L) | Removal (%) | RO Membrane | pH | Process Description | Reference |
|---|---|---|---|---|---|
8.6–13.2 |
99% |
15 membrane modules |
|
|
Wang et al. (2011) |
Sb(III) = 10 |
Polyamide |
3–10 |
|
Kang et al. (2000) |
|
Polyvinyl alcohol |
3 |
||||
60.2%Table 11 Footnote a |
7 |
||||
45.7%Table 11 Footnote a |
10 |
||||
Sb(V) = 10 |
Polyamide |
3–10 |
|||
Polyvinyl alcohol |
3–10 |
||||
RO – reverse osmosis.
|
|||||
Limitations of the RO process include possible membrane scaling, fouling, and failure, as well as higher energy use and capital costs. Calcium, barium, and silica can cause scaling and decrease membrane efficiency. Since RO completely removes alkalinity in water, it will continually lower product water pH and increase its corrosivity. Therefore, the product water pH must be adjusted and alkalinity may need to be increased to avoid corrosion issues in the distribution system such as the leaching of lead and copper (Schock and Lytle, 2011; U.S. EPA, 2012).
4.2.1.4 Coagulation followed by ultrafiltration membrane
Studies evaluating combined treatment processes (coagulation followed by an ultrafiltration membrane) were reviewed. Two bench-scale studies examined antimony removal using freshly prepared hydrolytic flocs (Du et al., 2014; Ma et al., 2017a, b) (see Table 12). The studies that examined Sb(V) (Ma et al., 2017a, b) showed that:
- With no coagulant, there was low removal and high transmembrane pressure (TMP).
- For four iron and aluminum-based coagulants, hydrated ferric chloride (FeCl3H2O) had the best performance.
- Continuous coagulant injection resulted in better Sb(V) removal as compared to injections every 2 days (however with higher TMP).
- As pH was lowered to 6, the Sb(V) was better removed and the TMP was lower.
- Sludge discharge frequency (5 days versus 10 days) had little effect on Sb(V) removal.
- Aeration had little effect on removal but TMP decreased as aeration increased.
The study that examined Sb(III) (Du et al., 2014) showed that:
- When no coagulant was used, the Sb(III) removal was low;
- pH (in range 5 to 9) had only slight effect on Sb(III) removal;
- Removal increased with dose (optimum at 0.4 mM); and
- Greater than 95% Sb(III) removal was obtained (dose = 0.4 mM; pH 8.5 ± 0.2; all initial concentrations).
Given only bench-scale studies are available it is critical to conduct bench- and pilot-scale studies prior to full-scale implementation to ensure the effectiveness of combined treatment and its optimal operating conditions.
| Influent (μg/L) |
Coagulant Type | Coagulant dose | Removal | Effluent (μg/L) |
TMP (kPa) |
pH | Conditions | Process Description |
|---|---|---|---|---|---|---|---|---|
| Sb(V) removal (Ma et al., 2017a, b) | ||||||||
5.4–19.8 |
None |
0 |
10.7% |
N/A |
74.6 |
7.5 |
|
|
AlCl3·H2O |
10 mM/inj |
27.2% |
N/A |
61.3 |
7.5 |
|||
FeCl3·H2O |
92.8% |
N/A |
3.7 |
6 |
||||
40.7% |
N/A |
17.9 |
7.5 |
|||||
15.9% |
N/A |
22.3 |
9 |
|||||
50 mM over 10 days |
53.6% |
N/A |
31.2 |
7.5 |
||||
5 mM/inj |
N/A |
26.8 |
||||||
4.1–13.7 |
10 mg/L |
N/A |
0.32 |
36.1 |
7.3-7.6 |
|
||
10 mg/L |
N/A |
0.26 |
33.7 |
|||||
| Sb(III) removal (Du et al., 2014) | ||||||||
62.5 ± 0.1 |
Ferric coag. |
0 |
9% |
N/A |
Not given |
6 |
|
|
12% |
N/A |
8 |
||||||
0.2 mM |
76% |
N/A |
6 |
|||||
66% |
N/A |
8 |
||||||
0.4 mM |
96% |
N/A |
6 |
|||||
94% |
N/A |
8 |
||||||
> 0.6 mM |
96% |
N/A |
6 and 8 |
|||||
100 ± 3.6 |
0.4 mM |
97% |
N/A |
5–8 |
||||
94% |
N/A |
9 |
||||||
30, 54, 89, 124 and 158 |
0 |
5%–7.5%Table 12 Footnote a |
N/A |
8.5 ± 0.2 |
||||
0.4 mM |
> 95%Table 12 Footnote a |
N/A |
||||||
HRT – hydraulic retention time; inj – injection; N/A – not available; TMP – transmembrane pressure; TOC – total organic carbon.
|
||||||||
4.2.2 Residential-scale
In cases where antimony removal is desired at the household level -- for example, when a household obtains its drinking water from a private well -- a residential drinking water treatment unit may be an option for decreasing antimony concentrations in drinking water. Systems classified as residential scale may have a rated capacity to treat volumes greater than that needed for a single residence, and thus may also be used in small systems.
Before a treatment unit is installed, the water should be tested to determine the general water chemistry and antimony concentration in the source water. Periodic testing by an accredited laboratory should be conducted on both the water entering the treatment unit and the treated water to verify that the treatment unit is effective. Units can lose removal capacity through use and time and need to be maintained and/or replaced. Consumers should verify the expected longevity of the components in the treatment unit according to the manufacturer’s recommendations and service it when required.
Health Canada does not recommend specific brands of drinking water treatment units, but it strongly recommends that consumers use units that have been certified by an accredited certification body as meeting the appropriate NSF International Standard/American National Standards Institute (NSF/ANSI) for drinking water treatment units. The purpose of these standards is to establish minimum requirements for the materials, design and construction of drinking water treatment units that can be tested by a third party. This ensures that materials in the unit do no leach contaminants into the drinking water (i.e., material safety). In addition, the standards include performance requirements that specify the removal that must be achieved for specific contaminants (for example, reduction claim) that may be present in water supplies.
Certification organizations (i.e., third parties) provide assurance that a product conforms to applicable standards and must be accredited by the Standards Council of Canada (SCC). Accredited organizations in Canada (SCC, 2020) include:
- CSA Group
- NSF International
- Water Quality Association
- UL LLC
- Bureau de normalisation du Québec (available in French only)
- International Association of Plumbing and Mechanical Officials
- Truesdail Laboratories Inc.
An up-to-date list of accredited certification organizations can be obtained from the SCC.
The drinking water treatment units that are expected to be effective for antimony removal at the residential-scale include RO (U.S. EPA, 1998). Distillation should also be effective based on physical and chemical properties of antimony. Currently, antimony is not included in the performance requirements (for example, reduction claims) of NSF/ANSI standards. However, use of a treatment unit that is certified to the standards for RO or distillation will ensure that the material safety of the units has been tested. These standards are NSF/ANSI Standard 58 (Reverse Osmosis Drinking Water Treatment Systems) (NSF International, 2022a) and NSF/ANSI Standard 62 (Drinking Water Distillation Systems) (NSF International, 2022b).
The effectiveness of RO units for antimony removal is dependent on the membrane (filter) type and pH of the water, and anticipated removals range from 46% to 99% (based on municipal-scale data). Therefore, an RO system will need to be carefully selected, considering influent water antimony concentrations and relevant water composition, to achieve treated water concentrations below the MAC. In addition, it may be necessary to pre-treat the water to reduce fouling and extend the service life of the RO membrane. Although there is a lack of data regarding the use of distillation for removal of antimony from drinking water, it is expected to perform adequately because it is effective for the reduction of other inorganic contaminants. However, this process requires a high electrical energy input. Consumers may want to consult a water treatment professional for advice on available treatment systems, as well as installation and maintenance costs, based on their specific water quality.
Water that has been treated using RO and distillation is more likely to be corrosive to internal plumbing components. Also, as large quantities of influent water are needed to obtain the required volume of treated water, these devices are generally not practical for point-of-entry installation. Therefore, these devices should be installed only at the point-of-use.
A consideration for limiting exposure to antimony is to specify that drinking water materials (i.e., components and treatment chemicals) meet health-based standards. These standards ensure that materials meet health-based requirements and are safe for use in potable water applications. NSF/ANSI Standards 61 (NSF International, 2022c) and 60 (NSF International, 2022d) require that the concentration of antimony does not exceed the single product allowable concentration of 0.0006 mg/L in components and treatment chemicals, respectively.
4.3 Distribution system considerations
Treated water, accumulation in the distribution system and leaching from brass and solder materials are potential sources of antimony in both distribution and household plumbing systems. Distribution systems are complex and the related water quality concerns are inherently more challenging to manage effectively than those for the water leaving the treatment plant. Additional information on distribution system issues and maintenance can be found elsewhere (Health Canada, 2022a, b).
4.3.1 Antimony deposition and accumulation
The accumulation of trace inorganic contaminants in the drinking water distribution system is a complex function of numerous factors, including contaminant concentration in treated water, pH, redox conditions and pipe material. Iron oxyhydroxides and hydrous manganese oxides are significant sinks for trace inorganic contaminant accumulation because of their adsorptive affinity for them. Water quality changes or physical disruptions in the distribution system can remobilize contaminants into the bulk water. Indicators of this include the presence of discoloured water or increased turbidity.
Total and dissolved antimony during water flushing trials of distribution systems were examined (Friedman et al., 2016). The results consistently showed that particulate antimony concentrations were higher in flushed samples than bulk water. The study indicates a lack in adsorption and release mechanisms of antimony within the distribution system.
Friedman et al. (2010) identified several key water quality conditions that should be controlled in order to maintain water stability for deposited trace inorganic contaminants. These include pH, the oxidation-reduction potential and the corrosion-control measures. It is also important to avoid the uncontrolled blending of surface water with groundwater and of chlorinated water with chloraminated water. Indicators of potential release of trace inorganic contaminants in the distribution system may include discoloured water and increased turbidity.
In a study of scale and sediment samples collected from the distribution systems of 20 U.S. drinking water utilities supplied by groundwater, surface water and blended water sources, antimony was found to be the tenth most concentrated of the 12 inorganics analyzed (Friedman et al., 2010). The authors reported that antimony was found in all solids but that its concentration was significantly lower than other metals. The median antimony concentration of all scale deposits and sediment samples combined was 0.14 μg/g (1.4 x 10-5 weight %), with 10th percentile and 90th percentile of 0.05 μg/g (5 x 10-6 weight %) and 0.86 μg/g (8.6 x 10-5 weight %), respectively. The median antimony concentrations in scale deposits and hydrant-flush solids were 0.13 μg/g and 0.17 μg/g (1.3 x 10-5 weight % and 1.7 x 10-5 weight %), respectively. It was noted that, of the six samples with higher antimony content (> 0.9 μg/g), there were no obvious commonalities to other co-occurring contaminants. The authors also reported an estimated antimony mass of 0.1 lb (0.05 kg) accumulated on a 100-mile pipe length (160 km) (based on a 12-in. diameter pipe [30.5 cm]). Theoretically, 60%–85% of the scale deposit would need to be released to exceed the U.S. EPA drinking water standard for antimony of 0.006 mg/L. Based on these results, the accumulation of antimony (and its potential release) in distribution systems is not considered significant relative to other inorganic contaminants.
Scale from lead service lines (N = 5) ranged from 2.54 mg/kg to 100 mg/kg and from an iron service line (N = 1) was 0.78 mg/kg (Schock, 2005). Scale from 23 lead pipes from older installations (dating from 1880 to 1947, and 2 unknown dates) had a range of 11.3 mg/kg to 292 mg/kg (Kim and Herrara, 2010). Friedman et al. (2016) analyzed 13 opportunity samples (those that became available including those from water meters, pipes and filters) with a range of 0 to 168 mg/kg. Antimony accumulation is thought to be due to surface adsorption and co-precipitation reaction with soluble Sb(OH)6- which is in anionic form at the pH typically found in the distribution system (Friedman et al., 2010).
Clement and Carlson (2004) investigated the distribution system of the Park City, Utah drinking water system discussed previously (see sections 4.2.1.1 and 4.2.1.2). At the time of this study, the drinking water system was supplied by several sources, including one of the historic mine tunnels (not specified). The source water had an antimony concentration of 0.0066 mg/L, mainly in soluble form, and had no physical treatment but was chlorinated prior to entering the distribution system. Samples from the distribution system were taken during a flushing exercise. It was found that the antimony concentration continued to rise throughout the system and reached 0.027 mg/L after ten minutes, when sampling was discontinued and maximum was not reached. The flushing concentration was 4 times greater than the source water concentration. Scanlan (2003) analyzed sediment samples from one reservoir from this same water system and had an average antimony content of 48 mg/kg. Another study examined 45 homes using random daytime sampling, with presence of lead in at least part of the service line. A total of 135 samples were collected and analyzed for antimony with a detection limit of 0.03 μg/L, and results showed a maximum of 0.23 μg/L (Deshommes et al., 2010). A third study investigated 16 water pipes, in which all 401 samples were below the detection limit of 0.5 μg/L (Zietz et al., 2015). Deshommes et al. (2012) investigated 45 taps in a large facility, including 9 basement taps that simulated dead ends due to their location and infrequent use. The water was sampled after twelve hours of stagnation and samples from the basement taps had antimony concentration of 8 μg/L, which authors stated was due to low frequency of use and no flushing prior to sampling. The remaining taps had antimony concentration of 5 μg/L. The authors noted a correlation between lead concentration and antimony that may be attributed to lead release from brass and leaded solder.
4.3.2 Leaching of antimony from non-lead based solder
Non-lead-containing solders are a potential source of antimony in drinking water (Fuge et al., 1992; U.S. EPA, 2006). Two studies assessed the leaching of metals from copper pipes or copper coupons with non-lead-based solders (tin/antimony, tin/silver and tin/copper/silver) (Subramanian et al., 1991, 1994). Different waters were tested and the tests were run over long periods to evaluate the leaching under different scenarios. Subramanian et al. (1991) evaluated three waters using copper pipes and took samples over a 90-day period. The tests that were run with high-purity water and well water had antimony concentrations below the detection limit of 1.2 μg/L for all solders tested. The test using tap water had no detected antimony up to day 7, with antimony concentration then increasing from day 14 to day 90 for tin/antimony solder. Another study by these same authors using copper coupons with various non-lead-based solders was run with four different test waters over a 28-day period (Subramanian et al., 1994). For all waters tested and all types of solder, the antimony concentration remained below the detection limit of 1.2 μg/L. These studies overall showed no antimony leaching for most waters, with the exception of tap water in Subramanian et al. (1991) which had some antimony leaching after long contact times.
Accumulation of lead and other heavy metals in two distribution systems was studied (Fuge et al., 1992). Three samples had antimony content ranging from 0.4 mg/kg to 1.7 mg/kg. The authors stated that the antimony in the pipe solid was due to the solder present in the system.
4.3.3 Leaching of antimony from brass materials
Plumbing devices that contain brass used in distribution systems or in premise plumbing have the potential to impact drinking water quality. Non-leaded brass and lead-free brass generally contain a maximum of 0.25% antimony and in some cases as high as 0.5% (Sandvig et al., 2007). A few studies were conducted to determine levels of antimony leaching from different brasses (see Table 13) (Sandvig et al., 2007, 2012; Turković et al., 2014). Two of the bench-scale studies showed no antimony leaching with a detection limit of 6 µg/L (Sandvig et al., 2007) and 0.08 µg/L (Turković et al., 2014). Turković et al. (2014) stated that this may be due to the low antimony concentration in the brasses investigated. A study using the NSF/ANSI Standard 61 testing protocol evaluated 10 non-leaded and lead-free brass devices (Sandvig et al., 2012). The tests were conducted using 11 test waters and mainly had results below the detection limit. The two test waters with the highest occurrence of antimony leaching, up to 0.4 μg/L, were the NSF/ANSI Standard 61 Section 9 synthetic test water in which one was chlorinated and the other was chloraminated.
| Material tested | Waters tested | Testing Procedure | Results | Reference |
|---|---|---|---|---|
7 brasses |
|
|
N = 42 |
Sandvig et al. (2007) |
6 devices NSF/ANSI 61 Section 8 devicesTable 13 Footnote c |
11 test waters with varying pH, alkalinity. Either chlorine or chloramine. |
NSF/ANSI Standard 61 testing protocol |
Control run (N = 66) – all < 0.5μg/L Runs with test waters (DL = 0.1μg/L):
|
Sandvig et al. (2012) |
4 devices NSF/ANSI 61 Section 9 devicesTable 13 Footnote d |
Control run (N = 32) – all < 0.5μg/L Runs with test waters (DL = 0.01–0.5μg/L):
|
|||
5 brasses (4 non-leaded and 1 leaded) Antimony content: < 0.001% to 0.003% |
NSF/ANSI 61 Section 9 synthetic test waterTable 13 Footnote a |
Short-term leaching tests in accordance with NSF/ANSI Standard 61 Section 9 test protocol. |
Detection limit = 0.08 μg/L No antimony leaching which the authors stated may be due to low antimony content in the brasses. |
Turković et al. (2014) |
5 “corner waters”
|
Long-term leaching tests (experimental protocol of DIN EN 15664-1)Table 13 Footnote e 26-week period. |
Detection limit = 0.08 μg/L No antimony leaching which the authors stated may be due to low antimony content in the brasses. |
||
ANSI – American National Standards Institute; DL – detection limit; N – sample size; NSF – NSF International.
|
||||
4.4 Residuals management
Treatment technologies may produce a variety of residuals that contain antimony (for example, backwash water, reject water/concentrate and media waste). The appropriate authorities should be consulted to ensure that the disposal of liquid and solid waste residuals from the treatment of drinking water meet applicable regulations. Guidance can be found elsewhere (CCME, 2003, 2007).
5.0 Management Strategies
All water utilities should implement a comprehensive, up-to-date risk management water safety plan. A source-to-tap approach should be taken that ensures water safety is maintained (CCME, 2004; WHO, 2011, 2012). This approach requires a system assessment to characterize the source water; describe the treatment barriers that prevent or reduce contamination; identify the conditions that can result in contamination; and implement control measures. Operational monitoring is then established, and operational/management protocols are instituted (for example, standard operating procedures, corrective actions and incident responses). Compliance monitoring is determined and other protocols to validate the water safety plan are implemented (for example, record keeping and consumer satisfaction). Operator training is also required to ensure the effectiveness of the water safety plan at all times (Smeets et al., 2009).
5.1 Control strategies
In water sources with higher than acceptable antimony concentrations, one or more treatment options (see section 4.2) may be implemented. Non-treatment strategies such as blending or alternative water supplies can also be considered. When the option of a treatment technology is chosen, the species of antimony should be identified and pilot-scale testing is recommended to ensure the source water can be successfully treated and process design is established. Attention must be given to the water quality of a new source prior to making any changes (i.e., switching, blending, and interconnecting) to an existing water supply. For example, if the new water source is more aggressive, it may cause leaching of lead or copper in the distribution system.
As it is difficult to control the accumulation and release of antimony and other contaminants of health concern in the distribution system, the control strategy should minimize the antimony concentration that enters the distribution system from the treatment plant. Generally, the distribution system should be managed such that drinking water is transported from the treatment plant to the consumer with minimum loss of quality. As source waters, treatment plants and distribution systems can differ significantly, a system-specific control strategy would be necessary.
5.2 Monitoring
5.2.1 Source water characterization
Water sources should be characterized to determine if antimony is present. Monitoring of source water should be conducted yearly. Authorities may consider reduced monitoring when it has been demonstrated that antimony is not present and/or appropriate treatment is in place.
5.2.2 Treatment
Where treatment is required to remove antimony, operational monitoring should be implemented to confirm whether the treatment process is functioning as required (i.e., paired samples of source and treated water to confirm the efficacy of treatment). The frequency of operational monitoring will depend on the treatment process. For example, if adsorption is used, at least quarterly monitoring should be conducted or a method to estimate BVs to breakthrough should be used to predict the need for media replacement.
5.2.3 Compliance monitoring
When treatment is in place for antimony removal, it is recommended that compliance monitoring for total antimony be conducted annually, at a minimum, to confirm the MAC is not exceeded. Samples should be collected after treatment prior to distribution (typically at the entry point prior to the distribution system) and analyzed by an accredited laboratory.
5.2.4 Distribution system
Like other inorganics, antimony can accumulate in distribution systems and later be released. Consequently, monitoring should also be conducted throughout the distribution system when antimony is or was historically present in the source and/or distributed water. Monitoring programs should be designed on a system-specific basis to verify that control strategies are operating as intended and consider risk factors that contribute to the likelihood that antimony may be elevated within the drinking water system. Factors that influence antimony accumulation and mobilization, such as changes to water chemistry and physical/hydraulic disturbances in the distribution system, could be used as indicators of when and where to monitor for antimony release.
Monitoring for total antimony and other contaminants (for example, iron, manganese, arsenic and lead) should be conducted when water quality changes or physical disruptions occur in the system. The release of antimony and other contaminants may be indicated by the presence of discoloured water or increased turbidity resulting from the release of deposits or scales present on the pipe wall. Customer complaints related to discoloured water episodes can also be an indication of the need to undertake water quality sampling. The number and location of sites for monitoring of antimony in the distribution system, including sampling at the tap, should take into consideration the site-specific accumulation and release risk factors. However, the absence of discoloured water does not mean that there are no metals being released.
Water utilities that have baseline data indicating that antimony is not present within the distribution system may conduct less frequent monitoring.
5.2.5 Residential
Households with private wells are encouraged to have their water tested for total antimony to ensure that the concentration in their water supply is below the MAC. In addition, homeowners with private wells using residential treatment devices should conduct routine testing on both the water entering the treatment device and the treated water to verify that the treatment device is effective.
6.0 International considerations
Other national and international organizations have drinking water guidelines, standards and/or guidance values for antimony in drinking water. Variations in these values can be attributed to the age of the assessments or to differing policies and approaches, including the choice of key study and the use of different consumption rates, body weights and source allocation factors.
| Agency (Year) |
Value (mg/L) |
Key Endpoint (Reference) |
NOAEL/ LOAEL (mg/kg bw per day) |
UF | TDI (mg/kg bw per day) |
BW (kg) |
DW Intake (L/d) |
AF (%) |
|---|---|---|---|---|---|---|---|---|
| Health Canada - MAC (2022) |
0.006 | Liver effects: anisokaryosis; biochemical changes related to liver histological changes (Poon et al., 1998) | 0.06 (NOAEL) | 300 | 0.0002 | 74 | 1.53 | 30 |
| U.S. EPA-MCL (1992, 2018) |
0.006 | Decreased lifespan, increased blood glucose and cholesterol (Schroeder, 1970) | 0.43 (LOAEL) | 1 000 | 0.0004 (RfD) | 70 | 2 | 40 |
| WHO (2003) |
0.02 | Reduced body weight gain and reduced food and water intake in rats (Poon et al., 1998) | 6 (NOAEL) | 1 000 | 0.006 | 60 | 2 | 10 |
| Australia (NHMRC and NRMMC, 2011) |
0.003 | Decreased lifespan, altered blood glucose and cholesterol (Schroeder, 1970). | 0.43 (LOEL) | 500 | N/A | 70 | 2 | 10 |
| EU (2020) |
0.01 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| AF – Allocation factor; BW – Body weight; DW -- Drinking water; LOAEL – Lowest observed adverse effect level; LOEL – Lowest observed effect level; MAC – Maximum acceptable concentration; MCL – Maximum contaminant level; N/A – Not available; NOAEL – No observed adverse effect level; RfD – Reference dose; TDI – Tolerable daily intake; UF – Uncertainty factor. | ||||||||
7.0 Rationale for maximum acceptable concentration
Antimony naturally occurs in the environment in the form of organic and inorganic compounds. Antimony enters the environment from natural emissions and anthropogenic activities, with coal combustion, mining and smelting being the most important sources of anthropogenic release. Antimony may enter drinking water from plumbing solders in drinking water distribution systems.
Oral exposure to antimony may induce adverse effects mainly on the gastrointestinal tract and the liver. Kidney, cardiovascular, metabolic, and developmental adverse effects have also been reported in the literature.
The derivation of the HBV of 0.003 mg/L (3 µg/L) is based on a form of antimony that is more soluble (and thus more bioavailable) than those forms occurring in drinking water. It is not possible, based on the available science, to quantify the potential health benefits from reducing the current MAC of 0.006 mg/L (6 µg/L) to the updated HBV, but it is not expected to significantly increase health protection. Given this fact and the anticipated treatment challenges associated with lowering the MAC, particularly for private wells and for small systems, the Federal-Provincial-Territorial Committee on Drinking Water used a risk managed approach that reaffirms the MAC of 0.006 mg/L (6 µg/L) for total antimony in drinking water.
The MAC is expected to be protective of all forms of antimony in drinking water. As part of its ongoing guideline review process, Health Canada will continue to monitor new research in this area and recommend any changes to this guideline technical document that it deems necessary.
8.0 References
- Adam, G.K., Abdulla, M.A., Ahmed, A.A. and Adam, I. (2009). Maternal and perinatal outcomes of visceral leishmaniasis (kala-azar) treated with sodium stibogluconate in eastern Sudan. Int. J. Gynaecol. Obstet., 107(3): 208–210.
- AGAT Laboratories Ltd. (2019a). Personal communication with A. Sekera. Calgary, AB.
- AGAT Laboratories Ltd. (2019b). Personal communication with S. Ortiz. Edmonton, AB.
- AGAT Laboratories Ltd. (2019c). Personal communication with M. Kellosalmi. Burnaby, BC.
- Alvarez, M., Malecot, C.O., Gannier, F. and Lignon, J.M. (2005). Antimony-induced cardiomyopathy in guineapig and protection by L-carnitine. Br. J. Pharmacol., 144(1): 17–27.
- Andersen, E.M., Cruz-Saldarriaga, M., Llanos-Cuentas, A., Luz-Cjuno, M., Echevarria, J., Miranda-Verastegui, C., Colina, O. and Berman, J.D. (2005). Comparison of meglumine antimoniate and pentamidine for peruvian cutaneous leishmaniasis. Am J Trop Med Hyg 72, 133–137.
- Anderson, C.G. (2012). The metallurgy of antimony. Antimony. Geochemistry, 72: 3–8.
- Andrewes, P. and Cullen, W.R. (2003). Organoantimony compounds in the environment. In: Organometallic compounds in the environment. Craig, P.J. (ed.). 2nd. John Wiley & Sons, Chichester, England. DOI: 10.1002/0470867868, pp. 277–303.
- Andrewes, P., Kitchin, K.T. and Wallace, K. (2004). Plasmid DNA damage caused by stibine and trimethylstibine. Toxicol. Appl. Pharmacol., 194(1): 41–48.
- APHA, AWWA and WEF (2017). Standard methods for the examination of water and wastewater. 23rd Ed. (Online version). Standard methods for the examination of water and wastewater. American Public Health Association. Available at https://www.standardmethods.org/
- ASTM (2016). ASTM D5673–16 Standard test method for elements in water by inductively coupled Plasma—Mass spectrometry. ASTM International, West Conshohocken, Pennsylvania.
- ATSDR (2019). Toxicological Profile for Antimony and compounds. Agency for Toxic Substances and Disease Registry. Atlanta (GA): U.S. Department of Health and Human Services, Public Health Service. Available at https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=332&tid=58
- AWWA (2017). Internal Corrosion Control in Water Distribution Systems: Manual of Water Supply Practices, M58. Second edition. American Water Works Association, Denver, CO.
- Bailly, R., Lauwerys, R., Buchet, J.P., Mahieu, P. and Konings, J. (1991). Experimental and human studies on antimony metabolism: Their relevance for the biological monitoring of workers exposed to inorganic antimony. Br. J. Ind. Med., 48(2): 93–97.
- Barloková, D., Ilavsky, J. and Munka, K. (2017). Removal of antimony from water using GEH sorption material at different filter bed volumes. Anonymous Lithuania.
- Barrera, C., Lopez, S., Aguilar, L., Mereado, L. and Bravo, M. (2016). Pentavalent antimony uptake pathway through erythrocyte membranes: molecular and atomic florescence approaches. Anal Bioanl Chem 408: 2937–2944.
- Belzile, N., Chen, Y. and Filella, M. (2011). Human exposure to antimony: I. sources and intake. Crit. Rev. Environ. Sci. Technol., 41(14): 1309–1373.
- Bento, D.B., de Souza, B., Steckert, A.V., Dias, R.O., Leffa, D.D., Moreno, S.E., Petronilho, F., de Andrade, V.M., Dal-Pizzol, F. and Romao, P.R. (2013). Oxidative stress in mice treated with antileishmanial meglumine antimoniate. Res. Vet. Sci., 95(3): 1134–1141.
- Borborema, S.E., Osso Jr, J.A., Andrade Jr, H.F. and Nascimento, N. (2013). Biodistribution of meglumine antimoniate in healthy and leishmania (leishmania) infantum chagasi-infected BALB/c mice. Mem. Inst. Oswaldo Cruz, 108(5): 623–630.
- British Columbia Ministry of Health (2019). Personal communication with D. Fishwick.
- Cai, Y., Li, L. and Zhang, H. (2015). Kinetic modeling of pH-dependent antimony (V) sorption and transport in iron oxide-coated sand. Chemosphere, 138: 758–764.
- Carneado, S., Hernandez-Nataren, E., Lopez-Sanchez, J.F. and Sahuquillo, A. (2015). Migration of antimony from polyethylene terephthalate used in mineral water bottles. Food Chem., 166: 544–550.
- CCME (2003). Guidance on the site-specific application of water quality guidelines in Canada: Procedures for deriving numerical water quality objectives. Canadian Council of Ministers of the Environment, Winnipeg, Manitoba.
- CCME (2004). From source to tap: Guidance on the multi-barrier approach to safe drinking water. Canadian Council of Ministers of the Environment, Winnipeg, Manitoba.
- CCME (2007). A protocol for the derivation of water quality guidelines for the protection of aquatic life. Canadian Council Ministers of the Environment, Winnipeg, Manitoba.
- CFIA (2016). 2012-2014 Antimony in Selected Foods. Canadian Food Inspection Agency, Ottawa, Canada. Available at https://inspection.canada.ca/food-safety-for-industry/food-chemistry-and-microbiology/food-safety-testing-bulletin-and-reports/antimony-in-selected-foods/eng/1645636000965/1645636001387 (accessed October 2019).
- CH2M (2016). Judge and Spiro tunnels mining – influenced water treatment evaluation: draft pilot testing report, CH2M Hill Engineering, Inc., Taylorsville, UT.
- Chen, R., Chen, J., Cheng, S., Qin, J., Li, W., Zhang, L., Jiao, H., Yu, X., Zhang, X., Lahn, B.T. and Xiang, A.P. (2010). Assessment of embryotoxicity of compounds in cosmetics by the embryonic stem cell test. Toxicol. Mech. Methods, 20(3): 112–118.
- Choe, S.Y., Kim, S.J., Kim, H.G., Lee, J.H., Choi, Y., Lee, H. and Kim, Y. (2003). Evaluation of estrogenicity of major heavy metals. Sci. Total Environ., 312(1–3): 15–21.
- Clement, B. and Carlson, G. (2004). Contaminant accumulation in the distribution system: Case histories (as cited in U.S. EPA, 2006).
- Coelho, D.R., De-Carvalho, R.R., Rocha, R.C.C., Saint’Pierre, T.D. and Paumgartten, F.J.R. (2014a). Effects of in utero and lactational exposure to SbV on rat neurobehavioral development and fertility. Reproductive Toxicology, 50(Supplement C): 98–107.
- Coelho, D.R., Miranda, E.S., Saint'Pierre, T.D. and Paumgartten, F.J. (2014b). Tissue distribution of residual antimony in rats treated with multiple doses of meglumine antimoniate. Mem. Inst. Oswaldo Cruz, 109(4): 420–427.
- Cooper, R.G. and Harrison, A.P. (2009). The exposure to and health effects of antimony. Indian J. Occup. Environ. Med., 13(1): 3–10.
- Cruz, A., Rainey, P.M., Herwaldt, B.L., Stagni, G., Palacios, R., Trujillo, R. and Saravia, N.G. (2007). Pharmacokinetics of antimony in children treated for leishmaniasis with meglumine antimoniate. J. Infect. Dis., 195(4): 602–608.
- Cumming, L.J., Chen, A.S.C. and Wang, L. (2009). Arsenic and antimony removal from drinking water by adsorptive media U.S. EPA demonstration project at South Truckee Meadows General Improvement District (STMGID), NV Final performance evaluation report. Water Supply and Water Resources Division. National Risk Management Research Laboratory, EPA/600/R-09/016, Cincinnati, Ohio.
- Darbre, P.D. (2006). Metalloestrogens: An emerging class of inorganic xenoestrogens with potential to add to the oestrogenic burden of the human breast. J. Appl. Toxicol., 26(3): 191–197.
- Deng, R., Jin, C., Ren, B., Hou, B. and Hursthouse, A.S. (2017). The potential for the treatment of antimony-containing wastewater by iron-based adsorbents. Water (Switzerland), 9(10).
- Deshommes, E., Laroche, L., Nour, S., Cartier, C. and Prévost, M. (2010). Source and occurrence of particulate lead in tap water. Water Res., 44(12): 3734–3744.
- Deshommes, E., Nour, S., Richer, B., Cartier, C. and Prévost, M. (2012). POU devices in large buildings: Lead removal and water quality. J. AWWA: E282-E297.
- DFG (2007). Antimony and its inorganic compounds* (inhalable fraction). The MAK-Collection Part I: MAK value documentations, vol. 23. DFG, deutsche forschungsgemeinschaft. ISBN: 978-3-527-31595-6. WILEY-VCH Verlag GmbH & Co., KGaA, Weinheim.
- Du, X., Qu, F., Liang, H., Li, K., Yu, H., Bai, L. and Li, G. (2014). Removal of antimony (III) from polluted surface water using a hybrid coagulation-flocculation-ultrafiltration (CF-UF) process. Chem. Eng. J., 254: 293–301.
- Dzamitika, S.A., Falcao, C.A., de Oliveira, F.B., Marbeuf, C., Garnier-Suillerot, A., Demicheli, C., Rossi-Bergmann, B. and Frezard, F. (2006). Role of residual Sb(III) in meglumine antimoniate cytotoxicity and MRP1-mediated resistance. Chem. Biol. Interact., 160(3): 217–224.
- ECCC (2010). Screening Assessment for the Challenge - Antimony trioxide (Antimony oxide). Environment and Climate Change Canada. Available at https://www.ec.gc.ca/ese-ees/9889ABB5-3396-435B-8428-F270074EA2A7/batch9_1309-64-4_en.pdf
- ECCC (2017). National long-term water quality monitoring data. Environment and Climate Change Canada. Available at http://Donnees.ec.gc.ca/data/substances/monitor/national-long-term-water-quality-monitoring-data/.
- ECCC and Health Canada (2020). Screening Assessment - Antimony-containing substances. Environment and Climate Change Canada and Health Canada. Government of Canada, Ottawa, Canada. Available at https://www.canada.ca/content/dam/eccc/documents/pdf/pded/antimony-containing-substances/FSAR-Antimony-EN.pdf
- ECHA (2014). Sodium hexahydrooxoantimonate. CAS RN 33908-66-6. European Chemicals Agency [accessed 2019 april].
- Edel, J., Marafante, E., Sabbioni, E. and Manzo, L. (1983). Metabolic behaviour of inorganic forms of antimony in the rat. Heavy Met. Environ. Int. Conf. 4th 1: 574–577.
- Ettler, V., Mihaljevic, M., Sebek, O. and Nechutny, Z. (2007). Antimony availability in highly polluted soils and sediments - a comparison of single extractions. Chemosphere, 68(3): 455–463.
- EU (2008). European Union Risk Assessment Report: Diantimony Trioxide. Available at https://echa.europa.eu/documents/10162/553c71a9-5b5c-488b-9666-adc3af5cdf5f
- EU (2020). Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption. Available at https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020L2184&from=EN
- Felicetti, S.W., Thomas, R.G. and McClellan, R.O. (1974). Metabolism of two valence states of inhaled antimony in hamsters. Am. Ind. Hyg. Assoc. J., 35: 292–300.
- Filella, M., Williams, P.A. and Belzile, N. (2009). Antimony in the environment: Knowns and unknowns. Environmental Chemistry, 6: 95–105.
- Filella, M. and Williams, P.A. (2010). Antimony biomethylation in culture media revisited in the light of solubility and chemical speciation considerations. Environ. Toxicol., 25(5): 431–439.
- Filella, M. (2020). Antimony and PET bottles: Checking facts. Chemosphere, 261, 127732. ISSN 0045-6535. Available at: https://doi.org/10.1016/j.chemosphere.2020.127732.
- Forns, J., Fort, M., Casas, M., Caceres, A., Guxens, M., Gascon, M., Garcia-Esteban, R., Julvez, J., Grimalt, J.O. and Sunyer, J. (2014). Exposure to metals during pregnancy and neuropsychological development at the age of 4 years. Neurotoxicology, 40: 16–22.
- Franke, E.D., Wignall, F.S., Cruz, M.E., Rosales, E., Tovar, A.A., Lucas, C.M., Llanos-Cuentas, A. and Berman, J.D. (1990). Efficacy and toxicity of sodium stibogluconate for mucosal leishmaniasis. Ann. Inter. Med., 113, 934–940.
- Frezard, F., Demicheli, C., Ferreira, C.S. and Costa, M.A. (2001). Glutathione-induced conversion of penatvalentantimony to trivalent antimony in meglumine antimoniate. Antimicrob. Agents Chemother., 45(3): 913–916.
- Frezard, F., Demicheli, C. and Ribeiro, R.R. (2009). Pentavalent antimonials: New perspectives for old drugs. Molecules, 14(7): 2317–2336.
- Friedman, M.J., Hill, A.S., Reiber, S.H., Valentine, R.L., Larsen, G., Young, A., Korshin, G.V. and Peng, C.Y. (2010). Assessment of inorganics accumulation in drinking water systems scales and sediments. Water Research Foundation, Denver, Colorado.
- Friedman, M.J., Hill, A.S., Booth, S., Hallett, M., McNeill, L., McLean, J., Stevens, D., Sorensen, D., Hammer, T., Kent, W., De Haan, M., MacArthur, K. and Mitchell, K. (2016). Metals accumulation and release within the distribution system: evaluation and mitigation. Report 4509. Water Research Foundation, Denver, Colorado.
- Friedrich, K., Vieira, F.A., Porrozzi, R., Marchevsky, R.S., Miekeley, N., Grimaldi, G.,Jr and Paumgartten, F.J. (2012). Disposition of antimony in rhesus monkeys infected with leishmania braziliensis and treated with meglumine antimoniate. J. Toxicol. Environ. Health A, 75(2): 63–75.
- Fuge, R., Pearce, N.J.G. and Perkins, W.T. (1992). Unusual sources of aluminium and heavy metals in potable waters. Environ. Geochem. Health, 14(1): 15–18.
- Garbarino, J.R. and Struzeski, T.M. (1998). Methods of analysis by the US Geological Survey National Water Quality Laboratory – Determination of elements in whole-water digests using inductively coupled plasma-optical emission spectrometry and inductively coupled plasma-mass spectrometry. United States Geological Survey, Denver, Colorado (Report No. 98-165). Available at http://nwql.usgs.gov/pubs/OFR/OFR-98-165.pdf
- Gerhardsson, L., Brune, D., Nordberg, G.F. and Wester, P.O. (1982). Antimony in lung, liver and kidney tissue from deceased smelter workers. Scand. J. Work Environ. Health, 8(3): 201–208.
- Gerhardsson, L., Brune, D., Nordberg, G.F. and Wester, P.O. (1988). Multielemental assay of tissues of deceased smelter workers and controls. Sci. Total Environ., 74: 97–110.
- Guo, X., Wu, Z. and He, M. (2009). Removal of antimony(V) and antimony(III) from drinking water by coagulation-flocculation-sedimentation (CFS). Water Res., 43(17): 4327–4335.
- Guo, X., Wu, Z., He, M., Meng, X., Jin, X., Qiu, N. and Zhang, J. (2014). Adsorption of antimony onto iron oxyhydroxides: Adsorption behavior and surface structure. J. Hazard. Mater., 276: 339–345.
- Guo, W., Fu, Z., Wang, H., Liu, S., Wu, F. and Giesy, J.P. (2018). Removal of antimonate (Sb(V)) and antimonite (Sb(III)) from aqueous solutions by coagulation-flocculation-sedimentation (CFS): Dependence on influencing factors and insights into removal mechanisms. Sci. Total Environ., 644: 1277–1285.
- Gupta, S., Raychaudhury, B, and Datta, S.C. (2009). Host peroxisomal properties are not restored to normal after treatment of visceral leishmaniasis with sodium antimony gluconate. Exp. Parasitol., 123, 140–145.
- Hammond, C.R. and Lide, D.R. (2019). “The elements,” in CRC Handbook of Chemistry and Physics, 100th edition (internet version 2019), John R. Rumble, ed., CRC Press/Taylor & Francis, Boca Raton, FL. Pp. 1–7.
- Hashemzaei, M.,Pourahmad, J., Safaeinejad, F., Tabrizian, K., Akbari, F., Bagheri, G.,Hosseini, M-J. and Shahraki, J. (2015). Antimony induces oxidative stress and cell death in normal hepatocytes. Toxicol. Environ. Chem., 97(2): 256–265.
- He, Z., Liu, R., Liu, H. and Qu, J. (2015). Adsorption of Sb(III) and Sb(V) on freshly prepared ferric hydroxide (FeOxHy). Environ. Eng. Sci., 32(2): 95–102.
- Health Canada (2013). Second report on human biomonitoring of environmental chemicals in Canada. Results of the Canadian Health Measures Survey Cycle (2009–2011). HC Pub.: 130019.
- Health Canada (2015). Guidelines for Canadian drinking water quality: Guideline technical document — pH. Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. (Catalogue No H144-28/2016E-PDF).
- Health Canada (2017). Personal communication with Anca-Maria Tugulea, Environmental and Health Sciences Research Bureau.
- Health Canada (2020) Canadian Total Diet Study – Trace Elements 2016-2018. Available at https://open.canada.ca/data/en/dataset/83934503-cfae-4773-b258-e336896c2c53
- Heath Canada (2021). Canadian exposure factors used in human health risk assessments. Fact Sheet. Health Canada, Ottawa, Ontario. Available at: https://www.canada.ca/en/health-canada/services/chemical-substances/fact-sheets/canadian-exposure-factors-human-health-risk-assessments.html
- Health Canada (2022a). Guidance on monitoring the biological stability of drinking water in distribution systems. Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. (Catalogue No H144-96/2022E-PDF).
- Health Canada (2022b). Guidance on sampling and mitigation measures for controlling corrosion. Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario.
- Hepburn, N.C., Siddique, I., Howie, A.F., Beckett, G.J. and Hayes, P.C. (1993). Hepatotoxicity of sodium stibogluconate in leishmaniasis. Lancet, 342(8865): 238–239.
- Hepburn, N.C., Siddique, I., Howie, A.F., Beckett, G.J. and Hayes, P.C. (1994). Hepatotoxicity of sodium stibogluconate therapy for american cutaneous leishmaniasis. Trans. R. Soc. Trop. Med. Hyg., 88(4): 453–455.
- Herath, I., Vithanage, M. and Bundschuh, J. (2017). Antimony as a global dilemma: Geochemistry, mobility, fate and transport. Environ. Pollut., 223: 545–559.
- Hext, P.M., Pinto, P.J. and Rimmel, B.A. (1999). Subchronic feeding study of antimony trioxide in rats. J. Appl. Toxicol., 19(3): 205–209.
- Hjortenkrans, D.S., Bergback, B.G. and Haggerud, A.V. (2007). Metal emissions from brake linings and tires: Case studies of Stockholm, Sweden 1995/1998 and 2005. Environ. Sci. Technol., 41(15): 5224–5230.
- Hockmann, K., Tandy, S., Lenz, M., Reiser, R., Conesa, H.M., Keller, M., Studer, B. and Schulin, R. (2015). Antimony retention and release from drained and waterlogged shooting range soil under field conditions. Chemosphere, 134: 536–543.
- Hopper, J.F. and Barrie, L.A. (1988). Regional and background aerosol trace elemental composition observed in eastern Canada. Tellus, 40B 446–462.
- Howe, C.G., Claus Henn, B., Farzan, S.F., Habre, R., Eckel, S.P., Grubbs, B.H., Chavez, T.A., Faham, D., Al-Marayati, L., Lerner, D., Quimby, A., Twogood, S., Richards, M.J., Meeker, J.D., Bastain, T.M. and Breton, C.V. (2021). Prenatal metal mixtures and fetal size in mid-pregnancy in the MADRES study. Environ. Res., 196:110388.
- Howe, C.G., Nozadi, S.S., Garcia, E., O'Connor, T.G., Starling, A.P., Farzan, S.F., Jackson, B.P., Madan, J.C., Alshawabkeh, A.N., Cordero, J.F., Bastain, T.M., Meeker, J.D., Breton, C.V. and Karagas, M.R. (2020). Prenatal metal(loid) mixtures and birth weight for gestational age: A pooled analysis of three cohorts participating in the ECHO program, Environment International, Volume 161, 107102, ISSN 0160-4120. Available at: https://www.sciencedirect.com/science/article/pii/S0160412022000289
- IARC (2023). Trivalent antimony trioxide . IARC Monographs on the Identification of Carcinogenic Hazards to Humans. International Agency for Research on Cancer. In prep. Available at: https://monographs.iarc.who.int/list-of-classifications
- ICRP (1981). Limits on intakes of radionuclides for workers. ICRP Publication 30, Part 3. International Commission on Radiological Protection. Ann. ICRP 6(2/3).
- ICRP (1995). Age-dependent doses to members of the public from intake of radionuclides, Part 3. Ingestion dose coefficients. ICRP Publication 69. International Commission on Radiological Protection. Ann. ICRP 25(1).
- ICRP (2017). Occupational intakes of radionuclides: Part3. ICRP publication 137. International Commission on Radiological Protection. Annals of the ICRP, 46(3/4). Available at https://journals.sagepub.com/doi/pdf/10.1177/ANIB_46_3-4
- Iffland, R. and Bösche, G. (1987). Therapie und klinisch-toxikologische Verlaufskontrolle einer Brechweinstein-Vergiftung durch ein Ameisenvernichtungsmittel bei einem Kind. [Therapy and clinical-toxicological development and control of a child’s poisoning with emetic tartar from an anticide.] Monatsschrift für Kinderheilkunde. 135: 227–230.
- Ilavský, J., Barloková, D., Hudec, P. and Munka, K. (2014). Iron-based sorption materials for the removal of antimony from water. J. Water Supply Res. Technol. Aqua, 63(6): 518–524.
- Ilavský, J., Barloková, D., Hudec, P. and Munka, K. (2015). READ-As and GEH sorption materials for the removal of antimony from water. Water Sci. Technol. Water Supply, 15(3): 525–532.
- Ilgen, A.G., Majs, F., Barker, A.J., Douglas, T.A. and Trainor, T.P. (2014). Oxidation and mobilization of metallic antimony in aqueous systems with simulated groundwater. Geochim. Cosmochim. Acta, 132: 16–30.
- Imai, K. and Nakamura, M. (2006). In vitro embryotoxicity testing of metals for dental use by differentiation of embryonic stem cell test. Congenit Anom (Kyoto), 46(1): 34–38.
- Inam, M.A., Khan, R., Park, D.R., Lee, Y.-. and Yeom, I.T. (2018). Removal of Sb(III) and Sb(V) by ferric chloride coagulation: Implications of Fe solubility. Water (Switzerland), 10(4): 418.
- Inam, M.A., Khan, R., Park, D.R., Khan, S., Uddin, A. and Yeom, I.T. (2019). Complexation of antimony with natural organic matter: Performance evaluation during coagulation-flocculation process. Int. J. Environ. Res. Public Health, 16(7): 1–16.
- Indigenous Services Canada. (2019). Personal communication with X. Redhead.
- Kang, M., Kawasaki, M., Tamada, S., Kamei, T. and Magara, Y. (2000). Effect of pH on the removal of arsenic and antimony using reverse osmosis membranes. Desalination, 131(1-3): 293-298.
- Kang, M., Kamei, T. and Magara, Y. (2003). Comparing polyaluminum chloride and ferric chloride for antimony removal. Water Res., 37(17): 4171–4179.
- Kato, K.C., Morais-Teixeira, E., Reis, P.G., Silva-Barcellos, N.M., Salaun, P., Campos, P.P., Dias Correa-Junior, J., Rabello, A., Demicheli, C. and Frezard, F. (2014). Hepatotoxicity of pentavalent antimonial drug: Possible role of residual Sb(III) and protective effect of ascorbic acid. Antimicrob. Agents Chemother., 58(1): 481–488.
- Khosravi, A., Sharifi, I., Tavakkoli, H., Derakhshanfar, A., Keyhani, A.R., Salari, Z., Mosallanejad, S.S. and Bamorovat, M. (2018). Embryonic toxico-pathological effects of meglumine antimoniate using a chick embryo model. PLoS One, 13(5): e0196424.
- Kim, E.J. and Herrera, J.E. (2010). Characteristics of lead corrosion scales formed during drinking water distribution and their potential influence on the release of lead and other contaminants. Environ. Sci. Technol., 44(16): 6054–6061.
- Kip, A.E., Schellens, J.H.M., Beijnen, J.H. and Dorlo, T.P.C. (2017). Clinical pharmacokinetics of systemically administered antileishmanial drugs. Clin. Pharmacokinet.
- Kobayashi, A. and Ogra, Y. (2009). Metabolism of tellurium, antimony and germanium simultaneously administered to rats. J. Toxicol. Sci., 34(3):295–303.
- Krishnan, K. and Carrier, R. (2008). Approaches for evaluating the relevance of multiroute exposures in establishing guideline values for drinking water contaminants. J. Environ. Sci. Health. C. Environ. Carcinog. Ecotoxicol. Rev., 26(3): 300–316.
- Lauwers, L.F., Roelants, A., Rosseel, P.M., Heyndrickx, B. and Baute, L. (1990). Oral antimony intoxications in man. Crit. Care Med., 18(3): 324–326.
- Lecureur, V., Lagadic-Gossmann, D. and Fardel, O. (2002a). Potassium antimonyl tartrate induces reactive oxygen species-related apoptosis in human myeloid leukemic HL60 cells. Int. J. Oncol., 20(5): 1071–1076.
- Lecureur, V., Le Thiec, A., Le Meur, A., Amiot, L., Drenou, B., Bernard, M., Lamy, T., Fauchet, R. and Fardel, O. (2002b). Potassium antimonyl tartrate induces caspase- and reactive oxygen species-dependent apoptosis in lymphoid tumoral cells. Br. J. Haematol., 119(3): 608–615.
- Leuz, A.-K., Mönch, H. and Johnson, C.A. (2006). Sorption of Sb(III) and Sb(V) to goethite: Influence on Sb(III) oxidation and mobilization. Environ. Sci. Technol., 40(23): 7277–7282.
- Lewis, R.J. (2012). Sax's Dangerous Properties of Industrial Materials. 12th Edition. Wiley-Interscience, Wiley & Sons, Inc. Hoboken, NJ. p. V2: 338.
- Li, A., Zhuang, T., Shi, J., Liang, Y. and Song, M. (2019). Heavy metals in maternal and cord blood in beijing and their efficiency of placental transfer. J. Environ. Sci. (China), 80: 99–106.
- Lippincott, S.W., Ellerbrook, L.D., Rhees, M., and Mason, P. (1947). A study of the distribution and fate of antimony when used as tartar emetic and fuadin in the treatment of American soldiers with Schistosomiasis japonica. J Clin Invest 26(3): 370–378.
- Liu, C., Huang, L., Huang, S., Wei, L., Cao, D., Zan, G., Tan, Y, Wang, S., Yang, M., Tian, L., Tang, W., He, C., Shen, C., Luo, B., Zhu, M., Liang, T., Pang, B., Li, M., Mo, Z. and Yang, X. (2022). Association of both prenatal and early childhood multiple metals exposure with neurodevelopment in infant: A prospective cohort study. Environmental Research, 205. 112450, ISSN 0013-9351. Available at: https://www.sciencedirect.com/science/article/pii/S0013935121017515
- López, S., Aguilar, L., Mercado, L., Bravo, M. and Quiroz, W. (2015). Sb(V) Reactivity with Human Blood Components: Redox Effects. PLoS ONE 10(1): 1–12.
- Lynch, B.S., Capen, C.C., Nestmann, E.R., Veenstra, G. and Deyo, J.A. (1999). Review of Subchronic/Chronic Toxicity of Antimony Potassium Tartrate, Regulatory Toxicology and Pharmacology, 30(1): 9-17.
- Ma, B., Wang, X., Liu, R., Jefferson, W.A., Lan, H., Liu, H. and Qu, J. (2017a). Synergistic process using Fe hydrolytic flocs and ultrafiltration membrane for enhanced antimony(V) removal. J. Membr. Sci., 537: 93–100.
- Ma, B., Wang, X., Liu, R., Qi, Z., Jefferson, W.A., Lan, H., Liu, H. and Qu, J. (2017b). Enhanced antimony(V) removal using synergistic effects of Fe hydrolytic flocs and ultrafiltration membrane with sludge discharge evaluation. Water Res., 121: 171–177.
- Manitoba Sustainable Development (2019). Personal communication with Dr. H.D. Coulibaly.
- McCallum, R.I. (2005). Occupational exposure to antimony compounds. J. Environ. Monit., 7(12): 1245–1250.
- McKee, J.E. and Wolf, H.W. (1963). Water quality criteria. 2nd edition. Resources Agency of California, State Water Quality Control Board. pp. 138–139.
- Miao, Y., Han, F., Pan, B., Niu, Y., Nie, G. and Lv, L. (2014). Antimony(V) removal from water by hydrated ferric oxides supported by calcite sand and polymeric anion exchanger. J. Environ. Sci., 26(2): 307–314.
- Miekeley, N., Mortari, S.R. and Schubach, A.O. (2002). Monitoring of total antimony and its species by ICP-MS and onl-line ion chromatography in biological samples from patients treated for leishmaniasis. Anal Bioanal Chem 372: 495–502.
- Ministère de l’Environnement et de la Lutte contre les changements climatiques (2020). Bilan de mise en œuvre du Règlement sur la qualité de l’eau potable 2013-2018. Available at : http://www.environnement. gouv.qc.ca/eau/potable/bilans/bilan-2013-2018.pdf
- Ministère du Développement durable, de l'Environnement, de la Faune et des Parcs (2019). Personal communication with P. Cantin.
- Miranda, E.S., Miekeley, N., De-Carvalho, R.R. and Paumgartten, F.J. (2006). Developmental toxicity of meglumine antimoniate and transplacental transfer of antimony in the rat. Reprod. Toxicol., 21(3): 292–300.
- Mitrakas, M., Mantha, Z., Tzollas, N., Stylianou, S., Katsoyiannis, I. and Zouboulis, A. (2018). Removal of antimony species, Sb(III)/Sb(V), from water by using iron coagulants. Water (Switzerland), 10(10).
- Mlika, R.B., Hamida, M.B., Hammami, H., Jannet, S.B., Badri, T., Fenniche, S. and Mokhtar, I. (2012). Should we continue to indicate meglumine antimoniate as first-line treatment for cutaneous leishmaniasis in Tunisia. Dermat. Ther. 25: 615–618.
- Mueller, M., Balasegaram, M., Koummuki, Y., Ritmeijer, K., Santana, M.R. and Davidson, R. (2006). A comparison of liposomal amphotericin B with sodium stibogluconate for the treatment of visceral leishmaniasis in pregnancy in sudan. J. Antimicrob. Chemother., 58(4): 811–815.
- Multani, R.S., Feldmann, T. and Demopoulos, G.P. (2016). Antimony in the metallurgical industry: A review of its chemistry and environmental stabilization options. Hydrometallurgy, 164(Supplement C): 141–153.
- Najm, I., Clark, T., Hanson, K. (2010). Performance & cost of a full-scale antimony removal plant. AWWA WQTC & Exposition, Savannah, GA.
- Najm, I., Romero, O., Gallagher, B., DeHaan, M., Busch, C., Swaim, P., Emerson, B. (2017). Heavy metals removal to ultra-low levels – a bench-scale study. AWWA ACE, Philadelphia, PA.
- New Brunswick Department of Environment and Local Government (2019). Personal communication with K. Gould.
- Newfoundland and Labrador Department of Municipal Affairs and Environment (2019). Personal communication with H. Khan.
- Newton, P.E., Bolte, H. F., Daly, I.W., Pillsbury, B.D., Terrill, J.B., Drew, R.T., Ben-Dyke, R., Sheldon, A.W. and Rubin, L.F. (1994). Subchronic and chronic inhalation toxicity of antimony trioxide in the rat. Fundam Appl Toxicol 22(4): 561–576.
- NHMRC and NRMMC (2011). Australian drinking water guidelines - paper 6, version 3.5 updated August 2018. National Water Quality Management Strategy. National Health and Medical Research Council, National Resource Management Ministerial Council. Commonwealth of Australia, Canberra.
- Nova Scotia Environment (2019). Personal communication with A. Polegato.
- NPRI (2017). Antimony (and its compounds). National Pollutant Release Inventory. Government of Canada. Available at https://pollution-waste.canada.ca/national-release-inventory/archives/index.cfm?lang=en
- NSF International (2022a). NSF/ANSI Standard 58: Reverse osmosis drinking water treatment systems. NSF International/American National Standards Institute. NSF International, Ann Arbor, Michigan.
- NSF International (2022b). NSF/ANSI Standard 62: Drinking water distillation systems. NSF International/American National Standards Institute. NSF International, Ann Arbor, Michigan.
- NSF International (2022c). NSF/ANSI/CAN Standard 61: Drinking water system components—health effects. NSF International/American National Standards Institute/Standards Council of Canada, Ann Arbor, Michigan
- NSF International (2022d). NSF/ANSI/CAN Standard 60: Drinking water treatment chemicals – health effects. NSF International/American National Standards Institute/Standards Council of Canada, Ann Arbor, Michigan
- NTP (1992). NTP report on the toxicity studies of antimony potassium tartratein F344/N rats and B6C3F1 mice (drinking water and intraperitoneal injection studies). NTP TOX 11. National Toxicological Program. National Institutes of Health (NIH), No 92-3130, Research Triangle Park, NC.
- NTP (2018). Report on carcinogens. National Toxicology Program. National Institute of Environmental Health Sciences. U.S. Department of Health and Human Services, Monograph on Antimony Trioxide.
- OEHHA (2016). Public Health Goal for Antimony in drinking water. Office of Environmental Health Hazard Assessment. California Environmental Protection Agency, U.S.A. Available at https://oehha.ca.gov/media/downloads/water/chemicals/phg/antimonyphg092316_0.pdf
- Okkenhaug, G., Zhu, Y.G., He, J., Li, X., Luo, L. and Mulder, J. (2012). Antimony (Sb) and arsenic (As) in Sb mining impacted paddy soil from xikuangshan, china: Differences in mechanisms controlling soil sequestration and uptake in rice. Environ. Sci. Technol., 46(6): 3155–3162.
- Oliveira, A.L., Brustoloni, Y.M., Fernandes, T.D., Dorval, M.E., Cunha, R.V. and Bóia, M.N. (2009). Severe adverse reactions to meglumine antimoniate in the treatment of visceral leishmaniasis: a report of 13 cases in the southwestern region of Brazil. Trop. Doct. 39: 180–182.
- Oliveira, L.F., Schubach, A.O., Martins, M.M., Passos, S.L., Oliveira, R.V., Marzochi, M.C. and Andrade, C.A. (2011). Systematic review of the adverse effects of cutaneous leishmaniasis treatment in the NewWorld. Acta Trop. 118: 87–96.
- Omura, M., Tanaka, A., Hirata, M. and Inoue, N. (2002). Testicular toxicity evaluation of two antimony compounds, antimony trioxide and antimony potassium tartrate, in rats and mice. Environ. Health. Prev. Med., 7(1): 15–18.
- Ontario Ministry of the Environment, Conservation and Parks (2020). Personal communication with S. Deshpande.
- Oorts, K., Smolders, E., Degryse, F., Buekers, J., Gasco, G., Cornelis, G. and Mertens, J. (2008). Solubility and toxicity of antimony trioxide (Sb2O3) in soil. Environ. Sci. Technol., 42(12): 4378–4383.
- Paracel Laboratories Ltd. (2019). Personal communication with D. Robertson. Ottawa, ON.
- Paumgartten, F.J. and Chahoud, I. (2001). Embryotoxicity of meglumine antimoniate in the rat. Reprod. Toxicol., 15(3): 327-331.
- PEI Department of Communities, Land and Environment (2019). Personal communication with G. Somers.
- Pieper, K. (2021). Dept. Civil & Environmental Engineering, Northeastern University. Personal communication.
- Poon, R., Chu, I., Lecavalier, P., Valli, V.E., Foster, W., Gupta, S. and Thomas, B. (1998). Effects of antimony on rats following 90-day exposure via drinking water. Food Chem. Toxicol., 36(1): 21–35.
- Porquet, A. and Filella, M. (2007). Structural evidence of the similarity of Sb(OH)3 and As(OH)3 with glycerol: Implications for their uptake. Chem. Res. Toxicol., 20(9): 1269–1276.
- Qi, P. and Pichler, T. (2016). Sequential and simultaneous adsorption of Sb(III) and Sb(V) on ferrihydrite: Implications for oxidation and competition. Chemosphere, 145: 55–60.
- Qi, P. and Pichler, T. (2017). Competitive adsorption of As(III), As(V), Sb(III) and Sb(V) onto ferrihydrite in multi-component systems: Implications for mobility and distribution. J. Hazard. Mater., 330: 142–148.
- Quiroz, W., Aguilar, L., Barría, M., Veneciano, J., Martínez, D., Bravo, M., Lobos, M.G. and Mercado, L. (2013). Sb(V) and Sb(III) distribution in human erythrocytes: Speciation methodology and the influence of temperature, time and anticoagulants. Talanta 115: 902–910.
- Reimann, C., Matschullat, J., Birke, M. and Salminen, R. (2010). Antimony in the environment: Lessons from geochemical mapping. Applied Geochemistry, 25(2): 175–198.
- Ribeiro, R.R., Ferreira, W.A., Martins, P.S., Neto, R.L.M., Rocha, O.G.F., Le Moyec, L., Demicheli, C. and Frézard, F. (2010). Prolonged absorption of antimony(V) by the oral route from non-inclusion meglumine antimoniate-beta-cyclodextrin conjugates. Biopharm. Drug Dispos. 31(2-3): 109–119.
- Roper, S.C. and Stupart, L. (2006). The in vitro percutaneous absorption of antimony trioxide through human skin. Charles River Laboratories Rep No. 25985. 2006; pp 1-112. Charles River Laboratories, Edinburgh. (as cited in EU, 2008).
- Saenz, R.E., de Rodriguez, C.G., Johnson, C.M. and Berman, J.D. (1991). Efficacy and toxicity of pentostam against Panamanian mucosal leishmaniasis. Am. J. Tro. Med. Hyg. 44: 394–398.
- Sandvig, A., Boyd, G., Kirmeyer, G., Edwards, M., Triantafyllidou, S. and Murphy, B.M. (2007). Performance and Metal Release of Non-Leaded Brass Meters, Components, and Fittings. AWWA Research Foundation (Denver, Colorado) and EPA (Washington D.C.).
- Sandvig, A., Martel, K., Beggs, K., Jaffe Murray, A., Greiner, P., Kneen, K., McLellan, C., Bennet, D. and Terrell, J. (2012). Is NSF 61 Relevant for Chloraminating Utilities? Water Research Foundation, 4243, Denver, Colorado.
- Saskatchewan Water Security Agency (2019). Personal communication with S. Ferris.
- Sazakli, E., Zouvelou, S.V., Kalavrouziotis, I. and Leotsinidis, M. (2015). Arsenic and antimony removal from drinking water by adsorption on granular ferric oxide. Water Sci. Technol., 71(4): 622–629.
- Scanlan, L. (2003). Distribution system water quality changes: A challenge. Proc. 2003 AWWA ann. conf. (as cited in U.S. EPA, 2006).
- SCC (2020). Directory of accredited product, process and service certification bodies. Standards Council of Canada, Ottawa, Ontario. Available at https://www.scc.ca/en/accreditation/product-process-and-service-certification/directory-of-accredited-clients
- Schock, M.R. (2005). Distribution systems as reservoirs and reactors for inorganic contaminants. In: Distribution system water quality challenges in the 21st century: A strategic guide. MacPhee, M.J. (Ed.). AWWA, pp. 105.
- Schock, M. and Lytle, D. (2011). Chapter 20: Internal corrosion and deposition control. In: Water quality and treatment: a handbook on drinking water, 6th edition. J.K. Edzwald (Ed.). McGraw Hill and American Water Works Association, Denver, Colorado.
- Schroeder, H.A., Mitchener, M. and Nason, A.P. (1970). Zirconium, niobium, antimony, vanadium and lead in rats: Life term studies. J. Nutr., 100(1): 59–68.
- Scinicariello, F. and Buser, M.C. (2016). Urinary antimony and leukocyte telomere length: An analysis of NHANES 1999-2002. Environ. Res., 150: 513–518.
- Shotyk, W., Krachler, M. and Chen, B. (2006). Contamination of Canadian and European bottled waters with antimony from PET containers. J. Environ. Monit., 8(2): 288–292.
- Skeaff, J.M., Beaudoin, R., Wang, R. and Joyce, B. (2013). Transformation/dissolution examination of antimony and antimony compounds with speciation of the transformation/dissolution solutions. Integr. Environ. Assess. Manag., 9(1): 98–113.
- Smeets, P.W.M.H., Medema, G.J. and van Dijk, J.C. (2009). The Dutch secret: how to provide safe drinking water without chlorine in the Netherlands. Drink. Water Eng. Sci., 2: 1–14.
- Subramanian, K.S., Connor, J.W. and Meranger, J.C. (1991). Leaching of antimony, cadmium, copper, lead, silver, tin and zinc from copper piping with non-lead-based soldered joints. J. Environ. Sci. Health Part A Environ. Sci. Eng. Toxic Hazard. Subst. Control, 26(6): 911–929.
- Subramanian, K.S., Connor, J.W. and Sastri, V.S. (1994). Drinking water quality: Impact of non-lead-based plumbing solders. Toxicol. Environ. Chem., 44(1-2): 11–20.
- Sunagawa, S. (1981). Experimental studies on antimony poisoning [author’s translation]. Igaku Kenkyu 3: 129–142. (as cited in ECCC and Health Canada, 2020).
- Sundar, S. and Chakravarty, J. (2010). Antimony toxicity. Int. J. Environ. Res. Public. Health, 7(12): 4267–4277.
- Swaim, P., Busch, C. and DeHaan, M. (2017). Removal of heavy metals to ultra-low levels with conventional treatment technologies: pilot-scale demonstration. AWWA, ACE 2017 Philadelphia, PA.
- Sztajnkrycer, M.D. (2017). Antimony and Nickel. In: Critical care toxicology. chap. 79. Brent, J.e.a. (Ed.). AG 2017. Springer International Publishing. DOI 10.1007/978-3-319-17900-1_46, pp. 1619–1637.
- Tirmenstein, M.A., Plews, P.I., Walker, C.V., Woolery, M.D., Wey, H.E. and Toraason, M.A. (1995). Antimony-induced oxidative stress and toxicity in cultured cardiac myocytes. Toxicol. Appl. Pharmacol., 130(1): 41–47.
- Turković, R., Werner, W. and Klinger, J. (2014). The Performance of nonleaded brass materials. Water Research Foundation, Denver, Colorado.
- Tylenda, C.A., Sullivan, D.W. and Fowler, B.A. (2015). Antimony. In: Handbook on the toxicology of metals - chapter 27. Nordberg, G.F., Fowler, B.A. and Nordberg, M. (Eds.). 4th. Academic Press, San Diego, pp. 565–579.
- U.S. EPA (1992). Drinking water criteria document for antimony (final report). United States Environmental Protection Agency, Washington, DC. January 1992. Available at https://nepis.epa.gov/Exe/ZyPDF.cgi/901H0800.PDF?Dockey=901H0800.PDF
- U.S. EPA (1994a). Method 200.8 revision 5.4 Determination of trace elements in waters and wastes by inductively coupled plasma - mass spectrometry. Environmental Monitoring Systems Laboratory. Office of Research and Development, Cincinnati, Ohio.
- U.S. EPA (1994b). Method 200.9 revision 2.2 Determination of trace elements by stabilized temperature graphite furnace atomic absorption. Environmental Monitoring Systems Laboratory. Office of Research and Development. EPA, Cincinnati, Ohio.
- U.S. EPA (1998). Small system compliance technology list for the non-microbial contaminants regulated before 1996. Office of Water. EPA 815-R-98-002.
- U.S. EPA (2003). Method 200.5 revision 4.2 Determination of trace elements in drinking water by axially viewed inductively coupled plasma - atomic emission spectrometry. National Exposure Research Laboratory. Office of Research and Development. EPA 600-R-06-115, Cincinnati, Ohio.
- U.S. EPA (2006). Inorganic contaminant accumulation in potable water distribution systems. Office of Water. Office of Ground Water and Drinking Water. Washington, DC.
- U.S. EPA (2012). Radionuclides in Drinking Water, Compliance Options: Treatment Technology Descriptions. Available at http://cfpub.epa.gov/safewater/radionuclides/radionuclides.cfm
- U.S. EPA (2014). Method 6020B revision 2. Inductively coupled plasma - mass spectrometry. SW-846 Online: Test Methods Update V available online at: https://www.epa.gov/hw-sw846/sw-846-compendium#6000series.
- U.S. EPA (2018). 2018 edition of the drinking water standards and health advisories. Office of Water. U.S. Environmental Protection Agency, EPA 822-F-18-001, Washington, DC.
- U.S. GS (2016). 2016 Minerals yearbook. Antimony. [advance release]. U.S. Geological Survey and U.S. Department of the Interior. Available at https://prd-wret.s3-us-west-2.amazonaws.com/assets/palladium/production/atoms/files/myb1-2016-antim.pdf
- U.S. GS (2020). Mineral commodity summaries. U.S. Geological Survey and U.S. Department of the Interior. Available at https://pubs.usgs.gov/periodicals/mcs2021/mcs2021.pdf
- Valli, V.E., Poon, R., Chu, I., Gupta, S. and Thomas, B.H. (2000). Comment: Subchronic/Chronic Toxicity of Antimony Potassium Tartrate. Reg. Toxicol. and Pharm., 32, 337–338.
- Vigeh, M., Yunesian, M., Matsukawa, T., Shamsipour, M., Jeddi, M.Z., Rastkari, N., Hassanvand, M.S., Shariat, M., Kashani, H., Pirjani, R., Effatpanah, M., Shirazi, M., Shariatpanahi, G., Ohtani, K. and Yokoyama, K. (2021). Prenatal blood levels of some toxic metals and the risk of spontaneous abortion. J. Environ. Health Sci. Eng., 2021 Feb 26;19(1): 357–363.
- Wang, L., Lewis, G.M. and Chen, A.S.C. (2011). Arsenic and antimony removal from drinking water by point-of-entry reverse osmosis coupled with dual plumbing distribution U.S. EPA Demonstration Project at Carmel Elementary School in Carmel, ME Final performance evaluation report. Water Supply and Water Resources Division. National Risk Management Research Laboratory EPA/600/R-11/026, Cincinnati, Ohio.
- Westerhoff, P., Prapaipong, P., Shock, E. and Hillaireau, A. (2008). Antimony leaching from polyethylene terephthalate (PET) plastic used for bottled drinking water. Water Res., 42(3): 551–556.
- WHO (2003). Antimony in drinking-water. Background document for development of WHO guidelines for drinking-water quality. World Health Organization, WHO/SDE/WSH/03.04/74.
- WHO (2011). Guidelines for drinking-water quality. Fourth edition. World Health Organization, Geneva, Switzerland. Available athttps://www.who.int/publications/i/item/9789241548151
- WHO (2012). Water safety planning for small community water supplies. World Health Organization, Geneva, Switzerland. Available at https://apps.who.int/iris/handle/10665/75145
- Wise, E.S., Armstrong, M.S., Watson, J. and Lockwood, D.N. (2012). Monitoring toxicity associated with parenteral sodium stibogluconate in the day-case management of returned travelers with New World cutaneous leishmaniasis. PLoS Negl. Trop. Dis. 6:e1688.
- Wu, Z., He, M., Guo, X. and Zhou, R. (2010). Removal of antimony (III) and antimony (V) from drinking water by ferric chloride coagulation: Competing ion effect and the mechanism analysis. Sep. Purif. Technol., 76(2): 184-190.
- Xi, J., He, M., Wang, K. and Zhang, G. (2013). Adsorption of antimony(III) on goethite in the presence of competitive anions. J. Geochem. Explor., 132: 201–208.
- Zietz, B.P., Richter, K., Laß, J., Suchenwirth, R. and Huppmann, R. (2015). Release of metals from different sections of domestic drinking water installations. Water Qual. Expos. Health, 7(2): 193–204.
Appendix A: List of abbreviations
- ANSI
- American national Standards Institute
- APT
- Antimony potassium tartrate
- As
- Arsenic
- ATO
- Antimony trioxide
- ATSDR
- Agency for Toxic Substances and Disease Registry
- BV
- Bed volume
- BW
- Body weight
- CAS RN
- Chemical Abstract Service registration number
- ECCC
- Environment and Climate Change Canada
- Fe
- Iron
- FC
- Ferric chloride
- GI
- Gastrointestinal
- HA
- Humic acid
- HBV
- Health-based value
- IARC
- International Agency for Research on Cancer
- ICP-MS
- Inductively coupled plasma mass spectrometry
- In-situ FeOxHy
- Freshly prepared ferric hydroxide
- LD50
- Oral median lethal dose
- LOAEL
- Lowest observed adverse effect level
- LOEL
- Lowest observed effect level
- MAC
- Maximum acceptable concentration
- MCL
- Maximum contaminant level
- MDL
- Method detection limit
- Mn
- Manganese
- MRL
- Method reporting limit
- N
- Sample size
- NC
- Not calculated
- NOAEL
- No observed adverse effect level
- NOM
- Natural organic matter
- NSF
- NSF International
- NTU
- Nephelometric turbidity unit
- OEHHA
- Office of Environmental Health Hazard Assessment (California EPA)
- Pb
- Lead
- PET
- Polyethylene terephthalate
- POD
- Point of departure
- RO
- Reverse osmosis
- ROS
- Reactive oxygen species
- SA
- Spontaneous abortion
- Sb
- Antimony
- Sb(III)
- Trivalent antimony
- Sb(V)
- Pentavalent antimony
- SCC
- Standards Council of Canada
- TDI
- Tolerable daily intake
- TiO2
- Titanium dioxide
- TMP
- Transmembrane pressure
- U.S. EPA
- United States Environmental Protection Agency
- WHO
- World Health Organization
Appendix B: Canadian water quality data
| Region | River Basin | Number of Samples | Number of DetectsTable B1 Footnote a | Median (μg/L) |
Mean (μg/L) |
90th Percentile (μg/L) |
Maximum (μg/L) |
|---|---|---|---|---|---|---|---|
| East | Maritime Coast | 2 139 | 1 669 | 0.03 | 0.03 | 0.05 | 0.29 |
| Newfoundland-Labrador | 2 138 | 1 669 | 0.015 | 0.0365 | 0.093 | 1.41 | |
| North Shore-Gaspé | 52 | 51 | 0.007 | 0.0095 | 0.021 | 0.057 | |
| Saint John-St. Croix | 158 | 156 | 0.04 | 0.0436 | 0.06 | 0.22 | |
| Central | Winnipeg | 109 | 109 | 0.059 | 0.0635 | 0.076 | 0.181 |
| Prairie | Assiniboine-Red | 1 036 | 1 035 | 0.27 | 0.2938 | 0.4423 | 3.00 |
| Churchill | 346 | 346 | 0.024 | 0.0318 | 0.0433 | 0.861 | |
| Lower Saskatchewan-Nelson | 448 | 448 | 0.1335 | 0.1415 | 0.1941 | 0.398 | |
| Missouri | 145 | 145 | 0.116 | 0.1303 | 0.2082 | 0.431 | |
| North Saskatchewan | 545 | 540 | 0.081 | 0.0969 | 0.2342 | 0.438 | |
| South Saskatchewan | 880 | 874 | 0.037 | 0.0796 | 0.1858 | 1.04 | |
| Pacific | Columbia | 4 191 | 4 135 | 0.047 | 0.0617 | 0.119 | 0.693 |
| Fraser | 3 944 | 3 916 | 0.047 | 0.051 | 0.083 | 3.39 | |
| Okanagan-Similkameen | 1 076 | 1 070 | 0.053 | 0.0555 | 0.069 | 0.495 | |
| Pacific Coastal | 2 931 | 2 903 | 0.022 | 0.0589 | 0.142 | 1.32 | |
| Peace-Athabasca | 817 | 809 | 0.069 | 0.0892 | 0.1852 | 0.952 | |
| Arctic | Arctic Coast | 220 | 201 | 0.019 | 0.0443 | 0.1258 | 0.581 |
| Keewatin-Southern Baffin Island | 66 | 63 | 0.01 | 0.0324 | 0.1095 | 0.215 | |
| Lower Mackenzie | 1 415 | 1 409 | 0.116 | 0.1495 | 0.255 | 6.72 | |
| Yukon | 807 | 807 | 0.102 | 0.1251 | 0.1882 | 0.967 | |
Source: ECCC (2017).
|
|||||||
| Jurisdiction (MDL μg/L) |
Years | Source | # Detects/ Samples | % Detection | Mean (μg/L) |
90th Percentile (μg/L) |
Max (μg/L) |
|---|---|---|---|---|---|---|---|
British ColumbiaTable B2 Footnote 1 |
2014–2018 |
Untreated tap water as well as samples from disturbed locations (for example, mines) |
31/64 |
48 |
0.96 |
0.55 |
11.80 |
ManitobaTable B2 Footnote 2 |
2000–2018 |
Production and observation wells |
330/646 |
51 |
0.50 |
0.70 |
26.00 |
Nova ScotiaTable B2 Footnote 3 |
1990–2019 |
Groundwater studies |
45/1 886 |
2.4 |
NC |
< DL |
89.00 |
QuebecTable B2 Footnote 4 |
1941–2014 |
Various research projects (possible sampling in groundwater of poor quality) |
82/909 |
9.0 |
NC |
< DL |
9 400 |
DL – detection limit; < DL – below detection limit (when 90th percentile with < 10% detects); MDL -- method detection limit; NC – not calculated (when % Detection is < 40%).
|
|||||||
