Draft screening assessment nitro musks group
Official title: Draft screening assessment nitro musks group
Chemical Abstracts Service Registry Numbers
81-14-1, 81-15-2
Environment and Climate Change Canada
Health Canada
September 2018
Synopsis
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of two substances referred to collectively as the Nitro Musks Group. Substances in this group were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns. The Chemical Abstracts Service Registry Numbers (CAS RNsFootnote 1 ), their Domestic Substances List (DSL) names and their common names and abbreviations are listed in the table below.
| CAS RN | DSL name | Common name (and abbreviation) |
|---|---|---|
81-14-1 |
Ethanone, 1-[4-(1,1-dimethylethyl)-2,6-dimethyl-3,5-dinitrophenyl]- |
Musk ketone (MK) |
81-15-2a |
Benzene, 1-(1,1-dimethylethyl)-3,5-dimethyl-2,4,6-trinitro- |
Musk xylene (MX) |
a This substance was not identified under subsection 73(1) of CEPA but was included in this screening assessment as it was considered a priority on the basis of other human health concerns.
Musk ketone and musk xylene do not occur naturally in the environment. They are used primarily as fragrances or fragrance ingredients. In Canada, musk ketone is found in some cosmetics, and musk xylene is found in some air fresheners, personal care products and cleaning products. According to a survey conducted for the year 2008, musk ketone and musk xylene were both imported into Canada in quantities between 1 000 and 10 000 kg. In the same year, musk ketone and musk xylene were manufactured in quantities of less than 100 kg and of between 100 and 1 000 kg, respectively.
Musk ketone and musk xylene are principally released into wastewater after the use of products available to consumers and, therefore, can enter the environment through wastewater discharges to surface water. Releases to surface water via wastewater effluent could also occur as a result of the formulation of cleaning and personal care products or when wastewater is generated from industrial operations such as cleaning. Releases to soil can occur from land application of biosolids derived from wastewater treatment.
Musk ketone and musk xylene have low water solubility and are very persistent in the environment, with an overall persistence in the order of years. When released to water—their primary mode of entry to the environment—they will biodegrade and hydrolyze slowly. The presence of musk ketone and musk xylene metabolites in effluents from wastewater treatment systems indicates that biodegradation during wastewater treatment could be occurring. Musk ketone and musk xylene are persistent in air, but significant releases to that medium are not expected. Musk ketone and musk xylene have, respectively, moderate and high bioaccumulation potential in aquatic organisms.
Empirical and modelled data suggest that both musk ketone and musk xylene are hazardous to aquatic organisms at relatively low concentrations. The few soil studies available indicate that musk ketone is not hazardous to soil-dwelling organisms. No soil studies were available for musk xylene, but given the similarity of its structure and physical-chemical properties to those of musk ketone, it is assumed that its effects on soil-dwelling organisms are similar.
Several studies that examined concentrations of musk ketone and musk xylene in Canadian wastewater treatment systems reported low but measurable concentrations in influents, effluents and biosolids. On the basis of the uses and quantities of musk ketone and musk xylene reported for 2008, as well as their expected release pathways and fate, three environmental exposure scenarios were developed: industrial releases to wastewater by formulators who use them; down-the-drain releases from products available to consumers that contain them; and land application of biosolids that contain them. Risk quotients for the industrial release scenario, as well as quantitative risk analyses for the other two scenarios, indicate that levels of musk ketone and musk xylene in the environment are below levels of concern to aquatic and soil-dwelling organisms.
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from musk ketone and musk xylene. It is proposed to conclude that musk ketone and musk xylene do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
In terms of potential effects on human health, decreased growth and food consumption and/or effects on the liver were observed in repeated dose laboratory studies conducted with musk ketone or musk xylene via the oral or dermal routes.
Both substances have been measured in environmental media in Canada. Estimates of exposure to Canadians were derived on the basis of levels of musk ketone and musk xylene in environmental media and in products used by consumers, such as cosmetics, air fresheners, and cleaning products. On the basis of a comparison of estimates of exposure to musk ketone and musk xylene with critical effect levels identified from laboratory studies, margins of exposure are considered to be adequate to address uncertainties in health effects and exposure data used to characterize risk.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that musk ketone and musk xylene do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that musk ketone and musk xylene do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of two substances referred to collectively as the Nitro Musks Group to determine whether they present or may present a risk to the environment or to human health. The substances in this group, musk ketone and musk xylene, were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns (ECCC, HC [modified 2017]).
Musk ketone and musk xylene were reviewed internationally by the European Commission (EC 2005a,b,c). Musk xylene was also reviewed by the International Agency for Research on Cancer (IARC) (1996). These assessments undergo rigorous review and endorsement by international governmental authorities. Environment and Climate Change Canada and Health Canada are active participants or observers in this process and consider these assessments reliable. The European Commission documents were used to inform the ecological and health effects sections of this screening assessment. The IARC document was additionally used to inform the health effects section of the screening assessment.
This draft screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to October 2017. However, more recent studies or information provided via internal and external peer consultation may also be cited. Empirical data from key studies as well as some results from models were used to reach proposed conclusions. Given the potential for these substances to be used in similar ways and applications, potential risk was assessed using similar exposure assumptions across the group.
This draft screening assessment was prepared by staff in the CEPA Risk Assessment Program at Environment and Climate Change Canada and Health Canada and incorporates input from other programs within these departments. The ecological portions of this assessment have undergone external review. Comments on the technical portions relevant to the environment were received from the United States Environmental Protection Agency (US EPA), the Australian National Industrial Chemicals Notification and Assessment Scheme (NICNAS), and the Research Institute for Fragrance Materials (RIFM). While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Environment and Climate Change Canada and Health Canada.
This draft screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight-of-evidence approach and precaution.Footnote 2 This draft screening assessment presents the critical information and considerations on which the proposed conclusions are based.
2. Identity of substances
The Nitro Musks Group consists of two substances, musk ketone and musk xylene. When referred to as“nitromusks” in this document, it includes additional substances (musk ambrette, CAS RN 83-66-9; musk moskene CAS RN 116-66-5; and musk tibetene, CAS RN 145-39-1). Those additional substances are not assessed in this document and the term “nitromusks” is used only in reference to the NICNAS (2016) review.
The Chemical Abstracts Service Registry Numbers (CAS RNootnote 3 ), Domestic Substances List (DSL) names, common names, and abbreviations for the individual substances in the Nitro Musks Group are presented in Table 2-1. In this assessment, the substances will hereinafter be identified by their abbreviations, MK and MX.
| CAS RN | DSL name (common name (abbreviation)) |
Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 81-14-1 |
Ethanone, 1-[4-(1,1-dimethylethyl)-2,6-dimethyl-3,5-dinitrophenyl]-(Musk ketone (MK)) | ![Ethanone, 1-[4-(1,1-dimethylethyl)-2,6-dimethyl-3,5-dinitrophenyl]-(Musk ketone (MK))](/content/dam/eccc/images/pded/nitro-musks-group/20180829-table2-1.1.jpg) |
294.30 |
| 81-15-2 |
Benzene, 1-(1,1-dimethylethyl)-3,5-dimethyl-2,4,6-trinitro-(Musk xylene (MX)) | 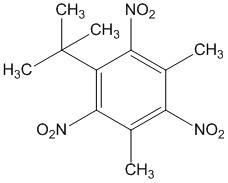 |
297.3 |
3. Physical and chemical properties
A summary of physical and chemical property data for the substances in the Nitro Musks Group is presented in Table 3-1. When experimental information was limited or not available, data from quantitative structure-activity relationship (Q)SAR models were used to generate predicted values for the properties of the substance.
Propertya |
MK |
MX |
References (MK; MX) |
Physical state |
yellow crystals |
solid powder |
Lide 2007; EC 2005b |
Melting point (oC) |
135.5 |
112-114 |
Lide 2007; EC 2005b |
Boiling point (oC) |
NAb |
NAb |
EC 2005c; EC 2005b |
Water solubility (mg/L) |
0.46 |
0.15 |
Schramm et al. 1996 |
Vapour pressure (Pa) |
7.78 × 10-5
|
7.87 × 10-5 (reported as 6.35 × 10-7 mm Hg) |
EPI Suite c2000-2012c; MPBPWIN 2010c |
Henry’s law constant (Pa m3/mol) |
1.92 × 10-4
|
7.84 × 10-4 (reported as 7.7 × 10-9 atm x m3/mol) |
EPI Suite c2000-2012 |
Dissociation constant pKa (dimensionless) |
NAd |
NAd |
NA |
log Kow (dimensionless) |
4.3 |
4.9 |
Schramm et al. 1996; EC 2005b |
log Koc (dimensionless) |
3.94 |
4.07 |
KOCWIN 2010c |
log Kaw (dimensionless) |
-7.07 |
-7.37 |
EPI Suite c2000-2012 |
Abbreviations: Kow, octanol-water partition coefficient; Koc, organic carbon-water partition coefficient; Kaw, air-water partition coefficient; NA, not applicable.
a All values are modelled, except for melting point, water solubility, and log Kow.
b The boiling points could not be determined because MK and MX decompose before reaching the boiling point.
c Calculated with user input (experimental value adjustment (EVA) method).
d The MK and MX molecules have no ionizing groups.
4. Sources and uses
MK and MX do not occur naturally in the environment. They are used primarily as fragrances or fragrance ingredients.
The two substances in the Nitro Musks Group were included in a survey issued pursuant to a CEPA section 71 survey (Environment Canada 2009). Table 4-1 presents a summary of information reported on the total manufacture and total import quantities for MK and MX in 2008. More recent information indicates that quantities in commerce have decreased since 2008 (personal communications, emails from stakeholders to Ecological Assessment Division, Environment and Climate Change Canada; dated November/December 2017; unreferenced).
Substance |
Quantity manufactured (kg)a |
Quantity imported (kg)a |
MK |
< 100 |
1 000–10 000 |
MX |
100–1 000 |
1 000–10 000 |
a Values reflect quantities reported in response to the surveys conducted under section 71 of CEPA (Environment Canada 2009, 2013). See surveys for specific inclusions and exclusions (schedules 2 and 3).
In the United States, the production volume of MK was 25 000 to 100 000 pounds in 2014 and production volume of MK was less than 25 000 pounds in 2015, as indicated in the Chemview database (US EPA 2018). A number of jurisdictions, including the EU and Japan, have taken actions to restrict or ban the use of MK and MX. For example, as of 2005, there has not been any production of MK or MX in the EU (EC 2005a, 2005b).
Results of a survey for the 2008 calendar year show that MX is used in Canada in the manufacture of some laundry and dishwashing products, odour agents, cleaning and furnishing care products, and personal care productsFootnote 4 and as an ingredient in fragrance/perfumes (Environment Canada 2009). According to publicly available product material safety data sheets (MSDSs), MK is found in some perfumes and MX is found in some air fresheners in Canada (MSDS 2015a; 2015b). Additional uses for MK and MX in Canada are listed in Table 4-2.
Use |
MK |
MX |
Food additivea |
N |
N |
Food packaging materialsb |
N |
N |
Internal Drug Product Database as (DPD) medicinal or non-medicinal ingredients in disinfectant, human or veterinary drug products in Canadac |
N |
N |
Natural Health Products Ingredients Database(NHPID)d |
N |
N |
Licensed Natural Health Products Database (LNHPD) as medicinal or non-medicinal ingredients in natural health products in Canadae |
N |
N |
List of Prohibited and Restricted Cosmetic Ingredientsf |
N |
N |
Notified to be present in cosmetics, on the basis of notifications submitted under the Cosmetic Regulations to Health Canadag |
Y |
N |
Formulant in pest control products registered in Canadah |
Y |
Y |
Abbreviations: Y, yes; N, no.
a Personal communication from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated November 2016; unreferenced.
b Personal communication from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated November 2016; unreferenced.
c DPD 2016.
d NHPID [modified 2017].
e LNHPD [modified 2016].
f Health Canada [modified 2015].
g Personal communication from Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated November 2016.
h Personal communication with the Pest Management Regulatory Agency, Health Canada, dated November 2016; unreferenced.
Globally, MK and MX are used as fragrance ingredients in cosmetics, detergents, fabric softeners, household cleaning products and other fragranced products (EC 2005a, 2005b; Nakata et al. 2015). Their concentrations in end products are reported to be up to 1% with typical concentrations of approximately 0.02% to 0.6% in cosmetics, including perfumes, 0.02% in detergents, and 0.07% in air fresheners (NICNAS 2016). In 2009, the European Union established maximum authorized concentrations of MK and MX in cosmetics (up to 1.4% for MK and up to 1.0% for MX, depending on the cosmetic product) (European Parliament 2009).
5. Releases to the environment
MK and MX are released into wastewater after the use of products available to consumers and, therefore, can enter the environment through wastewater discharges to surface water or by the land application of biosolids (Smyth et al. 2007). Releases to surface water via wastewater effluent could also occur as a result of the formulation of cleaning and personal care products or when wastewater is generated from industrial operations such as cleaning.
6. Environmental fate and behaviour
6.1 Environmental distribution and long-range transport potential
Table 6-1 presents the results of the Level III fugacity modelling for MK and MX showing ranges of percent partitioning into each environmental medium on the basis of a theoretical release to each medium.
Released to |
Air (%) |
Water (%) |
Soil (%) |
Sediment (%) |
Air (100%) |
0.84–1.00 |
4.33–4.46 |
94.4–94.7 |
0.12–0.16 |
Water (100%) |
negligible |
96.40–97.40 |
negligible |
2.60–3.60 |
Soil (100%) |
negligible |
negligible to 0.15 |
99.80–100 |
negligible |
MK and MX are predicted to partition mainly to water when released to that medium. This behaviour is consistent with their low vapour pressure. Partitioning from water to sediment could be higher than predicted by EQC, given their high log Kow and log Koc values and their low water solubility. Partitioning from water to air is expected to be negligible, considering the modelled air-water partition coefficient (log Kaw) of -7.07 for MK and -7.37 for MX, respectively (EPI Suite c2000-2012).
The Transport and Persistence Level III Model (TaPL3 2003) can be used to predict long-range transport (LRT) in water, a concept developed by Beyer et al. (2000). The characteristic travel distance (CTD) is defined as the maximum distance travelled by 63% of the substance after being released into the environment. Zarfl et al. (2011) have proposed a threshold CTD of 5200 km for classifying organic substances as having long-range transport potential in water. Using TaPL3, CTDs of MK and MX in water were calculated. They are, respectively, 19 400 km and 15 800 km, assuming a river with a current of 3.6 km/h and depth of 5 metres. This means that releases of MK and MX to a river would likely result in their transport along the full length of the river, and dilution, rather than degradation, will be the main factor affecting exposure concentrations. Therefore, chronic exposures could be expected far from the source of release.
The TaPL3 model can also be used to predict the CTDs of MK and MX in air. Beyer et al. (2000) have proposed CTDs of >2000 km as representing high long-range atmospheric transport potential (LRATP), 700 to 2000 km as moderate LRATP, and <700 km as low LRATP. The estimated CTDs for MK and MX in air from TaPL3 are 10 km and 39 km, respectively. Even though the half-lives in air are greater than 2 days for both MK and MX, the low CTDs are due to high transfer rates from air to water and soil, but not the reverse. The CTD values indicate that MK and MX are not expected to be transported through the atmosphere a significant distance from their emission sources.
6.2 Environmental persistence
6.2.1 Abiotic degradation
For MK and MX, laboratory experiments indicate that photolysis in water can occur (Butte et al. 1999). Modelled results indicate that MX and MK do not degrade rapidly by photodegradation with OH- radicals. Table 6-2 presents the key data available regarding the abiotic degradation of substances in the Nitro Musks Group.
Substance(s) |
Fate process |
Degradation endpoint or prediction |
Extrapolated half-life (t1/2 = days) |
Reference |
MK |
photodegradation with OH- radicals |
half-life |
8.27 |
AOPWIN 2010 |
MX |
photodegradation with OH- radicals |
half-life |
12.87 |
AOPWIN 2010 |
MK and MX |
ozone reaction |
N/Aa |
N/A |
AOPWIN 2010 |
MK and MX |
hydrolysis |
half-lifeb |
N/A |
N/A |
Abbreviations: N/A, not applicable.
a AOPWIN does not give an estimation for this type of substance
b No studies were found for the hydrolysis of MK and MX, but given their structures, it can be assumed that they hydrolyze very slowly (EC 2005a).
6.2.2 Biodegradation
Tables 6-3 and 6-4 summarize the key experimental and modelled data, respectively, for the aerobic biodegradation of substances in the Nitro Musks Group. These data support the assertion that MK and MX are not readily biodegradable. There is some evidence that MX biodegrades anaerobically (Käfferlein et al. 1998).
Studies have found that significant amounts of MK (62% to 92%) and MX (95% to 99%) are removed during wastewater treatment (Simonich et al 2000; Simonich et al. 2002; Sabiliunas et al. 2001; Smyth et al. 2007). Removal is by adsorption to sludge and metabolic transformation.
When MK and MX are transformed in wastewater treatment systems (WWTSsFootnote 5 ), the main products (metabolites) are 4-amino-MX (4-MX), 2-amino-MX (2-MX), and 2-amino-MK (2-MK). In a 1998 study (Gatermann et al.), the authors found that the concentration of 4-MX in WWTS effluent was up to 10 times higher than that of the parent compound and the concentration of 2-MK in WWTS effluent was up to 40 times higher than that of the parent compound. In a 1999 study, concentrations of the three aforementioned metabolites of MK and MX, as well as the parent compounds, were determined in several media in Germany. In the effluents of two WWTSs, low MK and MX concentrations and comparatively high levels of the metabolites were found (Rimkus et al 1999).
Substance |
Fate process |
Test conditions |
Degradation endpoint or prediction |
Reference |
MK |
Aerobic biodegradation (inherent), wastewater |
MITI II (OECD Guideline 302C) |
0% BOD |
Calame and Ronchi 1989 |
MX |
Aerobic biodegradation (ready), wastewater |
MITI I (OECD Guideline 301C) |
0% BOD |
Calame and Ronchi 1989 |
MX |
Aerobic biodegradation, wastewater |
ThCO2 (Modified Sturm) |
0% ThCO2 |
Marks and Marks 1987 |
Abbreviations: BOD (biological oxygen demand) is the amount of dissolved oxygen needed by aerobic biological organisms to break down organic material present in a given water sample at certain temperature over a specific time period; ThCO2 (theoretical carbon dioxide) is amount of CO2 produced by aerobic biological organisms when breaking down organic material present in water.
Substance |
Fate process |
Degradation prediction |
Reference |
MK |
Aerobic primary biodegradation, wastewater |
weeks-months (biodegrades slowly) |
BIOWIN 2008 Sub-model 4 (Expert Survey) |
MK |
Aerobic biodegradation (ready), wastewater |
0% BOD |
CATALOGIC 2014 |
MX |
Aerobic primary biodegradation, water |
weeks-months (biodegrades slowly) |
BIOWIN 2008 Sub-model 4 (Expert Survey) |
MX |
Aerobic biodegradation (ready), wastewater |
0% BOD |
CATALOGIC 2014 |
Abbreviations: BOD (biological oxygen demand) is the amount of dissolved oxygen needed by aerobic biological organisms to break down organic material present in a given water sample at certain temperature over a specific time period.
MK and MX are persistent in all media. The estimated half-lives of MK and MX, based on the experimental and modelled data, are 6 months in water and 9 to 13 days in air. To determine half-lives in soil and sediments, estimations based on ready and inherent biodegradability tests, as well as scientific judgement, were made (US EPA 2000). The resulting half-lives for MK and MX are 6 months in soil and 2 years in sediments.
6.3 Potential for bioaccumulation
The experimental bioconcentration data for MK and MX are summarized below in Table 6-5. These data indicate that MK and MX have, respectively, moderate and high bioaccumulation potential.
Substance |
Test organism |
Experimental concentration (duration) |
BCF (L/kg) |
Reference |
MK |
Rainbow trout (Onchorhynchus mykiss) |
5–47 µg/L (8 days) |
1380a |
Van Dijk and Burri 1995 |
MK |
Carp (Cyprinus carpio) |
not reported |
1100b |
Yamagishi et al. 1983 |
MX |
Bluegill sunfish (Lepomis macrochirus) |
0.98–13 µg/L (16 days) |
1600c |
Paradice and Suprenent 1984 |
MX |
Carp (Cyprinus carpio) |
Not reported |
4100d |
Yamagishi et al. 1983 |
MX |
Carp (Cyprinus carpio) |
1 µg/L (10 weeks) |
1440 to 6740e |
MITI 1992 |
MX |
Rainbow trout (Onchorhynchus mykiss) |
0.023 µg/L (several months) |
4200 5100f |
Kuhlmann et al.1997 |
a This value is for the radiolabelled parent (MK) and polar metabolites. The value for the parent compound alone will be lower.
b It is unknown whether steady state was reached.
c Based on radiolabelled residue in fish.
d It is unknown whether steady state was reached. Also, re-calculation with original data did not yield the same results (EC 2005b).
e It is unknown whether steady state was reached. Also, there is no discussion about the relatively large variability in BCF values.
f According to a personal communication between the study authors and the European Union, the study meets all requirements for a reliable estimate of BCF (EC 2005b).
Few experimental data were found for the bioconcentration factor (BCF) of MK, so modelled results were also obtained. The modelled BCF values for MK are 920 L/kg without biotransformation and 72 L/kg with biotransformation (BCFBAF 2010). Biota-sediment accumulation factor (BSAF) values for MK in bivalves from freshwater and coastal environments (water and sediments) in Korea ranged from 0.04 to 0.09, indicating that MK has low potential to bioaccumulate in sediment-dwelling organisms (Lee et al. 2014). A biomagnification factor (BMF) of 1 for MK was proposed for the aquatic route, because it has a log Kow < 4.5 and a BCF (fish) < 2000, indicating that it has low potential to biomagnify in aquatic organisms (EC 2005a).
The estimated BCF of MK for soil-dwelling organisms (earthworm) is 3.6 kgsoil/kgworm (EC 2005b). No BSAF data were found for MX. BMF data for MX are mixed. The European Union Technical Guidance Document (EC 2003) prescribes the use of a BMF for the aquatic route. MX falls in the category where a BMF of 2 is applicable (log Kow 4.5 to <5 and BCF (fish) 2000 to 5000). However, in a 2012 study (Inoue et al.), the authors developed linear regression between the 5% lipid normalized BCF (BCFL) and the lipid-corrected BMF (BMFL) and found an experimental BMFL of 0.38 for MX.
7. Potential to cause ecological harm
7.1 Ecological effects assessment
7.1.1 Mode/mechanism of action
The mode of action (MoA) of MK and MX is uncertain. While there is some evidence that MK and MX might have a non-polar narcotic mechanism of action (EC 2005a; EC2005b), MoA predictions from both the Verhaar scheme (Verhaar et al. 1992) and OASIS MoA scheme (OECD QSAR Toolbox 2016) indicate, respectively, that the MoA could not be classified or that it consists of an unspecified reactive MoA. ASTER (1999) indicates that MK and MX are both dinitroaromatic compounds, which have generally been associated with an intoxication syndrome that is consistent with a chemical reactivity-based MoA. This reactivity might result in oxidative stress.
Protein-binding is a molecular mechanism associated with a potentially higher hazard than narcosis in aquatic organisms. Although there is no conclusive evidence that MK and MX are protein-binders, ACD/Percepta model predictions for protein-binding indicate that substances like MK and MX tend to bind to plasma lipoproteins and, to a lesser extent, albumin. ACD/Percepta models indicate plasma protein-binding properties from lipophilicity and structural descriptors of the compounds (ACD/Percepta c1997-2012).
The OECD and OASIS DNA binding profilers (OECD QSAR Toolbox 2016; CATALOGIC 2014) suggest that MK and MX are not DNA binders.
No effects of MK or MX on the endocrine system have been identified by high throughput screening efforts (US EPA [updated Aug. 18, 2016]) or from other sources (EC 2005a; EC2005b). Effects on the endocrine system were found for metabolites of MK and MX (Chou and Dietrich 1999). Binding to the estrogen receptor (ER) was observed for 2-MK, 2-MX, and 4-MX in rainbow trout (Oncorhynchus mykiss) and the South African clawed frog (Xenopus laevis). The ER binding affinity of these metabolites was up to 375 times lower than that of estradiol and up to 150 times lower than that of bisphenol A (Taylor et al. 2014). Also, binding in both species occurred after exposure to the chemicals for 20 hours at concentrations of 10−6 to 10−3 M (0.265 mg/L - 265 mg/L) in surrounding media, which is well above levels that are expected to be found in Canadian surface water (see Section 7.2, below).
ER binding predictions from ACD/Percepta (ACD/Percepta c1997-2012) were obtained for the principal metabolites of MK and MX (2-MK, 2-MX, 4-MX). Results indicate that the probability of binding to estrogen receptor alpha is low.
7.1.2 Effects on aquatic organisms
Summaries of the experimental results for the toxicity of MK and MX to aquatic organisms are presented in EC (2005a), EC (2005b), and Appendix A (Tables A-1 and A-2) of this assessment. Three additional studies were found that are more recent than those referenced in the European Union assessment (i.e., Luckenbach and Epel 2005; Mottaleb et al. 2008; Schnell et al. 2009).
For the effects of MK in fish, no reliable acute lethality data (i.e., LC50s) were found. Chronic toxicity values for fish and invertebrates, including no observed effect concentrations (NOECs) and lowest observed effect concentrations (LOECs), range from 0.01 to 0.68 mg/L. The critical toxicity value (CTV) for effects on aquatic organisms is the EC10 of 0.01 mg/L for inhibition of larval development in the marine copepod Acartia tonsa (Wollenberger et al. 2001), which is the lowest value in the dataset from a reliable study. In this case, the CTV is already a low-level, non-lethal effect, so no acute-to-chronic extrapolation was needed. An assessment factor (AF) of 5 was used to account for interspecies variation given that the available dataset is relatively large, consisting of six species from three categories (fish, invertebrates, algae). After dividing the CTV by the AF of 5, the resulting aquatic predicted no-effect concentration (PNEC) for MK is 0.002 mg/L, indicating high aquatic toxicity.
For MX, most of the experimental LC50 values fall within the range of 0.4 to 3.75 mg/L. However, there is also one study which reported a 96-hour LC50 in rainbow trout of >1000 mg/L (Boleas et al. 1996). Chronic toxicity values, mostly NOECs and LOECs, range from 0.01 to 1.00 mg/L. Low water solubility is an issue when attempting to determine the toxicity of nitro musks, especially for MX. All experimental studies for MX are unreliable or unavailable. Consequently, no CTV was selected from among them. The closest analogue for MX is MK but its water solubility is higher than that of MX. Therefore, there are no usable analogues for selecting an experimental CTV for the effects of MX on aquatic organisms. For that reason, a modelled value was selected, specifically, the modelled 96 hour LC50 for fish of 0.2 mg/L (EPISuite c2000-2012). In this case, a factor of 10 was applied to extrapolate from the short-term median lethal effect CTV to a long-term low-level non-lethal effect level. Another factor of 10 was applied to account for interspecies variation because there are modelled data for only three species from three categories (fish, invertebrates, algae). After dividing the CTV by the AF of 100, the resulting aquatic PNEC for MX is 0.002 mg/L, indicating high aquatic toxicity.
7.1.3 Effects on soil-dwelling organisms
The limited data for effects on soil-dwelling organisms (springtail, earthworm) are presented in a European Union risk assessment report (EC 2005a). The CTV for effects of MK on soil-dwelling organisms is the maximum acceptable toxicant concentration (MATC) based on the 8-week NOEC (32 mg/kg) and LOEC (100 mg/kg) for reproductive effects on earthworms (Goßman and Petto 1997). The calculated MATC is 57 mg/kg. No acute-to-chronic extrapolation is needed, since the CTV is for long-term, low-level, sub-lethal effects. However, since only two species were available in the dataset—both invertebrates—a species variation factor of 50 was applied to account for interspecies variation. After dividing the CTV by the AF of 50, the resulting soil PNEC for MK is 1.14 mg/kg, indicating that MK has moderate toxicity to soil-dwelling organisms.
7.1.4 Effects on wildlife and sediment-dwelling organisms
No studies were found for effects of MK or MX on birds, non-laboratory mammals, or sediment-dwelling organisms. Therefore, no PNECs were derived for these types of organisms.
7.1.5 Metabolites
No experimental toxicity data were found for metabolites of MK. There is one reliable experimental study (Giddings et al. 2000) for a metabolite of MX (4-MX), which was conducted on behalf of the Research Institute for Fragrance Materials (RIFM). The authors report a 48-hour EC10 for immobilization of Daphnia magna at a 4-MX concentration between 0.27 and 0.3 mg/L. In the absence of experimental data, modelled results were obtained and are presented in Appendix A (Table A-3).
Given the high variability of the modelled results, the critical toxicity value (CTV) chosen for effects of 4-MX on aquatic organisms is the experimental 48-hour EC10 of 0.27 mg/L for 4-MX. An assessment factor (AF) of 5 was used because the CTV is a low-level effect of short duration, and immobilization is considered a surrogate for lethality in daphnids. An additional AF of 10 accounts for interspecies variation given that the available dataset is very small, consisting of three species from two categories (fish and invertebrates). Therefore, the overall AF is 50. After dividing the CTV by the AF of 50, the resulting aquatic PNEC for 4-MX is 0.0054 mg/L, indicating that 4-MX has high aquatic toxicity.
7.2 Ecological exposure assessment
Few data are available for concentrations of MK and MX in the Canadian environment. In a 1999 study (Gatermann et al.), the authors reported concentrations of MK and MX in Canadian aquatic biota. The highest concentrations found were in clams (lipid-based), at 0.11 µg/g for MK and 17.7 µg/g for MX. In a study conducted by ECCC researchers, concentrations of nitro musks were measured in air at four WWTSs in Ontario. Preliminary results show that concentrations of MK and MX in air are very low: the highest concentration of MK found off-site (~100 to 150 m away from the active area on the premises of the WWTS) was 22 pg/m3, and results for MX, off-site, were all non-detectable (personal communication; unpublished data received in 2017 by Ecological Assessment Division, ECCC, from Air Quality Research Division, ECCC, unreferenced). These results indicate that air is not likely to be a significant exposure pathway for MK and MX.
Several monitoring studies were conducted between 1997 and 2005 in Canada, the United States and Europe to measure the influent and effluent concentrations and removal rates of nitro musks in WWTSs (Simonich et al. 2000; Smyth et al. 2007; Smyth et al. 2008). There were also several monitoring studies conducted between 1997 to 2000 in the United States and Europe that specifically measured the effluent concentrations and removal rates of MK and MX, covering 17 WWTSs (Simonich et al. 2002). The influent and effluent concentrations and removal rates found in these studies were used in the exposure calculations of this assessment for estimating the predicted environmental concentrations (PECs) of MK and MX in the environment.
On the basis of the uses and quantities of MK and MX reported in the CEPA section 71 survey (Environment Canada 2009), as well as their expected release pathways and fate, three exposure scenarios were developed. Detailed descriptions of the scenarios, as well as the PEC calculations, are found in ECCC (2017).
7.2.1 Product formulation
Conservative estimates were derived for MK and MX PECs in the aquatic environment from the formulation of products. The PEC estimations are based on industrial releases to wastewater by formulators who use MK and MX as fragrance ingredients in the production of consumer and commercial products for cleaning. A number of representative facilities were selected. These facilities were determined to be representative because they indicated direct involvement with the substances. Site-specific characteristics as well as generic assumptions were used to define site conditions and to estimate the PECs in the aquatic environment. The upper limit of the quantities of MK and MX reported in the survey was used in the exposure calculations. Also, certain generic assumptions about the formulation and blending process were used. For example, these processes are highly variable but it is assumed that the concentration of MK, as well as the concentration of MX that is used during the formulation and blending process of all products is 3.5%. This value is adopted from EU 2005a and represents the maximum concentration of MK for blending/formulating fragrance compounds. This value is also used for MX since there was no literature available on the blending concentration for MX. The PECs of MK and MX in receiving (surface) water were estimated from the amounts of these substances released via wastewater treatment effluent (after WWTS removal), the daily wastewater flow rate and the dilution in the receiving water. Although site-specific characteristics were used to define site conditions, some assumptions were made about the release conditions. For example, companies that are part of the product formulation sector are highly variable in size, but it is assumed that the percentage of MK and MX that is lost to wastewater during the cleaning process at all facilities is 0.3%. This value is the generic release factor for typical large-scale industrial operations and is adopted from EC (2003). The calculated MK and MX PECs are 0.000065 mg/L and 0.00053 mg/L, respectively.
7.2.2 Product use and disposal
The primary entry of MK and MX into the environment is the result of the use and disposal of products available to consumers containing MK and MX (Simonich 2002). The exposure resulting from down-the-drain releases was investigated using measured influent and effluent concentrations of MK and MX. These concentrations were measured at six WWTSs in Ontario between 2003 and 2004 (Smyth et al. 2008), and the results are summarized in Table 7-1. These effluent concentrations, as conservative representations of exposure levels, were compared with the aquatic PNECs to estimate the potential risk to aquatic organisms.
Influent/effluent |
MK concentration (mg/L) |
MX concentration (mg/L) |
Influent |
0.00003–0.0004 (30–400 ng/L) |
0.00002–0.00022 (20–220 ng/L) |
Effluent |
0.000005–0.00015 (5–150 ng/L) |
0.000002– 0.00003 (2–30 ng/L) |
7.2.3 Land application of biosolids
Exposure resulting from the land application of biosolids from WWTSs was investigated using measured concentrations of MK and MX in biosolids from Canadian WWTSs. In Canada, WWTSs produce more than 660 000 tonnes of biosolids annually, of which 33% are applied to agricultural/forestry land (Kim et al. 2013). Two studies were found that report concentrations of biosolids from WWTSs in Canada (Lee et al. 2003; Guerra et al. 2015). The study by Lee et al. presented concentrations of MK and MX in biosolids from 19 WWTPs across Canada between 1996 and 2002; the reported values, however, are much lower than those in the Guerra study. Therefore, to be conservative, the median MK and MX concentrations in biosolids that were reported by Guerra were chosen. Table 7-2 summarizes the median concentrations of MK and MX in biosolids according to sludge treatment type (aerobic vs. anaerobic). As a conservative exposure scenario, these biosolids concentrations were compared with the PNEC for soil-dwelling organisms.
Substance |
Median concentration in biosolids: aerobic system (mg/kg) |
Median concentration in biosolids: anaerobic system (mg/kg) |
MK |
0.066 |
0.098 |
MX |
0.063 |
not detected |
7.3 Characterization of ecological risk
The approach taken in this ecological screening assessment was to examine assessment information and develop proposed conclusions using a weight-of-evidence approach and precaution. Lines of evidence considered include those evaluated in this assessment that directly support the characterization of ecological risk in the Canadian environment. Secondary or indirect lines of evidence are considered when available, including regulatory decisions and classification of hazard or fate characteristics made by other regulatory agencies.
7.3.1 Risk quotients and other quantitative risk analyses
Risk quotient (RQ) analyses were performed by comparing the various realistic worst-case estimates of exposure (PECs; see the Ecological Exposure Assessment section) with ecotoxicity information (PNECs; see the Ecological Effects section) to determine whether there is potential for ecological harm in Canada. RQs were calculated by dividing the PEC by the PNEC for relevant environmental compartments and associated exposure scenarios.
For the product formulation scenario, the RQs for effects on aquatic organisms are 0.033 for MK and 0.27 for MX.
Compound |
PEC (mg/L) |
PNEC (mg/L) |
RQ |
MK |
0.000065 |
0.002 |
0.033 |
MX |
0.00053 |
0.002 |
0.27 |
For the product use and disposal scenario, a conservative approach was used by comparing the concentrations measured in effluents with the aquatic PNECs. The highest measured effluent concentrations of MK and MX are many orders of magnitude lower than the aquatic PNECs for MK (0.002 mg/L) and MX (0.002 mg/L). MK and MX concentrations in the environment (PECs) would be even lower, so no further refinement of this scenario was done.
Similarly, for land application of biosolids, the highest concentration in biosolids (0.098 mg/kg) was compared directly with the soil PNEC (1.14 mg/kg). As these concentrations would be even lower once applied to soils, no further refinement of this scenario was done.
Metabolites
The assumption is made that 4-MX is present in WWTS effluent at approximately four times the concentration of the parent compound (MX), on the basis of a monitoring study by Gatermann et al. (1998).
Therefore, the PEC of 4-MX in surface water as a result of the product formulation is 0.0021 mg/L (4 x 0.00053 mg/L) and the RQ is 0.4 (0.0021/0.0054). The highest concentration of 4-MX in effluent as a result of product use and disposal is 0.00012 mg/L (0.00003 mg/L x 4). This is an order of magnitude below the PNEC for 4-MX of 0.0054 mg/L.
7.3.2 Consideration of lines of evidence
To characterize the ecological risk of MK and MX, technical information for various lines of evidence was considered (as discussed in the relevant sections of this report) and qualitatively weighted. The key lines of evidence supporting the assessment conclusion are presented in Table 7-4, with an overall discussion of the weight of evidence provided in section 7.3.3. The level of confidence refers to the combined influence of data quality and variability, data gaps, causality, plausibility and any extrapolation required within the line of evidence. The relevance refers to the impact the line of evidence has when determining the potential to cause harm in the Canadian environment. Qualifiers used in the analysis ranged from low to high, with the assigned weight having five possible outcomes.
Line of evidence |
Level of confidencea |
Relevance in assessmentb |
Weight assignedc |
Persistence in the environment |
high |
high |
high |
Long-range transport |
moderate |
low |
low to moderate |
Bioaccumulation in aquatic organisms |
moderate |
high |
moderate to high |
Mode of action and other non-apical data |
low to moderate |
moderate |
low to moderate |
PNEC for aquatic organisms |
moderate |
high |
moderate to high |
PNEC for soil-dwelling organisms (MK only) |
low |
high |
moderate |
Biotransformation (metabolites) |
low |
moderate |
low to moderate |
Monitoring data for concentrations in wastewater influent and effluent |
high |
high |
high |
Data for concentrations in wastewater biosolids |
high |
high |
high |
PEC in water |
moderate |
high |
moderate to high |
Risk quotient for water |
moderate |
moderate |
moderate |
Risk analysis for soil (applies to MK only) |
moderate |
moderate |
moderate |
a Level of confidence is determined according to data quality, data variability, and data gaps, i.e. are the data fit for purpose or suitable to characterize the endpoint or property.
b Relevance refers to the overall impact of the evidence on the assessment.
c Weight is assigned to each line of evidence according to the combined level of confidence and relevance in the assessment.
7.3.3 Weight of evidence for determining potential to cause harm to the Canadian environment
Evidence presented in this assessment indicates that MK and MX are slightly water soluble and very persistent in the environment. Structural and empirical evidence, as well as modelled results, mutually support the assertion that MK and MX have an overall persistence (Pov) in the environment in the order of years. When released to water—their primary mode of entry to the environment—MK and MX are likely to reside in the water column and undergo long-range transport in water, becoming distributed throughout a river system. Therefore, dilution by surface water bodies becomes the governing factor controlling environmental concentrations relevant to organism exposure.
Given their multimedia fate, MK and MX are both expected to undergo significant removal in WWTSs. Therefore, high rates of removal from wastewater effluents are expected, and the transfer of MK and MX to the terrestrial environment from biosolids application is expected to occur. Studies have shown that, when MK and MX are degraded in WWTSs, transformation products (metabolites) are formed. There are, however, no data for concentrations of the metabolites in Canadian surface water and almost no ecotoxicity data for them. Consequently, the presence and effects of the metabolites of MK and MX are of only moderate relevance in this assessment.
There is relatively consistent evidence that MK and MX have, respectively, moderate and high bioaccumulation potential in aquatic species. MK and MX are not expected to biomagnify significantly in aquatic organisms.
There are numerous empirical studies for MK and MX which report acute and chronic effects in aquatic organisms.However, only a few of these studies are considered reliable. Empirical and modelled data suggest that both MK and MX are hazardous to aquatic organisms. The few soil studies available indicate that MK is not hazardous to soil-dwelling organisms. No soil studies were available for MX, but given the similarity of its structure and physical-chemical properties to those of MK, it is assumed that effects on soil-dwelling organisms would be similar to those for MK.
A number of jurisdictions have taken actions to restrict or ban the use of MX. MX is identified as a Substance of Very High Concern (SVHC) in the European Union and is categorized as very persistent (vP) and very bioaccumulative (vB). It is listed on Annex XIV of the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) legislation, and authorization must be sought if this chemical is to be used in the European Union. In Japan, MX is a Monitoring Chemical Substance under the Act on the Evaluation of Chemical Substances and Regulation of Their Manufacture, etc. because it has been identified as a persistent and highly bioaccumulative chemical, with unclear long-term toxicity to humans and predator animals at higher trophic levels. In Australia, MX has been categorized as a PBT chemical and is prioritized for a Tier III assessment under the NICNAS Inventory Multi-tiered Assessment and Prioritisation (IMAP) framework (personal communication: email from NICNAS to the Ecological Assessment Division, ECCC, dated November 28, 2017; unreferenced).
The shortage of reliable experimental acute and chronic effects data for MK and MX and their metabolites, and the variability in the modelled data for the metabolites, reduced the confidence in the PNECs derived for MK, MX, and the 4-MX metabolite. Similarly, uncertainties associated with the exposure analyses lowered the level of confidence with the PECs. However, conservative assumptions were used to derive the PECs, so although there is some uncertainty in the PEC calculations, the actual environmental concentrations are likely lower than predicted. A comparison of the PECs resulting from product formulation to levels of concern is not indicative of risk. A conservative approach used to assess exposure to MK and MX from product use and disposal or land application of biosolids, whereby concentrations for MK and MX in WWTS effluent or biosolids were compared to levels that would cause adverse effects, showed that environmental concentrations from these sources would be well below levels of concern.
This information indicates that MK and MX have low potential to cause ecological harm in Canada. While exposure of the environment to MK and MX is not a concern at current levels, these substances are considered to have an environmental effect of concern given their potential to cause adverse effects in aquatic organisms at low concentrations. Therefore, there may be a concern for the environment if exposures were to increase.
7.3.4 Sensitivity of conclusion to key uncertainties
There is uncertainty about the use pattern information used in the exposure assessment. The manufacture and import quantities reported in the section 71 survey for 2008 might not reflect the current situation. Trends indicate that the use of MK and MX has been dropping steadily for a number of years (personal communication; email from the Research Institute for Fragrance Materialsto the Ecological Assessment Division, ECCC, dated March 10, 2017; unreferenced), meaning that RQs derived using current use quantities would be lower than those derived in this assessment.
There is uncertainty about the fate of MK and MX released to surface water. The EQC modelling results indicate only a small percentage will partition to sediment but, given the moderately high log Koc values of MK and MX, a higher percentage of partitioning than predicted is possible. However, no toxicity data for sediment-dwelling organisms were found, so it is uncertain how these organisms would be affected by an increase in partitioning of these substances to sediment.
There is uncertainty about the MoA, as well as acute and chronic effects of MK and MX. A number of studies have been conducted, mainly for acute effects, but few of them are reliable. AFs were applied to CTVs to reduce uncertainty by accounting for factors such as interspecies variation. There is evidence for the occurrence of some non-apical effects (e.g., protein-binding) at lower concentrations than those reported in the chronic effect studies. However, the data are limited (i.e., results from one model) and are inconclusive.
In the absence of data about certain aspects of the product formulation process, assumptions were made in developing the exposure scenarios. For product formulation, the uncertainties include the following: the exact concentrations of MK and MX in products and the subsequent concentrations in releases from product formulation; the concentration of MK and MX that is used during the formulation and blending process of all products; the number of operating days and the daily quantities; the percentage of MK and MX lost to wastewater during the cleaning process of the formulation stage. In the product use and disposal scenario, there is uncertainty associated with the potentially high variability of MK and MX levels used in different regions of Canada. Using the measured effluent concentrations of nitromusks from six WWTSs in Ontario might not be representative of the situation in Canada as a whole. For the land application of biosolids, the uncertainties include the estimated concentrations of MK and MX in biosolids, which may not be representative or may be influenced by other unknown factors, such as operating conditions of the WWTS generating the biosolids.
There is sufficient experimental and modelled evidence to support the assertion that MK and MX are, respectively, moderately hazardous and highly hazardous to aquatic organisms. Evidence, mostly modelled, indicates that non-apical effects, such as ER binding, are not significant. Therefore, further studies for the persistence, bioaccumulation potential, and toxicity of these substances to aquatic organisms are unlikely to impact the assessment conclusion. Given that significant releases to air and sediment are not expected, fate and effects studies for air, terrestrial organisms, sediment, and sediment-dwelling organisms are unlikely to impact the assessment conclusion. Releases to soil are expected, so further studies for the fate and effects of MK and MX in soil and soil-dwelling organisms could, pending the study outcomes, impact the assessment conclusion.
Most of the uncertainties in this assessment pertain to the exposure assessment for product formulation. Reducing uncertainties associated with this exposure scenario could impact the RQ and the assessment conclusion. Considering the conservatism of the approaches used for the other two scenarios, there are fewer uncertainties, and consequently, additional exposure information for these sources are unlikely to impact the assessment conclusion.
Although current use patterns and quantities in commerce indicate low concern at current levels, there may be concern if quantities of MK and MX were to increase in Canada.
8. Potential to cause harm to human health
8.1 Exposure assessment
Environmental media and food
There are no environmental monitoring data for MK or MX in Canada except for one study that reports concentrations in biota. MK was measured and detected in house dust collected in 13 cities across Canada during 2007-2010 (Kubwabo et al. 2012). No reports of either substance in Canadian food were identified.
Internationally, MK was measured in soil in Lubbock, Texas (Chase et al. 2012), and both MK and MX were reported in ambient air in Iowa and Wisconsin and over lakes Ontario, Erie and Michigan in the United States (Peck and Hornbuckle 2004; Peck and Hornbuckle 2006), in indoor air in Turkey (Sofuoglu et al. 2010), and in house and motel dust in the United States (Peck et al. 2007). Homem et al. (2016) did not detect either substance in tap water in Spain. However, both substances were detected in Lake Michigan surface water, as well as in river or lake surface water in Europe (Peck and Hornbuckle 2004; Rimkus 1999). Both substances were measured in fish, mussels and crustaceans in Europe (Rimkus 1999; Hajslova and Setkova 2004; Deudahl-Olesen et al. 2005). Peck et al. (2007) analyzed two single tissue samples from lake trout from the Great Lakes, one mussel tissue sample and one blubber sample from a pilot whale collected in Massachusetts, but neither MK nor MX were detected in any of the samples. Aguirre et al. (2014) measured MK in lettuce in Spain, but it was not detected in samples of carrots or peppers.
Biomonitoring
Total measured concentrations of substances in blood in individuals may provide a measure of integrated exposure for individuals from all routes (oral, dermal and inhalation) and all sources (including environmental media, diet, and frequent or daily use products to which they were exposed). In Canada, a preliminary study of women diagnosed with breast cancer showed MK levels of greater than 0.05 (limit of detection) to 1.52 µg/L in the blood serum of 8 of the 10 women (median = 0.46 µg/L) compared to levels greater than 0.05 to 0.76 µg/L found in 4 of 20 healthy women (median < 0.05 µg/L). MX was not detected in either the healthy women or the women diagnosed with breast cancer. Blood samples were obtained between 2004 and 2016 (personal communication, Exposure and Biomonitoring Division, Health Canada, to ESRAB, Health Canada, dated February 2017; unreferenced).
Internationally, concentrations of both MK and MX have been measured in human breast milk in many studies in Europe since the early 1990s, as well as starting more recently in the United States and China (Hajslova and Setkova 2004; EC 2005a, 2005b; Reiner et al. 2007; Lignell et al. 2008; Raab et al. 2008; Zhang et al. 2011, 2015; Zhou et al. 2012; Yin et al. 2012, 2016). During the 2000s in Europe and the United States, maximum concentrations of MK and MX ranged from 6.0 to 212 ng/g lipid weight and 150 to 240 ng/g lipid weight, respectively (Hajslova and Setkova 2004; Reiner et al. 2007; Raab et al. 2008). In addition, MK and MX have been measured in adipose tissue in Germany, Switzerland and Korea (EC 2005a, 2005b; Moon et al. 2002), blood plasma in Germany and Vietnam (EC 2005a; Hutter et al. 2009, 2010), and umbilical cord blood in China (Zhang et al. 2015).
Environmental media and food intake estimates
Daily intake estimates of MK and MX from environmental media, including food and breast milk, is shown in Appendix B (Tables B-1 and B-2). Based on maximum or 95th percentile values for each medium, the intake for MK ranged from 0.00003 mg/kg bw/day (60+ year adults) to 0.00087 mg/kg bw/day (breast milk fed infants aged 0 to 0.5 years), and the intake for MX ranged from 0.000004 mg/kg bw/day (60+ year adults) to 0.00062 mg/kg bw/day (breast milk fed infants aged 0 to 0.5 years) in the general population.
Products available to consumers
MK and MX are used primarily as fragrances or fragrance ingredients. In Canada, musk ketone is found in some cosmetics (such as hair conditioner and eau de toilette), and musk xylene is found in some air fresheners, personal care productsFootnote 6 (such as body lotion), cleaning products, some laundry and dishwashing products, and odour agents.
Product scenarios that result in the highest levels of potential exposure for each substance by the inhalation and dermal routes (when applicable) are presented in Tables 8-1 and 8-2. Potential exposures were estimated using conservative assumptions and default values from sentinel exposure scenarios (see Appendix C for details). Direct exposures from use of products by adults were evaluated, and in children if there was evidence that they could be exposed to some of the products. For estimated potential exposures via the dermal route, dermal absorption was 14% for MK and 10% for MX based on experimental data (see below).
Dermal absorption potential
In humans, dermal absorption rates of 14% for MK and 10% for MX were estimated on the basis of an in vivo human study and in vitro experiments with human and rat skin that showed that even after removal of the test substance, the skin acts as a depot from which MK or MX continues to be systemically released (EC 2005 a,b).
EC (2005a,b,c) had previously estimated that dermal bioavailability of MX was 20% of oral bioavailability using a simple calculation (10% dermal absorption /50% oral absorption). A recent pharmacokinetic model for MX developed by Hays et al. (2017) supports the evidence that dermal bioavailability is not as significant as previously thought. Using the toxicokinetic data for MX in both rats and humans, they developed a two-compartment model that was consistent with both oral and dermal dosing scenarios. Dermal bioavailability was found to be 2% of the oral bioavailability and was consistent with the relative difference in bioavailabilities calculated by Riedel and Dekant (1999) in their pharmacokinetic study of MX in humans. The lower dermal bioavailability estimated by Hays et al. (2017) suggests that in vivo dermal absorption of MX in humans may be lower than 10%.
Substance |
Product scenario |
Route of exposure |
Per event exposure (mg/kg bw) |
Mean event concentration (mg/m3) |
Daily systemic exposure (mg/kg bw/ day) |
MK |
Hair conditioner |
Dermal |
0.018
|
N/A |
0.0028 a |
MK |
Fragrance (eau de toilette) |
Dermal |
0.0047
|
N/A |
0.00011a |
MK |
Fragrance (eau de toilette) |
Inhalation |
0.0000012 |
0.0015 |
0.0000012 |
Abbreviation: N/A; not applicable.
a Values adjusted for 14% dermal absorption in humans (EC 2005a).
Substance |
Product scenario |
Route of exposure |
Per event exposure (mg/kg bw) |
Mean event concentration (mg/m3) |
Daily systemic exposure (mg/kg bw/ day) |
MX |
Body lotion |
Dermal |
0.068 |
N/A |
0.0068 a |
MX |
Air freshener -Adult |
Inhalation |
0.003 |
0.013 |
0.003 |
MX |
Air freshener -Child |
Inhalation |
0.008 |
0.013 |
0.008 |
Abbreviation: N/A; not applicable.
a Value adjusted for 10% dermal absorption in humans (EC 2005b).
8.2 Health effects essessment
Both MK and MX have been assessed internationally by the European Commission (EC 2005a,b,c,d). MX was also reviewed by IARC (1996). There is also a journal review of human exposure to nitro musks and evaluation of their potential toxicity by Taylor et al. (2014). Australia’s NICNAS (2016) summarized information on nitromusks from the more detailed reviews of the European Commission, IARC and Taylor et al. (2014). Salient information and critical effect levels determined from the European Commission assessments and Taylor et al. (2014) are presented below.
Absorption, distribution, detabolism, excretion
In both rats and humans, metabolism of MK involves glucuronide conjugation (EC 2005c). In an in vivo human study, only 0.5% of the applied dose was excreted in urine and feces within 120 hours. However, 14% of the dose was not recovered (EC 2005c). Metabolism studies for MX in rats involve both reduction of a nitro group to an amine and hydroxylation of methyl groups. Hydroxymethyl-MX is the main metabolite in bile. In humans, urine contained a single metabolite that was chromatographically distinct from both MX and hydroxymethyl-MX, or the presence of p-NH2-MX (EC 2005d).
In terms of sensitization potential, MK and MX were determined not to be skin sensitizers (up to 5% concentration) and were considered as weak photoallergens by the EC (EC 2005a,b).
Subchronic toxicity
Mice (10/sex/group) received a diet containing 0.0375% to 0.6% MX for 17 weeks (equivalent to 54 to 857 mg/kg bw/day, respectively). Eight of 10 males and all females at 429 mg/kg bw/day died, and all mice at 857 mg/kg bw/day died during the study. Enlargement and irregularity of liver cells were observed in both sexes at 214 mg/kg bw/day. From these results, 0.075% and 0.15% (equivalent to 107 and 214 mg/kg bw/day, respectively) were selected as appropriate dose levels for the carcinogenicity study. Because no other parameters were studied (e.g., clinical chemistry or hematology), no NOAEL can be derived (EC 2005b).
Rats (15/sex/dose) received dermal applications of 7.5, 24, 75 or 240 mg/kg bw/day MK or MX in phenylethyl alcohol for 13 weeks. For MK, females showed a dose-related significant decrease in body weight gain at 75 and 240 mg/kg bw/day and males showed a significant decrease at the top dose, despite similar or greater food consumption than that of controls. Relative liver weights were increased at 240 mg/kg bw/day in males (not statistically significant) and in females (statistically significant). For MX, relative liver weights were statistically significantly increased at 75 mg/kg bw/day in females and at 240 mg/kg bw/day in males (Ford et al. 1990). No gross necropsy or microscopic changes, including the neuropathological parameters studied, were observed for either substance. The NOAEL was determined to be 24 mg/kg bw/day for both MK and MX, based on decreased body weight gain and increased relative liver weight at 75 and 240 mg/kg bw/day (EC 2005a,b).
No inhalation studies were identified for MK other than a 13-week inhalation study in female rats and hamsters in which MK was administered as part of a fragrance mixture. This study was not used for risk characterization because of the low MK concentration in the mixture (EC 2005a).
Carcinogenicity/chronic toxicity
IARC (1996) classified MX as a Group 3 carcinogen (not classifiable as to its carcinogenicity to humans) on the basis of limited evidence in experimental animals and inadequate evidence in humans. In 2004, the European Union classified MX as a Category 3 carcinogen on the basis of limited evidence of a carcinogenic effect (EC 2005d). This was based on its similarity to phenobarbital in producing liver tumours. The Scientific Committee on Consumer Safety [SCCS] (2004) did not consider that the evidence for establishing a threshold was sufficient using the approach described in EC (2005b). On the basis of the SCCS (2004) opinion, both MX and MK were listed as a Category 2 carcinogen (suspected of causing cancer) in 2009 in the European Union (EC 2009; ECHA 2016).
For MX, an oral LOAEL of 70 mg/kg bw/day for carcinogenicity was determined from a 80-week mouse study on the basis of an increased incidence of liver tumours in both sexes (incidence of liver carcinomas was mechanistically related to microsomal enzyme induction) and an increased incidence of Harderian gland tumours in males. The relevance of the Harderian gland tumours to humans is unknown because this gland is not present in humans. The carcinogenicity of musk xylene was not investigated in the rat (EC 2005b).
Although there are no carcinogenicity data for MK, EC (2005c) stated that there is a concern that MK may be hepatocarcinogenic in mice as well. Just like MX, MK is a phenobarbital-like inducer of liver enzymes, but EC (2005c) considered it to be somewhat less potent than MX. EC (2005c) stated that because of the similarity of MK to MX in terms of physico-chemical and toxicokinetic properties and induction method of liver enzymes, it is felt that the carcinogenicity data available on MX can be safely used for the characterization of carcinogenic risk of MK to humans.
Genotoxicity
Available data indicate that MK is not genotoxic. Several in vitro tests showed negative results for MK (bacterial gene mutation tests, SOS [gene box] chromotests, a mammalian gene mutation test, tests for micronuclei induction and sister chromatid exchanges [SCEs] in mammalian cells, and an unscheduled DNA synthesis [UDS] test), while a chromosome aberration test in mammalian cells was equivocal. However, an in vivo mouse micronucleus test was negative in polychromatic erythrocytes up to an intraperitoneal dose of 1000 mg/kg bw (EC 2005c).
Available data indicate that MX is not genotoxic. Several in vitro tests showed negative results for MX (bacterial gene mutation tests, SOS chromotests, a mammalian gene mutation test, tests for chromosome aberrations and SCEs in mammalian cells, a micronucleus test in mammalian cells and an UDS test). Negative results were also observed in an in vivo-in vitro rat hepatocyte UDS test (EC 2005d).
Reproductive/developmental toxicity
In oral peri/postnatal toxicity studies similar in protocol to developmental neurotoxicity studies, MX or MK in corn oil were administered by gavage at doses of 0, 2.5, 7.5 or 25 mg/kg bw/day to rats (28 females/dose) from day 14 of pregnancy (end of organogenesis) through to postnatal day 21 (28-29 day exposure period). For MK, the NOAEL for maternal toxicity was 7.5 mg/kg bw/day based on decreased body weight gain and food consumption at 25 mg/kg bw/day. The NOAEL for developmental toxicity was 2.5 mg/kg bw/day based on lower body weight gains up to post-natal week 20 in F1 males from F0 dams receiving 7.5 (in the absence of maternal toxicity) and 25 mg/kg bw/day. For MX, the NOAEL for maternal toxicity was 7.5 mg/kg bw/day based on slightly decreased body weight gain and food consumption (not statistically significant) at 25 mg/kg bw/day. The NOAEL for developmental toxicity was 7.5 mg/kg bw/day based on lower body weight gains up to weaning (not statistically significant) and the behavioural effect of later day of attainment for air righting reflexesFootnote 7 in pups from F0 dams receiving 25 mg/kg bw/day (in the presence of maternal toxicity) (EC 2005a,b). EC (2005b) stated that the maternal and developmental NOAELs for MX are conservative based on the fact that the effects at 25 mg/kg bw/day were not statistically significant, but that the effects in pups may be biologically relevant because the same effects (lower body weight gains) were observed for MK.
In an oral developmental toxicity study, pregnant rats (25 females/group) received 0, 15, 45 or 150 mg/kg bw/day MK in corn oil by gavage during gestation days (GD) 7 to 17 (11-day exposure period) and then sacrificed on gestation day 20. The NOAEL for maternal toxicity was 15 mg/kg bw/day based a dose-related decrease in food consumption and body weight at 45 and 150 mg/kg bw/day. The NOAEL for developmental toxicity was 45 mg/kg bw/day based on increased post-implantation loss and reduced fetal body weight at 150 mg/kg bw/day (in the presence of maternal toxicity) (EC 2005a).
In an oral developmental study, pregnant rats (25females/dose) were gavaged with MX in corn oil at doses of 0, 20, 60 or 200 mg/kg bw/day during gestation days 7 to 17 (11-day exposure period) and then sacrificed on gestation day 20. The NOAEL for maternal toxicity was 20 mg/kg bw/day based on decreased body weight gain and food consumption at 60 and 200 mg MX/kg bw/day. One to 3 females at 60 mg/kg bw/day and 12 to 25 females at 200 mg/kg bw/day showed tremors on one or more days during the first 4 days of dosing. The NOAEL for developmental toxicity was 60 mg/kg bw/day based on an increased incidence of skeletal effects (extra thoracic ribs and increased ossification) in fetuses at 200 mg/kg bw/day (in the presence of maternal toxicity) (EC 2005b).
8.3 Characterization of risk to human health
Tables 8-3 and 8-4 provide all relevant exposure and hazard values for MK and MX, as well as resultant margins of exposure (MOEs), for determination of risk.
Exposure scenario |
Exposure concentration |
Critical effect level |
Critical health effect endpoint |
MOE |
Environmental media and food, daily exposure |
0.00087 mg/kg bw/day |
Oral LOAEL = 70 mg/kg bw/day based on 80-week mouse study (with MX) |
Increased incidence of liver tumours in both sexes |
80 500 |
Dermal exposure to hair conditionera (adult) |
0.018 mg/kg bw
|
Dermal NOAEL = 24 mg/kg bw/day based on 90-day rat study |
Decreased body weight gain at 75 mg/kg bw/day |
1 300 |
Dermal exposure to eau de toilette fragrance (adult) |
0.0047 mg/kg bw
|
Dermal NOAEL = 24 mg/kg bw/day based on 90-day rat study |
Decreased body weight gain at 75 mg/kg bw/day |
5 100 |
Per event inhalation exposure via eau de toilette fragrance (adult) |
0.0015 mg/m3 |
Oral maternal NOAEL = 15 mg/kg bw/day (equivalent to 48.4 mg/m3)b in rat developmental toxicity study (GD 7 to 17) |
Decreased body weight and food consumption in dams at 45 mg/kg bw/day |
32 300 |
a Exposure to hair shampoo yielded a similar exposure concentration.
b 1 mg/m3 in air is equal to 0.31 mg/kg bw/day in rats (Health Canada 1994).
Exposure scenario |
Exposure concentration |
Critical effect level |
Critical health effect endpoint |
MOE |
Environmental media and food, daily exposure |
0.00063 mg/kg bw/day |
Oral LOAEL = 70 mg/kg bw/day based on 80-week mouse study |
Increased incidence of liver tumours in both sexes |
111 100 |
Dermal exposure to body lotion |
0.068 mg/kg bw |
Dermal NOAEL = 24 mg/kg bw/day in 90-day rat study |
Increased relative liver weight at 75 mg/kg bw/day |
350 |
Per event inhalation exposure via air freshener (child and toddler) |
0.013 mg/m3 |
Oral NOAEL = 7.5 mg/kg bw/day (equivalent to 24.2 mg/m3)a in peri/postnatal rat toxicity study (GD 14 to postnatal day 21) |
Decreased body weight gain and delayed attainment of righting reflexes in pups from dams receiving 25 mg/kg bw/day (in the presence of maternal toxicity) |
1 860 |
a 1 mg/m3 in air is equal to 0.31 mg/kg bw/day in rats (Health Canada 1994).
For the environmental media and food scenarios, given the potential for an increased incidence of liver tumours for both MK and MX as discussed by EC (2005a,b) and the negative genotoxicity results for both substances, the MOEs shown in Tables 8-3 and 8-4 for MK and MX, respectively, are considered adequate to account for uncertainties in the health effects and exposure data used to characterize risk. The MOEs for all other scenarios listed in Tables 8-3 and 8-4 are also considered adequate to account for uncertainties in the health effects and exposure data used to characterize risk.
8.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below (Table 8-5).
Key source of uncertainty |
Impact |
Lack of Canadian data for both substances in environmental media. |
+/- |
Lack of chronic studies for MK by any route of exposure. |
+/- |
Lack of repeated dose studies for MK and MX by the inhalation route. |
+/- |
+/- = unknown potential to cause over or under estimation of risk.
9. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from musk ketone and musk xylene. It is proposed to conclude that musk ketone and musk xylene do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this draft screening assessment, it is also proposed to conclude that musk ketone and musk xylene do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that musk ketone and musk xylene do not meet any of the criteria set out in section 64 of CEPA.
10. References
ACD/Percepta [prediction module]. c1997-2012. Toronto (ON): Advanced Chemistry Development, Inc.
Adema DMM, Langerwerf JSA. 1985. The subchronic (14-d exposure) toxicity of E-2642.01 (musk xylene) to Brachydanio rerio. Private communication to RIFM. TNO, Delft. Report R 85/127. [cited in EC 2005b].
Aguirre J, Bizkarguenaga E, Iparraguirre A, Fernández LA, Zuloaga O, Prieto A. 2014. Development of stir-bar sorptive extraction–thermal desorption–gas chromatography–mass spectrometry for the analysis of musks in vegetables and amended soils. Anal Chim Acta. 812:74-82.
[AIEPS] Artificial Intelligence Expert Predictive System. c2010-2012. Ver. 3.0. Gatineau (QC): Environment Canada. Model developed by Stephen Niculescu.
[AOPWIN] Atmospheric Oxidation Program for Microsoft Windows [estimation model]. 2010. Ver. 1.92a. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[ASTER] Assessment Tools for the Evaluation of Risk. 1999. Duluth (MN): US Environmental Protection Agency, Mid-Continent Ecology Division.
[BCFBAF] Bioaccumulation Program for Microsoft Windows [estimation model]. 2010. Ver. 3.01. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Beyer A, Mackay D, Matthies M, Wania F, Webster E. 2000. Assessing long-range transport potential of persistent organic pollutants. Environ Sci Technol. 34:699-703.
[BIOWIN] Biodegradation Probability Program for Microsoft Windows [estimation model]. 2008. Ver. 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Boleas S, Fernandez C, Tarazona JV. 1996. Toxicological and kinetic study of musk xylene in rainbow trout, Onchorhynchus mykiss. Bull Environ Contamin Toxicol. 57:217-222. [cited in Tas et al. 1997].
Breitholtz M, Wollenberger L, Dinan L. 2003. Effects of four synthetic musks on the life cycle of the harpacticoid copepod Nitocra spinipes. Aquatic Toxicol. 63:103-118.
Butte, W, Schmidt S, Schmidt A. 1999. Photochemical degradation of nitrated musk compounds. Chemosphere. 38(6):1287-1291.
Calame R, Ronchi W. 1989. Musk xylene: determination of ready biodegradability. Private communication to RIFM. Givadan-Roure, Switzerland. Test report 33-89. [cited in EC 2010].
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c. 33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2009. Canadian Environmental Protection Act, 1999: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 143, no. 40, p. 2945-2956.
Carlsson G, Norrgren L. 2004. Synthetic musk toxicity to early life stages of zebrafish (Danio rerio). Arch Environ Contam Toxicol. 46:102-105.
CATALOGIC [environmental fate and ecotoxicity model]. 2014. Ver. 5.11.15. Bourgas (BG): University “Prof. Dr. Assen Zlatarov”, Laboratory of Mathematical Chemistry.
Chase DA, Karnjanapiboonwong A, Fang Y, Cobb GP, Morse AN, Anderson TA. 2012. Occurrence of synthetic musk fragrances in effluent and non-effluent impacted environments. Sci Total Environ. 416:253-260.
Chou Y-J, Dietrich DR. 1999. Interactions of nitromusk parent compounds and their amino-metabolites with the estrogen receptors of rainbow trout (Oncorhynchus mykiss) and the South African clawed frog (Xenopus laevis). Toxicol Lett. 111:27-36.
[CITI] Chemicals Inspection and Testing Institute Japan. 1992. Data of existing chemicals based on the CSCL Japan, Japan chemicals industry ecology-toxicology & information center. [cited in Rimkus et al. 1997].
ConsExpo Web [Consumer Exposure Web Model]. 2016. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
[DPD] Drug Product Database [database]. [modified 2016 Sep 27]. Ottawa (ON): Health Canada. [accessed 2017 Jan.].
Duedahl-Olesen L, Cederberg T, Pedersen KH, Hojgard A. 2005. Synthetic musk fragrances in trout from Danish fish farms and human milk. Chemosphere. 61:422-431.
[EC] European Commission. 2003. Technical guidance document on risk assessment: Part II. Luxembourg: Office for Official Publications of the European Communities.
[EC] European Commission. 2005a. European Union Risk Assessment Report. CAS RN 81-14-1: 4’-tert-butyl-2’,6’-dimethyl-3’,5’- dinitroacetophenone (musk ketone). Luxembourg: Office for Official Publications of the European Communities. Report No.: EUR 21507 EN 128 pp.
[EC] European Commission. 2005b. European Union Risk Assessment Report. CAS RN 81-15-2: 5-tert-butyl-2,4,6-trinitro-m-xylene (musk xylene). Luxembourg: Office for Official Publications of the European Communities. Report No.: EUR 21506 EN 151 pp.
[EC] European Commission. 2005c. Summary Risk Assessment Report. CAS RN 81-14-1: 4’-tert-butyl-2’,6’-dimethyl-3’,5’- dinitroacetophenone (musk ketone). Special Publication I.05.14. Joint Research Centre, European Chemicals Bureau, Ispra, Italy. 27 pp.
[EC] European Commission. 2005d Summary Risk Assessment Report. CAS RN 81-15-2: 5-tert-butyl-2,4,6-trinitro-m-xylene (musk xylene). Special Publication I.05.15. Joint Research Centre, European Chemicals Bureau, Ispra, Italy. 30 pp.
[EC] European Commission. 2009. Commission Regulation (EC) No 790/2009 of 10 August 2009 amending, for the purposes of its adaptation to technical and scientific progress, Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures. Official Journal of the European Union L235/1. Pp. 1-349. [accessed 2017 Jun 7].
[EC] European Commission. 2010. PBT assessment of musk xylene: addendum to the final report (2005) of the risk assessment.
[EC] European Commission. 2011. Chemicals/REACH: six dangerous substances to be phased out by the EU.
[ECCC] Environment and Climate Change Canada. 2017. Supporting documentation: nitromusks grouping: information in support of the screening assessment. Gatineau (QC): Environment Canada. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment Canada, Health Canada. [modified 2015 Sept]. Categorization. Ottawa (ON): Government of Canada.
[ECHA] European Chemicals Agency. 2016. C&L Inventory; search results for CAS RNs 81-14-1 and 81-15-2.. Helsinki (FI): ECHA. [accessed 2016 December].
[ECOSAR] ECOlogical Structure Activity Relationships Class Program [estimation model]. 2012. Ver. 1.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Environment Canada. 2009. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
European Parliament, 2009. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Official Journal of the European Union, L342, pp. 59-209. [accessed 2017 Jun 7].
Ford RA, Api AM, Newberne PM. 1990. 90-Day dermal toxicity study and neurotoxicity of nitromusks in the albino rat. Food Chem Toxicol. 28:55-61.
Gatermann R, Hüfhnerfuss H, Rimkus G, Attar A, Kettrup A. 1998. Occurrence of musk xylene and musk ketone metabolites in the aquatic environment. Chemosphere. 36(11):2535-2547.
Gatermann R, Hellou J, Hüfhnerfuss H, Rimkus G, Zitko V. 1999. Polycyclic and nitro musks in the environment: a comparison between Canadian and European aquatic biota. Chemosphere. 38(14): 3431-3441.
Giddings JM, Salvito D, Putt AE. 2000. Acute toxicity of 4-amino musk xylene to Daphnia magna in laboratory water and natural water. Wat Res. 34:14 3686-3689.
Goßman A, Petto R. 1997. Effects of musk ketone on reproduction and growth of earthworms in Eisenia fetida (Savigny 1826) in artificial soil. Report to RIFM. Ibacon, Germany. Project No. 2092022. [cited in EC 2005a].
Guerra P, Kleywegt S, Payne M, Svoboda ML, Lee H-B, Reiner E, Kolic T, Metcalfe C, Smyth SA. 2015. Occurrence and fate of trace contaminants during aerobic and anaerobic sludge digestion and dewatering. J Environ Qual. 44:1193-1200.
Hajslova J, Setkova L. 2004. Synthetic musks in bioindicators: Monitoring data of fish and human milk samples from the Czech Republic. Handbook Environ Chem. 3 (Part X):151-188.
Hays SM, Aylward LL, Kirman C. 2017. Biomonitoring equivalents for musk xylene. Report prepared under Health Canada Contract No. 4600001169. February 15, 2017. 24 pp. [restricted access].
Health Canada. 1994. Human health risk assessment for priority substances. Ottawa (ON): Minister of Supply and Services Canada. Cat. No.: En40-215/41E. Available upon request.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Government of Canada.
Health Canada. [modified 2013 Jun 27]. List of Permitted Food Additives. Ottawa (ON): Health Canada. [accessed 2015 Nov 18].
Health Canada. [modified 2015 Dec 14]. Cosmetic ingredient hotlist: list of ingredients that are prohibited for use in cosmetic products. Ottawa (ON): Health Canada, Consumer Product Safety Directorate. [accessed 2017 January].
[HMDB] Human Metabolome Database. 2016. Showing metabocard for 4’tertButyl2’, 6’dimethyl3’, 5’dinitroacetophenone (HMDB31865). [accessed 2016 Jul 19].
Homem V, Alves A, Alves A, Santo L. 2016. Ultrasound-assisted dispersive liquid–liquid microextraction for the determination of synthetic musk fragrances in aqueous matrices by gas chromatography–mass spectrometry. Talanta 148:84-93.
Hutter H-P, Wallner P, Moshammer H, Hartl W, Sattelberger R, Lorbeer G, Kundi M. 2009. Synthetic musks in blood of healthy young adults: Relationship to cosmetics use. Sci Total Environ. 407:4821-4825.
Hutter H-P, Wallner P, Hartl W, Uhl M, Lorbeer G, Gminski R, Mersch-Sundermann V, Kundi M. 2010. Higher blood concentrations of synthetic musks in women above fifty years than in younger women. Int. J Hyg Environ. Health 213:124-130.
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1996. Musk ambrette and musk xylene. IARC Monogr Eval Carcinog Risks Hum. 65:477-495.
[IFRA] International Fragrance Association. 2012. REACH exposure scenarios for fragrance substances. IFRA Operations, Brussels, Belgium. 126 pp.
Inoue Y, Hashizume N, Yoshida T, Murakami H, Suzuki Y, Koga Y,Takeshige R, Kikushima E,Yakata N, Otsuka M. 2012. Comparison of bioconcentration and biomagnification factors for poorly water-soluble chemicals using common carp (Cyprinus carpio L.). Arch Environ Contam Toxicol. 63:241-248.
Kim M, Guerra P, Theocharides M, Barclay K, Smyth SA, Alaee M. 2013. Polybrominated diphenyl ethers in sewage sludge and treated biosolids: effect factors and mass balance. Wat Res. 47:6496-6505.
[KOCWIN] Organic Carbon Partition Coefficient Program for Microsoft Windows [estimation model]. 2010. Ver. 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Kubwabo C, Fan X, Rasmussen PE. 2012. Determination of synthetic musk compounds in indoor dust by gas chromatography-ion trap mass spectrometry. Anal Bioanal Chem. 404:467-477.
Kuhlmann H, Rimkus GG, Butte W. 1997. Long-term bioconcentration and bioaccumulation of musk xylene and bromocyclen in rainbow trouts (Oncorhynchus mykiss). Private communication to the Food and Veterinary Institute of Schleswig-Holstein, Unpublished results. [cited in Käfferlein et al. 1998].
Lee I-S, Kim U-J, Oh J-E, Choi M, Hwang D-W. 2014. Comprehensive monitoring of synthetic musk compounds from freshwater to coastal environments in Korea: With consideration of ecological concerns. Sci Total Environ. 470-471: 1502-1508.
Lide DR. 2007. CRC Handbook of Chemistry and Physics 88th ed. Boca Raton (FL): CRC Press, Taylor & Francis, p. 3-78 [cited in HSDB 2012].
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2016 Aug 10]. Ottawa (ON): Health Canada. [accessed 2017 Jan 25].
Loretz LG, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, Rothenstein A, Scrafford CG, Sewall C. 2005. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol. 43: 279-291.
Luckenbach T, Epel D. 2005. Nitromusk and polycyclic musk compounds as long-term inhibitors of cellular xenobiotic defense systems mediated by multidrug transporters. Environ Health Perspect. 113(1):17-24.
Marks K, Marks P. 1987. Biodegradation of test substances (X0438.01R, musk xylene) and controls in activated sludge. Private communication to RIFM. Weston, USA. Project No. 87-009. [cited in EC 2010].
[MITI] Ministry of International Trade and Industry. 1992. MITI-List. Tokyo, Japan. [cited in EC 2005b].
[MSDS] Material Safety Data Sheet. 2015a. Red Door EDT Spray Naturel. Stamford (CT): Elizabeth Arden Inc. [accessed 2016 Jul 19]. [restricted access].
[MSDS] Material Safety Data Sheet. 2015b. Coronado Cherry Odor Eliminator Gel. Air freshener. Irvine (CA): California Scents. [accessed 2016 Jul 19].
Moon H-B, Lee D-H, Lee YS, Kannan K. 2012. Occurrence and accumulation patterns of polycyclic aromatic hydrocarbons and synthetic musk compounds in adipose tissues of Korean females. Chemosphere. 86:485-490.
Mottaleb MA, Zimmerman JH, Moy TW. 2008. Biological transformation, kinetics and dose-response assessments of bound musk ketone hemoglobin adducts in rainbow trout as biomarkers of environmental exposure. J Environ Sci. 20:878-884.
Nakata H, Hinosaka M, Yanagimoto H. 2015. Macrocyclic-, polycyclic-, and nitro musks in cosmetics, household commodities and indoor dusts collected from Japan: Implications for their human exposure. Ecotoxicol Environ Saf. 111:248-255.
[New EQC] New Equilibrium Criterion Model 2011. Ver. 1.00 (Beta). Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
[NHW] Department of National Health and Welfare. 1990. Present patterns and trends in infant feeding in Canada. Ottawa: NHW. Catalogue Number H39-199/1990E. ISBN 0-662-18397-5. 9 pp. [cited in Health Canada 1998].
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2017 Jan 10]. Ottawa (ON): Health Canada. [accessed 2017 Jan 25].
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme. 2016. Human Health Tier II Assessment for nitromusks. CAS RNs 81-14-1; 81-15-2; 116-66-5; 145-39-1. Inventory multi-tiered assessment and prioritisation (IMAP). Australian Government Department of Health and Ageing. [accessed 2016 July].
[OECD] Organisation for Economic Cooperation and Development. 2007. OECD Guideline for the Testing of Chemicals. Test Guideline No. 426: Developmental Neurotoxicity. Adopted 16 October 2007. 26 pp.
OECD QSAR Toolbox. 2016. Ver. 3.4. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Paradice AP, Suprenant DC. 1984. Accumulation and elimination 14C-residues by bluegill (Lepomis macrochirus) exposed to P1618.01R (musk xylene). Private communication to RIFM. [cited in Tas et al. 1997].
Peck AM, Hornbuckle KC. 2004. Synthetic musk fragrances in Lake Michigan. Environ Sci Technol. 38:367-372.
Peck AM, Hornbuckle KC. 2006. Synthetic musk fragrances in urban and rural air of Iowa and the Great Lakes. Atmos Environ. 40:6101-6111.
Peck AM, Kucklick JR, Schantz MM. 2007. Synthetic musk fragrances in environmental Standard Reference Materials. Anal Bioanal Chem. 387:2381-2388.
Raab U, Preiss U, Albrecht M, Shahin N, Parlar H, Fromme H. 2008. Concentrations of polybrominated diphenyl ethers, organochlorine compounds and nitro musks in mother’s milk from Germany (Bavaria). Chemosphere. 72:87-94.
Reiner JL, Wong CM, Arcaro KF, Kannan K. 2007. Synthetic musk fragrances in human milk from the United States. Environ Sci Technol. 41(11):3815-3820.
Riedel J, Dekant W. 1999. Biotransformation and toxicokinetics of musk xylene in humans. Toxicol Appl Pharmacol. 157(2):145-55.
[RIFM] Research Institute for Fragrance Materials. 2001. RIFM/FEMA database. Hackensack NJ 07601
Rimkus GG. 1999. Polycyclic musk fragrances in the aquatic environment. Toxicol Lett. 111:37-56.
Rimkus GG, Butte W, Geyer HJ. 1997. Critical considerations on the analysis and bioaccumulation of musk xylene and other synthetic nitromusks in fish. Chemosphere. 35(7):1497-1507.
[SCCS] Scientific Committee on Consumer Safety. 2004. Opinion of the scientific committee on cosmetic products and non-food products intended for consumers concerning musk xylene and musk ketone. Adopted by the SCCNFP during the 28th plenary meeting of 25 May 2004. Report no. SCCNFP/0817/04. 22 pp.
[SCCS] Scientific Committee on Consumer Safety. 2012. The SCCS’s Notes of Guidance for the Testing of Cosmetic Ingredients and their Safety Evaluation, 8th Revision.
Schnell S, Bols NC, Barata C, Porte C. 2009. Single and combined toxicity of pharmaceuticals and personal care products (PPCPs) on the rainbow trout liver cell line RTL-W1. Aquat Toxicol. 93:244-252.
Schramm KW, Kaune A, Beck B, Thumm W, Behechti A, Kettrup A, Nickolova P. 1996. Acute toxicities of five nitromusk compounds in daphnia, algae and photoluminescent bacteria. Wat Res. 30(10): 2247-2250.
Simonich SL, Begley WM, Debaere G, Eckoff WS. 2000. Trace analysis of fragrance materials in wastewater and treated wastewater. Environ Sci Technol. 34:954-965.
Simonich SL, Federle TW, Rottiers A, Webb S, Sabaliunas D, Wolf W. 2002. Removal of fragrance materials during US and European wastewater treatment. Environ Sci Technol. 36:2839-2847.
Smyth SA, Lishman LA, McBean EA, Kleywegt S, Yang J-J, Svoboda ML, Ormonde S, Pileggi V, Lee H-B, Seto P. 2007. Polycyclic and nitromusks in Canadian municipal wastewater: occurrence and removal in wastewater treatment. Water Qual Res J. Canada 42(3):138-152.
Smyth SA, Lishman LA, McBean EA, Kleywegt S, Yang J-J, Svoboda ML, Lee H-B, Seto P. 2008. Seasonal occurrence and removal of polycyclic and nitromusks from wastewater treatment plants in Ontario, Canada. Environ Eng Sci. 7:299-317.
Sofuoglu A, Kiymet N, Kavcar P, Sofuoglu SC. 2010. Polycyclic and nitro musks in indoor air: a primary school classroom and a women’s sport center. Indoor Air. 20:515-522.
Sousa V, Suprenant DC. 1984. Acute toxicity of P1618.02 (musk xylene) to bluegill (Lepomis macrochirus). Private communication to RIFM. Bionomics, USA. Report No. BW-84-2-1549. [cited in EC 2005b].
[TaPL3] Long Range Transport and Persistence Level III Model. 2003. Ver. 3.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
Tas JW, Balk F, Ford RA, van de Plassche EJ. 1997. Environmental risk assessment of musk ketone and musk xylene in the Netherlands in accordance with the EU-TGD. Chemosphere. 35(12):2973-3002.
Taylor M, Weisskopf M, Shine J. 2014. Human exposure to nitro musks and the evaluation of their potential toxicity: an overview. Environ Health. 13(14).
[US EPA] United States Environmental Protection Agency. 2000. Interim Guidance for Using Ready and Inherent Biodegradability Tests to Derive Input Data for Multimedia Models and Wastewater Treatment Plants (WWT) Models (9/1/2000).
[US EPA] US Environmental Protection Agency. [updated Aug 18 2016]. Endocrine Disruption Screening Program for the 21st Century (EDSP21). [accessed 2017 May 16].
[US EPA] US Environmental Protection Agency. [updated Dec 2 2016]. Toxicity Forecasting: ToxCast Dashboard. Washington (DC): US EPA. [accessed 2017 Apr 13].
[US EPA] United States Environmental Protection Agency. 2018. Chemview, Chemical Data Reporting [database on the Internet]. Updated 2018 Jan 1. [cited 2018 Jan 10].
Van Dijk A, Burri R. 1995. Accumulation and elimination of 14C-musk ketone by rainbow trout in a dynamic flow-through system. Report to RIFM. RCC Switzerland Project 379664. [cited in Tas et al. 1997].
Verhaar HJM, Van Leeuven C, Hermens JLM. 1992. Classifying environmental pollutants. 1: Structure-activity relationships for prediction of aquatic toxicity. Chemosphere. 25(4):471-491.
Wilson R, Jones-Otazo H, Petrovic S, Mitchell I, Bonvalot Y, Williams D, Richardson GM. 2013. Revisiting dust and soil ingestion rates based on hand-to-mouth transfer. Hum Ecol Risk Assess. 19(1):158-188.
Wollenberger L, Breitholtz M, Kusk KO, Bengtsson B-E. 2001. Inhibition of larval development of the marine copepod Acartia tonsa by four synthetic musk substances. Sci Total Environ. 305:53-64.
Yamagishi T, Miyazaki T, Horii S, Akiyama K. 1983. Synthetic musk residues in biota and water from Tama River and Tokyo Bay (Japan). Arch Environ Contam Toxicol. 12:83-89.
Yin J, Wang H, Zhang J, Zhou N, Gao F,Wu Y, Xiang J, Shao B. 2012. The occurrence of synthetic musks in human breast milk in Sichuan, China. Chemosphere. 87:1018–1023.
Yin J, Wang H, Li J, Wu Y, Shao B. 2016. Occurrence of synthetic musks in human breast milk samples from 12 provinces in China. Food Addit Contam Part A. 33(7):1219-1227.
Zarfl C, Scheringer M, Matthies M. 2011. Screening criteria for long-range transport potential of organic substances in water. Environ Sci Technol. 45:10075-10081.
Zhang X, Liang G, Zeng X, Zhou J, Sheng G, Fu J. 2011. Levels of synthetic musk fragrances in human milk from three cities in the Yangtze River Delta in Eastern China. J Environ Sci. 23:983–990.
Zhang X, Jing Y, Ma L, Zhou J, Fang X, Zhang X, Yu Y. 2015. Occurrence and transport of synthetic musks in paired maternal blood, umbilical cord blood, and breast milk. Int J Hyg Environ Health. 218:99-106.
Zhou J, Zeng X, Zheng K, Zhu X, Ma L, Xu Q, Zhang X, Yu Y, Sheng G, Fu J. 2012. Musks and organochlorine pesticides in breast milk from Shanghai, China: Levels, temporal trends and exposure assessment. Ecotoxicol Environ Saf. 84:325-333.
Appendix A. Aquatic toxicity data for MK, MX and their principal metabolites
Test organism |
Endpoint |
Valuea (mg/L) |
Reference |
Rainbow trout (Oncorhynchus mykiss) |
21 d LC50 |
> 0.50 |
Grutzner et al. 1995 |
Rainbow trout (O. mykiss) |
21 d NOEC (clinical signs) |
0.063 |
Grutzner et al. 1995 |
Rainbow trout (O. mykiss) |
21 d LOEC (clinical signs) |
0.13 |
Grutzner et al. 1995 |
Zebra fish (Danio rerio): (embryo larvae) |
48 h NOEC (heart rate) |
0.003 |
Carlsson and Norrgren 2004 |
Zebra fish (Danio rerio): (embryo larvae) |
48 h LOEC (heart rate) |
0.01 |
Carlsson and Norrgren 2004 |
Daphnid (Daphnia magna) |
48 h EC50 |
> 0.46 |
Schramm et. al. 1996 |
Daphnid (Daphnia magna) |
21 d EC50 (immobilization) |
0.34–0.68 |
Grutzner et al. 1995 |
Daphnid (Daphnia magna) |
21 d EC50 (reproduction) |
0.17–0.34 |
Grutzner et al. 1995 |
Daphnid (Daphnia magna) |
21 d NOEC (reproduction) |
0.17 |
Grutzner et al. 1995 |
Daphnid (Daphnia magna) |
21 d LOEC (reproduction) |
0.34 |
Grutzner et al. 1995 |
Marine copepod (Acartia tonsa) |
5 d EC50 (inhibition of larval development) |
0.066 |
Wollenberger et al. 2001 |
Marine copepod (Acartia tonsa) |
5 d EC10 (inhibition of larval development) |
0.01 |
Wollenberger et al. 2001 |
Brackish water copepod (Nitocra spinipes) |
22 d NOEC (larval development rate) |
0.01 |
Breitholtz et al. 2003 |
Brackish water copepod (Nitocra spinipes) |
22 d LOEC (larval development rate) |
0.03 |
Breitholtz et al. 2003 |
Green algae (Selenastrum capricornutum) |
72 h NOEC |
0.088 |
Grutzner et al. 1996 |
Green algae (Selenastrum capricornutum) |
72 h EbC50 |
0.12 |
Grutzner et al. 1996 |
Green algae (Selenastrum capricornutum) |
72 h ErC50 |
0.24 |
Grutzner et al. 1996 |
Green algae (Scenedesmus sub- spicatus) |
72 h EbC50 |
> 0.46 |
Grutzner et al. 1996 |
Abbreviations: LC50, the lethal concentration required to kill 50% of the population; d, day; EC, effect concentration; EC50, the concentration which induces a response halfway between the baseline and maximum; EbC50, the concentration at which 50% reduction of algal biomass is observed; ErC50, the concentration at which a 50% inhibition of algal growth rate is observed; h, hour; IC, inhibition concentration; LOEC, lowest observed effect concentration; NOEC; no effect concentration.
a In some studies, endpoint values are expressed in ppm, ppb, or µM/L. For consistency in the table, all such values were converted to mg/L.
Test organism |
Endpoint |
Value (mg/L) |
Reference |
Rainbow trout (Oncorhynchus mykiss): alevins |
96 h LC50 |
> 1000 |
Boleas et al. 1996 |
Rainbow trout (Oncorhynchus mykiss) |
24 h EC50 (liver cell line RTL-W1: cytotoxi-city) |
40.9–62.2 |
Schnell et al. 2009 |
Zebra fish (Danio rerio): embryo larvae |
48 h NOEC (heart rate) |
0.01 |
Carlsson and Norrgren 2004 |
Zebra fish (Danio rerio): embryo larvae |
48 h LOEC (heart rate) |
0.033 |
Carlsson and Norrgren 2004 |
Zebra fish (Danio rerio) |
96 h LC50 |
> 0.4 |
Chou and Deitrich 1999 |
Zebra fish (Danio rerio) |
48 h LC50 |
3.75 |
MITI 1992 |
Zebra fish (Danio rerio) |
14 d LC50 |
0.40 |
Adema and Langerwerf 1985 |
Zebra fish (Danio rerio) |
EC50 (embryo malformations) |
> 0.4 |
Chou and Deitrich 1999 |
Zebra fish (Danio rerio) |
EC50 (growth inhibition) |
> 0.4 |
Chou and Deitrich 1999 |
Bluegill sunfish (Lepomis macrochirus) |
24–72 h LC50 (calc.) |
> 0.78 |
Sousa and Suprenant 1984 |
Bluegill sunfish (Lepomis macrochirus) |
96 h LC50 (calc.) |
1.2 |
Sousa and Suprenant 1984 |
Carp (Cyprinus carpio) |
IC50 (Interference with metabolizing enzyme CYP1A: EROD activity) |
11 |
Schnell et al. 2009 |
Clawed frog (Xenopus laevis) |
96 h LC50 |
> 0.4 |
Chou and Deitrich 1999 |
Clawed frog (Xenopus laevis) |
96 h EC50 (growth inhibition) |
> 0.4 |
Chou and Deitrich 1999 |
Clawed frog (Xenopus laevis) |
96 h EC50 (embryo malformations) |
> 0.4 |
Chou and Deitrich 1999 |
Clawed frog (Xenopus laevis) |
11 d EC50 (growth inhibition) |
> 0.4 |
Chou and Deitrich 1999 |
Clawed frog (Xenopus laevis) |
11 d EC50 (embryo malformations) |
> 0.4 |
Chou and Deitrich 1999 |
Daphnid (Daphnia magna) |
48 h LC50 (est.) |
0.68 |
Adema and Langerwerf 1985 |
Daphnid (Daphnia magna) |
48 h EC50 |
> 0.79 |
Schramm et al. 1996 |
Daphnid (Daphnia magna) |
48 h EC50 |
> 1.0 |
Adema and Langerwerf 1985 |
Daphnid (Daphnia magna) |
48 h NOEC (swimming behavior) |
0.32 |
Adema and Langerwerf 1985 |
Daphnid (Daphnia magna) |
48 h NOEC (swimming behavior) |
0.32 |
Adema and Langerwerf 1985 |
Daphnid (Daphnia magna) |
21 d LC50 |
0.68 |
Adema and Langerwerf 1985 |
Daphnid (Daphnia magna) |
21 d NOEC (rep.) |
0.056 |
Adema and Langerwerf 1985 |
Green algae (Selenastrum capricornutum) |
5 d ECb50 |
> 10.0 |
Payne and Hall 1979 |
Green algae (Selenastrum capricornutum) |
5 d NOEC |
> 1.0 |
Payne and Hall 1979 |
Green algae (Scenedesmus subspicarus) |
72 h ECb50 |
> 0.15 |
Schramm et al. 1996 |
Blue-green algae (Microcystis aeruginosa) |
5 d NOEC |
> 10.0 |
Hughes and Krishnaswami 1985 |
Blue-green algae (Microcystis aeruginosa) |
5 d ECb50 |
> 1.0 |
Hughes and Krishnaswami 1985 |
Abbreviations: LC50, the lethal concentration required to kill 50% of the population; d, day; EC, effect concentration; EC50, the concentration which induces a response halfway between the baseline and maximum; EbC50, the concentration at which 50% reduction of algal biomass is observed; ErC50, the concentration at which a 50% inhibition of algal growth rate is observed; h, hour; IC, inhibition concentration; LOEC, lowest observed effect concentration; NOEC; no effect concentration.
a In some studies, endpoint values are expressed in ppm, ppb, or µM/L. For consistency in the table, all such values were converted to mg/L.
Metabolite abbreviation |
Species |
Endpoint |
Value (mg/L) |
Reference |
2-MK |
Fathead minnow |
96 h LC50 |
6.0 |
ACD/Percepta c1997-2012 |
2-MK |
Daphnid |
48 h LC50 |
0.931 |
ECOSAR 2012 |
2-MK |
Fish |
96 h LC50 |
2.883 |
ECOSAR 2012 |
2-MK |
Fish |
14 d LC50 |
0.133 |
ECOSAR 2012 |
2-MK |
Fathead minnow |
96 h LC50 |
29.34 |
AIEPS c2010-2012 |
2-MK |
Daphnia |
48 h LC50 |
18.35 |
AIEPS c2010-2012 |
2-MK |
Tetrahymena pyriformis |
ICG50 |
103 |
AIEPS c2010-2012 |
2-MX |
Fathead minnow |
96 h LC50 |
2.3 |
ACD/Percepta c1997-2012 |
2-MX |
Fish |
96 h LC50 |
0.587 |
ECOSAR 2012 |
2-MX |
Fathead minnow |
96 h LC50 |
323 |
AIEPS c2010-2012 |
2-MX |
Daphnia |
48 h LC50 |
8.58 |
AIEPS c2010-2012 |
2-MX |
Tetrahymena pyriformis |
ICG50 |
178 |
AIEPS c2010-2012 |
2-MX and 4-MX |
Daphnid |
48 h LC50 |
0.489 |
ECOSAR 2012 |
4-MX |
Fathead minnow |
96 h LC50 |
2.3 |
ACD/Percepta c1997-2012 |
4-MX |
Fathead minnow |
96 h LC50 |
29.34 |
AIEPS c2010-2012 |
4-MX |
Daphnia |
48 h LC50 |
8.58 |
AIEPS c2010-2012 |
4-MX |
Tetrahymena pyriformis |
ICG50 |
178 |
AIEPS c2010-2012 |
Abbreviations: LC50, the lethal concentration required to kill 50% of the population; d, day; h, hour; ICG50, the concentration required to inhibit population growth by 50%.
Appendix B. Upper-bounding estimates of daily intake (µg/kg bw/d) of MK and MX by various age groups within the general population in Canada
Route of exposure |
0–6 moa(breast milk fed)b |
0–6 moa (formula fed)c |
0–6 moa (not formula fed)d |
0.5–4 yre |
5–11 yrf |
12–19 yrg |
20–59 yrh |
60+ yri |
Ambient airj |
0.0007 |
0.0007 |
0.0007 |
0.0015 |
0.0012 |
0.0007 |
0.0006 |
0.0005 |
Indoor airk |
0.0001 |
0.0001 |
0.0001 |
0.0002 |
0.0002 |
0.0001 |
<0.0001 |
<0.0001 |
Drinking waterl |
N/A |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
Foodm |
0.86 |
N/A |
0.0004 |
0.10 |
0.082 |
0.047 |
0.045 |
0.029 |
Dustn |
0.0028 |
0.0028 |
0.0028 |
0.0015 |
0.0006 |
<0.0001 |
<0.0001 |
<0.0001 |
Soilo |
N/A |
N/A |
N/A |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
Total intake |
0.870 |
0.004 |
0.004 |
0.100 |
0.084 |
0.047 |
0.045 |
0.029 |
Abbreviations: N/A = not applicable; mo = months; yr = years.
a Assumed to weigh 7.5 kg, to breathe 2.1 m3 of air per day, to drink 0.8 L of water per day (formula fed) or 0.3 L/day (not formula fed, respectively (Health Canada 1998), and to ingest 38 and 0 mg of dust and soil per day, respectively (Wilson et al. 2013).
b Exclusively for breast-milk-fed infants, assumed to consume 0.742 L of breast milk per day (Health Canada 1998), and breast milk is assumed to be the only dietary source. The concentration for whole (breast) milk of 8.73 µg/L was based on the maximum value of 212 ng/g lipid weight x 4% lipid (average lipid concentration in breast milk) x 1.03 g/ml (density of breast milk) identified from 39 samples of human breast milk collected in 2004 from nursing women in Massachusetts, USA (Reiner et al. 2007).
c Exclusively for formula-fed infants, assumed to drink 0.8 L of water per day (Health Canada 1998), where water is used to reconstitute formula. No monitoring data on musk ketone in formula were identified; therefore dietary intakes are only those from water. See footnote on water for details.
d Exclusively for not formula-fed infants, assumed to drink 0.7 L of water per day (Health Canada 1998), and approximately 50% of non-formula-fed infants are introduced to solid foods by 4 months of age, and 90% by 6 months of age (NHW 1990).
e Assumed to weigh 15.5 kg, to breathe 9.3 m3 of air per day, to drink 0.7 L of water per day, to consume 54.7 g of fish per day, and 162.2 g of cereal products per day (Health Canada 1998), and to ingest 41 and 14 mg of dust and soil per day, respectively (Wilson et al. 2013).
f Assumed to weigh 31.0 kg, to breathe 14.5 m3 of air per day, to drink 1.1 L of water per day, to consume 89.8 g of fish per day, and 290.1 g of cereal products per day (Health Canada 1998), and to ingest 31 and 21 mg of dust and soil per day, respectively (Wilson et al. 2013).
g Assumed to weigh 59.4 kg, to breathe 15.8 m3 of air per day, to drink 1.2 L of water per day, to consume 97.3 g of fish per day, and 320.9 g of cereal products per day (Health Canada 1998), and to ingest 2.2 and 1.4 mg of dust and soil per day, respectively (Wilson et al. 2013).
h Assumed to weigh 70.9 kg, to breathe 16.2 m3 of air per day, to drink 1.5 L of water per day, to consume 111.7 g of fish per day, and 248.4 g of cereal products per day (Health Canada 1998), and to ingest 2.5 and 1.6 mg of dust and soil per day, respectively (Wilson et al. 2013).
I Assumed to weigh 72.0 kg, to breathe 14.3 m3 of air per day, to drink 1.6 L of water per day, to consume 72.9 g of fish per day, and 229.0 g of cereal products per day (Health Canada 1998), and to ingest 2.5 and 1.5 mg of dust and soil per day, respectively (Wilson et al. 2013).
j No monitoring data of ambient air in Canada were identified. The maximum value recorded in Milwaukee, Wisconsin (0.000141 gas phase + 0.020 particle phase µg/m3 = 0.0201 µg/m3) was used for deriving upper-bounding estimates of daily intake for ambient air exposure. Concentration of musk ketone from this urban location was considered to be representative for Canada. Canadians are assumed to spend 3 hours outdoors each day (Health Canada 1998).
k No monitoring data of indoor air in Canada were identified. The 95th percentile concentration of musk ketone (0.00044 µg/m3) measured in indoor air samples from a school classroom in Turkey (Sofuoglu et al. 2011) was selected for deriving upper-bounding estimates of daily intake for indoor air exposure. Canadians are assumed to spend 21 hours indoors each day (Health Canada 1998).
l No monitoring data of drinking water in Canada were identified. The 95th percentile concentration of musk ketone (0.000133 µg/L) measured in Lake Michigan surface water during 1999-2000 (Peck and Hornbuckle 2004) was selected for deriving upper-bounding estimates of daily intake for drinking water exposure.
m No monitoring data on marketed foods in Canada were identified. However, sufficient environmental fish data and limited vegetables data from Europe were available. The concentration of 28.34 µg/kg ("fresh" or "wet" weight) was based on a maximum mean concentration of 659 µg/kg lipid wt X 4.3% lipid in 9 pooled samples of Barbel from the Moldau River, Czech Republic, during 1998-2000. Ranges for all species sampled were not stated in Hajslova and Setkova (2004), but the Barbel value was the highest mean of all species sampled during 1997-2000 (Bream: 412 µg/kg lipid wt X 3.2% lipid = 13.18 µg/kg; Chub: 222 µg/kg lipid wt X 1.9% lipid = 4.22 µg/kg; Trout: 200 µg/kg lipid wt X 1.6% lipid = 3.20 µg/kg; Perch: 296 µg/kg lipid wt X 0.7% lipid = 2.07 µg/kg). A 95th percentile value of 0.036 µg/kg in lettuce bought from a supermarket in Bilbao, Spain, on or before 2013 was also included. Carrot and pepper from the same supermarket were also analyzed but musk ketone was not detected (Aguirre et al. 2014). Amounts of foods consumed on a daily basis by each age group over 12 food groups were obtained from the 1970-1972 Nutrition Canada Survey (Health Canada 1998).
n The maximum concentration of musk ketone (559 ng/g) in house dust from 13 cities across Canada during 2007-2010 (Kubwabo et al. 2012) was selected for deriving upper-bounding estimates of daily intake for dust exposure.
o No soil concentration data for Canada were identified. The maximum concentration of 1.57 µg/kg in soil from wastewater treatment plant effluent applied via irrigation to a land application site in Lubbock, Texas, during 2009-2010 (Chase et al. 2012) was selected for deriving upper-bounding estimates of daily intake for soil exposure.
Route of exposure |
0–6 moa (breast milk fed)b |
0–6 moa (formu-la fed)c |
0–6 moa (not formula fed)d |
0.5–4 yre |
5–11 yrf |
12–19 yrg |
20–59 yrh |
60+ yri |
Ambient airj |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
Indoor airk |
0.0038 |
0.0038 |
0.0038 |
0.0081 |
0.0063 |
0.0036 |
0.0031 |
0.0027 |
Drinking waterl |
N/A |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
Foodm |
0.61 |
N/A |
N/A |
0.0046 |
0.0038 |
0.0021 |
0.0020 |
0.0013 |
Dustn |
0.011 |
0.011 |
0.011 |
0.0056 |
0.0021 |
<0.0001 |
<0.0001 |
<0.0001 |
Soilo |
N/A |
N/A |
N/A |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
Total intake |
0.630 |
0.015 |
0.015 |
0.018 |
0.012 |
0.006 |
0.005 |
0.004 |
Abbreviations: N/A = not applicable; mo = months; yr = years.
a Assumed to weigh 7.5 kg, to breathe 2.1 m3 of air per day, to drink 0.8 L of water per day (formula fed) or 0.3 L/day (not formula fed, respectively (Health Canada 1998), and to ingest 38 and 0 mg of dust and soil per day, respectively (Wilson et al. 2013).
b Exclusively for breast milk-fed infants, assumed to consume 0.742 L of breast milk per day (Health Canada 1998), and breast milk is assumed to be the only dietary source. The concentration for whole (breast) milk of 6.18 µg/L was based on the maximum value of 150 ng/g lipid weight x 4% lipid (average lipid concentration in breast milk) x 1.03 g/ml (density of breast milk) identified from 39 samples of human breast milk collected in 2004 from nursing women in Massachusetts, USA (Reiner et al. 2007).
c Exclusively for formula-fed infants, assumed to drink 0.8 L of water per day (Health Canada 1998), where water is used to reconstitute formula. No monitoring data on musk xylene in formula were identified; therefore dietary intakes are only those from water. See footnote on water for details.
d Exclusively for not formula-fed infants, assumed to drink 0.7 L of water per day (Health Canada 1998), and approximately 50% of non-formula-fed infants are introduced to solid foods by 4 months of age, and 90% by 6 months of age (NHW 1990).
e Assumed to weigh 15.5 kg, to breathe 9.3 m3 of air per day, to drink 0.7 L of water per day, to consume 54.7 g of fish per day, and 162.2 g of cereal products per day (Health Canada 1998), and to ingest 41 and 14 mg of dust and soil per day, respectively (Wilson et al. 2013).
f Assumed to weigh 31.0 kg, to breathe 14.5 m3 of air per day, to drink 1.1 L of water per day, to consume 89.8 g of fish per day, and 290.1 g of cereal products per day (Health Canada 1998), and to ingest 31 and 21 mg of dust and soil per day, respectively (Wilson et al. 2013).
g Assumed to weigh 59.4 kg, to breathe 15.8 m3 of air per day, to drink 1.2 L of water per day, to consume 97.3 g of fish per day, and 320.9 g of cereal products per day (Health Canada 1998), and to ingest 2.2 and 1.4 mg of dust and soil per day, respectively (Wilson et al. 2013).
h Assumed to weigh 70.9 kg, to breathe 16.2 m3 of air per day, to drink 1.5 L of water per day, to consume 111.7 g of fish per day, and 248.4 g of cereal products per day (Health Canada 1998), and to ingest 2.5 and 1.6 mg of dust and soil per day, respectively (Wilson et al. 2013).
I Assumed to weigh 72.0 kg, to breathe 14.3 m3 of air per day, to drink 1.6 L of water per day, to consume 72.9 g of fish per day, and 229.0 g of cereal products per day (Health Canada 1998), and to ingest 2.5 and 1.5 mg of dust and soil per day, respectively (Wilson et al. 2013).
j No monitoring data of ambient air in Canada were identified. The maximum value recorded in Iowa City, Iowa (0.00013 gas phase µg/m3) was used for deriving upper-bounding estimates of daily intake for ambient air exposure. Concentration of musk xylene from this urban location was considered to be representative for Canada. Canadians are assumed to spend 3 hours outdoors each day (Health Canada 1998).
k No monitoring data of indoor air in Canada were identified. The 95th percentile concentration of musk xylene (0.01541 gas phase + 0.00009 particle phase = 0.0155 µg/m3) measured in indoor air samples from a school classroom in Turkey (Sofuoglu et al. 2011) was selected for deriving upper-bounding estimates of daily intake for indoor air exposure. Canadians are assumed to spend 21 hours indoors each day (Health Canada 1998).
l No monitoring data of drinking water in Canada were identified. The 95th percentile concentration of musk xylene (0.00007 µg/L) measured in Lake Michigan surface water during 1999-2000 (Peck and Hornbuckle 2004) was selected for deriving upper-bounding estimates of daily intake for drinking water exposure.
m No monitoring data on marketed foods in Canada were identified. However, sufficient environmental fish data from Europe were available. The concentration of 1.3 µg/kg "fresh" or "wet" weight was based on a maximum concentration in 87 pooled samples of Trout from Denmark during 2003-2004 (Duedahl-Olesen et al. 2005). A 95th percentile value of 0.036 µg/kg in lettuce bought from a supermarket in Bilbao, Spain on or before 2013 was also included. Carrot and pepper from the same supermarket were also analyzed but Musk ketone was not detected (Aguirre et al. 2014). [Although Aguirre et al. (2014) analyzed musk ketone in 3 different vegetables from Spain, they did not analyze for musk xylene in these vegetables]. Amounts of foods consumed on a daily basis by each age group over 12 food groups were obtained from the 1970–1972 Nutrition Canada Survey (Health Canada 1998).
n The maximum concentration of musk xylene (2130 ng/g) in house dust from 13 cities across Canada during 2007-2010 (Kubwabo et al. 2012) was selected for deriving upper-bounding estimates of daily intake for dust exposure.
o No soil concentration data for Canada were identified. Musk xylene was not detected in soil from WWTP effluent applied via irrigation to a land application site in Lubbock, Texas during 2009-2010 (Chase et al. 2012). One half the detection limit from that study (0.015 ng/g) was selected for deriving upper-bounding estimates of daily intake for soil exposure.
Appendix C. Estimated potential exposures to substances in the Nitro Musks Group
Sentinel exposure scenarios were used to estimate the potential exposure to substances in the Nitro Musks Group; scenario assumptions are summarized in Table C-1. Exposures were estimated on the basis of the assumed weight (70.9 kg) of an adult (Health Canada 1998), inhalation rate of an adult (16.2 m3/day) and use behaviours of an adult, unless noted otherwise. Exposures were estimated using ConsExpo Web version or algorithms from the model (ConsExpo Web 2016). For estimated potential exposures via the dermal route, dermal absorption was 14% for MK and 10% for MX (See section 8.1). An overall retention factor of 1 was used unless otherwise specified.
Exposure scenario |
Assumptions |
Air freshener – Adult (aged 20+ years) and Child (aged 0.5 to 4 years) |
Concentration of MX: 0.07% (NICNAS 2016) Exposure to vapour, instantaneous release Exposure duration: 24 hrs (expert judgment) Product amount: 5.4 g (expert judgment) Room volume: 20 m3 (IFRA 2012) Ventilation rate: 0.6 per hour (ConsExpo Web 2016) Inhalation rate: 16.2 m3/day (Adult; Health Canada 1998) and 9.3 m3/day (Child; Health Canada 1998) Body weight of child: 15.5 kg (Health Canada 1998) |
Hair conditioner |
Concentration of MK: 1% (personal communication, email from Consumer Product Safety Directorate [CPSD], Health Canada, to Existing Substance Risk Assessment Bureau [ESRAB], Health Canada, dated November 2016; unreferenced)
Direct product contact, instant application Product amount: 13.13 g (Loretz et al. 2005) Frequency: 1.1 times per day (Loretz et al. 2005) Surface area: 1092.5 cm2 (Health Canada 1998) Retention factor: 0.01 (SCCS 2012) |
Fragrance (eau de toilette) |
Concentration of MK: 0.1% (expert judgment)
Inhalation: Exposure to spray, spraying Frequency: 1.7 time per day (Loretz et al. 2006) Inhalation rate: 16.2 m3/day (Health Canada 1998)
Dermal: Direct product contact, instant application Surface area: 100 cm2 (ConsExpo Web 2016) Product amount: 0.33 g (Loretz et al. 2006) |
Body lotion |
Concentration of MX 0.1% (expert judgment) Frequency: 1.1 times per day (Loretz et al. 2005)
Dermal: Direct product contact, instant application Product amount: 4.4 g (Loretz et al. 2005) Surface area: 16 925 cm2 (Health Canada 1998) |
