Screening assessment arenes
Official title: Screening assessment arenes
Chemical Abstracts Service Registry Numbers 57-97-6, 98-82-8
Environment and Climate Change Canada
Health Canada
March 2019
Cat. No.: En14-362/2019E-PDF
ISBN 978-0-660-29636-4
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health conducted a screening assessment on two of nine substances referred to collectively under the Chemicals Management Plan as the Arenes Group. These two substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA. Seven of the nine substances were subsequently determined to be of low concern through other approaches, and decisions for these substances are provided in separate reportsFootnote 1 Footnote 2 . Accordingly, this screening assessment addresses the two substances listed in the table below. The two substances addressed in this screening assessment will hereinafter referred to as the Arenes Group.
| CAS RNa | Domestic Substances List (DSL) name | Common name (abbreviation) |
|---|---|---|
| 98-82-8 | Benzene, (1-methylethyl)- | Cumene; isopropylbenzene |
| 57-97-6 | Benz[a]anthracene, 7,12-dimethyl- | 7,12- Dimethylbenz[a]anthracene (DMBA) |
a The Chemical Abstracts Service Registry Number (CAS RN) is the property of the American Chemical Society and any use or redistribution, except as required in supporting regulatory requirements and/or for reports to the Government of Canada when the information and the reports are required by law or administrative policy, is not permitted without the prior, written permission of the American Chemical Society.
In 2011, between 100 000 and 1 000 000 kg of cumene (CAS RN 98-82-8) were reported to be manufactured in Canada, and the same quantity was imported into Canada. There were no reports of manufacture or import for 7, 12- dimethylbenz[a]anthracene (DMBA, CAS RN 57-97-6) above the reporting threshold of100 kg for the same year. In Canada, cumene is primarily used as a chemical intermediate, but it is also used in products available to consumers, including adhesives, paints, automotive-related products, and lubricants. In Canada, DMBA is used as a research chemical in laboratories. It can also be produced unintentionally from industrial processes as a by-product.
The ecological risks of cumene and DMBA were characterized using the Ecological Risk Classification of Organic Substances (ERC). The ERC is a risk-based approach that employs multiple metrics for both hazard and exposure based on weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are established principally on the basis of metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances based on their hazard and exposure profiles. The ERC identified cumene and DMBA as having low potential to cause ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from cumene and DMBA. It is concluded that cumene and DMBA do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
The risks to human health for cumene and DMBA were characterized on the basis of available health effects and exposure information.
For cumene, laboratory studies have identified carcinogenicity as a critical health effect following long-term exposure by the inhalation route. As well, following oral administration for 6 months or inhalation for 3 months, non-cancer effects were observed in laboratory studies on the kidney (6-month oral study) and liver (3-month inhalation study). Systemic adverse effects were not observed in acute and short-term laboratory studies following inhalation or dermal administration. General population exposure to cumene from environmental media and from its possible presence in food was characterized using measured levels. The predominant source of exposure was indoor air. On the basis of a comparison of exposure estimates and critical effect levels identified in health effects studies, margins of exposure were considered to be adequate to address uncertainties in the human health effects and exposure databases.
Estimates of exposure to cumene from the use of various products available to consumers were derived and were not identified as a concern for human health, as available laboratory studies do not identify acute and short-term exposures to be of concern.
DMBA is genotoxic and laboratory studies have identified carcinogenicity to multiple organs as a critical health effect following administration by the oral and dermal routes. It is considered that the substance could be carcinogenic via the inhalation route, and estimates of potency were derived on the basis of route-specific potencies of a well-studied polycyclic aromatic hydrocarbon. General population exposure to DMBA was characterized using measured levels in ambient air in Canada. On the basis of a comparison of exposure estimates and the derived critical effect level in health effects studies, the margin of exposure was considered adequate to address uncertainties in the human health effects and exposure databases.
On the basis of the information presented in this screening assessment, it is concluded that cumene and DMBA do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that cumene and DMBA do not meet any of the criteria under section 64 of CEPA.
1. Introduction
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment on two of nine substances (cumene and DMBA), referred to collectively under the Chemicals Management Plan as the Arenes Group, to determine whether these two substances present or may present a risk to the environment or to human health. These two substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA (ECCC, HC [modified 2017]).
The other seven substances (listed in Table 1-1, below) were considered in the Ecological Risk Classification of Organic Substances (ERC) Science Approach Document (ECCC 2016a), and in either the Threshold of Toxicological Concern (TTC)- based Approach for Certain Substances Science Approach Document (Health Canada 2016a), or via the Rapid Screening Approach (ECCC, HC 2017a), and were identified as being of low concern to both human health and the environment. As such, they are not further addressed in this report. Conclusions for these seven substances are provided in the Substances Identified as Being of Low Concern based on the Ecological Risk Classification of Organic Substances and the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances Screening Assessment Report (ECCC, HC 2017b) and the Rapid Screening of Substances with Limited General Population Exposure Screening Assessment (ECCC, HC 2017a).
| CAS RNa | Domestic Substances List name | Approach under which the substance was addressed | References |
|---|---|---|---|
| 632-51-9 | Benzene, 1,1',1'',1'''- (1,2-ethenediylidene) tetrakis- | ERC/TTC | ECCC, HC 2017b |
| 29036-02-0 | Quaterphenyl | ERC/TTC | ECCC, HC 2017b |
| 38640-62-9 | Naphthalene, bis(1- methylethyl)- | ERC/TTC | ECCC, HC 2017b |
| 64800-83-5 | Benzene, ethyl(phenylethyl)- | ERC/TTC | ECCC, HC 2017b |
| 68398-19-6 | Benzene, ethyl(phenylethyl)-, mono-ar-ethyl deriv. | ERC/TTC | ECCC, HC 2017b |
| 68953-80-0 | Benzene, mixed with toluene, dealkylation product | ERC/Rapid Screening | ECCC, HC 2017a |
| 68987-42-8 | Benzene, ethylenated, residues | ERC/Rapid Screening | ECCC, HC 2017a |
a The Chemical Abstracts Service Registry Number (CAS RN) is the property of the American Chemical Society and any use or redistribution, except as required in supporting regulatory requirements and/or for reports to the Government of Canada when the information and the reports are required by law or administrative policy, is not permitted without the prior, written permission of the American Chemical Society.
The two other substances (cumene and DMBA) are addressed directly in this screening assessment.
The ecological risks of cumene and DMBA were characterized using the ERC approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics including mode of action, chemical reactivity, food-web derived internal toxicity threshold, bioavailability, and chemical and biological activity, and it considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of factors including potential emission rates, overall persistence and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposure, as well as additional information submitted by stakeholders. Relevant data were identified up to July 2016. Additional clarification/follow-up with stakeholders was conducted up to October 2016. Empirical data from key studies and some results from models were used to reach conclusions. When available and relevant, the information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was peer-reviewed and subject to a 60-day public comment period. The human health portions of this assessment have undergone external peer review and/or consultation. Comments on the technical portions relevant to human health were received from Tetra Tech Inc. (Theresa Lopez, Jennifer Flippin, Katherine Super and Gary Drendel). Additionally, the draft of this screening assessment (published on July 21, 2017) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precautionFootnote 3 . This screening assessment presents the critical information and considerations on which the conclusions are made.
2. Characterization of ecological risk
The ecological risks of cumene and DMBA were characterized using the ecological risk classification of organic substances (ERC) (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure based on weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization as compared to an approach that relies on a single metric in a single medium (e.g., LC50) forcharacterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, fish bioconcentration), acute fish ecotoxicity, and chemical import and manufacture volumes in Canada were either collected from the scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox), and from responses to surveys under section 71 of CEPA or were generated using selected quantitative structure-activity relationship (QSAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were established principally on the basis of metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance based on its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low potential risk classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under- classification of hazard and exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC 2016a. The following describes two of the more substantial areas of uncertainty. Error in empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from QSAR models. However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue (CBR) analysis. Error in underestimation of acute toxicity will be mitigated through the use of other hazard metrics, such as structural profiling of mode of action, reactivity and/or estrogen-binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada based on what is believed to be the current use quantity and may not reflect future trends.
3. Cumene
3.1 Substance identity
The substance identity is presented in Table 3-1, including its CAS RN, Domestic Substances List (DSL) name, structure, and molecular weight. This substance is commonly known as cumene or isopropylbenzene.
| CAS RN | DSL name (common name) | Chemical structure and molecular formula | Molecular weight (Da) |
|---|---|---|---|
| 98-82-8 | Benzene, (1-methylethyl)- (Cumene; isopropylbenzene) |
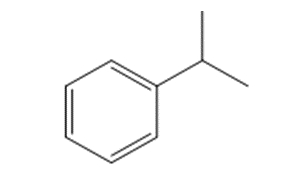 C9H12 C9H12 |
120 |
3.2 Physical and chemical properties
| Property | Empirical value(s) | Empirical reference(s) |
|---|---|---|
| Physical state | Liquid | (ECB 2001; Lide 2016) |
| Melting point (°C) | -96.01 | (ECB 2001; Lide 2016) |
| Boiling point (°C) | 152.4 | (ECB 2001; Lide 2016) |
| Vapour pressure (Pa) | 600 | (Daubert & Danner 1985 cited in PhysProp 2006) |
| Henry’s law constant (Pa·m3/mol) | 1.46 x 103 | (Lide 2016) |
| Water solubility (mg/L) | 50 |
(ECB 2001; Lide 2016) |
| Water solubility (mg/L) | 61.3 | (Sanemasa et al. 1982 cited in PhysProp 2006) |
| log Kow (dimensionless) | 3.66 | (Hansch et al. 1995 cited in PhysProp 2006) |
Additional physical and chemical properties are presented in ECCC (2016b).
3.3 Sources and uses
Cumene is primarily produced via alkylation of benzene with propene or distillation of coal tar (ECB 2001). According to information submitted pursuant to a survey under section 71 of CEPA, total reported manufactured quantity of cumene in Canada in 2011 was between 100 000 and 1 000 000 kg and the import quantity was in the same rangeFootnote 4 . In the United States, total manufacturing quantity in 2012 was more than 10 million tonnes (US EPA 2013).
According to National Pollutant Release Inventory (NPRI), in 2014 less than 50 tonnes of cumene releases, disposals and transfers for recycling were reported by industries. Among the reporting sectors were pulp and paper mills, oil and gas extraction, and chemical manufacturing.
Cumene can be found in crude oil, plants, marsh grasses and food (WHO 1999; ECB 2001; IARC 2012). It is not clear whether the occurrence in food is naturally-occurring or is the result of the presence of cumene in environmental media (e.g., soil). Cumene has been detected in foods, such as fruits, vegetables, cooked foods, and dairy products (ECB 2001; Hiatt and Pia 2004; FDA 2006). Cumene also occurs in cigarette smoke and vehicle emissions (WHO 1999; IARC 2012).
Cumene is primarily used in industrial applications but is also present in products available to consumers. It is used as an intermediate for phenol and acetone production and is present as a minor component in gasoline fuel and petroleum solvent (ECB 2001; IARC 2012). According to the information submitted pursuant to a section 71 survey under CEPA (Environment Canada 2013), consumer-related uses in Canada include paints and coatings, lubricants and greases, fuel and automotive-related products, adhesives and sealants. An additional literature search indicated that cumene can also be used in specialty cleaning products, such as fuel injector cleaner and concrete surface cleaner (UGL 2005; Prestone 2013), and in an anti-gelling agent for diesel fuel (Power Service 2014). All of these products are available on Canadian markets.
In Canada, cumene has been identified as a component in the manufacture of certain food packaging materials. The contribution from such use to overall human exposure is expected to be negligible (personal communication from the Food Directorate of Health Canada to the Existing Substances Risk Assessment Bureau, May 2016; unreferenced). In addition, cumene can be found in pest control products as a permitted formulant (personal communication with the Pest Management Regulatory Agency of Health Canada, May 2016; unreferenced). Cumene is not listed in the Therapeutic Products Directorate’s internal Drug Product Database as a medicinal or non-medicinal ingredient in final pharmaceutical, disinfectant or veterinary drug products in Canada. Cumene is not listed in the Natural Health Products Ingredients Database (NHPID) or the Licensed Natural Health Products Database (LNHPD) as a medicinal or non-medicinal ingredient in natural health products in Canada (DPD 2016; NHPID 2018; LNHPD 2018; personal communication, May 2016). The United States Pharmacopoeia (USP 2016) and the European Medicines Agency (EMA 2016) lists cumene as a class 2 residual solvent with a permitted daily exposure (PDE) level of 0.7 mg/day; and consequently, exposure from its potential use as solvent/intermediate in the processing/manufacturing of, and subsequent residual presence in, such products or their ingredient(s) would be expected to be lower than the USP and EMA PDE. The PDE value of 0.7 mg/day is also adopted by Health Canada (2016b). Cumene is not listed either as a prohibited or restricted ingredient on the Cosmetic Ingredient Hotlist, and is not notified to be present in cosmetics, based on notifications submitted under the Cosmetic Regulations to Health Canada (personal communication from Consumer Product Safety Directorate to Existing Substances Risk Assessment Bureau, July 2016).
3.4 Potential to cause ecological harm
Critical data and considerations used in ERC to develop the substance-specific profiles for cumene as well as the hazard, exposure and risk classification results are presented in ECCC (2016b).
Given the low hazard and low exposure classifications determined by ERC for cumene, this substance was classified as having a low potential for ecological risk. It is therefore unlikely that this substance results in concerns for the environment in Canada.
3.5 Potential to cause harm to human health
Exposure assessment
Due to the volatility of cumene, inhalation is considered to be the primary route of exposure.
Environmental media and food
Cumene can be present in ambient air, indoor air and food and, since 2000, it has been monitored in drinking water in three Canadian municipalities. No information was found on cumene presence in either dust or soil.
Cumene has been measured in several ambient air studies in Canada. It was monitored at various stations by the National Air Pollution Surveillance (NAPS) program and the average concentrations in 2010–2013 were below 0.02 µg/m3 (NAPS 2016). It was also measured in both outdoor air and indoor air in Windsor (2005, 2006), Regina (2007), Halifax (2009) and Edmonton (2010) during the summer and winter seasons by four different studies; the arithmetic mean concentrations in outdoor air ranged from lessthan 0.011 µg/m3 to 0.048 µg/m3 on the basis of data collected during the study periods in four regions (Health Canada 2010a, 2010b, 2012 and 2013a). Given the coverage of monitoring stations across Canada and the date of the data, the upper limit of NAPS data in 2010–2013, i.e., 0.02 µg/m3, was used in characterizing exposure of the general population to cumene via ambient air in this assessment report (Table 3-4).
Levels of cumene measured in indoor air are generally higher than those detected in outdoor air. As part of the 2009–2011 Canadian Health Measures Survey (CHMS), Zhu et al. (2013) conducted a national indoor air survey on over 3800 Canadian residential dwellings (e.g., houses, apartments) across Canada. With a detection frequency of nearly 80%, cumene was measured at concentrations of 0.25 µg/m3 as an arithmetic mean, 0.08 µg/m3 as a median value, and 2.52 µg/m3 as the 99th percentile value. The four different indoor air studies conducted in Windsor (2005, 2006), Regina (2007), Halifax (2009) and Edmonton (2010) reported arithmetic means of 0.092 to 0.564 µg/m3 and geometric means of 0.061 to 0.239 µg/m3 on the basis of data collected during the study periods in four regions (Health Canada 2010a, 2010b, 2012 and 2013a). As a conservative approach, the highest geometric mean of 0.239 µg/m3 and arithmetic mean of 0.564 µg/m3 for cumene from the Windsor study (45 homes, during the summer of 2005) (Health Canada 2010a) were selected for characterizing exposure of the general population to cumene via indoor air (Table 3-4).
Cumene has been detected in drinking water in the United States (EWG 2016), Japan (Shiraishi et al. 1985) and Europe (IRAC 2012). Cumene has been monitored in municipal drinking water systems in some cities across Canada since 2000, including Montreal, Regina and Toronto, but has not been detected in these water systems at detection limits of 0.03 to 0.2 µg/L (City of Montreal 2000–2012; City of Regina 2008–2013; City of Toronto 2003–2012).
There are no Canadian-specific data on levels of cumene in food. A summary of pesticide and industrial chemical analyses in food from the US FDA’s Total Diet Study (TDS) Program (1991–2003) reported that cumene was detected in 36 different food items, including vegetables (e.g., carrots), fruits (e.g., oranges), dairy products (e.g., cream cheese) and processed foods (e.g., meatloaf, chicken nuggets) (FDA 2006). The mean levels varied from 0.00005 ppm to 0.0031 ppm and the maximum levels ranged from 0.002 ppm to 0.063 ppm. However, for a given food type the number of samples for analysis was limited (i.e., 4 to 44), and more than 90% of the analytical results were below the limit of quantification.
Total intakes of cumene from environmental media and foods have been estimated to range from 0.10 to 0.31 µg/kg-bw/day, with children aged 6 months to 4 years having the highest intakes (i.e., 0.31 µg/kg-bw/day) (Appendix A-1). Dietary estimates derived using the US FDA’s TDS data are considered to be very conservative. Although this dataset provided empirical data for cumene in foods, cumene was detected in a limited number of foods as well as a limited number of samples of a given food type. Further, cumene was reported at quantifiable concentrations in few staple foods. Compared to the potential intake via food or drinking water, inhalation exposure via air (mainly indoor air) accounts for more than 95% of the total intake, and is considered to be the primary exposure source.
The European Union published a risk assessment on cumene (ECB 2001) and presented total intake estimates from air, drinking water and food based on modelled concentrations in each environmental compartment associated with the release of cumene into the environment. The ECB assessment also concluded that the primary source of human exposure (representing approximately 97% of total intake) is from air, which is consistent with the results of this assessment report.
Products available to consumers
Cumene is present in various products available to consumers, including adhesives, paints and coating products (liquid or spray), automotive-related cleaners, and lubricants. Sentinel exposure scenarios were selected. Because cumene is a volatile component, inhalation and to a lesser extent dermal contact are the predominant routes of exposure to cumene from products available to consumers.
Exposures were modelled using ConsExpo version 4.1 (ConsExpo 2006) and were considered to be short-term or acute. The conservative estimates are summarized in Table 3-3. Detailed input parameters for each sentinel scenario are provided in Table A-2 of Appendix A.
| Product category | Sentinel product scenario | Concentration of cumene (wt%)a | Inhalation, mean concentration on day of exposure (mg/m3) | Dermal exposure, (mg/kg- bw/event) |
|---|---|---|---|---|
| Adhesive | Flange/thread sealant | 1 | 0.96 | 0.21 |
| Paints and coatings | Liquid paints (brush and roller) | 3 | 35 | 1.52 |
| Paints and coatings | Spray paint | 1 | 0.55 | 0.21 |
| Paints and coatings | Craft paint, spray | 0.2 | 0.19 | 0.097 |
| Paints and coatings | Tile coating | 5 | 0.0087 (pre- application); 11.8 (application) |
0.035 (pre- application); 1.27 |
| Automotive- related products | Fuel injector cleaner | 5 | 0.24 | 0.007 |
| Lubricants and greases | Garage door/lock/ hinge lubricant, spray | 1 | 0.44 | 0.0024 |
a Based on the concentration values reported or in the case of a range, the upper limit was used.
Health effects assessment
The health effects of cumene have been reviewed in the Integrated Risk Information System (US EPA 1997) and assessed by joint international organizations (WHO 1999) and the European Commission’s Joint Research Centre (ECB 2001). No animal data on the carcinogenicity of cumene were available at the time those assessments were conducted. In 2009, the US National Toxicology Program (US NTP) published studies on the carcinogenicity of cumene in experimental animals (US NTP 2009). On the basis of these studies, the International Agency for Research on Cancer (IARC 2012) has classified cumene as “possibly carcinogenic to humans” (Group 2B) because of “inadequate evidence in humans” and “sufficient evidence in experimental animals”. Furthermore, the NTP found that cumene is “reasonably anticipated to be a human carcinogen” on the basis of sufficient evidence of carcinogenicity in studies in experimental animals (NTP 2012).
A two-year inhalation cancer study conducted in male and female mice and rats demonstrated the carcinogenicity of cumene (NTP 2009). In that study, groups of B6C3F1 mice (50/sex/group) were exposed to cumene at concentrations of 0, 125 (female only), 250, 500, or 1000 ppm (male only) (0, 614, 1229, 2458, 4916 mg/m3) for 6 hours per day, 5 days per week for 105 weeks. At the end of the study, complete necropsies and microscopic examinations were conducted the day following the last exposure. The incidence of alveolar/bronchiolar adenomas and carcinomas were found to increase in a dose-dependent manner in both male and female mice starting at the lowest treatment doses (LOAEL = 614 mg/m3). At the concentration of 2458 mg/m3, cumene also significantly increased the incidences of hepatocellular adenoma or carcinoma in female mice. The development of alveolar/bronchiolar adenomas and carcinomas in female mice was identified as the critical endpoint and was considered to be relevant to humans. This study was used for developing a benchmark dose (BMD) in this assessment report. A BMD for 10% extra risk level (BMD10) of 17.1 ppm and the lower confidence limit (BMDL10) of 14.1 ppm (equivalent to 12.4 mg/m3 as a continuous exposure) were computed using the cancer multistage model which assures low-dose linearity (Figure B-1 in Appendix B).
In the same two-year inhalation study (NTP 2009), groups of F344 rats (50/sex/group) were exposed to cumene vapour at concentrations of 0, 250, 500 or 1000 ppm (0, 1229, 2458, 4916 mg/m3) for 6 hours per day, 5 days per week for 105 weeks. The treatment resulted in a significant increase in the incidence of nasal epithelial adenomas in a dose groups in males and only at 1229 mg/m3 in females, and kidney tumours (renal tubule adenoma or carcinoma) in all dose groups in males. The kidney tumours were related to a rat-specific protein, alpha 2u-globulin, which is of no relevance to humans (NTP 2012).
On the basis of the available evidence, cumene as a parent chemical is generally considered to be non-genotoxic. Cumene was reported negative in most in vitro and in vivo genotoxicity assays, including the Ames test (Florin et al. 1980; NTP 2009, 2012), micronuclei test (NTP 2012), chromosome aberration assay (Putman DL 1987; Yang LL 1987), unscheduled DNA synthesis test (Curren RD 1987), and cell transformation assay (NTP 2009). However, while cumene resulted in negative Comet assays in target tissues (male rat kidney, male mouse lung, female mouse liver), there was also evidence cumene-induced DNA damage in male rat liver cells and female mouse lung cells in vivo (NTP 2012).
Though cumene is not considered genotoxic, a-methylstyrene oxide, a metabolite of cumene, is genotoxic. a-Methylstyrene oxide was found to be mutagenic in Salmonella (Rosman et al., 1986). Animal studies provided sufficient evidence for carcinogenicity of a-methylstyrene (NTP 2007). Increased frequencies of mutation in K-ras and Tp53 gene in cumene-induced lung cancer were observed (Hong et al. 2008) and might be the result of the conversion of cumene to this genotoxic metabolite. Therefore, as suggested by IARC (IARC 2012), a mutational mechanism is a likely mode of action by which cumene could produce lung tumours in rodents. Some organizations have proposed a non-genotoxic MOA (Bolt, 2016; Jahnke 2016).
Non-cancer endpoints were examined in repeated-dose studies through both oral and inhalation routes of exposure. In a sub-chronic oral study (Wolf et al. 1956), female Wistar rats (20 in the control group and 10 in each of the treatment groups) were administered cumene by gavage at 0, 154, 462 or 769 mg/kg-bw/day for 5 days a week for 6 months. A dose-dependent increase in kidney weight was observed in the 462 and 769 mg/kg-bw/day treatment groups (relative or absolute weight was not indicated in the study). No compound-related biochemical or histopathological changes were noted at any dose level. By adjusting to continuous dosing, the NOAEL and LOAEL were identified as 110 mg/kg-day and 330 mg/kg-day, respectively.
In a three-month inhalation study (NTP 2009), groups of 10 male and 10 female F344 rats and B6C3F1 mice were exposed to cumene vapour at concentrations of 0, 62.5, 125, 250, 500, or 1000 ppm (0, 307, 615, 1229, 2458, 4916 mg/m3) for 6 hours per day, 5 days per week for 14 weeks. Absolute and relative organ weights for both kidney and liver were increased in a concentration-dependent manner. Biologically (>10%) and statistically (p<0.05) significant increases in absolute and relative liver weight were observed at 1229 mg/m3 or greater in male rats (NTP 2009). Similarly, increased liver weight (both relative and absolute) was observed in both male and female mice exposed to cumene at 2458 mg/m3 or above. Clinical biochemistry data showed the serum total bile acid concentrations irreversibly increased at 2458 and 4916 mg/m3 in males, suggesting liver functions were affected by perturbation in bile acid uptake or excretion (NTP 2009). A complete histopathological examination (heart, right kidney, liver, lung, testis, and thymus) was performed on all control groups (mice and rats) and in the treatment groups at 2458 mg/ m3 (female mice only) and 4916 mg/m3 (mice and rats, male and female). No exposure-related gross lesions or tumour-related histopathological changes in the lungs or other organs were observed following 3 months of exposure. The NOAEC and LOAEC were identified at 615 and 1229 mg/m3, respectively, on the basis of increased liver weight in this assessment, and were used for risk characterization.
In a developmental study, a group of female SD rats were exposed to cumene for 6 hours per day during gestational days (GD) 6-15 at concentrations of 0, 100, 500, or 1200 ppm (0, 492, 2458, 5899 mg/m3). Pregnant dams were sacrificed and pups were examined at GD21 (Bushy Run Research Center 1989). No developmental effects were observed in any of the treatment groups.
In an acute inhalation study, groups of F344 rats (10/sex/group) were exposed to cumene at 100, 500 or 1200 ppm (492, 2458, 5899 mg/m3) for 6 hours (Cushman et al. 1995). A functional observational battery was conducted prior to the acute exposure and at 1, 6 and 24 hours post-exposure. No irreversible effects were observed in this study, despite transient ataxia observed 1 hour post-exposure. The author concluded that cumene vapour was neither neurotoxic nor ototoxic in rats. In a dermal study, cumene (unspecified amount) was routinely applied to the ear and on the shaved abdomen of rabbits (unspecified number of animals) over a period of two to four weeks (Wolf et al. 1956). Moderate skin irritation and transient development of thin devitalized tissue were reported; however, no systemic toxicological effects were observed. These results were supported by unpublished data from Procter and Gamble (1985), which showed no evidence of systemic toxicity in rabbits after 500 mg/kg-bw/day of cumene was applied to the animals’ back for 5 days a week for 4 weeks.
Characterization of risk to human health
On the basis of the studies and assessments reported by several international and national agencies (IARC 2012; NTP 2009, 2012, 2013), carcinogenicity was identified as the critical effect for characterization of risk to human health. The most sensitive carcinogenic effect is the development of alveolar/bronchiolar adenomas and carcinomas in female mice in a two-year cancer study (NTP 2009). Based on this effect, a BMDL10 was established (14.1 ppm; equivalent to 1.24 x 104 µg/m3 of continuous exposure).
Available evidence suggests a possible genotoxic mode of action for cumene-induced lung cancer.
Table 3-4 below provides the relevant exposures and hazard values, as well as resulting margin of exposure (MOEs), for the characterization of risk to human health from exposure to cumene.
| Exposure scenario | Systemic exposure | Critical effect level | Critical health effect endpoint | MOE |
|---|---|---|---|---|
| Inhalation of indoor air | 0.239 µg/m3 (highest geometric mean)a 0.564 µg/m3 (highest arithmetic mean)a | BMDL10=1.24 x 104 µg/m3 | Carcinogenicity (increased incidence of alveolar/bronchiolar adenomas and carcinomas from two- year inhalation study in mice) | 22000 - 51800 |
| Inhalation of indoor air | 0.239 µg/m3 (highest geometric mean)a 0.564 µg/m3 (highest arithmetic mean) | NOAEC=110 mg/m3 b | Increased liver weight | 190000 - 460000 |
| Total intake from environment al media (air, food and drinking water) | 0.31 µg/kg- bw/day c | NOAEL=110 mg/kg-bw/day | Increased renal weights | 355000 |
Abbreviation: MOE = margin of exposure
a Based on cumene based on samples collected from 45 homes in Windsor (Health Canada 2010a).
b NOAEC at 110 mg/m3 as a continuous exposure was converted from the concentration of 125 ppm (615 mg/m3) for the exposure of 6 hours per day and 5 days per week.
c See Appendix Table A-1, based on the 6-month to 4-year age group, which has the highest estimated exposure.
The MOEs listed above are considered adequate to account for uncertainties in the health effects and exposure databases.
For products available to consumers (see Table 3-3), the highest short-term exposure estimates based on the use of liquid paint products containing a maximum level of 3% cumene were 35 mg/m3 for inhalation (within 24 hours) and 1.52 mg/kg-bw/event for dermal exposure. Relevant critical adverse effect levels were not identified because of the low hazard observed for cumene following short-term exposures in experimental animals. Specifically, no critical adverse effects were identified in rats following acute inhalation exposure to cumene vapour at concentrations up to 1200 ppm (5899 mg/m3) for 6 hours. Exposure-related gross lesions or tumour-related histopathological changes were not observed in a three-month inhalation study in mice and rats (NTP 2009). For dermal exposure, no adverse health effects were identified from multiple cumene applications to the back of rabbits (500 mg/kg-bw/day) (Procter and Gamble 1985). Therefore, risk to human health from short-term exposure of the general population to cumene is expected to be low.
While exposure of the general population to cumene is not of concern at current levels, this substance is considered to have a health effect of concern owing to its potential carcinogenicity. Therefore, there may be a concern for human health if exposures to cumene were to increase.
Uncertainties in evaluation of risk to human health
Although the lack of chronic toxicity and carcinogenicity studies for cumene through oral and dermal routes constitutes an uncertainty, the predominant route of exposure is inhalation.
4. 7,12-Dimethylbenz[a]anthracene (DMBA)
4.1 Substance identity
DMBA is a polycyclic aromatic hydrocarbon (PAH). Its identity is presented in Table 4-1, including its CAS RN, DSL name, and structure.
| CAS RN (acronym) | DSL name (common name) | Chemical structure and molecular formula | Molecular weight (Da) |
|---|---|---|---|
| 57-97-6 | Benz[a]anthracene, 7,12-dimethyl- (7,12-Dimethylbenz[a]anthracene) |
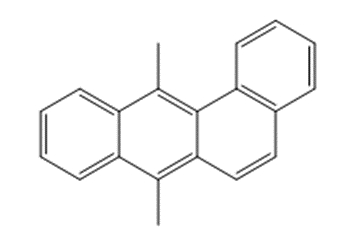 C20H16 C20H16 |
256 |
4.2 Physical and chemical properties
| Property | Empirical value(s) | Empirical reference(s) | Model value(s) | Model reference |
|---|---|---|---|---|
| Physical state | powder | (Sigma Aldrich 2016a) | N/A | N/A |
| Melting point (°C) | 123 | (Lide 2016) | 153.88 | (MPBPVP 2010) |
| Boiling point (°C) | N/A | N/A | 422.39 | (MPBPVP 2010) |
| Vapour pressure (Pa) | N/A | N/A | 3 x 10-5 | (MPBPVP 2010) |
| Henry’s law constant (Pa·m3/mol) | N/A | N/A | 0.206 | (HENRYWIN 2010) |
| Water solubility (mg/L) | 0.061 | (Mackay & Shiu 1977 cited in PhysProp 2006) | 0.019 | (WSKOWWIN 2010) |
| log Kow (dimensionless) | 5.8 | (Hansch et al. 1995 cited in PhysProp 2006) | 6.62 | (KOWWIN 2010) |
Additional physical and chemical properties are presented in ECCC (2016b).
4.3 Sources and uses
On the basis of the available information, manufacture and import quantities of DMBA in Canada are limited. According to information submitted pursuant to a section 71 survey under CEPA, there were no reports of DMBA manufacture or import above the reporting threshold of 100 kg in 2011Footnote 5 .In the United States, there were no reports of manufacture or import of DMBA above the reporting threshold of 25 000 lb (11 340 kg) in 2011 under the Chemical Data Reporting program (US EPA 2012).
DMBA originates primarily from anthropogenic activities. Information submitted pursuant to a section 71 survey under CEPA indicates that DMBA can be produced unintentionally from industrial processes as a by-product (Environment Canada 2013). It can be potentially present in carbon black (CAS RN 1333-86-4) or furnace black products. According to the carbon black assessment conducted under the Chemicals Management Plan (Environment Canada, Health Canada 2013), the presence of PAHs (e.g., DMBA) on the carbon black particle is not considered relevant in terms of carcinogenicity because of limited bioavailability. More specifically, little or no elution of PAHs has been observed from the surface of carbon black by biological fluids, while carbon black exposure does not appear to result in the formation of PAH-DNA adducts (JECFA 1987; Borm et al. 2005; OECD 2006). Therefore, the potential exposure to DMBA through the use of carbon black is not considered further in this assessment.
DMBA is used primarily as a research or experimental chemical. A literature search and follow-up with various programs within Health Canada did not identify any consumer- related uses.
4.4 Potential to cause ecological harm
Critical data and considerations used to develop the substance-specific profiles for DMBA and the hazard, exposure and risk classification results are presented in ECCC (2016b).
According to ERC, DMBA was classified as having a low hazard and low exposure potential; however, structural alerts from OECD toolbox identified this substance as being potential DNA binder. The potential effects and how they may manifest in the environment were not further investigated due to the low exposure of this substance. On the basis of current use patterns, this substance is unlikely to result in concerns for the environment in Canada.
4.5 Potential to cause harm to human health
Exposure assessment
No consumer uses were identified for DMBA.
Environmental media
In Canada, studies for monitoring of DMBA in ambient air in Alberta were available. Hsu et al. (2015) reported that in 2012–2013, mean concentrations of DMBA of 0.19 to 0.30 ng/m3 were detected at four ambient air monitoring stations in the Athabasca oil sands region of Northeastern Alberta, Canada. The monitoring stations were located in the residential or downtown areas of three communities with populations of approximately 560, 61 300 and 580 in 2011. The communities were located 6 to 35 km away from industry operation areas. Twenty-four-hour sampling was conducted every 6 days from January 2012 to December 2013. In total, approximately 120 samples were collected at each monitoring station over the specified period. DMBA levels from the same monitoring stations as described by Hsu et al. (2015) were also reported annually by the Wood Buffalo Environmental Association (WBEA 2010–2014), with average concentrations of 0.09 to 0.42 ng/m3. More recently, Zielinska et al. (2014) reported lower mean DMBA levels of 0.022 to 0.058 ng/m3 in residential ambient air in the United States adjacent to natural gas production sites in the Barnett Shale area. Alberta’s airdata warehouse, a repository of ambient air quality data, contains reports of DMBA levels of 0.3 ng/m3 in the period from January 2010 to December 2015 (Alberta Government 2016). Given the lack of DMBA monitoring data across Canada, the highest mean level of 0.3 ng/m3 reported by Hsu et al. (2015) was selected for characterizing chronic exposure of the general population to DMBA via ambient air.
According to NPRI data from 2011 to 2015, DMBA was reported to be released into the air in a total quantity of 2 to 4 kg/year from a few industry sectors, such as iron and steel mills, alumina and aluminum production, oil and gas extraction, pulp mills, and waste treatment (NPRI 2011–2015). DMBA has a release quantity at least 1000-fold lower than other PAHs reported under NPRI. According to the NPRI data (i.e., approximately 2 kg/year/site of DMBA released into the air), the ambient air DMBA concentrations predicted by a conservative air dispersion model are in the order of magnitude of ng/m3 in the vicinity of a facility (100 to 3000 m). Such modelled results are comparable to the monitoring data described above.
Health effects assessment
The health effects of DMBA have not been reviewed or assessed by any jurisdictions. The Government of Canada previously completed a human health risk assessment of certain PAHs (benzo[a]pyrene, benzo[b]fluoranthene, benzo[j]fluoranthene, benzo[k]fluoranthene and indeno[1,2,3-cd]pyrene), under the Priority Substances List Program (Environment Canada, Health Canada 1994). PAHs were added to the List of Toxic Substances in Schedule 1 of CEPA. Carcinogenicity of PAHs has also been classified by IARC (IARC 2010). However, DMBA was not included in the classification.
DMBA is reported to be a potent carcinogen in animals, showing induction of cancers in multiple systems by multiple routes (Medina et al. 1980; Currier et al. 2005). Groups of 10 female SD rats were administered a single dose of DMBA (0, 6.7, 16.7, 33.3, 66.7, 100 or 133 mg/kg-bw) in sesame oil by gavage and were examined frequently for palpable tumours for 150 days (Huggins et al. 1961a). The incidence of mammary cancer was 1/10, 2/10, 5/10, 8/10, 10/10, and 10/10 under single-dose exposure at 6.7, 16.7, 33.3, 66.7, 100 and 133 mg/kg-bw of DMBA, respectively. LOAEL was identified to be 6.7 mg/kg-bw, the lowest dose tested. The data indicates that a single administration of DMBA can be highly effective in the induction of mammary cancer in female rats.
Several short-term repeated-dose studies were conducted to identify its carcinogenicity. Mice administered 1.0 mg DMBA by gavage weekly for 6 weeks developed mammary tumours at an incidence of 69% (24 of 35) compared to zero (0 of 43) in untreated mice (Medina et al. 1980). In another study by Currier et al. (2003), 20 virgin female FVB/N mice were given 1.0 mg DMBA by oral gavage weekly for 6 weeks. By 23 weeks after the final dose, all mice had developed single or multiple tumours (75% had mammary tumours, 15% had lung tumours, 10% had lymphomas and 5% had skin tumours) (Currier et al. 2003). In a dermal study by Iversen (1991), a single dose of 51.2 mg DMBA induced a tumour rate of approximately 40%, and the same dose divided into 50 doses of 1 mg daily induced a tumour rate of almost 100% (Iversen 1991).
In a 14-month oral repeated-dose cancer study by Chouroulinkov et al. (1967), 144 female mice were administered DMBA in a diet dissolved in olive oil (15 µg DMBA/day). Gastric and mesenteric tumours were observed in 41 (28.4%) and 99 (68.7%) mice treated with DMBA, respectively. As a comparison, 0 out of 117 mice fed the diet alone (control group 1) developed tumours, and only 1 mesenteric tumour was observed in 158 mice fed the diet containing olive oil (control group 2). Using this data, Collins et al. (1998) established a cancer slope factor of 250 (mg/kg-day)-1 for oral exposure to DMBA.
As no cancer studies of DMBA through inhalation exposure were identified, dose extrapolation from oral to inhalation routes was conducted on the basis of potency equivalency relative to benzo[a]pyrene (BaP). This approach is commonly applied in cancer risk assessment on PAHs (Collins et al. 1998; OEHHA 1994). A tumorigenic concentration for inhalation exposure to DMBA associated with a 5% increase in incidence or mortality from tumours (TC05) was estimated by considering its relative potency to BaP and the TC05 identified for inhalation exposure to BaP. Collins et al. (1988) determined that the relative potency of DMBA to BaP was 22. This was derived by comparing the oral cancer potency factor of 250 (mg/kg-day)-1 for DMBA with the oral cancer potency factor of 11.5 (mg/kg-bw/day)-1 for BaP reported by Neal and Rigdoon (1967). The TC05 for inhalation exposure to BaP was determined to be 1.57 mg/m3 by Health Canada (1994) on the basis of the cancer study by Thyseen et al (1981) on inhalation exposure to BaP. Therefore, the estimated TC05 for inhalation exposure to DMBA was estimated at 0.071 mg/m3 (1.57 mg/m3 /22), assuming that the relative potency of DMBA to BaP by inhalation is the same as that by oral exposure (i.e., 22).
Available data indicates that DMBA is genotoxic. An Ames test was conducted with Salmonella typhimurium strain TA100 in the presence S9 isolated from the mouse, rat, hamster, pig and human. With the exception of the rat and pig, all animal species activated DMBA to mutagens (Phillipson and Loannides 1989). DMBA induced sister- chromatid exchanges in lymphocytes in female swines(McFee and Sherrill 1983) and bone marrow cells in rats (van Kesteren-van Leeuwen and Natarajan 1980). DMBA- DNA adducts were persistently observed up to 42 weeks in epidermis and dermis of mice applied with a single dose of 1.2 µmol DMBA on the shaved skin of the back (Randerath et al. 1985). A dose-dependent induction of unscheduled DNA synthesis was observed in mice with dermal application of DMBA at all applied doses (0, 0.24, 0.5 or 1%) (Mori et al. 1999).
For non-cancer endpoints, studies on rodents with a single administration of DMBA revealed that DMBA induced adverse effects on the adrenal gland in rats at 120 mg/kg- bw by gavage (Huggins et al. 1961b), on the testis at 8.6 mg/kg-bw by intravenous injection (Ford and Huggins 1963), on the ovary in mice at 0.1 mg/kg-bw by intraperitoneal injection (Weitzman et al. 1992) and on the bone marrow in mice at 19 mg/kg-bw by oral or intraperitoneal injection (N’jai et al. 2010). In a two-week repeated- dose study, B6CF31 mice (4 per group) were administered DMBA daily by gavage at 0.1, 1.0 and 10 mg/kg-bw/day for 14 days. On the basis of inhibition of lymphocyte activation, the LOAEL was determined to be 0.1 mg/kg-bw/day, the lowest dose tested (Burchiel et al. 1990).
A developmental study (Sanyal et al. 2007) in rats treated intraperitoneally with DMBA demonstrated that DMBA is toxic to placental tissues and to differentiating fetal blood system and results in significant fetal growth restriction.
DMBA clearance in mice was examined after one-time administration with oral gavage (N’jai et al. 2010). The study showed that the blood levels of DMBA peaked 1.5 to 3 hours following oral administration, dropped substantially at 6 hours and were not detectable at 24 hours. There is a lack of pharmacokinetic studies on inhalation of DMBA.
Characterization of risk to human health
On the basis of currently available information, exposure of the Canadian general population to DMBA is expected to be limited to ng/m3 levels in the ambient air. In information submitted pursuant to a section 71 survey under CEPA, there were no reports of manufacture or import of DMBA above the reporting threshold of 100 kg in 2011. DMBA was not identified as being present in products available to consumers.
DMBA is a potent carcinogen in animal studies. Comparison of the mean level of DMBA (i.e., 0.3 ng/m3) in ambient air reported by Hsu et al. (2015) and the TC05 estimate of 0.071 mg/m3 for DMBA results in an MOE of 2.4 x 105. Given the low total quantity of 2 to 4 kg/year released into the air (NPRI 2011–2015), low volatility and water solubility, it is likely that the magnitude of the actual MOE based on inhalation data would be larger. The derived MOE for inhalation is therefore considered to be adequate to address uncertainties in the health effects and exposure databases.
While exposure of the general population to DMBA is not of concern at current levels, this substance is considered to have health effects of concern because of its potential carcinogenicity. Therefore, there may be a concern for human health if exposure to DMBA were to increase.
Uncertainties in evaluation of risk to human health
Uncertainty exists regarding representativeness of Alberta monitoring data to the general population of Canada, although the monitoring data are in line with the conservative modelling results based on NPRI release information.
An uncertainty in the health effects database is the lack of comprehensive information on the potential health effects following inhalation exposure to DMBA, as the cancer studies were conducted through oral, dermal or subcutaneous (SC) injection routes.
5. Conclusions
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from cumene and DMBA. It is concluded that cumene and DMBA do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this screening assessment, it is concluded that cumene and DMBA do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that cumene and DMBA do not meet any of the criteria set out in section 64 of CEPA.
References
Alberta Government. 2016. Alberta’s ambient air quality data warehouse. [database on the internet]. Data reports [accessed 2016 Oct].
Bolt HM, Nielsen GD, Papameletiou C, Klein CL. 2016. 2-Phenylpropane (Cumene): Recommendation from the Scientific Committee on Occupational Exposure Limits (SCOEL/REC/029).
Borm P, Cakmak G, Jermann E, Weishaupt C, Kempers P, van Schooten FJ, Oberdörster G, Schins RP.
2005. Formation of PAH-DNA adducts after in vivo and in vitro exposure of rats and lung cells to different commercial carbon blacks. Toxicol Appl Pharmacol. 205:157–167.
Brugnone F, Perbellini L, Faccini GB, Pasini F, Maranelli G, Romeo L, Gobbi M, Zedde A. 1989. Breath and blood levels of benzene, toluene, cumene and styrene in non-occupational exposure. Int Arch Occup Environ Health. 61:303-311.
Burchiel SW, Davis DA, Gomez MP, Montano RM, Barton SL, Seamer LC 1990. Inhibition of lymphocyte activation in splenic and gut-associated lymphoid tissues following oral exposure of mice to 7,12- dimethylbenz[a]anthracene. Toxicol Appl Pharmacol. 105:434–442.
Bushy Run Research Center. 1989. Developmental toxicity study of inhaled cumene vapor in CD (Sprague-Dawley) rats. Final project report 52-621. TSCATS/0522881;EPA/OTS Doc. No. 40-8992172.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c. 33. Canada Gazette Part III, vol. 22, no. 3.
Chouroulinkov I, Gentil A, Guerin M. 1967. Study of the carcinogenic action of orally administered 9, 10- dimethylbenzanthracene and 3,4-benzopyrene. Bull. Cancer 54:67–78.
City of Montreal. 2000-2009. Montreal drinking water quality annual reports.
City of Regina. 2008-2013. Buffalo Pound water administration board annual reports.
City of Toronto, 2003-2012. Annual drinking water quality analysis summary.
Collins JF, Brown JP, Alexeeff GV, Salmon AG. 1998. Potency equivalency factors for some polycyclic aromatic hydrocarbons and polycyclic aromatic hydrocarbon derivatives. Regul Toxicol Pharmacol. 28:45–54.
Conkle JP, Camp BJ, Welch BE. 1975. Trace composition of human respiratory gas. Arch Environ Health 30:290-295. [cited in ECB 2001].
[ConsExpo] Consumer Exposure Model. 2006. Version 4.1. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. [cited 2016 Jul 22].
Currier N, Solomon SE, Demicco EG, Chang DL, Farago M, Ying H, Dominguez I, Sonenshein GE, Cardiff RD, Xiao ZX, Sherr DH, Seldin DC. 2005. Oncogenic signaling pathways activated in DMBA- induced mouse mammary tumors. Toxicol Pathol. 33:726–737.
Cushman JR, Norris JC, Dodd DE, Darmer KI, Morris CR. 1995. Subchronic Inhalation Toxicity and Neurotoxicity Assessment of Cumene in Fischer 344 Rats. J Am Coll Toxicol. 14:129–147.
[ECB] European Chemicals Bureau. 2001. European Union Risk assessment report: Cumene.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Data used to create substance-specific hazard and exposure profiles and assign risk classifications in the Ecological Risk Classification of organic substances. Gatineau (QC). Available from: substances@ec.gc.ca
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2017 Dec 20].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2017a. Rapid screening of substances with limited human health exposure. Ottawa (ON): Government of Canada.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2017b. Draft screening assessment: Substances identified as being of low concern based on the ecological risk classification of organic substances and the threshold of toxicological concern (TTC)-based approach for certain substances.
[EMA] European Medicines Agency. 2016. ICH guideline Q3C (R5) on impurities: guideline for residual solvents.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada, Health Canada 2013. Final Screening Assessment for Carbon Black. Ottawa (ON): Environment Canada; Health Canada. [cited 2016 Jul].
[ESIS] European Chemical Substances Information System [database on the Internet]. 2008. Database developed by the European Chemicals Bureau (ECB). [cited 2011 Aug 29].
[EWG] Environmental Working Group. National drinking water database on isopropylbenzene. [accessed 2016 Jul 21].
[FDA] US Food and Drug Administration. 2006. Total diet study market baskets 1991-3 through 2003-4.
Florin I, Rutberg L, Curvall M, Enzell CR. 1980. Screening of Tobacco Smoke Constituents for Mutagenicity using the Ames' Test. Toxicology 15:219–232.
Ford E, Huggins C. 1963. Selective destruction in testis induced by 7,12-dimethylbenz [a] anthracene. J Exp Med. 118:27–40.
Health Canada. 1994. Polycyclic aromatic hydrocarbons: Canadian Environmental Protection Act Priority Substances List Assessment Report. Environment Canada and Health Canada: Ottawa (ON).
Health Canada. 1998 Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Environmental Health Directorate, Health Canada: Ottawa (ON).
Health Canada. 2009. Guidelines for Canadian Drinking Water Quality: Guideline Technical Document Benzene, Health Canada, Ottawa.
Health Canada. 2010a. Windsor exposure assessment study (2005-2006): Data summary for volatile organic compound sampling. Water, Air and Climate Change Bureau, Healthy Environments and Consumer Safety Branch. Ottawa (ON): Health Draft Screening Assessment Liquefied Petroleum Gases 53 Canada. 81 pp. [cited 2014 Feb].
Health Canada. 2010b. Regina indoor air quality study (2007): Data summary for volatile organic compound sampling. Water, Air and Climate Change Bureau, Healthy Environments and Consumer Safety Branch. Ottawa (ON): Health Canada. 157 pp. [cited 2014 Feb.]
Health Canada. 2012. Halifax indoor air quality study (2009): VOC sampling data summary. Water, Air and Climate Change Bureau, Healthy Environments and Consumer Safety Branch. Ottawa (ON): Health Canada. 48 pp.
Health Canada 2013a. Edmonton Indoor Air Quality Study (2010): Volatile Organic Compounds (VOC) Data Summary. Ottawa (ON): Health Canada, Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch.
Health Canada. 2013b. Guidance for Benzene in Residential Indoor Air, Health Canada, Ottawa.
Health Canada. 2016a. Science approach document: threshold of toxicological concern (TTC)-based approach for certain substances. Ottawa (ON): Government of Canada.
Health Canada. 2016b. Guidance document impurities: guideline for residual solvents ICH Topic Q3C(R5). [cited 2017].
[HENRYWIN] Henry’s Law Constant Program for Microsoft Windows [estimation model]. 2008. Ver. 3.20. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Hiatt MH, Pia JH. 2004. Screening processed milk for volatile organic compounds using vacuum distillation/gas chromatography/mass spectrometry. Arch Environ Contam Toxicol. 46:189–196.
Hong HH, Ton TV, Kim Y, Wakamatsu N, Clayton NP, Chan PC, Sills RC, Lahousse SA. 2008. Genetic alterations in K-ras and p53 cancer genes in lung neoplasms from B6C3F1 mice exposed to cumene. Toxicol Pathol. 36:720–726.
Hsu Y, Harner T, Li H, Fellin P. 2015. PAH measurements in Air in the Athabasca Oil Sands Region. Environ Sci Technol. 49:5584–5592.
Huggins C, Grand LC, Brillantes FP. 1961a. Mammary cancer induced by a single feeding of polymucular hydrocarbons, and its suppression. Nature 189:204–207.
Huggins C, Morii S. 1961b. Selective adrenal necrosis and apoplexy induced by 7,12- dimethylbenz(a)anthracene. J Exp Med. 114:741–760.
Huggins CB, Yoshida H, Bird CC. 1974. Hormone-dependent stem-cell rat leukemia evoked by a series of feedings of 7,12-dimethylbenz(a)anthracene. J Natl Cancer Inst. 52:1301–1305.
[IARC] International Agency for Research on Cancer. 2010. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr Eval Carcinog Risks Hum. 92:1–853.
[IARC] International Agency for Research on Cancer. 2012. Cumene. In: Some Chemicals in Industrial and Consumer Products, Food Contaminants and Flavourings, and Water Chlorination By-Products. IARC Monogr Eval Carcinog Risks Hum. 101.
[IARC] International Agency for Research on Cancer. 2013. Cumene. In: Some chemicals present in industrial and consumer products, food and drinking-water. [PDF, 6.49 MB] IARC Monogr Eval Carcinog Risks Hum. 101. p. 325-348.
Iversen OH. 1991. The skin tumorigenic and carcinogenic effects of different doses, numbers of dose fractions and concentrations of 7,12-dimethylbenz[a]anthracene in acetone applied on hairless mouse epidermis. Possible implications for human carcinogenesis. Carcinogenesis 12:493–502.
Jahnke G, Hamann I, Laube B, Greim H, Hartwig A, Commission MAK 2016. Isopropyl benzene (cumene) [MAK Value Documentations, 2013]. In The MAK-Collection for Occupational Health and Safety. Wiley-VCH Verlag GmbH & Co. KGaA.
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 1987. Evaluation of certain food additives and contaminants. World Health Organization Technical Report Series 759. Geneva (CH): World Health Organization.
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [estimation model]. 2010. Ver. 1.68. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Krotoszynski B, Gabriel G. O’Neill H. 1977. Characterization of human expired air: a promising investigative and diagnostic technique. J Chromatogr Sci. 15:239–244.
Lide DR, editor. 2016. CRC handbook of chemistry and physics. 96th ed. Boca Raton (FL): CRC Press. [accessed 2016 Jun 3].
Lillie MA, Ambrus CM, Pickren JW, Akhter S, Islam A, Ambrus JL. 2004. Breast cancer in intraductal carcinogen-treated non-human primates. J Med. 35:271–275.
[LNHPD] Licensed Natural Health Products Database. [database] [modified 2018 Feb 06] Ottawa (ON): Government of Canada. [Accessed 2018 Jan 24].
McFee AF, Sherrill MN. 1983. Sister-chromatid exchanges induced in swine lymphocytes by chronic oral doses of dimethylbenzanthracene. Mutat Res. 116:349–359.
Medina D, Butel JS, Socher SH, Miller FL. 1980. Mammary tumorigenesis in 7,12- dimethybenzanthracene-treated C57BL x DBA/2f F1 mice. Cancer Res. 40:368–373.
Mori M, Kobayashi H, Sugiyama C, Katsumura Y, Furihata C. 1999. Induction of unscheduled DNA synthesis in hairless mouse epidermis by skin carcinogens. J Toxicol Sci. 24:217–226.
[MPBPVP] Melting Point Boiling Point Program for Microsoft Windows [estimation model]. 2010. Ver.1.43. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics
[NAPS] National Air Pollution Surveillance Program. 2016. NAPS annual raw data for 2010, 2011, 2012, 2013. Ottawa (ON): Environment and Climate Change Canada. [accessed 2016 Jul]
[NCI] National Chemical Inventories. Issue 1. Columbus (OH): American Chemical Society, Chemical Abstracts Service. [accessed 2016 Jul].
Neal J, Rigdon RH. 1967. Gastric tumors in mice fed benzo(a)pyrene: a quantitative study. Tex Rep Biol Med. 25:553–557.
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2018 Aug 29]. Ottawa (ON): Government of Canada. [accessed 2018 Jan 24].
N’jai AU, Larsen M, Shi L, Jefcoate CR Czuprynski CJ. 2010. Bone marrow lymphoid and myeloid progenitor cells are suppressed in 7,12-dimethylbenz(a)anthracene (DMBA) treated mice. Toxicology 271:27–35.
[NPRI] National Pollutant Release Inventory [database on the Internet]. 2011-2015. Gatineau (QC): Environment and Climate Change Canada. [accessed 2016 Oct].
[NTP] National Toxicology Program. 2009. Toxicology and carcinogenesis studies of cumene (CAS No. 98-82-8) in F344/N rats and B6C3F1 mice (inhalation studies). Natl Toxicol Program Tech Rep Ser. 542:1–200.
[NTP] National Toxicology Program. 2007. Toxicology and carcinogenesis studies of alpha-methylstyrene (CAS No. 98-83-9) in F344/N rats and B6C3F1 mice (inhalation studies). Natl Toxicol Program Tech Rep Ser. 543:1–210.
[NTP] National Toxicology Program. 2012. Final Report on the Cumene (CAS RN 98-82-8) Genotoxicity Studies. Research Triangle Park, NC: National Toxicology Program.
[NTP] National Toxicology Program. 2013. Report on carcinogens monograph on cumene. Rep Carcinog Monogr. 13-5983:1–166.
[OECD] Organisation for Economic Co-operation and Development. 2006. Carbon black, CAS 1333-86-4; SIDS initial assessment report for carbon black; CAS No. 1333-86-4. SIDS initial assessment meeting 21, Washington (DC), 18–21 October 2005 (includes SIDS Initial Assessment Profile, SIDS Initial Assessment Report, and IUCLID Data Set) [Internet]. Geneva (CH): United Nations Environment Programme. [last updated 2006 Jun 6].
[OEHHA] California Air Resources Board and the Office of Environmental Health Hazard-Assessment. 1994. Benzo[a]pyrene as a toxic air contaminant. Executive summary.
Phillipson CE, Loannides C. 1989. Metabolic activation of polycyclic aromatic hydrocarbons to mutagens in the Ames test by various animal species including man. Mutat Res. 211:147–151.
[PhysProp] Interactive PhysProp Database [database]. c2013. Syracuse (NY): SRC, Inc. [accessed 2016 Jun 3].
Prestone 2013. Safety Data Sheet Prestone® Fuel System Cleaner.
Procter & Gamble Company (1985) 28-day subacute percutaneous toxicity with cover letter. OTS 0206749, Doc ID 878215029.
Power Service. 2014. Material Safety Data Sheet for Diesel fuel supplement+ cetane boost.
Putman DL. 1987. Chromosome Aberrations in Chinese Hamster Ovary (CHO) Cells. NTIS/OTS 0522852. Microbiological Associates, Inc. (as cited in EC 2001).
Randerath E, Agrawal HP, Weaver JA, Bordelon CB, Randerath K. 1985. 32P-postlabeling analysis of DNA adducts persisting for up to 42 weeks in the skin, epidermis and dermis of mice treated topically with 7,12-dimethylbenz[a]anthracene. Carcinogenesis 6:1117–1126.
Rosman LB, Beylin VG, Gaddamidi V, Hooberman BH, Sinsheimer JE. 1986. Mutagenicity of para- substituted alpha-methylstyrene oxide derivatives with Salmonella. Mutat Res. 171:63–70.
Sanyal MK, Li YL. 2007. Deleterious effects of polynuclear aromatic hydrocarbon on blood vascular system of the rat fetus. Birth Defects Res B Dev Reprod Toxicol. 80:367–373.
[SCREEN3] Screening Tool Program for Windows [Screening Model]. 1996. Version 4.10. Research Triangle Park (NC): US Environmental Protection Agency, Office of Air Quality Planning and Standards Emissions, Monitoring, and Analysis Division.
Schneider K, Roller M, Kalberlah F, Schuhmacher-Wolz U. 2002. Cancer risk assessment for oral exposure to PAH mixtures. J Appl Toxicol. 22:73–83.
Shiraishi H. Pilkington NH, Otsukl A. Fuwa K. 1985. Occurrence of chlorinated polynuclear aromatic hydrocarbons in tap water. Environ Sci Technol. 19(7):585–590.
Thyssen J, Althoff, J, Kimmerle G, Mohr U. 1981. Inhalation studies with benzo[a]pyrene in Syrian golden hamsters. J Natl Cancer Inst. 66:575–577.
[UGL] United Gilsonite Laboratories. 2005. Material Safety Data Sheet for Concrete Cleaner and Degreaser.
[US EPA] US Environmental Protection Agency. 1997. Toxicological Review of Cumene (CAS No. 98-82-8). In Support of Summary Information on the Integrated Risk Information System. Washington, D.C.: U.S. Environmental Protection Agency.
[US EPA] US Environmental Protection Agency. 2011. Exposure factors handbook. Chapter 17.
[US EPA] US Environmental Protection Agency. 2012 Chemical Data Reporting Results. [accessed 2013 Apr].
[USP] United States Pharmacopoeia. 2016. The United States Pharmacopeia and the National Formulary, published by United States Pharmacopoeial Convention.
van Kesteren-van Leeuwen, AC, Natarajan AT. 1980. Localisation of 7-12-dimethylbenz(a)anthracene induced chromatid breaks and sister chromatid exchanges in chromosomes 1 and 2 of bone marrow cells of rat in vivo. Chromosoma 81:473–481.
[WEBA] Wood Buffalo Environmental Association. Annual reports. 2010-2014.
Weitzman GA, Miller MM, London SN, Mattison DR. 1992. Morphometric assessment of the murine ovarian toxicity of 7,12-dimethylbenz(a)anthracene. Reprod Toxicol. 6(2):137–41.
[WHO] World Health Organization. 1999. Concise international chemical assessment document 18: Cumene.
Wolf MA, Rowe VK, Mccollister DD, Hollingsworth RL, Oyen F. 1956. Toxicological studies of certain alkylated benzenes and benzene; experiments on laboratory animals. AMA Arch Ind Health. 14:387–398.
[WSKOWWIN] [Water Solubility for Organic Compounds Program for Microsoft Windows [estimation model]. 2010. Ver. 1.42. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Yang LL. 1987. CHO/HGPRT Mutation Assay. NTIS/OTS 0522853. Microbiological Associates, Inc.
Zhu J, Wong SL, Cakmak S. 2013. Nationally representative level of selected volatile organic compounds in Canadian residential indoor air: population-based survey. Environ Sci Technol. 47:13276–13283.
Zielinska B, Campbell D, Samburova V. 2014. Impact of emission from natural gas production facilities on ambient air quality in the Barnett Shale area: a pilot study. J Air Waste Manag Assoc. 64:1369–1383.
Appendices
Appendix A. Estimated exposures of the general population to cumene
| Environmental media | 0-0.5 yra | 0.5-4 yrb | 5-11 yrc | 12-19 yrd | 20-59 yre | 60+ yrf |
|---|---|---|---|---|---|---|
| Ambient air (µg/m3)g | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Indoor air (µg/m3)g | 0.564 | 0.564 | 0.564 | 0.564 | 0.564 | 0.564 |
| Drinking water intake (µg/kg- bw/day)h | 0.0032 (FF), 0.0012 (non FF) | 0.0014 | 0.0011 | 6.06 x 10-4 | 6.35 x 10-4 | 6.67 x 10-4 |
| Total food intake (µg/kg- bw/day)i | 0.0057 | 0.0105 | 0.0087 | 0.0055 | 0.0032 | 0.0027 |
| Total oral intake (drinking water and food, µg/kg-bw/day) | 0.0032 (FF), 0.0069 (non FF) | 0.012 | 0.0098 | 0.0061 | 0.0039 | 0.0034 |
| Total intake (air, drinking water and food, µg/kg-bw/day) | 0.14 | 0.31 | 0.24 | 0.14 | 0.12 | 0.10 |
Abbreviations: FF = Formula-fed infants
a assumed to weigh 7.5 kg, to breathe 2.1 m3/day of air. Formula-fed infants are assumed to exclusively consume 0.8 L/day of liquid infant formula reconstituted by drinking water. Non formula-fed infants are assumed to consume 0.3 L/day of drinking water
b assumed to weigh 15.5 kg, to breathe 9.3 m3 /day of air, to consume 0.7 L/day of drinking water
c assumed to weigh 31.0 kg, to breathe 14.5 m3/day of air, to consume 1.1 L/day of drinking water
d assumed to weigh 59.4 kg, to breathe 15.8 m3/day of air, to consume 1.2 L/day of drinking water
e assumed to weigh 70.9 Kg, to breath 16.2 m3/day of air, to consume 1.5 L/day of drinking water
f assumed to weigh 72.0 kg, to breath 14.3 m3/day of air, to consume 1.6 L/day of drinking water.
g To calculate inhalation dose, assuming 21-h inhalation of indoor air at 0.564 µg/m3 and 3-h inhalation of outdoor air at 0.02 µg/m3, multiplied by inhalation rate value and divided by body weight value for each age group as specified by Health Canada (1998)
h The daily intake value of cumene from drinking water was determined by multiplying the level of 0.03 µg/L with the daily water consumption rate value reported by Health Canada (1998).
i High-end estimates for daily intake of cumene were generated using the mean concentrations reported in the US FDA TDS (2006) data set for 36 food items, multiplied by the corresponding food consumption rate values from the 1970-1972 Nutrition Canada Survey as specified by Health Canada (1998). A high-end food intake estimate was then generated by aggregating the intake estimates from all 36 food items.
| Product Scenario | Model parameter | Estimated exposure |
|---|---|---|
| Flange/thread sealant, based on joint sealant scenario described in ConsExpo (2006) | Use frequency: 8.9/year (US EPA 2011) Exposure duration: 45 min. Application duration: 30 min. Product amount: 75 g Weight fraction: 0.01 Inhalation module: evaporation from increasing area Mass transfer rate: Langmuir’s method Molecular weight matrix: 3000 g/mol Room volume: 10 m3 Ventilation: 2 air changes/h Release area: 250 cm2 Contact rate: 50 mg/min. Update fraction: 1 |
Inhalation: Mean event concentration: 30.6 mg/m3 Mean concentration on the day of event: 0.956 mg/m3 Dermal: Acute, per event: 0.212 mg/kg-bw |
| Paint (liquid), based on brush/roller solvent rich paint scenario described in ConsExpo (2006) | Use frequency: 1/year Exposure duration: 132 min. Application duration: 120 min. Product amount: 1000 g Weight fraction: 0.03 Inhalation module: evaporation from increasing area Mass transfer rate: Langmuir’s method Molecular weight matrix: 300 g/mol Room volume: 20 m3 Ventilation rate: 0.6 air changes/h Release area: 10 m2 Contact rate: 30 mg/min. Update fraction: 1 |
Inhalation: Mean event concentration: 382 mg/m3 Mean concentration on the day of event: 35 mg/m3 Dermal: Acute, per event: 1.52 mg/kg-bw |
| Spray paint, based on a spray can scenario described in ConsExpo (2006) | Use frequency: 2/year Exposure duration: 20 min. Application duration: 15 min. Product amount: 280 g (assuming one 10-oz can is used) Weight fraction: 0.01 Inhalation module: evaporation from increasing area Mass transfer rate: Langmuir’s method Molecular weight matrix: 300 g/mol Room volume: 34 m3 (assuming the work is done in garage) Ventilation rate: 1.5 air changes/h Release area: 1.5 m2 Contact rate: 100 mg/min. Update fraction: 1 |
Inhalation: Mean event concentration: 39.4 mg/m3 Mean concentration on the day of event: 0.547 mg/m3 Dermal: Acute, per event: 0.212 mg/kg-bw |
| Spray paint used by children aged 5 to 11 years, based on a spray can scenario described in ConsExpo (2006) | Use frequency: 2/year Exposure duration: 20 min. Application duration: 15 min. Product amount: 280 g (assuming one 10-oz can is used) Weight fraction: 0.002 Inhalation module: evaporation from increasing area Mass transfer rate: Langmuir’s method Molecular weight matrix: 300 g/mol Room volume: 20 m3 Ventilation rate: 0.6 air changes/h Release area: 1.5 m2 Contact rate: 100 mg/min. Update fraction: 1 |
Inhalation: Mean event concentration: 13.7 mg/m3 Mean concentration on the day of event: 0.19 mg/m3 Dermal: Acute, per event: 0.0968 mg/kg-bw |
| Tub and tile coating, based on “two- component paints” scenario for pre- application, and waterborne paints (brush and roller) scenario for application. Both scenarios are described in ConsExpo (2006). | Pre-application, mixing and loading: Use frequency: 0.33/year Exposure duration: 5 min. Application duration: 5 min. Product amount: 12700 g (assuming one 1-qt refinishing kit is used) Weight fraction: 0.05 Inhalation module: evaporation from constant release area Mass transfer rate: Thibodeaux’s method Molecular weight matrix: 45 g/mol Room volume: 1 m3 (personal space) Ventilation rate: 0.6 air changes/h Release area: 95 cm2 Product spilled amount: 50 mg/event Update fraction: 1 Application: Use frequency: 0.33/year Exposure duration: 70 min. Application duration 60 min. Product amount: 12700 g Weight fraction: 0.05 Inhalation module: evaporation from increasing area Mass transfer rate: Thibodeaux’s method Molecular weight matrix: 45 g/mol Room volume: 10 m3 Ventilation rate: 2 air changes/h Release area: 3 m2 Contact rate: 30 mg/min. Update fraction: 1 |
Pre-application: Inhalation: Mean event concentration: 2.52 mg/m3 Mean concentration on the day of event: 0.00874 mg/m3 Dermal: Acute, per event: 0.0353 mg/kg-bw Application: Inhalation: Mean event concentration: 243 mg/m3 Mean concentration on the day of event: 11.8 mg/m3 Dermal: Acute, per event: 1.27 mg/kg-bw |
| Automotive fuel injector cleaner. Assuming the product is to load into the fuel system all at once, exposure only occurs during the loading process | Use frequency: 2/year Exposure duration: 1 min. Application duration: 1 min. Product amount: 280 g (assuming one 10-oz bottle is used) Weight fraction: 0.05 Inhalation module: evaporation from constant release area Mass transfer rate: Langmuir’s method Molecular weight matrix: 3000 g/mol Room volume: 1 m3 (personal space) for event exposure and 34 m3 (garage) for estimating mean concentration over the day Ventilation rate: 1.5 air changes/h Release area: 0.002 m2 Product spilled amount: 0.01g/event Update fraction: 1 |
Inhalation: Mean event concentration: 7030 mg/m3 (over 1 min.) Mean concentration on the day of event: 0.24 mg/m3 Dermal: Acute, per event: 0.00705 mg/kg-bw |
| Lubricant (spray), assuming an adult user lubricants door hingesb | Use frequency: 10.7 (US EPA 2011) Exposure duration: 60 min. Application duration: 10 seconds Product amount: 28 g Weight fraction: 0.01 Inhalation module: evaporation from increasing area Mass transfer rate: Langmuir’s method Molecular weight matrix: 3000 g/mol Room volume: 20 m3 Ventilation rate: 0.6 air changes/h Release area: 0.01 m2 (based on part of two door hinges) Contact rate: 100 mg/min. Update fraction: 1 |
Inhalation: Mean event concentration: 10.5 mg/m3 Mean concentration on the day of event: 0.437 mg/m3 Dermal: Acute, per event: 0.0024 mg/kg-bw |
a Assuming body weight of 70.9 kg and inhalation rate of 16.2 m3/day for an adult and body weight of 31 kg and inhalation rate of 14.5 m3/day for children aged 5 to 11 years.
b Due to the volatility of cumene, inhalation exposure is based on “evaporation from increasing area” module in ConsExpo (2006).
Appendix B. BMD estimation for cumene

Figure B-1. Incidences of alveolar/bronchiolar adenoma or carcinoma in response to inhalation concentrations of cumene in female mice (NTP 2009).
The dose-response curve was plotted with multistage cancer model by using BMD2.5 program (US EPA) in determining BMD10/BMDL10. BMD10 and BMDL10 were determined at 17.1 and 14.1 ppm respectively. BMDL10 based on a duration exposure (6 hours/day and 5 days/week) in this study was converted to a continuous exposure with the units of mg/m3 via the following equation:
BMDL10 =14.1 ppm x (120.2 g/mol /24.45 L/mol) x (6/24) x (5/7) =12.4 mg/m3
(where 120.2 is molecular mass of cumene; 24.45 is the volume of 1 mol substance in the gas phase at 25 °C)
