Draft screening assessment - Decenes Group
Official title: Draft screening assessment - Decenes Group
Chemical Abstracts Service Registry Numbers
68649-11-6
68649-12-7
Environment and Climate Change Canada
Health Canada
January 2021
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of two substances referred to collectively as the Decenes GroupFootnote 1 . Substances in this group were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA. The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 2 ), their Domestic Substances List (DSL) names and their common names are listed in the table below.
| CAS RN | DSL name | Common name or abbreviation |
|---|---|---|
| 68649-11-6a | 1-Decene, dimer, hydrogenated | Hydrogenated didecene |
| 68649-12-7a | 1-Decene, tetramer, mixed with 1-decene trimer, hydrogenated | Hydrogenated trimer and tetramer of decene or HTTD |
a This CAS RN is a UVCB (unknown or variable composition, complex reaction products, or biological materials).
According to information submitted in response to a CEPA section 71 survey, imported quantities of hydrogenated didecene and hydrogenated trimer and tetramer of decene (HTTD) in Canada in 2011 were in the range of 10 000 to 100 000 kg and 203 742 kg, respectively. In the same year, no Canadian manufacturing activities were reported for hydrogenated didecene above the reporting threshold of 100 kg, while HTTD was reported to be manufactured in quantities between 1 000 and 10 000 kg. Hydrogenated didecene is used in lubricants and greases, self-care products, and mining applications. HTTD is used in lubricants and greases for automotive care and in the automotive, aircraft and transportation sectors.
The ecological risks of the substances in the Decenes Group were characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances based on their hazard and exposure profiles. Based on the outcome of the ERC analysis, the substances in the Decenes Group are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from hydrogenated didecene and HTTD. It is proposed to conclude that hydrogenated didecene and HTTD do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
With respect to the human health assessment, no critical health effects were identified for hydrogenated didecene or HTTD via the oral or dermal routes of exposure. This was based on the available health effects data on these substances as well as on analogues. Therefore, oral exposure to substances in the Decenes Group resulting from potential releases to surface water and exposure to lipsticks are not a concern. In addition, dermal exposure from use of automotive and self-care products containing these substances are not of concern. With respect to inhalation exposure, the critical health effect is histopathological effects observed in the nasal cavity and lungs of rats exposed to aerosolized hydrogenated didecene. Hydrogenated didecene and HTTD were identified in products used as sprays for firearm maintenance (i.e., cleaner/lubricant/preservative (CLP)). Margins of exposure between concentrations of hydrogenated didecene and HTTD following use of CLP spray products for firearm maintenance and critical effects levels in laboratory studies are considered potentially inadequate to address the uncertainties in the health effects and exposure databases.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that hydrogenated didecene and HTTD meet the criteria under paragraph 64(c) of CEPA as they are entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that hydrogenated didecene and HTTD meet one or more of the criteria set out in section 64 of CEPA.
It is also proposed that HTTD meets the persistence criteria but not the bioaccumulation criteria and that hydrogenated didecene does not meet the persistence and bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
1. Introduction
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of two substances referred to collectively as the Decenes Group to determine whether these substances present or may present a risk to the environment or to human health. The substances in this group were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA (ECCC, HC [modified 2017]).
The ecological risks of the substances in the Decenes Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This draft screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to June 2019. Empirical data from key studies as well as results from models were used to reach proposed conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This draft screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The human health portions of this assessment have undergone external review and consultation. Comments on the technical portions relevant to human health were received from Theresa Lopez, Jennifer Flippin and Joan Garey of Tetra Tech, Owings Mills, Maryland, USA. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this draft screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This draft screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight-of-evidence approach and precaution.Footnote 3 This draft screening assessment presents the critical information and considerations on which the proposed conclusion is based.
2. Identity of substances
The Decenes Group consists of two polyalphaolefin (PAO) substances, both of which are of unknown or variable composition, complex reaction products or biological material (UVCB). PAOs are produced through catalytic polymerization reactions with decene and consist of branched, long-chain hydrocarbons (C20-C40) in which the carbon-carbon double bonds were hydrogenated to form single bonds (ECHA 2017). Kapur et al (2006) and Scheuermann et al (2011) noted that the resultant hydrogenated PAO mixtures consist of various structural isomers. Hydrogenated didecene is composed of 98% decene-dimer, 1% decene-monomer and 1% decene-trimer, while hydrogenated trimer and tetramer of decene or HTTD is composed of 85% decene-trimer, 13% decene-tetramer, and 2% decene-pentamer and higher (MAK 2011). The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 4 ), Domestic Substances List (DSL) names, common names, representative structures for the predominant dimer (hydrogenated didecene) and trimer (HTTD), and the molecular weight for the individual substances in the Decenes Group are presented in Table 2‑1.
| CAS RN | DSL name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol)a |
|---|---|---|---|
| 68649-11-6 | 1-Decene, dimer, hydrogenated (hydrogenated didecene) | 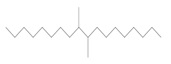 (Representative dimer structure) (Representative dimer structure)C20H42 | 282.556 |
| 68649-12-7 | 1-Decene, tetramer, mixed with 1-decene trimer, hydrogenated (hydrogenated trimer and tetramer of decene or HTTD) | 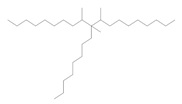 (Representative trimer structure) (Representative trimer structure)C30H62 | 422.81 |
a Molecular weight is based on representative structures.
2.1 Selection of analogues and use of (Q)SAR models
A read-across approach using data from analogues and the results of (quantitative) structure-activity relationship ((Q)SAR) models, where appropriate, has been used to inform the human health assessments. Analogues were selected that were structurally similar and/or functionally similar to substances within this group (similar physical-chemical properties) and that had relevant empirical data that could be used to read across to substances with limited empirical data. Details of the read-across data chosen to inform the human health assessments of the Decenes Group are further discussed in Appendices A and B, in addition to the relevant sections of this report.
Six branched, long-chain hydrocarbons (CAS RNs 68037-01-4, 151006-58-5, 151006-60-9, 151006-62-1, 163149-28-8, 1000172-11-1) were considered as analogues of both substances in the Decenes Group. Their selection was based on structural similarity, physical-chemical properties, profiler results from the OECD QSAR Toolbox (OECD QSAR Toolbox 2019), similarity to acute toxicity results and available health effect endpoint data. Although differences in the physical-chemical properties of the analogues are acknowledged (see Appendix B), the analogues selected were part of the ECHA REACH dossier for both hydrogenated didecene and HTTD (ECHA c2007-2019a, c2007-2019b), as well as the hazard characterization of HTTD by the US EPA under the High Production Volume (HPV) Challenge ProgramFootnote 5 (US EPA 2010).
Information on the identities and representative chemical structures of the analogues used to inform this assessment is presented in Table 2‑2.
| CAS RNa | DSL or other name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 68037-01-4 | 1-Decene, homopolymer, hydrogenated | 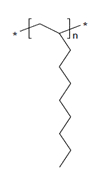 n is unknown n is unknown(US EPA 2010) C30H62 and C40H82 (ECHA 2019a)b | 422-562b |
| 151006-58-5 | 1-Dodecene, dimer with 1-decene, hydrogenated |  (ECHA) (ECHA)C20-22H42-46 (ECHA 2019b)b | 282-310b |
| 151006-60-9 | 1-Dodecene, polymer with 1-decene, hydrogenated |  n and m are unknown n and m are unknown(US EPA 2010) C32-34H66-70 (ECHA 2019c)b | 450-478b |
| 151006-62-1 | 1-Dodecene, trimer, hydrogenated |  (US EPA 2010) (US EPA 2010)C36H74 (ECHA 2017) | 506.0 |
| 163149-28-8 | 1-Dodecene, polymer with 1-decene and 1-octene, hydrogenated |  n, m and p are unknown n, m and p are unknown(US EPA 2010) C26-30H54-62 (ECHA 2019d)b | 366-422b |
| 1000172-11-1 | Pentadecane, 7-methylene-, mixed with 1-tetradecene, dimers and trimers, hydrogenated | 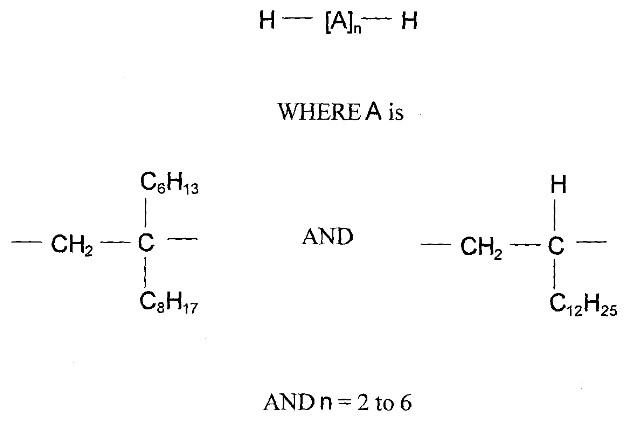 (ECHA c2007-2019c) (ECHA c2007-2019c)C28-80H58-162 (ECHA c2007-2019c)b | 394-1122b |
a This substance is a UVCB
b Estimated molecular formula and molecular weight based on depiction provided in ECHA Brief Profile or Registered substances database.
3. Physical and chemical properties
A summary of available physical and chemical properties data of the substances in the Decenes Group is presented in Table 3‑1. Additional physical and chemical properties are reported in ECCC (2016b).
| Property | Hydrogenated didecene | HTTD | Key reference(s) |
|---|---|---|---|
| Physical state | Liquid | Liquid | US EPA 2019; ECHA c2007-2019a, c2007-2019b |
| Melting point (°C) | -73 (experimental) | -73 (experimental) -20 (experimental)a | ECHA c2007-2019a; c2007-2019b; US EPA 2010, 2019 |
| Vapour pressure (Pa) | 1.889 (experimental) | < 13 (experimental) 2.5 x 10-7 (experimental)b | ECHA c2007-2019b; US EPA 2019; |
| Henry’s law constant (Pa·m3/mol) | 9.14 x 106 (predicted – Bond Method) | 1.55 x 108 (predicted – Bond Method) | EPISuite c2000-2012 |
| Water solubility (mg/L) | < 0.1 at 20°C (experimental) | < 1.0 x10-6 (experimental)c | ECHA c2007-2019a; c2007-2019b; US EPA 2010, 2019 |
| log Kow (dimensionless) | > 6.5 (experimental) | > 5 (experimental) | ECHA c2007-2019a, c2007-2019b |
| log Koc (dimensionless) | 5.713 (predicted – MCI method) | 8.284 (predicted – MCI method) | EPISuite c2000-2012 |
Abbreviations: Kow, octanol-water partition coefficient; Koc, octanol-carbon partition coefficient
a This value is read across from a standard study with dodec-1 -ene trimer hydrogenated (Hogg and Bartlett 1995), as cited in ECHA c2007-2019b.
b This value is read across from a standard study with dodec-1 -ene trimer hydrogenated (Howarth et al. 1995), as cited in ECHA c2007-2019b.
c This value is read- across from dodec-1-ene trimer hydrogenated (Seary 2000), as cited in ECHA c2007-2019b.
4. Sources and uses
The substances in the Decenes Group do not occur naturally. Both of the substances in the Decenes Group have been included in a survey issued pursuant to a CEPA section 71 notice (Environment Canada 2013). Table 4‑1 presents a summary of the information reported on total manufacture and total import quantities for the Decenes Group for the year 2011.
| Common name | Total manufacturea (kg) | Total importsa (kg) | Reporting year | Survey reference |
|---|---|---|---|---|
| Hydrogenated didecene | NR | 10 000 – 100 000 | 2011 | Environment Canada 2013 |
| HTTD | 1 000 – 10 000 | 203 742 | 2011 | Environment Canada 2013 |
Abbreviations: NR, not reported
a Values reflect quantities reported in response to a survey conducted under with section 71 of CEPA (Environment Canada 2013). See survey for specific inclusions and exclusions (schedules 2 and 3).
Table 4‑2 presents a summary of the major uses of substances in the Decenes Group according to information submitted in response to a CEPA section 71 survey (Environment Canada 2013). Table 4-3 summarizes additional Canadian uses.
| Major usesa | Hydrogenated didecene | HTTD |
|---|---|---|
| Self-care productsb | Y | N |
| Lubricants and greases | Y | Y |
| Automotive care | N | Y |
| Automotive, aircraft and transportation | N | Y |
| Mining applications | Y | N |
Abbreviations: Y, yes this use was reported for this substance; N, no this use was not reported for this substance
a Non-confidential uses reported in response to the survey conducted under section 71 of CEPA (Environment Canada 2013). See survey for specific inclusions and exclusions (schedules 2 and 3).
b Self-care products are products available for purchase without a prescription from a doctor and fall into one of three broad categories: cosmetics, natural health products, and non-prescription drugs.
| Use | Hydrogenated didecene | HTTD |
|---|---|---|
| Incidental additivesa,b | Y | Y |
| Medicinal or non-medicinal ingredients in licensed natural health productsc | Y | N |
| Notified to be present in cosmetics under the Cosmetic Regulations to Health Canadad | Y | N |
a Personal communication, e-mail from Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated July 31, 2018; unreferenced.
b While not defined under the Food and Drugs Act (FDA), incidental additives may be regarded, for administrative purposes, as those substances which are used in food processing plants and which may potentially become adventitious residues in foods (e.g., cleaners, sanitizers).
c LNHPD [modified 2018 Feb 6], Personal communication, e-mail from Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated August 27, 2018; unreferenced.
d Personal communication, e-mail from Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, July 25, 2018; unreferenced
Hydrogenated didecene and HTTD were identified in various lubricants and greases available to consumers including gear oil and transmission oil (SDS 2013a; MSDS 2015) and engine oil and gear oil (MSDS 2004 a,b; MSDS 2009, 2010, 2012a,b; SDS 2013b), respectively. Both substances were identified in cleaner/lubricant/preservative (CLP) oil used for firearm maintenance (SDS 2016; SDS 2018). As an ingredient in PAO fluids, hydrogenated didecene can also be used as components of aircraft hydraulic fluids and coolants for aircraft systems (Mattie et al. 2017).
5. Environmental fate and behaviour
5.1 Environmental persistence
According to models used in ERC (ECCC 2016b), hydrogenated didecene is not expected to be persistent in water, air, sediment or soil, whereas HTTD is expected to be persistent water, sediment and soil, but not in air.
5.2 Potential for bioaccumulation
Although Kow values are high, hydrogenated didecene and HTTD have very low bioconcentration factors (ECCC 2016b) and are not expected to significantly bioaccumulate in organisms.
6. Potential to cause ecological harm
6.1 Characterization of ecological risk
The ecological risks of the substances in the Decenes Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox 2014), and from responses to surveys issued pursuant to section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under-classification of hazard and exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for the substances in the Decenes Group and the hazard, exposure and risk classification results are presented in ECCC (2016b).
On the basis of low hazard and low exposure classifications according to information considered under ERC, hydrogenated didecene was classified as having a low potential for ecological risk. It is unlikely that this substance is resulting in concerns for the environment in Canada.
According to information considered under ERC, HTTD was classified as having a moderate exposure potential on the basis of long overall persistence and a moderate reported use volume according to information submitted in response to a CEPA section 71 survey (Environment Canada 2013). HTTD was classified as having a low hazard potential. HTTD was classified as having a low potential for ecological risk. Considering current use patterns, HTTD is unlikely to be resulting in concerns for the environment in Canada.
7. Potential to cause harm to human health
7.1 Health effects assessment
Toxicity information for the hazard assessment of both hydrogenated didecene and HTTD was informed by data submitted to the European Chemicals Agency (ECHA) dossier under the REACH program (ECHA c2007-2019a, c2007-2019b) and a United States Environmental Protection Agency (US EPA) Screening-Level Hazard Characterization of HTTD under the HPV Challenge Program (US EPA 2010). A search of the literature to June 2019 was conducted. An acute and two-week inhalation study conducted by the United States Air Force Research Laboratory (Mattie et al 2017; Wegner et al 2018) was identified for hydrogenated didecene and informs the hazard characterization.
Relevant, though limited, information specific to the Decenes Group is presented below, in addition to health effects information on analogues. Information on analogues informed a number of endpoints: genetic, repeated dose, reproductive and developmental toxicity. Additional information on analogue selection can be found in Section 2.1 and Appendix A. Appendix B provides a summary of physical-chemical properties and available health effects information for the Decenes Group substances and analogues. The toxicity studies identified for analogues of the Decenes Group were often conducted using products as the test material; therefore, there may be variability in the composition of the test substances between different products.
7.1.1 Genotoxicity
Overall, genotoxicity to the Decenes Group substances is not expected based on the negative in vivo and in vitro results identified from analogues (see Appendix B).
7.1.2 Oral route of exposure
Available toxicological data for oral exposure to hydrogenated didecene and HTTD were limited to acute toxicity, where LD50Footnote 6 values were determined to be greater than 4 100 mg/kg or above (tested as Emery 3002, SF-0203-41, SF-0201-3, Emery 3004) (ECHA c2007-2019a). Several repeated dose studies in rats were identified (Appendix B) for analogues of the Decenes Group (CAS RNs 68037-01-4, 151006-58-5, 151006-62-1). Study durations were from 28 to 91 days through dietary or gavage administration. Reproductive and developmental toxicity studies in rats via gavage (combined repeated dose/reproductive; one-generation; two-generation) were identified for some of the analogues of the Decenes Group (CAS RN 68037-01-4, 151006-62-1, 1000172-11-1). The analogue studies identified no-observed-adverse-effect levels (NOAELs) at the highest tested dose of 1000 mg/kg bw and above (ECHA c2007-2019a, c2007-2019c; Mobil 1990a, 1990b; Stonybrook 1995a; US EPA 2010). Using a read-across approach, the Decenes Group was considered to be of low concern with respect to human health through oral exposure.
7.1.3 Dermal route of exposure
Available toxicological data for dermal exposure to hydrogenated didecene and HTTD were limited to acute toxicity. A dermal LD50 was identified for hydrogenated didecene and was greater than 3000 mg/kg bw in rabbits (ECHA c2007-2019a, c2007-2019b). Dermal studies on rats (with the application site unoccluded) were identified for analogues of the Decenes Group (Appendix B). A 28-day repeated dose study (CAS RN 163149-28-8) and a developmental study conducted from rat gestational day 0 to 19 (CAS RN 68037-01-4) resulted in NOAEL values at the highest tested dose of 2000 mg/kg bw/day (Mobil 1995, 1988; US EPA 2010). A study of induction of skin tumours in mice did not result in an increased incidence after application of 1-Decene, homopolymer, hydrogenated (CAS RN 68037-01-4), an analogue of the Decenes Group, for 104 weeks (Kettering 1990; US EPA 2010). Although longer-term dermal repeated dose studies of systemic effects were not identified for the target or analogue substances in this group, based on the lack of adverse effects observed in animals in the available subchronic oral toxicity studies, substances in the Decenes Group are considered to be of low concern with respect to human health via dermal exposure.
7.1.4 Inhalation route of exposure
Appendix B provides a summary of the inhalation toxicity identified from acute studies that can be attributed to Decene Group substances. Excess mortalities, gross pathology effects and clinical signs were noted in several acute whole-body aerosol studies in rats and can be attributed to hydrogenated didecene (ECHA c2007-2019a).
Acute and short-term inhalation toxicity studies conducted by the United States Air Force Research Laboratory (Mattie et al. 2017; Wegner et al. 2018) were identified for hydrogenated didecene.
The acute range-finding study was conducted according to the Organisation for Economic Cooperation and Development (OECD) Test Guideline 403 (acute inhalation toxicity), where groups of male and female Fischer 344 rats (10/sex/dose) were exposed to 0, 100, 500 or 1000 mg/m3 PAO fluid aerosol for six hours. The PAO fluid tested is composed primarily of hydrogenated didecene (80% to 99.5%) with a proprietary additive (0.5% to 20%). As a modification to the study design, a separate group of rats was evaluated after a 2-week recovery period. Neurobehavioural observations, gross pathology, clinical chemistry, hematology and histopathological effects were evaluated. After exposure, neurobehavioural testing in 500 and 1000 mg/m3 males and females showed a significant reduction in motor activity compared to controls. After a 14-day recovery period, neurobehavioural testing results were observed to be similar to control groups. When evaluating body weight changes, a ratio of post-exposure day 1 weight to the weight on the day of exposure was used. Compared to controls, a significant decrease in the body weight ratios was observed for the 500 mg/m3 females and 1000 mg/m3 male and female groups. No body weight effects were reported at the end of the recovery period. Food and water consumption was also reported to be significantly decreased compared to controls 1 day after exposure in the groups noted to have lost body weight. Any changes in organ weight are considered to be due to differences in food and water consumption leading to body weight changes in 1000 mg/m3 group rats. No adverse clinical chemistry or hematology effects were reported between treated and control animals. With regard to histopathology, no lesions were observed in the lungs or nasal cavity of control rats, or in the first and second nasal cavity sections (NL1, NL2) after acute exposure. All concentrations were reported to cause lesions in both the posterior nasal cavity (NL3, NL4) and lungs of rats. A dose-response was reported with regard to the incidence and severity of the lesions, as noted in Table 7‑1 and Table 7-2. At 100 mg/m3, lesions were reported in the nasal cavity (NL4; multifocal degeneration, necrosis) and lungs (inflammation), with minimal severity overall in both tissues, whereas at higher concentrations, the incidence was 100% in the nasal cavity and 90% in the lungs. In the nasal cavity, severity of response increased as the histological sections progressed to the posterior end of the nasal cavity, as section NL3 had mild severity overall and section NL4 had marked severity overall for both the 500 mg/m3 and 1000 mg/m3 concentration groups. Olfactory epithelial lesions increased in severity with increasing concentration from 500 mg/m3 to 1000 mg/m3, progressing from multifocal degeneration with necrosis and edema to multifocal lesions involving necrosis, neutrophilic inflammation, edema, and loss of epithelium. In the lungs, inflammation was reported in two rats (one focal, one multifocal) at 100 mg/m3. At 500 mg/m3, overall severity was minimal, but some rats had mild responses. At 1000 mg/m3, individual rat severity ranged from minimal to mild. Pulmonary inflammation was observed in all rats with lung histopathology incidence, with some in the 500 and 1000 mg/m3 groups showing accumulation of neutrophils as well as lymphocytes, macrophages and debris (Mattie et al 2017; Wegner et al 2018).
| Tissue section | Control | 100 mg/m3 | 500 mg/m3 | 1000 mg/m3 |
|---|---|---|---|---|
| Nasal cavity NL3a | 0 | 0 | 16/20 | 18/20 |
| Nasal cavity NL4a | 0 | 10/20 | 20/20 | 20/20 |
| Lunga | 0 | 2/20 | 18/20 | 18/20 |
a Incidence measured as number of rats with lesions/number of rats in exposure group (Mattie et al 2017).
| Tissue section | Control | 100 mg/m3 | 500 mg/m3 | 1000 mg/m3 |
|---|---|---|---|---|
| Nasal cavity NL3a | 0 | 0 | 1.10 ± 0.16 | 1.00 ± 0.01 |
| Nasal cavity NL4a | 0 | 0.90 ± 0.22 | 3.20 ± 0.14 | 3.10 ± 0.10 |
| Lunga | 0 | 0.11 ± 0.08 | 1.21 ± 0.12 | 1.47 ± 0.16 |
a Severity = mean ± standard error of the mean; severity rating: 0 = normal; 1 = minimal; 2 = mild; 3 = moderate; 4 = marked; 5 = severe (Mattie et al 2017).
Fewer animals were affected and severity decreased after 14 days of recovery, but not all affected animals recovered completely. At 100 mg/m3, 95% of rats recovered from nasal cavity lesions, whereas 1 out of 20 had multifocal degeneration in the nasal cavity. When evaluating the NL3 tissue section, nearly all rats recovered (95% and 100% at mid- and high-concentration). However, NL4 nasal cavity tissue recovery was only reported for 42% and 70% of rats at 500 mg/m3 and 1000 mg/m3, respectively. Overall severity was reported to be minimal across all concentrations after recovery. Some 1000 mg/m3 rats showed signs of regeneration after recovery, but degeneration, necrosis, edema and inflammation were still present in affected rats. The lungs showed similar recovery incidence and minimal overall severity across concentrations. After recovery, inflammation, necrosis or degeneration were not reported in the lungs after 100 mg/m3 acute exposure. However, multifocal neutrophilic inflammation was reported in 500 mg/m3 and 1000 mg/m3 rats, in addition to granulomatous lungs at 1000 mg/m3 (Mattie et al 2017; Wegner et al 2018). The study authors identified a 6-hour NOAEL value of 100 mg/m3 for the observed respiratory tract histopathology effects (Mattie et al 2017). Based on the observed histopathology effects and incomplete recovery after 14 days reported in exposed rats, an acute lowest observed adverse effect concentration (LOAEC) value of 100 mg/m3 was determined for risk characterization in this screening assessment.
A repeated dose inhalation study with the PAO fluid was conducted according to the OECD Test Guideline 412, subsequent to the above-noted acute range-finding study. Groups of male and female Fischer 344 rats (10/sex/concentration) were exposed to an aerosol of 0, 20, 100 or 300 mg/m3 PAO fluid for 6 hours a day, 5 days a week over 2 weeks (10 exposures). Neurobehavioural testing was conducted on days 9 and 10 of exposure, whereas clinical chemistry, histopathology, hematology and gross pathology were conducted after necropsy on day 10 of exposure. No significant effects on neurobehavioural tests, food and water consumption, body weight or organ weight were reported for treated rats compared to controls. The only clinical chemistry result (significant increase in aspartate aminotransferase in females exposed to 20 mg/m3 compared to controls) was not considered to be indicative of a dose-response relationship. A significant decrease in percent lymphocytes as well as a significant increase in percent neutrophils were reported with increasing dose in female rats at all concentrations; a similar response was observed in males starting at 100 mg/m3. Significant but sporadic changes were reported in the number of white blood cells (100 mg/m3) and platelets (20 mg/m3) in females as well as mean platelet volume in male (100 mg/m3) and female (20 mg/m3) rats, when compared to controls. Histopathologic lesions were reported in the posterior nasal cavity (NL4) as well as in the lungs of animals exposed to 300 mg/m3. A nasal cavity lesion was reported at 100 mg/m3, but was determined to be unrelated to exposure. At a concentration of 300 mg/m3, lesions were observed in the posterior nasal cavity (NL4) and lungs of male and female rats. Nasal cavity lesions were reported in 12 of 20 rats with minimal severity overall (mean score = 0.71 ± 0.13). Inflammation, often described as being neutrophilic with multifocal origin, as well as necrotic debris, loss of olfactory epithelium and necrosis were noted among the affected rats at 300 mg/m3. Epithelial regeneration in some animals was also reported. At the same concentration (300 mg/m3), lung lesions were reported in 19 of 20 rats, the severity of which was considered to be mild overall (mean score = 1.25 ± 0.12; 6 animals scored mild, 13 scored minimal severity). Pulmonary inflammation was reported in the majority of affected animals exposed to 300 mg/m3, whereas only two rats had necrotic debris and single cell death in addition to inflammation. The authors derived a no observed adverse effect concentration (NOAEC) of 100 mg/m3 in rats based on the respiratory histopathology effects observed at 300 mg/m3 (Mattie et al 2017; Wegner et al 2018).
7.2 Exposure assessment
This exposure assessment focuses on the route of exposure where critical health effects have been identified, namely non-cancer effects in the lungs following inhalation of hydrogenated didecene and HTTD from use of spray products available to consumers.
7.2.1 Environmental media
No data were identified for hydrogenated didecene and HTTD in air, water, or soil in Canada. However, these substances may be released to the environment from Canadian facilities that manufacture or process them. No results for monitoring of hydrogenated didecene or HTTD in ambient air or water around manufacturing or processing facilities have been reported.
On the basis of fugacity modelling, hydrogenated didecene and HTTD are more likely to partition to soil and/or water than to air (EPISuite c2000-2012). Although the substances in the Decenes Group could be released to water from industrial activities in Canada, exposure to the general population of Canada by the oral route (i.e., drinking water) was not quantified, as no critical health effects from this route of exposure have been identified (refer to section 6.2).
Hydrogenated didecene and HTTD may be present as components in incidental additives, specifically in lubricants used in food processing establishments with no potential for food contact (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated August 2018; unreferenced). Therefore, exposure to these substances via food is not expected.
7.2.2 Products available to consumers
In Canada, hydrogenated didecene is present in approximately 45 cosmetic products including moisturizers, cleansers, lipsticks and make-up products (personal communication, email from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated August 2018; unreferenced) and as a non-medicinal ingredient in one active natural health product used topically as a skin conditioning agent (personal communication, email from the Natural and Non-Prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated August 2018; unreferenced). Hydrogenated didecene is also present in lubricants and greases available to consumers including gear oil and transmission oil (Environment Canada 2011; SDS 2013a; MSDS 2015). The primary route of exposure to hydrogenated didecene from use of products available to consumers is expected to be dermal (except for lipstick which is oral) as a result of direct skin contact. However, exposure from the dermal and oral routes was not quantified, as no critical health effects via these routes of exposure have been identified (refer to section 7.1).
In Canada, products available to consumers that contain HTTD are primarily lubricants and greases, such as engine oil and gear oil (Environment Canada 2011; MSDS 2004a, 2004b; MSDS 2009, 2010, 2012a,b; SDS 2013b). The primary route of exposure to HTTD from use of these products is expected to be via the dermal route as a result of direct skin contact during use. However, exposure from the dermal route was not quantified, as no critical health effects via this route have been identified.
Hydrogenated didecene and HTTD may also be present in Canada as ingredients in oil used for firearm maintenance (cleaning/lubricating/preserving). Hydrogenated didecene was identified in a liquid form of the product, which can be poured or applied using a trigger spray (SDS 2018), while both hydrogenated didecene and HTTD were identified in an aerosol spray form of the CLP product (SDS 2016). Given that the critical health effects are via inhalation of aerosols, only inhalation exposure from use of aerosol products and from use of trigger spray products was estimated. Estimates of peak air concentrations of hydrogenated didecene and HTTD from the use of CLP aerosol spray and CLP trigger spray for firearm maintenance were modelled using ConsExpo Web (ConsExpo Web 2016). Refer to Appendix C for details on the specific model and parameters used.
| Product | Chemicals | Product concentration (wt%) | Peak concentration on day of exposureb (mg/m3) |
|---|---|---|---|
| CLP aerosol spray | Mixture of hydrogenated didecene and HTTD | 40% 60% 100%c | 26 39 65 |
| CLP trigger spray | Hydrogenated didecene | 10% – 40% | 0.70 – 2.80 |
a Peak concentration is of both inhalable and respirable droplet sizes given effects are on nasal tissue.
b Highest estimated concentration over a 20-minute application period (ConsExpo Web).
c Given that both hydrogenated didecene and HTTD are found in the same product, peak concentrations were also derived using a maximum product concentration of 100%.
7.3 Characterization of risk to human health
No critical adverse health effects were identified via the oral or dermal routes of exposure for the Decenes Group on the basis of the health effects information on analogues. Therefore, oral and dermal exposures to hydrogenated didecene and HTTD from use of products available to consumers or from environmental media are not of concern to human health.
Critical effects have been identified for the Decenes Group via the inhalation route. From the available toxicological studies conducted with hydrogenated didecene, a LOAEC of 100 mg/m3 was selected from an acute rat study based on histopathologic effects observed in the nasal cavity and lungs. Neurobehavioural effects were observed at higher concentrations.
Table 7‑4 provides all relevant exposure and hazard values for the Decenes Group, as well as resultant margins of exposure (MOE), for determination of risk.
| Exposure scenario | Product concentration (wt%) | Peak concentration on day of exposure (mg/m3) | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|---|
| CLP aerosol for firearm (hydrogenated dideceneand HTTD) | 40% to 100% | 26 – 65 | LOAEC = 100 mg/m3 | Histopathological changes in nasal cavity and lungs | 2 – 3 |
| CLP trigger spray for firearm (hydrogenated didecene) | 10% to 40% | 0.70 – 2.80 | LOAEC = 100 mg/m3 | Histopathological changes in nasal cavity and lungs | 35 – 142 |
The calculated margins between the critical effect and the estimates of exposure from use of CLP sprays used for firearm maintenance are considered potentially inadequate to address uncertainties in the health effects and exposure databases.
7.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| Lack of data on exposure to hydrogenated didecene and HTTD from environmental media. | +/- |
| No information on the amount and size of respirable particles containing hydrogenated didecene and HTTD released during use of the CLP sprays for firearm maintenance. | + |
| There are no reproductive, developmental, sub-chronic or chronic animal studies for inhalation exposure to Decene Group substances. In addition, there are no repeated dose animal studies for inhalation exposure where recovery to aerosol exposure is observed. | +/- |
| The inhalation study on which the endpoint is based was conducted with a substance containing a proprietary additive (0.5% to 20%) in addition to the substance of interest (80% to 99% hydrogenated didecene). | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over or under estimation of risk.
8. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from hydrogenated didecene and HTTD. It is proposed to conclude that hydrogenated didecene and HTTD do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that hydrogenated didecene and HTTD meet the criteria under paragraph 64(c) of CEPA as they are entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that hydrogenated didecene and HTTD meet one or more of the criteria set out in section 64 of CEPA.
It is also proposed that HTTD meets the persistence criteria but not the bioaccumulation criteria and that hydrogenated didecene does not meet the persistence and bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
References
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c. 33. Canada Gazette Part III, vol. 22, no. 3.
[ConsExpo Web] Consumer Exposure Web Model. 2016. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment
Daniel EM. 1994. An oral (gavage) 91-day toxicity study of EthylFlo 166 in rats with an in utero exposure phase. Report by Springborn Laboratories, Inc., Spencerville OH, conducted for Albermarle Corporation, Baton Rouge LA. [accessed 2019 Jun 10] [Summary; Study 4 in Repeated-dose toxicity in HPVIS (US EPA 2019)].
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2019 Sep 12].
[ECHA] European Chemicals Agency. 2017. CLH report: Proposal for harmonised classification and labelling based on Regulation (EC) No 1272/2008 (CLP Regulation), Annex VI, Part 2: Substance name: Branched hexatriacontane Synonym: Alkane 4 or 1-Dodecene trimer, hydrogenated. [accessed 2019 Jun].
[ECHA] European Chemicals Agency. c2007-2019a. Registered substances database; search results for CAS RN 68649-11-6. Helsinki (FI): ECHA. [updated 2019 Mar 25; accessed 2019 Jun 19].
[ECHA] European Chemicals Agency. c2007-2019b. Registered substances database; search results for CAS RN 68649-12-7. Helsinki (FI): ECHA. [updated 2018 Nov 9; accessed 2019 Jun 19].
[ECHA] European Chemicals Agency. c2007-2019c. Registered substances database; search results for CAS RN 1000172-11-1. Helsinki (FI): ECHA. [updated 2018 Nov 9; accessed 2019 Jun 25].
[ECHA] European Chemicals Agency. c2007-2019d. Registered substances database; search results for CAS RN 68037-01-4. Helsinki (FI): ECHA. [updated 2019 Apr 29; accessed 2019 Sep 16].
[ECHA] European Chemicals Agency. 2019a. Brief profile: Dec-1-ene, homopolymer, hydrogenated Dec-1-ene, oligomers, hydrogenated; CAS RN 68037-01-4. Helsinki (FI): ECHA. [updated 2019 July 8; accessed 2019 Jul 11].
[ECHA] European Chemicals Agency. 2019b. Brief profile: 1-Dodecene dimer with 1-Decene, hydrogenated; CAS RN 151006-58-5. Helsinki (FI): ECHA. [updated 2019 July 8; accessed 2019 Jul 11].
[ECHA] European Chemicals Agency. 2019c. Brief profile: Reaction products of 1-decene and 1-dodecene, hydrogenated; CAS RN 151006-60-9. Helsinki (FI): ECHA. [updated 2019 Jul 8; accessed 2019 Jul 11].
[ECHA] European Chemicals Agency. 2019d. Brief profile: Reaction products of 1-decene, 1-dodecene and 1-octene, hydrogenated; CAS RN 163149-28-8. Helsinki (FI): ECHA. [updated 2019 Jul 7; accessed 2019 Jul 11].
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Health Canada. 1994. Human health risk assessment for priority substances. Ottawa (ON): Minister of Supply and Services Canada. Cat. No.: En40-215/41E.
Kapur GS, Sarpal AS, Sarin R, Jain SK, Srivastava SP, Bhatnagar AK. 2006. Detailed characterisation of polyalphaolefins and their branched structures using multi-pulse NMR techniques. J Synth Lubric. 15-3, 191 0265-6582.
[Kettering] Kettering Laboratory. 1990. Dermal Carcinogenicity Study on Decene Homopolymer. Performed by Mobil Environmental and Health Science Laboratory. [accessed 2019 Jun 10] [Summary; Study 5 Genetic toxicity in vivo in US EPA HPVIS (US EPA 2019)].
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2018 Feb 6]. Ottawa (ON): Government of Canada. [accessed 2018 Aug 27].
[MAK] 2011. The MAK Collection for Occupational Health and Safety. Polyalphaolefine, MAK, 51.[PDF] [MAK Value Documentation in German].
Mattie DR, Wegner MD, Wong BA, James RA, Mumy KL, McInturf SM, Marcel BJ, Sterner TR. 2017. Acute and two-week inhalation toxicity studies in rats for polyalphaolefin (PAO) fluid AFRL-RH-WP-TR-2017-0078. Air Force Research Laboratory 711th Human Performance Wing; Airman Systems Directorate; Bioeffects Division; Molecular Bioeffects Branch; Wright-Patterson AFB OH 45433-5707 [accessed 2019 Jul 4].
[Mobil] Mobil Environmental Health and Safety Department. 1985. Micronucleus assay of bone marrow and peripheral red blood cells from rats treated via dermal administration of Synthetic Hydrocarbon-Hydrogenated Polyolefins. [accessed 2019 Jun 10] [Summary; Study 1 Genetic toxicity in vivo in HPVIS (US EPA 2019)].
[Mobil] Mobil Environmental Health and Safety Department. 1988. Developmental toxicity screen in rats exposed dermally to decene homopolymer. [accessed 2019 Jun 10] [Summary; Developmental toxicity/teratogenicity in HPVIS (US EPA 2019)].
[Mobil] Mobil Environmental Health and Safety. 1990a. Range-finding study: oral administration of unadditized decene homopolymer to rats. Performed by Mobil Environmental Health and Safety Department [accessed 2019 Jun 10] [Summary; Study 1 Repeated-dose toxicity in HPVIS (US EPA 2019)].
[Mobil] Mobil Environmental Health and Safety Department. 1990b. 90-day oral administration of unadditized decene homopolymer to rats. [accessed 2019 Jun 10] [Summary; Study 2 Repeated-dose toxicity in HPVIS (US EPA 2019)].
[Mobil] Mobil Chemical Co. 1995. Four-week systemic toxicity study following daily dermal administration to rats. [accessed 2019 Jun 10] [Summary; Study 5 in Repeated-dose toxicity in HPVIS (US EPA 2019)].
[MSDS] Material Safety Data Sheet. 2004a. Castrol Syntec Synthetic Motor Oil (All SAE Grades) 0W-30, 5W-20, 5W-30, 5W-40, 10W-30, 10W-40, 20W-50, 5W-50. Castrol Canada Inc.
[MSDS] Material Safety Data Sheet. 2004b. PC Ultra Disc Brake Caliper Lube 59ML (Lubricant). Permatex Canada, Inc.
[MSDS] Material Safety Data Sheet. 2009. Mystik® JT-4® Synthetic ATV/UTV Engine Oil (Motor Oil), SAE 0W-40. CITGO Petroleum Corporation.
[MSDS] Material Safety Data Sheet. 2010. Mystik® JT-7® Synthetic Blend Gear Lubricant, SAE 75W-90 (Gear Oil). CITGO Petroleum Corporation.
[MSDS] Material Safety Data Sheet. 2012a. Tufoil for Engines (Engine lubricant). Fluoramics Inc.
[MSDS] Material Safety Data Sheet. 2012b. Tufoil for Engines (Engine lubricant). Marine Engine Oil; SAE 25W-40 Synthetic Blend. Mercury Marine.
[MSDS] Material Safety Data Sheet. 2015. Quaker State Full Synthetic 75W-140 (GL-5) – Transmission Oil. Shell Canada Products.
OECD QSAR Toolbox [Read across tool]. 2014. Ver. 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
OECD QSAR Toolbox [read across tool]. 2019. Ver. 4.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment (NL)]. 2009. The ConsExpo Spray Model. Modelling and experimental validation of the inhalation exposure of consumers to aerosols from spray cans and trigger sprays. Bilthoven (NL): RIVM. Report No.: 320104005/2009.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment (NL)]. 2010. New default values for the spray model. Bilthoven (NL): RIVM, March 2010.
[SafePharm] SafePharm Laboratories Ltd. 1995a. Twenty-eight day sub-acute oral (gavage) toxicity study in the rat – Limit test, including recovery groups. Conducted for Chevron Research and Technology Company, unpublished report. [accessed 2019 Jun 10] [Summary; Study 6 in Repeated-dose toxicity in HPVIS (US EPA 2019)]
[SafePharm] SafePharm Laboratories Limited (1995b). Micronucleus test in the mouse. Conducted for Chevron Research and Technology Company, unpublished report. [accessed 2019 Jun 10]. [Summary; Study 4 Genetic toxicity in vivo in HPVIS (US EPA 2019)].
[SafePharm] SafePharm Laboratories Limited. 1995c. Micronucleus test in the mouse. Conducted for Chevron Research and Technology Company. [Unpublished report]. [accessed 2019 Jun 10] [Summary; Study 3 Genetic toxicity in vivo in HPVIS (US EPA 2019)].
[SafePharm] SafePharm Laboratories Limited. 1995d. Salmonella typhimurium and Escherichia coli/Mammalian-Microsome Reverse Mutation Assay. Conducted for Chevron Research and Technology Company, unpublished report. [accessed 2019 Jun 10] [Summary; Study 6 Genetic toxicity in vitro in HPVIS (US EPA 2019)].
[SafePharm] SafePharm Laboratories Limited. 1995e. Chromosome aberration test in human lymphocytes. Conducted for Chevron Research and Technology Company, unpublished report. [accessed 2019 Jun 10] [Summary; Study 4 Genetic toxicity in vitro in HPVIS (US EPA 2019)].
Scheuermann SS, Eibl S, Bartl P. 2011. Detailed characterisation of isomers present in polyalphaolefin dimer and the effect of isomeric distribution on bulk properties. Lubric Sci. 23:221–232.
[SDS] Safety Data Sheet. 2013a. Mystik JT-7 Extended Ranged Synthetic Gear Oil, SAE 75W-140. CITGO Petroleum Corporation.
[SDS] Safety Data Sheet. 2013b. MotoMaster Synthetic Gear Oil SAE 75W-90. CITGO Petroleum Corporation.
[SDS] Safety Data Sheet. 2016. CLP Aerosol. [PDF] Jacksonville (FL): Safariland, LLC.
[SDS] Safety Data Sheet. 2018. CLP Liquid. [PDF] Jacksonville (FL): Safariland, LLC.
SITEK Research Laboratories. 2001. Test for chemical induction of gene mutation at the HGPRT locus in cultured Chinese hamster ovary (CHO) cells with and without metabolic activation with a confirmatory assay. Conducted for Chevron Research and Technology Company, unpublished report. [accessed 2019 Jun 10] [Summary; Study 3 Genetic toxicity in vitro in HPVIS (US EPA 2019)].
[Stonybrook] Stonybrook Laboratories, Inc.. 1995a. 90-day oral feeding study in Fischer 344 rats with hydrogenated polyalpha Decene in the diet, (1995) Performed by Stonybrook Laboratories, Inc. for Mobil Corporation. [accessed 2019 Jun 10] [Summary; Study 3 Repeated-dose toxicity in HPVIS (US EPA 2019)]
[Stonybrook] Stonybrook Laboratories, Inc.. 1995b. An Ames Salmonella/mammalian microsome mutagenesis assay. [accessed 2019 Jun 10] [Summary; Study 1 Genetic toxicity in vitro in HPVIS (US EPA 2019)]
[Stonybrook] Stonybrook Laboratories, Inc.. 1995c. Assay for induction of chromosomal aberrations in cultured Chinese hamster ovary (CHO) cells. Performed by Stonybrook Laboratories, Inc. [accessed 2019 Jun 10] [Summary; Study 2 Genetic toxicity in vitro in HPVIS (US EPA 2019)]
Thompson PW. (1995) Salmonella typhimurium and Escherichia coli/mammalian-microsome reverse mutation assay. Report by Safepharm Laboratories Limited, Derby U.K. conducted for Chevron Research & Technology Company, Richmond, CA. [accessed 2019 Jun 10] [Summary; Study 5 Genetic toxicity in vitro in HPVIS (US EPA 2019)].
[US EPA] United States Environmental Protection Agency. 2010. Screening-level hazard characterization. Sponsored chemical. 1-Decene, tetramer, mixed with 1-decene trimer, hydrogenated (CAS RN 68649-12-7). [PDF] [accessed 2019 May 14].
[US EPA] United States Environmental Protection Agency. 2019. ChemView [database on the Internet]. Search results for CAS RN 68649-12-7. [Updated 2019 Aug 9].
Wegner MD, Sterner TR, Mumy KL, Wong BA, Mattie DR. 2018. Histopathology results for acute and two-week inhalation toxicity studies with polyalphaolefin (PAO) fluid in rats. Interim report. Air Force Research Laboratory; 711th Human Performance Wing; Airman Systems Directorate; Human-centered ISR Division; Molecular Mechanisms Branch; Wright-Patterson AFB OH 45433. [accessed 2019 Jul 4].
Appendix A. Read-across approach
| Consideration | Rationale |
|---|---|
| 1. Chemical structure. Emphasis was placed on analogues with branched, long-chain hydrocarbons (C20-C40) in which the carbon-carbon double bonds were hydrogenated to form single bonds. No additional functional groups were included. | Analogues that have similar chemical structure are expected to have similar toxicity profiles. |
| 2. Common OECD QSAR Toolbox profiler/structural alerts | Analogues with similar structural alerts are expected to share greater similarity in terms of physical-chemical properties, exposure pathway, bioavailability and toxicity. |
| 3. Similar physical-chemical properties. Emphasis was placed on chemical structures with similar molecular weight, water solubility, vapour pressure, and log Kow. | Analogues with similar physical-chemical properties may potentially share similar toxicological profiles. |
| 4. Similar health effects. Emphasis was placed on analogues with health effects data available for both the target (Decenes Group) and analogue substances: acute toxicity, irritation, sensitization (anchor data). | Analogues with similar anchor data results will share similar toxicological profiles for other hazard endpoints. |
Appendix B. Hazard summary of hydrogenated didecene, HTTD and analogue substances
| Chemical name | 1-Decene, dimer, hydrogenated (hydrogenated didecene) | 1-Decene, tetramer, mixed with 1-decene trimer, hydrogenated (HTTD) | 1-Decene, homopolymer, hydrogenated | 1-Dodecene dimer with 1-decene, hydrogenated |
|---|---|---|---|---|
Role | Target substance | Target substance | Analogue 1 | Analogue 2 |
CAS RN | 68649-11-6 | 68649-12-7 | 68037-01-4 | 151006-58-5 |
Chemical structure | 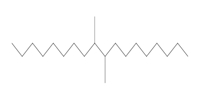 (Representative dimer structure) (Representative dimer structure)
| 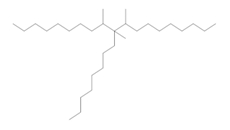 (Representative trimer structure) (Representative trimer structure) |  n is unknown n is unknown | 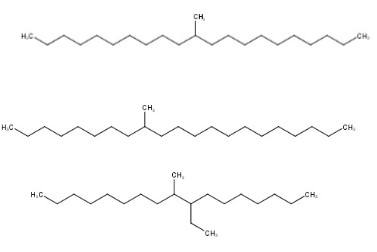 |
Molecular formula | C20H42 | C30H62 | C30H62 and C40H82 (ECHA 2019a) | C20-22H42-46 (ECHA 2019b) |
Molecular weight (g/mol) | 282.556 | 422.81 | 422-562 (ECHA 2019a) | 282-310 (ECHA 2019b) |
Melting point (°C) | -73 (experimental) (ECHA c2007-2019a) | -73 (experimental) (US EPA 2010, 2019) | < -57 (ECHA 2019a) | NA |
Boiling point (°C) | NA | NA | 217 – 596 at 101.3 kPa (ECHA 2019a) | NA |
Vapour pressure (Pa) | 1.889 (experimental) (ECHA c2007-2019a) | < 13 (experimental) (US EPA 2010, 2019) | < 0.545 Pa at 20°C (ECHA c2007-2019d) | NA |
Water solubility (mg/L) | < 0.1 at 20°C (experimental) (ECHA c2007-2019a) | NA | 0.1 (100 ug/L) at 20°C and pH 7 (measured; OECD TG 105) [Nexbase 2006] (ECHA c2007-2019d) | NA |
log KOW (dimensionless) | > 6.5 (experimental) [Nexbase 2002] (ECHA c2007-2019a) | > 5 (experimental) (ECHA c2007-2019b) | > 6.5 at 20°C and pH 7 (measured; OECD TG 117) [Nexbase 2006] (ECHA c2007-2019d) | NA |
Acute toxicity (oral) | LD50 > 4 100 mg/kg or 5 mL/kg bw in rats [SF-0203-41] (ECHA c2007-2019a) LD50 > 5 000 mg/kg in rats [Emery 3002] (ECHA c2007-2019a) LD50 > 15 380 mg/kg bw in rats [Synfluid SF-0201-3] (ECHA c2007-2019a) | LD50 > 5 000 mg/kg bw in rats [Emery 3004] (ECHA c2007-2019a) | LD50 > 5 000 mg/kg bw in rats [Emery 3006] (ECHA c2007-2019a) | LD50 > 5 000 mg/kg bw in rats [Oronite XS 101] (ECHA c2007-2019a) |
Acute toxicity (inhalation) | LOAEC = 100 mg/m3 based on histopathology effects in the nasal cavity and lungs of rats (6 hours; whole body exposure with 14 d recovery) (Mattie et al 2017; Wegner et al 2018) 4 hour LC50 = 900–1 400 mg/m3 (female); 1 400 – 2 000 mg/m3 (male) in rats [SF-0203-41]; death (various concentrations); lung effects at gross necropsy in all animals that died (ECHA c2007-2019a) 1 hour LC50: unable to determine LC50 due to mortality in rats within 3 days of exposure; clinical signs, body weight loss, change in lung and trachea weight reported [Oronite Synfluid PAO 2cSt] (ECHA c2007-2019a) 4 hour LC50 < 10 000 mg/m3 in rats; 6/10 rats died within 12 hours of exposure [Synfluid SF 0201-3] (ECHA c2007-2019a) |
| 4 hour LC50 > 5.2 mg/L (5200 mg/m3) in rats [MRD-05465] (ECHA c2007-2019a) 4 hour LC50 > 2.5 mg/L (2 500 mg/m3) in rats (US EPA 2010) | NA |
Acute toxicity (demal) | LD50 > 3 000 mg/kg bw in rabbits [Synfluid SF 0201-3] (ECHA c2007-2019a) | NA | LD50 > 2 ml/kg bw or 1 640 mg/kg in rabbits [SF-0802-37; 1-decene homopolymer hydrogenated] (ECHA c2007-2019b) | LD50 > 2 000 mg/kg bw in rats [Oronite XS 101] (ECHA c2007-2019a) |
Skin sensitization | Not a dermal sensitizer in guinea pigs [Ethylflo 362NF; Ethylflo 364NF; Oronite Synfluid PAO 2cST] (ECHA c2007-2019a) | NA | Not a dermal sensitizer in guinea pigs [Silkflo 366 NF] (ECHA c2007-2019a) | NA |
Short-term repeated dose toxicity (oral) | NA | NA | Study in rats by gavage (28 d): NOAEL = 5 000 mg/kg-day (HTD) (Mobil 1990a; US EPA 2010) Study in rats by diet (28 d): NOAEL = 6 245 mg/kg bw/day (male); 6 771 mg/kg bw/day (female); (HTD) [Nexbase 2006 FG] (ECHA c2007-2019a) | Study in rats by gavage (29 d): NOAEL = 1 000 mg/kg bw/day [Oronite XS 101] (ECHA c2007-2019a)
|
Short-term repeated dose toxicity (dermal) | NA | NA | NA | NA |
Subchronic repeated dose toxicity (oral) | NA | NA | Study in rats by gavage (91 d combined repeated dose/reproductive toxicity study): NOAEL = 1 000 mg/kg-day (HTD) [Ethylflo 166] (Daniel 1994; ECHA c2007-2019a; US EPA 2010) Study in rats by diet (90 d with 4 wk recovery): NOAEL = 4 159.4 mg/kg bw/day (male); 4 619.9 mg/kg bw/day (female); [Nexbase 2006 FG] (ECHA c2007-2019a) Study in rats by diet (90d): NOAEL = 20 000 ppm or 1 000 mg/kg bw/daya (HTD) (Stonybrook Laboratories 1995a; US EPA 2010) Study in rats by diet (90d): NOAEL = 20 000 ppm or 1 000 mg/kg bw/day a (HTD) (Mobil 1990b; US EPA 2010) | NA |
Reproductive/ developmental toxicity (oral) | NA | NA | Repeated dose/ reproductive study in rats by gavage: Reproductive NOAEL = 1 000 mg/kg-day (HTD) [Ethylflo 166] (Daniel 1994; ECHA c2007-2019a; US EPA 2010) | NA |
Reproductive/ developmental toxicity (dermal) | NA | NA | Developmental study in rats (GD 0-19): Developmental NOAEL = 2 000 mg/kg-day (HTD) (Mobil 1988; US EPA 2010) | NA |
Genotoxicity | NA | NA | Negative in vivo micronucleus assay (ECHA c2007-2019a; Mobil 1985; US EPA 2010) Negative in vitro mutagenicity (ECHA c2007-2019a) | Negative in vivo micronucleus assay (ECHA c2007-2019a) |
Carcinogenicity | NA | NA | Dermal study in mice (104 wk): 50 uL/application 2x/week did not induce skin tumour incidence(Kettering 1990; US EPA 2010) | NA |
a Dose conversion based on 1 ppm in food = 0.05 mg/kg bw/day in rats (Health Canada 1994).
Abbreviation: NA = not available; HTD = highest tested dose
| Chemical name | 1-Decene, dimer, hydrogenated (hydrogenated didecene) | 1-Decene, tetramer, mixed with 1-decene trimer, hydrogenated (HTTD) | 1-Dodecene, polymer with 1-decene, hydrogenated | 1-Dodecene, trimer, hydrogenated |
|---|---|---|---|---|
Role | Target substance | Target substance | Analogue 3 | Analogue 4 |
CAS RN | 68649-11-6 | 68649-12-7 | 151006-60-9 | 151006-62-1 |
Chemical structure | 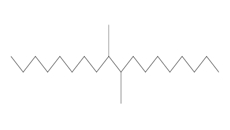 |  (Representative trimer structure) (Representative trimer structure) |  n and m are unknown n and m are unknown |  |
Molecular formula | C20H42 | C30H62 | C32-34H66-70 (ECHA 2019c) | C36H74 (ECHA 2017) |
Molecular weight (g/mol) | 282.556 | 422.81 | 450-478 (ECHA 2019c) | 506.0 (ECHA 2017) |
Melting point (°C) | -73 (experimental) (ECHA c2007-2019a) | -73 (experimental) (US EPA 2010, 2019) | NA | NA |
Boiling point (°C) | NA | N/A | NA | NA |
Vapour pressure (Pa) | 1.889 (experimental) (ECHA c2007-2019a) | < 13 (experimental) (US EPA 2010, 2019) | NA | 2.5x10-7 at 25 °C (EC 2017) |
Water solubility (mg/L) | < 0.1 at 20°C (experimental) (ECHA c2007-2019a) | NA | NA | < 0.1-0.482 mg/L at 20°C; < 1E-6 mg/L (EC 2017) |
log KOW (dimensionless) | > 6.5 (experimental) [Nexbase 2002](ECHA c2007-2019a) | > 5 (experimental) (ECHA c2007-2019b) | NA | >3.87 at 20°C; > 6.5 at 20°C; > 7.64 (EC 2017) |
Acute toxicity (oral) | LD50 > 4 100 mg/kg or 5 mL/kg bw in rats [SF-0203-41] (ECHA c2007-2019a) LD50 > 5 000 mg/kg in rats [Emery 3002] (ECHA c2007-2019a) LD50>15 380 mg/kg bw in rats [Synfluid SF-0201-3] (ECHA c2007-2019a) | LD50 > 5 000 mg/kg bw in rats [Emery 3004] (ECHA c2007-2019a) | LD50>5 000 mg/kg-bw in rats [Alkane 5] (ECHA c2007-2019a; US EPA 2010) | LD50 > 5 000 mg/kg-bw in rats [Alkane 4] (ECHA c2007-2019a; US EPA 2010) |
Acute Toxicity (inhalation) | LOAEC = 100 mg/m3 based on histopathology effects in the nasal cavity and lungs of rats (6 hours; whole body exposure with 14 d recovery) (Mattie et al 2017; Wegner et al 2018) 4 hour LC50 = 900-1400 mg/m3 (female); 1 400 – 2 000 mg/m3 (male) in rats [SF-0203-41]; death (various concentrations); lung effects at gross necropsy in all animals that died (ECHA c2007-2019a) 1 hour LC50: unable to determine LC50 due to mortality in rats within 3 days of exposure; clinical signs, body weight loss, change in lung, trachea weight reported [Oronite Synfluid PAO 2cSt] (ECHA c2007-2019a) 4 hour LC50 < 10 000 mg/m3 in rats;6/10 rats died within 12 hours of exposure [Synfluid SF 0201-3] (ECHA c2007-2019a) | N/A | 4 hour LC50 > 5.0 mg/L (5 000 mg/m3) in rats [Alkane 5] (ECHA c2007-2019a; US EPA 2010) | 4 hour LC50 > 5.06 mg/L (5 060 mg/m3) in rats [Alkane 4] (ECHA c2007-2019a ; US EPA 2010) |
Acute toxicity (dermal) | LD50 > 3 000 mg/kg bw in rabbits [Synfluid SF 0201-3] (ECHA c2007-2019a) | NA | LC50 > 2 000 mg/kg-bw in rats [Alkane 5] (ECHA c2007-2019a; US EPA 2010) | LC50 > 2 000 mg/kg-bw in rats [Alkane 4] (ECHA c2007-2019a; US EPA 2010) |
Skin sensitization | Not a dermal sensitizer in guinea pigs [Ethylflo 362NF; Ethylflo 364NF; Oronite Synfluid PAO 2cST] (ECHA c2007-2019a) | NA | NA | NA |
Short-term repeated dose toxicity (oral) | NA | NA | NA | Study in rats by gavage (28 d): NOEL = 1 000 mg/kg (single dose) bw/day[Alkane 4] (ECHA c2007-2019a; SafePharm Laboratories 1995a; US EPA 2010) |
Short-term repeated dose toxicity (dermal) | NA | NA | NA | NA |
Subchronic repeat dose toxicity (oral) | NA | NA | NA | Oral study in rats by gavage (10 weeks): NOAEL = 1 000 mg/kg bw/day (HTD) [Alkane 4] (ECHA c2007-2019a) |
Subchronic repeated dose toxicity (dermal) | NA | NA | NA | NA |
Long-term repeated dose toxicity (oral) | NA | NA | NA | NA |
Reproductive/ developmental toxicity (oral) | NA | NA | NA | One-generation reproduction study in rats by gavage: Reproductive and developmental NOAEL = 1 000 mg/kg bw (HTD) [Alkane 4] (ECHA c 2007-2019b) |
Reproductive/ developmental toxicity (dermal) | NA | NA | NA | NA |
Genotoxicity | NA | NA | Negative in vivo micronucleus assay (ECHA c2007-2019c; SafePharm 1995c) Negative in vitro mutagenicity (Thompson 1995; US EPA 2010) | Negative in vivo micronucleus assay (SafePharm 1995b; ECHA c2007-2019a) Negative in vitro mutagenicity (ECHA c2007-2019a; SafePharm 1995d; SITEK Research Laboratories 2001; US EPA 2010) Negative in vitro clastogenicity (ECHA c2007-2019a; SafePharm 1995e; US EPA 2010) |
Carcinogenicity | NA | NA | NA | N/A |
a Dose conversion based on 1 ppm in food = 0.05 mg/kg bw/day in rats (Health Canada 1994).
Abbreviation: HTD = highest tested dose
| Chemical name | 1-Decene, dimer, hydrogenated (hydrogenated didecene) | 1-Decene, tetramer, mixed with 1-decene trimer, hydrogenated (HTTD) | 1-Dodecene, polymer with 1-decene and 1-octene, hydrogenated | Pentadecane, 7-methylene-, mixed with 1-tetradecene, dimers and trimmers, hydrogenated |
|---|---|---|---|---|
Role | Target substance | Target substance | Analogue 5 | Analogue 6 |
CAS RN | 68649-11-6 | 68649-12-7 | 163149-28-8 | 1000172-11-1 |
Chemical structure |  |  (Representative trimer structure) (Representative trimer structure) |  n, m and p are unknown n, m and p are unknown | 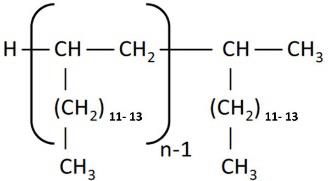 where n = 2-4 where n = 2-4 |
Molecular formula | C20H42 | C30H62 | C26-30H54-62 (ECHA 2019d) | C28-80H58-162 (ECHA c2007-2019c) |
Molecular weight (g/mol) | 282.556 | 422.81 | 366-422 (ECHA 2019d) | 394-1122 (ECHA c2007-2019c) |
Melting point (°C) | -73 (experimental) (ECHA c2007-2019a) | -73 (experimental) (US EPA 2010, 2019) | NA | -63 to -39 [Durasyn 124] (ECHA c2007-2019c) |
Boiling point (°C) | N/A | N/A | NA | 187-570 [Durasyn 124] (ECHA c2007-2019c) |
Vapour pressure (Pa) | 1.889 (experimental) (ECHA c2007-2019a) | < 13 (experimental) (US EPA 2010, 2019) | NA | < 0.013 at 20°C (0.0001 mmHg at 68°F [Durasyn 124] (ECHA c2007-2019c) |
Water solubility (mg/L) | < 0.1 at 20°C (experimental) (ECHA c2007-2019a) | NA | < 0.4 mg/L at 24°C and pH 7 (tested as MCP 1602) (ECHA 2019d) | NA |
log KOW (dimensionless) | > 6.5 (experimental) [Nexbase 2002] (ECHA c2007-2019a) | > 5 (experimental) (ECHA c2007-2019b) | NA | NA |
Acute toxicity (oral) | LD50 > 4 100 mg/kg or 5 mL/kg bw in rats [SF-0203-41] (ECHA c2007-2019a) LD50 > 5 000 mg/kg in rats [Emery 3002] (ECHA c2007-2019a) LD50 > 15 380 mg/kg bw in rats [Synfluid SF-0201-3] (ECHA c2007-2019a) | LD50 > 5 000 mg/kg bw in rats [Emery 3004] (ECHA c2007-2019a) | LD50 > 2 000 mg/kg-bw in rats [Octene, decene, dodecene copolymer] (US EPA 2010) | NA |
Acute toxicity (inhalation) | LOAEC = 100 mg/m3 based on histopathology effects in the nasal cavity and lungs of rats (6 hours; whole body exposure with 14 d recovery) (Mattie et al 2017; Wegner et al 2018) 4 hour LC50 = 900 – 1 400 mg/m3 (female); 1 400 – 2 000 mg/m3 (male) in rats [SF-0203-41]; death (various concentrations); lung effects at gross necropsy in all animals that died (ECHA c2007-2019a) 1 hour LC50: unable to determine LC50 due to mortality in rats within 3 days of exposure; clinical signs, body weight loss, change in lung, trachea weight reported [Oronite Synfluid PAO 2cSt] (ECHA c2007-2019a) 4 hour LC50 < 10 000 mg/m3 in rats; 6/10 rats died within 12 hours of exposure [Synfluid SF 0201-3] (ECHA c2007-2019a) | NA | NA | NA |
Acute toxicity (dermal) | LD50 > 3 000 mg/kg bw in rabbits [Synfluid SF 0201-3] (ECHA c2007-2019a) | NA | NA | NA |
Skin sensitization | Not a dermal sensitizer in guinea pigs [Ethylflo 362NF; Ethylflo 364NF; Oronite Synfluid PAO 2cST] (ECHA c2007-2019a) | NA | NA | NA |
Short-term repeated dose toxicity (oral) | NA | NA | NA | NA |
Short-term repeated dose toxicity (dermal) | NA | NA | Dermal study in rats (4 weeks with 2 week observation): NOAEL = 2 000 mg/kg-day (HTD) [Octene, decene, dodecene copolymer] (Mobil 1995; US EPA 2010) | NA |
Subchronic repeated dose toxicity (oral) | NA | NA | NA | Oral study in rats by gavage (from reproductive toxicity study): NOAEL = 1 000 mg/kg bw/day (HTD) [Durasyn 164X] (ECHA c2007-2019c) |
Reproductive/ developmental toxicity (oral) | NA | NA | NA | Oral 2-generation reproduction study in rats by gavage: Reproductive and developmental NOAEL = 1 000 mg/kg bw/day (HTD) [Durasyn 164X] (ECHA c2007-2019c) |
Reproductive/ developmental toxicity (dermal) | NA | NA | NA | NA |
Genotoxicity | NA | NA | Negative in vitro mutagenicity (Stonybrook 1995b; US EPA 2010) Negative in vitro clastogenicity (Stonybrook 1995c; US EPA 2010) | Negative in vitro mutagenicity (ECHA c2007-2019c) |
Carcinogenicity | NA | NA | NA | NA |
a Dose conversion based on 1 ppm in food = 0.05 mg/kg bw/day in rats (Health Canada 1994).
Abbreviation: HTD = highest tested dose
Appendix C. Inhalation exposures to humans from spray products used for firearm maintenance
| Exposure scenario | Parameters and assumptions |
|---|---|
Common parameters for an adult individual cleaning a single disassembled firearm (pistol) with CLP. | ConsExpo Web (2016) was used to estimate inhalation of aerosol. All parameters are from ConsExpo Web (2016) unless otherwise stated. Model: exposure to spray Mode of release: spraying (targeted) Exposure duration: 20 minutes (professional judgement based on observations from user videos where spraying of lubricant/cleaner on firearm might take place intermittently over 2 to 5 minute periods over a 20-minute cleaning period) Spray duration: 20 seconds (professional judgement on observations from user videos of product sprayed on a small firearm) Room volume: 20 m3 (utility/unspecified room); Room height: 2.5 m; Ventilation rate: 0.6/hr Age group: 19 years and older |
Aerosol spray | Airborne fraction: 0.2 g/g for lubricants, spray can, penetrating spray (RIVM 2009) Median aerosol diameter: 23.3 µm (lognormal distribution; arithmetic coefficient of variation: 1.3 for lubricants, spray can, penetrating spray (RIVM 2009) Mass generation rate: 1.5 g/s for lubricants, spray can, penetrating spray (Tuinman et al. 2007 as cited in RIVM 2009) Density of non-volatile: 1.8 g/cm3 (from defaults for pest control products, sprays, targeted spot, spray can as cited in ConsExpo Web 2016) Inhalation cut-off diameter: 49.9 µm (to include larger particle sizes that may be in the nasal cavity) |
Trigger spray | Airborne fraction: 0.008 g/g (based on ready-to-use, targeted spot, crack and crevice) (RIVM 2010) Median aerosol diameter: 7.7 µm (lognormal distribution; arithmetic coefficient of variation: 1.9; based on ready-to-use, targeted spot, crack and crevice RIVM 2010) Mass generation rate: 1.6 g/s; default for all trigger sprays in RIVM (2009) Density of non-volatile: 1.8 g/cm3 (from defaults for pest control products, sprays, targeted spot, trigger spray in ConsExpo Web 2016) Inhalation cut-off diameter: 49.9 µm (to include larger particle sizes that may be in the nasal cavity) |
