Ecological State of the Science Report on Decabromodiphenyl Ether (decaBDE): chapter 1
1. Introduction
The purpose of this report is to provide an updated analysis of the bioaccumulation and environmental transformation of decabromodiphenyl ether (decaBDE), to be considered in the context of the information and analyses already published in the final screening assessment on polybrominated diphenyl ethers (PBDEs) (Canada 2006). This evaluation is considered a state of the science review. While this report does not critique individual studies, it considers the reliability of individual studies when forming a weight of evidence for persistence, bioaccumulation or inherent toxicity to non-human biota. This report considers materials published up to August 25, 2009.
1.1 Background
In July 2006, the ministers of Environment and Health published their final screening assessment on PBDEs (Canada 2006). The environmental screening assessment examined various supporting information and developed conclusions based on a weight-of-evidence approach as required under subsection 76.1 of the Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999). As the term “screening assessment” implies, this was not an exhaustive review of all available data, but rather, it presented the most critical studies and lines of evidence supporting the conclusions (e.g., relating to persistence, bioaccumulation, inherent toxicity, risk quotients and long-range transport to remote regions like the Arctic). The PBDE screening assessment concluded that PBDEs--tetrabromodiphenyl ether (tetraBDE), pentabromodiphenyl ether (pentaBDE), hexabromodiphenyl ether (hexaBDE), heptabromodiphenyl ether (heptaBDE), octabromodiphenyl ether (octaBDE), nonabromodiphenyl ether (nonaBDE) and decaBDE--which are found in commercial pentaBDE, octaBDE and DecaBDE technical formulations, are entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity and thus meet the criterion under paragraph 64(a) of CEPA 1999. In addition, it was concluded that all of the PBDEs assessed met criteria for persistence, but only tetra- to hexaBDEs met the criterion for bioaccumulation as identified in the Persistence and Bioaccumulation Regulations (Canada 2000) under CEPA 1999. It also noted that higher brominated PBDEs, and decaBDE in particular, could accumulate to some degree in biota and debrominate to bioaccumulative and persistent transformation products.
Information obtained as of October 2004 was considered for inclusion into the environmental screening assessment (Environment Canada 2006a; Environment Canada 2006b), while information received between November 2004 and October 2005 was reviewed but not generally added to the assessment, as these studies supported the conclusions of the draft assessment published for public comment in 2004. Since 2004, a large amount of information has been and continues to be published on PBDEs and on the issues of decaBDE bioaccumulation and transformation. This report examines the new data concerning decaBDE and examines the development of lines of evidence respecting this substance’s bioaccumulation and transformation. In addition, a Notice of Objection, dated February 14, 2007, was submitted to the Minister of the Environment by the Sierra Legal Defence Fund (now known as Ecojustice Canada) on behalf of the David Suzuki Foundation, Environmental Defence, and the Canadian Environmental Law Association, requesting the establishment of a Board of Review to clarify the Canadian regulatory approach on PBDEs and to recommend changes to the proposed Polybrominated Diphenyl Ethers Regulations. In order to consider information and issues raised in the Notice of Objection and to inform a decision respecting the formation of a Board of Review, this detailed evaluation was prepared to consider the current science respecting the bioaccumulation and transformation of decaBDE to bioaccumulative products. This report considers published information up to August 25, 2009, identified in original literature and review documents. In addition to retrieving the references from a literature database search, direct contacts were made with researchers, academics, industry and other government agencies to obtain relevant information on decaBDE.
The analysis presented herein summarizes relevant information on bioaccumulation and transformation examined in the PBDE screening assessment (Canada 2006; Environment Canada 2006b), and examines how the new science builds upon existing lines of evidence respecting bioaccumulation and transformation. Ecological considerations rather than those relating to human health are presented. While the screening assessment of PBDEs (Health Canada 2004) concluded that worst-case estimates of Canadians’ exposure to PBDEs were much lower than the levels of exposure which caused health effects in laboratory animals, in light of uncertainties regarding the available database, Health Canada supports Environment Canada’s actions to limit the use of PBDEs so that levels do not reach a point where they could potentially harm the health of Canadians. The evaluation does not consider decaBDE accumulation in humans; however, consideration is given to laboratory rodent studies that are used in human health risk assessment since such studies also provide insight into impacts relating to mammalian wildlife.
This state of science report has undergone substantial internal and external written peer review/consultation. While external comments were taken into consideration, the final contents remain the responsibility of Environment Canada. Additionally, the draft of this evaluation was subject to a 60-day public comment period. The critical information and considerations upon which the evaluation is based are summarized below.
1.1.1 Composition of Commercial DecaBDE
In this evaluation, information on both the congener form of decaBDE (BDE209) and the commercial product also known as Decabromodiphenyl Ether (DecaBDE) are considered. Since nona- and octaBDEs are also found in DecaBDE formulations, these congeners are considered if appropriate.
According to the World Health Organization (WHO 1994), current manufactured formulations of DecaBDE typically contain
- decaBDE, 97-98% w/w, and
- other PBDEs (mainly nonaBDE), 0.3-3.0 w/w.
Older commercial DecaBDE products contained a higher proportion of lower brominated PBDEs (mainly nonaBDE and octaBDE isomers) than more recent formulations. For instance, FR-300-BA, produced in the 1970s and no longer commercially available, contained 77.4% decaBDE, 21.8% nonaBDE and 0.8% octaBDE (Norris et al. 1975).
La Guardia et al. (2006) analyzed compositions of the currently manufactured DecaBDE product, Saytex 102E, and compared it with that of Bromkal 82-ODE, which has not been manufactured for more than a decade (Table 1-1). They found that Saytex 102E and Bromkal 82-ODE contained 96.8% and 91.6% BDE209, respectively. Both formulations contained nonaBDEs, with BDE206 in the highest quantity, followed by BDE207 then -208. In addition, Bromkal 82-ODE contained 0.56% octaBDEs, with BDE196, -203 and -197 identified. octaBDEs were not identified in the Saytex 102E product.
Commercial octaBDE products (no longer in production) also contained decaBDE (La Guardia et al. 2006). For instance, Bromkal 79-8DE contained 49.6% decaBDE as well as significant nonaBDE (Table 1-1). In comparison, DE-79 contained lesser amounts of deca- and nonaBDE (i.e., 1.31 and 13.07%, respectively).
Table 1-1: Concentrations (%, w/w) of PBDEs in Selected Commercial Octa- and DecaBDE Products (La Guardia et al. 2006)
| PBDE Congener | Commercial octaBDE Products | Commercial DecaBDE Products | ||
|---|---|---|---|---|
| DE-79 | Bromkal 79-8DE | Saytex 102E | Bromkal 82-ODE | |
| BDE154 | 1.07 | 0.04 | ndFootnote b | nd |
| BDE144 | 0.1 | 0.12 | nd | nd |
| hexaBDEFootnote a | < 0.02 | nd | nd | nd |
| BDE153 | 8.66 | 0.15 | nd | nd |
| BDE139 | nd | nd | nd | nd |
| BDE140 | < 0.02 | nd | nd | nd |
| BDE138 | 0.62 | nd | nd | nd |
| BDE184 | < 0.02 | < 0.02 | nd | nd |
| heptaBDEFootnote a | < 0.02 | nd | nd | nd |
| BDE175/183 | 42 | 12.6 | nd | nd |
| BDE191 | < 0.02 | nd | nd | nd |
| BDE180 | 1.7 | nd | nd | nd |
| BDE171 | 1.81 | 0.17 | nd | nd |
| BDE201 | 0.78 | < 0.02 | nd | nd |
| BDE197 | 22.2 | 10.5 | nd | 0.03 |
| BDE203 | 4.4 | 8.14 | nd | 0.07 |
| BDE196 | 10.5 | 3.12 | nd | 0.46 |
| BDE194 | < 0.02 | nd | nd | nd |
| octaBDEFootnote a | < 0.02 | nd | nd | nd |
| BDE208 | 0.19 | < 0.02 | nd | 0.07 |
| BDE207 | 11.5 | 11.2 | 0.24 | 4.1 |
| BDE206 | 1.38 | 7.66 | 2.19 | 5.13 |
| BDE 209 | 1.31 | 49.6 | 96.8 | 91.6 |
1.2 Definitions and Rationale
For the purposes of this document, it is important to define and distinguish between bioaccumulation, bioconcentration and biomagnification. The term “bioaccumulation” has been recently summarized by Arnot and Gobas (2006):
Bioaccumulation is a process in which a chemical substance is absorbed in an organism via all routes of exposure as typically occurs in the natural environment, i.e., dietary and ambient environment sources. Bioaccumulation is the net result of competing processes of chemical uptake and elimination including the diet, respiratory exchange, fecal egestion, metabolic transformation of the “parent” compound and growth dilution… Growth dilution is considered a “pseudo- elimination” route since the chemical is not actually eliminated by the organism but the concentration can be diluted by an increase in the volume of tissue.
Thus, it is reasonable to expect that if a chemical is detectable in organism tissues, it is there as the result of the process of bioaccumulation. Bioaccumulation can be of concern because when organisms take up chemicals, the likelihood that those organisms will be harmed generally increases with the amount accumulated. In addition, as bioaccumulation increases, there is increased likelihood that contaminated organisms will cause indirect harm to predators that consume them. Chemicals that are highly bioaccumulative are of particular concern because they tend to biomagnify, i.e., increase in concentration from prey to predator across several trophic levels. Thus, relatively low ambient concentrations of highly bioaccumulativesubstances have the potential to cause both direct toxicity and indirect toxicity due to biomagnification.
It is important to distinguish between the process of “bioaccumulation” and the term “bioaccumulative” as used in the Persistence and Bioaccumulation Regulations (Canada 2000), a regulation made under CEPA 1999 and as described in Canada’s Toxic Substances Management Policy (Canada 1995a, 1995b). According to the Persistence and Bioaccumulation Regulations:
A substance is bioaccumulative
(a) when its bioaccumulation factor is equal to or greater than 5000;
(b) if its bioaccumulation factor cannot be determined in accordance with a method referred to in section 5, when its bioconcentration factor is equal to or greater than 5000; and
(c) if neither its bioaccumulation factor nor its bioconcentration factor can be determined in accordance with a method referred to in section 5, when the logarithm of its octanol-water partition coefficient is equal to or greater than 5.
The Regulations go on to indicate that “[t]he determination of persistence and bioaccumulation…must be made…taking into account the intrinsic properties of the substance, the ecosystem under consideration and the conditions in the environment.” Thus, determining whether these criteria are met involves professional judgement which considers the intrinsic properties of the substance and ecosystem under consideration.
These criteria were first proposed as part of the federal Toxic Substances Management Policy ( Canada 1995a, 1995b) and were intended to address lipophilic substances with the potential to bioaccumulate and biomagnify in aquatic organisms to levels causing effects at the top of the food web. Application of a criterion of 5000 for bioaccumulation factors (BAFs) or bioconcentration factors (BCFs), or 5 for log Kow (octanol-water partition coefficient), are recommended in the Policy. Substances that undergo significant bioaccumulation and/or biomagnification are considered “bioaccumulative.” As noted above, professional judgement is required in the application of these criteria. Notably, the Policy indicates that log Kow should be used with caution in predicting the bioconcentration and bioaccumulation potential of organic substances ( Canada 1995b). For instance, many substances can be metabolized and this can function to decrease bioaccumulation potential of the parent compound, and thus many substances with a very high log Kow (e.g., >5) can have very low bioaccumulation/bioconcentration potential.
Based on the definition in CEPA 1999 and its regulations which have their basis in the Toxic Substances Management Policy, the term “bioaccumulative” is not synonymous with the term “bioaccumulation.” Rather, “bioaccumulation” is the process which may lead to a substance being considered “bioaccumulative.” Evidence that a substance is both persistent and bioaccumulative when combined with evidence of toxicity and release or expected release into the environment provides a significant indication of its potential to cause ecological harm (Environment Canada 2007).
Gobas and Morrison (2000) provide a concise definition of the process of “biomagnification” as it is currently understood:
This is the process in which the chemical concentration in an organism achieves a level that exceeds that in the organism’s diet, due to dietary absorption. The extent of chemical biomagnification in an organism is best determined under laboratory conditions, where organisms are administered diets containing a known concentration of chemical, and there is no change in chemical uptake through other exposure routes (e.g., respiratory surface, dermis).
Biomagnification also can be determined under field conditions, based on chemical concentrations in the organism and its diet. Biomagnification factors derived under controlled laboratory conditions, which exclude uptake through routes other than the diet, are different from those determined under field conditions, because field-based biomagnification factors are inevitably the result of chemical uptake by all routes of chemical uptake, rather than dietary absorption alone.
The terms “trophic magnification,” “food web magnification” and “food web biomagnification” are often used interchangeably to refer to the same phenomenon. This is the phenomenon whereby chemical concentrations in organisms increase with trophic level, resulting in higher concentrations in predators than in prey (i.e., biomagnification through successive trophic levels). Gobas and Morrison (2000) describe “food-chain” bioaccumulation of neutral organic substances as
…the process in which chemical concentrations in organisms increase with each step in the food-chain, resulting in chemical concentrations in predators that are greater than those in their prey. Because concentrations of many hydrophobic chemicals in organisms increase as the lipid content of the organism increases, the occurrence of food-chain bioaccumulation is detected best by comparing chemical concentrations in predators and prey on a lipid weight basis. An increase in lipid-based concentrations in organisms with increasing trophic level indicates food-chain bioaccumulation.
Potential indicators of biomagnification or trophic magnification include field-measured biomagnification factors (BMFs--the ratio in concentration between predator and prey) for known predator-prey relationships, or trophic magnification factors (TMFs--sometimes called food web magnification factors) which examine the incremental magnification with each trophic level for the food web as a whole. TMFs also represent the average ratio of concentrations between predator and prey through an entire food web. A BMF or TMF exceeding 1 indicates that biomagnification or trophic magnification is occurring. The use of BMFs and TMFs are generally thought to be more appropriate for terrestrial and marine mammals which breathe air rather than water. Note that in laboratory feeding studies, dietary BAFs are often estimated by representing the relationship between the test organisms and their diet (i.e., synonymous with the definition of a BMF). For the purposes of this report, dietary BAFs are referred to as BMFs.
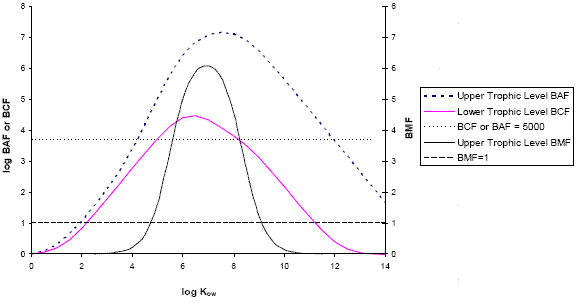
The BAF criterion of 5000 as identified in the Persistence and Bioaccumulation Regulations denotes chemicals which are highly bioaccumulative at the organism level and may also suggest the potential for biomagnification or trophic magnification. It should be noted that BAFs measured or estimated from total water concentrations are dependent on the quantity and quality of organic carbon and particulate matter in the water column. The available fraction of a chemical in water determines the uptake potential of a substance and subsequently the bioaccumulation potential. The BAF-QSAR model of Arnot and Gobas (2003) provides bioaccumulation predictions for a generic aquatic food web (with lower, middle and upper trophic levels) based on the current understanding of bioaccumulation processes in aquatic organisms, accounting for the available fraction in water. Figure 1-1 illustrates the model-predicted relationship for hydrophobic, non-metabolized substances between log Kow, BAF, BCF and BMF for aquatic lower and upper trophic levels in the model. The model predictions suggest that as log Kow increases toward and above 4-5, the BAF increases toward and above 5000. Also, in this range of log Kow, the BMF begins to significantly exceed 1, indicating that biomagnification may occur.
The underlying mechanism for this phenomenon is described by Kelly et al. (2004). For non-metabolized chemicals in aquatic organisms, the BMF results from competing processes of chemical uptake (respiratory and dietary) and chemical elimination (respiratory and gastrointestinal), which depend on organism physiology and chemical hydrophobicity. For aquatic organisms, respiratory elimination is significant for less hydrophobic chemicals, and elimination processes override gastrointestinal absorption processes. However, as hydrophobicity increases (i.e., log Kow > 5), respiratory elimination slows and gastrointestinal absorption becomes significant, resulting in biomagnification (i.e., a BMF exceeding 1). At very high log Kow, dietary chemical absorption efficiency tends to decline because chemicals are too strongly sorbed to the food matrix to be absorbed into the organism, resulting in a decline in both the BAF and the BMF (Arnot and Gobas 2003, 2004). Regardless of log Kow, when sufficient, chemical metabolism often sustains the total elimination rate at a high enough level to prevent chemical biomagnification, even for higher log Kow substances (Arnot and Gobas 2003; Kelly et al. 2004).
This relationship between log Kow, BAF and BMF for non-metabolized substances, which is based on current knowledge of bioaccumulation and biomagnification in aquatic organisms, supports the use of a BAF criterion of 5000 to indicate when bioaccumulation may occur. Note that the BCF is a less conservative endpoint since it does not increase as quickly with log Kow as the BAF. However, a lower trophic level BCF of 5000 generally corresponds to a log Kow of 5, which is consistent with the log Kow criterion of the Persistence and Bioaccumulation Regulations.
Figure 1 - 1: The Relationship Between BAF, BCF and BMF for Upper and Lower Trophic Levels Predicted by the BAF-QSAR Model for Hydrophobic, Non-metabolized Substances
Two additional measures which can also provide evidence of biomagnification include biota-sediment accumulation factors (sediment BSAFs) and biota-soil accumulation factors (soil BSAFs). The BSAF represents the steady-state concentration ratio between an organism and soil/sediment, on a lipid-normalized and organic-carbon-normalized basis. Assuming equal chemical sorptive capacities between lipid and organic carbon, sediment BSAFs of 1 are expected when organisms are in equilibrium with sediments (i.e., in the absence of food web biomagnification or trophic dilution). However, given the observed difference in sorptive capacity between lipid and organic carbon (see Seth et al. 1999), BSAFs up to approximately 3 are still consistent with equilibrium conditions. Alternatively, the American Society for Testing Materials (ASTM 1997) recommends a “cut-off” value of 1.7 to represent equilibrium conditions. Thus, sediment BSAFs that exceed approximately 1.7 to 3 suggest that food web biomagnification or an alternative magnification process is increasing organism chemical concentrations above equilibrium. Although there are currently no guidelines for evaluating soil BSAFs, a similar range would likely be appropriate to assess whether a soil BSAF value provides evidence that food web biomagnification or an alternative magnification process is increasing organism chemical concentrations above equilibrium.
The evaluation of bioaccumulation data in this report mainly relies on a combination of measured and model-predicted BAFs, BCFs, BMFs and TMFs, and in a few cases, measured sediment and soil BSAFs. Concentrations of decaBDE in organisms are also considered subjectively; however, without quantification of exposure concentrations, definite conclusions respecting the capacity for decaBDE to bioaccumulate cannot be made from biota concentrations alone. Consideration is also given to chemical uptake and elimination and metabolism. An organism’s metabolic capacity can reduce the bioaccumulation potential of a substance; however, this metabolic capacity can also result in the formation of bioaccumulative transformation products. Full evaluation of BAFs, BCFs, BMFs and TMFs would consider the bioaccumulation potential of both the parent substance and its metabolic product(s). Further discussion on metabolic transformation is found later in this report.