About the ozone layer
Content
The ozone gas
Oxygen occurs in three different forms in the atmosphere: as oxygen atoms (O), as oxygen molecules (O2), which is the oxygen we breath, and as ozone (O3). Ozone is a colourless gas that has a very harsh odour.
Oxygen atom (O)
Oxygen molecule (O 2 )
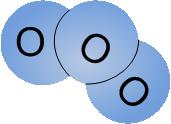
Ozone molecule (O 3 )
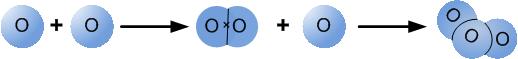
Relationship between oxygen atoms, oxygen molecules and ozone molecules
The formation of the ozone layer
Ozone is created in a section of the atmosphere, known as the stratosphere, when highly energetic solar rays strike molecules of oxygen (O2) and cause the two oxygen atoms to split apart. If a freed oxygen atom bumps into another O2, it joins up, forming ozone (O3). Ozone is also naturally broken down in the stratosphere by sunlight and by a chemical reaction with various compounds containing nitrogen, hydrogen and chlorine. These chemicals all occur naturally in the atmosphere in very small amounts.
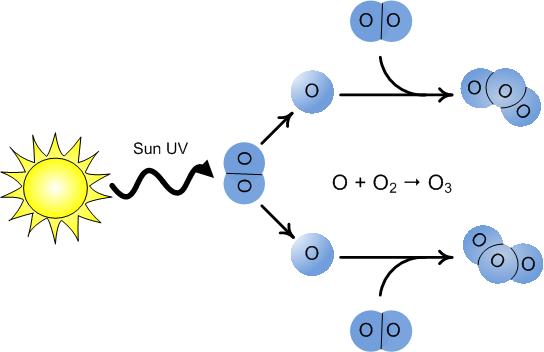
In an unpolluted atmosphere there is a balance between the amount of ozone being produced and the amount of ozone being destroyed. As a result, the total concentration of ozone in the atmosphere remains relatively constant.
Did you know : Four billion years ago, the planet was filled with volcanoes and the atmosphere consisted mainly of carbon dioxide and methane. There was no oxygen and no ozone. When life occurred in the oceans two billion years later, it began producing oxygen, which slowly became abundant in the atmosphere. As the oxygen rose slowly upward, the extreme ultraviolet radiation broke it down and ozone was formed.
The location of the ozone layer
Approximately 90 per cent of all ozone is produced naturally in the stratosphere. While ozone can be found through the entire atmosphere, the greatest concentration occurs at an altitude of about 25 km. This band of ozone-rich air is known as the "ozone layer". The amount of ozone above a location on the Earth varies naturally with latitude, season, and from day-to-day. Under normal circumstances, the ozone layer is thickest over the poles and thinnest around the equator. The ozone layer over Canada is normally thicker in winter and early spring, it can vary naturally by about 25 per cent.
Did you know : The ozone molecules in the upper atmosphere are spread so thinly that if they were compressed to pure ozone at ground level, they would create a band only 3 mm thick, or about the thickness of three dimes.
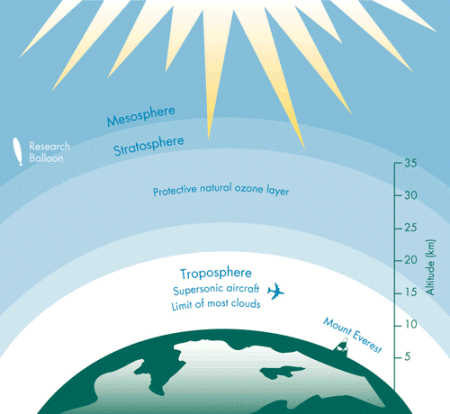
The role of the ozone layer
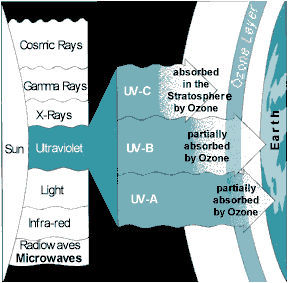
All life on Earth is protected by the ozone layer. This layer of gas acts as an invisible filter that protects all life forms from over-exposure to the sun’s harmful UV rays. Most incoming UV radiation is absorbed by ozone and prevented from reaching the Earth's surface. Without the protective effect of ozone, life on Earth would not have evolved the way it has.
Ground-level ozone and smog
Even though stratospheric ozone and ground-level ozone are exactly the same substance, their presence in different parts of the atmosphere has very different consequences. Stratospheric ozone blocks harmful solar radiation - all life on Earth has adapted to this filtered solar radiation. Ground-level ozone, in contrast, is a major health concern that can cause serious eye, nose and respiratory problems in humans and animals. The effect on the environment includes damage to plants, field crops, forests, and material damage. On the other hand, smog will absorb some incoming solar radiation, but it cannot make up for the loss of stratospheric ozone.