Screening assessment certain organic flame retardants substance grouping 1H-isoindole-1,3(2H)-dione, 2,2'-(1,2-ethanediyl)bis[4,5,6,7-tetrabromo- (EBTBP)
Official title: Screening assessment certain organic flame retardants substance grouping 1H-isoindole-1,3(2H)-dione, 2,2'-(1,2-ethanediyl)bis[4,5,6,7-tetrabromo- (EBTBP)
Chemical Abstracts Service Registry Number 32588-76-4
Environment and Climate Change Canada Health Canada
May 2019
Cat. No.: En14-367/2019E-PDF
ISBN 978-0-660-29860-3
Synopsis
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of 1H-isoindole-1,3(2H)-dione, 2,2'-(1,2-ethanediyl)bis[4,5,6,7-tetrabromo- (CAS RN 32588-76-4), commonly known as ethylene bis(tetrabromophthalimide) and denoted with the abbreviation EBTBP. EBTBP is a substance within the Certain Organic Flame Retardants (OFR) Substance Grouping, which includes ten organic substances having a similar function: application to materials to slow the ignition and spread of fire. This substance was identified as a priority for assessment on the basis of possible human health concerns (related to potential for exposure).
EBTBP does not occur naturally in the environment.
Results from an industry survey conducted for the year 2011 indicated that EBTBP was not manufactured in Canada in 2011; however, 1000 to 10 000 kg of neat EBTBP substance, 10 000 to 100 000 kg of formulation and 100 000 to 1 000 000 kg of EBTBP in manufactured items were imported into Canada.
EBTBP is used in Canada solely as a flame retardant, including in plastic and rubber materials and in the automotive sector. This substance is used as an alternative to decabromodiphenyl ether (decaBDE). Globally, EBTBP is used as a flame retardant in plastics, rubbers and textiles. This substance is also used in electronic applications and components.
Releases to the environment are likely to occur as a result of manufacture, transport, use, and disposal of EBTBP or materials containing EBTBP.
Few measured physical and chemical data are available on EBTBP. EBTBP is characterized by low modelled water solubility, and very low modelled vapour pressure and Henry’s Law constant and very high modelled values for the octanol-water partition coefficient. Modelled physical and chemical properties indicate that EBTBP will likely distribute into sediment and soil, binding to the organic fraction of particulate matter. Also, long-range transport in water is not likely for EBTBP because of its limited water solubility and high organic carbon-water partition coefficient. EBTBP is characterized by a short gas phase modelled half-life of 6.5 hours; however, greater than 99% of the chemical is expected to partition to the particulate aerosol phase, where degradation in air would be very limited. When adsorbed to atmospheric aerosols, EBTBP is expected to reside in air long enough to be transported through the atmosphere at a significant distance from its emission sources.
There are limited empirical data on persistence, bioaccumulation and environmental toxicity available for EBTBP. Few analogous structures with empirical data are available for EBTBP. However, some experimental persistence and environmental toxicity data for the closest analogue, decabromodiphenyl ethane (DBDPE), were considered as read-across information for these endpoints, which in turn are partly considering read-across information from its structural analogue decaBDE.
According to the modelled and limited experimental biodegradation data, EBTBP is expected to be subject to limited biodegradation. Overall, EBTBP may persist in water, sediment, soil, atmospheric aerosols, but not in air.
According to the only available fish bioconcentration study, EBTBP has a low to moderate potential for bioconcentration. However, this empirical result was not reliable because the concentrations in this study were higher than the water solubility of EBTBP. Nevertheless, EBTBP has a very high octanol-water partition coefficient and very low water solubility resulting in limited bioavailability even through dietary exposure. Thus, EBTBP is expected to have a low potential to bioaccumulate in organisms.
It is expected that EBTBP may be released to the Canadian environment as a result of industrial processing activities. Although EBTBP can be found in commercial products or products available to consumers, information on releases to the environment from this route is limited, and releases are expected to be diffused and minimal compared to industrial release. Industrial scenarios, on the basis of available site information, were developed to estimate releases to water. Predicted sediment concentrations were determined according to the equilibrium partitioning. EBTBP exposure in soils was estimated on the basis of a scenario of biosolids application.
Risk quotient analyses, integrating conservative estimates of exposure with toxicity information, were performed for the sediment and terrestrial compartments (soil). The limited available empirical toxicity data for EBTBP are indicative of a low level of acute toxicity to aquatic and mammalian (rodent) organisms. Considering EBTBP’s low bioavailability, very low water solubility and very high octanol-water partition coefficient, EBTBP is unlikely to have acute toxicity effects on aquatic organisms. Thus, a risk analysis was not performed for aquatic organisms. An equilibrium sediment-water partition approach was used to estimate the concentration of EBTBP in bottom sediment. Sediment exposure scenarios were developed as an extension of the industrial aquatic release scenarios to determine equilibrium sediment PECs (predicted environmental concentrations). Soil exposure scenarios were developed as an extension of the aquatic scenarios using biosolids concentration and production rates on the basis of site specific wastewater treatment plants.
While empirical and modelled biodegradation data suggest EBTBP is very stable in water, soil and sediment, EBTBP is not expected to be highly bioavailable or to highly accumulate in organisms, and is not expected to present risk in the environment on the basis of current estimated exposures.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from EBTBP. It is concluded that EBTBP does not meet the criteria under paragraph 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or may constitute a danger to the environment on which life depends.
No classifications of the health effects of EBTBP by national or international regulatory agencies were identified. No chronic or carcinogenicity studies on EBTBP were found. On the basis of the available information regarding genotoxicity, EBTBP is not genotoxic in vitro.
No adverse effects were observed in experimental animals exposed orally to EBTBP at the highest doses tested in short-term and sub-chronic studies. In developmental toxicity studies, no treatment-related maternal or developmental effects were observed in experimental animals exposed to EBTBP via the oral route up to the highest dose tested.
The highest doses tested in experimental animal studies, with no treatment related effects, are six orders of magnitude higher than the estimates of EBTBP intake from environmental media for the Canadian general population. This margin is considered to be adequate to account for uncertainties in the health effects and exposure databases.
On the basis of the information presented in this screening assessment, it is concluded that EBTBP does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Overall Conclusion
It is concluded that EBTBP does not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to sections 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health conduct screening assessments of substances to determine whether these substances present or may present a risk to the environment or to human health.
The Substance Groupings Initiative is a key element of the Government of Canada’s Chemicals Management Plan (CMP). The Organic Flame Retardant (OFR) substance grouping is part of the Groupings Initiative of the Government of Canada’s Chemical Management Plan (CMP). The grouping consists of ten substances identified as priorities for action as they met the categorization criteria under section 73 of CEPA, or were considered as a priority on the basis of ecological or human health concerns (ECCC, HC [modified 2017]). All of these substances have a similar function: the application to materials to slow the ignition and spread of fire. Also, these substances are potential alternatives for other flame retardants which are presently subject to regulatory controls or phase-out globally, and/or in Canada.
This screening assessment concerns the substance 1H-isoindole-1,3(2H)-dione, 2,2'-(1,2-ethanediyl)bis[4,5,6,7-tetrabromo- (CAS RN 32588-76-4), commonly known as ethylene bis(tetrabromophthalimide) and denoted with the abbreviation EBTBP. This substance was identified in the categorization of the Domestic Substance List (DSL) under subsection 73(1) of CEPA as a priority for assessment on the basis of other human health concerns. At categorization, the substance also met criteria for persistence, but was uncertain with respect to meeting criteria for bioaccumulation and inherent toxicity to non-human organisms.
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposure, including additional information submitted by stakeholders. Relevant data were identified up to January 2017 for the ecological and human health sections. Empirical data from key studies as well as some results from models were used to reach the conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
The ecological and human health portions of this assessment have undergone external written peer review and/or consultation. Comments on the technical portions relevant to the environment were received from Dr. Jon Arnot, Arnot Research and Consulting Inc., Dr. Adiran Covaci, Department of Biology at University of Antwerp, Dr. Laurence Deydier at European Chemicals Agency, and Dr. Marcia L. Hardy, Senior Toxicology Advisor at Albemarle Corporation. Comments on the technical portions relevant to human health were received from Michael Jayjock, the Lifeline Group, Penny Fenner-Crisp, Independent consultant, and John Reichard, Toxicology Excellence for Risk Assessment (TERA). Additionally, the draft of this screening assessment was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada (ECCC).
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precautionFootnote 1 . This screening assessment presents the critical information and considerations on which the conclusion is based.
2. Substance identity
This screening assessment focuses on the substance 1H-Isoindole-1,3(2H)-dione, 2,2'-(1,2-ethanediyl)bis[4,5,6,7-tetrabromo- (CAS RN 32588-76-4), also known as ethylene bis(tetrabromophthalimide) (NCI 2013). For this assessment, ethylene bis(tetrabromophthalimide) will be referred to as EBTBP. The substance identity of EBTBP is presented in Table 2-1. Other names for the substance are also available (ECCC 2017a).
| CAS RN | Chemical structure | Molecular mass | Chemical formula |
|---|---|---|---|
| 32588-76-4 | 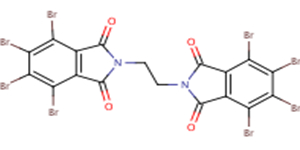 |
951.47 g/mol | C18H4Br8N2O4 |
2.1 Selection of analogues and use of (Q)SAR models
Structural analogues having relevant empirical data may be used to help assess those substances that lack empirical data. Structural analogues are chemicals that are structurally similar to one another and are therefore expected to have similar physical and chemical properties, behave similarly in the environment, and demonstrate similar toxicities in non-human organisms (as a function of bioavailability and chemical reactivity). Where there are experimental data for a given property of an analogue substance and the analogue has a very similar structure, chemical reactivity and bioavailability, data can be used directly (as read-across data) or with adjustment as an estimate of that property value for the substance under assessment. If there are slight to moderate differences in structure, adjustments to property estimates can be made to account for these differences using quantitative methods (e.g., Experimental Value Adjustment method in EPI Suite) or qualitative methods (e.g., using the analogue as a baseline from which to extrapolate). However, where there are differences in reactivity (specific mode of action, for example), an analysis is conducted to determine if the structural analogue has suitable read-across potential for the biological endpoint or property.
For the ecological section of this assessment, two analogues having closest chemical structures to EBTBP (having two aromatic rings that are fully brominated even though they do not contain the indoline-1,3-dione moiety) were found using the Organisation for Economic Co-operation and Development Quantitative Structure Activity Relationship (OECD QSAR) Application Toolbox 2012 (Table 2-2). These are decabromodiphenyl ether (decaBDE; CAS RN 1163-19-5) and decabromodiphenyl ethane (DBDPE; CAS RN 84852-53-9).
Given the structural difference relating to the indoline-1,3-dione moiety (imide group) of EBTBP in comparison with DBDPE and decaBDE, these substances are not considered to be suitable analogues for EBTBP in relation to persistence and transformation. In this regard, the imide group imparts significant uncertainty respecting the pathway of transformation and whether it would be analogous to decaBDE or DBDPE. However, as explained below, DBDPE and decaBDE are considered suitable analogues for read-across to EBTBP for physical and chemical properties and toxicity. The OECD QSAR Toolbox v3.0 indicates that these are all considered to elicit a base surface narcotic mode of toxic action, and none show differences in reactivity. Since decaDBE has more empirical physical-chemical properties than DBDPE, data on decaBDE are considered for potential read-across to EBTBP (Environment Canada 2006a, 2010). Like EBTBP, DBDPE is used as an alternative flame retardant for decaBDE. Because the applications of DBDPE are similar to EBTBP, likely related to similiarities in their properties, the environmental fate data for DBDPE are compared with the environmental fate of EBTBP. The toxicity data used for DBDPE assessment are also considered when assessing the ecological effects and exposure scenarios of EBTBP.
| CAS RN or acronym | Substance name | Chemical structure | Available empirical data (endpoints) |
|---|---|---|---|
| EBTBP 32588-76-4 | Ethylene bis(tetrabromophthal-imide) | 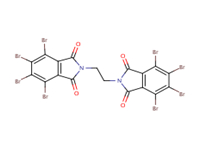 |
Biodegrada-tion, BCF, aquatic toxicity |
| decaBDE 1163-19-5 | Decabromodiphenyl ether | 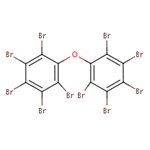 |
WS, MP, biodegrada-tion, BCF |
| DBDPE 84852-53-9 | Decabromodiphenyl ethane | 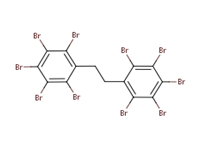 |
VP, WS, Log Kow, sediment and soil toxicity data |
3. Physical and chemical properties
Selected key physical and chemical property data for EBTBP are presented in Table 3-1 (ECCC 2017a).
There are limited experimental physical and chemical properties for EBTBP; therefore, read-across of relevant empirical information from its analogue decaBDE and modelled results are considered.
Prediction of the environmental fate of organic compounds requires knowledge of their tendency to stay in the water phase. At room temperature, EBTBP is in a solid state. The subcooled fugacity ratio correction for solids was conducted using a fugacity (F) ratio. The F ratio was determined using the melting point of EBTBP for this assessment (ECCC 2017a). The subcooled fugacity ratio was applied to solubility in octanol, water solubility and vapour pressure (ECCC 2017a). Subsequently, physical-chemical properties of EBTBP were checked for internal consistency and relative variance of these physical-chemical properties was accounted for in the Least-Square Adjustment (LSA) approach as per Schenker et al. (2005) (ECCC 2017a). These physical chemical properties resulting from LSA were then used as input to EPI Suite v4.1 to obtain Henry’s Law constant and organic carbon-water partition coefficient (Koc). Accordingly, the physical and chemical properties used in this assessment are presented in Table 3-1.
EBTBP is a white or a light yellow powder at room temperature (Albemarle 2005, 1999a, b) and has a high melting point. EBTBP is characterized by low water solubility, vapour pressure and Henry’s Law constant (modelled), and a high modelled octanol-water partition coefficient (Kow), Koc and octanol-air partition coefficient (Koa) (Table 3-1, ECCC 2017a).
| Property | Type | Value | Temperature | Reference |
|---|---|---|---|---|
| Physical form | Experimental | White to light yellow powder | NS | Albemarle 2005 |
| Melting point (°C) | Experimental | 445 | NA | EPI Suite 2012 [MPBPVP 2010] Experimental database structure match: Saytex BT 93 |
| Boiling point (°C) | Experimental | 827.9 (at 760 mmHg) | NA | ChemNet 1997 World of Chemicals 2013 (empirical) |
| Vapour pressure (Pa) | Estimated | 1.81 x 10-25 (liquid subcooled 2.59 x 10-21a) | NS | Geomeana; Subcooled fugacity ratio calculations using melting point and LSAa |
| Henry’s Law constant (Pa•m3/mol) | Modelled | 4.15 x 10-21 (4.21 x 10-16 atm•m3/mole) | NS | EPI Suite 2012 [HENRYWIN 2011]a |
| Log Kow (dimensionless) | Estimated | 8.90 | NS | Average value of estimates and LSAa |
| Log Koc (dimensionless) | Modelled | 5.34 (Koc = 2.22x 105 L/kg) | NS | EPI Suite 2012 [KOCWIN 2010]a |
| Log Koa (dimensionless) | Modelled | 27.52 (Koa = 3.29 x 1027) | NS | LSAa |
| Log Kaw (dimensionless) | Modelled | -18.62 (Kaw = 2.40 x 10-19) | NS | LSAa |
| Water solubility (mg/L) | Modelled | 2.88 x 10-7 (liquid subcooled 0.00413a) | NS | Geomeana; Subcooled fugacity ratio calculations using melting point and LSAa |
| pKa (dimensionless) | NA | NA | NA | NA |
Abbreviations: Log Kow, octanol-water partition coefficient;
Log Koc, organic carbon-water partition coefficient;
Log Koa, octanol-air partition coefficient;
Log Kaw, air-water partition coefficient;
pKa, acid dissociation constant;
NA, not applicable. NS, not specified.
a ECCC 2017a
4. Sources
EBTBP is not naturally occurring in the environment. EBTBP is commercially produced in Arkansas, U.S. (NIEHS 1999) and in Connecticut, U.S. (European Commission 2000b). It also appears to be manufactured in China (Unibrom 2014). Most environmental EBTBP may be the result of release from industrial processes that use this substance.
In 1986, it was reported that from 1000 to 1 000 000 kg of EBTBP were imported into Canada (Environment Canada 1986). In 2000, information on EBTBP submitted in response to the Notice with respect to Certain Substances on the DSL (Canada 2001) issued pursuant to Section 71 of CEPA indicated that no manufacture of this substance was occurring in Canada; however, it was reported as being imported in a quantity ranging from 10 000 to 100 000 kg. Information submitted in response to a 2013 section 71 Notice with respect to certain organic flame retardant substances (ECCC 2013 to 2014) issued pursuant to Section 71 of CEPA indicated that EBTBP was not manufactured in Canada in 2011, although amounts in the range of 100 000 to 1 000 000 kg were imported into the country. This amount of EBTBP was mostly imported to Canada in commercial products or products available to consumers (100 000 to 1 000 000 kg) with lesser amounts imported in a formulation (10 000 to 100 000 kg) and as a neat substance (1000 to 10 000 kg) (ECCC 2013 to 2014).
In the United States, production of EBTBP was reported to be less than 450 tonnes in 1990, and greater than 450 tonnes in 1986 and 1994 (NIEHS 1999). In 1994, EBTBP was listed on the U.S. High Production Volume chemical list. According to U.S. Environmental Protection Agency (US EPA 2006) Inventory Update Reporting, EBTBP was reported to be manufactured in the U.S. in annual quantities ranging from approximately 450 to 4500 tonnes. In Europe, the total tonnage per year has previously been reported at 5250 tonnes (United Kingdom Environment Agency 2003). EBTBP is also listed as an OECD HPV substance (OECD 2011). EBTBP has been used in Nordic countries since 1999 (SPIN 2006); however, because of information confidentiality, the annual quantity of manufacture or use is not available.
5. Uses
EBTBP is used as an additive flame retardant in plastics, rubbers and textiles. EBTBP has been identified as having increased deflection temperature (heat distortion temperature) under load and better UV stability than other flame retardants (OECD 1995). Its thermal and UV stability, as well as its resistance to bloom, make it favourable as a flame retardant (NIEHS 1999). Specifically, it is used as an additive flame retardant in high impact polystyrene (HIPS), polyethylene, polypropylene, thermoplastic polyesters, polyamide, ethylene propylenediene terpolymers (EPDM rubbers) and other synthetic rubbers, polycarbonate, ethylene copolymers, ionomer resins, epoxies, and textile treatments (Radloff et al. 1996, IPCS 1997, Tice 1999, Covaci et al. 2011). Manufactured items that may contain EBTBP include electrical and electronic components such as wire and cable insulation, switches and connectors, construction materials, storage and distribution products, automotive products, and waterborne emulsions and coatings (IPCS 1997, US EPA 2014, Albemarle 1999a, b, Albemarle 2004, Covaci et al. 2011).
EBTBP is considered a replacement for decaBDE, which was used as a flame retardant in electronic products (Kolic et al 2009). It is also suggested that EBTBP is a replacement for decaBDE in the production of HIPS (US EPA 2014).
In Canada, uses of EBTBP were reported in response to a survey issued pursuant to Section 71 of CEPA (Canada 2013). EBTBP is used in Canada as a flame retardant in plastic and rubber materials, in electrical and electronics, and in the automotive sector (ECCC 2013 to 2014). Less than 10 000 kg of EBTBP was reported as imports of the neat substance (ECCC 2013 to 2014). The main use of EBTBP in products available to consumers is its addition to plastics to make up wire and cable coatings and electronic housing. Hot melt adhesives used by the general consumer in hobby pursuits may also contain EBTBP (MSDS 2010).
EBTBP is not listed as an approved food additive in the Lists of Permitted Food Additives, which have been incorporated by reference into their respective Marketing Authorizations issued under the Food and Drugs Act (Health Canada [modified 2017]), nor has it been identified as being used/present in formulations of food packaging materials or incidental additives (2013 email from Food Directorate, Health Canada, to Risk Management Bureau, Health Canada; unreferenced). EBTBP is not listed in the Drug Product Database (DPD [modified 2017]), the Therapeutic Products Directorate's internal Non-Medicinal Ingredient Database, the Natural Health Products Ingredients Database (NHPID [modified 2017]) or the Licensed Natural Health Products Database (LNHPD [modified 2016]) as a medicinal or a non-medicinal ingredient present in final pharmaceutical products, natural health products or veterinary drugs (2013 email from Therapeutic Products Directorate, Natural Health Products Directorate and Veterinary Drugs Directorate, Health Canada, to Risk Management Bureau, Health Canada; unreferenced). On the basis of the notification submitted under the Cosmetic Regulations to Health Canada, EBTBP is not anticipated to be used in cosmetics in Canada (personal communication, email from the Consumer Product Safety Directorate [Health Canada] to the Existing Substances Risk Assessment Bureau [Health Canada], dated 2013; unreferenced).
6. Releases to the environment
Anthropogenic releases to the environment depend upon various losses occurring during the manufacture, industrial use, consumer/commercial use, service life and disposal of a substance.
In consideration of submissions made under section 71 (Canada 2013), EBTBP is imported into Canada in a neat form, as a formulation component, and in manufactured items (Canada 2013). Although not specifically identified by importers of EBTBP in Canada, the uses considered for EBTBP for this assessment are assumed to be similar to DBDPE because of their common usage profiles, only with lower use quantities than DBDPE. Therefore, exposure scenarios for EBTBP were informed by those developed for DBDPE (see assessment on DBDPE; ECCC, HC 2018). The following processes were used as a basis to develop exposure scenarios for EBTBP in Canada: textile manufacturing, rubber compounding, plastic extrusion, plastic injection molding, and textile coating (ECCC 2017b).
Similar to DBDPE, EBTBP release to the environment is expected to occur during the manufacturing, formulation and/or industrial use stages of these sectors. Releases to the environment are expected to occur primarily through wastewater, with some release to water directly from industrial sites. Release to the soil (i.e., to agricultural land) could also occur through the application of biosolids. In terms of migration from manufactured items, as an additive brominated flame retardant that is blended with the polymer product (rather than a reactive flame retardant chemically bonded to the polymer product), there is the possibility for some release from products available to consumers to the environment (Andersson et al. 2006; Guerra et al. 2011). However, it is expected that releases to the environment via this route are minimal and diffuse.
Emissions to air can result in atmospheric deposition to soil and water. When a substance is unintentionally transferred to land, it may be washed into sewers or surface water or transferred by wind or rain to nearby soil. However, because of the low volatility of EBTBP, the atmospheric pathway of release is expected to be very limited. Finally, landfills that do not collect and treat their leachate may potentially release substances to ground or surface water via leachate.
This information is used to further develop exposure characterization scenarios to estimate resulting environmental concentrations.
7. Environmental fate and behaviour
Data concerning concentrations of EBTBP in the Canadian environment are lacking in the available literature. Although EBTBP has been imported into the country for some time, there is no report of this substance being found in the Canadian environment.
In other jurisdictions, only one environmental concentration is available. EBTBP was detected in one of the 3 replicate seepage water samples at a concentration of 0.035 g/L at a metal recycling factory’s discharge point into the Loselva River in Norway (Nyholm et al. 2013).
7.1 Environmental distribution
EBTBP is expected to be released primarily to wastewater where it is expected to sequester into biosolids. Level III fugacity modelling (Table 7-1), using the updated EQC model (v1.0, 2012), was applied to describe the fate for possible modes of entry into the environment.
| Release of EBTBP to each compartment | Air (%) | Water (%) | Soil (%) | Sediment (%) |
|---|---|---|---|---|
| 100% to Air | 0.3 | 0.4 | 90.5 | 8.8 |
| 100% to Water | Negligible | 4.6 | Negligible | 95.4 |
| 100% to Soil | Negligible | Negligible | 99.8 | 0.2 |
a EQC v1.00 2012.
b Physical Chemical properties and half-lives (t1/2) of EBTBP in environmental media are required for modelling and are available (ECCC2017a)
The results of Level III fugacity modelling (EQC 2012, see Table 7-1) indicate that EBTBP will predominantly reside with the solid phase of the medium it is released to or be deposited back to soil.
When EBTBP is released to air, less than 0.3% of the substance is expected to reside in air. In the gas phase, EBTBP is quickly degraded through reactions with hydroxyl radicals (t ½ of less than or equal to 1 day) (see Table 7-3) and will partition to the particulate phase in air (i.e., Log Koa of 27.52). Therefore, in the gas phase, EBTBP is not expected to undergo long-range transport to remote regions in air. The particulate phase is deposited to land and water as wet and dry deposition. For the amount transferred from air to soil, the majority (90.5%) will remain in soil while a smaller fraction can be further transported as surface runoff to aqueous systems and, when combined with atmospheric inputs, result in approximately 8.8% of the mass fraction in sediment.
The OECD POPs Screening Model can be used to help identify chemicals with high persistence and long-range transport potential (Scheringer et al. 2009). The Characteristic Travel Distance (CTD) calculated for EBTBP using the OECD model is 2 860 km indicating that EBTBP has a significant potential for transport in air (with 100% of mass in air partitioned to particles/aerosols), but this is below the boundary (5 097 km, CTD of PCB 28) suggested for global pollutants by Klasmeier et al. (2006). The model also calculates an overall persistence (Pov) of 547 days, and the transfer efficiency (TE), which is the percentage of emission flux to air that is deposited to the surface (water and soil) in a remote region. The TE for EBTBP was calculated to be 12.7%, which is above the boundary of 2.248% (PCB-28). The high TE means that EBTBP will likely be deposited to some degree to Earth’s surface in remote regions.
Some organic flame retardants, such as certain polybrominated diphenyl ethers (PBDEs), are known or strongly suspected to undergo long-range transport in air associated with fine suspended particulates (Harner and Shoeib 2002, Muir et al. 2006). In general, while EBTBP (based on physical and chemical properties and modelling) might not be expected to be a high concern for long-range transport, when considering the high predicted transfer efficiency, the role of particle (aerosols) bound transport suggests that long-range transport of EBTBP is possible.
Results from both the AEROWIN Program (v1.00) and the OECD POPs Screening Model suggest that 100% of the fraction released to air will be associated with the particulate phase largely owing to a high estimated Log Koa value. In addition, the estimated overall persistence of 547 days in aerosols (6.5 hours in gaseous phase) by the OECD POPs Screening Model suggests that photodegradation for EBTBP may be insignificant or greatly reduced. Model estimates of CTD, Pov and TE; however, are not reliable for this substance because of uncertainty associated with key modelled partition coefficients (Koa, Kow, Kaw) required as input.
When released to surface water, the vast majority (95.0%) of EBTBP is expected to strongly adsorb to suspended solids and eventually sink to sediment. Volatilization from surface water to air is very low. Thus, loss of EBTBP from aqueous systems would primarily be a result of sediment burial and from biodegradation that is expected to be very slow (see Table 7-2 and Table 7-3).
When EBTBP is released to soil the majority of the mass fraction is expected to become adsorbed to soil (99.9%) owing to its very hydrophobic nature. Evaporation from soil into air is not expected owing to its extremely low vapour pressure. EBTBP is also expected to be very stable in soil and resistant to mineralization (t ½ of greater than 182 days) (see Table 7-2 and Table 7-3), and thus the loss process in soil will also be driven mainly by soil burial or surface runoff as described above.
7.2 Environmental persistence
Based on likely EBTBP releases and partitioning characteristics, environmental persistence is most relevant for the soil and sediment compartments where the majority of the substance is expected to be found. However, because of the potential particle transport of EBTBP in air and water, all media are considered in this section. Empirical and modelled data were considered in the weight-of-evidence for EBTBP persistence. Data were also compared to the analogue, DBDPE.
Results of empirical and modelled biodegradation data indicate that the half-life in water is likely in the order of many months to perhaps more than one year. EBTBP would also be very stable in soil and sediment and is likely to present long-term exposures in these media.
Tables 7-2 and 7-3 present empirical and modelled degradation data for EBTBP.
7.2.1 Empirical persistence data
Table 7-2 presents the empirical data for degradation of EBTBP. Empirical data on persistence for EBTBP is scarce in the available literature; therefore, persistence data on DBDPE is also considered for read-across to EBTPB (also shown in Table 7-2).
One study on aerobic biodegradation by the Chemical Biotesting Center in Japan was reported by the Albemarle Corporation (US EPA 2004, US EPA 2014). A ready biodegradation test in accordance with (MITI) guidelines was conducted in 1981 in Japan. In this study, standard activated sludge was inoculated with 100 mg/L of EBTBP and incubated at a temperature of 25 ±1°C under 14-day contact time. Measurement of biochemical oxygen demand (BOD) was 0% on day-28. Thus, EBTBP was not readily biodegradable in this study.
| Sub-stance | Medium | Fate process | Degrada-tion value | Degradation endpoint / units | Method | Reference |
|---|---|---|---|---|---|---|
| EBTBP | Standard Activated Sludge | Ready Bio-degradation Test (Aerobic Biodegrada-tion) | 0% | % degradation by BOD or UV after 28 days | OECD 301C (Modified MITI I test) | US EPA 2004; US EPA 2014 |
| DBDPE | Activated sludge | Bio-degradation | 2% | 28-day Biodegradation BOD/% | OECD 301C (Modified MITI I test) | CITI 1991 |
| DBDPE | Mineral salts aqueous media | Bio-degradation | 2.2% | 90-day Biodegradation/% | OECD 302D (CONCAWE test) | Schaefer and Carpenter 2010 |
| DBDPE | Anaerobic digester biosolids | Biotic/ Abiotic Anaerobic mineraliza-tion | 0 (biotic) 0 (abiotic) | 63-day anaerobic mineralization /% | OECD 314C (Anaerobic digester biosolids) | Schaefer and Matthews 2011 |
a Robust study summaries (RSS) were conducted for EBTBP to determine the quality of the study and are either appended (for critical studies) or are available upon request
The available empirical ready-biodegradation data for DBDPE also generally show 0% or close to 0% biodegradation over 28 days (US EPA 2004, US EPA 2014). Considering the available persistence data on EBTBP and DBDPE, it is concluded that EBTBP is resistant to biodegradation. These studies also showed no evidence of hydrolysis and this is substantiated by the lack of hydrolysable functional groups present in the molecules.
Two predicted degradation products of EBTBP with quantities of 0.1257 and 0.1745 moles/1 mole parent are identified by CATALOGIC 2012. However, these quantities are considered insignificant.
7.2.2 Modelling of persistence
Table 7-3 summarizes the results of available QSAR models for degradation in various environmental media.
A QSAR-based weight-of-evidence approach was also applied using the degradation models outlined in Table 7-3. Given the ecological importance of the soil and sediment compartments and the fact that EBTBP is expected to reside mainly in these compartments, it is considered reasonable and relevant to examine biodegradation in soil and sediment.
The probability of biodegradation using the TOPKAT model (2004) could not be obtained as the results are not within the optimum prediction space or the structural domain of the model. Therefore, these results are not reported here since they are considered unreliable. The CATALOGIC model (2012) also did not recognize 56% of EBTBP’s fragments, and thus, the estimate is considered to have low reliability. Nevertheless, both TOPKAT and CATALOGIC suggest very slow rates of mineralization from biodegradation consistent with empirical data and other models (Table 7-3). Predicted (modelled) persistence data from BIOWIN 3 Expert-Survey, BIOWIN 5 MITI Linear Probability, and BIOWIN 6 MITI Non-Linear Probability suggest that EBTBP is highly stable in water.
In summary, results of empirical and modelled biodegradation data indicate that the half-life in water is likely in the order of many months to perhaps more than one year. Environment Canada has adopted a half-life extrapolation procedure according to Boethling et al. (1995) using a ratio of 1:1:4 for water, soil, sediment. The results of this approach show that EBTBP would also be very stable in soil and sediment and is likely to present long-term exposures in these media.
| Fate process | Model and model basis | Model result and prediction | Extrapola-ted half-life (days) |
|---|---|---|---|
| Atmospheric oxidation (air) | AOPWIN 2010a,e | t 1/2 = 0.271days | ≤ 2 |
| Ozone reaction (air) | AOPWIN 2010a | NAb | NA |
| Hydrolysis (water) | HYDROWIN 2010a | NAb | NA |
| Primary biodegradation (aerobic) | BIOWIN 2010 a Sub-model 4: Expert Survey (qualitative results) | 1.2469c “recalcitrant” | ≥ 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2010 a Sub-model 3: Expert Survey (qualitative results) | 0.0085c “recalcitrant” | ≥ 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2010 a Sub-model 5: MITI linear probability | -0.6854d “does not biodegrade fast” | ≥ 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2010 a Sub-model 6: MITI non-linear probability | 0.0000d “does not biodegrade fast” | ≥ 182 |
| Ultimate biodegradation (aerobic) | TOPKAT 2004 Probability | 0.000d “biodegrades very slowly” | ≥ 182 |
| Ultimate biodegradation (aerobic) | CATALOGIC 2012 % BOD (biological oxygen demand) | % BOD = 9.5 “biodegrades very slowly” Ultimate half-life: 6 months 13 days | ≥ 182 |
a EPI Suite 4.1. (2012)
b Model does not provide an estimate for this type of structure.
c Output is a numerical score from 0 to 5.
d Output is a probability score.
e 8 760 hours instead of 6 months 13 days (CATALOGIC 2012) and EPIWIN prediction of 4 320 hours is selected as the model input for half-life in this assessment for biodegradation time longer than 6 months.
7.3 Potential transformation products
There is no empirical evidence respecting potential transformation products of EBTBP. One could speculate that transformation might occur because of debromination (e.g., similar to decaBDE) and/or ring cleavage followed by debromination, or some other unknown pathway. However, EBTBP contains an imide bridge between the two brominated rings that differs from DBDPE and decaBDE. This suggests susceptibility to biodegradation and ring cleavage, which is unlike the pathway of debromination expected for DBDPE or decaBDE (ECCC, HC 2018; Environment Canada 2010).
The predicted biodegradation products of EBTBP, via ring cleavage with a quantity of 0.01635 moles/1 mole parent eventually leaving a stable degradation product of 4,5-dibromophthalic acid (C8H4Br2O4; CAS RN 24063-28-3) with a quantity of 0.6595 moles/1 mole parent, is identified by CATALOGIC 2012. QSAR modelling predicted that this brominated phthalic acid has low bioaccumulation potential (BAF of 6.177 L/kg ww for middle trophic level fish) and low to moderate inherent toxicity (chronic toxicity of 16.261 mg/L estimated for mysid shrimp).
7.4 Bioaccumulation
The discussion on the potential for bioaccumulation examines several potential parameters, including properties of the substance (i.e., log Kow, log Koa, molecular size and cross-sectional diameters), bioconcentration factor (BCF), biomagnification factor (BMF), trophic magnification factor (TMF) and bioaccumulation factor (BAF). Thederivation and role of metabolism rate constants in determining bioaccumulation potential are also examined.
7.4.1 Physical and chemical properties
With the high modelled log Kow and log Koc values of 8.9 and 5.34, respectively, it is expected that EBTBP will have a strong propensity to sorb to solids like sediments/suspended particulates and soils, resulting in bound residues in the environment. This adsorption would likely limit the bioavailability of this substance, particularly to aquatic organisms. Furthermore, with the high log Kow, it is expected that EBTBP will have low gastro-intestinal tract assimilation efficiency (Kelly et al. 2004). While there is uncertainty with the estimated partition coefficients, it is reasonable to assume that EBTBP is highly hydrophobic and has a very high log Kow on the basis of its chemical structure (i.e., no water solubilizing functional groups and presence of multiple halogen atoms).
7.4.2 Empirically determined bioaccumulation
7.4.2.1 Bioconcentration factor (BCF)
A fish bioconcentration study performed at two water concentrations (Table 7-4) has been reported by Albemarle Corporation (2004). The study concluded that EBTBP did not bioconcentrate in fish (Cyprinus carpio) when tested over an 8-week period. At the highest concentration tested, 2 mg/L, a bioconcentration factor (BCF) of 1.3 was obtained when Cyprinus carpio (freshwater carp) were exposed to EBTBP over a period of 56 days at 25°C. At the lowest concentration tested, 0.2 mg/L, a BCF of less than 3.3 was found for Cyprinus carpio. The study was conducted according to MITI Guidelines, with a test substance purity of greater than or equal to 99%. HCO-40 was used to disperse the test material in water. However, the treatment concentrations of these tests are higher than the water solubility of EBTBP and these tests are therefore considered of low reliability, which causes difficulty in interpreting the calculated BCFs. Although the available experimental BCF data on EBTBP were tested at concentrations exceeding the substance’s water solubility limit (0.00413 mg/L), it is reasonable to conclude that the studies showed a very low level of bioconcentration in fish.
While this study supports a lower tendency for the substance to bioconcentrate from water, it is expected that diet and solids would be the more relevant pathway for exposure to fish given this substance’s hydrophobic nature.
| Test organism | steady-state value (L/kg)a, b | Reference |
|---|---|---|
| Freshwater Carp Cyprinus carpio | <0.3 – 1.3 (2 mg/L) | Albemarle Corporation 2004 |
| Freshwater Carp Cyprinus carpio | <3.3 (0.2 mg/L) | Albemarle Corporation 2004 |
a Values in parentheses represent the test concentrations at which the BCFs were derived.
b Robust study summaries (RSS) were conducted to determine the quality of the study and are either appended (for critical studies) or are available upon request
Information regarding molecular size and cross-sectional diameters are useful to consider and are commonly used by international jurisdictions such as the European Union (ECHA 2014) as weight-of-evidence for bioaccumulation potential. Recent investigations relating fish BCF data and molecular size parameters (Dimitrov et al. 2002, 2005) suggest that the probability of a molecule crossing cell membranes as a result of passive diffusion declines significantly with increasing maximum diameter (Dmax). The probability of passive diffusion decreases appreciably when the maximum diameter is greater than ~1.5 nm and much more so for molecules having a maximum diameter of greater than 1.7 nm. Sakuratani et al. (2008) have also investigated the effect of cross-sectional diameter on passive diffusion in a BCF test set of about 1200 new and existing chemicals. They observed that substances that do not have a very high bioconcentration potential (BCF of less than 5 000) often have a Dmax of greater than 2.0 nm and an effective diameter (Deff) greater than 1.1 nm.
As Arnot et al. (2010) have noted, there are uncertainties associated with the thresholds proposed by Dimitrov et al. (2002, 2005) and Sakuratani et al. (2008) since the BCF studies used to derive them were not critically evaluated. Arnot et al. (2010) point out that molecular size influences solubility and diffusivity in water and organic phases (membranes), and larger molecules may have slower uptake rates. However, these same kinetic constraints apply to diffusive routes of chemical elimination (i.e., slow in = slow out). Thus, significant bioaccumulation potential may remain for substances that are subject to slow absorption processes, if they are slowly biotransformed or slowly eliminated by other processes.
On the basis of the 3D analysis of 30 EBTBP conformers calculated using the BCFmax Model with Mitigating Factors (Dimitrov et al. 2005), the maximum diameters of EBTBP range from 1.48 nm to 2.01 nm and the effective diameter is 1.02 nm. This suggests that EBTBP is more likely to experience a reduced rate of uptake from steric effects at the gill surface allowing elimination processes to mitigate accumulation. This may explain, in part, the low observed empirical BCF values.
7.4.2.2 Biomagnification factor (BMF)
BMF values describe the process in which the concentration of a chemical in an organism reaches a level that is higher than that in the organism’s diet, owing to dietary absorption (Gobas and Morrison 2000). No experimental BMF studies were found in the available literature at the time of this analysis.
7.4.2.3 Trophic magnification factor (TMF)
The TMF is a measure of the biomagnification potential of a substance within a studied food web under field conditions. It is estimated by correlating the normalized substance concentrations in biota at different trophic levels. No TMF values were available for EBTBP in the literature at the time of this analysis.
7.4.2.4 Bioaccumulation factor (BAF)
Bioaccumulation factors are measured under field conditions as the ratio of the whole body chemical concentration taken up from all exposures to that of the ambient water concentrations. Measures of BAF are a preferred metric for assessing the bioaccumulation potential of substances because it incorporates all chemical exposures including the diet, which predominates for substances with log Kow of greater than ~4.0 (Arnot and Gobas 2003a).
No empirical BAF values are available for EBTBP.
7.4.3 Modelling bioaccumulation
Arnot and Gobas (2006) critically evaluated available bioaccumulation data (BCF and BAF) for fish and other organisms and created an empirical database of quality BCF and BAF values that Canada has used for categorization of the DSL and is now using for screening assessments under the Chemicals Management Plan (Arnot and Gobas 2003b). In Arnot and Gobas (2006) and Environment Canada’s own BCF/BAF database, the empirical distribution of “acceptable” fish BCF and BAF data shows that there are practically no recorded values for substance with log Kow above approximately 8.2 (i.e., only one or two highly halogenated biphenyls). The measurement of log Kow above 8 and its consistency with other properties becomes increasingly uncertain owing to the difficulty of measuring partitioning properties accurately for super hydrophobic compounds. Additionally, what little BCF testing that has been conducted beyond this limit always uses solubilizing agents to perform the test, reducing the test’s strength of inference. Finally, the relationship between dietary absorption efficiency and substance log Kow has been investigated by several authors and summarized in Kelly et al. (2004).
Kelly et al. (2004) demonstrated that the absorption of ingested chemical in fish (and other wildlife) decreases with increasing log Kow starting with a log Kow ~ 7 to 7.5 because the diffusion of hydrophobic substances, such as the EBTBP, across an unstirred water layer to the luminal membrane (i.e. gastrointestinal tract) of an organism is rate limiting for very high log Kow with very low solubilities in the water layers. Although Arnot and Gobas (2003a, 2004, 2006) do state that the log Kow domain of the model ranges from 1-9, there is considered to be insufficient empirical field evidence (i.e., BAF) to support model estimates beyond log Kow 8.2. Therefore, the log Kow of 8.90 for EBTBP is considered out of the model domain for the mass-balance three trophic level BCFBAF model (Arnot and Gobas 2003a) and the QSAR based Dimitrov et al. (2005) model. Importantly, lack of empirical BCF and BAF data for chemicals with log Kow greater than 8.3 does not allow for benchmarking of predicted results. Consequently, EBTBP was not modelled in this assessment.
In summary, empirical data on the bioaccumulation potential of EBTBP is limited and the high log Kow for EBTBP has precluded its BCF/BAF modelling. The physical-chemical properties of EBTBP suggest that this substance will have limited bioavailability in the environment owing to high log Kow, low water solubility and the high potential for the formation of bound residues. Even when bioavailability is enhanced, as in the BCF tests conducted by Japan, little bioconcentration was observed. Nonetheless, lack of field measurements of other bioaccumulation metrics (such as BMF or TMF) and lack of modelling capability suggests that some uncertainty remains, particularly with biomagnification potential. EBTPB is judged to have a limited potential for bioconcentration and biomagnification in the environment which will mitigate body burdens of this substance in organisms and reduce overall ecotoxicity potential.
7.5 Summary of environmental fate
EBTBP is expected to be released from industrial sources primarily through wastewater. A strong tendency to sorb to the solid phase in various media (including suspended air particles) means that this chemical will reside in biosolids, sediments, suspended air particles and will be transferred to soil from dry deposition and application of biosolids to land. Exposure to organisms in water is expected to be minimal. EBTBP’s high intrinsic persistence suggests that long-term exposures can be expected in sediment and soil with a potential for significant build-up in near-field environments from continuous emissions. Removal process from the environment would include sediment and soil burial. EBTBP might be expected to undergo long-range transport in air and deposition to remote environments because of fine particle transport, as has occurred with other hydrophobic flame retardants. Even with long-term exposure to EBTBP in terrestrial and aquatic environments, this substance is not expected to be highly bioavailable and thus tissue residue levels in organisms and migration in food webs is not expected to be significant.
8. Potential to cause ecological harm
8.1 Ecological effects assessment
Empirical data for EBTBP, and the structural analogue, DBDPE, were considered in a weight-of-evidence for assessing the ecological effects of EBTBP.
The limited available empirical toxicity data for EBTBP are indicative of a low level of acute toxicity to aquatic and mammalian (rodent) organisms. Given the lack of empirical effects data for EBTBP, studies using DBDPE were also considered. The available data set for DBDPE toxicity includes endpoint values for aquatic, sediment and terrestrial species. Overall, EBTBP is expected to have low toxicity to aquatic organisms because of its high log Kow (8.90) and a very low water solubility (2.88 x 10-7 mg/L) that suggests low bioavailability and limited dietary uptake for EBTBP.
While ECOSAR (2012) modelling was undertaken for EBTBP, both acute and chronic toxicity predictions were considered unreliable and not presented herein because the log Kow value for EBTBP is out of the domain. The suggested log Kow domain limit for acute predictions in ECOSAR is ~5.0 and for chronic toxicity it is ~8.0.
The results of fate modelling indicate that a negligible amount of EBTBP released to water will remain in water and, depending on the media of release, most will partition to sediments or soils. Of the fraction remaining in water (less than 5%), the high modelled log Kow and log Koc and very low water solubility will limit its bioavailability to pelagic organisms. On the basis of the limited amount of acute toxicity data (discussed in the Empirical Studies for the Aquatic Compartment section), EBTBP is not expected to cause effects at saturation in water. For these reasons, predicted no effect concentrations (PNECs) are determined for sediments and soils, but not for water.
8.1.1 Empirical studies for the aquatic compartment
Albemarle has reported an acute toxicity data of, 48-h LC50 of greater than 500 mg/L in orange-red Killifish (Oryzias latipes), a freshwater fish (European Commission 2000a). The study was performed according to MITI Guidelines, with an EBTBP purity of greater than or equal to 99%. No effects at saturation were observed in water and the treatment concentration is many orders of magnitude above water solubility limit for EBTBP in this study. Therefore, this study is not considered reliable and is not considered in this assessment. No other empirical aquatic toxicity data can be found in the available literature. For its analogue, results from the available empirical aquatic toxicity studies for DBDPE also have a high uncertainty. These studies were also characterized by treatment concentrations exceeding DBDPE’s limit of water solubility owing to the use of solubilizers and/or the results were indicative of no effects at saturation.
8.1.2 Empirical studies for other environmental compartments
No other empirical EBTBP toxicity data are available for consideration in this assessment. The available data set for DBDPE toxicity includes endpoint values for benthic and soil organisms. On the basis of the results of soil and sediment chronic toxicity testing, DBDPE has the potential to cause reproductive effects at high soil concentrations to earthworms and to affect plant survival and growth (Aufderheide 2003, Hardy et al. 2011).
Sediment organism toxicity tests have been performed for EBTBP’s analogue, DBDPE, with chironomids (Chironomus riparius) and oligochaetes (Lumbriculus variegates) (Krueger et al. 2003a, b; Hardy et al. 2012) (Table 8-1). Chironomids (midge) were exposed to DBDPE in sediment with overlying water over 28 days under static conditions. For oligochaete tests, 10 oligochaetes per test concentration were exposed to DBDPE for 28 days under flow-through conditions. In both studies, potential effects were noted, but endpoints did not show a significant effect. Therefore, EC50 values and NOECs for all measured endpoints were reported to be above the highest concentration level of greater than5000 mg/kg for both the chironomid and oligochaete studies. As the test sediment contained 1.8% organic carbon, the maximum ”solubility” (ECCC 2017b) of DBDPE in sediment was 298 mg/kg dry weight (dw). The sediment solubility limit, therefore, may have been exceeded under the conditions of the study, although no adverse effects were observed in the test organisms. Therefore, a Critical Toxicity Value (CTV) of 5000 mg/kg is selected for EBTBP in sediment, representing the only EBTBP toxicity endpoint available, although this value is unbounded with no effects observed at this concentration.
| Test Organism | Test Type | Endpoint | Value (mg/kg dw) | Reference |
|---|---|---|---|---|
| Midge (Chironomus riparius) | Prolonged sediment toxicity: survival, emergence and development | 28d EC50 LOEC NOEC | >5000 >5000 5000 | Krueger et al. 2003a, Hardy et al. 2012 |
| Oligochaete (Lumbriculus variegates) | Prolonged sediment toxicity: survivorship and growth | 28d EC50 LOEC NOEC | >5000 >5000 >5000 | Krueger et al. 2003b, Hardy et al. 2012 |
Abbreviations: EC, effective concentration; LOEC, lowest-observed effect concentration; NOEC, no-observed effect concentration
Terrestrial soil toxicity tests for EBTBP’s analogue, DBDPE, were undertaken with wastewater and soil bacteria, earthworms, and plants (Hardy et al. 2011) (Table 8-2). The effects of DBDPE on terrestrial plant seedling emergence and growth were evaluated by Hardy et al. (2011) in a 21-day study. Corn (Zea mays), onion (Allium cepa) and ryegrass (Lolium perenne) represented monocotyledons, while cucumber (Cucumis sativa), soybean (Glycine max) and tomato (Lycopersicon esculentum) represented the dicotyledons. No adverse effects on any endpoint were reported for corn, ryegrass or soybean, resulting in EC25 values greater than 6250 mg/kg. Cucumber’s group mean survival was reduced by 18% at the highest test concentration (LOEC =6250, NOEC=3125 mg/kg). Reductions in onion plant mean height of 22% and 24% and weight reductions of 32% and 30% respectively, were observed at the two highest concentrations (LOEC=3125, NOEC=1563 mg/kg). Effects on tomato height and weight at the highest concentration of DBDPE resulted in reductions of 37% and 40% compared to the controls (LOEC=6250, NOEC=3125 mg/kg). An EC25 for onion was reported as 2440 mg/kg.
| Test Organism | Test Type | Endpoint | Value (mg/kg dw) | Reference |
|---|---|---|---|---|
| Earthworms (Eisenia fetida) | 28-day Survival | LC50 | >3720 | Aufderheide 2003 |
| Earthworms (Eisenia fetida) | 56-day Reproduction | EC10 EC50 LOEC NOEC |
1860 3180 3720 (reproduction reduced 60%) 1910 |
Aufderheide 2003 |
Plants: Monocoty-ledons Onion (Allium cepa) |
21-day Survival / Reproduction | LOEC NOEC EC25 |
3091 (3125 nominal) 1722 (1563 nominal) 2440 (22% and 24% height reduction at 3125, 32% and 30% weight reduction at 6250 respectively) |
Porch and Krueger 2005 |
Plants: Dicotyledons Tomato (Lycopersicon esculentum) |
21-day Survival / Reproduction | LOEC NOEC EC25 |
6076 (6250 nominal) 2677 (3125 nominal) 4990 (37% height reduction and 40% weight reduction at 6250) |
Porch and Krueger 2005 |
Abbreviations: LC, Lethal concentration; EC, effective concentration; LOEC, lowest-observed effect concentration; NOEC, no-observed effect concentration
The lowest concentration at which a clear effect was reported from among the available soil toxicity studies is the EC10 value for earthworm reproduction of 1860 mg/kg DBDPE in soil and the EC25 for decreased onion weight of 2440 mg/kg (onion NOEC = 1563 mg/kg, but low EC values are preferred over NOEC values). For the purposes of this assessment, the value of 2440 mg/kg (EC25 for decreased onion weight of 2440 mg/kg) is selected as the CTV for EBTBP.
8.1.3 Derivation of the predicted no-effect concentrations
The only available empirical study for EBTBP was conducted well above the water solubility limit, thus limiting the usefulness of this result. As well, aquatic toxicity model predictions are not reliable and cannot be used for the predicted no effects concentration (PNEC). However, given EBTBP’s low predicted water solubility (2.88 x 10-7 mg/L) and high log Kow (8.90), it is expected that exposure via water would be minimal, and much less relevant than exposure in sediments and soil. For this reason, no pelagic predicted no effects concentration (PNEC) is determined for EBTBP.
For sediment, there are limited data clearly measuring organism effect levels for EBTBP’s analogue, DBDPE. Sediment organism toxicity tests for DBDPE have been performed with chironomids (Chironomus riparius) and oligochaetes (Lumbriculus variegates) (Krueger et al. 2003a, b). In both studies, effects were noted only at very high test concentrations, far exceeding that expected in the environment. EC50 values and NOECs for all measured endpoints were reported to be above the highest treatment concentration level of 5000 mg/kg dw for both the chironamid and oligochaete studies.
Using analogue data on DBDPE, a CTV of 5000 mg/kg is selected for EBTBP in sediment even though this value is unbounded with no effects observed at this concentration. When this value is adjusted from test organic carbon content (1.8%) to standard sediment organic carbon content (4%) (Webster et al. 2004), the CTV for sediment organisms is 11 100 mg/kg dw. An assessment factor of 100 is applied to account for extrapolation from laboratory to field conditions and interspecies and intraspecies variations in sensitivity, and the resulting PNEC for sediment organisms is 111 mg/kg dw. It is acknowledged that this value is already a no effects value; however, a higher application factor is also considered appropriate given that EBTBP may be somewhat more bioavailable than DBDPE.
Based on endpoints from a range of soil toxicity studies for DBDPE (soil bacteria, earthworms, and six plant species) (Aufderheide 2003, Hardy et al. 2011, Porch et al. 2005), the 21-d EC25 value for decreased onion (Allium cepa) weight of 2440 mg/kg dw is selected as the CTV. When this value is adjusted from test organic carbon content (2.7%) to standard soil (2%) organic carbon content, the CTV for soil organisms is 1807 mg/kg dw. An assessment factor of 100 is applied to account for extrapolation from laboratory to field conditions and interspecies and intraspecies variations in sensitivity, and the resulting PNEC for soil organisms is 18.07 mg/kg dry soil.
8.2 Ecological exposure assessment
Concentrations of EBTBP in water in Canada have not been identified. Therefore, environmental concentrations have been estimated from available information, including estimated substance quantities, estimated industrial release rates, and characteristics of the receiving environment. Environmental concentrations have been estimated for industrial release scenarios, as described in the following sections.
8.2.1 Exposure scenarios and predicted environmental concentrations
8.2.1.1 Industrial release
Scenarios for industrial release of EBTBP consider knowledge of EBTBP and DBDPE use (ECCC 2017b). Because use profiles and applications of EBTBP and DBDPE are considered very similar, and owing to limited information on industrial uses of EBTBP in Canada, exposure to EBTBP in the environment is assessed on the basis of information specific to EBTBP and DBDPE. While the uses of EBTBP and DBDPE are similar, use quantities of EBTBP are much less than those identified for DBDPE.
8.2.1.2 Water media
The aquatic exposure to EBTBP is expected if the substance is released from industrial manufacture, formulation either directly or to a wastewater system that discharges its effluent to a receiving surface water body. The concentration of the substance in the receiving water near the discharge point of the wastewater system is used as the predicted environmental concentration (PEC) to develop sediment and soil PECs in evaluating the sediment and soil risk of the substance. It can be calculated using the equation (ECCC 2017b):
Cwater-ind = [1000 x Q x L x (1-R)] / [N x F x D]
Where
Cwater-ind: aquatic concentration resulting from industrial releases, mg/L
Q: total substance quantity used annually at an industrial site, kg/yr
L: loss to wastewater, fraction
R: wastewater system removal rate, fraction
N: number of annual release days, d/yr
F: wastewater system effluent flow, m3/d
D: receiving water dilution factor, dimensionless
Several aquatic industrial release scenarios are developed to cover a range of different potential industrial activities in Canada. The scenarios include rubber compounders; plastic compounders, plastic injection molders, textile manufacturing and plastic extrusion facilities, textile back-coating facilities (for the ecological assessment only), and facilities using this substance for unspecified industrial activity. Table 8-3 presents the range of inputs used to estimate resulting aquatic concentrations close to the industrial points of discharge. On the basis of these assumptions, the industrial scenarios yield predicted environmental aquatic concentrations (PEC) of 2.20 x 10-8 to 2.88 x 10-7 mg/L (ECCC 2017b). These PEC values represent the total EBTBP concentrations in the receiving water near the point of the discharge at each site, and in some cases exceed the water solubility of EBTBP (i.e., dissolved EBTBP limit) by 1 to 4 orders of magnitude. The highest PECs result from industrial scenarios associated with high releases which are also uncertain (e.g., typically textile), and therefore are considered more conservative.
| Input | Value | Justification and reference |
|---|---|---|
| Quantity used per site (kg) | 1 000 to 100 000 | Section 71 survey (Canada 2013) |
| Loss to wastewater (%) | 0.001 to 1.0 | OECD 2010 |
| Wastewater system removal efficiency (%) | 0, 57.3, or 82.5 | Predicted with ASTreat 1.0 (2006) for no treatment, primary treatment, secondary treatment |
| Number of annual release days (days) | 250 to 350 | National Pollutant Reporting Inventory data or Environment Canada standard assumption |
| Wastewater system effluent flow (m3/d) | 2 908 to 400 000 | Site specific wastewater treatment system data |
| Dilution factor (–) | 1 to 10 | Site specific wastewater treatment system flow rate/receiving environment flow rate. When a dilution factor was greater than 10, a maximum default value of 10 was used. |
8.2.1.3 Sediment
An equilibrium sediment-water partition approach was used to estimate the concentration of EBTBP in bottom sediment. This approach is involves a partitioning principle described by the European Chemicals Agency (ECHA 2010) and incorporates two additional calculation methods. The first method is to estimate the substance’s concentration in the aqueous phase (dissolved) of the overlying water from its total concentration, according to studies by Gobas (2007 and 2010). The second method is to estimate a substance’s concentration in bottom sediment from its concentration in the aqueous phase of the overlying water using an equilibrium partitioning assumption between bottom sediment and overlying water described by the US EPA’s National Center for Environmental Assessment (US EPA 2003). At equilibrium, the predicted environmental concentration (PEC) in bottom sediment can linearly correlate with the concentration in the aqueous phase of the overlying water. Sediment exposure scenarios were developed as an extension of the industrial aquatic release scenarios described above to determine equilibrium sediment PECs, standardized to 4% organic carbon (a typical organic carbon content in bottom sediment for rivers and lakes). The resulting PEC values ranged from 0.00015 to 0.020 mg/kg dw.
8.2.1.4 Soil
An approach described by the European Chemicals Agency (ECHA 2010) was used to estimate predicted environmental concentrations in soil (soil PECs) resulting from the land application of biosolids. This approach employed the quantity of biosolids accumulated within the top 20 cm layer (ploughing depth) of soil over 10 consecutive years. An underlying assumption of the approach was that substances were subject to no loss because of degradation, volatilization, leaching and soil run-off upon their entry into soil via biosolids land application. This assumption, therefore, yielded conservative soil PECs. Soil exposure scenarios were developed as an extension of the aquatic exposure scenarios described above, using biosolids concentration and production rates from site specific wastewater treatment systems (WWTS). The estimated concentration in biosolids ranged from 0.0030 to 73.44 mg/kg dw. Soil PECs were standardized to 2% organic carbon and the resulting PEC values ranged from 1.00 x 10-4 to 2.54 mg/kg dw.
8.2.1.5 Consumer or commercial release
Although EBTBP can be found in commercial products or products available to consumers, it is expected that release from these products to the environment is minimal. Additive use of EBTBP in products suggests diffuse releases may occur from commercial products or products available to consumers, and although there are uncertainties, the rate may be low. While service life release rates were not found for EBTBP, a study by Kemmlein et al. (2003) determined a specific air emission rate of 0.3 ng/m2/h for decaBDE (in the technical Octabromodiphenyl Ether formulation) during a 105-day test of television set housing (23 ᵒC). Furthermore, many products made with EBTBP will not be in contact with water on a regular basis, e.g., electronics, wiring and appliances. The potential release during service life is estimated at 0.05% per year to water if the substance is for indoor use or 0.16% per year if use is outside (OECD 2009). Overall, releases from products are expected to be geographically dispersed and spread out over the duration of the service life and end-of-life of these products.
Therefore, the worst-case scenario for the diffuse release of EBTBP throughout Canada (via WWTS and disperse release directly to the environment), using the indoor release rate of 0.05% over service life information from OECD 2009, was estimated at 555 kg. This scenario includes a number of assumptions: the maximum values from each range of import (1 000 000 kg for commercial products or products available to consumers; 100 000 kg for formulation; and 10 000 kg for neat substances); complete use of EBTBP in products; low exposure to water over the service lifetime and indoor use. This result suggests that significant release of EBTBP products is unlikely. The scenario result is considered to be highly uncertain.
8.3 Characterization of ecological risk
8.3.1 Risk quotient analysis
The approach taken in this ecological screening assessment was to examine various supporting information and develop conclusions involving a weight-of-evidence approach, using precaution as required under CEPA. Lines of evidence considered include results from a conservative risk quotient calculation, as well as information on persistence, bioaccumulation, inherent or ecological toxicity, sources and fate of the substance.
A risk quotient analysis, integrating conservative estimates of EBTBP exposure with toxicity information from its analogue DBDPE, was performed for the sediment and soil media to determine whether there is potential for ecological harm in Canada. A risk quotient analysis was not conducted for the pelagic aquatic environment because of low relevance, and unreliable empirical and predicted toxicity data.
The industrial scenarios presented above yielded predicted environmental concentrations (PEC) from 2.20 x 10-8 to 2.88 x 10-7 mg/L (ECCC 2017b) for surface water. These PEC values represent the level of exposure in the receiving water near the point of the discharge. Using aquatic PECs in water to determine equilibrium sediment PECs, standardized to 4% OC, the resulting PEC values ranged from 0.00015 to 0.020 mg/kg dw. A predicted no-effect concentration (PNEC) was derived from the chronic sediment organism toxicity values for DBDPE to give a value of 111 mg/ kg dw (see Ecological Effects Assessment section). The resulting risk quotients (PEC/PNEC) = 1.3 x 10-6 to 0.00018 (Table 8-4).
Using a similar PEC/PNEC approach, predicted soil PECs resulting from biosolids applications to land (standardized to 2% OC) ranged from 1.0 x 10-4 to 2.54 mg/kg dw. The PNEC for soil organisms is 18.07 mg/kg dry soil (see the Ecological Effects Assessment section). The resulting risk quotients (PEC/PNEC) = 5.8 x 10-6 to 0.14 (Table 8-4). Therefore, harm to soil organisms is unlikely for these scenarios.
| Media | Scenario | PNEC | PEC | RQ |
|---|---|---|---|---|
| Sediment | Industrial release to water | 111 mg/kg dw | 0.00015 to 0.020 mg/kg dw | 1.3 x 10-6 to 0.00018 |
| Soil | Biosolids application to soil | 18.07 mg/kg dw | 1.0 x 10-4 to 2.54 mg/kg dw | 5.8 x 10-6 to 0.14 |
8.3.2 Consideration of the lines of evidence and conclusion
EBTBP is expected to be persistent in water, soil and sediment, but not in air (gas phase). EBTBP is expected to have limited bioaccumulation potential, but owing to a lack of empirical data, biomagnification in food webs cannot be ruled out. The importation volumes of EBTBP into Canada, along with information on its uses, indicate low potential for widespread release into the Canadian environment. Once released into the environment, EBTBP will be found mainly in sediment and soil, where it may persist for long periods of time. There are no experimental toxicity data available for EBTBP aside from one aquatic toxicity study performed at concentrations above EBTBP solubility in water, and the acute and chronic toxicity predictions are unreliable because EBTBP has a very high Log Kow of 8.90, which is outside of the modelling domain of available models. Toxicity data of sediment and soil organisms for DBDPE is used as read-cross for EBTBP. Results of the risk quotient analysis indicate that EBTBP has very low predicted risk quotients to sediment (RQ of less than 0.00018) and soil (RQ of less than 0.14) organisms. On the basis of these studies and the weight-of-evidence (Appendix A), EBTBP shows a low potential for harm to aquatic sediment and soil organisms.
The information indicates that EBTBP has low potential to cause ecological harm in Canada.
8.3.3 Uncertainties in evaluation of ecological risk
Uncertainties are present owing to the lack of information on the environmental concentrations in Canada, including in wastewater effluent and associated biosolids, soils, sediments and biota. In addition, there is some uncertainty with the estimated physical chemical properties. Even though the Least Square Adjustment was used in this assessment and the input values were adjusted as best as possible, the physical chemical properties are still uncertain because most QSAR values and estimates from other models were used as input values to the LSA methodology. In particular, the correction for log Kow is 194%; therefore, log Kow is likely underestimated. At the same time, however, there is no information on the extent to which it may be underestimated. Generally, confidence in EBTBP physical chemical properties is low to moderate.
Model estimates of CTD, Pov and TE needs to be analyzed with caution because of the uncertainties with key modelled partition coefficients (Koa, Kow, Kaw) required as input. Exposure scenarios for use in risk analysis were developed using the best available information, and utilizing some reference data from DBDPE given apparent similar usages. Nevertheless, they are considered to conservatively characterize potential risks from releases of EBTBP to the Canadian environment.
The assessment recognizes that there is no information characterizing potential releases from commercial products or products available to consumers in use and during disposal/recycling at the end of their service life. This assessment has not considered the EBTBP release to the environment resulting from leaching from products or from the degradation of products containing EBTBP in landfills owing to the lack of data, and because the emissions during use are expected to be low. While most landfills are expected to collect and treat their leachate in Canada, no Canadian EBTBP landfill leachate data have been reported to date, but such data could help interpret end-of-life releases. Generally, confidence in EBTBP exposure scenarios is moderate.
Even with conservative assumptions of small EBTBP quantities in use at industrial sites, risk quotients were much less than one, suggesting low risk. Finally, this assessment recognizes that there are information gaps on the toxicity of EBTBP to sediment, soil, and wildlife species. Use of DBDPE as a sediment and soil toxicity analogue for EBTBP is an additional source of uncertainty because DBDPE is less bioavailable than EBTBP (DBDPE has a higher log Koc than EBTBP), and the toxicity data presented in this assessment are the worst case; thus, the uncertainty on effects leans towards being overly conservative. With the high assessment factor (100), the analysis is considered sufficiently conservative to adequately characterize the potential effects which could result from EBTBP. Generally, there is moderate confidence in the EBTBP toxicity results.
Finally, there is no empirical evidence of EBTBP transformation in the environment and there is no information characterizing potential pathways of degradation. Therefore, there is low confidence in the analysis of hypothetical transformation products of EBTBP.
9. Potential to cause harm to human health
9.1 Exposure assessment
9.1.1 Environmental media and food
EBTBP is an additive flame retardant found in electronic housing, cable and wire coating and adhesives in Canada. As an additive flame retardant, EBTBP is not considered to be chemically bound to the polymer matrix that contains it, and therefore may be released into the environment over the service life of a product (Andersson et al. 2006; Guerra et al. 2011).
In studies where EBTBP is monitored, it is not often detected because of analytical method limitations (Nyholm et al. 2013). EBTBP, on the basis of its estimated low volatility and water insolubility, is expected to partition primarily to dust and sediment when released, and concentration in water is expected to be low. Exposure of the general population from environmental media is therefore expected to be mainly from dust or soil. Upper-bounding estimates of daily intake of EBTBP from environmental media for the general population of Canada are presented in Appendix B and range from 0.0004 and 0.39 µg/kg-bw per day.
9.1.1.1 Air
No reports were identified which measured EBTBP in ambient or indoor air in Canada or elsewhere.
9.1.1.2 Dust
No studies monitoring EBTBP in dust in Canada were identified. Due to the use of EBTBP in household electronics, it would be expected to be found in dust in the home. Abrasion of plastic cable coatings containing EBTBP may be released as dust, representing a potential source of exposure via incidental dust ingestion. Given the lack of dust monitoring data for EBTBP, dust concentrations from flame retardants with similar use patterns and physical chemical properties, i.e., decaBDE and DBDPE, were used to predict EBTBP dust concentrations. DecaBDE (CAS RN 1163-19-5) and DBDPE (CAS RN 84852-53-9) are both brominated flame retardants used in plastics for electronic products found in the home, resulting in potential releases to the dust stream from general wear or abrasion. In 2006, dust was collected from 19 homes in Boston and analyzed for DBDPE. For this study, the geometric mean was 153 ng/g and the maximum concentration was 11 070 ng/g (Stapleton et al. 2008). In 2012, dust was collected from 30 homes in Boston and analyzed for EBTBP (Stapleton et al., 2014). For this study, the geometric mean was 1720 ng/g and the maximum concentration was 76 130 ng/g. As a conservative approach for EBTBP, the maximum house dust concentrations for decaBDE (BDE209) and DBDPE (76 130 ng/g and 11 070 ng/g, respectively) reported in Stapleton et al. (2008, 2014) were used to represent a potential range of exposure from dust. Of note, this concentration is higher than the maximum estimated EBTBP soil concentration of 2.54 mg/kg dw resulting from the land application of biosolids (see section 8.2.1.4).
9.1.1.3 Soil and sediment
No appropriate or relevant soil and sediment studies on EBTBP monitoring in North America were identified. Based on its physical chemical properties, this substance is expected to partition to soil and sediment. Assuming biosolids from wastewater treatment systems are applied to soil in an agricultural field, the BASL4 model was used to predict a soil concentration of 2.54 mg/kg dw (section 8.2.1.4). Daily intake estimates of exposure to EBTBP from the ingestion of soil for the general population of Canada were derived based on this PEC estimate.
9.1.1.4 Water
There are no reports of EBTBP sampling in water or precipitation in Canada. In a Norwegian study, EBTBP was detected in seepage water outside a metal recycling facility at a concentration of 35 ng/L (Nyholm et al. 2013). In all but one sample, EBTBP concentrations were not detected (method’s detection limit of 6 ng/L). The authors reported analytical interference when analyzing the water samples and postulated that this concentration of EBTBP found in the water may be a false positive (Nyholm et al. 2013).
Based on its predicted insolubility in water (<1mg/L) and its strong propensity to partition to solids (dust or soil), along with the PEC values presented in section 8.2.1.2, it is considered unlikely that EBTBP would be present in water at the level (35 ng/L) reported by Nyholm et al. (2013). Therefore, water was not identified as a potential source of exposure for derivation of the total daily intake estimate for Canadians.
9.1.1.5 Food
No reports of EBTBP in food were identified in Canada or other areas of the world.
9.1.2 Products available to consumers
EBTBP is a flame retardant used in plastic and rubber materials, in electrical and electronics, and in the automotive sector in adhesives in Canada (see Section 5) (ECCC 2013-2014, MSDS 2010).
EBTBP is not sold directly to the public, but is added to various manufactured items such as wire and cable insulation and electronic equipment, which may result in potential exposure in the home.
When used for electronic equipment housing (i.e. computers or printers) or electrical cable insulation, exposure to EBTBP is not expected to occur to any significant extent. Low vapour pressure and vaporization make inhalation exposure unlikely. The negligible water solubility of EBTBP limits the potential for any water- or sweat-mediated dermal transfer, and dermal uptake from physical contact with electronics is expected to be low. Abrasion to the plastic surfaces may result in EBTBP adhering to dust particles in the surrounding area. Dust intake is discussed in the Environmental Media and Food section (see 9.1.1 above).
Around the home, infants and children may have access to electronics and electrical cables that they can put in their mouth. In a study of young children’s mouthing behaviour, unintentional mouthing of various products including cordless phone antenna, electrical cords, extension cords, and TV remote controls was observed (Juberg et al. 2001). However, this behaviour is considered to result in lower exposures than those expected from mouthing of toys or other products intended for children, given the incidental nature of exposure and the low water solubility of EBTBP.
EBTBP is also a component of hot melt adhesives which are used in glue guns. Potential exposure during use of a glue gun was considered to be low given its low vapour pressure. The potential for an increase in volatilization of EBTBP due to the increase in temperature during the glue application is not expected to result in a significant increase in concentration in the air. Dermal exposure is not expected as skin contact is naturally avoided to protect from burns. Based on current information regarding EBTBP’s use profile and physical-chemical properties, potential for exposure of the Canadian general population to EBTBP from manufactured items is low; therefore, exposure estimates were not derived. Any exposure from manufactured items is expected to be accounted for through estimates of EBTBP intake from soil and dust (see sections 9.1.1.2 and 9.1.1.3).
9.2 Health effects assessment
No classifications of the health effects of EBTBP by national or international regulatory agencies were identified. No chronic or carcinogenicity studies on EBTBP were found. Results of mutagenicity studies with EBTBP in Salmonella typhimurium (TA 98, TA 100, TA 1535, TA 1537, and TA 1538 strains), Escherichia coli (WP2 uvrA strain) and Saccharomyces cerevisiae were negative when tested with or without metabolic activation at concentrations ranging from 0 to 5000 μg/plate in DMSO (Cannon Laboratories Inc. 1978c; Chemical Inspection and Testing Institute 1982; Zeiger et al. 1985; Albemarle Corporation 2008). Results of a chromosomal aberration test in Chinese hamster ovary (CHO) cells were also negative at concentrations up to 750 μg/ml for 20 hours with or without metabolic activation (Albemarle Corporation 2008). No in vivo studies were identified.
In the absence of chronic and carcinogenicity studies on EBTBP, Health Canada conducted an analysis of quantitative structure-activity relationships (QSAR) model predictions for carcinogenicity. QSAR model predictions supported the findings from the in vitro assays that EBTBP is not genotoxic, but were inconclusive as to its potential for carcinogenicity. However, based on the negative results in the genotoxicity studies, lack of adverse effects in experimental animals up to the highest dose tested (see repeated-dose studies presented below), and the limited solubility and large molecular size of EBTBP that would lead to limited uptake, EBTBP is considered unlikely to be carcinogenic.
No adverse effects were observed in experimental animals administered EBTBP in their diet in several repeated-dose studies.
In a sub-chronic study, EBTBP was administered to male and female Sprague-Dawley rats (15 animals /sex/group) at doses of 0, 0.01, 0.1 or 1% of the diet for 90 days, followed by 46 days during which the rats were fed a control diet. No exposure related changes in hematology, serum chemistry or urinalysis were observed. No statistically significant differences were found in mean relative and absolute organ weights (liver, kidney, heart, and thyroid) between the control and the exposed groups. No exposure-related histopathological changes or lesions were present in organs and tissues among any dose group examined at the conclusion of the study. The highest dose tested was 1% of EBTBP in diet, which was equal to 692 mg/kg bw/day in males and 775 mg/kg bw/day in females (Cannon Laboratories Inc. 1978b; Albemarle Corporation 2004).Footnote 2
One short-term study was identified in which EBTBP was administered to male Sprague-Dawley (SD) rats (10 animals /group) at doses of 0, 0.01, 0.1 or 1% of the feed for 28 days. No clinical signs of toxicity were observed. Mean body weights, body weight gains, food consumption and organ weights (liver, heart, spleen, kidneys and testes) were not affected by exposure to EBTBP. No exposure-related changes in hematology and serum chemistry were observed, and no gross or microscopic tissue alterations were found. The highest dose tested was1% of the diet which was equal to 1267 mg/kg bw/day (WARF Institute Inc. 1976; Albemarle Corporation 2004).
Developmental effects of EBTBP in rats and rabbits were investigated. EBTBP was administered to 4 groups of mated female Sprague Dawley rats (25 per group) via gavage in corn oil at dose levels of 0, 100, 500, or 1000 mg/kg bw/day from gestation day (GD) 6 through 15. Animals were observed daily for clinical signs of toxicity. Body weights and food consumption were measured on GD 0, 6, 9, 12, 16 and 20. All female rats were sacrificed on GD 20 and subjected to caesarean section. Foetuses were individually weighed, sexed, and examined for external, visceral and skeletal abnormalities. No maternal mortality or exposure-related clinical signs of toxicity were observed. No exposure-related differences were observed among the groups with respect to maternal body weights, food consumption and morphopathological lesions. Intrauterine survival and fetal weights were similar among all study groups. The incidence of developmental effects in the treated groups was comparable to that of the control group. Overall, EBTBP was found to be neither maternally toxic nor to have developmental effects when administered orally to pregnant rats at a dosage level as high as 1000 mg/kg bw/day (Rodwell 1988a; Albemarle Corporation 2004).
In another developmental toxicity study, EBTBP was administered to 2 groups of mated female New Zealand White rabbits (20 per group) by gavage in carboxymethyl cellulose at dose levels of 0 or 1000 mg/kg bw/day from GD 7 through 19. Animals were observed daily for clinical signs of toxicity. Body weights were measured on GD 0, 7, 10, 13, 19, 24 and 29. Food consumption was measured daily. All female rabbits were sacrificed on GD 29 and subjected to caesarean section. Foetuses were individually weighed, sexed, and examined for external, visceral and skeletal abnormalities. No maternal mortality, abortions, or exposure-related clinical signs of toxicity were observed. Maternal body weights, weight gain, food consumption, necropsy observations and caesarean section parameters were comparable between the 2 study groups. Intrauterine survival and fetal weights were not adversely affected by exposure to EBTBP. The incidence of developmental effects in the treatment group was similar to that of the control group. Therefore, EBTBP was neither found to be maternally toxic nor to have developmental effects when administered orally to pregnant rabbits at a dose level of 1000 mg/kg bw/day (Rodwell 1988b; Albemarle Corporation 2004).
Information on the toxicokinetics of EBTBP is limited. One study was identified during which 14C-labelled EBTBP (equivalent to 0.67 mg/kg bw/day) was administered to five female Sprague-Dawley rats by gavage in corn oil for 14 consecutive days. Approximately 80% of the total dose was recovered in the feces (~ 65%) and urine (~15%) during the 14 days of dosing. Negligible 14C-activity was detected in expired air. All organs contained radioactive residues. After 14 days, the highest levels were present in liver and kidney. Lower levels of radioactivity were detected in muscle, fat and brain. By 30 days post-treatment, 14C-activity levels continued to drop with the highest level found in muscle (0.05ppm) (Cannon 1978a; European Commission 2000a; Albemarle Corporation 2004).
9.3 Characterization of risk on human health
No classifications of the health effects of EBTBP by national or international regulatory agencies were identified. No chronic or carcinogenicity studies on EBTBP were found. On the basis of the available information regarding genotoxicity, EBTBP is not considered genotoxic in vitro.
No toxicologically significant adverse effects were observed in rats exposed orally to EBTBP at doses up to 692 and 1267 mg/kg bw/day for 90 or 28 days, respectively. No reproductive studies were identified. In the developmental toxicity studies, no treatment- related maternal effects were observed in rats or rabbits exposed to EBTBP via the oral route; and no developmental effects occurred in the offspring at doses up to 1000 mg/kg bw/ day, although the rabbit study might not have dosed the animals for the full term.
The highest doses tested (of 692 and 1267 mg/kg bw/day in the 90- and 28-day oral rat studies, respectively), with no treatment-related effects, are 6 orders of magnitude higher than the EBTBP intake estimates for the Canadian general population from dust and soil ingestion (0.0004 to 0.39 µg /kg bw/day). This margin is considered to be adequate to account for uncertainties in the health effects and exposure databases.
9.4 Uncertainties in the evaluation of risk to human health
Overall, the confidence in the exposure database for environmental media is considered to be low, given that no data on the concentration of EBTBP in any environmental media were identified and that this could be related to challenges encountered in its detection using analytical methods.
There are uncertainties associated with the estimate of human exposure to EBTBP from environmental media since it is based on dust concentrations for related flame retardants. Although DBDPE and decaBDE have use patterns and physical-chemical properties similar to those of EBTBP, there is uncertainty in using these substances as surrogates for dust concentration.
Confidence in the health effects database for EBTBP is considered to be moderate as there is a lack of data on carcinogenicity and reproductive toxicity, and the oral repeated-dose toxicity database was limited.
10. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to organisms and the broader integrity of the environment from EBTBP. It is concluded that EBTBP does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Based on the adequacy of the margin between the estimates of exposure to the general population and the highest dose tested without adverse effects observed in experimental animals, it is concluded that EBTBP does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is concluded that EBTBP does not meet any of the criteria set out in section 64 of CEPA.
References
[AEROWIN] AEROWIN Program for Windows [Estimation Model]. 2010. Version 1.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [accessed 2014 January]
Albemarle Corporation. 1999a. Product Sheet: Saytex BT-93 Flame Retardant. Baton Rouge (LA): Albemarle Corporation.
Albemarle Corporation. 1999b. Product Sheet: Saytex BT-93W Flame Retardant. Baton Rouge (LA): Albemarle Corporation.
Albemarle Corporation. 2004. HPV Data Summary and Test Plan for 1H-Isoindole-1,3(2H)-dione,2,2’-(1,2-ethanediyl)bis(4,5,6,7-tetrabromo- (a.k.a Ethylene bis tetrabromophthalimide) CAS No. 32588-76-4. Report no. 201-15090. Baton Rouge (LA): Albemarle Corporation.
Albemarle Corporation. 2005. Material Safety Data Sheet: Saytex BT-93W Flame Retardant. 2005. Baton Rouge (LA): Albemarle Corporation.
Albemarle Corporation. 2008. Summary: Chromosome aberration assay in Chinese hamster ovary cells [PDF]. US EPA High Production Volume (HPV) Challenge Program, Revised Summaries for 1H-Isoindole-1,3(2H) -dione, 2,2'-(1,2-ethanediyl) bis(4,5,6,7-tetrabromo-, 17-8-2008.
Andersson P, Oberg K, Orn U. 2006. Chemical characterization of brominated flame retardants and identification of structurally representative compounds. Environ Tox and Chem 25(5): 1275-1282.
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2010. Version 1.92a. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [accessed 2014 January].
Arnot JA, Gobas FAPC. 2003a. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci 22(3):337–345.
Arnot JA, Gobas FAPC. 2003b. Categorization of organic substances on the Domestic Substances List for bioaccumulation potential. Report to Environment Canada, Existing Substances Branch. June 2003. Gatineau (QC): Environment Canada.
Arnot JA, Gobas FAPC. 2004. A food web bioaccumulation model for organic chemicals in aquatic ecosystems. Environ Toxicol Chem 23(10): 2343–2355.
Arnot JA, Gobas FAPC. 2006. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ Rev 14:257–297.
Arnot JA, Arnot MI, Mackay D, Couillard Y, MacDonald D, Bonnell M, Doyle P. 2010. Molecular size cutoff criteria for screening bioaccumulation potential: fact or fiction? Integr Environ Assess Manage 6(2):210–224.
ASTreat 1.0 (2006). A computer program developed by The Procter & Gamble Company for sewage treatment plant removal predictions, revised and released in 2006, available from Dr. Drew C. McAvoy, The Procter & Gamble Company, P.O. Box 538707, Cincinnati, OH 45253-8707, USA. Most conservative removal rate of the 4 STP models (STP-EX SimpleTreat 3.0 STP Model 2.1 ASTreat 1.0)
Aufderheide J. 2003. Effects of Saytex 8010 on the survival and reproduction of the earthworm, Eisenia fetida. ABC Laboratories, Columbia, Missouri (USA): Study number 47634.
[BCFBAF] Bioaccumulation Program for Windows [Estimation Model]2010. Version 3.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [accessed 2014 January].
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2010. Version 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4):741−752.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada 2000. Canadian Environmental Protection Act: Persistence and Bioaccumulation Regulations [PDF]. P.C. 2000-348, March 23, 2000, SOR/2000-107, Canada Gazette Part II, vol. 134, no. 7, p. 607-612. [accessed 2017 August]
Canada 2001. CAS RN 32588-76-4 – Preliminary Report of Section 71 Notice. Unpublished report, Gatineau (QC): Environment Canada, Ecological Assessment Division.
Canada. 2013. Canadian Environmental Protection Act, 1999: Notice with respect to certain organic flame retardant substances. Canada Gazette, Part I, vol. 147, no. 13, p. 613–633. March 30, 2013. [accessed 2017 August 17]
Cannon Laboratories, Inc. (1978a). Excretion and tissue distribution of 14C-RW-4-178B administered orally to rats. In: Ethyl Corporation (1990). Excretion and tissue distribution of 14C-RW-4-178B administered orally to rats with attachments and cover letter dated 030890. EPA/OTS 0522911: #86-900000347. Testing laboratory: Cannon Laboratories, Inc. Report date: 1978-05-23.
Cannon Laboratories, Inc. (1978b). 90-day feeding study in rats evaluating Cities Service compound RW-4-178B. In: Ethyl Corporation (1990). 90-day feeding evaluation study in rats with cover letter dated 030890. EPA/OTS 0522927: #86-900000363. Testing laboratory: Cannon Laboratories, Inc. Report date: 1978-09-19.
Cannon Laboratories, Inc. (1978c). Mutagenicity evaluation of RW-4-178B (BT-93) in the Ames Salmonella/microsome plate test. In: Ethyl Corporation (1990). Mutagenicity evaluation of RW-4-178B (BT-93) in the Ames Salmonella/microsome plate test with cover letter dated 030890. EPA/OTS 0522926: #86-900000362. Testing laboratory: Cannon Laboratories, Inc. Report date: 1978-03-09.CATALOGIC [Computer Model]. 2012. Version 5.11.6. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry.
Chemical Inspection and Testing Institute. 1982. Mutagenicity evaluation of ethylene-1,2-bis(3,4,5,6-tetrabromophthalimide) in Ames Salmonella/microsome assay with cover letter dated 030890. Testing laboratory: Chemical Inspection and Testing Institute. Report date: 1982-02-18.
ChemNet [online database]. 1997. CAS search, CAS: 32588-76-4 [online database]. ChemNet. [accessed 2012 January1]
[CITI] Chemicals Inspection and Testing Institute. 1991. Test of biodegradability of Saytex-402 by microorganisms. Kurume Research Laboratories, Chemical Biotesting Center. Test number 11893.
Covaci A, Harrad S, Abdallah M, Ali N, Law R, Herzke D, de Wit C. 2011. Novel brominated flame retardants: a review of their analysis, environmental fate and behaviour. Envi Int. 37:532-556.
Dimitrov S, Dimitrova N,Walker J, Veith G, Mekenyan O. 2002. Predicting bioconcentration potential of highly hydrophobic chemicals. Effect of molecular size. Pure and Appl Chem. 74(10):1823–1830.
Dimitrov S, Dimitrova N, Parkerton T, Comber M, Bonnell M, Mekenyan O. 2005. Base-line model for identifying the bioaccumulation potential of chemicals. SAR QSAR Environ Res 16(6):531–554.
[DPD] Drug Product Database [database]. [modified 2017 Feb 17]. Ottawa (ON): Government of Canada. [accessed 2014 April 24].
[ECHA] European Chemicals Agency. 2014. Guidance on information requirements and chemical safety assessment. Chapter R.11: PBT/vPvB assessment. Version 2.0, November 2014, p. 53. Guidance for the implementation of REACH. Helsinki (FI): European Chemicals Agency.
[ECHA] European Chemicals Agency. 2012. Guidance on information requirements and chemical safety assessment. Chapter R.16:Environmental exposure estimation. Version 2.1, October 2012. Guidance for the implementation of REACH. Helsinki (FI): European Chemicals Agency.
[ECOSAR] Ecological Structure Activity Relationships Class Program [Estimation Model]. 2012. Version 1.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [accessed January 2014].
Environment Canada. 1986. DSL main_Quantity and Use codes_07-06-07.xls. Gatineau (QC): Environment Canada, Ecological Assessment Division.
Environment Canada. 2006a. Ecological screening assessment report on polybrominated diphenyl ethers (PBDEs) [PDF], CEPA, June 2006. Environment Canada, Ecological Assessment Division. [accessed 2006 June]
Environment Canada. 2010. Ecological State of the Science Report on Decabromodiphenyl Ether (decaBDE)-Bioaccumulation and Transformation [Internet]. [accessed 2010 August]
[ECCC] Environment and Climate Change Canada. 2013-2014. Data for certain organic flame retardants substance grouping collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain organic flame retardant substances. Data prepared by: Environment and Climate Change Canada, Health Canada; Existing Substances Program.
[ECCC 2017a] Environment and Climate Change Canada. 2017a. Supporting documentation: Summary tables of substance identity, physical chemical properties and environmental fate and behaviour for Ethylene bis(tetrabromophthalimide). Gatineau (QC): Environment Canada. Information in support of the Screening Assessment for 1H-isoindole-1,3(2H)-dione, 2,2'-(1,2-ethanediyl)bis[4,5,6,7-tetrabromo-. Available on request from: ec.substances.ec@canada.ca
[ECCC 2017b] Environment and Climate Change Canada. 2017b. Supporting documentation: Summary tables of ecological exposure information for Ethylene bis(tetrabromophthalimide). Gatineau (QC): Environment Canada. Information in support of the Screening Assessment for 1H-isoindole-1,3(2H)-dione, 2,2'-(1,2-ethanediyl)bis[4,5,6,7-tetrabromo-. Available on request from: ec.substances.ec@canada.ca
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2017 June 20].
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2018. Screening Assessment for Benzene, 1,1’-(1,2-ethanediyl)bis[2,3,4,5,6-pentabromo- , or Decabromodiphenyl ethane (DBDPE). Ottawa (ON): Environment Canada, Health Canada; Existing Substances Program.
[EPI Suite] Estimation Programs Interface Suite for Microsoft Windows [Estimation Model]. 2000-2012. Version 4.1. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[EQC] Equilibrium Criterion Model [Estimation Model]. 2012. Version 1.00. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre.
[EC] European Commission. 2000a. IUCLID Dataset, N,N’-ethylenebis(3,4,5,6-tetrabromophthalimide), CAS No. 32588-76-4 Year 2000 CD-ROM edition. [place unknown]: European Chemicals Agency, European Commission. [accessed 2000 August 18]
[EC] European Commission. 2000b. IUCLID Dataset. N,N'-ethylenebis(3,4,5,6)-tetrabromophthalimide. European Commission Joint Research Centre, Institute for Health and Consumer Protection, European Chemical Substances Information System. Report. pp. 20.
Gobas FACP, Morrison HA. 2000. Bioconcentration and biomagnification in the aquatic environment. In Boethling RS, Mackay D, editors. Handbook of property estimation methods for chemicals, environmental and health sciences. Boca Raton (FL): CRC Press. p. 189–231.
Gobas F. 2007. Development and review of a generic water-sediment modelling framework for organic chemicals. Prepared for Environment Canada, March 26, 2007.
Gobas F. 2010. Comments on Approach to Sediment Approach. Prepared for Environment Canada, March 25, 2010.
Guerra P, Alaee M, Eljarrat E,Barcelo D. 2011. Introduction to Brominated Flame Retardants:Commercially Products, Applications, and Physicochemical Properties. In Eljarrat E, Barceló D. editors. 2011. Brominated Flame Retardants. Series: The Handbook of Environmental Chemistry. Volume 16. Heidelberg Berlin: Springer. 290 pp.
Hardy ML, Aufderheide J, Krueger HO, Mathews ME, Porch JR, Schaefer EC, Stenzel JI, Stedeford T. 2011. Terrestrial toxicity evaluation of decabromodiphenyl ethane on organisms from three trophic levels. Ecotox and Environ Safety 74:703-710.
Hardy ML, Krueger HO, Blankinship AS, Thomas S, Kendall TZ, Desjardins D. 2012. Studies and evaluation of the potential toxicity of decabromodiphenyl ethane to five aquatic and sediment organisms. Ecotox and Environ Safety 75:73-79.
Harner T, Shoeib M. 2002. Measurements of octanol-air partition coefficients (KoA) for polybrominated diphenyl ethers (PBDEs): Predicting partitioning in the environment. J Chem Eng Data 47:228-232.
Health Canada. [modified 2017 May 3]. Lists of Permitted Food Additives. Ottawa (ON): Health Canada Food Directorate. [accessed 2014 April 24].
[HENRYWIN] Henry’s Law Constant Program for Microsoft Windows [Estimation Model]. 2011. Version 3.20. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [accessed 2014 January].
[HYDROWIN] Hydrolysis Rates Program for Microsoft Windows [Estimation Model]. 2010. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [accessed 2014 January].
[IPCS] International Programme on Chemical Safety. 1997. Flame Retardants: A General Introduction, Environmental Health Criteria 192.
International Research and Development Corporation. 1981. Acute inhalation (one hour) toxicity study in rats (14 days). Testing laboratory: IRDC. Report no.: 254-028.
Juberg D, Alfano K, Coughlin R, Thompson K. 2001. An observational study of object mouthing behaviour by young children. Pediatrics 107(1):135-142.
Kelly BC, Gobas FAPC, McLachlan MS. 2004. Intestinal absorption and biomagnification of organic contaminants in fish, wildlife and humans. Environ Toxicol Chem 23(10):2324–2336.
Kemmlein S, Hahn O, Jann O. 2003. Emissions of organophosphate and brominated flame retardants from selected consumer products and building materials. Atmos Environ 37: 5485–5493.
Klasmeier J, Matthies M, MacLeod M, Fenner K, Scheringer M, Stroebe M, Le Gall AC, McKone TE, van de Meent D, Wania F. 2006. Application of multimedia models for screening assessment of long-range transport potential and overall persistence. Environ Sci Technol 40:53–60.
[KOCWIN] Organic Carbon Partition Coefficient Program for Windows [Estimation Model]. 2010. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [accessed 2014 January].
Kolic T, Shen L, MacPherson K, Fayez L, Gobran T, Helm P, Marvin C, Arsenault G, Reiner E. 2009. The analysis of halogenated flame retardants by GC-HRMS in environmental samples. J Chromatographic Science 47:83-91.
Krueger H, Thomas S, Kendall T. 2003a. S8010: A prolonged sediment toxicity test with Chironomus riparius using spiked sediment. Wildlife International, Ltd. Easton, Maryland (USA): Project number 471A-110.
Krueger H, Thomas S, Kendall T. 2003b. S8010: A prolonged sediment toxicity test with Lumbriculus variegatus using spiked sediment with a total of 2% organic carbon. Wildlife International, Ltd. Easton, Maryland (USA): Project number 471A-109.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2016 Aug 10]. Ottawa (ON): Government of Canada. [accessed 2014 April 24].
[MPBPVP] Melting Point Boiling Point Program for Microsoft Windows [Estimation Model]. 2010. Version 1.43. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. [accessed 2014 January].
[MSDS] Material Safety Data Sheet. 2010. 3M Scotch-Weld hot melt adhesive [PDF].
Muir DCG, Backus S, Derocher AE, Dietz R, Evans TJ, Gabrielsen GW, Nagy J, Norstrom RJ, Sonne C, Stirling I, Taylor MK and Lectcher RJ. 2006. Brominated Flame Retardants in polar Bears (Ursus maritimus) from Alaska, the Canadian Artic, East Greenland, and Svalbard. Environ. Sci. & Technol. 40(2):449-455.
[NCI] National Chemical Inventories [database on CD-ROM]. 2013. Issue 1. Columbus (OH): American Chemical Society.
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2017 June 21]. Ottawa (ON): Government of Canada. [accessed 2014 April 24].
[NIEHS] National Institute of Environmental Health Sciences. 1999. Ethylenebis(tetrabromophthalimide) [CASRN 32588-76-4] Review of Toxicological Literature. Research Triangle Park (North Carolina): Integrated Laboratory Systems.
Nyholm JR, Grabic R, Arp HP, Moskeland T, Andersson PL. 2013. Environmental occurrence of emerging and legacy brominated flame retardants near suspected sources in Norway. Science of the Total Environment 443:307-314.
[OECD] Organisation for Economic Co-operation and Development. 1995. OECD Environment Monograph Series No. 102. Risk Reduction Monograph No. 3 - Selected brominated flame retardants background and national experience with reducing risk [PDF].
[OECD] Organisation for Economic Co-operation and Development. 2010. Emission scenario document on plastics additives [PDF]. Paris (FR): OECD, Environment Directorate. Series on Emission Scenario Documents No. 3. Report No. ENV/JM/MONO(2004)8/REV1, JT00166678.
[OECD] Organisation for Economic Co-Operation and Development. 2011. OECD guidelines for testing of chemicals bioaccumulation in fish: Aqueous and dietary exposure. Draft document V.10. Paris (FR): OECD, Environment Directorate.
OECD QSAR Toolbox. [Read across tool]. 2012. Version 3.0. Paris (FR): Organisation for Economic Co-oporation and Development, Environment Directorate.
Porch JR and Krueger HO. 2005. S8010: A toxicity test to determine the effects of the test substance on seedling emergence of six species of plants. Wildlife International, Ltd. Easton, Maryland (USA): Project number 471-101A.
Radloff D, Spiess HW, Books JT, Dowling KC. 1996. Interaction Between Polybrominated Flame Retardants and High Impact Polystyrene. Journal of Applied Polymer Science 60: 715-720.
Rodwell DE. 1988a. Teratology study in rabbits with BT-93 (final report). Spring Life Sciences, Inc. Conducted for Ethyl Corporation. Study number: 3196.5. Report date: 1988-12-16.
Rodwell DE. 1988b. Teratology study in rats with BT-93 (final report). Spring Life Sciences, Inc. Conducted for Ethyl Corporation. Study number: 3196.4. Report date: 1988-12-16.
Sakuratani Y, Noguchi Y, Kobayashi K, Yamada J, Nishihara T. 2008. Molecular size as a limiting characteristic for bioconcentration in fish. J Environ Biol 29(1):89–92.
Schaefer EC, Carpernter K. 2010. Saytex 8010 : An evaluation of Inherent Biodegradability using the CONCAWE Test. Wildlife International Ltd. Project No. 471E-104. Draft OECD Guideline 302D. Wildlife International Lt Maryland. 45pp.
Schaefer EC, Matthews ME. 2011. Saytex 8010 : Biodegradation in anaerobic digester sludge. Wildlife International Ltd. Project No. 471E-105. OECD Guideline 314C. Wildlife International Lt Maryland. 56pp.
Schenker U, MacLeod M, Scheringer M, Hungerbuhler K. 2005. Improving data quality for environmental fate models: A least-squares adjustment procedure for harmonizing physicochemical properties of organic compounds. Environ Sci Technol 39(21): 8434-8441.
Scheringer M, MacLeod M and Wegmann F. 2009. The OECD Pov and LRTP Screening Tool, Version 2.2, A manual for the OECD Pov and LRTP Screening Tool 2.2 that is a follow-up version of the software POPorNot 1.0 distributed at the OECD/UNEP Workshop on Application of Multimedia Models for Identification of Persistent Organic Pollutants. ETH Zurich, April 2009. Version 2.2 fixes a bug introduced by changes in Excel 2007.
[SPIN] Substances in Preparations in Nordic Countries [database]. 2006. Copenhagen (DK): Nordic Council of Ministers.
Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, McClean MD, Webster TF. 2008. Alternate and new brominated flame retardants detected in U.S. house dust. Environ Sci Technol 42:6910-6916.
Stapleton HM, Misenheimer J, Hoffman K, Webster T. 2014. Flame retardant associations between children’s handwipes and house dust. Chemosphere 116:54-60.
Tice R. 1999. Ethylenebis (tetrabromopnthalimide) review of toxicological literature [PDF]. Prepared for National Institute of Environmental Health Sciences.
[TOPKAT] Toxicity Prediction by Komputer Assisted Technology [prediction module]. 2004. Version 6.2. San Diego (CA): Accelrys Software Inc.
UNIBROM. 2014. Unibrom Corp. products: EcoFlame B-951 [internet]. [accessed 2014 May 26]
United Kingdom Environment Agency. 2003. Prioritisation of flame retardants for environmental risk assessment.Peter Fish Associates for United Kingdom Environment Agency. ISBN: 978-1-84432-956-4.
[US EPA] US Environmental Protection Agency. 2003. “Exposure and human health reassessment of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds, Part I: Estimating exposure to dioxin-like compounds, Volume 4: Site-specific assessment procedures, Chapter 4: Estimating exposure media concentrations,” EPA/600/P-00/001Cb, U.S. Environmental Protection Agency, National Center for Environmental Assessment, Washington, DC, December 2003.
[US EPA] US Environmental Protection Agency. 2004. High Production Volume (HPV) Track. Sponsor: Albemarle Corporation. CAS Number: 32588764. Phthalimide, N,N'-ethylenebis(tetrabromo-.
[US EPA] US Environmental Protection Agency. 2006. Inventory Update Reporting for CAS RN: 32588-76-4. [US EPA] US Environmental Protection Agency. 2014. An Alternative Assessment for the Flame retardant Decarbromodiphenyl Ether (DecaBDE) (final report) [PDF]. 901 pp.
WARF Institute, Inc. 1976. Rat - 28 day feeding study - study code T-626. Testing laboratory: WARF Institute, Inc. Study number: T-626. Report date: 1976-09-15.
Webster E, Mackay D, Di Guardo A, Kane D,and Woodfine D. 2004. Regional differences in chemical fate model outcome. Chemosphere 55:1361–1376.
Wilson R, Jones-Otazo H, Petrovic S, Mitchell I, Bonvalot Y, Williams D, Richardson GM. 2013. Revisiting dust and soil ingestion rates based on hand-to-mouth transfer. Human and Ecological Risk Assessment 19(1):158-188. Available from:
World of Chemicals [database]. 2013. CAS search, CAS: 32588-76-4. Ethylenebistetrabromophthalimide. Singapore: WOC Labs Pte. Limited.
Zeiger E, Haworth S, Mortelmans K, Speck W. 1985. Mutagenicity testing of di(2-ethylhexyl) phthalate and related chemicals in Salmonella. Environmental Mutagenesis 7: 213-232. Testing laboratory: SRI International.
Appendix A. Weight-of-evidence table for ecological risk characterization
| Line of evidence | Level of confidencea | Relevance in assessmentb | Weight assignedc |
|---|---|---|---|
| Physical and chemical properties (water solubility and Log Kow | Low to Moderate | High | Moderate |
| Persistence | High | Moderate to high | High |
| Bioaccumulation | Moderate | High | Moderate to High |
| Exposure analysis | Low to Moderate | High | Moderate to High |
| Risk analysis | Low to Moderate | High | Moderate to High |
| Transformation products (photo-transformation products & biodegradation products) | Low | Low | Low |
a Level of confidence is determined according to data quality, data variability, data gaps and if the data are fit for purpose.
b Relevance refers to the impact of the evidence in the assessment.
c Weight is assigned to each line of evidence according to the combined level of confidence and relevance in the assessment.
Appendix B. Estimates of daily intake of EBTBP by various age groups within the general population of Canada
| Route of exposure | 0–6 moa | 0.5–4 yrb | 5–11 yrc | 12–19 yrd | 20–59 yre | 60+ yrf |
|---|---|---|---|---|---|---|
| Dustg | 0.06-0.39 | 0.03-0.20 | 0.011-0.076 | 4.1E-04-2.82E-03 | 3.9E-04-2.7E-03 | 3.8E-04 -2.6E-03 |
| Soilh | 0.0E+00 | 4.6E-05 | 1.7E-03 | 6.0E-05 | 5.7E-05 | 5.3E-05 |
| Total intake | 3.9E-01 | 2.0E-01 | 7.8E-02 | 2.9E-03 | 2.7E-03 | 2.7E-03 |
a Assumed to weigh 7.5 kg (Health Canada 1998), and to ingest 38 and 0 mg of dust and soil per day, respectively (Wilson et al. 2013).
b Assumed to weigh 15.5 kg (Health Canada 1998), and to ingest 41 and 14 mg of dust and soil per day, respectively (Wilson et al. 2013).
c Assumed to weigh 31.0 kg (Health Canada 1998), and to ingest 31 and 21 mg of dust and soil per day, respectively (Wilson et al. 2013).
d Assumed to weigh 59.4 kg (Health Canada 1998), and to ingest 2.2 and 1.4 mg of dust and soil per day, respectively (Wilson et al. 2013).
e Assumed to weigh 70.9 kg (Health Canada 1998), and to ingest 2.5 and 1.6 mg of dust and soil per day, respectively (Wilson et al. 2013).
f Assumed to weigh 72.0 kg (Health Canada 1998), and to ingest 2.5 and 1.5 mg of dust and soil per day, respectively (Wilson et al. 2013).
g No Canadian data was found for EBTBP in soil or dust studies. EBTBP was not measured in soil or dust elsewhere. A range of dust concentrations using surrogate flame retardants, DecaBDE and DBDPE, was used to estimate dust exposure to EBTBP. A maximum concentration of 76 130 ng/g was reported for DecaBDE from 30 homes in Boston, Massachusetts (Stapleton et al. 2014). DBDPE was measured in 20 homes in Boston at a maximum concentration of 11 070 ng/g (Stapleton et al. 2008). Both concentrations were selected to derive a range of estimated intake from dust for EBTBP.
h No appropriate or relevant soil and sediment studies on EBTBP monitoring in North America were identified. Therefore, the soil maximum predicted environmental concentration (PEC) of 2540 µg/kg dw was selected for deriving intake estimates from the ingestion of soil.
