Draft screening assessment - Parabens group
Official title: Draft screening assessment - Parabens group
Chemical Abstracts Service Registry Numbers: 94-13-3, 94-18-8, 94-26-8, 99-76-3, 120-47-8, 4191-73-5, 4247-02-3
Environment and Climate Change Canada
Health Canada
March 2020
Synopsis
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of seven substances referred to collectively as the Parabens Group. Substances in this group were identified as priorities for risk assessment as part of the Identification of Risk Assessment Priorities (IRAP) approach’s 2015 review on the basis of human health concerns. The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 1 ), their Domestic Substances List (DSL) names and their common names are listed in the table below.
| CAS RN | DSL name | Common name |
|---|---|---|
| 94-13-3 | Benzoic acid, 4-hydroxy-, propyl ester | Propylparaben |
| 94-18-8 | Benzoic acid, 4-hydroxy-, phenylmethyl ester | Benzylparaben |
| 94-26-8 | Benzoic acid, 4-hydroxy-, butyl ester | Butylparaben |
| 99-76-3 | Benzoic acid, 4-hydroxy-, methyl ester | Methylparaben |
| 120-47-8 | Benzoic acid, 4-hydroxy-, ethyl ester | Ethylparaben |
| 4191-73-5 | Benzoic acid, 4-hydroxy-, 1-methylethyl ester | iso-Propylparaben |
| 4247-02-3 | Benzoic acid, 4-hydroxy-, 2-methylpropyl ester | iso-Butylparaben |
According to information submitted in response to a survey under section 71 of CEPA, methylparaben was reported to be manufactured and imported in Canada in 2011 in volumes of 981 kg and 563 000 kg, respectively. In a separate survey, ethylparaben, propylparaben, butylparaben, iso-propylparaben, and iso-butylparaben were not reported to be manufactured in Canada above the reporting threshold of 100 kg, but were reported to be imported into Canada in 2016 at volumes of 4 000 kg, 8 500 kg, 100 to1 000 kg, 280 kg, and 230 kg, respectively. Benzylparaben was not reported to be imported or manufactured above threshold values in 2016.
Parabens are widely used as preservatives and fragrance ingredients in cosmetic products, such as moisturizers, make-up, toothpaste, hair/shaving products, and are used as antimicrobial preservatives, fragrance ingredients and flavour enhancers in natural health products (NHPs). Parabens are also used in pest control products, consumer products, and in prescription and non-prescription drugs. Methylparaben and propylparaben are permitted for use as preservatives in certain foods and beverages sold in Canada. Parabens are also naturally present in foods, such as berries, fruit, wine and vanilla.
The ecological risks of the substances in the Parabens Group were characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that uses multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food-web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence and long-range-transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances based on their hazard and exposure profiles. According to the outcome of the ERC analysis, substances in the Parabens Group are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from methylparaben, ethylparaben, propylparaben, butylparaben, benzylparaben, iso-propylparaben, and iso-butylparaben. It is proposed to conclude that methylparaben, ethylparaben, propylparaben, butylparaben, benzylparaben, iso-propylparaben, and iso-butylparaben do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Animals exposed to methylparaben in a repeat dose study showed clinical signs of ill-health, stomach erosion, spleen and thyroid atrophy, and mortality at the highest dose. Adverse effects were not reported in reproductive and prenatal developmental toxicity studies or in a study of male reproductive development. Predominant sources of exposure of the general population of Canada to methylparaben include cosmetics, NHPs, and prescription and non-prescription drugs. Margins of exposure based on biomonitoring data from the general population aged 3 to 79 years were considered adequate. Margins of exposure between the critical effect level and estimates of exposure to certain NHPs are considered potentially inadequate to account for uncertainties in the health effects and exposure databases.
Repeated exposure to ethylparaben at high doses resulted in depression, decreased motor activity and mortality in animal studies. Gestational exposure to ethylparaben resulted in enlargement of brain ventricles and hydronephrosis in fetuses. Prenatal development of the male reproductive system and male pubertal development were not affected by ethylparaben exposure. The general population of Canada is predominantly exposed to ethylparaben via cosmetics, NHPs, and non-prescription drugs. Margins of exposure based on biomonitoring data from the general population were considered adequate to address uncertainties in the health effects and exposure databases.
Propylparaben did not demonstrate significant adverse effects in repeat dose dietary toxicity studies. Adverse effects were not reported in a reproduction and developmental toxicity screen, or in studies of male and female pubertal and reproductive development. Predominant sources of exposure of the general population of Canada to propylparaben include cosmetics, NHPs, and prescription and non-prescription drugs. Margins of exposure based on biomonitoring data from the general population were considered adequate to address uncertainties in the health effects and exposure databases. Margins of exposure between the critical effect level and estimates of oral exposure to certain NHPs (at the highest dose and frequency recommended in the directions of use) are considered inadequate to address uncertainties in the health effects and exposure databases.
The critical effect for butylparaben was prenatal development of the reproductive system. Gestational exposure to butylparaben was associated with delayed onset of puberty, altered morphology of reproductive organs and reduced sperm count and motility in offspring. The general population of Canada is predominantly exposed to butylparaben via cosmetics, NHPs, and non-prescription drugs. Margins of exposure based on biomonitoring data from the general population aged 3 to 79 years were considered adequate. Margins of exposure between critical effects and estimated of exposure to certain cosmetics, non-prescription drugs and NHPs are considered potentially inadequate to account for uncertainties in the health effects and exposure databases.
The health effects database for benzylparaben is limited. A read-across approach was employed to select a critical effect of prenatal reproductive development based on butylparaben. No sources of exposure of the Canadian population to benzylparaben were identified. However, a biomonitoring study reported that benzylparaben was identified in the urine of pregnant Canadian women. Margins of exposure based on biomonitoring data from Canada, the United States and Europe are considered adequate to address uncertainties in the health effects and exposure databases.
Repeated exposure to iso-propylparaben resulted in changes in serum histochemistry, as well as renal and hepatic effects. The predominant source of exposure to iso-propylparaben is via the use of cosmetics, NHPs, and non-prescription drugs. Margins of exposure between the critical effect level and estimates of exposure to iso-propylparaben are considered adequate to address uncertainties in the health effects and exposure databases.
The critical effect identified for iso-butylparaben was reduced sperm motility and reduced epididymal sperm count in young males after maternal dosing (gestational and postnatal). The predominant source of exposure to iso-butylparaben is via use of cosmetics, NHPs and non-prescription drugs. Margins of exposure between the critical effect level and estimates of exposure to cosmetics, non-prescription drugs and NHPs containing iso-butylparaben are considered potentially inadequate to address uncertainties in the health effects and exposure databases.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that methylparaben, propylparaben, butylparaben and iso-butylparaben meet the criteria under paragraph 64(c) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that ethylparaben, benzylparaben and iso-propylparaben do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that methylparaben, propylparaben, butylparaben and iso-butylparaben meet one or more of the criteria set out in section 64 of CEPA and that ethylparaben, benzylparaben and iso-propylparaben do not meet any of the criteria set out in section 64 of CEPA.
It is also proposed that methylparaben, propylparaben, butylparaben, and iso-butylparaben do not meet the persistence or bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
1. Introduction
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of seven substances referred to collectively as the Parabens Group to determine whether they present or may present a risk to the environment or to human health. The substances in this group were identified as priorities for risk assessment in the Identification of Risk Assessment Priorities (IRAP) 2015 review because of human health concerns (ECCC, HC 2015).
The ecological risks of substances in the Parabens Group were characterized using the ERC approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This draft screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to April 2018. Empirical data from key studies as well as results from models were used to reach proposed conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This draft screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The human health portions of this assessment have undergone external review and/or consultation. Comments on the technical portions relevant to human health were received from Dr. Philippa Darbre (University of Reading, UK), Dr. Kurunthachalam Kannan (State University of New York at Albany, N.Y.), Dr. Po-Chin Huang (National Institute of Environmental Health Sciences, Taiwan), and Chris Kirman and Dr. Sean Hays (both from Summit Toxicology). The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This draft screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precaution.Footnote 2 This draft screening assessment presents the critical information and considerations on which the proposed conclusions are based.
2. Identity of substances
The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 3 ), Domestic Substances List (DSL) names and common names for the individual substances in the Parabens Group are presented in Table 2‑1.
| CAS RN | DSL name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 94-13-3 | Benzoic acid, 4-hydroxy-, propyl ester (propylparaben) | 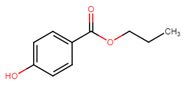 C10H12O3 C10H12O3 |
180.20 |
| 94-18-8 | Benzoic acid, 4-hydroxy-, phenylmethyl ester (benzylparaben) | 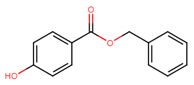 C14H12O3 C14H12O3 |
228.25 |
| 94-26-8 | Benzoic acid, 4-hydroxy-, butyl ester (butylparaben) | 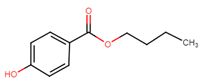 C11H14O3 C11H14O3 |
194.23 |
| 99-76-3 | Benzoic acid, 4-hydroxy-, methyl ester (methylparaben) | 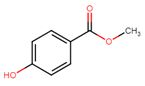 C8H8O3 C8H8O3 |
152.15 |
| 120-47-8 | Benzoic acid, 4-hydroxy-, ethyl ester (ethylparaben) | 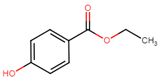 C9H10O3 C9H10O3 |
166.17 |
| 4191-73-5 | Benzoic acid, 4-hydroxy-, 1-methylethyl ester (iso-propylparaben) | 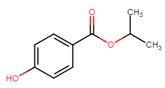 C10H12O3 C10H12O3 |
180.20 |
| 4247-02-3 | Benzoic acid, 4-hydroxy-, 2-methylpropyl ester (iso-butylparaben) | 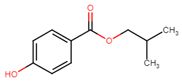 C11H14O3 C11H14O3 |
194.23 |
3. Physical and chemical properties
A summary of physical and chemical properties of the substances in the Parabens Group is presented in Table 3‑1 and Table 3‑2. Additional physical and chemical properties are reported in ECCC 2016b.
| Property | Methyl-paraben | Ethyl-paraben | Propyl-paraben | Butyl-paraben | Key reference(s) |
|---|---|---|---|---|---|
| Melting point (°C), exp. | 127–128 | 116–117 | 96–97 | 68–69 | Grant et al. 1984; Dymicky and Hutanen 1979 |
| Boiling point (°C), exp. | 275 (decompn.) | 297.5 | N/A | N/A | EPI Suite c2000-2012 |
| Vapour pressure (mm Hg, 25 °C), est.a | 2.37 × 10–4 | 9.29 × 10–5 | 5.55 × 10–4 |
1.86 × 10–4 |
EPI Suite c2000-2012 |
| Henry’s law constant (atm·m3/mol, 25 °C), est.b | 2.23 × 10–9 | 4.79 × 10–9 | 6.37 × 10–9 |
8.45 × 10–9 |
EPI Suite c2000-2012 |
| Water solubility (g/L, 25 °C), exp. | 2.5 | 0.75 | 0.50 | 0.17 | Dymicky and Hutanen 1979 |
| log KOW (dimensionless), exp. | 1.96 | 2.47 | 3.04 | 3.57 | Hansch 1995 |
| pKa (dimensionless), exp. | 8.17 | 8.22 | 8.35 | 8.37 | Dymicky and Hutanen 1979 |
Abbreviations: N/A, Not available; decompn., decomposition; est., estimated; exp., experimental.
a Calculated estimates of vapour pressure for the specified parabens are obtained using MPBPWIN software (EPISuite).
b Calculated estimates of Henry’s law constant are obtained using the HENRYWIN program (EPISuite) based on the bond contribution method of Meylan and Howard (1991).
| Property (unit), type | Benzylparaben | iso-Propylparaben | iso-Butylparaben | Key reference(s) |
|---|---|---|---|---|
| Melting point (°C),exp. | 111 | 86 | 73 | Cavill 1947; US EPA 2017 |
| Boiling point (°C), est.a | 355 | 276 | 291 | ChemSpider 2017a,b,c |
| Vapour pressure (mm Hg, 25 °C), est.a | 3.37 × 10–6 | 1.16 × 10–3 | 3.81 × 10–4 | EPI Suite c2000-2012 |
| Henry’s law constant (atm·m3/mol, 25 °C), est.b | 2.92 × 10–10 | 6.37 × 10–9 | 8.45 × 10–9 | EPI Suite c2000-2012 |
| Water solubility (mg/L, 25 °C), est.c | 1.07 × 102 | 6.90 × 102 | 2.24 × 102 | EPI Suite c2000-2012g |
| log KOW (dimensionless), est.d | 3.56 (exp.) | 2.91 | 3.4 | EPI Suite c2000-2012; Lehner 1993 |
Abbreviations: est., estimated; exp., experimental
a Calculated estimates of boiling point and vapour pressure for the specified parabens are obtained using MPBPWIN program (EPISuite).
b Calculated estimates of Henry’s law constants are obtained using the HENRYWIN program (EPISuite) based on the bond contribution method of Meylan and Howard (1991).
c Calculated estimates of water solubility are obtained using the WSKOWWIN program (EPISuite) based on the method of Meylan et al. (1996).
d Estimated values unless otherwise noted. Calculated estimates of log octanol–water partition coefficient are obtained using the KOWWIN program (EPISuite) based on the atom/fragment contribution method of Meylan and Howard (1995).
d Calculated estimates of log soil adsorption coefficient are obtained using the PCKOCWIN program (EPISuite).
4. Sources and uses
All of the substances in the Parabens Group have been included in surveys issued pursuant to a CEPA section 71 notice (Canada 2012, 2017). Table 4‑1 presents a summary of information reported on the total manufacture and total import quantities for the Parabens group. No Canadian manufacturing or import of benzylparaben was reported above the reporting threshold of 100 kg.
| Common name | Total manufacturea (kg) | Total importsa (kg) | Reporting year | Survey reference |
|---|---|---|---|---|
| Methylparaben | 981 | 563 190 | 2011 | Canada 2012 |
| Ethylparaben | Not reportedb | 4 029 | 2016 | Canada 2017 |
| Propylparaben | Not reportedb | 8 526 | 2016 | Canada 2017 |
| Butylparaben | Not reportedb | 100–1 000 | 2016 | Canada 2017 |
| iso-Propylparaben | Not reportedb | 284 | 2016 | Canada 2017 |
| iso-Butylparaben | Not reportedb | 232 | 2016 | Canada 2017 |
a Values reflect quantities reported in response to the surveys conducted under section 71 of CEPA (Canada 2012, 2017). See surveys for specific inclusions and exclusions (schedules 2 and 3).
b Not reported above the reporting threshold of 100 kg.
Table 4‑2 presents a summary of the major uses of the substances in the Parabens Group according to information reported pursuant to a CEPA section 71 surveys (Canada 2012, 2017).
| Common name | Personal carea | Natural healtha | Drugsa | Food and beveragea | Reporting year |
|---|---|---|---|---|---|
| Methylparaben | Yes | Yes | Yes | No | 2011 |
| Ethylparaben | Yes | No | Yes | No | 2016 |
| Propylparaben | Yes | Yes | Yes | Yes | 2016 |
| Butylparaben | Yes | No | Yes | No | 2016 |
| iso-Propylparaben | Yes | No | No | No | 2016 |
| iso-Butylparaben | Yes | No | No | No | 2016 |
a Non-confidential uses reported in response to the surveys conducted under section 71 of CEPA (Environment Canada 2013, 2017). See surveys for specific inclusions and exclusions (schedules 2 and 3).
Parabens are used in a wide variety of products. Members of this group may be used in Canada in food (additives), food packaging materials, prescription and non-prescription drugs, NHPs, cosmetics, products available to consumers and pest control products. Methylparaben, ethylparaben, propylparaben, and butylparaben are listed in the Personal Care Products Council Ingredient Database with reported functions of preservative and fragrance in a wide range of products; iso-propylparaben and iso-butylparaben have reported functions of preservative in a wide variety of products (PCPC 2018). Currently, there are no concentration restrictions for the use of parabens in cosmetic products in Canada. However, according to the European Commission Directive, methylparaben and ethylparaben are allowed to a maximum concentration of 0.4% (w/w) as single esters and a total maximum concentration of 0.8% for mixtures of esters (w/w). Butylparaben and propylparaben are allowed to a maximum concentration of 0.14% for the sum of the individual concentrations, and 0.8% for mixtures of methylparaben, ethylparaben, propylparaben and butylparaben. Benzylparaben, iso-propylparaben and iso-butylparaben are prohibited in cosmetics, in the EU (CosIng 2019). Parabens are restricted in Canada in NHPs to an oral upper limit of 10 mg/ kg bw/day exposure of the sum of methylparaben, ethylparaben, and propylparaben. Ethylparaben is also restricted to a total of 0.4% in topical products, and up to 0.8% for parabens in mixture. Methylparaben and propylparaben are listed in the Natural Health Products Ingredients Database (NHPID) with non-medicinal purposes of fragrance ingredient and preservative antimicrobial; butylparaben is listed with non-medicinal purposes of flavour enhancer and preservative antimicrobial; ethylparaben, benzylparaben, iso-propylparaben, and iso-butylparaben are listed with the non-medicinal ingredient purpose of preservative antimicrobial (NHPID 2019).
Based on notifications submitted under the Cosmetic Regulations to Health Canada from 2014 to 2017, methylparaben, ethylparaben, propylparaben, butylparaben, iso-propylparaben and iso-butylparaben are used in cosmetic products in Canada. Methylparaben, ethylparaben, propylparaben, butylparaben, and iso-butylparaben are used in a wide range of products including lotions, make-up, cleansers, oral care products, hair conditioner and shampoo. iso-Propylparaben is used in products including lotions, make-up, cleansers, hair colour, conditioner and shampoo. Methylparaben is the most prevalent in cosmetics, followed by propylparaben, ethylparaben, butylparaben, iso-butylparaben, and iso-propylparaben (internal data, Consumer Product Safety Directorate, Health Canada, dated August 18, 2017; unreferenced).
Methylparaben, ethylparaben, propylparaben, butylparaben, iso-propylparaben, and iso-butylparaben are reported as non-medicinal ingredients in non-prescription drug products. Methylparaben, ethylparaben, and propylparaben are also reported as non-medicinal ingredients in prescription drug products. Methylparaben is reported in prescription and non-prescription drug products including oral medications, intravenous/intramuscular medications, sunscreen, creams and ointments. Ethylparaben is reported in prescription and non-prescription drug products including oral medications, anti-infective wipes, sunscreen, medicated shampoo, balms and creams. Propylparaben is reported in prescription and non-prescription drug products including oral medications, intramuscular/intravenous medications, anti-infective wipes, sunscreen, medicated creams and ointments. Butylparaben is reported in non-prescription drug products including oral medications, anti-infective wipes, sunscreen, and medicated creams. iso-Propylparaben is reported in non-prescription drug products such as moisturizer and facial make-up that contain sunscreen. iso-Butylparaben is reported in non-prescription drug products, including acne treatments, sunscreen and medicated balms (personal communication, email from Therapeutic Products Directorate, Health Canada, to Consumer Product Safety Directorate, Health Canada, dated May 25, 2017; unreferenced).
Methylparaben, ethylparaben, propylparaben, butylparaben, iso-propylparaben, and iso-butylparaben are also reported as non-medicinal ingredients in NHPs, Methylparaben is reported in licensed NHPs, including oral medications and supplements, toothpastes, and sunscreen. Ethylparaben is reported in NHPs including oral traditional medicines, supplements, sunscreen, cleansers, medicated creams and ointments. Propylparaben is reported in NHPs including oral medications, supplements, oral care products, sunscreen, medicated creams and ointments. Butylparaben is reported in NHPs including cough medicines, traditional medicines, sunscreen, acne treatments, medicated creams and ointments. iso-Propylparaben is reported in one licensed NHP, an acne treatment. iso-Butylparaben is reported in licensed NHPs, including acne treatments, chemical peels, sunscreen and medicated creams (personal communication, emails from Natural and Non-Prescription Health Products Directorate, Health Canada, to Consumer Product Safety Directorate, Health Canada, dated February 20, 2017 and March 20, 2019; unreferenced).
Products available to consumers that contain methylparaben include cleaning wipes and children’s arts and craft supplies (washable markers and glue stick) (SDS 2010, 2008a, 2008b). Propylparaben was also identified in cleaning wipes (SDS 2010). No other parabens in this assessment were identified in products available to consumers.
Methylparaben has been identified as a component in the manufacture of food packaging materials, with no direct food contact, and as a component in incidental additivesFootnote 4 used in food processing establishments with potential food contact. Propylparaben has been identified as a component in the manufacture of food packaging materials, with and without direct food contact, and as a component in incidental additives used in food processing establishments with potential food contact. Methylparaben and propylparaben may also be used as a component in hand cleaners and skin products used by employees in food processing establishments followed by a potable water rinse treatment; therefore, contact with food is not expected. Ethylparaben and iso-butylparaben may be used as a component in hand cleaners used by employees in food processing establishments followed by potable water rinse treatment; therefore, contact with food is not expected.
Parabens are naturally occurring in foods, including berries, fruits, wine and vanilla (Soni et al. 2005). Methylparaben and propylparaben are included in the List of Permitted Preservatives and are each permitted as a preservative at up to 1 000 ppm in certain foods (personal communication, email from Food Directorate, Health Canada, to Consumer Product Safety Directorate, Health Canada, dated March 13, 2017; unreferenced).
Methylparaben, ethylparaben, propylparaben, and butylparaben are formulants in pest control products regulated under the Pest Control Products Act. They are used in Canada in a wide range of pest control products (personal communication, email from Pest Management Regulatory Agency, Health Canada, to Consumer Product Safety Directorate, Health Canada, dated March 13, 2017; unreferenced).
Benzylparaben is included in the NHPID, but is not present in licensed NHPs (personal communication, email from Natural and Non-Prescription Health Products Directorate, Health Canada, to Consumer Product Safety Directorate, dated February 20, 2017; unreferenced). According to notifications submitted under the Cosmetic Regulations to Health Canada from 2014 to 2017, benzylparaben is not present as an ingredient in cosmetic products in Canada. No other uses for benzylparaben were identified in Canada.
| Use | Methyl-paraben | Ethyl-paraben | Propyl-paraben | Butyl-paraben |
|---|---|---|---|---|
| Food additivea | Yes | No | Yes | No |
| Food packaging materialsa | Yes | No | Yes | No |
| Incidental additivesa | Yes | Yes | Yes | No |
| Internal Drug Product Database as medicinal or non-medicinal ingredients in disinfectant, human or veterinary drug products in Canadab | Yes | Yes | Yes | Yes |
| Natural Health Products Ingredients Databasec | Yes | Yes | Yes | Yes |
| Licensed Natural Health Products Database as medicinal or non-medicinal ingredients in natural health products in Canadad | Yes | Yes | Yes | Yes |
| Notified to be present in cosmetics, on the basis of notifications submitted under the Cosmetic Regulations to Health Canadae | Yes | Yes | Yes | Yes |
| Formulant in pest control products registered in Canadaf | Yes | Yes | Yes | Yes |
a Personal communication, email from Food Directorate, Health Canada, to Consumer Product Safety Directorate, Health Canada, dated March 13, 2017; unreferenced.
b DPD 2017.
c NHPID 2019.
d LNHPD 2018.
e Internal data, Consumer Product Safety Directorate, Health Canada, dated August 18, 2017; unreferenced
f Personal communication, email from Pest Management Regulatory Agency, Health Canada, to Consumer Product Safety Directorate, Health Canada, dated March 13, 2017; unreferenced.
| Use | Benzylparaben | iso-Propylparaben | iso-Butylparaben |
|---|---|---|---|
| Food additivea | No | No | No |
| Food packaging materialsa | No | No | No |
| Incidental additivea | No | No | Yes |
| Internal Drug Product Database as medicinal or non-medicinal ingredients in disinfectant, human or veterinary drug products in Canadab | No | Yes | Yes |
| Natural Health Products Ingredients Databasec | Yes | Yes | Yes |
| Licensed Natural Health Products Database as medicinal or non-medicinal ingredients in natural health products in Canadad | No | Yes | Yes |
| Notified to be present in cosmetics, on the basis of notifications submitted under the Cosmetic Regulations to Health Canadae | No | Yes | Yes |
| Formulant in pest control products registered in Canadaf | No | No | No |
a Personal communication, email from Food Directorate, Health Canada, to Consumer Product Safety Directorate, Health Canada, dated March 13, 2017; unreferenced.
b DPD 2017.
c NHPID 2019.
d LNHPD 2018.
e Internal data, Consumer Product Safety Directorate, Health Canada, dated August 18, 2017; unreferenced.
f Personal communication, email from Pest Management Regulatory Agency, Health Canada, to Consumer Product Safety Directorate, Health Canada, dated March 13, 2017; unreferenced.
5. Environmental fate and behaviour
5.1 Environmental persistence
According to models used in ERC (ECCC 2016b), substances in the Parabens Group are expected to degrade and not be persistent in water, air, sediment or soil.
5.2 Potential for bioaccumulation
Given their low Kow and low bioconcentration factors (ECCC 2016b), substances in the Parabens Group are not expected to significantly bioaccumulate in organisms.
6. Potential to cause ecological harm
6.1 Characterization of ecological risk
The ecological risks of the substances in the Parabens Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration [LC50]) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox), and from responses to surveys conducted under section 71 of CEPA, or they were generated using selected quantitative structure-activity relationship (QSAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under-classification of hazard, exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC 2016a. The following describes two of the more substantial areas of uncertainty. Error with empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2016). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue (CBR) analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics, such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada based on what is considered to be the current use quantity and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for the substances in the Parabens Group, as well as the hazard, exposure and risk classification results, are presented in ECCC (2016b).
The hazard and exposure classifications for the seven substances in the Parabens Group are summarized in Table 6-1.
| Substance | ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| Methylparaben | low | low | low |
| Ethylparaben | low | low | low |
| Propylparaben | low | low | low |
| Butylparaben | low | low | low |
| Benzylparaben | low | low | low |
| iso-Propylparaben | low | low | low |
| iso-Butylparaben | low | low | low |
On the basis of low hazard and low exposure classifications according to information considered under ERC, methylparaben, ethylparaben, propylparaben, butylparaben, benzylparaben, iso-propylparaben, and iso-butylparaben were classified as having a low potential for ecological risk. According to structural alerts from the OECD Toolbox, propylparaben, butylparaben, benzylparaben, and iso-propylparaben are identified as being potential endocrine receptor binders. Endocrine disruption is further considered in some health effects sections, and Appendix C provides a review of some of the estrogenic effects of parabens. The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of these substances. It is therefore unlikely that these substances will result in concerns for the environment in Canada.
7. Potential to cause harm to human health
7.1 Methylparaben
7.1.1 Exposure assessment
Methylparaben is naturally occurring in some foods and has been identified in environmental media. Methylparaben is also present in a number of products, including cosmetics, prescription and non-prescription drugs, NHPs, limited products available to consumers, and pest control products. It may also be used as a food additive or as a component in food packaging materials. Many of these sources contribute to total daily exposure to methylparaben. Urinary concentrations and estimated exposures of Canadians to methylparaben are presented in the following section.
Biomonitoring
Biomonitoring data collected in Cycle 4 (2014-2015) of the Canadian Health Measures Survey (CHMS) indicated a geometric mean urinary concentration of 17 µg/L (95% confidence interval of 13 to 22 µg/L) of methylparaben in Canadians aged 3 to 79 years, based on spot sampling (n = 2 564) (Health Canada 2017a). Females (3 to 9 years; n = 1 289) had a higher geometric mean urinary concentration of 30 µg/L (95% confidence interval of 21 to 43 µg/L) compared to males (3 to 79 years, n = 1 275) with 9.4 µg/L (geometric mean, 95% confidence interval 6.9 to 13 µg/L). Among the different age groups, the group with the highest urinary concentration was adults aged 40 to 59 years (n = 312), with a geometric mean urinary concentration of 21 µg/L (95% confidence interval of 11 to 38 µg/L).Footnote 5 The age group with the lowest urinary concentration was children aged 6 to 11 years (n = 514), who had a geometric mean urinary concentration of 7.6 µg/L (95% confidence interval of 6.4 to 9.1 µg/L) (Health Canada 2017a). In the whole population (aged 3 to 79 years), 8.39% of samples were below the limit of detection (1.3 ng/mL; Health Canada 2017b); the highest proportion of samples below the limit of detection was 11.22%, was in adults aged 40 to 59 years. In two other, smaller scale Canadian studies, a mean urinary concentrations in females of 101.3 µg/L (n = 28 including 9 pregnant patients) and a geometric mean of 94.86 µg/L in pregnant females (n = 31 females); the medians were 25.45 and 27.21 µg/L, respectively (Genuis et al. 2013; Fisher et al. 2017). The mean urinary concentration in males (n = 11), reported by Genuis et al. (2013) was 95.53 µg/L (median of 25.95 µg/L). The difference in adult concentrations (either mean or geometric mean) reported by CHMS versus Genuis et al. (2013) and Fisher et al. (2017) may be due to differences in the population assayed (i.e., small local populations and a population of pregnant women versus a large nationally representative sample of the general population).
Methylparaben was detected at a median concentration of 97.0 µg/L (free plus conjugated paraben, 25 to 75 percentile range of 39.9 to 272.3 µg/L) in urine collected from Korean infants (n = 46) within 48 hours of delivery (Kang et al. 2013). Forty-one low birth weight neonates in the NICU had a geometric mean urinary concentration of 203 µg/L total methylparaben (Calafat et al. 2009), this geometric mean is higher than that reported for the youngest child populations in the CHMS (geometric mean (GM) of 12 µg/L total methylparaben for children aged 3 to 5 years) or NHANES (GM of 33.5 µg/L total methylparaben for children aged 6 to 11 years) (Health Canada 2017a; Calafat et al. 2010).
Methylparaben was detected in breast milk at a median concentration of 0.22 µg/L in 56 Canadian women (GM = 0.0672 µg/L, 95th percentile = 6.792 µg/L), 3 months post-partum (Fisher et al. 2017). Methylparaben was detected in placenta samples of 12 women from Barcelona (maximum = 11.77 ng/g fresh weight) and in amniotic fluid in 40 pregnant women in a study in India (GM = 8.01 µg/L) (Valle-Sistac et al. 2016; Shekhar et al. 2017). Methylparaben was detected in cord blood at a mean of 0.037 µg/L in 50 mother-child pairs in the United States (Towers et al. 2015). In a study by Mulla et al. (2015), 181 neonates who received medicines containing methylparaben as an excipient had a median blood concentration of 12 µg/L.
Age-specific biomonitoring equivalents for parabens were derived on the basis of previously published acceptable daily intakes (ADIs) (Aylward et al. 2017a). Using a similar methodology, estimated daily intakes were derived from CHMS biomonitoring data for the general population, pregnant females and neonates (Health Canada 2017a; Fisher et al. 2017; Kang 2017). In each of the three biomonitoring studies, total methylparaben (i.e., free and conjugated species) was measured in urine by HLPC, after treatment with β -glucuronidase and sulfatase. Neonates were included because the detection of methylparaben in cord blood, placenta and amniotic fluid indicates that methylparaben passes through the placenta and that exposure may occur in utero (Shekhar et al. 2017; Valle-Sistac et al. 2016; Towers et al. 2015). Urinary concentrations from Korean neonates were used in the absence of data from a Canadian population; this is considered a conservative choice because the 95th percentile urinary concentrations of methylparaben in the general Korean population exceed those of the general Canadian population (Honda et al. 2018).
In a study of human pharmacokinetics in response to oral exposure to methylparaben, butylparaben and iso-butylparaben, three adults (two males and one female) ingested 10 mg each (0.12 to 0.19 mg/kg bw) of radiolabelled paraben (Moos et al. 2016). The elimination half-life of methylparaben was 6.9 hours, and 83.4% of the applied dose was eliminated in urine in 24 hours as methylparaben, ring-hydroxylated methylparaben, para-hydroxybenzoic acid (PHBA) and para-hydroxyhippuric acid (PHHA, a glycine conjugate of PHBA). After 48 hours, 17.4% of the orally administered dose was recovered as total methylparaben (free, plus conjugated species; Moos et al. 2016). High recovery of the radiolabelled administered dose in urine indicates that this is the primary mode of excretion for methylparaben, conjugates and metabolites. Methylparaben administered via the oral route has a short half-life and is not the major metabolite recovered. PHBA (and PHHA) was detected as the main metabolite of all three parabens tested. However, PHBA is an unsuitable biomarker as it is produced by the hydrolysis of all parabens and does not reflect differences in the toxicokinetics of each compound (Ye et al. 2006; Wang et al. 2013b). Methylparaben, in contrast, is a specific biomarker of exposure, and the fractional urinary excretion (FUE) values reported among study participants showed little variation (ranging from 0.155 to 0.192). The use of an oral fractional urinary excretion value may under- or over-estimate dermal exposure, and the use of the parent paraben as a biomarker provides a less robust measure of exposure as it is not the major metabolite. Due to the widespread use of methylparaben, the use of the parent compound as a biomarker may be also be susceptible to contamination during sampling and analysis, which may over-estimate exposure (Aylward et al. 2017a).
Estimated daily intake values were calculated using the following equation (Saravanabhavan et al. 2014):
EDI = UER/ FUE
and;
UER = [UCCr * CER]/ BW;
where EDI is the estimated daily intake in µg/kg bw/day, UER is the urinary excretion rate in µg/kg bw/day, FUE is the fractional urinary excretion based on oral exposure, UCCr is the creatinine-adjusted urinary concentration in µg/g creatinine, CER is the creatinine excretion rate in mg/day, and BW is body weight in kg. The fractional urinary excretion value of 0.174, or 17.4%, for methylparaben was reported in Moos et al. (2016), and creatinine excretion rate was calculated using the Mage equation (Saravanabhavan et al. 2014).
Urinary concentration values used to calculate estimated daily intake were the 95th percentile 95% confidence interval values (or highest available values) for creatinine-adjusted urinary concentration (µg/g Cr) for each age group presented. Methylparaben was below the limit of detection in 8% of all samples. However, fully validated methods were used to measure methylparaben. A high level of variability was associated with CHMS biomonitoring data for methylparaben in age-stratified groups at the 95th percentile and, in some cases, for the geometric mean (Health Canada 2017a). This may be due to the use of spot sampling, which is subject to variability based on time of sampling or variations in methylparaben exposure (e.g., due to variations in personal care product usage) and may underestimate exposure due to the short half-life of methylparaben (Fisher et al. 2017). These uncertainties were addressed by selecting the upper bound of the 95th percentile 95% confidence interval as a conservative upper bound value for deriving estimated daily intake. This is considered a conservative estimate that addresses the variation inherent in spot sampling, as it has been demonstrated that spot sample concentrations of methylparaben at the 95th percentile were similar to the 95th percentile of the distribution of 24-hour composite void concentrations, and this principle is considered to be generally applicable to spot samples of parabens (Aylward et al. 2017b). Where this value was not reported for an age group, the upper bound value of the 95% confidence interval for the highest available value was used in its place. The use of CHMS biomonitoring data allows increased confidence in the estimated daily intakes as it is based on a large, nationally representative population.
Estimated daily intakes and key parameters are presented in Table 7‑1. The values calculated here for the Canadian population are similar to daily intakes of methylparaben calculated at the 95th percentile for an adult German population (ranging from 26.9 to 56.5 µg/kg bw/day), reported in Moos et al. (2017). See Appendix A for further details of the derivation of estimated daily intake values.
| Source, location | Age (years)a | CER (mg/day)b | UCCr, P95 (CI) (µg/g Cr)c | FUEd | EDI, P95 (CI) (µg/kg bw/day) |
|---|---|---|---|---|---|
| CHMS (Cycle 4, 2014-2015)c, Canada | 3–5 | 130 | 430 (200–660)e | 0.174 | 21 (10–32) |
| CHMS (Cycle 4, 2014-2015)c, Canada | 6–11 | 418 | 620 (340–890)f | 0.174 | 48 (26–69) |
| CHMS (Cycle 4, 2014-2015)c, Canada | 12–19 | 1182 | 370 (100–640)e | 0.174 | 42 (11–73) |
| CHMS (Cycle 4, 2014-2015)c, Canada | 20–59g | 1248 | 310 (130–490)e | 0.174 | 31 (13–50) |
| CHMS (Cycle 4, 2014-2015)c, Canada | 60–79 | 1017 | 620 (340–890) | 0.174 | 50 (28–72) |
| Fisher et al. 2017, Canada | Pregnant women | - | 403h | 0.174 | 46 |
| Kang et al. 2013, Korea | Neonates | 9.6 | 106i | 0.174 | 14 |
Abbreviations: CER, creatinine excretion rate; UCCr, creatinine-adjusted urinary concentration; P95, 95th percentile; CI, confidence interval; FUE, fractional urinary excretion; EDI, estimated daily intake.
a Age groups are defined on the basis of the age groups reported by CHMS (Health Canada 2017a).
b Creatinine excretion rate was calculated using the Mage equation [0.993*1.64 [140 – Age] (Wt^1.5 Ht^0.5)/1000]. See Appendix A for values used for age weight and height.
c Health Canada 2017a.
d Moos 2016.
e These values were associated with high sampling variability (i.e., coefficient of variation between 16.6% and 33.3%). Health Canada recommends that this data be used with caution (Health Canada 2017a).
f CHMS data for the 95th percentile in this age stratum was suppressed due to high variability and “Age 60 to 79 years” was used as a surrogate. Although the 95th percentile value for this group is not known, this approach is considered conservative because the value used to estimate daily intake is the highest reported 95th percentile value for methylparaben.
g The “20–39” and “40–59” year age groups are presented together. The higher reported 95th percentile value of the two groups is presented here.
h This value is the specific gravity-adjusted urinary paraben concentration (µg/L) at the 95th percentile; creatinine-adjusted values were not reported in Fisher et al. 2017. Confidence intervals were not reported. EDI was calculated using the following equation: EDI = (UC*UFR)/FUE (Saravanabhavan et al. 2014), where UC is the urinary concentration, UFR is the urinary flow rate (0.20 L/kg bw/day, Aylward et al. 2015) and FUE is the fractional urinary excretion.
i This value is the 75th percentile creatinine-adjusted urinary paraben concentration; the 95th percentile was not reported (Kang et al. 2013). Confidence intervals were not reported.
Environmental media
Methylparaben has been identified in agricultural soil and house dust in Canada (Viglino et al. 2011; Fan et al. 2010), and in drinking water (Blanco et al. 2009), agricultural, industrial and forestry soil (Perez et al. 2011; Nunez et al. 2008), outdoor air (Moreau-Guigon et al. 2016; Ramirez et al. 2010), indoor (residential) air (Laborie et al. 2016; Alliot et al. 2014; Moreau-Guigon et al. 2016) and house dust (Wang et al. 2012; Ramirez et al. 2011; Tran et al. 2016; Canosa et al. 2007) in other countries. Average estimates of daily intake of methylparaben by the general population based on international studies range from 0.002 to 0.011 µg/kg bw/day for all age groups from infants to adults over 60 years.
In the absence of Canadian monitoring data, exposure from water, soil and air was modelled using ChemCAN (ChemCAN 2003). Methylparaben was found to partition primarily to water and sediments. Modelled data indicate that general population exposure due to environmental media in Canada is negligible.
Food
Methylparaben (identified as methyl paraben, methyl-ρ-hydroxy benzoate) and its sodium salt (identified as sodium salt of methyl-ρ-hydroxy benzoic acid) are permitted for use as food additives (preservatives) in foods sold in Canada. The foods to which they may be added and their maximum levels of use in those foods are set out in Part 2 of the List of Permitted Preservatives (personal communication, email from the Food Directorate, Health Canada, to the Consumer Product Safety Directorate, Health Canada, dated April 18, 2018; unreferenced). However, these uses were approved many years ago, and the results of a 2017 Health Canada survey of current uses of methylparaben by the food industry suggest that use in food is limited. The main use in foods as a preservative is currently in certain colouring preparations or dispersions, which are subsequently incorporated into a limited number of food products, such as certain confectionery products, marinades and unstandardized beverages, including some flavoured and carbonated or concentrated (including frozen) beverages. Additionally, methylparaben and its sodium salt are currently used in enzyme preparations as preservatives and as antimicrobial agents to destroy the production organisms used to produce various enzymes, after fermentation. Any methylparaben levels present in the finished enzyme preparation would be low, such that it would have no function in the finished food (personal communication, email from the Food Directorate, Health Canada, to the Consumer Product Safety Directorate, Health Canada, dated April 18, 2018; unreferenced). For the general public (aged 1 to over 71 years), conservative estimates of dietary exposure to methylparaben from its use in certain colour preparations range from 0.013 to 0.073 mg/kg bw/day at the 90th percentile. Estimates of dietary exposure from its use in certain enzyme preparations range from 0.053 to 0.240 mg/kg w/day at the 90th percentile. Biomonitoring data (Fisher et al. 2017) indicate that breastfed infants are expected to be exposed to 98.04 ng methylparaben/kg bw/day (0.000098 mg/kg bw/day) via breast milk.Footnote 6
Cosmetics and products available to consumers
Methylparaben has been identified in a wide variety of cosmetics, including lotions, make-up, cleansers, oral care products, hair conditioner and shampoo as well as in cleaning wipes (SDS 2010), washable markers (SDS 2008a), and glue sticks (SDS 2008b). Exposures from daily use of cosmetics and products available to consumers were considered to be addressed by biomonitoring data. Due to the use of spot sampling and the short metabolic half-life of methylparaben, exposures from products with intermittent uses were potentially not addressed by biomonitoring data. Exposure estimates for intermittent uses that result in the highest levels of potential exposure to methylparaben by the dermal route, hereinafter referred to as sentinel scenarios, are presented in Table 7‑2.
For potential exposure by the dermal route, experimentally determined dermal absorption and metabolism coefficients from an in vitro study of dermal absorption and metabolism in human skin were used to estimate an internal dose (Charles River Laboratories 2018). The mean (plus two standard deviations) for each parameter (percent absorption or amount absorbed) was used to estimate the systemic dose resulting from dermal exposure to each paraben. If the mean plus two standard deviations exceeded the maximum measured value for that parameter, then the maximum was used to estimate dermal exposure. The proportion of applied paraben identified by HPLC in the receptor fluid fraction (pooled from fresh and frozen samples) was used to conservatively estimate the amount of applied paraben that remains after hydrolysis to PHBA in the skin. Further details of the study and of how results were applied to the modelling of systemic dose due to exposure by the dermal route are available upon request (Health Canada 2018b).
Potential exposures were estimated using conservative assumptions and default values. See Appendix B for details on default values and models used for generating exposure estimates. Exposure estimates for each scenario are expressed on a per-event and/or daily basis, depending on exposure frequency (see section 7.1.3, Characterization of risk to human health).
| Product scenario | Concentration in product (%) | Age group (years) | Dermal load (µg/cm2/24 h) | Systemic exposure (mg/kg bw/day) |
|---|---|---|---|---|
| Hair perm/ straightener (per event) |
1–3a | Child (5–11) |
124–372 | 0.44–1.33b,c |
| Hair perm/ straightener (per event) |
1–3a | Adult (> 20) |
125–376 | 0.21–0.62b,c |
a Internal data, Consumer Product Safety Directorate, Health Canada, dated August 18, 2017; unreferenced.
b Calculated using 40.80% dermal absorption.
c Calculated using a metabolism refinement of 44.97% methylparaben.
Drugs and NHPs
Methylparaben is present as a non-medicinal ingredient in prescription and non-prescription drugs and NHPs administered by multiple routes. Exposures from daily use of prescription and non-prescription drugs and NHPs are considered to be addressed by biomonitoring data. Sentinel scenarios from non-prescription drugs and NHPs with intermittent use patterns are presented in Table 7‑3. Oral and dermal exposures to methylparaben in prescription drugs were not addressed in this assessment as the level of methylparaben in Canadian-approved pharmaceutical products is within standard use and is considered in the risk-benefit paradigm of the Therapeutic Products Directorate. Default values and models used in exposure scenarios are presented in Appendix B.
| Product scenario | Amount in producta,b | Age group (years) |
Dermal load (µg/cm2/24 h) | Systemic exposure (mg/kg bw/day) |
|---|---|---|---|---|
| Anti-diarrheal medicationa (oral, per day) |
1.5%c | Toddler (0.5–4) |
N/A | 50.81 |
| Anti-diarrheal medicationa (oral, per day) |
3.0%c | Teen (12–19) |
N/A | 106.1 |
| Cough lozengea (oral, per day) | 5 mg/ lozengec | Child (5–11) |
N/A | 1.61 |
| Heartburn medicationa (oral, per day) |
1.14%c | Adult (> 20) |
N/A | 51 |
| Motion sickness medicationb (oral, per day) |
0.15%d | Toddler (0.5–4) |
N/A | 2.4 |
| Radiological contrast mediaa (oral, per event) | 0.05%c | Infants (0–0.5) |
N/A | 14.0 |
| Radiological contrast mediaa (oral, per event) | 0.05%c | Toddler (0.5–4) |
N/A | 29.0 |
| Radiological contrast mediaa (oral, per event) | 0.05%c | Adults (> 20) |
N/A | 6.35 |
| Sunscreena,b (dermal, per day) | 0.44%c,d | Toddler (0.5–4) |
17.16 | 1.21e,f |
| Sunscreena,b (dermal, per day) | 0.44%c,d | Adult (> 20) |
17.16 | 0.91e,f |
a Natural health product.
b Non-prescription drug.
c Personal communication, email from Natural and Non-Prescription Health Products Directorate, Health Canada, to Consumer Product Safety Directorate, Health Canada, dated February 20, 2017; unreferenced.
d Personal communication, email from Therapeutic Products Directorate, Health Canada, to Consumer Product Safety Directorate, Health Canada, dated May 25, 2017; unreferenced.
e Calculated using a maximum dermal amount of 8.50 µg/cm2/24 h.
f Calculated using a metabolism refinement of 44.97% methylparaben.
7.1.2 Health effects assessment
Methylparaben has been extensively reviewed, and key risk assessments have been conducted by the Cosmetics Ingredient Review (CIR) (Andersen 2008), the EU Scientific Committee for Consumer Safety (SCCP 2005a, 2005b, 2006, 2008; SCCS 2010, 2011, 2013), the European Food Safety Authority (EFSA 2004), the European Medicine Agency (EMA 2015), the Australian National Industrial Chemicals Notification and Assessment Service (NICNAS 2016), and independent scientists (Soni et al. 2005). The literature published until March 2017 was searched for relevant information to supplement the data used in the international reviews and assessments.
Toxicokinetics
Methylparaben is highly metabolized in animals by oral and dermal routes of exposure (Jones et al. 1956; Kiwada et al. 1979, 1980; Tsukamoto and Terada 1964; Aubert et al. 2012). In rats, methylparaben is absorbed at a higher level than propylparaben and butylparaben when administered by oral and dermal routes. A single radiolabeled dose of 100 mg/kg methylparaben was administered to rats by oral and dermal routes (Aubert et al. 2012). Maximum plasma concentrations (Cmax) were achieved in less than 1 hour and 8 hours, respectively, with oral and dermal dosing. All administration routes produced a single peak in the plasma, corresponding to that of PHBA, and methylparaben was not detected. Over 70% of the oral dose was excreted in 24 hours, with <1% detected in feces and <1% in tissues. Approximately 50% of the applied dermal dose of methylparaben was not absorbed after 24 hours, 14% to 26% was excreted in urine and <2% was excreted in feces; the remainder was thought to be in external tissues (e.g., hair, nails) (Aubert et al. 2012). When a single dose of methylparaben was administered orally (1 g/kg) or intravenously (50 mg/kg) to dogs, the parent compound was not detected in plasma, and PHBA was detected within 1 hour (Jones et al. 1956). The majority of the applied dose (66.1%) was excreted within 24 hours as PHBA and other metabolites; the parent compound was nearly undetectable (0.014%). As observed in rats, shorter-chain parabens were metabolized more rapidly and completely than longer-chain species (methylparaben > ethylparaben > propylparaben > butylparaben). Urinary excretion increased to 96% after 24 hours when dogs were fed 1 g/kg bw/day of methylparaben daily for 1 year. At sacrifice, small amounts of methylparaben were detected in the brain and spleen; PHBA was detected in all tissues (Jones et al. 1956).
In humans, parabens are eliminated rapidly in urine and recovered predominantly as hydrolyzed and conjugated isoforms. Methylparaben was not detected in the urine or plasma of a single male volunteer following oral administration of 70 mg/kg methylparaben; 50% of administered methylparaben was recovered within 12 hours, 11% was recovered as free PHBA (Jones et al. 1956). Moos et al. (2016) reports the ingestion of 10 mg of methylparaben by 3 adult volunteers (equivalent to 0.12 to 0.19 mg/kg bw). The elimination half-life was 6.9 hours, with 83.4% of the administered dose recovered in urine 24 hours. After 48 hours, 17.4% of the administered dose was recovered in urine as free and conjugated methylparaben. Approximately 64% of the administered dose was recovered as PHHA, the main metabolite. Ye et al. (2006), who reported on the urinary concentration of free and conjugated parabens in 100 adults, and Wang and Kannan (2013) and Wang et al. (2013), who reported similar results in children and adults, found that 95% to 98% of parabens recovered from urine were in conjugated form. The major isoform was sulphate-conjugated paraben, which accounted for 67% of recovered methylparaben (Ye et al. 2006). Although the majority of parabens appears to be rapidly eliminated from the body, methylparaben has been detected at low levels in tumorous breast tissue, human adipose tissue, and in the brain (Barr et al. 2012; Wang et al. 2015; van der Meer 2017). It was not reported whether the parabens were free or conjugated. In a human study, methylparaben was shown to accumulate in the stratum corneum of the forearm with daily application, but did not persist 48 hours after application ceased (Ishiwatari et al. 2007).
Ex vivo studies have shown that parabens are metabolized by carboxylesterases in human liver, skin, keratinocytes, subcutaneous fat, and blood, and by UDP-glucuronosyltransferases in liver microsomes. Hydrolysis in human liver cells is significantly greater than in skin cells, by approximately 2 orders of magnitude (Jewell et al. 2007b; Harville et al. 2007), but both are more efficient than plasma (Prusakiewicz et al. 2006). In human liver and skin subcellular fractions, methylparaben is hydrolyzed 2 to 10 times faster than propylparaben and butylparaben (Jewell et al. 2007a, 2007b; Harville et al. 2007; Lobemeier et al. 1996; Abbas et al. 2010; Prusakiewicz et al. 2006). In human plasma, methylparaben was stable after 24 hours. In contrast, propylparaben was reduced to 47% after 6 hours, and butylparaben and benzylparaben were reduced to half within 1 hour. Hydrolysis of methylparaben in liver microsomes is more rapid than in blood, with a half-life of 22 minutes, compared to 87 minutes for butylparaben (Abbas et al. 2010).
Unlike human kinetics, rat skin and liver cell fractions hydrolyze parabens at roughly the same rate in in vitro studies (Harville et al. 2007). However, rat skin cells hydrolyze parabens at a rate of 2 to 3 orders of magnitude higher than that of human skin cells, which is consistent with overall rates of hydrolysis by carboxylesterases in these species (Harville et al. 2007; Prusakiewicz et al. 2006). In these assays, hydrolysis in rat tissue also increases with increasing chain length, unlike humans. Shorter chain parabens (methylparaben and ethylparaben) are metabolized in human liver cell fractions at a rate comparable to that in rat liver. However, butylparaben was metabolized in rat liver at a rate 10 times greater than that in human liver (Harville et al. 2007).
Repeat dose studies
In a study compliant with OECD guideline 407 and Good Laboratory Practices (GLP), Wistar rats (5/sex/dose) were exposed to 0, 50, 250 and 1 000 mg/kg bw/day of methylparaben in propylene glycol by gavage for 28 days. An additional 5 animals in the control and high-dose groups were allowed 14 days of recovery. Two animals in the high-dose group were sacrificed due to ill health and showed slight erosion to the stomach and atrophy of the spleen and thymus. Additional signs in the sacrificed animals included lethargy, hunched posture, laboured respiration, rales, swelling of the abdomen, piloerection, diarrhea, ptsosis, hypothermia, dehydration and a lean appearance. Surviving animals in the high-dose group demonstrated piloerection and hunched posture. Animals in the high-dose group and one female in the mid-dose group also showed laboured respiration, rales and gasping. Spleen-to-body-weight ratio, and/or spleen weight were higher for all methylparaben-treated males at the end of treatment. These changes resolved by the end of the recovery period and were not supported by related histopathological findings. No other toxicologically significant changes were noted in observed parameters or functional observations, ophthalmoscopy, body weight, organ weight, food consumption, hematology parameters, estrous cycle and spermatogenesis (Beerens-Heijnen 2009; REACH 2018a). A no observed adverse effect level (NOAEL) of 250 mg/kg bw/day was selected for this study based on clinical signs and systemic toxicity at 1 000 mg/kg bw/day.
Similar effects were not reported in studies of dietary administration of methylparaben. Sixteen male Wistar rats per dose were administered methylparaben in diet at 0, 100, 1 000 or 10 000 ppm per day (equivalent to 11.2 ± 0.5, 110.0 ±3.3, 1141.1 ± 58.9 mg/kg bw/day, based on animal consumption and body weight) for 8 weeks beginning at postnatal day (PND) 22. Parameters evaluated included clinical signs, feed consumption, body weight, organ weights, and gross pathology of the thoracic, abdominal and pelvic viscera. None of the parameters evaluated showed compound- or dosage-dependent adverse effects. A statistically significant increase in mean relative liver weight was observed in the 10 000 ppm methylparaben group, although absolute liver weight was not significantly increased. One rat in the 10 000 ppm methylparaben exposure group was found dead on study day 31. Urine-stained abdominal fur occurred in 3 rats in the 10 000 ppm methylparaben exposure group (Hoberman et al. 2008). In a similar repeat dose study, 8 Crj:Wistar rats per group were administered methylparaben in diet at 0%, 0.1%, and 1.0% per day (102 ± 2.75 and 1 030 ± 38.0 mg/kg bw/day, calculated on the basis of food consumption) for 8 weeks starting at PND 25. No statistically significant differences in body weights were reported, and food consumption values were similar among all test groups (Oishi 2004). The NOAEL in each of the 8-week studies was the highest dose tested, 10 000 ppm or 1% (1 030 to 1 141 mg/kg bw/day).
Twelve weanling albino rats per dose were fed 2% or 8% methylparaben (equivalent to 900 to 1 200 and 5 500 to 5 900 mg/kg bw/day) in diet for 96 weeks. Animals receiving 8% methylparaben showed a slower weight gain than negative controls in early stages of the experiment. The effect was greater in males and diminished by the end of the experiment. At 2%, no signs of toxicity were observed in treated animals compared to controls. The NOAEL in this study was 2% (900 to 1 200 mg/kg bw/day; Matthews et al. 1956).
In a 13-week dermal toxicity study, albino rats (10/dose) were exposed to daily topical doses of 0.7% methylparaben in a medicated cream on anterior dorsal shaved skin that comprised 10% to 15% of total body surface area (equivalent to 4.12 g/kg bw of cream or 288 mg/kg bw methylparaben). A significant decrease in body weight was observed in males in the test group, and significant gross and histopathological changes were noted at the application site. Slight changes to hematologic and blood chemistry were not considered toxicologically significant, and the authors concluded that there were no cumulative systemic toxicity effects (CFTA 1981f, as cited in Andersen 2008). In a 3-month dermal toxicity study, a cosmetic product formulation containing 0.2% methylparaben was administered to groups of 5 male and 5 female rabbits at 6.6 or 11 mg/cm2 over 8.4% of the body surface area. The product caused erythema, edema and desquamation at the application site, but body weight gain, food consumption, hematologic and blood chemistry values, urinalysis values, and organ weights were negative for toxicologically significant changes, and there was no mortality in the test groups (CTFA 1980g, as cited in Andersen 2008).
Genotoxicity
Methylparaben is not mutagenic in Ames assays, with or without metabolic activation (REACH 2018a; Andersen 2008; Ishidate et al. 1984; Prival et al. 1982, 1991; Blevins and Taylor 1982; Kawachi and Yahagi 1980; Morita et al. 1981), but induced a significant number of chromosomal aberrations in Chinese hamster ovary (CHO) cells at 0.5 to 1.0 mg/mL (REACH 2018a; Ishidate et al. 1978). In human dermal fibroblasts, methylparaben had low cytotoxicity, but induced apoptosis and necrosis at a concentration of 1% (Carvalho et al. 2012). However, methylparaben was negative in in vivo genotoxicity assays (REACH 2018a; Andersen 2008; Kawachi and Yahagi 1980). Overall, the weight of evidence indicates that methylparaben is not likely to be genotoxic in vivo (EFSA 2004; Andersen 2008).
Carcinogenicity
Male Fischer 344 rats (8/group) were exposed to methylparaben at 4% in diet (approximately 4 000 mg/kg bw/day) for 9 days. Methylparaben did not induce any changes in histopathology or methyl-3[H]thymidine-labeling in the forestomach epithelium. (Rodrigues et al. 1986). The NOAEL for methylparaben was 4% (~4 000 mg/kg bw/day), the highest tested dose.
In a carcinogenicity assay, Fischer 344 rats (80/high dose, 60/high mid-level dose, 40/low mid-level dose, 20/low dose, 100/control group) were injected subcutaneously twice a week for 52 weeks with 0.6, 1.1, 2.0, and 3.5 mg/kg bw/day methylparaben. Weight gain and mortality in the methylparaben groups was similar to vehicle and negative control groups, and no test substance-related organ pathology was reported. The total number of tumour-bearing animals in the methylparaben groups was also in the same range as in control groups (methylparaben: 5/100 males, 17/100 females; negative control: 10/50 males, 12/50 females; vehicle control: 6/50 males, 14/50 females). In females treated with methylparaben (all doses) tumours were found at the injection site (1/100), mammary tissue (8/100), uterus (9/100) and other sites (7/50). In males, tumours were found at the injection site (2/100) and other sites (3/100). In the negative and vehicle controls, tumours were found in females at the injection site (0/50, 1/50, respectively), mammary tissue (1/50, 3/50, respectively), uterus (5/50, 5/50, respectively) and other sites (7/50, 8/50, respectively). The total tumour-bearing animals in the methylparaben-treated groups, from highest to lowest dose, was 11%, 18%, 8% and 25%, indicating a lack of dose-dependence (Mason et al. 1971). No statistical analysis was reported in this study, but the authors stated that tumour incidence in the treatment groups was not significantly different from the control group.
Reproductive and developmental effects
Potential estrogenic effects of parabens are reviewed in Appendix C.
A series of studies similar in design to OECD Test Guideline 414 (Prenatal Developmental Toxicity Study) were performed in rabbits, hamsters, rats and mice (REACH 2018a). Although similar to OECD TG 414, these studies predate GLP and OECD guidelines and did not include statistical analysis. Mated female Dutch-belted rabbits (9 to 11 animals per group) and mated female Golden hamsters (21 to 22 pregnant animals per group) were administered methylparaben by gavage at 0, 3, 14, 65 and 300 mg/kg bw/day from gestational day (GD) 6 to GD 18 (rabbits) and from GD 6 to GD 10 (hamsters). Mated female Wistar rats (22 to 24 pregnant animals per group) and mated female CD-1 mice (21 to 25 pregnant females per group) were administered methylparaben at 0, 5.5, 25.5, 118 and 550 mg/kg bw/day from GD 6 to GD 15. No effects on body weight, clinical signs, mortality or the genital tract of dams were observed at any dose in any of the studies. The number of corpora lutea, implantation sites per dam, resorption sites and live and dead fetuses were also not affected by the treatment. Sex ratio in pups and fetal body weight were not significantly altered by treatment; skeletal or soft tissue abnormalities were not observed in pups. The NOAEL for maternal and developmental effects for all four studies is the highest dose tested, 300 or 550 mg/kg bw/day.
In a study of female pubertal development, pre-pubertal Sprague Dawley rats (10 animals per group) were orally administered 0, 62.5, 250, 1 000 mg/kg bw/day of methylparaben in corn oil from PND 21 to 40. Ethinylestradiol (EE, 1 mg/kg bw/day) was administered as a positive control. A statistically significant delay in vaginal opening was observed in animals in the high-dose methylparaben group (PND 36.8 ± 1.96), compared to the vehicle control group (PND 33.6 ± 3.23); vaginal opening occurred at PND 21.4 ± 0.53 in the ethinylestradiol group (Vo et al. 2010). The biological significance of this effect is not clear, as historical controls have been reported to range from PND 33.0 to PND 36.6 (Stump et al. 2014). Vo et al. (2010) also reported that serum thyroxine (T4) levels were significantly reduced to 1.38 ± 0.07 ng/mL in the methylparaben high-dose group compared to 3.00 ± 0.32 ng/mL in the vehicle control and 2.73 ± 0.50 ng/mL in the EE control. A significant decrease in the number of 4-day estrous cycles was observed in the high-dose group, but the number of days of each stage in the 4-day cycle (proestrus, estrus, metestrus, and diestrus) was not significantly different. Significantly decreased ovary and increased adrenal, thyroid and liver weights were also observed in the high-dose methylparaben group. Qualitative histopathological changes occurred in the methylparaben mid-dose group, but were not observed at the high dose. No significant changes were observed in mean body weight between methylparaben and the vehicle control. However, a high level of variation was observed in body weight at the 1 000 mg/kg bw/day dose (110.3 ± 20.59 g, compared to 118.69 ± 6.4 g in the vehicle control; Vo et al. 2010). The sample size (10 animals per dose) is less than the number (15 per dose) recommended in the EPA Guideline for Female Pubertal Assays (2009). It has been reported elsewhere that in feed restriction studies, reductions of 9% and 12% in female body weight have resulted in alterations of the number of 4–5 day estrous cycles, significant reductions in ovary weights and a significant decrease in T4 levels (Stump et al. 2014). Differences in T4 levels may also be related to stage of the estrous cycle. Considering the variation in animal weight at the highest dose of methylparaben in combination with the reduced sample size, it is unclear whether the reported effects are secondary to reduced body weight or variation in a small sample. Furthermore, if the date of initiation of monitoring of the estrous cycle occurred at vaginal opening day, per the EPA Guideline for Female Pubertal Assays (2009), or if monitoring began mid-cycle, there would not have been sufficient monitoring time to observe two full estrous cycles before animal sacrifice on PND 41. It is also within normal variation for young animals to cycle abnormally and require up to 8 weeks for normal cycles to occur, leading the EPA to comment that estrous cyclicity may not be established within the duration of the pubertal assay, even in control animals.
Vo et al. (2010) reports a significant decrease in serum T4 level in pubescent female rats at 1 000 mg/kg/d methylparaben, yet the authors did not report a dose-dependent effect on T4 level with other parabens tested in the same study. This finding is inconsistent with two other studies of paraben effects on thyroid function. In an in vitro study, Taxvig et al. (2011) reported that methylparaben, ethylparaben and butylparaben were agonists of the thyroid receptor and, based on T3-induced proliferation of GH3 cells in an in vitro study, and determined that butylparaben and ethylparaben were more potent than methylparaben. In an epidemiological study, Koeppe et al. (2013) reported that methylparaben did not demonstrate a statistically significant association with thyroid hormone levels in the NHANES 2007-2008 population (although some associations in adult females neared significance). Ethylparaben, propylparaben and butylparaben each demonstrated statistically significant inverse associations with T3 and T4, yet their urinary concentrations were 1 to 2 orders of magnitude lower than that of methylparaben. The available evidence indicates that methylparaben has the least potent effect of any of the parabens tested, thus the absence of a dose-dependent effect on T4 levels in the other parabens tested in Vo et al. (2010) is problematic. Due to the concerns detailed here regarding the effects reported in this study (Vo et al. 2010), a NOAEL was not selected.
Two studies of male pubertal development were identified for methylparaben. Methylparaben was administered in diet to male Crj:Wistar rats (8 animals per group) at 0%, 0.1% and 1.0% (equivalent to 102 ± 2.75, and 1030 ± 38.0 mg/kg bw/day) for 8 weeks starting at PND 25 to 27. Treatment-related effects were not observed for reproductive organ weights (testes, epididymides, prostates, seminal vesicles and preputial glands) in any of the dose groups. Changes in testosterone, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels relative to controls were not observed at any dose level. Sperm counts in cauda epididymis and testis were normal, as were distribution of spermatogonia, spermatocytes, round spermatids and elongated spermatids in stage VII–VIII tubules. The NOAEL for this study was 1030 mg/kg bw/day, the highest dose tested (Oishi 2004). It should be noted that the SCCS (SCCP 2008; SCCS 2011) has expressed doubt as to the quality of this and other studies produced by the Oishi laboratory, which reported (i) mean values for some parameters that fell far outside the historical control ranges and (ii) standard deviations for parameters including epididymal sperm concentrations and testosterone levels that were far less than the normal biological variability that has been observed by other groups. The SCCS has concluded that the quality of the Oishi studies cannot be properly assessed as the full test description and the complete raw data packages were no longer available.
In a subsequent study, methylparaben was administered in the diet to male Wistar rats (15 to 16 animals per dose) at 0, 100, 1 000 and 10 000 ppm (equivalent to 0, 11.2 ± 0.5, 110.0 ± 3.3, and 1141.1 ± 58.9 mg/kg bw/day) for 8 weeks, from PND 22. Parameters evaluated included organ weights and histopathology of reproductive tissues, as well as sperm production, motility, and morphology. None of the parameters evaluated showed significant effects in the treatment groups compared to the control diet. No effect was observed on reproductive organ weights (testes, epididymides, ventral prostate, and seminal vesicles). A statistically significant increase was observed in mean relative liver weight in the 10 000 ppm group, although absolute liver weight was not significantly increased. No effect was observed on sperm motility, cauda epididymal or testicular sperm concentration, or daily sperm production. Methylparaben exposure resulted in a significantly higher incidence of abnormal sperm in the 1 000 ppm and 10 000 ppm exposure groups of 5.0 ± 3.1% and 4.0 ± 1.7%, compared to 2.3 ± 1.5% in the control group. These were mostly composed of sperm with no head. The authors stated that they did not consider the effect to be treatment-related, given the low incidence of these effects, lack of dose-dependence and the fact that no other reproductive parameters were altered by methylparaben (Hoberman et al. 2008). A NOAEL of 1 141 mg/kg bw/day, the highest dose tested, was identified for this study.
Epidemiology
A literature search was conducted to identify epidemiological data for methylparaben, with a focus on reproductive and endocrine effects. Studies were evaluated and scored for quality using the Newcastle-Ottawa Quality Assessment Scale (NOS) and the Quality Assessment Tool for Observational Cohort and Cross-sectional Studies from the National Heart, Lung, and Blood Institute of the National Institutes of Health (RSI 2018). Urinary concentrations of methylparaben have been assessed in epidemiology studies addressing fertility, reproductive health, hormone levels, child and adolescent growth and development, allergic sensitization and body weight. The majority of studies did not find an association between methylparaben levels and the endpoints of interest. Weak positive associations were identified for increased time-to-pregnancy, lower odds of live birth in intrauterine insemination, select hormone levels in pregnant females, growth rates in male neonates and toddlers, and allergic sensitization (Smarr et al. 2017; Dodge et al. 2015; Aker et al. 2016, 2018; Wu 2017; Philippat et al. 2014; Guo et al. 2017; Savage et al. 2012; Spanier et al. 2014; Lee-Sarwar et al. 2017; Vernet et al. 2017). A weak inverse association with body mass index (BMI) and body weight was detected in adolescents, pregnant women, and adults (Den Hond et al. 2013; Smith et al. 2012; Kang et al. 2016; Polinski et al. 2018), and a weak inverse association was found between methylparaben levels in cord blood and fetal testosterone levels (Kolatorova et al. 2018). Other than growth rates in neonates and toddlers and fetal testosterone levels, different studies of similar quality gave conflicting results for these or similar endpoints. More detail is provided in Health Canada (2018a).
7.1.3 Characterization of risk to human health
A NOAEL of 250 mg/kg bw/day was reported for oral (gavage) exposure to methylparaben in a 28-day repeat dose study based on clinical signs of ill-health, stomach erosion, spleen and thyroid atrophy, and mortality at 1 000 mg/kg bw/day (Beerens-Heijnen 2009). Similar effects were not reported in dietary repeat dose studies with dosing up to 1 140 mg/kg bw day (Hoberman et al. 2008; Oishi 2004; Matthews et al. 1956). The weight of evidence indicates that methylparaben is not genotoxic. A carcinogenesis study (Mason et al. 1971) indicated that methylparaben is not carcinogenic. However, the dose level of 3.5 mg/kg bw/day may have been too low to detect an effect.
Vo et al. (2010) have reported several effects on female pubertal development at 1 000 mg/kg/d methylparaben. As discussed above, the reported effects are problematic for several reasons. A high level of variation was reported in the body weight of the high-dose group, and the sample size per dose (n = 10) was lower than that recommended by the EPA Guideline for Female Pubertal Assays (2009). As it has been reported that many of the effects reported for methylparaben at the high dose are also associated with reduced body weight, the biological relevance of these effects was not clearly established. In addition to this, the lack of similar effects reported for other parabens, particularly with respect to serum thyroid hormone levels contradicts the prevailing trend identified in other studies. Results from in vitro and epidemiology studies indicate a gradient of effect of parabens on thyroid function, where effects seen in response to methylparaben exposure are of a significantly lower magnitude than exposure to longer chain parabens, such as butylparaben (Taxvig et al. 2011; Koeppe et al. 2013). Other jurisdictions have reported mixed opinions on this study. The European Medical Association (EMA 2015) concluded that no consistent effects were observed with methylparaben up to the highest dose tested. The SCCS has noted the key results related to methylparaben are effects on organ weights, and concluded that this study could not be used to determine a NOAEL as it is not a guideline study and effects were not dose related (SCCS 2010, 2013). RIVM selected a NOAEL of 250 mg/kg bw/day based on delayed vaginal opening, estrous cycle length and organ weights (RIVM 2017), but states that it partially agrees with the SCCS and further study of the reported effects are needed. Health Canada concurs with the SCCS and the EMA that this study does not provide an appropriate endpoint for selection of a quantitative point of departure.
In a series of prenatal developmental toxicity studies, gestational exposure of methylparaben up to the highest tested dose of 550 mg/kg bw/day did not result in adverse effects (REACH 2018a). Two studies of male pubertal development also did not identify any effects of methylparaben up to the highest tested dose (Oishi 2004; Hoberman et al. 2008). However, the SCCS has identified significant concerns with the results of the Oishi study. The NOAEL for male reproductive effects is therefore 1141 mg/kg bw/day, the highest tested dose from the higher quality study. Hoberman et al. (2008) also reported toxicological endpoints of a repeat dose study (clinical signs, feed consumption, body weight, organ weights, and gross pathology of the thoracic, abdominal and pelvic viscera) and found no significant effects at the highest dose. A point of departure of 250 mg/kg bw/day, based on systemic toxicity in a repeat dose study, was selected for methylparaben and is expected to be protective of reproductive effects.
The Canadian population is exposed to methylparaben via cosmetics, prescription and non-prescription drugs, NHPs, food, and limited products available to consumers. Monitoring data available for some environmental media sources and worldwide data suggest that Canadians are also exposed to methylparaben via these sources.
The assessment of general population exposure to methylparaben is based on biomonitoring data from the CHMS, the Plastics and Personal-Care Product Use in Pregnancy (or P4) Study, and a study of Korean neonates within 48 hours of birth (Health Canada 2017a; Fisher et al. 2017; Kang et al. 2013). Biomonitoring data provide actual internal measures of exposure because they include specific measurements of methylparaben in urine. They are considered reliable estimates of urinary concentration of methylparaben resulting from multiple routes and sources, including most products used by consumers that contain methylparaben. Urine is the major route of excretion for methylparaben and the biomarker (free and conjugated methylparaben) is specific to the parent compound. Methylparaben has a relatively short half-life and a low fractional urinary excretion. To address the uncertainty associated with the robustness of the use of methylparaben as a biomarker and the use of spot sampling, the upper bound of the 95% confidence interval of the 95th percentile urinary concentration (or highest available general population value) for each age band was used to estimate daily intake and are therefore considered a conservative scenario. The use of CHMS biomonitoring data allows increased confidence in the estimated daily intakes as it is based on a large, nationally representative population. Margins of exposure resulting from estimated daily intakes are presented in Table 7‑4.
| Source, location | Age group (years) | Upper-bound estimated daily intake at P95 (mg/kg bw/day) | Critical effect levela (mg/kg bw/day) |
MOE |
|---|---|---|---|---|
| CHMS (Cycle 4, 2014-2015), Canada | 3–5 | 0.032 | NOAEL 250 | 7 866 |
| CHMS (Cycle 4, 2014-2015), Canada | 6–11 | 0.069 | NOAEL 250 | 3 629 |
| CHMS (Cycle 4, 2014-2015), Canada | 12–19 | 0.073 | NOAEL 250 | 3 415 |
| CHMS (Cycle 4, 2014-2015), Canada | 20–59 | 0.050 | NOAEL 250 | 5 043 |
| CHMS (Cycle 4, 2014-2015), Canada | 60–79 | 0.072 | NOAEL 250 | 3 461 |
| Fisher et al. 2017, Canada | Pregnant women | 0.046 | NOAEL 250 | 5 325 |
| Kang et al. 2013, Korea | Neonates | 0.014 | NOAEL 250 | 17 785 |
Abbreviations: NOAEL, no observed adverse effect level
a Critical effect: clinical signs of ill-health, stomach erosion, spleen and thyroid atrophy, and mortality.
On the basis of conservative parameters used to model estimated daily intake, the margins of exposure are considered adequate to address the risk to populations aged 3 to 79 years, pregnant women and neonates. Biomonitoring data were not available to address children under 3 years (other than neonates). With the exception of breast milk consumption, exposure for this group is not expected to differ significantly from the youngest age group surveyed by CHMS or from the neonatal group presented here. Breast milk consumption was modelled using Canadian data and is not expected to pose a risk. Therefore, the reported urinary concentrations and estimated daily intakes across all age groups indicate that exposure in children under 3 years is not expected to pose a risk.
Due to the short half-life of methylparaben administered by the oral route and the spot sampling methodology employed in biomonitoring studies, it is not clear that biomonitoring data adequately addresses high exposures to cosmetics, NHPs and non-prescription drugs with intermittent use patterns (i.e., which are not used daily). Sentinel scenarios are presented in Table 7‑5.
| Exposure scenario | Age group (years) |
Systemic exposure (mg/kg bw/day) | Critical effect levela (mg/kg bw/day) |
MOE |
|---|---|---|---|---|
| Hair perm/ straightenerb (dermal, per event) |
Child (5–11) |
0.44–1.33 | NOAEL 250 | 188–568 |
| Hair perm/ straightenerb (dermal, per event) |
Adult (> 20) |
0.21–0.62 | NOAEL 250 | 403–1 190 |
| Anti-diarrheal medicationc (oral, per day) |
Toddler (0.5–4) |
50.81 | NOAEL 250 | 5 |
| Anti-diarrheal medicationc (oral, per day) |
Teen (12–19) |
106.1 | NOAEL 250 | 2 |
| Cough lozengec (oral, per day) |
Child (5–11) |
1.61 | NOAEL 250 | 155 |
| Heartburn medicationc (oral, per day) |
Adult (> 20) |
51 | NOAEL 250 | 5 |
| Motion sickness medicationd (oral, per day) |
Toddler (0.5–4) |
2.4 | NOAEL 250 | 104 |
| Radiological contrast mediac (oral, per event) |
Infants (0–0.5) |
14.0 | NOAEL 250 | 18 |
| Radiological contrast mediac (oral, per event) |
Toddler (0.5–4) |
29.0 | NOAEL 250 | 9 |
| Radiological contrast mediac (oral, per event) |
Adult (> 20) |
6.35 | NOAEL 250 | 39 |
| Sunscreenc,d (dermal, per day) |
Toddler (0.5–4) |
1.21 | NOAEL 250 | 207 |
| Sunscreenc,d (dermal, per day) |
Adult (> 20) |
0.91 | NOAEL 250 | 275 |
Abbreviations: NOAEL, no observed adverse effect level.
a Critical effect: clinical signs of ill-health, stomach erosion, spleen and thyroid atrophy, and mortality.
b Cosmetic.
c Natural health product.
d Non-prescription drug.
The margins of exposure for oral exposure to anti-diarrheal medication, heartburn medication, and radiological contrast media are potentially inadequate to address uncertainties in the health effects and exposure databases.
7.2 Ethylparaben
7.2.1 Exposure assessment
Ethylparaben is naturally occurring in some foods and has been identified in environmental media. Ethylparaben is present in a number of products, including cosmetics, prescription and non-prescription drugs, NHPs, and pest control products; and may be used as a component in incidental additives used in food processing establishments. All of these sources may contribute to total daily exposure to ethylparaben. Urinary concentrations and estimated exposures of Canadians to ethylparaben are presented in the following section.
Biomonitoring
Ethylparaben has been measured by CHMS and in other Canadian biomonitoring studies. In biomonitoring data collected in Cycle 4 of the CHMS, 64.86% of Canadians tested had a urinary concentration of ethylparaben that was below the level of detection. The 95th percentile urinary concentration of ethylparaben in Canadians aged 3 to 79 years was 73 µg/LFootnote 7 (n = 2564, 95% confidence interval 33 to 110 µg/L). The highest frequency of detection was reported in adults aged 40 to 59 years, in which ethylparaben was below the limit of detection in 52.56% of samples and the 95th percentile was 98 µg/LFootnote 7 312, 95% confidence interval 44 to 150 µg/L). The lowest frequency of detection was reported in children aged 6 to 11 years (n = 514, in which 78.79% of samples had urinary concentrations below the level of detection and the 95th percentile was 3.4 µg/LFootnote 7 (95% confidence interval of 1.3 to 5.5 µg/L) (Health Canada 2017a). In regional studies conducted in Canada, the geometric mean urinary concentration of ethylparaben in pregnant females was 15.13 µg/L (n = 31) and the mean urinary concentration in adult females was 24.4 µg/L (n = 28 including 9 pregnant patients, median = 10.17 µg/L). The mean urinary concentration in males (n = 11) was 12.87 µg/L (median = 10.37 µg/L; Genuis et al. 2013).
Ethylparaben was detected in breast milk at a geometric mean concentration of 0.0023 µg/L in 56 Canadian women (95th percentile = 0.614 µg/L), 3 months post-partum (Fisher et al. 2017). Ethylparaben has also been detected in placenta, amniotic fluid and cord blood. The geometric mean in amniotic fluid was 1.87 µg/L (India, Shekhar et al. 2017), the mean detected in cord blood was 0.68 µg/L (United States, Geer et al. 2017) and in the maximum detected in placenta was 0.62 ng/g fresh weight (Spain, Valle-Sistac et al. 2016). Ethylparaben was detected at a median concentration of 2.9 µg/L (free plus conjugated paraben, range of 1.0 to 8.0 µg/L) in urine collected from Korean infants within 48 hours of delivery (Kang et al. 2013).
Using a methodology similar to that described for methylparaben (see section 7.1.1), daily estimated intakes were derived for the general population, pregnant females and neonates, based on biomonitoring data (CHMS, Health Canada 2017a; Fisher et al. 2017; Kang 2017). In each of the three biomonitoring studies, total ethylparaben (i.e., free and conjugated species) was measured in urine by HLPC, after treatment with β-glucuronidase and sulfatase. Neonates were included because the detection of ethylparaben in cord blood, placenta and amniotic fluid indicate that ethylparaben passes through the placenta and exposure may occur in utero (Shekhar et al. 2017; Geer et al. 2017; Valle-Sistac et al. 2016). Urinary concentrations from Korean neonates were used in the absence of data from a Canadian population. This is considered an appropriate choice because the 95th percentile urinary concentration of ethylparaben in the general Korean population is roughly equivalent to that of the general Canadian population (Honda et al. 2018).
Data from a human pharmacokinetics study of methylparaben, butylparaben, and iso-butylparaben (Moos et al. 2016) was used to model the fractional urinary excretion of ethylparaben (Moos et al. 2017). The authors observed that in a previous study (Moos et al. 2016), the fractional urinary excretion of methylparaben, butylparaben, and iso-butylparaben decreases with increasing length of the alkyl chain; conversely, the log KOW increases with increasing alkyl chain length. A fractional urinary excretion value for ethylparaben (and other parabens, including propylparaben) was derived using linear regression of the log KOW against the experimentally derived fractional urinary excretion values for methylparaben, butylparaben and iso-butylparaben. A fractional urinary excretion of 0.137 was thus calculated for ethylparaben and is used here to calculate estimated daily intake. As with methylparaben, ethylparaben is a specific biomarker of the parent compound, but is not expected to be the major metabolite in urine. Although there are no human studies of ethylparaben metabolism, in a study of oral and intravenous exposure in dogs, 64% of the applied dose was excreted in urine within 24 hours as PHBA and other metabolites (Jones et al. 1956). This is consistent with a relatively low fractional urinary excretion of ethylparaben in humans. The use of an oral fractional urinary excretion value may under- or over-estimate dermal exposure, and the use of the parent paraben as a biomarker provides a less robust measure of exposure as it is not the major metabolite. As with methylparaben, the use of the parent compound as a biomarker may be susceptible to contamination during sampling or analysis due to its widespread use as a preservative (Aylward et al. 2017a).
Estimated daily intake values were calculated using the 95th percentile 95% confidence interval values (or highest available values) for creatinine-adjusted urinary concentration (µg/g Cr) for each age group, as described for methylparaben (section 7.1.1). A high level of variability was associated with CHMS biomonitoring data for ethylparaben in age-stratified groups at the 95th percentile, and the geometric mean was not calculated for the whole population or for age-stratified groups because almost 65% of samples were below the limit of detectionFootnote 8 (Health Canada 2017a). The prevalence of samples below the limit of detection may be due to rapid metabolism or to low exposure. According to data submitted to Health Canada, ethylparaben is used in approximately 75% fewer products (i.e., cosmetics, NHPs and drugs) than methylparaben and is not permitted for use as a food additive, indicating that low exposure can be expected to account for some, if not all, of the lower detection levels of ethylparaben. As with methylparaben, variability may be due to variations in use patterns of products that contain ethylparaben or to the use of spot sampling, which is subject to variability based on the time of sampling and may underestimate exposure of substances with short half-lives. Both variability and potentially rapid metabolism were addressed by selecting upper bound of the 95th percentile 95% confidence interval as a conservative upper bound value for deriving estimated daily intake. This is considered a conservative estimate that addresses the variation inherent in spot sampling. The use of CHMS biomonitoring data allows increased confidence in the estimated daily intakes as it is based on a large, nationally representative population. The fractional urinary excretion value of 0.137 or 13.7% for ethylparaben was extrapolated in Moos et al. (2017), and creatinine excretion rate was calculated using the Mage equation (Saravanabhavan et al. 2014).
Estimated daily intakes and key parameters are presented in Table 7‑6. The values calculated here for the Canadian population are similar to daily intakes of ethylparaben calculated at the 95th percentile for an adult German population (ranging from 3.4 to 17.4 µg/kg bw/day), as reported in Moos et al. (2017). See Appendix A for further details of the derivation of estimated daily intake values.
| Source, location | Age (years)a |
CER (mg/day)b | UCCr, P95 (CI) (µg/g Cr)c | FUEd | EDI, P95 (CI) (µg/kg bw/day) |
|---|---|---|---|---|---|
| CHMS (Cycle 4, 2014-2015)c, Canada | 3–5 | 130 | 120 (54–90)e | 0.137 | 7.4 (3.4–12) |
| CHMS (Cycle 4, 2014-2015)c, Canada | 6–11 | 418 | 4.6 (2.2–7.1)f | 0.137 | 0.46 (0.22–0.71) |
| CHMS (Cycle 4, 2014-2015)c, Canada | 12–19 | 1 182 | 120 (54–90)e | 0.137 | 18 (8.0–28) |
| CHMS (Cycle 4, 2014-2015)c, Canada | 20–59g | 1 248 | 120 (54–90)e | 0.137 | 16 (7.0–25) |
| CHMS (Cycle 4, 2014-2015)c, Canada | 60–79 | 1 017 | 70 (29–110)f | 0.137 | 7.3 (3.0–12) |
| Fisher et al. 2017, Canada | Pregnant women | - | 84h | 0.137 | 12 |
| Kang et al. 2013, Korea | Neonates | 9.6 | 15i | 0.137 | 0.32 |
Abbreviations: CER, creatinine excretion rate; UCCr, creatinine-adjusted urinary concentration; P95, 95th percentile; CI, confidence interval; FUE, fractional urinary excretion; EDI, estimated daily intake
a Age groups are defined based on age groups reported by CHMS (Health Canada 2017a).
b Creatinine excretion rate was calculated using the Mage equation [0.993*1.64 [140 – age] (Wt^1.5 Ht^0.5)/1000]. See Appendix A for values used for age weight and height.
c Health Canada 2017a.
d Moos et al. 2017.
e CHMS data for the 95th percentile in this age stratum was suppressed due to high variability and “females 3 to 79” was used as a surrogate. Although the 95th percentile value for this group is not known, this approach is considered conservative because the value used to estimate daily intake is the highest reported 95th percentile value for ethylparaben.
f These values were associated with high sampling variability (i.e., coefficient of variation between 16.6% and 33.3%). Health Canada recommends that this data be used with caution (Health Canada 2017a).
g The “20-39” and “40-59” age groups are presented together. When 95th percentile values were reported for both age groups, the higher value is presented here; when one value was suppressed, the value from the other group is presented here.
h This value is the specific gravity-adjusted urinary paraben concentration (µg/L) at the 95th percentile; creatinine-adjusted values were not reported in Fisher et al. 2017. Confidence intervals were not reported. EDI was calculated using the following equation: EDI = (UC*UFR)/FUE (Saravanabhavan et al. 2014), where UC is the urinary concentration, UFR is the urinary flow rate (0.20 L/kg bw/day, Aylward et al. 2015) and FUE is the fractional urinary excretion.
i This value is the 75th percentile creatinine-adjusted urinary paraben concentration; the 95th percentile was not reported (Kang et al. 2013). Confidence intervals were not reported.
Environmental media
Ethylparaben has been identified in agricultural soil and house dust in Canada (Viglino et al. 2011; Fan et al. 2010). In other countries, it has been identified in drinking water (Carmona et al. 2014), agricultural and forestry soil (Perez et al. 2011; Nunez et al. 2008), outdoor air (Ramirez et al. 2010; Moreau-Guigon et al. 2016), residential indoor air (Laborie et al. 2016; Alliot et al. 2014; Moreau-Guigon et al. 2016), and house dust (Wang et al. 2012; Ramirez et al. 2011; Tran et al. 2016; Canosa et al. 2007). Average estimates of daily intake of ethylparaben by the general population based on international studies range from <0.001 to 0.013 µg/kg bw/day for all age groups from infants to adults over 60 years.
In the absence of Canadian monitoring data, exposure from water, soil and air was modelled using ChemCAN (ChemCAN 2003). Ethylparaben was found to partition primarily to sediments and water. Modelled data indicate that general population exposure due to environmental media in Canada is negligible.
Food
Ethylparaben exposure from food is expected to be negligible (personal communication, email from the Food Directorate, Health Canada, to the Consumer Product Safety Directorate, Health Canada, dated April 18, 2018; unreferenced). Biomonitoring data (Fisher et al. 2017) indicate that breastfed infants are expected to be exposed to 12.17 ng ethylparaben/kg bw/day (0.000012 mg/kg bw/day) via breast milkFootnote 9.
Cosmetics
Ethylparaben was identified in a wide variety of cosmetics, including lotions, make-up, oral care products, cleansers, and hair care products. Exposures from daily use of cosmetics were considered to be addressed by biomonitoring data. Due to the use of spot sampling and the short metabolic half-life of ethylparaben, exposures from products with intermittent uses were potentially not addressed by biomonitoring data. Sentinel scenarios from products with intermittent use patterns are presented in Table 7‑7.
For potential exposure by the dermal route, experimentally determined dermal absorption and metabolism coefficients from an in vitro study of dermal absorption and metabolism in human skin were used to estimate an internal dose, as described in section 7.1.1, and further details are available on request (Charles River Laboratories 2018; Health Canada 2018b).
Potential exposures were estimated using conservative assumptions and default values. See Appendix B for details on default values and models used for generating exposure estimates. Exposure estimates for each scenario are expressed on a per-event and/or daily basis, depending on exposure frequency (see section 7.2.3, Characterization of risk to human health).
| Product scenario | Concentration in product (%)a | Age group (years) |
Dermal load (µg/cm2/24 h) | Systemic exposure (mg/kg bw/day) |
|---|---|---|---|---|
| Face Paint (per event) |
0.1–0.3 | Toddler (0.5–4) |
2.99–8.97 | 0.04–0.12b,c |
| Face Paint (per event) |
0.1–0.3 | Adult (> 20) |
2.98–8.94 | 0.013–0.038b,c |
a Internal data, Consumer Product Safety Directorate, Health Canada, dated August 18, 2017; unreferenced.
b Calculated using the product dermal load as the maximum dermal amount.
c Calculated using a metabolism refinement of 47.93% ethylparaben.
Drugs and NHPs
Ethylparaben is present as a non-medicinal ingredient in prescription and non-prescription drugs and NHPs administered by multiple routes. Exposures from daily use of prescription and non-prescription drugs and NHPs are considered to be addressed by biomonitoring data. Sentinel scenarios from products with intermittent use patterns are presented in Table 7‑8. Default values and models used in exposure scenarios are provided in Appendix B.
| Product scenario | Amount in product (%) | Age group (years) |
Dermal load (µg/cm2/24 h) | Systemic exposure (mg/kg bw/day) |
|---|---|---|---|---|
| Traditional Chinese medicine (oral, per day) | 10 mg/capsulea | Adult (> 20) |
N/A | 1.69 |
| Hand sanitizer (dermal, per event) | 62%a | Toddler (0.5–4) |
1240.0 | 5.42a,b |
| Hand sanitizer (dermal, per event) | 62%a | Adult (> 20) |
1383.0 | 3.44b,c |
| Sunscreen (dermal, per day) | 0.4%a,b | Toddler | 15.60 | 1.54c,d |
| Sunscreen (dermal, per day) | 0.4%a,b | Adult | 15.60 | 1.17c,d |
a Personal communication, email from Natural and Non-Prescription Health Products Directorate, Health Canada, to Consumer Product Safety Directorate, Health Canada, dated February 20, 2017; unreferenced.
b Calculated using 40.38% dermal absorption.
c Calculated using a metabolism refinement of 47.93% ethylparaben.
d Calculated using a maximum dermal amount of 10.18 µg/cm2/24 h.
7.2.2 Health effects assessment
Ethylparaben has been extensively reviewed, and key risk assessments have been conducted by the CIR (Andersen 2008), the EU SCCS (SCCP 2005a, 2005b, 2006, 2008; SCCS 2010, 2011, 2013), EFSA (2004), NICNAS (2016), and independent scientists (Soni et al. 2005). The literature published until March 2017 was searched for relevant information to supplement the data used in the international reviews and assessments.
Toxicokinetics
A single dose of ethylparaben was administered orally (1 g/kg) or intravenously (50 mg/kg) to dogs. The parent compound was not detected in plasma at any time, and PHBA was detected within 1 hour (Jones et al. 1956). The majority of the applied dose (64%) was excreted within 24 hours as PHBA and other metabolites; only 0.034% of the parent compound was excreted. At sacrifice, small amounts of all ethylparaben were detected in the brain and pancreas (Jones et al. 1956). Ethylparaben has also been detected at low levels in tumorous breast tissue, human adipose tissue and in the brain (Barr et al. 2012; Wang et al. 2015; van der Meer 2017).
In ex vivo studies, ethylparaben is metabolized in human liver, skin, subcutaneous fat and blood. Hydrolysis in human liver cells is significantly greater than in skin cells (Jewell et al. 2007b; Harville et al. 2007), but both are more efficient than in plasma (Prusakiewicz et al. 2006). In liver and skin subcellular fractions, ethylparaben was hydrolyzed 2 to 10 times faster than propylparaben and butylparaben (Jewell et al. 2007a, 2007b; Harville et al. 2007; Lobemeier et al. 1996; Abbas et al. 2010; Prusakiewicz et al. 2006). In human plasma, ethylparaben was stable after 24 hours compared to propylparaben, butylparaben and benzylparaben, which were reduced to half within 1 to 6 hours. Ethylparaben hydrolysis in liver microsomes is more rapid than in blood, with a half-life of 35 minutes (Abbas et al. 2010). Esterases identified in keratinocytes and subcutaneous fat act on ethylparaben, but with less efficiency than on longer-chain parabens (Lobemeier et al. 1996).
Unlike human kinetics, rat skin and liver cell fractions hydrolyze parabens at roughly the same rate in in vitro studies (Harville et al. 2007). However, rat skin cells hydrolyze ethylparaben at a rate 2 to 3 orders of magnitude higher than that of human skin cells (Harville et al. 2007; Prusakiewicz et al. 2006). In these assays, hydrolysis in rat tissue also increases with increasing chain length, unlike humans. Ethylparaben is metabolized in human liver cell fractions at a rate 2- to 3-fold slower than in rat liver (Harville et al. 2007).
Repeat dose studies
In a 25-week oral toxicity study, SD-JCL rats (5/sex/test dose, 12/control) were fed 0.2%, 1.0% and 2.0% ethylparaben in their diet (equivalent to approximately 120, 600 and 1 200 mg/kg bw/day). Decreases were observed in male body weight in the mid- and high-dose groups during weeks 19 to 25 and 22 to 23, respectively, while increased male body weight was observed in the low-dose group from week 22 to 25. Alkaliphosphatase was significantly increased in males in all treatment groups, but was not dose-dependent and was not considered by the authors to be biologically significant. No significant differences were noted in clinical signs, food consumption, hematological effects, serum protein, cholesterol alkaliphosphatase levels (in females), organ weights, or pathological or histological findings. A NOAEL of 1 200 mg/kg bw/day (the highest dose tested) was identified (Sado 1973, as cited in Soni et al. 2005 and REACH 2018b).
Twelve weanling albino rats per dose were fed 2% or 8% ethylparaben (equivalent to 900 to 1 200 and 5 500 to 5 900 mg/kg bw/day) in diet for 12 weeks. Animals receiving 8% ethylparaben showed a slower weight gain than negative controls in early stages of the experiment. Signs of toxicity- such as depression and decreased motor activity as well as mortality were also observed in this group. Kidneys, liver, heart, lung, spleen and pancreas of all rats surviving the study were normal. The only significant finding was mortality in the high-dose group. These animals had extensive pulmonary consolidation and pneumonia, which was also observed in control animals. At 2%, no signs of toxicity were observed in treated animals compared to controls. The NOAEL in this study was 2% ethylparaben (900 to 1 200 mg/kg bw/day), based on effects observed at 8% (Matthews et al. 1956).
Genotoxicity
Ethylparaben is not mutagenic in Ames assays, but is positive in chromosomal aberration assays in Chinese hamster lung fibroblasts at 0.25 mg/mL and in mouse lymphoma and micronucleus assays, with and without metabolic activation (Finot et al. 2017; Ishidate et al. 1978, 1984; Ishidate and Yoshikawa 1980; Kawachi and Yahagi 1980). Ethylparaben was negative in in vivo assays including a chromosome aberration assay in mouse bone marrow (Kawachi and Yahagi 1980). Overall, the weight of evidence indicates that ethylparaben is not likely to be genotoxic in vivo (EFSA 1994; Andersen 2008).
Carcinogenicity
Male Fischer 344 rats (8/group) were exposed to PHBA, methylparaben, ethylparaben, propylparaben, and butylparaben at 4% in the diet (approximately 4 000 mg/kg bw/day) for 9 days. Ethylparaben caused a 2.9-fold increase in methyl-3[H]thymidine-labeling in the forestomach epithelium, indicating cell proliferation. Treatment with all parabens resulted in lesions visible under light microscopy (mucosal thickening, rete pegs and papillae) that increased in severity with increasing chain length (Rodrigues et al. 1986). The lowest observed adverse effect level (LOAEL) in this study was 4%, based on cell proliferation and lesions in the forestomach epithelium.
Reproductive and developmental effects
Potential estrogenic effects of parabens are reviewed in Appendix C.
Pregnant Wistar rats (8 to 12/group) were fed 0%, 0.1%, 1.0% or 10% ethylparaben (equivalent to 54–63, 517–658 and 2 970–3 260 mg/kg bw/day) from GD 8 to 15. At GD 21, dams were sacrificed and 305 fetuses were divided into two groups and prepared for observation of visceral features and osseous features. Bleeding of the parietal brain area was observed in 12/64 fetuses in the high-dose group, in 13/40 fetuses in the mid-dose group and in 1/44 fetuses in the low-dose group, as compared to 2/32 fetuses in the control group. Abnormal enlargement of the lateral and third ventricles of the brain was present in 2/32 control fetuses and in 8/64 fetuses in the high-dose group. Hydronephrosis was observed in 4/64 fetuses at the high dose, 1/40 at the mid-dose, 1/44 at the low dose and 1/32 in the control group. Bone malformations were observed in the high- (18/61) and low-dose groups (27/46) as well as in the control (8/32). However, only 4/44 animals in the mid-dose group showed osseous effects, suggesting that the effect was not specific to the test substance. Maternal body weight was lower in all treatment groups, particularly in the high-dose group, (363.8 ± 50.1, 363.2 ± 41.5, and 326.6 ± 39.5 g, respectively in low, mid- and high-dose groups) at PND 20, as compared to control (412.0 ± 36.2 g). In a second stage of the study, 6 pregnant rats in two groups were given 0.1% or 10% of ethylparaben in feed from GD 8 to GD 15; they were allowed to deliver spontaneously, and neonates were nursed for approximately 1 month. The authors report that no cases of teratogenesis were observed at 1 month in 98 pups from dams treated with ethylparaben in the second stage of the experiment, and there were no apparent differences from controls in pup sex ratio and body weight. The offspring of mothers treated with ethylparaben were reported to have a normal growth rate and normal behaviour. The proportion of live births was greater in the test groups than in the control, and there was no difference in maternal body weight after delivery (Moriyama et al. 1975; REACH 2018b). Statistical analysis was not conducted in this study. The authors concluded that ethylparaben did not cause any significant ill effects. This position was supported by the CIR (Andersen 2008). NICNAS (2016) determined that on the basis of bone malformations, hydronephrosis and enlargement of the third ventricle of the brain, a NOAEL of 517 to 658 mg/kg bw/day was determined for developmental toxicity and a NOAEL of 517 to 658 mg/kg bw/day was determined for maternal toxicity. A NOAEL of 517 to 658 mg/kg bw/day was selected for this study on the basis of enlargement of the lateral and third ventricle of the brain and hydronephrosis.
In a study of early reproductive development, pregnant Wistar rats (14/test group) were subcutaneously administered 0 or 400 mg/kg bw/day ethylparaben from GD 7 to GD 21. No effect was observed on maternal toxicity or reproductive parameters, including number of implantations, number of fetuses, post-implantation loss, resorptions, or sex ratio. No significant toxic effect was observed in fetuses, and no effect was observed on birth weight or anogenital distance in pups. Testes, adrenals, and ovaries from exposed fetuses were histologically similar to controls. Fetal (progesterone, testosterone, thyroxine) and maternal (triiodothyronine, thyroxine, progesterone, 17α-hydroxyprogesterone) serum, fetal testicular (testosterone, progesterone) and fetal ovary (estradiol) hormone levels were also unchanged in the test group (Taxvig et al. 2008). The NOAEL of this study is 400 mg/kg bw/day, the highest dose tested.
Ethylparaben was administered in the diet to male Crj:Wistar rats (8 animals per group) at 0%, 0.1% and 1.0% (equivalent to 102 ± 2.75 and 1 030 ± 38.0 mg/kg bw/day) for 8 weeks starting at PND 25 to 27. Treatment-related effects were not observed for reproductive organ weights (testes, epididymides, prostates, seminal vesicles and preputial glands) in any of the dose groups. Changes in testosterone, LH and FSH levels relative to controls were not observed at any dose level. Sperm counts in cauda epididymis and testis were normal, as were distribution of spermatogonia, spermatocytes, round spermatids and elongated spermatids in stage VII–VIII tubules. The NOAEL for this study was 1 030 mg/kg bw/day, the highest dose tested (Oishi 2004). As described in the methylparaben section, the SCCS (2008, 2011) has expressed doubt as to the quality of this and other studies produced by the Oishi laboratory, which reported (i) mean values for some parameters that fell far outside the historical control ranges and (ii) standard deviations for key parameters including epididymal sperm concentrations and testosterone levels that were far less than the normal biological variability that has been observed by other groups. The SCCS has concluded that the quality of the Oishi studies cannot be properly assessed as the full test description and the complete raw data packages were no longer available.
Epidemiology
A literature search was conducted to identify epidemiological data for ethylparaben, with a focus on reproductive and endocrine effects. Studies were evaluated and scored for quality using the Newcastle-Ottawa Quality Assessment Scale (NOS) and the Quality Assessment Tool for Observational Cohort and Cross-sectional Studies from the National Heart, Lung, and Blood Institute of the National Institutes of Health (RSI 2018). Epidemiology studies were identified that address the relationship between urinary concentration of ethylparaben and fertility, reproductive health, serum hormone levels, growth and development in children and adolescents, body weight, allergic sensitization and respiratory health in children. The majority of studies did not report an association between ethylparaben and the endpoint of interest. Weak positive associations were identified between ethylparaben levels and abnormal sperm morphology among patients of a fertility clinic, birth weight and height in male neonates, and allergic sensitization (Jurewicz et al. 2017a; Philippat et al. 2014; Guo et al. 2017; Savage et al. 2012; Spanier et al. 2014; Vernet et al. 2017). A weak inverse association was identified between ethylparaben concentration and serum thyroid hormone levels in adult females (Koeppe et al. 2013) and BMI in pregnant women (Polinski et al. 2018). Other than growth rates in neonates and toddlers and serum thyroid hormone levels, different studies of similar quality gave conflicting results for these or similar endpoints. More detail is provided in Health Canada (2018a).
7.2.3 Characterization of risk to human health
Ethylparaben demonstrated low toxicity in repeat dose studies, with reported NOAELs of 900 to 1 200 mg/kg bw/day (Sado 1973; Matthews et al. 1956). A NOAEL of 900 mg/kg bw/day was reported for dietary exposure to ethylparaben in a 12-week repeat dose study based on depression, decreased motor activity, and lethality at 5 500 mg/kg bw/day (Matthews et al. 1956). The weight of evidence indicates that ethylparaben is not genotoxic, and in vivo studies indicate that ethylparaben is not carcinogenic.
Ethylparaben administered in the diet at up to 1 030 mg/kg bw/day did not result in observed effects on male pubertal development in rats (Oishi 2004). However, the SCCS has expressed significant concerns about the quality of studies performed by the Oishi lab. In a study of prenatal reproductive development, no effects were observed on reproductive endpoints in pups after gestational subcutaneous dosing up to 400 mg/kg bw/day (Taxvig et al. 2008). In an older study of prenatal developmental toxicity, ethylparaben exposure resulted in enlargement of the lateral and third ventricles of the brain and hydronephrosis at the highest dose tested (2 970 to 3 260 mg/kg bw/day (Moriyama et al. 1975). Bleeding of the parietal brain was also noted in the mid- and high-dose groups, but was not dose-dependent as the effect was observed in 13/40 fetuses at the mid dose, and in 12/64 fetuses at the high dose. Maternal body weight was notably decreased at the high dose; however, the effect on fetal brain ventricles is considered to be independent of maternal body weight. A NOAEL of 517 mg/kg bw/day was therefore selected as a point of departure for ethylparaben, based on prenatal developmental toxicity. A NOAEL of 900 mg/kg bw/day was selected as a point of departure for general toxicity, based on repeat dose studies.
The Canadian population is exposed to ethylparaben via cosmetics, prescription and non-prescription drugs and NHPs. Monitoring data available for some environmental media sources and worldwide data suggest that Canadians are also exposed to ethylparaben via these sources.
The assessment of general population exposure to ethylparaben is based on biomonitoring data from the CHMS, the Plastics and Personal-Care Product Use in Pregnancy (or P4) Study, and a study of Korean neonates within 48 hours of birth (Health Canada 2017a; Fisher et al. 2017; Kang et al. 2013). These data encompass exposures to ethylparaben from multiple sources and routes, reflecting the integrated exposure due to use of most products used by consumers that contain ethylparaben. Biomonitoring data are therefore considered reliable estimates of exposure of the general population in Canada to ethylparaben. Urine is the major route of excretion for ethylparaben, and the biomarker (free and conjugated ethylparaben) is specific to the parent compound. However, ethylparaben has a relatively low fractional urinary excretion and the majority of samples were below the limit of detection. To address the uncertainty associated with the robustness of the use of ethylparaben as a biomarker, estimates of daily intake were based on the upper-bound of 95th percentile (or highest available) exposure value for each age band and are therefore considered a conservative scenario. Margins of exposure resulting from estimated daily intakes are presented in Table 7‑9.
| Source, Location | Age group (years) | Upper-bound estimated daily intake at P95 (mg/kg bw/day) | Critical effect level (mg/kg bw/day) |
MOE |
|---|---|---|---|---|
| CHMS (Cycle 4, 2014-2015), Canada | 3–5 | 0.012 | NOAEL 900a | 76 322 |
| CHMS (Cycle 4, 2014-2015), Canada | 6–11 | 0.00071 | NOAEL 900a | 1 270 729 |
| CHMS (Cycle 4, 2014-2015), Canada | 12–19 | 0.028 | NOAEL 517b | 18 458 |
| CHMS (Cycle 4, 2014-2015), Canada | 20–59 | 0.025 | NOAEL 517b | 20 866 |
| CHMS (Cycle 4, 2014-2015), Canada | 60–79 | 0.012 | NOAEL 900a | 78 212 |
| Fisher et al. 2017, Canada | Pregnant women | 0.012 | NOAEL 517b | 41 466 |
| Kang et al. 2013, Korea | Neonates | 0.00031 | NOAEL 900a | 2 827 403 |
Abbreviations: NOAEL, no observed adverse effect level.
a Critical effects: depression, decreased motor activity, and mortality.
b Critical effects: fetal hydronephrosis and enlargement of the lateral and third ventricles of the fetal brain.
On the basis of conservative parameters used to model estimated daily intake, the margins of exposure are considered adequate to address the risk to populations aged 3 to 79 years, pregnant women and neonates. Biomonitoring data were not available to address children under 3 years (other than neonates). With the exception of breast milk consumption, exposure for this group is not expected to differ significantly from the youngest age group surveyed by CHMS or from the neonatal group presented here. Breast milk consumption was modelled using Canadian data and is not expected to pose a risk. Therefore, the reported urinary concentrations and estimated daily intakes across all age groups indicate that exposure in children under 3 years is not expected to pose a risk.
Due to the short half-life of ethylparaben administered by the oral route and the spot sampling methodology employed in biomonitoring studies, it is not clear that biomonitoring data adequately addresses high exposures to cosmetics and NHPs with intermittent use patterns. Sentinel scenarios are presented in Table 7‑10.
| Exposure scenario | Age group (years) | Systemic exposure (mg/kg bw/day) | Critical effect level (mg/kg bw/day) | MOE |
|---|---|---|---|---|
| Face paintc (dermal, per event) |
Toddler (0.5–4) |
0.04–0.12 | NOAEL 900b | 7 500–22 500 |
| Traditional Chinese medicined (oral, per day) |
Adult (> 20) |
1.69 | NOAEL 517a | 306 |
| Hand sanitizerd (dermal, per event) | Toddler (0.5–4) |
5.42 | NOAEL 900b | 166 |
| Hand sanitizerd (dermal, per event) | Adult (> 20) |
3.44 | NOAEL 517a | 150 |
| Sunscreend (dermal, per day) |
Toddler (0.5–4) |
1.54 | NOAEL 900b | 584 |
| Sunscreend (dermal, per day) |
Adult (> 20) |
1.17 | NOAEL 517a | 442 |
Abbreviations: NOAEL, no observed adverse effect level>
a Critical effects: fetal hydronephrosis and enlargement of the lateral and third ventricles of the fetal brain
b Critical effects: depression, decreased motor activity, and mortality.
c Cosmetic
d Natural health product
The margins of exposure of all products are considered adequate to address uncertainties in the health effects and exposure databases.
7.3 Propylparaben
7.3.1 Exposure assessment
Propylparaben is naturally occurring in some foods and has been identified in environmental media. Propylparaben is also present in a number of products, including prescription and non-prescription drugs, NHPs, cosmetics, pest control products, and limited products available to consumers. It may be used as a food additive or a component in food packaging materials. Many of these sources contribute to total daily exposure to propylparaben. Urinary concentrations and estimated exposures of Canadians to propylparaben are presented in the following section.
Biomonitoring
Biomonitoring data collected in Cycle 4 of the Canadian Health Measures Survey indicated a geometric mean urinary concentration of 2.5 µg/L (95% confidence interval of 1.8 to 3.5 µg/L) of propylparaben in Canadians aged 3 to 79 years (n = 2 564). Key values from Canadian biomonitoring studies are presented in Table 7-18. Females had a higher geometric mean urinary concentration of 4.9 µg/L[1] (95% confidence interval of 3.2 to 7.6 µg/L, n = 1 289) compared to males, with 1.3 µg/L (95% confidence interval of 0.96 to 1.8 µg/L, n = 1 275). Among different age groups, the group with the highest reported exposure was adults aged 60 to 79 years, with a geometric mean urinary concentration of 3.0 µg/LFootnote 10 (95% confidence interval of 2.0 to 4.6 µg/L). Children aged 6 to 11 years had the lowest reported exposure with a geometric mean urinary concentration of 1.2 µg/L (95% confidence interval of 0.99 to 1.6 µg/L) (Health Canada 2017a). In the whole population (aged 3 to 79 years), 20.83% of samples were below the limit of detection; the highest proportion of samples below the limit of detection was 26.39% in adults aged 60 to 79 years. In two smaller scale Canadian studies, the mean urinary concentration in females was 26.45 µg/L (n = 28 including 9 pregnant patients, median = 2.8 µg/L) and the geometric mean urinary concentration in pregnant females was 25.50 µg/L (n = 31) (Genuis et al. 2013; Fisher et al. 2017). The mean urinary concentration in males (n = 11), reported by Genuis et al. (2017), was 61.9 µg/L (median of 3.09 µg/L). The difference in mean and geometric mean urinary concentration levels reported by CHMS versus Genuis et al. (2013) and Fisher et al. (2017) may be due to differences in the population assayed (i.e., small local populations and a population of pregnant women versus a large nationally representative sample of the general population).
Propylparaben was detected at a median concentration of 4.0 µg/L (free plus conjugated paraben, range of 0.84 to 15.2 µg/L) in urine collected from Korean infants within 48 hours of delivery (Kang et al. 2013). Low-birth-weight neonates in the NICU (n = 41 aged ≤ 4 weeks) had a urinary concentration of 2.6 µg/L free propylparaben and 16.8 µg/L total paraben (GMs reported; Calafat et al. 2009), a level higher than the youngest child populations reported by CHMS or NHANES (Health Canada 2017a; Calafat et al. 2010).
Propylparaben was detected in breast milk at a geometric mean concentration of 0.0277 µg/L in 56 Canadian women (95th percentile = 1.320 µg/L), 3 months post-partum (Fisher et al. 2017). Propylparaben was detected in placenta in a sample of 12 women (Barcelona, maximum = 1.28 ng/g fresh weight) and in amniotic fluid in 40 pregnant women (India, GM = 7.84 µg/L) (Valle-Sistac et al. 2016; Shekhar et al. 2017). Propylparaben was detected in cord blood at a mean of 5.59 µg/L in 34 samples in the United States (Geer et al. 2017). Propylparaben was undetectable in the blood of 73.9% of neonates (n = 181) that received medicines containing propylparaben as an excipient; the maximum detected in blood was 147 µg/L (Mulla et al. 2015).
Estimated daily intakes of propylparaben were calculated for the general population, pregnant females and neonates, based on biomonitoring data and according to the method described for methylparaben in section 7.1.1 (CHMS, Health Canada 2017a; Fisher et al. 2017; Kang 2017). In each of the three biomonitoring studies, total propylparaben (i.e., free and conjugated species) was measured in urine by HLPC, after treatment with β-glucuronidase and sulfatase. The fractional urinary excretion value of 0.097 or 9.7% for propylparaben was extrapolated in Moos et al. (2017) based on measured values for methylparaben and butylparaben in adults. Creatinine excretion rate was calculated using the Mage equation (Saravanabhavan et al. 2014). Neonates were included because the detection of propylparaben in cord blood, placenta and amniotic fluid indicate that propylparaben passes through the placenta and exposure may occur in utero (Shekhar et al. 2017; Geer et al. 2017; Valle-Sistac et al. 2016). Urinary concentrations from Korean neonates were used in the absence of data from a Canadian population; this is considered a conservative choice because the 95th percentile urinary concentrations of propylparaben in the general Korean population exceed those of the general Canadian population (Honda et al. 2018).
As described for ethylparaben (see section 7.2.1), Moos et al. (2017) used a linear regression of fractional urinary excretion values from a human pharmacokinetics study (Moos et al. 2016) of methylparaben, butylparaben and iso-butylparaben and log KOW to model the fractional urinary excretion of propylparaben as 0.097 (Moos et al. 2017). A low recovery of propylparaben in urine is consistent with other studies. In human studies in which 20 mg/kg bw/day of propylparaben was administered orally, PHBA is detected in blood within 60 minutes, but the parent paraben is not detected at any point (Heim 1960, as cited in Andersen 2008). In another study, a single volunteer ingested 2 g of propylparaben per day for 5 days and excreted 17.4% as PHBA in urine. Only conjugated-propylparaben was identified in urine; unmetabolized paraben was not detected (the majority of the dose was unaccounted for) (Sabalitschka and Neufeld-Crzelliter 1954, as cited in Soni et al. 2005). See section 7.3.2 for further discussion of propylparaben toxicokinetics. The value of 9.7% was therefore used as the fractional urinary excretion value in the calculation of biomonitoring equivalents and estimated daily intake for propylparaben. As with other parabens, propylparaben is a specific biomarker of the parent compound and is primarily excreted in urine, but is not the major metabolite. The use of an oral fractional urinary excretion value may under- or over-estimate dermal exposure, and the use of the parent paraben as a biomarker provides a less robust measure of exposure as it is not the major metabolite. Due to its widespread use as a preservative, the use of the parent compound as a biomarker may be susceptible to contamination during sample collection and analysis (Aylward et al. 2017a).
Estimated daily intake values were calculated using the 95th percentile 95% confidence interval values (or highest available values) for creatinine-adjusted urinary concentration (µg/g Cr) for each age group, as described for methylparaben (section 7.1.1). Propylparaben was below the limit of detection in approximately 20% of all CHMS samples. However, fully validated methods were used to measure propylparaben, and human biomonitoring data provide realistic exposure in the general population. The number of samples with undetectable levels of propylparaben may be due to rapid metabolism or to low exposure. According to data submitted to Health Canada, propylparaben is used in approximately 35% fewer products (i.e., cosmetics, NHPs and prescription and non-prescription drugs) than methylparaben, indicating that lower exposure may account for some, if not all, of the lower detection levels of propylparaben. A high level of variability (based on the coefficient of variation) was associated with CHMS biomonitoring data for propylparaben in age-stratified groups at the 95th percentile, and in some age groups, the geometric mean. Variability was addressed by selecting the lower and upper bounds of the 95th percentile 95% confidence interval as conservative lower and upper bound values for deriving estimated daily intake. The use of CHMS biomonitoring data allows increased confidence in the estimated daily intakes as they are based on a large, nationally representative population.
Estimated daily intakes and key parameters are presented in Table 7‑11. The values calculated here for the Canadian population are similar to daily intakes of propylparaben calculated at the 95th percentile for an adult German population (ranging from 9.8 to 26.9 µg/kg bw/day), reported in Moos et al. (2017). See Appendix A for further details of the derivation of estimated daily intake values.
| Source, Location | Age (years)a |
CER (mg/day)b | UCCr, P95 (CI) (µg/g Cr)c | FUEd | EDI, P95 (CI) (µg/kg bw/day) |
|---|---|---|---|---|---|
| CHMS (Cycle 4, 2014-2015)c, Canada | 3–5 | 130 | 68 (20–120)e | 0.097 | 5.9 (1.7–10) |
| CHMS (Cycle 4, 2014-2015)c, Canada | 6–11 | 418 | 190 (110–280)f | 0.097 | 26 (15–39) |
| CHMS (Cycle 4, 2014-2015)c, Canada | 12–19 | 1 182 | 85 (42–130)e | 0.097 | 17 (8.6–27) |
| CHMS (Cycle 4, 2014-2015)c, Canada | 20–59g | 1 248 | 96 (29–160)e | 0.097 | 17 (5.3–29) |
| CHMS (Cycle 4, 2014-2015)c, Canada | 60–79 | 1 017 | 190 (110–280)e | 0.097 | 28 (16–41) |
| Fisher et al. 2017, Canada | Pregnant women | - | 111h | 0.097 | 23 |
| Kang et al. 2013, Korea | Neonates | 9.6 | 37.3i | 0.097 | 1.1 |
Abbreviations: CER, creatinine excretion rate; UCCr, creatinine-adjusted urinary concentration; P95, 95th percentile; CI, confidence interval; FUE, fractional urinary excretion; EDI, estimated daily intake.
a Age groups are defined based on age groups reported by CHMS (Health Canada 2017a).
b Creatinine excretion rate was calculated using the Mage equation [0.993*1.64 [140 – Age] (Wt^1.5 Ht^0.5)/1000]. See Appendix A for values used for age weight and height.
c Health Canada 2017a.
d Moos et al. 2016.
e These values were associated with high sampling variability (i.e., coefficient of variation between 16.6% and 33.3%). Health Canada recommends that this data be used with caution (Health Canada 2017a).
f CHMS data for the 95th percentile in this age stratum was suppressed due to high variability and ”Age 60 to 79” was used as a surrogate. Although the 95th percentile value for this group is not known, this approach is considered conservative because the value used to estimate daily intake is the highest reported 95th percentile value for propylparaben.
g The “20-39” and “40-59” age groups are presented together. The 95th percentile value for age 20-39 years was suppressed due to high variability; the 95th percentile value for Age 40-59 years is presented here.
h This value is the specific gravity-adjusted urinary paraben concentration (µg/L) at the 95th percentile; creatinine-adjusted values were not reported in Fisher et al. 2017. Confidence intervals were not reported. EDI was calculated using the following equation: EDI = (UC*UFR)/FUE (Saravanabhavan et al. 2014), where UC is the urinary concentration, UFR is the urinary flow rate (0.20 L/kg bw/day, Aylward et al. 2015) and FUE is the fractional urinary excretion.
i This value is the 75th percentile creatinine-adjusted urinary paraben concentration; the 95th percentile was not reported (Kang et al. 2013). Confidence intervals were not reported.
Environmental media
Propylparaben has been identified in agricultural soil and house dust in Canada (Viglino et al. 2011; Fan et al. 2010). In other countries, it has been identified in drinking water (Carmona et al. 2014), garden, agricultural, industrial and forestry soil (Ferreira 2011; Perez et al. 2011; Nunez et al. 2008), outdoor air (Moreau-Guigon et al. 2016), residential indoor air (Laborie et al. 2016; Alliot et al. 2014; Moreau-Guigon et al. 2016), and house dust (Wang et al. 2012; Ramirez et al. 2011; Tran et al. 2016; Canosa et al. 2007). Average estimates of daily intake of propylparaben by the general population of Canada based on these studies range from 0.004 to 28 µg/kg bw/day for all age groups from infants to adults over 60 years.
In the absence of Canadian monitoring data, exposure from water and air was modelled using ChemCAN (ChemCAN 2003). Propylparaben was found to partition primarily to sediments and water. Modelled data indicate that general population exposure due to environmental media is negligible.
Food
Propylparaben (identified as propyl paraben, propyl-ρ-hydroxy benzoate) and its sodium salt (identified as sodium salt of propyl-ρ-hydroxy benzoic acid) are permitted for use as food additives (preservatives) in foods sold in Canada. The foods to which they may be added and their maximum levels of use in those foods are set out in Part 2 of the List of Permitted Preservatives (personal communication, email from the Food Directorate, Health Canada, to the Consumer Product Safety Directorate, Health Canada, dated April 18, 2018; unreferenced). However, these uses were approved many years ago, and the results of a 2017 Health Canada survey of current uses of propylparaben by the food industry suggest that use in food is limited. The main use in foods as a preservative is currently in certain colouring preparations or dispersions, which are subsequently incorporated into a limited number of food products such as certain confectionery products, marinades and unstandardized beverages, including some flavoured and carbonated or concentrated (including frozen) beverages (personal communication, email from the Food Directorate, Health Canada, to the Consumer Product Safety Directorate, Health Canada, dated April 18, 2018; unreferenced). For the general public (age bands from 1 to over 71 years), conservative estimates of exposure to propylparaben from its use in certain colour preparations range from 0.013 to 0.073 mg/kg bw/day at the 90th percentile. Biomonitoring data (Fisher et al. 2017) indicate that breastfed infants are expected to be exposed to 33.04 ng/kg bw/day (0.000033 mg/kg bw/day) via breast milk.
Cosmetics and products available to consumers
Propylparaben was identified in cleaning wipes (SDS 2010), and a wide variety of cosmetics, including lotions, make-up, cleansers, and hair care products. Exposures from daily use of cosmetics and products available to consumers were considered to be addressed by biomonitoring data. Due to the use of spot sampling and the short metabolic half-life of propylparaben, exposures from products with intermittent uses were potentially not addressed by biomonitoring data. Sentinel exposures from cosmetics with intermittent use patterns are presented in Table 7‑12.
For potential exposure by the dermal route, experimentally determined dermal absorption and metabolism coefficients from an in vitro study of dermal absorption and metabolism in human skin were used to estimate an internal dose, as described in section 7.1.1, and further details are available on request (Charles River Laboratories; Health Canada 2018b).
Potential exposures were estimated using conservative assumptions and default values. See Appendix B for details on default values and models used for generating exposure estimates. Exposure estimates for each scenario are expressed on a per-event and/or a daily basis, depending on exposure frequency (see section 7.3.3, Characterization of risk to human health).
| Product scenario | Concentration in product (%) | Age group (years) |
Dermal load (µg/cm2/24 h) | Systemic exposure (mg/kg bw/day) |
|---|---|---|---|---|
| Face Paint (per event) |
0.1–0.3a | Toddler (0.5–4) |
2.99–8.97 | 0.031–0.93b,c |
| Face Paint (per event) |
0.1–0.3a | Adult (> 20) |
2.98–8.94 | 0.0099–0.030b,c |
aInternal data, Consumer Product Safety Directorate, Health Canada, dated August 18, 2017; unreferenced.
bCalculated using the product dermal load as the maximum dermal amount.
cCalculated using a metabolism refinement of 36.97% propylparaben.
Drugs and NHPs
Propylparaben is present as a non-medicinal ingredient in prescription and non-prescription drugs and NHPs administered by multiple routes. Exposures from daily use of prescription and non-prescription drugs and NHPs are considered to be addressed by biomonitoring data. Sentinel scenarios from products with intermittent use patterns are presented in Table 7‑13. Default values and models used in exposure scenarios are provided in Appendix B.
| Product scenario | Concentration in product (%) | Age group (years) |
Dermal load (µg/cm2/24 h) | Systemic exposure (mg/kg bw/day) |
|---|---|---|---|---|
| Heartburn medicationa (per day) |
0.65c | Adult (> 20) |
N/A | 29.33 |
| Fluoride treatmenta (per event) | 0.2c | Child (5–11) |
N/A | 0.52 |
| Fluoride treatmenta (per event) | 0.2c | Adult (> 20) |
N/A | 0.23 |
| Sunscreenb (dermal, per day) |
0.1d | Infant (0–0.5) |
4.59 | 1.84e,g |
| Sunscreena (dermal, per day) |
0.4c | Toddler (0.5–4) |
15.6 | 0.78df,h |
| Sunscreena (dermal, per day) |
0.4c | Adult (> 20) |
15.6 | 0.58f,h |
Abbreviations: N/A, not applicable.
a Natural health product.
b Non-prescription drug.
c Personal communication, email from Natural and Non-Prescription Health Products Directorate, Health Canada, to Consumer Product Safety Directorate, Health Canada, dated February 20, 2017; unreferenced.
d Personal communication, email from Natural and Non-Prescription Health Products Directorate, Health Canada, to Consumer Product Safety Directorate, Health Canada, dated February 20, 2017; unreferenced.
e Calculated using the product dermal load as the maximum dermal amount.
f Calculated using a maximum dermal amount of 8.23 µg/cm2/24 h.
g A metabolism refinement was not applied to the infant age group.
h Calculated using a metabolism refinement of 29.86% propylparaben.
7.3.2 Health effects assessment
Propylparaben has been extensively reviewed, and key risk assessments have been conducted by the CIR (Andersen 2008), the EU SCCS (SCCP 2005a, 2005b, 2006, 2008; SCCS 2010, 2011, 2013), EFSA (2004), EMA (2015), NICNAS (2016), and independent scientists (Soni et al. 2005). The literature published until March 2017 was searched for relevant information to supplement the data used in the international reviews and assessments.
Toxicokinetics
A single radiolabeled dose of 100 mg/kg propylparaben was administered to rats by oral and dermal routes (Aubert et al. 2012). Maximum plasma concentrations (Cmax) was achieved in less than 1 hour and 8 hours, respectively with oral and dermal dosing. All administration routes produced a single peak in the plasma, corresponding to that of PHBA, and parent paraben was not detected. Over 70% of the oral dose was excreted in 24 hours, with <4% detected in feces and <1% in tissues. Approximately 60% of the applied dermal dose was not absorbed after 24 hours, 17% to 20 % was excreted in urine and <2% in feces, the remainder was thought to be in external tissues (e.g., hair, nails) (Aubert et al. 2012). When a single dose of propylparaben was administered orally (1 g/kg) or intravenously (50 mg/kg) to dogs, the parent compound was not detected in plasma at any time, and PHBA was detected within 1 hour (Jones et al. 1956). Fifty-three percent of the applied dose was excreted within 24 hours as PHBA and other metabolites; the parent compound was excreted at 0.042%. Urinary excretion increased to 96% after 24 hours when dogs were fed 1g/kg bw/day of propylparaben daily for 1 year. At sacrifice, small amounts of all propylparaben were detected in the brain (Jones et al. 1956).
When human subjects were orally administered up to 20 mg/kg bw propylparaben in a single dose, the parent compound was not detected in blood, but PHBA was detected within 60 minutes (Heim 1960, as cited in Andersen 2008). A single volunteer orally administered 2 g of propylparaben for 5 days excreted 17.4% as PHBA. Fifty-five percent of excreted propylparaben was conjugated with sulfuric acid but the parent form was not detected in urine. In this study, the majority of the administered dose was unaccounted for (Sabalitschka and Neufeld-Crzelliter 1954, as cited in Soni et al. 2005). These findings are consistent with those of Ye et al. (2006) who reported that parabens in urine were found predominantly in a conjugated form (95% to98% of detected parabens). The major isoform was sulphate-conjugated paraben, which accounted for 55% of recovered propylparaben. Although the majority of propylparabens appears to be rapidly eliminated from the body in humans, propylparaben have been detected at low levels in tumorous breast tissue, adipose tissue, and in the brain (Barr et al. 2012; Wang et al. 2015; van der Meer 2017). It was not reported whether the parabens were free or conjugated.
In liver and skin subcellular fractions, propylparaben is metabolized up to an order of magnitude less efficiently than methyl paraben and ethylparaben (Jewell et al. 2007a, 2007b; Harville et al. 2007; Lobemeier et al. 1996; Abbas et al. 2010; Prusakiewicz et al. 2006). In human plasma, propylparaben was reduced to 47% after 6 hours. Propylparaben had a half-life of 67 minutes in human liver microsomes (Abbas et al. 2010). Esterases identified in keratinocytes and subcutaneous fat demonstrate a preference for longer chain parabens (i.e., propylparaben, and butylparaben) with decreasing affinity for parabens with decreasing chain length (Lobemeier et al. 1996).
Unlike human kinetics, rat skin and liver cell fractions hydrolyze parabens at roughly the same rate in in vitro studies (Harville et al. 2007). However, rat skin cells hydrolyze propylparaben at a rate 3 orders of magnitude greater than that of human skin cells, consistent with overall rates of hydrolysis by carboxylesterases in these species (Harville et al. 2007; Prusakiewicz et al. 2006). Hydrolysis in rat tissue also increases with increasing chain length, unlike humans. Unlike shorter chain parabens, propylparaben was metabolized in rat liver at a rate approximately 10 times greater than that in human liver (Harville et al. 2007).
Repeat dose studies
In an OECD TG 422-compliant study (Combined Repeat Dose Toxicity and Reproduction/Developmental Toxicity Screening Test), Wistar rats (11/sex/group) were administered 0, 1 500, 4 500 or 15 000 ppm propylparaben in the diet (corresponding to 59.3–98.0, 178.3–305.1, and 605.0–980.9 mg/kg bw/day for the males and 116.0–137.3, 341.9–431.8, and 1 076.4–1 380.0 mg/kg bw/day for females) for up to 7 weeks. Male rats were treated for 28 days, and female rats were treated for 14 days prior to pairing, through the pairing and gestation periods until first filial (F1) PND 4. No signs of adverse toxicity were observed in the treated males and females at any dose. Reduced body weight gain was observed in males of the high-dose group, but there was no statistically significant change in the resulting body weights. In the high-dose group, there was also an increase in serum triglyceride concentration, with no further related changes (REACH 2018c). Reproductive effects are discussed in another section, below. Full details for this study were not available in the REACH dossier. A NOAEL of 1 076 mg/kg bw/day is identified for chronic toxicity.
Twelve weanling albino rats per dose were fed 2% or 8% propylparaben (equivalent to 900 to 1 200 and 5 500 to 5 900 mg/kg bw/day) in the diet for 96 weeks. Animals receiving 8% propylparaben had slower weight gain than negative controls in early stages of the experiment. The effect was greater in males and diminished by the end of the experiment. At 2%, no signs of toxicity were observed in treated animals compared to controls. The NOAEL identified for this study is 2% propylparaben (900 to 1 200 mg/kg bw/day, Matthews et al. 1956).
Genotoxicity
Propylparaben was negative in Ames assays and negative in chromosomal aberration assays in Chinese hamster lung fibroblasts at concentrations up to 0.45 mg/mL (REACH 2018c; McCann et al. 1975; Andersen 2008; Kier et al. 1986; Morita et al. 1981; Kawachi and Yahagi 1980; Odashima 1976). Propylparaben induced cytotoxicity and apoptosis/necrosis at 1% in human dermal fibroblasts and diminished cell proliferation and induced DNA breaks in Vero cells at 500 µM, and induced sister chromatid exchanges, chromosomal aberrations and positive comet assays at 1 to 1.5 mM in CHO-K1 cells (Tayama et al. 2008; Carvalho et al. 2012; Martin et al. 2010). Propylparaben was negative in in vivo genotoxicity assays, including a dominant lethal assay (Ayar and Uysal 2013; Odashima 1976; EFSA 2004). Overall, the weight of evidence indicates that propylparaben is not likely to be genotoxic in vivo (EFSA 1994; Andersen 2008).
Carcinogenicity
Male Fischer 344 rats (8/group) were exposed to PHBA, methylparaben, ethylparaben, propylparaben, and butylparaben at 4% in diet (approximately 4 000 mg/kg bw/day) for 9 days. Propylparaben a 6.7-fold increase in methyl-3[H]thymidine-labeling in the forestomach epithelium, indicating cell proliferation. Propylparaben caused a thickening of mucosa and treatment with all parabens resulted in lesions visible under light microscopy (mucosal thickening, rete pegs and papillae) that increased in severity with increasing chain length (Rodrigues et al. 1986).
Male Fischer 344 rats (5/group) were treated with 0%, 1% or 4% propylparaben in diet (approximately 1 000 and 4 000 mg/kg bw/day) for 9 days. A 1.5-fold increase in labeling in the prefundic region of the forestomach was observed in the 1% group and a 2.5-fold increase in the 4% treatment group. Histologically, hyperplasia and lesions were observed in both groups, with increased severity in the 4% group (Nera 1984). In contrast to these results, male Fischer 344 rats (5/group) were fed 3% propylparaben in diet for 8 weeks and did not show any labeling or hyperplastic effects in the forestomach or glandular stomach (Shibata et al. 1990). Likewise, male Golden hamsters administered 3% propylparaben in the diet for 20 weeks did not cause proliferative lesions, hyperplasia or increase in labeling indices in the forestomach (Hirose et al. 1986). Taken together, the results from cell proliferation studies suggest that there is a threshold effect at greater than 3% propylparaben exposure in diet.
Reproductive and developmental effects
Potential estrogenic effects of parabens are reviewed in Appendix C.
In an OECD TG 422-compliant study (Combined Repeat Dose Toxicity and Reproduction/Developmental Toxicity Screening Test), Wistar rats (11/sex/group) were administered 0, 1 500, 4 500 or 15 000 ppm propylparaben in the diet (corresponding to 59.3–98.0, 178.3–305.1, and 605.0–980.9 mg/kg bw/day for the males and 116.0–137.3, 341.9–431.8, and 1 076.4–1 380.0 mg/kg bw/day for females) for up to 7 weeks. Male rats were treated for 28 days and female rats were treated for 14 days prior to pairing, through pairing and gestation periods until F1 PND 4. Signs of general toxicity were discussed in a previous section. No effect was observed in the parental generation on reproductive parameters (sperm motility, morphology and sperm count, estrus cycle, mating performance, fertility, duration of gestation, corpora lutea count, implantation rate, post implantation and postnatal loss and litter size) and no test substance–related findings were observed in the offspring, either in the first 4 days post-partum, or at necropsy (REACH 2018c). Full details for this study were not available in the REACH dossier. A NOAEL of 1076 mg/kg bw/day is identified for reproductive effects.
In a study of male and female pubertal development and reproduction, juvenile Crl:CD rats (25/sex/dose) were treated by gavage with 0, 10, 100 or 1 000 mg/kg bw/day propylparaben from PND 4 to 90. At approximately PND 100, treated males and females were mated with untreated females/ males The F1 generation were sacrificed on PND 91 (end of dose) or following a treatment-free recovery period by PND 132 (25–27 days after cohabitation for males and on lactation day 5 to 7 for females). The F2-generation pups were evaluated for survival, sex, gross external dysmorphology, and body weight and euthanized thereafter on PND 4 to 6. In the F1 generation, no adverse effects were observed on body weights, body weight gains, food consumption, clinical pathology parameters, organ weights, or gross or histopathologic findings in reproductive tissues (ovaries, uterus testes, epididymides, prostate and seminal vesicles) at any dose level. No effects were noted on age of sexual development, estrous cycling, mating and fertility indices, mean number of days to mating, conception rate, gestation index, or length of gestation. At PND 24, uterine weights were examined in 6 animals per dose. No treatment-related gross uterine observations and no changes absolute or relative uterine weight were observed at any dose. In comparison, all rats treated with E2 (1 µg/kg bw/day) had a fluid filled uterus at necropsy and statistically significant increase in absolute and relative uterine weights, compared to control (4.3- and 4.2-fold, respectively). At the end of dosing (PND 91) there was a significantly increase in relative uterine weight in the 1 000 mg/kg bw/day group. This group also had an increased number of animals in proestrus and estrus (4/10 and 2/10, respectively) compared to controls (3/10 and 1/10) which increased the endometrial and luminal fluid (a natural feature of these stages of estrus) and thus contributed to the increased weight. Estrous data indicates that these animals were cycling normally at PND 89 and no differences were identified in the durations of estrous cycles, the duration of proestrus and estrus, or the mean numbers of cycles relative to those in control females. Early vaginal opening was observed in the high-dose group (day 31.2) when compared with controls (day 33.9), but was within the range of historical controls for the test facility (29 to 33.9 days). In the control group, 7/25 females displayed late development (35 to 43 days) that was outside of the range of historical controls resulting in a high control mean value. Small, soft testes were observed in 3/75 animals (1/25 at 10, 2/25 at 100 and 0/25 at 1 000 mg/kg bw/day), but were considered unrelated to treatment, as they are known to occur spontaneously in this animal strain. A single case of mammary adenocarcinoma in 1 female at 1 000 mg/kg bw/day at end of dose necropsy was considered incidental due to no observation of precursor lesions in end of recovery group, within historical range for testing facility, known to occur spontaneously in juvenile and adult rats. In treated females mated to naïve males, no effects were observed on the sex ratio of offspring, number of live and dead pups at birth, number of implantations, and live births. In naïve females mated with treated males, the number of corporea lutea, implantation sites, live embryos, dead embryos, early resorptions, pre- and post-implantation losses were not different in treatment groups compared to controls. In the offspring of treated/naïve pairings, no clinical signs, malformations, effects on viability, or body weight were observed (Sivaraman et al. 2018; Pouliot 2013). The NOAEL identified for this study is 1 000 mg/kg bw/day.
In a study of male pubertal development, Wistar rats (20 per group) were administered 0, 3, 10, 100 or 1 000 mg/kg bw/day propylparaben by gavage for 8 weeks starting at PND 21. Ten animals per group were sacrificed at the end of treatment, and the remaining 10 were sacrificed after a 26-week washout period. Hypersalivation was observed in the high-dose animals through the end of the treatment period. No other treatment-related clinical signs and no effect on mean body weight were observed. No effects were observed on the date of onset of puberty (as measured by balanopreputial separation) the weight of the male reproductive organs (epididymis, prostate and seminal vesicle, and testis), epididymal sperm parameters, hormone levels (LH, FHS and testosterone), or histopathology at the end of treatment or the recovery period. Propylparaben did not affect mean testicular spermatid counts nor epididymal sperm counts or mean motility parameters in any group at the end of either treatment or recovery. Reduced sperm counts were noted between the end of treatment (77 days) and the end of recovery (258) in 1/10 animals given 10 mg/kg bw/day and 1/10 animals given 1 000 mg/kg bw/day. The same animals exhibited severe testicular atrophy/hypoplasia or hypospermatogenesis. These effects were considered sporadic occurrences and at the end of the recovery period, the mean testicular spermatid count was not significantly different from control (Gazin et al. 2013). A NOAEL of 1 000 mg/kg bw/day, the highest dose tested, was identified for this study.
Immature male Crj:Wistar rats (8 animals per group) were treated with 0%, 0.01%, 0.1% and 1.0% propylparaben administered in diet (equivalent to 12.4 ±3.04, 125 ±30.0, and 1290± 283 mg/kg bw/day) for 4 weeks starting on PND 19 to 21. A significant reduction in body weight was observed at the highest dose. Treatment-related effects on the testes, epididymides, ventral prostates, seminal vesicles and preputial glands (either absolute or relative to body weight) were not observed. Cauda epididymal sperm reserves (number of sperm/cauda epididymis) and sperm concentrations (number of sperm/g cauda epididymis) decreased in a dose-dependent manner, significant at 0.1% and above. Daily sperm production and its efficiency in the testis also decreased dose-dependently and the decrease was significant in all treated groups. Serum testosterone concentration decreased in a dose-dependent manner. The decrease was significant at a dose of 1.00% and the testosterone concentration at this dose was 64.6% of the control value (Oishi 2004). The SCCS (2008, 2011) has expressed doubt as to the ability of the Oishi laboratory to evaluate the parameters measured and has concluded that the quality of the Oishi studies could not be properly assessed as the full test description and the complete raw data packages were not available. Specifically, in this and other studies, the Oishi laboratory has reported (i) mean values for some parameters that fell far outside the historical control ranges and (ii) standard deviations for parameters including epididymal sperm concentrations and testosterone levels that were far less than the normal biological variability that has been observed by other groups. Furthermore, the effects described by Oishi (2004) were not observed in Sivaraman et al. (2018) or Gazin et al. (2013) each of which considered a larger group size for a longer dosing period; furthermore, the sperm-related parameters observed were less precise than those reported in Gazin et al. (2013) and historical controls were not reported. The reproductive success reported in Sivaraman et al. (2018) also indicates that the effects reported here were not biologically significant.
In a study of female pubertal development, pre-pubertal Sprague Dawley rats (10 animals per group) were orally administered 0, 62.5, 250, 1 000 mg/kg bw/day of propylparaben in corn oil from postnatal day 21 to 40. Ethinylestradiol (1 mg/kg bw/day) was administered as a positive control. Treatment-related effects were not observed in body weight, organ weights (with the exception of adrenal) vaginal opening day, or estrous cycling. A significant increase in adrenal weight was observed at the highest dose. Serum T4 levels were significantly reduced in the mid-dose group, but not the high or low dose. Myometrial hypertrophy and a significant increase in thickness of the uterine wall were observed in the high-dose group, although there was no corresponding increase in uterine weight (Vo et al. 2010). The estrous day of animals at sacrifice was not reported and no effect was reported for relative uterine weight. As noted by Stump et al. (2014) and Sivaraman et al. (2018), estrous stage at termination correlates with uterine histological effects and the natural variability of estrous stage at terminal stage imparts variability on uterine weight, therefore, it is not clear that the change in uterine wall thickness is test substance-related.
Epidemiology
A literature search was conducted to identify epidemiological data for propylparaben, with a focus on reproductive and endocrine effects. Studies were evaluated and scored for quality using the Newcastle-Ottawa Quality Assessment Scale (NOS) and the Quality Assessment Tool for Observational Cohort and Cross-sectional Studies from the National Heart, Lung, and Blood Institute of the National Institutes of Health (RSI 2018). Epidemiology studies were identified that addressed exposure to propylparaben in relation to fertility, reproductive health, serum hormone levels, growth and development in children, allergic sensitization, and respiratory health. The majority of studies did not report an association between propylparaben and the endpoint of interest. Positive associations were reported between propylparaben concentrations and decreased odds of live birth in intrauterine insemination, birth weight in males, and allergic sensitization (Dodge et al. 2015; Philippat et al. 2014; Savage et al. 2012; Spanier et al. 2014). An inverse association was identified between propylparaben concentrations and serum thyroid hormone levels in adult females (pregnant and general population), BMI in a general population and pregnant women (Koeppe et al. 2013; Kang et al. 2016; Aker et al. 2018; Smith et al. 2012; Polinski et al. 2018), and between propylparaben levels in cord blood and fetal testosterone levels (Kolatorova et al. 2018). For all endpoints with an identified association, other than fetal testosterone levels, different studies of similar quality gave conflicting results for these or similar endpoints. More detail is provided in Health Canada (2018a).
7.3.3 Characterization of risk to human health
Propylparaben demonstrated low toxicity in repeat dose studies in which dietary doses in the range of 900 to 1 200 mg/kg bw/day did not result in adverse effects (REACH 2018c; Matthews et al. 1956). Weight of evidence indicates that propylparaben is not genotoxic or carcinogenic.
In a study of male and female pubertal development and reproduction, oral (gavage) doses of propylparaben up to 1 000 mg/kg bw/day did not induce effects on a wide range of reproductive development and fertility endpoints in male and female rats (Sivaraman et al. 2018; Pouliot 2013). An OECD TG 422 study reported in the REACH dossier supports these results (REACH 2018c). No effects were observed on male pubertal development and sperm parameters in an additional study, at doses of up to 1 000 mg/kg bw/day (Gazin et al. 2013). Two studies (Oishi 2004 and Vo et al. 2010) reported effects on male and female pubertal development. However, the reports by Sivaraman et al. and Pouliot effectively refute these findings and, as discussed elsewhere, multiple concerns with the Oishi papers have been reported by the SCCS. Considering the totality of evidence, a NOAEL of 1 000 mg/kg bw/day, the highest dose tested in Sivaraman et al. (2008), was selected as a point of departure for propylparaben.
The Canadian population is exposed to propylparaben via food, products available to consumers, cosmetics, prescription and non-prescription drugs and NHPs. Monitoring data available for some environmental media sources and worldwide data suggest that Canadians are also exposed to propylparaben via these sources.
The assessment of general population exposure to propylparaben is based on biomonitoring data from the CHMS, the Plastics and Personal-Care Product Use in Pregnancy (or P4) Study, and a study of Korean neonates within 48 hours of birth (Health Canada 2017a; Fisher et al. 2017; Kang et al. 2013). These data encompass exposures to propylparaben from multiple potential sources and routes, and are considered reliable estimates of exposure of the general population in Canada to propylparaben. Biomonitoring data provide an internal measures of exposure, because they include specific measurements of propylparaben in urine, and because they reflect the integrated exposure to propylparaben from multiple sources, including propylparaben-containing products used by consumers. Urine is the major route of excretion for propylparaben and the biomarker (free and conjugated propylparaben) is specific to the parent compound. However, propylparaben has a low fractional urinary excretion and a short half-life. To address the uncertainty associated with the robustness of the use of propylparaben as a biomarker, estimates of daily intake were based on the upper-bound of 95th percentile (or highest available) exposure value for each age band and are therefore considered a conservative scenario. Margins of exposure resulting from estimated daily intakes are presented in Table 7‑14.
| Source location | Age group (years) | Upper-bound estimated daily intake at P95 (mg/kg bw/day) | Critical effect levela (mg/kg bw/day) |
MOE |
|---|---|---|---|---|
| CHMS (Cycle 4, 2014-2015), Canada | 3–5 | 0.010 | NOAEL 1 000 (HDT) | 96 476 |
| CHMS (Cycle 4, 2014-2015), Canada | 6–11 | 0.039 | NOAEL 1 000 (HDT) | 25 725 |
| CHMS (Cycle 4, 2014-2015), Canada | 12–19 | 0.027 | NOAEL 1 000 (HDT) | 37 493 |
| CHMS (Cycle 4, 2014-2015), Canada | 20–59 | 0.029 | NOAEL 1 000 (HDT) | 34 436 |
| CHMS (Cycle 4, 2014-2015), Canada | 60–79 | 0.0541 | NOAEL 1 000 (HDT) | 24 530 |
| Fisher et al. 2017, Canada | Pregnant women | 0.023 | NOAEL 1 000 (HDT) | 43 668 |
| Kang et al. 2013, Korea | Neonates | 0.0011 | NOAEL 1 000 (HDT) | 895 645 |
Abbreviations: HDT, Highest dose tested; NOAEL, no observed adverse effect level.
a Critical effect: no effect on reproduction, reproductive development, or prenatal development.
On the basis of conservative parameters used to model estimated daily intake, the margins of exposure are considered adequate to address the risk to populations aged 3 to 79 years, pregnant women and neonates. Biomonitoring data were not available to address children under 3 years (other than neonates). With the exception of breast milk consumption, exposure for this group is not expected to differ significantly from the youngest age group surveyed by CHMS or from the neonatal group presented here. Breast milk consumption was modelled using Canadian data and is not expected to pose a risk. Therefore, the reported urinary concentrations and estimated daily intakes across all age groups indicate that exposure in children under 3 years is not expected to pose a risk.
Due to the short half-life of propylparaben administered by the oral route and the spot sampling methodology used in biomonitoring studies, it is not clear that biomonitoring data adequately addresses high exposures to cosmetics, prescription and non-prescription drugs and NHPs with intermittent use patterns. Sentinel scenarios are presented in Table 7‑15.
| Exposure scenario | Age group (year) |
Systemic exposure (mg/kg bw/day) | Critical effect levela (mg/kg bw/day) |
MOE |
|---|---|---|---|---|
| Face Paintb (dermal, per event) |
Toddler (0.5–4) |
0.031–0.93 | NOAEL 1 000 (HDT) | 1 075–32 258 |
| Heartburn medicationc (oral, per day) |
Adult (> 20) |
29.33 | NOAEL 1 000 (HDT) | 34 |
| Fluoride treatmentc (oral, per event) | Child (5–11) |
0.52 | NOAEL 1 000 (HDT) | 1 923 |
| Fluoride treatmentc (oral, per event) | Adult (> 20) |
0.23 | NOAEL 1 000 (HDT) | 4 348 |
| Sunscreend (dermal, per day) |
Infant (0–0.5) |
1.84 | NOAEL 1 000 (HDT) | 544 |
| Sunscreenc (dermal, per day) |
Toddler (0.5–4) |
0.78 | NOAEL 1 000 (HDT) | 1 282 |
| Sunscreenc (dermal, per day) |
Adult (> 20) |
0.58 | NOAEL 1 000 (HDT) | 1 724 |
Abbreviation: HDT, Highest dose tested; NOAEL, no observed adverse effect level.
a The critical effect was no effect on reproduction, reproductive development, or prenatal development.
b Cosmetic
c Natural health product.
d Non-prescription drug.
The margins of exposure for oral exposure to heartburn medication are considered potentially inadequate to address uncertainties in the health effects and exposure databases.
Data from an in vitro study of cutaneous metabolism of propylparaben suggested that propylparaben may be hydrolyzed to ethylparaben and methylparaben in the skin (Health Canada 2018b). This potential exposure is incorporated into biomonitoring data for methylparaben and ethylparaben. Potential risk from this putative route of exposure is addressed in Sections 7.1.3 and 7.2.3.
7.4 Butylparaben
7.4.1 Exposure assessment
Butylparaben is naturally occurring in some foods and has been identified in environmental media. It is also present in a number of products, including non-prescription drugs, NHPs, cosmetics, and pest control products. All of these sources may contribute to total daily exposure to butylparaben. Urinary concentrations and estimated exposure of Canadians to butylparaben are presented in the following section.
Biomonitoring
Biomonitoring data collected in Cycle 4 of the CHMS indicated over 83% of Canadians tested had a urinary concentration of butylparaben that was below the level of detection (0.30 ng/mL). The 95th percentile urinary concentration in Canadians aged 3 to 79 years was 4.3 µg/LFootnote 11 (95% confidence interval of 2.0 to 6.6 µg/L, n = 2 564) of butylparaben. Butylparaben was more frequently detected in females than in males, with 76.26% of samples below the level of detection compared to 90.12%. Among different age groups, the group with the highest frequency of detection was adults aged 60 to 79 years, in which 80.0% of samples had butylparaben concentrations below the level of detection and the 95th percentile was 6.8 µg/L (95% confidence interval of 4.4 to 9.1 µg/L). The age group with the lowest frequency of detection was children aged 6 to 11 years, in which 90.08% had urinary concentrations below the level of detection and the 95th percentile was 1.1 µg/L9 (95% confidence interval of 0.3 to 1.8 µg/L) (Health Canada 2017a). In Canadian regional studies, the mean urinary concentration of butylparaben in females was 2.08 µg/L (n = 28 including 9 pregnant patients, median = 0.3 µg/L) and the geometric mean in pregnant females (n = 31) was 3.28 µg/L The mean urinary concentration in males (n = 11) was 0.36 µg/L (media n = 0.35 µg/L; Genuis et al. 2013).
Butylparaben was detected at a maximum concentration of 1.4 µg/L (free plus conjugated paraben, median was “not detectable”) in urine collected from Korean infants within 48 hours of delivery (Kang et al. 2013). Butylparaben was not detected in breast milk in 56 Canadian women (LOD 0.1 µg/L), 3 months post-partum (Fisher et al. 2017). Butylparaben was detected in placenta in a sample of 12 women (Barcelona, maximum = 0.90 ng/g fresh weight) and in amniotic fluid in 40 pregnant women (India, GM = 1.79 µg/L) (Valle-Sistac et al. 2016; Shekhar et al. 2017). Butylparaben was detected in cord blood at a mean of 0.033 µg/L in 50 mother-child pairs and at a mean of 0.07 µg/L in 34 samples in two studies in the United States (Towers et al. 2015; Geer et al. 2017).
Using the methodology described for methylparaben (section 7.1.1), estimated daily intakes were derived for the general population, pregnant females and neonates, based on biomonitoring data (CHMS, Health Canada 2017a; Fisher et al. 2017; Kang 2017). In each of the three biomonitoring studies, total butylparaben (i.e., free and conjugated species) was measured in urine by HLPC, after treatment with β-glucuronidase and sulfatase. Neonates were included because the detection of butylparaben in cord blood, placenta and amniotic fluid indicate that butylparaben passes through the placenta and exposure may occur in utero (Shekhar et al. 2017; Valle-Sistac et al. 2016; Towers et al. 2015; Geer et al. 2017). Urinary concentrations from Korean neonates were used in the absence of data from a Canadian population; this is considered a conservative choice because the 95th percentile urinary concentrations of butylparaben in the general Korean population exceed those of the general Canadian population (Honda et al. 2018).
In a study of pharmacokinetics in response to oral exposure to methylparaben, butylparaben and iso-butylparaben in humans, three adults (two males and one female) ingested 10 mg each of radiolabelled paraben (Moos et al. 2016). The elimination half-life of butylparaben was 3.7 hours, and 80.8% of the applied dose was eliminated in urine in 24 hours as butylparaben, ring-hydroxylated butylparaben, PHBA and PHHA. After 48 hours, 5.6% of the administered dose was recovered as total butylparaben (free, plus conjugated species; Moos et al. 2016). High recovery of the administered dose in urine indicates that this is the primary mode of excretion for butylparaben. However, butylparaben has a short half-life and is not the major metabolite recovered. Nevertheless, butylparaben is a specific biomarker of exposure and the fractional urinary excretion values reported among study participants showed little variation (ranging from 0.052 to 0.064). The value of 5.6% was used as the fractional urinary excretion value in the calculation of biomonitoring equivalents and estimated daily intake for butylparaben, based on the results of Moos et al. (2016). The use of an oral fractional urinary excretion value may under- or over-estimate dermal exposure, and the use of the parent paraben as a biomarker provides a less robust measure of exposure as it is not the major metabolite. As with other commonly used parabens, the use of the parent compound as a biomarker may be susceptible to contamination during collection and analysis, which could further inflate the estimate (Aylward et al. 2017a).
Estimated daily intake values were calculated using the 95th percentile 95% confidence interval values (or highest available values) for creatinine-adjusted urinary concentration (µg/g Cr) for each age group, as described for methylparaben (section 7.1.1). A high level of variability was associated with CHMS biomonitoring data for butylparaben in age-stratified groups at the 95th percentile and the geometric mean was not calculated for the whole population, or for age-stratified groups because over 83% of samples were below the limit of detectionFootnote 12 (Health Canada 2017a). Given the short half-life of butylparaben (Moos et al. 2016), the preponderance of samples below the limit of detection may be due to rapid metabolism or to low exposure. According to data submitted by Health Canada, butylparaben is used in approximately 85% fewer products (i.e., cosmetics, NHPs and non-prescription drugs) than methylparaben and is not permitted for use as a food additive, indicating that low exposure can be expected to account for a portion of the lower detection levels of butylparaben. Uncertainty due to variability and potential rapid metabolism were addressed by selecting the lower and upper bounds of the 95th percentile 95% confidence interval as a conservative lower and upper bound values for deriving estimated daily intake, a conservative estimate that addresses the variation inherent in spot sampling. The fractional urinary excretion value of 0.056 or 5.6% for butylparaben was reported in Moos et al. (2016) and age group-specific values for 24-hour urinary volumes were estimated in Aylward et al. (2015).
Estimated daily intakes and key parameters are presented in Table 7‑16. The values calculated here for the Canadian population are similar to daily intakes of butylparaben calculated at the 95th percentile for an adult German population (ranging from 2.2 to 7.6 µg/kg bw/day), as reported in Moos et al. (2017). See Appendix A for further details of the derivation of estimated daily intake values.
| Source, Location | Age (years)a |
CER (mg/day)b | UCCr, P95 (CI) (µg/g Cr)c | FUEd | EDI, P95 (CI) (µg/kg bw/day) |
|---|---|---|---|---|---|
| CHMS (Cycle 4, 2014-2015)c, Canada | 3–5 | 130 | 3.1 (<LOD–5.1)e | 0.056 | 0.46 (NC–0.8) |
| CHMS (Cycle 4, 2014-2015)c, Canada | 6–11 | 418 | 0.8 (0.3–1.3)e | 0.056 | 0.19 (0.07–0.31) |
| CHMS (Cycle 4, 2014-2015)c, Canada | 12–19 | 1182 | 9.2 (<LOD–15)f | 0.056 | 3.3 (NC–5.3) |
| CHMS (Cycle 4, 2014-2015)c, Canada | 20–59g | 1248 | 9.2 (<LOD–15)f | 0.056 | 2.9 (NC–4.72) |
| CHMS (Cycle 4, 2014-2015)c, Canada | 60–79 | 1017 | 6.7 (2.1–11)e | 0.056 | 1.7 (0.53–2.8) |
| Fisher et al. 2017, Canada | Pregnant women | - | 12.20h | 0.056 | 4.4 |
| Kang et al. 2013, Korea | Neonates | 9.6 | 3.4i | 0.056 | 0.18 |
Abbreviations: CER, creatinine excretion rate; UCCr, creatinine-adjusted urinary concentration; P95, 95th percentile; CI, confidence interval; FUE, fractional urinary excretion; EDI, estimated daily intake; NC, not calculated.
a Age groups are defined based on age groups reported by CHMS (Health Canada 2017a).
b Creatinine excretion rate was calculated using the Mage equation [0.993*1.64 [140 – Age] (Wt^1.5 Ht^0.5)/1000]. See Appendix A for values used for age weight and height.
c Health Canada 2017a.
d Moos et al. 2016.
e These values were associated with high sampling variability (i.e., coefficient of variation between 16.6% and 33.3%). Health Canada recommends that this data be used with caution (Health Canada 2017a).
f CHMS data for the 95th percentile in this age stratum was suppressed due to high variability and “females 3 to 79” was used as a surrogate. Although the 95th percentile value for this group is not known, this approach is considered conservative because the value used to estimate daily intake is the highest reported 95th percentile value for ethylparaben.
g The “20-39” and “40-59” age groups are presented together. When 95th percentile values were reported for both age groups, the higher value is presented here; when one value was suppressed, the value from the other group is presented here.
h This value is the specific gravity-adjusted urinary paraben concentration (µg/L) at the 95th percentile; creatinine-adjusted values were not reported in Fisher et al. 2017. Confidence intervals were not reported. EDI was calculated using the following equation: EDI = (UC*UFR)/FUE (Saravanabhavan et al. 2014), where UC is the urinary concentration, UFR is the urinary flow rate (0.20 L/kg bw/day, Aylward et al. 2015) and FUE is the fractional urinary excretion.
i This value is the 75th percentile creatinine-adjusted urinary paraben concentration; the 95th percentile was not reported (Kang et al. 2013). Confidence intervals were not reported.
Environmental media
Butylparaben has been identified in agricultural soil and house dust in Canada (Viglino et al. 2011; Fan et al. 2010). In studies carried out in other countries, it has been identified in drinking water (Carmona et al. 2014), agricultural, industrial and forestry soil (Perez et al. 2011; Nunez et al. 2008), outdoor air (Moreau-Guigon et al. 2016), residential indoor air (Laborie et al. 2016; Moreau-Guigon et al. 2016), and house dust (Wang et al. 2012; Ramirez et al. 2011; Tran et al. 2016; Canosa et al. 2007). Average estimates of daily intake of butylparaben by the general population of Canada based on these studies range from < 0.001 to 0.003 µg/kg bw/day for all age groups from infants to adults over 60 years.
In the absence of Canadian monitoring data, the total reported import volume of butylparaben in Canada (up to 1 000 kg) was used as conservative input to estimate theoretical environmental concentrations of butylparaben using the level III fugacity model ChemCAN version 6.00 (ChemCAN 2003). The theoretical environmental concentrations derived from this approach were used to estimate potential exposures from environmental media for the general population of Canada. This results in an estimated intake of 0.342 ng/kg bw/day from air, water and soil. This approach indicates that general population exposure due to environmental media in Canada is potentially negligible.
Food
Butylparaben exposure from food is expected to be negligible (personal communication, email from the Health Canada Food Directorate, Health Canada, to the Health Canada Consumer Product Safety Directorate, Health Canada, dated April 18, 2018; unreferenced). According to biomonitoring data (Fisher et al. 2017), butylparaben was not detected in breast milk in 56 Canadian women (LOD 0.1 µg/L), 3 months post-partum; breastfed infants are therefore not expected to be exposed to butylparaben via breast milk.
Cosmetics
Butylparaben was identified in a variety of cosmetics including lotions, shaving products, hair care products, oral care products, and cleansers. Exposures from daily use of cosmetics and products available to consumers were considered to be addressed by biomonitoring data. Due to the use of spot sampling and the short metabolic half-life of butylparaben, exposures from products with intermittent uses were potentially not addressed by biomonitoring data. Sentinel scenarios from products with intermittent use patterns are presented in Table 7‑17.
Experimentally determined dermal absorption and metabolism coefficients from an in vitro study of dermal absorption and metabolism in human skin were used to estimate an internal dose from potential exposure by the dermal route, as described in section 7.1.1, and further details are available on request (Charles River Laboratories 2018; Health Canada 2018b).
Potential exposures were estimated using conservative assumptions and default values as outlined in Appendix B. Exposure estimates for each scenario are expressed on a per-event and/or a daily basis, depending on exposure frequency (see section 7.4.3, Characterization of risk to human health).
| Product scenario | Concentration in product (%)a | Age group (years) |
Dermal load (µg/cm2/24 h) | Systemic exposure (mg/kg bw/day) |
|---|---|---|---|---|
| Hair dye (permanent, per event) | 1–3 | Teen (12–19) |
137.0–411.0 | 0.36–1.09b,c |
| Hair dye (permanent, per event) | 1–3 | Adult (> 20) |
156.9–470.59 | 0.30–0.91b,c |
a Internal data, Consumer Product Safety Directorate, Health Canada, dated August 18, 2017; unreferenced.
b Calculated using 54.71% dermal absorption.
c Calculated using a metabolism refinement of 39.35% butylparaben.
Non-prescription drugs and NHPs
Butylparaben is present as a non-medicinal ingredient in non-prescription drugs and NHPs administered by multiple routes. Exposures from daily use of non-prescription drugs and NHPs are considered to be addressed by biomonitoring data. Sentinel scenarios from products with intermittent use patterns are presented in Table 7‑18. Default values and models used in exposure scenarios are provided in Appendix B.
| Product scenario | Concentration in product (%) | Age group (years) |
Dermal load (µg/cm2/24 h) | Systemic exposure (mg/kg bw/day) |
|---|---|---|---|---|
| Herbal cough medicinea (oral, per day) |
0.02c | Teen (12–19) |
N/A | 0.14 |
| Herbal cough medicinea (oral, per day) |
0.02c | Adult (> 20) |
N/A | 0.12 |
| Children’s analgesic suspensionb (oral, per day) |
0.13d | Toddler (0.5–4) |
N/A | 2.10 |
| Antacidb (oral, per day) |
0.033d | Teen (12–19) |
N/A | 0.44 |
| Antacidb (oral, per day) |
0.033d | Adult (> 20) |
N/A | 0.37 |
| Analgesic creama (dermal, per day) | 0.0088–0.044c | Child (5–11) |
0.16–0.78 | 0.012–0.060e,f |
| Analgesic creama (dermal, per day) | 0.0088–0.044c | Adult (> 20) |
0.16–0.78 | 0.011–0.053e,f |
| Medicated hand creama (dermal, per day) |
0.06c | Child (5–11) |
3.36 | 0.020e,f |
| Medicated hand creama (dermal, per day) |
0.06c | Adult (> 20) |
3.36 | 0.017e,f |
| Sunscreena (dermal, per day) | 0.4c | Toddler (0.5–4) |
15.6 | 0.44g,h |
| Sunscreena (dermal, per day) | 0.4c | Adult (> 20) |
15.6 | 0.33g,h |
a Natural health product
b Non-prescription drug
c Personal communication, email from Health Canada Natural and Non-Prescription Health Products Directorate, Health Canada, to Consumer Product Safety Directorate, Health Canada, dated February 20, 2017; unreferenced.
d Personal communication, email from Therapeutic Products Directorate, Health Canada, to Consumer Product Safety Directorate, Health Canada, dated May 25, 2017; unreferenced.
e Calculated using the product dermal load as the maximum dermal amount.
f Calculated using a metabolism refinement of 47.93% ethylparaben.
g Calculated using a maximum dermal amount of 3.94 µg/cm2/24 h.
h Calculated using a metabolism refinement of 35.33% butylparaben.
7.4.2 Health effects assessment
Butylparaben has been extensively reviewed, and key risk assessments have been conducted by the CIR (Andersen 2008), the EU SCCS (SCCP 2005a, 2005b, 2006, 2008; SCCS 2010, 2011, 2013), EFSA (2004), NICNAS (2016) and independent scientists (Soni et al. 2005). The literature published until March 2017 was searched for relevant information to supplement the data used in the international reviews and assessments.
Toxicokinetics
Butylparaben is highly metabolized in animals by oral, dermal and subcutaneous routes of exposure (Jones et al. 1956; Kiwada et al. 1979, 1980; Tsukamoto and Terada 1964; Aubert et al. 2012). A single radiolabeled dose of 100 mg/kg butylparaben was administered to rats by oral, dermal and subcutaneous routes (Aubert et al. 2012). Maximum plasma concentrations (Cmax) were achieved in less than 1 hour, 8 hours, and 2 to 4 hours, respectively with oral, dermal and subcutaneous dosing. All administration routes produced a single peak in the plasma, corresponding to that of PHBA, and parent parabens were not detected. Over 70% of the oral dose was excreted in urine in 24 hours, with ≤3% detected in feces and a negligible amount in tissues. More than 50% of the applied dermal dose was not absorbed after 24 hours, approximately 25% was excreted in urine and <2% in feces, the remainder was thought to be in mainly in external tissues (e.g., hair, nails) (Aubert et al. 2012). When a single doses of butylparaben was administered orally (1 g/kg) or intravenously (50 mg/kg) to dogs, the parent compound was not detected in plasma for any of the parabens tested, and PHBA was detected within 1 hour (Jones et al. 1956). The majority of the applied dose (40% to 47%) was excreted within 24 hours as PHBA; less than 1% of the applied compound was excreted as butylparaben. At sacrifice, small amounts of butylparaben was detected in the brain, pancreas, and spleen (Jones et al. 1956). In rats, <1% of oral doses of butylparaben remained in the tissues at 24 or 72 hours post-dosing (Mathews 1956).
Moos et al. (2016) report the ingestion of 10 mg of butylparaben by 3 adult volunteers (equivalent to 0.12–0.19 mg/kg bw). The elimination half-life was 3 to 4 hours, with 80% of administered dose recovered in urine in 24 hours. After 48 hours, 5.6% of free and conjugated butylparaben was recovered in urine. PHHA, the main metabolite, comprised 62% of the applied dose recovered in urine. 26 healthy males received a whole body application of 2 mg/cm2 of a standard cream containing 2% butylparaben (approximately equivalent to 11 mg/kg bw/day), as well as 2% each of diethyl phthalate and dibutyl phthalate. The mean recovery of butylparaben in urine was 0.32% of the applied dose, of which the majority was conjugated to glucuronate and sulphate and 2.1% was free paraben. Butylparaben has been detected at low levels in tumorous breast tissue and in human adipose tissue, although it was not reported whether the parabens were free or conjugated (Barr et al. 2012; Wang et al. 2015).
In human liver and skin subcellular fractions, butylparaben is hydrolyzed 2 to 10 times less efficiently than shorter-chain parabens (Jewell et al. 2007a, 2007b; Harville et al. 2007; Lobemeier et al. 1996; Abbas et al. 2010; Prusakiewicz et al. 2006). In ex vivo experiments using human plasma, the half-life of butylparaben was approximately 1 hour. In liver microsomes, butylparaben has a half-life of 87 minutes (Abbas et al. 2010). Esterases identified in keratinocytes and subcutaneous fat demonstrate a preference for butylparaben with decreasing affinity for parabens with decreasing chain length (Lobemeier et al. 1996).
Unlike human kinetics, rat skin and liver cell fractions hydrolyze butylparaben at roughly the same rate in in vitro studies (Harville et al. 2007). However, rat skin cells hydrolyze butylparaben at a rate approximately 3 orders of magnitude higher than that of human skin cells (Harville et al. 2007; Prusakiewicz et al. 2006). Hydrolysis in rat tissue also increases with increasing chain length, unlike humans. Shorter chain parabens (methylparaben and ethylparaben) are metabolized in human liver cell fractions at a rate comparable to that in rat liver. However, butylparaben was metabolized in rat liver at a rate 10 times greater than that in human liver (Harville et al. 2007).
Repeat dose studies
In a 6-week study, ICR/Jcl mice (10/sex/group) were exposed to 0%, 0.6%, 1.25%, 2.5%, 5% or 10% butylparaben in the diet (corresponding to approximately 900, 1 875, 3 750, 7 500 or 15 000 mg/kg bw/day). All rats in the two highest dose groups died within 2 weeks, and weight gain in the next two highest doses was less than 10% that of the control group. All doses higher than 0.6% lead to marked atrophy of lymphoid tissue in organs including the spleen, thymus and lymph nodes and multifocal degeneration and necrosis in the liver parenchyma. No significant changes in the visceral organs were observed in the 0.6% dose group or the controls (Inai et al. 1985). Statistical analysis was not reported. The NOAEL identified in this study was 0.6% (approximately 900 mg/kg bw/day).
Twelve weanling albino rats per dose (6 male and 6 female) were fed 2% or 8% butylparaben (equivalent to 900 to 1 200 and 5 500 to 5 900 mg/kg bw/day) in diet for 12 weeks. Animals receiving 8% butylparaben showed a slower weight gain than negative controls in early stages of the experiment. Signs of toxicity such as depression and decreased motor activity as well as mortality were also observed in this group. All males in the 8% group died before the end of the 12-week treatment. Similar, but less severe effects were seen in females. At 2%, no signs of toxicity were observed in treated animals compared to controls. Statistical analysis was not reported. The NOAEL in this study was 2% butylparaben (900 to 1 200 mg/kg bw/day), based on effects observed at 8% (Matthews et al. 1956).
Genotoxicity
Butylparaben was negative in Ames assays and in chromosomal assays up to 0.06 mg/mL, but induced positive responses in a sister chromatid exchange, chromosome aberration and comet assay at 0.4 to 0.75 mM (Ishizaki 1978; Ishidate et al. 1978, 1984; Tayama et al. 2008; Morita et al. 1981; Kawachi and Yahagi 1980). Butylparaben was not genotoxic in in vivo assays, including dominant lethal and in vivo chromosome aberration assays (EFSA 2004; Kawachi and Yahagi 1980). Overall, the weight of evidence indicates that butylparaben is not likely to be genotoxic in vivo (EFSA 1994; Andersen 2008).
Carcinogenicity
Male Fischer 344 rats (8/group) were exposed to PHBA, methylparaben, ethylparaben, propylparaben, and butylparaben at 4% in diet (approximately 4 000 mg/kg bw/day) for 9 days. Butylparaben caused an 11.3-fold increase in methyl-3[H]thymidine-labeling in the forestomach epithelium, indicating cell proliferation. Butylparaben caused a thickening of mucosa and treatment with all parabens resulted in lesions visible under light microscopy (mucosal thickening, rete pegs and papillae) that increased in severity with increasing chain length (Rodrigues et al. 1986).
ICL/Jcr mice (50/group) were fed 0%, 0.15%, 0.3% or 0.6% butylparaben in diet for 102 weeks (equivalent to approximately 235, 494 and 964 mg/kg bw/day in males and 172, 373 and 772 mg/kg bw/day in females). High numbers of animals died in all groups (control and test groups) before 78 weeks. Tumour incidences were similar in test groups compared with the control, and there were no significant differences between dose groups. Twenty-one total neoplasms were observed in the control group; 21, 19 and 33 in the butylparaben low-, mid-, and high-dose groups, respectively. The authors report that there were no apparent differences in incidence or time-to-death from neoplasms between test and control groups (Inai et al. 1985). Statistical analysis was not reported. The NOAEL identified for this study is 772 mg/kg bw/day, the highest dose tested.
Reproductive and developmental effects
Potential estrogenic effects of parabens are reviewed in Appendix C.
In a study of early male and female reproductive system development, pregnant Wistar rats (18 dams/group; 12 to 18 pups/group) were orally administered 0, 10, 100 or 500 mg/kg bw/day butylparaben from GD 7 to PND 22. Exposure to butylparaben did not affect maternal reproductive parameters, including maternal body weight gain, gestation length, litter size, pre-, or perinatal offspring survival, or pup body weight either at birth or in the postnatal period. Reduced anogenital distance in newborns of both sexes, reduced ovary weight and increased mammary gland growth were observed in the mid- and high-dose groups; effects demonstrated dose-dependence. Epididymal sperm count in males was significantly reduced at 10 mg/kg bw/day and higher. Prostate histology was altered at pre-puberty and adult prostate weights (at PND 90) were reduced in the high-dose group. The testicular expression of markers of steroidogenesis CYP 19a1 (aromatase) and Nr5a1 were reduced at all doses in prepubertal and adult animals, respectively (Boberg et al. 2016). The NOAEL identified for this study was 100 mg/kg bw/day based on reduced anogenital distance in both sexes, reduced ovary weight and mammary gland growth. Epididymal sperm count was altered in male offspring of the 10 mg/kg bw/day group, but no other effect was observed on sperm or reproductive tissues at this dose.
In a study of early male reproductive system development, pregnant Sprague Dawley rats (6-8 dams/group) were injected subcutaneously with 0, 100 or 200 mg/kg bw/day butylparaben from GD 6 to PND 20. In the high-dose group, the number of pups born alive and the proportion surviving to weaning was significantly reduced. The weights of testes, seminal vesicles and prostate glands were significantly decreased in the male offspring of the 100 mg/kg bw/day group at PND 49. Sperm count and motility in the epididymis, and the number of round and elongated spermatids in the seminiferous tubule at stage VII were significantly decreased in offspring of both test groups. In offspring of the high-dose group, testicular expression of ER-α and ER-β mRNA were significantly increased at PND 90 (Kang et al. 2002). The LOAEL identified in this study was 100 mg/kg bw/day based on reduced reproductive organ weights and reduced sperm count and motility.
Pregnant Wistar rats (7 to 8 dams per group) were orally administered 0, 64, 160, 400 or 1 000 mg/kg bw/day butylparaben from GD 12 to PND 21. Pups were observed until day 180 (n = 1 pup per litter, 7-8 litters).No effects were observed on maternal toxicity, fertility parameters (number of pups, pups per litter, live birth rate, pup gender proportion). Maternal serum FSH and LH levels were higher than controls and alanine aminotransferase and aspartate aminotransferase were significantly increased at 400 and 1 000 mg/kg bw/day. In pups, significant decreases in body weight were observed in the 400 mg/kg bw/day group from PND 0 to PND 49 and in the 1 000 mg/kg bw/day group from PND 0 to PND 77. Reduced anogenital distance and delayed preputial separation were observed in male F1 at 400 and 1 000 mg/kg bw/day. Reduced testes weight at PND 21-90, reduced epididymis weight at all time points except PND 35, and reduced seminal vesicle weights at PND 21, were observed at the mid and high dose. Altered testicular histopathology was observed in the 400 and 1 000 mg/kg bw/day groups, characterized by reduced and loosely arranged germ cells, and reduced layers of seminiferous tubules. Dose dependent reductions in epididymal cauda sperm counts and daily sperm production were observed and were statistically significant at 400 and 1 000 mg/kg bw/day. In pups, serum testosterone, estradiol, progesterone, LH and FSH levels were significantly altered on 1 to 3 observation days (PND 21, 35, 49, 90 and 180) in the 400 mg/kg bw/day group and on 2 to 5 observation days in the 1 000 mg/kg bw/day, but were not altered below these levels. The LOAEL identified in this study is 400 mg/kg bw/day, based on delayed onset of puberty, altered testicular morphology and reduced epididymal cauda sperm counts and production (Zhang et al. 2014).
In two studies of male reproductive development, butylparaben was administered in diet to male Wistar rats (8 animals per group) at 0%, 0.01%, 0.1% and 1.0% (equivalent to 10.4 ± 3.07, 103 ± 31.2, and 1 026 ± 310 mg/kg bw/day) for 8 weeks starting at approximately PND 21 and to Crj:CD-1 mice (8 animals per group) at 0%, 0.01%, 0.1% and 1.0% (equivalent to 14.4 ± 3.60, 146 ± 35.9, and 1 504 ± 357mg/kg bw/day) for 10 weeks starting at approximately PND 28. In rats, no significant changes in body weight were noted at any dose. The absolute and relative weights of epididymides were significantly decreased at the high, and mid and high doses, respectively; the absolute weights of the seminal vesicles were significantly decreased at the high dose. No changes were noted in the weights of the testes, ventral prostate, or preputial glands. The cauda epididymal sperm reserve (number of sperm/cauda) of all treated groups was significantly decreased, and the sperm count (number of sperm/g) of the group receiving the highest dose was significantly lower than control values (91.8 ± 29.4 x 107/g, compared to 169.8 ± 91.4 x 107/g). The daily sperm production (DSP) and efficiency (DSP/g testis) in the testis was also significantly lower in all treated groups when compared to controls. Serum testosterone concentration was reduced dose-dependently and was significant at 0.1% or more (Oishi 2001). In mice, no treatment-related effects were observed at any dose on body weight or the weight of the liver, ventral prostate, seminal vesicles, or preputial glands. However, the absolute and relative weights of the epididymides were significantly higher than control in the high-dose group. A decrease of both round spermatid counts in stages VII–VIII seminiferous tubules was observed in the high-dose group, and elongated spermatid counts were decreased in all of the treated groups, in a dose-dependent manner. Serum testosterone concentration significantly decreased at the highest dose (Oishi 2002). The LOAEL for each of these studies is 0.01% in diet (equivalent to 10.4 mg/kg bw/day in rats and 14.4 mg/kg bw/day in mice). As discussed in the methylparaben, ethylparaben, and propylparaben sections, the SCCS (2008, 2011) has expressed doubt as to the ability of the Oishi laboratory to evaluate the parameters measured in this and other studies. Specifically, the Oishi laboratory has reported (i) mean values for some parameters that fell far outside the historical control ranges and (ii) standard deviations for parameters including epididymal sperm concentrations and testosterone levels that were far less than the normal biological variability that has been observed by other groups. The SCCS has concluded that the quality of the Oishi studies could not be properly assessed as the full test description and the complete raw data packages were not available.
In another male reproductive development study, butylparaben was administered in diet to male Wistar rats (15 to 16 animals per dose) at 0, 100, 1 000 and 10 000 ppm (equivalent to 0, 10.9 ± 0.4, 109.3 ± 8.2, and 1087.6 ± 67.8 mg/kg bw/day) for 8 weeks, from PND 22. Eye lesions secondary to orbital bleeding were observed in the butylparaben groups, likely as a result of blood collection from the orbital sinus. Parameters evaluated included organ weights, and histopathology of reproductive tissues, as well as sperm production, motility, and morphology. None of the parameters evaluated showed significant effects in the treatment groups, compared to the control diet. No effect was observed on reproductive organ weights (testes, epididymides, ventral prostate, and seminal vesicles). No effect was observed on sperm motility, cauda epididymal or testicular sperm concentration, daily sperm production, or the number of abnormal sperm. Serum testosterone levels were significantly reduced in the mid- and high-dose groups at week 3, and in the high-dose group at week 9; FSH level was increased in the high-dose group at week 9 and LH was reduced in the high- and low-dose groups at week 5. The authors reported a NOAEL of 1087.6 mg/kg bw/day, the highest dose tested (Hoberman et al. 2008).
Epidemiology
A literature search was conducted to identify epidemiological data for butylparaben, with a focus on reproductive and endocrine effects. Studies were evaluated and scored for quality using the Newcastle-Ottawa Quality Assessment Scale (NOS) and the Quality Assessment Tool for Observational Cohort and Cross-sectional Studies from the National Heart, Lung, and Blood Institute of the National Institutes of Health (RSI 2018). Epidemiology studies were identified that address the relationship between urinary butylparaben concentration and fertility, reproductive health, serum hormone levels, growth and development in children, body weight, allergic sensitization and respiratory health. The majority of studies did not report an association between butylparaben and the endpoint of interest. Positive associations between butylparaben and reduced sperm motility, abnormal sperm morphology, select hormones in pregnant women (decreased estradiol and increased thyroid hormone, T4), male birth weight, and allergic sensitization (Jurewicz et al. 2017a; Aker et al. 2016; Philippat et al. 2014; Savage et al. 2012; Spanier et al. 2014). An inverse association was identified between butylparaben concentration and menstrual cycle length in young women, and serum thyroid hormone (T3) level in adult females (pregnant and general population), and BMI in pregnant women (Nishihama et al. 2016; Koeppe et al. 2013; Aker et al. 2018; Polinski et al. 2018). For all endpoints with an identified association, different studies of similar quality gave conflicting results for these or similar endpoints. More detail is provided in Health Canada (2018a).
7.4.3 Characterization of risk to human health
In repeat dose studies, dietary dosing of butylparaben did not lead to adverse effects at doses ranging from 900 to 1 200 mg/kg bw/day, but doses of 1 875 mg/kg bw/day and higher were associated with organ atrophy, necrosis, and mortality at higher doses (Inai et al. 1985; Matthews et al. 1956). The weight of evidence indicates that butylparaben is not genotoxic and not carcinogenic.
Young male rats administered up to 1 087 mg/kg bw/day for 8 weeks starting at PND 22 did not demonstrate effects on reproductive organ weights or on sperm quality (Hoberman et al. 2008). However, when butylparaben was administered to dams during gestation (GD 6 or 7 to PND 20 or 21), exposure was associated with effects on male and female reproductive development in offspring, including delayed onset of puberty, altered reproductive organ morphology and growth, and reduced sperm count and morphology (Boberg et al. 2016; Kang et al. 2002; Zhang et al. 2014). Reduced sperm count was observed at 10 mg/kg bw/day in Boberg et al. (2016). However, in the absence of accompanying effects on reproductive tissues or reproductive success, this effect was not considered adverse. A point of departure of 100 mg/kg bw/day, the LOAEL identified in the studies conducted by Boberg et al. (2016) and Kang et al. (2002), is considered to be protective of these effects.
The Canadian population is exposed to butylparaben via cosmetics, non-prescription drugs and NHPs. Monitoring data available for some environmental media sources and worldwide data suggest that Canadians are also exposed to butylparaben via these sources. Exposure from food is expected to be negligible.
The assessment of general population exposure to butylparaben is based on biomonitoring data from the CHMS, the Plastics and Personal-Care Product Use in Pregnancy (or P4) Study, and a study of Korean neonates within 48 hours of birth (Health Canada 2017a; Fisher et al. 2017; Kang et al. 2013). Biomonitoring data provide actual internal measures of exposure because they include specific measurements of butylparaben in urine. They are considered reliable estimates of urinary concentration of butylparaben resulting from multiple routes and sources, including most products used by consumers that contain butylparaben. Urine is the major route of excretion for butylparaben and the biomarker (free and conjugated butylparaben) is specific to the parent compound. Butylparaben has a short half-life and a low fractional urinary excretion. To address the uncertainty associated with the robustness of the use of butylparaben as a biomarker, estimates of daily intake were based on the upper-bound of 95th percentile (or highest available) exposure value for each age band and are therefore considered a conservative scenario. Margins of exposure resulting from estimated daily intakes are presented in Table 7‑19.
| Source location | Age group (years) | Upper-bound estimated daily intake at P95 (mg/kg bw/day) | Critical effect levela (mg/kg bw/day) |
MOE |
|---|---|---|---|---|
| CHMS (Cycle 4, 2014-2015), Canada | 3–5 | 0.00076 | LOAEL 100 | 131 053 |
| CHMS (Cycle 4, 2014-2015), Canada | 6–11 | 0.00031 | LOAEL 100 | 319 875 |
| CHMS (Cycle 4, 2014-2015), Canada | 12–19 | 0.0053 | LOAEL 100 | 18 759 |
| CHMS (Cycle 4, 2014-2015), Canada | 20–59 | 0.0047 | LOAEL 100 | 21 206 |
| CHMS (Cycle 4, 2014-2015), Canada | 60–79 | 0.0028 | LOAEL 100 | 36 048 |
| Fisher et al. 2017, Canada | Pregnant women | 0.0044 | LOAEL 100 | 22 950 |
| Kang et al. 2013, Korea | Neonates | 0.00018 | LOAEL 100 | 567 260 |
Abbreviations: LOAEL, lowest observed adverse effect level.
a Critical effect: Reduced anogenital distance in both sexes, reproductive organ morphology, sperm count and motility.
On the basis of conservative parameters used to model estimated daily intake, the margins of exposure are considered adequate to address the risk to populations aged 3 to 79 years, pregnant women and neonates. Biomonitoring data were not available to address children under 3 years (other than neonates). With the exception of breast milk consumption, exposure for this group is not expected to differ significantly from the youngest age group surveyed by CHMS or from the neonatal group presented here. Based on Canadian biomonitoring data, infants are not expected to be exposed to butylparaben via breast milk. Therefore, the reported urinary concentrations and estimated daily intakes across all age groups indicate that exposure in children under 3 years is not expected to pose a risk.
Butylparaben is present in environmental media at negligible levels in all age groups, other than formula-fed infants. In this age group, exposure is estimated to be 3.34 ng/kg bw/day, which results in a margin of exposure of 2.99 x 107. The margin of exposure is considered adequate to address the potential risk.
Due to the short half-life of propylparaben administered by the oral route and the spot sampling methodology used in biomonitoring studies, it is not clear that biomonitoring data adequately address high exposures to cosmetics, non-prescription drugs and NHPs with intermittent use patterns (i.e., products that are not used daily). Sentinel scenarios are presented in Table 7‑20.
| Exposure scenario | Age group (years) |
Systemic exposure (mg/kg bw/day) | Critical effect levela (mg/kg bw/day) |
MOE |
|---|---|---|---|---|
| Hair dyeb (permanent, dermal, per event) | Teen (12–19) |
0.36–1.09 | LOAEL 100 | 92–276 |
| Hair dyeb (permanent,dermal, per event) | Adult (> 20) |
0.30–0.91 | LOAEL 100 | 110–330 |
| Herbal cough medicinec (oral, per day) |
Teen (12–19) |
0.14 | LOAEL 100 | 691 |
| Herbal cough medicinec (oral, per day) |
Adult (> 20) |
0.12 | LOAEL 100 | 4 122 |
| Children’s analgesic suspensiond (oral, per day) |
Toddler (0.5–4) |
2.10 | LOAEL 100 | 48 |
| Antacidd (oral, per day) |
Teen (12–19) |
0.44 | LOAEL 100 | 225 |
| Antacidd (oral, per day) |
Adult (> 20) |
0.37 | LOAEL 100 | 269 |
| Analgesic creamc (dermal, per day) | Child (5–11) |
0.012–0.060 | LOAEL 100 | 5 015–25 072 |
| Analgesic creamc (dermal, per day) | Adult (> 20) |
0.011–0.053 | LOAEL 100 | 5 686–28 431 |
| Medicated hand creamc (dermal, per day) |
Child (5–11) |
0.020 | LOAEL 100 | 14 644 |
| Medicated hand creamc (dermal, per day) |
Adult (> 20) |
0.017 | LOAEL 100 | 17 667 |
| Sunscreenc (dermal, per day) |
Toddler (0.5–4) |
0.44 | LOAEL 100 | 227 |
| Sunscreenc (dermal, per day) |
Adult (> 20) |
0.33 | LOAEL 100 | 303 |
Abbreviations: LOAEL, lowest observed adverse effect level.
a Critical effect: Reduced anogenital distance in both sexes, reproductive organ morphology, sperm count and motility.
b Cosmetic
c Natural health product
d Non-prescription drug
The calculated margins of exposure to hair dye, herbal cough medicine, children’s analgesic suspension, antacid, and sunscreen are considered potentially inadequate to address uncertainties in the health effects and exposure databases.
Data from an in vitro study of cutaneous metabolism of butylparaben suggested that butylparaben may be hydrolyzed to ethylparaben and methylparaben in the skin. This potential exposure is incorporated into biomonitoring data for methylparaben and ethylparaben. Potential risk from this putative route of exposure is addressed in Sections 7.1.3 and 7.2.3.
7.5 Benzylparaben
7.5.1 Exposure assessment
Benzylparaben has been identified in environmental media and biomonitoring studies outside of Canada, and other exposures have not been identified in Canada. Potential concentrations of benzylparaben in Canadians are presented in the following section.
Biomonitoring
Benzylparaben was not a target of the Canadian Health Measures Survey. In a large scale study in Germany (n = 660 adults), the detection rate was 1.4% (limit of quantification (LOQ) = 0.5 µg/L), with a maximum urinary concentration of 1.2 µg/L (Moos et al. 2015). In a small scale, regional study in the United States (n = 100 adults), benzylparaben was detected in 39% of participants, with a 95th percentile urinary concentration of 0.5 µg/L (Ye et al. 2006). Benzylparaben was detected in the urine of 40.96% pregnant women ((LOD = 0.2 µg/L, n = 31 women, 532 samples) in a Canadian study, with a 95th percentile urinary concentration of 0.57 µg/L (Fisher et al. 2017). In the same study, benzylparaben was not detectable (LOD = 0.1 µg/L) in the breast milk of 56 women at 3 months post-partum. In a Danish study of mother-child pairs (n = 143), benzylparaben was detected in 2.1% of mothers and 4.2% of children (aged 6 to 11 years), with maximum detection levels of 1.1 and 0.65 µg/L, respectively (Frederiksen et al. 2013). In a regional study in the United States, benzylparaben was detected (LOQ = 0.03 µg/L) in the urine of 15% of children aged 3 to 10 years (n = 40); the 95th percentile was 0.42 µg/L (Wang et al. 2013). Benzylparaben was detected in placenta at a maximum level of 0.12 ng/g fresh weight (Spain, n = 12) (Valle-Sistac 2016).
Daily estimated intakes were derived for the populations represented by key biomonitoring data (Fisher et al. 2017; Moos et al. 2015; Ye et al. 2006; Frederiksen et al. 2013; Wang et al. 2013). In the absence of Canadian data for general populations, data from US and European sources were used to estimate daily intake. Urinary concentrations from Canadian pregnant women were also modelled. In each of the propylparaben studies, total benzylparaben (i.e., free and conjugated species) was measured in urine by HLPC, after treatment with β -glucuronidase and sulfatase.
As with other parabens, the use of benzylparaben as a biomarker is specific to the parent compound. Benzylparaben is less widely used than other parabens (e.g., no uses were reported in Canada), thus the risk of contamination during sample collection and analysis is considered minimal or negligible. A fractional urinary excretion of 1% of the applied dose was estimated by Aylward (2017a), based on the log KOW of 3.56 and the regression equation reported by Moos et al. (2017). However, based on the structural differences between benzylparaben and paraben species with measured fractional urinary excretion (on which the linear regression was based), Aylward et al. (2017a) report very low confidence in the estimated value for benzylparaben.
Estimated daily intake values were estimated using the 95th percentile or highest available values for creatinine-adjusted urinary concentration (µg/g Cr) or urinary concentration (µg/L) for each population, as described for methylparaben (section 7.1.1). In all populations reported here, the concentration of benzylparaben was below the limit of detection in the majority of samples. The prevalence of samples below the limit of detection (ranging from 0.1 to 0.5 µg /L) may be due to rapid metabolism, low exposure, or both. The use of the 95th percentile or highest available value was based on data availability and is considered a conservative estimate that addresses the variation inherent in spot sampling.
Estimated daily intakes and key parameters are presented in Table 7‑21.
| Source, location | Age (years) | % <LOD or LOQ | LOD or LOQ (µg/L) | UFR (L/kg bw/ day)a | CER (mg/ day)b | UC | FUEc | EDI, P95 (CI) (µg/kg bw/day) |
|---|---|---|---|---|---|---|---|---|
| Moos et al. 2015, Germany | Adults (20-30) (n = 660) |
98.6 | 0.5 | N/A | 1440 | 3.1 µg/g Cr (max) | 0.01 | 6.3 |
| Frederiksen et al. 2013, Denmark | Children (6-11) (n = 143) |
95.8 | 0.18 | N/A | 418 | 1.0 µg/g Cr (max) | 0.01 | 1.3 |
| Fisher et al. 2017, Canada | Pregnant women (n = 31) |
59.0d | 0.20 | 0.020 | N/A | 0.57 µg/L (P95) | 0.01 | 1.1e |
| Ye et al. 2006, USA | Adults (n = 100) |
61.0 | 0.10 | 0.020 | N/A | 0.5 µg/L (P95) | 0.01 | 1.0e |
| Wang et al. 2013, USA | Children (3-10) (n = 40) |
85.0 | 0.03 | 0.030 | N/A | 0.42 µg/L (P95) | 0.01 | 1.3e |
Abbreviations: LOD, limit of detection; LOQ, limit of quantification; CER, creatinine excretion rate; UFR, urinary flow rate; UC, urinary concentration; FUE, fractional urinary excretion; EDI, estimated daily intake; N/A, not applicable; max, maximum reported value; P95, 95th percentile
a Aylward et al. 2015
b Creatinine excretion rate was calculated using the Mage equation [0.993*1.64 [140 – Age] (Wt^1.5 Ht^0.5)/1000].
c Aylward et al. 2017a
d %<LOD is based on 542 samples from 31 subjects (Fisher et al. 2017).
e When creatinine-adjusted concentrations were not reported, EDI was calculated based on urinary concentration (µg/L) using the following equation: EDI = (UC*UFR)/FUE (Saravanabhavan et al. 2014), where UC is the urinary concentration (µg/L), UFR is the urinary flow rate (L/kg bw/ day) and FUE is the fractional urinary excretion.
Environmental media
Benzylparaben was below the limit of detection in house dust (8 ng/g) and agricultural soil (2.8 ng/g) in Canadian monitoring studies (Fan 2010; Viglino 2011). In studies carried out elsewhere, benzylparaben has been identified in drinking water (Azzouz and Ballesteros 2014) and house dust (Wang et al. 2012; Ramirez et al. 2011; Tran et al. 2016; Canosa et al. 2007). In the absence of Canadian monitoring data, as no import or manufacture in Canada was reported in a Section 71 survey, an upper bound release value of 100 kg was used as a conservative input to estimate theoretical environmental concentrations of benzylparaben using the level III fugacity model ChemCAN version 6.00 (ChemCAN 2003). The theoretical environmental concentrations derived from this approach were used to estimate potential exposures from environmental media for the general population of Canada. This results in an estimated intake of 0.034 ng/kg bw/day from air, water and soil. This approach indicates that general population exposure due to environmental media in Canada is potentially negligible, which is supported by data from international monitoring studies.
Food
Benzylparaben exposure from food is expected to be negligible (personal communication, email from the Health Canada Food Directorate, Health Canada, to the Health Canada Consumer Product Safety Directorate, Health Canada, dated April 18, 2018; unreferenced).
According to biomonitoring data (Fisher et al. 2017), benzylparaben was not detected in breast milk in 56 Canadian women (LOD 0.1 µg/L), 3 months post-partum; breastfed infants are therefore not expected to be exposed to benzylparaben via breast milk.
7.5.2 Health effects assessment
Benzylparaben has been addressed in several reviews and key risk assessments of parabens, including those conducted by the CIR (Andersen 2008), the EU SCCS (2010, 2011), EFSA (2004) and independent scientists (Soni et al. 2005). The literature published until March 2017 was searched for relevant information to supplement the data used in the international reviews and assessments.
Toxicokinetics
Two human volunteers administered 2 g of benzylparaben per day for 5 days excreted in urine approximately 6% as unchanged parent compound, 87% as a sulphate conjugate and small quantities as forms of PHBA and benzoic acid (Sabalitschka and Neufeld-Crzelliter 1954, as cited in Andersen 2008). In a report of the urinary concentration of parabens in 100 adults, Ye et al. (2006) has reported that free benzylparaben was below the limit of detection in all subjects, but conjugated (glucuronidated and sulfated) benzylparaben was detected at low levels in 39% of samples. Benzylparaben has been detected at low levels in humans in tumorous breast tissue, adipose tissue, and in brain, although it was not reported whether benzylparaben was free or conjugated (Barr et al. 2012; Wang et al. 2015; van der Meer 2017).
The rate of hydrolysis of benzylparaben in human skin and liver cells has been reported to be either similar to that of butylparaben (Jewell et al. 2007a, 2007b) or intermediate to the rates of short and long chain parabens (Abbas et al. 2010). In human liver cells, benzylparaben is hydrolyzed at a rate 5 to 10 times slower than methylparaben; in human skin cells, benzylparaben is metabolized at a rate roughly 5-fold slower than methylparaben (Jewell et al. 2007a, 2007b). In another in vitro assay, benzylparaben had a hydrolysis half-life of 43 minutes in human liver microsomes, approximately double that of methylparaben (Abbas et al. 2010).
Repeat dose studies
The CIR reports that guinea pigs fed 1 g of benzylparaben per day for 19 days showed no signs of toxicity (Ishizeki 1955, as cited in Andersen 2008).
Genotoxicity
Benzylparaben was estimated to be negative in an Ames assay, with and without metabolic activation, based on QSAR prediction (REACH 2018d).
Reproductive and developmental effects
See Appendix C for a review of in vitro estrogenic effects of benzylparaben.
Benzylparaben was administered to female Sprague Dawley rats by gavage at 0.0064, 0.032, 0.16, 0.8, 4, 20, and 100 mg/kg bw/day from PND 21 to 23. Estradiol was administered as a positive control at 1, 5, 25, 100, 400 ug/kg bw/day. No effect was observed on mortality, physiological stress or body weight in animals at any dose. Relative uterine weight was significantly increased in a dose dependent manner to 107% to 136% of control weights at 0.16 mg/kg bw/day benzylparaben and above. Administration of 5 to 400 µg/kg bw/day estradiol induced at 155% to 440% increase in relative uterine weight, compared to control. The NOAEL identified for this study is 0.032 mg/kg bw/day, based on uterotrophic effects at 0.16 mg/kg bw/day (Hu et al. 2013).
Epidemiology
Epidemiology studies were identified addressing the relationship between benzylparaben and fertility, body weight and growth in children. No association was identified between benzylparaben concentration and time-to-pregnancy, growth in toddlers, or BMI in obese children and adolescents (Smarr et al. 2017; Guo et al. 2017; Xue et al. 2015). More detail is provided in Health Canada (2018a).
7.5.3 Characterization of risk to human health
Read-across hazard data
There is a lack of reliable studies for benzylparaben, particularly for reproductive endpoints. Based on similar physical chemical properties, toxicokinetics and functionality in in vitro assays (see Appendix F), butylparaben was selected as an appropriate analogue for benzylparaben, with respect to this endpoint. Therefore, as a point of departure, a LOAEL of 100 mg/kg bw/day for butylparaben based on effects on early reproductive system development (i.e., reduced anogenital distance in both sexes, reproductive organ weight and morphology, sperm count and motility) was applied to benzylparaben (Boberg et al. 2016; Kang et al. 2002; Zhang et al. 2014).
Benzylparaben was not identified in products available to consumers, cosmetics, prescription or non-prescription drugs, NHPs, or pesticides. Exposure from food and environmental media is expected to be negligible. Benzylparaben was detected in the urine of pregnant women in a Canadian biomonitoring study and has been detected in adults and children in biomonitoring studies in Europe and the United States. These data encompass exposures to benzylparaben from multiple potential sources and routes, known or unknown, and may be considered reliable estimates of exposure of the general population. Urine is expected to be the major route of excretion for benzylparaben as it is for other parabens, and the biomarker (free and conjugated benzylparaben) is specific to the parent compound. There is uncertainty associated with the use of the modelled fractional urinary excretion factor, and the half-life in humans is unknown. To address the uncertainty associated with the robustness of the use of benzylparaben as a biomarker, estimates of daily intake were based on the upper-bound of 95th percentile (or highest available) exposure value for each age band and are therefore considered a conservative scenario. Margins of exposure resulting from estimated daily intakes are presented in Table 7‑22.
| Source location | Age group (years) | Estimated daily intake at P95 (mg/kg bw/day) | Critical effect levela (mg/kg bw/day) |
MOE |
|---|---|---|---|---|
| Moos et al. 2015, Germany | Adults (20–30) |
0.0063 | LOAEL 100 | 15 875 |
| Frederiksen et al. 2013, Denmark | Children (6–11) |
0.0013 | LOAEL 100 | 74 257 |
| Fisher et al. 2017, Canada | Pregnant women | 0.0011 | LOAEL 100 | 87 719 |
| Ye et al. 2006, United States | Adults | 0.001 | LOAEL 100 | 100 000 |
| Wang et al. 2013, United States | Children (3–10) |
0.0013 | LOAEL 100 | 79 365 |
Abbreviations: LOAEL, lowest observed adverse effect level.
a Critical effect: Reduced anogenital distance in both sexes, reproductive organ weight, sperm count and motility.
On the basis of conservative parameters used to model estimated daily intake, the margins of exposure are considered adequate to address the risk to adults, pregnant women and children. The reported urinary concentrations and estimated daily intakes across all age groups indicate that exposure in children under 3 years is not expected to pose a risk.
7.6 iso-Propylparaben
7.6.1 Exposure assessment
iso-Propylparaben has been identified in environmental media and in biomonitoring studies outside of Canada. iso-Propylparaben is present in non-prescription drugs, NHPs, and cosmetics. All of these sources may contribute to total daily exposure to iso-propylparaben. Urinary concentrations and estimated exposure of Canadians to iso-propylparaben are presented in the following section.
Biomonitoring
iso-Propylparaben was not a target of the Canadian Health Measures Survey. In a large scale study in Germany (n = 660 adults), the detection rate was 4%, with a maximum urinary concentration of 99.5 µg/L (Moos et al. 2015). In another, smaller study of adults and children in Germany (n = 59 females, 39 males and 59 children aged 6 to 8 years), the detection rate in urine was 3% and the maximum concentration was 175 µg/L (Moos et al. 2014). In a Danish study of mother-child pairs (n = 143), iso-propylparaben was detected in 15% of mothers and 3% of children (aged 6 to 11 years), with mean detection levels of 0.63 and 0.13 µg/L, respectively (Frederiksen et al. 2013). In a study of Greek children aged 4.24 ± 0.24 years (n = 500), iso-propylparaben was detected in 3.8% of participants with a maximum detection of 15.0 µg/L (Myridakis et al. 2016).
Environmental media
No Canadian monitoring studies were identified for iso-propylparaben. In studies carried out in other countries, iso-propylparaben has been identified in drinking water (Azzouz and Ballesteros 2014), agricultural and forestry soils (Nunez 2008), and house dust (Ramirez et al. 2011). In the absence of Canadian monitoring data, the total reported import volume of iso-propylparaben in Canada (284 kg) was used as a conservative input to estimate theoretical environmental concentrations of iso-propylparaben using the level III fugacity model ChemCAN version 6.00 (ChemCAN 2003). The theoretical environmental concentrations derived from this approach were used to estimate potential exposures from environmental media for the general population of Canada. This results in an estimated intake of 0.097 ng/kg bw/day from air, water and soil. This approach indicates that general population exposure from environmental media in Canada is potentially negligible, which is supported by data from international monitoring studies.
Food
iso-Propylparaben exposure from food is not expected (personal communication, email from the Health Canada Food Directorate, Health Canada, to the Health Canada Consumer Product Safety Directorate, Health Canada, dated April 18, 2018; unreferenced). No Canadian studies were identified reporting iso-propylparaben concentration in breast milk.
Cosmetics
iso-Propylparaben was identified in cosmetics, including lotions, cleansers and hair care products. Exposure estimates for the oral and dermal routes based on sentinel scenarios associated with the use of cosmetics containing iso-propylparaben were evaluated, and sentinel scenarios are presented in Table 7‑23 and Table 7‑24, respectively. Potential exposures were estimated using conservative assumptions and default values as outlined in Appendix B. Exposure estimates for each scenario are expressed on a per-event and/or a daily basis, depending on exposure frequency (see section 7.6.3, Characterization of risk to human health).
| Product scenario | Concentration in product (%)a | Age group (years) |
Systemic exposure (mg/kg bw/day) |
|---|---|---|---|
| Lip balm (per day) | 0.1–0.2 | Teen (12–19) |
0.00040–0.00081 |
a Internal data, Consumer Product Safety Directorate, Health Canada, dated August 18, 2017; unreferenced
Experimentally determined dermal absorption and metabolism coefficients from an in vitro study of dermal absorption and metabolism in human skin were used to estimate and internal dose from potential exposure by the dermal route, as described in section 7.1.1, and further details are available on request (Charles River Laboratories 2018; Health Canada 2018b). Exposure estimates from the dermal route are presented in Table 7‑24. Default values and models used in exposure scenarios are provided in Appendix B.
| Product scenario | Concentration in product (%)a | Age group (years) |
Dermal load (µg/cm2/24 h) | Systemic exposure (mg/kg bw/day) |
|---|---|---|---|---|
| Body lotion (per event) |
0.05–0.1 | Toddler (0.5–4) |
0.42–0.84 | 0.11–0.22b,d |
| Sunless tanning product (per event) |
0.3–1 | Adult (> 20) |
0.78–2.60 | 0.16–0.54b,e |
| Body scrub (per event) |
0.3–1 | Teen (12–19) |
0.57–1.89 | 0.14–0.47b,e |
| Hair dye (permanent, per event) | 0–0.01 | Teen (12–19) |
13.70 | 0.12c,e |
| Face moisturizer (per day) |
0.3–1 | Teen (12–19) |
8.88–29.59 | 0.089b,d–0.11c,d |
| Body cleanser (liquid, per day) | 3–10 | Toddler (0.5–4) |
0.06–0.19 | 0.016–0.052b,e |
a Internal data, Consumer Product Safety Directorate, Health Canada, dated August 18, 2017; unreferenced.
b Calculated using the product dermal load as the maximum dermal amount.
c Calculated using a maximum dermal amount of 11.09 µg/cm2/24 h.
d Calculated using a metabolism refinement of 81.98% iso-propylparaben (based on moisturizer formulation).
e Calculated using a metabolism refinement of 86.98% iso-propylparaben (based on non-moisturizer formulation).
Non-Prescription Drugs and NHPs
iso-Propylparaben is present as a non-medicinal ingredient in non-prescription drugs and NHPs, primarily in make-up with sun protection claims. Sentinel scenarios via the oral and dermal routes are presented in Table 7‑25. Default values and models used in exposure scenarios are provided in Appendix B.
| Product scenario | Concentration in product (%)a | Age group (years) |
Dermal load (µg/cm2/24 h) | Systemic exposure (mg/kg bw/day) |
|---|---|---|---|---|
| Face make-up (per day) |
0.12 | Teen (12–19) |
1.22 | 0.01b,c |
a Personal communication, email from Therapeutic Products Directorate, Health Canada, to Consumer Product Safety Directorate, Health Canada, dated May 25, 2017; unreferenced.
b Calculated using the product dermal load as the maximum dermal amount.
c Calculated using a metabolism refinement of 86.98% iso-propylparaben.
7.6.2 Health effects assessment
iso-Propylparaben has been addressed in several reviews and key risk assessments of parabens, including those conducted by the CIR (Andersen 2008), the EU SCCS (SCCP 2005b; SCCS 2010, 2011), and independent scientists (Soni et al. 2005). The literature published until March 2017 was searched for relevant information to supplement the data used in the international reviews and assessments.
Toxicokinetics
iso-Propylparaben is detected in human biomonitoring samples primarily in conjugated form, suggesting that it is metabolized by the same pathways as other parabens. iso-Propylparaben was detected primarily in conjugated form in human urine and breast milk (Azzouz et al. 2016). In urine samples from 14 human volunteers, free iso-propylparaben was detected, in only 2 samples, at 0.15 to 0.18 µg/L, but iso-propylparaben conjugated to glucuronic acid was detected in 7 samples (total paraben range of 0.21 to 2.1 µg/L). In breast milk samples from 7 human volunteers, free iso-propylparaben was detected, in only 1 sample, at 0.73 µg/L; iso-propylparaben conjugated to glucuronic acid was detected in 2 samples (total paraben range of 0.90 to 1.22 µg/L).
Repeat dose studies
Fischer 344 rats (10/sex/group) were fed 0%, 0.25%, 1.25%, 2.5% or 5% iso-propylparaben (corresponding to approximately 0, 128, 640, 1 280 and 2 560 mg/kg bw/d) in diet for 13 weeks. Reduced weight was observed in males at 2.5 and 5.0%, and in females at 1.25% and above. Increases in serum gamma-GTP and total cholesterol were reported in males at 2.5% or more and increases in gamma-GTP, ALP and BUN in females at 1.25% or more. Centrilobular hepatocellular swelling and hepatocytes filled with vacuoles were observed in the liver in males in the two highest treatment groups and in females in the highest treatment group. Renal histological effects (increased severity of intracytoplasmic eosinophilic globule formation in the renal proximal tubular epithelia) were also reported in males in the highest dose group. The NOAEL selected for this study was 1.25% (corresponding to approximately 640 mg/kg bw/day) (Onodera 1994).
5-week-old Sprague Dawley rats (10/group) were treated dermally with 0, 50, 100, 300 or 600 mg/kg bw/day of either iso-propylparaben or iso-butylparaben daily for 28 days in an OECD 410 repeat dose study. The test article was dissolved in ethanol and applied in cream to shaved dorsal skin at 50 µL/cm2 on 10% of the body surface area. No effects were observed on body weight, organ weight, or serum levels of triiothyronine, thyroid-stimulating hormone, insulin, estrogen, follicle-stimulating hormone, or testosterone. The iso-propylparaben groups did not show any specific application site lesions in either male or female rats (Kim 2015). The NOAEL identified for this study is 600 mg/kg/d, the highest dose tested.
Genotoxicity
iso-Propylparaben was negative in Ames assays, as well as in chromosomal aberration assays and sister chromatid exchange assays in Chinese hamster and human embryo fibroblasts (REACH 2018e; Ishidate and Odashima 1977; Ishidate et al. 1984; Kawachi and Yahagi 1980; Sasaki 1980). It was also negative in an in vivo chromosomal aberration assay in silkworms (Kawachi and Yahagi 1980). iso-Propylparaben is not expected to be genotoxic in vivo.
Reproductive and developmental effects
See Appendix C for a review of in vitro estrogenic effects of iso-propylparaben.
In a modified uterotrophic assay, immature female Sprague-Dawley rats (8 animals per group) were subcutaneously administered iso-propylparaben at 0, 62.5, 250, and 1 000 mg/kg bw/day for 3 days starting at PND 14. Significantly increased uterine wet weight relative to body weight was observed at the 1 000 mg/kg/d dose of iso-propylparaben. This effect was reduced, although still significantly different from the vehicle control, with the addition of ICI 182,780, an antagonist of the estrogen receptor (Vo and Jeung 2009). The NOAEL identified for this study is 250 mg/kg bw/day, based on uterotrophic effects at 1 000 mg/kg/d.
In a study of female pubertal development, pre-pubertal Sprague Dawley rats (10/ group) were orally administered 0, 62.5, 250, 1 000 mg/kg bw/d of iso-propylparaben in corn oil from postnatal day 21 to 40. Ethinylestradiol (1 mg/kg bw/day) was administered as a positive control. A statistically significant decrease in body weight was not reported, although at the highest dose, body weight is 102.45 ± 4.38 g, compared to the control group values of 118.69 ± 6.4 g. A statistically significant delay in vaginal opening was observed in animals in the mid- and high-dose iso-propylparaben groups (PND 36.2 ± 1.03 and 36.7 ± 0.71), compared to the control group (PND 33.6 ± 3.23); vaginal opening occurred at PND 21.4 ± 0.53 in the ethinylestradiol group. The vaginal opening dates in the mid- and high-dose groups are within the range of historical controls (PND 33.0 to 36.6, Stump et al. 2014). A significant decrease in the number of 4-day estrous cycles was also observed in the high-dose group, but the number of days of each stage in the 4-day cycle (proestrus, estrus, metestrus, and diestrus) were not significantly different. Serum estradiol levels were significantly reduced in the high dose-group (16.23 ± 6.86 pg/mL, compared to 47.07 ± 14.72 pg/mL in the control) and T4 was reduced in the mid-dose group (1.73 ± 0.34 ng/mL compared to 3.00 ± 0.32 ng/mL in the control), but not in the high-dose group. Significantly decreased ovary and kidney weights were observed in the high-dose iso-propylparaben group. Histopathological changes in the ovaries and uterus were also observed at the high dose of iso-propylparaben (Vo et al. 2010). As discussed in the methylparaben section, some effects described here have been shown to result from body weight reductions of 9% to 12% (Stump et al. 2014). The reported mean body weight of animals in the highest dose group is reduced by 13.7% compared to the control group, suggesting that the changes in estrous cycling, reduction in organ weights, as well as uterine histology may be secondary to reduced body weight. Due to the problematic nature of several results reported in this study, and a lack of dose-dependent effects, a NOAEL was not selected.
Epidemiology
No epidemiological studies addressing iso-propylparaben were identified.
7.6.3 Characterization of risk to human health
In a 13-week study, dietary exposure to iso-propylparaben resulted in changes in serum histochemistry and renal and hepatic organ effects at 2.5% (approximately 1 280 mg/kg bw/day) and higher (Onodera 1994). Dermal exposure to iso-propylparaben up to 600 mg/kg bw/day for 28 days did not result in application site effects or effects on organ weight or serum hormone levels (Kim 2015). A NOAEL of 640 mg/kg bw/day was therefore selected as a point of departure for exposure to iso-propylparaben, based on serum histochemistry and organ effects in a 13-week study.
The Canadian population is exposed to iso-propylparaben via cosmetics, non-prescription drugs and NHPs. Exposure from food and environmental media is expected to be negligible. To address the potential risk associated with exposure to iso-propylparaben from products, margins of exposure resulting from modelled exposures in sentinel scenarios are presented in Table 7‑26.
| Product scenario | Age group | Systemic exposure (mg/kg bw/day) | Critical effect levela (mg/kg bw/day) |
MOE |
|---|---|---|---|---|
| Lip balmb (oral, per day) |
Teen (12–19) |
0.00040–0.00081 | NOAEL 640 | 7.9 x105 –1.6 x106 |
| Body lotionb (dermal, per event) |
Toddler (0.5–4) |
0.11–0.22 | NOAEL 640 | 2909–5818 |
| Sunless tanning productb (dermal, per event) |
Adult (> 20) |
0.16–0.54 | NOAEL 640 | 1 185–4 000 |
| Body scrubb (dermal, per event) |
Teen (12–19) |
0.14–0.47 | NOAEL 640 | 1 362–4 571 |
| Hair dyeb (permanent, per event) | Teen (12–19) |
0.12 | NOAEL 640 | 5333 |
| Face moisturizerb (per day) |
Teen (12–19) |
0.089–0.11 | NOAEL 640 | 5 818–7 191 |
| Body cleanserb (liquid, per day) | Toddler (0.5–4) |
0.016–0.052 | NOAEL 640 | 12 300–40 000 |
| Face make-upc (per day) |
Teen (12–19) |
0.01 | NOAEL 640 | 64 000 |
Abbreviations: NOAEL, no observed adverse effect level.
a Critical effect; Changes in serum histochemistry, renal and hepatic organ effects.
b Cosmetic.
c Non-prescription drug.
The calculated margins of exposure are considered adequate to address uncertainties in the health effects and exposure databases.
7.7 iso-Butylparaben
7.7.1 Exposure assessment
iso-Butylparaben has been identified in environmental media outside of Canada and in biomonitoring studies both in Canada and elsewhere. iso-Butylparaben is present in non-prescription drugs, NHPs, and cosmetics, and it has been identified as a component in incidental additives used in food processing establishments. Many of these sources contribute to total daily exposure to iso-butylparaben. Urinary concentrations and estimated exposure of Canadians to iso-butylparaben are presented in the following section.
Biomonitoring
iso-Butylparaben was not a target of the Canadian Health Measures Survey. In Canadian regional study, the mean urinary concentrations of iso-butylparaben was 0.22 µg/L in females (n = 28 including 9 pregnant patients) and 0.29 µg/L in males (Genuis et al. 2013). In a Canadian study of pregnant females (n = 31), iso-butylparaben was detected in 38.93% of samples and had a 95th percentile concentration in urine of 2.96 µg/L. In the same study, iso-butylparaben was not detected in breast milk in 56 Canadian women, 3 months post-partum (Fisher et al. 2017). In a large scale study in Germany (n = 660 adults), the detection rate was 24%, with a 95th percentile urinary concentration of 3.1 µg/L (Moos et al. 2015). In a Danish study of mother-child pairs (n = 143), iso-butylparaben was not detected in mothers or children (aged 6 to 11 years) (Frederiksen et al. 2013). However, in a study of Greek children aged 4.24 ± 0.24 years (n = 500), iso-butylparaben was detected in 10% of participants with a maximum detection of 50.4 µg/L (Myridakis et al. 2016).
Environmental media
iso-Butylparaben has been identified in drinking water (Azzouz and Ballesteros 2014) and agricultural soil (Perez 2011) in other countries. Canadian monitoring studies were not identified for iso-butylparaben.
In the absence of Canadian monitoring data, the total reported import volume of iso-butylparaben in Canada (232 kg) was used as a conservative input to estimate theoretical environmental concentrations of iso-butylparaben using the level III fugacity model ChemCAN version 6.00 (ChemCAN 2003). The theoretical environmental concentrations derived from this approach were used to estimate potential exposures from environmental media for the general population of Canada. This results in an estimated intake of 0.079 ng/kg bw/day from air, water and soil. This approach indicates that general population exposure due to environmental media in Canada is potentially negligible, which is supported by data from international monitoring studies.
Food
iso-Butylparaben exposure from food is not expected (personal communication, email from the Health Canada Food Directorate, Health Canada, to the Health Canada Consumer Product Safety Directorate, Health Canada, dated April 18, 2018; unreferenced). Biomonitoring data (Fisher et al. 2017) indicate that iso-butylparaben was not detected in breast milk in 56 Canadian women (LOD 0.1 µg/L), 3 months post-partum; breastfed infants are therefore not expected to be exposed to iso-butylparaben via breast milk.
Cosmetics
iso-Butylparaben was identified in cosmetics, such as lotions, cleansers, make-up and hair care products. Sentinel scenarios for exposure to cosmetics by the oral and dermal routes are presented in Table 7‑27 and Table 7‑28, respectively. See Appendix B for details on assumptions and default values used for generating exposure estimates. Exposure estimates for each scenario are expressed on a per-event and/or a daily basis, depending on exposure frequency and the critical health effects (see section 7.7.3, Characterization of risk to human health).
| Product scenario | Concentration in product (%)a | Age group (years) |
Systemic exposure (mg/kg bw/day) |
|---|---|---|---|
| Lip balm (per event) | 0–0.1 | Toddler (0.5–4) |
6.5x10-4 |
| Lipstick (per day) | 0.1–0.3 | Teen (12–19) |
1.2x10-3–4.0x10-4 |
a Internal data, Consumer Product Safety Directorate, Health Canada, dated August 18, 2017; unreferenced
Experimentally determined dermal absorption and metabolism coefficients from an in vitro study of dermal absorption and metabolism in human skin were used to estimate an internal dose from potential exposure by the dermal route, as described in section 7.1.1, and further details are available on request (Charles River Laboratories 2018; Health Canada 2018b). Exposure estimates from the dermal route are presented in Table 7‑28Table 7‑28. Estimated potential dermal exposures to iso-butylparaben from the use of cosmetics on an age group-specific basis. Default values and models used in exposure scenarios are provided in Appendix B.
| Product scenario | Concentration in product (%)a | Age group (years) |
Dermal load (µg/cm2/24 h) | Systemic exposure (mg/kg bw/day) |
|---|---|---|---|---|
| Body lotion (per event) |
0.3–1 | Toddler (0.5–4) |
2.51–8.35 | 0.46–1.54b,e |
| Body oil (per event) |
1–3 | Adult (> 20) |
2.23–6.68 | 0.26–0.79b,e |
| Body scrub (per event) |
0.3–1 | Teen (12–19) |
0.57–1.89 | 0.094–0.31b,e |
| Face make-up (per day) |
0.1–1.5 | Teen (12–19) |
1.02–15.26 | 0.0064b,e–0.079d,e |
| Facial moisturizer (per day) |
1–3 | Teen (12–19) |
29.59–88.77 | 0.090d,e–0.15c,e |
| Eye lotion (per day) |
3–10 | Teen (12–19) |
54–180 | 0.0061–0.020c,e |
| Sunless tanning product (per event) |
0–0.1 | Adult (> 20) |
0.26 | 0.036b,e |
| Shampoo (per day) |
0.1–0.5 | Adult (> 20) |
0.12–0.59 | 0.0011–0.0053b,e |
| Hair conditioner (rinse-off, per event) | 0.3–1 | Toddler (0.5–4) |
0.61–2.05 | 0.010–0.033b,e |
| Body cleanser (liquid, per day) | 0.1–0.5 | Infant (0–0.5) |
0.0031–0.016 | 0.0012–0.0062b,f |
a Internal data, Consumer Product Safety Directorate, Health Canada, dated August 18, 2017; unreferenced.
b Calculated using the product dermal load as the maximum dermal amount.
c Calculated using 23.18% dermal absorption.
d Calculated using a maximum dermal amount of 12.64 µg/cm2/24 h.
e Calculated using a metabolism refinement of 58.25% iso-butylparaben.
f A metabolism refinement was not applied to the infant age group.
Non-Prescription Drugs and NHPs
iso-Butylparaben is present as a non-medicinal ingredient in non-prescription drugs and NHPs, primarily in acne therapy products, make-up with sun protection claims, and sunscreen. With the exception of the products in Table 7‑29, these exposures are exceeded by cosmetic exposures for lotions, makeup and cleansing products, presented in Table 7‑28. Sentinel scenarios via the oral and dermal routes are presented in Table 7‑28. Default values and models used in exposure scenarios are provided in Appendix B.
| Product scenario | Concentration in product (%) | Age group (years) |
Dermal load (µg/cm2/24 h) | Systemic exposure (mg/kg bw/day) |
|---|---|---|---|---|
| Sunscreena (per day) |
0.0115c | Infants (0–0.5) |
0.45 | 0.18e,f |
| Analgesic creamb (per day) |
0.015d | Adult (12–19) |
0.27 | 0.027e,g |
| Acne spot treatmentb (per day) |
0.01d | Teen (12–19) |
0.17 | 0.00026e,g |
a Natural health product.
b Non-prescription drug.
c Personal communication, email from Therapeutic Products Directorate, Health Canada, to Consumer Product Safety Directorate, Health Canada, dated May 25, 2017; unreferenced.
d Personal communication, email from Health Canada Natural and Non-Prescription Health Products Directorate, Health Canada, to Consumer Product Safety Directorate, Health Canada, dated February 20, 2017; unreferenced.
e Calculated using the product dermal load as the maximum dermal amount.
f A metabolism refinement was not applied to the infant age group.
g Calculated using a metabolism refinement of 48.95% iso-butylparaben.
7.7.2 Health effects assessment
iso-Butylparaben has been addressed in several reviews and key risk assessments of parabens, including those conducted by the CIR (Andersen 2008), the EU SCCS (SCCP 2005a, 2005b; SCCS 2010, 2011), EFSA (2004), NICNAS (2016) and independent scientists (Soni et al. 2005). The literature published until March 2017 was searched for relevant information to supplement the data used in the international reviews and assessments.
Toxicokinetics
Moos et al. (2016) report the ingestion of 10 mg of iso-butylparaben by 3 adult volunteers (equivalent to 0.12-0.19 mg/kg bw). The elimination half-life for all participants was 3 to 4 hours, with 85% of the administered dose recovered in 24 hours. After 48 hours, 6.8% of free and conjugated iso-butylparaben was recovered in urine. PHHA was the main metabolite, comprising 57% of the applied dose recovered in urine. iso-Butylparaben was detected primarily in conjugated form in human urine, blood, and breast milk, although to a lesser extent than other parabens (Azzouz et al. 2016). In urine samples from 14 human volunteers, free iso-butylparaben was detected in only 1 sample, at 0.13 µg/L, but iso-butylparaben conjugated to glucuronic acid was detected in 9 samples (total paraben range of 0.13 to 0.99 µg/L). In breast milk samples from 7 human volunteers, free and conjugated iso-butylparaben was detected in only one sample, free paraben at 0.15 µg/L and total paraben (free +conjugated) at 0.75 µg/L. In blood samples from 8 human volunteers, free paraben was not detected in any samples, but conjugated paraben was detected in two samples (total paraben range of 0.14 to 0.29 µg/L).
Repeat dose studies
In a 6-week study, ICR/Jcl mice (10/sex/group) were exposed to 0%, 0.6%, 1.25%, 2.5%, 5% or 10% iso-butylparaben in the diet (corresponding to approximately 900, 1 875, 3 750, 7 500 or 15 000 mg/kg bw/day). All rats in the two highest dose groups died within 2 weeks, and weight gain in the next two highest doses was less than 10% that of the control group. All doses higher than 0.6% lead to marked atrophy of lymphoid tissue in organs, including the spleen, thymus and lymph nodes and multifocal degeneration and necrosis in the liver parenchyma. No significant changes in the visceral organs were observed in the 0.6% dose group or the controls (Inai et al. 1985). The NOAEL identified for this study is 0.6% (approximately 900 mg/kg bw/day).
Five-week-old Sprague Dawley rats (10/group) were treated dermally with 0, 50, 100, 300 or 600 mg/kg bw/day iso-butylparaben daily for 28 days in an OECD 410 repeat dose study. The test article was dissolved in ethanol and applied in cream to shaved dorsal skin at 50 µL/cm2 on 10% of the body surface area. No effects were observed on body weight, organ weight, or serum levels of triiothyronine, thyroid-stimulating hormone, insulin, estrogen, follicle-stimulating hormone, or testosterone. Epidermal hyperplasia and other local dermal effects were observed in animals from the 100 mg/kg bw/day and higher iso-butylparaben group (Kim 2015). The NOAEL identified for this study is 50 mg/kg bw/day based on epidermal hyperplasia observed at 100 mg/kg bw/day.
Genotoxicity
iso-Butylparaben was negative in Ames assays and in chromosomal aberration assays in Chinese hamster and human embryo fibroblasts (Kawachi and Yahagi 1980, Ishidate et al. 1984). It was also negative in an in vivo chromosomal aberration assay in silkworms, but had equivocal results in an in vivo chromosome aberration assay in rat bone marrow (Kawachi and Yahagi 1980). iso-Butylparaben is not expected to be genotoxic in vivo.
Carcinogenicity
ICL/Jcr mice (50/group) were fed 0%, 0.15%, 0.3% or 0.6% iso-butylparaben in the diet for 102 weeks (equivalent to 259, 470 and 940 mg/kg bw/day in males and 223, 372 and 887 mg/kg bw/day in females). High numbers of animals died in all groups (control and test groups) before 78 weeks. Tumour incidences were similar in test groups compared with the control, and there were no significant differences between dose groups. Twenty-one total neoplasms were observed in the control group, and 26, 22, and 27 were observed in iso-butylparaben low-, mid-, and high-dose groups, respectively. There were no significant differences in incidence or time-to-death from neoplasms between test and control groups (Inai et al. 1985). Statistical analysis was not reported. The NOAEL identified for this study is 0.6% (887 mg/kg bw/day), the highest dose tested.
Reproductive and developmental effects
See Appendix C for a review of in vitro estrogenic effects of iso-butylparaben.
iso-Butylparaben administered subcutaneously caused an increase in uterine weight in ovariectomized Crj:CD mice at 250 mg/kg bw/day (Koda et al. 2005). However, in a female pubertal assay, the same effect was not observed when assayed slightly later in development. In a study of female pubertal development, pre-pubertal Sprague Dawley rats (10/group) were orally administered 0, 62.5, 250, and 1 000 mg/kg bw/day of iso-butylparaben in corn oil from PND 21 to 40. No significant effects were observed in organ weights, vaginal opening day, or estrous cycling. No significant differences in uterine weight were observed on PND 41 at any tested dose, relative to vehicle control. A significant increase in uterine wall thickness was observed in animals treated with all doses of iso-butylparaben. However, the significance of this effect is not clear as the estrous cycle at termination was not reported. Significant changes were also observed in the number of corpora lutea and cystic follicles, at all doses (Vo et al. 2010).
Pregnant Sprague Dawley rats (3 dams/group; 8 pups/group) were administered 0 or 2.5 mg/kg bw/day iso-butylparaben by gavage from GD 5 to PND 21. No effect was reported on key developmental events in pups (pinna detachment, eruption of the incisors, separation of the eyelids, nipple retention, descent of the testis, and separation of the prepuce), pup body weight, relative testis or epididymis weight, or serum hormone levels (17β-estradiol, testosterone, and follicle stimulating hormone). Male offspring had significantly reduced sperm motility and epididymal sperm count (Yang 2016). The LOAEL identified for this study was 2.5 mg/kg bw/day, the only dose tested.
Epidemiology
In a cross-sectional study of male patients at a fertility clinic, urinary iso-butylparaben concentration was positively associated with high DNA stainability, indicative of DNA damage. No association was observed with other semen quality parameters, such as morphology, motility and concentration, nor with serum levels of FSH, testosterone or estradiol (Jurewicz et al. 2017).
7.7.3 Characterization of risk to human health
In a 6-week study, dietary doses of iso-butylparaben at greater than 0.6% (approximately equal to 900 mg/kg bw/day) resulted in organ atrophy and necrosis (Inai et al. 1985). Dermal exposure of up to 600 mg/kg bw/day did not result in effects on organ weight or serum hormone levels. Epidermal hyperplasia was observed at the application site at 100 mg/kg bw/day (Kim 2015). No evidence was identified to suggest that iso-butylparaben is genotoxic or carcinogenic.
In a study of female reproductive development, significant changes in the number of ovarian corpora lutea and cystic follicles and in uterine wall thickness were observed at all doses (Vo et al. 2010). When administered to dams from GD 5 to PND 21, no significant effect was observed on several developmental endpoints in pups; however significantly reduced sperm motility and epididymal sperm count was observed in male pups (Yang 2016). This effect is consistent with the finding that urinary iso-butylparaben concentration is positively associated with DNA stainability in male patients at a fertility clinic (Jurewicz et al. 2017). This study shares a similar dosing regimen and investigated similar endpoints as Boberg et al. (2016) and Kang et al. (2002), which were the key studies used to determine the point of departure for butylparaben. However, only one dose level of iso-butylparaben was administered in Yang (2016) and it was lower than the lowest dose of butylparaben (10 mg/kg bw/day) administered in Boberg et al. (2016). Several in vitro studies have shown that iso-butylparaben exhibits estrogenic activity that is equivalent or greater than that of butylparaben (Terasaki et al. 2009; van Meeuwen et al. 2008) suggesting that similar effects may be expected in vivo. Taken together, this analysis suggests that, although reduced sperm concentration and motility are not clearly adverse in the absence of other effects on reproductive organs and development, in this case, they may be considered a critical effect for determining a point of departure. Therefore, a point of departure of 2.5 mg/kg bw/day, the LOAEL from Yang et al. (2016) was selected for iso-butylparaben.
The Canadian population is exposed to iso-butylparaben from cosmetics, non-prescription drugs and NHPs. Exposure from food and environmental media is expected to be negligible. To address the potential risk associated with exposure to iso-butylparaben from products, margins of exposure resulting from modelled exposures in sentinel scenarios are presented in Table 7‑30.
| Product scenario | Age group (years) | Systemic exposure (mg/kg bw/day) | Critical effect levela (mg/kg bw/day) |
MOE |
|---|---|---|---|---|
| Lip balmb (oral, per event) |
Toddler | 6.5x10-4 | LOAEL 2.5 | 3846 |
| Lipstickb (oral, per day) |
Teen | 1.2x10-3- 4.0x10-4 |
LOAEL 2.5 | 2083–6250 |
| Body lotionb (dermal, per event) |
Toddler | 0.46–1.54 | LOAEL 2.5 | 1.6–5.4 |
| Body oilb (dermal, per event) |
Adult | 0.26–0.79 | LOAEL 2.5 | 3.2–9.5 |
| Body scrubb (dermal, per event) |
Teen | 0.094–0.31 | LOAEL 2.5 | 8–27 |
| Face make-upb (dermal, per day) |
Teen | 0.0064 –0.079 | LOAEL 2.5 | 32–393 |
| Face lotionb (dermal, per day) |
Teen | 0.090 –0.15 | LOAEL 2.5 | 17–28 |
| Eye lotionb (dermal, per day) |
Teen | 0.0061–0.020 | LOAEL 2.5 | 122–407 |
| Sunless tanning productb (dermal, per event) |
Adult | 0.036 | LOAEL 2.5 | 69 |
| Shampoob (dermal, per day) |
Adult | 0.0011–0.0053 | LOAEL 2.5 | 469–2344 |
| Hair conditionerb (rinse-off, dermal, per event) | Toddler | 0.010–0.033 | LOAEL 2.5 | 75–249 |
| Body cleanserb (liquid, dermal, per day) | Infant | 0.0012–0.0062 | LOAEL 2.5 | 401–2005 |
| Sunscreenc (dermal, per day) | Infant | 0.18 | LOAEL 2.5 | 14 |
| Analgesic creamd (dermal, per day) |
Adult | 0.027 | LOAEL 2.5 | 94 |
| Acne spot treatmentd (dermal, per day) | Teen | 0.00026 | LOAEL 2.5 | 9442 |
Abbreviations: LOAEL, lowest observed adverse effect level
a Critical effect: Reduced sperm count and motility
b Cosmetic
c Natural health product
d Non-prescription drug
The calculated margins for exposure to body lotion, body oil, body scrub, face make-up, face lotion, eye lotion, sunless tanning product, shampoo, hair conditioner, body cleanser (infant exposure), sunscreen and analgesic cream are considered potentially inadequate to address uncertainties in the health effects and exposure databases.
7.8 Uncertainties in the evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Substance | Key source of uncertainty | Impact |
|---|---|---|
| Methylparaben, ethylparaben, propylparaben, butylparaben and benzylparaben | Biomonitoring data used to estimate exposures to parabens is based on spot urine samples, which reflect recent exposures and may not be not accurate reflections of longer-term average exposures. Use of the upper confidence limit of the 95th percentile of urinary concentration among spot urine samples is a conservative approach to characterize longer-term average exposures. | + |
| Methylparaben, ethylparaben, propylparaben, butylparaben and benzylparaben | The fractional urinary excretion used to estimate daily intake from biomonitoring data is based on oral exposure and may not accurately address dermal exposure. | +/- |
| Benzylparaben | No biomonitoring data for benzylparaben levels in urine or plasma in the general population of Canada were found. | +/- |
| Methylparaben and butylparaben | The human fractional urinary excretion used to calculate daily intake from biomonitoring data was based on a small sample size, addressed by additional consideration of uncertainty in the MOE. | + |
| Ethylparaben and propylparaben | The human fractional urinary excretion used to calculate daily intake from biomonitoring data was extrapolated from human pharmacokinetics data for other parabens, addressed by additional consideration of uncertainty in the MOE. | + |
| Benzylparaben | There is low confidence in the estimated fractional urinary excretion of benzylparaben. | +/- |
| Ethylparaben | Approximately 65% of CHMS samples contained ethylparaben at a concentration below the limit of detection. | - |
| Propylparaben | Approximately 20% of CHMS samples contained propylparaben at a concentration below the limit of detection. | - |
| Butylparaben | Approximately 83% of CHMS samples contained butylparaben at a concentration below the limit of detection. | - |
| Propylparaben and butylparaben | CHMS sample analysis did not differentiate n- and iso-forms of propyl and butylparaben | + |
| Propylparaben | Toxicokinetic studies indicate that humans may metabolize propylparaben less efficiently than rats. | - |
| Methylparaben | Chronic animal studies for oral or dermal exposure to methylparaben were not identified. Reliable studies for female pubertal development were not identified for methylparaben. | +/- |
| Ethylparaben | Chronic animal studies for oral or dermal exposure to ethylparaben were not identified. Reliable studies for male/female pubertal development were not identified for ethylparaben. | +/- |
| Propylparaben | Chronic animal studies for oral or dermal exposure to propylparaben were not identified. | |
| Butylparaben | Chronic animal studies for oral or dermal exposure to butylparaben were not identified. Reliable studies for female pubertal development were not identified for butylparaben. | +/- |
| Benzylparaben | There is a limited database of hazard studies for all endpoints for benzylparaben. | +/- |
| iso-Propylparaben | Chronic animal studies for oral or dermal exposure to iso-propylparaben were not identified. Reliable studies for male/female pubertal development, reproduction or prenatal development were not identified for iso-propylparaben. | +/- |
| iso-Butylparaben | Chronic animal studies for oral or dermal exposure to iso-butylparaben were not identified. Reliable studies for male/female pubertal development, reproduction or prenatal development in females were not identified for iso-butylparaben. | +/- |
| All parabens | Confirmed concentrations in cosmetics indicate that exposure modelling based on notifications made to Health Canada may overestimate exposure to parabens from cosmetics. | + |
- + = uncertainty with potential to cause over-estimation of exposure/risk;
- - = uncertainty with potential to cause under-estimation of exposure risk;
- +/- = unknown potential to cause over or under estimation of risk.
8. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from methylparaben, ethylparaben, propylparaben, butylparaben, benzylparaben, iso-propylparaben, and iso-butylparaben. It is proposed to conclude that methylparaben, ethylparaben, propylparaben, butylparaben, benzylparaben, iso-propylparaben, and iso-butylparaben do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that methylparaben, propylparaben, butylparaben and iso-butylparaben meet the criteria under paragraph 64(c) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that ethylparaben, benzylparaben and iso-propylparaben do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that methylparaben, propylparaben, butylparaben and iso-butylparaben meet one or more of the criteria set out in section 64 of CEPA and that ethylparaben, benzylparaben and iso-propylparaben do not meet any of the criteria set out in section 64 of CEPA.
It is also proposed that methylparaben, propylparaben, butylparaben and iso-butylparaben do not meet the persistence or bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
References
Abbas S, Greige-Gerges H, Karam N, Piet MH, Netter P, Magdalou J. 2010. Metabolism of parabens (4-hydroxybenzoic acid esters) by hepatic esterases and UDP-glucuronosyltransferases in man. Drug Metab Pharmacokinet. 25(6):568-577.
Adler-Hradecky C, Kelenty B. 1960. On the toxicity and local analgesic activity of p-hydroxybenzoic acid esters. Archives Internationales de Pharmacodynamie et de Therapie. 128:135-142. [as cited by Andersen 2008].
Adoamnei E, Mendiola J, Moñino-García M, Vela-Soria F, Iribarne-Durán LM, Fernández MF, Olea N, Jørgensen N, Swan SH, Torres-Cantero AM. 2018. Urinary concentrations of parabens and reproductive parameters in young men. Sci Total Environ. 621:201-209.
Aker AM, Johns L, McElrath TF, Cantonwine DE, Mukherjee B, Meeker JD. 2018. Associations between maternal phenol and paraben urinary biomarkers and maternal hormones during pregnancy: A repeated measures study. Environ Int. 113:341-349.
Aker AM, Watkins DJ, Johns LE, Ferguson KK, Soldin OP, Del Toro LVA, Alshawabkeh AN, Cordero JF, Meeker JD. 2016. Phenols and parabens in relation to reproductive and thyroid hormones in pregnant women. Environ Res. 151:30-37.
Alliot F, Moreau-Guigon E, Bourges C, Desportes A, Teil MJ, Blanchard M, Chevreuil M. 2014. A multi-residue method for characterization of endocrine disruptors in gaseous and particulate phases of ambient air. Atmos Environ. 92:1-8.
Andersen FA. 2008. Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int J Toxicol. 27(4):1-82.
Aubert N, Ameller T, Legrand JJ. 2012. Systemic exposure to parabens: Pharmacokinetics, tissue distribution, excretion balance and plasma metabolites of [14C]-methyl-, propyl- and butylparaben in rats after oral, topical or subcutaneous administration. Food Chem Toxicol. 50:445-454.
Ayar A, Uysal H. 2013. Examination of the genotoxic effects of various parabens used as food additives with the Drosophila wing spot test (SMART). J Appl Biol Sci. 7(2):83-88.
Aylward LL, Hays SM, Kirman C, Summit Toxicology, LLP. 2017a. Derivation of Biomonitoring Equivalents for Selected Esters of para-Hydroxybenzoic Acid (Parabens). Health Canada Contract No. 4500354002.
Aylward LL, Hays SM, Zidek A. 2017b. Variation in urinary spot sample, 24 h samples, and longer-term average urinary concentrations of short-lived environmental chemicals: Implications for exposure assessment and reverse dosimetry. J Expo Sci Environ Epidemiol 27:582-590.
Aylward LL, Vezina A, Deveau M, St. Amand A, Nong A, Hays S. 2015. Biomonitoring Equivalents for interpretation of urinary fluoride. Regul. Toxicol Pharmacol. 72:158-167.
Azzouz A, Ballesteros E. 2014. Trace analysis of endocrine disrupting compounds in environmental water samples by use of solid-phase extraction and gas chromatography with mass spectrometry detection. J Chromatogr A. 1360:248-257.
Azzouz A, Rascon AJ, Ballesteros E. 2016. Determination of free and conjugated forms of endocrine-disrupting chemicals in human biological fluids by GC−MS. Bioanalysis. 8(11):1145-1158.
Barr L, Metaxas G, Harbach CAJ, Savoy LA, Darbre PD. 2012. Measurement of paraben concentrations in human breast tissue at serial locations across the breast from axilla to sternum. J Appl Toxicol. 32(3):219-232.
Beerens-Heijnen CGM. 2009. Repeated dose 28-day oral toxicity study with methyl 4-hydroxybenzoate by daily gavage in the rat followed by a 14-day recovery period. 25 September 2009. NOTOX Project 489456.
Blanco E, Casais MC, Mejuto MC, Cela R. 2009. Combination of off-line solid-phase extraction and on-column sample stacking for sensitive determination of parabens and p-hydroxybenzoic acid in waters by non-aqueous capillary electrophoresis. Anal Chim Acta. 647:104-111.
Blevins RD, Taylor DE. 1982. Mutagenicity screening of twenty-five cosmetic ingredients with the salmonella/microsome test. J Environ Sci Health. A17(2) 217-239.
Boberg J, Axelstad M, Svingen T, Mandrup K, Christiansen S, Vinggaard AM, Hass U. 2016. Multiple endocrine disrupting effects in rats perinatally exposed to butylparaben. Toxicol Sci. 152(1):244-256.
Boberg J, Taxvig C, Christiansen S, Hass U. 2010. Possible endocrine disrupting effects of parabens and their metabolites. Reprod Toxicol. 30:301-312.
Bremmer HF, Prud’homme de Lodder LCH, van Engelen JGM. 2006. Cosmetics Fact Sheet [PDF]. RIVM report 320104001/2006.
Byford JR, Shaw LE, Drew MGB, Pope GS, Sauer MJ, Darbre PD. 2002. Oestrogenic activity of parabens in MCF7 human breast cancer cells. J Steroid Biochem Mol Biol. 80:4-60.
Calafat AM, Weuve J, Xiaoyun Y, Jia LT, Hu H, Ringer S, Huttner K, Hauser R. 2009. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect. 117(4):639-644.
Calafat AM, Ye X, Wong LY, Bishop AM, Needham LL. 2010. Urinary concentrations of four parabens in the US population: NHANES 2005–2006. Environ Health Perspect. 118(5):679-685.
Canada. 1978. Food and Drug RegulationsC.R.C., c. 870.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c. 33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
Canada, Dept. of the Environment. 2017. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances included as part of the 2017 Inventory Update. Canada Gazette, Part I, vol. 151, no. 2.
Canosa P, Rodrgues I, Rubi E, Cela R. 2007. Determination of parabens and triclosan in indoor dust using matrix solid-phase dispersion and gas chromatography with tandem mass spectrometry. Anal Chem. 79:1675-1681.
Carmona E, Andreu V, Pico Y. 2014. Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: From waste to drinking water. Sci Total Environ. 484:53-63.
Carvalho CM, Menezes PFC, Letenski GC, Praes CEO, Feferman IHS, Lorencini M. 2012. In vitro induction of apoptosis, necrosis and genotoxicity by cosmetic preservatives: Application of flow cytometry as a complementary analysis by NRU. Int J Cosmet Sci. 34:176-182.
Charles River Laboratories. 2018. The In Vitro Percutaneous Absorption and Metabolism of Radiolabelled Iso-Propylparaben, Radiolabelled Iso-Butylparaben, Radiolabelled Methylparaben, Radiolabelled Ethylparaben, Radiolabelled Propylparaben and Radiolabelled Butylparaben in Two Test Preparations Through Human Skin. Test Facility Study No. 781278, Report No. 39507.
ChemCAN [level III fugacity model of 24 regions of Canada]. 2003. Version 6.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
ChemSpider. 2017a. ChemSpider ID: 6912. [accessed 2017 Aug 22].
ChemSpider. 2017b. ChemSpider ID: 18995. [accessed 2017 Aug 22].
ChemSpider. 2017c. ChemSpider ID: 19066. [accessed 2017 Aug 22].
[CoRAP] ECHA Community Rolling Action Plan. 2016. Substance Evaluation Report: 4-hydroxybenzoic acid. June 7, 2016. EC 202-804-9.
CosIng. 2019. Cosmetic Ingredient Database, European Commission, Health and Consumers – Cosmetics. [accessed 2019 Apr 17].
[CTFA] Cosmetic, Toiletry and Fragrance Association. 1980a. Safety data test summary: Acute oral toxicity, Ethylparaben. Unpublished data submitted by CTFA (January 25, 1980; CTFA Code No. 2-7-89) [as cited in Andersen 2008].
[CTFA] Cosmetic, Toiletry and Fragrance Association. 1980b. Safety data test summary: Oral, dermal, and ocular testing of product containing Butylparaben. Unpublished data submitted by CTFA (CTFA Code No. 2-7-12) [as cited in Andersen 2008].
[CTFA] Cosmetic, Toiletry and Fragrance Association. 1980g. Subchronic (three month) dermal toxicity study in rabbits with product AI-0025 containing Methylparaben, study B-7049. Unpublished data submitted by CTFA (Oct. 1980; CTFA Code No. 2-7-75) [as cited in Andersen 2008].
[CTFA] Cosmetic, Toiletry and Fragrance Association. 1981f. Thirteen-week subchronic dermal toxicity study in albino rats with medicated cream containing Methylparaben and medicated lotion containing Propylparaben. Study project code ATOI65. Unpublished data submitted by CTFA (April 24, 1981; CfFA Code No. 2-7-113). [as cited in Andersen 2008].
[CTFA] Cosmetic, Toiletry and Fragrance Association. 1985. Benzylparaben: Acute oral toxicity, acute dermal irritation/corrosion, acute eye irritation/corrosion, and COLIPA summary. Unpublished data submitted by CTFA. [as cited in Andersen 2008].
[CTFA] Cosmetic, Toiletry and Fragrance Association. 1983. Summary for the results of surveys of the amount and frequency of use of cosmetic products by women. Report prepared by Pitkin B, Rodericks JV, Turnbull D. Environ Corporation, Washington, DC.
[ConsExpo] Consumer Exposure Model [Internet]. 2006. Version 4.1. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu (National Institute for Public Health and the Environment).
[Danish EPA] Danish Environmental Protection Agency. 2008. Survey and health assessment of chemical substances in hobby products for children. Survey of Chemical Substances in Consumer Products, No. 93. Danish Ministry of the Environment.
Deierlein AL, Wolff MS, Pajak A, Pinney SM, Windham GC, Galvez MP, Rybak M, Calafat AM, Kushi LH, Biro FM, Teitelbaum SL. 2017. Phenol concentrations during childhood and subsequent measures of adiposity among young girls. Am J Epidemiol 186(5):581-592.
Den Hond E, Paulussen M, Geens T, Bruckers L, Baeyens W, David F. 2013. Biomarkers of human exposure to personal care products: Results from the Flemish Environment and Health Study (FLEHS 2007-2011). Sci Total Environ. 463-464:102-110.
Dodge LE, Williams PL, Williams MA, Missmer SA, Toth TL, Calafat AM, Russ H. 2015. Paternal urinary concentrations of parabens and other phenols in relation to reproductive outcomes among couples from a fertility clinic. Environ Health Perspect. 123(7):665-671.
[DPD] Drug Product Database [database]. [modified 2015 Jul 17]. Ottawa (ON): Government of Canada. [accessed 2017 Mar 7].
Dymicky M, Hutanen CN. 1979. Inhibition of Clostridium botulinum by p-hydroxybenzoic acid n-alkyl esters. Antimicrob Agents Chemother. 15(6):798-801.
[EC] European Commission. 2003. Technical Guidance Document on Risk Assessment, Part I. 1998. Institute for Health and Consumer Protection, European Chemicals Bureau.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC). ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2015. Identification of risk assessment priorities: results of the 2015 review. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada.
[EFSA] European Food Safety Authority. 2004. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a Request from the Commission related to para hydroxybenzoates. The EFSA Journal 83:1-26.
Elder DP, Crowley PJ. 2012. Antimicrobial preservatives part two: Choosing a preservative. American Pharmaceutical Review.
[EMA] European Medicines Agency. 2015. Reflection paper on the use of methyl- and propylparaben as excipients in human medicinal products for oral use. 22 October 2015. EMA/CHMP/SWP/272921/2012.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada. 2017. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[EPA] United States Environmental Protection Agency. 2009. Endocrine Disruptor Screening Program Test Guidelines OPPTS 890.1450: Pubertal Development and Thyroid Function in Intact Juvenile/ Peripubertal Female Rats. October 2009. EPA 740-C-09-009.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Fahrenholz JM, Simon RA. 1996. Adverse reactions to benzoates and parabens. In: Metcalfe DD, Sampson HA, Simon RA, editors. Food allergy: Adverse reactions to food and food additives. 2nd ed., p. 375-386.
Fan X, Kubwabo C, Rasmussen P, Jones-Otazo H. 2010. Simultaneous quantitation of parabens, triclosan, and methyl triclosan in indoor house dust using solid phase extraction and gas chromatography-mass spectrometry. J Environ Monit. 12:1891-1897.
Ferreira AMC, Moder M, Laespada MEF. 2011. Stir bar sorptive extraction of parabens, triclosan and methyl triclosan from soil, sediment and sludge with in situ derivatization and determination by gas chromatography–mass spectrometry. J Chromatogr A. 1218:3837-3844.
Ficheux AS, Chevillotte G, Wesolek N, Morisset T, Dornic N, Bernard A, Bertho A, Romanet A, Leroy L, Mercat AC, Creusot T, Simon E, Roudot AC. 2016. Consumption of cosmetic products by the French population second part: Amount data. Food Chem Toxicol. 90:130-141.
Ficheux AS, Wesolek N, Chevillotte G, Roudot AC. 2015. Consumption of cosmetic products by the French population. First part: Frequency data. Food Chem Toxicol. 78:159-169.
Finot F, Kaddour A, Morat L, Mouche I, Zaguia N, Cuceu C, Souverville D, Négrault S, Cariou O, Essahli A et al. 2017. Genotoxic risk of ethyl-paraben could be related to telomere shortening. J Appl Toxicol. 37:758-771.
Fisher M, MacPherson S, Braun JM, Hauser R, Walker M, Feeley M, Mallick R, Bérubé R, Arbuckle TE. 2017. Paraben concentrations in maternal urine and breast milk and its association with personal care product use. Environ Sci Technol. 51(7):4009-4017.
Frederiksen H, Nielsen JKS, Morck TA, Hansen PW, Jensen JF, Nielsen O, Andersson AM, Knud LE. 2013. Urinary excretion of phthalate metabolites, phenols and parabens in rural and urban Danish mother-child pairs. Int J Hyg Environ Health. 216:772-783.
Furia TE. 1972. Handbook of Food Additives, Vol.1, 2nd ed.
Gazin V, Marsden E, Marguerite F. 2013. Oral propylparaben administration to juvenile male Wistar rats did not induce toxicity in reproductive organs. Toxicol Sci. 136(2):392-401.
Geer LA, Pycke BFG, Waxenbaum J, Sherer DM, Abulafia O, Halden RU. 2017. Association of birth outcomes with fetal exposure to parabens, triclosan and triclocarban in an immigrant population in Brooklyn, New York. J Hazard Mater. 323:177-183.
Genuis SJ, Birkholz D, Curtis L, Sandau C. 2013. Paraben Levels in an Urban Community of Western Canada. ISRN Toxicology 2013 (Article ID 507897).
Giulivo M, de Alda ML, Capri E, Barceló D. 2016. Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environ Res. 151:251-264.
Gomez E, Pillon A, Fenet H, Rosain D, Duchesne MJ, Nicolas JC, Balaguer P, Casellas C. 2005. Estrogenic activity of cosmetic components in reporter cell lines: Parabens, UV screens and musks. J Toxicol Environ Health A. 68:239-251.
Gomez-Berrada MP, Gautier F, Parent-Massin D, Ferret PJ. 2013. Retrospective exposure data for baby and children care products: An analysis of 48 clinical studies. Food Chem Toxicol 57:185-194.
Grant DJW, Mehdizadeh M, Chow AHL, Fairbrother JE. 1984. Non-linear van't Hoff solubility - temperature plots and their pharmaceutical interpretation. Int J Pharm. 18 25-38.
Guo J, Wu C, Lu D, Jiang S, Liang W, Chang X, Xu H, Wang G, Zhou Z. 2017. Urinary paraben concentrations and their associations with anthropometric measures of children aged 3 years. Environ Poll 222:307-314.
Harville HM, Voorman R, Prusakiewicz JJ. 2007. Comparison of paraben stability in human and rat skin. Drug Metab Lett. 1:17-21.
Haman C, Dauchy X, Rosin C, Munoz JF. 2015. Occurrence, fate and behavior of parabens in aquatic environments: A review. Water Res. 68:1-11.
Hays SM, Aylward LL, LaKind JS, Bartels MJ, Barton HA, Boogaard PJ, Brunk C, DiZio S, Dourson M, Goldstein DA, Kilpatrick ME, Krewski D, Krishnan, K, Lipscomb J, Nordberg M, Okino M, Tan Y-M, Viau C, Yager JW. 2008. Guidelines for the derivation of biomonitoring equivalents: Report from the Biomonitoring Equivalents Expert Workshop. Regul Toxicol. Pharmacol. 51:S4-S15.
Health Canada. 1995. Investigating human exposure to contaminants in the environment: A handbook for exposure calculations. Ottawa (ON): Health Canada, Great Lakes Health Effects Program.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate
Health Canada. [modified 2015 Dec 14]. Cosmetic Ingredient Hotlist. Ottawa (ON): Government of Canada. [accessed 2017 Aug 18].
Health Canada. 2015. Food Consumption Table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004), Share file. Ottawa.
Health Canada. 2017a. Fourth Report on Human Biomonitoring of Environmental Chemicals in Canada. Results of the Canadian Health Measures Survey Cycle 4 (2014–2015) [PDF].
Health Canada. 2017b. Standard Operating Procedure: Analysis of parabens in human urine by UPLC MS MS. Food Directorate - Western Region Laboratory. WES-MET-0185, Revision 9.
Health Canada. 2018a. Supporting Documentation: Evaluation of Epidemiologic Studies on Parabens. Ottawa (ON): Health Canada. Available from: substances@ec.gc.ca.
Health Canada. 2018b. Supporting Documentation: Results and interpretation of an in vitro dermal absorption and metabolism study of methyl, ethyl, propyl, butyl, iso-propyl, and iso-butylparaben. Ottawa (ON): Health Canada. Available from: substances@ec.gc.ca.
Heim F, Leuschner F, Wunderlich G. 1957. Metabolism of phydroxybenzoic acid ethyl ester. Klin. Wochenschr. 35:823-825. [as cited in Andersen 2008].
Hirose M, Inoue T, Asamoto M, Tagawa Y, Ito N. 1986. Comparison of the effects of 13 phenolic compounds in induction of proliferative lesions of the forestomach and increase in the labelling indices of the glandular stomach and urinary bladder epithelium of Syrian golden hamsters. Carcinogenesis. 7(8):1285-1289.
Hoberman AM, Schreur DK, Leazer T, Daston GP, Carthew P, Re T, Loretz L, Mann P. 2008. Lack of effect of butylparaben and methylparaben on the reproductive system in male rats. Birth Defects Res B Dev Reprod Toxicol. 83:123-133.
Honda M, Robinson M, Kannan K. 2018. Parabens in human urine from several Asian countries, Greece, and the United States. Chemosphere. 201:13-19.
Hossaini A, Larsen JJ, Larsen JC. 2000. Lack of oestrogenic effects of food preservatives (parabens) in uterotrophic assays. Food Chem Toxicol. 38:319-323.
Hu P, Chen X, Whitener RJ, Boder ET, Jones JO, Porollo A, Chen J, Zhao L. 2013. Effects of Parabens on Adipocyte Differentiation. Toxicol Sci. 131(1):56-70.
Inai K, Aoki Y, Akamizu H, Eto R, Nishida T, Tokuoka S. 1985. Tumorgenicity study of butyl and isobutyl p-hydroxybenzoates administered orally to mice. Food Chem Toxicol. 23(6):575-578.
Ishidate M, Hayashi M, Sawada M, Yoshikawa K, Ono M, Nakadate M. 1978. Cytotoxicity test on medical drugs—chromosome aberration tests with Chinese hamster cells in vitro. Eisei Shikenjo Hokoku. 96:55-61.
Ishidate M, Odashima S. 1977. Chromosome tests with 134 compounds on Chinese hamster cells in vitro – a screening for chemical carcinogens. Mutat Res. 48:337-354.
Ishidate M, Sofuni T, Yoshikawa K, Hayashi M, Nohmi T, Sawada M, Matsuoka A. 1984. Primary mutagenicity screening of food additives currently in use in Japan. Food Chem Toxicol. 22(8):623-636.
Ishidate M, Yoshikawa K. 1980. Chromosome aberration tests with Chinese hamster cells in vitro with and without metabolic activation - A comparative study on mutagens and carcinogens. Arch. Toxicol. 4:41-44.
Ishiwatari S, Suzuki T, Hitomi T, Yoshino T, Matsukuma S, Tsuji T. 2007. Effects of methyl paraben on skin keratinocytes. J Appl Toxicol. 27:1-9.
Ishizaki M, Ueno S, Oyamada N, Katsumura K. 1978. Studies on degradation of food additives by irradiation (IV): Reaction of butyl p-hydroxybenzoate with potassium nitrate or sodium nitrite by irradiation of ultra violet ray and mutagenicity of its reaction products. Food Hyg Safety Sci. 19(3):305-10.
Ishizeki C, Aoyama S, Hatta Y, Fujita Y, Oda Y, Urabe M. 1955. Studies on food antiseptics. I. Bull. Natl. Hyg. Lab. Tokyo. 73:237-243. [as cited in Andersen 2008].
Jakimska A, Huerta B, Bargańska Z, Kot-Wasik A, Rodríguez-Mozaz S, Barceló D. 2013. Development of a liquid chromatography–tandem mass spectrometry procedure for determination of endocrine disrupting compounds in fish from Mediterranean rivers. J Chromatogr A. 1306:44-58.
Janjua NR, Mortensen GK, Andersson AM, Kongshoi Brian, Skakkebaek NE, Wulf HC. 2007. Systemic uptake of diethyl phthalate, dibutyl phthalate, and butyl paraben following whole-body topical application and reproductive and thyroid hormone levels in humans. Environ Sci Technol. 41(15):5564-5570.
Jewell C, Bennett P, Mutch E, Ackermann C, Williams FM. 2007a. Inter-individual variability in esterases in human liver. Biochem Pharmacol. 74:932-939.
Jewell C, Prusakiewicz JJ, Ackermann C, Payne NA, Fate G, Voorman R, Williams FM. 2007b. Hydrolysis of a series of parabens by skin microsomes and cytosol from human and minipigs and in whole skin in short-term culture. Toxicol Appl Pharmacol. 225:221-228.
Jones PS, Thispen D, Morrison JL, Richardson AP. 1956. p-Hydroxybenzoic acid esters as preservatives III. The physiological disposition of p-hydroxybenzoic acid and its esters. J Am Pharm Assoc 45(4):268-273.
Jurewicz J, Radwan M, Wielgomas B, Dziewirska E, Karwacka A, Klimowska A, Kaluzny P, Radwan P, Bochenek M, Hanke W. 2017a. Human semen quality, sperm DNA damage, and the level of reproductive hormones in relation to urinary concentrations of parabens. J Occup Environ Med. 59(11):1034-1040.
Jurewicz J, Radwan M, Wielgomas B, Klimowska A, Kaluzny P, Radwan P, Jakubowski L, Hanke W. 2017b. Environmental exposure to parabens and sperm chromosome disomy. Int J Environ Health Res. 27(5):332-343.
Kang H, Kyung MS, Ko A, Park JH, Hwang MS, Kwon JE, Suh JH, Lee HS, Moon GI, Hong JH et al. 2016. Urinary concentrations of parabens and their association with demographic factors: A population-based cross-sectional study. Environ Res 146:245-251.
Kang S, Kim S, Park J, Kim HJ, Lee J, Choi G, Choi S, Kim S, Kim SY, Moon HB, et al. 2013. Urinary paraben concentrations among pregnant women and their matching newborn infants of Korea, and the association with oxidative stress biomarkers. Sci Total Environ 461-462:214-221.
Kang S, Che JH, Ryu DY, Kim TW, Li GX, Lee YS. 2002. Decreased sperm number and motile activity on the F1 offspring maternally exposed to butyl p-hydroxybenzoic acid (butyl paraben). Sci Total Environ. 461-462:214-221.
Kanno J, Onyon L, Peddada S, Ashby J, Elard J, Owens W. 2003. The OECD program to validate the rat uterotrophic bioassay. Phase 2: Dose-response studies. Environ Health Perspect. 111(12):1530-1549.
Kawachi T, Yahagi T. 1980. Cooperative programme on short-term assays for carcinogenicity in Japan. IARC Sci Publ. 27:323-30.
Kier LE, Brusick DJ, Auletta AE, Von Halle ES, Brown MM, Simmon VF, Dunkel V, McCann J, Mortelmans K, et al. 1986. The Salmonella typhimurium/mammalian microsomal assay: A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat Res 168:69-240.
Kim JW, Ramaswamy BR, Chang KH, Isobe T, Tanabe S. 2011. Multiresidue analytical method for the determination of antimicrobials, preservatives, benzotriazole UV stabilizers, flame retardants and plasticizers in fish using ultra high performance liquid chromatography coupled with tandem mass spectrometry. J Chromatogr A. 1218(22):3511-3520.
Kim S, Kwack SJ, Lim SK, Kim YJ, Roh TH, Choi SM, Kim HS, Lee BM. 2015. Toxicological evaluation of isopropylparaben and isobutylparaben mixture in Sprague Dawley rats following 28 days of dermal exposure. Regul Toxicol Pharmacol 73:544-551.Kim S, Kim S, Won S, Choi K. 2017. Considering common sources of exposure in association studies - Urinary benzophenone-3 and DEHP metabolites are associated with altered thyroid hormone balance in the NHANES 2007–2008. Environ Int. 107:25-32.
Kiwada H, Awazu S, Hanono M. 1979. The study on the biological fate of paraben at the dose of practical usage in rat. I. The metabolism and excretion of ethyl p-hydroxybenzoate (ethyl paraben) and p-hydroxybenzoic acid. J Pharmacobiodyn. 2:356-364.
Kiwada H, Awazu S, Hanono M. 1980. The study on the biological fate of paraben at the dose of practical usage in rat. II. The pharmacokinetic study on the blood concentration after the administration of ethyl paraben or p-hydroxybenzoic acid. J Pharmacobiodyn. 3:353-363.
Koda T, Umezu T, Kamata R, Morohoshi K, Ohta T, Morita M. 2005. Uterotrophic effects of benzophenone derivatives and a p-hydroxybenzoate used in ultraviolet screens. Environ Res. 98:40-45.
Koeppe ES, Ferguson KK, Colacino JA, Meeker JD. 2013. Relationship between urinary triclosan and paraben concentrations and serum thyroid measures in NHANES 2007–2008. Sci Total Environ. 445-446:299-305.
Kolatorova L, Viktu J, Hampl R, Adamcova K, Skodova T, Simkova M, Parizek A, Starka L, Duskova M. 2018. Exposure to bisphenols and parabens during pregnancy and relations to steroid changes. Environ Res. 163:115-122.
Laborie S, Moreau-Guigon E, Alliot F, Desportes A, Oziol L. 2016. A new analytical protocol for the determination of 62 endocrine-disrupting compounds in indoor air. Talanta Elsevier 147:132-141.
Lee-Sarwar K, Hauser R, Calafat AM, Ye X, O'Connor GT, Sandel M, Bacharier LB, Zeiger RS, Laranjo N, Gold DR, et al. (2017). Prenatal and early-life triclosan and paraben exposure and allergic outcomes. J Allergy Clin Immunol. 142(1):269-278.
Lemini C, Hernandez A, Jaimez R, Franco Y, Avila ME, Castell A. 2004. Morphometric analysis of mice uteri treated with the preservatives methyl, ethyl, propyl, and butylparaben. Toxicol Ind Health. 20:123-132.
Lemini C, Jaimez R, Avila ME, Franco Y, Larrea F, Lemus AE. 2003. In vivo and in vitro estrogen bioactivities of alkyl parabens. Toxicol Ind Health. 19:69-79.
Lemini C, Silva C, Timossi D Luque A, Valverde M, Gonzalez-Martinez A, Hernandez C, Rubio-Poo C, Chavez Lara B, Valenzuela F. 1997. Estrogenic effects of p-hydroxybenzoic acid in CD1 mice. Environ Res. 75:130-134.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2018 Feb 6]. Ottawa (ON): Government of Canada. [accessed 2019 Mar 26].
Lobemeier C, Tschoetschel C, Westie S, Heymann E. 1996. Hydrolysis of parabenes by extracts from differing layers of human skin. Biol Chem. 377:647-651.
Loretz LG, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, Rothenstein A, Scrafford CG, Sewall C. 2005. Exposure data for cosmetic products: Lipstick, body lotion, and face cream. Food Chem Toxicol. 43:279-291.
Loretz L, Api AM, Barraj L, Burdick J, Davis DA, Dressler W, Gilberti E, Jarrett G, Mann S, Pan YHL, Re T, Renskers K, Scrafford C, Vater S. 2006. Exposure data for personal care products: Hairspray, spray perfume, liquid foundation, shampoo, body wash, and solid antiperspirant. Food Chem Toxicol. 44:2008-2018.
Loretz LG, Api AM, Babcock L, Barraj LM, Burdick J, Cater KC, Jarrett G, Mann S, Pan YHL, Re TA, Renskers KJ, Scrafford CG. 2008. Exposure data for cosmetic products: Facial cleanser, hair conditioner, and eye shadow. Food Chem Toxicol. 46:1516-1524.
Martin JMP, Peropadre A, Herrero O, Freire PF, Labrador V, Hazen MJ. 2010. Oxidative DNA damage contributes to the toxic activity of propylparaben in mammalian cells. Mutat Res. 702:86-91.
Mason MM, Cate CC, Baker J. 1971. Toxicology and carcinogenesis of various chemicals used in the preparation of vaccines. Clin Toxicol. 4(2):185-204.
Matthews C, Davidson J, Bauer E, Morrison JL, Richardson AP. 1956. p-Hydroxybenzoic acid esters as preservatives II. Acute and chronic toxicity in dogs, rats, and mice. J Am Pharm Assoc. 45(4):260-267.
McCann J, Choi E, Yamasaki E, Ames BN. 1975. Detection of carcinogens as mutagens in the Salmonella/microsome test: Assay of 300 chemicals. Proc. Nat. Acad. Sci. USA 72(12):5135-5139.
Meeker JD, Yang T, Ye X, Calafat AM, Hauser R. 2011. Urinary concentrations of parabens and serum hormone levels, semen quality parameters, and sperm DNA damage. Environ Health Perspect. 119(2):252-257.
Meylan W, Howard PH. 1991. Bond contribution method for estimating Henry’s Law constants. Environ Toxicol Chem. 10:1283-1293.
Meylan W, Howard PH. 1993. Computer estimation of the atmospheric gas-phase reaction rate of organic compounds with hydroxyl radicals and ozone. Chemosphere. 26(12):2293-2299.
Minguez-Alarcon L, Chiu YH, Messerlian C, Williams PL, Sabatini ME, Toth TL, Ford JB, Calafat AM, Hauser R; EARTH Study Team. 2016. Urinary paraben concentrations and in vitro fertilization outcomes among women from a fertility clinic. Fertil Steril. 105(3):714-721.
Moos RK, Angerer J, Dierkes G, Brüning T, Koch HM. 2016. Metabolism and elimination of methyl, iso- and n-butyl paraben in human urine after single oral dosage. Arch Toxicol 90:26992709.
Moos RK, Angerer J, Wittsiepe J, Wilhelm M, Brüning T, Koch HM. 2014. Rapid determination of nine parabens and seven other environmental phenols in urine samples of German children and adults. Int J Hyg Environ Health. 217:845-853.
Moos RK, Apel P, Schroter-Kermani C, Kolossa-Gehring M, Bruning T, Koch HM. 2017. Daily intake and hazard index of parabens based upon 24h urine samples of the German Environmental Specimen Bank from 1995 to 2012. J Expo Sci Environ Epidemiol. 27(6):591-600.
Moos RK, Koch HM, Angerer J, Apel P, Schröter-Kermani C, Brüning T, Kolossa-Gehring M. 2015. Parabens in 24 h urine samples of the German Environmental Specimen Bank from 1995 to 2012. Int J Hyg Environ Health. 218:666-674.
Moreau-Guigon E, Alliot F, Gasperi J, Blanchard M, Teil MJ, Mandin C, Chevreuil M. 2016. Seasonal fate and gas/particle partitioning of semi-volatile organic compounds in indoor and outdoor air. Atmos Environ. 147:423-433.
Morita K, Ishigaki M, Abe T. 1981. Mutagenicity of materials related with cosmetics. J Soc Cosmet Chem Jpn. 15(3):243-253.
Moriyama I, Hiraoka K, Yamaguchi R. 1975. Teratogenic effects of food additive ethyl-p-hydroxy benzoate studies in pregnant rats. Acta Obst Gynaec Jpn. 22(2):94-106.
Mulla H, Yakkundi S, McElnay J, Lutsar I, Metsvaht T, Varendi H, Nellis G, Nunn A, Duncan J, Pandya H, et al. 2015. An observational study of blood concentrations and kinetics of methyl- and propyl-parabens in neonates. Pharm Res 32:1084-1093.
Myridakis A, Chalkiadaki G, Fotou M, Kogevinas M, Chatzi L, Stephanou EG. 2016. Exposure of preschool-age Greek children (RHEA cohort) to bisphenol A, parabens, phthalates, and organophosphates. Environ Sci Technol. 50:932-941.
Nera EA, Lok E, Iverson F, Ormsby E, Karpinski KF, Clayson DB. Short term pathological and proliferative effects of butylated hydroxyanisole and other phenolic antioxidants in the forestomach of Fischer 344 rats. Toxicology. 32:197-213.
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2019 Mar 21]. Ottawa (ON): Government of Canada. [accessed 2019 Mar 26].
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme. 2016. Parabens: Human health tier II assessment. Australian Government, Department of Health.
Nishihama T, Kawakami N, Hatanaka T, Kawakami J, Adachi I. 2003. Age dependency of esterase activity in rat and human keratinocytes. Biol Pharm Bull. 26(9):1311-1314.
Nishihama Y, Yoshinaga J, Iida A, Konishi S, Imai H, Yoneyama M, Nakajima D, Shiraishi H. 2016. Association between paraben exposure and menstrual cycle in female university students in Japan. Reprod Toxicol. 63:107-113.
[NTP] National Toxicology Program. 2005. Butylparaben [CAS No. 94-26-8]. Review of Toxicological Literature.
Nunez L, Tadeo JL, Garcia-Valcarcel AI, Turiel E. 2008. Determination of parabens in environmental solid samples by ultrasonic-assisted extraction and liquid chromatography with triple quadrupole mass spectrometry. J Chromatogr A. 1214:178-182.
Odashima S. 1976. The cooperative development in Japan of methods for screening chemicals for carcinogenicity. In: Montesano R, Bartsch H, Tomatis L, editors. Screening Tests in Chemical Carcinogenesis. IARC Scientific Publication No. 12, p. 61-79.
[OECD] Organisation for Economic Co-operation and Development. 2007. Test No. 440: Uterotrophic bioassay in rodents: A short-term screening test for oestrogenic properties. OECD Guidelines for the Testing of Chemicals, Section 4, OECD Editions, Paris.
OECD QSAR Toolbox. 2016. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Ohta R, Tagaki A, Ohmukai H, Marumo H, Ono A, Matsushima Y, Inoue T, Ono H, Kanna J. 2012. Ovariectomized mouse uterotrophic assay of 36 chemicals. J Toxicol Sci. 37(5):879-889.
Oishi S. 2001. Effects of butylparaben on the male reproductive system in rats. Toxicol Ind Health. 17:31-39.
Oishi S. 2002. Effects of propyl paraben on the male reproductive system. Food Chem Toxicol. 40:1807-1813.
Oishi S. 2004. Lack of spermatotoxic effects of methyl and ethyl esters of p-hydroxybenzoic acid in rats. Food Chem Toxicol. 42:1845-1849.
Okubo T, Yokoyama Y, Kano K, Kano I. 2001. ER-dependent estrogenic activity of parabens assessed by proliferation of human breast cancer MCF-7 cells and expression of ERa and PR. Food Chem Toxicol. 39:1225-1232.
Onodera H, Mitsumori K, Yasuhara K, Shimo T, Kurokawa N, Takahashi M. 1994. 13 week subchronic oral toxicity study of isopropyl p-hydroxybenzoate in F344 rats. Eisei Shikenjo Hokoku. 112:82-88.
[PCPC] Personal Care Products Council. 2018. Cosmetic Ingredient Identification Database (International Cosmetic Ingredient Dictionary (INCI)). [accessed 2018 May 25].
Perez RA, Albero B, Miguel E, Sanchez-Brunete C. 2011. Determination of parabens and endocrine-disrupting alkylphenols in soil by gas chromatography-mass spectrometry following matrix solid-phase dispersion or in-column microwave-assisted extraction: A comparative study. Anal Bioanal Chem. 402:2347-2357.
Petersen B, Wulf HC. 2014. Application of sunscreen – theory and reality. Photodermatol Photoimmunol Photomed. 30:96-101.
Philippat C, Botton J, Calafat AM, Ye X, Charles MA, Slama R, EDEN Study Group. 2014. Prenatal Exposure to Phenols and Growth in Boys. Epidemiology. 25:625-635.
Polinski KJ, Dabelea D, Hamman RF, Adgate JL, Calafat AM, Ye X, Starling AP. 2018. Distribution and predictors of urinary concentrations of phthalate metabolites and phenols among pregnant women in the healthy start study. Environ Res 162:308-317.
Pouliot L. 2013. Propylparaben: Three-month oral developmental study in juvenile rats. Test Facility Study No. 9000049. BMS Reference No. DN12114. 30 October 2013. Charles River Laboratories Preclinical Services.
Prival MJ, Sheldon AT, Popkin D. 1982. Evaluation, using Salmonella typhimurium, of the mutagenicity of seven chemicals found in cosmetics. Food Chem Toxicol. 20:427-432.
Prival MJ, Simmon VF, Mortelmans KE. 1991. Bacterial mutagenicity testing of 49 food ingredients gives very few positive results. Mutat Res. 260:321-329.
Prusakiewicz JJ, Ackermann C, Voorman R. 2006. Comparison of skin esterase activities from different species. Pharm Res. 23(7):1517-1524.
Pugazhendhi D, Pope GS, Darbre PD. 2005. Oestrogenic activity of p-hydroxybenzoic acid (common metabolite of paraben esters) and methylparaben in human breast cancer cell lines. Journal of Applied Toxicology 25:301-309.
Ramírez N, Marcé RM, Borrull F. 2010. Development of a thermal desorption-gas chromatography–mass spectrometry method for determining personal care products in air. J Chromatogr A. 1217:4430-4438.
Ramírez N, Marcé RM, Borrull F. 2011. Determination of parabens in house dust by pressurised hot water extraction followed by stir bar sorptive extraction and thermal desorption–gas chromatography–mass spectrometry. J Chromatogr A. 1218:6226-6231.
[REACH] Registration, Evaluation, Authorisation and Restriction of Chemicals. 2018a. A registration dossier for Methyl 4-hydroxybenzoate [CAS RN 99-76-3] submitted to REACH. [cited 2018 May].
[REACH] Registration, Evaluation, Authorisation and Restriction of Chemicals. 2018b. A registration dossier for Ethyl 4-hydroxybenzoate [CAS RN 120-47-8] submitted to REACH. [cited 2018 May].
[REACH] Registration, Evaluation, Authorisation and Restriction of Chemicals. 2018c. A registration dossier for Propyl 4-hydroxybenzoate [CAS RN 94-13-3] submitted to REACH. [cited 2018 May].
[REACH] Registration, Evaluation, Authorisation and Restriction of Chemicals. 2018d. A registration dossier for Benzyl 4-hydroxybenzoate [CAS RN 94-18-8] submitted to REACH. [cited 2018 May].
[REACH] Registration, Evaluation, Authorisation and Restriction of Chemicals. 2018e. A registration dossier for Isopropyl 4-hydroxybenzoate [CAS RN 4191-73-5] submitted to REACH. [cited 2018 May].
Reddy A, Norris DF, Momeni SS, Waldo B, Ruby JD. The pH of beverages in the United States. The Journal of the American Dental Association 147(4):255-63.
Renz L, Volz C, Michanowicz D, Ferrar K, Christian C, Lenzner D, El-Hefnawy T. 2013. A study of parabens and bisphenol A in surface water and fish brain tissue from the Greater Pittsburgh Area. Ecotoxicology. 22(4):632-41.
[RIVM] The Netherlands National Insititute for Public Health and the Environment. Exposure to and toxicity of methyl-, ethyl- and propylparaben: A literature review with a focus on endocrine-disruption properties. RIVM Report 2017-0028.
Rodrigues C, Lok E, Nera E, Iverson F, Page D, Karpinski K, Clayson DB. 1986. Short-term effects of various phenols and acids on the Fischer 344 male rat forestomach epithelium. Toxicology. 38:103-117.
Routledge EJ, Parker J, Odum J, Ashby J, Sumpter JP. 1998. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 153:12-19.
[RSI] Risk Sciences International. 2018. Final Report: Reproductive and Endocrine Effects Associated with Parabens. Prepared for Health Canada. 13 April 2018.
Sabalitschka T, Neufeld-Crzellitzer. 1954. Zum Verhalten der p-oxybenzosaureester im menschlichen Korper (translated from the original German. Arzneimittelforsch 4:575-579. [as cited in Andersen 2008 and Soni et al. 2005].
Sado I. 1973. Synergistic toxicity of official permissible preservative food additives. Japanese Journal of Hygiene. 28:463-476. [as cited in Soni et al. 2005 and REACH 2018b].
Sasaki M, Sugimura K, Yoshida A, Abe S. 1980. Cytogenetic effects of 60 chemicals on cultured human and Chinese hamster cells. La Kromosomo 2(20):574-584.
Savage JH, Matsui EC, Wood RA, Keet CA. 2012. Urinary levels of triclosan and parabens are associated with aeroallergen and food sensitization. J Allergy Clin Immunol. 130(2):453-460.
Saravanabhavan G, Walker M, Guay M, Aylward L. 2014. Urinary excretion and daily intake rates of diethyl phthalate in the general Canadian population. Sci Total Environ. 500-501:191-198.
[SCCP] European Commission Scientific Committee on Consumer Products. 2005a. Extended opinion on the safety evaluation of parabens. 28 January 2005. SCCP/0873/05.
[SCCP] European Commission Scientific Committee on Consumer Products. 2005b. Extended opinion on parabens, underarm cosmetics and breast cancer. 28 January 2005. SCCP/0874/05
[SCCP] European Commission Scientific Committee on Consumer Products. 2006. Opinion on parabens. COLIPA no. P82. 10 October 2006. SCCP/1017/06.
[SCCP] European Commission Scientific Committee on Consumer Products. 2008. Opinion on parabens. COLIPA no. P82. 24 June 2008. SCCP/1183/08.
[SCCS] European Commission Scientific Committee on Consumer Safety. 2010. Opinion on parabens. COLIPA no. P82. 14 December 2010. SCCS/1348/10.
[SCCS] European Commission Scientific Committee on Consumer Safety. 2011. Clarification on Opinion SCCS/1348/10 in the light of the Danish clause of safeguard banning the use of parabens in cosmetic products intended for children under three years of age. 10 October 2011. SCCS/1446/11.
[SCCS] European Commission Scientific Committee on Consumer Safety. 2013. Opinion on parabens: Updated request for a scientific opinion on propyl- and butylparaben. COLIPA n° P82. 3 May 2013. SCCS/1514/13.
[SCCS] European Commission Scientific Committee on Consumer Safety. 2015. The SCCS’s notes of guidance for the testing of cosmetic substances and their safety evaluation. 9th Revision. 29 September 2015. SCCS/1564/15.
Schlumpf M, Kypke K, Wittassek M, Angerer J, Mascher H, Mascher D, Vökt C, Birchler M, Lichtensteiger W. 2010. Exposure patterns of UV filters, fragrances, parabens, phthalates, organochlor pesticides, PBDEs, and PCBs in human milk: Correlation of UV filters with use of cosmetics. Chemosphere 81(10):1171-1183.
[SDS] Safety Data Sheet 2008a. Washable Ink. Hauppauge, New York, USA. Liqui-Mark
[SDS] Safety Data Sheet 2008b. Embellishment Glue Stick. Chatsworth, California, USA. Pioneer Photo Albums.
[SDS] Safety Data Sheet 2010. All Purpose Wipe. Lin’an Hangzhou, Zhejiang, China. Lin’an Thumb Cleaning Products Co. Ltd.
Shekhar S, Sood S, Showkat S, Lite C, Chandrasekhar A, Vairamani M, Barathi S, Santosh W. 2017. Detection of phenolic endocrine disrupting chemicals (EDCs) from maternal blood plasma and amniotic fluid in Indian population. Gen Comp Endocrinol. 241:100-107.
Shibata MA, Yamada M, Hirose M, Asakawa E, Tatematsu M, Ito N. 1990. Early proliferative responses of forestomach and glandular stomach of rats treated with five different phenolic antioxidants. Carcinogenesis. 11(3):425-429.
Sivaraman L, Pouliot L, Wang B, Brodie T, Graziano M, McNerney ME. 2018. Safety assessment of propylparaben in juvenile rats. Regul Toxicol Pharmacol. 92:370-381.
Smarr MM, Sundaram R, Honda M, Kannan K, Buck LG. 2017. Urinary concentrations of parabens and other antimicrobial chemicals and their association with couples' fecundity. Environ Health Perspect. 125(4):730-736.
Smith KW, Braun JM, Williams PL, Ehrlich S, Correia KF, Calafat AM, Ye X, Ford J, Keller M, Meeker JD, et al. 2012. Predictors and variability of urinary paraben concentrations in men and women, including before and during pregnancy. Environ Health Perspect. 120(11):1538-1543.
Smith KW, Souter I, Dimitriadis I, Ehrlich S, Williams PL, Calafat AM, Hauser R. 2013. Urinary paraben concentrations and ovarian aging among women from a fertility center. Environ Health Perspect. 121(11-12):1299-1305.
Soni MG, Burdock GA, Taylor SL, Greenberg NA. 2001. Safety assessment of propyl paraben: a review of the published literature. Food Chem Toxicol. 39(6):513-532.
Soni MG, Carabin IG, Burdock GA. 2005. Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem Toxicol. 43(7):985-101
Spanier AJ, Fausnight T, Camacho TF, Braun JM. 2014. The associations of triclosan and paraben exposure with allergen sensitization and wheeze in children. Allergy Asthma Proc. 35:475-481.
Statistics Canada. 2012. Custom tabulation of grooming products data from the Canadian Health Measures Survey Cycle 1 (2007-2009). Prepared for Existing Substances Risk Assessment Bureau, Health Canada by Statistics Canada. Unpublished.
Strittholt CA, McMillan DA, He T, Baker RA, Barker ML 2016. A randomized clinical study to assess ingestion of dentrifrice by children. Regul Toxicol Pharmacol. 75:66-71.
Stump DG, Connor JCO, Lewis JM, Marty MS. 2014. Key lessons from performance of the U.S. EPA Endocrine Disruptor Screening Program (EDSP) Tier 1 male and female pubertal assays. Birth Defects Res B Dev Reprod Toxicol. 101:43-62.
Sun L, Yu T, Guo J, Zhang Z, Hu Y, Xiao X, Sun Y, Xiao H, Li J, Zhu D, Sai L, Li J. 2016. The estrogenicity of methylparaben and ethylparaben at doses close to the acceptable daily intake in immature Sprague-Dawley rats. Scientific Reports 6(25173):1-9.
Taxvig C, Olesen PT, Nellemann C. 2011. Use of external metabolizing systems when testing for endocrine disruption in the T-screen assay. Toxicol Appl Pharmacol 250:263-269.
Taxvig C, Vinggaard AM, Hass U, Axelstad M, Boberg J, Hansen PR, Frederiksen H, Nellemann C. 2008. Do parabens have the ability to interfere with steroidogenesis? Toxicol Sci. 106(1)206-213.
Tayama S, Nakagawa Y, Tayama K. 2008. Genotoxic effects of environmental estrogen-like compounds in CHO-K1 cells. Mutat Res. 649:114-125.
Terasaki M, Kamata R, Shiraishi F, Makino M. 2009. Evaluation of estrogenic activity of parabens and their chlorinated derivatives by using the yeast two-hybrid assay and the enzyme-linked immunosorbent assay. Environ Toxicol Chem. 28(1):204-208.
Towers CV, Terry PD, Lewis D, Howard B, Chambers W, Armistead C, Weitz B, Porter S, Borman CJ, Kennedy RC, et al. 2015. Transplacental passage of antimicrobial paraben preservatives. J Expo Sci Environ Epidemiol 25:604-607.
Tran TM, Minh TB, Kumosani TA, Kannan K. 2016. Occurrence of phthalate diesters (phthalates), p-hydroxybenzoic acid esters (parabens), bisphenol A diglycidyl ether (BADGE) and their derivatives in indoor dust from Vietnam: Implications for exposure. Chemosphere. 144:1553-1559.
Tsukamoto H, Terada S. 1964. Metabolism of drugs. XLVII. Metabolic fate of p-hydroxybenzoic acid and its derivatives in rabbits. Chem Pharm Bull (Tokyo). 12(7):765-769.
Valle-Sistac J, Molins-Delgado D, Díaz M, Ibáñez L, Barceló D, Díaz-Cruz MS. 2016. Determination of parabens and benzophenone-type UV filters in human placenta. First description of the existence of benzyl paraben and benzophenone-4. Environment International 88:243-249.
van Meeuwen JA, van Son O, Piersma AH, de Jong PC, van den Berg M. 2008. Aromatase inhibiting and combined estrogenic effects of parabens and estrogenic effects of other additives in cosmetics. Toxicol Appl Pharmacol. 230:372-382.
Vernet C, Pin I, Giorgis-Allemand L, Philippat C, Benmerad M, Quentin J, Calafat AM, Ye X, Annesi-Maesano I, Siroux V, et al. 2017. In utero exposure to select phenols and phthalates and respiratory health in five-year-old boys: A prospective study. Environ Health Perspect. 125(9):097006.
Viglino L, Prevost M, Sauve S. 2011. High throughput analysis of solid-bound endocrine disruptors by LDTD-APCI-MS/MS. J Environ Monit. 13:583-590.
Vo TTB, Jeung EB. 2009. An evaluation of estrogenic activity of parabens using uterine calbindin-d9k gene in an immature rat model. Toxicol Sci. 112(1):68-77.
Vo TTB, Yoo YM, Choi KC, Jeung EB. 2010. Potential estrogenic effect(s) of parabens at the prepubertal stage of a postnatal female rat model. Reprod Toxicol. 29:306-316.
Wang L, Asimakopoulos AG, Kannan K. 2015. Accumulation of 19 environmental phenolic and xenobiotic heterocyclic aromatic compounds in human adipose tissue. Environ Int 78:45-50.
Wang L, Liao C, Liu F, Wu Q, Guo Y, Moon HB, Nakata H, Kannan K. 2012. Occurrence and human exposure of p-hydroxybenzoic acid esters (parabens), bisphenol A diglycidyl ether (BADGE), and their hydrolysis products in indoor dust from the United States and three East Asian countries. Environ Sci Technol. (46):11584-11593.
Wang L, Kannan K. 2013. Alkyl protocatechuates as novel urinary biomarkers of exposure to p-hydroxybenzoic acid esters (parabens). Environ Int. 59:27-32.
Wang L, Wu Y, Zhang W, Kannan K. 2013. Characteristic profiles of urinary p-hydroxybenzoic acid and its esters (parabens) in children and adults from the United States and China. Environ Sci Technol. 47:2069-2076.
Wilson R, Jones-Otazo H, Petrovic S, Mitchell I, Bonvalot Y, Williams D, Richardson GM. 2013. Revisiting dust and soil ingestion rates based on hand-to-mouth transfer. Hum Ecol Risk Assess. 19(1):158-188.
Wolff MS, Pajak A, Pinney SM, Windham GC, Galvez M, Rybak M, Silva MJ, Ye X, Calafat AM, Kushi LH, et al. 2017. Associations of urinary phthalate and phenol biomarkers with menarche in a multiethnic cohort of young girls. Reprod Toxicol. 67:56-64.
Wolff MS, Teitelbaum SL, McGovern K, Pinney SM, Windham GC, Galvez M, Pajak A, Rybak M, Calafat AM, Kushi LH, et al. 2015. Environmental phenols and pubertal development in girls. Environ Int. 84:174-180.
Wormuth M, Scheringer M, Vollenweider M, Hungerbuhler K. 2006. What are the sources of exposure to eight frequently used pthalic acid esters in Europeans? Risk Anal 26(3):803-824.
Wu C, Huo W, Li Y, Zhang B, Wan Y, Zheng T, Zhou A, Chen Z, Qian M, Zhu Y, et al. 2017. Maternal urinary paraben levels and offspring size at birth from a Chinese birth cohort. Chemosphere. 172:29-36.
Wu X, Bennett DH, Ritz B, Cassady DL, Lee K, Hertz-Picciotto I. 2010. Usage pattern of personal care products in California households. Food Chem Toxicol 48:3109-3119.
Xue J, Wu Qian, Sakthivel S, Pavithran PV, Vasukutty JR, Kannan K. 2015. Urinary levels of endocrine-disrupting chemicals, including bisphenols, bisphenol A diglycidyl ethers, benzophenones, parabens, and triclosan in obese and non-obese Indian children. Environ Res. 137:120-128.
Yang Y, Hong Y, Chae SA. 2016. Reduction in semen quality after mixed exposure to bisphenol A and isobutylparaben in utero and during lactation periods. Hum Exp Toxicol. 35(8):902-911.
Ye X, Bishop AM, Reidy JA, Needham LL, Calafat AM. 2006. Parabens as urinary biomarkers of exposure in humans. Environ Health Perspect. 114(12):1843-1846.
Zhang L, Dong L, Ding S, Qiao P, Wang C, Zhang M, Zhang L, Du Q, Li Y, Tang N, et al. 2014. Effects of n-butylparaben on steroidogenesis and spermatogenesis through changed E2 levels in male rat offspring. Environ Toxicol Pharmacol. 37:705-717.
Appendix A. Estimated daily intake of parabens from biomonitoring data
The estimated daily intake of parabens was calculated from biomonitoring data using the following equation:
Estimated daily intake (µg/kg bw/day) = UER (µg/kg bw/day)/FUE
where UER is the urinary excretion rate and FUE is the fractional urinary excretion.
UER was calculated using the following equation (Saravanabhavan et al. 2014):
UER (µg/kg bw/day) = [UCCr (µg/g Cr) x CER (mg/day)]/ BW (kg)
where UCCr is the creatinine-adjusted urinary concentration, CER is the creatinine excretion rate and BW is body weight.
CER was calculated using the Mage equation:
CER = [0.993*1.64 [140 – Age] (Wt^1.5 Ht^0.5)/1000]
Default values used to calculate CER are presented in Table A‑1.
| Age band from sourcea | Age (year)b | Weight (kg)c | Height (cm)d |
|---|---|---|---|
| 3–5 | 2.5 | 15.5 | 90 |
| 6–11 | 8 | 31 | 127 |
| 12–19 | 15.5 | 59.4 | 162 |
| 20–59 | 39.5 | 70.9 | 163 |
| 60–79 | 60 | 72 | 163 |
| Neonates | 0 | 3.3e | 50e |
a Health Canada 2017a, Kang et al. 2013.
b With the exception of neonates, ages were selected to align age bands reported in literature with CMP default age bands.
c With the exception of neonates, weights were based on CMP exposure scenario defaults.
d Heights are the 50th percentile from WHO height-for-age growth Child Growth Standards (http://www.who.int/childgrowth/standards/en/).
e Neonatal height and weight are the 50th percentile from WHO Child Growth Standards (http://www.who.int/childgrowth/standards/en/).
| Substance | Age (years)a | CER (mg/ day)b | Metric | UCCr, P95 (CI) (µg/g Cr)c | UER (µg/kg bw/day) |
FUEd | EDI, P95 (CI) (µg/kg bw/day) |
|---|---|---|---|---|---|---|---|
| Methylparaben | 2.25 | 130 | GM | 21 (16–27) | 0.18 (0.13–0.23) | 0.174 | 1.0 (0.77–1.3) |
| Methylparaben | 2.25 | 130 | P95 | 430 (200–660)e | 3.6 (1.7–5.5) | 0.174 | 21 (10–32) |
| Methylparaben | 8 | 418 | GM | 8.4 (7.1–9.8) | 0.11 (0.10–0.13) | 0.174 | 0.65 (0.55–0.76) |
| Methylparaben | 8 | 418 | P95 | 620 (340–890)f | 8.4 (4.6–12) | 0.174 | 48 (26–69) |
| Methylparaben | 15.5 | 1182 | GM | 9.9 (6.7–15)e | 0.20 (0.13–0.30) | 0.174 | 1.1 (0.77–1.7) |
| Methylparaben | 15.5 | 1182 | P95 | 370 (100–640)e | 7.4 (2.0–13) | 0.174 | 42 (11–73) |
| Methylparaben | 39.5 | 1248 | GM | 19 (10–35)e | 0.33 (0.18–0.62) | 0.174 | 1.9 (1.0–3.54) |
| Methylparaben | 39.5 | 1248 | P95 | 310 (130–490)e | 5.5 (2.3–8.6) | 0.174 | 31 (13–50) |
| Methylparaben | 60 | 1017 | GM | 20 (16–23) | 0.28 (0.23–0.32) | 0.174 | 1.6 (1.3–1.9) |
| Methylparaben | 60 | 1017 | P95 | 620 (340–890) | 8.8 (4.8–13) | 0.174 | 50 (28–72) |
| Ethylparaben | 2.25 | 130 | GM | NCg | NC | 0.137 | NC |
| Ethylparaben | 2.25 | 130 | P95 | 120 (54–90)h | 1.0 (0.45–1.6) | 0.137 | 7.4 (3.4–12) |
| Ethylparaben | 8 | 418 | GM | NCg | NC | 0.137 | NC |
| Ethylparaben | 8 | 418 | P95 | 4.6 (2.2–7.1)e | 0.06 (0.03–0.10) | 0.137 | 0.46 (0.22–0.71) |
| Ethylparaben | 15.5 | 1182 | GM | NCg | NC | 0.137 | NC |
| Ethylparaben | 15.5 | 1182 | P95 | 120 (54–90)h | 2.4 (1.1–3.8) | 0.137 | 18 (8.0–28) |
| Ethylparaben | 39.5 | 1248 | GM | NCg | NC | 0.137 | NC |
| Ethylparaben | 39.5 | 1248 | P95 | 120 (54–90)h | 2.1 (0.95–3.3) | 0.137 | 16 (7.0–25) |
| Ethylparaben | 60 | 1017 | GM | NCg | NC | 0.137 | NC |
| Ethylparaben | 60 | 1017 | P95 | 70 (29–110)e | 0.99 (0.41–1.6) | 0.137 | 7.3 (3.0–12) |
| Propylparaben | 2.25 | 130 | GM | 2.6 (2.0–3.4) | 0.02 (0.02–0.03) | 0.097 | 0.22 (0.17–0.29) |
| Propylparaben | 2.25 | 130 | P95 | 68 (20–120)e | 0.57 (0.17–1.0) | 0.097 | 5.9 (1.7–10) |
| Propylparaben | 8 | 418 | GM | 1.4 (1.1–1.7) | 0.02 (0.01–0.02) | 0.097 | 0.19 (0.15–0.24) |
| Propylparaben | 8 | 418 | P95 | 190 (110–280)f | 2.6 (1.5–3.8) | 0.097 | 26 (15–39) |
| Propylparaben | 15.5 | 1182 | GM | 1.7 (1.2–2.4) | 0.03 (0.02–0.05) | 0.097 | 0.35 (0.25–0.49) |
| Propylparaben | 15.5 | 1182 | P95 | 85 (42–130)e | 1.7 (0.8–2.6) | 0.097 | 17 (8.6–27) |
| Propylparaben | 39.5 | 1248 | GM | 5.1 (3.0–8.5)i | 0.09 (0–.05–0.15) | 0.097 | 0.93 (0.54–1.5) |
| Propylparaben | 39.5 | 1248 | P95 | 96 (29–160)e | 1.7 (0.5–2.8) | 0.097 | 17 (5.3–29) |
| Propylparaben | 60 | 1017 | GM | 2.9 (2.0–4.2)e | 0.04 (0.03–0.06) | 0.097 | 0.42 (0.29–0.61) |
| Propylparaben | 60 | 1017 | P95 | 190 (110–280)e | 2.7 (1.6–4.0) | 0.097 | 28 (16–41) |
| Butylparaben | 2.25 | 130 | GM | NCg | NC | 0.056 | NC |
| Butylparaben | 2.25 | 130 | P95 | 3.1 (<LOD–5.1)e | 0.03 (NC–0.04) | 0.056 | 0.46 (NC–0.8) |
| Butylparaben | 8 | 418 | GM | NCg | NC | 0.056 | NC |
| Butylparaben | 8 | 418 | P95 | 0.8 (0.3–1.3)e | 0.01 (0.00–0.02) | 0.056 | 0.19 (0.07–0.31) |
| Butylparaben | 15.5 | 1182 | GM | NCg | NC | 0.056 | NC |
| Butylparaben | 15.5 | 1182 | P95 | 9.2 (<LOD–15)h | 0.18 (NC–0.30) | 0.056 | 3.3 (NC–5.3) |
| Butylparaben | 39.5 | 1248 | GM | NCg | NC | 0.056 | NC |
| Butylparaben | 39.5 | 1248 | P95 | 9.2 (<LOD–15)h | 0.16 (NC–0.26) | 0.056 | 2.9 (NC–4.72) |
| Butylparaben | 60 | 1017 | GM | NCg | NC | 0.056 | NC |
| Butylparaben | 60 | 1017 | P95 | 6.7 (2.1–11)e | 0.09 (0.03–0.16) | 0.056 | 1.7 (0.53–2.8) |
Abbreviations: CER, creatinine excretion rate; UCCr, creatinine-adjusted urinary concentration; UER, Urinary excretion rate; FUE, fractional urinary excretion; EDI, estimated daily intake; GM, geometric mean; P95, 95th percentile; CI, confidence interval; NC, not calculated; LOD, limit of detection.
a Age used to calculate creatinine clearance was the midpoint of the age bands used in exposure scenarios.
b Creatinine excretion rate was calculated using the Mage equation [0.993*1.64 [140 – Age] (Wt^1.5 Ht^0.5)/1000].
c Urinary concentration values for GM and P95 values (Health Canada 2017a) were from age groups reported in CHMS that closely matched the age groups for UFR estimates.
d Moos et al. 2016, 2017.
e These values were associated with high sampling variability (i.e., coefficient of variation between 16.6% and 33.3%). Health Canada recommends that this data be used with caution (Health Canada 2017a).
f CHMS data for the 95th percentile in this age stratum was suppressed due to high variability and “Age 60 to 79” was used as a surrogate. Although the 95th percentile value for this group is not reported, this approach is considered conservative because the value used to estimate daily intake is the highest reported 95th percentile value for this substance.
g Geometric means were not calculated as >40% of samples were below the limit of detection (Health Canada 2017a).
h CHMS data for the 95th percentile in this age stratum was suppressed due to high variability and “females 3 to 79” was used as a surrogate. Although the 95th percentile value for this group is not known, this approach is considered conservative because the value used to estimate daily intake is the highest reported 95th percentile value for this substance.
i CHMS data for the geometric mean in this age stratum was suppressed due to high variability and 'Females, 3 to 79 years' was used as a surrogate. Although the geometric mean value for this group is not known, this approach is considered conservative because the value used to estimate daily intake is the highest reported geometric mean for this substance.
| Population | Sample year(s) | Methyl-paraben* | Ethyl-paraben* | Propyl-paraben* | Butyl-paraben* | n | Source |
|---|---|---|---|---|---|---|---|
| Korea general population, P95 | 2006-2007 | 165.5 | 16.7 | 88.6 | 16.7 | 26 | Honda et al. 2018 |
| U.S. general population, P95 | 2005-2006 | 112.0 | 8.5 | 62.9 | 7.0 | 2548 | Calafat et al. 2010 |
| China general population, P95 | 2010-2012 | 101.5 | 9.9 | 75.2 | 0.6 | 47 | Honda et al. 2018 |
| Canada general population aged 3–79, upper bound CI of P95 | 2014-2015 | 73.6 | 16.3 | 37.9 | 2.4 | 2564 | Health Canada 2017a |
| German females aged 20–30 years, P95 | 1995-2012 | 52.7 | 10.1 | 26.9 | 7.0 | 330 | Moos et al. 2017 |
| Pregnant women in Korea, P75 | 2011 | 51.9 | 30.0 | 13.8 | 0.2 | 46 | Kang et al. 2013 |
| Korean neonates, P75 | 2011 | 46.9 | 1.8 | 4.7 | 0.9 | 46 | Kang et al. 2013 |
| Pregnant women in Canada, P95 | 2009-2010 | 46.4 | 12.3 | 22.9 | 4.4 | 31 | Fisher et al. 2017 |
| Japan general population, P95 | 2010-2012 | 34.3 | 14.6 | 20.7 | 6.7 | 36 | Honda et al. 2018 |
Abbreviations: P95, 95th percentile; CI, confidence interval; P75, 75th percentile.
* Estimated daily intake (µg/kg bw/day)
Appendix B. Parameters for estimating oral and dermal exposures to products
Exposure to products was estimated using ConsExpo Web (2016) combined with specific parameters obtained from published literature. Exposure estimates were calculated using default body weights of 70.9 kg for adults (20 years and older), 59.4 kg for adolescents (12 to 19 years old), 15.5 kg for toddlers (6 months to 4 years old), and 7.5 kg for infants (Health Canada 1998). The estimated inhalation and dermal exposure parameters for cosmetics and other products available to consumers are described in Table A-3. Unless specified otherwise, the parameter values are taken from the relevant ConsExpo Fact Sheet (Bremmer et al. 2006) for the scenario presented.
| Exposure scenario |
Assumptions |
|---|---|
| Lipstick/ Lip balm (oral) |
Toddler: Frequency of use: 4.1/week (Wu et al. 2010) Amount per use: 0.01 g (Loretz et al. 2005) Teen/Adult: Frequency of use: 2.4/day (Loretz et al. 2005) Amount per use: 0.01 g (Loretz et al. 2005) Ingestion factor: 1.0 |
| Body lotion (dermal) |
Toddler: Frequency of use: 0.8/day (Ficheux et al. 2015) Amount per use: 4.1 g (Ficheux et al. 2015) Exposed area: 4 910 cm2 (Health Canada 1995) Adult: Frequency of use:1/day (Ficheux et al. 2015) Amount per use: 10 g (Ficheux et al. 2015) Exposed area: 16 925 cm2 (Health Canada 1995) Retention factor: 1.0 |
| Hair perm/straightener (dermal) |
Child: Frequency of use: 0.1/month (Wu et al. 2010) Amount per use: 75 g (surface area adjustment from adult value) Exposed area: 605 cm2 (Health Canada 1995) Adult: Frequency of use: 0.5/month (Wu et al. 2010) Amount per use: 80 g Exposed area: 637.5 cm2 (Health Canada 1995) Retention factor: 0.1 |
| Facial moisturizer (dermal) |
Teen: Frequency of use: 1.8/day (Loretz et al. 2005) Amount per use: 1.2 g (Loretz et al. 2005) Exposed area: 730 cm2 (Health Canada 1995) Adult: Frequency of use: 1.8/day (Loretz et al. 2005) Amount per use:1.2 g (Loretz et al. 2005) Exposed area: 637.5 cm2 (Health Canada 1995) Retention factor: 1.0 |
| Eye lotion (dermal) |
Teen: Frequency of use: 1.8/day (facial moisturizer, Loretz et al. 2005) Amount per use:0.09 g (surface area adjustment from facial moisturizer, Loretz et al. 2005) Exposed area: 50 cm2 (Health Canada 1995) Adult: Frequency of use: 1.8/day (facial moisturizer, Loretz et al. 2005) Amount per use:0.09 g (surface area adjustment from facial moisturizer, Loretz et al. 2005) Exposed area: 50 cm2 (Health Canada 1995) Retention factor: 1.0 |
| Face make-up (dermal) |
Teens and Adults: Frequency of use: 1.2/day (Loretz et al. 2006) Amount per use: 0.54 g (Loretz et al. 2006) Exposed area: 637 cm2 (Health Canada 1995) Retention factor: 1.0 |
| Hair conditioner (rinse-off, dermal) |
Toddler: Frequency of use:13.5/month (Wu et al. 2010) Amount per use: 8.9 g (surface area adjustment from Loretz et al. 2008) Exposed area: 605 cm2 (Health Canada 1995) Adult: Frequency of use: 33/month (Loretz et al. 2008) Amount per use: 13.1 g (Loretz et al. 2008) Exposed area: 1 092.5 cm2 (Health Canada 1995) Retention factor: 0.01 |
| Hair dye (permanent, dermal) |
Teen: Frequency of use: 0.3/month (Statistics Canada 2012) Amount per use: 100 g Exposed area: 730 cm2 (Health Canada 1995) Adult: Frequency of use: 0.67/month (Statistics Canada 2012) Amount per use: 100 g Exposed area: 637.5 cm2 (Health Canada 1995) Retention factor: 0.1 |
| Body cleanser (liquid, dermal) |
Infant: Frequency of use: 0.85/day (Ficheux et al. 2015) Amount per use: 4.6 g (Ficheux et al. 2016) Exposed area: 3 350 cm2 (Health Canada 1995) Toddler: Frequency of use: 0.85/day (Ficheux et al. 2015) Amount per use: 4.6 g (Ficheux et al. 2016) Exposed area: 4 910 cm2 (Health Canada 1995) Adult: Frequency of use: 1.4/day (Loretz et al. 2006) Amount per use: 11 g (Loretz et al. 2006) Exposed area: 16 925 cm2 (Health Canada 1995) Retention factor: 0.01 |
| Body oil (dermal) |
Adult: Frequency of use: 1/day or less (professional judgement) Amount per use: 3.2 (same as massage oil, Ficheux et al. 2016) Exposed area: 14 380 cm2 (Health Canada 1995) Retention factor: 1.0 |
| Shampoo (dermal) |
Infant: Frequency of use: 3.0/month (CTFA 1983) Amount per use: 0.5 g (CTFA 1983) Exposed area: 330 cm2 (Health Canada 1995) Adult: Frequency of use: 33/month (Loretz et al. 2006) Amount per use: 11.8 g (Loretz et al. 2006) Exposed area: 1 092 cm2 (Health Canada 1995) Retention factor: 0.01 |
| Face Paint (dermal) |
Toddler: Frequency of use: 12/year (Bremmer et al. 2006) Amount per use: 1.3 g (Bremmer et al. 2006) Exposed area: 435 cm2 (Health Canada 1995) Adult: Frequency of use: 6/year (Bremmer et al. 2006) Amount per use: 1.9 g (Bremmer et al. 2006) Exposed area: 638 cm2 (Health Canada 1995) Retention factor: 1.0 |
| Sunless tanning product (dermal) |
Adult: Frequency of use: 1.4/week (professional judgement) Amount per use: 4.4g (body lotion, Loretz et al. 2005) Exposed area: 16 925 cm2 (Health Canada 1995) Retention factor: 1.0 |
| Body scrub (dermal) |
Teen: Frequency of use: 2/week (face exfoliation, ConsExpo) Amount per use: 28 g (surface area adjustment from facial exfoliant, ConsExpo) Exposed area: 14 740 cm2 (Health Canada 1995) Adult: Frequency of use: 2/week (face exfoliation, ConsExpo) Amount per use: 32 g (surface area adjustment from facial exfoliant, ConsExpo) Exposed area: 16 925 cm2 (Health Canada 1995) Retention factor: 0.1 |
| Anti-diarrheal medication (oral)a |
Toddler: Dose: 7.5 mL Frequency: up to 7 times per day Teen: Dose: 30 mL Frequency: up to 7 times per day |
| Cough lozenge (oral) a |
Child: Dose: 1 lozenge Frequency: 1 to 10 per day |
| Heartburn medication (oral) a |
Adult: Dose: 10 to 20 mL Frequency: up to 16 times per day |
| Motion sickness medication (oral)b |
Toddler: Dose 8.3 mL (25 mg of 15 mg/5 mL solution) Frequency: up to 3 times per day |
| Radiological contrast media (oral) a |
Infant: Dose: 180-210 mL Frequency: once Toddler: Dose: 900 mL Frequency: once Adult: Dose: 900 mL Frequency: once |
| Antiviral agent (oral)b,c |
Toddler: Dose: 20 mL Frequency: 4 times daily |
| Anticonvulsant (oral)b,c |
Chilld: Dose: 80 mL (maximum daily dose) Frequency: 1 |
| Respiratory smooth muscle relaxant (oral)b,c |
Child: Dose: 87.19 mL Frequency: 1 |
| Antitussive solution (oral)b,c |
Teen : Dose: 30 mL Frequency: 6 times daily (maximum dose) |
| Traditional Chinese medicine (oral) a |
Adult: Dose: 3 to 4 capsules Frequency: 3 times per day |
| Fluoride treatment (oral) a |
Child: Dose: 8 mL Frequency: 1/year Adult: Dose: 8 mL Frequency: 1/year |
| Herbal cough medicine (oral)a |
Teens and Adults: Dose:5-10 mL Frequency: 1 to 5 times per day |
| Children’s analgesic suspension (oral)b |
Toddler: Dose: 5 mL Frequency: up to 5 times per day |
| Antacid (oral)b |
Adult: Dose: 10-20 mL Frequency: up to 4 times per day |
| Sunscreen (dermal) |
Infant: Frequency of use: 3/day (professional judgement) Amount per use: 3.9 g (1.3 mg/cm2, Petersen 2014) Exposed area: 3 020 cm2 (Health Canada 1995) Toddler: Frequency of use: 3/day (professional judgement) Amount per use: 6.8 g (1.3 mg/cm2, Petersen 2014) Exposed area: 4 910 cm2 (Health Canada 1995) Adult: Frequency of use: 3/day (professional judgement) Amount per use: 22.0 g (1.3 mg/cm2, Petersen 2014) Exposed area: 16 925 cm2 (Health Canada 1995) Retention factor: 1.0 |
| Antibacterial/ antifungal treatment (dermal)b |
Infant (diaper cream scenario): Frequency of use: 1.1/day (Gomez-Berrada et al. 2013) Amount per use: 3.5 g (Gomez-Berrada et al. 2013) Exposed area: 258 cm2 (Health Canada 1995) |
| Hand sanitizer (dermal) |
Toddler: Frequency of use: 0.8/day (Wu et al. 2010) Amount per use: 0.7 g (Health Canada 2015) Exposed area: 350 cm2 (Health Canada 1995) Adult: Frequency of use:2.9/day (Wu et al. 2010) Amount per use: 0.7 g (Health Canada 2015) Exposed area: 910 cm2 (Health Canada 1995) Retention factor: 1.0 |
| Analgesic cream (dermal)a,b |
Child: Frequency of use: up to 3 times per day Amount per use: 3.6 g (extrapolated from body lotion, Wu et al. 2010) Exposed area: 6 035 cm2 (trunk and legs, Health Canada 1995) Adult: Frequency of use: up to 3 times per day Amount per use: 7.2 g (surface area adjustment from body lotion, Ficheux et al. 2016) Exposed area: 12 190 cm2 (trunk and legs, Health Canada 1995) Retention factor: 1.0 |
| Medicated hand cream (dermal)b |
Child: Frequency of use: up to 3 times per day Amount per use: 0.9 g (surface area adjustment from hand cream, Loretz et al. 2005) Exposed area: 480 cm2 (Health Canada 1995) Adult: Frequency of use: up to 3 times per day Amount per use: 1.7 g (Loretz et al. 2005) Exposed area: 910 cm2 (Health Canada 1995) Retention factor: 1.0 |
| Acne spot treatmenta (dermal) |
Teen: Frequency of use: 2 times per day Amount per use: 0.14 g (surface area adjustment from face make-up, Loretz et al. 2006) Exposed area: 159 cm2 (1/4 face, Health Canada 1995) Retention factor: 1.0 |
a Personal communication, email from Health Canada Natural and Non-Prescription Health Products Directorate, Health Canada, to Consumer Product Safety Directorate, Health Canada, dated February 20, 2017; unreferenced.
b Personal communication, email from Therapeutic Products Directorate, Health Canada, to Consumer Product Safety Directorate, Health Canada, dated May 25, 2017; unreferenced.
Appendix C. Review of estrogenic effects of parabens
In vitro assays
Several reviews have suggested that estrogenic activity is a common mode of action for parabens (Soni et al. 2005, Boberg et al. 2010). Parabens have been shown to bind to the estrogen receptor in vitro, to activate transcription of estrogen-dependent genes, and estrogen-dependent cell proliferation. Paraben estrogenic potency ranges from 3 to 6 orders of magnitude lower than 17-β-estradiol and increases with chain length, such that methylparaben<ethylparaben <propylparaben <butylparaben <iso-propylparaben ≈iso-butylparaben ≈benzylparaben (Byford et al. 2002; Routledge et al. 1998; Terasaki et al. 2009; Okubo 2001; van Meeuwen et al. 2008), as summarized in Table C‑1. Methylparaben potency is the lowest of parabens when tested in parallel and is frequently undetectable in assays. In MCF7 human breast cancer cells, at 1 000 000-fold molar excess, parabens competitively inhibited 21% (methylparaben) to 86% (butylparaben)17-β estradiol from binding to ER-α (Byford et al. 2002). However, in a transcriptional assay in HELN reporter cell lines, methylparaben was unable to activate ER-mediated transcription (Gomez 2005). Methylparaben was also inactive in yeast-2-hybrid and ELISA assays of ER-binding, in which other tested parabens had the relative potency of ethylparaben <propylparaben <butylparaben ≈iso-propylparaben ≈iso-butylparaben ≈benzylparaben, ranging from 2.0 x 10-5 to 3.5x 10-3 –fold lower affinity than 17-β-estradiol (Terasaki et al. 2009). In a cell proliferation study in MCF 7 cells, iso-propyl- and iso-butylparaben induced the highest level of proliferation, with a relative proliferative potency that was 170 000 lower than that of 17-β-estradiol; the relative proliferative potency of methylparaben was 6 000 000 times lower than 17-β-estradiol (Okubo 2001) In a similar assay in MCF7 cells, all parabens except for methylparaben, were able to induce 100% cell proliferation relative to 17-β-estradiol. In this study, the relative potencies of parabens ranged from 10-5 for butylparaben, benzylparaben, iso-propylparaben and iso-butylparaben, 10-6 for ethylparaben and propylparaben and to 10-7 for methylparaben (van Meeuwen et al. 2008). para-Hydroxybenzoic acid (PHBA), the major metabolite of parabens, has been shown to produce weak estrogenic effects in human breast cancer cell lines, including binding to the estrogen receptor, inducing gene expression and cell proliferation. However, these effects were of equal or lower magnitude than those of methylparaben (Pugazhendhi et al. 2005). In multiple binding assays, PHBA binding to the estrogen receptor is low to absent, and generally considered to be < 1 000 000 times less potent than estradiol (Routledge et al. 1998; Lemini et al. 2003; Soni et al. 2005).
| Paraben | Estrogen receptor binding assaya | MCF7 Cell proliferation assayb |
|---|---|---|
| Methylparaben | ≤ 10-7 | 10-7 |
| Ethylparaben | ≤ 10-5 | 10-6 |
| Propylparaben | 10-5 to 10-4 | 10-6 |
| Butylparaben | 10-5 to 10-3 | 10-6 to 10-5 |
| Benzylparaben | 10-4 to 10-3 | 10-5 |
| iso-Propylparaben | 10-5 to 10-3 | 10-5 |
| iso-Butylparaben | 10-4 to 10-3 | 10-5 |
a Routledge et al. 1998; Terasaki et al. 2009
b Okubo 2001; van Meeuwen et al. 2008
Uterotrophic assays
In uterotrophic assays performed in immature (PND 18 to 23) rats or ovariectomized rats and mice, all parabens administered orally and subcutaneously were positive (Table C‑1). However, the effective dose for methylparaben, ethylparaben, propylparaben and butylparaben varies widely in different studies. Methylparaben induced a statistically significant increase in absolute and relative uterine weights in immature Sprague Dawley rats at 20 mg/kg bw/day administered orally (Sun 2016), but also induced no effect at oral doses up to 800 mg/kg bw/day in immature rats (Routledge et al. 1998). Similarly, when administered subcutaneously, a dose of 165 mg/kg bw/day induced uterine weight changes in ovariectomized CD1 mice (Lemini et al. 2003), but a dose of up to 800 mg/kg bw/day did not induce an effect in ovariectomized rats (Routledge et al. 1998). Ethylparaben induced uterotrophic effects at 4 mg/kg bw/day (oral) in immature Sprague Dawley rats (Sun 2016), but caused no effect with oral doses up to 1 000 mg/kg bw/day in ovariectomized mice (Ohta et al. 2012). When administered subcutaneously to ovariectomized mice, ethylparaben induced uterotrophic effects at 18 mg/kg bw/day (Lemini et al. 2003) and failed to induce effects at 1 000 mg/kg bw/day (Ohta et al. 2012). Propylparaben induced increased uterine weights in ovariectomized CD1 mice at a subcutaneous dose of 20 mg/kg bw/day (Lemini et al. 2003) and had no effect at up to 1 000 mg/kg bw/day administered orally or subcutaneously to ovariectomized mice (Ohta et al. 2012). Butylparaben induced a statistically significant increase in uterine weight in ovariectomized CD1 mice at 21 mg/kg bw/day (subcutaneous dose) (Lemini et al. 2003) and failed to induce an effect in the same model at 300 mg/kg bw/day (Ohta et al. 2012). Butylparaben has induced uterotrophic effects in immature rats at subcutaneous doses ranging from 70 to 600 mg/kg bw/day (Lemini et al. 2003; Routledge et al. 1998; Hossaini et al. 2000) and in ovariectomized rats at 800 mg/kg bw/day (Routledge et al. 1998) and had no effect in immature rats at oral doses up to 1 200 mg/kg bw/day (Routledge et al. 1998). PHBA, the major metabolite of parabens, induced a dose-dependent increase in uterine weight in ovariectomized CD1 mice at 5 mg/kg bw/day (subcutaneous dose) (Lemini et al. 1997), but did not induce uterotrophic effects in immature Wistar rats at subcutaneous doses of up to 150 mg/kg bw/day in two separate studies (Lemini et al. 2003; Hossaini et al. 2000), suggesting a species-specific effect. Uterotrophic assay results are summarized in Table C‑2.
| Paraben | Uterotrophic model | Route of exposure | LOAEL (mg/kg bw/day) | NOAEL (mg/kg bw/day) | Reference |
|---|---|---|---|---|---|
| Methylparaben | Immature rat | Oral | 20 | 4 | Sun 2016 |
| Methylparaben | Immature rat | Oral | N/A | 800 (HDT) | Routledge et al. 1998 |
| Methylparaben | Immature rat | SC | 55 | 16.5 | Lemini et al. 2003 |
| Methylparaben | Immature rat | SC | N/A | 80 (HDT) | Routledge et al. 1998 |
| Methylparaben | OVX mice | SC | 165 | 55 | Lemini et al. 2003 |
| Methylparaben | OVX mice | SC | 55 (LDT) | N/A | Lemini et al. 2004 |
| Methylparaben | OVX rats | SC | N/A | 800 (HDT) | Routledge et al. 1998 |
| Ethylparaben | Immature rat | Oral | 4 | 0.8 | Sun et al. 2016 |
| Ethylparaben | Immature rat | SC | 180 | 60 | Lemini et al. 2003 |
| Ethylparaben | OVX mice | Oral | N/A | 1 000 (HDT) | Ohta et al. 2012 |
| Ethylparaben | OVX mice | SC | 18 | 6 | Lemini et al. 2003 |
| Ethylparaben | OVX mice | SC | 60 (LDT) | N/A | Lemini et al. 2004 |
| Ethylparaben | OVX mice | SC | N/A | 1 000 (HDT) | Ohta et al. 2012 |
| Propylparaben | Immature rat | SC | 65 | 20 | Lemini et al. 2003 |
| Propylparaben | OVX mice | Oral | N/A | 1 000 (HDT) | Ohta et al. 2012 |
| Propylparaben | OVX mice | SC | 20 | 6.5 | Lemini et al. 2003 |
| Propylparaben | OVX mice | SC | 65 (LDT) | N/A | Lemini et al. 2004 |
| Propylparaben | OVX mice | SC | N/A | 1 000 (HDT) | Ohta et al. 2012 |
| Butylparaben | Immature rat | Oral | N/A | 1 200 (HDT) | Routledge et al. 1998 |
| Butylparaben | Immature rat | SC | 70 | 21 | Lemini et al. 2003 |
| Butylparaben | Immature rat | SC | 400 | 200 | Routledge et al. 1998 |
| Butylparaben | Immature rat | SC | 600 | 400 | Hossaini et al. 2000 |
| Butylparaben | OVX mice | Oral | N/A | 1 000 (HDT) | Ohta et al. 2012 |
| Butylparaben | OVX mice | SC | 21 | 7 | Lemini et al. 2003 |
| Butylparaben | OVX mice | SC | 70 (LDT) | N/A | Lemini et al. 2004 |
| Butylparaben | OVX mice | SC | 1 000 | 300 | Ohta et al. 2012 |
| Butylparaben | OVX rat | SC | 800 | N/A | Routledge et al. 1998 |
| PHBA | OVX mice | SC | 5 | 0.5 | Lemini et al. 1997 |
| PHBA | Immature rat | SC | N/A | 5 (HDT) | Hossani 2000 |
| PHBA | Immature rat | SC | N/A | 150 (HDT) | Lemini et al. 2003 |
Abbreviations: OVX, ovariectomized; SC, subcutaneous; HDT, highest dose tested; LDT, lowest dose tested
In a female pubertal assay, uterotrophic effects were not observed when assayed slightly later in development. In a study of female pubertal development, pre-pubertal Sprague Dawley rats (10/group) were orally administered 0, 62.5, 250, 1 000 mg/kg bw/day of methylparaben, ethylparaben, propylparaben, butylparaben, iso-propylparaben, or iso-butylparaben in corn oil from PND 21 to 40. Although no significant differences in uterine weight were observed on PND 41 in animals exposed to parabens at any tested dose relative to vehicle control, a significant increase in uterine wall thickness was observed in animals treated with 1 000 mg/kg bw/day propylparaben, 1 000 mg/kg bw/day iso-propylparaben, and all doses of butylparaben and iso-butylparaben (Vo et al. 2010). However, it is not clear that the effect on uterine wall thickness is treatment related, as Sivaraman et al. (2018) has shown that uterine histology and weight vary with natural variations in estrous cycle, which was not reported by Vo et al. (2010).
In a reflection paper on the development of OECD Test Guideline 440 for uterotrophic assay, Kanno et al. (2003) noted that there was significant variation in the dose required to produce a statistically significant effect, depending on the laboratory performing the assay. Interpretation of these studies is further hindered by deficiencies in study protocol and reporting of key factors that impact assay outcome. Very low effect levels were reported for all parabens tested in Lemini et al. (2003, 2004) and Sun et al. (2016). However, Lemini et al. (2003) and Sun et al. (2016) failed to report body weight, particularly of control animals, which is critical to interpretation of results in immature animals where uterine weight and body weight are interdependent (OECD 2007). In addition, Lemini et al. (2003, 2004) failed to report whether ovariectomized animals had ceased estrus, which is critical to interpretation of assay results in ovariectomized animals. Much higher NOAELs, frequently at the highest doses tested, were reported by Routledge et al. (1998), Hossaini et al. (2000) and Ohta et al. (2012). However, these authors also did not report body weights, and Ohta et al. (2012) did not report uterine weight (only effect levels) and used a longer dosing regime than recommended by the OECD test guideline (OECD 2007). Given these limitations, uterotrophic assays were not considered quantitative endpoints for methylparaben, ethylparaben, propylparaben and butylparaben. Uterotrophic assays were considered in the hazard and risk characterization of benzylparaben, iso-propylparaben and iso-butylparaben due to a lack of other endpoints.
Estrogenicity of PHBA
PHBA was evaluated in the ECHA Community Rolling Action Plan (CoRAP) (2016). The CoRAP evaluation concluded that concerns about the potential endocrine activity and the systemic, reproductive, and developmental toxicity of PHBA are unjustified. Although some data indicate that PHBA can exert a trace level of estrogenic activity and receptor binding, this level is as low, or lower than that of methylparaben and did not result in measurable effects in an OECD 422-equivalent study (Combined Repeated Dose Toxicity Study with the Reproduction/Developmental Toxicity Screening Test), at doses of up to 1 000 mg/kg bw/day. As noted by the SCCS (2013), PHBA is a common metabolite of all parabens addressed in this assessment, yet these substances have different levels of estrogenic receptor binding and activity. Such differential effects would not be possible if PHBA exerted a significant estrogenic effect.
