Notice of objection: Goodyear Canada Inc.
Goodyear Canada Inc.
450 Kipling Avenue, Toronto, Ontario M8Z 5E1
Telephone (416)201-4300
Robin M. Hunter
Secretary
Direct dial: (416) 201-7708
Facsimile No.: (416) 201-4249
Email: robin.hunter@goodyear.com
December 14, 2011
The Honorable Peter Kent
Minister of the Environment
c/o The Executive Director
Program Development and Engagement Division
Department of Environment
Gatineau, Quebec K1A 0H3
Re: Notice of Objection and Request for Board of Review in relation to the Proposed Order to add N,N'-mixed phenyl and tolyl derivatives of 1,4-benzenediamine [CAS No. 68953-84-4; "N,N'-mixed phenyl and tolyl derivatives of 1,4-benzenediamine (BENPAT)"] to Schedule I to the Canadian Environmental Protection Act 1999; Canada Gazette Vol. 145, No. 42 -- October 15, 2011
Dear Minister:
This submission responds to the October 15, 2011, Gazette Notice ("Notice") in which the Governor in Council, on the recommendation of the Minister of the Environment ("Minister"), proposed an Order to add N,N'-mixed phenyl and tolyl derivatives of 1,4-benzenediamine (BENPAT) to Schedule 1 of the Canadian Environmental Protection Act, 1999 ("CEPA") (hereafter referred to as "Proposed Order").Footnote 1 As provided for by section 3 32(2) of the Canadian Environmental Protection Act, 1999 (CEPA 1999), Goodyear Canada Inc.Footnote 2 ("Goodyear") is filing this notice of objection and respectfully requests that a board of review be established pursuant to section 333 of CEPA "to inquire into the nature and extent of danger"Footnote 3 posed by BENPAT.
Goodyear maintains that a Board of Review is warranted as the Proposed Order to add BENPAT to Schedule 1 is based on a final screening assessment ("Assessment") that has been conducted in a manner that is not consistent with the best available science. Use of the best available science would not have resulted in the conclusion that BENPAT "is entering or may enter the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity."Footnote 4 Inappropriately, the approach used in the Assessment erroneously:
- assumes BENPAT does not degrade quickly in the environment and is persistent in water, soil, and sediments so to pose a risk;
- uses overstated industrial release factors to calculate overstated exposure scenarios resulting in risk quotients ("RQs") erroneously indicating BENPAT has the potential to cause ecological harm; and
- uses combinations of release and dilution factors to generate overstated exposure scenarios from consumer releases that appear unlikely and physically impossible to occur, which erroneously indicates BENPAT has the potential to cause ecological harm.
Important new data relevant to Environment Canada's Assessment have become available that challenge Environment Canada's persistence designation of BENPAT. Since the release of the Assessment, recently completed degradation studies show that the material, when bio-available, is readily degradable by bacteria. An ongoing study, to be concluded in early 2012, shows evidence of rapid degradation for BENPAT. These studies, using radio-labeled material, demonstrate that the majority of the bio-available material is mineralized to carbon dioxide (CO2) in days and the biomass-bound material is so tightly bound as to be immobilized, biologically unavailable, and thus posing no risk of immediate or long term harmful effect to the environment or its biological diversity.
Environment Canada's Assessment conclusion for industrial releases was based on the risk quotients derived from two emissions scenarios applied to three unidentified sites, but neither scenario considered current risk management measures. In July of 2010, Goodyear submitted a sample calculation with emissions factors based on European Tyre and Rubber Manufacturers' Association (ETRMA) guidance and consistent with what was ultimately used in Scenario 2 of the Assessment, but noted that ETRMA was in the process of completing an emission factor study for European tire facilities which would provide more reliable information. Emission rates inclusive of current industry-wide risk management measures are now available online (ETRMA, 2010)Footnote 5. As discussed in more detail below, risk quotients (RQs) extrapolated from these factors recommended by ETRMA are all less than 1 and conclude no risk of immediate or long term harmful effect to the environment or its biological diversity.
In the Assessment, it is reported that predicted environmental concentrations (PECs) for BENPAT under two scenarios may exceed the PNEC (predicted no effect concentrations) in a small percentage of the water bodies receiving wastewater across Canada under lowest flow conditions (10% quartile). Releases from many consumer products are equally likely in dry and wet times due to downthe- drain sources, but not for tires. Tire wear particles deposited along the roadway represent the primary possible release source, and substantial roadway runoff is unlikely during dry periods. Environment Canada in its Assessment used a proprietary spreadsheet, Mega Flush, to calculate potential environmental concentrations from consumer use of tires. It is not clearly apparent that the inherent differences between tires and other consumer products are taken into account in the modeling. A traditional application of a down-the-drain model would be inappropriate for tire modeling releases and generate overstated risk results. Conditions that cannot physically occur should not be included in the risk assessment.
Background
Underlying the Proposed Order is a finding by the Minister of the Environment that BENPAT meets the CEPA section 64 definition of "toxic". Under Section 64 of CEPA, a substance is "toxic" if it is entering or may enter the environment in a quantity or concentration or under conditions that:
- have or may have an immediate or long-term harmful effect on the environment or its biological diversity; or
- constitute or may constitute a danger to the environment on which life depends; or
- constitute or may constitute a danger in Canada to human life or health.
Pursuant to section 74 of CEPA, the Ministers of the Environment and Health prepared an Assessment for BENPAT, and the conclusion of the Assessment forms the basis of the Proposed Order. With respect to potential human health impacts, the Ministers concluded that BENPAT is not "entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health."Footnote 6 Thus, BENPAT was not deemed to meet the CEPA section 64 definition of toxic based on human health concerns.
With respect to environmental concerns, the Ministers identified BENPAT as meeting the persistence criteria established in the Persistence and Bioaccumulation Regulations in certain environmental compartments. But the final Assessment concluded that there is sufficient consistent evidence that the material does not meet the bioaccumulation criterion. The Ministers concluded that long term exposure "may cause adverse effects to aquatic organisms in certain Canadian environments."Footnote 7 BENPAT was deemed to satisfy the CEPA section 64 definition of toxic based on an assertion that they are "entering the environment in a quantity or concentration or under conditions that have or may have an immediate long-term harmful effect on the environment or its biological diversity".
Objection to the proposed order
Goodyear believes the conclusion regarding environmental concerns and the resulting Proposed Order are not consistent with the best available science. Further information concerning the basis for Goodyear's objection and request for a board of review follows.
1. The proposed order is inconsistent with the Cabinet Directive on Streamlining Regulations as it is not based on the best available science.
The Cabinet Directive on Streamlining Regulations specifies that the Government shall make decisions on "the best available knowledge and science in Canada and worldwide".Footnote 8 In this case, the assessment prepared by Environment Canada is not based on the best available data and scientific knowledge regarding the environmental properties of BENPAT.
To accurately evaluate the ecological impacts of BENPAT, an understanding of whether BENPAT will actually be present and bio-available in which compartments (e.g., air, water, sediment) and at what levels is required. In its Assessment, Environment Canada relied on overly conservative modeling that predicts BENPAT will not degrade in the aquatic compartment and that material in the soil/sediment compartments is bio-available so to pose a risk. New data have become available from best-practice scientific studies demonstrating BENPAT's fate using radio-labeling. These data are reviewed below. Preliminary data are provided from one study that is expected to be completed in early 2012.
a. BENPAT is rapidly biodegradable.
Biodegradation studies for BENPAT constituent 14C-R898, conducted at 2 suspended solids concentrations, indicate half-lives of <10 days at both levels (see Figure 1 in Addendum). While it may be questioned whether BENPAT achieves ready biodegradation status per Organisation for Economic Co-operation and Development (OECD) guidelines, it now meets "inherent biodegradable" status and should not be classified as persistent. Globally Harmonized System (GHS) has established criteria for substances tested at low concentrations in test media as was the case for BENPAT (10- and 100 ug R898/L). GHS guidance states that chemicals that degrade when tested at low concentrations will likely exhibit first-order kinetics, and a rate that achieves 70% degradation in 28 days is one that exhibits a half-life of <16 days. The half-life estimates for BENPAT in two studies (Figures 1 & figure 2) demonstrate support for conclusions that criteria are met for "rapidly biodegradable" according to GHS, and "inherent biodegradability" per OECD.
In contrast, Environment Canada indicated that through means of modeling, half-lives in aqueous media for the components of BENPAT would exceed 182 days while soil half-lives would exceed 360 days. The new data derived from biodegradation tests of a BENPAT constituent R898 clearly demonstrates both a significant degree of mineralization and a half-life below 10 days in aqueous media. This evidence refutes the conclusions contained in the final assessment of BENPAT regarding prolonged persistence of this chemical in the environment.
b. BENPAT'S low water solubility and its influence on biodegradation rates, aquatic toxicity, and partitioning to biomass (Figure 3).
BENPAT was found to exhibit toxicity towards aquatic species (algae, daphnia, fish) with EC5Os <0.5 mg/L. Exposures in these tests were chemically analyzed with data representing watersoluble chemical concentrations in the aquatic environments. In contrast, toxicity results for soil and/or sediment species (earthworm and chironomid) indicated negligible effects on these target organisms at levels -- 1000 mg/kg. A measurement of soluble levels of BENPAT in the overlying water layer in the chironomid study demonstrated an absence of soluble BENPAT, which comports with the assertion that the chemical partitions from water to the solid phase of the sediment. These observations in combination with the elevated Koc values for the constituents of BENPAT suggest that there is extensive adsorption of BENPAT to sediment to a degree that negates toxic activity in resident species.
Another test that characterizes biological behavior of soluble phase BENPAT is the biodegradation assay. It is reasonable to expect that only soluble fraction chemical is susceptible to microbial bioaccumulation with potential biodegradation in the STP incubation environment. Pilot data have been submitted to Canada that show ultimate biodegradation (to 14CO2) of BENPAT up to levels exceeding 30% of applied chemical within 28 days (Figure 1). The biodegradation rates achieve plateau levels with evidence that the microbial population remains viable. This latter observation is confirmed through addition of test compound to the incubation media with resumption of biodegradative activity. Preliminary evidence from mass balance trials shows that a substantial fraction of the administered BENPAT not degraded is partitioned to biomass within the incubation chambers. The nature of this partitioning was characterized as non-extractable material using the strong organic solvent methylene chloride. It is therefore logical to deduce that radioactive R898 from BENPAT added to biodegradative media has the following 3 disposition options:
- It is ultimately biodegraded to 14CO2,
- It is subject to primary biodegradation that leads to metabolite binding to biomass without a potential for further degradation, or
- Primary degradation occurs with incorporation of '4C into intermediary metabolic pathways leading to lipid, protein or other biomolecules.
It is possible that the parent test chemical could adhere to biomass without bond creation, but this type of non-covalent adherence would certainly be such that test compound radioactivity would be extractable by organic solvents, which in the case of BENPAT was not.
Other published studies have shown that aromatic amines bind covalently to humic acids under ambient conditions, particularly to quinone componentsFootnote 9,Footnote 10. It is opined by the latter paper's authors that this type of binding between aromatic amines and humic acid may reduce the chemicals' hioavailability and toxicity to soil-dwelling species. While evidence for potential binding between sediment-soil/humic acids and BENPAT is limited and circumstantial, further support could be developed through conduct of acute aquatic toxicity (daphnia) in presence and absence of sediment. This testing should employ analytical assessments of aquatic levels and time-course of soluble concentrations of BENPAT in the test media. Attenuation of toxicity in the presence of sediment in parallel with a decrease in water-soluble levels of BENPAT would provide prima facie evidence for the ameliorative impact of BENPAT in the real-world environmental conditions in which the universal presence of sediment is a constant for aquatic species.
In concert with consideration of solvent non-extractability of chemicals from soil and sediment matrices, the German UBA (German Federal Environment Agency) is developing a structure that addresses mobility of these substances and the associated risks resulting from attenuated accessibility of chemicals to biological species. Categories are being developed according to risk associated with levels of mobility of chemicals based upon solvent extractability. In essence, the first category represents substances that can be removed from environmental substrates (soil, sediment) through mild extraction methods, and are considered to pose some biological risk as a result of a substances' mobilization from the matrix, ie., they are bioavailable. A second chemical category is for materials that cannot he mobilized from soil/sediment through a protocol of extractive measures that demonstrate strong binding and a lack of mobility. This suggests minimal risks as a result of low bioavailability. Further characterization may determine whether there is covalent binding of the test compound or biogenic incorporation into biomass structures such as lipids, membranes, carbohydrates, etc. Extraction results to date as well as mass balance determinations in progress for BENPAT and R898 indicate a high degree of non-mobility from biomass. If confirmed, this information indicates the chemical exists in low risk situations when present in biological environments such as those soil, sediment or aquatic matrices.
c. Weight of evidence and environmental risks of BENPAT
Data development for BENPAT has progressed in recent years through the use of a radioactive constituent plus methodologies to maximize the fraction of chemical in the water-soluble phase in biodegradation testing. This has led to significant enhancements of the characterizations of this chemical. To summarize:
- Biodegradation tests clearly show substantial degree of ultimate biodegradation:
- Half-life estimate values fall within ranges considered to be "rapidly biodegradable" (GHS) and "inherent biodegradability" [Registration, Evaluation and Authorization of Chemicals (REACH)]; and
- Elevated Koc values plus disparities for toxicity potencies found for aquatic species (tests in soluble fractions of BENPAT) versus those for soil and sediment species (presence of solid substrates) indicate strong evidence for presence of attenuating influence of real world conditions (e.g., presence of sediment in aquatic environment) towards toxicity of BENPAT in aquatic species.
It is reasonable to conclude that according to best available data for BENPAT as cited above, weight-of-evidence and scientific judgment for this chemical point to the absence of threat to the environment under use conditions.
Figure 1
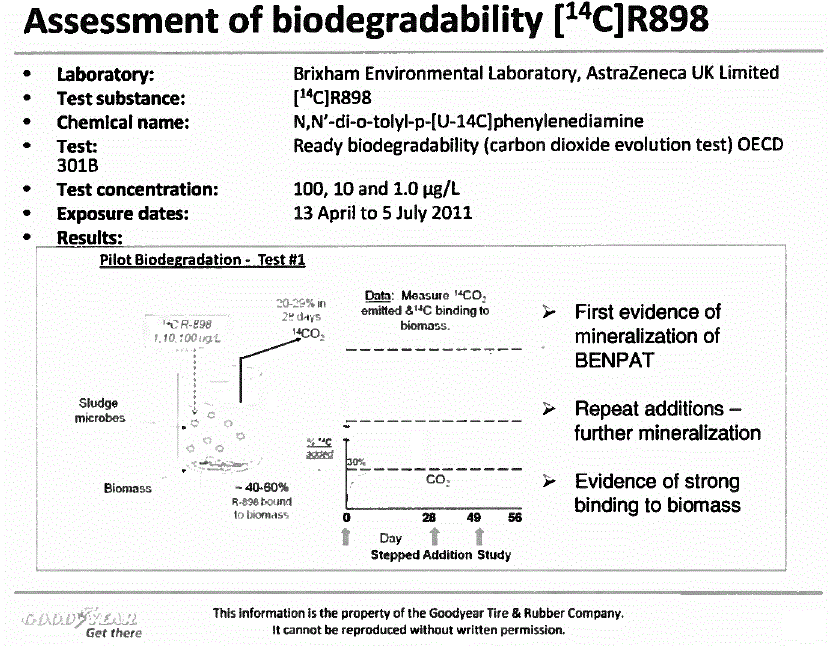
Description of figure 1
Figure 1. Assessment of biodegradability [14C]R898 - Laboratory: Brixham Environmental Laboratory, AstraZeneca UK Limited; Test substance: [14C]R898; Chemical name: N,N'-di-o-tolyl-p-[U-l4C]phenylenediamine; Test: 301B: Ready biodegradability (carbon dioxide evolution test) OECD; Test concentration: 100, 10 and 1.0 µg/L; Exposure dates: 13 April to 5 July 2011;
Figure 2
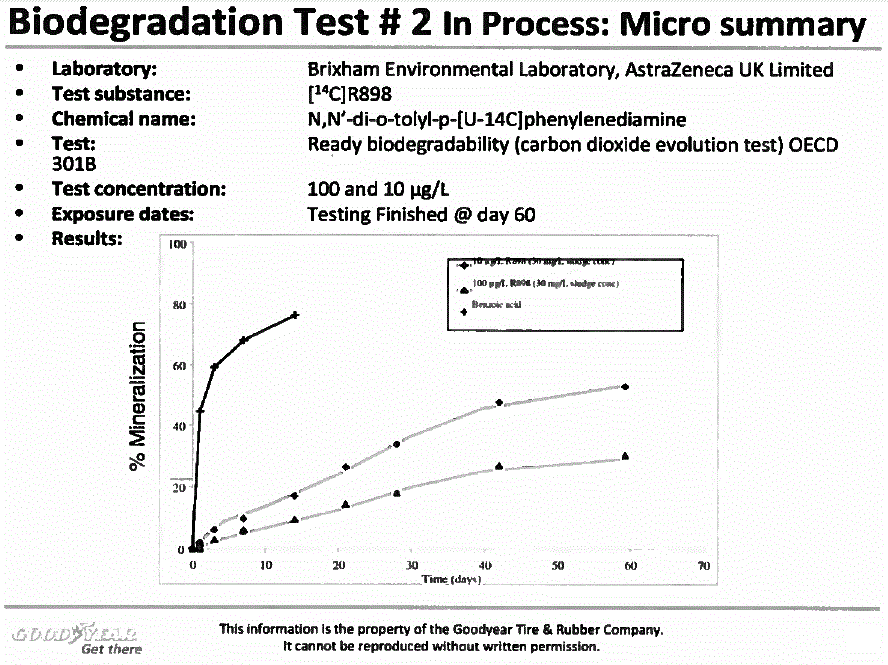
Description of figure 2
Figure 2. Biodegradation Test # 2 In Process: Micro summary. Laboratory: Brixham Environmental Laboratory, AstraZeneca UK Limited; Test substance: [14C]R898; Chemical name: N,N'-di-o-tolyl-p-[U-l4C]phenylenediamine; Test: 301B: Ready biodegradability (carbon dioxide evolution test) OECD; Test concentration: 100 and 10 µg/L; Exposure dates: Testing Finished @ day 60;
Figure 3. BENPAT binding to biomass
- Evidence of non-extractability of radioactivity from biomass
- 40-60% in trials to date
- Other notable observations:
- [14C]R898 shown to be ultimately biodegradable
- Radio-TLC results showed there was no [14C]R898 remaining in the aqueous phase after 28 days incubation.
- At the end of exposure, a large fraction of the radioactivity was not extractable with organic solvents from sludge solids.
- Recently learned Germany is addressing issue of chemical binding to b iomass
- UBA (German Federal Environment Agency) identify three types of NER:
- NER 1 -- remobilizable and a potential source of risk
- NER 2 -- non-mobilizable and of low risk
- Material incorporated into biomass (low risk as with NER 2)
- Proposed use of a battery of extraction methods, which should (1) determine the biogenic components and (2) distinguish between NER1 and NER2 through sequential extraction.
- Our test rotocols and approaches we are following for R898 are following this USA position.
This information is the property of Goodyear Tire & Rubber Compapy. It cannot be reproduced without written permission.
2. The underlying assessments rely upon overly precautionary assumptions for industrial releases.
The Government's own Framework for the Application of Precaution in Science-based Decision Making about Risk mandates that "sound scientific information and its evaluation must be the basis for applying precaution."Footnote 11 Instead, the assessors of BENPAT relied upon overly conservative assumptions, inconsistent with the best available data (e.g., emission rates inclusive of current industrywide risk management measures).
With respect to industry releases, the Assessment conclusion was based on the risk quotients derived from two emissions scenarios in the screening assessment. RQs for Scenario 1 were 1.9 to 150.7 and were 0.1 to 7.5 for Scenario 2. In a July 22, 2010, comment document, Goodyear submitted a sample calculation with emissions factors consistent with Scenario 2, but noted that ETRMA was in the process of completing an emission factor study for European tire facilities which would provide more reliable information. The ETRMA study was published in August of 2010 and is now available online (ETRMA, 2010)Footnote 12. The study included analysis of 6-PPD, CBS and DPG. which provide a reasonable basis for read-across of emissions factors. The ETRMA emission factor approach was favorably peer-reviewed by ÖKOPOL on behalf of the German Federal Ministry of the Environment (ÖKOPOL, 20 11)Footnote 13. The review (p. 22) indicated the preparation of emission factor guidance was completed in a transparent and scientifically sound manner. Based on the methodology used and review process, these guidance represent the best available data representative of the current controls and should have be included in the Assessment.
The ETRMA guidance provides three tiers of emission factors. The guidance was organized in tiers to fit within the framework that had been proposed for registration, evaluation and authorization of chemicals (REACH) exposure assessment. The first tier (Tier 0) is based on the REACH default for Environmental Release Categories (ERCs), which are considered to be beyond worst case in most cases. The second tier (Tier 1) is based on the European Risk Assessment Technical Guidance Document A-Tables. These factors do not take into account risk management measures. The final tier (Tier 2) emission factors are based on measured European data collected in 2010, and considered to be most reliable for use in risk assessment. These factors reflect actual measured emission rates inclusive of industry-wide risk management measures.
We note that ETRMA did not adopt the emission factors corresponding to Environment Canada Scenario 1, which were derived from the OECD Emission Scenario Document for the Rubber Industry. As discussed by ETRMA (2010), these factors were determined based on a survey by the Association of the German Rubber Industry that asked about the fraction of additives remaining after processing and curing. These factors are believed in the industry to greatly overstate the true emission rate because they are not based on measured data and the approach used was neither well described nor transparent.
The ETRMA Tier 2 factors were not considered in the Environment Canada screening assessment. Given that, the assessment did not utilize measured emission factors recommended by ETRMA, which are the best available. The RQs that would have been derived using ETRMA Tier 2 emission factors are presented in Table 1. Some emission factors depend on annual usage. The annual usage of each site is unknown but has been assumed as indicated in the table. Based on the Tier 2 emission factors recommended by ETRMA, the extrapolated RQs for Sites 1, 2 and 3 are all less than 1, ranging from 0.03 to 0.2.
Goodyear requests that Environment Canada consider ETRMA Tier 2 factors as the best available data and evaluate the calculated RQs.
| Site | Usage | Scenario | Emission factor to water | RQ |
|---|---|---|---|---|
| 1 | >100 tons/year | 1 (OECD ESD) |
1%
|
28
|
| 1 | >100 tons/year | 2 (ETRMA Tier 1) |
0.05%
|
1.4
|
| 1 | >100 tons/year | 3* (ETRMA Tier 2) |
0.001%
|
0.03
|
| 2 | ≤100 tons/year | 1 (OECD ESD) |
1%
|
1.9
|
| 2 | ≤100 tons/year | 2 (ETRMA Tier 1) |
0.05%
|
0.1
|
| 2 | ≤100 tons/year | 3* (ETRMA Tier 2) |
0.02%
|
0.04
|
| 3 | >100 tons/year | 1 (OECD ESD) |
1%
|
151
|
| 3 | >100 tons/year | 2 (ETRMA Tier 1) |
0.05%
|
7.5
|
| 3 | >100 tons/year | 3* (ETRMA Tier 2) |
0.001%
|
0.2
|
* Scenario 3 supplements Scenario 1 and 2 already considered in the screening assessment.
3. The underlying assessments rely upon consumer release scenarios not based on the best available science.
As stated previously, the Cabinet Directive on Streamlining Regulations specifies that the Government shall make decisions on "the best available knowledge and science in Canada and worldwide" and the Government's own Framework for the Application of Precaution in Science-based Decision Making about Risk mandates that "sound scientific information and its evaluation must be the basis for applying precaution."Footnote 14 Instead, the modeling of BENPAT fate for Consumer releases was not conducted based on recognized scientific principles, but rather on unreasonable, physically impossible scenarios.
In general terms it appears the proprietary Environment Canada (EC) Mega Flush spreadsheet was used and a range of PECs was calculated by considering 1000 release sites. It is stated that the dilution factor for the release sites conservatively ranged from 1 to 10. The fraction of release sites with no primary and secondary treatment was difficult to discern from the screening assessment. PECs ranged over three orders of magnitude from 7.4 x 10-6 to 2.2 x 10-3 mg/L. Taken as a whole, the results of the modeling indicate that the RQ is unlikely to be greater than 1 during the service life in the vast majority of cases. When 67% of the emissions were assumed to be directed to water, with treatment at 50% of those sites, the RQ was greater than 1 at 10th percentile flow at only 4% of sites. When 50% of emission were assumed to be directed to water with the Mega Flush database of release sites, 11% of the water bodies had RQ's greater than 1. Because the amount of emissions to water is lower in Scenario 2 as compared to Scenario 1, we assumed that the increase in RQ in Scenario 2 is attributable to the fraction flow that receives treatment.
The results of the modeling, substance properties, and consideration of the possible chemical transformation of the substance during the service life indicate that BENPAT is unlikely to be detected in freshwater. It is important to consider that the model is characterizing low flow conditions, however under low flow conditions, tread from road surfaces is unlikely to be flushed to surface waters or STPs.
We note that the discussion of the Mega Flush modeling is not transparent and it is unclear whether the combinations of release and dilution factor are physically possible. For example, it is unclear whether emissions of tread at specific release points are consistent with local traffic load or population. Additionally, a traditional application of a down-the-drain model would be to assess consumer product usage, where loading to the surface water at low flow conditions may be typical. For example, substantial roadway runoff is unlikely during dry periods, hut dish soap usage is equally likely in dry and wet times. It is unclear how these differences have been taken into account in the modeling. We question whether 25th percentile or geometric mean flow might have been a better metric to characterize the balancing of first-flush loading with that of increasing stream-flow as compared to 10th percentile flow.
In light of these considerations, moving forward on a Proposed Order that is based on overly precautionary assumptions and modeling using overly conservative parameters as compared to the best and most current science would be inconsistent with the Government's own Framework for the Application of Precaution in Science-based Decision Making about Risks, which mandates that "sound scientific information and its evaluation must be the basis for applying precaution."Footnote 15
For the forgoing reasons, Goodyear objects to the Proposed Order and requests that a Board of Review be convened.
Sincerely,
Robin M. Hunter
Secretary