Screening Assessment - Appendices
Aromatic Azo and Benzidine-based Substance Grouping
Certain Benzidine-based Dyes and Related Substances
Environment Canada
Health Canada
November 2014
Table of Contents
- Appendix A: Supplementary Data Tables
- Appendix B: Aquatic PEC Calculations for Benzidine-based Acid and Direct Dyes Used in Textile Dyeing
- Appendix C: Soil PEC Calculations for Benzidine-based Acid and Direct Dyes Used in Textile Dyeing
- Appendix D: Estimated Exposures to 3,3′-DMB from Polyamide Cooking Utensils
- Appendix E: Estimates of Exposure to Acid Red 97 from Textile and Leather Products
- Appendix F: Benchmark Dose Calculations for 3,3′-DMOB·2HCl
- Appendix G: Benchmark Dose Calculations for 3,3′-DMB·2HCl
- Appendix H: Benzidine Derivatives and Benzidine-based Substances with Human Health Effects of Concern
- Back to the Screening Assessment - Synopsis
- Back to the Screening Assessment - Part 1
- Back to the Screening Assessment - Part 2
Appendix A: Supplementary Data Tables
| CAS RN | Chemical structure | Chemical formula (molecular weight in g/mol) |
|---|---|---|
| 3701-40-4 | 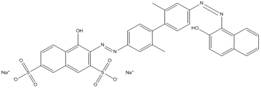 |
C34H24N4O8S2Na2 (726.69) |
| 6358-57-2 | 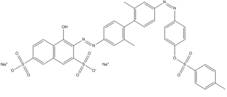 |
C37H30N4O10S3, 2Na (830.82) |
| 6459-94-5 |  |
C37h38N4O10S3, 2Na (830.82) |
| 6470-20-8 |  |
C32H22N6O8S2Na2 (728.67) |
| 6548-30-7 | 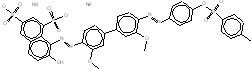 |
C37h38N4012S3Na2 (862.81) |
| 68318-35-4 |  |
C36H26N7012S3Na3 (913.80) |
| 68400-36-2 | 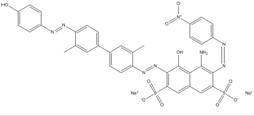 |
C36H26N8O10S2Na2 (840.75) |
| 83221-63-0 | 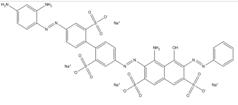 |
C34H26N9O13S4Na (919.87) |
| 89923-60-4 | 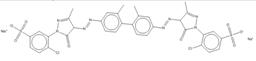 |
C34H26Cl2N8O8S2Na2 (855.64) |
| 10169-02-5 |  |
C32H20N4O8S2Na2 (698.64) |
| CAS RN | Chemical structure | Chemical formula (molecular weight in g/mol) |
|---|---|---|
| 72-57-1 | 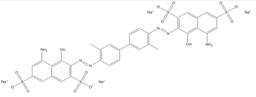 |
C34N6O14S4Na4 (960.80) |
| 573-58-0 | 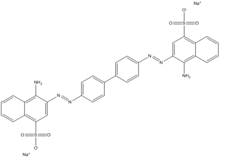 |
C32H22N6O6S2Na2 (696.67) |
| 992-59-6 | 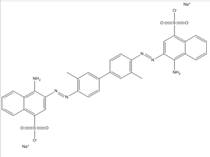 |
C34H26N6O6S2Na2 (724.72) |
| 1937-37-7 | 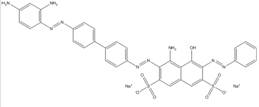 |
C34H27N9O7S2 (737.77) |
| 2150-54-1 |  |
C34H22N4O8S2Na4 (770.65) |
| 2429-71-2 | 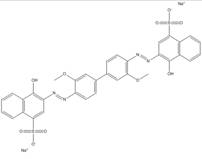 |
C34N4O9S2Na2 (742.69) |
| 2429-74-5 | 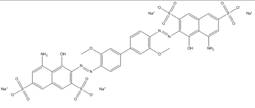 |
C34h38N5O10S2Na4 (922.75) |
| 6420-06-0 | 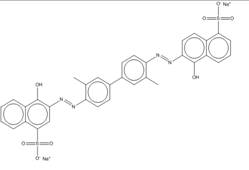 |
C34N4O8S2Na2 (726.69) |
| 6420-22-0 |  |
C34H25N6O11S3Na3 (858.76) |
| 6449-35-0 | 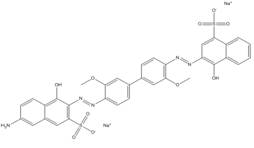 |
C34H25N5O10S2Na2 (773.70) |
| 6548-29-4 | 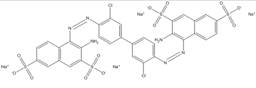 |
C32H20CL2N6O6S2Na2 (765.56) |
| 6655-95-4 | 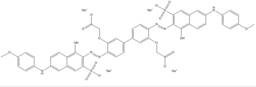 |
C50H36N6O16S2Na4 (1132.95) |
| 67923-89-1 | 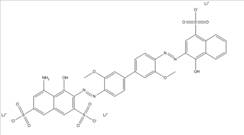 |
C34N5O13S3Li3 (827.60) |
| 70210-28-5 | 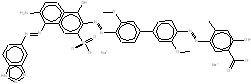 |
C38h38N10O9SNa2 (846.75) |
| 71215-83-3 | 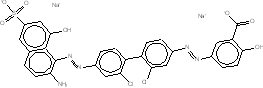 |
C29h27Cl2N5O7SNa2 (696.43) |
| 71550-22-6 | 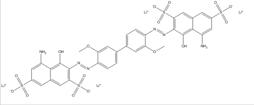 |
C34N6O16S4Li4 (928.60) |
| 72252-59-6 |  |
C47H31N9O16S2Na4 (1133.90) |
| 75659-72-2 |  |
C34N6O16S4Na3Li (976.75) |
| 75659-73-3 | 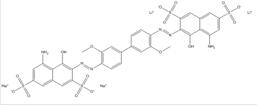 |
C34N6O16S4Na2Li2 (960.70) |
| 75673-18-6 | 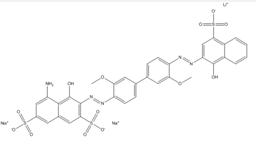 |
C34H25N5O13S3Na2 (860.76) |
| 75673-19-7 | 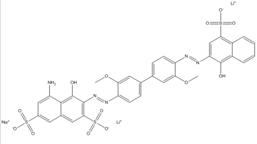 |
C34H26N5O13S3Na (831.78) |
| 75673-34-6 | 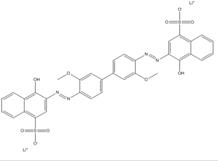 |
C34N4O10S2Li2 (726.59) |
| 75673-35-7 | 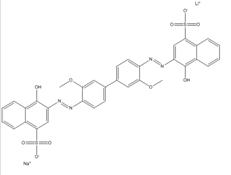 |
C34N4O10S2NaLi (742.64) |
| 75752-17-9 |  |
C34N6O16S4NaLi3 (944.65) |
| 16071-86-6 | 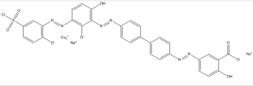 |
C31h28N6O9SNa2Cu (760.11) |
| CAS RN | Chemical structure | Chemical formula (molecular weight in g/mol) |
|---|---|---|
| 298-83-9 |  |
C40H30Cl2N10O6 (817.65) |
| 1871-22-3 | 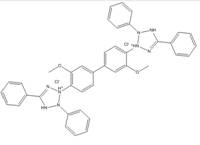 |
C40H30N8O2Cl2 (654.74) |
| CAS RN | Property | Value | Reference |
|---|---|---|---|
| Acid Red 111 | Physical state | Red Powder (formulation of Lanasyn Scarlet F-3GL 103) | Study Submission 2007 |
| Acid Red 111 | Melting point (°C) | 170–190 (formulation of Lanasyn Scarlet F-3GL 103) | Study Submission 2007 |
| Acid Red 111 | Density (kg/m3) | 390 | Study Submission 2007 |
| Acid Red 111 | Water solubility (mg/L) | 65 000 | SMS Technology Co., Ltd. 2012 |
| Acid Red 111 | Water solubility (mg/L) | 25 000 (at 80°C) | Study Submission 2007 |
| Acid Red 114 | Melting point (°C) | 185 | MITI 1992 |
| Acid Red 114 | Water solubility (mg/L) | greater than 500 | MITI 1992 |
| Acid Yellow 23 (read-across for log Kow) | Melting point (°C) | greater than 300 | Acros Organics 2006 |
| Acid Yellow 23 (read-across for log Kow) | Water solubility (mg/L) |
200 000 | Marmion 1991 |
| Acid Yellow 23 (read-across for log Kow) | Water solubility (mg/L) |
300 000 | Green 1990 |
| Acid Yellow 23 (read-across for log Kow) | Water solubility (mg/L) |
greater than 2% | MITI 1992 |
| Acid Yellow 23 (read-across for log Kow) | Log Kow | −0.017 | CITI 1992 |
| Acid Yellow 36 (read-across for log Kow) | Water solubility | Soluble | Ricca Chemical Co. 2008; Acros Organics 2009a |
| Acid Yellow 36 (read-across for log Kow) | Log Kow | 0.7 | Tonogai et al. 1982 |
| Acid Orange 7 (read-across for log Kow) |
Melting point (°C) | 164 | Acros Organics 2009b |
| Acid Orange 7 (read-across for log Kow) |
Log Kow | 0.57 | Tonogai et al. 1982 |
| Acid Orange 7 (read-across for log Kow) |
Water solubility (mg/L) |
116 000 | Acros Organics 2009b |
| Acid Orange 7 (read-across for log Kow) |
Water solubility (mg/L) |
50 000 | Merck Index 1989 |
| CAS RN | Property | Value | Reference |
|---|---|---|---|
| Direct Blue 14 | Physical state | Bluish-grey powdered solid | ChemicalBook 2008a |
| Direct Blue 14 | Melting point (°C) | greater than 300 (decomposes) | ChemicalBook 2008a |
| Direct Blue 14 | Melting point (°C) | greater than 300 (decomposes) | CHRIP ©2002-2012 |
| Direct Blue 14 | Melting point (°C) | 300 | Øllgaard et al. 1998 |
| Direct Blue 14 | Water solubility (mg/L) | 20 000 | CHRIP ©2002-2012 |
| Direct Blue 14 | Water solubility (mg/L) | 10 000 | ChemicalBook 2008a |
| Direct Black 38 | Melting point (°C) | 109–110 | ChemicalBook 2008b |
| Direct Black 38 | Water solubility (mg/L) | 93 000 | Isik and Sponza 2004 |
| Direct Red 28 | Physical state | Brown-red powder | ChemicalBook 2008c |
| Direct Red 28 | Melting point (°C) | greater than 360 | ChemicalBook 2008c; Alfa Aesar ©2011 |
| Direct Red 28 | Density (kg/m3) | 995 | ChemicalBook 2008c |
| Direct Red 28 | Log Kow | 0.77 | Tonogai et al. 1982 |
| Direct Red 28 | Water solubility (mg/L) | 116 000 | Dehn 1917 |
| Direct Brown 95 | Physical state | Dark brown microcrystals or charcoal black powder | ChemicalBook 2008d |
| Direct Blue 15 | Physical state | Deep purple to dark blue microcrystalline powder | ChemicalBook 2008e |
| Direct Blue 15 | Water solubility (mg/L) | 30 000 | Brown 1992 |
| Direct Red 2 | Melting point (°C) | ~290 (decomposes) | Chemexper 2012 |
| Direct Blue 8 | Physical state | Bluish-black powder | ChemicalBook 2008f |
| Direct Violet 28 | Physical state | Bluish-black powder | ChemicalBook 2008g |
| Direct Blue 151 | Physical state | Bluish-black powder | ChemicalBook 2008h |
| CAS RN | Property | Value | Reference |
|---|---|---|---|
| TDBD | Physical state | Yellow crystalline solid | ChemicalBook 2008i |
| TDBD | Melting point (°C) | 255 | ChemicalBook 2008i |
| TDBD | Melting point (°C) | ~190 | Alfa Aesar ©2011 |
| TDBD | Water solubility (mg/L) | 9000 | Green 1990 |
| TDBPD | Physical state | Yellow crystals | ChemicalBook 2008j |
| TDBPD | Melting point (°C) | 189 | Sigma-Aldrich 2012a |
| TDBPD | Melting point (°C) | 200 | Chemical Book 2008j |
| TDBPD | Water solubility (mg/L) | 10 000 | Green 1990 |
| Basic Dyes | Log Kow | Low | Øllgaard et al. 1998 |
| CAS RN | Property | Value | Reference |
|---|---|---|---|
| Naphthol AS-BR | Melting point (°C) | 246 | MPBPWIN 2010 |
| Naphthol AS-BR | Melting point (°C) | 350 | MPBPWIN 2010 |
| Naphthol AS-BR | Boiling point (ºC) | 927.49 | MPBPWIN 2010 |
| Naphthol AS-BR | Vapour pressure (Pa) | 7.7 × 10−25 | MPBPWIN 2010 |
| Naphthol AS-BR | Henry’s Law constant (Pa·m3/mol) | 1.96 × 10−15 | HENRYWIN 2011 |
| Naphthol AS-BR | Log Kow | 7.75 | KOWWIN 2010 |
| Naphthol AS-BR | Log Koc | 1.43 × 105 (MCI method) | KOCWIN 2010 |
| Naphthol AS-BR | Log Koc | 8.27 × 105 (Kow method) | |
| Naphthol AS-BR | Log Koa | 25.853 | KOAWIN 2010 |
| Naphthol AS-BR | Water solubility (mg/L) | 8.97 × 10−6 | WSKOWWIN 2010 |
| Naphthol AS-BR | Water solubility (mg/L) | 1.44 × 10−5 | WATERNT 2010 |
| TCDB | Melting point (°C) | 250.21 | MPBPWIN 2010 |
| TCDB | Boiling point (ºC) | 580.51 | MPBPWIN 2010 |
| TCDB | Vapour pressure (Pa) | 1.12 × 10−10 | MPBPWIN 2010 |
| TCDB | Henry’s Law constant (Pa·m3/mol) | 5.81 × 10−9 | HENRYWIN 2011 |
| TCDB | Log Kow | 5.13 | KOWWIN 2010 |
| TCDB | Log Koc | 2.2 (MCI method) | KOCWIN 2010 |
| TCDB | Log Koc | 5.47 (Kow method) | |
| TCDB | Log Koa | 16.760 | KOAWIN 2010 |
| TCDB | Water solubility (mg/L) | 0.2588 | WSKOWWIN 2010 |
| TCDB | Water solubility (mg/L) | 32.801 | WATERNT 2010 |
| Chemical | Property | Value or range | Reference |
|---|---|---|---|
| 3,3′-DMB | Physical state | Light brown powder | Sigma-Aldrich 2012b |
| 3,3′-DMB | Melting point (°C) | 128–132 | Alfa Aesar ©2011 |
| 3,3′-DMB | Melting point (°C) | 131.5 | PhysProp 2006 |
| 3,3′-DMB | Melting point (°C) | 129–131 | Merck Index 2006 |
| 3,3′-DMB | Melting point (°C) | 147.85 | MPBPWIN 2010 |
| 3,3′-DMB | Boiling point (ºC) | 200 | ACGIH 1986 |
| 3,3′-DMB | Boiling point (ºC) | 339 | PhysProp 2006 |
| 3,3′-DMB | Boiling point (ºC) | 300 | Hawley 1981 |
| 3,3′-DMB | Boiling point (ºC) | 393.08 | MPBPVPWIN 2010 |
| 3,3′-DMB | Density (kg/m3) | 1234 | ICSC 1998 |
| 3,3′-DMB | Vapour pressure (Pa) |
9.23 × 10−5 (6.92 × 10−7 mmHg) |
Neely and Blau 1985 |
| 3,3′-DMB | Vapour pressure (Pa) |
2.74 × 10−2 (2.06 × 10−5 mmHg) |
MPBPWIN 2010 |
| 3,3′-DMB | Henry’s Law constant (Pa·m3/mol) |
6.38 × 10−6 (Bond estimation method) 8.21 × 10−6 (Group contribution method) |
HENRYWIN 2011 |
| 3,3′-DMB | Henry’s Law constant | 6.37 × 10−7 (6.29 × 10−11 atm·m3/mol) |
Meylan and Howard 1991 |
| 3,3′-DMB | (Pa·m3/mol) | 2.59 × 10−2 (2.56 × 10−7 atm·m3/mol) (EVA method)Footnote Appendix A Table A8 [a] |
HENRYWIN 2011 |
| 3,3′-DMB | Log Kow | 2.34 | Hansch et al. 1995 |
| 3,3′-DMB | Log Kow | 2.39 | MITI 1992 |
| 3,3′-DMB | Log Kow | 3.02 | KOWWIN 2010 |
| 3,3′-DMB | Log Kow | 2.43 (EVA method)Footnote Appendix A Table A8 [b] | KOWWIN 2010 |
| 3,3′-DMB | Log Koc | 2.17 (from log Kow) 3.50 (from MCI) |
KOCWIN 2010 |
| 3,3′-DMB | Log Koa | 10.93 | KOAWIN 2010 |
| 3,3′-DMB | Water solubility (mg/L) |
50 | MITI 1992 |
| 3,3′-DMB | Water solubility (mg/L) | 1300 | Bowman et al. 1976 |
| 3,3′-DMB | Water solubility (mg/L) | 27.1 | WATERNT 2010 |
| 3,3′-DMB | Water solubility (mg/L) | 134 | MPBPWIN 2010 |
| 3,3′-DMB | Water solubility (mg/L) | 51.263 (EVA method)Footnote Appendix A Table A8 [c] | WATERNT 2010 |
| 3,3′-DMB | pKa | 4.6 | Kawakami et al. 2010 |
| 3,3′-DMB | pKa | pKa1 = 4.5 pKa2 = 3.4–3.5 |
Perrin 1965 |
| 3,3′-DMB | pKa | pKa1 = 3.3 | Kubota and Ezumi 1980 |
| 3,3′-DMB·2HCl | Physical state | Light red powder | Sigma Aldrich 2012c |
| 3,3′-DMB·2HCl | Melting point (°C) | 340 | Sigma Aldrich 2012c |
| 3,3′-DMB·2HCl | Melting point (°C) | 210 | Beilstein 1984 |
| 3,3′-DMB·2HCl | Water solubility (mg/L) | Soluble in water | CHRIP ©2002-2012 |
| 3,3′-DMB·2HCl | Water solubility (mg/L) | 10 000 – 50 000 | ChemBioFinder ©1998–2013 |
| 3,3′-DMOB | Physical state | Beige brown crystalline powder | Acros Organics 2007 |
| 3,3′-DMOB | Melting point (°C) | 137 | Lewis 1997 |
| 3,3′-DMOB | Melting point (°C) | 136–137 | Alfa Aesar ©2011 |
| 3,3′-DMOB | Melting point (°C) | 137–138 | Merck Index 2006 |
| 3,3′-DMOB | Melting point (°C) | 161.6 | MPBPWIN 2010 |
| 3,3′-DMOB | Boiling point (°C) | 356 | SRC 2011 |
| 3,3′-DMOB | Boiling point (°C) | 417.2 | MPBPWIN 2010 |
| 3,3′-DMOB | Vapour pressure (Pa) |
9.45 × 10−4 (7.09 × 10−6 mmHg) |
MPBPWIN 2010 |
| 3,3′-DMOB | Vapour pressure (Pa) |
1.66 × 10−5 (1.25 × 10−7 mmHg) |
Neely and Blau 1985 |
| 3,3′-DMOB | Henry’s Law constant (Pa·m3/mol) |
1.83 × 10−8 (1.81 × 10−13 atm·m3/mol) (Bond estimation method) 4.72 × 10−6 (4.66 × 10−11 atm·m3/mol) (Group contribution method) |
HENRYWIN 2011 |
| 3,3′-DMOB | Henry’s Law constant (Pa·m3/mol) |
4.762 × 10−6 (4.7 × 10−11 atm·m3/mol) |
Meylan and Howard 1991 |
| 3,3′-DMOB | Henry’s Law constant (Pa·m3/mol) |
7.45 × 10−5 (7.35 × 10−10 atm·m3/mol) (EVA method)[a] |
HENRYWIN 2011 |
| 3,3′-DMOB | Log Kow | 1.81 | Debnath and Hansch 1992 |
| 3,3′-DMOB | Log Kow | 2.08 | KOWWIN 2010 |
| 3,3′-DMOB | Log Kow | 1.5 (EVA method)Footnote Appendix A Table A8 [d] | KOWWIN 2010 |
| 3,3′-DMOB | Log Koc | 1.99 (from log Kow) 2.71 (from MCI) |
KOCWIN 2010 |
| 3,3′-DMOB | Log Koa | 13.211 | KOAWIN 2010 |
| 3,3′-DMOB | Water solubility (mg/L) | 60 mg/L at 25°C | Bowman et al. 1976 |
| 3,3′-DMOB | Water solubility (mg/L) | Insoluble | NIOSH 2012 |
| 3,3′-DMOB | Water solubility (mg/L) | Slightly soluble | Chemical Book 2008k |
| 3,3′-DMOB | Water solubility (mg/L) | 77.54 | WATERNT 2010 |
| 3,3′-DMOB | Water solubility (mg/L) | 146.8 (EVA method)e | WATERNT 2010 |
| 3,3′-DMOB | Water solubility (mg/L) | 351 | WSKOWWIN 2010 |
| 3,3′-DMOB | pKa | 4.7 | Kawakami et al. 2010 |
| 3,3′-DMOB | pKa | 4.2 (estimated) | PhysProp 2006 |
| TODI | Physical state | Colourless to pale yellow flakes | Sigma-Aldrich 2012d |
| TODI | Melting point (°C) | 70–72 | Chemical Book 2008l |
| TODI | Melting point (°C) | 70 | Woolrich 1973 |
| TODI | Melting point (°C) | 71 | PhysProp 2006 |
| TODI | Melting point (°C) | 71.7 | ECHA 2012 |
| TODI | Melting point (°C) | 115.98 | MPBPWIN 2010 |
| TODI | Boiling point (°C) |
371–373 | ECHA 2012 |
| TODI | Boiling point | 314 | Kirk-Othmer 1981 |
| TODI | (°C) | 364.35 | MPBPVPWIN 2010 |
| TODI | Density (kg/m3) | 1330 | ECHA 2012 |
| TODI | Density (kg/m3) | 1156 (at 80°C) | Kirk-Othmer 1981 |
| TODI | Vapour pressure (Pa) |
2.95 × 10−3 (2.21 × 10−5 mmHg) |
MPBPWIN 2010 |
| TODI | Henry’s Law constant (Pa·m3/mol) |
NA | NA |
| TODI | Log Kow | NA | NA |
| TODI | Log Koc | NA | NA |
| TODI | Log Koa | 10.466 | KOAWIN 2010 |
| TODI | Water solubility (mg/L) | NA | NA |
| TODI | pKa | Not applicable | Not applicable |
| 4N-TMB | Physical state | Tan-coloured powder | Acros Organics 2008 |
| 4N-TMB | Melting point (°C) | 193–195 | Acros Organics 2008 |
| 4N-TMB | Melting point (°C) | 193 | ChemSpider ©2011 |
| 4N-TMB | Melting point (°C) | 194 | SRC 2011 |
| 4N-TMB | Melting point (°C) | 108.5 | MPBPWIN 2010 |
| 4N-TMB | Boiling point (°C) |
353.7 | MPBPWIN 2010 |
| 4N-TMB | Vapour pressure (Pa) |
2.17 × 10−3 (1.63 × 10−5 mmHg) |
Neely and Blau 1985 |
| 4N-TMB | Vapour pressure (Pa) |
2.41 × 10−4 (1.08 × 10−7 mmHg) |
MPBPWIN 2010 |
| 4N-TMB | Henry’s Law constant (Pa·m3/mol) |
1.06 × 10−2 (Bond estimation method) (1.05 × 10−7 atm·m3/mol) |
HENRYWIN 2011 |
| 4N-TMB | Henry’s Law constant (Pa·m3/mol) |
4.94 × 10−1 (4.88 × 10−6 atm·m3/mol) (EVA method)[a] |
HENRYWIN 2011 |
| 4N-TMB | Log Kow | 4.11 | KOWWIN 2010 |
| 4N-TMB | Log Kow | 3.53 (EVA method)[b] | KOWWIN 2010 |
| 4N-TMB | Log Koc | 3.17 (from MCI) 3.07 (from log Kow) 2.75 (from corrected log Kow) |
KOCWIN 2010 |
| 4N-TMB | Log Koa | 9.48 | KOAWIN 2010 |
| 4N-TMB | Water solubility (mg/L) | 8.23 | Meylan et al. 1996 |
| 4N-TMB | Water solubility (mg/L) | 0.65 | WSKOWWIN 2010 |
| 4N-TMB | Water solubility (mg/L) | 25.85 (EVA method)[d] | WSKOWWIN 2010 |
| 4N-TMB | Water solubility (mg/L) | 17.87 | WATERNT 2010 |
| 4N-TMB | Water solubility (mg/L) | 33.833 (EVA method)[c] | WATERNT 2010 |
| Fate process | Model and model basis | Model result and prediction | Extrapolated half-life (days) |
|---|---|---|---|
| Atmospheric oxidation (air) | AOPWIN 2010Footnote Appendix A Table A9a [b] | t½ = 0.05–1.38 days | less than or equal to 2 |
| Ozone reaction (air) | AOPWIN 2010[b] | N/AFootnote Appendix A Table A9a [c] | N/A |
| Hydrolysis (water) | HYDROWIN 2010[b] | Not in training set | N/A |
| Primary degradation: Biodegradation (aerobic) (water) | BIOWIN 2008[b] Submodel 4: Expert Survey (qualitative results) |
2.15–2.92Footnote Appendix A Table A9a [d] (biodegrades slowly) |
greater than or equal to 182 |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | BIOWIN 2008[b] Submodel 3: Expert Survey (qualitative results) |
0.48–1.55[d] (biodegrades very slowly) |
greater than or equal to 182 |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | BIOWIN 2008[b] Submodel 5: MITI linear probability |
−2.29 to −1.01Footnote Appendix A Table A9a [e] (biodegrades very slowly) |
greater than or equal to 182 |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | BIOWIN 2008[b] Submodel 6: MITI non-linear probability |
0[e] (biodegrades very slowly) |
greater than or equal to 182 |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | DS TOPKAT c2005–2009 Probability |
N/A | |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | CATALOGIC ©2004–2011 % BOD |
% BOD = 0–20 (biodegrades slowly) |
greater than or equal to 182 |
| Fate process | Model and model basis | Model result and prediction | Extrapolated half-life (days) |
|---|---|---|---|
| Atmospheric oxidation (air) | AOPWIN 2010Footnote Appendix A Table A9b [b] | t½ = 0.21–0.71 days | less than or equal to 2 |
| Ozone reaction (air) | AOPWIN 2010[b] | N/AFootnote Appendix A Table A9b [c] | N/A |
| Hydrolysis (water) | HYDROWIN 2010[b] | N/A, not in training set | N/A |
| Primary biodegradation: Biodegradation (aerobic) (water) | BIOWIN 2008[b] Submodel 4: Expert Survey (qualitative results) |
2.29–3.2Footnote Appendix A Table A9b [d] (biodegrades slowly) |
greater than or equal to 182 |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | BIOWIN 2008[b] Submodel 3: Expert Survey (qualitative results) |
0.37–1.37[d] (biodegrades very slowly) |
greater than or equal to 182 |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | BIOWIN 2008[b] Submodel 5: MITI linear probability |
−2.01 to −0.79Footnote Appendix A Table A9b [e] (biodegrades very slowly) |
greater than or equal to 182 |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | BIOWIN 2008[b] Submodel 6: MITI non-linear probability |
0[e] (biodegrades very slowly) |
greater than or equal to 182 |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | DS TOPKAT c2005–2009 Probability |
N/A | |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | CATALOGIC ©2004–2011 % BOD |
% BOD = 0–8 (biodegrades very slowly) |
greater than or equal to 182 |
| Fate process | Model and model basis | Model result and prediction | Extrapolated half-life (days) |
|---|---|---|---|
| Atmospheric oxidation (air) | AOPWIN 2010Footnote Appendix A Table A9c [b] | t½ = 0.143–0.16 days | less than or equal to 2 |
| Ozone reaction (air) | AOPWIN 2010[b] | N/AFootnote Appendix A Table A9c [c] | N/A |
| Hydrolysis (water) | HYDROWIN 2010[b] | N/A, not in training set | N/A |
| Primary biodegradation: Biodegradation (aerobic) (water) | BIOWIN 2008[b] Submodel 4: Expert Survey (qualitative results) |
2.62–3.08Footnote Appendix A Table A9c [d] (biodegrades slowly) |
greater than or equal to 182 |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | BIOWIN 2008[b] Submodel 3: Expert Survey (qualitative results) |
0.98–1.72[d] (biodegrades very slowly) |
greater than or equal to 182 |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | BIOWIN 2008[b] Submodel 5: MITI linear probability |
−1.51 to −0.63Footnote Appendix A Table A9c [e] (biodegrades very slowly) |
greater than or equal to 182 |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | BIOWIN 2008[b] Submodel 6: MITI non-linear probability |
0[e] (biodegrades very slowly) |
greater than or equal to 182 |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | DS TOPKAT c2005–2009 Probability | N/A | |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | CATALOGIC ©2004–2011 % BOD |
% BOD =7 (biodegrades very slowly) |
greater than or equal to 182 |
| Fate process | Model and model basis | Model result and prediction | Extrapolated half-life (days) |
|---|---|---|---|
| Atmospheric oxidation (air) | AOPWIN 2010Footnote Appendix A Table A9d [b] | t½ = 0.08–0.09 days | less than or equal to 2 |
| Ozone reaction (air) | AOPWIN 2010[b] | N/AFootnote Appendix A Table A9d [c] | N/A |
| Hydrolysis (water) | HYDROWIN 2010[b] | N/A, not in training set | N/A |
| Primary biodegradation: Biodegradation (aerobic) (water) | BIOWIN 2008[b] Submodel 4: Expert Survey (qualitative results) |
3.50–3.65Footnote Appendix A Table A9d [d] (may biodegrade fast) |
less than or equal to 182 |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | BIOWIN 2008[b] Submodel 3: Expert Survey (qualitative results) |
1.80–2.31[d] (biodegrades slowly) |
greater than or equal to 182 |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | BIOWIN 2008[b] Submodel 5: MITI linear probability |
−0.11 to 0.11Footnote Appendix A Table A9d [e] (biodegrades very slowly) |
greater than or equal to 182 |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | BIOWIN 2008[b] Submodel 6: MITI non-linear probability |
0–0.01[e] (biodegrades very slowly) |
greater than or equal to 182 |
| Ultimate biodegradation: Biodegradation (aerobic) | DS TOPKAT c2005–2009 Probability | NA | |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | CATALOGIC ©2004–2011 % BOD |
% BOD = 7–26 (biodegrades slowly) |
greater than or equal to 182 |
| Fate process | Model and model basis | Model result and prediction | Extrapolated half-life (days) |
|---|---|---|---|
| Atmospheric oxidation (air) | Meylan and Howard 1993Footnote Appendix A Table A9e [b] (calculated) |
t½ = 0.167–0.25 day (1.3 × 10−10 to 1.9 × 10−10 cm3 molecule – sec) |
less than or equal to 2 |
| Atmospheric oxidation (air) | AOPWIN 2010Footnote Appendix A Table A9e [c] | t½ = 0.052–0.079 day | less than or equal to 2 |
| Ozone reaction (air) | AOPWIN 2010[c] | N/AFootnote Appendix A Table A9e [d] | N/A |
| Hydrolysis (water) (CAS RN 91-97-4) |
HYDROWIN 2010[c] | t½ = less than 10 days (even at low pHs) | N/A |
| Primary biodegradation: Biodegradation (aerobic) (water) | BIOWIN 2008[c] Submodel 4: Expert Survey (qualitative results) |
2.925–3.433Footnote Appendix A Table A9e [e] “may biodegrade fast” |
less than or equal to 182 |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | BIOWIN 2008[c] Submodel 3: Expert Survey (qualitative results) |
2.158–2.31[e] “biodegrades slowly” |
greater than or equal to 182 |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | BIOWIN 2008[c] Submodel 5: MITI linear probability |
−0.105 to 0.111Footnote Appendix A Table A9e [f] “biodegrades very slowly” |
greater than or equal to 182 |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | BIOWIN 2008[c] Submodel 6: MITI non-linear probability |
0.006–0.027[f] “biodegrades very slowly” |
greater than or equal to 182 |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | DS TOPKAT c2005–2009 Probability |
0–0.3[f] “biodegrades slowly” |
greater than or equal to 182 |
| Ultimate biodegradation: Biodegradation (aerobic) (water) | CATALOGIC ©2004–2011 % BOD |
% BOD = 0.6–15.85 “biodegrades slowly” |
greater than or equal to 182 |
| CAS RN | Test organism | Type of test (duration) | Endpoint | Value (mg/L)b | Reference |
|---|---|---|---|---|---|
| 91-97-4 | Rainbow trout Oncorhynchus mykiss |
Acute (96 h) | NOEC | 0.18–0.19 | ECHA 2012 |
| 91-97-4 | Rainbow trout Oncorhynchus mykiss |
Acute (96 h) | LC50 | 0.25 | ECHA 2012 |
| 119-93-7 | Alga Pseudokircheneriella subcapitata |
Chronic (72 h) | NOEC (growth area under the curve) | 0.32 | MITI 2000 |
| 119-93-7 | Alga | Chronic (72 h) | NOEC (growth rate) | 0.45 | MITI 2000 |
| 119-93-7 | Pseudokircheneriella subcapitata | Chronic (72 h) | EC50 (growth area under the curve) | 2 | MITI 2000 |
| 119-93-7 | Alga | Chronic (72 h) | EC50 (growth rate) | 6.3 | MITI 2000 |
| 119-93-7 | Daphnia | Chronic (21 days) | NOEC (reproduction) |
0.16 | Kuhn et al. 1989 |
| 119-93-7 | Daphnia | Acute (24 h) | EC0 (behaviour) | 1.5 | Kuhn 1989 |
| 119-93-7 | Daphnia | Acute (24 h) | EC50 (behaviour) | 3.2 | Kuhn 1989 |
| 119-93-7 | Daphnia | Chronic (21 days) | NOEC | 0.26 | MITI 2000 |
| 119-93-7 | Daphnia | Chronic (21 days) | EC50 | 0.64 | MITI 2000 |
| 119-93-7 | Daphnia | Acute (48 h) | EC50 (immobilization) |
4.5 | MITI 2000 |
| 119-93-7 | Fish Oryzias latipes |
Acute (96 h) | LC50 | 13 | MITI 2000 |
| 119-93-7 | Fish Oryzias latipes |
Acute (48 h) | LC50 | 55.8 | MITI 1992 |
| 119-93-7 | Green alga Desmodesmus subspicatus |
Chronic (72 h) (growth rate) |
NOEC | greater than or equal to 1.5 | ECHA 2012 |
| 119-93-7 | Green alga Desmodesmus subspicatus |
Chronic (72 h) (growth rate) |
EC50 | greater than 1.5 | ECHA 2012 |
| 119-93-7 | Daphnia magna | Chronic (48 h) | NOEC | greater than or equal to 1.2 | ECHA 2012 |
| 119-93-7 | Daphnia magna | Chronic (48 h) | EC50 | greater than 1.2 | ECHA 2012 |
Appendix B: Aquatic PEC Calculations for Benzidine-based Acid and Direct Dyes Used in Textile Dyeing
The method used for the stepwise estimation of the aquatic PECs from the textile wet processing sector is described as follows.
Step 1: Maximum annual quantity of the Benzidine-based Acid or Direct Dyes used by the textile wet processing sector
There are 10 acid dyes in the Benzidine-based Acid Dyes group. Survey data showed that one acid dye was reported in an annual quantity of 100–1000 kg/year, and no quantities were reported for each of the remaining nine acid dyes with the reporting threshold of 100 kg/year. The maximum annual quantity of the Benzidine-based Acid Dyes would then be 1900 kg/year by adding together the upper end of one reported quantity (1000 kg/year) and 9 times the 100 kg/year threshold.
Maximum annual quantity of Benzidine-based Acid Dyes used by the textile sector = 1900 kg/year
There are 25 direct dyes in the Benzidine-based Direct Dyes group. Survey data showed that one direct dye was reported in an annual quantity of 0–100 kg/year, and no report was received for each of the remaining 24 direct dyes with the reporting threshold of 100 kg/year. The maximum annual quantity of the Benzidine-based Direct Dyes would then be 2500 kg/year by adding together the upper end of one reported quantity (100 kg/year) and 24 times the 100 kg/year threshold.
Maximum annual quantity of Benzidine-based Direct Dyes used by the textile sector = 2500 kg/year
Step 2: Maximum annual quantity of the Benzidine-based Acid or Direct Dyes used at one mill
The highest quantity of the Benzidine-based Acid Dyes sold to one single textile mill was 300 kg/year according to industry surveys conducted for the years 2005 and 2006 under Canada Gazette notices issued pursuant to section 71 of CEPA 1999 (Canada 2006b, 2008b). This highest quantity is selected as the maximum quantity of the Benzidine-based Acid Dyes used at any given single mill. No survey data are available on the highest quantity of the Benzidine-based Direct Dyes sold to one single textile mill above the 100 kg/year reporting threshold. Thus, the maximum quantity of the Benzidine-based Direct Dyes used at any given single mill is assumed to be 100 kg/year.
Maximum annual quantity of the Benzidine-based Acid Dyes used at one mill = 300 kg/year
Maximum annual quantity of the Benzidine-based Direct Dyes used at one mill = 100 kg/year
Step 3: Daily use quantity at one mill
The daily use quantity of the Benzidine-based Acid or Direct Dyes at one mill is estimated based on a typical daily quantity of textile dyed and a typical dye use rate. Typically, a dyelot is completed within 6 hours from batch dyeing or 8 hours from continuous dyeing (US EPA 1994). When a mill operates three shifts or 24 hours/day, the maximum number of dyelots completed per day would be four dyelots, as determined for batch dyeing. One dyelot typically consists of 454 kg of textile, so the daily quantity of textile dyed would be 1816 kg/day (454 kg/dyelot × 4 dyelots/day). For a typical dye use rate of 0.02 kg dyes per kilogram of textile (Cai et al. 1999), the daily quantity of the Benzidine-based Acid or Direct Dyes used at one mill is estimated as:
Daily quantity of the Benzidine-based Acid or Direct Dyes used at one mill = 1816 kg/day × 0.02 kg/kg = 36 kg/day
Step 4: Number of annual release days from one mill
The number of annual release days from one mill is assumed to be the same as the number of the annual operation days, since the wastewater resulting from dyeing (spent bath and rinse water) is generally not stored on site and is released to municipal sewers soon after it is generated. The number of annual release days is then estimated as 8.3 days for the Benzidine-based Acid Dyes and 2.8 days for the Benzidine-based Direct Dyes by dividing the maximum annual quantity (300 kg/year for the Benzidine-based Acid Dyes or 100 kg/year for the Benzidine-based Direct Dyes) used at one mill by their daily use quantity (36 kg/day). These values represent the maximum durations for the continuous release of the Benzidine-based Acid or Direct Dyes via wastewater.
Number of annual release days from one mill for the Benzidine-based Acid Dyes = 8.3 days
Number of annual release days from one mill for the Benzidine-based Direct Dyes = 2.8 days
Step 5: Daily release to sewers from one mill
The daily release of the Benzidine-based Acid or Direct Dyes to sewers is estimated based on their respective emission factors to wastewater. On average, the emission factor is 10% for acid dyes and 12% for direct dyes (OECD 2004). The daily release to sewers of the Benzidine-based Acid or Direct Dyes from one mill is then calculated by multiplying the daily use quantity by the emission factor.
Daily release of the Benzidine-based Acid Dyes to sewers from one mill = 36 kg/day × 10% = 3.6 kg/day
Daily release of the Benzidine-based Direct Dyes to sewers from one mill = 36 kg/day × 12% = 4.3 kg/day
These release estimates are based on the assumption of zero removal for on-site wastewater treatment, because specific information is not available on the type of on-site wastewater treatment at each of the mills evaluated. The use of the zero removal assumption yields conservative release estimates.
Step 6: Estimated wastewater influent concentration
The concentration of the Benzidine-based Acid or Direct Dyes in wastewater influent is calculated by dividing the daily release quantity (3.6 kg/day for the Benzidine-based Acid Dyes or 4.3 kg/day for the Benzidine-based Direct Dyes) by the wastewater flow (L/day) of a municipal wastewater treatment system. The wastewater flow varies from location to location. For example,
Wastewater flow in Arthur, ON = 1 041 600 L/day
Wastewater flow in Montréal, QC = 2 786 797 997 L/day
The concentrations of the Benzidine-based Acid or Direct Dyes in wastewater influent at these two locations are determined as:
Wastewater influent concentration for the Benzidine-based Acid Dyes in Arthur, ON
= 3.6 kg/day / 1 041 600 L/day = 3.46 × 10−6 kg/L = 3460 mg/L
Wastewater influent concentration for the Benzidine-based Acid Dyes in Montréal, QC
= 3.6 kg/day / 2 786 797 997 L/day = 1.29 × 10−9 kg/L = 1.29 mg/L
Wastewater influent concentration for the Benzidine-based Direct Dyes in Arthur, ON
= 4.3 kg/day / 1 041 600 L/day = 4.13 × 10−6 kg/L = 4130 mg/L
Wastewater influent concentration for the Benzidine-based Direct Dyes in Montréal, QC
= 4.3 kg/day / 2 786 797 997 L/day = 1.54 × 10−9 kg/L = 1.54 mg/L
Step 7: Removal by off-site wastewater treatment systems
No suitable model was available to estimate the removal of the Benzidine-based Acid or Direct Dyes through wastewater treatment systems. The models used by Environment Canada (e.g., ASTreat 2006; STP 2006) are designed for neutral substances and are not suitable for ionic chemicals. Since both Benzidine-based Acid Dyes and Benzidine-based Direct Dyes are water-soluble anionic compounds (US EPA 1996), they fall outside the domain of applicability for the above-mentioned models.
Literature data are available on the wastewater treatment removal of azo dyes in general and can be used to provide removal estimates for the Benzidine-based Acid or Direct Dyes, since they are azo dyes. In a Danish survey report (Øllgaard et al. 1998), removal rates of 40–80% were found for azo dyes. This removal range is a result of adsorption to sludge alone, without accounting for any additional removal by abiotic or biotic degradation. This range is therefore expected to occur with all three common wastewater treatment types (primary, secondary and lagoons), since all these systems provide sludge removal or settling. As an approximation, an average (60%) of this removal range is selected for the Benzidine-based Acid or Direct Dyes. The average is judged to be more statistically representative than any other value of the different wastewater treatment systems involved and the different individual azo dye substances in the Benzidine-based Acid or Direct Dyes.
Wastewater treatment removal for the Benzidine-based Acid or Direct Dyes = 60%
Step 8: Lagoon dilution
Many textile mills are located in municipalities served by lagoons. These lagoons contain large volumes of water and have long hydraulic retention times. The retention time of a lagoon is measured in weeks to months, according to field data collected through the CMP Monitoring and Surveillance Program at Environment Canada (Smyth 2012). The implication of a long retention time is that a substance entering a lagoon within a relatively short duration is subject to not only removal, but also dilution. As a result, the substance concentration in the lagoon effluent is reduced by both removal and dilution. This is the case with the release of the Benzidine-based Acid or Direct Dyes. The duration of the release within a year was estimated previously as 8.3 days for the Benzidine-based Acid Dyes or 2.8 days for the Benzidine-based Direct Dyes (see Step 4 above). These durations are short compared with a lagoon’s residence time. Dilution is therefore justified. Such dilution is, however, not expected in primary or secondary treatment systems, because their hydraulic retention times are short, typically measured in hours.
No quantitative method is available to determine the degree of lagoon dilution. Nevertheless, the ratio of a lagoon’s retention time to a substance’s release duration can be considered as the maximum dilution, because the ratio is equivalent to the full dilution or the volume ratio of the entire lagoon water to the wastewater containing a specific substance. As an estimate, the lagoon retention time in weeks to months is interpreted as 42 days (6 weeks) to 84 days (12 weeks). The full dilution is then determined to be 5- to 10-fold for the Benzidine-based Acid Dyes or 15- to 30-fold for the Benzidine-based Direct Dyes by dividing the retention time (42–84 days) by the release duration (8.3 days for the Benzidine-based Acid Dyes or 2.8 days for the Benzidine-based Direct Dyes). As an approximation, an average is selected from each range for lagoon dilution, 7.5-fold for the Benzidine-based Acid Dyes and 22.5-fold for the Benzidine-based Direct Dyes.
Lagoon dilution for the release of the Benzidine-based Acid Dyes = 7.5
Lagoon dilution for the release of the Benzidine-based Direct Dyes = 22.5
Step 9: Wastewater effluent concentration
The concentration of the Benzidine-based Acid or Direct Dyes in wastewater effluent is determined by applying the wastewater treatment removal to the influent concentration. Dilution is also considered for lagoons. For example, the wastewater from a mill in Montréal, QC, is discharged to a primary system, and only the 60% removal is used to estimate the effluent concentration.
Wastewater effluent concentration for the Benzidine-based Acid Dyes in Montréal, QC
= influent concentration × (1 − removal)
= 1.29 µg/L × (1 − 60%) = 0.52 µg/L
Wastewater effluent concentration for the Benzidine-based Direct Dyes in Montréal, QC
= influent concentration × (1 − removal)
= 1.54 µg/L × (1 − 60%) = 0.62 µg/L
For a mill in Arthur, ON, the mill wastewater is discharged to a lagoon, and the concentration of the Benzidine-based Acid or Direct dyes in the effluent is estimated as:
Wastewater effluent concentration for the Benzidine-based Acid Dyes in Arthur, ON
= influent concentration × (1 − removal) / lagoon dilution for the Benzidine-based Acid Dyes
= 3460 µg/L × (1 − 60%) / 7.5 = 185 µg/L
Wastewater effluent concentration for the Benzidine-based Direct Dyes in Arthur, ON
= influent concentration × (1 − removal) / lagoon dilution for the Benzidine-based Direct Dyes
= 4130 µg/L × (1 − 60%) / 22.5 = 73.4 µg/L
Step 10: Predicted aquatic environmental concentration
The predicted aquatic environmental concentration (aquatic PEC) is determined by applying the receiving water dilution to the effluent concentration. Since the aquatic PEC is assessed near the discharge point, the receiving water dilution selected should also be applicable to this condition. The full dilution potential of a river is considered appropriate if it is between 1 and 10. Otherwise, the dilution is kept at 10 for both large rivers and still waters.
For the wastewater treatment system (a lagoon) in Arthur, ON, the receiving water is the Conestogo River, and its dilution potential is determined to be 7.64 (ratio of the 10th percentile river flow 7 957 160 L/day to the wastewater effluent flow 1 041 600 L/day). The aquatic PEC for the Benzidine-based Acid or Direct Dyes at the site of Arthur, ON, is then estimated as:
Aquatic PEC for the Benzidine-based Acid Dyes at site of Arthur, ON
= Wastewater effluent concentration / Receiving water dilution
= 185 µg/L / 7.64 = 24.2 µg/L
Aquatic PEC for the Benzidine-based Direct Dyes at site of Arthur, ON
= Wastewater effluent concentration / Receiving water dilution
= 73.4 µg/L / 7.64 = 9.6 µg/L
For the wastewater treatment system (primary) in Montréal, QC, the receiving water, the St. Lawrence River, has a very large flow, so the dilution is limited to 10 near the discharge point. The aquatic PEC for the Benzidine-based Acid or Direct Dyes at the site of Montréal, QC, is then estimated as:
Aquatic PEC for the Benzidine-based Acid Dyes at site of Montréal, QC
= Wastewater effluent concentration / Receiving water dilution
= 0.52 µg/L / 10 = 0.052 µg/L
Aquatic PEC for the Benzidine-based Direct Dyes at site of Montréal, QC
= Wastewater effluent concentration / Receiving water dilution
= 0.62 µg/L / 10 = 0.062 µg/L
Although there are sites where multiple textile mills are identified to discharge to one single wastewater treatment system, the chance of more than one mill at any of these sites using and releasing the same acid or direct dyes is expected to be low. This is because mills are operated year-round, while the release from one single mill occurs only for 8.3 days for the Benzidine-based Acid Dyes and 2.8 days for the Benzidine-based Direct Dyes. The release overlapping within these short periods is therefore a low possibility. As a result, the aquatic PEC resulting from each single mill can be considered to reflect the level of exposure near the discharge point, although there are two or more mills identified at a site.
The aquatic PECs calculated for the Benzidine-based Acid and Direct Dyes are summarized in Table 12 in the section on Characterization of Ecological Risk.
Appendix C: Soil PEC Calculations for Benzidine-based Acid and Direct Dyes Used in Textile Dyeing
The method used for the stepwise estimation of the soil PECs from the textile wet processing sector and biosolids application is described as follows.
Step 1: Biosolids quantity
The quantity of biosolids produced from the wastewater treatment systems at the 33 sites evaluated for the aquatic exposure can be approximately assumed to equal the quantity of sludge generated. The quantity of sludge generated can be estimated from the per capita sludge production rate and the population served by the wastewater treatment systems. The per capita sludge production rate is reported as 0.090 kg/day per person from primary treatment and 0.115 kg/day per person from secondary treatment (Droste 1997). The combined population served by the wastewater treatment systems at the 33 sites is determined to be 5 661 000 persons based on the population served by each individual treatment system. This combined population is broken down into 1 810 000 persons serviced by primary treatment and 3 851 000 persons serviced by secondary treatment. The quantity of sludge generated or the quantity of biosolids produced is then estimated as:
Biosolids quantity = 0.090 kg/day per person × 1 810 000 persons + 0.115 kg/day per person × 3 851 000 persons = 605 765 kg/day = 221 104 000 kg/year
Step 2: Quantity of Benzidine-based Acid or Direct Dyes in biosolids
The quantity of Benzidine-based Acid or Direct Dyes in biosolids is estimated based on the maximum quantity of Benzidine-based Acid or Direct Dyes used for textile dyeing and the removal efficiency by wastewater treatment. The maximum quantity used for textile dyeing was estimated previously as 1900 kg/year for Benzidine-based Acid Dyes and 2500 kg/year for Benzidine-based Direct Dyes. The wastewater treatment removal by sludge sorption in the range of 40–80%, as reported for azo dyes by the Danish Environmental Protection Agency (Øllgaard et al. 1998), is considered applicable to both Benzidine-based Acid and Direct Dyes. An average removal rate of 60% is judged to be statistically representative of a large number of wastewater treatment operations across the sites of the 75 mills involving different treatment types and different individual azo dye substances. This removal rate is therefore used to estimate the quantity of Benzidine-based Acid or Direct Dyes in biosolids.
Quantity of Benzidine-based Acid Dyes in biosolids = 1900 kg/year × 60% = 1140 kg/year
Quantity of Benzidine-based Direct Dyes in biosolids = 2500 kg/year × 60% = 1500 kg/year
These estimated quantities are conservative, since they are not corrected for the amounts released to lagoons. In general, lagoons do not produce biosolids, and the amounts released to lagoons therefore do not end up in biosolids.
Step 3: Concentration of Benzidine-based Acid or Direct Dyes in biosolids
The concentration of the Benzidine-based Acid or Direct Dyes in biosolids is calculated by dividing the quantity in biosolids by the quantity of biosolids produced.
Concentration of Benzidine-based Acid Dyes in biosolids
= 1140 kg/year / 221 104 000 kg/year = 0.000 005 2 kg/kg = 5.2 mg/kg
Concentration of Benzidine-based Direct Dyes in biosolids
= 1500 kg/day / 221 104 000 kg/day = 0.000 006 8 kg/kg = 6.8 mg/kg
Step 4: Land application rate
The land application rate of municipal wastewater sludge (or biosolids) is regulated by the provinces and territories. The allowable annual limits on a dry weight basis are 1.6 tonnes/ha in Ontario, 3.4 tonnes/ha in British Columbia, 4.4 tonnes/ha in Quebec and 8.3 tonnes/ha in Alberta (Crechem 2005). The limit in Alberta is the highest in Canada and is used for soil exposure calculations.
Annual land application rate = 8.3 tonnes/ha = 0.83 kg/m2
Step 5: Quantity of Benzidine-based Acid or Direct Dyes over 10 years of biosolids application
The European Chemicals Agency (ECHA 2010) suggests using 10 consecutive years as a length of accumulation in evaluating soil exposure resulting from biosolids application. The quantity of the Benzidine-based Acid or Direct Dyes received per square metre of the amended soil during this 10-year period would be:
Quantity of Benzidine-based Acid Dyes per square metre of soil
= biosolids application rate × 10 years × concentration of Benzidine-based Acid Dyes in biosolids
= 0.83 kg/m2 per year × 10 years × 5.2 mg/kg = 43.2 mg/m2
Quantity of Benzidine-based Direct Dyes per square metre of soil
= biosolids application rate × 10 years × concentration of Benzidine-base Direct Dyes in biosolids
= 0.83 kg/m2 per year × 10 years × 6.8 mg/kg = 56.4 mg/m2
Step 6: Mass of ploughing-layer soil per square metre
The European Chemicals Agency (ECHA 2010) also suggests using 20 cm (i.e., 0.2 m) as the ploughing depth in determining a mixing layer. Using a dry soil density of 1200 kg/m3 (Williams 1999), the mass of the top 20 cm soil layer per square metre is:
Mass of ploughing layer per 1 m2 = 1200 kg/m3 × 1 m2 × 0.2 m = 240 kg/m2
Step 7: Soil PEC
The soil PEC is determined by dividing the quantity of the Benzidine-based Acid or Direct Dyes upon 10-year land application by the mass of ploughing-layer soil on a per square metre basis.
Soil PEC for Benzidine-based Acid Dyes = 43.2 mg/m2 / 240 kg/m2 = 0.18 mg/kg
Soil PEC for Benzidine-based Direct Dyes = 56.4 mg/m2 / 240 kg/m2 = 0.24 mg/kg
Appendix D: Estimated Exposures to 3,3′-DMB from Polyamide Cooking Utensils
Exposures to 3,3′-DMB from use of black polyamide cooking utensils were estimated, based on information indicating that this substance can leach from the utensil to soup or sauce during use. Estimated exposures are based on the following assumptions: that an individual uses a polyamide black cooking utensil every day, that the leaching of 3,3′-DMB remains constant over multiple uses and that the utensil remains in the hot soup or sauce (while cooking) for a long period of time. Estimated daily intakes were derived using a detailed intake of foods (Health Canada 1998) and the median leaching level of 3,3′-DMB (based on the third extraction levels, using the LOD for non-detect utensils and an average volume:area ratio when not indicated) calculated from the Danish study (McCall et al. 2012).
Estimates are considered to be conservative, as leaching test conditions (3% volume per volume [v/v] aqueous acetic acid, 100°C, 30 minutes to 4 hours) are not truly representative of real use conditions; it is unlikely that all soups or sauces will be stirred continually for the entire duration of this length of time or at this temperature. As shown in the study, the concentration leaching out of these utensils is highly variable.
Estimated intake from a food item = [Chemical in food (µg/g) × Consumption (g/day)] / Body weight
3,3′-DMB in food (median leaching level):
3,3′-DMB in food = 1.4 µg/kg
Body weights (Health Canada 1998):
Infant (0–6 months): 7.5 kg
Toddler (0.5–4 years): 15.5 kg
Child (5–11 years): 21.0 kg
Teenager (12–19 years): 59.4 kg
Adult (20–59 years): 70.9 kg
Senior (60+ years): 72.0 kg
Conservative estimates of daily intakes of 3,3′-DMB from use of black polyamide cooking utensils are presented in Table D-1.
Table D-1. Consumption and estimated daily intakes of 3,3′-DMB from use of black polyamide cooking utensils
| Food item | 0–6 months: Consumption (g/day) | 0–6 months: Intake (µg/kg-bw per day) |
0.5–4 years: Consumption (g/day) | 0.5–4 years: Intake (µg/kg-bw per day) |
|---|---|---|---|---|
| Soups, meat, canned | 5.36 | 0.0010 | 41.64 | 0.0037 |
| Soups, vegetable | 4.97 | 0.0009 | 8.16 | 0.0007 |
| Soups, tomato | 1.91 | 0.0004 | 6.50 | 0.0006 |
| Soups, dehydrated | 0.33 | 0.0001 | 10.43 | 0.0009 |
| Sauces and gravies | 0.68 | 0.0001 | 5.64 | 0.0005 |
| Total | 13.24 | 0.0025 | 72.38 | 0.0065 |
| Food item | 5–11 years: Consumption (g/day) | 5–11 years: Intake (µg/kg-bw per day) |
12–19 years: Consumption (g/day) | 12–19 years: Intake (µg/kg-bw per day) |
|---|---|---|---|---|
| Soups, meat, canned | 41.76 | 0.0019 | 35.12 | 0.0008 |
| Soups, vegetable | 10.99 | 0.0005 | 21.88 | 0.0005 |
| Soups, tomato | 11.67 | 0.0005 | 6.95 | 0.0002 |
| Soups, dehydrated | 7.98 | 0.0004 | 7.91 | 0.0002 |
| Sauces and gravies | 8.98 | 0.0004 | 14.29 | 0.0003 |
| Total | 81.38 | 0.0036 | 86.15 | 0.0020 |
| Food item | 20–59 years: Consumption (g/day) | 20–59 years: Intake (µg/kg-bw per day) |
60+ years: Consumption (g/day) | 60+ years: Intake (µg/kg-bw per day) |
|---|---|---|---|---|
| Soups, meat, canned | 55.29 | 0.0011 | 54.16 | 0.0010 |
| Soups, vegetable | 15.03 | 0.0003 | 18.17 | 0.0004 |
| Soups, tomato | 6.92 | 0.0001 | 7.93 | 0.0002 |
| Soups, dehydrated | 8.33 | 0.0002 | 5.70 | 0.0001 |
| Sauces and gravies | 14.82 | 0.0003 | 10.76 | 0.0002 |
| Total | 100.40 | 0.0020 | 96.72 | 0.0019 |
Appendix E: Estimates of Exposure to Acid Red 97 from Textile and Leather Products
| Product scenario | Daily exposure (mg/kg-bw per day) |
|---|---|
| Textiles; personal apparel (adult; dermal) | 0.002 6 |
| Textiles; baby sleeper (infant; dermal) | 0.004 0 |
| Textiles (infant; oral) | 2.7 × 10−5 |
Dermal Exposure from Textile
Exposure estimate = [SA × AW × SCF × C × M × DA × F × P] / BW
Dermal exposure was estimated based on a scenario of full (100%) body coverage from wearing clothing to account for exposures from multiple pieces of apparel that cover the entire surface area of the body.
Oral Exposure from Textile
Exposure estimate = [SA × AW × SCF × C × M × F × P] / BW
Oral exposure to Acid Red 97 is estimated based on a scenario assuming that the infant is mouthing a textile object (e.g., blanket, textile toy) that may release Acid Red 97.
Parameters
SA: Total surface area = 18 200 cm2 (dermal; adult; personal apparel) and 3020 cm2 (dermal; infant; baby sleeper) (Health Canada 1998); 20 cm2 (oral; infant Zeilmaker et al. 2000).
AW: Area weight of textile = 20 mg/cm2 (US EPA 2012).
SCF: Skin contact factor = 1.
C: Concentration = 0.01 (unitless) (BfR 2007). Based on the default model developed by the “Textiles” Working Group established at the German Federal Institute for Risk Assessment (BfR 2007), assuming that a standard textile garment of 100 g/m2 is dyed with 1% active dye ingredient.
M: Migration fraction = 0.0005 (BfR 2007). The migration of azo dyes from textiles varies considerably depending on the type of fibre, the type of dye used, the dye load, dyeing technology and colour intensity and after treatment. The exposure from textiles is partly dictated by the amount of dye that migrates from textile material onto human skin (ETAD 1983) or via mouthing. The “Textiles” Working Group (BfR 2007) uses a peak initial migration of 0.5% to estimate exposure to dyes from newly bought unwashed garments, and the chronic migration rate is assumed to be one tenth of the value measured for the first migration to reflect exposure after initial washes. It is assumed that the sweat migration rate is similar to the salivary migration rate; this is consistent with observations of leaching behaviours of dyes from textiles reported by Zeilmaker et al. (1999). Accordingly, the fraction of dye that migrates from a textile material per wear is assumed to be 0.0005 for both dermal and oral exposure.
DA: Dermal absorption = 100%.
F: Frequency = 1×/day.
P: Probability that Acid Red 97 is present in textiles = 10%. In the RIVM risk assessment of azo dyes and aromatic amines from garments and footwear (Zeilmaker et al. 1999), the authors derived a chance of 8% for the appearance of carcinogenic azo dyes and aromatic amines in garments based on four European studies. The congener of Acid Red 97 is not an EU22 amine; the prevalence of this dye is not clear because there is limited product testing and monitoring on non-EU22 amines and associated dyes. From the limited data available (Danish EPA 1998; Brüschweiler et al. 2014), the detection of most non-EU22 amines in textiles is usually less than 10%. Accordingly, the presence of associated dyes in textiles would be the same or lower. The chances of an individual’s outfit containing Acid Red 97 every day are low. Given the conservatism used in other parameters in this exposure scenario (e.g. full body coverage), the probability that Acid Red 97 is present in a textile is assumed to be 10% in this screening assessment based on professional judgement.
BW: Body weight = 7.5 kg for infant, 70.9 kg for adult (Health Canada 1998).
| Product scenario | Per event exposure (mg/kg-bw) |
|---|---|
| Shoes | 5.8 × 10−2 |
| Boots | 1.9 × 10−2 |
| Gloves | 2.1 × 10−3 |
| Jackets and coats | 7.7 × 10−2 |
| Trousers | 5.0 × 10−2 |
| Furniture | 2.3 × 10−2 |
| Toys | 4.0 × 10−2 |
Dermal Exposure from Leather
Exposure estimate = [SA × AW × SCF × C × M × DA] / BW
Direct skin contact with articles of leather can result in dermal exposure to dyes used in leather dyeing. Of all the leather products considered, the potential drivers for exposure are presented below; furniture, apparel (e.g., jackets, trousers and gloves), footwear (e.g., shoes and boots) and toys, where it is assumed that direct contact with the infant’s palms can occur when playing with the toy. The exposure estimates presented below are considered upper-bounding based on conservative assumptions as well as not taking into account of a final application of a polyurethane sealant coating which would further reduce the consumer’s dermal exposure to the leather dye.
Parameters
SA: Surface area of skin contact (Health Canada 1998; Therapeutic Guidelines Ltd. 2008)
- Shoes: 1275 cm2 (adult feet)
- Boots: 4185 cm2 (adult legs and feet)
- Gloves: 455 cm2 (adult hands)
- Jackets and coats: 8920 cm2 (adult trunk and arms)
- Trousers: 5820 cm2 (adult lower body)
- Furniture: 5005 cm2 (adult back, buttocks and back of thighs)
- Toys: 92.5 cm2 (infant palms)
AW: Area weight of leather = 0.15 g/cm2 (Danish EPA 2012)
SCF: Skin contact factor
- Shoes: 1
- Boots: 0.1
- Gloves: 0.1
- Jackets and coats: 0.19
- Trousers: 0.19
- Furniture: 0.1
- Toys: 1
When the entire leather article is in direct contact with the skin, SCF is assumed to be 1. When the leather article is in indirect contact with the skin (e.g., shielding due to interior lining), SCF is assumed to be 0.1, which is a default value used to account for exposure due to diffusion of sweat-extracted dye from the leather material through the shielding fabric onto the skin (Zeilmaker et al. 1999). When a portion of the leather article is in direct contact and the remaining portion is in indirect contact, a weighted SCF is calculated: [(SAdirect × 1) + (SAindirect × 0.1)]/(SAtotal).
C: Concentration = 0.02 (unitless weight fraction) (Øllgaard et al. 1998)
M: Migration fraction = 0.1% (i.e., 39% over 365 days).
The dermal exposure to dyes from leather is partly dictated by the amount of dye that migrates from leather material onto human skin. Zeilmaker et al. (1999) measured the experimental leaching of azo dyes from leather footwear material to be 15% and 39%. The leaching was determined by extracting from 1 g of unwashed material from the upper side of a newly bought leather shoe with 100 mL sweat stimulant (extraction conditions: 16 hours at 37°C while shaking). These extraction conditions are expected to overestimate the migration of dyes from sweat. In estimating exposure to dyes from leather articles, it is assumed that 39% of the dye content leaches over one year and is available for dermal exposure, which would be equivalent to 0.1% leaching in one day.
DA: Dermal absorption = 100%.
BW: Body weight = 7.5 kg for infant, 70.9 kg for adult (Health Canada 1998).
Appendix F: Benchmark Dose Calculations for 3,3′-DMOB·2HCl
| Tumours | 0 ppm | 80 ppm | 170 ppm | 330 ppm |
|---|---|---|---|---|
| Equivalent dose for male rats (mg/kg-bw per day) | 0 | 6 | 12 | 21 |
| Skin basal cell or sebaceous gland neoplasms | 2/59 | 33/44 | 56/72 | 41/56 |
| Skin squamous cell neoplasms | 0/59 | 13/42 | 28/65 | 22/48 |
| Zymbal gland neoplasms | 0/58 | 10/45 | 25/75 | 30/60 |
| Preputial gland adenoma or carcinoma | 16/59 | 12/42 | 33/73 | 29/59 |
| Oral papilloma or carcinoma | 1/59 | 8/44 | 10/73 | 11/57 |
| Small intestine neoplasms | 0/59 | 4/44 | 7/75 | 5/60 |
| Large intestine neoplasms | 0/59 | 1/44 | 8/73 | 8/57 |
| Liver neoplasms | 1/58 | 4/39 | 7/54 | 8/35 |
| Mesothelium | 2/59 | 1/44 | 7/72 | 6/56 |
| Equivalent dose for female (mg/kg-bw per day) | 0 | 7 | 14 | 23 |
| Zymbal gland neoplasms | 1/60 | 12/45 | 21/74 | 16/59 |
| Clitoral gland neoplasms | 7/58 | 27/44 | 48/74 | 41/55 |
| Mammary gland adenocarcinomas | 1/60 | 2/45 | 14/73 | 20/57 |
| Tumours | Model name | # of groups | AIC | P-value | SRI | BMR | BMD | BMDL |
|---|---|---|---|---|---|---|---|---|
| MR - Skin basal cell or sebaceous gland neoplasmsFootnote Appendix F Table F2 [b] | LogLogistic | 3 | 148.6 | 0.235 | −0.015 | 0.1 | 0.32 | 0.22 |
| MR - Skin squamous cell neoplasms | Multistage | 4 | 211.2 | 0.518 | 0 | 0.1 | 1.96 | 1.49 |
| MR - Zymbal gland neoplasms | Multistage cancer | 4 | 225.6 | 0.952 | 0 | 0.1 | 2.98 | 2.44 |
| MR - Preputial gland neoplasms | Multistage cancer | 4 | 306.7 | 0.572 | −0.77 | 0.1 | 5.47 | 3.47 |
| MR - Oral cavity neoplasms | LogLogistic | 4 | 174.1 | 0.097 | −0.38 | 0.1 | 9.06 | 5.82 |
| MR - Small intestine neoplasms | LogLogistic | 4 | 113.35 | 0.258 | 0.38 | 0.1 | 15.08 | 9.99 |
| MR - Large intestine neoplasms | Quantal-linear | 4 | 109.3 | 0.811 | 0.63 | 0.1 | 13.63 | 9.37 |
| MR - Liver neoplasms | LogLogistic | 4 | 119.4 | 0.880 | −0.37 | 0.1 | 8.95 | 5.66 |
| MR - Mesothelium | Quantal-linear | 4 | 116.55 | 0.528 | −0.14 | 0.1 | 24.36 | 13.14 |
| FR - Zymbal gland neoplasms | LogLogistic | 4 | 229.4 | 0.045 | 1.9 | 0.1 | 4.74 | 3.44 |
| FR - Clitoral gland neoplasms | LogLogistic | 4 | 265.5 | 0.414 | −0.11 | 0.1 | 0.91 | 0.66 |
| FR - Mammary gland adenocarinomas | LogProbit | 4 | 177.9 | 0.692 | 0.26 | 0.1 | 10.70 | 8.21 |
Appendix G: Benchmark Dose Calculations for 3,3′-DMB·2HCl
| Tumours | 0 ppm | 30 ppm | 70 ppm | 150 ppm |
|---|---|---|---|---|
| Equivalent dose for male rats (mg/kg-bw per day) | 0 | 1.8 | 4.0 | 11.2 |
| Skin basal cell neoplasms | 0/60 | 11/44 | 54/72 | 30/45 |
| Skin sebaceous cell adenoma | 0/60 | 0/44 | 7/72 | 5/49 |
| Skin keratoacanthomas | 1/60 | 1/44 | 8/67 | 5/27 |
| Skin squamous cell neoplasms | 0/60 | 2/45 | 17/74 | 27/59 |
| Zymbal gland neoplasms | 1/60 | 3/45 | 32/74 | 36/60 |
| Preputial gland neoplasms | 2/60 | 4/44 | 6/72 | 9/49 |
| Liver neoplasms | 0/60 | 0/45 | 35/72 | 33/55 |
| Oral cavity neoplasms | 0/60 | 0/44 | 4/67 | 5/32 |
| Small intestine neoplasms | 0/60 | 0/45 | 4/74 | 8/59 |
| Large intestine neoplasms | 0/60 | 0/45 | 6/67 | 15/38 |
| Lung neoplasms | 1/60 | 0/45 | 8/73 | 6/57 |
| Equivalent dose for female (mg/kg-bw per day) | 0 | 3.0 | 6.9 | 12.9 |
| Skin basal cell neoplasms | 0/60 | 3/45 | 10/69 | 9/46 |
| Skin squamous cell neoplasms | 0/60 | 3/45 | 9/72 | 12/55 |
| Zymbal gland neoplasms | 0/60 | 6/45 | 32/74 | 42/59 |
| Clitoral gland neoplasms | 0/60 | 14/45 | 42/73 | 32/58 |
| Oral cavity neoplasms | 0/60 | 3/45 | 9/73 | 13/59 |
| Small intestine neoplasms | 0/60 | 1/45 | 3/72 | 5/57 |
| Large intestine neoplasms | 0/60 | 1/45 | 7/70 | 4/46 |
| Tumours | Model name | # of groups | AIC | P-value | SRI | BMR | BMD | BMDL |
|---|---|---|---|---|---|---|---|---|
| MR - Skin basal cell neoplasmsFootnote Appendix G Table G2 [b] | Multistage | 3 | 134.5 | 1 | 0 | 0.1 | 1.07 | 0.51 |
| MR - Skin sebaceous cell adenoma | LogLogistic | 4 | 85.17 | 0.24 | −0.78 | 0.1 | 7.60 | 4.74 |
| MR - Skin keratoacanthomas | Multistage | 4 | 100.2 | 0.48 | 0.81 | 0.1 | 5.24 | 3.24 |
| MR - Skin squamous cell neoplasms | Quantal-linear | 4 | 181.6 | 0.62 | 1.15 | 0.1 | 1.91 | 1.51 |
| MR - Preputial gland neoplasms | LogLogistic | 4 | 137.0 | 0.723 | −0.35 | 0.1 | 7.11 | 3.87 |
| MR - Oral cavity neoplasms | Quantal-linear | 4 | 62.3 | 0.748 | 0.267 | 0.1 | 7.83 | 4.74 |
| MR - Small intestine neoplasms | Quantal-linear | 4 | 82.0 | 0.777 | 0.18 | 0.1 | 8.64 | 5.56 |
| MR - Large intestine neoplasms | LogProbit | 4 | 96.4 | 0.732 | 0.439 | 0.1 | 4.57 | 3.45 |
| FR - Skin basal cell neoplasms | LogLogistic | 4 | 126.9 | 0.961 | 0.33 | 0.1 | 5.06 | 3.50 |
| FR - Skin squamous cell neoplasms | LogLogistic | 4 | 136.0 | 0.998 | −0.11 | 0.1 | 5.16 | 3.62 |
| FR - Zymbal gland neoplasms | LogLogistic | 4 | 211.4 | 0.999 | −0.023 | 0.1 | 2.51 | 1.56 |
| FR - Clitoral gland neoplasms | LogLogistic | 4 | 241.2 | 0.239 | 0 | 0.1 | 0.76 | 0.59 |
| FR - Oral cavity neoplasms | LogLogistic | 4 | 140.8 | 0.996 | −0.15 | 0.1 | 5.16 | 3.64 |
| FR - Small intestine neoplasms | Quantal-linear | 4 | 70.5 | 0.997 | 0.1 | 0.1 | 15.48 | 9.37 |
| FR - Large intestine neoplasms | LogLogistic | 4 | 85.9 | 0.645 | 0.75 | 0.1 | 10.18 | 6.39 |
Appendix H: Benzidine Derivatives and Benzidine-based Substances with Human Health Effects of Concern
Some of the Benzidine Derivatives, Benzidine-based Acid Dyes, Benzidine-based Direct Dyes, and Benzidine-based Precursors in this assessment have human health effects of concern based on potential carcinogenicity. The details for supporting the potential carcinogenicity for these substances are outlined in section 7.2 Health Effects Assessment (see specific sub-sections), and generally based on one or more of the following lines of evidence:
- Classifications by national or international agencies for carcinogenicity (may be a group classification).
- Evidence of carcinogenicity in animal studies and/or human epidemiology based on the specific substance.
- Potential to release one or more of the EU22 aromatic amines by azo bond cleavage.
- Read-across to related substances for which one of the above lines of evidence apply.
| Substance Name/ acronym and CAS RN | Classification for carcinogenicityFootnote Appendix H Table H1 [a] | Evidence of carcino-genicity from animal studies and/or human epidemiology | Release of EU22 aromatic amine by azo bond cleavageFootnote Appendix H Table H1 [b] | Read-across |
|---|---|---|---|---|
| Acid Red 128 6548-30-7 |
EU Category 1B carcinogenFootnote Appendix H Table H1 [c], NTP “Reasonably anticipated to be a human carcinogen”[c] |
3,3′-DMOB | ||
| Acid Red 114 6459-94-5 |
IARC 2B, EU Category 1B carcinogen[c], NTP “Reasonably anticipated to be a human carcinogen”[c] |
x | 3,3′-DMB | |
| Acid Black 209 68318-35-4 |
EU Category 1B carcinogen[c], NTP “Reasonably anticipated to be a human carcinogen”[c] |
3,3′-DMB | ||
| NAAHD 68400-36-2 |
EU Category 1B carcinogen[c], NTP “Reasonably anticipated to be a human carcinogen”[c] |
3,3′-DMB | ||
| Acid Red 99 3701-40-4 |
release 2,2′-DMB by azo bond cleavageFootnote Appendix H Table H1 [d] | |||
| BADB 89923-60-4 |
release 2,2′-DMB by azo bond cleavage[d] | |||
| Direct Red 28 573-58-0 |
IARC 1[c], EU Category 1B carcinogen[c], NTP “Known to be a human carcinogen”[c] |
Benzidine | ||
| Direct Brown 95 16071-86-6 |
IARC 1[c], EU Category 1B carcinogen[c], NTP “Known to be a human carcinogen”[c] |
x | Benzidine | |
| Direct Blue 8 2429-71-2 |
EU Category 1B carcinogen[c], NTP “Reasonably anticipated to be a human carcinogen”[c] | 3,3′-DMOB | ||
| Direct Blue 15 2429-74-5 |
IARC 2B, EU Category 1B carcinogen[c], NTP “Reasonably anticipated to be a human carcinogen”[c] |
x | 3,3′-DMOB | |
| Direct Blue 151 6449-35-0 |
EU Category 1B carcinogen[c], NTP “Reasonably anticipated to be a human carcinogen”[c] | 3,3′-DMOB | ||
| NAAH·3Li 67923-89-1 | EU Category 1B carcinogen[c], NTP “Reasonably anticipated to be a human carcinogen”[c] | 3,3′-DMOB | ||
| BABHS 70210-28-5 |
EU Category 1B carcinogen[c], NTP “Reasonably anticipated to be a human carcinogen”[c] | 3,3′-DMOB | ||
| NADB·4Li 71550-22-6 | EU Category 1B carcinogen[c], NTP “Reasonably anticipated to be a human carcinogen”[c] | 3,3′-DMOB | ||
| NADB·Li·3Na 75659-72-2 | EU Category 1B carcinogen, NTP “Reasonably anticipated to be a human carcinogen” | 3,3′-DMOB | ||
| NADB·2Li·2Na 75659-73-3 | EU Category 1B carcinogen[c], NTP “Reasonably anticipated to be a human carcinogen”[c] | 3,3′-DMOB | ||
| NAAH·Li·2Na 75673-18-6 | EU Category 1B carcinogen[c], NTP “Reasonably anticipated to be a human carcinogen”[c] | 3,3′-DMOB | ||
| NAAH·2Li·Na 75673-19-7 | EU Category 1B carcinogen[c], NTP “Reasonably anticipated to be a human carcinogen”[c] | 3,3′-DMOB | ||
| NADB·2Li 75673-34-6 | EU Category 1B carcinogen[c], NTP “Reasonably anticipated to be a human carcinogen”[c] | 3,3′-DMOB | ||
| NADB·Li·Na 75673-35-7 | EU Category 1B carcinogen[c], NTP “Reasonably anticipated to be a human carcinogen”[c] | 3,3′-DMOB | ||
| NADB·3Li·Na 75752-17-9 | EU Category 1B carcinogen[c], NTP “Reasonably anticipated to be a human carcinogen”[c] | 3,3′-DMOB | ||
| Direct Blue 14 72-57-1 |
IARC 2B, EU Category 1B carcinogen[c], NTP “Reasonably anticipated to be a human carcinogen”[c] |
x | 3,3′-DMB | |
| Direct Red 2 992-59-6 |
EU Category 1B carcinogen[c], NTP “Reasonably anticipated to be a human carcinogen”[c] |
3,3′-DMB | ||
| Direct Blue 25 2150-54-1 |
EU Category 1B carcinogen[c], NTP “Reasonably anticipated to be a human carcinogen”[c] |
3,3′-DMB | ||
| Direct Violet 28 6420-06-0 |
EU Category 1B carcinogen[c], NTP “Reasonably anticipated to be a human carcinogen”[c] |
3,3′-DMB | ||
| Direct Blue 295 6420-22-0 |
EU Category 1B carcinogen[c], NTP “Reasonably anticipated to be a human carcinogen”[c] |
3,3′-DMB | ||
| Direct Red 46 6548-29-4 |
3,3′-DCB | |||
| BAHSD 71215-83-3 |
release 2,2′-DCB by azo bond cleavage[d] | |||
| TCDB 93940-21-7 |
EU Category 1B carcinogen[c], NTP “Reasonably anticipated to be a human carcinogen”[c] |
3,3′-DMOB | ||
| 3,3′-DMOB 119-90-4 |
IARC 2B, EU Category 1B carcinogen, NTP “Reasonably anticipated to be a human carcinogen |
x | N/A (EU22) |
|
| 3,3′-DMB 119-93-7 |
IARC 2B, EU Category 1B carcinogen, NTP “Reasonably anticipated to be a human carcinogen” |
x | N/A (EU22) |
|
| 3,3′-DMB-2HClFootnote Appendix H Table H1 [e] 612-82-8 |
NTP “Reasonably anticipated to be a human carcinogen” | x | N/A (HCl salt of EU22) |