Risk management approach for hexanoic acid, 2-ethyl-, 2-ethylhexyl ester
Official title: Risk management approach for hexanoic acid, 2-ethyl-, 2-ethylhexyl ester (2-ethylhexyl-2-ethylhexanoate)
Chemical abstracts service registry number: 7425-14-1
Environment and Climate Change Canada Health Canada
December 2018
Summary of proposed risk management
This document outlines the proposed risk management actions for the substance 2-ethylhexyl-2-ethylhexanoate. In particular, the Government of Canada is proposing:
Adding 2-ethylhexyl-2-ethylhexanoate as a restricted ingredient on the Health Canada Cosmetic Ingredient Hotlist;
Applying Significant New Activity provisions under CEPA to 2-ethylhexyl-2-ethylhexanoate.
The risk management options outlined in this Risk Management Approach document may evolve through consideration of assessments and risk management options published for other Chemicals Management Plan (CMP) substances as required to ensure effective, coordinated, and consistent risk management decision-making.
Note: The above summary is an abridged list of options under consideration to manage this substance and to seek information on identified information gaps and uncertainties. Refer to section 3 of this document for more complete details in this regard.
1. Context
The Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999) provides the authority for the Minister of the Environment and the Minister of Health (the ministers) to conduct assessments to determine if substances are toxic to the environment and/or harmful to human health as set out in section 64 of CEPA,Footnote 1, Footnote 2 and if so to manage the associated risks.
The substance hexanoic acid, 2-ethyl-, 2-ethylhexyl ester, Chemical Abstracts Service Registry Number (CAS RN)Footnote 3 7425-14-1, referred to throughout this document as “2-ethylhexyl-2-ethylhexanoate”, is included in the assessment of calcium 2-ethylhexanoate and 2-ethylhexyl-2-ethylhexanoate, as part of the CMP (Canada 2018).
2. Issue
2.1 Final screening assessment conclusion
Health Canada and Environment and Climate Change Canada conducted a joint screening assessment relevant to the evaluation of 2-ethylhexyl-2-ethylhexanoate and calcium 2-ethylhexanoate (hexanoic acid, 2-ethyl-, calcium salt, CAS RN 136-51-6, referred to throughout this document as calcium 2-ethylhexanoate) in Canada. A notice summarizing the scientific considerations of the draft screening assessment for these substances was published in the Canada Gazette, Part I, on March 25, 2017 (Canada 2017a).
Based on the information available, the final screening assessment concludes that 2-ethylhexyl-2-ethylhexanoate meets the criteria under paragraph 64(c) of CEPA as it is entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health (Canada 2018). It is concluded that calcium 2-ethylhexanoate does not meet any of the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is concluded that 2-ethylhexyl-2-ethylhexanoate and calcium 2-ethylhexanoate are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity, or that constitute or may constitute a danger to the environment on which life depends under paragraphs 64(a) or 64(b) of CEPA, respectively (Canada 2018).
The exposure source of concern, identified in the final screening assessment, is dermal exposure to 2-ethylhexyl-2-ethylhexanoate from foot lotion and face make-up (refer to section 5).
Of note, the proposed risk management actions described in this document may be subject to change. For further information, refer to the final screening assessment for 2-ethylhexyl-2-ethylhexanoate and calcium 2-ethylhexanoate.
2.2 Recommendation under CEPA
Based on the findings of the final screening assessment conducted under CEPA, the ministers recommend that 2-ethylhexyl-2-ethylhexanoate be added to the List of Toxic Substances in Schedule 1 of the Act.Footnote 4
As the ministers finalize the recommendation to add 2-ethylhexyl-2-ethylhexanoate to Schedule 1, a risk management instrument will be proposed and finalized within a set period of time (refer to section 8.2 of this document for targeted publication timelines applicable to this substance).
2.3 Public comment period on the risk management scope
The Risk Management Scope document for 2-ethylhexyl-2-ethylhexanoate, which summarized the proposed risk management actions under consideration at that time, was published on March 25, 2017 (Canada 2017b). Industry and other interested stakeholders were invited to submit comments on the Risk Management Scope document during a 60-day comment period. No public comments were received for 2-ethylhexyl-2-ethylhexanoate.
3. Proposed risk management
3.1 Proposed human health objective
Proposed human health objectives are quantitative or qualitative statements of what should be achieved to address human health concerns.
The proposed human health objective for 2-ethylhexyl-2-ethylhexanoate is to reduce exposure of the general population to the substance to levels that are protective of human health.
3.2 Proposed risk management objective and proposed action
Proposed risk management objectives set quantitative or qualitative targets to be achieved by the implementation of risk management regulation(s), instrument(s) and/or tool(s) for a given substance or substances. In this case, the proposed risk management objectives for 2-ethylhexyl-2-ethylhexanoate are:
- to reduce dermal exposure to the sources of greatest concern, specifically cosmetics containing 2-ethylhexyl-2-ethylhexanoate; and
to prevent increases in exposure to 2-ethylhexyl-2-ethylhexanoate.
To achieve the proposed risk management objectives and to work towards achieving the proposed human health objective, the risk management actions being considered are:
To add 2-ethylhexyl-2-ethylhexanoate as a restricted ingredient on Health Canada’s Cosmetic Ingredient Hotlist, which is an administrative tool that Health Canada uses to communicate to manufacturers and others that certain substances may contravene the general prohibition found in section 16 of the Food and Drugs Act (F&DA) or may contravene one or more provisions of the Cosmetic Regulations. Section 16 of the F&DA states, among other things, that “No person shall sell any cosmetic that has in or on it any substance that may cause injury to the health of the user.” In addition, the Cosmetic Ingredient Hotlist includes certain substances that may make it unlikely for a product to be classified as a cosmetic under the F&DA. Compliance with the provisions of section 16 are monitored, in part, through the mandatory notification provisions of section 30 of the Cosmetic Regulations of the F&DA, which requires that all manufacturers and importers provide a list of the cosmetic’s ingredients to Health Canada.
To apply Significant New Activity (SNAc) provisions under CEPA to 2-ethylhexyl-2-ethylhexanoate that would require that any proposed new manufacture, import or use be subject to further assessment and that would determine if the new activity requires further risk management consideration.
Following the publication of this Risk Management Approach document, additional information obtained from the public comment period and from other sources will be considered, along with the information presented in this document, in the instrument selection and development process.Footnote 5 The risk management options outlined in this document may evolve through consideration of assessments and risk management options published for other CMP substances to ensure effective, coordinated, and consistent risk management decision-making.
3.3 Risk management information gaps
At this time, no additional information is required from industry.
4. Background
4.1 General information on 2-ethylhexyl-2-ethylhexanoate
2-Ethylhexyl-2-ethylhexanoate is an organic substance and is based on the parent structure 2-ethylhexanoic acid (2-EHA), CAS RN 149-57-5, which was evaluated by Health Canada and Environment and Climate Change Canada as part of the Challenge initiative (Health Canada 2011). 2-Ethylhexyl-2-ethylhexanoate is the ester of 2-EHA and 2-ethylhexanol.
4.2 Current uses and identified sectors
Responses to a 2011 survey conducted under section 71 of CEPA indicated that there were no reports of manufacture or import of 2-ethylhexyl-2-ethylhexanoate in Canada above the reporting threshold of 100 kg in that year (Environment Canada 2013).
According to notifications submitted under the Cosmetic Regulations to Health Canada (Canada 2018), 2-ethylhexyl-2-ethylhexanoate is present in certain cosmetic products in Canada, namely foot lotions and face make-ups. No occurrence data regarding 2-ethylhexyl-2-ethylhexanoate in food in Canada were identified. It was reported as a volatile component of certain foreign food samples; however, exposure of the general population of Canadians to 2-ethylhexyl-2-ethylhexanoate from these food sources is considered negligible (Canada 2018).
5. Exposure source and identified risk
General population exposure to 2-ethylhexyl-2-ethylhexanoate may occur from the use of certain cosmetics, namely foot lotions and face make-ups. According to the final screening assessment of 2-ethylhexyl-2-ethylhexanoate (Canada 2018), dermal exposure was estimated to be 0.37-1.10 mg/kg/d from the use of foot lotion containing 1-3% w/w of 2-ethylhexyl-2-ethylhexanoate and 0.91-3.05 mg/kg/d from the use of face make-up containing 3-10% w/w of 2-ethylhexyl-2-ethylhexanoate.
Substance-specific health effects data were not identified for 2-ethylhexyl-2-ethylhexanoate. However, 2-EHA and 2-ethylhexanol (i.e., CAS RNs 149-57-5 and 104-76-7, respectively) are considered relevant to 2-ethylhexyl-2-ethylhexanoate, and were used as analogues where critical health effects data were required. No dermal studies were available for 2-EHA; however, based on oral studies, 2-EHA was determined to be the more potent of the two analogues. Thus, an oral study for 2-EHA was selected to be conservative. The oral study conducted on laboratory animals revealed developmental health effects at the lowest dose (100 mg/kg-bw/day) following repeated exposure to 2-EHA in rat dams. Margins of exposure comparing effect levels from the oral dosing of 2-EHA in laboratory animals and estimates of dermal exposure from the use scenarios of foot lotion and face-makeup are considered potentially inadequate to account for uncertainties in the health effects and exposure databases for 2-ethylhexyl-2-ethylhexanoate (Canada 2018).
6. Risk management considerations
6.1 Alternatives and alternate technologies
With respect to foot lotion and face make-up, alternative cosmetic products are available that do not use 2-ethylhexyl-2-ethylhexanoate.
6.2 Socio-economic and technical considerations
Socio-economic factors will be considered in the selection process for a regulation and/or instrument respecting preventive or control actions, and in the development of the risk management objective(s). Socio-economic factors will also be considered in the development of regulations, instrument(s) and/or tool(s) as identified in the Cabinet Directive on Regulatory Management (TBS 2012a) and the guidance provided in the Treasury Board document Assessing, Selecting, and Implementing Instruments for Government Action (TBS 2007).
7. Overview of existing risk management
7.1 Related Canadian risk management context
2-Ethylhexyl-2-ethylhexanoate is not currently subject to any substance-specific risk management in Canada.
Food and drugs act (F&DA)
Based on notifications submitted under the Cosmetic Regulations, 2-ethylhexyl-2-ethylhexanoate is present in cosmetics. It is not currently included on Health Canada’s Cosmetic Ingredient Hotlist.
7.2 Pertinent international risk management context
United States:
Federal food, drug and cosmetic act (FD&C Act)
2-Ethylhexyl-2-ethylhexanoate is not currently included on the United States Food and Drug Administration’s List of Prohibited and Restricted Ingredients from use in cosmetics.
Europe:
Regulations on cosmetic products
2-Ethylhexyl-2-ethylhexanoate is subject to European Commission Regulations (EC) No 1223/2009 as a substance which is prohibited for use in cosmetics due to its classification as a Category 2 reprotoxic substance (EU 2008), unless an evaluation by the Scientific Committee on Consumer Safety (SCCS) has found the substance safe for use in cosmetic products (EU 2009). As of December 2017, the SCCS has not carried out a safety evaluation of 2-ethylhexyl-2-ethylhexanoate in cosmetics.
Australia:
Poisons standard June 2017
As an alkyl ester of 2-ethylhexanoic acid, 2-ethylhexyl-2-ethylhexanoate is subject to labelling requirements as per the Australian Poisons Standard June 2017, based on the following listing under Schedule 6: “2-ethylhexanoic acid and its alkyl esters except in preparations containing 5 per cent or less calculated as 2-ethylhexanoic acid”. The warning statement required for products that fall under this listing is "CAUTION (name of substance) should not be used by pregnant women" (Australia 2017).
8. Next steps
8.1 Public comment period
Industry and other interested stakeholders are invited to submit comments on the content of this Risk Management Approach or other information that would help to inform decision-making (such as outlined in sections 3.2). Please submit additional information and comments prior to February 12, 2019.
Comments and information submissions on the Risk Management Approach should be submitted to the address provided below:
Environment and Climate Change Canada
Chemicals Management Division
Gatineau Quebec K1A 0H3
Tel: 1-800-567-1999 | 819- 938-3232
Fax: 819-938-5212
Email: substances@ec.gc.ca
Companies that have a business interest in 2-ethylhexyl-2-ethylhexanoate are encouraged to identify themselves as stakeholders. Stakeholders will be informed of future decisions regarding 2-ethylhexyl-2-ethylhexanoate and may be contacted for further information.
8.2 Timing of actions
Action |
Date |
Publication of the final screening assessment and the Risk Management Approach document |
December 15, 2018
|
Electronic consultation on the Risk Management Approach |
December 15, 2018 to February 12, 2019
|
Submission of additional studies or information on 2-ethylhexyl-2-ethylhexanoate |
on or before February 12, 2019
|
Publication of responses to public comments on the Risk Management Approach, if applicable, and publication if required, of the proposed instrument(s) |
24-months from the publication of the final screening assessment |
Consultation on the proposed instrument(s), if required |
60-day public comment period starting upon publication of the proposed instrument(s) |
Publication of the final instrument(s), if required |
18-months from the publication of the proposed instrument(s)
|
References
Australia. 2017. Poisons Standard June 2017. Australian Government Department of Health. Therapeutic Goods Administration.
Canada. 2015. Red Tape Reduction Act.
Canada. 2017a. Dept. of the Environment, Dept. of Health. Draft Screening Assessment for Calcium 2-ethylhexanoate and 2-Ethylhexyl-2-ethylhexanoate.
Canada. 2017b. Dept. of the Environment, Dept. of Health. Risk Management Scope for 2-Ethylhexyl-2-ethylhexanoate.
Canada. 2018. Dept. of the Environment, Dept. of Health. Final Screening Assessment for Calcium 2-ethylhexanoate and 2-Ethylhexyl-2-ethylhexanoate.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment and Climate Change Canada, Health Canada; Existing Substances Program.
[EU] European Union. 2008. Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. Off J Eur Union L. 353:1-1355.
[EU] European Union. 2009. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off J Eur Union L. 342:59-209..
Health Canada. 2011. Screening Assessment for the Challenge. Hexanoic acid, 2-ethyl-. Ottawa (ON): Health Canada.
[TBS] Treasury Board of Canada Secretariat. 2007. Assessing, Selecting, and Implementing Instruments for Government Action.
[TBS] Treasury Board of Canada Secretariat. 2012a. Cabinet Directive on Regulatory Management.
[TBS] Treasury Board of Canada Secretariat. 2012b. Red Tape Reduction Action Plan.
[US EPA] United States Environmental Protection Agency. Substance Registry Services (SRS) Database. 2016.
Appendix A. Substance targeted for risk management
CAS RN |
DSL name (common name) |
Chemical structure and molecular formula |
Molecular weight (g/mol) |
7425-14-1 |
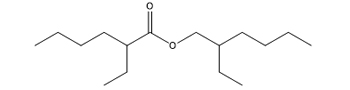
Hexanoic acid, 2-ethyl-, 2-ethylhexyl ester |
C16H32O2 |
256.43 |