Draft screening assessment - triclocarban
Official title: Draft screening assessment - urea, N-(4-chlorophenyl)-N'-(3,4-dichlorophenyl)- (triclocarban)
Chemical Abstracts Service Registry Number 101-20-2
Environment and Climate Change Canada
Health Canada
October 2020
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and Climate Change and the Minister of Health have conducted a screening assessment of urea, N-(4-chlorophenyl)-N'-(3,4-dichlorophenyl)-, hereinafter referred to as triclocarban. The Chemical Abstracts Service Registry Number (CAS RNFootnote 1 ) for triclocarban is 101-20-2. This substance was identified as a priority for assessment as it met categorization criteria under subsection 73(1) of CEPA.
According to information submitted in response to surveys under section 71 of CEPA, triclocarban was reported to be imported into Canada in volumes in the range of 10 000 to 100 000 kg and 1000 to 10 000 kg in 2008 and 2015, respectively, but was not reported to be manufactured in Canada above the reporting threshold of 100 kg. Triclocarban was reported to be used in Canada as an antibacterial ingredient in cosmetic and drug products such as bar soaps and facial cleansers.
The ecological risk of triclocarban was characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. The ERC approach resulted in an exposure classification of low for triclocarban, based on its reported use patterns, and in a hazard classification of moderate. As this substance is a known anti-bacterial agent, its hazard classification was reviewed using a broader set of data than considered under the initial ERC analysis. Based on this additional analysis, triclocarban is considered to have a high hazard based on its inherent toxicity in aquatic organisms and high potential for bioaccumulation in aquatic invertebrates and gastropods. However, due to its limited exposure potential, triclocarban is considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this draft screening assessment, there is a low risk of harm to the environment from triclocarban. It is proposed to conclude that triclocarban does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
The critical health effect identified for triclocarban was reduced absolute and relative organ weights (spleen, kidney, liver, adrenal, heart, and pituitary) with changes in organ histology in animal studies. Triclocarban exposure also produced effects on fecal microbial diversity, body weight and organ weight in repeat dose studies. Effects on male reproductive tissues, reproduction, live births, reduced rat pup body weight and reduced pup survival were observed in animal studies. Canadians are mainly exposed to triclocarban via cosmetics, and drug products. Canadian biomonitoring data indicated that the majority of the population have a low exposure to triclocarban. Margins of exposure were considered adequate to address uncertainties in the health effects and exposure databases.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that triclocarban does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that triclocarban does not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of triclocarban to determine whether this substance presents or may present a risk to the environment or to human health. This substance was identified as a priority for assessment under Canada’s Chemicals Management Plan (CMP) as it met categorization criteria under subsection 73(1) of CEPA (ECCC, HC [modified 2017]).
The ecological risk of triclocarban was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics including mode of action, chemical reactivity, food-web derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This draft screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant ecological data were identified from literature searches conducted up to July 2018. Relevant health data were identified up to October 2018. Empirical data from key studies as well as results from models were used to reach proposed conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This draft screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The human health portion of this assessment has undergone external review and/or consultation. Comments on the technical portions relevant to human health were received from Dr R.S. Prosser (University of Guelph, Canada), Dr Hongbo Ma (Univeristy of Wisconsin –Milwaukee, USA), Dr Ndeke Musee (University of Pretoria, South Africa), and Dr Rolf Halden (Arizona State University, USA). The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external peer-review as well as a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this draft screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This draft screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precaution.Footnote 2 This draft screening assessment presents the critical information and considerations on which the proposed conclusion is based.
2. Substance identity
The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 3 ), Domestic Substances List (DSL) name and common name for triclocarban are presented in Table 2‑1.
| CAS RN | DSL name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 101-20-2 | Urea, N-(4-chlorophenyl)-N'-(3,4-dichlorophenyl)- (Triclocarban) | 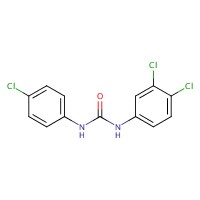 C13H9Cl3N2O
C13H9Cl3N2O | 315.59 |
Synonyms:1-(3',4'-Dichlorophenyl)-3-(4'-chlorophenyl)urea;3,4,4'-Trichlorocarbanilide;3,4,4'-Trichlorodiphenylurea;Carbanilide,3,4,4'-trichloro-;N-(3,4-Dichlorophenyl)-N'-(4-chlorophenyl)urea;N-(4-Chlorophenyl)-N'-(3,4-dichlorophenyl)urea;Trichlocarban;Triclocarban;Triclocarbanum;Urea, N-(4-chlorophenyl)-N'-(3,4-dichlorophenyl)-;Carbanilide, 3,4,4'-trichloro-;N-(4-Chlorophenyl)-N'-(3,4-dichlorophenyl)urea;Triclocarban;Urea, N-(4-chlorophenyl)-N'-(3,4-dichlorophenyl)-;Carbanilide, 3,4,4'-trichloro- (ChemIDplus, 2016).
Triclocarban is a carbanilide composed of mono- and a di-chlorinated benzene rings linked by urea (also known as a carbamide).
3. Physical and chemical properties
Physical and chemical property data of triclocarban are presented in Table 3‑1. Additional physical and chemical properties are reported in ECCC 2016b.
| Property | Value | Data type | Key reference |
|---|---|---|---|
| Physical state | Solid | Experimental | O’Neill 2013 |
| Melting point (°C) | 255.6°C | Experimental | Bradley 2014 |
| Vapour pressure (Pa, 25°C) | 3.6 x10-4 | Modelled | PubChem 2019 |
| Henry’s law constant (Pa·m3/mol) | 4.6 x 10-6 | Modelled | PubChem 2019 |
| Water solubility (mg/L, 25°C) | <0.01 | Experimental | REACH 2019 |
| Log Kow (dimensionless) | 4.32 | Experimental | REACH 2019 |
| pKa (dimensionless, 20°C) | 1.6 x 10-14 | Experimental | REACH 2019 |
Abbreviations: Kow, octanol-water partition coefficient; pKa, acid dissociation constant
4. Sources and uses
Triclocarban was included in surveys issued pursuant to a CEPA section 71 notice (Canada 2009, 2017Footnote 4 ). Triclocarban was not reported to be manufactured in Canada in the reporting years of 2008 and 2015. Respondents reported importing 10 000 to 100 000 kg and 1 000 to 10 000 kg of triclocarban into Canada in 2008 and 2015, respectively. Triclocarban was reported to be used as an active ingredient in natural health products, an antibacterial agent in soaps, and as an antibacterial agent to prevent body odor (Canada 2009, 2017).
Triclocarban is listed in the Natural Health Products Ingredients Database as a non-NHP, and is not reported in licensed natural health products in Canada (NHPID 2019; LNHPD 2018). Triclocarban is not a food additive, incidental additive, or component used to manufacture food packaging materials (Personal communication, email from Food Directorate, Health Canada to Consumer and Hazardous Products Safety Directorate, Health Canada, dated August 31, 2018; unreferenced). Triclocarban is not an active ingredient or formulant in registered pest control products (Personal communication, email from Pest Management Regulatory Agency, Health Canada to Consumer and Hazardous Products Safety Directorate, Health Canada, dated August 31, 2018; unreferenced).
Triclocarban is currently not listed on the Cosmetic Ingredient Hotlist (Health Canada 2018). Based on notifications submitted under the Cosmetic Regulations to Health Canada from December 2015 to December 2018, triclocarban is used in Canada in seven cosmetic products including in bar soaps and facial cleansers (internal data, Consumer and Hazardous Products Safety Directorate, Health Canada, dated January 7, 2019; unreferenced). Triclocarban is also reported as an active ingredient in a single over-the-counter medicated soap which is approved but not currently marketed (personal communication, email from Therapeutic Products Directorate, Health Canada to Consumer and Hazardous Products Safety Directorate, Health Canada, dated August 31, 2018; unreferenced).
Triclocarban is listed in the Personal Care Products Council Ingredient Database with reported functions of cosmetic biocide, deodorant agent and preservative and with reported use in bath oils, tablets and salts, bath soaps and detergent, cleansing products, deodorants and powders (PCPC 2018).
Triclocarban has been identified in Europe in product categories including air care products, coatings and paints, thinners, paint removers, fillers, putties, plasters, modelling clay, finger paints, ink and toners, pharmaceuticals, washing and cleaning products (CoRAP 2018). Triclocarban was not identified in these or other products available to consumers in Canada, other than those described above.
Triclocarban is restricted in cosmetics in Europe to less than 1.5% in rinse-off products when used for purposes other than as a preservative (Annex III/100, EC 2018a) and is restricted to no more than 0.2% in cosmetics when used as a preservative (Annex V/23, EC 2018b). The US FDA has published a final rule stating that triclocarban (and 18 other active ingredients) are not generally recognized as safe or effective (GRAS/GRAE) in consumer antiseptic washes (hand and body) based on a lack of data to support safety and efficacy in this context (US FDA 2016).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risk of triclocarban was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based prioritization approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration [LC50]) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox 2016), and from responses to surveys conducted under section 71 of CEPA, or they were generated using selected quantitative structure-activity relationship (Q)SAR or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over and under classification of hazard and exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC 2016a. The following describes two of the more substantial areas of uncertainty. Error with empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values derived using (Q)SAR models (OECD QSAR Toolbox 2016). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue (CBR) analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity, and may not reflect future trends. Critical data and considerations used to develop the substance-specific profiles for triclocarban, and the hazard, exposure and risk classification results, are presented in ECCC (2016b).
The ERC approach resulted in an exposure classification of low, based in part on its reported use patterns and quantities in commerce (1000 – 10 000 kg, Canada 2017), and in a hazard classification of moderate. Based on this combination, triclocarban was classified as having low potential for ecological risk. Available measured surface water data in Canada indicate that triclocarban concentrations were below the reported detection limit of 0.006 µg/L (Garcia-Ac et al 2009) supporting the ERC classification result of low exposure to wildlife. The moderate hazard was determined by the classification rules applied under ERC, specifically those associated with the aquatic Hazard Assessment Factor (HAF)Footnote 5 and bioavailability.
As triclocarban is a known anti-bacterial agent with a potentially higher hazard profile, an additional ecological hazard characterization was conducted that made use of a broader set of data than were applied under the ERC approach. Empirical toxicity data for triclocarban suggest a high hazard (rather than a moderate hazard) for aquatic species, particularly for aquatic invertebrates (toxicity values considering all species range from 0.13 – 910 µg/L); Appendix A, Table A-1). Empirical bioaccumulation data suggest a high potential for bioaccumulation in aquatic invertebrates, particularly in daphnids (BCF/BAF: 1240 – 82 900) and gastropods (BCF/BAF: 1600 – 7943) (Appendix A, Table A-2), two organisms which were not accounted for in the metrics considered under ERC.
Based on consideration of this additional information, the hazard of triclocarban is likely greater than predicted based on the metrics considered under ERC. While current exposures of triclocarban to the Canadian environment are unlikely to be of concern, triclocarban is considered to have a high hazard based on its inherent toxicity to aquatic species and its high potential for bioaccumulation in aquatic invertebrates and gastropods. As such, there may be a concern for the Canadian environment should exposures increase.
6. Potential to cause harm to human health
6.1 Exposure assessment
6.1.1 Environmental media and food
Environmental media
Environmental media studies have measured triclocarban in drinking water, soil, and house dust. Health Canada analysed 65 drinking water treatment systems across Canada in the National Survey of Disinfection By-Products and Selected New and Emerging Contaminants in Canadian Drinking Water (2009-2010). Triclocarban levels in both treated and untreated water sourced from well water, river water, or lake water were below the minimum detection level (4 ng/L) in 92% of the available sampling sites. Where detected (in four samples), levels found in well water ranged from 9.2 to 29.3 ng/L in untreated samples and from 109.9 to 160.5 ng/L in treated samples, with 160.5 ng/L being the highest level found in all samples. These data indicate that triclocarban levels may be higher in treated water than untreated; the reasons for this are unclear (Personal communication, email from Environmental and Radiation Health Sciences Directorate, Health Canada to Consumer and Hazardous Products Safety Directorate, Health Canada, dated September 20, 2018; unreferenced). Triclocarban was below the limit of detection (LOD) in a study of drinking water in three boroughs of Montreal, QC (LOD=3 ng/L; Garcia-Ac 2009). Triclocarban was not detected in drinking water in an early monitoring study in 12 metropolitan areas in the USA (LOD=10 ng/L; Monsanto 1980); however, this study may predate modern usage practised and had a high LOD. In a more recent study, triclocarban was detected in Spain in mineral water and tap water at 53 and 56 ng/L, respectively (limit of quantification (LOQ)=0.1 ng/L; Carmona 2014).
Triclocarban has been measured in agricultural soil, after application of biosolids. Reported concentrations vary widely with location, potentially due to the extent of prior biosolid application or background levels of contamination. In Quebec, Canada, soil samples from two regions which had received 12 and 11 applications of municipal biosolids between 1991 and 2006 had mean triclocarban concentrations of 53 and 13 ng/g, respectively (Viglino 2011). In the mid-Altantic region and Northern Virginia, USA, fields that received a single application of biosolids over the last 3 to 13 years had a mean triclocarban concentration of 107.1 ng/g (dry weight). Fields that received multiple applications in the same time period had a slightly higher mean of 131.9 ng/g (dry) (Lozano 2018). In Illinois, fields in which biosolids had been applied for 33 years had a maximum triclocarban concentration of 1251 ng/g (dry), and soil in control plots had a maximum of 744 ng/g (dry; Xia 2010).
Canadian environmental monitoring data was not identified for triclocarban in house dust. A median concentration of 200 ng/g triclocarban was reported in a study of dust samples from a mixed-use athletic and educational facility in the USA (Hartmann 2016). A study of dust samples from 19 athletic facilities and 27 single-family detached homes in Oregon reported a mean concentration of 497 ng/g, and a maximum concentration of 9760 ng/g triclocarban (Chen 2018).
Environmental monitoring studies for triclocarban in indoor and outdoor air were not identified. As triclocarban has a low vapour pressure, it is not expected to partition to air.
Food
The agricultural use of municipal biosolids and reclaimed wastewater have been reported in various countries, including Canada and the United States, to contain triclocarban from its use in cosmetics, drugs and natural health products. Both biosolids and reclaimed wastewater may be potential sources of triclocarban in foods (NICNAS 2017, SCCP 2005, U.S. EPA 2009 & 2002).
Available studies from the scientific literature focus primarily on estimated uptake of triclocarban by fruits, vegetables, cereal grains, or animal products from soil and water; however, these studies are limited to experimental trials or modelling. The only measured concentrations of triclocarban in retail foods identified, in Canada or elsewhere, were samples of leafy and root vegetables purchased from a market in Spain (Aparicio et al. 2018), all of which contained detectable concentrations of triclocarban. For the purpose of this assessment, the maximum reported triclocarban concentration of 14.6 ppb (ng/g dry matter) reported by Aparicio et al. (2018) in lettuce, calculated to be 0.79 ppb on a wet weight basis, was conservatively assumed to represent all foods within the broad 'vegetable' category.
The single-day 'eaters only' food consumption rate for the 'vegetables' category from the 2004 Canadian Community Health Survey (CCHS) was used for 6-month to 3-year-old children (Statistics Canada 2004), and consumption data from the 2015 CCHS were employed for all other age groupsFootnote 6 (Statistics Canada 2015). Dietary exposure to triclocarban was conservatively estimated by multiplying the maximum concentration of triclocarban in lettuce, described above, with mean and 90th percentile consumption rates for vegetables from the CCHS surveys. Mean and 90th percentile exposure estimates from food ranged from 2.31 to 6.84 ng/kg bw/day and from 4.69 to 13.71 ng/kg bw/day, respectively (personal communication, email from Food Directorate, Health Canada to Consumer and Hazardous Products Safety Directorate, Health Canada, dated March 5, 2019; unreferenced).
Triclocarban was not detected in breastmilk (n=56, LOD=0.86 µg/L) in a regional study in Ottawa that is part of the Plastics and Personal-care Products use in Pregnancy (P4) Study (Arbuckle 2015). Exposures from breastfeeding were estimated using the LOD from this study as a conservative approach and were included in the human daily intake value below for breastfed infants.
Considering all identified sources of exposure from environmental media and food, estimates of human daily intake range from 7.8 ng/kg bw/day for adolescents aged 14 to 18 years, to 113.8 ng/kg bw/day for breastfed infants (0 to 5 months).
Table B‑1 in Appendix B summarizes the potential intake of triclocarban from environmental media and food.
6.1.2 Biomonitoring
Total triclocarban in urine provides a measure of integrated exposure for individuals, from all routes of exposure and all sources (including environmental media, diet and daily use products) to which they were exposed. In human studies, 27% of the ingested dose was excreted in urine over 3 days after oral exposure, and triclocarban (free and metabolites) can be detected in urine after dermal exposure as well (Hiles 1978a; Scharpf 1975; Schebb 2011b). Elimination following oral dosing is biphasic, with half-lives of 2.4 and 20 hours (Hiles 1978a). Elimination after dermal exposure is monophasic with a half life of 8 to 10 hours (Scharpf 1975).The primary metabolites of triclocarban detected in urine are glucuronidated forms of either triclocarban or hydroxylated triclocarban (2’ or 3’-hydroxy-triclocarban). Total triclocarban is detected after enzymatic deconjugation and acid hydrolysis; free triclocarban is rarely detected in human urine (Birch 1978, Ye 2011, Zhou 2012). See Section 6.2.1 for further details of triclocarban metabolism and excretion.
Triclocarban was a biomonitoring target in Cycle 2 (2009-2011) of the Canadian Health Measures Survey (CHMS). In this study, total triclocarban was detected in urine after enzymatic deconjugation and acid hydrolysis. Triclocarban was detected in the urine of less than 4% of a nationally representative sample of 2549 Canadians, aged 3 to 79 years (limit of detection, LOD= 1 µg/L). The 95th percentile was less than the LOD in all age groups including children, with the exception of the 40 to 59 year age group, which was not reported due to high variation (Health Canada 2013). Total triclocarban was detected in only 4% of urine samples (LOD = 1.1 µg/L) from pregnant women (n=80) in a regional study in Ottawa that is part of the Plastics and Personal-care Products use in Pregnancy (P4) Study (Arbuckle 2015).
Total triclocarban (after enzymatic deconjugation and acid hydrolysis) was detected in 37% of urine samples (LOD = 0.1 µg/L) of a general population aged 6 years and over in the US National Health and Nutrition Examination Survey (NHANES, n=2686) in 2013-2014, with a 95th percentile value of 13.4 µg/L and a maximum value of 588 µg/L (Ye 2016). The difference in concentration at the 95th percentile suggests more widespread or heavier use of triclocarban in the US population. However, the lower frequency of detection in Canada can be partly attributed to the lower LOD in NHANES compared to CHMS. The highest reported detection rate identified for triclocarban in urine was >99% in a group of 209 healthy adult volunteers in China (LOD=0.005 µg/L). The maximum value reported in this study was 192 µg/L (Yin 2016).
Triclocarban was detected in 22% of urine samples from children in NHANES in 2013-2014, compared to 37% in adults, with a 95th percentile urinary concentration of 0.9 µg/L in children (Ye 2016). However, in a smaller US study (n=181), triclocarban was detected in 37% of urine samples (LOD= 0.1 µg/L) from children aged 3 to 6 years, with a maximum reported value of 8.5 µg/L (Hoffman 2018). Worldwide, triclocarban detection frequency in children’s urine was 28% in Denmark (ages 6 to 11 years, LOD = 0.01 µg/L), undetected in Germany (LOQ=1.0 µg/L), and up to 70% in Brazil (6 to 14 years, LOD=0.004 µg/L) (Fredericksen 2013; Moos 2014; Rocha 2018a, 2018b). The maximum measured concentrations were 1.0 µg/L in Denmark and 0.94 µg/L in Brazil (Fredericksen 2013; Rocha 2018a).
Triclocarban was detected in umbilical cord blood in 22% of samples from 33 US neonates (LOD not reported) and in 65% of 92 Chinese neonates (LOD=0.002 µg/L) (Pycke 2014; Wei 2017). The maximum reported concentration in the latter study was 0.82 µg/L. Triclocarban was not detected in meconium (n=54, LOD=0.53 ng/g) or breastmilk (n=56, LOD=0.86 µg/L) samples in the P4 study, and was not detected in breastmilk samples (n=20, LOD=1.2 µg/L) in a US study (Arbuckle 2015; Ye 2006).
Estimated daily intakes of triclocarban were derived based on biomonitoring data from the CHMS and NHANES studies (Health Canada 2013; Ye 2006). In a study of human pharmacokinetics, in response to oral exposure to triclocarban, human volunteers (n=6 males, aged 20 to 40 years) were administered a single dose of 2.2 µmol of 14C labelled triclocarban per kg bw (Hiles 1978b). Triclocarban was absorbed rapidly and a maximum plasma level of 3.7 nmol/g was achieved in less than 3 hours. Twenty-seven percent of the applied dose was excreted in urine over 80 hours. Metabolism of triclocarban does not result in breaking the basic structure, thus the recovery of 14C label in the urine is a reliable estimate of excretion of the original dose by the route and can be considered a specific biomarker. CHMS and NHANES biomonitoring studies detected total triclocarban in urine after acid hydrolysis and enzyme deconjugation, which is considered a specific measure of triclocarban (Health Canada 2013; Ye 2006).
Estimated daily intakes were derived from the 95th percentile values from CHMS and NHANES studies, using a fractional urinary excretion value of 27%, based on Hiles (1978b). The 95th percentile concentrations reported by CHMS were below the LOD, and a value of 1.0 µg/L was used as a conservative estimate of urinary concentration. See Appendix C for further details on default values and models used to calculate estimated daily intakes. Estimated daily intakes based on Canadian biomonitoring data range from 0.07 to 0.11 µg/kg bw/day. Intakes are presented in Table 6‑1.
| Source | Age group (y) | UC or UCCr, P95 | FUE | Estimated daily intake (mg/kg bw/day) |
|---|---|---|---|---|
| CHMS Cycle 2, 2009-2011 (Health Canada 2013) | 3 to 5 | 1.0 µg/L | 0.27 | 0.00011 |
| CHMS Cycle 2, 2009-2011 (Health Canada 2013) | 6 to 11 | 1.0 µg/L | 0.27 | 0.000093 |
| CHMS Cycle 2, 2009-2011 (Health Canada 2013) | 12 to 19 | 1.0 µg/L | 0.27 | 0.000074 |
| CHMS Cycle 2, 2009-2011 (Health Canada 2013) | 20 to 39 | 1.0 µg/L | 0.27 | 0.000074 |
| CHMS Cycle 2, 2009-2011 (Health Canada 2013) | 40 to 59 | 1.0 µg/L | 0.27 | 0.000074 |
| CHMS Cycle 2, 2009-2011 (Health Canada 2013) | 60 to 79 | 1.0 µg/L | 0.27 | 0.000074 |
| NHANES, 2013-2014 (Ye 2006) | 6 to 11 | 0.778 µg/g Cr | 0.27 | 0.000033 |
| NHANES, 2013-2014 (Ye 2006) | 12 to 19 | 1.97 µg/g Cr | 0.27 | 0.00015 |
| NHANES, 2013-2014 (Ye 2006) | 20+ | 17.6 µg/g Cr | 0.27 | 0.0012 |
| NHANES, 2013-2014 (Ye 2006) | All | 14.6 µg/g Cr | 0.27 | 0.0010 |
Abbreviations: UC, urinary concentration, UCCr, creatinine-adjusted urinary concentration; Cr, creatinine; FUE, fractional urinary excretion
6.1.3 Cosmetics and drugs
Triclocarban has been reported in body bar soaps and facial cleansers for use on the body and face, which are classified as cosmetics or drug products in Canada. Reported concentrations of triclocarban in these products range from less than 0.1% to 3% (internal data, Consumer and Hazardous Products Safety Directorate, Health Canada, dated January 7, 2019; unreferenced; personal communication, email from Therapeutic Products Directorate, Health Canada to Consumer and Hazardous Products Safety Directorate, Health Canada, dated August 31, 2018; unreferenced). Potential exposures were estimated based on conservative assumptions and default values. See Appendix C for details on default values and models used for generating exposure estimates. Sentinel exposure scenarios are presented in Table 6‑2.
Dermal absorption values from various human studies were used to estimate an internal dose. Dermal absorption was assayed in static and flow through in vitro skin cell systems using adult and newborn human skin (Wester 1985). Triclocarban was applied at a surface load of 27 µg/cm2. At 37°C, 0.26% of the applied dose was absorbedFootnote 7 by newborn abdominal skin and 0.23% by adult abdominal skin in a static cell. In a continuous flow model, 6% was absorbed by adult abdominal skin. In an in vivo trial, 14C-labelled triclocarban was applied to a skin surface area of 500 cm2 at 4µg/cm2 in 5 human male volunteers. Over a period of 7 days, 7.0% of the applied dose penetrated the skin, based on urinary excretion (Wester 1985). In two separate studies, triclocarban absorption was measured in human volunteers after showering with triclocarban-containing soap. In the first study, 6 adult male subjects used approximately 7 g of soap containing 2% triclocarban (equivalent to a surface load of approximately 8 µg/cm2 before rinsing, based on default values for the 19+ years age group). The total average recovery in urine and feces was 0.39% of the applied dose (0.16% in urine over 2 days and 0.23% in feces over 6 days) (Scharpf 1975). In the second study, 6 adult volunteers (5 male, 1 female) used soap containing 0.6% triclocarban, applying an average maximal dose of 4 µg/cm2. After lathering with the soap, the volunteers let the foam stand for 15 minutes before rinsing. The average urinary excretion over 72 hours was 0.6% of the applied dose, or 0.5 mg per shower per person (Schebb 2011b). In each of these studies, the reported applied dose was prior to rinsing. Based on these studies, the dermal absorption of triclocarban applied in soap at a surface loading of >8 µg/cm2 (prior to rinsing) can be conservatively estimated at 0.39% of the applied dose (based on Scharpf 1975). The dermal absorption of triclocarban applied in soap at 4 µg/cm2 or less (before rinsing), can be estimated at >0.6% of the applied dose, based on Schebb (2011b) as fecal excretion was not reported. In the interest of a conservative estimate, a value of 1.0% absorption was applied to scenarios with a surface load of <4 µg triclocarban/cm2.
| Product Scenario | Upper limit of concentration (%) | Age group | Surface loada (µg/cm2) | Dermal absorption (%) | Systemic exposure (mg/kg bw/day) |
|---|---|---|---|---|---|
| Body soap (solid) | 3.0b | 19+ years | 2.3 | 1.0 | 0.0053 |
| Body soap (solid) | 3.0b | 9 to 13 years | 2.2 | 1.0 | 0.0067 |
| Facial cleanser | 0.4c | 19+ years | 36 | 0.39 | 0.0011 |
| Facial cleanser | 0.4c | 9 to 13 years | 43 | 0.39 | 0.0014 |
a Surface load is prior to rinsing.
b Internal data, Consumer and Hazardous Products Safety Directorate, Health Canada, dated January 7, 2019; unreferenced.
c Personal communication, email from Therapeutic Products Directorate, Health Canada to Consumer and Hazardous Products Safety Directorate, Health Canada, dated August 31, 2018; unreferenced.
6.2 Health effects assessment
Triclocarban has been reviewed by the European Commission Scientific Committee on Consumer Products, the Australian Department of Health National Industrial Chemicals Notification and Assessment Scheme and as part of the US EPA High Production Volume Challenge (SCCP 2005; NICNAS 2017; US EPA 2002). Some data from these sources have been considered in this assessment.
6.2.1 Toxicokinetics
Triclocarban is readily absorbed and metabolised via oral and intravenous routes in humans, rats and other species. Triclocarban is less readily absorbed by the dermal route, but doses absorbed by this route are readily metabolised and excreted. Once absorbed, metabolism does not break the basic structure; triclocarban undergoes hydroxylation followed by conjugation with glucuronic acid and sulfates in varying proportions, depending on the tissue. Conjugation can be to either triclocarban or to hydroxylated species. Very little of the absorbed dose (<1%) is distributed to tissues in animal studies (Hiles 1977, 1978b). In humans, rats, and monkeys, over 90% of the absorbed oral dose is excreted in urine and feces, with the greatest portion in feces (Hiles 1978a, 1978b, 1978c). Urinary excretion occurs over up to 80 hours and fecal excretion of triclocarban occurs for up to 12 days (Hiles 1977, 1978a; Scharpf 1975; Schebb 2011b).
Human studies
In humans, triclocarban was rapidly absorbed after oral dosing, reaching a maximum plasma concentration after less than 3 hours (Hiles 1978a). After dermal application by showering with a soap containing up to 2% triclocarban, triclocarban and metabolites were below detection level (10 ppb) in blood at all times sampled (Scharpf 1975; Taulli 1977). Following intravenous administration, triclocarban underwent a very short distribution phase in plasma, with a half-life of less than 5 minutes, followed by an elimination phase with a half-life of 8.6 hours (Scharpf 1975). After a single oral dose, two thirds to three quarters of triclocarban in blood is sulfonated within 3 hours, and less than 10% is glucuronidated; within 24 hours, over 95% of triclocarban represents in plasma is sulfonated (Taulli 1977;Birch 1978). Triclocarban metabolites were eliminated from plasma in two phases: glucuronides were eliminated with a half-life of 1.8 hours and sulfates were eliminated with a half-life of 20.2 hours (Hiles 1978a). Very little evidence was found describing the organ distribution of triclocarban in humans. However, triclocarban was identified in the hypothalamus in 1 of 24 samples, and in white matter in 2 of 10 samples in a biomonitoring study; and in cord blood in additional studies (Van Der Meer 2017; Wei 2017; Pycke 2014).
After dermal exposure from showering with a triclocarban-containing soap, excreted metabolites are mainly glucuronidated and little parent triclocarban was detected in urine. The highest concentration of N-triclocarban glucuronides in urine was observed 10-24 hours after showering with 0.6% triclocarban soap and demonstrated a large amount of inter-individual variation. Repeated daily showering resulted in a steady state of triclocarban glucuronides in urine (Schebb 2011b; Scharpf 29175). After a single dermal exposure, the majority of triclocarban was excreted in urine over up to 36 hours, comprising up to 0.6% of the applied dose and a further 0.24% of the applied dose was excreted in feces (Scharpf 1975; Schebb 2011b). After intravenous dosing, 18% of the absorbed dose was excreted in urine after 24 hours and 20% after 4 days. An additional 10% was excreted in feces in the after two days and 55% after 12 days (Scharpf 1975). After oral dosing, 27% was eliminated in urine in 80 hours and 70% was eliminated in feces in 120 hours indicating potential route-specific differences (Hiles 1978a).
Animal studies
In adult rhesus monkeys, plasma concentrations increased rapidly up to 12 hours after intravenous injection and increased relatively slowly from 12 to 24 hours, suggesting first order kinetics (Hiles 1978b). In male Sprague Dawley rats, 43% of a gavage dose of 14C-triclocarban was recovered in urine, bile and tissue over 72 hours (Hiles 1977). In the same study, 7.8% was recovered in feces, bile, urine and tissues over 72 hours after dermal exposure to14C-triclocarban in a 10% soap solution (Hiles 1977). After intravenous, oral or dermal administration in male rats, the only tissues with more than 0.01% of the administered 14C were liver, kidney, testes and lung, in order of relative accumulation. However, quantities were very small, ranging from 0.072% to 0.04% of the administered dose for liver and lungs, respectively (Hiles 1977). In a study of reproductive and post-natal dosing in female CD-1 mice using ad libitum dosing in drinking water, triclocarban translocated across the placenta and was transferred through breastmilk. Triclocarban-related compounds were 7 to 18% of the absorbed dose was detected in the brain, heart, fat and female gonads in offspring and much lower levels (<1 to 7% of absorbed dose) were found in the brain, muscle and heart of dams (Enright 2017).
As with humans, the primary metabolites detected in plasma after intravenous and oral administration in animals to adult rhesus monkeys were sulfonated forms of triclocarban; in bile, the majority of triclocarban species were glucuronidated (Hiles 1978b; Taulli 1977; Birch 1978). After dermal exposure in rats, glucuronide conjugates were only detected in plasma in higher dose groups (Schebb 2011b). In monkeys, removal from plasma also occurred in two phases: fast elimination of glucuronide species followed by slower removal of sulfate-conjugated species (Hiles 1978b). Following oral or intravenous administration to rats, approximately 90% of the administered dose was excreted in feces or bile, and 4.3% in urine (Hiles 1977). After dermal administration, the absorbed dose was steadily excreted over 72 hours, 15.6% in urine, and 77% in bile (Hiles 1977). In rhesus monkeys, approximately 20% of the absorbed dose was excreted in urine after intravenous administration, with the remainder eliminated in feces (Hiles 1978b).
6.2.2 Acute studies, irritation and sensitization
Triclocarban is of low acute toxicity by the oral and dermal routes (SCCP 2005). Studies were not available by the inhalation route, however, inhalation exposure is not expected due to low vapour pressure. Triclocarban is not irritating and is not a sensitizer in animal and human studies (SCCP 2005).
6.2.3 Genotoxicity
Triclocarban was negative in Ames assays, with and without metabolic activation, in Salmonella typhimurium strains TA98, TA100, TA1535, TA1537, and TA1538 at doses up to 5000 µg/plate (Bayer AG 1992; Bonin 1982; REACH 2019). Triclocarban was also negative in an in vitro chromosome aberration test in Chinese hamster ovary cells, with and without metabolic activation, at concentrations up to 2000 µg/mL (Soap and Detergent Association 2002). In Tox21 assays, triclocarban was identified as genotoxic in cell lines deficient in DNA repair pathways (Kim 2019).
6.2.4 Repeat dose studies
In a repeat dose study, weaned female Sprague Dawley rats at PND 22 (4 per group) were exposed to 0, 0.2 or 0.5% triclocarban in diet (equivalent to approximately 103 and 257 mg/kg bw/day, respectively) for 28 days, followed by a 28-day washout period (Kennedy 2018). No significant differences were observed in body weight or body weight gain. Fecal samples were collected throughout the study and 16S rRNA was sequenced from extracted total fecal DNA to determine the diversity of microbiota. Phylogenetic diversity decreased significantly over time in both dose groups in the treatment phase (compared to day 0) over the entire treatment period. The decreasing trend in phylogenetic diversity (compared to day 0) was statistically significant in the low dose group at treatment day 28 and at days 5, 12, and 28 in the high dose group. Phylogenetic diversity increased in the washout period, and on washout day 8 (and thereafter) was significantly different in both groups from day 2. A statistically significant microbial community shift compared to control groups occurred in both treatment groups on treatment day 2 and continued throughout the treatment phase. During the washout period, the microbial communities became more similar to control microbiota over time. In the low dose group, differences were statistically significant at day 2 of the washout period, but were no longer significant at day 8 and thereafter; in the high dose group differences were statistically significant up to washout day 11, but were no longer significant at day 28. There were no significant differences in phylogenetic diversity or microbial community between the treatment groups in either phase of the study. During the treatment phase, Firmicutes was the dominant phyla present in both treatment groups, and Bacteroidetes was the dominant species in the control group and on day 0 in treatment groups. In the washout phase, the relative abundance of Bacteroidetes and Firmicutes in the treatment groups recovered to levels that were not significantly different from the control group (Kennedy 2018).
Groups of 12 adult male C57BL/6 mice were exposed to 0, 3, 10, 30 and 90 mg/kg bw/day triclocarban by intragastric intubation for 35 days in a study of short-term effects on cardiac function (Xie 2018). Animals were sacrificed on day 35 and their hearts removed for histological and metabolomic analysis. A statistically significant decrease in body weight compared to controls was observed at 10, 30 and 90 mg/kg bw/day. A statistically significant decrease in absolute heart weight was observed at 30 and 90 mg/kg bw/day and a statistically significant decrease in heart weight relative to body weight was observed in all test groups. Histopathological examination revealed that cardiac fibres were thicker with less staining in animals from the two highest dose groups. Metabolomic data indicated multiple effects on cardiac metabolism including changes in levels of endogenous metabolites and the levels of cardiac enzymes involved in fatty acid synthesis and metabolism (Xie 2018). The biological significance of metabolic effects was not clearly established. Metabolic changes induced by triclocarban are mediated by the constitutive androstane receptor (CAR), of which triclocarban is an established activator. CAR plays a central role in CYP and phase II enzyme induction, as well as lipid and glucose metabolism, among other processes. However, CAR is poorly conserved across species and the CAR receptors of different species vary considerably in their ability to bind and become activated by CAR-activating chemicals (Omiecinski 2011). Therefore, the CAR-mediated alterations in metabolism and subsequent cardiac physiology observed by Xie and colleagues are unlikely to be of human relevance.
In a two-year chronic study performed based on a protocol approved by the U.S. Food and Drug Administration, groups of 80 Sprague Dawley rats we exposed to 0, 25, 75 and 250 mg/kg bw/day triclocarban in diet (Monsanto 1981). Clinical signs, body weight, and food consumption were monitored throughout the study. Ophthalmoscopic examinations were conducted regularly and clinical evaluations of hematology, clinical chemistry, and urinalysis were conducted at 6, 12, 20, 23 (males) and 25 (females) months. Necropsy and pathological examination were conducted at termination. Gross lesions were examined microscopically for neoplastic changes. No treatment-related clinical signs or mortality were observed throughout the study. No differences were observed with regard to ophthalmic observations, food consumption, or urinalysis. Signs of laboured breathing, emaciation, rales and mortality were observed among control and treated males in weeks 64 to 86 and 70 to 83, respectively, due to a respiratory infection. The mean body weight of males at 250 mg/kg bw/day and females at 75 and 250 mg/kg bw/day was slightly reduced compared to controls for most of the study duration. Anemia was observed in males at 75 and 250 mg/kg bw/day and in females at 250 mg/kg bw/day. A slight increase in serum alkaline phosphatase, blood urea nitrogen, glucose and total bilirubin was observed in high-dose males at various time points. Statistically significant changes in organ weights included increased liver weights in both sexes at 75 and 250 mg/kg bw/day, increased spleen weights at 75 (males) and 250 mg/kg bw/day (males and females), and increased testes and heart weights in males at 250 mg/kg bw/day. No microscopic changes were observed to account for increased organ weights, and the authors stated that the organ weight changes may therefore not be biologically significant. An increase in incidence of small and flaccid testes was observed in males at 250 mg/kg bw/day that died spontaneously or were killed moribund between 12 and 23 months. A similar treatment-related increase was not apparent at terminal sacrifice. There was no evidence for dose-related increases in tumour incidence at any site (Monsanto 1981). A NOAEL of 25 mg/kg bw/day was selected by the SCCP (2005) for this study based on anemia, organ weight changes, and body weight changes observed at 75 mg/kg bw/day.
6.2.5 In vitro studies
In prostate cancer-derived cells, co-treatment of androgen with triclocarban increased activation of a luciferase reporter with an androgen response element (ARE) promoter compared to androgen alone. This effect was suppressed by an androgen receptor-binding inhibitor (bicalutamide) (Duleba 2011). Co-exposure of triclocarban with estrogen or dihydrotestosterone enhances estrogenic and androgenic activation of luciferase reporters in cell lines such as HeLa 9908 and MDA-2kb (Tarnow 2013; Huang 2014; Christen 2010; Chen 2008; Blake 2010; Ahn 2008). In MCF-7 breast cancer cells, triclocarban promotes cell proliferation, reduces ERα RNA and protein expression, and stimulates AhR expression when co-expressed with estrogens (Huang 2014; Tarnow 2013). In non-cancerous breast cells (MCF10A), triclocarban induced premalignant cancer-like characteristics including reduced dependence on growth factors, anchorage-independent growth, and increased cell proliferation (Sood 2013). Triclocarban exposure resulted in significant changes in the abundance of thyroid hormone-responsive transcripts in rat GH3 cells, inhibited iodide uptake and inhibited thyroid peroxidase activity in celluar thyroid models (Hinther 2011; Wu 2016).
Triclocarban induced ATP depletion at non-cytotoxic concentrations and significant arrhythmic beating in human-induced pluriopotent stem cell-derived cardiomyocites (Chaudari 2018). Triclocarban was identified in a Tox21 in vitro screen for chemicals affecting mitochondrial function. (Xia 2018).
6.2.6 Reproduction and development studies
In a three-generation reproductive study, triclocarban was administered to groups of 12 male and 24 female Charles River CD rats in diet at 0, 250, 500, 1000, and 3000 ppm (corresponding to uptake of 0, 23, 50, 95, and 280 mg/kg bw/day, respectively) (Monsanto 1983). Triclocarban was administered at least 60 days before mating and continuously thereafter. Each parent generation was mated to produce two litters and some F2 animals were mated to produce a third litter. Offspring from the second litters of F0 and F1 parents were selected to be parents of subsequent generations. The F2 and F3 generations received the test substance for and 80-day growth period before mating, then continuously thereafter. Throughout the study, there were no treatment-related clinical observations, effects on body weight or food consumption in the adult generations during growth or between mating periods. There were no consistent trends in effects on body weight or food consumption in parents during gestation or lactation phases of the study. Mating indices and male fertility were not adversely affected by treatment in any of the generations other than F1. The pregnancy rate was unusually low in the 3000 ppm group during the second litter of the F1 generation. In a small satellite study, of the animals from the 3000 ppm group that did not demonstrate fertility, 1/3 males and 3/10 females were not fertile. The mean number of live pups at birth was lower than controls for both litters in the highest dose group of the F0 generation; a similar effect was not observed in the F1 or F2 generations. Mean pup weight was significantly reduced at PND 21 in both litters of the highest dose group in the F0 generation. Reduced spleen and liver weights compared to controls were observed in second litter F3 pups at of 1000 ppm and above, and the kidney/bodyweight ratio was lower than control in the 3000-ppm group. Histological effects were observed in the kidneys of first litter F1 pups at 500 ppm and higher. Splenic congestion was observed in F3 females pups at 3000 ppm. In the adult generation, differences were observed in absolute and relative spleen, kidney, liver, adrenal, heart and/or pituitary weights at 500 ppm and above. Histopathological evaluation of selected tissues from adult animals at 3000 ppm revealed effects in the spleen, liver, kidneys and bone marrow (Monsanto 1983). A NOAEL of 250 ppm (23 mg/kg bw/day) was reported by the SCCP (2005) for systemic effects in the parental generation based on changes in absolute and relative organ weights at 500 ppm, which were supported by histological changes at 3000 ppm (Monsanto 1983). A NOAEL for reproductive and developmental toxicity of 1000 ppm (95 mg/kg bw/day) was reported by the SCCP (2005), based on reduced pregnancy rate, reduced live pups at birth, and reduced pup weight at PND 21 observed at 3000 ppm (280 mg/kg bw/day).
In a modified developmental study, pregnant and lactating Sprague Dawley rats were exposed to triclocarban in diet at 0, 0.2% or 0.5% (approximately 0, 103 and 257 mg/kg bw/day, respectively) for a period during gestation only, gestation and lactation/nursing, or lactation/nursing only (Kennedy 2015). In the first part of the study, pregnant rats were administered 0 (n=4), 0.2% (n=5), or 0.5% (n=5) triclocarban in diet from GD 5 to 19. Dams were sacrificed on GD 19. Triclocarban was detected in maternal serum and amniotic fluid. A statistically significant decrease in body weight gain and in serum T3 was observed in dams in the 0.5% group. There were no observed effects on survival, implantation number, systemic or sex organ weight, gross physiological or histological evaluation of organs (liver, kidney, adrenal, and ovaries), circulating estradiol, testosterone, progesterone, thyroxine (T4) and thyroid-stimulating hormone. The second arm of the study was divided into parts A and B, in which pregnant females were exposed to triclocarban in diet from GD 5 to PND 21 (weaning), or PND 14, respectively. In part A of this study arm, pregnant rats were exposed to 0 (n=5) or 0.5% (n=5) from GD5 to PND 21. Dams were terminated either on PND 21 or on the day when remaining pups died. At birth, there were no differences in the number of live births or birthweights between the groups. Neonates born to and nursed by dams in the 0.5% triclocarban group did not survive past PND 8. All neonates born to and nursed by control animals survived beyond weaning. Milk bands were observed in pups from the 0.5% group (indicating milk intake), however mammary glands collected from 0.5% w/w dams had evidence of involution. In part B of this study arm, pregnant females were exposed to 0 (n=5) or 0.5% (n=5) from GD5 to PND 14. In this part of the study, litters from dams in the 0.5% group were culled to 6 pups on PND 0 and 3 pups were replaced by control pups. At PND 3, control pups were replaced by new, healthy pups and on PND 6, all pups born to treated dams were replaced by new control pups. On PND 9, the control pups added to the litter on PND 3 were replaced with healthy pups. Milk band scores were similar among control and treated groups on PND 1 and PND3, but milk bands were absent on PND 6 in pups. born/raised by 0.5% dams. Mammary glands from treated dams on PND 14 were not involuted when additional healthy pups were continuously provided to maintain normal suckling activity. In the third arm of the study, pregnant female rats were fed 0 (n=5), 0.2% (n=5) or 0.5% (n=5) in diet from GD 5 to PND21. Litters were culled to 6 pups and cross-fostered: each dam carried and nursed 2 pups from her own litter, and 2 from each of the other test groups. All dam groups (n=5) raised 30 pups: 10 pups born to 0.5%-treated dams, 10 pups born to 0.2%-treated dams, and 10 pups born to control dams. At birth, there were no differences in live births or the average birth weight per litter. At PND 3, the average body weight was 16 and 25% lower than controls in pups raised by 0.2% and 0.5%-treated dams, respectively. Within each dam group there was no difference between the body weights of pups with different in utero exposure. No pups raised by 0.5% triclocarban-treated dams survived beyond PND 5 regardless of in utero exposure status (n=30). Twenty-seven of thirty pups raised by 0.2% -treated dams survived to PND 6, but only 4 animals in this group survived beyond weaning day. All pups raised by control dams survived the study period, regardless of in utero exposure. At weaning, the average body weight of the 4 surviving offspring raised by the 0.2%-treated dam was approximately half that of offspring raised by control dams (statistical analysis was not possible as all 4 pups were raised by the same dam). The abdomens of all pups raised by dams exposed to either triclocarban concentration were distended and all pups had diarrhea. On PND 4 and 5, gross pathological examination of pups raised by the 0.5%-treated dams showed small acute gastric ulcers and fatty vacuolation of hepatocytes. In utero status had no effect on anogential distance (AGD), vaginal opening (VO) date, or first date of estrus after VO, or organ weight. Dam-raising had no effect on AGD (Kennedy 2015). The LOAEL selected for this study is 0.2% triclocarban (103 mg/kg bw/day, lowest tested dose) based on reduced body weight and survival in pups nursed by dams treated at this dose and above.
In a reproductive and teratogenic study, female New Zealand Rabbits (n=20/group) were administered 0 (untreated), 0 (vehicle only), 250, 500 or 1000 mg/kg bw/day of a 2:1 mixture of triclocarban and 3-trifluoromethyl-4,4’-dichlorocarbaniliinde (TFC) by the dermal route from gestational day 7 to 18 (Nolan 1979). Triclocarban and TFC were administered in a 1% soap solution applied to a clipped 14x24 cm area on the back of each doe and rinsed off after 4 hours. Animals were sacrificed on day 29 and fetuses removed by Caesarian section. No significant differences were reported in the number of live/dead fetuses, resorptions, implantations, copora lutea, the weights of fetuses, or malformations (based on gross, soft tissue, and skeletal examinations). Maternal toxicity was not observed, but mild skin irritation was seen in all treated animals (Nolan 1979).
Castrated male Sprague Dawley rats were treated with triclocarban in diet and/or testosterone propionate injection over 10 days (Chen 2008). Animals were divided into four groups (n=12/group) based on treatment. Group 1 received a sham injection and normal diet, Group 2 received an injection of 0.2 mg/kg bw/day testosterone propionate and normal diet, Group 3 received sham injection and 0.25% triclocarban in diet (equivalent to 123 mg/kg bw/day) and Group 4 received an injection of 0.2 mg/kg bw/day testosterone propionate and 0.25% triclocarban in diet. No significant difference was detected in total body weight, kidney or liver weight between the groups. No significant differences were observed for the weights of the seminal vesicles, Cowper’s gland, levator ani-bulbocavernosus muscle (LABC), and glans penis between control rats (Group 1) and rats receiving only triclocarban (Group 3). An increase in ventral prostate weight was observed in rats treated with only triclocarban only (Group 3), compared with control rats (Group 1). Treatment with testosterone propionate alone (Group 2) significantly increased the weights of accessory sex organs, compared with controls (Group 1) and triclocarban alone (Group 3). Treatment with both testosterone propionate with triclocarban resulted in a significant increase in the weights of all accessory sex organs, compared with testosterone propionate treatment alone, indicating a potential synergism between testosterone propionate and triclocarban in vivo (Chen 2008).
In a study of male reproductive toxicity, male Sprague Dawley rats (aged 48 to 52 days) were divided into groups of 12 and treated with 0 or 0.25% triclocarban (equivalent to 129 mg/kg bw/day) in diet for 10 days (Duleba 2011). Animals in the treatment group had significantly more weight gain (5.1% higher final weight) compared to controls. Treated animals also had higher absolute and relative liver weights compared to controls, but kidney, adrenal and testes weights were not affected. Significantly higher absolute and relative weights were also observed in seminal vesicles (42%), ventral prostate (42%), LABC (136%) and glans penis (35%). Significantly higher dry weights of seminal vesicles, LABC, and glans penis were also observed, although no visible abnormalities or histological differences were found in accessary sex glands, penis, or testes. Hyperplasia was observed in vesicular glands which were variably distended with fluid and formed numerous complex folds that extended in to the lumen and in acini of prostate gland which were also distended compared to controls. Significantly greater protein and DNA content were observed in the ventral prostate, LABC, and glans penis compared to controls. Serum lutenizing hormone and testosterone levels were not significantly altered by triclocarban treatment (Duleba 2011).
6.2.7 Epidemiology
In epidemiological studies, potential associations were identified between urinary concentrations of triclocarban and hormone levels during pregnancy, and decreased gestational age at birth (Aker 2018, Geer 2017). In a case-control sample (nested within a cohort study) of 439 pregnant women, a small but statistically significant decrease in total serum triiodothyronine (T3) (based on samples taken at up to 4 time points in pregnancy) was observed in relation to an inter-quartile range increase in urinary triclocarban levels (measured as a binary variable, either above or below the LOD). A non-significant increase in thyroid stimulating hormone (TSH) was also associated with triclocarban levels above the LOD. However, the association with T3 level was no longer significant in a sensitivity analysis conducted among women with term births (>37 weeks gestation) (Aker 2018). In a group of 34 neonates, triclocarban concentration in umbilical cord blood was associated with increased odds of decreased gestational age at birth. In a sensitivity analysis, 2’-hydroxy-triclocarban was marginally significantly associated with decreased body length at birth, but cord blood triclocarban was no longer associated with gestational age at birth (Geer 2017).
No association was reported between urinary concentrations of triclocarban and fetal growth, fetal malformation, DNA damage in children, diabetes incidence, fecundity (time-to-pregnancy), and adult semen quality parameters (Ferguson 2018; Wei 2017; Rocha 2018a; Li 2018; Smarr 2017, 2018).
6.3 Characterization of risk to human health
Triclocarban has low mammalian toxicity in acute studies, is minimally irritating to eyes and skin, and is not a sensitizer. In a dietary two-year study, anemia, reduced body weight, and increased organ weights were observed in rats at doses of 75 mg/kg bw/day and above, with a NOAEL of 25 mg/kg bw/day (Monsanto 1981). This NOAEL was selected as a point of departure by the European Commission SCCP in their Opinion on Triclocarban (2005). In a dietary three-generation reproductive study, reduced pregnancy rate in the F1 generation, reduced live pups at birth in the F0 generation and reduced body weight in pups in the F0 generation and reduced organ weight in F3 pups were reported at 280 mg/kg bw/day, (although none of these effects were present in all generations) resulting in a NOAEL of 95 mg/kg bw/day reported by the SCCP (2005) for reproductive effects (Monsanto 1983). In the same study, a NOAEL of 23 mg/kg bw/day was reported by the SCCP (2005) for changes in absolute and relative organ weights (spleen, kidney, liver, adrenal, heart, and pituitary) in parents, supported by histological changes. However, no significant effects on reproduction, teratogenicity, or maternal toxicity were reported in rabbits when up to 1000 mg/kg bw/day of a 2:1 mixture of triclocarban and TFC was applied dermally during gestation (Nolan 1979).
Effects were also observed at the lowest oral dose tested (103 to 129 mg/kg bw/day) in one repeat dose and three developmental and reproductive toxicity studies of shorter duration. In a 28-day dietary study, significant changes in fecal microbial diversity were observed at doses of 103 mg/kg bw/day and higher (lowest tested dose; Kennedy 2018). In a modified developmental study, reduced body weight and survival was observed in pups (rats) nursed by dams treated at 103 mg/kg bw/day in diet (lowest dose tested) and above (Kennedy 2015). A significant increase in the weights of multiple accessory sex organs was observed in castrated males rats when testosterone was co-administered with a dietary dose of 123 mg/kg bw/day triclocarban (Chen 2008). In a related study of male reproductive toxicity, male accessory sex organs in male rats treated with 129 mg/kg bw/day in diet showed increased absolute and relative weights, hyperplasia and altered morphology (Duleba 2011). Effects on the male reproductive system are consistent with in vitro studies that demonstrate an amplification of testosterone signalling in the presence of triclocarban.
Sentinel exposure scenarios for triclocarban are based on daily topical use of cosmetic products and oral exposure to environmental media and food. In consideration of critical effects and the long term nature of the sentinel exposure scenarios, the NOAEL of 23 mg/kg bw/day for systemic toxicity in a dietary three-generation reproductive study was selected as a point of departure. The resulting margins of exposure are expected to be protective of other systemic and reproductive effects reported in studies of shorter duration and in a two-year chronic toxicity study.
The Canadian population is exposed to triclocarban via environmental media (including food, drinking water and dust), cosmetics and drug products. Biomonitoring data indicates that over 96% of the Canadian population has a urinary concentration of less than 1 µg/L triclocarban. Triclocarban was not detected in breastmilk or meconium in a Canadian study. To address the potential risk associated with exposure to triclocarban from environmental media and products, margins of exposure resulting from modelled exposures in sentinel scenarios are presented in Table 6‑3.
| Exposure scenario | Systemic exposure (mg/kg bw/day) | Critical effect level (mg/kg bw/day) | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Environmental media and food (0-5 months, breast fed) | 1.1 x 10-4 | NOAEL 23 | Reduced absolute and relative organ weights; altered organ histology | 200 000 |
| Body soap (solid, 9 to 13 years) | 0.0067 | NOAEL 23 | Reduced absolute and relative organ weights; altered organ histology | 3430 |
| Facial cleanser (9 to 13 years) | 0.0014 | NOAEL 23 | Reduced absolute and relative organ weights; altered organ histology | 16 400 |
Abbreviations: MOE, Margin of Exposure; NOAEL, No Observed Adverse Effect Level
On the basis of the conservative parameters used in modelling exposure, the calculated margins are considered adequate to address uncertainties in the health effects and exposure databases.
6.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| No identified Canadian or North American data for triclocarban in retail foods. The maximum triclocarban concentration reported in the scientific literature for lettuce was used to represent all vegetables in the food intake assessment. | +/- |
| Few repeat dose dermal studies were available for triclocarban | +/- |
+ = uncertainty with potential to cause over-estimation of exposure/risk; - = uncertainty with potential to cause under-estimation of exposure risk; +/- = unknown potential to cause over or under estimation of risk.
7. Conclusion
Considering all available lines of evidence presented in this draft screening assessment, there is low risk of harm to the environment from triclocarban. It is proposed to conclude that triclocarban does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this draft screening assessment, it is proposed to conclude that triclocarban does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore proposed to conclude that triclocarban does not meet any of the criteria set out in section 64 of CEPA.
References
Ahn KC, Zhao B, Chen J, Cherednichenko G, Sanmarti E, Denison MS, Lasley B, Pessah IN, Kultz D, Chang DPY, Gee SJ, Hammock BD. (2008) In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: receptor-based bioassay screens. Environ Health Perspect 116: 1203-10.
Albanese KA, Lanno RP, Hadad CM, Chin Y-P. 2017. Photolysis- and dissolved organic matter-induced
toxicity of triclocarban to Daphnia magna. Environ Sci Technol Lett 4: 457-462
Aker AM, Johns L, McElrath TF, Cantonwine DE, Mukherjee B, Meeker JD. (2018) Associations between maternal phenol and paraben urinary biomarkers and maternal hormones during pregnancy: A repeated measures study. Environ Int 113: 341-349.
Aparicio I, Martín J, Abril C, Santos JL, Alonso E. 2018. Determination of household and industrial chemicals, personal care products and hormones in leafy and root vegetables by liquid chromatography-tandem mass spectrometry. Journal of Chromatography A 1533: 49–56.
Arbuckle TE, Weiss L, Fisher M, Hauser R, Dumas P, Berube R, Neisa A, LeBlanc A, Lang C, Ayotte P, Walker M, Feeley M, Koniecki D, Tawagi G. (2015) Maternal and infant exposure to environmental phenols as measured in multiple biological matrices. Science of the Total Environment 508: 575–584.
Arnot JA, Mackay D. 2008. Policies for chemical hazard and risk priority setting: Can persistence, bioaccumulation, toxicity and quantity information be combined? Environ. Sci. Technol. 42(13): 4648-4654.
Aylward LL, Vezina A, Deveau M, St. Amand A, Nong A, Hays S. 2015. Biomonitoring Equivalents for interpretation of urinary fluoride. Reg. Toxicol. Pharmacol. 72:158-167.
Barros S, Montes R, Quintana JB, Rodil R, Oliveira JMA, Santos MM, Neuparth T. 2017. Chronic effects of triclocarban in the amphipod Gammarus locusta : behavioural and biochemical impairment. Ecotoxicology and Environmental Safety 135: 276-283.
Bayer AG (1992) Preventol SB - Salmonella/microsome test. Report no. 21078. [As cited in SCCP 2005].
Birch CG, Hiles RA, Eichhold TH, Jeffcoat AR, Handy RW, Hill JM, Willis SL, Hess TR, Wall ME. (1978)Biotransformation products of 3,4,4'-trichlorocarbanilide in rat, monkey, and man. Drug Metab Dispos 6: 169-76,
Blake LS, Martinovic D, Gray LE, Wilson VS, Regal RR, Villeneuve DL, Ankley GT. (2010) Characterization of the androgen-sensitive MDA-kb2 cell line for assessing complex environmental mixtures. Environ Toxicol Chem 29: 1367-76.
Bonin AM, Arkauskas AP, Angus DS, Baker RSU, Gallagher CH, Greenoak G, Lane Brown MM, Meher-Homji KM, Reeve V. (1982) UV-absorbing and other sunprotecting substances: genotoxicity of 2-ethylhexyl P-methoxycinnamate. Mut. Res. 105, 303-308. [As cited in SCCP 2005].
Bradley, Jean-Claude; Lang, Andrew; Williams, Antony (2014): Jean-Claude Bradley Double Plus Good (Highly Curated and Validated) Melting Point Dataset. figshare. Dataset.
Brausch JM, Rand GM. 2011. A review of personal care products in the aquatic environment: environmental concentrations and toxicity. Chemosphere 82: 1518-1532.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2009. Canadian Environmental Protection Act, 1999: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List. Canada Gazette, Part I, vol. 143, no. 40, p. 2945-2956.
Canada, Dept. of the Environment. 2017. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances included as part of the 2017 Inventory Update. Canada Gazette, Part I, vol. 151, no. 2.
Carmona E, Andreu V, Pico Y. (2014) Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: From waste to drinking water. Science of the Total Environment 484: 53–63.
[CCHS] Statistics Canada. 2004. Canadian Community Health Survey – Nutrition (CCHS). Detailed information for 2004 (Cycle 2.2). Ottawa (ON): Statistics Canada.
Chaudhari U, Nemade H, Sureshkumar P, Vinken M, Ates G, Rogiers V, Hescheler J, Hengstler JG, Sachinidis A. (2018) Functional cardiotoxicity assessment of cosmetic compounds using human-induced pluripotent stem cell-derived cardiomyocytes. Arch Toxicol 92: 371-381.
Chen J, Ahn KC, Gee NA, Ahmed MI, Duleba AJ, Zhao L, Gee SJ, Hammock BD, Lasley BL. (2008) Triclocarban enhances testosterone action: a new type of endocrine disruptor? Endocrinology 149: 1173-9.
Chen J, Hartmann EM, Kline J, Van Den Wymelenberg, Halden RU. (2018) Assessment of human exposure to triclocarban, triclosan and five parabens in U.S. indoor dust using dispersive solid phase extraction followed by liquid chromatography tandem mass spectrometry. Journal of Hazardous Materials 360: 623–630.
Chiaia-Hernandez AC, Ashauer R, Moest M, Hollinghaus T, Jeon J, Spaak P, Hollender J. 2013. Bioconcentration of organic contaminants in Daphnia resting eggs. Environ Sci Technol 47: 10667-10675.
Christen V, Crettaz P, Oberli-Schrammli A, Fent K. (2010) Some flame retardants and the antimicrobials triclosan and triclocarban enhance the androgenic activity in vitro. Chemosphere 81:1245-1252.
Coogan MA, Edziyie RE, La Point TW, Venables BJ. 2007. Algal bioaccumulation of triclocarban, triclosan, and methyl-triclosan in a North Texas wastewater treatment plant receiving stream. Chemosphere 67: 1911-1918.
Coogan MA and La Point TW. 2008. Snail bioaccumulation of triclocarban, triclosan, and methyltriclosan in a north Texas, USA, stream affected by wastewater treatment plant runoff. Environ Toxicol Chem 27 (8): 1788-1793
[CoRAP] ECHA Community Rolling Action Plan. 2018. Justification Document for the Selection of a CoRAP Substance: Triclocarban. March 20 2018. EC 202-924-1.
Dom N, Knapen D, Benoot D, Nobels I, Blust R. 2010. Aquatic multi-species acute toxicity of (chlorinated) anilines: experimental versus predicted data. Chemosphere 81:177-186.
Dong X, Xu H, Wu X, Yang L. 2018. Multiple bioanalytical method to reveal developmental biological responses in zebrafish embryos exposed to triclocarban. Chemosphere 193: 251-258.
[DPD] Drug Product Database [database]. [modified 2015 Jul 17]. Ottawa (ON): Government of Canada. [accessed 2018 Aug 29].
Duleba AJ, Ahmed MI, Sun M, Gao AC, Villanueva J, Conley AJ, Turgeon JL, Benirschke K, Gee NA, Chen J, Green PG, Lasley BL. (2011) Effects of triclocarban on intact immature male rat: augmentation of androgen action. Reprod Sci 18:119-27.
[ECCC, HC] Environment Canada, Health Canada. 2014. Approach for identification of chemicals and polymers as risk assessment priorities under Part 5 of the Canadian Environmental Protection Act, 1999 (CEPA 1999). Ottawa (ON):
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2015. Identification of risk assessment priorities: results of the 2015 review. Ottawa (ON): Government of Canada.
[ECCC and HC] Environment Canada and Health Canada. 2016. Final assessment: triclosan: Chemical Abstracts Service Registry Number 3380-34-5. Ottawa (ON): Environment Canada, Health Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2017 Mar 12].
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Data used to create substance-specific hazard and exposure profiles and assign risk classifications in the Ecological Risk Classification of organic substances. Gatineau (QC). Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2017a. Rapid screening of substances with limited general population exposure. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2017b. Draft screening assessment: substances identified as being of low concern using the ecological risk classification of organic substances and the threshold of toxicological concern (TTC)-based approach for certain substances. Ottawa (ON): Government of Canada.
[EC] European Commission. 2018a. Annex III: List of substances which cosmetics must not contain except subject to the restrictions laid down. [Updated 24 October 2018; cited 31 May 2019].
[EC] European Commission. 2018b. Annex V: List of preservatives allowed in cosmetics products. [Updated 23 November 2018; cited 31 May 2019].
Enright HA, Falso MJS, Malfatti MA, Lao V, Kuhn EA, Hum N, Shi Y, Sales AP, Haack KW, Kulp KS, Buchholz BA, Loots GG, Bench G, Turteltaub KW, (2017) Maternal exposure to an environmentally relevant dose of triclocarban results in perinatal exposure and potential alterations in offspring development in the mouse model. PLoS ONE 12: e0181996.
Environment Canada, Health Canada. 2007. Categorization. Ottawa (ON): Government of Canada. [updated 2007 April 20; cited 31 May 2019].
Environment Canada. 2009. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Ferguson KK, Meeker JD, Cantonwine DE, Mukherjee B, Pace GG, Weller D, McElrath TF. (2018) Environmental phenol associations with ultrasound and delivery measures of fetal growth. Environ Int 112: 243-250.
Ficheux AS, Chevillotte G, Wesolek N, Morisset T, Dornic N, Bernard A, Bertho A, Romanet A, Leroy L, Mercat AC, Creusot T, Simon E, Roudot AC. 2016. Consumption of cosmetic products by the French population second part: Amount data. Food and Chemical Toxicology 90: 130-141.
Ficheux AS, Wesolek N, Chevillotte G, Roudot AC, 2015. Consumption of cosmetic products by the French population. First part: Frequency data. Food and Chemical Toxicology 78: 159-169.
Frederiksen H, Nielsen JKS, Morck TA, Hansen PW, Jensen JF, Nielsen O, Andersson, A-M, Knudsen LE. (2013) Urinary excretion of phthalate metabolites, phenols and parabens in rural and urban Danish mother-child pairs. Int J Hyg Environ Health 216: 772-83.
Gao L, Yuan T, Cheng P, Bai Q, Zhou C, Ao J, Wang W, Zhang H. 2015. Effects of triclosan and triclocarban on the growth inhibition, cell viability, genotoxicity and multixenobiotic resistance responses of Tetrahymena theromphila. Chemosphere 139: 434-440.
Garcia-Ac A, Segura PA, Viglino L, Fϋrtos A, Gagnon C, Prévost M, Sauvé S. 2009. On-line solid-phase extraction of large-volume injections coupled to liquid chromatography-tandem mass spectrometry for the quantitation and confirmation of 14 selected trace organic contaminants in drinking and surface water. Journal of Chromatography A 1216: 8518-8527.
Geer LA, Pycke BFG, Waxenbaum J, Shere DM, Abulafia O, HaldenRU. (2017) Association of birth outcomes with fetal exposure to parabens, triclosan and triclocarban in an immigrant population in Brooklyn, New York. J Hazard Mater 323: 177-183.
Giudice BD, Young TM. 2010. Microbial triclocarban stimulates embryo production in the freshwater mudsnail Potamopyrgus antipodarium. Environ Toxciol Chem 29 (4): 966-970.
Han J, Won E-J, Hwang U-K, Kim I-C, Yim J-H, Lee J-S. 2016. Triclosan (TCS) and triclocarban (TCC) cause lifespan reduction and reproductive impairment through oxidative stress-mediated expression of the defensome in the mongonot rotifer (Brachionus koreanus). Comparative Biochemistry and Physiology Part C 185-186: 131-137.
Hartmann EM, Hickey R, Hsu T, Roman CMB, Chen J, Schwager R, Kline J, Brown GZ, Halden RU, Huttenhower C, Green JL. (2016) Antimicrobial Chemicals Are Associated with Elevated Antibiotic Resistance Genes in the Indoor Dust Microbiome. Environ. Sci. Technol. 50: 9807−9815.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Health Canada. (2013) Second Report on Human Biomonitoring of Environmental Chemicals in Canada. Results of the Canadian Health Measures Survey Cycle 2 (2009-2011). Ottawa (ON): Government of Canada. [accessed 2019 Apr 17].
Health Canada. (2018) Cosmetic Ingredient Hotlist: List of substances that are restricted and prohibited in cosmetics. Ottawa (ON): Government of Canada. [accessed 2019 Feb 2].
Higgins CP, Paesani Z, Abbot Chalew TE, Halden RU. 2009. Bioaccumulation of triclocarban in Lumbriculus variegatus. Environ Toxicol Chem 28 (12): 2580-2586.
Hiles RA, Birch CG. (1978a) The absorption, excretion and biotransformation of 3,4,4'-trichlorocarbanilde in humans. Drug Metabolism and Distribution 6: 177-183.
Hiles RA, Caudill D, Birch CG, Eichhold T. (1978b) The Metabolism and Disposition of 3,4,4’-Trichlorocarbanilide in the Intact and Bile Duct-Cannulated Adult and in the Newborn Rhesus Monkey (M. mulatfa). Toxicology and Applied Pharmacology 46:593-608
Hiles RA. (1977) Metabolism and toxicity of halogenated carbanilides: Absorption, distribution and excretion of radioactivity from 3,4,4'-trichloro[14C]carbanilide (TCC) and 3-trifluoromethyl-4-4'-dichloro[14C]carbanilide (TFC) in rats. Fd Cosmet Toxicol 15:205-211.
Hinther A., Bromba CM, Wulff JE, Helbing CC. (2011) Effects of triclocarban, triclosan, and methyl triclosan on thyroid hormone action and stress in frog and mammalian culture systems. Environmental Science & Technology 45: 5395-5402.
Hoffman K, Hammel SC, Phillips AL, Lorenzo AM, Chen A, Calafat AM, Ye X, Webster TF, Stapleton HM. (2018) Biomarkers of exposure to SVOCs in children and their demographic associations: The TESIE Study. Environ Int 119: 26-36.
Huang H, Du G, Zhang W, Hu J, Wu D, Song L, Xia Y, Wang X. The in vitro estrogenic activities of triclosan and triclocarban. J Appl Toxicol 34: 1060-7.
Hyland KC, Blaine AC, Higgins CP. 2015. Accumulation of contaminants of emerging concern in food crops – part 2: plant distribution. Environ Toxicol Chem 34(10): 2222-2230.
Ismail NS, Muller CE, Morgan RR, Luthy RG. 2014. Uptake of contaminants of emerging concern by the bivalves Anodonta californiensis and Corbicula fluminea. Environ Sci Technol 48: 9211-9219.
Kennedy RC, Fling RR, Robeson MS, Saxton AM, Schneider LG, Darcy JL, Bemis DA, Zhao L, Chen J. (2018) Temporal dynamics of gut microbiota in triclocarban-exposed weaned rats. Environ Sci Pollut Res Int 25: 14743-14751.
Kennedy RCM, Menn FM, Healy L, Fecteau KA, Hu P, Bae J, Gee NA, Lasley BL, Zhao L, Chen J. (2015) Early life triclocarban exposure during lactation affects neonate rat survival. Reprod Sci 22: 75-89.
Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, Li Q, Shoemaker BA, Thiessen PA, Yu B, Zaslavsky L, Zhang J, Bolton EE. PubChem 2019 update: improved access to chemical data. Nucleic Acids Res. 2019 Jan 8; 47(D1):D1102-1109. doi:10.1093/nar/gky1033. [PubMed PMID: 30371825]
Lawrence JR, Zhu B, Swerhone GDW, Roy J, Wassenaar LI, Topp E, Korber DR. Comparative microscale analysis of the effects of triclosan and triclocarban on the structure and function of river biofilm communities. Science of the Total Environment. 407: 3307-3316.
Lenz KA, Pattison C, Ma H. 2017. Triclosan and triclocarban induce systemic toxic effects in a model organism in the nematode Caenorhabditis elegans. Environmental Pollution 231: 462-470.
Li H, ZhaoY, Chen L, Su Y, Li X, Jin L, Ge RS. (2017) Triclocarban and Triclosan Inhibit Human Aromatase via Different Mechanisms. Biomed Res Int 2017: 8284097.
Li AJ, Xue J, Lin S, Al-Malki AL, Al-Ghamdi MA, Kumosani TA, Kannan K. (2018) Urinary concentrations of environmental phenols and their association with type 2 diabetes in a population in Jeddah, Saudi Arabia. Environ Res 166: 544-552.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2018 Feb 06]. Ottawa (ON): Government of Canada. [accessed 2019 Jan 30].
Loretz LG, Api AM, Babcock L, Barraj LM, Burdick J, Cater KC, Jarrett G, Mann S, Pan YHL, Re TA, Renskers KJ, Scrafford CG. 2008. Exposure data for cosmetic products: Facial cleanser, hair conditioner, and eye shadow. Food Chem Toxicol 46: 1516-1524.
Lozano N, Rice CP, Ramirez M, Torrents A (2018) Fate of triclocarban in agricultural soils after biosolid applications. Environ Sci Pollut Res 25:222–232.
Macherius A, Lapen DR, Reemtsma T, Rombke J, Topp E, Coors A. 2014. Triclocarban, triclosan, and its transformation product methyl triclosan in native earthworm species four years after a commercial scale biosolids application. Science of the Total Environment 472: 235-238.
Marzulli FN, Maibach HI. (1973) The use of graded concentrations in studying skin sensitizers: experimental contact sensitization in man. Food Cosmet. Toxicol. 12, 219-227. [As cited in SCCP 2005].
Monsanto (1980) Study # MSL-1264 (9/22/1980) TCC Environmental Monitoring-Surface Waters and Drinking Waters. [as cited by EPA HPVIS].
Monsanto (1981) A twenty-four month dietary toxicity/carcinogenicity study of TCC in rats. Report no. BDN-77-280. [As cited in SCCP 2005].
Monsanto (1983) A three-generation reproduction study with TCC rats. Report no. BD-79-058. [As cited in SCCP 2005].
Moos RK, Angerer J, Wittsiepe J, Wilhelm M, Bruning T, Koch HM. (2014) Rapid determination of nine parabens and seven other environmental phenols in urine samples of German children and adults. Int J Hyg Environ Health 217: 845-53.
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2016 Nov 1]. Ottawa (ON): Government of Canada. [accessed 2019 Jan 30].
[NHW] Department of National Health and Welfare. 1990. Present patterns and trends in infant feeding in Canada. Ottawa: NHW. Catalogue Number H39-199/1990E. ISBN 0-662-18397-5. 9 pp. [cited in Health Canada 1998].
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme. 2017. Urea, N-(4-chlorophenyl)-N'-(3,4-dichlorophenyl)-: Environment tier II assessment. Australian Department of Health. Accessed: 31 Jan 2019.
Nolen GA, Dierckman TA. 1979. Reproduction and teratogenic studies of a 2:1 mixture of 3,4,4'-trichlorocarbanilide and 3-trifluoromethyl-4,4'-dichloro-carbanilide in rats and rabbits. Toxicol Appl Pharmacol 51:417-425.
OECD QSAR Toolbox. 2016. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Omiecinski CJ, Coslo DM, Chen T, Laurenzana EM, Peffer RC. 2011. Multi-species Analyses of Direct Activators of the Constitutive Androstane Receptor. Toxicological Sciences 123 (2):550–562.
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Cambridge, UK: Royal Society of Chemistry, 2013., p. 1789.
[PCPC] Personal Care Products Council. 2018. Cosmetic Ingredient Identification Database (International Cosmetic Ingredient Dictionary (INCI)). [Accessed 2019 Jan 30].
Prosser RS, Lissemore L, Shahmohamadloo RS, Sibley PK. 2015. Effect of biosolids-derived triclosan and triclocarban on the colonization of plant roots by arbuscular mycorrhizal fungi. Science of the Total Environment 508: 427-434.
Prosser RS, Lissemore L, Solomon KR, Sibley PK. 2014. Toxicity of biosolids-derived triclosan and triclocarban to six crop species. Environ Toxicol Chem 33(8): 1840-1848.
[PubChem] National Center for Biotechnology Information. PubChem Compound Database; CID=7547 (accessed Mar. 5, 2019).National Center for Biotechnology Information. PubChem Compound Database; CID=7547, https://pubchem.ncbi.nlm.nih.gov/compound/7547 (accessed Mar. 5, 2019).
Pycke BFG, Geer LA, Dalloul M, Abulafia O, Jenck AM, Halden RU. (2014) Human fetal exposure to triclosan and triclocarban in an urban population from Brooklyn, New York. Environ Sci Technol 48: 8831-8.
[REACH] Registration, Evaluation, Authorisation and Restriction of Chemicals. 2019. A registration dossier for Triclocarban [CAS RN 101-20-2] submitted to REACH. [Cited 2019 February 2].
Rocha BA, Asimakopoulos AG, Honda M, da Costa NL, Barbosa RM, Barbosa F, Kannan K. (2018a) Advanced data mining approaches in the assessment of urinary concentrations of bisphenols, chlorophenols, parabens and benzophenones in Brazilian children and their association to DNA damage. Environ Int 116: 269-277.
Rocha BA, de Oliveira ARM, Barbosa F. (2018b) A fast and simple air-assisted liquid-liquid microextraction procedure for the simultaneous determination of bisphenols, parabens, benzophenones, triclosan, and triclocarban in human urine by liquid chromatography-tandem mass spectrometry. Talanta 183: 94–101.
Sabourin L, Duenk P, Bonte-Gelok S, Payne M, Lapen DR, Topp E. 2012. Uptake of pharmaceuticals, hormones, and parabens into vegetables grown in soil fertilized with municipal biosolids. Science of the Total Environmemnt 431: 233-236.
Saravanabhavan G, Walker M, Guay M, Aylward L. 2014. Urinary excretion and daily intake rates of diethyl phthalate in the general Canadian population. Science of the Total Environment 500-501: 191-198.
Satyro S, Saggioro EM, Verissimo F, Buss DF, de Paiva Magalhaes D, Oliviera A. 2017. Triclocarban: UV photolysis, wastewater disinfection, and ecotoxicity assessment using molecular biomarkers. Environ Sci Pollut Res 24: 16077-16085.
[SCCP] European Commission Scientific Committee on Consumer Products. (2005) Opinion on Triclocarban for uses other than as a preservative. Colipa no P29. SCCP/0851/04.
Scharpf LGJ, Hill ID, MaibachHI. (1975) Percutaneous penetration and disposition of triclocarban in man: body showering. Arch Environ Health 30: 7-14.
Schebb NH, Flores I, Kurobe T, Franze B, Ranganathan A, Hammock BD, Teh, SJ. 2011a. Bioconcentration, metabolism and excretion of triclocarban in larval Qurt medaka (Oryzias latipes). Aquatic Toxicology 105: 448-454.
Schebb NH, Inceoglu B, Ahn KC, Morisseau C, Gee SJ, Hammock BD. 2011b. Investigation of human exposure to triclocarban after showering and preliminary evaluation of its biological effects. Environ Sci Technol 45: 3109-15.
Sherburne JJ, Anaya AM, Fernie KJ, Forbey JS, Furlong ET, Kolpin DW, Dufty AM, Kinney CA. 2016. Occurrence of triclocarban and triclosan in an agro-ecosystem following application of biosolids. Environ Sci Technol 50: 13206-13214.
Smarr MM, Honda M, Kannan K, Chen Z, Kim S, Louis G, Buck M. (2018) Male urinary biomarkers of antimicrobial exposure and bi-directional associations with semen quality parameters. Reprod Toxicol 77: 103-108
Smarr MM, Sundaram R, Honda M, Kannan K, Buck LG. (2017) Urinary concentrations of parabens and other antimicrobial chemicals and their association with couples' fecundity. Environ Health Perspect 125: 730-736.
Sreevidya VS, Lenz KA, Svoboda KR, Ma H. 2018. Benzalkonium chloride, benzethonium chloride, and chloroxylenol – three replacement antimicrobials are more toxic than triclosan and triclocarban in two model organisms. Environmental Pollution 235:814-824.
Synder EH, O’Connor GA, McAvoy DC. 2010. Fate of 14-C triclocarban in biosolids-amended soils. Science of the Total Environment 408: 2726-2732
Snyder EH, O’Connor GA, McAvoy DC. 2011.Toxicity and bioaccumulation of biosolid-borne triclocarban (TCC) in terrestrial organisms. Chemosphere 82: 460-467.
Soap and Detergent Association (2002) In vitro mammalian chromosome aberration test. Report no. 2002-01-TCC. [As cited in SCCP 2005].
Sood S, Choudhary S, Wang HCR. (2013) Induction of human breast cell carcinogenesis by triclocarban and intervention by curcumin. Biochemical and Biophysical Research Communications 438: 600-606.
Sultana T, Murray C, Ehsanul Hoque M, Metcalfe C. 2017. Monitoring contaminants of emerging concern from tertiary wastewater treatment plants using passive sampling modelled with performance reference compounds. Environ Monit Assess 189:1
Tamura I, Kagota K, Yasuda Y, Yoneda S, Morita J, Nakada N, Kameda Y, Kimura K, Tatarazako N, Yamamoto H. 2013. Ecotoxicity and screening level ecotoxicological risk assessment of five antimicrobial agents: triclosan, triclocarban, resorcinol, phenoxyethanol and p-thymol. J Appl Toxicol 33: 1222-1229.
Tanoue R, Nomiyama K, Nakamura H, Kim J-W, Isobe T, Shinohara R, Kunisue T, Tanabe S. 2015. Uptake and tissue distribution of pharmaceuticals and personal care products in wild fish from treated-wastewater-impacted streams. Environ Sci Technol 49: 11649-11658.
Tarnow P, Tralau T, Hunecke D, Luch A. (2013) Effects of triclocarban on the transcription of estrogen, androgen and aryl hydrocarbon receptor responsive genes in human breast cancer cells. Toxicol In Vitro 27: 1467-75.
Taulli TA, Hill JT, Pounds GW. (1977) High-pressure liquid chromatographic studies of TCC and metabolites in experimental animals and man. J Chromatogr Sci 15: 111-8.
Torres T, Cunha I, Martins R, Santos MM. 2016. Screening the toxicity of selected personal care products using embryo bioassays : 4-MBC, propylparaben, and triclocarban. Int J Mol Sci 17: 1762
[US EPA] United States Environmental Protection Agency. 2002. High Production Volume (HPV) Chemical Challenge Program Data Availability and Screening Level Assessment for Triclocarban. 201-14186A. Prepared for the HPV Challenge Program by the triclocarban Consortium.
[US EPA] United States Environmental Protection Agency. 2009. Targeted National Sewage Sludge Survey Statistical Analysis Report. Office of Water (4301T), Washington, DC. EPA-822-R-08-018.
[US EPA] United States Environmental Protection Agency. 2011. Exposure Factors Handbook: 2011 Edition. Washington (DC): National Center for Environmental Assessment, Office of Research and Development, U.S. Environmental Protection Agency.
[US FDA] United States Food and Drug Administration. 2016. Safety and Effectiveness of Consumer Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use. 21 CFR Part 310.
Viglino L, Prevost M, Suave S. (2011) High throughput analysis of solid-bound endocrine disruptors by LDTD-APCI-MS/MS. J. Environ. Monit. 13: 583-590.
Vingskes AK and Spann N. 2018. The toxicity of a mixture of two antiseptics, triclosan and triclocarban, on reproduction and growth of the nematode Caenorhabditis elegans. Ecotoxicology 27: 420-429
Wei L, Qiao P, Shi Y, Ruan Y, Yin J, Wu Q, Shao B. (2017) Triclosan/triclocarban levels in maternal and umbilical blood samples and their association with fetal malformation. Clin Chim Acta 466:133-137.
Wester RC, Maibach HI, Surichak J, Bucks DAW. (1985) Predictability of In Vitro Diffusion Systems: Effect of Skin Types and Ages on Percutaneous Absorption of Triclocarban. Dermatology 6: 223-226. [As cited in SCCP 2005].
Wilson R, Jones-Otazo H, Petrovic S, Mitchell I, Bonvalot Y, Williams D, Richardson GM. 2013. Revisiting dust and soil ingestion rates based on hand-to-mouth transfer. Human and Ecological Risk Assessment 19(1): 158-188.
Witorsch RJ and Thomas JA. 2010. Personal care products and endocrine disruption: a critical review of the literature. Critical Reviews in Toxicology 40(S3): 1-30.
Wu Y, Beland FA, Fang JL. (2016) Effect of triclosan, triclocarban, 2,2',4,4'-tetrabromodiphenyl ether, and bisphenol A on the iodide uptake, thyroid peroxidase activity, and expression of genes involved in thyroid hormone synthesis. Toxicol In Vitro 32: 310-9.
Xia K, Hundal LS, Kumar K, Armbrust K, Cox AE, Granato TC. (2010) Triclocarban, triclosan, polybrominated diphenyl ethers, and 4-nonylphenol in biosolids and in soil receiving 33-year biosolids application. Environmental Toxicology and Chemistry 29: 597–605.
Xia M, Huang R, Shi Q, Boyd WA, Zhao J, Sun N, Rice JR, Dunlap PE, Hackstadt AJ, Bridge MF, Smith MV, Dai S, Zheng W, Chu PH, Gerhold, D, Witt KL, DeVito M, Freedman JH, Austin CP, Houck KA, Thomas RS, Paules RS, Tice RR, Simeonov A. (2018) Comprehensive Analyses and Prioritization of Tox21 10K Chemicals Affecting Mitochondrial Function by in-Depth Mechanistic Studies. Environ Health Perspect 126: 077010
Xie W, Zhang W, Ren J, Li W, Zhou L, Cui Y, Chen H, Yu W, Zhuang X, Zhang Z, Shen G, Li H. (2018) Metabonomics Indicates Inhibition of Fatty Acid Synthesis, β-Oxidation, and Tricarboxylic Acid Cycle in Triclocarban-Induced Cardiac Metabolic Alterations in Male Mice. J Agric Food Chem 66: 1533−1542.
Xu X, Lu Y, Zhang D, Wang Y, Zhou X, Xu H, Mei Y. 2015. Toxic assessment of triclosan and triclocarban on Artemia salina. Bull Environ Contam Toxicol 95:728-733.
Yang L-H, Ying G-G, Su H-C, Stauber JL, Adams M-S, Binet MT. 2008. Growth inhibiting effects of 12 antibacterial agents and their mixtures on the freshwater microalga Pseudokirchneriella subcaptiata. Environ Toxicol Chem 27(5): 1201-1208.
Ye X, Wong LY, Dwivedi P, Zhou X, Jia T, Calafat AM. (2016) Urinary Concentrations of the Antibacterial Agent Triclocarban in United States Residents: 2013−2014 National Health and Nutrition Examination Survey. Environ Sci Technol 50: 13548−13554.
Ye X, Zhou X, Furr J, Ahn KC, Hammock BD, Gray EL, Calafat AM. (2011) Biomarkers of exposure to triclocarban in urine and serum. Toxicology 286: 69-74.
Yin J, Wei L, Shi Y, Zhang J, Wu Q, Shao B. (2016) Chinese population exposure to triclosan and triclocarban as measured via human urine and nails. Environ Geochem Health 38: 1125-35.
Zarate FM Jr, Schulwitz SE, Stevens KJ, Venables BJ. 2012. Bioconcentration of triclosan, methyl-triclosan, and triclocarban in the plants and sediments of a constructed wetland. Chemosphere 88: 323-329.
Zhou X, Ye X, Calafat AM. (2012) Automated on-line column-switching HPLC-MS/MS method for the quantification of triclocarban and its oxidative metabolites in human urine and serum. J Chromatogr B Analyt Technol Biomed Life Sci 881-882: 27-33.
Appendices
Appendix A. Summary of ecological data for triclocarban
| Test organism | Endpoint | Value (µg/L or µg/g dw) | Observations | Reference |
|---|---|---|---|---|
Water flea (Daphnia magna) | 96h LC50 | 27.4 | - | Albanese et al 2017 |
Water flea (Daphnia magna) | 48h LC50 | 10 | - | cited from Brausch and Rand 2011 |
Water flea (Ceriodaphnia dubia) | 48h LC50 | 3.1 | - | cited Brausch and Rand 2011 |
Water flea (Daphnia magna) | 21d LOEC (growth) 21d NOEC (growth) | 4.7 2.9 | - | cited Brausch and Rand 2011 |
Water flea (Daphnia magna) | 48h EC50 | 10 | - | Tamura et al 2013 |
Water flea (Ceriodaphnia dubia) | 8d NOEC | 1.9 | - | Tamura et al 2013 |
Water flea (Daphnia similis) | 48h EC50 | 14 | - | Satyro et al 2017 |
Brine shrimp (Artemia salina) | 24h LC50 | 17.8 | - | Xu et al 2015 |
Mysid shrimp (Mysidopsis bahia) | 28d LOEC (reproduction) 28 d NOEC (reproduction) | 0.13 0.06 | - | cited in Brausch and Rand 2011 |
Mysid shrimp (Mysidopsis bahia) | 48h LC50 96h LC50 | 15 10 | - | cited in Brausch and Rand 2011 |
Rotifer (Brachionus koreanus) | 24h LC50 | 388 | 200 µg/L : Population growth reduced 100 µg/L: oxidative stress transcriptional regulation of detoxification, antioxidant and heat shock proteins resulting in changes in lifespan and fecundity | Han et al 2016 |
Marine amphipod (Gammarus locusta) | 60 d LOEC (biochemical markers) | 2.5 | No impact seen in survival/growth/ reproduction at any concentration ; monotonic response 0.1 µg/L: higher LPO levels in females compared to males 0.1 and 0.5 µg/L: significant CAT/GST activity 2.5 µg/L: similar or lower levels CAT/GST compared to control ; 65% increase AChE for males and females | Barros et al 2017 |
Freshwater protozoan (Tetrahymena thermophila) | 24h EC50 (growth) | 295 | 1.0 µg/L: DNA damage 0.316 µg/L : downregulate MXR gene expression 0.79 µg/L: inhibition of efflux transporter activities | Gao et al 2015 |
Nematode (Caenorhabditis elegans) | 24h LC50 | 910 | 10 µg/L: reduction in brood size, delayed hatching | Lenz et al 2017 |
Nematode (Caenorhabditis elegans) | 96h EC50 (reproduction and growth) | 119 | - | Vingskes and Spann 2018 |
California blackworm (Lumbriculus variegatus) | 10 day (mortality) | 100 (ug/g dw) | No mortality seen | Higgins et al 2009 |
Japanese medaka (Oryzias latipes) | 96h LC50 | 85 | - | Tamura et al 2013 |
Zebrafish (Danio rerio) | 9d NOEC | 24 | - | Tamura et al 2013 |
Zebrafish (Danio rerio) | 32 and 80 hours post-fertilization LOEC | 350 | - | Torres et al 2016 |
Zebrafish (Danio rerio) | 96h LC10 96h LC50 | 133.3 6.6 147.5 215.8 | 133.3 µg/L: inhibition of thyroid hormone, altered expression of thyroid hormone responsive genes 6.6 µg/L: altered expression of proteins related to binding, metabolism, skeletal muscle development, nervous system development, and immune response | Dong et al 2018 |
Rainbow trout (Oncorhynchus mykiss) | 96h LC50 | 120 | - | cited in Brausch and Rand 2011 |
Bluegill (Lepomis macrochirus) | 96h LC50 | 97 | - | cited in Brausch and Rand 2011 |
Fathead minnow (Pimephales promelas) | 22 days NOEC 22 days LOEC (reproduction) | 1 5 | 1 µg/L: no effect on reproduction 5 µg/L: Reduced fecundity No effect on body mass or gonadosomatic index | Villeneuve et al 2017 |
Green algae (Scenedesmus subspicatus) | 72h LC50 (growth) | 20 | - | cited in Brausch and Rand 2011 |
Green algae (Pseudokirchneriella subcapitata) | 72h LC50 (growth) | 0.017 | - | cited in Brausch and Rand 2011 |
Green algae (Pseudokirchneriella subcapitata) | 72h IC50 NOEC LOEC | 17 <10 10 | Growth inhibition | Yang et al 2008 |
Green algae (Pseudokirchneriella subcapitata) | 14d LOEC (growth) 14d NOEC (growth) | 10 36 | - | cited in Brausch and Rand 2011 |
Green algae (Pseudokirchneriella subcapitata) | 72h EC50 | 29 | - | Tamura et al 2013 |
Green algae (Pseudokirchneriella subcapitata) | 72h NOEC | 5.7 | - | Tamura et al 2013 |
Green algae (Pseudokirchneriella subcapitata) | 72h IC50 | 319 | - | Satyro et al 2017 |
River biofilm community structure | 8 weeks (biomass) | 10 | Significant reduction in algal biomass Suppressed carbon utilization Community altered from autotrophic processes to heterotrophic processes Significant changes in community composition and bacterial communities | Lawrence et al 2009 |
Sea urchin (Paracentrotus lividus) | 8 and 80 hours post-fertilization LOEC | 0.64 | Decreased larval length; morphological abnormalities | Torres et al 2016 |
Freshwater mudsnail (Potamopyrgus antipodarum) | 2 – 4 weeks NOEC LOEC EC10 EC50 | 0.5 0.2 0.5 2.5 | 2.5 µg/L: embryo production stimulated | Giudice and Young 2010 |
American bullfrog (Rana catesbeiana) | 48h cultured frog tadpole tail fin bioassay | 0.315 | No effect on TRß transcript levels RLK1 transcript levels decreased IHSP30 and CAT transcript levels increased | Hinther et al 2011 |
Lettuce Corn | 65 days (symbiosis: arbuscular mycorrhizal fungi with plant roots) | 0 – 0.304 (µg/g dw) | No inhibition of colonization of crop plant roots by arbuscular mycorrhizal fungi | Prosser et al 2015 |
Radish Carrot Soybean Lettuce Wheat | 81 days (seed emergence, growth) | 14 – 304 (µg/g dw) | Negligible effects | Prosser et al 2014 |
Soil microbial community | 31 days (soil microbial community respiration, ammonification, and nitrification) | 6 – 717 (µg/g dw) | Negligible effects | Synder et al 2011 |
Abbreviations: LC50, concentration of a substance that is estimated to be lethal to 50% of the test organisms; LOEC, lowest-observed-effect concentration is the lowest concentration in a toxicity test that caused a statistically significant effect in comparison to the controls; NOEC, no-observed-effect concentration is the highest concentration in a toxicity test not causing a statistically significant effect in comparison to the controls; EC50, concentration of a substance that is estimated to cause an effect on 50% of the test organisms; EC10, concentration of a substance that is estimated to cause an effect on 10% of the test organisms; CAT, catalase; GST, glutathione-stransferase; LPO, lipid peroxidation; AChE, acetylcholinesteras
| Test organism | Triclocarban concentration (duration) | BCF/BAF/BSAF/RCF/SCF (L/kg or ng/g) | Reference |
|---|---|---|---|
Medaka (Oryzias latipes) | Lab reconstituted water (20 µg/L) (24 h) | BCF: 724 (TCC was oxidatively metabolized to sulfate and glucuronic acid conjugates) | Schebb et al 2011a |
Crucian carp (Carassius carassius) | WWTP effluent (0.000023 – 0.000044 µg/ml) | Plasma BAF: <0.1 – 8.6 | Tanoue et al 2015 |
Green algae (Cladophora spp) | WWTP effluent (0.191 µg/L) | BAF: 1900
| Coogan and La Point 2008 |
Green algae (Cladophora spp) | (WWTP effluent) 0.08 – 0.20 µg/L | BAF: 1600 – 2700
| Coogan et al 2007 |
Water flea (Daphnia longispina-galeata resting eggs) | Lake Greifensee (Switzerland) sediment (0.0024 – 0.0152 µg/g dw) (120 h) | BCF: 1240 – 82 900
| Chiaia-Hernandez et al 2013 |
California blackworm (Lumbriculus variegatus) | Lab spiked sediment (22.4 µg/g dw) (56 days) | BSAF: 1600 - 2200 | Higgins et al 2009 |
Earthworm (Eisenia foetida) | biosolids (707 µg/gdw) (31 days) | BSAF: 2.2 – 20
| Synder et al 2011 |
Earthworm (Eisenia foetida) | biosolids (7.6 – 10.8 µg/g dw) (28 days) | BSAF: 0.22 – 0.71 | Higgins et al 2011 |
Freshwater snail (Helisoma trivolvis)
| WWTP effluent (0.191 µg/L) (2 weeks) | BAF: 1600
| Coogan and La Point 2008 |
Clam (Corbicula fluminea) | WWTP effluent (14 days) | BCF: 7943
| Ismail et al 2014 |
Mussel (Anodonta californiensis) | WWTP effluent (14 days) | BCF: 7943
| Ismail et al 2014 |
Lettuce (Lactuca sativa) | Irrigation water (0.35 µg/L) | RCF <10 (harvested at maturity) | Hyland et al 2015 |
Strawberry (Fragaria ananassa) | Irrigation water (0.35 µg/L) | RCF <100 SCF <100 (harvested at maturity) | Hyland et al 2015 |
Sweet corn (Zea mays) Carrot (Daucus carota) Tomato (Solanum lycopersicum) Potato (Solanum tuberosum) | biosolids (6.03 µg/g dw) | No uptake detected at harvest | Sabourin et al 2012 |
biosolids →soil→ earthworms→ deer mice (Peromyscus maniculatus)→ European starling eggs (Sturnus vulgaris)→ American kestrel eggs (Falco sparverius) | biosolids (1.25 µg/g ww)
| BSAF earthworm: 0.79 (estimated as earthworms not depurated)
BSAF deer mouse liver: 0.20
BSAF starling egg: 0.25
BSAF kestrel egg: 0.05 | Sherburne et al 2016 |
Abbreviations: dw, dry weight; RCF, root concentration factor; SCF, shoot concentration factor; BSAF, biota-to-soil or sediment accumulation factor; BAF, bioaccumulation factor; BCF, bioconcentration factor; WWTP, wastewater treatment plant
Appendix B. Human exposure from environmental media and food
| Route of exposure | 0-5 moa (breast fed)b | 0-5 moa (formula fed)c | 6 to 11 mod | 1 yre | 2 to 3 yrf | 4 to 8 yrg | 9 to 13 yrh | 14 to 18 yri | 19+ yrj |
|---|---|---|---|---|---|---|---|---|---|
| Ambient airk | NI | NI | NI | NI | NI | NI | NI | NI | NI |
| Indoor airl | NI | NI | NI | NI | NI | NI | NI | NI | NI |
| Drinking waterm | NA | 21.0 | 13.5 | 5.3 | 4.6 | 3.7 | 2.8 | 2.8 | 3.3 |
| Food and beveragesn | 80.3 | NI | 53.6 | 13.7 | 12.5 | 11.9 | 6.7 | 4.7 | 5.1 |
| Soilo | NA | NA | 0.05 | 0.05 | 0.02 | 0.02 | 0.01 | 0.001 | 0.001 |
| Dustp | 33.5 | 33.5 | 29.0 | 31.1 | 13.9 | 10.4 | 5.5 | 0.33 | 0.34 |
| Total intake | 113.8 | 54.5 | 96.0 | 50.1 | 31.1 | 26.0 | 15.1 | 7.8 | 8.7 |
Abbreviations: NA, not applicable; NI, data not identified in the literature.
a Assumed to weigh 6.3 kg (Health Canada 2015), to breathe 3.7 m3 of air per day (US EPA 2011 [modified]), and to ingest 21.6 mg of dust per day (Wilson and Meridian 2015 [modified]). It is assumed that no soil ingestion occurs due to typical caregiver practices.
b Exclusively for breast milk-fed infants, assumed to consume 0.744 L of breast milk per day (Health Canada 2018), and breast milk is assumed to be the only dietary source. Triclocarban was not detected in breast milk samples from 80 Canadian women at two to three months post-partum (Arbuckle 2015); the limit of detection of 0.68 µg/L from this study was used to estimate an upper-bound exposure level.
c Exclusively for formula-fed infants, assumed to drink 0.826 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote on drinking water for details.
d Assumed to weigh 9.1 kg (Health Canada 2015), to breathe 5.4 m3 of air per day (US EPA 2011 [modified]), to drink 0 L of water per day (Health Canada 2017), to ingest 7.3 mg of soil per day, and to ingest 27.0 mg of dust per day (Wilson and Meridian 2015 [modified]). For breast milk-fed infants, assumed to consume 0.632 L of breast milk per day (Health Canada 2018). For formula-fed infants, assumed to drink 0.764 L of water per day (Health Canada 2018), where water is used to reconstitute formula. See footnote on drinking water for details.
e Assumed to weigh 11.0 kg (Health Canada 2015), to breathe 8.0 m3 of air per day (US EPA 2011 [modified]), to drink 0.36 L of water per day (Health Canada 2017), to ingest 8.8 mg of soil per day, and to ingest 35.0 mg of dust per day (Wilson and Meridian 2015 [modified]).
f Assumed to weigh 15 kg (Health Canada 2015), to breathe 9.2 m3 of air per day (US EPA 2011 [modified]), to drink 0.43 L of water per day (Health Canada 2017), to ingest 6.2 mg of soil per day, and to ingest 21.4 mg of dust per day (Wilson and Meridian 2015 [modified]).
g Assumed to weigh 23 kg (Health Canada 2015), to breathe 11.1 m3 of air per day (US EPA 2011 [modified]), to drink 0.53 L of water per day (Health Canada 2017), to ingest 8.7 mg of soil per day, and to ingest 24.4 mg of dust per day (Wilson and Meridian 2015 [modified]).
h Assumed to weigh 42 kg (Health Canada 2015), to breathe 13.9 m3 of air per day (US EPA 2011 [modified]), to drink 0.74 L of water per day (Health Canada 2017), to ingest 6.9 mg of soil per day, and to ingest 23.8 mg of dust per day (Wilson and Meridian 2015 [modified]).
i Assumed to weigh 62 kg (Health Canada 2015), to breathe 15.9 m3 of air per day (US EPA 2011 [modified]), to drink 1.09 L of water per day (Health Canada 2017), to ingest 1.4 mg of soil per day, and to ingest 2.1 mg of dust per day (Wilson and Meridian 2015 [modified]).
j Assumed to weigh 74 kg (Health Canada 2015), to breathe 15.1 m3 of air per day (US EPA 2011 [modified]), to drink 1.53 L of water per day (Health Canada 2017), to ingest 1.6 mg of soil per day, and to ingest 2.6 mg of dust per day (Wilson and Meridian 2015 [modified]).
k No monitoring data for triclocarban in ambient (outdoor) air were identified, in Canada or elsewhere.
l No monitoring data for triclocarban in indoor air were identified, in Canada or elsewhere.
m A maximum value of 160.5 ng/L triclocarban in treated water from Canadian water treatment plants was reported (Personal communication, email from Environmental and Radiation Health Sciences Directorate, Health Canada to Consumer and Hazardous Products Safety Directorate, Health Canada, dated September 20, 2018; unreferenced).
n Food consumption rates are described in Health Canada (2015). 90th percentile values provided by Food Directorate were used, except for the 0-5 month formula-fed age band, which was suppressed due to small sample size and the 6-11 month age band, which incorporated both breastfeeding and the 90th percentile food value. Sources and values for exposure to triclocarban via food are described in Section 6.1.1 provided by the Food Directorate, Health Canada (personal communication, email from Food Directorate, Health Canada to Consumer and Hazardous Products Safety Directorate, Health Canada, dated March 5, 2019; unreferenced).
o A mean value of 53 ng/g triclocarban was reported in agricultural soil in Quebec (Viglino, 2011).
p No monitoring data on house dust in Canada were identified. A maximum value of 9760 ng/g triclocarban was reported in a study of dust collected from athletic facilities and single-family detached homes in Oregon (Chan 2018).
Appendix C. Estimated daily intake of triclocarban from biomonitoring data
The estimated daily intake of triclocarban was calculated from CHMS biomonitoring data based on the equation Daily intake (µg/kg bw/d) = UER (µg/kg bw/d)/FUE, where UER is urinary excretion rate and FUE is fractional urinary excretion.
UER was calculated based on the equation UER (µg/kg bw/d) = UC (µg/L) x UFR (L/kg bw/d), where UC is urinary concentration and UFR is urinary flow rate (Saravanabhavan 2014).
| Age group (y) | UFRa (L/kg bw/d) | UC, P95b (µg/L) | UER, P95 (µg/kg bw/ day) | FUEc | Estimated daily intake (µg/kg bw/day) |
|---|---|---|---|---|---|
| 3 to 5 | 0.030 | 1.0 | 0.030 | 0.27 | 0.11 |
| 6 to 11 | 0.025 | 1.0 | 0.025 | 0.27 | 0.09 |
| 12 to 19 | 0.020 | 1.0 | 0.020 | 0.27 | 0.07 |
| 20 to 39 | 0.020 | 1.0 | 0.020 | 0.27 | 0.07 |
| 40 to 59 | 0.020 | 1.0 | 0.020 | 0.27 | 0.07 |
| 60 to 79 | 0.020 | 1.0 | 0.020 | 0.27 | 0.07 |
Abbreviations: UFR, urinary flow rate; UC, urinary concentration; UER, urinary excretion rate; P95, 95th percentile; FUE, fractional urinary excretion
a Urinary flow rates from Aylward (2015).
b Urinary concentrations are from Health Canada (2013). The values at the 95th percentile were reported as <LOD, so 1.0 µg/L was used as a surrogate value.
c Hiles 1978b
The estimated daily intake of triclocarban was calculated from NHANES biomonitoring data based on the equation Estimated daily intake (µg/kg bw/day) = UER (µg/kg bw/day)/FUE, where UER is the urinary excretion rate and FUE is the fractional urinary excretion.
UER was calculated using the equation UER (µg/kg bw/day) = [UCCr (µg/g Cr) x CER (mg/day)]/ bw (kg) where UCCr is the creatinine-adjusted urinary concentration, CER is the creatinine excretion rate and BW is body weight (Saravanabhavan 2014).
CER was calculated using the Mage equation: CER= [0.993*1.64 [140 – Age] (Wt^1.5 Ht^0.5)/1000].
Default values used to calculate CER are presented in Table C-2.
| Age band from sourcea (y) | Age (year)b | Weight (kg)c | Height (cm)d |
|---|---|---|---|
| 6 to11 | 8 | 23 | 127 |
| 12 to 19 | 15.5 | 62 | 162 |
| 20+ | 39.5 | 75 | 163 |
a Ye 2016
b Ages were selected to align to age groups reported in literature with CMP default age groups.
c Weights were based on CMP exposure scenario defaults.
d Heights are the 50th percentile from WHO height-for-age growth Child Growth Standards.
| Age group (y) | CER (mg/day) | UCCr, P95a (µg/g Cr) | UER (µg/kg bw/day) | FUEb | Estimated daily intake (µg/kg bw/day) |
|---|---|---|---|---|---|
| 6 to 11 | 267.2 | 0.778 | 0.01 | 0.27 | 0.033 |
| 12 to 19 | 1276.6 | 1.97 | 0.04 | 0.27 | 0.15 |
| 20+ | 1390.1 | 17.6 | 0.33 | 0.27 | 1.21 |
| All | 1390.1 | 14.6 | 0.27 | 0.27 | 1.00 |
Abbreviations: CER, creatinine exchange rate; UCCr, creatinine-adjusted urinary concentration; UER, urinary excretion rate; P95, 95th percentile; FUE, fractional urinary excretion
a Urinary concentrations are from Ye (2016).
b Hiles 1978b
Appendix D. Parameters for estimating dermal exposures to products
Exposure to products was estimated using specific parameters obtained from literature. The estimated dermal exposure parameters for cosmetics and drug products are presented in Table D-1.
| Exposure scenario | Assumptions |
|---|---|
| Body soap (solid) | 9 to 13 years: 19+ years: Dermal absorption: 1.0% |
| Facial cleanser | 9 to 13 years: 19+ years: Dermal absorption: 0.39% |
