Draft screening assessment - Selected C3-C5 Alcohols Group
Official title: Draft screening assessment - Selected C3-C5 Alcohols Group
Chemical Abstracts Service Registry Numbers
71-23-8
67-63-0
57-55-6
78-83-1
75-65-0
71-41-0
Environment and Climate Change Canada
Health Canada
June 2023
Synopsis
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of six substances referred to collectively under the Chemicals Management Plan as the Selected C3-C5 Alcohols Group. The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 1 ), their Domestic Substances List (DSL) names, and their common names are listed in the table below. or administrative policy, is not permitted without the prior written permission of the American Chemical Society.
| CAS RN | DSL name | Common name |
|---|---|---|
| 71-23-8 | 1-Propanol | Propyl alcohol |
| 67-63-0 | 2-Propanol | Isopropanol |
| 57-55-6 | 1,2-Propanediol | Propylene glycol |
| 78-83-1 | 1-Propanol, 2-methyl- | Isobutanol |
| 75-65-0a | 2-Propanol, 2-methyl- | tert-Butanol |
| 71-41-0 | 1-Pentanol | NA |
Abbreviation: NA, not available
a This substance did not meet categorization criteria under subsection 73(1) of CEPA, and was prioritized through other mechanisms.
1-Propanol and 2-propanol naturally occur in the environment. The remaining substances in the Selected C3-C5 Alcohols Group do not naturally occur in the environment.
The substances in the Selected C3-C5 Alcohols Group, except for propylene glycol, were included in a survey issued pursuant to section 71 of CEPA. In 2011, manufacturing quantities in Canada were reported for 1-propanol (1 410 kg), 2-propanol (over 10 000 000 kg), isobutanol (17 800 kg), and tert-butanol (over 10 000 000 kg), while 1-pentanol was not reported to be manufactured above the reporting threshold of 100 kg. In the same year, import quantities in Canada were reported for 1-propanol (8 285 724 kg), 2-propanol (17 934 589 kg), isobutanol (over 10 000 000 kg), tert-butanol (10 000 to 100 000 kg), and 1-pentanol (104 863 kg). According to the Canadian International Merchandise Trade Web Application, 24 199 865 kg of propylene glycol was imported into Canada in 2021.
According to information submitted in response to a CEPA section 71 survey for these substances (except propylene glycol), the primary reported use is in paints and coatings. Other main uses include ink, toners and colourants; cleaning and furnishing care (1-propanol, 2-propanol, and isobutanol); automotive, aircraft and transportation (2-propanol and 1-pentanol); personal-care products; adhesives and sealants; and oil and natural gas extraction (2-propanol). Substances in the Selected C3-C5 Alcohols Group may also be used in cosmetics (all except 1-pentanol), as food additives (2-propanol and propylene glycol), food flavouring agents (1-propanol, 2-propanol, isobutanol, and 1-pentanol), components in the manufacture of food packaging materials (all), incidental additives (tert-butanol), medicinal or non-medicinal ingredients in drugs (all except 1-pentanol) including natural health products (1-propanol, 2-propanol, propylene glycol, and tert-butanol), as active ingredients in pest control products (2-propanol and propylene glycol), as formulants in pest control products (all), and in other products available to consumers.
The ecological risks of the substances in the Selected C3-C5 Alcohols Group were characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, the 6 substances in the Selected C3-C5 Alcohols Group are considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from the 6 substances in the Selected C3-C5 Alcohols Group. It is proposed to conclude that the 6 substances in the Selected C3-C5 Alcohols Group do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
1-Propanol, 2-propanol, propylene glycol, isobutanol, and 1-pentanol are not identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity, or reproductive toxicity. They are assessed using the approach outlined in the Science approach document for substances with low human health hazard potential. As these five substances are considered to be of low hazard potential, quantitative exposure estimates for these substances were not derived, and the risk to human health from these substances is considered to be low.
Exposure to the general population to tert-butanol is expected from air and drinking water, and from the use of various products available to consumers, such as cosmetics and drugs including natural health products. In laboratory studies with tert-butanol, there were increased non-cancer kidney effects (nephropathy). There were increased incidences of kidney and thyroid tumours, although the relevance of increased kidney tumours to humans was unclear. tert-Butanol has not been shown to be genotoxic. A comparison between critical cancer and non-cancer effects and levels to which the general population may be exposed to tert-butanol from environmental media, cosmetics (such as teeth whitener, body lotion, and hair spray), natural health products (such as hand sanitizer), non-prescription drugs (such as sunscreen lotion), markers, and multi-purpose odour eliminator spray, resulted in margins of exposure that are considered adequate to account for uncertainties in the health effects and exposure data used to characterize risk.
The human health assessment took into consideration those groups of individuals within the Canadian population who, due to greater susceptibility or greater exposure, may be more vulnerable to experiencing adverse health effects from exposure to substances. The potential for increased susceptibility during development and reproduction was assessed and age specific exposure estimates were derived. Infants and young children are expected to have higher exposure to tert-butanol than adults. In addition, people living near industrial releases were considered in the screening assessment of tert-butanol. All of these populations were taken into consideration while assessing the potential harm to human health.
Considering all the information presented in this draft screening assessment, it is proposed to conclude that the 6 substances in the Selected C3-C5 Alcohols Group do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that the 6 substances in the Selected C3-C5 Alcohols Group do not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of six substances referred to collectively under the Chemicals Management Plan as the Selected C3-C5 Alcohols Group to determine whether these substances present or may present a risk to the environment or to human health. The substances in this group were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were prioritized through other mechanisms (ECCC, HC [modified 2017]).
The ecological risks of the substances in the Selected C3-C5 Alcohols Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics including mode of toxic action, chemical reactivity, food-web derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and it considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of factors including potential emission rates, overall persistence and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
Some substances in the Selected C3-C5 Alcohols Group in this draft screening assessment have been reviewed internationally by the Organisation for Economic Cooperation and Development (OECD) and there are OECD Screening Information Data Sets (SIDSs) and SIDS Initial Assessment Reports available. These assessments undergo rigorous review (including peer-review) and endorsement by international governmental agencies. Health Canada and Environment and Climate Change Canada are active participants in this process and consider these assessments to be reliable. In addition, the health effects of some substances in the Selected C3-C5 Alcohols Group have been reviewed by the International Programme on Chemical Safety (IPCS), the United States Environmental Protection Agency (US EPA), the United States National Toxicology Program (NTP), the European Commission (EC), and the European Chemicals Agency (ECHA). Health Canada’s Pest Management Regulatory Agency (PMRA) reviewed 2-propanol (Health Canada 2017a, 2018b) and propylene glycol as active ingredients in pesticides (Health Canada 2008a, 2008b). . Reviews conducted by these institutions are used to inform the health effects characterization in this draft screening assessment. There are also reviews by the European Food Safety Authority (EFSA) and the Australian Industrial Chemicals Introduction Scheme.
This draft screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses, and exposures, including additional information submitted by stakeholders. Relevant data were identified up to December 2021. Empirical data from key studies as well as some results from models were used to reach proposed conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This draft screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this draft screening assessment remain the responsibility of Environment and Climate Change Canada and Health Canada.
This draft screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight-of-evidence approach and precaution.Footnote 2 This draft screening assessment presents the critical information and considerations on which the proposed conclusions are based.
2. Identity of substances
The Chemical Abstracts Service Registry Numbers (CAS RN), Domestic Substances List (DSL) names, and common names for the individual substances in the Selected C3-C5 Alcohols Group are presented in Table 2-1. In this draft screening assessment, the common name propyl alcohol could be applicable to 1- or 2-propanol. To reduce ambiguity, common names are used to refer to the substances where available, except for 1- and 2-propanol where the DSL names are used to refer to the substances.
| CAS RN | DSL name (common name) |
Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 71-23-8 | 1-Propanol (propyl alcohol) |
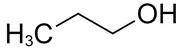 C3H8O |
60.09 |
| 67-63-0 | 2-Propanol (isopropanol) |
 C3H8O |
60.09 |
| 57-55-6 | 1,2-Propanediol (propylene glycol) |
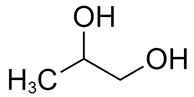 C3H8O2 |
76.09 |
| 78-83-1 | 1-Propanol, 2-methyl- (isobutanol) |
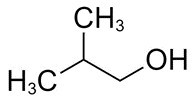 C4H10O |
74.12 |
| 75-65-0 | 2-Propanol, 2-methyl- (tert-butanol) |
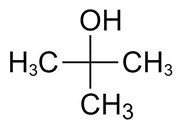 C4H10O |
74.12 |
| 71-41-0 | 1-Pentanol | 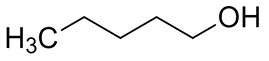 C5H12O |
88.15 |
2.1 Selection of analogues
A read-across approach using data from analogues has been used to inform the human health assessments for 1-propanol, isobutanol and 1-pentanol. Analogues were selected that were structurally similar and/or functionally similar to substances within this group (similar physical-chemical properties) and that had relevant empirical data that could be used to read across to substances with limited empirical data. 2-Propanol was considered suitable for use as an analogue to inform the health effects of 1-propanol, since the two substances are isomeric to each other and have the most similar physical-chemical properties. Information on the identities and chemical structures of the other analogues used to inform the human health assessment is presented in Table 2‑2.
| CAS RN | DSL name (common name) |
Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 71-36-3 | 1-Butanol (n-butyl alcohol) |
 C4H10O |
74.12 |
| 123-51-3 | 1-Butanol, 3-methyl- (isoamyl alcohol) |
 C5H12O |
88.15 |
a PubChem 2004.
In this draft screening assessment, these analogues will be referred to as 1-butanol and isoamyl alcohol. 1-Butanol was considered a suitable analogue by OECD for its ecological risk assessment of isobutanol (OECD 2004a); it is included in this draft screening assessment to support the human health assessment of isobutanol. Isoamyl alcohol was used as an analogue to inform the human health assessment of 1-pentanol. The health effects and physical-chemical properties of the analogues are presented in Appendix A. Details of the read-across data chosen to inform the human health assessments of substances in the Selected C3-C5 Alcohols Group are further discussed in the relevant sections of this report.
3. Physical and chemical properties
A summary of physical and chemical property data of the substances in the Selected C3-C5 Alcohols Group is presented in Table 3-1. Additional physical and chemical properties are provided in ECCC (2016b).
| Substance | Melting point (°C) | Vapour pressure (Pa) | Henry’s law constant (Pa·m3/mol) | Water solubility (mg/L) | Log Kow (dimensionless) |
|---|---|---|---|---|---|
| 1-Propanol | −126 | 2798 | 0.75 | 1.0 × 106 | 0.25 |
| 2-Propanol | −90 | 6063 | 0.82 | 1.0 × 106 | 0.05 |
| Propylene glycol | −60 | 17 | 0.00013 | 1.0 × 106 | −0.92 |
| Isobutanol | −108 | 1400 | 0.99 | 8.5 × 104 | 0.76 |
| tert-Butanol | 25 | 5426 | 0.92 | 1.0 × 106 | 0.35 |
| 1-Pentanol | −78 | 293 | 1.32 | 2.2 × 104 | 1.51 |
Abbreviation: Kow, octanol–water partition coefficient.
a ChemIDPlus 1993.
4. Sources and uses
1-Propanol and 2-propanol naturally occur in the environment (IPCS 1990a, b). 1-Propanol is produced in nature by the decomposition of organic materials by a variety of microorganisms, and occurs in plants and fuel oil (IPCS 1990a). 1-Propanol is found as a fermentation product in alcoholic beverages such as beer, wine, and spirits, and as a naturally occurring flavour in various non-alcoholic drinks (such as kefir culture, cream culture, milk, and apple, tomato, and orange juices) and foods (such as apples, tomatoes, white breads, butters, cheeses, and soybeans) (IPCS 1990a). 2-Propanol is present as a product of microbial metabolism in some foods (such as cheese), and as a naturally occurring flavour in some foods (such as apples, tomatoes, mushrooms, endives, and soybeans) (IPCS 1990b).
All of the substances in the Selected C3-C5 Alcohols Group except for propylene glycol were included in a survey issued pursuant to section 71 of CEPA (Canada 2012). Table 4-1 presents a summary of information reported on the total manufacture and total import quantities for the Selected C3-C5 Alcohols Group in 2011 (Environment Canada 2013), including import quantities for propylene glycol according to the Canadian International Merchandise Trade Web Application.
| Substance | Total manufacturea (kg) | Total importsa (kg) |
|---|---|---|
| 1-Propanol | 1 410 | 8 285 724 |
| 2-Propanol | Over 10 000 000 | 17 934 589 |
| Propylene glycol | NA | 24 199 865b |
| Isobutanol | 17 800 | Over 10 000 000 |
| tert-Butanol | Over 10 000 000 | 10 000 to 100 000 |
| 1-Pentanol | NR | 104 863 |
Abbreviations: NA, not surveyed pursuant to section 71 of CEPA; NR, not reported above the reporting threshold of 100 kg.
a Values reflect quantities reported in response to a CEPA section 71 survey (Environment Canada 2013) except for propylene glycol which was not surveyed. See survey for specific inclusions and exclusions (Canada 2012 -Schedules 2 and 3).
b Annual Canadian import data for propylene glycol (propane-1,2-diol) for 2021 were obtained from the Canadian International
Merchandise Trade Web Application (Statistics Canada [modified 2022]).
According to the information submitted in response to a CEPA section 71 survey (Canada 2012), the substances in the Selected C3-C5 Alcohols Group, except for propylene glycol which was not surveyed, are used in various consumer, commercial, and industrial applications (Environment Canada 2013). The primary use for substances in the Selected C3-C5 Alcohols Group, except for propylene glycol, is in paints and coatings. Other main applications of 1-propanol, 2-propanol, and isobutanol include ink, toners and colourants; and cleaning and furnishing care. 2-Propanol and 1-pentanol are also used in automotive, aircraft and transportation. 2-Propanol is also used in self-care products; adhesives and sealants; oil and natural gas extraction; automotive care; lubricants and greases; drugs; and antifreeze and de-icing products (Environment Canada 2013). Additional consumer uses of substances in the Selected C3-C5 Alcohols Group in Canada are listed in Table 4‑2.
| Use | 1-Propanol | 2-Propanol | Propylene glycol | Isobutanol | tert-Butanol | 1-Pentanol |
|---|---|---|---|---|---|---|
| Food additivea | N | Y | Y | N | N | N |
| Food flavouring agentsa | Y | Y | N | Y | N | Y |
| Incidental additivea,b | N | N | N | N | Y | N |
| Food packaging materialsa | Y | Y | Y | Y | Y | Y |
| Medicinal or non-medicinal ingredients in disinfectant, human or veterinary drug productsc | Y | Y | Y | Y | Y | N |
| Medicinal or non-medicinal ingredients in licensed natural health productsd | Y | Y | Y | N | Y | N |
| Notified to be present in cosmetics under the Cosmetic Regulationse | Y | Y | Y | Y | Y | N |
| Active ingredient or formulant in registered pest control productsf | Y(F) | Y(AI, F) | Y(AI, F) | Y(F) | Y(F) | Yg |
Abbreviations: AI = active ingredient; F = formulant; N = use was not reported for this substance; Y = use was reported for this substance.
a Personal communication, email from the Food Directorate, Health Canada (HC), to the Existing Substances Risk Assessment Bureau (ESRAB), HC, dated October 5, 2021; unreferenced.
b While not defined under the Food and Drugs Act, incidental additives may be regarded, for administrative purposes, as those substances which are used in food processing plants and which may potentially become adventitious residues in foods (such as cleaners, sanitizers).
c Personal communication, email from the Pharmaceutical Drugs Directorate, HC, to the ESRAB, HC, dated September 28, 2021; unreferenced.
d Personal communication, email from the Natural and Non-prescription Health Products Directorate, HC, to the ESRAB, HC, dated October 14, 2021; unreferenced.
e Personal communication, emails from the Consumer and Hazardous Products Safety Directorate, HC, to the ESRAB, HC, dated September 29, 2021 and May 4, 2022; unreferenced.
f Personal communication, email from the Pest Management Regulatory Agency (PMRA), HC, to the ESRAB, HC, dated September 22, 2021; unreferenced.
g Although 1-pentanol is in the PMRA database as a formulant, it is not currently used in any pest control products (Personal communication, email from the PMRA, HC, to the ESRAB, HC, dated September 22, 2021; unreferenced).
In addition to the uses presented above, additional major uses of substances in the Selected C3-C5 Alcohols Group have been identified in products available to consumers in Canada. Propylene glycol is mainly found in cleaning and furnishing care (SDS 2013a), laundry and dishwashing (SDS 2016a), and paints and coatings (SDS 2014). tert-Butanol is found in glow sticks (SDS 2007), markers (SDS 2016b), automotive engine cleaner (SDS 2013b), and odour eliminator spray (SDS 2015).
Four of the substances in the Selected C3-C5 Alcohols Group, 1-propanol, 2-propanol, isobutanol, and tert-butanol, are reportable under the National Pollutant Release Inventory. For 2018, total releases (air, water, land), disposals, transfers for treatment, and transfers for recycling were reported for 1-propanol (1800 tonnes released to air), 2-propanol (2200 tonnes transferred for treatment, 1700 tonnes released to air, 800 tonnes transferred for recycling), isobutanol (350 tonnes transferred for recycling, 180 tonnes released to air, 80 tonnes transferred for treatment), and tert-butanol (2.3 tonnes transferred for recycling, 1.2 tonnes transferred for treatment) (NPRI 2022).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risks of the substances in the Selected C3-C5 Alcohols Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (for example, median lethal concentration) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, from available empirical databases (for example, OECD QSAR Toolbox 2014), from responses to surveys issued pursuant to section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (such as classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over and under classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (for example, mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for the substances in the Selected C3-C5 Alcohols Group and the hazard, exposure and risk classification results are presented in ECCC (2016b).
The hazard and exposure classifications for the six substances in the Selected C3-C5 Alcohols Group are summarized in Table 5‑1.
| Common name | ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| 1-Propanol | low | low | low |
| 2-Propanol | low | low | low |
| Propylene glycol | low | low | low |
| Isobutanol | low | low | low |
| tert-Butanol | low | high | low |
| 1-Pentanol | low | low | low |
According to information considered under ERC, 1-propanol, 2-propanol, propylene glycol, isobutanol and 1-pentanol were classified as having low hazard potential. Although these substances have high reported use quantities according to information submitted in response to a CEPA section 71 survey (except propylene glycol which was not surveyed) (Canada 2012), 1-propanol, 2-propanol, propylene glycol, isobutanol and 1-pentanol are considered to have short overall persistence of less than 13 days and are not expected to undergo long-range transport in air. As such, these substances were classified as having low exposure potential. On the basis of low hazard and low exposure classifications according to information considered under ERC, 1-propanol, 2-propanol, propylene glycol, isobutanol and 1-pentanol were classified as having a low potential for ecological risk. It is therefore unlikely that these substances are resulting in concerns for the environment in Canada.
According to information considered under ERC, tert-butanol was classified as having a low hazard potential. tert-Butanol was classified as having a high exposure potential on the basis of a critically long half-life in air and high reported use quantities according to information submitted in response to a CEPA section 71 survey (Environment Canada 2013). Given the low hazard potential, tert-butanol was classified as having a low potential for ecological risk. Although the current use patterns result in a high exposure potential, considering the low hazard potential, tert-butanol is unlikely to be resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
Potential exposures to substances in the Selected C3-C5 Alcohols Group from environmental media, food, and products available to consumers are presented in this section.
6.1.1 1-Propanol, 2-propanol, propylene glycol, isobutanol and 1-pentanol
Oral, inhalation, and dermal exposures to 1-propanol, 2-propanol, propylene glycol, isobutanol and 1-pentanol may occur from environmental media, food, and the use of products available to consumers containing these substances. Quantitative estimates of exposure to the general population were not derived for 1-propanol, 2-propanol, propylene glycol, isobutanol and 1-pentanol as these substances are considered to have a low hazard potential following the approach outlined in the Science approach document for substances with low potential for human health hazard (Health Canada 2019).
Results from a level III fugacity model (ChemCAN 2003) suggests that 2-propanol partitions primarily to air and to a lesser extent water, whereas 1-propanol, isobutanol, and 1-pentanol partition primarily to water and to a lesser extent air. In contrast, propylene glycol partitions primarily to water and to a lesser extent soil and air. In Canada, 1-propanol and 2-propanol levels were reported in indoor and outdoor air monitoring studies in Edmonton (Health Canada 2013), Regina (Health Canada 2010a), Windsor (Health Canada 2010b) and Halifax (Health Canada 2012) (Table 6‑1).
| Alcohol | Season | Indoor geometric mean (µg/m3) |
Indoor P5-P95 (µg/m3) |
Outdoor geometric mean (µg/m3) |
Outdoor P5-P95 (µg/m3) |
|---|---|---|---|---|---|
| 1-Propanol | Summer | 2.102 | 0.400 – 7.713 | 0.178 | 0.074 – 0.375 |
| 1-Propanol | Winter | 1.580 | 0.453 – 4.793 | 0.072 | 0.031 – 0.226 |
| 2-Propanol | Summer | 25.69 | 1.148 – 8227.0 | 4.111 | 0.328 –18.60 |
| 2-Propanol | Winter | 26.11 | 3.444 – 456.66 | 2.107 | 0.369 – 9.41 |
a The highest geometric mean among the four cities is listed for each season, along with the 5th to 95th percentile [P5-P95] range [µg/m3] for that city.
Food is a potential source of 1-propanol and 2-propanol (IPCS 1990a, b). Propylene glycol is used in the manufacture of certain food packaging materials with potential for direct food contact, which may result in dietary exposure to the general population. 1-Propanol, 2-propanol, isobutanol, and 1-pentanol may also be used in the manufacture of certain food packaging materials as solvents, but there is no potential for direct food contact. 1‑Propanol, 2-propanol, isobutanol, and 1-pentanol are identified as being used as food flavouring agents internationally (Burdock 2010), and it is possible that these substances are present as flavouring agents in foods sold in Canada . There may also be exposure to 2-propanol and propylene glycol from use of these substances as food additives. 2‑Propanol is permitted as a carrier or extraction solvent in fish protein, food flavouring preparations and extracts, as well as meat and egg marking inks that are applied directly on the meat or the shell for branding or other designation. Propylene glycol is permitted as a carrier or extraction solvent in various food additive and flavouring preparations and extracts. Propylene glycol is also permitted for use as an anticaking agent in salt and as a humectant in a variety of foods (Personal communication, email from the Food Directorate, Health Canada (HC), to the Existing Substances Risk Assessment Bureau, HC, dated October 5, 2021; unreferenced).
6.1.2 tert-Butanol
For tert-butanol, sentinel exposure scenarios resulting in the highest exposures for relevant age groups are presented to characterize risk.
Environmental media and food
There were no Canadian or international drinking water, ambient (outdoor) air, soil, or dust data identified for tert-butanol. Based on results from a level III fugacity model (ChemCAN 2003), tert‑butanol partitions primarily to water (84.5%) and air (15.3%), with minimal partitioning to soil (0.2%). ChemCAN (2003) was used to derive concentrations of tert‑butanol in drinking water, ambient air, and soil using total quantities in commerce (Table 4‑1). Maximum estimated concentrations of tert-butanol in drinking water, ambient air, and soil were 3.06 µg/L, 0.72 µg/m3, and 13.5 µg/kg, respectively. These modelled concentrations were used to estimate general population exposures. The potential for tert-butanol to be released to the environment from industrial manufacturing activities was also considered and is not expected to be of concern. As a result, exposure to the general population from this source is not expected.
tert-Butanol was measured in 29 indoor air samples from 50 homes in Quebec City between 2008 and 2010 with a geometric mean concentration of 0.18 µg/m3 and a maximum concentration of 2.37 µg/m3 (Won and Lustyk 2011). tert-Butanol was measured in cycles 2 and 3 of the Canadian Health Measures Survey (Zhu et al. 2013 and Li et al. 2019, respectively). For cycles 2 and 3, respectively, the detection rates of tert-butanol were 90% and 96%, the geometric means were 0.20 µg/m3 and 0.19 µg/m3, and the 95th percentile concentrations were 2.49 µg/m3 and 1.35 µg/m3. The highest indoor air concentration among these studies (95th percentile, 2.49 µg/m3, Zhu et al. 2013) was used to estimate general population exposures to indoor air.
Estimates of exposure for tert-butanol from environmental media ranged from 0.53 µg/kg bw/day for adults (19 years and older) to 1.75 µg/kg bw/day for infants (1 year old). Total daily intake for other age categories and parameters for estimating exposure to environmental media are described in Appendix B.
Although tert-butanol may be used as a solvent in the manufacture of certain food packaging materials with potential for direct food contact, dietary exposure from this use is expected to be low. tert-Butanol may also be present in incidental additives, with no potential for direct food contact, and exposure is not expected (Personal communication, email from the Food Directorate, Health Canada (HC), to the Existing Substances Risk Assessment Bureau, HC, dated October 5, 2021; unreferenced).
Exposure from products available to consumers
tert-Butanol is used primarily as an intermediate, additive (Environment Canada 2013), fragrance ingredient, solvent, and denaturant for ethanol (Personal communication, email from the Natural and Non-prescription Health Products Directorate, Health Canada (HC), to the Existing Substances Risk Assessment Bureau, HC, dated October 14, 2021; unreferenced) in products available to consumers.
According to the information submitted in response to a CEPA section 71 survey (Canada 2012), tert-butanol is used in paints and coatings (Environment Canada 2013). Based on notifications submitted under the Cosmetic Regulations to Health Canada, tert-butanol is used in various cosmetics in Canada, including teeth whitener, make-up, fragrance spray, deodorant, self-tanner, aftershave, hair products, and body and face creams, cleansers, and masks (Personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada (HC), to the Existing Substances Risk Assessment Bureau, HC, dated September 29, 2021 and May 4, 2022; unreferenced). tert-Butanol is also present as a non-medicinal ingredient in various topical natural health products, including hand sanitizers (Personal communication, email from the Natural and Non-prescription Health Products Directorate, HC, to the Existing Substances Risk Assessment Bureau, HC, dated October 14, 2021; unreferenced), and non-prescription drugs, including sunscreens (Personal communication, email from the Pharmaceutical Drugs Directorate, HC, to the Existing Substances Risk Assessment Bureau, HC, dated September 28, 2021; unreferenced). tert-Butanol is also used in products available to consumers such as glow sticks (SDS 2007), markers (SDS 2016b), automotive engine cleaner (SDS 2013b), and odour eliminator spray (SDS 2015).
Although dermal exposure would be expected to contribute to the overall exposure during use of products available to consumers, the primary route is considered to be inhalation or oral depending on the product use scenario. The dermal absorption of tert‑butanol was investigated in a toxicokinetic study in which male Sprague-Dawley (SD) rats were dermally exposed (semi-occluded) to a single dose of [14C]-tert-butanol for 6 hours (Huntington Life Sciences 1998 as cited in ECHA c2020b). Most of the applied dose (89% by 1 hour post-dosing) evaporated and was retained in carbon filters above the dose site. By 72 hours post-dosing, less than 1.5% of the applied dose was detected in urine, feces and tissues. Additional radioactive volatile organic material (4.4 to 8.4% by 1 hour post-dosing) was measured and assumed to be the result of evaporation from the dosing site which was not retained by the carbon filters (Huntingdon Life Sciences 1998 as cited in ECHA c2020b). Based on the 1.5% absorption reported in this study, a toxicological review of tert-butanol described the dermal absorption of tert-butanol as poor (McGregor 2010). Since the source of the volatile material was not definitively determined, ECHA (c2020b) assumed it may have been expired by the rats and included the volatile material in the absorption calculations, resulting in a dermal absorption value of 6 to 11% (ECHA c2020b). Given the available dermal absorption data and the high volatility of tert-butanol, dermal exposure is expected to be minimal in comparison to that of oral and inhalation exposures; therefore, only oral and inhalation estimates are presented.
Per event exposure estimates were not derived for tert-butanol since there were no adverse human health endpoints identified relevant to per event exposure. To characterize daily exposures to tert‑butanol from the use of products available to consumers, exposure estimates were derived using ConsExpo Web (2021) or product- and route-specific exposure algorithms (Appendix C). Internal exposure estimates were derived assuming 100% oral or inhalation absorption. Estimates for the product scenarios that result in the highest level of potential exposure (referred to as sentinel scenarios) for each major product type are presented in Table 6‑2. Exposure to other products available to consumers is expected to be lower than the sentinel exposure estimates presented in this assessment.
| Scenario | Age (years) | Maximum concentration in product (%) | Exposure estimate (mg/kg bw/day)a |
|---|---|---|---|
| Teeth whitener (oral) | 19 and above | 3b | 2.9 x 10-1 |
| Markers (oral) | 0.5 to 1 | 5c | 2.7 x 10-1 |
| Body lotion (inhalation) | 9 to 13 | 0.1b | 2.6 x 10-3 |
| Hair spray (inhalation) | 19 and above | 0.3b | 3.2 x 10-3 |
| Hand sanitizer (inhalation) | 4 to 8 | 1e | 6.2 x 10-3 |
| Hand sanitizer (inhalation) (scenarios of public health concern)d | 2 to 3 | 1e | 1.4 x 10-1 |
| Sunscreen lotion (inhalation) | 4 to 8 | 0.2f | 3.0 x 10-2 |
| Multi-purpose odour eliminator spray (inhalation) |
19 and above | 1g | 4.2 x 10-3 |
a Exposure estimates are derived from the “internal dose on day of exposure” calculated using ConsExpo Web (2021). This value represents the sum of internal doses for multiple events that take place on the same day, where applicable. See Appendix C for more details.
b Personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada (HC), to the Existing Substances Risk Assessment Bureau (ESRAB), HC, dated September 29, 2021 and May 4, 2022; unreferenced.
c SDS 2016b.
d For situations of public health concern, the use of hand sanitizers among the general population may increase up to 25 uses per day (personal use by adults, increased use by children in schools and child care facilities) (RIVM 2021).
e SDS 2020.
f Personal communication, email from the Pharmaceutical Drugs Directorate, HC, to the ESRAB, HC, dated September 28, 2021; unreferenced.
g SDS 2015.
6.1.3 Consideration of subpopulations who may have greater exposure
There are groups of individuals within the Canadian population who, due to greater exposure, may be more vulnerable to experiencing adverse health effects from exposure to substances. The potential for elevated exposure within the Canadian population was examined. Exposure estimates are routinely assessed by age to take into consideration physical and behavioural differences during different stages of life. In the assessment of background exposure from environmental media, food and drinking water, young children had higher exposure than adults. Formula-fed infants had higher exposure than human-milk fed infants (from drinking water used to reconstitute formula) and than adults. In the assessment of exposure to tert-butanol in products available to consumers, products used by children that were assessed included markers, body lotion, hair spray, sunscreen lotion, and hand sanitizer. In addition, people living near industrial releases were considered in the assessment of tert-butanol.
6.2 Health effects assessment
1-Propanol (IPCS 1990a; EC 2008; US EPA 2005, 2007a), 2-propanol (US EPA 2007b, 2014; EC 2015), propylene glycol (OECD 2001; US EPA 2008), isobutanol (OECD 2004a) and tert-butanol (ECHA 2019; US EPA 2021) have been reviewed internationally. Health Canada’s PMRA also has reviews of 2-propanol (under the name of isopropanol) (2017a, 2018b) and propylene glycol (2008a, 2008b). 2-Propanol is not classified as to its carcinogenicity to humans (IARC group 3) (IARC 1999). The draft screening assessment of the Alcohols Group presents information on the health risks of 1-butanol (ECCC, HC 2022). There is also a 2018 ECHA evaluation report review of 1‑butanol, the analogue for isobutanol. tert-Butanol was also reviewed by the NTP (1995, 1997). These reviews have been used to inform the health effects characterization in this draft screening assessment. None of these substances have been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity, or reproductive toxicity.
In contrast to tert-butanol, five substances (1-propanol, 2-propanol, propylene glycol, isobutanol, and 1-pentanol) were assessed using the approach outlined in the Science approach document for substances with low human health hazard potential (Health Canada 2019). These substances are considered to have a low hazard potential, given that no adverse effects were observed after oral or inhalation systemic exposures up to 1000 mg/kg bw/day (no relevant dermal toxicity studies were available), and given the available information indicating a low concern for reproductive, developmental or genotoxic effects.
6.2.1 1-Propanol
1-Propanol is rapidly distributed throughout the body following oral exposure. It is metabolized to propionaldehyde and propionic acid by alcohol and aldehyde dehydrogenases. 1-Propanol is rapidly eliminated from the body; its half-life in the rat is 45 minutes after administration of 1000 mg/kg bw by the oral route. It is excreted (unchanged and/or as metabolites) via expired air or urine (IPCS 1990a).
In a 4-month oral study in male rats exposed to 0 or 60.1 g/L (3300 mg/kg bw/day) of 1‑propanol in drinking water (Lington and Bevan 1991 as cited in IPCS 1990a and US EPA 2005), there were no effects observed.
In a “behavioural teratology study” in SD rats, animals were exposed to 0, 8 700 or 17 000 mg/m3 (equivalent to 0, 2 870 or 5 608 mg/kg bw/day) of 1-propanol by whole‑body inhalation (7 hours/day, 7 days/week) through the gestation period in females and for six weeks in male rats. Seven tests spanning post-natal day (PND) 10 to “approximately 90” were used to evaluate central nervous system (CNS) functions such as neuromuscular ability, activity, and learning in pups. No difference between the exposed and control groups was observed in any of the tests. No difference in the number of live pups per litter, gestational length, birth weight or neonatal survival was observed in any of the groups compared to controls. Infertility in male rats and crooked tails in pups was observed at the highest dose. However, all infertile males were able to produce litters by 13 weeks post-exposure; therefore, regaining fertility (Nelson et al. 1989a as cited in IPCS 1990a).
In a developmental study, SD rats were exposed by whole-body inhalation to 0, 8 600, 17 000 or 26 000 mg/m3 (7 hours/day) (equivalent to 0, 2 837, 4 948, or 7 917 mg/kg bw/day) of 1-propanol during gestation days (GDs) 1-19. No maternal toxicity was observed up to the highest dose tested. At 17 000 mg/m3 and above, there was decreased fetal body weight and reduced feed intake. At 26 000 mg/m3, there were external malformations (short or missing tail, or extrodactyly), skeletal malformations (rudimentary cervical ribs), and visceral malformations (cardiovascular or urinary defects) (Nelson et al. 1988 as cited in IPCS 1990a).
Overall, a genotoxic potential was identified for 1-propanol based on overall negative in vitro reverse mutation assays (Hudolei et al. 1987 as cited in IPCS 1990a), and negative in vitro sister chromatid exchange and micronucleus assays (Hilscher 1969 as cited in IPCS 1990a). No carcinogenicity studies with 1‑propanol were identified.
6.2.2 2-Propanol
While there are PMRA (Health Canada 2017a, 2018b) and US EPA reviews (2007b, 2014) available for 2-propanol, the EC’s review (2015) was used as a basis to describe the human health effects since more study details were reported.
Over 99% of 2-propanol is absorbed following a single dose administration by oral (Slauter et al. 1995 as cited in EC 2015) and inhalation exposure (Laham et al. 1980 as cited in EC 2015). Oxidation yields acetone as the major metabolite; approximately 60% of the administered 2-propanol dose is recovered in the form of acetone via exhaled air, and 4% in urine. The proportion of 2-propanol excreted was 15% in air and less than 1% in urine (Slauter et al. 1994 as cited in EC 2015).
In a 12-week drinking water study, exposure to 0, 870 or 1280 mg/kg bw/day of 2‑propanol did not produce any adverse effects in Wistar rats (Pilegaard and Ladefoged 1993 as cited in EC 2015).
In a 13-week whole-body inhalation exposure to 2-propanol at 0, 246, 1 229, 3 687 or 12 300 mg/m3 for 6 hours/day, 5 days/week in Fischer 344 (F344) rats (equivalent to 0, 97, 487, 2 433, or 4 865 mg/kg/day) or CD-1 mice (equivalent to 0, 132, 659, 3 295, or 6 589 mg/kg/day), increased relative kidney weights in male rats were observed at the highest dose, along with increased size and frequency of kidney hyaline droplets at all doses. Increased mean corpuscular erythrocyte volume (MCV) and mean corpuscular haemoglobin (MCH) were observed in treated male rats, and increased MCV and water consumption was reported for female rats at 1229 mg/m 3 and above. Elevated MCV and MCH values were also observed in female mice at the highest dose (Burleigh-Flayer et al. 1994 as cited in EC 2015). In accordance with the US EPA’s guidance on alpha 2u-globulin, the kidney effects in male rats were not considered toxicologically significant as there are not enough data available to determine the relative contribution of alpha 2u-globulin nephropathy to the kidney effects and their relevance to humans (US EPA 2021).
In a two-generation reproductive toxicity study, no adverse effects were observed in SD rats which were administered a daily gavage dose of 2-propanol at 0, 100, 500 or 1000 mg/kg bw/day (Bevan 1995 as cited in EC 2015). In a developmental study, exposure to 2-propanol in female rats via gavage on GDs 6-15 at 0, 400, 800 or 1200 mg/kg bw/day did not cause developmental effects. Likewise, exposure to female New Zealand White rabbits from GDs 6-18 in the same study at doses (gavage) of 0, 120, 240 or 480 mg/kg bw/day of 2-propanol did not cause any developmental toxicity in fetuses (Tyl et al. 1994 as cited in EC 2015). Furthermore, in a developmental neurotoxicity study, no adverse effects were observed in developing nervous system in offspring following oral administration (gavage) to SD rats of 0, 200, 700 or 1200 mg/kg bw/day of 2-propanol through GD 6 to PND 21 (Bates et al. 1994 as cited in EC 2015).
2-Propanol was not mutagenic in bacterial and in vitro mammalian cell systems with and without metabolic activation (reverse mutation assay, SOS chromotest, mammalian cell sister chromatid exchange and mammalian cell HGPRT) or in vivo (micronucleus) (EC 2015).
There was a 2-year whole-body inhalation combined chronic/carcinogenicity study in which F344 rats (65/sex/dose, with 10/sex/dose interim sacrificed after 54 weeks) were exposed to 0, 1 239, 6 167, or 12 380 mg/m3 (equivalent to 0, 490, 2 441, or 4 901 mg/kg bw/day) of 2-propanol (6 hours/day, 5 days/week). At 490 mg/kg bw/day, increased testes weight was observed in males, with increased absolute and relative liver weights in both males and females. At 2441 mg/kg bw/day and above, increased body weight or body weight gain, increased absolute and/or relative kidney weights were observed in both males and females. Kidney effects were observed in males (increases of tubular proteinosis, glomerulosclerosis, interstitial nephritis, interstitial fibrosis, mineralization, tubular dilation, glomerulosclerosis, hydronephrosis, and transitional cell hyperplasia). At the same doses, increased interstitial cell adenoma of the testes was also observed in males, although the EC (2015) did not consider this to be treatment-related due to an unusually low incidence in the control group. The US EPA (2007b) attributed this to marked hyperplasia rather than autonomous growth. Decreased osmolality and increased total protein, volume, and glucose were also observed in males at the two highest doses, and in females at 4901 mg/kg bw/day (Burleigh-Flayer et al. 1997 as cited in EC 2015).
In a 78-week whole-body inhalation combined chronic/carcinogenicity study, groups of 45 CD-1 mice (55/sex/dose, with 10/sex/dose sacrificed after 54 weeks) were exposed to 0, 1 239, 6 167, or 12 380 mg/m3 (equivalent to 0, 664, 3 306, or 6 638 mg/kg bw/day) of 2-propanol. Increased tubular proteinosis in the kidneys was observed at all experimental doses for males and females. At 3306 mg/kg/day, an increased incidence of seminal vesicle enlargement was observed in males (Burleigh-Flayer et al. 1997 as cited in EC 2015).
6.2.3 Propylene glycol
Propylene glycol is absorbed through the gastrointestinal tract following oral administration. Propylene glycol is rapidly cleared from the bloodstream, with a half-life of approximately 2 hours (OECD 2001).
A repeated-dose dietary exposure to 0 or 50 000 ppm (2 500 mg/kg bw/day) of propylene glycol for 15 weeks did not cause any toxicity in SD rats (Gaunt et al. 1972 as cited in ECHA c2020a).
In a 140-day drinking water study, rats of an unspecified strain were exposed to 0, 1 600, 3 680, 7 700, 13 200, 21 000 or 37 000 mg/kg bw/day of propylene glycol. Limited parameters were examined. All animals at the 2 highest doses died. There were no adverse effects observed at the other doses (Seidenfeld and Hanzlik 1932 as cited in ECHA c2020a). In a 2-year chronic toxicity study, Beagle dogs were fed 0, 2000 or 5000 mg/kg bw/day of propylene glycol. At the highest dose tested, there were effects (such as decreased haemoglobin, haematocrit and erythrocytes and increased reticulocytes) indicative of erythrocyte destruction with accelerated replacement from the bone marrow. No other adverse effects were observed (Wiel et al. 1971 as cited in ECHA c2020a).
In a two-generation reproductive toxicity study, rats were exposed to propylene glycol in drinking water at 0, 1 820, 4 800 or 10 100 mg/kg bw/day. No parental, reproductive or offspring effects were observed (Morrissey et al. 1989 as cited in ECHA c2020a).
There was no evidence of genotoxicity in vitro (bacterial and mammalian cells and cultures) or in vivo (micronucleus, dominant lethal, chromosome aberration) studies with propylene glycol (OECD 2001).
In a 2-year carcinogenicity study, groups of 30 SD rats were fed 0, 6 250, 12 500, 25 000 or 50 000 ppm (equivalent to 0, 312, 625, 1 250, or 2 500 mg/kg bw/day) propylene glycol in the diet. Neither adverse effects nor carcinogenic potential were observed (Gaunt et al. 1972 as cited in ECHA c2020a).
6.2.4 Isobutanol
Isobutanol is rapidly absorbed following oral and inhalation exposure. It is metabolized to isobutyraldehyde and isobutyric acid by alcohol and aldehyde dehydrogenases. Isobutanol is rapidly cleared from the bloodstream (OECD 2004a).
No significant effects were observed in a 13-week oral (gavage) exposure to isobutanol at 0, 100, 316 or 1000 mg/kg bw/day in SD rats. Transient hypoactivity and ataxia at the highest dose tested (1000 mg/kg bw/day) were not considered adverse as the incidence decreased at week 4, and was only seen sporadically thereafter (US EPA 1986). Similarly, in a 13-week neurotoxicity study via whole-body inhalation, there were no effects observed from exposure to 0, 760, 3030 or 7580 mg/m3 (equivalent to 0, 860, 3427, or 8573 mg/kg bw/day) of isobutanol in SD rats (Branch 1996 as cited in OECD 2004a).
In a two-generation reproductive toxicity study in SD rats, there was no parental, reproductive or offspring toxicity observed after whole-body inhalation exposure to isobutanol at 0, 1520, 3030 or 7580 mg/m3 (6 hours/day, 7 days/week) (equivalent to 0, 430, 857, or 2143 mg/kg bw/day) (WIL Res. Labs 2003 as cited in OECD 2004a). In developmental toxicity studies, inhalation exposure in SD rats (GDs 6-15) and rabbits (GDs 7-19) at 0, 500, 1 000 or 10 000 mg/m3 (6 hours/day, 7 days/week) (equivalent to 0, 141, 283, or 2828 mg/kg bw/day in rats), there were no effects observed in dams or fetuses (BASF 1990a, 1990b).
In a developmental toxicity study in SD rats, dams were given 0, 316, 1454, or 5654 mg/kg bw/day of the analogue 1-butanol via drinking water throughout (GDs 0-20) pregnancy (Ema et al. 2005 as cited in ECHA 2018). A no observed adverse effect level (NOAEL) of 1454 mg/kg bw/day for both maternal and developmental effects was based on decreased maternal body weight gain, decreased fetal weight, and increased skeletal variations (primarily short supernumerary ribs) at 5654 mg/kg bw/day, which exceeds the limit dose (ECHA 2018).
Isobutanol was not mutagenic in several in vitro studies and in an in vivo mouse micronucleus assay (OECD 2004a). No carcinogenicity studies were identified, but there is low concern for its carcinogenicity based on negative genotoxicity and lack of carcinogenicity identified for 1-propanol, 2-propanol and propylene glycol.
6.2.5 1-Pentanol
1-Pentanol is rapidly distributed throughout the body following a single inhalation exposure (Oxo Process Panel – ACC 2004 as cited in ECHA c2020c). It is metabolized to pentanoic acid by alcohol dehydrogenase (Ehrig et al. 1988 as cited in ECHA c2020c). 1-Pentanol is excreted via expired air or urine (Haggardet et al. 1945 as cited in ECHA c2020c).
In 13-week repeated dose study, Ash/CSE rats were exposed to 0, 50, 150 or 1000 mg/kg bw/day 1-pentanol via oral gavage. There were no treatment-related effects observed (Butterworth et al. 1978 as cited in ECHA c2020c).
In a whole-body inhalation developmental study, SD rats were exposed to 0 or 14 000 mg/m3 (equivalent to 0 or 4618 mg/kg bw/day) of 1-pentanol for 7 hours/day from GD 1‑19 (Nelson et al. 1989b). No maternal or developmental effects were observed.
In a combined repeated dose toxicity study with the reproduction / developmental toxicity screening test with the analogue isoamyl alcohol, Wistar rats were exposed to 0, 77, 254 or 842 mg/kg bw/day (males), or 0, 177, 372, or 1239 mg/kg bw/day (females) in drinking water. No adverse effects were observed up to the highest dose tested (ECHA c2020c).
1-Pentanol was not genotoxic in a reverse mutation assay. Additionally, its analogue isoamyl alcohol was negative in in vitro (Szybalski 1958 as cited in ECHA c2020c) and in vivo studies (reverse mutation, mammalian cell gene mutation, and micronucleus assays) (ECHA c2020c). No carcinogenicity studies were identified for 1-pentanol or isoamyl alcohol, but there is low concern for the carcinogenicity of 1-pentanol based on negative genotoxicity and lack of carcinogenicity identified for 1-propanol, 2-propanol and propylene glycol.
6.2.6 tert-Butanol
tert-Butanol is a CNS depressant that affects the kidneys (LeBlanc and Kalant 1975 as cited in NTP 1997). The interpretation of kidney effects in male F344/N rats is confounded by the unclear contribution of alpha 2u-globulin, since alpha 2u-globulin nephropathy in male kidneys is not considered relevant to humans. The US EPA (2021) considered that overall, the available information demonstrates that tert-butanol causes alpha 2u–globulin nephropathy, while ECHA (2019) did not discount that chronic progressive nephropathy to be an alternative potential mechanism of action. This difference impacted their point of departure (POD) selections, which are presented when available. This assessment is in agreement with the PODs selected by the US EPA and are overall in agreement with those selected by ECHA, except where noted.
The US EPA (2021) reported that tert-butanol is rapidly absorbed following oral and inhalation exposure, with studies indicating that the substance is 99% absorbed after oral administration. Observed tert-butanol blood levels are comparable after acute oral and inhalation exposure. The substance is distributed throughout the body following exposure (ARCO 1983 as cited in US EPA 2021). According to McGregor (2010), tert‑butanol is not metabolized via the alcohol dehydrogenase pathway, contrary to other alcohols, but via the microsomal oxidase (cytochrome P450) system. In human studies with methyl tert-butyl ether and ethyl tertiary butyl ether that metabolize to tert‑butanol, urinary excretion of tert-butanol was less than 1% of absorption with an excretion half-life of approximately 8 hours (Nihlén et al. 1998 as cited in US EPA 2021). Other studies report that at higher doses, the urinary excretion route becomes saturated, and tert-butanol is then excreted through exhaled air: elimination shifted from being primarily in the urine at 500 ppm (equivalent to 1401 mg/kg bw/day) to occurring primarily by exhalation at 5000 ppm (equivalent to 14 003 mg/kg bw/day) in F344/N rats (Borghoff and Asgharian 1996 as cited in US EPA 2021).
In a 13-week oral study, adult F344/N rats (10/sex/group) were exposed to tert-butanol in drinking water at 0, 230/290, 490/590, 840/850, 1520/1560, or 3610/3620 mg/kg bw/day (males/females) (NTP 1995). At 230/290 mg/kg bw/day and above, there was nephropathy in males and increased absolute and relative kidney weights in females. At 490 mg/kg bw/day and above, there were increased relative liver weights and accumulation of kidney hyaline droplets in males. At 840/850 mg/kg bw/day and above, there were general and urinary system effects, such as decreased urine volume in both sexes, increased mineralization of kidneys and decreased body weight in males, nephropathy in females with severity similar to controls. At 1520 mg/kg bw/day and above in males and 3620 mg/kg bw/day in females, there was increased hyperplasia of transitional cells and inflammation of bladder mucosa. At 3610/3620 mg/kg bw/day, there was mortality in both sexes and decreased body weight in females. ECHA (2019) selected a lowest observed adverse effect level (LOAEL) of 230 mg/kg bw/day for this study based on kidney effects in male rats. In this assessment, its relevance to humans is considered to be confounded by the potential contribution of alpha 2u-globulin-mediated nephropathy, an outcome of uncertain relevance to humans (as discussed previously and see below). An increased liver weight in males at 490 mg/kg bw/day and increased kidney weights in females at 290 and 590 mg/kg bw/day were not considered adverse in the absence of other supportive changes. In this assessment, the NOAEL was considered 490 mg/kg bw/day.
In a 13-week oral study, adult B6C3F1 mice (10/sex/group) were exposed to tert-butanol in drinking water at 0, 350/500, 640/820, 1 590/1 660, 3 940/6 430, or 8 210/11 620 mg/kg bw/day (males/females) (NTP 1995). Decreased final mean body weights and increased hyperplasia of transitional cells and inflammation of bladder mucosa were observed at 3 940 mg/kg bw/day and above in males and at 11 620 mg/kg bw/day in females. In both sexes, there was mortality at the highest dose. ECHA (2019) selected a NOAEL of 1590 mg/kg bw/day.
In an 18-day whole-body inhalation study, F344/N rats were exposed to 0, 406, 825, 1 643, 3274 or 6369 mg/m3 tert-butanol (equivalent to 0, 463, 926, 1852 or 3703 mg/kg bw/day) (NTP 1997). Rats at 825 mg/m3 and above had ataxia, hyperactivity and hypoactivity. At 3274 mg/m3, there were decreased body weight and thymus weight in both sexes. All rats died at the highest dose. For this study, ECHA (2018) selected a no observed adverse effect concentration (NOAEC) of 406 mg/m3.
In a 13-week whole-body inhalation study, F344/N rats were exposed to tert-butanol at 0, 406, 825, 1643, 3274 or 6369 mg/m3 for 6 hours/day, 5 days/week (equivalent to 0, 162, 324, 648, 1296 or 2520 mg/kg bw/day) (NTP 1997). An increase in severity of nephropathy in males was observed at all experimental doses. At 3274 mg/m3 and above, there were decreased hematocrit values, hemoglobin concentrations, and erythrocyte counts, and increased absolute and relative kidney weights in males. In females, relative liver weights were increased at 3274 mg/m3 and above, and the relative kidney weights increased at 6369 mg/m3. Decreased alkaline phosphatase activity was observed at 3274 mg/m3 and above in both sexes. There was also a decrease in urine pH in female rats at 3274 mg/m3 and above and at 6369 mg/m3 in males. ECHA (2019) selected a lowest observed adverse effect concentration (LOAEC) of 406 mg/m3 based on nephropathy in males although the role of alpha-2u-globulin in contributing to kidney toxicity in males was uncertain. In contrast, the US EPA (2021) selected a NOAEC of 6369 mg/m3 based on kidney effects in females since the relevance of kidney effects in male rats to humans was complicated by the unclear contribution of alpha 2u-globulin. In this assessment, effects in females at 3274 mg/m3 (increased relative liver weights, decreased alkaline phosphatase activity, decreased urine pH) were not considered adverse in the absence of other changes, and the NOAEC is considered to be 6369 mg/m3, which is in agreement with the US EPA (2021).
In an 18-day whole-body inhalation study, B6C3F1 mice were exposed to 0, 1 385, 2 759, 5 305, 10 683 or 21 294 mg/m3 tert-butanol (equivalent to 0, 627, 1 254, 2 508, 5 015 or 9 752 mg/kg bw/day) (NTP 1997). There was, ataxia, urogenital wetness, hypoactivity or hyperactivity in both sexes at 5305 mg/m3 and above. At 10 683 mg/m3, there was also rapid breathing and increased relative liver weight in both sexes, death in one male, and decreased absolute and relative thymus weight in females. All mice died at the highest dose. For this study, ECHA (2019) selected a NOAEC of 2759 mg/m3.
In a 13-week whole-body inhalation study, B6C3F1 mice were exposed to tert-butanol at 0, 406, 825, 1643, 3274 or 6369 mg/m3 for 6 hours/day, 5 days/week (equivalent to 0, 219, 439, 878, 1755 or 3413 mg/kg bw/day). In females, decreased mean body weight gain and final mean body weight was observed at 3274 mg/m3 and above and at 6369 mg/m3, respectively. Increased liver weight in females was also observed at 3274 mg/m3 and above. Increased segmented neutrophil count was observed in males at 6369 mg/m3.There was mortality in males at 3274 mg/m3 and above (NTP 1997). For this study, ECHA (2019) selected a NOAEC of 1643 mg/m3.
In a reproduction/developmental toxicity study, SD rats were exposed to tert-butanol via oral gavage at 0, 64, 160, 400 or 1000 mg/kg bw/day for 4 weeks prior to mating, then sacrificed after 9 weeks for parental males, PND 21 for parental females and PND 27 for offspring. At 160 mg/kg bw/day and above, increased relative kidney weights in parental males was observed. At 400 mg/kg bw/day, reduced feed consumption and reduced weight gain were observed in parents. At 1000 mg/kg bw/day, CNS effects (lethargy, ataxia, increased vocalization, and rapid breathing) were observed in parental animals, and increased organ weights in parental males (increased absolute kidney weights, relative liver weight, and relative testis weight). No adverse effects on mating performance, fertility, or reproductive organs were observed. At 1000 mg/kg bw/day, there were reduced mean litter size and number of live born per pregnancy, increased number of stillborn pups and pup mortality, and decreased mean pup body weight at birth which persisted to weaning (ECHA c2020b). For this study, ECHA (2019) selected a NOAEL of 64 mg/kg bw/day for parental effects, a NOAEL of 1000 mg/kg bw/day for reproductive toxicity, and a NOAEL of 400 mg/kg bw/day for offspring effects.
ECHA (2019) described a non-guideline dietary prenatal developmental toxicity study in which Swiss Webster mice were exposed to 0, 3270, 4521 or 6250 mg/kg bw/day tert‑butanol on GDs 6-20 (Daniel and Evans 1982 as cited in ECHA 2019). Maternal weight was recorded on day 5, 10, 15 and 20 of pregnancy. Physiological measurements consisting of length of gestation, gross structural anomalies and number of deaths were recorded within 24 hours. Maternal weight was recorded on day 5, 10, 15 and 20 of pregnancy. Physiological measurements consisting of length of gestation, gross structural anomalies and number of deaths were recorded within 24 hours of parturition. Litters were selected for behavioural studies (righting reflex, negative geotaxis, open field behaviour, cliff avoidance, roto-rod performance). Test scores with the exception of the roto-rod were recorded every second day from day 2 through day 10. Roto-rod evaluations were made every other day from day 14 to day 22, inclusive. Maternal body weight was decreased at day 20 of pregnancy 4521 mg/kg bw/day and above. A reduction in litter size, number, weight, and viability were observed at 3270 mg/kg bw/day and above. At 4521 mg/kg bw/day and above, there were also effects in offspring, such as delayed righting reflex (days 4-6), open field performance differences (days 4-6), delays in cliff avoidance (days 6-10), and reduced roto-rod performance (days 18-22). The study was limited by a lack of details (for example, when fetuses were sacrificed). ECHA (2019) did not determine a NOAEL or LOAEL for this study.
In a whole-body inhalation prenatal developmental toxicity study, SD rats were exposed to 0, 6 063, 10 610 or 15 158 mg/m3 tert-butanol for 7 hours/day on GDs 1-19. ECHA (2019) selected a LOAEC of 6 063 mg/m3 for both maternal and developmental toxicity based on fetal body weight in the presence of unsteady gait at 6 063 mg/m3 and above, skeletal malformations and variations at 10 610 mg/m3, and decreased maternal body weight, decreased food consumption and clinical signs at 15 158 mg/m3 (Nelson 1989b; ECHA 2019).
Although gene mutation assays had mixed results (McGregor 2010), overall genotoxicity studies with tert-butanol were considered negative in vitro (gene mutation, sister chromatid exchange, and chromosomal aberration assays) (NTP 1995; McGregor 2010; US EPA 2021). When mice were administered tert-butanol via drinking water for 13 weeks (0, 350/500, 640/820, 1 590/1 660, 3 940/6 430, or 8 210/11 620 mg/kg bw/day [males/females]), there was no increase in micronucleated erythrocytes (NTP 1995; McGregor 2010; US EPA 2021).
In a 2-year combined chronic/carcinogenicity study in drinking water, groups of 50 F344/N rats were exposed to 0, 90/180, 200/330 or 420/650 mg/kg bw/day (males/females) (NTP 1995). At 90 mg/kg bw/day and above, there were decreased bodyweight and kidney effects in males (increased incidence of focal renal tubule hyperplasia adenomas and carcinomas, nephropathy, transitional cell hyperplasia, of the kidney). At 180 mg/kg bw/day and above, increased absolute and relative kidney weights were observed in exposed female rats, with increased severity of nephropathy. In male rats, a presence of hyaline droplets and regeneration of renal tubular epithelium, along with increased relative kidney weights were observed at 200 mg/kg bw/day and above. Inflammation of the kidneys was observed at 330 mg/kg bw/day and above in females. At 420/650 mg/kg bw/day, males had decreased bodyweight gain, and females had decreased bodyweight. Renal tubule hyperplasia was also observed in one female. ECHA (2019) selected a LOAEL of 90 mg/kg bw/day for this study based on kidney effects in male rats, but in this assessment its relevance to humans is considered to be confounded by the potential contribution of alpha 2u-globulin-mediated nephropathy, an outcome of uncertain relevance to humans (as discussed previously and see below).
In a 2-year chronic/carcinogenicity study in drinking water, groups of 60 B6C3F1 mice were exposed to 0, 540/510, 1040/1020, or 2070/2110 mg/kg bw/day (males/females) tert-butanol (NTP 1995). At 540 mg/kg bw/day and above in males, there was increased thyroid gland follicular cell hyperplasia, which was not observed in females until 1020 mg/kg bw/day. There were also transitional epithelium hyperplasia and chronic inflammation of the urinary bladder, which was not observed in females until 2110 mg/kg bw/day. At 1040 mg/kg bw/day, there were increased incidences of thyroid gland follicular cell adenoma or carcinoma (combined) in males. At 2070/2110 mg/kg bw/day (males/females), there were increased incidences of benign thyroid gland follicular cell carcinoma in males and adenoma in females (US EPA 2021). There were also decreased bodyweight in females and decreased bodyweight gain in both sexes. Although effects were only seen in males at the lowest dose, ECHA (2019) selected a LOAEL of 510 mg/kg bw/day for this study based on kidney and thyroid effects in male mice. An oral cancer slope factor (SF) of 5 × 10-4 (mg/kg bw/day)-1 was based on increased incidences of thyroid follicular cell adenoma or carcinoma in male or female B6C3F1 mice (US EPA 2021).
tert-Butanol exposure induces male rat-specific nephropathy associated with tert-butanol binding to alpha 2u-globulin (Williams and Borghoff 2001 as cited in US EPA 2021). In accordance with the US EPA’s guidance on alpha 2u-globulin, male rat kidney tumours are unsuitable for quantitative analysis because not enough data are available to determine the relative contribution of alpha 2u-globulin nephropathy and other processes to the overall kidney tumour response (US EPA 2021). Chronic progressive nephropathy might also be involved in some non-cancer effects, but these data are complicated by alpha 2u-globulin nephropathy in males (US EPA 2021; NTP 1995). In agreement with the US EPA (2021), in this health effects assessment the renal effects in male F344/N rats were not considered for the risk characterization as there are not enough data available to determine the relative contribution of alpha 2u-globulin nephropathy to the kidney effects and their relevance to humans.
6.2.7 Consideration of subpopulations who may have greater susceptibility
There are groups of individuals within the Canadian population who, due to greater susceptibility, may be more vulnerable to experiencing adverse health effects from exposure to substances. The potential for susceptibility during different life stages or by sex are considered from the available studies. In this health effects assessment, studies included examinations of different sexes of laboratory animals, as well as developmental and neurotoxicity effects in the young, reproductive effects in pregnant animals, and chronic/carcinogenicity effects in older individuals. There was not a particular life stage or sex that was considered more vulnerable for any of the substances in this group based on the available information.
6.3 Characterization of risk to human health
6.3.1 1-Propanol, 2-propanol, propylene glycol, isobutanol and 1-pentanol
1-Propanol, 2-propanol, propylene glycol, isobutanol and 1-pentanol are considered to have a low hazard potential given that no adverse effects were observed as a result of oral exposures or equivalent systemic inhalation doses up to the limit dose of 1000 mg/kg bw/day, and given the available information indicating a low concern for reproductive, developmental toxicity, or genotoxic effects. Although there were no carcinogenicity studies identified for isobutanol and 1-pentanol, there was low concern for their carcinogenicity based on negative genotoxicity and lack of carcinogenicity identified for 1-propanol, 2-propanol and propylene glycol. As such, quantitative exposure estimates for these substances were not derived and the risk to human health from 1-propanol, 2-propanol, propylene glycol, isobutanol and 1-pentanol is considered to be low.
6.3.2 tert-Butanol
Non-cancer health effects
For the risk estimation of non-cancer endpoints of tert-butanol, a LOAEL of 180 mg/kg bw/day was selected based on increased absolute and relative kidney weights and increased severity of nephropathy in female rats in a 2-year combined chronic/carcinogenicity study in drinking water (NTP 1995). It was selected for comparison to long-term oral and inhalation exposures.
Although the ECHA (2019) evaluation report identified a LOAEL of 90 mg/kg bw/day for the chronic carcinogenicity drinking water study based on kidney effects in male rats, and a NOAEL of 64 mg/kg bw/day for the gavage reproductive/developmental toxicity screening test based on increased kidney weights in parental male rats at 160 mg/kg bw/day, in this human health assessment these were not selected for use in risk characterization because of the potential confounding effect of alpha 2u globulin to male renal effects.
The use of the LOAEL of 180 mg/kg bw/day for estimating the risk to daily oral and inhalation exposures is consistent with the US EPA (2021). However, for oral exposures, the US EPA (2021) further applied a body weight to the ¾ power scaling to convert the animal oral dose to an oral human equivalent of 43.2 mg/kg bw/day. For inhalation exposures, the US EPA (2021) converted this LOAEL to a human equivalent concentration using a physiologically based pharmacokinetic (PBPK) modelling and simulation model by assuming the same average blood concentration of tert-butanol in both species, resulting in a concentration of 491 mg/m3 (188 mg/kg bw/day) (US EPA 2021).
Based on uncertainties regarding the use of a PBPK model to extrapolate across routes of exposure, the LOAEL of 180 mg/kg bw/day was used in this human health assessment to consider the risk from daily oral and inhalation exposures. In addition, for the inhalation route a LOAEL of 180 mg/kg bw/day with a target margin of exposure (MOE) of 300 (10-fold for both intraspecies extrapolation and interspecies variation, 3-fold for use of a LOAEL) is within the same range as the US EPA’s approach to using an oral human equivalent dose of 43.2 mg/kg bw/day. Per event exposure estimates and MOEs were not derived for tert-butanol since there were no adverse human health endpoints identified relevant to per event exposure.
Table 6‑3 provides relevant daily exposure estimates and critical effect levels for tert‑butanol, as well as resultant MOEs, for determination of risk to non-cancer health effects.
| Exposure scenario | Age group (years) |
Systemic exposure (mg/kg bw/day)a | Critical effect level (LOAEL) (mg/kg bw/day) | Critical health effect endpoint | MOE |
|---|---|---|---|---|---|
| Environmental media (oral and inhalation) | 1 | 1.8 x 10-3 | 180 | Kidney effects (increased absolute and relative kidney weights, and increased severity of nephropathy) in female rats in a 2-year chronic/carcinogenicity study in drinking water. | 100 000 |
| Teeth whitener (oral) | 19 and above | 2.9 x 10-1 | 180 | Kidney effects (increased absolute and relative kidney weights, and increased severity of nephropathy) in female rats in a 2-year chronic/carcinogenicity study in drinking water. | 620 |
| Markers (oral) | 0.5 to 1 | 2.7 x 10-1 | 180 | Kidney effects (increased absolute and relative kidney weights, and increased severity of nephropathy) in female rats in a 2-year chronic/carcinogenicity study in drinking water. | 670 |
| Body lotion (inhalation) | 9 to 13 | 2.6 x 10-3 | 180 | Kidney effects (increased absolute and relative kidney weights, and increased severity of nephropathy) in female rats in a 2-year chronic/carcinogenicity study in drinking water. | 69 000 |
| Hair spray (inhalation) | 19 and above | 3.2 x 10-3 | 180 | Kidney effects (increased absolute and relative kidney weights, and increased severity of nephropathy) in female rats in a 2-year chronic/carcinogenicity study in drinking water. | 56 000 |
| Hand sanitizer (inhalation) | 4 to 8 | 6.2 x 10-3 | 180 | Kidney effects (increased absolute and relative kidney weights, and increased severity of nephropathy) in female rats in a 2-year chronic/carcinogenicity study in drinking water. | 29 000 |
| Hand sanitizer (inhalation) (scenarios of public health concern) | 2 to 3 | 1.4 x 10-1 | 180 | Kidney effects (increased absolute and relative kidney weights, and increased severity of nephropathy) in female rats in a 2-year chronic/carcinogenicity study in drinking water. | 1300 |
| Sunscreen lotion (inhalation) | 4 to 8 | 3.0 x 10-2 | 180 | Kidney effects (increased absolute and relative kidney weights, and increased severity of nephropathy) in female rats in a 2-year chronic/carcinogenicity study in drinking water. | 6000 |
| Multi-purpose odour eliminator spray (inhalation) | 19 and above | 4.2 x 10-3 | 180 | Kidney effects (increased absolute and relative kidney weights, and increased severity of nephropathy) in female rats in a 2-year chronic/carcinogenicity study in drinking water. | 43 000 |
Abbreviations: LOAEL, lowest observed adverse effect level; MOE, margin of exposure.
a Exposure estimates are derived from the “internal dose on day of exposure” calculated using ConsExpo Web (2021). This value represents the sum of internal doses for multiple events that take place on the same day, where applicable. See Appendix C for more details.
With respect to exposure to tert-butanol from environmental media, teeth whitener, markers, body lotion, hair spray, hand sanitizer, sunscreen lotion, and multi-purpose odour eliminator spray, the calculated margins for non-cancer health effects are considered adequate to address the uncertainties in the health effects and exposure databases.
Cancer health effects
An oral cancer SF of 5 x 10-4 (mg/kg bw/day)-1 for increased thyroid tumours in mice derived by US EPA (2021) was used to calculate quantitative cancer risk estimates for tert-butanol for the Canadian population. The US EPA (2021) did not present an SF for calculating inhalation cancer risk due to a lack of chronic inhalation studies and PBPK data in mice. Given the high volatility of tert-butanol, quantitative cancer risk estimates for inhalation exposure were calculated in this human health assessment using the oral cancer SF derived by the US EPA (2021). The lifetime average daily doses (LADDs) for tert-butanol were calculated for exposures to environmental media, teeth whitener, markers, body lotion, hair spray, hand sanitizer, sunscreen lotion, and multi-purpose odour eliminator spray (Appendix D), in order to derive cancer risk values. Table 6‑4 presents the LADDs, critical effect levels, and resulting cancer risk values for the determination of risk to tert-butanol for cancer effects.
| Exposure scenario | Lifetime average daily dose (mg/kg bw/day)a | Critical effect level (oral slope factor) (mg/kg bw/day)-1 | Critical health effect endpoint | Cancer risk value |
|---|---|---|---|---|
| Environmental media (oral and inhalation) | 6.4 × 10-4 | 5 × 10-4 | Increased thyroid tumours in mice in a 2-year drinking water chronic/carcinogenicity study | 3.2 × 10-7 |
| Teeth whitener (oral) | 1.7 x 10-2 | 5 × 10-4 | Increased thyroid tumours in mice in a 2-year drinking water chronic/carcinogenicity study | 8.5 x 10-6 |
| Markers (oral) | 2.4 x 10-3 | 5 × 10-4 | Increased thyroid tumours in mice in a 2-year drinking water chronic/carcinogenicity study | 1.2 x 10-6 |
| Body lotion (inhalation) | 2.0 x 10-3 | 5 × 10-4 | Increased thyroid tumours in mice in a 2-year drinking water chronic/carcinogenicity study | 1.0 x 10-6 |
| Hair spray (inhalation) | 2.6 x 10-3 | 5 × 10-4 | Increased thyroid tumours in mice in a 2-year drinking water chronic/carcinogenicity study | 1.3 x 10-6 |
| Hand sanitizer (inhalation) | 5.0 x 10-3 | 5 × 10-4 | Increased thyroid tumours in mice in a 2-year drinking water chronic/carcinogenicity study | 2.5 x 10-6 |
| Hand sanitizer (inhalation) (scenarios of public health concern) | 9.8 x 10-3 | 5 × 10-4 | Increased thyroid tumours in mice in a 2-year drinking water chronic/carcinogenicity study | 4.9 x 10-6 |
| Sunscreen lotion (inhalation) | 1.0 x 10-2 | 5 × 10-4 | Increased thyroid tumours in mice in a 2-year drinking water chronic/carcinogenicity study | 5.0 x 10-6 |
| Multi-purpose odour eliminator spray (inhalation) | 4.5 x 10-4 | 5 × 10-4 | Increased thyroid tumours in mice in a 2-year drinking water chronic/carcinogenicity study | 2.3 x 10-7 |
a Exposure estimates are derived from the “internal year average dose” calculated using ConsExpo Web (2021). See Appendix C for more details.
b Adjusted incremental lifetime cancer risk = sum of (SF (mg/kg bw/day)-1 x LADDage group)
Based on the negative genotoxicity and the benign nature of the thyroid tumours in tert‑butanol chronic/carcinogenicity studies, products with a cancer risk below 1 x 10-5 were considered to not represent a concern to human health. With respect to exposure to tert-butanol from environmental media, teeth whitener, markers, body lotion, hair spray, hand sanitizer, sunscreen lotion, and multi-purpose odour eliminator spray, the adjusted incremental lifetime cancer risk values do not represent a concern for human health.
6.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty in the risk characterization for the Selected C3-C5 Alcohols Group are presented in Table 6‑5.
| Key source of uncertainty | Impact |
|---|---|
| tert-Butanol may be used in hand sanitizers. There is uncertainty regarding the duration that increased hand sanitizer use may occur in a situation of public health concern. | +/- |
| For tert-butanol, there are no available long-term inhalation studies. | +/- |
| For isobutanol and 1-pentanol, there are no available carcinogenicity studies. | +/- |
+/- = unknown potential to cause over or under estimation of risk.
7. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from the 6 substances in the Selected C3-C5 Alcohols Group. It is proposed to conclude that the 6 substances in the Selected C3-C5 Alcohols Group do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Considering all the information presented in this draft screening assessment, it is proposed to conclude that the 6 substances in the Selected C3-C5 Alcohols Group do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that the 6 substances in the Selected C3-C5 Alcohols Group do not meet any of the criteria set out in section 64 of CEPA.
References
[AGDH] Australian Government Department of Health. 2013. 1-Propanol: IMAP (inventory multi-tiered assessment and prioritisation) tier II assessment. Sydney (AU): Department of Health, National Industrial Chemicals Notification and Assessment Scheme (NICNAS). [accessed 2022 Jul 13].
[AGDH] Australian Government Department of Health. 2013. 2-Propanol: IMAP (inventory multi-tiered assessment and prioritisation) tier II assessment. Sydney (AU): Department of Health, National Industrial Chemicals Notification and Assessment Scheme (NICNAS). [accessed 2022 Jul 13].
[AGDH] Australian Government Department of Health. 2013. 2-Propanol, 2-methyl: IMAP (inventory multi-tiered assessment and prioritisation) tier II assessment. Sydney (AU): Department of Health, National Industrial Chemicals Notification and Assessment Scheme (NICNAS). [accessed 2022 Jul 13].
Bansaghi S, Soule H, Guitart C, Pittet D, Haidegger T. 2020. Critical Reliability Issues of Common Type Alcohol-Based Handrub Dispensers. Antimicrob Resist Infect Control. 9 (90).
BASF AG. 1990a. Product Safety, Prenatal Toxicity of 2-methyl-1-propanol in rats after inhalation. Project No. 37R0057/88047. BASF AG. 1990b. Product Safety, Prenatal toxicity of 2-methyl-1-propanol in rabbits after Inhalation. BASF Project No. 90R0057/88048, Experimental Toxicology and Ecology, BASF AG, Ludwigshafen, Germany, unpublished report, 1990.
BASF AG. 1990b. Product Safety, Prenatal toxicity of 2-methyl-1-propanol in rats after inhalation. BASF Project No. 90R0057/88048, Experimental Toxicology and Ecology, BASF AG, Ludwigshafen, Germany, unpublished report, December 14, 1990.
Burdock, GA. 2010. Fenaroli’s handbook of flavor ingredients. 6th ed. Boca Raton (FL): CRC Press.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
ChemCAN [level III fugacity model of 24 regions of Canada]. 2003. Version 6.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
ChemIDplus [database]. 1993. Bethesda (MD): US National Library of Medicine. [accessed 2023 Feb 02].
[ConsExpo Web] Consumer Exposure Web Model. 2021. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
[EC] European Commission. 2008. European Union Risk Assessment Report: Propan-1-ol: CAS No. 71-23-8 [PDF]. Luxembourg (LU): Office for Official Publications of the European Communities. Report No: EUR 22159 EN. [accessed 2022 Mar 03].
[EC] European Commission. 2015. European Union Evaluation of Active Substances; Assessment Report: Propan-2-ol [PDF]. Luxembourg (LU): Office for Official Publications of the European Communities. Regulation (EU) No 528/2012 concerning the making available on the market and use of biocidal products [accessed 2022 Jan 10].
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Data used to create substance-specific hazard and exposure profiles and assign risk classifications in the Ecological Risk Classification of organic substances. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [Modified 2011 Dec 15]. Status of Prioritized Substances. Ottawa (ON): Government of Canada. [accessed 2015 Sep 25].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [Modified 2017 Mar 12]. Categorization of chemical substances. Ottawa (ON): Government of Canada. [accessed 2022 Jan 10].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2022. Draft screening assessment - Alcohols Group. Ottawa (ON): Government of Canada. [accessed 2022 Jun 30].
[ECHA] European Chemical Agency. 2018. Substance evaluation conclusion document. Evaluation report for Butan-1-ol (CAS No 71-36-3). Evaluating Member State: Hungary. Helsinki (FI): ECHA. Report No.: EC No 200-751-6. [accessed 2022 Feb 04].
[ECHA] European Chemical Agency. 2019. Substance evaluation conclusion document. Evaluation report for 2-methylpropan-2-ol (tertiary butyl alcohol) (CAS No 75-65-0). Evaluating Member State: United Kingdom. Helsinki (FI): ECHA. Report No. EC No 200-889-7. [accessed 2022 Mar 03].
[ECHA] European Chemicals Agency [database]. c2020a. Registered Substances Database; Search Results for CAS RN 57-55-6. Helsinki (FI): ECHA. [updated 2021 Sep 28; accessed 2022 Jan 07].
[ECHA] European Chemicals Agency [database]. c2020b. Registered Substances Database; Search Results for CAS RN 75-65-0. Helsinki (FI): ECHA. [updated 2021 Aug 9; accessed 2022 Jan 07].
[ECHA] European Chemicals Agency [database]. c2020c. Registered Substances Database; Search Results for CAS RN 71-41-0. Helsinki (FI): ECHA. [updated 2021 Aug 9; accessed 2022 Feb 04].
[ECHA] European Chemicals Agency [database]. c2020d. Registered Substances Database; Search Results for CAS RN 123-51-3. Helsinki (FI): ECHA. [updated 2021 Aug 9; accessed 2022 Feb 04].
[EFSA] European Food Safety Authority. 2009. Scientific Opinion on the evaluation of substances as acceptable previous cargoes for edible fats and oils. EFSA Panel on Contaminants in the Food Chain (CONTAM). EFSA J. 7(11):1391.
[EFSA] European Food Safety Authority. 2011. Scientific Opinion on the evaluation of the substances currently on the list in the Annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils – Part I of III. EFSA J. 9(12):2482.
[EFSA] European Food Safety Authority. 2012. Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils – Part II of III. EFSA J. 10(5):2703.
[EFSA] European Food Safety Authority. 2018. Scientific Opinion. Re-evaluation of propane-1,2-diol (E 1520) as a food additive. EFSA J. 16(4): e05235.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Ficheux AS, Wesolek N, Chevillotte G, Roudot AC. 2015. Consumption of cosmetic products by the French population. First part: Frequency data. Food Chem Toxicol. 78:159-169.
Ficheux AS, Chevillotte G, Wesolek N, Morisset T, Dornic N, Bernard A, Bertho A, Romanet A, Leroy L, Mercat AC, Creusot T, Simon E, Roudot AC.et al. 2016. Consumption of cosmetic products by the French population. Second part: Amount data. Food Chem Toxicol. 90:130-141.
Hansen PL, Tønning K, Malmgren-Hansen B. 2008. Survey and health assessment of chemical substances in hobby products for children [PDF]. Survey of Chemical Substances in Consumer Products, No. 93. Denmark: Danish Ministry of the Environment, Environmental Protection Agency. [accessed 2022 Mar 03].
Health Canada. 2008a. Proposed Re-evaluation Decision. Propylene Glycol [PDF]. Ottawa (ON): Government of Canada. HC Pub. No.: PRVD2008-17. [accessed 2022 Mar 03].
Health Canada. 2008b. Re-evaluation Decision. Propylene Glycol [PDF]. Ottawa (ON): Government of Canada. HC Pub. No.: RVD2008-31. [accessed 2022 Mar 03].
Health Canada. 2010a. Regina Indoor Air Quality Study (2007): Data Summary for Volatile Organic Compound Sampling. Ottawa (ON): Government of Canada. [accessed 2017 June].
Health Canada. 2010b. Windsor Exposure Assessment Study (2005-2006): Data Summary for Volatile Organic Compound Sampling. Ottawa (ON): Government of Canada. [accessed 2017 June].
Health Canada. 2012. Halifax Indoor Air Quality Study (2009): Volatile Organic Compounds (VOC) Data Summary. Ottawa (ON): Government of Canada. [accessed 2017 June].
Health Canada. 2013. Edmonton Indoor Air Quality Study (2010): Volatile Organic Compounds (VOC) Data Summary. Ottawa (ON): Government of Canada. [Accessed 2018 Feb 14].
Health Canada. 2015. Food Consumption Table Derived from Statistics Canada’s 2004 Canadian Community Health Survey, Nutrition, Share File. Ottawa (ON): Government of Canada.
Health Canada. 2017a. Proposed Re-evaluation Decision. Isopropyl alcohol [PDF]. Ottawa (ON): Government of Canada. HC Pub. No.: PRVD2017-09. [accessed 2022 Mar 03].
Health Canada. 2017b. Water Consumption Table Derived from Statistics Canada’s 2004 Canadian Community Health Survey, Nutrition, Share File. Ottawa (ON): Government of Canada. [accessed 2022 Mar 03].
Health Canada. 2018a. Draft backgrounder document on default values for breast milk and formula intakes. Ottawa (ON): Government of Canada.
Health Canada. 2018b. Re-evaluation Decision. Isopropyl alcohol and its Associated End-use Products [PDF]. Ottawa (ON): Government of Canada. HC Pub. No.: RVD2018-14. [accessed 2022 Mar 03].
Health Canada. 2019. Science Approach Document. Science approach document for substances with low human health hazard potential [PDF]. Ottawa (ON): Health Canada. [accessed 2022 Mar 03].
Health Canada. 2020. Personal Care Products Workbook. Recommended Defaults. Last updated: October 19, 2020. Internal Draft. Unpublished report. Ottawa (ON): Existing Substances Risk Assessment Bureau, Health Canada.
Health Canada. [modified 2022 February 11]. Canadian exposure factors used in human health risk assessments. Ottawa (ON): Government of Canada. [accessed 2022 March 03].
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1999. Isopropanol. IARC Monogr Eval Carcinog Risks Hum. 71:1034.
[IPCS] International Programme on Chemical Safety. 1990a. 1-Propanol. Geneva (CH): United Nations Environment Programme, International Labour Organisation, World Health Organization. [Accessed 2022 Jan 10].
[IPCS] International Programme on Chemical Safety. 1990b. 2-Propanol. Geneva (CH): United Nations Environment Programme, International Labour Organisation, World Health Organization. [Accessed 2022 Jan 10].
Kampf G, Ruselack S, Eggerstedt S, Nowak N, Bashir M. 2013. Less and less–influence of volume on hand coverage and bactericidal efficacy in hand disinfection. BMC Infect Dis. 13:472.
KWF (Koningin Wilhelmina Fonds voor de Nederlandse Kankerbestrijding). 2018. Slim met de zon. Zonprotocol, 2018.
Li Y, Cakmak S, Zhu J. 2019. Profiles and monthly variations of selected volatile organic compounds in indoor air in Canadian homes: Results of Canadian national indoor air survey 2012–2013. Environ Int. 126:134-144.
Lington AW, Bevan C. 1991. Alcohols, in Patty's Industrial Hygiene and Toxicology, 4th edition. John Wiley & Sons, Inc.
Loretz LG, Api AM, Babcock L, Barraj LM, Burdick J, Cater KC, Jarrett G, Mann S, Pan YHL, Re TA, et al. 2008. Exposure data for cosmetic products: Facial cleanser, hair conditioner, and eye shadow. Food Chem Toxicol 46:1516-1524.
Macinga DR, Edmonds SL, Campbell E, Shumaker BS, Arbogast, JW. 2013. Efficacy of Novel Alcohol-Based Hand Rub Products at Typical In-Use Volumes. Inspection Control and Hospital Epidemiology. 34 (3):299-301.
McGregor D. 2010. Tertiary-Butanol: A toxicological review. Crit. Rev. Toxicol. 40(8):697-727.
Nelson BK, Brightwell WS, Taylor BJ, Khan A, Burg JR, Krieg EF JR, Massari VJ. 1989a. Behavioral teratology investigation of propan-1-ol administered by inhalation to rats. Neurotoxicol Teratology. 11:153-159.
Nelson BK, Brightwell WS, Khan A, Burg JR, Goad PT. 1989b. Developmental toxicology evaluation of 1-pentanol, 1-hexanol, and 2-ethyl-1-hexanol administered by inhalation to rats. J Am Coll Toxicol. 8(2):405–410.
[NTP] National Toxicology Program (US). 1995. NTP Technical Report Series: Toxicology and carcinogenesis studies of t-butyl alcohol (CAS no. 75-65-0) in F344/N rats and B6C3F1 mice (Drinking water studies). Research Triangle Park, (NC): US Department of Health and Human Services, National Toxicology Program.
[NTP] National Toxicology Program (US). 1997. NTP Technical Report Series: Toxicity of t-butyl alcohol (CAS no. 75-65-0) Administered by Inhalation to F344/N rats and B6C3F1 mice. Research Triangle Park, (NC): US Department of Health and Human Services, National Toxicology Program.
[NPRI] National Pollutant Release Inventory. 2022. Pollutant Release and Transfer Data Reported by Facilities, Single Year Tabular Format – National Pollutant Release Inventory (NPRI). NPRI Dataset for 2018 [Internet]. Gatineau (QC): Environment and Climate Change Canada. [accessed 2022 March 03].
[OECD] Organisation for Economic Cooperation and Development. 2001. SIDS Initial Assessment Report 1,2-dihydroxypropane: CAS No. 57-55-6. SIAM [SIDS Initial Assessment Meeting] 11; 2001 January; DC, USA. [accessed 2022 Jan 07].
[OECD] Organisation for Economic Cooperation and Development. 2004a. SIDS Initial Assessment Report Isobutanol: CAS No. 78-83-1. SIAM [SIDS Initial Assessment Meeting] 19; 2004 October; Berlin, Germany. [accessed 2022 Jan 07].
[OECD] Organisation for Economic Cooperation and Development. 2004b. SIDS Initial Assessment Report n-Butyl Alcohol: CAS No. 71-36-3. SIAM [SIDS Initial Assessment Meeting] 19; 2004 October; Berlin, Germany. [accessed 2022 Jan 07].
OECD QSAR Toolbox [Read-across tool]. 2014. Version 3.3. Paris (FR): Organisation for Economic Co‑operation and Development, Laboratory of Mathematical Chemistry.
PubChem [database]. 2004. Bethesda (MD): US National Library of Medicine, National Center for Biotechnology Information. [accessed 2021 Nov 16].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2021. Health Risk Assessment when using ethanol-containing hand gel. Bilthoven (NL): RIVM. Report No.: 2021-0026.
[SDS] Safety Data Sheet. 2007. ACTIVATED” Chemiluminescent Product (Reverse Oxalate Component) All Colors [PDF]. Indian Orchard [MA]: Omniglow LLC. [accessed 2022 April 25].
[SDS] Safety Data Sheet. 2013a. Weiman Silver Wipes [PDF]. Gurnee [IL]: Weiman Products. [accessed 2022 April 25].
[SDS] Safety Data Sheet. 2013b. Wynn’s Hot Shot Injector Cleaner With Octane Booster [PDF]. Mississauga [ON]: Wynn’s Canada LTD. [accessed 2022 April 25].
[SDS] Safety Data Sheet. 2014. Origins By Benjamin Moore Ultimate Interior [PDF]. Montvale [NJ]: Benjamin Moore & Cie. [accessed 2022 April 25].
[SDS] Safety Data Sheet. 2015. General Formula [PDF]. Litchfield (CT): Zero Odor LLC. [accessed 2022 April 25].
[SDS] Safety Data Sheet. 2016a. Tide Pods [PDF]. Toronto [ON]: Procter & Gamble Inc. [accessed 2022 April 25].
[SDS] Safety Data Sheet. 2016b. ColorBlast! Markers [PDF]. Westport (CT): Melissa & Doug, LLC. [accessed 2022 April 25].
[SDS] Safety Data Sheet. 2020. Scott® Pro Moisturizing Foam Hand Sanitizer [PDF]. Roswell [GA]: Kimberly-Clark Corporation. [accessed 2023 Feb 2].
Statistics Canada. [Modified Feb 8 2022]. [Canadian International Merchandise Trade Web Application]. Search Results for 2905.32.00.00 — Propylene glycol (propane-1,2-diol) imports. Ottawa (ON): Statistics Canada. [accessed 2022 July 27].
[US EPA] United States Environmental Protection Agency. 1986. Rat oral subchronic toxicity study. Final report. Compound: isobutyl alcohol. TRL Study # 032-002. Toxicity Research Laboratories, Ltd., Muskegon, MI
[US EPA] United States Environmental Protection Agency. 2005. Inert Reassessment-n-propanol, CAS# 71-23-8. US EPA, Office of Prevention, Pesticides and Toxic Substances. Washington (DC). [accessed 2022 Jan 07].
[US EPA] United States Environmental Protection Agency. 2007a. Provisional Peer Reviewed Toxicity Values for n-Propyl Alcohol, CASRN 71-23-8. US EPA, Office of Prevention, Pesticides and Toxic Substances. Washington (DC). [accessed 2022 Jun 29].
[US EPA] United States Environmental Protection Agency. 2007b. Reregistration Eligibility Decision (RED) Aliphatic Alcohols. US EPA, Office of Prevention, Pesticides and Toxic Substances. Washington (DC). [accessed 2022 Mar 08].
[US EPA] United States Environmental Protection Agency. 2008. Provisional Peer Reviewed Toxicity Values for Propylene Glycol, CASRN 57-55-6. US EPA, Office of Research and Development, National Center for Environmental Assessment. Washington (DC). [accessed 2022 Mar 03].
[US EPA] US Environmental Protection Agency. 2011. Exposure Factors Handbook: 2011 Edition. National Center for Environmental Assessment, Office of Research and Development. Washington (DC). [accessed 2022 Mar 03].
[US EPA] United States Environmental Protection Agency. 2014. Provisional Peer Reviewed Toxicity Values for Isopropanol, CASRN 67-63-0. US EPA, Office of Research and Development, National Center for Environmental Assessment. Washington (DC). [accessed 2022 Mar 03].
[US EPA] US Environmental Protection Agency. 2021. Toxicological Review of tert-Butyl Alcohol (tert-Butanol) [CASRN 75-65-0]. US EPA, Office of Research and Development, Integrated Risk Information System Center for Public Health and Environmental Assessment. Washington (DC). [accessed 2022 Mar 03].
Won D, Lusztyk E. 2011. Data gathering on chemicals released to indoor air of residences from building materials and furnishings. Final Report. Ottawa (ON): NRC. 158 pp. Report No.: B3332.2.
Wu X, Bennett DH, Ritz B, Cassady DL, Lee K, Hertz-Picciotto I. 2010. Usage pattern of personal care products in California households. Food Chem Toxicol. 48:3109-3119.
Zhu J, Wong SL, Cakmak S. 2013. Nationally representative levels of selected volatile organic compounds in Canadian residential indoor air: Population-based survey. Environ Sci Technol. 47:13276-13283.
Appendix A. Summary table of read-across for health effects endpoints
| Property or health effect | Isobutanol (target) | 1-Butanol (analogue) |
|---|---|---|
| Structure |  |
 |
| Melting point (°C) | −108 | −89.8 |
| Vapour pressure (Pa) | 1400 | 893 |
| Henry’s law constant (Pa·m3/mol) | 0.99 | 0.89 |
| Water solubility (mg/L) | 8.5 × 104 | 6.32 × 104 |
| Log Kow (dimensionless) | 0.76 | 0.88 |
| Acute oral | Rat LD50 > 2830 mg/kg (males); = 3350 mg/kg (females) (OECD 2004a). | Rat LD50 = 2290 mg/kg bw (females) (ECHA 2018). |
| Acute dermal | Rabbit LD50 > 2000 mg/kg bw (males); = 2460 mg/kg bw (females) (OECD 2004a). | Rabbit LD50 = 3402 mg/kg (OECD 2004b). |
| Acute inhalation | Rat LC50 > 6000 ppm (18 120 mg/m3) (OECD 2004a). | Rat LC0 = 8000 ppm (24 000 mg/m3) (OECD 2004b). |
| Short-term oral | N/A | Increased activation of alcohol dehydrogenase and catalase in serum, membrane lesions of hepatocytes and lysosomes and a decrease of adrenal weight at 0.2 mg/kg bw/day in 30-day gavage study in male rats. No details on clinical observations and other endpoints (ECHA 2018). |
| Short-term inhalation | N/A | N/A |
| Short-term dermal | N/A | Drying of the skin, cracking, furrowing and exfoliation of the epidermis when 1-butanol was applied to rabbit skin at 100% concentration under occlusive conditions for 12 times for a 5-hour duration within 21 days (ECHA 2018). |
| Subchronic oral | No adverse effects (13-week gavage study in SD rats; HDT = 1000 mg/kg bw/day). Transient hypoactivity and ataxia at 1000 mg/kg bw/day (not 316 mg/kg bw/day or lower) but they were not considered adverse as they were reversible by the fourth week of study (US EPA 1986). | NOAEL = 125 mg/kg bw/day (ataxia and hypoactivity at 500 mg/kg bw/day in a 13-week gavage study in rats) (US EPA 1986). |
| Subchronic inhalation | NOAEC = 7580 mg/m3 (13-week neurotoxicity study in SD rats; whole-body inhalation) (Branch et al. 1996 as cited in OECD 2004a). | N/A |
| Genotoxicity | Not genotoxic in vitro and in vivo (OECD 2004a). | Not genotoxic in vitro and in vivo (OECD 2004b). |
| Carcinogenicity | N/A | N/A |
| Reproductive and/or developmental toxicity | No parental, reproductive or offspring toxicity (two-generation inhalation study in SD rats; HDT = 7 580 mg/m3); no maternal or developmental effects (two-generation inhalation study in SD rats and rabbits; HDT = 10 000 mg/m3) (WIL Res Lab 2003 as cited in OECD 2004a). | LOAEL = 5654 mg/kg bw/day (drinking water developmental toxicity study in SD rats; decrease in fetal body weight and increase incidence of skeletal variations) (Ema et al. 2005 as cited in ECHA 2018). |
| Neurotoxicity | No effects observed (13-week neurotoxicity study in SD rats, whole-body inhalation; NOAEC = 7580 mg/m3 (HDT)) (Branch et al. 1996 as cited in OECD 2004a). | NOAEC = 6 000 ppm/18 000 mg/m3 (HDT). No effects on parental general toxicity or pregnancy rate observed (behavioural peri-, postnatal developmental neurotoxicity study in SD rats, whole-body inhalation (ECHA 2018). |
Abbreviations: HDT, highest dose tested; Kow, octanol–water partition coefficient; LOAEL, lowest observed adverse effect level; N/A, not available.
a ChemIDPlus 1993
| Property or health effect | 1-Pentanol (target) | Isoamyl alcohol (analogue) |
|---|---|---|
| Structure |  |
 |
| Melting point (°C) | −78 | −117 |
| Vapour pressure (Pa) | 293 | 316 |
| Henry’s law constant (Pa·m3/mol) | 1.32 | 1.43 |
| Water solubility (mg/L) | 2.2 × 104 | 2.67 × 104 |
| Log Kow (dimensionless) | 1.51 | 1.16 |
| Acute oral | Osborne-Mendel rat LD50 = 3030 mg/kg bw/day (ECHA c2020c). | SD rat LD50 > 5000 mg/kg bw (ECHA c2020d). |
| Acute dermal | Albino rabbit LD50 = 2292 mg/kg bw (male) (ECHA c2020c). | NZ White rabbit LD50 = 3216 mg/kg bw (male) (ECHA c2020d). |
| Acute inhalation | SD rat LC0 = 8.29 mg/L air (ECHA c2020c). | SD rat LC0 = 11.05 mg/L air (ECHA c2020d) |
| Short-term oral | N/A | NOAEL = 250 mg/kg bw/day (14-day gavage (1% CMC-Na solution containing 1% Tween 80) study in SD rats, mortality at 1 000 mg/kg bw/day) (ECHA c2020d). |
| Subchronic oral | No adverse effects (13-week gavage study of Ash/CSE rats; HDT = 1000 mg/kg bw/day) (ECHA c2020c). | No adverse effects (13-week drinking water study in Wistar rats; HDT = 1250 mg/kg bw/day) (ECHA c2020c). |
| Genotoxicity | Not genotoxic in vitro (ECHA c2020c). | Not genotoxic in vitro and in vivo (ECHA c2020c). |
| Carcinogenicity | N/A | N/A |
| Reproductive and/or developmental toxicity | No developmental effects (whole-body inhalation study of SD rats; HDT = 14 000 mg/m3) (ECHA c2020c). | No adverse effects (combined repeated dose toxicity study with the reproduction / developmental toxicity screening test in Wistar rats; HDT = 875/1273 mg/kg bw/day (males/females)) (ECHA c2020c). |
Abbreviations: HDT, highest dose tested; Kow, octanol–water partition coefficient; N/A, not available.
a ChemIDPlus 1993
Appendix B. Deterministic estimates of daily human exposure to tert-butanol in environmental media
| Age group (years) | Inhalation rate (m3/day) | Body weight (kg) | Water drinking rate (L/day) |
|---|---|---|---|
| 0 to 0.5 | 3.7 | 6.3 | 0-0.8 |
| 0.5 to 1 | 5.4 | 9.1 | 0-0.8 |
| 1 | 8 | 11 | 0.36 |
| 2 to 3 | 9.2 | 15 | 0.43 |
| 4 to 8 | 11.1 | 23 | 0.53 |
| 9 to 13 | 13.9 | 42 | 0.74 |
| 14 to 18 | 15.9 | 62 | 1.09 |
| 19 and above | 15.1 | 74 | 1.53 |
| Route of exposure | 0-0.5a | 0-0.5b | 0.5-1a | 0.5-1b | 1 | 2-3 | 4-8 | 9-13 | 14-18 | 19+ |
|---|---|---|---|---|---|---|---|---|---|---|
| Ambient airc | 0.05 | 0.05 | 0.05 | 0.05 | 0.07 | 0.06 | 0.04 | 0.03 | 0.02 | 0.02 |
| Indoor aird | 1.28 | 1.28 | 1.29 | 1.29 | 1.58 | 1.34 | 1.05 | 0.72 | 0.56 | 0.44 |
| Drinking watere | N/A | 0.40 | N/A | 0.26 | 0.10 | 0.09 | 0.07 | 0.05 | 0.05 | 0.06 |
| Total Intake | 1.33 | 1.73 | 1.35 | 1.60 | 1.75 | 1.48 | 1.17 | 0.80 | 0.64 | 0.53 |
Abbreviation: N/A, not applicable.
a Human milk is assumed to be the only dietary source for human milk-fed infants.
b Formula is assumed to be the only dietary source for formula-fed infants.
c Intake estimated using maximum estimated concentration of 0.72 ng/m3 in ambient air, modelled using ChemCAN (2003).
d Intake estimated using highest measured concentration of 2.49 µg/m3 in indoor air in Canadian homes (P95, Zhu et al. 2013).
e Intake estimated using maximum estimated concentration of 3.06 µg/L in drinking water, modelled using ChemCAN (2003).
Appendix C. Parameters used to estimate human exposures to tert-butanol from products available to consumers
Unless otherwise specified, human exposure from the use of products available to consumers containing tert-butanol was estimated using ConsExpo Web (2021) using the highest concentration of tert-butanol found per product type or scenario. Unless otherwise specified, the values for exposure duration, emission duration, room volume, and room ventilation rate were based on the relevant ConsExpo Fact Sheet for the scenario presented. Based on the high volatility of tert-butanol, the inhalation-exposure to vapour-evaporation-constant release area model was used to estimate inhalation exposures from the use of products available to consumers (including spray products), assuming 100% of the substance in the used product amount is available for volatilization. Absorption from the oral and inhalation routes was assumed to be 100%. Unless specified, the “product is substance in pure form” option was selected for the molecular weight matrix, and the application temperature was 32°C.
The inhalation rates, skin surface areas, and body weights used to estimate exposures to products available to consumers are described in the Canadian exposure factors used in human health risk assessments fact sheet (Health Canada [modified 2022]). Unless otherwise specified, the values for product amount and frequency of use in self- care product estimates were assumed from internal defaults, which were developed through a process established for Chemicals Management Plan (CMP) assessments (Health Canada 2020). This process includes a review of available data on product amount and frequency of use of self-care products for comprehensiveness of the study or survey, the relevance of the data collected, and the type of information collected. The highest central tendency value from the studies with the highest quality rating is selected for use in CMP assessments, and underlying studies are cited. The parameters used in the estimation of oral and inhalation exposures from the use of products available to consumers are described in Table C-1.
| Product scenario | Assumptions |
|---|---|
| Teeth whitener | Age: 19 years and above (product packaging) Maximum concentration: 3%a Frequency: 0.15/day (product packaging) Product amount: 0.36 g/use (product packaging) Estimated daily oral exposure = (frequency (/day) x concentration (%) x product amount (mg))/(body Weight (kg)) |
| Markers | Age: 0.5 to 1 years, 1 year, 2 to 3 years, 4 to 8 years, 9 to 13 years, 14 to 18 years, 19 years and above Maximum concentration: 5%b Estimated amount of ink per exposure: 50 mg (Hansen et al. 2008) Estimated daily oral exposure (assuming worst-case event occurs daily) = (concentration (%) x estimated amount of ink per exposure (mg))/(body Weight (kg)) |
| Body lotion | Age: 0 to 0.5 years, 0.5 to 1 years, 1 year, 2 to 3 years, 4 to 8 years, 9 to 13 years, 14 to 18 years, 19 years and above Maximum concentration: 0.1%a Frequency: 0.8/day (0 to 0.5 years, 0.5 to 1 year, 1 year, 2 to 3 years; Ficheux et al. 2015), 0.8/day (4 to 8 years, 9 to 13 years, 14 to 18 years; Wu et al. 2010); 1/day (19 years and above; Ficheux et al. 2015; Wu et al. 2010) Product amount: 2 g/use (0 to 0.5 years); 2.5 g/use (0.5 to 1 years); 3.1 g/use (1 year) (Ficheux et al. 2016; surface area adjustment); 4.1 g/use (2 to 3 years; Ficheux et al. 2016); 5 g/use (4 to 8 years); 7.7 g/use (9 to 13 years) (Ficheux et al. 2016; surface area adjustment); 10 g/use (14 to 18 years, 19 years and above; Ficheux et al. 2016) Exposure and emission duration: 20 minutes (time in bathroom) Room volume: 10 m3 (bathroom) Room ventilation rate: 2 changes per hour (bathroom) Release area: 2860 cm2 (0 to 0.5 years); 3680 cm2 (0.5 to 1 year); 4 430 cm2 (1 year); 5950 cm2 (2 to 3 years); 8290 cm2 (4 to 8 years); 12 700 cm2 (9 to 13 years); 16 460 cm2 (14 to 18 years,); 17 530 cm2 (19 years and above) (total body minus head) |
| Hair spray (pump) | Age: 4 to 8 years, 9 to 13 years, 14 to 18 years, 19 years and above
Maximum concentration: 0.3%a Frequency: 0.63/day (4 to 8 years, 9 to 13 years, 14 to 18 years) (Wu et al. 2010); 1.5/day (19 years and above) (Loretz et al. 2008) Product amount: 1.1 g/use (4 to 8 years, 9 to 13 years, 14 to 18 years) (Ficheux et al. 2016); 3.6 g/use (19 years and above) (Loretz et al. 2008) Exposure and emission duration: 20 minutes (time in bathroom) Room volume: 10 m3 (bathroom) Room ventilation rate: 2 changes per hour (bathroom) Release area: 305 cm2 (4 to 8 years, half head); 350 cm2 (9 to 13 years, half head); 370 cm2 (14 to 18 years, half head); 585 cm2 (19 years and above, half head) |
| Hand sanitizer | Age: 2 to 3 years, 4 to 8 years, 9 to 13 years, 14 to 18 years, 19 years and above Maximum concentration: 1%c Frequency: 0.8/day (2 to 3 years); 1.4/day (4 to 8 years, 9 to 13 years, 14 to 18 years); 2.9/day (19 years and above) (Wu et al. 2010) Product amount: 1.5 g/use (Bansaghi et al. 2020, Kampf et al. 2013, Macinga et al. 2013) Exposure and emission duration: 20 minutes Room volume: 20 m3 (unspecified room) Room ventilation rate: 0.6 changes per hour (unspecified room) Release area: 310 cm2 (2 to 3 years); 430 cm2 (4 to 8 years); 610 cm2 (9 to 13 years); 770 cm2 (14 to 18 years,); 910 cm2 (19 years and above) (hands) |
| Hand sanitizer (scenarios of public health concern) | Age: 2 to 3 years, 4 to 8 years, 9 to 13 years, 14 to 18 years, 19 years and above Maximum concentration: 1%c Frequency: 25/day (Wu et al. 2010; RIVM 2021) Product amount: 1.5 g/use (Bansaghi et al. 2020, Kampf et al. 2013, Macinga et al. 2013) Exposure and emission duration: 20 minutes Room volume: 20 m3 (unspecified room) Room ventilation rate: 0.6 changes per hour (unspecified room) Release area: 310 cm2 (2 to 3 years); 430 cm2 (4 to 8 years); 610 cm2 (9 to 13 years); 770 cm2 (14 to 18 years,); 910 cm2 (19 years and above) (hands) |
| Sunscreen lotion | Age: 0.5 to 1 years, 1 year, 2 to 3 years, 4 to 8 years, 9 to 13 years, 14 to 18 years, 19 years and above Maximum concentration: 0.2%d Frequency: 1.4/day (0.5 to 1 year, 9 to 13 years, 14 to 18 years, 19 years and above); 1.6/day (1 year, 2 to 3 years, 4 to 8 years) (Ficheux et al. 2015) Product amount: 5.4 g/use (0.5 to 1 year, 1 year, 2 to 3 years); 6.3 g/use (4 to 8 years, 9 to 13 years); 18.2 g/use (14 to 18 years, 19 years and above) (Ficheux et al. 2016) Exposure and emission duration: 30 minutes (KWF 2018) Room volume: 20 m3 (unspecified room) Room ventilation rate: 0.6 changes per hour (unspecified room) Release area: 3680 cm2 (0.5 to 1 year); 4 430 cm2 (1 year); 5 950 cm2 (2 to 3 years); 8 290 cm2 (4 to 8 years); 12 700 cm2 (9 to 13 years); 16 460 cm2 (14 to 18 years,); 17 530 cm2 (19 years and above) (total body minus head) |
| Multi-purpose odour eliminator spray | Age: 19 years and above Maximum concentration: 1%e Inputs based on personal communication with RIVM (2021) unless otherwise noted. Generic exposure scenario for spray applications Frequency: 0.14 / day (52/year) (assume similar use frequency to interior fabric freshener spray to treat furniture) Product amount: 4.2 g/use (assume similar amount used to toilet spray) Exposure and emission duration: 20 minutes Room volume: 20 m3 (unspecified room) Room ventilation rate: 0.6 changes per hour (unspecified room) Release area: 2190 cm2 (hands and forearms) |
a Personal communication, emails from the Consumer and Hazardous Products Safety Directorate, Health Canada (HC), to the Existing Substances Risk Assessment Bureau (ESRAB), HC, dated September 29, 2021 and May 4, 2022; unreferenced.
b SDS 2016b.
c SDS 2020.
d Personal communication, email from the Pharmaceutical Drugs Directorate, HC, to the ESRAB, HC, dated September 28, 2021; unreferenced.
e SDS 2015.
Appendix D. Lifetime average daily dose calculations for tert-butanol
For estimating the risk of cancer from exposure to tert-butanol, lifetime average daily doses (LADDs) from environmental media and from oral and inhalation exposures to products available to consumers were calculated as follows.
LADD (mg/kg bw/day) = [Sum of (daily exposure rate (mg/kg bw/day)age group) × (exposure duration (years)age group)] / (Lifetime duration (78 years (US EPA 2011)); (Health Canada 2013)
For situations of public health concern, the LADD for hand sanitizer was estimated assuming increased exposure during a situation of public health concern with a duration of 3 years per lifetime. The highest 3-year exposure estimates for situations of public health concern were for individuals 2 to 5 years of age.
