Appendix of the Screening Assessment for the Challenge
This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Archived
Rosin, hydrogenated
Chemical Abstracts Service Registry Number
65997-06-0
Resin acids and Rosin acids, hydrogenated, esters with pentaerythritol
Chemical Abstracts Service Registry Number
64365-17-9
Resin acids and Rosin acids, hydrogenated, esters with glycerol
Chemical Abstracts Service Registry Number
65997-13-9
Resin acids and Rosin acids, hydrogenated, esters with triethylene glycol
Chemical Abstracts Service Registry Number
68648-53-3
Environment Canada
Health Canada
January 2011
Appendix
- Appendix 1: Robust Study Summary
- Appendix 2a: Analogue Comparison of HR with Rosin for Ecological Hazard Estimation.
- Appendix 2b: Analogue Comparison of HRPE and HRGE with RPE for Ecological Hazard Estimation.
- Appendix 2c: Analogue Comparison of HRTE with RDE for Persistence Estimation
- Appendix 3a: Upper-BoundingEstimates of Daily Intakes of HR for Various Age Groups
- Appendix 3b: Upper-BoundingEstimates of Daily Intakes of HRPE for Various Age Groups
- Appendix 3c: Upper-BoundingEstimates of Daily Intakes of HRGE for Various Age Groups.
- Appendix 3d: Upper-BoundingEstimates of Daily Intakes of HRTE for Various Age Groups
- Appendix 4: Upper-Bounding Exposure Estimates for HR, HRPE, HRGE and HRTE for Consumer Product Scenarios using ConsExpo v4.1 (ConsExpo 2006)
- Appendix 5: Summary of Health Effects Information for HR (CAS RN 65997-06-0), HRGE (CAS RN 65997-13-9) and HRPE (CAS RN 64365-17-9).
- Appendix 6: Summary of Health Effects Information for Analogues Rosin (CAS RN 8050-09-7), Resin acids and Rosin acids, esters with glycerol (RGE)(CAS RN 8050-31-5) and Resin acids and Rosin acids, esters with pentaerythritol (RPE) (CAS RN 8050-26-8)
- Appendix 7a: PBT Model Inputs Summary Table for HR
- Appendix 7b: PBT Model Inputs Summary Table for HRPE
- Appendix 7c: PBT Model Inputs Summary Table for HRGE
- Appendix 7d: PBT Model Inputs Summary Table for HRTE
Robust Study Summaries: Aquatic iT
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 1 | Reference: Solubility and Toxicity of Resin Acids, G. Peng and J.C. Roberts, 2000, Water Research, Vol. 4, No. 10, pp. 2779-2785. | |||
| 2 | Substance identity: CAS RN | n/a | N | |
| 3 | Substance identity: chemical name(s) | n/a | Y | |
| 4 | Chemical composition of the substance | 2 | Y | |
| 5 | Chemical purity | 1 | Y | Study evaluated the concentration of resin acids in standard solutions using GC-FID |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | Y | Solubility over 72 hrs was monitored by GC-FID |
| Method | ||||
| 7 | Reference | 1 | Y | ISO 6341 and British standard BS 6068 |
| 8 | OECD, EU, national, or other standard method? | 3 | Y | |
| 9 | Justification of the method/protocol if not a standard method was used | 2 | n/a | |
| 10 | GLP (Good Laboratory Practice) | 3 | N | |
| Test organism | ||||
| 11 | Organism identity: name | n/a | Y | D. Magna |
| 12 | Latin or both Latin & common names reported? | 1 | Y | |
| 13 | Life cycle age / stage of test organism | 1 | Y | Less than 24 hours old |
| 14 | Length and/or weight | 1 | n/a | |
| 15 | Sex | 1 | n/a | |
| 16 | Number of organisms per replicate | 1 | Y | Five |
| 17 | Organism loading rate | 1 | N | |
| 18 | Food type and feeding periods during the acclimation period | 1 | N | |
| Test design / conditions | ||||
| 19 | Test type (acute or chronic) | n/a | Y | Acute |
| 20 | Experiment type (laboratory or field) | n/a | Y | Laboratory |
| 21 | Exposure pathways (food, water, both) | n/a | Y | Water |
| 22 | Exposure duration | n/a | Y | 24 hours |
| 23 | Negative or positive controls (specify) | 1 | Y | Negative + dose response |
| 24 | Number of replicates (including controls) | 1 | Y | Six |
| 25 | Nominal concentrations reported? | 1 | N | |
| 26 | Measured concentrations reported? | 3 | N | |
| 27 | Food type and feeding periods during the long-term tests | 1 | n/a | |
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | N | |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for the metal toxicity - pH, DOC/TOC, water hardness, temperature) | 3 | Y | |
| 30 | Photoperiod and light intensity | 1 | N | |
| 31 | Stock and test solution preparation | 1 | Y | |
| 32 | Was solubilizer/emulsifier used, if the chemical was poorly soluble or unstable? | 1 | N | |
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | n/a | |
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | n/a | |
| 35 | Monitoring intervals (including observations and water quality parameters) reported? | 1 | N | |
| 36 | Statistical methods used | 1 | N | |
| Information relevant to the data quality | ||||
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by organism’s health (e.g. when mortality in the control >10%) or physical effects (e.g. 'shading effect')? | n/a | Y | |
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | |
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Y | |
| 40 | Does system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and organism's nature/habits? | 2 | Y | |
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | Y | |
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | |
| 43 | Was toxicity value below the chemical’s water solubility? | 3 | Y | |
| Results | ||||
| 44 | Toxicity values (specify endpoint and value) | n/a | n/a | |
| 45 | Other endpoints reported - e.g. BCF/BAF, LOEC/NOEC (specify)? | n/a | N | |
| 46 | Other adverse effects (e.g. carcinogenicity, mutagenicity) reported? | n/a | N | |
| 47 | Score: ... % | 66.7 | ||
| 48 | EC Reliability code: | 2 | ||
| 49 | Reliability category (high, satisfactory, low): | Satisfactory Confidence | ||
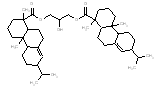
An analogue approach to estimate ecological hazard for HR was performed using Rosin (Chemical Abstracts Service Registry Number 8050-09-7) due to a lack of empirical data for HR. The analogue Rosin had a measured log D of 1.9 – 7.7 (pH = 2) and water solubility of 0.95 mg/L, which compared well to that of HR (log D of 2.5-7.6 and water solubility of 1.18 mg/L) (USEPA 2008a). In addition, a comparison of structural features, molecular weight and size is presented in the Table below. Overall, the bioavailability values of components in HR and Rosin are expected to be similar, thus making Rosin a good analogue for HR in this assessment.
Analogue comparison table for HR vs. Rosin.
| HR (CAS RN 65997-06-0) | Rosin (CAS RN 8050-09-7) | ||
|---|---|---|---|
| Log D (pH =2) | 2.5-7.6 | Log D (pH =2) | 1.9-7.7 |
| Water solubility (mg/L) |
1.18 | Water solubility (mg/L) |
0.95 |
| Representative structures | mw* (g/mol) / size (nm) | Representative structures | mw (g/mol) / size (nm) |
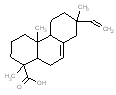 |
302.5 / 1.15-1.43 | 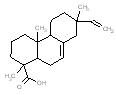 |
302.5 / 1.15-1.43 |
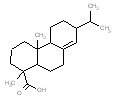 |
304.5 / 1.25-1.44 | 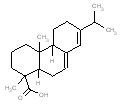 |
302.5 / 1.31-1.46 |
 |
306.5 / 1.23-1.44 |  |
302.5 / 1.29-1.46 |
An analogue approach to estimate ecological hazard for HRPE and HRGE was performed using Resin acids and Rosin acids, esters with pentaerythritol (RPE; Chemical Abstracts Service Registry Number 8050-26-8) due to lack of empirical data for HRPE and HRGE. The analogue RPE had a measured log Kow of 3.5-7.1 and water solubility of 0.38 mg/L, which compared reasonably well to that measured for HRPE and HRGE of log Kow 4.6-7.3 and 4.7-5.8 and water solubility of <0.22 mg/L and 0.15 mg/L (USEPA 2008b). In addition, a comparison of structural features, molecular weight and size is presented in the analogue comparison tables. Overall, the bioavailability of components in RPE and HRPE are expected to be similar, thus making RPE a good analogue for HRPE in this assessment work. Also, the bioavailability of components in RPE and HRGE may be similar enough for the analogue approach. Both RPE and HRGE are thought to be enriched in triester components of molecular weight 951.5 g/mol (1.98-2.72) and 988.7 g/mol (2.05-2.86) and thus, serve as close analogues to be used for the purposes of this assessment. Monoester components (<500 g/mol) which would be more bioavailable than the larger components also, show similarity between RPE (analogue) and HRGE, thus further supporting the notion that both substances would have a similar bioavailability. Finally, RPE would also contain a significant amount of tetraester (>1000 g/mol) which would not be present in the HRGE. However, it is not expected that that the overall bioavailability of the RPE substance would be influenced due to the larger molecular weight and size of this component.
Analogue comparaison table for HRPE vs RPE
| HRPE (CAS RN 64365-17-9) | RPE (CAS RN 8050-26-8) | ||
|---|---|---|---|
| Log Kow | 4.6-7.3 | Log Kow | 3.5-7.1 |
| Water solubility(mg/L) | <0.22 | Water solubility(mg/L) | 0.38 |
| Representatives structures | mw* (g/mol) / size (nm) | Representative structures | mw (g/mol) / size (nm) |
 |
422.6 / 1.36-1.84 |  |
420.3 / 1.30-1.90 |
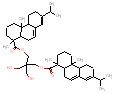 |
709.1 / 1.74-3.04 | 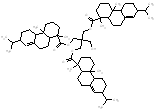 |
704.5 / 1.73-3.01 |
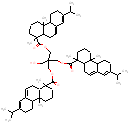 |
995.5 / 2.09-2.94 | 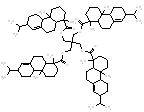 |
988.7 / 2.05-2.86 |
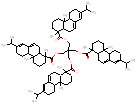 |
1282 / 2.28-2.88 | 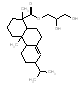 |
1273 / 2.21-3.03 |
Analogue comparaison table for HRGE vs RPE
| HRGE (CAS RN 65997-13-9) | RPE (CAS RN 8050-26-8) | ||
|---|---|---|---|
| Log Kow | 4.7-5.8 | Log Kow | 3.5-7.1 |
| Water solubility (mg/L) |
0.15 | Water solubility (mg/L) |
0.38 |
| Representatives structures | mw* (g/mol) / size (nm) | Representative structures | mw (g/mol) / size (nm) |
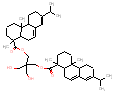
 |
378.6 / 1.27-1.90 |
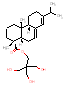
 |
420.3 / 1.30-1.90 |
 |
665.0 / 1.59-3.05 |
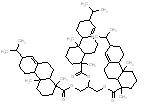 |
704.5 / 1.73-3.01 |
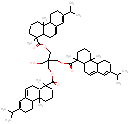 |
951.5 / 1.98-2.72 |
 |
988.7 / 2.05-2.86 |
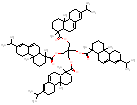
| - | - | 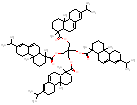 |
1273 / 2.21-3.03 |
An analogue approach to estimate environmental persistence for HRTE was performed using Resin acids and Rosin acids, esters with diethylene glycol (RDE; Chemical Abstracts Service Registry Number 68153-38-8) due to lack of empirical data on the persistence of HRTE. The analogue RDE had a measured log Kow of 4.0-5.8 and water solubility of <2.38 mg/L, which were some what similar to the values predicted for representatives structures of HRTE , that is log Kow >5.31 and water solubility of <0.62 mg/L. In addition, a comparison of structural features and molecular weight is presented in the table below.
Analogue comparison table for HRTE vs. RDE
| HRTE (CAS RN 68648-53-3) | RDE (CAS RN 68153-38-8) | ||
|---|---|---|---|
| Log Kow | ≥5.31 | Log Kow | 4.0-5.8 |
| Water solubility(mg/L) | <0.62** | Water solubility(mg/L) | <2.38 |
| Representatives structures | mw*(g/mol) / | Representative structures | mw (g/mol) / size (nm) |
 |
436.6 | 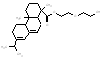 |
390.4 |
| |
723.1 |  |
648.8 |
** based on modelled data taken from Table 2d.
| Route of exposure | Estimated intake (µg/kg-bw per day) of HR by various age groups | |||||||
|---|---|---|---|---|---|---|---|---|
| 0–0.5 years1,2,3 | 0.5–4 years4 | 5–11 years5 | 12–19 years6 | 20–59 years7 | 60+ years8 | |||
| Breast milk fed | Formula fed | Not formula fed | ||||||
| Air9 | 9.6 × 10-5 | 9.6 × 10-5 | 9.6 × 10-5 | 2.0 × 10-4 | 1.6 × 10-4 | 9.1 × 10-5 | 7.8 × 10-5 | 6.8 × 10-5 |
| Drinking water10 | N/A | 4.4 × 10-3 | 1.6 × 10-3 | 1.9 × 10-3 | 1.5 × 10-3 | 8.4 × 10-4 | 8.8 × 10-4 | 9.2 × 10-4 |
| Food and beverages11 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Soil12 | 8.7 × 10-3 | 8.7 × 10-3 | 8.7 × 10-3 | 1.4 × 10-2 | 4.6 × 10-3 | 1.1 × 10-3 | 9.2 × 10-4 | 9.1 × 10-4 |
| Total intake | 8.8 × 10-3 | 1.3 × 10-2 | 1.0 × 10-2 | 1.6 × 10-2 | 6.3 × 10-3 | 2.0 × 10-3 | 1.9 × 10-3 | 1.9 × 10-3 |
| Maximum total intake from all routes of exposure: 1.6 × 10-2 µg/kg-bw per day | ||||||||
1 No quantitative data were identified for concentrations of HR in breast milk.
2 Assumed to weigh 7.5 kg, to breathe 2.1 m3 of air per day, to drink 0.8 L of water per day (formula fed) or 0.3 L/day (not formula fed) and to ingest 30 mg of soil per day (Health Canada 1998).
3 For exclusively formula-fed infants, intake from water is synonymous with intake from food. No quantitative data on concentrations of HR in drinking water or formula were identified for Canada. The concentration of HR in drinking water was estimated using ChemCAN v6.00 at 41.4 ng/L (ChemCAN 2003). For non-formula-fed infants, approximately 50% are introduced to solid foods by four months of age and 90% by six months of age (NHW 1990).
4 Assumed to weigh 15.5 kg, to breathe 9.3 m3 of air per day, to drink 0.7 L of water per day and to ingest 100 mg of soil per day (Health Canada 1998).
5 Assumed to weigh 31.0 kg, to breathe 14.5 m3 of air per day, to drink 1.1 L of water per day and to ingest 65 mg of soil per day (Health Canada 1998).
6 Assumed to weigh 59.4 kg, to breathe 15.8 m3 of air per day, to drink 1.2 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998).
7 Assumed to weigh 70.9 kg, to breathe 16.2 m3 of air per day, to drink 1.5 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998).
8 Assumed to weigh 72.0 kg, to breathe 14.3 m3 of air per day, to drink 1.6 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998).
9 No quantitative data were identified for concentrations of HR in air. The concentration of HR in air was estimated using ChemCAN v6.00 at 0.342 ng/m3 (ChemCAN 2003).
10 No quantitative data were identified for concentrations of HR in drinking water. The concentration of HR in drinking water was estimated using ChemCAN v6.00 at 41.4 ng/L (ChemCAN 2003).
11 No quantitative data were identified for concentrations of HR in food or beverages.
12 No quantitative data were identified for concentrations of HR in soil. The concentration of HR in soil was estimated using ChemCAN v6.00 at 2179 ng/g solids (ChemCAN 2003).
| Route of exposure | Estimated intake (µg/kg-bw per day) of HRPE by various age groups | |||||||
|---|---|---|---|---|---|---|---|---|
| 0–0.5 years1,2,3 | 0.5–4 years4 | 5–11 years5 | 12–19 years6 | 20–59 years7 | 60+ years8 | |||
| Breast milk fed | Formula fed | Not formula fed | ||||||
| Air9 | 6.9 × 10-5 | 6.9 × 10-5 | 6.9 × 10-5 | 1.5 × 10-4 | 1.2 × 10-4 | 6.6 × 10-5 | 5.7 × 10-5 | 4.9 × 10-5 |
| Drinking water10 | N/A | 7.0 × 10-3 | 2.6 × 10-3 | 3.0 × 10-3 | 2.3 × 10-3 | 1.3 × 10-3 | 1.4 × 10-3 | 1.4 × 10-3 |
| Food and beverages11 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Soil12 | 8.4 × 10-3 | 8.4 × 10-3 | 8.4 × 10-3 | 1.4 × 10-2 | 4.4 × 10-3 | 1.1 × 10-3 | 8.9 × 10-4 | 8.8 × 10-4 |
| Total intake | 8.5 × 10-3 | 1.5 × 10-2 | 1.1 × 10-2 | 1.7 × 10-2 | 6.8 × 10-3 | 2.5 × 10-3 | 2.3 × 10-3 | 9.4 × 10-4 |
| Maximum total intake from all routes of exposure: 1.7 × 10-2 µg/kg-bw per day | ||||||||
1 No quantitative data were identified for concentrations of HRPE in breast milk.
2 Assumed to weigh 7.5 kg, to breathe 2.1 m3 of air per day, to drink 0.8 L of water per day (formula fed) or 0.3 L/day (not formula fed) and to ingest 30 mg of soil per day (Health Canada 1998).
3 For exclusively formula-fed infants, intake from water is synonymous with intake from food. No quantitative data on concentrations of HR in drinking water or formula were identified for Canada. The concentration of HRPE in drinking water was estimated using ChemCAN v6.00 at 65.5 ng/L (ChemCAN 2003). For non-formula-fed infants, approximately 50% are introduced to solid foods by four months of age and 90% by six months of age (NHW 1990).
4 Assumed to weigh 15.5 kg, to breathe 9.3 m3 of air per day, to drink 0.7 L of water per day and to ingest 100 mg of soil per day (Health Canada 1998).
5 Assumed to weigh 31.0 kg, to breathe 14.5 m3 of air per day, to drink 1.1 L of water per day and to ingest 65 mg of soil per day (Health Canada 1998).
6 Assumed to weigh 59.4 kg, to breathe 15.8 m3 of air per day, to drink 1.2 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998).
7 Assumed to weigh 70.9 kg, to breathe 16.2 m3 of air per day, to drink 1.5 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998).
8 Assumed to weigh 72.0 kg, to breathe 14.3 m3 of air per day, to drink 1.6 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998).
9 No quantitative data were identified for concentrations of HRPE in air. The concentration of HRPE in air was estimated using ChemCAN v6.00 at 0.248 ng/m3(ChemCAN 2003).
10 No quantitative data were identified for concentrations of HRPE in drinking water. The concentration of HRPE in drinking water was estimated using ChemCAN v6.00 at 65.5 ng/L (ChemCAN 2003).
11 No quantitative data were identified for concentrations of HRPE in food or beverages.
12 No quantitative data were identified for concentrations of HRPE soil. The concentration of HRPE in soil was estimated using ChemCAN v6.00 at 2114 ng/g solids (ChemCAN 2003).
| Route of exposure | Estimated intake (µg/kg-bw per day) of HRGE by various age groups | |||||||
|---|---|---|---|---|---|---|---|---|
| 0–0.5 years1,2,3 | 0.5–4 years4 | 5–11 years5 | 12–19 years6 | 20–59 years7 | 60+ years8 | |||
| Breast milk fed | Formula fed | Not formula fed | ||||||
| Air9 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Drinking water10 | N/A | 0.11 | 0.04 | 0.05 | 0.04 | 0.02 | 0.02 | 0.02 |
| Food and beverages11 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Soil12 | 0.06 | 0.06 | 0.06 | 0.10 | 0.03 | 0.01 | 0.01 | 0.01 |
| Total intake | 0.06 | 0.17 | 0.11 | 0.15 | 0.07 | 0.03 | 0.03 | 0.03 |
| Maximum total intake from all routes of exposure: 0.17 µg/kg-bw per day | ||||||||
1 No quantitative data were identified for concentrations of HRGE in breast milk.
2 Assumed to weigh 7.5 kg, to breathe 2.1 m3 of air per day, to drink 0.8 L of water per day (formula fed) or 0.3 L/day (not formula fed) and to ingest 30 mg of soil per day (Health Canada 1998).
3 For exclusively formula-fed infants, intake from water is synonymous with intake from food. No quantitative data on concentrations of HRGE in drinking water or formula were identified for Canada. The concentration of HRGE in drinking water was estimated using ChemCAN v6.00 at 1.020 µg/L (ChemCAN 2003). For non-formula-fed infants, approximately 50% are introduced to solid foods by four months of age and 90% by six months of age (NHW 1990).
4 Assumed to weigh 15.5 kg, to breathe 9.3 m3 of air per day, to drink 0.7 L of water per day and to ingest 100 mg of soil per day (Health Canada 1998).
5 Assumed to weigh 31.0 kg, to breathe 14.5 m3 of air per day, to drink 1.1 L of water per day and to ingest 65 mg of soil per day (Health Canada 1998).
6 Assumed to weigh 59.4 kg, to breathe 15.8 m3 of air per day, to drink 1.2 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998).
7 Assumed to weigh 70.9 kg, to breathe 16.2 m3 of air per day, to drink 1.5 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998).
8 Assumed to weigh 72.0 kg, to breathe 14.3 m3 of air per day, to drink 1.6 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998).
9 No quantitative data were identified for concentrations of HRGE in air. The concentration of HRGE in air was estimated using ChemCAN v6.00 at 0.238 ng/m3(ChemCAN 2003).
10 No quantitative data were identified for concentrations of HRGE in drinking water. The concentration of HRGE in drinking water was estimated using ChemCAN v6.00 at 1.02 µg/L (ChemCAN 2003).
11 No quantitative data were identified for concentrations of HRGE in food or beverages.
12 No quantitative data were identified for concentrations of HRGE in soil. The concentration of HRGE in soil was estimated using ChemCAN v6.00 at 16.223 µg/g solids (ChemCAN 2003).
| Route of exposure | Estimated intake (µg/kg-bw per day) of HRTE by various age groups | |||||||
|---|---|---|---|---|---|---|---|---|
| 0–0.5 years1,2,3 | 0.5–4 years4 | 5–11 years5 | 12–19 years6 | 20–59 years7 | 60+ years8 | |||
| Breast milk fed | Formula fed | Not formula fed | ||||||
| Air9 | 5.0 × 10-5 | 5.0 × 10-5 | 5.0 × 10-5 | 1.1 × 10-4 | 8.4 × 10-5 | 4.8 × 10-5 | 4.1 × 10-5 | 3.6 × 10-5 |
| Drinking water10 | N/A | 1.8 × 10-3 | 6.9 × 10-4 | 7.8 × 10-4 | 6.1 × 10-4 | 3.5 × 10-4 | 3.6 × 10-4 | 3.8 × 10-4 |
| Food and beverages11 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Soil12 | 1.0 × 10-3 | 1.0 × 10-3 | 1.0 × 10-3 | 1.7 × 10-3 | 5.5 × 10-4 | 1.3 × 10-4 | 1.1 × 10-4 | 1.1 × 10-4 |
| Total intake | 1.1 × 10-3 | 2.9 × 10-3 | 1.8 × 10-3 | 2.6 × 10-3 | 1.2 × 10-3 | 5.3 × 10-4 | 5.1 × 10-4 | 5.3 × 10-4 |
| Maximum total intake from all routes of exposure: 2.9 × 10-3 µg/kg-bw per day | ||||||||
1 No quantitative data were identified for concentrations of HRTE in breast milk.
2 Assumed to weigh 7.5 kg, to breathe 2.1 m3 of air per day, to drink 0.8 L of water per day (formula fed) or 0.3 L/day (not formula fed) and to ingest 30 mg of soil per day (Health Canada 1998).
3 For exclusively formula-fed infants, intake from water is synonymous with intake from food. No quantitative data on concentrations of HRTE in drinking water or formula were identified for Canada. The concentration of HRTE in water was estimated using ChemCAN v6.00 at 17.2 ng/L (ChemCAN 2003). For non-formula-fed infants, approximately 50% are introduced to solid foods by four months of age and 90% by six months of age (NHW 1990).
4 Assumed to weigh 15.5 kg, to breathe 9.3 m3 of air per day, to drink 0.7 L of water per day and to ingest 100 mg of soil per day (Health Canada 1998).
5 Assumed to weigh 31.0 kg, to breathe 14.5 m3 of air per day, to drink 1.1 L of water per day and to ingest 65 mg of soil per day (Health Canada 1998).
6 Assumed to weigh 59.4 kg, to breathe 15.8 m3 of air per day, to drink 1.2 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998).
7 Assumed to weigh 70.9 kg, to breathe 16.2 m3 of air per day, to drink 1.5 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998).
8 Assumed to weigh 72.0 kg, to breathe 14.3 m3 of air per day, to drink 1.6 L of water per day and to ingest 30 mg of soil per day (Health Canada 1998).
9 No quantitative data were identified for concentrations of nitromethane in air. The concentration of HRTE in air was estimated using ChemCAN v6.00 at 0.180 ng/m3 (ChemCAN 2003).
10 No quantitative data were identified for concentrations of HRTE in drinking water. The concentration of HRTE in water was estimated using ChemCAN v6.00 at 17.2 ng/L (ChemCAN 2003).
11 No quantitative data were identified for concentrations of HRTE in food or beverages.
12 No quantitative data were identified for concentrations of HRTE in soil. The concentration of HRTE in soil was estimated using ChemCAN v6.00 at 263 ng/g solids (ChemCAN 2003).
Appendix 4: Upper-Bounding Exposure Estimates for HR, HRPE, HRGE and HRTE for Consumer Product Scenarios using ConsExpo v4.1 (ConsExpo 2006)
| Substance | Consumer product | Assumptions1 | Exposure estimate |
|---|---|---|---|
| HRGE & HRTE | Depilatory wax (used to remove hair) | Common assumptions:
HRGE assumption:
HRTE assumption:
|
HRGE:
HRTE:
|
| HRGE | Water-based adhesive (used to connect laminate to furniture) | The scenario assumptions are based on a ConsExpo default scenario for bottled glue – moderate size surfaces (construction uses) (RIVM 2007) The dermal scenario used uptake by diffusion:
|
acute dermal deposition 0.353 mg/kg-bw per event |
| HRPE | Lipstick |
uptake fraction: 1 |
oral chronic dose 0.0564 mg/kg bw per day |
| HR | Hair wax/pomade | The scenario assumptions are based on a ConsExpo default scenario for hair gel (RIVM 2006a)
|
chronic dermal deposition 1.36 mg/kg-bw per day |
| HRPE | Mascara | - maximum weight fraction: 0.08 (Environment Canada 2010a)
|
chronic dermal deposition 0.0282 mg/kg-bw per day |
| HR | Hot melt adhesive | The inhalation component of the scenario assumes instantaneous release (due to the high temperature of application, ~150-180°C) (RIVM 2007):
The dermal scenario used uptake by diffusion:
|
mean inhalation event concentration: 0.0186 mg/m3 acute dermal deposition: 0.141 mg/kg-bw per event |
Appendix 5: Summary of Health Effects Information for HR (CAS RN 65997-06-0), HRGE (CAS RN 65997-13-9) and HRPE (CAS RN 64365-17-9)
| Endpoint | Lowest effect levels1/Results | ||
|---|---|---|---|
| HR CAS RN (65997-06-0) | HRGE (CAS RN 65997-13-9) | HRPE (CAS RN 64365-17-9) | |
| Laboratory animals and in vitro | |||
| Acute toxicity | Oral LD50 (rat) > 2000 mg/kg-bw (European Commission 2000c) Inhalation LC0 (non-lethal concentration) (rat) > 2480 ppm (31 000 mg/m3) (European Commission 2000c) No dermal studies identified. |
Oral LD50 (rat) > 2000 mg/kg-bw (European Commission 2000a) Inhalation LC0 (non-lethal concentration) (rat) > 0.158 mg/l (6 hours exposure) (European Commission 2000a) No dermal studies identified. |
Oral LD50 (rat) > 2000 mg/kg-bw (European Commission 2000b) No dermal nor inhalation studies identified. |
| Short-term repeated-dose toxicity | No data identified. | (Lowest) Oral LOEL = 500 mg/kg-bw per day (1.0%) based on reduced body weight in females when both sexes of Sprague-Dawley rats (10 per sex per group) were exposed to 0, 0.2 or 1.0% (equivalent to 0, 100 or 500 mg/kg-bw per day, respectively) of HRGE in diet for 28 days. No weight changes were reported in males. Food consumption and gross and microscopic pathology were unaffected by treatment. (US EPA 2008a). No other studies identified. |
No data identified. |
| Sub-chronic toxicity | (Lowest) Oral LOEL = 1000 mg/kg-bw per day (1%) based on decreased mean body weight and body weight gain (food consumption related) in weanling Sprague-Dawley rats (10 per sex per group) exposed to hydrogenated rosin at 0, 0.01, 0.05, 0.2, 1 or 5% (approximately 0, 10, 50, 200, 1000 or 5000 mg/kg-bw per day respectively) in diet for 90 days. A statistically significant increase in absolute liver weight and relative weights of liver, kidney, spleen in males, and liver in females at 1000 mg/kg-bw per day of HR were observed but were not considered to be toxicologically significant according by the author. All high dose animals died between days 4 and 12 (author claimed that the death was related to starvation associated with food refusal) (Calandra 1960a). No other studies identified. |
Highest Oral NOEL =2500 mg/kg-bw per day (5% ) based on no treatment-related effects in Sprague Dawley rats (20 to 25 per sex per group) treated with 0, 0.2, 1.0 or 5.0% of HRGE in diet (equivalent to 0, 100, 500 or 2500 mg/kg bw per day, respectively) for 90 days. Parameters measured were body weight, food consumption, haematology, clinical chemistry, urinalysis, ophthalmology, fecal parameters, organ weights and gross and microscopic pathology in all dose groups (US EPA 2008a). No treatment-related effects were also reported in another 90-day study on the same strain of rats (both sexes) treated with HRGE in diet at 0, 2000, 5000, or 10000 ppm for 90 days (equivalent to 0, 100, 250 or 500 mg/kg-bw per day, respectively) (Laveglia 1987). Other study identified: Calandra 1967. |
No data identified. |
| Chronic toxicity/ carcinogenicity | Both sexes of Sprague-Dawley rats (30 per sex per group) were exposed to dietary concentrations of 0, 0.05, 0.2 or 1% of HR (equivalent to 0, 0, 50, 200, or 1000 mg/kg-bw per day, respectively) for two years. No evidence of carcinogenicity was noted at any dose level. No effects on haematology, urinalysis, organ weights, and gross and microscopic pathology were reported. (Lowest) Oral LOELfor non-neoplastic effects = 1000 mg/kg-bw per day (1%) based on the decreased weight gain in both sexes at the interim sacrifice (12 months) (the only effect observed in this study, the reliability of this unaudited IBT study is questionable according to US EPA 2008b) (Kay 1962a). No other studies identified. |
No data identified. | No data identified. |
| Sensitization | In a maximization test, female Dunkin-Hartley guinea pigs (15-21/ treated group, 15-20/control group) were challenged with 10, 3, or 1% of HR for 24 hours following the induction of Rosin or HR (5% intradermally and 25% epidermally). A significant response was found at the high concentration with the hydrogenated rosin induction groups. However, no such effect was found with the Rosin induction groups. In Freund’s complete adjuvant test, no significant response was observed in female Dunkin-Hartley guinea pigs challenged with 10, 3, 1, or 0.3% of HR following induction of three intradermal injections of a 5% solution of HR (Karlberg et al. 1988). | No sensitization noticed in guinea pigs in a maximization test (no study details provided) (European Commission 2000a). | No data identified. |
| Irritation | No skin irritation noticed in rabbits (no study details provided) (European Commission 2000a). | No data identified. | No data identified. |
| Human | |||
| Sensitization | In a patch test, Rosin or HR was applied to 11 human subjects with known sensitivity to Rosin (sex and age not identified) at concentrations of 0.001%, 0.01, 0.1, 1, 10, and 20%. Fewer reactions to HR than to Rosin were observed. No patient was found to react to the hydrogenated rosin without reacting to the unmodified rosin at the same concentration. Although the positive reactions were observed in 1 or 2 subjects at 0.001% to 0.1% HR, 5 out of 11 subjects showed positive reactions at 1% and 10% concentrations. At 20% HR, only 7 subjects were tested and 3 out of 7 subjects showed positive reactions compared to all 7 subjects who showed positive reactions to 20% Rosin (Karlberg et al. 1988). | In a patch test, 51 subjects of both sexes (ages 17-75 years old) were exposed to 0.2 mL of the test material containing 20% Hydrogenated Purified Ester Gum-2-Octyldodecyl Myristate and 80% petrolatum (effective concentration of HRGE in the test material was 10%). No evidence of skin irritation or sensitization was noted in any of the subjects (Johnson 2004). In three follow up patch tests, HRGE 20% in petrolatum was applied to human patients with clinical symptoms of sensitization or irritation as a result of previously used HRGE containing consumer products. Positive skin sensitization reactions were observed (Ota et al. 2007; Foti et al. 2006; Bonamonte et al. 2001). |
In three follow up patch tests, an unspecified amount of HRPE was applied to human patients with clinical symptoms of sensitization or irritation as a result of previously using HRPE containing wound dressings (contain approximately up 20% of HRPE used as adhesive in final products). Positive skin sensitization reactions were observed (Pereira et al. 2007; Sasseville et al. 1997; Schliz et al. 1996). |
Appendix 6: Summary of Health Effects Information for Analogues Rosin (CAS RN 8050-09-7), Resin acids and Rosin acids, esters with glycerol (RGE)(CAS RN 8050-31-5) and Resin acids and Rosin acids, esters with pentaerythritol (RPE) (CAS RN 8050-26-8)
| Endpoint | Lowest effect levels1/Results | |||
|---|---|---|---|---|
| Rosin (CAS RN 8050-09-7) | Resin acids and Rosin acids, esters with glycerol (RGE) (CAS RN 8050-31-5) | Resin acids and Rosin acids, esters with pentaerythritol (RPE) (CAS RN 8050-26-8) | ||
| Laboratory animals and in vitro | ||||
| Acute toxicity | Lowest Oral LD50 (mouse, guinea pig) = 4100- 4600 mg/kg-bw (US EPA 2008b). Other Oral LD50 (rat) = 7600 - 8400 mg/kg-bw (US EPA 2008b) No dermal nor inhalation studies identified. |
Oral LD50 (rat) > 2000 mg/kg-bw (European Commission 2000d) No dermal nor inhalation studies identified. |
Oral LD50 (rat) > 2000 mg/kg-bw (US EPA 2008b) No dermal nor inhalation studies identified. |
|
| Sub-chronic toxicity | (Lowest) Oral LOEL = 1000 mg/kg-bw per daybased on reduced body weight and increased relative organ (liver, kidney and spleen) weights observed in five separate 90-day studies in Sprague-Dawley rats (10 per sex per group) exposed to 0, 0.01, 0.05, 0.2, 1.0 or 5.0% (equivalent to 0, 10, 50, 200, 1000 or 5000 mg/kg-bw per day, respectively) of Rosin in diet. No treatment-related effects on haematology or urinalysis parameters were reported in any study. At necropsy, no treatment-related changes were noted. An increase in absolute liver weights was reported in three of the five studies: relative organ weights were reported as “altered” in two studies and increases in relative organ weights (liver, kidney and/ or spleen) were reported in three studies. (Calandra 1960b). No other studies identified. |
(Lowest) Oral LOEL = 1000 mg/kg-bw per day based on dose-related, statistically significant increases in relative liver weights in mid- and high-dose males of Sprague-Dawley rats (15 per sex per group) exposed to RGE in diet at 0, 0.2, 1, or 5% (equivalent to 0, 200, 1 000, or 5 000 mg/kg-bw per day respectively) for 90 days. Other effects observed were statistically significant increases in relative liver weights in high-dose females, statistically significantly decreased food consumption in high-dose males and females, and very slight periportal hepatocytic vacuolation in high-dose females (Mann et al. 1982). Other studies: Blari 1991. No dermal nor inhalation studies identified. |
(Lowest) Oral LOAEL = 2500 mg/kg-bw per day) based on a significant increase in absolute and relative liver weights in the high-dose Sprague Dawley rats observed in both sexes of (10 per sex per dose group) exposed to RPE at 0.01, 0.05, 0.2, 1, and 5% in diet (equivalent to 0, 5, 25, 100, 500 and 2500 mg/kg-bw per day respectively) for 90 days. Not treatment related effect on body weight, body weight gain, clinical signs, haematology, urinalysis, or gross pathology were reported (Calandra 1960c). | |
| Chronic toxicity/ carcinogenicity | Both sexes of Sprague-Dawley rats (30 per sex per dose) were exposed to dietary concentrations of 0, 0.05 or 1% of Rosin (equivalent to 0, 50 or 1000 mg/kg-bw per day, respectively) for two years. No evidence of carcinogenicity was noted at any dose level. (Lowest) Oral LOELfor non-neoplastic effects = 1000 mg/kg-bw per day (1%) based on decreased mean body weight and body weight gain (associated with decreased food consumption); and increased relative liver weight were observed in high dose groups(US EPA 2008b; Kay 1962b). No other studies identified. |
No data identified. | Both sexes of Sprague-Dawley rats (30 per sex per group) were exposed to RPE in the diet at 0 or 0.05% (equivalent to 0 or 50 mg/kg-bw per day) for two years. Treatment did not affect body weight, body weight gain, food consumption, food utilization, haematology, urinalysis, gross pathology, organ weights or microscopic pathology. The number of tumour bearing animals was 0 males and 5 females in the first control group; 0 males and 9 females in the second control group; and 2 males and 7 females in the 0.05% group. In all groups, the tumours were primarily subcutaneous fibroadenomas or adenofibromas. No other treatment-related effects were reported (Kay 1962c). | |
Genotoxicity and related endpoints: in vitro |
No data identified. | Gene mutation Negative: Salmonella typhimuriumstrains TA98, TA100, TA1535, TA1537 and TA1538 at 2.5 to 500 µg/plate with or without metabolic activation (Jagannath 1988); strains TA92, TA94, TA98, TA100, TA1535 and TA1537 at 10 000 µg/plate with or without metabolic activation (Ishidate et al. 1984). Chromosome aberration Negative: Chinese hamster ovary (CHO) cells at 50.7- 507 µg/mL or 127-507 µg/ml with or without metabolic activation (Murli 1988). Fibroblast of Chinese hamster at 8000 µg/ml (Ishidate et al. 1984). Unscheduled DNA synthesis Negative: Rat primary hepatocyte at 5.08-102 µg/mL (Cifone 1988). |
No data identified. | |
Reproductive/ developmental toxicity |
Sprague-Dawley rats (10 per sex per group) were administered Rosin via diet at 0, 1000, 3000 or 10 000 ppm (equivalent to 0, 105, 275 or 825 mg/kg-bw per day, respectively; males dosed for at least four weeks, starting from two weeks prior to mating while the females dosed from two weeks prior to mating until at least day four of lactation). (Lowest) LOEL for reproductive/developmental toxicity = 825 mg/kg-bw per day (10 000 ppm) based on reduced mean litter and pup weight (considered as a secondary effect to reduced food intake and subsequent body weight loss in dams). There were no effects of treatment on mating performance, fertility or duration of gestation. (Lowest) LOEL for systemic toxicity = 275 mg/kg-bw per day (3 000 ppm) based on slightly reduced body-weight gain in males. Other effects observed at the high dose was a treatment related decreased body weight gain in both sexes associated with decreased food consumption in the first few weeks of treatment (Clubb and Sutherland 2002). No other studies identified. |
No data identified. | Sprague-Dawley rats (10 per sex per group) were administered RPE via diet at 0, 1000, 5000 and 20 000 ppm (equivalent to 0, 95, 475 or 1900 mg/kg-bw per day respectively; males dosed for at least four weeks, starting from two weeks prior to mating while the females dosed from two weeks prior to mating until at least day four of lactation). A slightly lower male fertility index in mid- and high-dose groups was reported (within the historical background range). There was no obvious effect of treatment at any dose level on mean number of live pups and implants compared with control. Mean litter and pup weights were slightly lower than control over day 1 to 4 of lactation in all treated groups without clear dose relationship, although weight gain from days 1to 4 was comparable with controls. No treatment related abnormalities noted among pups, no obvious effect of treatment on epididymis or testes weights in any treatment group and no histology or necropsy findings that could be attributed to treatment at any dose level. There were no obvious effects of treatment with RPE noted at any of the dose levels. The NOEL for systemic and reproductive/developmental effects for RPE was greater than 1900 mg/kg-bw per day (20 000 ppm) (US EPA 2008a) | |
| Sensitization | Several resin acid and related products were tested in guinea pigs. Ambiguous or positive sensitization reactions were reported (European Commission 2000e). | No sensitization noticed in guinea pigs in a maximization test (no study details provided) (European Commission 2000d). | In a maximization test, female Dunkin-Hartley guinea pigs (10-15 per group) were challenged with 15% of RPE following the intradermal induction with 5% of the same substance. No sign of allergic skin sensitization were detected (Andersen 1998). | |
| Irritation | Slight skin and eye irritation noticed in rats (no study details provided) (European Commission 2000e). | Slight skin irritation noticed in rabbits (no study details provided) (European Commission 2000d). In an ocular irritation test, Purified Ester Gum-2-Octyldodecyl Myristate (contains 50% RGE and 50% octyldodecyl myristate) was evaluated using six New Zealand white rabbits. The test substance was instilled (0.1 ml) into the conjunctival sac of one eye of each animal. No ocular irritating reaction was reported (Johnson 2004). |
In a Draize test, 10% of RPE was instilled into the conjunctival sac of the eye of rabbits. No ocular irritation was observed (Andersen 1998). In a single insult occlusive patch test, RPE was tested on the skin of rabbits (strain and sex not specified). Minimal irritation was observed at a dose level of 25% of RPE. Moderate reactions were observed with a mascara (containing 9.2% of RPE) and an eyeliner (containing 7% of RPE) (Andersen 1998). |
|
| Human | ||||
Sensitization/ Irritation |
Positive sensitization reactions in human subjects were reported in patch tests with Rosin in Germany and Sweden. Several Rosin containing consumer products or Rosin related products used occupationally, were associated with dermal or inhalation allergic reactions (European Commission 2000e). Colophony (Rosin) is well recognized as a skin sensitizer and is also the third-highest cause of occupational asthma. However, the specific allergens involved particularly in occupational asthma have not been comprehensively assessed or identified (Sadhra et al. 1994) In a patch test, Rosin was applied to 11 human subjects with known sensitivity to Rosin (sex and age not identified) at concentrations of 0.001, 0.01, 0.1, 1, 10, and 20% in petrolatum. Positive reactions appeared at 0.001% and 0.01% in two test subjects and 9 of 11 test subjects reported positive reaction at 0.1% and 1% and all 11 test subject reacted to 10% of Rosin. Only 7 subjects were tested at 20% of Rosin, but all showed positive reactions (Karlberg et al. 1988). |
No dermal sensitization was found with a RGE related product when tested in human (no study details provided) (European Commission 2000d). | In a repeated insult patch test, an eyeliner containing 0.1 mL of 7% of RPE was applied to 50 human subjects (both sexes). No sensitization reactions were observed. A mascara and an eyeshadow (containing 9.6% and 9.2% of RPE, respectively) were also tested on human subjects. No sensitization reactions or dermal irritation were observed (Andersen 1998). | |
| Phys-Chem/Fate | Fate | Fate | Fate | PBT Profiling | Ecotoxicity | |
|---|---|---|---|---|---|---|
| Model Input Parameters | EPIWIN Suite (all models, including: AOPWIN, PCKOCWIN, BCFBAF BIOWIN and ECOSAR) |
ASTreat | EQC | Arnot- Gobas BCF/BAF Model |
Canadian-POPs (including: Catabol, BCF Mitigating Factors Mode) |
Artificial Intelligence Expert System (AIEPS)/TOPKAT |
| SMILES Code | CC1(CCC2 C(C1)=CC C1C2(C)C CCC1(C)C (O)=O)C=C |
CC(C)C1CC C2C(CCC3 C2(C)CCCC 3(C)C(O)= O)C1 |
- | CC1(CCC2 C(C1)=CC C1C2(C)C CCC1(C)C (O)=O)C=C |
CC1(CCC2 C(C1)=CC C1C2(C)C CCC1(C)C (O)=O)C=C |
CC1(CCC2 C(C1)=CC C1C2(C)C CCC1(C)C (O)=O)C=C |
| CC(C)C1CC C2C(CCC3 C2(C)CCCC 3(C)C(O)= O)=C1 |
- | - | CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (O)=O)=C1 |
CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (O)=O)=C1 |
CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (O)=O)=C1 |
|
| CC(C)C1CC C2C(CCC3 C2(C)CCCC 3(C)C(O)= O)C1 |
- | - | CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (O)=O)C1 |
CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (O)=O)C1 |
CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (O)=O)C1 |
|
| Molecular weight (g/mol) | 302.5, 304.5, 306.5 |
306.5 | 302.5, 304.5, 306.5 |
|||
| Melting point (ºC) | 160, 147, 142 | |||||
| Boiling point (ºC) | ||||||
| Data temperature (ºC) | 20 | |||||
| Density (kg/m3) | 1.2x10-3 | |||||
| Vapour pressure (Pa) | 7.54x10-5, 1.05x10-41.5x10-4 | |||||
| Henry’s Law constant (dimensionless) | 3.2x10-4 | |||||
Log Kaw (Air-water partition coefficient; dimensionless) |
||||||
Log Kow (Octanol-water partition coefficient; dimensionless) |
5.5, 6.0, 6.3 | 5.5, 6.0, 6.3 | 5.5, 6.0, 6.3 | |||
Kow (Octanol-water partition coefficient; dimensionless) |
||||||
Log Koc (Organic carbon-water partition coefficient – L/kg) |
||||||
| Water solubility (mg/L) | 0.06 | 2.42, 2.69, 2.00 | ||||
Log Koa (Octanol-air partition coefficient; dimensionless) |
||||||
| Soil-water partition coefficient (L/kg)1 | ||||||
| Sediment-water partition coefficient (L/kg)1 | ||||||
| Suspended particles-water partition coefficient (L/kg)1 | 96694 | |||||
| Fish-water partition coefficient (L/kg)2 | ||||||
| Aerosol-water partition coefficient; dimensionless3 | ||||||
| Vegetation-water partition coefficient; dimensionless1 | ||||||
| Enthalpy (Kow) | ||||||
| Enthalpy (Kaw) | ||||||
| Half-life in air (days) | 1.00E+115 | |||||
| Half-life in water (days) | 1.00E+115 | |||||
| Half-life in sediment (days) | 1.00E+115 | |||||
| Half-life in soil (days) | 1.00E+115 | |||||
| Half-life in vegetation (days)4 | ||||||
| Metabolic rate constant (1/days) | ||||||
| Biodegradation rate constant (1/days) | 1.37 | |||||
| Biodegradation half-life in primary clarifier (t1/2-p) (hr) | ||||||
| Biodegradation half-life in aeration vessel (t1/2-s) (hr) | ||||||
| Biodegradation half-life in settling tank (t1/2-s) (hr) |
2 derived from BCF data
3 default value
4 derived from half-life in water
5 Negligible (default value)
| Phys-Chem/Fate | Fate | Fate | PBT Profiling | Ecotoxicity | |
| Model Input Parameters | EPIWIN Suite (all models, including: AOPWIN, PCKOCWIN, BCFBAF BIOWIN and ECOSAR) |
EQC | Arnot- Gobas BCF/BAF Model |
Canadian-POPs (including: Catabol, BCF Mitigating Factors Model) |
Artificial Intelligence Expert System (AIEPS)/ TOPKAT |
| SMILES Code | CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (CO)(CO) CO)=C1 |
- | CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (CO)(CO) CO)=C1 |
CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC | (CO)(CO) CO)=C1 |
CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (CO)(CO) CO)=C1 |
| CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (CO)(CO) COC(=O) C2(C)CCC C3(C)C4C CC(C=C4C CC23)C(C) C)=C1 |
- | CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (CO)(CO)C OC(=O)C2 (C)CCCC3 (C)C4CCC (C=C4CCC 23)C(C)C) =C1 |
CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (CO)(CO) COC(=O) C2(C)CCC C3(C)C4C CC(C=C4 CCC23)C (C)C)=C1 |
CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (CO)(CO) COC(=O) C2(C)CCC C3(C)C4C CC(C=C4 CCC23)C (C)C)=C1 |
|
| CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (CO)(COC (=O)C2(C) CCCC3(C) C4CCC(C= C4CCC23) C(C)C)COC (=O)C2(C) CCCC3(C) C4CCC(C= C4CCC23)C (C)C)=C1 |
- | CC(C)C1C CC2C(CCC 3C2(C)CCC C3(C)C(=O) OCC(CO)(C OC(=O)C2 (C)CCCC3 (C)C4CCC (C=C4CCC 23)C(C)C) COC(=O)C 2(C)CCCC 3(C)C4CCC (C=C4CCC 23)C(C)C) =C1 |
CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (CO)(COC (=O)C2(C) CCCC3(C) C4CCC(C= C4CCC23) C(C)C)CO C(=O)C2(C) CCCC3(C) C4CCC(C= C4CCC23) C(C)C)=C1 |
CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (CO)(COC (=O)C2(C) CCCC3(C) C4CCC(C =C4CCC2 3)C(C)C) COC(=O)C 2(C)CCCC 3(C)C4CC C(C=C4C CC23)C(C) C)=C1 |
|
| CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (COC(=O) C2(C)CCC C3(C)C4C CC(C=C4C CC23)C(C) C)(COC(=O) C2(C)CCCC 3(C)C4CCC (C=C4CCC 23)C(C)C)C OC(=O)C2 (C)CCCC3 (C)C4CCC (C=C4CCC 23)C(C)C) =C1 |
- | CC(C)C1C CC2C(CCC 3C2(C)CCC C3(C)C(=O) OCC(COC (=O)C2(C)C CCC3(C)C4 CCC(C=C4 CCC23)C (C)C)(COC (=O)C2(C) CCCC3(C) C4CCC(C= C4CCC23) C(C)C)COC (=O)C2(C)C CCC3(C)C4 CCC(C=C4 CCC23)C (C)C)=C1 |
CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (COC(=O) C2(C)CCC C3(C)C4C CC(C=C4C CC23)C(C) C)(COC(= O)C2(C)C CCC3(C)C 4CCC(C= C4CCC23) C(C)C)CO C(=O)C2(C) CCCC3(C) C4CCC(C= C4CCC23) C(C)C)=C1 |
CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (COC(=O) C2(C)CCC C3(C)C4C CC(C=C4 CCC23)C (C)C)(CO C(=O)C2 (C)CCCC3 (C)C4CCC (C=C4CC C23)C(C) C)COC(=O) C2(C)CCC C3(C)C4C CC(C=C4C CC23)C(C) C)=C1 |
|
| Molecular weight (g/mol) | 422.6, 709.1, 995.5, 1282 | 422.6, 709.1, 995.5, 1282 | |||
| Melting point (ºC) | 223, 309, 350, 350 | ||||
| Boiling point (ºC) | |||||
| Data temperature (ºC) | 20 | ||||
| Density (kg/m3) | |||||
| Vapour pressure (Pa) | 3.32x10-4, 1.33x10-15, 3.35x10-25, 1.85x10-26 | ||||
| Henry’s Law constant (Pa·m3/mol) | |||||
Log Kaw (Air-water partition coefficient; dimensionless) |
|||||
Log Kow (Octanol-water partition coefficient; dimensionless) |
5.70, 12.2, 19.2, 27.7 | 5.70, 12.2, 19.2, 27.7 | 5.70, 12.2, 19.2, 27.7 | ||
Kow (Octanol-water partition coefficient; dimensionless) |
|||||
Log Koc (Organic carbon-water partition coefficient – L/kg) |
|||||
| Water solubility (mg/L) | 8.52, 4.17x10-6, 9.97x10-7, 1.28x10-6 | ||||
Log Koa (Octanol-air partition coefficient; dimensionless) |
|||||
| Soil-water partition coefficient (L/kg)1 | |||||
| Sediment-water partition coefficient (L/kg)1 | |||||
| Suspended particles-water partition coefficient (L/kg)1 | |||||
| Fish-water partition coefficient (L/kg)2 | |||||
| Aerosol-water partition coefficient; dimensionless3 | |||||
| Vegetation-water partition coefficient; dimensionless1 | |||||
| Enthalpy (Kow) | |||||
| Enthalpy (Kaw) | |||||
| Half-life in air (days) | 1.00E+115 | ||||
| Half-life in water (days) | 1.00E+115 | ||||
| Half-life in sediment (days) | 1.00E+115 | ||||
| Half-life in soil (days) | 1.00E+115 | ||||
| Half-life in vegetation (days)4 | |||||
| Metabolic rate constant (1/days) | |||||
| Biodegradation rate constant (1/days) or (1/hr)-specify | |||||
| Biodegradation half-life in primary clarifier (t1/2-p) (hr) | |||||
| Biodegradation half-life in aeration vessel (t1/2-s) (hr) | |||||
| Biodegradation half-life in settling tank (t1/2-s) (hr) |
2 Derived from BCF data
3 Default value
4 Derived from half-life in water
5Negligible (default value)
| Phys-Chem/Fate | Fate | Fate | PBT Profiling | Ecotoxicity | |
|---|---|---|---|---|---|
| Model Input Parameters | EPIWIN Suite (all models, including: AOPWIN, PCKOCWIN, BCFBAF, BIOWIN and ECOSAR) |
EQC | Arnot- Gobas BCF/BAF Model |
Canadian-POPs (including: Catabol, BCF Mitigating Factors Model) |
Artificial Intelligence Expert System (AIEPS)/ TOPKAT |
| SMILES Code | CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (O)CO)=C1 |
- | CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (O)CO)=C1 |
CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (O)CO)=C1 |
CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (O)CO)=C1 |
| CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (O)COC(=O) C2(C)CCC C3(C)C4CC C(C=C4CC C23)C(C)C) =C1 |
- | CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (O)COC(=O) C2(C)CCC C3(C)C4C CC(C=C4C CC23)C(C) C)=C1 |
CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (O)COC(=O) C2(C)CCC C3(C)C4CC C(C=C4CC C23)C(C)C) =C1 |
CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (O)COC(=O) C2(C)CCCC 3(C)C4CCC (C=C4CCC 23)C(C)C) =C1 |
|
| CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (COC(=O) C2(C)CCC C3(C)C4C CC(C=C4C CC23)C(C) C)OC(=O) C2(C)CCC C3(C)C4C CC(C=C4C CC23)C(C) C)=C1 |
- | CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (COC(=O) C2(C)CCC C3(C)C4C CC(C=C4C CC23)C(C) C)OC(=O) C2(C)CCC C3(C)C4C CC(C=C4C CC23)C(C) C)=C1 |
CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (COC(=O) C2(C)CCC C3(C)C4C CC(C=C4C CC23)C(C) C)OC(=O) C2(C)CCC C3(C)C4CC C(C=C4CC C23)C(C)C) =C1 |
CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCC (COC(=O) C2(C)CCC C3(C)C4CC C(C=C4CC C23)C(C)C) OC(=O)C2 (C)CCCC3 (C)C4CCC (C=C4CCC 23)C(C)C) =C1 |
|
| Molecular weight (g/mol) | 378.6, 665.0, 951.5 | 378.6, 665.0, 951.5 | |||
| Melting point (ºC) | |||||
| Boiling point (ºC) | |||||
| Data temperature (ºC) | 20 | ||||
| Density (kg/m3) | |||||
| Vapour pressure (Pa) | 4.03x10-9, 1.54x10-15, 4.76x10-19 | ||||
| Henry’s Law constant (Pa·m3/mol) | |||||
Log Kaw (Air-water partition coefficient; dimensionless) |
|||||
Log Kow (Octanol-water partition coefficient; dimensionless) |
5.23, 12.3, 19.7 | 5.23, 12.3, 19.7 | 5.23, 12.3, 19.7 | ||
Kow (Octanol-water partition coefficient; dimensionless) |
|||||
Log Koc (Organic carbon-water partition coefficient – L/kg) |
|||||
| Water solubility (mg/L) | 2.54, 1.30x10-6, 9.51x10-7 | ||||
Log Koa (Octanol-air partition coefficient; dimensionless) |
|||||
| Soil-water partition coefficient (L/kg)1 | |||||
| Sediment-water partition coefficient (L/kg)1 | |||||
| Suspended particles-water partition coefficient (L/kg)1 | |||||
| Fish-water partition coefficient (L/kg)2 | |||||
| Aerosol-water partition coefficient; dimensionless3 | |||||
| Vegetation-water partition coefficient; dimensionless1 | |||||
| Enthalpy (Kow) | |||||
| Enthalpy (Kaw) | |||||
| Half-life in air (days) | 1.00E+115 | ||||
| Half-life in water (days) | 1.00E+115 | ||||
| Half-life in sediment (days) | 1.00E+115 | ||||
| Half-life in soil (days) | 1.00E+115 | ||||
| Half-life in vegetation (days)4 | |||||
| Metabolic rate constant (1/days) | |||||
| Biodegradation rate constant (1/days) or (1/hr)-specify | |||||
| Biodegradation half-life in primary clarifier (t1/2-p) (hr) | |||||
| Biodegradation half-life in aeration vessel (t1/2-s) (hr) | |||||
| Biodegradation half-life in settling tank (t1/2-s) (hr) |
2 Derived from BCF data
3 Default value
4 Derived from half-life in water
5Negligible (default value)
| Phys-Chem/Fate | Fate | Fate | Fate | PBT Profiling | Ecotoxicity | |
|---|---|---|---|---|---|---|
| Model Input Parameters | EPIWIN Suite (all models, including: AOPWIN, PCKOCWIN, BCFBAF BIOWIN and ECOSAR) |
SimpleTreat | EQC | Arnot- Gobas BCF/BAF Model |
Canadian-POPs (including: Catabol, BCF Mitigating Factors Model) |
Artificial Intelligence Expert System (AIEPS)/ TOPKAT |
| SMILES Code | CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCCO CCOCCO) =C1 |
CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCCO CCOCCO) =C1 |
- | CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCCO CCOCCO) =C1 |
CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCCO CCOCCO) =C1 |
CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCCO CCOCCO) =C1 |
| CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCCO CCOCCOC (=O)C2(C) CCCC3(C) C2CCC2CC C(C=C32)C (C)C)=C1 |
- | - | CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCCO CCOCCOC (=O)C2(C) CCCC3(C) C2CCC2C CC(C=C32) C(C)C)=C1 |
CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCCO CCOCCOC (=O)C2(C) CCCC3(C) C2CCC2CC C(C=C32)C (C)C)=C1 |
CC(C)C1C CC2C(CC C3C2(C)C CCC3(C)C (=O)OCCO CCOCCOC (=O)C2(C) CCCC3(C) C2CCC2C CC(C=C32) C(C)C)=C1 |
|
| Molecular weight (g/mol) | 436.6, 723.1 | 438.65 | 436.6, 723.1 | |||
| Melting point (ºC) | 201, 291 | |||||
| Boiling point (ºC) | ||||||
| Data temperature (ºC) | 20 | |||||
| Density (kg/m3) | ||||||
| Vapour pressure (Pa) | 1.09x10-9, 1.84x10-13 | |||||
| Henry’s Law constant (Pa·m3/mol) | 2.9x10-6 | |||||
Log Kaw (Air-water partition coefficient; dimensionless) |
||||||
Log Kow (Octanol-water partition coefficient; dimensionless) |
5.4 | 5.31, 12.8 | 5.31, 12.8 | 5.31, 12.8 | ||
Kow (Octanol-water partition coefficient; dimensionless) |
251189 | |||||
Log Koc (Organic carbon-water partition coefficient – L/kg) |
||||||
| Water solubility (mg/L) | 7.23x10-7 | |||||
Log Koa (Octanol-air partition coefficient; dimensionless) |
||||||
| Soil-water partition coefficient (L/kg)1 | ||||||
| Sediment-water partition coefficient (L/kg)1 | ||||||
| Suspended particles-water partition coefficient (L/kg)1 | 18707 | |||||
| Fish-water partition coefficient (L/kg)2 | ||||||
| Aerosol-water partition coefficient; dimensionless3 | ||||||
| Vegetation-water partition coefficient; dimensionless1 | ||||||
| Enthalpy (Kow) | ||||||
| Enthalpy (Kaw) | ||||||
| Half-life in air (days) | 1.00E+115 | |||||
| Half-life in water (days) | 1.00E+115 | |||||
| Half-life in sediment (days) | 1.00E+115 | |||||
| Half-life in soil (days) | 1.00E+115 | |||||
| Half-life in vegetation (days)4 | ||||||
| Metabolic rate constant (1/days) | ||||||
| Biodegradation rate constant (1/hr) | 0.007 | |||||
| Biodegradation half-life in primary clarifier (t1/2-p) (hr) | ||||||
| Biodegradation half-life in aeration vessel (t1/2-s) (hr) | ||||||
| Biodegradation half-life in settling tank (t1/2-s) (hr) |
2 Derived from BCF data
3 Default value
4 Derived from half-life in water
5 Negligible (default value)