Draft screening assessment flame retardants group
Official title: Draft Screening Assessment - Flame Retardants Group
Chemical Abstracts Service Registry Numbers:
- 78-40-0
- 78-51-3
- 78-42-2
- 298-07-7
- 115-86-6
- 56803-37-3
- 68937-41-7
- 29761-21-5
- 65652-41-7
- 58965-66-5
Environment and Climate Change Canada
Health Canada
November 2021
Synopsis
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of 10 of 13 substances referred to collectively as the Flame Retardants Group. These 13 substances were identified as priorities for assessment. Three of the 13 substances were determined to be of low concern through a separate approach, and decisions for these substances are provided in a separate report.Footnote 1 Accordingly, this screening assessment addresses the 10 substances listed in the table below, which will hereinafter be referred to as the Flame Retardants Group. The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 2), their Domestic Substances List (DSL) names and their common names and abbreviations are listed in the table below. The substances in this group have been assessed under two subgroups (aryl organophosphates and alkyl organophosphates), with the exception of one substance, which has been assessed as an individual substance, as shown in the table below.
| CAS RN | DSL name | Common names (abbreviation) | Subgroup |
|---|---|---|---|
| 115-86-6a | Phosphoric acid, triphenyl ester | Triphenyl phosphate (TPHP) | Aryl organophosphate |
| 56803-37-3 | Phosphoric acid, (1,1-dimethylethyl)phenyl diphenyl ester | Tert-butylphenyl diphenyl phosphate (BPDP) | Aryl organophosphate |
| 65652-41-7a | Phosphoric acid, bis[(1,1-dimethylethyl)phenyl] phenyl ester | Bis(tert-butylphenyl) phenyl phosphate (BDMEPPP) | Aryl organophosphate |
| 29761-21-5 | Phosphoric acid, isodecyl diphenyl ester | Isodecyl diphenyl phosphate (IDDP) | Aryl organophosphate |
| 68937-41-7c | Phenol, isopropylated, phosphate (3:1) | Isopropylated triphenyl phosphate (IPPP) | Aryl organophosphate |
| 78-40-0b | Phosphoric acid, triethyl ester | Triethylphosphate; Triethyl phosphate (TEP) | Alkyl organophosphate |
| 78-51-3 | ethanol, 2-butoxy-, phosphate (3:1) | Tris(2-butoxethyl) phosphate (TBOEP) | Alkyl organophosphate |
| 78-42-2b | Phosphoric acid, tris(2-ethylhexyl) ester | Tris(2-ethylhexyl) phosphate (TEHP) | Alkyl organophosphate |
| 298-07-7b | Phosphoric acid, bis(2-ethylhexyl) ester | Bis(2-ethylhexyl) phosphate; bis(2-ethylhexyl) hydrogen phosphate (BEHP) | Alkyl organophosphate |
| 58965-66-5a | Benzene, 1,2,4,5-tetrabromo-3,6-bis(pentabromophenoxy)- | Tetradecabromo-1,4-diphenoxybenzene; perbromo-1,4-diphenoxybenzene (TDBDPB) | Not applicable |
a This substance did not meet categorization criteria, but was included in this assessment because it was determined to be a priority as a result of the approach described for Identification of Risk Assessment Priorities.
b This CAS RN is a UVCB (unknown or variable composition, complex reaction products, or biological materials).
c This substance was not identified under subsection 73(1) of CEPA, but was included in this assessment as it was considered a priority on the basis of other human health concerns.
None of the substances in this group occur naturally. According to information submitted to surveys issued pursuant to section 71 of CEPA for the reporting years of 2008, 2011, and/or 2015, none of the aryl organophosphates (OP) were manufactured in Canada. The ranges for total imports into Canada during these years were from 100 000 kg to 1 000 000 kg for TPHP, BPDP and IPPP and from 10 000 kg to 100 000 kg for BDMEPPP and IDDP. For the alkyl OP substances, TBOEP and BEHP were each reported to be manufactured in Canada in a quantity ranging from 1 000 kg to 10 000 kg in 2011. For that same year, reported total TEP imports into Canada ranged from 100 000 kg to 1 000 000 kg, while reported total TBOEP, TEHP and BEHP imports into Canada ranged from10 000 kg to 100 000 kg. TDBDPB was not reported to be manufactured in Canada, but was imported and used in Canada in 2008 (< 10 000 kg). The major North American producer of TDBDPB is reported to have discontinued manufacture of this substance prior to 2012, and TDBDPB is not currently imported or used in Canada.
In Canada, the alkyl and aryl OPs subject to this assessment are primarily used as either additive flame retardants or plasticizers in various applications involving hydraulic fluids, plastics, rubber products, textiles, foam, paints, adhesives and sealants, and building materials. Some of these substances are also used in food packaging applications (TPHP, IDDP, TEP, TEHP, TBOEP), foam (TEP) and as formulants in pest control products (TPHP, IPPP, TEHP). TPHP is also used in nail care products in Canada. TDBDPB is an additive flame retardant that has been used in plastic and rubber in Canada.
Aryl OP subgroup substances are not expected to be persistent in water, soil, sediment or air based on modelled and experimental data. However, TPHP has been measured in remote locations (e.g., Canadian and European Arctic) possibly due to particle bound atmospheric transport and is considered persistent in air. Aryl OPs bioconcentration studies, metabolism studies, and bioaccumulation modelling suggest that TPHP, BPDP, and IDDP will have low to moderate bioaccumulation potential. For the more hydrophobic BDMEPPP, and possibly IPPP (depending on UVCB mixture), moderate to high bioaccumulation potential is identified by measured and predicted data.
Based on the available empirical ecotoxicity studies and modelled data, aryl OPs subject to this assessment are considered to have high toxicity to aquatic organisms, with acute and chronic effects demonstrated at less than 1 mg/L. Sediment and soil toxicity data are limited for the individual substances. However, TPHP/BPDP/BDMEPPP mixture and IPPP UVCB tests provide evidence of moderate to high toxicity in those media as well. There are limited toxicity data on terrestrial wildlife that clearly measure organism effect levels for aryl OPs. Recent studies suggest that these substances may induce neurobehavioural and developmental effects in biota, as well as other effects, including disruption to reproductive and endocrine systems.
It is expected that the aryl OPs in this group may be released to the Canadian environment through industrial processing activities, releases from products used by consumers, wastewater discharges to surface waters, and biosolids application to land. Given the likelihood of the substances being used together or interchangeably for the identified uses, and considering their common properties, aryl OP substance quantities were combined into a total quantity for each ‘use’ to develop combined ecological exposure scenarios. Risk quotient analyses integrating estimates of exposure and toxicity information were performed for scenarios involving industrial releases and releases from products used by consumers. These analyses indicated that there is risk of harm to aquatic and sediment organisms and to wildlife consuming aryl OP subgroup substances via fish. Based on current aryl OP uses, the analysis indicated low risk of harm to soil organisms.
For the ecological assessment of the alkyl OP subgroup, TEP, TEHP, and BEHP were characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of ERC analysis, substances in the alkyl OP subgroup, TEP, TEHP and BEHP are considered unlikely to be causing ecological harm. Therefore, only TBOEP is addressed in the ecological portion of the alkyl OP assessment.
TBOEP is not shown to be persistent in water, soil, sediment or air based on modelled and limited experimental data. However, it has been measured in the Canadian Arctic indicating sufficient persistence for long-range transport. TBOEP shows a low potential to bioaccumulate or biomagnify in biota and is considered to have a moderate to high level of toxicity to aquatic organisms, with acute and chronic effects demonstrated from approximately less than 1.0 to 100 mg/L and a moderate level of toxicity to soil organisms based on limited data. Exposure scenarios were developed for releases from industrial activities and for releases of products available to consumers to surface water. Risk quotient analyses, comparing conservative estimates of exposure with the available toxicity information, were performed and showed a low potential for risk to aquatic and soil organisms from TBOEP.
TDBDPB is extremely hydrophobic and persistent but may be susceptible to photolytic degradation producing lower brominated polybrominated diphenoxybenzenes (PBDPBs). TDBDPB is considered to have limited bioavailability and bioaccumulation potential. However, model-based bioconcentration and bioaccumulation factors for two phototransformation products of TDBDPB (PBDPBs with four and five bromine atoms) indicate a very high potential to bioaccumulate in aquatic organisms. The bioavailable/bioaccumulative products of debromination (e.g., PBDPBs with four and five bromine atoms) of TDBDPB are expected to have a much higher potential for inherent toxicity. There is a low potential for risk to the Canadian environment from TDBDPB given that there is no known importation or use of this substance in Canada at this time.
Considering all available lines of evidence presented in this draft screening assessment, there is a risk of harm to the environment from TPHP, BPDP, BDMEPPP, IDDP and IPPP. It is proposed to conclude that TPHP, BPDP, BDMEPPP, IDDP and IPPP meet the criteria under paragraphs 64(a) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity. However, it is proposed to conclude that TPHP, BPDP, BDMEPPP, IDDP and IPPP do not meet the criteria under paragraph 64(b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger to the environment on which life depends. Also, it is proposed to conclude that TBOEP, TEP, TEHP, BEHP and TDBDPB do not meet the criteria under paragraph 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
For the human health assessment, BDMEPPP was evaluated using the approach applied in the Rapid Screening of Substances with Limited General Population Exposure. The potential for exposure of the general population to BDMEPPP was considered to be negligible. Therefore, BDMEPPP is considered to be a low concern for human health at current levels of exposure.
Based on laboratory studies, the critical health effects for the aryl OP subgroup include decreased body weight gain for TPHP, organ weight changes for BPDP, adverse adrenal gland and liver effects for IPPP, and liver effects for IDDP. No reproductive or developmental effects were observed for TPHP, BPDP or IDDP. Reproductive effects were observed after exposure to IPPP. The general population in Canada is exposed to TPHP and IPPP from environmental media and food, to BPDP from environmental media, and to all three of these substances from lying on foam-containing mattresses or furniture. Children may also be exposed to these substances while mouthing toys and foam in products such as nap mats and changing table pads. For IDDP, Canadians are expected to be exposed from environmental media only. TPHP is also present in various nail care products such as nail polish. Comparisons of estimated levels of exposure to TPHP, BPDP and IDDP and critical effect levels result in margins that are considered to be adequate to address uncertainties in the exposure and health effects databases. For IPPP, the resulting margins associated with exposures from contact with environmental media and food, as well as from mouthing certain foam-containing products, such as foam toys, and from sitting in infant or child restraint seats, are considered adequate. However, the margins associated with prolonged skin contact to IPPP for infants and children lying on foam-containing mattresses or furniture are considered potentially inadequate to account for uncertainties in the exposure and health effects databases.
For the alkyl OP subgroup, based on laboratory studies, the critical health effects include liver effects for TEP, liver effects in males for TBOEP, thyroid effects for TEHP, and liver effects for BEHP. The general population in Canada may be exposed to these substances from dust (TEP, TBOEP, TEHP), indoor air (TEP, TBOEP, TEHP), drinking water (TEP, TBOEP, BEHP), food (TBOEP), and breast milk (TBOEP), and from the use of products available to consumers, including foam-containing mattresses or furniture (TEP, TBOEP ), infant and child restraint seats (TEP, TBOEP), and all-purpose remover for oven cleaning (TEP), floor sealant (TEHP) and gear oil (BEHP). Children may also be exposed from mouthing foam in toys or products available to consumers containing TEP or TBOEP. A comparison of estimated levels of exposure to TBOEP, TEHP and BEHP and critical effect levels results in margins of exposure that are considered adequate to address uncertainties in the exposure and health effects databases. For TEP, the resulting margins of exposure associated with the exposure to environmental media and food as well as use of all-purpose remover and foam sealant are considered adequate. However, the margins associated with prolonged skin contact to TEP from lying on foam-containing mattresses or furniture (all ages) and from sitting in infant or child restraint seats are considered potentially inadequate to account for uncertainties in the exposure and health effects databases.
Exposure of the general population to TDBDPB through environmental media, food, or the use of products available to consumers is not expected. Accordingly, the potential risk to human health is considered to be low.
Considering all the information presented in this draft screening assessment, it is proposed to conclude that IPPP and TEP meet the criteria under paragraph 64(c) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Considering all the information presented in the draft screening assessment, it is proposed to conclude that TPHP, BPDP, BDMEPPP, IDDP, TBOEP, TEHP, BEHP and TDBDPB do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that TPHP, BPDP, BDMEPPP, IDDP, IPPP and TEP meet one or more of the criteria set out in section 64 of CEPA, and that TBOEP, TEHP, BEHP and TDBDPB do not meet any of the criteria set out in section 64 of CEPA. It is also proposed that TPHP and TEP meet the persistence criteria but do not meet the bioaccumulation criteria; BPDP and IDDP do not meet the persistence criteria or the bioaccumulation criteria; and BDMEPPP and IPPP do not meet the persistence criteria but do meet the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
List of abbreviations
- AchE
- Acetylcholinesterase
- AHR
- Aryl hydrocarbon receptor
- ATSDR
- Agency for Toxic Substances and Disease Registry
- BAF
- Bioaccumulation factor
- BBOEHEP
- Bis(2-butoxyethyl) hydroxyethyl phosphate
- BCF
- Bioconcentration factor
- BDMEPPP
- Bis(tert-butylphenyl) phenyl phosphate
- BEHP
- Bis(2-ethylhexyl) phosphate
- BMD(L)
- Benchmark dose (lower confidence limit)
- BPDP
- Tert-Butylphenyl diphenyl phosphate
- BW
- Body weight
- CAS RN
- Chemical Abstracts Service Registry Number
- CBR
- Critical body residue
- CCHS
- Canadian Community Health Survey
- CDR
- Chemical data reporting
- CEPA
- Canadian Environmental Protection Act
- CHDS
- Canadian House Dust Study
- CMP3
- Chemicals Management Plan (phase 3)
- CPSD
- Consumer Product Safety Directorate
- CTD
- Characteristic travel distance
- CTV
- Critical toxicity value
- DA
- Dermal absorption
- DSL
- Domestic Substances List
- EA
- Environment Agency (UK Government)
- EC50
- Median effective concentration
- ECCC
- Environment and Climate Change Canada
- ECHA
- European Chemicals Agency
- ED
- Exposure duration
- ERC
- Ecological Risk Classification of Organic Substances
- EROD
- Ethoxyresorufin O-deethylase
- ESIS
- European Chemical Substance Information Systems
- ESRAB
- Existing Substances Risk Assessment Bureau
- FD
- Food Directorate
- FM550
- Firemaster 550
- HC
- Health Canada
- IARC
- International Agency for Research on Cancer
- IC
- Inhibition concentration
- IDDP
- Isodecyl diphenyl phosphate
- IPCS
- International Programme on Chemical Safety
- IPPP
- Isopropylated triphenyl phosphate
- Koa
- Octanol-air partition coefficient
- Koc
- Organic carbon–water partition coefficient
- Kow
- Octanol–water partition coefficient
- LC50
- Lethal concentration (kills 50% of test population)
- LOEC
- Lowest observed effect concentration
- LO(A)EL
- Lowest observed (adverse) effect level
- LRAT
- Long-range atmospheric transport
- LSA
- Least-squares adjustment procedure
- M
- Migration rate
- MATC
- Maximum allowable toxicity concentration
- MDL
- Method detection limit
- MOA
- Mode of action
- MOE
- Margin of exposure
- MRL
- Minimal risk level
- MSDS
- Material safety data sheet
- MUR
- Most used room
- MW
- Molecular weight
- ND
- Not detected
- NO(A)EL
- No observed (adverse) effect level
- NOEC
- No observed effect concentration
- OC
- Organic carbon
- OECD
- Organisation for Economic Co-operation and Development
- OH-PB-DiPhOBz
- Tetradecabromo-1,4-diphenoxybenzene
- OPFR
- Organophosphate flame retardant
- PB-DiPhOBz
- Polybrominated-diphenoxybenzene
- PBT
- Polybutylene terephthalate
- PEC
- Predicted environmental concentration
- PET
- Polybutylene terephthalate
- PMRA
- Pest Management Regulatory Agency
- PNEC
- Predicted no effect concentration
- Pov
- Overall persistence
- PUF
- Polyurethane foam
- PVC
- Polyvinylchloride
- (Q)SAR
- (Quantitative) structure activity relationship
- RIVM
- Netherlands National Institute for Public Health and Environment
- RQ
- Risk quotients
- SCF
- Skin contact factor
- SD
- Sprague-Dawley
- SMILES
- Simplified molecular-input line-entry system
- T2IPPP
- Tris(2-isopropylphenyl) phosphate
- T3IPPP
- Tris(3-isopropylphenyl) phosphate
- TBOEP
- Tris(2-butoxethyl) phosphate
- TDBDPB
- Tetradecabromo-1,4-diphenoxybenzene
- TDI
- Total daily intake calculation
- TE
- Transfer efficiency
- TEHP
- Tris(2-ethylhexyl) phosphate
- TEP
- Triethylphosphate
- TESIE
- Toddler’s exposure to SVOCs in the indoor environment
- TMF
- Trophic Magnification Factor
- TPF
- Textile penetration factor
- TPHP
- Triphenyl phosphate
- TRV
- Toxicity reference value
- TTC
- Threshold of Toxicological Concern
- US EPA
- United States Environmental Protection Agency
- UVCB
- Unknown or variable composition, complex reaction products, or biological materials
- WHO
- World Health Organization
- WWTS
- Wastewater treatment system
1. Introduction
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment on 10 of 13 substances, referred to collectively under the Chemicals Management Plan as the Flame Retardants Group, to determine whether these 10 substances present or may present a risk to the environment or to human health. These 10 substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns (ECCC, HC [modified 2017]) or were included in this assessment because they were determined to be priorities for assessment as a result of the approach described for Identification of Risk Assessment Priorities (ECCC, HC 2015; Environment Canada, Health Canada 2014).
The other three substances (the CAS RN are listed in Table 1‑1, below) were considered in the Ecological Risk Classification of Organic Substances (ERC) Science Approach Document (ECCC 2016a) and via the approach applied in the Rapid Screening of Substances with Limited General Population Exposure (ECCC, HC 2018) and were identified as being of low concern to both human health and the environment. As such, they are not further addressed in this report. Conclusions for these three substances are provided in the Rapid Screening of Substances with Limited General Population Exposure (ECCC, HC 2018).
The 10 substances addressed in this screening assessment will hereinafter be referred to as the Flame Retardants Group.
| CAS RN | Domestic Substances List name | Approach under which the substance was addressed | References |
|---|---|---|---|
| 26446-73-1 | Phenol, 4-(1,1-dimethylethyl)-, phosphate (3:1) | ERC/Rapid Screening | ECCC, HC 2018 |
| 68527-01-5 | Alkenes, C12-30 α-, bromo chloro | ERC/Rapid Screening | ECCC, HC 2018 |
| 68527-02-6 | Alkenes, C12-24, chloro | ERC/Rapid Screening | ECCC, HC 2018 |
All but one of the substances in the Flame Retardant Group have been addressed in two subgroups (aryl organophosphates and alkyl organophosphates), based on similar chemical structure, physical and chemical properties or toxicity, while TDBDPB has been assessed as an individual substance. Given the potential for these substances to be used in similar ways and applications, potential risk is assessed using similar exposure assumptions across each group.
Three substances in the Flame Retardant Group (TEP, TEHP and BEHP) were identified as having a low potential to be causing ecological harm based on the ERC approach (ECCC 2016b; Appendix A). These results are considered in support of the conclusions made under section 64 of CEPA in this screening assessment. Therefore, only TBOEP is addressed in the ecological portion of the alkyl OP assessment.
The human health risk of BDMEPPP was considered under the approach applied in the Rapid Screening of Substances with Limited General Population Exposure (ECCC, HC 2018). The potential for direct exposure was evaluated on the basis of considerations such as evidence of the substance being present in a product used by the general population, and the potential for indirect exposure was adopted from the general approach reported in the Rapid Screening of Substances with Limited General Population Exposure document (ECCC, HC 2018). On the basis of the evaluation of both direct and indirect exposure conducted as part of this approach, exposure of the general population to BDMEPPP was considered to be negligible. Therefore, this substance is considered to be a low concern for human health at current levels of exposure.
Some substances currently being evaluated in the Flame Retardant Group have been reviewed internationally through the Cooperative Chemicals Assessment Programme of the Organisation for Economic Cooperation and Development (OECD). These assessments undergo rigorous review (including peer review) and endorsement by international governmental authorities. Health Canada and Environment and Climate Change Canada are active participants in this process and consider these assessments to be reliable. Some of the substances have also been reviewed by the Agency for Toxic Substances and Disease Registry (ATSDR), the International Programme on Chemical Safety (IPCS), the UK government’s Environment Agency and the United States Environmental Protection Agency (US EPA) and there are existing assessments available. These assessments undergo rigorous review (including peer review). Health Canada considers these assessments to be reliable. These assessments were used to inform the health effects characterization in this screening assessment.
This draft screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to March 2018. Targeted literature searches were conducted up to December 2019. Empirical data from key studies as well as results from models were used to reach proposed conclusions.
This draft screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological and human health portions of this assessment have undergone external review and/or consultation. Comments on the technical portions concerning the organophosphate substances relevant to the environment were received from Dr. Alana Greaves (Carleton University), Dr. Ian Doyle (United Kingdom Environment Agency), Dr. Miriam Diamond (University of Toronto), Dr. Pamela Campbell (ToxEcology – Environmental Consulting Ltd) and Dr. Royi Mazor (ICL Group). Comments on the technical portions relevant to human health were received from scientists selected by Risk Sciences International, including Dr. Supratik Kar (Jackson State University), Dr. Ole Jakob Nøstbakken (Institute of Marine Research, Norway), and Dr. Kevin Crofton (US Environmental Protection Agency). The ecological portions of this assessment for TEP, TEHP and BEHP are based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. For BDMEPPP, the health portion of this assessment uses the approach applied in the Rapid Screening of Substances with Limited General Population Exposure (published June 10, 2017), which was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This draft screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight-of-evidence approach and precaution.Footnote 3 This draft screening assessment presents the critical information and considerations on which the proposed conclusions are based.
2. Assessment of the aryl organophosphate subgroup (TPHP, BPDP, BDMEPPP, IDDP, IPPP)
2.1 Identity of substances
The CAS RN, Domestic Substances List (DSL) names, common names and abbreviations for the individual discrete substances in the aryl organophosphate (OP) subgroup are presented in Table 2‑1. Information on the identity of the substances and their components is presented below.
| CAS RN (abbreviation) | DSL name (common name) | Representative chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 115-86-6 (TPHP) | phosphoric acid, triphenyl ester (triphenyl phosphate) | 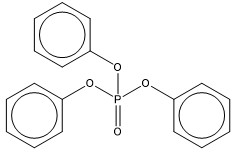 C18H15O4P C18H15O4P
|
326.29 |
| 56803-37-3 (BPDP) | phosphoric acid, (1,1-dimethylethyl)phenyl diphenyl ester (tert-Butylphenyl diphenyl phosphate) | 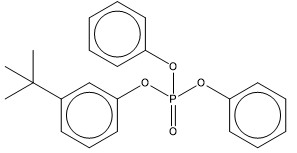 C22H23O4P C22H23O4P
|
382.40 |
| 65652-41-7 (BDMEPPP) | phosphoric acid, bis[(1,1-dimethylethyl)phenyl] phenyl ester (bis(tert-butylphenyl) phenyl phosphate) | 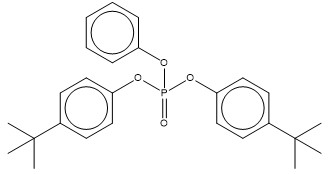 C26H31O4P C26H31O4P
|
438.5 |
| 29761-21-5 (IDDP) | phosphoric acid, isodecyl diphenyl ester (isodecyl diphenyl phosphate) | 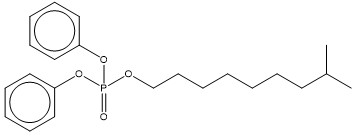 C22H31O4P C22H31O4P
|
390.46 |
Abbreviations: CAS RN, Chemical Abstracts Service Registry Number; DSL, Domestic Substances List
Substances within this subgroup are organophosphate esters with two or three aryl groups (i.e., diaryl or triaryl, respectively), and varying degrees of alkylation (Table 2‑1). Included within this group are four discrete substances and one substance of unknown or variable composition, complex reaction products, or biological materials (UVCB) (IPPP). One of the discrete substances, TPHP, is the base structure for BPDP, BDMEPPP and a component of IPPP. TPHP purity is reported as > 99.6% (OECD 2002b). TPHP is also commonly found in commercial flame retardant mixtures (McGee et al. 2013; Phillips et al. 2017). BPDP and BDMEPPP are variations of tert-butyl-triphenyl phosphates and are typically found together, along with TPHP, in commercial mixtures (Mihajlovic 2015; Phillips et al. 2017). CAS RNs 68937-40-6 and 220352-35-2 may sometimes be used to identify commercial mixtures of BPDP, BDMEPPP, and TPHP (EA 2009b; MSDS 2013). BPDP is an isomeric mixture where a single tert-butyl group may be bound at one of three points on one of the phenyl rings. The remaining discrete substance, IDDP, is an alkyldiaryl phosphate ester, which is generally > 90% pure in commercial IDDP (EA 2009a).
IPPP is a UVCB that is a mixture of potentially over 50 isomers of isopropylated triphenyl phosphates (US EPA 2010). Commercial IPPP formulations contain TPHP in varying quantities from about 5% to 50%, depending on the grade of the product, as well as relative amounts of isopropylated isomers (Appendix A) (EA 2009d; Sjögren et al. 2009). The various IPPP commercial products are manufactured from feedstocks containing different ratios of isopropylated phenols to phenol and contain the same isomers but at different ratios, reflecting the different degrees of isopropylation (EA 2009d). Other CAS RNs have been associated with IPPP, either for the UVCB (e.g., 26967-76-0) or for specific isomers within the UVCB (e.g., CAS RN 72668-27-0, 26967-76-0, and 68937-41-7) (EA 2009d; Sjögren et al. 2009). For example, the IPPP CAS RN 68937-41-7 for this assessment has also been used to represent a tris(isopropylphenyl) phosphate isomer within IPPP (e.g., tris(4-isopropylphenyl) phosphate, tris(4-propan-2-ylphenyl) phosphate) (Sjögren et al. 2009).
For the ecological risk assessment, the approach used for IPPP was to select two representative chemicals that represent the range of water solubility, hydrophobicity and bioaccumulation potential (Table 2‑2). TPHP, which contains no alkylation, is the most water soluble component and was selected to represent fate and toxicity in water. This component represents the highest proportion (up to 50%) of commercial IPPP. A tris(isopropylphenyl) phosphate isomer was selected to represent the largest (steric) IPPP component, with the highest degree of alkylation, hydrophobicity and potential for bioaccumulation. Of the tris(isopropylphenyl) phosphate isomers within IPPP, tris(3-isopropylphenyl) phosphate (T3IPPP) (CAS RN 72668-27-0) has been detected and documented in IPPP mixtures (ECHA c2007-2018d; Phillips et al. 2017) and is selected as the representative IPPP structure for modelling fate (including bioaccumulation) and toxicity in sediment.
The human health risk assessment used a similar approach to that described above when modelling exposures of the general population of Canada to IPPP, in that TPHP was considered representative of the more water soluble components of IPPP, while isomers with the highest degrees of alkylation (such as T3IPPP) were considered representative of the less water soluble components. Limited monitoring data were identified for some isopropylated isomers within IPPP and they were supplemented with modelled data for T3IPPP. Monitoring and modelled data for TPHP were also considered. This is described in more detail in section 2.7.1.4.
| CAS RN | Substance name (abbreviation) | Representative structure name and chemical formula | Proportion in the UVCB | Use in assessment |
|---|---|---|---|---|
| 115-86-6 | Triphenyl-phosphate (TPHP) |  C18H15O4P C18H15O4P
|
5%–50% | Representative structure for more water soluble components of IPPP |
| 72668-27-0 | tris(3-isopropylphenyl) phosphate (T3IPPP) | 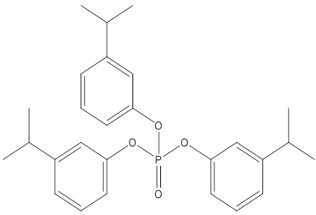 C27H33O4P C27H33O4P
|
< 1%–11%a | Representative structure for least water soluble components of IPPP |
a < 1% for T3IPPP isomer specifically reported in a commercial mixture (Phillips et al. 2017). 11% for tris(isopropylphenyl) phosphate isomers generally (EA 2009d).
Abbreviations: CAS RN: Chemical Abstracts Service Registry Number; DSL: Domestic Substances List
2.1.1 Selection of analogues and use of (Q)SAR models
For the ecological risk assessment, all substances within the aryl OP subgroup were considered analogues for one another due to their structural and functional (e.g., toxicokinetics) similarity. Within the ecological effects and risk analysis sections of this assessment, a read-across approach, using data from the aryl OP substances that had relevant empirical data to represent substances with limited empirical data, was conducted for critical toxicity value (CTV) selection.
In the human health risk assessment, a read-across approach using data from an analogue has been used in the exposure assessment of IPPP. The substance 2-propanol, 1,3-dichloro-, phosphate (3:1), herein referred to as TDCPP, was selected as an analogue for dermal absorption based on structural and functional similarity and data availability, as limited data on dermal absorption were available for the substances in the aryl OP subgroup. Like the components of IPPP, TDCPP is an organophosphate ester. TDCPP has three alkyl groups with two chlorine atoms apiece and is commonly used as an additive flame retardant and a plasticizer. More details of the read-across approach are discussed in section 2.7.1.4. Information on the identity and chemical structure of TDCPP is presented in Table 2‑3.
| CAS RN (abbreviation) | DSL name | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 13674-87-8 (TDCPP) | 2-propanol, 1,3-dichloro-, phosphate (3:1) | 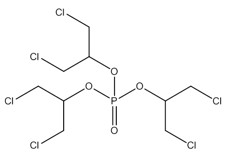 C9H15Cl6O4P C9H15Cl6O4P
|
430.91 |
Abbreviations: CAS RN: Chemical Abstracts Service Registry Number; DSL: Domestic Substances List
2.2 Physical and chemical properties
A summary of physical and chemical property data of the substances in the aryl OP subgroup is presented in Table 2‑4. Experimental data were gathered from published literature, industry reports, as well as international assessments (e.g., EA 2009a; EA 2009b; EA 2009c; EA 2009d; IPCS 1991; OECD 2002b). (Q)SAR models were also used to generate predicted values for the substances. Substance-specific physical-chemical information is provided in ECCC (2020a).
Where more than one appropriate model or valid empirical result was available for a given property, the mean was taken as the key value for that parameter. The three-solubilities approach (Schenker et al. 2005) was used to quantitatively check the final mean values for internal consistency.
| Property | TPHP | BPDP | BDMEPPP | IDDP | IPPP (T3IPPP representative structure) |
|---|---|---|---|---|---|
| Melting point (°C) (selected values) | 50 | 89 | 75 | -50 | -26 |
| Vapour pressure (Pa) (mean of modelled and experimental data) | 1.68 x 10-4 a,b | 1.41 x 10-6 a | 1.07 x 10-6 a | 2.82 x 10-6 a | 1.07 x 10-6 a |
| Henry’s law constant (Pa·m3/mol) (mean of modelled data) | 0.03 c | 1.56 x 10-2 | 0.05501 c | 1.42 x 10-2 c | 2.97 x 10-2 c |
| Water solubility (mg/L) (mean of modelled and experimental data) | 2.25 f | 0.13 e | 8.53 x 10-3 e | 2.42 x 10-1 g | 3.39 x 10-3 f |
| log Kow(dimensionless) (mean of modelled and experimental data) | 4.42 h | 5.68 i | 7.29 i | 6.34 i | 7.55 g |
| log Koc (dimensionless) (mean of modelled data) | 3.59 j | 4.36 j | 5.22 j | 4.60 j | 5.89 j |
| log Koa (dimensionless) (mean of modelled data) | 9.39 k | 10.80 k | 13.49 k | 11.10 k | 13.0 k |
Abbreviations: Kow, octanol–water partition coefficient; Koc, organic carbon–water partition coefficient; Koa, octanol-air partition coefficient.
a MPBPWIN 2010 (Antoine method, modified Grain method, and Mackay method).
b Dobry and Keller 1957, Huckins et al. 1991).
c HENRYWIN 2008, New EQC 2011.
d New EQC 2011, HENRYWIN 2008, Muir 1984.
e Study Submission 2013, KOWWIN 2010, WATERNT 2010, TEST 2012, ACD/Percepta c1997-2012.
f Hollifield 1979, Saeger et al. 1979, Ofstad and Sletten 1985, WSKOWWIN 2010, WATERNT 2010, TEST 2012, ACD/Percepta c1997-2012.
g Saeger et al. 1979, KOWWIN 2010, ACD/Percepta c1997-2012, WATERNT 2010, TEST 2012.
h Study Submission 2013, Saeger et al. 1979, Hansch et al. 1995, Kenmotsu et al. 1980, Sasaki et al. 1981, KOWWIN 2010, ACD/Percepta c1997-2012, ACD/Labs.
i Study Submission 2013, KOWWIN 2010, ACD/Percepta c1997-2012.
j KOCWIN 2010.
k KOAWIN 2010, EPI Suite c2000-2012.
2.3 Sources and uses
The substances within the aryl OP subgroup are produced from synthetic alcohols (Mihajlovic 2015) and do not occur naturally in the environment. Triaryl phosphate flame retardants were originally developed for use in flammable plastics (EA 2009c; Weil 1993).
All of the substances in the aryl OP subgroup have been included in surveys issued pursuant to section 71 of CEPA (Canada 2009a; 2012). A follow-up survey with key industry stakeholders (substance manufacturers and users) was also conducted to further refine quantity and use estimates of the aryl OP substances (ECCC 2016b). Manufacturing of the substances in the aryl OP subgroup was not reported in Canada during any of the years for which data were collected (see Table 2‑5 for specific reporting years by substance) (ECCC 2016b; Environment Canada 2009b, 2013). However 30 companies reported importing a total of 100 000 to 10 000 000 kg of substances in the aryl OP subgroup into Canada in each of 2008, 2011 and 2015 (Table 2‑5). The extent to which the reported values represent quantities present in manufactured goods entering Canada from other parts of the world is unknown, as these uses would be unlikely to meet the reporting criteria for these surveys.
| Substance acronym | Total importsa (kg) | Reporting year | Reference |
|---|---|---|---|
| TPHP | 100 000 to 1 000 000 | 2011, 2015 | Environment Canada 2013, ECCC 2016b |
| BPDP | 100 000 to 1 000 000 | 2008, 2015 | Environment Canada 2009b, ECCC 2016b |
| BDMEPPP | 10 000 to 100 000 | 2011, 2015 | Environment Canada 2013, ECCC 2016b |
| IDDP | 10 000 to 100 000 | 2008, 2015 | Environment Canada 2009b, ECCC 2016b |
| IPPP | 100 000 to 1 000 000 | 2011, 2015 | Environment Canada 2013, ECCC 2016b |
a Values reflect quantities reported in response to the surveys conducted under section 71 of CEPA (Environment Canada 2009b, 2013; ECCC 2016b). See surveys for specific inclusions and exclusions (schedules 2 and 3).
In the United States, estimates of the production (manufacture and import) quantities were greater than 31 500 000 lb/yr (14 300 000 kg/yr) for the total of these five aryl OPs for 2012. The estimates are based on the Toxic Substances Control Act (TSCA) Chemical Data Reporting (CDR) reporting period. The North American market for flame retardants, which is driven by the US market, is expected to grow at an average annual rate of 2.5% to 3% during 2016–2121. The fastest growing North American flame retardant segment is for organophosphorus compounds (IHS 2018).
TPHP and IDDP are manufactured and/or imported in the European Economic Area in the range of 100 000 to 1 000 000 kg/yr per substance, and IPPP is manufactured and/or imported in the European Economic Area in the range of 1 000 000 to 10 000 000 kg/yr (ECHA c2007-2018d). TPHP, IDDP and IPPP are all identified as high production volume (HPV) chemicals by the OECD (ECHA c2007-2018c, c2007-2018d, c2007-2018e).
Organophosphate flame retardant substances (including aryl OP substances) are most commonly used as flame retardants in electronics, lubricants, plastics, rubbers, resins, textiles, elastomers, adhesives and sealants. However, they are also commonly used as plasticizers in many of the same applications (IHS 2018). As plasticizers, aryl OPs are applied to improve the flexibility and durability of certain materials, such as polyvinylchloride (PVC), flexible and rigid polyurethane foams (PUF) and thermoplastic materials (Marklund 2005).
In Canada, the aryl OPs in this assessment are primarily used as either additive flame retardants and/or plasticizers in products available to consumers and commercial products, such as hydraulic fluids, plastics, synthetic rubber, textiles, paints, adhesives, and building materials (ECCC 2016b; Environment Canada 2009b, 2013). Table 2‑6 presents a summary of the major uses of the aryl OP subgroup according to information submitted in response to CEPA section 71 surveys (ECCC 2016b; Environment Canada 2009b, 2013). Additional Canadian uses are presented in Table 2‑7.
Globally, substances in the aryl OP subgroup are used in printed circuit boards, photographic films, resin, furniture, foam seating or bedding, rubber products, lubricants and greases, hydraulic liquids, base fluids for power generation fluids, adhesives, textile coatings, paints and pigment dispersions, inks, and coatings, flooring, toys, construction materials, curtains, foot-wear, leather products, and paper and cardboard products (EA 2009b, EA 2009c; ECHA c2007-2018c, c2007-2018d, c2007-2018e; OECD 2002b).
| Major usesa | TPHP | BPDP | BDMEPPP | IDDP | IPPP |
|---|---|---|---|---|---|
| Adhesives and sealants | Y | N | N | N | Y |
| Paints and coatings | Y | Y | Y | Y | Y |
| Lubricants and greases | Y | Y | Y | Y | Y |
| Plastic and rubber formulation | Y | N | N | N | Y |
Abbreviations: Y = yes this use was reported for this substance; N = no this use was not reported for this substance.
a Non-confidential uses of aryl OPs reported in response to the surveys conducted under section 71 of CEPA (Environment Canada 2009b, 2013; ECCC 2016b). See surveys for specific inclusions and exclusions (schedules 2 and 3).
| Use | TPHP | IDDP | BPDP | IPPP |
|---|---|---|---|---|
| Food packaging materialsa | Y | Y | N | N |
| Present in cosmetics, based on notifications submitted under the Cosmetics Regulationsb | Y | N | N | N |
| Formulant in registered pest control productsc | Y | N | N | Y |
Abbreviations: Y = yes, use was reported for this substance; N = no, use was not reported for this substance.
a Personal communication, e-mail from Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated January 11, 2017; unreferenced.
b Personal communication, e-mail from Consumer and Hazardous Products Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated January 12, 2017; unreferenced.
c Personal communication, e-mail from Pest Management Regulatory Agency, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated December 21, 2017 and February 8, 2018; unreferenced.
In Canada, TPHP and IDDP may be used as a component in the manufacture of certain printing inks that may be applied on the outside layer of laminated plastic structures for food packaging applications (Environment Canada 2013-2014). In the United States, TPHP is approved for use as an additive in adhesives (US CFR 2017a).
In Canada, TPHP and IPPP can be used as formulants in pest control products and are currently registered in a few products, including anti-fouling paints with marine applications for domestic and/or commercial use (personal communication, e-mail from Pest Management Regulatory Agency, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated February 8, 2018; unreferenced).
2.4 Releases to the environment
Anthropogenic releases to the environment depend on various losses occurring during the manufacture, industrial use, consumer or commercial use, service life and disposal of a substance. Releases of substances in the aryl OPs subgroup to the Canadian environment from their use as additive flame retardants and/or plasticizers are expected from point sources (e.g., from industrial processing facilities, wastewater treatment systemsFootnote 4). The aryl OPs may also enter the environment from the use and disposal of consumer products (e.g., leaching of PVC plastics and PUF that contain these substances) (Gad 2014). Releases from products available to consumers and commercial products may occur in both indoor and outdoor environments.
Releases of substances in the aryl OP subgroup to the environment from industrial activity (e.g., plastic and rubber compounding, lubricant blending) are expected to occur primarily to the water and sediment compartment via wastewater. Releases to soil could also occur through the application of biosolids to agricultural and pasture lands. Releases to air from industrial activities are not expected to be significant.
2.5 Environmental fate and behaviour
2.5.1 Environmental distribution
Table 2‑8 presents the range in results of Level III fugacity modelling for the substances in the aryl OP subgroup.
| Substance: | Substances released to: | Air (%) | Water (%) | Soil (%) | Sediment (%) |
|---|---|---|---|---|---|
| TPHP | Air (100%) | 4.7 | 5.2 | 89.5 | < 1 |
| Aryl OPs excluding TPHP | Air (100%) | 1.6–2.6 | 2.4–4.1 | 87.7– 90.5 | 2.8–8.2 |
| TPHP | Water (100%) | < 1 | 88.4 | <1 | 11.5 |
| Aryl OPs excluding TPHP | Water (100%) | < 1 | 22.6–58.9 | <1 | 41.1–77.3 |
| TPHP and other aryl OPss | Soil (100%) | < 1 | < 1 | 99.8–100 | < 1 |
These data indicate that when released to air, these aryl OPs will largely distribute to soil, with a minor percentage (< 5%) remaining in air or distributing to water, with at most 8.2% ending up in sediment. When released to water, most of these aryl OPs will distribute to water or sediment, and when released to soil, the aryl OPs will remain in soil. Of the aryl OP subgroup substances, TPHP distributes most to water, and the other OP substances distribute less to water and more to sediments.
2.5.2 Persistence
2.5.2.1 Abiotic and biotic degradation
Given the likely releases and partitioning characteristics of the aryl OP subgroup substances, environmental persistence is most relevant for the water, sediment and soil compartments. Empirical and modelled data were considered in the weight of evidence for persistence. Several degradation studies were identified for TPHP, as well as for BPDP, IDDP and IPPP (Table 2‑9 and Table 2‑10). Many of the available persistence studies (pre-2009) have been considered in other international assessments (EA 2009a, 2009b, 2009c, 2009d; ECHA c2007-2018c, c2007-2018d, c2007-2018e; OECD 2002b), as summarized in (ECCC 2020b). When available and relevant, information presented in these assessments was considered and is included below.
Modelled predictions for atmospheric oxidation of the aryl OP subgroup substances in air indicate a half-life of less than 1 day (gas phase) (Table 2‑9) (AOPWIN 2010; OECD 2009a). However, Liu et al. (2014) found that TPHP demonstrated persistence when bound to aerosols. They estimated an atmospheric lifetime of 5.6 days for TPHP adsorbed to particulates exposed to OH radicals. Since modelling by AOPWIN (2010) indicates that BPDP, BDMEPPP and IDDP are predominantly associated with particulates in air and since monitoring has shown that TPHP is also associated with atmospheric particulates, it is reasonable to expect that gas phase predictions concerning persistence for aryl OPs likely underestimate persistence in air.
The aryl OP substances in this assessment may undergo hydrolysis under alkaline conditions (e.g., to diphenyl phosphate and phenol), but this process is not expected to have a significant effect on the fate of aryl OPs under typical environmental pH conditions in Canada, i.e., pH 6 to 8 (Table 2‑9) (Anderson et al. 1993; David and Seiber 1999; EA 2009d; HYDROWIN 2010; Muir et al. 1983; Su et al. 2016a).
Generally, empirical and modelled biodegradation data indicate that TPHP, BPDP and IDDP are readily to inherently biodegradable (Jurgens et al. 2014; Muir et al. 1985, 1989; Saeger et al. 1979) and are not persistent in water (Table 2‑10 and Table 2‑11). Experimental studies suggest that alkylated aryl OPs with fewer and shorter chain lengths are more biodegradable than those with longer and more alkyl chains and that biodegradation begins with the initial hydrolysis of the phosphate group (Saeger et al. 1979). Transformation to diphenyl phosphate was reported by Quintana et al. (2006), who also reported its complete removal after 47 days in an aerobic biodegradation study with activated sludge. BPDP is not considered to be persistent in sludge. Complete biodegradation occurred in 11 days in water, and 84% to 93% degradation occurred over 1 day in domestic activated sludge (Heitkamp et al. 1986). Empirical and modelled data are in agreement indicating that IDDP is non-persistent. Empirical half-lives in water and sediment were reported to be 0.44 and 39 days, respectively (Muir et al. 1985). This coincides with a modelled ultimate biodegradation value of 37.5 days.
The available (Q)SAR models predict primary biodegradation on the order of days to months, with ultimate degradation on the order of weeks to years (Table 2‑10, ECCC 2020b). Modelling for BPDP, BDMEPPP and IPPP suggests slower ultimate biodegradation (up to years) (ECCC 2020b). Overall, the modelled and empirical data provide sufficient weight of evidence that the aryl OPs undergo significant primary degradation and, therefore, are not persistent in water. Application of a half-life extrapolation procedure based on Boethling et al. (1995) using a ratio of 1:1:4 for water:soil:sediment suggests that the aryl OP substances will also break down relatively quickly in soil and sediment.
| Substance | Fate process | Test conditions | Degradation endpoint (half-life) unless otherwise noted | Reference |
|---|---|---|---|---|
| TPHP | Biodegradation | Aerobic activated sludge, 28 d, lab | Living sludge: 2.8 d Sterilized sludge: 8 d | Jurgens et al. 2014 |
| TPHP | Biodegradation | Aerobic (CO2) and anaerobic, loamy sand soil, 25 °C, lab, up to 102 d incubation period | 32–37 d | Anderson et al. 1993 |
| TPHP | Biodegradation | Pond and river samples of water and sediment, lab, up to 64 d, aerobic |
Pond: River: |
Muir et al. 1989 |
| TPHP | Biodegradation | Water and sediment, mesocosm, 15 weeks, aerobic | 30 d | Muir et al. 1982 |
| BPDP | Biodegradation | Pond and river samples of water and sediment, lab, up to 64 d, aerobic |
Pond: River: |
Muir et al. 1989 |
| BPDP | Biodegradation | River water, lab, aerobic | Degradation (84%–93%) in 10–21 d | Saeger et al. 1979 |
| BPDP | Biodegradation | Activated sludge, aerobic | Complete mineralization in 11 d | Heitkamp et al. 1986 |
| IDDP | Biodegradation | River water, lab, aerobic | Degradation (20%–54%) in 10–21 d | Saeger et al. 1979 |
| IPPPa (28%–32% TPHP, 70% IPPP) | Biodegradation | Activated sludge inoculum, 28 d, aerobic | 74%–80%b (as CO2 evolution) | IUCLID 2000 |
| IPPPa (28%–32% TPHP, 70% IPPP) | Biodegradation | Aerobic aqueous, 26 d | 94% | IUCLID 2000 |
Abbreviations: NA, Not available; N/A, Not applicable; d, day.
a commercial product containing components of TPHP and IPPP by percentage.
b degradation at two solutions, 10 and 20 mg/L, respectively.
| Medium | Fate process | Degradation endpoint / units | Model | Reference |
|---|---|---|---|---|
| Water | Aerobic ultimate biodegradation |
% BOD = 1–84 Primary half-life: 4.45 d–2 m 17 d Ultimate half-life: 10.44 d–6 yr 5 m 15 d |
CATALOGIC 2014 % BOD (biological oxygen demand) | CATALOGIC 2014 |
| Water | Aerobic primary biodegradation | Value = 3.167–3.9 "biodegrades fast" (days - weeks) |
BIOWIN 4.10 Sub-model 4 |
BIOWIN 2010a |
| Water | Aerobic ultimate biodegradation | 1.96–2.7 "biodegrades fast" (weeks – months) |
BIOWIN 4.10 Sub-model 3 |
BIOWIN 2010b |
| Water | Aerobic ultimate biodegradation | -0.4076–0.0835 "does not biodegrade fast" |
BIOWIN 4.10 Sub-model 5: MITI linear probability |
BIOWIN 2010c |
| Water | Aerobic ultimate biodegradation | 0.0009–0.0302 "does not biodegrade fast" |
BIOWIN 4.10 Sub-model 6: MITI non-linear probability |
BIOWIN 2010d |
a Sub-model 4b: Expert survey (qualitative results).
b Sub-model 3: Expert survey (qualitative results).
c Sub-model 5: MITI linear probability.
d Sub-model 6: MITI non-linear probability.
2.5.2.2 Long-range transport
The OECD POPs Screening Model can be used to help identify chemicals with high persistence and long-range transport potential (Scheringer et al. 2006). The characteristic travel distance (CTD) calculated for aryl OP subgroup substances ranges from 397 to 2441 km, indicating some potential for transport in air to northern regions in Canada, but this is below the boundary (5097 km, CTD of PCB 28) suggested for global pollutants by Klasmeier et al. (2006). The model also calculates an overall persistence (Pov) of 54 to 87 days, and the transfer efficiency (TE), which is the percentage of emission flux to air that is deposited to the surface (water and soil) in a remote region. The TE for these aryl OP substances ranges from 0.19% to 9.19%, with TPHP, BPDP and IDDP values below and BDMEPPP and T3IPPP (representative structure) above the boundary of 2.248% (PCB-28) established based on the model’s reference substances empirically known to be deposited from air to soil or water. The high TE suggests that the higher log Kow aryl OP substances, such as BDMEPPP and IPPP, might be deposited in remote regions.
Of the substances within this subgroup, monitoring and surveillance studies in remote areas have identified environmental concentrations of TPHP only. TPHP (particle phase) has been detected in air in the remote Canadian Arctic (ship-based mean of 84 ± 264 pg/m3; land based mean of 22 ± 26 pg/m3) (Sühring et al. 2016). TPHP has also been measured in air in other international Arctic locations at concentrations up to 60 pg/m3 (Möller et al. 2012; Salamova et al. 2014a). Although TPHP has been measured in remote Arctic locations, it is unknown whether local sources such as hydraulic fluid used on ships/aircrafts, influenced the measured concentrations. TPHP has also been measured in ocean sediment in remote locations ranging from the north Pacific to the central Arctic Ocean (non-detect to 105 pg/g dw) (Ma et al. 2017). As well, TPHP has been measured in Arctic fish, birds and mammals from the Svalbard Archipelago, Norway (Hallanger et al. 2015), and in mammals from Eastern Greenland (Strobel et al. 2018a, b).
Therefore, while TPHP is not expected to be subject to long-range atmospheric transport based on modelling, empirical measurements suggest that TPHP is reaching remote regions. Whether this substance is reaching remote areas due to particle-bound long-range transport (Liu et al. 2014) and/or to local sources is not well understood, but the long-range transport potential of TPHP cannot be discounted.
2.5.3 Potential for bioaccumulation
The log Kow range of 4.42 to 7.55 of the substances in the aryl OP subgroup suggests some potential to bioaccumulate or biomagnify in biota. Empirical and modelled data were considered in the weight of evidence for bioaccumulation. Several bioconcentration studies were identified for TPHP, but few empirical studies are available for BPDP, IDDP and IPPP. Many of the bioconcentration studies (pre-2009) have been reviewed and summarized in other international assessments (EA 2009a, 2009b, 2009c, 2009d; OECD 2002b). When available and relevant, information presented in assessments from other jurisdictions was considered and is included below and in Table 2‑12.
Low to moderate bioconcentration factors (BCFs) are generally reported in these studies (e.g., approximate BCF in the range of a few hundred to a few thousand, Table 2‑11). A few older studies are available for commercial mixtures containing the more hydrophobic aryl OPs (BDMEPPP, IPPP). They suggest that BCFs determined for fish (fathead minnow) are above this range for IPPP. However, these studies have limitations for data interpretation due to the use of a mixture and to treatment-related organism mortality and are therefore not considered further.
Laboratory studies reporting bioaccumulation factors (BAFs) are not available for any of the aryl OP substances. Bengtsson et al. (1986) conducted a long-term dietary accumulation study with TPHP and Eurasian minnows (Phoxinus phoxinus) that demonstrated very low accumulation in fish. Modelled BCFs and BAFs generally support low to moderate bioaccumulation potential for most of the substances in the aryl OP subgroup, with high bioaccumulation potential predicted for BDMEPPP and IPPP (Table 2‑12).
Of the aryl OPs, only TPHP has been considered in studies of trophic transfer and food web dynamics (Brandsma et al. 2015; Eulaers et al. 2014; Greaves and Letcher 2014; Greaves et al. 2016b; Guo et al. 2017; Kim et al. 2011). Although the results are variable, they generally suggest limited biomagnification and/or even trophic dilution for TPHP (Brandsma et al. 2015; Greaves et al. 2016; Hallanger et al. 2015). Studies of trophic transfer and food web dynamics could be important for BDMEPPP and IPPP given their greater hydrophobicity and potential for bioaccumulation, as suggested by BCF/BAF modelling (see Table 2‑12).
Low to moderate concentrations of TPHP in biota (e.g., up to 25 ng/g ww), suggest that metabolism may limit its bioaccumulation (EA 2009d; van der Veen 2012; Wei et al. 2015).
Aryl OP metabolism rates and pathway, while likely species specific, are structurally dependent and are generally slower as a function of alkyl group substitution (Muir et al. 1983; Strobel et al. 2018a, 2018b). Generally, aryl OPs can be metabolized in biota (Greaves et al. 2016a; Sasaki et al. 1984). For example, measured half-lives for TPHP in biota range from 5 hours to greater than 4 days (Hou et al. 2016; Sasaki et al. 1981; Su et al. 2014, 2015a; Wang et al. 2017) (Table 2‑11). However, some aryl OP metabolites (e.g., DPHP) are considered more stable than their parent compounds, and the unknown effects of these stable metabolites represent a data gap (Crump et al. 2012; Su et al. 2014; Strobel et al. 2018a). Empirical BCF data are not available for BDMEPPP.
| Substance | Test organism | Exposure concentration (mg/L) | BCF (L/kg wet weight (ww) whole fish unless otherwise noted) | Reference |
|---|---|---|---|---|
| TPHP | Zebrafish (Danio rerio) | 0.0042 | 46 (muscle) - 224 (gills) | Wang et al. 2017 |
| TPHP | Zebrafish (Danio rerio) | 0.021 | 45 (muscle) - 182 (gills) | Wang et al. 2017 |
| TPHP | Fathead minnow (Pimephales promelas) | 0.001 to 0.021 | 700 to 2468 | Cleveland et al. 1986 |
| TPHP | Killifish (Oryzias latipes) | 0.25 | 84 to 390 | Sasaki et al. 1981, 1982 |
| TPHP | Killifish (Oryzias latipes) | 1.0 | 250 to 500 | Sasaki et al. 1981, 1982 |
| BPDP (Santicizer 154) | Bluegill (Lepomis macrochirus) | 0.0172 (mean) | 1850 | Hamelink and Ea to n 1979 |
| BPDP | Fathead minnow (Pimephales promelas) | 0.017 to 0.315 | 1997 to 4535 | Cleveland et al. 1986 |
| BPDP |
Rainbow trout (Salmo gairdneri) Fathead minnow (Pimephales promelas) |
0.005 and 0.050 |
Rainbow trout: 1096 Fathead minnow: 1010 (calculated by use of to tal radioactivity, static test method) |
Muir et al. 1983 |
| IDDP | Fathead minnow (Pimephales promelas) | 0.017 to 0.315 | 441 to 862 | Cleveland et al. 1986 |
| IPPP (Reofos 35) | Bluegill (Lepomis macrochirus) | 0.0031a | 512 | ECHA c2007 to 2018d |
| IPPP (Reofos 35) | Bluegill (Lepomis macrochirus) | 0.024a | 634 | ECHA c2007 to 2018d |
a mean measured water concentration.
| Substance | Test organism | BCF (L/kg ww) | BAF (L/kg ww) | kM (/day) | Model |
|---|---|---|---|---|---|
| TPHP | Fish | 96.8 a | 97.1 a | 3.60 | BCFBAF 2012 |
| TPHP | Fish | 323.6 b | NA | 0.009 | BCF-baseline, CATALOGIC 2014 |
| BPDP | Fish | 1010a | 1638 a | 0.346 | BCFBAF 2012 |
| BPDP | Fish | 1380.4 b | NA | 0.022 | BCF-baseline, CATALOGIC2014 |
| BDMEPPP c | Fish | 575a | 26131 a | 0.238 | Arnot et al. 2008a, 2008b |
| BDMEPPP | Fish | 47.9b | NA | 0.022 | BCF-baseline, CATALOGIC 2014 |
| IDDP | Fish | 289.2 a | 578.7 | 0.922–0.955 | BCFBAF 2012 |
| IDDP | Fish | 691.8 b | NA | 0.019 | BCF-baseline, CATALOGIC2014 |
| IPPP (T3IPPP) | Fish | 355 a | 22508 a | 0.238 d | Arnot et al. 2008a, 2008b |
| IPPP (T3IPPP) | Fish | 11.22 b | NA | 0.022 | BCF-baseline, CATALOGIC 2014 |
Abbreviations: ww, wet weight; NA, not available; kM, metabolic rate constant
a BCF and BAF reported for mid-trophic level fish and adjusted for biotransformation (metabolism).
b BCF corrected with mitigating factors such as molecular size, metabolism of parent chemical, water solubility, and ionization.
c Read-across from IPPP kM to BDMEPPP, due to lack of bioaccumulation data available for BDMEPPP.
d kM estimated from empirical BCF fish study for IPPP (ECHA c2007–2018d, Table 2‑12).
2.6 Potential to cause ecological harm
2.6.1 Ecological effects assessment
2.6.1.1 Mode/mechanism of action
The aryl OP subgroup substances share similar modes of action (MOA) in that they are considered generally reactive or non-narcotic. Profiling of the aryl OPs in this assessment and their potential metabolites (> 20% occurrence rate) in the OECD Toolbox for structural alerts associated with toxicity and mode of action revealed alert profiles including neurotoxicity, estrogen binding activity, reproductive effects and developmental effects (DNA, RNA and protein interactions).
QSAR MOAs identified for the subgroup include: Verhaar MOA - Class 5, - phosphate esters, esters, neutral organic; OASIS MOA- ‘reactive unspecified’; and TEST MOA – acetylcholinesterase (AchE) inhibition (ASTER 1999; OECD QSAR Toolbox 2016; TEST 2016). OECD alerts include in vivo mutagenicity, although IDDP is also identified with protein/DNA binding.
Shi et al. (2018) reported that TPHP has adverse effects on developing neurons, including induction of developmental neurotoxicity through pathways involved in cytoskeleton regulation, axon growth, neuron maturation, and nervous system differentiation. The authors also reported marked inhibition of total AChE activity in zebrafish larvae, which is considered a biomarker of neurotoxicant exposure. The authors also suggest that TPHP is a neurotoxin because of its structural similarity to neurotoxic organophosphate pesticides, including chlorpyrifos. The similarity in chemical structure may point to possible effects similar to organophosphate pesticides on neurotransmitter-mediated morphogenesis. While summaries of earlier studies suggest conflicting conclusions regarding aryl OP substance neurotoxicity (van der Veen and de Boer 2012), more recent studies identify neurotoxicity concerns (Behl et al. 2015; Jarema et al. 2015; Shi et al. 2018).
Studies have also highlighted endocrine disruption as a concern for OPs including TPHP, BPDP, IDDP and IPPP, with evidence of disruption to reproductive and thyroid systems in fish (Liu et al. 2013; Zhang et al. 2014; Kim et al. 2015).
2.6.1.2 Effects on aquatic organisms
Table 2‑13 and Table 2‑14 summarize the range of effect concentrations determined by experimental aquatic toxicity studies. Many of the acute and chronic toxicity studies (pre-2009) have been reviewed and summarized in other international assessments (EA 2009a, 2009b, 2009c, 2009d; OECD 2002b). When available and relevant, information presented in assessments from other jurisdictions was considered and is included below.
Although experimental values are slightly higher than those predicted by QSAR modelling (ECOSAR 2012, see ECCC 2020c), there is overall agreement between experimental and modelled results.
TPHP, IPPP and BPDP have elicited developmental toxicity on post fertilization in zebrafish embryos (Behl et al. 2015). Developmental toxicity endpoints included curved spine, edema, small head, and small eyes at concentrations of 0.65, 2.2 and 3.7 mg/L.
It should be noted that some toxicity testing reported in this assessment has involved the use of commercial mixtures (e.g., for BPDP, BDMEPPP, and IPPP) (Table 2‑14), and some studies used solvents, which at times resulted in treatment concentrations exceeding the substances’ water solubility limits. However, the studies selected for the aquatic effects assessment were performed using standard testing guidelines and/or showed sufficiently reliable results (ECHA c2007-2018d, c2007-2018e; OECD 2002b). These factors affect the overall comparability of the experimental data, as well as the comparability of the modelled experimental data. Nevertheless, almost all aquatic toxicity data (both experimental and modelled) for all categories of organisms, suggest that all of the aryl OP subgroup substances have high aquatic toxicity (i.e., < 1 mg/L).
Of the aryl OP substances in this assessment, TPHP has undergone the most extensive aquatic toxicity testing (OECD 2002b) and is identified as increasing the toxicity of commercial mixtures, as well as UVCBs (i.e., IPPP) of which it is a component (Cleveland et al. 1986; ECHA c2007-2018d). In recent aquatic studies, TPHP has been associated with effects on survival, growth and reproduction, as well as effects on endocrine activity, metabolism, genotoxicity, neurotoxicity, and cardiotoxicity (Behl et al. 2015; Du et al. 2015; Du et al. 2016; Liu et al. 2013; McGee et al. 2013; Sun et al. 2016; Yuan et al. 2018; Zhang et al. 2014). McGee et al. (2013) reported that TPHP and an isomer of IPPP (mono-isopropylated triaryl phosphate) resulted in effects on cardiac looping and function during zebrafish (Danio rerio) embryogenesis, although through different mechanisms. The IPPP isomer induced cardiotoxicity via an aryl hydrocarbon receptor (AHR)-dependent pathway, while the TPHP cardiotoxicity induced by TPHP was AHR-independent. Du et al. (2015) also identified specific cardiac developmental defects in zebrafish caused by TPHP.
Studies suggest that some of the aryl OP substances in this assessment result in disruptions to reproductive and thyroid systems. For example, in a 21-day study in zebrafish, Liu et al. (2013) found TPHP effects (0.04 to 1.0 mg/L) on the reproductive system relating to steroid hormone and vitellogenin (VTG) perturbations and reduced fecundity. VTG is essential for oocyte maturation and successful reproduction of female fish and is synthesized in the liver of female fish in response to estrogens (Liu et al. 2009). Zhang et al. (2014) reported evidence that TPHP behaves as an estrogenic receptor α agonist (ERα). Kim et al. (2015) observed that thyroid hormone concentrations increased and the levels of several genes responsible for thyroid hormone synthesis were upregulated in zebrafish larvae, supporting the thyroid hormone-disrupting potential of TPHP. Also, expressional changes of various genes in thyroid cells (GH3 and FRTL-5 cells) indicate that TPHP can stimulate thyroid hormone synthesis in vitro. A chronic study on the body length, fecundity and survival of less than 12-h old Daphnia magna exposed to 0, 5, 50 or 500 μg/L TPHP for 21 days resulted in significantly decreased body lengths of both F0 and F1 generations and inhibited the fecundity of F0 generation at the 500 μg/L nominal concentration (Yuan et al. 2018).
Developmental and neurobehavioural effects of IDDP, BPDP and IPPP on zebrafish embryos from 0 to 5 days post-fertilization through to 7 months were reported by Glazer et al. (2018). The authors reported that the IPPP and BPDP solutions contained high percentages of TPHP, namely 54.8% and 35.5%, respectively. Treatment with 0.13 mg/L IPPP resulted in spinal curvature in over 50% of individuals by 6 days post-fertilization (dpf). Exposure to 1.3 mg/L IPPP resulted in pericardial edema in all individuals at 4–5 dpf. Spinal curvature was observed in over 50% of individuals exposed to 1.14 mg/L BPDP at 4 to 5 dpf. IDDP exposure did not cause elevation in mortality or deformities at any concentration up to 3.96 mg/L. At 5 to 7 months of age, the fish were tested on a battery of behavioural tests to assess sensorimotor response, social interaction and predator evasion. At 0.045 and 0.0045 mg/L, IPPP baseline activity (behavioural test) was significantly reduced in the 0.0045 mg/L treatment. Exposure to BPDP resulted in significant treatment effects characterized by the reduction in activity at concentrations of 0.1 and 0.01 mg/L. Larval and adult behaviours were not found to be affected by exposure to IDDP at any treatment concentrations.
Jarema et al. (2015) and Behl et al. (2015) evaluated the neurobehavioural effects of acute and chronic exposure of zebrafish larvae to OP substances, including TPHP, BPDP, IDDP, and IPPP. TPHP and IPPP are reported to cause behavioural changes at 6 dpf (TPHP lowest observed effect level (LOEL) = 0.13 mg/L, IPPP LOEL = 0.54 mg/L). All four aryl OPs caused behavioural changes at 6 dpf after acute exposure (LOELs ranged from 0.22 to 4.68 mg/L). Noyes et al. (2015) also demonstrated similar zebrafish neurological and morphological sensitivities to aryl OPs (TPHP, BPDP, IDDP, and IPPP isomers). Exposure to TPHP at 1 and 5 dpf resulted in reduced survival with high concentration edemas and caused hypoactive locomotor responses. Sun et al. (2016) evaluated the developmental neurotoxicity of TPHP in the early life stages of Japanese medaka (Oryzias latipes) larvae (Sun et al. 2016). The total AChE activity of larval medaka from the TPHP‐exposed groups was significantly inhibited at 0.12 mg/L and 0.62 mg/L. The results provide evidence of potential developmental effects on the vertebrate nervous system.
With the intent of estimating aquatic effects in this assessment, TPHP was used as a read-across to the aryl OP subgroup given the availability of toxicity studies for this substance and given that TPHP has the highest likelihood of partitioning to water. The 30-d EC10 (growth) of 0.037 mg/L (Sitthichaikasem 1978) for TPHP was selected as the critical toxicity value (CTV) as it represents the lowest effect value based on reliable experimental aquatic toxicity data for the substances in the aryl OP subgroup.
The uncertainty associated with the read-across of TPHP to the other four aryl OP substances for aquatic toxicity was qualitatively considered based on an analysis and comparison of parent compounds and stable metabolites (estimated by CATALOGIC with greater than 20% probability of occurrence). The analysis considered similarity in chemical structures, physical and chemical properties, toxicodynamics and toxicokinetics of TPHP and metabolites in comparison with parent and metabolites for the other four aryl OP substances (using ACD Percepta, EPI Suite and the OECD Toolbox). The comparison of physical and chemical properties revealed sizable differences in orders of magnitude for parameters including log Kow and Henry’s law constant. In addition, differences were also evident for potential neurotoxicity and behavioural effects, reproductive and developmental effects expressed in toxicodynamic parameters, such as DNA binding, protein binding, and toxicity under repeated dose conditions (Hazard Evaluation Support System database (HESS 2020), as evident between metabolites of TPHP and at least one or more metabolites of the other four aryl OPs. Thus, not only are there potential differences in potential toxicokinetics among metabolites, but the current endpoint used as the CTV may not be sensitive enough to cover effects including neurotoxicity, thyroid and developmental effects. As a result, the approach described above was used to derive the assessment factor (AF) to account for the differences between the potential metabolites of TPHP and the other four aryl OPs.
To derive the predicted no effect concentration (PNEC) for aquatic organisms, an overall assessment factor of 100 was applied. The assessment factor of 100 is determined with consideration given to species sensitivity and mode of action for aquatic organisms. Specifically, a factor of 1 was applied to the CTV to account for inter- and intra-species variation as a reasonably large dataset was available (seven species from three categories, i.e., vertebrates, invertebrates and algae) and a factor of 1 to account for mortality to sublethal extrapolation. Also, as noted above, an analysis of the substances in the aryl OP subgroup suggests that the aquatic toxicity may be elicited by both the parent compounds and their respective metabolites. The predicted metabolites include both narcotic and reactive and/or specifically acting substances (i.e., AChE inhibitors) indicating high potential variation in toxicity outcomes among species and substances. The main endpoints of interest are neurotoxicity and behavioural/reproductive/developmental effects resulting from DNA/RNA/protein interactions (e.g., genotoxicity). In order to account for the reactivity of the metabolites, an assessment factor of 100 was thus selected to rationalise the differences in the mode of action of the metabolites in the aryl OP subgroup that may elicit effects (including neurotoxicity and AChE inhibition, which can be an irreversible effect) at lower concentrations than TPHP and other parent substances. Therefore, the PNEC for the aryl OP subgroup is calculated using the three application factors, 1 x 1 x 100, as 0.037 mg/L = 0.00037 mg/L.
| Common name | Test organism | Endpoint | Range / value (mg/L) | Reference |
|---|---|---|---|---|
| TPHP |
Rainbow trout (Oncorhynchus mykiss) Killifish (Oryzias latipes) Goldfish (Carassius auratus) |
96-h LC50 | 0.4–1.2 | ECHA c2007-2018c; Mayer et al. 1981; Sasaki et al. 1981 |
| TPHP | Rainbow trout (Oncorhynchus mykiss) | 30-d EC10 | 0.037 | Sitthichaikasem 1978 |
| TPHP | Rainbow trout (Oncorhynchus mykiss) | 30-d LOEC | 0.055 | Sitthichaikasem 1978 |
| TPHP | Mysid shrimp (Americamysis bahia) | 96-h LC50 | ≥ 0.18 to ≤ 0.32 | ECHA c2007-2018c |
| TPHP | Daphnids (Daphnia magna) | 96-h EC50 | 1.0 | Mayer et al. 1981 |
| TPHP | Daphnids (Daphnia magna) | 21-d NOEC | 0.254–0.831 | ECHA c2007-2018c |
| TPHP | Daphnids (Daphnia magna) | 21-d LOEC | 0.254–0.831 | ECHA c2007-2018c |
| TPHP |
Algae (Pseudokirch-neriella subcapitata) |
72-h LOEC | 0.5–5 | Millington et al. 1988 |
| TPHP | Algae (Desmodesmus subspicatus) | 72-h LOEC | 0.5–5 | Millington et al. 1988 |
| TPHP | Algae (Desmodesmus subspicatus) | 72-h NOEC | 0.25–2.5 | Millington et al. 1988 |
| TPHP (15%–20%)a, BPDP (35%–40%)b, BDMEPPP (23%–28%)b | Fathead minnow (Pimephales promelas) | 96-h LC50 | 0.65–2.3 | Adams et al. 1983; Cleveland et al. 1986 |
| TPHP (15%–20%)a, BPDP (35%–40%)b, BDMEPPP (23%–28%)b | Fathead minnow (Pimephales promelas) | 60–90-d MATC | > 0.14 to < 0.83 | Adams et al. 1983; Cleveland et al. 1986 |
| TPHP (27%–32%)b, BPDP (35%–40%)b, BDMEPPP (23%–28%)b | Daphnids (Daphnia magna) | 48-h EC50 | 0.202–2.9 | Adams et al. 1983; Sanders et al. 1985 |
| TPHP (27%–32%)b, BPDP (35%–40%)b, BDMEPPP (23%–28%)b | Daphnids (Daphnia magna) | 21-d MATC | 0.010 to < 0.226 | Adams et al. 1983; Sanders et al. 1985 |
| IDDP (91%)a, TPHP (6%)a | Rainbow trout (Oncorhynchus mykiss) | 96-h EC50 | > 0.15 | ECHA c2007-2018e |
| IDDP (91%)a, TPHP (6%)a | Fathead minnow (Pimephales promelas Rafinesque) | 90-d MATC | > 0.19 to < 0.38 | Cleveland et al. 1986 |
| IDDP (91%)a, TPHP (6%)a | Daphnids (Daphnia magna) | 48-h EC50 | 0.042–0.48 | ECHA c2007-2018e; Adams and Heindolph 1985 |
| IDDP (91%)a, TPHP (6%)a | Daphnids (Daphnia magna) | 21-d NOEC | 0.0038 to > 0.0062 | ECHA c2007-2018e |
| IDDP (91%)a, TPHP (6%)a | Algae (Pseudokirch-neriella subcapitata) | 72-h–96-h EC50 | > 0.1 | ECHA c2007-2018e; EG and G Bionomics 1979a |
| IPPP (TPHP > 5%) | Fathead minnow (Pimephales promelas Rafinesque) | 30-d EC10 | 0.0031 | ECHA c2007-2018d |
| IPPP (TPHP > 5%) | Fathead minnow (Pimephales promelas Rafinesque) | 30-d LOEC | 0.0082 | ECHA c2007-2018d |
| IPPP (TPHP > 5%) | Fathead minnow (Pimephales promelas Rafinesque) | 30-d MATC | 0.005 | ECHA c2007-2018d |
| IPPP | Midge (Chironomus plumosus) | 48-h LC50 | 1.5–2.2 | ECHA c2007-2018d; Sanders et al. 1985 |
| IPPP | Daphnids (Daphnia magna) | 21-d NOEC | 0.019–0.0415 | ECHA c2007-2018d; Sanders et al. 1985 |
| IPPP | Daphnids (Daphnia magna) | 21-d LOEC | 0.106 | ECHA c2007-2018d; Sanders et al. 1985 |
| IPPP | Daphnids (Daphnia magna) | 21-d MATC | 0.0663 | ECHA c2007-2018d; Sanders et al. 1985 |
| IPPP | Algae (Selenastrum capricornutum Printz) | 72-h EC50 | > 2.5 | ECHA c2007-2018d |
| IPPP | Algae (Selenastrum capricornutum Printz) | 72-h NOEC | 0.31 | ECHA c2007-2018d |
Abbreviations: LC: lethal concentration, EC: effective concentration, MATC: maximum allowable toxicity concentration. LOEC: lowest-observed effect concentration, NOEC: no observed effect concentration, h: hours, d: days.
a Composition reported in study.
b Composition reported in publicly available MSDS 2012.
| Common name | Test organism | Endpoint | Value (mg/kg) | Reference |
|---|---|---|---|---|
| IPPP (TPHP < 5%)a | Midge (Chironomus riparius) | 28-d EC50 emergence | 87 | ECHA 2007-2018 |
| IPPP (TPHP < 5%)a | Midge (Chironomus riparius) | 28-d EC10/LOEC | 37 | ECHA 2007-2018 |
| IPPP (TPHP < 5%)a | Midge (Chironomus riparius) | 28-d NOEC | < 37 | ECHA 2007-2018 |
Abbreviations: EC: effective concentration, LOEC: lowest-observed effect concentration, NOEC: no observed effect concentration
a Composition reported in study.
For the substances within the aryl OP subgroup, only one sediment toxicity study on IPPP was identified that could be a CTV (Table 2-14). A chironomid toxicity test using spiked sediment (OECD Test Guideline No. 218) measured the effects of commercial IPPP on midges (Chironomus riparius) exposed for 28 days to sediment concentrations ranging from 37 to 688 mg/kg and examined the total number of adults that emerged and the development rate (ECHA c2007-2018d). The EC50 (emergence) was determined to be 87 mg/kg dw. Based on the effects observed on development rate, the LOEC (and EC10) for the study was 37 mg/kg and the NOEC was less than 37 mg/kg. A second study by Huckins et al. (1991), which examined TPHP toxicity in sediment and overlying water, was available, but effect levels were not determined for sediment exposure, and thus that study was not considered further.
While the overall approach within the assessment is to use representative structures (TPHP or T3IPPP) for IPPP (rather than testing on the whole UVCB), given the lack of sediment toxicity data for this subgroup and from potential analogues, the experimental UVCB study (i.e., ECHA c2007-2018d) was selected for the CTV. Further, given the expected homogeneity of the IPPP UVCB (components are all aryl OP esters ranging from TPHP to various isomers of isopropylated TPHP (Appendix A)), as well as close similarity of these UVCB components to substances within the aryl OP subgroup, it was considered that results from toxicity testing would have relevance to the subgroup as a whole. It is expected that the hydrophobic components of IPPP (e.g., isopropylated components such as T3IPPP) should partition to sediments more so than the TPHP component.
Using IPPP to represent sediment toxicity for the aryl OP subgroup, an assessment factor of 100 was applied to the chronic CTV of 37 mg/kg (28 d EC10). This represents an assessment factor of 50 to account for inter- and intra-species variation as there is only one species (Chironomus riparius), from one category (invertebrates) in the toxicity dataset. Additionally, an assessment factor of 2 was applied to account for the reactive mode of action of the aryl OP. This calculation resulted in a PNEC value of 0.37 mg/kg based on application factors of 50 x 2 x 1.
2.6.1.3 Effects on soil-dwelling organisms
Soil toxicity studies were identified for commercial mixtures containing combinations of substances in the aryl OP subgroup (Table 2‑15), as well as studies for IPPP (the UVCB), TPHP and BPDP. While preference is for single substance testing, given the lack of soil toxicity data for individual substances in this subgroup and from potential analogues, and the fact that the commercial mixtures consist of approximately 99% substances within the aryl OP subgroup, these studies were considered for the assessment and relevant to the subgroup as a whole.
| Mixture composition | Test organism | Endpoint | Value (mg/kg) | Reference |
|---|---|---|---|---|
| TPHP(40.6%), BPDP (47.5%), BDMEPPP (10.9%)a | Plants (tomato (Lycopersicon esculentum), turnip (Brassica rapa), ryegrass (Lolium perenne), onion (Allium cepa), cucumber (Cucumis sativus) and lettuce (Lactuca sativa)) |
21-d EC50 growth/emergence |
182–787 | Study Submission 2015 |
| TPHP(40.6%), BPDP (47.5%), BDMEPPP (10.9%)a | Plants (tomato (Lycopersicon esculentum), turnip (Brassica rapa), ryegrass (Lolium perenne), onion (Allium cepa), cucumber (Cucumis sativus) and lettuce (Lactuca sativa)) |
21-d NOEC growth/emergence |
12.3–111 | Study Submission 2015 |
| TPHP (45.7%), BPDP (40%–46%), BDMEPPP (12%–18%)a | Earthworm (Eisenia fetida) |
28-d NOEC mortality/weight 56-d NOEC reproduction |
> 100 | Study Submission 2014 |
| IPPP (TPHP < 5%)a |
Wheat(Triticum aestivum) Radish (Raphanus sativus) Mung bean (Phaseolus aureus) |
19-d LC50 | > 100 | ECHA c2007-2018d |
| IPPP (TPHP < 5%)a | Earthworm (Eisenia fetida) |
28-d EC50 reproduction |
> 1000 | ECHA c2007-2018d |
| IPPP (TPHP < 5%)a | Earthworm (Eisenia fetida) |
28-d LOEC reproduction |
500 | ECHA c2007-2018d |
| IPPP (TPHP < 5%)a | Earthworm (Eisenia fetida) |
28-d NOEC reproduction |
250 | ECHA c2007-2018d |
Abbreviations: LC: lethal concentration, EC: effective concentration, LOEC: lowest-observed effect concentration, NOEC: no observed effect concentration
a Composition from reference cited.
The available studies include 19- to 21-day plant studies examining survival and growth (height, weight, emergence) and 28-day earthworm studies of aryl OP effects on survival and reproduction.
Study Submission (2015) examined effects of a commercial mixture with TPHP (40.6%), BPDP (47.5%), BDMEPPP (10.9%) on seedling emergence and growth of two monocot and four dicot species of terrestrial plants: tomato (Lycopersicon esculentum), turnip (Brassica rapa), ryegrass (Lolium perenne), onion (Allium cepa), cucumber (Cucumis sativus) and lettuce (Lactuca sativa). Planting seeds into soils with test substance concentrations ranging from 4.1 to 1000 mg/kg dry soil resulted in adverse effects on the seedling emergence and/or early growth of all six species tested at reported concentrations. The NOEC values ranged from 12.3 to 111 mg/kg, the LOEC values from 37 to 1000 mg/kg, and the EC50 values from 181.7 to 786.9 mg/kg.
To calculate an aryl OP subgroup PNEC for soil organisms, the lowest measured effect level (21‑d EC50 of 182 mg/kg for reduced tomato growth) was selected as the CTV. An assessment factor of 20 was used to calculate the PNEC for the aryl OP subgroup in soil. This assessment factor includes a factor of 5 to extrapolate from a median effect level (EC50) to a low- or no-effect and an assessment factor of 2 to account for inter- and intra-species variation as only two categories of species (primary producers and invertebrates) were represented in the toxicity dataset (including at least seven species). A factor of 2 was also applied to account for the reactive mode of action of the aryl OPs. With an overall assessment factor of 20 (i.e., 5 x 2 x 2), this calculation resulted in a PNEC of 9.09 mg/kg dw.
2.6.1.4 Effects on wildlife
Toxicity to mammals was examined using a wildlife toxicity reference value (TRV) approach (Sample et al. 1996), where effects in rats were normalized to a typical body weight of mink (Mustela vison) and river otter (Lontra canadensis) (Appendix C). TRVs represent predicted no effect intake rates in the mammalian receptor. For the purpose of this assessment, the mink and the river otter are considered to be representative of Canadian mammalian wildlife. For the assessment of wildlife, the substance with a high bioaccumulation potential which also had relevant mammalian toxicity data, IPPP (T3IPPP representative structure), was selected for the estimation of the TRV because it is considered to be representative of an aryl OP subgroup substance with high potential for exposure to wildlife.
Due to lack of wildlife toxicity data for the substances in the aryl OP subgroup, rodent data considered in the human health component of the assessment were also considered in the ecological assessment. For IPPP, in a 91-day gavage study in which rats were administered a commercial mixture containing 65% IPPP and 35% TPHP (Reofos 35) (EA 2009b), the LOAEL was considered to be 25 mg/kg/day based on adverse histopathological changes observed in the adrenal glands at all dose levels (see section 2.7.3.4). In an oral reproductive study in which rats were exposed to another commercial mixture of IPPP, the no observed adverse effect level (NOAEL) for reduced male and female reproductive performance was 25 mg/kg bw/day. The NOAEL for F1 neonatal toxicity was considered to be 25 mg/kg/day (ECHA c2007-2018d). A LOAEL of 25 mg/kg bw/day was considered based on adverse histopathological changes observed in the adrenal glands, and the NOAEL for reduced male and female reproductive performance was 25 mg/kg bw/day (ECHA c2007-2018d).
An assessment factor of 10 was applied to the wildlife CTV in order to account for inter- and intra-species variation. Wildlife CTV estimates for IPPP of 14.05 mg/kg bw/day for mink and 8.36 mg/kg bw/day for otter were determined from the LOAEL toxicity study in rats presented above. The resulting TRVs were 1.4 and 0.84 mg/kg bw day for mink and river otter, respectively (Table 2‑16).
| Common name | CTV (mg/kg bw/day) | TRV (mg/kg bw/day) |
|---|---|---|
| Mink (Mustela vison) | 14.05 | 1.4 mg/kg bw/d |
| River otter (Lontra canadensis) | 8.36 | 0.84 mg/kg bw/d |
2.6.2 Ecological exposure assessment
While the exposure analysis considers measured concentrations of the substances in the aryl OP subgroup in the Canadian environment, these are insufficient to assess exposure for the risk analysis. Therefore, the exposure analysis also estimates aryl OP substance releases to water from industrial facilities, focusing on the major reported uses: formulation of plastics and rubber products, formulation of lubricants and lubricant additives, and formulation of paints and coatings. These predicted environmental concentrations are estimated using available Canadian information, including substance quantities, estimated emission factors, and characteristics of the receiving environment. Specific industrial exposure scenarios are described below.
As the leaching of aryl OPs from various commercial and consumer products (e.g., plastics, rubber, other manufactured items) could occur, the assessment also examines pathways and predicted environmental concentrations in water that could result from commercial products and products available to consumers.
2.6.2.1 Measured concentrations in environmental media and wastewater
Canadian monitoring data exist for TPHP in most environmental media: air, water, marine sediment, wastewater, and biota (Table 2‑17). Recent environmental monitoring data for the other aryl OPs subject to this assessment are lacking for both Canada and other jurisdictions. Environmental concentrations have been estimated from the available Canadian information, including estimated substance quantities, estimated release rates, and characteristics of the receiving environment. Environmental concentrations have been estimated for industrial release scenarios, as described in section 2.6.2.2.
| Media (units) | Location | Concentration maximum, mean, median or range | Reference(s) |
|---|---|---|---|
| Air (pg/m3) | Toronto |
< 87–2220 827 (mean) 700 (median) |
Shoeib et al. 2014 |
| Air (pg/m3) | Arctic |
22 – 84 (average) ND–1930 (median) |
Sühring et al. 2016 |
| Air (pg/m3) | Toronto | 1063 (mean) | Abdollahi et al. 2017 |
| Air (pg/m3) | Quebec City | ND–512 (median) | Sühring et al. 2016 |
| Rain (mg/L) | Toronto | ND –3.4 x 10-8 | Truong 2016 |
| Surface water (mg/L) | Lakes Huron, Erie and Michigan, Don River, Etobicoke Creek, Highland Creek |
1.3 x 10-7 – 2.39 x 10-4 (max high flow, urban stream) < LOD–2.1 x 10-5 |
Venier et al. 2014, Truong 2016 |
| Sediment (marine surface sediment)(mg/kg dw) | Canada Basin margin, Arctic Ocean |
ND – 7.4 x 10-5 (mean =1.1 x 10-5) |
Ma et al. 2017 |
| Fish (ng/g ww) | Great Bear Lake, Great Lakes (Canada and US) | 0.10–25 | McGoldrick et al. 2014, Guo et al. 2017 |
| Bird tissue (bird eggs) (ng/g ww) | Lake Huron |
ND–4.18 (ND–0.81) |
Chen et al. 2012, Greaves et al. 2014, Su et al. 2014 |
|
Wastewater (median values): influent (mg/L) effluent (mg/L) biosolids (mg/kg dw) |
8 WWTS |
Influent: 8.1 x 10-5 – 7.5x10-4 median: 1.8 x 10-4 Effluent: 1.1 x 10-5 – 7.2 x 10-4 median: 9.1 x 10-5 Biosolids: 0.012–0.83 |
ECCC 2016d, Truong 2016 |
Abbreviations: LOD, limit of detection; ND, not detected; WWTS, wastewater treatment system
2.6.2.2 Calculation of PECs and general assumptions
To inform the risk characterization of the aryl OP subgroup substances, predicted environmental concentrations (PECs) in relevant media were calculated. Releases to surface water may result from industrial discharges to sewers, followed by treatment at a wastewater treatment facility. Application of biosolids from WWTSs to agricultural land results in soil exposure, and equilibrium processes occur in the water column resulting in partitioning and exposure to sediment located near discharge points. Additionally, wildlife PECs were derived for two piscivorous species (mink (Mustela vison) and river otter (Lontra canadensis)) from a total daily intake calculation (TDI) using estimated levels of the aryl OP substance IPPP in fish.
Based on the major uses of aryl OPs, the following exposure scenarios resulting from industrial formulation activities are considered in this assessment: formulation of lubricant additives, lubricants, plastic products, rubber products and paints and coatings. An exposure scenario was not developed for the use of industrial, commercial or consumer lubricants and greases as it was determined that there would likely be limited environmental exposure from this use. It is anticipated that products are contained during use and that programs are in place to collect these products at end of life for recycling or disposal at waste facilities. Potential releases via container cleaning and transport, including loading and unloading, are not considered in this assessment. As leaching of aryl OPs from various commercial and consumer products (e.g., plastics, rubber, other manufactured items) could occur, the assessment also examines PECs in surface water that could result from leaching of these products. Exposure resulting from releases from commercial and consumer products was evaluated using measured concentration data from surface water. PECs for each exposure scenario in surface water, sediment and soil are presented in Table 2‑19.
Scenarios were developed based on available information using a combination of representative site and generic considerations. The substance quantity used annually at a facility is based on the average of the total quantity of aryl OPs reported by companies. For a few scenarios, a user reporting the highest quantity of aryl OP by an individual facility was also considered (ECCC 2016b; Environment Canada 2009b, 2013). Daily dilution volumesFootnote 5 are either specific to the representative site or, when locations of facilities are unknown, based on a distribution of daily dilution volumes associated with facilities relevant to the industrial sector. Emission factors to wastewater and number of release days at a facility are based on assumptions from generic sources of information. The approach assumes that industrial effluents from indirect dischargers are typically sent to a secondary WWTS, the most common treatment type in Canada. All parameters used for PEC derivations are provided in ECCC (2020d).
For all PEC calculations, the total substance quantity used annually at a site was assumed to be the sum of all five individual substance quantities reported by a company (Environment Canada 2009, 2013). This assumption was made as it is expected that, given their similar uses and properties, the five aryl OP substances could be used in combination or interchangeably. In fact, many commercial formulations contain more than one of the aryl OP substances considered in this assessment and so are used concurrently. An average representative secondary WWTS removal efficiency of 86% is used for the derivation of the aquatic, sediment and soil PECs. That figure is based on measured removal rates for TPHP (ECCC 2016c) and a modelled removal rate for IPPP (SimpleTreat 2003).
A sediment–water equilibrium partition approach was used to estimate the PECs of aryl OPs in sediment. This approach assumes that suspended sediment in the water column is in chemical equilibrium with the aqueous phase (ECHA 2016). The approach also assumes that bottom sediment is in equilibrium with suspended sediment according to US EPA (2003). On the basis of these two assumptions, the bottom sediment, suspended sediment and aqueous phase of a water body are all in equilibrium with one another. Typical characteristics of suspended and bottom sediments, as suggested by Gobas (2007 and 2010), were used in the estimation. The PECs were standardized to 3% organic carbon (OC), which is typical for Canadian river and lake bottom sediments.
Estimation of soil exposure was based on an approach described by the European Chemicals Agency (ECHA 2016) involving land application of biosolids from WWTSs. This approach estimates the concentration of the accumulated substance (soil PEC) within the top 20 cm soil layer after 10 years of biosolids application. However, given that TPHP may degrade quickly in soil, the approach was modified to consider degradation in soil. The soil biodegradation half-life for TPHP is 37 days (Andersen et al. 1993). Concentrations were determined on a yearly basis immediately after application and at the end of the year (365 days) prior to the subsequent application, over a 10-year period. Given the half-life, the concentration of TPHP in soil does not accumulate much over the 10-year period, and soil concentrations are maximal immediately after application (decreasing significantly over the year). Quantity and removal rate assumptions used in the PEC soil calculations are the same as those used for the aquatic PEC.
Estimates of exposure of piscivorous wildlife to the aryl OP subgroup substances are based on the wildlife exposure model (US EPA 1993) (ECCC 2020c). This model involves estimating a total daily intake (TDI) for mink (Mustela vison) and river otter (Lontra canadensis) (ECCC 2020c), which were chosen as representative local wildlife species in a riverine environment in Canada. This scenario is intended to assess the exposure of mink and river otter living near a discharge area. In calculating the TDI, a water concentration (Cw) of 2.7 x 10-3 mg/L was selected, based on the most conservative water PEC of all exposure scenarios (Table 2‑18). The TDI was then estimated using parameters for IPPP (T3IPPP representative chemical) given that, based on the bioaccumulation assessment. this is the substance that is the most likely to accumulate in organisms and to transfer through the food chain. This results in TDIs of 1.65 mg/kg bw/day and 1.78 mg/kg bw/day for mink and river otter, respectively.
Exposure scenarios were developed for the manufacturing and industrial use of the Flame Retardants Group substances.
Formulation of lubricant additives
According to information submitted in response to CEPA section 71 surveys, (Environment Canada 2009, 2013; ECCC 2016b), the aryl OP substances subject to this assessment are imported as a mixture or as pure substances and blended with other substances to prepare lubricant additive packages which are sold to industrial lubricant formulators. Two PECs are derived in this scenario: one depicting a representative high quantity industrial user and the other a representative generic industrial user. For the latter, as different companies are considered as possible formulators of additives, a distribution of daily dilution volumes associated with secondary and tertiary WWTS receiving effluent from the chemical manufacturers sector was used. A representative composition of 5% aryl OPs in lubricant additive packages was selected based on available safety data sheets.
Formulation of lubricants
According to information submitted in response to CEPA section 71 surveys, (Environment Canada 2009, 2013; ECCC 2016b), the aryl OP substances are imported for use in lubricant products. As different companies are considered possible formulators of lubricants, a representative low end value of the daily dilution volumes for WWTS receiving effluent from the lubricant blending facilities was used. Based on available safety data sheets for lubricants containing substances in this subgrouping ranging from 0.1% to 1%, a representative concentration was selected for the calculations to represent the amount of aryl OPs in the final lubricant products. The main source of release during formulation activities is from overspill during packaging of the final additive package (OECD 2004a). An on-site representative removal rate of 50% was applied. This removal rate accounts for the common use of oil/water separators at lubricant blending facilities (OECD 2011).
Formulation of plastic products
According to information submitted in response to CEPA section 71 surveys, (Environment Canada 2009, 2013; ECCC 2016b), aryl OPs are imported in plastic additive packages by plastic manufacturers and blended with a polymer resin to produce various plastic products, such as PUF and PVC plastics. These substances function as plasticizers and flame retardants in plastic products. Based on available information, formulation of plastic additive packages is not a major use of aryl OPs in Canada (Environment Canada 2009b, 2013; ECCC 2016b). This scenario covers the compounding and conversion of plastics. As all locations of companies potentially using these additive packages are unknown, a distribution of daily dilution volumes associated with secondary and tertiary WWTS receiving effluent from the plastic manufacturing sector was used. Plasticizers and flame retardant additives may be found at concentrations of 1% to 50% and 5% to 40%, respectively, in various types of plastic products (OECD 2009b). A representative value of 5% was selected for the calculations.
Formulation of rubber products
This scenario represents the formulation of rubber compounds containing aryl OP additives. According to information submitted in response to CEPA section 71 surveys, aryl OPs are imported as flame retardants and plasticizers by rubber compounding facilities (Environment Canada 2009b, 2013; ECCC 2016b). Based on the information available, this activity is not expected to be widespread, and PECs were therefore calculated for a representative site. Plasticizers and flame retardants may be found at concentrations of 1% to 10% in various types of rubber products (OECD 2004b). A representative value of 5% was selected for the calculations.
Formulation of paints and coatings
According to information submitted in response to CEPA section 71 surveys, the aryl OPs are imported by distributors who sell these substances to paint and coating formulators for use as plasticizer and flame retardant additives in their formulated products (Environment Canada 2009b, 2012, 2013; ECCC 2016b). As all locations of companies potentially using these substances to formulate paint and coating products are unknown, a distribution of daily dilution volumes associated with secondary and tertiary WWTS receiving effluent from the paints and coatings formulation sector was used. The final users of paint products are not considered for this scenario as they are unknown, and the potential customers are likely highly dispersed. It is expected that the aryl OP substances may be found in both water- and solvent-based paints and coatings. However, the scenario focused on water-based (latex) paints as they have the highest potential releases to wastewater. Based on available safety data sheets, a representative concentration of 1% was selected to represent the amount of aryl OPs in final paint and coating products.
Industrial use of adhesives in the automotive sector
These substances are found in automotive sealers used in vehicle assembly. The sealer is applied between two sheets of metal and cured in an oven. Once hardened, it is affixed to the sheet metal (Environment Canada 2013). Nevertheless, aryl OPs could enter a wastewater stream before the sealer is cured, during the cleaning process (phosphating). No information is available on the amount of body shop sealers, and their constituents, that could be potentially lost to wastewater during the phosphating process. Due to the lack of necessary information on the amounts of sealants applied and potential losses to wastewater, a quantitative exposure analysis cannot be performed. However, it is expected that aryl OP release to the environment and the subsequent risk resulting from this activity would be low.
Commercial and consumer product release
Flame retardants may be released to indoor air and dust from household products, such as electronics, plastics and rubber products (see section 2.7.3), and may subsequently accumulate on clothing and enter laundry wastewater during washing. Flame retardants may then be transported to WWTSs and released to the aquatic environment (Schreder and La Guardia 2014; Saini et al. 2016; Haglund et al. 2016). Ecological exposure resulting from releases of aryl OPs from commercial products and products available to consumers was evaluated using surface water monitoring data (Truong 2016). The highest median value of TPHP measured in urban streams was 0.04 µg/L and was selected as a representative aquatic PEC resulting from release of aryl OPs from consumer products.
2.6.3 Characterization of ecological risk
The approach taken in this ecological screening assessment was to examine the assessment information and develop proposed conclusions using a weight-of-evidence approach and precaution as required under CEPA. Evidence was gathered to determine the potential for substances in the aryl OP subgroup to cause harm in the Canadian environment. Lines of evidence considered include those that directly support the characterization of ecological risk (e.g., measured endpoints or properties), as well as indirect lines of evidence (e.g., classification of hazard or fate characteristics by other regulatory agencies).
2.6.3.1 Risk quotient analysis
Risk quotient (RQ) analyses were performed by integrating realistic worst-case estimates of exposure (PECs; see the Ecological Exposure Assessment section) with ecological toxicity information (PNECs; see the Ecological Effects Assessment section) to determine whether there is potential for ecological harm in Canada. Table 2‑18 presents the range of RQs for the substances in the aryl OP subgroup in water, sediment, soil and wildlife. In the aquatic scenarios, RQs for water are greater than 1 for all except two scenarios—i.e., formulation of lubricant additives and consumer product releases—suggesting risk to the aquatic environment under current releases and near sources of release. In all soil scenarios, RQs for soil are less than 1, suggesting low risk to soil organisms under current releases. For sediment, RQs are greater than 1 for all scenarios, suggesting some risk to sediment organisms under current aryl OP releases.
Critical body residues (CBRs) were calculated in fish for the five aryl OP substances using the highest estimated PECs and BAFs (Appendix B). The analysis showed that the OP substances do not have the potential to accumulate sufficiently in tissues to exceed the threshold for lethality after acute exposure (2 to 8 mmol/kg) or chronic exposure (0.2 to 0.8 mmol/kg) (Appendix B, Table B-1 BAFs) (McCarty et al. 1992; Escher et al. 2011). However, this does not rule out the potential for effects at body burdens lower than the identified thresholds for lethality. Such effects are reasonable to expect for substances like those in the aryl OP subgroup, which have a reactive mode of action.
Modelled BAFs indicate low to moderate bioaccumulation potential for TPHP, BPDP, and IDDP (97.1) and a high bioaccumulation potential for BDMEPPP and IPPP (26 131 and 22 508, respectively). Thus, mammalian piscivores can be expected to be more exposed to these latter two aryl OPs from the consumption of fish than the other aryl OP subgroup substances. A conservative risk analysis was also conducted for two piscivorous wildlife species—mink and river otter—for IPPP (T3IPPP component) to represent the aryl OP substances more likely to result in exposure to fish-eating mammals. Predicted no-effect intake rates (TRV, section 2.6.1.1) are compared to total daily intake values (TDI, section 2.6.1.4) for mink and river otter. The risk quotient calculations for mink and river otter indicate a potential for risk for the IPPP substance.
| Compartment | PEC or TDI | PNEC or TRV | RQ |
|---|---|---|---|
| Water | 4.0 x 10-5 to 0.0027 mg/L | 0.00037 mg/L | 0.1 to 7.11 |
| Sediment | 0.5 to 9.5 mg/kg | 0.37 mg/kg | 1.22 to 25.57 |
| Soil | 0.2 to 2.4 mg/kg | 9.09 mg/kg | 0.02 to 0.27 |
| Wildlifea (mink) | 1.65 mg/kg bw/day | 1.4 mg/kg bw/day | 1.18 |
| Wildlifea (otter) | 1.78 mg/kg bw/day | 0.83 mg/kg bw/day | 2.14 |
a IPPP (T3IPPP component)
Abbreviations: PEC, Predicted Environmental Concentration; TDI, total daily intake calculation;PNEC, Predicted No Effect Concentration; TRV, Toxicity Reference Value; RQ, Risk Quotient.
| Exposure scenario | Aquatic PEC (mg/L) | RQ aquatic (PNEC: 0.00037 mg/L) | Sediment PEC (mg/kg) | RQ sediment (PNEC: 0.37 mg/kg dw) | Soil PEC (mg/kg) | RQ soil (PNEC: 9.09 mg/kg dw) |
|---|---|---|---|---|---|---|
| Formulation of lubricant additives (high quantity user) | 2.5 x 10-4 | 0.67 | 0.45 | 1.22 | 0.2 | 0.02 |
| Formulation of lubricant additives (generic user) | 6.4 x 10-4 | 1.72 | 1.18 | 3.2 | 0.86 | 0.01 |
| Formulation of lubricants (generic user) | 1.37 x 10-3 | 3.70 | 2.82 | 7.62 | 0.38 | 0.04 |
| Formulation of plastic products (generic user) | 2.43 x 10-3 | 6.57 | 6.78 | 18.33 | 1.52 | 0.17 |
| Formulation of rubber roducts (high quantity user) | 2.7 x 10-3 | 7.11 | 9.46 | 25.57 | 1.18 | 0.13 |
| Formulation of paints and coatings (generic user) | 1.45 x 10-3 | 3.91 | 4.04 | 10.92 | 2.4 | 0.27 |
| Consumer product release (based on measured data) | 4.0 x 10-5 | 0.10 | N/A | N/A | N/A | N/A |
NA: Not applicable
a It is unlikely that biosolids from the industrial WWTS would be applied to land.
Abbreviations: PEC, predicted environmental concentration; PNEC, predicted no effect concentration; RQ, risk quotient
2.6.3.2 Consideration of the lines of evidence
To characterize the ecological risk of substances in the aryl OP subgroup, technical information for various lines of evidence was considered (as discussed in the relevant sections of this report) and qualitatively weighted. The key lines of evidence supporting the assessment conclusion are presented in Table 2‑20, with an overall discussion of the weight of evidence provided in section 2.6.3.3. The level of confidence refers to the combined influence of data quality and variability, data gaps, causality, plausibility and any extrapolation required within the line of evidence. The relevance refers to the impact the line of evidence has when determining the potential to cause harm in the Canadian environment. Qualifiers used in the analysis ranged from low to high, with the assigned weight having five possible outcomes.
| Line of evidence | Level of confidencea | Relevance in assessmentb | Weight assignedc |
|---|---|---|---|
| Persistence in the environment | moderate | moderate | moderate |
| Long-range transport | low | moderate | low-moderate |
| Bioaccumulation: TPHP, BPDP, IDDP | moderate | moderate | moderate |
| Bioaccumulation: BDMEPPP, IPPP (T3IPPP representative structure) | low | moderate | moderate |
| Mode of action and/or other non-apical data | moderate | high | moderate-high |
| PNEC for aquatic, sediment and soil organisms | moderate | high | moderate-high |
| TRV for wildlife | low | high | moderate |
| CBR for fish | low | moderate | low-moderate |
| Measured concentrations in the Canadian environment | low | high | moderate |
| PEC(s) in aquatic industrial scenarios | moderate | high | moderate-high |
| PEC(s) in sediment and soil industrial scenarios, TDI in wildlife industrial scenario | low | moderate | low-moderate |
| RQ(s) for water | moderate | high | moderate-high |
| RQ(s) for sediment, soil and wildlife | low | high | moderate |
a Level of confidence is determined according to data quality, data variability, data gaps and if the data are fit for purpose.
b Relevance refers to the impact of the evidence in the assessment.
c Weight is assigned to each line of evidence according to the combined level of confidence and relevance in the assessment.
Abbreviations: PNEC, predicted no effect concentration; TRV, toxicity reference value; CBR, critical body residue; PEC, predicted environmental concentrations; RQ, risk quotient
2.6.3.3 Weighted lines of key evidence considered to determine the potential for substances in the aryl OP subgroup to cause harm in the Canadian environment
Although the substances in the aryl OP subgroup are generally not expected to be highly persistent in water, soil or sediment, relatively slow ultimate degradation has been identified for BDMEPPP and IPPP. While modelled predictions for atmospheric oxidation of the aryl OP subgroup substances indicate a half-life of less than 1 day (gas phase), empirical data for TPHP demonstrate persistence when bound to aerosols (lifetime of 5.6 days for TPHP adsorbed to particulates exposed to OH radicals). There are no equivalent empirical data available for BPDP, BDMEPPP, IDDP or IPPP, but it is possible that the persistence of these substances in air is underpredicted if adsorption to particulates were to be considered. Furthermore, TPHP has been measured in remote Arctic locations, supporting the assumption that TPHP can be considered persistent in air.
The limited bioconcentration studies and QSAR modelling for BDMEPPP and possibly IPPP (T3IPPP representative component) predict moderate to higher bioaccumulation potential. The BAF estimates were highly relevant to the assessment because of their use in the CBR analysis and wildlife risk analysis. While an estimated kM, derived from an empirical IPPP BCF study, was used for the modelling of BAF, an equivalent value for BDMEPPP could not be derived due to the lack of empirical bioconcentration studies. Thus, the derived kM for IPPP was read across to BDMEPPP, an approach that is considered reasonable given the similarities between the two substances in terms of chemical structure and physical-chemical properties. As a result, measured BAF data for both IPPP and BDMEPPP would improve the lower confidence in the data relating to the bioaccumulation potential ascribed to these substances as well as the lower confidence in the CBR and wildlife TDI analyses.
Metabolites of the aryl OP subgroup substances have largely been identified as their respective diesters and a variety of hydroxylated metabolites, which appear more stable than their respective parent substances. Using the OECD Toolbox, the predicted metabolites contained at least one alert profile of interest, e.g., neurotoxicity, reproductive effects, and developmental effects (DNA, RNA and protein interactions). There are differences in the reactivity and mechanism of action of the parent aryl OPs and their metabolites (as indicated by OECD Toobox hazard profiles). Industry and literature studies, QSAR modelling, and CBR analysis provide evidence for high acute and chronic aquatic toxicity.
For the aquatic effects assessment, TPHP was used as a read-across to the aryl OP subgroup given the availability of toxicity studies for this substance and because TPHP has the highest likelihood of partitioning to water. The uncertainty associated with the read-across of TPHP to the other four aryl OP substances for aquatic toxicity was qualitatively considered based on an analysis and comparison of parent compound and stable metabolites with respect to chemical structures, physical and chemical properties, toxicodynamics, and toxicokinetics. Differences were identified in all areas of comparison. These differences were recognized when determining the assessment factor for aquatic life (100), which is considered sufficiently protective of the other aryl OP substances.
Although the PNECs were derived from toxicity studies using mixtures, the selected studies are of acceptable quality, consider sensitive endpoints, and use mixtures consisting of substances in the aryl OP subgroup, including BDMEPPP and IPPP, which are considered most hydrophobic and likely to partition to organic solids. With the use of assessment factors, the derived PNECs are considered to provide adequate protection to the soil and sediment organisms for the aryl OP subgroup.
The exposure assessment provides a key line of evidence presenting both estimated and measured exposure to the aryl OPs in the Canadian environment. However, environmental monitoring is limited for all aryl OP substances subject to this assessment except for TPHP. The quantities of the individual substances in the aryl OP subgroup were totalled for major industrial uses to estimate PECs (water, sediment soils, and TRV for wildlife) representing scenarios of combined usage. This is considered reasonable because of their common uses and thus their potential use as alternatives for each other and combined presence in commercial mixtures/formulations. While there is moderate confidence in surface water PECs, confidence in soil and sediment PECs is lower given the generic nature of the approaches and the lack of measured soil and sediment concentrations in Canada.
The risk quotient analysis found that PECs for surface water and sediments exceed PNECs derived for these media, thereby indicating a potential risk of harm to aquatic and benthic organisms under current total use of the substances in the aryl OP subgroup. For the assessment of wildlife, IPPP (T3IPPP representative structure), with a high bioaccumulation potential, was used to represent the exposure and toxicity of the aryl OP substances. Risk quotients suggest risk to wildlife for IPPP (T3IPPP representative structure) using estimates of exposure and TDI values. CBR analyses indicate that the aryl OPs do not have the potential to accumulate sufficiently in tissues to result in mortality due to acute or chronic exposure at the highest predicted surface water concentration.
2.6.3.4 Sensitivity of conclusion to key uncertainties
There is uncertainty associated with using TPHP as a read-across for aquatic toxicity to all of the aryl OP substances. The OP substances are known to metabolize, and the modelling analysis suggests that toxicity may be caused not only by the parent compounds, but also by their respective metabolites. A large assessment factor was used to account for the level of uncertainty associated with using TPHP as a read-across to the other aryl OP substances and the various pathways for adverse effects of the aryl OP potential metabolites. There are differences among the OPs in the reactivity and mode and mechanism of action of metabolites.
Environmental monitoring data are lacking for all aryl OP subgroup substances except for TPHP. Canadian monitoring data could help to refine the exposure assessment, which estimated exposures based on reported use quantities and on measured data for TPHP.
The exposure scenarios identified for the aryl OP subgroup are developed on the basis of information submitted in response to CEPA section 71 surveys and follow-up with stakeholders. In the absence of particular data, realistic assumptions are made in order to estimate PECs. For the industrial exposures, refinement of industrial users after distribution would help increase the certainty in the PECs.
There is a data gap with respect to the release of aryl OP substances from the use and disposal of products available to consumers. However, potential exposures determined using Toronto-area stream water concentrations for TPHP are 10 to 1000 times lower than those determined for the industrial exposure scenarios. Additional information on exposure resulting from releases from product use or disposal would not change the conclusion, which is based on exposure scenarios developed for industrial formulation activities.
There are limited bioaccumulation data for BDMEPPP and IPPP, and modelled results suggest a high potential for these substances to bioaccumulate in aquatic organisms. Results of empirical data and BAF modelling for the aryl OPs were used to estimate exposure via fish to piscivorous mammals. The model results are considered conservative in the context of other lines of evidence, such as metabolic biotransformation. Additional information on the bioaccumulative potential of these substances could clarify this aspect of the assessment.
Uncertainties exist in the sediment exposure estimates due to the use of equilibrium partitioning, particularly for the highly hydrophobic aryl Ops, including BDMEPPP and IPPP. The partitioning estimates using sediment organic carbon partition coefficient (KOC) tends to over-predict exposures, resulting in conservative PECs. Given the predicted partitioning behaviour of the aryl OPs, excluding TPHP, and the lack of measured concentrations, the significance of sediment as an important media of exposure is a data gap.
Within the ecological effects assessment, studies show that the aryl OP substances may have a potential for adverse effects in fish, including neurotoxicity, reproductive and developmental effects and endocrine disruption. A key area of uncertainty includes the lack of data characterizing toxicity to wildlife, especially for substances that suggest potential to bioaccumulate in biota, such as BDMEPPP and IPPP.
2.7 Potential to cause harm to human health
BDMEPPP
The human health risk of BDMEPPP was characterized using the Rapid Screening of Substances with Limited General Population Exposure (ECCC, HC 2017). The potential for exposure of the general population to BDMEPPP was considered to be negligible and not to be of concern to human health. Therefore, BDMEPPP is considered to be of low concern for human health at current levels of exposure.
2.7.1 Exposure assessment of the aryl organophosphate subgroup (TPHP, BPDP, IPPP, IDDP)
2.7.1.1 TPHP
Environmental media and food
Canadian monitoring data for TPHP in ambient air and surface water are summarized in section 2.6.2.1 (Table 2‑17). As a conservative approach, the maximum ambient air concentration (2 220 pg/m3) from the Shoeib et al. (2014) study was used to estimate general population exposures.
TPHP has also been measured in indoor air in Canada (Vykoukalová et al. 2017; Yang et al. 2019). Yang et al. (2019) measured TPHP in indoor air in homes in the Greater Toronto Area (n = 32) and Ottawa (n = 19) in 2015 over a 3-week sampling period. Data were collected in bedrooms in all of the homes (n = 51) as well as in the most used room (MUR) in 26 of the homes. TPHP was measured with detection frequencies of 100% for bedrooms and 96% for MURs. Concentrations of TPHP ranged from 0.34 to 17 000 pg/m3 (MDL = 0.34), with geometric means of 3 480 and 3 440 pg/m3 and 95th percentile concentrations of 11 700 and 15 200 pg/m3 in bedrooms and MURs, respectively (Yang et al. 2019). TPHP has also been measured globally in residences (e.g., Cequier et al. 2014; Luongo and Östman 2016; Takeuchi et al. 2014; Vykoukalová et al. 2017) as well as in childcare centres in the United States (Stubbings et al. 2018) and Europe (Bergh et al. 2011; Fromme et al. 2014). The 95th percentile value of 15 200 pg/m3 for MURs in the Yang et al. (2019) study was considered most appropriate for estimating general population exposures for Canadians.
Two Canadian drinking water studies that included TPHP were identified. Williams et al. (1982) reported a mean TPHP concentration of 1.3 ng/L for 24 samples of potable drinking water from 12 water treatment plants that drew water from the Great Lakes system (detection frequency not reported; concentrations ranged from not detected to 4.8 ng/L). The City of Toronto targeted TPHP in a municipal drinking water monitoring program from 2002-2003 and it was not found to be present above the detection limit of 200 ng/L throughout this time period (e.g., City of Toronto 2002a, b, c, d, 2003a, b, c). Given the lack of recent Canadian potable water data, as a conservative approach, the maximum estimated aquatic PEC for all of the aryl OPs of 2.7 µg/L (section 2.6.2.2, Table 2‑19) was used to derive general population intakes of TPHP from drinking water.
TPHP levels in house dust in Canada have been measured in several studies. TPHP was measured in archived house dust samples (n = 818) collected in 2007-2008 from various Canadian cities within the Canadian House Dust Study (CHDS) (personal communication, emails from Environmental Health Science and Research Bureau, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated October 28, 2013; unreferenced). TPHP was detected in 99.4% of the baseline study samples, with concentrations ranging from not detected (method detection limit, MDL = 130 ng/g) to 91 000 ng/g and a 95th percentile of 11 000 ng/g. Yang et al. (2019) measured TPHP in dust in homes in the Greater Toronto Area (n = 32) and Ottawa (n = 19) in 2015 over a 3-week sampling period. Data were collected in bedrooms in all of the homes (n = 51) as well as in the MUR in 26 of the homes, with detection frequencies of 100% in both cases. Concentrations of TPHP ranged from 2.16 to 75 600 ng/g (MDL = 0.25 ng/g) in dust from bedrooms, with a geometric mean of 4 760 ng/g and a 95th percentile of 20 700 ng/g. Concentrations in the MURs ranged from 996 to 16 900 ng/g, with a geometric mean of 4 880 ng/g and a 95th percentile of 15 800 ng/g. Vykoukalová et al. (2017) and Harrad et al. (2016) also measured TPHP in dust from homes in Toronto within the concentration ranges found by Yang et al. (2019) and in the CHDS. TPHP levels in dust in homes and daycare centres globally have also been reported at similar concentrations (Brommer and Harrad 2015; Castorina et al. 2017; Fromme et al. 2014; Stubbings et al. 2018; Vykoukalová et al. 2017). The 95th percentile concentration of 20 700 ng/g from the Yang et al. (2019) study was used to estimate general population exposure to TPHP from dust.
Given the lack of recent Canadian data for soil, as a conservative approach, the maximum estimated PEC of 2 400 ng/g for soil for all of the aryl OPs (see section 2.6.2.2) was used to estimate general population exposure to TPHP from soil and resulted in negligible exposures for all age groups.
Potential exposure to TPHP from its use as a component in the manufacture of food packaging materials (see section 2.3) is not expected since the substance does not come into contact with food (personal communication, emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated August 8, 2014; unreferenced). Any unexpected contribution to overall dietary exposure from these uses would be accounted for in the occurrence data for processed foods that were employed in the assessment described below.
As a result of various anthropogenic uses, TPHP has been found at generally low levels in foods. TPHP has been detected in one fish study in Canada (see Table 2‑17). As very limited Canadian occurrence data for TPHP were identified, data employed in the dietary exposure assessment were predominantly from the US Food and Drug Administration’s Total Diet Study and, to a lesser extent, from studies from Europe and China (Appendix E, Table E-1). Overall, limited data were available for TPHP in foods, and some studies also reported low detection frequencies. The maximum TPHP concentrations in foods and beverages used to estimate exposures in the present assessment ranged from 0.27 µg/kg in eggs to 290 µg/kg in confectionary and sugar-based foods (Appendix E, Table E-1). Mean and 90th percentile “all person” exposures to TPHP across all age groups ranged from 0.72 to 3.11 µg/kg bw/day and from 1.27 to 5.64 µg/kg bw/day, respectively (Appendix E, Table E-3).
There are no Canadian data for TPHP in breast milk, but TPHP has been detected in breast milk in Sweden, Japan, Vietnam, and the Philippines (Sundkvist et al. 2010; Kim et al. 2014). In the study that examined TPHP in breast milk in Asian countries (Kim et al. 2014), an overall detection frequency of 86% was reported, and TPHP concentrations in the breast milk of women living in urban areas or close to an e-waste recycling site or waste dumping site ranged from non-detected (method dection limit between 0.01 to 0.08 ng/g lipids) to 140 ng/g lipids. In the Swedish study, breast milk samples were obtained from 286 women in four Swedish towns, and TPHP was measured in all six samples tested (five of which were composites of samples collected from 1997 to 2003) (Sundkvist et al. 2010). A concentration of 10 ng/g lipids was found for the pooled samples from 90 women in Uppsala in 1998, and only one sample collected from one woman in Umeå in 2007 gave a higher level of TPHP in this study, at 11 ng/g lipids. Given the higher sample size in the Swedish study, the value of 10 ng/g lipids from the composite sample measured in Swedish women was used to estimate exposure to breast-fed infants (0.035 µg/kg bw/day Appendix F, Table F-1).
Estimates of exposure for TPHP from environmental media and food for the general population of Canada ranged from 0.14 µg/kg bw/day for breast-fed infants to 5.82 µg/kg bw/day for 0.5 to 4-year-olds, with the main sources of exposure being dust for infants and food for ages 0.5 years and up (Appendix F).
Products available to consumers
Cosmetics:
Based on notifications submitted under the Cosmetic Regulations to Health Canada, TPHP is used in various nail care products in Canada such as base coats, top coats, and nail polish (personal communication, e-mail from Consumer and Hazardous Products Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated January 12, 2017; unreferenced). The function of TPHP in these products is as a plasticizer (Mendelsohn et al. 2016). Given that TPHP has a low vapour pressure (1.68 x 10-4 Pa), the primary route of exposure is considered to be through the skin (Mendelsohn et al. 2016). Therefore, only dermal estimates are presented. Limited dermal absorption data were identified for TPHP (Frederiksen et al. 2018), but given that a dermal toxicological study was available, only external dermal exposures were derived (see section 2.7.3.1). Table 2‑21 summarizes the external dermal doses for nail care products containing TPHP that are available to consumers. Given that these products may be used together, the estimated external dermal exposures for the three products were aggregated for adults and teens. Details on the method and parameters used to estimate external dermal exposures to TPHP from nail care products are available in Appendix G, Table G-1.
| Age group | Base coata | Nail polish | Top coata | Total |
|---|---|---|---|---|
| 20+ years | 0.06 | 0.68 | 0.10 | 0.84 |
| 12-19 years | 0.07 | 0.81 | 0.12 | 0.99 |
| 5-11 years | N/A | 0.58 | N/A | 0.58 |
Abbreviations: N/A, not applicable
a Product not anticipated to be used by children younger than 12 years old
Other products:
In Canada, TPHP was reported as an ingredient in lubricants and greases, but exposures to consumers are not expected (personal communication, email from Consumer and Hazardous Products Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated June 6, 2014; unreferenced).
Manufactured items:
TPHP is one of several flame retardants that is incorporated into flexible PUF during foam production (Marklund 2005). TPHP is commonly found in commercial flame retardant mixtures, one of which is Firemaster 550 (or FM550) (McGee et al. 2013; Phillips et al. 2017). Some studies report the level of FM550 as a sum of TPHP and the two brominated substances (benzoic acid, 2,3,4,5-tetrabromo-, 2-ethylhexyl ester or TBB and 1,2-benzenedicarboxylic acid, 3,4,5,6-tetrabromo-, bis(2-ethylhexyl) ester or TBPH) that make up most of this blend (Stapleton et al. 2012), while others used an authentic standard of FM550 for its quantification (Cooper et al. 2016). TPHP has been detected in several products available to consumers in Canada, such as foam-containing products, including furniture such as couches, and children’s products such as nap mats, baby slings, baby mattresses, infant and child restraint seats, changing table pads, portable mattresses, and rocking chairs (CEH 2013a,b; CEC 2015b; Cooper et al. 2016; Danish EPA 2015; Stapleton et al. 2011).
A study by the Commission for Environmental Cooperation (CEC) examined the presence of 16 flame retardants in foam-containing furniture purchased in Canada, the United States and Mexico between December 2014 and April 2015 (CEC 2015b). TPHP (reported as TPP) was the third most frequently detected flame retardant out of the six that were measured and was found at levels ranging from 200 to 11 500 ppm (0.02% to 1.15% w/w). TPHP had the highest detection frequencies for products purchased in Canada. TPHP was detected in 5%, 0.7% and 2% of products purchased in Canada, Mexico and the United States, respectively, and was predominantly present in foam samples as opposed to fabric (CEC 2015b). Prolonged dermal exposure to TPHP from lying or sitting on foam-containing mattresses or furniture containing TPHP was estimated (Table 2‑22) and represents the sentinel exposure scenario covering dermal exposure to other product types, such as infant and child restraint seats and changing table pads. Details for the scenario are described in Appendix G.
No studies of children’s products in Canada that contained TPHP were identified. TPHP has, however, been identified in toys made of plastic, rubber, wood, foam and textiles in Antwerp, Belgium (Ionas et al. 2014) and in various children’s products in the United States (Stapleton et al. 2011). It is expected that some of the same types of children’s products as those found to contain TPHP in Europe and the United States would be present in Canada. Therefore, estimates of exposure to TPHP via mouthing were also derived (Table 2‑22) as described in Appendix G.
| Exposure route and duration | Source | Age group | Exposure estimate (mg/kg bw/day) |
|---|---|---|---|
| Dermal (daily) | Children’s foam-containing furniture or mattresses | 0–6 months | 0.021–0.13 |
| Dermal (daily) | Children’s foam-containing furniture or mattresses | 0.5–4 years | 0.015–0.10 |
| Dermal (daily) | Foam containing furniture or mattresses | 20+ years | 5.6×10-3 – 4.7×10-2 |
| Oral (daily) | Foam in toys and children’s products | 0–6 months | 1.33×10-5 – 2.47×10-5 |
| Oral (daily) | Foam in toys and children’s products | 0.5–4 years | 1.29×10-5 – 2.39×10-5 |
2.7.1.2 BPDP
Environmental media and food
The concentration of BPDP in air was predicted on the basis of environmental modelling estimates using ChemCAN (2003), whereby a conservative scenario was derived from a maximum Canadian import quantity of approximately 100 000 kg. Given the lack of recent Canadian data for soil, the maximum estimated PEC for soil of 2400 ng/g for all of the aryl OPs (see section 2.6.2.2) was used to estimate general population exposure to BPDP from soil. Predicted concentrations of BPDP in air and soil resulted in negligible estimates of exposure via these sources for the general population of Canada.
Given the lack of Canadian potable water data for BPDP, as a conservative approach, the maximum estimated aquatic PEC of 2.7 µg/L for all of the aryl OPs (section 2.6.2.2, Table 2‑19) was used to derive general population intakes of BPDP from drinking water.
BPDP (reported as tBPDPP, tert-butylphenyl diphenyl phosphate, CAS RN 56803-37-3) was measured in active floor dust samples collected from 144 homes in 13 cities (population > 100,000) across Canada under the CHDS (personal communication, e-mail from Environmental Health Science and Research Bureau, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated December 18, 2018; unpublished). BPDP was detected in 91.7% of the samples, with concentrations ranging from below the method detection limit (MDL) (15.26 ng/g) to a maximum of 3 329 ng/g. The limit of quantitation (LOQ) was 48.55 ng/g, and the 50th and 95th percentiles were 51.73 ng/g and 340 ng/g, respectively. One of the isomers that comprise BPDP (4-tert-butylphenyl diphenyl phosphate, CAS RN 981-40-8, reported as 4tBPDPP) was reported in indoor dust samples collected in 190 homes as part of the Toddler’s Exposure to SVOCs in the Indoor Environment (TESIE) study in North Carolina, USA (Phillips et al. 2018). BPDP was detected in 94.7% of 188 samples over a concentration range of less than 2.4 ng/g (method limit of detection) to 85 986 ng/g. The geometric mean was 510.9 n/g and the 90th percentile was 2 544 ng/g. The 95th percentile concentration of BPDP (340 ng/g) in the CHDS was used to estimate general population exposures to BPDP.
No data on the presence of BPDP in food or breast milk were identified.
Estimates of exposure for BPDP from environmental media for the general population of Canada ranged from 1.7 ng/kg bw/day for breast-fed infants to 290 ng/kg bw/day for formula-fed infants, with dust and water, respectively, as the sources of exposure (Appendix F, Table F-2).
Products available to consumers
Manufactured items:
BPDP is one of several flame retardants that is used in commercial flame retardant mixtures that have been measured in flexible PUF samples from a number of foam-containing products (Cooper et al. 2016; Phillips et al. 2017; Stapleton et al. 2012). Firemaster 600 (or FM600) is a blend containing two brominated substances, TPHP, and several tert-butyl-triphenyl phosphates, including at least one isomer of BPDP (4-tert-butylphenyl diphenyl phosphate reported as 4tBPDPP, reportedly makes up approximately 2.6% of this blend out of the accounted percentage of 95.3%) (Phillips et al. 2017). This same BPDP isomer (4tBPDPP) is also present in a mixture of TPHP and tert-butyl-triphenyl phosphates, referred to in the literature as TBPP mixture, with 4tBPDPP reportedly having a mass fraction of about 36% of the 79.5% of this blend that was accounted for (Phillips et al. 2017). One or both of these flame retardant mixtures were measured in PUF samples collected in the United States between February 2014 and June 2016 from children’s mattresses, other children’s products, sofas and love seats, chairs, rocking chairs and recliners, and mattress pads (Cooper et al. 2016). TBPP mixture has also been detected in nap mats (CEH 2013b), couches (Stapleton et al. 2012), and a children’s foam chair (CEH 2013a) bought in the United States. As discussed previously (section 2.7.1.1), TPHP has also been found in a number of foam-containing products, including mattresses, furniture, children’s products, and toys, and it is possible that some of these products could contain BPDP given that commercial mixtures of BPDP are reported to contain some percentage of residual TPHP (EA 2009b; Phillips et al. 2017).
Although no studies that investigated levels of BPDP in foam-containing products purchased in Canada have been identified, it is expected that some of the same types of products found to contain flame retardant blends that include BPDP elsewhere would also be present in Canada. Estimates of dermal and oral exposures to BPDP from foam-containing products were therefore derived (Table 2‑23). Prolonged dermal exposure to BPDP could occur through lying or sitting on foam-containing mattresses or furniture containing BPDP. No dermal absorption data were identified for BPDP, and therefore, as a conservative approach, a dermal absorption of 100% was assumed. Exposures were estimated as described in Appendix G, and Table 2‑23 summarizes exposure scenarios for manufactured items containing BPDP. Estimates of exposure to BPDP via mouthing were estimated as described in Appendix G.
| Exposure route and duration | Source | Age group | Exposure estimate (mg/kg bw/day) |
|---|---|---|---|
| Dermal (daily)a | Children’s foam-containing furniture or mattresses | 0–6 months | 0.018–0.098 |
| Dermal (daily)a | Children’s foam-containing furniture or mattresses | 0.5–4 years | 0.012–0.074 |
| Dermal (daily)a | Foam containing furniture or mattresses | 20+ years | 0.005–0.034 |
| Oral (daily) | Foam in children’s products | 0–6 months | 1.11×10-5 – 1.81×10-5 |
| Oral (daily) | Foam in children’s products | 0.5–4 years | 1.07×10-5 – 1.75×10-5 |
a Assumed dermal absorption is equivalent to that of oral.
2.7.1.3 IDDP
Environmental media and food
No environmental monitoring data were identified for IDDP in air, water, or soil in Canada. Concentrations of IDDP in air were predicted from environmental modelling estimates using ChemCAN (2003), where a scenario was derived on the basis of the maximum quantity of IDDP imported into Canada of approximately 1 000 000 kg (see Table 2‑5). Given the lack of recent Canadian data for soil, the maximum estimated PEC for soil of 2 400 ng/g for all the aryl OPs (see section 2.6.2.2) was used to estimate general population exposure to IDDP from soil. Predicted concentrations of IDDP in air and soil resulted in negligible estimates of exposure via these sources for the general population of Canada.
As a conservative approach, the maximum estimated PEC for water of 2.7 µg/L for all the aryl OPs (section 2.6.2.2, Table 2‑19) was used to derive general population intakes of IDDP from drinking water.
IDDP (reported as IDDPHP, isodecyl diphenyl phosphate, CAS RN 29761-21-5) was measured in active floor dust samples collected from 144 homes in 13 cities (population > 100,000) across Canada under the CHDS (personal communication, e-mail from Environmental Health Sciences Research Bureau, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated December 18, 2018; unreferenced). IDDPHP was detected in 100% of the samples, with a concentration range of below the MDL (22.32 ng/g) to a maximum of 17 851 ng/g. The limit of quantitation (LOQ) was 71.03 ng/g, and the 50th and 95th percentiles were 1 225 ng/g and 7 582 ng/g, respectively. IDDP has also been measured at similar concentrations in indoor house dust in the UK and Norway (Kademoglou et al. 2017), in homes, offices and cars in Spain and the Netherlands (Bjornsdotter et al. 2018) and in car interiors in Greece (Christia et al. 2018). The 95th percentile concentration of 7 582 ng/g from the CHDS was used to estimate general population exposure to IDDP from dust.
Potential exposure to IDDP from its use as a component in food packaging materials is not expected since the substance does not come into direct contact with food (Personal communication, e-mail from Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated January 11, 2017; unreferenced). No Canadian data on the presence of IDDP in food were identified. IDDP (reported as IDPP) was quantified in 6 of 12 fish samples in Spain, with a maximum concentration of 851 ng/g lipid weight (lw) measured in a single barbel (method limit of detection = 2.13 ng/g lw) (Santin et al. 2016). Estimates of IDDP intake for the general population based on the consumption of fish with this maximum concentration of IDDP were negligible.
Estimates of exposure for IDDP from environmental media for the general population of Canada ranged from 38 ng/kg bw/day for breast-fed infants to 326 ng/kg bw/day for formula-fed infants, with dust and water, respectively, as the sources of exposure (Appendix F, Table F-3).
Products available to consumers
IDDP was identified as a component of a water-based marine paint available to consumers in Canada (MSDS 2011). Dermal exposure to IDDP was estimated to be 2.2 mg/kg bw/day using ConsExpo Web (2016). Details regarding this scenario are described in Appendix G, Table G-1. Given that IDDP has a low vapour pressure (2.82x10-6 Pa), inhalation exposure is expected to be negligible.
2.7.1.4 IPPP
Commercial IPPP is a mixture of relatively similar isopropylated isomers and varying amounts of TPHP (see section 2.1). As discussed above, TPHP has other applications, and data have been identified with concentrations of TPHP in many environmental media (section 2.6.2.1), food, and products available to consumers (see section2.7.1.1). Some of the TPHP measured in these studies could originate from the IPPP mixture. Therefore, data on TPHP, which is considered to be the most water soluble component of IPPP, in combination with limited data identified for bulkier and less water soluble isopropylated isomers and modelled data for a tris(isopropylphenyl) phosphate (T3IPPP, see Table 2‑2 for isomer identity), will be taken into consideration when estimating exposure for the general population of Canada to IPPP.
Environmental media and food
As discussed in section 2.1, tris(isopropylphenyl) phosphate isomers represent the largest (steric) components of IPPP, with the highest degree of alkylation and hydrophobicity. One of these isomers, tris(3-isopropylphenyl) phosphate (T3IPPP), was selected as a representative IPPP isomer for estimating ambient air and soil concentrations for the isopropylated species within IPPP and was compared to the limited monitoring data available, as discussed below. ChemCAN (2003) was employed to model concentrations of T3IPPP in air using the maximum quantity of IPPP imported into Canada of approximately 1 000 000 kg (see Table 2‑5).
According to Salamova et al. (2014), T2IPPP was not detected in ambient air measurements at two remote locations, but was measured at between 3.6 and 5.3 pg/m3 (particle concentrations) at two urban locations (Chicago and Cleveland) and one rural location (Sturgeon Point). This same isomer was not detected (method detection limit = 13.8 ppb) in 40 atmospheric particulate matter samples collected in 2013 across four sites in Houston, Texas (Clark et al. 2017). T2IPPP was also detected in air samples collected in 2014 at eight sites in Bursa, Turkey (Kurt-Karakus et al. 2018). None of the reported concentrations of T2IPPP are higher than the ambient air concentration estimated for T3IPPP using ChemCAN. Therefore the modelled concentration was used as a more conservative approach to estimating exposure of the general population of Canada to the isopropylated isomers of IPPP.
The T2IPPP isomer (reported as TIPPP) was also monitored in indoor air in a study by Vykoukalová et al. (2017) in 23 homes in Toronto as well as in 20 homes in each of Indiana, US, and Brno, Czech Republic. T2IPPP was detected in 97% of the samples collected in Toronto over a range of concentrations of 0.002 to 0.034 ng/m3 with a median of 0.007 ng/m3. The maximum concentration of T2IPPP (0.034 ng/m3) in this study was used to estimate general population exposures to the isopropylated components of IPPP.
Given the lack of Canadian potable water data, as a conservative approach, the maximum estimated aquatic PEC of 2.7 µg/L for all the aryl OPs (section 2.6.2.2) was chosen for deriving general population intakes of IPPP from drinking water.
T2IPPP has been measured in house dust in Toronto (Vykoukalová et al. 2017) and in Vancouver (Shoeib et al. 2019). Samples were collected in 2013 from 23 homes in Toronto in the Vykoukalová et al. (2017) study and T2IPPP (reported as TIPPP) was measured over a concentration range of 4.26 to 74.3 ng/g, with a median of 20.4 ng/g and a detection frequency of 100%. Samples in Vancouver (n = 92) were collected in 2007-2008 for the Shoeib et al. (2019) study, and T2IPPP was measured over a concentration range of 10 to 1 500 ng/g (method detection limit = 0.5 ng/g), with a mean of 57 ng/g and a detection frequency of 25%. IPPP (reported as TIPPP, tris(isopropylphenyl) phosphate, CAS RN 64532-95-2) was measured in active floor dust samples collected from 144 homes in 13 cities (population of over 100,000) across Canada under the CHDS (personal communication, e-mail from Environmental Health Sciences Research Bureau, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated December 18, 2018; unreferenced). TIPPP was detected in 76.4% of the samples, with concentrations ranging from below the method detection limit (42.40 ng/g) to a maximum of 52 713 ng/g. The limit of quantitation (LOQ) was 135 ng/g, and the 50th and 95th percentiles were 72.45 ng/g and 518 ng/g, respectively. As part of the Toddler’s Exposure to SVOCs in the Indoor Environment (TESIE) study in North Carolina, USA, Phillips et al. (2018) measured six isopropylated IPPP isomers in indoor dust samples collected in 190 homes. The most substituted isomer that was measured in dust samples in this study was bis(2,4-diisopropylphenyl) phenyl phosphate (B24DIPPPP) in 8% of samples over a concentration range from less than 3.5 ng/g (method detection limit) to 29 637 ng/g. The 95th percentile concentration of TIPPP (518 ng/g) in the CHDS project was used to estimate general population exposures to IPPP.
Given the lack of recent Canadian data on soil, the maximum estimated PEC for soil of 2 400 ng/g for all of the aryl OPs (see section 2.6.2.2) was used to estimate general population exposure to TIPPP from soil.
IPPP (isopropyl phenyl phosphate) was detected in 9 of 12 fish samples in Spain, with a maximum concentration of 601 ng/g lipid weight (lw) measured in a single carp (method detection limit of 51.6 ng/g lw) (Santin et al. 2016). Estimates of IPPP intake for the general population based on the consumption of fish with this maximum concentration of IPPP were negligible. Gunderson (1988) estimated total dietary exposure to ‘isopropylphenyl phenyl phosphates, mixed’ as part of the US FDA Total Diet Study (1982-1984). For all population age groups considered between 6 months and 65 years, exposures ranged from 0.1 to 0.7 ng/kg bw/day. As TPHP is a component of IPPP and the literature reports either neglibible exposure or estimates that are notably dated, estimates of exposure to IPPP from food were derived from estimates based on TPHP (see section 2.7.1.1 and Appendix E, Table E-3).
Intake estimates for TPHP (Appendix F, Table F-1) and those for the isopropylated components of IPPP were added together to derive conservative estimates of exposure to IPPP from environmental media and food (Appendix F, Table F-4). Estimates of exposure from ambient air and soil were negligible. Intake estimates of IPPP for the general population ranged from 0.14 µg/kg bw/day for breast-fed infants, where dust was the main source of exposure, to 5.79 µg/kg bw/day for 0.5- to 4-year-olds, where food was the main source of exposure (Appendix F, Table F-4).
Products available to consumers
Manufactured items:
IPPP, like TPHP, is one of several flame retardants that is incorporated into flexible PUF during foam production (Marklund 2005). IPPP is a constituent of the commercial flame retardant mixture Firemaster 550 (FM550), which contains approximately 40% IPPP, approximately 20% TPHP and approximately 40% to 50% of the two brominated substances (TBB and TBPH), as discussed above (see section 2.7.1.1) (McGee et al. 2013; Stapleton et al. 2008; Phillips et al. 2017, 2018). Studies that analyze furniture and other foam products for flame retardants often screen for FM550 as a flame retardant blend. Levels of FM550 might be reported as a sum of TPHP and the two brominated substances, without including the portion made up of IPPP (Stapleton et al. 2012). A standard of FM550 has also been used for identification of this commercial mixture in furniture, which would then also include the IPPP component (Cooper et al. 2016).
As noted previously (section 2.7.1.1), a study by the Commission for Environmental Cooperation (CEC) measured 16 flame retardants in furniture products available to consumers in Canada, the United States and Mexico between December 2014 and April 2015, and IPPP (reported as PIP) was not identified in any of the samples. However, TPHP (not a component of IPPP and reported as TPP) was the third most frequently detected flame retardant of the six that were detected in samples (CEC 2015b). TPHP was detected in about 5% of samples tested from Canada, while 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (TBB, CAS RN 183658-27-7) and bis(2-ethylhexyl) 3,4,5,6-tetrabromophthalate (TBPH, CAS RN 26040-51-7) were each detected in less than 0.5% of samples tested from Canada (CEC 2015b). A US study analyzed foam from a number of product types for flame retardants, including FM550 (e.g., sofas, chairs, rocking chairs, mattresses, mattress pads, child mattresses, child restraint seats, pillows, other child products). FM550 was detected in 10% of all samples tested and was the second most detected flame retardant in this study, both overall and specifically for US sofas purchased between 1985 and 2010 (Cooper et al. 2016).
Prolonged dermal exposures to IPPP from lying or sitting on furniture and from sitting in an infant or child restraint seat manufactured with foam treated with IPPP or a commercial blend (e.g., FM550) that contains IPPP were estimated (Table 2‑24). Exposures were estimated as described in Appendix G for both TPHP and for T3IPPP, a bulky and less water soluble representative isopropylated IPPP isomer. The exposure estimates summarized in Table 2‑24 represent the range calculated for each of these substances. Limited dermal absorption data were identified for TPHP, and no dermal absorption data were identified for IPPP or the T3IPPP isomer. Frederiksen et al. (2018) reported flux and permeability coefficients for TPHP and other organophosphate ester flame retardants, but did not incorporate residues in the epidermis into the reported values. Some substances can remain partly in the skin and later be released systemically (Roberts et al. 2004, as cited in WHO 2006), so the use of the values from the Frederiksen et al. (2018) study might underestimate systemic exposure to the TPHP component of the commercial IPPP. In vitro dermal absorption data have been reported for other organophosphate flame retardants, so a read-across approach to TDCPP (see section 2.1.1) was used. The Frederiksen et al. (2018) study suggests that TDCPP absorbed through the skin slightly more than TPHP, as it had flux and permeability values just higher than those reported for TPHP; this approach is therefore considered to be conservative. An adjusted dermal absorption value of 30% for TDCPP (EU RAR 2008b) was used to represent dermal absorption of IPPP.
No Canadian specific studies measuring flame retardants in children’s products were identified, but it is expected that the same types of foam-containing children’s products as those found in the United States and Europe would be present in Canada and that some of these products would contain IPPP (CEC 2015b; Cooper et al. 2016; Danish EPA 2015; Ionas et al. 2014; Stapleton et al. 2011). Exposure to IPPP via mouthing was estimated as described in Appendix G based on intakes calculated for TPHP and T3IPPP (Table 2‑24). Exposure of children to IPPP through mouthing of such products is considered to be minimal, based in part on the low water solubilities of TPHP and IPPP (see Table 2‑4).
| Exposure route and duration | Source | Age group | Exposure estimatea (mg/kg bw/day) |
|---|---|---|---|
| Dermal (daily) | Children’s foam-containing furniture or mattresses | 0–6 months | 0.0052–0.040 |
| Dermal (daily) | Children’s foam-containing furniture or mattresses | 0.5–4 years | 0.0036–0.030 |
| Dermal (daily) | Children’s foam-containing furniture or mattresses | 5–11 years | 0.0024–0.021 |
| Dermal (daily) | Foam containing furniture or mattresses | 12–19 years | 0.0020–0.019 |
| Dermal (daily) | Foam containing furniture or mattresses | 20+ years | 0.001–0.014 |
| Dermal (daily) | Foam in infant restraint seats | 0–6 months | 5.1×10-4 – 1.2×10-3 |
| Dermal (daily) | Foam in child restraint seats | 0.5–4 years | 4.1×10-4 – 9.4×10-4 |
| Oral (daily) | Foam in children’s products | 0–6 months | 1.08–2.47 x10-5 |
| Oral (daily) | Foam in children’s products | 0.5–4 years | 1.04–2.39 x10-5 |
a Range of exposure estimates represents exposure to T3IPPP isomer (lowest exposure) and TPHP (highest exposure). See Appendix G for more details.
2.7.2 Biomonitoring data for the aryl OP subgroup
Diphenyl phosphate (DPHP) has been measured in numerous human biomonitoring studies, including some from Canada (Kosarac et al. 2016; Yang et al. 2017), the United States (Ospina et al. 2018; Butt et al. 2014, 2016; Hoffman et al. 2017; Thomas et al. 2016) and elsewhere (Cequier et al. 2015; Fromme et al. 2014; He et al. 2018). DPHP has also been measured in serum (Li et al. 2017; Ma et al. 2017), and hair and nails (Alves et al. 2017; Liu et al. 2016). DPHP is a primary metabolite for several organophosphate flame retardants, including TPHP and BPDP among others (Kosarac et al. 2016; He et al. 2018; Bjornsdotter et al. 2018). DPHP may also be used itself in various industrial applications, and therefore the origin of the compound in urine is unknown (Kosarac et al. 2016; Bjornsdotter et al. 2018). As a result, neither TPHP nor BPDP have appropriate biomarkers or sufficient information on the kinetics and metabolism of these substances from which to derive exposure estimates. A more specific biomarker for TPHP has been identified by Su et al. (2016c), but more information and measured levels of this metabolite in humans are required before it can be used to estimate human exposures.
Limited human biomonitoring data have been identified for certain IPPP isomers (Phillips et al. 2018) and IDDP (Gibson et al. 2018). However, there is insufficient information on appropriate biomarkers as well as information on the kinetics and metabolism for these substances to derive exposure estimates.
2.7.3 Health effects assessment of the aryl OP subgroup (TPHP, BPDP, IPPP, IDDP)
2.7.3.1 TPHP
TPHP has been assessed by ATSDR (2012) and OECD (2002b). These reviews provide a basis for the health effects characterization in this draft screening assessment. Targeted literature searches were conducted from a year prior to the ATSDR (2012) report to October 2018. No health effect studies that would impact the risk characterization (i.e., result in different critical endpoints or lower points of departure than those stated in ATSDR 2012) were identified.
A safety assessment of TPHP as used in cosmetics was also available (CIR 2018).
Toxicokinetics
Triphenyl phosphate (TPHP) is degraded by hydrolysis in rat liver homogenate to diphenyl phosphate as the major metabolite (OECD 2002b). The dermal uptake and percutaneous penetration of TPHP was studied using human skin in Franz diffusion cells. TPHP tended to build up in the skin tissues, primarily in the upper layers. Only “smaller amounts” of TPHP permeated the skin and reached the receptor fluid within 72 hours (Frederiksen et al. 2018). TPHP has the potential to be absorbed dermally following cosmetic application in humans (Mendelsohn et al. 2016, as cited in CIR 2018). A mean of 41% TPHP is retained in the lungs when inhaled at a flow rate of 18 L/minute. This retention rate is increased with increasing particle size and flow rate (Landhal et al. 1951, 1952, as cited in ATSDR 2012).
Carcinogenicity and genetic toxicity
No carcinogenicity studies were identified for TPHP. TPHP is not considered to be genotoxic based on negative evidence of mutagenicity in in vitro tests with Salmonella typhimurium, with and without metabolic activation (ATSDR 2012).
Repeated dose and reproductive/developmental toxicity
Two 4-month oral studies were available. Rats were exposed to TPHP at doses of 0, 161, 345, 517 or 711 mg/kg bw/day in diet. A statistically significant reduction in growth weight was detected at levels of 345 mg/kg bw/day and higher in Sobotka et al. (1986 as cited in OECD 2002b). This change was only observed at 711 mg/kg bw/day in Hinton et al. (1987 as cited in OECD 2002b). However, only limited data are reported and a number of standard parameters of repeated dose toxicity are missing, such as organ weight measurement and histopathology of organs other than lymphoid organs (spleen, thymus, lymph nodes) as well as hematology and clinical chemistry other than serum proteins. Based on the available data, the overall NOAEL is considered to be 161 mg/kg bw/day. TPHP had no effect in another 4-month fertility and developmental study up to the highest dose tested of 690 mg/kg bw/day (Welsh et al. 1987, as cited in OECD 2002b).
In a 90-day oral study, Wistar rats were fed TPHP at doses of 0, 20, 105 and 583 mg/kg bw/day for males and 0, 22, 117 and 632 mg/kg bw/day for females (ECHA c2007-2018c). Treatment-related increases in liver weight in the high dose groups (approximately 30% and 21% for males and females, respectively) were considered adverse in nature, although no supportive adverse histopathological changes were noted in the liver. The LOEL for this study was 105 mg/kg bw/day.
In a short-term oral study, Wistar rats were exposed in the diet to TPHP at doses of 0, 23.5, 161.4 or 701 mg/kg bw/day for 28 days (ECHA c2007-2018c). In males, decreased body weight gain was observed at 161.4 mg/kg bw/day. At 701 mg/kg bw/day, there was an increase in food consumption in both males and females. A statistically significant increase in liver weights was also observed at 701 mg/kg bw/day in both sexes. Males displayed a higher frequency of enlarged livers. This trend correlates with the occurrence of slight hypertrophy/cytoplasmic change of periportal hepatocytes observed in males at 161.4 mg/kg bw/day, compared to females at 701 mg/kg bw/day. A distinct change in liver function was also observed at 701 mg/kg bw/day in females. The NOAEL was established to be 161.4 mg/kg bw/day, based on decrease in body weight gain and liver effects at the next tested dose. In another study, only a slight depression of body weight gain and an increase of liver weights at a level of 350 mg/kg bw/day were observed after 35 days of treatment (Sutton et al. 1960, as cited in OECD 2002b).
In a short-term dermal study, rabbits were exposed to TPHP on clipped and intact or abraded skin at doses of 0, 100 or 1000 mg/kg bw/day for 3 weeks. No effect on body weight, hematology and clinical chemistry, organ weights or histopathology was observed. The only treatment-related effect was a dose-related depression (only statistically significant at 1000 mg/kg bw/day) of acetyl cholinesterase in plasma, erythrocytes and the brain of the TPHP treated rabbits, with no clinical signs of increased cholinergic activity. The toxicological relevance of these effects is unclear. No effects were observed in the reproductive organs of rabbits administered TPHP up to the highest dose of 1000 mg/kg bw/day. The NOAEL was considered to be 1000 mg/kg bw/day (Monsanto 1979, as cited in OECD 2002b).
No developmental or reproductive effects were observed in the 4-month oral fertility and developmental study in rats up to doses of 690 mg/kg bw/day (Welsh et al. 1987, as cited in OECD 2002b).
2.7.3.2 BPDP
Literature searches to identify health effects were conducted up to October 2018. An environmental risk evaluation report by the UK Environment Agency (EA 2009a) and a US EPA (2009) report were available for BPDP. Targeted literature searches were conducted up to March 2018. No new health effects studies that would impact the risk characterization (i.e., result in different critical endpoints or lower points of departure than those stated herein) were identified. The majority of studies carried out in the late 1970s and early 1980s were conducted on Phosflex 51B, a commercial mixture of phosphorus flame retardants, while more recent studies have been conducted on other commercial preparations, such as Durad 220B and Phosflex 61B. It is unclear how these commercial products differ in composition. The IUCLID (2001) and US EPA (2009) dossiers describe the compositions of these substances as containing 75% to 80% w/w BPDP and 20% to 25% w/w TPHP (EA 2009a).
Toxicokinetics
No information on absorption, distribution, metabolism and excretion of BPDP is available.
Carcinogenicity and genetic toxicity
No carcinogenicity studies were identified for BPDP. BPDP is not considered to be genotoxic based on available data as it did not induce mutations in in vitro mutagenicity studies and it did not induce chromosomal aberrations or sister chromosome exchanges in a mouse lymphoma L5178Y cytogenetic assay (EA 2009a).
Repeated dose toxicity
In a subchronic oral study, Sprague-Dawley (SD) rats were fed Phosflex 51B for 90 days at doses corresponding to 0, 6.6, 26.7 and 107.5 mg/kg bw/day for males and 0, 7.7, 30.0 and 124.8 mg/kg bw/day for females. At the highest dose, a significant increase in absolute and relative liver weights in both sexes, a significant increase in kidney weights in males, and an increase in adrenal glands weights in females were observed. However, there was no corresponding increase in histopathological changes in these organs. A LOEL of 107.5 mg/kg bw/day was established for this study (Freudenthal et al. 2001; EA 2009a).
No short-term oral or dermal repeat dose toxicity studies were identified for BPDP. No systemic toxicity could be observed in a 15-day developmental toxicity study or an 8-week reproductive study up to doses of 1000 mg/kg bw/day (Experimur 2003; Stauffer Chemical Company 1981, both cited in EA 2009a). The only effect observed, increased liver weight at the highest dose in the 8-week study, was considered by the authors (Stauffer Chemical Company 1981, as cited in EA 2009a) to be an adaptive response (enzyme induction) rather than due to the toxicity of the compound. No histopathology examination of the liver was performed.
Reproductive and developmental toxicity
In an oral reproductive study, SD rats were exposed by gavage to Phosflex 61B (purity not stated) at doses of 0, 50, 250 or 1000 mg/kg bw/day for 8 weeks (including 2 weeks prior to mating, a 2-week mating period, and through gestation and lactation, for a total of 8 weeks). No changes in clinical signs of toxicity, food consumption, body weight or organ weights were observed, and no treatment-related histological changes were observed in the reproductive organs. There were no significant differences in litter size or in the number of live pups (Experimur 2003, as cited in EA 2009a).
In another oral developmental study, female pregnant rats were exposed to Phosflex 51B at doses of 0, 100, 400 or 1000 mg/kg bw/day from gestation day (GD) 6 to 20. Reduced body weights of dams and fetuses (8%) in the high-dose group, possibly due to reduced food consumption, was observed. A significant dose-related increase in absolute and relative liver weights was also observed in all treatment groups, which was considered by the authors to be an adaptive response (enzyme induction). The NOAEL for developmental toxicity is 1000 mg/kg bw/day (Stauffer Chemical Company 1981, as cited in EA 2009a).
No dermal reproductive toxicity studies were identified for BPDP.
2.7.3.3 IDDP
Literature searches to identify health effects were conducted up to March 2018. An environmental risk evaluation report by the UK Environment Agency (EA 2009c) was available for IDDP. No health effect studies that would impact the risk characterization (i.e., result in different critical endpoints or lower points of departure than those stated herein) were identified.
Toxicokinetics
IDDP can be absorbed after oral and respiratory exposure, but the amount of absorption cannot be predicted (ECHA c2007-2018e). No information is available on the distribution, metabolism or excretion of this substance.
Carcinogenicity and genetic toxicity
No carcinogenicity studies were available for IDDP. IDDP is not considered to be genotoxic (EA 2009c).
Repeated dose toxicity
In a short-term oral study with limited reporting, rats were exposed to IDDP at doses of 3, 9, 30, 90 or 900 mg/kg bw/day in the diet for 28 days (Monsanto 1984 cited in EA 2009c). Decreased serum cholinesterase was observed at 90 mg/kg bw/day, while hypertrophy of liver centrilobular cells, cholangitis of the liver, as well as an increase in total cholesterase and cholesterol were observed at 900 mg/kg bw/day. The NOAEL was considered to be 90 mg/kg bw/day. In another 28-day oral study, a LOAEL of 250 mg/kg bw/day was derived based on an enlarged liver observed at all doses (Monsanto 1979a, as cited in EA 2009c).
In a subchronic oral study, SD rats were administered IDDP in the diet for 90 days at doses of 9.3, 93.8 or 465 mg/kg bw/day for males and 11.1, 110 or 533 mg/kg bw/day for females. A statistically significant decrease in body weight and food consumption was observed in both sexes at the high-dose level. An increase in liver weights was also observed in mid- and high-dose animals. The combined incidence of hepatocellular hypertrophy and/or hyperplasia increased in a dose-related manner in both sexes. High-dose females also demonstrated an increase in hepatocellular brown pigment. Hematological and biochemical parameters were also altered in all treatment groups in both sexes. At terminal sacrifice, hematological changes included increased hematocrit, decreased hemoglobin and increased red blood cell count in both sexes and reduced white blood cell count and increased platelet count in females. The LOAEL for systemic toxicity was considered to be 9.3 mg/kg bw/day, which was the lowest dose tested. No NOAEL could be derived (Monsanto 1983b, as cited in EA 2009c).
Reproductive and developmental toxicity
In an oral developmental study, SD rats were exposed to IDDP at doses of 300, 1000 or 3000 mg/kg bw/day on days 6 to 15 of gestation. A dose-related decrease in body weight was observed in dams at 1000 and 3000 mg/kg bw/day. The maternal NOAEL was 300 mg/kg bw/day. The developmental NOAEL was 3000 mg/kg bw/day (Monsanto 1980, as cited in EA 2009c). In a similar study, Robinson et al. (1986) also determined a fetal NOAEL of 3000 mg/kg bw/day based on no effects on fetal body weight, on fetal sex ratio or on the number of live and dead fetuses.
2.7.3.4 IPPP
Literature searches to identify health effects were conducted up to October 2018. An environmental risk evaluation report by the UK Environment Agency (EA 2009b) and a US EPA screening-level hazard characterization report (2010) were available for IPPP. A registration dossier submitted to ECHA (ECHA c2007-2018d) was also available. Available health effect studies that would impact the risk characterization are presented below. IPPP is a commercially produced mixture of isopropylated triphenyl phosphate isomers with an unspecified number of isopropyl groups, and it covers a range of substances with differing degrees of alkylation. The test substance composition is not always clearly described in the available literature.
Toxicokinetics
No information on absorption, distribution, metabolism or excretion of IPPP is available.
Carcinogenicity and genetic toxicity
No carcinogenicity studies were identified for IPPP. IPPP is not considered genotoxic (EA 2009b).
Repeated dose toxicity
In a 91-day gavage study, where Reofos 35Footnote 6 was administered to rats at 0, 25, 100 or 325 mg/kg/day, no NOAEL was identified and the LOAEL was considered to be 25 mg/kg/day based on adverse histopathological changes observed in the adrenal glands at all dose levels. Macroscopic and organ weight changes in the adrenal glands correlated to diffuse vacuolation of the zona fasciculata and were characterized by enlarged cells with foamy cytoplasm. In addition, the zona fasciculata cells present near the zona reticularis had large single clear vacuoles that expanded outwardly depending on severity. At recovery, the diffuse vacuolation was not present; however, cells near the zona reticularis had large single cytoplasmic vacuoles (identified as increased vacuolation) (ECHA c2007-2018d).
In a short-term developmental study described below, the LOAEL for parental systemic toxicity from oral exposure to Reofos 65Footnote 7 was considered to be 25 mg/kg/day. Effects were observed on food consumption (females only), organ weights, hematology and/or serum chemistry. At dose levels of 25 mg/kg/day and above, mean adrenal gland weights in both males and females and liver weights in males were increased. The effects noted on adrenal gland and liver weights corresponded to the increased severity of macroscopic and histopathological findings for these organs (pale and/or enlarged adrenal glands and adrenal cortex vacuolization and centrilobular hepatocellular hypertrophy) (ECHA c2007-2018d).
In two briefly reported 4-week dermal studies, Kronitex 50 (at 100, 500 or 2000 mg/kg bw/day) or Reolube HYD 46 (a commercial IPPP product) (at 40, 200 or 1000 mg/kg bw/day) was applied to the shaved skin of rats for 6 hours per day, 5 days a week, for 4 weeks (EA 2009b). In the Kronitex study, increased adrenal weights were noted in mid- and high-dose males (no further details). Histopathological examination revealed slight fatty change of the adrenal cortex in males given 500 mg/kg bw/day (2/5) and 2000 mg/kg bw/day (3/5) Kronitex 50. Based on the limited information provided, the NOAEL for Kronitex 50 was 100 mg/kg bw/day. For Reolube HYD 46, slightly higher absolute and relative adrenal weights were noted in treated animals, but this finding was reported not to correlate with any microscopic findings (no further details given; EA 2009b). Lower testicular weights were also noted in high-dose males, while histopathology showed slight testicular tubular atrophy in control and treated rats (no further details). Based on the limited information available, a NOAEL of 200 mg/kg bw/day was established for Reolube HYD 46. Therefore, the overall NOAEL for dermal exposure is considered to be 200 mg/kg bw/day.
In a subchronic inhalation study using MIL-H-19457B (Durad MP280) as the test substance (no details on composition or purity), rats, hamsters and rabbits were exposed to doses of 10 and 100 mg/m3 via hydraulic fluid aerosol on a continuous basis for 90 days. General toxicity was evidenced by effects on organ weights, hematology and/or serum chemistry. A no observed adverse effect concentration (NOAEC) could not be established (ECHA c2007-2018d).
Reproductive and developmental toxicity
In an oral reproductive study, Crl:CD(SD) IGS BR rats were exposed to Reofos 65 by gavage at doses of 0, 25, 100 or 400 mg/kg bw/day. Rats were exposed to Reofos 65 for 15 days prior to pairing. Males received the test article throughout the mating period and through the day prior to necropsy for a total of 29 doses. Females received the test article through lactation day 4, post-mating day 25 or post-cohabitation day 25, for a total of 41 to 54 doses (ECHA c2007-2018d). At dose levels of 25 mg/kg bw/day and above, parental effects included increased ovary/oviduct, adrenal gland (males and females) and liver (males only) weights and decreased epididymal weights. Corresponding macroscopic and/or microscopic changes in these tissues were generally noted. Male and female reproductive performance was also adversely affected at dose levels of 100 and 400 mg/kg bw/day, manifested by significant reductions in fertility and copulation/conception indices. Early postnatal development was also affected at dose levels of 100 and 400 mg/kg bw/day. The number of pups born and live litter size were decreased in these groups, while the numbers of pups found dead or euthanized in extremis were increased; all pups from five of six litters in the 400 mg/kg/day group were either found dead or euthanized in extremis prior to postnatal day (PND 4). Based on the results of this study, the NOAEL for male and female reproductive performance was 25 mg/kg bw/day. The NOAEL for neonatal toxicity was considered to be 25 mg/kg bw/day (ECHA c2007-2018d).
In an oral developmental study, CD female rats were administered Reofos 35 by gavage at doses of 0, 100, 200 or 400 mg/kg bw/day once daily from GD 0 to 19. A statistically significant decrease in body weight gain was observed at 400 mg/kg bw/day. This decrease correlated with a test material-related decrease in food consumption and was considered adverse. Red or white foci and swollen mucosa of the nonglandular portion of the stomach were observed at the high dose. Reofos 35 was not found to be teratogenic. The NOAEL was established at 200 mg/kg bw/day for maternal toxicity and 400 mg/kg bw/day for developmental toxicity (ECHA c2007-2018d).
In a 28-day dermal study in which RIAF rats were treated with 40, 200 or 1000 mg/kg bw/day of Reolube HYD 46, a decrease in testicular weight was observed in males receiving 1000 mg/kg bw/day. Microscopic examination of the testes showed slight tubular atrophy in both controls and treated groups. The NOAEL was determined to be 200 mg/kg bw/day (ECHA c2007-2018d).
2.7.4 Characterization of risk to human health of the aryl OP subgroup (TPHP, BPDP, IPPP, IDDP)
2.7.4.1 TPHP
No carcinogenicity studies were identified for TPHP. TPHP is not expected to be genotoxic. Based on the available studies, the NOAEL for repeated oral exposure to TPHP of 161 mg/kg bw/day for decreased body weight and liver effects (ECHA c2007-2018c; OECD 2002b) was selected as the critical effect level to characterize the risk to human health from oral exposures to TPHP. No developmental toxicity was observed up to a dose of 690 mg/kg bw/day (Welsh et al. 1987, as cited in OECD 2002b). No adverse effects were observed after a 3-week dermal exposure to TPHP (Monsanto 1979, as cited in OECD 2002b). The NOAEL from this study was used to characterize the risk to human health from per-event or intermittent dermal exposures to TPHP. Given the duration of the dermal study, the oral NOAEL for repeated dose was also considered when determining the adequacy of the calculated margins of exposure (MOEs).
Table 2‑25 provides all relevant exposure estimates and hazard points of departure as well as the resultant margins of exposure (MOEs) for the characterization of risk for exposures to TPHP.
| Exposure scenario | Systemic exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Environmental media and food | 0.00014 – 0.0058 mg/kg bw/day | NOAEL = 161 mg/kg bw/day | Decreased body weight gain in 4-month oral studies | 27 759–1 150 000 |
| Mouthing foam in toys and children’s products (oral) | 1.33x10-5 – 2.47x10-5 mg/kg bw/day (0–6 months) | NOAEL = 161 mg/kg bw/day | Decreased body weight gain in 4-month oral studies | > 1 million |
| Mouthing foam in toys and children’s products (oral) | 1.29x10-5 – 2.39x10-5 mg/kg bw/day (0.5–4 years) | NOAEL = 161 mg/kg bw/day | Decreased body weight gain in 4-month oral studies | > 1 million |
| Children’s foam-containing furniture or mattresses (dermal) | 0.021–0.13 mg/kg bw/day (0–6 months) | NOAEL = 1000 mg/kg bw/day | No adverse effects in 3-week dermal study | 7 692–47 619 |
| Children’s foam-containing furniture or mattresses (dermal) | 0.015–0.10 mg/kg bw/day (0.5–4 years) | NOAEL = 1000 mg/kg bw/day | No adverse effects in 3-week dermal study | 10 000–66 667 |
| Foam containing furniture or mattresses (dermal) | 5.6×10-3 – 4.7×10-2 mg/kg bw/day (20+ years) | NOAEL = 1000 mg/kg bw/day | No adverse effects in 3-week dermal study | 21 277–178 571 |
| Nail polish (dermal) | 0.84–0.99 mg/kg-bw per event | NOAEL = 1000 mg/kg bw/day | No adverse effects in 3-week dermal study | 1 010–1 190 |
Abbreviations: NOAEL, no observed adverse effect level.
The calculated MOEs are considered adequate to address uncertainties in the health effects and exposure databases.
2.7.4.2 BPDP
No carcinogenicity studies were identified for BPDP. BPDP is not expected to be genotoxic. Based on the available studies, a LOEL for repeated oral exposure to BPDP of 107.5 mg/kg bw/day based on organ weight changes (liver, kidney and adrenal glands) in a 90-day study conducted with a mixture of BPDP and TPHP (Freudenthal et al. 2001) was considered to be the most relevant endpoint for characterization of human health risk from exposure to BPDP. No oral or dermal short-term studies were identified for BPDP. In the two reproductive and developmental studies, no adverse systemic effects could be observed up to the dose of 1000 mg/kg bw/day (Experimur 2003; Stauffer Chemical Company 1981, both cited in EA 2009a). No developmental or reproductive effect was observed in these studies. The LOEL of 107.5 mg/kg bw/day has been selected as the critical effect level to characterize the risk to human health from environmental media and foam-containing manufactured items.
Table 2‑26 provides all relevant exposure and hazard points of departure as well as the resultant MOEs for the characterization of risk for exposures to BPDP.
| Exposure scenario | Systemic exposure | Critical effect levelb | MOE |
|---|---|---|---|
| Environmental media | 1.72×10-6 – 2.90×10-4 mg/kg bw/day | LOEL = 107.5 mg/kg bw/day | 370 690–62 500 000 |
| Mouthing foam in children’s products (oral) | 1.11×10-5 – 1.81×10-5 mg/kg bw/day (0–6 months) | LOEL = 107.5 mg/kg bw/day | > 1 million |
| Mouthing foam in children’s products (oral) | 1.07×10-5 – 1.75×10-5 mg/kg bw/day (0.5–4 years) | LOEL = 107.5 mg/kg bw/day | > 1 million |
| Children’s foam-containing furniture or mattresses (dermal)a | 0.018–0.098 mg/kg bw/day (0–6 months) | LOEL = 107.5 mg/kg bw/day | 1 097–5 972 |
| Children’s foam-containing furniture or mattresses (dermal)a | 0.012–0.074 mg/kg bw/day (0.5–4 years) | LOEL = 107.5 mg/kg bw/day | 1 453–8 958 |
| Foam-containing mattresses (dermal)a | 0.005–0.034 mg/kg bw/day (20+ years) | LOEL = 107.5 mg/kg bw/day | 3 162–21 500 |
Abbreviations: LOEL, lowest observed effect level
a Assumed dermal absorption is equivalent to that of oral.
b Organ weight changes (liver, kidney and adrenal glands in 90-day).
The calculated MOEs are considered adequate to address uncertainties in the health effects and exposure databases.
2.7.4.3 IDDP
No carcinogenicity studies were identified for IDDP. IDDP is not expected to be genotoxic. No NOAEL for systemic effects could be identified in a subchronic oral repeated dose study (Monsanto 1983b, as cited in EA 2009c). Liver histological changes were observed in both sexes of rats, as well as a decrease in body weight and food consumption. Hematological and biochemical parameters were also altered in all treatment groups in both sexes. At terminal sacrifice, hematological changes included increased hematocrit, decreased hemoglobin and increased red blood cell count in both sexes and reduced white blood cell count and increased platelet count in females. The LOAEL for systemic toxicity was considered to be 9.3 mg/kg bw/day (Monsanto 1983b, as cited in EA 2009c).
No dermal studies were available for IDDP. The NOAEL of 300 mg/kg bw/day based on a dose-related decrease in body weight from the oral developmental study was used to characterize the risk to human health from short-term dermal exposure to IDDP from the use of marine paint.
Table 2‑27 provides all relevant exposure estimates and hazard points of departure as well as the resultant margins of exposure (MOEs) for the characterization of risk for exposures to IDDP.
| Exposure scenario | Systemic exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Environmental media | 3.84×10-5 – 3.26×10-4 mg/kg bw/day | LOAEL = 9.3 mg/kg bw/day | Liver histological changes, decreased body weight and food consumption and hematological changes | 28 528–242 188 |
| Marine paint (dermal)a | 2.2 mg/kg bw/day | NOAEL= 300 mg/kg bw/day | Dose-related decrease in body weight | 136 |
Abbreviations: LOAEL, lowest observed adverse effect level; NOAEL, no observed adverse effect level
a Assumed dermal absorption is equivalent to that of oral.
On the basis of the conservative approaches used in estimating exposures to IDDP from environmental media and from products available to consumers, the calculated MOE is considered adequate to address any uncertainties in the health effects and exposure databases.
2.7.4.4 IPPP
No carcinogenicity studies were identified for IPPP. IPPP is not expected to be genotoxic (EA 2009b). Based on the available studies, no NOAEL was identified. In a 91-day oral study, adverse histopathological changes were observed in the adrenal glands at the lowest dose tested (25 mg/kg bw/day), and in a short-term developmental and reproductive toxicity study, effects were observed on the adrenal glands and liver in parental animals (ECHA c2007-2018d). Developmental effects were also observed at higher doses, starting at 100 mg/kg bw/day. The dermal studies that were available were limited in their reporting and therefore were not used to characterize the risk to human health from exposures to IPPP from foam-containing furniture. A LOAEL of 25 mg/kg bw/day was therefore considered to be the most relevant endpoint to characterize the risk to human health from all potential exposures to IPPP.
Table 2‑28 provides all relevant exposure and hazard points of departure as well as the resultant MOEs for the characterization of risk for exposures to IPPP.
| Exposure scenario | Systemic exposure | Critical effect levelb | MOE |
|---|---|---|---|
| Environmental media and food | 1.4×10-4 – 5.79×10-3 mg/kg bw/day | LOAEL = 25 mg/kg bw/day (LDT) | 4 318–178 571 |
| Mouthing foam in children’s products (oral) | 1.08×10-5 – 2.47×10-5 mg/kg bw/day (0–6 months)a | LOAEL = 25 mg/kg bw/day (LDT) | > 1 million |
| Mouthing foam in children’s products (oral) | 1.04×10-5 – 2.39×10-5 mg/kg bw/day (0.5–4 years)a | LOAEL = 25 mg/kg bw/day (LDT) | > 1 million |
| Children’s foam-containing furniture or mattresses (dermal)c | 0.0052–0.040 mg/kg bw/day (0–6 months)a | LOAEL = 25 mg/kg bw/day (LDT) (oral) | 625–4 808 |
| Children’s foam-containing furniture or mattresses (dermal)c | 0.0036–0.030 mg/kg bw/day (0.5–4 years)a | LOAEL = 25 mg/kg bw/day (LDT) (oral) | 833–6 944 |
| Children’s foam-containing furniture or mattresses (dermal)c | 0.0024–0.021 mg/kg bw/day (5–11 years)a | LOAEL = 25 mg/kg bw/day (LDT) (oral) | 1 190–10 417 |
| Foam-containing furniture or mattresses (dermal)c | 0.0020–0.019 mg/kg bw/day (12–19 years)a | LOAEL = 25 mg/kg bw/day (LDT) (oral) | 1 316–12 500 |
| Foam-containing furniture or mattresses (dermal)c | 0.0014–0.014 mg/kg bw/day (20+ years)a | LOAEL = 25 mg/kg bw/day (LDT) | 1 786–17 857 |
| Foam in infant restraint seats (dermal)c | 5.1×10-4 – 1.2×10-3 mg/kg bw/day (0–6 months)a | LOAEL = 25 mg/kg bw/day (LDT) | 20 833–49 020 |
| Foam in child restraint seats (dermal)c | 4.1×10-4 – 9.4×10-4 mg/kg bw/day (0.5–4 years)a | LOAEL = 25 mg/kg bw/day (LDT) | 26 596–60 976 |
Abbreviations: LOAEL, lowest observed adverse effect level; LDT, lowest dose tested
a Range of exposure estimates represents exposure to T3IPPP isomer (lowest exposure) and TPHP (highest exposure). See Appendix G for more details.
b Adverse histopathological changes observed in the adrenal glands at all dose levels in a 91-day study as well as adrenal glands and liver effects in a reproductive and developmental study.
c An adjusted dermal absorption of 30% for TDCPP (EU RAR 2008b) was used to represent dermal absorption of IPPP.
MOEs for exposure to IPPP in environmental media and food, mouthing foam toys/products, sitting in infant or child restraint seats as well as exposure to IPPP from lying on foam-containing mattresses for children, teens and adults are considered adequate to address any uncertainties in the health effects and exposure databases. The calculated MOEs from prolonged skin contact with IPPP from lying on foam-containing furniture or mattresses for infants and children are considered potentially inadequate to account for uncertainties in the databases.
2.7.5 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| IPPP is a UVCB with varying proportions of isomers in different blends, making it difficult to model exposures or to identify studies measuring concentrations of IPPP or one of its components. | +/- |
| Limited data measuring TPHP in foods sold in Canada were available. Data from other countries were used to derive exposure estimates. | +/- |
| The maximum concentrations reported in a given food item were assumed to be representative of a broader category as a whole. | + |
| The conservative dietary exposure estimates for TPHP were applied to IPPP. | + |
| Limited data measuring TPHP, BPDP and IPPP in foam-containing mattresses and/or furniture sold in Canada. Data from other countries were used to support derivation of exposure estimates. | +/- |
| Limited data on reported uses of IDDP in Canada given high detection frequencies in homes. | +/- |
| Absence of data on migration of TPHP, BPDP, and IPPP out of foam materials. | +/- |
| Dermal absorption data for TPHP, BPDP, and IPPP were limited and therefore an analogue approach was used or 100% dermal absorption was assumed. | +/- |
| There are no chronic toxicity studies for TPHP, BPDP, IPPP or IDDP, and the overall database on BPDP and IPPP is limited. | +/- |
| No data were available on dermal (BPDP, IPPP, IDDP) or inhalation toxicity (TPHP). | +/- |
| Use of commercial mixture containing various quantities of the assessed chemical and unknown purity in toxicological studies (BPDP and IPPP) | +/- |
3. Assessment of the alkyl organophosphate subgroup (TEP, TBOEP, TEHP, BEHP)
3.1 Identity of substances
The four substances of the alkyl organophosphate (OP) subgroup assessed in this screening assessment are organophosphorus flame retardants with the general formula shown in Figure 3‑1.

The CAS RN, DSL names and common names, and/or acronyms for the individual substances in the alkyl OP subgroup are presented in Table 3‑1. A list of additional chemical names (e.g., trade names) is available from the National Chemical Inventories (NCI 2015).
|
CAS RN (abbreviation) |
DSL name (common name) |
Representative chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
|
78-40-0 (TEP) |
phosphoric acid, triethyl ester (triethylphosphate; triethyl phosphate) |
 C6H15O4P C6H15O4P
|
182.15 |
|
78-51-3 (TBOEP) |
ethanol, 2-butoxy-, phosphate (3:1) (tris(2-butoxethyl) phosphate) |
 C18H39O7P C18H39O7P
|
398.47 |
|
78-42-2 (TEHP) |
phosphoric acid, tris(2-ethylhexyl) ester (tris(2-ethylhexyl) phosphate) |
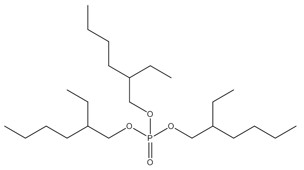 C24H51O4P C24H51O4P
|
434.64 |
|
298-07-7 (BEHP) |
phosphoric acid, bis(2-ethylhexyl) ester (bis(2-ethylhexyl) phosphate; bis (2-ethylhexyl) hydrogen phosphate |
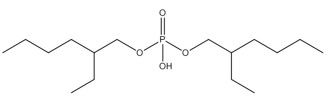 C16H35O4P C16H35O4P
|
322.42 |
Abbreviations: CAS RN, Chemical Abstracts Service Registry Number; DSL, Domestic Substances List
3.1.1 Selection of analogues and use of (Q)SAR models
In the human health risk assessment, a read-across approach using data from an analogue has been used in the exposure assessment of TBOEP. The substance 2-propanol, 1-chloro-, phosphate (3:1), herein referred to as TCPP, was selected as an analogue for dermal absorption based on structure, functional similarity and data availability, as limited data on dermal absorption were available for TBOEP. Like TBOEP, TCPP is an organophosphate ester and has three alkyl groups. TCPP is a halogenated organophosphate ester, however, and each alkyl group contains one chlorine atom. TCPP is commonly used as an additive flame retardant and a plasticizer. More details of the read-across approach are discussed in section 3.7.1.2. Information on the identity and chemical structure of TCPP is presented in Table 3‑2.
|
CAS RN (abbreviation) |
DSL name | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
|
13674-84-5 (TCPP) |
2-propanol, 1-chloro-, phosphate (3:1) | 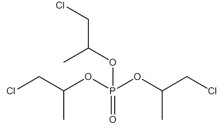 C9H18Cl3O4P C9H18Cl3O4P
|
327.57 |
Abbreviations: CAS RN, Chemical Abstracts Service Registry Number; DSL, Domestic Substances List
3.2 Physical and chemical properties
A summary of physical and chemical property data of the substances in the alkyl OP subgroup is presented in Table 3‑3. When experimental information was limited or not available for a property, data from the quantitative structure activity relationship (QSAR) models were used to generate predicted values for the substance. Substance-specific physical and chemical information is provided in ECCC 2020e.
| Property | TEP | TBOEP | TEHP | BEHP |
|---|---|---|---|---|
| Melting point (°C) |
-56.4 c (experimental) |
-70 l (experimental) |
-74 g (experimental) |
-50 k (not specified) |
| Vapour pressure (Pa) |
5.2x10+01 at 25 °C a (calculated) |
6.14x10-06 b,l (calculated) |
1.1x10-5 at 25 °C h (experimental) |
4.65x10-08 at 25 °C a (calculated) |
| Henry’s law constant (Pa·m3/mol) |
3.60x10-08 at 20 °C d (experimental) |
3.09x10-06 b (calculated) |
7.96x10-03 at 25 °C i (not specified) |
4.15x10-03 at 25 °C d (calculated) |
| Water solubility (mg/L) |
5.00x10+05 at 25 °C d (experimental) |
793 b,m (calculated) |
0.6 at 24 °C d (experimental) |
182 at 25 °C d (experimental) |
| log Kow (dimensionless) |
0.8 d (experimental) |
3.87 b,l (calculated) |
9.49 a (estimated) |
6.07 a (calculated) |
| log Koc (dimensionless) |
1.81 f (not specified) |
2.90 a (calculated) |
6.87 e (not specified) |
4.23 f (not specified) |
| log Koa (dimensionless) |
2.4 at 25 °C j (calculated) |
12.77 b,a (calculated) |
8.5 at 25 °C j (calculated) |
11.18 a (calculated) |
a EPI Suite c2000-2012.
b The value was least-squares adjusted according to Schenker et al. 2005.
c Lide 2005.
d TOXNET 2015.
e Dandan et al. 2017.
f PubChem 2018.
g Lewis 2012.
h Hinckley et al. 1990.
I J-CHECK 2014.
j Kanazawa et al. 2010.
k Verschueren 2001.
l WHO 2000.
m MITI 1992.
3.3 Sources and uses
The substances within the alkyl OP group are commercially produced and do not occur naturally in the environment. All of the substances in the alkyl OP subgroup were included in a recent survey issued pursuant to section 71 of CEPA (Canada 2012). Table 3‑4 presents a summary of information reported on the total manufacture and total import quantities for the reporting year of 2011 for the alkyl OP subgroup.
| Common name | Total manufacturea (kg) | Total importsa (kg) |
|---|---|---|
| TEP | 0 | 100 000–1 000 000 |
| TBOEP | 1 000–10 000 | 10 000–100 000 |
| TEHP | 0 | 10 000–100 000 |
| BEHP | 1 000–10 000 | 10 000–100 000 |
a Values reflect quantities reported for 2011 in response to the survey conducted under section 71 of CEPA (Canada 2012). See survey for specific inclusions and exclusions (schedules 2 and 3).
Table 3‑5 presents a summary of the major uses of the alkyl OP subgroup according to information reported in response to the CEPA section 71 survey (Environment Canada 2013). Table 3‑6 presents additional uses identified in Canada.
| Major usesa | TEP | TBOEP | TEHP | BEHP |
|---|---|---|---|---|
| Adhesives and sealants | Y | N | N | N |
| Paints and coatings | Y | Y | Y | N |
| Building or construction materials | Y | N | N | N |
| Lubricants and greases | N | N | Y | Y |
| Floor coverings | N | Y | Y | N |
| Industrial use | N | N | N | Y |
Abbreviations: Y = yes this use was reported for this substance; N = no this use was not reported for this substance.
a Non-confidential uses reported in response to the survey conducted under section 71 of CEPA (Environment Canada 2013). See survey for specific inclusions and exclusions (schedules 2 and 3).
| Use | TEP | TBOEP | TEHP | BEHP |
|---|---|---|---|---|
| Food packaging materialsa | Y | Y | Y | N |
| Formulant in pest control products registered in Canadab | N | N | Y | N |
Abbreviations: Y = yes this was reported for this substance; N = no this was not reported for this substance
a Personal communication, e-mail from Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated January 11, 2017; unreferenced).
b Personal communication, e-mail from Pest Management Regulatory Agency, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated February 8, 2018; unreferenced).
In general, TEP, TBOEP and TEHP are most notably used as flame retardants and plasticizers, whereas BEHP is used as a lubricant and an extreme pressure additive in metalworking fluids, among other applications (Ash and Ash 2009). Globally, TBOEP is also commonly used in floor polishes and as a plasticizer in rubber and plastics (WHO 2000). The alkyl diphenyl phosphate products were originally developed to improve low-temperature flexibility in PVC, relative to triaryl phosphates (EA 2009a; Weil 1993).
In Canada, TEP may be used as a component in the manufacture of a limited number of laminated films for food packaging materials, TBOEP may be used as a component in the manufacture of a limited number of adhesives used in the middle layers of food packaging materials and TEHP may be used as a component in the manufacture of printing inks (personal communication, e-mail from Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated January 11, 2017; unreferenced). In the United States, TEP, TBOEP and TEHP are permitted as components of adhesives intended for use in food packaging materials, TBOEP and TEHP are permitted as components of defoaming agents used in the manufacture of paper and paperboard and TEHP is also permitted as a component of paper and paperboard in contact with dry food (US CFR 2017a,b).
In Canada, TEHP, TEP and TBOEP can be used as formulants in pest control products and are currently registered in a few products for applications for domestic and/or commercial use (personal communication, e-mail from Pest Management Regulatory Agency, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated February 8, 2018; unreferenced).
3.4 Releases to the environment
Releases of TBOEP to the environment can occur through losses during manufacture, industrial use, consumer or commercial use, service life and disposal of a substance. TBOEP usage by industrial facilities in Canada is likely to result in point source releases to the environment. Releases of TBOEP to the Canadian environment from its consumer use in a variety of products are expected to be diffuse. Dispersive releases from commercial products and products available to consumers occur in both indoor and outdoor environments. Although TBOEP was previously reported to be manufactured in Canada, current available information indicates that this substance is not being manufactured in Canada at this time (Environment Canada 2013).
Releases to the environment occur primarily to the water compartment via wastewater. Release to soil occurs through the application of biosolids containing TBOEP to agricultural and pasture lands (Yager et al. 2013, 2014).
3.5 Environmental fate and behaviour
3.5.1 Environmental distribution
Table 3‑7 presents the range in results of Level III fugacity modelling for TBOEP. The modelling results indicate that when released to air, TBOEP will largely partition to soil, with a minor percentage (less than 1%) to sediment and less than 3% to water. When released to water, TBOEP will distribute to water (84%) with approximately 16% partitioning to sediment, and less so to soil or air (less than 1%). When released to soil, TBOEP will remain almost completely in soil (99.7%).
| Environmental medium | Air (%) | Water (%) | Soil (%) | Sediment (%) |
|---|---|---|---|---|
| Air (100%) | < 1 | 2.8 | 96.7 | < 1 |
| Water (100%) | < 1 | 84.4 | < 1 | 15.6 |
| Soil (100%) | < 1 | < 1 | 99.7 | < 1 |
a Physical and chemical properties and environmental half-lives (t1/2) of TBOEP in environmental media are required for modelling and are listed in ECCC 2020e.
3.5.2 Persistence
3.5.2.1 Abiotic and biotic degradation
Based on the likely releases and partitioning characteristics of TBOEP, environmental persistence is most relevant for the water, sediment and soil compartments. Empirical and modelled data were considered in the weight of evidence for persistence for this substance (Table 3‑8).
Modelled predictions for TBOEP in air suggest a half-life of less than 1 day (gas phase) and or an overall persistence (Pov) of 137 days (OECD Pov and LRTP Screening Tool 2009a). However, TBOEP has been reported to be frequently detected in ambient airborne particles, including in the Great Lakes atmosphere (51 to 262 pg/m3) (Salamova et al. 2014).
Results from the HYDROWIN model (2010) suggest that the rate of hydrolysis for TBOEP is relatively steady at pH ranging from 5 (95.46 days) to 9 (92.87 days). However, it increases at pH 10 (74.67 days). The half-life for the more environmentally-relevant pH of 8 (pH 8.2 in natural water of Lake Ontario) (Howard and Deo 1979) of 95.2 days was used in this assessment to predict the fate of TBOEP in water. Andresen et al. (2007) estimated in situ half-lives for TBOEP based on dilution-corrected concentrations reported for surface water of the German Bight (North Sea). The authors reported half-lives for TBOEP of less than 14 days (range of 8 to 30 days considering uncertainty in flows and determination of concentrations).
Cao et al. (2017) reported on sediment cores from Lake Michigan with measured TBOEP concentrations at depths from 0.5 cm to 29.5 cm. The analysis indicated inputs of TBOEP since as early as 1860. Core depths associated with more recent deposition showed that concentrations of TBOEP remained relatively constant, with the highest concentrations of 19.34 and 14.98 ng/g dw recorded at depths of 8.5 and 0.5 cm in the core, respectively. The former corresponds to TBOEP deposition in 1968, and the latter corresponds to TBOEP deposition in 2009. The measurement of TBOEP at depths indicate the potential for this substance to persist in sediments, potentially under anaerobic conditions.
Quintana et al. (2006) conducted an aerobic laboratory degradation test with activated sludge as inoculum and powdered milk as additional carbon source and found that 16 days was required for the complete removal of TBOEP with a half-life of approximately 5 days. The researchers concluded that microbial degradation of trialkyl phosphates starts with the microbial hydrolysis of one of the ester linkages to form dialkyl phosphates, which are then further degraded along a yet unknown pathway.
The CATALOGIC model (2014) predicts that 7% of TBOEP biodegrades under aerobic conditions within 28 days and estimates a primary half-life of 70 days. Biodegradation was further predicted using BIOWIN 3 and BIOWIN 4 models, which also suggest that TBOEP is not persistent in water. While this is not consistent with predictions from BIOWIN 5 or BIOWIN 6 (TBOEP is not predicted to biodegrade quickly by these submodels), the overall results generally support fast initial primary degradation, but slower ultimate biodegradation.
The results from ERC (ECCC 2016b) indicate that the other three substances in the alkyl OP subgroup (TEP, TEHP and BEHP) are not considered persistent.
While there is some variability among the results with respect to biodegradation modelling, overall there is sufficient weight of evidence that TBOEP undergoes significant degradation within 182 days and is therefore not considered persistent in water. Application of a half-life extrapolation procedure based on Boethling et al. (1995) using a ratio of 1:1:4 for water:soil:sediment suggests that TBOEP will break down in soil and sediment and will not present a long-term exposure in these media.
| Fate process | Test method or model basis | Degradation endpoint or prediction | Reference |
|---|---|---|---|
| Atmospheric oxidation | Expert system | Half-life: 1.994a | AOPWIN 2010a,b |
| Ozone reaction | Expert system | NA | AOPWIN 2010b |
| Hydrolysis | Expert system |
Half-life: 95.46 days (pH 6) 95.43 days (pH 7) 95.2 days (pH 8) |
HYDROWIN 2010b |
| Aerobic primary biodegradation, water | Sub-model 4: Expert system |
Value = 4.5162 “biodegrades fast” (hours-days) |
BIOWIN 2010b |
| Aerobic ultimate biodegradation, water | Sub-model 3: Expert system |
Value of 3.3413 “biodegrades fast” (days-weeks) |
BIOWIN 2010b |
| Aerobic biodegradation, water | Sub-model 5: MITI linear probability |
Value of 0.4283 “does not biodegrade fast” |
BIOWIN 2010b |
| Aerobic biodegradation, water | Sub-model 6: MITI nonlinear probability |
Probability value of 0.1587 “does not biodegrade fast” |
BIOWIN 2010b |
| Biological oxygen demand | % BOD (biological oxygen demand) |
7±6 Primary half-life: 2 months 10 days Ultimate half-life: 8 months 24 days |
CATALOGIC 2014 |
Abbreviations: NA, not applicable.
a Based on a day length of 12 hours, a hydroxyl radical concentration of 1.5×106 molecules/cm3 (12-hour annual average), and a system temperature of 25 °C.
b EPI Suite c2000-2012
3.5.2.2 Long-range transport
Modelling was conducted to determine the long-range atmospheric transport potential for TBOEP. The OECD POPs Screening Model (OECD QSAR Toolbox 2016; Scheringer et al. 2009) estimates a characteristic travel distance (CTD) of 47 km in air. This, along with low vapour pressure (9.47 x 10-6 Pa), indicates that TBOEP should have a low potential for transport in air. However, there are few models available for predicting LRTP (e.g., OECD Pov and LRTP Screening Tool 2009a), and because they do not consider substances associated with the particle phase, they would be considered to underestimate overall transport potential to remote locations.
While modelling for TBOEP suggests limited long-range transport potential for TBOEP, measured concentrations in remote locations indicate that TBOEP may travel long distances. For instance, Möller et al. (2012) reported the occurrence of TBOEP in airborne particles during two polar expeditions in 2010-2011—one from East Asia to the high Arctic and the other from East Asia toward the Indian Ocean to the Antarctic—thereby providing evidence of the potential of TBOEP for long-range transport to the Arctic and Antarctica. TBOEP was detected at a frequency of 45% and 40% of the samples, respectively, with concentrations ranging from not detected to 81 pg/m3 and from not detected to 77 pg/m3, respectively.
De Silva et al. (2016a) evaluated the long-range transport potential of various organophosphate substances, including TBOEP, by examining their profile in high Arctic snow and ice. An ice cap was selected in the Canadian Arctic archipelago, at extreme northern latitude 75°N and the highest point of the ice cap, at an elevation of 2200 m. In order to perform a robust and continuous time series, a longer ice core with a bottom depth dating to 1979 was selected. Organophosphate substances, including TBOEP, were detected in every annual layer of ice from 2014 to 1979. In 2015, TBOEP was one of six analytes reported and comprised 7.3% of the total organophosphate concentration in the ice core.
De Silva et al. (2016b) further investigated the deposition and transport of TBOEP in the Arctic. The presence of organophosphate substances, including TBOEP, was reported in surface water taken from the Lake Hazen glacier-fed watershed (82°N) in Quttinirpaaq National Park on northern Ellesmere Island, Canada. A sample taken at a water column depth of 260 m in Lake Hazen was dominated by TBOEP (0.89 to 8.4 ng/L). Of all organophosphates subject to analysis, concentrations for TBOEP were the highest and most frequently detected not only in water samples taken from Lake Hazen, but also from outflow streams taken from the Ruggles and Turnabout Rivers.
3.5.3 Potential for bioaccumulation
The discussion on the potential for bioaccumulation of TBOEP examines several parameters, including physical and chemical properties of the substance (i.e., log Kow, log Koa, molecular size and cross-sectional diameters), bioconcentration factor (BCF), biomagnification factor (BMF), trophic magnification factor (TMF) and bioaccumulation factor (BAF). The potential derivation and role of metabolic biotransformation rate constants in determining bioaccumulation potential are also examined.
The log Kow value of 3.87 for TBOEP suggests low potential to bioaccumulate or biomagnify in biota.
In a study assessing the fate of emerging organic contaminants in a freshwater catchment impacted by small- and large-scale sewage treatment plants in Sweden, TBOEP was detected in surface water, wastewater effluent and perch (Perca fluviatilis) from September 2014 to December 2015 (Blum et al. 2018). A log BAF of 3.5 lipid weight (lw) and log BAF of 1.2 wet weight (ww) were estimated based on concentrations of TBOEP in fish of 230 ng/g lw and 1.1 ng/g ww, respectively.
One empirical study on TBOEP bioconcentration was identified. It indicated a low potential for TBOEP accumulation in fish. Bioconcentration of TBOEP in freshwater common carp (Cyprinus carpio) was studied at 25 °C under flow-through water conditions at a pH of 6.0 to 8.5 over 42 days in 1990 (J-CHECK c2010-). A mixture of male and female carp (average 3.80% lipid) were exposed to TBOEP concentrations of 0.2 mg/L and 0.02 mg/L (less than the empirical water solubility value of 1670 mg/L). Bioconcentration factors (BCFs) of 4.10 L/kg ww and less than 5.79 L/kg ww, respectively, were determined for these two concentrations.
Based on 3D analysis of 30 TBOEP conformers calculated using the BCFmax model with mitigating factors (Dimitrov et al. 2005), the maximum diameters of TBOEP range from 10.18 nm to 18.39 nm and the effective diameter is 12.16 nm. The model used a subset of chemicals, including halogenated benzenes, biphenyls and dioxins, and a cross-sectional diameter cut-off of 0.95 nm (9.5 Å) was proposed for membrane permeation of molecules. This suggests that TBOEP may experience a reduced rate of uptake from steric effects at the gill surface, allowing elimination processes to mitigate accumulation.
TBOEP has been reported to have limited trophic magnification in Great Lakes food webs (TMF of 0.59 to 1.6), although results were not conclusive because benthic organisms were not sampled or represented in the TMF calculations (Greaves et al. 2016b).
The BCF and BAF of TBOEP was also estimated using structure-based models, QSARS, and a three-trophic-level kinetic mass-balance model (Arnot and Gobas 2003a). All estimates of BCF and BAF, except those derived using sub-model 1 of the BCFBAF model in EPIWIN v4.0, were corrected for metabolism as it is a fundamental elimination pathway for many chemicals, including TBOEP. The results of the BCF and BAF modelling for middle trophic level fish for TBOEP are 24.9 and 60.0 L/kg wet weight, respectively (ECCC 2020e).
In summary, the log Kow value of 3.87 for TBOEP combined with indications of steric hindrance, metabolic transformation, low measured BCFs and low predicted BCFs/BAFs, indicate a low potential to bioaccumulate or biomagnify in biota. According to the outcome from ERC (ECCC 2016b), the other three substances in the aryl OP subgroup (TEP, TEHP and BEHP) also have a low bioconcentration potential and are not expected to significantly bioaccumulate in organisms.
3.6 Potential to cause ecological harm
Based on the outcome of ERC analysis, TEP, TEHP and BEHP are considered unlikely to be causing ecological harm (ECCC 2016a, 2016b; Appendix A). Therefore, no ecological analysis of TEP, TEHP or BEHP is presented in this chapter.
3.6.1 Ecological effects assessment
Empirical ecotoxicity data were considered for assessing the ecological effects of TBOEP. TBOEP is expected to be predominantly released from industrial sources and products available to consumers via wastewater. Modelled results suggest that exposure to aquatic organisms is mainly expected because, once released to water, a high proportion (96%) of TBOEP is expected to remain in water. While some TBOEP may partition to sediments (~4%), and by analogy to biosolids, the aqueous phase is considered the main medium of concern for this assessment. There are also limited soil and sediment toxicity data. Given the limited accumulation potential of TBOEP in organisms and analysis of aquatic/soil-food chain exposure, analysis of aquatic/soil and wildlife are not warranted.
3.6.1.1 Mode/mechanism of action
TBOEP is considered to have a reactive and specifically acting mode of action (MOA). OECD QSAR Toolbox (2016) alerts identified for this substance include DNA and protein binding, in vivo mutagenicity, and hepatotoxicity. In addition, ASTER (1999) determined an OP mediated AChE inhibition MOA for this substance. Using the OECD QSAR Toolbox (2016), results suggest that TBOEP also does not exhibit estrogen and androgen binding activities.
3.6.1.2 Effects on aquatic organisms
The aquatic toxicity of TBOEP determined from empirical studies is summarized in Table 3‑9. The modelling results are summarized in Environment and Climate Change Canada (2020e).
Aquatic toxicity of TBOEP has been associated with effects on survival, growth, reproduction, endocrine activity, and metabolism. TBOEP also exhibits genotoxicity, neurotoxicity, and cardiotoxicity in aquatic organisms.
A 21-day chronic study showed that exposure to a range of nominal sublethal concentrations of TBOEP (0.0147 to 1.47 mg/L) did not impact growth, survival or reproduction of Daphnia magna, although there was a trend towards a decreased reproductive output between the lowest and the highest dose (Giraudo et al. 2015). Gene transcription profiling in this study revealed that 101 genes were differentially transcribed in response to TBOEP. Most of the responding genes were involved in protein metabolism, biosynthesis and energy metabolism, indicating that TBOEP could have chronic effects by disrupting these pathways. The authors also reported a 48‑h acute LC50 value of 147 mg/L for Daphnia magna in the same study.
In a follow-up paper, Giraudo et al. (2017) exposed Daphnia magna to an environmentally relevant concentration of TBOEP (0.01 mg/L) over three 21-d generations and evaluated effects on gene transcription profiling, protein metabolism, and life history (i.e., survival, reproduction and growth). Chronic exposure to TBEOP did not impact survival or reproduction of Daphnia magna but affected the growth output in the form of reduced molting, which could lead to decreased size of daphnids over multiple generations. The effects observed suggest the potential for endocrine disruption by TBOEP in Daphnia magna.
A range of tests were conducted on various organisms to determine the inhibition concentration (IC), lethal concentration (LC) and effective concentration (EC) of TBOEP (Douville et al. 2016). The 72-h IC25 and IC50 values for growth inhibition in the green alga Pseudokirshneriella subcapitata were reported at 6.02 mg/L and 30 mg/L, respectively. In addition, the 96-h LC50 and EC50 (morphological effects) values of TBOEP in the invertebrate Hydra attenuata were determined to be 44.2 mg/L and 17.5 mg/L, respectively. When exposed to TBOEP, the 24-h LC50 for beavertail fairy shrimp (Thamnocephalus platyurus) was found to be 55.8 mg/L. The reported 48-h LC50 for TBOEP in Daphnia magna was 147 mg/L.
The toxicity of TBOEP on the hatching of zebrafish embryos (0 to 96 h post-fertilization) was investigated by Du et al. (2015). They reported a 96-h EC50 of 4.1 mg/L for impaired cardiac function (pericardium edema) and a 96-h LC50 of 3.34 mg/L.
A study on zebrafish larvae (Liu et al. 2017) reported a 96-h LC50 of 3.49 mg/L. TBOEP did not significantly affect hatching or survival rates. However, the authors reported developmental effects, namely malformation, growth delay, and decreased heart rate, in zebrafish caused by TBOEP at concentrations of 1, 1, and 0.2 mg/L, respectively, starting from 2 h post-fertilization. TBOEP exposure also had a significant impact (i.e., LOEC range of 0.02 to 2 mg/L) on mRNA abundance of genes involved in the growth hormone/insulin-like growth factor (GH/IGF) axis at 144 h post-fertilization. Extensive studies on the GH/IGF axis system suggest that these signaling pathways are a key neuroendocrine regulator of growth in fish (Wood et al. 2005).
In a study of the effects on reproduction and endocrine function, Xu et al. (2017) exposed adult zebrafish pairs to TBOEP at concentrations of 0, 5, 50 and 500 µg/L for 21 days. Reproduction effects were observed at 50, 5, 500, and 500 µg/L for decreased total egg production, egg diameter, F1 generation survival rate, and F1 generation hatching rate, respectively. The authors suggest that TBOEP could disturb the sex hormone balance by altering regulatory circuits of the hypothalamic-pituitary-gonadal axis, affect gonadal development, and eventually lead to disruption of reproductive performance.
The most sensitive LOEC of 0.01 mg/L (reduced molting) determined for Daphnia magna exposed to TBOEP over three 21-d generations (Giraudo et al. 2017) was selected as the critical toxicity value (CTV). A lower LOEC of 0.005 mg/L was reported in the literature, but the effects at 0.005 mg/L were not considered to be readily translated to the organismal or population level. Additional assessment factors were then considered to address the potential uncertainties associated with extrapolating the CTV to an environmental concentration that will be protective to most species in water. With a CTV selected from a long-term and sublethal endpoint, no additional assessment factor was applied for short-term to long-term and mortality to sublethal endpoints extrapolation. Due to the presence of abundant (seven species) and diverse (three categories of trophic groups) test species in the data set, no additional assessment factor was applied for species variation extrapolation. However, since TBOEP is considered to have a reactive and specifically acting MOA, an additional assessment factor of 2 was applied to address the potential differences in species sensitivity. Collectively, this results in an assessment factor of 2 (based on application factor calculations of 1 x 1 x 2), and the resulting PNEC is 0.005 mg/L.
| Test organism | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|
| Zebrafish larvae (Danio rerio) | 96-h LC50 | 0.29 | Ma et al. 2016 |
| Zebrafish larvae (Danio rerio) | 96-h LC50 | 3.49 | Liu et al. 2017 |
| Zebrafish larvae (Danio rerio) | 96-h LC50 | 3.34 | Du et al. 2015 |
| Zebrafish larvae (Danio rerio) | 96-h EC50 | 4.1 | Du et al. 2015 |
| Zebrafish larvae (Danio rerio) | 96-h NOEC (decreased heart rate) | 0.02 | Liu et al. 2017 |
| Zebrafish larvae (Danio rerio) | 96-h LOEC (decreased heart rate) | 0.2 | Liu et al. 2017 |
| Zebrafish larvae (Danio rerio) | 120-h NOEC (developmental defects) | 2.55 | Noyes et al. 2015 |
| Zebrafish (Danio rerio) | 21-d NOEC (decreased egg production) | 0.005 | Xu et al. 2017 |
| Zebrafish (Danio rerio) | 21-d LOEC (decreased egg production) | 0.05 | Xu et al. 2017 |
| Zebrafish (Danio rerio) | 21-d LOEC (decreased egg diameter) | 0.005 | Xu et al. 2017 |
| Zebrafish (Danio rerio) | 21-d NOEC (decreased F1 generation survival) | 0.05 | Xu et al. 2017 |
| Zebrafish (Danio rerio) | 21-d LOEC (decreased F1 generation survival) | 0.5 | Xu et al. 2017 |
| Zebrafish (Danio rerio) | 21-d NOEC (decreased F1 generation hatching) | 0.05 | Xu et al. 2017 |
| Zebrafish (Danio rerio) | 21-d LOEC (decreased F1 generation hatching) | 0.5 | Xu et al. 2017 |
| Killifish (Oryzias latipes) | 48-h LC50 | 6.8 | Tsuji et al. 1986 |
| Rainbow trout (Oncorhynchus mykiss) | 96-h LC50 | 24 | ECHA c2007-2018f |
| Daphnids (Daphnia magna) | 48-h EC50 (immobility) | 53 | ECHA c2007-2018f |
| Daphnids (Daphnia magna) | 48-h LC50 | 147 | Douville et al. 2016 |
| Daphnids (Daphnia magna) | 48-h LC50 | 147 | Giraudo et al. 2015 |
| Daphnids (Daphnia magna) | 21-d NOEC (inhibition of growth, survival, or reproduction) | 1.47 | Giraudo et al. 2015 |
| Daphnids (Daphnia magna) | 21-d LOEC (reduced molting) | 0.01 | Giraudo et al. 2017 |
| Algae (Pseudokirshneriella subcapitata) | 72-h IC25 (growth inhibition) | 6.02 | Douville et al. 2016 |
| Algae (Pseudokirshneriella subcapitata) | 72-h IC50 (growth inhibition) | 30 | Douville et al. 2016 |
| Algae (Pseudokirshneriella subcapitata) | 72-h EC50 (growth inhibition) | 61 | ECHA c2007-2018f |
| Hydra (Hydra attenuata) | 96-h EC50 (morphological changes) | 17.5 | Douville et al. 2016 |
| Hydra (Hydra attenuata) | 96-h LC50 | 44.2 | Douville et al. 2016 |
| Thamnotoxkit (Thamnocephalus platyurus) | 24-h LC50 | 55.8 | Douville et al. 2016 |
Abbreviations: EC50, the concentration of a substance that is estimated to cause some effect on 50% of the test organisms; LC50, the concentration of a substance that is estimated to be lethal to 50% of the test organisms; IC50, the inhibiting concentration that causes a 50% reduction in a quantitative biological measurement such as growth rate; IC25, the inhibiting concentration that causes a 25% reduction in a quantitative biological measurement such as growth rate; LOEC, lowest observed effect concentration; NOEC, no observed effect concentration; h, hours; and d, days.
a Effect over three consecutive generations of Daphnia magna.
3.6.1.3 Effects on sediment and soil-dwelling organisms
There are no available data characterizing the toxicity of TBOEP to benthic organisms.
Two studies were identified for effects of TBOEP on terrestrial organisms. Standardized acute toxicity tests were performed with TBOEP on earthworms (Eisenia fetida) and three different plant species, Lolium perenne, Lactuca sativa and Brassica rapa (ECHA c2007-2018f).
Available data were retrieved from an earthworm study on Europe’s registered substances database (ECHA c2007-2018f). In an earthworm reproduction test conducted according to OECD guideline 207, adult earthworms (Eisenia fetida) were exposed to TBOEP at nominal concentrations of 62.5, 125, 250, 500 and 1000 mg/kg artificial soil, for 14 days. A 14d-LC50 of 544 mg/kg dry soil, with 95% confidence limits of 250 and 1000 mg/kg dry soil, was determined.
Seedling emergence and seedling growth tests (OECD 2006) were conducted to assess the effects of exposure to TBOEP on ryegrass (Lolium perenne), lettuce (Lactuca sativa) and mustard (Brassica rapa) (ECHA c2007-2018f). Growth (dry weight) was the most sensitive endpoint for TBOEP exposure for all three plant species. Of the three species, Lactuca sativa was the most sensitive to TBOEP, with 21-d effective rate (ER)10 and ER50 of 22.7 and 238.7 mg/kg, respectively, after organic carbon normalization to reflect natural soil conditions (i.e., 3.4%). Similarly, ER10 and ER50 values of 70.5 and 165.8 mg/kg, respectively, were determined for Brassica rapa. Lolium perenne was the least sensitive to TBOEP, with ER10 and ER50 values of 109.8 and 572.3 mg/kg, respectively.
Similarly to the aquatic PNEC derivation process, the most sensitive endpoint was the 21-d ER10 of 22.7 mg/kg (growth – dry weight) for Lactuca sativa exposed to TBOEP (ECHA c2007-2018f). This endpoint was selected as the CTV for terrestrial receptors. Additional assessment factors were then considered to address the potential uncertainties associated with extrapolating the CTV to an environmental concentration that will be protective to most species in soil. An assessment factor of 5 was applied to account for uncertainties associated with short-term to long-term extrapolation. Given the limited availability and diversity of studies, a species sensitivity factor of 5 was also applied. Since TBOEP is considered to have reactive and specifically acting MOA, an additional mode of action factor of 2 was applied to address the potential differences in species sensitivity. Collectively, this results in an assessment factor of 50 (based on application factor calculations of 5 x 5 x 2), and the resulting PNEC is 0.45 mg/kg.
| Test organism | Endpoint | Value (mg/kg dw)a |
|---|---|---|
| Earthworm (Eisenia fetida) | 14-d LC50 (reproduction) | 544 |
| Plant (Lolium perenne) | 21-d ER50 (seedling emergence) | 885.4 |
| Plant (Lolium perenne) | 21-d ER50 (growth – height) | 610.2 |
| Plant (Lolium perenne) | 21-d ER50 (growth – dry weight) | 572.3 |
| Plant (Lolium perenne) | 21-d ER10 (seedling emergence) | 476.0 |
| Plant (Lolium perenne) | 21-d ER10 (survival) | 380.0 |
| Plant (Lolium perenne) | 21-d ER10 (growth – height) | 206.5 |
| Plant (Lolium perenne) | 21-d ER10 (growth – dry weight) | 110.0 |
| Plant (Brassica rapa) | 21-d ER50 (survival) | 893.6 |
| Plant (Brassica rapa) | 21-d ER50 (growth – height) | 283.3 |
| Plant (Brassica rapa) | 21-d ER50 (growth – dry weight) | 165.8 |
| Plant (Brassica rapa) | 21-d ER10 (seedling emergence) | 165.0 |
| Plant (Brassica rapa) | 21-d ER10 (survival) | 950.2 |
| Plant (Brassica rapa) | 21-d ER10 (growth – height) | 132.1 |
| Plant (Brassica rapa) | 21-d ER10 (growth – dry weight) | 70.5 |
| Plant (Lactuca sativa) | 21-d ER50 (growth – height) | 723.2 |
| Plant (Lactuca sativa) | 21-d ER50 (growth – dry weight) | 238.7 |
| Plant (Lactuca sativa) | 21-d ER10 (seedling emergence) | 1057.2 |
| Plant (Lactuca sativa) | 21-d ER10 (growth – height) | 333.6 |
| Plant (Lactuca sativa) | 21-d ER10 (growth – dry weight) | 22.7 |
Abbreviations: dw, dry weight; LC50, the concentration of a substance that is estimated to be lethal to 50% of the test organisms; ERX: effect rate at which the tested species shows an effect at the x% level.
a Toxicity values from plants were normalized to 3.4% organic carbon to reflect natural soil conditions from 0.96% (experimental conditions).
3.6.1.4 Effects on wildlife
Egloff et al. (2014) injected TBOEP into the air cell of chicken embryos at concentrations ranging from 0 to 45 400 ng/g and subsequently studied pipping success (breaking open an eggshell using an egg tooth), development, hepatic mRNA expression of 9 target genes, thyroid hormone levels, and circulating bile acid concentrations. The results from exposure to the highest dose of TBOEP showed negligible detection of the parent compounds in embryonic contents at pipping, indicating their complete metabolic degradation. It was also found that TBOEP exposure had limited effects on chicken embryos, with the exception of hepatic CYP3A37 mRNA induction. However, TBOEP did cause a relatively small, but significant, decrease in chicken embryonic body mass of 9% (Egloff et al. 2014). A slight mRNA upregulation of CYP3A37 was observed with a maximum fivefold increase. Induction of CYP3A37 suggests the activation of the chicken xenobiotic receptor, which is the analog to the mammalian PXR and constitutive androstane receptor (Egloff et al. 2014).
Crump et al. (2016) compared the effects of several environmental chemicals on cytotoxicity, ethoxyresorufin O-deethylase (EROD) activity, and mRNA expression in a wild piscivorous bird species, double-crested cormorant (Phalacrocorax auritus). Significantly decreased viability of embryonic hepatocytes occurred at 300 μM (119.54 mg/L) after 24 hours, whereas no effects were observed at 1 μM (0.39 mg/L). However, actual concentrations were not quantified in cormorant cells or culture medium in this study. The authors noted that this would be an important addition for future studies in order to determine actual uptake and resulting exposure levels in wild avian species. The inclusion of a full concentration response curve was not possible for the compound due to the limited pool of cormorant hepatocytes. The highest concentration was selected because it decreased cell viability of chicken and/or herring gull hepatocytes in previous studies and the lowest concentration had no effect on viability. Results of an in vitro avian embryonic hepatocyte assay indicated significantly decreased viability, with LC50 values ranging from 61.7 to 94.6 mM (0.15 to 0.23 mg/L) (Porter et al. 2013). However, results from cluster analysis with array data for TBOEP showed that TBOEP did not affect target genes.
Fernie et al. (2015) reported on the in vivo uptake and effects of chronic dietary exposure to TBOEP in captive American kestrels (Falco sparverius), focusing on physiological systems (e.g., hepatic oxidative status, thyroid function, and hepatic function). TBOEP was one of four substances that significantly affected hepatic T4-outer ring deiodinase activity at environmentally relevant concentrations. The authors also suggest that TBOEP may be implicated in altered thyroid status in American kestrels as the triiodothyronine (T3) plasma concentrations overall were altered with increased T3 under 7d exposure. Fernie et al. (2017) also reported that TBOEP was detected in the plasma of peregrine falcon (Falco peregrinus) nestlings at the highest frequency relative to all four of the OP flame retardants studied, at concentrations of 1.0 to 7.5 ng/g ww (Fernie et al. 2017).
3.6.2 Ecological exposure assessment
To inform the risk characterization of TBOEP, predicted environmental concentrations (PECs) in relevant media were calculated. Environmental release of TBOEP is likely to occur during industrial formulation activities and through use of household products. Release from these sources will result in exposure of surface waters and soil. Because of limited ecotoxicity data for TBOEP in sediments, a risk quotient is not derived for this media. The predominant exposure medium is water.
Releases to surface water from industrial activities result from indirect discharge to the sewer and subsequent release from wastewater treatment systems (WWTSs). Release to soil is an extension of the aquatic scenario and is a result of biosolids application to agricultural lands. Additionally, ecological exposure of surface waters resulting from releases of TBOEP from commercial and consumer products was evaluated using measured data from surface water (Truong 2016).
According to information reported in response to surveys under section 71 of CEPA, (Environment Canada 2013; ECCC 2016b), exposure scenarios for TBOEP were developed for the major uses of alkyl OPs (i.e., formulation of cleaning and furnishing care products and the formulation of paints and coatings). Potential releases via container cleaning and transport, including loading and unloading, are not considered in this assessment. PECs for each exposure scenario in surface water and soil are presented in Table 3‑13.
Scenarios were developed on the basis of available information and generic considerations. The total substance quantity used annually at a facility is based on the average of the quantity of TBOEP reported by companies (Environment Canada 2013; ECCC 2016b). Daily dilution volumes are based on distributions of daily dilution volumes associated with facilities relevant to the industrial sector. Emission factors to wastewater and number of release days at a facility are based on assumptions from generic sources of information. The approach assumes that industrial effluents are sent to a secondary WWTS, the most common treatment type in Canada. A secondary WWTS removal efficiency of 82% is used in the PEC calculations; it is based on measured removal rates for TBOEP (ECCC 2016c).
Estimation of soil exposure to TBOEP was done using an approach described by the European Chemicals Agency (ECHA 2016) involving the land application of biosolids from WWTSs. This approach estimates the concentration of the accumulated substance (soil PEC) within the top 20 cm soil layer after 10 years of biosolids application. However, given that TBOEP may degrade quickly in soil, the approach was modified to consider degradation in soil. The soil biodegradation half-life for TBOEP is 70 days (CATALOGIC 2014). Concentrations were determined on a yearly basis immediately after application and at the end of the year (365 days) prior to the subsequent application, over a 10-year period. Given the half-life, the concentration of TBOEP in soil does not accumulate much over the 10-year period, and soil concentrations are maximal immediately after application (decreasing significantly over the year).
As TBOEP is imported in bulk in a pure liquid form or as part of a liquid mixture, residues may form in transport containers, and container cleaning operations may lead to environmental releases of these substances. Although environmental concentrations of TBOEP resulting from such releases may be high, they would likely be episodic in nature and probably of short duration. Given these considerations and the current data gaps associated with container cleaning operations and practices, a quantitative exposure characterization was not developed for these releases.
3.6.2.1 Measured concentrations in environmental media and wastewater
Canadian concentrations for TBOEP are available for air, precipitation, surface water, lake sediment, biosolids and biota. TBOEP concentrations in Canada are summarized in Table 3‑11, with further details found in ECCC (2020f).
In a study of Canadian Arctic air, ship- and land-based sampling was conducted from 2007 to 2013 to analyze long-range transport of organophosphate esters. TBOEP was detected in air at concentrations ranging from below the limit of detection to 157 pg/m3, with a 6.6% increase in concentration over the study period (Sühring et al. 2016). Air samples were also collected in the Great Lakes to determine TBOEP concentrations (personal communication, email from Jantunen et al. dated 2018; unreferenced). Detected TBOEP air concentrations ranged from 15 to 138 pg/m3, with the highest level reported in Lake Erie (29 to 138 pg/m3).
Globally, TBOEP was detected in air (ND to 3.8 x 105 pg/m3), water (ND to 0.013 mg/L), sediment (ND to 1.97 mg/kg dw), soil (ND to 0.45 mg/kg dw), biota (ND to 8.84 mg/kg ww) and other media samples in the United States, Germany, the Arctic, Sweden, Norway, Switzerland, Austria, China, Japan, Philippines, Spain, Czech Republic, New Zealand, Australia, Antarctica and Serbia from 1980 to 2014 (Fries and Puttman 2001; Fries and Mihajlovic 2011; Wang et al. 2011; Bollman et al. 2012; Rodil et al. 2012; Esteban et al. 2014; Eulaers et al. 2014; Salamova et al. 2014; Liu et al. 2014; Cui et al. 2017; Giulivo et al. 2017).
| Media (units) | Location | Concentration mean or rangea | Reference(s) |
|---|---|---|---|
| Air (pg/m3) | Great Lakes | 15–262 | Salamova et al. 2014, Jantunen et al. 2018 (unpublished) |
| Air (pg/m3) | Arctic | ND–157 | Suhring et al. 2016 |
| Surface water (mg/L) | Toronto, Ontariob | 1.0 x 10-4–1.03 x 10-2 | Hao et al. 2018 |
| Surface water (mg/L) | Don River | 2.4 x 10-4–5.2 x 10-3 | Truong 2016 |
| Surface water (mg/L) | Lake Erie | 7.5 x 10-5 ± 3.9 x 10-5 | Venier et al. 2014 |
| Precipitation (mg/L) | Great Lakes basin, Toronto, Ontario | ND–2.32 x 10-3 | Truong 2016 |
| Precipitation (mg/L) | Southern Ontario | 7.0 x 10-6–1.0 x 10-4 | Jantunen et al. 2013 |
| Sediment (mg/kg dw) | Lake Superior | ND–4.6 x 10-4 | Cao et al. 2017 |
| Sediment (mg/kg dw) | Lake Ontario | ND–2.37 x 10-2 | Cao et al. 2017 |
| Wastewater (mg/L) | Toronto area wastewater treatment systems (WWTS) | Effluent: 2.9 x 10-4–1.02 x 10-2 | Hao et al. 2018 |
| Wastewater (mg/L) | 3 WWTS in Toronto area | Effluent: 4.0 x 10-4–5.6 x 10-3 | Truong 2016 |
| Wastewater (mg/L) | 8 WWTS from across Canada |
Influent: 0.0011–0.031 Effluent: 8.4 x 10-5 – 1.3 x 10-2 |
ECCC 2016c |
| Biosolids (mg/kg dw) | 8 WWTS from across Canada | Biosolids: 0.62–13.5 | ECCC 2016c |
| Wastewater (mg/L) | secondary WWTS in western Canada |
Influent: 3.29 x 10-3c Effluent: 5.47 x 10-4c |
Woudneh et al. 2015 |
| Biosolids (mg/kg dw) | secondary WWTS in western Canada | Biosolids: 2.24c | Woudneh et al. 2015 |
| Fishd (mg/kg ww) | Lake Ontario, Lake Erie | ND–1.35 x 10-2 | Chu and Letcher 2015, Greaves et al. 2016b, McGoldrick et al. 2014, McGoldrick and Murphy 2016, |
| Fishd (mg/kg ww) |
Great Lakes |
ND–9.8 x 10-3 | Chu and Letcher 2015, Greaves et al. 2016b, McGoldrick et al. 2014, McGoldrick and Murphy 2016, |
| Fishd (mg/kg ww) | Great Bear Lake, Kusawa lake, Cold Lake, Lake Athabasca | < 2.6 x 10-4–3.2 x 10-3 (median) | Chu and Letcher 2015, Greaves et al. 2016b, McGoldrick et al. 2014, McGoldrick and Murphy 2016, |
| Birdse (mg/kg ww) | Great Lakes | ND–1.3x10-2 | Chen et al. 2012, Greaves and Letcher 2014, Greaves et al. 2016b, Letcher et al. 2011, Su et al. 2015b |
Abbreviations: ND, not detected; ww, wet weight; dw, dry weight
a In cases where concentration ranges are provided, data represent minimum to maximum concentrations, unless otherwise specified.
b Urban streams sampled in Toronto area were Etobicoke Creek, Don River and Highland Creek, and the nearshore waters are Humber Bay and Toronto Harbour. Truong (2016) reported that Don River had the highest TBOEP concentration of 5.220 µg/L.
c Influent, effluent and biosolids are reported as the mean of duplicate measurements (Woudneh et al. 2015).
d The biota samples collected were from a variety of fish species using egg or animal tissues of lake trout (Salvelinus namaycush), rainbow smelt (Osmerus mordax), slimy sculpin (Cottus cognatus), round goby (Neogobius melanostomus), deepwater sculpin (Myoxocephalus thompsonii), alewife (Alosa pseudoharengus), walleye (Sander vitreus), trout perch (Percopsis omiscomaycus), yellow perch (Perca flavescens), or white perch (Morone americana).
eBird samples were collected from herring gull (Larus argentatus) eggs in the Great Lakes.
3.6.2.2 Formulation of cleaning and furnishing care products
TBOEP functions as a plasticizer/coalescence agent in cleaning and furnishing care products. A representative concentration of 3% TBOEP in final products was selected on the basis of available product information. According to information submitted in response to CEPA section 71 surveys, (Environment Canada 2013; ECCC 2016b), all-purpose cleaners, degreasers, floor care products, sanitizers and disinfectants are identified as final products containing TBOEP. As all locations of companies potentially using this substance to formulate cleaning and furnishing care products are unknown, a distribution of daily dilution volumes associated with secondary and tertiary WWTS receiving effluent from the cleaning and furnishing care formulation sector was used.
Formulation of paints and coatings
According to information submitted in response to CEPA section 71 surveys, (Environment Canada 2013; ECCC 2016b), formulators of paints and coatings import TBOEP as a plasticizer, which is then blended in the formulation process to produce the final paint and coating products. As all locations of companies potentially using this substance to formulate paint and coating products are unknown, a distribution of daily dilution volumes associated with secondary and tertiary WWTS receiving effluent from the paints and coating formulation sector was used.
The final users of paint and coating products are not considered in this scenario as they are unknown and the potential customers are disperse and widespread. It is expected that TBOEP may be found in different types of paints and coatings. However, the scenario focused on water-based (latex) paints as these paints have the highest potential releases to wastewater.
Commercial and consumer products
Recent studies show that flame retardants, like TBOEP, are released from household products (e.g., electronics, plastics, rubber, etc.) to indoor air and dust and subsequently accumulate on fabrics, such as clothing and drapes, acting as passive filters. During washing, flame retardants may be released from clothing and other washed fabrics to domestic wastewater and are transported to wastewater treatment facilities before reaching the aquatic environment (Schreder and La Guardia 2014; Saini et al. 2016). Flame retardants may also be released to domestic wastewater during washing of floors, carpets, walls, windows and other indoor items. Ecological exposure resulting from releases of TBOEP from consumer products was evaluated using surface water monitoring data (Truong 2016). The highest median value of TBOEP measured in urban streams was 0.00118 mg/L (Truong 2016), and this value was selected as a representative aquatic PEC resulting from release of TBOEP from consumer products.
3.6.3 Characterization of ecological risk
The approach taken in this ecological screening assessment was to examine assessment information and develop proposed conclusions using a weight-of-evidence approach and precaution as required under CEPA. Evidence was gathered to determine the potential for TBOEP to cause harm in the Canadian environment. Lines of evidence considered include those that directly support the characterization of ecological risk (e.g., measured endpoints or properties), as well as indirect lines of evidence (e.g., classification of hazard or fate characteristics by other regulatory agencies).
3.6.3.1 Risk quotient analysis
Risk quotient analyses were performed by integrating realistic estimates of exposure (PECs; see the Ecological Exposure Assessment section) with ecological toxicity information (PNECs; see the Ecological Effects Assessment section) to determine whether there is potential for ecological harm in Canada. Risk quotients (RQs) were calculated by dividing the PEC by the PNEC for relevant environmental compartments and associated exposure scenarios. RQs were derived for aquatic environmental compartments and other relevant exposure scenarios for TBOEP (Table 3‑12).
The PEC values presented in Table 3‑12 represent the level of exposure in the receiving water near the point of the discharge of the wastewater treatment system at each site. An aquatic PNEC of 0.005 mg/L was derived from the three-generation 21-d LOEC of 0.01 mg/L for daphnids (Daphnia magna) (Giraudo et al. 2017, section 3.6.1.2). The resulting risk quotients range from 0.02 to 0.56. Similarly, soil RQs of 0.06 and 0.79 were derived for the formulation of cleaning and furnishing care products and the formulation of paints and coatings scenarios, respectively. This analysis indicates that harm to pelagic and terrestrial organisms is unlikely to occur in the Canadian environment based on current levels of use of TBOEP.
| Exposure scenario | Aquatic PEC (mg/L) | Aquatic PNEC (mg/L) | Aquatic RQ | Soil PEC (mg/kg) | Soil PNEC (mg/kg) | Soil RQ |
|---|---|---|---|---|---|---|
| Formulation of cleaning and furnishing care products | 2.82x10-3 | 0.005 | 0.56 | 0.36 | 0.45 | 0.79 |
| Formulation of paints and coatings | 8.1x10-5 | 0.005 | 0.02 | 0.03 | 0.45 | 0.06 |
| Commercial and consumer product release | 1.18x10-3 | 0.005 | 0.24 | N/A | N/A | N/A |
3.6.3.2 Consideration of the lines of evidence
To characterize the ecological risk of TBOEP, technical information for various lines of evidence was considered (as discussed in the relevant sections of this report) and qualitatively weighted. The key lines of evidence supporting the assessment conclusion are presented in Table 3‑13, with an overall discussion of the weight of evidence provided in section 3.6.3.4. Level of confidence refers to the combined influence of data quality and variability, data gaps, causality, plausibility and any extrapolation required within the line of evidence. Relevance refers to the impact the line of evidence has when determining the potential to cause harm in the Canadian environment. Qualifiers used in the analysis ranged from low to high, with the assigned weight having five possible outcomes.
| Line of evidence | Level of confidencea | Relevance in assessmentb | Weight assignedc |
|---|---|---|---|
| Physical and chemical properties | moderate | moderate | moderate |
| Persistence in the environment | moderate | moderate | moderate |
| Long-range transport | low | high | moderate |
| Bioaccumulation in aquatic, terrestrial organisms | moderate | low | low-moderate |
| Mode of action and/or other non-apical data | moderate | high | moderate-high |
| PNEC for aquatic and soil organisms | moderate | high | moderate-high |
| Monitoring data for concentrations in surface water, wastewater effluents, sediments, air, biota | moderate | moderate | moderate |
| PEC(s) in water | moderate | high | moderate-high |
| PEC(s) in soil | low | high | moderate |
| RQ(s) for water | moderate | high | moderate-high |
| RQ(s) for soil | low | high | moderate |
a Level of confidence is determined according to data quality, data variability, data gaps and if the data are fit for purpose.
b Relevance refers to the impact of the evidence in the assessment.
c Weight is assigned to each line of evidence according to the combined level of confidence and relevance in the assessment.
Abbreviations: PEC, predicted environmental concentration; PNEC, predicted no effect concentration; RQ, risk quotient.
3.6.3.3 Weight of evidence for determining potential to cause harm to the Canadian environment
The reported low manufacture and import volumes of TBOEP in Canada and the modelled low long-range transport results are inconsistent with the potential for widespread release into the Canadian environment evident from reported elevated levels in the environmental media. TBOEP has been detected frequently in samples, including air, water, sediment, biosolids and soil found near sources and demonstrates a potential for persistence. Therefore, the main concern for TBOEP is for both near- and far-field exposure.
Although concentrations of TBOEP in sediment appeared to remain relatively constant over approximately 40 years in one field study based on what was considered historical input of TBOEP, these data are uncertain given that variables are not controlled in field studies. Overall, there is sufficient weight of evidence that indicates that TBOEP undergoes significant degradation in water, sediment, and soil based on empirical studies and modelling that show that TBOEP is not persistent.
There is low confidence in the data concerning long-range transport due to the variability in modelled and empirical findings. Based on modelling, TBOEP would not be considered highly persistent in air would not be considered highly amenable to long-range atmospheric transport. However, the substance is found associated with atmospheric particulates which have been shown to increase the persistence of other OP substances. TBOEP was detected in some samples at very low concentrations in remote areas, such as the Arctic and Antarctic.
The selected log Kow value of 3.81, the fast rate of metabolic biotransformation (kM), and the low BCF and BAF for TBOEP indicate a limited potential to bioaccumulate and biomagnify in biota. At current levels of use and based on low potential to bioaccumulate or biomagnify in biota, TBOEP is not expected to have food chain effects and or to result in harm due to food chain transfer.
Based on computational modelling, TBOEP is considered to have reactive and specifically acting MOA. This line of evidence is supported with empirical findings where TBOEP is shown to be capable of exerting adverse acute (e.g., mortality) and chronic (e.g., behavioural and physiological) effects on a number of aquatic (e.g., fish and daphnids) and terrestrial (e.g., earthworms and plants) organisms at low concentrations. While there are some uncertainties due to the limitations in the available toxicity data, particularly for soil organisms, the derived PNECs are considered adequately protective of aquatic and soil organisms in the environment.
Site-specific industrial scenarios were developed to cover a range of potential industrial activities known to exist for TBOEP in Canada. The scenarios estimated exposure to TBOEP in the receiving water near the point of discharge of the wastewater treatment system at each site and in soils. The exposure assessment is assigned a high weight as it is a key line of relevant evidence supporting the proposed conclusion for TBOEP. The estimated PECs from industrial release scenarios for water are in the range of measured TBOEP concentrations in Canada. Since these predicted industrial PECs involved the use of some conservative assumptions, it is considered that measurements of TBOEP in soil and surface water would further improve the confidence of exposure estimates. The importance of releases from products is an area of uncertainty due to the lack of data characterizing environmental exposures from these sources. However, based on the available evidence, the risk analysis indicates that present levels are not likely to result in risk. While current exposures of the Canadian environment to TBOEP are unlikely to be of concern, TBOEP is considered to have a high hazard based on its inherent toxicity to aquatic species. There may therefore be a concern for the Canadian environment should exposures increase.
3.6.3.4 Sensitivity of conclusion to key uncertainties
An uncertainty identified for TBOEP is the absence of data to quantify environmental exposure due to the release of TBOEP during the life cycle and disposal of products available to consumers. The amounts of TBOEP in products available to consumers is a significant area of uncertainty as import quantities are not considered to adequately characterize quantities of TBOEP imported in products. Frequent measurement of TBOEP in wastewater treatment effluent and biosolids as well as surface water further suggest widespread release of this substance. PECs characterizing release from products available to consumers were estimated based on measurements considering multiple sources and were not shown to result in potential risk. Thus, the impact of the absence of data concerning releases from products on this assessment is considered neutral.
Key areas of uncertainty for this assessment include the lack of data characterizing TBOEP toxicity to sediment and soil organisms (and a lack of data from appropriate analogues). This assessment has shown the potential for exposure to TBOEP in sediments based on measured concentrations and in soils due to potential land application of biosolids. In sediments, one study supports potential high persistence, thus suggesting chemical build-up over time. However, without toxicity data to evaluate whether predicted concentrations are harmful to sediment organisms, there is a potential that this assessment may underestimate overall risk to the environment.
This assessment did not consider risk to mammalian wildlife receptors due to consumption of prey containing accumulated concentrations of TBOEP. However, there is a moderate level of confidence that this substance will not bioaccumulate appreciably and will be metabolized, and exposure and risk due to food chain transfer of this substance is therefore considered to be low. This uncertainty is unlikely to have an effect on this risk assessment.
3.7 Potential to cause harm to human health
3.7.1 Exposure assessment of the alkyl OP subgroup (TEP, TBOEP, TEHP, BEHP)
3.7.1.1 TEP
Environmental media and food
No data on levels of TEP in outdoor air were identified in Canada. Based on the limited data identified elsewhere (Aragon et al. 2012, 2013; Liu et al. 2016), ambient air is not considered to be an important source of exposure for TEP.
In a Canadian study by Yang et al. (2017), TEP was not detected in indoor air of 51 homes in Toronto and Ottawa above the detection limit (0.34 pg/m3). However, TEP was measured in indoor air of homes in Sweden (Staaf and Ostman 2005; Bergh et al. 2011; Luongo and Östman 2016), Germany (Zhou et al. 2017a), and Japan (Saito et al. 2007; Kanazawa et al. 2010; Takeuchi et al. 2014, 2015). The lack of detection of TEP in the Canadian study may be due to differences in sampling protocol, detection limits, as well as sample size and location. As a conservative approach, and to account for potential transient TEP emissions from building and construction materials, human exposure to TEP from indoor air was characterized using the maximum value of 297 ng/m3 from the Swedish study by Luongo and Östman (2016). This study measured TEP in indoor air of 62 apartments in Stockholm in 2009 and reported the highest concentration of TEP in indoor air.
TEP was detected in Ontario and Quebec in Canadian drinking water surveys from 1978 to 1980 (Lebel et al. 1981; Williams and Lebel 1981; Williams et al. 1982). Concentrations were reported to be up to 23 ng/L. However, the recoveries for TEP from the methodology in these surveys were poor (i.e., around 25%), and actual TEP concentrations were probably higher when TEP was detected. Researchers in other countries (e.g., Spain, China, Korea) have also measured TEP in drinking water (Rodil et al. 2012; Ding et al. 2015; Lee et al. 2016). A study of surface waters in Ontario reported TEP concentrations of up to 105 ng/L (Truong 2016). Samples were collected between 2014 and 2015 and, depending on the sampling location, reported detection frequencies ranged from 11% to 100%. TEP in surface waters was also reported in Germany (Bollman et al. 2012; Wolschke et al. 2015), Spain (Rodil et al. 2012), and China (Wang et al. 2011; Yan et al. 2012). The use of the Canadian surface water data (maximum value of 105 ng/L) was considered to be most applicable and conservative for estimating human exposure to TEP from drinking water in Canada.
Based on a 2013 study of dust from 818 homes across Canada, TEP was measured in 16% of the dust samples with concentrations ranging from not detected (MDL = 0.09 µg/g) to 2.83 µg/g and a 95th percentile of 0.32 µg/g (personal communication, emails from the Environmental Health Science and Research Bureau, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated October 28, 2013; unreferenced). In two other Canadian studies with smaller sample sizes (i.e., 134 and 51), TEP was not detected above the method detection limits of 90 ng/g and 0.25 ng/g (Fan et al. 2014; Yang et al. 2017). As a conservative approach, the 95th percentile value from the Canadian House Dust Study was used for characterizing human exposure to TEP from dust from residential environments. Concentrations of TEP in residential dust in Canada are similar to those reported globally (Ali et al. 2012; Dodson et al. 2012; Luongo and Östman 2016; Wu et al. 2016; Zhou et al. 2017b).
No data on levels of TEP in soil were identified in Canada. TEP was detected in one study from China (Cui et al. 2017), and the maximum (0.011 mg/kg dw) concentration of TEP in soil samples collected from park areas and residential areas resulted in negligible exposures across all age groups in the Canadian population.
Tomizawa et al. (2004) reported higher levels of TEP in food packaging materials than in food contents in samples collected from the United Kingdom, Italy and France. According to the study, levels of TEP in oatmeal and oatmeal packaging from the United Kingdom was 270 ng/g and 470 ng/g, respectively, and levels of TEP in pasta and pasta packaging from Italy was 90 ng/g and 150 000 ng/g, respectively. In France, two separate samples of pasta and pasta packaging had TEP levels of 80 ng/g and 4 700 ng/g and 90 ng/g and 130 000 ng/g, respectively. The authors assumed that TEP leached into the food from the packaging. Potential exposure to TEP from its use as a component in the manufacture of food packaging materials (see section 3.3) is not expected when the substance is separated from food with an effective functional barrier (personal communication, emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated April 10, 2019; unreferenced).
TEP was measured in breast milk of women in Japan, Philippines, and Vietnam (Kim et al. 2014). The study reported an overall detection frequency for TEP in breast milk of 16% with concentrations of TEP in breast-milk ranging from not detected (method detection limit between 0.01 to 0.08 ng/g lipids) to 15 ng/g lipids (Kim et al. 2014). Given the low detection frequency and small sample size, this study was not used to estimate exposure of the Canadian general population to TEP from breast milk.
Estimated total daily intakes of TEP from its presence in environmental media were derived and range from 0.054 µg/kg bw/d (60+ year-olds) to 0.16 µg/kg bw/d (0.5- to 4-year-olds) (Appendix F, Table F-5).
Products available to consumers
Manufactured items:
TEP was found in the foam and fabric components of 10 out of 15 popular infant and child restraint seats tested in the United States (Miller and Gearhart 2016). In one child restraint seat, it was also found in a Velcro part. TEP has also been found in the foam of an infant crib wedge, in mattress toppers, and in infant and child restraint seats in Canada (personal communication, email from the Consumer Product Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated May 2019; unreferenced). Estimates of prolonged dermal exposure to TEP for the general population lying on foam-containing mattresses or furniture and for 0 to 6 months old and 0.5 to 4 year olds sitting in restraint seats are presented in Table 3‑14. Details on the parameters used to estimate these exposures can be found in Appendix G.
Other products:
TEP was reported as an ingredient in a limited number of products available to consumers including an all-purpose remover (MSDS 2015) and in foam sealant products (MSDS 2014a). Acute dermal exposure from the use of an all-purpose remover product containing TEP was estimated using a representative scenario of oven cleaning. Limited dermal absorption data for TEP were identified (Marzulli et al. 1965; Thors et al. 2016). The Marzulli et al. (1965) paper does not include any details regarding the study design, and thus it is not possible to determine the quality of the study. Thors et al. (2016) included TEP in a study that demonstrated that water acts as a skin penetration enhancer for organophosphorus compounds and that lower dilution solutions of the compounds tested, such as a 10% solution of TEP, have higher dermal penetration than more concentrated solutions. No suitable analogues for dermal absorption of TEP were identified given its very high water solubility and considering other physical-chemical properties. A dermal absorption of 100% was therefore assumed (i.e., equivalent to oral absorption). Use of foam sealant products containing TEP that are available at consumer retail stores in Canada is a potential source of inhalation exposure. It is expected that most of the retail sales are to professionals, while a small fraction can be to Do-It-Yourself consumers. Inhalation exposure may occur during application and was estimated using a representative scenario. Estimated exposures from all relevant routes are summarized in Table 3‑14 and details on the parameters used to estimate these exposures can be found in Appendix G.
| Exposure route and duration | Source | Age group | Exposure estimatea |
|---|---|---|---|
| Oral (daily) | Foam in children’s products | 0–6 months | 0.018–0.54 mg/kg bw/day |
| Oral (daily) | Foam in children’s products | 0.5–4 years | 0.017–0.53 mg/kg bw/day |
| Dermal (daily) | Infant restraint seatsb | 0–6 months | 0.28–8.60 mg/kg bw/day |
| Dermal (daily) | child restraint seatsb | 0.5–4 years | 0.22–6.91 mg/kg bw/day |
| Dermal (daily) | Foam containing furniture or mattresses | 0–6 months | 2.8–294 mg/kg bw/day |
| Dermal (daily) | Foam containing furniture or mattresses | 0.5–4 years | 2.0–224 mg/kg bw/day |
| Dermal (daily) | Foam containing furniture or mattresses | 5–11 years | 1.3–156 mg/kg bw/day |
| Dermal (daily) | Foam containing furniture or mattresses | 12–19 years | 1.1–134 mg/kg bw/day |
| Dermal (daily) | Foam containing furniture or mattresses | 20+ years | 0.75–103 mg/kg bw/day |
| Dermal (per event) | Oven cleaning using an all-purpose remover (combined spraying and cleaning)c | 20+ years | 1.28 mg/kg bw per event |
| Inhalation (per event) | Foam sealantd | 20+ years | 500 mg/m3 |
a Dermal absorption value of 100% was used to estimate dermal exposures.
b TEP identified in the foam and fabric components of infant and child restraint seats (Miller and Gearhart 2016).
c Potential inhalation exposure to TEP from use of oven cleaner is covered by the inhalation of foam sealant scenario.
d Potential dermal exposure to TEP from use of foam sealant is covered by the dermal exposure from use of oven cleaners.
3.7.1.2 TBOEP
Environmental media and food
Canadian monitoring data for TBOEP in ambient air and surface water are summarized in section 3.6.2.1 (Table 3‑11). Estimated intakes of TBOEP from ambient air are negligible (≤ 2.5 ng/kg bw/day).
In Canada, there is one study that reported TBOEP concentrations in indoor air (Yang et al. 2019). Sampling of indoor air in 51 residences in the cities of Toronto and Ottawa showed concentrations up to 6 040 pg/m3; the detection frequency of TBOEP was 84% in samples from bedrooms and 81% in the most used rooms. TBOEP concentrations in indoor air were also reported in residences in Europe and Asia (Kanazawa et al. 2010; Bergh et al. 2011; Takeuchi et al. 2014; Cequier et al. 2014; Luongo and Östman 2016). Elevated concentrations of TBOEP in indoor air (maximum values > 10 ng/m3) were reported in child care centres and schools (Fromme et al. 2014; Cequier et al. 2014; Bergh et al. 2011; Zhou et al. 2017a). It was shown that TBOEP concentrations in dust are correlated with TBOEP concentrations in indoor air (Cequier et al. 2014; Fromme et al. 2014). Therefore, and in the absence of studies on alkyl organophosphate ester flame retardants in indoor air in Canadian child care centres and schools, the 95th percentile concentration of 833 ng/m3 (833 000 pg/m3) for TBOEP in indoor air from the German study by Fromme et al. (2014) (mentioned above) was used to estimate exposure of the younger population to TBOEP from indoor air in child care centres and schools. For adults, the 95th percentile value of 5 640 pg/m3 from the Canadian study was most appropriate for characterizing exposure to TBOEP from indoor air.
TBOEP was measured in potable water in Canada in various water surveys from 1978 to 1983 (Lebel et al. 1981; Williams and Lebel 1981; Williams et al. 1982; Lebel et al. 1983; Lebel et al. 1987). Most samples were obtained at municipal treatment plants, but some were obtained from residential taps. Concentrations ranged from below the limit of detection to 6 000 ng/L. Elevated concentrations were measured after periods of non-use where water had been standing in the pipes. Certain components of the tap pipes (i.e., rubber gaskets, washers, O-ring and seals) were identified as sources of TBOEP (Lebel et al. 1981; Williams and Lebel 1981; Williams et al. 1982; Lebel et al. 1983; Lebel et al. 1987). Concentrations of TBOEP were also measured in drinking water in Europe (Esteban et al. 2014; Rodil et al. 2012) and Asia (Ding et al. 2015; Lee et al. 2016), and in surface waters in Canada (Truong 2016; Venier et al. 2014) and globally (Christensen et al. 2010; Elliot and VanderMeulen 2017; Bollman et al. 2012; Gorga et al. 2015; Zushi et al. 2016). Exposure of the Canadian general population to TBOEP from drinking water was estimated using the maximum TBOEP concentration of 560 ng/L reported in the national water survey of 29 municipalities across Canada (Williams and Lebel 1981).
TBOEP was measured in indoor dust in Canadian studies at concentrations ranging from 0.73 µg/g up to 275 µg/g (CHDS, n = 818; Won and Lusztyk 2011; Fan et al. 2014; Yang et al. 2017; personal communication, emails from Environmental Health Science and Research Bureau, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated October 28, 2013; unreferenced). Detection frequency of TBOEP in these studies ranged from 88% to 100%. Data from the CHDS study were considered to be most appropriate for characterizing exposures to TBOEP from dust from residential environments. The 95th percentile concentration of TBOEP in dust was reported to be 104 µg/g (personal communication, email from Environmental Health Science and Research Bureau, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated October 28, 2013; unreferenced). Concentrations of TBOEP in residential dust in Canada are similar to those reported elsewhere, except for Japan, which has reported higher levels.
Elevated concentrations of TBOEP in dust (maximum values > 1 000 µg/g) were reported in childcare centres and schools in the United States, Europe and Asia (Bergh et al. 2011; Cequier et al. 2014; Fromme et al. 2014; Mizouchi et al. 2015; Langer et al. 2016; Dodson et al. 2017; Zhou et al. 2017b). Considering this pattern of elevated TBOEP concentrations in dust of educational environments, and in the absence of studies on alkyl phosphate ester flame retardants in dust in Canadian childcare centres and schools, data from the German study by Fromme et al. (2014) were used in this assessment to estimate exposure of the younger population (aged 6 months to 4 years) to TBOEP from dust in childcare centres and schools. This study analysed analyzed dust samples from 63 daycares from 2011 to 2012 and reported the highest 95th percentile concentration of 3 633 µg/g across relevant daycare studies for TBOEP in dust.
No data on levels of TBOEP in soil were identified in Canada. TBOEP was detected in one study from China (maximum of 0.15 mg/kg in surface soil from residential area) (Cui et al. 2017). Given the lack of Canadian data, the maximum estimated PEC for soil for TBOEP (formulation of cleaning and furnishing care products exposure scenario) of 360 ng/g was used to estimate intakes of TBOEP through soil ingestion. Intake levels were negligible across all age groups.
Potential exposure to TBOEP from its use as a component in the manufacture of food packaging materials (see section 3.3) is not expected since the substance does not come into direct contact with food. Any unexpected contribution to overall dietary exposure from food packaging uses would be accounted for in the occurrence data for processed foods that were employed in the assessment.
TBOEP has been measured in fish in Canada (McGoldrick et al. 2014; Greaves et al. 2016b) and has been reported in certain foods (i.e., oatmeal, whole wheat bread, caramel candy, apple juice, baby food, canned peach and fruit-flavored popsicle) in the results of the Total Diet Study (US FDA 2006). In a study conducted in China, TBOEP was measured in dairy products, meat and vegetables (Zhang et al. 2016). As very limited Canadian occurrence data for TBOEP were identified, data employed in the dietary exposure assessment were predominantly from US total diet studies and, to a lesser extent, the Chinese study. Overall, limited data were available for TBOEP in foods, and some studies also reported low detection frequencies. The maximum TBOEP concentrations in foods and beverages used in the present assessment ranged from 0.29 ppb in dairy products to 110 ppb in grain products (Appendix E, Table E-2). Mean and 90th percentile “all person” exposures to TBOEP across all age groups ranged from 0.38 to 1.65 µg/kg bw/day and 0.66 to 2.73 µg/kg bw/day, respectively (Appendix E, Table E-4).
Based on results from two studies, TBOEP was measured in breast milk of women in Sweden, Japan, Philippines, and Vietnam (Sundkvist et al. 2010; Kim et al. 2014). The study that examined breast milk of women in the Asian countries reported an overall detection frequency of 39% and concentrations of TBOEP in breast milk of women living in urban areas or who lived close to an e-waste recycling site, or near a waste dumping site, ranged from not detected (method detection limit between 0.01 to 0.08 ng/g lipids) to 206 ng/g lipids (Kim et al. 2014). In the Swedish study, TBOEP was measured in 4 of 6 samples of which 5 of these were pooled composites of breast milk collected from 1997 to 2003 (Sundkvist et al. 2010). Collectively, the samples represented breast milk from 286 women, and a maximum concentration of 63 ng/g lipids was measured in a composite sample from 69 women from Uppsala in 1997. Given the higher sample size in the Swedish study, the value of 63 ng/g lipids from the composite sample measured in Swedish women was used to estimate exposure to breast-fed infants (0.22 µg/kg bw/day; see Appendix F, Table F-6).
Estimated total daily intakes of TBOEP from its presence in environmental media and food were derived and range from 0.7 µg/kg bw/d (60+ years) to 18.3 µg/kg bw/d (breast-fed 0-to-6-month olds) (Appendix F, Table F-6).
Products available to consumers
TBOEP was found in the foam of two sofas out of 132 furniture products purchased across Canada, the United States and Mexico between December 2014 and April 2015; both sofas were purchased in Canada (CEC 2015b). TBOEP was also found in 9 of 15 popular infant and child restraint seats tested in the United States. In all 9 cases, it was found in the foam component, and in 5 of the 9 cases, it was also found in the fabric (Miller and Gearhart 2016). Prolonged dermal exposures were estimated for these products and are summarized in Table 3‑15 (see Appendix G, Table G-5, for details on parameters). Limited dermal absorption data were identified for TBOEP. Frederiksen et al. (2018) reported flux and permeability coefficients for TBOEP and other organophosphate ester flame retardants but did not incorporate residues in the epidermis into the reported values. As discussed in section 2.7.1.4, this method may underestimate systemic exposure. Furthermore, the data for TBOEP are considered to be semi-quantitative by the authors, and the permeability coefficient that incorporated residues in the dermis was not recovery-corrected (Frederiksen et al. 2018). In vitro dermal absorption data have been reported for other organophosphate flame retardants (Frederiksen et al. 2018; EU RAR 2008a). Data from the Frederiksen et al. (2018) study suggest that TCPP has an equivalent or higher ability to penetrate the skin than TBOEP, so a read-across approach to TCPP was used (see section 3.1.1). Dermal absorptions of 23% and 40% were reported for TCPP based on in vitro dermal absorption studies using human skin membranes with direct application of radiolabelled TCPP (TNO Quality of Life 2005, 2006b, cited in EU RAR 2008a). The two values were derived from studies testing different doses; the dermal absorption of 40% was based on experiments using lower concentrations considered more representative of exposure from dermal contact with foam (EU RAR 2008a). Therefore 40% dermal absorption was used for exposures to TBOEP from foam-containing infant and child restraint seats and furniture.
In Europe, TBOEP has been measured in toys made of plastic, rubber, wood, and foam and textiles (Ionas et al. 2014). No studies of children’s products in Canada that included TBOEP were identified, but it could be expected that some of the same types of toys as those found to contain TBOEP in Europe may be present in Canada. Estimates of exposure to TBOEP via mouthing were estimated as described in Appendix G.
In Canada, TBOEP was reported as an ingredient in a limited number of products (MSDS 2014b) available to consumers. Exposure from the use of rust paint containing TBOEP was identified as the sentinel exposure scenario. Dermal exposure was estimated and is summarized in Table 3‑15 (see Appendix G, Table G-1 for details on parameters). Because TBOEP has a low vapour pressure, inhalation exposure during use of products available to consumers is expected to be negligible.
| Exposure route and duration | Source | Age group | Systemic exposure estimate |
|---|---|---|---|
| Oral (daily) | Foam in toys and children’s products | 0–6 months | 0.003–0.008 mg/kg bw/day |
| Oral (daily) | Foam in toys and children’s products | 0.5–4 years | 0.003–0.008 mg/kg bw/day |
| Dermal (daily)a | Foam in infant restraint seats and furniture | 0–6 months | 0.021–0.049 mg/kg bw/day |
| Dermal (daily)a | Foam in child restraint seats and furniture | 0.5–4 years | 0.017–0.040 mg/kg bw/day |
| Dermal (daily)a | Foam-containing furniture | 20+ years | 0.029–0.068 mg/kg bw/day |
| Dermal (per event)a | Rust paint | 20+ years | 0.76 mg/kg bw per event |
a Dermal absorption was assumed to be 40%.
3.7.1.3 TEHP
Environmental media and food
TEHP has been measured at low levels (< 1 ng/m3) in ambient air in Canada and elsewhere (Suhring et al. 2016; Salamova et al. 2014; Li et al. 2017; Möller et al. 2012; Zhou et al. 2017a). Estimated intakes of TEHP from ambient air are negligible.
There are two studies that measured TEHP in samples of dust and indoor air from Canadian homes (Vykoukalová et al. 2017; Yang et al. 2019). The study by Vykoukalová et al. (2017) had a sample size of 23 homes, which were located in Toronto, and was conducted in 2013. The study by Yang et al. (2019) had a sample size of 51 homes, which were located in Toronto and Ottawa, and was conducted in 2015. Yang et al. (2019) reported higher concentrations of TEHP in dust and indoor air, but lower detection frequencies than the study by Vykoukalová et al. (2017). As a conservative approach, the 95th percentile concentrations of TEHP in dust (1 780 ng/g) and indoor air (11.4 ng/m3) from the study by Yang et al. (2019) were used for characterizing general population exposure to TEHP in this screening assessment. Concentrations of TEHP in dust and indoor air in Canadian homes are similar to those reported in American and European homes (Coelho et al. 2016; Langer et al. 2016; Luongo and Östman 2016; Vykoukalová et al. 2017; Zhou et al. 2017a,b).
TEHP was measured in drinking water from one out of six water treatment plants tested in Eastern Ontario in 1978 (Lebel et al. 1981). The concentration of TEHP was reported to be 0.3 ng/L. TEHP was also measured in surface waters in Toronto, Lake Erie and Lake Huron (Venier et al. 2014; Truong 2016). However, concentrations were not reported because the detection frequency of TEHP was low (i.e., less than 20%). A similar pattern of low detection frequencies or absence of TEHP was reported in surface waters in the United States, Europe and China (Bohlen et al. 1989; Weber and Ernst 1983; Ernst 1988; Rodil et al. 2012; Yan et al. 2012; Venier et al. 2014). Estimated intakes of TEHP from drinking water are considered to be negligible.
No data on levels of TEHP in soil were identified in Canada. TEHP was detected in one study from China (Cui et al. 2017), and the maximum concentration of TEHP in soil collected from park areas, residential areas and paddy/vegetable fields was 0.015 mg/kg dw (range = ND to 0.015 mg/kg dw; MDL = 0.0004 mg/kg). Based on these values, exposures to TEHP from soil are expected to be negligible across all age groups in the Canadian population.
Potential exposure to TEHP from its use as a component in the manufacture of food packaging materials is not expected since the substance does not come into direct contact with food (personal communication, e-mail from the Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated January 19, 2018; unreferenced). TEHP was previously reported in a limited group of foods in the United States based on data from the FDA Total Diet Study for 1980 to 1991 (Gartrell et al. 1986a, 1986b; KAN-DO Office and Pesticides Team 1995). However, based on later Total Diet Study data from 1991 to 2005, TEHP was not detected (US FDA 2006). TEHP was not detected in fish in Canada (McGoldrick et al. 2014) or in Europe, for the most part (Giulivo et al. 2017). This substance was not found in a food survey in Sweden (Poma et al. 2017), but was found in some foods at low concentrations in Belgium (Poma et al. 2018) and China (Ma et al. 2013; Zhang et al. 2016). However, the applicability of these results to exposure of the general population in Canada is limited. Based on the overall weight of evidence, exposure of the Canadian general population to TEHP from foods is expected to be minimal.
Estimated total daily intakes of TEHP from its presence in environmental media were derived and range from 0.002 µg/kg bw/d (adults aged 25+ years) to 0.012 µg/kg bw/d (infants aged 0 to 6 months) (Appendix F, Table F-7).
Products available to consumers
In Canada, TEHP was reported as an ingredient in a floor joint sealant (Ash and Ash 2009, as cited in Knovel; Hoffmann Mineral 2018). Dermal exposure may occur during use and was estimated to be 0.21 to 1.3 mg/kg (see Appendix G, Table G-1 for details).
3.7.1.4 BEHP
Environmental media and food
No environmental monitoring data were identified for BEHP in air, dust or soil. Exposure to BEHP from these media is not expected to be a significant contributer to overall exposure to this substance given its physical-chemical properties and its limited presence in products available to consumers.
Hoa et al. (2018) reported BEHP concentrations in surface water ranging from 0.13 to 0.48 µg/L (streams, n = 20, detection frequency = 4/20, LOD = 1.5–30 ng/L) from samples collected in the Toronto area in 2014 to 2015. The maximum value from this study was used to estimate general population intakes from drinking water.
No data on the presence of BEHP in food or breast milk were identified.
Using the drinking water intakes for Canadians outlined in Appendix F, estimated total daily intakes of BEHP from its presence in drinking water ranged from 0.0097 µg/kg bw/d (adolescents aged 12–19 years) to 0.051 µg/kg bw/d (0- to 6-month-old formula-fed infants).
Products available to consumers
In Canada, BEHP was reported as an ingredient in gear oil, a type of auto care product that is available to consumers (Environment Canada 2013; MSDS 2013). Dermal exposure to BEHP may occur during use of gear oil and was estimated to be 0.13 mg/kg bw per event for adults (see Appendix G, Table G-1 for details).
3.7.1.5 Biomonitoring data for the alkyl OP subgroup
Generally, organophosphate esters are metabolized to the corresponding diester or hydroxylated metabolites and conjugates (Van den Eede et al. 2013; Dodson et al. 2014; Fromme et al. 2014; Su et al. 2015b; Kosarac et al. 2016; Völkel et al. 2018).
Diethylphosphate, a metabolite of TEP, was measured in urine samples in the US National Health and Nutrition Examination Survey (NHANES 2000-2004) and in a human biomonitoring study in Japan (Yoshida 2012). However, while diethylphosphate is a metabolite of TEP, it is also a metabolite of several organophosphate pesticides and therefore is not a suitable biomarker of exposure to TEP. TEP has also been measured in other biological matrices (e.g., placenta, hair, serum, and whole blood) in Asia (Ding et al. 2016; Qiao et al. 2016; Zhao et al. 2017).
Typical metabolites of TBOEP include bis(2-butoxyethyl) phosphate (BBOEP), desbutyl tris(2-butoxyethyl) phosphate (BBOEHEP) and tris(2-(3-hydroxy)butoxyethyl) phosphate (OH-TBOEP). Due to lack of specificity and low percentage of excretion in urine, BBOEP may not be a suitable biomarker to estimate exposure to TBOEP via reverse dosimetry (Völkel et al. 2018). Similarly, the urinary excretion rate of OH-TBOEP is even lower. For these reasons, BBOEHEP was proposed as the most suitable biomarker for exposure to TBOEP. The fractional urinary excretion was determined to be 0.0838 based on a toxicokinetic study in three male and three female volunteers following a single oral administration (Völkel et al. 2018). According to the results in this study, the maximum concentrations of BBOEHEP were observed between the first and third hour after administration of TBOEP. Taking the average across all participants, the half-life for BBOEHEP was determined to be 3.9 hours.
There are two biomonitoring studies in Canada that screened for BBOEHEP in urine. In one study, BBOEHEP was not detected above the detection limit of 0.2 ng/mL in the urine of 20 Canadian pregnant women (Kosarac et al. 2016). In the other study, BBOEHEP was detected in 18% of the urine samples from 44 premenopausal women from Toronto and Ottawa (Yang et al. 2019). The mean and 95th percentile creatinine-adjusted concentrations of BBOEHEP in urine were reported to be 4.86 ng/g creatinine and 33.7 ng/g creatinine, respectively. Reverse dosimetry was used to derive estimates of daily intakes based on these urinary concentrations. Accordingly, the daily intakes were estimated to be 9.8 × 10-4 µg/kg bw/day (based on the mean concentration) and 6.8 × 10-3 µg/kg bw/day (based on the 95th percentile concentration). Details regarding reverse dosimetry are provided in Appendix G.
BEHP was measured as a urinary metabolite of TEHP in a methodology study (Su et al. 2015b). However, BEHP itself is a substance that may be present in the environment and in products available to consumers, and therefore the origin of the compound in urine is unknown.
3.7.2 Health effects assessment of the alkyl OP subgroup (TEP, TBOEP, TEHP, BEHP)
3.7.2.1 TEP
TEP has been reviewed by the OECD in a Screening Information Data Set (SIDS) dossier (OECD 2002a). This review was used to inform the health effects characterization of TEP. Targeted literature searches were conducted from a year prior to the OECD (2002a) report to March 2018. No health effect studies that would impact the risk characterization (i.e., result in different critical endpoints or lower points of departure) were identified.
Toxicokinetics
TEP administered orally to rodents is rapidly excreted in urine (90% within 16 hours).
Carcinogenicity and genotoxicity
No carcinogenicity studies were reported for TEP.
Genotoxicity studies were mostly negative. TEP was found to be weakly mutagenic in some non-standard bacteria, virus and yeast assays (OECD 2002a). A follow up hypoxanthine-guanine phosphoribosyltransferase (HPRT) test in V79 cell cultures was negative (OECD 2002a). TEP showed no DNA-damaging effects. The results for Drosophilia melanogaster in the limited documented recessive-lethal tests are contradictory, while in vivo studies on the mouse (cytogenetics in the bone marrow, dominant lethal test) were negative (OECD 2002a). On the basis of all the available data on genotoxicity, TEP is not expected to be mutagenic.
Repeated dose toxicity
In a 90-day gavage study, rats were exposed to TEP at concentrations of 60, 200 or 700 mg/kg bw/day (ECHA c2007-2018a). Increased liver weight and minimal to slight hepatocellular hypertrophy were observed in the high-dose group. Hyaline droplet accumulation and increased kidney weight (also observed as enlarged kidney) and increased potassium and calcium levels in blood also accompanied these findings. The NOAEL for this study was established at 200 mg/kg bw/day (ECHA c2007-2018a).
In a repeated dose gavage study, Wistar rats were administered TEP at doses of 0, 10, 100 or 1000 mg/kg bw/day for 28 days. Changes were noted in the 1000 mg/kg bw/day group, such as slight hepatocellular hypertrophy in both sexes, increased absolute liver weights (only significant in females) and decreased body weight gain in males (10%). The effects were caused by an increase in metabolic activity in the liver and a sign of adaption of liver function. The NOAEL was therefore considered to be 1000 mg/kg bw/day (Bayer 1992, as cited in OECD 2002a).
Other oral studies on TEP were also available. In one study, no adverse effect was observed in a single dose 4-week study in mice (274 mg/kg bw/day; 1/5 LD50) (Pyatlin 1968, as cited in US EPA 1985). In another study, only non-significant changes in organ weight of rats compared to controls were observed after a 9-week exposure to a dose of 335 mg/kg bw/day (Oishi et al. 1982, as cited in US EPA 1985). TEP has also been demonstrated to have the potential to have a depressive effect on the central nervous system and slight inhibition of cholinesterases at high doses (BUA 37 1989, as cited in US EPA 1985).
In a short-term inhalation study, rats were exposed to TEP 12 times (5 hours/day, 5 days/week) at concentrations of 0, 366 or 1786 mg/m3 as an aerosol. About 35% of the aerosol was respirable. Effects observed in the high-dose group consisted of lethargy, decreased aural investigatory reflex, unsteadiness of gait, and porphyrin-like nasal discharge, all of which occurred daily but subsided prior to subsequent exposure. However, clinical signs, organ weights, clinical chemistries, and hematology were normal, and no gross or microscopic pathology was observed (Eastman Kodak 1984, as cited in OECD 2002a; US EPA 1985). In another study, no acute inhalation toxicity, other than transient narcosis effects, was observed after a single 4-hour exposure up to 8817 mg/m3. A NOEC was established at 1400 mg/m³, based on marginal retardation in weight gain in male rats in the first week (ECHA c2007-2018a). The available information indicates that there are no critical systemic effects following single or periodic or intermittent acute exposures to TEP via the inhalation route.
Reproductive and developmental toxicity
In a reproductive toxicity study, SD rats were fed TEP at dietary levels of 0.1%, 0.5%, 1.0%, 5.0% or 10% by weight in the diet (corresponding to 67, 335, 670, 3350 or 6700 mg/kg bw/day, respectively). Males were administered TEP 92 days before mating and up to 120 days in total, and females received TEP for 150 days (until weaning at day 21). Females and their offspring were subsequently examined for reproductive effects (Gumbmann et al. 1968). Severe anorexia in the high-dose group made it necessary to exclude this group from consideration of TEP toxicity. Reproduction was adversely affected at 1% TEP and was completely prevented at 5% TEP. Growth of the young was slightly retarded (though not significantly) in all groups receiving TEP when adjustment of weaning weights to a constant litter size was performed. Neither testicular weights nor the histological investigation of the testes revealed remarkable findings in this study. The NOAEL was determined to be 335 mg/kg bw/day for fertility effects. Significant retardation in weight gain, elevated liver and adrenals weights, and liver lesions were observed at 3350 mg/kg bw/day. The maternal NOAEL was considered to be 670 mg/kg bw/day.
In a developmental study, Wistar rats were exposed by gavage to TEP from day 6 to 15 post coital at doses of 0, 25, 125 or 625 mg/kg bw/day (Bayer 1995). There were no developmental effects up to the highest dose tested. Reduction of body weight gain, food intake, and feces excretion as a sign of maternal toxicity was observed at 625 mg/kg bw/day (NOAELmat = 125 mg/kg bw/day).
In a 28-day reproductive toxicity study with doses up to 1000 mg/kg bw/day, no testicular weight changes were observed (Bayer 1992, as cited in OECD 2002a).
3.7.2.2 TBOEP
TBOEP has been reviewed by ATSDR (2012), IPCS (WHO 2000) and ECETOC (1992b). These reviews were used to inform the health effects characterization of TBOEP. A search of the literature from a year prior to the 2012 ATSDR report to March 2018 was conducted. No health effect studies that would impact the risk characterization (i.e., result in different critical endpoints or lower points of departure than those stated in ATSDR 2012) were identified.
Toxicokinetics
Typical metabolites of TBOEP include bis(2-butoxyethanol, butoxyethyl) phosphate (BBOEP), desbutyl tris(2-butoxyethyl) phosphate (BBOEHEP) and tris(2-(3-hydroxy)butoxyethyl) phosphate (OH-TBOEP) (Völkel et al. 2018). Absorption of TBOEP may occur via dermal, oral, and respiratory routes. The metabolites have been detected in urine and may also be excreted in exhaled air and in the feces. The unchanged parent compounds have been detected in the urine in trace amounts only (US EPA 1985).
Carcinogenicity and genotoxicity
No carcinogenicity studies were reported for TBOEP. Bacterial and mammalian cell tests for gene mutation gave negative results, but no tests for chromosomal damage have been reported. Therefore, TBOEP is not expected to be genotoxic (ATSDR 2012).
Repeated dose toxicity
In an 18-week study, SD rats were fed diets containing TBOEP at doses corresponding to 0, 17, 173 or 578 mg/kg bw/day for males and to 0, 21, 209 or 698 mg/kg bw/day for females (Monsanto 1987a, as cited in WHO 2000; Reyna and Thake 1987, as cited in ATSDR 2012). Microscopic examination showed mild periportal hepatocellular hypertrophy and periportal vacuolization in males at mid- and high-dose levels. No significant histopathology was reported in female rats. Liver weight was increased in the highest dose group, but not significantly. Body weight, food intake and clinical observations were similar in treated and control rats. Hematological and clinical chemistry parameters were normal, except for increased platelet counts in the highest dose group. The LOAEL was considered to be 173 mg/kg bw/day. A BMDL10 of 8.88 mg/kg bw/day for hepatocyte vacuolization in males was derived by the ATSDR (2012) in order to calculate an oral intermediate minimal risk level (MRL).
Mixed results were observed in two other oral subchronic studies. No adverse effects were observed at 200 mg/kg bw/day after 14-week exposure to TBOEP, and a LOAEL of 2000 mg/kg bw/day was identified based on moderate periportal hepatocyte swelling in male rats (Tsuda et al. 1993; Saitoh et al. 1994, both cited in WHO 2000). However, no NOAEL could be identified in another 18-week study where liver and kidney weights increased (20% relative to the control) and narcosis effects were observed at 255 mg/kg bw/day (Laham et al. 1984a, 1985a, as cited in WHO 2000).
Short-term exposure
In a 10-day developmental toxicity study (see details below), a significant decrease in weight gain (35%) was observed in dams at 1500 mg/kg bw/day (Monsanto Co 1985b, as cited in ATSDR 2012). Ataxia and lethargy were also observed. The systemic NOAEL was 500 mg/kg bw/day in this study. A BMDL of 477 mg/kg bw/day was derived by the ATSDR (2012) for an acute-duration oral exposure (14 days or less) to TBOEP.
In the other two available oral studies (14-day and 4-week diet rat studies), effects observed were limited to a slight decrease in body weight and food consumption in females receiving diets containing 375 or 750 mg/kg bw/day (no effect in males). No compound-related changes were observed at necropsy (Komsta 1989, as cited in WHO 2000; Monsanto 1985a, as cited in WHO 2000).
In a 21-day dermal study, New Zealand rabbits (6/sex/group) were exposed to 0, 10, 100 or 1000 mg/kg bw/day of TBOEP for 3 weeks (5 days/week; unabraded clipped skin; occluded for 6 hours/day) (Monsanto 1985d, as cited in ATSDR 2012). There was no indication that dermal exposure to TBOEP resulted in any adverse systemic effects. Local irritation, edema, atonia and desquamation occurred at all dose levels.
There were several studies that measured red blood cell cholinesterase activity. However, a statistically significant decrease (magnitude unspecified) was observed in only one study in rats after 9 weeks of treatment. This effect was not found after 18 weeks of treatment. There were no clinical signs associated with the decrease in cholinesterase activity (ATSDR 2012).
Reproductive and developmental toxicity
In a teratology study in which 25 mated female rats were administered TBOEP by gavage at doses of 0, 250, 500 or 1500 mg/kg bw/day on GD 6 to 15, no developmental effects were observed (Monsanto Co 1985b, as cited in ATSDR 2012). The treatment had no effect on fetal resorption, fetal viability, post-implantation loss, total implantations or the incidence of fetal malformations. Maternal toxicity such as ataxia and lethargy and significant decrease in weight gain (35%) were observed at 1500 mg/kg bw/day. Therefore, the NOAEL for developmental toxicity is 1500 mg/kg bw/day and the NOAEL for maternal toxicity is 500 mg/kg bw/day (ATSDR 2012).
3.7.2.3 TEHP
TEHP has been reviewed by the US EPA (2009), WHO (2000) and ECETOC (1992a). These reviews were used to inform the health effects characterization of TEHP. A search of the literature from a year prior to the US EPA (2009) report to March 2018 was conducted.
Toxicokinetics
TEHP is expected to be metabolized to BEHP and mono(2-ethylhexyl) phosphate (CAS RN 1070-03-7). Based on the evidence for dealkylation as the primary metabolic pathway, 2-ethylhexanol is expected to be the primary metabolite of TEHP (ACC 2005; Kluwe, et al. 1985).
TEHP and/or its metabolites were distributed into the lungs (13% of total radioactivity after 5 minutes), stomach contents (64% after first hour), brain (9%) and liver (16%). Lower amounts (less than 2%) were found in other organs or tissues (spleen, kidney, bone, muscle and fat) (MacFarland and Punte 1966, as cited in WHO 2000).
Carcinogenicity and genotoxicity
TEHP was tested for chronic toxicity and carcinogenicity in rats and mice (US NTP 1984, as cited in WHO 2000). TEHP was administered to 50 male (2000 mg/kg bw/day and 4000 mg/kg bw/day) and 50 female (1000 mg/kg bw/day and 2000 mg/kg bw/day) Fischer-344 rats by gavage for 5 days/week for 103 weeks. TEHP was also similarly administered to 50 male and 50 female B6C3F1 mice at 500 mg/kg bw/day and 1000 mg/kg bw/day. The US NTP concluded that there was some evidence of carcinogenicity based on an increased incidence of hepatocellular carcinomas in female mice at the high-dose level (1000 mg/kg bw/day) and equivocal evidence of carcinogenicity based on an increased incidence of adrenal pheochromocytomas in male rats at both dose levels. However, pheochromocytomas show a variable background incidence in rats, and the incidence of these tumours in two previous NTP bioassays was equal to the incidence observed in the TEHP bioassay. The only other significant neoplastic finding was hepatocellular carcinomas in the high-dose group of female mice. Therefore, these results indicate that TEHP does not present a significant carcinogenic risk to humans (WHO 2000). In the chronic study in mice, the LOAEL for thyroid follicular cell hyperplasia was 357 mg/kg bw/day. A NOAEL in mice was not established. The NOAEL for chronic toxicity in male rats was 2857 mg/kg bw/day and in female rats was 1428 mg/kg bw/day.
TEHP is not expected to be genotoxic (US EPA 2009).
Repeated dose toxicity
Only a small suppression in body weight gain was observed in rats receiving 0, 250, 500, 1000, 2000 or 4000 mg/kg bw/day and in mice receiving 0, 500, 1000, 2000, 4000 or 8000 mg/kg bw/day of TEHP by gavage (5 days/week) for 13 weeks. No deaths, toxic effects or induced histological alteration were attributed to TEHP administration at any of these treatment dosage levels (US NTP 1984, as cited in WHO 2000; Kluwe et al. 1985). The suppression of body weight gain at the highest dose in male and female rats was 5%; in male and female mice the suppression of weight gain at the highest dose was 7% and 5%, respectively. However, these minimal changes were not considered biologically significant. Hence, the NOAELs in rats and mice were identified to be 2860 mg/kg bw/day and 5710 mg/kg bw/day, respectively (WHO 2000). This study did not examine thyroid effects.
Preliminary results obtained from an oral study in which rats were administered TEHP at dose levels of 0, 300, 1000 or 3000 mg/kg bw/day for 28 consecutive days indicate that only rat weight gains in males were affected in the 3000 mg/kg bw/day treatment group. Limited effects in the high-dose group on serum chemistry and hematological parameters, such as reduced mean platelet volume (both sexes) and higher white blood cell counts (females only) were observed. Apart from in the testis and epididymis (3000 mg/kg bw/day) (described in next section), no histological changes or lesions were observed in treated rats. Therefore, the NOAEL for this study is considered to be 1000 mg/kg bw/day for TEHP based on decreased body weight gain (Pelletier et al. 2020).
In other available oral studies, reported health effects of TEHP were primarily related to decreased body weight at high concentrations. No deaths, toxic effects or induced histological alterations were attributed to TEHP administration at any of the treatment levels other than a slight to moderate suppression in body weight gain (US NTP 1984; Kluwe et al. 1985; Smyth and Carpenter 1948, as cited in WHO 2000).
In a limited repeated dose dermal study in rabbits, TEHP was applied to clipped, intact skin of the upper back of male rabbits (MacFarland and Punte 1966, as cited in WHO 2000). Animals received a total of 10 (4/6 animals) or 20 applications (2/6 animals). The animals appeared normal and gained weight; no alterations were observed at necropsy. Moderate erythema following the first application (250 mg undiluted TEHP) was observed. Following subsequent applications of 0.1 mL on 5 days/week (10 or 20 applications), the erythema did not increase in intensity, but a gradual increase in the size of the affected zone was seen. Desquamation, hemorrhagic areas, and thickening of the skin were also observed. Microscopic examination revealed hyperkeratosis and parakeratosis.
A 3-month inhalation study exposed guinea pigs, dogs and rhesus monkeys to TEHP for 6 hours/day, 5 days/week for a total of 60 exposures and revealed no significant effects up to a concentration of 85 mg/m3. High mortality was observed in the guinea pigs study due to respiratory infections and it was therefore considered invalid. In dogs,mild chronic inflammatory changes in the lungs and deterioration of conditional avoidance performance in relation to the concentration administered were observed (MacFarland and Punte 1966).
Reproductive and developmental toxicity
In a developmental toxicity/teratogenicity study, Wistar rats were exposed daily by gavage to 0, 500 and 1000 mg/kg bw/day of TEHP on days 6 to 20 post coitum (ECHA c2007-2018b). No maternal toxicity was noted in any of the treatment groups. Fetal pathology (external, visceral, and skeletal examinations) did not reveal any test item-related findings. Based on the results of this study, the NOEL for maternal and developmental effects was considered to be 1000 mg/kg bw/day.
Reproductive toxicity to TEHP was observed in the 28-day rat study described above. Exposure to high doses of TEHP (3000 mg/kg bw/day) resulted in the retention of mature spermatids in testes. Additional disturbances of testis and epididymis histology were also observed (Pelletier et al. 2020). The NOAEL for reproductive effects is therefore 1000 mg/kg bw/day for TEHP.
3.7.2.4 BEHP
BEHP has been reviewed by the US EPA (US EPA 2009) and ECETOC (1992a). These reviews were used to inform the health effects characterization of BEHP. A search of the literature from a year prior to the US EPA review (2009) to March 2018 was conducted. No health effect studies that would impact the risk characterization (i.e., result in different critical endpoints or lower points of departure) were identified.
Toxicokinetics
No information on the distribution, absorption, metabolism or excretion of BEHP is available. BEHP is a primary metabolite of TEHP (ACC 2005; Kluwe et al. 1985).
Carcinogenicity and genetic toxicity
No carcinogenicity studies were reported for BEHP.
BEHP was not genotoxic in several in vivo and in vitro tests for mutagenicity (US EPA 2009).
Repeated dose toxicity
In a 28-day study, Wistar rats were exposed by gavage to 0, 30, 150 or 750 mg/kg bw/day of BEHP (ECHA c2007-2018b). The NOAEL of the study is 30 mg/kg bw/day based on evidence of treatment-related effects on liver function at 150 mg/kg bw/day. In males, protein concentration was significantly decreased at 750 mg/kg bw/day, relative liver weights were increased starting at 30 mg/kg bw/day in males and at 750 mg/kg bw/day in females, and hepatocellular hypertrophy was present starting at 150 mg/kg bw/day. Hypertrophy of the follicular epithelia in the thyroid gland starting at 150 mg/kg bw/day was noted secondary to findings of liver metabolism activation. At histopathologic evaluation, diffuse hyperplasia of the zona fasciculata in the adrenal gland was noted starting at 150 mg/kg bw/day in males and 750 mg/kg bw/day in females. At necropsy, absolute and relative weights of adrenals were increased in males at the dose of 750 mg/kg bw/day. In a preliminary 28-day oral study in Fischer rats dosed with 20, 60 and 180 mg/kg bw/day BEHP by gavage, effects on body weight gain (males), organ weights, stomach histopathology, hematology and serum chemistry were observed starting at 60 mg/kg bw/day (Pelletier et al. 2020).
No dermal studies were available for BEHP.
Reproductive and developmental toxicity
No developmental studies were available for BEHP.
In the 28-day Wistar rat study described above, the weight of seminal vesicles was decreased correlating to reduced secretion in the prostate and seminal vesicle at histopathology at 750 mg/kg bw/day. Additionally, a minimally increased proportion of round spermatids in the epididymitis were noted. The study authors note that these findings might indicate a primary systemic effect, but that it is more likely that they reflect delayed sexual maturation as a sequel of poor condition of these animals. The NOAEL for reproductive effects was identified to be 750 mg/kg bw/day for BEHP (ECHA c2007-2018b).
3.7.3 Characterization of risk to human health of the alkyl subgroup (TEP, TBOEP, TEHP, BEHP)
3.7.3.1 TEP
No carcinogenicity studies were identified for TEP. Considering all the available data on genotoxicity, TEP is not expected to be genotoxic. Based on the available studies, two oral rat studies have been selected as the critical studies to characterize risks to human health from exposures to TEP. A 90-day oral study in which liver effects were observed and the NOAEL was 200 mg/kg bw/day was used to characterize risk from repeated exposure (ECHA c2007-2018a). This is protective of reproductive effects observed at higher doses (NOAEL of 335 mg/kg bw/day). The second study is an oral 28-day toxicity study in rats by Bayer Co. (1995) with a NOAEL of 1000 mg/kg bw/day. These studies were selected on the basis of the endpoints examined, the quality of the methodology, and the identification of the most appropriate NOAEL values.
No dermal studies were available, and for that reason, the NOAEL of 1000 mg/kg bw/day from the aforementioned oral 28-day study was used as the point of departure to characterize risk from short-term exposure. The risk to human health related to potential dermal exposures to TEP from use of products available to consumers, such as a foam sealant or all-purpose remover, is therefore considered to be low.
The available information from inhalation studies indicates that the effects observed (narcosis) following acute exposure to high concentrations of TEP are mild, transient and reversible once the subject is removed from the exposure source. Therefore, the risk to human health related to potential exposures to TEP via the inhalation route from use of products available to consumers, such as a foam sealant or all-purpose remover, is considered to be low.
Table 3‑16 provides all relevant exposure estimates and hazard points of departure as well as the resultant margins of exposure (MOEs) for the characterization of risk for exposures to TEP.
| Exposure scenario | Systemic exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Environmental media | 0.054–0.16 µg/kg bw/day | NOAEL = 200 mg/kg bw/day | Liver effects in 90-day study | > 1 million |
| Mouthing foam in children’s products (oral) | 0.018–0.54 mg/kg bw/day (0–6 months) | NOAEL = 200 mg/kg bw/day | Liver effects in 90-day study | 370–11111 |
| Mouthing foam in children’s products (oral) | 0.017–0.53 mg/kg bw/day (0.5–4 years) | NOAEL = 200 mg/kg bw/day | Liver effects in 90-day study | 377–11765 |
| Foam or fabric in infant restraint seats (dermal) | 0.28–8.60 mg/kg bw/day (0–6 months; 100% absorption) | NOAEL = 200 mg/kg bw/day (oral) | Liver effects in 90-day study | 23–714 |
| Foam or fabric in child restraint seats (dermal) | 0.22–6.91 mg/kg bw/day (0.5–4 years; 100% absorption) | NOAEL = 200 mg/kg bw/day (oral) | Liver effects in 90-day study | 29–909 |
| Foam containing mattress or furniture (dermal) | 2.8–294 mg/kg bw/day (0–6 months; 100% absorption) | NOAEL = 200 mg/kg bw/day (oral) | Liver effects in 90-day study | < 1–71 |
| Foam containing mattress or furniture (dermal) | 2.0–224 mg/kg bw/day (0.5–4 years; 100% absorption) | NOAEL = 200 mg/kg bw/day (oral) | Liver effects in 90-day study | < 1–100 |
| Foam containing mattress or furniture (dermal) | 1.3–156 mg/kg bw/day (5–11 years; 100% absorption) | NOAEL = 200 mg/kg bw/day (oral) | Liver effects in 90-day study | 1–154 |
| Foam containing mattress or furniture (dermal) | 1.31–134 mg/kg bw/day (12–19 years; 100% absorption) | NOAEL = 200 mg/kg bw/day (oral) | Liver effects in 90-day study | 1–153 |
| Foam containing mattress or furniture (dermal) | 0.75–103 mg/kg bw/day (20+ years; 100% absorption) | NOAEL = 200 mg/kg bw/day (oral) | Liver effects in 90-day study | 2–267 |
Abbreviations: NOAEL, no observed adverse effect level; 100% dermal absorption is assumed for the dermal scenarios because dermal absorption is considered in this assessment to be equivalent to oral absorption, given that an oral toxicity study is being used for the risk characterization.
On the basis of the conservative approaches used in estimating exposures to TEP from environmental media, from mouthing foam items, and from the use of all-purpose remover and foam sealant, the calculated MOEs are considered adequate to address any uncertainties in the health effects and exposure databases. However, the calculated MOEs from prolonged dermal exposure to TEP from lying on foam-containing mattresses or furniture (all age groups) and sitting in infant or child restraint seats are considered potentially inadequate to account for uncertainties in the databases.
3.7.3.2 TBOEP
No carcinogenicity studies were identified for TBOEP. TBOEP is not expected to be genotoxic (ATSDR 2012). An 18-week oral study was selected as the most relevant study to characterize the risk to human health from exposures to TBOEP (Reyna and Thake 1987, as cited in ATSDR 2002). A BMDL10 of 8.88 mg/kg bw/day was derived by the ATSDR (2012) based on periportal hepatocellular vacuolization in males at 173 to 209 mg/kg bw/day (NOAEL = 17-21 mg/kg bw/day). Since no long-term dermal study was available, this BMDL10 was used as the point of departure for characterization of the risk from daily exposures via the dermal route. No reproductive or developmental effects were observed after dermal exposure to doses up to 1000 mg/kg bw/day of TBOEP (Monsanto 1985d, as cited in ATSDR 2012). The NOAEL of 1000 mg/kg bw/day was used to characterize the risk to human health from short-term dermal exposure to TBOEP from the use of rust paint.
The Canadian general population may be exposed to TBOEP from dust, indoor air, water and food, including breast milk. The highest exposure to TBOEP from the use of products available to consumers was considered to be associated with sitting on a sofa or in an infant or child restraint seat containing TBOEP, or with using rust paint. Table 3‑17 provides all relevant exposure estimates and hazard points of departure as well as the resultant MOEs from the characterization of risk for exposure to TBOEP.
| Exposure scenario | Systemic exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Environmental media and food | 0.7–18.7 µg/kg bw/day | BMDL= 8.88 mg/kg bw/day | Periportal hepatocellular vacuolization in males in a 18-week rat study | 475–12686 |
| Mouthing foam in toys and children’s products (oral) | 0.003–0.008 mg/kg bw/day (0–6 months) | BMDL= 8.88 mg/kg bw/day | Periportal hepatocellular vacuolization in males in a 18-week rat study | 1110–2960 |
| Mouthing foam in toys and children’s products (oral) | 0.003–0.008 mg/kg bw/day (0.5–4 years) | BMDL= 8.88 mg/kg bw/day | Periportal hepatocellular vacuolization in males in a 18-week rat study | 1100–2960 |
| Foam in an infant restraint seat or on a sofa (dermal)a | 0.021–0.049 mg/kg bw/day (0–6 months) | BMDL= 8.88 mg/kg bw/day | Periportal hepatocellular vacuolization in males in a 18-week rat study | 181–423 |
| Foam in a child restraint seat or on a sofa (dermal) a | 0.017–0.040 mg/kg bw/day (0.5–4 years) | BMDL= 8.88 mg/kg bw/day | Periportal hepatocellular vacuolization in males in a 18-week rat study | 222–522 |
| Foam containing furniture (dermal)a | 0.029–0.068 mg/kg bw/day (20+ years) | BMDL= 8.88 mg/kg bw/day | Periportal hepatocellular vacuolization in males in a 18-week rat study | 131–306 |
| Rust paint (dermal) a | 0.76 mg/kg bw per event | NOAEL = 1000 mg/kg bw/day (HDT) | No effect in a 3-week rabbit study | 1316 |
| Biomonitoring data (adult women) | 0.0068 µg/kg bw/day | BMDL= 8.88 mg/kg bw/day | Periportal hepatocellular vacuolization in males in a 18-week rat study | > 1 million |
Abbreviations: BMDL, benchmark dose level; NOAEL, no observed adverse effect level; HDT, highest dose tested
a An adjusted dermal absorption of 40% for TCPP (EU RAR 2008a) was used to represent dermal absorption of TBOEP.
On the basis of the conservative approaches used in estimating exposures to TBOEP from environmental media and food and from products available to consumers, the calculated MOEs are considered adequate to address any uncertainties in the health effects and exposure databases.
3.7.3.3 TEHP
TEHP was tested for chronic toxicity and carcinogenicity in rats and mice. There were increases in adrenal pheochromocytomas in both dose groups of male rats and in hepatocellular carcinomas in female mice in the high-dose group. In rats, pheochromocytomas show a variable background incidence. Taking into account the low incidence of hepatocellular carcinomas, its occurrence in only one sex of one species, and the lack of evidence of genetic toxicity, TEHP is not considered to present a significant carcinogenic risk to humans (US NTP 1984, as cited in WHO 2000). TEHP is not expected to be genotoxic (US EPA 2009). The LOAEL of 357 mg/kg bw/day, based on thyroid follicular cell hyperplasia in the chronic study in mice, was selected as the critical effect level to characterize the risk to human health from daily exposures to TEHP. The dermal study available was limited, and therefore the NOAEL of 1000 mg/kg bw/day from a 28-day oral repeated-dose study was used to characterize the risk from acute dermal exposure to products available to consumers containing TEHP (Pelletier et al. 2020).
Table 3‑18 provides all relevant exposure estimates and hazard points of departure as well as the resultant MOEs for the characterization of risk for exposures to TEHP.
| Exposure scenario | Systemic exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Environmental media | 0.002–0.012 µg/kg bw/day | LOAEL = 357 mg/kg bw/day | Thyroid follicular cell hyperplasia in a 103-week study | > 1 million |
| Floor joint sealant (dermal)a | 0.21 to 1.3 mg/kg bw per event | NOAEL = 1000 mg/kg bw/day | Body weight gain decrease in oral 28-day study | 769–4762 |
Abbreviations: LOAEL, lowest-observed-adverse-effect-level; NOAEL, no observed adverse effect level.
a Assumed dermal absorption is equivalent to oral.
On the basis of the conservative approaches used in estimating exposures to TEHP from environmental media and from products available to consumers, the calculated MOEs are considered adequate to address any uncertainties in the health effects and exposure databases.
3.7.3.4 BEHP
No carcinogenicity studies were identified for BEHP; however, it could be assumed that BEHP does not likely pose a carcinogenic risk to humans based on the carcinogenicity study on TEHP. BEHP is not expected to be genotoxic. A NOAEL of 30 mg/kg bw/day based on liver effects in a 28-day oral study (ECHA c2007-2018b) was identified as the most relevant endpoint for characterization of risk from exposure to BEHP.
The predominant source of exposure to BEHP is expected to be from drinking water and from the use of gear oil. Table 3‑19 provides all relevant exposure estimates and hazard points of departure as well as the resultant MOEs for the characterization of risk for exposures to BEHP.
| Exposure scenario | Systemic exposure | Critical effect levelb | MOE |
|---|---|---|---|
| Drinking water | 0.0097–0.051 µg/kg bw/day | NOAEL = 30 mg/kg bw/day | > 588235 |
| Gear oil (dermal)a | 0.13 mg/kg bw per event | NOAEL = 30 mg/kg bw/day | 231 |
Abbreviations: NOAEL, no observed adverse effect level
aAssumed 100% dermal absorption. Given we are using an oral toxicity study for the risk characterization we consider dermal absorption to be equivalent to that of oral.
b Effects on the liver function (28-day study)
On the basis of the conservative approaches used in estimating exposures to BEHP from drinking water and from the use of a limited number products available to consumers, the calculated MOEs are considered adequate to address any uncertainties in the health effects and exposure databases.
3.7.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| Limited Canadian environmental monitoring data (including food) were identified. Data from other countries were used to derive exposure estimates when applicable. | +/- |
| The primary source of occurrence data (United States and China) employed in the dietary exposure assessment reported very low positive detection rates of TBOEP in foods, and this information was not quantitatively considered in the exposure assessment. | + |
| The maximum TBOEP concentrations reported were applied to all food categories and were assumed to be representative of a broader category as a whole. | +/- |
| Limited available data on the presence of TEP and TBOEP in foam in infant and child restraint seats and TBOEP in foam-containing furniture in Canada. | + |
| Absence of data on migration of TBOEP and TEP out of foam materials. | +/- |
| Dermal absorption data for TEP, TBOEP, TEHP and BEHP are limited; therefore an analogue approach or 100% dermal absorption was assumed. | + |
| There are no chronic toxicity studies for TEP or TBOEP, and the overall database on BEHP is limited. | - |
| The 13-week NTP study for oral exposure in rats did not examine thyroid effects for TEHP. | - |
| The database on the dermal toxicity of TEP, TBOEP, TEHP and BEHP is limited. | +/- |
4. Assessment of tetradecabromo-1,4-diphenoxybenzene (TDBDPB)
4.1 Substance identity
The CAS RN, common name, chemical structure and molecular formula and molecular weight for benzene, 1,2,4,5-tetrabromo-3,6-bis(pentabromophenoxy)- are presented in Table 4‑1. The substance will hereinafter be referred to by its abbreviation, TDBDPB.
|
CAS RN (acronym) |
DSL name (common name) |
Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
|
58965-66-5 (TDBDPB) |
benzene, 1,2,4,5-tetrabromo-3,6-bis(pentabromophenoxy)- (tetradecabromo-1,4-diphenoxybenzene) |
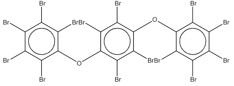 C18Br14O2 C18Br14O2
|
1366.85 |
Abbreviations: CAS RN, Chemical Abstracts Service Registry Number; DSL, Domestic Substances List
Impurities of up to 10% may occur for this substance and may include decabromodiphenyl ether (decaBDE; CAS RN 1163-19-5), octabromodibenzo-p-dioxin (CAS RN 2170-45-8) and hexabromo benzene (CAS RN 87-82-1). DecaBDE has been previously assessed and managed under the Existing Substances Program (EC 2006; EC 2010).
4.1.1 Selection of analogues and use of (Q)SAR models
A read-across approach using data from an analogue substance and the results of (quantitative) structure-activity relationship ((Q)SAR) models, where appropriate, has been used to inform the ecological and human health assessments. The analogue was selected based on structural and/or functional similarity to TDBDPB and presence of relevant empirical data. The applicability of (Q)SAR models was determined on a case-by-case basis. Details of the read-across data and (Q)SAR models chosen to inform the ecological assessment of TDBDPB are further discussed in the relevant sections of this report.
The analogue used to inform the ecological assessment is presented in Table 4‑2. Decabromodiphenyl ether (decaBDE) represents the closest structural analogue available and is considered appropriate for certain physical-chemical properties, transformation and ecotoxicity when reliable data on TDBDPB are not available.
|
CAS RN (abbreviation) |
DSL name (common name) |
Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
|
1163-19-5 (decaBDE) |
bis(pentabromophenyl) ether (benzene, 1,1'-oxybis[2,3,4,5,6-pentabromo]) (decabromodiphenyl ether) |
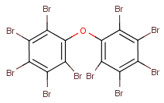 C12Br10O C12Br10O
|
959.171 |
a Environment Canada 2010.
Abbreviations: CAS RN, Chemical Abstracts Service Registry Number; DSL, Domestic Substances List
4.2 Physical and chemical properties
A summary of physical and chemical property data for TDBDPB is presented in Table 4‑3, with the range in values indicated for each property. Empirical physical and chemical property data for this substance have not been identified; therefore, read-across of relevant empirical information from its analogue, decaBDE, and any available modelled results were considered. However, there are only a few reliable modeled values for TDBDPB available as this substance has limited representation (due to large molecular size and chemistry) within model training sets. Since the physical-chemical properties listed in Table 4‑3 for TDBDPB are based on model domain extremes and values for decaBDE, rather than on TDBDPB itself, these values should be interpreted with caution. Given its structural characteristics (fully brominated three phenol rings), it is reasonable to expect that TDBDPB would be more hydrophobic than decaBDE.
| Property | Value or range | Type of data | Key reference(s) |
|---|---|---|---|
| Physical state | Solid | Experimental | Study Submission 1984 |
| Melting point (°C) | 350a–419 | Modelled | MPBPWIN 2010; TEST 2016 |
| Vapour pressure (Pa) | 4.63 x 10-6 | Experimental (read-across decaBDE) | EC 2006 |
| Henry’s law constant (Pa·m3/mol) | 6.63 x 10-7a | Modelled | HENRYWIN v3.20 2011 (bond contribution method) |
| Water solubility (mg/L) | < 0.0001 | Experimental (read-across decaBDE) | EC 2006; EC 2010 |
| log Kow (dimensionless) | 8.7 | Experimental (read-across decaBDE) | EC 2010 |
| log Kow (dimensionless) | > 10a | Modelled | ACD/Percepta c1997-2012 |
| log Koc (dimensionless) | 7.43a | Modelled | KOCWIN 2010 |
| Dmin (nm) | 1.80 | Modelled | CATALOGIC 2014 |
| Dmax (nm) | 1.98 | Modelled | CATALOGIC 2014 |
Abbreviations: Dmax, effective maximum cross-sectional diameter; Dmin, effective minimum cross-sectional diameter; Kow, octanol–water partition coefficient; Koc, organic carbon–water partition coefficient.
aPrediction is uncertain as structure and/or predicted value are at the limits of the domain of the model.
4.3 Sources and uses
According to information reported in response to surveys under section 71 of CEPA, TDBDPB was not manufactured in Canada in 2008, but was imported and used in Canada in 2008 (< 10 000 kg), namely in plastic and rubber materials (Environment Canada 2009b). However, TDBDPB shows no current importation to and/or use in Canada. According to Hardy et al. (2012), the major North American discontinued manufacture of the substance, with limited distribution through 2010 and January 2011. In addition, online suppliers of commercial TDBDPB products indicate that the main North American product has been discontinued and is no longer available and that it has been replaced by decabromodiphenyl ethane (DBDPE) (SpecialChem 2000). However, Chen et al. (2013) noted other suppliers in Asia continue to market TDBDPB or products containing this substance. TDBDPB is listed as a Low Production Volume Chemical (under 1 000 000 kg/yr) on the European Chemical Substance Information Systems (ESIS) website (searched November 2016) (ESIS 1995-2012).
TDBDPB is considered to be an additive flame retardant (Su et al. 2016a) and has been identified as a potential replacement for commercial decabromodiphenyl ether (decaBDE) (Kierkegaard et al. 2004; Covaci et al. 2011; EFSA 2012). General use information indicates that this substance may be utilized in engineering resins and other systems where high temperatures are employed during processing, including nylon, polybutylene terephthalate (PET), polybutylene terephthalate (PBT), and styrenic resins (SpecialChem 2000). Assuming it is a replacement for decaBDE and has been replaced by decabromodiphenyl ethane (DBDPE), TDBDPB might have similar uses to decaBDE and DBDPE in electrical and electronic products (e.g., televisions, computers, household appliances, cables and wires) and in the production of textiles (e.g., such as upholstery and drapery fabrics). While there are no identified imports or manufacturing of this substance in Canada, it is possible that the substance may be found in commercial products or products available to consumers sold in Canada.
4.4 Releases to the environment
Currently, there is no empirical information on releases of TDBDPB in Canada. Because current data indicate that the substance is not manufactured, imported or used in Canada, releases to the Canadian environment of TDBDPB are likely very low.
4.5 Environmental fate and behaviour
4.5.1 Environmental fate
Given the physical and chemical properties of TDBDPB and considering the environmental fate of decaBDE (EC 2006), TDBDPB is expected to reside predominantly in the organic fraction of particulate matter, soil and/or sediment, depending on the compartment of release.
The 2006 assessment report on PBDEs (Environment Canada 2006) indicated that if 100% of decaBDE were released to water, 98.1% would be associated with sediments and 1.9% would partition to water, with negligible amounts partitioning to air and soil. If 100% of decaBDE were released to air, 80.1% would partition to soil, 19% to sediment, and 0.6% and 0.4% to air and water, respectively. If released to soil, 99.9% would stay in soil, with very small amounts partitioning to other media.
Although model prediction (New EQC 2011) indicates that some level of uncertainty still remains, given the tendency for TDBDPB to bind to organic matter in the water column, along with its high persistence, its long-range transport potential in the water column is likely to be high. Limited amounts of TDBDPB would be expected to reach air, and long-range transport potential is expected to be low, as shown by the estimated characteristic travel distance (CTD) of 480 to 735 km for decaBDE (EC 2006). The CTD was defined as the distance a parcel of air has travelled until approximately 63% of the chemical has been removed by degradation or deposition processes (Klasmeier et al. 2006).
4.6 Persistence and bioaccumulation
Empirical studies on persistence and bioaccumulation of TDBDPB are scarce. The overall understanding of the persistence of TDBDPB is that the substance is not readily biodegradable, may be susceptible to hydrolysis based on its chemical structure, and is empirically shown to undergo photolytically stepwise reductive debromination to some extent, although many of the scenarios under which the experiments were conducted did not reflect natural environmental conditions (Chen et al. 2013). There are no available empirical data for bioaccumulation, biomagnification, or trophic magnification for TDBDPB. The high log Kow (> 10) and large molecular size with a cross-sectional diameter of 1.8 to 2.0 nm indicate that this substance likely has very limited bioavailability and bioaccumulation potential. In a fish study, TDBDPB was not shown to bioconcentrate, even when the substance was dissolved in water with a carrier solvent that allowed the test concentrations to go beyond the estimated solubility limit (Study Submission 1988). However, computational models showed possible phototransformation products of TDBDPB that may be of environmental relevance, namely Br4-, Br5-, and Br6-polybrominated diphenoxybenzene (PBDPB) (Chen et al. 2011), which could potentially be bioaccumulative (BCF values range from 3 200 to 10 700 L/kg, with BAF values all above 106 L/kg). Additional data on persistence and bioaccumulation are summarized in Environment and Climate Change Canada (2020g).
4.7 Potential to cause ecological harm
4.7.1 Ecological effects assessment
TDBDPB is predicted to have a base surface narcotics mode of action (OECD QSAR Toolbox 2016). There is some preliminary evidence that the substance may also act through specific mode(s) of action. Structural alerts for reproduction and developmental potential were found via DART scheme (OECD QSAR Toolbox 2016). Metabolites of lower bromination products of photodegradation of TDBDPB, such as hydroxylated Br4-PBDPB, are similarly known to elicit reproductive and/or developmental effects and have been shown to have a mild thyroid disruption potential both in silico and in vitro testing (Hill et al. 2018).
As stated in the substance identity section, technical products of TDBDPB are known to contain minor amounts of decaBDE and octabromodibenzo-p-dioxin (CAS RN 2170-45-8) impurities (< 10%). In addition, sunlight irradiation of dissolved and powder forms of TDBDPB and decaBDE have been shown to result in the formation of lower brominated congeners, along with polybrominated polybenzofurans and dibenzofurans (Su et al. 2016a, 2016b). Su et al. (2014, 2016b) investigated the effects of photolytic degradation of TDBDPB on chicken embryonic hepatocytes and showed that degradation of TDBDPB by natural sunlight generates by-products that induce the in vitro expression of genes, especially the aryl hydrocarbon receptor (AhR)-mediated CYP1A4, but to a much lesser degree than the by-products formed during irradiation of decaBDE. Su et al. (2016b) showed that sunlight irradiation can transform solvent-dissolved TDBDPB and decaBDE into toxic photoproducts with thirtyfold and threefold lower predicted relative potencies than those of dioxin-like PCBs based on in vitro CYP1A4/5 mRNA expression. Overall, several uncertainties exist, including how well the TDBDPB photodegradation studies (based on solvent dissolved or powdered substance) represent natural environmental conditions. In addition, as only gene level in vitro effects have been reported, it is not clear if these would translate to adverse effects in whole organisms for TDBDPB. However, the ability of polychlorinated dioxins and furans (e.g., PCDDs and PCDFs) or other halogenated aromatic hydrocarbons (e.g., PCBs) to bind to the AhR correlates well with their ability to induce gene expression and to produce toxicity (Abbot 1998).
Empirical aquatic toxicity study results available for TDBDPB and decaBDE in water, sediment and soil are summarized in Table 4‑4 and Table 4‑5. A detailed analysis of decaBDE aquatic toxicity is found in the assessment of this substance (Environment Canada 2006).
| Common name | Test organism | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|
| TDBDPB | Water flea (Daphnia magna) | 48-h EC50 | 680b | Study Submission 1986b |
| TDBDPB | Rainbow trout (Oncorhynchus mykiss) | NOEC | 1000a,b | Study submission 1986c |
| TDBDPB | Orange-red killifish (Oryzias latipes) | 48-h LC50 | 200c | Study Submission 1988 |
| DecaBDE | African clawed frog (Xenopus laevis) | MATC (delayed metamorphosis) | 0.001032d | Qin et al. 2010 |
Abbreviations: LC50 = median lethal concentration; EC50 = median effects concentration; NOEC = no observed effects concentration; MATC, maximum acceptable toxicant concentration.
a The study found no mortality or adverse effects at any treatment concentration with the highest concentration of 1000 mg/L.
b The test substance was added directly to the dilution water and tested as a suspension; therefore, the toxicity result is prone to underestimation.
c Hydrogenated castor oil used as a dispersive in water resulted in a test material with much higher experimental concentrations (2, 20, 200 mg/L) than the water solubility limit for TDBDPB (< 0.0001 mg/L) and thus, this study is considered of lower reliability.
d The MATC value was derived by taking a geomean of NOEC (0.0001 mg/L) and LOEC (0.001 mg/L).
| Medium | Test organism | Endpoint | Value (mg/kg dry weight (dw)) | Reference |
|---|---|---|---|---|
| Sediment |
Oligichaete (Lumbriculus variegatus) |
28-d NOEC | 5000a | ACCBFRIP 2001a, 2001b |
| Soil | Earthworm (Eisenia fetida) | NOEC (28-day survival and 56-day reproduction) | 5000a | ACCBFRIP 2001c |
Abbreviations: NOEC = no observed effects concentration.
a Based on nominal concentrations.
With respect to decaBDE, early hazard assessments suggested that significant acute or chronic toxic effects were not likely to occur in aquatic organisms at concentrations below water solubility limits (e.g., EC 2006; UKEA 2009). However, more recent aquatic toxicity studies of the substance have reported effects on aquatic organisms, fish and amphibians, such as effects on growth, reproduction, development, thyroid hormone disruption, and neurobehavioural alterations after exposure to low concentrations of decaBDE (Kuo et al. 2010; Noyes et al. 2011; Qin et al. 2010; UKHSE 2012). The lowest aquatic NOEC for exposure via water is reported to be below 0.001 mg/L for delayed metamorphosis in amphibians (Qin et al. 2010). DecaBDE exposure to fish via diet (feeding studies) with fathead minnows conducted at environmentally relevant concentrations showed that the substance may interfere with the thyroid hormone system in juvenile fish (Noyes et al. 2011, 2013). Thus, there is still a potential for TDBDPB to elicit developmental or reproductive effects in organisms at very low concentrations. However, given the low bioavailability predicted for TDBDPB, these effects may be minimal. The more bioavailable/bioaccumulative products of debromination (e.g., Br4- PBDPB) are expected to have a much higher potential to elicit reproductive and developmental toxic effects. It should be noted that prediction of aquatic toxicity for the Br4 to Br6 PBDPB congeners was considered using ECOSAR. However, the log Kow for these substances (estimated at 7.2 to 8.3) are considered outside of the model domain (up to log Kow of 5) and so are considered uncertain and are not reported. Lastly, dioxin-like derivatives produced via sunlight-mediated photodegradation of TDBDPB dissolved in solvents may increase the potential for toxic effects of TDBDPB in the environment should they form under natural conditions.
4.7.2 Ecological exposure assessment
TDBDPB is considered an additive flame retardant and has been used in plastics and rubber products. TDBDPB is not manufactured in Canada and is not currently importated into and/or used in Canada. While there are no identified current imports or manufacturing of this substance in Canada, it is possible that the substance may be found in commercial products or products available to consumers sold in Canada, but there are no data on actual quantities in such products in Canada. According to Hardy et al. (2012), the major North American producer of this substance discontinued manufacture of the product some time ago, with limited distribution through 2010 and January 2011.
Sampling studies have been conducted to find TDBDPB and its debrominated products in the environment. Trouborst et al. (2015) reported no detections of TDBDPB and lower brominated PBDPB substances in sediment samples collected in 2012 and 2013 in the Great Lakes ecosystem. Recovery rates for TDBDPB were found to be less than 50% (26% to 40%) and it was shown that with increasing degree of bromination above 10 bromine atoms, there was increasing difficulty in extracting and isolating PBDPBs from the spiked sediment matrix. Thus, the reliability of these results may have been impacted. Su et al. (2017) also reported no detections of PBDPBs or methoxylated polybrominated diphenoxybenzene (MeO-PBDPBs) in any of the 65 analyzed water or sediment samples collected in 2010 from sites in Lakes Ontario and Erie.
Chen et al. (2011) sampled herring gull (Larus argentatus) eggs in 2009 from 14 colony sites in the Great Lakes and identified MeO-PBDPBs. Specifically, they identified a number of methoxylated Br4 to Br6-PBDPBs in the egg homogenates. Chen et al. (2011) suggest that these methoxylated congeners may be metabolites of debrominated TDBDPB products. Total MeO-PBDBP concentrations ranging from less than 0.2 to 36.8 ng/g ww in pooled egg homogenates (collected in 2009) were reported from 14 herring gull colony sites across the Great Lakes, with the highest concentration being for Channel-Shelter Island in Saginaw Bay (Lake Huron).
Based on industrial releases to water from plastic manufacturing, one would expect exposure from TBDPB in sediments and soils. However, because there is no currently identified manufacture, import or use of TDBDPB in Canada, and because sampling (albeit limited) has not detected the substance in the Canadian environment, its exposure potential is considered negligible and therefore is not pursued further.
4.7.3 Characterization of ecological risk
The potential for widespread release of TDBDPB to the Canadian environment is presently considered negligible based on the lack of known current manufacturing/use in Canada, and the lack of detected concentrations in the environment. The very low water solubility of TDBDPB (estimated from analogue information at < 0.0001 mg/L) and its high log Kow (estimated from analogue information at 8.7; modelled at > 10), likely limit its bioavailability. This substance is not expected to accumulate in organisms to any appreciable extent. TDBDPB has low toxicity potential based on empirical toxicity data. However, analogous toxicity data for decaBDE suggest potential for effects to amphibians at low concentrations. There is uncertainty regarding the chronic reproductive and developmental toxicity potential of TDBDPB.
While present exposure of the environment to TDBDPB is not of concern, this substance has been considered an alternative for decaBDE and DBDPE. Although there is some uncertainty with respect to this substance’s potential for photodegradation to form lower brominated congeners of PBDPBs and dioxin-like products, these products may be significantly more bioavailable, bioaccumulative and thus inherently toxic than TDBDPB. While current exposures of TDBDPB to the Canadian environment are unlikely to be of concern, TDBDPB is considered to have a high hazard based on its potential photodegradation products. There may therefore be a concern for the Canadian environment should exposures increase.
4.7.4 Sensitivity of conclusion to key uncertainties
TDBDPB has been shown in empirical studies to have the potential to photolytically undergo stepwise reductive debromination that follows first-order kinetic degradation models when exposed to UV or natural sunlight radiation and when dissolved in the solvents (e.g., tetrahydrofuran, methanol, or n-hexane). The potential for debrominated forms to be more bioavailable and/or inherently toxic than the parent, TDBDPB, are a concern. There are also concerns regarding the formation of brominated dioxin/furan compounds from TDBDPB in natural sunlight irradiation. However, there are no data available to substantiate the potential for this transformation to occur in the aquatic or terrestrial environments.
Assuming photolysis of TDBDPB does occur in a natural environment, there are uncertainties with regards to the extent that transformation products (i.e., PBDPB, dioxin and furan products) may be formed. Given the lack of detection of TDBDPB in sediments (an expected sink for this substance), the occurrence of MeO-PBDPBs in herring gull eggs suggests that herring gulls might have accumulated these contaminants through exposure to materials in solid waste facilities. Further work is needed, however, to determine the source of accumulated MeO-PBDPBs in order to draw any conclusions with regards to TDBDPB exposure in the Canadian environment.
4.8 Potential to cause harm to human health
4.8.1 Exposure assessment of TDBDPB
According to information reported in response to surveys under section 71 of CEPA (Environment Canada 2009b), TDBDPB was previously imported into Canada. However, subsequent information indicates that TDBDPB is no longer manufactured by the major North American producer (Hardy et al. 2012), and the main North American product has been discontinued (SpecialChem 2000).
Based on general use information for TDBDPB, the substance may be used as a chemical additive in wire, cables, certain plastic materials, and styrenic resins. TDBDPB exhibits non-blooming properties (Weil and Levchik 2003), and significant release of this substance from any plastic or polymeric material is therefore not expected.
Based on the information available, exposure of the general population in Canada to TDBDPB is not expected.
4.8.2 Health effects assessment of TDBDPB
TDBDPB was not identified as posing a high hazard to human health on the basis of classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity, or reproductive toxicity. It is also not on the European Chemicals Agency’s Candidate List of Substances of Very High Concern for Authorisation (ECHA 2018). Further investigation of health effects is not warranted at this time given that exposure of the general Canadian population is not expected.
4.8.3 Characterization of risk to human health of TDBDPB
Exposure of the general population to TDBDPB through environmental media, food, or the use of products available to consumers is not expected and it has not been identified as posing a high hazard based on national or international agencies. Given these factors, the potential risk to human health is considered to be low.
4.8.4 Uncertainties in evaluation of risk to human health
Although there are some limitations in the exposure database (e.g., no environmental monitoring), because TDBDPB is not expected to be used in Canada, a qualitative approach to risk characterization is considered appropriate for this assessment.
5. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is risk of harm to the environment from TPHP, BPDP, BDMEPPP, IDDP and IPPP. It is proposed to conclude that TPHP, BPDP, BDMEPPP, IDDP and IPPP meet the criteria under paragraph 64(a) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity. However, it is proposed to conclude that TPHP, BPDP, BDMEPPP, IDDP and IPPP do not meet the criteria under paragraph 64(b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger to the environment on which life depends.
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from TEP, TBOEP, TEHP, BEHP and TDBDPB. It is proposed to conclude that TEP, TBOEP, TEHP, BEHP and TDBDPB do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that IPPP and TEP meet the criteria under paragraph 64(c) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that TPHP, BPDP, BDMEPPP, IDDP, TBOEP, TEHP, BEHP, and TDBDPB do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that TPHP, BPDP, BDMEPPP, IDDP, IPPP and TEP meet one or more of the criteria set out in section 64 of CEPA.
It is also proposed to conclude that TBOEP, TEHP, BEHP, and TDBDPB do not meet any of the criteria set out in section 64 of CEPA.
It is also proposed that TPHP and TEP meet the persistence criteria but do not meet the bioaccumulation criteria, that BPDP and IDDP do not meet the persistence criteria or the bioaccumulation criteria, and that BDMEPPP and IPPP do not meet the persistence criteria but do meet the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
References
Abbott B, Birnbaum L. 1998. Dioxin and teratogenesis. In: Puga A, Wallace K, editors. Molecular biology of the toxic response. Taylor and Francis. p. 439-449.
Abdollahi A, Eng A, Jantunen L, Ahrens L, Shoeib M, Parnis J, Harner T. 2017. Characterization of polyurethane foam (PUF) and sorbent impregnated PUF (SIP) disk passive air samplers for measuring organophosphate flame retardants. Chemosphere. 167:212-219.
[ACC] American Chemistry Council. 2005. Phosphoric Acid Derivatives Category Test Plan and Data Assessment.
[ACCBFRIP] American Chemistry Council Brominated Flame Retardant Industry Panel. 2001a. Decabromodiphenyl ether: A prolonged sediment toxicity test with Lumbriculus variegatus using spiked sediment with 2% total organic carbon. Final report. Project No. 439A-113, Wildlife International, Ltd., February.
[ACCBFRIP] American Chemistry Council Brominated Flame Retardant Industry Panel. 2001b. Decabromodiphenyl ether: A prolonged sediment toxicity test with Lumbriculus variegatus using spiked sediment with 5% total organic carbon. Final report. Project No. 439A-114, Wildlife International, Ltd., February.
ACD/Percepta [prediction module]. c1997-2012. Toronto (ON): Advanced Chemistry Development, Inc.
Adams WF, Kimerle RA, Heidolph BB, Michael PR. 1983. Field comparison of laboratory-derived acute and chronic toxicity data. Aquatic Toxicology and Hazard Assessment: Sixth Symposium, ASTM STP 802. In: Bishop RD, Cardwell RD, Heidolph BB, editors. American Society for Testing and Materials. p. 367-385.
Adams WJ, Heidoph BB. 1985. Short-cut chronic toxicity estimates using Daphnia magna. Aquatic Toxicology and Hazard Assessment: Seventh Symposium, ASTM STP 854. In: Cardwell RD, Purdy R, Bahner RC, editors. American Society for Testing and Materials. p. 87-103.
Ali N, Dirtu A, Van den Eede N, Goosey E, Harrad S, Neels H, Mannetje A, Coakley J, Douwes J, Covaci A. 2012. Occurrence of alternative flame retardants in indoor dust from New Zealand: Indoor sources and human exposure assessment. Chemosphere. 88:1276-1282.
Alves A, Covaci A, Voorspoels S. 2017. Method development for assessing the human exposure to organophosphate flame retardants in hair and nails. Chemosphere 168:692-698.
Anderson C, Wischer D, Schmieder A, Spiteller M. 1993. Fate of triphenyl phosphate in soil. Chemosphere. 27(5):869-879.
Andresen JA, Muir D, Ueno D, Darling C, Theobold N, Bester K. 2007. Emerging pollutants in the North Sea in comparison to Lake Ontario, Canada data. Environ Toxicol Chem. 26 (6):1081-1089.
[AOPWIN] Atmospheric Oxidation Program for Microsoft Windows [estimation model]. 2010. Ver. 1.92a. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Aragon M, Marce RM, Borrull F. 2012. Determination of phthalates and organophosphate esters in particulated material from harbour air samples by pressurised liquid extraction and gas chromatography–mass spectrometry. Talanta. 101:473-478.
Aragon M, Borrull F, Marce RM. 2013. Thermal desorption-gas chromatography-mass spectrometry method to determine phthalate and organophosphate esters from air samples. J Chromatogr A. 1303:76-82.
Arnot JA, Gobas FA. 2003a. A Generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci. 22(3):337-345.
Arnot JA, Gobas FAPC. 2003b. Categorization of organic substances on the Domestic Substances List for bioaccumulation potential. Report to Environment Canada, Existing Substances Branch. June 2003. Gatineau (QC): Environment Canada.
Arnot JA, Mackay D, Parkerton TF, Bonnell M. 2008a. A database of fish biotransformation rate for organic chemicals. Environ Toxicol Chem. 27(11):2263-2270.
Arnot JA, Mackay D, Bonnell M. 2008b. Estimating metabolic biotransformation rates in fish from laboratory data. Environ Toxicol Chem. 27(2):341-351.
Ash M, Ash I. 2009. Specialty Chemicals - Source Book. 4th ed. Endicott (NY). Synapse Information Resources, Inc. [accessed 2018 May 4 from Knovel].
[ASTER] Assessment Tools for the Evaluation of Risk. 1999. Duluth (MN): US Environmental Protection Agency, Mid-Continent Ecology Division.[restricted access].
[ATSDR] Agency for Toxic Substances and Disease Registry. 2012. Toxicological profile for Phosphate Ester Flame Retardants [PDF]. Atlanta (GA): US Department of Health and Human Services, Public Health Service [accessed 2017 Oct 26].
Bayer AG. 1995. Triethylphosphate. Developmental toxicity study in rats after oral administration, with cover letter dated 10/24/95. Triethylphosphate (78-40-0). Fiche No OTS0558192.
[BCFBAF] Bioaccumulation Program for Windows [estimation model]. 2012. Ver. 3.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Behl M, Hsieh JH, Shafer TJ, Mundy WR, Rice JR, Boyd WA, Freedman JH, Hunter ES 3rd, Jarema KA, Padilla S, et al. 2015. Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicol Teratol. 52:181-193.
Bengtsson GE, Tarkpea M, Sletten T, Carlberg GE, Kringstad A. 1986. Bioaccumulation and effects of some technical triaryl phosphate products in fish and Nitocra spinipes. Environ Toxicol Chem. 5:853-861.
Berger M, Schwarzbauer J. 2016. Historical deposition of riverine contamination on terrestrial floodplains as revealed by organic indicators from an industrial point source. Water Air Soil Pollut. 227:1-14.
Bergh C, Torgrip R, Emenius G, Ostman C. 2011. Organophosphate and phthalate esters in air and settled dust – a multi-location indoor study. Indoor Air. 21:67-76.
[BIOWIN] Biodegradation Probability Program for Microsoft Windows [estimation model]. 2008. Ver. 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Bjornsdotter MK, Romera-Garcia E, Borrull J, de Boer J, Rubio S, Ballesteros-Gomez A. 2018. Presence of diphenyl phosphate and aryl-phosphate flame retardants in indoor dust from different microenvironments in Spain and the Netherlands and estimation of human exposure. Environ Int. 112:59-67.
Blum KM, Andersson PL, Ahrens L, Wiberg K, Haglund P. 2018. Persistence, mobility and bioavailability of emerging organic contaminants discharged from sewage treatment plants. Sci Total Environ. 612:1532-1542.
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolation in biodegradability assessment. Chemosphere. 30:741-752.
Bohlen H, Hicke K, Stoebel AO, Zierorr M, Thiemann W. 1989. Die Belastung der Unterweser im bremischen Raum mit Halogenorganika und Phosphorsaeureestern I. Vom Wasser. 72:185-197 [cited in ECETOC 1992a].
Bollmann UE, Moller A, Xie Z, Ebinghaus R, Einax JW. 2012. Occurrence and fate of organophosphorus flame retardants and plasticizers in coastal and marine surface waters. Water Res. 46:531-538.
Brandsma SH, Leonards PEG, Leslie HA, de Boer J. 2015. Tracing organophosphorus and brominated flame retardants and plasticizers in an estuarine food web. Sci Total Environ. 505:22-31.
Brommer S, Harrad S. 2015. Sources and human exposure implications of concentrations of organophosphate flame retardants in dust from UK cars, classrooms, living rooms, and offices. Environ Int. 83:202-207.
Butt C, Congleton J, Hoffman K, Fang M, Stapleton H. 2014. Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate (EH-TBB) in urine from paired mothers and toddlers. Environ Sci Technol. 48:10432-10438.
Butt C, Hoffman K, Chen A, Lorenzo A, Congleton J, Stapleton H. 2016. Regional comparison of organophosphate flame retardant (PFR) urinary metabolites and tetrabromobenzoic acid (TBBA) in mother-toddler pairs from California and New Jersey. Environ Int. 94:627-634.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Cao D, Guo J, Wang Y, Li Z, Liang K, Corcoran MB, Hosseini S, Bonina SM, Rockne KJ, Sturchio NC, et al. 2017. Organophosphate esters in sediment of the Great Lakes. Environ Sci Technol. 51(3):1441-1449.
Castorina R, Bradman A, Stapleton H, Butt C, Avery D, Harley K, Gunier R, Holland N, Eskenazi B. 2017. Current-use flame retardants: Maternal exposure and neurodevelopment in children of the CHAMACOS cohort. Chemosphere 189:574-580.
CATALOGIC [environmental fate and ecotoxicity model]. 2014. Ver. 5.11.15. Bourgas (BG): University “Prof. Dr. Assen Zlatarov”, Laboratory of Mathematical Chemistry.
[CEC] Commission for Environmental Cooperation. 2015a. Enhancing Trilateral Understanding of Flame Retardants and Their Use in Manufactured Items: Supply Chain Analysis of Select Flame Retardants Contained in Manufactured Items Used in Indoor Environments [PDF]. Montreal (QC): CEC. 33 p. [accessed 2018 Mar 23].
[CEC] Commission for Environmental Cooperation. 2015b. Enhancing Trilateral Understanding of Flame Retardants and Their Use in Manufactured Items: Analysis of Select Flame Retardants Contained in Office and Household Furniture [PDF]. Montreal (QC): CEC. 16 p. [accessed 2018 Mar 23].
[CEH] Center for Environmental Health. 2013a. Playing on poisons: Harmful flame retardants in children’s furniture [PDF]. Oakland (CA): Center for Environmental Health. 19 p. [accessed 2016 Mar 17].
[CEH] Center for Environmental Health. 2013b. Naptime nightmares: toxic flame retardants in child care nap mats [PDF]. Oakland (CA): CEH. 18 p. [accessed 2016 Mar 17].
Cequier E, Ionas AC, Covaci A, Marcé RM, Becher G, Thomsen C. 2014. Occurrence of a broad range of legacy and emerging flame retardants in indoor environments in Norway. Environ Sci Technol. 48:6827-6835.
Cequier E, Sakhi AK, Marce RM, Becher G, Thomsen C. 2015. Human exposure pathways to organophosphate triesters — A biomonitoring study of mother–child pairs. Environ Int. 75:159-165.
Chau HC, Kadokami K, Duong HT, Kong L, Nguyen T, Nguyen TQ, Ito Y. 2018. Occurrence of 1153 organic micropollutants in the aquatic environment of Vietnam. Environ Sci Pollut Res. 25:7147-7156.
ChemCAN [level III fugacity model of 24 regions of Canada]. 2003. Version 6.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
ChemIDplus [database]. 1993– . Bethesda (MD): US National Library of Medicine. [accessed 2018 Feb 16].
Chen D, Letcher RJ, Gauthier LT, Chu S, McCrindle R, Potter D. 2011. Novel methoxylated polybrominated diphenoxybenzene congeners and possible sources in herring gull eggs from the Laurentian Great Lakes of North America. Environ Sci Technol. 45:9523-9530.
Chen D, Letcher RJ, Chu S. 2012. Determination of non-halogenated, chlorinated and brominated organophosphate flame retardants in herring gull eggs based on liquid chromatography–tandem quadrupole mass spectrometry. J Chromatogr A. 1220:169-174.
Chen D, Letcher RJ, Gauthier LT, Chu S. 2013. Tetradecabromodiphenoxybenzene flame retardant undergoes photolytic debromination. Environ Sci Technol. 47:1373−1380.
Christensen V, Lee K, Kieta K, Elliott S. 2010. Presence of selected chemicals of emerging concern in water and bottom sediment from the St. Louis River, St. Louis Bay, and Superior Bay, Minnesota and Wisconsin. Final Report. Reston (VA): U.S. Geological Survey Scientific Investigations Report 2012-5184. 23 p.
Christia C, Poma G, Besis A, Samara C, Covaci A. 2018. Legacy and emerging organophosphorus flame retardants in car dust from Greece: Implications for human exposure. Chemosphere. 196:231-239.
Chu S, Letcher RJ. 2015. Determination of organophosphate flame retardants and -plasticizers in lipid-rich matrices using dispersive solid-phase extraction as a sample cleanup step and ultra-high performance liquid chromatography with atmospheric pressure chemical ionization mass spectrometry. Anal Chim Acta. 885:183-190.
[CIR] Cosmetic Ingredient Review. 2018. Safety Assessment of Triphenyl Phosphate as Used in Cosmetics. Final Report. September 18, 2018. Washington (DC): CIR. [accessed 2018 Oct 23].
City of Toronto. 2002a. Water quality quarterly report (January–March 2002). Quarterly Report. Toronto (ON): Works and Emergency Services. 23 p.
City of Toronto. 2002b. Water quality quarterly report (April–June 2002). Quarterly Report. Toronto (ON): Works and Emergency Services. 22 p.
City of Toronto. 2002c. Water quality quarterly report (July–September 2002). Quarterly Report. Toronto (ON): Works and Emergency Services. 22 p.
City of Toronto. 2002d. Water quality quarterly report (October–December 2002). Quarterly Report. Toronto (ON): Works and Emergency Services. 22 p.
City of Toronto. 2003a. Water quality quarterly report (January–March 2003). Quarterly Report. Toronto (ON): Works and Emergency Services. 18 p.
City of Toronto. 2003b. Water quality quarterly report (April–June 2003). Quarterly Report. Toronto (ON): Works and Emergency Services. 19 p.
City of Toronto. 2003c. Water quality quarterly report (July–September 2003). Quarterly Report. Toronto (ON): Works and Emergency Services. 20 p.
Clark A, Yoon S, Sheesley RJ, Usenko S. 2017. Spatial and temporal distributions of organophosphate ester concentrations from atmospheric particulate matter samples collected across Houston, TX. Environ Sci Technol. 51:4239−4247.
Cleveland L, Mayer FL, Buckler DR, Palawski DU. 1986. Toxicity of five alkyl-aryl phosphate ester chemicals to four species of freshwater fish. Environ Toxicol Chem. 5:273-282.
Coelho SD, Sousa AC, Isobe T, Kim JW, Kunisue T, Nogueira AJ, Tanabe S. 2016. Brominated, chlorinated and phosphate organic contaminants in house dust from Portugal. Sci Total Environ. 569-570:442-449.
[ConsExpo Web] Consumer Exposure Web Model. 2016. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
Cooper EM, Kroeger G, Davis K, Clark C, Ferguson PL, Stapleton H. 2016. Results from screening polyurethane foam based consumer products for flame retardant chemicals: Assessing impacts on the change in the furniture flammability standards. Environ Sci Technol. 50:10653-10660.
Covaci A, Harrad S, Abdallah M A-E, Ali N, Law RJ, Herzke D, de Wit C. 2011. Novel brominated flame retardants: A review of their analysis, environmental fate and behaviour. Environ Int. 37:532-556.
Crump D, Chiu S, Kennedy SW. 2012. Effects of tris(1,3-dichloro-2-propyl) phosphate and tris(1-chloropropyl) phosphate on cytotoxicity and mRNA expression in primary cultures of avian hepatocytes and neuronal cells. Toxicol Sci. 126:140-8.
Crump D, Farhat A, Chiu S, Williams K, Jones S, Langlois V. 2016. Use of a novel double-crested cormorant ToxChip PCR array and the EROD assay to determine effects of environmental contaminants in primary hepatocytes. Environ Sci Technol. 50:3265−3274.
Cui K, Wen J, Zeng F, Li S, Zhou X, Zeng Z. 2017. Occurrence and distribution of organophosphate esters in urban soils of the subtropical city, Guangzhou, China. Chemosphere. 175:514-520.
Dandan C, Jiehong G, Yawei W, Zhuona L, Kang L, Margaret C, Soheil H, Solidea B, Karl R, Neil S, John G, Jingfu L, An L, Guibin J. 2017. Organophosphate esters in sediment of the Great Lakes. Environ Sci Technol. 51:1441−1449.
[Danish EPA] Danish Environmental Protection Agency. 2015. Chemical substances in car safety seats and other textile products for children. Survey of chemical substances in consumer products No. 135. Copenhagen (DK): Danish Ministry of the Environment. 159 p. [accessed 2018 Feb 13].
David MD, Seiber JN. 1999. Accelerated hydrolysis of industrial organophosphates in water and soil using sodium perborate. Environ Pollut. 105:121-128.
De Silva AO, Young C, Spencer C, Muir DC, Criscitiello A, Sharp M, Cook C, Pickard H. 2016a. Long range transport of organophosphate flame retardants and plasticizers: Deposition in a High Arctic ice cap. In: 7th SETAC World Congress, SETAC North America 37th Annual Meeting / Society of Environmental Toxicology and Chemistry Abstract Book. Pensacola [FL]. p. 311-312.
De Silva AO, St. Pierre K, St. Louis V, Spencer C, Lehnherr I, Muir DC. 2016b. Organophosphate flame retardants and plasticizers in the High Arctic: Deposition and transport in Lake Hazen. In: 7th SETAC World Congress, SETAC North America 37th Annual Meeting / Society of Environmental Toxicology and Chemistry Abstract Book. Pensacola[FL]. p. 311.
Dimitrov S, Dimitrova N, Parkerton T, Comber M, Bonnell M, Mekenyan O. 2005. Base-line model for identifying the bioaccumulation potential of chemicals. SAR QSAR Environ Res. 16(6):531-554.
Ding J, Shen X, Liu W, Covaci A, Yang F. 2015. Occurrence and risk assessment of organophosphate esters in drinking water from Eastern China. Sci Total Environ. 538:959-965.
Ding J, Xu Z, Huang W, Feng L, Yang F. 2016. Organophosphate ester flame retardants and plasticizers in human placenta in Eastern China. Sci Total Environ. 554-555:211-217.
Dobry A, Keller R. 1957. Vapor pressures of some phosphate and phosphonate esters. J Phys Chem. 61:1448-1449.
Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, Brody JG, Rudel RA. 2012. After the PBDE phase-out: A broad suite of flame retardants in repeat house dust samples from California. Environ Sci Technol. 46(24):13056-13066.
Dodson RE, Van den Eede N, Covaci A, Perovich LJ, Brody JG, Rudel RA. 2014. Urinary biomonitoring of phosphate flame retardants: Levels in California adults and recommendations for future studies. Environ Sci Technol. 48:13625-13633.
Dodson RE, Rodgers KM, Carey G, Laurent JGC, Covaci A, Poma G, Malarvannan G, Spengler JD, Rudel RA, Allen JG. 2017. Flame retardant chemicals in college dormitories: Flammability standards influence dust concentrations. Environ Sci Technol. 51(9):4860-4869.
Douville M, Jean K, Houde M. 2016. Multitrophic aquatic toxicity of emerging brominated and phosphorus flame retardants. Fresenius Environ Bull. 25(5):1-8.
Du Z, Wang G, Gao S, Wang Z. 2015. Aryl organophosphate flame retardants induced cardiotoxicity during zebrafish embryogenesis: By disturbing expression of the transcriptional regulators. Aquat Toxicol. 161:25-32.
Du Z, Zhang Y, Wang G, Peng J, Wang Z, Gao S. 2016. TPhP exposure disturbs carbohydrate metabolism, lipid metabolism, and the DNA damage repair system in zebrafish liver. Sci Rep. 6, 21827.
[EA] Environment Agency. 2009a. Environmental risk evaluation report: tertbutylphenyl diphenyl phosphate (CAS no. 56803-37-3) [PDF]. Bristol (UK): Environment Agency. 94 p. [accessed 2018 Apr 9].
[EA] Environment Agency. 2009b. Environmental risk evaluation report: Isopropylated triphenyl phosphate (CAS no. 28108-99-8, 26967-76-0 & 68937-41-7)) [PDF]. Bristol (UK):Environment Agency. 93 p. [accessed 2018 Apr 12].
[EA] Environment Agency. 2009c. Environmental risk evaluation report: Isodecyl diphenyl phosphate (CAS no. 29761-21-5) [PDF]. Bristol (UK): Environment Agency. 93 p. [accessed 2018 Apr 10].
[EA] Environment Agency. 2009d. Environmental risk evaluation report: Triphenyl phosphate (CAS no. 115-86-6) [PDF]. Bristol (UK): Environment Agency. 93 p. [accessed 2018 Apr 10].
[ECCC] Environment and Climate Change Canada. 2013-2014. Data for certain organic flame retardants substance grouping collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain organic flame retardant substances. Data prepared by: Environment and Climate Change Canada, Health Canada; Existing Substances Program.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2015. Identification of risk assessment priorities: Results of the 2015 review. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: Ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC] Environment and Climate Change Canada. 2016c. Data collected under the Canadian Environmental Protection Act, 1999: Early stakeholder engagement to help inform the plan to address the remaining 1 550 substances under the Chemicals Management Plan. Data prepared by ECCC, Health Canada; Existing Substances Program.
[ECCC] Environment and Climate Change Canada. 2016d. Occurrence and Fate of Certain Organic Flame Retardants in Municipal Wastewater Treatment Systems: Organophosphorus. Unpublished report. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2018 Oct 15].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018. Rapid screening of substances with limited general population exposure [PDF]. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2020a. Supporting documentation: Physical and Chemical Properties for the Aryl Organophosphate Subgroup. Gatineau (QC): ECCC. Information in support of the Draft Screening Assessment for the Flame Retardants Group. Available from: substances@ec.gc.ca.
[ECCC] Environment and Climate Change Canada. 2020b. Supporting documentation: Ecological Persistence for the Aryl Organophosphate Subgroup. Gatineau (QC): ECCC. Information in support of the Draft Screening Assessment for the Flame Retardants Group. Available from: substances@ec.gc.ca.
[ECCC] Environment and Climate Change Canada. 2020c. Supporting documentation: Ecotoxicity for the Aryl Organophosphate Subgroup. Information in support of the Draft Screening Assessment Report for the Flame Retardants Group. Gatineau (QC): ECCC. Information in support of the draft screening assessment report for the Flame Retardants Group. Available from: substances@ec.gc.ca.
[ECCC] Environment and Climate Change Canada. 2020d. Supporting documentation: Ecological exposure for the Aryl Organophosphate Subgroup. Gatineau (QC): ECCC. Information in support of the draft screening assessment for the Flame Retardants Substances Group. Available from: substances@ec.gc.ca.
[ECCC] Environment and Climate Change Canada. 2020e. Supporting Documentation: Substance Identity, Physical/Chemical Properties and Environmental Fate and Behaviour for Ethanol, 2-butoxy-, phosphate (3:1). Gatineau (QC): ECCC. Information in support of the Draft Screening Assessment for the Flame Retardants Group. Available from: substances@ec.gc.ca.
[ECCC] Environment and Climate Change Canada. 2020f. Supporting documentation: Environmental Concentrations. Flame Retardants Group: Ethanol, 2-butoxy-, phosphate (3:1), Chemical Abstracts Service Registry Number 78-51-3. Gatineau (QC): ECCC. Information in support of the draft screening assessment for the Flame Retardants Group. Available from: substances@ec.gc.ca.
[ECCC] Environment and Climate Change Canada. 2020g. Supporting documentation: Potential for persistence and bioccumulation for benzene, 1,2,4,5-tetrabromo-3,6-bis(pentabromophenoxy). Information in support of the draft screening assessment report for the Flame Retardants Group. Gatineau (QC): ECCC. Available from: substances@ec.gc.ca.
[ECETOC] European Chemical Industry Ecology and Toxicology Center. 1992a. Joint Assessment of Commodity Chemicals (JACC) No 20. Tris(2-ethylhexyl)phosphate, bis(2-ethylhexyl)phosphate, mono(2- ethylhexyl)phosphate [PDF]. Brussels (BE): European Chemical Industry Ecology and Toxicology Centre Report.ISSN-0773-6339-20. [accessed 2018 Mar 22].
[ECETOC] European Chemical Industry Ecology and Toxicology Center. 1992b. Joint Assessment of Commodity Chemicals (JACC) No 21. Tris-(2-Butoxyethyl)-Phosphate [PDF] CAS 78-51-3. Brussels (BE): European Chemical Industry Ecology and Toxicology Centre Report. ISSN-0773-6339-21. [accessed 2017 Oct 26].
[ECHA] European Chemicals Agency.c2007-2018a. Registered substances database; search results for CAS RN 78-40-0. Helsinki (FI): ECHA. [accessed 2018 Apr 4].
[ECHA] European Chemicals Agency. c2007-2018b. Registered substances database; search results for CAS RN 78-42-2. Helsinki (FI): ECHA. [accessed 2018 Apr 4].
[ECHA] European Chemicals Agency. c2007-2018c. Registered substances database; search results for CAS RN 115-86-6. Helsinki (FI): ECHA. [accessed 2018 Apr 4].
[ECHA] European Chemicals Agency. c2007-2018d. Registered substances database; search results for CAS RN 68937-41-7. Helsinki (FI): ECHA. [accessed 2018 Apr 9].
[ECHA] European Chemicals Agency. c2007-2018e. Registered substances database; search results for CAS RN 29761-21-5. Helsinki (FI): ECHA. [accessed 2018 Apr 9].
[ECHA] European Chemicals Agency. c2007-2018f. Registered substances database; search results for CAS RN 78-51-3. Helsinki (FI): ECHA. [accessed 2020 Jun 10].
[ECHA] European Chemicals Agency. [modified 2015 Jun 15]. Candidate List of Substances of Very High Concern for Authorisation [Internet]. Helsinki (FI): European Chemicals Agency. [accessed 2018 Jun 28].
[ECHA] European Chemicals Agency. 2016. Guidance on information requirements and chemical safety assessment. Chapter R.16: Environmental exposure estimation, Version 3.0 [PDF]. Helsinki (FI): ECHA.
[ECHA] European Chemicals Agency. 2018. Candidate List of Substances of Very High Concern for Authorisation [Internet]. Helsinki (FI): European Chemicals Agency.
[ECOSAR] ECOlogical Structure Activity Relationships Class Program [estimation model]. 2012. Ver. 1.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[EFSA] European Food Safety Authority. 2012. Scientific Opinion on Emerging and Novel Brominated Flame Retardants (BFRs) in Food. EFSA Panel on Contaminants in the Food Chain. EFSA Journal. 10(10):2908.
EG AND G BIONOMICS, 1979a. The chronic toxicity of S-148 (BN-79-1384345-2) to the water flea (Daphnia magna). EG and G Bionomics Report Number BW-19-10-549. Research Report Submitted to Monsanto Company, BN-81-170. [cited in EA 2009c].
EG AND G BIONOMICS, 1979b. Toxicity of S-148 (BN-79-1384345-ld) to the freshwater alga Selenastrum capricornutum. EG and G Bionomics Report Number BP-79-4-61. Toxicity Test Report Submitted to Monsanto Industrial Chemicals Company, BN-79-122. [cited in EA 2009c].
Egloff C, Crump D, Porter E, Williams K, Letcher R, Gauthier L, Kennedy S. 2014. Tris(2-butoxyethyl)phosphate and triethyl phosphate alter embryonic development, hepatic mRNA expression, thyroid hormone levels, and circulating bile acid concentrations in chicken embryos. Toxicol Appl Pharmacol. 279:303-310.
Elliott S, VanderMeulen D. 2017. A regional assessment of chemicals of concern in surface waters of four Midwestern United States national parks. Sci Total Environ. 579:1726-1735.
Environment Canada. 2006. Ecological Screening Assessment Report on Polybrominated Diphenyl Ethers (PBDEs). Ottawa (ON): Government of Canada.
Environment Canada. 2009a. Management of Toxic Substances. Substance Detail – Polybrominated Diphenyl Ethers.
Environment Canada. 2009b. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada. 2010. Ecological State of the Science Report on Decabromodiphenyl Ether (decaBDE)-Bioaccumulation and Transformation. Ottawa(ON): Government of Canada.
Environment Canada. 2012. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada, Health Canada. 2014. Approach for identification of chemicals and polymers as risk assessment priorities under Part 5 of the Canadian Environmental Protection Act, 1999 (CEPA 1999). Ottawa (ON): Environment Canada, Health Canada.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Ernst W. 1988. Evaluation of contaminants of low degradability in estuaries. Biotechnology. 129:60-63. [cited in WHO 2000].
Escher BI, Ashauer R, Dyer S, Hermens J, Lee JH, Leslie HA, Mayer P, Meador J, Warne MSJ. 2011. Crucial role of mechanisms and modes of toxic action for tissue residues of organic chemicals. Integr Environ Assess Manag. 7:28-49.
[ESIS] European Chemical Substances Information System [database]. c1995-2012. Joint Research Centre (JRC). European Commission ESIS: European Inventory of Existing Commercial Chemical Substances. [accessed 2016 Nov 24].
Esteban S, Gorga M, Gonzalez-Alonso S, Petrovic M, Barcelo D, Valcarcel Y. 2014. Monitoring endocrine disrupting compounds and estrogenic activity in tap water from Central Spain. Environ Sci Pollut Res Int. 21:9297-9310.
Eulaers I, Veerle L, Jaspers B, Halley DJ, Lepoint G, Nygård T, Rianne Pinxten T, Adrian Covaci A, Eens M. 2014. Brominated and phosphorus flame retardants in White-tailed Eagle Haliaeetus albicilla nestlings: Bioaccumulation and associations with dietary proxies (δ13C, δ15N and δ34S. Sci Total Environ. 478:48-57.
[EU RAR] European Union Risk Assessment Report. 2008a. Tris(2-chloro-1-methylethyl)phosphate (TCPP) [PDF]. Luxembourg: Office for Official Publications of the European Communities. [accessed 2018 Apr 30].
[EU RAR] European Union Risk Assessment Report. 2008b. Tris[2-chloro-1-(chloromethyl)ethyl]phosphate (TDCP) [PDF].Luxembourg: Office for Official Publications of the European Communities. [accessed 2018 Oct 1].
Fan X, Kubwabo C, Rasmussen PE, Wu F. 2014. Simultaneous determination of thirteen organophosphate esters in settled indoor house dust and a comparison between two sampling techniques. Sci Total Environ. 491-492:80-86.
Fernie K, Palace V, Peters L, Basu N, Letcher R, Karouna-Renier N, Schults S, Lazarus R, Rattner R. 2015. Investigating endocrine and physiological parameters of captive American kestrels exposed by diet to selected organophosphate flame retardants. Environ Sci Technol. 49(12):7448-7455.
Fernie KJ, Chabot D, Champoux L, Brimble S, Alaee M, Marteinson S, Chen D, Bird DM, Letcher RJ. 2017. Spatiotemporal patterns and relationshhips among the diet, biochemistry, and exposure to flame retardants in an apex avian predator, the peregrine falcon. Environ Res 158: 43-53.
Ficheux AS, Morriset T, Chevillotte G, Postic C, Roudot AC. 2014. Probabilistic assessment of exposure to nail cosmetics in French consumers. Food Chem Toxicol. 66:36-43.
Frederiksen M, Stapleton HM, Vorkamp K, Webster TF, Jensen NM, Sørensen JA, Nielsen F, Knudsen LE, Sørensen LS, Clausen PA, et al. 2018. Dermal uptake and percutaneous penetration of organophosphate esters in a human skin ex vivo model. Chemosphere. 197:185-192.
Freudenthal RI, Brandwene D, Clous W. 2001. A subchronic toxicity study of Phosflex 51B in Sprague-Dawley rats. Int J Toxicol. 20(5):269-274.
Fries E, Püttmann W. 2001. Occurrence of organophosphate esters in surface water and ground water in Germany. J Environ Monit. 3:621-626.
Fries E, Mihajlovic I. 2011. Pollution of soils with organophosphorus flame retardants and plasticizers. J Environ Monit. 13:2692-2694.
Fromme H, Lahrz T, Kraft M, Fembacher L, Mach C, Dietrich S, Burkardt R, Völkel W, Göen T. 2014. Organophosphate flame retardants and plasticizers in the air and dust in German daycare centers and human biomonitoring in visiting children (LUPE 3). Environ Int. 71:158-163.
Gad SC. 2014. Phosphate Ester Flame Retardants. Encyclopedia of Toxicology. 3rd ed. Elsevier Academic Press. p. 909-912.
Gartrell MJ, Craun JC, Podrebarac DS, Gunderson EL. 1986a. Pesticides, selected elements, and other chemicals in adult total diet samples, October 1980-March 1982. J Assoc Off Anal Chem. 69:146-161 [cited in ECETOC 1992a].
Gartrell MJ, Craun JC, Podrebarac DS, Gunderson EL. 1986b. Chemical contaminants monitoring. Pesticides, selected elements, and other chemicals in infant and toddler total diet samples, October 1980-March 1982. J Assoc Off Anal Chem. 69:123-145 [cited in ECETOC 1992a].
Gibson E, Stapleton H, Calero L, Holmes D, Burke K, Martinez R, Cotes B, Nematollahi A, Evans D, Herbstman J. 2018. Flame retardant exposure assessment: Findings from a behavioural intervention study. J Expo Sci Environ Epidemiol. 29:33-48DOI.
Giraudo M, Douville M, Houde M. 2015. Chronic toxicity evaluation of the flame retardant tris (2-butoxyethyl) phosphate (TBOEP) using Daphnia magna transcriptomic response. Chemosphere. 132:159-165.
Giraudo M, Dubé MM, Gagnon P, Douville M, Houde M. 2017. Multigenerational effects evaluation of the flame retardant tris(2-butoxyethyl) phosphate (TBOEP) using Daphnia magna. Aquat Toxicol. 190:142-149.
Giulivo M, Capri E, Kalogianni E, Milacic R, Majone B, Ferrari F, Eljarrat E, Barcelo D. 2017. Occurrence of halogenated and organophosphate flame retardants in sediment and fish samples from three European river basins. Sci Total Environ. 586:782-791.
Glazer L, Hawkey AB, Wells CN, Drastal M, Odamah KA, Behl M, Levin ED. 2018. Developmental exposure to low concentrations of organophosphate flame retardants causes life-long behavioral alterations in zebrafish. Toxicol Sci. 165(2):487-498.
Gorga M, Insa S, Petrovis M, Barcelo D. 2015. Occurrence and spatial distribution of EDCs and related compounds in waters and sediments of Iberian rivers. Sci Total Environ. 503-504:69-86.
Greaves AK, Letcher RJ. 2014. Comparative body compartment composition and in ovo transfer of organophosphate flame retardants in North American Great Lakes herring gulls. Environ Sci Technol. 48:7942-7950.
Greaves AK, Su G, Letcher RJ. 2016a. Environmentally relevant organophosphate triesters in herring gulls: In vitro biotransformation and kinetics and diester metabolite formation using a hepatic microsomal assay. Toxicol Appl Pharmacol. 308:59-65.
Greaves AK, Letcher RJ, Chen D, McGoldrick DJ, Gauthier LT, Backus SM. 2016b. Retrospective analysis of organophosphate flame retardants in herring gull eggs and relation to the aquatic food web in the Laurentian Great Lakes of North America. Environ Res. 150:255-263.
Gumbmann MR, Gagne WE, Williams SN. 1968. Short-term toxicity studies of rats fed triethylphosphate in the diet. Toxicol Appl Pharmacol. 12(3):360-371 [cited in OECD 2002a].
Gunderson EL. 1988. FDA Total Diet Study, April 1982–April 1984, dietary intakes of pesticides, selected elements, and other chemicals. J Assoc Off Anal Chem. 71(6):1200−1209.
Guo J, Venier M, Salamova A, 2017. Bioaccumulation of dechloranes, organophosphate esters, and other flame retardants in Great Lakes fish. Sci Total Environ. 583:1-9.
Haglund P, Renberg M, Alsberg, T, Lundin L, Kaj L, Lilielind P, Hjelt M. 2016. Hazardous compounds released from textiles and the associated load they place on Swedish sewage treatment plants [PDF]. Umeå Universitet. 67 p.
Hallanger IG, Sagerup K, Evenset A, Kovacs KM, Leonards P, Fuglei E, Routti H, Aars J, Strøm H, Lydersen C, Gabrielsen GW. 2015. Organophosphorous flame retardants in biota from Svalbard, Norway. Mar Pollut Bull. 101:442-447.
Hamelink JL, Eaton JG. 1979. Proposed standard practice for conducting bioconcentration tests with fishes and saltwater bivalve molluscs. ASTM Draft No. 9.
Hansch C, Leo A, Hoekman D. 1995. Exploring QSAR: Hydrophobic, electronic and steric constants. Washington (DC): American Chemical Society. 348 p.
Hao C, Helm P, Morse D, Reiner E. 2018. Liquid chromatography-tandem mass spectrometry direct injection analysis of organophosphorus flame retardants in Ontario surface water and wastewater effluent. Chemosphere. 191:288-295.
Hardy ML, Hall T, Le Van ST. 2012. Comment on “Novel methoxylated polybrominated diphenoxybenzene congeners and possible sources in herring gull eggs from the Laurentian Great Lakes of North America”. Environ Sci Technol. 46:3587−3588.
Harrad S, Brommer S, Mueller JF. 2016. Concentrations of organophosphate flame retardants in dust from cars, homes, and offices: An international comparison. Emerg Contam. 2:66-72.
Hays SM, Aylward LL, Gagne M, Nong A, Krishnan K. 2010. Biomonitoring equivalents for inorganic arsenic. Regul Toxicol Pharmacol. 58:1-9.
He C, Toms LM, Thai P, Van den Eede N, Want X, Li Y, Baduel C, Harden F, Heffernan A, Hobson P, et al. 2018. Urinary metabolites of organophosphate esters: Concentrations and age trends in Australian children. Environ Int. 111:124-130.
Health Canada. 1995. Investigating human exposure to contaminants in the environment: A handbook for exposure calculations [PDF]. Ottawa (ON): Great Lakes Health Effects Program, Health Protection Branch, Health Canada. [accessed 2018 May 2].
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Heitkamp MA, Cerniglia CE. 1986. Microbial degradation of t-butylphenyl diphenyl phosphate: a comparative microcosm study among five diverse ecosystems. Toxicity Assessment: An International Quarterly. 1(1):103-122.
[HENRYWIN] Henry’s Law Constant Program for Microsoft Windows [estimation model]. 2008. Ver. 3.20. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[HESS] Hazard Evaluation Support System. 2020. Tokyo (JP): Safety Assessment Division, Chemical Management Center, National Institute of Technology and Evaluation (NITE).
Hill KL, Mortensen AK, Techlechiel D, Willmore WG, Sylte I, Jenssen BM, Letcher RJ. 2018. In vitro and in silico competitive binding of brominated polyphenyl ether contaminants with human and gull thyroid hormone transport proteins. Environ Sci Technol. 52:1533-1541.
Hinckley DA, Bidleman TF, Foreman WT. 1990. Determination of vapor pressures for nonpolar and semipolar organic compounds from gas chromatographic retention data. J Chem Eng Data. 35:232-237.
Hoa C, Helm PA, Morse D, Reiner EJ. 2018. Liquid chromatography-tandem mass spectrometry direct injection analysis of organophosphorus flame retardants in Ontario surface water and wastewater effluent. Chemosphere. 191:288-295.
Hoffman K, Lorenzo A, Butt CM, Adair L, Herring AH, Stapleton HM, Daniels JL. 2017. Predictors of urinary flame retardant concentration among pregnant women. Environ Int. 98:96-101.
Hoffmann Mineral. 2018. Guide Formulation – 2K-PU joint sealant for floor joints, pourable good mechanical properties / improved adhesive strength 40 Shore A. Neuburg (Donau): Hoffmann Mineral GmbH. [accessed 2018 Apr 13].
Hollifield HC. 1979. Rapid nephelometric estimate of water solubility of highly insoluble organic chemicals of environmental interest. Bull Environ Contam Toxicol. 23:579-586.
Hou R, Xu Y, Wang Z. 2016. Review of OPFRs in animals and humans: Absorption, bioaccumulation, metabolism, and internal exposure research. Chemosphere. 153:78-90
Howard PH, Deo PG. 1979. Degradation of aryl phosphates in aquatic environments. Bull Environ Contam Toxicol. 22:337-344.
Huckins JN, Fairchild JF, Boyle TP. 1991. Role of exposure mode in the bioavailability of triphenyl phosphate to aquatic organisms. Arch Environ Contam Toxicol. 21:481-485.
[HYDROWIN] Hydrolysis Rates Program for Microsoft Windows [estimation model]. 2010. Ver. 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[IHS] IHS Markit. 2018. Flame Retardants. Specialty Chemicals Update Program. [accessed 2018 Nov 30].
Ionas AC, Dirtu AC, Anthonissen T, Neels H, Covaci A. 2014. Downsides of the recycling process: Harmful organic chemicals in children’s toys. Environ Int. 65:54-62.
[IPCS] International Programme on Chemical Safety. 1991. Environmental Health Criteria 112: tri-n-butyl phosphate [PDF]. Geneva (CH): United Nations Environment Programme, International Labour Organization, World Health Organization. [accessed 2017 Oct 26].
[IUCLID] International Uniform Chemical Information Database. 2000. IUCLID Data set on phenol, isopropylated, phosphate (3:1). CAS No. 68937-41-7. Year 2000 CD-ROM Edition. European Chemicals Bureau, European Commission. [cited in EA 2009b].
[IUCLID] International Uniform Chemical Information Database. 2001. IUCLID Data set for tris(methylphenyl) phosphate. Great Lakes Chemical Corporation. [cited in UKEA 2009].
[J-CHECK] Japan CHEmicals Collaborative Knowledge database [database]. c2010– . Tokyo (JP): National Institute of Technology and Evaluation (NITE). [accessed 2014 Nov 7].
Jantunen L, Struger J, Backus S, Kraft J, Brommer S. 2013. Organophosphate flame retardants in southern Ontario tributaries and precipitation. Poster presentated at the Eastern Canada Trace Organic Workshop. Saskatoon, 2012 April 22-25.
Jarema KA, Hunter DL, Shaffer RM, Behl M, Padilla S. 2015. Acute and developmental behavioral effects of flame retardants and related chemicals in zebrafish. Neurotoxicol Teratol. 52:194-209.
Jurgens SS, Helmus R, Waaijers SL, Uittenbogaard D, Dunnebier D, Vleugel M, Kraak MHS, de Voogt P, Parsons JR. 2014. Mineralisation and primary biodegradation of aromatic organophosphorus flame retardants in activated sludge. Chemosphere. 111:238-242.
Kademoglou K, Xu F, Padilla-Sanchez JA, Haug L, Covaci A, Collins CD. 2017. Legacy and alternative flame retardants in Norwegian and UK indoor environment: Implications of human exposure via dust ingestion. Environ Int. 102:48-56.
Kanazawa A, Saito I, Araki A, Takeda M, Ma M, Saijo T, Kishi R. 2010. Association between indoor exposure to semi-volatile organic compounds and building-related symptoms among the occupants of residential dwellings. Indoor Air. 20:72-84.
KAN-DO Office and Pesticides Team. 1995. Accumulated pesticide and industrial chemical finding from a ten-year study of ready-to-eat foods. J Assoc Off Anal Chem. 78(3):614-631 [cited in WHO 2000].
Kierkegaard A, Bjorkland J, Fridean U. 2004. Identification of the flame retardant decabromodiphenyl ethane in the environment. Environ Sci Technol. 38:3247-3253.
Kim JW, Ramaswamy BR, Chang KH, Isobe T, Tanage S. 2011. Multiresidue analytical method for the determination of antimicrobials, preservatives, benzotriazole UV stabilizers, flame retardants and plasticizers in fish using ultra high performance liquid chromatography coupled with tandem mass spectrometry. J Chromatogr A. 1218:3511-3520.
Kim JW, Isobe T, Muto M, Tue NM, Katsura K, Marlarvannan G, Sudaryanto A, Chang KH, Prudente M, Viet PH, et al. 2014. Organophosphorus flame retardants (PFRs) in human breast milk from several Asian countries. Chemosphere. 116:91-97.
Kim S, Jung J, Lee I, Jung D, Youn H, Choi K. 2015. Thyroid disruption by triphenyl phosphate, an organophosphate flame retardant, in zebrafish (Danio rerio) embryos/larvae, and in GH3 and FRTL-5 cell lines. Aquat Toxicol. 160:188-196.
Klasmeier J, Matthies M, MacLeod M, Fenner K, Scheringer M, Stroebe M, Le Gall AC, McKone TE, van de Meent D, Wania F. 2006. Application of multimedia models for screening assessment of long-range transport potential and overall persistence. Environ Sci Technol. 40:53-60.
Kluwe WM, Huff JE, Matthews HB, Irwin R, Haseman JK. 1985. Comparative chronic toxicities and carcinogenic potentials of 2-ethylhexyl-containing compounds in rats and mice. Carcinogenesis. 6:1577-1583.
[KOAWIN] Octanol-Air Partition Coefficient Program for Microsoft Windows [estimation model]. 2010. Ver. 1.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[KOCWIN] Organic Carbon Partition Coefficient Program for Microsoft Windows [estimation model]. 2010. Ver. 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Kosarac I, Kubwabo C, Foster WG. 2016. Quantitative determination of nine urinary metabolites of organophosphate flame retardants using solid phase extraction and ultra-performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS). J Chromatogr B. 1014:24-30.
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [estimation model]. 2010. Ver. 1.68. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Kuo YM, Sepúlveda MS, Sutton TM, Ochoa-Acuῆa HG, Muir AM, Miller B, Hua I. 2010. Bioaccumulation and biotransformation of decabromodiphenyl ether and effects on daily growth in juvenile lake whitefish (Coregonus clupeaformis). Ecotoxicology. 19(4):751-60.
Kurt-Karakus P, Alegria H, Birgul A, Gungormus E, Jantunen L. 2018. Organophosphate ester (OPEs) flame retardants and plasticizers in air and soil from a highly industrialized city in Turkey. Sci Total Environ. 625:555-565.
Langer S, Fredricsson M, Weschler CJ, Beko G, Strandberg B, Remberger M, Toftum J, Clausen G. 2016. Organophosphate esters in dust samples collected from Danish homes and daycare centers. Chemosphere. 154:559-566.
LeBel GL, Williams DT, Benoit FM. 1981. Gas chromatographic determination of trialkyl/aryl phosphates in drinking water, following isolation using macroreticular resin. J Assoc Off Anal Chem. 64(4):991-998.
LeBel GL, Williams DT, Benoit FM. 1983. Problems in collection of representative samples for determination of tributoxyethyl phosphate in potable water. J Assoc Off Anal Chem. 66(1):202-203 [cited in WHO 2000].
LeBel GL, Williams DT, Benoit FM. 1987. Use of large volume resin cartridges for the determination of organic contaminants in drinking water derived from the Great Lakes. Adv Chem Ser. 214:309-325 [cited in WHO 2000].
Lee S, Jeong W, Kannan K, Moon HB. 2016. Occurrence and exposure assessment of organophosphate flame retardants (OPFRs) through the consumption of drinking water in Korea. Water Res. 103:182-188.
Lewis RJ. 2012. Sax's Dangerous Properties of Industrial Materials. 12th ed. Hoboken, (NJ): Wiley-Interscience, Wiley & Sons, Inc. p. V5:4461.
Li P, Jin Y, Hu J, Xu J, Sun Y, Ma Y. 2017. Concentrations of organophosphorus, polybromobenzene, and polybrominated diphenyl ether flame retardants in human serum, and relationships between concentrations and donor ages. Chemosphere. 171:654-660.
Lide DR, editor. 2005. CRC Handbook of Chemistry and Physics. 86th ed. Boca Raton (FL): CRC Press, Taylor & Francis.
Liu CS, Yu LQ, Deng J, Lam PKS, Wu RSS, Zhou B. 2009. Waterborne exposure to fluotelomer alcohol 6:2 FTOH alters plasma sex hormone and gene transcription in the hypothalamic-pituitary-gonadal (HPG) axis of zebrafish. Aquat Toxicol. 93:131-137.
Liu X, Ji K, Choi K. 2012. Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquat Toxicol. 114-115:173-181.
Liu X, Ji K, Jo A, Moon HB, Choi K. 2013. Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (Danio rerio). Aquat Toxicol. 134-135:104-111.
Liu Y, Liggio J, Harner T, Jantunen L, Shoeib M, Li SM. 2014. Heterogeneous OH initiated oxidation: A possible explanation for the persistence of organophosphate flame retardants in air. Environ Sci Technol. 48:1041−1048.
Liu LY, He K, Hites R, Salamova A. 2016. Hair and nails as noninvasive biomarkers of human exposure to brominated and organophosphate flame retardants. Environ Sci Technol. 50:3065−3073.
Liu Y, Wu D, Xu Q, Yu L, Liu C, Wang J. 2017. Acute exposure to tris (2-butoxyethyl) phosphate (TBOEP) affects growth and development of embryo-larval zebrafish. Aquat Toxicol. 191:17-24.
Luongo G, Östman C. 2016. Organophosphate and phthalate esters in settled dust from apartment buildings in Stockholm. Indoor Air. 26:414-425.
Ma Y, Cui K, Zeng F, Wen J, Liu H, Zhu F, Ouyang G, Luan T, Zeng Z. 2013. Microwave-assisted extraction combined with gel permeation chromatography and silica gel cleanup followed by gas chromatography–mass spectrometry for the determination of organophosphorus flame retardants and plasticizers in biological samples. Anal Chim Acta. 786:47-53.
Ma Y, Xie Z, Lohmann R, Mi W, Gao G. 2017. Organophosphate ester flame retardants and plasticizers in ocean sediments from the North Pacific to the Arctic Ocean. Environ Sci Technol. 51:3809−3815.
Ma Z, Tang S, Su G, Miao Y, Liu H, Xie Y, Giesy JP, Saunders DMV, Hecker M, Yu H. 2016. Effects of tris (2-butoxyethyl) phosphate (TBOEP) on endocrine axes during development of early life stages of zebrafish (Danio rerio). Chemosphere. 144:1920-1927.
Marklund A. 2005. Levels and sources of organophosphorus flame retardants and plasticizers in indoor and outdoor environments [PDF]. Umea (SE): Umea University. [accessed 2016 Mar 23].
Marzulli FN, Callahan JF, Brown DWC. 1965. Chemical structure and skin penetrating capacity of a short series of organic phosphates and phosphoric acid. J Invest Dermatol. 44(5):339-344.
Mayer FL, Adams WJ, Finley MT, Michael PR, Mehrle PM, Saeger VW. 1981. Phosphate ester hydraulic fluids: An aquatic environmental assessment of Pydrauls 50E and 115E. In: Branson D, Dickson K, editors. American Society for Testing and Materials. p. 103-123 [cited in EA 2009d].
McCarty LS, Dixon DG, Mackay D, Smith AD. 1992. Residue‐based interpretation of toxicity and bioconcentration QSARs from aquatic bioassays: Neutral narcotic organics. Environ Toxicol Chem. 11(7):917-930.
McGee SP, Konstantinov A, Stapleton HM, Volz DC. 2013. Aryl phosphate esters within a major PentaBDE replacement product induce cardiotoxicity in developing zebrafish embryos: Potential role of the aryl hydrocarbon receptor. Toxicol Sci. 133(1):144-156.
McGoldrick DJ, Murphy EW. 2016. Concentration and distribution of contaminants in lake trout and walleye from the Laurentian Great Lakes (2008-2012). Environ Pollut. 217:85-96.
McGoldrick DJ, Letcher RJ, Barresi E, Keir JM, Small J, Clark MG, Sverko E, Backus SM. 2014. Organophosphate flame retardants and organosiloxanes in predatory freshwater fish from locations across Canada. Environ Pollut. 193:254-261.
Mendelsohn E, Hagopian A, Hoffman K, Butt CM, Lorenzo A, Congleton J, Webster TF, Stapleton HM. 2016. Nail polish as a source of exposure to triphenyl phosphate. Environ Int. 86:45-51.
Mihajlovic I. 2015. Recent development of phosphorus flame retardants in thermoplastic blends and nanocomposites. In: Visakh PM, Arao Y, editors. Flame retardants: Polymer blends, composites and nanocomposites. Switzerland: Springer International. p. 79-114.
Miller GZ, Gearhart J. 2016. Traveling with toxics: Flame retardants & other chemicals in children’s car seats. Michigan (US): HealthyStuff, Ecology Center. [accessed 2018 Mar 29].
[MITI] Ministry of International Trade & Industry (Jpn). 1992. Biodegradation and bioaccumulation data of existing chemicals based on the CSCL Japan. Tokyo (Jpn): Japan Chemical Industry Ecology-Toxicology & Information Centre.
Mizouchi S, Ichiba M, Takigama H, Kajiwara N, Takumuku T, Miyajima T, Kodama H, Someya T, Ueno D. 2015. Exposure assessment of organophosphorus and organobromine flame retardants via indoor dust from elementary schools and domestic houses. Chemosphere. 123:17-25.
Möller A, Sturm R, Xie Z, Cai M, He J, Ebinghaus R. 2012. Organophosphorus flame retardants and plasticizers in airborne particles over the Northern Pacific and Indian Ocean toward the polar regions: Evidence for global occurrence. Environ Sci Technol. 46:3127−3134.
[MPBPWIN] Melting Point Boiling Point Program for Microsoft Windows [estimation model]. 2010. Ver. 1.43. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[MSDS] Material Safety Data Sheet. 2011. Aquagard II Aluminum Koat [PDF]. Lakewood (NJ): Flexabar Corporation, USA. [accessed 2019 Apr 17].
[MSDS] Material Safety Data Sheet. 2012. Fyrquel GT. St Louis (MO): ICL Industries Products, USA. [accessed 2019 Jun 18].
[MSDS] Material Safety Data Sheet. 2013. Mystik JT-7 Extended Range Synthetic Gear Oil, SAE 75W-140. Houston (TX): CITGO Petroleum Corporation. [accessed 2016 Jun 16].
[MSDS] Material Safety Data Sheet. 2014a. 1.75 Froth Polyol HFC INT. Calgary (AB): Dow Chemical Canada ULC. [accessed 2016 Jun 16].
[MSDS] Material Safety Data Sheet. 2014b. TRMCLD 2X3.78L WATER BASE GLOSS BLACK. Concord (ON): Rust-Oleum Consumer Brands Canada. [accessed 2016 Jun 20].
[MSDS] Material Safety Data Sheet. 2015. EZ STRIP all purpose remover [PDF]. Windsor (ON): EZ Strip USA Inc. [accessed 2018 Apr 9].
Muir DCG, Grift NP, Lockhart WL. 1982. Comparison of laboratory and field results for prediction of the environmental behavior of phosphate esters. Environ Toxicol Chem. 1:113-119.
Muir DCG, Grift NP. 1983. Extraction and cleanup procedures for determination of diaryl- phosphates in fish, sediment and water samples. J Assoc Off Anal Chem. 66:684-690.
Muir DCG, Yarechewski AL, Grift NP. 1983. Environmental dynamics of phosphate esters. III. Comparison of the bioconcentration of four triaryl phosphates by fish. Chemosphere. 12:155-166 [cited in EA 2009a].
Muir DG. 1984. Phosphate esters. Anthropogenic Compounds. In: The Handbook of Environmental Chemistry. vol. 3. Springer-Verlag. p. 41-66.
Muir DCG, Lint D, Grift NP. 1985. Fate of three phosphate ester flame retardants in small ponds. Environ Toxicol Chem. 4:663-675.
Muir DCG, Yarechewski AL, Grift NP. 1989. Biodegradation of four triaryl/alkyl phosphate esters in sediment under various temperature and redox conditions. Toxicol Environ Chem. 18:269-286 [also cited in EA 2009c, OECD SIDS]
[NCI] National Chemical Inventories [software]. 2015. Issue 2. Columbus (OH): American Chemical Society, Chemical Abstracts Service.
[New EQC] New Equilibrium Criterion Model. 2011. Ver. 1.00 (Beta). Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
[New EQC] New Equilibrium Criterion Model. 2012. Ver. 1.01 (Beta). Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
[NHANES 2000-2004] Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, [2000-2004].
Norris B, Smith S. 2002. Research into the mouthing behaviour of children up to 5 years old. Consumer and Competition Policy Directorate.
Noyes P, Haggard D, Gonnerman G, Tanguay R. 2015. Advanced morphological – behavioral test platform reveals neurodevelopmental defects in embryonic zebrafish exposed to comprehensive suite of halogenated and organophosphate flame retardants. Toxicol Sci. 145:177-195.
Noyes PD, Lema SC, Macaulay LJ, Douglas NK, Stapleton HM. 2013. Low level exposure to the flame retardant BDE-209 reduces thyroid hormone levels and disrupts thyroid signaling in fathead minnows. Environ. Sci. Technol. 47:10012-10021.
Noyes PD, Hinton DE, Stapleton HM. 2011. Accumulation and debromination of decabromodiphenyl Ether (BDE-209) in juvenile fathead minnows (Pimephales promelas) induces thyroid disruption and liver alterations. Toxicol Sci. 122(2):265-274.
[OECD] Organisation for Economic Co-operation and Development. 2002a. SIDS initial assessment report Triethylphosphate: CAS No. 78-40-0. SIAM [SIDS Initial Assessment Meeting] 1 [PDF], 1993 & 3, 1995; Germany. [accessed 2017 Oct 26].
[OECD] Organisation for Economic Co-operation and Development. 2002b. SIDS initial assessment profile Triphenyl phosphate: CAS No. 115-86-6. SIAM [SIDS Initial Assessment Meeting] 15, 2002; Germany. [accessed 2018 Apr 5].
[OECD] Organisation for Economic Co-operation and Development. 2004a. Emission scenario document on lubricants and lubricant additives [PDF]. Paris (FR): OECD, Environment Directorate. (Series on Emission Scenario Documents No. 10; Report No.: ENV/JM/MONO(2004)21, JT00174617). [accessed 2019 Feb 15].
[OECD] Organisation for Economic Co-operation and Development. 2004b. Emission scenario document on additives in rubber industry [PDF]. Paris (France): OECD, Environment Directorate. (Series on Emission Scenario Documents No. 6; Report No.: ENV/JM/MONO(2004)11, JT00166668). [accessed 2019 Feb 15].
[OECD] Organisation for Economic Co-operation and Development. 2011. Emission scenario document on the use of metalworking fluids [PDF]. Paris (FR): OECD, Environment Directorate. (Series on Emission Scenario Documents No. 28; Report No.: ENV/JM/MONO(2011)18, JT03304938). [accessed 2019 Feb 15].
OECD Pov and LRTP Screening Tool. 2009a. Ver. 2.2. Paris (FR): Organisation for Economic Cooperation and Development (OECD). A software model for estimating overall persistence (Pov) and long-range transport potential (LRTP) of organic chemicals.
[OECD] Organisation for Economic Co-operation and Development. 2009b. Emission scenario document on plastic additives [PDF]. Paris (FR): OECD, Environment Directorate. (Series on Emission Scenario Documents No. 3; Report No.: ENV/JM/MONO(2004)8/REV1, JT03267870). [accessed 2019 Feb 15].
OECD QSAR Toolbox. 2016. Ver. 3.4. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Ofstad EB, Sletten T. 1985. Composition and water solubility determination of a commercial tricresylphosphate. Sci Total Environ. 43:233-241.
Ospina M, Jayatilaka N, Wong LY, Restrepo P, Calafat A. 2018. Exposure to organophosphate flame retardant chemicals in the U.S. general population: Data from the 2013–2014 National Health and Nutrition Examination Survey. Environ Int. 110:32-41.
Pelletier G, Rigden M, Wang GS, Caldwell D, Siddique S, Leingartner K, Kosarac I, Cakmak S, Kubwabo C. 2020. Comparison of tris(2-ethylhexyl) phosphate and di(2-ethylhexyl) phosphoric acid toxicities in a rat 28-day oral exposure study. J Appl Toxicol. 40(5):600-618.
Phillips AL, Hammel SC, Hoffman K, Lorenzo AM, Chen A, Webster TF, Stapleton HM. 2018. Children's residential exposure to organophosphate ester flame retardants and plasticizers: Investigating exposure pathways in the TESIE study. Environ Int. 116:176-185.
Phillips AL, Hammel SC, Konstantinov A, Stapleton HM. 2017.Characterization of individual isopropylated and tert-butylated triarylphosphate (ITP and TBPP) isomers in several commercial flame retardant mixtures and house dust standard reference material SRM 2585. Environ Sci Technol. 51:13443-13449.
Poma G, Glynn A, Malarvannan G, Covaci A, Darnerud PO. 2017. Dietary intake of phosphorus flame retardants (PFRs) using Swedish food market basket estimations. Food Chem Toxicol. 100:1-7.
Poma G, Malysheva SV, Goscinny S, Malarvannan G, Voorspoels S, Covaci A, Van Loco J. 2018. Occurrence of selected halogenated flame retardants in Belgian foodstuff. Chemosphere. 194:256-265.
Porter E, Crump D, Egloff C, Chiu S, Kennedt SW. 2013. Use of an avian hepatocyte assay and the avian toxchip polymerse chain reaction array for testing prioritization of 16 organic flame retardants. Environ Toxicol Chem. 33(3):573-582.
PubChem [database]. 2004– . Bethesda (MD): US National Library of Medicine, National Center for Biotechnology Information. [updated 2018 Nov 17, accessed 2018 Nov 16].
Qiao L, Zheng XB, Zheng J, Lei WX, Li HF, Wang MH, He CT, Chen SJ, Yuan JG, Luo XJ, et al. 2016. Analysis of human hair to assess exposure to organophosphate flame retardants: Influence of hair segments and gender differences. Environ Res. 148:177-183.
Qin X, Xia X. 2010. Thyroid disruption by technical decabromodiphenyl ether (DE-83R) at low concentrations in Xenopus laevis. J Environ Sci. 22(5):744-751.
Quintana JB, Rodil R, Reemtsma T. 2006. Determination of phosphoric acid mono- and diesters in municipal wastewater by solid-phase extraction and ion-pair liquid chromatography-tandem mass spectrometry. Anal Chem. 78:1644-1650.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2008. Chemicals in Toys A general methodology for assessment of chemical safety of toys with a focus on elements [PDF]. Bilthoven (NL): RIVN. Report 320003001/2008. [accessed 2018 Jul 9].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2007. Do-it-yourself products fact sheet: to assess the risks for the consumer [PDF]. Bilthoven (NL): RIVM. Report No.: 320104007/2007. [accessed 2018 Mar 7].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2016. Cleaning products fact sheet: Default parameters for estimating consumer exposure [PDF]. Bilthoven (NL): RIVM. Report 2016-0179. [accessed 2018 Jan 31].
Robinson EC, Hammond BG, Johannsen FR, Levinskas GJ, Rodwell DE. 1986. Teratogenicity studies of alkylaryl phosphate ester plasticizers in rats. Fundam Appl Toxicol. 7(1):138-143 [cited in EA 2009b].
Rodil R, Quintana JB, Concha-Grana E, Lopez-Mahia P, Muniategui-Lorenzo S, Prada-Rodriguez D. 2012. Emerging pollutants in sewage, surface and drinking water in Galicia (NW Spain). Chemosphere. 86:1040-1049.
Saeger VW, Hicks O, Kaley RG, Michael PR, Mieure JP, Tucker ES. 1979. Environmental fate of selected phosphate esters. Environ Sci Technol. 13(7):840-844.
Saini A, Thaysen C, Jantunen L, McQueen RH, Diamond ML. 2016. From clothing to laundry water: Investigating the fate of phthalates, brominated flame retardants, and organophosphate esters. Environ Sci Technol. 50:9280-9297.
Saito I, Onuki A, Seto H. 2007. Indoor organophosphate and polybrominated flame retardants in Tokyo. Indoor Air. 17:28-36.
Saitoh M, Umemura T, Kawasaki Y, Momma J, Matsushima Y, Matsumoto M, Eshita N, Isama K, Kaniwa M. 1994. Subchronic toxicity study of tributoxyethyl phosphate in Wistar rats. Eiser Shikensko Hokoku. 112:27-39 [cited in WHO 2000].
Salamova A, Ma Y, Venier M, Hites R. 2014. High levels of organophosphate flame retardants in the Great Lakes atmosphere. Environ Sci Technol Lett. 1:8-14.
Sample BE, Opresko DM, Suter GW. 1996. Toxicological Benchmarks for Wildlife: 1996 Revision. Oak Ridge (TN): Oak Ridge National Laboratory. Report no. ES/ER/TM-86/R3. [accessed 2019 Apr 26].
Sanders HO, Hunn JB, Robinson-Wilson E, Mayer FL Jr. 1985. Toxicity of seven potential polychlorinated biphenyl substitutes to algae and aquatic invertebrates. Environ Toxicol Chem. 4:149-154.
Santin G, Elijarrat E, Barcelo D. 2016. Simultaneous determination of 16 organophosphorus flame retardants and plasticizers in fish by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 1441:34-43.
Sasaki K, Takeda M, Uchiyama M. 1981. Toxicity, absorption and elimination of phosphoric acid triesters by killifish and goldfish. Bull Environ Contam Toxicol. 27:775-782.
Sasaki K, Suzuki T, Takeda M, Uchiyama M. 1982. Bioconcentration and excretion of phosphoric acid triesters by killifish (Oryzeas latipes). Bull Environ Contam Toxicol. 28:752-759 [cited in OECD SIDS TPP 2002, Environmental Health Criteria 111].
Sasaki K, Suzuki T, Takeda M, Uchiyama M. 1984. Metabolism of phosphoric acid triesters by rat liver homogenate. Bull Environ Contam Toxicol. 33:281-288.
Schenker U, MacLeod M, Scheringer M, Hungerbuhler K. 2005. Improving data quality for environmental fate models: A least-squares adjustment procedure for harmonizing physicochemical properties of organic compounds. Environ Sci Technol. 39:8434-8441.
Scheringer M, MacLeod M, Wegmann F. 2006. The OECD Pov and LRTP Screening Tool. Version 2.0. Organization for Economic Cooperation and Development. Zurich (CH): Swiss Federal Institute of Technology.
Scheringer M, MacLeod M, Wegmann F. 2009. The OECD Pov and LRTP Screening Tool, Version 2.2. Zurich (CH): ETH Zurich.
Schreder ED, La Guardia MJ. 2014. Flame retardant transfers from US households (dust and laundry wastewater) to the aquatic environment. Environ Sci Technol. 48:11575-11583.
[SDS] Safety Data Sheet. 2018a. Reofos® 35 [PDF]. CT (USA): Lanxess Solutions US In. [accessed 2018 Nov 1].
[SDS] Safety Data Sheet. 2018b. Reofos® 65 [PDF]. Ontario (Canada): Lanxess Canada [accessed 2018 Nov 1].
Shi Q, Wang M, Shi F, Yang L, Guo Y, Feng C, Liu J, Zhou B. 2018. Developmental neurotoxicity of triphenyl phosphate in zebrafish larvae. Aquat Toxicol. 203:80-87.
Shoeib M, Ahrens L, Jantunen L, Harner T. 2014. Concentrations in air of organobromine, organochlorine and organophosphate flame retardants in Toronto Canada. Atmos Environ. 99:140-147.
Shoeib T, Webster G, Hassan Y, Tepe S, Yalcin M, Turgut C, Kurt-Karakus PB, Jantunen L. 2019. Organophosphate esters in house dust: A comparative study between Canada, Turkey and Egypt. Sci Total Environ. 650:193-201.
SimpleTreat [sewage treatment plant removal model]. 2003. Ver. 3.1. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu (RIVM) [National Institute for Public Health and the Environment]. Laboratory for Ecological Risk Assessment.
Sitthichaikasem S. 1978. Some toxicological effects of phosphate esters on rainbow trout and bluegill [dissertation]. Ames (IA): Iowa State University. 6473.
Sjögren B, Iregren A, Järnberg J. 2009. Phosphate triesters with flame retardant properties. The Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals. University of Gothenburg.
Smyth HF, Carpenter CP. 1948. Further experience with the range finding test in the industrial toxicology laboratory. J Ind Hyg Toxicol. 30:63-68.
SpecialChem - Universal Selector [database]. 2000. Search results for CAS RN 58965-66-5. [accessed 2018 Feb 28].
Staaf T, Ostman C. 2005.Organophosphate trimesters in indoor environments. J Environ Monit. 7:883-887.
Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, McClean MD, Webster TF. 2008. Alternate and new brominated flame retardants detected in U.S. house dust. Environ Sci Technol. 42:6910-6916.
Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, Webster TF, Blum A. 2011. Identification of flame retardants in polyurethane foam collected from baby products. Environ Sci Technol. 45:5323-5331.
Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A. 2012. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ Sci Technol. 46:13432-13439.
Statistics Canada. 2015. Canadian Community Health Survey (CCHS). Ottawa (ON): Statistics Canada.
Strobel A, Willmore WG, Sonne C, Dietz R, Letcher RJ. 2018a. Organophosphate esters in East Greenland polar bears and ringed seals: Adipose tissue concentrations and in vitro depletion and metabolite formation. Chemosphere. 196:240-250.
Strobel A, Letcher RJ, Willmore WG, Sonne C, Dietz R. 2018b. Structure-dependent in vitro metabolism of alkyl-substituted analogues of triphenyl phosphate in East Greenland polar bears and ringed seals. Environ Sci Technol Lett. 5(4):214-219.
Stubbings WA, Schreder ED, Thomas MB, Romanak K, Venier M, Salamova A. 2018. Exposure to brominated and organophosphate ester flame retardants in U.S. childcare environments: Effect of removal of flame-retarded nap mats on indoor levels. Environ Pollut. 238:1056-1068.
Study Submission. 1984. Unpublished confidential submission for Saytex® 120 submitted to Environment and Climate Change Canada under the Chemicals Management Plan initiative. Gatineau (QC): ECCC, Program Development and Engagement Division.
Study Submission. 1986a. Unpublished confidential submission for Saytex® 120 submitted to Environment and Climate Change Canada under the Chemicals Management Plan initiative. Gatineau (QC): ECCC, Program Development and Engagement Division.
Study Submission. 1986b. Unpublished confidential submission for Saytex® 120 submitted to Environment and Climate Change Canada under the Chemicals Management Plan initiative. Gatineau (QC): ECCC, Program Development and Engagement Division.
Study Submission. 1986c. Unpublished confidential submission for Saytex® 120 submitted to Environment and Climate Change Canada under the Chemicals Management Plan initiative. Gatineau (QC): ECCC, Program Development and Engagement Division.
Study Submission. 1988. Unpublished confidential submission for Saytex® 120 submitted to Environment and Climate Change Canada under the Chemicals Management Plan initiative. Gatineau (QC): ECCC, Program Development and Engagement Division.
Study Submission. 2003a. Unpublished confidential submission for PHOSFLEX 61B submitted to Environment and Climate Change Canada under the Chemicals Management Plan initiative. Gatineau (QC): ECCC, Program Development and Engagement Division.
Study Submission. 2003b. Unpublished confidential submission for PHOSFLEX 31L submitted to Environment and Climate Change Canada under the Chemicals Management Plan initiative. Gatineau (QC): ECCC, Program Development and Engagement Division.
Study Submission. 2003c. Unpublished confidential submission for PHOSFLEX 61B submitted to Environment and Climate Change Canada under the Chemicals Management Plan initiative. Gatineau (QC): ECCC, Program Development and Engagement Division.
Study Submission. 2009. Unpublished confidential submission for PHOSFLEX 71B submitted to Environment and Climate Change Canada under the Chemicals Management Plan initiative. Gatineau (QC): ECCC, Program Development and Engagement Division.
Study Submission. 2013. Unpublished confidential submission for PHOSFLEX 71B submitted to Environment and Climate Change Canada under the Chemicals Management Plan initiative. Gatineau (QC): ECCC, Program Development and Engagement Division.
Study Submission. 2014. Unpublished confidential submission for PHOSFLEX 71B submitted to Environment and Climate Change Canada under the Chemicals Management Plan initiative. Gatineau (QC): ECCC, Program Development and Engagement Division.
Study Submission. 2015. Unpublished confidential submission for PHOSFLEX 71B submitted to Environment and Climate Change Canada under the Chemicals Management Plan initiative. Gatineau (QC): ECCC, Program Development and Engagement Division.
Su G, Letcher RJ, Crump D, Farmahin R, Giesy JP, Kennedy SW. 2014. Photolytic degradation products of two highly brominated flame retardants cause cytotoxicity and mRNA expression alterations in chicken embryonic hepatocytes. Environ Sci Technol. 48:12039−12046.
Su G, Letcher RG, Crump D, Gooden DM, Stapleton HM. 2015a. In vitro metabolism of the flame retardant triphenyl phosphate in chicken embryonic hepatocytes and the importance of the hydroxylation pathway. Environ Sci Technol Lett. 2:100-104.
Su G, Letcher RJ, Moore JN, Williams LL, Martin PA, de Solla SR, Bowerman WW. 2015b. Spatial and temporal comparisons of legacy and emerging flame retardants in herring gull eggs from colonies spanning the Laurentian Great Lakes of Canada and United States. Environ Res. 142:720-730.
Su, G, Letcher, RJ, Yu, H, Gooden, DM, Stapleton, HM. 2016. Determination of glucuronide conjugates of hydroxyl triphenyl phosphate (OH-TPHP) metabolites in human urine and its use as a biomarker of TPHP exposure. Chemosphere. 149:314-319.
Su G, Greaves AK, Teclechiel D, Letcher RJ. 2016a. In vitro metabolism of photolytic breakdown products of tetradecabromo-1,4-diphenoxybenzene flame retardant in herring gull and rat liver microsomal assays. Environ Sci Technol. 50:8335−8343.
Su G, Letcher RJ, Crump D, Farmahin R, Giesy JP, Kennedy SW. 2016b. Sunlight irradiation of highly brominated polyphenyl ethers generates polybenzofuran products that alter dioxin-responsive mRNA expression in chicken hepatocytes. Environ Sci Technol. 50:2318−2327.
Su G, Letcher RJ, Yu H. 2016c. Organophosphate flame retardants and plasticizers in aqueous solution: pH-dependent hydrolysis, kinetics, and pathways. Environ Sci Technol. 50:8103-8111.
Su G, Letcher RJ, McGoldrick DJ, Backus SM. 2017. Halogenated flame retardants in predator and prey fish from the Laurentian Great Lakes: Age-dependent accumulation and trophic transfer. Environ Sci Technol. 51:8432−8441.
Sühring R, Diamond ML, Scheringer M, Wong F, Pućko M, Stern G, Burt A, Hung H, Fellin P, Li H, Jantunen LM. 2016. Organophosphate esters in Canadian Arctic air: Occurrence, levels and trends. Environ Sci Technol. 50:7409-7415.
Sun S, Tan H, Peng T, Wang S, Xu W, Qian H, Jin Y, Fu Z. 2016. Developmental neurotoxicity of organophosphate flame retardants in early life stages of Japanese medaka (Oryzias latipes). Environ Toxicol Chem. 35(12):2931-2940.
Sundkvist A, Olofsson U, Haglund P. 2010. Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. J Environ Monit. 12:943-951.
Takeuchi S, Kojima H, Saito I, Jin K, Kobayashi S, Tanaka-Kagawa T, Jinno H. 2014. Detection of 34 plasticizers and 25 flame retardants in indoor air from houses in Sapporo, Japan. Sci Total Environ. 491-492:28-33.
Takeuchi S, Tanaka-Kagawa T, Saito I, Kojima H, Jin K, Satoh M, Kobayashi S, Jinno H. 2015. Differential determination of plasticizers and organophosphorus flame retardants in residential indoor air in Japan. Environ Sci Pollut Res Int. 25(8):7113-7120.
[TEST] Toxicity Estimation Software Tool. 2016. Ver. 4.2. Washington (DC): US Environmental Protection Agency.
Thomas MB, Stapleton H, Dills RL, Violtte H, Christakis D, Sathyanarayama S. 2016. Demographic and dietary risk factors in relation to urinary metabolites of organophosphate flame retardants in toddlers. Chemosphere. 185:918-925.
Thors L, Koch B, Koch M, Hägglund L, Bucht A. 2016. In vitro human skin penetration model for organophosphorus compounds with different physicochemical properties. Toxicol In Vitro. 32:198-204.
TNO Quality of Life. 2005. In vitro percutaneous absorption of [14C]tris(2-chloro-1-methylethyl)phosphate (TCPP) through human skin membranes using flow-through diffusion cells. Unpublished report. [cited in EU RAR 2008a].
TNO Quality of Life. 2006a. In vitro percutaneous absorption of neat [1,3-14C]TDCP (Tris (1,3-chloro-2-propyl) phosphate) through human skin membranes using flow-through diffusion cells. Unpublished report [cited in EU RAR 2008b].
TNO Quality of Life. 2006b. In vitro percutaneous absorption of neat [14C]TCPP (Tris(2-chloro-1-methylethyl)phosphate) through human skin membranes using flow-through diffusion cells. Unpublished report. [cited in EU RAR 2008a].
Tomizawa S, Takano I, Kobayashi M, Tamura Y, Tateishi Y, Sakai N, Kitayama K, Nagayama T, Kamata K, Saito K. 2004. Identification of unknown peaks in foods during residual pesticide analysis. Shokuhin Eiseigaku Zasshi. 45:259-263.
[TOXNET] Toxicology Data Network. 2015. National Library of Medicine HSDB Database. [Access on 2018 Nov 16].
Trouborst L, Chu S, Chen D, Letcher R. 2015. Methodology and determination of tetradecabromo-1, 4-diphenoxybenzene flame retardant and breakdown by-products in sediments from the Laurentian Great Lakes. Chemosphere. 118:342-349.
Truong JW. 2016. Organophosphate esters (OPEs) as emerging contaminants in the environment: Indoor sources and transport to receiving waters [Master’s thesis]. Toronto (ON): University of Toronto.
Tsuda M, Saito M, Umemura T, Kawasaki Y, Momma J, Matsushima Y, Matsumoto M, Isama K, Kawiwa M, Kurokawa Y. 1993. A 14-week oral toxicity study of tributoxyethyl phosphate (TBEP) in rats. J Toxicol Sci. 18(4):421-429 [cited in WHO 2000].
Tsuji S, Tonogai Y, Ito Y. 1986. The influence of rearing temperatures on the toxicity of various environmental pollutants for Killifish (Oryzias latipes). Eisei Kagaku. 32 (1):46-53.
[US CFR] US Code of Federal Regulations. 2017a. Washington (DC): National Archives and Records Administration’s Office of the Federal Register (OFR); Government Publishing Office. Title 21, Vol. 3, b. I, Part 175.105: Adhesives. [accessed 2018 May 4].
[US CFR] US Code of Federal Regulations. 2017b. Washington (DC): National Archives and Records Administration’s Office of the Federal Register (OFR); Government Publishing Office. Title 21, Vol. 3, b. I, Part 176.210: Defoaming agents used in the manufacture of paper and paperboard. [accessed 2018 May 4].
[US CPSC] US Consumer Product Safety Commission, 2005. Migration of Flame Retardant Chemicals in Upholstered Furniture Foam [PDF]. Washington (DC): Division of Chemistry, US CPSC. [accessed 2018 Mar 29].
[US CPSC] US Consumer Product Safety Commission. 2006. CPSC Staff Preliminary risk assessment of flame retardant chemicals in upholstered furniture foam [PDF]. Bethesda (MD): US CPCS. [accessed 2018 Mar 29].
[US EPA] United States Environmental Protection Agency. 1985. Chemical Hazard Information Profile: Triethyl Phosphate, Oak Ridge National Lab.
[US EPA] United States Environmental Protection Agency. 1993. Wildlife Exposure Factors Handbook. Washington (DC): Office of Research and Development, US EPA. Volumes I and II. [accessed 2020 Jan 21].
[US EPA] United States Environmental Protection Agency. 2009. Screening-level hazard characterization of high production volume chemicals: Phosphoric acid derivatives (INTERIM). Washington (DC): High Production Volume Chemicals Branch, Risk Assessment Division, Office of Pollution Prevention and Toxics, US EPA,. pp. 32. [accessed 2020 Jun 4]
[US EPA] United States Environmental Protection Agency. 2010. Screening-level hazard characterization of isopropylated triphenyl phosphate (3:1) (CASRN 68937-41-7). Washington (DC): High Production Volume Chemicals Branch, Risk Assessment Division, Office of Pollution Prevention and Toxics, US EPA,. pp. 32. [accessed 2020 Jun 4]
[US EPA] United States Environmental Protection Agency. 2012. Chemical Data Reporting data collected under the Environmental Protection Agency’s Toxic Substances Control Act. Washington (DC): US EPA. [accessed 2020 Jun 4]
[US FDA] US Food and Drug Administration. 2006. Total Diet Study Market Baskets 1991-3 through 2003-4 [PDF]. College Park (MD): Center for Food Safety and Applied Nutrition. [accessed 2018 Jan 17].
Van den Eede N, Maho W, Erratico C, Neels H, Covaci A. 2013. First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicol Lett. 223(1):9-15.
Van der Veen I, de Boer J. 2012. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 88:1119-1153.
[VCCLab] Virtual Computational Chemistry Laboratory. ALOGPS [non-Java interface]. 2005. Ver. 2.1. Munich (DE): VCCLab. [Tetko IV, Gasteiger J, Todeschini R, Mauri A, Livingstone D, Ertl P, Palyulin VA, Radchenko EV, Zefirov NS, Makarenko AS, Tanchuk VY, Prokopenko VV. 2005. Virtual computational chemistry laboratory - design and description. J Comput Aided Mol Des. 19:453-463.]. http://www.vcclab.org/lab/alogps/.
Venier M, Dove A, Romanak K, Backus S, Hites R. 2014. Flame retardants and legacy chemicals in Great Lakes’ water. Environ Sci Technol. 48:9563-9572.
Versar Inc. 1986a. Standard scenarios for estimating exposure to chemical substances during use of consumer products. Washington (DC): Versar Inc. Vol I. Prepared for the U.S. Environmental Protection Agency, Office of Toxic Substances. [accessed 2015 Jun 24].
Versar Inc. 1986b. Standard scenarios for estimating exposure to chemical substances during use of consumer products. Washington (DC): Versar Inc. Vol II. Prepared for the U.S. Environmental Protection Agency, Office of Toxic Substances. [accessed 2015 Jun 24].
Verschueren, K. 2001. Handbook of environmental data on organic chemicals. 4th ed. Volumes 1-2. New York (NY). John Wiley & Sons. 855 p.
Völkel, W, Fuchs, V, Wöckner, M, Fromme, H. 2018. Toxicokinetic of tris(2-butoxyethyl) phosphate (TBOEP) in humans following single oral administration. Archives Toxicol. 92(2):651-660.
Vykoukalová M, Venier M, Vojta S, Melymuk L, Becanova J, Romanak K, Prokes R, Okeme J, Saini A, Diamond M, et al. 2017. Organophosphate esters flame retardants in the indoor environment. Environ Int. 106:97-104.
Wang X-W, Liu J-F, Yin Y-G. 2011. Development of an ultra-high-performance liquid chromatography–tandem mass spectrometry method for high throughput determination of organophosphorus flame retardants in environmental water. J Chromatogr A. 1218:6705-6711.
Wang G, Shi H, Du Z, Chen H, Peng J, Gao S. 2017. Bioaccumulation mechanism of organophosphate esters in adult zebrafish. Environ Pollut. 229:177-187.
[WATERNT] Water Solubility Program [estimation model]. 2010. Ver. 1.01. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Weber K, Ernst W. 1983. Occurrence and fluctuation of organic chemicals in major German estuaries. Vom Wasser. 61:111-123 [cited in ECETOC 1992a].
Wei G, Li D, Zhuo M, Liao Y, Xie Z, Guo T, Li J, Zhang S, Liang Z. 2015. Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity and human exposure. Environ Pollut. 196:29-46.
Weil ED, Levchik S. 2003. Current practice and recent commercial developments in flame retardancy of polyamides. J Fire Sci. 22:251-264.
Weil ED. 1993. Flame retardants (Phosphorus). In: Kirk-Othmer Encyclopedia of Chemical Technology. Online version. New York (NY): John Wiley and Sons, Inc. [restricted access].
[WHO] World Health Organization. 2000. Environmental Health Criteria 218: Flame retardants: tris(2-butoxyethyl) phosphate, tris(2-ethylhexyl) phosphate and tetrakis(hydroxymethyl) phosphonium salts [PDF]. Geneva: World Health Organization [accessed 2017 October 26].
[WHO] World Health Organization. 2006. Environmental Health Criteria 235: Dermal Absorption. Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals [PDF]. Geneva: World Health Organization. [accessed 2018 Nov 9].
Williams DT, LeBel GL. 1981. A national survey of tri(haloalkyl)-, trialkyl-, and triarylphosphates in Canadian drinking water. Bull Environ Contam Toxicol. 27:450-457.
Williams DT, Nestmann ER, LeBel GL, Benoit FM, Otson R. 1982. Determination of mutagenic potential and organic contaminants of Great Lakes Drinking Water. Chemosphere. 11(3):263-276.
[Wilson and Meridian] Wilson Scientific Consulting Inc. and Meridian Environmental Inc. 2015 [modified]. Critical review of soil ingestion rates for use in contaminated site human health risk assessments in Canada. Contractor report prepared for the Contaminated Sites Division, Safe Environments Programme, Health Canada: Ottawa (ON).
Wilson R, Jones-Otazo H, Petrovic S, Mitchell I, Bonvalot Y, Williams D, Richardson GM. 2013. Revisiting dust and soil ingestion rates based on hand-to-mouth transfer. Hum Ecol Risk Assess.19(1):158-188. [accessed 2014 Dec 18].
Wolschke H, Sühring R, Xie Z, Ebinghaus R. 2015. Organophosphorus flame retardants and plasticizers in the aquatic environment: A case study of the Elbe River, Germany. Environ Pollut. 206:488-493.
Wood AW, Duan C, Bern HA. 2005. Insulin-like growth factor signaling in fish. Int Rev Cytol. 243:215-286.
Won D, Lusztyk E. 2011. Data gathering on chemicals released to indoor air of residences from building materials and furnishings. Ottawa (ON): NRC-Institute for Research in Construction. Report no. B3332.2. Prepared for Health Canada.
Woudneh MB, Benskin JP, Wang G, Grace R, Hamilton MC, Cosgrove JR. 2015. Quantitative determination of 13 organophosphorous flame retardants and plasticizers in a wastewater treatment system by high performance liquid chromatography tandem mass spectrometry. J Chromatogr A. 1400:149-155.
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [estimation model]. 2010. Ver. 1.42. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Wu M, Yu G, Cao Z, Wu D, Liu K, Deng S, Huang J, Wang B, Wang Y. 2016. Characterization and human exposure assessment of organophosphate flame retardants in indoor dust from several microenvironments of Beijing, China. Chemosphere. 150:465-471.
Xu F, Garcia-Bermejo A, Malarvannan G, Gomara B, Neels H, Covaci A. 2015. Multi-contaminant analysis of organophosphate and halogenated flame retardants in food matrices using ultrasonication and vacuum assisted extraction, multi-stage cleanup and gas chromatography-mass spectrometry. J Chromatogr A. 1401:33-41.
Xu Q, Wu D, Dang Y, Yu L, Liu C, Wang J. 2017. Reproduction impairment and endocrine disruption in adult zebrafish (Danio rerio) after waterborne exposure to TBOEP. Aquat Toxicol. 182:163-171.
Yager TJB, Crock JG, Smith DB, Furlong ET, Hageman PL, Foreman WT, Gray JL, ReVello RC. 2013. Effects of Surface Applications of Biosolids on Groundwater Quality and Trace-Element Concentrations in Crops near Dear Trail, Colorado, 2004-2010 [PDF]. Reston (VA): U.S. Geological Survey and U.S. Department of the Interior. Scientific Investigations Report. [accessed 2018 Jul 26].
Yager TJB, Furlong ET, Kolpin DW, Kinney CA, Zaugg SD, Burkhardt MR. 2014. Dissipation of contaminants of emerging concern in biosolids applied to nonirrigated farmland in eastern Colorado: Journal of the American Water Resources Association. 50 (2):343-357.
Yalkowsky SH, He Y, Jain P. 2010. Handbook of Aqueous Solubility Data. Second ed. Boca Raton (FL): CRC Press. p 1185.
Yan XJ, He H, Peng Y, Wang XM, Gao ZQ, Yang SG, Sun C. 2012. Determination of organophosphorus flame retardants in surface water by solid phase extraction coupled with gas chromatography-mass spectrometry. Fenxi Huaxue/ Chin J Anal Chem. 40:1693-1697.
Yang C, Diamond ML, Latifovic L, Tsirlin D, Harris SA, Jantunen L. 2017. Semivolatile organic compounds in Canadian homes; A comprehensive household exposure study. Unpublished report. Toronto (ON): University of Toronto, Cancer Care Ontario, and Environment and Climate Chage Canada.
Yang C, Harris SA, Jantunen LM, Sidiqque S, Kubwabo C, Tsirlin D, Latifovic L, Fraser B, St-Jean M, De La Campa R, You H, Kulka R, Diamond ML. 2019. Are cell phones an indicator of personal exposure to organophosphate flame retardants and plasticizers? Environ Int. 122:104-116.
Yoshida T, Yoshida J. 2012. Simultaneous analytical method for urinary metabolites of organophosphorus compounds and moth repellents in general population. J Chromatogr B. 880:66-73.
Yuan S, Li H, Dang Y, Liu C. 2018. Effects of triphenyl phosphate on growth, reproduction and transcription of genes of Daphnia magna. Aquat Toxicol. 195:58-66.
Zhang Q, Lu M, Dong X, Wang C, Zhang C, Liu W, Zhao M. 2014. Potential estrogenic effects of phosphorus-containing flame retardants. Environ Sci Technol. 48:6995−7001.
Zhang X, Zou W, Mu Li, Chen Y, Ren C, Hu X, Zhou Q. 2016. Rice ingestion is a major pathway for human exposure to organophosphate flame retardants (OPFRs) in China. J Hazard Mater. 318:686-693.
Zhao F, Chen M, Gao F, Shen H, Hu J. 2017. Organophosphorus flame retardants in pregnant women and their transfer to chorionic villi. Environ Sci Technol. 51:6489-6497.
Zhou L, Hiltscher M, Gruber D, Puttmann W. 2017a. Organophosphate flame retardants (OPFRs) in indoor and outdoor air in the Rhine/Main area, Germany: Comparison of concentrations and distribution profiles in different microenvironments. Environ Sci Pollut Res Int. 24:10992-11005.
Zhou L, Hitscher M, Puttmann W. 2017b. Occurrence and human exposure assessment of organophosphate flame retardants in indoor dust from various microenvironments of the Rhine/Main region, Germany. Indoor Air. 27:1113-1127.
Zushi Y, Hashimoto S, Tanabe K. 2016. Nontarget approach for environmental monitoring by GCxGC-HRTOFMS in the Tokyo Bay basin. Chemosphere. 156:398-406.
Appendix A. The ecological risk classification of organic substances (ERC)
The ecological risks of three of the alkyl OP subgroup substances (BEHP, TEHP and TEP) addressed in this assessment were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure on the basis of weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration [LC50]) for characterization.
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox 2016), and from responses to surveys under section 71 of CEPA, or they were generated using selected quantitative structure-activity relationship (QSAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also composed of multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under-classification of hazard and exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC 2016a. The following describes two of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2016). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue used for critical body residue (CBR) analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is considered to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for these substances, and the hazard, exposure and risk classification results, are presented in ECCC (2016b).
The hazard and exposure classifications for three alkyl OP subgroup substances are summarized in Table A-1.
| Substance | ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| BEHP | moderate | low | low |
| TEHP | moderate | low | low |
| TEP | high | low | moderate |
According to information considered under ERC, BEHP was classified as having a low exposure potential. It was further classified as having a moderate hazard potential on the basis of its reactive mode of action and its potential to cause adverse effects in aquatic and terrestrial food webs given its moderate bioaccumulation potential. Considering current use patterns, BEHP is unlikely to be resulting in concerns for the environment in Canada.
According to information considered under ERC, TEHP was classified as having a low exposure potential. It was further classified as having a moderate hazard potential on the basis of its reactive mode of action and its potential to cause adverse effects in aquatic food webs given its moderate bioaccumulation potential. In addition, structural alerts from the OECD toolbox identified TEHP as being a potential protein binder. The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of this substance. Considering current use patterns, TEHP is unlikely to be resulting in concerns for the environment in Canada.
According to information considered under ERC, TEP was classified as having a low exposure potential. It was further classified as having a high hazard potential on the basis of the agreement between its reactive mode of action (reactive) and elevated ecotoxicity ratio, both of which suggest that this chemical is likely of high potency. In addition, structural alerts from the OECD toolbox identified TEHP as being a potential protein binder. TEP was classified as having moderate potential for ecological risk. Considering current use patterns, this substance is unlikely to be resulting in concerns for organisms or the broader integrity of the environment in Canada.
Appendix B. Components of IPPP
| IPPP components – Example 1a | Percent | IPPP components – Example 2b | Percent | |
|---|---|---|---|---|
|
24 | TPHP | 44.6 | |
| Ortho-isopropylphenyl diphenyl phosphate | 24 | 2-isopropylphenyl diphenyl phosphate | 26.9 | |
| Ortho-para diisopropylphenyl diphenyl phosphate | 18 | 3-isopropylphenyl diphenyl phosphate | 0.3 | |
| Di(ortho-isopropylphenyl) phenyl phosphate | 10 | 4-isopropylphenyl diphenyl phosphate | 4.9 | |
| Di(isopropylphenyl) phenyl phosphate | 10 | 2,4-diisopropylphenyl diphenyl phosphate | 7.2 | |
| Para-isopropylphenyl diphenyl phosphate | 6 | bis(2-isopropylphenyl) phenyl phosphate | 11.1 | |
| Isopropylphenyl diisopropylphenyl phenyl phosphate | 7 | bis(3-isopropylphenyl) phenyl phosphate | 0.8 | |
| Di(diisopropylphenyl) phenyl phosphate | < 1 | bis(4-isopropylphenyl) phenyl phosphate | 1.1 | |
| - | - | tris(3-isopropylphenyl) phosphate (T3IPPP) | 0.1 |
a As identified in Sjogren et al. 2009.
b As identified in Phillips et al. 2017.
Appendix C. Critical body residue (CBR)
| CAS RN | log Kow | BAFa (or BCF) (L/Kg) | CBR (mmol/kg) |
Meets acute CBR effects (2–8 mmol/kg) |
Meets chronic CBR effects (0.2–0.8 mmol/kg) |
|---|---|---|---|---|---|
| TPHP | 4.42 | 573 | 0.004 | no | no |
| BPDP | 5.68 | 1638 | 0.01 | no | no |
| BDMEPPP | 7.29 | 26131 | 0.15 | no | no |
| IDDP | 6.34 | 862 | 0.006 | no | no |
| IPPP | 7.55 | 22508 | 0.13 | no | no |
a BAF (or BCF) value represents the highest identified for the substance (empirical or modelled) (ECCC 2019c).
Appendix D. Total daily intake formula used in mammalian wildlife risk analysis for the aryl OP subgroup
A wildlife PEC was derived from a total daily intake (TDI) for mink (Mustela vison) and river otter (Lontra canadensis) consuming fish following the approach of the US EPA (1993).
TDI = (FMR x (Ci x Pi / GEi x AEi) + (Cs x IRs) + (Cw x IRw)) x ED x Pt
where:
TDI = total daily intake (mg/kg bw/day)
FMR = normalized free metabolic rate of wildlife receptor of interest (kcal/kg bw/day)
Ci = concentration of contaminant in the ith prey species (mg/kg)
Pi = proportion of the ith prey species in the diet (%)
GEi = gross energy of the ith prey species (kcal/kg prey)
AEi = assimilation efficiency of the ith prey species by the wildlife receptor of interest
Cs = concentration of contaminant in soil or sediments (mg/kg dw)
IRs = intake rate of soil or sediments (kg dw/kg bw/day)
Cw = concentration of contaminant in water (mg/L)
IRw = intake rate of water (L/kg bw/day)
ED = the dietary assimilation efficiency of the contaminant by the predator (%)
Pt = proportion of the time the receptor spends in the contaminated area (%)
Appendix E. Data used in dietary exposure estimates
The food consumption data used in the present assessment were from the Canadian Community Health Survey (CCHS) (Statistics Canada 2015). Health Canada's Food Directorate conservatively estimated dietary exposure to TPHP and TBOEP by multiplying the maximum concentrations assumed for each food category (Table E-1 and Table E-2) with the quantity of that food reportedly consumed by each respondent in the CCHS survey. This yielded a full distribution of TPHP and TBOEP exposure estimates for various age groups. As TPHP and TBOEP were found to occur in many of the main food groups that are a regular part of the diet of Canadians, mean and 90th percentile ‘all persons’ (AP) dietary exposure estimates for TPHP and TBOEP were calculated (Table E-3 and Table E-4).
| Food category used in dietary exposure assessment | Maximum TPHP concentration (ppb) | Food with the maximum concentration | Reference |
|---|---|---|---|
| Alcohol | 30 | wine, dry table, red/white | US FDA 2006 |
| Baby foods | 176 | baby food, zwieback toast | US FDA 2006 |
| Beverages | 18 | lemonade, frozen concentrated, reconstituted | US FDA 2006 |
| Condiments and sauces | 50 | white sauce | US FDA 2006 |
| Confectionary and sugar-based foods | 290 | candy, caramels | US FDA 2006 |
| Dairy | 0.73 | dairy | Zhang et al. 2016 |
| Eggs | 0.27 | hen's egg | Xu et al. 2015 |
| Fats and oils | 267 | margarine, regular | US FDA 2006 |
| Fish and seafood | 1.56 | fresh, frozen, canned, shellfish, and fish products | Poma et al. 2017 |
| Fruits | 12 | strawberries | US FDA 2006 |
| Grains | 110.5 | rice | Zhang et al. 2016 |
| Juices | 130 | apple-banana juice | US FDA 2006 |
| Meat | 1.54 | meat | Poma et al. 2017 |
| Soups and stews | 39 | beef and vegetable stew, canned | US FDA 2006 |
| Vegetables | 10 | vegetables | US FDA 2006 |
| Food category used in dietary exposure assessment | Maximum TBOEP concentration (ppb) | Food with the maximum concentration | Reference |
|---|---|---|---|
| Beverages | 8 | apple juice | US FDA 2006 |
| Confectionary and sugar based foods | 90 | popsicle, fruit-flavoured | US FDA 2006 |
| Dairy | 0.29 | dairy | Zhang et al. 2016 |
| Fish and seafood | 13.53 | white perch (Morone americana) | Greaves et al. 2016b |
| Fruits | 17 | peach, canned in light/medium syrup | US FDA 2006 |
| Grains | 110 | oatmeal, plain, cooked | US FDA 2006 |
| Meat | 0.34 | meat | Zhang et al. 2016 |
| Vegetables | 3.54 | vegetables | Zhang et al. 2016 |
Of the available occurrence data, the maximum concentration identified for a food item within a broader food category was conservatively assumed to represent the category as a whole. For example, margarine was found to contain the highest concentration of TPHP for all foods in the ‘fats and oils’ category for which data were available. Therefore, its maximum concentration was assumed to be representative of this entire category.
The maximum concentrations in foods and beverages used in the present assessment ranged from 0.27 ppb in eggs to 290 ppb in confectionary and sugar-based foods for TPHP and from 0.29 ppb in dairy products to 110 ppb in grain products for TBOEP (Table E-1, Table E-2).
Mean and 90th percentile ‘all persons’ exposures to TPHP across all age-sex groups ranged from 0.72 to 3.11 µg/kg bw/day and 1.27 to 5.64 µg/kg bw/day, respectively (Table E-3). For TBOEP, mean and 90th percentile ‘all persons’ exposures across all age-sex groups ranged from 0.38 to 1.65 µg/kg bw/day and 0.66 to 2.73 µg/kg bw/day, respectively (Table E-4).
| Age group, males and females (years) | ‘All persons’ dietary exposure to TPHP (µg/kg bw/day) - Mean | ‘All persons’ dietary exposure to TPHP (µg/kg bw/day) - p90 |
|---|---|---|
| 1-3 | 3.11 | 5.64 |
| 4-8 | 2.73 | 4.81 |
| 9-13 | 1.63 | 2.87 |
| 14-18 | 1.08 | 1.97 |
| 19-30 | 0.90 | 1.65 |
| 31-50 | 0.79 | 1.39 |
| 51-70 | 0.72 | 1.27 |
| 71+ | 0.74 | 1.29 |
Abbreviations: p90, 90th percentile
| Age group, males and females (years) | ‘All persons’ dietary exposure to TBOEP (µg/kg bw/day) - Mean | ‘All persons’ dietary exposure to TBOEP (µg/kg bw/day) - p90 |
|---|---|---|
| 1-3 | 1.65 | 2.73 |
| 4-8 | 1.32 | 2.12 |
| 9-13 | 0.83 | 1.43 |
| 14-18 | 0.55 | 1.05 |
| 19-30 | 0.47 | 0.83 |
| 31-50 | 0.44 | 0.81 |
| 51-70 | 0.38 | 0.67 |
| 71+ | 0.38 | 0.66 |
Abbreviations: p90, 90th percentile
Appendix F. Estimates of daily intake by various age groups within the general population of Canada
Assumptions for various age groups within the general population of Canada are as follows:
- 0–6 months: Assumed to weigh 7.5 kg, to breathe 2.1 m3 of air per day (Health Canada 1998), and to ingest 38 mg of dust per day, respectively (Wilson et al. 2013). Assumed that 0-6 months old infants do not ingest soil due to typical caregiver practices.
- Exclusively for breast -fed infants: Assumed to consume 0.742 L of breast milk per day (Health Canada 1998), and breast milk is assumed to be the only dietary source.
- Exclusively for formula-fed infants: assumed to drink 0.8 L of water per day (Health Canada 1998), where water is used to reconstitute formula.
- 0.5–4 years: Assumed to weigh 15.5 kg, to breathe 9.3 m3 of air per day, to drink 0.7 L of water per day, to ingest 41 mg of dust per day (Wilson et al. 2013) and to ingest 14 mg of soil per day (Wilson and Meridian 2015 [modified]).
- 5–11 years: Assumed to weigh 31.0 kg, to breathe 14.5 m3 of air per day, to drink 1.1 L of water per day, to ingest 31 mg of dust per day (Wilson et al. 2013) and to ingest 21 mg of soil per day (Wilson and Meridian 2015 [modified]).
- 12–19 years: Assumed to weigh 59.4 kg, to breathe 15.8 m3 of air per day, to drink 1.2 L of water per day, to ingest 2.2 mg of dust per day (Wilson et al. 2013) and to ingest 1.4 mg of soil per day (Wilson and Meridian 2015 [modified]).
- 20–59 years: Assumed to weigh 70.9 kg, to breathe 16.2 m3 of air per day, to drink 1.5 L of water per day, to ingest 2.5 mg of dust per day (Wilson et al. 2013) and to ingest 1.6 mg of soil per day (Wilson and Meridian 2015 [modified]).
- ≥ 60 years: Assumed to weigh 72.0 kg, to breathe 14.3 m3 of air per day, to drink 1.6 L of water per day, to ingest 2.5 mg of dust per day (Wilson et al. 2013) and to ingest 1.5 mg of soil per day (Wilson and Meridian 2015 [modified]).
- Negligible exposures are defined as < 2.5 ng/kg bw/day.
| Route of exposure | 0–6 months (breast-fedb) | 0–6 months (formula-fed) | 0.5–4 yr | 5–11 yr | 12–19 yr | 20–59 yr | ≥ 60 yr |
|---|---|---|---|---|---|---|---|
| Indoor airc | 3.8E-03 | 3.8E-03 | 8.1E-03 | 6.4E-03 | 3.6E-03 | 3.1E-03 | 2.7E-03 |
| Drinking waterd | N/A | 2.9E-01 | 1.2E-01 | 9.6E-02 | 5.5E-02 | 5.7E-02 | 6.0E-02 |
| Foode | 3.5E-02 | N/A | 5.6E+00 | 4.8E+00 | 2.9E+00 | 1.7E+00 | 1.3E+00 |
| Dustf | 1.0E-01 | 1.0E-01 | 5.5E-02 | 2.1E-02 | 7.7E-04 | 7.3E-04 | 7.2E-04 |
| Soil | N/A | N/A | 2.17E-03 | 1.63E-03 | 5.66E-05 | 5.42E-05 | 5.00E-05 |
| Total intake | 1.43E-01 | 3.97E-01 | 5.82E+00 | 4.93E+00 | 2.93E+00 | 1.71E+00 | 1.35E+00 |
Abbreviations: N/A, not applicable; yr, years.
a Estimated intakes of TPHP from ambient air were negligible when using the maximum concentration of TPHP in outdoor air (0.0022 µg/m3; Shoeib et al. 2014). No monitoring data of soil in North America were identified. An estimated PEC for soil representing the worst case scenario (i.e., the maximum PEC for all the aryl OPs of 2 400 ng/g) and a maximum concentration of 46 µg/kg from a soil study for commercial areas in China (Cui et al. 2017) was available, but resulted in negligible exposures.
b No Canadian monitoring of TPHP in breast milk was identified. The concentration of TPHP in breast milk, 0.35 µg/L, was based on a reported 10 ng/g lipid x 3.4% (lipid content of breast milk) x 1.03 g/mL (density of breast milk) identified in pooled breast milk samples collected in 1998 from 90 women in Sweden (Sundkvist et al. 2010).
c The 95th percentile indoor air concentration of TPHP (0.0152 µg/m3, from Toronto and Ottawa, ON) (Yang et al. 2018) was used for deriving upper-bounding estimates of daily intake for indoor air exposure. Canadians are assumed to spend 21 hours indoors each day (Health Canada 1998).
d The maximum estimated aquatic PEC for all the aryl OPs of 2.7 µg/L was chosen as a conservative approach for deriving general population intakes of TPHP from drinking water.
e See Appendix E for details of the assessment of dietary exposure to TPHP.
f The 95th percentile concentration of 20 700 µg/kg of TPHP in dust from bedrooms in Toronto and Ottawa, ON (Yang et al. 2019) was selected for deriving upper-bounding estimates of daily intake for dust exposure.
| Route of exposure | 0–6 months (breast-fed) | 0–6 months (formula-fed) | 0.5–4 yr | 5–11 yr | 12–19 yr | 20–59 yr | ≥ 60 yr |
|---|---|---|---|---|---|---|---|
| Drinking waterb | N/A | 2.9E-01 | 1.2E-01 | 9.6E-02 | 5.5E-02 | 5.7E-02 | 6.0E-02 |
| Dustc | 1.7E-03 | 1.7E-03 | 9.0E-04 | 3.4E-04 | 1.3E-05 | 1.2E-05 | 1.2E-05 |
| Total intake | 1.7E-03 | 2.9E-01 | 1.2E-01 | 9.6E-02 | 5.5E-02 | 5.7E-02 | 6.0E-02 |
Abbreviations: N/A, not applicable; yr, years.
a Intakes of BPDP for other media (indoor air, food, ambient air and soil) were negligible.
b No monitoring data for BPDP in water were identified, and the highest aquatic PEC of 2.7 µg/L (described in section 2.6.2.2 and reported in Table 2‑19) for the aryl OPs was therefore used to estimate water intakes of BPDP.
c The 95th percentile concentration of BPDP measured in house dust in Canada (340 ng/g) was used to estimate intakes of BPDP from dust (personal communication, e-mail from Environmental Health Science and Research Bureau, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated December 18, 2018; unreferenced).
| Route of exposure | 0–6 months (breast-fed) | 0–6 months (formula-fed) | 0.5–4 yr | 5–11 yr | 12–19 yr | 20–59 yr | ≥ 60 yr |
|---|---|---|---|---|---|---|---|
| Drinking waterb | N/A | 2.9E-01 | 1.2E-01 | 9.6E-02 | 5.5E-02 | 5.7E-02 | 6.0E-02 |
| Dustc | 3.8E-02 | 3.8E-02 | 2.0E-02 | 7.6E-03 | 2.8E-04 | 2.7E-04 | 2.6E-04 |
| Total intake | 3.8E-02 | 3.3E-01 | 1.4E-01 | 1.0E-01 | 5.5E-02 | 5.7E-02 | 6.0E-02 |
Abbreviations: N/A, not applicable; yr, years.
a Intakes of IDDP for other media (indoor air, food, ambient air and soil) were negligible.
b No monitoring data for IDDP in water were identified, and the highest aquatic PEC of 2.7 µg/L (described in section 2.6.2.2 and reported in Table 2‑19) for the aryl OPs was therefore used to estimate water intakes of IDDP.
c The 95th percentile concentration of IDDP measured in house dust in Canada (7 582 ng/g) was used to estimate intakes of IDDP from dust (personal communication, e-mail from Environmental Health Science and Research Bureau, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated December 18, 2018; unreferenced).
| Route of exposure | 0–6 months (breast-fedb) | 0–6 months (formula-fed) | 0.5–4 yr | 5–11 yr | 12–19 yr | 20–59 yr | ≥ 60 yr |
|---|---|---|---|---|---|---|---|
| Indoor airc | 3.8E-03 | 3.8E-03 | 8.1E-03 | 6.4E-03 | 3.6E-03 | 3.1E-03 | 2.7E-03 |
| Drinking waterd | N/A | 2.9E-01 | 1.2E-01 | 9.6E-02 | 5.5E-02 | 5.7E-02 | 6.0E-02 |
| Foode | 3.5E-02 | N/A | 5.6E+00 | 4.8E+00 | 2.9E+00 | 1.7E+00 | 1.3E+00 |
| Dustf | 1.0E-01 | 1.0E-01 | 5.6E-02 | 2.2E-02 | 7.9E-04 | 7.5E-04 | 7.4E-04 |
| Soilg | N/A | N/A | 2.2E-03 | 1.6E-03 | 5.7E-05 | 5.4E-05 | 5.0E-05 |
| Total intake | 1.4E-01 | 4.0E-01 | 5.8E+00 | 4.9E+00 | 3.0E+00 | 1.8E+00 | 1.4E+00 |
Abbreviations: N/A, not applicable; yr, years.
a Estimated intakes of IPPP were calculated by adding the estimated intakes of TPHP (Table F-1) and those for the isopropylated components of IPPP (data described in section 2.6.2.2) to represent the range of physical-chemical properties of the components of this UVCB (see section 2.1 and Table 2‑2). Intakes for TPHP were estimated using monitoring data as detailed in Table F-1, but without the highest aquatic PEC (2.7 µg/L) for the aryl OPs to prevent double counting as this was applied for TIPPP. Intakes for the isopropylated isomers were estimated using monitoring data and modelled environmental concentrations. Ambient air concentrations were modelled using ChemCAN (2003), where a scenario was derived from a maximum Canadian import quantity of approximately 1 000 000 kg (range imported presented in Table 2‑5). The highest aquatic PEC of 2.7 µg/L (described in section 2.6.2.2 and reported in Table 2‑19) for the aryl OPs was used to estimate water intakes of TIPPP. The highest soil PEC (2 400 ng/g) for the aryl OPs was not used to prevent double counting as this was applied for TPHP.
b No monitoring data for any of the isopropylated IPPP isomers in breast milk were identified, and therefore only data for TPHP were considered in estimating intakes of IPPP.
c The maximum indoor air concentration of T2IPPP from a study by Vykoukalová et al. (2017) (0.034 ng/m3) was used to estimate exposures to the isoproylated components of IPPP. The estimated intakes where then added to those estimated for TPHP as described in Table F-1.
d No relevant monitoring data for TIPPP or other isopropylated IPPP isomers in water were identified, and the highest aquatic PEC of 2.7 µg/L (described in section 2.6.2.2 and reported in Table 2‑19) for the aryl OPs was therefore used to estimate water intakes of the isopropylated components of IPPP.
e Only estimated intakes for TPHP were considered in estimating exposure to IPPP from food.
f The 95th percentile of 518 ng/g of an isopropylated IPPP isomer in dust (TIPPP) from the CHDS project (personal communication, e-mail from Environmental Health Science and Research Bureau, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated December 18, 2018; unreferenced) was used to estimate intake values of the isopropylated components of IPPP from dust. The estimated intakes were then added to those estimated for TPHP as described in Table F-1.
g No monitoring data of soil in North America for TPHP were identified. A maximum concentration of 46 µg/kg of TPHP from a soil study for commercial areas in China (Cui et al. 2017) was available, but resulted in negligible exposures. Estimated intakes of T3IPPP from ambient air and soil were negligible when modelled environmental concentrations derived using ChemCAN (2003) were considered (223 pg/m3 for ambient air, 4.45 ng/g for soil). Estimated intakes of TPHP from ambient air were negligible when using the maximum concentration of TPHP in outdoor air (0.0022 µg/m3; Shoeib et al. 2014).
| Route of exposure | 0–6 months (breast-fed) | 0–6 months (formula-fed) | 0.5–4 yr | 5–11 yr | 12–19 yr | 20–59 yr | ≥ 60 yr |
|---|---|---|---|---|---|---|---|
| Indoor airb | 7.3×10-2 | 7.3×10-2 | 0.16 | 0.12 | 6.9×10-2 | 5.9×10-2 | 5.2×10-2 |
| Drinking waterc | N/A | 1.1×10-2 | 4.7×10-3 | 3.7×10-3 | 2.1×10-3 | 2.2×10-3 | 2.3×10-3 |
| Dustd | 1.6 ×10-3 | 1.6×10-3 | 8.5×10-4 | 3.2×10-4 | 1.2×10-5 | 1.1×10-5 | 1.1×10-5 |
| Total intake | 7.5×10-2 | 8.6×10-2 | 0.16 | 0.13 | 7.1×10-2 | 6.2×10-2 | 5.4×10-2 |
Abbreviations: N/A, not applicable
a Intakes of TEP for other media (ambient air, food and soil) were negligible.
b The maximum indoor air concentration from Stockholm, Sweden of 297 ng/m3 (Luongo and Östman 2016) was selected for deriving upper-bounding estimates of daily intake for indoor air exposure. Canadians are assumed to spend 21 hours indoors each day (Health Canada 1998).
c The maximum concentration of TEP (105 ng/L) in surface water from streams in Toronto, Ontario, Canada (Truong 2016) was selected for deriving upper-bounding estimates of daily intake for drinking water exposure.
d The 95th percentile concentration of TEP (0.32 µg/g) in the Canadian baseline study (Canadian House Dust Study preliminary data; Kubwabo et al. (in prep), Environmental Health Science and Research Bureau, Health Canada, dated December 13, 2013; unreferenced), measured in various Canadian cities, was selected for deriving upper-bounding estimates of daily intake for dust exposure.
| Route of exposure | 0–6 months (breast-fed)b | 0–6 months (formula-fed) | 0.5–4 yr | 5–11 yr | 12–19 yr | 20–59 yr | ≥ 60 yr |
|---|---|---|---|---|---|---|---|
| Indoor airc | 7.9E-02 | 7.9E-02 | 1.7E-01 | 1.3E-01 | 7.5E-02 | 1.1E-03 | 9.8E-04 |
| Drinking waterd | N/A | 6.0E-02 | 2.5E-02 | 2.0E-02 | 1.1E-02 | 1.2E-02 | 1.2E-02 |
| Foode | 2.2E-01 | N/A | 2.7E+00 | 2.1E+00 | 1.4E+00 | 8.3E-01 | 6.7E-01 |
| Dustf | 1.8E+01 | 1.8E+01 | 9.6E+00 | 3.6E+00 | 1.3E-01 | 3.7E-03 | 3.6E-03 |
| Total intake | 18.3 | 18.1 | 12.5 | 5.9 | 1.6 | 0.85 | 0.69 |
Abbreviations: N/A, not applicable
a Intakes of TBOEP for other media (ambient air and soil) were negligible.
b No Canadian monitoring of TBOEP in breast milk was identified. The concentration of TBOEP in breast milk, 2.21 µg/L, was based on the maximum TBOEP concentration of 63 ng/g lipid x 3.4% (lipid content of breast milk) x 1.03 g/mL (density of breast milk) identified in pooled breast milk samples collected in 1997 from 69 women in Sweden (Sundkvist et al. 2010).
c The 95th percentile indoor air concentration of TBOEP (5 640 pg/m3) from homes in Toronto and Ottawa, Canada (Yang et al. 2019) was selected for deriving upper-bounding estimates of daily intake for indoor air exposure. For age groups below 20 years (i.e., children and adolescents), the 95th percentile indoor air concentration of TBOEP (833 ng/m3 or 833 000 pg/m3) from daycare centres in Germany (Fromme et al. 2014) was also used to account for exposures from elevated concentrations of TBOEP in childcare or educational environments during 8 hours of the day. Canadians are assumed to spend 21 hours indoors each day (Health Canada 1998).
d The maximum concentration of TBOEP (560 ng/L) in potable water from municipal water treatment plants across Canada (Williams and Lebel 1981) was selected for deriving estimates of daily intake for drinking water exposure.
e Used 90th percentile estimates for all persons from Appendix E, Table E-4. The highest estimated intake from relevant age groups was used (e.g., P90 value for 1- to 3-year-olds was used for 0.5- to 4-year-olds).
f For age groups above 20 years, the 95th percentile concentration of TBOEP (104 µg/g) in the Canadian baseline study (personal communication, emails from the Environmental Health Science and Research Bureau, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated October 28, 2013; unreferenced), measured in various Canadian cities, was selected for deriving estimates of daily intake for dust exposure. For age groups below 20 years (i.e., children and adolescents), the 95th percentile dust concentration of TBOEP (3 633 µg/g) from daycare centres in Germany (Fromme et al. 2014) was selected for deriving upper-bounding estimates of daily intake for dust exposure.
| Route of exposure | 0–6 months | 0.5–4 yr | 5–11 yr | 12–19 yr | 20–59 yr | ≥ 60 yr |
|---|---|---|---|---|---|---|
| Indoor airb | 2.8×10-3 | 6.0×10-3 | 4.7×10-3 | 2.7×10-3 | 2.3×10-3 | 2.0×10-3 |
| Dustc | 9.0×10-3 | 4.7×10-3 | 1.8×10-3 | 6.6×10-5 | 6.3×10-5 | 6.2×10-5 |
| Total intake | 1.2×10-2 | 1.1×10-2 | 6.5×10-3 | 2.8×10-3 | 2.4×10-3 | 2.1×10-3 |
a Intakes of TEHP for other media (drinking water, ambient air, food and soil) were negligible
b The 95th percentile indoor air concentration of TEHP (11400 pg/m3) from homes in Toronto and Ottawa, Canada (Yang et al. 2019) was selected for deriving upper-bounding estimates of daily intake for indoor air exposure. Canadians are assumed to spend 21 hours indoors each day (Health Canada 1998).
c The 95th percentile concentration of TEHP in dust (1 780 ng/g) from homes in Toronto and Ottawa, Canada (Yang et al. 2019) was selected for deriving estimates of daily intake for dust exposure.
Appendix G. Parameters used to estimate human exposure from use of products and manufactured items available to consumers
Products
Sentinel exposure scenarios were used to estimate the potential exposure to substances in the Flame Retardants Group. Exposure estimates were calculated based on default body weights of 70.9 kg, 59.4 kg, 31.0 kg, and 15.5 kg for 20 years and older, 12 to 19 year olds, 5 to 11 year olds, and 6 month to 4 year olds respectively (Health Canada 1998). Exposures were estimated using ConsExpo Web (ConsExpo Web 2016) or algorithms (see below for more details). Scenario-specific assumptions are provided in Table G‑1.
| Product (substance) | Assumptions |
|---|---|
|
Top coat (assume put on finger and toe nails)a (TPHP) |
Concentration of TPHP: 10% Dermal: |
|
Nail polish (2 coats on finger and toe nails)a (TPHP) |
Concentration of TPHP: 30% Dermal: |
|
Base coat (assume put on finger and toe nails)a (TPHP) |
Concentration of TPHP: 10% Dermal: |
|
Water-based paint (IDDP) |
Weight fraction of substance: 1.5% Scenario: Brush and Roller Painting in Paint Products Fact Sheet (RIVM 2007). Dermal: |
|
Oven cleaning using all-purpose remover (TEP) |
Concentration of TEP: ≤ 7% (MSDS 2015) Scenario: Oven cleaner in Cleaning Products Fact Sheet (RIVM 2016). Dermal – during spraying Dermal – during cleaning |
|
Foam sealant (TEP) |
Concentration of TEP: ≤ 5% (MSDS 2014a) Scenario: Insulation foam in Do-It-Yourself Products Fact Sheet (RIVM 2007). Inhalation – Exposure to vapour – instantaneous release |
|
Rust paint (TBOEP) |
Concentration: ≤ 5% (MSDS 2014b) Adapted from scenario for Solvent-Based Varnish in VERSAR (1986a) Dermal: Estimated Exposure = (SA × PCR × Concentration) / Body Weight |
|
Floor joint sealant (TEHP) |
Concentration: ≤ 6% (Hoffmann Mineral 2018) Scenario: General Coatings in Do-It-Yourself Products Fact Sheet (RIVM 2007). Dermal: Scenario: Joint Sealant in Do-It-Yourself Products Fact Sheet (RIVM 2007). Dermal: |
|
Gear oil (BEHP) |
Concentration: ≤ 5% (MSDS 2013) Adapted from scenarios for Motor Oil and Lubricating Grease in VERSAR (1986b) Surface area of skin contact (SA): 12 cm2 (2 fingers and 2 thumbs, Health Canada 1998) Estimated Exposure = (Concentration × SA × T × DSY) / Body weight |
a A retention factor of 1 and an absorption factor of 1 were used.
b Not factored in per event exposures.
Manufactured Items
Based on the available information, dermal exposure was estimated for direct contact with foam-containing mattresses and related manufactured items for all age groups and oral exposures are estimated for 0 to 6 months and 0.5 to 4 year olds. The exposure parameters and values used to estimate exposures are presented below and are based on conservative assumptions.
Dermal uptake = [SA × SCF × TPF × M × ED × DA] / BW
Oral intake = [SA × M × ED] / BW
| Age group | Surface area of skin contacta (SA) | Exposure durationb (ED) | Body weightc (BW) |
|---|---|---|---|
| 0–6 months | 545–1840 cm2 | 12 hr/d | 7.5 kg |
| 0.5–4 years | 792–2890 cm2 | 12 hr/d | 15.5 kg |
| 5–11 years | 1258–4830 cm2 | 10 hr/d | 31.0 kg |
| 12–19 years | 1972–8100 cm2 | 10 hr/d | 59.4 kg |
| 20+ years | 2033–9100 cm2 | 8 hr/d | 70.9 kg |
a For this scenario, a range in surface areas (SA) were used to represent dermal contact with a mattress. For the lower SA used, it is assumed that an individual is wearing shorts and a t-shirt that cover half of the limbs. The surface area of exposure is based on exposure to a fraction of the lower half of the limbs (arms and legs) and the back of the head. The surface areas of the limbs (Health Canada 1995) were multiplied by one half to account for clothing coverage and then were multiplied by one third to account for the triangular shape of limbs, where only one side is directly in contact with the mattress (US CPSC 2006). The surface area of the head (Health Canada 1995) was multiplied by a factor of 0.5 to represent exposure to the back of the head only. For the higher SA used, it was assumed that half of the body was in dermal contact with the mattress (US EPA 2012).
b Exposure duration for lying was adjusted from durations reported in US CPSC (2006) for leisure sitting to account for longer lying durations relative to sitting.
c Health Canada (1998).
| Parameter | TDCPP | TBB | TPHP | BPDP | T3IPPP (isomer in IPPP) |
|---|---|---|---|---|---|
| Water solubility (mg/L) | 18.1 | 0.00282 | 2.25 | 0.13 | 0.00339 |
| Log Kow (dimensionless) | 3.69 | 7.71 | 4.42 | 5.68 | 7.55 |
| Rate of migration from covered foam (mg/cm2/hr) a | 5.62x10-5 | 1.97x10-5 |
2.45x10-5 b 4.54x10-5 c |
2.03x10-5 b 3.32x10-5 c |
2.00x10-5 b 1.98x10-5 c |
a The migration rates for TDCPP and TBB were determined in migration studies performed on treated furniture foam by the US CPSC (US CPSC 2005). In the study, a furniture mini-seat mock-up consisting of a block of foam covered with cotton fabric and attached to plywood was prepared. The mini-seat was wetted with a saline solution, to mimic sweat, and pressure was applied to imitate the action of sitting. The migration rates of TDCPP and TBB were determined based on the reported maximum daily amount extracted for each substance (8 and 2.8 µg, respectively) onto a filter paper (5-cm diameter) over the course of the migration testing period (6 hours) (US CPSC 2005).
b Calculated based on plotting a straight line between water solubilities and migration rates for TDCPP and TBB with equation y = (2×10-6) × water solubility + 2×10-5
c Calculated using differential equations based on TDCPP and TBB migration rates, water solubilities, and log Kow values using the equation migration rate = x (log of water solubility) + y (log Kow). For TDCPP this is 0.0000562 mg/cm2/hr = x (1.26) + y (3.69) and for TBB this is 0.0000197 = x (-2.55) + y (7.71). Solving for x and y results in: x = 0.0000188 and y = 0.0000088. For TPHP, migration rate = 0.0000188 (log of water solubility) + 0.0000088 (log Kow) = 0.0000188 (0.35) + 0.0000088 (4.42) = 4.54 × 10-5 mg/cm2/hr. For BPDP, migration rate = 0.0000188 (log of water solubility) + 0.0000088 (log Kow) = 0.0000188 (-89) + 0.0000088 (5.68) = 3.32 × 10-5 mg/cm2/hr. For T3IPPP, migration rate = 0.0000188 (log of water solubility) + 0.0000088 (log Kow) = 0.0000188 (-2.47) + 0.0000088 (7.55) = 1.98 × 10-5 mg/cm2/hr.
| Parameter | TPHP | BPDP | IPPP (T3IPPP) | IPPP (TPHP / T3IPPP) |
|---|---|---|---|---|
| Skin contact factora (SCF) | 1 | 1 | 1 | 1 |
| Textile penetration factorb (TPF) | N/A | N/A | N/A | N/A |
| Migration ratec (M) (mg/cm2/hr) | 2.45×10-5 – 4.54×10-5 | 2.03×10-5 – 3.32×10-5 | 1.98×10-5 – 2.00×10-5 | Refer to TPHP and T3IPPP |
| Dermal absorption (DA) | N/A | 100%d | 30%e | 30%e |
| Exposure estimatesf – 0 –6 months (mg/kg bw/day) | 0.021–0.13 | 0.018–0.098 | 0.0052–0.018 | 0.0052–0.040g |
| Exposure estimatesf – 0.5–4 years (mg/kg bw/day) | 0.015–0.10 | 0.012–0.074 | 0.0037–0.013 | 0.0036–0.030g |
| Exposure estimatesf – 5–11 years (mg/kg bw/day) | 0.010–0.071 | 0.0082–0.052 | 0.0024–0.0093 | 0.0024–0.021g |
| Exposure estimatesf – 12–19 years (mg/kg bw/day) | 0.0081–0.062 | 0.0067–0.045 | 0.0020–0.0081 | 0.0020–0.019g |
| Exposure estimatesf – 20+ years (mg/kg bw/day) | 5.6×10-3 – 4.7×10-2 | 0.005–0.034 | 0.001–0.006 | 0.0014–0.014g |
Abbreviations: N/A, not applicable
a No substance-specific skin contact factors, i.e., the fraction of substance on a surface adhering to skin, were identified in the literature for TPHP, BPDP or IPPP. A value of 1 was therefore selected, with the implicit assumption that all of the chemical in contact with the skin is available for absorption.
b A textile penetration factor (TPF) was not applied since the migration rates used for extrapolation were found using covered foam samples (see Table G-3).
c Refer to Table G-3 for derivation of migration rates.
d Dermal absorption of BPDP was assumed to be 100% due to limited substance-specific data.
e Dermal absorptions of TPHP and T3IPPP were adjusted to 30% based on the use of TDCPP as an analogue for IPPP (EU RAR 2008b).
f Derived using the following equation: Dermal uptake = [SA × SCF × TPF × M × ED × DA] / BW. Refer to Table G-2 for SA, ED and BW, and Table G-3 for migration rates (M).
g Range of exposure estimates represents exposure to T3IPPP isomer (lowest exposure) and TPHP (highest exposure x 30% DA).
| Age group | Surface area of skin contacta (SA) | Exposure durationb (ED) | Body weightc (BW) |
|---|---|---|---|
| 0–6 months | 215 cm2 | 3 hr/d | 7.5 kg |
| 0.5–4 years | 357 cm2 | 3 hr/d | 15.5 kg |
| 20+ years | 1395 cm2 | 6 hr/d | 70.9 kg |
a For this scenario, it is assumed that an individual is wearing shorts and a t-shirt that cover half of the limbs. The surface area of exposure is based on exposure to a fraction of the lower half of the limbs (arms and legs). The surface areas of the limbs (Health Canada 1995) were multiplied by one half to account for clothing coverage and then were multiplied by one third to account for the triangular shape of limbs, where only one side is directly in contact with the foam item (i.e., infant or child restraint seat or sofa) (US CPSC 2006).
b Exposure duration for sitting was adjusted from durations reported in US CPSC (US CPSC 2006) for leisurely sitting.
c Health Canada (1998).
| Parameter | TDCPP | TCEP | TBOEP | TEP |
|---|---|---|---|---|
| Water solubility (mg/L) | 18.1 | 7820 | 1670 | 500 000 |
| log Kow (dimensionless) | 3.69 | 1.78 | 3.81 | 0.8 |
| Rate of migration from covered foam (mg/cm2/hr)a | 0.00297 | 0.0207 |
0.00624b 0.0143c |
1.0b 0.0325c |
a The migration rates for TDCPP and TCEP were determined in migration studies performed on treated furniture foam by the Danish EPA (2015) as reported by ECHA (2018). The migration rates of TCEP and TDCPP were determined using children’s products (i.e., infant or child restraint seats, baby slings, baby mattresses) by submerging pieces of foam from these products (usually with some of the fabric covering included in the samples) in sweat simulant and incubating them at 37°C for 3 hours (Danish EPA 2015). The migration rate for TDCPP used here is the average of the rates found across all samples for this flame retardant while the migration rate for TCEP was from a single item (ECHA 2018).
b Calculated based on plotting a straight line between water solubilities and migration rates for TDCPP and TCEP with equation y = (2×10-6) × water solubility + 0.0029
c Calculated using differential equations based on TDCPP and TCEP migration rates, water solubilities, and log Kow values using the equation migration rate = x (log of water solubility) + y (log Kow). For TDCPP this is 0.00297 mg/cm2/hr = x (1.26) + y (3.69) and for TCEP this is 0.0207 = x (3.89) + y (1.78). Solving for x and y results in: x = 0.0059 and y = -0.0012. For TBOEP, migration rate = 0.0059 (log of water solubility) – 0.0012 (log Kow) = 0.0059 (3.22) – 0.0012 (3.81) = 1.43 × 10-2 mg/cm2/hr. For TEP, migration rate = 0.0059 (log of water solubility) – 0.0012 (log Kow) = 0.0059 (5.70) – 0.0012 (0.8) = 3.25 × 10-2 mg/cm2/hr.
| Parameter | TEP (infant or child restraint seat) | TEP (sitting on a foam-containing mattress or furniture) | TBOEP (infant or child restraint seat or sofa) | IPPP (TPHP / T3IPPP) (infant or child restraint seat) |
|---|---|---|---|---|
| Skin contact factor a (SCF) | 1 | 1 | 1 | 1 |
| Textile penetration factor b (TPF) | 0.1 | 0.1 | 0.1 | N/A |
| Migration rate c (M) (mg/cm2/hr) | 3.25×10-2 – 1.0 | 3.25×10-2 – 1.0 | 6.24×10-3 – 1.43×10-2 | 1.98×10-5 – 4.54×10-5 |
| Dermal absorption (DA) | 100% d | 100% d | 40% e | 30% f |
| Exposure estimates – 0–6 months (mg/kg bw/day) | 0.28–8.60 g | 2.8–294 h | 0.021–0.049 g | 5.1×10-4 – 1.2×10-3 g |
| Exposure estimates – 0.5–4 years (mg/kg bw/day) | 0.22–6.91 g | 2.0–224 h | 0.017–0.040 g | 4.1×10-4 – 9.4×10-4 g |
| Exposure estimates – 5–11 years (mg/kg bw/day) | N/A | 1.3–156 h | N/A | N/A |
| Exposure estimates – 12–19 years (mg/kg bw/day) | N/A | 1.1–134 h | N/A | N/A |
| Exposure estimates– 20+ years (mg/kg bw/day) | N/A | 0.75–103 h | N/A | N/A |
Abbreviations: N/A, not applicable
a No substance-specific skin contact factors, i.e., the fraction of substance on a surface adhering to skin, was identified in the literature for TEP or TBOEP. A value of 1 was therefore selected, with the implicit assumption that all of the chemical in contact with the skin is available for absorption.
b A textile penetration factor (TPF) was applied for TEP and TBOEP to account for the migration rates used for extrapolation (i.e., TDCPP and TCEP) being determined using uncovered foam (ECHA 2018). No substance-specific textile penetration data were identified in the literature. A value of 0.1 (Driver et al. 2007 as cited in ECHA 2018) was therefore used for the TPF for both TEP and TBOEP. A textile penetration factor (TPF) was not applied to exposure estimates of IPPP since the migration rates used for extrapolation were found using covered foam samples (see Table G-3).
c Refer to Table G-6 for derivation of migration rates for TEP and TBOEP and Table G-3 for IPPP.
d Dermal absorption of TEP was assumed to be 100% due to limited substance-specific data.
e Dermal absorption of TBOEP was adjusted to 40% based on the use of TCPP as an analogue (EU RAR 2008a).
f Dermal absorptions of TPHP and T3IPPP were adjusted to 30% based on the use of TDCPP as an analogue (EU RAR 2008b).
g Derived using the following equation: Dermal uptake = [SA × SCF × TPF × M × ED × DA] / BW. Refer to Table G-5 for SA, ED and BW, and Table G-6 for migration rates (M).
hDerived for TEP using the following equation: Dermal uptake = [SA × SCF × TPF × M × ED × DA] / BW. Refer to Table G-2 for SA, ED and BW, and Table G-6 for migration rates (M).
| Age Group | Surface area moutheda (SA) | Exposure durationb (ED) | Body weightc (BW) |
|---|---|---|---|
| 0–6 months | 10 cm2 | 24.5 min/d | 7.5 kg |
| 0.5–4 years | 20 cm2 | 24.5 min/d | 15.5 kg |
a Surface area for 0 to 6 month olds is based on multiple references (RIVM 2008). Surface area for 0.5 to 4 years olds is based on professional judgment reflecting twice the surface area of the opening of a 0.5 to 4 years olds’ mouth.
b The mouthing duration for children’s foam products such as nap mats, infant or child restraint seats and small furniture was based on the duration for “other objects” in Norris and Smith (2002) [cited in US EPA (2011)].
c Health Canada (1998).
| Substance | 0–6 months (mg/kg bw/day) | 0.5–4 years (mg/kg bw/day) |
|---|---|---|
| TPHP | 1.33×10-5 – 2.47×10-5 | 1.29×10-5 – 2.39×10-5 |
| BPDP | 1.11×10-5 – 1.81×10-5 | 1.07×10-5 – 1.75×10-5 |
| IPPPb | 1.08 ×10-5 – 2.47×10-5 | 1.04 ×10-5 – 2.39×10-5 |
| TEP | 0.018–0.54 | 0.017–0.53 |
| TBOEP | 3.40×10-3 – 7.79×10-3 | 3.29×10-3 – 7.53×10-3 |
a Derived using the following equation: Oral intake = [SA × M × ED] / BW. Refer to Table G-8 for SA and ED, and Table G-3 and Table G-6 for migration rates (M). It is assumed that each substance is completely absorbed in exposure through the oral route and that a textile covering on a foam object would not affect migration (i.e., no textile penetration factor, TPF, applied).
b Range of exposure estimates represents exposure to T3IPPP isomer (lowest exposure) and TPHP (highest exposure).
Appendix H. TBOEP intake estimate from urinary BBOEHEP biomonitoring reverse dosimetry
Reverse dosimetry was used to derive estimates of daily intakes from urine concentrations for Canadian premenopausal women (age 18 to 44 years) (Yang et al. 2019). Daily intakes based on creatinine-adjusted concentrations were calculated using reverse dosimetry as shown in the equation below; see Table H-1.
Daily Intake = ([Urine]CR × CR ) / (BW × FUE)
| Symbol | Description | Value |
|---|---|---|
| [Urine]CR | Creatinine-adjusted urinary concentration of BBOEHEP (ng/mL)a |
4.86 (mean) 33.7 (95th percentile) |
| CR | 24 hr creatinine excretion (g/day) | 1.2 (20+ years men/women)b |
| BW | Body weight (kg) | 70.9 (20+ years)c |
| FUE | Fractional urine excretion | 0.0838d |
| Daily Intake | Intake (µg/kg bw/day) |
9.8 × 10-4 (based on mean [Urine]CR) 6.8 × 10-3 (based on 95th percentile [Urine]CR) |
a Yang et al. 2019
b Hays et al. 2010
c Health Canada 1998.
d Following the oral administration of TBOEP to human subjects (n=6) in a toxicokinetic study (Völkel et al. 2018), the average percentage of excretion of BBOEHEP within 39 h was 8.38%.
