Draft screening assessment - Salicylates group
Official title: Draft screening assessment - Salicylates group
Chemical Abstracts Service Registry Numbers 69-72-7; 87-22-9; 118-56-9; 68917-75-9; 84012-15-7
Environment and Climate Change Canada
Health Canada
March 2020
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of five substances referred to collectively as the Salicylates Group. Substances in this group were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA. The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 1 ), their Domestic Substances List (DSL) names and their common names are listed in the table below.
| CAS RN | DSL name | Common name |
|---|---|---|
| 68917-75-9a | Oils, wintergreen | Wintergreen oil |
| 69-72-7 | Benzoic acid, 2-hydroxy- | Salicylic acid |
| 118-56-9 | Benzoic acid, 2-hydroxy-, 3,3,5-trimethylcyclohexyl ester | Homosalate |
| 87-22-9 | Benzoic acid, 2-hydroxy-, 2-phenylethyl ester | Phenethyl salicylate |
| 84012-15-7a | Birch, Betula alba, ext. | Betula alba extract |
a This CAS RN is a UVCB (unknown or variable composition, complex reaction products, or biological materials).
In this assessment, the substances in the group are discussed in the order outlined in the table above as the health effects data for methyl salicylateFootnote 2 (found in wintergreen oil) and salicylic acid form the basis of the discussion for some of the other substances.
With the exception of Betula alba extract, the substances in this screening assessment are structurally similar insofar as they contain a salicylate moiety comprised of a carboxyl group occupying the ortho position of a phenol. These four substances differ in the substituents forming the ester bond with the carboxyl carbon. Betula alba extract is a substance of unknown or variable composition, complex reaction products, or biological material (UVCB) derived primarily from Betula alba, a species of the family Betulaceae. Major components of this substance vary depending on the part of the plant extracted, the extraction method, and the type of extract. Although wintergreen oil is also considered a UVCB, spectroscopic analysis has demonstrated that the oil typically contains up to 99% methyl salicylate, a discrete chemical.
Wintergreen oil, salicylic acid, and Betula alba extract are naturally present in the environment, whereas homosalate and phenethyl salicylate are not known to naturally occur. All of the substances in the Salicylates Group have been included in surveys issued pursuant to CEPA section 71 notices. Wintergreen oil, salicylic acid, and homosalate were reported to be imported into Canada in quantities of 100 to 1 000 kg, 87 437 kg, and 100 000 to 1 000 000 kg, respectively. Phenethyl salicylate and Betula alba extract were not reported to be imported into Canada above the 100 kg reporting threshold during the 2011 reporting year. None of the substances were reported to be manufactured in Canada, according to information submitted under section 71.
Wintergreen oil is reported to be used in Canada as an ingredient in cosmetics and pesticides and as a non-medicinal (NMI) and medicinal ingredient (MI) in natural health products (NHPs). The substance is a component of wintergreen extract, wintergreen essence, and wintergreen flavour, which are standardized flavouring preparations in Canada.
In Canada, salicylic acid is used as an ingredient in cosmetics and food packaging materials and as an NMI and MI in NHPs and drugs. The substance was also identified in cleaning products such as dishwashing detergents.
Homosalate is primarily used as an active ingredient in sunscreen formulations, where it functions as a UV-filter. The substance was reported to be used in a variety of cosmetic products in Canada.
In Canada, phenethyl salicylate is used as an ingredient in cosmetics and may be used as an NMI in NHPs. The substance may also be used as a food flavouring ingredient.
Betula alba extract was reported to be used in Canada as an ingredient in self-care products.Footnote 3
The ecological risks of the substances in the Salicylates Group were characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, substances in the Salicylates Group are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from wintergreen oil, salicylic acid, homosalate, phenethyl salicylate and Betula alba extract. It is proposed to conclude that wintergreen oil, salicylic acid, homosalate, phenethyl salicylate and Betula alba extract do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
With respect to human health, effects on the liver and developmental effects were identified to be the critical effects associated with exposure to wintergreen oil on the basis of information available for its main component, methyl salicylate. A comparison of the estimated oral exposure to wintergreen oil from use as a food flavouring ingredient and as an ingredient in antacid tablets and toothpastes resulted in margins of exposure that are considered adequate to address uncertainties in the health effects and exposure databases.
A comparison of the estimated exposure levels of wintergreen oil from its use as an ingredient in cosmetics, including massage oils and face moisturizers, resulted in margins of exposure that are considered potentially inadequate to account for uncertainties in the health effects and exposure databases. A comparison of the estimated exposure level of wintergreen oil from its use as an ingredient in analgesic creams resulted in margins of exposure that are also considered potentially inadequate to address uncertainties in the health effects and exposure databases.
Critical effects associated with salicylic acid are effects on the liver and kidney, as well as developmental effects. Comparison of exposure to salicylic acid from its presence in environmental media and food to the critical effect levels resulted in margins of exposure that are considered adequate to address the uncertainties in the health effects and exposure databases. A comparison of estimated exposure levels of salicylic acid from its use in a hair spray resulted in margins of exposure that are considered adequate to account for uncertainties in the health effects and exposure databases.
With respect to dermal exposure to salicylic acid from the use of certain cosmetics, a comparison of the critical effect levels to the estimated exposures resulted in margins of exposure that are considered potentially inadequate to address uncertainties in the health effects and exposure databases.
With respect to dermal and oral exposure to salicylic acid from the use of certain NHPs and drugs, including sunscreens, liquid antacids, analgesic creams, acne creams, and after-shave creams, a comparison of the estimated exposure to the critical effect levels resulted in margins of exposure that are considered potentially inadequate to address uncertainties in the health effects and exposure databases.
On the basis of the available information, the critical effects associated with exposure to homosalate are reproductive effects and effects on the kidneys, thymus and thyroid. A comparison of exposure to homosalate from the use of tanning products resulted in a margin of exposure that is considered potentially inadequate to address uncertainties in the health effects and exposure databases. Use of face moisturizers (creams and aerosols) resulted in margins of exposure that are considered adequate to address these uncertainties.
On the basis of toxicity data from structurally related analogues (i.e., methyl salicylate), the critical effects associated with exposure to phenethyl salicylate are effects on the liver and developmental effects. A comparison of the estimates of exposure to phenethyl salicylate from its use as an ingredient in cosmetics and as a food flavour ingredient to the critical effect level resulted in margins of exposure that are considered adequate to address uncertainties in the health effects and exposure databases.
The available health effects information on Betula alba extract and its main components indicate that this substance is of low hazard potential, and therefore the risk to human health is considered to be low.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that wintergreen oil, salicylic acid, and homosalate meet the criteria under paragraph 64(c) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that phenethyl salicylate and Betula alba extract do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is proposed to conclude that wintergreen oil, salicylic acid, and homosalate meet one or more of the criteria set out in section 64 of CEPA.
It is also proposed to conclude that phenethyl salicylate and Betula alba extract do not meet any of the criteria set out in section 64 of CEPA, and it is proposed that salicylic acid does not meet the persistence or bioaccumulation criteria, that homosalate meets the bioaccumulation criteria but not the persistence criteria, and that wintergreen oil meets the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
1. Introduction
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of five substances, referred to collectively as the Salicylates Group, to determine whether these substances present or may present a risk to the environment or to human health. The substances in this group were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA (ECCC, HC [modified 2017]).
The ecological risks of the substances in the Salicylates Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This draft screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to November 2017. Empirical data from key studies as well as results from models were used to reach proposed conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This draft screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological and human health portions of this assessment have undergone external review and/or consultation. Comments on the technical portions relevant to human health were received from Risk Sciences International and Sandrine Charles, Agence Nationale de Sécurité Sanitaire. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This draft screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precautionFootnote 4 . This draft screening assessment presents the critical information and considerations on which the proposed conclusions are based.
2. Identity of substances
The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 5 ), Domestic Substances List (DSL) names and common names for the individual substances or representative substances in the Salicylates Group are presented in Table 2‑1.
| CAS RN | DSL name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 69-72-7 | Benzoic acid, 2-hydroxy- (Salicylic acid) | 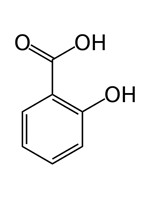 C7H6O3 C7H6O3 |
138.1 |
| 87-22-9 | Benzoic acid, 2-hydroxy-, 2-phenylethyl ester (Phenethyl salicylate) | 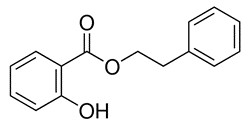 C13H9O3 C13H9O3 |
242.3 |
| 118-56-9 | Benzoic acid, 2-hydroxy-, 3,3,5-trimethylcyclohexyl ester (Homosalate) | 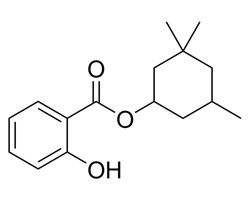 C16H21O3 C16H21O3 |
262.3 |
| CAS RN | DSL name (Common name) | Representative chemical name (formula) | Representative chemical structure |
|---|---|---|---|
| 68917-75-9 | Oils, wintergreen (Wintergreen oil) | ~99%: Methyl salicylate (C8H8O3) |  |
| 84012-15-7 | Birch, Betula alba, ext. (Betula alba extract) | 10.5-82%: Betulin (C30H50O2) | 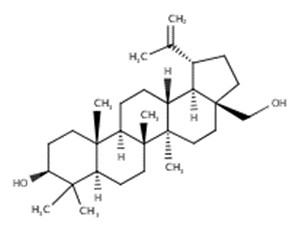 |
a UVCB is an acronym for Unknown or Variable composition Complex reaction products and Biological material.
In this assessment, the substances in the group are discussed in the following order: wintergreen oil, salicylic acid, homosalate, phenethyl salicylate, and Betula alba extract. The reason for this is that the health effects data for methyl salicylate (found in wintergreen oil) and salicylic acid form the basis of the discussion for some of the other substances.
Wintergreen oil represents a chemical substance of UVCB nature derived from either Gaultheria procumbens or Betula lenta. Under the United States’ Toxic Substances Control Act (TSCA) Chemical Substance Inventory, wintergreen oil (CAS RN 68917-75-9) is defined as “extractives and their physically modified derivatives Gaultheria procumbens, Ericacenae or Betula lenta, Betulaceae” (TSCA 2017). Despite being a UVCB, spectroscopic studies have revealed that wintergreen oil can contain up to 99% methyl salicylate, a discrete substance (El-Obeid et al. 1979). Tisserand and Young (2014) have noted that the toxicity of methyl salicylate and wintergreen oil were “essentially identical.”
Betula alba extract represents a chemical substance of UVCB nature derived from Betula alba. According to the Integrated Taxonomic Information System (ITIS) established by federal agencies in the United States and Canada, “Betula alba L.” is currently a synonym for Betula pubescens ssp. pubescens Ehrh. (ITIS 2017a, ITIS 2017b). In the European Inventory of Existing Commercial Chemical Substances (EINECS), Betula alba extract (CAS RN 84012-15-7) is defined as “extractives and their physically modified derivatives such as tinctures, concretes, absolutes, essential oils, oleoresins, terpenes, terpene-free fractions, distillates, residues, etc., obtained from Betula alba, Betulaceae” (ECHA 2017a).
The composition of constituents in Betula alba extract vary depending on the plant part used (e.g., buds, leaves, bark), the type of extract, and the extraction process. In products available to consumers, the ingredient identification may be specific (e.g., “Betula alba leaf extract”) or unspecific (e.g., “Betula alba”). Spectroscopic studies have found that birch extracts contain terpenoids and their esters, ether oils, hydrocarbons and their epoxides, steroids, tannins, flavonoids, hydroxycoumarins, vitamins, polymeric proanthocyanidins, and a number of unidentified compounds. However, triterpenoids and hydrocarbons were found to be the major components (Abyshev et al. 2007). Some of the major components (≥ 10%) that have been investigated in the literature include betulin (10.5% to 82%) and its derivatives (e.g., betulinic acid) (Laszczyk et al. 2006; Abyshev et al. 2007; Orav et al. 2011; EMEA 2014).
2.1 Selection of analogues
The Salicylates Group is comprised of salicylic acid and its esters with the exception of Betula alba extract. The salicylic acid esters are expected to be hydrolyzed to salicylic acid and their corresponding alcohols, which undergo subsequent metabolism (JECFA 2002a). The rate of hydrolysis of the ester bond depends on the chain length or the bulkiness of the substituent group attached to salicylic acid (Dittert et al. 1968). Information on the physical-chemical properties and toxicity of the salicylates and their analogues can be found in Table A-2, Appendix A.
Since some of the substances were associated with limited data for certain endpoints, a read-across approach using data from analogues was used to inform the human health assessments for the Salicylates Group. Analogues were selected on the basis of structural, functional, or toxicological similarity (e.g., similar physical-chemical properties, toxicokinetics, reactivity) and the availability of pertinent empirical data. Due to the inherent similarity in chemistry, metabolism, and toxicity among the majority of members in the Salicylates Group, there were instances where one substance in the group was identified to be an analogue for another substance.
Details of the read-across data chosen to inform the human health assessments of the salicylates are further discussed in the relevant sections of this report and in Appendix A. A list of the analogues used to inform the health effects assessment for the Salicylates Group is presented in Table 2‑3.
| CAS RN | Common name | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 119-36-8 | Methyl salicylate | 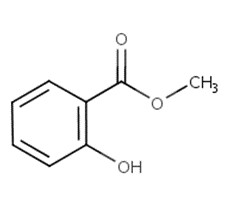 C8H8O3 C8H8O3 |
152.15 |
| 118-55-8 | Phenyl salicylate | 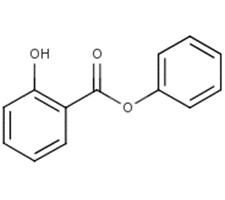 C13H10O3 C13H10O3 |
214.22 |
| 118-58-1 | Benzyl salicylate | 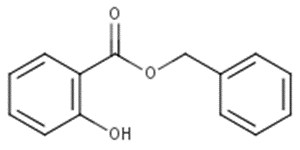 C14H12O3 C14H12O3 |
228.25 |
3. Physical and chemical properties
A summary of physical and chemical properties of the substances in the Salicylates Group is presented in Table 3‑1. When experimental information was limited or not available, data from analogues were used for read-across and/or (Q)SAR models were used to generate predicted values. Additional physical and chemical properties are presented in ECCC (2016b).
| Property | Wintergreen oilc | Salicylic acida | Homosalateb | Phenethyl salicylatea | Betula alba extract |
|---|---|---|---|---|---|
| Physical state | colourless liquid | white crystals | colourless liquid | white powder | white powderd |
| Melting point (°C) | - 8 | 158 | < -20 | 123e | 210-495d |
| Vapour pressure (Pa) | 4.57 | 0.01 | 0.013 | 1.85E-04e | 4.2E-11d |
| Henry’s law constant (atm·m3/mol) | 7.9E-08e | 7.34 E-09 | 1.93E-05a,e | 4.87E-07e | 5.34E-06d |
| Water solubility (mg/L) | 700 | 2 240 | 0.4 | 7.2 – 7.8e | 0.02f |
| Log Kow (dimensionless) | 2.55 | 2.26 | > 6 (at 40C) | 4.80e | 8.18d |
| pKa (dimensionless) | N/A | 2.98 | N/A | N/A | N/A |
a EpiSuite 2012.
b ECHA 2017b.
c Information on methyl salicylate (CAS RN 119-36-8) was used to inform wintergreen oil, as it is a major component of the wintergreen oil and constitutes greater than 95% of the oil. Properties of methyl salicylate were obtained from EpiSuite (2012).
d Information on Betula alba extract is based on one of its main component, betulin. Data from EpiSuite (2012).
e Predicted value.
f Jäger et al. 2008, for betulin
4. Sources and uses
4.1 Natural sources
Salicylic acid is present in plants as a free phenolic acid or in conjugated forms, generated through metabolic processes such as glucosylation, methylation or hydroxylation of the aromatic ring (Raskin 1992; Lee et al. 1995, as cited in Bandurska 2013). It is commonly found as the derivative salicin, occurring in species of willow trees (Salix alba, S. purpurea, S. daphnoides, and S. fragilis). High contents of free salicylic acid were reported in the species S. laponum and S. plantifolia (Raskin 1992; Lee et al. 1995, as cited in Bandurska 2013). Salicylic acid is present in humans primarily from the consumption of fruits and vegetables (Paterson et al. 2006) and may also be present from the breakdown of other chemicals, such as benzoic acid.
Wintergreen oil is an essential oil derived primarily from the Gaultheria genus of plants, including Gaultheria procumbens, as well as Betula lenta (TSCA 2017). Available information indicate that the major constituent of wintergreen oil is methyl salicylate (CAS RN 119-36-8), which can comprise up to 99% of the wintergreen oil components (Tisserand and Young 2014; El-Obeid et al. 1979).
Betula alba extract is derived from the Betulaceae family with a complex component profile. Major components of this substance have been identified as terpenoids and their esters, ether oils, hydrocarbons and their epoxides, steroids, tannins, flavonoids, hydroxycoumarins, vitamins, polymeric proanthocyanidins, and a number of unidentified compounds. However, triterpenoids and hydrocarbons were found to be the major components (Jäger et al. 2008; Abyshev et al. 2007). Levels of components that make up the extract vary depending on the type of birch tree, type of extract, and other factors (Jäger et al. 2008).
Homosalate and phenethyl salicylate are not known to be naturally occurring substances and are synthesized from salicylate derivatives.
4.2 Anthropogenic sources
All of the substances in the Salicylates Group have been included in surveys issued pursuant to section 71 of CEPA (Environment Canada 2013). Table 4‑1 presents a summary of the total reported import quantities for the Salicylates Group during the 2011 reporting year. None of the substances in the Salicylates Group were reported to be manufactured in Canada above the reporting threshold of 100 kg during the 2011 reporting year (Environment Canada 2013). Phenethyl salicylate and Betula alba extract were not reported to be imported in Canada above the 100 kg reporting threshold (Environment Canada 2013; ECCC 2017).
| Common name | Total importsa,b (kg) |
|---|---|
| Wintergreen oil | 100 – 1 000 |
| Salicylic acid | 87 437 |
| Homosalate | 100 000 – 1 000 000 |
| Phenethyl salicylate | < 100 |
| Betula alba extract | <100 |
a Values reflect quantities reported in response to a survey conducted under section 71 of (CEPA Environment Canada 2013). See survey for specific inclusions and exclusions (Schedules 2 and 3).
b ECCC 2017
4.3 Uses
Wintergreen oil is classified as an NHP under Schedule 1 item 2 (extract) of the Natural Health Product Regulations (NHPR) and is listed as an MI and NMI in a variety of licensed NHPs in Canada. Types of NHPs that contain the substance include analgesic creams, ointments, and antacid tablets. The substance is not a permitted food additive in Canada; however wintergreen essence, wintergreen extract, or wintergreen flavour are standardized flavouring preparations set out in B.10.027 of the Food and Drug Regulations. They are prepared from wintergreen oil and must contain not less than 3.0% by volume of wintergreen oil to meet the standard. Wintergreen oil may also be added to foods directly. Although there is no definitive information readily available concerning the use of wintergreen oil in food flavouring agents in Canada, the substance may be present in foods in Canada as a flavour ingredient or as a component of flavouring preparations (personal communication, emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated December 2017; unreferenced). The Flavour and Extract Manufacturers Association (FEMA) has classified wintergreen oil as GRAS (Generally Recognized as Safe) with uses in foods such as baked goods, beverages, candy, chewing gum, ice cream, and syrups (Hall and Oser 1965). In Canada, wintergreen oil was reported to be used as an ingredient in a variety of cosmetics, such as massage oils, moisturizers, and toothpastes, among others.
According to information pursuant to a survey under section 71 of CEPA and searches of public information in Canada, salicylic acid was reported to be used in the following sectors: paints and coatings, personal care, drugs, lubricants and greases, natural health, and laundry and dishwashing (ECCC 2013, SDS 2015). Salicylic acid may have a variety of functions depending on the type of product which contains it. The substance can be used as an anti-acne agent, anti-dandruff agent, exfoliant, and preservative in a variety of self-care products (ECCC 2013). Salicylic acid is widely used in the treatment of many common dermatological conditions because of its keratolytic properties (SCCP 2002).
Salicylic acid is included on the List of Prohibited and Restricted Cosmetic Ingredients (Cosmetic Ingredient Hotlist) and is restricted for use in cosmetics at a maximum permitted concentration of 2% (Health Canada [modified 2015]). Salicylic acid is reported to be used in many diverse cosmetic products, including shampoos and conditioners, cleansers and soaps, exfoliants, hair dyes, hair products, body and face moisturizers, tanning products, massage products, antiperspirants/deodorants, fragrances, make-up and make-up removers, lip balms, and shaving products.
According to information available in Health Canada’s internal Drug Product Database (DPD), salicylic acid is an active ingredient in human and veterinary drugs. The substance is also present as an NMI in many diverse drug products. Topical products reported to contain salicylic acid as an NMI include SPF and non-SPF creams and moisturizers, shampoos, and after-shave products. Products taken orally reported to contain salicylic acid are antacids, absorbents and antidiarrheals. Salicylic acid is listed in the Natural Health Products Ingredients Database (NHPID) with a medicinal role and is classified as an NHP substance falling under Schedule 1, Item 2 (an isolate), of the NHPR. It is also listed as an NMI for topical use only. Types of NHPs for which salicylic acid is listed as an NMI include acne treatment products, facial make-up and concealers, and sunscreens.
Salicylic acid may be used as a component in the manufacture of food packaging materials such as epoxy-based hardeners of beer tank linings, caulking agents for floor coating systems, and adhesives with no direct food contact application. The substance may be present as an incidental additive in foods based on its potential use in the formulation of cleaners. In the United States, salicylic acid is used as an indirect food additive, specifically as a component in adhesives, coatings, and other rubber articles intended for repeated use under sections CFR Title 21 175.105, 175.300, and 177.2600, respectively (US eCFR [modified 2017]). Salicylic acid was evaluated by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) for its use as a food flavour ingredient (JECFA 2002a,b). The EU permits the use of salicylic acid as a flavouring agent (FL No. 08.112) in all categories of flavoured foods (EU Food Flavouring Database). Therefore, while there is no definitive information available concerning the use of salicylic acid as a food flavouring agent in Canada, it is possible that the substance may be present in foods as a flavouring agent in Canada.
Homosalate is primarily used as a broad-spectrum UV filter in sunscreens in Canada (Environment Canada 2013; SCCP 2007). The use of homosalate as a medicinal ingredient in sunscreens is outlined in Health Canada’s Primary and Secondary Sunscreen Monographs (Health Canada 2018a,b), which indicate that homosalate is an acceptable medicinal ingredient at concentrations of ≤ 15% that can be market authorized without the submission of additional evidence to Health Canada. The substance is listed in the NHPID as a non-NHP because this is not a naturally occurring substance included in Schedule 1 of the NHPR, and it is not listed in any licensed NHPs in Canada. Homosalate is used as an ingredient in cosmetics and it is present in such products as moisturizers, massage products, tanning products, fragrances, and bath products, among others.
Phenethyl salicylate was reported to be used in Canada as an ingredient in a limited number of cosmetics, cleaning products, and hand sanitizers (ECCC 2017). In a 2007 fragrance material review on phenethyl salicylate, Lapszynski et al. reported uses of phenethyl salicylate in decorative cosmetics, fine fragrances, shampoos, toilet soaps and other toiletries, as well as in non-cosmetic products such as household cleaners and detergents. The European Union lists phenethyl salicylate as a cosmetic ingredient where it functions as a perfuming agent (CosIng [modified 2017]). Phenethyl salicylate is listed in the NHPID with a non-medicinal role for oral use as a flavour enhancer, but it is not reported as an ingredient in any licensed NHPs in Canada. In the United States, phenethyl salicylate is listed in Title 21, CFR 172.515, as a synthetic flavour that may be used at a minimum quantity required to produce the intended effect and in accordance with good manufacturing practice (GMP) (eCFR [modified 2017]). The substance was evaluated by JECFA for its use as a food flavour ingredient (JECFA 2002a,b). The European Union permits the use of phenethyl salicylate as a flavouring agent (FL No. 09.753) in foods (EU Food Flavourings Database [modified 2012]). Therefore, while there is no definitive information available concerning the use of phenethyl salicylate as a food flavouring agent in Canada, it is possible that the substance may be present in foods as a flavouring agent in Canada.
Information on Betula alba extract obtained from a CEPA section 71 survey indicates that the substance is used as a filler and odour agent in cosmetics available to consumers in Canada (ECCC 2017). Betula alba extract is used in cosmetic products including shampoos and conditioners, moisturizers, antiperspirant/deodorant, cleansers, exfoliants, fragrance products, massage products, styling products, sunless tanning products (non-SPF), make-up and make-up removers, and hair colouring products (personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated December 2016; unreferenced). According to information available on Health Canada’s internal DPD, Betula alba extract is used as an NMI in sunscreens and skin tanning products. Betula alba extract is also listed in the NHPID as Betula alba bark extract and Betula alba leaf extract, with both extracts being used as NMIs in NHPs (NHPID [modified 2018]). The leaf extract is reported to be non-medicinally used as a cosmetic astringent, fragrance ingredient, and skin-conditioning agent, and the bark extract as a fragrance ingredient and skin-conditioning agent (NHPID [modified 2018]). Betula alba extract is an NMI in a limited number of NHPs, including creams, sunscreens, oral capsules, and hand sanitizers.
Table 4‑2 below summarizes the uses in Canada for substances in the Salicylates Group.
| Use | Wintergreen oil | Salicylic acid | Homosalate | Phenethyl salicylate | Betula alba extract |
|---|---|---|---|---|---|
| Food packaging materialsa | No | Yes | No | No | No |
| Incidental additivea | No | Yes | No | No | No |
| Internal Drug Product Database as medicinal (MI) or non-medicinal ingredient (NMI) in disinfectant, human or veterinary drug products in Canadab | No | MI, NMI | MI | No | NMI |
| Natural Health Products Ingredients Databasec | Yes | Yes | Yes | Yes | Yes |
| Licensed Natural Health Products Database as medicinal (MI) or non-medicinal ingredient (NMI) in natural health products in Canadac | MI, NMI | MI, NMI | No | No | NMI |
| List of Prohibited and Restricted Cosmetic Ingredientsd | No | Yes | No | No | No |
| Notified to be present in cosmetics, on the basis of notifications submitted under the Cosmetic Regulations to Health Canadad | Yes | Yes | Yes | No | Yes |
| Formulant or active ingredient in pest control products registered in Canadae | formulant and activef | formulant | No | formulant | No |
a Personal communication, emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated December 2017; unreferenced.
b Personal communication, emails from the Therapeutic Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated December 2017; unreferenced.
c Personal communication, emails from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated December 2016; unreferenced.
d Personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated December 2016; unreferenced.
e Personal communication, emails from the Pest Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated December 2016; unreferenced.
f Health Canada 2014.
5. Environmental fate and behaviour
5.1 Environmental persistence
According to models used in ERC (ECCC 2016b), salicylic acid, phenethyl salicylate, homosalate and Betula alba extract are expected to degrade and not be persistent in air, water, sediment, or soil. Wintergreen oil is expected to be persistent in water, sediment and soil, but not in air (ECCC 2016b).
5.2 Potential for bioaccumulation
Given their low Kows and low bioconcentration factors (ECCC 2016b), salicylic acid, phenethyl salicylate, wintergreen oil and Betula alba extract are not expected to significantly bioaccumulate in organisms.
Given its high Kow and high bioconcentration factor (ECCC 2016b), homosalate is expected to significantly bioaccumulate in organisms.
6. Potential to cause ecological harm
6.1 Characterization of ecological risk
The ecological risks of the substances in the Salicylates Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration [LC50]) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from scientific literature, from available empirical databases (e.g., OECD [Q]SAR Toolbox [OECD 2016]), and from responses to surveys under section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency and margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance based on its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under- classification of hazard, exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC 2016a. The following describes two of the more substantial areas of uncertainty. Error in empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from QSAR models. However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue (CBR) analysis. Error in underestimation of acute toxicity will be mitigated through the use of other hazard metrics, such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada based on what is believed to be the current use quantity and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for the substances in the Salicylates Group and the hazard, exposure and risk classification results are presented in ECCC (2016b).
The hazard and exposure classifications for the substances in the Salicylates Group are summarized in Table 6-1.
| Substance | ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| Wintergreen oil | low | low | low |
| Salicylic acid | low | low | low |
| Homosalate | high | low | moderate |
| Phenethyl salicylate | high | low | low |
| Betula alba extract | low | low | low |
On the basis of low hazard and low exposure classifications according to information considered under ERC for wintergreen oil, salicylic acid and Betula alba extract, these substances were classified as having low potential for ecological risk. It is therefore unlikely that these substances are resulting in concerns for the environment in Canada.
According to information considered under ERC, homosalate was classified as having a high hazard potential due to structural alerts from the OECD (Q)SAR Toolbox (OECD 2016), which identified this substance as being a potential endocrine receptor binder and having potential to cause adverse effects in aquatic foodwebs given its bioaccumulation potential. Homosalate was classified as having moderate potential for ecological risk due to low exposure. The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of this substance. Considering current use patterns, this substance is unlikely to be resulting in concerns for the environment in Canada.
According to information considered under ERC, phenethyl salicylate was classified as having a high hazard potential due to structural alerts from the OECD (Q)SAR Toolbox (OECD 2016), which identified this substance as being a potential endocrine receptor binder. Phenethyl salicylate was initially classified as having a moderate potential for ecological risk, but the risk classification was decreased to low potential for ecological risk following the adjustment of risk classification based on current use quantities (see section 7.1.1 of ECCC 2016a). The potential effects and how they may manifest in the environment were not further investigated due to the very low exposure of this substance. Considering current use patterns, this substance is unlikely to be resulting in concerns for the environment in Canada.
7. Potential to cause harm to human health
7.1 Wintergreen oil
7.1.1 Exposure assessment
Monitoring data on wintergreen oil in indoor and outdoor air, water, soil, or dust in Canada or elsewhere were not identified. However, considering the low quantities (100 to 1000 kg) of the substance reported in Canada (Environment Canada 2013), the potential exposure to wintergreen oil from environmental media is expected to be minimal.
Wintergreen oil may be present in some foods as a food flavouring agent or as a component in flavouring preparations in Canada. Under Canadian regulations, flavouring preparations containing wintergreen oil must contain no less than 3% by volume of wintergreen oil. No specifications were set out by JECFA for wintergreen oil, but specifications were set out for methyl salicylate (CAS RN 119-36-8), which it identified as “synthetic wintergreen oil”, with “assay min %” of 98%. In the assessment of methyl salicylate, JECFA concluded “no safety concern” at the current levels of intake when methyl salicylate is used as a flavouring agent (JECFA 2002a,b).
In the absence of definitive data readily available on the use of wintergreen oil in food flavourings in Canada, an individual consumption intake of 0.0023 mg/kg bw/day can be used as a suitable surrogate for Canadian consumption (Burdock 2009). Individual consumption intakes are a per capita estimate of intake (maximum survey-derived daily intake or MSDI) based on “disappearance data” from periodic surveys conducted by the National Academy of Sciences under contract to the FDA (NRC 1989, as cited in Burdock 2009). The assumption is made for a 60 kg individual.
Cosmetics and natural health products
In notifications submitted under the Cosmetic Regulations, wintergreen oil is reported to be present as an ingredient in a variety of cosmetics and NHPs. The substance was reported as an ingredient in massage oils, bath products, conditioners, toothpastes, and face moisturizers. It was also reported as an ingredient in NHPs such as analgesic creams and antacid tablets.
“Sentinel” scenarios were selected to evaluate the potential for exposure to wintergreen oil from cosmetics and NHPs. They are scenarios that resulted in the highest level of potential exposure to wintergreen oil via the oral or dermal route, taking into consideration frequencies of use and reported concentrations of wintergreen oil.
Studies on the percutaneous absorption of methyl salicylate (a major component of wintergreen oil, comprising up to 99%) were examined to evaluate the systemic exposure to wintergreen oil from dermally applied products. Studies of human in vivo percutaneous absorption have noted significant esterase activity following topical administration of formulations containing methyl salicylate (Cross et al. 1998; Megwa et al. 1995; Behrendt and Kampfmeyer 1989). A human in vivo study demonstrated that methyl salicylate is readily absorbed following topical administration of analgesic creams, that repeated exposure may cause skin irritant effects (e.g., puritis, erythema, stinging), and that absorption may increase following repeated exposures (Morra et al. 1996). In a single-dose human in vitro dermal absorption study, methyl salicylate (in an acetone vehicle) was applied for approximately 30 minutes (Moody et al. 2007). At doses comparable to the exposure scenarios considered as part of this screening assessment, absorption was reported to be approximately 40% of the applied dose within the 30-minute exposure duration. Another in vivo dermal absorption study using porcine skin reported 15% to 55% dermal absorption of neat methyl salicylate over 6 hours (Duncan et al. 2002). The dermal absorption potential of wintergreen oil took into consideration the relatively high fractions of methyl salicylate absorbed reported from in vitro studies in short time periods (30 minutes or 6 hours), the notion that repeated exposure may enhance penetration, and the overall variability in absorption of the substance cited elsewhere (human in vivo studies ranging from 12% to 93% (Cross et al. 1998; Yano et al. 1986, as cited in Belsito et al. 2007). The dermal absorption for wintergreen oil (based on methyl salicylate) is conservatively assumed to be equivalent to oral absorption for all dermal scenarios presented below.
Estimated sentinel exposure scenarios for cosmetics that contain wintergreen oil are presented in Table 7‑1. Massage oils and face moisturizers were selected as the sentinel dermal scenarios for cosmetics containing wintergreen oil, and non-fluorinated toothpastes were selected as the sentinel oral exposure. Details on the derivation of exposure estimates can be found in Appendix B.
| Product | Reported concentration (%)a | Infant | Toddler | Child | Teen | Adult |
|---|---|---|---|---|---|---|
| Massage oil b,c | 10 | N/A | 11.6 | 8.06 | 4.89 | 4.51 |
| Massage oil b,c | 3 | 7.2 | N/A | N/A | N/A | N/A |
| Toothpaste | 3 | N/A | 0.57 | 0.27 | 0.081 | 0.067 |
| Face moisturizerb | 3 | N/A | N/A | N/A | 1.1 | 0.91 |
a Based on notifications reported to Health Canada under the Cosmetic Regulations. For massage oils, notifications at maximum concentration of 10% were contraindicated for use on children < 2 years. Therefore, notifications at a maximum concentration of 3% were used to estimate exposure to infants.
b Dermal absorption assumed to be equivalent to oral absorption.
c Exposure estimated on a per-event basis.
Wintergreen oil is also reported to be used as an NMI in topical analgesic creams for the treatment of unspecified muscle or joint pain. Oral exposure may arise from its use in herbal teas and antacid tablets (personal communication, emails from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated December 2016-February 2019; unreferenced). The use of analgesic creams and antacids are considered intermittent exposures. Estimated dermal and oral exposures to wintergreen oil from the use of NHPs are presented in Table 7-2 below. Concentrations of wintergreen oil in these types of products ranged from less than 1 to 20% (personal communication, emails from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated December 2016; unreferenced)
| Product | Reported concentration (%)a | Route | Toddler | Child | Teen | Adult |
|---|---|---|---|---|---|---|
| Analgesic creamb | 20 | Dermal | N/A | 22.2 | 19.4 | 19.8 |
| Antacid tabletc | 0.29 | Oral | N/A | N/A | 0.39 | 0.32 |
a Based on notifications reported to Health Canada under the NHPR.
b This row includes estimates of systemic exposure, based on the assumption that dermal absorption is equivalent to oral absorption.
c Product label contraindicates use by children or younger age groups.
Exposure to wintergreen oil from consumption of herbal tea was not quantified because data on the amount of wintergreen oil that would be extracted through steeping was not identified. However, it is expected that the wintergreen oil content in brewed tea would be low, as current laboratory extraction methods for wintergreen oil such as steam distillation have reported low yields (less than 0.7%) (The Essential Oil Company 2017).
7.1.2 Health effects assessment
There are currently no hazard classifications designated by the US EPA, ECHA, or IARC for wintergreen oil. Wintergreen oil represents a chemical substance of UVCB nature, which can contain up to 99% methyl salicylate (El-Obeid et al. 1979). Therefore, health effects studies conducted with methyl salicylate will be used to inform the assessment of wintergreen oil. Harmonized classification and labelling for methyl salicylate has recently been proposed for future entry into Annex VI of the Classification, Labelling, and Packaging (CLP) Regulation (ECHA 2018). Methyl salicylate was identified as being harmful if swallowed (acute toxicity category 4 [Acute Tox 4]), as having the potential to cause an allergic skin reaction (skin sensitizer category 1B [Skin Sens 1B]), and as having the potential to damage the unborn child (reproductive toxicity category 1B [Repr 1B]). These classifications may be subject to change until the opinion is adopted by the ECHA Committee for Risk Assessment (RAC).
Repeated-dose toxicity
The effects of subchronic administration of methyl salicylate have been investigated in weanling Osborne-Mendel rats (n=10/sex/group) fed diets containing 0%, 0.1%, and 1% synthetic methyl salicylate (99% purity) for 17 weeks (Webb and Hansen 1963). This was equivalent to approximately 0, 50, and 500 mg/kg bw/day (Health Canada 1994). Animals at the highest dose exhibited significantly reduced body weight gain, but no gross or microscopic findings were observed.
Synthetic methyl salicylate was also administered to beagle dogs (n=3/sex/group) by gelatin capsules at doses of 0, 150, 300, 500, and 800 mg/kg/day for 7.5 months (Abbott and Harrisson 1978). Clinical signs, hematology, urinalyses, gross pathology, and histological examination were performed on all major organs. At 150 mg/kg bw/day, mean relative liver and kidney weights were in excess of those for negative control, but were within normal variation. At higher dose levels, these parameters exceeded normal variation values, while other effects such as reduced body weights and mortality were observed. A subsequent study was conducted by the same authors, whereby beagle dogs (n=4-6/sex/group) were administered 0, 50, 100, and 167 mg/kg bw/day methyl salicylate in capsules for 6 months. Only the liver and kidneys, the prime organs of interest, were subjected to histological examination. No treatment-related adverse effects were identified up to the highest dose tested.
The effects of chronic exposure to methyl salicylate have also been examined in multiple species being treated for a period of 2 years (Webb and Hansen 1963). Weanling Osborne-Mendel rats (n=25/sex/group) were administered 0%, 0.1%, 0.5%, 1%, and 2% methyl salicylate in the diet, equivalent to approximately 0, 50, 250, 500, and 1000 mg/kg bw/day, respectively (Health Canada 1994). At levels greater or equal to 0.5% (250 mg/kg bw/day), there were findings of gross pituitary lesions, significant growth inhibition, rough hair coats, significantly increased heart and kidney weights, and mortality. In addition, there was a dose-dependent increase in the number of animals with an increased amount of cancellous bone, along with decreased length of certain bones (femur, humerus, tibia, and radius). The effects of methyl salicylate administration on bones have been reproduced in rats in a series of short-term experiments conducted by Abbott and Harrisson (1978). In these experiments, there were observations of increased density in the femur, humerus, tibia and radius. However, the authors noted that the effects on bone appeared to be species-specific as they were only observed in rats and not in other species.
Webb and Hansen (1963) also investigated the effects of chronic exposure in beagle dogs (n=2/sex/group), whereby methyl salicylate was administered as oral capsules containing 0, 50, 150, 350 mg/kg/day, 6 days a week for 2 years. The administration of 150 and 350 mg/kg bw/day resulted in reduced body weight gains and enlarged livers. Microscopically, these livers had larger hepatic cells than those in controls. However, fatty metamorphosis was not greater in the livers of the treated dogs than the very small amounts seen in the livers of control dogs. The no-observed-adverse-effect-level (NOAEL) was determined to be 50 mg/kg bw/day on the basis of reduced body weight and liver effects observed at the next dose of 150 mg/kg bw/day.
With respect to the dermal route of administration, Webb and Hansen (1963) conducted a study whereby 0.5, 1, 2, and 4 mL/kg/day of methyl salicylate (99% purity) was applied to the skin of rabbits (n=3/group), 5 days a week, 6.5 h a day, for 96 days. This was equivalent to approximately 585, 1170, 2360, and 4680 mg/kg bw/day (assuming a density of 1.17 g/mL). At 2 mL/kg bw/day, slight sloughing of epidermal scales was observed in two out of three rabbits. At 4 mL/kg bw/day, all of the animals died within 28 days, accompanied by signs of anorexia, weight loss, and decreased activity. In the surviving animals of the other dose groups, there were reduced weight gains and the incidence of spontaneous nephritis and mild hepatitis appeared to be increased compared to historical data. There was also slight to very slight dermatitis. The lowest-observed-adverse-effect-level (LOAEL) for systemic effects was determined to be the lowest dose tested of 0.5 mL/kg bw/day (585 mg/kg bw/day) on the basis of reduced weight gains, nephritis, and mild hepatitis observed in the animals. A control group was not included in this study.
With respect to the inhalation route of administration, two studies examining the effects of methyl salicylate were identified. In the first study, female Alderley Park specific-pathogen-free rats (n=4/group) were exposed to 700 mg/m3 (120 ppm) of saturated vapour of methyl salicylate. The animals were exposed 20 times, each with a duration of 7 h per day, over a period of approximately 3 to 4 weeks (Gage 1970). The authors reported “no toxic signs” (i.e., the animals remained in good condition) and “organs normal” (i.e., histopathological examinations revealed no changes that could be attributed to the treatment). A NOAEL of 700 mg/m3 (120 ppm) was determined.
In another inhalation study, male rats were exposed to 1.2, 8, or 40 mg/m3 methyl salicylate, 4 h a day for 4 months (Rumyantsev et al 1992, as cited in CIR 2003). In the report published by the Cosmetic Ingredient Review Expert Panel (CIR 2003), it was noted that the high dose resulted in changes in nervous system functioning, a decrease in hemoglobin content and number of erythrocytes, and a change in serum leucine aminopeptidase and urinary creatinine content. At microscopic examination, pulmonary focal hemorrhages and hyperplasia were observed in the peribronchial lymphoid tissue, and the number of plasmatic cells in the lymphoid follicles was increased. In the kidneys, scaling of the epithelium of the convoluted tubules, focal infiltration, and focal hemorrhages were seen. The original article by Rumyantsev et al. (1992) was in Russian and thus the results could not be interpreted. However, the English abstract indicated a “threshold level of 8 mg/m3.”
Carcinogenicity and genotoxicity
Although guideline studies examining the carcinogenic potential of wintergreen oil have not been identified, the available information indicates that wintergreen oil is not likely to be carcinogenic. In a chronic study whereby mice were administered wintergreen oil in the diet beginning at approximately 11.7 months of age (when spontaneous tumours are known to manifest), it was observed that wintergreen oil could delay the time at which tumours would normally develop (Strong 1932). Other studies examining larger doses or the effects of wintergreen oil on the survival/prognosis of tumour-bearing animals have yielded similar results or found no significant differences when compared to control animals (Strong 1934; Strong 1935; Strong 1936; Boyland and Mawson 1938).
Chronic treatment with the main constituent of wintergreen oil, methyl salicylate, also did not reveal any carcinogenic effects (Webb and Hansen 1963). With respect to genotoxicity, wintergreen oil was not mutagenic in an in vitro assay incubated with rat neuronal cells (Celik and Turkez 2016). Methyl salicylate was also negative for genotoxicity in Rec-assays (Oda et al. 1978; Kuboyama and Fujii 1992), in bacterial mutagenicity assays (Mortelmans et al. 1986; Kuboyama and Fujii 1992; Ishidate et al. 1984; Study 15-32, as cited in FDA 2006), and in chromosome aberration assays conducted on mammalian cells (Ishidate et al. 1984; FDA 2006). Methyl salicylate also tested negative for clastogenic potential in an in vivo rat micronucleus test (FDA 2006).
Reproductive and developmental toxicity
Studies examining the potential reproductive and developmental effects of wintergreen oil were not identified. However, these effects were examined for methyl salicylate. In a two-generation study, Wistar rats (n=25/sex/group) were administered methyl salicylate at dietary levels of 0%, 0.25%, and 0.5% throughout the study period, starting 60 days prior to mating (Abbott and Harrisson 1978). This was equivalent to approximately 0, 211, and 422 mg/kg bw/day. The diets were fed to all of the animals (parents and young). Stillborn, viability, lactation, and reproduction indices were calculated. The authors noted that the litter size was lower in the treated groups and that there was reduced pup viability in the high-dose group. However, these findings were not found to be statistically significant. The authors indicated that “all pups were examined closely for physical abnormalities.” None of the offspring exhibited any gross abnormalities when the parental animals were exposed to up to 0.5% (422 mg/kg bw/day) methyl salicylate in the diet. The authors concluded that methyl salicylate did not produce any gross teratology or any significant adverse effects on reproduction.
A parallel two-generation study was conducted in mice (n=25/sex/group) at dietary levels of 0%, 0.25%, and 0.5% methyl salicylate for a period of 30 days prior to mating and throughout the study period (Abbott and Harrison 1978). This was equivalent to approximately 0, 300, and 600 mg/kg bw/day. Conception rate was higher for the treated groups compared to controls. Furthermore, viability, lactation, and reproduction indices were comparable to or better than those in the control animals. No physical abnormalities were observed in the pups, and all pups survived to weaning with normal development with respect to body growth, appearance, and behaviour. The authors concluded that reproduction was not adversely affected and “no teratology was observed.”
In another study, methyl salicylate was administered in the diet to Osborne-Mendel rats (n=20/sex/dose) at levels of 0, 500, 1500, 3000, or 5000 ppm over three generations (Collins et al. 1971). This was equivalent to 0, 25, 75, 150, and 250 mg/kg bw/day (Health Canada 1994). After 100 days of treatment, animals were mated and fertility was recorded. Observations in the offspring (F1a) included the number of stillborn, live born, and gross abnormalities. The litters were observed similarly on day 4 and counts were made on the number and condition of the pups. The F1a pups were sacrificed at weaning. The parental animals (F0) were re-mated to generate F1b litters and the same observations were made. However, at weaning, 20 litter-mated pairs were selected at each dose level to produce the next generation (F2a and F2b). The same procedure was followed for succeeding generations. No treatment-related adverse effects were noted in the F0 parental animals (“appeared healthy”), and no grossly visible abnormalities were detected in parental animals of the subsequent generations. In addition, fertility was not significantly affected at any dose (p>0.05) in any generation, although decreases were observed in the matings at the 5000 ppm level. None of the litters revealed any gross abnormalities when parental animals were exposed to up to 5000 ppm (250 mg/kg bw/day). However, there were dose-related reductions in litter size, number of liveborn pups, pup survival (i.e., alive at day 4), and number of offspring weaned (i.e., alive at day 21). These effects occurred in the second generation matings and were statistically significant at doses of 3000 and 5000 ppm (150 and 250 mg/kg bw/day, respectively). Details regarding the onset and time of pup death between postnatal day (PND) 4 and 21 were not reported in the study and it is therefore unclear as to whether it could be associated with direct exposure to the substance in the milk, diet (pups can start to eat solid food by PND 21) or other factors. These effects were not evident in the first or third generation matings, and occurred in the absence of maternal toxicity. A NOAEL of 75 mg/kg bw/day was determined on the basis of reduced litter size, pup survival, and number of offspring weaned at the next dose level.
The effect of methyl salicylate has also been examined in continuous breeding assays conducted by the National Toxicology Program (NTP 1984a). In a first study, CD-1 mice (n=8/sex/dose) were administered 0, 25, 50, or 100 mg/kg bw/day methyl salicylate (≥99% purity, in corn oil) by gavage for 7 days pre-mating and for about 100 days during cohabitation. Body weight, fertility, number of litters produced, proportion of live pups per litter, and mean body weight of offspring were recorded. No effects on fertility, the number of litters, pups per litter, or proportion of pups born alive were observed. Live pup weights, when adjusted for litter size, were significantly greater at 100 mg/kg bw/day, but the authors noted that this effect could be due to chance since no other indications of toxicity were observed. The offspring from the control and high-dose groups were continued on treatment and subsequently mated as well to assess their fertility and reproductive performance. The same parameters were examined, with the addition of histopathological examination of the liver, brain, pituitary gland, and reproductive tract. No treatment-related adverse effects were noted for any of the parameters examined up to the highest dose tested (100 mg/kg bw/day).
In a second study, CD-1 mice (n=20/sex/dose, n=40/sex in control group) were administered 0, 100, 250, or 500 mg/kg bw/day methyl salicylate (≥99%, in corn oil) by gavage under a similar protocol as the previous study (NTP 1984b). The highest dose tested (i.e., 500 mg/kg bw/day) was previously determined in a 2-week dose range-finding study to be the maximum tolerated dose (MTD) on the basis of mortality observed at the next dose level (1000 mg/kg bw/day). The MTD is defined as the highest dose that could be administered without significantly reducing body weight or depressing weight gain by more than 10% or resulting in significant mortality (≤ 10%). The animals were sacrificed and the liver, brain, pituitary gland, and reproductive tract were subjected to histopathological examinations. The authors noted that there were no distinct symptoms of toxicity throughout the study. In addition, there were no treatment-related effects on body weight or fertility. However, there was a significant decrease in the mean number of litters, pups per litter, proportion of pups born alive, and mean live pup weights at the highest dose tested, which occurred in the absence of overt maternal toxicity. A significant reduction in live pup weights was also observed at 250 mg/kg bw/day when an adjustment was made for litter size. A NOAEL of 100 mg/kg bw/day was determined on the basis of reduction in adjusted mean pup weight at the next dose level (250 mg/kg bw/day).
Reproductive and developmental effects were not observed for methyl salicylate following dermal application at doses similar to the aforementioned oral studies. When a petroleum-based grease containing 3% methyl salicylate was dermally applied to pregnant rats at doses of 0, 1, 3, and 6 g/kg bw/day from gestational day (GD) 6 to 15, there were no signs of maternal toxicity (as measured by food consumption, body weight, and clinical signs), no alterations in reproductive parameters, and no malformations or variations in the fetuses in any of the doses tested (Infurna et al. 1990, abstract only).
7.1.3 Characterization of risk to human health
The hazard dataset for wintergreen oil was considered to be limited, and available information on health effects of methyl salicylate, its main component, was used to inform the risk characterization where relevant.
For chronic exposure scenarios, the three-generation dietary study conducted by Collins et al. (1971) on Osborne-Mendel rats with methyl salicylate was considered to be the most relevant study for the characterization of human health risk to adults and teens (of child-bearing age). A NOAEL of 75 mg/kg bw/day was selected as the critical effect level on the basis of developmental effects, such as reduced litter size, reduced pup viability, reduced pup survival, and reduced number of weanlings observed at the next dose level (150 mg/kg bw/day), in the absence of maternal toxicity. These effects manifested in the second generation matings following long-term daily treatment of parental animals in the previous generations.
The three-generation study conducted by Collins et al. (1971) was also considered to be the most relevant study for the characterization of risk to infants from any duration of exposure, on the basis of the effects observed on pups of the second generation matings at PND4 and PND21 and because exposure to wintergreen oil via breast milk could not be dismissed as a potential cause of these effects. The NOAEL of 75 mg/kg bw/day was selected on the basis of pup death observed at the next dose level (150 mg/kg bw/day) in the absence of maternal toxicity.
The NOAEL of 75 mg/kg bw/day from the three-generation study conducted by Collins et al. (1971) was also considered to be relevant for the characterization of risk to children and toddlers from chronic exposure to wintergreen oil. This is on the basis that a dose of 75 mg/kg bw/day did not result in any apparent adverse effects on any of the parental animals from any generation. This is supported by the results of the available chronic studies by Webb and Hansen (1963) in which effects were observed at 150 mg/kg bw/d in beagle dogs (liver effects and reduced body weights) and at 250 mg/kg bw/day in rats (gross pituitary lesions and bone lesions), following administration of wintergreen oil via the oral route for 2 years, resulting in a NOAEL of 50 mg/kg bw/d.
For short-term intermittent exposure scenarios in teens and adults, the continuous breeding assay conducted by NTP (1984b) on CD-1 mice with methyl salicylate was considered to be the most relevant study for the characterization of risk. In this study, methyl salicylate is administered for approximately 107 days, and a NOAEL of 100 mg/kg bw/day was selected as the critical effect level on the basis of a significant reduction in adjusted mean pup weight observed at the next level (250 mg/kg bw/day).
For short-term intermittent exposure scenarios for toddlers and children, the short-term study by Abbott and Harrisson (1978) on beagle dogs with methyl salicylate was considered to be the most relevant study for the characterization of risk. This study administered methyl salicylate as oral capsules for 7.5 months. A NOAEL of 150 mg/kg bw/day was selected as the critical effect level on the basis of increased relative liver weights at the next dose (300 mg/kg bw/day). This critical effect level was applicable for dermal and oral routes of exposure.
Table 7‑3 provides all relevant exposure estimates, critical effect levels, and resulting margins of exposure for characterization of risk to human health for wintergreen oil from use in cosmetics.
| Scenarioa | Systemic exposure (mg/kg bw/day) | Critical effect | MOE |
|---|---|---|---|
| Massage oilb,c (infant) (Short-term intermittent dermal exposure) | 7.2 | NOAEL = 75d | 10 |
| Massage oil (toddlerb,c,e ) (Short-term intermittent dermal exposure), | 11.6 | NOAEL = 150f | 13 |
| Massage oil (teenb,c,g) (Short-term intermittent dermal exposure) | 4.89 | NOAEL=100h | 20 |
| Face moisturizer (teenb,g) (Daily dermal exposure) | 1.1 | NOAEL = 75i | 68 |
| Toothpaste (toddlere) (Daily oral exposure) | 0.57 | NOAEL = 75d | 131 |
| Toothpaste (teeng) (Daily oral exposure) | 0.081 | NOAEL = 75i | 926 |
a Based on notifications reported to Health Canada under the Cosmetic Regulations.
b Dermal absorption assumed to be equivalent to oral absorption.
c Exposure estimated on a per-event basis.
d NOAEL = 75 mg/kw bw/day; on the basis of reduced pup viability, pup survival, and number of weanlings at the next dose of 150 mg/kg bw/day in a three-generation dietary study.
e Age group with highest exposure relative to body weight among toddlers and children.
f NOAEL = 150 mg/kg bw/day; on the basis of increased relative liver weights at the next dose of 300 mg/kg bw/day in a short-term oral study.
g Age group with highest exposure relative to body weight, among teens and adults.
h NOAEL = 100 mg/kg bw/day; (on the basis of reduction in adjusted mean pup weight at the next dose of 250 mg/kg bw/day in a continuous breeding assay).
I NOAEL=75 mg/kg bw/day on the basis of reduced litter size, pup viability, pup survival, number of weanlings at the next dose of 150 mg/kg bw/day in a three-generation dietary study.
With respect to dermal exposure to wintergreen oil from the use of massage oils or face moisturizers, comparisons of the critical effect level to the estimated levels of exposure resulted in margins of exposure (MOEs) ranging from 10 to 68, which are considered potentially inadequate to address uncertainties in the health effects and exposure databases.
With respect to oral exposure to wintergreen oil from the use of toothpastes, comparison of the estimated exposure to the critical effect level resulted in MOEs of 131 and 926 for toddlers and teens, respectively, which are considered adequate to address uncertainties in the health effects and exposure databases.
| Scenarioa | Systemic exposureb (mg/kg bw/day) | Critical effect level (mg/kg bw/day) | MOE |
|---|---|---|---|
| Analgesic cream (childc) (Short-term intermittent dermal exposure) | 22.2 | NOAEL = 150d | 7 |
| Analgesic cream (adulte) (Short-term intermittent dermal exposure) | 19.8 | NOAEL = 100f | 5 |
| Antacid (teene) (Short-term intermittent oral exposure) | 0.39 | NOAEL = 100f | 256 |
| Food flavouring use (adult) (Daily oral exposure) | 0.0023 | NOAEL = 75g | > 30 000 |
a Assuming dermal and inhalation absorption is equivalent to oral absorption, where relevant.
b Estimated based on the day of exposure.
c Age group with highest exposure relative to body weight, among toddlers and children.
d NOAEL = 150 mg/kg bw/day, based on increased relative liver weights at the next dose of 300 mg/kg bw/day in a short-term oral study.
e Age group with highest exposure relative to body weight, among teens and adults.
f NOAEL = 100 mg/kg bw/day, based on a reduction in adjusted mean pup weight at the next dose of 250 mg/kg bw/day in a continuous breeding assay.
g NOAEL=75 mg/kg bw/day on the basis of reduced litter size, pup viability, pup survival, and number of weanlings at the next dose of 150 mg/kg bw/day in a three-generation dietary study
With respect to dermal exposure to wintergreen oil from the use of analgesic creams by teens or adults, comparison of the critical effect levels to the estimated level of exposure resulted in a MOE of 5, which is considered potentially inadequate to address uncertainties in the health effects and exposure databases. With respect to dermal exposure to wintergreen oil from the use of analgesic creams by children, comparison of the critical effect levels to the estimated level of exposure resulted in an MOE of 7, which is also considered potentially inadequate to address uncertainties in the health effects and exposure databases.
A comparison of the critical effect level to the estimated level of oral exposure from the use as an ingredient in food flavourings and antacid tablets resulted in MOEs of > 30 000 and 256, respectively, which are considered adequate to address uncertainties in the health effects and exposure databases.
7.1.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the Table 7‑5 below.
| Key source of uncertainty | Impact |
|---|---|
| Although the composition of wintergreen oil is almost entirely comprised of methyl salicylate, minor components of the oil may affect dermal exposure estimates | +/- |
| There are no animal studies examining the repeated-dose toxicity of wintergreen oil for any of the relevant routes of exposure (i.e., dermal, oral, inhalation). Hazard data from the major component, methyl salicylate, was used to inform the health effects assessment, where applicable. | +/- |
| There is uncertainty in the use of the study conducted by Collins et al (1971) in that it is a three-generation study but observations of reduced litter size, pup viability, pup survival, and number of weanlings occurred only in the second generation matings. However, since salicylic acid (a metabolite of methyl salicylate) is also associated with developmental effects at similar dose levels, the findings in the Collins et al. (1971) study were considered relevant for human health risk characterization. | + |
| The continuous breeding assay conducted by NTP (1984b) using methyl salicylate was identified as the critical study for short-term, intermittent exposure scenarios. This study was conducted on CD-1 mice, which may represent a less sensitive species compared to rats. | - |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over or under estimation of risk.
7.2 Salicylic acid
7.2.1 Exposure assessment
Environmental media and food
Salicylic acid was not identified in outdoor or indoor air in Canada. Estimates on the distribution of salicylic acid in the environment suggest that over 90% of the substance would distribute to the aquatic compartment, and approximately 0.03% would be found in air, on the basis of level 1 fugacity modelling (Anonymous 2001, as cited in ECHA 2017c). Salicylic acid has a very low estimated air-water partition coefficient (7.34 x 10-9 atm-m3/mol), indicating that partitioning from the aquatic compartment to air is unlikely for this substance.
In a 2013 review by Kone et al., the authors examined the occurrence of pharmaceuticals in Canadian sewage treatment plant (STP) effluents and surface waters. Salicylic acid was reported as the analgesic detected at the highest concentration among other analgesics, with a peak measured concentration of 59.6 µg/L in STP effluents (Toronto, Ontario)and of 17 µg/L in surface waters (Gander, Newfoundland). Salicylic acid has been measured elsewhere in Canada in surface waters and waters near sewage plants at concentration ranges in the ng/L levels to low µg/L (Comeau et al. 2008; Berryman et al. 2014; Brun et al. 2006; Servos et al. 2007; Verenitch et al. 2006; Crouse et al. 2012). The authors attributed the varying concentrations of salicylic acid among these studies to specific sampling sites and water characteristics (depth, temperature, flow rate) (Kone et al. 2013).
In a 2011 publication, the National Research Council Canada (NRC) examined the presence of 954 organic chemicals from four databases on building materials, indoor air, and dust samples. A subset of data from a 2010 NRC study involving indoor air and dust samples from 115 homes in Quebec City was re-analyzed to identify certain chemicals, including substances in the Salicylates Group. Salicylic acid was identified during the re-examination of chromatograms from this Quebec study in dust samples. The geometric mean for samples containing salicylic acid greater than the method detection limit was reported to be 2.75 µg salicylic acid/g dust (NRC 2011).
JECFA evaluated a flavouring group of hydroxy- and alkoxy-substituted benzyl derivatives, including the re-evaluation of salicylic acid at its 59th meeting (JECFA 2002a,b,c). As part of the evaluation, it estimated the per capita intake (PCI) of salicylic acid from its use as a food flavouring agent by means of a maximized survey-derived daily intake (MSDI) approach. Using this approach, JECFA estimated an intake of 0.03 µg/day for the US population (0.0005 µg/kg bw/day, based on an average body weight of 60 kg for the general population) for salicylic acid. JECFA concluded there is “no safety concern at estimated levels of intake” for salicylic acid when used as a food flavouring agent (JECFA 2002a,b,c). Considering the low estimated intake of salicylic acid from foods when used as a flavouring agent (approximately 0.5 ng/kg bw/day), the potential exposure from this use is considered negligible.
No data on the natural occurrence of salicylic acid, or salicylates, in the Canadian diet was readily available. An estimate of exposure to naturally occurring salicylic acid (as total salicylates) was conducted using the highest reported analytical values for spices, fruits, vegetables, and several other food categories (personal communication, emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated December 2017; unreferenced). Data used to inform this estimate was sourced from Swain et al. (1985), Paterson et al. (2006), and Wood et al. (2011). The highest reported analytical values were applied to the relevant food codes in the Canadian Community Health Survey (CCHS) Cycle 2.2 (2004) dataset. These estimates are conservative insofar as they are based on the highest reported analytical levels of total salicylates as a surrogate for the exposure from naturally occurring salicylic acid in foods (personal communication, emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated December 2017; unreferenced).
An estimate of intake of salicylic acid from its presence in water, dust, and food was carried out using the maximum reported concentration from the review of STP effluents and surface waters by Kone et al. (2013) (59.6 µg /L), the reported mean concentration in dust from the 2011 NRC report (2.75 µg salicylic acid/g dust), and the estimated median intakes from presence in food. The intake values for each age group are presented in Table 7‑6 below.
| Route of exposure | 0-0.5 yra (formula fed)b,c | 0.5-4 yrd | 5-11 yre | 12-19 yrf | 20-59 yrg | 60 + yrh |
|---|---|---|---|---|---|---|
| Drinking wateri | 6.4 | 2.7 | 2.1 | 1.2 | 1.3 | 1.3 |
| Dustj | 1.4E-02 | 7.3E-03 | 2.8E-03 | 1.0E-04 | 9.7E-05 | 9.5E-0 |
| Foodk | N/A | 106 | 70.6 | 46.4 | 38.7 | 38.7 |
| Total intake | 6.4 | 108.7 | 72.7 | 47.6 | 40 | 40 |
a Assumed to weigh 7.5 kg, to breathe 2.1 m3 of air per day (Health Canada 1998), and to ingest 38 and 0 mg of dust and soil per day, respectively (Wilson et al. 2013).
b Exclusively for formula-fed infants, assumed to drink 0.8 L of water per day (Health Canada 1998), where water is used to reconstitute formula. See footnote on drinking water for details. Highest exposed infant group among breast milk fed infants, and non-formula fed infants (not presented above).
c Exclusively for not formula-fed infants, assumed to drink 0.7 L of water per day (Health Canada 1998), with approximately 50% of non-formula-fed infants introduced to solid foods by 4 months of age, and 90% by 6 months of age (NHW 1990).
d Assumed to weigh 15.5 kg, to breathe 9.3 m3 of air per day, to drink 0.7 L of water per day (Health Canada 1998), and to ingest 41 mg of dust per day (Wilson et al. 2013).
e Assumed to weigh 31.0 kg, to breathe 14.5 m3 of air per day, to drink 1.1 L of water per day (Health Canada 1998), and to ingest 31 mg of dust per day (Wilson et al. 2013).
f Assumed to weigh 59.4 kg, to breathe 15.8 m3 of air per day, to drink 1.2 L of water per day (Health Canada 1998), and to ingest 2.2 mg of dust per day (Wilson et al. 2013).
g Assumed to weigh 70.9 kg, to breathe 16.2 m3 of air per day, to drink 1.5 L of water per day (Health Canada 1998), and to ingest 2.5 mg of dust per day (Wilson et al. 2013).
h Assumed to weigh 72.0 kg, to breathe 14.3 m3 of air per day, to drink 1.6 L of water per day (Health Canada 1998), and to ingest 2.5 of dust per day (Wilson et al. 2013).
i The maximum concentration of salicylic acid (59.6 ug/L) from measured environmental concentrations in a sewage treatment plant (STP) effluent (Toronto, Ontario) from Kone et al. (2013) was selected for deriving estimates of daily intake from drinking water.
j The geometric mean for salicylic acid from the Quebec field study in dust samples (2.75 ug salicylic acid/g dust, NRC 2011) was selected for deriving estimates of intake for dust exposure.
k Data modified from personal communication, emails from Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada, dated December 2017; unreferenced, using the highest reported analytical values for salicylates reported in spices, fruits, vegetables, and several other food categories.
a Assumed to weigh 7.5 kg, to breathe 2.1 m3 of air per day (Health Canada 1998), and to ingest 38 and 0 mg of dust and soil per day, respectively (Wilson et al. 2013).
b Exclusively for formula-fed infants, assumed to drink 0.8 L of water per day (Health Canada 1998), where water is used to reconstitute formula. See footnote on drinking water for details. Highest exposed infant group among breast milk fed infants, and non-formula fed infants (not presented above).
c Exclusively for not formula-fed infants, assumed to drink 0.7 L of water per day (Health Canada 1998), with approximately 50% of non-formula-fed infants introduced to solid foods by 4 months of age, and 90% by 6 months of age (NHW 1990).
d Assumed to weigh 15.5 kg, to breathe 9.3 m3 of air per day, to drink 0.7 L of water per day (Health Canada 1998), and to ingest 41 mg of dust per day (Wilson et al. 2013).
e Assumed to weigh 31.0 kg, to breathe 14.5 m3 of air per day, to drink 1.1 L of water per day (Health Canada 1998), and to ingest 31 mg of dust per day (Wilson et al. 2013).
f Assumed to weigh 59.4 kg, to breathe 15.8 m3 of air per day, to drink 1.2 L of water per day (Health Canada 1998), and to ingest 2.2 mg of dust per day (Wilson et al. 2013).
g Assumed to weigh 70.9 kg, to breathe 16.2 m3 of air per day, to drink 1.5 L of water per day (Health Canada 1998), and to ingest 2.5 mg of dust per day (Wilson et al. 2013).
h Assumed to weigh 72.0 kg, to breathe 14.3 m3 of air per day, to drink 1.6 L of water per day (Health Canada 1998), and to ingest 2.5 of dust per day (Wilson et al. 2013).
i The maximum concentration of salicylic acid (59.6 ug/L) from measured environmental concentrations in a sewage treatment plant (STP) effluent (Toronto, Ontario) from Kone et al. (2013) was selected for deriving estimates of daily intake from drinking water.
j The geometric mean for salicylic acid from the Quebec field study in dust samples (2.75 ug salicylic acid/g dust, NRC 2011) was selected for deriving estimates of intake for dust exposure.
k Data modified from personal communication (emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated December 2017; unreferenced) using the highest reported analytical values for salicylates reported in spices, fruits, vegetables, and several other food categories.
Products available to consumers
Searches of material safety data sheets, the U.S. Household Products Database, the GoodGuide (GoodGuide 2018), notifications submitted to Health Canada under the Cosmetic Regulations, the Licenced Natural Health Products Database, Health Canada’s internal drug product database, and data pursuant to a section 71 survey indicate that salicylic acid is found in thousands of products available to Canadians. The presence of salicylic acid in cosmetics, in NHPs and drugs as an NMI, and in cleaning products can result in direct exposure to Canadians during use.
To evaluate the potential for exposure to salicylic acid from these products, “sentinel” scenarios were selected, i.e., scenarios that resulted in the highest level of potential exposure to salicylic acid via the oral, inhalation or dermal route, taking into consideration frequencies of use and reported concentrations of the substance.
Dermal exposure
According to notifications submitted under the Cosmetic Regulations, salicylic acid is found in approximately 2000 cosmetic products in Canada (personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated December 2016; unreferenced). The concentration of salicylic acid in these products ranges from less than 0.1% to its maximum permissible concentration of 2%. Types of cosmetics reported to contain salicylic acid include body, face, and foot moisturizers, hair care products (hair dyes, perms/straighteners), tanning products, massage oils, antiperspirants/deodorants, and shaving products, among others.
Table 7‑7 and Table 7‑8 summarize the dermal exposure to the above noted sentinel cosmetic products containing salicylic acid, for all relevant age groups.
In a 1997 human in vivo percutaneous absorption study, 2% salicylic acid formulated in a hydroalcoholic formulation (63% water, 35% ethanol and 2% salicylic acid) and a cream formulation consisting of 80% water, 2% salicylic acid, and 18% cosmetic excipient mixture (PPG-14 butyl ether, glycerin, cetyl and stearyl alcohols, polyquaternium37, mineral oil, dimethicone, Steareth-21, cyclomethicone, and triethanolamine) was topically applied to the face and neck of 10 healthy female volunteers. Plasma salicylate levels were measured at intermittent intervals during the 14-day application period (1 application per day). Plasma salicylate levels from dermal administration were compared to plasma salicylate levels after oral administration of 81 mg of acetylsalicylic acid (ASA) to determine a relative dermal bioavailability (comparison of area under the curve). The authors reported bioavailabilities of 57.6% for the hydroalcoholic formulation and 44.0% for the cream formulation, highlighting apparent differences based on application vehicle. In view of the high variability of dermal penetration of salicylic acid based on different formulations and the large number of self-care products that may contain the substance, a dermal absorption value of 60% was selected to characterize exposure to salicylic acid from dermally applied products.
| Scenarioa | Maximum reported concentrationb (%) | Systemic exposurec (mg/kg bw/day) |
|---|---|---|
| Permanent hair dye | 0.3 | 0.30 |
| Body moisturizer | 2 | 1.76 |
| Body moisturizer (infant)d | 1 | 2.00 |
| Tanning product (adult) | 1 | 0.37 |
| Foot moisturizer | 2 | 0.73 |
| Hand moisturizer | 2 | 0.64 |
| Massage oil (infant)d | 0.3 | 0.43 |
| Face moisturizer | 2 | 0.44 |
| Hair conditioner (leave-in) | 2 | 0.29 |
| Shaving lotion | 2 | 0.24 |
| Deodorant/ antiperspirant (adult) | 2 | 0.13 |
a Scenarios presented in the table above describe exposure to teens (the age group with the highest exposure relative to body-weight), unless otherwise indicated.
b Based on notifications submitted to Health Canada under the Cosmetic Regulations.
c This represents a systemic exposure, using a dermal absorption value of 60% (Davis et al. 2007).
d Age group with the highest exposure relative to body weight, among infants, teens and adults.
| Scenarioa | Maximum reported concentrationb (%) | Systemic exposurec (mg/kg bw/day) |
|---|---|---|
| Body moisturizer | 1 | 1.59 |
| Massage oil | 0.3 | 0.21 |
| Hair conditioner (leave-in) | 2 | 0.69 |
a Scenarios in the table above describe exposure to toddlers unless otherwise indicated (the age group with the highest exposure relative to body weight between toddlers and children).
b Based on notifications submitted to Health Canada under the Cosmetic Regulations.
c This represents a systemic exposure, using a dermal absorption value of 60% (Davis et al. 2007).
Given the large number and variety of cosmetic products reported to contain salicylic acid, an estimate of combined exposure to salicylic acid from multiple products was considered, taking into consideration the product types outlined in the SCCS Guidance for the Testing of Cosmetic Ingredients and their Safety Evaluation document (SCCS 2016). Estimates are presented in Table 7‑9 using a dermal absorption value of 60%, where relevant.
| Product | Maximum reported concentrationa (%) | Infant | Toddler | Child | Teen | Adult |
|---|---|---|---|---|---|---|
| Body moisturizerb | 1-2 | 2.00 | 1.59 | 0.97 | 1.76 | 1.70 |
| Face moisturizerb | 2 | N/A | N/A | N/A | 0.44 | 0.37 |
| Antiperspirant /deodorantb | 2 | N/A | N/A | 0.13 | 0.11 | 0.13 |
| Hair styling productb | 1 | N/A | N/A | 0.06 | 0.03 | 0.04 |
| Eye make-upb | 1 | N/A | N/A | N/A | 0.002 | 0.002 |
| Bath productb | 2 | 0.074 | 0.036 | 0.023 | 0.022 | 0.026 |
| Shampoob | 2 | 0.008 | 0.063 | 0.05 | 0.026 | 0.022 |
| Hair conditionerb (leave-in) | 2 | N/A | 0.69 | 0.51 | 0.29 | 0.24 |
| - |
Total combined exposure (mg/kg bw/day) | 2.08 | 2.34 | 1.74 | 3.11 | 2.53 |
a Based on notifications submitted to Health Canada under the Cosmetic Regulations.
b This represents a systemic exposure, using a dermal absorption value of 60% (Davis et al. 2007).
“N/A”: Not applicable as these age groups are not expected to use these products.
The estimates of exposure from the use of salicylic acid as an NMI in NHP’s and drugs, including acne-creams, analgesics creams, sunscreens, and after-shave creams are presented in Table 7‑10 below. The estimates of exposure to salicylic acid from use of sunscreens presented below derive from survey data and are used to characterize longer-term use (Ficheux et al. 2015, 2016). Concentrations of salicylic acid as an NMI in these types of products ranged from less than 1 to 5% (personal communication, emails from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated December 2016; unreferenced)
| Scenario | Reported concentrationa (%) | Toddlers | Children | Teens | Adults |
|---|---|---|---|---|---|
| Sunscreenb | 0.14 | 0.47 | 0.24 | 0.36 | 0.30 |
| Acne creamb,c | 2.07 | N/A | N/A | 0.75 | 0.63 |
| Analgesic creamb,d | 4.29 | N/A | 2.8 | 2.5 | 2.5 |
| After-shave creamb,e | 2.0 | N/A | N/A | 0.97 | 0.81 |
a Based on notifications reported to Health Canada under the NHPR.
b This represents a systemic exposure using a dermal absorption value of 60% (Davis et al. 2007).
c Teens and adults were assumed to weigh 59.4 and 70.9 kg (Health Canada 1998), respectively. The applied product amount was assumed to be 1.2 g (Loretz et al. 2005) and a use frequency of 3x per day (personal communication, emails from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated December 2016; unreferenced).
d Assuming area exposed is approximately ½ surface of a trunk from Health Canada (1998). Product amounts estimated based on surface area adjustment factors derived from the use of a body moisturizer (Ficheux et al. 2016). Frequency assumed to be 4x per day based on product labels (personal communication, emails from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated December 2016; unreferenced).
e Teens and adults were assumed to weigh 59.4 and 70.9 kg (Health Canada 1998), respectively. The applied product amount was assumed to be 1.2 g (Wormuth et al. 2006) and a use frequency of 4 times per day (personal communication, emails from the Therapeutics Product Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated December 2016; unreferenced).
N/A: Not applicable.
Inhalation exposure
Use of hair spray was identified as the sentinel scenario associated with the highest potential for inhalation exposure relative to other cosmetics reported to contain salicylic acid. The average air concentration during the exposure event was estimated to be 0.56 mg/m3, which corresponds to 0.00077 mg/kg bw for a teen (highest exposed relative to adults on a body-weight basis) and 0.0014 mg/kw bw/day for a child. Details on the parameters used in ConsExpo Web to estimate this exposure is presented in Appendix B.
Oral exposure
A limited number of drug products taken orally were reported to contain salicylic acid as an NMI and were antacid and absorbent products, according to available information on Health Canada’s DPD. Concentrations of salicylic acid in these products ranged from less than 0.1% to 0.2% (personal communication, emails from the Therapeutic Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated December 2016; unreferenced). Using available information on dosing regime, oral exposure to salicylic acid at its highest reported concentration in these types of products was estimated to be 3.5 mg/kg bw over a 24-hour period for children aged 10 to 12 years and 3.6 mg/kg bw/day for individuals 12 years and older (teens).
7.2.2 Health effects assessment
There are currently no hazard classifications designated for salicylic acid by the US EPA or the IARC. The ECHA RAC recently adopted an opinion proposing harmonized classification and labelling at the EU level for salicylic acid (ECHA 2016). The following classifications were adopted by consensus and have been included as part of Annex VI to the CLP Regulation as an amendment: suspected of damaging the unborn child (Repr 2), harmful if swallowed (Acute Tox 4), and causes serious eye damage (Eye Dam 1) (EU 2018).
Repeated-dose toxicity
The short-term effects of salicylic acid have been documented in a series of publicly-available reports with limited details. Lower doses of orally-administered salicylic acid were associated with minimal toxicity in different experimental animals. For example, when mice (n=10) were administered 100 mg/kg bw/day salicylic acid by gavage for 57 days, no changes in weight gain and food intake were observed when compared to control animals. In addition, no histopathological changes in the liver and kidneys were observed (Herz et al. 1951, as cited in JECFA 1962). Similarly, when rats were administered approximately 9, 69, or 237 mg/kg bw/day for 28 days, there were no mortalities, significant gross lesions, or changes in liver, kidney, adrenal, or testes weights (Anonymous 1971, as cited in ECHA 2017c). At higher doses, signs of toxicity begin to manifest. For example, when mice were given 300 mg/kg bw/day salicylic acid by gavage for 34 days, 60% of the animals died, with histopathological examinations showing significant degenerative changes of the liver and kidney, necrosis of the liver cells, and fatty infiltration of the liver (Herz et al. 1951, as cited in JECFA 1962). Necrosis of the liver and kidneys was also found after the administration of 300 mg/kg bw/day for 2 weeks in dogs. In rats that received 400, 500, or 600 mg/kg bw/day salicylic acid for 4-21 days, mortality was observed in the mid- and high-dose groups within 10 days. Beyond these doses, moderate to severe necrosis of the liver and kidneys were observed (Barbour et al. 1933, as cited in JECFA 1962). In another rat study, animals were administered 1% or 5% salicylic acid in the diet for 8 weeks (Shporn et al. 1958, as cited in JECFA 1962), which was equivalent to approximately 500 and 2500 mg/kg bw/day, respectively (Health Canada 1994). The 5% high dose was declared to be “finitely toxic” in the report in JECFA 228. The 1% level appeared to be associated with effects near the end of the experiment.
The effects of salicylic acid have also been investigated in dermal and inhalation studies. In a one-year photocarcinogenesis study, a cream containing 0, 2, or 4% salicylic acid was applied to the dorsal skin of mice (2 mg/cm2) (NTP 2007). Examination of clinical signs, body weights, the spleen, lungs, and bone marrow did not reveal significant differences compared to controls. Examinations were considered to be limited and insufficient details were provided to identify doses.
In a Health Hazard Evaluation conducted by the National Institute for Occupational Safety and Health (NIOSH 1973), medical interviews were conducted on employees at a glass manufacturing plant who were exposed to dust containing up to 0.355 mg/m3 salicylic acid. Workers reported acute symptoms of eye, nose, and throat irritation. Blood tests performed on these workers indicated negligible absorption of salicylic acid. NIOSH concluded air concentration levels of 0.07 mg/m3 salicylic acid did not cause significant blood levels of salicylic acid. However, this air level did not protect workers from the aforementioned site-of-contact symptoms.
Hazard data associated with the effects of salicylic acid following chronic administration were not identified for any of the relevant routes of exposure. The Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers (SCCNFP) conducted an assessment on the safety of salicylic acid and used hazard information from acetylsalicylic acid to supplement its review of chronic oral toxicity (SCCNFP 2002). In a 200-day study, acetylsalicylic acid was administered to rats (n=10) at a dose of 200 mg/kg bw/day (Thomas et al. 1977, as cited in SCCNFP 2002). Clinical signs, body weight observations, hematology, urinalysis, gross pathology, and histopathology were performed. No significant treatment-related effects were observed compared to control animals. The SCCNFP notes that oral doses of acetylsalicylic acid of 100 mg/kg bw or higher induce toxicity symptoms in humans (e.g., lethargy, nausea, vomiting, tinnitus, and dizziness) appearing at plasma levels of 0.35 mg/mL or greater (Cawley et al. 1953, as cited in SCCNFP 2002).
Methyl salicylate was identified as an appropriate analogue with relevant hazard data to inform the assessment of salicylic acid where there was limited data on salicylic acid itself. Methyl salicylate represents an ester of salicylic acid and contains an additional methyl group in its chemical structure. Methyl salicylate is readily metabolized to salicylic acid (Davidson et al. 1961) and they both exhibit similar physical-chemical properties and acute toxicity profiles (Table A-2, Appendix A). The effects of methyl salicylate following chronic administration have been described in the Health Effects Assessment for Wintergreen Oil (Section 7.1.2).
Carcinogenicity and genotoxicity
There is one oral carcinogenicity study conducted with salicylic acid. In this study, Saitama strain rats (n=34) were administered 0.5% to 1% salicylic acid in the diet for the duration of their lifetime (Umeda 1957). This is equivalent to approximately 250 to 500 mg/kg bw/day (Health Canada 1994). The stomachs of the experimental rats showed fibrosis, and there was one incidence of ulcer that was accompanied by liver hypertrophy, fibrotic spleen, hemosiderosis, and atrophy. However, none of the animals developed tumours in any organs during the course of the experiment. The authors concluded that salicylic acid did not show carcinogenic activity.
The carcinogenic potential from dermal application of salicylic acid has been partially informed by a photocarcinogenesis study conducted by the National Toxicology Program (NTP 2007). In this study, creams containing 0%, 2%, or 4% salicylic acid were applied to the dorsal skin of SKH-1 hairless mice (n=18/sex/group) that were subsequently exposed to filtered simulated solar light (SSL), 5 days/week for 40 weeks. The mice were examined weekly for the presence of skin lesions consistent with the development of skin tumours. Necropsies and microscopic examinations were performed for all mice on a selected number of tissues/organs: gross lesions, skin, skin tumours (squamous cell papillomas, carcinoma in situ, and squamous cell carcinoma), spleen, lungs, and the right femur (bone marrow). Mean body weights of all treated male and female mice were similar to the control group. The animals treated with salicylic acid exhibited comparable or improved survival as the concurrent controls throughout the study. No significant differences in terms of non-cancer skin effects were observed in the salicylic acid groups compared to concurrent controls. In addition, animals treated with salicylic acid had comparable or decreased incidence of skin carcinomas during exposure to SSL compared to concurrent controls. The study authors concluded that salicylic acid had some protective effect against the photocarcinogenicity of light at lower intensities.
Methyl salicylate, an analogue of salicylic acid, was evaluated under the health effects assessment for wintergreen oil and was not found to be carcinogenic in different animal models following chronic administration. Furthermore, studies conducted on a similar substance, acetylsalicylic acid, did not reveal carcinogenic potential when it was administered to mice at 1% and 5% and to rats at 0.25% and 2% in drinking water for 115 weeks (Odashima 1979, as cited in SCCNFP 2002). Overall, the available information suggests that salicylic acid is not expected to be carcinogenic.
With respect to genotoxicity, salicylic acid was negative in a series of in vitro bacterial mutagenicity assays conducted in both Samonella typhimurium and Bacillus subtillis strains (McCann et al. 1975; Kuboyama and Fujii. 1992; Japan Chemical Industry Ecology, as cited in CCRIS). In an in vivo study in male Swiss albino mice, the ability for salicylic acid to elicit sister chromatid exchange and chromosome aberrations was examined (Giri et al. 1996). Salicylic acid was administered by gavage (in 2% gum acacia, 350 mg/kg bw) or intraperitoneally (in DMSO, 25, 50, 100, and 200 mg/kg bw), followed by the extraction of bone marrow. The authors concluded that salicylic acid induced neither sister chromatid exchange nor chromosome aberrations and was not genotoxic in bone marrow cells of mice.
Reproductive and developmental toxicity
The potential for salicylic acid to cause developmental toxicity has been investigated in an oral gavage study in which pregnant Sprague-Dawley rats (n=10/group) received 0 or 20 mg/kg bw/day of salicylic acid (in distilled water) from GD 20 to 21 (Waltman 1973). Salicylic acid significantly increased the duration of gestation compared to concurrent controls (539.4 ± 2.66 vs 521.8 ± 4.36 h, respectively, p<0.01). The duration of parturition was normal for all control animals (1 h), but was 2 h for 10% of the animals treated with salicylic acid. Bleeding was normal in all control animals, but was slightly increased in 20% of the animals, and heavy bleeding occurred in another 20% of the animals treated with salicylic acid. The authors noted that typical Sprague-Dawley rats usually litter within 1 hour with little or no blood loss at parturition. No dead pups were born from any of the dams treated with salicylic acid (0/106 vs. 1/117 in control), but there was a decrease in the mean number of pups delivered per dam (10.6 vs. 11.7 in control; statistical analyses were not performed). As only one dose was tested, a dose-response relationship could not be established.
In another study, pregnant Wistar rats (n=20/group) were fed 0%, 0.06%, 0.1%, 0.2%, and 0.4% dietary salicylic acid for one week from GD 8 to 14 (Tanaka et al. 1973a). This was equivalent to 0, 50, 77, 165, and 205 mg/kg bw/day. On GD 20, 15 of the dams were sacrificed for fetal examination. Half of the fetuses were assessed for skeletal bone anomalies, while the other half were examined for internal organ anomalies. The remaining dams were allowed to give birth. The offspring were weaned on PND 21 and autopsied for visceral and skeletal anomalies. During this period, general appearance, behaviour and survival were examined daily, and body weights were recorded every 3 days. No external, internal, or skeletal bone anomalies were observed in the fetuses up to 77 mg/kg bw/day. At 165 mg/kg bw/day however, there was a significant reduction in tail length and a tendency of reduced body weight in the fetuses. Malformations such as spina bifida, deformed limbs, and cervical bone shortening were also observed in some of the fetuses and offspring. At the highest dose (205 mg/kg bw/day), there were signs of maternal toxicity such as marked body weight loss, decreased in food intake, low uterine weight, and significantly low placental weight. No live fetuses were obtained in 9 of the 15 dams and the average litter size was low. In fetuses, there was a reduced body weight and significantly reduced body and tail lengths. There was also a high incidence of severe malformations (exposed skull, spina bifida, missing digits on limbs, deformed limbs, unilateral enlargement and/or dislocated kidneys, and bone defects). The NOAEL for maternal toxicity was determined to be 165 mg/kg bw/day on the basis of weight loss, decreased food consumption, and decreased uterine/placental weights at the next dose level (205 mg/kg bw/day). The NOAEL for developmental toxicity was determined to be 77 mg/kg bw/day on the basis of reduced fetal body weights and malformations observed at the next dose level (165 mg/kg bw/day).
The same authors conducted a similar study with identical methodology, except animals were administered salicylic acid via oral gavage rather than via the diet (Tanaka et al. 1973b). Female Wistar rats (n=20/group) received 0, 75, 150, or 300 mg/kg/d salicylic acid (in 0.5% sodium carboxymethylcellulose) from GD 8 to 14 (6 days). At the lowest dose (75 mg/kg bw/day), 1.8% of the fetuses examined exhibited external anomalies (pes varus, taillessness) and 2.5% exhibited skeletal anomalies (cervical, sacral, caudal vertebrae) compared to controls in the absence of maternal toxicity. In the offspring, one case of hydronephrosis (2.5%) and one case of hydrouterus (2.5%) were observed. These observations have been observed historically in Wistar rats at similar or slightly lower frequencies (Liberati et al. 2002; Noritake et al. 2013) and thus were not considered to be treatment-related. At 150 mg/kg bw/day, there were statistically significant decreases in uterus weight, but there were no marked changes in body weight gain, general appearance, or mortality in the dams. There was significantly lower litter size, fetal body weight, and body and tail lengths. The incidence of external, internal, and skeletal anomalies was 27.8%, 12.7%, and 65.7%, respectively, compared to 0% in the control group. The pups from the pregnant animals that were allowed to rear exhibited reduced body weight, body and tail lengths, external anomalies (pes varus, syndactyly, closed eyelid, closed vaginal orifice), internal anomalies (17.2%, hydronephrosis, derformed kidney, absence of ovary, absence of uterine horn, retardation of uterine horn), and skeletal anomalies (high incidence of cervical and thoracic vertebrae showing either deformity or fusion with adjacent bone). The authors also noted that after 8 weeks, the weaning rate was below 60%. At the highest dose (300 mg/kg bw/day), signs of maternal toxicity were evident, including decreased food/water consumption, salivation, piloerection, marked weight loss, reduced uterus and placental weights, and mortality. No live fetuses were obtained in this dose group for examinations. The NOAEL for maternal toxicity was determined to be 150 mg/kg bw/day on the basis of general toxicity (weight loss, decreased food/water consumption, clinical signs) and mortality at the next dose level. The NOAEL for developmental toxicity was determined to be 75 mg/kg bw/day on the basis of decreased litter size, fetal body weight, and the observation of external, internal, and skeletal anomalies at the next dose level (150 mg/kg bw/day). This effect level is consistent with that declared by the Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers (SCCNFP 2002). The ECHA Committee for Risk Assessment (RAC) also considered this information as part of its assessment and considered the classification of salicylic acid as Repr 2 (suspected of damaging the unborn child) to be justified (ECHA 2016).
The potential for salicylic acid to cause developmental toxicity has also been investigated in single-dose studies with different routes of administration. The effects of salicylic acid when administered early or late in gestation have been examined in a study whereby pregnant NMRI mice (n=5-13) were administered 0, 500 mg/kg, or 1000 mg/kg salicylic acid (in 1% sodium carboxymethylcellulose) by oral gavage on GD 9 or GD 17 (Cekanova 1974). When salicylic acid was administered early during gestation (GD 9), there was a decrease in the number of implantations and an increase of resorbed fetuses compared to controls (no statistical analyses performed), even at the low dose that was considered to be well tolerated without mortality. Furthermore, there were increased incidences of rib and vertebral malformations when compared to control (4.5% vs 0%). When salicylic acid was administered late in gestation (GD 17), the authors noted that in addition to the previous observations, there was a very high frequency of fetal death. The results of this study confirmed earlier reports on salicylate-induced skeletal malformations when administered during early organogenesis, while when administered in late pregnancy, it caused a higher incidence of fetal death (Larsson and Eriksson 1966) and induced premature birth (Eriksson and Larsson 1968). Similar findings have been observed in Sprague-Dawley rats administered either 0 or 380 mg/kg bw salicylic acid by subcutaneous injection (Koshakji and Schulert 1973). When these animals were administered salicylic acid on GD 9, there was a high incidence of fetal malformations, resorptions, and significantly reduced fetal weights. However, in dams that were administered salicylic acid later in gestation (e.g., GD 16), there were 3 incidents of hematuria, a fetal hemorrhage along the brain/spine, and a high rate of fetal mortality. Although the authors concluded that salicylic acid caused the developmental effects, the authors also noted that salicylic acid was associated with marked maternal body weight loss, loss of appetite, complete relaxation, weakness, drowsiness, muscular limpness, inactivity, accelerated respiration rate, and occasionally elevated water intake and urinary excretion.
In a dermal study conducted by E.I. Dupont de Nemours & Co (1973), 0, 450, 670, 1000, 1500, and 2250 mg/kg bw, salicylic acid (in DMSO) was applied as a single dose to the back of CD rats (n=3) on GD 12. Animals were sacrificed on GD 20 and the following observations were made: gross examination of uterus and fetuses, number of implantation sites, number of live fetuses, number of early/late resorptions, fetal weight, and fetal crown-rump length. No statistical analyses was performed. A dose level of 1500 mg/kg bw/day was lethal to the pregnant animals. Compared to the control group, treatment with salicylic acid resulted in reduced number of implantation sites, reduced number of live fetuses, and increased number of early resorptions, although statistical analyses were not performed. Only one fetus with exencephaly was detected at the lowest dose. However, it was unknown whether this effect was treatment-related since there was no dose-dependence or statistical analyses to compare with concurrent or historical controls.
With respect to data on acetylsalicylic acid, the CIR (2003) noted that low-dose acetylsalicylic acid (81 mg) was “an exposure generally considered to not present a reproductive or toxicity risk.” However, Henderson et al. (2014) prepared a systematic evidence review of low-dose acetylsalicylic acid for the prevention of morbidity and mortality from preeclampsia and found that there was a suggestion of a higher likelihood of harm when analyses were limited to low- or average-risk women for preeclampsia. In particular, there was a tendency of an increased risk of perinatal mortality and placental abruption. Comparison of other maternal and fetal bleeding outcomes provided no evidence of harm from low-dose acetylsalicylic acid use beginning during the second trimester of pregnancy. The California Environmental Protection Agency (2016) has listed acetylsalicylic acid as causing developmental and female reproductive toxicity.
7.2.3 Characterization of risk to human health
On the basis of the data available, salicylic acid is not likely to be carcinogenic or genotoxic. Developmental toxicity was identified to be the critical effect, and the study conducted by Tanaka et al. (1973b) was considered to be the most relevant study for use in the risk characterization of salicylic acid following exposure in adults, teens, and infants. A NOAEL of 75 mg/kg bw/day was selected on the basis of statistically significant reductions in litter size, fetal body weights, and fetal body/tail lengths, and a high incidence of developmental effects and decreased weaning rate at the next dose level (150 mg/kg bw/day). These effects occurred at a dose at which no maternal toxicity was observed.
A short-term study described in the report by JECFA (1962) was considered to be the most relevant study for the characterization of risk from exposure to salicylic acid for toddlers and children. In that study (Herz et al. 1951, as cited in JECFA 1962), a NOAEL of 100 mg/kg bw/day was determined to be the critical effect level on the basis of mortality, liver damage, and kidney damage observed at 300 mg/kg bw/day. This NOAEL of 100 mg/kg bw/day is consistent with effect levels associated with the analogue methyl salicylate. Doses greater than 100 mg/kg bw/day were associated with reduced body weights, liver hypertrophy, increased kidney weights, reduced uterus/placental weights, reproductive/developmental effects, and mortality (Webb and Hansen 1963; Collins et al. 1971; Abbott and Harrisson 1978; NTP 1984a,b).
Table 7‑11 to 7-14 present relevant exposure estimates, critical effect levels, and resulting margins of exposure for characterization of risk to human health from exposure to salicylic acid.
| Scenarioa,b | Systemic exposure (mg/kg bw/day) | Critical effect (mg/kg bw/day)c | MOE |
|---|---|---|---|
| Permanent hair dye | 0.30 | NOAEL = 75 | 250 |
| Body moisturizer | 1.76 | NOAEL = 75 | 43 |
| Body Moisturizer (infant) | 2.00 | NOAEL = 75 | 38 |
| Tanning product (adult) | 0.37 | NOAEL = 75 | 203 |
| Foot moisturizer | 0.73 | NOAEL = 75 | 102 |
| Hand moisturizer | 0.64 | NOAEL = 75 | 117 |
| Massage oil (infant) | 0.43 | NOAEL = 75 | 175 |
| Face moisturizer | 0.44 | NOAEL = 75 | 170 |
| Hair conditioner (leave-in) | 0.29 | NOAEL = 75 | 259 |
| Shaving product | 0.24 | NOAEL = 75 | 312 |
| Deodorant/ antiperspirant (adult) | 0.13 | NOAEL = 75 | 577 |
| Inhalation exposure from aerosol hair product (teen) | 0.00077d (converted from 0.56 mg/m3) | NOAEL = 75 | > 90 000 |
| Dermala,b combined exposure from cosmetics (teen) | 3.11 | NOAEL = 75 | 24 |
a All exposure estimates above are for teens as they were the highest exposed relative to adults on a per kg body weight basis, unless otherwise specified in parenthesis.
b A dermal absorption value of 60% was used (Davis et al. 1997).
c NOAEL = 75 mg/kg bw/day, based on significant reductions in litter size, fetal body weights, and fetal body/tail lengths, high incidence of malformations, reduced weaning rate at the next dose of 150 mg/kg bw/day in a prenatal developmental toxicity study.
d Inhalation exposure converted to a systemic exposure using default inhalation rates and body weights corresponding to a teen. Inhalation rate: 14.5 m3/day; body weight: 59.4 kg
With respect to dermal exposure from the use of cosmetics outlined in Table 7‑11 by infants, teens, or adults, a comparison of the critical effect level to estimated exposures resulted in MOEs ranging from 38 to 577, which are considered potentially inadequate to address uncertainties in the health effects and exposure databases. Inhalation exposure from the use of an aerosol hair product resulted in an MOE of > 90 000 (when converted to a systemic exposure), which is considered adequate to address uncertainties in the health effects and exposure databases.
| Scenarioa | Exposure estimate (mg/kg bw/day) | Critical effect (mg/kg bw/day)b | MOE |
|---|---|---|---|
| Body moisturizer (toddler) | 1.59 | NOAEL=100 | 63 |
| Massage oil (toddler) | 0.21 | NOAEL=100 | 476 |
| Hair conditioner (leave in, toddler) | 0.69 | NOAEL=100 | 145 |
| Inhalation exposure from aerosol hair product (child) | 0.0014c | NOAEL=100 | > 70 000 |
| Dermal combined exposure from cosmetics (toddler) | 2.34 | NOAEL=100 | 43 |
a For the estimation of systemic exposure from use of body moisturizer, massage oil, and hair conditioner, it was assumed that 60% of salicylic acid applied to the skin would be absorbed (Davis et al. 1997).
b NOAEL = 100 mg/kg bw/day, based on liver damage, kidney damage, and mortality at the next dose of 300 mg/kg bw/day in a 57-day oral gavage study.
c Exposure concentration of 0.56 mg/m3 converted to a systemic exposure dose using default inhalation rate (14.5 m3/day ) and body weight (31 kg) of a child (Health Canada 1998).
With respect to dermal exposure from the use of body moisturizers and hair conditioners by toddlers or children, a comparison of the critical effect level to the estimate exposures resulted in MOEs of 63 and 145, respectively, which are considered potentially inadequate to address uncertainties in the health effects and exposure databases. Dermal exposure from the use of massage oils and inhalation exposure from aerosol hair products, when compared to the critical effect levels, resulted in MOEs of 476 and > 70 000, respectively, which are considered adequate to address uncertainties in the health effects and exposure databases.
| Scenarioa | Systemic exposure (mg/kg bw/day) | Critical effect (mg/kg bw/day) | MOE |
|---|---|---|---|
| Sunscreen (toddler) | 0.47 | NOAEL = 100b | 213 |
| NMI in liquid antacid (oral, short term, child) | 3.5 | NOAEL = 100b | 29 |
| Analgesic cream (child) | 2.8 | NOAEL = 100b | 36 |
| Sunscreen (teen) | 0.36 | NOAEL = 75c | 208 |
| Acne cream (teen) | 0.75 | NOAEL = 75c | 100 |
| Analgesic cream (teen) | 2.5 | NOAEL = 75c | 30 |
| After-shave cream | 0.97 | NOAEL = 75c | 77 |
| NMI in liquid antacid (oral, short-term, adult) | 3.6 | NOAEL = 75c | 21 |
a For the estimation of systemic exposure from use of sunscreen, analgesic cream, acne cream, and after-shave cream, it was assumed that 60% of salicylic acid applied to the skin would be absorbed (Davis et al. 1997).
b NOAEL = 100, based on liver damage, kidney damage, and mortality at the next dose of 300 mg/kg bw/day in a 57-day oral gavage study.
c NOAEL=75, based on significant reductions in litter size, fetal body weights, and fetal body/tail lengths, high incidence of malformations and decreased weaning rate at the next dose level, 150 mg/kg bw/day in a prenatal developmental toxicity study.
With respect to dermal and oral exposure from the use of sunscreens, liquid antacids, and analgesic creams by toddlers and children, comparison of the critical effect levels to the estimated exposure resulted in MOEs ranging from 29 to 213, which are considered potentially inadequate to address uncertainties in the health effects and exposure databases.
With respect to dermal and oral exposure form the use of sunscreens, acne creams, analgesic creams, after-shave creams, and liquid antacids by teens or adults, comparison of the critical effect levels to the estimate exposure resulted in MOEs ranging from 21 to 208, which are considered potentially inadequate to address uncertainties in the health effects and exposure databases.
| Scenario | Intake estimate (mg/kg bw/day) | Critical effect (mg/kg bw/day) | MOE |
|---|---|---|---|
| Intake from presence in environmental media (water, dust) and food for toddlers (highest exposed group relative to body weight) | 0.109 | NOAEL = 100a | 917 |
| Intake from presence in environmental media (water, dust) and food for adults | 0.040 | NOAEL = 75b | 1 875 |
a NOAEL=100 mg/kg bw/day, based on liver damage, kidney damage, and mortality at the next dose of 300 mg/kg bw/day in a 57-day oral gavage study.
b NOAEL=75 mg/kg bw/day, based on significant reductions in litter size, fetal body weights, and fetal body/tail lengths, high incidence of malformations at the next dose of 150 mg/kg bw/day in a prenatal developmental toxicity study.
Comparison of the intake of salicylic acid from its presence in the environmental media and food and the critical effect levels resulted in MOEs of 917 for toddlers and 1 875 for adults, which are considered adequate to address the uncertainties in the health effects and exposure databases.
7.2.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| There is lack of Canadian occurrence data for salicylic acid in foods; values used to derive dietary intakes of salicylic acid were obtained from measurements of total salicylate as a surrogate for salicylic acid. | + |
| Potential exposure to salicylic acid may arise from the breakdown of other salicylate esters present in products available to consumers. | - |
| The combined exposure to salicylic acid from cosmetics uses the highest reported maximum concentration data obtained under the Cosmetic Regulations and assumes all products are used together. | + |
| There were no chronic/carcinogenicity studies on salicylic acid for any of the routes of exposure. Hazard data from the analogues methyl salicylate and acetylsalicylic acid were used to inform the assessment of these endpoints. | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over- or under-estimation of risk.
7.3 Homosalate
7.3.1 Exposure assessment
Environmental media
Homosalate was not identified in indoor air or outdoor air in Canada. However, considering its low vapour pressure (0.015 Pa) and use profile (ingredient in sunscreens and lotions), exposure to the general population from its presence in outdoor or indoor air is expected to be minimal.
Homosalate was identified in dust samples from 115 homes in Quebec City analyzed in the NRC study (2011) mentioned above. The geometric mean for all data samples reporting homosalate greater than the method detection limit was reported in the study to be 6.50 µg homosalate/g dust.
Studies on the presence of homosalate in drinking water or surface waters in Canada were not identified. Homosalate was identified in several surface water samples from various locations globally. In their analysis of existing literature, Hopkins and Blaney (2016) reported homosalate concentrations in surface waters to range from less than 0.001 µg/L to less than 1.0 µg/L, with a 75th percentile concentration of approximately 0.07 µg/L (n = 35, reported from studies sampling surface waters in Japan and Slovenia (Tashiro et al. 2013, Kameda et al. 2011, Cuderman et al. 2007, all as cited in Hopkins and Blaney 2016). Some studies examined water samples from beaches, for which the homosalate concentrations may be attributed to the use of sunscreens during beach activities (Tashiro et al. 2013, Kameda et al. 2013, both as cited in Hopkins and Blaney 2015; Rodriguez et al. 2015). Homosalate in surface waters is not expected to persist as the substance has a short hydrolysis half-life of approximately 9 days at neutral pH (ECHA 2017e).
Modelling using the quantities reported in Canadian commerce for 2011 (Environment Canada 2013) was performed with ChemCAN (ChemCAN 2003) to estimate environmental concentrations of homosalate in soil (as the substance may persist in soil due to its high octanol-water partition coefficient). Using the modelled estimates for homosalate in soil and the measured concentrations in dust (6.50 ug/g dust) and surface water (0.07 ug/L, maximum concentration reported from a search of available data), intakes were derived and estimated to be in the nanogram range, which demonstrate a minimal exposure potential (approximately 40 ng/kg bw/day) to homosalate from environmental media.
Products available to consumers
According to notifications under the Cosmetic Regulations, homosalate is found in face moisturizers, massage products, fragrance products, make-up (non-permanent), bath products, shampoos, and tanning products. To assess the potential for dermal and inhalation exposure from the use of these products, “sentinel” scenarios were selected, i.e., scenarios that resulted in the highest level of potential exposure to homosalate, taking into consideration frequencies of use and reported concentrations of the substance. Tanning products and face moisturizers were used as representative products.
Available information on homosalate suggests that the substance has a low dermal absorption potential (NICNAS 2015; SCCP 2007). In an in vitro dermal absorption study (OECD Guideline 428) of homosalate using dermatomed human skin, absorption of the substance was measured in unoccluded conditions over a 24-hour period (ECHA 2017e). Homosalate was applied (0.5449 mg/cm2) to skin samples, and a potentially absorbable dose of 7.63 µg/cm2 was reported for a standard sunscreen formulation containing 10.1% by weight of radiolabelled homosalate. This dose describes the amount of homosalate that is potentially absorbable per unit area applied to the skin surface over a 24-hour period. Mean recovery of human skin samples was 92.4%. To assess exposure to homosalate from face moisturizers and tanning products, the dose of 7.63 µg/cm2 (corresponding to a sunscreen preparation) was used owing to the similarity between product type and experimental formulations, the duration of experimental conditions, and the duration of exposure scenarios estimated. Table 7-16 below summarizes the daily systemic exposure to homosalate from these products.
| Product | Maximum reported concentration (%)a | Infant | Toddler | Child | Teen | Adult |
|---|---|---|---|---|---|---|
| Face moisturizerb,c | 30% | N/A | N/A | N/A | 0.082 | 0.069 |
| Tanning product (spray)c,d | 10% | N/A | N/A | N/A | N/A | 1.20 |
a Based on notifications submitted under the Cosmetic Regulations.
b This represents a systemic exposure using a potentially absorbable dose of 7.63 µg/cm2 (ECHA 2017e) and default body and face surface areas from Health Canada (1998).
c This represents a daily exposure.
d This represents a per-event exposure.
“N/A“: Not applicable as these age groups are not expected to use these products.
Homosalate is also present in a moisturizer formulated as an aerosol for facial application (personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated December 2016; unreferenced). Exposure from this use may result in incidental inhalation exposure. As no product-specific exposure parameters were available for aerosol moisturizers, default values for a deodorant spray were modified to represent this exposure scenario and can be found in Appendix B. The air concentration during exposure to homosalate at maximum concentration of 3% in an aerosol moisturizer was estimated to be 4.0 mg/m3. This value represents the average air concentration of homosalate over the estimated exposure duration of 5 minutes. The potential inhalation exposure from the use of a tanning product in a spray formulation is addressed in the assessment of the aerosol facial moisturizer.
7.3.2 Health effects assessment
There are currently no hazard classifications designated by the European Chemicals Agency (ECHA), the United States Environmental Protection Agency (US EPA) or the International Agency for Research on Cancer (IARC) for homosalate.
Repeated-Dose toxicity
The short-term effects of homosalate have been described in a report by the Scientific Committee on Consumer Products (SCCP) (unpublished data 2005, as cited in SCCP 2007). In a 2-week range-finding, non-GLP-compliant study, rats (n=5/sex/group) were administered 0, 100, 300, and 1000 mg/kg bw/day homosalate by gavage for 2 weeks. Clinical signs, body weight, food consumption, hematology, and clinical chemistry were performed. At the end of the study, all animals were sacrificed and macroscopically examined. With the exception of slightly retarded body weight gain and a corresponding reduction of food efficiency at 1000 mg/kg bw/day, there was no effect on body weight or food consumption or efficiency in the other groups. Increases in blood coagulation times were observed in males at greater than or equal to 300 mg/kg bw/day and in females at 1000 mg/kg bw/day. Bilirubin was reduced at doses greater than or equal to 100 mg/kg bw/day in males and at doses greater than or equal to 300 mg/kg bw/day in females, while triglycerides were increased for both sexes at the highest dose. However, these effects were considered as not adverse or only potentially adverse by the author (no data or further information supplied). The author established a NOAEL of 100 mg/kg bw/day on the basis of coagulation in males at ≥ 300 mg/kg bw/day and in females at 1000 mg/kg bw/day.
The short-term effects of homosalate exposure via the oral route have also been examined in a combined repeated-dose and reproduction/developmental screening study (OECD TG 422), whereby homosalate (in corn oil) was administered by gavage at doses of 0, 60, 120, 300, and 750 mg/kg bw/day to male rats (n=10/group) for 67 days and to female rats (n=10/group) for 14 days prior to pairing, during the pairing and gestation periods, and until the offspring reached day 3 post-partum (approximately 7 weeks) (unnamed study report 2013; ECHA 2017f). Clinical signs, mortality, food consumption, body weights, number of implantation sites, corpora lutea, litter size, live births, still births, and gross anomalies were recorded. All parental animals and pups were examined macroscopically. Qualitative assessment of male reproductive organs was performed, with special emphasis on the first stages of spermatogenesis and histopathology of interstitial cell structure. Histological examination of the ovaries was also carried out on any females that did not give birth and on the reproductive organs of infertile males. At 300 mg/kg bw/day, there were statistically significant increases in absolute liver weight, liver-to-brain weight, kidney weight, and kidney-to-brain weight in females. In addition, there was a higher incidence and/or severity of diffuse hypertrophy of the follicular epithelium of the thyroid glands in female animals and a greater incidence and severity of decreased cortical lymphocytes in the thymus of males. At a dose of 750 mg/kg bw/day, one animal died and one was sacrificed due to severe toxicity. In the surviving animals, there were significantly higher concentrations of albumin, significantly increased absolute and relative liver weights, reduced food consumption and reduced body weight. In males, absolute weights of the prostate and seminal vesicles were significantly reduced. In addition, there was a higher incidence and/or severity of diffuse hypertrophy of the follicular epithelium of the thyroid glands and a greater incidence and severity of decreased cortical lymphocytes in the thymus in both sexes.
With respect to the liver effects, the authors indicated that the increased relative liver weights were accompanied by mild centrilobar hepatocyte hypertrophy. In the absence of related changes in clinical biochemistry parameters, findings in the liver were considered to be an adaptive reaction to increased metabolic burden caused by the treatment. With respect to the kidney effects, the authors noted that although increased kidney weights were observed in females, these effects were not considered to be adverse due to the absence of any microscopic changes. For the findings in the thymus, the authors indicated that these effects were considered to be a nonspecific response to stress rather than an effect of immunosuppression due to the absence of any effects in other lymphatic tissues. For the findings in the thyroid gland, the authors indicated that they were most probably associated with the presence of enzyme induction in the liver and consequent increased hepatic clearance of thyroid hormone. The authors established a NOAEL of 300 mg/kg bw/day on the basis of reduced food consumption, reduced body weight, and mortality at the next dose level. However, since the kidney represents an endocrine organ, changes in kidney weights should not be dismissed. With respect to the thymus, Elmore (2006) has noted that a decrease in size or weight of the thymus is one of the first measures of compound-induced effects, with cortical lymphocytes (thymocytes) being especially susceptible. Without further analyses to determine the cause of the decreased cortical lymphocytes or further analyses on other immune parameters, the effects on the thymus are considered relevant to health effects characterization. Similarly, the effects on the thyroid gland could not be dismissed given the lack of hormone analyses and liver enzyme assessment. On the basis of these considerations, the 300 mg/kg bw/day dose level was considered to be a LOAEL instead, which differs from the NOAEL of 300 mg/kg bw/day established by the authors. It was noted that there was a disturbance of the light/dark cycle resulting in constant lighting of the experimental room. The impact of this methodological error on the study results was not clear.
Carcinogenicity and genotoxicity
Carcinogenicity studies for homosalate were not identified. Methyl salicylate was identified as an appropriate analogue with relevant hazard data to inform the assessment of carcinogenicity for homosalate. Both methyl salicylate and homosalate represent esters of salicylic acid and are expected to share the common metabolite salicylic acid (TIMES 2017). The available data on methyl salicylate suggests that carcinogenicity is not likely to be a concern (Section 7.1.2). With respect to genotoxicity, homosalate has consistently resulted in negative findings in bacterial mutagenicity assays (Bonin et al. 1982; Zeiger et al. 1987; Anonymous 2005, as cited in ECHA 2017f). In addition, homosalate did not induce gene mutations or chromosome aberrations in mammalian cells in vitro, with and without metabolic activation up to cytotoxic concentrations (Anonymous 2005, eAnonymous 2006, eAnonymous 2013, all as cited in ECHA 2017f). Although no in vivo genotoxicity assays have been conducted, the available information indicates that homosalate is unlikely to be genotoxic.
Reproductive and developmental toxicity
In the combined repeated-dose and reproduction/developmental screening study (OECD TG 422) noted previously, homosalate was administered by gavage at doses of 0, 60, 120, 300, and 750 mg/kg bw/day to rats prior to pairing, during the pairing and gestation periods, and until the offspring reached day 3 post-partum (unnamed study report 2013; ECHA 2017f). The litters were examined for litter size, live births, still births, sex ratio and any gross anomalies. All parental animals and pups were examined macroscopically for any structural changes at necropsy or after death. No treatment-related adverse effects were noted up to 120 mg/kg bw/day. At 300 mg/kg bw/day, a statistically significant increase in post-implantation loss was noted, which resulted in a lower birth index (79.5% vs 91.8% in controls). At the 750 mg/kg bw/day dose level, there was a statistically significant decrease in the number of normal complete sperm (89.% compared to 97.3% in controls), reduced sperm motility, and reduced mean number of progressive motile sperms (52.4% vs 72.7%), although sperm count was unaffected. Absolute weights of prostate and seminal vesicles were also significantly reduced. In females, the number of corpora lutea in pregnant females was lower than the control value. In one female, post-implantation loss was very high and only one pup was found in the litter. The two remaining pregnant females in this group did not give birth to live pups, resulting in a significantly reduced birth index (12.5% vs 91.8% in control). It should be noted that infertility was recorded across all groups resulting in low numbers of pregnant females, which could have been due to a disturbance of the light/dark cycle during the conduct of this study. The investigators noted that there was no indication of a reproductive effect at doses of 60 and 120 mg/kg bw/day. However, because of low numbers of pregnant females, none of these dose levels could be conclusively confirmed as a NOAEL. No effect on development was observed up to 300 mg/kg-bw/day. At the highest dose (750 mg/kg-bw/day), the body weight of the single pup was lower compared to the mean pup body weight in the control group. This occurred at the dose where maternal toxicity was evident, as demonstrated by reduced body weights, reduced food consumption, and mortality. Given the small number of pregnant females however, these results were considered to be equivocal. Overall, a LOAEL of 300 mg/kg bw/day was determined in this screening assessment on the basis of increased post-implantation loss and decreased birth index observed in the reproduction/developmental component of this study and on the basis of histopathological changes in the thyroid gland and thymus observed in the repeated-dose component of the study. As mentioned previously, the impact of the disturbance in the light/dark cycle resulting in constant lighting of the experimental room and how this methodological error could affect the results of the study are not fully understood.
There were also studies examining the effects of homosalate on estrogenic activity. With respect to in vitro studies, the results were considered to be equivocal (Schlumpf et al. 2001; Schreurs et al. 2002; Ma et al. 2003; Schlumpf et al. 2004; Gomez et al. 2005; Kunz et al. 2005; Schreurs et al. 2005; Kunz and Fent 2006; Kunz et al. 2006; Jimenez-Diaz et al. 2013). However, findings of estrogenic activity were not detected in vivo. In a 3-day uterotrophic bioassay administering up to 1000 mg/kg bw/day homosalate by subcutaneous injection to juvenile Wistar rats (n=6/group), no treatment-related adverse effects were recorded (Anonymous 2002, as cited in ECHA). Homosalate was also found to be inactive in other uterotrophic assays when doses of up to 2100 mg/kg bw/day were administered to Long Evans rats (n=4 to 6/group) in the diet for 3 to 4 days (Schumpf et al. 2001; Schlumpf et al. 2004).
7.3.3 Characterization of risk to human health
On the basis of the data available, homosalate is not likely to be carcinogenic or genotoxic. The combined repeated-dose and reproduction/developmental screening study (OECD TG 422) was identified to be the most appropriate study for the risk characterization of exposure to homosalate in infants, teens, and adults. A LOAEL of 300 mg/kg bw/day was selected as the critical effect level on the basis of increased kidney weights, histopathological changes in the thyroid gland and thymus, increased post-implantation loss, and a decreased birth index. This study was conducted after the publication of the report generated by the SCCP (2007), in which a margin of safety was derived from a NOAEL of 100 mg/kg bw/day on the basis of coagulation effects observed at the next dose level (i.e., 300 mg/kg bw/day) in a 14-day range-finding study.
Table 7‑17 provides all relevant exposure estimates, critical effect levels and resulting margins of exposure for characterization of risk to human health for homosalate.
| Scenario | Systemic exposure (mg/kg bw/day) | Critical effect (mg/kg bw/day) | MOE |
|---|---|---|---|
| Tanning product (spray) (10%, adult)a | 1.20 | LOAEL = 300b | 250 |
| Face moisturizer (30%, teen)a | 0.082 | LOAEL = 300b | 3 658 |
| Aerosol facial moisturizer (3.0%, toddler) | 0.015c (converted from 4 mg/m3) | LOAEL = 300b | 20 000d |
| Aerosol facial moisturizer (3.0%, adult) | 0.0057c (converted from 4 mg/m3) | LOAEL = 300b | 52 632d |
a This represents a systemic exposure using a potentially absorbable dose of 7.63 µg/cm2 (ECHA 2017e) and default body and face surface areas from Health Canada (1998).
b LOAEL = 300 mg/kg bw/day, based on increased kidney weights, histopathological changes in the thyroid glands and thymus, increased post-implantation loss, and decreased birth index in a combined repeated-dose and reproductive, developmental toxicity study.
c Converted to a systemic exposure. Toddler: Inhalation rate 9.3 m3/day and body weight of 15.5 kg; adult: inhalation rate of 16.2 m3/day and body weight of 70.9 kg (Health Canada 1998).
d Assuming equivalent oral and inhalation absorption.
With respect to dermal exposure to homosalate from the use of tanning products, comparison of the critical effects to the estimated exposures resulted in an MOE of 250, which is considered potentially inadequate to address uncertainties in the exposure and health effects databases. Exposure from the use of a face moisturizer, when compared to the critical effect level, results in an MOE of 3658, which is considered adequate to address uncertainties in the exposure and health effects databases.
Inhalation exposure to homosalate from the use of an aerosol face moisturizer was converted to a systemic exposure and compared to the critical effect levels for adults and toddlers. Respective MOEs were greater than 20 000 and are considered adequate to address the uncertainties in the health effects and exposure database.
7.3.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| No chronic studies have been conducted using homosalate for any relevant routes of exposure. Short-term data was used to inform the assessment. | +/- |
| There is uncertainty with respect to the hazard dataset for homosalate. The combined repeated-dose and reproductive/developmental toxicity study was associated with methodological errors (i.e., disturbance in the light cycle), which may have affected the interpretation of the effects and effect levels. | +/- |
7.4 Phenethyl salicylate
7.4.1 Exposure assessment
Environmental media and food
No measured concentrations of phenethyl salicylate in air, water, or soil were identified. In consideration of the low quantities (<100 kg) of the substance reported to be used in Canada (ECCC 2017), exposure to phenethyl salicylate from potential releases to the environment is not expected.
As phenethyl salicylate is permitted for use as a food flavouring agent in the United States and Europe, it is possible that the substance may be present in foods as a flavouring agent in Canada. JECFA evaluated a flavouring group of hydroxy- and alkoxy-substituted benzyl derivatives, including a re-evaluation of phenethyl salicylate at its 59th meeting (JECFA 2002a,b,c). As part of the evaluation, the it estimated the per capita intake of phenethyl salicylate from its use as a food flavouring agent by means of an MSDI approach. Using this approach, JECFA estimated an intake of 4 µg/day for the US population (0.07 µg/kg bw/day based on an average body weight of 60 kg for the general population) for phenethyl salicylate. The JECFA concluded there is “no safety concern at estimated levels of intake” for phenethyl salicylate when used as a food flavouring agent.
Products available to consumers
According to information obtained pursuant a section 71 survey, phenethyl salicylate was identified as a fragrance ingredient in cosmetics and products available to consumers in Canada (ECCC 2017). Types of cosmetics that were reported to contain phenethyl salicylate include hair shampoos and conditioners, body washes, antiperspirants/deodorants, skin creams, and shave gels (ECCC 2017). Concentrations in these products were below 1 ppm. Phenethyl salicylate was also reported to be present at less than 2 ppm in laundry detergents, laundry scent beads, fabric enhancer liquids & sheets, and dishwashing liquids and is present at a maximum level of 500 ppm in plug-in air fresheners (ECCC 2017).
In a 2007 fragrance material review on phenethyl salicylate, Lapczynski et al. (2007) estimated maximum daily exposures on the skin of phenethyl salicylate from its presence as a fragrance ingredient in 10 representative cosmetic product types (body lotion, face cream, eau de toilette, fragrance cream, antiperspirant, shampoo, bath products, shower gel, toilet soap, and hair spray). The authors used information generated from a 2002 International Fragrance Association (IFRA) survey to derive 97.5th percentiles for phenethyl salicylate levels in fragrance mixtures in general (1.8827%) and typical concentration levels of these of fragrance mixtures in each type of cosmetics (0.3% to 8%) (Lapczynski et al. 2007).
Although the identified cosmetic products in Canada containing phenethyl salicylate report the substance to be present at low concentrations, it may be present in other cosmetics available to consumers in Canada at higher concentrations, considering that information from Lapzynski et al. (2007) reported 97.5th percentile levels of 1.8827% in the fragrance component of cosmetics. On the basis of this information, estimates of exposure to phenethyl salicylate were derived for “sentinel” scenarios, i.e., scenarios that resulted in the highest level of potential exposure to phenethyl salicylate by inhalation or dermal route, using concentration data from Lapczynski et al. (2007).
Dermal exposure to phenethyl salicylate was estimated using the data from the IFRA on phenethyl salicylate concentrations in representative cosmetic products (as these values were higher than those reported under a section 71 survey). The sentinel scenario to describe dermal exposure to the substance was the use of a body moisturizer containing phenethyl salicylate at a concentration of 0.0076% based on the 2002 IFRA survey data, as cited in Lapczynski et al. (2007). The resultant daily dermal exposure for infant, toddler, child, teenager, and adult age groups were 0.025, 0.02, 0.012, 0.011, and 0.011 mg/kg bw/day, respectively. Inhalation exposure to phenethyl salicylate was estimated on the basis of its use in a plug-in air freshener (ECCC 2017). Using ConsExpo Web (2016), the daily air concentration from the use of this product was estimated to be 0.0027 mg/m3 (0.0016 mg/kg bw/day, toddler)
7.4.2 Health effects assessment
There are currently no hazard classifications designated for phenethyl salicylate by the US EPA, ECHA or the IARC. The hazard dataset for phenethyl salicylate was considered to be limited. Therefore, data from the analogues benzyl salicylate, phenyl salicylate, and methyl salicylate were used to inform the health effects assessment of phenethyl salicylate. The former two analogues differ from phenethyl salicylate by one or two carbon atoms at the aromatic ring and thus represent more closely related analogues with respect to physical-chemical properties (Table A-2, Appendix A). However, phenethyl salicylate and its analogues are all expected to be metabolized to the common product, salicylic acid (Davison et al. 1961; TIMES 2017). As a result, these substances are predicted to share similar toxicological profiles.
Repeated-dose toxicity
Studies examining the effects of phenethyl salicylate following repeated administration have not been identified. Furthermore, no studies on repeated-dose toxicity were identified for benzyl salicylate, the closest analogue of phenethyl salicylate. The hazard data available for phenyl salicylate was limited to an abstract of a 51-day study in beagle dogs (Kociba et al. 1976, abstract only). In this study, the dogs were administered phenyl salicylate orally by capsules. Doses of 250 mg/kg bw/day and 500 mg/kg bw/day were not tolerated by the dogs, which showed decreased appetite, body weight, and activity. The urine and feces were darkened and there were transient increases in the percentage of neutrophilic leukocytes. Serum glutamic pyruvic transaminase and glutamic oxaloacetic transaminase activities were elevated. When the dose was adjusted to 125 mg/kg bw/day, values for all of the affected parameters returned to within normal limits. Subsequent examination of hematologic, urinary, chemical, clinical, and morphological parameters revealed no treatment-related alterations. The NOAEL was determined to be 125 mg/kg bw/day on the basis of adverse effects at the next dose levels.
Methyl salicylate is another analogue of phenethyl salicylate. The effects of methyl salicylate following repeated administration have been described previously in the Health Effects Assessment for Wintergreen Oil. For further details, refer to the section Wintergreen Oil above.
Carcinogenicity and genotoxicity
There were no studies identified pertaining to chronic toxicity or carcinogenicity for phenethyl salicylate, benzyl salicylate or phenyl salicylate. However, studies in experimental animals that administered methyl salicylate for a period of 2 years did not reveal any carcinogenic effects. In the study conducted by Webb and Hansen (1963), Osborne-Mendel rats (n=25/sex/group) were administered 0%, 0.1%, 0.5%, 1%, or 2% methyl salicylate in the diet while beagle hounds (n=2/sex/group) were given methyl salicylate as oral capsules containing 0, 50, 150, 350 mg/kg/day, 6 days a week for 2 years. No carcinogenic effects were reported. Further details on these studies can be found in the section Wintergreen Oil, above. On the basis of this information, phenethyl salicylate is not expected to be carcinogenic.
Studies investigating genotoxicity have not been identified for phenethyl salicylate. However, the data available for the analogues benzyl salicylate, phenyl salicylate, and methyl salicylate suggest that genotoxicity is unlikely. In a bacterial mutagenicity assay, benzyl salicylate did not reveal mutagenic effects in a series of Salmonella typhimurium strains (TA100, TA1535, TA1537, TA98) (Zeiger et al. 1987). When phenyl salicylate was tested, negative results were obtained for all strains except TA100, where the response was considered questionable (dose-related increase was insufficiently high to declare mutagenicity or a non-dose-related increase was seen). However, subsequent follow-up testing demonstrated negative results in TA100. The analogue methyl salicylate was also negative for genotoxicity in several in vitro assays such as Rec-assays (Oda et al. 1978; Kuboyama and Fujii 1992) in bacterial mutagenicity assays (Mortelmans et al. 1986; Kuboyama and Fujii 1992; Ishidate et al. 1984), and in mammalian cell mutagenicity assays (Ishidate et al. 1984).
Reproductive and developmental toxicity
The dataset for reproductive and developmental toxicity following the administration of phenethyl salicylate is limited. No studies using benzyl salicylate were identified. For phenyl salicylate, one study was identified whereby pregnant Wistar rats were either administered 100, 200, and 300 mg/kg bw/day phenyl salicylate (in 0.5% carboxymethylcellulose) by gavage from GD 7 to 12 or they were administered 200, 300, and 400 mg/kg bw/day from GD 7 to 9 (Baba et al. 1966). Fetuses were removed for examinations on GD 20. At 200 mg/kg bw/day, seven fetuses exhibited retarded costal ossification with dorsal waved ribs. Maternal mortality was observed at 300 and 400 mg/kg bw/day associated with a high incidence of resorption and maceration in the fetuses. The fetuses from the highest dose group also had Y-shaped, fused and waved ribs. The authors noted that reduced fetal weights were observed following a high dose or a long administration, but no data were presented within the report. Exencephaly was also reported, but it was unknown at which dose these observations occurred. Although no statistical analyses were performed on any of the outcomes, the authors concluded that administration of phenyl salicylate resulted in a high rate of resorption and maceration and a low rate of anomalies in fetuses.
Studies examining the potential developmental toxicity for methyl salicylate have been previously discussed. Further details can be found in the health effects assessment of wintergreen oil, above. Overall, although no studies investigating the effects of phenethyl salicylate on reproductive or developmental toxicity, information on the analogues suggest that developmental effects are expected to occur following exposure to this substance.
With respect to estrogenic activity, the effects of phenethyl salicylate in vivo were considered to be inconclusive. In an immature mouse uterotrophic assay, immature female CD-1 mice (n=12/group) at an age of PND 19 were administered 0, 11.1, 33.3, 100, or 300 mg/kg/day phenethyl salicylate (in peanut oil) by gavage for 3 days beginning on PND 21. Body weights were recorded daily throughout the study. At PND 24, the mice were weighed and sacrificed, and their uteri dissected. Each uterus was blotted, and the wet weight was recorded. No mortality occurred in any of the dosing groups. The uterine weights were significantly increased in mice that received 33.3 mg/kg/day, corresponding to 115% of the control. Although the authors stated that results from the immature mouse uterotrophic assay suggest that phenethyl salicylate could exert estrogenic activities in vivo, no dose-dependent relationship on the uterine weights was observed (Zhang et al. 2012).
7.4.3 Characterization of risk to human health
Studies investigating chronic toxicity, carcinogenicity, or genotoxicity for phenethyl salicylate were not identified. However, information from the analogues benzyl salicylate, phenyl salicylate, and methyl salicylate suggest the carcinogenicity and genotoxicity of the substance is unlikely.
A three-generation study conducted with methyl salicylate by Collins et al. (1971) on Osborne-Mendel rats was considered to be the most relevant study for the characterization of risk for phenethyl salicylate, for all exposure scenarios, for both children and adult age groups. A NOAEL of 75 mg/kg bw/day was selected as the critical effect level on the basis of reduced litter size, reduced pup viability, reduced pup survival, and reduced number of weanlings observed at the next dose level (150 mg/kg bw/day), in the absence of maternal toxicity.
Table 7‑19 provides all relevant exposure estimates, critical effect levels and resulting margins of exposure for characterization of risk to human health for phenethyl salicylate.
| Scenario | Systemic exposurea (mg/kg bw/day) | Critical effect level (mg/kg bw/day) | Margin of exposure (MOE) |
|---|---|---|---|
| Dermal exposure from the use of a body lotion (infant) | 0.025 | NOAEL=75b | 3 000 |
| Dermal exposure from the use of a body lotion (adult) | 0.011 | NOAEL=75b | 6 818 |
| Oral intake from use as a food flavouring agent (adult) | 0.00007 | NOAEL=75b | > 1 000 000 |
| Inhalation exposure from a plug-in air freshener (toddler)b | 0.0016 (converted from 0.0027 mg/m3) | NOAEL=75b | 46 875 |
a Converted to a systemic exposure, assuming dermal absorption is equivalent to oral absorption
b NOAEL = 75 mg/kg bw/day, based on reduced litter size, pup viability, pup survival, number of weanlings at the next dose level, 150 mg/kg bw/day, in a three-generation dietary study
These MOEs are considered adequate to address uncertainties in the health effects and exposure databases. As the substance is typically used as a fragrance ingredient, there may be a potential for its presence in multiple types of products available to consumers, resulting in a potential for aggregate exposure (Lapzynski et al. 2007; ECCC 2017). However, the large margin of exposure for phenethyl salicylate from the use of a body lotion (several orders of magnitude) is considered sufficient to address this uncertainty.
While exposure of the general population to phenethyl salicylate is not of concern at current levels, this substance is considered to have a health effect of concern because of its potential to elicit developmental toxicity (based on read-across from the analogue methyl salicylate). Furthermore, salicylic acid, one of the predicted metabolites of phenethyl salicylate (TIMES 2017), has been classified by ECHA as “suspected of damaging the unborn child” (Repr 2). Therefore, there may be a concern for human health if exposures were to increase.
7.4.4 Uncertainties in evaluation of risk to human health
| Key source of Uncertainty | Impact |
|---|---|
| 95th percentile data from the IFRA were used to inform the potential use of phenethyl salicylate in the absence of substantive Canadian data. | + |
| Dermal and oral absorption were assumed to be equivalent for the risk characterization of phenethyl salicylate. | + |
| Due to the lack or limited health effects data for phenethyl salicylate for relevant routes and durations of exposure for phenethyl salicylate, the use of analogue data on related substances (methyl salicylate) were used to inform the hazard characterization of this substance. | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over or under estimation of risk.
7.5 Betula alba extract
7.5.1 Exposure assessment
The following section provides information on exposure to Betula alba extract. As the substance is considered to be of low hazard potential (Section 7.5.2), quantitative estimates of exposure to the general population were not estimated.
No Canadian or recent international data on levels of Betula alba extract in ambient air, indoor air, drinking water, dust, or soil were identified. However, according to information submitted pursuant to a section 71 survey (ECCC 2013), this substance was not reported to be imported or manufactured in Canada above 100 kg, and the potential for exposure to Betula alba extract from environmental media is therefore not expected.
Given there are no uses reported for Betula alba extract as a food additive or as an ingredient in food packaging materials or incidental additives (see Uses – Section 4.3), exposures to the general population from food are not expected.
Information on specific uses of Betula alba extract in Canada can be found in the Uses section of this assessment report (Section 4.3). Dermal exposure of the general population to Betula alba extract is expected given its use as an ingredient in cosmetics, drugs, and natural health products.
7.5.2 Health effects assessment
Betula alba extract represents a chemical substance of UVCB nature derived from Betula alba. The composition of constituents in Betula alba extract vary depending on the plant part used (e.g., buds, leaves, bark), the type of extract, and the extraction process. Some of the major components that have been identified include betulin and its derivatives (10.5% to 82%) (Laszczyk et al. 2006; Abyshev et al. 2007; Orav et al. 2011; EMEA 2014). Although methyl salicylate represents a major component in a birch species known as Betula lenta, it has not been identified in Betula alba on the basis of the information available.
There are currently no hazard classifications designated for Betula alba extracts or the main components by the US EPA, ECHA, or IARC. Since the hazard dataset for Betula alba extracts was limited, information on the main components was also considered in the health effects assessment for this substance.
Studies examining the short-term effects of a Betula alba extract have demonstrated a lack of treatment-related, systemic adverse effects following intraperitoneal (i.p.) and subcutaneous (s.c.) routes of administration (Jäger et al. 2008). No histopathological or morphological effects were observed in rats and dogs injected with doses of up to 520 mg/kg bw/day (i.p.) and 300 mg/kg bw/day (s.c.), respectively, of birch extract for 28 days. In a study investigating the effects of birch extracts in rats administered a high-fat diet, animals administered up to 2500 mg/kg bw/day of Betula alba extract for 64 days did not appear to exhibit any signs of toxicity or mortality (Boqué et al. 2012).
Other studies examining the effects of Betula alba extracts or its components on immunosuppression (Koffuor et al. 2014), liver disease (Di et al. 2009), sepsis (Zhao et al. 2016), arsenic-induced nephrotoxicity (Prakash et al. 2018), and malaria (Steele et al. 1999) also did not identify or report any adverse effects.
The epidemiological data examining the use of Betula alba extracts or its components on liver disease, skin conditions, cancer, and immunosuppression was associated with a low incidence of side effects (Huyke et al. 2006; Huyke et al. 2009; Wolters Kluwer Health Inc. 2010; Shikov et al. 2011; Koffuor et al. 2014; Barret et al. 2017). However, these effects were considered to be mild and transient.
Chronic studies on Betula alba extracts and its components are currently limited. However, this substance is not expected to be carcinogenic or genotoxic on the basis of the information available (Boyland and Mawson 1938; Pisha et al. 1995; Zuco 2002; Zhanataev et al. 2004; Jäger et al. 2008; Ciurlea et al. 2010). Some of the components appear to exhibit anti-tumorigenic activity and have been selected by the National Cancer Institute for addition into the RAID (Rapid Access to Intervention Development) program, which provides a formalized process to quickly transition novel molecules from the laboratory to the clinic for clinical trial testing. Although reproductive or developmental toxicity studies were not identified in the literature, treatment-related adverse effects on the reproductive system have not been identified in the short-term studies mentioned previously.
7.5.3 Characterization of risk to human health
Exposure of the general population to Betula alba extract may occur through a wide range of products, including cosmetics (moisturizers, shampoos, cleansers, sunless tanning products, styling products, etc.) and an NHP used in the alleviation of headache symptoms. Exposures are expected to occur via the oral and dermal routes.
Overall, this substance is considered to be of low hazard potential given the lack of systemic effects observed in the available studies by different routes of administration (subcutaneous, intraperitoneal, oral, and dermal). As a result, characterization of exposure (i.e., derivation of exposure estimates) was not considered to be warranted and the risk to human health is considered low.
7.5.4 Uncertainties in evaluation of risk to human health
Although there are some uncertainties in the health effects database (e.g., use of hazard information from main components, lack of data on relevant routes of administration, lack of chronic or developmental studies), and some limitations in the exposure databases, given that Betula alba extract is not associated with health effects of concern, a qualitative approach to risk characterization is considered appropriate for this assessment.
8. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from salicylic acid, homosalate, wintergreen oil, phenethyl salicylate and Betula alba extract. It is proposed to conclude that salicylic acid, homosalate, wintergreen oil, phenethyl salicylate, and Betula alba extract do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that wintergreen oil, salicylic acid and homosalate meet the criteria under paragraph 64(c) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that phenethyl salicylate and Betula alba extract do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore it is proposed to conclude that wintergreen oil, salicylic acid and homosalate meet one or more of the criteria set out in section 64 of CEPA, and it is also proposed to conclude that phenethyl salicylate and Betula alba extract do not meet any of the criteria set out in section 64 of CEPA.
It is proposed that salicylic acid does not meet the persistence or bioaccumulation criteria, that homosalate meets the bioaccumulation criteria but not the persistence criteria and that wintergreen oil meets the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
References
Abbott DD, Harrisson JWE. 1978. Methyl salicylate: studies of osseous changes in the rat, reproduction in the rat and mouse, and liver and kidney effects in the dog. Unpublished report to the Flavor and Extract Manufacturers Association, Washington (DC), USA.
Abyshev AZ, Agaev EM, Guseinov AB. 2007. Studies of the chemical composition of birch bark extracts (Cortex betula) from the Betulaceae family. Pharm Chem J. 41(8):22-26.
[AGDH] Australian Government Department of Health. 2017. Human health tier II assessment for salicylic acid and its salts. Sydney (AU): Department of Health, National Industrial Chemicals Notification and Assessment Scheme (NICNAS).
Ahangarpour A, Oroojan AA, Khorsandi L, Arzani G, Afshari G. 2016. Effects of betulinic acid on the male reproductive system of a streptozotocin-nicotinamide-induced diabetic mouse model. World J Mens Health. 34(3):209-216.
Alpen EL, Mandel HG, Rodwell VW, Smith PK. 1951. The metabolism of C14 carboxyl salicylic acid in the dog and in man. J Pharmacol Exp Ther. 102(3):150-155.
Baba T, Nagahama M, Akiyama N, Miki T. 1966. Experimental production of malformations due to acetyl salicylate and phenyl salicylate in rats. Osaka City Med J. 12(1):23-29.
Bandurska H. 2013. Salicylic acid: An update on biosynthesis and action in plant response to water deficit and performance under drought. In: Hayat S, Ahmad A, Alyemeni MN, editors. Salicylic acid: Plant growth and development. New York (NY): Springer. p. 1-10.
Barret JP, Podmelle F, Lipovy B, Rennekampff HO, Schumann H, Schwieger-Briel A, Zahn TR, Metelmann HR. 2017. Accelerated re-epithelialization of partial-thickness skin wounds by a topical betulin gel: results of a randomized phase III clinical trials program. Burns. 43(6):1284-1294.
Behrendt H, Kampffmeyer HG. 1989. Absorption and ester cleavage of methyl salicylate by skin of single-pass perfused rabbit ears. Xenobiotica. 19(2):131-141.
Belsito D, Bickers D, Bruze M, Calow P, Greim H, Hanifin JM, Rogers AE, Saurat JH, Sipes IG, Tagami H. 2007. A toxicologic and dermatologic assessment of salictlates when used as fragrance ingredients. Food Chem Toxicol. 45:S318-S361.
Berryman D, Rondeau M, Trudeau V. 2014. Concentrations of medications, hormones, and other emergent contaminants in the St. Lawrence and three of its tributaries [PDF]. Ottawa (ON): Environment Canada. [accessed 2017 Dec 10].
Bonin AM, Arlauskas AP, Angus DS, Baker RSU, Gallagher CH, Greenoak G, Brown MM, Meher-Homji KM, Reeve V. 1982. UV-absorbing and other sun-protecting substances: genotoxicity of 2-ethylhexyl p-methoxycinnamate. Mutat Res. 105(5):303-308.
Boqué N, Campión J, de la Iglesia R, de la Garza AL, Milagro FI, San Román B, Banuelos O, Martínez JA. 2012. Screening of polyphenolic plant extracts for anti-obesity properties in Wistar rats. J Sci Food Agric. 93(5):1226-1232.
Boyland E, Mawson EH. 1938. CCLVII. Experiments on the chemotherapy of cancer. II. The effect of aldehydes and glucosides. Biochemistry. 32:1982-1987.
Brun GL, Bernier M, Losier R, Doe K, Jackman P, Lee HB. 2006. Pharmaceutically active compounds in Atlantic Canadian sewage treatment plant effluents and receiving waters, and potential for environmental effects as measured by acute and chronic aquatic toxicity. Environ Toxicol Chem. 25(8):2163-2176.
Burdock GA. 2009. Fenaroli's Handbook of Flavor Ingredients, 6th Edition. Boca Raton (FL): CRC Press. [accessed 2018 Mar 20].
California Environmental Protection Agency. 2016. Aspirin. California: Office of Environmental Health Hazard Assessment.
Canada. [1978]. Food and Drug Regulations, C.R.C., c. 870.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c. 33. Canada Gazette Part III, vol. 22, no. 3.
[CCRIS] Chemical Carcinogenesis Research Information System [database]. 2017. Bethesda (MD): US National Library of Medicine. [updated 2010 Jun 2].
Cekanova E, Larsson KS, Mörck E, Aberg G. 1974. Interactions between salicylic acid and pyridyl-3-methanol: anti-inflammatory and teratogenic effects. Acta Pharmacol Toxicol (Copenh). 35(2):107-118.
Celik K, Turkez H. 2016. Investigation of in vitro cytotoxicity and genotoxicity of wintergreen oil in rat primary neurons and N2a neuroblastoma cells. J Essent Oil Bear Pl. 19(6):1340-1350.
[CTFA] Cosmetic, Toiletry and Fragrance Association. 1983. Summary of the results of surveys of the amount and frequency of use of cosmetic products by women. Report prepared by Pitkin B, Rodericks JV, Turnbull D. Environ Corporation, Washington, DC.
ChemCAN [level III fugacity model of 24 regions of Canada]. 2003. Ver. 6.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
[ChemID] ChemIDplus [database]. 1993-. Bethesda (MD): US National Library of Medicine.
[ChemView] ChemView [database]. [modified 2017 Aug 18]. Washington (DC): U.S. Environmental Protection Agency. [accessed 2017 Aug 22].
Cheng X, Shin YG, Levine BS, Smith AC, Tomaszewski JE, Breemen RB. 2003. Quantitative analysis of betulinic acid in mouse, rat, and dog plasma using electrospray liquid chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 17(18):2089-2092.
[CIR] Cosmetic Ingredient Review Expert Panel. 2003. Safety assessment of salicylic acid, butyloctyl salicylate, calcium salicylate, C12-15 alkyl salicylate, capryloyl salicylic acid, hexyldodecyl salicylate, isocetyl salicylate, isodecyl salicylate, magnesium salicylate, MEA-salicylate, ethylhexyl salicylate, potassium salicylate, methyl salicylate, myristyl salicylate, sodium salicylate, TEA-salicylate, and tridecyl salicylate. Int J Toxicol. 22 Suppl. 3:1-108.
Ciurlea SA, Tiulea C, Csanyi E, Berko S, Toma CC, Dehelean CA, Loghin F. 2010. A pharmacotoxicological evaluation of a betulin topical formulation tested on C57BL/6J mouse experimental nevi and skin lesions. Studia Universitatis “Vasile Goldis”, Seria Stiintele Vietii. 20(4):5-9.
Clayton GD, Clayton FE, editors. 1981. Patty’s industrial hygiene and toxicology. New York (NY): Wiley.
Clydesdale F. 1997. Food Additives: Toxicology, Regulation and Properties. Boca Raton (FL): CRC Press.
Collins TFX, Hansen WH, Keeler HV. 1971. Effect of methyl salicylate on rat reproduction. Toxicol Appl Pharmacol. 18(4):755-765.
[CosIng] Cosmetic Ingredients and Substances. [database]. 2017. v. 2. Brussels (BE). DG Internal Market, Industry, Entrepreneurship and SMEs. [accessed 2017 Aug 29].
Comeau F, Surette C, Brun GL, Losier. 2008. The occurrence of acidic drugs and caffeine in sewage effluents and receiving waters from three coastal watersheds in Atlantic Canada. Sci Total Environ. 396:132-146.
Cross SE, Anderson C, Roberts MS. 1998. Topical penetration of commercial salicylate esters and salts using human isolated skin and clinical microdialysis studies. Br J Clin Pharmacol. 46(1):29-35.
Crouse BA, Ghoshdastidar AJ, Tong AZ. 2012. The presence of acidic and neutral drugs in treated sewage effluents and receiving waters in the Cornwallis and Annapolis River watersheds and the Mill CoveStewage Treatment Plant in Nova Scotia, Canada. Environ Res. 112:92-99.
Davis DAP, Kraus AL, Thompson GA, Olerich M, Odio MR. 1997. Percutaneous absorption of salicylic acid after repeated (14-day) in vivo administration to normal, acnegenic, or aged human skin. J Pharm Sci. 86(8):896-9.
Davison C, Zimmerman EF, Smith PK. 1961. On the metabolism and toxicity of methyl salicylate. J Pharmacol Exp Ther. 132:207-211.
Dencker L. 1976. Tissue localization of some teratogens at early and late gestation related to fetal effects. Acta Pharmacol Toxicol (Copenh). 39 Suppl 1:1-131.
Di YB, Zhang GY, Wu GJ, Wang BG, Gong SL, Zhao G. 2009. Protective effects of betulin on alcoholic liver disease in rats. Journal of Jilin University (Medicine Edition). 35(2):210-213.
Dittert LW, Caldwell HC, Ellison T, Irwin GM, Rivard DE, Swintosky JV. 1968. Carbonate ester prodrugs of salicylic acid. Synthesis, solubility characteristics, in vitro enzymatic hydrolysis rates, and blood levels of total salicylate following oral administration to dogs. J Pharm Sci. 57(5):828-831.
[DPD] Drug Product Database [database]. [modified 2015 Jul 17]. Ottawa (ON): Health Canada.
Duncan EJS, Brown A, Lundy P, Sawyer TW, Hamilton M, Hill I, Conley JD. 2002. Site-specific percutaneous absorption of methyl salicylate and VX in domestic swine. J Appl Toxicol. 22:141-148.
[EC] European Commission. 2003. Technical guidance document on risk assessment, Part I. 1998. Institute for Health and Consumer Protection, European Chemicals Bureau.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization of chemical substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016a. Ecological science approach: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Data used to create substance-specific hazard and exposure profiles and assign risk classifications in the Ecological Risk Classification of organic substances. Gatineau (QC). Available from: substances@ec.gc.ca.
[ECCC] Environment and Climate Change Canada. 2017. Data collected from a targeted information gathering initiative for assessments under the Chemicals Management Plan (August 2017). Data prepared by: ECCC, Health Canada; Existing Substances Program.
[ECCC] Environment and Climate Change Canada. 2017b. Pharmaceuticals and Personal Care Products Surveillance Network. [modified 2017 Feb 16; accessed 2017 Dec 10].
[ECHA] European Chemicals Agency. 2016. Committee for Risk Assessment (RAC). Opinion: Proposing harmonised classification and labelling at EU level of salicylic acid [PDF]. EC Number: 200-712-3. CAS Number: 69-72-7. CLH-O-0000001412-86-110/F.
[ECHA] European Chemicals Agency. 2017a. Substance information. Birch, Betula alba, ext. CAS RN 84012-15-7. Helsinki (FI): ECHA. [updated 2017 Dec 7].
[ECHA] European Chemicals Agency. 2017b. Registered substances database: Registered dossier for homosalate CAS RN 118-56-9. Helsinki (FI): ECHA [updated 2018 Oct 13; accessed 2018 Nov 1].
[ECHA] European Chemicals Agency. 2017c. Brief profile: Salicylic acid; CAS RN 69-72-7. Helsinki (FI): ECHA. [updated 2017 Aug 16; accessed 2017 Aug 22].
[ECHA] European Chemicals Agency. 2017d. Guidance on safe use: Salicylic acid; CAS RN 69-72-7. Helsinki (FI): ECHA. [updated 2017 Oct 4].
[ECHA] European Chemicals Agency. 2017e. Brief profile: Homosalate; CAS RN 118-56-9. Helsinki (FI): ECHA. [updated 2017 Aug 16; accessed 2017 Aug 22].
[ECHA] European Chemicals Agency. 2017f. Guidance on safe use: Homosalate; CAS RN 118-56-9. Helsinki (FI): ECHA. [updated 2017 Apr 23].
[ECHA] European Chemicals Agency. 2018. Harmonised classification and labelling public consultations. Methyl Salicylate. Helsinki (FI): ECHA [updated 2018 Dec; accessed 2019 Jan 9].
E.I. Dupont de Nemours & Co. 1973. Maternal toxicity, embryotoxicity and teratogenic potential of neoprene accelerators applied to skin of rats during organogenesis. Microfiche No. OTS0556789. Old Doc ID. 86940000194.
Elmore SA. 2006. Enhanced histopathology of the thymus. Toxicological Pathology. 34(5):656-665.
El-Obeid HA, Hassan MMA. 1979. PMR assay of essential oils: II. Assay of methylsalicylate in wintergreen oil. Spectrosc Lett. 12(7&8):555-557.
[EMEA] European Medicines Agency. 2014. European Union herbal monograph on Betula pendula Roth and/or Betula pubescens Ehrh. as well as hybrids of both species, folium [PDF]. Committee on Herbal Medicinal Products.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Eriksson M, Larsson KS. 1968. Premature birth induced in mice by salicylate. Nature. 220(5165):385.
[EU] European Union. 2018. Commission Regulation (EU) 2018/1480 of 4 October 2018 amending, for the purposes of its adaptation to technical and scientific progress, Regulation (EC) No 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures and correcting Commission Regulation (EU) 2017/776 [PDF]. Official Journal of the European Union. 5.10.2018.
EU Food Flavourings Database [database]. [modified 2016]. Brussels (BE): European Commission Directorate-General for Health and Consumers. [Accessed 2018-03-01].
[FDA] Food and Drug Administration. 2006. Application Number 22-029. Pharmacology Review(s) [PDF]. Silver Spring (MD): US Department of Health & Human Services.
Ficheux AS, Wesolek N, Chevillotte G, Roudot AC. 2015. Consumption of cosmetic products by the French population. First part: Frequency data. Food Chem Toxicol. 78:159-169.
Ficheux AS, Chevillotte G, Wesolek N, Morisset T, Dornic N, Bernard A, Bertho A, Romanet A, Leroy L, Mercat AC, Creusot T, Simon E, Roudot AC. 2016. Consumption of cosmetic products by the French population second part: Amount data. Food Chem Toxicol. 90:130-141.
Gage JC. 1970. The subacute inhalation toxicity of 109 industrial chemicals. Br J Ind Med. 27(1):1-18.
Giri AK, Adhikari N, Khan KA. 1996. Comparative genotoxicity of six salicylic acid derivatives in bone marrow cells of mice. Mutat Res. 370(1):1-9.
Goggelmann W, Schimmer O. 1986. Mutagenic activity of phytotherapeutical drugs. Prog Clin Biol Res. 206:63-72.
Gomez E, Pillon A, Fenet H, Rosain D, Duchesne MJ, Nicolas JC, Balaguer P, Casellas C. 2005. Estrogenic activity of cosmetic components in reporter cell lines: parabens, UV screens, and musks. J Toxicol Environ Health A. 68(4):239-251.
GoodGuide. 2018. Salicylic Acid. [accessed 2018 Mar 20].
Hall RL, Oser BL. 1965. Recent progress in the consideration of flavoring ingredients under the food additives amendment. Food Technol. 19(2):151-197.
Health Canada. 1994. Canadian Environmental Protection Act: Human Health Risk Assessment for Priority Substances. Ottawa (ON): Government of Canada.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Health Canada. 2013. Sunscreen Monograph. Ottawa (ON): Health Canada Health Products and Food branch. [accessed 2017 Oct 23].
Health Canada. 2013. Supporting documentation: summary tables of health effects information for methylenediphenyl diisocyanates and diamines. Ottawa (ON): Health Canada. Information in support of the Screening Assessment for the MDI/MDA Substance Grouping. Available from: substances@ec.gc.ca.
Health Canada. 2014. Thyme Oil and Wintergreen Oil [PDF]. Registration Decision RD2014-22. Ottawa (ON): Health Canada. [accessed 2017 Dec 10].
Health Canada. [modified 2015 Dec 14]. Cosmetic ingredient hotlist: list of ingredients that are prohibited for use in cosmetic products. Ottawa (ON): Health Canada, Consumer Product Safety Directorate. [accessed 2017 Dec 10].
Health Canada. 2016. Acne therapy. Ottawa (ON): Government of Canada.
Health Canada. 2018a. Primary Sunscreen Monograph. Health Products and Food Branch. Ottawa (ON): Government of Canada. [accessed 2019 Jan 22]
Health Canada. 2018b. Secondary Sunscreen Monograph. Health Products and Food Branch. Ottawa (ON): Government of Canada. [accessed 2019 Jan 22]
Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. 2014. Low-dose aspirin for the prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Evidence synthesis No. 112. AHRQ Publication No. 14-05207-EF-1. Rockville (MD): Agency for Healthcare Research and Quality.
Hopkins ZR, Blaney L. 2016. An aggregate analysis of personal care products in the environment: Identifying the distribution of environmentally-relevant concentrations. Environ Int. 92-93:301-316.
[HPFB] Health Products and Food Branch. 2013. Sunscreen Monograph, version 2.0. Ottawa (ON): Health Canada. [accessed 2017 Oct 3].
Huyke C, Laszczyk M, Scheffler A, Ernst R, Schempp CM. 2006. Treatment of actinic keratosis with birch bark extract: a pilot study. J Dtsch Dermatol Ges. 4(2):132-137.
Huyke C, Reuter J, Rödig M, Kersten A, Laszczyk M, Scheffler A, Nashan D, Schempp C. 2009. Treatment of actinic keratosis with a novel betulin-based oleogel. A prospective, randomized, comparative pilot study. J Dtsch Dermatol Ges. 7(2):128-133.
Infurna R, Beyer B, Twitty L, Koehler G, Daughtrey W. 1990. P47. Evaluation of the dermal absorption and teratogenic potential of methyl salicylate in a petroleum based grease. [abstract only]. Teratology Society Abstracts. p. 566.
Ishidate M. Sofuni JR, Yoshikawa K, Hayashi M, Nohmi T, Sawada M, Matsuoka A. 1984. Primary mutagenicity screening of food additives currently used in Japan. Food Chem Toxicol. 22(8):623-636.
[ITIS] Integrated Taxonomic Information System [database]. 2017a. Search results for Betula alba L.
[ITIS] Integrated Taxonomic Information System [database]. 2017b. Search results for Betula pubescens ssp. pubescens Ehrh.
Jäger S, Laszczyk MN, Scheffler A. 2008. A preliminary pharmacokinetic study of betulin, the main pentacyclic triterpene from extract of outer bark of birch (Betulae alba cortex). Molecules. 13(12):3224-3235.
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 1962. Evaluation of the toxicity of a number of antimicrobials and antioxidants [PDF]. Geneva (CH): World Health Organization. (WHO Technical Report Series No. 228.). Sixth report of the JECFA.
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2002a. Safety evaluation of certain food additives and contaminants: hydroxyl- and alkoxy-substituted benzyl derivatives. Geneva (CH): World Health Organization. (WHO Food Additives Series: 48).
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2002b. Evaluation of certain food additives and contaminants [PDF]. Fifty-seventh report of the Joint FAO/WHO Expert Committee on Food Additives. Geneva (CH): World Health Organization. (WHO Technical Report Series 909).[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2002c. Evaluation of certain food additives [PDF]. Fifty-ninth report of the Joint FAO/WHO Expert Committee on Food Additives. Geneva (CH): World Health Organization. (WHO Technical Report Series 913).
Jiménez-Díaz I, Molina-Molina JM, Zafra-Gómez A, Ballesteros O, Navalón A, Real M, Sáenz JM, Fernández MF, Olea N. 2013. Simultaneous determination of the UV filters benzyl salicylate, phenyl salicylate, octyl salicylate, homosalate, 3-(4-methylbenzylidene) camphor and 3-benzylidene camphor in human placental tissue by LC-MS/MS. Assessment of their in vitro endocrine activity. J Chromatogr B. Analyt Technol Biomed Life Sci. 936:80-87.
Jine Y, Lis M, Szczypka M, Obminska-Mrukowicz B. 2012. Influence of betulinic acid on lymphocyte subsets and humoral immune response in mice. Pol J Vet Sci. 15(2):305-313.
Kim TH, Shin BS, Kim KB, Shin SW, Seok SH, Kim MK, Kim EJ, Kim D, Kim MG, Park ES, Kim JY, Yoo SD. 2014. Percutaneous absorption, disposition, and exposure assessment of homosalate, a UV filtering agent, in rats. J Toxicol Environ Health A. 77(4):202-213.
Kociba RJ, Kalnins RV, Wade CE, Garfield EL, Fishbeck WA. 1976. Elevated urinary phenol levels in dogs ingesting Salol [abstract]. Toxicol Appl Pharmacol. 37:93-192.
Koffuor GA, Dickson R, Gbedema SY, Ekuadzi E, Dapaah G, Otoo LF. 2014. The immunostimulatory and antimicrobial property of two herbal decoctions used in the management of HIV/AIDS in Ghana. Afr J Tradit Complement Altern Med. 11(3):166-172.
Kone M, Cologgi DL, Lu W, Smith DW, Ulrich AC. 2013. Pharmaceuticals in Canadian sewage treatment plant effluents and surface waters: occurrence and environmental risk assessment. Environ Technol Rev. 2(1):17-27.
Koshakji RP, Schulert AR. 1973. Biochemical mechanisms of salicylate teratology in the rat. Biochem Pharmacol. 22(3):407-416.
Krause M, Klit A, Blomberg Jensen M, Soeborg T, Frederiksen H, Schlumpf M, Lichtensteiger W, Skakkebaek NE, Drzewieck KT. 2012. Sunscreens: are they beneficial for health? An overview of endocrine disrupting properties of UV filters. Int J Androl. 35(3):424-436.
Kuboyama N, Fujii A. 1992. Mutagenicity of analgesics, their derivatives, and anti-inflammatory drugs with S9 mix of several animal species. J Nihon Univ Sch Dent. 34(3):183-195.
Kunz PY, Fent K. 2006. Multiple hormonal activities of UV filters and comparison of in vivo and in vitro estrogenic activity of ethyl-4-aminobenzoate in fish. Aquat Toxicol. 79(4):305-324.
Kunz PY, Galicia HF, Fent K. 2006. Comparison of in vitro and in vivo estrogenic activity of UV filters in fish. Toxicol Sci. 90(2):349-361.
Lapczynski A, McGinty D, Jones L, Bhatia S, Letizia CS, Api AM. 2007. Fragrance material review on phenethyl salicylate. Food Chem Toxicol. 45:467-471.
Laszcyzk M, Jäger S, Simon-Haarhaus B, Scheffler A, Schempp CM. 2006. Physical, chemical and pharmacological characterization of a new oleogel-forming triterpene extract from the outer bark of birch (Betulae cortex). Planta Med. 72(15):1389-1395.
Larsson KS, Eriksson M. 1966. Salicylate-induced fetal death and malformations in two mouse strains. Acta Paediatr Scand. 55(6):569-576.
Liberati TA, Roe BJ, Feuston MH. 2002. An oral (gavage) control embryo-fetal development study in the Wistar Hannover rat. Drug Chem Toxicol. 25(1):109-130.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2018 Feb 6]. Ottawa (ON): Health Canada.
Loretz LG, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, Rothenstein A, Scrafford CG, Sewall C. 2005. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol. 43:279-291.
Loretz L, Api AM, Barraj L, Burdick J, Davis DA, Dressler W, Gilberti E, Jarrett G, Mann S, Pan YHL, Re T, Renskers K, Scrafford C, Vater S. 2006. Exposure data for personal care products: Hairspray, spray perfume, liquid foundation, shampoo, body wash, and solid antiperspirant. Food Chem Toxicol. 44: 2008-2018.
Loretz LG, Api AM, Babcock L, Barraj LM, Burdick J, Cater KC, Jarrett G, Mann S, Pan YHL, Re TA, Renskers KJ, Scrafford CG. 2008. Exposure data for cosmetic products: Facial cleanser, hair conditioner, and eye shadow. Food Chem Toxicol. 46:1516-1524.
Ma R, Cotton B, Lichtensteiger W, Schlumpf M. 2003. UV filters with antagonistic action at androgen receptors in the MDA-kb2 cell transcriptional-activation assay. Toxicol Sci. 74(1):43-50.
McCann J, Choi E, Yamasaki E, Ames BN. 1975. Detection of carcinogens as mutagens in the Salmonella / microsome test: Assay of 300 chemicals. Proc Natl Acad Sci U S A. 72(12):5135-5139.
Megwa SA, Benson HAE, Roberts MS. 1995. Percutaneous absorption of salicylates from some commercially available topical products containing methyl salicylate or salicylate salts in rats. J Pharm Pharmacol. 47:891-896.
Moody RP, Akram M, Dickson E, Chu I. 2007. In vitro dermal absorption of methyl salicylate, ethyl parathion, and malathion: First responder safety. J Toxicol Environ Health, A. 70:985-999.
Morra P, Bartle WR, Walker SE, Lee SN, Bowles SK, Reeves RA. 1996. Serum concentrations of salicylic acid following topically applied salicylate derivatives. The Annals of Pharmacotherapy. 30:935-940.
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, Zeiger E. 1986. Salmonella mutagenicity tests: II. Results from the testing of 270 chemicals. Environ Mutagen. 8 Suppl 7:1-119.
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2019 Jul 3]. Ottawa (ON): Health Canada. [accessed 2017 Sep 21].
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme. 2015. Public Report: Benzoic Acid, 2-hydroxy, 2-butyloctyl ester. SYD (AUS): National Industrial Chemicals Notification and Assessment Scheme.
[NIOSH] National Institute for Occupational Safety and Health. 1973. Health Hazard Evaluation Report 71-10-48. Hazard Evaluation Services Branch, Division of Technical Services. Cincinnati (OH): US Department of Health, Education, and Welfare. [restricted access].
Noritake K, Ikeda T, Ito K, Miwa Y, Senuma M, Takashima H, Tateishi T, Hisada S, Maki E. 2013. A study for collecting background data on Wistar Hannover [Crl:WI(Han)] rats in embryo-fetal development studies – comparative data to Sprague-Dawley rats. J Toxicol Sci. 38(6):847-854.
[NRC] National Research Council of Canada. 2011. Data gathering on chemicals released to indoor air of residences from building materials and furnishings. NRC-Institute for Research in Construction Report no. B3332.2. Prepared for Health Canada, Ottawa (ON).
[NTP] National Toxicology Program (US). 1984a. Methyl salicylate: reproduction and fertility assessment in CD-1 mice when administered by gavage. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program.
[NTP] National Toxicology Program (US). 1984b. Methyl salicylate: fertility assessment in CD-1 mice when administered by gavage. Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program.
[NTP] National Toxicology Program (US). 2007. NTP technical report on the photocarcinogenesis study of glycolic acid and salicylic acid (CAS Nos 79-14-1 and 69-72-7) in SKH-1 mice (simulated solar light and topical application study). Research Triangle Park (NC): US Department of Health and Human Services, National Toxicology Program.
Oda Y, Hamono Y, Inoue K, Yamamoto H, Niihara T, Kunita N. 1978. Mutagenicity of food flavors in bacteria. Shokuhin Eisei Hen. 9:177-181.
OECD QSAR Toolbox. [read across tool]. 2016. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Opdyke DLJ, editor. 1968. Monographs on fragrance raw materials. A collection of monographs originally appearing in Food and Cosmetics Toxicology. An International Journal: Methyl salicylate. Oxford (UK): Pergamon Press.
Opdyke DLJ. 1978. Fragrance raw materials monographs: Phenethyl salicylate. Food Cosmet Toxicol. 16 (Suppl 1):849.
Orav A, Arak E, Boikova T, Raal A. 2011. Essential oil in Betula spp. leaves naturally growing in Estonia. Biochem Syst Ecol. 39:744-748.
Ozaki H, Sugihara K, Tamura Y, Fujino C, Watanabe Y, Uramaru N, Sone T, Ohta S, Kitamura S. 2015. Hydrolytic metabolism of phenyl and benzyl salicylates, fragrances and flavoring agents in foods, by microsomes of rat and human tissues. Food Chem Toxicol. 86:116-123.
Packman EW, Abbott DD, Wagner BM, Harrisson JWE. 1961. Chronic oral toxicity of oil sweet birch (methyl salicylate) [abstract]. Pharmacologist. 3:62.
Paterson JR, Srivastava R, Baxter GJ, Graham AB, Lawrence JR. 2006. Salicylic acid content of spices and its implications. J Agric Food Chem. 54:2891-2896.
Pisha E, Chai H, Lee IS, Chagwedera TE, Farnsworth NR, Cordell GA, Beecher CWW, Fong HHS, Kinghorn D, Brown DM, Wani MC, Wall ME, Hieken TJ, Gupta TKD, Pezzuto JM. 1995. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat Med. 1(10):1046-1051.
Prakash B, Surendran A, Chandraprabha VR, Pettamanna A, Nair HNR. 2018. Betulinic acid, natural pentacyclic triterpenoid prevents arsenic-induced nephrotoxicity in male Wistar rats. Comp Clin Pathol. 27(1):37-44. [published online 2017 Sep 13].
[REACH] Registration, Evaluation, Authorisation and Restriction of Chemicals. 2005. Registration dossier submission.
Rodriguez AS, Sanz MR, Betancort, Rodriguez JRB. 2015. Occurrence of eight UV filters in beached of Gran Canaria. An approach to environmental risk assessment. Chemosphere. 131:85-90.
Rumiantsev GI, Novikov SM, Andrusov VE, Mel’nikova NN, Semenovykh LN. 1992. Hygienic standardization of methyl salicylate in the air of workplace. Gigiena Sanitariia. 7-8:28-31. [Article in Russian; English abstract available].
[SCCNFP] Scientific Committee on Consumer Products and Non-Food Products Intended for Consumers. 2002. Opinion on salicylic acid [PDF]. Brussels (BE): European Commission. [accessed 2017 Oct 10].
[SCCP] Scientific Committee on Consumer Products. 2007. Opinion on homosalate [PDF], COLIPA No. S12. Brussels (BE): European Commission. [accessed 2017 Oct 10].
[SCCS] European Scientific Committee on Consumer Safety. 2016. The SCCS notes of guidance for the testing of cosmetic ingredients and their safety evaluation [PDF]. Document No. SCCS/1564/15. Brussels (BE): Directorate-General Health and Food Safety. [accessed 2017 Dec 10].
Schreurs R, Lanser P, Seinen W, Burg B. 2002. Estrogenic activity of UV filters determined by an in vitro reporter gene assay and an in vivo transgenic zebrafish assay. Arch Toxicol. 76(5-6):257-261.
Schreurs RHMM, Sonneveld E, Jansen JHJ, Seinen W, Burg B. 2005. Interaction of polycyclic musks and UV filters with the estrogen receptor (ER), androgen receptor (AR), and progesterone receptor (PR) in receptor gene bioassays. Toxicol Sci. 83(2):264-272.
Schlumpf M, Cotton B, Conscience M, Haller V, Steinmann B, Lichtensteiger W. 2001. In vitro and in vivo estrogenicity of UV screens. Environ Health Perspect. 109(3):239-244.
Schlumpf M, Schmid P, Durrer S, Conscience M, Maerkel K, Henseler M, Gruetter M, Herzog I, Reolon S, Ceccatelli R, Faass O, Stutz E, Jarry H, Wuttke W, Lichtensteiger W. 2004. Endocrine activity and developmental toxicity of cosmetic UV filters – an update. Toxicology. 205(1-2):113-122.
[SDS] Safety Data Sheet. 2015. Sunlight Ultra Liquid Dish Detergent - Orange Antibacterial 750mL. Wilton (CT): Sun Products. [accessed 2017 Dec 10].
Servos MR, Smith M, McInnis R, Burnison BK, Lee, BH, Seto P, Backus S. 2007. The presence of selected pharmaceuticals and the antimicrobial triclosan in drinking water in Ontario, Canada. Water Qual. Res. J. 42(2):130-137.
Sexton WJ, Jarow JP. 1997. Effect of diabetes mellitus upon male reproductive function. Urology. 49(4):508-513.
Shikov AN, Djachuk GI, Sergeev DV, Pozharitskaya ON, Esaulenko EV, Kosman VM, Mararov VG. 2011. Birch bark extract as therapy for chronic hepatitis C – a pilot study. Phytomedicine. 18(10):807-810.
Steele JCP, Warhurst DC, Kirby GC, Simmonds MSJ. 1999. In vitro and in vivo evaluation of betulinic acid as an antimalarial. Phytother Res. 13(2):115-119.
Strong LC. 1932. Possible effect of oil of Gaultheria in diet of mice susceptible to spontaneous carcinoma of the breast. I. A suggestion. Proc Soc Exp Biol Med. 30(3):386-390.
Strong LC. 1934. Possible effect of oil of Gaultheria in diet of mice susceptible to spontaneous carcinoma of the breast. II. A latent period. Am J Cancer Res. 20(2):387-389.
Strong LC. 1935. Possible effect of oil of Gaultheria in diet of mice susceptible to spontaneous carcinoma of the breast. III. Survival time. Am J Cancer Res. 25(4):797-801.
Strong LC. 1936. Possible effect of oil of Gaultheria in diet of mice susceptible to spontaneous carcinoma of the mammary gland. V. Growth rate and certain retrogressive changes of tumours after onset of malignancy. Am J Cancer Res. 28(3):550-558.
Swain AR, Dutton SP, Truswell AS. 1985. Salicylates in foods. J Am Diet Assoc. 85(8):950-960.
Tanaka S, Kawashima K, Nakaura S, Nagao S, Kuwamura T, Takanaka A, Omori Y. 1973a. Studies on the teratogenicity of food additives (3). Teratogenic effect of dietary salicylic acid in rats. Food Hyg Safe Sci. 14(6):549-557.
Tanaka S, Kawashima K, Nakaura S, Nagao S, Kuwamura T, Takanaka A, Omori Y. 1973b. Studies on teratogenic effects of salicylic acid and aspirin in rats as related to fetal distribution. Cong. Anom. 13(2):73-84.
Tashiro Y, Kameda Y. 2013. Concentration of organic sun-blocking agents in seawater of beaches and coral reefs of Okinawa Island, Japan. Mar Pollut Bull. 77:333-340.
The Essential Oil Company. 2017. Percent yield guide for essential oil distillation. [accessed 2017 Dec 10].
[TIMES] Tissue Metabolism Simulator [prediction module]. 2017. Ver. 2.27.19. Bourgas (BG): University “Prof. Dr. Assen Zlatarov”, Laboratory of Mathematical Chemistry.
Tisserand R, Young R. 2014. Essential Oil Safety. 2nd ed. London (UK): Churchill Livingstone.
[TSCA] Toxic Substances Control Act Chemical Substance Inventory [database]. [updated 2017 Jun]. Search results for CAS RN 68917-75-9. Washington (DC): United States Environmental Protection Agency.
Udeani GO, Zhao GM, Geun Shin Y, Cooke BP, Graham J, Beecher CW, Kinghorn AD, Pezzuto JM. 1999. Pharmacokinetics and tissue distribution of betulinic acid in CD-1 mice. Biopharm Drug Dispos. 20(8):379-383.
Umeda M. 1957. Screening tests of various chemical substances as carcinogenic hazards (report 1). Gann: Jpn J Cancer Res. 48(1):57-64.
Unnamed study report. 2013. Unpublished confidential study submitted to Health Canada. Ottawa (ON): Existing Substances Risk Assessment Bureau. Submission received on February 23, 2018.
[US eCFR] Code of Federal Regulations Title 21. [database]. [modified 2017 Apr 1]. Silver Springs (MD): U.S. Food and Drug Administration. [accessed 2017 Dec 10].
Verenitch SS, Christopher JL, Mazumder A. 2006. Determination of acidic drugs and caffeine in municipal wastewaters and receiving waters by gas chromatography—ion trap tandem mass spectrometry. J Chromatogr. 1116:193-203.
Walker RM, Chang PK, Martin RA. 1989. Effects of salicylate on rat liver in short-term toxicity studies. Biochem Pharmacol. 38(2):382-384.
Waltman R, Tricomi V, Shabanah EH, Arenas R. 1973. The effect of anti-inflammatory drugs on parturition parameters in the rat. Prostaglandins. 4(1):93-106.
Webb WK, Hansen WH. 1963. Chronic and subacute toxicology and pathology of methyl salicylate in dogs, rats, and rabbits. Toxicol Appl Pharmacol. 5(5):576-587.
Wilson R, Jones-Otazo H, Petrovic S, Mitchell I, Bonvalot Y, Williams D, Richardson GM. 2013. Revisiting dust and soil ingestion rates based on hand-to-mouth transfer. Hum Ecol Risk Assess. 19(1):158–188.
Wolowich WR, Hadley CM, Kelley MT, Walson PD, Casavant MJ. 2003. Plasma salicylate from methyl salicylate cream compared to oil of wintergreen. J Toxicol Clin Toxicol. 41(4):355-358.
Wolters Kluwer Health Inc. 2010. Antineoplastics/antibacterials: cutaneous toxicity, treated with betulin emulsions: 15 case reports. Reactions Weekly, 2010 October 16, p9. Academic OneFile. [Accessed 12 June 2017].
Wood A, Baxter G, Thies F, Kyle J, Duthie G. 2011. A systematic review of salicylates in foods: Estimated daily intake of a Scottish Population. Mol. Nutr. Food Res. 55:7-14.
Wormuth M, Scheringer M, Vollenweider M, Hungerbuhler K. 2006. What are the sources of exposure to eight frequently used pthalic acid esters in Europeans? Risk Anal 26(3):803-824.
Wu X, Bennett DH, Ritz B, Cassady DL, Lee K, Hertz-Picciotto I. 2010. Usage pattern of personal care products in California households. Food Chem Toxicol. 48:3109-3119.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, Speck W. 1987. Salmonella mutagenicity tests: III. Results from the testing of 255 chemicals. Environ Mutagen. 9 (Suppl 9):1-110.
Zhanataev AK, Presnova GA, Chistyakov AN, Durnev AD. 2004. Effect of betula bark extract on spontaneous and induced mutagenesis in mice. Bull Exp Biol Med. 138(5):475-478.
Zhang Z, Jia C, Hu Y, Sun L, Jiao J, Zhao L, Zhu D, Li J, Tian Y, Bai H, Li R, Hu J. 2012. The estrogenic potential of salicylate esters and their possible risks in foods and cosmetics. Toxicology Letters. 209(2):146-153.
Zhao H, Liu Z, Liu W, Han X, Zhao M. 2016. Betulin attenuates lung and liver injuries in sepsis. Int Immunopharmacol. 30:50-56.
Zuco V, Supino R, Righetti SC, Cleris L, Marchesi E, Gambacorti-Passerini C, Formelli F. 2002. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 175(1):17-25.
Appendix A. Read-across approach
| Consideration | Rationale |
|---|---|
| 1) Chemical structure. Emphasis was placed on analogues that represented salicylic acid esters. | Analogues that have similar chemical structure are expected to possess similar physical-chemical properties, undergo similar metabolic pathways, and have similar reactivity. |
| 2) Similar metabolites (predicted or observed). The salicylic acid esters are all expected undergo hydrolysis to yield salicylic acid and its corresponding alcohol. The rate of hydrolysis of the ester bond depends on the chain length or the bulkiness of the substituent group attached to salicylic acid (Dittert et al. 1968). | Analogues that are metabolized through similar pathways to similar degradation products are expected to have similar toxicity profiles. |
| 3) Common structural alerts | Analogues with similar structural alerts are expected to share greater similarity in terms of toxicity. |
| 4) Similar physical-chemical properties. Emphasis was placed on chemical structures with similar molecular weight, water solubility, vapour pressure, and log Ko/w. | Analogues with similar physical chemical properties may potentially share similar toxicological profiles. |
| 5) Availability of health effects data | Only analogues with hazard data of sufficient quality were considered applicable for read-across purposes. |
| - | Salicylic acid | Methyl salicylate | Phenyl salicylate | Benzyl salicylate | Phenethyl salicylate | Homosalate |
|---|---|---|---|---|---|---|
| CAS RN | 69-72-7 | 119-36-8 | 118-55-8 | 118-58-1 | 87-22-9 | 118-56-9 |
| Structure |  |
 |
 |
 |
 |
 |
| MW (g/mol) | 138.12 | 152.15 | 214.22 | 228.25 | 242.27 | 262.35 |
| Vapour pressure (Pa) | 0.01 | 4.6 | 1.1E-3 | 4.5E-4 | 1.9E-04 | 0.013 |
| Henry’s law constant (atm·m3/mol) | 7.34E-9 | 7.9E-8 | 1.68E-6 | 3.67E-7 | 4.87E-07 | 1.93E-05 |
| Water solubility (mg/L) | 2240 | 700 | 150 | 24.6 | 7.2 – 7.8 | 0.4 |
| logKo/w | 2.26 | 2.55 | 3.8 | 4.3 | 4.80 | > 6 |
| Oral LD50 (g/kg) | 0.5-1.3 | 0.7-2.1 | 3 | 2.2 | >5 | >5 |
| Dermal LD50 (g/kg) | >2-10 | - | >5 | >5–14 | >5 | >5 |
| Genotoxicity | Negative | Negative | Equivocal | Negative | - | Negative |
| Carcinogenicity | - | Negative | - | - | - | - |
| Reproductive/ developmental toxicity (mg/kg bw/day) | NOAEL: 75 (reductions in litter size, fetal body weights, and fetal body/tail lengths, high incidence of malformations, reduced weaning rate at the next dose of 150 mg/kg bw/day, in the absence of maternal toxicity)a | NOAEL: 75 (reduced pup viability, pup survival, number of weanlings at the next dose of 150 mg/kg bw/day, in absence of maternal toxicity)b NOAEL: 100 (reduction in adjusted mean pup weight at the next dose of 250 mg/kg bw/day, in the absence of maternal toxicity)c | LOAEL: 100 (Exencephaly was reported, but it was unknown at which dose these observations occurred. Therefore, assumed to occur at the lowest dose.)d | - | - | LOAEL: 300 (increased post-implantation loss and decreased birth index)e |
* Unless otherwise specified, data was retrieved from ChemID (1993-) and from the Physical and Chemical Properties section of this report.
a Tanaka et al. 1973b;
b Collins et al. 1971;
c NTP 1984b;
d Baba et al. 1966;
e Unnamed study report 2013.
Appendix B. Estimated potential human exposures to substances in the Salicylates Group
Dermal, oral, and inhalation exposures to cosmetics, drugs, or natural health products were estimated for relevant age groups, and parameters and assumption used are presented in Table B-1, Table B-2 and Table B-3. Exposure was assumed to be daily for products used once per day or more. For products used less than once per day, exposure on the day of use was estimated (i.e., for a frequency < 1, it was assumed to be 1 to estimate exposure on the day of use). Dermal absorption for wintergreen oil and phenethyl salicylate are assumed to be equivalent to oral absorption. Dermal absorption of salicylic acid was assumed to be 60% (Davis et al. 1997).
Estimates of exposure for the relevant age groups were based on the following body weights (Health Canada 1998):
Infants (0-0.5 years): 7.5 kg
Toddlers (0.5-4 years): 15.5 kg
Children (5-11 years): 31.0 kg
Teens (12-19 years): 59.4 kg
Adults (20-59 years): 70.9 kg
| Exposure scenario | Assumptions |
|---|---|
| Body moisturizer | Exposure frequency (per day): Product amount(g)c: |
| Tanning product (salicylic acid) | Product amount (Adult, g): 4d |
| Face moisturizer | Product amount (g): Exposure frequency (per day): |
| Hand moisturizer | Exposure frequency (per day): Product amount (g): |
| Foot moisturizer | Exposure frequency (per day): Product amount (g): |
| Massage oil | Product amount (g)c: Teens: 2.8 Children: 2.3 Toddlers: 1.8 |
| Antiperspirant/ deodorant (dermal exposure to spray) | Exposure frequency (per day)b: Product amount(g)d: |
| Shaving product | Product amount (g): 1.6f |
| Hair styling product (dermal exposure to spray, pump) | Exposure frequency (per day) Product amount(g): Retention factor: 0.1 |
| Permanent hair dye | Product amount (g): Retention factor: 0.1h |
| Eye make-up (make-up remover) | Exposure frequency (per day): Product amount (g): |
| Bath product (liquid body soap) | Exposure frequency (per day)a Product amount (g)a: |
| Shampoo | Exposure frequency (per day) Product amount (g): |
| Hair conditioner (leave-in) | Exposure frequency (per day) Product amount (g)k: Retention factor: 0.1 |
| Hair spray, salicylic acid (inhalation)h | Concentration: 0.1% Exposure model: instantaneous release Exposure duration: 5 min Room volume: 10 m3 (bathroom) Ventilation rate : 2.5/hr (bathroom) Room height: 2.5 m Non-respirable uptake fraction: 1 |
| Facial moisturizer, homosalate (aerosol, inhalation)h | Concentration of homosalate: 3.0% Exposure duration: 5 min Room volume: 10 m3 (bathroom) Ventilation rate : 2.5/hr (bathroom) Room height: 2.5 m (standard room height) Non-respirable uptake fraction: 1 |
a Ficheux et al. 2015
b Wu et al. 2010
c Ficheux et al. 2016
d Loretz et al. 2005
e ConsExpo Web 2016
f Wormuth et al. 2006
g Loretz et al. 2006
h ConsExpo Web 2016
i CTFA 1983
j EC 2003
k Loretz et al. 2008
| Exposure scenario | Assumptions |
|---|---|
Analgesic creama (salicylic acid and wintergreen oil) |
Surface area: Assuming area exposed is approximately ½ surface of a trunk from Health Canada (1998)
Product amount: Product amounts estimated based on surface area adjustment factors derived from the use of a body moisturizer (Ficheux et al. 2016)
Frequency: 4x/daya Dermal absorption: 60% (Davis et al. 1997, for salicylic acid) |
Antacid tablets/liquid antacids (salicylic acid and wintergreen oil)a,b |
Antacid tableta Frequency: 3x/day
Liquid antacidb Frequency of use: Adults/children: up to 8 doses per 24 hr |
Sunscreen (salicylic acid)a |
Concentration: 0.14%a Product amount per dayc (g): Toddlers: 5.4 Children: 6.3 Teens: 18.2 Adults: 18.2
Frequency (/day)d: Toddlers: 1.6 Children: 1.4 Teens: 1.4 Adults: 1.4 |
Plug-in air freshener (phenethyl salicylate)e
|
Exposure model: exposure to vapour – constant rate Product amount: 1.7 g Concentration: < 1ppmf Exposure duration: 24 hours Room volume: 20 m3 Ventilation rate: 0.6 per hour Emission duration: 24 hours |
a Personal communication, emails from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated December 2016; unreferenced.
b Personal communication, emails from the Therapeutic Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated December 2016; unreferenced.
c Ficheux et al. 2016.
d Ficheux et al. 2015.
e ConsExpo Web 2016.
f ECCC 2017.
Dermal exposure to homosalate
The potential absorbable dose of homosalate (HMS) from the OECD Guideline 428 dermal absorption study (ECHA 2017e) was used to characterize systemic exposure to homosalate via the dermal route for different scenarios. The following algorithms were used:
Per-Event Systemic Exposure = (AV x PAA)/BW
and
(Total) Dermal Load = Conc x Product Amount x RF x F)/AV
Where:
AV = skin surface area exposed
PAA = potential absorbable dose (over 24 hr of exposure)
F = exposure frequency (incorporated only if >1)
Conc = concentration
RF = retention factor
If the PAA was less than the (total) dermal load, the per-event systemic exposure was used to characterize systemic exposure given the lack of full dose depletion, otherwise the (total) dermal load was used (due to full dose depletion). Where “F” is greater than once per day, the per-event systemic exposure can be used as a daily systemic exposure estimate. In other words, no adjustment for exposure frequency would be needed regardless of the number of product applications within a 24-hour period given that PAA represents the cumulative amount absorbed over 24 hours.
| Sentinel exposure scenario | Age group | Dermal load (mg/cm2) | Potential absorbable dose (mg/cm2)a | Systemic exposure (mg/kg bw) |
|---|---|---|---|---|
| Face moisturizer (30%) | Teen | 1.012b | 0.00763 | 0.082c |
| Tanning product (10%) | Adult | 0.123d | 0.00763 | 1.20c |
a Where potential absorbable dose = amount of HMS applied in the dermal absorption study (0.5449 mg/cm2, ECHA 2017e) * % absorption observed in the study (1.4% over 24 hr, ECHA 2017e). There might be slight differences due to rounding
b Where dermal load = 1.2g (Loertz et al. 2005) * HMS concentration * 1000 mg/g (unit conversion) * 1.8 applications per day (Loretz et al. 2005) / 637 cm2 (corresponding to application of product on face based on defaults from Health Canada 1998). There may be slight differences due to rounding.
c Systemic exposure = (PAA * AV)/BW
d Where dermal load = 5.2g (Ficheux et al. 2015) * HMS concentration * 1000 mg/g * 4 applications per day (professional judgement)/ 11 192.5 cm2 (corresponding to application of product on face, arms, legs, hands, and feet, based on defaults from Health Canada 1998). There may be slight differences due to rounding.
