Draft screening assessment - Heptamethylnonane
Official title: Draft Screening Assessment - Nonane, 2,2,4,4,6,8,8-heptamethyl-(Heptamethylnonane)
Chemical Abstracts Service Registry Number: 4390-04-9
Environment and Climate Change Canada
Health Canada
January 2020
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of nonane, 2,2,4,4,6,8,8-heptamethyl-, hereinafter referred to as heptamethylnonane (HMN). The Chemical Abstracts Service Registry Number (CAS RNFootnote 1) for HMN is 4390-04-9. This substance is among those substances identified as priorities for assessment as it met categorization criteria under subsection 73(1) of CEPA.
HMN is a highly-branched aliphatic hydrocarbon and is not known to naturally occur in the environment. The substance is primarily used as a skin conditioning agent, emollient, or solvent in self-care products. According to information obtained in a survey issued pursuant to a CEPA section 71 notice, the substance was reported to be imported into Canada in quantities ranging from 10 000 to 100 000 kg and was not reported to be manufactured in Canada above the reporting threshold of 100 kg.
The ecological risk of HMN was characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, HMN is considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from HMN. It is proposed to conclude that HMN does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
HMN is considered to be of low human hazard potential due to the lack of genotoxic, reproductive or developmental effects, and other adverse effects relevant to human health up to 1000 mg/kg bw/day on the basis of oral studies conducted on HMN, and up to 1393 mg/m3 on the basis of inhalation studies conducted on a structurally-related substance. As HMN is considered to be of low hazard potential, and the risk to human health is considered to be low,
estimates of exposure to the general population were not derived.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that HMN does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that HMN does not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of nonane, 2,2,4,4,6,8,8-heptamethyl-, hereinafter referred to as heptamethylnonane (HMN), to determine whether this substance presents or may present a risk to the environment or to human health. This substance was identified as a priority for assessment as it met categorization criteria under subsection 73(1) of CEPA (ECCC, HC [modified 2017]).
The ecological risk of HMN was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This draft screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to February 2019. Targeted literature searches were conducted up to June 2019. Empirical data from key studies as well as results from models were used to reach proposed conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This draft screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The human health portions of this assessment have undergone external review and/or consultation. Comments on the technical portions relevant to human health were received from Jennifer Flippin, Theresa Lopez, and Joan Garey, all affiliates of Tetra Tech. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this draft screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This draft screening assessment focuses on information critical to determining whether this substance meets the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precautionFootnote 2. This draft screening assessment presents the critical information and considerations on which the proposed conclusion is based.
2. Substance identity
The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 3), Domestic Substances List (DSL) name and common name for HMN is presented in Table 2-1.
| CAS RN | DSL name (common names, acronym) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 4390-04-9 | Nonane,2,2,4,4,6,8,8-heptamethyl- (heptamethylnonane [HMN], isohexadecanea) |
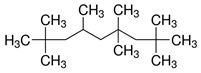 C16H34 |
226.45 |
a The common name “isohexadecane” represents a general term used to describe branched, saturated hydrocarbon substances with 16 carbon atoms (CIR 2012).
2.1 Selection of analogues
A read-across approach using data from an analogue was used to inform the human health assessment. The analogue was selected on the basis of structural and/or functional similarity to HMN (similar physical-chemical properties, toxicokinetics, reactivity) and had relevant empirical data that could be used to read across to endpoints with limited empirical data. Table 2-2 provides the identity of the analogue used to inform the assessment of HMN. Appendix A provides further details on the factors considered in the identification of potential analogues. For further information on the physical-chemical properties and health effects data available on the analogue, refer to Appendix B. Details of the read-across data to inform the human health assessment of HMN are further discussed in the relevant sections of this report
| CAS RN | Common names | Chemical structure | Molecular weight (g/mol) |
|---|---|---|---|
| 13475-82-6 |
|
UVCB (representative structure)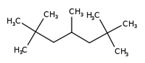 C12H26 |
UVCB (based on representative structure) 170 |
a The common name “isododecane” represents a group of linear and/or branched saturated hydrocarbons composed exclusively of hydrogen and carbon (general formula C12H26). Theoretically, there can be 355 different isododecane isomers (ANSES 2013).
b UVCB substance. UVCB is an acronym for Unknown or Variable composition Complex reaction products and Biological material. These materials are derived from natural sources or complex reactions and cannot be characterized in terms of constituent chemical compounds because their composition is too complex or variable. A UVCB is not an intentional mixture of discrete substances and is considered a single substance
3. Physical and chemical properties
A summary of physical and chemical properties of HMN is presented in Table 3-1, with a range in values indicated for each property where applicable. When experimental information was limited or not available for a property, data from analogues were used for read-across and/or (Q)SAR models were used to generate predicted values for the substance. Additional physical and chemical properties are reported in ECCC (2016b).
| Property | Value or range | Type of data | Key reference(s) |
|---|---|---|---|
| Physical state | White liquid | Experimental | Sigma Aldrich 2015 |
| Boiling point (°C) | 240 | Experimental | Sigma Aldrich 2015 |
| Vapour pressure (Pa) | 130 (20°C) | Experimental | Sigma Aldrich 2015 |
| Henry’s law constant (atm·m3/mol) | 2.90 x101 – 1.12 x102 | Modelled | EPI Suite c2000-2012 |
| Water solubility (mg/L) | 0.00205 | Modelled | EPI Suite c2000-2012 |
| Log Kow (dimensionless) | 7.79 | Modelled | EPI Suite c2000-2012 |
| Log Koc (dimensionless) | 4.15 – 6.76 | Modelled | EPI Suite c2000-2012 |
| Log Koa (dimensionless) | 4.72 | Modelled | EPI Suite c2000-2012 |
4. Sources and uses
HMN is a highly-branched aliphatic hydrocarbon and is not known to naturally occur in the environment. The substance may be produced through alkylation processes by combining shorter chain alkane substances, followed by isomerization to produce the branched structure (CIR 2012). According to information submitted in response to a survey pursuant to section 71 of CEPA during the 2011 reporting year, HMN was not manufactured in Canada above the reporting threshold of 100 kg and was imported into Canada in quantities ranging from 10 000 to 100 000 kg (Environment Canada 2013)
In Canada, HMN is an ingredient in a variety of self-care productsFootnote 4 available to consumers and is used in personal care and pharmaceutical sectors (Environment Canada 2013). Functions of the substance as a cosmetic ingredient include; skin conditioning agent, emollient, or solvent (CIR 2012). HMN is reported as an ingredient in thousands of cosmetics notified to Health Canada under the Cosmetic Regulations of the Food and Drugs Act and is most commonly reported to be present in moisturizers, make-up products, cleansers, and hair conditioners (personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated July 2018; unreferenced).
HMN is listed in the Natural Health Products Ingredients Database (NHPID) with a non-medicinal role as a skin-conditioning agent – emollient and solvent and listed in the Licensed Natural Health Products Database (LNHPD) as an ingredient in many licensed natural health products, most commonly found in skin creams/lotions and sunscreens (personal communication, emails from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated December 2018; unreferenced)
According to information available in Health Canada’s internal Drug Product Database, HMN is a non-medicinal ingredient (NMI) in non-prescription drugs, most notably in sunscreens (personal communication, emails from the Therapeutic Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated January 2019; unreferenced).
HMN is not an approved food additive in Canada and there are no reports of its use as a component in food packaging materials or incidental additives in Canada, according to information obtained from Health Canada’s Food Directorate (personal communication, emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated January 2019; unreferenced). According to information from Health Canada’s Pest Management Regulatory Agency, HMN is not reported to be used as a pesticide active ingredient or formulant in Canada (personal communication, emails from the Pest Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated July 2018; unreferenced).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risk of HMN was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration [LC50]) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, available empirical databases (e.g., OECD QSAR Toolbox 2017), and from responses to surveys conducted under section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over and under classification of hazard and exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2016). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue (CBR) analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profile for HMN, and the hazard, exposure and risk classification results, are presented in ECCC (2016b).
According to information considered under ERC, HMN was classified as having a low exposure potential. HMN was classified as having a moderate hazard potential on the basis of an elevated ecotoxicity ratio and a high potential to cause adverse effects in aquatic food webs given its bioaccumulation potential. The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of this substance. HMN was classified as having a low potential for ecological risk. On the basis of current use patterns, this substance is unlikely to be resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
As HMN is considered to be of low hazard potential (see health effects assessment below), quantitative estimates of exposure to the general population were not derived. This section provides general information on exposure to HMN.
No Canadian or recent international data on the levels of HMN in ambient air, indoor air, drinking water, dust, or soil were identified. Releases of HMN to water may result from industrial formulation activities of the substance in self-care products or down-the-drain releases from the use of these products.
Exposures to the general population from food are not expected.
Inhalation and dermal exposure to HMN is expected for the general population of Canada based on its vapour pressure and its use as an ingredient in large number of certain types of self-care products in Canada (e.g., moisturizers, make-up products, cleansers, hair conditioners, sunscreens, and acne-creams).
6.2 Health effects assessment
The general principles outlined in the science approach document for substances with low human health hazard potential (HC 2017) were taken into consideration for this health effects assessment. HMN is considered to have a low hazard potential due to the lack of genotoxic, reproductive or developmental effects, and other adverse effects relevant to human health up to 1000 mg/kg bw/day on the basis of oral studies conducted on HMN, and up to 1393 mg/m3 on the basis of inhalation studies conducted on an analogue (as detailed below).
Toxicokinetics
In a rat study examining absorption, dietary ingestion of 100 mg/kg bw/day HMN resulted in an apparent absorption of 98% based on the difference between ingested dose and the fraction eliminated in feces (Tulliez and Bories 1975). No toxicokinetic studies examining the distribution and elimination of HMN were identified. With respect to metabolism, it was noted that HMN is metabolized to 2,2,4,4,6,8,8-heptamethylnonane dicarboxylic acid following short-term, oral exposure in rats (Ikeda et al. 1988). This major metabolite was isolated from the liver and represents a stable lipophilic anion that is not expected to be metabolized via β-oxidation due to the presence of two adjacent α-methyl groups at the terminal carbon atoms. Thus, it is expected to be slowly excreted and accumulate at the site of formation.
Repeated-dose toxicity
Studies examining the health effects from repeated doses of HMN were limited to four short-term oral studies (5, 14, 28, and approximately 49 days). A 5-day oral gavage study, with limited observations, was conducted in male Wistar rats (n=5/group) administered 250 mg/kg bw/day HMN (in peanut oil) (Bomhard et al. 1990). Microscopic examinations were conducted on the kidney tissues to grade the occurrence of hyaline droplets on a scale of 0-4. The activity score was determined to be 2.0 for HMN, which corresponds to a “mild response [hyaline droplets] in a large number of proximal tubules.” Since a concurrent control group was not included in this study however, the toxicological relevance of these findings is unknown.
In a 14-day dietary study examining the effects of HMN on the liver, male Wistar-Imamichi rats (n=6/group) were given 0 or 1% (w/w) of HMN in the diet, equivalent to 0 or approximately 1300 mg/kg bw/day (Ikeda et al. 1988). On day 15, animals were sacrificed and the livers were extracted. Enzyme activity (aniline hydroxylase, aminopyrine demethylase, carnitine acetyltransferase, carnitine palmitoyltransferase, catalase, cyanide-insensitive fatty acyl-CoA oxidase, lauric acid hydrolase), cytochrome (CYP) P450 enzyme content, and protein levels were measured. Microscopic examinations of the liver tissues were also conducted. In the treated animals, there was a significant decrease in body weight (-10%), and significant increases in liver weights (absolute and relative, 21 and 32% respectively), catalase activity, cyanide-insensitive fatty acyl-CoA oxidizing activity, peroxisomal enoyl-CoA hydralase activity, and mitochondrial enzyme activity (carnitine palmitoyl transferase, carnitine acetyl transferase). Furthermore, the content of CYP P450 enzymes was increased by 57%, with associated increases in aniline hydroxylase, aminopyrine demethylase, and lauric acid oxidase activity. There was also a significant decrease in the serum levels of triglycerides, phospholipids, free/total cholesterol. Histopathological examinations revealed that the liver tissues had a higher abundance of peroxisomes, accompanied with an induction of smooth endoplasmic reticulum. The authors indicated that all of these effects suggest that HMN may be associated with the induction of peroxisome proliferation in the liver. Although the dose administered in this study was high (i.e., 1300 mg/kg bw/day), which may not be relevant to human exposures, this study provided insight on the potential mode-of-action associated with the liver effects (i.e., peroxisome proliferation).
The previous studies focused on specific organs (i.e., kidney and livers), but did not provide further toxicity information on other vital systems. Data on the other systems were available in a 28-day oral gavage study, whereby Crj:CD(SD) rats (n=5/sex/dose) were administered 0, 100, 300, or 1000 mg/kg bw/day HMN (99.9% purity, in corn oil) for 28 days. Additional animals (n=5/sex) were included in the control and high-dose groups, for which the exposure period was followed by a 14-day recovery period (Hatano Research Institute 1991). The methods were comparable to the Organisation for Economic Co-operation and Development Testing Guideline (OECD TG) 407. All of the dose groups, including the control group, exhibited signs of transient salivation, with a dose-dependent increase in incidence at higher doses. At 300 mg/kg bw/day, there was a significant increase in liver weights (19 to 22% relative to control) that was accompanied with centrilobar hypertrophy of hepatocytes and a decrease in triglyceride levels (-40% relative to control). As these effects were no longer observed after the recovery period, not associated with degenerative or necrotic changes (e.g., necrosis, fibrosis, inflammation), and not accompanied by a biologically significant change in markers of liver damage (e.g., alanine aminotransferase, aspartate aminotransferase), they were considered to be adaptive and non-adverse, consistent with the guidelines proposed by the European Society of Toxicologic Pathology (Hall et al. 2012) and the United States Environmental Protection Agency (US EPA 2002). At 1000 mg/kg bw/day, the animals also exhibited prolonged prothrombin and activated thromboplastin times (70 and 27% relative to control, respectively), which persisted into the recovery period. However, the extent of these effects was less severe at the end of the recovery period (17 and 12% relative to control, respectively) and was accompanied by a significant increase in the number of platelets, suggestive of reversibility. The authors also indicated that there were no pathological findings associated with the haematological and clinical chemistry changes, and that these aforementioned changes did not markedly exceed the range of physiological fluctuations. There were 3 cases of calculi in the kidneys of female animals at the high dose, but these observations were no longer present at the end of the recovery period. A no-observed-effect level (NOEL) of 100 mg/kg bw/day was identified by the authors (no further details provided). However, given that the organ effects were adaptive, reversible, and non-adverse and that the haematological and clinical chemistry changes fell within historical control ranges, a NOAEL of 1000 mg/kg bw/day was considered, representing the highest tested dose (HTD).
In another short-term study investigating the reproductive and developmental toxicity of HMN, Crl:CD(SD) rats (n=12/sex/group) were administered 0, 100, 300, 1000 mg/kg bw/day of HMN (98.4% purity, in corn oil) by gavage for at least 49 days in males and approximately 5 to 7 weeks in females (14 days prior to mating up to post-natal day [PND] 3 of the pups) (MHLW 2007). The study was conducted in accordance with OECD TG 421. Clinical signs, mortality, body weight, and food consumption were recorded. Gross pathology was conducted on all major organs and tissues, but histopathology was only conducted on reproductive organs and those with macroscopic abnormalities. A low incidence of salivation (1-2 cases) was observed at doses equal to or greater than 300 mg/kg bw/day, but was considered to be transient since it disappeared immediately after administration. At 1000 mg/kg bw/day, 70% of the male animals had liver centrilobar hypertrophy of hepatocytes. The authors established a NOEL of 300 mg/kg bw/day for male animals on the basis of liver effects observed at the next dose level of 1000 mg/kg bw/day, although they noted that these changes were not accompanied by necrosis of the hepatic cells or “changes that would suggest harm.” On the basis of this information, this study was not considered to reveal any adverse effects up to the highest tested dose of 1000 mg/kg bw/day.
Studies of longer duration and on other routes of administration were not identified for HMN. However, three subchronic studies using inhalation and oral routes of administration were available for the analogue isododecane. According to the available data, isododecane represents a commercial/technical grade product having a boiling point range from 160°C to 170°C, with the following composition: less than 2 ppm in total C4 hydrocarbons, 0.3% (w/w) total C8 hydrocarbons, 97.7% (w/w) total C12 hydrocarbons (consisting of 82% 2,2,4,6,6-pentamethylheptane and 17.7% others), less than 0.1% (w/w) total C16 hydrocarbons, less than 10 ppm aromatics content, 10 ppm water content, and 2 mg bromium/100 g material (Appelman et al. 1982). Isododecane and HMN exhibit structural similarity, similar branching patterns, and are comparable in terms of carbon length. On this basis, they are expected to undergo similar metabolic pathways (TIMES 2016), exhibit similar reactivity (OECD QSAR Toolbox 2017), and have similar physical-chemical properties (Appendix B; ECHA 2019a).
The effects of isododecane through the inhalation route of exposure was examined in a 13-week study whereby rats (n=20/sex/group) were exposed to 0, 12.5, 50, 100, or 200 ppm isododecane (equivalent to 0, 87, 348, 697, 1393 mg/m3) by whole-body for 6h/day, 5d/week (Appelman et al. 1982, raw data not available). Observations were made for general appearance, growth, hematology, clinical chemistry, and urine composition. Gross pathology was conducted on all major organs, but histopathology was only performed on the kidneys. At concentrations greater than or equal to 348 mg/m3, dose-dependent increases in the incidence of tubular nephrosis in the kidneys of male animals were observed. The lesions were characterized by a loss of cytoplasmic eosinophilia, striation, loss of brush border, increased cellular and nuclear size of epithelium (mainly the proximal tubules). These changes were occasionally accompanied by very small to small aggregates of mononuclear inflammatory cells. The authors noted that there were differences in hematology, blood chemistry, urine analyses, and organ weights, but indicated that they were within the range of biological variability without clear dose-response relationships. Therefore, these differences were not considered to be toxicologically relevant. Macroscopic examination at autopsy did not reveal any gross lesions that could be attributed to treatment. The authors established a “no-toxic-effect level” at 12.5 ppm (87 mg/m3). However, there is evidence that the administration of hydrocarbon solvents is associated with alpha-2u-globulin accumulation and nephropathy in male rats (Bomhard et al. 1990; CIR 2002; McKee et al. 2015). Alpha-2u-globulin-induced nephropathy in male rats is considered to be a gender- and species-specific effect and is not considered to be relevant to human health (US EPA 1991; Hard et al. 1993).
Renal effects were also reported in an abstract of a 13-week inhalation study on rats (unspecified strain, n=20/sex/group) exposed to 0, 200, 600, 1800 ppm (0, 1400, 4200, 12600 mg/m3) isododecane for 6h/day, 5d/week (Appelman et al. 1980, full study not available). Increased relative kidney weights and increased urine with low density were observed in males at concentrations equal to or greater than 4200 mg/m3, and in females at the highest concentration (12600 mg/m3). However, macroscopic and histopathological changes (i.e., greenish kidneys, tubular nephrosis) were only observed in male animals. On the basis of the evidence associating hydrocarbon solvent exposure to alpha-2u-globulin-induced nephropathy (Bomhard et al. 1990; CIR 2002; McKee et al. 2015), these effects were not considered to be relevant to human health. No route-specific effects (e.g., respiratory sensitization) or treatment-related site-of-contact effects were reported in the inhalation studies.
In a 13-week oral study, Wistar rats (n=20/sex/group) were administered 0, 330, 1000, 3000 mg/kg bw/day isododecane by gavage (Bayer Corp. 1983). The lowest dose was associated with decreased α-globulin levels and increased absolute/relative organ weights (liver, spleen, kidney). However, histopathological changes were only observed in the kidneys of male animals (i.e., mild to severe tubular degeneration). At 1000 mg/kg bw/day, there was reduced body weight (-9%) and changes in relative organ weights (i.e., increased heart, lungs, liver, spleen, kidneys, adrenals, testes/ovaries). However, histopathological changes were only observed in the liver (slight to mild degeneration with inflammation in 6/10 animals) and in male kidneys (tubular degeneration and inflammation). There were also clinical chemistry changes (e.g., increased urea, protein, albumin, glutamic oxaloacetic transaminase, calcium levels and decreased α-, β-globulin levels), but these were considered to be secondary to the changes observed in the liver and kidneys. In addition to these effects, the 3000 mg/kg bw/day dose level was associated with haematological changes (e.g., decreased erythrocyte count [-5%], haemoglobin content [-9%], and hematocrit [-8%]), which were within historical control ranges for this strain (Envigo 2019). The authors noted that isododecane “was tolerated by female rats with the 330 mg/kg dose without damage” (no further details provided). Presumably, the authors considered 330 mg/kg bw/day to be the lowest-observed-adverse-effect level (LOAEL) in male rats on the basis of tubular degeneration. However, the reported kidney effects are commonly observed following hydrocarbon solvent exposure and have been associated with alpha-2u-globulin-induced nephropathy, a gender- and species-specific phenomenon in male rats (CIR 2002; McKee et al. 2015). Thus, they are not relevant to human health. A NOAEL of 330 mg/kg bw/day was interpreted on the basis of liver effects observed at the next dose level of 1000 mg/kg bw/day. It was noted that although degenerative changes in the liver were observed at dose levels equal to or greater than 1000 mg/kg bw/day, these effects were characterized as “slight” or “mild.” Furthermore, although the incidence of degeneration/inflammation increased at 3000 mg/kg bw/day, the severity did not change (with the exception of 1/10 animals that was characterized as “moderate”). This suggests that the actual NOAEL is closer to 1000 mg/kg bw/day than 330 mg/kg bw/day. In this study, the majority of the effects occurred at higher doses, equal to or greater than 1000 mg/kg bw/day. This dose level is typically considered by the OECD and the U.S Food and Drug Administration (FDA) to be a limit dose in oral toxicity studies (OECD 2018; FDA 2010; HC 2017). Effects observed beyond this dose limit may not be relevant for typical human exposures.
The Cosmetic Ingredient Review (CIR) Expert Panel conducted a safety assessment of isoparaffins used in cosmetics, in which HMN (referred to as “isohexadecane”) was included (CIR 2012). The assessment consisted of isoparaffins ranging in carbon chain length from C7 to C70. The CIR indicated that no significant toxicity was identified in the available oral and inhalation studies. However, the expert panel was concerned with nephrotoxicity and noted the involvement of alpha-2u-globulin in the mechanism for isoparaffin-induced nephrotoxicity and renal tubule cell proliferation in male rats of various strains, which were not observed in the NCI-Black-Reiter strain of rats that lacked the alpha-2u-globulin protein. The panel agreed that the findings associated with alpha-2u-globulin in rats were not relevant to humans, consistent with the recommendations outlined by the US EPA (1991). The CIR Expert Panel concluded that the aforementioned isoparaffins (including HMN) were safe in the present practices of use and concentrations defined in the assessment (mainly solvents, emollients between 0.0001 and 90%).
Reproductive and developmental toxicity
The potential for HMN to result in reproductive and developmental toxicity was examined in a screening test conducted in accordance with OECD TG 421 (MHLW 2007). In this study, Crl:CD(SD) rats (n=12/sex/group) were administered 0, 100, 300, 1000 mg/kg bw/day of HMN (98.4% purity, in corn oil) by gavage for at least 49 days in males and approximately 5 to 7 weeks in females (14 days prior to mating up to post-natal day [PND] 3 of the pups). There were no treatment-related effects on the reproductive organs, reproductive performance (e.g., estrous cyclicity, number of animals copulated, number of animals impregnated, number of animals pregnant, duration of mating) or development (number of corpora lutea, implantations, gestation days, number of newborns, number of stillborn, viability, external anomalies, sex ratio). With respect to parental toxicity, reproductive, and developmental toxicity, a NOEL of 1000 mg/kg bw/day was identified by the authors, the highest tested dose (HTD). On the basis of this information, HMN is not expected to be a reproductive or developmental toxicant.
Carcinogenicity and genotoxicity
Studies investigating the effects of HMN (or its analogues) following chronic exposure have not been identified. However, no structural alerts related to carcinogenicity were identified for HMN using QSAR modelling software (OECD QSAR Toolbox 2017; Derek Nexus 2016).
With respect to genotoxicity, HMN was not mutagenic in bacterial strains such as Salmonella typhimurium TA100, TA1535, TA98, TA1537, and Escherichia coli uvrA with or without metabolic activation (J-CHECK c2010-2019). HMN was also negative in an in vitro chromosome aberration assay in Chinese Hamster Lung (CHL) cells (J-CHECK c2010-2019). It did not induce any chromosomal aberrations or polyploidy in the cells up to cytotoxic concentrations with or without metabolic activation. Although no in vivo genotoxicity data was available, HMN was not associated with any mutagenicity structural alerts (OECD QSAR Toolbox 2017; Derek Nexus 2016). On the basis of this information, HMN is not expected to be genotoxic.
6.3 Characterization of risk to human health
Exposure of the general population to HMN may occur via the inhalation and dermal routes primarily through its use as an ingredient in self-care products in Canada. Indirect exposure via environmental media is expected to have a minimal contribution to exposure relative to the potential for direct exposure from self-care products.
On the basis of the health effects data available, HMN is not expected to be genotoxic, carcinogenic, or result in reproductive and developmental toxicity. With respect to repeated-dose toxicity, the only effects observed following oral doses up to 1000 mg/kg bw/day HMN were morphological changes observed in the liver that are considered to be non-adverse, adaptive physiological responses as they are not observed following a recovery period.
Although no inhalation studies on HMN were identified, available data on the analogue isododecane indicated that the only treatment-related effect observed was tubular nephrosis in the kidneys of male animals at concentrations up to 1393 mg/m3. Since this effect is commonly observed following hydrocarbon solvent administration, which is considered to be a gender- and species-specific phenomenon in male rats, it is not considered to be relevant for the characterization of risk to human health. No route-specific effects or treatment-related site-of-contact effects were identified.
Overall, HMN is considered to represent a substance with low hazard potential due to the lack of genotoxic, reproductive or developmental effects, and other adverse effects relevant to human health up to 1000 mg/kg bw/day on the basis of oral studies conducted on HMN and up to 1393 mg/m3 on the basis of inhalation studies conducted on an analogue.
6.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| Studies examining in vivo genotoxicity and the carcinogenicity of HMN were not identified. | +/- |
| Studies examining the toxicokinetics of HMN were limited. In a short-term oral study, HMN was found to be biotransformed to a stable metabolite, which could potentially accumulate at the site of formation (e.g., liver). Data regarding the metabolism of HMN in humans has not been identified. However, the studies conducted on experimental animals using HMN suggest that the effects on the liver are expected to be adaptive, reversible, and non-adverse. | +/- |
| Sub-chronic and chronic animal studies via the oral and dermal routes of administration were not identified for HMN. | +/- |
| Inhalation studies on HMN were not identified. A read-across approach using available data on the analogue isododecane was applied. The inhalation studies on isododecane revealed nephropathy in male rats, which was not considered to be relevant to human health. | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over or under-estimation of risk.
7. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from HMN. It is proposed to conclude that HMN does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that HMN does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that HMN does not meet any of the criteria set out in section 64 of CEPA.
References
[ANSES] Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail. 2013. Opinion of the French Agency for Food, Environmental and Occupational Health & Safety on the application for authorisation to use isododecane in the manufacture of organic materials coming into contact with water intended for human consumption. ANSES Opinion Request no 2012-SA-0143 [PDF]. Maisons-Alfort Cedex (FR): ANSES [accessed 2019 May 23].
Appelman LM, Bosland MC, Bruyntjes JP. 1982. Subchronic (13-week) inhalation toxicity study with isododecane in rats. Report no. V82.275/212294. Submitted to TSCA on 11/18/99 by Bayer Corporation. Microfiche No. OTS0559847. New Doc ID: 88000000040. Old Doc ID: 8EHQ-1199-14600.
Appelman LM, Bosland MC, Bruyntjes JP. 1980. Subchronic (13-week) inhalation toxicity study with isododecane in rats. Report no. V81.069/292473. Submitted to TSCA on 12/8/99 by Bayer Corporation. Microfiche No. OTS0559857. New Doc ID: 88000000050. Old Doc ID: 8EHQ-1299-14610.
Bayer Corporation. 1983. Isododekan, subchronische toxikologische untersuchungen an ratten (versuch mit schlundsondenapplikation uber 3 monate). Submitted to TSCA on 11/18/99 by Bayer Corporation. Microfiche No: OTS0559846. New Doc ID: 88000000039. Old Doc ID: 8EHQ-1199-14599. [article in German].
Bharati A, King CM. 2004. Allergic contact dermatitis from isohexadecane and isopropyl myristate. Contact Dermatitis. 50(4): 252-263.
Bomhard E, Marsmann M, Ruhl-Fehlert C, Zywietz A. 1990. Relationships between structure and induction of hyaline droplet accumulation in the renal cortex of male rats by aliphatic and alicyclic hydrocarbons. Arch Toxicol. 64(7): 530-538.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
ChemIDplus [database]. 1993- . Bethesda (MD): US National Library of Medicine. [accessed 2019 March 4].
[CIR] Cosmetic Ingredient Review. 2012. Safety assessment of IsoParaffins as used in Cosmetics. [PDF] Int J Toxicol. 31(6): 270S-295S. [accessed 2019 Jan 10]
Derek Nexus [toxicity prediction module]. 2016. Ver. 5.0.2. Leeds (UK): Lhasa Limited. [restricted access].
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2015. Identification of risk assessment priorities: results of the 2015 review. Ottawa (ON): Government of Canada..
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada.
[ECHA] European Chemicals Agency 2019a. Guidance on safe use: 2,2,4,6,6-pentamethylheptane; CAS RN 13475-82-6. Helsinki (FI): ECHA. [updated 5 February, 2019; accessed 2019 March 4].
[ECHA] European Chemicals Agency 2019b. Guidance on safe use: 2,2,4,6,8,8-heptamethylnonane; CAS RN 4390-04-9. Helsinki (FI): ECHA. [updated 20 February 2019; accessed 2019 March 4].
Envigo. 2019. Historical control data of haematological data in HsdRccHanTM: WIST, Wistar Hannover rats. Compiled from toxicity studies performed at RCC Ltd. Itingen/Switzerland [PDF]. New Jersey: Envigo.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[FDA] Food and Drug Administration. 2010. Guidance for industry: M3(R2) Nonclinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals. Rockville (MD): US Department of Health and Human Sciences.
Hall AP, Elcombe CR, Foster JR, Harada T, Kaufmann W, Knippel A, Kϋttler K, Malarkey DE, Maronpot RR, Nishikawa A, Nolte T, Schulte A, Strauss V, York MJ. 2012. Liver hypertrophy: a review of adaptive (adverse and non-adverse) changes – conclusions from the 3rd international ESTP expert workshop. Toxicol Pathol. 40(7): 971-994.
Hard GC, Rodgers IS, Baetcke KP, Richards WL, McGaughy RE, Valcovic LR. 1993. Hazard evaluation of chemicals that cause accumulation of α2μ-globulin, hyaline droplet nephropathy, and tubule neoplasia in the kidneys of male rats. Environ Health Perspect. 99: 313-349.
Hatano Research Institute. 1991. 28-day repeat oral administration toxicity study of 2,2,4,4,6,8,8-heptamethylnonane in rats (14-day recovery period). Japan: Food and Drug Safety Center, Hatano Research Institute. Entrusted by the Environmental Health Bureau of the Ministry of Health and Welfare.
[HC] Health Canada. 2017. Science Approach Document. Science approach document for substances with low human health hazard potential. Ottawa (ON): Health Canada. December 2017
Ikeda T, Ida-Enomoto M, Mori I, Fukada K, Iwabuchi H, Komai T, Suga T. 1988. Induction of peroxisome proliferation in rat liver by dietary treatment with 2,2,4,46,8,8-heptamethylnonane. Xenobiotica. 18(11): 1271-1280.
[J-CHECK] Japan CHEmicals Collaborative Knowledge database [database]. c2010-2019. Tokyo (JP): National Institute of Technology and Evaluation (NITE). [accessed 2019 March 4].
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2018-02-06]. Ottawa (ON): Government of Canada. [accessed 2018-02-06]
McKee RH, Adenuga MD, Carrillo JC. 2015. Characterization of the toxicological hazards of hydrocarbon solvents. Crit Rev Toxicol. 45(4):273-365.
[MHLW] Ministry of Health, Labour and Welfare (Japan). 2007. Oral administration simple reproduction study of 2,2,4,6,8,8-heptamethylnonane employing rats. Japan: Chemical Substance Safety Measures Office, Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour, and Welfare.
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2019-01-18]. Ottawa (ON): Government of Canada. [accessed 2019 February 4].
[OECD] Organisation for Economic Co-operation and Development. 2018. OECD Guidelines for the Testing of Chemicals, Section 4 Health Effects. Test No. 408: Repeated dose 90-day oral toxicity study in rodents [PDF].
OECD QSAR Toolbox. 2016. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
[PSS] Product Safety Summary. 2016. IsoparTM M Fluid [PDF]. ExxonMobil. [accessed 2019 January 31].
Sigma Aldrich. 2015. Safety Data Sheet for 2,2,4,4,6,8,8-Heptamethylnonane [PDF]. St. Louis (MO). [accessed 2019 March 10]
[TIMES] TIssue MEtabolism Simulator [prediction module]. 2016. Ver. 2.27.19. Bourgas (BG): University “Prof. Dr. Assen Zlatarov”, Laboratory of Mathematical Chemistry.
Tulliez J and Bories G. 1975. Metabolism of paraffinic and naphthalenic hydrocarbons in higher animals. I. Retention of paraffins (normal, cyclo and branched) in rats. Ann Nutr Aliment. 29(3): 201-211. [article in French].
[US EPA] US Environmental Protection Agency. 1991. Alpha2u-globulin: association with chemically induced renal toxicity and neoplasia in the male rat. Washington (DC): US EPA, Risk Assessment Forum.
[US EPA] US Environmental Protection Agency. 2002. Hepatocellular hypertrophy. HED Guidance Document #G2002.01. Washington (DC): US EPA, Health Effects Division, Office of Pesticide Programs.
Appendix A. Read-Across Approach
| Consideration | Rationale |
|---|---|
| 1) Chemical structure. Emphasis was placed on analogues with similar branching patterns and similar carbon chain lengths. | Analogues that have similar chemical structure are expected to have similar toxicity profiles. |
| 2) Similar metabolites (predicted or observed). The metabolism of HMN mainly occurs through hydroxylation and subsequent carboxylation. In rats, the major metabolite is 2,2,4,4,6,8,8-heptamethylnonane dicarboxylic acid. | Analogues that are metabolized through similar pathways to similar degradation products are expected to have similar toxicity profiles. |
| 3) Common structural alerts | Analogues with similar structural alerts are expected to share greater similarity in terms of toxicity. |
| 4) Similar physical-chemical properties. Emphasis was placed on chemical structures with similar molecular weight, water solubility, vapour pressure, and log Ko/w. | Analogues with similar physical chemical properties may potentially share similar toxicological profiles. |
Appendix B. Physical-Chemical Properties and Summary Health Effects Data on HMN and Isododecane
| - | Isododecane (CAS 13475-82-6) | HMN (CAS 4390-04-9) |
|---|---|---|
| Chemical structure | 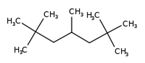 |
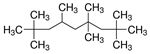 |
| MW (g/mol) | 170 | 226 |
| Melting point (°C) | -67a | -70f |
| Boiling point (°C) | 178a | 240 |
| Vapour pressure (Pa) | 100b | 130 |
| Water solubility (mg/L) | 4.86x10-3b | 2.05x10-3 |
| Log Ko/w (unitless) | 6.96b | 7.79 |
| Oral acute tox (mg/kg bw/day) | NA | LD50>2 g/kg bwg |
| Repeated-dose tox (mg/kg bw/day) | Oral study in rats (13 wks)c:
Inhalation study in rats (13 wks)d:
Inhalation study in rats (13 wks)e:
|
Oral study in rats (28 days)g:
|
| Repro/devo tox | NA | NOAEL=1000 mg/kg bw/day (HTD)h |
| Cancer | NA | NA |
| In vitro genotoxicity | Negative | Negative |
| In vivo genotoxicity | NA | NA |
Abbreviations: Devo, developmental; HMN, heptamethylnonane; HTD, highest tested dose; MW, molecular weight; NA, not available; NOAEL, no-observed-adverse-effect-level; repro, reproductive; tox, toxicity
References: aChemID 1993-; bECHA 2019a; cBayer Corp. 1983, study in German; dAppelman et al. 1982, raw data not available; eAppelman et al. 1980, full study not available; fECHA 2019b; gHatano Research Institute 1991; hMHLW 2007
