Draft screening assessment - Phosphoric acid derivatives group
Official title: Draft screening assessment - Phosphoric acid derivatives group
Chemical Abstracts Service Registry Numbers
25155-23-1, 37310-83-1, 119345-01-6
Environment and Climate Change Canada
Health Canada
July 2019
Synopsis
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment on three of six substances referred to collectively under the Chemicals Management Plan as the Phosphoric Acid Derivatives Group. These three substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns. The three other substances were determined to be of low concern through other approaches, and proposed decisions for these substances are provided in a separate report.Footnote 1 Accordingly, this screening assessment addresses the three substances listed in the table below, which are hereinafter referred to as the Phosphoric Acid Derivatives Group. The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 2 ), their Domestic Substances List (DSL) names and their common names are listed in the table below.
| CAS RN | DSL name | Common name |
|---|---|---|
| 25155-23-1 | Phenol, dimethyl-, phosphate (3:1) | Trixylyl phosphate |
| 37310-83-1a | 9-Octadecen-1-ol, (Z)-, phosphate | Oleyl phosphate |
| 119345-01-6ab | Phosphorous trichloride, reaction products with 1,1-biphenyl and 2,4-bis(1,1-dimethylethyl)phenol | NA |
Abbreviations: NA, Not Available.
a This substance is a UVCB (unknown or variable composition, complex reaction products, or biological materials).
b This substance was not identified under subsection 73(1) of CEPA but was included in this assessment as it was considered a priority on the basis of other human health concerns.
The substances in the Phosphoric Acid Derivatives Group do not occur naturally in the environment. According to information reported in response to surveys under section 71 of CEPA, trixylyl phosphate was not reported to be manufactured in Canada above the reporting threshold of 100 kg but between 100 000 and 1 000 000 kg was imported into Canada in 2008. In 2011, no Canadian manufacturing or importing activities were reported for oleyl phosphate above the reporting threshold of 100 kg. CAS RN 119345-01-6 was reported to be imported into Canada in 2011 in quantities ranging from 10 000 to 100 000 kg but was not reported to be manufactured above the reporting threshold.
Reported uses of trixylyl phosphate in Canada include as a flame retardant, in lubricants and greases. Other potential uses of trixylyl phosphate include as food packaging materials, a plasticizer, in hydraulic fluids and for wire and cabling insulation. Oleyl phosphate is used in cosmetics such as permanent hair dye in Canada. CAS RN 119345-01-6 is used in plastic and rubber materials and may be used in food packaging materials.
The ecological risks of the substances in the Phosphoric Acid Derivatives Group were characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure based on weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are established based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances based on their hazard and exposure profiles. Based on the outcome of the ERC analysis, trixylyl phosphate, oleyl phosphate, and CAS RN 119345-01-6 are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from trixylyl phosphate, oleyl phosphate and CAS RN 119345-01-6. It is proposed to conclude that trixylyl phosphate, oleyl phosphate and CAS RN 119345-01-6 do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Trixylyl phosphate has been reviewed internationally by the European Union under REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) and by the Australian’s National Industrial Chemicals Notification and Assessment Scheme (NICNAS); these evaluations informed the health effects characterization of trixylyl phosphate. Trixylyl phosphate is on the ECHA (European Chemicals Agency) List of Substances of Very High Concern for reproductive toxicity (ECHA 2013) and has been classified as a reproductive toxicant (H360F: “may damage fertility”). The available health effects information on trixylyl phosphate indicates that the critical effects are in reproductive organs (testes, epididymis ovaries), and adrenal glands (decreased adrenal weights, vacuolation) in both sexes. The general population of Canada may be exposed to trixylyl phosphate through environmental media as a result of its presence in dust and through certain products available to consumers. A comparison of levels of trixylyl phosphate that Canadians may be exposed to in environmental media and through products available to consumers with levels associated with adverse effects results in margins that are considered adequate to address uncertainties in exposure and health effects data used to characterize risk.
Oleyl phosphate is considered to be of low hazard potential and the risk to human health related to the presence of oleyl phosphate in cosmetics is considered to be low.
The available health effects information on CAS RN 119345-01-6 indicates developmental effects in laboratory studies. The predominant source of exposure to CAS RN 119345-01-6 for the general population is through the diet primarily from its potential use in food packaging materials. A comparison of estimated levels of exposure to CAS RN 119345-01-6 and critical effect levels results in margins that are considered adequate to address uncertainties in exposure and health effects data used to characterize risk.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that trixylyl phosphate, oleyl phosphate and CAS RN 119345-01-6 do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that trixylyl phosphate, oleyl phosphate and CAS RN 119345-01-6 do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment on three of six substances, referred to collectively under the Chemicals Management Plan as the Phosphoric Acid Derivatives Group, to determine whether these three substances present or may present a risk to the environment or to human health. These three substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other human health concerns (ECCC, HC [modified 2017]).
The other three substances in the group (Chemical Abstracts Service Registry Number (CAS RNsFootnote 3 ) 68604-99-9, fatty acids, C18-unsatd., phosphates; 68952-35-2, tar acids, cresylic, Ph phosphates; 111174-61-9, alcohols, C8-16, reaction products with phosphorus oxide (P2O5), compds. with 2-ethyl-1-hexanamine) were considered in the ecological risk classification (ERC) of organic substances Science Approach Document (ECCC 2016a) and via the approach applied in the Rapid Screening of Substances with Limited General Population Exposure (ECCC, HC 2018), and were identified as being of low concern to both human health and the environment. As such, they are not further addressed in this report. Conclusions for these three substances are provided in the Substances Identified as Being of Low Concern based on the Ecological Risk Classification of Organic Substances and the Rapid Screening of Substances with Limited General Population Exposure Screening Assessment (ECCC, HC 2018). The three substances addressed in this screening assessment will hereinafter be referred to as the Phosphoric Acid Derivatives Group.
The ecological risks of the substances in the Phosphoric Acid Derivatives Group were characterized using the ERC approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
Only trixylyl phosphate has been reviewed internationally through the European Chemical Agency’s Community Rolling Action Plan (ECHA CoRAP) under REACH and the National Industrial Chemicals Notification and Assessment Scheme (NICNAS). Reviews conducted by these institutions are used to inform the health effects characterization in this screening assessment.
This draft screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to July 2018. Empirical data from key studies as well as results from models were used to reach proposed conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This draft screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The human health portions of this assessment have undergone external review and/or consultation. Comments on the technical portions relevant to human health were received from scientific experts [Dr. Judy LaKind (University of Maryland School of Medicine), Dr. Kefeni (Tshwane University of Technology), Dr. Michael Hughes (United States Environmental Protection Agency at the National Health and Environmental Effects Research Laboratory) and Dr. Mohamed Abou-Elwafa Abdallah (University of Birmingham)] from Risk Sciences International. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This draft screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precautionFootnote 4 . This draft screening assessment presents the critical information and considerations on which the proposed conclusions are based.
2. Identity of substances
The CAS RN, Domestic Substances List (DSL) names and common names for the individual substances in the Phosphoric Acid Derivatives Group are presented in Table 2‑1.
| CAS RN | DSL name (common name) | Molecular formula | Chemical Structure (Figure 1) | Molecular weight (g/mol) | Reference |
|---|---|---|---|---|---|
| 25155-23-1 | Phenol, dimethyl-, phosphate (3:1) (Trixylyl phosphate) | C24H27O4P | 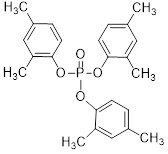 |
410.45 | PubChem 2004- |
| 37310-83-1 | 9-Octadecen-1-ol, (Z)-, phosphate (Oleyl phosphate) | C18H36Ox-H3O4P (UVCBa) | (O)=O](/content/dam/eccc/images/pded/phosphoric-acid-derivatives/20190604-t2-1b.jpg) |
348.46 – 366.48 | Chemical Book 2016a, ChemIDplus 1993- |
| 119345-01-6 (EC 432-130-2b) | Phosphorous trichloride, reaction products with 1,1-biphenyl and 2,4-bis(1,1-dimethylethyl)phenol | C14H22O C12H10 Cl3P (UVCBa) |  |
497.87 – 1035.4 | Chemical Book 2016b, ChemIDplus 1993- |
a UVCB is an acronym for Unknown or Variable composition Complex reaction products and Biological material. These materials are derived from natural sources or complex reactions and cannot be characterized in terms of constituent chemical compounds because their composition is too complex or variable. A UVCB is not an intentional mixture of discrete substances and is considered a single substance.
b European Community Number assigned by ECHA (c2007-2018c) and for the purposes of this assessment is considered to be equivalent to CAS RN 119345-01-6.
3. Physical and chemical properties
A summary of physical and chemical property data of the substances in the Phosphoric Acid Derivatives Group is presented in Table 3‑1. When experimental information was limited or not available for a property, data from analogues were used for read-across and/or (Q)SAR models were used to generate predicted values for the substance. Additional physical and chemical properties are presented in ECCC (2016b).
| Property | Trixylyl phosphate | Oleyl phosphate | CAS RN 119345-01-6 | Key reference(s) |
|---|---|---|---|---|
| Physical state | liquid | liquid | solid | PubChem 2004-, ECHA c2007-2018a, Chemical Book2016b |
| Melting point (°C) | -20 to -15 | -77 to 53 (no standard melting point found) | 75 to 95 | ECHA c2007-2018b, ECHA c2007-2018a, Wypych 2015 |
| Vapour pressure (Pa) | 210 @ 20 deg. C | 8 x 10-9 to 2 x 10-6 (estimated) | Not available | ECHA c2007-2018b |
| Henry’s law constant (Pa·m3/mol) | 3.1X10-8 atm-cu m/mole (estimated) | 2.64X10-10 atm-cu m/mole (estimated) | Not available | HSDB 1983-, ChemSpider 2015 |
| Water solubility (mg/L) | < 0.02 | 0.06 | wt%: <0.01 | ECHA c2007-2018b, ECHA c2007-2018a, Wypych 2015, |
| Log Kow (dimensionless) | > 6.2 | 7.42 (estimated) | Not available | ECHA c2007-2018b, ChemSpider 2015 |
Abbreviations: Kow, octanol-water partition coefficient.
4. Sources and uses
The substances in the Phosphoric Acid Derivatives Group do not occur naturally. All of the substances in the Phosphoric Acid Derivatives Group have been included in surveys issued pursuant to a CEPA section 71 notice (Canada 2009, Canada 2012). Table 4‑1 presents a summary of information reported on the total manufacture and total import quantities for the Phosphoric Acid Derivatives Group.
| Common name | Total manufacturea (kg) | Total importsa (kg) | Reporting year | Survey reference |
|---|---|---|---|---|
| Trixylyl phosphate | NR | 100 000 – 1 000 000 | 2008 | Environment Canada 2009 |
| Oleyl phosphate | NR | NR | 2011 | Environment Canada 2013 |
| CAS RN 119345-01-6 | NR | 10 000 – 100 000 | 2011 | Environment Canada 2013 |
Abbreviations: NR = Not reported above the DSL IU reporting threshold of 100 kg threshold.
a Values reflect quantities reported in response to the surveys conducted under section 71 of CEPA (Canada 2009, Canada 2012). See surveys for specific inclusions and exclusions (schedules 2 and 3).
Table 4‑2 presents a summary of the major uses of the Phosphoric Acid Derivatives Group according to information reported pursuant to CEPA section 71 surveys (Environment Canada 2009, Environment Canada 2013). Table 4‑3 presents additional Canadian uses for this group of substances.
| Major usesa | Trixylyl phosphate | Oleyl phosphateb | CAS RN 119345-01-6 |
|---|---|---|---|
| Plastic and rubber materials | N | N/A | Y |
| Lubricants and greases | Y | N/A | N |
| Flame retardant | Y | N/A | N |
Abbreviations: N/A, Not Applicable; Y = yes this use was reported for this substance; N = no this use was not reported for this substance.
a Non-confidential uses reported in response to the surveys conducted under section 71 of CEPA (Canada 2009, Canada 2012). See surveys for specific inclusions and exclusions (schedules 2 and 3).
b In 2011, no Canadian manufacturing or importing activities were reported for oleyl phosphate above the reporting threshold of 100 kg.
| Use | Trixylyl phosphate | Oleyl phosphate | CAS RN 119345-01-6 |
|---|---|---|---|
| Food packaging materialsa | Y | N | Y |
| Notified to be present in cosmetics, on the basis of notifications submitted under the Cosmetic Regulations to Health Canadab | N | Y | N |
Abbreviations: Y = yes this use was reported for this substance; N = no this use was not reported for this substance.
a Personal communication, e-mail from the Food Directorate, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada dated August 31, 2016 ,December 1, 2017, and June 6, 2018, unreferenced)
b Personal communication, e-mail from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated Dec. 5, 2017 and April 13, 2018, unreferenced)
Globally, trixylyl phosphate may be used as a flame retardant, a plasticizer or as/in hydraulic fluids, for wire and cabling insulation, in lubricants and greases, in power generation fluids, in metal working fluids and in various plastics including polyvinyl chloride, rigid and flexible polyurethane, ethylene propylene diene monomer (M-class) rubber (EPDM), copolymer of polycarbonate and acrylonitrile-butadiene-styrene (PC/ABS) (alloys), phenolic resins (ECHA 2018, HSDB 1983-2018, Danish EPA 2016, United Kingdom Environment Agency 2009).
In Canada, trixylyl phosphate may be used in food packaging as an antioxidant in natural rubber-polyterpene based adhesives. However, direct food contact is not expected and migration of the component is unlikely (Personal communication, e-mail from the Food Directorate, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada dated June 6, 2018, unreferenced).
Based on notifications submitted under the Cosmetic Regulations to Health Canada, oleyl phosphate is used in certain cosmetic products in Canada such as hair dyes, bleach and straightening, waving and curling products (personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated April 13, 2018; unreferenced). Oleyl phosphate is used as a surfactant, emulsifying agent in cosmetics (Cosmetic Ingredients & Substances [accessed 2018]).
CAS RN 119345-01-6 may be used in food packaging materials as an antioxidant and stabilizer in polyethylene, polypropylene, acrylonitrile-butadiene-styrene, polystyrene, polyamide, polycarbonate and polyethylene/polypropylene copolymers with potential for direct exposure (Personal communication, e-mail from the Food Directorate, Health Canada to Existing Substances Risk Assessment Bureau, Health Canada dated December 1, 2017, unreferenced). This substance may also be used as a stabilizer and secondary antioxidant for non-food packaging polymers including filled polyolefins, acrylonitrile-butadiene-styrene, polystyrene, polybutylene, polybutylene terephthalate, polycarbonate, thermoplastic polyester, nitrile barrier resins and fibers, as an antioxidant and colour suppressant for powder coatings, in plastics manufacturing as a peroxide decomposer and reduces equipment corrosion in flame-retardant applications (Ash and Ash 2008, Ash and Ash 2013).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risks of the substances in the Phosphoric Acid Derivatives Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure on the basis of weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration [LC50]) for characterization. Since oleyl phosphate and CAS RN 119345-01-6 are UVCB substances and could not be suitably represented by single chemical structures, a manual judgement-based approach to classification was used. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from scientific literature, from available empirical databases (e.g., OECD [Q]SAR Toolbox 2016), and from responses to surveys under section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also composed of multiple metrics including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure. However, in the case of oleyl phosphate and CAS RN 119345-01-6, hazard and exposure could not be fully profiled due to the lack of a representative structure to estimate needed properties, and the lack of empirical data for these properties. Therefore, manual classification of hazard and exposure was performed by examining the UVCB constituents and DSL Inventory Update information and making decisions on the basis of consideration of similar substances and application of expert judgement.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances which had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over and under classification of hazard and exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC 2016a. The following describes two of the more substantial areas of uncertainty. Error with empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2016). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue used for critical body residue (CBR) analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is believed to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for the substances in the Phosphoric Acid Derivatives Group, and the hazard, exposure and risk classification results are presented in ECCC (2016b).
The hazard and exposure classifications for the substances in the Phosphoric Acid Derivatives Group are summarized in Table 5-1.
| Substance | ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| Trixylyl phosphate | moderate | low | low |
| Oleyl phosphate | high | low | low |
| CAS RN 119345-01-6 | low | low | low |
According to information considered under ERC, trixylyl phosphate was classified as having a low exposure potential. It was classified as having a moderate hazard potential on the basis of agreement between the reactive mode of action and elevated toxicity ratio, both of which suggest that this chemical is likely of high potency, as well as a moderate potential to cause adverse effects in aquatic food webs given its bioaccumulation potential. However, the potential effects and how they may manifest in the environment were not further investigated due to the low exposure of this substance. Trixylyl phosphate was classified as having a low potential for ecological risk. On the basis of current use patterns, trixylyl phosphate is unlikely to be resulting in concerns for the environment in Canada.
According to information considered under ERC, oleyl phosphate was classified as having a low exposure potential. It was classified as having a high hazard potential on the basis of a reactive mode of action as well as a high potential to cause adverse effects in aquatic food webs given its bioaccumulation potential. However, the risk classification was decreased to low potential for ecological risk following the adjustment of risk classification based on current use quantities (see section 7.1.1. of the ERC approach document [ECCC 2016a]). The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of this substance. On the basis of current use patterns, oleyl phosphate is unlikely to be resulting in concerns for the environment in Canada.
On the basis of low hazard and low exposure classifications according to information considered under ERC, CAS RN 119345-01-6 was classified as having a low potential for ecological risk. It is therefore unlikely that this substance is resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
6.1.1 Trixylyl phosphate
No Canadian monitoring data was identified. The only empirical data identified is on the presence of trixylyl phosphate in dust. Based on physical-chemical properties this substance is expected to adsorb to suspended solids and sediments and is expected to have low mobility in soil (Brooke et al. 2009a).
Trixylyl phosphate was identified in indoor dust samples from homes in the United Kingdom (UK) (n = 10) and in Norway (n = 10) in 2013 and 2014 (Kademoglou et al. 2017). The maximum reported concentrations of trixylyl phosphate in house dust were 105 and 537 ng/g in Norway and UK, respectively. Trixylyl phosphate was also detected in stores (n=6) and offices (n=6) located in Reading, UK with concentrations ranging from 20.8 to 5820 ng/g (Kademoglou et al. 2017).
ChemCAN (2003), a level III fugacity model, was used to derive potential environmental concentrations of trixylyl phosphate in Canada using the upper-end volume data from Table 4‑1 (i.e., 1 000 000 kg). The estimated concentrations from air and soil were 3.02ng/m3 and 15.2 ng/g, respectively. Given the absence of surface water and drinking water monitoring data for trixylyl phosphate in Canada, an industrial release scenario based on Health Canada (2015a) was used to derive the theoretical concentration of trixylyl phosphate in surface water as a surrogate for drinking water. Total annual usage corresponding to the maximum import quantities identified through section 71 survey data (i.e., 1 000 000 kg), removal percentage by wastewater treatment plants of 84% (ECCC 2016b), and a conservative maximum loss percent release to wastewater of 2% (for industrial release scenarios) (OECD 2004), and a flow rate of 21.33 m3/s (50th percentile) were used as inputs. The resulting surface water concentration was 6.95 x 10-3 mg/L. These concentrations were used to estimate exposure to trixylyl phosphate from environmental media (air, water, and soil) for the general population of Canada. No occurrence data for trixylyl phosphate in food were identified in Canada or elsewhere.
The highest calculated exposure estimate for trixylyl phosphate for the general population of Canada from environmental media was 0.91 µg/kg bw/d for infants (see Appendix A).
Trixylyl phosphate may be present in automotive fluids, such as power steering fluid, in concentrations ranging from 0.1% to 1% by weight (SDS 2010). Dermal exposure was derived using exposure factors taken from the US EPA Versar Manual and based on a thin film approach (US EPA 1986, 1987). Parameters used in the model are outlined in Appendix B. No information on dermal absorption was identified; therefore, dermal absorption was considered equivalent to oral absorption. However, dermal absorption is likely less based on the physical-chemical properties of trixylyl phosphate and the relatively short duration of use (less than 1 hour) for this product scenario. The systemic exposure was estimated to be 0.01 mg/kg-bw/day.
6.1.2 Oleyl phosphate
No data were identified on the presence of oleyl phosphate in environmental media and food in Canada or elsewhere. Given that oleyl phosphate is not manufactured or imported in Canada in quantities greater than 100 kg (see section 4.0), and taking into account its physical and chemical properties, exposure to this substance from environmental media and food is not expected.
Oleyl phosphate is present in certain cosmetic products in Canada such as permanent and temporary hair dyes, bleach, and straightening, waving and curling products (personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated April 13, 2018; unreferenced). Thus, there is potential for dermal exposure to oleyl phosphate. However, exposure has not been quantified as no health effects of concern have been identified (see Section 6.3.2).
6.1.3 CAS RN 119345-01-6
No data were identified on the presence of CAS RN 119345-01-6 in environmental media and food in Canada or elsewhere. However, an industrial release scenario based on Health Canada (2015a) was used to derive the theoretical concentration of CAS RN 119345-01-6 in surface water as a surrogate for drinking water. Inputs included a total annual usage corresponding to the maximum import quantities identified through section 71 survey data (i.e., 100 000kg), a conservative worst-case removal percentage for this substance by wastewater treatment plants of 0%, a conservative worst-case percent release to wastewater of 100% (for industrial release scenarios), and a flow rate of 21.33 m3/s (50th percentile). The resulting surface water concentration was 2.17 x 10-1 mg/L. As such, the estimated theoretical intake from drinking water is negligible.
CAS RN 119345-01-6 may be used in food packaging materials as an antioxidant and stabilizer in polyethylene, polypropylene, acrylonitrile-butadiene-styrene, polystyrene, polyamide(PA), polycarbonate and polyethylene/polypropylene copolymers with potential for direct exposure. The Probable Daily Intake (PDI) ranges from 9.73 μg/kg-bw/day for children 9 – 13 years of age to 23.37 μg/kg-bw/day for children 2 – 3 years of age (see Appendix C). The substance is not reported to be used in infant formula food packaging materials (personal communication, e-mail from Food Directorate, Health Canada to Existing Substances Risk Assessment Bureau (ESRAB), Health Canada, dated Dec. 4, 2017, unreferenced).
6.2 Health effects assessment
6.2.1 Trixylyl phosphate
Trixylyl phosphate has been reviewed internationally by the European Union under REACH and by Australia’s National Industrial Chemicals Notification and Assessment Scheme (NICNAS). Targeted literature searches were conducted until July 2018. No additional health effect studies that would impact the risk characterization were identified (i.e., result in different critical endpoints or lower points of departure than those stated in NICNAS 2017). Trixylyl phosphate is on the ECHA List of Substances of Very High Concern for reproductive toxicity (ECHA 2013) and has been classified as a reproductive toxicant (H360F: ‘’may damage fertility’’) (ECHA 2016).
No carcinogenicity studies were identified for trixylyl phosphate. Trixylyl phosphate is not considered to be genotoxic (NICNAS 2017).
In a combined repeated-dose and reproductive/developmental toxicity study, Sprague Dawley (SD) rats were given trixylyl phosphate via gavage at 0, 25, 200, or 1000 mg/kg bw/day for 2 weeks prior to mating, during mating, through gestation and lactation for a total of approximately 33 days of dosing for males and 48 days for females (ECHA 2010; NICNAS 2017). Successful gestation occurred in 100% of control and low-dose animals, 18% in the mid-dose group and 0% in the high-dose group. There was no statistical difference in the pups’ survival rate, mean litter size, number of stillborn pups or offspring body weight. Analysis of the uterus revealed only two gravid animals at 1000 mg/kg bw/day and none in the mid-dose group (except the two that underwent parturition). These results suggest that the reduction in successful gestation is mainly due to decreased fertility rather than post-implantation loss (ECHA 2010; NICNAS 2017). Changes in the weights of the adrenals, testes, heart, epididymis, liver and ovaries, starting at the low dose in females and at the mid dose in males, were observed. Treatment-related histological findings were found in adrenal glands, liver and in the testes, epididymis and ovaries at all doses. The incidence and severity of effects were completely reversed in the recovery animals (from control and high-dose treated animals). Mating of recovery animals showed complete reversal of the effects seen on reproductive performance. Therefore, the functional deficit in reproductive performance was reversed in males and females after the recovery period. Since the reversal was observed in both sexes, it was not possible to determine if the effects on reproductive performance were male- or female-mediated (ECHA 2010).
The lowest observed adverse effect level (LOAEL) for systemic and reproductive toxicity was 25 mg/kg-bw/day, based on organ weight and histopathological changes in the testes, epididymis, ovaries and adrenal glands observed at the lowest dose tested. No developmental no observed adverse effect level (NOAEL) or LOAEL could be established due to fertility effects observed and limited evaluations in offspring during the recovery period of the study.
6.2.2 Oleyl phosphate
Oleyl phosphate has also been reviewed in the Safety Assessment of Alkyl Phosphates as Used in Cosmetics (CIR 2014). A registration dossier has also been submitted to ECHA under REACH (ECHA c2007-2018b)
No carcinogenicity studies were identified for this substance. It is not considered to be genotoxic (ECHA c2007-2018b).
No effects were observed after a 14-day or 28-day oral exposure to oleyl phosphate up to doses of 1000 mg/kg bw/day in rats (ECHA c2007-2018b). No significant changes in body weight, food consumption, histopathology and hematology or clinical chemistry parameters were observed. Only a slightly higher mean activity of ALT (alanine aminotransferase) in the 1000 mg/kg bw/day males group was observed, but was determined to be toxicologically not relevant as there were no changes in other clinical pathology parameters and there was no correlation with any histological findings.
In a reproductive/developmental oral study, rats exposed by gavage up to 1000 mg/kg bw/day for 41-46 days, no toxicological effects in dams or pups were identified (ECHA c2007-2018b).
Wistar rats were exposed by gavage to 0, 100, 300 or 1000 mg/kg bw/day of oleyl phosphate for 14 days prior to mating, and throughout mating. Oleyl phosphate was administrated to males after mating up to the day of necropsy (for a total of 41 days) and to females up to lactation day 3, 4 or 5 (for a total of 41-46 days). Observations included mortality, clinical signs, body weight, food consumption, mating, pregnancy and delivery process, as well as development of pups. Oleyl phosphate did not cause adverse effects and did not influence reproductive performance (gonad function, mating behaviour, conception, pregnancy, parturition) in parental male and female rats or development of the F1 offspring (ECHA c2007-2018b).
6.2.3 CAS RN 119345-01-6
CAS RN 119345-01-6 has not been assessed by other agencies. A registration dossier has been submitted to ECHA for the reaction products of phosphorous trichloride, with 1,1′-biphenyl and 2,4-bis(1,1- dimethylethyl) phenol (EC Number 432-130-2; ECHA c2007-2018c), which is considered equivalent to CAS RN 119345-01-6 for this assessment.
No carcinogenicity studies were identified for this substance. It is not considered to be genotoxic (ECHA c2007-2018c).
No adverse systemic effects were observed in rats fed with Sandostab P-ENQ (identified as CAS RN 119345-01-6) up to doses of 497 mg/kg bw/day (males) and 812 mg/kg bw/day (females) for 13 and 18 weeks (Hazleton Laboratories 1978).
In the one-generation toxicity study, rats were treated with Sandostab P-ENQ (identified as CAS RN 119345-01-6) in the diet at doses of 63, 172 or 469 mg/kg bw/day for males and 74, 202 or 560 mg/kg bw/day for females, for 30 days prior to mating (males were sacrificed after mating), through gestation and lactation, and the F1 generation (57-76, 200-253 and 497-812 mg/kg bw/day (for males and females, respectively) for 13 weeks after weaning (Hazleton Laboratories 1978). Another F1 group from untreated parents was added and received 466 mg/kg bw/day for males and 672 mg/kg bw/day for females, respectively, for 13 weeks. At the high dose, there was a significant increase in female to male ratios. Mean pup weight was also significantly lower at 10 days, but normal on days 1, 4, and 21 or at any time in the other dose groups. Two males showed exophthalmos during treatment and six neonates showed exophthalmos, corneal dulling, and intra-ocular opacity or blackening. These changes were not seen in the mid and low dose groups. Similar changes were noted in several F1 weaned progeny selected, but it was considered to be related to the experimental manipulations (e.g., orbital sinus puncture for blood samplings). Body weight gain, food and water consumption were not affected during the entire experiment. Therefore, the NOAEL for this study was established at 200 mg/kg bw/day (Hazleton Laboratories 1978).
In another study, the oral dietary administration of EC Number 432-130-2 to Wistar rats at dose levels of 145, 305 or 608 mg/kg bw/day for males and 190, 366 or 794 mg/kg bw/day for females (two weeks prior to mating, during the mating period and three weeks post mating for males and two weeks prior to mating, during time to conception, during pregnancy and four days after delivery, up to the day before scheduled sacrifice for females), was well tolerated (ECHA c2007-2018c). There were no toxicologically significant effects on body weight, food consumption, and reproductive parameters during the treatment period. There was no effect on the pups. The systemic NOAEL is considered to be 608 mg/kg bw/day for males and 794 mg/kg bw/day for females (ECHA c2007-2018c).
No adverse effects were observed in either study described above (Hazleton Laboratories 1978; ECHA c2007-2018c). No adverse effects were found after exposure for 28 days to EC 432-130-2 up to a dose of 1000 mg/kg bw/day (ECHA c 2007-2018c).
6.3 Characterization of risk to human health
6.3.1 Trixylyl phosphate
No NOAEL could be established for effects on fertility from exposure to trixylyl phosphate. The reproductive LOAEL was 25 mg/kg bw/day based on histological changes in reproductive organs observed at the lowest dose level in both sexes. Overall, the results of the available combined study on repeated dose toxicity and reproduction/developmental toxicity show a clear reduction in fertility (significant and dose-related weight changes in testes, epididymides and ovaries), accompanied by histological changes in these organs. Effects in adrenal glands (diffuse cytoplasmic vacuolation) and clinical chemistry parameters were also observed (Expedimur 2004 cited in ECHA 2010).
The general population of Canada may be exposed to trixylyl phosphate through environmental media. It may also be present in a limited number of products available to consumers. Table 6-1 provides relevant exposure estimates, critical effect levels as well as resulting margins of exposure (MOEs) for the characterization of risk to human health from exposure to trixylyl phosphate.
| Exposure scenario | Systemic exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Environmental media | 0.00091 mg/kg bw/day | LOAEL = 25 mg/kg-bw/day (LDT) | organ weight and histopathological changes in the testes, epididymides, ovaries and adrenal glands | 27 472 |
| Power steering fluid (dermal) | 0.0113 mg/kg-bw/daya | LOAEL = 25 mg/kg-bw/day (LDT) | organ weight and histopathological changes in the testes, epididymides, ovaries and adrenal glands | 2212 |
Abbreviations: LDT, lowest dose tested; LOAEL, lowest observed adverse effect level
a considering that dermal absorption is equivalent to oral absorption.
These margins of exposure are considered adequate to address uncertainties in the health effects and exposure databases.
While exposure of the general population to trixylyl phosphate is not of concern at current levels, this substance is considered to have a health effect of concern on the basis of its potential to cause reproductive effects. Therefore, there may be a concern for human health if exposures were to increase.
6.3.2 Oleyl phosphate
No reproductive/developmental effects or general toxicity were observed after exposure to oleyl phosphate up to a dose of 1000 mg/kg-bw/day. As such, oleyl phosphate is considered to be of low hazard potential.
Given the low hazard potential of oleyl phosphate, risk to human health related to the presence of oleyl phosphate in products available to consumers is considered to be low.
6.3.3 CAS RN 119345-01-6
A NOAEL of 200 mg/kg bw/day based on a decrease in sex ratio and ocular defects was determined for this substance (Hazleton Laboratories 1978). Overall, the results of the available studies showed that this substance is of low systemic toxicity. Mortality observed in the one generation study is not considered to be treatment related.
The European Commission’s Scientific Committee for Food has also derived a tolerable daily intake (TDI) of 0.3 mg/kg bw (European Commission 1995).
The general population of Canada may be exposed to CAS RN 119345-01-6 through its potential presence in food packaging materials. Comparison of the highest PDI of 0.023 mg/kg bw/day with the NOAEL of 200 mg/kg bw/day results in a MOE of 8557 which is considered adequate to address uncertainties in the health effects and exposure databases.
6.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| Limited environmental monitoring data. | +/- |
| There is limited information on the physical-chemical properties of the grouping and estimations are used to fill data gaps. | +/- |
| There are no chronic or carcinogenicity animal studies for all routes of exposure and use of short-term studies were used to characterize risk. | +/- |
| No chemical-specific dermal absorption, information for trixylyl phosphate, oleyl phosphate and CAS RN 119345-06-1are available | + |
| There are no or few animal studies examining the repeated-dose toxicity of the substances for the relevant routes of exposure (i.e., dermal), and therefore route-to-route extrapolation was used when required. | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over or under estimation of risk.
7. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from trixylyl phosphate, oleyl phosphate and CAS RN 119345-06-1. It is proposed to conclude that trixylyl phosphate, oleyl phosphate and CAS RN 119345-06-1 do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that trixylyl phosphate, oleyl phosphate and CAS RN 119345-06-1 do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that trixylyl phosphate, oleyl phosphate and CAS RN 119345-06-1 do not meet any of the criteria set out in section 64 of CEPA.
References
Ash M, Ash I. 2008. Specialty Chemicals, Source Book. Third edition. Endicott (NY): Synapse Information Resources, Inc. [accessed through Knovel subscription]
Ash M, Ash I. 2013. Handbook of Paint and Coating Raw Materials. Second edition. Endicott (NY): Synapse Information Resources, Inc. [accessed through Knovel subscription]
Brooke D N, Crookes M J, Quarterman P and Burns J. 2009a. An overview of the environmental risk evaluation reports for aryl phosphate esters. Environment Agency. Bristol, United Kingdom.
Brooke D N, Crookes M J, Quarterman P and Burns J. 2009b. Environmental risk evaluation report: Trixylenyl phosphate (CAS no. 25155-23-1). Environment Agency. Bristol, United Kingdom.
Canada. [1978]. Food and Drug Regulations. C.R.C., c.870.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2009. Canadian Environmental Protection Act, 1999: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 143, no. 40, p. 2945-2956.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
Chemical Book. 2016a. Oleyl Phosphate (Mono- And Di- Ester Mixture). [accessed 2018 June 15]
Chemical Book. 2016b. IRGAFOS P-EPQ . [accessed 2018 June 15]
ChemCAN [level III fugacity model of 24 regions of Canada]. 2003. Version 6.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
ChemIDplus [database]. 1993- . Bethesda (MD): US National Library of Medicine. [accessed 2018 June 15].
ChemSpider. 2015. Search results for CAS RN 37310-82-1, CSID: 74160 . Royal Society of Chemistry [accessed 2018 June 12]
[CIR] Cosmetic Ingredient Review. 2014. Safety Assessment of Alkyl Phosphates as Used in Cosmetics. Final Report. September 29, 2014. Washington DC. [accessed 2018 June 27]
[CosIng] Cosmetic Ingredients & Substances [database]. Ingredient: Oleyl Phosphate. Brussels (BE): European Commission. [accessed 2018 June 15]
[Danish EPA] Danish Environmental Protection Agency. 2016. Environmental and health screening profiles of phosphorous flame retardants. [PDF] A LOUS follow-up project. Environmental project No. 1823, 2016.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Data used to create substance-specific hazard and exposure profiles and assign risk classifications in the Ecological Risk Classification of organic substances. Gatineau (QC). Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization of chemical substances. Ottawa (ON): Government of Canada. [accessed 2018 June 15].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018. Rapid screening of substances with limited general population exposure. Ottawa (ON): Government of Canada.
[ECHA] European Chemicals Agency. c2007-2018a. Registered substances database; search results for CAS RN 25155-23-1. Helsinki (FI): ECHA. [Updated 2017 July 10; [accessed 2018 June 19].
[ECHA] European Chemicals Agency. c2007-2018b. Registered substances database; search results for CAs RN 37310-83-1. Helsinki (FI): ECHA. [Updated 2018 May 18; [accessed 2018 June 25].
[ECHA] European Chemicals Agency. c2007-2018c. Registered substances database; search results for EC number 432-130-2. Helsinki (FI): ECHA. [Updated 2017 July 10; [accessed 2018 June 21].
[ECHA] European Chemicals Agency, 2010. Committee for Risk Assessment. Background document to the opinion of the committee for risk assessment on a proposal for harmonised classification and labelling of trixylyl phosphate (CAS no 25155-23-1) [PDF]. [Accessed June 2018].
[ECHA] European Chemicals Agency, 2013. Support document for identification of trixylyl phosphate as a substance of very high concern because of its CMR properties.[PDF] [Accessed June 2018].
[ECHA] European Chemicals Agency, 2014. Substance Evaluation – Community rolling action plan (CoRAP). Trixylyl phosphate. CAS RN 25155-23-1. Institute of Health. Italy [Accessed August 2018].
[ECHA] European Chemicals Agency, 2016. Recommendation of the European Chemicals Agency of 10 November 2016 for the inclusion of substances in Annex XIV to REACH (List of Substances subject to Authorisation). [PDF] [Accessed June 2018].
[ECHA] European Chemicals Agency. 2018. Brief profile: Trixylyl phosphate; CAS RN 25155-23-1. Helsinki (FI): ECHA. [updated 2018 Feb 2; accessed 2018 June 15].
Environment Canada. 2009. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada, Health Canada. 2014. Approach for identification of chemicals and polymers as risk assessment priorities under Part 5 of the Canadian Environmental Protection Act, 1999 (CEPA 1999). Ottawa (ON):
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
European Commission. 1995. Food Science and Techniques. Reports of the Scientific Committee for Food. Thirty-third series. First report on certain additives used in the manufacture of plastic materials intended to come into contact with foodstuffs (Opinions expressed until 3 May 1992). Luxembourg: Office for Official Publications of the European Communities
Hazleton Laboratories. 1992. Initial submission: Phosphorous Trichloride: Generation Short-Term Toxicity Study in the Rat with cover letter dated 041092. OTS0536229. Doc ID: 88-920001967. CIBA-GEIGY Corporation. Hazleton Laboratories Europe Ltd. Report No.: 1373-252/9. Date June 1978.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Government of Canada.
Health Canada. 2015a. Environmental Assessment Unit Drinking Water Spreadsheets. [Excel format]. Ottawa (ON): Health Canada.
Health Canada. 2015b. Food Consumption Table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004), Share file. Ottawa.
Health Canada. 2016. Science approach document: threshold of toxicological concern (TTC)-based approach for certain substances. Ottawa (ON): Government of Canada.
Health Canada. 2017. Water Consumption Table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004). Share file. Ottawa.
Health Canada. 2018. Draft backgrounder document on default values for breast milk and formula intakes. Unpublished report. Ottawa (ON): Government of Canada.
[HSDB] Hazardous Substances Data Bank [database]. 1983- . Bethesda (MD): National Library of Medicine (US). [accessed 2018 June 12].
[HSDB] Hazardous Substances Data Bank [database]. 1983- 2018. Search results for Trixylyl Phosphate CAS RN 25155-23-1. Bethesda (MD): National Library of Medicine (US). [updated 2013 June 17; accessed 2018 June 15].
Kademoglou K, Xu F, Padilla-Sanchez JA, Haug LS, Covaci A, Collins CD. 2017.Legacy and alternative flame retardants in Norwegian and UK indoor environment: Implications of human exposure via dust ingestion. Environ Int. 102:48-56
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2016 Aug 10]. Ottawa (ON): Government of Canada. [accessed 2017 Oct].
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2017 Oct 23]. Ottawa (ON): Government of Canada. [accessed 2017 Oct].
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme. 2017. Human health Tier II assessment for xylyl phosphate esters (CAS No 25155-23-1). Australian Government Department of Health. [Accessed June 2018].
[OECD] Organisation for Economic Co-operation and Development. 2004. Emission scenario document on lubricants and lubricant additives. [PDF] Paris (FR): OECD, Environment Directorate. (Series on Emission Scenario Documents No. 10; Report No.: ENV/JM/MONO(2004)21, JT00174617). [accessed 2018 August 27].
OECD QSAR Toolbox. [read across tool]. 2016. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
PubChem [database]. 2004- . Bethesda (MD): US National Library of Medicine, National Center for Biotechnology Information. [accessed 2018 June 15].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu. 2006. General Fact Sheet: Limiting conditions and reliability, ventilation, room size, body surface area. Updated version for ConsExpo 4 [Internet]. Report No.: 320104002/2006. Bilthoven (NL): RIVM (National Institute for Public Health and the Environment) (PDF).
Saliya RG, Yokoyama A. 2017. US patent number: US9540533B2, US Patent and Trademark Office.
SDS. 2010. Weir Parts Center Material Safety Data Sheet. [PDF] Binder 1. Weir Parts Center.
Sormani P, Sunkara H, O’Neil J, Kurian J. 2004. US patent number: US20040185263A1, US Patent and Trademark Office.
[US EPA] United States Environmental Protection Agency. 1986. Standard scenarios for estimating exposure to chemical substances during use of consumer products, vol 1 & 2. Washington (DC): prepared for the US EPA, Office of Toxic Substances, Exposure Evaluation Division, by Versar, Inc. Contract no. 68-02-3968.
[US EPA] United States Environmental Protection Agency. 1987. Methods for Assessing Exposure to Chemical Substances Volume 7 Methods for Assessing Consumer Exposure to Chemical Substances. Washington (DC): prepared for the US EPA, Office of Toxic Substances, Exposure Evaluation Division, by Patricia D. Jennings, Karen A. Hammerstrom, Leslie Coleman Adkins, Thompson Chambers, Douglas A. Dixon. Contract no. 68-02-3968.
[US EPA] US Environmental Protection Agency. 2011. Chapter 6: Inhalation Rates. Exposure Factors Handbook 2011 Edition (Final). U.S. Environmental Protection Agency: Washington, DC. EPA/600/R-09/052F.
[Wilson and Meridian] Wilson Scientific Consulting Inc. and Meridian Environmental Inc. 2006. Critical review of soil ingestion rates for use in contaminated site human health risk assessments in Canada. Contractor report prepared for the Contaminated Sites Division, Safe Environments Programme, Health Canada: Ottawa (ON).
Wypch G, editor. 2015. Handbook of UV Degradation and Stabilization (Second Edition). ChemTec Publishing. Pages 121
| Route of exposure | 0 to 5 monthsa (breast milk-fed)b | 0 to 5 monthsa (formula fed)c | 6 to 11 monthsd | 1 yeare | 2 to 3 yearsf | 4 to 8 yearsg | 9 to 13 yearsh | 14 to 18 yearsi | Greater than or equal to 19 yearsj |
|---|---|---|---|---|---|---|---|---|---|
| Airk | 1.77E-03 | 1.77E-03 | 1.79E-03 | 2.20E-03 | 1.85E-03 | 1.46E-03 | 9.99E-04 | 7.75E-04 | 6.16E-04 |
| Drinking waterl | N/A | 0.91 | 0.58 | 0.23 | 0.20 | 0.16 | 0.12 | 0.12 | 0.14 |
| Food and beveragesm | N/I | N/I | N/I | N/I | N/I | N/I | N/I | N/I | N/I |
| Soiln | N/A | N/A | 1.22E-5 | 1.22E-5 | 6.28E-6 | 5.75E-6 | 2.50E-6 | 3.43E-7 | 3.29E-7 |
| Dusto | 1.84E-3 | 1.84E-3 | 1.59E-3 | 1.71E-3 | 7.66E-4 | 5.70E-4 | 3.04E-4 | 1.82E-5 | 1.89E-5 |
| Total intake | 3.61E-3 | 0.91 | 0.59 | 0.23 | 0.20 | 0.16 | 0.12 | 0.12 | 0.14 |
Abbreviations: N/A, not applicable; N/I, data not identified in the literature.
a Assumed to weigh 6.3 kg (Health Canada 2015b), to breathe 3.7 m3 of air per day (US EPA 2011 [modified]), and to ingest 21.6 mg of dust per day (Wilson and Meridian 2015 [modified]). It is assumed that no soil ingestion occurs due to typical caregiver practices.
b Exclusively for breast milk-fed infants, assumed to consume 0.744 L of breast milk per day (Health Canada 2018), and breast milk is assumed to be the only dietary source.
c Exclusively for formula-fed infants, assumed to drink 0.826 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote on drinking water for details.
d Assumed to weigh 9.1 kg (Health Canada 2015b), to breathe 5.4 m3 of air per day (US EPA 2011 [modified]), to drink 0 L of water per day (Health Canada 2017), to ingest 7.3 mg of soil per day, and to ingest 27.0 mg of dust per day (Wilson and Meridian 2015 [modified]). For breast milk-fed infants, assumed to consume 0.632 L of breast milk per day (Health Canada 2018). For formula-fed infants, assumed to drink 0.764 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote on drinking water for details.
e Assumed to weigh 11.0 kg (Health Canada 2015b), to breathe 8.0 m3 of air per day (US EPA 2011 [modified]), to drink 0.36 L of water per day (Health Canada 2017), to ingest 8.8 mg of soil per day, and to ingest 35.0 mg of dust per day (Wilson and Meridian 2015 [modified]).
f Assumed to weigh 15 kg (Health Canada 2015b), to breathe 9.2 m3 of air per day (US EPA 2011 [modified]), to drink 0.43 L of water per day (Health Canada 2017), to ingest 6.2 mg of soil per day, and to ingest 21.4 mg of dust per day (Wilson and Meridian 2015 [modified]).
g Assumed to weigh 23 kg (Health Canada 2015b), to breathe 11.1 m3 of air per day (US EPA 2011 [modified]), to drink 0.53 L of water per day (Health Canada 2017), to ingest 8.7 mg of soil per day, and to ingest 24.4 mg of dust per day (Wilson and Meridian 2015 [modified]).
h Assumed to weigh 42 kg (Health Canada 2015b), to breathe 13.9 m3 of air per day (US EPA 2011 [modified]), to drink 0.74 L of water per day (Health Canada 2017), to ingest 6.9 mg of soil per day, and to ingest 23.8 mg of dust per day (Wilson and Meridian 2015 [modified]).
i Assumed to weigh 62 kg (Health Canada 2015b), to breathe 15.9 m3 of air per day (US EPA 2011 [modified]), to drink 1.09 L of water per day (Health Canada 2017), to ingest 1.4 mg of soil per day, and to ingest 2.1 mg of dust per day (Wilson and Meridian 2015 [modified]).
j Assumed to weigh 74 kg (Health Canada 2015b), to breathe 15.1 m3 of air per day (US EPA 2011 [modified]), to drink 1.53 L of water per day (Health Canada 2017), to ingest 1.6 mg of soil per day, and to ingest 2.6 mg of dust per day (Wilson and Meridian 2015 [modified]).
k Empirical data were not available; therefore, the concentration of trixylyl phosphate in air was estimated to be 3.02 x 10-3 µg/m3 using ChemCAN (2003) and the upper-end volume data from Table 4-1 (i.e., 1 000 000 kg).
l Empirical data were not available; therefore, the concentration of trixylyl phosphate in drinking water was estimated to be 6.95 µg/L using the EAU Drinking Water Spreadsheet (Health Canada 2015a) and the upper-end volume data from Table 4-1 (i.e., 1 000 000 kg).
m No data were identified for trixylyl phosphate in food.
n Empirical data were not available; therefore, the concentration of trixylyl phosphate in soil was estimated to be 15.20 ng/g using ChemCAN (2003) and the upper-end volume data from Table 4-1 (i.e., 1 000 000 kg).
o No Canadian data on levels of trixylyl phosphate in dust were identified. The highest concentration of 537 (µg/kg) of trixylyl phosphate measured in 10 samples of house dust in Reading, United Kingdom was used (Kademoglou et al. 2017).
Appendix B. Parameters used to estimate exposures
Exposure estimates were calculated based on a default body weight of 74 kg for adults (19 years and older) (Health Canada 2015b). The estimated dermal exposure parameters are described in Table B-1.
| Exposure scenario | Assumptions |
|---|---|
| Power steering fluid | Maximum reported concentration: 1% Scenario: Dermal exposure to fluid during transfer to vehicle Thin-film thickness estimation with the following default values: Estimate dose (adapted from US EPA 1987) for one event = (6 cm2 ) (0.015 88 cm) (0.88 g/cm3) (0.01) (1)/ 74 kg =1.13 x 10-5 g/kg-bw/day or 0.0113 mg/kg-bw/day |
Appendix C. Probable daily intake estimates from migration data for the use of CAS RN 119345-01-6 in food packaging materials.
| Age group | Body weight (kg) | PDI µg/kg bw/day |
|---|---|---|
| 0-5 mo | 6.3 | N/A |
| 6-11 mo | 9.1 | N/A |
| 1 yr | 11 | N/A |
| 2-3 yr | 15 | 23.37 |
| 4-8 yr | 23 | 16.34 |
| 9-13 yr | 42 | 9.73 |
| 14-18 yr | 62 | 12.39 |
| 19+ yr | 74 | 10.38 |
Abbreviations: N/A, not applicable.
