Risk management scope - Salicylates group
Official title: Risk management scope for salicylic acid; homosalate; wintergreen oil (Salicylates group)
Chemical Abstracts Service Registry Number
69-72-7; 118-56-9; 68917-75-9
Environment and Climate Change Canada
Health Canada
March 2020
Summary of proposed risk management
This document outlines the risk management options under consideration for the substances wintergreen oil, salicylic acid and homosalate. In particular, the Government of Canada is considering:
- Communicating measures to reduce exposures to wintergreen oil and homosalate from certain cosmetics by describing wintergreen oil and homosalate as prohibited or restricted ingredients on the Health Canada Cosmetic Ingredient Hotlist;
- Communicating measures to reduce exposures to salicylic acid from certain cosmetics by modifying the current description for this substance on the Health Canada Cosmetic Ingredient Hotlist. It is currently described as having a restriction;
- Prohibiting or restricting wintergreen oil in certain natural health products, including changes to the recommended conditions of use;
- Prohibiting or restricting salicylic acid in certain natural health products and non-prescription drugs, including changes to the recommended conditions of use;
- Further risk mitigation for homosalate in certain non-prescription drugs under the Food and Drugs Act.
The risk management options outlined in this Risk Management Scope document may evolve through consideration of assessments and risk management options published for other Chemicals Management Plan (CMP) substances as required to ensure effective, coordinated, and consistent risk management decision-making.
Note: The above summary is an abridged list of options under consideration to manage this substance and to seek information on identified information gaps and uncertainties. Refer to section 3 of this document for more complete details in this regard.
1. Context
The Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999) provides the authority for the Minister of the Environment and the Minister of Health (the ministers) to conduct assessments to determine if substances are toxic to the environment and/or harmful to human health as set out in section 64 of CEPAFootnote 1 ,Footnote 2 and if so to manage the associated risks.
The 3 substances, listed in Annex A and referred to throughout this document as “wintergreen oil,” “salicylic acid” and “homosalate,” are included in the Salicylates group assessment as part of the CMP (Canada 2020a).
2. Issue
2.1 Draft screening assessment conclusion
Health Canada and Environment and Climate Change Canada conducted a joint screening assessment relevant to the evaluation of certain salicylates, including information relevant to the evaluation of wintergreen oil, salicylic acid and homosalate in Canada. A notice summarizing the scientific considerations of the draft screening assessment for these substances was published in the Canada Gazette, Part I, on [March 14, 2020] (Canada 2020b).
Based on the information available, the draft screening assessment proposes that wintergreen oil, salicylic acid and homosalate are harmful to human health under section 64(c) of CEPA because they are entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health (Canada 2020a).
It is proposed that wintergreen oil, salicylic acid and homosalate are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity, or that constitute or may constitute a danger to the environment on which life depends under section 64(a) or 64(b) of CEPA, respectively (Canada 2020a).
The draft screening assessment also proposes that the other substances in the Salicylates group, Benzoic acid, 2-hydroxy-, 2-phenylethyl ester (phenethyl salicylate) and Birch, Betula alba, ext (Betula alba extract) do not meet any of the criteria set out in section 64 of CEPA 1999. However, phenethyl salicylate has effects of concern based on its potential to elicit developmental toxicity (based on read-across data from the analogue methyl salicylate). While available information does not indicate a risk to human health for Canadians at current levels of exposure, there may be a concern if exposures were to increase.
The exposure sources of concern for wintergreen oil, as identified in the draft screening assessment, is dermal exposures to certain cosmetics and natural health products. For salicylic acid, dermal exposures to certain cosmetics as well as dermal and oral exposures to certain natural health products and non-prescription drugs were identified as the exposures of concern. For homosalate, dermal exposure to certain cosmetics were identified as a concern in the draft screening assessment (refer to section 5).
Of note, the proposed risk management options described in this document and the proposed conclusion outlined in the draft screening assessment are preliminary and may be subject to change. For further information on the draft screening assessment for the salicylates group, refer to the draft screening assessment of salicylates group.
2.2 Proposed recommendation under CEPA
Based on the findings of the draft screening assessment conducted as per CEPA, the ministers propose to recommend that wintergreen oil, salicylic acid and homosalate be added to the List of Toxic Substances in Schedule 1 of the Act.Footnote 3
The ministers will take into consideration comments made by stakeholders during the 60-day public comment period on the draft screening assessment and Risk Management Scope document in the preparation of the final screening assessment and Risk Management Approach document, if required. If it is concluded that wintergreen oil, salicylic acid and homosalate meets one or more of the criteria under section 64 of CEPA at the time of the final screening assessment and the Ministers recommend the addition of wintergreen oil, salicylic acid and homosalate to Schedule 1, risk management instrument(s) will be proposed within 24 months of the date on which the final screening assessment is published and will be finalized within 18 months of the date on which the risk management instrument(s) are proposed.
3. Proposed risk management
3.1 Proposed human health objective
Proposed human health objectives are quantitative or qualitative statements of what should be achieved to address human health concerns.
The proposed human health objective for wintergreen oil, salicylic acid and homosalate is to reduce exposures from certain wintergreen oil, salicylic acid or homosalate containing cosmetics, natural health products and/or non-prescription drugs available to consumers to levels that are protective of human health.
3.2 Proposed risk management objective and options under consideration
Proposed risk management objectives set quantitative or qualitative targets to be achieved by the implementation of risk management regulations, instrument(s) and/or tool(s) for a given substance or substances.
In the case of wintergreen oil, the proposed risk management objective is to reduce exposures of the general population to wintergreen oil in certain cosmetic products and natural health products to levels which are protective of human health.
To achieve the proposed risk management objective and to work towards achieving the proposed human health objective, the risk management options under consideration are:
- Communicate measures to reduce exposures to wintergreen oil from certain cosmetics by describing wintergreen oil as a prohibited or restricted ingredient on the Health Canada Cosmetic Ingredient HotlistFootnote 4 and
- Prohibit or restrict wintergreen oil in certain natural health products, including changes to the recommended conditions of use.
In the case of salicylic acid, the proposed risk management objective is to reduce exposure of the general population to salicylic acid in certain cosmetic products, natural health products, and non-prescription drugs to levels which are protective of human health.
To achieve the proposed risk management objective and to work towards achieving the proposed human health objective, the risk management options under consideration are:
- Communicate measures to reduce exposures to salicylic acid from certain cosmetics by modifying the current description for this substance on the Health Canada Cosmetic Ingredient Hotlist. It is currently described as a restricted ingredientFootnote 4 ; and
- Prohibit or restrict salicylic acid in certain natural health products and non-prescription drugs, including changes to the recommended conditions of use.
For homosalate, the proposed risk management objective is to reduce exposure of the general population to homosalate in certain cosmetic products, to levels which are protective of human health.
To achieve the proposed risk management objective and to work towards achieving the proposed human health objective, the risk management option under consideration is:
- Communicate measures to reduce exposures to homosalate from certain cosmetics by describing homosalate as a prohibited or restricted ingredient on the Health Canada Cosmetic Ingredient HotlistFootnote 4 .
The Government of Canada may also consider:
- Further risk mitigation for homosalate in certain non-prescription drugs under the Food and Drugs Act.
Following the publication of this Risk Management Scope document, additional information obtained from the public comment period and from other sources will be considered, along with the information presented in this document, in the instrument selection and development processFootnote 5 . The risk management options outlined in this document may evolve through consideration of assessments and risk management options published for other CMP substances to ensure effective, coordinated, and consistent risk management decision-making.
3.3 Risk management information gaps
Interested stakeholders are invited to provide further information, to inform risk management decision-making for wintergreen oil, salicylic acid and homosalate:
- Changes to wintergreen oil, salicylic acid and homosalate use patterns and economic impacts:
- Anticipated economic impacts if the import and/or use of wintergreen oil, salicylic acid and homosalate is prohibited or restricted in certain applications in Canada; and
- Ongoing or anticipated changes in use of wintergreen oil, salicylic acid and homosalate.
- Chemical and non-chemical alternatives to wintergreen oil and salicylic acid:
- Details on chemical alternatives and/or technologies to wintergreen oil and salicylic acid and their feasibility, as applicable to Canadian importers.
- Chemical and non-chemical alternatives to homosalate as an active ingredient in sunscreens:
- Details on chemical alternatives and/or technologies to homosalate as a UV filter and their feasibility, as applicable to Canadian manufacturers and importers.
Should stakeholders have further information to help address these gaps, they should provide it to the contact identified in section 8 of this document on or before May 13, 2020 to inform the risk management decision-making process).
4. Background
4.1 General information on wintergreen oil, salicylic acid and homosalate
Wintergreen oil, salicylic acid and homosalate are structurally similar insofar as they contain a salicylate moiety comprised of a carboxyl group occupying the ortho position of a phenol. These substances differ in the substituents forming the ester bond with the carboxyl carbon.
Wintergreen oil is an essential oil and represents a chemical substance of UVCB (unknown or variable composition, complex reaction products, or biological materials) nature derived primarily from Gaultheria genus of plants including the Gaultheria procumbens as well as Betula lenta (TSCA 2017). Despite being a UVCB, spectroscopic studies have revealed that wintergreen oil can contain up to 99% methyl salicylate, a discreet substance (Tisserand and Young 2014; El-Obeid et al. 1979).
Salicylic acid is naturally present in some plant species as a free phenolic acid or in conjugated forms (Raskin 1992; Lee et al. 1995, as cited in Bandurska 2013). It is commonly found as the derivative salicin, occurring in species of willow trees (Salix alba, S. purpurea, S. daphnoides, and S. fragilis).
Homosalate is a synthetic substance which is primarily used as a broad-spectrum UV filter in primary and secondary sunscreens; this substance is also found in cosmetics (Environment Canada 2013; SCCP 2007).
All of the substances in the Salicylates group have been included in surveys issued under section 71 of CEPA (Environment Canada 2013). Wintergreen oil, salicylic acid and homosalate were reported to be imported into Canada in quantities of 100 to 1000 kg, 87 437 kg and 100 000 to 1 000 000 kg, respectively, during the 2011 reporting year. None of the substances were reported to be manufactured in Canada, according to information from section 71.
4.2 Current uses and identified sectors
Wintergreen oil is reported to be used in Canada as an ingredient in cosmetics and pesticides, and as a non-medicinal and medicinal ingredient in natural health products. The substance is a component of wintergreen extract, wintergreen essence, and wintergreen flavour, which are standardized flavouring preparations in Canada (Canada 2020a).
Salicylic acid is naturally present in some plant species and may be present in food as a flavouring agent. In Canada, salicylic acid is widely used in cosmetics, natural health products, drugs, and has limited uses in some food packaging materials and incidental additives. The substance was also identified in a limited number of cleaning products, including dishwashing detergents.
Homosalate is primarily used as an active ingredient in sunscreen formulations, where it functions as a UV-filter. The use of homosalate as a medicinal ingredient in sunscreens is outlined in Health Canada’s Primary and Secondary Sunscreen Monographs (Health Canada 2018a, b) which identify that homosalate is an acceptable medicinal ingredient at concentrations of ≤ 15% to be market authorized without the submission of additional evidence to Health Canada. The substance was reported to be used in a limited number of cosmetics products in Canada.
5. Exposure sources and identified risk
According to the draft screening assessment, the general population in Canada may be exposed to wintergreen oil via exposure to certain cosmetics and natural health products. Increased relative liver weights and developmental effects were identified to be the critical effects associated with exposure to wintergreen oil on the basis of information available for its main component, methyl salicylate.
A comparison of the estimated exposure levels of wintergreen oil from its use as an ingredient in cosmetics such as massage oils and face moisturizers resulted in margins of exposure that are considered potentially inadequate to account for uncertainties in the health effects and exposure databases. A comparison of the estimated exposure level of wintergreen oil from its use as an ingredient in analgesic creams resulted in margins of exposure that are also considered potentially inadequate to address uncertainties in the health effects and exposure databases. Exposure to wintergreen oil from use as a food flavouring ingredient and as an ingredient in antacid tablets and toothpaste resulted in margins of exposure that are considered adequate to address these uncertainties (Canada 201Xa).
For salicylic acid, the draft screening assessment identified general population exposure through the use of certain cosmetics, natural health products and non-prescription drugs and environmental media and food. On the basis of the available information, the critical effects of salicylic acid are developmental, liver, and kidney effects.
With respect to dermal exposure to salicylic acid from the use of certain cosmetics, a comparison of the critical effect levels to the estimated exposures resulted in margins of exposure that are considered potentially inadequate to address uncertainties in the health effects and exposure databases.
With respect to dermal and oral exposure to salicylic acid from the use of certain natural health products and non-prescription drugs including sunscreens, liquid antacids, analgesic creams, acne creams, and after-shave creams, a comparison of the estimated exposure to the critical effect levels resulted in margins of exposure that are considered potentially inadequate to address uncertainties in the health effects and exposure databases.
Exposure to salicylic acid from its presence in environmental media and food and from its use in a hair spray resulted in margins of exposure that are considered adequate to address uncertainties and were not identified as a concern in the draft screening assessment.
Based on the draft screening assessment, general population exposure to homosalate may occur through the use of certain cosmetics. On the basis of the available information, the critical effects associated with exposure to homosalate are reproductive effects and effects on the kidneys, thymus and thyroid glands. A comparison of exposure to homosalate from the use of tanning products resulted in margins of exposure that are considered potentially inadequate to address uncertainties in the health effects and exposure databases. Use of face moisturizers resulted in margins of exposure that are considered adequate to address these uncertainties.
6. Risk management considerations
6.1 Alternatives and alternate technologies
No information on alternatives to wintergreen oil was identified. As stated above, wintergreen oil is used in a variety of cosmetic and natural health products including massage oils, moisturizers and analgesic creams. In general, there are products that do not contain wintergreen oil for each of these product categories. If more specific information on alternatives is known, we ask that stakeholders please submit this information.
Salicylic acid may have a variety of functions depending on the type of product which contains it. Information from the section 71 survey indicates that the substance can be used as an anti-acne, antidandruff, exfoliant, and preservative agent in a variety of personal care products (Environment Canada 2013). Therefore, depending on the product category, there are alternatives available. If more specific information on alternatives in the different product categories is known, we ask that stakeholders please submit this information.
For homosalate use in cosmetics, such as tanning products, there are alternative products which do not use homosalate. If more specific information on alternatives in cosmetics is known, we ask that stakeholders please provide this information.
6.2 Socio-economic and technical considerations
Socio-economic factors will be considered in the selection process for a regulation and/or instrument respecting preventive or control actions, and in the development of the risk management objectives(s). Socio-economic factors will also be considered in the development of regulations, instrument(s) and/or tool(s) as identified in the Cabinet Directive on Regulatory Management (TBS 2012a) and the guidance provided in the Treasury Board document Assessing, Selecting, and Implementing Instruments for Government Action (TBS 2007).
7. Overview of existing risk management
7.1 Related Canadian risk management context
Wintergreen Oil
Existing risk management for wintergreen oil in Canada relates to its presence in food, natural health products and pesticides and is summarized below.
- Wintergreen essence, wintergreen extract, or wintergreen flavour are standardized flavouring preparations set out in B.10.027 of the Food and Drug Regulations. They are prepared from wintergreen oil, and must contain not less than 3.0% by volume of wintergreen oil to meet the standard (Canada 2018a). Wintergreen oil may also be used alone as a food flavouring agent (that is, not part of a preparation). The safety of wintergreen oil as a food flavouring agent (alone or in a preparation) is subject to the provisions of section 4(1)(a) of the Food and Drugs Act (Canada 2018b).
- Wintergreen oil is listed in the Natural Health Products Ingredients Database with a medicinal role, classified as a Natural Health Product substance falling under Schedule 1 item 2 (extract) of the Natural Health Product Regulations (Canada 2018c). It is also listed in the Natural Health Products Ingredients Database with a non-medicinal role (Canada 2018d).
- Wintergreen Oil is registered as an active pesticide ingredient under the authority of the Pest Control Products Act as well as a List 4B formulantFootnote 6 (Canada 2017, Canada 2014).
- Wintergreen Oil is classified as an approved feed ingredient under Schedule V (flavouring agents for livestock feed) of the Feeds Regulations, 1983 (Canada 2009).
Salicylic Acid
Existing risk management for salicylic acid in Canada relates to its presence in drugs, food, natural health products, cosmetics and pesticides and is summarized below.
- Salicylic acid is an active ingredient and a non-medicinal ingredient in many diverse drug products. Salicylic acid is subject to the Food and Drug Regulations with various requirements for cautionary statements on labels and child resistant packages (Canada 2018e).
- The safety of salicylic acid when used in food packaging materials is subject to the provisions of Division 23 of the Food and Drug Regulations (Canada 2018a) and section 4(1)(a) of the Food and Drugs Act (Canada 2018b).
- The safety of salicylic acid as a food flavouring agent or when used in incidental additives is subject to the provisions of section 4(1)(a) of the Food and Drugs Act (Canada 2018b).
- Salicylic acid is listed in the Natural Health Products Ingredients Database with a medicinal role, classified as a Natural Health Product substance falling under Schedule 1, item 2 (an isolate) of the Natural Health Product Regulations (Canada 2018c). It is also listed in the Natural Health Products Ingredients Database with a non-medicinal role for topical use and permitted at concentrations equal to or less than 2%. This ingredient is also assigned the role of non-Natural Health Product (non-NHP), as listed on the Prescription Drug List: When sold in topical formulations containing salicylic acid at concentrations greater than 20% and/or with a pH of less than 3.0, except when sold to be applied to warts, corns or calluses (Canada 2018d).oui
- Salicylic acid is included on the List of Prohibited and Restricted Cosmetic Ingredients (Cosmetic Ingredient Hotlist) and is described as restricted for use in cosmetics at a maximum permitted concentration of 2% (Health Canada modified 2015).
- Salicylic acid is a List 3 formulant in pesticides (formulants that do not meet the criteria of any other lists) (Canada 2017).
Homosalate
Existing risk management for homosalate in Canada relates to its presence in natural and non-prescription health products and are summarized below.
- The use of homosalate as a medicinal ingredient in sunscreens is outlined in Health Canada’s Primary and Secondary Sunscreen Monographs (Health Canada 2018a, b) which identify that homosalate is an acceptable medicinal ingredient at concentrations of ≤ 15% to be market authorized without the submission of additional evidence to Health Canada (Health Canada 2018a,b).
- Homosalate is listed in the Natural Health Products Ingredients Database as a non-NHP substance which does not fall under Schedule 1 to the Natural Health Product Regulations (Canada 2018c).
7.2 Pertinent international risk management context
Wintergreen Oil
Internationally, there is a risk management measure in place for wintergreen oil in pesticides. Wintergreen Oil is a U.S. EPA inert pesticide ingredient which is classified as a List 4A (Minimum Risk Inerts) (U.S. EPA 2018a) as well as a registered active ingredient (PAN 2016).
Salicylic Acid
Internationally, there are risk management measures in place for salicylic acid in drugs, foods, cosmetics, environmental media and pesticides as shown below.
- Salicylic acid is subject to various requirements for Over the Counter (OTC) drugs under the U.S. Food and Drug Administration (FDA) Regulations. The Regulations identify specific salicylic acid concentrations in certain products and various labelling requirements (U.S. FDA 2017).
- Salicylic acid is subject to the U.S. FDA Regulations under Part 175 Indirect Food Additives: Adhesives and Components of Coatings and Part 177 Indirect Food Additives: Polymers (U.S. FDA 2017).
- The U.S. FDA has established a zero tolerance for salicylic acid residue in milk from dairy animals (U.S. FDA 2017).
- Salicylic acid is listed on the Register of Flavouring Substances in the European Union, which outlines procedures for these substances used or intended for use in or on foodstuffs (EC 2012).
- Salicylic acid is approved for use as a component in certain food packaging materials as per Regulation (EU) No 10/2011 (Consolidated-2018-06-26): Plastic materials and articles intended to come into contact with food (EC 2011).
- The European Commission has restrictions in place for salicylic acid in cosmetics with a maximum concentration of 3% in rinse-off products, 2% in other products and 0.5% when used as a preservative. As well, it is not to be used in preparations for children under 3 years old except for shampoos with specific labelling requirements (CosIng 2016).
- In Japan, salicylic acid is restricted to 0.2% in all types of cosmetics as per Japan’s Standards for Cosmetics (Japan 2000).
- Under the U.S. EPA Clean Air Act, salicylic acid is produced, as an intermediate or final product, by process units that are subject to certain emission standards of 40 CFR Part 60 (U.S. EPA 2018b).
- Salicylic acid is listed on the German Water Hazard Class Substances List under the Federal Water Act which requires that facilities using these substances be built and operated to protect from water contamination (Umweltbundesamt 2018).
- Salicylic acid is a U.S. EPA pesticide inert ingredient which is classified as a List 3 (inerts of unknown toxicity) (U.S. EPA 2018a).
- Salicylic acid is subject to the European Union Biocidal Products Regulations as an active biocidal product (Regulation EU 528/2012) (ECHA 2018).
Homosalate
International risk management measures for homosalate relate to its uses in sunscreens, environmental media and pesticides, as outlined below.
- In the U.S., homosalate up to 15% in over-the-counter topical sunscreen products is generally recognized as safe and effective and is not misbranded if it meets each condition in Part 352 and each general condition established in 330.1 of 31CFR352 (U.S. FDA 2017).
- In the European Commission and in Japan, homosalate is restricted in cosmetics at a maximum concentration of 10% in ready to use preparations where it functions as a UV filter (CosIng 2016; Japan 2000).
- Homosalate is listed on the German Water Hazard Class Substances List under the Federal Water Act which requires that facilities using these substances be built and operated to protect from water contamination (Umweltbundesamt 2018).
- Homosalate is a U.S. EPA inert pesticide ingredient which is classified as a List 3 (inerts of unknown toxicity) (U.S. EPA 2018).
8. Next steps
8.1 Public comment period
Industry and other interested stakeholders are invited to submit comments on the content of this Risk Management Scope or other information that would help to inform decision-making (such as outlined in sections 3.2). Please submit additional information and comments prior to May 13, 2020. The Risk Management Approach document, which will outline and seek input on the proposed risk management instrument(s), will be published at the same time as the final screening assessment. At that time, there will be further opportunity for consultation.
Comments and information submissions on the Risk Management Scope should be submitted to the address provided below:
Environment and Climate Change Canada
Chemicals Management Division
Gatineau Quebec K1A 0H3
Tel: 1-800-567-1999 | 819- 938-3232
Fax: 819-938-5212
Email: ec.substances.ec@canada.ca
Companies that have a business interest in the salicylates group are encouraged to identify themselves as stakeholders. Stakeholders will be informed of future decisions regarding wintergreen oil, salicylic acid and homosalate and may be contacted for further information.
8.2 Timing of actions
| Action | Date |
|---|---|
| Electronic consultation on the Risk Management Scope | March 14 to May 13, 2020 |
| Submission of additional studies or information on wintergreen oil, salicylic acid and/or homosalate | on or before May 13, 2020 |
| Publication of responses to public comments on the draft screening assessment and Risk Management Scope | on or before Spring 2021 |
| Publication of the final screening assessment and, if required, the Risk Management Approach document | on or before Spring 2021 |
| Publication of responses to public comments on the Risk Management Approach, if applicable, and publication if required, of the proposed instrument(s) | 24-months from the publication of the final screening assessment |
| Consultation on the proposed instrument(s), if required | 60-day public comment period starting upon publication of the proposed instrument(s) |
| Publication of the final instrument(s), if required | 18-months from the publication of the proposed instrument(s) |
References
Bandurska H. 2013. Salicylic acid: An update on biosynthesis and action in plant response to water deficit and performance under drought. In: Hayat S, Ahmad A, Alyemeni MN, editors. Salicylic acid: Plant growth and development. New York (NY): Springer. p. 1-10.
Canada. 2020a. Dept. of the Environment, Dept. of Health. Draft Screening Assessment for the Salicylates Group.
Canada. 2018a. Food and Drugs Regulations. C.R.C., c. 870. Part B Foods.
Canada. 2018b. Food and Drugs Act. R.S.C., 1985, c. F-27.
Canada. 2018c. Natural Health Products Regulations. SOR/2003-196.
Canada. 2018d. Natural Health Products Ingredients Database. Ottawa (ON): Health Canada.
Canada. 2018e. Food and Drugs Regulations. C.R.C., c. 870. Part C Drugs.
Canada. 2017. Pest Management Regulatory Agency (PMRA) List of Formulants. Ottawa (ON): Health Canada.
Canada. 2015. Red Tape Reduction Act. (S.C. 2015, c. 12).
Canada. 2014. Pest Management Regulatory Agency (PMRA). Registration Decision RD2014-22, Thyme Oil and Wintergreen Oil. Ottawa (ON): Health Canada.
Canada. 2009. Feeds Regulations, 1983. SOR/83-593.
[CosIng] Cosmetic Ingredients & Substances. 2016. European Commission. European Union.
[EC] European Commission. 2012. Commission Implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC. OJ L 267, 2.10.2012, p. 1–161.
[EC] European Commission. 2011. Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. OJ L 12, 15.1.2011, p. 1–89.
[ECHA] European Chemicals Agency. 2018. Substance Information – Salicylic Acid. Helsinki (FI): European Chemicals Agency.
El-Obeid HA and Hassan MMA. 1979. PMR assay of essential oils: II. Assay of methylsalicylate in wintergreen oil. Spectroscopy Letters. 12(7&8):555-557).
Environment Canada. 2013. DSL Inventory Update data collected under theCanadian Environmental Protection Act, 1999, section71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Health Canada. [modified 2015 Dec 14]. Cosmetic ingredient hotlist: list of ingredients that are prohibited for use in cosmetic products. Ottawa (ON): Health Canada, Consumer Product Safety Directorate.
Health Canada. 2018a. Primary Sunscreen Monograph. Ottawa (ON): Health Canada.
Health Canada. 2018b. Secondary Sunscreen Monograph. Ottawa (ON): Health Canada.
[Japan] Ministry of Health and Welfare. 2000. Standards for Cosmetics [pdf]. Provisional Translation. Notification No.331. Tokyo (JP): Ministry of Health and Welfare.
[PAN] Pesticide Action Network. 2016. Pesticide Database. Oakland (CA).
[SCCP] Scientific Committee on Consumer Products. 2007. Opinion on homosalate, COLIPA No. S12 [PDF]. Brussels (BE): European Commission.
[TBS] Treasury Board of Canada Secretariat. 2007. Assessing, Selecting, and Implementing Instruments for Government Action. Catalogue No. BT58-2/2007. Ottawa (ON): Treasury Board of Canada.
[TBS] Treasury Board of Canada Secretariat. 2012a. Cabinet Directive on Regulatory Management. Ottawa (ON): Treasury Board of Canada.
[TBS] Treasury Board of Canada Secretariat. 2012b. Red Tape Reduction Action Plan. Ottawa (ON): Treasury Board of Canada.
Tisserand R, Young R. 2014. Essential Oil Safety. 2nd ed. London (UK): Churchill Livingstone.
[TSCA] Toxic Substances Control Act Chemical Substance Inventory [database]. [updated June 2017]. Search results for CAS RN 68917-75-9. Washington (DC): United States Environmental Protection Agency.
Umweltbundesamt. 2018. Substances hazardous to waters. Berlin (DE): Umweltbundesamt.
[U.S. EPA]. U.S. Environmental Protection Agency. 2018a. InertFinder. Washington (DC): United States Environmental Protection Agency.
[U.S. EPA]. U.S. Environmental Protection Agency. 2018b. Electronic Code of Federal Regulations. Washington (DC): United States Environmental Protection Agency.
[U.S. FDA]. U.S. Food and Drug Administration. 2017. CFR – Code of Federal Regulations Title 21. Washington (DC): United States Food and Drug Administration.
Appendix A. Substances targeted for risk management
| CAS RN | DSL name | Common name | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|---|
| 68917-75-9a | Oils, wintergreen | Wintergreen oil | 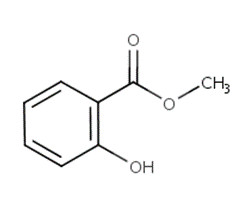 |
N/A |
| 69-72-7 | Benzoic acid, 2-hydroxy- | Salicylic acid | 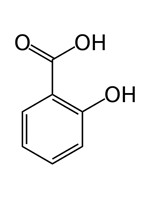 C7H6O3 C7H6O3 |
138.1 |
| 118-56-9 | Benzoic acid, 2-hydroxy-, 3,3,5-trimethylcyclohexyl ester | Homosalate | 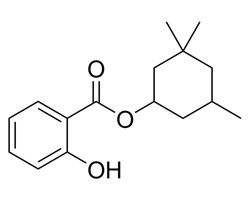 C16H21O3 C16H21O3 |
262.3 |
a This CAS RN is a UVCB (unknown or variable composition, complex reaction products, or biological materials).
