Draft screening assessment - phenol, methylstyrenated
Official title: Draft screening assessment - phenol, methylstyrenated
Chemical Abstracts Service Registry Numbers
68512-30-1
Environment and Climate Change Canada
Health Canada
November 2021
Synopsis
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of phenol, methylstyrenated (CAS RNFootnote 1 68512-30-1), hereinafter referred to as MSP.
MSP was previously assessed as part of the Final Screening Assessment Report for Potentially Toxic substances in 2008. As no exposure to humans or the environment was expected based on information available at the time, it was concluded that MSP did not meet any of the criteria set out in section 64 of CEPA as it was not posing a risk to humans or the environment. However, it was determined that new activities could result in exposure to humans or the environment, potentially causing a risk. Therefore, this substance has been subject to the Significant New Activity (SNAc) provisions specified under subsection 81(3) of CEPA since 2008.
From 2015 to 2018, there were multiple Significant New Activity Notifications (SNANs) received in response to the SNAc provisions applied to MSP. These notifications have not indicated intent to manufacture this substance in Canada, but the total notified imports are in the range of 10 000 to 100 000 kg per year. The major proposed use of this substance specified in these notifications is for paints and coatings on ships and large equipment. Outcomes from evaluation of the SNANs suggest that releases of MSP may pose a risk to the environment. Given indication of increasing use in Canada it was determined that potential risk to the environment and human health should be further evaluated in a screening assessment, pursuant to section 68 of the Act.
MSP is an organic Unknown or Variable composition, Complex reaction products and Biological material (UVCB) substance, which consists of oligomerisation and alkylation reaction products of 2-phenylpropene (C9 monomer) and phenol. More significant components of MSP are expected to be a phenol with 1 to 3 methylstyrenated substituents, and dimers and trimers of C9 monomer. The proportions of these components can vary in commercially manufactured MSP under the same CAS RN. In MSP imported into Canada, the composition is dominated by three major components: mono- and dimethylstyrenated phenol and dimers of C9 monomer.
On the basis of empirical data and model predictions, the major components of MSP are not expected to degrade rapidly in the environment; dimethylstyrenated phenol and dimers of C9 monomer are also expected to bioaccumulate in organisms. Empirical effects data suggest that these major components are highly toxic to aquatic organisms. Some components are also associated with endocrine estrogenic activity and endocrine effects on organisms. Environmental exposure associated with the notified uses was predicted on the basis of data submitted in notifications. Outcomes from the ecological risk characterization for MSP indicate that releases of this substance from notified uses may pose a risk to aquatic organisms.
Considering all available lines of evidence presented in this draft screening assessment, there is a risk of harm to the environment from MSP. It is proposed to conclude that MSP meets the criteria under paragraph 64(a) of CEPA as it is entering or may enter the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity. However, it is proposed to conclude that MSP does not meet the criteria under paragraph 64(b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger to the environment on which life depends.
The general population is not directly exposed to MSP from its use in industrial applications; however, the substance may be released to surface water and the general population may be exposed via drinking water consumption. A comparison of the estimated exposure to MSP from drinking water and critical effect levels results in margins of exposure that are considered adequate to address uncertainties in the health effects and exposure databases.
Considering all the information presented in this draft screening assessment, it is proposed to conclude that MSP does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that MSP meets one or more of the criteria set out in section 64 of CEPA. It is also proposed that MSP meets the criteria for persistence and bioaccumulation as set out in Persistence and Bioaccumulation Regulations of CEPA.
1. Introduction
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of phenol, methylstyrenated (CAS RN 68512-30-1), hereinafter referred to as MSP, to determine whether this substance presents or may present a risk to the environment or to human health.
MSP was previously assessed as part of the Final Screening Assessment Report for Potentially Toxic Substances (Canada 2008a). This substance was included in this previous screening assessment having been identified as a high priority for assessment as it met the s.73(1) criteria for persistence, bioaccumulation potential, and inherent toxicity. Information obtained from a survey issued pursuant to section 71 of CEPA reported no industrial activity (import or manufacture) of this substance in Canada above the reporting threshold of 100 kg for the reporting year 2005. As there was no exposure to the general population or in the environment, it was concluded that the substance did not meet any criteria set out in section 64 of CEPA as it was not posing a risk to humans or the environment (Canada 2008a). However, given the characteristics of the substance, i.e., persistence, potential for bioaccumulation, and inherently toxicity (PBiT), there was a concern that new activities could lead to exposure to humans or the environment, potentially causing a risk. Therefore, this substance has been subject to the Significant New Activity (SNAc) provisions specified under subsection 81(3) of CEPA since 2008 (Canada 2008b).
From 2015 to 2018, there were multiple Significant New Activity Notification (SNAN) submissions received in response to the SNAc provisions associated with this substance. These notifications have not indicated an intent to manufacture this substance in Canada, but the total notified imports are in the range of 10 000 to 100 000 kg per year. The major use of this substance specified in these notifications is for paints and coatings on ships and large equipment. Outcomes from the evaluation for the SNANs suggest that releases of MSP may pose a risk to the environment. Given indications of increasing use in Canada, it was determined that potential risk to the environment and human health should be more thoroughly evaluated in a screening assessment, pursuant to section 68 of CEPA.
This draft screening assessment considered relevant data identified in the literature up to October 2018 and information submitted by stakeholders, including as part of Significant New Activity Notifications.
This draft screening assessment was prepared by the staff of the CEPA Risk Assessment Program at Environment and Climate Change Canada and Health Canada. The ecological portion of this assessment has undergone external review. Comments on the technical portions relevant to the environment were received from Dr. Valérie Langlois of the Institut national de la recherche scientifique and Dr. Connie Gaudet. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Environment and Climate Change Canada and Health Canada.
This draft screening assessment focuses on information critical to determining whether the substance meets the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precaution.Footnote 2 This draft screening assessment presents the critical information and considerations on which the proposed conclusion is based.
2. Identity of the substance
Phenol, methylstyrenated is an organic Unknown or Variable composition, Complex reaction products and Biological materials (UVCB) substance. For the purpose of the screening assessment, this substance is referred to as MSP, derived from the name methylstyrenated phenol.
MSP consists of oligomerisation and alkylation reaction products of 2-phenylpropene (C9 monomer) and phenol. Components of MSP include mono-, di-, and trimethylstyrenated phenol (Table 2-1), and dimers (Table 2-2) and trimers of C9 monomer (Table 2-3) (proportions of components are listed in Table 2-4). Dimers and trimers do not contain the hydroxyl (-OH) group.
| CAS RN | Chemical name on DSL | Common name | Chemical structure |
|---|---|---|---|
| 599-64-4 | Phenol, 4-(1-methyl-1-phenylethyl)- | Monomethyl-styrenated phenol | 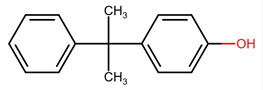
|
| 2772-45-4 | Phenol, 2,4-bis(1-methyl-1-phenylethyl)- | Dimethyl-styrenated phenol | 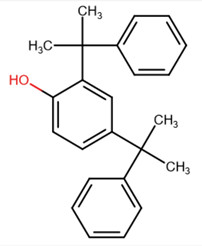
|
| 30748-85-7 | 2,4,6-Tris(1-methyl-1-phenylethyl)phenol | Trimethyl-styrenated phenol | 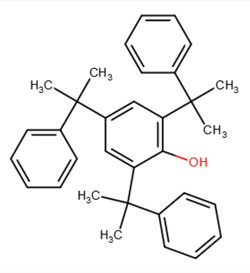
|
Abbreviations: CAS RN, Chemical Abstracts Service Registry Number; DSL, Domestic Substances List
| CAS RN | Chemical name | Chemical structure |
|---|---|---|
| 3910-35-8 | 2,3-Dihydro-1,1,3-trimethyl-3-phenyl-1H-indene | 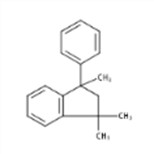
|
| 6258-73-7 | Benzene, 1,1'-(1,3,3-trimethyl-1-propene-1,3-diyl)bis- | 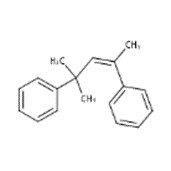
|
| 6362-80-7 | Benzene, 1,1'-(1,1-dimethyl-3-methylene-1,3-propanediyl)bis- | 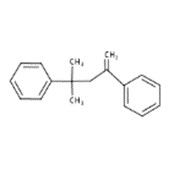
|
Abbreviations: CAS RN, Chemical Abstracts Service Registry Number
| CAS RN | Chemical name | Chemical structure |
|---|---|---|
| 19303-34-5 | Benzene, 1,1',1''-(1,3,5,5-tetramethyl-1-pentene-1,3,5-triyl)tris- | 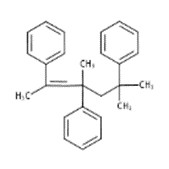
|
| 41906-71-2 | 1H-Indene, 2,3-dihydro-1,3-dimethyl-1-(2-methyl-2-phenylpropyl)-3-phenyl- | 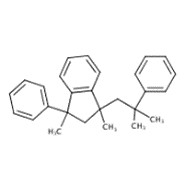
|
| 62604-62-0 | Benzene, 1,1',1''-(1,1,3-trimethyl-5-methylene-1,3,5-pentanetriyl)tris- | 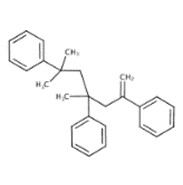
|
Abbreviations: CAS RN, Chemical Abstracts Service Registry Number
For the purpose of this screening assessment, the specific name and CAS RN of an identified component in MSP are used when information is applicable or relevant to that component. Such component specific information is then used to inform the assessment of the whole substance.
The relative proportions of mono-, di-, and trimethylstyrenated phenol and dimers/trimers of C9 monomer (without OH) vary in commercially manufactured substance under the same CAS RN. Based on data provided by notifiers of significant new activity notifications (ECCC 2018) and information available for a few commercial products under this CAS RN in the global market (ECHA c2007-2017), major components in MSP imported into Canada are monomethylstyrenated phenol, dimethylstyrenated phenol and dimers of C9 monomer; while trimethylstyrenated phenol and trimers of C9 monomer are present at very low concentrations. Therefore, the ecological risk assessment focuses on major components of MSP, namely monomethylstyrenated phenol, dimethylstyrenated phenol and dimers of C9 monomer (see Table 2-4).
| Component | Proportion in MSP (%) |
|---|---|
| Monomethylstyrenated phenol | 3.5-21 |
| Dimethylstyrenated phenol | 10-50 |
| Dimers of C9 monomer | 31-50 |
2.1 Selection of analogues and use of (Q)SAR models
The results of (quantitative) structure-activity relationship ((Q)SAR) models and a read-across approach using data from analogues have been used to inform the ecological assessment, where appropriate. The applicability of (Q)SAR models was determined on a case-by-case basis. Details of the read-across data and (Q)SAR models chosen to inform the ecological and human health assessments of MSP are further discussed in the relevant sections of this report.
Phenol, styrenated (CAS RN 61788-4-1), referred to as styrenated phenol in this report, was identified as a structural analogue. Styrenated phenol is also a UVCB that includes mono-, di-, and tristyrenated phenol components. Its representative chemical structure is presented in Fig 2-1. The relevant data for each component of the analogue UVCB are used to assess the corresponding component in MSP in this assessment as presented in Table 2‑5, along with an indication of the read-across data available for different parameters.
| CAS RN | DSL name | Uses | Physical-chemical property data | Fate data | Ecotoxicity data |
|---|---|---|---|---|---|
| 61788-44-1 | Phenol, styrenated | Yes | Yes | Yes | Yes |

3. Physical and chemical properties
The empirical physical and chemical property data for MSP are limited and the available data are summarized in Table 3‑1.
| Property | Valuea | Key reference(s) |
|---|---|---|
| Melting point (°C) | -14 (at 1013 hPa) | ECHA c2007-2017 |
| Boiling point (°C) | ≥300 (at 1013 hPa) | ECHA c2007-2017 |
| Vapour pressure (Pa) | 0.03 – 0.056 (at 20°C) | ECHA c2007-2017 |
| Vapour pressure (Pa) | 0.05 – 0.09 (at 25°C) | ECHA c2007-2017 |
| Water solubility (mg/L) | 0.58-8.1 (originally reported as 0.5-7 mg TOC/L) | ECHA c2007-2017 |
Acronym: TOC, total organic carbon.
a Value is the empirical measurement.
The physical and chemical properties for major components of MSP (monomethylstyrenated phenol, dimethylstyrenated phenol, and dimers of C9 monomer) are also compiled in the following tables. When experimental information was limited or not available for a property for a component in MSP, read-across from the experimental measurements of the corresponding component in the analogous UVCB was used (noted in Table 3-3). In some cases, (Q)SAR models were used to generate predicted values.
| Property | Valuea | Key reference(s) |
|---|---|---|
| Melting point (°C) | 74-76 | EPI Suite c2000-2012 |
| Boiling point (°C) | 335 | EPI Suite c2000-2012 |
| Vapour pressure (Pa) | 0.00792 | EPI Suite c2000-2012 |
| Henry’s law constant (Pa·m3/mol) | 0.023 (calculatedb) | Not applicable |
| Water solubility (mg/L) | 72 at pH = 6-7 | ECHA c2007-2017 |
| Log KOW (dimensionless) | 3.7 at 23°C and pH = 5.3 | ECHA c2007-2017 |
| Log KOC (dimensionless) | 3.4 | ECHA c2007-2017 |
| Log KOA (dimensionless) | 9.14 (modelled, using log KOW = 3.7) | EPI Suite c2000-2012 |
| pKa (dimensionless) | 10.0±0.4 (modelled) | ACD/Percepta c1997-2012 |
Abbreviations: KOW, octanol-water partition coefficient; KOC, organic carbon-water partition coefficient; KOA, octanol-air partition coefficient; pKa, acid dissociation constant
a Value is the empirical measurement at the standard temperature, unless specified.
b Henry’s law constant is calculated from vapour pressure × molecular weight ÷ water solubility.
| Property | Value | Key reference(s) |
|---|---|---|
| Melting point (°C) | 172 (modelled) | EPI Suite c2000-2012 |
| Boiling point (ºC) | 436 (modelled) | EPI Suite c2000-2012 |
| Vapour pressure (Pa) | 7.78×10-8 | EPI Suite c2000-2012 |
| Henry’s law constant (Pa·m3/mol) | 1.12×10-4 (calculateda) | Not applicable |
| Water solubility (mg/L) | 0.665 (read-across from distyrenated phenol) | Brooke et al. 2009 |
| Log KOW (dimensionless) | 6.2 (read-across from distyrenated phenol) | Brooke et al. 2009 |
| Log KOC (dimensionless) | 4.52 (modelled, based on log KOW=6.2) | EPI Suite c2000-2012 |
| Log KOA (dimensionless) | 12.45 (modelled, using log KOW = 6.2) | EPI Suite c2000-2012 |
| pKa (dimensionless) | 10.0±0.4 (modelled) | ACD/Percepta c1997-2012 |
Abbreviations: KOW, octanol-water partition coefficient; KOC, organic carbon-water partition coefficient; KOA, octanol-air partition coefficient; pKa, acid dissociation constant
a Henry’s law constant is calculated from vapour pressure × molecular weight ÷ water solubility.
| Property | Value | Key reference(s) |
|---|---|---|
| Melting point (°C) | 67.0 | ECHA c2007-2017 |
| Boiling point (°C) | 312.1 | ECHA c2007-2017 |
| Vapour pressure (Pa) | 0.063 | ECHA c2007-2017 |
| Henry’s law constant (Pa·m3/mol) | 64.7 (calculateda) | Not applicable |
| Water solubility (mg/L) | 0.23 | ECHA c2007-2017 |
| Log KOW (dimensionless) | 6.2 | ECHA c2007-2017 |
| Log KOC (dimensionless) | 4.82 | ECHA c2007-2017 |
| Log KOA (dimensionless) | 7.68 (modelled, using log KOW = 6.2) | EPI Suite c2000-2012 |
| pKa (dimensionless) | No predicted value | Not applicable |
Abbreviations: KOW, octanol-water partition coefficient; KOC, organic carbon-water partition coefficient; KOA, octanol-air partition coefficient; pKa, acid dissociation constant
a Henry’s law constant is calculated from vapour pressure × molecular weight ÷ water solubility.
4. Sources and uses
MSP was included in a survey issued pursuant to section 71 of CEPA in 2006 (Canada 2008a). There were no reports of manufacturing of this substance or import into Canada above the 100 kg reporting threshold in the 2005 calendar year.
In response to the SNAc provision on this substance (Canada 2008b), starting in 2015, stakeholders have submitted multiple Significant New Activity Notifications (SNANs) and indicated anticipated imports of the substance into Canada at a total quantity in the range of 10 000 to 100 000 kg per year. There was no report of manufacturing of this substance above the 100 kg reporting threshold in Canada.
In Canada, MSP was notified to be used in paints and coatings for ships and large equipment.
In addition, internationally, MSP is known to be used as synthetic resin and for adhesives, sealants, coatings, printing inks and rubber goods (SDS 2019). It is also used as an intermediate in formation of fuel additives and fuel blends and in polymer production (ECHA c2007-2017). In Nordic countries (SPIN c2017), the reported use quantities were in the range of 100 000 to 1 000 000 kg per year from 2010 to 2016 and above 1 000 000 kg in 2017; major applications included uses in anti-corrosion surface treatment, paints, laquers and varnishes, adhesives, and construction materials (SPIN c2017).
On the basis of the use information on the analogous UVCB, styrenated phenol (Brooke et al. 2009), and a few structural analogues notified under the New Substances Program, MSP could also be used as an antioxidant in rubber or as a reactant to produce polymeric surfactants.
5. Releases to the environment
Point source releases are expected to occur during the formulation of products containing MSP and its use in large volume or in a non-contained environment. Water is expected to be the main receiving compartment.
Disposal of end-use products containing MSP is not addressed in this assessment because the substance is covalently bound with the polymeric matrices of cured paints and coatings following application. The release of the substance is unlikely when and after those cured paints and coatings are disposed of. For coated metal equipment and parts, the substance is expected to be destroyed during the recycling using high temperature metallurgical processes.
6. Environmental fate and behaviour
6.1 Environmental distribution
The environmental fate of a substance describes the processes by which it moves and is transformed in the environment. Given that MSP is a UVCB consisting of a number of components, if released to the environment, each component would be distributed into environmental media separately. Therefore, the environmental distribution of MSP is characterized by the distribution of its components.
Using the physical and chemical properties of each major component, the environmental distribution was predicted using Level III fugacity modelling (New EQC 2011) assuming steady-state emissions to water, or soil. Substantial direct release of MSP to air is not expected. The Level III EQC model assumes non-equilibrium conditions between environmental compartments, but equilibrium within compartments. The results represent the net effects of chemical partitioning, inter-media transport and loss by both advection (out of the modelled region), and degradation/transformation processes, i.e., relative steady-state distribution in the physical environmental compartments. Outcomes are summarized in Tables 6-1 and 6-2.
If released to water, the major components of MSP are expected to mainly remain in water or adsorb in sediment. The ratio of partitioning into these two compartments vary for each component, depending on the water solubility and the organic carbon partition coefficient. Volatilization from surface water is expected to be negligible.
| Component | Partitioning in air (%) | Partitioning in water (%) | Partitioning in soil (%) | Partitioning in sediment (%) |
|---|---|---|---|---|
| Monomethylstyrenated phenol | Negligible | 84 | Negligible | 16 |
| Dimethylstyrenated phenol | Negligible | 26 | Negligible | 74 |
| Dimers of C9 monomer | Negligible | 6 | Negligible | 94 |
If released to soil, all major components in MSP are expected to remain in this compartment. Volatilization from surface soil to air and partitioning from soil to water are expected to be low to negligible.
| Component | Partitioning in air (%) | Partitioning in water (%) | Partitioning in soil (%) | Partitioning in sediment (%) |
|---|---|---|---|---|
| Monomethylstyrenated phenol | Negligible | 0.4 | 99.6 | 0.1 |
| Dimethylstyrenated phenol | Negligible | Negligible | 99.9 | 0.1 |
| Dimers of C9 monomer | Negligible | Negligible | 99.9 | 0.1 |
6.2 Environmental persistence
6.2.1 Degradation in the environment
Empirical biodegradation data have been identified for MSP and some of its components (ECHA c2007-2017). These data are summarized in Table 6-3. These data show that MSP does not undergo significant biodegradation in water.
| Substance name (CAS RN) | Fate process | Test inoculum and method | Degradation data and conclusion |
|---|---|---|---|
| MSP (68512-30-1) | Biodegradation (ready biodegradability) | Activated sludge, non-adapted OECD Guideline 310 and ISO Guideline No 14593 | 28-day degradation = 4% (CO2 evolution) No biodegradation was observed under test conditions. |
| Monomethyl styrenated phenol (599-64-4) | Biodegradation (ready biodegradability) | Activated sludge, non-adapted OECD Guideline 301 C | 28-day degradation = 0% (O2 consumption) 28-day degradation = 7% (HPLC) No biodegradation was observed under test conditions. |
| Dimers of C9 monomer (6362-80-7) | Biodegradation (ready biodegradability) | Activated sludge, non-adapted OECD 301C | 28-day degradation = 0 (O2 consumption) 28-day degradation = 3%(HPLC) |
| Dimers of C9 monomer (6362-80-7) | Biodegradation (ready biodegradability) | Activated sludge, non-adapted Test Method Relating to New Chemical Substances | 28-day degradation = 0% (DOC removal, O2 consumption, HPLC) No biodegradation was observed under test conditions. |
| Dimers of C9 monomer (6362-80-7) | Biodegradatio (inherent biodegradability) | Activated sludge and micro-organisms OECD Guideline 302 C | 28-day degradation = 65% (O2 consumption) 28-day degradation = 82% (chemical analysis) |
6.2.2 Long-range transport potential in air
Long-range transport potential (LRTP) was predicted using TaPL3 (2003) and the OECD POV and LRTP Screening Tool (2009). Outcomes are summarized in Table 6-4. The characteristic travel distance (CTD) predicted by both models for all major components in MSP are below the cut-off values defined for the models, suggesting a low potential for long-range transport for the substance.
| Component | CTDa (km) predicted by TaPL3 | CTD (km) predicted by OECD POV and LRTP Screening Tool | Potential for LRTPb |
|---|---|---|---|
| Monomethylstyrenated phenol | 58 | 477 | Low |
| Dimethylstyrenated phenol | 37 | 770 | Low |
| Dimers of C9 monomer | 42 - 244 | 154 - 245 | Low |
a CTD, characteristic travel distance.
b Different values were defined by models associated with the LRTP. For TaPL3, the cut-off values of CTD are <700 km for low LRTP, 700 – 2000 km for moderate LRTP, and > 2000 km for high LRTP. For the OECD POV and LRTP Screening Tool, the cut-off value is 5098 km associated with LRTP.
Based on the empirical data available and model predictions, the major components of MSP and the UVCB substance itself are expected to persist in the environment. These components of MSP are expected to have a low potential for long‑range transport in air.
6.3 Potential for bioaccumulation
The monomethylstyrenated phenol component of MSP is considerably more water soluble than are the other major components (dimethylstyrenated phenol and dimers of C9 monomer) of the substance. Monomethylstyrenated phenol possesses a moderate log KOW and its bioavailability in water is high. Therefore, the bioconcentration factor (BCF) is used to characterize the bioaccumulation for this component. However, for dimethylstyrenated phenol and dimers of C9 monomer that possess high log KOW (6.2) and low water solubilities, it becomes important to consider exposure via food in addition to uptake via water. Given that, the bioaccumulation factor (BAF) is considered to be more appropriate to characterize the bioaccumulation potential by taking into account the uptake of these components from food.
Some empirical bioaccumulation data have been identified (see Table 6-5). A measured BCF of 60-190 L/kg whole body wet weight for monomethylstyrenated phenol was reported in a 60-day study on Cyprinus carpio (J-CHECK c2010-). A measured BCF range of 427 to 4410 L/kg for a dimer of C9 monomer (CAS RN 6362-80-7) was reported in a 60-day study in the same aquatic organism (C. carpio) (ECHA c2007-2017).
Models were used to produce estimates for BCF and BAF for all major components in MSP, where there is a lack of empirical data, or as supplemental information (Table 6-5).
| Component | Type of data (experimental vs. modelled) | Endpoint and value | Reference |
|---|---|---|---|
| Monomethylstyrenated phenol | Experimental | BCF = 60-190 L/kg | ECHA c2007-2017 |
| Monomethylstyrenated phenol | Modelled | BCF = 279.9 L/kg(mid trophic) | EPI Suite c2000-2012 |
| Monomethylstyrenated phenol | Modelled | BAF = 281.7 L/kg(mid trophic) | EPI Suite c2000-2012 |
| Monomethylstyrenated phenol | Modelled | BCF (corrected) = 53.70 L/kg | BCF base-line model in OASIS CATALOGIC 2014 |
| Dimethylstyrenated phenol | Modelled | BCF = 976.6 L/kg(mid trophic) | EPI Suite c2000-2012 |
| Dimethylstyrenated phenol | Modelled | BAF = 11,860 L/kg(mid trophic) | EPI Suite c2000-2012 |
| Dimethylstyrenated phenol | Modelled | BCF (corrected) = 489.78 L/kg | BCF base-line model in OASIS CATALOGIC 2014 |
| Dimers of C9 monomer | Experimental | BCF = 427-4410 L/kg | ECHA c2007-2017 |
| Dimers of C9 monomer | Modelled | BCF = 2362 - 3333 L/kg(mid trophic) | EPI Suite c2000-2012 |
| Dimers of C9 monomer | Modelled | BAF = 15,560 - 45,710 L/kg (mid trophic) | EPI Suite c2000-2012 |
| Dimers of C9 monomer | Modelled | BCF (corrected) = 4466.84 - 12589.25 L/kg | BCF base-line model in OASIS CATALOGIC 2014 |
| Dimers of C9 monomer | Experimental | BMF = 0.07 | ECHA c2007-2017 |
Acronyms: BCF, bioconcentration factor; BAF, bioaccumulation factor; BMF, biomagnification factor.
It is noted that MSP, as typically imported into Canada, is mainly composed of monomethylstyrenated phenol, dimethylstyrenated phenol and dimers of C9 monomer. Considering both empirical data and model predictions, dimethylstyrenated phenol and dimers of C9 monomer are considered to possess high potential for bioaccumulation in organisms, while monomethylstyrenated phenol possesses low potential for bioaccumulation.
Based on the empirical data available and model predictions, with consideration of the read-across data for the analogue UVCB, one component in MSP (monomethylstyrenated phenol) possesses low potential for bioaccumulation in organisms. However, the other two major components in MSP (dimethylstyrenated phenol and dimers of C9 monomer) possess high potential for bioaccumulation in organisms. Since dimethylstyrenated phenol and dimers of C9 monomer components comprise a very large fraction of the composition of MSP, MSP is expected to significantly bioaccumulate in organisms.
7. Potential to cause ecological harm
7.1 Ecological effects assessment
7.1.1 Mode/mechanism of action
There is indication for an endocrine-mediated mode of action for monomethylstyrenated phenol (CAS RN 599-64-4) and dimethylstyrenated phenol (CAS RN 2772-45-4), where estrogenic responses were observed in various test systems (CoRAP 2014; Terasaki et al. 2005; Matsushima et al. 2008; Sanseverino et al. 2009; Ogawa et al. 2006; Okuda et al. 2011; Biggers and Laufer 2004). Furthermore, estrogenic activity via induction of the biomarker vitellogenin was observed in fish following exposure to MSP. In a 14-day study, fish (Pimephales promelas) were exposed to MSP via food (500 μg/g wet weight) (ECHA c2007-2017). Vitellogenin was measured in fish blood at days 0, 7, and 14 of exposure. Results indicated an increase in vitellogenin in treated male fish compared to the controls but no effects were seen in females (ECHA c2007-2017).
Ogawa et al. (2006) also reported estrogenic activity for a dimer of C9 monomer (CAS RN 6362-80-7) and found it to be similar to that of Bisphenol A. The other two dimers of C9 monomer (CAS RNs 3910-35-8 and 6258-73-7) were not included in the study (Ogawa et al. 2006).
7.1.2 Effects on aquatic organisms
Empirical toxicity data have been identified for MSP. The available data indicate low to moderate toxicity to aquatic organisms.
| Organism | Test Method | Endpoint and Result | Reference |
|---|---|---|---|
| Fish(Danio rerio) | OECD 203 | 96-hour LC50 = 3.46 mg/L (originally reported as 3 TOC mg/L) | ECHA c2007-2017 |
| Aquatic invertebrate(Daphnia magna) | OECD 202 | 48-hour EC50 above the water solubilitya (read-across from styrenated phenol) | Brooke et al. 2009 |
Acronyms: EC50, concentration of a substance that is estimated to cause some sublethal effect on 50% of the test organisms; LC50, concentration of a substance that is estimated to be lethal to 50% of the test organisms; TOC, total organic carbon.
a The test substance consisted of 20% distyrenated phenol and 80% tristyrenated phenol.
Empirical toxicity data have also been identified for major components of MSP and their analogues. These data are summarized in Tables 7-2 to 7-4, indicating that some of the major components possess moderate-to-high toxicity to aquatic organisms.
| Organism (species, if specified) | Test Method | Endpoint and Result | Reference |
|---|---|---|---|
| Fish (Oncorhynchus mykiss) | OECD 203 | 24-96-hour LC50 = 0.9 mg/L | ECHA c2007-2017 |
| Fish (Oryzias latipes) | Not specified | 96-hour LC50 = 1.6 mg/L | J-CHECK c2010- |
| Fish | Not specified | 96-hour LC50 = 1.2 mg/L | J-CHECK c2010- |
| Invertebrate(Daphnia magna) | OECD 202 | 48-hour EC50 = 0.9 mg/L | ECHA c2007-2017 |
| Invertebrate | Not specified | 48-hour EC50 = 1.7 mg/L | J-CHECK c2010- |
| Algae (Pseudokirchnerella subcapitata) | OECD 201 | 72-hour EC50 = 1.4 mg/L(measured)(growth rate) | ECHA c2007-2017 |
| Algae (Pseudokirchnerella subcapitata) | OECD 201 | 72-hour NOEC = 0.9 mg/L(estimated)(growth rate) | ECHA c2007-2017 |
| Algae | Not specified | 72-hour EC50 = 1.4 mg/L (growth rate) | J-CHECK c2010- |
| Algae | Not specified | 72-hour NOEC = 0.33 mg/L (growth rate) | J-CHECK c2010- |
| Algae | Not specified | 72-hour EC50 = 0.60 mg/L (areas under the growth curves) | J-CHECK c2010- |
| Algae | Not specified | 72-hour NOEC = 0.33 mg/L (areas under the growth curves) | J-CHECK c2010- |
Acronyms: EC50, concentration of a substance that is estimated to cause some sublethal effect on 50% of the test organisms; LC50, concentration of a substance that is estimated to be lethal to 50% of the test organisms; NOEC, the highest concentration in a toxicity test not causing a statistically significant effect in comparison with the controls.
There is a lack of empirical data for dimethylstyrenated phenol. Therefore, read-across from the analogue (distyrenated phenol) has been used to characterize its effects on aquatic organisms (see Table 7-3).
| Organism | Test Method | Endpoint and Result |
|---|---|---|
| Fish (Oryzias latipes) | Not specified | 96-hour LC50 = 5.6 mg/L |
| Aquatic invertebrate (Daphnia magna) | Not specified | 48-hour EC50 = 4.6 mg/L |
| Fish (Oryzias latipes) | Not specified | 14-day LC50 = 3.8 mg/L |
| Fish (Oryzias latipes) | Not specified | 14-day NOEC = 1.9 mg/L |
| Aquatic invertebrate (Daphnia magna) | OECD 211 | 21-day NOEC = 0.115 mg/L(reproduction and parental immobilisation) |
| Aquatic invertebrate (Daphnia magna) | Not specified | 21-day EC50 = 1.5 mg/L(reproduction) |
| Aquatic invertebrate (Daphnia magna) | Not specified | 21-day NOEC = 0.2 mg/L (reproduction) |
Acronyms: EC50, concentration of a substance that is estimated to cause some sublethal effect on 50% of the test organisms; LC50, concentration of a substance that is estimated to be lethal to 50% of the test organisms; NOEC, the highest concentration in a toxicity test not causing a statistically significant effect in comparison with the controls.
Empirical toxicity data for a dimer of C9 monomer have been summarized in Table 7-4.
| Organism | Test Method | Endpoint and Result |
|---|---|---|
| Fish (Oryzias latipes) | Japan Methods for Testing of New Chemical Substances | 96-hour LC50 > 0.0092 mg/L |
| Invertebrate (Daphnia magna) | Japan Methods for Testing of New Chemical Substances | 48-hour EC50 = 0.057 mg/L |
| Algae (Pseudokirchnerella subcapitata) | Japan Methods for Testing of New Chemical Substances | 72-hour NOEC > 0.059 mg/L |
Acronyms: EC50, concentration of a substance that is estimated to cause some sublethal effect on 50% of the test organisms; LC50, concentration of a substance that is estimated to be lethal to 50% of the test organisms; NOEC, the highest concentration in a toxicity test not causing a statistically significant effect in comparison with the controls.
7.1.3 Predicted No Effect Concentration (PNEC) for the aquatic compartment
PNECs were established from the critical toxicity values (CTV) through the application of an assessment factor (AF) (see Table 7-5). Predicted no effect concentrations (PNECs) for the aquatic compartment were calculated for the major components of MSP, i.e., mono- and dimethylstyrenated phenol and dimers of C9 monomer (Table 7-5).
| Component | CTVa (mg/L) | AFb | FESc | FSVd | FMOAe | Aquatic PNEC (µg/Lf) |
|---|---|---|---|---|---|---|
| Monomethyl styrenated phenol | Fish (Oncorhynchus mykiss) 96-hour LC50 = 0.9 mg/L | 100 | 10 | 2(empirical data identified for 5 species in 3 categories of organisms) | 5 | 9 |
| Dimethyl styrenated phenol | Aquatic invertebrate (Daphnia magna) 21-day NOEC = 0.115 mg/L(reproduction and parental immobilisation)(read-across from distyrenated phenol) | 100 | 1 | 50(empirical data identified for 1 species in 1 category of organism) | 2g | 1.2 |
| Dimers of C9 monomer | Aquatic invertebrate (Daphnia magna) 48-hour EC50 = 0.057 mg/L | 250 | 10 | 5(empirical data identified for 3 species in 3 categories of organisms) | 5 | 0.23 |
Acronyms: EC50, concentration of a substance that is estimated to cause some sublethal effect on 50% of the test organisms; LC50, concentration of a substance that is estimated to be lethal to 50% of the test organisms; NOEC, the highest concentration in a toxicity test not causing a statistically significant effect in comparison with the controls.
a Critical Toxicity Value (CTV); the effect endpoint identified from a reliable and relevant toxicity study as representative of the potential adverse effects level in the available dataset.
b An assessment factor (AF) is determined on the basis of consideration of the endpoint standardization (FES), the species variation (FSV), and the mode of action (FMOA), as following: AF = FES × FSV × FMOA.
c The endpoint standardization factor (FES) is used to account for extrapolations from short-term to long-term exposure, mortality to sub-lethal effects, and median to low effects.
d The species variation factor (FSV) is determined on the basis of the number of different organisms for which empirical data are available in the dataset.
e The mode of action factor (FMOA) is applied to address a known or suspected non-narcotic MoA that the substance possesses. A higher value of FMOA is applied to substances whose mode of action is not expressed in the acute toxicity data when chronic toxicity data are not available for the substance.
f For the purpose of the risk characterization, the aquatic PNEC is in µg/L.
g It is noted that dimethylstyrenate phenol possessed endocrine-mediated MoA. Given that a chronic toxicity data has been selected as the CTV, it is considered that the specific MoA has been well expressed in the chronic study; hence, an FMoA of 2 (instead of 5) has been chosen in extrapolation of the aquatic PNEC.
7.1.4 Effects on organisms in non-aquatic compartments
For non-aquatic compartments (soil and sediment), no empirical data were identified for the substance or the analogous UVCB substance.
7.2 Ecological exposure assessment
Measured concentrations of MSP components in the Canadian environment or elsewhere have not been identified.
7.2.1 Determination of exposure scenarios
As specified in the significant new activity notifications, MSP is being imported into Canada for multiple industrial applications. These applications include protective coatings for routine maintenance on ships and during fabrication of large equipment. Two exposure scenarios have been developed for these applications: 1) protective coating applied on ships and 2) industrial coating of large equipment. These scenarios are used in characterizing risk of MSP in the environment. Details for these two scenarios are presented in section 7.2.3 and Appendix A.
It is noted that there are other known international uses of MSP, as outlined in section 4, which could lead to future exposures if these uses were to be notified in Canada.
7.2.2 The approach of calculating the predicted environmental concentration in surface water
Predicted environmental concentration (PEC) in surface water is calculated to represent the environmental exposure that could result from either direct entry into receiving waters in the case of routine maintenance coating on ships, or indirect entry via wastewater treatment effluent from industrial applications and manufacture.
Key factors that are considered in PEC calculations are estimates of daily release quantities and estimates of daily dilution water volumes. The derived PEC represents the level of exposure near the point of discharge of MSP to receiving water.
Where
PEC: predicted environmental concentration in receiving water near discharge point; μg/L
109: conversion factor from kg to mg, mg/kg
Q: annual quantity of MSP used or manufactured at a facility; kg/year
X: proportion of a major component in MSP; fraction
E: emission factor to wastewater; fraction
R: wastewater treatment removal; fraction
N: number of annual release days related to MSP; day(s)/year
V: daily dilution water volume; L/d.
For scenarios other than routine maintenance coating on ships, daily dilution volumes are calculated by multiplying the effluent flow of the wastewater treatment system (WWTS) or facility discharging to a receiving water body by the dilution factor of the receiving water body. In most cases, aquatic PECs were derived using a dilution factor based on the 10th percentile low flow of the receiving water body and capped at a maximum dilution factor of 10, while the approach used to determine daily dilution volumes for routine maintenance coating on ships is described in section 7.2.3 below.
MSP consists mainly of three major components (mono- and dimethylstyrenated phenol and dimers of C9 monomer) (ECHA c2007-2017; ECCC 2018) but in somewhat variable proportions (see Table 7-6). In selecting proportion values for PEC calculations, more weight is given to the more toxic component. Specifically, the upper end of the range (50%) was assigned to dimers of C9 monomer that possess the highest toxicity among the three major components. A relatively high proportion (40%) is assigned to dimethylstyrenated phenol and a near-average value (10%) to monomethylstyrenated phenol as they possess moderate and the lowest toxicity, respectively, among the three major components. These assigned proportions are to ensure that the toxicity of each major component is adequately taken into account in estimating exposure in a manner that is protective of aquatic organisms.
The removal of major components of MSP in wastewater treatment systems was estimated using SimpleTreat 3.1 (2003) and is summarized in Table 7-6. It is assumed that the treatment level of wastewater treatment systems in municipalities associated with each industrial sector identified is mainly biological (secondary or lagoons). The treatment level is thus assumed to be biological throughout all scenarios.
| Component | Proportion in MSP (%) | Proportion (X) selected for exposure calculations (%) | Wastewater treatment removal (R) |
|---|---|---|---|
| Monomethylstyrenated phenol | 3.5-21 | 10 | 0.172 |
| Dimethylstyrenated phenol | 10-50 | 40 | 0.873 |
| Dimers of C9 monomer | 31-50 | 50 | 0.873 |
For routine maintenance coating on ships, releases of MSP are expected to enter surface water directly with no treatment.
Other parameters, such as the use quantity (Q), the emission factor to wastewater (E), the number of annual days of operation (N), and the dilution water volume (V) depend on each industrial activity. Determination of these values is discussed for each scenario in the following sections.
7.2.3 Exposure scenarios
As specified in significant new activity notifications, paints and coatings containing MSP are used on ships for repair and maintenance purposes (ECCC 2018). These paints and coatings can be applied when ships are moving or docked. When ships are moving, releases are diluted by a large volume of water in the path of the moving ship; hence, the environmental concentrations are expected to be low and are not quantified here. The PEC is only calculated for the exterior coating application on ships that are docked. In the calculation, V is assumed to equal the volume of water displaced on the day when the ship moves away from the dock. This volume is the displacement volume below the ship’s waterline. A typical ship size is selected for this approximation (224 m in length, 28 m in width and 7 m in depth below waterline) (CruiseMapper 2018). For the purpose of the screening assessment, the entire quantity used in a year was assumed to be applied in one day. This assumption yields a maximum estimate for exposure. The actual case may involve multiple days of operation; therefore, the quantity used each day would be less, resulting in lower daily release and exposure.
Based on information specified in notifications, MSP is also present in coatings that are applied to large equipment in fairly high quantities (10 000 – 100 000 kg/year) (ECCC 2018). Assumptions used in the calculations are presented in Appendix A. Given this information and assumptions proposed, the PECs associated with these notified uses are summarized in Table 7-7.
| Scenario | Monomethyl-styrenated phenol PEC (µg/L) | Dimethyl-styrenated phenol PEC (µg/L) | Dimer of C9 monomer PEC (µg/L) |
|---|---|---|---|
| Protective maintenance coating on ships at dock | 1.4 | 5.5 | 6.8 |
| Industrial coating of large equipment | 5.8 | 5.0 | 6.2 |
7.3 Characterization of ecological risk
The approach taken in this ecological screening assessment was to examine assessment information and apply a weight-of-evidence approach and precaution when proposing a conclusion. Evidence was gathered to determine the potential for MSP to cause harm in the Canadian environment. Secondary or indirect lines of evidence are considered when available, including classification of hazard or fate characteristics made by other regulatory agencies.
Risk characterization for MSP focuses on its releases to surface water from the industrial applications associated with uses identified in significant new activity notifications. Potential uses are presented in section 7.3.3 to inform pollution prevention activities. It is noted that both the dimethyl styrenated phenol and C9 monomer components of the substance may partition to sediment significantly after it enters surface waters. Additionally, the application of biosolids from wastewater treatment systems that contain this substance may cause releases to soil. However, due to a lack of data for effects to soil and sediment organisms, risk to these media is not quantified.
7.3.1 Risk quotient analysis
For characterizing risk associated with the notified uses in repair and maintenance to ships at dock and in industrial coating of large equipment, the risk quotient (RQ) was calculated by dividing the PECs from each scenario by the PNECs derived from the toxicity data for each component. Outcomes are summarized in Table 7-8. RQs associated with dimethylstyrenated phenol and dimers of C9 monomer are above 1, indicating that aquatic exposure to MSP could cause harm.
| Notified scenario | Major component | PEC (µg/L) | Aquatic PNEC (µg /L) | RQ (=PEC/PNEC) |
|---|---|---|---|---|
| Repair and maintenance coating to ships at dock | Monomethylstyrenated phenol | 1.4 | 9 | 0.16 |
| Repair and maintenance coating to ships at dock | Dimethylstyrenated phenol | 5.5 | 1.2 | 4.6 |
| Repair and maintenance coating to ships at dock | Dimers of C9 monomer | 6.8 | 0.23 | 29.6 |
| Industrial coating of large equipment | Monomethylstyrenated phenol | 5.8 | 9 | 0.64 |
| Industrial coating of large equipment | Dimethylstyrenated phenol | 5.0 | 1.2 | 4.2 |
| Industrial coating of large equipment | Dimers of C9 monomer | 6.2 | 0.23 | 27 |
7.3.2 Consideration of the lines of evidence
To characterize the ecological risk of MSP, technical information for various lines of evidence was considered (as discussed in the relevant sections of this assessment report) and qualitatively weighted. The key lines of evidence informing the assessment conclusion are presented in Table 7-9. The level of confidence refers to the combined influence of data quality and variability, data gaps, causality, plausibility and any extrapolation required within the line of evidence. The relevance refers to the impact the line of evidence has when determining the potential to cause harm in the Canadian environment. Qualifiers used in the analysis range from low to high, with the assigned weight having five possible outcomes.
| Line of evidence | Level of confidencea | Relevance in assessmentb | Weight assignedc |
|---|---|---|---|
| Similarity in chemical structure for read-across purposes | high | high | high |
| Persistence in the environment | high | high | high |
| Long-range transport | moderate | low | low-moderate |
| Bioaccumulation in aquatic organisms | high | high | high |
| Mode of action and other non-apicald data | high | high | high |
| PNEC (derived from the toxicity data) for aquatic organisms | moderate | high | moderate-high |
| Aquatic PECs in scenarios developed for the notified uses | moderate | high | moderate-high |
| RQs based on the toxicity data for water | moderate | high | moderate-high |
a Level of confidence is determined according to data quality, data variability, data gaps, causality, plausibility and any extrapolation required within the line of evidence.
b Relevance refers to the impact of the evidence in the assessment.
c Weight is assigned to each line of evidence according to the overall combined weights for level of confidence and relevance in the assessment.
d Non-apical endpoints refer to endpoints other than mortality, growth, reproduction (i.e., those endpoints identified with population-level effects).
7.3.3 Weight of evidence for determining the potential to cause harm to the Canadian environment
MSP is an organic UVCB, consisting of reactive components (mono-, di-, and trimethylstyrenated phenol) and non-OH containing components (dimers and trimers of C9 monomer). Based on the available information, MSP imported into Canada is typically composed of three major components: mono- and dimethylstyrenated phenol and dimers of C9 monomer.
Based on empirical data and model predictions, monomethylstyrenated phenol is expected to persist in the environment but is unlikely to bioaccumulate in organisms. The other two major components of MSP (dimethylstyrenated phenol and dimers of C9 monomer) are expected to persist and to significantly bioaccumulate in organisms; each of these two components comprises a very large fraction of the composition of MSP that is imported into Canada. None of the components are expected to have high potential for long-range transport in air.
Empirical data suggest that major components of MSP are highly toxic to aquatic organisms. Outcomes from studies on organisms and yeast assay suggest that the major components (monomethylstyrenated phenol, dimethylstyrenated phenol and a dimer of C9 monomer) are associated with the estrogenic activity.
Exposure assessment focuses on uses that were identified in significant new activity notifications in response to the SNAc provisions of CEPA. Environmental exposure was predicted on the basis of uses and quantities identified in the notifications. Given the potential for persistence, the substance is expected to remain in the environment over a long period of time; potential increases in environmental concentrations cannot be fully captured by the predicted environmental concentrations.
In the risk quotient analysis for MSP for the notified uses in paint for repair and maintenance coating of ships and industrial coating of large equipment, RQs > 1 were determined for 2 of its components, dimethylstyrenated phenol and dimers of C9 monomer, indicating that releases from these notified uses of MSP pose a risk to aquatic organisms.
In addition, according to information available on uses of structurally similar substances, MSP has the potential for a broader use pattern. It could potentially be used as an antioxidant in tire manufacturing, a reactant to manufacture polymeric surfactants, or for formulation into coating products. Such uses could prompt increases in domestic demand of this substance, which could lead to its manufacture taking place in Canada. Considering Canadian volumes reported for other substances with similar applications, a number of exposure scenarios were developed to estimate releases from these potential uses of MSP to the environment to inform pollution prevention activities. According to the risk characterization, releases from the potential uses of MSP may also pose a risk to the environment.
7.3.4 Sensitivity of conclusion to key uncertainties
MSP is an organic UVCB consisting of a number of components, the proportions of which may vary under the CAS RN. Risk characterization of MSP focused on the major components identified in the UVCB. Considering the magnitude of the RQs, moderate differences in proportion of components are not likely to influence outcomes of the assessment as to their potential to cause harm to organisms.
Most components of MSP possess high log KOW (>5). As predicted by the new EQC model, if released to water, their partitioning to sediment will be significant. In addition, these components may be captured in biosolids during the wastewater treatment process, consequently resulting in exposure in soil via the application of biosolids. Due to a lack of effects data, the potential ecological risk from exposure to major components of MSP in sediment and soil could not be addressed.
In the exposure scenario for the notified use in industrial coating of large equipment, the daily dilution water volume distribution is also a source of uncertainty. The distribution is generic and provides an overall profile for all indirect industrial dischargers. Namely, the daily dilution volume is not specific to any individual industrial sector. However, the deviation from the actual conditions is not expected to be large considering that the geographically disperse distribution of potential locations of use can suitably be approximated by a generic distribution.
8. Potential to cause harm to human health
8.1 Exposure assessment
MSP does not occur naturally and no reports of the substance being measured in the environment were identified in the scientific literature.
As described in Section 4, MSP is not reported to be manufactured in Canada; however, stakeholders have submitted multiple SNANs since 2015 indicating this substance may be imported into the country at a total volume in the range of 10 000 to 100 000 kg per year for use in industrial applications. On the basis of information reported in Canada (2008a) and information received from multiple SNANs, the general population is not directly exposed to MSP from its use in industrial applications. However, the substance may be released to surface water and the general population may be exposed via drinking water consumption.
Aquatic PEC values for the major components of MSP (i.e., monomethylstyrenated and dimethylstyrenated phenols and dimers of C9 monomer) discussed in Section 7.2 were used to inform estimates of potential exposure to MSP to the general population from drinking water. To estimate overall potential human exposure to MSP, the PECs developed for each major component of MSP were summed to obtain an overall PEC for MSP, for scenarios involving notified uses. Daily intakes resulting from potential releases to water from use as a maintenance coating of ships are 0.2 to 1.8 µg/kg bw/day, while estimated intakes were slightly greater for the release scenario for use as an industrial coating of large machinery (0.3 to 2.2 µg/kg bw/day). In both scenarios, infants less than 6 months old had the highest estimated exposure to MSP, compared to all other age groups based on their size and drinking water intake rates (summarized in Appendix B).
The estimated intakes for both scenarios assume no additional removal or dilution of the substance before or during the drinking water purification processes. Exposure from other environmental media is not expected.
8.2 Health effects assessment
MSP is not genotoxic in the Bacterial Reverse Mutation Assay (OECD TG 471) or the Mammalian Erythrocyte Micronucleus Test (OECD TG 474) (ECHA c2007-2017). This is consistent with the assessments of other members of the related styrenated phenols group conducted by other international jurisdictions (Brooke et al. 2009; US EPA HPVIS 2018).
In a 100-day oral extended one-generation reproductive toxicity study (OECD Guideline 443), rats were administered MSP orally via feed at 0, 12, 40 or 122 mg/kg bw/day (corresponding to 0, 150, 500 or 1500 mg/kg diet nominal). According to study authors, a systemic no observed adverse effect level (NOAEL) of 40 mg/kg bw/day was identified based on a decrease in mean body weight at 122 mg/kg bw/day (males and females). Effects on the liver were observed in both sexes at the high dose; however, these were deemed to be adaptive. No effects on reproductive toxicity were observed up to the highest dose tested (ECHA c2007-2017). This study was provided as a response to a study request from ECHA (CoRAP 2014) to address potential endocrine toxicity concerns identified as part of the evaluation under the European Community Rolling Action Plan (CoRAP). Therefore, the study included additional evaluations of the F1 generation that were relevant for the detection of endocrine disrupting effects (OECD TG 408). No such effects were observed in the tested rats, nor were there any effects on developmental neurotoxicity or developmental immunotoxicity. In particular, histopathological examination of the peripheral and central nervous system did not reveal any treatment related changes; and, no treatment-related effects were identified on anogenital distance, sperm parameters (motility, morphology and sperm counts), or thyroid-hormone levels (Unnamed Study Report 2018).
In an oral repeated dose study, rats were administered 24.5, 97.1 or 337.6 mg/kg bw/day for 28 days (males) or 42 days (females) in their diet (OECD TG 422). According to study authors, a significant reduction in body weight and food consumption was observed in the high dose group (NOAEL 97.1 mg/kg bw/day) (Unnamed Study Report 2018).
In a prenatal developmental toxicity study (OECD TG 414), rats were administered 60, 150 or 300 mg/kg bw/day via oral gavage from gestation days 6 to 19. According to study authors, no embyrotoxicity or foetotoxicity were observed up to the highest does tested. A lowest observed adverse effect level (LOAEL) of 150 mg/kg bw/day was identified for maternal toxicity based on reduced body weight gain and reduced food consumption. The study authors reported these reductions observed during gestation days 6 to 20 in maternal body weight gain and food intake to be “relatively mild signs of maternal toxicity” and identified a NOEL of 60 mg/kg bw/day (Unnamed Study Report 2017).
Additional data, from the components of MSP and the analogue listed in section 2, were also considered, where available. The results from available repeat dose, reproductive and developmental studies for these substances did not identify effect levels more conservative than the values described above (e.g. Brooke et al 2009, CoRAP 2014).
8.3 Characterization of risk to human health
Margins of exposure (MOEs) were calculated for exposure via drinking water by comparing the NOAEL of 40 mg/kg bw/day (which corresponds to decreased mean body weight) to the total daily intake (which is based on an aquatic PEC value at the 90th percentile). All MOEs for notified uses of MSP were 18 000 or greater which is considered adequate to address uncertainties in the health effects and exposure databases.
8.4 Uncertainties in evaluation of risk to human Health
| Key source of uncertainty | Impact |
|---|---|
| No measured data were identified for drinking water in Canada or elsewhere | +/- |
| All of the toxicological studies identified are unpublished | +/- |
| No chronic oral toxicity studies were identified | +/- |
+/- = unknown potential to cause over or under estimation of risk.
9. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is risk of harm to the environment from MSP. It is proposed to conclude that this substance meets the criteria under paragraph 64(a) of CEPA as it is entering or may enter the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity. However, it is proposed to conclude that it does not meet the criteria under paragraph 64(b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger to the environment on which life depends.
Considering all the information presented in this draft screening assessment, it is proposed to conclude that MSP does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that MSP meets one or more of the criteria set out in section 64 of CEPA.It is also proposed that MSP meets the persistence and bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
References
ACD/Percepta [prediction module]. c1997-2012. Toronto (ON): Advanced Chemistry Development, Inc.
Biggers WJ, Laufer H. 2004. Identification of juvenile hormone-active alkylphenols in the lobster Homarus am ericanus and in marine sediments. Bioi. Bull. 206: 13-24. Cited in ECHA 2014.
Brooke D, Burns J, Cartwright C, Pearson A. 2009. Environmental risk evaluation report: Styrenated phenol [PDF]. Published by United Kingdom Environment Agency, Rio House, Waterside Drive, Aztec West, Almondsbury, Bristol, BS32 4UD.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada. 2000. Canadian Environmental Protection Act, 1999: Persistence and Bioaccumulation Regulations. P.C. 2000-348, 23 March, 2000, SOR/2000-107.
Canada. 2008a. Final screening assessment report for potentially toxic substances. Government of Canada
Canada. 2008b. Final decision on the Screening assessment of 145 substance on the Domestic Substances List (subsection 77(6) of the Canadian Environmental Protection Act, 1999) [PDF]. Canada Gazette Part I, vol. 142, no. 23.
CATALOGIC [environmental fate and ecotoxicity model]. 2014. Ver. 5.11.15. Bourgas (BG): University “Prof. Dr. Assen Zlatarov”, Laboratory of Mathematical Chemistry.
[CoRAP]. Community rolling action plan. European Chemicals Agency (ECHA). 2014. Decision on substance evaluation pursuant to article 46(1) of regulation (EC) No 1907/2006. Helsinki, Finland. [accessed 14 May 2019]
CruiseMapper. 2018. Cruise ship size, comparison, dimensions. [accessed December 2018]
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2018 December].
[ECCC] Environment and Climate Change Canada. 2018. Data collected under New Substances Program of Environment and Climate Change Canada.
[ECHA] European Chemicals Agency. c2007-2017. Registered substances database; search results for CAS RN 68512-30-1. Helsinki (FI): ECHA. [accessed 2018 December].
[ECHA] European Chemicals Agency. 2014. Decision on substance evaluation pursuant to article 46(1) of regulation (EC) No 1907/2006 for Oligomerisation and alkylation reaction products of 2-phenylpropene and phenol (EC No. 700-960-7), previously registered as Phenol, methylstyrenated, CAS No 68512-30-1 (EC No 270-966-8). [accessed 2015 August]
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
European Chemicals Bureau. 2003. Technical guidance document on risk assessment in support of Commission Directive 93/67/EEC on risk assessment for new notified substances and Commission Regulation (EC) No 1488/94 on risk assessment for existing substances [PDF]. Luxembourg City (LU): European Chemicals Bureau.
Health Canada. 2015. Food Consumption Table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004), Share file. Ottawa.
Health Canada. 2018. Updated default values for breast milk and formula intake for use in ESRAB assessments. Unpublished report. Ottawa (ON): Government of Canada.
[J-CHECK] Japan CHEmicals Collaborative Knowledge database [database]. c2010- . Tokyo (JP): National Institute of Technology and Evaluation (NITE). [accessed 2018 December].
Matsushima A, Teramoto T, Okada H, Liu X, Tokunaga T, Shimohigashi Y. 2008. ERRgamma tethers strongly bisphenol A and 4-alpha-cumylphenol in an induced-fit manner. Biochem. Biophys. Res. Commun. 373(3): 408-413. Cited in ECHA 2014.
[New EQC] New Equilibrium Criterion Model. 2011. Ver. 1.00 (Beta). Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
[OECD] Organisation for Economic Co-operation and Development. 2009. Emission scenario documents on coating industry (paints, lacquers and varnishes). ENV/JM/MONO(2009)24. Environment Directorate, Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology. July 8.
OECD Pov and LRTP Screening Tool. 2009. Ver. 2.2. Paris (FR): Organisation for Economic Cooperation and Development (OECD). A software model for estimating overall persistence (Pov) and long-range transport potential (LRTP) of organic chemicals.
Ogawa Y, Kawamura Y, Wakui C, Mutsuga M, Nishimura T, Tanamoto K. 2006. Estrogenic activities of chemicals related to food contact plastics and rubbers tested by the yeast two-hybrid assay. Food Additives and Contaminants, 23(4), 422-430.
Okuda K, Fukuuchi T. Takiguchi M, Yoshihara S. 2011. Novel pathway of metabolic activation of bispehol A-related compounds for estrogenic activity. Drug Metabolism and Disposition 39(9): 1696-1703. Cited in ECHA 2014.
Sanseverino J, Eldrige ME, Layton AC, Schultz TW. 2009. Screening of potentially hormonally active chemicals using bioluminescent yeast bioreporters. Toxicological Sciences: an official journal of the society of Toxicology 107: 122-134. Cited in ECHA 2014.
[SDS]. 2019. Safety data sheet CAS RN 68512-30-1. RÜTGERS Germany GmbH, Varziner Straße 49, D-47138 Duisburg. [restricted access].
SimpleTreat [Sewage Treatment Plant Removal Model]. 2003. version 3.1. Bilthoven (NL): National Institute for Public Health and the Environment (RIVM). Available from: National Institute for Public Health and the Environment (RIVM), Laboratory for Ecological Risk Assessment, Bilthoven, the Netherlands.
[SPIN] Substances in Preparations in Nordic Countries [database]. c2017. Copenhagen (DK): Nordic Council of Ministers. [accessed 2019 July].
[TaPL3] Long Range Transport and Persistence Level III Model. 2003. Ver. 3.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
Terasaki M, Shiraishi F, Nishikawa T, Edmonds JS, Makino M. 2005. Estrogenic activity of impurities in industrial grade bisphenol A. Environ. Sci. Technol. 39(10): 3703-7. Cited in ECHA 2014.
Unnamed Study Report 2017. Developmental Study. OECD Guideline 414 (Prenatal Developmental Toxicity Study). Cited in ECHA c2007-2017]. [accessed 2018 December]
Unnamed Study Report 2018. Extended one-generation reproductive study; with both developmental neuro- and immunotoxicity (Cohorts 1A, 1B without extension, 2A, 2B, and 3). OECD Guideline 408 combined with OECD 443). Cited in ECHA c2007-2017]. [accessed 2018 December]
[US EPA HPVIS] United States Environmental Protection Agency High Production Volume Information System. 2018. Styrenated Phenols Category. Submitted by American Chemistry Council (ACC) Rubber and Plastic’s Additives (RAPA) Panel. [accessed 30 Oct 2018].
Appendix A. Ecological exposure assessment: Summary of assumptions
| Variable name | Value | Units | Additional comments |
|---|---|---|---|
| Use quantity per ship (Q) | 31.6 | kg/yr | Quantity of paint used was estimated by the notifiers to range from 10 to 100 kg of MSP per year per ship. The logarithmic mean of the range is used to represent a typical quantity applied on a ship. |
| Emission factor (E) | 0.018 | fraction | Emission factor estimated for maintenance coating on ships (OECD emission scenario document on coating industry (OECD 2009). |
| Days of release (N) | 1 | days/yr | Assumed to be 1 day per year during which the annual quantity (Q) of 31.6 kg would be used on a single ship (European Chemicals Bureau 2003). The one-day-per-year maintenance schedule represents the realistic worst case scenario for environmental releases within a day. |
| Daily dilution volume (V) | 41 600 000 | L/day | Daily dilution volume based on the volume of water displaced below the ship’s waterline for a typical ship size of 224 m in length, 28 m in width and 7 m in depth below waterline (CruiseMapper 2019). |
| Variable name | Value | Units | Additional comments |
|---|---|---|---|
| Use quantity (per facility) (Q) | 31 623 | kg/yr | The annual use quantity was estimated based on the notifications. The logarithmic mean of the range (10,000 – 100,000 kg) is used to represent a typical quantity applied on large industrial equipment per year. |
| Emission factor (to wastewater) (E) | 0.02 | fraction | Emission factor estimated by European Chemicals Bureau (2003) |
| Days of release (N) | 300 | days/yr | Estimated by European Chemicals Bureau 2003). |
| Removal rate at secondary WWTS (R) | 0.38-0.87 | fraction | Component-specific; SimpleTreat 2003 |
| Daily dilution volume (V) | 23 000 000 | L/day | 10th percentile of the distribution of daily dilution volumes for industrial facilities discharging to WWTS; representing the realistic worst case scenario |
Appendix B. Estimated daily intake from oral exposure to humans to MSP
| Age categories | Ship coatinga | Industrial coatinga |
|---|---|---|
| 0 to 5 monthsb | 1.8 | 2.2 |
| 6 to 11 monthsc | 1.2 | 1.4 |
| 1 yeard | 0.5 | 0.6 |
| 2 to 3 yearse | 0.4 | 0.5 |
| 4 to 8 yearsf | 0.3 | 0.4 |
| 9 to 13 yearsg | 0.2 | 0.3 |
| 14 to 18 yearsh | 0.2 | 0.3 |
| Greater than or equal to 19 yearsi | 0.3 | 0.4 |
a Concentration of MSP in water (µg/L) based on the aquatic PECs determined for the following use scenarios: ship coating, 13.7; industrial coating, 17.0. See 7.2.3 for details).
b Assumed to weigh 6.3 kg (Health Canada 2015). Exclusively for formula-fed infants, assumed to drink 0.826 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote (a) for drinking water for details.
c Assumed to weigh 9.1 kg (Health Canada 2015), for breast milk-fed infants, assumed to consume 0.632 L of breast milk per day (Health Canada 2018). For formula-fed infants, assumed to drink 0.764 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote (a) for drinking water for details.
d Assumed to weigh 11 kg (Health Canada 2015), and to drink 0.36 L of water per day (Health Canada 2018).
e Assumed to weigh 15 kg (Health Canada 2015), and to drink 0.43 L of water per day (Health Canada 2018).
f Assumed to weigh 23 kg (Health Canada 2015), and to drink 0.5. L of water per day (Health Canada 2018).
g Children 9 to 13 years old assumed to weigh 42 kg (Health Canada 2015), and to drink 0.74 L of water per day (Health Canada 2018).
h Children 14 to 18 years old assumed to weigh 62 kg (Health Canada 2015), and to drink 1.09 L of water per day (Health Canada 2018).
i Assumed to weigh 74 kg (Health Canada 2015), and to drink 1.53 L of water per day (Health Canada 2018).
