Screening assessment - Chlorhexidine and its salts
Official title: Screening Assessment - Chlorhexidine and its Salts
Environment and Climate Change Canada
Health Canada
June 2019
Cat. No. En14-379/2019E-PDF
ISBN 978-0-660-31299-6
Synopsis
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of chlorhexidine and its salts, including (but not limited to) the salts listed in the table below. These substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other concerns. In July 2013, a draft screening assessment for chlorhexidine diacetate (then referred to as chlorhexidine acetate) was published proposing that it was not harmful to human health, but was harmful to the environment. Significant new information subsequently became available regarding other potential sources of exposure to the chlorhexidine moiety. As a result, a subsequent draft screening assessment was published on August 19, 2017 that assessed the chlorhexidine moiety to consider potential impacts on the environment and human health with respect to exposures from other potential sources of chlorhexidine.
The Chemical Abstracts Service Registry Numbers (CAS RNsFootnote 1), Domestic Substances List (DSL) names or chemical names, and common names of chlorhexidine and its salts are listed in the table below.
| CAS RN | DSL name or chemical name | Common name |
|---|---|---|
| 55-56-1a,b | 2,4,11,13-Tetraazatetradecanediimidamide, N,N''-bis(4-chlorophenyl)-3,12-diimino- | Chlorhexidine |
| 56-95-1 | 2,4,11,13-Tetraazatetradecanediimidamide, N,N''-bis(4-chlorophenyl)-3,12-diimino-, diacetate | Chlorhexidine diacetate |
| 3697-42-5b | 2,4,11,13-Tetraazatetradecanediimidamide, N,N''-bis(4-chlorophenyl)-3,12-diimino-, dihydrochloride | Chlorhexidine dihydrochloride |
| 18472-51-0a | D-Gluconic acid, compound with N,N''-bis(4-chlorophenyl)-3,12-diimino-2,4,11,13-tetraazatetradecanediimidamide | Chlorhexidine digluconate |
a This substance was not identified under subsection 73(1) of CEPA but was included in this assessment as it was considered a priority on the basis of other concerns.
b This substance is on the revised In Commerce List of Food and Drugs Act substances. Chlorhexidine dihydrochloride is not on the DSL or the Non Domestic Substances List.
Chlorhexidine and its salts do not occur naturally. Surveys have been conducted under section 71 of CEPA for chlorhexidine (reporting year 2011), chlorhexidine diacetate (reporting years 2005, 2006 and 2011), chlorhexidine digluconate (reporting year 2011), chlorhexidine dihydrochloride (reporting year 2015), with voluntary information being submitted for chlorhexidine dihydrochloride for 2013. None of these substances were reported to be manufactured in Canada for the years reported. Chlorhexidine diacetate and chlorhexidine dihydrochloride were reported to be imported into Canada in quantities of 1000 to 10 000 kg, while imports of chlorhexidine digluconate were reported to be in the range of 10 000 to 100 000 kg. No data on measured concentrations in the Canadian environment have been identified for any of these substances. Chlorhexidine and its salts are used in Canada as broad-spectrum antiseptics and antimicrobial preservatives in such products as cosmetics, natural health products, prescription and non-prescription drugs for human or veterinary uses, and hard-surface disinfectants.
Releases of chlorhexidine and its salts to the Canadian environment come from consumer use and formulation of chlorhexidine-based products. Releases are expected to be diffuse (i.e. down-the-drain from the use of products containing chlorhexidine or its salts), as well as from point sources (e.g. from sites formulating products containing chlorhexidine or its salts). When released to the aquatic environment, chlorhexidine salts dissociate in water to release chlorhexidine. Information on the fate and behaviour in the environment of chlorhexidine indicates that this substance tends to persist in water, sediment and soil, and it has a low potential to bioaccumulate in aquatic organisms. Experimental acute and chronic toxicity data for chlorhexidine and its salts show that chlorhexidine has the potential to cause adverse effects to aquatic organisms at low concentrations. Ecological exposure scenarios were developed for down-the-drain releases from uses of products containing these substances, as well as for releases from industrial sites formulating products containing these substances using a combination of results from monitoring and modelling. Risk quotient analyses were conducted to compare aquatic concentrations of chlorhexidine to adverse effect concentrations on aquatic and benthic organisms. The results indicate that chlorhexidine and its salts pose a risk to aquatic and benthic organisms when released as a result of industrial use, but not from the use of products containing these substances (down-the-drain releases).
Considering all available lines of evidence presented in this screening assessment, there is a risk of harm to the environment from chlorhexidine and its salts. It is concluded that chlorhexidine and its salts meet the criteria under paragraph 64(a) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity. However, it is concluded that chlorhexidine and its salts do not meet the criteria under paragraph 64(b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger to the environment on which life depends.
General population exposures to chlorhexidine and its salts from environmental media are expected to be low. Considering current use patterns, exposures are not expected from the diet. General population exposures can occur from use of cosmetics and natural health products containing chlorhexidine or its salts.
No evidence of carcinogenicity or genotoxicity was observed in the available health effects database for chlorhexidine and its salts. The margins between estimates of exposures from environmental media and from use of products available to consumers and levels associated with effects in laboratory studies are considered adequate to address uncertainties in the health effects and exposure databases. On the basis of available information for human health considerations, it is concluded that chlorhexidine and its salts do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that chlorhexidine and its salts meet one or more of the criteria set out in section 64 of CEPA. It has also been determined that the chlorhexidine moiety meets the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
1. Introduction
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of chlorhexidine (CAS RNFootnote 2 55-56-1) and its salts to determine whether these substances present or may present a risk to the environment or to human health. The salts include, but are not limited to, chlorhexidine diacetate (CAS RN 56-95-1), chlorhexidine digluconate (CAS RN 18472‑51-0), and chlorhexidine dihydrochloride (CAS RN 3697-42-5). Chlorhexidine, chlorhexidine diacetate and chlorhexidine digluconate are on the Domestic Substances List (DSL). Chlorhexidine and chlorhexidine dihydrochloride are on the revised In Commerce List (ICL) of Food and Drugs Act substances (Canada 1978). These substances were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA or were considered a priority on the basis of other concerns (ECCC, HC [modified 2017]). Chlorhexidine and its salts are being assessed as a group because they dissociate in water to release chlorhexidine, the moiety of toxicological concern.
A draft screening assessment for chlorhexidine diacetate was published in July 2013 (then referred to as chlorhexidine acetate) (Environment Canada, Health Canada [modified 2013]). It proposed that the substance was harmful to the environment and met the criteria under paragraph 64(a) of CEPA, but that it was not harmful to human health. No public comments were received on that draft assessment. However, significant new information subsequently became available regarding other potential sources of exposure to chlorhexidine. This information included quantities of chlorhexidine salts in commerce, presence in products sold in Canada, and elucidation of industry details related to the formulation of chlorhexidine-based products. As a result, a subsequent draft screening assessment was published on August 19, 2017 that assessed the chlorhexidine moiety to consider potential impacts on the environment and human health with respect to exposures from all potential sources of chlorhexidine.
This screening assessment on the chlorhexidine moiety considers potential impacts on the environment and human health with respect to exposures from all potential sources of chlorhexidine, and it includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data that were available were reviewed and evaluated up to June 2016, with limited and focused searches performed up to March 2019. Empirical data from key studies and monitoring as well as some results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological and human health portions of this assessment have undergone external review and/or consultation. Comments on the technical portions relevant to the environment were received from Dr. Jules Blais (University of Ottawa) and Dr. Connie Gaudet (consultant). Comments on the technical portions relevant to human health were received from scientific experts selected and directed by Toxicology Excellence for Risk Assessment (TERA)/University of Cincinnati, including Dr. Cynthia Bearer from University of Maryland School of Medicine (US), Dr. Simeon West from University College Hospital (UK), Dr. Michael Jayjock from The Lifeline Group (US), and Dr. Bernard Gadagbui from TERA/University of Cincinnati (US). Additionally, the draft of this screening assessment (published August 19, 2017) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight-of-evidence approach and precautionFootnote 3. This screening assessment presents the critical information and considerations on which the conclusions are based.
2. Identity of substances
The Chemical Abstracts Service Registry Numbers (CAS RNs), Domestic Substances List (DSL) names (or chemical names) and common names for chlorhexidine and certain chlorhexidine salts are presented in Table 2‑1.
| CAS RN | Common name | DSL name or chemical name |
|---|---|---|
| 55-56-1a,b | Chlorhexidine | 2,4,11,13-Tetraazatetradecanediimidamide, N,N''-bis(4-chlorophenyl)-3,12-diimino- |
| 56-95-1 | Chlorhexidine diacetate | 2,4,11,13-Tetraazatetradecanediimidamide, N,N''-bis(4-chlorophenyl)-3,12-diimino-, diacetate |
| 3697-42-5b | Chlorhexidine dihydrochloride | 2,4,11,13-Tetraazatetradecanediimidamide, N,N''-bis(4-chlorophenyl)-3,12-diimino-, dihydrochloride |
| 18472-51-0a | Chlorhexidine digluconate | D-Gluconic acid, compd. with N,N''-bis(4-chlorophenyl)-3,12-diimino-2,4,11,13-tetraazatetradecanediimidamide |
a This substance was not identified under subsection 73(1) of CEPA but was included in this assessment as it was considered a priority on the basis of other concerns.
b This substance is on the revised In Commerce List of the Food and Drug Act substances. Chlorhexidine dihydrochloride is not on the DSL or the Non Domestic Substances List.
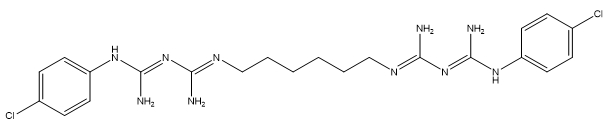
As a cationic broad-spectrum antimicrobial substance, chlorhexidine belongs to the bis(biguanide) family (Sigma Aldrich 2015). Its functional groups include guanidines, anilines, secondary aromatic amines, and aliphatic amines. Its structural configuration is a significant contributor to its bactericidal properties (Tanzer et al. 1977). In a review of cationic antiseptics, the structure of chlorhexidine contains cationic phospholipid binding sites and a hydrophobic hexamethylene group (Gilbert and Moore 2005), which contribute to its mode of action as a biocide.
Table 2‑2 presents the chemical structure information for chlorhexidine and certain chlorhexidine salts.
| Substance | Chlorhexidine structure, counterion and molecular formula | Molecular weight (g/mol) |
|---|---|---|
| Chlorhexidine |
C22H30Cl2N10 |
505.5 |
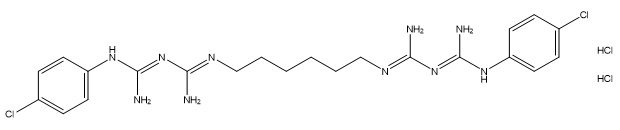
| Chlorhexidine dihydrochloride |
C22H30Cl2N10•2(HCl) |
578.4 |
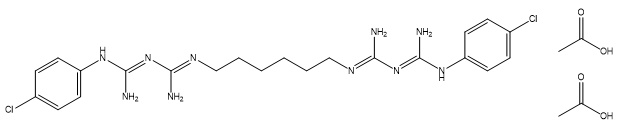
| Chlorhexidine diacetate |
C22H30Cl2N10•2(C2H4O2) |
625.6 |
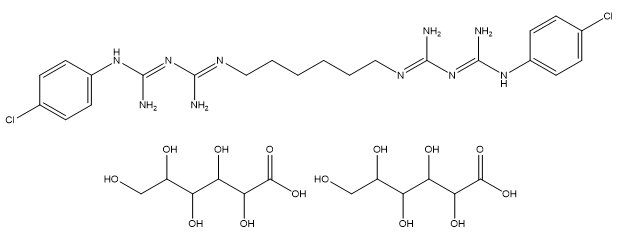
| Chlorhexidine digluconate |
C22H30Cl2N10•2(C6H12O7) |
897.8 |
3. Physical and chemical properties
Chlorhexidine salts dissociate in water to produce the associated counterions and chlorhexidine. Chlorhexidine is a strong base (pKa = 11.3) and is predicted to ionize in water as a base whereby protons are attracted to the amine groups (ACD/Percepta c1997-2012). It is expected to protonate in water at pH 4 to 9, such that virtually all (98% to 100%) of the substance will exist with two of its amine groups positively charged. The speciation of chlorhexidine in biological fluids will also be dependent on pH. Because the chlorhexidine (i.e., free base) is of toxicological concern, its physical and chemical properties are important to this assessment.
Table 3‑1 presents experimental and modelled data on the physical and chemical properties of chlorhexidine. Detailed substance-specific information for the chlorhexidine salts is available in ECCC (2018). Models based on quantitative structure-activity relationships (QSARs) were used to generate data for vapour pressure, Henry’s law constant, and log Koc. These models are mainly based on fragment addition methods (i.e., they rely on the structure of the chemical) and accept only the neutral (i.e., un-ionized) form of a chemical as input (in SMILES form or simplified molecular-input line-entry system).
| Property | Type | Value | Descriptor | Reference |
|---|---|---|---|---|
| Density (g/cm3) | Estimation via calculation | 1.39 | 20°C | ChemSpider 2011 |
| Vapour pressure (Pa) | Modelled (Modified Grain Method) | 2.6 x 10-12 | - | MPBPWIN 2010 |
| Water solubility (mg/L) | Experimental | 800a | 20°C | O'Neil 2013 |
| Henry’s law constant (Pa·m3/mol) | Modelled (VP/WSol estimate) | 1.7 x 10-12 | - | HENRYWIN 2008 |
| Log Dow (distribution coefficient; dimensionless) | Experimental (log Kow; octanol-water) | 0.08b | (ionized pH 5) | Hansch et al.1995 |
| Koc (organic carbon-water partition coefficient) | Modelled (log Koc; MCI estimate) | 5.9 | - | KOCWIN 2010 |
| Deffective (nm) | Calculated | 1.1 (average) | - | CPOPs 2014 |
| Dmaximum (nm) | Calculated | 2.1 (average) | - | CPOPs 2014 |
| pKa (dimensionless) | Modelled | 11.3 | Strongest pKa (base) | ACD/Percepta c1997-2012 |
a Values selected in modelling with EPI Suite (c2000-2010). The SMILES for chlorhexidine is used in this model along with the experimental water solubility and log Kow values shown here, and experimental melting point 134°C (HSDB 1983- ), as user inputs.
b The distribution coefficient or log D takes into account the presence of the ionic species; it represents the net amount of the neutral and ionic forms expected to partition into lipid and water at a given pH.
The ionic nature of chlorhexidine is an important consideration in interpreting its physical and chemical properties as they relate to environmental fate and behaviour (see the Environmental Fate and Behaviour section for further discussion). As chlorhexidine is ionic, it has a negligible vapour pressure and Henry’s law constant. Experimental data for chlorhexidine indicate a high solubility in water (800 mg/L, O’Neil 2001), as do data for the salts. Experimental water solubility values of 1.0 x 104 to 3.3 x 103 mg/L at pH 4 to 7 (Anusavice et al. 2006) and 1.9 x 104 mg/L at 20°C and unknown pH (O’Neil 2001) are reported for chlorhexidine diacetate. The water solubility of chlorhexidine digluconate has been documented as >70% w/v at 20°C (Senior 1973). Chlorhexidine diacetate and chlorhexidine digluconate have also been found to be soluble to some degree in other solvents (O’Neil 2001; US EPA 1996; US EPA 2011b). The experimental log Kow value for chlorhexidine (0.08 at pH 5) is low and accounts for the ionizing characteristics of the substance. The modelled data for chlorhexidine indicate a very high log Koc (5.9). However, it is recognized that there is uncertainty in modelling such parameters for ionizing substances and that electrostatic interactions may be more important than organic carbon in determining partitioning characteristics.
Additionally, chlorhexidine may have surface-acting characteristics. Its surface tension (approximately 50 dynes/cm; ECHA c2007-2015a) is below the 60 dynes/cm threshold indicative of surface active properties (European Union 1998–2016). It has been reported that chlorhexidine diacetate forms micelles in solution with a critical micellar concentration of 6256 to 6882 mg/L (molar critical micellar concentration of 0.010 to 0.011) at 25°C (Block 2001; Heard and Ashworth 1968), while another study found it does not (Attwood and Natarajan 1979). A critical micellar concentration of 5925 mg/L (molar critical micellar concentration of 0.0066) has also been reported for chlorhexidine digluconate (Heard and Ashworth 1968).
4. Sources and uses
Chlorhexidine and its salts do not naturally occur in the environment.
Surveys have been conducted under section 71 of CEPA for chlorhexidine (reporting year 2011), chlorhexidine diacetate (reporting years 2005, 2006 and 2011), chlorhexidine digluconate (reporting year 2011), and chlorhexidine dihydrochloride (reporting year 2015) (Canada 2006; Canada 2009; Canada 2012; Canada 2017). Voluntary information was submitted for chlorhexidine dihydrochloride for 2013 (Environment Canada 2015). None of these substances were reported to be manufactured in Canada above the 100 kg per year threshold for the years reported. All of the chlorhexidine salts (diacetate, digluconate and dihydrochloride) were imported into Canada during one or more of the reporting years and were also identified as being used in products available to consumers. Fewer than five companies reported importing chlorhexidine diacetate into Canada in 2005 (Environment Canada 2007), as well as in 2006 (Environment Canada 2010). All reported import quantities were in the range of 100 to 1000 kg for each company. For the 2011 reporting year (Environment Canada 2015), the total quantity of chlorhexidine diacetate imported in a product or for processing/formulation (pure salt) was in the range of 100 to 1000 kg. Nine companies reported importing chlorhexidine digluconate in 2011 (including imports of a product and pure salt for processing/formulation), with total imports in the range of 10 000 to 100 000 kg. There were no imports of chlorhexidine above the 100 kg per year threshold in 2011. Fewer than five companies reported importing 100 to 1000 kg of chlorhexidine dihydrochloride in 2013 (for processing/formulation in the form of pure salt), as well as 1000 to 10 000 kg in 2015 with almost half of that being subsequently exported (Environment Canada 2015, 2018).
Chlorhexidine is included on the 2007 Organisation for Economic Co-operation and Development (OECD)’s list of high production volume (HPV) chemicals (OECD 2009), indicating that it is produced or imported at levels greater than 1000 tonnes per year in at least one member country or region. The annual consumption of chlorhexidine in the European Union was reported to be 10 000 to 50 000 tonnes in 2000, while the estimated use of the digluconate salt was 7.9 tonnes in 2009 in Sweden (SWECO Environment 2011). Certain chlorhexidine substances have since been registered as part of the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) program. In particular, chlorhexidine has been registered for intermediate use only, and the digluconate salt has been registered for manufacturing and/or importation (10 to 100 tonnes per year; ECHA c2007-2015a,b).
Chlorhexidine and its salts are broad spectrum antiseptics used for sterilization, cleaning skin and hands, disinfecting wounds, and oral health and are generally effective against a wide variety of bacteria, viruses and yeasts (Chemicalland21 2010, Cheminfo Services Inc. 2014). In Canada, they are used as broad-spectrum antiseptics and antimicrobial preservatives in such products as cosmetics, natural health products, prescription and non-prescription drugs for human or veterinary uses, and hard-surface disinfectants. Permitted uses are further described as per the relevant lists and databases administered in Canada detailed below.
Chlorhexidine digluconate and chlorhexidine diacetate (as ‘chlorhexidine acetate salt’) are listed in the Drug Product Database (DPD) as active ingredients in prescription and non-prescription drugs for human or veterinary use and hard-surface disinfectants (DPD [modified 2015]). Within dairy applications, chlorhexidine is mainly used for the prevention of mastitis in cows and is manufactured as teat dips and wipes as well as udder washes (DPD [modified 2015]; Westagro Canada 2014a,b); chlorhexidine diacetate is the dominant salt utilized, with very few products registered that are based on the digluconate salt (DPD [modified 2015]; Cheminfo Services Inc. 2014). When chlorhexidine digluconate and chlorhexidine diacetate are used as active ingredients in prescription and non-prescription drugs, human exposure to chlorhexidine from use of these products is addressed under the Food and Drugs Act and is not considered further in this screening assessment.
Chlorhexidine, chlorhexidine digluconate and chlorhexidine diacetate are listed in the Natural Health Products Ingredients Database (NHPID) with a non-natural health product role; thus, they cannot be used as medicinal ingredients in natural health products (NHPID [modified 2019]). Chlorhexidine, chlorhexidine digluconate and chlorhexidine diacetate are listed in the NHPID with a non-medicinal role for topical use as antimicrobial preservatives in natural health products and are associated with upper limits of 0.14%, 0.20%, and 0.19%, respectively. They are also associated with an upper limit for ophthalmic use of 0.01% (calculated as chlorhexidine free base). Chlorhexidine and chlorhexidine digluconate are listed in the Licensed Natural Health Products Database (LNHPD) as being present as a non-medicinal ingredient in currently licensed natural health products, including acne therapy products, medicated skin care products, oral care products, and sunscreens (LNHPD [modified 2019]).
Chlorhexidine and its salts are not listed in the lists of permitted food additives (Health Canada [modified 2013]) under the Food and Drugs Act and associated marketing authorizations, nor have they been identified as being used or present in formulations of food packaging materials. Chlorhexidine digluconate and chlorhexidine diacetate may be present in cleaning products that are not in direct contact with food, such as hand soaps, sanitizers and general purpose cleaners used in food preparation facilities (personal communication, emails from the Food Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 2016 and February 2019; unreferenced).
Chlorhexidine and its salts are included on the List of Prohibited and Restricted Cosmetic Ingredients, which is an administrative tool used by Health Canada to communicate to manufacturers and others that products containing certain substances are unlikely to be classified as a cosmetic under the Food and Drugs Act, and in addition, that certain substances, when present in a cosmetic at certain concentrations, may contravene the general prohibition found in section 16 of the Act, or may contravene one or more provisions of the Cosmetic Regulations. Chlorhexidine and its salts are listed as restricted to concentrations equal to or less than 0.14%, calculated as chlorhexidine free base; which corresponds to 0.19%, calculated as chlorhexidine diacetate; 0.20%, calculated as chlorhexidine digluconate; and 0.16%, calculated as chlorhexidine dihydrochloride (Health Canada [modified 2015]). According to notifications submitted under the Cosmetic Regulations to Health Canada, chlorhexidine digluconate and to a lesser extent chlorhexidine dihydrochloride are used in certain cosmetics in Canada, such as make-up, hair products, skin care products, and aftershaves (personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 2016; unreferenced).
Chlorhexidine and its salts are not listed on the Pest Management Regulatory Agency (PMRA) Pesticide Formulants List or on PMRA’s List of Active Pesticide Ingredients (Health Canada 2010; personal communication, emails from the Pest Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated December 2014; unreferenced; Pesticide Label Search [modified 2016]). Chlorhexidine diacetate has been identified as a component of footwear baths for farm visitors in Canada (OMAFRA 2009).
Chlorhexidine digluconate is used in eyewash stations as a bacteriostatic additive at a concentration of 5300 ppm or 0.53% (SDS 2015).
5. Environmental fate and behaviour
Given the sources and uses of chlorhexidine and its salts, there is the potential for these substances to be released to the environment. The following sections focus on the environmental fate and behaviour of chlorhexidine, as the moiety of concern. As the cationic form of chlorhexidine dominates at environmentally relevant pH, its environmental mobility and fate will be strongly dependent on its sorption to suspended particulates in water and air and to sediment and soil particles (Droge and Goss 2012).
5.1 Environmental distribution
Chlorhexidine is not expected to be released to air given its intended uses and physical-chemical properties. Chlorhexidine has a very low vapour pressure and Henry’s law constant, high water solubility, and its existence in a protonated form in the environment indicate that volatilization would be negligible from either dry or moist soil surfaces or surface waters.
If released to the aquatic environment, chlorhexidine salts will dissociate, releasing chlorhexidine and the associated counterions. Chlorhexidine will have an affinity for negatively charged particles in the water column (e.g., humic and fulvic acids, clay materials). The sorption processes would be dominated by electrostatic interactions as a result of the negatively charged sorption sites on dissolved organic carbon and suspended solids, although organic carbon may also play a small role (Kah and Brown 2006; Droge and Goss 2012, 2013). Suspended solids may eventually settle to bed sediment, where the sorbed chlorhexidine is likely to remain unless mixing and transport of the bed sediment occurs. Considering its persistence (see section 5.2), chlorhexidine may be present in water and sediment both near and far from source.
Although no direct releases to soil are anticipated, indirect releases may result from the application to land of biosolids from wastewater treatment systemsFootnote 4 (WWTSs) receiving wastewater that contains chlorhexidine. The degree to which chlorhexidine is removed from the wastewater will be determined by the characteristics of the WWTS and the affinity of chlorhexidine for negatively charged suspended solids, but it is expected to be associated with dissolved and suspended solids to a large degree.
The fate of chlorhexidine in soils will be dictated by its cationic nature. While cation-exchange is complex and not fully understood (Droge and Goss 2012, 2013), it is expected that chlorhexidine would have an affinity for negatively charged particles and may or may not be mobile depending on the soil moisture content, soil type (e.g., it would likely be less mobile in soils with high organic matter or high clay content) (Droge and Goss 2012, 2013), and soil erosion or runoff. In addition, for organic cations such as chlorhexidine, the sorption affinity further depends on competition with other organic cations present in soils (Droge and Goss 2012). Both positive and negative relationships between adsorption and ionic strength (electrolyte composition and concentration) have been reported in the case of ionic pesticides, and results suggest that application of large amounts of phosphorus and lime to agricultural fields could decrease sorption and increase pesticide concentration in solution, particularly in weathered soils (Kah and Brown 2006). Addition of lime to a field could result in considerable increase in bioavailability of organic cations (Droge and Goss 2012). In addition to ionic strength, other factors influencing adsorption of ionisable compounds in soils include soil properties, water content and, to a minor degree, temperature (Kah and Brown 2006).
5.2 Environmental persistence
Abiotic degradation of chlorhexidine is not expected to be significant. Chlorhexidine does not contain functional groups expected to undergo hydrolysis (HYDROWIN 2010). Although it is not expected to be released to air or reside in air if released to it, reactions with hydroxyl radicals would be the most important fate process in the atmosphere (estimated half-life of 25 minutes; AOPWIN 2010). The substance is not expected to react appreciably with other photo-oxidative species in the atmosphere (such as O3; AOPWIN 2010). Some studies indicate that chlorhexidine salts (diacetate and digluconate) undergo photodegradation (Revelle et al. 1993; Freitag et al. 1985; Zong and Kirsch 2012).
Several ready and inherent biodegradation studies investigating the microbial degradation of chlorhexidine and its salts are available. Many of the studies show limited or no biodegradation, and results are consistent with model results. This result is also consistent with a study on river microbial biofilm development, where no mineralization of chlorhexidine was observed after 120 days of incubation with [14C]chlorhexidine (Lawrence et al. 2008). A few studies using activated sludge report some biodegradation as a result of resistant strains of bacteria. However, environmental conditions would be quite different from laboratory test conditions (i.e., lower bacterial concentrations, varying temperatures, other environmental conditions), and would limit bioaccessibility. Thus microbial degradation is not anticipated to be a dominant degradation pathway for chlorhexidine and its salts in the environment.
No degradation was observed in an activated sludge die-away test conducted using freshly collected activated sludge dosed with 50 μg/L 14C chlorhexidine dihydrochloride (Study Submission 2010). A second test was conducted using acclimated activated sludge continuously exposed to wastewater amended with 200 μg/L chlorhexidine dihydrochloride for a 31-day period. Both tests were conducted according to the test procedures of OECD 314B (for determining rates of primary and ultimate degradation rates), and used test concentrations of 50 μg/L 14C chlorhexidine dihydrochloride and a biosolids concentration of 2500 mg/L. The results from both die-away tests showed no significant primary degradation of the test material (Study Submission 2010).
A closed bottle test using an activated sludge inoculum (1.5 mg/L) and chlorhexidine (5.35 ppm) resulted in 0% chemical oxygen demand (COD) after 28 days (De Waart and Van der Most 1986, as cited in HSDB 1983- ). In another test, 14C-labelled chlorhexidine was incubated at 0.05 ppm in an activated sludge for 5 days, with results of 0.1% CO2 evolution, 94.3% non-extractable residues (amount retained in sludge), and 0.2% volatilization (Freitag et al. 1982).
Kodama et al. (1988) evaluated the effect of treatment by activated sludge on chlorhexidine concentrations in hospital and domestic wastewaters before and after pretreatment with hydrochloride and celite. Results by colorimetric and high-performance liquid chromatography (HPLC) methods show comparable chlorhexidine concentrations in both the inflow and outflow of treatments systems, indicating low removal rates.
Sugio and Kojima (1992) investigated the characteristics of chlorhexidine digluconate-resistant activated sludge acclimatized to wastewater containing the substance by isolating strains of the resistant bacteria and inoculating acclimated activated sludge with 100 ppm chlorhexidine digluconate. Biological oxygen demand (BOD) results showed that both the un-acclimated and acclimated sludge could not degrade chlorhexidine or chlorhexidine digluconate.
In a biodegradation study of chlorhexidine (12 mg/L) in wastewater, no degradation was observed after 21 days in OECD minimal media tests for detergents in both aerobic and anaerobic conditions (Voets et al. 1976). Meanwhile, the OECD activated sludge organic medium test showed no degradation under anaerobic conditions, but 60% to 100% degradation was reported under aerobic conditions.
A few studies indicate that chlorhexidine may be biodegraded in activated sludge (Sakagami and Yokoyama 1983; Kido et al. 1988). Kido et al. suggest that 2 out of 7 bacterial strains isolated from activated sludge utilize chlorhexidine as a sole nitrogen source for growth in aerobic conditions. In more recent studies, Tanaka et al. (2005, 2006) have reported microbial degradation of chlorhexidine digluconate by a particular strain of bacteria under laboratory conditions conducive to its growth (i.e., 37°C). Although degradation was not quantified, the authors reported “significant” degradation of chlorhexidine within 7 days on the basis of results of the HPLC chromatograms. These findings indicated a possible resistance mechanism of some bacterial strains to disinfectants through biodegradation.
Biodegradation was modelled using EPI Suite (c2000-2010; see ECCC 2018 for modelling results). Results for the primary biodegradation model (sub-model 4; BIOWIN 2008) and the three ultimate biodegradation models (sub-models 3, 5 and 6; BIOWIN 2008) indicate that biodegradation is slow. The extrapolated half-life in water is predicted to be more than 182 days. On the basis of an extrapolation ratio of 1:1:4 for a water:soil:sediment biodegradation half-life (Boethling et al. 1995), the ultimate biodegradation half-life in water is used to extrapolate the half-lives in these other media. The estimated ultimate degradation half-life in aerobic soil is therefore expected to be greater than or equal to 182 days, and the half-life in aerobic sediment is expected to be greater than or equal to 365 days.
A potential degradation product of chlorhexidine is p-chloroaniline. Different routes of chlorhexidine degradation to p-chloroaniline have been reported under laboratory conditions, including hydrolysis and decarboxylation reactions (Sigma Aldrich 2003; Revelle et al. 1993), and bacterial degradation (Ogase et al. 1992). Chlorhexidine has also been shown to degrade under test conditions of heating (IPCS 2003; Revelle et al. 1993), and in acidic and alkaline conditions when subjected to high temperatures (Zong and Kirsch 2012; Revelle et al. 1993). Residual p-chloroaniline content in chlorhexidine is reported to be less than 500 mg/kg (<0.05%), but may reach 2000 mg/L (0.2%) if chlorhexidine solutions are stored for 2 years or more at high (tropical) ambient temperatures or if inadvertently heat sterilized (IPCS 2003). A national screening program that investigated the occurrence of chlorhexidine and p-chloroaniline in Sweden did not measure p-chloroaniline in any of the environmental samples collected (SWECO Environment 2011). It is unlikely that p-chloroaniline will be formed in the Canadian environment, given the low potential for biodegradation of chlorhexidine and the fact that conditions necessary for the formation of p-chloroaniline are not environmentally relevant.
In summary, the available information indicates that chlorhexidine tends to persist in water, sediment and soil. Half-lives in water and soil are greater than 182 days and in sediment is greater than 365 days. As a result of its persistence, there is a potential for prolonged exposure to chlorhexidine both near and far from points of discharge to the environment. There is also the potential for increased spatial exposure in the aquatic environment as a result of its affinity for negatively charged particles and transport via suspended solids and sediment. However, as chlorhexidine associates with negatively charged particles, it may become less bioavailable over time.
5.3 Potential for bioaccumulation
The molecular weight of chlorhexidine (505.5 g/mol) and calculated cross-sectional diameters (Deffective and Dmaximum of 1.1 nm and 2.1 nm, respectively; CPOPs 2014) indicate that it is a relatively large molecule. Investigations on fish bioconcentration factors (BCFs) and molecular size parameters show that the probability of passive diffusion via the gills decreases appreciably when the effective diameter of a chemical is greater than 1.1 nm and when the maximum diameter of a chemical is greater than about 1.5 nm (and much more so for molecules having a maximum diameter greater than 1.7 nm) (Dimitrov et al., 2002, 2003; Sakuratani et al. 2008).
There were a few studies that investigated the potential for the bioconcentration of chlorhexidine in aquatic organisms. Two studies evaluated the bioconcentration potential of chlorhexidine in golden eye (Leuciscus idus melanotus), exposing fish to chlorhexidine at 0.05 mg/L for 3 days (Freitag et al. 1982, 1985). Evaluation of the concentration of chlorhexidine in fish compared with the concentration in water resulted in BCFs of 42 and 40, indicating low bioconcentration potential. Details on the methods used were limited in these studies, but results are consistent with what would be expected given the low experimental log Kow of 0.08 (ionized pH 5) and log D (0.47) of chlorhexidine. The same study (Freitag et al. 1985) derived a BCF from the distribution of chlorhexidine between algae and water. Green algae (Chlorella fusca var. vacuolata) were exposed to chlorhexidine at 0.05 mg/L for 24 hours, and a moderate BCF of 2560 was determined. This moderate BCF is likely attributed to the strong association of chlorhexidine to exposed anionic sites on the cell surfaces.
A study using soft X-ray scanning transmission X-ray microscopy to map chlorhexidine relative to major biochemical components in natural river biofilms showed the bioaccumulation of chlorhexidine in the lipid rich regions of diatoms and bacteria after an 8 week exposure to chlorhexidine digluconate (Dynes et al. 2006). This method showed chlorhexidine was sorbed or chemically associated with lipids in the diatoms and bacteria. The community composition of the river biofilms studied was also altered in the presence of chlorhexidine, with the most significant observation being the suppression of grazers. Given the cationic nature of chlorhexidine, it is likely to interact with the negatively charged, phospholipid bilayer of cell membranes.
As chlorhexidine has structural alerts for potential protein binding (OECD QSAR Toolbox 2015), it could bioaccumulate through this route. Princz et al. (2014) studied the bioaccumulation potential of phloxine B, an ionic substance that, like chlorhexidine, would be expected to have a low potential to bioaccumulate. However, phloxine B is ionized at environmentally relevant pH and was shown to bind to dermal and internal protein tissues within earthworms, resulting in high observed biota-to-soil accumulation factors (BSAFs). In their review of cationic antiseptics, Gilbert and Moore (2005) report that, like quaternary ammonium compounds (QACs), the biguanide groupings of bisbiguanide antiseptics associate strongly with exposed anionic sites on the cell membrane and cell wall of bacteria, particularly acidic phospholipids and proteins. However, unlike QAC biocides, the hydrophobic 6-carbon-long regions of chlorhexidine do not become solubilized within the hydrophobic core of the cell membrane, as it is somewhat inflexible and incapable of folding to interlock into the bilayer. Instead, chlorhexidine bridges between pairs of adjacent phospholipid head-groups (Gilbert and Moore 2005).
Considering the above information, chlorhexidine is expected to have a low potential to bioaccumulate given its high water solubility, low experimental log Kow and predicted log Dow, and the results of experimental BCF studies. Modelled data (BCFs and bioaccumulation factors or BAFs) are consistent with experimental results (ECCC 2018). Chlorhexidine and its salts could potentially bioaccumulate through protein binding, similar to phloxine B, but no empirical data on this was found. Overall, the chlorhexidine moiety likely has a low potential to bioaccumulate.
6. Potential to cause ecological harm
6.1 Ecological effects assessment
Known as a membrane disruptor, the toxic mode of action for chlorhexidine has been studied in bacteria and is due to the strong association of biguanide groupings to exposed anionic sites on the cell membrane and cell wall (particularly to acidic phospholipids and proteins) (Broxton et al. 1984; Fraud et al. 2003; Gilbert and Moore 2005). Using scanning transmission X-ray microscopy, Dynes et al. (2006) demonstrated that chlorhexidine was sorbed or perhaps chemically associated with the lipids in diatoms and bacteria. Chlorhexidine has been reported in some studies to cause cellular leakage, inhibition of respiration and solute transport, and loss of structural integrity through damage to the cellular envelope (Gilbert and Moore 2005; O’Driscoll et al. 2014). The Profiler function of the OECD QSAR Toolbox (2015) identified structural alerts for protein binding, suggesting that chlorhexidine exerts adverse effects beyond a baseline narcotic mode of action. In assessing the potential for ecotoxicity, physical-chemical properties and the bioavailability of chlorhexidine were considered, as well as its mode of action in bacteria and predicted reactive mode of action.
Key empirical aquatic and terrestrial toxicity data are summarized below for chlorhexidine and its salts. Detailed information on all available studies is tabulated in ECCC (2018). Since chlorhexidine is the moiety of concern, experimental toxicity data have been expressed in chlorhexidine equivalent values through application of a molecular weight ratio with the associated salt.
6.1.1 Water
Key experimental data for acute (or short-term) and chronic (or long-term) aquatic toxicity are summarized in Table 6‑1 and Table 6‑2, respectively. Studies are listed in increasing order of chlorhexidine equivalent effect concentrations.
| Test organism | Test compound | Endpoint | Chlorhexidine equivalent valuea mg/L | Reference |
|---|---|---|---|---|
Green algae (Scenedesmus subspicatus) |
Chlorhexidine digluconate | 72-h EC50 (biomass) | 0.0062b (0.011) |
ECHA c2007-2015b |
Green algae (S. subspicatus) |
Chlorhexidine digluconate | 72-h EC50 (biomass) | 0.021 (0.038) |
ECHA c2007-2015b |
| Pseudokirchneriella subcapitata | Chlorhexidine digluconate | 24-h EC50 (growth inhibition) | 0.0233 (0.0413) |
Jesus et al. 2013 |
| Daphnia magna | Chlorhexidine digluconate | 48-h EC50 (immobilization) |
0.025 (0.045) |
Jesus et al. 2013 |
| D. magna | Chlorhexidine diacetate | 48-h EC50 (immobilization) |
0.05 (0.06) |
Murphy and Smith 1991a |
| D. magna | Chlorhexidine digluconate | 48-h EC50 (immobilization) | 0.049 (0.087) |
ECHA c2007-2015b |
| D. magna | Chlorhexidine digluconate | 48-h EC50 | 0.24 (0.42) |
US EPA 2011b |
| Bluegill sunfish (Lepomis macrochirus) |
Chlorhexidine digluconate | 96-h LC50 | 0.29 (0.51) |
US EPA 2011b |
Zebrafish embryos (Danio rerio) |
Chlorhexidine digluconate | 96-h LC50 | 0.453 (0.804) |
Jesus et al. 2013 |
| Bluegill sunfish (L. macrochirus) |
Chlorhexidine diacetate | 96-h LC50 | 0.5 (0.6) |
Murphy and Smith 1991b |
Zebrafish (D. rerio) |
Chlorhexidine digluconate | 96-h LC50 | 1.17 (2.08) |
ECHA c2007-2015b |
Rainbow trout (Oncorhynchus mykiss) |
Chlorhexidine digluconate | 96-h LC50 | 1.3 (2.3) |
US EPA 2011b |
Zebrafish (D. rerio) |
Chlorhexidine | 96-h LC50 | 1.4 (1.4) |
ECHA c2007-2015a |
Rainbow trout (O. mykiss) |
Chlorhexidine diacetate | 96-h LC50 | 1.5 (1.9) |
Murphy and Smith 1991c |
a Values in parenthesis are original values reported for the corresponding test compound.
LC50: The concentration of a substance that is estimated to be lethal to 50% of the test organisms.
EC50: The concentration of a substance that is estimated to cause some effect on 50% of the test organisms.
b Critical toxicity value.
| Test organism | Test compound | Endpoint | Chlorhexidine equivalent valuea mg/L | Reference |
|---|---|---|---|---|
Green algae (S. subspicatus) |
Chlorhexidine digluconate | 72-h EC10 (biomass) | 0.002 (0.003) |
ECHA c2007-2015b |
Green algae (S. subspicatus) |
Chlorhexidine digluconate | 72-h NOEC (biomass) | 0.0042 (0.0075) |
ECHA c2007-2015b |
| P. subcapitata | Chlorhexidine digluconate | 24-h EC20 (growth inhibition) | 0.0116 (0.0206) |
Jesus et al. 2013 |
| D. magna | Chlorhexidine digluconate | 21-d NOEC (mortality) | 0.0116 (0.0206) |
ECHA c2007-2015b |
| Monoraphidium griffithii | Chlorhexidine digluconate | 10-d IC10 (growth rate) | 0.29 (0.52) |
ECHA c2007-2015b |
a Values in parenthesis are original values reported for the corresponding test compound.
EC10: The concentration of a substance that is estimated to cause some effect on 10% of the test organisms.
EC20: The concentration of a substance that is estimated to cause some effect on 20% of the test organisms.
NOEC: No observable effects concentration.
IC10: Inhibitory concentration.
Acute toxicity data for chlorhexidine and its salts indicate that chlorhexidine is toxic to aquatic organisms at low concentrations, with adverse effects reported in key studies below 0.1 mg/L chlorhexidine. Jesus et al. (2013) reported acute toxicity for chlorhexidine digluconate to algae (EC50=0.0233 mg/L) and Daphnia magna (EC50=0.025 mg/L). This is consistent with algae studies submitted to ECHA (c2007-2015b) for chlorhexidine digluconate, and for D. magna studies submitted to the US EPA (2011b) and ECHA (c2007-2015b) for chlorhexidine digluconate and chlorhexidine diacetate. Toxicity data for chlorhexidine and its salts also indicate that chlorhexidine causes acute adverse effects in fish, including bluegill sunfish, zebrafish, and rainbow trout (US EPA 2011b; ECHA c2007-2015b; US EPA 2011b, respectively). Fish appear to be less sensitive to the effects of chlorhexidine than are algae and daphnids, with the exception of the more sensitive life stage of zebrafish embryos, as indicated by the LC50 of 0.453 mg/L reported by Jesus et al. (2013). Original studies submitted to the US EPA and ECHA were not available for review in the context of the current assessment.
Chronic toxicity data for chlorhexidine salts also indicate that algae are particularly sensitive to the effects of chlorhexidine, likely due to its mode of action as a membrane disruptor, binding to anionic sites on cell surfaces. Values of 0.002 mg/L (EC10) and 0.0042 mg/L (NOEC) for Scenedesmus subspicatus have been reported in two studies submitted to ECHA (c2007-2015b). Jesus et al. (2013) reported an EC20 of 0.0116 mg/L for Pseudokirchneriella subcapitata when exposed to chlorhexidine digluconate, raising concerns about its potential effects in aquatic food webs. In a long-term study, microbial community composition was shown to be sensitive to the presence of low levels of chlorhexidine (10 µg/L and 100 µg/L treatments) over an 8-week period (Lawrence et al. 2008). The introduction of chlorhexidine at 100 µg/L resulted in the elimination of protozoans and metazoans in the biofilms, in addition to significant changes in algal, cyanobacterial, and bacterial biomass, and carbon utilization. Alteration in community composition of river biofilms was also observed by Dynes et al. (2006) in the presence of chlorhexidine, with suppression of grazers. These studies provide useful community-level effects data due to the presence of chlorhexidine, and provide insight on changes to community population and dynamics that might be seen in the natural environment.
The lowest acute effects concentration of 0.0062 mg/L chlorhexidine for S. subspicatus was identified from the data set as the critical toxicity value (CTV) to be used in deriving a predicted no-effects concentration (PNEC). The two lower chronic effects concentrations were not chosen as the CTV because they would have resulted in a less sensitive PNEC. The CTV of 0.0062 mg/L chlorhexidine was divided by an assessment factor of 30, considering the endpoint type and the need to estimate a long-term no-effects concentration. This factor also accounts for inter-species and intra-species variability in sensitivity as well as a predicted reactive mode of action, in consideration of the dataset available for chlorhexidine. The resulting PNEC value is 0.21 µg/L (0.00021 mg/L), indicating that chlorhexidine has the potential to cause adverse effects in aquatic organisms at low concentrations.
6.1.2 Sediment
Only one sediment toxicity study was available as a submission to ECHA (the original study was not available for review in the context of this assessment). The chronic 28-day study tested the effects of chlorhexidine digluconate on the harlequin fly (Chironomus riparius), with a reported chlorhexidine equivalent NOEC value of 2.44 mg/kg sediment dry weight (2% organic carbon content as set by the guidelines followed), calculated using emergence rate (ECHA c2007-2015b).
This chronic study was used to derive a PNEC for sediment. An assessment factor of 100 was applied to account for inter- and intra-species variation as well as its predicted reactive mode of action (OECD QSAR Toolbox 2015), given the lack of effects data for benthic organisms. After standardizing to an organic carbon (OC) content of 4% (a typical OC content in bottom sediment for rivers and lakes used in characterizing risk; see the Characterization of Ecological Risk section), the resulting PNEC is 0.049 mg/kg dry weight (dw).
6.1.3 Soil
One soil toxicity study has been submitted to ECHA (the original study was not available for review in the context of this assessment). The results indicate that chlorhexidine digluconate has a low potential to adversely affect terrestrial plants (Brassica napus, Avena sativa, Glycine max) with respect to seedling growth (ECHA c2007-2015b). Table 6‑3 summarizes these key soil toxicity studies for chlorhexidine digluconate. In another study, no mortality was reported for the redworm, Eisenia fetida, after 14 days of exposure to a single chlorhexidine digluconate test concentration of 1000 mg/kg soil dw (ECHA c2007-2015b). A dose-response relationship was not demonstrated.
| Test organism | Test compound | Endpoint | Chlorhexidine equivalent valuea (mg/kg soil dw) | Reference |
|---|---|---|---|---|
| Brassica napus | Chlorhexidine digluconate | 21-d NOEC (shoot fresh weight) | 35.2 (62.5) |
ECHA c2007-2015b |
| Avena sativa | Chlorhexidine digluconate | 21-d NOEC (shoot height) | 70 (125) |
ECHA c2007-2015b |
| Glycine max | Chlorhexidine digluconate | 21-d NOEC (shoot fresh weight) | 281 (500) |
ECHA c2007-2015b |
a Values in parenthesis are original values reported for the corresponding test compound.
NOEC: No observable effects concentration.
The 21-d NOEC of 35.2 mg/kg soil dw (1.18% OC content) for B. napus was chosen as the chronic CTV, and an assessment factor of 100 was applied to account for inter- and intra-species variation (as there are only three species from one taxonomic group) as well as its predicted unknown reactive mode of action (OECD QSAR Toolbox 2015) given a lack of effects data for soil organisms. After standardizing to an OC content of 3.1% (OC content used in the BASL4 model to characterize risk; see the Characterization of Ecological Risk section), the resulting PNEC is 0.93 mg/kg dw.
6.1.4 Wildlife
Toxicological data on avian species (including the northern bobwhite and mallard) for chlorhexidine diacetate and chlorhexidine digluconate show that chlorhexidine has low toxicity to these species (Campbell et al. 1991; OPP Pesticide Ecotoxicity Database 1991; Long et al. 1991a,b; US EPA 2011b). The lowest subacute dietary NOEL value is 1438 mg/kg chlorhexidine (OPP Pesticide Ecotoxicity Database 1991) and the lowest acute (single dose, oral) effect value is 1627 mg/kg chlorhexidine (Campbell et al. 1991). These data indicate that dietary exposure to chlorhexidine is not likely to result in adverse effects in avian species.
Mammalian toxicity studies on chlorhexidine and its salts are discussed in detail in the Health Effects Assessment section. Various studies have been conducted (including oral, dermal, inhalation, dietary studies) on rats, mice, dogs, rabbits, marmosets, and rhesus monkeys. A lowest observed effect level of 5 mg/kg bw per day was reported in oral chronic studies when rats were exposed to chlorhexidine digluconate through drinking water and diet (Case 1977; Block 2001; ECHA c2007-2015b). The US EPA review of the toxicology data (US EPA 1996) concluded that chlorhexidine diacetate is mildly to moderately toxic to mammals when administered by inhalation, oral or dermal routes.
Although there is the potential for birds and animals to be exposed to chlorhexidine in environmental media via their drinking water, diet or dermal contact, they are not likely to be exposed to levels that would result in adverse effects. There is also evidence that chlorhexidine is poorly absorbed through skin and the gastrointestinal tract (see the Health Effects Assessment section). Therefore, this pathway is not considered further in exposure analyses.
6.2 Ecological exposure assessment
6.2.1 Measured concentrations in environmental media and wastewater
Data concerning concentrations of chlorhexidine in the Canadian environment have not been identified. However, different wastewater systems in Canada were sampled in 2016-17 and 2017-2018 as part of the Chemicals Management Plan Monitoring and Surveillance program. Influent and effluent samples were analyzed for chlorhexidine at 24 different WWTSs over the 2 year period (personal communication, from the Emerging Priorities Division, ECCC to the Ecological Assessment Division, ECCC, dated November 2018; unreferenced). These WWTSs were selected to represent typical Canadian treatment systems and geographic variations. Either grab samples or 24-h composite samples were collected. Of the 96 influent samples analyzed, chlorhexidine was measured in 79 samples with concentrations ranging from 0.0339 to 4.470 µg/L (reporting limits 0.0153 to 0.0230 µg/L). A total of 96 effluent samples were analyzed and chlorhexidine was measured in 19 of these samples. Concentrations ranged from 0.0188 to 0.448 µg/L (reporting limits 0.0115 to 0.0121 µg/L). Removal rates for different treatment technologies were also estimated using paired influent and effluent samples. Median per cent removals of chlorhexidine were 93.7% for facultative lagoons, 94.4% for aerated lagoons, 83.6% for primary treatment systems, 98.0% for secondary treatment systems and 98.2% for advanced treatments (personal communication, from the Emerging Priorities Division, ECCC to the Ecological Evaluation Division, ECCC, dated November 2018; unreferenced).
Sampling was also conducted at some other wastewater treatment systems in Canada that receive industrial wastewater from facilities producing chlorhexidine-based products (personal communication, from the Emerging Priorities Division, ECCC to the Ecological Assessment Division, ECCC, dated November 2018; unreferenced). Either grab samples or 24-h composite samples were collected. These results do not necessarily correspond with production of chlorhexidine-based products at the time of sampling or, where production was confirmed, did not likely represent peak concentrations being released in a pulse (i.e., non-continuously). Measured concentrations of chlorhexidine in influent samples ranged from 0.130 to 0.429 µg/L (n=7). Measured concentrations of chlorhexidine in effluent samples ranged from 0.152 to 0.668 µg/L (n=15). The reporting limit for influent and effluent samples was 0.0115 µg/L.
Other jurisdictions have identified chlorhexidine as a potential concern in the environment due to its widespread use and have noted the need for further information on environmental concentrations (Boxall et al. 2005). Chlorhexidine was selected for inclusion in a national screening program to measure and report on its occurrence in Sweden (SWECO Environment 2011). WWTSs were chosen as sampling locations because of their general potential to release household chemicals into the aquatic environment. Only one WWTS was identified as receiving wastewater from a pharmaceutical company using chlorhexidine. The study did not find chlorhexidine (or its potential degradation product, p-chloroaniline) in any of the samples taken, including those taken from: influent, effluent and sludge at WWTSs; surface waters, sediment and fish in streams receiving effluents from WWTSs; wastewater from hospitals; or agricultural soils that had received sludge amendment. The limits of quantification for chlorhexidine were 0.010 µg/L for influent and effluent at WWTSs, recipient water, and background water, 0.010 mg/kg for sewage sludge (dry weight), sediment, and agricultural soil receiving sludge, and 0.10 mg/kg (wet weight, muscle) for fish. Detailed descriptions of the analytical methods were not provided (including whether total or dissolved fractions were measured).
Chlorhexidine has been measured in wastewaters in Japan. Kodama et al. (1988) reported a range of 1.62 to 10.30 mg/L (originally reported as µg/ml) for chlorhexidine concentrations in wastewater. Matsushima and Sakurai (1984) reported chlorhexidine concentrations in wastewater from a medical wastewater treatment plant ranging from 0.085 to 1.94 mg/L. The same authors cited another study (Yamayoshi et al. 1981) that reported concentrations of chlorhexidine in medical wastewater in the range of hundreds of µg/L. In another study, the authors reported concentrations of chlorhexidine in wastewater samples of approximately 2 to 7 mg/L (originally reported as µg/mL) (Kido et al. 1988).
6.2.2 Releases to the environment
Releases of substances to the environment depend on various losses occurring during the manufacture, industrial use, consumer or commercial use, service life and disposal of a substance. Releases of chlorhexidine and its salts to the Canadian environment may result from the consumer use and formulation of chlorhexidine-based products. Releases are expected to be diffuse (i.e., down the drain from use of products containing chlorhexidine) and from point sources (e.g., from sites formulating products containing chlorhexidine). Releases associated with formulation may also occur in pulses due to batch processes or periodic release of accumulated waste.
Releases of chlorhexidine and its salts are expected to occur primarily to municipal and industrial wastewater. Since treatment technologies may only partially remove chlorhexidine, it may be released to surface water, and also to soil through the application of biosolids (from WWTSs) to agricultural and pasture lands. Chlorhexidine contained in products and manufactured items that are disposed of in landfills may leach out of these materials and end up in landfill leachate. No chlorhexidine landfill leachate data have been reported to date, but such data could help interpret end-of-life releases. Whether released to water or soil, chlorhexidine will eventually partition to negatively charged particulates because of its cationic nature.
6.2.3 Exposure scenarios and predicted environmental concentrations
As no data on measured chlorhexidine concentrations in environmental media in Canada have been identified, environmental concentrations were estimated from available information on quantities of chlorhexidine and its salts imported and used in Canada. Quantitative exposure characterization is typically focused on scenarios representing the greatest and/or most representative exposure situation(s) for the substance being released. In general, the magnitude of release is a direct function of either the quantity of a substance manufactured or used in industrial applications or the quantity used in products for consumer/commercial use (along with product use patterns and its disposal).
The focus of this exposure assessment is on the estimated releases of chlorhexidine and its salts as a result of both the industrial formulation of products containing chlorhexidine and the consumer/commercial use of products containing this substance (i.e., releases down the drain). Releases from veterinary products containing chlorhexidine used on dairy farms across Canada are not considered, as total quantities used during any given period at a farm and resultant exposure concentrations are expected to be lower than those releases evaluated in the key exposure scenarios presented below.
6.2.4 Exposure scenario 1 – Industrial formulation of products containing chlorhexidine
The aquatic exposure of organisms to chlorhexidine is expected from the release of the substance during its industrial use. The formulation of chlorhexidine-based products generates wastewater during the cleaning of mixing and packaging equipment. The chlorhexidine-containing wastewater is discharged to a WWTS, which removes a certain fraction of the chlorhexidine. The chlorhexidine that is not removed is subsequently released to a receiving water body via wastewater effluent. The concentration of the substance in the receiving water body near the discharge point of the WWTS is used as the predicted environmental concentration (PEC). It can be calculated using the following equation:
Cwater-ind = (1000(Q)(L)(1-R)) / (N(F)(D))
Where:
Cwater-ind: aquatic concentration resulting from industrial releases, mg/L
1000: factor combining conversion from kg to mg and m3 to L
Q: total substance quantity used annually at an industrial site, kg/year
L: loss to wastewater, fraction (% shown in table)
R: WWTS removal rate, fraction (% shown in table)
N: number of annual release days, days/year
F: WWTS effluent flow, m3/day
D: receiving water dilution factor, dimensionless
Predicted aquatic environmental concentrations (PECaquatic industrial) were calculated for a number of industrial sites that formulate chlorhexidine-based products and its salts in a quantity above 100 kg per year. These sites were identified following analysis of information submitted from mandatory and voluntary surveys regarding the manufacture, import and use of chlorhexidine and its salts (Environment Canada 2007; Environment Canada 2010; Environment Canada 2015). A summary of input values used in estimating these PECs is provided in Table 6‑4.
| Input description | Value | Justification |
|---|---|---|
| Yearly quantity of chlorhexidine (kg/year) used at each site | Q = <10 000 kg/year | Total quantity used at each formulation site. Data for salts were converted to a chlorhexidine equivalent quantity.a |
| Loss to wastewater (%) | L = 0.426% | Calculation based on results of voluntary sampling of waste storage tanks conducted in 2015 by one of the formulators of chlorhexidine-based products.a Assumption is that this loss to wastewater would be the same for other formulators. |
| WWTS removal rate (efficiency; %) | R = 84% (primary) 98% (secondary) 94% (lagoon) |
Removal rate for primary level treatment, secondary level treatment, and lagoonb is chosen based on the type of treatment used at the WWTS to which the industrial facilities are connected (personal communication, from the Emerging Priorities Division, ECCC to the Ecological Evaluation Division, ECCC, dated November 2018; unreferenced). |
| Number of annual release days (d/year) | N = 1 to 350 | Days per year that the substance is released to wastewater. As reported during a voluntary survey of importers and formulators of chlorhexidine and chlorhexidine-based products for the reporting years of 2011 and 2013. |
| WWTS effluent flow (m3/d) | F = 1750 to 2 240 000 | Site specific data for the WWTS that receive wastewater from industrial facilities. |
| Receiving water dilution factor (unitless) | D = 10 | Assuming an instantaneous dilution of the effluent, the dilution factor of a receiving water course was calculated by dividing the flow of the WWTS effluent (connected to the facility) by the 10th percentile of the annual distribution of the flow of the receiving water course. When this dilution factor was greater than 10, a maximum default value of 10 was used. In all cases the dilution factor was above 10 and capped at 10. This dilution factor represents exposures near the discharge point of the effluent. |
a Based on information received from formulators and their customers as a result of mandatory surveys conducted under CEPA as well as follow-up voluntary surveys (Environment Canada 2015).
b Removal rate for the lagoon is used in the consumer release scenario.
The calculated PECs in water (PECaquatic industrial) for facilities formulating chlorhexidine-based products range from 0.0074 to 0.309 µg/L. Consideration was given to situations where formulators were discharging to the same WWTS, and in these cases the PECs in the receiving water body were summed. These PECs are used in risk quotient analyses for water (see the Characterization of Ecological Risk section). This calculation assumes continuous release averaged over the number of release days per year. Pulse release of larger quantities of chlorhexidine associated with batch processing or release of accumulated waste could result in higher acute exposures.
An equilibrium sediment-water partitioning approach was used to estimate the concentration of chlorhexidine in bottom sediment. This approach is based on a partitioning principle described by the European Chemicals Agency (ECHA 2010) and incorporates two additional calculation methods. The first method is to estimate the substance’s concentration in the aqueous phase (truly dissolved) of the overlying water from its total concentration, as in studies by Gobas (2007 and 2010). The second method is to estimate a substance’s concentration in bottom sediment from its concentration in the aqueous phase of the overlying water using an equilibrium partitioning assumption between bottom sediment and overlying water, as described by the US EPA’s National Center for Environmental Assessment (US EPA 2003). At equilibrium, the PEC in bottom sediment can linearly correlate with the concentration in the aqueous phase of the overlying water. Sediment exposure scenarios were developed as an extension of the industrial aquatic release scenarios described above to determine equilibrium sediment exposure concentrations, standardized to 4% OC (a typical OC content in bottom sediment for rivers and lakes). The resulting concentrations in bottom sediment (PECsediment) were 0.005 to 0.305 mg/kg dw. These PECs are used in risk quotient analyses for sediment (see the Characterization of Ecological Risk section).
Indirect releases to soil may result from the application of biosolids from WWTSs receiving wastewater that contains chlorhexidine. As has been reported in the case of ionic pesticides, the application of large amounts of phosphorus and lime to agricultural fields could decrease sorption and increase concentration in solution, particularly in weathered soils (Kah and Brown 2006). When the dissolved electrolytes of lime added to fields are considered, the bioavailability of organic cations may be considerably decreased (Droge and Goss 2012). To be conservative, it has been assumed that the maximum soil concentration calculated (PEC) is 100% bioavailable, even though this may not be the case if lime has been applied to the fields. The BASL4 model (2011), a fugacity-based model, was used to estimate a PEC in soil. Soil exposure scenarios were developed as an extension of the aquatic release scenarios described above, using a chlorhexidine concentration in biosolids (0.0007 to 0.034 g/kg) and biosolids production rates (400 to 321 000 kg/day) based on information from specific WWTSs. Assumptions included an application rate of 8300 kg/ha, with an application frequency of once per year over a 10-year period, and a half-life in soil of 8640 hours (360 days; EPI Suite c2000-2010). The maximum soil concentration (PECsoil biosolids) was estimated to be 0.46 mg/kg dw (0.37 mg/kg ww), with an organic carbon content of 2.7%. This PEC is used in a risk quotient analysis for soil (see the Characterization of Ecological Risk section).
6.2.5 Exposure scenario 2 – Down-the-drain releases from commercial and consumer uses of chlorhexidine-based products
Chlorhexidine may be released to WWTSs through the commercial and consumer use of chlorhexidine-based products (see the Sources and Uses section for further details). PECs for sediment and soil were not calculated for the down-the-drain scenario because they are expected to be lower than the industrial scenario.
In order to estimate the level of aquatic exposure resulting from these down-the-drain releases, a probabilistic approach based on per capita use and information on Canadian WWTSs was used. Distribution information including dilution factors (derived from the 10th percentile flow of the receiving water body), WWTS treatment levels and per capita water discharge were used. Other parameters, such as per capita consumption of products containing chlorhexidine, are considered deterministically. A summary of input values is presented in Table 6‑5.
| Input description | Value | Justification |
|---|---|---|
| Yearly quantity of chlorhexidine (kg/year) used in products | Q = <10 000 kg/year | Total chlorhexidine in products (excluding products used on farms). Data for salts were converted to a chlorhexidine equivalent quantity.b |
| Loss to wastewater (%) | L = 100% | Conservative assumption is that the total quantity of a substance containing chlorhexidine is sent to a WWTS. |
| Receiving water dilution factor (unitless) | D = 1-10 | Assuming an instantaneous dilution of the effluent, the dilution factor of a receiving water course was calculated by dividing the flow of the WWTS effluent (connected to the facility) by the 10th percentile of the annual distribution of the flow of the receiving water course. When this dilution factor was greater than 10, a maximum default value of 10 was used. This dilution factor represents exposures near the discharge point of the effluent. |
a WWTS removal rates are the same as those used in Exposure scenario 1: Industrial formulation of products containing chlorhexidine (see Table 6‑4).
b Based on information received from formulators and their customers as a result of mandatory surveys conducted under CEPA, as well as follow-up voluntary surveys (Environment Canada 2015).
The total mass of chlorhexidine (including the proportion from its salts) in various products was estimated using data received from surveys and follow up with importers and formulators of chlorhexidine and chlorhexidine-based products for the year 2011 and 2013. It was conservatively assumed that this total mass (excluding 10% of the total mass representing products used on farms) would ultimately be released down the drain. Aquatic PECs were estimated for water bodies receiving effluent from each WWTS. The 95th percentile of this probabilistic distribution of PECs is 0.07 µg/L and was selected as a realistic worst-case scenario of exposure, given the nature of the input parameters and level of confidence associated to them. This scenario can be interpreted as follows: if surface water was sampled close to a random WWTS discharge point in Canada, 95% of the time the concentration of chlorhexidine in this sample is estimated to be lower than or equal to 0.07 µg/L.
The concentration of chlorhexidine in water bodies receiving effluents from 24 Canadian WWTSs was also estimated using measured WWTS effluent concentrations (from 2016-17 and 2017-2018) (personal communication, from the Emerging Priorities Division, ECCC to the Ecological Evaluation Division, ECCC, dated November 2018; unreferenced). Using a dilution factor of 10 and all individual effluent sample results (including the reporting limit for those with no chlorhexidine measured), the PECaquatic: down-the-drain ranged from 0.00115 to 0.0448 µg/L. These PECs are within the range of those estimated for down-the-drain releases.
6.3 Characterization of ecological risk
This ecological screening assessment presents conclusions developed on the basis of a weight-of-evidence approach and using precaution. Various lines of evidence have been considered for chlorhexidine and its salts. The volumes of chlorhexidine and its salts imported into Canada, along with information on its uses, indicate potential for both periodic and continual releases into the Canadian environment. Chlorhexidine is expected to be persistent in environmental media (water, sediment and soil). Half-lives are greater than 182 days for water and soil and greater than 365 days for sediment. Thus, the potential for organisms to be exposed both spatially and temporally to this moiety in the environment is increased. Chlorhexidine salts released to the aquatic environment will dissociate to release chlorhexidine, the moiety of concern. Chlorhexidine will partition to negatively-charged dissolved and suspended solids in the aquatic environment, may settle in bed sediment, or may be transported far from sources of release to the environment. Indirect release of chlorhexidine to soils may occur through the application of biosolids, where biosolids contain chlorhexidine.
Chlorhexidine and its salts are used as broad-spectrum antiseptics and antimicrobial preservatives in a wide range of products. The structure of chlorhexidine contains cationic phospholipid binding sites and a hydrophobic group that contribute to its mode of action as a biocide. It is known to act as a membrane disruptor in bacteria due to the strong association of the biguanide groupings to exposed anionic sites on the cell membrane and cell wall (particularly to acidic phospholipids and proteins). The structural characteristics of chlorhexidine also explain its strong binding to skin and mucosa, which results in its poor absorption through the skin and gastrointestinal tract in mammals. Although the available information indicates that chlorhexidine has a low potential to bioaccumulate in aquatic organisms, the toxicity data demonstrate that chlorhexidine has the potential to cause adverse effects in aquatic organisms (including benthic organisms) at low concentrations. Algae are particularly sensitive to the effects of chlorhexidine, likely due to its mode of action as a membrane disruptor. Alteration in community composition of river biofilms has also been observed, with suppression of grazers and elimination of protozoans and metazoans in the community.
Risk quotient analyses were performed by integrating realistic worst-case estimates of exposure (PECs) with ecological toxicity information (PNECs) to determine whether there is potential for ecological harm in Canada. Risk quotients (RQs) were calculated on the basis of the key ecotoxicity studies presented (see the Ecological Effects Assessment section), by dividing the PEC by the PNEC for the associated environmental compartment. Table 6‑6 shows resulting risk quotients (RQs) for exposure scenarios developed for releases from industrial uses (including aquatic, sediment and soil biosolids) and down-the-drain releases.
| PEC exposure scenariob | PEC range | PNEC | PEC and PNEC units | RQ range |
|---|---|---|---|---|
| PECaquatic: industrial | 0.0074 – 0.309 | 0.21 | µg/L | 0.04–1.5 |
| PECsediment | 0.005 – 0.305 | 0.049 | mg/kg dw | 0.1–6.3 |
| PECsoil biosolids | 0.46 (maximum) | 0.93 | mg/kg dw | 0.6 |
| PECaquatic: down the drain | 0.07 (95th percentile) | 0.21 | µg/L | 0.4 |
a PECs for sediment (PECsediment) have been standardized to 4% OC (a typical OC content in bottom sediment for rivers and lakes) and PECs for soil (PECsoil biosolids) have been modelled at 2.7% OC content. Therefore, PNECs for sediment and soil have been standardized to the corresponding OC contents for comparison with calculated PECs to determine risk.
b PECaquatic industrial, PECsediment, and PECsoil biosolids have been estimated from the scenario for industrial formulation of chlorhexidine-based products.
The results indicate that chlorhexidine and its salts pose a risk to aquatic and benthic organisms from the industrial formulation of chlorhexidine-based products. An analysis of predicted concentrations in soil indicates that the potential for risk to soil-dwelling organisms is low (RQs less than 1). The exposure scenario from down-the-drain releases through commercial and consumer uses of chlorhexidine-based products indicates that chlorhexidine does not pose a risk to the aquatic environment (RQ less than 1) at current levels of use.
In summary, this information indicates that chlorhexidine and its salts have the potential to cause ecological harm in Canada. It has also been determined that the chlorhexidine moiety meets the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA (Canada 2000).
6.3.1 Consideration of the lines of evidence and uncertainties
As the cationic form of chlorhexidine dominates at environmentally relevant pH, its environmental fate and behaviour will be strongly dependent on its sorption to dissolved organic carbon and suspended solids in the water column or to sediment and soil particles. These sorption processes will be dominated by electrostatic interactions with negatively charged sorption sites, with organic carbon playing a role. However, cation-exchange is complex and difficult to predict in the environment given the various factors that can influence sorption. Therefore, there is uncertainty in determining the degree to which chlorhexidine would be sorbed or desorbed, particularly with respect to removal during wastewater treatment. It is expected that chlorhexidine will strongly sorb to sludge during wastewater treatment. The removal rates used in this assessment (to estimate the fraction of chlorhexidine that may be removed from wastewater) are calculated from results of monitoring of paired influents and effluents at 24 Canadian WWTSs.
There is no information on environmental concentrations (e.g., monitoring data) of chlorhexidine in the Canadian environment. Therefore, exposure concentrations in water, sediment, and soil were estimated using models. Quantities of substances in commerce reflect imported quantities reported in response to surveys for certain years/substances and may not reflect actual quantities of the substances in Canada, particularly given the use of chlorhexidine in various imported products. Although there is always some uncertainty with the use of models when there is limited data to use for input parameters, conservative approaches or realistic worst-case scenarios were chosen.
Toxicity studies for chlorhexidine and its salts are limited for sediment and soil species. While the toxic mode of action for chlorhexidine as a cellular membrane disruptor in bacteria has been well studied, there is also the potential for a reactive mode of action, including possible protein binding. Assessment factors have been applied to the sediment and soil critical toxicity values to address these sources of uncertainty. However, there is uncertainty in the degree to which chlorhexidine would be bioaccessible to organisms living in soil or sediment environments, due to its sorption characteristics. Therefore, there is some uncertainty in the risk quotients derived for soil and sediment, as it is assumed that the predicted environmental concentrations of chlorhexidine are 100% bioavailable, thus providing protective estimates of risk for these media.
7. Potential to cause harm to human health
7.1 Exposure assessment
Environmental media and food
Empirical data on concentrations of chlorhexidine and its salts in environmental media or food in Canada were not identified. Chlorhexidine digluconate and chlorhexidine diacetate are potential incidental additives because of their presence in a limited number of cleaning products for use in food preparation facilities. Dietary exposure is not expected since the cleaning products are not in direct contact with food as their use is followed by a potable water rinse.
Chlorhexidine is not expected to be released to air given its very low vapour pressure and high water solubility, and its existence in a protonated form in the environment indicate that volatilization would be negligible from either dry or moist soil surfaces, or surface waters.
While there are no available data on environmental concentrations of chlorhexidine in Canada, chlorhexidine has been measured in wastewaters in Japan (see Ecological exposure assessment section).
Chlorhexidine can be released into water as a result of its use as a disinfectant and/or as an antimicrobial preservative in a number of products, including cosmetics, natural health products and drugs. A down-the-drain scenario was used to derive a concentration in surface water for potential contaminant ingestion through water.
In the Ecological exposure assessment section, an exposure scenario for the commercial and consumer uses of chlorhexidine-based products released down the drain was presented and aquatic PECs for water bodies receiving effluent from the WWTS were estimated. The scenario uses a probabilistic approach based on per capita use and information on Canadian WWTSs. The 95th percentile of this probabilistic distribution of PECs is 0.07 µg/L and was selected as a realistic worst-case scenario of ecological exposure. This PEC value was used to generate intake estimates from drinking water that range from 1.41 x10-6 mg/kg bw per day for teens (aged 12 to 19 years) to 7.47 x10-6 mg/kg bw per day for formula-fed infants (aged 0 to 6 months).
Products available to consumers
Chlorhexidine, as the digluconate, and to a lesser extent, as the dihydrochloride salt, is found in a variety of cosmetics in Canada, including, but not limited to, make-up, hair products (e.g., dyes, conditioners, and hair grooming products), skin care products (e.g., moisturizers, cleansers, and exfoliants) and aftershaves. This information is based on notifications submitted under the Cosmetic Regulations to Health Canada (personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 2016; unreferenced).
Chlorhexidine and its digluconate salt are listed in the LNHPD as being present as a non-medicinal ingredient for the purpose of antimicrobial preservative in currently licensed natural health products (personal communication, emails from the Natural and Non-prescription Health Products Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 2016; unreferenced; LNHPD [modified 2019]).
Cosmetics considered for a per application exposure scenario are listed in Table 7‑1, while products (cosmetics and natural health products) considered to contribute to daily exposures are listed in Table 7‑2. The generic default parameters applied to each of the exposure scenarios are provided in Appendix A. Dermal absorption was not factored into the exposure estimates because these estimates will be compared to a dermal toxicity study (see the Characterization of Risk to Human Health section).
| Exposure scenario from a per application use of cosmetics | Concentrationa (%) | Per application exposureb,c (mg/kg bw) |
|---|---|---|
| Hair dye (permanent) | 0.1 | 0.122 |
| Genitalia lubricant | 0.2 | 0.158 |
a Concentrations are based on notifications submitted under the Cosmetic Regulations to Health Canada (personal communication, emails from the Consumer Product Safety Directorate, Health Canada to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 2016; unreferenced) and the maximum permitted concentrations of chlorhexidine and its salts on the List of Prohibited and Restricted Cosmetic Ingredients (Health Canada [modified 2015]).
b Dermal deposition.
c Estimated per application exposure expressed as chlorhexidine equivalent.
| Exposure scenario for daily use | Concentration (%) | Source | Route | Daily exposurea (mg/kg bw per day) |
|---|---|---|---|---|
| Body moisturizer (adults) | 0.2b | NCR | Dermal | 0.0788 |
| Body moisturizer (infants) | 0.2b | NCR | Dermal | 0.355 |
| Leave-in hair conditioner (adults) | 0.1–0.2b | NCR | Dermal | 0.0113–0.023 |
| Lipstick (adults) | 0.1b | NCR | Oral | 0.000191 |
| Lip balm (toddlers) | 0.1b | NCR | Oral | 0.000214 |
| Mouthwash (adults) | 0.2c | NNHPD | Oral | 0.0270 |
| Mouthwash (children) | 0.2c | NNHPD | Oral | 0.0310 |
| Sunscreen (adults) | 0.05 | NNHPD | Dermal | 0.048 |
| Sunscreen (toddlers) | 0.05 | NNHPD | Dermal | 0.054 |
Abbreviations: NCR, Notifications submitted under the Cosmetic Regulations (personal communication, emails from the Consumer Product Safety Directorate, Health Canada to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 2016; unreferenced); NNHPD, Natural and Non-prescription Health Products Directorate (personal communication, emails from the Natural and Non-prescription Health Products Directorate, Health Canada to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 2016; unreferenced).
a Estimated daily exposure expressed as chlorhexidine equivalent.
b Cosmetic concentrations are based on notifications submitted under the Cosmetic Regulations to Health Canada (personal communication, emails from the Consumer Product Safety Directorate, Health Canada to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 2016; unreferenced) and the maximum permitted concentrations of chlorhexidine and its salts on the List of Prohibited and Restricted Cosmetic Ingredients (Health Canada [modified 2015]).
c Concentration based on products with a Natural Product Number in Oraldent 2015.
7.2 Health effects assessment
Health effects information on chlorhexidine and its salts, including chlorhexidine diacetate, chlorhexidine digluconate and chlorhexidine dihydrochloride, were taken into consideration in the assessment of the health effects of the chlorhexidine moiety. The speciation of the chlorhexidine moiety in biological fluids is dependent on pH, but independent of the original form. The anion component of the chlorhexidine salts, i.e., diacetate, dihydrochloride and digluconate, are considered to be of low concern and pose no unreasonable risk to human health (NICNAS 2014). Therefore, the chlorhexidine moiety is expected to be responsible for the health effects of these substances.
Multiple toxicology studies on chlorhexidine as the digluconate salt not otherwise identified in the public domain were cited from the Registration, Evaluation, Authorisation and Restriction of Chemicals’ dossier on this substance (ECHA c2007-2015b).
Long-term studies in rats and mice did not identify any treatment-related increases in neoplasms when animals were exposed to chlorhexidine as the digluconate salt up to the maximum tolerated dose in either their feed or drinking water (Case 1977; ICI 1992; ECHA c2007-2015b).
Overall, in vitro and in vivo genotoxicity data indicate that chlorhexidine and its salts are not genotoxic (Suessmuth et al. 1979; Farrow 1983; Myhr 1983; Cifone 1984; COLIPA 1984; Sakagami et al. 1986; Ribeiro et al. 2004; Hikiba et al. 2005; Miyachi and Tsutsui 2005; Grassi et al. 2007; McEvoy 2010; Li et al. 2012; ECHA c2007-2015b).
The acute toxicity of chlorhexidine is considered to be low by the oral and dermal routes of exposure given the high lethal dose (LD50) of different formulations of chlorhexidine in various species (Miller 1993a,b; Shapiro 1993; ECHA c2007-2015b; NICNAS 2014).
Chlorhexidine was not irritating to the skin of rabbits in acute skin irritation studies (Greener et al. 1985; ECHA c2007-2015b).
Several cases of severe irritant contact dermatitis in human preterm newborn infants have been reported with the use of chlorhexidine digluconate solutions for skin antisepsis prior to invasive procedures such as insertion of central vascular catheters. The most severe and life-threatening of these chemical skin injuries (including ulcerations and burns requiring skin grafts), were observed in very premature neonates (less than 34 weeks gestation) who were less than 14 days old and likely to have functionally and morphologically immature skin (Health Canada 2015). As chlorhexidine digluconate is present as a medicinal ingredient in such solutions, exposures from this source were considered to be outside the scope of the assessment.
The toxicokinetics of chlorhexidine and its salts have been investigated in humans and in a number of laboratory animals (Magnusson and Heyden 1973; Winrow 1973; Case 1977; Willis 1993; EMEA 1996; Block 2001; Xue et al. 2009, 2012; US FDA 2013). Owing to their cationic nature, the chemicals bind strongly to skin and mucosa; thus, they are poorly absorbed through the skin and the gastrointestinal tract (EMEA 1996; US FDA 2013). The oral bioavailability was reported to be approximately 1% in a recent human health tier II assessment of chlorhexidine by NICNAS (2014). Similarly, dermal absorption was reported to be less than 1% to 4% in in vitro, animal and human studies for chlorhexidine and its salts (Chow et al. 1978; Cowen et al. 1979; Case 1980; O’Neill et al. 1982; Gongwer et al. 1980; Willis 1993; EMEA 1996; Lafforgue et al. 1997; Karpanen et al. 2008).
Appearance of giant cells in the cortical and paracortical areas of the mesenteric lymph nodes were observed in Wistar rats exposed to 5, 25 or 40 mg/kg bw per day of chlorhexidine as the digluconate salt in drinking water for 2 years. They were also observed in an earlier study of the same duration where rats were exposed to 125 or 158 mg/kg bw per day of chlorhexidine as the digluconate salt in drinking water and in a 90-day study where rats received 50, 100 or 200 mg/kg bw per day of chlorhexidine in their drinking water (Case 1977, Block 2001). These changes were described as reactive, non-progressive and reversible and were attributed to local effects in the intestine from the uptake of the substance (Case 1977; ECHA c2007-2015b). Pigment-laden macrophages (grade II severity) were also observed in the mesenteric lymph nodes of Wistar rats exposed to 5, 25 and 50 mg/kg bw per day of chlorhexidine as the digluconate salt in their feed for 2 years (ECHA c2007-2015b). In a rat developmental study whose main focus was to detect effects in the developing lymph nodes, mild to moderate histocytosis in the mesenteric lymph nodes of dams and offspring were observed at 0.5 mg/kg bw per day when dams were exposed to chlorhexidine as the digluconate salt for 50 days from gestational day 15 and the pups from post-natal day 21 to 14 weeks or 6 months of age by gavage. The severity increased with exposure duration, with pups appearing to be less susceptible than the dams (ECHA c2007-2015b). These effects were not observed in the long-term oral studies in mice and dogs (ECHA c2007-2015b).
Hepatic damage following oral exposure to chlorhexidine was observed in beagle dogs administered chlorhexidine as the digluconate salt in capsules at doses of 0.5, 5 or 25 mg/kg bw per day for up to 1 year (ECHA c2007-2015b). A no-observed adverse effect level (NOAEL) of 0.5 mg/kg bw per day and a lowest observed adverse effect level (LOAEL) of 5 mg/kg bw per day were derived based on hepatic centrilobular fibrosis. Focal degeneration, irregular areas of liver necrosis, loss of hepatocytes and increased serum levels of liver enzymes were observed at the high dose. There was, however, no histological evidence of liver toxicity in the long-term studies in rats and mice (NICNAS 2014; ECHA c2007-2015b).
In a subchronic dermal study, a systemic NOAEL of 250 mg/kg bw per day was derived based on increased liver enzyme activity and degenerative changes in the liver that were observed at the next dose when rabbits were topically exposed to 250, 500 or 1000 mg/kg bw per day, under occlusive dressing, for 13 weeks. Liver necrosis, however, was graded as minimal in all treated animals. This dose is also considered a dermal lowest observed effect level (LOEL) based on skin irritation (Henwood 1988). There was no treatment-related effect in newborn rhesus monkeys exposed daily to a skin cleanser containing 8% chlorhexidine digluconate for 5 minutes over a 3-month period (Gongwer et al. 1980).
No significant treatment-related effects were reported in inhalation repeated dose studies with the digluconate salt or diacetate salt of chlorhexidine (Andrews and Paul 1977; Willis 1993).
In a reproductive study reported by EMEA (1996), an oral NOAEL of 4.9 mg/kg bw per day and a LOAEL of 44.4 mg/kg bw per day were identified based on decreased pup weight at postpartum day 4, decreased maternal body weight gain and decreased number of viable foetus when female rats were gradually exposed to 0, 4.9 and 44.4 mg/kg bw per day of chlorhexidine for 2 weeks and then treated with the intended doses for 14 days prior to mating with untreated males. The intended doses of 0, 5 and 50 mg/kg bw per day were not reached due to a dose-related decrease in water consumption caused by the substance. Another reproductive study that tested a number of related compounds reported that chlorhexidine reduced the number of litters by half in mice that were exposed to the test substances at 400 mg/kg bw per day for a week (information on maternal toxicity of chlorhexidine was not provided) (Cutting et al. 1964).
In an oral developmental toxicity study, a developmental NOAEL of 30 mg/kg bw per day and a developmental LOAEL of 100 mg/kg bw per day were identified based on a significant increase in the incidence of delayed skeletal development at the highest dose when pregnant rats were exposed by gavage to 0, 10, 30 or 100 mg/kg bw per day of chlorhexidine as the digluconate salt from gestational days 6 to 19. Other effects at this dose included increases in early, late and total resorptions and decreased number of fetuses. The NOAEL for maternal toxicity in this study is 10 mg/kg bw per day (ECHA c2007-2015b). No treatment-related embryotoxic effects were observed when pregnant Wistar rats were exposed to 0, 0.1, 0.5 and 5 mg/kg bw per day of chlorhexidine as the digluconate salt from gestational day 15 through lactation and to their offspring (0–2.5 mg/kg bw per day) from weaning for periods up to 6 months. There were also no treatment-related effects on the number, litter size, sex ratios or growth rate of the F1 pups (ECHA c2007-2015b). Similarly, no adverse effects were reported in the fetuses of pregnant rats that were exposed to a dose of 68.5 mg/kg bw per day by gavage on gestation days 6-15 or to those orally exposed to 10, 25 or 50 mg/kg bw per day of chlorhexidine as the digluconate salt (Case 1977; Gilman and De Salva 1979). A developmental study on chlorhexidine as the diacetate salt did not identify adverse developmental effects in rats that were exposed orally to 0, 15.63, 31.26 or 62.5 mg/kg bw per day on gestation day 6 through 15 (Lamb 1991). Maternal toxicity including dose-related reduced body weight gain, rales and increased salivation were observed at 31.25 mg/kg bw per day (Lamb 1991).
A number of human studies on mouthwashes containing chlorhexidine digluconate were described in the Cosmetic Ingredient Review, including a 6 month study in school children (10 to 12 year olds) exposed to up to 6 times per week up to a 1% solution, a six month study in adults exposed to 0.12% solution, a 9-month study in adults exposed to 0.12% solution and long-term studies (1 to 2 years) in adults exposed to 0.12% and 0.2% solution (Willis 1993). Only reversible effects, such as change in taste perception, minor irritation, superficial desquamation of the epithelium of the oral mucosa and teeth staining were observed. No allergic reactions were reported. Following long-term exposure, no significant treatment-related effects were reported in either blood parameters or oral mucosa.
Several cases of sensitization were reported in the literature in humans in patch or prick tests with chlorhexidine (Broeckx et al. 1987; Nagendran et al. 2009; ECHA c2007-2015a), chlorhexidine diacetate (Andersen and Brandrup 1985, Reynolds and Harman 1990; Evans 1992; Wong et al. 1990; Leow and Goh 1999) and chlorhexidine digluconate (Roberts et al. 1981; Bechgaard et al. 1985; Bergovist-Karlsson 1988; Okano et al. 1989; Osmundsen 1982; Liippo et al. 2011). However, these sensitization cases were mostly observed in individuals with pre-existing skin disorders or when applied to mucous membranes. Similarly, in a summary safety review conducted on topical antiseptic non-prescription chlorhexidine products, a potential for serious allergic reactions including anaphylaxis was identified under certain conditions when used in the mouth, on open wounds, or immediately before or during surgery. Of the 53 reports of serious allergic reactions Health Canada had received from the use of such products, 3 were anaphylactic reactions. Health Canada’s Antiseptic Skin Cleansers monograph does require labelling for such products to include a warning statement to minimize the risk of allergic reactions (Health Canada 2016).
In a study investigating cosmetic intolerance, only 15 out of 5202 patients (0.3%) tested for contact dermatitis using computer analysis of medical histories and epicutaneous patch tests showed an allergic contact dermatitis caused by chlorhexidine (Broeckx et al. 1987). Garvey et al. (2003) investigated the prevalence of sensitization and allergy to chlorhexidine in health care workers. None of the 104 doctors, nurses and auxiliary staff had any reactions to skin patches containing chlorhexidine diacetate (1%) and chlorhexidine digluconate (1%) in water (Garvey et al. 2003). In another two studies involving health care workers; however, 3% to 4% were diagnosed with IgE-mediated chlorhexidine allergy following serological or skin prick tests or showed positive reactions to patch test with 0.5% chlorhexidine diacetate or 0.5% chlorhexidine digluconate (Nagendran et al. 2009; Toholka and Nixon 2013). Similarly, as part of a retrospective study, 82 out of 8497 patients (1%) patch tested with chlorhexidine during 2003-2013 at the Department of Dermato-Allergology at the Copenhagen University Hospital were positive. Of these 82 patients, 43 (0.5%) had a positive test reaction to chlorhexidine diacetate, 11 (0.1%) had a positive test reaction to chlorhexidine digluconate, and 28 (0.3%) had positive test reactions to both chlorhexidine salts. Known causes of the allergy were reported by 19 patients (40%) and were mainly attributed to products used in the healthcare setting (Opstrup et al. 2016). In a multicenter, cluster-randomized study evaluating daily bathing with chlorhexidine impregnated washcloths on the acquisition of multidrug-resistant organisms and the incidence of hospital-acquired bloodstream infections, the overall incidence of skin reactions among patients assigned to chlorhexidine bathing was 2.0% (78 of 3970 patients), as compared with 3.4% (130 of 3842) among those assigned to bathing with the control product (Climo et al. 2013).
7.3 Characterization of risk to human health
No evidence for carcinogenicity or genotoxicity was observed in the available empirical data for chlorhexidine and its salts. Therefore, characterization of risk in this screening assessment is based on non-cancer effects.
There is no significant absorption when chlorhexidine is applied to intact skin and, similarly, the oral bioavailability was reported to be approximately 1% in a recent human health tier II assessment of chlorhexidine by NICNAS (2014). These properties have led to the development of chlorhexidine principally as a topical antiseptic.
Chlorhexidine can be released into water as a result of its use as disinfectant and/or as an antimicrobial preservative in a number of products, including cosmetics, natural health products and drugs. A down-the-drain scenario was used to derive a concentration in surface water for potential contaminant ingestion through water and resulted in intake estimates from drinking water that range from 1.41 x10-6 mg/kg bw per day for teens (aged 12 to 19 years) to 7.47 x10-6 mg/ kg bw per day for formula-fed infants (aged 0 to 6 months).
A LOEL of 5 mg/kg bw per day based of the appearance of giant cells in the mesenteric lymph nodes were derived from two oral chronic studies when rats were exposed to chlorhexidine as the digluconate salt through drinking water and diet. These effects were described as reactive, non-progressive and reversible and were attributed to localized effects in the intestine from the uptake of the substance (Case 1977; Block 2001; ECHA c2007-2015b).
Comparison of intake estimates from drinking water (7.47 x10-6 mg/ kg bw per day for formula-fed infants aged 0 to 6 months) and the chronic oral critical effect level (LOEL of 5 mg/kg bw per day based on localized effects in the intestine in rats exposed to chlorhexidine for 2 years) results in margin of exposure (MOE) of 670 000. This margin of exposure is considered adequate to address uncertainties in the health effects and exposure databases.
Exposures of the general population in Canada to chlorhexidine and its salts occur predominantly through products by the dermal route. No chronic dermal toxicity study was identified for chlorhexidine and its salts. A systemic NOAEL of 250 mg/kg bw per day was derived based on liver effects when rabbits were topically exposed, under occlusive dressing, to chlorhexidine as the diacetate salt for 13 weeks (Henwood 1988). This dose is also considered a dermal LOEL based on minimal skin irritation. No treatment-related effects were observed in another subchronic study where newborn rhesus monkeys were exposed daily to a skin cleanser containing 8% chlorhexidine as the digluconate salt (Gongwer et al. 1980).
Estimates of risk associated with daily use of cosmetics and natural health products that resulted in the greatest exposures to chlorhexidine are presented in Table 7‑3.
Comparison of the estimates of dermal exposure to chlorhexidine and its salts from the daily use of cosmetics and natural health products that resulted in the greatest exposures to chlorhexidine with the critical effect level (subchronic systemic NOAEL of 250 mg/kg bw per day) results in MOEs of 704 to 22 100, which are considered adequate to address uncertainties in the health effects and exposure databases. Although a study of shorter duration is used and a MOE of less than 1 000 was obtained for the infant body moisturizer scenario, the MOE is still considered protective. This is based on the use of conservative default values and algorithms in estimating exposures and the use of a systemic NOAEL as the point of departure for a dermal exposure. In addition, given that minimal skin irritation was also observed in some animals at that dose, exposure is expected to be self-limiting.
| Exposure scenario | Source | Daily exposure (mg/kg bw per day) | Critical effect level (mg/kg bw per day) | MOE |
|---|---|---|---|---|
| Body moisturizer (adults) | NCR | 0.0788 | Systemic NOAEL = 250 | 3 170 |
| Body moisturizer (infants) | NCR | 0.355 | Systemic NOAEL = 250 | 704 |
| Leave-in hair conditioner (adults) | NCR | 0.0113–0.023 | Systemic NOAEL = 250 | 22 100–10 900 |
| Sunscreen lotion (adults) | NNHPD | 0.048 | Systemic NOAEL = 250 | 5 210 |
| Sunscreen lotion (toddlers) | NNHPD | 0.054 | Systemic NOAEL = 250 | 4 630 |
Abbreviations: MOE, margin of exposure; NOAEL, no-observed-adverse-effect level; NCR, Notifications submitted under the Cosmetic Regulations; NNHPD, Natural and Non-prescription Health Products Directorate.
Although concurrent or sequential use of products containing chlorhexidine and its salts may occur, simultaneous exposures from a number of products containing these substances would not be of concern based on the conservatism nature of the exposure scenario and the very low dermal absorption (less than 1% to 4%) consistently observed for these substances (Chow et al. 1978; Case 1980; EMEA 1996; Lafforgue et al. 1997; Karpanen et al. 2008; NICNAS 2014).
Exposure of the general population in Canada to chlorhexidine and its salts also occurs through a per application use of cosmetics by the dermal route. Exposure scenarios for the use of permanent hair dyes and genitalia lubricants resulted in the greatest exposure to chlorhexidine. Estimates of risk associated with a per application use of permanent hair dye and genitalia cream are presented in Table 7‑4.
Comparison of estimates of dermal exposure to chlorhexidine and its salts from the per application use of permanent hair dyes and genitalia lubricants with the critical effect level (subchronic systemic NOAEL of 250 mg/kg bw per day) results in MOEs of greater than or equal to 2 050 and of greater than or equal to 1 600, respectively, which are considered adequate to address uncertainties in the health effects and exposure databases.
| Exposure scenario | Per application exposure (mg/kg bw) | Critical effect level (mg/kg bw per day) | MOE |
|---|---|---|---|
| Hair dye (permanent) (adults) | 0.122 | Systemic NOAEL = 250 | 2 050 |
| Genitalia lubricant (adults) | 0.158 | Systemic NOAEL = 250 | 1 600 |
Abbreviations: MOE, margin of exposure; NOAEL, no-observed-adverse-effect level
Exposure to chlorhexidine and its salts can also occur orally through use of a limited number of cosmetics (i.e., lipsticks and lip balms), as well as of natural health products (i.e., mouthwashes). These product types only encompass very few products each (LNHPD [modified 2019]; personal communication, emails from the Natural and Non-prescription Health Products Directorate, Health Canada to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 2016, unreferenced; personal communication, emails from the Consumer Product Safety Directorate, Health Canada to the Existing Substances Risk Assessment Bureau, Health Canada, dated June 2016, unreferenced). Given the limited number of products available to the general population and the small market share of these products, exposure of the general population of Canada to chlorhexidine and its salts through the oral route is therefore limited.
Estimates for oral exposure are 1.91 x 10-4 mg/kg bw per day for lipstick (adults) and 2.14 x 10-4 mg/kg bw per day for lip balm (toddlers), respectively. Exposure to mouthwashes in adults and children resulted in the highest exposure by the oral route; estimates are 0.0270 mg/kg bw per day and 0.0310 mg/kg bw per day for adults and children, respectively.
A number of human studies on mouthwashes containing chlorhexidine digluconate were described in the Cosmetic Ingredient Review, including a 6-month study in school children (aged 10 to 12 years) and long-term studies (1 to 2 years) in adults exposed to up to a 1% solution (Willis 1993). The authors noted only reversible effects, such as change in taste perception, minor irritation, superficial desquamation of the epithelium of the oral mucosa, and teeth staining were observed. The authors also noted no allergic reactions were reported and following long-term exposure, no significant treatment-related effects were reported in either blood parameters or oral mucosa.
Given that no critical effect level was identified from the mouthwash studies carried out in humans, risk to human health of the general population from oral exposures to lipsticks, lip balms, and mouthwashes is expected to be low at current levels of exposure.
7.4 Uncertainties in evaluation of risk to human health
There is uncertainty regarding the estimation of exposure due to the lack of representative measured concentrations of the chlorhexidine moiety in Canadian surface water or drinking water and the use of a model for estimating risk to human health. However, confidence is high that actual exposures to chlorhexidine in Canadian drinking water would be lower than the exposures estimated using the model. The uncertainty in the human risk estimates could be reduced significantly by the use of measured concentration data in environmental media.
The confidence in the health effects assessment for chlorhexidine and its salts is considered moderate to high. The modes of action of chlorhexidine and its salts for the induction of the health effects observed in animals have not been fully elucidated. Empirical data has been identified for the relevant toxicological endpoints. Many of the toxicity studies identified were based on unpublished study reports described through secondary sources, including ECHA (c2007-2015b), Willis (1993) and EMEA (1996). According to ECHA (c2007-2015b), however, many of those studies, including those on chronic toxicity, were conducted according to guidelines that are similar or equivalent to OECD guidelines and were based on good laboratory practices. Furthermore, given the clinical use of chlorhexidine as an antiseptic and disinfectant for over 50 years, human empirical data is available for adults, children and infants (EMEA 1996; Willis 1993).
There is uncertainty associated with the hazard characterization regarding the duration of the study selected to characterize the risks following daily use of cosmetics and natural health products. As no chronic dermal toxicity study was identified, a subchronic dermal toxicity study was used to derive MOEs for chronic dermal exposures.
It is recognized that chlorhexidine and its salts may result in sensitization in some individuals and they have in rare cases caused serious allergic reactions as was reported following patch and prick tests with the substances and documented in a Summary Safety Review by Health Canada (see the Health effects assessment section).
8. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is risk of harm to the environment from chlorhexidine and its salts. It is concluded that chlorhexidine and its salts meet the criteria under paragraph 64(a) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity. However, it is concluded that chlorhexidine and its salts do not meet the criteria under paragraph 64(b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information currently available on its potential to cause harm to human health, it is concluded that chlorhexidine and its salts do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that chlorhexidine and its salts meet one or more of the criteria set out in section 64 of CEPA. It has also been determined that the chlorhexidine moiety meets the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
References
ACD/Percepta [prediction module]. c1997-2012. Toronto (ON): Advanced Chemistry Development, Inc.
Andersen BL, Brandrup F. 1985. Contact dermatitis from chlorhexidine. Contact Dermatitis. 13:307-309.
Andrews JJ, Paul JW. 1977. Chlorhexidine fogging: a safety study in dogs. Vet Med Small Anim Clin. 72(8):1330-1334.
Anusavice KJ, Zhang NZ, Shen C. 2006. Controlled release of chlorhexidine from UDMW-TEGDMA resin. J Dental Res. 85(10):950-954.
[AOPWIN] Atmospheric Oxidation Program for Microsoft Windows [estimation model]. 2010. Ver. 1.92a. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
ASTreat Model [sewage treatment plant removal model]. c1997. Ver. 1.0. Cincinnati (OH): Procter & Gamble Company.
Attwood D, Natarajan R. 1979. Micellar properties and surface activity of some bolaform drugs in aqueous solution. J Pharm Pharmacol. 32:460-462.
[BASL4] Biosolid-Amended Soil: Level IV Model. 2011. Ver. 2. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry (CCEMC). Prepared for Environment Canada by CCEMC (Contract No.: K8A43-10-0015).
Bechgaard E, Ploug E, Hjorth N. 1985. Contact sensitivity to chlorhexidine? Contact Dermatitis. 13:53-55.
Bergovist-Karlsson A. 1988. Delayed and immediate-type hypersensitivity to chlorhexidine. Contact Dermatitis. 18:84-88.
[BIOWIN] Biodegradation Probability Program for Microsoft Windows [estimation model]. 2008. Ver. 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Block SS. 2001. Disinfection, Sterilization, and Preservation, 5th ed. Philadelphia (PA): Lippincott Williams & Wilkins. p. 1481.
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere. 30(4):741-752.
Boxall ABA, Fogg LA, Baird DJ, Lewis C, Telfer TC, Kolpin D, Gravell A, Pemberton E, Boucard T. 2005. Targeted monitoring study for veterinary medicines in the environment [PDF]. Environment Agency Science Report SC030183/SR. Bristol (UK): Environment Agency.
Broeckx W, Blondeel A, Dooms-Goossens A, Achten G. 1987. Cosmetic intolerance. Contact Dermatitis. 16:189-194. [as cited in ECHA c2007-2015a]
Broxton P, Woodcock PM, Heatley F, Gilbert P. 1984. Interaction of some polyhexamethylene biguanides and membrane phospholipids in Escherichia coli. J Appl Bacteriol. 57:115-124.
Calabrese EJ, Kenyon EM. 1991. Air toxics and risk assessment. Chelsea (MI): Lewis Publishers, Inc.
Campbell S, Grimes J, Smith G. 1991. Chlorhexidine diacetate: an acute oral toxicity study with the Northern Bobwhite: lab project number: 277-103. Unpublished study prepared by Wildlife International, Ltd. [cited in US EPA 1996].
Canada. [1978]. Food and Drug Regulations, C.R.C., c. 870.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33. Canada Gazette. Part III, vol. 22, no. 3.
Canada. 2000. Canadian Environmental Protection Act, 1999: Persistence and Bioaccumulation Regulations. P.C. 2000-348, 29 March, 2000, SOR/2000-107.
Canada, Dept. of the Environment. 2006. Canadian Environmental Protection Act, 1999: Notice with respect to selected substances identified as priority for action [PDF]. Canada Gazette, Part I, vol. 140, no. 9, p. 435-459.
Canada, Dept. of the Environment. 2009. Canadian Environmental Protection Act, 1999: Notice with respect to Batch 12 Challenge substances [PDF]. Canada Gazette, Part I, vol. 143, no. 52, p. 3813-3836.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement, p. 3-94.
Canada, Dept. of the Environment. 2017. Canadian Environmental Protection Act, 1999: Notice with respect to substances included as part of the 2017 Inventory Update [PDF]. Canada Gazette, Part I, vol. 151, no. 2, p. 89-161.
Case DE. 1977. Safety of hibitane I. Laboratory experiments. J Clin Periodontol. 4(5):66-72.
Case DE. 1980. Chlorhexidine: attempts to detect percutaneous absorption in man. In: Newsom SWB, Caldwell ADS, editors. Problems in the control of hospital infection. (London: Royal Society of Medicine International Congress and Symposium Series, No. 23.) London (UK): RSM/Academic Press, 1980. p. 39‑43 [cited in Block 2001].
[Chemicalland21] Worldwide chemical information [database]. 2010. [accessed 2010 Aug].
Cheminfo Services Inc. 2014. Chemical Management Plan 2 (CMP2) scoping project for substance information on chlorhexidine and chlorhexidine digluconate, Final report. November 28, 2014. Unpublished report prepared for Environment Canada. Markham (ON).
ChemSpider [database]. 2011. London (UK): Royal Society of Chemistry. [cited in ECHA c2007-2015a].
Chow CP, Buttar HS, Downie RH. (1978). Percutaneous absorption of chlorhexidine in rats. Toxicol Lett.1(4): 213-216.
Cifone M. 1984. Mouse lymphoma forward mutation assay: chlorhexidine hydrochloride: LBI Project No. 20989.
Climo MW, Yokoe DS, Warren DK, Perl TM, Bolon M, Herwaldt LA, Weinstein RA, Sepkowitz KA, Jernigan JA, Sanogo K, Wong ES. 2013. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 368:533-542.
[COLIPA] The European Cosmetics Association. 1984. Submission I to the EEC. Chlorhexidine and its digluconate, diacetate and dihydrochloride salts. COLIPA, p. 35. EEC: Annex VI, part 2. n. 31: 2 [cited in Willis 1993].
[ConsExpo] Consumer Exposure Model. 2006. Version 4.1. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
Cowen J, Ellis SH, McAinsh. 1979. Absorption of chlorhexidine from the intact skin of newborn infants. Arch Dis Child. 54:379-383.
Cutting WC, Cutting JW, Tabar P. 1964. Studies on the chloroguanide antifertility effect. Med Exp 10:361-8 [cited in Willis 1993].
De Waart J, Van der Most MM. 1986. Biodegradation test for microbicides. Int Biodeterior. 22:113-120 [cited in HSDB 1983- ].
Dimitrov SD, Dimitrova NC, Walker JD, Veith GD, Mekenyan OG. 2002. Predicting bioconcentration factors of highly hydrophobic chemicals. Effects of molecular size. Pure Appl Chem. 74(10):1823-1830.
Dimitrov SD, Dimitrova NC, Walker JD, Veith GD, Mekenyan OG. 2003. Bioconcentration potential predictions based on molecular attributes – an early warning approach for chemicals found in humans, birds, fish and wildlife. QSAR Comb Sci. 22:58-68.
[DPD] Drug Product Database [database]. [modified 2015 Jul 17]. Ottawa (ON): Health Canada. [accessed 2016 Jun].
Droge S, Goss K. 2012. Effect of sodium and calcium cations on the ion-exchange affinity of organic cations for soil organic matter. Environ Sci Technol. 46:5894-5901.
Droge ST, Goss K. 2013. Development and evaluation of a new sorption model for organic cations in soil: contributions from organic matter and clay minerals. Environ Sci Technol. 47:14233-14241.
Dynes JJ, Lawrence JR, Korber DR, Swerhone GDW, Leppard GG, Hitchcock AP. 2006. Quantitative mapping of chlorhexidine in natural river biofilms. Sci Total Environ. 369(1-3):369-363.
[ECCC] Environment and Climate Change Canada. 2018. Supporting documentation: Chlorhexidine and its salts. Information in support of the screening assessment for chlorhexidine and its salts. Gatineau (QC): Environment and Climate Change Canada, Ecological Assessment Division. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2017 Dec 19].
[ECHA] European Chemicals Agency. c2007-2015a. Registered substances database. Search results for CAS RN [55-56-1]. Helsinki (FI): ECHA. [updated 2013 Jul 10; accessed 2016 Jan 14].
[ECHA] European Chemicals Agency. c2007-2015b. Registered substances database. Search results for CAS RN [18472-51-0]. Helsinki (FI): ECHA. [updated 2013 Jul 10; accessed 2016 Jan 14].
[ECHA] European Chemicals Agency. 2010. Guidance on information requirements and chemical safety assessment [PDF]. Chapter R.16: Environmental exposure estimation, Ver. 2. Helsinki (FI): European Chemicals Agency.
[EMEA] European Agency for the Evaluation of Medicinal Products. 1996. Committee for Veterinary Medicinal Products Chlorhexidine Summary Report [PDF].
Environment Canada. 2007. Data for selected substances collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to selected substances identified as priority for action. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada. 2010. Data for the Batch 12 substance, CAS RN 56-95-1, collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to Batch 12 Challenge substances. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada. 2015. DSL Inventory Update data collected for CAS RN 55-56-1 and CAS RN 18472-51-0 under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada. 2018. DSL Inventory Update data collected for CAS RN 3697-42-5 under the Canadian Environmental Protection Act, 1999, section 71 notice: Notice with respect to substances included as part of the 2017 Inventory Update. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada, Health Canada. 2013. Draft screening assessment for the Challenge: Chlorhexidine acetate: Chemical Abstracts Service Registry Number 56-95-1. Ottawa (ON): Environment Canada, Health Canada. [accessed 2015 Jul].
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2010. Ver. 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[ESRAB] Existing Substances Risk Assessment Bureau. 2017. DRAFT Recommended Default Values for Personal Care Product Exposure Scenarios: Module V, Sunscreens. Ottawa (ON): Health Canada, Existing Substances Risk Assessment Bureau. Internal reference.
European Union. 1998–2016. Comission directive 92/69/EEC of 31 July 1992 adapting to technical progress for the seventeenth time Council Directive 67/548/EEC on the approximation of laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances. A.5. Surface Tension. Official Journal L 383 A, 29/12/1992. p. 0113-0115. Luxembourg: Publications Office of the European Union. Document 31992L0069. [accessed 2016 Jun].
Evans RJ. 1992. Acute anaphylaxis due to topical chlorhexidine acetate. Brit Med J. 304(6828):686.
Farrow M. 1983. In-vitro chromosome aberrations in Chinese hamster ovary cells assay: chlorhexidine hydrochloride: Hazleton Project No. 2191-101. Unpublished study prepared by Hazleton Laboratories America [cited in US EPA 1996].
Ficheux AS, Wesolek N, Chevillotte G, Roudot AC. 2015. Consumption of cosmetic products by the French population. First part: Frequency data. Food Chem Toxicol. 78:159-169.
Ficheux AS, Chevillotte G, Wesolek N, Morisset T, Dornic N, Bernard A, Bertho A, Romanet A, Leroy L, Mercat AC, Creusot T, Simon E, Roudot AC. 2016. Consumption of cosmetic products by the French population second part: Amount data. Food Chem Toxicol. 90:130-141.
Fraud S, Hann AC, Maillard JY, Russell AD. 2003. Effects of ortho-phthalaldehyde, glutaraldehyde and chlorhexidine diacetate on Mycobacterium chelonae and Mycobacterium abscessus strains with modified permeability. J Antimicrob Chemoth. 51:575-584.
Freitag D, Geyer H, Kraus A, Viswanathan R, Kotzias D, Attar A, Klein W, Korte F. 1982. Ecotoxicological profile analysis: VII. Screening chemicals for their environmental behavior by comparative evaluation. Ecotoxicol Environ Saf. 6(1):60-81 [cited in HSDB 1983- ].
Freitag D, Ballhorn L, Geyer H, Korte F. 1985. Environmental hazard profile of organic chemicals. An experimental method for the assessment of the behaviour of organic chemicals in the ecosphere by means of simple laboratory tests with carbon-14-labeled chemicals. Chemosphere. 14:1589-1616.
Garvey LH, Roed-Petersen J, Husum B. 2003. Is there a risk of sensitization an allergy to chlorhexidine in health care workers? Acta Anaesthesiol Scand. 47:720-724.
Gilbert P, Moore LE. 2005. Cationic antiseptics: diversity of action under a common epithet. J Appl Microbiol. 99:703-715.
Gilman MR, De Salva SJ. 1979. Teratology studies on benzelthonium chloride, cetyl pyridinium chloride and chlorhexidine in rats. Toxicol Appl Pharmacol 48:A35 [cited in Willis 1993].
Gobas F. 2007. Development and review of a generic water–sediment modelling framework for organic chemicals. Report prepared for Environment Canada. Burnaby (BC): Simon Fraser University, Faculty of Environment. March 26, 2007.
Gobas F. 2010. Comments on approach to sediment exposure approach. Report prepared for Environment Canada. Burnaby (BC): Simon Fraser University, Faculty of Environment. March 25, 2010.
Gongwer LE, Hubben K, Lenkiewicz RS, Hart ER, Cockrell BY. 1980. The effects of daily bathing of neonatal rhesus monkeys with an antimicrobial skin cleanser containing chlorhexidine gluconate. Toxicol Appl Pharmacol. 52:255-261.
Grassi TF, Camargo EA, Salvadori DM, Marques ME, Ribeiro DA. 2007. DNA damage in multiple organs after exposure to chlorhexidine in Wistar rats. Int J Hyg Environ Health. 210(2):163-7.
Greener Y, McCartney M, Jordan L, Schmitt D, Youkilis EJ. 1985. Assessment of the systemic effects primary dermal irritation and ocular irritation of chlorhexidine acetate solutions. J Am Coll Toxicol. 4(6):309-320.
Hansch C, Leo A, Hoekman D. 1995. Exploring QSAR - Hydrophobic, Electronic, and Steric Constants. Washington (DC): American Chemical Society p. 178 [cited in HSDB 1983- ].
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Government of Canada.
Health Canada. 2010. PMRA List of Formulants [PDF]. Ottawa (ON): Health Canada, Pest Management Regulatory Agency (PMRA). HC Pub. No. 100460. Cat. No.: H114-22/2010E. [accessed 2016 Jun 9].
Health Canada. [modified 2013 Jun 27]. Lists of permitted food additives. Ottawa (ON): Government of Canada. [accessed 2016 Jun 15].
Health Canada. 2015. Signal assessment: chemical skin injuries (including burns) in neonates with the use of non-prescription, chlorhexidine gluconate-containing skin antiseptics. Unpublished report. Ottawa (ON): Government of Canada. [restricted access].
Health Canada. [modified 2015 Dec 14]. Cosmetic Ingredient Hotlist: Prohibited and Restricted Ingredients. Ottawa (ON): Government of Canada. [accessed 2017 Dec 21].
Health Canada. 2016. Summary safety review - topical antiseptic non-prescription chlorhexidine products - assessing the potential risk of serious allergic reactions (hypersensitivity reactions). Ottawa (ON): Government of Canada. [accessed 2017 May 17].
Heard DD, Ashworth RW. 1968. The colloidal properties of chlorhexidine and its interaction with some macromolecules. J Pharm Pharmacol. 20(7): 505-512.
[HENRYWIN] Henry’s Law Constant Program for Microsoft Windows [estimation model]. 2008. Ver. 3.20. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Henwood S. 1988. 13-week dermal toxicity study with chlorhexidine acetate in rabbits: final report: Laboratory Project ID HLA 6247-102. Unpublished study prepared by Hazleton Laboratories America, Inc. [cited in US EPA 1996 and ECHA c2007-2015b].
Hikiba H, Watanabe E, Barrett JC, Tsutsui T. 2005. Ability of fourteen chemical agents used in dental practice to induce chromosome aberrations in Syrian hamster embryo cells. J Pharmacol Sci. 97:146-152.
[HSDB] Hazardous Substances Data Bank [database]. 1983- . Bethesda (MD): US National Library of Medicine. [updated 2015 Feb 18; accessed 2015 July].
[HYDROWIN] Hydrolysis Rates Program for Microsoft Windows [estimation model]. 2010. Ver. 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[ICI] ICI Pharmaceuticals Group. 1992. Submission of unpublished data on the carcinogenicity of chlorhexidine [cited in Willis 1993].
[IPCS] International Programme on Chemical Safety. 2003. Concise International Chemical Assessment Document 48: 4-chloroaniline [PDF]. Geneva (CH): United Nations Environment Programme; International Labour Organization; World Health Organization. [accessed 2015 Jun]. On the cover: First draft prepared by Boehncke A, Kielhorn J, Könnecker G, Pohlenz-Michel C, and Mangelsdorf I, Fraunhofer Institute of Toxicology and Aerosol Research, Drug Research and Clinical Inhalation, Hanover, Germany.
Jesus FT, Oliveira R, Silva A, Catarino AL, Soares AM, Nogueira AJ, Domingues I. 2013. Lethal and sub lethal effects of the biocide chlorhexidine on aquatic organisms. Ecotoxicology. 22:1348-1358.
Kah M, Brown CD. 2006. Adsorption of ionisable pesticides in soils. Rev Environ Contam T.188:149-217.
Karpanen TJ, Worthington T, Conway BR, Hilton AC, Elliott TSJ, Lambert PA. 2008. Penetration of chlorhexidine into human skin. Antimicrob Agents Ch. 52(10):3633-3636.
Kido Y, Kodama H, Uraki F, Uyeda M, Tsuruoka M, Shibata M. 1988. Microbial degradation of disinfectants. I. Chlorhexidine-degrading bacteria isolated from activated sludge. Eisei Kagaku. 34(1):10-14.
[KOCWIN] Organic Carbon Partition Coefficient Program for Microsoft Windows [estimation model]. 2010. Ver. 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Kodama H, Hashimoto T, Tsuruoka M, Kido Y, Uyeda M, Shibata M. 1988. Microbial degradation of disinfectants. IV. Treatment by activated sludge of chlorhexidine. Eisei Kagaku. 34(5):408-413. [cited in SWECO Environment 2011].
Lafforgue C, Carret L, Falson F, Reverdy ME, 1997. Freney J. percutaneous absorption of a chlorhexidine digluconate solution. Int J Pharm. 147:243-246.
Lamb I. 1991. A developmental toxicity study of chlorhexidine diacetate in rats: final report: Lab Project Number: WIL-173001. Unpublished study prepared by WIL Research Labs [cited in US EPA 1996].
Lawrence JR, Zhu B, Swerhone GDW, Topp E, Roy J, Wassenaar LI, Rema T, Korber DR. 2008. Community-level assessment of the effects of the broad-spectrum antimicrobial chlorhexidine on the outcome of river microbial biofilm development. Appl Environ Microbiol. 74(11):3541-3550.
Leow Y-H, Goh C-L. 1999. Contact allergy in Singapore. Asian Pac J Allergy Immunol. 17(3):207-217.
Li YC, Kuan YH, Huang FM, Chang YC. 2012. The role of DNA damage and caspase activation in cytotoxicity and genotoxicity of macrophages induced by bisphenol‐A‐glycidyldimethacrylate. Int Endod J. 45(6):499-507.
Liippo J, Kousa P, Lammintausta K. 2011. The relevance of chlorhexidine contact allergy. Contact Dermatitis. 64:229-234.
Loretz LG, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, Rothenstein A, Scrafford CG, Sewall C. 2005. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol. 43:279–291.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2018 Feb 06]. Ottawa (ON): Government of Canada. [accessed 2018 Jan 23].
Long R, Hoxter K, Smith G. 1991a. Chlorhexidine diacetate: a dietary LC50 study with the Northern Bobwhite: Lab Project Number: 277-101. Unpublished study prepared by Wildlife International, Ltd. [cited in US EPA 1996].
Long D, Hoxter K, Smith G. 1991b. Chlorhexidine diacetate: a dietary LC50 study with the Mallard: lab project number: 277-102. Unpublished study prepared by Wildlife International, Ltd. [cited in US EPA 1996].
Magnusson B, Heyden G. 1973. Autoradiographic studies of 14C-chlorhexidine given orally in mice. J Periodont Res. 8(12):49-54.
Masson P. 2002. Exposure to cosmetic products: General consideration—Calculation of exposure according to the SCCNFP Notes of Guidance. Bordeaux (FR): EVIC France.
Matsushima H, Sakurai N. 1984. A selected ion monitoring assay for chlorhexidine in medical waste water. Biomed Mass Spectrom. 11(5):203-206.
McEvoy GK, editor. 2010. Chlorhexidine gluconate (EENT). American Hospital Formulary Service – Drug Information (2010). Bethesda (MD): American Society of Health-System Pharmacists. [restricted access].
Miller E. 1993a. Acute oral toxicity evaluation of chlorhexidine diacetate technical in rats: Lab Project Number: 9096-92: P952-1: KVR-P952-1. Unpublished study prepared by Stillmeadow, Inc. [cited in US EPA 1996].
Miller E. 1993b. Acute dermal toxicity evaluation of chlorhexidine diacetate technical in rabbits: Lab Project Number: 9097-92: KVR-P952-2. Unpublished study prepared by Stillmeadow Inc. [cited in US EPA 1996].
Miyachi T, Tsutsui T. 2005. Ability of 13 chemical agents used in dental practice to induce sister chromatid exchanges in Syrian hamster embryo cells. Odontology. 93:24-29.
[MPBPWIN] Melting Point Boiling Point Program for Microsoft Windows [estimation model]. 2008. Ver. 1.43. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Murphy D, Smith G. 1991a. Chlorhexidine diacetate: a 48-hour static acute toxicity test with the cladoceran (Daphnia magna): Lab Project Number: 227A-103. Unpublished study prepared by Wildlife International, Ltd. [cited in US EPA 1996].
Murphy D, Smith G. 1991b. Chlorhexidine diacetate: a 96-hour static acute toxicity test with the bluegill (Lepomis macrochirus): Lab Project Number: 277A-102. Unpublished study prepared by Wildlife International, Ltd. [cited in US EPA 1996].
Murphy D, Smith G. 1991c. Chlorhexidine diacetate: a 96-hour static acute toxicity test with the rainbow trout (Oncorhynchus mykiss): Lab Project Number: 277A-101. Unpublished study prepared by Wildlife International, Ltd. [cited in US EPA 1996].
Myhr B. 1983. Primary rat hepatocyte unscheduled DNA synthesis assay: chlorhexidine hydrochloride: LBI Project No. 20991. Unpublished study prepared by Litton Bionetics, Inc. [cited in US EPA 1996].
Nagendran V, Wicking J, Ekbote A, Onyekwe T, Garvey LH. 2009. IgE-mediated chlorhexidine allergy: a new occupational hazard? Occup Med (Lond). 59:270-272.
[NCI] National Chemical Inventories [database on CD-ROM]. 2015. Issue 1. Columbus (OH): American Chemical Society. [accessed 2015 Aug].
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme. 2014. Inventory multi-tiered assessment and prioritization. Human health tier II assessment for chlorhexidine. Sydney (NSW): Australian Government.
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2019 Feb 22]. Ottawa (ON): Government of Canada. [accessed 2018 Jan 23].
O’Driscoll NH, Labovitiadi O, Cushnie TT, Matthews KH, Lamb AJ. 2014. Potassium loss from chlorhexidine-treated bacterial pathogens is time-and concentration-dependent and variable between species. Curr Microbiol. 68(1):6-11.
[OECD] Organisation for Economic Co-operation and Development. 2009. The 2007 OECD list of high production volume chemicals. Paris (FR): OECD, Environment Directorate. (Series on Testing and Assessment No. 112). Report No.: ENV/JM/MONO(2009)40, JT03272769. [accessed 2015 Jun].
OECD QSAR Toolbox. [read across tool]. 2015. Ver. 3.3.2. Paris (FR): Organisation for Economic Co-oporation and Development, Laboratory of Mathematical Chemistry.
Ogase H, Nagal I, Kameda K, Kume S, Ono S. 1992. Identification and quantitative analysis of degradation products of chlorhexidine with chlorhesidine-resistant bacteria with three-dimensional high performance liquid chromatography. J Appl Bacteriol. 73:71-78.
Okano M, Nomura M, Hata S, Okada N, Sato K, Kitano Y, Tashiro M. 1989. Anaphylactic symptoms due to chlorhexidine glugconate. Arch Dermatol. 125:50-52.
[OMAFRA] Ontario Ministry of Agriculture, Food and Rural Affairs. 2009. Biosecurity: health protection and sanitation strategies for cattle and general guidelines for other livestock. Ontario Ministry of Agriculture, Food and Rural Affairs. [accessed 2010 Aug 9]. .
O’Neil MJ, editor. 2001. The Merck Index: An encyclopedia of chemicals, drugs, and biologicals. 13th edition, Whitehouse Station (NJ): Merck and Co., Inc. p. 361.
O'Neil MJ, editor. 2013. The Merck Index – An encyclopedia of chemicals, drugs, and biologicals. Cambridge (UK): Royal Society of Chemistry, p. 371 [cited in HSDB 1983- ].
O’Neill J, Hosmer M, Challop R, Driscoll J, Speck W, Sprunt K. 1982. Percutaneous absorption potential of chlorhexidine in neonates. Curr Ther Res Clin Exp. 31:485-489.
OPP Pesticide Ecotoxicity Database [database]. 1991. Washington (DC): US Environmental Protection Agency, Office of Pesticide Programs, Ecological Fate and Effects Division. [accessed 2015 Jun].
Opstrup MS, Johansen JD, Zachariae C, Garvey LH. 2016. Contact allergy to chlorhexidine in a tertiary dermatology clinic in Denmark. Contact Dermatitis 74(1):29-36.
Oraldent Pharma. 2015. Oraldent Pharma products. [accessed 2015 Dec 1].
Osmundsen PE. 1982. Contact dermatitis to chlorhexidine. Contact Dermatitis 8(2):81-83.
Pesticide Label Search [database]. [modified 2016 Oct 4]. Ottawa (ON): Health Canada. [accessed 2017 Dec 19].
Princz J, Bonnell M, Ritchie E, Velicogna J, Robidoux P, Scroggins R. 2014. Estimation of the bioaccumulation potential of a nonchlorinated bisphenol and an ionogenic xanthene dye to Eisenia andrei in field-collected soils, in conjunction with predictive in silico profiling. Environ Toxicol Chem. 33(2):308-316.
Revelle LK, Doub, WH, Wilson RT, Harris MH, Rutter AM. 1993. Identification and isolation of chlorhexidine digluconate impurities. Pharm Res.10(12):1777-1784.
Reynolds NJ, Harman RR 1990. Allergic contact dermatitis chlorhexidine diacetate in a skin swab. Contact Dermatitis. 22(2):103-4.
Ribeiro DA, Bazo AP, da Silva Franchi CA, Marques MEA, Salvadori DMF. 2004. Chlorhexidine induces DNA damage in rat peripheral leukocytes and oral mucosal cells. J Periodont Res. 39:358–361.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2006a. Cleaning products fact sheet: to assess the risks for the consumer [PDF]. Bilthoven (NL): RIVM Report 320104003/2006. [accessed 2010 Jul].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment (NL)]. 2006b. Cosmetics fact sheet: to assess the risks for the consumer: updated version for ConsExpo 4 [PDF]. Bilthoven (NL): RIVM Report No.: 320104001/2006.
Roberts DL, Summerly R, Byrne JPH. 1981. Contact dermatitis due to the constituents of Hibiscrub. Contact Dermatitis. 7:326-328.
Sakagami Y, Yokoyama H. 1983. Degradation of some disinfectants by activated sludge. Eisei Kagaku. 29(6):342-351.
Sakagami Y, Yokoyama H, Ose Y, Sato T. 1986. Screening test for carcinogenicity of chlorhexidine digluconate and its metabolites. J Hyg Chem. 32(3):171-5.
Sakagami Y, Yamasaki K, Yokoyama H, Ose Y, Sato T. 1988. DNA repair test of disinfectants by liquid rec-assay. Mutat Res. 193(1):21-30.
Sakuratani Y, Noguchi Y, Kobayashi K, Yamada J, Nishihara T. 2008. Molecular size as a limiting characteristic for bioconcentration in fish. J Environ Biol. 29(1):89-92.
[SCCS] Scientific Committee on Consumer Safety. 2015. The SCCS’s notes of guidance for the testing of cosmetic ingredients and their safety evaluation [PDF]. 9th Revision. European Commission. Report No. SCCS/1564/15, Revised version of 25 April 2016.
[SDS] Safety Data Sheet. 2015. Encon Hydrosep Potable Water Additive [PDF]. Houston (TX): Encon Safety Products. [accessed 2019 Mar 11]
Senior N. 1973. Some observations on the formulation and properties of chlorhexidine. J Soc Cosmet Chem. 24:259-278.
Shapiro R. 1993. EPA acute inhalation toxicity--defined LC50: chlorohexidine-diacetate: Lab Project Number: T-1813. Unpublished study prepared by Product Safety Labs [cited in US EPA 1996].
Sigma Aldrich. 2003. Product information sheet: Chlorhexidine diacetate salt hydrate – C6143. [accessed 2015 Jun].
Sigma Aldrich. 2015. C6143 - Chlorhexidine diacetate salt hydrate. [accessed 2015 Jun].
Statistics Canada. 2012. Custom tabulation of grooming products data from the Canadian Health Measures Survey Cycle 1 (2007-2009). Prepared for Existing Substances Risk Assessment Bureau, Health Canada, by Statistics Canada. Unpublished.
[STP-EX] Sewage Treatment Plant Expanded Model. c2000-2013. Ver. 1.0. Windsor (ON): University of Windsor, Dept. of Civil and Environmental Engineering. [Model described in Seth R, Webster E, Mackay D. 2008. Continued development of a mass balance model of chemical fate in a sewage treatment plant. Water Res. 42:595-604].
Study Submission. 2010. Unpublished confidential study submitted to Environment Canada under the Chemicals Management Plan Challenge initiative. Gatineau (QC): Environment Canada, Program Development and Engagement Division.
Suessmuth R, Ackermann B, Lingens F. 1979. Mutagenic effect of 1.1′-hexamethylene-bis-[(5-p-chlorophenyl)-biguanide]. Chem-Biol Interact. 28(2):249-58.
Sugio N, Kojima S. 1992. Biological treatment of chlorhexidine digluconate-containing waste water. II. Chlorhexidine digluconate-acclimated bacteria. Jpn J Tox Env Health. 38(4):329-333.
SWECO Environment. 2011. SWECO Environment Screening Report. Chlorhexidine and p-chloroaniline. Report commissioned by the Swedish Environmental Protection Agency. Stockholm (Sweden): SWECO. Project number: 1270481000.
Tanaka T, Murayama S, Tuda N, Mishiyama M, Nakagawa K, Matsuo Y, Isohama Y, Kido Y. 2005. Microbial degradation of disinfectants. A new chlorhexidine degradation intermediate (CHDI), CHDI-C, produced by Pseudomonas sp. Strain No. A-3. J Health Sci. 51:357-361.
Tanaka T, Ishii M, Nakano S, Mori Y, Yano Y, Iijima T, Takeda K, Kido Y. 2006. Microbial degradation of disinfectants: two new aromatic degradation products of chlorhexidine, chlorhexidine aromatic degradation product (CHADP)-4 and (CHADP)-6, produced by Pseudomonas sp. Strain No. A-3. J Health Sci. 52(1):58-62.
Tanzer JM, Slee AM, Kamay BA. 1977. Structural requirements of guanide, biguanide, and bisbiguanide agents for antiplaque activity. Antimicrob Agents Chemother. 12(6):721-729.
Toholka R, Nixon R. 2013. Allergic contact dermatitis to chlorhexidine. Australas J Dermatol. 54(4):303-306.
[US EPA] United States Environmental Protection Agency. 1996. Reregistration eligibility decision. Chlorhexidine diacetate. Washington (DC): US EPA. EPA738-R-96-025.
[US EPA] United States Environmental Protection Agency. 1997. Exposure factors handbook. Washington (DC): US EPA, National Center for Environmental Assessment, Office of Research and Development.
[US EPA] United States Environmental Protection Agency. 2003. Exposure and human health reassessment of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds. Part I: Estimating exposure to dioxin-like compounds. Volume 3: Site-specific assessment procedures. Chapter 4: Estimating exposure media concentrations. EPA/600/P-00/001Cb. Washington (DC): US EPA, National Center for Environmental Assessment.
[US EPA] United States Environmental Protection Agency. 2011a. Chlorhexidine derivatives summary document: Registration review – Initial docket March 2011 (Case #3038). Washington (DC): US EPA, Chemical Safety and Pollution Prevention. [accessed 2011 Jun]. .
[US EPA] United States Environmental Protection Agency. 2011b. Memorandum – Summary of product chemistry, environmental fate, and ecotoxicity data for the chlorhexidine derivatives registration review decision document. Washington (DC): US EPA, Chemical Safety and Pollution Prevention. .
[US FDA] United States Food and Drug Administration. 2013. Peridex-chlorhexidine gluconate mouthwash [PDF]. 3M. Reference ID: 3246401. [accessed 2015 Mar].
Voets JP, Pipyn P, van Lancker P, Verstraete W. 1976. Degradation of microbicides under different environmental conditions. J Appl Microbiol. 40(1):67-72 [cited in HSDB 1983- ].
Westagro Canada. 2014a. Sani-Wash Lave-Pis 1.6%. Westagro Canada. [accessed 2014 Jan 24].
Westagro Canada. 2014b. Agro Blue. Westagro Canada. [accessed 2014 Jan 24].
Willis L. 1993. Final report on the safety assessment of chlorhexidine/chlorhexidine diacetate/chlorhexidine dihydrochloride/chlorhexidine digluconate. J Am Coll Toxicol. 12(3):201-223.
Winrow MJ. 1973. Metabolic studies with radiolabelled chlorhexidine in animals and man. J Periodont Res. 12:45-48.
Wong WK, Goh CL, Chan KW. 1990. Contact urticaria from chlorhexidine. Contact Dermatitis. 22:52.
Wormuth M, Scheringer M, Vollenweider M, Hungerbuhler K. 2006. What are the sources of exposure to eight frequently used pthalic acid esters in Europeans? Risk Anal. 26(3):803-824.
Wu X, Bennett DH, Ritz B, Cassady DL, Lee K, Hertz-Picciotto I. 2010. Usage pattern of personal care products in California households. Food Chem Toxicol. 48:3109-3119.
Xue Y, Tang M, Hieda Y, Fujihara J, Takayama K, Takatsuka H, Takeshita H. 2009. High-performance liquid chromatographic determination of chlorhexidine in whole blood by solid-phase extraction and kinetics following an intravenous infusion in rats. J. Anal Toxicol. 33:85-91.
Xue Y, Zhang S, Tang M, Zhang T, Wang Y, Hieda Y, Takeshita H. 2012. Comparative study on toxic effects induced by oral or intravascular administration of commonly used disinfectants and surfactants in rats. J Appl Toxicol. 32:480-487.
Yamayoshi T, Doi H, Tatsumi N. 1981. The effect of disinfectants on waste water from medical centers. Jpn J Infect Dis. 55(6):385-399.
Zong Z, Kirsch LE. 2012. Studies on the instability of chlorhexidine, part 1: Kinetics and mechanisms. J Pharm Sci. 101(7): 2417-2427.
Appendix A. Estimated human exposures from use of products
Exposures were estimated for different age groups based on body weights from Health Canada’s exposure factors for the general population of Canada (Health Canada 1998):
Infants (0-6 months): 7.5 kg
Toddlers (0.5-4 years): 15.5 kg
Children (5-11 years): 31.0 kg
Adults (20-59 years): 70.9 kg
All assumptions are listed below for dermal exposure parameters (including exposure scenarios for cosmetics and natural health products) and were ConsExpo default assumptions (RIVM 2006b) unless otherwise noted. An overall retention factor of 1 was used unless otherwise stated. Exposures were estimated for an adult unless otherwise specified.
Body moisturizer (infant):
Exposure frequency: 1.7 per day (Wormuth et al. 2006)
Product amount: 1.4 g per application (Wormuth et al. 2006)
Body moisturizer:
Exposure frequency: 1.13 per day (Loretz et al. 2005)
Product amount: 4.4 g per application (mean) (Loretz et al. 2005)
Genitalia lubricant:
Exposure frequency: 0.005 per day (personal communication, email from the New Substances Assessment and Control Bureau, Health Canada to the Existing Substances Risk Assessment Bureau, Health Canada, dated July 5, 2016; unreferenced)
Product amount: 10 g per application (US EPA 1997)
Hair dye (non-spray/wash-in; permanent):
Exposure frequency: 0.02 per day (7.99 per year) (Statistics Canada 2012)
Product amount: 100 g per application
Overall retention factor: 0.10 (SCCS 2015)
Leave-in hair conditioner:
Exposure frequency: 1.1 per day
Product amount: 13.1 g per application
Overall retention factor: 0.1 (professional judgment)
Sunscreen lotion:
Product amount: 12.3 g per day based on 177 use days (ESRAB 2017)
Sunscreen lotion (toddler):
Product amount: 3 g per day based on 177 use days (ESRAB 2017)
All assumptions listed below are for oral exposure parameters for cosmetics and natural health products, and were ConsExpo default assumptions (RIVM 2006b) unless otherwise noted. All product scenarios are for adults unless otherwise indicated.
Lip balm (toddler):
Exposure frequency: 0.59 per day (Wu et al. 2010)
Product amount: 0.01 g per application
Lipstick:
Exposure frequency: 2.4 per day (Loretz et al. 2005)
Product amount: 0.01 g per application
Mouthwash (child):
Exposure frequency: 0.85 per day (Ficheux et al. 2015)
Product amount: 10 g per application (Product labels on children’s mouthwash recommend children between 6 and 12 years old consume 10 ml per use. Assuming a density of 1 g/ml).
Overall retention factor: 0.1 (SCCS 2015)
Mouthwash:
Exposure frequency: 1.0 per day (Ficheux et al. 2015)
Product amount: 17 g per application (Ficheux et al. 2016)
Overall retention factor: 0.1 (SCCS 2015)