Draft screening assessment - piperazine
Official title: Draft screening assessment - piperazine
Chemical Abstracts Service Registry Number
110-85-0
Environment and Climate Change Canada
Health Canada
March 2021
Synopsis
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of piperazine. The Chemical Abstracts Service Registry Number (CAS RNFootnote 1) for piperazine is 110-85-0. This substance was identified as a priority for assessment on the basis of other human health concerns.
Piperazine does not occur naturally in the environment. According to information obtained from a survey issued pursuant to section 71 of CEPA, for the 2008 reporting year, respondents indicated that no company manufactured the substance above the 100 kg reporting threshold. However, between 10 000 and 100 000 kg of piperazine was imported into Canada for commercial use in paints and coatings, and as a chemical intermediate in industrial settings, including use in carbon capture and storage systems. Information obtained from other programs within Health Canada as well as product safety data sheets identified additional uses in Canada, including as a medicinal ingredient in human and veterinary antiparasitic drugs, and as a co-monomer in epoxy adhesives. It is possibly used as a flavouring agent in foods sold in Canada.
The ecological risk of piperazine was characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, piperazine is considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from piperazine. It is proposed to conclude that piperazine does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Piperazine was not detected in a Canadian indoor air study and no other monitoring data of piperazine in the environment was identified. Piperazine is expected to partition to water if released to the environment and is not expected to be stable in air. Consequently, estimates of piperazine exposure to Canadians from environmental media were calculated based on potential wide-disperse releases to surface water and point source releases to air.
Neurological effects were identified by the Organisation for Economic Co-operation and Development and in the European Union Risk Assessment Report as the critical health effect, based on clinical reports and studies of humans receiving piperazine administered as an antiparasitic drug. Piperazine is also classified by the European Chemicals Agency as a reproductive toxicant and a respiratory sensitizer.
Piperazine may be released to the environment (i.e., air and water), when used in industrial applications, including use in carbon capture and storage systems (also referred to as gas scrubbers). Based on a comparison of the estimates of exposure to piperazine from environmental media, and levels at which critical effects are observed, margins are considered to be adequate to address uncertainties in the health effects and exposure databases. On the basis of the Joint Food and Agriculture Organization of the United Nations and World Health Organization Expert Committee on Food Additives per capita intake estimate for the population of the United States, exposure to Canadians from piperazine and its use as a food flavouring agent is considered negligible and the risk to human health is considered to be low.
Exposures to piperazine for the general population of Canada can occur from its use in epoxy adhesive products available to consumers. Based on a comparison of estimated dermal and inhalation exposures to piperazine with levels at which critical effects are observed, margins are considered adequate to address uncertainties in the health effects and exposure databases.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that piperazine does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that piperazine does not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of piperazine to determine whether this substance presents or may present a risk to the environment or to human health. This substance was identified as a priority for assessment on the basis of other human health concerns (ECCC, HC [modified 2017]).
The ecological risk of piperazine was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
The substance currently being evaluated has been reviewed internationally through the Organisation for Economic Co-operation and Development (OECD), and there is an OECD Screening Information Dataset (SIDS) Initial Assessment Profile (SIAP) available. These assessments undergo rigorous review (including peer-review) and endorsement by international governmental authorities. Health Canada and Environment and Climate Change Canada are active participants in these processes, and consider these assessments to be reliable. The final assessment report from the OECD process was published as a European Union Risk Assessment Report (EU RAR). The OECD SIAP and EU RAR were used to inform the health effects characterization in this screening assessment.
This draft screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to August 2019. Empirical data from key studies as well as results from models were used to reach proposed conclusions.
This draft screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period.
This draft screening assessment focuses on information critical to determining whether a substance meets the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precaution.Footnote 2 This draft screening assessment presents the critical information and considerations on which the proposed conclusion is based.
2. Substance identity
The Chemical Abstracts Service Registry Number (CAS RNFootnote 3 ), and Domestic Substances List (DSL) name for piperazine are presented in Table 2‑1.
| CAS RN | DSL name | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 110-85-0 | Piperazine | 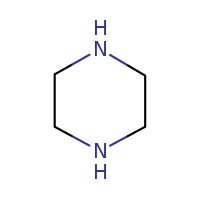 C4H10N2 C4H10N2 | 86.1 |
3. Physical and chemical properties
A summary of physical and chemical properties of piperazine is presented in Table 3‑1. Additional physical and chemical properties are reported in ECCC (2016b).
| Property | Value | Key reference |
|---|---|---|
| Physical state | Solid | ECHA c2007-2019 |
| Melting point (°C) | 106 @ 101.325 kPa | ECHA c2007-2019 |
| Vapour pressure (Pa) | 39 | ECHA c2007-2019 |
| Water solubility (mg/L) | 150 000 | ECHA c2007-2019 |
| Log Kow (dimensionless) | -1.24 | ECHA c2007-2019 |
| pKa1 (dimensionless) | 9.7 | ECHA c2007-2019 |
| pKa2 (dimensionless) | 5.3 | ECHA c2007-2019 |
Abbreviations: Kow, octanol-water partition coefficient; pKa, acid dissociation constant.
4. Sources and uses
Piperazine was included in a survey issued pursuant to section 71 of CEPA (Canada 2009). The substance was not reported to be manufactured above the reporting threshold of 100 kg during the 2008 calendar year in Canada. Total import quantities during that same period were reported in the range of 10 000 to 100 000 kg for commercial use in paints and coatings, and as a chemical intermediate in industrial settings, including use in carbon capture and storage systems (Environment Canada 2009). The substance is also used in industrial adhesives (SDS 2017b; SDS 2017c; SDS 2019), plastic bonder epoxy (SDS 2016) and automotive adhesive (SDS 2017a) available to consumers as part of automotive repair kits. In Canada, piperazine is also used as a medicinal ingredient in one human and seven veterinary antiparasitic drugs (personal communication, email from the Therapeutic Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated January 2019) and as a component in coatings used in flooring applications in food processing establishments (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated February 2019). No definitive information is available concerning the potential use of piperazine as a food flavouring agent in foods sold in Canada. However, since this substance is known to be used as a food flavouring agent internationally, it is possible that it is present as a flavouring agent in foods sold in Canada (personal communication, email from Food Directorate, Health Canada to Safe Environments Directorate, Health Canada, dated February 2019; unreferenced).
Internationally, piperazine is used in the manufacture of polyamide resins to modify certain physical properties of the polyamide, such as melting point. Polyamide resins may be used in hot-melt adhesives, and as binders used in flexographic printing (Product brochure 2007); however, piperazine is incorporated into the polyamide structure with only trace amounts remaining (EU 2005).
5. Potential to cause ecological harm
The ecological risk of piperazine was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data for physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox 2014), and from responses to surveys issued pursuant to section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over and under classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for piperazine and the hazard, exposure and risk classification results are presented in ECCC (2016b).
On the basis of low hazard and low exposure classifications according to information considered under ERC, piperazine was classified as having a low potential for ecological risk. It is unlikely that this substance is resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
6.1.1 Environmental media and food
Piperazine was not detected in a study of indoor air sampled from 50 residential homes in Quebec City conducted from 2008 to 2010 (NRC 2011). No other Canadian or international environmental monitoring data for piperazine were identified.
Piperazine may be released to the environment when used in industrial applications, including use in carbon capture and storage systems (also referred to as gas scrubbers). Gas scrubbers remove carbon dioxide from industrial flue gases by passing the flue gas through a solvent blend of amines containing piperazine. The amine functional groups react readily with carbon dioxide to produce (in the case of piperazine) a piperazine-carbamate. While the cleaned flue gas is released into the atmosphere, the carbamate is circulated to a heating chamber where the piperazine is regenerated for reuse and the carbon dioxide is diverted to storage. During this process, formation of the piperazine-carbamate reduces the amount of free piperazine in the scrubber solvent (and thereby the partial-pressure/volatility of piperazine) resulting in reduced fugitive emissions of piperazine (Closmann et al. 2009; Shell 2013).
Potential exposure to Canadians living in the vicinity of industrial facilities with gas scrubbers have been modelled as a point source release, as piperazine has a calculated half-life in air of 0.8 hours and is not expected to disperse widely in the atmosphere. A maximum 1-hour concentration of 2.67 µg/m3 (at a distance of 1200 m from the source) was derived using SCREEN3 (2013), to estimate air intakes for residents within the vicinity of an industrial facility equipped with a gas scrubber. Input parameters and outputs for SCREEN3 dispersion modelling scenarios are described in Appendix A.
Piperazine is unstable in air and is unlikely to result in exposures beyond those near a point source release. However, it is highly soluble and stable in water. Therefore, industrial releases to surface water have the potential to travel further distances and have the potential to enter drinking water systems. To determine potential environmental exposure to piperazine by the general population of Canada (not necessarily in the vicinity of industrial gas scrubbers), estimates were derived based on potential wide-disperse releases to surface water using the Environmental Assessment Unit’s Drinking Water Workbook (Health Canada 2015a). Input parameters included the maximum total import quantities received in response to a survey issued pursuant to section 71 of CEPA (i.e., 100 000 kg), a conservative loss percent release to wastewater of 5% (for industrial release scenarios), a removal percentage by wastewater treatments systems of 83% (ECCC 2016b), and a receiving water body flow rate of 0.823 m3/s. The resulting estimate of the concentration of piperazine in surface water was 48.6 µg/L and was used to estimate exposure to piperazine from drinking water consumption.
No reports of piperazine in Canadian foods were identified. In Canada, piperazine is used as a component in coatings for flooring in food processing establishments. Exposure to piperazine by the general population is not expected to occur from this use (personal communication, email from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated February 2019).
No definitive information is available concerning the potential use of piperazine in foods sold in Canada. However, since this substance is recognized as a food flavouring agent internationally, it is possible that it is present as a flavouring agent in foods sold in Canada. The Joint (Food and Agriculture Organization/World Health Organization [FAO/WHO]) Expert Committee on Food Additives (JECFA) evaluated piperazine for its use as a food flavouring agent and estimated the per capita intake of this substance to be 0.09 µg/day (0.001 µg/kg bw/day based on a 60 kg body weight) for the United States population 1 year old and older. This estimate was based on annual production volumes reported by the food industry (EFSA 2015; Gavin 2007 as presented in JECFA 2009). JECFA determined that this substance presents no safety concern at current levels of intake when used as a flavouring agent. In the absence of data on the actual use, if any, of piperazine as a food flavouring agent in foods sold in Canada, the JECFA (2009) per capita intake estimate for the United States population is considered an acceptable estimate of potential Canadian dietary exposure to this substance from this use in food. Estimated aggregated exposure to piperazine from environmental media and food for the general population ranged from 1.54 to 7.94 µg/kg bw/day, with formula-fed infants less than 6 months of age having the greatest estimated exposure, predominantly from drinking water (see Appendix B for input parameters and estimates of daily intake).
6.1.2 Products available to consumers
When used as a medicinal ingredient in veterinary drugs, treatment for domestic animals usually involves the oral administration of up to two tablets, monthly. When administered in liquid form, the drug is applied directly onto the animal’s feed on a similar schedule. Potential human exposure is considered to be low for this use, given the short handling time, minimal contact with the medication, and frequency of administering the antiparasitic.
Piperazine may also be used in automotive repair kits (SDS 2017a), and plastic bonder epoxy intended for smaller projects (SDS 2016). When formulated into epoxy adhesives, piperazine is reported to be present at concentrations less than 1%, and to be entirely consumed upon curing. All consumer epoxy adhesive products identified as being formulated with piperazine come supplied with a pre-loaded syringe equipped to deliver parts A and B of the epoxy simultaneously. The two-part epoxy mixes during application as a result of multiple, offset baffles located in the interior of the syringe barrel. As a result, potential exposure to piperazine is eliminated during the mixing/loading stage and the potential for exposure during application is reduced. The two-component glue fact sheet (RIVM 2007) on ConsExpo Web (2019) was used to estimate exposure to piperazine via the dermal and inhalation routes (see Appendix C for scenario details and input parameters). The resulting estimated systemic exposures to an adult 19 years of age or older to piperazine from an epoxy glue used for automotive repairs (the sentinel scenario) was 33.4 µg/kg bw/event (via inhalation route) and 6.8 µg/kg bw/event (via dermal route).
6.2 Health effects assessment
The OECD SIAP (OECD 2004), which was presented at the OECD SIAMFootnote 4 18, and the EU RAR (EU 2005) were used to inform the health effects characterization for this screening assessment.
A literature search was conducted from the year prior to the OECD SIAM 18, from January 2003 to August 2019. No health effects studies that could impact the risk characterization as published by the OECD (2004) and EU (2005) were identified. Piperazine has been classified by the European Chemicals Agency (ECHA c2007-2019) as a reproductive toxicant (Repr 2), as well as a category 1 skin and respiratory sensitizer (ECHA 2008).
The EU RAR (2005) indicated that piperazine is readily absorbed through the gastrointestinal tract in pigs. The principal route of excretion of piperazine and its metabolites is through urine. The kinetics of uptake and excretion in humans and pigs were found to be similar (OECD 2004). Because there are no data on uptake via dermal or inhalation routes of exposure, 100% absorption was assumed for both routes (EU 2005).
Based on the evaluation of data on the use of piperazine as an antiparasitic in humans, the EU (EU 2005) identified an acute lowest observed adverse effect level (LOAEL) of 110 mg/kg for neurological effects (EU 2005, OECD 2004). The critical study used to derive the acute LOAEL was from Padelt et al. (1966). This study was conducted on 89 children (41 boys and 48 girls), who were treated with piperazine following diagnosis of pinworm infection. Piperazine was administered orally at a dose of 90 to 130 mg/kg twice in one day, with doses administered 12 hours apart. Changes in electroencephalographic (EEG) measures were taken one day before and after treatment. Abnormalities in EEG readings were observed in 33 of 89 children. However, because no other signs of neurotoxicity were observed, the degree of adversity related to the EEG abnormalities is expected to be minimal (EU 2005). Consequently, this was not considered to be a key study for risk characterization in the current assessment.
The EU (2005) identified a LOAEL of 30 mg/kg bw/day for piperazine based on neurological effects observed in human patients treated orally for 3 to 7 days. The EU (2005) derived this LOAEL based on an evaluation of a number of clinical reports and studies dealing with neurological findings, including abnormal effects on EEG measures. The assessment from the OECD (2004) indicates that this LOAEL is based on “documentation of (rare cases) of neurotoxicity from human clinical practice”. The EU (2005) used the LOAEL of 30 mg/kg bw/day as the key repeat dose toxicity study result for characterizing risk for humans exposed via the environment (e.g. drinking water, air), as well as for occupational exposures (EU 2005)Footnote 5.
Piperazine is not expected to be genotoxic. The substance tested negative in the Ames assay and in the in vitro Mammalian Chromosome Aberration test (Chinese hamster ovary cells), with and without metabolic activation. Piperazine also tested negative in the in vivo micronucleus assay (OECD 2004). It was further determined that piperazine is unlikely to be carcinogenic (OECD 2004).
Piperazine has been identified and classified as a reproductive toxicant. The basis for this classification is from a two-generation study in Sprague Dawley CD rats (OECD test guideline 416) by Wood and Brooks (1994), which was also identified as the key reproductive study by the OECD (2004). In this study, a no observed adverse effect level (NOAEL) of 125 mg/kg bw/day was identified based on effects on fertility at 300 mg/kg bw/day (EU 2005; OECD 2004). The LOAEL of 300 mg/kg bw/day was identified based on reduced pregnancy index, decreased number of implantation sites, and decreased litter sizes observed in rats at this dose (EU 2005). Developmental effects were observed in a study in rabbits; however, effects were only observed at doses that were maternally toxic (EU 2005; Ridgeway 1987).
Based on animal data, as well as human observations and epidemiological studies from occupational settings, exposure to piperazine and its salts have been demonstrated to cause allergic dermatitis as well as respiratory sensitisation, but no points of departure could be determined from these studies (EU 2005).
6.3 Characterization of risk to human health
Piperazine has been classified by ECHA as a suspected reproductive toxicant (category 2) under the Global Harmonized System of Classification and Labelling of Chemicals (GHS). ECHA has also classified piperazine as a category 1 skin and respiratory sensitizer (ECHA 2008).
The critical effect for repeat-dose toxicity was determined to be a LOAEL of 30 mg/kg bw/day, based on neurological effects identified from the evaluation of a number of clinical reports and studies undertaken as part of the EU RAR (EU 2005).
Exposure of the general population to piperazine may occur through environmental media and dietary intake. The estimated systemic exposure to piperazine from environmental media and dietary intake was in the range of 1.54 to 7.94 µg/kg bw/day, with formula-fed infants less than 6 months of age having the highest estimated exposure, predominantly from drinking water (see Appendix B for details). Comparison of the estimated oral exposures to piperazine with the critical effect dose level (30 mg/kg bw/day) results in margins of exposure (MOEs) of 3700 or higher. The MOEs are considered adequate to address uncertainties in the health effects and exposure databases. As the neurological endpoint is lower than the NOAEL of 125 mg/kg bw/day identified for reproductive toxicity, the calculated MOEs are also adequate for this effect.
Acute exposure to piperazine by the general population of Canada may also occur through the use of certain epoxy adhesives available to consumers. Exposures from this use were estimated to be 33.4 µg/kg bw/event (inhalation), and 6.8 µg/kg bw/event (dermal), for adults 19 years of age or older. Comparison of the combined estimated systemic exposures with the critical effect dose level (30 mg/kg bw/day) results in a MOE of 746. The MOE for reproductive effects would be even larger. The MOEs for both neurological and reproductive effects are considered adequate to address uncertainties in the health effects and exposure databases. While exposure of the general population to piperazine is not of concern at current levels, this substance is considered to have a health effect of concern on the basis of its potential to cause reproductive effects. Therefore, there may be a concern for human health if exposures were to increase.
6.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
Table 6‑1. Sources of uncertainty in the risk characterization
| Key source of uncertainty | Impact |
|---|---|
| There is a lack of Canadian occurrence data for piperazine in foods; therefore, values used to derive dietary intakes of piperazine were taken exclusively from JECFA (2009) using a maximized survey-derived intake (MSDI) approach.a | +/- |
| There is a lack of Canadian occurrence data for piperazine in drinking water; therefore, values used to derive oral exposure from drinking water were modelled based on the maximum reported quantity in-commerce in Canada. | + |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over or under estimation of exposure/risk
a JECFA estimated exposure from possible use as a flavouring using the MSDI approach, which is based on the reported amount of the flavouring agent introduced into the food supply per year in a specific region (in this case, the United States). It is corrected for under-reporting by assuming that only 80% of the annual production amount was reported, and assumes that 10% of the relevant population would consume foods containing the flavouring agent.
7. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from piperazine. It is proposed to conclude that piperazine does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that piperazine does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that piperazine does not meet any of the criteria set out in section 64 of CEPA.
References
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2009. Canadian Environmental Protection Act, 1999: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 143, no. 40, p. 2945-2956.
Closmann F, Nguyen T, Rochelle GT. 2009. MDEA/Piperazine as a solvent for CO2 capture [PDF]. Energy Procedia. Vol. 1, no. 1, p. 1351-1357.
[ConsExpo Web] Consumer Exposure Web Model. 2019. Ver. 1.0.6. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. [accessed 2019 Aug 27].
Curry P, Kramer G, Newhook R, Sitwell J, Somers D, Tracy B, Oostdam JV. 1993. Reference values for Canadian populations. Prepared by the Environmental Health Directorate Working Group on reference values. Health Canada. 1988 (updated in 1993).
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2019 September 9].
[ECHA] European Chemicals Agency. c2007-2019. Registered substances database; search results for CAS RN 110-85-0. Helsinki (FI): ECHA. [updated 2019 Jul 29; accessed 2019 Aug 22].
[ECHA] European Chemicals Agency. 2008. Summary of Classification and Labelling [for CAS RN 110‑85‑0]. Helsinki (FI): ECHA. [accessed 2020 Dec 2].
[EFSA] European Food Safety Authority. 2015. Scientific Opinion on Flavouring Group Evaluation 86, Revision 2 (FGE.86Rev2): Consideration of aliphatic and arylalkyl amines and amides evaluated by JECFA (65th meeting). EFSA Journal: 13(1). 3998.
[EFFA] European Flavour and Fragrance Association. 2005. European inquiry on volume use. Private communication to the Flavor and Extract Manufacturers Association, Washington, DC, USA. Submitted to WHO by the International Organization of the Flavor Industry, Brussels, Belgium.
Environment Canada. 2009. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[EU] European Union. 2005. EU Risk Assessment Report (RAR): Piperazine CAS No 110-85-0. Final report. Brussels (BE): EU. [accessed 2019 Aug 20].
Gavin CL, Williams MC, Hallagan JB. 2007. FEMA 2005 poundage and technical effects update survey. Washington (DC): Flavor and Extract Manufacturers Association.
Health Canada. 1996. Labelling Standard- Drug Product: Anthelmintics [PDF]. Ottawa (ON): Government of Canada [accessed 2019 Oct 1].
Health Canada. 2015a. Environmental Assessment Unit drinking water spreadsheets. [Excel format]. Ottawa (ON): Government of Canada. [accessed 2019 Aug 26].
Health Canada. 2015b. Food Consumption Table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004), Share file. Ottawa (ON): Government of Canada.
Health Canada. 2017. Water Consumption Table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004). Share file. Ottawa (ON): Government of Canada.
Health Canada. 2018. Draft backgrounder document on default values for breast milk and formula intakes. Unpublished report. Ottawa (ON): Government of Canada.
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2006. Evaluation of Certain Food Additives. WHO Technical Report Series No. 934. Sixty-fifth report. Geneva (CH): World Health Organization. [accessed 26 Aug 2019].
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2009. Evaluation of Certain Food Additives. WHO Technical Report Series No. 952. Sixty-ninth report. Geneva (CH): World Health Organization. [accessed 2019 Oct 25].
[NRC] National Research Council. Won D, Lusztyl E. 2011. Data gathering on chemicals released to indoor air of residences from building materials and furnishings. Final Report. Ottawa (ON): National Research Council Canada. Report No.: B3332.2.
[OECD] Organisation for Economic Co-operation and Development. 2004. SIDS Initial Assessment Profile (SIAP). Piperazine. Paris (FR): OECD, Environment Directorate. SIAM 18, 20-23 April 2004. [accessed 2019 Aug 20].
OECD QSAR Toolbox [Read-across tool]. 2014. Version 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Product brochure. 2007. Ethyleneamines A global profile of products and services [PDF]. The Woodlands (TX): Huntsman Corporation [accessed 2021 Jan 28].
Padelt B, Bruhn B, Nicolai A. 1966. Das Hirnstrombild vor und nach Kurzzeitbehandlung der Enterobiasis mit Piperazinderivaten. Padiatr. Grenzgeb. 5, 1-9. [cited in EU 2005].
Ridgway P. 1987. Piperazine phosphate. Rabbit teratology study. Report to Reckitt and Coleman from Toxicol Laboratories Ltd., Ledbury, Herefordshire. Unpublished. [cited in EU 2005].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2007. Do-it-yourself products fact sheet: to assess the risks for the consumer [PDF]. Bilthoven (NL): RIVM. Report No.: 320104007/2007. [accessed 2019 Aug 27].
SCREEN3 [computer model]. 2013. Ver. 3.5.0. Research Triangle Park (NC): US Environmental Protection Agency, Office of Air Quality Planning and Standards, Emissions, Monitoring, and Analysis Division.
[SDS] Safety Data Sheet. 2016. Plastic Bonder (Black) – Syringe – Parts B [PDF]. Sulphur Springs (TX): J-B Weld Company, LLC [accessed 2019 Jul 15].
[SDS] Safety Data Sheet. 2017a. 3M Universal Adhesive Plastic bonding adhesive [PDF]. London (ON): 3M Canada Company [accessed 2019 Jun 12].
[SDS] Safety Data Sheet. 2017b. LOCTITE E-30UT Epoxy [PDF]. Mississauga (ON): Henkel Canada Corporation [accessed 2019 Jun 5].
[SDS] Safety Data Sheet. 2017c. PF7770 Pliogrip Urethane structural adhesive [PDF]. Milton (ON): Pro Form Products Ltd. [accessed 2019 Jun 5].
[SDS] Safety Data Sheet. 2019. Plastic bonding adhesive [PDF]. Oakville (ON): Ford Motor Company of Canada [accessed 2019 Jun 5]
Shell. 2013. Shell global solutions technology portfolio. Hague (NL): Shell Inc. [accessed 2020 Jan 10].
SimpleTreat [sewage treatment plant removal model]. 1997. Ver. 3.0. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu (RIVM) [National Institute for Public Health and the Environment], Laboratory for Ecological Risk Assessment.
[US EPA] United States Environmental Protection Agency. 1992 [modified]. Screening procedures for estimating the air quality impact of stationary sources [PDF]. Revised. Research Triangle Park (NC): US EPA, Office of Air and Radiation, Office of Air Quality Planning and Standards. EPA-454/R-92-019. [accessed 2019 Nov 26].
[US EPA] United States Environmental Protection Agency. 1999. Estimates of stack heights and exit gas velocities for TRI facilities in OPPT’s risk-screening environmental indicators model [PDF]. Washington (DC): US EPA, Office of Pollution Prevention and Toxics.[accessed 2019 Nov 26].
[US EPA] United States Environmental Protection Agency. 2011 [modified]. Chapter 6: Inhalation Rates. Exposure Factors Handbook 2011 Edition (Final) [PDF]. Washington (DC): US EPA. EPA/600/R-09/052F.
Wood E, Brooks PN. 1994. Piperazine hydrochloride: Dietary two generation reproduction study in the rat. Report to Akzo Nobel from Safepharm Laboratories Ltd., Derby. Unpublished. [cited in EU 2005].
Appendix A. Input parameters to SCREEN3 for dispersion modelling and estimated piperazine concentrations in the vicinity of industrial gas scrubbers
| Input parameter | Parameter value |
|---|---|
| Source type | Point |
| Emission ratea (g/s) | 0.317 |
| Source release heightb (m) | 15 |
| Stack inside diameterb (m) | 0.1 |
| Stack gas exit velocityc (m/s) | 4.0 |
| Emission temperatureb (K) | 358 |
| Ambient air temperatured (K) | 293 |
| Receptor heighte (m) | 1.74 |
| Urban-rural optiond | Urban |
| Consider building downwash | No |
| Consider terrain above stack height | No |
| Consider terrain above stack base | No |
| Meteorologyd | 1 (full meteorology) |
| Minimum and maximum distance to useb | 1200 to 5000 m |
| Adjustment factor for annual exposuref | 0.2 |
a Based on an assumed emission rate of 10 000 kg/year (10% of volume [professional judgement] reported in-commerce in Canada) (Environment Canada 2009) occurring over 365 days (at 24 hours per day).
b Professional judgement.
c Professional judgement; using the median exit gas velocity reported in US EPA (1999) resulting in the greatest release estimate.
d Default value in SCREEN3.
e Curry et al. (1993).
f An adjustment factor of 0.2 is used for estimation of maximum piperazine concentration over a one-year period, based on the resultant SCREEN3 output (which is an estimate for a 1-hour period). This factor takes into account temporal variations in wind and meteorological conditions (US EPA 1992 [modified]).
| Distance (m) | 1 hour concentration (µg/m3)a | Annual concentration (µg/m3)b |
|---|---|---|
| 1200 | 13.36 | 2.67 |
| 1300 | 11.93 | 2.39 |
| 1400 | 10.75 | 2.15 |
| 1500 | 9.76 | 1.95 |
| 2000 | 6.56 | 1.31 |
| 2500 | 4.87 | 0.97 |
| 3000 | 3.84 | 0.77 |
| 4000 | 2.66 | 0.53 |
| 5000 | 2.03 | 0.41 |
a SCREEN3 (2013).
b An adjustment factor of 0.2 is used for estimation of maximum piperazine concentration over a one-year period, based on the resultant SCREEN3 output (which is an estimate for a 1-hour period). This factor takes into account temporal variations in wind and meteorological conditions and is based on professional judgement (US EPA 1992 [modified]).
Appendix B. Estimated daily intake from oral exposure to piperazine to humans
| Route of exposure | 0 to 5 monthsa(breast milk-fed)b | 0 to 5 monthsa (formula fed)c | 6 to 11 monthsd | 1 yeare | 2 to 3 yearsf | 4 to 8 yearsg | 9 to 13 yearsh | 14 to 18 yearsi | Greater than or equal to 19 yearsj |
|---|---|---|---|---|---|---|---|---|---|
| Airk | 1.57 | 1.57 | 1.59 | 1.94 | 1.63 | 1.29 | 0.88 | 0.69 | 0.55 |
| Drinking waterl | N/A | 6.37 | 4.08 | 1.59 | 1.39 | 1.12 | 0.86 | 0.85 | 1.00 |
| Food and beveragesm | N/A | N/A | N/A | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 |
| Total intake | 1.57 | 7.94 | 5.66 | 3.53 | 3.03 | 2.41 | 1.74 | 1.54 | 1.55 |
Abbreviations: N/A, not applicable.
a Assumed to weigh 6.3 kg (Health Canada 2015b).
b Exclusively for breast milk-fed infants, assumed to consume 0.744 L of breast milk per day (Health Canada 2018), and breast milk is assumed to be the only dietary source.
c Exclusively for formula-fed infants, assumed to drink 0.826 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote on drinking water for details.
d Assumed to weigh 9.1 kg (Health Canada 2015b). For breast milk-fed infants, assumed to consume no drinking water and 0.632 L of breast milk per day (Health Canada 2018). For formula-fed infants, assumed to drink 0.764 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote on drinking water for details.
e Assumed to weigh 11.0 kg (Health Canada 2015b), and to drink 0.36 L of water per day (Health Canada 2017).
f Assumed to weigh 15 kg (Health Canada 2015b), and to drink 0.43 L of water per day (Health Canada 2017).
g Assumed to weigh 23 kg (Health Canada 2015b), and to drink 0.53 L of water per day (Health Canada 2017).
h Assumed to weigh 42 kg (Health Canada 2015b), and to drink 0.74 L of water per day (Health Canada 2017).
i Assumed to weigh 62 kg (Health Canada 2015b), and to drink 1.09 L of water per day (Health Canada 2017).
j Assumed to weigh 74 kg (Health Canada 2015b), to breathe 15.1 m3 of air per day (US EPA 2011 [modified]), and to drink 1.53 L of water per day (Health Canada 2017).
k 2.67 µg/m3 as derived using SCREEN3 (2013) to determine ambient and indoor air exposure intakes for residents within the vicinity of an industrial facility equipped with a gas scrubber. Input parameters and outputs for SCREEN3 modelling scenarios are described in Appendix A.
l No monitoring data on drinking water in Canada were identified. Five percent of the maximum reported quantity (100 000 kg) of piperazine in-commerce in Canada was assumed to be released to a body of surface water. Following a wastewater treatment process (removal rate 82.7%), a piperazine concentration in drinking water was calculated to be 48.6 µg/L (Health Canada 2015a; SimpleTreat 1997).
m No food occurrence data in Canada were identified. Intake from piperazine use as a food flavouring agent was estimated as a per capita intake (for the United States population 1 year old and older) to be 0.09 µg/day (0.0015 µg/kg bw/day based on a 60 kg body weight). This estimate was based on annual production volumes reported by the food industry (EFSA 2005; Gavin 2007 as presented in JECFA 2009).
Appendix C. Exposure scenario parameters for two-component epoxies
ConsExpo Web (2019) was used to estimate inhalation and dermal exposures to piperazine from two-component epoxies. The Do-it-yourself products fact sheet (RIVM 2007) was used as guidance in determining the exposure intake estimates, with some refinements based on product details. The mixing and loading exposure scenario was excluded since all products available to consumers that were identified used a variation of a device that pre-mixed the epoxy during application. Therefore, only the application exposure scenario was considered. A use scenario for automotive epoxy was chosen as it was considered to result in the greatest potential of exposure on the basis of the amount of product used.
| Input parameter | Parameter value |
|---|---|
| Concentration (%) | 0.5 (SDS 2017a) |
| Product amount (g) | 200 |
| Frequency (per year) | 3 |
| Exposure duration (min) | 240 |
| Inhalation model | Exposure to vapour, evaporation |
| Room volume (m3) | 20 |
| Ventilation rate (air exchanges per hour) | 0.6 |
| Inhalation rate (m3/day)a | 15.1 |
| Inhalation uptake (%) | 100 |
| Release area (m2) | 0.5 |
| Mass transfer coefficient (m/h) | 10 |
| Molecular weight matrix (g/mol) | 3 000 |
| Dermal model | Direct contact, instant application |
| Product amount (g) | 0.1 |
| Dermal uptake (%) | 100 |
a Consumer assumed to be an adult 19 years or older.
