Appendices of Draft Screening Assessment
Aromatic Azo and Benzidine-based Substance Grouping
Certain Azo Metal Complexes and Other Azo Substances
Environment Canada
Health Canada
May 2014
Table of Contents
Appendix A: Supplementary Data Tables
| CAS RN C.I. name |
Chemical structure and formula | % similarityFootnote Appendix A Table A1a[a] | Read-across to |
|---|---|---|---|
| 1787-61-7 Mordant Black 11 |
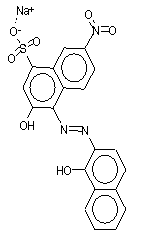 |
60–70; 70–80; 80–90 |
85029-57-8 a; 94276-35-4 a; 72391-06-1 a |
| 5610-64-0 Acid Black 52 |
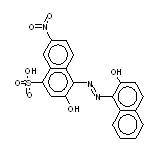 |
60–70; 70–80; 80–90 |
85029-57-8 a; 94276-35-4 a; 72391-06-1 a |
| CAS RN C.I. name |
Chemical structure and formula | % similarityFootnote Appendix A Table A1b[a] | Read-across to |
|---|---|---|---|
| Disperse Yellow 7 6300-37-4 |
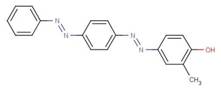 |
NA | 6708-61-8 and 63224-47-5 |
| Substance identity | Property | Value | Reference |
|---|---|---|---|
| Substance A | Melting point (°C) | greater than 269 | Confidential data |
| Substance A | Water solubility (g/L) | 20 | Confidential data |
| Substance A | Log Kow (dimensionless) | −2.8 | Confidential data |
| Substance A | Molecular weight (g/mol) | NA | Confidential data |
| Substance B | Melting point (°C) | less than 300 | Confidential data |
| Substance B | Water solubility (mg/L) | 0.5 | Confidential data |
| Substance B | Log Kow (dimensionless) | −1.4 | Confidential data |
| Substance B | Molecular weight (g/mol) | NA | Confidential data |
| Substance C | Melting point (°C) | less than 300 | Confidential data |
| Substance C | Water solubility (mg/L) | 0.002 | Confidential data |
| Substance C | Log Kow (dimensionless) | NA | Confidential data |
| Substance C | Molecular weight (g/mol) | NA | Confidential data |
| Property | Value(s) or range (for more than three data points) | Pivotal value(s) for this assessment (basis for selection) |
|---|---|---|
| Melting point and decomposition (°C) | 211 | 211 (sole experimental value) |
| Water solubility (mg/L) | 0.0096–0.35 | 0.18 (mean value) |
| Log Kow (dimensionless) | NA | NA |
| Dmin (nm) | 0.92 | 0.92 (sole estimated value) |
| Dmax (nm) | 1.10 | 1.10 (sole estimated value) |
| pKa (dimensionless) | NA | NA |
| Property | Value(s) or range (for more than three data points) | Pivotal value(s) for this assessment (basis for selection) |
|---|---|---|
| Melting point and decomposition (°C) | NA | NA |
| Water solubility (mg/L) | NA | NA |
| Log Kow (dimensionless) | NA | NA |
| Dmin (nm) | 1.54 | 1.54 (sole estimated value) |
| Dmax (nm) | 1.54 | 1.54 (sole estimated value) |
| pKa (dimensionless) | NA | NA |
| Property | Substance | Type of data | Value(s) or range (for more than three data points) | Pivotal value(s) for this assessment (basis for selection) |
|---|---|---|---|---|
| Melting point and decomposition (°C) | 72391-06-1 a and analogues | Experimental | NA | NA |
| Melting point and decomposition (°C) | 72391-06-1 b | Modelled | 248.14 | 248.14 (sole estimated value) |
| Water solubility (mg/L) | 72391-06-1 a and analogues | Experimental | NA | NA |
| Water solubility (mg/L) | 72391-06-1 b | Modelled | 2.80 × 10−4 | 2.80 × 10−4 (sole estimated value) |
| Log Kow (dimensionless) | 72391-06-1 a and analogues | Experimental | NA | NA |
| Log Kow (dimensionless) | 72391-06-1 b | Modelled | 6.63 | 6.63 (sole estimated value) |
| Dmin (nm) | 72391-06-1 a and analogues | Experimental | 1.25 | 1.25 (sole estimated value) |
| Dmax (nm) | 72391-06-1 a and analogues | Experimental | 1.25 | 1.25 (sole estimated value) |
| pKa (dimensionless) | 72391-06-1 a and analogues | Experimental | NA | NA |
| Property | Value(s) or range (for more than three data points) | Pivotal value(s) for this assessment (basis for selection) |
|---|---|---|
| Melting point and decomposition (°C) | NA | NA |
| Water solubility (mg/L) | NA | NA |
| Log Kow (dimensionless) | NA | NA |
| Dmin (nm) | 0.96 | 0.96 (sole estimated value) |
| Dmax (nm) | 1.30 | 1.30 (sole estimated value) |
| pKa (dimensionless) | NA | NA |
| Property | Substance | Type of data | Value(s) or range (for more than three data points) | Pivotal value(s) for this assessment (basis for selection) |
|---|---|---|---|---|
| Melting point and decomposition (°C) | 94276-35-4 a and analogues | Experimental | 165 | 165 (sole value) |
| Water solubility (mg/L) | 94276-35-4 a and analogues | Experimental | 1.2 × 104 | 1.2 × 104 (sole value) |
| Log Kow (dimensionless) | 94276-35-4 a and analogues | Experimental | 1.95 | 1.95(sole value) |
| Dmin (nm) | 94276-35-4 a and analogues | Experimental | 0.89 | 0.89 (sole estimated value) |
| Dmax (nm) | 94276-35-4 a and analogues | Experimental | 1.14 | 1.14 (sole estimated value) |
| pKa (dimensionless) | 94276-35-4 a and analogues | Experimental | NA | NA |
| Melting point and decomposition (°C) | 94276-35-4 b and analogues | Experimental | 217.85 | 217.85 (sole experimental value) |
| Melting point and decomposition (°C) | 94276-35-4 b | Modelled | 217.85 | 217.85 (sole estimated value) |
| Water solubility (mg/L) | 94276-35-4 b and analogues | Experimental | 1.946 | 1.946 (sole experimental value) |
| Water solubility (mg/L) | 94276-35-4 b | Modelled | 1.946 | 1.946 (sole estimated value) |
| Log Kow (dimensionless) | 94276-35-4 b and analogues | Experimental | 4.81 | 4.81 (sole experimental value) |
| Log Kow (dimensionless) | 94276-35-4 b (989-38-8) |
Modelled | 4.81 | 4.81 (sole estimated value) |
| Dmin (nm) | 94276-35-4 b and analogues | Experimental | NA | NA |
| Dmax (nm) | 94276-35-4 b and analogues | Experimental | NA | NA |
| pKa (dimensionless) | 94276-35-4 b and analogues | Experimental | NA | NA |
Table A-3: Summary of modelled data for degradation of chromium-containing Azo Metal ComplexesFootnote Appendix A Table A3[a]
| Fate process | Model and model basis | Model result and prediction | Extrapolated half-life (days) |
|---|---|---|---|
| Atmospheric oxidation | AOPWIN 2010Footnote Appendix A Table A3[b] | t½ = 0.01–0.73 day | less than or equal to 2 |
| Ozone reaction | AOPWIN 2010[b] | N/AFootnote Appendix A Table A3[c] | N/A |
| Fate process | Model and model basis | Model result and prediction | Extrapolated half-life (days) |
|---|---|---|---|
| Hydrolysis | HYDROWIN 2010[b] |
| Fate process | Model and model basis | Model result and prediction | Extrapolated half-life (days) |
|---|---|---|---|
| Biodegradation (aerobic) | BIOWIN 2010[b] Submodel 4: Expert Survey (qualitative results) |
2.7859–3.5638Footnote Appendix A Table A3[d] (borderline) |
greater than or equal to 182 |
| Fate process | Model and model basis | Model result and prediction | Extrapolated half-life (days) |
|---|---|---|---|
| Biodegradation (aerobic) | BIOWIN 2010[b] Submodel 3: Expert Survey (qualitative results) |
1.5815–2.47[d] (borderline) |
greater than or equal to 182 |
| Biodegradation (aerobic) | BIOWIN 2010[b] Submodel 5: MITI linear probability |
−0.7244 to 0.1139Footnote Appendix A Table A3[e] (biodegrades slowly) |
greater than or equal to 182 |
| Biodegradation (aerobic) | BIOWIN 2010[b] Submodel 6: MITI non-linear probability |
0–0.0038[e] (biodegrades slowly) |
greater than or equal to 182 |
| Biodegradation (aerobic) | DS TOPKAT ©2005–2009 Probability |
N/A | N/A |
| Biodegradation (aerobic) | CATALOGIC 2012 % BOD |
N/A | N/A |
| CAS RN C.I. name |
Test organism | Duration of test (h) | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|---|
| 1787-61-7 Mordant Black 11 |
Fathead minnow Pimephales promelas | 24 | LC50 | 10 | Little et al. 1974 |
| 1787-61-7 Mordant Black 11 |
Fathead minnow Pimephales promelas | 48 | LC50 | 6 | Little et al. 1974 |
| 1787-61-7 Mordant Black 11 |
Fathead minnow Pimephales promelas | 96 | LC50 | 6 | Little et al. 1974 |
| 1787-61-7 Mordant Black 11 |
Fathead minnow Pimephales promelas | 96 | LC50 | 6 | Little and Lamb 1973 |
| 5610-64-0 Acid Black 52 |
Fathead minnow Pimephales promelas | 24 | LC50 | 7 | Little et al. 1974 |
| 5610-64-0 Acid Black 52 |
Fathead minnow Pimephales promelas | 48 | LC50 | 6.2 | Little et al. 1974 |
| 5610-64-0 Acid Black 52 |
Fathead minnow Pimephales promelas | 96 | LC50 | 6.2 | Little et al. 1974 |
| 5610-64-0 Acid Black 52 |
Fathead minnow Pimephales promelas | 96 | LC50 | 7 | Little and Lamb 1973 |
| Substance A | Daphnia magna | 24 | EC50 (immobilization) | 480.7 | Confidential information |
| Substance A | Carp (Cyprinus carpio) | 96 | LC50 | greater than 1200 | Confidential information |
| Substance B | Daphnia magna | 48 | EC50 (immobilization) | greater than 100 | Confidential information |
| Substance B | Rainbow trout Oncorhynchus mykiss | 48 | LC50 | 100 | Confidential information |
| Substance C | Daphnia magna | 72 | EC50 (immobilization) | 1.7 | Confidential information |
| Substance C | Rainbow trout Oncorhynchus mykiss | 96 | LC50 | 0.4 | Confidential information |
| Substance C | Fathead minnow Pimephales promelas | 96 | LC50 | 37.0 | Confidential information |
| CAS RN C.I. name |
Test organism | Endpoint | Reference |
|---|---|---|---|
| Disperse Yellow 7 6300-37-4 |
Hyalella | 7 d LC50 = 0.18 mg/L 14 d LC50 = 0.16 mg/L 20 d LC50 = 0.025 mg/L 21 d LC50 = 0.12 mg/L 28 d LC50 = 0.12 mg/L 28 d EC50 greater than 0.2 mg/L |
Bartlett 2013 |
Appendix B: Ecological Aquatic Exposure Calculations for the Azo Metal Complex CAS RN 85029-57-8
The predicted environmental concentrations (PECs) of the only in-commerce substance (CAS RN 85029-57-8) were estimated in water for the 10 paint/coating formulation sites identified. The estimates were based on several parameters: annual use quantity, emission factor for emissions to wastewater, removal by on-site wastewater treatment and dilution by wastewater and receiving water.
The annual use quantity of the substance at each facility was obtained from a survey issued pursuant to section 71 of CEPA 1999 (Canada 2011). This quantity was between 10 and 1000 kg for the 2010 calendar year:
Annual use quantity of substance CAS RN 85029-57-8 at a facility
= Actual annual quantity found from CEPA section 71 survey (Canada 2011)
= 10 to 1000 kg/year
The use of the actual quantities found from the survey in these exposure calculations is intended to provide more accurate release and exposure estimates whenever possible with the data available. This would improve the certainty in risk characterization and avoid false risk conclusions. However, conservative assumptions are still needed as a precautionary measure in the case of data gaps and data uncertainties.
The number of annual operation days involved with the substance at each facility was unknown. This parameter was conservatively assumed to be 1 day/year:
Number of annual operation days = 1 day/year
The daily use quantity of the substance at each facility was then calculated by dividing the annual use quantity by the number of annual operation days. As an example, for an annual use quantity of 100 kg/year, the daily use quantity was calculated as
Daily use quantity of substance CAS RN 85029-57-8 at a facility
= Annual use quantity of substance CAS RN 85029-57-8 at a facility / Number of annual operation days
= 100 kg/year / 1 day/year
= 100 kg/day
The emission factor for emissions to wastewater from the cleaning of paint/coating formulation facilities was obtained from an analysis of industry data (Environment Canada 2012). This parameter was found to be 0.3%:
Emission factor to wastewater = 0.3%
The daily release quantity of the substance to wastewater from a facility was estimated by multiplying a facility’s daily use quantity of the substance by the emission factor to wastewater. As an example, for a daily use quantity of 100 kg/day, the daily quantity of the substance released to wastewater from a facility was estimated as follows:
Daily release quantity of substance CAS RN 85029-57-8 to wastewater at a facility
= Daily use quantity of substance CAS RN 85029-57-8 at a facility × Emission factor to wastewater
= 100 kg/day × 0.3%
= 0.3 kg/day
Several site visits to paint/coating formulation facilities indicated that settling tanks were used as on-site wastewater treatment for solids removal (Crechem 2003). These site visits also indicated that the treated wastewater was treated further by off-site local wastewater treatment systems before being discharged to the aquatic environment. Both on-site and off-site wastewater treatment were therefore assumed to occur, and both are reflected in the exposure calculations.
Dyes with water solubility under 1 mg/L are expected to be removed by 90% via settling tanks (OECD 2009). The removal mechanism is precipitation when a substance is present in water at concentrations above its water solubility. Since the substance CAS RN 85029-57-8 has a water solubility below 1 mg/L (0.002–0.5 mg/L) and on-site wastewater treatment systems are expected to have settling tanks, the removal efficiency is expected to be 90% for the on-site treatment:
On-site wastewater treatment removal = 90%
The quantity of the substance released from a facility to sewer was then determined from the daily release quantity to wastewater and the on-site wastewater treatment removal. As an example, for a daily release quantity of 0.3 kg/day to wastewater, the daily release quantity to sewer was estimated as:
Daily release quantity of substance CAS RN 85029-57-8 to sewer at a facility
= Daily release quantity of substance CAS RN 85029-57-8 to wastewater at a facility × (1 – On-site wastewater treatment removal)
= 0.3 kg/day × (1 – 0.9)
= 0.03 kg/day
The concentration of the substance in influent to a local municipal wastewater treatment system depends upon the influent flow. The concentration of the substance in influent was estimated by dividing the daily release quantity to sewer at a facility by the influent flow. The example below is used to illustrate the calculations for a daily release quantity of 0.03 kg/day to sewer and an influent flow of 200 000 L/day:
Concentration of substance CAS RN 85029-57-8 in influent
= Daily release quantity of substance CAS RN 85029-57-8 to sewer at a facility / Influent flow
= 0.03 kg/day / 200 000 L/day
= 1.5 × 10−7 kg/L
= 150 µg/L
It should be noted that the influent and effluent flows of a local wastewater treatment system were assumed to be equal in all concentration calculations. For the sake of convenience, they were also referred to as the flow of a wastewater treatment system.
The removal efficiencies of the off-site local wastewater treatment systems for the 10 paint/coating formulation sites are assumed to be negligible. This is because the substance has low affinity for solids due to its low octanol–water partition coefficient (log Kow = −2.8 to −1.4). Thus, the removal by sludge sorption is expected to be insignificant. In addition, the substance is not volatile, so the removal by volatilization is negligible. The substance is assumed to be non-biodegradable under wastewater treatment conditions due to a lack of biodegradation data. The overall removal is therefore assumed to be 0%.
Two of the off-site local wastewater treatment systems were identified as lagoons. Lagoons contain large volumes of water and have long hydraulic retention times. The retention time of a lagoon was in weeks to months, according to field data collected through the Chemicals Management Plan’s Monitoring and Surveillance Program at Environment Canada (Smyth 2012). The implication of a long retention time is that the substance entering a lagoon within a relatively short duration is subject to dilution, although the removal is negligible. As a result, the concentration of the substance in lagoon effluent is reduced by such dilution. The duration of the aquatic release of the substance within a year was 1 day, as assumed in this analysis:
Duration of aquatic release of substance CAS RN 85029-57-8 at a facility = 1 day
This duration was relatively short compared with a lagoon’s residence time. Dilution was therefore justified. Such dilution was, however, not expected in primary or secondary treatment systems, because their hydraulic retention times were short, typically in hours.
No quantitative method was available to determine the degree of lagoon dilution. Nevertheless, the ratio of a lagoon’s retention time to a substance’s release duration could be used as an approximation to the lagoon dilution, because the ratio represented the full dilution or the volume ratio of the entire lagoon water to the wastewater containing a specific substance. As an estimate, the lagoon retention time in weeks to months was interpreted as 42 days (6 weeks) to 84 days (12 weeks), with an average of 63 days. The full dilution was then determined by dividing this average by the release duration (1 day):
Lagoon dilution
= Average lagoon hydraulic retention time / Release duration
= 63 day / 1 day
= 63
The concentration of the substance in lagoon effluent was estimated by considering lagoon dilution. As an example, for a concentration of 150 µg/L in influent, the concentration of the substance in effluent was estimated as:
Concentration of substance CAS RN 85029-57-8 in lagoon effluent
= Concentration of substance CAS RN 85029-57-8 in lagoon influent / Lagoon dilution
= 150 µg/L / 63
= 2.4 µg/L
For primary or secondary systems, the concentration of the substance in effluent equals the concentration in influent.
The aquatic PEC was determined by applying the receiving water dilution to the concentration in effluent. Since the aquatic PEC was assessed near the discharge point, the receiving water dilution selected should also be applicable to this condition. The full dilution potential of a river was considered appropriate if it was between 1 and 10, based on its 10th percentile flow. Otherwise, the dilution was kept at 10 for both large rivers and still waters.
As an example, for a concentration of 2.4 µg/L in effluent discharging at 200 000 L/day to a river with a 10th percentile flow of 34 000 000 L/day, the full dilution of the receiving water was calculated as 170 (34 000 000 L/day / 200 000 L/day). The dilution factor of 10 was therefore selected in determining the concentration near the discharge point:
Aquatic PEC
= Concentration of substance CAS RN 85029-57-8 in effluent / Receiving water dilution factor
= 2.4 µg/L / 10
= 0.24 µg/L