Screening assessment dinoseb
Official title: Screening assessment - Phenol, 2-(1-methylpropyl)-4,6-dinitro- (Dinoseb)
Chemical Abstracts Service Registry Number 88-85-7
Environment and Climate Change Canada
Health Canada
February 2021
Cat. No.: En14-398/2020E-PDF
ISBN 978-0-660-33888-0
Synopsis
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of phenol, 2-(1-methylpropyl)-4,6-dinitro-, commonly known as dinoseb. The Chemical Abstracts Service Registry Number (CAS RNFootnote 1) for dinoseb is 88-85-7. This substance was identified as a priority for assessment on the basis of human health concerns.
Dinoseb was used in Canada as an herbicide until 2001, when all herbicidal uses were discontinued. The largest current use in Canada is as a polymerization retarder in the production of styrene monomer. Information obtained under the export notification provisions of the Rotterdam Convention and from follow-up discussions with industry indicates that between 100 000 and 1 000 000 kg of dinoseb was imported into Canada in 2015. Data obtained from the Canadian Border Services Agency indicates that smaller quantities of a related substance, dinoseb acetate, were imported into Canada between 2011 and 2015 by several companies for other unknown uses. Dinoseb acetate will dissociate to dinoseb in the environment and therefore could be contributing to total dinoseb exposure levels.
Releases of dinoseb to surface water are possible and, according to information on use patterns, these releases could be continuous. In water, dinoseb will hydrolyze slowly, and it is not readily biodegradable. Degradation by photolysis can occur at a moderate rate, but will vary depending on factors such as water depth and turbidity. Overall, it is expected to persist in water. Dinoseb is slightly persistent in air, although significant releases to that medium are not expected. Dinoseb is not expected to bioaccumulate in aquatic organisms.
Dinoseb is a reactive chemical whose principal mode of action is the uncoupling of oxidative phosphorylation, which results in the interference of energy synthesis. Dinoseb is hazardous to various forms of aquatic organisms, as well as to birds and mammals. Dinoseb has effects on reproduction (embryotoxicity), survival and growth (changes in metabolism and abnormal development), and binds to protein and DNA. Empirical studies, in vitro assays, and quantitative structure-activity relationship (QSAR) modelling all indicate potential for adverse effects in aquatic organisms at low concentrations.
There are historical environmental monitoring data for dinoseb from the time it was used as an herbicide, as well as from shortly after it was discontinued for that use. However, there are limited current environmental monitoring data for dinoseb in surface water, and no current data for dinoseb in air, sediment or soil in Canada. An exposure analysis was conducted to estimate the predicted environmental concentration of dinoseb in surface water due to industrial releases. A risk quotient analysis for this scenario indicates that there is possible risk of harm to aquatic organisms from dinoseb. The potential for harm is supported by other lines of evidence, including persistence and long-range transport in water. Given the high hazard of dinoseb to aquatic organisms, even very low levels of exposure may pose a risk to the environment.
Dinoseb has previously been assessed through the Organisation for Economic Co-operation and Development (OECD) Cooperative Chemicals Programme, and the OECD Screening and Information Dataset Initial Assessment Report (SIAR) was used to inform the health effects section of this screening assessment. The main endpoints of concern for dinoseb are reproductive and developmental toxicity, based on effects on sperm parameters in male rats and the subsequent decrease in gestation index in an oral study, and maternal and fetal toxicity, as determined from an oral study in rats and a dermal study in rabbits. Dinoseb is no longer used as a pesticide, nor is it used in products available to consumers. Recent drinking water monitoring data from various municipalities across Canada show no detection of dinoseb. Exposure of the general population in Canada to dinoseb through environmental media, food, or the use of products is not expected. Any population exposures resulting from potential releases to surface waters from industrial uses would still be several orders of magnitude less than levels associated with health effects. Given these considerations, the potential risk to human health is deemed to be low.
Considering all available lines of evidence presented in this screening assessment, there is risk of harm to the environment from dinoseb. It is concluded that dinoseb meets the criteria under paragraph 64(a) of CEPA as it is entering or may enter the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity. However, it is concluded that dinoseb does not meet the criteria under paragraph 64(b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger to the environment on which life depends. It is also concluded that dinoseb does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute a danger in Canada to human life or health.
Therefore, it is concluded that dinoseb meets one or more of the criteria set out in section 64 of CEPA.
It has also been determined that dinoseb meets the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
1. Introduction
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of phenol, 2-(1-methylpropyl)-4,6-dinitro-, commonly known as dinoseb, to determine whether this substance presents or may present a risk to the environment or to human health. This substance was identified as a priority for assessment on the basis of human health concerns (ECCC, HC [modified 2007]).
Dinoseb was reviewed internationally through the Organisation for Economic Co-operation and Development (OECD) Cooperative Chemicals Assessment Programme, and an OECD Screening Information Dataset Initial Assessment Report (SIAR) is available (OECD 2007). These assessments undergo rigorous review (including peer review) and endorsement by international governmental authorities. Environment and Climate Change Canada and Health Canada are active participants in this process and consider these assessments reliable. The health assessment section of the OECD SIAR was used to inform the health effects section of this screening assessment. Additionally, the ecological assessment section of the OECD SIAR was reviewed, and relevant information from it was considered in the ecological section of this assessment along with other sources of ecological hazard information.
This screening assessment includes consideration of information on chemical properties, uses, environmental fate, hazards, and exposures, including additional information submitted by stakeholders. Relevant data were identified up to December 2016. Empirical data from key studies, as well as some results from models, were used to reach the conclusion.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Environment and Climate Change Canada and Health Canada (including the Pest Management Regulatory Agency) and incorporates input from other programs within these departments. The ecological portion of this screening assessment has undergone external review. Comments on the technical portions relevant to the environment were received from officials at the European Chemicals Agency (ECHA), and the United States Environmental Protection Agency (US EPA). Additionally, the draft of this screening assessment (published June 2, 2018) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remains the responsibility of Environment and Climate Change Canada and Health Canada.
This screening assessment focuses on information critical to determining whether a substance meets the criteria as set out in section 64 of CEPA, by examining scientific information and incorporating a weight of evidence approach and precautionFootnote 2. This screening assessment presents the critical information and considerations on which the conclusion is based.
2. Identity of substance
Substance identity information, including the CAS RNFootnote 3, Domestic Substances List (DSL) name, and common name, is presented in Table 2-1. In this assessment, the substance will be identified by its common name, dinoseb.
A list of additional chemical names (e.g., trade names) for dinoseb is available from the National Chemical Inventories (NCI 2017). One of the most common abbreviations, which is used in commerce and experimental studies, is DNBP.
| CAS RN | DSL name (common names and abbreviation) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 88-85-7 | Phenol, 2-(1-methylpropyl)-4,6-dinitro- (Dinoseb, DNBP) |
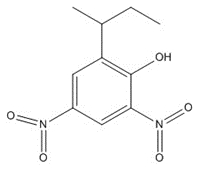
C10H12N2O5 |
240.24 |
Dinoseb forms salts and esters, some of which are water soluble, with inorganic and organic bases (Worthing and Walker (eds.) 1983; Kearney and Kaufman 1976). This assessment focuses on dinoseb (CAS RN 88-85-7) which is on the DSL and is known to be in commerce in Canada. However, it is recognized that dinoseb salts, in particular dinoseb acetate, once released to the environment, will dissociate to produce dinoseb itself, and therefore could be contributing to total dinoseb exposure levels. Therefore, potential dinoseb exposure resulting from dinoseb acetate has also been considered.
3. Physical and chemical properties
A summary of relevant experimental and modelled physical and chemical property values or ranges of values for dinoseb are presented in Table 3-1.
| Property | Value | Reference |
|---|---|---|
| Physical state | Yellow crystals or orange solid, with a pungent odour | Hartley and Kidd 1983; Worthing and Walker (eds.) 1983; WSSA 1979 |
| Melting point (°C) | 39.74–41.94 | ECHA c2007-2015a |
| Boiling point (°C) | >230a | ECHA c2007-2015a |
| Vapour pressure (Pa) | 0.007 (@ 20 ºC ) | IPCS 2011 |
| Water solubility (mg/L) | 52 (average) | Barbash and Resek 1996 |
| Water solubility (mg/L) | 25.8 | MITI 1992 |
| Other solubilities (mg/L): ethanol; n-heptane | 480 000; 270 000 | WSSA 1979 |
| Henry’s law constant (Pa·m3/mol) | 4.5 x 10-1 | Tremp et al. 1993 |
| log Kow (dimensionless) | 3.00 (avg. value, at pH 7)*–3.69 | Bromilow et al. 1991; MITI 1992; de Bruijn et al. 1989; IPCS 2011 |
| log Koc | 3.82 (at pH 3)b, 1.9 (at pH 7)* | Hodson and Williams 1988; calculatedc |
| log Koa | 8.29 (modelled) | EPI Suite c2000-2012 |
| log Kaw | 2 x 10-5 (modelled) | EPI Suite c2000-2012 |
| pKa (dimensionless) | 4.47 *–4.65 | Schwarzenbach et al. 1988; Worthing and Walker (eds.) 1983; ECHA c2007-2015a |
Abbreviations: Kow, octanol–water partition coefficient; Koc, organic carbon–water partition coefficient; pKa, acid dissociation constant.
* Indicates that this value was chosen for use in modelling.
a The boiling point could not be determined because the sample started to decompose before reaching the boiling point (onset temperature approximately 230°C; ECHA c2007-2015a). When dinoseb decomposes on heating, it produces harmful fumes of nitrogen oxides (IPCS 2011).
b Reported as Koc of 6607.
c Koc at pH 7 calculated from the pKa using an equation from Franco and Trapp (2008) for ionizing substances.
4. Sources, uses, and releases
Dinoseb is not known to occur naturally in the environment.
Dinoseb and its salts and esters are listed under the Rotterdam Convention as chemicals that require prior informed consent (PIC) before they can be exported from one Party to another (UNEP 2010). Chemicals and pesticides can be listed under the Rotterdam Convention when two or more Parties, located in different geographical regions of the world, have taken regulatory action to prohibit or severely restrict the substance as a consequence of a risk to health and/or the environment. Canada is a Party to the Rotterdam Convention and does not consent to the import of dinoseb and its salts and esters for pesticidal use (Environment Canada 2015). Although the Convention and its PIC procedure do not explicitly apply to exports of these substances for other uses, such as industrial uses, Environment and Climate Change Canada (ECCC) receives export notifications from some Parties who choose to notify importing countries when companies intend to export dinoseb for industrial uses. Since 2013, ECCC has received notifications about intended exports to Canada of dinoseb under the PIC classification of dinoseb and its salts and esters.
According to the export notification information described above and to follow-up discussions with industry, dinoseb was imported into Canada in the range of 100 000 to 1 000 000 kg in 2015 for use as a polymerization retarder in the production of styrene monomer. Although this use involves a closed industrial process, waste effluents from this process are sent off-site to a wastewater treatment system (WWTS)Footnote 4 and, after treatment, discharged to surface water. Therefore, there is a potential for release of dinoseb to surface water; however, there are limited monitoring data available to confirm what quantities, if any, are being released.
Information on the import of dinoseb was also obtained from the Canadian Border Services Agency (CBSA). Data were received for imports between the years 2011 and 2015 under the Harmonized System (HS) code for “Dinoseb (ISO) acetate”. These data indicated that eight different companies imported dinoseb acetate over the five-year period at low quantities, typically less than 100 kg per company per year (CBSA 2016). It is not known how these smaller imported quantities are being used.
Historically, dinoseb was imported into Canada for use as an herbicide, specifically as a pre-emergent or contact spray and as a desiccant. It was available commercially for these purposes as an aqueous solution and also as an emulsifiable concentrate (NCBI [accessed 2020]). The registration of all non-essential pesticidal (in this case, herbicidal) uses of dinoseb was suspended by Agriculture Canada in 1990 when health concerns about dinoseb were raised. No further uses were registered after December 31, 2000. The use of dinoseb as an herbicide has been discontinued as of December 31, 2001 (PMRA 2000). Historical releases of dinoseb in Canada were due to its use as an herbicide.
No other uses of dinoseb were identified (Table 4-1).
| Database | Dinoseb |
|---|---|
| Food additivea | No |
| Food packaging materialsb | No |
| Drug Product Databasec | No |
| Natural Health Products Ingredients Databased | No |
| Licensed Natural Health Products Database being present as a medicinal or non-medicinal ingredient in natural health products in Canadae | No |
| List of Prohibited and Restricted Cosmetic Ingredientsf | No |
| Notified to be present in cosmetics, on the basis of notifications submitted under the Cosmetic Regulations Health Canadag | No |
| Formulant in pest control products registered in Canadah | No |
a Personal communications, emails from Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada; dated November 2016; unreferenced.
b Personal communications, emails from Food Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada; dated November 2016; unreferenced.
c DPD (modified 2015).
d NHPID (modified 2016).
e LNHPD (modified 2016).
f Health Canada (modified 2015).
g Personal communications, emails from Consumer Product Safety Directorate, Health Canada, to Existing Substances Risk Assessment Bureau, Health Canada; dated December 2014; unreferenced.
h Health Canada 2010.
5. Environmental fate and behaviour
5.1 Environmental distribution
Table 5-1 presents the results of the Level III fugacity modelling for the neutral form of dinoseb, showing percent partitioning into each environmental medium for three release scenarios. The neutral form of dinoseb was used as the model (EQC) cannot make predictions for charged substances. Therefore, these results should be interpreted with caution as the dissociated (charged) form of dinoseb may behave differently in some media.
| Dinoseb released to | Air (%) | Water (%) | Soil (%) | Sediment (%) |
|---|---|---|---|---|
| Air (100%) | 29.22 | 16.96 | 53.70 | 0.12 |
| Water (100%) | 0.20 | 98.74 | 0.36 | 0.69 |
| Soil (100%) | 0.12 | 7.01 | 92.82 | 0.05 |
Fugacity modelling indicates that, when released into water, the neutral form of dinoseb is predicted to largely remain in that medium. However, the pKa for dinoseb (4.47) indicates that it will be present in water largely in the dissociated form at environmentally relevant pH values (6 to 9). Therefore, partitioning to sediment could vary from that predicted by fugacity modelling because natural sediments have a net negative charge (Blaskó 2008). On the basis of the modelled air-water partition coefficient (Kaw) of 2 x 10-5 (EPI Suite c2000-2012), partitioning from water to air is expected to be negligible.
The Transport and Persistence Level III Model (TaPL3 2003) can be used to predict long-range transport (LRT) in water, a concept developed by Beyer et al. (2000). Using TaPL3, the characteristic travel distance (CTD) of dinoseb in water was calculated. The CTD is defined as the maximum distance travelled by 63% of the substance after being released into the environment. Zarfl et al. (2011) have proposed a threshold CTD of 5200 km for classifying organic substances as having long-range transport potential in water. The predicted CTD for dinoseb is approximately 17 000 kilometres, assuming a river with a current of 3.6 km/h and depth of 20 metres. This means that releases of dinoseb to a river would likely result in its transport along the full length of the river, and dilution, rather than degradation, will be the main factor affecting exposure concentrations. Chronic exposures could therefore be expected in the far-field.
The TaPL3 model can also be used to predict the CTD of dinoseb in air. The estimated CTD in air from this model, and the estimated CTD from the OECD Pov and LRTP Screening Tool (OECD 2009), are 900 km and 1065 km, respectively. These values indicate that dinoseb, if released to air, is expected to be transported through the atmosphere moderate distances from its emission sources.
The fate of dinoseb in soil depends on many factors, including the form of dinoseb (neutral or dissociated), the type of soil, the form and concentration of ionic species in the soil (e.g., Ca2+), and especially, the pH of the soil (Aharonson 1987; Saltzman and Yariv 1974; Tulp et al. 2009; US EPA 1987; Cornell University 1987; Agriculture Canada 1991).
In terms of loss from plant surfaces, Menzie (1978) reported that 72% of dinoseb was lost 28 days after topical application to apples. Although the author indicates that the loss was likely due to volatilization, it was more likely due to photolysis or water run-off, given that the vapour pressure for dinoseb is relatively low. Translocation of dinoseb in plants does not appear to occur because no residues have been traced to foliar or root uptake (WSSA 1983; Kearney and Kaufman 1976).
5.2 Environmental persistence
The key experimental and modelled data for the abiotic degradation of dinoseb are summarized in Table 5-2.
| Medium and fate process | Degradation end-point or prediction | Value | Reference |
|---|---|---|---|
| Air; photo-oxidation | Half-life | 2.65 days | AOPWIN 2010 |
| Water; photolysis | Half-life | 12 days | ECHA c2007-2015a |
| Water; hydrolysis (pH = 4, 7, and 9) | Half-life | Uncertain (but stable for 5 days @ 50°C)* | CERI 2003a |
| Water; hydrolysis (pH = 5-9) | Half-life | Uncertain (but stable for 5 days @ 50°C)* | US EPA 1987 |
| Soil surfaces; photolysis in soil | Half-life (predicted) | 6–102 days | ECHA c2007-2015a; Stevens et al. 1989 |
| Plant surfaces; photolysis | Half-life | <1 hour to 6 days | Matsuo and Casida 1970; Hawkins and Saggers 1974 |
* The dinoseb molecule does not have any hydrolysable groups.
In air, dinoseb reacts with photochemically produced hydroxyl radicals. Dinoseb is not expected to react with other photo-oxidative species in the atmosphere, such as ozone, but it could react with nitrate radicals (AOPWIN 2010). However, it is expected that reactions with hydroxyl radicals will be the most important fate process in the atmosphere for dinoseb. With a half-life of 2.65 days via reactions with hydroxyl radicals, dinoseb is considered to be persistent in air. When present in air, dinoseb is expected to largely (> 80%) remain in the gaseous phase and to not partition significantly to airborne particulates (AEROWIN 2010).
Photolysis of dinoseb on plant surfaces could be a significant fate process for the degradation of dinoseb. Photolysis of dinoseb on soil surfaces could also be significant, but there is a great deal of uncertainty given the wide range of modelled half-lives that have been predicted (see Table 5-2).
In water, hydrolysis does not appear to be a significant fate process. Under certain conditions, photolysis in water can result in moderate degradation rates. However, photolysis is expected to vary considerably with water depth and turbidity and thus was not factored into the consideration of the residence time of dinoseb in water.
Table 5-3 summarizes the key experimental and modelled data for the biodegradation of dinoseb. The results of tests using OECD Guidelines 301B and 301C indicate that dinoseb is not readily biodegradable (ECHA c2007-2015a; CERI 2003b). Therefore, given these results, dinoseb is unlikely to undergo significant biodegradation in most natural waters (NCBI [accessed 2020]; OECD 2007). The experimental data are supported by modelled results for the neutral form of dinoseb (EPI Suite c2000-2012)
| Fate process | Test conditions | Degradation endpoint or prediction | Reference |
|---|---|---|---|
| Aerobic biodegradation | OECD 301B (activated sludge) | CO2 evolution 24% (after 28 days) | ECHA c2007-2015a |
| Aerobic biodegradation | OECD 301C (activated sludge, non-adapted) | BOD 0% (after 28 days) | CERI 2003b |
| Aerobic biodegradation | NA | Ready biodegradability prediction: No | EPI Suite c2000-2012 |
Abbreviations: BOD, biological oxygen demand; NA, Not Available
Test results for the biodegradation of dinoseb in soil are variable. Factors affecting biodegradation include the concentration of dinoseb, previous exposure to dinoseb, soil conditions (e.g., type of soil, pH), and the sorption of dinoseb to soil surfaces (Stevens et al. 1990; Stojanovic et al. 1972; Kearney and Kaufman 1976). Organisms that have been found to degrade dinoseb under aerobic conditions include Pseudomonas aeruginosa and Pseudomonas putida (Doubos and Reid 1956; Stevens et al. 1990), as well as Azotobacter (Wallnöfer et al. 1978) and Clostridium bifermentans (KMR-1) (Hammill and Crawford 1996). A number of studies report that dinoseb can also be degraded anaerobically (Hammill and Crawford 1996; Stevens et al. 1991; Kaake et al. 1992).
Dinoseb has been classified as unlikely to be degraded by even extended exposure to conventional biological sewage treatment processes (Verschuren 1983). This assertion is supported by modelling results for the neutral form, which show an overall WWTS removal rate of 15.4% (SimpleTreat 2003), and by the results of treatability studies by Monnig and Zweidinger (1980). However, Monnig and Zweidinger also discovered that a treatment system involving activated carbon filtration removed dinoseb. Specifically, after passage through a carbon-filled column, no dinoseb was detected in the water collected from the column, even though water samples had an initial dinoseb concentration of 750 mg/L.
The evidence for persistence, presented above, indicates that dinoseb is a relatively persistent chemical under many conditions. It has a predicted overall persistence (Pov) in the environment of 195 days (OECD 2009). It is persistent in air, according to modelled results, with a predicted half-life of 2.56 days. It is not readily biodegradable and does not hydrolyze rapidly in water. While photolysis in water could occur relatively quickly under the appropriate conditions, it is expected to vary considerably with water depth and turbidity and was therefore not factored into the consideration of the residence time of dinoseb in water. Similarly, degradation in soil could occur relatively quickly, but will vary considerably depending on conditions such as soil type and pH.
5.3 Potential for bioaccumulation
Table 5-4 summarizes the key experimental data for the bioconcentration of dinoseb in aquatic organisms. On the basis of these experimental results, dinoseb is considered to have a low potential for bioaccumulation. However, it should be noted that dinoseb will bind predominantly to plasma and protein (Luk’yanchuk et al. 1983; Rutherford et al.1984), not lipids, and will therefore distribute throughout an organism differently than a lipophilic substance. This phenomenon, and the resulting body burden in non-fatty tissue, might not be accounted for by some tests for bioaccumulation potential.
Care must also be taken for chemicals classified as polar non-volatiles, such as dinoseb, with log Kow > 2 and log Koa > 5. This group of substances has a low bioaccumulation potential in aquatic organisms, but a high bioaccumulation potential in air-breathing organisms, unless they are rapidly metabolized (Kelly 2006). Phenolic pesticides appear to be readily assimilated by animals, but excreted slowly over a period of many weeks (Kearney and Kaufman 1976).
| Test organism | Experimental concentration (duration) | BCF (L/kg) | Reference |
|---|---|---|---|
| Common carp (Cyprinus carpio) | 10 mg/L (6 weeks) | < 0.3–1.0 | CERI 1985a |
| Common carp (Cyprinus carpio) | 1 mg/L (6 weeks) | < 2.5 | CERI 1985a |
| Fathead minnow (Pimephales promelas) | 0.62 µg/L (24 days) | 61.5 | Call et al. 1983b |
| Fathead minnow (Pimephales promelas) | 7.22 µg/L (24 days) | 64.1 | Call et al. 1983b |
| Fathead minnow (Pimephales promelas) | 7.76 µg/L (28 days) | 56.2 | Call et al. 1983b |
a Test conditions not specified.
b Tests were whole body.
6. Potential to cause ecological harm
6.1 Ecological effects assessment
6.1.1 Mode/mechanism of action
Dinoseb is known to be a reactive, non-narcotic chemical. It interferes with energy synthesis through the process of uncoupling oxidative phosphorylation (Escher et al. 2010). This mechanism of action is consistent with what is generally expected for polynitroaromatic compounds (US EPA 2010). Uncoupling occurs when the transport of electrons (derived from carbohydrate or fat metabolism) into mitochondria is delinked from the production of adenosine triphosphate (ATP), which is an energy carrying molecule in the cell. This mechanism of action can occur in plants, animals, and fungi because they have similar biochemical pathways for creating energy (Felsot 1998).
Uncoupling can also stimulate metabolism, leading to the production of reactive oxygen species and enhancement of oxidative damage. Oxidative damage is a possible factor that contributes to embryotoxicity, potentially due to rapid cellular growth and incomplete metabolic development during embryogenesis (Paskova et al. 2011). In medaka (Oryzias latipes) embryos, significant changes in metabolism and increased abnormal development and post-exposure mortality were observed at low concentrations of dinoseb (Viant et al. 2006b). In mammals, an increase in oxidative metabolism can lead to various adverse effects, including the depletion of carbohydrate and fat stores (Morgan 1982; WSSA 1983; NRC 1983; Toxipedia 2014).
Supporting evidence for the mechanism of action of dinoseb was obtained from the ToxCast and Tox21 high-throughput in vitro assays (US EPA [updated 2016]). This included one assay addressing mitochondrial membrane depolarization, which underlies uncoupling (Sakamuru et al. 2012), and zebrafish assays addressing developmental effects (Padilla et al. 2012; Truong et al. 2014).
Protein and DNA binding, which are molecular mechanisms associated with high hazard in aquatic organisms, have also been noted for dinoseb (Call et al. 1983; ACD/Percepta c1997-2012; NCBI [accessed 2020]).
While some effects of dinoseb on the endocrine system have been found, including abnormal sperm and decreased thyroid weight (Linder et al. 1992; Van den Berg et al. 1991), dinoseb is not expected to be a binder of estrogen or androgen steroid receptors given its structure-activity relationships (ACD/Percepta c1997-2012, CATALOGIC 2014). Dinoseb appears in the European Union’s updated ranked endocrine disruptor priority list and is currently designated a Category 3b substance, meaning a substance with no or insufficient information gathered (EC-Environment 2016).
6.1.2 Effects on aquatic organisms
The acute and chronic toxicity of dinoseb to aquatic organisms is well characterized. The key experimental aquatic toxicity studies are summarized in Appendix A. Results show that dinoseb is harmful to fish, aquatic invertebrates, and algae.
On the basis of the available data set, freshwater invertebrates appear to be less sensitive to dinoseb than fish. The toxicity of dinoseb to fish is dependent on species and life stage, and as dinoseb is an ionizing substance, its toxicity is also influenced by pH, water hardness, and temperature (Johnson and Finley 1980; Woodward 1976; Lipschuetz and Cooper 1961; McCorkle et al. 1977; Skelley 1989).
In fish, acute median lethal concentration (LC50) values range from 0.032 to 0.96 mg/L. Chronic toxicity values, mostly no observed effect concentrations (NOECs) and lowest observed effect concentrations (LOECs), range from 0.0005 to 0.059 mg/L (Call et al. 1983, 1984; Call 1987; Woodward 1976; see Appendix A). The range of the observed chronic toxicity values is supported by other toxicity metrics. For example, these values, when converted to body residues (at 0.00012 to 0.01 mmol/kg), correspond to the range of critical body residues (CBR) for respiratory uncouplers (0.00015 to 0.094 mmol/kg) identified in McCarty and Mackay (1993). Similarly, ToxCast high-throughput zebrafish assays show embryonic toxicity between 0.0001 and 0.0004 mmol/L (reported as 0.137 to 0.430 µmol/L) from lethal and sublethal observations (Padilla et al. 2012; Truong et al. 2014). Assuming that extracellular and intracellular concentrations are comparable in the zebrafish assays, the observed embryonic toxicity follows the range for the chronic CBR.
The critical toxicity value selected for effects to aquatic organisms is the 60-day (post-hatch) LOEC of 0.5 µg/L (5 x 10-4 mg/L) for effects on the length and weight of lake trout fry (Woodward 1976). An assessment factor (AF) of 3 was selected to account for extrapolation from a 35% reduction in weight and length of fish fry, to a no-effect concentration. No extrapolation to account for interspecies variation was required because there are effects data available for a large number of species (i.e., greater than 10). After application of the AF, the predicted no-effect concentration (PNEC) for effects of dinoseb on aquatic organisms is 0.17 µg/L (1.7 x 10-4 mg/L).
6.1.3 Effects on birds and mammals
The effects of dinoseb on birds are summarized in OECD (2007). Studies pertain to toxicity by dietary exposure only. For example, the 5-day LC50 for Mallard duck (Anas platyrhynchos) is 410 ppm (Hill et al. 1975).
Studies from the 1980s that found reproductive and developmental effects in laboratory mammals were the initial reason why regulatory actions were undertaken in many countries, including Canada, to control the use of dinoseb as an herbicide. Studies reporting such effects on laboratory animals are summarized in the human health section of this assessment. Recently, the adverse reproductive and developmental effects of dinoseb have been acknowledged by a number of international jurisdictions. For example, dinoseb has been identified as a substance of very high concern (SVHC) because of its reprotoxic properties and has been added to the Candidate List (for eventual inclusion in Annex IV to the Registration, Evaluation, Authorisation and Restriction of Chemicals [REACH] regulation) under article 57(c) of REACH (ECHA c2007-2015b).
The Canadian Water Quality Guidelines for the Protection of Agricultural Water Uses (CCME 1999) summarizes other effects of dinoseb on laboratory and domestic mammals. PNECs for effects on birds and mammals were not derived because exposure in air and soil in Canada are not likely to be significant given the current use of dinoseb. Additionally, it is expected that food web transfers to birds will be low given its low bioaccumulation potential.
6.1.4 Effects on plants
Most studies of the effects of dinoseb on higher plants are efficacy field trials conducted when dinoseb was used as an herbicide. The results of these studies, which pertain mainly to effects on seed emergence and growth, are summarized in OECD (2007). No additional information for effects on plants was found.
6.1.5 Effects on soil-dwelling organisms
In a 2006 study (Staempfli et al.), the authors found that, after 6 days of exposure at 15 to 30 mg of dinoseb/g dry soil, the weight, lipid, and protein content of the exposed springtails (Folsomia candida) were higher than the controls. This suggests that growth increased in order to improve reproduction, which was confirmed by the greater number of eggs laid in exposed organisms. However, after 21 days, all measured parameters decreased and lethality increased.
As a protein and DNA binder, dinoseb could also be potentially quite toxic to skin-breathing organisms such as earthworms or frogs, potentially acting as a skin sensitizer (Princz et. al. 2014). However, there are currently no studies available on exposure of dinoseb to these types of organisms.
6.2 Ecological exposure assessment
Current monitoring data for environmental concentrations of dinoseb in Canada are limited. There are historical environmental monitoring data for dinoseb in Canada from the time it was used as an herbicide (Environment Canada 2011; Frank et al. 1979; Wan 1989; O’Neill et al. 1989; Milburn et al. 1991), as well as from 2003–2005, shortly after it was banned for that use (Environment Canada 2011). The results for dinoseb from the monitoring of surface water in Quebec from 2003 to 2005 were all non-detects, at a method detection limit (MDL) of 0.04 µg/L (Environment Canada 2011). More recently, analysis of water samples collected from three locations in the St. Clair River in 2018 showed no detection of dinoseb at a detection limit of 4.0 x 10-4 µg/L (personal communication, presentation from the Water Quality Monitoring and Surveillance Division, Environment and Climate Change Canada (ECCC), October 24, 2018; unreferenced).
Industry data on dinoseb concentrations measured daily for a number of years in untreated wastewater from a facility that uses dinoseb were provided to Environment and Climate Change Canada. All results show that dinoseb is not present at or above the MDL of 50 µg/L. This MDL, for the method used by the facility, is high; analytical methods with much lower detection limits are available. If it is assumed that dinoseb is present at half the MDL, then the concentration of dinoseb in untreated wastewater would be 25 µg/L. Process information provided by the user indicates that dilution will occur in the wastewater treatment system; therefore, a dilution factor of 10 was applied. Assuming a 15.4% removal in the treatment systems (as estimated with SimpleTreat 2003), the resulting concentration of dinoseb in the treated wastewater discharged to the environment is estimated to be 2.1 µg/L. A further dilution factor of 10 was applied to account for dilution once the treated wastewater is released to surface water. Therefore, a concentration of dinoseb in surface water of 0.21 µg/L is estimated as an upper bound of potential environmental concentrations resulting from use in a large facility.
More recently, industry data on dinoseb concentrations measured using a more sensitive analytical method in several samples of untreated wastewater from a facility that uses large volumes of dinoseb were provided to Environment and Climate Change Canada. Out of ten samples, dinoseb was not present at levels above the method detection limit of 0.05 µg/L in nine of them. There was one sample where a concentration of 0.117 µg/L was measured (personal communication, confidential data provided by email to Environment and Climate Change Canada, dated October 24, 2018; unreferenced). Given that these more recent data were collected over a short time period and comprise a small number of samples, it is not known how representative they might be. Therefore, the older and more extensive dataset is still considered relevant, despite the higher detection limit.
For other low-volume uses that may be occurring (as indicated by CBSA data; CBSA 2016), a generic analysis was conducted to determine the predicted environmental concentrations (PECs) in surface water resulting from releases from small industrial facilities. Multiple PECs were calculated by varying the inputs for certain parameters to capture a range of potential situations. Calculations were based on a total quantity of 85 kg of dinoseb used per year (the mass of dinoseb that would be present in 100 kg of dinoseb acetate), representing a small-sized operation. The number of days of release was varied between 50, 100 and 350 days per year. An emission factor of 0.3% was used, which is a default industrial release emission factor for generic operations (EC 2003). Releases from industrial facilities were assumed to occur via wastewater treatment systems (WWTS). A wastewater treatment removal estimation model (SimpleTreat 2003) was used to estimate the removal of dinoseb at a WWTS via sorption, volatilization and degradation. Using the water solubility, Henry’s law constant, organic-carbon partitioning coefficient and biodegradation rate constant for dinoseb as inputs to SimpleTreat, a 15.4% removal rate was estimated; this percent removal was used in all of the PEC calculations. The daily dilution volume of the receiving water (calculated as the WWTS effluent flow multiplied by the dilution factor of the receiving water body) was based on either the 10th or 50th percentile of the distribution of daily dilution volume for selected WWTS receiving industrial effluents, in Canada. Based on these inputs, 6 different generic PECs were calculated, representing a range of potential conditions. The PECs ranged from 0.01 to 0.82 µg/L, with a median of 0.09 µg/L.
There are no monitoring data for dinoseb concentrations in air in Canada. Significant releases to air would not be expected from the current use given that dinoseb is used in what is considered to be a closed process (OECD 2007)Footnote 5. However, minor releases could be possible. For example, small releases of dinoseb to air at chemical manufacturing facilities have been reported under the US EPA’s Toxics Release Inventory (TRI) program (US EPA 2016) as recently as 2015.
There are also no current monitoring data for dinoseb concentrations in soil and sediment in Canada.
6.3 Characterization of ecological risk
The approach taken in this ecological screening assessment was to examine assessment information and develop conclusions based on a weight-of-evidence approach and precaution. Evidence was gathered to determine the potential for dinoseb to cause harm to the Canadian environment. Lines of evidence considered include those evaluated in this assessment that support the characterization of ecological risk in the Canadian environment. Secondary or indirect lines of evidence are considered when available, including regulatory decisions and classification of hazard or fate characteristics made by other regulatory agencies.
6.3.1 Risk quotient analysis
Risk quotient analyses were performed by comparing the various realistic worst-case estimates of exposure (PECs; see the Ecological Exposure Assessment section) with ecotoxicity information (PNECs; see the Ecological Effects Assessment section) to determine whether there is potential for ecological harm in Canada. Risk quotients (RQs) were calculated by dividing the PEC by the PNEC for relevant environmental compartments and associated exposure scenarios.
Given its current main use in the chemical sector, any releases of dinoseb are expected to occur to surface water. Once released to surface water, dinoseb is expected to primarily remain in that medium because of its water solubility and low partitioning to sediments. Therefore, the RQ analysis for dinoseb focussed on the aquatic ecosystem. Potential releases of dinoseb acetate to surface water were also considered, as this substance will dissociate in the environment to produce dinoseb.
For the exposure scenario that considered releases via wastewater from large facilities using dinoseb, a PEC of 0.21 µg/L was estimated. Comparing this PEC with the PNEC for effects on aquatic organisms of 0.17 µg/L, the resulting RQ is 1.2. This indicates that facilities using high volumes of dinoseb could potentially pose a risk to the environment, depending on the handling practices used at the facility.
On the basis of potential releases of wastewater containing dinoseb to surface water, from the use of dinoseb acetate in small industrial facilities, PECs for dinoseb in surface water were calculated to range from 0.01 to 0.82 µg/L. Dividing these PECs by the PNEC of 0.17 µg/L, the resulting RQs for harm to aquatic organisms (PEC/PNEC) range from 0.06 to 4.8, with a median of 0.54. Of the 6 PECs estimated, 2 resulted in risk quotients greater than one. These results indicate the potential for exposure concentrations resulting from industrial activities to exceed chronic no-effect thresholds in the receiving environment, even when dinoseb is used in very low quantities.
6.3.2 Consideration of the lines of evidence
To characterize the ecological risk of dinoseb, technical information for various lines of evidence was considered (as discussed in the relevant sections of this report) and qualitatively weighted. The key lines of evidence supporting the assessment conclusion are presented in Table 6-1, with an overall discussion of the weight of evidence provided in section 6.3.3. The level of confidence refers to the combined influence of data quality and variability, data gaps, causality, plausibility, and any extrapolation required within the line of evidence. The relevance refers to the impact the line of evidence has when determining the potential to cause harm to the Canadian environment. Qualifiers used in the analysis ranged from low to high, with the assigned weight having five possible outcomes.
Direct lines of evidence presented in Table 6-1 relate to environmental fate and distribution, ecotoxicity, environmental release and concentrations, and the result from the risk quotient analysis. Indirect lines of evidence, such as regulatory decisions in other jurisdictions (e.g., SVHC candidate listing under REACH, multi-national pesticide restrictions, Rotterdam Convention listing) were also considered, but they were not given a qualitative weight because of the regulatory context of these decisions in other jurisdictions.
| Line of evidence | Level of confidencea | Relevance in assessmentb | Weight assignedc |
|---|---|---|---|
| Persistence in the environment | moderate | high | moderate to high |
| Long-range transport | moderate | moderate | moderate |
| Bioaccumulation in aquatic organisms | moderate | moderate | moderate |
| Mode of action and other non-apical data | high | high | high |
| PNEC for aquatic organisms | high | high | high |
| PEC in water (releases from large facilities using dinoseb) | low | high | moderate |
| PECs in water (releases from small facilities using dinoseb acetate) | low | high | moderate |
| Risk quotient for water | low | high | moderate |
a Level of confidence is determined according to data quality, data variability, data gaps and if the data are fit for purpose.
b Relevance refers to the impact of the evidence in the assessment.
c Weight is assigned to each line of evidence according to the combined level of confidence and relevance in the assessment.
6.3.3 Weight of evidence for determining potential to cause harm to the Canadian environment
Evidence presented in this assessment indicates that dinoseb is water soluble and relatively persistent in the environment. Structural and empirical evidence, as well as modelled results based on consistent high-quality measured physicochemical properties, mutually support the assertion that dinoseb has an overall persistence (Pov) in the environment in the order of months. When released to water (i.e., its primary potential mode of entry to the environment), dinoseb is likely to reside in the water column and undergo long-range transport in water where it will become distributed throughout a river system. Therefore, dilution by surface water bodies becomes the governing factor controlling environmental concentrations relevant to organism exposure. There is some uncertainty with half-life estimates as well as model estimates, which are mostly limited to the neutral form of dinoseb. A primary degradation pathway involves photolysis in surface water, which is expected to vary considerably with water depth and turbidity and thus was not factored into the consideration of residence time. Dinoseb is not expected to partition significantly to air from surface water; release to air at industrial facilities is uncertain. Furthermore, the evidence for CTD in air is modelled on the neutral form, and its accuracy is uncertain. Consequently, fate and transport of dinoseb in air is of low relevance in this assessment. Given its multimedia fate, dinoseb is not expected to be highly removed in waste treatment systems. Therefore, a low rate of removal from waste effluents (estimated at 15.4% by SimpleTreat for secondary treatment) is expected, while transfer of dinoseb to the terrestrial environment from biosolids application is not expected to be significant.
There is relatively consistent evidence indicating that dinoseb has low bioaccumulation potential in aquatic species. Dinoseb is not expected to biomagnify significantly in aquatic organisms. There is uncertainty about bioaccumulation in terrestrial organisms because dinoseb is internally distributed in blood plasma and structural proteins and is not rapidly metabolized and excreted.
Several lines of evidence of high weight mutually support the assertion that dinoseb is a highly reactive toxicant, with acute and chronic effects observed in all organisms tested. There are many reliable and mutually supportive empirical studies showing acute and chronic effects in aquatic organisms in the µg/L range, which is consistent with the mode of action. Mutually supportive evidence from in silico, in vitro and in vivo studies, shows that dinoseb interacts with biological tissues (e.g., proteins and DNA) at very low internal or external exposure concentrations resulting in embryo toxicity and chronic reproductive effects ultimately affecting organisms at the population level. The evidence indicates that exposure to dinoseb can result in more than one adverse outcome (death, growth reduction, embryo toxicity, reproductive effects). Dinoseb was identified as a substance of very high concern (SVHC) in the European Union and appears on the Candidate List (ECHA c2007-2015b) as a CMR (carcinogenic, mutagenic or toxic for reproduction) substance, specifically for reproductive effects. Little ecotoxicity data exist for dinoseb in soil organisms, and no sediment toxicity data were found for this assessment. Given the reactivity of dinoseb, it is likely that it would also be hazardous to soil and sediment organisms. However, given the lack of exposure in these media, soil and sediment scenarios were not considered in this assessment.
The PNEC calculated for dinoseb reflects a high level of confidence from several mutually supportive and highly weighted lines of evidence. There is some uncertainty in the PEC that was derived for large facilities using dinoseb, due to the high detection limit that was used for effluent monitoring data. More recent effluent monitoring data using a lower detection limit did not find dinoseb at concentrations above the PNEC for a small number of samples. Therefore, a low weight was given to this PEC.
There is also some uncertainty in the PECs that were determined for small facilities using dinoseb acetate given that they were derived using generic industrial release assumptions. The lower weight given to the aquatic PECs reflects these uncertainties. Nonetheless, a low annual usage rate was assumed in all calculations, and still indicated risk in two cases. The RQ values would be sensitive to any errors associated with the PECs. The moderate weight assigned to the RQs reflects these potential weaknesses.
On the basis of the above lines of evidence, dinoseb is considered to have potential to cause ecological harm in Canada. It has also been determined that dinoseb meets the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
6.3.4 Sensitivity of conclusion to key uncertainties
Multiple lines of evidence mutually support the understanding of the fate and effects of dinoseb in the aquatic environment, which is the primary media of concern in this assessment. Thus, the conclusion is not sensitive to refinement of fate or ecotoxicity determination in water or other media and would not change with additional information on these aspects.
There is some uncertainty in the estimates of exposure concentrations. However, even assuming very low use quantities, two estimated PECs indicated a potential for risk. Given the very high hazard of this substance, any releases of dinoseb to the environment could pose a concern. The limited sampling of surface waters and industrial effluents indicate that dinoseb concentrations are very low at these locations. More extensive wastewater effluent monitoring or monitoring for dinoseb in receiving waters could help reduce exposure uncertainty. However, monitoring for dinoseb at distances from source emissions (e.g., >1 km) may not result in its detection where the receiving water body has a high dilution capacity.
7. Potential to cause harm to human health
7.1 Exposure assessment
Dinoseb was used in the past as an herbicide, but that use has been discontinued in Canada since 2001 (PMRA 2000). There have been no identified consumer uses for dinoseb, and the main industrial use of this substance in a closed industrial system is not expected to result in exposure of the general population. Dinoseb (including any dinoseb formed as a result of the dissociation of dinoseb acetate) is routinely tested for in drinking water and has not been found above the limit of detection (0.1 to 1.0 µg/L) in recent surveys (Exova 2010; AGAT Laboratories 2013; WSH Labs 2015; City of Markham 2015; City of Barrie 2016; City of Guelph 2016; Regional Municipality of Wood Buffalo 2015). Dinoseb was included in the Guidelines for Canadian Drinking Water established by the Federal-Provincial-Territorial Committee on Drinking Water in 1996, but this guideline has since been withdrawn because dinoseb is no longer registered for use as a pesticide in Canada and it is no longer found in Canadian drinking water supplies “at levels that could pose a risk to human health” (Health Canada 2019). Dinoseb is not expected in air, drinking water or food and is not used in products; therefore, exposure of the general population is not expected.
7.2 Health effects assessment
Dinoseb was previously assessed by the US EPA Integrated Risk Information System (IRIS 1997-) and the OECD (2007). The OECD SIAR was used to inform the health effects characterization in this screening assessment. Dinosed is classified by the European Commission as a category 1B reproductive toxicant (may damage the unborn child; suspected of damaging fertility) (EU 2008). A literature search was conducted from the year prior to the OECD SIDS initial assessment meeting (SIAM) (i.e., April 2006) to September 2016. No health effects studies that could impact the risk characterization (i.e., result in different critical endpoints or lower points of departure than those stated in OECD 2007) were identified. This section provides critical endpoints and corresponding effect levels for dinoseb, as cited directly from OECD 2007.
In a combined repeated dose toxicity study and reproduction/developmental toxicity screening test (OECD TG 422), rats were administered dinoseb by gavage at doses of 0, 0.78, 2.33 or 7 mg/kg bw/day (MHLW, Japan, 2005, as cited in OECD 2007). Males were dosed for a total of 42 days from 14 days before mating, and females were dosed from 14 days before mating throughout the mating and pregnancy period to day 6 of lactation. Males in the 7.0 mg/kg bw/day dose group had significantly decreased motile sperm rate, progressive sperm rate, path velocity and viability rate. In addition, the amplitude of lateral head displacement, abnormal sperm rate and abnormal tail rate were significantly increased in the males of this dose group. Females in the 7.0 mg/kg bw/day dose group had a significantly lower gestation index compared with controls. On the basis of the above findings, the NOEL for reproductive and developmental toxicity was determined to be 2.33 mg/kg bw/day. At doses of 0.78 mg/kg bw/day and higher, a significant increase in hematocrit count was observed in males. At doses of 2.33 mg/kg bw/day and higher, a significant decrease in extramedullary hematopoiesis in the spleen was observed in females. Mortalities occurred in females administered the 7.0 mg/kg bw/day dose. On the basis of these observations, the LOAEL for males and the NOAEL for females were considered to be 0.78 mg/kg bw/day (MHLW, Japan, 2005, as cited in OECD 2007).
In another reproductive toxicity study, dinoseb was administered in the diet of male rats, equivalent to 0, 3.8, 9.1, 15.6 or 22.2 mg/kg bw/day, for up to 77 days (Linder et al. 1982, as cited in OECD 2007). At doses of 9.1 mg/kg bw/day and higher, animals displayed a significant decrease in sperm counts and a significant increase in atypical spermatozoa. The NOAEL was determined to be 3.8 mg/kg bw/day.
In a developmental toxicity study, dinoseb was applied dermally to pregnant rabbits for 6 hours per day on gestation days 7 through 19 at doses of 0, 1, 3, 9 or 18 mg/kg bw/day (Johnson et al. 1988, as cited in OECD 2007). There was an increased incidence of hydrocephaly and anophthalmia in fetuses from dams exposed to 3 mg/kg bw/day and higher. In dams exposed to 9 mg/kg bw/day, there were a decreased number of live fetuses in addition to an increased incidence of fetuses with cleft palate, microcephaly and microphthalmia. Maternal mortality and hyperthermia were observed at doses of 3 mg/kg bw/day and higher. On the basis of the above findings, the NOEL for maternal toxicity and reproductive and developmental toxicity is considered to be 1 mg/kg bw/day.
In another developmental toxicity study, dinoseb was administered to pregnant rats either by gavage at doses of 0, 2.5, 5, 10 or 15 mg/kg bw/day, or in the diet at approximately 15 mg/kg bw/day, between gestation days 6 and 15 (Giavini et al. 1986, as cited in OECD 2007). At gavage doses of 10 mg/kg bw/day and higher, there was an increased incidence of fetuses with skeletal variations, and at gavage doses of 15 mg/kg bw/day, offspring displayed delayed ossification, significantly decreased body weight and an increased incidence of fetuses with skeletal variations. In addition, offspring from dams administered 15 mg/kg bw/day through diet (the only tested dose) displayed microphthalmia and significantly decreased body weight. Maternal body weight gain was reduced at gavage doses of 10 mg/kg bw/day and higher. The NOAEL for maternal and developmental toxicity was considered to be 5 mg/kg bw/day.
In vitro studies showed that dinoseb was not mutagenic in bacteria. In addition, dinoseb did not induce chromosomal aberrations in cultured mammalian cells (MHLW Japan 2005, as cited in OECD 2007). The limited carcinogenicity studies in rats and mice available gave no indication of carcinogenic effect (US EPA 1987, unpublished, as cited by OECD 2007).
7.3 Characterization of risk to human health
Exposure of the general population in Canada to dinoseb through environmental media, food, or the use of products is not expected. Any population exposures resulting from potential releases of dinoseb or dinoseb acetate to surface waters from industrial uses would still be several orders of magnitude less than levels associated with health effects. Given these considerations, the potential risk to human health is considered to be low.
While exposure of the general population to dinoseb is not of concern at current levels, this substance is considered to have a health effect of concern because of its potential reproductive and developmental toxicity. Therefore, there may be a concern for human health if exposure were to increase.
7.4 Uncertainties in evaluation of risk to human health
Overall, given that the uses and properties of dinoseb have been well characterized, a qualitative approach to risk characterization is considered appropriate for this assessment.
8. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is risk of harm to the environment from dinoseb. It is concluded that dinoseb meets the criteria under paragraph 64(a) of CEPA as it is entering or may enter the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity. However, it is concluded that dinoseb does not meet the criteria under paragraph 64(b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger to the environment on which life depends. It is also concluded that dinoseb does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that dinoseb meets one or more of the criteria set out in section 64 of CEPA.
It has also been determined that dinoseb meets the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
9. References
ACD/Percepta [prediction module]. c1997-2012. Toronto (ON): Advanced Chemistry Development, Inc.
[AEROWIN] Atmospheric Oxidation Program for Microsoft Windows [estimation model]. 2010. Ver. 1.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
AGAT Laboratories. 2013. Drinking water certificate of analysis. Calgary (AB): Mountain View Regional Water Services Commission.
Agriculture Canada. 1991. Guideline for the maximum acceptable concentration (MAC) for dinoseb in drinking water [PDF].
Aharonson N. 1987. Potential contamination of ground water by pesticides. Pure Appl Chem. 59(10):1419-1446.
[AOPWIN] Atmospheric Oxidation Program for Microsoft Windows [estimation model]. 2010. Ver. 1.92a. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Barbash JE, Resek EA. 1996. Pesticides in ground water: distribution, trends, and governing factors. US Geological Survey, Chelsea (MI): Ann Arbor Press. (Pesticides in the Hydrologic System, Vol. 2).
Beyer A, Mackay D, Matthies M, Wania F, Webster E. 2000. Assessing long-range transport potential of persistent organic pollutants. Environ Sci Technol. 34:699-703.
Blaskó L. 2008. Soil science [PDF]. Digital textbook.
Brack W, Frank H. 1998. Chlorophyll a fluorescence: a tool for the investigation of toxic effects in the photosynthetic apparatus. Ecotoxicol Environ Saf. 40:34-41.
Bromilow RH, Chamberlain K. 1991. Pathways and mechanisms of transport of herbicides in plants. In: Kirkwood R, editor. Target sites for herbicide action. Springer US. p. 245-284.
Brown LS, Lean DRS. 1995. Toxicity of selected pesticides to lake phytoplankton measured using photosynthetic inhibition compared to maximal uptake rates of phosphate and ammonium. Environ Toxicol Chem. 14(1):93-98.
Call DJ, Brooke LT, Kent RJ. 1983. Toxicity, bioconcentration, and metabolism of five herbicides in freshwater fish. US Department of Commerce, National Technical Information Service.
Call DJ, Brooke LT, Kent RJ, Poirier SH, Knuth ML, Shubat PJ, Slick EJ. 1984. Toxicity, uptake, and elimination of the herbicides alachlor and dinoseb in freshwater fish. J Environ Qual. 13:493-498.
Call DJ. 1987. Memorandum July 6 Memo to L.J. Larson. Center for Lake Superior. Environmental Studies, University of Wisconsin-Superior, p. 1-4.
Canada 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c. 33. Canada Gazette Part III, vol. 22, no. 3.
CATALOGIC [environmental fate and ecotoxicity model]. 2014. Ver. 5.11.15. Bourgas (BG): University “Prof. Dr. Assen Zlatarov”, Laboratory of Mathematical Chemistry.
[CBSA] Canada Border Services Agency. 2016. Information gathered on the import of commodities corresponding to the code HS 291536. Confidential information.
[CCME] Canadian Council of Ministers of the Environment. 1999. Canadian Water Quality Guidelines for the Protection of Agricultural Water Uses: Protocols for Deriving Water Quality Guidelines for the Protection of Agricultural Water Uses (Irrigation and Livestock Water) [PDF].
[CERI] Chemicals Evaluation and Research Institute, Japan. 1985. Study of bioaccumulation of 2,4-dinitro-6-(1-methylpropyl)phenol, Report Number 205008.
[CERI] Chemicals Evaluation and Research Institute, Japan. 2003a. Study of hydrolysis as a function of pH of 2,4-dinitro-6-(1-methylpropyl)phenol, Report Number 805054.
[CERI] Chemicals Evaluation and Research Institute, Japan. 2003b. Study of biodegradability of 2,4-dinitro-6-(1-methylpropyl)phenol, Report Number 205008.
Chèvre N, Brazzale AR, Beker-van Slooten K, Behra R, Tarradellas J, Guettinger H. 2005. Modeling the concentration-response function of the herbicide dinoseb on Daphnia magna (survival time, reproduction) and Pseudokirchneriella subcapitata (growth rate). Ecotoxicol Environ Saf. 62:17-25.
City of Barrie 2016. Drinking Water System 2016 Annual Report [PDF]. Section 11, O. Reg. 170/03. For the Period of January 1 to December 31, 2016.
City of Guelph. 2016. Annual and Summary Report For the period of Jan 1, 2015 – Dec 31, 2015 [PDF]. For Guelph Drinking Water System and Gazer Mooney Subdivision Distribution System. Prepared by City of Guelph Water Services Environmental Services Department.
City of Markham. 2015. City of Markham Water Sampling Program for the Period of January 1 to December 31, 2015. Annual Report.
Cornell University: [PMEP] Pesticide Management Education Program. 1987. Dinoseb (Premerge, Dinitro) Herbicide Profile 4/87.
de Bruijn J, Busser F, Seinen W, Hermens J. 1989. Determination of octanol/water partition coefficients for hydrophobic organic chemicals with the “slow-stirring” method. Environ Toxicol Chem. 8:499-512.
Doubos JD, Reid JJ. 1956. Decomposition of certain herbicides in soil microflora. Bacteriol Proc. p. 23.
[DPD] Drug Product Database [database]. [modified 2015 Jul 17]. Ottawa (ON): Government of Canada. [accessed 2016 June].
[EC] European Commission. 2003. Technical Guidance Document on Risk Assessment. Part II [PDF]. Luxembourg: Office for Official Publications of the European Communities.
Environment Canada. 2011. Presence and levels of priority pesticides in selected Canadian aquatic ecosystems [PDF]. Ottawa (ON): Environment Canada. Water Science and Technology Directorate.
Environment Canada. 2015. Export Control List, CEPA Schedule 3.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2007 Apr 20]. Categorization. Ottawa (ON): Government of Canada. [accessed 2016 Jun 10].
[ECHA] European Chemicals Agency. c2007-2015a. Substance information: Dinoseb Helsinki (FI): ECHA. [accessed 2016 Dec 1].
[ECHA] European Chemicals Agency. c2007-2015b. Inclusion of Substances of Very High Concern in the Candidate List.
[EC-Environment] European Commission – Environment. 2016. Study on enhancing the Endocrine Disruptor list with a focus on low production volume chemicals [PDF]. Available, with EU background information.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Escher BI, Ashauer R, Dyer S, Hermens JLM, Lee J-H, Leslie HA, Mayer P, Meador JP, Warne MSJ. 2010. Crucial role of mechanisms and modes of toxic action for understanding tissue residue toxicity and internal effect concentrations of organic chemicals. Integr Environ Assess Manag. 7(1):28-49.
[EU] European Union. 2008. Regulation (Ec) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006 [PDF].
Exova. 2010. Drinking water analytical report. Turner Valley Water Treatment Plant. Calgary (AB): Pure Elements Environmental.
Fabacher DL, Chambers H. 1974. Resistance to herbicides in insecticide- resistant mosquitofish, Gambusia affinis. Environ Lett. 7(1):15-20.
Felsot AS. 1998. Dinoseb--banned but not forgotten: the tale of an unusually hazardous pesticide. Agrichemical and Environmental News, Issue 146. Washington State University Cooperative Extension newsletter.
Franco A, Trapp S. 2008. Estimation of the soil–water partition coefficient normalized to organic carbon for ionizable organic chemicals. Environ Toxicol Chem 27:1995-2004.
Frank R, Sirons GJ, BD Ripley BD. 1979. Herbicide contamination and decontamination of well waters in Ontario, Canada, 1969-78. Pestic Monit J. 13(3):120-27.
Geiger DL, Northcott CE, Call DJ, Brooke LT. 1984. Acute toxicities of organic chemicals to fathead minnows (Pimephales promelas): Volume II. Center for Lake Superior Environmental Studies, University of Wisconsin-Superior.
Gersich FM, Mayes MA. 1986. Acute toxicity tests with Daphnia magna Straus and Pimephales promelas Rafinesque in support of national pollutant discharge elimination permit requirements. Water Res. 20:939-941.
Hammill TB, Crawford RL. 1996. Degradation of 2-sec-butyl-4,6-dinitrophenol (dinoseb) by Clostridium bifermentans KMR-1. Appl Environ Microbiol. 62(5):1842-1846.
Hartley D, Kidd H, editors. 1983. The agrochemicals handbook. Surrey (UK): Royal Society of Chemistry/Unwin Brothers Ltd. p. A159.
Hawkins DR, Saggers VH. 1974. The fate of dinobuton and dinoseb on growing apples. Pest Manag Sci. 5(4):497-504.
Hawxby K, Tubea B, Ownby J, Basler E. 1977. Effects of various classes of herbicides on four species of algae. Pest Biochem Physiol. 7:203-209.
Health Canada. 2010. PMRA list of formulants [PDF]. Ottawa (ON): Health Canada, Pest Management Regulatory Agency (PMRA). HC Pub. No.: 100460, Cat. No.: H114-22/2010E. [accessed 2016 June].
Health Canda. 2019. Guidelines for Canadian Drinking Water Quality-Summary Table [PDF]. Ottawa (ON): Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada.
Health Canada. [modified 2015 Dec 14]. Cosmetic Ingredient Hotlist: list of ingredients that are prohibited for use in cosmetic products. Ottawa (ON): Health Canada, Consumer Product Safety Directorate. [accessed 2016 June].
Hill, EF, Heath RG, Spann JW, Williams JD. 1975. Lethal dietary toxicities of environmental pollutants to birds. US Fish and Wildlife Service, Special Scientific Report-Wildlife 191, p. 1-61.
Hodson J, Williams NA. 1988. The estimation of the adsorption coefficient (Koc) for soils by high performance liquid chromatography. Chemosphere. 17(l):67-77.
[IPCS] International Programme on Chemical Safety. 2011. INCHEM: International Chemical Safety Cards (ICSCs): Dinoseb.
[IRIS] Integrated Risk Information System [database]. 1997-. Dinoseb. Bethesda (MD): US National Library of Medicine. [updated 2003 Oct 10; accessed 2015 Dec 17].
Johnson WW, Finley MT. 1980. Handbook of acute toxicity of chemicals to fish and aquatic invertebrates. United States Department of the Interior; Fish and Wildlife Service. Resource Publication 137. Washington (DC): US Government Printing Office.
Kaake RH, Roberts DJ, Stevens TO, Crawford RL, Crawford DL. 1992. Bioremediation of soils contaminated with the herbicide 2-sec-butyl-4,6-dinitrophenol (dinoseb). Appl Environ Microbiol. 58(5):1683-1689.
Kearney PC, Kaufman DD, editors. 1976. Herbicides: chemistry, degradation and mode of action. Volume 2. 2nd edition. New York: Marcel Dekker, Inc.
Kelly BC. 2006. Bioaccumulation potential of organic contaminants in an arctic marine food web. Thesis submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy, Simon Fraser University.
Kratky BA, Warren GF. 1971. A rapid bioassay for photosynthetic and respiratory inhibitors. Weed Sci 19(6):658-661.
Linder RE, Strader LF, Slott VL, Suarez JD. 1992. Endpoints of spermatotoxicity in the rat after short duration exposures to fourteen reproductive toxicants. Reprod Toxicol. 6(6):491-505.
Lipschuetz M, Cooper AM. 1961. Toxicity of 2-secondary-butyl-4,6-dinitrophenol to blacknose dace and rainbow trout. NY Fish Game J. 8:110-121.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2016 Aug 10]. Ottawa (ON): Government of Canada. [accessed 2016 June].
Lorz HW, Glenn SW, Williams RH, Kunkel CM, Norris LA, Loper BR. 1979. Effects of selected herbicides on smolting of coho salmon. EPA-600/3-79-071, Corvallis (OR): US EPA.
Luk'yanchuk VD, Luik AI. 1983. Mechanisms of conformational adaptation of albumin to ligands of differing chemical-structure. Biochemistry (Moscow) 48.4 (1983):559-565.
Matsuo H, Casida JE. 1970. Photodegradation of two dinitrophenolic pesticide chemicals, dinobuton and dinoseb, applied to bean leaves. Bull Environ Contam Toxicol. 5(1):72-78.
Mayer FL Jr., Ellersieck MR. 1986. Manual of Acute Toxicity: Interpretation and Data Base for 410 Chemicals and 66 Species of Freshwater Animals.
McCarty LS, Mackay D. 1993. Enhancing ecotoxicological modeling and assessment: body residues and modes of toxic action. Environ Sci Technol. 27(9):1719-28.
McCorkle FM, Chambers JE, Yarbrough JD. 1977. Acute toxicities of selected herbicides to fingerling channel catfish, Ictalurus punctatus. Bull Environ Contam Toxicol. 18(3):267-270.
McLeese DW, Zitko V, Peterson MR. 1979. Structure-lethality relationships for phenols, anilines and other aromatic compounds in shrimp and clams. Chemosphere. 8(2):53-57.
Menzie CM. 1978. Metabolism of Pesticides, Update II. US Department of the Interior, Fish Wildlife Service, Special Scientific Report - Wildlife No. 212. Washington (DC): US Government Printing Office.
Milburn P, O’Neill H, Gartley C, Pollock T, Richards JE, Bailey H. 1991. Leaching of dinoseb and metribuzin from potato fields in New Brunswick [PDF]. Canadian Agricultural Engineering.
[MITI] Ministry of International Trade and Industry (Japan). 1992. Hazard Data Book for Chemical Substances: (2-(1-methylpropyl)-4,6-dinitrophenol).
[MOE] Ministry of the Environment (Japan). 2015. Results of Eco-toxicity tests of chemicals conducted by Ministry of the Environment in Japan (March 2015) [PDF].
Monnig E, Zweidinger RA. 1980. Treatability studies of pesticide manufacturing wastewaters: dinoseb and atrazine. US Environmental Protection Agency Technical Report no. 600/2-80-077c.
Morgan DP. 1982. Recognition and Management of Pesticide Poisonings. EPA 540/9-80-005. Washington (DC): US Government Printing Office.
[NCBI] National Center for Biotechnology Information. PubChem Database. Dinoseb, CID=6950. [accessed 2020 Feb 10]
[NCI] National Chemical Inventories Global [database]. 2017. Columbus (OH): American Chemical Society, Chemical Abstracts Service. [accessed 2017 12 18].
[New EQC] New Equilibrium Criterion Model. 2011. Ver. 1.00 (Beta). Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2016 Nov 1]. Ottawa (ON): Government of Canada. [accessed 2016 June].
[NRC] National Research Council (USA). 1983. Drinking Water & Health. Volume 5. Washington (DC): National Academy Press. p. 46.
[OECD] Organisation for Economic Co-operation and Development. 2007. SIDS initial assessment report for 2-sec-butyl-4,6-dinitrophenol: CAS No. 88-85-7 [PDF] SIAM [SIDS Initial Assessment Meeting] 17-20; 2007 April; Paris, France. [accessed 2016 Sep 14].
[OECD] Organisation for Economic Co-operation and Development. 2009. OECD Pov and LRTP Screening Tool. Ver. 2.2. Paris (FR): OECD. A software model for estimating overall persistence (Pov) and long-range transport potential (LRTP) of organic chemicals.
O’Neill HJ, Pollock TL, Bailey HS, Milburn P, Gartley C, Richards JE. 1989. Dinoseb presence in agricultural subsurface drainage from potato fields in northwestern New Brunswick, Canada. Bull. Environ Contam Toxicol. 43:935-940.
Padilla S, Corum D, Padnos B, Hunter DL, Beam A, Houck KA, Sipes N, Kleinstreuer N, Knudsen T, Dix DJ, Reif DM. 2012. Zebrafish developmental of the ToxCast Phase I chemical library. Repro Toxicol. 33:174-187.
Pašková V, Hilscherová K, Bláha L. 2011. Teratogenicity and embryotoxicity in aquatic organisms after pesticide exposure and the role of oxidative stress. Rev Environ Contam Toxicol. 211: 25-61.
[PMRA] Pest Management Regulatory Agency. 2000. Clean Crop Dinoseb 300 Restricted Emulsifiable Concentrate (PCP 15086) [PDF]. Ottawa (ON): Health Canada, PMRA. [accessed 2016 Sep 26].
Princz J, Bonnell M, Ritchie E, Velicogna J, Robidoux P-Y, Scroggins R. 2014. Estimation of the bioaccumulation potential of a non-chlorinated bisphenol and an ionogenic xanthene dye to Eisenia andrei in field-collected soils, in conjunction with predictive in silico profiling. Environ Toxicol Chem. 33(2): 308-316.
Regional Municipality of Wood Buffalo. 2015. Annual Report Conklin Waterworks System [PDF]. Submitted to Alberta Environment and Parks.
Rutherford AW, Zimmermann JL, Mathis P. 1984. The effect of herbicides on components of the PS II reaction centre measured by EPR. FEBs Lett. 165(2):156-162.
Saarikoski, J, Viluksela M. 1981. Influence of pH on the toxicity of substituted phenols to fish. Arch Environ Contam Toxicol. 10:747-753.
Sakamuru S, Li X, Attene-Ramos MS, Huang R, Lu J, Shou L, Shen M, Tice RR, Austin CP, Xia M. 2012. Application of a homogenous membrane potential assay to assess mitochondrial function. Physiol Genomics. 1;44(9):495-503.
Saltzman S, Yariv S. 1974. Infrared study of the sorption of phenol and p-nitrophenol by montmorillonite. Soil Sci Soc Am Proc. 39:474-9.
Sanders HO. 1970. Toxicities of some herbicides to six species of freshwater crustaceans. J Water Pollut Control Fed. 42(8):1544-1550.
Schwarzenbach RP, Stierli R, Folsom BR, Zeyer J. 1988. Compound properties relevant for assessing the environmental partitioning of nitrophenols. Environ Sci Technol. 22:83-92.
[SimpleTreat 3.1] Sewage Treatment Plant Removal Model version 3.1. 2003. Bilthoven (NL): National Institute for Public Health and the Environment (RIVM). Available from: National Institute for Public Health and the Environment (RIVM), Laboratory for Ecological Risk Assessment, Bilthoven, the Netherlands.
Skelley JR. 1989. Toxicity of 2-sec-butyl-4,6-dinitrophenol (Dinoseb) and monosodium methane arsonate (MSMA),individually and in a mixture, to Channel Catfish (Ictalurus punctatus) and Fathead Minnows (Pimephales promelas). Environ Toxicol Chem. 8:623-628.
Staempfli C, Tarradellas J, Becker-van Slooten K. 2007. Effects of Dinoseb on energy reserves in the soil arthropod Folsomia candida. Ecotoxicol Environ Saf. 68:263-271.
Stevens DK, Grenney WJ, Yan Z, Sims RC. 1989. Sensitive parameter evaluation for a vadose zone fate and transport model. US EPA Project Summary: EPA/600/S2-89/039.
Stevens TO, Crawford RL, Crawford DL. 1990. Biodegradation of dinoseb (2-sec-butyl-4,6-dinitrophenol) in several Idaho soils with various dinoseb exposure histories. Appl Environ Microbiol. 56(1):133-139.
Stevens TO, Crawford RL, Crawford DL. 1991. Selection and isolation of bacteria capable of degrading Dinoseb ( 2.sec-butyl-4,6.dinitrophenol). Biodegradation. 2:1-13.
Stojanovic BJ, Kennedy MV, Shuman FL. 1972. Edaphic aspects of the disposal of unused pesticides, pesticides wastes, and pesticides containers. J Environ Qual. 1(1):54-62.
[TaPL3] Long Range Transport and Persistence Level III Model. 2003. Ver. 3.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
Toxipedia. 2014. Dinoseb.
Tremp J, Mattrel P, Fingler S, Giger W. 1993. Phenols and nitrophenols as tropospheric pollutants: emissions from automobile exhausts and phase transfer in the atmosphere. Water Air Soil Pollut. 68:113-123.
Truong L, Reif DM, St Mary L, Geier MC, Truong HD, Tanguay RL. 2014. Multidimensional in vivo hazard assessment using zebrafish. Toxicol Sci. 137(1):212-233.
Tulp HC, Fenner K, Schwarzenbach RP, Goss K-U. 2009. pH-dependent sorption of acidic organic chemicals to soil organic matter. Environ Sci Technol. 43:9189-9195.
[UNEP] United Nations Environment Program. 2010. Rotterdam Convention.
[US EPA] US Environmental Protection Agency. 1987. Dinoseb: Health advisory. Washington (DC): US EPA, Office of Drinking Water.
[US EPA] US Environmental Protection Agency. 2010. TSCA New Chemicals Program (NCP) Chemical Categories [PDF].
[US EPA] US Environmental Protection Agency. 2016. TRI (Toxics Release Inventory).
[US EPA] US Environmental Protection Agency. [updated 2016 Dec 2]. Toxicity Forecasting: Advancing the Next Generation of Chemical Evaluation. Washington (DC): US EPA. [accessed 2016 Dec 9].
Van den Berg KJ, van Raaij JAGM, Bragt PC, Notten WRF. 1991. Interactions of halogenated industrial chemicals with transthyretin and effects on thyroid hormone levels in vivo. Arch Toxicol. 65(1):15-19.
Verschuren K. 1983. Handbook of environmental data on organic chemicals. 2nd ed. New York (NY): Van Nostrand Reinhold. p. 570.
Viant MR, Pincetich CA, Tjeerdema RS. 2006a. Effects of dinoseb, diazinon and esfenvalerate in eyed eggs and alevins of Chinook salmon (Oncorhynchus tshawytscha) determined by 1H NMR metabolomics. Aquat Toxicol. 77:359-371.
Viant MR, Pincetich CA, Hinton DE, Tjeerdema RS. 2006b. Toxic actions of dinoseb in medaka (Oryzias latipes) embryos as determined by in vivo 31P NMR, HPLC-UV and 1H NMR metabolomics. Aquat Toxicol. 76:329-342.
Wallnöfer PR, Ziegler W, Engelhardt G, Rothmeier H. 1978. Transformation of dinitrophenol-herbicides by Azotobacter sp. Chemosphere. 12:967-972.
Wan MT. 1989. Levels of selected pesticides in farm ditches leading to rivers in the lower mainland of British Columbia. J Environ Sci Health B. 24(2):183-203.
Woodward DF. 1976. Toxicity of the herbicides dinoseb and picloram to cutthroat (Salmo clarki) and lake trout (Salvelinus namaycush). J Fish Res Board Can. 33:1671-1676.
Worthing CR, Walker SB, editors. 1983. The pesticide manual - a world compendium. 7th ed. Lavenham, Suffolk (UK): The Lavenham Press Limited. p. 213.
[WSSA] Weed Science Society of America. 1979. Herbicide handbook. 4th ed. Champaign (IL): Weed Science Society of America. p. 174.
[WSSA] Weed Science Society of America. 1983. Herbicide handbook. 5th ed. Champaign (IL): Weed Science Society of America.
Zarfl C, Scheringer M, Matthies M. 2011. Screening criteria for long-range transport potential of organic substances in water. Environ Sci Technol. 45:10075-10081.
Zitko V, McLeese DW, Carson WG, Welch HE. 1976. Toxicity of alkyldinitrophenols to some aquatic organisms. Bull Environ Contam Toxicol. 16(5):508-515.
Appendices
Appendix A. Aquatic toxicity data
| Test organism | Endpoint | Value (mg/L)a | Reference |
|---|---|---|---|
| Fathead minnow (Pimephales promelas) | 96 h LC50 | 0.088 | Skelly 1989 |
| Fathead minnow (Pimephales promelas) | 96 h LC50 | 0.13 | Gersich and Mayes 1986 |
| Fathead minnow (Pimephales promelas) | 96 h LC50 | 0.17 | Gersich and Mayes 1986 |
| Fathead minnow (Pimephales promelas) | 96 h LC50 | 0.41 | Geiger et al. 1984 |
| Fathead minnow (Pimephales promelas) | 96 h LC50 | 0.54 | Call 1987 |
| Fathead minnow (Pimephales promelas) | 96 h LC50 | 0.7 | Call et al. 1983 |
| Mosquitofish (Gambusia affinis) (insecticide-resistant) | 96 h LC50 | 0.96 | Fabacher and Chambers 1974 |
| Cutthroat trout (Salmo clarki) | 96 h LC50 | 0.071 | Mayer and Ellersieck 1986 |
| Cutthroat trout (Salmo clarki) | 96 h LC50 | 0.041 | Woodward 1976 |
| Lake trout (Salvelinus namaycush) | 96 h LC50 | 0.032 | Woodward 1976 |
| Cutthroat trout (Salmo clarki) | 96 h LC50 | 0.067 | Johnson and Finley 1980 |
| Lake trout (Salvelinus namaycush) | 96 h LC50 | 0.044 | Johnson and Finley 1980 |
| Coho salmon (Oncorhynchus kisutch) | 144 h LC50 | 0.088 | Lorz et al. 1979 |
| Chinook salmon (Oncorhynchus tshawytscha) | 96 h LC50 | 0.071 | Viant et al. 2006a |
| Channel catfish (Ictalurus punctatus) | 96 h LC50 | 0.058 | Skelley 1989 |
| Channel catfish (Ictalurus punctatus) | 96 h LC50 | 0.118 | McCorkle et al. 1977 |
| Medaka (Oryzias latipes) | 96 h LC50 | 0.28 | MOE (Japan) 2015 |
| Guppy (Poecilia reticulata) | 96 h LC50 | 0.35 | Saarikoski and Viluksela 1981 |
| Fathead minnow (Pimephales promelas) | 60 d NOEC (mortality, fry weight) | 0.0145 – 0.0485 | Call et al. 1983; Call et al. 1984 |
| Fathead minnow (Pimephales promelas) | 32 d NOEC (growth) | 0.059 | Call 1987 |
| Lake trout (Salvelinus namaycush) | 60 d LOEC (fry weight and length) | 0.0005 | Woodward 1976 |
| Daphnid (Daphnia magna Strauss) | 96 h LC50 | 0.24 | Gersich and Mayes 1986 |
| Daphnid (Daphnia magna) | 48 h EC50 (reproduction) | 0.18 | Chèvre et al. 2005 |
| Daphnid (Daphnia magna) | 48 h EC50 | 0.40 | MOE (Japan) 2015 |
| Daphnid (Daphnia magna) | 48 h EC50 (immobilization) | 0.24 | MITI 1992 |
| Scud (Gammarus fasciatus) | 96 h EC50 | 1.8 | Sanders 1970 |
| Shrimp (Crangon septemspinosa) | 96 h LC50 | 5.1 | McLeese et al. 1979 |
| Soft-shelled clam (Mya arenaria) | 84 h LC50 | 2.6 | McLeese et al. 1979 |
| American lobster larvae (Homarus americanus) | 96 h LC50 | 0.0075 | Zitko et al. 1976 |
| Daphnid (Daphnia magna) | 21 d EC50 | 0.17 | MOE (Japan) 2015 |
| Green algae (Chlamydomonas reinhardtii) | EC5 (toxicity threshold) | 0.34 | Brack and Frank 1998 |
| Green algae (Chlorella pyrenoidosa) | EC50 (growth rate inhibition) | 1.03 | Hawxby et al. 1977 |
| Blue-green algae (Lyngbya sp.) | EC50 (growth rate inhibition) | 1.42 | Hawxby et al. 1977 |
| Green algae (Chlorella pyrenoidosa) | EC50 (inhibition of photosynthesis) | 0.43 | Hawxby et al. 1977 |
| Blue-green algae (Lyngbya sp.) | EC50 (inhibition of photosynthesis) | 0.74 | Hawxby et al. 1977 |
| Green algae (Pseudokirchneriella subcapitata) | 72 h EbC50 | 0.81 | MOE (Japan) 2015 |
| Green algae (Pseudokirchneriella subcapitata) | 72 h ErC50 | 1.4 | MOE (Japan) 2015 |
| Green algae (Pseudokirchneriella subcapitata) | 72 h ErC50 | 0.49 | Chèvre et al. 2005 |
| Green algae (Chlorella pyrenoidosa) | 18-36 h IC50 (inhibition of chlorophyll production) | 0.15 | Kratky and Warren 1971 |
| Green algae (Chlorella pyrenoidosa) | 60–120 minute IC50 (inhibition of O2 evolution) | 8.0 | Kratky and Warren 1971 |
| Natural plankton (Jack’s Lake, Ontario) | 2 d IC50 (depression of proportional carbon, assimilation rates) | 1 | Brown and Lean 1995 |
| Natural plankton (Jack’s Lake, Ontario) | 2 d IC50 (depression of proportional phosphate, assimilation rates) | 12 | Brown and Lean 1995 |
| Natural plankton (Jack’s Lake, Ontario) | 2 d EC50 (50% depression of proportional ammonium assimilation rates) | 5 | Brown and Lean 1995 |
Abbreviations: LC50, the lethal concentration required to kill 50% of the population; d, day; EC, effect concentration; EC50, the concentration which induces a response halfway between the baseline and maximum; EbC50, the concentration at which 50% reduction of algal biomass is observed; ErC50, the concentration at which a 50% inhibition of algal growth rate is observed; h, hour; IC, inhibition concentration; LOEC, lowest observed effect concentration; NOEC; no effect concentration.
a In some studies, endpoint values are expressed in ppm, ppb, or µM/L. For consistency in the table, all such values were converted to mg/L.
