Draft screening assessment - Benzoxazole, 2,2’-(1,4-naphthalenediyl)bis- (Fluorescent brightener 367)
Official title: Draft screening assessment - Benzoxazole, 2,2’-(1,4-naphthalenediyl)bis- (Fluorescent brightener 367)
Chemical Abstracts Service Registry Number 5089-22-5
Environment and Climate Change Canada
Health Canada
February 2020
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of benzoxazole, 2,2’-(1,4-naphthalenediyl)bis- (CAS RNFootnote 1 5089-22-5), hereinafter referred to as fluorescent brightener 367. This substance was identified as a priority for assessment as it met the categorization criteria under subsection 73(1) of CEPA.
Fluorescent brightener 367 does not occur naturally in the environment and, according to information submitted pursuant to a CEPA section 71 survey, was not manufactured in, or imported into, Canada above the reporting threshold of 100 kg per year during the 2011 calendar year. Under the Cosmetic Regulations, fluorescent brightener 367 was declared as present in certain nail polishes.
The ecological risk of fluorescent brightener 367 was characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of ERC analysis, fluorescent brightener 367 is considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this draft screening assessment, there is a low risk of harm to the environment from fluorescent brightener 367. It is proposed to conclude that fluorescent brightener 367 does not meet the criteria under paragraph 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitutes or may constitute a danger to the environment on which life depends.
As no empirical information was identified on the toxicological characteristics of fluorescent brightener 367, the potential health effects of this substance were based on toxicological data available for an analogue, fluorescent brightener 184 (CAS RN 7128-64-5). In studies with fluorescent brightener 184, no critical health effects were observed in subchronic and chronic studies up to the highest doses tested. The general population is not expected to be exposed to fluorescent brightener 367 from environmental media, food or drinking water. A comparison of levels of exposure from use of nail polish containing fluorescent brightener 367 with the highest dose tested in laboratory studies resulted in margins of exposure that are considered adequate to address uncertainties in the health effect and exposure databases.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that fluorescent brightener 367 does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that fluorescent brightener 367 does not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of benzoxazole, 2,2’-(1,4-naphthalenediyl)bis-, hereinafter referred to as fluorescent brightener 367, to determine whether this substance presents or may present a risk to the environment or to human health. This substance was identified as a priority for assessment as it met categorization criteria under subsection 73(1) of CEPA (ECCC, HC [modified 2017]).
The ecological risk of fluorescent brightener 367 was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This draft screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to May 2019. Empirical data from key studies as well as results from models were used to reach proposed conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This draft screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. The human health portions of this assessment have undergone external review and/or consultation. Comments on the technical portions relevant to human health were received from Jennifer Flippin, Joan Garey and Theresa Lopez of Tetra Tech Inc. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This draft screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precaution.Footnote 2 This draft screening assessment presents the critical information and considerations on which the proposed conclusions are based.
2. Substance identity
The Chemical Abstracts Service Registry Number (CAS RNFootnote 3), Domestic Substances List (DSL) name and common name for fluorescent brightener 367 are presented in Table 2-1.
| CAS RN | DSL name (common names) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 5089-22-5 | Benzoxazole, 2,2'-(1,4-naphthalenediyl)bis- (Fluorescent brightener 367, Fluorescent brightener KCB) |
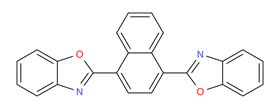
C24H14N2O2 |
362.39 |
2.1 Selection of analogue and use of (Q)SAR models
A read-across approach using data from one analogue and the results of (quantitative) structure-activity relationship ((Q)SAR) models, where appropriate, have been used to inform the human health assessment. Information on the identity and chemical structure of the selected analogue, 2,5-bis(5-tert-butyl-2-benzoxazolyl)thiophene (CAS RN 7128-64-5), hereinafter referred to as fluorescent brightener 184, was used to inform the human health assessment (summarized in Table 2‑2).
| CAS RN | DSL or other name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 7128-64-5 | 2,5-Bis(5-tert-butyl-2-benzoxazolyl)thiophene (Fluorescent brightener 184) |
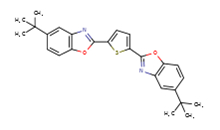
C26H26N2O2S |
430.56 |
Fluorescent brightener 184 was considered to be an appropriate analogue based on structural similarities with fluorescent brightener 367, in particular, the presence of two benzoxazole structural features. A number of other more structurally similar analogues were identified, however they lacked empirical data for use in a read-across approach. These other analogues were considered as part of QSAR modelling that was undertaken to ensure that the predictions were consistent across the broader group of benzoxazole-based substances.
Fluorescent brightener 184 has a number of structural features that differ from that of fluorescent brightener 367. These include a thiophene substituent between the two benzoxazole substructures, instead of a naphthalene substituent. In addition, fluorescent brightener 184 has tert-butyl substituent groups on both benzoxazole substructures; a structural feature not present on fluorescent brightener 367. Notwithstanding these differences both substances belong to the benzoxazolines grouping which has been assessed as a group by other jurisdictions, namely the Danish EPA (2018). In that assessment, the Danish EPA used the available empirical data from the analogue fluorescent brightener 184 to inform its human health evaluation of the benzoxazole-based substances more broadly.
3. Physical and chemical properties
A summary of physical and chemical properties of fluorescent brightener 367 is presented in Table 3‑1 and the analogue, fluorescent brightener 184 in Table 3‑2. No experimental data were identified for these substances so (Q)SAR and physicochemical prediction models were used to generate predicted values for the substance. Additional physical and chemical properties are reported in ECCC (2016b).
| Property | Predicted range | Key reference |
|---|---|---|
| Melting point (°C) | 162 to 244 | US EPA 2019a |
| Boiling point (°C) | 345 to 566 | US EPA 2019a |
| Vapour pressure (Pa) | 1.38 x 10-8 to 2.72 x 10-10 | US EPA 2019a |
| Henry’s law constant (Pa·m3/mol) | NA | US EPA 2019a |
| Water solubility (mg/L) | 2.27 x 10-3 to 9.93 x 10-1 | US EPA 2019a |
| Log Kow (dimensionless) | 3.70 to 7.46 | US EPA 2019a |
Abbreviations: Kow, octanol-water partition coefficient
| Property | Predicted range | Key reference |
|---|---|---|
| Melting point (°C) | 130 to 246 | US EPA 2019b |
| Boiling point (°C) | 319 to 571 | US EPA 2019b |
| Vapour pressure (Pa) | 1.04 x 10-8 to 7.08 x 10-8 | US EPA 2019b |
| Henry’s law constant (Pa·m3/mol) | NA | US EPA 2019b |
| Water solubility (mg/L) | 2.70 x 10-3 to 1.18 | US EPA 2019b |
| Log Kow (dimensionless) | 5.64 to 8.98 | US EPA 2019b |
Abbreviations: Kow, octanol-water partition coefficient
4. Sources and uses
Fluorescent brightener 367 was included in a survey issued pursuant to a CEPA section 71 notice (Canada 2012) and was not reported to be manufactured in, or imported into, Canada above the reporting threshold of 100 kg per year during the 2011 calendar year (Environment Canada 2013). No evidence of uses in licensed natural health products, therapeutic drugs, food packaging, or pest control products in Canada were identified (personal communications, emails from Health Products and Food Branch or Pesticide Management Regulatory Agency, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated September to October, 2018; unreferenced).
In Canada, fluorescent brightener 367 was notified as present in certain nail polishes based on notifications submitted under the Cosmetic Regulations (personal communication, email from Consumer and Hazardous Products Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated October 9, 2018; unreferenced). The substance also has potential commercial use as an additive in artificial hair used in toy dolls (Cordova 2006); however, based on available information, this use was not identified in Canada or elsewhere.
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risk of fluorescent brightener 367 was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration [LC50]) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox 2016), and from responses to surveys conducted under section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over and under classification of hazard and exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC 2016a. The following describes two of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2016). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue (CBR) analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for fluorescent brightener 367, and the hazard, exposure and risk classification results, are presented in ECCC (2016b).
According to information considered under ERC, fluorescent brightener 367 was classified as having a low exposure potential. Fluorescent brightener 367 was classified as having high hazard on the basis of its high potential to cause adverse effects in aquatic food webs given its bioaccumulation potential. Fluorescent brightener 367 was classified as having moderate potential for ecological risk; however, the risk classification was decreased to low potential for ecological risk following the adjustment of risk classification based on current use quantities (see section 7.1.1. of the ERC approach document [ECCC 2016a]).The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of this substance. On the basis of current use patterns, fluorescent brightener 367 is unlikely to be resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
Fluorescent brightener 367 was not identified in environmental media in Canada or elsewhere. Significant releases to the environment are not expected as fluorescent brightener 367 was not reported to be manufactured in, or imported into, Canada above the reporting threshold of 100 kg per year during the 2011 calendar year (Environment Canada 2013). On the basis of this information, exposure of the general population to fluorescent brightener 367 from environmental media is expected to be negligible.
Exposure to fluorescent brightener 367 may occur through the use of certain nail polishes (personal communication, email from Consumer and Hazardous Products Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated October 9, 2018; unreferenced). Exposure via inhalation is not expected because fluorescent brightener 367 has a very low predicted vapour pressure. The highest reported concentration of fluorescent brightener 367 in nail polish (0.3%) and a dermal absorption value of 100% were used for estimating exposure to the substance. The highest estimated exposure to consumers from the use of nail polish containing fluorescent brightener 367 ranged from 6.5 to 12 µg/kg bw/event, with the highest estimates for individuals aged 2 to 3 years old. See Appendix A for additional exposure parameters.
6.2 Health effects assessment
No empirical information was identified on the toxicological characteristics of fluorescent brightener 367. Fluorescent brightener 367 belongs to a class of optical brighteners called Benzoxazolines (Danish EPA 2018). Analysis for the purposes of read-across identified fluorescent brightener 184 as being the most structurally related analogue to fluorescent brightener 367 that had available toxicological data. As discussed in section 2.1, the Danish EPA (2018) also identified fluorescent brightener 184 as being the most tested of the benzoxazole-based substances.
No short-term toxicity studies were identified for the analogue fluorescent brightener 184. However, sub-chronic and chronic studies were identified.
The registration dossier submitted to the European Chemicals Agency (ECHA) contains two repeat dose OECD guideline studies for fluorescent brightener 184 (ECHA 2019). In the Unnamed study report (1991), 40 Beagle dogs, in treatment groups of 4 males and 4 females, were administered fluorescent brightener 184 in their diet at doses of 0, 500, 1500, 5000 or 50 000 ppm. The study authors calculated these doses to be the equivalent of 16.6, 49, 169 and 1682 mg/kg bw/day in females (doses were calculated to be slightly lower in males in the study). The dogs were treated once per day, 7 days per week, for 91 to 93 days. The authors found no treatment related effects at the highest dose, and therefore calculated the NOEL to be 1571 and 1682 mg/kg bw/day, the highest dose tested in male and female dogs, respectively.
In a second study, Sprague-Dawley rats were administered fluorescent brightener 184 in their diet for 13 weeks (Unnamed study report 1981). Treatment groups, comprised of 20 male and 20 female rats, were administered doses of 1000, 3000 or 10 000 ppm, calculated to be the equivalent of 78.3, 246 or 788.8 mg/kg bw/day (females). The study authors indicated that no adverse effects were observed at the highest dose, with the only treatment related changes observed being slightly higher absolute and body weight to liver weight ratios in both sexes at 10 000 ppm. These changes were deemed not significant by the study authors. Haematological examination (at 6 and 12 weeks) and post mortem macroscopic tissue examination did not identify any treatment related changes. The authors calculated a NOAEL of 788.8 mg/kg bw/day (10 000 ppm), based on the highest dose tested, with a NOEL of 246 mg/kg bw/day (3000 ppm) based on findings not considered adverse at the next dose level.
The ECHA dossier for fluorescent brightener 184 also includes a study investigating the reproductive toxicity of this substance in mice (Unnamed study report 1969). In this study, mice, in groups of 52 animals per sex, were dosed 1000 ppm via diet (calculated and reported in ECHA 2019 to be the equivalent of 150 mg/kg bw/day). The study also included untreated groups of males and females as controls. The reproductive toxicity of the parent generation (F0) was investigated based on treatment of the animals for 36 weeks prior to mating. Treatment continued throughout the gestation, mating and lactation periods. The study found no reproductive effects on either sex of F0 generation, concluding a NOEL of 150 mg/kg bw/day (1000 ppm), the only dose tested. The F1 generation also received a similar treatment regime as the F0 generation, including treatment through maturation and mating. The F2 generation was treated through to maturity, and then sacrificed. The study authors did not identify any treatment related effects in either the F1 or F2 generations and concluded a NOEL of 150 mg/kg bw/day (1000 ppm), the highest dose tested. The evidence of fluorescent material in adipose tissue, as identified under UV lighting, was considered the sole treatment-related finding (Unnamed study report 1969).
Data submitted to ECHA (2019) indicates that fluorescent brightener 184 was negative in four in vitro genotoxicity assays; including Ames, chromosomal aberration and mammalian cell micronucleus tests. An Unnamed study report (1968) cited in ECHA (2019) investigated the carcinogenic potential of fluorescent brightener 184 in rats, in a study cited as being the “equivalent or similar to” OECD Guideline 453 (combined chronic toxicity/carcinogenicity studies). In the study, groups of 35 male and 35 female rats were administered 1000 ppm (nominal) via diet for 104 weeks. The ECHA (2019) dossier summary of this study indicates 1000 ppm was the equivalent of 50 mg/kg bw/day. An untreated group of males and females was also examined. The study did not identify any evidence of carcinogenicity at 1000 ppm, nor any evidence of adverse effects to treatment more broadly, and determined that the NO(A)EL for this study was 50 mg/kg bw/day (1000 ppm), the only treatment dose administered (Unnamed study report 1968).
The Danish EPA (2018) found that the benzoxazole-based substances were not carcinogenic, mutagenic or reproductive toxicants, and were not sensitising by skin contact or inhalation, based on a review of (Q)SAR calculations and empirical data that were available. This is consistent with profiling work done by Health Canada using the OECD (Q)SAR Toolbox 4.2.1, where no alerts or flags were identified for fluorescent brightener 367,fluorescent brightener 184, or seven additional substances identified as structurally similar to fluorescent brightener 367 (OECD QSAR Toolbox 2018).
6.3 Characterization of risk to human health
The estimated systemic exposure from use of fluorescent brightener 367 in nail polish for all age groups ranged from 6.5 to 12 µg/kg bw/event, with individuals aged 2 to 3 years old having the greatest estimated exposure. To calculate margins of exposure (MOEs) for fluorescent brightener 367 via exposures from its use in nail polish, in the absence of toxicity data from shorter term studies, the critical effect was obtained from sub-chronic and chronic data available from the analogue, fluorescent brightener 184. A NOAEL of 788.8 mg/kg bw/day (highest dose tested) in a 13-week study was selected, based on the absence of statistically significant adverse effects observed at this dose. This critical effect level represents a conservative value, as effect levels from a sub-chronic study of shorter duration identified a NOEL greater than 1500 mg/kg bw/day.
Comparison of the estimated daily intake for fluorescent brightener 367 with the selected critical effect level identified in the health effects studies results in MOEs greater than 65 000 for all age groups, which are considered adequate to address uncertainties in the health effects and exposure databases
6.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in Table 6‑1.
| Key source of uncertainty | Impact |
|---|---|
| There were no empirical physicochemical properties available to inform exposure modelling. | +/- |
| There were no empirical short-term or chronic toxicity studies available for the substance. Read-across chronic study data from an analogue was required. There were no short-term toxicity studies available for the analogue. | +/- |
| There were no dermal studies available for either fluorescent brightener 367, or its analogue(s) | +/- |
Note: +/- = uncertainty with potential to cause over- or under-estimation of risk.
7. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from fluorescent brightener 367. It is proposed to conclude that fluorescent brightener 367 does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that fluorescent brightener 367 does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that fluorescent brightener 367 does not meet any of the criteria set out in section 64 of CEPA.
References
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
[ConsExpo] Consumer Exposure Web. 2019. Consumer exposure modelling software. Version 1.0.6 updated February 13, 2019. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
Cordova A, Kroskrity J, inventors; Mattel, Inc., assignee. 2006 September 19. Light reflecting polymeric articles containing benzoxazolyl-napthalene optical brighteners. United States Patent US 7,108,913 B1. [accessed 2019 Jan 30].
[Danish EPA]. 2018. Danish Environmental Protection Agency. Substitution of Optical Brightener Agents in Industrial Textiles [PDF]. Ministry of Environment and Food of Denmark. MUDP report. October 2018.. [accessed 2019 April 18].
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2019 Mar 20].
[ECHA] European Chemicals Agency. 2019. Registration Dossier: 2,5-thiophenediylbis(5-tert-butyl-1,3-benzoxazole) CAS RN 7128-64-5. Helsinki (FI): ECHA. [updated 2019 Feb 9; accessed 2019 Apr 23].
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Ficheux AS, Morisset T, Chevillotte G, Postic C, Roudot AC. 2014. Probabilistic assessment of exposure to nail cosmetics in French consumers. Food. Chem. Toxicol. 66:36-43.
Health Canada. 2015. Food consumption table derived from Statistics Canada, Canadian community health survey, cycle 2.2, Nutrition (2004), hare file. Ottawa (ON).
OECD QSAR Toolbox. 2016. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
OECD QSAR Toolbox. [read across tool]. 2018. Ver. 4.2.1. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment (NL)]. 2006. Cosmetics fact sheet: to assess the risks for the consumer [PDF]. Updated version for ConsExpo 4. Bilthoven (NL): RIVM. Report No.: 320104001/2006. [accessed 2019 Mar 20].
Unnamed study report. 1968. Combined Chronic Toxicity/Carcinogenicity Study in Rats. [as cited in ECHA 2019]. [accessed 2019 Apr 23].
Unnamed study report. 1969. One-generation Reproductive Study in Mice. [as cited in ECHA 2019]. [accessed 2019 Apr 23].
Unnamed study report. 1981. Short-term Repeated Dose Toxicity Study in Rats. [as cited in ECHA 2019]. [accessed 2019 Apr 23].
Unnamed study report. 1991. Short-term Repeated Dose Toxicity Study in Dogs. [as cited in ECHA 2019]. [accessed 2019 Apr 23].
[ECHA] European Chemicals Agency. 2019. Registration Dossier: 2,5-thiophenediylbis(5-tert-butyl-1,3-benzoxazole) CAS RN 7128-64-5. Helsinki (FI): ECHA. [updated 2019 Feb 9; accessed 2019 Apr 23].
[US EPA] US Environmental Protection Agency. 2019a. Chemistry Dashboard for Benzoxazole, 2,2'-(1,4-naphthalenediyl)bis-. [accessed 2019 Mar 20].
[US EPA] US Environmental Protection Agency. 2019b. 2,2'-(2,5-Thiophenediyl)bis[5-(1,1-dimethylethyl)-benzoxazole]. [accessed 2020 May 3].
Appendix A. Estimated dermal exposure to fluorescent brightener 367
Dermal exposure scenarios for nail polish application were used to estimate the potential exposure to fluorescent brightener 367 and are summarized in Table A-1. Exposures were estimated using ConsExpo (2019). The maximum concentration of fluorescent brightener 367 in nail polish reported (0.3%; personal communication, email from Consumer and Hazardous Products Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated October 9, 2018; unreferenced) was applied for all exposure scenarios. Dermal absorption was assumed to be 100%. Unless specified otherwise, all other parameter values are taken from the ConsExpo cosmetics fact sheet (RIVM 2006).
| Exposure scenario | Model input parameter | Estimated systemic exposure (µg/kg bw/event) |
|---|---|---|
| Nail polish (2 to 3 year olds) | Product amount: 0.06 g per application Body weight: 15 kg |
12 |
| Nail polish (4 to 8 year olds) | Product amount: 0.06 g per application Body weight: 23 kg |
7.8 |
| Nail polish (9 to 13 year olds) | Product amount: 0.16 g per application Body weight: 42 kg |
11 |
| Nail polish (14 to 18 year olds) | Product amount: 0.16 g per application Body weight: 62 kg |
7.7 |
| Nail polish (19 year olds and older) | Product amount: 0.16 g per application Body weight: 74 kg |
6.5 |
Sources: Product amount (Ficheux et al. 2014). Body weight (Health Canada 2015). Physico-chemical parameters (not shown) were selected from the predicted average value of the predicted range (US EPA 2019a).
