Draft screening assessment - silanamine, 1,1,1-trimethyl-N-(trimethylsilyl)-, hydrolysis products with silica (TMSS)
Official title: Draft screening assessment - silanamine, 1,1,1-trimethyl-N-(trimethylsilyl)-, hydrolysis products with silica (TMSS)
Chemical Abstracts Service Registry Number
68909-20-6
Environment and Climate Change Canada
Health Canada
September 2020
Synopsis
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of silanamine, 1,1,1-trimethyl-N-(trimethylsilyl)-, hydrolysis products with silica, hereinafter referred to as TMSS. The Chemical Abstracts Service Registry Number (CAS RNFootnote 1 ) for TMSS is 68909-20-6. This substance was identified as a priority for assessment on the basis of other human health concerns.
TMSS is an Unknown or Variable Composition, Complex Reaction Products, or Biological Materials (UVCBs) substance. This substance is produced by surface treatment of fumed synthetic amorphous silica (CAS RN 112945-52-5) using hexamethyldisilazane (CAS RN 999-97-3). In the present assessment, TMSS is represented by its major component (over 99%), surface-treated fumed synthetic amorphous silica.
TMSS does not naturally occur in the environment. According to information submitted in response to a CEPA section 71 survey, the total import quantity reported in Canada in 2011 was 212 498 kg and no manufactured quantity was reported above the reporting threshold of 100 kg.
In Canada, TMSS is primarily used as filler, suspending agent, emollient, and additive in the manufacturing of cosmetics, sunscreens, multipurpose cement adhesives, paints, silicone rubbers, inks and toners, and medical devices, as well as in industrial applications including automotive, electrical and electronic. TMSS may be used in food packaging materials and may be used as a component in an incidental additive used in food processing establishments. It is also a formulant in pest control products.
The ecological risk of TMSS was characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, TMSS is considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from TMSS. It is proposed to conclude that TMSS does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of available health effects information for TMSS and read across from substances with similar chemical structure and physical-chemical properties, no critical effects were identified via the oral or dermal routes. As such, oral or dermal exposures to TMSS from environmental media and food, or products available to consumers, are not of concern.
Based on laboratory studies, repeated inhalation exposures to TMSS have the potential to cause adverse effects in the lungs. Inhalation exposure from environmental media is expected to be minimal. The focus of the assessment is on inhalation exposure to TMSS from use of loose-powder products containing TMSS. Comparison of the estimates of inhalation exposure to TMSS from use of dry hair shampoo and facial blush to a critical effect level resulted in margins of exposure that were considered adequate to address uncertainties in the health effects and exposure databases.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that TMSS does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that TMSS does not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of silanamine, 1,1,1-trimethyl-N-(trimethylsilyl)-, hydrolysis products with silica, hereinafter referred to as TMSS, to determine whether this substance presents or may present a risk to the environment or to human health. This substance was considered a priority on the basis of other human health concerns (ECCC, HC [modified 2017]).
Engineered nanomaterials composed of, or containing, TMSS are not explicitly considered in exposure scenarios of this assessment, but products used by consumers considered in the exposure characterization may include a fraction of this substance within the nano-scale. In addition, health effects associated with nano-scale TMSS may not be explicitly considered in this assessment. Any potential risks posed by TMSS at the nano-scale may be subject to separate review at a future date.
The ecological risk of TMSS was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This draft screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data for TMSS were identified up to February 2019. Additional data were submitted by stakeholders up to April 2019. Empirical data from key studies as well as results from models were used to reach proposed conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This draft screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The human health portions of this assessment have undergone external review and/or consultation. Comments on the technical portions relevant to human health were received from Dr. Christine F. Chaisson, Dr. Judy S. LaKind, and Dr. Claudia Fruijtier-Pölloth through Risk Sciences International, Inc. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this draft screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This draft screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precaution.Footnote 2 This draft screening assessment presents the critical information and considerations on which the proposed conclusion is based.
2. Substance identity
For the purpose of this document, this substance will be referred to as TMSS, derived from the Domestic Substance List (DSL) name, silanamine, 1,1,1-trimethyl-N-(trimethylsilyl)-, hydrolysis products with silica. The Chemical Abstracts Service Registry Number (CAS RNFootnote 3 ), DSL name, and common name for TMSS are presented in Table 2-1.
| CAS RN | DSL name(common name) | Representative chemical structurea | Molecular weight (g/mol) |
|---|---|---|---|
| 68909-20-6 | Silanamine, 1,1,1-trimethyl-N-(trimethylsilyl)-, hydrolysis products with silica (TMSS) | 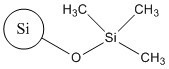 | N/A |
Abbreviation: N/A, Not Applicable
a This substance is a Unknown or Variable Composition, Complex Reaction Products, or Biological Materials (UVCB); i.e., it is not a discrete chemical and thus may be characterized by a variety of structures.
TMSS is a UVCBFootnote 4 substance and is produced by surface treatment of fumed synthetic amorphous silica (CAS RN 112945-52-5) using hexamethyldisilazane (HMDS, CAS RN 999-97-3) (Becker et al. 2013; SCCS 2015). In the present assessment, this substance is represented by its major component (over 99%), surface-treated fumed synthetic amorphous silica (Health Canada 2019).
Synthetic amorphous silica (SAS) is distinctive from, but shares the same CAS RN 7631-86-9 with, crystalline and natural amorphous silica (Martin 2007; Fruijtier-Pölloth 2016). In general, SAS may refer to various untreated forms of synthetic amorphous silica produced by the wet method (i.e., colloidal and precipitated SAS) or the thermal method (i.e., fumed SAS), or their surface-treated derivations (ECETOC 2006; Environment Canada, Health Canada 2013a). However in this assessment, the acronym SAS by itself refers only to untreated synthetic amorphous silica.
2.1 Selection of analogues and use of (Q)SAR models
A read-across approach using data from analogues has been used to inform the human health assessment. Analogues were selected that were structurally similar to substances within this group (i.e., similar physical and chemical properties and toxicokinetics) and that had relevant empirical data that could be used to read-across to substances with limited empirical data.
Details of the read-across data to inform the human health assessment of TMSS are further discussed in the relevant sections of this report. Information on the identities and chemical structures of the analogues used to inform the human health assessment is presented in Table 2-2.
| CAS RN | DSL or other name (common name) | Generic empirical formula or representative structure | Molecular weight (g/mol) |
|---|---|---|---|
| 112926-00-8 | Colloidal SAS, and precipitated SAS | nSiO2 | 60.08a |
| 112945-52-5 | Fumed SAS | nSiO2 | 60.08a |
| 68611-44-9b | Silane, dichlorodimethyl, reaction products with silica (silica dimethyl silylate) | 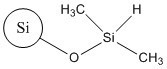 | N/A |
Abbreviation: N/A, Not Applicable
a The molecular weight of SiO2
b Silica dimethyl silylate is a UVCB substance and is not a discrete chemical, and thus may be characterized by a variety of structures. Shown in the table is the representative structure of its surface where some of the hydroxyl groups on the surface are replaced by dimethylsilyl groups.
Analogues include untreated colloidal and precipitated SAS, as well as untreated fumed SAS. The latter is a precursor compound of TMSS (target substance) and silica dimethyl silylate (analogue). Silica dimethyl silylate is a silica derivative in which the surface of the fumed silica has been modified by the addition of dimethylsilyl groups, in contrast to TMSS (target substance) whose surface has been modified by the addition of trimethylsiloxy groups (Becker et al. 2013). Physical and chemical properties and toxicological data for these analogues can be found in Appendix A.
3. Physical and chemical properties
A summary of physical and chemical property data of TMSS and its analogues are presented in Table 3-1. Additional physical and chemical properties are reported in ECCC (2016b).
The surface treatment of SAS does not change its solid properties such as primary particle size. However, surface treatment alters physical and chemical properties, such as hydrophobicity and reduced moisture uptake, depending on the type and amount of surface treatment (ECETOC 2006; Langer et al. 1958). The level of treatment of TMSS can vary and may result in a range of physical and chemical properties including rendering the substance more or less hydrophobic. Therefore, the use of analogues that capture this range allows for a more complete characterisation of the physical and chemical properties and environmental fate and behaviour of TMSS.
| Property | TMSS | Untreated SAS | Silica dimethyl silylate | Key references |
|---|---|---|---|---|
| Melting point (°C) | 1700 | 1700 | 1700 | Environment Canada, Health Canada 2013a; OECD 2004 |
| Boiling point (°C) | 2300 | 2230 | NA | Environment Canada, Health Canada 2013a; ESIS c1995–2009 |
| Vapour pressure (mm Hg at 20°C) | negligible | negligible | negligible | SCCS 2015; OECD 2004; US EPA 2011 |
| Water solubility (mg/L) | < 0.01 (negligible) | 15 - 68(at 20 °C,pH 5.5 - 6.6) | < 0.0001 (negligible) | Becker et al. 2013; OECD 2004; US EPA 2011 |
| Density (kg/m3 at 20°C) | 2200 | 2200 | 2000 | Environment Canada, Health Canada 2013a; OECD 2004; ESIS c1995–2009 |
| Bulk density (tapped, kg/m3) | 100 - 300 | 50 – 320 | 30 - 50 | Environment Canada, Health Canada 2013a; OECD 2004; ESIS c1995–2009 |
| Primary particle size (nm) | 5 – 20a | 5 – 50 (fumed) 5 – 100 (precipitated) | 10 – 50b | Fruijtier-Pölloth 2012; ECETOC 2006; SCCS 2015 |
| Aggregate size (nm) | 100 – 1000a | 100 – 1000 | < 5000b | Fruijtier-Pölloth 2012; ECETOC 2006; SCCS 2015 |
| Agglomerate size (nm) | mostly > 125 000a | 1000 – 250 000 | NA | Fruijtier-Pölloth 2012; ECETOC 2006 |
Abbreviation: NA, Not Available
a Particle size ranges of surface-treated SAS (Fruijtier-Pölloth 2012). The primary particle size of TMSS has also been reported to be 6.9 - 8.6 nm (ECHA 2016), which agrees with the generic size range for the primary particles of surface-treated SAS (5 – 20 nm).
b Particle size range of hydrophobic fumed silica (SCCS 2015).
The primary particle size of surface-treated SAS ranges from 5 nm to 20 nm (Fruijtier-Pölloth 2012). However, the primary particles are expected to form aggregates (100 – 1000 nm) or agglomerates (mostly > 125 000 nm) in the manufacturing and surface modification processes (Fruijtier-Pölloth 2012; Environment Canada, Health Canada 2013a).Footnote 5 ,Footnote 6 Thus, TMSS is expected to exist mainly as aggregates or agglomerates once manufactured.
4. Sources and uses
TMSS does not occur naturally in the environment. TMSS has been included in a survey issued pursuant to CEPA section 71 (Canada 2012). According to information submitted in the survey, the total import quantity reported in Canada in 2011 was 212 498 kg and no manufactured quantity was reported (Environment Canada 2013). In Canada, TMSS is primarily used as filler, suspending agent, emollient, and additive in the manufacturing of cosmetics, sunscreens, multipurpose cement adhesives, paints, silicone rubbers, inks and toners, and medical devices (Environment Canada 2013; MSDS 2016; CosIng 2019). It is also used in various industrial applications including automotive, electrical and electronic (Environment Canada 2013). TMSS provides thickening in pastes and ointments to inhibit the separation of components and to maintain flow properties in powder products (OECD 2004).
Table 4-1 presents a summary of additional uses of TMSS in Canada.
| Use | TMSS |
|---|---|
| Incidental additivea | Yes |
| Food packaging materialsa | Yes |
| Non-medicinal ingredient in disinfectant, human or veterinary drug productsb | Yes |
| Non-medicinal ingredient in licensed natural health productsc | Yes |
| Notified to be present in cosmetics under the Cosmetic Regulationsd | Yes |
| Formulant in registered pest control productse | Yes |
a Personal communication, e-mails from Food Directorate (FD), Health Canada (HC) to Existing Substance Risk Assessment Bureau (ESRAB), HC, dated October 8, 2018 and February 11, 2019; unreferenced.
b Personal communication, emails from Therapeutic Products Directorate (TPD), HC to ESRAB, HC, dated October 1, 2018 and February 14, 2019; unreferenced.
c Personal communication, emails from Natural and Non-Prescription Health Products Directorate (NNHPD), HC to ESRAB, HC, dated October 5, 2018 and February 25, 2019; unreferenced.
d Personal communication, emails from Consumer and Hazardous Products Safety Directorate (CHPSD), HC to ESRAB, HC, dated October 9, 2018 and February 14, 2019; unreferenced.
e Personal communication, email from the Pest Management Regulatory Agency (PMRA), HC to ESRAB, HC, dated October 22, 2018; unreferenced.
TMSS may be used as filler in silicone elastomers and a component in antifoam formulations in the manufacture of food packaging materials. TMSS may also be used as a component in an incidental additive (lubricant) used in food processing establishments (personal communication, e-mails from FD, HC to ESRAB, HC, dated October 8, 2018 and February 11, 2019; unreferenced). TMSS has a non-medicinal role in sunscreens as a sunscreen agent (personal communication, emails from TPD, HC to ESRAB, HC, dated October 1, 2018 and February 14, 2019; unreferenced) as well as an antifoaming, bulking, skin-conditioning, and suspending agent (personal communication, emails from NNHPD, HC to ESRAB, HC, dated October 5, 2018 and February 25, 2019; unreferenced). Based on notifications submitted under the Cosmetic Regulations to Health Canada, TMSS is used in certain cosmetics in Canada, primarily in makeups, moisturizers, hair styling or shampoo products, and nail polish. Main formulation types included loose powders, pressed powders, solid cakes, semi-solid ointment/balms, lotions, gels, creams, and liquid formulations (personal communication, emails from CHPSD, HC to ESRAB, HC, dated October 9, 2018 and February 14, 2019; unreferenced).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risk of TMSS was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration [LC50]) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox 2014), and from responses to surveys issued pursuant to section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over and under classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in (ECCC 2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue (CBR) analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for TMSS, and the hazard, exposure and risk classification results, are presented in ECCC (2016b).
According to information considered under ERC, TMSS was classified as having a high exposure potential on the basis of a long overall persistence and a large annual import quantity according to information submitted in response to a CEPA section 71 survey (Environment Canada 2013c). TMSS was classified as having a low hazard potential and as having a low potential for ecological risk. Although the current use patterns result in a high exposure potential, considering the low hazard potential TMSS is unlikely to be resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
This exposure assessment focuses on routes of exposure through which critical effects have been identified (i.e., inhalation). No critical health effects from the oral and dermal routes of exposure have been identified for TMSS, and thus exposures from these routes were not quantified.
6.1.1 Environmental media and food
No measured concentration data have been identified for TMSS in relevant environmental media or food in Canada or elsewhere. Based on its physical and chemical properties, TMSS is expected to be present predominantly in soils and sediments when released into the environment (OECD 2004). When released to air, TMSS is expected to be deposited to surface waters and soils based on its high density and negligible vapour pressure. If released to surface waters or the terrestrial environment, it is expected to partition to sediments or remain in soils, respectively, based on its negligible water solubility, and high density and chemical stability (Environment Canada, Health Canada 2013a). Thus, TMSS exposure from ambient air and drinking water is not expected to be significant.
TMSS may be used in the manufacture of food packaging materials with the potential for direct food contact. TMSS exposure from this source is expected to be negligible. TMSS may also be used as a component of an incidental additive (lubricant) used in food processing establishments, with no direct food contact; therefore, exposure from this source is not expected [personal communication, e-mails from FD, HC to ESRAB, HC, dated October 8, 2018 and February 11, 2019; unreferenced].
6.1.2 Products available to consumers
Exposure of the general population to TMSS can result from uses including in cosmetics, sunscreens, multipurpose cement adhesives, and paints. There is the potential for oral exposure resulting from the use of lip gloss. Likewise, there is the potential for dermal contact with TMSS from the use of cosmetics, sunscreens, multipurpose cement adhesives, and paints. However, exposure from the oral and dermal routes was not quantified as no critical health effects from these routes of exposure have been identified for TMSS.
The characterization of exposure is focused on products available to consumers that are associated with the potential for inhalation of insoluble respirable TMSS such as cosmetics formulated as loose powders (Personal communication, emails from CHPSD, HC to ESRAB, HC, dated October 9, 2018 and February 14, 2019; unreferenced).
The use of dry hair shampoo and facial blush were considered to be sentinel scenarios as these products contain the highest TMSS concentration among cosmetics available in Canada, and they are formulated as loose powders. Products formulated as pressed powders (e.g., face makeup) were not identified as a potential source of exposure of concern because the formation of a ‘dust cloud’ available for inhalation is not expected during their use. These products contain coarser particles and binders such as oils or waxes, which help bind the particles together (Kogel et al. 2006). In addition, products in liquid or paste form (e.g., adhesives and brush or roller paints) were not considered to be associated with a potential for inhalation exposure since TMSS is not expected to evaporate from the surface where the products are applied based on its negligible vapour pressure. Also, painting with a brush or roller does not produce large amounts of spray droplets, and most of the droplets would not be respirable (Environment Canada, Health Canada 2013b).
Anderson et al (2017) and Rasmussen (2019) measured air concentrations of particulate matter of an average aerodynamic diameter of 4 μm or less (PM4) during the use of face or body powder containing talc. This study was considered an appropriate surrogate study for derivation of exposure estimates for TMSS. This was considered appropriate based on the relevance of product application type and similarity of physical and chemical properties such as melting point, vapour pressure, and water solubility, between TMSS and talc.
Anderson et al. (2017) conducted a study to characterize airborne respirable dust concentrations during the use of historical talc products from the 1960s and 1970s. Cyclone air sampling devices capturing PM4 were attached to the breathing zone of five volunteers. Average PM4 concentrations over the 48-minute exposure simulation were calculated using the total measured mass (from 8 applications over 48 minutes) and the air volume over the entire 48-minute sampling period. Respirable PM4 concentrations ranged from 0.26 to 5.03 mg/m3, and the average was 1.46 mg/m3. The average air concentration by subject ranged from 0.44 to 3.28 mg/m3.
In 2018, Health Canada conducted a small study in order to measure the air concentrations of particles in the breathing zone of adult volunteer subjects while they were applying talc-containing self-care products (Rasmussen et al. 2019). Continuous, direct-reading, personal breathing-zone monitors (positioned beside the nose) measured average PM4 concentrations of 0.48 ± 0.18 mg/m3 and 1.80 ± 0.82 mg/m3 for two volunteers applying body powder (subject A) and loose face powder (subject B), respectively. Subjects repeated the application in triplicate. These average concentrations fall within the range of concentrations measured by Anderson et al. (2017). Average air concentrations by subject from Anderson et al. (2017) were combined with the body and face powder replicates from Rasmussen et al. (2019) to obtain an overall average air concentration of 1.36 ± 0.97 mg/m3 available for inhalation exposure during the use of cosmetics (Appendix B, Table B-1).
The average air concentration value of 1.36 mg/m3 was used to estimate adjusted air concentrations during use of dry hair shampoo and facial blush containing TMSS. The results are summarized in Table 6-1. The inputs and adjustment factors for these scenarios are outlined in Appendix B (Table B-1). These exposure estimates are considered conservative as the size of TMSS particles in cosmetics may not all be respirable (personal communication, emails from CHPSD, HC to ESRAB, HC, May 27, 2019; unreferenced). The distribution range of the TMSS particles in loose powders could span the respirable to the non-respirable size due to agglomeration.
| Product scenario | Maximum concentration | Concentration in air per event (mg/m3)b | Adjusted exposure concentration (mg/m3)c | Higher tier adjusted exposure concentration (mg/m3)d |
|---|---|---|---|---|
| Dry hair shampoo | 100%a | 1.36 | 0.019 (6 hour-TWA) | 0.0011 (continuous exposure) |
| Facial blush | 30%a | 0.408 | 0.0057 (6 hour-TWA) | 0.0014 (continuous exposure) |
Abbreviation: TWA = time weighted average
a Personal communication, emails from CHPSD, HC to ESRAB, HC, dated October 9, 2018 and February 14, 2019; unreferenced.
b Average measured air concentration value in mg/m3 (Anderson et al. 2017, Rasmussen et al. 2019) × maximum TMSS concentration (%) in product
c Per event inhalation exposure estimates were amortized over a 6-hour period by multiplying it by ‘exposure duration/6-hour’ to be aligned with the duration of treatment per day (via inhalation) in the toxicity study. Exposure duration for both scenarios is 5 minutes.
d The per event inhalation exposure estimates for TMSS were adjusted to a continuous exposure estimate (24 hours/day, 7 days/week) for the chronic exposure scenario according to US EPA guidance on inhalation risk assessment (US EPA 2009; see Appendix B for more details).
6.2 Health effects assessment
There is limited toxicological information available for TMSS. The health effects assessment of TMSS were also informed by its analogues, silica dimethyl silylate, and SAS (colloidal, precipitated and fumed SAS). Among other endpoints, these analogues were useful to inform the potential toxicity of TMSS by oral and dermal routes, as well as the clearance and short-term toxicity of TMSS by the inhalation route.
SAS and surface-treated SAS, including TMSS, were evaluated by the European Centre for Ecotoxicology and Toxicological of Chemicals (ECETOC 2006). Health effects of silica dimethyl silylate were characterized by the US Environmental Protection Agency (US EPA 2011). SAS and surface-treated SAS were reviewed internationally by the Organisation for Economic Co-operation and Development (OECD) Cooperative Chemicals Assessment Programme in a SIDS Initial Assessment Report (OECD 2004), the European Commission’s Scientific Committee on Consumer Safety (SCCS 2015). Silica dimethyl silylate is the most similar analogue to TMSS, since they are both surface-treated fumed SAS, but the associated database is limited (e.g., no dermal toxicity study is available). Colloidal, precipitated, and fumed SAS were also used as analogues.
TMSS
In a 13-week inhalation study, Wistar rats (10/sex/group) were exposed to TMSS (mean particle size from 2.8 to 4.5 µm) at concentration of 0, 0.51, 2.05 or 10.01 mg/m3 for 6 hours per day and 5 days per week by inhalation (whole body or nose-only not specified) (Wacker 1998 a,b, as cited in ECETOC 2006). At 10.01 mg/m3, there was increased total protein, aspartate-aminotransferase and alkaline phosphatase levels in lung lavage fluid, and increased neutrophils, macrophages/monocytes and lymphocytes. Exposure to 10.01 mg/m3 TMSS also increased the absolute and relative weights of the lungs and tracheobronchial lymph nodes. Histological examination showed accumulation of alveolar macrophages with few polymorphonuclear cells, bronchiolar-alveolar epithelial hyperplasia, interstitial inflammatory cell infiltrates in the lungs, and increased histiocytosis and macrophage aggregates in draining mediastinal lymph nodes, at 10.01 mg/m3 group. Treatment did not cause interstitial fibrosis. At 2.05 mg /m3, effects were limited to an increased relative number of neutrophils with a decrease in the relative number of macrophages/monocytes without influencing absolute cell numbers. This was not considered to be of toxicological significance. A no observed adverse effect concentration (NOAEC) of 2.05 mg/m3 was determined in this assessment.
In a repeated-dose inhalation study with limited details, rats and cynomolgus monkeys were exposed to TMSS at concentrations of 0, 10, 50, or 150 mg/m3 (particle sizes not stated) for 6 hours a day and 5 days a week for up to 12 months [Dow Corning, 1972, as cited in ECETOC 2006]. No effects were observed at 10 mg/m3. Exposure to 50 and 150 mg/m3 caused aggregation of foamy macrophages in alveoli in rats and interstitial fibrosis in monkeys.
TMSS was not genotoxic in in vitro bacterial mutation or in vitro chromosomal aberration assays (Cabot 1994a, b, c, 1995 as cited in ECETOC 2006).
Silica dimethyl silylate
Toxicokinetic studies were limited to clearance studies in rats. The clearance studies of silica dimethyl silylates (Degussa 1964 as cited in Becker et al. 2013) were conducted in rats exposed to aerosolized silica dimethyl silylate (200 mg/m3, particle sizes not provided) 5 hours per day for 3 days (Degussa 1964 as cited in Becker et al. 2013). The silicon contents in the lungs were detected at 24 hours after the last exposure, but none were detected at 1 month post-exposure. The test substance in the mediastinal lymph nodes was detected, but reduced gradually from 1 to 3 months post-exposure. At 3 months, 81% of the test substance had been eliminated from the lung.
In a 6-month oral study, Wistar rats (40/sex/dose) were administered silica dimethyl silylate in the diet at 0 or 500 mg/kg bw/day (US EPA 2011). Clinical signs and body weights throughout the study were recorded. Blood was drawn monthly from 10 rats/sex and haematological parameters were examined. All rats were subjected to necropsy and histopathological examination were performed at the end of the experiment. The no observed adverse effect level (NOAEL) was identified by the US EPA as the only tested dose of 500 mg/kg bw/day.
In a two-week inhalation study, Wistar rats (10/sex/group) were exposed to silica dimethyl silylate aerosol (particle size less than 10 μm) via whole-body inhalation at the concentrations of 0, 31, 87, or 209 mg/m3 for 6 hours per day, 5 days per week (US EPA 2011). Pathological changes (granulomata, focal increased septal cellularity, and accumulation of alveolar macrophages) in the lungs were observed in all treatment groups. Decreased body weight gain and changes of haematological parameters (increased red cell counts, packed cell volume and hemoglobin) were observed in animals at 87 and 209 mg/m3. The lowest observed adverse effect concentration (LOAEC) of 31 mg/m3 was identified by US EPA on the basis of histopathological findings in the lungs.
In a 13-week inhalation study, Wistar rats (10/sex/group) were exposed to silica dimethyl silylate aerosol (particle sizes below 10 μm) via whole body inhalation at the concentrations of 0 or 35 mg/m3 for 6 hours per day, 5 days per week (US EPA 2011). At 35 mg/m3, changes in the lungs, such as lesions (spongy tissue and spotted surface) and histopathological changes (granuloma, interstitial fibrosis), were observed. These were more severe in comparison to the lung effects observed in the aforementioned two-week inhalation study with silica dimethyl silylate, suggesting a durational response.
In an oral one-generation reproductive toxicity study, Wistar rats (10 females and 2 males/pre-mating group, 5 females to 1 male/dose group) were administered silica dimethyl silylate in the diet at 0 or 500 mg/kg bw/day for 8 or 17 weeks pre-mating, mating, gestation (females only) and lactation (females only) periods (US EPA 2011). The treatment had no effects on appearance, behaviour, body weight gain and food consumption in parent animals. Animals in the treatment groups (both those from mating after 8 weeks of administration or mating after 17 weeks of administration) had no difference in reproductive performance, reproductive organ weight and fertility in comparing controls. No effects in the offspring were observed.
Silica dimethyl silylate was not genotoxic in vitro in bacterial mutation or chromosomal aberration assays (US EPA 2011). In a limited two-year oral carcinogenicity study (there were no concurrent controls; single dose), silica dimethyl silylate was administered in the diet of Wistar rats (20/sex) at 100 mg/kg bw/day. Results were compared with historical control data, and no carcinogenicity or other treatment-related effects were observed (US EPA 2011).
SAS (colloidal, precipitated, and fumed SAS)
Toxicokinetic data for SAS were limited. The absorption of precipitated SAS (15 μm) and fumed SAS (crystalline-free, particle sizes from 0.010 to 0.040 μm) by oral administration was examined in humans (Langendorf and Lang 1967, as cited by EFSA 2018). Volunteers (5 males and 1 female, 22 to 28 years old, no control diet) ingested 2500 mg in apple juice. The total urine was collected for 3 days of pre-application (control values) and for 4 days of post-application for each person, and silicon contents in urine were determined. There were no significant changes of silicon contents in urine between pre- and post-application, suggesting no absorption of silicon after ingestion of SAS. The clearance of SAS in the lung through inhalation route of exposure was described in two studies.
In a 5-day inhalation study, Wistar rats (10/sex/group) were exposed to three types of SAS (colloidal SAS, precipitated SAS and fumed SAS) each (particle sizes for each type were from 1 to 4 μm) at 0, 1, 5, or 25 mg/m3 6 hours a day for 5 days via nose-only inhalation (Arts et al. 2007). Following exposure to all three types of SAS, increased absolute and relative lung and tracheobronchial lymph node weights were observed at 25 mg/m3. Histopathological examination revealed intra-alveolar accumulation of macrophages and granulocytes, and bronchial/bronchiolar hypertrophy at 5 and 25 mg/m3. No changes of the organ weights and no bronchial/bronchiolar hypertrophy were observed at 5 and 25 mg/m3 after 3 months of the recovery period. The silicon content was found to be significantly decreased in the lungs after 1 month of exposure in comparison to one day after exposure, and silicon was not detected after 3 months of exposure for all types of SAS. OECD (2004) identified the NOAEC at 1 mg/m3.
In a 13-week repeated-dose inhalation study, Wistar rats (10/sex/group) were exposed to fumed SAS particles (particle sizes not provided) at 0, 1.3, 5.9 or 31 mg/m3 via whole body inhalation for 6 hours per day, 5 days per week, and were kept for post-recovery periods of 13, 26, 39 and 52 weeks (Reuzel et al. 1991). The inhalation of 1.3 mg/m3 SAS resulted in a mild reversible pro-inflammatory cell proliferation rather than pathological relevant changes. In rats exposed to 5.9 and 31 mg/m3, gross lesions of the lung (spongy tissue and spotted surface) along with lung histopathological changes (such as accumulation of alveolar macrophages, interstitial fibrosis) were observed. Granuloma lesions of the lung were also observed in rats exposed to 31 mg/m3. During the post-exposure observation, some changes in the lungs (such as accumulation of alveolar macrophages) were recovered. The granuloma lesions were not progressive, i.e., no silicogenic nodules formed. Silica could be detected in the lung at the end of the exposure period at 31 mg/m3 groups but was not recovered from any animals during the post-exposure observation. The NOAEC of 1.3 mg/m3 was identified on the basis of observed inflammatory effects in the lungs at the LOAEC of 5.9 mg/m3.
In a 13-week repeated-dose dermal study, Sprague-Dawley rats (10/sex/group) were topically treated with colloidal SAS particles (0.020 μm) at 0 (water), 500, 1000 or 2000 mg/kg bw/day on the back of rats under a semiocclusive dressing (Ryu et al. 2014). No systemic toxicity was observed in treated animals on the basis of clinical observations and examinations of haematology, biochemistry, and histology.
Developmental toxicity of SAS (colloidal and precipitated SAS) was examined in four animal species (rat, mouse, hamster, and rabbit) at oral gavage doses up to 1600 mg/kg bw/day (Food and Drug Research Laboratories 1973 as cited in OECD 2004). No significant signs of maternal or developmental toxic effects were observed in any species tested.
SAS (colloidal and precipitated SAS) was also negative in in vitro and in vivo genotoxicity tests (OECD 2004). In a cancer study, SAS (colloidal and precipitated SAS) was administered to mice for 93 weeks and to rats for 103 weeks at 5% in the diet (equivalent to 6500 mg/kg bw/day in mice and 2500 mg/kg bw/day in rats; particle size not stated). No carcinogenic or other treatment-related systemic effects were observed (Takizawa et al. 1988 as cited in OECD 2004).
6.3 Characterization of risk to human health
No critical effects were identified via the oral or dermal routes of exposure to TMSS analogues. As such, oral or dermal exposures to TMSS from environmental media and food, or the use of products available to consumers are not of concern.
The observed inflammatory responses in the lungs of laboratory animals appeared to be a function of both concentration and duration of exposure, rather than by the peak or maximum concentration of exposure. This is supported by the observation of more severe histopathological changes, such as interstitial fibrosis, in the 13-week study, than in the 5-day study, for the analogue fumed SAS. However, the NOAECs and LOAECs (approximately 1 mg/m3 and 5 mg/m3, respectively) determined in both the 5-day (Arts et al. 2007) and 13-week studies (Reuzel et al. 1991) were similar, suggesting that a clearance mechanism prevented lung damage at 1 mg/m3 during longer exposures.
Table 6-2 provides the relevant exposure and hazard values for TMSS, as well as the resultant margin of exposure, for determination of risk. In this assessment, the NOAEC of 2.05 mg/m3 (adjusted to 0.37 mg/m3 as a continuous exposure), which was identified in the 13-week inhalation study with TMSS, was used as a critical effect level in risk characterization.
| Exposure scenario | Exposure estimate | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Daily inhalation exposure to dry hair shampoo (19+ years) | 0.0011 mg/m3 (continuous exposure)a | NOAEC[adj]b = 0.37 mg/m3 | Inflammatory responses in lungs in rats exposed to TMSS at 10.01 mg/m3 in a 13-week (6 hours/day, 5 days/week) inhalation study | 340 |
| Daily inhalation exposure to facial blush (19+ years) | 0.0014 mg/m3 (continuous exposure)a | NOAEC[adj]b = 0.37 mg/m3 | Inflammatory responses in lungs in rats exposed to TMSS at 10.01 mg/m3 in a 13-week (6 hours/day, 5 days/week) inhalation study | 264 |
Abbreviations: MOE, Margin of Exposure; adj, adjusted.
a To address the differences in exposure between the hazardous effect study and the actual use pattern, both the NOAEC and the exposure estimate for TMSS were adjusted to a continuous exposure scenario (24 hours/day, 7 days/week) according to US EPA guidance on inhalation risk assessment (US EPA 2009).
b The NOAEC was adjusted to 0.37 mg/m3 [=2.05 mg/m3 X (6 hours/24 hours) x (5 days/7days)] as a continuous exposure.
For the daily inhalation exposure to dry hair shampoo and facial blush, the MOE of 340 and 264 were calculated by comparing the NOAEC[adj] of 0.37 mg/m3 with the exposure estimates of 0.0011 mg/m3 and 0.0014 mg/m3, respectively. These exposure estimates are considered conservative, as the size of TMSS particles in cosmetics may not all be respirable (personal communication, emails from CHPSD, HC to ESRAB, HC, May 27, 2019; unreferenced), in comparison those in a 13-week inhalation study (OECD 2004).
Due to the inflammatory response induced by crystalline-free silica particles in the lungs that are dependent on the burden of total inhaled particles, the daily inhalation scenario is considered protective of the per event scenario and it was considered unnecessary to quantitatively characterize risk for the per event inhalation scenarios.
The calculated margins are considered adequate to address uncertainties in the health effects and exposure databases.
6.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| Particle size distribution, shape, and surface properties of TMSS in products are not available. | +/- |
| No Canadian monitoring data for TMSS levels in air, drinking water, soil, dust, or food were available. | - |
| Use of surrogate exposure study for dry hair shampoo and facial blush scenarios. The measured air concentration of PM4 from the use of body powder and face powder was used as a surrogate for TMSS based on their similarities in physical and chemical properties and product application type. | +/- |
Abbreviations: + = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of risk; +/- = unknown potential to cause over or under estimation of risk.
7. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from TMSS. It is proposed to conclude that TMSS does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that TMSS does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that TMSS does not meet any of the criteria set out in section 64 of CEPA.
References
Anderson EL, Sheehan PJ, Kalmes RM, Griffin JR. 2017. Assessment of Health Risk from Historical Use of Cosmetic Talcum Powder. Risk Anal. 37(5):918-928.
Arts JHE, Muijser H, Duistermaat E, Junker K, Kuper CF. 2007. Five-day inhalation toxicity study of three types of synthetic amorphous silicas in Wistar rats and post-exposure evaluations for up to 3 months. Food Chem Toxicol. 45(10):1856-67.
Becker LC, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler D, Marks JG Jr, Shank RC, Slaga TJ, Snyder PW, Andersen FA. 2013. Safety assessment of silylates and surface-modified siloxysilicates. Int J Toxicol.32(3 Suppl):5S-24S.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
Cabot. 1994a. Inhalation toxicity in rats, [Cab-O-Sil] TS-530, laboratory project MB 94-3664 E.Unpublished report. Moreno T. MB Research Laboratories, Spinnerstown, PA, USA. Cabot, Tuscola, Illinois, USA.
Cabot. 1994b. Cab-O-Sil TS-530, Salmonella plate incorporation mutagenicity assay (Ames test), laboratory study G94AZ74.501. Unpublished report. San RHC and Klug ML. Microbiological Associates, Rockville, Maryland, USA. Cabot, Tuscola, Illinois, USA.
Cabot. 1994c. Cab-O-Sil TS-530, chromosome aberrations in Chinese hamster ovary (CHO) cells, laboratory study G94AZ74.330. Unpublished report. Curry PT and Schadly E. Microbiological Associates, Rockville, Maryland, USA. Cabot, Tuscola, Illinois, USA.
Cabot. 1995. Cab-O-Sil TS-500, chromosome aberrations in Chinese hamster ovary (CHO) cells, laboratory study G94BN14.330. Unpublished report. Curry PT and Schadly E. Microbiological Associates, Rockville, Maryland, USA. Cabot, Tuscola, Illinois, USA.
ConsExpo Web [Consumer Exposure Web Model]. 2018. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
[CosIng] Cosmetic Ingredients & Substances [database]. Brussels (BE): European Commission. [accessed 2019 May 24].
[CTFA] Cosmetic, Toiletry and Fragrance Association. 1983. Summary for the Results of Surveys of the amount and Frequency of use of cosmetic products by Women. Report Prepared by Pitkin B, Rodericks JV, Turnbull D. Washington (DC): CTFA Inc.
Degussa AG. 1964. Gewerbehygienisch-toxicologische Untersuchung der Wesselinger hydrophoben ‘‘reaction products of dichlorodimethyl silane with silica"; 1964. Study submitted to the High Production Volume Information System of the US EPA.
Dement JM, Mangin JH, Wallingfor KM, Shuler PJ, Sumwalde RD. 1972. Fiber Exposure During Use of Baby Powders. Prelminary Report. Cincinnati(OH): Enviornmental Investigations Branch, National Institute for Occupational Safety and Health.
Dow Corning. 1972. One-year chronic dust inhalation toxicity study with J-DCA in albino rats and cynomolgus monkeys. Unpublished report. Industrial Bio-Test Laboratories, Northbrook, Illinois, USA. Dow Corning, Midland, Michigan, USA [Summary].
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2015. Identification of risk assessment priorities: results of the 2015 review. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada.
[ECETOC] European Centre for Ecotoxicology and Toxicology of Chemicals. 2006. Synthetic Amorphous Silica (CAS No. 7631-86-9). ECETOC Joint Assessment of Commodity Chemicals (JACC) No. 51. Brussels, Belgium.
[ECHA] European Chemicals Agency. 2016. Opinion on the application for approval of the active substance pyrogenic, synthetic amorphous silicon dioxide, nano, surface treated, Product type: 18. ECHA/BPC/122/2016. Helsinki (FI): ECHA. [accessed 2019 Feb 1].
EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS), 2018. Scientific Opinion on Re-evaluation of silicon dioxide (E 551) as a food additive. EFSA Journal 2018;16(1):5088. [accessed 2019 May 27]
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada, Health Canada. 2013a. Screening assessment for the Challenge: Silanamine, 1,1,1-trimethyl-N-(trimethylsilyl)-, reaction products with ammonia, octamethylcyclotetrasiloxane and silica: Chemical Abstracts Service Registry Number 68937-51-9. Ottawa (ON): Government of Canada. [accessed 2019 Feb 4].
Environment Canada, Health Canada. 2013b. Screening assessment for the Challenge: Quartz (CAS RN 14808-60-7) and Cristobalite (CAS RN 14464-46-1). [PDF] Ottawa (ON): Government of Canada. [accessed 2019 June 28].
[ESIS] European Chemical Substances Information System [database on the Internet]. c1995–2009. European Chemical Bureau (ECB). [cited 2010 Oct].
Ficheux AS, Wesolek N, Chevillotte G, Roudot AC. 2015. Consumption of cosmetic products by the French population. First part: Frequency data. Food Chem Toxicol. 78:159-169.
Ficheux AS, Chevillotte G, Wesolek N, Morisset T, Dornic N, Bernard A, Bertho A, Romanet A, Leroy L, Mercat AC, Creusot T, Simon E, Roudot AC. 2016. Consumption of cosmetic products by the French population. Second part: Amount data. Food Chem Toxicol. 90:130-141.
Food and Drug Research Laboratories, Inc. 1973: Teratologic Evaluation of FDA 71-48 (Syloid; silica aerogel). Prep. for: FDA, U. S. Food and Drug Administration; NTIS, National Technical Information Service, U. S. Department of Commerce, USA, PB-223 808
Fruijtier-Pölloth C. 2012. The toxicological mode of action and the safety of synthetic amorphous silica-a nanostructured material. Toxicology 294(2-3):61-79
Fruijtier-Pölloth C. 2016. The safety of nanostructured synthetic amorphous silica (SAS) as a food additive (E 551). Arch Toxicol. 90(12): 2885–2916.
Hallegot P and Grégoire S. 2011. Synthetic Amorphous Silica - L’Oréal Study No. PH/OD/11.0273/Si
Haz-Map [database]. [updated 2018 October]. Bethesda (MD): US National Library of Medicine. [accessed March 1, 2019].
Health Canada. 2015. Food consumption table derived from Statistics Canada, Canadian Community Health Survey, Cycle 2.2, Nutrition (2004), Share file. Ottawa.
Health Canada. 2017. Science approach document for substances with low human health hazard potential. Ottawa (ON): Government of Canada.
Health Canada. 2018a. Draft Inhalation Risk Assessment Guidance. October 2018. Internal Draft. Unpublished report. Ottawa (ON): Existing Substances Risk Assessment Bureau, Health Canada.
Health Canada. 2018b. Draft Personal Care Products Workbook August 2018. Internal Draft. Unpublished report. Ottawa(ON): Existing Substances Risk Assessment Bureau, Health Canada.
Health Canada. 2019. Data collected from a targeted information gathering initiative for assessments under the Chemicals Management Plan (March – May 2019). Data collected by ECCC, Health Canada; Existing Substances Program.
Heylings J.2013. Silica overview: Skin Penetration of Synthetic Amorphous Silica: analytical (quantitative) approach. January 15, 2013.
[ISO] International Organization for Standardization. 2007. Workplace atmospheres – Ultrafine, nanoparticle and nano-structured aerosols – inhalation exposure characterization and assessment. Geneva (SW): ISO 27628:2007.
IUCLID 2003. Data set for ID 68611-44-9, EC No. 271-893-4, U.S. Environmental Protection Agency.
Johnson IR. 2013. Synthetic Amorphous Silica - In vitro Absorption of Silica from Three Formulations through Dermatomed Human Skin. Dermal Technology Laboratory Study No. JV2218.
Kogel JE, Trivedi NC, Barker JM, Krukowski ST, editors. 2006. Industrial Minerals & Rocks. 7th ed: Commodities, Markets, and Uses. Littleton (Colorado): Society for Mining, Metallurgy, and Exploration, Inc. p. 1190-1191.
Langendorf H, Lang K. 1967. Der Einfluss polymerer Kieselsaeuren auf die renale SiO2-Ausscheidung beim Menschen. Zeitschrift Ernaehrungswissenschaft, 8, 27–32.
Langer SH, Connell S, Wender I. 1958. Preparation and properties of trimethylsilyl ethers and related compounds. J Org Chem 23(1): 50-58.
Loretz LG, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, Rothenstein A, Scrafford CG, Sewall C. 2005. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol 43: 279-291.
Martin KR. 2007. The chemistry of silica and its potential health benefits. The Journal of Nutrition, Health & Aging. 11 (2): 94-97.
[MSDS] Material Safety Data Sheet. 2013. Valspar paint 502 DARK GREEN/AQUA 6UC. Medina (OH): The Valspar Corporation. [accessed 2019 January 10].
[MSDS] Material Safety Data Sheet. 2015a. Silicon dioxide, amorphous, hexamethyldisilazane treated, CAS RN 68909-20-6. Morrisville (PA): Gelest, Inc. [accessed 2019 February 1].
[MSDS] Material Safety Data Sheet. 2015b. Elmer’s Stix-All. Westerville (OH): Elmer's Products, Inc. [accessed 2019 January 10].
[MSDS] Material Safety Data Sheet. 2016. AEROSIL® R 812 S. Parsippany (NJ): Evonik Corporation USA. [accessed 2019 May 24].
Nazarenko Y, Zhen H, Han T, Lioy PJ, Mainelis G. 2012. Potential for Inhalation Exposure to Engineered NanoparticlesNanomaterial inhalation exposure from Nanotechnology-Based Cosmetic Powders. Environmental Health Perspectives. 120(6): 885 – 892nanotechnology-based cosmetic powders: a quantitative assessment. J Nanopart Res 14(11) doi:10.1007/s11051-012-1229-2.
[OECD] Organisation for Economic Co-operation and Development. 2004. IDS Dossier on Synthetic Amorphous Silica and Silicates. October 2004. [accessed 2019 May 27]
OECD QSAR Toolbox. 2014 Version 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Rasmussen PE, Levesque C, Niu J, Gardner HD, Nilsson G, Macey K. 2019. Characterization of airborne particles emitted during application of cosmetic talc products. Manuscript in preparation. Ottawa (ON): Exposure and Biomonitoring Division, Health Canada.
Reuzel PGJ, Bruijntjes JP, Feron VJ, Woutersen RA. 1991. Subchronic inhalation toxicity of amorphous silicas and quartz dust in rats. Food Chem. Toxicol. 29,341–354.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2006. Cosmetics fact sheet: to assess the risks for the consumer: updated version for ConsExpo 4 [PDF]. Bilthoven (NL): RIVM. Report No.: 320104001/2006. [accessed 2019 06 17].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2007a. Paint products fact sheet: to assess the risks for the consumer. Updated version for ConsExpo 4. Bilthoven (NL): RIVM. Report No.: 320104008/2007. [accessed 2019 May 13] (PDF).
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2007b. Do-it-yourself products fact sheet: to assess the risks for the consumer. Bilthoven (NL): RIVM. Report No.: 320104007/2007. [accessed 2019 May 13] (PDF).
Ryu HJ, Seong NW, So BJ, Seo HS, Kim JH, Hong JS, Park MK, Kim MS, Kim YR, Cho KB, Seo MY, Kim MK, Maeng EH, Son SW. 2014. Evaluation of silica nanoparticle toxicity after topical exposure for 90 days. Int J Nanomedicine. 9 Suppl 2:127-36.
[SCCS] Scientific Committee on Consumer Safety. 2015. Opinion on Silica, Hydrated Silica, and Silica Surface Modified with Alkyl Silylates (nano form). Adopted by the SCCS on 20 March 2015, Revision of 29 September 2015. Report no. SCCS/1545/15
Science Direct [database]. [updated 2019]. Search results for silicone rubber. Elsevier B.V. [accessed 2019 June 6].
Statistics Canada. 2017. Custom tabulation of grooming products data from the Canadian Health Measures Survey Cycle 2 (2010-2011). Prepared for Existing Substances Risk Assessment Bureau, Health Canada by Statistics Canada. Unpublished.
Takizawa, Y; Hirasawa, F.; Noritomi, E.; Aida, M; Tsunoda, H.; Uesugi, S. 1988. Oral ingestion of syloid to mice and rats and its chronic toxicity and carcinogenicity. Acta Medica et Biologica, 36, 27-56
[US EPA] United States Environmental Protection Agency. 2009. Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part F, Supplemental Guidance for Inhalation Risk Assessment). Washington (D.C.): U.S. EPA, Office of Superfund Remediation and Technology Innovation.
[US EPA] US Environmental Protection Agency. 2011. Screening-level hazard characterization: Silane, Dichlorodimethyl-, Reaction Product with Silica (CAS RN 68611-44-9). Washington (DC): US EPA, Office of Pollution Prevention and Toxics.
Villota R and Hawkes JG. 1986. Food Applications and the Toxicological and Nutritional Implications of Amorphous Silicon Dioxide. Crit Rev Food Sci and Nutr 23(4): 289-321.
Wacker. 1998a. Sub-chronic (13-week) inhalation toxicity study with Wacker HDK SKS300 in rats, additional parameters: Annex 5, silicon content determination in lungs and tracheobronchial lymph nodes. Appendix 4.1, individual data pathology main study. Unpublished report V98.684. Arts JHE and Kuper CF, TNO Nutrition and Food Research Institute, Zeist, Netherlands. Wacker Chemie, Burghausen, Germany.
Wacker. 1998b. Sub-chronic (13-week) inhalation toxicity study with Wacker HDK SKS 300 in rats. Unpublished report V98.497. Arts JHE and Kuper CF, Part 1, 2 and 3. TNO Nutrition and Food Research Institute, Zeist NL. Wacker-Chemie, Burghausen, Germany.
Appendix A. Read-across for TMSS
| Role | Analogue | Analogue | Target |
|---|---|---|---|
| CAS RN(common name or acronym) | 68611-44-9 (silica dimethyl silylate) | CAS RN 112926-00-8 (colloidal SAS); CAS RN 112926-00-8 (precipitated SAS); CAN RN 112945-52-5 (fumed SAS) | 68909-20-6 (TMSS) |
| Representative structure | 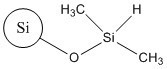
| SiO2 | 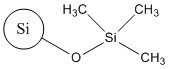
|
| Water solubility (mg/L) | <0.0001 (negligible) | approx. 15 - 68at 20 °C (pH 5.5 – 6.6) | negligible |
| Log Kow | N/A | N/A | N/A |
| Vapour pressure (mm Hg) | negligible | negligible | negligible |
| Primary particle size (nm) | 10 – 50b | 5 – 50 (fumed) 5 – 100 (precipitated) | 5 – 20a |
| Aggregate size (nm) | < 5000b | 100 – 1000 | 100 – 1000a |
| Toxicokinetics | In rats exposed to the aerosolized substance at 200 mg/m3 , 5 hours per day for 3 days, silicon was detected in the lungs at 24 hours post-exposure, but not at 1 month post-exposure, (Degussa AG 1964, as cited by Becker et al. 2013) | No silicon absorbed in volunteers who received SAS (precipitated and fumed) particles orally. In rats exposed to 25 mg/m3 of all types of untreated SAS for 6 hours per day for 5 days, the silicon content in lungs decreased significantly after 1 month of exposure (compared to one day after exposure) and was not detected after 3 months of exposure for all types of SAS. In rats exposed to 31 mg/m3 fumed silica for 13 weeks, silica could be detected in the lungs at the end of the exposure period but was not recovered from any animal during the 13, 26, 39 and 52 weeks of post exposure observation. (OECD 2004) | NA |
| Repeat dose toxicity (Oral) | No treatment-related effects in rats receiving 500 mg/kg bw/day in the diet for 6 months (US EPA 2011 ) | No systemic effects in mice receiving 6500 mg/kg bw/day in diet for 93 weeks (OECD 2004) | NA |
| Repeat dose toxicity (Dermal) | NA | No adverse effects at the highest dose tested of 2000mg/kg bw/day for 90day, in rats treated with colloidal SAS topically (Ryu et al. 2014) | NA |
| Repeat dose toxicity (Inhalation) | LOAEC=31 mg/m3(rats, two weeks whole-body inhalation, 6h/day, 5 days/week, pathological changes in the lungs at the lowest concentration tested, US EPA 2011) LOAEC=35 mg/m3(13-week whole-body inhalation, 6h/day, 5 days/week, pathological changes in the lungs in rats at the lowest concentration tested, US EPA 2011) | NOAEC=1 mg/m3(5-day inhalation, 6h/day, slight pathological changes in the lungs in rats exposed to colloidal SAS or precipitated SAS or fumed SAS at 5 mg/m3, Arts et al. 2007) NOAEC=1.3 mg/m3(13-week whole-body inhalation, 6 h/day, 5 days/week, pathological changes in the lungs in rats exposed to fumed SAS at 5.9 mg/m3, OECD 2004) | NOAEC=2.05 mg/m3, LOAEC=10.01 mg/m3 (13-week inhalation , 6 h/day, 5 days/week, pathological changes in the lungs in rats exposed to TMSS at 10.01 mg/m3 (ECETOC 2006) |
| Reproductive and/or develop-mental toxicity (oral) | No adverse effects observed in rats treated with 500mg/kg bw/day(one generation reproductive study , Pr-B 1965) | No maternal or developmental effects observed at doses up to 1600 mg/kg bw/day SAS (colloidal and precipitated SAS) in rats, mice, hamsters and rabbits (OECD 2004) | NA |
| Genetic toxicity | Negative (US EPA 2011) | Negative (OECD 2004) | Negative (ECETOC 2006) |
| Carcinogenicity | No carcinogenic effects observed in rats receiving 100 mg/kg bw/day in diet for two years (US EPA 2011) | No carcinogenic effects in mice receiving 6500 mg/kg bw/day in diet for 93 weeks (OECD 2004) | NA |
Appendix B. Parameters used to estimate human exposure to TMSS
Inputs and adjustment factors for the inhalation scenario of dry hair shampoo and facial blush are described in Table B-1.
| Scenario | Product conc.a | Average study conc. (mg/m3)b | Tier 1CA (mg/m3)b | ET (h/d)c | Tier 2 EC adjusted (mg/m3)d | EF (d/yr)e | ED (yr)f | Higher tier EC adjusted (mg/m3)g |
|---|---|---|---|---|---|---|---|---|
| Dry hair shampoo | 100% | 1.36 | 1.36 | 0.083 | 0.019 | 84 | 68 | 0.0011 |
| Facial blush | 30% | 1.36 | 0.408 | 0.083 | 0.0057 | 365 | 68 | 0.0014 |
Abbreviations: Conc., concentration; CA, concentration in air per event; d. day; ET, exposure time; EF, exposure frequency; ED, exposure duration; EC, adjusted exposure concentration; h, hour; yr, year.
a Highest concentration of TMSS found in dry hair shampoos (highest among all the loose-powder products) and in facial blush from notifications submitted under the Cosmetic Regulations to Health Canada for TMSS (Personal communication, emails from CHPSD, HC to ESRAB, HC, dated October 9, 2018 and February 14, 2019; unreferenced).
b CA = average study concentration × maximum TMSS concentration in product. Average study concentration is the average measured air concentration of PM4 by subject from Anderson et al. 2017 and Rasmussen et al. 2019 (unpublished).
c ET = exposure duration × number of applications/day = 5 minutes/application × 1 application/day × 1 hour/60 minutes. An exposure duration of 5 minutes/application is based on median time spent in the bathroom following a shower or bath (US EPA 2011, RIVM 2006) and 1 application/day was assumed for dry hair shampoo and facial blush. The exposure duration estimate is also based on a number of factors including the duration of the particle cloud measured in Rasmussen et al. 2019 (approximately 1 minute), the average sampling duration of 6 minutes from Anderson et al. (2017), the formation of secondary particle clouds as observed in Rasmussen et al. (2019) and by NIOSH in an earlier study (Dement et al. 1972), therefore there is a need to account for time spent in the vicinity of where the individual is conducting the activity.
d Tier 2 exposure concentration = CA x hours exposure / hours exposure in animal study (Health Canada 2018a). Exposure duration for both scenarios is 5 minutes.
e EF for dry hair shampoo is 0.23 times/day or 84 days/year (Ficheux et al. 2015, Health Canada 2018b) and EF for facial blush is assumed to be daily (Ficheux et al. 2015, Health Canada 2018b) as these values were the highest central tendency from the highest quality study available for each product type.
f Assumed adult exposure duration for dry hair shampoo and facial blush (80 years lifetime - 12 years as a child = 68 years as an adult).
g EC adjusted = (CA × ET × EF × ED)/AT, where AT (= averaging time) is on the basis of ED × 365 days/year × 24 hours/day. Adjusted exposure concentration is calculated as per Equation 8 in the US EPA 2009 guidance document “Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual”.
