Appendices of Screening Assessment Report
Phenol, 4,4'-(1-methylethylidene) bis[2,6-dibromo-
Chemical Abstracts Service Registry Number
79-94-7
Ethanol, 2,2'-[(1-methylethylidene)bis[(2,6-dibromo-4,1-phenylene)oxy]]bis
Chemical Abstracts Service Registry Number
4162-45-2
Benzene, 1,1'-(1-methylethylidene)bis[3,5-dibromo-4-(2-propenyloxy)-
Chemical Abstracts Service Registry Number
25327-89-3
Environment Canada
Health Canada
November 2013
Table of Contents
- Appendices
- Appendix 1a: PBT Model Inputs Summary Table- TBBPA
- Appendix 1b: PBT Model Inputs Summary Table- TBBPA bis(2-hydroxyethyl ether)
- Appendix 1c: PBT Model Inputs Summary Table- TBBPA bis (allyl ether)
- Appendix 2: Robust Study Summaries
- Appendix 3: Upper-bounding estimates of daily intake of TBBPA, TBBPA bis(2-hydroxyethyl ether) and TBBPA bis(allyl ether) by Canadians
- Appendix 4: TBBPA Levels in Indoor Air
- Appendix 5: TBBPA Levels in Dust
- Appendix 6: TBBPA Levels in Food
- Appendix 7: TBBPA Levels in Human Milk
- Appendix 8: TBBPA Levels in Human Serum and Adipose Tissue
- Appendix 9: Summary of health effects information for TBBPA and Derivatives
- Appendix 10: Supplementary Data from van der Ven et al. (2008)
- Back to the Screening Assessment
- Back to the Tables
Appendix 1a: PBT Model Inputs Summary Table- TBBPA
| Model Input Parameters | Phys-Chem/Fate EPISuite (all models, including: AOPWIN, KOCWIN, BCFBAF, BIOWIN and ECOSAR) |
Fate STP (1) ASTreat (2) SimpleTreat (3) (required inputs are different depending on model) |
Fate EQC (required inputs are different if Type I vs. Type II chemical) |
Fate TaPL3 (required inputs are different if Type I vs. Type II chemical) |
Fate OECD POPs Tool |
Fate Arnot- Gobas BCFBAF Model |
Fate BASL4 Model |
PBT Profiling Canadian-POPs (including: Catabol, BCF Mitigating Factors Model, OASIS Toxicity Model) |
Ecotoxicity Artificial Intelligence Expert System (AIES)/ TOPKAT/ ASTER) |
|---|---|---|---|---|---|---|---|---|---|
| SMILES Code | Oc(c(cc(c1) C(c(cc(c(O) c2Br)Br)c2) (C)C)Br)c1Br |
Oc(c(cc(c1) C(c(cc(c(O) c2Br)Br)c2) (C)C)Br)c1Br |
Oc(c(cc(c1) C(c(cc(c(O) c2Br)Br)c2) (C)C)Br)c1Br |
||||||
| Molecular weight (g/mol) | x (1, 2, 3) | 543.88 (I,II) | x (I,II) | X | 543.88 | ||||
| Melting point (ºC) | 181 (I) | x (I) | |||||||
| Boiling point (ºC) | |||||||||
| Data temperature (ºC) | 20 (I,II) | x (I,II) | |||||||
| Density (kg/m3) | x (2) | 14873 | |||||||
| Vapour pressure (Pa) | x (1, 3) | 1.19 × 10-5 (I) | x (I) | 1.19 x 10-5 | |||||
| Henry’s Law constant (Pa·m3/mol) | x (3) | ||||||||
| Log Kaw (air-water partition coefficient; dimensionless) |
x (2) | x (II) | x (II) | X | |||||
| Log Kow (octanol-water partition coefficient; dimensionless) |
5.9 | x (1) | 5.9 (I) | x (I) | X | 5.9 | 5.9 | 5.9 | 5.9 |
| Kow (octanol-water partition coefficient; dimensionless) |
x (2, 3) | ||||||||
| Log Koc (organic carbon-water partition coefficient – L/kg) |
5.43 | ||||||||
| Water solubility (mg/L) | x (1, 3) | 0.063a (I) | X | 0.063 | |||||
| Log Koa (octanol-air partition coefficient; dimensionless) |
|||||||||
| Soil-water partition coefficient (L/kg)Footnote Appendix 1a Table 11 | x (II) | x (II) | |||||||
| Sediment-water partition coefficient (L/kg)1 | x (II) | x (II) | |||||||
| Suspended particles-water partition coefficient (L/kg)1 | x (2) | x (II) | x (II) | ||||||
| Fish-water partition coefficient (L/kg)Footnote Appendix 1a Table 12 | x (II) | x (II) | |||||||
| Aerosol-water partition coefficient; dimensionlessFootnote Appendix 1a Table 13 | x (II) | x (II) | |||||||
| Vegetation-water partition coefficient; dimensionless1 | x (II) | ||||||||
| Enthalpy (Kow) | -20 (3) | ||||||||
| Enthalpy (Kaw) | 55 (3) | ||||||||
| Half-life in air (days) | 3.615 (I,II) | x (I,II) | X | ||||||
| Half-life in water (days) | 4166 (I,II) | x (I,II) | X | ||||||
| Half-life in sediment (days) | 4166 (I,II) | x (I,II) | |||||||
| Half-life in soil (days) | 4166 (I,II) | x (I,II) | X | 182 | |||||
| Half-life in vegetation (days)Footnote Appendix 1a Table 14 | x (I,II) | ||||||||
| Metabolic rate constant (1/days) | 1.12 | ||||||||
| Biodegradation rate constant (1/days) or (1/hr)–specify | x (3, 1/hr) (2, 1/days) |
||||||||
| Biodegradation half-life in primary clarifier (t1/2-p) (hr) | x (1) | ||||||||
| Biodegradation half-life in aeration vessel (t1/2-s) (hr) | x (1) | ||||||||
| Biodegradation half-life in settling tank (t1/2-s) (hr) | x (1) |
Appendix 1b: PBT Model Inputs Summary Table- TBBPA bis(2-hydroxyethyl ether)
| Model Input Parameters | Phys-Chem/Fate EPISuite (all models, including: AOPWIN, KOCWIN, BCFBAF, BIOWIN and ECOSAR) |
Fate STP (1) ASTreat (2) SimpleTreat (3) (required inputs are different depending on model) |
Fate EQC (required inputs are different if Type I vs. Type II chemical) |
Fate TaPL3 (required inputs are different if Type I vs. Type II chemical) |
Fate OECD POPs Tool |
Fate Arnot- Gobas BCFBAF Model |
PBT Profiling Canadian-POPs (including: Catabol, BCF Mitigating Factors Model, OASIS Toxicity Model) |
Ecotoxicity Artificial Intelligence Expert System (AIES)/ TOPKAT/ ASTER) |
|---|---|---|---|---|---|---|---|---|
| SMILES Code | OCCOc1c (Br)cc(cc1Br) C(C)(C)c2cc (Br)c(OCCO) c(Br)c2 |
OCCOc1c (Br)cc(cc1Br) C(C)(C)c2cc (Br)c(OCCO) c(Br)c2 |
OCCOc1c (Br)cc(cc1Br) C(C)(C)c2cc (Br)c(OCCO) c(Br)c2 |
|||||
| Molecular weight (g/mol) | x (1, 2, 3) | 631.98 (I,II) | x (I,II) | X | ||||
| Melting point (ºC) | 247.21 (I) | x (I) | ||||||
| Boiling point (ºC) | ||||||||
| Data temperature (ºC) | 25 (I,II) | x (I,II) | ||||||
| Density (kg/m3) | x (2) | |||||||
| Vapour pressure (Pa) | x (1, 3) | 1.29 x10-13 (I) | x (I) | |||||
| Henry’s Law constant (Pa·m3/mol) | x (3) | |||||||
| Log Kaw (air-water partition coefficient; dimensionless) |
x (2) | x (II) | x (II) | X | ||||
| Log Kow (octanol-water partition coefficient; dimensionless) |
5.48 | x (1) | 5.48 (I) | x (I) | X | 5.48 | 5.48 | 5.48 |
| Kow (octanol-water partition coefficient; dimensionless) |
x (2, 3) | |||||||
| Log Koc (organic carbon-water partition coefficient – L/kg) |
||||||||
| Water solubility (mg/L) | x (1, 3) | 0.031 (I) |
X | |||||
| Log Koa (octanol-air partition coefficient; dimensionless) |
||||||||
| Soil-water partition coefficient (L/kg)Footnote Appendix 1b Table 11.1 | x (II) | x (II) | ||||||
| Sediment-water partition coefficient (L/kg)1 | x (II) | x (II) | ||||||
| Suspended particles-water partition coefficient (L/kg)1 | x (2) | x (II) | x (II) | |||||
| Fish-water partition coefficient (L/kg)Footnote Appendix 1b Table 12.1 | x (II) | x (II) | ||||||
| Aerosol-water partition coefficient; dimensionlessFootnote Appendix 1b Table 13.1 | x (II) | x (II) | ||||||
| Vegetation-water partition coefficient; dimensionless1 | x (II) | |||||||
| Enthalpy (Kow) | -20 (3) | |||||||
| Enthalpy (Kaw) | 55 (3) | |||||||
| Half-life in air (days) | 0.418 (I,II) | x (I,II) | X | |||||
| Half-life in water (days) | 180 (I,II) | x (I,II) | X | |||||
| Half-life in sediment (days) | 720 (I,II) | x (I,II) | ||||||
| Half-life in soil (days) | 180 (I,II) | x (I,II) | X | |||||
| Half-life in vegetation (days)Footnote Appendix 1b Table 14.1 | x (I,II) | |||||||
| Metabolic rate constant (1/days) | 13.8 | |||||||
| Biodegradation rate constant (1/days) or (1/hr)–specify | x (3, 1/hr) (2, 1/days) |
|||||||
| Biodegradation half-life in primary clarifier (t1/2-p) (hr) | x (1) | |||||||
| Biodegradation half-life in aeration vessel (t1/2-s) (hr) | x (1) | |||||||
| Biodegradation half-life in settling tank (t1/2-s) (hr) | x (1) |
Appendix 1c: PBT Model Inputs Summary Table- TBBPA bis (allyl ether)
| Model Input Parameters | Phys-Chem/Fate EPISuite (all models, including: AOPWIN, KOCWIN, BCFBAF, BIOWIN and ECOSAR) |
STP (1) ASTreat (2) SimpleTreat (3) (required inputs are different depending on model) |
Fate EQC (required inputs are different if Type I vs. Type II chemical) |
Fate TaPL3 (required inputs are different if Type I vs. Type II chemical) |
Fate OECD POPs Tool |
Fate Arnot- Gobas BCFBAF Model |
PBT Profiling Canadian-POPs (including: Catabol, BCF Mitigating Factors Model, OASIS Toxicity Model) |
Ecotoxicity Artificial Intelligence Expert System (AIES)/ TOPKAT/ ASTER) |
|---|---|---|---|---|---|---|---|---|
| SMILES Code | C=CCOc1c (Br)cc(cc1Br) C(C)(C)c2cc (Br)c(OCC=C) c(Br)c2 |
C=CCOc1c (Br)cc(cc1Br) C(C)(C)c2cc (Br)c(OCC=C) c(Br)c2 |
C=CCOc1c (Br)cc(cc1Br) C(C)(C)c2cc (Br)c(OCC=C) c(Br)c2 |
|||||
| Molecular weight (g/mol) | x (1, 2, 3) | 624.01 (I,II) | x (I,II) | X | ||||
| Melting point (ºC) | 216.64 (I) | x (I) | ||||||
| Boiling point (ºC) | ||||||||
| Data temperature (ºC) | 25 (I,II) | x (I,II) | ||||||
| Density (kg/m3) | x (2) | |||||||
| Vapour pressure (Pa) | x (1, 3) | 2.9 x10-9 (I) | x (I) | |||||
| Henry’s Law constant (Pa·m3/mol) | x (3) | |||||||
| Log Kaw (air-water partition coefficient; dimensionless) |
x (2) | x (II) | x (II) | X | ||||
| Log Kow (octanol-water partition coefficient; dimensionless) |
x (1) | 8.71 (I) | x (I) | X | 8.71 | 8.71 | 8.71 | |
| Kow (octanol-water partition coefficient; dimensionless) |
x (2, 3) | |||||||
| Log Koc (organic carbon-water partition coefficient – L/kg) |
||||||||
| Water solubility (mg/L) | x (1, 3) | 0.000020 (I) | X | |||||
| Log Koa (octanol-air partition coefficient; dimensionless) |
||||||||
| Soil-water partition coefficient (L/kg)Footnote Appendix 1c Table 11.2 | X (II) | x (II) | ||||||
| Sediment-water partition coefficient (L/kg)1 | x (II) | x (II) | ||||||
| Suspended particles-water partition coefficient (L/kg)1 | x (2) | x (II) | x (II) | |||||
| Fish-water partition coefficient (L/kg)Footnote Appendix 1c Table 12.2 | x (II) | x (II) | ||||||
| Aerosol-water partition coefficient; dimensionlessFootnote Appendix 1c Table 13.2 | x (II) | x (II) | ||||||
| Vegetation-water partition coefficient; dimensionless1 | x (II) | |||||||
| Enthalpy (Kow) | -20 (3) | |||||||
| Enthalpy (Kaw) | 55 (3) | |||||||
| Half-life in air (days) | 0.159 (I,II) | x (I,II) | X | |||||
| Half-life in water (days) | 180 (I,II) | x (I,II) | X | |||||
| Half-life in sediment (days) | 360 (I,II) | x (I,II) | ||||||
| Half-life in soil (days) | 180 (I,II) | x (I,II) | X | |||||
| Half-life in vegetation (days)Footnote Appendix 1c Table 14.2 | x (I,II) | |||||||
| Metabolic rate constant (1/days) | 0.0018 | |||||||
| Biodegradation rate constant (1/days) or (1/hr)–specify | x (3, 1/hr) (2, 1/days) |
|||||||
| Biodegradation half-life in primary clarifier (t1/2-p) (hr) | x (1) | |||||||
| Biodegradation half-life in aeration vessel (t1/2-s) (hr) | x (1) | |||||||
| Biodegradation half-life in settling tank (t1/2-s) (hr) | x (1) |
Appendix 2: Robust Study Summaries
Description of the reliability evaluation
To evaluate the reliability of studies for key ecological endpoints (i.e., inherent toxicity to aquatic organisms, bioaccumulation potential, persistence), an approach analogous to that of Klimisch et al. (1997) has been developed. It involves the use of a standardized Robust Study Summary form, including a scoring system to quantitatively evaluate the studies. The Robust Study Summary (RSS) is an adaptation of the OECD Robust Study Summary templates (OECD 2009). It consists of a checklist of items or criteria (column 2 of the RSS) relating to identity of the substance, experimental protocol or method, test organism, specific test design/conditions, ecological relevance, and results. Most items are weighted according to their criticality to the quality and reliability of the study (column 3). The most important or critical items (which describe parameters/factors that have the most direct influence on the quality of the study) have been given a higher weight (3 points), while the less critical items have been given a lower score (1 or 2 points). For each item, the evaluator must indicate whether the item has been addressed appropriately in the study by answering “yes”, “no” or “non-applicable (n/a)” (column 4). Specific information relating to the items is provided in column 5 of the RSS.
Once answers to all the items have been provided in column 4, an overall Robust Study Summary score for the study is calculated as:
Overall Study Score (%) = [ΣWYes / ΣWYes+No] × 100%
-
Where:
W Yes - weight of applicable “Yes” answers;
- W Yes+No
- weight of applicable “Yes” and “No” answers.
The overall score’s corresponding reliability code and category is determined using the four categories adapted from the Klimisch approach and based on the score ranges as described in Table A.
| Reliability Code | Reliability Category | Overall Study Score Range |
|---|---|---|
| 1 | High confidence | greater than or equal to 80% |
| 2 | Satisfactory confidence | 60 – 79% |
| 3 | Low confidence | 40 – 59% |
| 4 | Not acceptable | less than 40% |
References
Klimisch HJ, Andreae M, Tillmann U. 1997. A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regulatory Toxicology and Pharmacology 25:1-5
[OECD] Organisation for Economic Co-operation and Development. 2009. Manual for the Assessment of Chemicals. Annex 1: Guidance for Completing a SIDS Dossier. [Internet]. Paris (FR): OECD, Environment Directorate. [cited July, 2011].Available from http://www.oecd.org/dataoecd/13/17/36045066.pdf
Robust Study Summary Forms For Key Ecotoxicological Studies
Reference: Springborn Laboratories Inc. 1989. Determination of the Biodegradability of Tetrabromobisphenol A in a Soil Under Aerobic Conditions. SLS Report: 88-11-2848; Study No.: 1199-1287-6103-760 p. 40. (Reference: Brominated Flame Retardants Industry Panel 1989d).
| Item | Yes | No |
|---|---|---|
| Chemical composition of the substance (including purity, by-products) | X |
| Item | Yes | No |
|---|---|---|
| References | X | |
| OECD, EU, national, or other standard method? | X | |
| Justification of the method/protocol if not a standard method was used | ||
| *GLP (Good Laboratory Practice) | X |
Test design / conditions
Study type (photodegradation, hydrolysis, biodegradation, other –specify, do not assess): Biodegradation
| Item | Yes | No |
|---|---|---|
| Information on stability of the substance in the media of concern is reported? | X | |
| Controls (positive or negative): Not mentioned | X | |
| Number of replicates (including controls) | X | |
| Temperature | X | |
| Duration of the experiment | X |
| Item | Yes | No |
|---|---|---|
| Light source (specify): | ||
| Light spectrum and relative intensity based on sunlight intensity: |
| Item | Yes | No |
|---|---|---|
| Measured concentrations reported? | ||
| Basic water properties (pH, hardness, etc.) |
| Item | Yes | No |
|---|---|---|
| Ready or inherent biodegradation (specify): Inherent | X | |
| Inoculum (concentration and source): Not mentioned | X |
Results
Endpoints: half-life (preferred); degradation, %; etc. (do not assess this item): 78.1% remaining (k @ 0.0039; t1/2 @ 179 days) (64-days - MASDLM); 42.2% remaining (k @ 0.013; t1/2 @ 51.3 days) (64-days - Clay Loam); 38% remaining (k @ 0.015; t1/2 @ 45.8 days) (64-days - Silty Loam). Average t1/2 @ 92 days
Information on breakdown products (do notassess this item): No
Overall score: 6/11 = 55 %
EC Reliability code: 3
Reliability category (high, satisfactory, low): Low
Comments: Score = 55%; Chemical purity – ‘99%’. Method as per “Protocol for Determining the Inherent Biodegradability in Soil under Aerobic Conditions” – in-house protocol. Temperature = 20-25°C. 64-day study; chemical measured by liquid scintillation counting. Maintained in darkness. Solvent – Acetone. 3 different soil types – Massachusetts Sandy Loam (MASDLM); Clay Loam; Silty Loam. “Only a limited amount ( less than 6%) of the applied radioactivity was recovered in the CO2 traps, suggesting merely partial biodegradation to unidentified products”. The absence of reported controls is a concern. Further, there is no mention of controls in the in-house protocol, thus the confidence that the reported percentages are a result of biodegradation only is low. It is hard to distinguish what factors cause the differences in biodegradation rates (e.g. OC content, pH etc) since there is also no documentation on inoculum. Low confidence; reliability code = 3.
| Soil Type | Organic Carbon (%) | pH | Field Moisture Capacity (%) | Sand (%) | Silty (%) | Clay (%) | Remaining TBBPA in Soil (%) | ½ life (d) (estimated by evaluator) |
|---|---|---|---|---|---|---|---|---|
| MASDLM | 4.4 | 7.0 | 74.8 | 83 | 13 | 4 | 74.3-81.9 | 179 |
| Clay Loam | 0.8 | 6.2 | 43.9 | 16 | 58 | 26 | 41.1-43.2 | 51.3 |
| Silty Loam | 1.8 | 7.6 | 75.9 | 43 | 24 | 33 | 35.9-40.1 | 45.8 |
Robust Study Summary - Persistence
Reference: Springborn Laboratories Inc. 1989. (Tetrabromobisphenol A) – Determination of the Biodegradability in a Sediment/Soil Microbial System. SLI Report: 89-8-3070; Study No.: 1199-1287-6102-785 p. 69. (Reference: Brominated Flame Retardants Industry Panel 1989f).
| Item | Yes | No |
|---|---|---|
| Chemical composition of the substance (including purity, by-products) | X |
| Item | Yes | No |
|---|---|---|
| References | X | |
| OECD, EU, national, or other standard method? | X | |
| Justification of the method/protocol if not a standard method was used | ||
| *GLP (Good Laboratory Practice) | X |
Test design / conditions
Study type (photodegradation, hydrolysis, biodegradation, other –specify, do not assess): Biodegradation
| Item | Yes | No |
|---|---|---|
| Information on stability of the substance in the media of concern is reported? | X | |
| Controls (positive or negative): Negative control | X | |
| Number of replicates (including controls) | X | |
| Temperature | X | |
| Duration of the experiment | X |
| Item | Yes | No |
|---|---|---|
| Light source (specify): | ||
| Light spectrum and relative intensity based on sunlight intensity: |
| Item | Yes | No |
|---|---|---|
| Measured concentrations reported? | ||
| Basic water properties (pH, hardness, etc.) |
| Item | Yes | No |
|---|---|---|
| Ready or inherent biodegradation (specify): Not specified | X | |
| Inoculum (concentration and source): Natural assemblages of microorganisms from sediment. 103 – 105 colony forming units per ml (final) | X |
Results
Endpoints: half-life (preferred); degradation, %; etc. (do not assess this item): (10 ug/L) t1/2 = 48 days (k = 0.0145 day-1), r2 = 0.67; (100 ug/L) t1/2 = 69 days (k = 0.0101 day-1), r2 = 0.87; (1,000 ug/L) t1/2 = 84 days (k = 0.00829 day-1), r2 = 0.79.
| Item | Yes | No |
|---|---|---|
| Information on breakdown products (do not assess this item): No |
Overall score: 9/11 = 82 %
EC Reliability code: 2
Reliability category (high, satisfactory, low): satisfactory
Comments: Score = 82%; Chemical purity – ‘96%’. Method as per “Protocol for Determining the Biodegradation of a Test Material in a Sediment/Water Microbial System Following Proposed TSCA Guidelines” – in-house protocol, but the actual reference is not provided. Temperature = 25°C. Stability of chemical monitored throughout the 56-day study by HPLC. Maintained in darkness. Solvent – Acetone. pH range 5.2 – 6.6. Sediment characteristics – total carbon=6.8%, sand=92%, silt=6%, clay=2%, field moisture capacity=15.9%. 3 concentrations of test chemical used – high (1,000 ug/L); medium (100 ug/L); and low (10 ug/L). Three replicates per sample time. “Only a limited amount ( less than 8%) of the applied radioactivity was recovered in the CO2 traps, suggesting merely partial biodegradation to unidentified products”. Although experiment conducted in water/sediment, half-lives and rates should be applied to sediment compartment, as this is where the majority of the chemical was detected. The increased concentration of test chemical appears to inhibit biodegradation rates perhaps by some effect on microbial populations. This was further investigated but was generally inconclusive (according to methods of OECD 209). Satisfactory confidence; reliability code = 2. Value could be selected based on concentration of chemical to align with these rates or suggested bold value.
Robust Study Summary - Persistence
Reference: Ronen, Z. and A. Abeliovich. 2000. Anaerobic-Aerobic Process for Microbial Degradation of Tetrabromobisphenol A. Applied and Environmental Microbiology 66:2372-2377.
| Item | Yes | No |
|---|---|---|
| Chemical composition of the substance (including purity, by-products) | X |
| Item | Yes | No |
|---|---|---|
| References | X | |
| OECD, EU, national, or other standard method? | X | |
| Justification of the method/protocol if not a standard method was used | ||
| *GLP (Good Laboratory Practice) | n/a | n/a |
Test design / conditions
Study type (photodegradation, hydrolysis, biodegradation, other –specify, do not assess): Biodegradation
| Item | Yes | No |
|---|---|---|
| Information on stability of the substance in the media of concern is reported? | X | |
| Controls (positive or negative): Negative control | X | |
| Number of replicates (including controls) | X | |
| Temperature | X | |
| Duration of the experiment | X |
| Item | Yes | No |
|---|---|---|
| Light source (specify): | ||
| Light spectrum and relative intensity based on sunlight intensity: |
| Item | Yes | No |
|---|---|---|
| Measured concentrations reported? | ||
| Basic water properties (pH, hardness, etc.) |
| Item | Yes | No |
|---|---|---|
| Ready or inherent biodegradation (specify): Not specified (~Inherent) | X | |
| Inoculum (concentration and source): Natural assemblages of microorganisms from sediment. | X |
Results
Endpoints: half-life (preferred); degradation, %; etc. (do not assess this item): EstimatedAnaerobic – t1/2 = 11.5 days (based on rate constant of 0.06/day estimated from graph from reviewer). No graphs or rates presented for aerobic degradation of BPA (this is also not the primary chemical).
| Item | Yes | No |
|---|---|---|
| Information on breakdown products (do not assess this item): No |
Overall score: 5/10 = 50 %
EC Reliability code: 3
Reliability category (high, satisfactory, low): low
Comments: Score = 50%; Chemical purity – not determined. No standard methods mentioned. Temperature = 30°C. Two experiments conducted from contaminated site sediment. (i) Anaerobic degradation of TBBPA and (ii) aerobic degradation of BPA. Medium for anaerobic conditions include peptone, tryptone, glucose and yeast extract. TBBPA is biodegradable under anaerobic conditions; however, BPA (primary degradation product - 88%) is not biodegradable under anaerobic conditions. BPA requires aerobic conditions to be degraded. pH range adjusted to 7.7. Rate and half-life estimated from a graph by reviewer. This rate is for the anaerobic biodegradation of TBBPA to BPA only. Low confidence; reliability code = 3.
Robust Study Summary - Inherent Toxicity
Reference: Surprenant, D.C. 1989. The toxicity of tetrabromobisphenol A (TBBPA) to Fathead Minnow (Pimphales promelas) embryos and larvae. Springborn Life Sciences, Inc. Report No: 89-2-2937. Study No: 1199-1287-6108-120. 52 pp..(Reference:Brominated Flame Retardants Industry Panel 1989i).
| Item | Yes | No |
|---|---|---|
| *Chemical composition of the substance (including purity, by-products) | X | |
| Persistence/stability of test substance in test system | X |
| Item | Yes | No |
|---|---|---|
| References | X | |
| *OECD, EU, national, or other standard method? | X | |
| Justification of the method/protocol if not a standard method was used | ||
| *GLP (Good Laboratory Practice) | X |
| Item | Yes | No |
|---|---|---|
| Latin or both Latin and common names reported? | X | |
| Life cycle age / stage of test organism | X | |
| Sex | X | |
| Length and weight of test organisms | X | |
| Number of test organisms per replicate | X | |
| Food type / feeding periods (acclimation/during test) | X |
Test design / conditions
| Item | Yes | No |
|---|---|---|
| Experiment type (laboratory or field) specified? | X | |
| System type (static, semi-static, flow through)? | X | |
| Negative or positive controls (specify)? Negative control | X | |
| Number of replicates (including controls) and concentrations | X | |
| Exposure pathways (food, water, both) | X | |
| Exposure duration | X | |
| *Measured concentrations reported? | X | |
| Exposure media conditions (temperature, pH, electrical conductivity, hardness, TOC, DOC, DO, major cations and anions; other) | X | |
| Was pH within 6-9 range? (do not assess this item) | X | |
| Was temperature within 5-28 °C range? (do not assess this item) | X | |
| Photoperiod and light intensity | X | |
| Stock and test solution preparation | X | |
| Use of emulgators / solubilizers (especially for poorly soluble / unstable substances) | X | |
| Analytical monitoring intervals | X | |
| Statistical methods used | X |
Results
Toxicity values (LC50, EC50, or IC50 - specify, do not assess this item): 35 d LOEC=0.31 mg/L, 35 d NOEC = 0.16 mg/L
Other endpoints reported - BCF/BAF, LOEC/NOEC (specify, do not assess this item): No
| Item | Yes | No |
|---|---|---|
| *Was toxicity value below the chemical’s water solubility? | X | |
| Other adverse effects (carcinogenicity, mutagenicity, etc. Do not assess this item) | X |
Score: major items – 5/5; overall score – (97%)
EC Reliability code: 1
Reliability category (high, satisfactory, low): high
Comments: The test was well-conducted and followed an established protocol based on EPA methods. Observations, both biotic and abiotic, were recorded daily and all WQ was within acceptable limits. Test concentrations were measured radiometrically throughout the test, with HPLC used to check the accuracy of the radiometric technique. Analysis of the test concentrations indicates they were reasonably well maintained. Control performance was good and there was a definitive dose-response.
Reference: Krueger, H.O., Kendall, T.Z. and M. Jaber. 2002. Tetrabromobisphenol A – A Prolonged Sediment Toxicity Test with Lumbriculus variegatus Using Spiked Sediment with 2% Total Organic Carbon. Wildlife International, Ltd, Easton Maryland Project No.: 439A-115, p. 103. (Reference: ACCBFRIP 2002c).
| Item | Yes | No |
|---|---|---|
| *Chemical composition of the substance (including purity, by-products) | X | |
| Persistence/stability of test substance in test system | X |
| Item | Yes | No |
|---|---|---|
| References | X | |
| *OECD, EU, national, or other standard method? | X | |
| *GLP (Good Laboratory Practice) | X |
| Item | Yes | No |
|---|---|---|
| Latin or both Latin and common names reported? | X | |
| Life cycle age / stage of test organism | X | |
| Sex | X | |
| Length and weight of test organisms | X | |
| Number of test organisms per replicate | X | |
| Food type / feeding periods (acclimation/during test) | X |
Test design / conditions
| Item | Yes | No |
|---|---|---|
| Experiment type (laboratory or field) specified? | X | |
| System type (static, semi-static, flow through)? | X | |
| Negative or positive controls (specify)? Negative control | X | |
| Number of replicates (including controls) and concentrations | X | |
| Exposure pathways (food, water, both) | X | |
| Exposure duration | X | |
| *Measured concentrations reported? | X | |
| Exposure media conditions (temperature, pH, electrical conductivity, hardness, TOC, DOC, DO, major cations and anions; other) | X | |
| Was pH within 6-9 range? (do not assess this item) | X | |
| Was temperature within 5-28 °C range? (do not assess this item) | X | |
| Photoperiod and light intensity | X | |
| Stock and test solution preparation | X | |
| Use of emulgators / solubilizers (especially for poorly soluble / unstable substances) | X | |
| Analytical monitoring intervals | X | |
| Statistical methods used | X |
Results
Toxicity values (LC50, EC50, or IC50 - specify, do not assess this item): EC50= 294 mg/Kg dry weight sediment (28 day)
Other endpoints reported - BCF/BAF, LOEC/NOEC (specify, do not assess this item): Yes, LOEC = 151 mg/Kg dry weight sediment (statistically different than control (p less than 0.05)); NOEC = 90 mg/Kg dry weight sediment.
| Item | Yes | No |
|---|---|---|
| *Was toxicity value below the chemical’s water solubility? | X | |
| Other adverse effects (carcinogenicity, mutagenicity, etc. Do not assess this item) | X |
Score: major items – 4/5; overall score – 22/25 (88%)
EC Reliability code: 1
Reliability category (high, satisfactory, low): high
Comments: Four major items reported “yes”; overall score 88%. Methods as per “A prolonged sediment toxicity test with Lumbriculus variegatus using spiked sediment with 5% total organic carbon” –in-house protocol based on ASTM E 1706-95b (1995) and OPPTS 850.1735 Guideline (US EPA 1996a). Flow through test design. Chemical purity – ‘98.91%’. Temperature 23°C. 95% C.L. = 140 - 391 mg/Kg dry wt. sediment. Effect – ‘survival/reproduction’. Hydrophobic chemical (log Kow ~4.5-7.0). Good documentation of test organism and conditions. The water, sediment and pore water are sampled during test to verify test concentrations but nominal concentrations used to determine endpoints. High confidence; reliability code = 1.
| Soil Type | Organic Matter (%) | Organic Carbon (%) | pH | Water Holding Capacity (%) | Sand (%) | Silt (%) | Clay (%) |
|---|---|---|---|---|---|---|---|
| Artificial Sediment | 4.4 | 2.5 | 8.1 | 10.7 | 83 | 8 | 9 |
Robust Study Summary - Inherent Toxicity
Reference: Krueger, H.O., Kendall, T.Z. and M. Jaber. 2002. Tetrabromobisphenol A – A Prolonged Sediment Toxicity Test with Lumbriculus variegates Using Spiked Sediment with 5% Total Organic Carbon. Wildlife International, Ltd, Easton Maryland Project No. 439A-116, p. 104. (Reference: ACCBFRIP 2002d).
| Item | Yes | No |
|---|---|---|
| *Chemical composition of the substance (including purity, by-products) | X | |
| Persistence/stability of test substance in test system | X |
| Item | Yes | No |
|---|---|---|
| References | X | |
| *OECD, EU, national, or other standard method? | X | |
| Justification of the method/protocol if not a standard method was used | ||
| *GLP (Good Laboratory Practice) | X |
| Item | Yes | No |
|---|---|---|
| Latin or both Latin and common names reported? | X | |
| Life cycle age / stage of test organism | X | |
| Sex | X | |
| Length and weight of test organisms | X | |
| Number of test organisms per replicate | X | |
| Food type / feeding periods (acclimation/during test) | X |
Test design / conditions
| Item | Yes | No |
|---|---|---|
| Experiment type (laboratory or field) specified? | X | |
| System type (static, semi-static, flow through)? | X | |
| Negative or positive controls (specify)? Negative control | X | |
| Number of replicates (including controls) and concentrations | X | |
| Exposure pathways (food, water, both) | X | |
| Exposure duration | X | |
| *Measured concentrations reported? | X | |
| Exposure media conditions (temperature, pH, electrical conductivity, hardness, TOC, DOC, DO, major cations and anions; other) | X | |
| Was pH within 6-9 range? (do not assess this item) | X | |
| Was temperature within 5-28 °C range? (do not assess this item) | X | |
| Photoperiod and light intensity | X | |
| Stock and test solution preparation | X | |
| Use of emulgators / solubilizers (especially for poorly soluble / unstable substances) | X | |
| Analytical monitoring intervals | X | |
| Statistical methods used | X |
Results
Toxicity values (LC50, EC50, or IC50 - specify, do not assess this item): EC50= 405 mg/Kg dry weight sediment (28 day)
Other endpoints reported - BCF/BAF, LOEC/NOEC (specify, do not assess this item): Yes, LOEC = 426 mg/Kg dry weight sediment (statistically different than control (p less than 0.05)); NOEC = 254 mg/Kg dry weight sediment.
| Item | Yes | No |
|---|---|---|
| *Was toxicity value below the chemical’s water solubility? | X | |
| Other adverse effects (carcinogenicity, mutagenicity, etc. Do not assess this item) | X |
Score: major items – 4/5; overall score – 22/25 (88%)
EC Reliability code: 1
Reliability category (high, satisfactory, low): high
Comments: Four major items reported “yes”; overall score 88%. Methods as per “A prolonged sediment toxicity test with Lumbriculus variegatus using spiked sediment with 5% total organic carbon” –in-house protocol based on ASTM E 1706-95b (1995) and OPPTS 850.1735 Guideline (US EPA 1996a). Flow through test design. Chemical purity – ‘99%’. Temperature 23°C. 95% C.L. = 314 - 869 mg/Kg dry wt. sediment. Effect – ‘survival/reproduction’. Hydrophobic chemical (log Kow ~4.5-7.0). Good documentation of test organism and conditions. The water, sediment and pore water are sampled during test to verify test concentrations but nominal concentrations used to determine endpoints. High confidence; reliability code = 1.
| Soil Type | Organic Matter (%) | Organic Carbon (%) | pH | Water Holding Capacity (%) | Sand (%) | Silty (%) | Clay (%) |
|---|---|---|---|---|---|---|---|
| Artificial Sediment | 10.1 | 5.9 | 8.0 | 13.9 | 80 | 14 | 6 |
Robust Study Summary - Inherent Toxicity
Reference: Aufderheide, J., Kendall, T. Z. and W.B. Nixon. 2003. Effect of Tetrabromobisphenol A on the Survival and Reproduction of the Earthworm, Eisenia fetida. ABC Study No. 47014 and Wildlife International, Ltd Project No. 439C-131, p. 109. (Reference: ACCBFRIP 2003).
| Item | Yes | No |
|---|---|---|
| *Chemical composition of the substance (including purity, by-products) | X | |
| Persistence/stability of test substance in test system | X |
| Item | Yes | No |
|---|---|---|
| References | X | |
| *OECD, EU, national, or other standard method? | X |
| Item | Yes | No |
|---|---|---|
| *GLP (Good Laboratory Practice) | X |
| Item | Yes | No |
|---|---|---|
| Latin or both Latin and common names reported? | X | |
| Life cycle age / stage of test organism | X | |
| Sex | X | |
| Length and weight of test organisms | X | |
| Number of test organisms per replicate | X | |
| Food type / feeding periods (acclimation/during test) | X |
Test design / conditions
| Item | Yes | No |
|---|---|---|
| Experiment type (laboratory or field) specified? | X | |
| System type (static, semi-static, flow through)? | X | |
| Negative or positive controls (specify)? Negative control | X | |
| Number of replicates (including controls) and concentrations | X | |
| Exposure pathways (food, water, both) | X | |
| Exposure duration | X | |
| *Measured concentrations reported? | X | |
| Exposure media conditions (temperature, pH, electrical conductivity, hardness, TOC, DOC, DO, major cations and anions; other) | X | |
| Was pH within 6-9 range? (do not assess this item) | X | |
| Was temperature within 5-28 °C range? (do not assess this item) | X | |
| Photoperiod and light intensity | X | |
| Stock and test solution preparation | X | |
| Use of emulgators / solubilizers (especially for poorly soluble / unstable substances) | n/a | n/a |
| Analytical monitoring intervals | X | |
| Statistical methods used | X |
Results
Toxicity values (LC50, EC50, or IC50 - specify, do not assess this item): Reproduction - EC50 = 1.7 mg/Kg dry weight soil (56 day); EC10 = 0.12 mg/Kg dry weight soil (56 day). Survival - EC50 = greater than 4,840 mg/Kg dry weight soil (28 day); EC10 = 0.12 mg/Kg dry weight soil (28 day).
Other endpoints reported - BCF/BAF, LOEC/NOEC (specify, do not assess this item): Yes, NOEC = 2.11 mg/Kg dry weight soil (reproduction); NOEC = 4,840 mg/Kg dry weight soil (survival).
| Item | Yes | No |
|---|---|---|
| *Was toxicity value below the chemical’s water solubility? | X | |
| Other adverse effects (carcinogenicity, mutagenicity, etc. Do not assess this item) | X |
Score: major items – 5/5; overall score – 23/24 (96%)
EC Reliability code: 1
Reliability category (high, satisfactory, low): high
Comments: Five major items reported “yes”; overall score 96%. Methods as per “US EPA OPPTS guideline 850.6200, OECD guideline No. 207, OECD proposed guideline “Earthworm Reproduction Test (Eisenia fetida)”. Two studies conducted (i) 28-d survival and (ii) 56-d reproduction. Initial mean weight of worms 450 – 520 mg/worm. Chemical purity – ‘99%’. Temperature 19.4 – 21.3°C. 95% C.L. = 0.46 – 3.7 mg/Kg dry wt. soil (56 day reproduction EC50). Effect – ‘reproduction’. Good documentation of test organism and conditions. High confidence; reliability code = 1.
| Soil Type | Organic Matter (%) | Organic Carbon (%) | pH | Moisture @ 60% of water holding capacity (%) | Sand (%) | Silt (%) | Clay (%) |
|---|---|---|---|---|---|---|---|
| Artificial Sandy Loam | 8.1 | 4.7 | 6.0 – 6.9 | 26 | 79 | 8 | 13 |
| Soil Type | Organic Matter (%) | Organic Carbon (%) | pH | Moisture @ 60% of water holding capacity (%) | Sand (%) | Silt (%) | Clay (%) |
|---|---|---|---|---|---|---|---|
| Artificial Sandy Loam | 7.7 | 4.5 | 5.8 – 7.5 | 22.3 | 78 | 10 | 12 |
Appendix 3: Upper-bounding estimates of daily intake of TBBPA, TBBPA bis(2-hydroxyethyl ether) and TBBPA bis(allyl ether) by Canadians
| Route of exposure | 0–6 monthsFootnote Appendix 31, Footnote Appendix 3 2, Footnote Appendix 33.3 breast fed |
0–6 months1, 2, 3 formula fed |
0–6 months1, 2, 3 greater than not formula fed |
0.5–4 yearsFootnote Appendix 34.3 | 5–11 yearsFootnote Appendix 35 | 12–19 yearsFootnote Appendix 36 | 20–59 yearsFootnote Appendix 37 | 60+ yearsFootnote Appendix 38 |
|---|---|---|---|---|---|---|---|---|
| Ambient airFootnote Appendix 39 | less than 0.0001 | less than 0.0001 | less than 0.0001 | less than 0.0001 | less than 0.0001 | less than 0.0001 | less than 0.0001 | less than 0.0001 |
| Indoor airFootnote Appendix 310 | 0.002 | 0.002 | 0.002 | 0.005 | 0.004 | 0.002 | 0.002 | 0.002 |
| Drinking waterFootnote Appendix 311 | 0.187 | 0.002 | 0.001 | 0.001 | 0.001 | less than 0.00001 | less than 0.0001 | less than 0.0001 |
| Food Footnote Appendix 312 | 0.187 | 0.002 | 0.081 | 0.050 | 0.030 | 0.017 | 0.011 | 0.009 |
| Soil/ DustFootnote Appendix 313 | 0.006 | 0.006 | 0.006 | 0.009 | 0.002 | 0.001 | 0.001 | 0.001 |
| Total intake | 0.195 | 0.010 | 0.090 | 0.065 | 0.038 | 0.020 | 0.014 | 0.012 |
Appendix 4: TBBPA Levels in Indoor Air
| Location | Sampling Period | Number of Samples | Detection Limit (mg/m3) | Mean ConcentrationFootnote Appendix 4 Table 11.4 (mg/m3) | Reference |
|---|---|---|---|---|---|
| United Kingdom | 2007 | 5 | LOQ n.s. | 1.6 x 10-8 (16 pg/m3) |
Abdallah et al. 2008 |
| Apartment and houses, Tokyo, Japan | March – May, 2003 | 48 | 1.0 x 10-7 (0.1 ng/m3) |
[3 x 10-7 – 8 x 10-7] (0.3 – 0.8 ng/m3) |
Inoue et al. 2003 |
| Location | Sampling Period | Number of Samples | Detection Limit (mg/m3) | Mean Concentration1(mg/m3) | Reference |
|---|---|---|---|---|---|
| USA | 2006-2007 | 10 buildings 18 samples |
n.s. | Vapor Range = [1.2 – 8.6 x 10-8 ] (12-86 pg/m3 ) Particulate matter range–1.1-1.2x10-8 (11-12 pg/m3 ) |
Batterman et al. 2010 |
| United Kingdom | 2007 | 5 | LOQ n.s. | 1.6 x 10-8 (16 pg/m3) |
Abdallah et al. 2008 |
| United Kingdom, public micro-environments | 2007 | 4 | LOQ n.s. | 2.6 x 10-8 (26 pg/m3) |
Abdallah et al. 2008 |
| Offices, Sweden | 1 working day in offices with 2 or 3 computers | 4 | n.s. | 3.6 x 10-8 (0.036 ng/m3) [1.0 x 10-8 – 7.0 x 10-8] (0.01-0.07 ng/m3) |
Sjödin et al. 2001 |
| Computer teaching hall, Sweden | 1 working day in hall with 20 computers | 2 | n.s. | 3.5 x 10-8 and 1.5 x 10-7 (0.035 and 0.15 ng/m3) |
Sjödin et al. 2001 |
| Computer room, Germany | 3 weeks | 1 | n.s. | 8.0 x 10-9 (8.0 pg/m3) |
Kemmlein 2000 |
| Computer School room Germany |
3 weeks 8 computers several printers |
1 | n.s. | 2.9 x 10-8 (29 pg/m3) |
Kemmlein 2000 |
| Electronic Recycling Plants (point sources) | - | - | - | [3 to 15 x 10-5] | Tollback et al. 2006 Sjödin et al. 2001 Morf et al. 2005 as cited in Xie et al. 2007 |
Appendix 5: TBBPA Levels in Dust
| Location | Sampling Period | No. of Samples | Detection Limit (mg/kg) | Mean Concentration (mg/kg) | Reference |
|---|---|---|---|---|---|
| Belgium | 2008 | 43 homes 10 offices |
3 x 10-4 to 5 x 10-4 | [ less than 0.003 to 0.419]Footnote Appendix 5 Table 11.5 | D’Hollander et al. 2010 |
| United States | 2006-2007 | 10 buildings | n.s. | [0.020 – 0.938] | Batterman et al. 2010 |
| United Kingdom | 2007-2008 | 45 homes 28 offices 20 cars |
n.s. | [0.017– 1.4] | Harrad et al. 2010 |
| Japan | n.s. TVs used until 2005- Manufactured from 1989-1998 |
Dust 5/5 Circuit board 5/5 Front cabinet 5/5 Rear cabinet 5/5 |
n.s. | Dust= 240 [5.5-680] Circuit board= 280 [7.9-1300] Front cabinet= 20 [0.24 -67] Rear cabinet= 1.9 x 104 [0.12-9.7 x 104] |
Takigami et al. 2008 |
| United Kingdom, dust, public micro-environments, 3 pubs and 1 restaurant |
2006-2007 | 4 | LOQ n.s. | 0.220 | Abdallah et al. 2008 |
| United Kingdom, House dust | 2006-2007 | 34/35 | LOQ n.s. | 0.087 | Abdallah et al. 2008 |
| United Kingdom, Office dust | 2006-2007 | 24/28 | LOQ n.s. | 0.049 | Abdallah et al. 2008 |
| United Kingdom, Car dust | 2006-2007 | 10/20 | LOQ n.s. | 0.006 | Abdallah et al. 2008 |
| Belgium, Flanders, House and office dust |
Spring 2008 | 20 (18 houses 2 offices) |
LOQ n.s. | 0.146+/-0.365 0.073+/-0.039 |
Geens et al. 2009 |
| House dust 10 regions of UK mainland |
October – November, 2002 | 70 (pooled into 10 final samples) Detected in 4 of 10 pooled samples | 0.5 to 3 x 10-3 | 0.116 [ less than 0.010 – 0.340] |
Santillo et al. 2003 |
| Finland | 2002 | 1 | 0.5 to 3 x 10-3 | 0.025 | Santillo et al. 2003 |
| Denmark | 2002 | 1 | 0.5 to 3 x 10-3 | 0.400 | Santillo et al. 2003 |
| Parliament The Hague, Netherlands |
June 2000 | 1 | 0.5 to 3 x 10-3 | 0.005 | Santillo et al. 2001 |
| Computer room and offices, Netherlands | June 2000 | 3 | 0.5 to 3 x 10-3 | less than 0.0005 - less than 0.001 | Santillo et al. 2001 |
| Parliament, Helsinki, Finland | May 2000 | 1 | 0.5 to 3 x 10-3 | less than 0.003 | Santillo et al. 2001 |
| Parliament, Stockholm, Sweden | May 2000 | 1 | 0.5 to 3 x 10-3 | less than 0.002 | Santillo et al. 2001 |
| Senato and Palazzo Marini, Italy | July 2000 | 2 | 0.5 to 3 x 10-3 | less than 0.001 | Santillo et al. 2001 |
| Eigtved Pakhus and Parliament, Copenhagen, Denmark | July 2000 | 2 | 0.5 to 3 x 10-3 | less than 0.001 | Santillo et al. 2001 |
| Parliament, Vienna, Austria | October 4, 2000 | 2 | 0.5 to 3 x 10-3 | 0.015 and 0.033 | Santillo et al. 2001 |
| Reichstag, Berlin, Germany | September and October, 2000 | 2 | 0.5 to 3 x 10-3 | 0.046 and 0.20 | Santillo et al. 2001 |
| Parliament, London, England | January 2001 | 2 | 0.5 to 3 x 10-3 | 0.012 and 0.047 | Santillo et al. 2001 |
Appendix 6: TBBPA Levels in Food
| Item Sampled | Sampling Period | No. of Samples | Detection Limit (μg/kg lipid) | Mean Concentration (μg/kg lipid) | Reference |
|---|---|---|---|---|---|
| Fish, England | Summer 2008 | 30 | 0.29 | ( less than 0.29-1.7)Footnote Appendix 6 Table 11.6 [ less than 0.29-1.7 ng/g] |
Ha.rrad et al. 2009 |
| Fish, Scotland | 2006 | n.s. | 0.3 wet wt. | less than LOQ | Russell et al. 2008 |
| Bull shark muscle, USA, East Coast of Florida | 1993-1994 2002-2004 |
6 7 |
n.s. | 5.17 [4.17-8.07] 13.2 [0.035-35.6] |
Johnson-Restrepo et al. 2008 |
| Atlantic sharpnose shark muscle, USA, Florida | 2004 | 3 | n.s. | 0.9 [0.5-1.4] | Johnson-Restrepo et al. 2008 |
| Harbor porpoise blubber, United Kingdom |
1994-2003 | 18/68 | n.s. | 6-35 (w.wt) | Law et al. 2006 |
| Harbor porpoise blubber, United Kingdom |
2003-2006 | 0/138 | n.s. | n.d. | Law et al. 2008 |
| Fish, Japan | - | 29/45 | - | [0.01-0.11 w.wt] (0.01-0.11 ng/g wet wt) |
Ashizuka et al. 2008 |
| Fish, Japan, Marine products from food market stores of 3 regions (Nagoya, Seto Inland Sea, Kyushu) | 2004-2005 | 45 | n.d. | 0.02 [n.d.-0.11] |
Nakagawa et al. 2006 |
| UK 2004 Total Diet Survey Data, Shellfish, oysters, mussels, scallops, Scotland | 2004 | 0/35 | 0.05 | n.d. | Driffield et al. 2008 |
| Food- Fourth Total Diet Study, China n=12 Provinces | 2007 | 48 | n.s. | [ less than LOD- 2.0] ( less than LOD- 2044 pg/g lipid) |
Shi et al. 2009b |
| Cow’s milk, Ireland | 2006 | 0/5 composite samples of 3 individual milk samples each | 0.2 | n.d. | Grümping et al. 2007 |
| Whole fish, fish muscle, Norway | 2003 | 16 | n.s. | [n.d.-9.0] (n.d.-9.0 ng/g lipid) |
Fjeld et al. 2004 |
| Cod liver, Norway | 2003 | 6 | n.s. | n.d. – 3.0 (n.d.-3.0 ng/g lipid) |
Fjeld et al. 2004 |
| 121 Food categories, UK | 2001 | 0/n.s., but expected to be hundreds | 1.4 to 30 | n.d. | Food Standards Agency 2004 |
| Eel, hatched and imported | 2002 | 3 | 0.1 ng/g wet wt | 0.2-3.4 ng/g wet wt | de Winter-Sorkina et al. 2003 |
| Herring | 2001 | 2 | 0.12 ng/g wet wt | nd and 0.6 ng/g wet wt | de Winter-Sorkina et al. 2003 |
| Whiting fish, North Sea |
1999 | 3 | 0.5 | 136 [ less than 0.97 -245] |
Morris et al. 2004 |
| Eel, rivers in Netherlands | 1999 | 11 | 0.5 | 0.3 [ less than 0.1-1.3] |
Morris et al. 2004 |
| Eel, Scheldt basin, Belgium | 2000 | 19 | 0.5 | 1.6 [ less than 0.1-13] |
Morris et al. 2004 |
| Harbour porpoise blubber, North Sea | n.s. | 4 | 0.5 | less than 11 | Morris et al. 2004 |
| Harbour porpoise blubber, UK | 1998 | 5 | 0.5 | 83 [0.1-418] |
Morris et al. 2004 |
| Blue mussel, Norway |
2002 | 6 | n.s. | 0.021 ng/g wet wt 0.01-0.03 ng/g wet wt |
Schlabach et al. 2002 |
| Cod liver, Norway | 2002 | 6 | n.s. | 0.11 ng/g wet wt 0.08-0.16 ng/g wet wt |
Schlabach et al. 2002 |
| Fish, 24 areas in Japan | 1987 – 2000 | 0/237 | 1 and 20,000 (0.001 and 20 μg/g) |
n.d. | MOE Japan 2003 |
| Hard cheese | 2002 | 2 | 0.1 ng/g cheese | 0.06 and 0.09 ng/g cheese | de Winter-Sorkina et al.2003 |
| Cow’s milk from Oslo, Norway | 2001 | 1 | 5 x 10-4 (0.5 pg/g milk) |
0.013 (13 pg/g) |
Thomsen et al. 2002a |
| Porpoises, multiple locations, UK |
1999-2001 | 4/8 | 1.1 μg/kg wet weight | (n.d. – 376 μg/kg wet weight) | Law et al. 2003 |
| Freshwater fish, Germany | 1998-1999 | 2 | 8.5 pg/μl for diacetyl TBBPA | 0.91 and 1.12 (0.91 and 1.12 ng/g) |
Kemmlein 2000 |
| Eel, Germany | 1998-1999 | 2/8 | 8.5 pg/μl for diacetyl TBBPA | 0.47 and 0.78 (0.47 and 0.78 ng/g) |
Kemmlein 2000 |
Appendix 7: TBBPA Levels in Human Milk
| Location | Sampling Period | No. of Samples | Detection Limit | Mean concentration (ng/g lipid) | Reference |
|---|---|---|---|---|---|
| Boston, MA (USA) | 2004-2005 | 43 | LOQ 30 pg/g lipid weight | Not reported due to low detection frequency | Carginan et al. 2012 |
| China | 2007 | 24 pooled | n.s | [ less than LOD-5.1] Levels in 75% of samples were less than 1 ng/g lw |
Shi et al. 2009b |
| France | 2004-2006 | 34/77 | LOQ less than 0.05 | 4.1 [0.06-37.3] |
Cariou et al. 2008 |
| France, mother/newborn dyads | 2005 | 23 | n.s. | 0.17 (median) 0.03-9.4 |
Antignac et al. 2006 |
| Pooled samples, Norway | 2001 | 1 | 5 x 10-4 | 0.067 (67 pg/g lipid) |
Thomsen et al. 2002a |
| Pooled and single samples, Germany | 1998-1999 | 2/10 | 8.5 pg/μl for diacetyl TBBPA | [0.29 -0.94] | Kemmlein 2000 |
| Pooled samples, Germany | Archived 1990 | 0/5 | 8.5 pg/μl for diacetyl TBBPA | n.d. | Kemmlein 2000 |
| Single sample, Faroe Islands, Denmark | Archived 1990 | 1 | 8.5 pg/μl for diacetyl TBBPA | 11.0 ng/g lipid | Kemmlein 2000 |
Appendix 8: TBBPA Levels in Human Serum and Adipose Tissue
| Location | Sampling Period | No. of Samples | Detection Limit | Mean concentrationFootnote Appendix 8 Table 11.7 (ng/g lipid) |
Reference |
|---|---|---|---|---|---|
| Belgium | 2008-2-11 | 515 | 0.015 ng/ml | Mean: less than LOQ P95: 0.022 ng/ml Max: 0.186 ng/ml |
Kicinski et al. 2012 |
| Alberta’s Biomonitoring Program | Jan-Dec 2005 | 0/50,599 | 0.03 ng/g serum | n.d. | Alberta Health and Wellness 2008 |
| Canadian Arctic, Nunavik (Northern Quebec) | Aug. to Oct. 2004 | 771 | 0.01 ng/ml | less than 0.01-0.48 ng/mL | Dallaire et al. 2009Footnote Appendix 8 Table 12.4 |
| Belgium | 7 in 2007, 14 pooled samples in 1999 |
7 14
|
0.05 ng/ml | 0.08 +/-0.02 ng/mL 0.09+/-0.03 ng/mL |
Dirtu et al. 20082 |
| France, Mothers | 2004-2006 | 29/91 | less than 0.05 | 19.9 +/-24.15 (0.23-93.22) |
Cariou et al. 2008 |
| France, Newborns | 2004-2006 | 27/90 | less than 0.05 | 103.5+/-149.73 (2.1- 649.45) |
Cariou et al. 2008 |
| France n=26 mother/newborn dyads | 2005 | 26 | n.s. | maternal serum mean=0.054) 30.4 ng/g lipid (cord serum mean= 0.152) |
Antignac et al. 2006 |
| Members of European Parliament | 2003 | 27/40 samples | n.s. | 0.33 ng/g whole blood (n.d. -330 pg/g whole blood) |
WWF 2004 |
| Computer technicians Sweden |
1999 | 8/10 | n.s. | [ less than 0.54-1.8] ( less than 1-3.4 pmol/g lipid) |
Jakobsson et al. 2002 |
| Pooled samples, Norwegian men, aged 40-50 yrs |
1977-1999 | 6 |
LOQ: 0.0004 ng/g plasma |
[n.d. – 0.65] | Thomsen et al. 2002b |
| Pooled samples, Norwegians aged birth to greater than 60 yrs | 1998 | 8 | LOQ: 0.0004 ng/g plasma | [0.31 - 0.71] | Thomsen et al. 2002b |
| Electronics dismantlers, Norway | n.s. | 39 | 0.0004 ng/g plasma | 1.3 [0.64 - 1.8] |
Thomsen et al. 2001 |
| Circuit board producers, Norway | n.s. | 50 | LOQ: 0.0004 ng/g serum | 0.54 [n.d. – 0.80] |
Thomsen et al. 2001 |
| Laboratory personnel | n.s. | 46 | 0.08 ng/g lipid (LOQ: 0.0004 ng/g serum) |
0.34 [n.d. – 0.52] 1 |
Thomsen et al, 2001 |
| Electronics dismantlers, Sweden | 1998 | 4 | n.s. | [0.41-1.3] (2.2 -7 pmol/g lipid) |
Hagmar et al. 2000 |
| Japanese men and women, aged 37- 49 yrs | 1998 | 8/14 | n.s. | median: 0.92 (920 pg/g lipid) [n.d. – 3.7] (n.d. – 3,700 pg/g lipid) |
Nagayama et al. 2000 |
| Location | Sampling Period | No. of Samples | Detection Limit | Mean concentration (ng/g lipid) |
Reference |
|---|---|---|---|---|---|
| France n=26 mother/newborn dyads | 2005 | 0/26 | n.s. | n.d. | Antignac et al. 2006 |
| France | 2004-2006 | 0/44 | less than 0.05 | n.d. | Cariou et al. 2008 |
| U.S.A. New York | 2003-2004 | 14/20 | 0.0033 | 0.048 +/- 0.102 ng/g l.wt [ less than 0.0033 – 0.464] |
Johnson-Restrepo et al. 2008 |
Appendix 9: Summary of health effects information for TBBPA and Derivatives
| Endpoint | TBBPA (CAS RN 79-94-7) |
TBBPA bis(allyl ether) TBBPA diallyl ether (CAS RN 25327-89-3) |
TBBPA bis (2-hyrdoxyethyl ether) TBBPA ethoxylated (CAS RN 4162-45-2) |
|---|---|---|---|
| % Similarity (ChemID) | 100% | 75% | 80% |
| Structure | 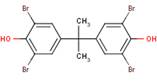 |
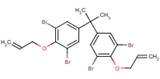 |
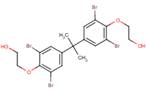 |
| Acute toxicity | LD50(oral, rat) greater than 50 000 mg/kg bw (International Bio-Research. Inc. 1967). LC50 (inhalation, rat) greater than 10,920 mg/m3 after 4 hr (Velsicol Chemical Corporation 1978e) (Decreased motor activity, eye squint, slight dyspnea and erythema). LD50 (dermal, rabbit) greater than 10 000 mg/kg bw (Hill Top Research, Inc. 1966). |
LD50(oral, rat) greater than 5000 mg/kg bw (Abbott et al. 1981). LD50 (dermal, rabbit) greater than 2000 mg/kg (Abbott et al. 1981). Slight to moderate erythema and edema. The skin reaction decreased in severity and area over time. No gross pathological findings were observed. |
LD50(oral, rat) greater than 5000 mg/kg bw (Goldenthal and Dean 1974a) LC50 (inhalation, rat) greater than 12.5 mg/L (12500 mg/m3) (Goldenthal and Dean 1974a) LD50 (dermal, rabbit) greater than 2000 mg/kg (Goldenthal and Dean 1974a) |
| Short-term Toxicity | Lowest oral LOAEL = 700 mg/kg-bw per day based on slight enlargement of hepatocytes, inflammatory cell infiltration and focal necrosis of hepatocytes at this dose and higher (Tada et al. 2007). Male ICR mice were administered 0, 350, 700 or 1400 mg/kg-bw per day TBBPA via gavage for 14 consecutive days. Absolute and relative liver weight was significantly increased at the highest dose. Other oral NOAEL (rats, 28 days) = 250 mg/kg-bw per day. A gavage study with Wistar rats showed no significant hepatic effects in the liver (Szymanska et al. 2000). The EU RAR (2006) noted that most of the few repeated-dose studies by oral exposure were limited or poorly reported (IRDC 1972; Sato et al. 1996; Szymanska 1995; Frydrych and Szymanska 2001). More recent studies by Germer et al. (2006) reported no effects upon hepatic mRNA or microsomes in Wister rats when exposed to dietary concentrations equivalent to intakes of 0, 30, 100 or 300 mg/kg-bw per day for 28 days. Lowest inhalation LOAEC (rats, 14 days) = 18 000 mg/m3 based on observations of local irritation in the upper respiratory tract (IRDC 1975). There were no toxicologically significant systemic effects (IRDC 1975). Lowest dermal NOAEL (rabbits, 3 weeks) = 2500 mg/kg-bw per day. No adverse effects were observed (IRDC 1979). |
No studies identified. | Lowest oral LOAEL = 1000 mg/kg/day based on increased bromine content in the liver. CD Rats (10/sex/group) were fed a diet containing 0, 100, or 1000 mg TBBPA bis (2-hydroxyethyl ether)/kg for 28 days. No changes in organ weights, pathological lesions, or histopathological changes were observed in liver, kidney, or thyroid of any animal (Goldenthal and Geil 1974b). |
| Sub-chronic toxicity | Lowest oral NOAEL (rats, 90 days) = 100 mg/kg bw per day (The Dow Chemical Company 1975). No gross or histopathological lesions were observed in rats exposed by diet to 0, 0.3, 3, 30 or 100 mg/kg-bw per day for 90 days. Other oral NOAEL (rats, 90 days) = 1000 mg/kg-bw per day (MPI Research 2002a). In a 13-week gavage study, rats were exposed to 0, 100, 300 or 1000 mg/kg-bw per day. There were no effects observed in either the functional observational battery or motor activity tests, no adverse histopathological changes in liver, thyroid, parathyroid or pituitary and no changes in serum levels of T3 or TSH. Although there was a significant decrease in serum T4 in both sexes, in the absence of any other relevant thyroid-related effects, this was not considered adverse. |
No studies identified. | No studies identified. |
| Chronic toxicity/ carcinogenicity | No studies identified. | No studies identified. | No studies identified. |
| Genotoxicity and related endpoints: in vitro | Negative: S.typhimurium strains (TA1535, TA 1537, TA1538, TA92 TA98, TA100) 0 to 10,000 μg/plate with and without metabolic activation (Litton Bionetics Inc 1976; Velsicol Chemical Corporation 1978a; Israel Institute for Biological Research 1978; Ethyl Corporation 1981; The Dow Chemical Company 1985; Mortelmans et al. 1986; Great Lakes Chemical Corporation 1986). Negative: in Yeast strains Saccharomyces cerevisiae D3 and D4 at TBBPA concentrations of 0 to 500 μg/plate with or without metabolic activation (Velsicol Chemical Corporation 1978a; The Dow Chemical Company 1985). |
Negative:Salmonella and Saccharomyces at 0.1 to 500 μg BE-51 applied per plate with and without metabolic activation (Brusick 1977). |
Negative: S.typhimurium strains (TA1535, TA 1537, TA1538, TA98, TA100) 0 to 1000 μg/plate with and without metabolic activation (Jagannath and Brusick 1979). |
| Genotoxicity: in vivo | No studies identified. | No studies identified. | No studies identified. |
| Developmental/Reproductive toxicity (post 2006) |
Lowest oral LO(A)EL (mice) = 140.5-379.9 mg/kg-bw per day based upon enlargement of hepatocytes, very slight focal necrosis of hepatocytes, and decreased serum triglyceride (quantitative data not presented) levels in female offspring at this dose (Tada et al. 2006). There was also an increase in total serum cholesterol (quantitative data not presented) in male offspring at this dose. Pregnant ICR mice fed a diet of 0%, 0.01%, 0.1%, or 1.0% TBBPA (0, 15.7-42.1, 140.5-379.9, or 1639.7-4155.9 mg/kg bw) from GD 0 to weaning at PND 27. Serum concentrations of total-cholesterol and triglycerides were also affected in dams and offspring. No effects upon litter size, litter weight, total number of offspring male or female offspring weight. The lowest benchmark dose, BMDL = 0.5 mg/kg-bw per day for increased F1 testis weight (critical effect dose of 1.4 mg/kg-bw per day) and increased F1 male pituitary weight (critical effect dose, 2.2 mg/kg-bw per day; BMDL, 0.6 mg/kg-bw per day). Wistar rats were administered TBBPA with calculated intakes of 0, 3, 10, 30, 100, 300, 1000 or 3000 mg/kg-bw per day in diet for 70 days (male) or 14 days (female) prior to mating and continuing during mating and throughout gestation and lactation (van der Ven et al. 2008; Lilienthal et al. 2008). There were no effects upon endpoints of reproduction. Other effects noted included delayed sexual development in females, and effects upon brainstem auditory evoked potentials. There were no exposure-related histopathological changes in the organs of the F1 animals. There were no effects upon sperm counts or morphology. There was no effect upon the immunisation response against sheep red blood cells in F1 males. Another “major effect” was the developmentally induced increase of hearing latency at low frequency, with BMDLs of 7.8 and 8.4 mg/kg-bw per day for males and females, respectively. It is noted that concerns have been published concerning the methodology employed in this assay (Banasik et al. 2009; Strain et al. 2009; Lilienthal et al. 2009; van der Ven et al. 2009). A previous study was performed on male Wistar rats which were administered TBBPA with calculated intakes of 0, 30, 100, or 300 mg/kg-bw per day in diet for 28 days. The only effects were a decrease in circulating T4 and increased T3 levels in male rats. Lowest oral LOAEL (rats) = 200 mg/kg bw per daybased on polycystic lesions associated with the dilation of the tubules in the kidneys of 2 of 6 males at this dose (Fukuda et al. 2004). Newborn rats were dosed by gavage from days 4 to 21 after birth, at doses of 0, 40, 200 or 600 mg/kg-bw per day. Effects on the kidney were observed at the two highest doses. Diarrhea observed in some male and some females treated with 200 and 600 mg/kg bw. In the same study, five-week old rats were dosed at levels of 0, 2000 or 6000 mg/kg-bw per day for 18 days. No similar histopathological renal effects were observed. The EU RAR (2006) selected the NOAEL of 40 mg/kg bw per day for the purpose of risk characterization. However, it is considered that the effect on the kidneys is the result of “unconventional direct gavage administration of very high doses of TBBPA to such young animals and the immature metabolic capability and/or immature kidneys. Therefore, the relevance to human health of this isolated finding is considered questionable”. Other oral LOEL = 100 mg/kg-bw per day based on a decrease in serum T4 levels in F0 and F1 offspring at this does and higher (MPI Research 2002b, 2003). Sprague Dawley rats were administered 0, 100, 200 or 1000 mg/kg-bw per day gavage for 10 weeks premating, 2 weeks mating and for females, throughout gestation and lactation. Serum T3 levels decreased significantly in F0 males in the 1000 mg/kg-bw dose group. No effects in either the F1 or F2 pups with regard to body weight, clinical findings, sex ratios, survival to weaning, macroscopic findings or organ weights. It was concluded that the effects in T3 and T4 levels were not toxicologically significant, as there was little impact on other parameters (MPI Research 2002b, 2003). Other oral LOEL = 818.9-2129.2 mg/kg-bw per day based on decreased relative uterine weight in female offspring at post-natal week 11 at this dose (Saegusa et al. 2009). No other histopathological changes were observed in the uterus or any other organs examined. Pregnant Sprague-Dawley rats were exposed to dietary levels of 0, 100 ppm (9.5 – 22.9 mg/kg-bw per day), 1000 ppm (86.8 – 202.1 mg/kg-bw per day) or 10,000 ppm (818.9 – 2129.2 mg/kg-bw per day) from gestational day 10 to post natal day 20 after delivery (weaning). TBBPA did not alter normal brain development. Relative kidney weights decreased significantly at 1000 ppm dose level, but not at the higher dose in female offspring. There were no significant dose-related effects on T3, T4 or TSH. No effects upon number of implantation sites, number of live offspring or sex ratio (Saegusa et al. 2009). A prepubertal exposure study examined the effects of TBBPA on susceptibility to thyroid tumours induced by a further exposure to DHPN or DMBA in Fisher 344 rats. Although the results of a complex exposure scenario are not taken into consideration in the assessment of TBBPA alone, the initial administration of 1% (1249 mg/kg-bw) TBBPA to dams from parturition to weaning (3 weeks) showed a statistically significant increase in thyroid weights and a decrease in relative liver weights (Imai et al. 2009). However, no histopathological changes were found in the liver and only one dam in 6 had diffuse follicular cell hyperplasia in the thyroid. No developmental or neurotoxicological effects were observed at doses up to 10,000 mg/kg-bw per day and 1000 mg/kg bw per day, respectively in various studies (Velsicol Chemical Corporation 1978c; Noda et al. 1985; Eriksson et al. 1998, 2001; MPI Research 2001, 2002b, 2003; Hass et al. 2003). |
No studies identified. | No studies identified. |
| Neurotoxicity in vivo | Acute oral LOEL = 11.5 mg/kg bw based on a decrease in binding sites of the nicotinic ligand cytisine in frontal cortex, but not in the parietal cortex or hippocampus in male neonate mice. Male NMRI mice (n=6-8) were given 11.5 mg TBBPA/kg bw (21 µmol) or 20% fat-emulsion vehicle/kg bw on PND 10 via a metal gastric-tube, as one single oral dose. The animals were killed 7 days after treatment and protein levels of CaMKII, GAP-43, synaptophysin and tau in hippocampus and cortex were measured using slot-blot analysis. [3H]-QNB binding (all subtypes of muscarinic receptors), [3H]-AFDX 384 binding (M2/M4 muscarinic receptors) and [3H]-cytisine binding (α4β2 nicotinic receptors) were assessed in the hippocampus, parietal and frontal cortex. The statistical evaluation was made using one-way ANOVA and pairwise testing using Newman–Keul’s post hoc test. TBBPA did not appear to affect the levels of proteins involved in maturation of the brain, neuronal growth or synaptogenesis in neonate mice. However, there was a decrease in binding sites of the nicotinic ligand cytisine in frontal cortex, (35.9 ± 10.4 pmol/g protein compared to 47.2 ± 7.7 pmol/g protein in controls), but not in the parietal cortex or hippocampus (Viberg and Eriksson 2011). Oral LOEL = 86.8-202.1 mg/kg bw/day based on a transient increase in reelin-expressing interneurons in the dentate hilus at this dose and above in offspring at PND 20, but not at PND 77. Pregnant Sprague–Dawley rats (n = 8/group) were exposed to 0, 100, 1000, or 10 000 ppm (0, 9.5 – 22.9, 86.8 – 202.1, 818.9 – 2129.2 mg/kg-bw per day) TBBPA in the diet from GD 10 through to day 20 after delivery (PND77). No major treatment-related changes were observed in dams during gestation and lactation. There were no dose-related changes in thyroid serum levels (See reproductive/developmental section above; Saegusa et al. 2009) and no effects on organ to body weight changes in the brain or the thyroid of offspring. A slight increase in apoptotic bodies in offspring (n ~ 20/sex/group) was observed at 818.9 – 2129.2 mg/kg-bw per day at PND 20, but this effect appeared reversible as there were very few apoptotic bodies at PND 77. An increase in reelin-expressing interneurons in the dentate hilus was observed at the mid and high doses of TBBPA, but again, these effects returned to control levels at PND77. There was an excess of mature neurons in the hilus later stages, but these effects were reversible. No changes were observed in the number of GAD67- immunoreactive cells in the highest dose groups compared with the untreated controls at both PND 20 and PND 77 nor any changes in EphA5- and Tacr3-immunoreactive cells in the hippocampal CA1 region (Saegusa et al. 2012). Behavioural changes were observed in the two lowest dose groups of exposed male mice to 0, 0.1, 5 or 250 mg/kg-bw once by gavage, but were not considered to be treatment related. It is noted that the authors proposed that a compensation mechanism may account for the lack of a dose-response relationship (Nakajima et al. 2009). |
No studies identified. | No studies identified. |
| Neurotoxicity in vitro | Cytotoxicity: TBBPA appeared to be cytotoxic at low micromolar concentrations (LC50 = 15 ± 4 µM) on SH-SY5Y human neuroblastoma cells, however the authors stated that it is unclear from this study if these results showed that TBBPA is neurotoxic (Al-Mousa and Michelangeli 2012). TBBPA caused activation of caspases (3/7) after the cells were exposed to TBBPA for 12 hours at a 1 to 5 µM concentration range. There was also a transient increase in intracellular [Ca2+] levels and reactive-oxygen-species (ROS) within these neuronal cells. Furthermore, TBBPA also caused rapid depolarization of the mitochondria and cytochrome c release in these neuronal cells (at 10 µM). Application of 3 and 10 µM of TBBPA for 12hrs caused increased b-amyloid peptide (Ab-42) processing and release from these cells with a few hours of exposure (Al-Mousa and Michelangeli 2012). TBBPA was also found to be acutely cytotoxic in primary cultures of rat cerebellar granule cells after 30 minute exposures to 10-50 µM of TBBPA (significant at 25 uM). According to the authors, TBBPA also induced an increase in intracellular Ca2+ concentrations, depolarization of mitochondria, and activation of ROS production (Zieminska et al. 2012). |
Cytotoxicity: Environmental fractions of TBBPA bis(allyl ether) induced high cytotoxicity in neuronal cells of primary cultured cerebellar granule cells from 7 day old Sprague Dawley pups. Neurotoxicity was measured as cell viability compared to the positive control of paraquat. Liquid chromatography quadrupole time-of-flight mass spectrometry (LC-Q-TOFMS) and gas chromatography coupled electron capture negative ionization mass spectrometry (GC-ECNI-MS) was optimized to confirm TBBPA bis(allyl ether) as the key neurotoxicant. Human liver carcinoma Hep G2, human breast cancer MCF-7, and mouse leukemic monocyte macrophage RAW 264.7 cell lines were also used to investigate the non-neurotoxic potencies. Results showed that none of the sediment fractions or pure TBBPA bis(allyl ether) standard significantly affected the activity of these cell lines (For details see Qu et al. 2011). | No studies identified. |
| Endocrine effects in vivo (post 2006) |
Kitamura et al. (2005b) exposed ovariectomized B6C3F1 mice to 0, 20, 100, 300 and 500 mg/kg bw of TBBPA by ip injection for three days. Although the uterus to body weight ratio was significantly increased in all exposed groups, there was a poor dose-response. Other oral LOEL: 150 mg/kg bw/day based on a decrease in T3-independent transcription activation of both Trh and Mc4r promoter genes in the hypothalamus of offspring on PND 2 (n greater than 10/group). Pregnant Swiss wild-type mice (n greater than 10/group) were administered 150 mg/kg bw of TBBPA daily via oral gavage from day 13 post conception for 7 days. The activity of Trh and Mc4r promoters was measured in the pup hypothalami using a reporter gene assay and TBBPA significantly reduced (P less than 0.001) T3-independent transcription from both Mc4r and Trh reporter constructs in pups. When pups were administered TBBPA in acute doses with one or two 2.1 g/kg injections of TBBPA, opposite effects were observed on T3-independent transcriptional activity of both promoters. In protocol no. 1, a single injection 48 h before sacrifice decreased Mc4r and Trh transcription in absence of T3 by 32.4% and 33.6% respectively. In contrast, administering TBBPA injections 48 and 24 h before sacrifice significantly increased transcription from both promoters: 28.7% and 37.5% for Mc4r and Trhconstructs, respectively (Decherf et al. 2010). Other oral NOEL: 1000 mg/kg bw/day based on the lack of change in uterine weight in adult female mice. TBBPA was administered daily via oral gavage and subcutaneous injection for 7 days using C57BL/6J ovariectomized adult female mice (n = 6) in accordance with OECD Test Guideline No. 440. For detection of agonistic activity, control, 4 doses at a half-log ratio, and EE as a positive control were tested. For antagonistic activity, a control and the same 4 doses were administered with a reference dose of EE. The LOEL was defined as the lowest dose that induced significant change in uterine weight. Results from this study showed that TBBPA was negative for agonistic and antagonistic estrogenic responses by both routes of exposure using concentrations up to 1000 mg/kg bw/day (Ohta et al 2012). |
No studies identified. | No studies identified. |
| Endocrine effects in vitro (post 2006) |
Strong PR antagonist/Weak ERα agonist: Li et al. (2010) measured ER and PR-mediated transcription of β-galactosidase in vitro in reporter yeasts. TBBPA also exhibited the ability to reverse the estrogen-related receptor (ERR) inhibition induced by 4-hydrooxytamoxifen. Yeast strains were tested with increasing concentrations of TBBPA (1 x 10-9to 1 x 10-4 mol per L) for 2hrs. Estrogenic activity of TBBPA was evident using ERE-luciferase reporter assay in MCF-7 breast cancer cells at 1x10-6 to 1x10-4 M. Anti-estrogenic acitivty was also measured in an E2 assay sysem in MCF-7 cells at 1x10-5 M (Kitamura et al. 2005b). Negative PR antagonist/ Negative ER agonist: No detectable antiprogestagenic or ER agonistic potency was measured in ER- and PR-CALUX assays in human breast cancer (ER) and human osteoblast (PR) cells at 10 and 12.5 µM concentrations, respectively (Hamers et al. 2006). No ER agonistic/antagonistic effects were found by Riu et al. (2011) using HGELN, HGELN-ERα, HGELN-ERβ reporter cell lines (10-9 to 10-5M). No effect was observed on cell growth in an E-screen assay in MCF-7 breast cancer cells even at a maximum concentration of 20 µM TBBPA did not induce estrogen receptor-mediated TFFl gene expression in vitro (Dorosh et al. 2010). TBBPA did not show any estrogenic activity at the estrogen receptor alpha (ERα) ligand at concentrations between 10-10 and 10-5 M in an OECD guideline stably transfected transcriptional activation (STTA) assay (Lee et al. 2012). Positive Inhibitor of E2 Sulfation: Hamers et al. (2006) reported that TBBPA was a potent inhibitor of E2 (estradiol) sulfation (IC = 0.016 uM) in an E2SULT assay. Positive competitor of T4 binding to TTR: TBBPA was a potent T4 competitor in a TTR-binding assay (IC50 less than 0.1 µM) with a 1.6 times higher TTR-binding potency than the natural ligand T4 (Hamers et al. 2006). Conflicting Thyroid Hormone agonist/antagonist: Administration of 25 µM TBBPA significantly suppressed TRβ activity (IC50 of 2.95 x 10-5 M). Authors developed a TRβ-1 mediated reporter gene assay by transiently transfecting Gal4-fused thyroid hormone receptor (TR) expressing vector and the Gal4 response reporter structure pUAS-tk-luc into HepG2 cells (1, 10, 25, 50 and 100 µM). When treated alone, TBBPA could not induce the expression of luciferase which indicated that it could not activate TR (Sun et al. 2009). Another TH-responsive luciferase–based reporter gene assay using the human hepatocarcinoma cell line HepG2 showed that TBBPA displayed agonistic effects at 10 µM, but was antagonistic when coincubated with T3 at 1 µM (concentrations ranged from 10-4 to 10 µM for 24 h; Hofman et al. 2009). TBBPA bound to the human TRα-LBD, activating transcription when applied alone (without T3) at 3 and 10µM. Further, TBBPA displaced physiological concentrations of T3 from TRα binding in HeLA cells using a reporter system based on fusion of the ligand-binding domain (LBD) from TRα to a GAL4 DNA– binding domain at 10 µM (Fini et al. 2012). TBBPA prevented binding (or induced dissociation) of NcoRp, but failed to promote SRC2p binding, and even inhibited T3-induced SRC2p binding in a coactivator/corepressor peptide binding assay. Increasing concentrations (2–50 µM) of TBBPA were incubated with GST-LBD and FITC-labeled corepressor peptide (NcoRp) or coactivator peptide (SRC2p) in the presence and absence of T3 (cell-free) (Lévy-Bimbot et al. 2012). At concentrations as high as 10 µM, TBBPA did not affect transcriptional activities of TRα and TRβ in any of the species studied (three frog species (Xenopus laevis, Silurana tropicalis and Rana rugosa), a fish (Oryzias latipes), an alligator (Alligatormississippiensis) and human (Homo sapiens). In order to examine whether T3-stimulated activity was inhibited by TBBPA, T3 was added at a concentration that generated an EC50 value for each receptor type (TRα: 4x10–10 M and TRb: 2x10–9 M). Results indicated that TBBPA inhibited T3-stimulated activation of TRα and TRβ from S. tropicalis andhuman cells at concentrations higher than 10µM (however, the cell viability after treatment with TBBPA was compromised at doses higher than 10 µM). Therefore, TBBPA can be evaluated at this concentration or less in this assay, which showed no effects on activity in human cells in vitro. Using this approach, authors noted that T3-induced transactivity of the O. latipes TRα was inhibited by 10 µM TBBPA, but this compound at this concentration did not antagonize TRβ activity (Oka et al. 2012). Deiodinase Inhibition: Almost complete inhibition of DI activity was observed at the highest dose tested (2.1µM) for TBBPA. A dose-response relationship was shown for TBBPA in inhibition of the formation of rT3 from T4. Inhibition of 3,3’-T2 formation was also observed at 1.9 µM. Thyroxine (T4) and reverse triiodothyronine (rT3) deiodination kinetics were measured by incubating pooled human liver microsomes with T4 or rT3 and monitoring the production of T3, rT3, 3,3’-diiodothyronine, and 3-monoiodothyronine by liquid chromatography tandem mass spectrometry (Butt et al. 2011). Equivocal AR antagonist: Weak antiandrogenic activity in MDA-kb2 cells in vitro between 10 and 50 µM for 24 hrs followed by a luciferase assay (Christen et al. 2010). Li et al. (2010) and Hamers et al. (2006) did not find any effect on AR activity in yeast cells (concentration not listed) or in human osteoblast cells at 10 µM concentrations using an AR-CALUX assay, respectively, after administration of TBBPA. No antiandrogenic activity was found in an AR responsive luciferase reporter gene system in NIH3T3 cells at 1x10-11 to 1x10-9 M concentration range (Kitamura et al. 2005b). Negative aromatase activity: TBBPA did not inhibit or induce aromatase (CYP19) activity and was not cytotoxic at concentrations of 2.5 µM and 7.5 µM in H295R human adrenocortical carcinoma cells after 24 hr incubation (Cantón et al. 2005). |
No studies identified. | No studies identified. |
| Sensitization | The EU RAR (2006) concluded that TBBPA is neither a skin nor a respiratory sensitizer. | No studies identified. | No studies identified. |
| Irritation | Not irritating: In skin (Hill Top Research 1966; Pharmakon Laboratories 1981a; Israel Institute for Biological Research 1978; IRDC 1979; EU RAR 2006) or eyes (Pharmakon Laboratories 1981b; Israel Institute for Biological Research 1978; Hill Top Research 1966; EU RAR 2006). | Mildly irritating: In eyes and skin of New Zealand Albino rabbits after applications of 100 mg and 500 mg of BE-51, respectively (Abbott et al. 1981). | Not irritating: In eyes or skin of New Zealand Albino rabbits after applications of 100 mg and 500 mg of TBBPA bis (2-hydroxyethyl ether), respectively (Goldenthal and Dean 1974a). |
| Special Studies | Immunotoxicity in vitro: Exposure of 0 to 10 µM of TBBPA to human natural killer cells decreases the lytic function at concentrations as low as 0.5 µM after 24 hrs exposure and 1.0 µM after 1 hr exposure to TBBPA (Kibakaya et al. 2009). A follow up study showed that the expression of cell-surface proteins CD2, CD11a, CD16, CD18 needed for attachment of NK cells to target cells was decreased after exposure to 5 µM TBBPA for 24 hr (Hurd and Whalen 2011). NK cells were exposed to TBBPA (0, 1, 2.5, 5 and 10 µM) for 24h, 48h, and 6 days or for 1 hr followed by 24h, 48 hr, and 6 days without TBBPA. Each experiment was completed four times using cells prepared from different blood donors. CD16 expression was decreased by greater than 35%, CD11a by 16%, and CD18 and CD56 by ~ 20%. Expression of CD18 was also decreased (14%) at 2.5 µM. Cell viability was compromised at 10 µM TBBPA (at all timepoints) and at 5 µM after 48 hrs and not evaluated. Expression of CD16 and CD56 proteins were similar at 24 and 48 hrs at 2.5 µM (Hurd and Whalen 2011). Han et al. (2009) reported that exposure to TBBPA (0- 50 µM) induced COX-2 transcription and proinflammatory cytokine expression through the P13-K/Akt/MAPK signalling pathway mediated via increased NF-κB and AP-1 activation in murine macrophages in vitro. |
Immunotoxicity in vitro: Mice splenocytes incubated with a TBBPA allyl ether (10 µmol/L) exhibited significantly reduced expression of the interleukin-2 receptor α chain (CD25), an antigen necessary for the production of activated T cells during an immune response (Pullen et al. 2003). Exposure of 2 uM and higher concentrations of TBBPA to human neutrophil granulocytes enhanced reactive oxygen species (ROS) production in a concentration dependent manner (Reistad et al. 2005). Mice splenocytes incubated with a TBBPA (3 µmol/L) exhibited significantly reduced expression of the interleukin-2 receptor α chain (CD25), an antigen necessary for the production of activated T cells during an immune response (Pullen et al. 2003). TBBPA showed no cytotoxic effect on either splenocytes or bone marrow-derived dendritic cells (BMDCs) from atopic prone NC/Nga mice. After exposure to TBBPA, cell surface molecule expression and cytokine production was measured using WST-1 assay, FACS and ELISA (three individual cultures obtained from three animals and two or three independent experiments were repeated). Spenocytes were exposed to 0.01–10 µg/ml TBBPA for 24 hrs and BM cells were exposed to 0.001 – 1 µM TBBPA for 6 days and BMDCs were collected. TBBPA did increase MHC class II and CD86 expression, and T-cell receptor (TCR) expression in splenocytes (1 and 10 µg/ml) and increased interleukin (IL)-4 production (but a chemical-dependent impact pattern was observed). There was no effect on expression of MHC class II, CD80, CD86, CD11c and DEC205) and chemokine production after 24-h exposureafter TBBPA exposure in BMDCs (Koike et al. 2012). Immunotoxicity in vivo: Exposure in mice to 1% TBBPA in the diet for 28 days (1887 mg/kg-bw per day) demonstrated that host immunity to respiratory syncytial virus was affected in lung and BALF samples while systemic immunity was not affected when spleen samples were examined (Watanabe et al. 2010). Hepatoxicity in vitro: TBBPA competitively inhibited the binding of [3H]-rosiglitazone to PPARγ with a half maximal inhibitory concentration (IC50) of 0.7 µM. HeLa cells transiently transfected with (GALRE)5-βglobin-luciferase and pSG5-GAL4-PPARγ (human and zebrafish) or (PPRE)3-TK-luciferase and pSG5-PPARγ (Xenopus laevis) plasmids were incubated with 10 μM TBBPA or 1 μM rosiglitazone (Rosi) to assess their agonist potential on PPARγ. The binding affinities to PPARγ by competitive binding assays with [3H]-rosiglitazone were measured (0.001–30 μM) and the impact of TBBPA on adipocyte differentiation using NIH3T3-L1 cells was investigated. At 10 μM, TBBPA also induced adipogenesis, whereas co-treatment with CD5477 inhibited the adipogenic action of TBBPA, indicating that TBBPA mediates adipogenesis via PPARγ (Riu et al 2011). Alternatively, when the ability of TBBPA was tested to activate human PXR or mouse PXR using a transfection assay, results were negative (Siu et al. 2012). HepG2 cells were transfected with either full-length hPXR together with CYP3A4-luc reporter or full-length mPXR together with (CYP3A2)3-luc reporter and CMX-ß-galactosidase control plasmid. Cells were treated with DMSO (control) or TBBPA for 24 h at 0-20 µM concentrattions. |
| Sensitization/ Irritation | Not sensitizing: TBBPA did not result in skin sensitization with humans in a multiple insult test after approximately 3 to 5 mg of TBBPA (50-70% concentration) was applied to 54 volunteers (IRDC 1978). | No studies identified. | No studies identified. |
| Toxicokinetics | Blood serum levels ranged from greater than 0.5 to 3.8 µg/kg lipid in various occupational groups in several studies in Sweden and Norway (EU RAR 2006). Levels in breast milk ranged from 0.01 to as high as 11 µg/kg lipid in one case in the Faroe Islands (EU RAR 2006). Five human subjects were administered a single oral dose of 0.1 mg/kg TBBPA. Two major metabolites of TBBPA, TBBPA glucuronide (5 of 5 subjects) and TBBPA-sulfate (2 of 5 subjects), were found in urine and blood samples while the parent TBBPA was not present in detectable levels in any of the human plasma samples. TBBPA-glucuronide plasma levels reached peak concentrations between 2 and 6 hrs after administration and was slowly eliminated in urine to reach the LOD 124 hrs after administration. TBBPA-sulfate (found in 2 of the 5 human subjects) was measured between 4 and 6 hrs after administration as was below the LOD in urine. The authors suggest that the major role of enterohepatic circulation is indicated by the slow elimination of TBBPA-glucuronide in urine in both humans and rats and this, along with efficient hepatic metabolism, would result in low systemic bioavailability of TBBPA in humans (Schauer et al. 2006). |
No studies identified. | No studies identified. |
Appendix 10: Supplementary Data from van der Ven et al. (2008)
| Group (mg/kg-bw per day) |
Body weight (g) | Testis weight (g) | Organ to body weight ratio (presented by Health Canada) |
|---|---|---|---|
| Control | 414 | 3.01 | 0.0073 |
| 3 | 433 | 3.25 | 0.0075 |
| 10 | 453 | 3.48 | 0.0077 |
| 30 | 461 | 3.50 | 0.0076 |
| 100 | 478 | 3.55 | 0.0074 |
| 300 | 454 | 3.56 | 0.0078 |
| 1000 | 461 | 3.41 | 0.0074 |
| 3000 | 472 | 3.32 | 0.0070 |
Critical effect dose, 1.4 mg/kg-bw per day (BMDL, 0.5 mg/kg-bw per day)
| Group (mg/kg-bw per day) |
Body weight (g) | Pituitary weight (g) | Organ to body weight ratio (presented by Health Canada) |
|---|---|---|---|
| Control | 414 | 0.011 | 0.000027 |
| 3 | 433 | 0.012 | 0.000028 |
| 10 | 453 | 0.014 | 0.000031 |
| 30 | 461 | 0.016 | 0.000035 |
| 100 | 478 | 0.014 | 0.000029 |
| 300 | 454 | 0.017 | 0.000037 |
| 1000 | 461 | 0.013 | 0.000028 |
| 3000 | 472 | 0.016 | 0.000034 |
Critical effect dose, 2.2 mg/kg-bw per day (BMDL, 0.6 mg/kg-bw per day)
| Group (mg/kg-bw per day) |
TT4 (nmol/L) (females) |
TT4 (nmol/L) (males) |
|---|---|---|
| Control | 34.3 | 53.4 |
| 3 | 33.5 | 40.7 |
| 10 | 38.0 | 45.7 |
| 30 | 41.2 | 47.6 |
| 100 | 27.1 | 43.0 |
| 300 | 23.2 | 31.5 |
| 1000 | 22.2 | 26.5 |
| 3000 | 18.4 | 27.9 |
Critical effect dose females: 35.6 mg/kg-bw per day (BMDL, 16.1 mg/kg-bw per day)
Critical effect dose, males: 99.5 mg/kg-bw per day (BMDL, 30.8 mg/kg-bw per day)