Draft screening assessment ethers group
Official title: Draft Screening Assessment - Ethers Group
Chemical Abstracts Service Registry Numbers:
- 60-29-7
- 101-84-8
- 115-10-6
- 34590-94-8
Environment and Climate Change Canada
Health Canada
March 2021
Synopsis
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of four substances referred to collectively under the Chemicals Management Plan as the Ethers Group. Substances in this group were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns. The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 1), their Domestic Substances List (DSL) names, common names and abbreviations are listed in the table below.
| CAS RN | DSL name | Common name | Abbreviation |
|---|---|---|---|
| 60-29-7 | Ethane, 1,1’-oxybis- | Diethyl ether | DEE |
| 101-84-8 | Benzene, 1,1’-oxybis- | Diphenyl ether | DPE |
| 115-10-6a | Methane, oxybis- | Dimethyl ether | DME |
| 34590-94-8 | Propanol, 1(or 2)-(2-methoxymethylethoxy)- | Dipropylene glycol methyl ether | DPGME |
a This substance was not identified under subsection 73(1) of CEPA but was included in this assessment as it was considered a priority on the basis of other human health concerns.
DEE, DPE and DME naturally occur at low levels in some foods, but DPGME does not occur naturally in the environment. All four substances in the Ethers Group were included in surveys issued pursuant to Section 71 of CEPA. The submitted information indicated that DEE and DPE are not manufactured in Canada above the reporting threshold of 100 kg, while 100 000 to 1 000 000 kg of DME and 10 000 to 100 000 kg of DPGME were manufactured in Canada in 2011. The four substances were also imported into Canada with quantities ranging from 487 199 to 1 287 772 kg. Reported uses are wide-ranging, with most substances being used in air care, automotive, aircraft and transportation, cleaning and furnishing care, fuels and related products, oil and natural gas extraction, and paints and coatings.
In Canada, the substances in the Ethers Group may also be used as components in food packaging materials, food processing aids, food flavouring agents, medicinal or non-medicinal ingredients in disinfectant, human or veterinary drug products and natural health products, cosmetics, and various other products available to consumers, and as formulants in pest control products.
The ecological risks of the four substances in the Ethers Group were characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, substances in the Ethers Group are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from DEE, DPE, DME and DPGME. It is proposed to conclude that DEE, DPE, DME and DPGME do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
For the general population of Canada, the predominant sources of exposure to substances in the Ethers Group are from use of products available to consumers that contain these substances, and from environmental media and food. Scenarios which result in the highest levels of exposure were used to characterize potential exposure of Canadians to substances in the Ethers Group through the use of products available to consumers, and from environmental media and food.
According to the available information, the general population is expected to be exposed to DEE from environmental media and from the use of various products available to consumers such as body lotions, corn and callus removers, and automotive starting fluids. Based on laboratory studies, the most critical effects for DEE were body weight changes, liver toxicity and increased mortality when exposed orally, and liver toxicity when exposed via the inhalation route. A structurally-similar substance, diisopropyl ether (DIPE), was also used to inform the health effects assessment of DEE.
Exposure of the general population to DPE is expected from environmental media and potential use as a food flavouring agent, and from the use of various products available to consumers such as air fresheners and hand creams. Based on laboratory studies, changes in body weight were noted as the most critical effects for oral and long-term inhalation exposure to DPE.
Exposure of the general population to DME is expected from environmental media and from the use of various products such as spray sunscreens. Based on laboratory studies, the critical effect for DME was decreased survival rates in rats exposed via long-term inhalation.
Although potential inhalation and dermal exposure to DPGME may occur from the use of products available to consumers, exposure to DPGME is characterized qualitatively as it is considered to be of low hazard potential. DPGME was not identified as inducing any adverse effects in any of the available studies. A structurally-similar substance, propylene glycol methyl ether (PGME), was also used to inform the health effects assessment of DPGME.
Comparisons of levels of exposure to DEE, DPE and DME from environmental media, and from the use of products available to consumers, as well as exposure to DPE from food from its potential use as a food flavouring agent, with levels at which health effects occur result in margins that are considered adequate to address uncertainties in the health effects and exposure databases. As DPGME is considered to be of low hazard potential, the risk to human health is considered to be low.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that DEE, DPE, DME and DPGME do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that DEE, DPE, DME and DPGME do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of four substances referred to collectively under the Chemicals Management Plan as the Ethers Group to determine whether these substances present or may present a risk to the environment or to human health. The substances in this group were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns (ECCC, HC [modified 2017]).
The ecological risks of the substances in the Ethers Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
DEE has been reviewed by the United States Environmental Protection Agency (US EPA) and the German Senate Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area (MAK Commission). DPE has been reviewed by the European Commission (EC), the European Food Safety Agency (EFSA), the Joint Food and Agricultural Organization/World Health Organization (WHO) Expert Committee on Food Additives (JECFA), the MAK Commission and US EPA. DME has been reviewed by the US EPA, MAK Commission and EFSA. DPGME has been reviewed by the MAK Commission, the Australian Government Department of Health (AGDH) and the Organisation for Economic Co-operation and Development (OECD) Cooperative Chemicals Assessment Programme; an OECD Screening Information Dataset (SIDS) International Assessment Report (SIAR) is available. These reviews were used to inform the health effects characterization in this screening assessment.
This draft screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to September, 2019. Empirical data from key studies as well as results from models were used to reach proposed conclusions.
This draft screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The human health portions of this assessment have undergone external review. Comments on the technical portions relevant to human health were received from Dr. Supratik Kar, Dr. Jerzy Leszczynski and Dr. Ole Jalob Nøstbakken at Risk Sciences International. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this draft screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This draft screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precaution.Footnote 2 This draft screening assessment presents the critical information and considerations on which the proposed conclusions are based.
2. Identity of substances
The Chemical Abstracts Service Registry Numbers (CAS RN), Domestic Substances List (DSL) names, common names and abbreviations for the individual substances in the Ethers Group are presented in Table 2-1. As part of the categorization exercise, DPGME was considered as a discrete substance under CEPA (ECCC, HC [modified 2017]). However, based on its mixture of isomers with the methyl substituent in either the 1 or 2 position, it is recognized that this substance possesses the characteristics of an Unknown or Variable composition Complex reaction products and Biological material (UVCB).
| CAS RN (abbreviation) | DSL name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 60-29-7 (DEE) | Ethane, 1,1’-oxybis- (Diethyl ether) | 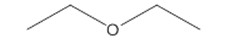 C4H10O C4H10O |
74.12 |
| 101-84-8 (DPE) |
Benzene, 1,1’-oxybis- (Diphenyl ether) | 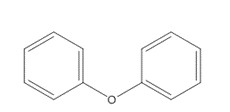 C12H10O C12H10O |
170.21 |
| 115-10-6 (DME) | Methane, oxybis- (Dimethyl ether) | 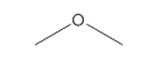 C2H6O C2H6O |
46.07 |
| 34590-94-8(DPGMEa, b) | Propanol, 1(or 2)-(2-methoxymethylethoxy-) (Dipropylene glycol methyl ether) | 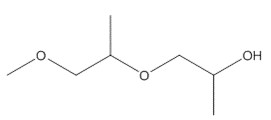 C7H16O3 C7H16O3 |
148.20 |
a DPGME has characteristics of an UVCB (Unknown or Variable composition Complex reaction products and Biological material).
b The chemical structure of the 1-isomer is shown.
2.1 Selection of analogues and use of (Q)SAR models
A read-across approach using data from analogues and the results of (quantitative) structure-activity relationship ((Q)SAR) models, where appropriate, has been used to inform the human health assessment. Analogues were selected that were structurally similar and/or functionally similar (e.g., similar physical-chemical properties, toxicokinetics) to substances within this group and that had relevant empirical data that could be used to read across to substances with limited empirical data. The applicability of (Q)SAR models was determined on a case-by-case basis. Details of the read-across data and (Q)SAR models chosen to inform the human health assessment of the Ethers Group are further discussed in the relevant sections of this report.
Information on the identities and chemical structures of the analogues used to inform this assessment is presented in Table 2-2. Information on the hazard and physical chemical properties of the analogues is presented in Appendix A.
| CAS RN (acronym/abbreviation) | DSL or other name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 108-20-3 (DIPE) | Diisopropyl ether | 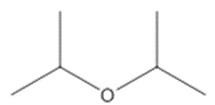 C6H14O C6H14O |
102.18 |
| 1320-67-8 (PGME) | Propylene glycol methyl ether | 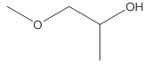 C4H10O2 C4H10O2 |
90.12 |
DIPE (CAS RN 108-20-3) was used as an analogue by the US EPA to inform the human health assessment of DEE, and was considered appropriate based on structural similarities, including the chain length and location of the ether group, and mechanism of action (US EPA 2014).
PGME (CAS RN 1320-67-8) was used as an analogue by the OECD to inform the human health assessment of DPGME, and was considered appropriate based on structural similarities, physical chemical properties, and toxicokinetics (OECD 2001).
3. Physical and chemical properties
A summary of physical and chemical property data of the substances in the Ethers Group is presented in Table 3-1. When experimental information was limited or not available for a property, (Q)SAR models were used to generate predicted values for the substance. Additional physical and chemical properties are reported in ECCC (2016b).
| Property | DEE | DPE | DME | DPGME | Reference |
|---|---|---|---|---|---|
| Physical state | Liquid | Solid | Gas | Liquid | N/A |
| Melting point (°C) | -116 | 27 | -142 | -83 | ChemIDplus 1993- |
| Vapour pressure (Pa) | 7.17 × 104 | 3.00 | 5.93 × 105 | 73.3 | ChemIDplus 1993- |
| Henry’s law constant (Pa·m3/mol) | 1.25 × 102 | 28.3 | 1.01 × 102 a | 1.08 × 10−2a | ChemIDplus 1993- |
| Water solubility (mg/L) | 6.04 × 104 | 18.0 | 4.60 × 104 | 1.00 × 106 | ChemIDplus 1993- |
| Log Kow (dimensionless) | 0.89 | 4.21 | 0.1 | -0.35a | ChemIDplus 1993- |
Abbreviations: N/A, not applicable; Kow, octanol-water partition coefficient
a Modelled values
4. Sources and uses
DEE, DME and DPE are reported to naturally occur at low levels in some foods (Nijssen 2018; WHO 2004). DPGME does not occur naturally.
All of the substances in the Ethers Group have been included in surveys issued pursuant to section 71 of CEPA (Canada 2009, 2012). Table 4-1 presents a summary of information reported on the total manufacture and total import quantities for the Ethers Group.
| Common name | Total manufacturea (kg) | Total importsa (kg) | Reporting year | Survey reference |
|---|---|---|---|---|
| DEE | NRb | 914298 | 2011 | Environment Canada 2013 |
| DPE | NRb | 100000 – 1000000 | 2008 | Environment Canada 2009 |
| DME | 100 000 – 1000000 | 487 199 | 2011 | Environment Canada 2013 |
| DPGME | 10000 – 100000 | 1287772 | 2011 | Environment Canada 2013 |
Abbreviation: NR, Not Reported
a Values reflect quantities reported in response to the surveys conducted under section 71 of CEPA (Environment Canada 2009, 2013). See surveys for specific inclusions and exclusions (schedules 2 and 3).
b No manufacturing quantities were reported for the substance above the reporting threshold of 100 kg for the specified reporting year.
Table 4-2 presents a summary of the major uses of substances in the Ethers Group according to information reported through a CEPA section 71 survey (Environment Canada 2009, 2013). Table 4-3 presents possible additional uses for substances in the Ethers Group identified in Canada.
| Major usesa | DEE | DPE | DME | DPGME |
|---|---|---|---|---|
| Adhesives and sealants | N | N | Y | N |
| Air care | N | N | Y | Y |
| Automotive, aircraft and transportation | N | N | Y | Y |
| Automotive Care | N | N | N | Y |
| Building or construction materials | N | N | N | Y |
| Cleaning and furnishing care | N | N | Y | Y |
| Electrical and electronics | N | N | N | Y |
| Explosive materials | Y | N | N | N |
| Fabric, textile and leather articles | N | N | N | Y |
| Fuels and Related Products, mixtures or manufactured items | Y | N | N | Y |
| Heat transfer fluid | N | Y | N | N |
| Ink, toners and colourants | N | N | N | Y |
| Lubricants and greases | N | N | N | Y |
| Oil and natural gas extraction | Y | N | N | Y |
| Paints and coatings | N | N | Y | Y |
| Personal care | N | N | Y | N |
| Plastic and rubber materials | N | N | N | Y |
| Solvent | N | N | N | Y |
Abbreviations: Y = yes this use was reported for this substance; N = no this use was not reported for this substance
a Non-confidential uses reported in response to the surveys conducted under section 71 of CEPA (Environment Canada 2009, 2013). See surveys for specific inclusions and exclusions (schedules 2 and 3).
| Use | DEE | DPE | DME | DPGME |
|---|---|---|---|---|
| Food packaging materialsa | N | N | N | Yb |
| Food processing aidsa | Y | N | Y | N |
| Food flavouring agenta | N | Y | N | N |
| Medicinal or non-medicinal ingredients in disinfectant, human or veterinary drug productsc | Y | N | Y | Y |
| Natural Health Products Ingredients Databased | Y | Y | Y | Y |
| Medicinal or non-medicinal ingredients in licensed natural health productse | Y | N | Y | Y |
| Notified to be present in cosmetics under the Cosmetic Regulationsf | Y | Y | Y | Y |
| Formulant in registered pest control productsg | N | Y | Y | Y |
Abbreviations: Y = use was indicated for this substance; N = use was not indicated for this substance
a Personal communications, emails from the Food Directorate (FD), Health Canada to Existing Substances Risk Assessment Bureau (ESRAB), Health Canada, dated 2018 and 2019; unreferenced
b Used as a solvent in the manufacturing of coatings and printing inks that have no potential for food contact
c Personal communication, email from the Therapeutic Products Directorate (TPD), Health Canada to ESRAB, Health Canada, dated 2018; unreferenced
d NHPID (modified 2019); Personal communications, emails from the Non-prescription and Natural Health Products Directorate (NNHPD), Health Canada to ESRAB, Health Canada, dated 2018; unreferenced
e LNHPD (modified 2018)
f Personal communication, email from the Consumer and Hazardous Products Safety Directorate (CHPSD), Health Canada to ESRAB, Health Canada, dated 2018; unreferenced
g Personal communication, email from the Pest Management Regulatory Agency (PMRA), Health Canada to ESRAB, Health Canada, dated 2018; unreferenced
In addition to the uses presented above, further uses of substances in the Ethers Group have been identified in Canada. DEE is found in automotive starting fluids (SDS 2017a). DPE is present in air fresheners (SDS 2016a) and a bathroom cleaner (SDS 2017b). DME is used in sealants, spray/marking chalk (SDS 2015a; SDS 2019a), wall repair sprays (SDS 2015a), building insulation foam (SDS 2017d), fabric spray paint (SDS 2018a), tire protectants (SDS 2015b), salt stain removers (SDS 2015c), and deer attractants (SDS 2018b). DPGME is present in a bathroom etching cream (SDS 2016e), leather care product (SDS 2016f), fabric treatment product (SDS 2014a), and pepper spray (SDS 2014b, 2016h).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risks of the substances in the Ethers Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox 2014), and from responses to surveys issued pursuant to section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over and under classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for the substances in the Ethers Group, and the hazard, exposure and risk classification results, are presented in ECCC (2016b).
The hazard and exposure classifications for the four substances in the Ethers Group are summarized in Table 5-1.
| Substance | ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| DEE | low | low | low |
| DPE | low | low | low |
| DME | low | high | low |
| DPGME | low | low | low |
On the basis of low hazard and low exposure classifications according to information considered under ERC, DEE, DPE and DPGME were classified as having a low potential for ecological risk. It is unlikely that these substances are resulting in concerns for the environment in Canada.
According to information considered under ERC, DME was classified as having a high exposure potential on the basis of a long overall persistence and a large annual import quantity according to information submitted in response to a CEPA Section 71 survey (Environment Canada 2013). DME was classified as having a low hazard potential and thus a low potential for ecological risk. Although current use patterns result in a high exposure potential, considering the low hazard potential, DME is unlikely to be resulting in concerns to the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
Potential exposures to substances in the Ethers Group from environmental media, food, and products available to consumers are presented in this section. As DPGME is considered to be of low hazard potential (see Section 6.2.4), quantitative estimates of exposure to the general population were not derived. For DEE, DPE, and DME, sentinel exposure scenarios resulting in the highest exposures for each age group are presented to characterize risk. Additional details regarding the exposure scenarios are summarized in Appendix B.
6.1.1 DEE
Environmental media and food
DEE has been previously found in the Canadian environment and internationally. Chan et al. (1990) measured DEE in indoor and ambient air in and around 12 Canadian homes. During the initial sampling in 1986, DEE was detected in indoor air samples from 5 of 12 homes with a maximum concentration of 2 µg/m3 but was not detected in ambient air samples. In the subsequent sampling in 1987, 6 homes were sampled and DEE was detected in one indoor air sample (647 µg/m3) and in one ambient air sample (24 µg/m3). In addition to its presence in air, DEE was separately detected in a monitoring well network and outwash aquifer near the Gloucester Landfill in Ontario, with a maximum concentration of 658 µg/L and a detection frequency of 68% (Lesage et al. 1990). In the absence of Canadian monitoring data for soils, level III fugacity modelling was performed using ChemCAN v6.00 to simulate release of DEE in Canada. Using the combined reported total manufacture and import quantities (Environment Canada 2013), the predicted concentration of this substance in soil was considered to be negligible. The maximum measured concentrations of DEE in indoor and ambient air around Canadian homes (647 µg/m3 and 24 µg/m3, respectively) and in a Canadian aquifer (658 µg/L) were used to characterize exposure of Canadians to DEE from environmental media. The highest estimated intake of DEE from environmental media for the Canadian population was 0.44 mg/kg bw/day, corresponding to the intake for 1-year old children (see Appendix C for details).
Internationally, DEE has been reported in air samples from various locations, including inside cars in Spain, Japan, and Germany (with concentrations up to 25.2 µg/m3), in indoor air of a multi-storey building after extensive renovations in Germany (with concentrations up to 330 µg/m3), and in ambient air in Spain (with concentrations up to 0.09 µg/m3) (Buters et al. 2007; Cadena et al. 2018; Chan et al. 1990; Hippelein et al. 2006; Moreno et al. 2019; Rabaud et al. 2003; Raboni et al. 2015; Ramirez et al. 2010; Smet et al. 1999; Tokumura et al. 2006; Veijanen et al. 2011). DEE was also detected in water samples from groundwater in Alaska, Colorado, Connecticut, Georgia and Kansas (with concentrations up to 17 µg/L) and at a wetland in Arizona (measured at a concentration of 0.1 µg/L) (Apodaca et al. 2002; Gonthier et al. 2011; Keefe et al. 2004; Lesage et al. 1990; Mullaney et al. 1999; Pope et al. 2002; Roy et al. 2001).
No definitive information is available concerning the use of DEE in foods sold in Canada. DEE is known to be used in food processing aids internationally and is potentially used in food processing aids in Canada; if used, dietary exposure to DEE from this use in Canada is expected to be negligible (Personal communications, emails from the Food Directorate (FD), Health Canada to Existing Substances Risk Assessment Bureau (ESRAB), Health Canada, dated February 2018 and September 2019; unreferenced).
DEE is also reported to occur naturally at low levels in some foods (Nijssen 2018; WHO 2004). The occurrence data used to estimate dietary exposure to DEE was sourced from the Volatile Compounds in Food Database (Nijssen 2018), which reported a concentration of 0.15 ppm in fresh apples and a qualitative presence of DEE in other foods. For each food and beverage category in the database, the highest concentration reported for DEE was conservatively applied to represent the food category (e.g., the maximum concentration reported in apples of 0.15 ppm was applied to all apples). Canadian dietary exposure to DEE from its natural occurrence in foods was estimated by multiplying the consumption of foods by the amount of DEE in those foods. Mean and 90th percentile food consumption estimates were based on individual one-day “eaters only” food intakes reported by respondents to the 2004 Canadian Community Health Survey (CCHS) for infants up to 12 months of ageFootnote 3 and the 2015 CCHS for all other age groups (Statistics Canada 2004a, 2015). The mean and 90th percentile dietary exposures estimated in this manner for various age groups are shown in Table 6‑1. For some age groups the number of survey respondents was insufficient to generate consumption figures and corresponding exposure estimates (Personal communications, emails from FD, Health Canada to ESRAB, Health Canada, dated 2018 and 2019; unreferenced).
| - | 0 – 5 months | 6 – 11 months | 1 year | 2 – 3 years | 4 – 8 years | 9 – 13 years | 14 – 18 years | 19+ years |
|---|---|---|---|---|---|---|---|---|
| Mean | NA | 1.61 | 1.43 | 1.43 | 1.12 | 0.62 | 0.44 | 0.33 |
| 90th Percentile | NA | 3.86 | 2.50 | 2.35 | 1.97 | 1.00 | 0.80 | 0.57 |
Abbreviation: NA, Not Available
The highest estimated intake of DEE as a result of its natural occurrence in food was for 6 to 11 month olds at 1.61 μg/kg bw/day (mean) or 3.86 μg/kg bw/day (90th percentile). The natural occurrence in food is not further considered, as the potential exposures are lower in comparison to the combined exposures from other environmental media sources.
Products available to consumers
DEE is present in a starting fluid for combustion engines (SDS 2017a) and self-care products such as body lotion (Personal communication, email Consumer and Hazardous Products Safety Directorate (CHPSD), Health Canada to ESRAB, Health Canada, dated 2018; unreferenced) and corn and callus remover and wart remover (Personal communications, emails from the Natural and Non-prescription Health Products Directorate (NNHPD), Health Canada to ESRAB, Health Canada, dated 2018 and 2019; unreferenced). Due to the very high vapour pressure of DEE (7.17 × 104 Pa), exposure to DEE through the use of products available to consumers is expected to be predominantly through the inhalation route; exposure through the oral route is not expected and systemic exposure through the dermal route is expected to be minimal in comparison to inhalation as DEE is not readily absorbed through the skin (MAK Commission 1996). Table 6‑2 summarizes the estimated exposures to DEE from the use of these products for the highest exposed age group.
| Product scenario (age group) | Product concentration | Inhalation exposurea (mg/kg bw(/day)) |
|---|---|---|
| Body lotion, daily exposure (aged 19 years or above) | 3%b | 3.2 × 10-2 |
| Corn and callus remover, per event exposure (aged 9 to 13 years) | 57%c | 7.8 × 10-2 |
| Starting fluid, per event exposure (aged 19 years or above) | 60%d | 0.63 |
a 100% absorption is assumed for inhalation exposures
b Personal communication, email from CHPSD, Health Canada to ESRAB, Health Canada, dated 2018; unreferenced
c DailyMed 2019
d SDS 2017a
6.1.2 DPE
Environmental media and food
DPE has been detected in environmental media in Canada and internationally. No measured levels of DPE in Canadian environmental media were identified, although Rogers et al. (1986) conducted a study at the wastewater treatment plant at Iona Island, British Columbia where DPE was identified as a component in base/neutral fractions of wastewater and sludge. Internationally, DPE was measured in indoor air in hair salons in Spain (with concentrations up to 130 µg/m3) (Ronda et al. 2009) and in water from river basins in the Slovak Republic (with concentrations up to 4.4 µg/L) (Slobodnik et al. 2010).
In the absence of Canadian monitoring data (where DPE was measured), level III fugacity modelling was performed using ChemCAN v6.00 to simulate release of DPE in Canada. Using the combined reported total manufacture and import quantities (Environment Canada 2013), the concentrations in air and water were predicted to be 0.00628 µg/m3 and 0.0552 µg/L, respectively. These concentrations were used to characterize exposure of Canadians to DPE from environmental media. The predicted concentration of this substance in soil was in the ng/g range and would result in negligible intake.
No definitive information is available concerning the use of DPE in foods sold in Canada. DPE is known to be used as a food flavouring agent internationally and it is possible that this substance is present as a flavouring agent in foods sold in Canada (Burdock 2010). The Joint FAO/WHO Expert Committee on Food Additives (JECFA) evaluated DPE for use as a food flavouring agent (WHO 2004), and estimated the per capita intake for the United States population to be 5 µg per person per day (approximately 0.1 µg/kg bw/day for a 60 kg person) based on annual production volumes reported by the food industry in poundage surveys (NAS 1989, IOFI 1995, Lucas 1999 as cited in WHO 2004). JECFA concluded that this substance presents “no safety concern at current levels of intake when used as a flavoring agent.” In the absence of data on the actual use, if any, of DPE as a food flavouring agent in foods sold in Canada, the per capita intake estimate for the US population derived by JECFA is an acceptable surrogate for possible Canadian dietary exposure to this substance from its use as a food flavouring agent (Personal communications, emails from FD, Health Canada to ESRAB, Health Canada, dated 2018 and 2019; unreferenced).
DPE is also reported to occur naturally at low levels in some foods (Nijssen 2018; WHO 2004). The occurrence data used to estimate dietary exposure to DPE was sourced from the Volatile Compounds in Food Database (Nijssen 2018), which reported a concentration of 0.5 ppm in capers and a qualitative presence of DPE in other foods. For each food and beverage category in the database, the highest concentration reported for DPE was conservatively applied to represent the food category (e.g., the maximum concentration reported in capers of 0.5 ppm was applied to all capers). Canadian dietary exposure to DPE from its natural occurrence in foods was estimated with the same method used for DEE (see section 6.1.1). The mean dietary exposure estimated in this manner is 0.061 µg/kg bw/day for Canadians aged 19 years or above; the corresponding 90th percentile dietary exposure cannot be estimated due to a lack of data. For the remaining age groups, the number of survey respondents was insufficient to generate consumption figures and corresponding exposure estimates (Personal communications, emails from FD, Health Canada to ESRAB, Health Canada, dated 2018 and 2019; unreferenced). The natural occurrence in food is not further considered in this assessment, as the potential exposures are lower in comparison to potential exposures from food flavouring use as well as the combined exposures from other environmental media sources.
The Joint FAO/WHO Expert Committee on Food Additives (JECFA) per capita intake estimate of 5 µg per person per day for the United States (based on annual production volumes reported by the food industry in poundage surveys) was used to derive daily intakes of DPE as a food flavouring agent.
The highest estimated intake of DPE from environmental media and food for the Canadian population was 0.557 µg/kg bw/day, corresponding to the intake for 6 – 11 months old infants (see Appendix C for details).
Products available to consumers
DPE is present in various products available to consumers including household air fresheners that can be used indoors (SDS 2016a) and in hand cream (Personal communication, email from CHPSD, Health Canada to ESRAB, Health Canada, dated 2018; unreferenced).
Canadians may be exposed to DPE on a daily basis during the use of air fresheners and hand cream. Table 6‑3 summarizes the estimated daily inhalation exposure to DPE from the use of these products for the most exposed age group.
Although there is a potential for intermittent exposure to DPE via the dermal and inhalation routes (e.g., from the use of bathroom cleaners) and a potential for daily dermal exposure (e.g., from the use of hand cream), these exposures were not quantified due to the lack of adverse effects observed in a 13-week dermal study in rats and a 33-day inhalation study in rats (see Section 6.2.2 for details).
| Product scenario (age group) | Product concentration | Inhalation exposurea (mg/kg bw/day) |
|---|---|---|
| Household air fresheners, daily exposure (aged 1 year) | 5%b | 2.3 × 10-2 |
| Hand cream, daily exposure (aged 19 years or above) | 5%c | 1.7 × 10-2 |
Abbreviation: N/A, Not Applicable
a 100% absorption is assumed for inhalation exposures
b SDS 2016a
c Personal communication, email from CHPSD, Health Canada to ESRAB, Health Canada, dated 2018; unreferenced
6.1.3 DME
Environmental media and food
DME has been reported to the National Pollutant Release Inventory (NPRI). The highest release from a single facility was reported to be 28 tonnes in air emissions in 2017 with releases for this substance identified as fugitive or storage emissions (NPRI 2017). SCREEN3 is a screening-level Gaussian air dispersion model for assessing pollutant concentrations from various sources (SCREEN3 2011). DME emissions were considered to originate from an area source based on facility-specific information. An emission rate of 5.73×10-5 g/m2·s (based on the NPRI air emission data and facility-specific information), receptor height of 1.74 m (Curry et al. 1993), effective emission area of 130 m x 119 m and source height of 6 m (professional judgement), and urban and full meteorology (as a default) were selected. For exposure events happening over the span of a year, it can be expected that the direction of the prevalent winds will be more variable and uncorrelated to the wind direction for a single event; thus, the maximum amortized exposure concentration for one year can be determined by multiplying the maximum 1-hour exposure by a scaling factor of 0.08 for point source emissions (US EPA 1992). However, such scaling factors are not used for non-point source emissions. To prevent overestimation of the exposures originating from area sources, a scaling factor of 0.2 was used to obtain the yearly amortized concentration from the value of the maximum 1-hour exposure concentration determined by SCREEN3 (SCREEN3 2011). This resulted in an estimated concentration of 123 µg/m3 in ambient air based on the distance (119 m) with the highest calculated concentration.
Internationally, DME was detected in indoor air in Spanish hair salons (with concentrations up to 90 µg/m3) and in ambient air in Switzerland (with a mean concentration of 0.60 µg/m3 and a concentration of 1.40 µg/m3 at the 95th percentile) (Gaeggeler et al. 2008; Ronda et al. 2009). Pellizzari et al. (1982) also reported the qualitative detection of DME in 1 of 12 breast milk samples in the United States.
In the absence of Canadian data on water and soil, level III fugacity modelling was performed using ChemCAN v6.00 to simulate release of DME in Canada. Using the combined reported total manufacture and import quantities (Environment Canada 2013), the concentration in water was predicted to be 0.058 µg/L, and the predicted concentration in soil was considered to be negligible.
Concentrations in ambient air from air dispersion model (123 µg/m3) and in water from fugacity modelling (0.058 µg/L) were used to characterize exposure of Canadians to DME from environmental media. The highest estimated intake of DME from environmental media for the Canadian population was 0.089 mg/kg bw/day, corresponding to the intake for 1 year old children (see Appendix C).
No definitive information is available concerning the use of DME in foods sold in Canada. DME is known to be used in food processing aids internationally. It is also potentially used in food processing aids in Canada; if used, dietary exposure to DME from this use in Canada is expected to be negligible (Personal communications, emails from FD, Health Canada to ESRAB, Health Canada, dated 2018 and 2019; unreferenced).
DME is reported to naturally occur at low levels in some foods such as potatoes and vinegar (Nijssen 2018; WHO 2004) but its presence was not quantified. As such, dietary exposure from its natural occurrence in foods was not estimated in this assessment (Personal communications, emails from FD, Health Canada to ESRAB, Health Canada, dated 2018 and 2019; unreferenced).
Products available to consumers
DME is present in aerosol products, including spray chalk paint (SDS 2017c), adhesives (e.g., general purpose, temporary for fabric, automotive headliner) (Environment Canada 2013; SDS 2018a), interior wood finish (SDS 2019b), interior wall repair spray (SDS 2015a), foam for building insulation (SDS 2017d), tire protectant (SDS 2015b), stain remover (SDS 2015c), coating for truck bed liner (SDS 2018d), automotive rust protector (SDS 2017e), fabric paint (SDS 2018b) and jewellery coating (SDS 2016b). DME is also found in self-care products such as hair spray, aerosol temporary hair colour, spray self-tanner (Personal communication, email from CHPSD, Health Canada to ESRAB, Health Canada, dated 2018; unreferenced), foaming hand sanitizer (Personal communications, emails from NNHPD, Health Canada to ESRAB, Health Canada, dated 2018 and 2019; unreferenced) and spray sunscreen (Personal communication, email from TPD, Health Canada to ESRAB, Health Canada, dated 2018; unreferenced). Based on available information, the main function of DME in such products is to act as a propellant.
DME is also an ingredient in spray animal attractant which may be used while in a stand or blind, when stalking deer, on scent trails, in mock scrapes, or when applied to a decoy (SDS 2018c). Exposure to DME during use is expected to be minimal as this product, that is available to consumers, is only used outdoors.
Due to the very high vapour pressure of DME (5.93 × 105 Pa) and its physical state as a gas at room temperature, exposure to DME through the use of products available to consumers is expected to be predominantly through the inhalation route; exposure through the oral route is not expected and exposure through the dermal route is expected to be minimal compared to inhalation as DME acts as a propellant and would revert to the gas phase upon discharge from the canister or container. The spray sunscreen scenario represents the highest estimated daily exposures to DME with a concentration in the product of 40% (Personal communication, email from TPD, Health Canada to ESRAB, Health Canada, dated 2018; unreferenced) and an estimated inhalation exposure of 0.61 mg/kg bw/day where 100% absorption via the inhalation route is assumed.
6.1.4 DPGME
As DPGME is considered to be of low hazard potential (see Section 6.2.4), quantitative estimates of exposure to the general population were not derived.
Environmental media and food
There were no Canadian environmental monitoring data identified for DPGME. Internationally, DPGME was measured in indoor air of Swedish homes (with concentrations up to 9.16 μg/m3) and in storage rooms of a German museum (with an average concentration of 12 μg/m3) (Choi et al. 2010; Schieweck et al. 2005). In addition, DPGME was measured at 0.6 μg/L in treated water from a wastewater treatment plant in France (Bruchet et al. 2007).
Although DPGME may be used as a solvent in the manufacturing of coatings and printing inks used in food packaging materials, these materials have no potential for food contact and exposure to DPGME from food is not expected (Personal communications, emails from FD, Health Canada to ESRAB, Health Canada, dated 2018 and 2019; unreferenced).
Products available to consumers
DPGME is present in products available to consumers, including air fresheners (SDS 2015d, 2017f), inks (SDS 2016g), degreasers and disinfectants (MSDS 2010, 2018e), cleaners (SDS 2016c, 2016d, 2015e), rust converters (SDS 2016e), bathroom etching cream (SDS 2016f), paints and coatings (SDS 2015f, 2018f), furniture restorers (SDS 2010), leather care products (SDS 2016g), lubricants (SDS 2015g), automotive products (SDS 2015h), fabric treatment products (SDS 2014a), and pepper spray (SDS 2014b, 2016i). DPGME is also present in various self-care products such as moisturizers and creams, shampoos and conditioners, cleansers, makeup, nail polish, and other hair styling products (Personal communication, email from CHPSD, Health Canada to ESRAB, Health Canada, dated February 2018; unreferenced). Potential inhalation and dermal exposures to DGPME may occur during the use of these products.
6.2 Health effects assessment
6.2.1 DEE
DEE has been evaluated internationally by the US EPA (1987, 2009a, 2014) and the German MAK commission (MAK Commission1996). A REACH dossier is also available (ECHA c2007-2019).
Repeat-dose toxicity
Sprague Dawley rats (8 for each concentration and sex), ICR mice (24 for each concentration and sex) and Hartley guinea pigs (8 for each concentration and sex) were exposed to 1000 or 10 000 ppm (3031, 30 031 mg/m3) DEE for 24 hours/day, for 35 days by whole-body inhalation (US EPA 2009a, MAK Commission 1996). Each exposure group had a concurrent control. No adverse effects were observed in rats. The only treatment-related effects in guinea pigs were a significant decrease in body weight gain and a high mortality rate (25%) in the highest tested group. Mice were the most sensitive species; at 10 000 ppm 25% of animals died within 20 days. At 1000 ppm, absolute and relative liver weights in mice were significantly increased in males (26% and 11% respectively), and at 10 000 ppm similar but more pronounced changes were observed in males and females (76% and 86% for males, 59% and 66% for females). Degenerative lesions were found in the liver of mice exposed to 10 000 ppm, but not 1000 ppm. No other organs were affected in any of the tested species. Based on these results, NOAECs of 10 000 ppm (30 031 mg/m3) for rats and 1000 ppm (3031 mg/m3) for guinea pigs, and a LOAEC of 1000 ppm for mice are determined.
In a sub-chronic oral study (US EPA 2009a), Sprague Dawley rats (30 for each concentration and sex) were administered DEE dissolved in corn oil by gavage at 0, 500, 2000 or 3500 mg/kg bw/day for 90 days. Mortalities occurred in the mid- and high-dose groups (7% and 25% respectively) with discoloration observed in the lung, liver and stomach, as well as alterations in the stomach smooth mucosa. Significantly reduced body weight gains were observed in males of the mid- and high-dose groups, as well as females in the high-dose groups. Additional effects in the high dose group include decreased food consumption, light anaesthesia, decreased organ absolute weights and changes in alanine aminotransferase (ALT) activity and cholesterol levels. Considering effects on body weight, hepatic changes and mortality in rats at 2000 mg/kg bw/day, a NOAEL of 500 mg/kg bw/day is determined.
Genotoxicity and carcinogenicity
Based on the available information, DEE is not considered genotoxic (MAK Commission 1996, US EPA 2009a).
No carcinogenicity studies for DEE are available. DIPE was used as a supporting chemical in the US EPA Screening Level Hazard Characterization of DEE (US EPA 2014). The US EPA used DIPE repeat-dose, developmental and genotoxicity endpoints to inform the health effects assessment of DEE, but did not use the information on carcinogenicity of DIPE. The US EPA did not derive a carcinogenicity reference dose (RfD) for DEE based on a lack of suitable data (US EPA 2009a).
Reproductive and developmental toxicity
A number of studies in rats and mice examined the effects of DEE inhalation on foetal development, sperm parameters and male reproductive organs, with conflicting results (MAK Commission 1996). However, none of them were conducted according to any established guideline and used concentrations that were close to or greater than reported inhalation LC50 values (95 000-220 000 mg/m3). Thus, the observed effects may be related to hypoxia or maternal stress (MAK Commission 1996).
The US EPA (2014) used the results of studies conducted with DIPE to inform the assessment of the developmental toxicity endpoints for DEE. Pregnant Sprague Dawley rats (22 for each concentration) were exposed to DIPE vapour by inhalation at 430, 3095 or 6745 ppm (1800, 12 940 or 28 200 mg/m3) for 6 hours/day, from gestational day (GD) 6 to15. Results showed that dams had a slight reduction in body weight gain and food consumption at the mid- and high concentrations, and the differences from controls were statistically significant only during the treatment period (GD 6-16), suggesting possible food aversion during the first week of the study (US EPA 2011). The only observation in the foetuses was a significant increase in the incidence of rudimentary (small, discrete ossification) or short (less than one half the length of the preceding ribs) 14th ribs in the mid- and high concentration groups. In the absence of other foetal abnormalities, these effects are not considered adverse.
6.2.2 DPE
Several international agencies have evaluated DPE, including the US EPA (2010, 2017), the EU council (EC 2012), EFSA (2011, 2012), JECFA (WHO 2004) and the German MAK commission (2004). In addition, a REACH dossier for DPE is available (ECHA c2007-2019).
Repeat-dose toxicity
Several repeat-dose studies in which DPE is administered via the inhalation, dermal or oral route were identified. In some studies, DPE was administered as part of a mixture known as either Therminol A or Dowtherm A, which contains 73.5% DPE and 26.5% diphenyl (CAS RN 92-52-4).
Male rats (20 for each concentration), male rabbits (4 for each concentration) and male dogs (2 for each) were exposed to 35 or 71 mg/m3 DPE in a whole-body exposure apparatus for 7 hours/day, 5 days/week for 33 days (US EPA 2017). A third group of male and female rats (10 for each sex) was exposed to 142 mg/m3 DPE for 27 days. Local irritation of the eye and nose was noted at 71 mg/m3 but no other treatment related effects were observed. Based on the lack of adverse effects, a NOAEC of 142 mg/m3 is determined, which is the highest tested concentration.
In a 13-week dietary study (Johnson et al. 1992, EC 2012), male and female Sprague Dawley rats (10 for each dose and sex) were fed 0, 200, 1000 or 5000 ppm of DPE (0, 11.7, 60.7 or 301.1 mg/kg bw/day for males and 0, 14.5, 73.9 or 334.8 mg/kg bw/day for females). Additional groups of rats (10 for each dose and sex) were retained for observation during a 4-week recovery period after the 13-week feeding period. Observed health effects included a significant decrease in body weight gain and food consumption at the highest tested dose in males, and at the mid- and high dose groups in females. However, as body weights and food consumption were significantly increased in the recovery period, the authors attributed the earlier effects to the unpalatability of the substance in the diet, rather than a treatment related effect. In consideration of the uncertainty associated with this study, as a result of insufficient reporting, a NOAEL of 15 mg/kg bw/day is determined based on decreased body weight gain in females at 74 mg/kg bw/day.
In a 13-week dermal study (Api and Ford 2003, US EPA 2017), male and female Sprague Dawley rats (12 for each dose and sex) were exposed to DPE daily by a semi-occlusive dressing 6 hours/day at 0, 100, 300 or 1000 mg/kg bw/day. No histopathological lesions were observed in any of the examined organs. A significant increase in relative and absolute liver weight was observed in males at 300 mg/kg bw/day, however this was not a dose-dependent effect. At 1000 mg/kg bw/day, significantly higher relative brain, kidney and liver weights were observed. In females, relative liver weights were significantly increased at 300 mg/kg bw/day, and both relative and absolute liver weights were increased in the highest dose tested. Considering the absence of histopathological effects of the affected organs, the lack of significant changes in blood or urine parameters, and the lack of dose-response relationships, the study authors concluded that DPE did not induce any adverse systemic effects and considered the organ weight changes to lack biological significance. Thus, the NOAEL is set at 1000 mg/kg bw/day, which is the highest dose tested.
A mixture of Therminol A (73.5% DPE) was administered by whole-body inhalation to Sprague Dawley rats (25 for each concentration and sex) as an aerosol at concentrations of 0, 7.4, 37.5 or 95.6 mg DPE/m3 for 6 hours/day, 5 days/week for 7 or 14 weeks (US EPA 2017). In animals exposed for 14 weeks, significantly reduced body weight gains in males and females were observed at 95.6 mg DPE/m3 in weeks 2-6. There were reduced white blood cell counts males in the low- and mid-concentration groups, but the effect was not dose-dependent. In addition, significantly lower relative weight of liver (in males and females), brain and spleen (in females) were observed in the highest concentration group, but these may be attributed to the reduced body weights. The authors assigned a NOAEC of 37.5 mg DPE/m3 based on body weight changes at 95.6 mg DPE/m3.
Genotoxicity and carcinogenicity
DPE is not considered genotoxic as demonstrated by several in vitro and in vivo assays (US EPA 2017).
In a 13-month carcinogenicity study, male albino rats (8 animals for each dose) were given diets containing DPE at 530 mg/kg bw/day, or as a mixture of Therminol A (73.5% DPE) at 396 mg DPE/kg bw/day. No tumours or treatment-related pathological effects were observed in animals treated with DPE alone, or as a mixture (WHO 2004).
Reproductive and developmental toxicity
In a rat dietary repeat-dose study, the reproductive organs of both genders were examined macroscopically and histopathologically; no adverse effects related to treatment were found (Johnson et al. 1992, EC 2012).
There are no reproductive or developmental toxicity studies for DPE available where DPE is not part of a mixture.
In a developmental toxicity study (US EPA 2017, ECHA c2007-2019), a mixture of Therminol A containing 73.5% DPE was given in corn oil to pregnant Sprague Dawley rats (24 animals per dose) by gavage at 0, 36.75, 147 and 367.5 mg DPE/kg bw/day between GD 6-15. Animals were sacrificed on GD 20 and both the dams and foetuses were examined for clinical and developmental toxicity. In the dams, a non-significant decrease in body weight gain was observed at the lowest dose. At 147 mg DPE/kg bw/day, significant reduction in body weight gain (20% less than control) and food consumption were noted, along with salivation and staining of the ano-genital area. These effects were more severe at the highest tested dose, where two dams died. In the foetuses, no overt toxicity was observed at any of the tested doses, including any skeletal, soft-tissue or external malformations. Thus, the NOAEL for maternal toxicity is 36.75 mg DPE/kg bw/day based on body weight changes at 147 mg DPE/kg bw/day. The NOAEL for developmental toxicity is 367.5 mg DPE/kg bw/day, which is the highest tested dose.
6.2.3 DME
Several international agencies have evaluated DME, including the US EPA (2009b), EFSA (2009, 2015) and the German MAK commission (MAK Commission 1990). In addition, a REACH dossier for DME is available (ECHA c2007-2019). In an Initial Risk-Based Prioritization of High Production Volume Chemicals Report, the US EPA recommended that DME be classified as low priority based on its low human health hazard (US EPA 2009b).
Repeat-dose toxicity
As DME is a gas at body temperature, animals were exposed to the substance via inhalation in all available repeat-dose toxicity studies.
No critical health effects via inhalation were identified in two short-term studies and one sub-chronic study in rats at concentrations up to 94 211 mg/m3 DME (MAK Commission 1990). In a chronic study (MAK Commission 1990), male and female Wistar rats (25 for each concentration and sex) were exposed to DME at 0, 197, 1964 or 18 830 ppm (0, 371, 3701 or 35 480 mg/m3) for 6 hours/day, 5 days/week for 30 weeks. No effects were noted on body weight, food consumption, clinical signs or the eye. No abnormalities in the haematology or urinalysis were observed except for a significantly higher level of AST activity in males exposed to 1964 ppm only and in in ALT activity in both males and females exposed to 18 830 ppm. In addition, there was a significant reduction in relative liver weight (8% less than control) in males of the highest tested concentration.
In a 2-year carcinogenicity GLP study (MAK Commission 1990), male and female Crl:CD rats (100 for each concentration and sex) were exposed to DME vapour at 0, 2000, 10 000 or 25 000 ppm (0, 3768, 18 842 or 47 105 mg/m3) for 6 hours/day, 5 days/week for 104 weeks. The most notable findings are a significant increase in body weight and a non-significant decrease in lifetime of male rats at the mid- and high-concentration groups at the end of the study period. In the highest concentration group, males showed a decrease in erythrocyte count, an increase in spleen weight and evidence of splenic congestion at 6 months, along with normal bone histology. In the same group, females showed a decrease in erythrocyte counts at 3 months. These changes, however, were not noted at later time-points. Aside from observations made in the spleen, no histopathological effects were observed in other organs. The number of observed masses in female rats was higher in all tested groups compared to control, including the presence of mammary tumours at the highest tested concentration. However, the study authors did not consider this effect to be treatment related as the incidence of masses and tumours in the control group were low compared to historical data. Thus, a NOAEC of 2000 ppm (3768 mg/m3) is determined based on reduced survival rate in male rats at the mid- and high concentrations.
Genotoxicity and carcinogenicity
DME is not considered genotoxic as demonstrated in several in vitro assays (MAK Commission 1990, US EPA 2009b, ECHA c2007-2019).
In the previously described 2-year carcinogenicity study in rats (MAK Commission 1990), it was considered that DME did not induce a carcinogenic effect.
Reproductive and developmental toxicity
No reproductive toxicity studies are available for DME. However, no adverse effects were observed in gross and histopathological examination of male and female reproductive organs in several short-term and long-term repeat exposure inhalation studies in rats and hamsters.
Two developmental studies for DME conducted in rats are available (CIVO 1981, Haskell Labs 1983, US EPA 2009b, MAK Commission 1990), where pregnant Wistar and Crl:CD rats were exposed to DME up to 28 000 ppm (52 758 mg/m3) for 6 hours/day from GD 6 to 16. No maternal toxicity was observed in any of the treatment groups. No treatment-related soft-tissue malformations were observed in the foetuses of all treatment groups. The incidence of supernumerary lumbar ribs (both uni- and bi-lateral) was significantly higher in the treatment groups compared to the controls, but the biological significance of this finding was considered uncertain by study reviewers (Haskell Labs 1983, MAK Commission 1990). Significant differences in the ossification of hind limb phalanges as well as cervical and thoracic vertebrae were also observed. However, the study authors and reviewers considered these differences not to be treatment-related due to the lack of a clear concentration-response relationship, and attributed them to normal variation in ossification of this animal strain (MAK Commission 1990).
6.2.4 DPGME
DPGME has been evaluated internationally by the OECD (2001) and the German MAK commission (MAK Commission 1993). Reviews by ECETOC (Gribble 2005) and the Cosmetic Ingredients Review (Robinson et al. 2009) on the health effects of glycol ethers, including DPGME, are also available. In addition, a REACH dossier for DPGME exists (ECHA c2007-2019). In a SIDS Initial Assessment Meeting (SIAM) the OECD recommended that DPGME be classified as low priority for risk evaluation based on its low hazard profile (OECD 2001). Similarly, in a multi-chemical Tier I human health risk assessment carried out by the Australian Government Department of Health (AGDH 2018), DPGME was listed as one of the chemicals that were not considered to pose an unreasonable risk to the health of workers or the general public.
Repeat-dose toxicity
Oral, dermal and inhalation repeat-dose studies are available for DPGME.
In eight separate inhalation studies, mice, rats, rabbits, guinea pigs and monkeys exposed to DPGME concentrations up to 300 ppm (1818 mg/m3) for 6-7 hours/day for 2 to 31 weeks did not experience any adverse effects (OECD 2001). It should be noted that 300 ppm is the highest concentration attainable of DPGME vapour at standard temperature and pressure.
In an oral short-term study (OECD 2001), Sprague Dawley rats (5 for each dose and sex) were given 0, 40, 200 or 1000 mg/kg bw/day DPGME by gavage for 28 days. The NOAEL is set at 1000 mg/kg bw/day due to the lack of biologically significant effects at the highest tested dose.
No adverse effects were observed in male Wistar rats (8 for each dose) exposed to 0, 100 or 1000 mg/kg bw/day DPGME dermally by occlusive and semi-occlusive applications for 4 hours/day, 5 days/week for four weeks (OECD 2001). In a semi-occlusive dermal study, male rabbits (5-7 for each dose) were exposed to 0, 950, 2850, 4750, or 9510 mg/kg bw/day DPGME for 5 days/week for 90 days (OECD 2001). Minor skin irritation, narcosis and some deaths were observed in animals exposed to 4750 mg/kg bw/day and above. Thus, the NOAEL is 2850 mg/kg bw/day.
Genotoxicity and carcinogenicity
DPGME is not considered to be genotoxic as demonstrated by several in vitro assays (OECD 2001).
No carcinogenicity studies for DPGME are available. However, sub-chronic and chronic studies on rats, rabbits, guinea pigs and monkeys did not report any significant tumour incidence when 300 ppm (the maximum attainable concentration) DPGME was administered by inhalation for 7 hours/day, 5 days/week up to 31 weeks (OECD 2001).
The OECD (2001) used a supporting chemical, PGME, to evaluate the carcinogenic potential of DPGME using a 2-year inhalation study conducted in Fischer rats and B6C3F1 mice which were exposed to 0, 300, 1000 or 3000 ppm (0, 1106, 3686, 11 058 mg/m3) of PGME for 6 hours/day, 5 days a week (OECD 2001). No evidence of carcinogenicity was observed in either species up to the highest tested concentration.
Reproductive and developmental toxicity
No reproductive toxicity studies are available for DPGME. However, no gross or histopathological effects were observed in the testes or ovaries of male and female rats and rabbits exposed to DPGME for 90 days by inhalation (OECD 2001).
No maternal or foetal toxicity was observed in two studies where pregnant Fischer 344 rats and New Zealand rabbits were exposed up to 300 ppm DPGME by whole-body inhalation from GD 6-15 (rats) and GD 7-19 (rabbits) for 6 hours/day (OECD 2001).
6.3 Characterization of risk to human health
Tables 6-4 to 6-6 provide relevant exposure and hazard values for DEE, DPE and DME, as well as resultant margins of exposure (MOE), for determination of risk.
A NOAEL of 500 mg/kg bw/day, based on changes in body weight gain, liver effects and increased mortality in rats given 2000 or 3500 mg/kg bw/day DEE by oral gavage (US EPA 2009a), was considered to be the most relevant endpoint for oral exposures. It should be noted that the mid- and high-doses are above the oral LD50 for DEE (1568 mg/kg bw; US EPA 2014).
A LOAEC of 3031 mg/m3 (LOAELadj= 4256 mg/kg bw/day) was considered the most relevant endpoint for exposure via inhalation, based on increased liver weights at 3031 mg/m3 DEE and increased liver degeneration and mortality at 30 031 mg/m3 DEE in mice exposed via inhalation for 35 days (US EPA 2009a). The mouse was the most sensitive species tested in this study (compared to the rat and guinea pig) and all animals were exposed continuously for 35 days. Exposure from the body lotion is expected to be from inhalation mainly due to the high vapour pressure of DEE, thus a comparison to the 35-day inhalation study was considered appropriate.
On the basis of the conservative parameters used in estimating exposure, the resulting MOEs for the oral and inhalation routes are considered adequate to address uncertainties in the health effects and exposure databases of DEE.
| Substance | Exposure scenario | Systemic exposure (mg/kg bw/day)a |
Critical effect level (mg/kg bw/day) |
Critical health effect | MOE |
|---|---|---|---|---|---|
| DEE | Environmental media, daily exposure (aged 1 year) e | 0.44 b | NOAEL = 500 (13-week oral gavage study in rats) | Changes in body weight gain, liver effects and increased mortality in rats at 2000 mg/kg bw/day | 1136 |
| DEE | Body lotion, daily exposure (aged 19 years or above) | 3.2 × 10-2 c | LOAELadj = 4256d (35-day inhalation study in mice) | Liver toxicity in mice at adjusted dose of 4256 mg/kg bw/dayd | 133 000 |
| DEE | Corn and callus remover, per event exposure (aged 9 to 13 years) | 7.8 × 10-2 c | LOAELadj = 4256d (35-day inhalation study in mice) | Liver toxicity in mice at adjusted dose of 4256 mg/kg bw/dayd | 54564 |
| DEE | Starting fluid, per event exposure (aged 19 years or above) | 0.63 c | LOAELadj = 4256d (35-day inhalation study in mice) | Liver toxicity in mice at adjusted dose of 4256 mg/kg bw/dayd | 6756 |
Abbreviations: MOE, Margin of Exposure; NOAEL, No Observed Adverse Effect Level; LOAELadj : Adjusted Lowest Observed Adverse Effect Level
a Systemic exposure is derived from the “external dose on day of exposure” calculated using ConsExpo Web (2016), conservatively assuming 100% absorption for inhalation and dermal exposures. This value represents the sum of external doses for multiple events that take place on the same day, where applicable. See Appendix C for more details.
b Represents the sum of oral and inhalation exposures.
c Represents inhalation exposure.
d Critical effect levels are calculated based on converting No Observed Adverse Effect Concentrations (NOAECs) or Low Observed Adverse Effect Concentrations (LOAECs) from inhalation toxicity studies into internal doses that account for animal inhalation rate (m3/day), body weight (kg), and time adjustment factors (hours of exposure/24 ; days of exposure in a week/7), unless specified otherwise. Animal inhalation rates were determined using the equation provided in Bide et al. (2000). Animal body weights were derived from the study reports if available; a default value as presented in Meek et al. (1994) was used otherwise
e Dietary exposure to DEE from use in Canada as food processing aids is expected to be negligible. The natural occurrence in food is not further considered as the potential exposures are lower in comparison to the combined exposures from other environmental media sources.
A NOAEL of 15 mg/kg bw/day, based on changes in body weight gain in female rats exposed to 74 mg/kg bw/day DPE in the diet for 13 weeks (Johnson et al. 1992, EC 2012), was considered the most relevant endpoint for oral exposure. Reduced food consumption due to palatability issues with the substance was possibly a contributing factor to the reduced body weight gains observed; however, limited information is available to validate this assumption. In addition, similar effects were observed in pregnant rats exposed to a mixture containing DPE by oral gavage, where significant body weight gain reductions occurred starting from 147 mg DPE/kg bw/day. Thus, a NOAEL of 15 mg/kg bw/day is considered appropriate and would be protective of any potential body weight effects that could occur at higher doses.
A NOAEC of 37.5 mg DPE/m3 (NOAELadj = 6 mg/kg bw/day) based on body weight changes in rats exposed via inhalation to 95.6 mg DPE/m3 for 6 hours/day, 5 days/week for 13-14 weeks (US EPA 2017), was considered the most relevant endpoint for long-term inhalation exposure. This study was considered appropriate to characterize the risk from exposure from hand cream as the most likely route of exposure for this substance would be via inhalation due to its moderate vapour pressure and low dermal absorption rate (approximately 20% of 1000 mg/kg bw/day of DPE was absorbed in rats over a 72-hour period as described in Api and Ford 2003, US EPA 2017), and lack of adverse effects in rats when DPE was administered dermally up to 1000 mg/kg bw/day for 13 weeks.
For characterizing the risk from dermal and short-term inhalation exposures, it was further considered that there was a lack of adverse effects in rats when DPE was administered dermally up to 1000 mg/kg bw/day for 13 weeks, and that there was also a lack of adverse effects by inhalation up to the highest tested concentration of 142 mg/m3 for 27 days. As such, margins of exposure for dermal and short-term inhalation exposures were not derived and the risk to human health from relevant scenarios is considered to be low.
As such, the resulting MOEs for the oral and long-term inhalation exposure scenarios are considered adequate to address uncertainties in the health effects and exposure databases of DPE.
| Substance | Exposure scenario | Systemic exposure (mg/kg bw/day)a |
Critical effect level (mg/kg bw/day) |
Critical health effect | MOE |
|---|---|---|---|---|---|
| DPE | Environmental media and food, daily exposure (aged 6 – 11 months) | 5.6 × 10-4 b | NOAEL = 15 (13-week diet study in rats) | Changes in body weight gain in female rats at 74 mg/kg bw/day | 27 000 |
| DPE | Household air freshener, daily exposure (aged 1 year) | 2.3 × 10-2 c | NOAELadj = 6d (14 week inhalation study in rats) | Changes in body weight gain in rats at adjusted dose of 15 mg/kg bw/dayd | 261 |
| DPE | Hand cream, daily exposure (aged 19 years or above) | 1.7 × 10-2 c | NOAELadj = 6d (14 week inhalation study in rats) | Changes in body weight gain in rats at adjusted dose of 15 mg/kg bw/dayd | 353 |
Abbreviations: MOE, Margin of Exposure; NOAELadj: Adjusted No Observed Adverse Effect Level
a Systemic exposure is derived from the “external dose on day of exposure” calculated using ConsExpo Web (2016), conservatively assuming 100% absorption for inhalation and dermal exposures. This value represents the sum of external doses for multiple events that take place on the same day, where applicable. See Appendix C for more details.
b Represents the sum of oral and inhalation exposures.
c Represents inhalation exposure.
d Critical effect levels are calculated based on converting No Observed Adverse Effect Concentrations (NOAECs) or Low Observed Adverse Effect Concentrations (LOAECs) from inhalation toxicity studies into internal doses that account for animal inhalation rate (m3/day), body weight (kg), and time adjustment factors (hours of exposure/24; days of exposure in a week/7), unless specified otherwise. Animal inhalation rates were determined using the equation provided in Bide et al. (2000). Animal body weights were derived from the study reports if available; a default value as presented in Meek et al. (1994) was used otherwise.
A NOAEC of 3768 mg/m3 (NOAECadj = 590 mg/kg bw/day) based on decreased survival rates in male rats exposed to 18 842 or 47 105 mg/m3 DME via inhalation for 6 hours/day, 5 days/week for 2 years (MAK Commission 1990) was selected as the most relevant endpoint for long-term inhalation exposures. The resulting MOEs are considered adequate to address uncertainties in the health effects and exposure databases of DME.
For short-term inhalation exposures, it was considered that there was a lack of adverse effects in rats exposed to DME for 10 days up to 94 211 mg/m3; as such, MOEs for short-term inhalation exposures were not derived and the risk to human health from relevant scenarios is considered to be low.
| Substance | Exposure scenario | Systemic exposure (mg/kg bw(/day))a |
Critical effect level (mg/kg bw/day) |
Critical health effect endpoint | MOE |
|---|---|---|---|---|---|
| DME | Environmental media, daily exposure (aged 1 year) | 8.9 × 10-2 b | NOAELadj = 590d (2-year inhalation study in rats) | Reduced survival rates in male rats at adjusted dose of 2955 mg/kg bw/dayd | 6600 |
| DME | Spray sunscreen, daily exposure (aged 1 year) | 0.61c | NOAELadj = 590d (2-year inhalation study in rats) | Reduced survival rates in male rats at adjusted dose of 2955 mg/kg bw/dayd | 967 |
Abbreviations: MOE, Margin of Exposure; NOAELadj : Adjusted No Observed Adverse Effect Level
a Systemic exposure is derived from the “external dose on day of exposure” calculated using ConsExpo Web (2016), conservatively assuming 100% absorption for inhalation and dermal exposures. This value represents the sum of external doses for multiple events that take place on the same day, where applicable. See Appendix C for more details.
b Represents the sum of oral and inhalation exposures.
c Represents inhalation exposure.
d Critical effect levels are calculated based on converting No Observed Adverse Effect Concentrations (NOAECs) or Low Observed Adverse Effect Concentrations (LOAECs) from inhalation toxicity studies into internal doses that account for animal inhalation rate (m3/day), body weight (kg), and time adjustment factors (hours of exposure/24; days of exposure in a week/7), unless specified otherwise. Animal inhalation rates were determined using the equation provided in Bide et al. (2000). Animal body weights were derived from the study reports if available; a default value as presented in Meek et al. (1994) was used otherwise.
DPGME is considered to have a low hazard potential given that no adverse effects were observed as a result of oral or dermal exposures up to the limit dose, or inhalation exposures up to the maximum concentration attainable, and given the available information indicating a low concern for reproductive, developmental or genotoxic effects. In addition, based on read-across from the carcinogenicity endpoint of the analogue PGME, DPGME is not likely to be carcinogenic. As such, exposure estimates were not derived and the risk to human health is considered to be low.
6.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| There is a lack of Canadian monitoring data for substances in the Ethers Group in environmental media (e.g., air and water). | +/- |
| There is a lack of carcinogenicity, reproductive toxicity and long-term repeat dose studies for DEE. | +/- |
| The use of a eutectic mixture (Therminol A) in some DPE hazard studies results in uncertainty in determining the component causing the observed health effects. | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over or under estimation of risk.
7. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from DEE, DPE, DME and DPGME. It is proposed to conclude that DEE, DPE, DME and DPGME do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that DEE, DPE, DME and DPGME do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that DEE, DPE, DME and DPGME do not meet any of the criteria set out in section 64 of CEPA.
References
[AGDH] Australian Government Department of Health. 2018. Tier I Human Health Assessments INVENTORY MULTI-TIERED ASSESSMENT AND PRIORITISATION (IMAP) FRAMEWORK. Sydney (AU): Department of Health, National Industrial Chemicals Notification and Assessment Scheme. [accessed 2019 September 11].
Api A and Ford R. 2003. Evaluation of the dermal subchronic toxicity of diphenyl ether in the rat. Food and Chemical Toxicology 41(2):259-64.
Apodaca L, Bails J, Smith M. 1997. Water quality in shallow alluvial aquifers, Upper Colorado Riwer Basin, Colorado, 1997. J Am Water Resour Assoc. 38(1):133-149.
Bide RW, Armour SJ, Yee E. 2000. Allometric respiration/body mass data for animals to be used for estimates of inhalation toxicity to young adult humans. J Appl Toxicol 20(4):273-90.
Burdock, GA. 2010. Fenaroli’s handbook of flavor ingredients. 6th ed. Boca Raton (FL): CRC Press.
Buters JT, Schober W, Gutermuth J, Jakob T, Aguilar-Pimentel A, Huss-Marp J, Traidl-Hoffmann C, Mair S, Mair S, Mayer F, Breuer K, Behrendt H. et al. 2007. Toxicity of parked motor vehicle indoor air. Environ Sci Technol. 41(7):2622-2629.
Cadena E, Adani F, Font X, Artola A. 2018. Including an odor impact potential in life cycle assessment of waste treatment plants. Int J Environ Sci Technol. 15:2193–2202.
Canada. [1978]. Food and Drug Regulations. C.R.C., c.870.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2009. Canadian Environmental Protection Act, 1999: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 143, no. 40, p. 2945-2967.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
Chan C, Vainer L, Martin J, Williams D. 1990. Determination of organic contaminants in residential indoor air using an adsorption-thermal desorption technique. J Air Waste Manage Assoc. 40(1):62-7.
ChemIDplus [database]. 1993- . Bethesda (MD): US National Library of Medicine. [updated 2016 Jul 20; accessed 2019 Sept 25].
ChemCAN [level III fugacity model of 24 regions of Canada]. 2003. Version 6.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
CIVO Institute. 1981. Initial submission: Inhalation embryotoxicity/teratogenicity studies witii dimethylether in rats with cover letfer dated 08/24/92. Netherlands: DuPont. Report No. OTS 0555244. Available from: NTIS, Springfield, VA.
Curry P, Kramer G, Newhook R, Sitwell J, Somers D, Tracy B, Oostdam JV. 1993. Reference values for Canadian populations. Prepared by the Environmental Health Directorate Working Group on reference values. Health Canada. 1988 (updated in 1993).
DailyMed [database]. 2019. Search result for Freezone. Bethesda (MD): US National Library of Medicine. [updated 2019 Sep 19; accessed 2019 Sep 27].
[DPD] Drug Product Database [database]. [modified 2015 Jul 17]. Ottawa (ON): Government of Canada. [accessed 2019 Sept 25].
[EC] European Commission. 2012. Recommendation from the scientific committee on occupational exposure limits for diphenyl ether. European Commission. Report No. SCOEL/SUM/182.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2019 Sept 25].
[ECHA] European Chemicals Agency. c2007-2019. Registered substances database; search results for CAS RNs 60-29-7, 101-84-8, 115-10-6, 34590-94-8. Helsinki (FI): ECHA. [accessed 2019 September 26].
[EFSA] European Food Safety Authority. 2009. Safety in use of dimethyl ether as an extraction solvent. EFSA Journal 7(3):984.
[EFSA] European Food Safety Authority. 2011. Scientific Opinion on Flavouring Group Evaluation 59, Revision 1 (FGE.59Rev1): Consideration of aliphatic and aromatic ethers evaluated by JECFA (61st meeting and 63rd meeting) structurally related to aliphatic, alicyclic and aromatic ethers including anisole derivatives evaluated by EFSA in FGE.23 Rev2 (2010). Parma, Italy: European Food Safety Authority. EFSA Journal, No. 2158.
[EFSA] European Food Safety Authority. 2012. Scientific Opinion on the safety and efficacy of aromatic ethers including anisole derivatives (chemical group 26) when used as feed nadditives for all animal species. EFSA Journal 2012;10(5):2678
[EFSA] European Food Safety Authority. 2015. Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific opinion on the safety of use of dimethyl ether as an extraction solvent under the intended conditions of use and the proposed maximum residual limits. EFSA Journal 13(7):4174.
Environment Canada. 2009. DSL Inventory Update Phase 1 data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Ficheux AS, Wesolek N, Chevillotte G, Roudot AC. 2015. Consumption of cosmetic products by the French population. First part: Frequency data. Food and Chemical Toxicology 78: 159-169.
Ficheux AS, Chevillotte G, Wesolek N, Morisset T, Dornic N, Bernard A, Bertho A, Romanet A, Leroy L, Mercat AC, Creusot T, Simon E, Roudot AC. 2016. Consumption of cosmetic products by the French population second part: Amount data. Food and Chemical Toxicology 90: 130-141.
Gaeggeler K, Prevot A, Dommena J, Legreid G, Reimann S, Baltensperger U. 2008. Residential wood burning in an Alpine valley as a source for oxygenated volatile organic compounds, hydrocarbons and organic acids. Atmos Environ 42:8278-87.
Gonthier G, Lawrence S, Peck M, Holloway G. 2011. Groundwater conditions and studies in the Augusta–Richmond County Area, Georgia, 2008-2009. Atlanta, Georgia: U.S. Geological Survey. 45 pages. Scientific Investigations Report 2011–5188.
Gribble M. 2005. The toxicology of glycol ethers and its relevance to man. ECETOC.
Haskell Labs. 1983. Initial submission: Evaluation of embryotoxicity and teratogenicity studies w/dimethylether (dme) in rats with cover letter dated 082492. Final report. Netherlands: DuPont. Report No. NTIS/OTS 0546404. Available from: NTIS, Springfield, VA, USA.
Health Canada. [modified 2015 Dec 14]. Cosmetic Ingredient Hotlist: list of ingredients that are prohibited for use in cosmetic products. Ottawa (ON): Government of Canada. [accessed 2019 Sept 25].
Health Canada. 2015a. Food Consumption Table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004), Share file. Ottawa (ON).
Health Canada 2015b. Keep calm and use hand sanitizer – how much and how often. Unpublished. Existing Substances Risk Assessment Bureau and New Substances Assessment and Control Bureau, Health Canada. Presented at Health Canada Science Forum 2015.
Hefner Jr R, Leong B, Kociba R, Gehring P. 1975. Repeated inhalation toxicity of diphenyl oxide in experimental animals. Toxicol Appl Pharmacol 33(1):78-86.
Hippelein M. 2006. Analysing selected VVOCs in indoor air with solid phase microextraction (SPME): a case study. Chemosphere. 65:271–277.
[IOFI] International Organization of the Flavour Industry, 1995. European Inquiry on Volume of Use. Submitted to WHO by the Flavor and Extract Manufacturers' Association of the United States, Washington DC, USA.
Johnson W, Ehrlich J, Hatoum N, Yermakoff J. 1992. Thirteen week oral (diet) toxicity study of diphenyl ether in rats. Toxicologist 12(1992):117.
Keefe SH, Barber LB, Runkel RL, Ryan JN. 2004. Fate of volatile organic compounds in constructed wastewater treatment wetlands. Environ Sci Technol. 38(7):2209-16.
Lesage S, Jackson R, Priddle M, Riemann P. 1990. Occurrence and fate of organic solvent residues in anoxic groundwater at the gloucester landfill, Canada, Environ Sci Technol. 24(4):559-566.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2018 Feb. 06]. Ottawa (ON): Health Canada.
Loretz LG, Api AM, Babcock L, Barraj LM, Burdick J, Cater KC, Jarrett G, Mann S, Pan YHL, Re TA, Renskers KJ, Scrafford CG. 2008. Exposure data for cosmetic products: Facial cleanser, hair conditioner, and eye shadow. Food Chem Toxicol 46: 1516-1524.
Lucas, C.D. et al., eds. 1999. 1995 Poundage and technical effects update summary. Washington, DC, Flavor and Extract Manufacturers’ Association of the Unites States.
MAK Commission. 1990. [Dimethyl ether MAK value documentation, 1990]. Wiley-VCH Verlag GmbH & Co.
MAK Commission. 1993. Dipropylene glycol monomethyl ether [MAK value documentation, 1993]. Wiley-VCH Verlag GmbH & Co.
MAK Comission. 1996. Diethyl ether [MAK value documentation, 1999]. Wiley-VCH Verlag GmbH & Co.
MAK Comission. 2004. Diphenylether (dampf) [MAK value documentation in german language, 2004]. Wiley-VCH Verlag GmbH & Co.
Meek M, Newhook R, Liteplo R, Armstrong V. 1994. Approach to assessment of risk to human health for priority substances under the canadian environmental protection act. Journal of Environmental Science & Health Part C 12(2):105-34.
Moreno T, Pacitto A, Fernandez A, Amato F, Marco E, Grimalt J, Buonanno G, Querol X. 2019. Vehicle interior air quality conditions when travelling by taxi. Environ Res.172:529-542.
[MSDS] Material Safety Data Sheet. 2010. Motorcycle Degreaser [PDF]. Hauppauge (NY): Finishi Line Technologies Inc. [accessed 2019 Sep 19].
Mullaney J, Mondazzi R, Stone J. 1999. Hydrogeology and water quality of the Nutmeg Valley Area, Wolcott and Waterbury, Connecticut. Denver: U.S. Geological Survey. 89 pages. Water-Resources Investigations Report 99-4081.
[NAS] National Academy of Sciences. 1989. 1987 Poundage and Technical Effects Update of
Substances Added to Food, Committee on Food Additives Survey Data, Food and Nutrition
Board, Institute of Medicine, National Academy of Sciences, Washington DC, USA.
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2019 Sept 26]. Ottawa (ON): Health Canada.
Nijssen LM, Ingen-Visscher CA van, Donders JJH [eds]. 1963-2018. VCF (Volatile Compounds in Food): database, Version 16.6.1, 2018. Triskelion B.V. Zeist, Netherlands.
[NPRI] National Pollutant Release Inventory. [database]. 2017. Gatineau (QC): Environment Canada.
[OECD] Organisation for Economic Co-operation and Development. 2001. SIDS initial assessment report: Dipropylene glycol methyl ether: CAS No. 34590-94-8. SIAM [SIDS Initial Assessment Meeting] 12. Paris, France.
OECD QSAR Toolbox. 2014. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Pellizzari E, Hartwell T, Harris B, Waddell R, Whitaker D.1982. Purgeable organic compounds in mother's milk. Bull Environ Contam Toxicol. 28(3):322-8.
Pope LM, Bruce BW, Rasmussen PP, Milligan CR. 2002. Quality of shallow ground water in areas of recent residential and commercial development, Wichita, Kansas, 2000. Denver: U.S. Geological Survey. 67 pages. Water-Resources Investigations Report 02–4228
PubChem. 2004- . Bethesda (MD): US National Library of Medicine, National Center for Biotechnology Information. [accessed 2019 September 26].
Rabaud N, Ebeler S, Ashbaugh L, Flocchini R. 2003. Characterization and quantification of odorous and non-odorous volatile organic compounds near a commercial dairy in California. Atmos Environ 37:933–940.
Raboni M, Torretta V, Urbini G, Viotti P. 2015. Automotive shredder residue: a survey of the hazardous organic micro-pollutants spectrum in landfill biogas. Waste Manag Res 33(1), 48-54.
Ramírez N, Cuadras A, Rovira E, Borrull F, Marcé R. 2010. Comparative study of solvent extraction and thermal desorption methods for determining a wide range of volatile organic compounds in ambient air. Talanta 82:719–727.
Robinson V, Bergfeld WF, Belsito DV, Klaassen CD, Marks JG, Shank RC, Slaga TJ, Snyder PW, Andersen FA. 2009. Final report on the safety assessment of PPG-2 methyl ether, PPG-3 methyl ether, and PPG-2 methyl ether acetate. Int J Toxicol 28(6_suppl):162S-74S.
Rogers IH, Birtwell IK, Kruzynski GM.1986. Organic extractables in municipal wastewater Vancouver, British Columbia. Water Qual Res J Can 21(2):187-204.
SCREEN3 [computer model]. 2011. Ver. 3.5.0. Research Triangle Park (NC): US Environmental Protection Agency, Office of Air Quality Planning and Standards, Emissions, Monitoring, and Analysis Division.
[SDS] Safety Data Sheet. 2010. Neutral Floor Cleaner [PDF]. Miramar (US): For Life Products, Inc. [accessed 2019 Sep 19].
[SDS] Safety Data Sheet. 2014a. Mace Pepper Gun - Refill Cartridge [PDF]. Cleveland (US): Mace Security International [accessed 2019 Sep 19].
[SDS] Safety Data Sheet. 2014b. Restorative Water Repellent for Outerwear and Apparel [PDF]. St. Paul (US): New Business Ventures [accessed 2019 Sep 19].
[SDS] Safety Data Sheet. 2015a. Homax Spray Spackling [PDF]. Longueuil CA): PPG Architectural Coatings Canada [accessed 2019 Sep 11].
[SDS] Safety Data Sheet. 2015b. Simoniz Tire Foam. Repentigny (QC): Auto-Chem Incoporated [accessed 2019 Sep 11].
[SDS] Safety Data Sheet. 2015c. Salt Eraser. Brampton (CA): Empack Spraytech Incorporated [accessed 2019 Sep 11].
[SDS] Safety Data Sheet. 2015d. Febreze Noticeables Air Freshener [PDF]. Toronto (CA): Procter & Gamble Inc [accessed 2019 Sep 19].
[SDS] Safety Data Sheet. 2015e. Ultra power remover [PDF]. Chicago (US): Rust-Oleum Corporation [accessed 2019 Sep 19].
[SDS] Safety Data Sheet. 2015f. Stainless Steel Basecoat [PDF]. St. Louis (US): Giani, Inc. [accessed 2019 Sep 19].
[SDS] Safety Data Sheet. 2015g. Teflon Penetrant [PDF]. Suffolk County (US): Finish Line Technologies, Inc. [accessed 2019 Sep 20].
[SDS] Safety Data Sheet. 2015h. Restore Black [PDF]. St. Paul (US): Automotive Aftermarket [accessed 2019 Sep 20].
[SDS] Safety Data Sheet. 2016a. Febreze Small Spaces Mediterranean Lavender [PDF]. Toronto (CA): Proctor & Gamble Incorporated [accessed 2019 Sep 11]
[SDS] Safety Data Sheet. 2016b. Tarnish-Me-Not [PDF]. Passaic County (US): Tarnish Me Not [accessed 2019 Sep 11].
[SDS] Safety Data Sheet. 2016c. Graffiti Remover [PDF]. Chicago (US): Rust-Oleum Corporation [accessed 2019 Sep 19].
[SDS] Safety Data Sheet. 2016d. Spa jet cleaner [PDF]. Alpharetta (US)[accessed 2019 Sep 19].
[SDS] Safety Data Sheet. 2016e. Rust converter [PDF]. Fulf Breeze (US): Radical Rafts, Inc. [accessed 2019 Sep 19].
[SDS] Safety Data Sheet. 2016f. Tub & Tile Etch [PDF]. Toronto (CA): Rust-Oleum Consumer Brands Canada [accessed 2019 Sep 19].
[SDS] Safety Data Sheet. 2016g. Herman Survivors Liquid Silicone [PDF]. Durham (US): Implus Footcare LLC [accessed 2019 Sep 19].
[SDS] Safety Data Sheet. 2016h. 1915 Classic Inks [PDF]. Cleveland (US): Mace Security International [accessed 2019 Sep 19].
[SDS] Safety Data Sheet. 2016i. 10% Pepper Stream [PDF]. Erie County(US): International Imaging Materials, Inc [accessed 2019 Sep 19].
[SDS] Safety Data Sheet. 2017a. Kleen-Start Starting Fluid [PDF]. Toronto (CA): Kleen-Flo Tumbler Industries Limited [accessed 2019 Sep 6].
[SDS] Safety Data Sheet. 2017b. Arm & Hammer Scrub Free Bathroom Cleaner [PDF]. Mercer County (US): Church & Dwight [accessed 2019 Sep 11].
[SDS] Safety Data Sheet. 2017c. Rust-Oleum Spray Chalk, yellow [PDF]. Vaughan (CA): Rust-Oleum Consumer Brands Canada [accessed 2019 Sep 11].
[SDS] Safety Data Sheet. 2017d. LePage Quad Window and Door Foam [PDF]. Hartford County (US): Henkel Corporation [accessed 2019 Sep 11].
[SDS] Safety Data Sheet. 2017e. Rust Check Rust Converter [PDF]. Mississauga (CA): Rust Check Corporation [accessed 2019 Sep 11].
[SDS] Safety Data Sheet. 2017f. Air Wick Liquid Electrical - Fresh Waters [PDF]. Mississauga (ON): Reckiit Benckiser LLC. [accessed 2019 Sep 19].
[SDS] Safety Data Sheet. 2018a. Headliner Adhesive [PDF]. St. Paul (US): 3M [accessed 2019 Sep 11].
[SDS] Safety Data Sheet. 2018b. Tulip Color Shot Instant Fabric Color [PDF]. Fresno (US): Duncan Enterprises [accessed 2019 Sep 11].
[SDS] Safety Data Sheet. 2018c. Buck Bomb Doe “P” Bomb [PDF]. Cedar Rapids (US): Buck Bomb Incorporated [accessed 2019 Sep 11].
[SDS] Safety Data Sheet. 2018d. EZ Liner [PDF]. Mississauga (CA): Dominion Sure Seal Limited [accessed 2019 Sep 11].
[SDS] Safety Data Sheet. 2018e. Disinfectant Degreaser. Montreal (CA): LAVO [accessed 2019 Sep 19].
[SDS] Safety Data Sheet. 2018f. WDCARE 4X177ML VARA POLY TUBE SEMI GLOSS [PDF]. Vaughan (ON): Rust-Oleum Canada [accessed 2019 Sep 19].
[SDS] Safety Data Sheet. 2019a. Krylon Professional Marking Chalk [PDF]. Mississauga (CA): Krylon Products Group [accessed 2019 Sep 11].
[SDS] Safety Data Sheet. 2019b. Varathane Diamond Wood Finish – Interior [PDF]. Vaughan (CA): Rust-Oleum Canada [accessed 2019 Sep 11].
Slobodnik J, Mrafkova L, Carere M, Ferrara F, Pennelli B, Schuurmann G, Carsten von der Ohe P. 2010. Identification of river basin specific pollutants and derivation of environmental quality standards: A case study in the Slovak Republic.Trends Analyt Chem 41:133-145.
Smet E, Langenhove H, Bo I. 1999.The emission of volatile compounds during the aerobic and the combined anaerobic/aerobic composting of biowaste. Atmos Environ 33:1295-1303.
Statistics Canada. 2004a. Canadian Community Health Survey (CCHS)—Cycle 2.2: Nutrition. Share File. Ottawa, ON. Canada. Estimates generated using SAS 9.3 [GRID] through SAS EG 5.1.
Statistics Canada. 2004b. Canadian Community Health Survey, Cycle 2.2: General Health Component. Share File.
Statistics Canada. 2015. Canadian Community Health Survey (CCHS) 2015 Share File. Ottawa, ON. Canada. Estimates generated using SAS 9.3 [GRID] through SAS EG 5.1.
Tokumura M, Hatayama R, Tatsu K, Naito T, Takeda T, Raknuzzaman M, Habibullah-Al-Mamun M, Masunaga S. 2006. Car indoor air pollution by volatile organic compounds and aldehydes in Japan. AIMS Environ Sci. 3(3): 362-381.
[US EPA]. 1992. Screening procedures for estimating the air quality impact of stationary sources, revised [PDF]. Washington (DC): U.S. Environmental Protection Agency, Office of Air Quality. Report No. EPA-454/R-92-019.
[US EPA]. 1987. Health Effects Assessment for Ethyl Ether. Washington, DC: U.S. Environmental Protection Agency. Report No. EPA/600/8-88/039.
[US EPA]. 2009a. Provisional peer-reviewed toxicity values for ethyl ether. Washington, DC: U.S. Environmental Protection Agency. Report No. EPA/690/R-09/022F.
[US EPA]. 2009b. Dimethyl ether Initial Risk-Based Prioritization of HPV Chemicals. Washington (DC): U.S. Environmental Protection Agency. [accessed 2019 Aug 28].
[US EPA]. 2010. Diphenyl oxide Screening Level Hazard Characterization. Washington (DC): U.S. Environmental Protection Agency. [accessed 2019 Aug 28].
[US EPA]. 2011. Exposure Factors Handbook: 2011 Edition. Washington (DC): National Center for Environmental Assessment, Office of Research and Development, U.S. Environmental Protection Agency.
[US EPA]. 2014. Diethyl ether Screening Level Hazard Characterization. Washington (DC): U.S. Environmental Protection Agency. [accessed 2019 Aug 28].
[US EPA]. 2017. Provisional peer-reviewed toxicity values for diphenyl ether. Washington (DC): U.S. Environmental Protection Agency. Report No. EPA/690/R-17/009F.
Veijanen A, Lehtinen J. 2011. Odour monitoring by combined TD–GC–MS–sniff technique and dynamic olfactometry at the wastewater treatment plant of low H2S concentration. Water Air Soil Pollut. 218:185–196.
[WHO] World Health Organization. 2004. Evaluation of certain food additives and contaminants: sixty-first meeting of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series: 922. Geneva. World Health Organization.
Wu X, Bennett DH, Ritz B, Cassady DL, Lee K, Hertz-Picciotto I. 2010. Usage pattern of personal care products in California households. Food Chem Toxicol 48: 3109-3119.
Appendices
Appendix A. Summary table of read-across for health effects endpoints
| Property | DEE (target) | DIPE (analogue) |
|---|---|---|
| Structure |  |
 |
| Physical state | Liquid | Liquid |
| Melting point (°C) | –116a | -86.8a |
| Vapour pressure (Pa) | 7.17 × 104 a | 1.99 x 104 a |
| Henry’s law constant (Pa·m3/mol) | 1.25 × 102 a | 2.31 x 102 a |
| Water solubility (mg/L) | 6.04 × 104 a | 8.8 x 104 a |
| Log Kow (dimensionless) | 0.89a | 1.52a |
| Genotoxicity | Negative | N/A |
| Short-term oral | NA | N/A |
| Short-term inhalation | LOAELadj = 4256 mg/kg bw/day (35 day inhalation study in mice; liver toxicity and mortality) | N/A |
| Short-term dermal | NA | N/A |
| Sub-chronic oral | NOAEL = 500 mg/kg bw/day (13-week oral gavage study in rats; changes in body weight and increased mortality) | N/A |
| Sub-chronic inhalation | N/A | N/A |
| Sub-chronic dermal | NA | N/A |
| Chronic oral | NA | N/A |
| Chronic inhalation | NA | N/A |
| Chronic dermal | NA | N/A |
| Carcinogenicity | N/A | N/A |
| Reproductive and/or developmental toxicity | NOAEC = 28200 mg/m3 (read-across from DIPE) | NOAEC = 28200 mg/m3 (inhalation developmental study in rats; no adverse effects; no adverse foetal effects) |
Abbreviations: N/A, not applicable; NA, not available
a ChemIDplus 1993-
| Property | DPGME (target) | PGME (analogue) |
|---|---|---|
| Structure |  |
 |
| Physical state | Liquid | Liquid |
| Melting point (°C) | –83b | -95c |
| Vapour pressure (Pa) | 7.33 × 101 b | 1.2c |
| Henry’s law constant (Pa·m3/mol) | 1.08 × 10−2 a | 9.3 x 10−2 c |
| Water solubility (mg/L) | 1.00 × 106 b | 1.00 x 105 c |
| Log Kow (dimensionless) | –0.35a | -0.49c |
| Genotoxicity | Negative | N/A |
| Short-term oral | NOAEL = 1000 mg/kg bw/day (28-day oral study in rats; no adverse effects) | N/A |
| Short-term inhalation | NOAEC = 1818 mg/m3 (2-week studies in rats, mice; no adverse effects) | N/A |
| Short-term dermal | NOAEL = 1000 mg/kg bw/day (4-week study in rats; no adverse effects) | N/A |
| Sub-chronic oral | NA | N/A |
| Sub-chronic inhalation | NOAEC = 1818 mg/m3 (13-week studies in rats, rabbits; no adverse effects) | N/A |
| Sub-chronic dermal | NOAEL = 2850 mg/kg bw/day (90-day study in rabbits; minor skin irritation, narcosis & death) | N/A |
| Chronic oral | NA | N/A |
| Chronic inhalation | NOAEC = 1818 mg/m3 (31-week studies in rats, rabbits, guinea pigs, monkeys; no adverse effects) | N/A |
| Chronic dermal | NA | N/A |
| Carcinogenicity | NOAEC = 11058 mg/m3 (read-across from PGME) | NOAEC = 11 058 mg/m3 (2-year inhalation carcinogenicity study in rats, mice; no carcinogenic effects) |
| Reproductive and/or developmental toxicity | NOAEC = 1818 mg/m3 (developmental inhalation studies in rats, rabbits; no adverse foetal effects) | N/A |
Abbreviations: N/A, not applicable; NA, not available
a Modelled values
b ChemIDplus 1993-
c PubChem 2004-
Appendix B. Parameters used to estimate human exposure to DEE, DPE and DME from the use products available to consumers
Human exposure estimates from the use of products available to consumers were estimated using ConsExpo Web (ConsExpo Web 2016). Unless otherwise specified in the exposure scenarios below, default parameters from the ConsExpo Web model were used. Default parameters were selected from the General Fact Sheet (RIVM 2014), Cleaning Product Fact Sheet (RIVM 2018) and ConsExpo spray model documentation (RIVM 2009) and the Cosmetics Fact Sheet (RIVM 2006). To be conservative, absorption from the inhalation route was assumed to be 100% for DEE, DPE and DME, and absorption from the dermal route was assumed to be 100% for DPE.
Inhalation rates and body weights of the users are specified in Health Canada 2015a and are summarized in Table A-1.
| Age group | Inhalation rate (m3/day) | Body weight (kg) |
|---|---|---|
| 19 years or above | 15.1 | 74 |
| 14 to 18 years | 15.9 | 62 |
| 9 to 13 years | 13.9 | 42 |
| 4 to 8 years | 11.1 | 23 |
| 2 to 3 years | 9.2 | 15 |
| 1 year | 8.0 | 11 |
| Exposure scenario, route of exposure and age group | Parameters used in ConsExpo Web |
|---|---|
| Body lotion (daily exposure), inhalation, aged 19 years or abovea |
Model: Exposure to vapour – instantaneous release Use frequency: 1/day (Ficheux 2015; Wu 2010) Exposure duration: 24 hours (RIVM 2006) Product amount: 10 g (Ficheux 2016) Weight fraction: 0.03 (Personal communication, email from the Consumer and Hazardous Products Safety Directorate, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada, dated February 2018; unreferenced) Room volume: 80 m3 (professional judgement and is expected to be representative of mixing in indoor air over the day) Ventilation rate: 1/h (professional judgement) |
| Corn and callus remover (per event exposure), inhalation, aged 9 to 13 years |
Model: Exposure to vapour – instantaneous release Exposure duration: 10 minutes (professional judgement) Product amount: 0.7 g (as per use instructions in Health Canada’s Licensed Natural Health Products Database, corresponds to 2 drops of 0.5 mL each with a density of 0.7 g/mL for DEE) Weight fraction: 0.57 (DailyMed 2019) Room volume: 10 m3 (default for bathroom, RIVM 2014) Ventilation rate: 2.0/h (default for bathroom, RIVM 2014) |
| Starting fluid (per event exposure), inhalation, aged 19 years or above |
Model: Exposure to vapour – instantaneous release Exposure duration: 30 minutes (professional judgement) Product amount: 12 g (assumed 5 sprays of 2 seconds each based on instructions for a similar product, with a mass generation rate of 1.2 g/s (RIVM 2009)) Weight fraction: 0.6 (SDS 2017c) Room volume: 34 m3 (default for garage, RIVM 2014) Ventilation rate: 1.5/h (default for garage, RIVM 2014) |
a The age group of 19 years and above was selected as the corresponding product is expected to be used by adults only. Younger age groups were considered for other similar products which resulted in lower exposures on a per kg basis.
| Exposure scenario, route of exposure and age group | Parameters used in ConsExpo Web |
|---|---|
|
Household air freshener (daily exposure), inhalation, aged 1 year |
Model: Exposure to vapour – instantaneous release Exposure duration: 24 hours (professional judgement) Product amount: 0.18 g (package indicates that 5.5 mL lasts 30 days, assumed a density of 1 g/mL (US EPA CEM user guide)) Weight fraction: 0.05 (SDS 2016a) Room volume: 20 m3 (default for unspecified room, RIVM 2014) Ventilation rate: 0.6/h (default for unspecified room, RIVM 2014) |
|
Hand cream (daily exposure), inhalation, aged 19 years or above |
Model: Exposure to vapour – instantaneous release Exposure duration: 24 hours (professional judgement) Use frequency: 1/day (adjustments made to the product amount to account for the conservative estimate that the exposure duration is for 24 hours; Ficheux 2015) Product amount: 3.2 g (as the product amount for one application is 1.6 g and hand cream is estimated to be used twice a day, surface area adjustment based on data in Ficheux 2016) Weight fraction: 0.05 (Personal communication, email from CHPSD, Health Canada to ESRAB, Health Canada, dated 2018; unreferenced) Room volume: 80 m3 (professional judgement and is expected to be representative of mixing in indoor air over the day) Ventilation rate: 1/h (professional judgement) Application temperature: 37°C |
| Exposure scenario, route of exposure and age group | Parameters used in ConsExpo Web |
|---|---|
|
Spray sunscreen (daily exposure), inhalation, aged 1 year |
Model: Exposure to vapour – instantaneous release Use frequency: 1/day (adjustments made to the product amount to account for the conservative estimate that the exposure duration is for 24 hours; Ficheux 2015) Exposure duration: 24 hours (professional judgement) Product amount: 4 g (as the product amount for one application is 2.5 g and the spray sunscreen is estimated to be used 1.6 times a day; Ficheux 2016) Weight fraction: 0.4 (Personal communication, email from TPD, Health Canada to ESRAB, Health Canada, dated 2018; unreferenced) Room volume: 80 m3 (professional judgement and is expected to be representative of mixing in indoor air over the day) Ventilation rate: 1/h (professional judgement) |
Appendix C. Estimates of daily intake by various age groups within the general population of Canada
| Route of exposure | 0 to 5 monthsa (breast milk-fed)b | 0 to 5 monthsa (formula fed)c | 6 to 11 monthsd | 1 yeare | 2 to 3 yearsf | 4 to 8 yearsg | 9 to 13 yearsh | 14 to 18 yearsi | Greater than or equal to 19 yearsj |
|---|---|---|---|---|---|---|---|---|---|
| Ambient airk | 1.76E+0 | 1.76E+0 | 1.78E+0 | 2.18E+0 | 1.84E+0 | 1.45E+0 | 9.93E-1 | 7.69E-1 | 6.12E-1 |
| Indoor airl | 3.32E+2 | 3.32E+2 | 3.36E+2 | 4.12E+2 | 3.47E+2 | 2.73E+2 | 1.87E+2 | 1.45E+2 | 1.16E+2 |
| Drinking waterm | N/A | 8.63E+1 | 5.52E+1 | 2.15E+1 | 1.89E+1 | 1.52E+1 | 1.16E+1 | 1.16E+1 | 1.36E+1 |
| Total intake | 3.34E+2 | 4.19E+2 | 3.92E+2 | 4.35E+2 | 3.67E+2 | 2.89E+2 | 2.00E+2 | 1.57E+2 | 1.29E+2 |
Abbreviations: N/A, not applicable.
a Assumed to weigh 6.3 kg (Health Canada 2015a), to breathe 3.7 m3 of air per day (US EPA 2011 [modified]), and to ingest 21.6 mg of dust per day (Wilson and Meridian 2015 [modified]). It is assumed that no soil ingestion occurs due to typical caregiver practices.
b Exclusively for breast milk-fed infants, assumed to consume 0.744 L of breast milk per day (Health Canada 2018), and breast milk is assumed to be the only dietary source.
c Exclusively for formula-fed infants, assumed to drink 0.826 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote on drinking water for details.
d Assumed to weigh 9.1 kg (Health Canada 2015a), to breathe 5.4 m3 of air per day (US EPA 2011 [modified]), to drink 0 L of water per day (Health Canada 2017), to ingest 7.3 mg of soil per day, and to ingest 27.0 mg of dust per day (Wilson and Meridian 2015 [modified]). For breast milk-fed infants, assumed to consume 0.632 L of breast milk per day (Health Canada 2018). For formula-fed infants, assumed to drink 0.764 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote on drinking water for details.
e Assumed to weigh 11.0 kg (Health Canada 2015a), to breathe 8.0 m3 of air per day (US EPA 2011 [modified]), to drink 0.36 L of water per day (Health Canada 2017), to ingest 8.8 mg of soil per day, and to ingest 35.0 mg of dust per day (Wilson and Meridian 2015 [modified]).
f Assumed to weigh 15 kg (Health Canada 2015a), to breathe 9.2 m3 of air per day (US EPA 2011 [modified]), to drink 0.43 L of water per day (Health Canada 2017), to ingest 6.2 mg of soil per day, and to ingest 21.4 mg of dust per day (Wilson and Meridian 2015 [modified]).
g Assumed to weigh 23 kg (Health Canada 2015a), to breathe 11.1 m3 of air per day (US EPA 2011 [modified]), to drink 0.53 L of water per day (Health Canada 2017), to ingest 8.7 mg of soil per day, and to ingest 24.4 mg of dust per day (Wilson and Meridian 2015 [modified]).
h Assumed to weigh 42 kg (Health Canada 2015a), to breathe 13.9 m3 of air per day (US EPA 2011 [modified]), to drink 0.74 L of water per day (Health Canada 2017), to ingest 6.9 mg of soil per day, and to ingest 23.8 mg of dust per day (Wilson and Meridian 2015 [modified]).
i Assumed to weigh 62 kg (Health Canada 2015a), to breathe 15.9 m3 of air per day (US EPA 2011 [modified]), to drink 1.09 L of water per day (Health Canada 2017), to ingest 1.4 mg of soil per day, and to ingest 2.1 mg of dust per day (Wilson and Meridian 2015 [modified]).
j Assumed to weigh 74 kg (Health Canada 2015a), to breathe 15.1 m3 of air per day (US EPA 2011 [modified]), to drink 1.53 L of water per day (Health Canada 2017), to ingest 1.6 mg of soil per day, and to ingest 2.6 mg of dust per day (Wilson and Meridian 2015 [modified]).
k The maximum ambient air concentration from Canadian homes (24 µg/m3) was used for deriving upper-bounding estimates of daily intake for ambient air exposure as a conservative estimate (Chan et al. 1990). Canadians are assumed to spend 3 hours outdoors each day (Health Canada 1998).
l The maximum indoor air concentration from Canadian homes (647 µg/m3) was used for deriving upper-bounding estimates of daily intake for indoor air exposure as a conservative estimate (Chan et al. 1990). Canadians are assumed to spend 21 hours indoors each day (Health Canada 1998).
m The maximum concentration measured in a monitoring well network and outwash aquifer (658 µg/L) at the Gloucester Landfill in Canada was used for deriving upper-bounding estimates of daily intake for drinking water exposure as a conservative estimate (Lesage et al. 1990).
| Route of exposure | 0 to 5 monthsa (breast milk-fed)b | 0 to 5 monthsa (formula fed)c | 6 to 11 monthsd | 1 yeare | 2 to 3 yearsf | 4 to 8 yearsg | 9 to 13 yearsh | 14 to 18 yearsi | Greater than or equal to 19 yearsj |
|---|---|---|---|---|---|---|---|---|---|
| Airk | 3.69E-3 | 3.69E-3 | 3.73E-3 | 4.57E-3 | 3.85E-3 | 3.03E-3 | 2.08E-3 | 1.61E-3 | 1.28E-3 |
| Drinking waterl | N/A | 7.24E-3 | 4.63E-3 | 1.81E-3 | 1.58E-3 | 1.27E-3 | 9.73E-4 | 9.70E-4 | 1.14E-3 |
| Food and beveragesm | N/A | N/A | 5.49E-1 | 4.55E-1 | 3.33E-1 | 2.17E-1 | 1.19E-1 | 8.06E-2 | 6.76E-2 |
| Total intake | 3.69E-3 | 1.00E-2 | 5.57E-1 | 4.61E-1 | 3.38E-1 | 2.21E-1 | 1.22E-1 | 8.32E-2 | 7.00E-2 |
Abbreviations: N/A, not applicable.
a Assumed to weigh 6.3 kg (Health Canada 2015a), to breathe 3.7 m3 of air per day (US EPA 2011 [modified]), and to ingest 21.6 mg of dust per day (Wilson and Meridian 2015 [modified]). It is assumed that no soil ingestion occurs due to typical caregiver practices.
b Exclusively for breast milk-fed infants, assumed to consume 0.744 L of breast milk per day (Health Canada 2018), and breast milk is assumed to be the only dietary source.
c Exclusively for formula-fed infants, assumed to drink 0.826 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote on drinking water for details.
d Assumed to weigh 9.1 kg (Health Canada 2015a), to breathe 5.4 m3 of air per day (US EPA 2011 [modified]), to drink 0 L of water per day (Health Canada 2017), to ingest 7.3 mg of soil per day, and to ingest 27.0 mg of dust per day (Wilson and Meridian 2015 [modified]). For breast milk-fed infants, assumed to consume 0.632 L of breast milk per day (Health Canada 2018). For formula-fed infants, assumed to drink 0.764 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote on drinking water for details.
e Assumed to weigh 11.0 kg (Health Canada 2015a), to breathe 8.0 m3 of air per day (US EPA 2011 [modified]), to drink 0.36 L of water per day (Health Canada 2017), to ingest 8.8 mg of soil per day, and to ingest 35.0 mg of dust per day (Wilson and Meridian 2015 [modified]).
f Assumed to weigh 15 kg (Health Canada 2015a), to breathe 9.2 m3 of air per day (US EPA 2011 [modified]), to drink 0.43 L of water per day (Health Canada 2017), to ingest 6.2 mg of soil per day, and to ingest 21.4 mg of dust per day (Wilson and Meridian 2015 [modified]).
g Assumed to weigh 23 kg (Health Canada 2015a), to breathe 11.1 m3 of air per day (US EPA 2011 [modified]), to drink 0.53 L of water per day (Health Canada 2017), to ingest 8.7 mg of soil per day, and to ingest 24.4 mg of dust per day (Wilson and Meridian 2015 [modified]).
h Assumed to weigh 42 kg (Health Canada 2015a), to breathe 13.9 m3 of air per day (US EPA 2011 [modified]), to drink 0.74 L of water per day (Health Canada 2017), to ingest 6.9 mg of soil per day, and to ingest 23.8 mg of dust per day (Wilson and Meridian 2015 [modified]).
i Assumed to weigh 62 kg (Health Canada 2015a), to breathe 15.9 m3 of air per day (US EPA 2011 [modified]), to drink 1.09 L of water per day (Health Canada 2017), to ingest 1.4 mg of soil per day, and to ingest 2.1 mg of dust per day (Wilson and Meridian 2015 [modified]).
j Assumed to weigh 74 kg (Health Canada 2015a), to breathe 15.1 m3 of air per day (US EPA 2011 [modified]), to drink 1.53 L of water per day (Health Canada 2017), to ingest 1.6 mg of soil per day, and to ingest 2.6 mg of dust per day (Wilson and Meridian 2015 [modified]).
k No monitoring data of air in Canada were identified. Based on an estimate of 6.28 ng/L on the basis of a 100% release scenario to air from ChemCAN v6.00 where the simulations conservatively assumed that total quantities were released into a single region of Canada, i.e., the Ontario Mixed-Wood Plain region.
l Rogers et al. (1986) identified DPE as a component in base/neutral fractions of wastewater and sludge at the Iona Island in British Columbia but the concentration of DPE was not determined. Therefore, no monitoring data where DPE was measured in Canada were identified. Based on an estimate of 0.0552 µg/L on the basis of a 100% release scenario to water from ChemCAN v6.00 where the simulations conservatively assumed that total quantities were released into a single region of Canada, i.e., the Ontario Mixed-Wood Plain region, at a 100% emission factor and assuming 0% removal for wastewater treatment processes (for water releases).
m No definitive information is available concerning the potential use of DPE in foods sold in Canada. The Joint FAO/WHO Expert Committee on Food Additives (JECFA) per capita intake estimate of 5 µg per person per day for the United States (based on annual production volumes reported by the food industry in poundage surveys) was used to derive daily intakes of DPE as a food flavouring agent (see section 6.1.2).
| Route of exposure | 0 to 5 monthsa (breast milk-fed)b | 0 to 5 monthsa (formula fed)c | 6 to 11 monthsd | 1 yeare | 2 to 3 yearsf | 4 to 8 yearsg | 9 to 13 yearsh | 14 to 18 yearsi | Greater than or equal to 19 yearsj |
|---|---|---|---|---|---|---|---|---|---|
| Airk | 7.21E+1 | 7.21E+1 | 7.28E+1 | 8.93E+1 | 7.53E+1 | 5.92E+1 | 4.06E+1 | 3.15E+1 | 2.50E+1 |
| Drinking waterl | N/A | 7.60E-3 | 4.87E-3 | 1.90E-3 | 1.66E-3 | 1.34E-3 | 1.02E-3 | 1.02E-3 | 1.20E-3 |
| Total intake | 7.21E+1 | 7.21E+1 | 7.28E+1 | 8.93E+1 | 7.53E+1 | 5.92E+1 | 4.06E+1 | 3.15E+1 | 2.50E+1 |
Abbreviations: N/A, not applicable.
a Assumed to weigh 6.3 kg (Health Canada 2015a), to breathe 3.7 m3 of air per day (US EPA 2011 [modified]), and to ingest 21.6 mg of dust per day (Wilson and Meridian 2015 [modified]). It is assumed that no soil ingestion occurs due to typical caregiver practices.
b Exclusively for breast milk-fed infants, assumed to consume 0.744 L of breast milk per day (Health Canada 2018), and breast milk is assumed to be the only dietary source.
c Exclusively for formula-fed infants, assumed to drink 0.826 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote on drinking water for details.
d Assumed to weigh 9.1 kg (Health Canada 2015a), to breathe 5.4 m3 of air per day (US EPA 2011 [modified]), to drink 0 L of water per day (Health Canada 2017), to ingest 7.3 mg of soil per day, and to ingest 27.0 mg of dust per day (Wilson and Meridian 2015 [modified]). For breast milk-fed infants, assumed to consume 0.632 L of breast milk per day (Health Canada 2018). For formula-fed infants, assumed to drink 0.764 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote on drinking water for details.
e Assumed to weigh 11.0 kg (Health Canada 2015a), to breathe 8.0 m3 of air per day (US EPA 2011 [modified]), to drink 0.36 L of water per day (Health Canada 2017), to ingest 8.8 mg of soil per day, and to ingest 35.0 mg of dust per day (Wilson and Meridian 2015 [modified]).
f Assumed to weigh 15 kg (Health Canada 2015a), to breathe 9.2 m3 of air per day (US EPA 2011 [modified]), to drink 0.43 L of water per day (Health Canada 2017), to ingest 6.2 mg of soil per day, and to ingest 21.4 mg of dust per day (Wilson and Meridian 2015 [modified]).
g Assumed to weigh 23 kg (Health Canada 2015a), to breathe 11.1 m3 of air per day (US EPA 2011 [modified]), to drink 0.53 L of water per day (Health Canada 2017), to ingest 8.7 mg of soil per day, and to ingest 24.4 mg of dust per day (Wilson and Meridian 2015 [modified]).
h Assumed to weigh 42 kg (Health Canada 2015a), to breathe 13.9 m3 of air per day (US EPA 2011 [modified]), to drink 0.74 L of water per day (Health Canada 2017), to ingest 6.9 mg of soil per day, and to ingest 23.8 mg of dust per day (Wilson and Meridian 2015 [modified]).
i Assumed to weigh 62 kg (Health Canada 2015a), to breathe 15.9 m3 of air per day (US EPA 2011 [modified]), to drink 1.09 L of water per day (Health Canada 2017), to ingest 1.4 mg of soil per day, and to ingest 2.1 mg of dust per day (Wilson and Meridian 2015 [modified]).
j Assumed to weigh 74 kg (Health Canada 2015a), to breathe 15.1 m3 of air per day (US EPA 2011 [modified]), to drink 1.53 L of water per day (Health Canada 2017), to ingest 1.6 mg of soil per day, and to ingest 2.6 mg of dust per day (Wilson and Meridian 2015 [modified]).
k Based on the NPRI air emission data and facility-specific information, the US EPA model SCREEN3 was selected to estimate concentration in ambient air (123 µg/m3).
l No monitoring data of water Canada were identified. Based on an estimate of 0.058 µg/L on the basis of a 100% release scenario to water from ChemCAN v6.00 where the simulations conservatively assumed that total quantities were released into a single region of Canada, i.e., the Ontario Mixed-Wood Plain region, at a 100% emission factor and assuming 0% removal for wastewater treatment processes (for water releases).
