Drinking water screening value for Iodide – Technical Summary
Download the alternative format
(PDF format, 818 KB, 11 pages)
Organization: Health Canada
Date published: May 27, 2022
A drinking water screening value of 0.24 mg/L (240 µg/L) is established for iodide.
Screening values
Health Canada's screening values identify limits for contaminants in water that could be used as a source of drinking water. A lifetime of exposure to these contaminants up to the screening value, both by drinking the water or by using it for showering or bathing, is not expected to increase health risks for any Canadian, including children.
Screening values are established for contaminants that are not commonly found in Canadian drinking water (either source or treated) and therefore Guidelines for Canadian Drinking Water Quality are not established. Health Canada establishes screening values for contaminants at the request of federal departments, provinces and territories (jurisdictions). These requests are usually made when there is a concern for human health because the presence of a contaminant is suspected or detected in local source water and that contaminant does not have an established limit in drinking water. Since 2020, the technical summaries for screening values are typically published online when Health Canada expects that screening values may be needed by more than one stakeholder or jurisdiction.
Screening values do not replace or supersede existing regulations. However, screening values may help jurisdictions and the public understand the potential health effects of a contaminant.
Screening values are based on a review of scientific research and international regulatory information available at the time of their development. In addition, screening values are externally peer-reviewed to ensure scientific integrity.
Health Canada is committed to keeping pace with new science, including the potential health risks from contaminants that are not typically found in drinking water and do not have Guidelines for Canadian Drinking Water Quality. To this end, Health Canada includes contaminants with screening values in its cyclical prioritization of contaminants for full guideline development.
Table of contents
- 1.0 Exposure considerations
- 2.0 Health considerations
- 3.0 Derivation of the screening value
- 4.0 International considerations
- 5.0 References
1.0 Exposure considerations
1.1 Identity and sources
Iodine (I) is a non-metallic trace element that exists as a bluish-black or violet-black lustrous solid. For the purposes of this screening value, iodine is used to refer to the element in any form while iodide refers to the anion I- and molecular iodine is used to refer to I2. Iodine occurs naturally in minerals, seawater, seafood, seaweed and underground brines (water containing dissolved salts and ions) associated with natural gas and oil deposits. Iodine is used in a number of applications including in photography, the formulation of inks and colouring agents, making batteries, fuels and lubricants, in pharmaceuticals and as an antiseptic or sanitising agent (ATSDR, 2004). In agriculture, iodine is added as a supplement to animal feed and is used in teat sanitizers for dairy herds (Borucki Castro et al., 2010). In some countries, including Canada, iodine is added to table salt in the form of potassium iodide to compensate for iodine-deficient diets (CFIA, 2015). Although not used for disinfecting larger drinking water supplies, iodine is used for emergency or short-term disinfection of drinking water (WHO, 2020). Iodine used for emergency or short-term disinfection of drinking water may result in exposures above the screening value. However, this is not expected to be a health concern since the screening value is based on lifetime exposure.
In water, iodine exists in its molecular form (I2) and as iodide (I-) and iodate (IO3-) (Fuge and Johnson, 1986). Most iodine compounds are moderately or highly soluble in water (Table 1). Iodine is generally mobile in the environment; it readily migrates in water and is transferred to the atmosphere primarily through photochemical oxidation (Fuge and Johnson, 1986). The taste and odour thresholds for iodine range from 0.147 to 0.204 mg/L in water (Ruth, 1986).
| Property | Iodine | Potassium Iodide |
|---|---|---|
| CAS RN | 1918-00-9 | 7681-11-0 |
| Molecular formula | I2 | KI |
| Molecular weight (g/mol) | 253.809 | 166.02 |
| Water solubility (g/L) at 25°C | 0.33 | 1,429 |
| Vapour pressure (volatility) | 0.305 mm Hg at 25°C | No data |
| Log octanol-water partition coefficient (Log Kow) | 2.49 | No data |
| Henry's Law constant | No data | No data |
| Source: ATSDR (2004) | ||
Exposure
The main source for Canadian's exposure to iodine is through food and iodized salt. Salt sold for general household use in Canada contains 0.01% potassium iodide and approximately 380 μg of iodine per teaspoon (Dieticians of Canada, 2014; CFIA, 2015). In terms of food, saltwater seafood is one of the largest sources of iodine.
To a lesser extent, Canadians are exposed to iodine through drinking water. In a study of 65 small, medium and large water treatment systems across Canada, concentrations of iodide ranged from < 0.018 to 131 μg/L in summer (median = 0.222 μg/L) and < 0.018 to 117 μg/L in winter (median = 0.075 μg/L) (Tugulea et al., 2018). Higher levels of iodide have been found in regions previously covered by the Champlain Sea (i.e., Ontario, Quebec). Levels in groundwater throughout southern Ontario ranged from below the detection limit (5 μg/L) to 6 650 μg/L in a total of 2 091 samples. The median value was 11.2 μg/L while the 90th percentile was 140 μg/L (Hamilton, 2015). In a follow-up study of locations known to have a greater potential for higher iodine concentrations in groundwater (n = 80), iodine concentrations ranged from below the detection limit (5 μg/L) to 2 100 μg/L with a mean concentration of 133 μg/L (Rogerson, 2018).
When water that contains iodine is disinfected with chlorine or chloramine, it can produce iodinated disinfection by-products.. Iodinated disinfection by-products are occasionally detected in drinking water from treatment plants located in coastal saltwater areas (Weinberg et al., 2002).
Exposure to iodine has been evaluated in the Canadian Health Measures Survey (CHMS) where iodine status is measured through median urinary iodine concentrations (UIC). During the 2009–2011 sampling period (Cycle 3 of the CHMS), children ages 6–11 years had a median UIC of 189 μg/L while adults 20–60 years old had median UICs of 122–126 μg/L (Statistics Canada, 2013; Hays et al., 2018). Biomonitoring equivalents have been calculated to help interpret biomonitoring data in light of recommended values. These values show that the median Canadian population values are above those required to prevent deficiency and well below the threshold for excessive intake (Hays et al., 2018). This interpretation is also consistent with World Health Organization (WHO) epidemiological criteria for assessing iodine nutrition status, which considers these iodine levels to be indicative of an adequate iodine status (WHO et al., 2007). An analysis of cycle five CHMS data (2016–2017 sampling period) also found adequate iodine intakes in school-aged children and other sex-age groups. However, the analysis did note that iodine intakes for some women of childbearing age were below estimated average requirements for pregnancy and lactation, thus indicating a possible insufficiency for women who are pregnant or breastfeeding (Bertinato et al., 2021).
2.0 Health considerations
Kinetics
Iodine can be ingested in a variety of chemical forms, although most ingested iodine is reduced to iodide in the gastrointestinal tract before being absorbed (Zimmermann, 2009). Based on studies in humans, iodide salts are close to completely absorbed (100%) once ingested (Fisher et al., 1965; Ramsden et al., 1967). The presence of perchlorate, thiocyanates, isothiocyanates, nitrates, fluorides, calcium, magnesium and iron in food and water has been shown to interfere with iodide absorption (Ubom, 1991; Cengiz et al., 2022). The absorption of iodine is similar in adults, adolescents and children. However, the absorption of iodine in infants maybe 2%–20% lower than in children and adults (ATSDR, 2004). Iodide is cleared from circulation primarily by the thyroid and the kidneys and is turned over rapidly. With a normally functioning thyroid, iodine has a half-life in the plasma of approximately ten hours (Chung, 2014). The biological half-life of iodine from the whole body varies considerably between individuals and has been measured as approximately 66 days in individuals with normally functioning thyroids (Kramer et al., 2002).
Essentiality
Iodine is an essential element required for normal thyroid function. Iodide is used in the synthesis of the thyroid hormones thyroxine (T4) and triiodothyronine (T3). Through these hormones, iodine plays an important role in energy-yielding metabolism and on the expression of genes that affect many physiological functions including embryogenesis, growth and development, and neurological and cognitive functions (EFSA, 2014). Worldwide, the greatest public health concerns regarding iodine are associated with deficiencies rather than excess exposures (Iodine Global Network, 2021). People at risk of deficiency include those who do not use iodized salt, people who are pregnant, vegans (i.e., no/low consumption of dairy, seafood, eggs) and people living in areas with iodine deficient soils (NIH, 2020). Deficiency is not generally expected in the Canadian population because of salt iodization.
Iodine is an essential element; therefore, recommended dietary allowances have been established by the Food and Nutrition Board of the US Institute of Medicine (IOM, 2001) and Health Canada (2010) (Table 2). In addition, tolerable upper intake levels have been set due to the potential for adverse health effects from ingestion of high amounts of iodine.
| Age Group | Adequate Intake or Recommended Dietary Allowance (μg per day)Footnote a | Tolerable Upper Intake Levels (μg per day) |
|---|---|---|
| 0–6 months | 110 | Not determinable |
| 7–12 months | 130 | Not determinable |
| 1–3 years | 90 | 200 |
| 4–8 years | 90 | 300 |
| 9–13 years | 120 | 600 |
| 14–18 years | 150 | 900 |
| 19 years and older | 150 | 1 100 |
| Pregnancy <18 | 220 | 900 |
| Pregnancy <19–50 years | 220 | 1 100 |
| Lactation <18 | 290 | 900 |
| Lactation <19–50 years | 290 | 1 100 |
|
||
Health Effects
The toxicity curve of iodine is U-shaped in that both too little and too much iodine can cause thyroid dysfunction. Exposure to excess levels of iodine is generally well tolerated, as a healthy thyroid gland is very adaptable to fluctuations in iodine intake (Pennington, 1990; Farebrother et al., 2019). Most healthy people can tolerate an excess of ≥ 1 500 μg per day without clinical symptoms. At these high concentrations, a persistent drop of serum T4 and T3 and a rise of thyroid stimulating hormone (TSH) has been observed, although levels remain in the normal range (Backer and Hollowell, 2000; Burgi, 2010).
The primary effects of excessive iodine ingestion are on the thyroid gland and regulation of thyroid hormone production and secretion. Disruption of the thyroid gland affects many organ systems including the skin, cardiovascular system, pulmonary system, kidneys, gastrointestinal tract, liver, blood, neuromuscular system, central nervous system, skeleton, reproductive systems, and numerous endocrine organs, including the pituitary and adrenal glands (ATSDR, 2004).
Chronic exposure (greater than 6 months) to greater than 0.03 mg/kg bw per day (e.g., 2 100 μg per day for a 70 kg adult) has been associated with many adverse health effects (ATSDR, 2004). Iodine-induced hypothyroidism (underactive thyroid, inadequate production of thyroid hormones) in humans can lead to neurological effects (delayed or deficient brain and neuromuscular development) in sensitive populations, particularly in the fetus and in newborn infants (Boyages, 2000) as well as reproductive effects including changes in the menstrual cycle, spontaneous abortions, stillbirths, and premature births (Dunn and Delange, 2001). Hyperthyroidism (overactive thyroid, overproduction of thyroxine) in humans has been associated with accelerated growth linked to accelerated pituitary growth hormone turnover or a direct effect of thyroid hormone on bone maturation and growth (Snyder, 2000). It has also been linked to reproductive effects including changes in gonadotropin release and sex hormone-binding globulin, and changes in the levels and metabolism of steroid hormones in both females and males (Krassas et al., 2010).
Iodine freely diffuses across the placenta and infants can be exposed to iodine in breast milk. Goitre and hypothyroidism have been reported in the offspring of mothers exposed to pharmacological doses of iodine and iodide (EGVM, 2003; Connelly et al., 2012).
Generally, acute, sub-chronic and chronic toxicity studies in animals support the findings from human studies. However, animal data are of limited value in assessing the toxicity of iodide because of significant species differences in basal metabolic rates and iodine metabolism (Hetzel and Maberly, 1986).
Iodine is not considered to be mutagenic (ATSDR, 2004). In humans, the link between iodine intake and the development of thyroid cancer has been explored in a number of investigations but the relationship remains uncertain (Prete et al., 2015; Zimmermann and Galetti, 2015; Cao et al. 2017; Lee et al., 2017). The International Agency for Research on Cancer (IARC) has not reviewed the carcinogenicity of iodide, nor has the United States Environmental Protection Agency (US EPA).
Mode of Action
Iodide is an essential element and is the rate-limiting chemical in the synthesis of thyroid hormones by the thyroid gland. Iodide is transferred into the thyroid cell by the sodium/iodine symporter where it combines with the amino acid tyrosine to make the hormones T4 and T3. These hormones are released into the blood stream and are circulated throughout the body where they are responsible for the regulation of metabolism. Feedback mechanisms exist and a healthy thyroid gland can adapt to varying levels of iodide. However, when the normal adaptive function of the thyroid fails, various morbidities may ensue. The mechanisms of action for various iodide-induced thyroid disorders are reviewed by Prete et al. (2015).
Selection of Key Studies
The critical effects associated with excessive iodine intake are on the thyroid gland and regulation of thyroid hormone production and secretion. Relevant toxicity studies, including those used by Health Canada and other international jurisdictions to set tolerable upper intake levels, are summarized in Table 3. Together, these studies indicate that the point of departure (POD) for iodine is approximately 0.01 mg/kg bw per day for both short and longer-term studies. This point of departure also applies to both adults and children.
| Reference | Population | Exposure duration | Critical effect(s) | POD |
|---|---|---|---|---|
| Gardner et al. (1988) | 30 men aged 22 to 40 years given iodine supplementation | 500, 1 500, or 4 500 μg NaI per day for 14 days | Significant decreases in T4 concentration and free thyroxine index values; significant increases in baseline and TRH-stimulated serum TSH | LOAEL = 1 800 μg per dayFootnote a NOAEL = 800 μg per day equivalent to 0.01 mg/kg bw per day |
| Paul et al. (1988) | 9 men aged 26 to 56 years and 23 women aged 23 to 44 years given iodine supplementation |
250, 500, or 1 500 μg NaI per day for 14 days | Significant decreases in T4 and T3 concentrations; significant increase in baseline and TRH-stimulated serum TSH | LOAEL = 1 700 μg per dayFootnote a NOAEL = 700 μg per day equivalent to 0.01 mg/kg bw per day |
| Chow et al. (1991) | Women aged 25–54 and thyroid antibody positive (n = 20) or antibody negative (n = 30), or aged 60–75 and from area with adequate iodine (n = 29) or from iodine deficient area (n = 35). |
500 μg KI per day or placebo for 28 days | Significant decreases in T4 levels and significant increases in TSH levels | 0.01 mg/kg bw per dayFootnote b |
| Li et al. (1987) | Children aged 7–15 years, who resided in two areas where iodide in drinking water were either 462 μg/L (n = 120) or 54 μg/L (n = 51) | Equivalent to 1 150 μg per day (0.029 mg/kg per day) and 400 μg per day (0.010 mg/kg per day) in the high and low iodide groups | Subclinical hypothyroidism in healthy human children | NOAEL = 0.01 mg/kg bw per day |
| Boyages et al. (1989) | Children aged 7–15 years, who resided in two areas where iodide in drinking water were either 462 μg/L (n = 29) or 54 μg/L (n = 26) | |||
KI: potassium iodide; LOAEL: lowest observed adverse effect level; NaI: sodium iodide; NOAEL: no observed adverse effect level; POD: point of departure; T3: triiodothyronine; T4: thyroxine; TRH: thyrotropin-releasing hormone; TSH: thyroid stimulating hormone |
||||
|
||||
3.0 Derivation of the Screening Value
Based on a weight of evidence approach, the tolerable daily intake of iodide is 0.01 mg/kg bw per day. No uncertainty factors were applied to the point of departure since the relevant studies were conducted in humans and the point of departure is similar for children, adults and the elderly (i.e., no need to account for human sensitivity) (Boyages et al.,1989; Chow et al., 1991).
Based on the above tolerable daily intake, a screening value can be derived as follows:
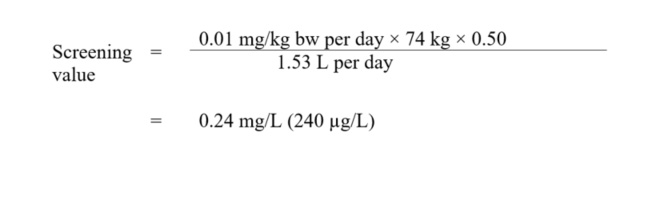
Figure 1 - Text Description
This equation calculates the drinking water screening value for iodide. The chronic oral reference dose for iodide is multiplied by the median body weight estimated for an adult and by a source allocation factor for drinking water, and then is divided by the estimated daily volume of tap water consumed by an adult. The result is a screening value of 0.24 mg/L (240 µg/L).
where:
- 0.01 mg/kg bw per day is the tolerable daily intake, as derived above;
- 74 kg is the average body weight for an adult (Health Canada, 2021);
- 0.50 is the allocation factor: the proportion of exposure to iodide from drinking water, as opposed to other media (i.e., food, consumer products, air, soil). Given that food and water represent the main source of exposure, a value of 0.50 (50%) was applied (Krishnan and Carrier, 2013);
- 1.53 L per day is the drinking water intake rate for a Canadian adult (Health Canada, 2021). Exposure to iodide from showering or bathing is unlikely to be significant. Consequently, a multi-route exposure assessment, as outlined by Krishnan and Carrier (2008), was not performed.
A screening value of 0.24 mg/L (240 µg/L) for iodide in drinking water is recommended by Health Canada. This is a conservative value as the critical effects identified in the relevant toxicity studies are biochemical in nature and not clinically adverse effects.
Although iodine is not recommended for use as a primary disinfectant in drinking water treatment, it can be used for emergency or short-term disinfection of drinking water. Use in these situations could result in exposures above the screening value. However, this is not expected to be a health concern since the iodide screening value is based on lifetime exposure. Because of the public health risk associated with microbiologically unsafe water, disinfection should not be compromised. Further information on the use of iodine as a drinking-water disinfectant is provided by the WHO (2018).
4.0 International considerations
Drinking water quality guidelines, standards and/or guidance established by foreign governments or international agencies may vary due to the science available at the time of assessment, as well as the utilization of different policies and approaches, such as the choice of key study, and the use of different consumption rates, body weights and allocation factors.
Australia has developed a drinking water guideline for iodide of 0.5 mg/L (500 μg/L) based on subtracting a background daily dietary intake of 200 μg per person from a tolerable daily intake of 1 100 μg per person then dividing by an adult drinking water consumption rate of 2 L per day (NHMRC, 2011). The US EPA, the European Union and the WHO do not have regulatory guidelines for iodide in drinking water.
5.0 References
ATSDR (2004). Toxicological profile for iodine. Agency for Toxic Substances and Disease Registry.
Backer, H. and Hollowell, J. (2000). Use of iodine for water disinfection: iodine toxicity and maximum recommended dose. Environ. Health Perspect., 108(8): 679–684.
Bertinato, J., Qiao, C. and L'Abbé, M.R. (2021). Iodine status of Canadian children, adolescents, and women of childbearing age. J. Nutr.,151(12):3710–3717.
Borucki Castro, S.I., Berthiaume, R., Laffey, P., Fouquet, A., Beraldin, F., Robichaud, A. and Lacasse, P. (2010). Iodine concentration in milk sampled from Canadian farms. J. Food Prot., 73(9): 1658–1663.
Boyages, S.C., Bloot, A.M., Maberly, G.F., Eastman, C.J., Li, M., Qian, Q.D., Liu, D.R., van der Gaag, R.D. and Drexhage, H.A. (1989). Thyroid autoimmunity in endemic goitre caused by excessive iodine intake. Clin. Endocrinol. (Oxf), 31(4): 453–465.
Boyages, S.C. (2000). The neuromuscular system and brain in thyrotoxicosis. In: Braverman RD, Utiger, RD, eds. Werner and Ingbar's The thyroid: A fundamental and clinical text. 8th edition. Philadelphia, PA, Lippincott-Raven.
Burgi, H. (2010). Iodine excess. Best Pract. Res. Clin. Endocrinol. Metab., 24(1): 107–115.
Cao, L.Z., Peng, X.D., Xie, J.P., Yang, F.H., Wen, H.L. and Li, S. (2017). The relationship between iodine intake and the risk of thyroid cancer: A meta-analysis. Medicine (Baltimore), 96(20): e6734.
Cengiz, M.M., Sen, F., Bilgin, A.K. and Boyaci-Gunduz, C.P. (2022). Determination of exposure to major iodide ion uptake inhibitors through drinking waters. Environ. Res., 204 (Part D):112345.
CFIA (2015). Product Specific Labelling Requirements. Labelling Requirements for Salt. Iodide Declaration. Canadian Food Inspection Agency. Available at: www.inspection.gc.ca/food/labelling/food-labelling-for-industry/salt/eng/1391790253201/1391795959629?chap=5#s6c5.
Chow, C.C., Phillips, D.I., Lazarus, J.H. and Parkes, A.B. (1991). Effect of low dose iodide supplementation on thyroid function in potentially susceptible subjects: Are dietary iodide levels in Britain acceptable? Clin. Endocrinol. (Oxf), 34(5): 413–416.
Chung, H.R. (2014). Iodine and thyroid function. Ann. Pediatr. Endocrinol. Metab., 19(1): 8–12.
Connelly, K.J., Boston, B.A., Pearce, E.N., Sesser, D., Snyder, D., Braverman, L.E., Pino, S. and LaFranchi, S.H. (2012). Congenital hypothyroidism caused by excess prenatal maternal iodine ingestion. J. Pediatr., 161(4): 760–762.
Dieticians of Canada (2014). Food sources of iodine. Available at: www.dietitians.ca/Your-Health/Nutrition-A-Z/Minerals/Food-Sources-of-Iodine.aspx.
Dunn, J.T. and Delange, F. (2001). Damaged reproduction: The most important consequence of iodine deficiency. 86(6): 2360–2363.
EFSA (2014). Scientific Opinion on Dietary Reference Values for Iodine. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA), European Food Safety Authority, Parma, Italy.
EGVM (2003). Safe Upper Levels for Vitamins and Minerals. Expert Group on Vitamins and Minerals. pp 203–212.
Farebrother, J., Zimmermann, M.B. and Andersson, M. (2019). Excess iodine intake: Sources, assessment, and effects on thyroid function. Ann. N. Y. Acad. Sci., 1446(1): 44–65.
Fisher, W.D., Voorhess, M.L. and Gardner, L.I. (1965). Congenital hypothyroidism in infant maternal I131 therapy: With a review of hazards of environmental radioiosotope contamination. J. Pediatr., 62: 132–146.
Fuge, R. and Johnson, C.C. (1986). The geochemistry of iodine - a review. Environ. Geochem. Health, 8(2): 31–54.
Gardner, D.F., Centor, R.M. and Utiger, R.D. (1988). Effects of low dose oral iodide supplementation on thyroid function in normal men. Clin. Endocrinol. (Oxf), 28(3): 283–288.
Hamilton, S.M. (2015). Ambient Groundwater Geochemistry Data for Southern Ontario, 2007–2014. Available at: http://www.geologyontario.mndm.gov.on.ca/mndmaccess/mndm_dir.asp?type=pub&id=MRD283-REV, Ontario Geological Survey, Miscellaneous Release - Data 283-Revised.
Hays, S.M., Poddalgoda, D., Macey, K., Aylward, L. and Nong, A. (2018). Biomonitoring equivalents for interpretation of urinary iodine. Regul. Toxicol. Pharmacol., 94: 40–46.
Health Canada (2021). Canadian exposure factors used in human health risk assessments. Fact sheet. Health Canada, Ottawa, Ontario. Available at: https://www.canada.ca/en/health-canada/services/chemical-substances/fact-sheets/canadian-exposure-factors-human-health-risk-assessments.html.
Health Canada (2010). Dietary reference intake tables. Available at: https://www.canada.ca/en/health-canada/services/food-nutrition/healthy-eating/dietary-reference-intakes/tables/reference-values-elements-dietary-reference-intakes-tables-2005.html.
Hetzel, B.S. and Maberly, G.F. (1986). Iodine. In: Trace elements in human and animal nutrition. Mertz, W. (ed.). Vol 2. Academic Press, Orlando, pp. 139–208.
Iodine Global Network. Global scorecard of iodine nutrition in 2020 in the general population based on school-age children (SAC). IGN: Ottawa, Canada. 2021. Available at: https://www.ign.org/cm_data/IGN_Global_Scorecard_2021_7_May_2021.pdf.
IOM (2001). Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium and Zinc. U.S. National Institute of Medicine, Available at: www.nap.edu/read/10026/chapter/1.
Kramer, G.H., Hauck, B.M. and Chamberlain, M.J. (2002). Biological half-life of iodine in adults with intact thyroid function and in athyreotic persons. Radiat. Prot. Dosimetry, 102(2): 129–135.
Krassas, G.E., Poppe, K. and Glinoer, D. (2010). Thyroid function and human reproductive health. Endocr. Rev., 31:702–55.
Krishnan, K. and Carrier, R. (2008). Approaches for evaluating the relevance of multiroute exposures in establishing guideline values for drinking water contaminants. J. Environ. Sci. Health. C. Environ. Carcinog. Ecotoxicol. Rev., 26(3): 300–316.
Krishnan, K. and Carrier, R. (2013). The use of exposure source allocation factor in the risk assessment of drinking-water contaminants. J. Toxicol. Environ. Health B Crit. Rev., 16(1): 39–51.
Lee, J.H., Hwang, Y., Song, R.Y., Yi, J.W., Yu, H.W., Kim, S.J., Chai, Y.J., Choi, J.Y., Lee, K.E. and Park, S.K. (2017). Relationship between iodine levels and papillary thyroid carcinoma: A systematic review and meta-analysis. Head Neck, 39(8): 1711–1718.
Li, M., Liu, D.R., Qu, C.Y., Zhang, P.Y., Qian, Q.D., Zhang, C.D., Jia, Q.Z., Wang, H.X., Eastman, C.J. and Boyages, S.C. (1987). Endemic goitre in central China caused by excessive iodine intake. Lancet, 2(8553): 257–259.
NHMRC (2011). National Water Quality Management Strategy. Australian Drinking Water Guidelines 6. Version 3.5 Updated August 2018. National Health and Medical Research Council (NHMRC), Canberra. Available at: https://www.Nhmrc.Gov.au/about-us/publications/australian-Drinking-Water-Guidelines.
NIH (2020). Iodine Fact Sheet for Health Professionals. National Institutes of Health, Office of Dietary Supplements. Available at: https://ods.od.nih.gov/factsheets/Iodine-HealthProfessional/.
Paul, T., Meyers, B., Witorsch, R.J., Pino, S., Chipkin, S., Ingbar, S.H. and Braverman, L.E. (1988). The effect of small increases in dietary iodine on thyroid function in euthyroid subjects. Metab. Clin. Exp., 37(2): 121.
Pennington, J.A.T. (1990). A review of iodine toxicity reports. J. Am. Diet. Assoc., 90(11): 1571–81.
Prete, A., Paragliola, R.M. and Corsello, S.M. (2015). Iodine supplementation: Usage "with a grain of salt". Int. J. Endocrinol., 2015: 312305.
Ramsden, D., Passant, F.H., Peabody, C.O. and Speight, R.G. (1967). Radioiodine uptakes in the thyroid. Studies of the blocking and subsequent recovery of the gland following the administration of stable iodine. Health Phys., 13(6): 633–646.
Rogerson, C.M. (2018). Groundwater as a source of high iodine levels in milk in eastern and southwestern Ontario dairy herds. MSc. thesis. University of Guelph, Guelph, ON.
Ruth, J.H. (1986). Odor thresholds and irritation levels of several chemical substances: A review. Am. Ind. Hyg. Assoc. J., 47(3): A142–51.
Statistics Canada (2013). Iodine status of Canadians, 2009 to 2011. Available at: www.statcan.gc.ca/pub/82-625-x/2012001/article/11733-eng.htm.
Snyder, P.J. (2000). The pituitary in thyrotoxicosis. In: Braverman L.E, Utiger R.D., eds. Werner and Ingbar's The thyroid: A fundamental and clinical text. 8th ed. Philadelphia, PA: Lippincott-Raven, 634–636.
Tugulea, A.M., Aranda-Rodriguez, R., Berube, D., Giddings, M., Lemieux, F., Hnatiw, J., Dabeka, L. and Breton, F. (2018). The influence of precursors and treatment process on the formation of iodo-THMs in Canadian drinking water. Water Res., 130: 215–223.
Tugulea, A.M., Aranda-Rodriguez, R., Giddings, M., Hnatiw, J. and Lemieux, F. (2015). CHAPTER 5. Iodo-THM Formation During Chlorination of Source Waters With Naturally Occurring Ammonium and High Sodium Content. In: Disinfection By-products in Drinking Water. Thompson, K.C., Gillespie, S. and Goslan, E.H., Eds., Royal Society of Chemistry. DOI: 10.1039/9781782622710-00046.
Ubom, G.A. (1991). The goitre-soil-water-diet relationship: case study in Plateau State, Nigeria. Sci. Total Environ., 107: 1–11.
Weinberg, S.H., Krasner, S.W. and Richardson, S.D. (2002). The occurrence of disinfection by-products (DBPs) of health concern in drinking water: results of a nationwide DBP occurrence study. Environmental Protection Agency, National Exposure Research Laboratory, Office of Research and Development. Athens, Ga., U.S.
WHO (2020). Iodine in drinking water. Background document for development of WHO Guidelines for drinking-water quality. WHO/HEP/ECH/WSH/2020.5. World Health Organization, Geneva.
WHO (2018). Alternative drinking-water disinfectants: bromine, iodine and silver. World Health Organization, Geneva.
WHO, United Nations Children's Fund and International Council for the Control of the Iodine Deficiency Disorders (2007). Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers (third ed.). World Health Organization, Geneva.
Zimmermann, M.B. (2009). Iodine deficiency. Endocr. Rev., 30(4): 376–408.
Zimmermann, M.B. and Galetti, V. (2015). Iodine intake as a risk factor for thyroid cancer: A comprehensive review of animal and human studies. 8: 8–8.