Screening assessment Cobalt and Cobalt-Containing substances
Table of contents
- Synopsis
- 1. Introduction
- 2. Scope of the assessment and substances identity
- 3. Physical and chemical properties
- 4. Sources, uses and releases to the environment
- 4.1 Natural sources
- 4.2 Anthropogenic sources, uses and releases
- 4.3 Cobalt production
- 4.4 Manufacture, import and uses of cobalt-containing substances, products and manufactured items
- 4.5 Incidental manufacture
- 4.6 Disposal and waste management of products, manufactured items and wastes containing cobalt
- 5. Environmental Fate
- 6. Potential for bioaccumulation
- 7. Ecological effects
- 8. Ecological exposure assessment
- 8.1 Ambient/background concentrations
- 8.2 Deriving environmental concentrations from anthropogenic activities
- 8.3 Sector-specific exposure scenarios
- 8.3.1 Rubber
- 8.3.2 Chemical manufacturing/Manufacture and use of catalysts
- 8.3.3 Paints and coatings
- 8.3.4 Plastic
- 8.3.5 Fertilizers
- 8.3.6 Animal feed manufacturing
- 8.3.7 Alloy and superalloy manufacturing
- 8.3.8 Metal mining
- 8.3.9 Base metals smelting and refining
- 8.3.10 Iron and steel
- 8.3.11 Electricity (power generation)
- 8.3.12 Petroleum refining
- 8.3.13 Oil sands
- 8.3.14 Pulp and paper mills
- 8.3.15 Electrical and electronic equipment
- 8.3.16 Battery recycling
- 8.3.17 Disposal and waste management
- 8.4 Exposure based on provincial or territorial-wide aquatic monitoring
- 9. Characterization of ecological risk
- 9.1 Industrial scenarios based on modelling of substance-specific information
- 9.2 Industrial scenarios based on incidental releases and monitoring
- 9.3 Provincial or territorial-wide aquatic monitoring
- 9.4 Summary of ecological risk characterization
- 9.5 Consideration of lines of evidence and uncertainties
- 9.6 Conclusion of ecological risk characterization
- 10. Potential to cause harm to human health
- 11. Conclusion
- References
- Appendices
List of tables and figures
- Table 6-1. summary of experimental data selected for estimating the trophic magnification potential of cobalt
- Figure 7-1. species sensitivity distribution (SSD) for cobalt based on hardness-corrected (100 mg/L CaCO3) chronic toxicity data for freshwater organisms. The Normal model fit to data is shown on the graph along with the 95% confidence intervals
- Figure 7-2. species sensitivity distribution (SSD) for cobalt based on chronic toxicity data for freshwater benthic organisms. The Gumbel model fit to data is shown on the graph along with the 95% confidence intervals
- Figure 7-3. general approach used for the incorporation of Co bioavailability in soils (adapted from CoRC, 2012)
- Table 8-1: concentrations of cobalt in surface waters of minimally impacted areas of Canada
- Table 8-2: summary of estimated aquatic concentrations (EAC) ranges, background and PEC ranges for substance-specific industrial exposure scenarios
- Table 8-3: summary of measured concentrations of cobalt in the vicinity of metal mines in Canada
- Table 8-4: summary of measured concentrations of cobalt in the vicinity of base metals smelters and refineries in Canada
- Table 8-5: concentrations of cobalt in the Wabamun Lake area, Alberta
- Table 8-6: concentrations of cobalt in the Athabasca region (Oil Sands), Alberta, Canada
- Table 8-7: concentrations of cobalt in the vicinity of pulp and paper mills in Canada
- Table 9-1: risk quotient (RQ) calculations ranges for substance-specific key industrial modeled exposure scenarios in the aquatic compartment
- Table 9-2: risk quotient (RQ) calculations for the metal mining sector in the surface water and sediment compartments
- Table 9-3: risk quotient (RQ) calculations for the base metals smelting and refining sector in the surface water, sediment and soil compartments
- Table 9-4: risk quotient (RQ) calculations in the surface water, sediment and soil compartments for a coal-fired power generation scenario in the Wabamun Lake area, Alberta
- Table 9-5: risk quotient (RQ) calculations in the surface water and sediment in the Athabasca Oil Sands, Alberta
- Table 9-6: risk quotient (RQ) calculations in the surface water and sediment in the vicinity of pulp and paper mills in Canada
- Table 9-7: risk quotient (RQ) calculations for the surface waters of Ontario and Yukon from the PWQMN and BISY databases
- Table 9-8: summary of sectors/activities of concern based on number of locations or areas and range in risk quotients
- Table 9-9: uncertainty characterization and analysis of the weight of evidence in the risk assessment for Cobalt
Synopsis
Pursuant to sections 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of cobalt and cobalt-containing substances, as part of the Substance Groupings Initiative of the Government of Canada's Chemicals Management Plan (CMP). Fifty cobalt-containing substances were identified during the categorization of the Domestic Substances List as priorities for action as they met categorization criteria under subsection 73(1) of CEPA and/or were considered as a priority based on other human health concerns.
Information was reported under section 71 of CEPA for 22 cobalt-containing substances that were manufactured, imported or used above reporting thresholds in Canada in recent years (2006-2011). Four substances were reported to be in commerce in quantities greater than 1 000 tonnes, while the others were in commerce in quantities ranging from tens to hundreds of tonnes. Activities and uses involving cobalt in Canada include its use as an intermediate in metallurgical processes, in non-ferrous metal smelting and refining, as a component in alloys and carbides, as feed supplements and fertilizers, as hard material tools, and as paints and coatings, plastic, rubber, and batteries.
There are natural and anthropogenic sources of cobalt to the environment. Anthropogenic sources include cobalt production (e.g., mining); the manufacture, import and use of cobalt-containing substances, products and manufactured items; fossil fuel combustion; and waste management. This assessment considers combined exposure to the cobalt moiety, from natural or anthropogenic sources, whether it is present in environmental media (e.g., water, sediment, soil, air), food or products. The assessment focuses on the cobalt moiety, and thereby considers cobalt in its elemental form, cobalt-containing substances and cobalt released in dissolved, solid or particulate form. As such, substances considered in this assessment are not limited to those having met the categorization criteria. All substances that have the potential to dissolve, dissociate and/or degrade to release cobalt through various transformation pathways can potentially contribute to the exposure of living organisms to bioavailable forms of cobalt.
Following releases to the environment, cobalt may enter the water, soil and air media. The water solubility of cobalt and cobalt-containing substances ranges widely, from sparingly soluble to greater than 106 mg/L. Therefore, to various extents, these substances will dissolve in contact with moisture in the aquatic and soil media and will yield a variety of dissolved cobalt species of varying proportions depending on the environmental conditions. Dissolved cobalt, as the bioavailable fraction, may be taken up by aquatic, soil and sediment-dwelling organisms and has been demonstrated to cause harm to these organisms at very low concentrations. Survival, growth, or reproduction of these organisms may be affected. The bioaccumulation potential of cobalt is relatively low, yet cobalt uptake may still lead to levels causing harm to sensitive species at body concentrations higher than required for essentiality.
Ecological exposure scenarios were developed for the various activities that may represent significant sources of release of cobalt or cobalt-containing substances to the environment. Exposure to cobalt was assessed based on modeled (predicted) or measured concentrations of total or dissolved cobalt in environmental media. Substance-specific exposure scenarios were developed to represent releases associated with the following sectors mainly involving manufacture: rubber, chemicals, paints and coatings, plastics (polyester resin), fertilizers, animal feed, alloys/superalloys and base metals smelting and refining. In addition, exposure was assessed for the following sectors based on their potential to release cobalt incidentally (as a by-product): metal mining, base metals smelting and refining, iron and steel, electricity (power generation), petroleum refining, oil sands, pulp and paper mills, electrical and electronic equipment, disposal and waste management.
Risk quotient analyses were performed comparing exposure concentrations to effects concentrations of dissolved or total cobalt. As a result, a likelihood of harm to aquatic, sediment or soil organisms is identified mainly in the vicinity of some facilities for a number of sectors. The metal mining and base metals smelting and refining sectors are of concern for cobalt. Releases of liquid effluent were found to be the most important source of exposure for aquatic organisms near these activities. Drainage from historical mining activities and, to a lesser extent, metal mining exploration were also found to be a cause for concern for cobalt. Other sectors or sources found to be of concern were pulp and paper mills and leachate from landfills.
Considering all available lines of evidence presented in this screening assessment, there is risk of harm to organisms, but not to the broader integrity of the environment, from cobalt and soluble cobalt compounds. It is concluded that cobalt and soluble cobalt compounds meet the criteria under paragraph 64(a) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity. However, it is concluded that cobalt and soluble cobalt compounds do not meet the criteria under paragraph 64(b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger to the environment on which life depends.
For the human health assessment, general population exposure was characterized using nationally representative biomonitoring data collected from 2009 to 2011 as part of the Canadian Health Measures Survey (CHMS). Whole blood cobalt concentrations are representative of daily exposure to natural and anthropogenic sources of bioavailable cobalt from all sources including environmental media, food and the use of frequent or daily use products. The results of the CHMS did not show statistically significant differences in blood concentrations of cobalt between the general population and subpopulations based on age or gender. Inhalation exposure to solid or particulate forms of the cobalt moiety was evaluated using concentrations of cobalt measured in personal air samplers and is considered most representative of typical daily exposures.
Based on the weight of evidence analysis, international agencies have classified cobalt-containing substances as carcinogens. These classifications are primarily based on the evidence of tumors observed in rodents exposed to some cobalt substances via the inhalation or injection route. Available short-term and subchronic oral studies in animals, or epidemiology studies in humans, do not provide evidence for potential systemic or site-specific carcinogenicity by the oral route. Genotoxicity of cobalt is likely mediated by indirect mechanisms, including generation of reactive oxygen species and inhibition of DNA repair enzymes. Lethal cardiomyopathy in malnourished individuals who consumed large quantities of beer containing cobalt sulphate was identified as a critical effect for risk characterization. Selection of this endpoint is considered conservative as the affected population may have been more susceptible than the general population due to dietary insufficiencies and prior cardiac damage from excessive alcohol consumption. Polycythemia (the increase of red blood cells and haemoglobin) observed in humans was identified as another critical health effect for the risk characterization of the general population. The critical effect identified for inhalation exposure was reduced lung function reported in individuals occupationally exposed to dust containing cobalt in the diamond polishing industry.
These endpoints were considered conservative and protective of potential harmful effects observed in the animal database, including developmental, reproductive and carcinogenic effects. The margins of exposure between cobalt levels in whole blood of Canadians from a nationally representative survey or cobalt levels in personal air samples and conservative effect levels are considered adequate to address uncertainties in the health effects and exposure databases.
Therefore, it is concluded that cobalt and cobalt from cobalt-containing substances, including the substances identified in Appendix A, do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Overall conclusion
It is concluded that cobalt and soluble cobalt compounds meet one of the criteria set out in section 64 of CEPA. In addition, cobalt and soluble cobalt compounds have been determined to meet the persistence criteria but do not meet the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
1. Introduction
Pursuant to section 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999) the Minister of the Environment and the Minister of Health conduct screening assessments of substances to determine whether these substances present or may present a risk to the environment or to human health.
The Substance Groupings Initiative is a key element of the Government of Canada's Chemicals Management Plan (CMP). The Cobalt-containing Substance Grouping includes 50 substances (listed in Appendix A) that were identified as priorities for action, as they met the categorization criteria under section 73 of CEPA (Environment Canada 2007a; Health Canada 2009a). Potential ecological effects of concern have been identified for cobalt during previous assessment activities conducted under the Challenge initiative of the CMP (Environment Canada, Health Canada 2011a).
This screening assessment focuses on the cobalt moiety, and thereby considers cobalt in its elemental form, cobalt-containing substances and cobalt released in dissolved, solid or particulate form. As such, it is not limited to consideration of the substances having met categorization criteria, and listed in Appendix A. All substances that have the potential to dissolve dissociate and/or degrade to release cobalt through various transformation pathways can potentially contribute to the exposure of living organisms to bioavailable forms of cobalt. This assessment considers combined exposure to the cobalt moiety, whether it is present in environmental media (e.g., water, sediment, soil, air), food or products.
Four cobalt-containing substances included in this grouping were assessed during the earlier Challenge initiative of the CMP (elemental cobalt, cobalt chloride, and two cobalt sulfates). Although potential ecological concerns were identified in the Challenge assessment, these substances were found as not meeting any of the criteria set out in section 64 of CEPA when considered as individual substances; however, these four substances are included in this assessment to consider combined exposure to cobalt.
A notice to industry was published under authority of section 71 of CEPA requiring that relevant data be submitted on 16 cobalt-containing substances. This information supplements data obtained through previous section 71 notice surveys, namely a 2009 survey for the Challenge initiative (four substances) and a survey for the 2009 Domestic Substances List Inventory Update (DSL IU) initiative (35 substances). As a result, submissions of information pertaining to the properties, hazard, uses and exposure of the substances were received.
Screening assessments focus on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA, by examining scientific information to develop conclusions by incorporating a weight of evidence approach and precautionFootnote1.
This screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including information submitted by stakeholders. Relevant data were identified up to September 2014 and targeted literature searches were conducted up to November 2016. Empirical data from key studies and certain results from models were used to reach these conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered. The screening assessment does not represent an exhaustive or critical review of all available data; rather, it presents the studies deemed most critical and the lines of evidence deemed most pertinent to the conclusion. Additional supporting information used for this assessment is summarized separately in supporting documentation, which is available upon request.
The screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological and human health portions of this assessment have undergone external written peer review and consultation. Comments on the technical portions relevant to the environment were received from Dr. Claude Fortin, Institut National de la Recherche Scientifique - Eau, Terre et Environnement (INRS-ETE); Dr. Kevin J. Wilkinson, Université de Montréal; Dr. Beverly Hale, University of Guelph; Dr. Scott Smith, Wilfrid Laurier University; Dr. William Stubblefield, Oregon State University; Dr. Peter Lepper, European Chemicals Agency (ECHA); Dr. José V. Tarazona, ECHA. Comments on the technical portions relevant to human health were received from Cathy Petito Boyce, Leslie Beyer, and Chris Long of Gradient Consulting. Additionally, the draft of this screening assessment was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
The critical information and considerations upon which the screening assessment is based are given below.
2. Scope of the assessment and substances identity
2.1 Scope of the assessment
This screening assessment focuses on the cobalt moiety, and thereby includes cobalt in its elemental form, cobalt-containing substances and cobalt released in dissolved, solid or particulate form. This assessment considers combined exposure of humans and other living organisms to the cobalt moiety, whether it is present in environmental media (e.g., water, sediment, soil, air), food or products. The presence of the cobalt moiety in these media, food or products may result from natural or anthropogenic sources. Anthropogenic sources include cobalt production (e.g., mining); the manufacture, import and use of cobalt-containing substances, products and manufactured items; fossil fuel combustion; and waste management.
2.2 Substances identity
Cobalt-containing substances, whether produced incidentally or commercially, belong to various categories including elemental cobalt, inorganic metal compounds, organic-metal salts, organometallic compounds, and unknown or variable composition, complex reaction products, or biological materials (UVCBs). Identities for all of the commercial cobalt-containing substances that had been identified as meeting the categorization criteria are presented in Appendix A.
Four cobalt-containing substances that are included in the grouping were assessed during the earlier Challenge initiative of the CMP (elemental cobalt, CAS RN 7440-48-4; cobalt chloride, CAS RN 7646-79-9; two cobalt sulfates, CAS RN 10124-43-3 and CAS RN 10393-49-4) (Environment Canada, Health Canada 2011a). These substances were found as not meeting any of the criteria set out in section 64 of CEPA when considered as individual substances; however, in order to consider combined exposure to cobalt, these four substances are included in this assessment.
This assessment only considers effects associated with the cobalt moiety, and does not address other elements that may be present in certain complex cobalt-containing substances that may release these other elements (such as cadmium, chromium, silver, and copper). Of note, some of these elements have already been addressed through previous assessments conducted as part of the Priority Substances List program under CEPA.
Cobalt-containing substances can include organic-metal salts, as well as organometallic compounds. These substances may dissolve, dissociate or degrade to release organic or organometallic transformation products and the cobalt moiety. The organic or organometallic transformation products or organic counter-ions from these substances were not specifically evaluated in this assessment. However, available human health effects data of the organic counterions were compared to the hazard database for the cobalt moiety. It was determined that the health effects database for cobalt is protective of the organic components.
Engineered nanomaterials composed of or containing cobalt were not explicitly considered in exposure scenarios of this assessment. However, measured cobalt concentrations in the environment could include engineered cobalt or cobalt-containing nanomaterials.
3. Physical and chemical properties
Substances included in this assessment have the potential to release cobalt through dissolution, dissociation, transformation and/or degradation when they reach certain environmental media (e.g., water, soil). As such, properties such as water solubility of cobalt-containing substances and acid dissociation constant (Ka) of the organic component of organic-metal salts are relevant to the environmental fate and ecotoxicity of these substances. Values for these properties are available in ECCC (2016a). Molecular weights are also provided and are used in the assessment to calculate quantities and concentrations on a cobalt molar basis. The values presented for the molecular weight of organometallic and organic-metal salt UVCBs are an approximation, based on simple addition of the named components. Indeed, by definition, molecular weight of UVCBs cannot be assigned. Other physical and chemical properties such as boiling and melting points, vapour pressure, Henry's Law constant were not documented as it is expected that substances in the grouping will be solid at environmental temperatures and will not be volatile. Certain partition coefficients that pertain to cobalt as an ion (as compared to bound cobalt within a substance) are available in ECCC (2016a), and are discussed in the environmental fate section of this report.
Data presented in ECCC (2016a) show that the water solubility of cobalt and cobalt-containing substances ranges widely, from sparingly soluble to greater than 106 mg/L. Some of these values are based on the transformation-dissolution protocol developed by the Organisation for Economic Co-operation and Development (OECD) for metals and metal compounds (OECD 2001, OECD 2008a,b), representing a relevant measure regarding the expected release of cobalt ions from elemental cobalt and sparingly soluble cobalt compounds. Even though some solubility or transformation-dissolution values are very low, they are within the range of the concentrations expected to be potentially harmful to sensitive aquatic organisms. It can be noted that there were no empirical water solubility or transformation-dissolution data available for a number of substances. In cases where solubility data were not available, a qualitative estimate was made based on equilibrium constants such as the solubility product constant (Ksp) and acid dissociation constant (Ka) for analogous substances. When such information was not available, estimates were based on professional judgement (2001 personal communication from Robert Burk, Carleton University, to Ecological Assessment Division, Environment Canada; unreferenced). There were no data available for water solubility for inorganic UVCB compounds. These substances are often complex matrices (e.g., sludges and slags) that contain numerous metals. If exposed to water, they may leach certain metals depending on exposure conditions. Based on an analogue approach, it was deemed that these compounds could potentially release cobalt. Thus, they were identified as being soluble (2001 personal communication from Robert Burk, Carleton University, to Ecological Assessment Division, Environment Canada; unreferenced). This is a conservative approach for some of the UVCB compounds (e.g., fritsFootnote2 chemicals).
In addition, the substance with the common name cobalt carbonyl (CAS RN 10210-68-1) is an organometallic compound that has reactivity with air and water, as acknowledged in several Material Safety Data Sheets. Carbon monoxide and cobalt hydroxide, a soluble compound, are the expected decomposition products which may be produced upon release of cobalt carbonyl to the environment. Thus, cobalt carbonyl was included in the original grouping even though the literature indicates that it is insoluble in water.
4. Sources, uses and releases to the environment
4.1 Natural sources
Cobalt is a naturally occurring element in the terrestrial crust. Cobalt concentrations in the upper continental crust have been determined to average about 25 ppm and to range between 0.1 and 110 ppm (Reimann and de Caritat 1998). Cobalt is not known to exist naturally in its elemental (metallic) form; naturally occurring cobalt is comprised of various minerals, oxide and salt forms (ECCC 2016a).
Global natural emissions to the atmosphere have been estimated to range between 690 and 11 000 tonnes of cobalt per year, with a median of 6100 tonnes (Nriagu 1989). Sources include wind-blown continental dusts, weathering of rocks, seawater sprays, forest fires and volcanoes (IPCS 2006). Atmospheric deposition and introduction of cobalt into surface water and soil as a result of these natural processes are reflected in the geochemical background levels in these media. These background levels are considered when estimating the exposure of ecological receptors to cobalt substances in the characterization of ecological risk section of this assessment.
4.2 Anthropogenic sources, uses and releases
Anthropogenic sources of cobalt and cobalt-containing substances include activities such as the production of cobalt (mining, smelting and refining); the manufacture, import and use of cobalt-containing substances, products or manufactured items; as well as the disposal and waste management of cobalt-containing substances, products or manufactured items. These various stages, parts of the life-cycle of cobalt-containing substances, are presented in the following sections, with an explanation of potential releases to the environment at each of these stages. Sources related to the incidental manufacture of cobalt-containing substances (i.e., as a by-product) in any form are also described, where applicable, with respect to releases to the environment. Unless otherwise stated, cobalt quantities, releases and emissions [(e.g., National Pollutant Release Inventory (NPRI) data)] are presented as total cobalt on an elemental basis.
4.3 Cobalt production
4.3.1 Metal mining
Elemental cobalt is rarely the exclusive metal isolated from a mine but it is mainly an additional product of copper or nickel mining (BGS 2009). Cobalt can also be found in association with silver, lead and iron ores. A total of 2275 tonnes of cobalt were mined in Canada in 2009 (Natural Resources Canada 2009a). The province with the largest quantity extracted was Ontario (779 tonnes) with the rest mined from Newfoundland and Labrador (626 tonnes), Manitoba (374 tonnes) and Quebec (496 tonnes) (Natural Resources Canada 2009a). There were 18 mines and mills which produced cobalt in 2009. These facilities were located in the following mining areas: Sudbury (Ontario), Voisey's Bay (Newfoundland and Labrador), Thompson (Manitoba) and Raglan (Quebec) (Natural Resources Canada 2009b). Operating mines are located in areas of increased mineralization and therefore may also have historical mines in proximity. Most of the mines which produce cobalt are underground mining operations.
Mines and mills, even if they do not purposefully extract cobalt as a product, may release cobalt to the environment given that this metal is present in a variety of ores. Cobalt can be released from mining facilities because, during the mining process, water comes into contact with cobalt-containing rock, ore and tailings. This cobalt can be dissolved into the contact water and can then be released mainly as part of the effluent of the mine at the final discharge point. Cobalt can continue to be released from mine waste storage facilities (waste rock and tailings) long after the mine has ceased operation. At some locations, the pH of the contact water can be lowered due to the presence of other substances such as sulphide minerals and, due to the lowered pH of this water, it commonly contains elevated concentrations of dissolved metals (including cobalt). The outflow of this acidic (low pH) water is called acid mine drainage (AMD) and all or most of it is treated for pH and metals at active mine sites.
In 2011, 44 mines and mills reported on cobalt and its compounds to the NPRI of Environment Canada. Reported on-site releases to air, water and land, for that year are 1.7, 1.3 and 1.3 tonnes respectively while on-site disposal amounted to 3 637 tonnes and transfers off-site for disposal was 88 kg (Table C-1, Appendix C). It should be noted that "disposal" includes information on the disposal of tailings and waste rock. There was no off-site recycling reported (NPRI 1995). Forty-three out of the 44 mines and mills reported total releases (air, water, soil) of cobalt of less than one tonne, while 15 reported having no releases of cobalt. The reporting threshold for "cobalt and its compounds" is 10 tonnes Manufactured, Processed or Otherwise used (MPO) at a concentration of 1% or greater. However, NPRI requires that cobalt in tailings and by-products be included in the calculation of the reporting threshold regardless of the concentration of cobalt in these materials (including less than 1%). All releases, disposals and transfers of cobalt (except for quantities in waste rock at less than 1%) must then be reported on to the NPRI if the threshold for reporting was met. The requirement to include all cobalt in tailings in the calculation of the MPO threshold may contribute to more extensive reporting from the metal mining sector compared to other sectors. While most of the 44 mines and mills that reported cobalt releases to the NPRI were metal mines, there were a few non-metal mines as well (phosphate, potash, diamond, and coal).
4.3.2 Base metals smelting and refining
Smelting is used to produce a number of products, including cobalt, from mined ore. The smelting process uses heat and chemical reduction to extract the metal from the ore. Similar to mines, smelters and refineries that do not smelt or refine cobalt may release cobalt to the environment given that this metal is present in a variety of ores or concentrates being processed. Smelters that produce nickel, specifically, often have ores containing cobalt. In Canada, nickel smelters are located in Sudbury (Ontario) and Thompson (Manitoba).
Cobalt can be further refined by hydrometallurgical and electrolytic processing to increase its purity. There are two cobalt refineries in Canada located in Port Colborne (Ontario) and Fort Saskatchewan (Alberta) (Natural Resources Canada 2009b).
Smelters and refineries may release cobalt to the environment, depending on the materials treated. In 2011, eight smelters and refineries reported to the NPRI total releases of 4.1 tonnes of cobalt, mainly to air (3.8 tonnes), while amounts reported to be disposed of on-site were -58.7 tonnes (Table C-1, Appendix C) (NPRI 1995). Reporting of a negative number for disposal of waste rock indicates that the quantity of a substance removed from the management area exceeded the quantity of the substance deposited in that area for a given year.
Thirteen of the 50 cobalt-containing substances included in the grouping are inorganic UVCBs that are or were generated solely by base metals smelters and refineries (Environment Canada 1988 and 2009) (Table A-1, Appendix A). These substances are intermediates or wastes that likely do not result in direct exposure to the environment (ECCC 2016b).
4.4 Manufacture, import and uses of cobalt-containing substances, products and manufactured items
Table B-1 of Appendix B presents a summary of information received on the quantities of the substances that were manufactured, imported and/or used in Canada for various reporting years. This information was acquired through three surveys issued pursuant to section 71 of CEPA (Canada 2009a, Canada 2009b, Canada 2011b). See appendix B for details about the surveys.
In Table B-1 of Appendix B, the quantities, activities and uses are presented for the cobalt-containing substances. Since each substance has a different molecular weight, the proportion of cobalt varies from one substance to the other. Hence, the substances having the highest quantities in commerce do not necessarily represent the highest quantities of cobalt in commerce.
Most of the highest quantities of substance-containing substances belong to the category of discrete inorganics and a few substances are organic-metal salts. One organic-metal salt UVCB (cobalt naphthenate) and one organometallic (cobalt carbonyl) are also in commerce in relatively high volumes. Three substances are manufactured, imported or used in quantities greater than 1000 tonnes. Twelve substances are manufactured, imported or used in quantities totalling from a few tens to a few hundreds of tonnes. Four substances belonging to the category of inorganic UVCBs generated as residues by base metals smelters and refineries are manufactured in high volumes (greater than 500 tonnes); given their nature, they were included at the bottom of Table B-1 to differentiate them from the discrete substances.
Table B-2 (Appendix B) presents the three activities or uses for which the highest quantities were reported for each substance that was in commerce in 2006, 2008 or 2011. Activities or uses reported for substances having the highest quantities in commerce are intermediates in metallurgical processes; non-ferrous metal smelting and refining; component in alloys and carbides; and batteries. Other activities or uses were reported in quantities in the order of a few tens of tonnes such as incidental production as a by-product; catalyst; rubber; paints and coatings; plastic; and automobile manufacturing. Major uses in Canada are comparable to major uses worldwide. Indeed, globally, the two largest uses of cobalt are in hard materials (such as superalloys, hard-facing and carbides) and batteries; when magnets are included this accounts for approximately 74% of total use (CDI 2011).
The substances that were below the reporting threshold used in the surveys are listed in Table B-3 in Appendix B (22 substances). Since no significant releases to the environment are expected for these substances given their expected low volumes, no further exposure characterization was conducted for these substances in this assessment.
Six other substances listed in Table B-3 of Appendix B were not included in any of the three surveys. Four of them are inorganic UVCB substances generated by smelters and refineries for which it was not deemed necessary to obtain additional information given their nature. This was also the case for one of the two other substances that were not surveyed (frits chemicals). Information on the last substance that was not surveyed (Pigment Blue 36) was obtained through a voluntary data submission.
Additional sources of information indicate that cobalt containing substances are also present in cosmetics (including skin products, hair products including dyes, deodorants (CoRC 2004; 2011 and 2013 emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced)), face paints (Sarantis 2009) and toy makeup (Corazza et al. 2009). In addition they have been found at trace levels in textiles as pigments (KEMI 2013) and children's products including toys and bedding (Uding and Schreder 2013).
Cobalt alloys, used in medical and dental implants, which are subject to pre-market review under the authority of the Food and Drugs Act and the Medical Devices Regulations (Canada 2013), are not assessed in this document. Several cobalt compounds are used in food packaging materials, including use in colourants in the form of colour concentrates (CAS RN 68187-11-1), coatings (CAS RN 1560-69-6), and as a catalyst or additive in manufacturing and modification of plastics, such as polypropylene and polyethylene (CAS RN 13586-84-0), polyester resins (CAS RN 71-48-7) and polyethylene terephthalate, PET, (CAS RNs 13455-36-2/ 71-48-7/ 27253-31-2/ 136-52-7). In addition cobalt compounds are also found as a result of impurities in glass jars and bottles (CAS RN 1307-96-6). Cobalt compounds are used in paints and primers (CAS RN 7440-48-4) and printing inks (CAS RN 136-52-7) with no direct food contact applications (2013 emails from Health Products and Food Branch (HPFB), Health Canada to Existing Substances Risk Assessment Bureau (ESRAB), Health Canada). The Food and Drug Regulations identify cobalt as a mineral nutrient which may be added to food, the Regulations also permit fortification of certain food products with Vitamin B12, which contains sequestered cobalt (i.e the cobalt is not bioavailable).
Cobalt is listed, with hydroxocobalamin, methylcobalamin, sierry clay, and Vitamin B12 (cyanocobalamin) as source ingredients, in the Natural Heath Products Ingredients Database (NHPID) with a medicinal role for use in natural health products (NHPID 2015). Cobalt is listed, with hydroxocobalamin, methylcobalamin, and vitamin B12 as souce ingredients, in the Natural and Non-prescription Health Products Directorate's Multi-Vitamin/Mineral Supplements monograph as medicinal ingredient with a maximum daily dose of 44 mcg/day for children 1 year and older, adolescents and adults. Cobalt and cobalt-containing compounds [i.e., CAS RNs 7440-48-4 (as cobaltum and cobaltum metallicum with minimum homeopathic potencies of 4D and 8D), 7646-79-9 (as cobaltum muriaticum with minimum homeopathic potencies of 4X and 12CH) and 10141-05-6 (as cobaltum nitricum with minimum homeopathic potencies of 6X and 12CH] are also listed in the NHPID with a homeopathic role for use in homeopathic medicines. Cobalt compounds are listed in the Licensed Natural Health Products Database (LNHPD) as being present as medicinal ingredient in currently licensed natural health products (LNHPD 2015). Four cobalt-containing substances (i.e., CAS RNs 7542-09-8; 7646-79-9, 7440-48-4, and 10124-43-3) are identified as an active ingredient in veterinary drugs (DPD 2013). Based on notifications submitted under the Cosmetic Regulations to the Cosmetics Program of Health Canada, categorized cobalt-containing substances (i.e., CAS RNs 7440-48-4, 136-52-7, and 7646-79-9) are used in certain cosmetic products in Canada (2011 and 2013 emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
Various agricultural products contain cobalt compounds; in particular pesticides, livestock feeds and fertilizers have been identified. Six formulants (CAS RNs 136-54-7, 7440-48-4 and 27253-31-2) and 3 trade name formulants have been registered with the Pest Management Regulatory Agency (PMRA) as cobalt containing substances. The uses include material preservative, wood preservative, anti-fouling paint and sanitizer. There are no cobalt-containing active ingredients (2015 email communication from PMRA to Risk Management Bureau, Health Canada, unreferenced). As cobalt is an essential nutrient for livestock and is found in low levels in forage, cobalt compounds are included in livestock feeds at concentrations of 0.1-10 ppm (most commonly at the lower levels) (personal communication, email from Canadian Food Inspection Agency to Risk Management Bureau, Health Canada, dated August 16, 2011, unreferenced). Cobalt is included in the standard for metals in fertilizers and supplements under Trade Memoranda T-4-093, there is a maximum acceptable cumulative metal addition to soil for cobalt of 30 kg/ha which are generally applicable to fertilizers or supplements applied to land or in crop production (CFIA 1997). However, cobalt is generally not considered to be an essential nutrient for plant growth and is only essential for some plant species (Canadian Fertilizer Institute 2013).
Cobalt is contained in electronic and electrical equipment (including batteries). The vast majority of these products used in Canada are manufactured outside of the country (2012 personal communication from Products Division, Environment Canada, to Ecological Assessment Division, Environment Canada; unreferenced). In addition, no releases of cobalt from these items are expected during their service/use life. These items could be a source of cobalt emissions at the end of their life, during their recycling for metals recovery. However, none of the major recyclers of electronic scrap in Canada reported releases of cobalt to the NPRI.
4.5 Incidental manufacture
For the purpose of this assessment, the term "manufacture" also includes the incidental production of cobalt-containing substances at any level or concentration as a result of the manufacturing, processing or other uses of other substances, mixtures, or products. In other words, the unintentional production of a substance as a by-product is considered incidental manufacture. This is the same definition as the one used by Environment Canada's NPRIFootnote3 (NPRI 2013). Sectors that may not be intentionally involved with the manufacture, import or use of one of the 50 substances listed in the grouping but that may incidentally produce cobalt-containing substances as a result of their activities are described below. The source of release for each sector is briefly described while detailed exposure scenarios leading to predicted environmental concentrations are provided later in this report for the sectors with the greatest expected releases of cobalt.
4.5.1 Iron and steel
Cobalt is likely associated in trace levels with the ilmenite ore feeding ilmenite smelters used in the iron and steel sector. Also, iron and steel mills usually burn coal, coke and fuel oil as energy sources for their industrial processes (Natural Resources Canada 2007). Combustion of these fuels may result in atmospheric emissions of cobalt. Cobalt is also contained in low levels in steel slags which are by-products of steel production. These slags can be disposed of or recycled as construction materials depending on their composition and leaching characteristics.
One facility in the iron and steel sector reported releases of cobalt (30 kg to air; no releases to water or land) to the NPRI for 2011; however, this facility does not use the fuels mentioned above. This facility also reported off-site disposal as tailings and off-site recycling (41 and 16 tonnes, respectively). The quantity sent for disposal actually results mainly from the production of titanium dioxide rather than from iron and steel making. Another facility in this sector did not report releases but did report off-site recycling (5 tonnes) (Table C-1, Appendix C).
4.5.2 Electricity (power generation)
Power generation may use fossil fuels such as coal or fuel oil as an energy source. Cobalt is naturally present in these materials. Canadian milled-coals contain 0.99 to 7.8 mg Co/kg (Evans 1985, Goodarzi 2013). As a result of the combustion of coal and fuel oil to produce electricity, a portion of the cobalt that they contain may be released to the atmosphere through stack emissions of flue gas and fly ash, depending on the efficacy of pollution control devices such as bag houses and electrostatic precipitators (Reddy et al. 2005, Goodarzi 2013). Cobalt is also found in bottom ash and boiler slag. Like the residues collected by pollution control devices, bottom ash and slag can be disposed of or recycled (US EPA 2013). Their reuse in construction materials and the potential for the metals that they contain to leach out of these materials is extensively studied (e.g., Siddique 2010).
There were 18 coal-fired and six heavy oil-fired electrical power generation plants in Canada in 2012 (2012 personal communication from Electricity and Combustion Division, Environment Canada, to Ecological Assessment Division, Environment Canada; unreferenced). Three of these facilities reported releases and disposal of cobalt to the NPRI for 2011 (Table C-1, Appendix C). Releases were mainly to air (69 kg). In response to a notice issued pursuant to section 71 of CEPA for the year 2006 (Canada 2009a), three facilities reported releases of cobalt to air (406 kg in total) as well as disposal of 16 300 kg of cobalt as fly ash and bottom ash (Environment Canada 2010a). Two of these facilities also reported releases to the NPRI for 2011. Information was also received from six facilities as part of a voluntary submission; together, these facilities reported releasing about 50 kg and 5 kg of cobalt to air and water, respectively, for 2012. These facilities sent about 40 tonnes of cobalt (as ash) for on-site or off-site disposal or recycling (Environment Canada 2012a).
4.5.3 Petroleum refining
Petroleum refining involves separation processes and techniques such as cracking and coking to convert crude oil into fuels (e.g., gasoline, heavy fuel oil), non-fuel products (e.g., lubricating oils, asphalt) and raw materials for the chemical industry.
The combustion of fossil fuels by petroleum refineries to meet their energy requirements can release cobalt to the atmosphere. Also, crude oil naturally contains metals including cobalt that can be emitted to water during some petroleum refining processes. Water is used within refineries for a number of purposes including cooling, steam generation, and washing products. It can come into direct contact with hydrocarbons or treating chemicals at a refinery (sour water, cooling tower and boiler blowdown) and becomes processed water. The properties of refinery effluents (flowrate, concentration of contaminants present) depend on a number of factors including the refinery configuration, the discharge point and the method of cooling (2013 personal communication from Oil, Gas, and Alternative Energy Division, Environment Canada, to Ecological Assessment Division, Environment Canada; unreferenced).
Petroleum refining processes, such as hydrotreatment to remove sulfur, may require the use of cobalt-containing catalysts such as cobalt-molybdenum oxide. The catalysts are not consumed in the process and spent catalysts are usually recycled. As such, this specific use is not expected to result in environmental releases. There were 19 petroleum refineries in Canada in 2012 (Canadian Fuels Association 2013). Three of these refineries reported to the NPRI no quantities released to the environment, a total of 0.076 tonnes of cobalt disposed of on-site and off-site and 20.1 tonnes of cobalt recycled off-site for 2011 (Table C-1, Appendix C).
4.5.4 Oil sands
Cobalt occurs naturally in the bitumen found in the Athabasca oil sands deposits in northern Alberta. Coking processes are used at industrial facilities located in this area to upgrade the bitumen to produce synthetic crude oil. These processes produce fly ash that closely resemble the fly ash formed from coal combustion in terms of overall bulk composition and physical characteristics (Holloway et al. 2005). Other types of fly ash are also produced when facilities burn fossil fuels such as coke to produce electricity. Hence, cobalt may be released to the atmosphere through stack emissions of flue gas and fly ash.
Cobalt may also be released during oil sands extraction when the hydrocarbon fraction they contain is extracted using a hot water process. A proportion of the metals contained in the bitumen partitions to water during the extraction process. Tailings, including process water, are stored in ponds close to facilities, along with sand, residual oil and clays. The water can also be partially recycled and re-used.
In response to a section 71 notice survey, one facility that processes oil sands reported stack releases of elemental cobalt for year 2006 (180 kg) as part of the fly ash generated during their coking process. The facility also reported the transfer of 660 kg of cobalt to an off-site waste management facility during that same year (Environment Canada 2010a). Of the four facilities that reported to the NPRI in 2011 for the non-conventional oil extraction (oil sands and heavy oil) sector, two reported releases of cobalt (total of 0.021 tonnes; Table C-1, Appendix C). Most of the disposal quantities of cobalt that were reported to the NPRI were for on-site disposal (530 of the total 531 tonnes).
4.5.5 Pulp and paper mills
The main energy sources for the pulp and paper industry are spent pulping liquor and solid wood waste (Statistics Canada 2007). These materials include trace levels of cobalt that may be released in an oxidized form in the particulate matter produced during combustion (Environment Canada 2012a). Pulp and paper mills also use fuels such as natural gas and heavy oil (Statistics Canada 2007). As explained earlier, the combustion of heavy oil may lead to atmospheric emissions of fly ash that could contain cobalt. In response to a s.71 notice survey, nine pulp and paper mills reported total releases of 2134 kg of cobalt (likely oxides) from air stacks as a result of the combustion of biomass, wastes (including ash and sludge) and fuel for the year 2006 (Environment Canada 2010a). Two of these mills also reported total releases of 22 kg of cobalt to water as part of the mill effluent. Another facility reported using elemental cobalt and cobalt sulfate (CAS RN 10124-43-3) as a micronutrient for anaerobic industrial waste water treatment; no releases of cobalt to water were reported by this facility. One pulp mill reported releases of cobalt to the NPRI for 2011 (264 kg to air; Table C-1, Appendix C); other companies may not have met the reporting requirements.
4.6 Disposal and waste management of products, manufactured items and wastes containing cobalt
In terms of recycling, cobalt is recovered from recycled scrap metal in Canada (Environment Canada 2010a). It is also recovered from recycled batteries (Environment Canada 2010b), spent catalysts and electronic scrap.
4.6.1 Disposal
Cobalt contained in products and manufactured items that are disposed of in landfills may leach out of the products and items and could end up in landfill leachate. However, if cobalt is encapsulated or trapped in a very stable matrix, leaching may be very limited or insignificant. In 94% of large landfill sites in Canada (permitted to receive 40 000 tonnes of municipal solid waste annually), leachate is collected and treated on-site and/or off-site (sent to nearby wastewater treatment plant (WWTP)) prior to being released to receiving water. However, leachate is most likely not treated in smaller landfills (Conestoga-Rovers and Associates 2009). At these sites, cobalt may potentially be released to ground or surface water via leachate.
4.6.2 Incineration
In Canada, 3% of wastes are incinerated; municipal solid waste incinerators have been shown to be a source of cobalt to the atmosphere (ATSDR 2004). Cobalt is also present in fly ash and bottom ash produced by incinerators. Air pollution control (APC) residues also contain cobalt; these residues are usually managed as hazardous wastes.
4.6.3 Waste management
Facilities that specialize in waste treatment reported to the NPRI for 2011 on-site and off-site disposal of 19.9 and 7.9 tonnes of cobalt (mostly to landfills), respectively, as well as 17.7 tonnes for off-site recycling (recovery of metals) (Table C-1, Appendix C). They did not report any releases to air, water or land.
4.6.4 Wastewater and biosolids
In general, wastewater (sewage) is a common point of entry of a substance to water and a potential point of entry to soil through the subsequent management of biosolids. One publicly owned WWTP reported releases of 14 tonnes of cobalt to water to the NPRI for 2011. Concentrations of cobalt measured in wastewater influent, effluent and biosolids for certain WWTPs in Canada are provided in the Ecological Exposure Assessment section of this report.
5. Environmental fate
Cobalt originating from natural or anthropogenic sources may have various forms in ambient air, surface water, sediments, soils and groundwater.
A metal ion is considered infinitely persistent because it cannot degrade any further, though it can transform into different chemical species and/or partition among different phases within an environmental medium. In other words, cobalt and cobalt ions will always be present in the environment; it is the form under which they are found that will determine their bioavailability and potential to be harmful to life. Biodegradation and photodegradation are not applicable to the inorganic metal-containing substances or to the inorganic cobalt released upon dissolution, dissociation or degradation. These processes can, however, be applicable to the organic metal salts and organometallics. The persistence of the parent organic metal salts and organometallics and their possible organic counter-ions or organic transformation products is not evaluated individually in the present assessment. However, the dissolution, dissociation and degradation capabilities (e.g., half-lives) of the parent organic metal salts and organometallics may be evaluated or estimated to determine the extent or potential for inorganic cobalt release.
In terms of partitioning, the fate of dissolved cobalt ions may in part be generally characterized by partition coefficients--namely soil-water (Ksw), suspended particles-water (Kspw) and sediment-water (Ksdw) partition coefficients (ECCC 2016a). Since cobalt tends to sorb to solid particles in aquatic media (median log Kspw = 5.33), a significant proportion of dissolved forms of this metal will end up in sediments through adsorption to settling suspended particles (Hamilton-Taylor and Willis 1984). Cobalt should then stay mostly in this compartment (median log Ksdw = 3.20) unless sediments become resuspended through bioturbation, dredging, seasonal floods or mixing by turnover events. In addition, cobalt may be remobilised to water following certain physical and chemical properties changes (e.g., pH, Eh). Thus, partition coefficients are dependent upon particular system conditions. Elemental cobalt and sparingly soluble compounds (e.g., cobalt oxide) that are released to surface water are not expected to be found in significant amounts in the water column, especially if their density is greater than that of water. A portion of these compounds may be found in sediments, or in soil if released to this compartment, in a non-dissolved, solid form.
5.1 Air
Being a non-gaseous element with a negligible vapour pressure, cobalt is emitted to air principally in the form of fine particulate matter (PM). Depending on the size of the PM with which cobalt is associated, it will travel for a certain distance in air before being deposited to aquatic or terrestrial environments. Particle size distributions of atmospheric aerosols in England showed that the majority of cobalt in relatively unpolluted areas is found in the 2 to 10 μm size fraction and that concentrations are greater in urban areas than in rural sites (Eleftheriadis and Colbeck 2001).
Long-Range Transport Potential (LRTP) was not quantified in this screening assessment as cobalt-containing substances or incidental releases are not expected to travel over very long distances and contribute significantly to environmental concentration in remote areas (e.g., arctic). As well, the environmental concentrations (in water, soil or sediments) near the major sources of releases were considered and included any cobalt deposited from air releases (see the Ecological Exposure Assessment section).
5.2 Freshwater
Data presented in ECCC (2016a) show that the water solubility of cobalt-containing substances ranges widely, from sparingly soluble to greater than 106 mg/L. Thus, if released to water bodies, some substances will release more cobalt ions than others upon dissolution or dissociation. Under typical pH and Eh (oxido-reduction potential) conditions, oxidation state (II) for cobalt is more stable than oxidation state (III) (Cotton and Wilkinson 1988), although under conditions of high pH and Eh, Co (III) may be more thermodynamically stable (Lee and Tebo 1994). Under conditions commonly found in oxic freshwaters (i.e., pH between 5 and 9; Eh between 0.5 and 1 V), Co2+, CoCO30, and CoHCO3+ will be the dominant inorganic species in solution (Brookins 1988; Takeno 2005). This result can be partly explained by Smith and Martell (2004) who have demonstrated high stability for the complex CoHCO3+ with a thermodynamic stability constant, log Kf of 12.9 when studying inorganic complexation of cobalt in solution at a temperature of 25°C and ionic strength (I) of 0 mole/L.
Cobalt is expected to be more mobile under oxidizing conditions than under reducing conditions (Garrett 2005), where it is mainly associated with the solid phase including particulates. In addition, environmental mobility will be higher under acidic conditions than under neutral to alkaline conditions (Reimann and de Caritat 1998; Garrett 2005) because cobalt is mostly present in the dissolved phase.
Interactions between metals and natural organic matter is a topic of interest linked particularly to the fate and bioavailability of cationic metals in aquatic systems. Over the years, a variety of physical and chemical techniques have been used for investigating complexes of cobalt with natural organic ligands in waters of different compositions. Conditional stability constants (log Kf) determined by some of these studies varied between 2.45 and 11.6 depending on the nature of the organic ligand and chemical composition of water (Lee and Joansson 1983; Ephraim et al. 1989; Pham and Garnier 1998; Kurk and Choppin 2000; Pandey et al. 2000; Hamilton-Taylor et al. 2002; Prado and Airoldi 2003; Qian et al. 1998; Alvarez-Puebla et al. 2004).
Given the great influence of chemical speciation on metal bioavailability in aquatic systems, the speciation of cobalt in natural water bodies was determined. The Windermere Humic Aqueous Model, version VI (WHAM VI: Tipping 2002) was used to model chemical speciation in Canadian water bodies of various physico-chemical characteristics. Modeled data indicate that the importance of inorganic cobalt complexation increases with pH and water hardness. Similarly, the proportion of cobalt bound to organic matter such as humic acids and fulvic acids generally increases with the concentration of dissolved organic carbon (DOC). Additional information on cobalt speciation is available in ECCC (2016a).
5.3 Sediments
It is known that sediments act as sinks for trace metals in aquatic systems (Förstner and Wittmann 1981). The suspended particulate flux in surface waters acts as a "conveyer-belt" mechanism whereby metals are "scavenged", being adsorbed by or incorporated into particles generated in situ or of allochthonous origin. In turn, these particles fall through the water column and eventually settle to bottom sediments (Santschi 1984). Consistent with Santschi's findings, an in situ experiment with cobalt-57 showed that about 80% of the radioisotope was transferred from the water column to bottom sediments 20 days after its initial introduction in a lake enclosure open to surface sediments (Diamond et al. 1990).
Once in sediments, and similarly for most trace metals, cobalt may be found in a variety of fractions in this compartment: dissolved in pore water; present in exchangeable fractions of clays, hydrated oxides of iron and manganese and humic acids; bound to carbonates; bound to iron and manganese oxides, bound to particulate organic matter; complexed with sulphides including acid volatile forms, and in the crystal lattice of primary and secondary minerals (Tessier et al. 1979; Förstner and Wittmann 1981; Di Toro et al. 1992).
5.4 Soils
Similar to sediments, soils are major sinks for metals released to air from natural and anthropogenic sources. After entry of metal compounds into soils, transformation processes will involve dissolution, partitioning, leaching and ageing. The latter designates reactions transferring metals from labile pools to relatively insoluble pools (Smolders et al. 2007). In general, metal bioavailability is governed by the mobility and solubility of different geochemical forms (Smolders et al. 2007). The behaviour of cobalt in soils is linked to chemical and physical properties of both the soil (e.g., pH, soil organic matter) and the cobalt-containing compound (e.g., water solubility) entering this compartment. These factors are further described in both the bioavailability and effects to terrestrial organisms sections below.
6. Potential for bioaccumulation
Bioaccumulation of metals--like that of organic substances--is of potential concern because of the possibility of reaching internal body concentrations that can cause harm to the organisms accumulating these substances in their tissues and/or to the predators that eat these organisms. The step immediately preceding the accumulation of metals in organisms is the uptake process, which depends on the forms of the metals that are actually bioavailable. Bioavailability and the uptake process are therefore discussed below as part of the bioaccumulation section, with a focus on the cobalt moiety. No attempt was made to assess the bioavailability and/or bioaccumulation of the organic counter ion or organic component of the organic cobalt salts and the organometallics included in the grouping.
The studies that investigate the bioavailability and bioaccumulation of cobalt are conducted with a variety of soluble cobalt-containing substances. All soluble cobalt-containing substances are expected to generate dissolved cobalt species that should behave similarly in a given environmental medium, depending on the physical and chemical conditions prevailing in this medium as well as on its composition.
6.1 Bioavailability
6.1.1 Water
The Biotic Ligand Model (BLM) was developed to predict metal biouptake in recognition that the bioavailability and bioreactivity of metals control their potential to cause adverse effects in organisms. Basically, the BLM incorporates the competition between the free metal ion and other naturally occurring cations (e.g., major cations and H+), together with the complexation by abiotic ligands (e.g., dissolved organic matter, chloride, carbonates, sulfate) for binding with the biotic ligand which is assumed to represent exposure at the site of toxic action for the organism (Paquin et al. 2002). In fact, it is well documented that the toxicity of metals depends on the pH and ionic strength of the external media (Parametrix 2010a; Di Toro et al. 2001). For cobalt, toxicity studies conducted to date suggest that increased water hardness reduces acute cobalt toxicity to aquatic organisms (Borgmann et al. 2005; Parametrix 2010b; Parametrix 2010c; Diamond et al. 1992; Rathore and Khangarot 2003), likely because of the existence of competitive interactions between Co2+ and hardness cations for binding with the biotic ligand which reduces bioavailability. While pH is also known to have an effect on cationic metal bioavailability, its influence on cobalt is deemed to be relatively limited and experimental results were contradictory (Parametrix 2010b; Parametrix 2010c; Khangarot et al. 2003; Macfie et al. 1994; Nautilus Environmental 2009).
Dissolved organic matter (DOM) is typically considered to reduce toxicity of metals by decreasing free metal ion concentration and thus decreasing metal bioavailability. Nonetheless, in his review, Campbell (1995) noted that quantitative studies on the subject are more or less evenly divided between examples of reduced and enhanced toxicity in the presence of DOM. The author suggested that it is imprudent to treat natural DOM as a simple hydrophilic ligand because this colloidal fraction is multifunctional and its role is not limited to complexing metals in the bulk solution.
Among pH, DOC and water hardness, the latter is the most influencial toxicity-modifying factor on cobalt uptake and accounts for approximately 85% of the variability in the response of freshwater organisms tested (Parametrix 2010b; Parametrix 2010c; 2013 personal communication from William Stubblefield, from Oregon State University to Ecological Assessment Division, Environment Canada; unreferenced).
6.1.2 Sediments
Cobalt may be found in a variety of fractions in sediments. Depending on the fraction, cobalt will be either weakly bound (e.g., to exchangeable fractions of clays) or strongly bound (e.g., complexed with sulphides). The bioavailability of cobalt to benthic organisms is controlled by various key factors including organic carbon, sulphides and clay concentrations which in turn control the proportions of the fractions in which cobalt is present in sediments as well as in the overlying water that can be brought in sediments by organisms activities (e.g., filtration-feeding, burrowing). For instance, cobalt dissolved in pore water or in the overlying water would likely be more bioavailable to organisms than cobalt bound to manganese oxides. No studies on the bioavailability of cobalt to benthic organisms could be found in the literature to further explore these interactions.
6.1.3 Soil
The development of a BLM for terrestrial organisms is conceivable for modelling biouptake and toxicity of cobalt in soil pore water. Efforts have been made in that direction in studies examining the effects of cobalt on the root growth of barley (Hordeum vulgare) in nutrient solution, and on the survival of the potworm Enchytraeus albidus exposed to nutrient solution added to acid washed and pre-combusted sand (Lock et al. 2006, 2007). The exposure media in these experiments were very well chemically defined but too simplistic to simulate the uptake and toxicity of cobalt to plants and earthworms in real soils, a caveat acknowledged by the authors. For example, uptake of cobalt by the worms by ingestion of soil particulate matter was not considered. These studies found that ions such as Ca2+, Mg2+, K+ or H+ increased LC50 values expressed as concentrations of freely dissolved cobalt ions, up to several times, for both test species. These results were explained by the existence of competitive interactions between these major ions and Co2+ for binding sites at the organism-water interface, the overall effect being a decreased toxicity of the free cobalt ion (Lock et al. 2007). Dissolved organic matter was not a variable tested in these experiments.
Using another approach, many soil toxicity tests were conducted in support of the regulatory assessment of nickel conducted by the European Union (EURAR 2008). Soils covering large ranges of pH and cation exchange capacities (CEC) were used. An empirical linear regression model was developed from this dataset of experimental toxicity values, having the general format:
Toxicity value = a + b × pH + c × CEC
An ageing factor was then applied to the predicted toxicity value, this factor being derived from the duration of ageing, soil pH and CEC (Vangheluwe et al. 2007). Smolders et al. (2009) demonstrated that this approach for evaluating the toxicity of nickel in soils can also be applied to cobalt. Further details specific to cobalt soil toxicity are provided in the Ecological Effects section of this report (terrestrial organisms).
6.2 Bioaccumulation
Cobalt is essential in small amounts for nitrogen fixation by bacteria, blue-green algae, and symbiotic systems such as those in the root of leguminous plants (IPCS 2006). It is also an essential micro-nutrient for animals and is required for the formation of vitamin B12 and for its participation in enzymatic processes (Adam et al. 2001; Gál et al. 2008; Mathews et al. 2008; Metian et al. 2009). Hence, cobalt will naturally be taken up and to some extent may be accumulated by certain species of organisms.
6.2.1 Water
Bioaccumulation potential is typically quantified by determining either a bioaccumulation factor (BAF) or a bioconcentration factor (BCF). However, these ratios are often criticized when applied to metals because they are considered of little usefulness in predicting metal hazards (Schlekat et al. 2007). For example, some metals may naturally be highly accumulated from the surrounding medium because of their nutritional essentiality (e.g., Ca; K). Furthermore, both essential and non-essential metals may be regulated within relatively narrow margins by the homeostatic and detoxification mechanisms that many organisms possess. It follows that when ambient concentrations of metals are low, BCFs and BAFs are often elevated. Conversely, when ambient metal concentrations are high, BCFs and BAFs tend to decrease (McGeer et al. 2003; DeForest et al. 2007). Thus, inverse relationships may be observed between BCF and BAF values and metal exposure concentrations, and this complicates the interpretation of BCF/BAF values. Natural background concentrations in organisms may contribute to these negative trends (e.g., Borgmann and Norwood 1995). In addition, inverse relationships can occur for non-essential elements as well because there are a finite number of binding sites for the transport of these metals on the organism (e.g., gill) that could become saturated at higher concentrations (e.g., Borgmann et al. 2004, MacLean et al. 1996).
To take into account these complicating factors, a mechanistically-based saturation model for the bioaccumulation of metals using the freshwater amphipod Hyalella azteca as a test organism has been developed (Borgmann et al. 2004; Norwood et al. 2007). This model can estimate a BCF based on background-corrected metal accumulation at low aqueous concentration, which avoids the above-mentioned concentration dependence. In addition, these authors have shown that (i) lethality occurs when tissue concentrations surpass a critical body concentration (CBC) and that (ii) CBCs appear relatively constant for a variety of different non-essential or marginally essential metals in spite of large differences in the waterborne concentrations that result in chronic toxicity (e.g., Schlekat et al. 2007). It can be deduced from these two points that when the uptake of a given metal is more efficient, the chronic toxicity threshold in tissue is reached at a lower water concentration. Consistent with this statement, these researchers have observed a strong negative relationship between estimates of chronic toxicity and BCF/BAF values for non- or marginally essential metals and metalloids (in laboratory: Norwood et al. 2007; Schlekat et al. 2007; in field settings: Couillard et al. 2008). This relationship holds because total metal body concentration in Hyalella is likely related to the concentration of the metal at the site of toxic action. In principle, animals with metal handling strategies not including important pools of metals stored in detoxified forms, may show close relationships between bioaccumulation ratios (BAFs and BCFs) and chronic toxicity (Couillard et al. 2008).
The selection of studies for assessing the bioaccumulation potential of cobalt builds on the above knowledge and on accepted methodologies for deriving BCFs and BAFs (OECD 1993; OECD 1996; Arnot and Gobas 2006). ECCC 2016a summarizes criteria and considerations used for BCF and BAF data quality assessment. In recognition that these ratios are less meaningful for organisms with large and inert metal-rich compartments, studies with such metal accumulators have been left aside. When information was available, only metal concentrations in soft tissues were considered for invertebrates with shells or important exoskeletons.
To characterize the bioaccumulation and biomagnification potential of cobalt, 38 studies were considered; 20 of these were considered appropriate to provide the data for this bioaccumulation assessment. A complete summary of all bioaccumulation data used is provided in ECCC (2016a). The data presented are for cobalt as an element and not for individual substances because, as explained in previous sections of this report, these substances will dissolve in water and will release cobalt ions. These ions are considered potentially bioavailable (mainly Co2+) and can be taken up by organisms.
Considering all aquatic data, 31 acceptable bioaccumulation factors were reported for various species of algae, invertebrates, fish, and zooplankton for marine and fresh water. These values ranged from 7.4 to 3110 L/kg, with a mean value of 878 L/kg (95% CI 611-1146) and a median value of 720 L/kg. No groups of organisms seemed to have higher BCF/BAF than others. Four biota-to-sediment accumulation factors (BSAF-sed.) were considered. BSAF-sed values ranged from 0.091 to 0.645, with a mean value of 0.232 (95% CI 0.024-0.441) and a median value of 0.138.
6.2.2 Soil
In terrestrial environments, four acceptable biota-to-soil accumulation factors (BSAF-soil) were identified for only two species, bay bolete (Xerocomus badius)and white mulberry (Morus alba). Values ranged from 0.007 to 0.81. One soil study considered the cobalt concentration in a soil solution, thus providing bioaccumulation factors. The BAF values obtained for the three species tested in this study ranged from 0.100 to 0.146, wet-weight (Li et al. 2009).
6.3 Biomagnification
Biomagnification Factor (BMF) values describe the process in which the concentration of a chemical in an organism reaches a level that is higher than that in the organism's diet, due to dietary absorption (Gobas and Morrison 2000). A BMF exceeding 1 indicates that biomagnification is potentially occurring.
Five BMFs were found in the literature for cobalt for four fish species and zooplankton in marine and freshwater environments. Values ranged from 0.004 to 0.087 with a mean value of 0.026 and a median value of 0.01. In addition, one study, not reporting specific BMF values for cobalt, estimated that they were less than 1 for the three fish species studied (Mathews et al. 2008). Details on studies consulted and results are available in ECCC (2016a).
A number of studies also attempted to quantify the trophic magnification factor of cobalt (TMF). The TMF is a measure of the biomagnification potential of a substance within a studied food chain under field conditions. The TMF value depends on the correlation between trace element concentrations and nitrogen isotopes (δ15N), measured in an array of members of a given food web (Nfon et al. 2009), and has the simple equation:
TMF = 10B,
where B is the slope of the regression of the log [trace element concentration (μg/g wet weight)] against δ15N (Nfon et al. 2009). As the δ15N count increases predictably with each trophic level in a food chain, a significant correlation between these two variables can be indicative of the potential for biomagnification (if TMF greater than 1) or biodilution (if TMF less than 1) (Nfon et al. 2009).
Table 6-1 summarizes all data considered for assessing trophic magnification factors for cobalt. All values are expressed on a wet-weight basis. In these studies, there was generally no statistically significant relationship between cobalt concentration and nitrogen isotopes in food webs. These results lead to the conclusion that cobalt does not present a risk for biomagnification.
| Food web | Study type | Study type | TMF value | Reference |
|---|---|---|---|---|
| Marine pelagic food chain (Baltic Sea) | Field | Su | 1.1a | Nfon et al. 2009 |
| Marine pelagic Arctic food chain (Baffin Bay) | Field | Su | 0.93b | Campbell et al. 2005 |
| Freshwater food chain (Mekong Delta) | Field | Su | 0.95 | Ikemoto et al. 2008 |
| Marine pelagic and benthic food chain (East China Sea) | Field | Su | 0.71; 1.45c |
Asante et al. 2008 |
Su - Field survey of organism, water, sediment, etc.; TMF: Trophic magnification factor.
a. Correlation between trophic level and Cobalt concentration demonstrated, but with no statistical significance.
b. Study reported that no relationship was demonstrated for cobalt in this food web, due to the small change in cobalt concentration at each trophic level and due to highly variable data. The TMF value is calculated as 10B, where B is the slope of the log [Co] vs. nitrogen isotope (δ15N) regression (Nfon et al. 2009). In this case, B was reported to be -0.03, with statistical significance (p less than 0.05). From the equation provided by Nfon et al. (2009), this corresponds to a TMF value of 0.93. The r2 value for this regression was 0.04.
c. Study reported contradictory results: a positive and a negative relationship were observed between cobalt concentration in the organism and trophic level. The TMFs were calculated according to Nfon et al. 2009. B was reported to be 0.16, with statistical significance (p less than 0.01, r2 = 0.15) for deep-water fish whereas B = -0.15 for all deep-water species (p less than 0.05, r2 = 0.50). The corresponding TMF values were respectively 1.45 and 0.71.
6.4 Potential for bioaccumulation summary
To summarize, there are several lines of evidence to suggest that the bioaccumulation potential of cobalt in natural ecosystems is relatively low. While certain toxicity modifying factors may affect its uptake, cobalt is an essential micronutrient and as such, certain forms are readily bioavailable to organisms. Nevertheless, low BAFs have been reported in eight laboratory (steady state) studies and four field studies; five BSAF-sediment values have been found to be low (less than 1); and, four average BSAF-soil values have been reported to be low (less than 1). In addition, results from six field investigations plus two laboratory studies indicate the absence of biomagnification of cobalt in natural food webs. Finally, cobalt is an essential micro-nutrient which is expected to be regulated to some extent by many organisms. It should to be noted that cobalt uptake could still lead to internal levels causing harm to sensitive species (e.g., Hyalella azteca) at body concentrations higher than required for essentiality (Norwood et al. 2007).
7. Ecological effects
7.1 Aquatic organisms
7.1.1 Freshwater
There is experimental evidence that cobalt causes harm to aquatic freshwater organisms following short-term (acute) and longer-term (chronic) exposure at very low concentrations. Many empirical data are available in the literature for acute and chronic toxicity of cobalt chloride, cobalt sulfate and other soluble cobalt compounds. Since these compounds all lead to the formation of potentially bioavailable dissolved cobalt species, in particular the free ion Co2+, all data from reliable chronic studies that were conducted with such compounds were considered in order to derive a critical toxicity value (CTV). The CTV is a quantitative expression of a low toxic effect (e.g., IC25) that relates to the most sensitive toxicity endpoint for receptor organisms in the medium of interest. Robust Study Summaries (RSS) were completed for all studies from which the toxicity data were used to derive a CTV. These RSS are available upon request.
Reliable acute studies were identified for 15 species including 12 invertebrate species and three fish species. Toxicity values range from 16 to 585 800 μg/L as dissolved cobalt (Nautilus Environmental 2009). Individual data for these studies are neither included nor further discussed in this assessment since they are not used to derive a CTV.
Chronic data are of greater relevance than acute data in this assessment because they are a more sensitive indicator of potential for harm from long-term exposures, which are most likely to occur. Releases to the environment from sectors considered in this assessment are on-going or chronic. Acute toxicity scenarios are typically used for intermittent releases or spills which were not needed or considered in this assessment. Tables D-1, D-2 and D-3 (Appendix D) summarize reliable chronic aquatic toxicity data for cobalt to freshwater organisms. Thirteen species were identified including 3 fish species, 6 invertebrate species and 4 plant/algae species. Toxicity values affecting survival growth or reproduction, range from 348 to 2171 μg/L for fish, from 0.76 to 167 μg/L for invertebrates and from 4.9 to 1120 μg/L for plants and algae. It should be noted that concentrations in the studies cited are expressed in micrograms of cobalt per litre (μg Co/L). Therefore, the CTV derived from these data is for dissolved cobalt rather than for the compounds tested (e.g., CoCl2). According to these studies, invertebrates is the most sensitive taxonomic group to cobalt followed by algae/plants and fish. The biological diversity and the stability of the food chain may be adversely affected by cobalt. For instance, considering many fish species feed on invertebrates, reductions in the quality and the quantity of this food source may have negative indirect impacts on fish (e.g., reduced growth) even at lower cobalt concentrations than where direct chronic effects are known to occur (see Tables D-1 and D-2). Some studies showed that these indirect effects of metal exposure to fish are indeed possible and can occur (Rasmussen et al. 2008; Sherwood et al. 2002).
7.1.2 Deriving predicted no-effect concentration (PNEC) using hardness as a modifying factor
As indicated in the Bioavailability section of this report, hardness appears to be the key toxicity modifying factor for cobalt among the various water quality parameters that could potentially influence metal uptake and toxicity. To quantitatively assess the influence of hardness on cobalt toxicity, log-log or ln-ln linear regressions can be used to characterise hardness-toxicity relationships (Stephan et al. 1985; US EPA 1987; Zajdlik and associates 2009). The standard methods used here to examine such relationships are well described by Stephan et al. (1985). Toxicity values for different species are plotted against all the water hardness values used in the bioassays to get a regression line for each species. Then, an analysis of covariance is performed to examine statistical differences between the species slopes, and, where none exists, to produce a pooled slope giving an all-species estimate of the relationship between hardness and toxicity.
Reliable data from two species were available to assess this relationship for cobalt in the present assessment. Ceriodaphnia dubia neonates were exposed for 7 days in nine water calcium concentrations with increasing levels of water hardness from 52 to 396 mg/L while keeping all other factors constant (Parametrix 2010b). The effect of cobalt on reproduction was assessed and toxicity values (EC20s) calculated. The regression line slope was 0.28 and its R2 coefficient was 0.50. Larval Fathead minnow (Pimephales promelas) were exposed for 7 days in four water calcium concentrations corresponding to increases in water hardness from 52 to 356 mg/L while also keeping all other factors constant (Parametrix 2010c). The effect of cobalt on growth was assessed and EC20s calculated. The regression line slope was 0.69 and its R2 coefficient was 0.83. The covariance analysis showed there was no difference between the two slopes (p greater than 0.05), therefore they have been pooled. The pooled slope value is 0.41 with a R2 coefficient of 0.98 and is significantly different from zero (p less than 0.05). These analyses were performed using SYSTAT 13 (SYSTAT 2013).
To best compare cobalt toxicity data from the different studies presented in Tables D-1, D-2 and D-3 (Appendix D), original values were standardized to a common hardness, using a statistically significant pooled hardness-toxicity slope that has been derived (0.41). To minimize interpolation corrections, the hardness chosen (100 mg/L as CaCO3) was close to the central tendency of the distribution of original hardness values. The original hardness values that were below 52 mg/L, were corrected to 52 mg/L instead of 100 mg/L, again to minimize the extrapolation correction that is outside the slope range for which the pooled slope was calculated. The corrected values are presented between brackets in Tables D-1, D-2 and D-3 (Appendix D).
Toxicity data used as input in a species sensitivity distribution (SSD) may be normalized for the effects of pH, ionic strength and hardness, and dissolved organic carbon depending on assessment needs (Vangheluwe et al. 2007). As such, a SSD was developed using the chronic toxicity data corrected for hardness (52 and 100 mg/L as CaCO3) shown in Tables D-1, D-2 and D-3 (Appendix D), for a total of 13 species: three fish, six invertebrates and four aquatic plant/algae species (Figure 7-1). When more than one value for an endpoint was available for a single species, the value to be used in the SSD was chosen following guidance from the Canadian Council of Ministers of the Environment (CCME 2007). For instance, when test conditions and parameters are similar among studies (e.g., endpoint, duration, pH, hardness, etc.), the geometric mean of the toxicity values may be calculated and used in the SSD. Otherwise, the lowest toxicity value for a given species is selected. While a geometric mean was calculated for a set of Hyalella azteca data, the lowest value was selected in all other cases for inclusion in the SSD (Appendix D).
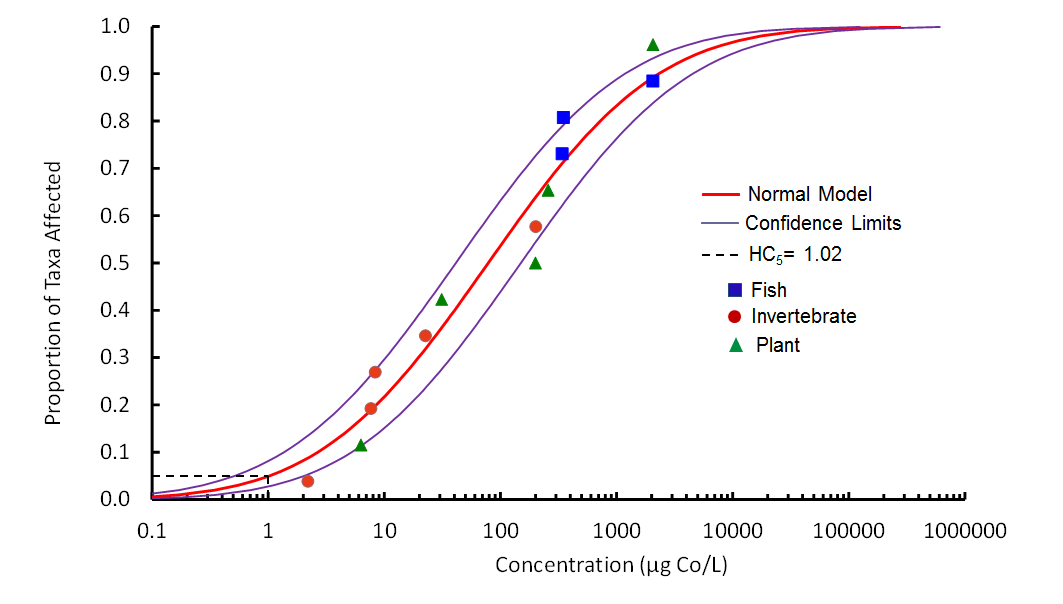
Long description for figure 7-1
Species sensitivity distribution (SSD) for dissolved cobalt based on selected chronic toxicity data for freshwater aquatic organisms. The Normal model fit to the data is shown on the graph along with the 95% confidence intervals. The data used in the SSD are identified in Appendix 4. This figure shows that the sensitivity of organisms to dissolved cobalt is distributed following an S-shaped curve. Of the 14 data points, 3 points fall outside the 95% confidence intervals of the curve fit. They are located at both extremities of the fit and in the middle.
The software SSD Master v3.0 (SSD Master 2010) was used to plot the SSD shown in Figure 7-1. Several cumulative distribution functions (CDFs) (Normal, Logistic, Extreme Value, and Gumbell) were fit to the data using regression methods. Model fit was assessed using statistical and graphical techniques. The best model was selected based on consideration of goodness-of-fit and model feasibility. Model assumptions were verified graphically and with statistical tests. The Normal model provided the best fit of the models tested upon visual inspection, lowest levels of statistical variability (residuals), even distribution of the residuals, lowest normalized confidence interval spread and best significance of the Anderson-Darling Statistic test (A2) = 0.384 (p less than 0.05). The 5th percentile (HC5), i.e., hazardous concentration to 5% of species, of the SSD plot is 1.02 μg/L with lower and upper confidence limits of 0.51 and 2.03 μg/L, respectively.
The HC5 of 1.02 μg/L calculated from the SSD is selected as the CTV for aquatic freshwater organisms at a hardness of 100 mg/L. The value is not likely below essential requirements considering that it is well above the 50th percentile of concentrations of minimally impacted areas that range from 0.04-0.16 μg/L (see Table 8-1, in section 8.1). In addition, since this value is based on a chronic SSD that covers multiple species and taxa, an assessment factor was not used to derive the predicted no-effect concentration (PNEC) for freshwater organisms. Hence, the PNEC is equivalent to the CTV. Because water hardness protects freshwater aquatic organisms from cobalt toxicity, it is preferable to have a PNEC value that can be adjusted on a site-specific basis depending on the hardness of the water at the location of interest. The long-term PNEC equation is based on the chronic or long-term toxicity-hardness relationship slope value of 0.414 that was calculated above. This slope represents the relationship between the natural logarithm of cobalt concentration (y-axis) and the natural logarithm of water hardness (x-axis). Because the slope of this line is known (0.41), as well as the x,y co-ordinates of one point on this line (ln(100), ln(1.02)), we can determine the general equation describing this line by solving for the y-intercept. If the equation ln(y) = m×ln(x) + b is rearranged to solve for b (i.e., the y-intercept), the following result is obtained:
y-intercept (b) = ln (5th percentile) - [slope × ln(hardness)]
= ln (1.02) - [0.414 × ln(100)]
= -1.887
Therefore, the resulting hardness-dependent equation to derive the long-term or chronic PNEC for toxicity to freshwater organisms is
PNEC = e{0.414[ln(hardness)] - 1.887}
where the PNEC is in μg/L and hardness is measured as CaCO3 equivalents in mg/L. The PNEC should not be extrapolated outside the range of hardness for which the slope was developed (52-396 mg/L). Therefore, based on the equation, the minimum PNEC is 0.78 μg/L and the maximum is 1.80 μg/L for hardness levels of 52 and 396 mg/L, respectively. This PNEC range is lower than the non-adjusted PNEC (2.5 μg/L) previously determined in the screening assessment of elemental cobalt, cobalt chloride and two cobalt sulphate substances conducted under the Challenge Initiative of the CMP (Environment Canada, Health Canada 2011a) and in the Canadian Federal Environmental Quality Guideline for Cobalt (Environment Canada 2013a). Due to advancements in the SSD Master software, a new model has been selected as the best fitting (Normal model instead of the Weibull model) to derive the HC5 and results in a significantly lower HC5 value. In addition, the consideration of hardness as a modifying factor further increases the difference at low hardness.
7.1.3 Other factors of less potential influence
The pH of the water used in the toxicity tests ranged from 6.5 to 8.5; Table D-1, D-2 and D-3 (Appendix D), which is very similar to representative Canadian waters (6.4 to 8.6; data not shown, ECCC 2016a). In addition, this factor has a low influence on cobalt effects to freshwater organisms as stated in the bioavailability section above. Also mentioned in this section, studies on the influence of DOM on toxicity show mixed and inconclusive results for cobalt. For these reasons, these two modifying factors were not integrated in correction equations for this assessment.
7.2 Benthic organisms
7.2.1 Freshwater
There is limited experimental evidence that cobalt causes harm to freshwater benthic (sediment-dwelling) organisms following long-term (chronic) exposure at relatively low concentrations. Only two studies were found in the literature for chronic toxicity of cobalt (using cobalt chloride) and no data were found for short-term (acute) toxicity. The focus is on chronic toxicity since exposure to cobalt in sediments is expected to be long term. Robust Study Summaries (RSS) were completed for these studies from which the toxicity data were used to derive a CTV. These RSS are available upon request.
Reliable chronic studies were identified for six invertebrate species. Hyallela azteca, Ephoron virgo, Chironomus riparius, Gammarus pulex, Tubifex tubifex and Lumbriculus variegatus were exposed to cobalt applied to the sediment phase in a laboratory sediment-water system for 28 days (Nguyen et al., 2009a; Nguyen et al., 2009b). Natural uncontaminated sediments were spiked with cobalt concentrations ranging from 32 to 5600 mg/kg dry sediment and were equilibrated for 35 days before the initiation of the tests. Cobalt chloride was used as it is a highly soluble cobalt compound that leads to the presence of bioavailable dissolved cobalt species in sediment pore water, in particular the free ion Co2+. The effects of cobalt on growth, emergence, survival or reproduction were assessed and toxicity values (EC10s) calculated. The values ranged from 86 to greater than 2170 mg of total Co/kg sediment dw and are summarized in Table D-4 (Appendix D).
A SSD was developed using the chronic toxicity data shown in Table D-4 (Appendix D) for a total of six invertebrate species (Figure 7-2). It is documented that the toxicity of metals in sediments depends on many factors (e.g., presence of sulfides, pH) as mentioned in the bioavailability section. As a result, toxicity data used as input in a SSD may be normalized for some of these factors depending on assessment needs. However, this was not done for this SSD as all the bioassays were performed under the same conditions and using the same sediments, thus offering the same bioavailability characteristics.
Long description for figure 7-2
Species sensitivity distribution (SSD) for cobalt based on selected chronic toxicity data for freshwater benthic organisms. The Gumbel model fit to the data is shown on the graph along with the 95% confidence intervals. The data used in the SSD are identified in Appendix 4. This figure shows that the sensitivity of organisms to cobalt is distributed following an S-shaped curve. All of the 6 data points fit inside the 95% confidence intervals of the curve fit.
The software SSD Master v3.0 (SSD Master 2010) was used to plot the SSD shown in Figure 7-2. Several cumulative distribution functions (CDFs) (Normal, Logistic, Extreme Value and Gumbell) were fit to the data using regression methods. Model fit was assessed using statistical and graphical techniques. The best model was selected based on consideration of goodness-of-fit and model feasibility. Model assumptions were verified graphically and with statistical tests. The Gumbell model provided the best fit of the models tested upon visual inspection, lowest level of statistical variability (residuals), even distribution of the residuals, lowest normalized confidence interval spread and best significance of the Anderson-Darling Statistic test (A2) = 0.284 (p less than 0.05). The 5th percentile (HC5), i.e., hazardous concentration to 5% of species, of the SSD plot is 50.1 mg Co/kg with lower and upper confidence limits of 22.2 and 113.4 mg Co/kg, respectively.
The HC5 of 50.1 mg Co/kg calculated from the SSD is selected as the CTV for freshwater benthic organisms. Since this value is based on a chronic SSD that only covers a low (6) number of species, an assessment factor (AF) of 3 was used to account for intraspecies and interspecies variability in sensitivity. A higher assessment factor was not deemed necessary since Hyallela azteca was also tested for this medium and is the most sensitive species among the pelagic species tested. Therefore, it could also be one of the most sensitive species for the sediments compartment. In addition, a higher application factor would result in a PNEC in the range of background cobalt concentrations. Consequently, the PNEC for toxicity to freshwater benthic organisms is 16.7 mg Co/kg dry wt. (PNEC = CTV/AF = 50.1/3).
7.2.2 Toxicity modifying factors
The current level of science for cobalt does not allow the correction of toxicity by modifying factor for sediments; therefore, the PNEC is generic. Nickel toxicity in sediments has been observed to vary at most by a factor of 5 (EURAR 2008) and can serve as a basis for comparison at this time for cobalt which has a similar chemistry to that of nickel (2013 personal communication from William Stubblefield from Oregon State University to Ecological Assessment Division, Environment Canada; unreferenced).
7.3 Terrestrial organisms
Many empirical data are available on the chronic toxicity of soluble cobalt compounds to terrestrial organisms such as plants and invertebrates. The focus in this document is on chronic toxicity since exposure to cobalt in soil is expected to be long term. As with the approach taken for the aquatic toxicity data, all data from reliable chronic studies for soil organisms were considered together since the soluble cobalt compounds used in these studies all lead to the presence of bioavailable dissolved cobalt species in soil pore water, in particular the free ion Co2+. The bioavailability of these species will vary from one test to the other, depending on the characteristics of the soil tested. The data were then used to derive a CTV. RSS were completed for all toxicity studies used to derive this CTV and are available upon request.
Chronic data are of greater relevance than acute data in this assessment because they are a more sensitive indicator of potential for harm from long-term exposures, most likely to occur. Reliable chronic data were identified for 11 species including seven plant species and four invertebrate species. In addition, cobalt toxicity to soil microorganisms was considered by including three key soil biochemical processes. The species were exposed to varying concentrations of cobalt in different types of European and North American soils. Toxicity values range from 6.3 to 2213 mg of total Co/kg soil dw (Tables D-5, D-6 and D-7, Appendix D).
7.3.1 Toxicity modifying factors
Available toxicity data demonstrate that for many terrestrial species, the range of effects in soils, expressed as the total cobalt concentration, vary across a very wide range (up to over 100 fold). This variation is mainly attributable to the differences in bioavailability of cobalt in different types of soils but also to intra-species variability due to the type of endpoint tested (e.g., radish, Table D-5, appendix D). Taking these differences into consideration is critical in characterizing effects and risk of cobalt in soils.
7.3.2 Ageing and leaching
It has been shown that freshly spiked soils with metal salts show greater bioavailability that result in greater toxicity than in field-collected soils that have been contaminated with metals progressively and over long periods where ageing processes have occurred (Redeker et al. 2008). Indeed, ageing will remove metals from the soil solution to the solid phases (on their surfaces and/or deeper inside) with time through various mechanisms (McLaughlin, 2001; Smolders et al. 2007) rendering them less bioavailable. At the same time, leaching, i.e. the loss of metals to lower soil horizons and groundwater by migration (Degryse et al. 2009), can lower the ionic strength and hence lower the toxicity in higher soil horizons (Redeker et al. 2008).
Soil properties (pH, % organic carbon, % clay, CEC)
The most important factors modifying the bioavailability of cobalt in soils include pH, organic carbon and clay content (ICMM 2007a). The cationic exchange capacity (CEC), which is defined by the total capacity of the soil to retain or bind cations, best integrates the variations of the factors mentioned previously (Redeker et al. 2008). The higher the CEC, the lower the bioavailable dissolved cobalt concentration will be in the pore water and vice-versa.
These toxicity modifying factors will determine the amount and type of metal species available for uptake and the resulting possible toxic response and/or bioaccumulation for plants, invertebrates, and soil microorganisms (ICMM 2007a).
7.3.3 Data transformation
In the EU report on Cobalt (CoRC 2012) raw toxicity data was transformed and normalized to account for the bioavailability of cobalt in soils (i.e. calculate a PNEC specific to a particular soil). Figure 7-3 shows the step by step approach used in that procedure.
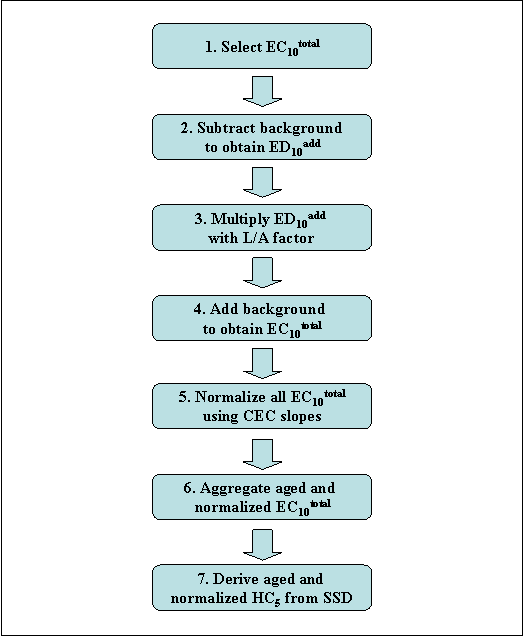
Long description for figure 7.3
General approach used for the incorporation of Co bioavailability in soils. The approach includes seven steps: Step 1: select EC10 total (total concentration of a substance that is estimated to cause some effect on 10% of the test organisms); Step 2: Subtract background to obtain ED10 add (the added dose of a substance that is estimated to cause some effect on 10% of the test organisms); Step 3: Multiply ED10 add (the added dose of a substance that is estimated to cause some effect on 10% of the test organisms) with Leaching/Ageing factor; Step 4: Add background to obtain EC10 total (total concentration of a substance that is estimated to cause some effect on 10% of the test organisms); Step 5: Normalize all EC10 total (total concentration of a substance that is estimated to cause some effect on 10% of the test organisms) total using CEC (Cationic Exchange Capacity) slopes; Step 6: Aggregate aged and normalized EC10 total (the added dose of a substance that is estimated to cause some effect on 10% of the test organisms); Step 7: Derive aged and normalized HC5 (Hazardous concentration to 5% (5th percentile) of the species) from the species sensitivity distribution (SSD).
In the first step, all reliable EC10/NOECstotal are selected from the literature (Tables D-.5, D-6 and D-.7, Appendix D). In the second step, the background concentration of cobalt found in the control soil is subtracted from the "total" reported toxicity value to obtain the "added" concentration. The third step involves the correction for the difference in Co bioavailability between laboratory conditions (Co freshly added as soluble salts) and field conditions, through application of a leaching-ageing factor (L/A factor). Because the background concentration is assumed to be already aged, the correction only applies to the "added" fraction. In step 4, the background is added back to get the "total" and "aged" concentration. The next step, (5) includes the correction of values according to the specific soil abiotic properties of a given site that affect cobalt toxicity. The normalization is based on the slopes of the organism specific regressions models involving CEC. In step 6, the corrected data are aggregated (e.g., take the lowest effect endpoint, apply geometric mean for the same endpoint for the same species) in order to get one value per species or microbial process for the soil of interest. Finally, in step 7 the SSD is drawn to determine the HC5 which is selected as the CTV for soil organisms. The CTV value is based on a chronic SSD that covers multiple species and taxa representing well the trophic levels of terrestrial organisms. However, a small assessment factor of 2 was used to derive the PNEC for soil organisms since there were no field/microcosm data available to evaluate the laboratory to field extrapolation (CoRC 2012). Hence, the PNEC is equivalent to the CTV/2. Soil PNECs are specific to each soil of interest. The soil PNEC calculator, an Excel-based software developed by ARCHE Consulting (2012), includes all of the data above and equations that account for modifying factors to calculate the PNEC (from the SSD) and risk quotients for a certain number of metals including cobalt in different types of soils. The user inputs the total concentration of cobalt and the CEC in the soil of interest to generate a PNEC result. This tool was used for this assessment to calculate site specific PNECs in the characterization of ecological risks section (details presented in ECCC 2016c). The quantification of the specific soil toxicity modifying factors (i.e. ageing, pH and leaching) including the data and equations considered are available in ECCC (2016c).
8. Ecological exposure assessment
8.1 Ambient/background concentrations
Concentrations of dissolved or total cobalt measured in water bodies across Canada are summarized in Table 8-1. While some of the high concentrations may be related to the contribution from point-source anthropogenic releases, concentrations up to the 50th percentile should be representative of the regional biogeochemical background concentration (ICMM 2007b). These data were collected between 2009 and 2012 as part of Environment Canada's Monitoring and Surveillance Program under the CMP (Environment Canada 2013c).
| Location | Concentration range (μg/L) |
Median (μg/L) |
Reference |
|---|---|---|---|
| Pacific and Yukon Region | 0.002 to 64 (dissolved) |
0.038 | Environment Canada 2013b, 2013c |
| Prairie and Northern Region | 0.014 to 0.49 (dissolved) |
0.061 | Environment Canada 2013c |
| Ontario Region (Hudson's Bay) |
0.004 to 2.4 (dissolved) |
0.09 | Environment Canada 2013c |
| Ontario Region (Erie-Superior-Ontario) |
0.002 to 1.1 (total) |
0.04 | Environment Canada 2013c |
| Quebec Region | 0.02 to 2.3 (total) |
0.16 | Environment Canada 2013c |
| Atlantic Region | 0.007 to 3.9 (total) |
0.083 | Environment Canada 2013c |
8.2 Deriving environmental concentrations from anthropogenic activities
Exposure scenarios were developed for the various activities that may represent significant sources of release of cobalt or cobalt-containing substances to the environment. These scenarios are presented in this section and are split by industrial/commercial sectors. For each scenario, a predicted environmental concentration (PEC) is estimated in order to assess exposure to cobalt for ecological receptors. Depending on the environmental media to which releases of cobalt-containing substances are expected to occur, PECs are estimated for surface water, sediments and/or soil. PECs for air were not developed since cobalt-containing substances are usually not volatile under normal conditions and since most cobalt forms travelling in this compartment are expected to be in the form of particulate matter that will ultimately deposit on soil or water bodies.
8.2.1 Exposure based on measured environmental concentrations
When available, adequate data on measured concentrations of cobalt in relevant environmental media were used to estimate PECs as they provide evidence for exposure of organisms in Canada. The adequacy of measured environmental concentrations was assessed considering factors such as the distance between sampling sites and the source of release, the year the samples were collected, and the analytical method used. Also, when data were available for both total and dissolved cobalt concentrations in water, only dissolved concentrations were considered since they comprise the fraction of cobalt that is likely bioavailable. When it was not specified whether concentrations were reported for total or dissolved cobalt, it was assumed that concentrations were dissolved in order to be conservative (i.e. assuming high bioavailability).
8.2.2 Exposure based on modeled environmental concentrations
When data on measured concentrations of cobalt were inadequate or not available, models were used to estimate PECs. These PECs were determined based on a tiered approach, i.e. using conservative assumptions for the first calculations and then refining these assumptions as needed (i.e. where a concern is identified) to increase the level of realism of the scenario.
For certain industrial sectors, the main medium of potential environmental concern is surface water. This is because substances are expected to be primarily released through wastewater systems for these sectors. For these sectors, an estimated aquatic concentration (EAC) was calculated for each site that may release cobalt in wastewater.
EACs for surface water near the discharge point of a wastewater system were estimated using the following generic aquatic equation:
EAC = 106 × Q × L × (1-R) / N × F × D
Where
EAC: estimated aquatic concentration resulting from industrial releases, μg Co/L
Q: total substance(s) quantity used annually at an industrial site, kg Co/yr
L: loss to wastewater, fraction
R: wastewater treatment system removal rate (on-site and/or off-site), fraction
N: number of annual release days, d/yr
F: wastewater treatment system effluent flow, m3/d
D: receiving water dilution factor, dimensionless
8.2.2.1 Quantity used (Q)
This value is based on the yearly quantities reported in response to the section 71 notice surveys. Occasionally, where a company did not report a quantity in response to a section 71 notice survey but had reported releases of cobalt to the NPRI, a quantity of 10 tonnes is presumed as being manufactured, processed or otherwise used (MPO) since this is the threshold reporting requirement for the NPRI. It is important to note that all data used in the calculations are for cobalt-converted values based on the ratio of molecular cobalt for the particular substances reported.
8.2.2.2 Loss to wastewater (L)
This value is usually calculated based on the cobalt released to a facility's waste water divided by the total quantity of cobalt used or manufactured at the facility. When this information is not available, a generic value is taken from emission scenario documents.
8.2.2.3 Wastewater treatment system removal rate (R)
The removal rate for off-site wastewater treatment is based on the type of treatment used at the WWTP to which the industrial facilities are connected. A median removal rate of 27% for primary treatment and of 62% for secondary treatment was selected based on measured concentrations of total cobalt in the influent and effluent of 11 publicly owned WWTPs located across Canada (two plants with primary treatment and nine plants with secondary treatment). These data are based on samples that were collected each summer and winter, from 2009 to 2012 (Environment Canada 2013c). This monitoring program was conducted under the Chemicals Management Plan. Sixteen other publicly owned WWTPs were also sampled as part of this program for which cobalt concentrations in the influent and effluent were below the detection limit of 6 ng/L. A removal rate of 90% for on-site wastewater treatment was selected for one facility where an ion exchange treatment is used.
8.2.2.4 Number of release days (N)
The number of days of release is based on the days of operation for each facility on a case-by-case basis. It is assumed that cobalt-containing substances are used and released every day of operation.
8.2.2.5 Wastewater treatment system effluent flow (F)
For off-site wastewater treatment, the wastewater treatment system effluent flow is the effluent flow of the WWTP into the receiving water that receives wastewater from industrial facilities. This information is available in an internal database used by Environment and Climate Change Canada.
8.2.2.6 Receiving water dilution factor (D)
Assuming an instantaneous dilution of the effluent, the dilution factor of a receiving water course was calculated by dividing the flow of either the facility effluent (in case of direct discharge to a water course) or the WWTP effluent (connected to the facility) by the 10th percentile of the annual distribution of the flow of the receiving water course. When this dilution factor was greater than 10, a maximum default value of 10 was used. A dilution factor of 10 was also used for those releases that occur in a lake, bay or basin. This maximum dilution factor represents exposures in the receiving watercourses near the discharge point of the effluent. This is based on the assumption that full dilution does not occur immediately upon release to large waterbodies.
8.2.2.7 Survey data on manufacture, import and uses
When estimating release and exposure associated with individual substances, the extent of detail in survey data was variable leading to some uncertainty (see section 4.4). However, for those substances of higher volume (e.g., elemental cobalt, cobalt sulfate, cobalt hydroxide and cobalt chloride), detailed survey data was available that provided information for major sources accounting for most of the use quantities.
8.2.3 Consideration of ambient/background concentrations
Whether PECs were based on measured or modeled data, the ambient and biogeochemical background concentrations of cobalt in water, sediments or soil were taken into account in order to assess total exposure to cobalt. To simplify referring to these two types of concentrations, the term "background concentration" is used through the rest of this assessment report. When data were modeled for releases to surface water, the PEC for a particular site was obtained by adding the median (50th percentile) background concentration of cobalt in water to the EAC (i.e., PEC = EAC + median background concentration). As a conservative approach, the highest median background concentration (0.16 μg/L, Table 8-1) was used. In cases where a concern was identified, the background concentration was refined using site- or area-specific data, when available, geochemically relevant to the site receiving releases. Given that organisms in aquatic ecosystems are exposed to both anthropogenic and natural sources of cobalt, their total exposure to this metal is captured in the PEC. When measured data were used to assess exposure, the background concentration of cobalt was not added since it is already reflected in the measurement, which represents the total exposure of organisms to cobalt. However, whenever possible, measured data in areas most likely impacted by anthropogenic releases of cobalt were compared to measured data from a similar area but in a zone likely not affected by anthropogenic releases (i.e., a reference site). This allowed an estimation of the proportion of cobalt exposure attributable to natural sources. Exposure for soil and benthic organisms was only based on measured data so the approach of adding a background concentration to a modeled concentration for a given release was not used.
8.3 Sector-specific exposure scenarios
8.3.1 Rubber
Cobalt propionate (CAS RN 1560-69-6), cobalt borate neodecanoate (CAS RN 68457-13-6), cobalt naphthenate (CAS RN 61789-51-3) and cobalt neodecanoate (CAS RN 27253-31-2) have been reported to be used as metal to rubber adhesion promoters (Environment Canada 2012a and 2009a, NPRI 1995). These substances are added during the compounding of rubber that is to be in contact with metal components. The formulation and processing lifecycle stages in the rubber industry are dry processes. However, incidental emissions to water could occur as a result of floor scrubbing, equipment washing/blowdown or compounds cooling (ETRMA 2010). Companies that reported using cobalt-containing substances for this use were mainly tire manufacturers. One company reported being involved in rubber compounding. Exposure scenarios were developed for both uses. A scenario for the release of cobalt from tire wear during service life was not considered because cobalt compounds are not added to the tread (Kreider et al. 2010).
Estimated aquatic concentrations (EACs) were calculated for four facilities in the rubber sector using the equation described in section 8.2.2. These EACs range from 0.0084 to 0.55 μg Co/L (Table 8-2). Additional details on how these values were obtained are available in ECCC (2016d).
8.3.2 Chemical manufacturing/manufacture and use of catalysts
Cobalt(II) hydroxide (CAS RN 21041-93-0) and cobalt oxide (CAS RN 1307-96-6) were reported to be imported in Canada in 2008 and/or 2011 for the manufacture and use of hydrotreatment catalysts for the removal of sulfur from feedstocks (Environment Canada 2009a and 2012a). During the manufacturing of these cobalt-containing substances, aqueous releases of cobalt are expected to occur during the filtration and washing of the precipitated material.
The mixture containing a hydrate of cobalt acetate (CAS RN 6147-53-1) that had been reported to be imported in Canada for use as a homogenous catalyst (Environment Canada 2012a) is reported to be no longer used by the facility. It has been replaced by another cobalt-containing catalyst for which restrictions on environmental releases are in place.
Exposure scenarios were developed for the use of cobalt hydroxide and cobalt oxide in the chemical sector and in the manufacture and/or use of catalysts. EACs were calculated for two sites using the equation described in the section "Exposure based on modeled environmental concentrations". These EACs range from 0.02 to 0.08 μg Co/L (Table 8-2). Additional details on how these values were calculated are available in ECCC (2016d). A scenario for the use of hydrotreatment catalysts was not developed because, in this process, cobalt is fixated on a solid support (e.g., alumina) and is likely not released from the catalyst (ATSDR 2004). However, a scenario pertaining to metal recovery from spent hydrotreatment catalysts was developed since this activity was reported to take place in Canada (Environment Canada 2012a). A scenario for the regeneration/rejuvenation of spent hydrotreatment catalysts was not developed because this activity is mostly done outside Canada (NPRI 1995).
Cobalt stearate (CAS RN 13586-84-0), cobalt (II) hydroxide (CAS RN 21041-93-0), cobalt (II) 2-ethylhexanoate (CAS RN 136-52-7), cobalt neodecanoate (CAS RN 27253-31-2) and cobalt naphthenate (CAS RN 61789-51-3) have been reported to be manufactured by two companies in Canada (Environment Canada 2009a and 2012a). These cobalt-containing substances are then used for either manufacturing rechargeable batteries, as metal to rubber adhesion promoters or as additives in paints and coatings. During the manufacturing of the cobalt compounds, aqueous releases of cobalt are expected to arise during the filtration and washing of the precipitated material.
Exposure scenarios were developed for the manufacture of these compounds. EACs were calculated for two sites using the equation described in the section "Exposure based on modeled environmental concentrations". The EACs obtained are 0.04 and 0.08 μg Co/L (Table 8-2). Additional details on how these two values were calculated are available in ECCC (2016d). Scenarios for the subsequent industrial use of these cobalt-containing substances in rubber and in paints and coatings are described in other sections of this report. A scenario for the manufacture of rechargeable batteries was not developed because no imports or uses of cobalt-containing substances were reported for this activity in Canada in response to the section 71 surveys for 2008 and 2011 (Environment Canada 2009a and 2012a). Potential emissions of cobalt during battery recycling for metal recovery are covered in another section of this report (Disposal and waste management).
8.3.3 Paints and coatings
Cobalt(II) 2-ethylhexanoate (CAS RN 136-52-7), cobalt neodecanoate (CAS RN 27253-31-2), cobalt(II) hydroxide (CAS RN 21041-93-0) and cobalt naphthenate (CAS RN 61789-51-3) have been reported to be used as driers in paints and coatings by several companies (Environment Canada 2009a and 2012a). Overall, the paints and coatings industry uses little process water. The only use of process water is for cleaning of plant surfaces and the exterior of equipment. The main source of release will be during the wet cleaning of the plant floors and equipments (OECD 2009).
EACs were calculated for this sector using the equation described in the section "Exposure based on modeled environmental concentrations". These EACs were calculated for seven facilities and they range from 0.0001 to 0.004 μg Co/L (Table 8-2). Additional details on how these values were obtained are available in ECCC (2016d).
8.3.4 Plastic
Cobalt neodecanoate (CAS RN 27253-31-2), cobalt(II) hydroxide (CAS RN 21041-93-0) and cobalt naphthenate (CAS RN 61789-51-3) have been reported to be used as promoters/accelerators for the curing of polyester resins for fibre-reinforced plastic (Environment Canada 2012a).
EACs were calculated for two facilities using the equation described in the section "Exposure based on modeled environmental concentrations". The values obtained are 0.0001 and 0.002 μg Co/L (Table 8-2). Additional details on how these values were obtained are available in ECCC (2016d).
8.3.5 Fertilizers
Cobalt oxide (CAS RN 1307-96-6) has been reported to be imported for use as a catalyst by a fertilizer manufacturer in 2008 (Environment Canada 2009a). An exposure scenario for this use was not developed because cobalt is likely not released from the catalyst during its use (see section above on Chemical manufacturing). However, cobalt is naturally present in the ore used to produce fertilizers (UNIDO and IFDC 1998). Hence, an exposure scenario was developed to assess releases of cobalt by the fertilizer manufacturer.
Even though only one facility was included in the exposure scenario, two EACs were estimated since the facility has two different points of discharge. The EACs obtained are 0.11 and 0.54 μg Co/L (Table 8-2). Additional details on how these values were calculated are available in ECCC (2016d).
8.3.6 Animal feed manufacturing
Cobalt(II) carbonate (CAS RN 513-79-1) and cobalt hydroxide carbonate (CAS RN 12602-23-2) have been reported to be used as additive in animal feed (Environment Canada 2009a and 2012a). Among the companies that have reported this activity, only one is actually using cobalt(II) carbonate to manufacture feed in Canada. An exposure scenario was developed for this use to assess releases to water. An EAC of 0.33 μg Co/L was calculated (Table 8-2). Additional details on how this value was calculated are available in ECCC (2016d).
Seven facilities that manufacture animal feed reported to the NPRI releases of 837 kg of cobalt to air for 2011 (NPRI 1995). The highest releases from an individual facility were reported as 335 kg. These releases could translate into elevated concentrations of cobalt in soil surrounding this facility. However, a follow-up with the facility indicated that the quantity of cobalt had mistakenly been reported as being released while it was actually being used by the facility. While the actual quantity released is expected to be much lower, no revised quantitative estimate was provided and the facilities have since changed ownership.
8.3.7 Alloy and superalloy manufacturing
Elemental cobalt (CAS RN 7440-48-4) was reported to be used by one alloy and superalloy manufacturer in Canada (excluding ferro-alloys) (Environment Canada 2010a). Elemental cobalt is predominantly used as a component in alloys and carbides for applications requiring high strength and temperature resistance (Donaldson and Beyersmann 2005). The manufacturing process of alloys and superalloys may release cobalt to water. An EAC of 0.031 μg Co/L was calculated for this activity (Table 8-2). Additional details on how this value was calculated are available in ECCC (2016d).
Automobile manufacturers use cobalt alloys for certain motor parts (CDI 2006). Welding activities may result in significant releases of cobalt oxides and elemental cobalt dusts to air. One facility from the motor vehicle parts manufacturing sector reported significant releases of cobalt to air to the NPRI. Between 2007 and 2011, this company reported average releases of 706 kg of cobalt and its compounds to air with a maximum of 1281 kg in 2011 (NPRI 1995).
| Use/sector | EAC (μg Co/L) |
Background (μg Co/L) |
PEC (μg Co/L) |
|---|---|---|---|
| Rubber | 0.0084-0.55 | 0.012-0.16 | 0.17-0.56 |
| Manufacture or use of catalysts | 0.02-0.08 | 0.16 | 0.18-0.24 |
| Manufacture of chemicals | 0.04-0.08 | 0.16 | 0.20-0.24 |
| Paints and Coatings | 0.0001-0.004 | 0.16 | 0.16 |
| Plastic (Polyester resin) | 0.0001-0.002 | 0.16 | 0.16 |
| Fertilizers | 0.11-0.54a | 0.16 | 0.26-0.70 |
| Animal feed manufacturing | 0.33 | 0.16 | 0.49 |
| Alloy and superalloy manufacturing | 0.031 | 0.1b | 0.13 |
| Base metals smelting and refining | 0.15-11.3 | 0.12-0.52 | 0.25-11.7 |
a. transfer to publicly owned WWTP.
b. site-specific background.
8.3.8 Metal mining
Cobalt may be released into the environment as a result of mining and the production of concentrates. Measured concentrations of cobalt in environmental media in the vicinity of operating metal mines across Canada are available in a variety of reports and databases (Table 8-3). Historical (abandoned or closed) metal mines may be present on some of these sites or nearby. These concentrations were used as Predicted Environmental Concentrations (PECs) for risk characterization (see next chapter). Additional details about these data are available in ECCC (2016d). Data are available for 41 locations (for water and/or sediments), including 19 reference (often upstream) locations, 21 exposure locations downstream of effluent discharge points and one location downstream of a historical mining site. For those sites where concentrations are available both upstream and downstream of an effluent discharge point, concentrations of cobalt in water and sediments are higher at downstream locations in about half of the cases (9 locations) indicating that some metal mining sites are likely contributing to elevated concentrations of cobalt in water and sediments.
| Medium and units | PEC range | Sampling period | References |
|---|---|---|---|
| Water, reference areas (μg/L) |
less than 0.05 to 11.8 | 1995-2012 | Couillard et al. 2008, Environment Canada 2013d |
| Water, exposure areas (μg/L) |
0.01 to 42 | 1995-2012 | Evans 2000, Couillard et al. 2008, Environment Canada 2013d |
| Water downstream historical mining site (Aldermac) (μg/L) |
0.08 to 176 | 2013 | 2013 personal communicationa |
| Sediments, reference areas (mg/kg) |
4.7 to 38 | 1995-2011 | Environment Canada 2013d |
| Sediments, exposure areas (mg/kg) |
6.0 to 64 | 1995-2011 | Environment Canada 2013d |
a. from Landis Hare, Institut National de la Recherche Scientifique - Eau, Terre et Environnement (INRS-ETE), to Ecological Assessment Division, Environment Canada; unreferenced.
8.3.9 Base metals smelting and refining
Canadian or imported ore concentrates that contain cobalt are processed in smelters and refineries, with the recovery of cobalt as an intermediate product, a residue or main product. Elemental cobalt (CAS RN 7440-48-4), cobalt sulfate (CAS RN 10393-49-4), cobalt chloride (CAS RN 7646-79-9), cobalt(II) hydroxide (CAS RN 21041-93-0), cobalt sulfide (CAS RN 1317-42-6) and residues, cobalt-refining (CAS RN 124222-15-7) were reported to be manufactured, imported and/or used for or during nickel smelting and refining steps (processes) (Environment Canada 2010a and 2012a).
Exposure scenarios were developed for the substances listed above and the facilities that reported manufacturing or using them. These facilities vary considerably due to their different processes and products. Hence, these facilities will have varied levels of cobalt in their effluents. EACs were calculated for five facilities using the equation described in the section "Exposure based on modeled environmental concentrations". The EACs obtained range from 0.15 to 11.3 μg Co/L (Table 8-2). Additional details on how these values were calculated are available in ECCC (2016d). These EACs only consider liquid effluents that would be discharged from smelters and refineries; releases that would occur through atmospheric emissions are discussed later in this section. Also, it is important to note that some smelters have combined effluents with mines. For the purpose of calculating the EACs presented above, only emissions from smelters and refineries were considered.
Additional scenarios were developed based on measured concentrations of cobalt in the environment. Indeed, because cobalt may be present in the ore that is mined and further processed, it may be emitted to the atmosphere from a smelter or refinery stack as part of fine particulate matter. Measured concentrations of cobalt in environmental media in the vicinity of smelters and refineries across Canada are available in a variety of reports and databases (Table 8-4). These concentrations were used as Predicted Environmental Concentrations (PECs) for risk characterization (see next chapter). These data are for samples collected in the following areas: Rouyn-Noranda (QC), Cobalt (ON), Port Colborne (ON), Sudbury (ON), Flin Flon (MB), Thompson (MB) and Trail (BC). Additional details about these data are available in ECCC (2016d).
Data are available for 22 sites (for water, sediments and or/soil), including 14 exposure sites and 8 reference sites. Overall, these data indicate that some base metals smelters and refineries are likely contributing to elevated concentrations of cobalt in water, sediments and soil, following deposition of atmospheric emissions. Cobalt concentrations in the vicinity of some of the smelters are higher than local natural background concentrations (10 sites). The scarcity of data for water for smelters is mainly due to the difficulty in finding data for water bodies located in the vicinity of smelters and that are only affected by atmospheric deposition and not by mining effluents and/or historical mines. The difference between the relatively low levels of cobalt in water versus the high levels in sediments can be explained by the fact that metals in the water column eventually settle to sediments. Indeed, concentrations in sediments reflect several years of metal deposition, depending on sedimentation rate and depth. Similarly, concentrations of cobalt in soil represent several years of deposition.
| Medium and units | PEC range | Sampling period | References |
|---|---|---|---|
| Water, reference areas (μg/L) |
0.015 to 0.058 | 1996 to 2008 | SARA 2009, Intrinsik Environmental Sciences Inc. 2010 |
| Water, exposure areas (μg/L) |
less than 0.05 to 60 | 1997-2012 | Keller et al. 2004, Szkokan-Emilson et al. 20141, Intrinsik Environmental Sciences Inc. 2010, Geological Survey of Canada 2001 |
| Sediments, reference areas (mg/kg) |
14 to 50 | 1993 to 2008 | SARA 2009, Intrinsik Environmental Sciences Inc. 2010 |
| Sediments, exposure areas (mg/kg) |
1 to 190 | 1993-2008 | SARA 2009, Intrinsik Environmental Sciences Inc. 2010, Geological Survey of Canada 2001 |
| Soil, reference areas (mg/kg) |
2 to 38 | 2001-2006 | OMOE 2004, Manitoba Conservation 2003, Manitoba Conservation 2007, Geological Survey of Canada and Teck Cominco Metals Ltd 2001 |
| Soil, exposure areas (mg/kg) |
0.5 to 195 | 1998-2007 | OMOE 2004, Manitoba Conservation 2003, Intrinsik Environmental Sciences Inc. 2010, OMOE 2000, Intrinsik Environmental Sciences Inc. et al. 2011 |
a. Concentrations data were provided by the author.
8.3.10 Iron and steel
Combustion of fossil fuels by iron and steel mills along with the steel-making process that takes place in furnaces may emit cobalt to air. These emissions could translate into deposition of cobalt to land and water bodies that surround a mill. Measured concentrations of cobalt in soil in the vicinity of iron and steel mills in Canada could not be found. However, given the low releases of cobalt to air reported to the NPRI for this sector (30 kg/year), it is expected that deposition of cobalt to soil would not be of concern for soil organisms. Similarly, deposition to water bodies is expected to be low (no releases reported to the NPRI). Water samples collected in Hamilton Harbour (there are two steel mills in Hamilton) during spring 2010 and 2011 under the Great Lakes Surveillance Program indicate that cobalt concentrations were 0.12 and 0.082 μg/L, respectively (Environment Canada 2012b).
In terms of releases to water as point sources, concentrations of cobalt in process effluent for the iron and steel sector are available in a status report produced under the Municipal/Industrial Strategy for Abatement (MISA) of the Ontario Ministry of the Environment. Even though they were collected many years ago (1989-1990) and the production of steel has decreased in the years 2000s due to a decline in certain sectors such as the automobile industry, these data were considered in this assessment since they are the only data that were found for this sector. It was assumed that the activity level of facilities in the iron and steel sector in Ontario are representative of the whole sector in Canada. Only two of the seven steel mills that reported data under MISA back in 1989-1990 detected levels of cobalt in their process effluent(s) (OMOE 1991). The regulation method of detection limit (RMDL) for cobalt was high at 20 μg/L which may explain why some of the mills did not report results for cobalt. For one of the two mills that detected cobalt in their effluent, measured data for cobalt was deemed to be unreliable based on the statistics provided (also the case for other metals). Measured data for the other mill, which reflected a 9-month period of effluent monitoring, indicated average concentrations of 6 to 13 μg Co/L for the various effluents discharged by the mill. The highest concentration measured during that period was 27 μg Co/L. However, cobalt was only detected in 11-13% of the samples. Effluents from this mill were discharged in Hamilton Harbour, which has a high dilution capacity but which also receives other industrial waste water effluents as well as wastewater effluent from publicly owned WWTPs. Concentrations of cobalt measured recently in Hamilton Harbour are quite low (from 0.082 to 0.12 μg/L, see above). In addition, two steel mills located in this area send some of their effluents to one of Hamilton's WWTPs. Recent data collected from 2010 to 2012 under the Chemicals Management Plan monitoring program indicate that total cobalt concentrations ranged from less than 0.006 to 1.1 μg/L (median of 0.49 μg/L) in the final effluent of this WWTP (2013 personal communication from Emerging Priorities Division, Environment Canada, to Ecological Assessment Division, Environment Canada; unreferenced).
8.3.11 Electricity (power generation)
Power generation is among the most important activities in terms of fossil fuel combustion in the country. This source emits cobalt to air in small-sized particulate matter, which can be expected to be deposited to terrestrial and aquatic ecosystems. Most of atmospheric releases of cobalt reported in recent years were for power plants located in Alberta, in the Wabamun Lake area (NPRI 1995, Environment Canada 2010a, Environment Canada 2012a). As such, an exposure scenario for this area was developed based on concentrations of cobalt measured in various environmental media and was used as a realistic worst-case scenario to assess releases from coal-fired power plants. Other than power plants and co-located coal mining, there are no other major industries along or close to Wabamun Lake; hence, above-background levels of cobalt measured in the various environmental media in this area can reasonably be attributed to the activities of these two sectors. No measured data were available to develop a realistic exposure scenario for oil-fired power plants. However, given that concentrations of cobalt in heavy fuel oil are usually lower than in coal (ATSDR 2004) and given the higher calorific value of heavy fuel oil as compared to coal (FAO 1990, Goodarzi 2013), it is expected that releases of cobalt from an oil-fired power plant would not be higher than those from the coal-fired power plants covered in the exposure scenario below.
An air deposition survey was conducted in the Wabamun Lake area between 1994 and 1997 by the Geological Survey of Canada (2002). Soil samples collected as part of this survey showed a median soil cobalt concentration of 7.5 mg/kg. The highest concentration, 13 mg Co/kg soil, was measured at a sampling station located downwind from all power plants located in the area (Table 8-5). However, no or low deposition of cobalt was measured in a moss metal biomonitor at this station during the 4-year study, suggesting that most of the cobalt was from natural sources. Generally, the low cumulative deposition rate of cobalt around Wabamun Lake (1.07 g/ha) suggests that power plants are not an important source of anthropogenic cobalt to land in that area. Even if these data are not recent, they are considered to be representative (or even overestimates) of current levels, considering that one of the plants in this area has since closed.
The Alberta Ministry of the Environment conducted water quality and sediment surveys of Wabamun Lake in 2002 and 2005 (Alberta Environment 2002, 2003, 2006). The highest measured concentration of cobalt in water in 2002 was 0.3 mg/L (dissolved) in a sample collected at the ash pond lagoon discharge (effluent) of a power plant. Concentrations in samples collected in the rest of the lake, including close to the local publicly-owned WWTP discharge, were all below the detection limit of 2 μg/L (Alberta Environment 2002). Samples collected in 2005 indicated a maximum cobalt concentration of 0.056 mg/L in the pelagic zone of the lake. The PEC for water for this risk assessment was based only on the data from 2005 since all data from 2002 were below the relatively high detection limit of 2 μg/L (except for the ash pond effluent) (Table 8-5). The highest cobalt concentration measured in sediments was 8 mg/kg in 2002 and 9.1 mg/kg in 2005. The PEC for sediments was based on the 2005 data since this value is for extractable cobalt (with nitric acid) which is considered to be more representative of the bioavailable fraction of cobalt to benthic organisms than total cobalt.
| Medium and units | PEC range | Sampling period | Reference |
|---|---|---|---|
| Water (μg/L) |
0.019 to 0.056 | 2005 | Alberta Environment 2006 |
| Sediments (mg/kg) |
1.4 to 9.1a | 2005 | Alberta Environment 2006 |
| Soil (mg/kg) |
3 to 13 | 1994 | Geological Survey of Canada (2002) |
a. extractable with nitric acid.
8.3.12 Petroleum refining
The burning of fossil fuels by petroleum refineries to meet their energy requirements may release cobalt as a component of fly ash. No exposure scenario was developed for these facilities for atmospheric emissions because releases of cobalt are expected to be similar to those from power generation plants.
In terms of releases to water, concentrations of cobalt in process effluent for the petroleum refining sector are available in a report produced for Environment Canada (Gentsia Consulting Inc. 2009). This report contains data for three refineries in Canada. The average concentrations of cobalt in the effluent of these refineries for year 2008 were 0.49, 2 and 2.8 μg/L. Two of these refineries discharge their effluent directly into a large river. Using a maximum default value of 10 as a dilution factor, the resulting EACs for these sites would be 0.049 and 0.2 μg/L. The third refinery sends its treated effluent to a publicly owned WWTP that uses a secondary treatment and that discharges its effluent into a large water body. Hence, the corresponding EAC for that refinery is expected to be below 0.1 μg/L.
The only other data that are available for this sector are from an older report produced under the Municipal/Industrial Strategy for Abatement (MISA) of the Ontario Ministry of the Environment (OMOE 1989). These data were collected in 1988 and 1989. Effluent monitoring for a 6-month period indicated average total concentrations of 2 and 9.8 μg Co/L for two of the seven petroleum refineries that monitored their process effluent and that are still active today. These refineries discharged their effluent into Lake Ontario and the St. Clair River, respectively. Using the highest average effluent cobalt concentration and a maximum default value of 10 as a dilution factor, the resulting EAC would be 0.98 μg/L. A short stretch (300 m) of a creek that receives this effluent before joining the St. Clair River could have had higher levels of cobalt due to lower dilution capacity.
8.3.13 Oil sands
Some facilities in the oil sands sector reported releases of cobalt to air (Environment Canada 2010a, NPRI 1995). Releases are expected to be lower than those from power generation plants (see Table C-1). Therefore an exposure scenario for atmospheric emissions and subsequent deposition to soil was not developed for the oil sands sector.
Deposition of cobalt to water bodies as well as potential leaching of process water out of tailing ponds could be a source of cobalt to surrounding water bodies. Measured concentrations of total and dissolved cobalt in water are available for nearly 40 water bodies located in the Athabasca area (Alberta) where most of the oil sand operations are located. Concentrations of cobalt in sediments are available for 21 water bodies in the same area. These data come from the Regional Aquatics Monitoring Program (RAMP) and were collected regularly between 1997 and 2011 (program still ongoing). Results are shown in Table 8-6 (RAMP 2012) and are used as realistic worst-case PECs to quantify exposure of aquatic and benthic organisms to cobalt for an oil sands scenario.
| Medium and units | PEC range | 5th a | 50th a | 95th a | Sampling period | Reference |
|---|---|---|---|---|---|---|
| Water (μg/L) |
less than 0.01 to 23 (dissolved) | 0.024 | 0.1 | 0.34 | 1997-2011 | RAMP 2012 |
| Water (μg/L) |
less than 0.01 to 27 (total) | 0.043 | 0.2 | 1.6 | 1997-2011 | RAMP 2012 |
| Sediments (mg/kg) |
0.2 to 13.5 | 0.9 | 5.0 | 10.0 | 1997-2011 | RAMP 2012 |
a. Percentiles; For values less than MDL, the value of the MDL was used by default to calculate percentiles.
8.3.14 Pulp and paper mills
Pulp and paper mills burn a variety of fuels to meet their energy demands, which may contribute to atmospheric emissions of cobalt. Cobalt may also be released to surface water and sediment from plant effluent as certain cobalt-containing substances are reportedly used in wastewater treatment (Environment Canada 2010a). Releases of cobalt were also reported to land for 2006 (2723 kg) and were mostly wastes like liquor dregs, boiler ash and sludge that were landfilled (Environment Canada 2010a).
Atmospheric emissions of cobalt can translate into deposition of this metal to land and water bodies that surround a mill. A few measured concentrations of cobalt in surface water close to pulp and paper mills are available and discussed below. Depending on the distance between the mills and the sampling sites, these concentrations would likely represent a mix of cobalt released through mill effluents and of cobalt present in atmospheric emissions. No measured concentrations of cobalt in soil in the vicinity of pulp and paper mills could be found. Therefore, exposure in this environmental medium could not be assessed for this sector.
Measured concentrations of cobalt in water and sediments in the vicinity of six pulp and paper mills in Canada are available in reports produced under Environment Canada's Environmental Effects Monitoring program (EEM) (Table 8-7). Data are available for 14 locations (for water and/or sediments), including 7 reference (upstream) locations and 7 exposure locations downstream (at effluent discharge point or near-field). Data for these mills indicate that they are not significant sources of cobalt releases to aquatic ecosystems. Additional details about these data are available in ECCC (2016d).
Due to the scarcity of environmental concentrations of cobalt available for aquatic ecosystems, concentrations in effluents were also analyzed. Mean total cobalt concentrations in effluents were available for six mills in the EEM reports, and they ranged from 0.34 to 4.4 μg Co/L for sampling conducted between 1990 and 2009. The effluent having the highest concentration (4.4 μg Co/L in 1990) was a combination of waste water from two mills, one of which is now closed. Considering dilution by the receiving watercourse, the concentration of cobalt in surface water downstream from the discharge point of the effluent is now predicted to be below 0.4 μg/L. Another mill had high cobalt concentrations in its final effluent (4.2 μg/L in 2009); this mill discharges its effluent into a ditch which then joins an estuary. Using a dilution factor of 10, the resulting concentration is not expected to be a concern.
Cobalt was detected between 1998 and 2004 in the effluents of 25 mills out of 52 mills that were sampled in the province of Quebec (NCASI 2006, MDDEP 2010). The former reference (NCASI 2006) is a draft unpublished report that was not finalized and published. Therefore, the data it includes has some uncertainty but was still deemed acceptable for use in this assessment. For 16 of the 27 mills for which cobalt was not detected in the effluent samples, the method detection limit was 10 μg Co/L or higher. The average concentrations in the final effluent from the 25 mills where cobalt was detected ranged from 0.17 to 48 μg Co/L (total cobalt). Some of these mills treated their effluent on-site (secondary or primary treatment) before releasing it to the environment. Other mills sent their effluent to a publicly owned WWTP, in some cases following on-site primary treatment. Four mills that discharged their effluent to the environment (following on-site secondary treatment) reported relatively high average concentrations of total cobalt in their final effluent (5.4 to 48 μg/L). Given that pulp and paper mills often discharge their effluent in large waterbodies, the default maximum value of 10 was used as a dilution factor to estimate EACs from these effluent concentrations. The calculated EACs ranged from 0.54 to 4.8 μg Co/L. Adding a default background concentration of 0.16 μg Co/L to the EACs result in PECs ranging from 0.70 to 5.0 μg Co/L (total cobalt). Pulp and paper mills in the province of Quebec are considered to be representative of this sector in Canada as they include a variety of pulping processes that are commonly used in Canada.
| Medium and units | PEC range | Sampling period | References |
|---|---|---|---|
| Water, reference areas (μg/L) |
0.1 to 0.3 | 1998 and 2009 | Environment Canada 2013d |
| Water, exposure areas (μg/L) |
0.1 to 0.81 | 1998 and 2009 | Environment Canada 2013d |
| Water (pulp and paper mills in Quebec) (μg/L) |
0.7-5.0 | 1998-2004 | NCASI 2006, MDDEP 2010 |
| Sediments, reference areas (mg/kg) |
3 to 41 | 1993-2002 | Environment Canada 2013d |
| Sediments, exposure areas (mg/kg) |
3.1 to 19 | 1993-2002 | Environment Canada 2013d |
8.3.15 Electrical and electronic equipment
No exposure scenario was developed for cobalt used in electrical and electronic equipment because the vast majority of these products are manufactured outside the country and no companies in this sector reported releases to the NPRI in recent years (NPRI 1995).
8.3.16 Battery recycling
Certain types of batteries contain cobalt that could be emitted during their recycling for metals recovery, or after their disposal in landfills. According to a study on battery recycling, two facilities in Canada are involved in this activity for cobalt recovery (Kelleher Environmental 2009). One of these facilities reported low releases of cobalt to the NPRI in recent years (maximum of 3 kg in 2008); hence no exposure scenario was developed for this facility. The second facility is a smelter for which an exposure scenario is already included in another section of this report (base metals smelting and refining).
8.3.17 Disposal and waste management
8.3.17.1 Disposal
Cobalt contained in products and manufactured items that are disposed of in landfills may leach out of these products and items and end up in landfill leachate. Monitoring data collected at 13 selected larger landfills between 2008 and 2012 under the Chemicals Management Plan monitoring program across Canada indicate that total cobalt concentrations in leachate range from less than 0.006 to 82 μg/L before any treatment (median of 12 μg/L). Five of the 13 landfills are treating their leachate on-site before either sending it to a WWTP or releasing it to the environment. For these landfills, total cobalt concentrations in leachate after treatment were 12 to 47 μg/L (median of 21 μg/L). In some instances, the concentration of cobalt in leachate was higher after treatment than before treatment, as illustrated by the low mean removal rate of 4% (Conestoga-Rovers and Associates 2013). For landfills that send their leachate (treated or not) to a WWTP, the dilution of the leachate in the WWTP influent in addition to the removal of cobalt during wastewater treatment followed by the dilution of the WWTP effluent in the receiving watercourse will likely result in the concentrations of cobalt below the levels of concern (PNEC) for aquatic ecosystems. For landfills that release their leachate (treated or not) to the environment, concentrations of cobalt could be of concern, if released directly to an aquatic ecosystem. For instance, one of the 13 landfills monitored discharged its treated leachate to a river. Total concentrations of cobalt in treated leachate at this landfill range from 15 to 47 μg/L. There could potentially be other landfills in similar situations.
8.3.17.2 Incineration
In Canada, 97% of wastes are landfilled and 3% are incinerated (Environment Canada 2007b; Statistics Canada 2013b). For those wastes that are incinerated, a mass-balance calculation was done to assess whether this source could be important in terms of releases of cobalt to the atmosphere. The municipal solid waste incinerator that is located in Burnaby, B.C., was chosen for the mass-balance calculation because it is the only incinerator for which emission data could be found. Considering that it is the second biggest incinerator in terms of quantity of wastes processed annually, it is likely a good representation of the other facilities in this sector (Environment Canada 2007b). Emissions from this facility for the sum of As, Co, Ni, Se and Te were 0.008 mg/m3 in 2007 based on periodic manual stack testing (Environment Canada 2011). MSW incinerators can produce volumes of flue gas ranging from 3.5 to 7 m3/kg of waste burned (Johnke 2000, UNEP 2002). Using the upper volume of this range and the quantity of wastes produced by the Burnaby incinerator, it is estimated that 1.91 Mm3 of gas were produced in 2006 by this incinerator. This would translate into releases of 15 kg for the sum of As, Co, Ni, Se and Te that would have been emitted to the atmosphere at this location in 2006. Based on this number, MSW incinerators in Canada are not considered to be an important source of cobalt in terms of atmospheric releases.
8.3.17.3 Wastewater, sludge and biosolids
Wastewater is a common point of entry of a substance to water through wastewater system effluent and a potential point of entry to soil through the subsequent management of biosolids. Data collected between 2009 and 2012 under the Chemicals Management Plan monitoring program for 27 WWTPs located across Canada indicate that total cobalt concentrations ranged from less than 0.006 to 43 μg/L in the raw influent and from less than 0.006 to 34 μg/L in the final effluent. Median values for both the influent and the effluent were less than 0.006 μg/L. Cobalt was detected in half of the wastewater samples; however, it was detected in all sludge and biosolids samples indicating its frequent presence in wastewater with a certain degree of partitioning to solids. Concentrations in primary sludge, waste biological sludge and treated biosolids ranged from less than 0.012 to 102 mg/kg dw (median of 1.8 mg/kg dw), less than 0.012 to 11 mg/kg dw (median of 1.9 mg/kg dw) and 0.060 to 138 mg/kg dw (median of 4.0 mg/kg dw) respectively (2013 personal communication from Emerging Priorities Division, Environment Canada, to Ecological Assessment Division, Environment Canada; unreferenced). As discussed earlier in this report, median wastewater removal rates of 27% for primary treatment and 62% for secondary treatment were calculated based on these data. In a study conducted for the Canadian Council of Ministers of the Environment to document the occurrence of certain substances in sludge and biosolids, samples were collected in 2009 at 11 WWTPs located across Canada. Cobalt was detected at seven of the eleven plants sampled, at median and maximum concentrations of 2.6 and 4.2 mg/kg dw, respectively (Hydromantis Inc. et al. 2010).
The highest concentrations (greater than 10 μg Co/L) measured in the final effluent of the WWTPs mentioned above were due to the presence of a cobalt-containing substance that had been previously used by an industrial facility. While recent monitoring data is not available for this WWTP, the facility's releases of cobalt are managed to an extent where current releases should be below levels of concern. All other concentrations in WWTPs were less than 1 μg Co/L (except one facility) suggesting that these effluents are unlikely to be a concern once diluted in receiving watercourses. One facility had effluent concentrations between 1.5 and 3.1 μg Co/L and releases it effluent to a watercourse for which a dilution capacity of 10 was used. The resulting concentrations in the receiving watercourse (PECs) range, therefore, from 0.31 to 0.47 μg Co/L after dilution and the addition of a background of 0.16 μg Co/L. It is recognized that there are numerous other WWTPs in Canada and that the data available represent only a small sample. However, it is expected that the highest concentrations of cobalt in effluent would be measured for WWTPs that receive wastewater from those industries that have reported using the cobalt compounds covered by this assessment and/or that release cobalt as a by-product of an industrial process. Exposure scenarios have been developed in this case and were presented earlier in this section.
Biosolids from WWTPs are sent to landfills, incinerated or spread on agricultural land. The equation below was used to estimate the input of cobalt to soils via spreading of biosolids containing cobalt.
EAC = [cobalt]sludge • application rate / depth • density
To simulate a worst-case scenario, a maximum application rate of 8300 kg dw/ha per year (based on the highest existing provincial regulatory limit; Environment Canada 2006), a mixing depth of 0.2 m (plough depth; ECHA 2012) and a soil density of 1200 kg/m3 were used (Williams 1999) along with the highest concentration of cobalt measured in biosolids in WWTPs in Canada, that are not incinerated (9.33 mg/kg dw). A period of 10 consecutive years was chosen as a length of accumulation (ECHA 2012). The cumulative cobalt concentration in soil at the end of this period is calculated to be 0.32 mg/kg dw. This EAC value is based on the conservative assumption that cobalt will not leach or run off, nor be taken up by plants and removed through harvest. Considering the median of background concentrations of cobalt in agricultural soils in Canada (8 mg Co/kg; NTE 2011), the spreading of biosolids to agricultural soils is not considered to be of concern for cobalt.
8.3.17.4 Additional sources of cobalt in agricultural soils
Biosolids are only one of the possible sources of cobalt in agricultural soils. In order to estimate several sources of cobalt simultaneously and to assess the relative importance of biosolids as a source, the National Agri-Environmental Health Analysis and Reporting Program (NAHARP) Trace Element (NTE) deterministic spreadsheet model was used (NTE 2011, Sheppard et al. 2009). The NTE model estimates concentrations of cobalt in soil expected at steady state, i.e. once inputs and losses have reached equilibrium. Additional details on this model are available in Sheppard et al. 2009 and ECCC (2016d).
The NTE model estimated the background level of cobalt to range between 5.4 and 22.1 mg/kg dw in soils, with a median of 8 mg/kg dw. This range is comparable to published values for Canadian soils. Total concentration of cobalt in soil resulting from atmospheric deposition, fertilizers, manure and biosolids was estimated to range between 0.025 and 0.77 mg/kg dw (median of 0.42 mg/kg dw). These results indicate that anthropogenic sources of cobalt to soil, including biosolids, are not significant in terms of exposure for soil organisms.
8.4 Exposure based on provincial or territorial-wide aquatic monitoring
8.4.1 Ontario
The Provincial Water Quality Monitoring Network (PWQMN) of the Ontario Ministry of Environment (OMOE 2013) includes measurement of many metals including cobalt and physical chemical parameters in surface water for 446 sites from 264 watercourses in Ontario. Data for total concentrations of cobalt from 2005 to 2011 were extracted as well as key toxicity modifying factors. 203 of the sites from 155 watercourses had cobalt concentrations that were above the detection limit (greater than or equal to 1.5 μg/L). Criteria were defined to select the sites with significant high concentrations. From the criteria, 14 sites from 12 watercourses were identified. The ranges of the cobalt concentrations (total) measured for Northern Ontario (8 sites) and Southern Ontario areas (6 sites) were from less than 1.5 to 146 μg/L and from less than 1.5 to 97 μg/L respectively. Details on data and criteria for selecting sites with high cobalt concentrations are available in ECCC (2016d).
8.4.2 Yukon
The Biomonitoring Information System for the Yukon (BISY) (Environment Canada 2013b) contains biological and physical chemical data on surface water and sediments in the Yukon Territory collected since 1973. All data for dissolved concentrations of cobalt in surface water (from 2002 to 2011) were extracted from the database as well as key toxicity modifying factors. Two separate method detection limits were used (greater than 0.005 or greater than 5 μg/L) and based upon these values, cobalt was detected at 281 of 702 sites where cobalt measurements were taken. The 702 sites covered 298 watercourses whereas the 281 sites covered 181 watercourses. At least 70 sites (approximately 25% of the sites where cobalt was detected) had cobalt concentrations exceeding the lowest hardness adjusted PNEC (0.78 μg/L). Most of these sites were found to be often relatively geographically close to each other, within 1 to 50 km approximately and thus formed clusters (n greater than or equal to 3) of monitoring sites which could be grouped together. Six areas (A to F), covering a total of 55 sites (24 watercourses) were identified as having high concentrations and possibly being under the influence of anthropogenic releases. Dissolved cobalt concentrations ranged from 1.3 to 1333 μg/L in these areas (n =55). For all other sites with concentrations mainly below 0.78 μg/L (n =211), an approximate geochemical background was calculated (median = 0.086 μg/L). Additional details on locations and data are available in ECCC (2016d).
9. Characterization of ecological risk
The approach taken in this ecological screening assessment was to examine supporting information and develop conclusions based on a weight-of-evidence approach and using precaution as required under CEPA. Lines of evidence considered include results from risk quotient calculations for key exposure scenarios, as well as information on persistence, bioaccumulation, toxicity, sources and fate of the substances. This section first presents the results of risk quotient analyses for exposure scenarios based on the various anthropogenic activities that may represent significant sources of release of cobalt to the environment. The environmental concentrations were either based on calculations using realistic estimations of releases (surface water) or field measurements (surface water, sediments and soils) with a focus on data collected in the vicinity of industrial or commercial facilities and settings. A summary section then brings together all the lines of evidence leading to a conclusion on the potential for ecological harm.
9.1 Industrial scenarios based on modelling of substance-specific information
Table 9-1 presents the risk quotient calculations for eight sectors using data on the cobalt content of the 13 non-UVCB cobalt-containing substances for which information on the manufacture, importation or use in Canada in recent years was received. Predicted environmental concentrations (PECs) were calculated by adding the highest median background value of 0.16 μg Co/L (see Table 8-2) to the modelled estimated aquatic concentrations (EACs) determined in the Ecological Exposure Assessment section. In cases where a concern was identified, the background concentration was either refined using site/area specific data geochemically relevant to the site receiving releases. If site or area specific information was not available, the highest median background concentration (0.16 μg/L) was used. The PNECs were adjusted for the hardness of the receiving watercourse (site-specific) or the hardness of the closest local or regional watercourse (see ECCC (2016c) for toxicity modifying factors correction details). Both PECs and PNECs have been expressed in terms of dissolved cobalt concentrations, and used to calculate the risk quotients (RQ = PEC/PNEC). Additional details on site-specific RQ calculations are available in ECCC (2016d).
| Use/sector | PEC (μg Co/L) |
Adjusted PNEC (μg Co/L) |
RQ range | Number of scenario(s) with RQ greater than 1 |
|---|---|---|---|---|
| Rubber | 0.17-0.56 | 0.78-1.30 | 0.13-0.55 | 0 |
| Manufacture or use of catalysts | 0.18-0.24 | 1.09-1.31 | 0.14-0.18 | 0 |
| Manufacture of chemicals | 0.20-0.24 | 0.93-0.98 | 0.20-0.26 | 0 |
| Paints and Coatings | 0.16 | 0.82-1.41 | 0.14-0.20 | 0 |
| Plastic (Polyester resin) | 0.16 | 0.82-1.13 | 0.14-0.20 | 0 |
| Fertilizers | 0.26-0.70 | 1.27 | 0.20-0.55 | 0 |
| Animal feed manufacturing | 0.49 | 1.60 | 0.31 | 0 |
| Alloy and superalloy manufacturing | 0.13 | 1.11 | 0.12 | 0 |
| Base metals smelting and refining | 0.25-11.8 | 0.82-1.80 | 0.20-6.56 | 2 |
Results show that for two scenarios, the RQs calculated from modelled aquatic PECs exceed one. The releases expected from the base metals smelting and refining (two out of five facilities) indicate that dissolved cobalt concentrations in waterbodies near sources of releases may exceed estimated no effect levels for aquatic organisms.
9.2 Industrial scenarios based on incidental releases and monitoring
The sections below, that include Tables 9-2 to 9-7, show the results of risk quotient calculations based on field measurement of concentrations in relevant environmental media for selected sites representing nine industrial sectors. The areas where these concentrations were measured are, for the vast majority, in the vicinity of facilities and installations where cobalt may be released in effluents or emitted to air. Total or dissolved cobalt concentrations were measured in receiving watercourses, sediments or soils where available and are presented as predicted environmental concentrations (PECs). These PECs include contributions from geochemical background (total risk approach). In parallel, PNECs were developed including the cobalt background in water, soil and sediment of the laboratory toxicity studies considered. Industrial releases to air which may be deposited to the water or soil compartments are included. PNECs were adjusted for the hardness of the receiving watercourse or for the CEC of the affected soil (site-specific). If the site-specific data were not available, the closest local watercourse or regional watercourses hardness was used for correction (same approach for soil CEC). See ECCC (2016c) for details regarding corrections applied to take into account toxicity modifying factors.
For the water compartment, PECs are expressed in terms of total or dissolved cobalt concentrations whereas PNECs are expressed as total. For sediments, both PECs and PNECs are expressed in terms of total cobalt concentrations. For soil, PECs are expressed in terms of total cobalt and the PNEC is adjusted using the CEC. Additional details on site-specific RQ calculations are available in ECCC (2016d).
9.2.1 Metal mining
Environmental concentration data (41 locations) associated with metal mining include 19 reference (often upstream) locations, 21 exposure (downstream) locations that are assumed to have the highest potential to be impacted by effluent discharges and one location affected by historical mining (Aldermac). Fifteen out of the 21 exposure locations were found to have RQs exceeding one (Table 9-2). The RQs calculated for the exposure locations, ranging from 0.01-23.3 for the water compartment and from 0.36-3.83 for sediments, are generally higher than RQs of corresponding reference locations. For the Aldermac location, RQs range from 0.09 to 193. RQs exceed 1 on 7 reference locations; although, 3 of the values were only slightly above 1 (ECCC 2016d). For the 4 remaining reference locations, RQs are significantly above one (ECCC 2016d). For 3 of these reference locations, with RQs significantly greater than 1, the presence of additional active mines and/or a smelter may have contributed to the cobalt concentrations measured. For the remaining reference location, the source responsible for the elevated RQ could not be identified. In summary, for this sector, dissolved or total cobalt concentrations in waterbodies and in sediment may exceed estimated no effect levels for aquatic organisms and sediment-dwelling organisms, respectively.
| Medium and units | PEC range | Adjusted PNECa | RQ range | Number of locations with RQ greater than 1 |
|---|---|---|---|---|
| Water, reference areas (μg/L) |
less than 0.05 to 11.8 | 0.78 | less than 0.06-15.1 | 3 |
| Water exposure areas (μg/L) |
0.01 to 42 | 1.80 | 0.01-23.3 | 9 |
| Water downstream historical mining/tailings site (Aldermac) (μg/L) |
0.08 to 176 | 0.91 | 0.09-193 | 1 |
| Sediments, reference areas (mg/kg) |
4.7 to 38 | 16.7 | 0.28-2.28 | 4 |
| Sediments, exposure areas (mg/kg) |
6.0 to 64 | 16.7 | 0.36-3.83 | 6 |
a. except for sediment where PNEC is generic; μg/L for water, mg/kg dry weight for sediment.
9.2.2 Base metals smelting and refining
Environmental concentration data were available for 22 locations (covering all media), including 14 exposure locations and 8 reference locations (Table 9-3). RQs exceeded one at the majority of exposure locations (10 out of 14 locations) and all media had exposure sites with RQs that surpassed one. The highest RQs were found for the water compartment (range less than 0.06-77), followed by sediments (range 0.06-2.69) and soils (0.06-1.63). RQs were found to exceed one at only one reference location out of eight. Thus, for this sector, dissolved or total cobalt concentrations in waterbodies, in sediment and soils near sources of releases may exceed estimated no effect levels for aquatic organisms, sediment-dwelling organisms and terrestrial organisms, respectively.
| Medium and units | PEC range | Adjusted PNECa | RQ range | Number of locations with RQ greater than 1 |
|---|---|---|---|---|
| Water, reference areas (μg/L) |
0.015 to 0.058 | 0.78 | 0.02-0.07 | 0 |
| Water, exposure areas (μg/L) |
less than 0.05 to 60 | 0.78 | less than 0.06-77 | 4 |
| Sediments, reference areas (mg/kg) |
14 to 50 | 16.7 | 0.84-2.99 | 1 |
| Sediments, exposure areas (mg/kg) |
1 to 190 | 16.7 | 0.06-11.4 | 3 |
| Soil, reference areas (mg/kg) |
2 to 38 | 70.7 | 0.03-0.54 | 0 |
| Soil, exposure areas (mg/kg) |
0.5 to 195 | 8.8-119.4 | 0.05-4.09b | 3 |
a. except for sediment where PNEC is generic; μg/L for water, mg/kg dry weight for sediment and soil.
b. 4.09 calculated with values not presented in table-PEC (36 mg/kg) and PNEC (8.8 mg/kg) (see ECCC 2016d).
9.2.3 Iron and steel
For this sector, it is expected that deposition of cobalt to soil would not be of concern for soil organisms. Similarly, deposition to waterbodies is expected to be low. Concentrations of cobalt monitored in 2010 and 2011 in the Hamilton Harbour, which has a high dilution capacity, were 0.12 and 0.082 μg/L respectively. This is significantly lower than the worst-case aquatic PNEC (lowest hardness) of 0.78 μg/L. Therefore, these concentrations, which reflect all sources of cobalt in the Hamilton Harbour area, are below the thresholds of concern for aquatic organisms. However, it was not possible to assess the risks to benthic organisms in Hamilton Harbour, because no measured concentration data were available for cobalt in the sediments. Considering the low measured concentrations in the water compartment, concentrations in sediments from this source are expected to be low. Therefore, risks to organisms are not expected to be a concern for this sector.
9.2.4 Electricity (power generation)
Coal fired and heavy oil fired electrical power generation plants represent sources of cobalt considered in this assessment. No measured data were available to develop a realistic exposure scenario for oil-fired power plants. However, it is expected that releases of cobalt from an oil-fired power plant would not be higher than those from the coal-fired power plants. Based on some of the measured environmental concentrations presented in section 8, realistic worst-case PEC ranges were chosen and RQs calculated to quantify exposure and risks of ecological receptors to cobalt for a coal-fired power generation scenario. Results are presented in Table 9-4. Risk quotients associated with electrical power generation are low and therefore risks to organisms are not expected in any media from this source.
| Medium and units | PEC range | Adjusted PNECa | RQ range |
|---|---|---|---|
| Water (μg/L) |
0.019 to 0.056 | 1.07 | 0.02-0.05 |
| Sediments (mg/kg) |
1.4 to 9.1 | 16.7 | 0.08-0.54 |
| Soil (mg/kg) |
3 to 13 | 30.2 | 0.10-0.43 |
a. Except for sediment where PNEC is generic; μg/L for water, mg/kg dry weight for sediment and soil.
9.2.5 Petroleum refining
Releases of cobalt to water from petroleum refineries' process effluents are possible. The highest average from the older data, (two refineries: 9.8 μg/L) could be of concern depending on the dilution factor expected for a short stretch (300 m) of the receiving water body before joining the St-Clair River. Assuming a dilution factor of 10 and a background cobalt concentration of 0.04 μg/L [Ontario Region (Erie-Superior-Ontario), Table 8-2], would give a PEC of 1.02 μg/L. Using the hardness adjusted PNEC for the site (0.94 μg/L) would give a calculated RQ of 1.09, slightly exceeding the threshold of possible risks to aquatic organisms.
More recent data from 2008 from three additional refineries in Canada revealed that resulting PECs (0.21, 0.26 and 0.36 μg/L) are all below the level of concern for aquatic organisms (lowest PNEC of 0.78 μg/L) following the addition of the highest background level of 0.16 μg/L to the EACs.
Overall, data are available for 6 out of the 15 refineries in Canada. Based on this information, the evidence of harm is weak considering that some of the concentration data are old (1988-1989), for the older data the exceedance is slight at only 1 site, and more recent data from 2008 indicate no concern.
9.2.6 Oil sands
Releases of cobalt to air from the Oil sands are expected to be lower than those from power generation plants. As such, no risks are expected from the cobalt releases to air. The contribution to the deposition to water bodies would likely be reflected in the concentration of cobalt considered below.
Measured concentrations of total and dissolved cobalt are available for nearly 40 water bodies located in the Athabasca area (Alberta) where most of the oil sand operations are located (RAMP 2012). Table 9-5 below presents the results of this monitoring in terms of PECs, PNECs and resulting RQs. Only the dissolved concentrations were considered for the calculation of RQs.
| Medium | PEC range | 5th a | 50th a | 95th a | Adjusted PNECb | RQ Percentilesc (5th-50th-95th) |
|---|---|---|---|---|---|---|
| Water (μg/L) |
less than 0.01-23 | 0.024 | 0.1 | 0.34 | 0.90 | 0.03-0.11-0.38 |
| Sediment (mg/kg) |
0.2-13.5 | 0.9 | 5.0 | 10.0 | 16.7 | 0.05-0.30-0.60 |
a. Percentiles.
b. except for sediment where PNEC is generic; μg/L for water, mg/kg dry weight for sediment.
c. Calculated risk quotients based on percentiles data: (5th-50th-95th).
Risk quotients are low and therefore risks to aquatic organisms and sediment-dwelling organisms are not expected.
9.2.7 Pulp and paper mills
Regarding releases of cobalt to water, two sources of data were considered: the EEM program where field data were collected and a regulatory program from the Ministère du développement durable, de l'environnement et des parcs du Québec where concentrations in raw effluents were measured. From the latter source, PECs were calculated (see the Ecological Exposure Assessment section for details). Table 9-6 below shows the risk quotients calculated from both sources of data.
| Medium and units | PEC range | Adjusted PNECa | RQ range | Number of locations with RQ greater than 1 |
|---|---|---|---|---|
| Water, reference areas (μg/L) |
0.1 to 0.3 | 0.78 | 0.13-0.38 | 0 |
| Water, exposure areas (μg/L) |
0.1 to 0.81 | 0.78-1.21 | 0.13-0.67 | 0 |
| Sediments, reference areas (mg/kg) |
3 to 41 | 16.7 | 0.18-2.46 | 1 |
| Sediments, exposure areas (mg/kg) |
3.1 to 19 | 16.7 | 0.19-1.14 | 1 |
| Water (pulp and paper mills in Quebec) (μg/L) |
0.7-5.0 | 0.78-0.94 | 0.90-6.41 | 3 |
a. except for sediment where PNEC is generic; μg/L for water, mg/kg dry weight for sediment and soil.
The risk quotients indicate that the six pulp and paper mills (EEM data) are not significant sources of cobalt releases to aquatic ecosystems. All mills show minimal or no difference in measured cobalt concentrations between reference sampling sites and effluent discharge point or downstream for both water and sediment. While a potential risk was identified in the sediments at one site, the source of cobalt is likely not attributable to the pulp and paper mills because the far field concentrations are higher than at the effluent discharge point (ECCC 2016d). Therefore, the source of this cobalt is of unknown origin. Potential risk to aquatic organisms was identified for three Quebec mills. The cobalt concentrations in the effluent are likely influenced by the type of pulping process and the use of cobalt-containing chemicals.
9.2.8 Disposal and waste management
Among the 13 landfills monitored under the CMP monitoring program across Canada for 2008-2012 one case needed further characterization as it released its treated leachate to a river and concentrations of cobalt in the leachate were relatively high, ranging between 15 to 47 μg/L. Using a dilution factor of 10 and adding 0.16 μg/L for background led to a predicted environmental concentrations of 1.66 to 4.86 μg/L. Using the worst-case PNEC (0.78 μg/L), calculated RQs would range from 2.13 to 6.23. Risk is therefore identified for aquatic organisms under this scenario.
9.2.9 Wastewater, sludge and biosolids
Data were collected between 2009 and 2012 under the Chemicals Management Plan monitoring program for 27 WWTPs located across Canada. PECs ranging from 0.31 to 0.47 μg Co/L were calculated for the facility showing the highest cobalt concentration in its effluent and not previously considered in other sectors or scenarios. Using the site-specific hardness adjusted PNECs (0.80 μg/L), the calculated RQs range from 0.39 to 0.59. There is no risk identified for aquatic organisms.
The calculations presented in the section "Ecological Exposure Assessment" have shown that spreading of biosolids to agricultural soils is not considered to be of concern to terrestrial organisms for cobalt.
9.3 Provincial or territorial-wide aquatic monitoring
9.3.1 Ontario
The Provincial Water Quality Monitoring Network (PWQMN) of the Ministry of Environment of Ontario (OMOE 2013) includes measurement of cobalt and physical chemical parameters in surface water for 446 sites from 264 watercourses in Ontario. Criteria were developed (ECCC 2016d) to select fourteen sites with the most elevated cobalt concentrations: eight sites for Northern Ontario and six sites for Southern Ontario. Table 9-7 below shows the results of risk quotients calculations ranges for the sites in the two areas.
Results show that more than half of the selected sites with elevated RQs (ranging from less than 0.83 to 81) are located in Northern Ontario, near metal mining, base metals smelting, and/or refining operations (6 sites) or historical mines (2 sites) and under the influence of significant releases of cobalt to the water compartment. These sites are in the vicinity of mining areas with active or historical mining operations. At historical metal and coal mines, AMD is usually released into the environment without treatment. Conversely, all or most of the AMD at operating mining sites is collected with other streams, such as process effluents and runoffs, and is released as part of the treated liquid effluent at the final discharge point. Some of the sites had elevated hardness when compared to nearby watercourses suggesting that some of the mining effluents may be treated with lime in order to precipitate some of the metals they contain before they are released to the environment. However, in spite of the possible treatments, cobalt releases are still significant at these locations and this is reflected in the high RQs calculated in the watercourses which receive these effluents.
Thus, effluents from the final discharge point of operating mines, base metal smelting, and/or refining operations and acid mine drainage from historical mines are likely the main sources of cobalt to the aquatic compartment in the vicinity of mining installations or tailings. Smelters may also contribute to the aquatic concentrations after deposition of particulates but likely to a lesser extent in the short-term because of the small direct surface area for deposition in comparison to soil. In addition, less soluble cobalt-containing particulates are more likely to settle out of the water column.
Six other sites located in Southern Ontario also show elevated RQs (ranging from 1.36 to 58) but are not in the vicinity of contributors of cobalt that could be identified.
9.3.2 Yukon
The Biomonitoring Information System for the Yukon (BISY) (Environment Canada 2013b) includes information on dissolved concentrations of cobalt in surface water for 702 sites covering 298 watercourses in the Yukon Territory. Criteria were developed to select six areas with the most elevated cobalt concentrations and are presented in the Ecological Exposure section. The results of risk quotient calculations for all six areas are presented in Table 9-7.
Risk quotients for surface waters in this region were high, ranging from 0.98 to 741, which indicate a high risk for aquatic organisms. Potential sources of cobalt were identified for all areas. Four of the six areas (A, B, E, F) are located in the proximity of identifiable individual or combined sources of cobalt including: metal mining operations (A and B), historical mines (E) (that may include abandoned mine tailings) and metal mining exploration (F). These areas are likely under the influence of considerable anthopogenic releases of cobalt to the water compartment. According to details found in the BISY database, the two remaining areas (C and D) are affected by runoff/seepage which has come into contact with natural rock with high concentration of cobalt, potentially contributing to the elevated cobalt concentrations. Although naturally-occurring instances of high cobalt concentrations in rock may, in certain rare circumstances, increase the natural background for the water compartment on certain specific locations, the Yukon-wide geochemical background is 1 order of magnitude lower than the aquatic worst-case PNEC and comparable to other Canadian regions (median of 0.086 μg/L). Elevated natural sources may exceptionally be present in this region. However, past or actual anthropogenic activity may still contribute to the cobalt loadings being measured. In addition, since there are no active smelters or refineries in the territory, releases likely result from mining facilities, historical mines and metal mining exploration (Environment Canada 2013b; Mining Association of Canada 2015). Metal mining exploration likely has a limited potential to release cobalt considering the smaller operations that have lower environmental disturbances. Therefore, area F may potentially also include natural mineralization runoff/seepage or historical sources, but no evidence was found to support this.
| Area | Possible contributing sectors/sources | PEC range (μg/L) |
Adjusted PNECa (μg/L) |
RQ range | Number of sites with RQ greater than 1 |
|---|---|---|---|---|---|
| Northern Ontario | metal mining | less than 1.5 to 146b | 1.80 | less than 0.83-81 | 3 |
| Northern Ontario | base metals smelting and refining | less than 1.5 to 2.92 | 0.78 | less than 1.92-3.72 | 2 |
| Northern Ontario | metal mining and base metals smelting and refining | less than 1.5 to 12.1b | 1.80 | less than 0.83-5.64 | 1 |
| Northern Ontario | historical mining activity | 4.12 to 46.4b | 1.14; 1.21 | 3.61-38.3 | 2 |
| Southern Ontario | unknown | 1.5 to 97b | 1.10-1.66 | 1.36-58 | 6 |
| Yukon, areas A and B | metal miningc | 1.3 to 225a | 1.32 | 0.98-137d | 2e |
| Yukon, area E | historical mining activity | 1.42 to 141a | 1.80 | 0.79-78.3 | 1f |
| Yukon, area F | metal mining exploration | 9 to 1221 | 1.80 | 5-67.8 | 1g |
| Yukon, areas C and D | runoff/seepageg | 3.08 to 1333a | 1.80 | 1.71-741 | 2i |
a. dissolved cobalt concentration.
b. total cobalt concentration.
c. may also include metal mining exploration as a lesser contributing source.
d. 137 is calculated with the 95th percentile of the concentration (181.4 μg/L).
e. areas A and B include a total of 35 sites.
f. area E includes a total of 6 sites.
g. area F includes a total of 7 sites.
h. from contact with natural rock with high cobalt concentration.
i. areas C and D include a total of 7 sites.
9.4 Summary of ecological risk characterization
This screening assessment focuses on the cobalt moiety, and thereby includes cobalt in its elemental form, cobalt-containing substances and cobalt released in dissolved, solid or particulate form. It considers all substances having the potential to dissolve, dissociate and/or degrade to release cobalt through various transformation pathways that can potentially contribute to the combined exposure of ecological receptors to the cobalt moiety of concern (i.e. dissolved cobalt). In turn, dissolved cobalt can cause harmful effects to organisms. Sources of cobalt include activities involving the fifty cobalt-containing substances that had been identified as meeting the categorization criteria, as well as incidental production and natural/ambient background concentration of cobalt. Data on manufacture, import and use of specific substances were used, where possible, to model releases to estimate PECs. As well, other anthropogenic incidental sources of the metal to the environment were systematically included using a sector-based approach and through the use of monitoring data to estimate PECs.
Risk quotients, which are based on either modeled or measured concentrations of cobalt (total or dissolved) were calculated for a variety of sectors and activities involving cobalt releases. Table 9-8 shows a summary of the sectors and activities of concern and the media affected. Locations with RQs exceeding 1 were associated with sectors/activities that are expected to contribute to cobalt concentrations in the vicinity to the extent possible. When both the reference location and the corresponding exposure location had RQs exceeding 1, risks were not attributed to the sector/activity in question unless additional sources could be identified. If additional sources could not be identified, the locations were added to the "unidentified sources" category.
| Sector/activity | Type of scenario (modeled/measured) | Number of locations with risk quotient(s) greater than 1 (water) |
Number of locations with risk quotient(s) greater than 1 (sediment) |
Number of locations with risk quotient(s) greater than 1 (soil) |
|---|---|---|---|---|
| Metal mininga | measured | 13 (0.56-137) |
5 (0.36-3.83) |
- |
| Base metals smelting and refining | modeled | 2 (6.562) |
- | - |
| Base metals smelting and refining | measured | 5 (less than 0.06-77) |
3 (0.06-11.4) |
3 (0.19-4.09) |
| Metal mining and/or base metals smelting and refining | measured | 3 (0.93-5.64) |
2 (0.43-1.06) |
- |
| Historical mining activity | measured | 4 (0.09-193) |
- | - |
| metal mining exploration | measured | 1 (5-67.8) |
- | - |
| Pulp and paper mills | modeled (water)c | 3 (0.90-6.41) |
- | - |
| Leachate (from landfills) |
modeledd | 1 (2.13-6.23) |
- | - |
| Unidentified sourcese | measured | 8 (0.13-58) |
4 (0.90-2.46) |
- |
| Total | 40 (0.09-193) |
14 (0.06-11.4) |
3 (0.19-4.09) |
a. Includes locations from Table 9-2 (EEM-water and sediments) and Table 9-7 (PWQMN and BISY - water).
b. maximum of range.
c. based on effluent concentrations.
d. based on leachate concentrations.
e. Include locations from the metal mining sector (water and sediments) and the pulp and paper mills sector (sediments).
A total of 40 locations or areas of concern for aquatic organisms covering four sectors were identified. These sectors include: metal mining, base metals smelting and refining, pulp and paper mills, and leachate (from landfills). In addition, historical mining and metal mining exploration activities were also identified with locations of concern. Fourteen locations of concern were identified for sediment-dwelling organisms covering two sectors (metal mining and base metals smelting and refining). Three locations showing risk to terrestrial organisms were identified in the base metals smelting and refining sector. A relatively small but significant proportion (~5% or 3 locations) of pulp and paper mills were identified as being of concern due to RQ results. The cobalt concentrations in the effluent are likely influenced by the type of pulping process and the use of cobalt-containing chemicals. However, there is insufficient evidence to conclude that the pulp and paper sector as a whole or a particular sub-sector (manufacturing process) is of concern. Another sector or source found to be of concern was leachate from landfills with 1 location for the water compartment. In addition, eight locations of concern to aquatic organisms and four locations for sediment-dwelling organisms were identified with uncharacterized sources.
The data indicate that the metal mining and base metals smelting and refining sectors are of concern for cobalt (in all media) at approximately half of the total number of locations/areas assessed. These sectors also show the highest number of locations with risk quotients greater than 1 identified in the assessment. Releases of liquid effluent at the final discharge point is the most important source of concern for aquatic organisms for these sectors. Releases to air can also be significant for the base metals smelting and refining sector and some of the soil monitoring studies were indicative that this is the case. Releases to air may also contribute to exposure to the water compartment by deposition; however, this contribution seems to be limited. Historical mining activities and metal mining exploration were also found to be a cause for concern for cobalt: the calcuted RQs for these activities are as high or higher as active mining facilities (ECCC 2016d). However, since the focus of the assessment was primarily on active facilities, the extent of consideration of historical mining activities and metal mining exploration was not as comprehensive, resulting in a lower number of locations identified. Even if these results are less extensive, historical mining activites and, to a lesser extent, metal mining exploration are also identified to be a cause for concern for cobalt. It is assumed that historical mining activities have a higher potential for releases than metal mining exploration.
9.5 Consideration of lines of evidence and uncertainties
A weight of evidence approach, where several lines of evidence for ecological risk (e.g., environmental fate, bioaccumulation, ecological effects) and precaution (as appropriate) were applied to develop a conclusion as required under CEPA. The lines of evidence and the weighting assigned to each line of evidence, based on considerations of uncertainty and relevance in the assessment, are described in Table 9-9 and described in this section.
The weight of evidence approach includes a qualitative assessment of the relevance of each line of evidence according to scientific or regulatory importance in the assessment. A weight is assigned to each line of evidence based on a function of level and direction of uncertainty and relevance of that line of evidence in the assessment. Qualifiers used in the analysis range from low to high. The weight of evidence serves to determine the overall confidence in the decision-making process as well as indicate the key lines of evidence supporting a risk outcome.
To assess the impacts of the identified uncertainties on the risk assessment of cobalt and cobalt-containing substances, both the level and direction of uncertainty were identified for each line of evidence. Uncertainties arise from data gaps due to limited, incomplete, or absence of data as well as data variability. Estimation of the level of uncertainty was based on the availability and quality of data and its suitability (e.g., representativeness of realistic environmental conditions considering natural background and adequate source identification). The direction of uncertainty was based on scientific analysis and judgement as to whether the specific uncertainty could lead to either an over or underestimation of risk or has negligible or unknown impact on the risk outcome. Relevance refers to the impact of the evidence in the assessment scientifically and/or from a regulatory perspective.
| Line of evidence | Level of uncertaintya | Direction of uncertaintyb | Relevance in assessmentc | Weight assignedd |
|---|---|---|---|---|
| Physical and chemical properties - water solubility | Low | +/- | Moderate | Low to moderate |
| Environmental Fate - persistence | Low | +/- | Moderate | Low to moderate |
| Bioaccumulation/biomagnification | Low | +/- | Moderate | Moderate |
| Ecotoxicity - PNEC aquatic | Low | +/- | High | High |
| Ecotoxicity - PNEC sediment | Moderate | +/- | High | Moderate to high |
| Ecotoxicity - PNEC soil | Low to moderate | +/- | High | Moderate |
| Environmental exposure - bioavailable concentration (total vs dissolved) | Low to moderate | + | High | Moderate to high |
| Environmental exposure - source identification - metal mining; base metals smelting and refining | Low to moderate | +/- | High | Moderate to high |
| Environmental exposure - source identification - pulp and paper; leachate | Low | +/- | High | Moderate to high |
| Risk quotient analysis - magnitude; locations/area impacted - metal mining; base metals smelting and refining | Low to moderate | +/- | High | Moderate to high |
| Risk quotient analysis -magnitude; locations/area impacted - pulp and paper; leachate | Moderate | +/- | High | Moderate |
a. Level of uncertainty is determined according to data quality, data variability, data gaps and if the data are fit for purpose.
b. Denotes if uncertainty may contribute to over-estimation of risk (+) or under-estimation of risk (-); +/- indicates little impact or direction is unknown.
c. Relevance refers to the impact of the evidence in the assessment scientifically and/or from a regulatory perspective.
d. Weight is assigned to each line of evidence and it is directly related to its relevance in the assessment as well as factors such as data suitability and quality.
Water solubility of cobalt-containing substances was attributed a low uncertainty since empirical data points exist for most discrete substances. However, in certain cases, solubility can vary as a result of environmental conditions. Solubility has a moderate relevance in the assessment as the values may be useful to confirm solubility in modeled scenarios; however, the relevance is lower for measured dissolved concentrations where the dissolved state is known and water solubility values are not needed. The weight assigned is therefore low to moderate due the fact that this information is occasionally important, even if only to qualitatively confirm the potential of discrete substances to contribute to environmental cobalt loadings.
There is high certainty that, once released into the environment, cobalt is expected to be infinitely persistent in water, soil and sediment. Cobalt can therefore accumulate in the environment year after year, resulting in increased exposure mainly in the latter two compartments. However, the bioavailability of cobalt in these compartments may be partially reduced by ageing processes. This line of evidence is more applicable as a prediction for the future trends of measured concentrations in the environment mainly in soil and sediment compartments. Indeed, actual measured concentrations incorporate this fate characteristic. Therefore, the attributed weight for persistence is low to moderate.
The bioaccumulation potential of cobalt is relatively low. However, cobalt uptake may still lead to levels causing harm to sensitive species at body concentrations higher than those required for essentiality. The relevance and weight assigned are thus moderate.
Cobalt has been demonstrated to have a high toxicity to sensitive aquatic organisms, sediment-dwelling organisms and terrestrial organisms. Survival, growth or reproduction of these organisms may be affected. In addition, the biological diversity and the stability of the food chain may be adversely impacted by cobalt (e.g., reduction in the quality and quantity of fish food sources). Considering the number of species, taxa and the extent of toxicity modifying factors inclusion, the aquatic PNEC has the lowest level of uncertainty followed by the soil and sediment PNECs. The relevance in the assessment is high for all three PNECs but considering the frequency of their use in the assessment, the weight assigned is highest for water, followed by sediment and soil.
While it is understood that additional abiotic modifying factors, beyond hardness (e.g., pH, DOC), may influence cobalt toxicity in surface waters under certain circumstances, they were not corrected for in this assessment. The rationale considered for this decision was the following: (i) based on current observations, water hardness is the key and most important factor explaining almost the entire variability in the organisms responses; (ii) there is evidence that in addition to Co2+ ions, cobalt complexes with particulate organic matter or dissolved humic and fulvic acids may be available for uptake by some types of aquatic organisms; and (iii) for most sites, risk quotients are elevated and adding additional toxicity modifying factors (e.g., BLM) may only have resulted in minor variations in toxicity, not significantly changing the majority of risk quotients and conclusions.
The consideration of bioavailablity of cobalt is an important aspect for environmental exposure. The measured environmental concentrations for many sectors including the province-wide PWQMN database present cobalt results as total cobalt rather than dissolved cobalt. The dissolved fraction represents a fraction of total cobalt which can vary depending on the quantity and binding strength of particles, and site-specific factors such as watershed and watercourse type, geology, biological productivity and other factors. The percentage dissolved fraction range calculated from the RAMP data varies from 10 to 50% (RAMP 2012). It was not possible to calculate a correction from the RAMP data as the site-specific factors are too complex and variable to build a reliable model. However, despite the differences between total to dissolved concentrations, it is still expected that most identified risk quotients for the aquatic compartment would remain above one. For example, the RQs calculated using the BISY database data (Yukon Territory) were based on dissolved concentrations in a similar metal mining context compared to northern Ontario (PWQMN, OMOE 2013). They were comparable and even more elevated. Thus, it was not deemed necessary or critical to integrate additional aquatic modifying factors at this time, for this screening assessment. This line of evidence was attributed a moderate to high weight considering that the level of uncertainly may vary from low to moderate depending on the data considered. The relevance in assessment is high.
There are three types of source identification/apportionment for cobalt and other metals present naturally in the environment: anthropogenic versus natural sources, historical versus recent sources and the type of activity/sector involved. The latter two are discussed below.
Historical contamination may be present and part of some of the measured concentrations in soils and sediment depending on depth. While top soil samples concentrations from 0-5 cm rather than 0-10 cm were used in the assessment and should be more consistent with recent contamination, it was not possible to account for historical versus recent contamination in these samples. Therefore, for these soil or sediment sites, risk quotients may represent total (historical and recent) estimates of harm. In the water compartment, however, the measured concentrations can reasonably be expected to represent recent or actual releases from active or historically used sites. The historical component consideration has a lower coverage in this assessment since the main focus was on active facilities, thus, the identification of this concern bears higher uncertainity.
Efforts were made throughout this ecological assessment to focus on all sources of cobalt and link assessment endpoints and exposure scenarios to industrial activities to the extent possible. While the vast majority of measured concentrations where related to identifiable sectors/activities, some relatively high concentrations found at certain sites in the provincial PWQMN databases or other sites are not linked to obvious sources. Hence, source apportionment was not possible at these sites. The cobalt concentrations measured at those sites may originate from multiple sources or one major source.
The level of uncertainty for source identification for the metal mining and base metals smelting and refining sectors is low to moderate. The weight assigned is moderate to high. Some of this information is used to help inform the potential risk management phase but is still needed to confirm anthropogenic sources of concern for the determination of toxic under CEPA.
For the following sectors/activities: pulp and paper, leachate (from landfills) and the manufacture or use of catalysts, the level of uncertainty is lower as these sources are easier to delineate because concentrations are measured in the effluent or leachate directly (pulp and paper, leachate) or the quantity treated by the WWTP is known and the modeled concentrations and the measured concentrations are relatively similar (manufacture or use of catalysts). For the same reasons as for the metal mining and base metals smelting and refining sectors, the weight assigned is moderate to high for the environmental exposure for these sectors.
Risk quotients, which are based on either modeled or measured concentrations of cobalt (total or dissolved) were calculated for a variety of sectors and activities involving cobalt releases. Magnitude and extent of impact in terms of number/proportions of sites/areas affected were assessed for the main sectors/activities of concern. The level of uncertainty for the metal mining and base metals smelting and refining sectors is lower than for the remaining of sectors/activities of concern. The latter have less estimates/measurements for environmental concentrations compared to the metal mining and base metals smelting and refining sectors for which concern was identified at approximately half of the total number of locations/areas assessed. The relevance in assessment of such lines of evidence is critical and was attributed a high ranking for all sectors/activities. The weight assigned to the metal mining and base metals smelting and refining sectors was moderate to high whereas, the remaining sectors were attributed a moderate ranking.
It is acknowledged that the calculation of RQs could have been expressed including the quantification of confidence intervals or margins based probabilistic approaches. It is acknowledged that the uncertainty on the determination of risk for RQs around 1 is very high compared to more elevated RQs. Where possible, other lines of evidence have been considered for cases where RQs were close to 1 (e.g., petroleum refining sector, see Characterization of Ecological Risk section).
9.6 Conclusion of ecological risk characterization
Available measured concentrations for all the sites where incidental releases could be significant were considered and results indicated that a significant proportion of these sites also have concentrations higher than the background for all media in Canada in particular for water. As well, the high manufacture and importation volumes of some of the cobalt-containing substances in Canada notably elemental cobalt, cobalt sulfate, cobalt chloride and cobalt hydroxide along with information on their uses and the calculated RQs support the likelihood that anthropogenic releases of these substances result in concentrations of the metal at levels higher than local background and effects thresholds concentrations in the Canadian environment (water, sediment and soil).
This cobalt moiety-based assessment examined the combined exposures from a wide range of sources covering all substances that could contribute to loadings of the moiety in the environment.
Considering the key lines of evidence such as infinite persistence, bioaccumulation, high inherent toxicity, the magnitude of RQs, the number of location/areas affected in all media and the established links with anthropogenic sources and releases, there is risk of harm to organisms from cobalt and soluble cobalt compounds in Canada. Cobalt and soluble cobalt compounds have been determined to meet the persistence criteria but do not meet the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
10. Potential to cause harm to human health
10.1 Health effects assessment
The human health risk of four cobalt-containing substances that are included in the grouping were assessed during the earlier Challenge initiative of the CMP (elemental cobalt, CAS RN 7440-48-4; cobalt chloride, CAS RN 7646-79-9; two cobalt sulfates, CASRN 10124-43-3 and CAS RN 10393-49-4) (Environment Canada, Health Canada 2011a). The details of the health effects database for these substances can be found in the Health Effects Assessment section and the Appendix VIII of the Cobalt screening assessment report (Environment Canada, Health Canada 2011a). While a tabulation of the health effects database considered in this cobalt and cobalt-containing substances screening assessment is provided in a supporting document (HC 2016), a summary of the health effects can be found in Tables E-1 and E-2 of Appendix E.
Justification for a moiety-based assessment
Stopford et al. (2003) and CoRC (2010a) conducted in vitro bioaccessibility studies to investigate the solubility of various cobalt compounds in artificial physiological fluids. Their findings demonstrated that almost all cobalt substances are completely soluble under physiologically relevant concentrations and pH conditions similar to those found in gastric juice (approximately pH 1.5). The studies also demonstrated that all the soluble cobalt substances release toxicologically equivalent divalent cobalt (Co2+) moiety. The divalent cobalt moiety is highly soluble and readily bioavailable compared to trivalent cobalt moiety, which is usually found in insoluble oxides or hydroxides. Accordingly, the systemic health effects of cobalt substances are expected to be primarily due to Co2+ while the local health effects are expected to result from the combination of released ions and parent compound at the point of contact (Reviewed in ATSDR 2004; IPCS 2006; IARC 2006). In addition, the in vitro studies available for a wide range of cobalt carboxylates indicated that bioavailability (under physiological conditions), pharmacokinetics and toxicicological effects were independent of the counter ion (Stopford et al. 2003; Firriolo et al. 1999). Consistent observations were made in an in vivo acute toxicity study in rats exposed to a series of soluble cobalt salts. The median lethal dose (LD50) values for the majority of the substances were in the range of 140 to 190 mg Co/kg bw. The investigators concluded that the LD50 values (calculated based on the dose of Co2+ ions) were within a factor of two for many of the bioavailable cobalt compounds, regardless of the counter ion (Speijers et al. 1982). However, LD50values for insoluble cobalt substances could be significantly different from the above observation (e.g.: LD50 for cobalt sulphide is greater than 3200 mg Co/kg bw).
The available data support the concept of using the health effects data from cobalt moiety to characterize health effects for bioavailable cobalt substances. Use of health effects data from highly bioavailable cobalt substances to characterize health effects of less bioavailable substances is considered conservative.
Toxicokinetics
Cobalt is an essential component of vitamin B12, which serves as a cofactor in the synthesis of methionine and metabolism of folates and purines. The recommended daily allowance of vitamin B12 in adults is 2.4 μg/day and the cobalt content in this dose is about 0.1 μg/day (ATSDR 2004; Health Canada 2010 and IOM 1998). Cobalt in vitamin B12 remains bound and does not affect the free cobalt levels in the body. As a result, cobalt in vitamin B12 does not contribute to any potential negative health effects of the free cobalt ion in humans or in animals. After oral exposure, cobalt salts completely or nearly completely dissociate into the Co2+ ion and the anionic component. The Co2+ ion is rapidly absorbed through the small intestine (Ayala-Fierro et al. 1999). While the absorption of ingested cobalt can vary from 3-97% from individual to individual depending on the dose, form and nutritional state; the majority of people have an absorption fraction in the range of 15-35% for soluble cobalt chloride and 1-3% for relatively insoluble cobalt oxide (Christensen et al. 1993, Leggett 2008). One study in humans observed that gastrointestinal (GI) uptake of cobalt was significantly higher in females than in males (Christensen et al. 1993). Studies have shown that fasting individuals or those who have low iron levels absorb more cobalt than healthy, adequately nourished individuals. It is considered that cobalt and iron share a common absorptive intestinal pathway, though the cobalt absorption does not require binding to ferritin,an iron binding protein in the body (ATSDR 2004; Harp and Scoular 1952; Smith et al. 1972; Sorbie et al. 1971; Reuber et al. 1994; Valberg et al. 1969).
Following inhalation exposure to cobalt, mainly in the metallic form in the occupational setting and as cobalt oxides in airborne dust, the deposition pattern in the respiratory track primarily depends on the particle size. Larger particles (greater than 5μm) deposit in the nasopharyngeal region of the upper respiratory track while smaller particles deposit in the tracheobronchiolar region (2-5 μm) and the alveolar sacs (1 μm or less) of the lower respiratory track (Klaassen 2001). Once deposited, larger particles and physiologically insoluble particles can undergo mechanical clearance or be transferred to the GI tract and therefore have low systemic absorption. Small particles which are more water soluble can be absorbed and distributed via lymphatic and vascular systems (Bailey et al. 1989; Collier et al. 1989). Studies in hamsters indicated that approximately 30% of inhaled cobalt oxide was absorbed through the lungs (Wehner et al. 1977).
In general, in vivo animal studies indicate that dermal absorption of cobalt is relatively low (ATSDR 2004; Horev-Azaria et al. 2011; Lauwerys and Lison 1994). However, some positive skin sensitization reactions and elevated urinary elimination of cobalt in human volunteers exposed under experimental settings demonstrate that cobalt can be absorbed through intact skin (Scansetti et al. 1994). In vitro studies of dermal absorption of highly soluble cobalt substances also indicate that dermal absorption of Co2+ is low and therefore, the authors concluded that "percutaneous uptake is a negligible route for risk characterization"(CoRC 2010a). Other in vitro absorption studies have shown that when cobalt powder dispersed in synthetic sweat (pH 4.5) was applied to outer skin, a substantial amount of cobalt can penetrate through damaged skin compared to intact skin (Filon et al. 2009).
Cobalt does not undergo metabolism within the body, but, rapidly transforms to Co2+ upon dissolution. Following absorption, cobalt is distributed throughout the body with the highest concentrations noted in the liver and kidney. Intravenous administration of radioactive cobalt chloride indicated that liver is the main repository soon after administration and could hold about 20% of the total body burden of cobalt (Elinder and Friberg 1986). The blood clearance appears to be triphasic, with half lives of 0.5, 2.7 and 59 days (Morsy et al. 1970). Cobalt is eliminated via both urine and faeces. In general, faecal elimination is the primary route of elimination for unabsorbed cobalt while for absorbed cobalt; renal elimination plays a predominant role (Leggett 2008). Human and animal studies demonstrated that cobalt elimination is multi-phased and most of the cobalt is eliminated rapidly from the body regardless of exposure route. Inhalation exposure of cobalt particles in occupational settings showed first rapid elimination (t½ in the range of 2-44 hrs) mainly due to mucociliary clearance of particles deposited in the tracheobronchial region, followed by slower elimination (t1/2 in the range of 10-78 days), which may represent macrophage mediated clearance of cobalt particles from the lung and finally long term retention of relatively insoluble cobalt (eg: cobalt oxide) in the deep regions of the lungs with the half-life in the order of years (Mosconi et al. 1994). Oral intakes of cobalt by human volunteers showed a long-term biological half-life extending up to 625 days (Beleznay and Osvay 1994; Leggett 2008). Smith et al. (1972) studied the total body retention of cobalt in healthy human volunteers after receiving radio-labelled cobalt chloride (60CoCl2) by intravenous injection. The biological half-lives and the retention were reported as 0.5 days (44%), 6 days (32%), 60 days (13%) and 800 days (11%). A similar retention pattern was reported by Letourneau et al. (1972) for exposure to 58CoCl2 via intravenous injection. Intra-peritoneal injection of rats with radiolabeled cobalt showed that the initial retention of the majority of cobalt was found in liver; but the long-term retention was primarily in skeleton followed by skeletal muscle, liver and kidney (Barnaby et al. 1968). Cobalt content in skeleton and skeletal muscles increases over time after initial exposure (Leggett 2008).
Health effects in humans
Cobalt is known to stimulate the production of red blood cells and is a potent erythropoietin transcription inducer (Davis and Fields 1958; Unice et al. 2012). A transient increase in red blood cell numbers and haemoglobin levels (polycythemia) was observed in a study of six adult male volunteers dosed orally with cobalt chloride at approximately 1 mg Co/kg bw per day for 3 weeks (Davis and Fields 1958). Similar effects were observed in patients on dialysis given cobalt chloride as treatment for anaemia at approximately 0.16 to 0.32 mg Co/kg bw per day for several months (Duckham and Lee 1976; Taylor et al. 1977). In contrast, pregnant women given cobalt chloride during the third trimester at 0.45 to 0.62 mg Co/kg bw per day did not have increased haemoglobin and red blood cells. In addition, no developmental effects of the fetuses were observed following treatment of pregnant women with cobalt chloride (Holly 1955). Children (ages 5 to 9 years old) dosed up to 1.8 mg Co/kg bw per day showed no change in hemoglobin levels (Jaimet and Thode, 1955 as reviewed by Finley et al. 2012). While polycythemia is considered a desirable effect in anaemic patients, long-term polycythemia may result in slower blood flow to vital organs due to increased thickness of blood. Some of the initial secondary symptoms may include headaches, blurred vision, a ruddy complexion, increased blood pressure, titinnus and dizziness. These patients may also experience itchy skin, especially after shower, due to the release of histamine by increased numbers of white blood cells. In severe cases, slow blood flow can also cause blood clots, which increase the risk of heart attack, stroke and blockage of lungs. Polycythemia is a reversible haematological effect, where exposed individuals returned to normal levels after cobalt treatment ended. It has also been suggested that large quantities of cobalt salt may be administered to athletes as an alternative blood doping agent due to its ability to induce erythropoietin (Simonsen et al. 2012). The lowest end of the dose range which did not result in polycythemia in pregnant women, that is 0.45 mg Co/kg bw per day (corresponding 290 μg/L whole blood cobalt concentration derived using the biokinetic model documented below) was identified as the suitable endpoint for risk characterization of oral exposure to cobalt by the general population.
Reversible thyroid effects have been reported in some individuals orally exposed to cobalt. Thyroid hyperplasia and enlargement were reported in some anaemic children who underwent cobalt therapy at doses of 2.8 to 3.9 mg Co/kg bw per day for 3 to 8 months (Gross et al. 1955; Kriss et al. 1955). Roche and Layrisse 1956 reported decreased iodine uptake in healthy adults administered 0.97 mg Co/kg bw per day for two weeks while Paley et al. 1958 reported similar observations in two out of 4 patients orally administered 0.54 mg Co/kg bw per day for 10-21 days. In contrast, thyroid effects have not been observed in some anemic children orally administered 1.8 mg Co/kg bw per day for 10 weeks or in any of the mothers or their offspring of pregnant women treated with cobalt chloride at 0.45 to 0.62 mg Co/kg bw per day (Holly 1955; Jaimet and Thode, 1955 in Finley et al. 2012).
In mid-1960s, a series of case reports were published on the mortalities among heavy beer drinkers in North America and Europe due to cardiomyopathy, which is a chronic disease of heart muscle, characterized by abnormal electrocardiograms, enlarged heart, left ventricular failure, diminished myocardial compliance and pericardial effusion, as well as by extensive intracellular changes, including alterations in the myofibrils, glycogen and cellular mitochondria (Paustenbach et al. 2013). The individuals in these studies chronically drank large quantities of beer containing cobalt sulfate, which was added by some breweries as a foam stabilizer. Thyroid hyperplasia was also reported in some of the heavy beer drinkers. The exposure to cobalt from beer in these individuals was estimated to be 0.04 to 0.14 mg Co/kg bw per day, based on a cobalt concentration in beer of 1 to 1.5 mg/L and consumption of 8 to 30 pints (4-14 liters) per day (Bonenfant et al. 1969; Alexander 1972; reviewed in ATSDR 2004; IPCS 2006). The reported dose estimates in these beer drinkers have a large degree of uncertainty, because the concentration of cobalt in the beer and amount of beer consumed by the individuals as well as their body weights are not well documented. These beer drinkers were malnourished and known to consume a protein-poor diet leading to low circulating blood proteins. As a result, it is considered that these subjects were increasingly susceptible for cobalt toxicity due to the presence of elevated levels of non-protein-bound cobalt (free cobalt) in blood and cardiac tissues. Kesteloot et al. 1968 reported that well-nourished beer drinkers who consumed similar quantities of cobalt sulphate-treated beer did not develop cardiac problems. Authors of a recent cross-sectional survey conducted in a cobalt production facility in Belgium concluded that there was no dose-response relationship between occupational cobalt exposure, as determined by urinary cobalt concentrations, and parameters reflecting dilated cardiomyopathy (Lantin et al. 2013).
While it is unlikely that a healthy population would be susceptible to similar cardiac damage, the lower range of the estimated intake, that is 0.04 mg Co/kg bw per day (corresponding to 26 μg/L whole blood cobalt concentration derived from the biokinetic model documented below) from the case studies involving cobalt exposure from beer consumption was selected as the most conservative endpoint for the risk characterization of the oral exposure of cobalt.
The lowest exposure level associated with respiratory effects were reported in a cross-sectional study of individuals occupationally exposed in the diamond industry in which the cohorts were exposed to airborne cobalt dust from the use of cobalt-containing polishing discs (Nemery et al. 1992). In this study, 194 diamond polishers (166 men and 28 women) from the diamond polishing industry in Belgium and 59 workers from other workshops (control group) in the diamond industry were examined. The individuals were divided into three exposure categories: control (mean environmental air concentration of 0.0004 ± 0.0006 mg Co/m3), low (mean environmental air concentration of 0.0053 ± 0.0032 mg Co/m3) and high (mean environmental air concentration of 0.0151 ± 0.0117 mg Co/m3). Exposure groups were defined based on air measurements at the time of the study, and exposure was confirmed by the measurement of cobalt in urine. The duration of employment was not noted. In the high exposure group, significant increases in the prevalence of eye, nose and throat irritation, cough and reduced lung function were reported. Although some symptoms, such as cough and phlegm were reported in the low exposure group, these symptoms were not statistically significant compared to control values. Air concentrations of 0.0053 mg Co/m3 and 0.0151 mg Co/m3were determined as the no- observed-adverse-effect-concentration (NOAEC) and the lowest-observed-adverse-effects-concentration (LOAEC), respectively. The LOAEC of 0.0151 mg Co/m3 was considered the critical effect level for the risk characterization of inhalation exposure of cobalt.
Cobalt has been classified for dermal sensitization by the EU ("may cause sensitization by skin contact"). Several assays for skin sensitization in mouse lymph node assay (LLNA) show that cobalt is a potential skin sensitizer (CoRC 2010d, f, CoRC 2013 a, b, Ikarashi et al. 1992a, b). Skin sensitization has been reported in studies with human volunteers, individuals occupationally exposed to cobalt and in clinical trials (CoRC 2010a; Pratt et al. 2002; Uter et al. 2005). In a human study, when volunteers were exposed to cobalt chloride at 0.02 mg/m2, positive results for skin sensitization was reported for 5 men and 30 women (CoRC 2010a). Similarly, positive results were observed for in vitro skin corrosion assay using the Human Skin Model, skin sensitization in mouse local lymph node assay and in vivo guinea pig maximization test and guinea pig adjuvant and patch test (Yamano et al. 2006; Yanagi et al. 2001). Contact dermatitis in humans is common: in several large studies, patch tests have detected sensitization to cobalt in up to 10% of patients (ATSDR 2004; IPCS 2006).
The EU has also classified cobalt as an inhalation sensitizer:"may cause sensitization by inhalation". Bronchial asthma has been described in workers exposed to various forms of cobalt, including 'pure' cobalt particles and other cobalt compounds including cobalt salts (Nemery et al. 1992; Swennen et al. 1993; ATSDR 2004).
Derivation of whole blood cobalt concentrations
Cobalt is one of the chemical substances measured as part of the Canadian Health Measures Survey (CHMS) biomonitoring study. Cobalt was measured in the blood and urine of over 6000 Canadians aged 3 to 79 years at 18 sites across Canada from 2009 to 2011(Health Canada 2013). In order to use the blood cobalt levels measured in the CHMS biomonitoring study in the general population for risk characterization, the corresponding whole blood cobalt concentrations at the points of departure in experimental animals and in epidemiology studies are required. As very few dosing studies in experimental animals or humans have measured blood cobalt concentrations, a biokinetic model was used. Biokinetic models describe the biological behavior of inorganic cobalt in humans and laboratory animals, and provide a method to convert oral doses to corresponding blood cobalt concentrations at the point of departure (Leggett 1998; Unice et al. 2012 and Finley et al. 2012). The model proposed by Leggett (2008) is one of the most comprehensive biokinetic models for cobalt, because this model is based on a large database of literature on the distribution of inorganic cobalt in humans and experimental animals. Furthermore, the model was developed with the intention of incorporating a physiologically realistic framework that represents the cycling of cobalt between blood and tissues and the transfer to the excretory pathways. Unice et al. (2012) used the Leggett (2008) biokinetic model along with a standard human alimentary track model (ICRP 2006). The objective of this model is to predict urinary or blood cobalt levels following different ranges of exposure: from acute or chronic oral occupational exposures, medicinal therapy, dietary and supplemental intake or other non-occupational exposures.
The blood cobalt concentrations are considered to be representative of steady-state exposure, while urine concentrations are representative of more recent exposure. Urinary levels decline rapidly within 24 hrs after exposure ceases (Alexandersson et al. 1988). Furthermore, cobalt does not undergo metabolism in the body. Since there are no metabolites, it is possible to assume that the blood cobalt concentrations are in a relative steady-state with tissue concentrations. Therefore, the estimation of blood cobalt concentrations is considered to be a better biomarker of cobalt exposure than the urine cobalt concentrations.
A recent publication by Finley et al. (2012) used the Unice et al. (2012) biokinetic model to predict the whole blood and urine cobalt concentrations for a number of health effect endpoints for cobalt. In this publication, the GI absorption fraction and body weight were assumed as 15% and 70 kg, respectively. The estimated whole blood cobalt concentrations for the oral dose levels (0.04 mg Co/kg bw per day) for cardiomyopathy in malnourished beer drinkers were in the range of 15 to 34 μg/L. The whole blood cobalt concentration at the LOAEL (approximately 1 mg Co/kg bw per day) for polycythemia in healthy male volunteers was 320 μg/L and the NOAEL (0.53 mg Co/kg bw per day) for polycythemia in pregnant women was 200 μg/L (Finley et al. 2012). Furthermore, the authors of this publication concluded that blood cobalt concentrations are a valuable predictor of adverse health effects.
Health Canada (Nong et al. 2013) used the same Unice et al. (2012) model with the fractional GI absorption of 25% to derive the whole blood cobalt concentration at the point of departure for beer drinkers' cardiomyopathy. Although there is an indication of gender variation in cobalt absorption, when the absorption fraction in both men and women were combined, most individuals have an absorption fraction in the range of 15%-35% for soluble cobalt (Christensen et al. 1993; Leggett 2008). The central tendency of this absorption range, which is approximately 25% was applied in deriving whole blood cobalt concentration. This range for soluble cobalt is also consistent with inorganic cobalt absorption in mature rats; with GI absorption ranges from 13% to 34% for cobalt chloride while only 1%-3% for insoluble cobalt oxide (ATSDR 2004). Unice et al. (2012) reported daily intakes and estimated average blood and urine concentrations of cobalt for short (1 day) to continuous daily long-term (365 days) exposure (Table 1 in Unice et al. 2012). This data was used to generate regression lines for predicting blood and urine concentrations based on oral intake. These blood and urine concentrations estimates were based on a 70-kg person and the authors assumed that the ingested cobalt had an average fractional absorption of 25%. The longest term of exposure (365 days) estimated values were used in this regression analysis. The linear relationship between chronic daily intake and the modeled blood or urine cobalt concentrations after one year can be described as follows:
- Cobalt blood concentration (μg/L) = 0.0092 × (cobalt oral intake μg/day) + 0.2374 (R2 0.99)
- Cobalt urine concentration (μg/L) = 0.1095 × (cobalt oral intake μg/day) + 0.3741 (R2 0.99)
These regression equations suggest that there is a linear relationship between oral intake of cobalt and corresponding blood and urine concentrations above an initial background level of cobalt. The background level is assumed to be the static essential level stored in the body found in healthy individuals before loss or intake of cobalt.
For oral dosing studies in which blood cobalt concentrations were not reported, the blood cobalt concentrations associated with the effects and no-effects doses were estimated using the above described regression equations derived based on the Unice et al. (2012) biokinetic model (as shown earlier).
Based on the regression equations described above, the estimated whole blood cobalt concentration for beer drinkers' cardiomyopathy based on the point of departure of 0.04 mg/kg bw per day (equivalent to 2800 μg per day for a 70 kg person), is 26 μg/L. Similarly, the estimated whole blood cobalt concentration for polycythemia based on the point of departure of 0.45 mg/kg bw per day (equivalent to 31 500 μg per day for a 70 kg person), is 290 μg/L.
Unice et al. (2012) and Nong et al. (2013) applied validation methods to characterize the accuracy of the model predictions. A detailed description of the validation method applied in Unice et al. (2012) can be found in Finley et al. (2012) and Tvermoes et al. (2013). In summary, 4 adult male volunteers were orally exposed to 400 μg/d of cobalt (0.005 mg Co/kg bw per day) in the form of cobalt chloride as a liquid ionic cobalt supplement (Mineralife) for 14 days. The blood samples were taken and analysed for cobalt before the dosing and several times during the dosing. Blood cobalt concentrations before dosing were less than the detection limit of 0.5 μg/L while the mean whole blood concentrations of volunteers at the end of dosing were 3.6 μg Co/L and the ranged from 1.8 to 5.1 μg Co/L. Blood monitoring data suggest that 2 of the volunteers reached the steady state after 8 or 9 days of supplement intake. However, it is unclear whether the rest of the volunteers reached the steady-state during dosing since blood cobalt levels were still increasing at the end of the treatment. When the biokinetic model was applied, for a mean oral absorption rate of 25%, the whole blood cobalt concentration at the end of 14-day exposure period was reported as 2.4 μg/L. When the mean blood cobalt concentration of the volunteers at the end of exposure period was compared with the model predictions, the blood Co levels of the volunteers were within 5% of the concentration range predicted by the biokinetic model assuming 15-35% GI absorption into systemic circulation. This indicates that the biokinetic model developed by Unice et al. (2012) accurately predict the central tendencies for cobalt whole blood concentrations during oral exposure to cobalt. The human biokinetic model (Unice et al. 2012) applied in this study indicates that for a mean oral absorption factor of 25%, whole blood cobalt concentration is expected to increase by less than 1 μg/L when dosing period is increased from 14 days to 1 year (Tvermoes et al. 2013).
The second validation method was used by Nong et al. (2013). First, estimates of daily intake (dietary) were used to predict blood and urine concentrations. Dietary intake is considered to be the primary source of exposure to cobalt for the general Canadian population and these estimations were obtained from the Food Directorate of Health Canada. Secondly, these dietary estimates were used in conjunction with the regression lines described (equations 1 and 2) above to predict the corresponding blood and urine concentrations. The predicted blood concentrations ranged from 0.28 to 0.40 μg/L while urine concentrations ranged from 0.14 to 1.51 μg/L. These were in turn compared with blood and urine concentrations measured in CHMS. It was reported that cobalt was detected in almost 100% of the population with a median whole blood concentration of 0.22 μg/L and a 95th percentile of 0.40 μg/L (Health Canada 2013). Median and 95th percentile urine concentrations were 0.25 and 0.97 μg/L respectively (Health Canada 2013). Hence, the predicted blood and urine concentrations from the regression equations were within the range of cobalt concentrations measured in Canadians.
The biokinetic models described here were applied to derive whole blood cobalt concentrations at the point of departure dose level of oral toxicity studies, where systemic toxicity was observed. Most of the inhalation toxicity studies on cobalt are associated with route of entry (pulmonary) effects. The derivation guidelines for biomonitoring equivalent (BE) (Hays et al. 2008) indicate that it is not appropriate to derive whole blood or urine chemical concentrations for site of entry or contact effects, in particular when exposures in the general population are either from multiple routes of entry. The guidelines published by Hays et al. (2008) state "Since biomonitoring data cannot distinguish among the routes of entry for chemical exposures, BEs derived from exposure guidance values established to protect against route of entry effects could be misleading unless the exposures for a given chemical are known to occur predominantly by the route of entry of concern". Therefore, whole blood or urine cobalt concentrations were not generated for the point of departure from inhalation toxicity studies.
Health effects in experimental animals and in vitro
Carcinogenicity and genotoxicity
Occupational exposure studies are insufficient to evaluate the carcinogenicity of cobalt substances in humans due to confounding effects (IARC 2006). However, in a 2-yr inhalation study on rats and mice exposed to aerosols of cobalt sulphate (CoSO4.7H2O) at 0.11, 0.38, or 1.14 mg Co/m3 significant increases in benign and malignant alveolar/bronchiolar tumours in both species and sexes were observed (significant at high concentration for male mice and rats and significant at mid and high concentration for female mice and rats) (Butcher et al. 1999; NTP 1998). Oral and dermal carcinogenicity studies were unavailable (IARC 2006). However, available short-term and subchronic oral studies in animals or human case studies do not provide evidence for potential site-specific or systemic carcinogenicity through oral route. Based on the results of 2-yr inhalation study in rodents, the International Agency for Research on Cancer (IARC) has classified elemental cobalt and soluble cobalt (II) salts as Group 2B carcinogens (possibly carcinogenic to humans) based on inadequate evidence in humans and sufficient evidence in experimental animals (IARC 2006). On similar basis, cobalt chloride and cobalt sulfate have been classified by the European Commission as Category 2 for carcinogenicity (should be regarded as if it is carcinogenic to man) (ESIS 2006; European Commission 2004a, b). The United States National Toxicology Program (NTP) considers cobalt sulfate to be "reasonably anticipated to be a human carcinogen" based on sufficient evidence of carcinogenicity in experimental animals (NTP 2005).
In 2014, NTP conducted a chronic inhalation study by exposing male and female rats and mice to particulate aerosol of cobalt metal at concentrations of 0, 1.25, 2.5, or 5 mg/m3 for two years. Similar to the above noted study which used cobalt sulfate, this study reported increased incidences of alveolar/bronchiolar adenoma and carcinoma in the lung. However an increased incidence of benign and malignant pheochromocytoma of the adrenal medulla, and an increased incidence of pancreatic islet adenoma or carcinoma (combined) were noted in this study, suggesting a toxicological profile, specific to the metal form. Further, the occurrences of cystic keratinizing epithelioma of the lung and of renal tubule adenoma or carcinoma (combined) were also considered potentially related to exposure. Based on theses evidence, the NTP (2014) concluded that there was "clear evidence of carcinogenic activity of cobalt metal" in male and female F344/NTac rats and in male and female B6C3F1/N mice. Consistent with these recent classifications, the NTP fourteenth report on carcinogens has listed the class of cobalt and cobalt compounds that release cobalt ions in vivo as "reasonably anticipated to be human carcinogens". This listing is based on sufficient evidence of carcinogenicity in experimental animals through inhalation exposure and exposure via injection (site-specific tumors) and supporting data from studies on mechanisms of carcinogenesis (NTP 2016).
In vitro and in vivo genotoxicity data indicate that elemental cobalt and cobalt-containing substances have the potential to cause DNA and chromosome damage. In 2004, cobalt chloride and cobalt sulfate are classified as Category 3 mutagens ("cause concern for man owing to possible mutagenic effects") by the European Commission (ESIS 2006; European Commission 2004a,b). In a recent review of all available mutagenicity and genotoxicity data of cobalt by the OECD Cooperative Chemicals Assessment Meeting (CoCAM), it was concluded that "soluble cobalt salts do not elicit any mutagenic activity either in bacterial or mammalian test systems" (OECD 2014). The observed in vitro genotoxicity of cobalt is likely mediated by indirect mechanisms including the generation of reactive oxygen species, increased oxidative stress, and inhibition of DNA repair enzymes (reviewed in IPCS 2006; OECD 2014).
While there are no oral studies on carcinogenicity of cobalt, the available inhalation studies on rats and mice (Butcher et al. 1999; NTP1998) indicate that the point of departure for carcinogenic effects were approximately 25 times higher than the dose levels that reported for non-carcinogenic effects such as reduced lung function in workers exposed to cobalt dust (Nemery et al. 1992).
Reproductive and developmental effects
Cobalt chloride and cobalt sulfate are classified by the EU as Category 2 Reproductive toxicants ("should be regarded as if they impair fertility in humans") (ESIS 2006; European Commission 2004a,b). No data on the potential for reproductive toxicity of elemental cobalt or soluble cobalt (II) salts in humans were available; however, effects on the male reproductive system have been observed in rodents. Male mice were administered cobalt chloride in the drinking water at 0, 200, 400 or 800 ppm (0, 9.9, 19.8, 39.7 mg Co/kg bw per day) for 12 weeks, and then mated with untreated females. At all doses, there were decreased implantations, increased number of resorptions, decreased number of viable fetuses, and decreased sperm counts; and at the two higher doses, there was also decreased relative testes weight, and testes necrosis and degeneration (Elbetieha et al 2008). Reduced fertility, decreased sperm concentration and motility, testicular atrophy, degeneration and necrosis were also reported in several other studies in male mice and rats given higher oral doses of cobalt chloride (Anderson et al. 1992, 1993; Corrier et al. 1985; Domingo et al. 1985; Mollenhaur et al. 1985; Nation et al. 1983; Pedigo et al. 1988; Pedigo and Vernon 1993). In a 13-week study in mice exposed to cobalt sulfate aerosols by inhalation, sperm motility was decreased at 1.14 mg Co/m3 and higher; and at 11.38 mg Co/m3, there was testicular atrophy, increased abnormal sperm, and decreased testes weight (Bucher et al. 1990; NTP 1991).
In pregnant rats administered cobalt sulfate by gavage at 5.2, 10.5, or 21 mg Co/kgbw per day during gestation, there was a statistically significant increase in frequency of skeletal malformations, decreased pup body weight at postnatal days 1 and 7, decreased survival from birth to postnatal day 5, and delays in postnatal developmental parameters (ear opening, incisor eruption, descending of testes, swimming performance and auditory reflex) at all dose levels relative to controls. By postnatal day 21, body weight, developmental parameters, and survival rates (day 5-21) had returned to control levels. Some maternal toxicity was observed at the high dose (increased relative weight of liver, adrenals and spleen; serum alterations) (Szakmary et al. 2001).
The reproductive and developmental effects of cobalt in experimental animals occur at dose levels more than 100 times higher than the dose level reported for beer drinkers' cardiomyopathy, which is the lowest dose level associated with adverse health effects. Therefore, reproductive and developmental effects were not considered as critical health effects for human health risk characterization.
Other health effects
In short term oral studies, when rats were treated with cobalt chloride hexahydrate for 8 weeks, increase in red blood cell (erythrocytes) numbers (polycythemia) was reported at 2.5 mg Co/kg bw per day (Stanley et al. 1947). In a subchronic study on cobalt chloride in rats, transient increase in red blood cells and increased haemoglobin were observed at doses of 0.5 mg Co/kg bw per day and persistent polycythemia at 2.5 mg Co/kg bw per day (Krasovskii and Fridlyand 1971). Similar effects have also been reported in humans exposed to cobalt substances.
In rats administered cobalt sulfate in the diet for 24 weeks at 8.4 mg Co/kgbw per day, cardiac enzyme activity and mitrochondrial ATP production were significantly reduced. The hearts of treated animals were isolated and were found to have left ventricular hypertrophy and impaired ventricular function (Haga et al. 1996; Clyne et al. 2001). Cardiomyopathy has been observed in guinea pigs exposed through oral gavage to cobalt sulfate at 20 mg Co/kg bw per day for 5 weeks (Mohiuddin et al. 1970) and also in rats exposed to a single gavage dose of 176.6 mg Co/kgbw (Speijers et al. 1982). Thyroid necrosis was observed in mice dosed by the oral route for 15 to 45 days with cobalt chloride at 26 mg Co/kg bw per day (Shrivastava et al. 1996).
Exposure of rats to cobalt substances resulted in neurological effects including mild to moderate reduction in spontaneous activity, muscle tone, touch response and respiration for single gavage exposure of 4.24 mg Co/kg as cobalt chloride (Singh and Junnarkar 1991). Long-term exposure of rats to cobalt chloride resulted in a significant increase in the latent reflex period at or above 0.5 mg Co/kgbw per day (Krasovskii and Fridlyand 1971).
In 13-week and 2-year inhalation studies, when rats and mice were administered cobalt sulfate in the dose range of 0 to 11.38 mg Co/m3, a 'spectrum of inflammatory, fibrotic and proliferative lesions in the nose, larynx and lung were reported. The severity of the effects was increased with the increasing dose levels in both species (Bucher et al. 1990, 1999; NTP 1991, 1998).
Confidence in health effects characterization
The confidence in the health effects database on cobalt moiety is moderate. Data on acute toxicity, repeated-dose toxicity, carcinogenicity, genetic toxicity, and reproductive and developmental toxicity in experimental animals are available, although no chronic oral studies are available. Studies in humans include occupational exposure to inhaled cobalt, short-term oral studies on cobalt salts in anaemic patients and volunteers, longer-term oral exposure to cobalt sulfate in beer drinkers, and in individuals taking cobalt compounds as health supplements. The database is limited by the small number of subjects in the oral studies on humans, as well as by the absence of healthy controls in those studies. Inhalation studies in humans are limited as most are in occupational scenarios where other substances are present (i.e. hard metals), and exposures are difficult to characterize.
Confidence in the use of a moiety approach for health effects characterization is moderate. Studies on solubility and bioavailability of several cobalt substances indicate that most inorganic cobalt substances are soluble and bioavailable under physiological conditions (Stopford et al. 2003; CoRC 2010a). Available rodent studies that compare the acute toxicity of several organic cobalt substances demonstrated that soluble cobalt substances have relatively similar acute toxicity regardless of the counter ions (Firriolo et al. 1999; and Speijers et al. 1982; Stopford et al. 2003). These observations support the application of health effects data of cobalt moiety to characterize the health effects of soluble cobalt substances within the grouping. However, limited toxicity data are available on less bioavailable cobalt substances. The applicability of this approach for less soluble cobalt substances is uncertain, as it may lead to greater conservatism for these substances. Similarly, limited data are available on organometallic and UVCB cobalt substances. However, the characterization of risk is based on the most conservative toxicological endpoints in the database, which is considered to account for uncertainties in the health effects database of the grouping.
Confidence in the biokinetic model used in predicting whole blood cobalt concentrations at the point of departure of beer drinkers' cardiomyopathy is moderate. The biokinetic model described in Leggett (2008) was based on a large database of literature on cobalt distribution in humans and animals and has also incorporated physiological factors necessary for the reliable estimation of the exchange of cobalt between key tissue compartments and blood. Furthermore, the validation methods suggest that the biokinetic model described in Unice et al. (2012) accurately predicts the central tendencies of whole blood cobalt concentrations for various exposure scenarios investigated. The confidence in use of blood cobalt as a measure of steady-state exposure is high as is confidence in use of blood cobalt levels, at the point of departure in the health effects study, as an effect level for cardiomyopathy and polycythemia in humans.
10.2 Exposure assessment
Cobalt is a naturally occurring element and is an element in the Earth's crust with an average concentration of 0.0025% (ATSDR 2004). As a result of both natural occurrence and anthropogenic use, cobalt is present in soil, household dust, airborne particulate matter, surface, ground and drinking water as well as food. The levels of cobalt in environmental media and food in Canada which were used to estimate cobalt intakes are described below; average estimates of daily intake and data tables are presented in Appendix G. In addition to environmental media and food, other sources of potential exposure to cobalt include use products containing cobalt and current or historical industrial point sources such as mines.
Biomonitoring data are available for the general Canadian population as well as individuals with elevated exposure. The measurement of cobalt in blood and urine represents exposure from all sources. Typical analytical methods used to measure inorganic substances such as cobalt are not able to identify the different cobalt oxidation states or substances (e.g. elemental, ionic, oxide, or specific salt) therefore, the biomonitoring data integrates exposure from not only all sources but also from all cobalt substances, both natural and anthropogenic, which release the cobalt moiety (including but not limited to the categorized substances listed in Table A-1). The summary of cobalt levels in environmental media and food in Canada are presented following the biomonitoring data.
Biomonitoring data
Cobalt has been measured in a wide variety of biological media including whole blood, serum, plasma, urine, human tissues, human milk, nails, and hair (ATSDR 2004); concentrations in blood and urine are presented in Appendix F, Table F-1 and F-2. In April 2013, the Government of Canada released national cobalt biomonitoring data collected from 2009 to 2011 as part of the CHMS. Cobalt was measured in the blood and urine of over 6000 Canadians aged 3 to 79 years at 18 sites across Canada from 2009 to 2011. In order that the results of the CHMS be representative of the entire Canadian population sample weights were applied. The weighted data represents 96.3% of the Canadian population. People living on reserves or in other Aboriginal settlements in the provinces, residents of institutions, full-time members of the Canadian Forces, persons living in certain remote areas, and persons living in areas with a low population density were excluded. The CHMS is not a targeted survey, thus did not target individuals with high cobalt exposures. Cobalt was detected in blood in almost 100% of the population with a median whole blood concentration of 0.22 μg/L and a 95th percentile of 0.40 μg/L (Health Canada 2013). Median and 95th percentile urine concentrations were 0.25 and 0.97 μg/L respectively with detection in 93% of the population (Health Canada 2013). There were no differences in cobalt blood concentrations by age. Urinary cobalt concentrations were significantly higher in children 3 - 19 over adults. Generally urine concentrations are observed to decline with age. Females had higher blood and urine concentrations than males, but this was only statistically significant at the 95th percentile concentrations. Females have higher blood and urine cobalt concentrations than the total population; however these differences were not statistically significant.
Concentrations of cobalt measured in adults in Quebec City, adults in the west coast of British Columbia, and children in Alberta are similar to cobalt concentrations in the general population of Canada as measured in the CHMS but lower than cobalt concentrations measured in pregnant women in Alberta (Alberta Health and Wellness 2008; Clark et al. 2007; INSPQ 2004; Government of Alberta 2010). Increases in cobalt blood concentrations during pregnancy, followed by a decline postpartum, have been observed in other studies (Hansen et al. 2011). Cobalt readily crosses the placenta and concentrations in cord blood are reflective of maternal blood concentrations (Ziaee et al. 2007). Cobalt sera concentrations have been shown to rise in infants immediately after birth upon initiation of feeding (human milk or formula) (Krachler et al. 1999). Generally, serum concentrations of cobalt are slightly higher than whole blood measurements. Urinary cobalt concentrations in Canadians are similar to those measured in the United States as part of the 2009-2010 National Health and Nutrition Examination Survey (NHANES) (US CDC 2013), in France 2006-2007 (Fréry et al. 2010), and in Germany (Heitland et al. 2006).
Elevated concentrations of cobalt in the blood have been measured in people following occupational exposure to cobalt and those with metal-on-metal hip replacement but these subpopulations are not within the scope of this assessment.
Total cobalt measured in whole blood is representative of cobalt exposure from all routes and all sources including environmental media, diet, frequent or daily use products, and vitamin B12. Due to its natural presence in vitamin B12 and considering that vitamin B12 is essential for human health, cobalt will always be present in humans. Vitamin B12 was measured in the serum of Canadians participating in the CHMS (MacFarlane et al. 2011, Statistics Canada 2013). Children (3 to 11 years) have significantly higher vitamin B12 concentrations than adults. Median and 95th percentile serum concentrations of vitamin B12 in Canadians aged 3 to 79 years were 0.42 and 0.85 μg/L respectively. Based on the cobalt and vitamin B12 measurements in the CHMS, vitamin B12 is estimated to account for approximately 10% of total cobalt measured in blood; this has also been reported in other studies (Hansen et al. 2011). The cobalt in vitamin B12 is sequestered and not bioavailable as the free cobalt ion. Cobalt blood concentrations from the CHMS are representative of steady-state exposure, while urine concentrations are representative of more recent exposure. Urinary levels decline rapidly within 24 hours after exposure ceases (Alexandersson et al. 1988). Inter-individual differences in cobalt absorption in the GI tract are large and are impacted by the form of cobalt, the dose of cobalt, the nutritional status and iron levels of the subjects (ATSDR 2004). Studies in animals have suggested that children may absorb more cobalt from foods and liquids containing cobalt than adults (ATSDR 2004) and that female have higher absorption than males (Christiansen et al. 1993 from ATSDR 2004). Once absorbed and distributed through the body, cobalt is excreted predominantly in the urine and to a lesser extent the feces (US CDC 2009). Unabsorbed cobalt is primarily excreted in the feces.
Environmental media and food
As described in the Sources, Uses and Releases to the Environment section of this report, there are natural and anthropogenic sources of airborne cobalt. In Canada between 2003 and 2008 over 4500 measurements of cobalt in outdoor (ambient) air were collected and analysed by the National Air Pollution Surveillance (NAPS) Program from 18 sites across Canada (Table G-4). Under this nation-wide program, filter-based samples of particulate matter (PM) having aerodynamic diameter less than 10 μm (PM10) are collected and analysed for a variety of elements, including cobalt. PM10 is inhalable and therefore potentially available for systemic absorption. Median concentrations at the 18 sites ranged from below the limit of detection (LOD), less than 0.05 to 0.14 ng/m3; 95th percentile concentrations ranged from 0.04 to 0.68 ng/m3 (Sable Island and Halifax, Nova Scotia, respectively). The maximum reported value was 5.5 ng/m3 (Dow Settlement, New Brunswick) (NAPS 2003 - 2008). Canadian outdoor air data are also available near several mines and smelters in the region surrounding Sudbury, Ontario. The Sudbury Area Risk Assessment (SARA 2008) reported that the vast majority of samples were orders of magnitude less than 10 ng/m3 and an absolute maximum concentration of cobalt in a 24h PM10 sample to be 60 ng/m3. These concentrations measured in Canada are similar to the United States where mean air concentrations in urban settings range from less than 1 to 2 ng/m3 and up to 10 ng/m3 near industrial sources (ATSDR 2004).
Health Canada has conducted air quality studies in Windsor, Ontario, Edmonton and Calgary, Alberta, and Halifax, Nova Scotia. These studies provide data on concentration of cobalt in a variety of size fractions (PM1, PM2.5, and PM10) and media personal (Windsor), ambient (Windsor, Calgary and Halifax) and indoor air (Windsor, Edmonton and Halifax), see Table G-4. While the studies are not directly comparable, due to differences in sample collection and analysis, when indoor and outdoor values are available in the same study, median concentrations in outdoor air were generally higher than indoor and personal air samples. Overall, for all studies the same PM fractions had similar cobalt concentrations and the outdoor PM10 concentrations measured in the Windsor exposure assessment study were within a factor of 2 from some of the NAPS sites (e.g., Montreal, Edmonton and Halifax).
Cobalt soil concentrations are highly variable and cobalt is often found in mineral deposits, often in association with nickel, silver, lead, copper and iron ores (ATSDR 2004). Soil and sediments act as primary environmental sinks for cobalt compounds. In Canada, background cobalt concentrations in soil sources from various geographical locations are available through the Natural Resources Canada's Geological Survey of Canada (GSC). The GSC has reported the concentration of cobalt in glacial till (geological background) ranges from 0.25 to 95 mg/kg, with a median and 95th percentile of 7 mg/kg and 23 mg/kg respectively (Rencz et al. 2006). These results are based on 7398 samples collected and analyzed between 1956 and 2006. Glacial till is considered to represent the background concentration (i.e., the normal abundance of cobalt in unmineralized soil that is unaffected by anthropogenic activities) (Rencz et al. 2006). The Geological Survey of Canada report indicates Canadian background cobalt levels are similar to the United States where the average concentration of cobalt in soil is reported to be 7.2 mg/kg (Barceloux 1999). In 1993, garden soil was collected within 15 m of 50 Ottawa, Ontario residences, and analyzed for multiple elements including cobalt (Rasmussen et al. 2001). The median and 95th percentiles garden soils were 8.05 and 11.58 mg/kg, respectively. In Toronto, Ontario, Wiseman et al. (2013), collected, analyzed and compared the concentration of cobalt from soil samples adjacent to moderate and high traffic roads as well as two sites not impacted by traffic. In that study, the median cobalt concentrations for all soil samples ranged from 6.0 to 10.0 mg/kg and the minimum and maximum concentrations were 2.3 mg/kg and 21.0 mg/kg, respectively. Based on this data, minor anthropogenic influences do not contribute significantly to the variability of cobalt concentrations in soil. The Canadian House Dust Study (CHDS) provided nationally representative data for the concentration of cobalt in homes across Canada (Rasmussen 2013). The CHDS included measurements of total and bioaccessible cobalt from 1 017 homes and is statistically representative of urban residential homes in Canada. Bioaccessible cobalt concentrations represent the concentrations of cobalt extracted from the sample under biologically relevant conditions (i.e., in simulated stomach fluids), in the CHDS cobalt bioaccessibility ranged from 7 to 98%, and the median was 35% (Rasmussen 2013). For total cobalt the median concentration was reported to be 5.6 mg/kg and the 95th percentile concentration was reported to be 18.9 mg/kg (Rasmussen 2013). These results are similar to an earlier study of 48 homes in Ottawa, Ontario, where the median and 95th percentile total cobalt concentrations were reported to be 8.77 and 13.10 mg/kg, respectively (Rasmussen et al. 2001). The median and 95th percentile concentrations of bioaccessible cobalt reported in the CHDS were 2.0 and 5.1 mg/kg respectively (Rasmussen 2013).
Mines are located to take advantage of geological formations enriched in minerals of interest, therefore soil concentrations of metals in the vicinity of active or historical mines as well as in the vicinity of refineries are typically higher than other areas. Site specific assessments have been conducted in several Ontario communities impacted by mining and refining activities (OMOE 2011, OMOE 2002, SARA 2005). The Ontario Ministry of Environment (OMOE) conducted a soil study around the Town of Cobalt and Coleman Township (OMOE 2011), which has been the site of mining activities since 1903. Cobalt concentrations in soil were measured in areas considered accessible to the general population (e.g., public green spaces, school yards, residential properties) The mean concentrations ranged from 6 mg/kg in samples collected on a walking trail to 340 mg/kg in samples collected from exposed tailings (95th percentile concentrations were reported to be 7 and 1200 mg/kg respectively). Of the 11 sites considered accessible to the general population, 9 of the mean concentrations were below 40 mg/kg. In Port Colborne, Ontario, which is located near a metal refinery, cobalt soil concentrations were reported to range from 5 - 262 mg/kg, with a mean concentration of 51 mg/kg (OMOE 2002). The cobalt in soil results from the long term atmospheric deposition of cobalt from local stack emissions (OMOE 2002). Several mines and processing facilities are located in Sudbury, Ontario and in a study of multiple substances including cobalt reported an arithmetic mean concentration of cobalt in dust sampled from 86 homes as 55.23 mg/kg (range 6.28 to 246.00 mg/kg) and 28.80 mg/kg (range 13.6 to 45.1 mg/kg) in ten elementary schools (SARA 2005).
While there is currently no Canadian guideline for cobalt in drinking water (Health Canada 2009c), cobalt is commonly measured in drinking water treatment facilities and distribution systems in Canada. Drinking water measurements of cobalt are representative soluble salts and suspended particulate matter. Provincial data was available from Alberta, Manitoba, Ontario, New Brunswick and Saskatchewan and the concentrations are reported in Table G-5 (2013 emails from the Water and Air Quality Bureau, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced). Data was also available from several Canadian cities including Toronto, Ontario, Montreal, Québec, Winnipeg, Manitoba, Victoria, Brithish Columbia and Ottawa, Ontario (City of Ottawa 2008, 2009, 2010; City of Winnipeg 2008, 2009, 2010; CRD Water Services 2008, 2009, 2010, 2011; Montreal 2008, 2009, 2011; Toronto Water 2008, 2009, 2010). A national survey was conducted in 1981 to determine the levels of certain inorganic substances, including cobalt, in Canadian distributed drinking water (Méranger et al. 1981). Based on the representative samples collected at the tap after 5 minutes of flushing at maximum flow rate, the survey concluded that cobalt levels did not increase to a significant degree in the drinking water at the tap when compared with raw and treated water (Health Canada 2009b).Therefore, it is considered that levels measured in treatment facilities and distribution systems are representative of tap water levels and the source of cobalt in drinking water is associated with the raw water entering treatment facilities. Cobalt is not commonly detected in drinking water, the highest median value above the detection limit was 0.11μg/L from the Province of Ontario and concentrations as high as 6.1 μg/L have been reported (2013 emails from the Water and Air Quality Bureau, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
Cobalt is an essential component of vitamin B12; however, as previously described, the analytical methods used to measure environmental cobalt concentrations and cobalt concentrations in foods do not distinguish the forms of cobalt. Cobalt is the only metal that is an inherent component of a vitamin in humans. The cobalt ion is essential for many other forms of life, such as soil- or rumen bacteria, where it is used to synthesize vitamin B12. As stated above, cobalt is a naturally occurring element in soil and it is used in certain fertilizers and animal supplements; therefore it can be present at low levels in a large number of foods via uptake by plants and livestock. Cobalt may also enter food through fortification, during processing, packaging or food preparation. Cobalt is present in food packaging materials and migration of cobalt from ceramic food contact materials increases with the acidity of the contents (Demont et al. 2012), it is also present as an impurity of colour concentrates used in polyethylene terephthalate PET trays (2013 emails from HPFB, Health Canada to ESRAB). Dietary intake estimates are derived by Health Canada and are based on prepared meals therefore incorporating natural and anthropengic sources of cobalt in food. Data for total cobalt levels in food, baby formula and drinking water were reported in the Canadian Total Diet Study (TDS) 1986 to 1988 (Dabeka 1989; Dabeka and McKenzie 1995), 1992 to 1999 (Health Canada 2009b) and 2000 to 2007 TDSs (Health Canada 2009b)). Based on the 1986-88TDS , Dabeka and McKenzie (1995) identified bakery goods and cereals and vegetables as the categories which were the most significant contributors to dietary cobalt intake. Consistent with these earlier findings, based on the 2007 TDS the major contributors are bakery goods, cereals and vegetables, additionally dairy products are notable contributors. The concentrations of cobalt measured in food and beverage items purchased and intake estimates based on these results are also available (Health Canada 2009b). The TDS estimates intakes for all ages and male and female combined, these combined intakes facilitate comparison on an annual basis. Studies conducted from 1993 to 1997 were combined in one estimate, 0.24 μg/kg body weight per day, and for each of the eight years from 2000 - 2008 estimated intakes were reported to be 0.21, 0.20, 0.20, 0.17, 0.26, 0.17, 0.32, and 0.21 μg/kg body weight per day (Health Canada 2009b), indicating intakes have been stable.
Several international reports and one Canadian report of cobalt concentrations in human milk were identified, see Table G-3 for details. A Canadian study reported levels of cobalt in the breast milk of 43 nursing mothers; the authors compare the composition of the milk from mothers of premature and full-term infants during the first three months and the range of median concentrations reported was 0 - 6 μg/L (Friel et al. 1999). Eighteen median concentrations were reported; seventeen median values were less than or equal to 2 μg/L and one was 6 μg/L. The central tendency (median or arithmetic mean) cobalt levels measured in breast milk samples measured in other countries around the world ranges from less than 0.001 to 1.40 μg/L.
Products
As detailed in the Sources Uses and the Releases to the Environment section of this report, cobalt substances are present in a range of products available to Canadians such as paints and coatings, cosmetics and natural health products, as well as being a component of manufactured items that the general population may come in contact with. The most common exposure route for the general population to cobalt from these uses is expected to be dermal, considering that cobalt from vitamin B12 is not bioavailable. The general population may have indirect exposure to cobalt (e.g., trace quantities of catalyst remaining in a plastic product, stack emissions from an industrial facility, or as a component of indoor house dust from paints and coatings containing cobalt) or directly (e.g., use of a cosmetic product containing a cobalt containing pigment, or painting a room with cobalt-containing paint). These exposures may occur on a daily basis or infrequently. Overall, the predominant exposure pathway for products is dermal, for example certain cobalt containing substances are present in eye-makeup (2011 and 2013 emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced) and as described in the Toxicokinetics Section, dermal absorption via intact skin is low. Absorbed cobalt from the use of frequent or daily use products is reflected in the CHMS biomonitoring data regardless of exposure route.
Summary of exposure
Although the cobalt blood concentration data capture total exposure to cobalt from all sources, the data does not identify what the individual sources are or how individual sources contribute to total exposure. Average estimates of daily intake of cobalt from air, water, food and beverages, household dust and soil for the general population of Canada were generated for the purposes of identifying key sources of exposure by comparing relative contributions from the different media and food to total intake and are presented in Table A7.1. The estimated intakes range from 0.64 μg/kg bw per day for non-breast fed infants declining with age to 0.19 μg/kg bw per day for those over 60 years of age. For all age groups food is estimated be the largest contributor, estimated to account for a minimum 98% of total intake; soil and dust are minor contributors to the estimated intakes of infants, toddlers and children, up to 1.7%, while air (indoor and ambient) and drinking water were not found to be significant contributors to total exposure. Canadians living near current or former point source emissions of cobalt such as mines and smelters are exposed to higher soil and dust concentrations than those living in areas not impacted by a point source; however the difference in central tendency between impacted and non-impacted soil and dust is approximately 4 and 10 fold, respectively. Therefore, even in locations affected by a point source of cobalt, dietary intake is expected to remain the most significant source of exposure. Overall, children have higher estimated cobalt intake and excretion than adults but similar blood concentrations and no statistically significant differences in blood concentrations have been observed between the general population and sub-populations based on age or gender.
Absorbed cobalt is reflected in the CHMS biomonitoring data regardless of exposure route or source.
Confidence in the exposure database
Confidence in the exposure database is high. Canadian data on cobalt concentrations in all media and food were identified from either multiple sites, or from nationally representative studies. These studies reported total cobalt which includes levels from all substances that contain cobalt, soluble and non-soluble, and both natural and anthropogenic sources. Additionally, cobalt concentrations in blood from the CHMS were available. Blood cobalt concentrations capture absorbed cobalt from all anthropogenic and natural sources, in environmental media and food as well as exposure resulting from the use of cobalt- containing products. The total daily intake of adult Canadians from environmental media and food can be converted into a blood equivalent (see Biokinetic Models section of the Hazard Assessment for details) for comparison with levels measured in Canadians as part of the CHMS. The estimated intake of 0.23 μg/kg bw per day equates to an estimated blood concentration of 0.39 μg/L (Table A6.1) which is quite close to the 95th percentile measured in the CHMS (0.40 μg/L). This close agreement gives confidence that the estimated intakes from environmental media and food account for the majority of exposure. The cobalt concentrations measured in blood reported by the CHMS represents 96.3% of the Canadian population from 3 to 79 years old. While no national cobalt blood concentration data exist for children less than 3 years, age related differences in blood concentrations are not expected. There were no observed age-related trends in the CHMS dataset. Comparison of population level blood concentrations with studies including children less than 5 years in Alberta, 2 to 6 years in Germany and studies of pregnant women, newborns and cord blood do not demonstrate age related differences in cobalt blood concentrations. Children have a higher estimated intake and urinary excretion of cobalt than adults and comparable blood concentrations. As a future source of data, cobalt was measured in the milk of mothers participating in the Maternal-Infant Research on Environmental Chemicals (MIREC) Study. The MIREC study included over 2000 participants from 10 cities across Canada, but data are not yet available.
10.3 Characterization of risk to human health
With the availability of cobalt biomonitoring data, risk to human health posed by cobalt is characterized based on comparisons of whole blood concentrations measured in the general population of Canada to the whole blood equivalent of critical health effects in experimental animal and human studies.
Total cobalt measured in whole blood reflects exposure to all forms of cobalt from all routes and all sources. Based on data from the CHMS, the median whole blood concentration of cobalt is 0.22 μg/L and the 95th percentile of cobalt is 0.40 μg/L (10% of measured cobalt is from vitamin B12) (Health Canada 2013).
Two endpoints were identified for the risk characterization. One of the endpoints was cardiomyopathy in chronic alcoholic beer drinkers and the other endpoint is polycythemia identified in cobalt-treated individuals. The minimum oral dose associated with chronic cobalt toxicity in humans is 0.04 mg Co/kg bwCo/kg bw per day, at which cardiomyopathy was observed in malnourished heavy beer drinkers. Using a biokinetic model, a whole blood concentration of 26 μg /L was derived for this point of departure. This critical endpoint is consistent with the previous evaluations of other regulatory bodies (ATSDR 2004; IPCS 2006). However, this endpoint is considered highly conservative, since well-nourished beer drinkers did not experience any cardiac effects at estimated whole blood cobalt concentration of 34 μg/L.
Since cardiomyopathy is only observed in people with severely deteriorated health conditions who chronically consumed high volumes of alcohol, the use of this endpoint may only be appropriate to protect an atypical sensitive sub-population. Therefore, polycythemia (the increase of red blood cells), observed in humans was identified as the the other critical endpoint for the characterization of the risk to the general population. Increased red blood cell numbers and hemoglobin levels have been observed in volunteers and anaemic patients given cobalt salts orally at doses near 1 mg Co/kg bw per day, for periods of several weeks to several months (Davis and Fields 1958). While this effect is desired in anaemic patients, long-term polycythemia may result in secondary adverse outcome in healthy people such as elevated risk of strokes and heart attacks due to the increased thickness of blood. Thus, the point of departure of 0.45 mg/kg bw per day (equivalent to a whole blood cobalt concentration of 290 μg/L), where polycythemia was not detected in pregnant women was selected for risk characterization (Holly 1955). In addition, polycythemia is a reversible haematological effect, where exposed individuals returned to normal levels after cobalt treatment ended.
Comparison of whole blood cobalt concentrations at the critical effect levels for repeated-dose toxicity, via oral route (i.e., 26 μg/L for cardiomyopathy) with median and 95th percentile of whole blood cobalt concentrations of general population (i.e., 0.22 and 0.40 μg/L, respectively) results in MOEs of approximately 118 and 65, respectively. The blood concentration estimated for the NOAEL for polycythemia was approximately 290 μg/L and the resulting MOEs based on mean and 95th percentile of whole blood cobalt concentrations in general population were 1318 and 725, respectively. The derived MOEs based on beer drinkers' cardiomyopathy, which is the lowest dose level in the human database, are considered adequate to protect humans from the harmful effects noted in the animal database, including reproductive and developmental effects as those effects were only observed at dose levels more than 100 times higher than the critical dose level used to derive the MOEs. As the points of departure considered in the risk characterization are based on effects in humans, interspecies variatibility would not be taken into consideration when determining the adequacy of the margins of exposure. In addition, recent publications by Finley et al. (2013) and Tvermoes et al. (2014) concluded that there were no clinically significant changes in health status of male and female volunteers exposed to approximately 1 mg Co per day for 31 and 90 days, respectively, and the resulted in peak blood cobalt concentrations in each study were 91 and 117 μg/L, respectively.
The respiratory system is the primary site of injury after inhalation exposure to cobalt in both experimental animals and humans. The critical effect level identified from human studies is the LOAEC of 0.0151 mg Co/m3 in individuals occupationally-exposed in diamond polishing workshops exposed to cobalt dust (Nemery et al. 1992). There was a significantly higher prevalence of eye, nose and throat irritation and cough, and reduced lung function in this exposed group (0.0151 mg Co/m3) compared to unexposed individuals. In chronic inhalation study, significant increase in benign and malignant alveolar/bronchiolar tumors was observed in both rats and mice (Bucher et al. 1999 and NTP 1998). The lowest dose at which tumours were reported (0.38 mg Co/m3) is 25 times higher than the critical effect level in humans (i.e. 0.0151 mg Co/m3).
A maximum daily intake via inhalation is estimated to be less than 0.001 μg/kg bw per day (less than 0.2% of the oral daily intake) based on the highest 95th percentile cobalt concentration in PM10 personal air samples collected in Windsor, Ontario (i.e. 2.8×10-7 mg/m3 A comparison of the critical effect level for inhalation toxicity (i.e. a LOAEC in humans of 0.0151 mg/m3) with the 95th percentile estimate of general population exposure (i.e. 2.8×10-7 mg /m3) results in a MOE of approximately 53 000. A MOE of 1 340 000 was obtained for the lung tumours noted in the rodent database. These MOEs are considered adequate to addresss uncertainties in the health effects and exposure databases. The MOEs between cobalt levels in whole blood of Canadians from a nationally representative survey and cobalt levels in personal air samples and conservative effect levels are considered adequate to address uncertainties in the health effects and exposure databases. Therefore, it is concluded that cobalt and cobalt from cobalt-containing substances do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
10.3.1 Uncertainty in evaluation of risk to human health
The primary route of exposure to cobalt for the general population is oral (inhalation exposure is estimated to be 0.16% of total exposure). There are limited toxicological data on the chronic effects of oral exposure to cobalt; however, available information from short-term and subchronic studies does not suggest that carcinogenicity would be an endpoint following long-term oral exposure.
Although chronic NTP studies with rodent species have indicated that respiratory tract is the primary target site of carconigenic activity of inhalation exposure to aerosols of cobalt substances, the recent NTP study with aerosols of cobalt metal have observed tumors in adrenal medulla and pancreatic islets of rats. However, the adrenal gland as well as the pancreas were not noted as target organs following oral exposures, suggesting that these effects were a direct result of the route of exposure. Alternatively, these effects were only noted in studies using metallic cobalt as a test material, suggesting a specific toxicological effect of this specific cobalt form. Long term oral exposures in humans, to various cobalt substances, have not noted the prevalence of any tumour formation.
The biokinetic model used to derive blood cobalt levels at the point of departure was based on a healthy adult male. Therefore, there are uncertainties associated with the applicability of the model to children and females. There are also uncertainties regarding the absorbed fraction of cobalt. The model predictions assume that 25% of the ingested cobalt is absorbed from the GI tract but absorption can vary depending on various factors. However, available biomonitoring data suggests that the average Canadian female has a similar blood concentration of cobalt as does an average male. The assumption that over time an average of 25% of ingested cobalt is absorbed is considered reasonable.
11. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is risk of harm to organisms from cobalt and soluble cobalt compounds.
It is concluded that cobalt and soluble cobalt compounds meet the criteria under paragraph 64(a) of CEPA as they are entering or may enter the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity. However, it is concluded that cobalt and soluble cobalt compounds do not pose a risk to the broader integrity of the environment and do not meet the criteria under paragraph 64(b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger to the environment on which life depends. Cobalt and soluble cobalt compounds have been determined to meet the persistence criteria but do not meet the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
The MOEs between cobalt levels in whole blood of Canadians from a nationally representative survey and cobalt levels in personal air samples and conservative effect levels are considered adequate to address uncertainties in the health effects and exposure databases. Therefore, it is concluded that cobalt and cobalt from cobalt-containing substances do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is concluded that cobalt and soluble cobalt compounds meet one of the criteria set out in section 64 of CEPA.
References
Abballe A, Ballard TJ, Dellatte E, Dellatte E, di Domenico A, Ferri F, Fulgenzi AR, Grisanti G, Iacovella N, Ingelido AM, Malisch R, Miniero R, Porpora MG, Risica S, Ziemacki G, De Felip E.. 2008. Persistent environmental contaminants in human milk: Concentrations and time trends in Italy. Chemosphere 73:S220-S227.
Adam C, Baudin JP, Garnier-Laplace J. 2001. Kinetics of 110mAg, 60Co, 137Cs, and 54Mn Bioaccumulation from water and Depuration by the Crustacean Daphnia Magna. Water Air Soil Pollut. 125: 171-188.
Alberta Environment. 2002. Lake Wabamun water quality and sediment survey. Preliminary report. Edmonton (AB): Alberta Environment. ISBN 0-7785-2296-0. 37 p.
Alberta Environment. 2003. A survey of metals and trace organic compounds in sediments from Wabamun Lake and other Alberta lakes. Edmonton (AB): Alberta Environment. ISBN 0-7785-2501-4. 147 p.
Alberta Environment. 2006. Wabamun Lake Oil Spill August 2005: Data report for Water and Sediment Quality in the Pelagic Area of the Lake (August 4-5 to September 15, 2005). Edmonton (AB): Alberta Environment. ISBN 0-7785-4589-X. 99 p.
Alberta Health and Wellness. 2008. The Alberta Biomonitoring Program: Chemical Biomonitoring in Serum of Pregnant Women in Alberta. Edmonton: Alberta Health and Wellness. ISBN 978-0-7785-6695-3. [cited 2013 May 2].
Alexander CS. 1969. Cobalt and the heart. Annals of Internal Medicine. 70: 411-413. [cited in IPCS 2006].
Alexander CS. 1972. Cobalt-beer cardiomyopathy. A clinical and pathologic study of twenty eight cases. Am. J. Med. 53: 395-417.
Alexandersson R. 1988. Blood and urinary concentrations as estimators of cobalt exposure. Arch Environ Health 43(4):299-303. [cited in US CDC 2009].
Alvarez-Puebla RA, Valenzuela-Calahorro C, Garrido JJ. 2004. Retention of Co(II), Ni(II), and Cu(II) on a purified brown humic acid. modeling and characterization of the sorption process. Langmuir 20: 3657-3664.
Anderson MB, Lepak K, Farinas V, George WJ. 1993. Protective action of zinc against cobalt-induced testicular damage in the mouse. Reprod Toxicol, 7:49-54.
Anderson MB, Pedigo NG, Katz RP, George WJ. 1992. Histopathology of testes from mice chronically treated with cobalt. Reprod Toxicol, 6:41-50.
ARCHE Consulting. 2012. Predicted No Effect Concentration (PNEC) calculator. August 2012. Belgium. ARCHE. [cited 2013 July-August].
Arnot JA, Gobas FAPC. 2006. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ Rev. 14: 257-297.
Asante KW, Agusa T, Mochizuki H, Ramu K, Inoue S, Kubodera T, Takahashi S, Subramanian A, Tanabe S. 2008. Trace elements and stable isotopes (δ13C and δ15N) in shallow and deep-water organisms from the East China Sea. Environ Pollut. 156:862-873.
[ATSDR] Agency for Toxic Substances and Disease Registry. 2004. Toxicological Profile for Cobalt. [Internet]. Atlanta (GA): U.S. Department of Health and Human Services, ATSDR.
Ayala-Fierro F, Firriolo JM, Carter DE. 1999. Disposition, toxicity, and intestinal absorption of cobaltous chloride in male Fischer 344 rats. J Toxicol Environ Health A, 56:571-591.
Bailey MR, Kreyling WG, Andre S, Bachelor A, Collier CG, Drosselmeyer E, Ferron GA, Foster P, Haider B, Hodgson A, et al. 1989. An interspecies comparison of the lung clearance of inhaled monodisperse cobalt oxide particles- Part 1: Objectives and summary of results. J Aerosol Sci. 20: 169-188.
Barceloux DG. 1999. Cobalt. Clin Toxicol 37(2): 201-216.
Barnaby CF, Smith T, Thompson BD. Dosimetry of the radioisotopes of cobalt. 1968. Phys Med Biol; 13:421-33.
Beleznay E, Osvay M.1994. Long-term clearance of accidentally inhaled 60Co aerosols in humans. Health Physics, 66:392-399. Biosearch Inc. 1980. Philadelphia, PA. (Study no. 80-1975A), study conducted for Tenneco Chemicals, Inc., Saddle Brook, NJ (In US HPV, 2005).
Bencko V, Wagner V, Wagnerova M, Reichrtova E. 1983. Immuno-biochemical findings in groups of individuals occupationally and non-occupationally exposed to emissions containing nickel and cobalt. Journal of Hygiene, Epidemiology, Microbiology and Immunology, 27(4):387-394. [cited in IPCS 2006].
[BGS] British Geological Survey. 2009. Cobalt Commodity Profile. Nottingham (UK): British Geological Survey [Internet]. [cited 2009 sept].
Borgmann U, Norwood WP. 1995. Kinetics of excess background copper and zinc in Hyalella azteca and their relationship to chronic toxicity. Can J Fish Aquat Sci. 52: 864-874.
Bonenfant JL, Auger C, Miller G, Chenard J, Roy PE. 1969. Quebec beer-drinkers' myocardosis: pathological aspects. Ann N Y Acad Sci. 156(1):577-582.
Borgmann U, Couillard Y, Doyle P, Dixon G. 2005. Toxicity of sixty-three metals and metalloids to Hyalella azteca at two levels of water hardness. Environ Toxicol Chem. 24: 641-652.
Borgmann U, Norwood WP, Dixon DG. 2004. Re-evaluation of metal bioaccumulation and chronic toxicity in Hyalella azteca using saturation curves and the biotic ligand model. Environ Pollut. 131: 469-484.
Bowie E A, Hurley P.J. 1975. Cobalt chloride in the treatment of refractory anaemia in patients undergoing long-term haemodialysis. Aust N Z J Med. 5: 306-314.
Brewitz E, Larsson CM Larsson M. 1996. Responses of nitrate assimilation and N translocation in tomato (Lycopersicon esculentum Mill) to reduced ambient air humidity. J Exp Bot. 47 (300): 855-861.
Brodner W, Bitzan P, Meisinger V, Kaider A, Gottsauner-Wolf F, Kotz R. 2003. Serum Cobalt Levels After Metal-on-Metal Total Hip Arthroplasty. J Bone Joint Surg Am. 85A(11):2168-2713.
Brookins DG. 1988. Eh-pH diagrams for geochemistry. Berlin, Heidelberg: Springer-Verlag. 175 p.
Bucher JR, Elwell MR, Thompson MB, Chou BJ, Renne R, Ragan HA. 1990. Inhalation toxicity studies of cobalt sulfate in F344/N rats and B6C3F1 mice. Fundam and Appl Toxicol, 15:357-372.
Bucher JR, Hailey JR, Roycroft JR, Haseman JK, Sills RC, Grumbein SL, Mellick PW, Chou BJ. 1999. Inhalation toxicity and carcinogenicity studies of cobalt sulfate. Toxicol.Sci. 49: 56-67.
Camarasa JMG. 1967. Cobalt contact dermatitis. Acta Dermato-Venereologica, 47(5): 287-292.
Camner, P., Boman, A., Johansson, A., Lundborg, M., Wahlberg, J.E. 1993. Inhalation of cobalt by sensitised guinea pigs: Effects on the lungs. Br. J. ind. Med. 50: 753-757.
Campbell LM, Norstrom RJ, Hobson KA, Muir DCG, Backus S, and Fisk AT. 2005 Mercury and other trace elements in a pelagic Arctic marine food web (Northwater Polynya, Baffin Bay). Sci Total Environ. 351-352: 247-263.
Campbell PGC. 1995. Interactions between trace metals and aquatic organisms: A critique of the free-ion activity model. In: Tessier A, Turner Dr (eds.) Metal speciation and bioavailability in aquatic systems. Chichester (England): John Wiley and Sons Ltd. p 45-102.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33, Canada Gazette. Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2009a. Canadian Environmental Protection Act, 1999: Notice with respect to Batch 10 Challenge substances. Canada Gazette, Part I, vol. 143, no. 25, p. 1796-1810.
Canada, Dept. of the Environment. 2009b. Canadian Environmental Protection Act, 1999: Notice with respect to certain inanimate chemicals (substances) on the Domestic Substances List. Canada Gazette, Part I: Vol. 143, No. 40 - October 3, 2009.
Canada, Dept. of the Environment. 2011a. Canadian Environmental Protection Act, 1999: Announcement of planned actions to assess and manage, where appropriate, the risks posed by certain substances to the health of Canadians and the environment. Canada Gazette, Part I, vol. 145, no. 41.
Canada, Dept. of the Environment. 2011b. Canadian Environmental Protection Act, 1999: Notice with respect to certain cobalt-containing substances. Canada Gazette, Part I: Vol. 146, No. 22 - June 2, 2012.
Canada. 2013. Food and Drugs Act: Medical Devices Regulations, 25 June 2013, SOR/98-282.
Canadian Fertilizer Institute. 2013. Frequently Asked Questions [Internet]. Ottawa (ON): Canadian Fertilizer Institute. [cited: 2013 August].
Canadian Fuels Association. 2013. The Fuels Industry. [Modified in 2012, cited 2013 Oct 7].
[CCME] Canadian Council of Ministers of the Environment. 2007. A protocol for the derivation of water quality guidelines for the protection of aquatic life 2007. In: Canadian environmental quality guidelines, 1999, Canadian Council of Ministers of the Environment, 1999.
[CDI] The Cobalt Development Institute. 2006. Cobalt Fact Sheet 8: Electronics. Surrey, United Kingdom, [cited on 2010 Feb 5].
[CDI] The Cobalt Development Institute. 2011. Cobalt Fact Sheet 10: Supply and Demand. Surrey, United Kingdom, [2013 Jul].
[CFIA] Canadian Food Inspection Agency. 1997. T-4-093 - Standards for Metals in Fertilizers and Supplements[Internet]. Ottawa (ON): Canadian Food Inspection Agency. [cited: 2013 August].
Christensen JM, Poulsen OM, Thomsen M. 1993. A short-term crossover study on oral administration of soluble and insoluble cobalt compounds: sex differences in biological levels. Int Arch Occup Environ Health, 65(4):233-40.
City of Montreal, Environment and Infrastructures Services. 2008. Municipal drinking water produced by Atwater et Charles-J.-des-Baillets Water Plants.
City of Montreal, Environment and Infrastructures Services. 2009. Municipal drinking water produced by Atwater et Charles-J.-des-Baillets Water Plants.
City of Montreal, Environment and Infrastructures Services. 2011. Municipal drinking water produced by Atwater et Charles-J.-des-Baillets Water Plants.
City of Ottawa. 2008. City of Ottawa Drinking Water Quality: Physical, Chemical, Microbiological & Radiological.
City of Ottawa. 2009. City of Ottawa Drinking Water Quality: Physical, Chemical, Microbiological & Radiological.
City of Ottawa. 2010. City of Ottawa Drinking Water Quality: Physical, Chemical, Microbiological & Radiological.
City of Toronto. 2008. Drinking Water Analysis Summary for All Plants and Distribution for January 1, 2008 to December 31, 2008.
City of Toronto. 2009. Drinking Water Analysis Summary for All Plants and Distribution for January 1, 2009 to December 31, 2009.
City of Toronto. 2010. Drinking Water Analysis Summary for All Plants and Distribution for January 1, 2010 to December 31, 2010.
City of Winnipeg, Water and Waste Department. 2008. City of Winnipeg Water Supply System Annual Report 2008.
City of Winnipeg, Water and Waste Department. 2009. City of Winnipeg Water Supply System Annual Report 2009.
City of Winnipeg, Water and Waste Department. 2010. City of Winnipeg Water Supply System Annual Report 2010.
Clark NA, Teschke K, Rideout K. Copes R.. 2007. Trace element levels in adults from the west coast of Canada and associations with age, gender, diet, activities, and levels of other trace elements. Chemosphere, 70: 155-164.
Clyne N, Hofman-Bang C, Haga Y, Hatori N, Marklund SL,Pehrsson SK, Wibom R. 2001. Chronic cobalt exposure affects antioxidants and ATP production in rat myocardium. Scand J Clin Lab Invest 61(8):609-614.
Collier CG, Bailey MR, Hodgson A. 1989. An interspecies comparison of the lung clearance of inhaled monodisperse cobalt oxide particles - Part V: Lung clearance of inhaled cobalt oxide particles in hamsters, rats and guinea-pigs. J Aerosol Sci. 20(2):233-247.
Conestoga-Rovers and Associates. 2009. Baseline information on major municipal solid waste landfills in Canada. Unpublished report prepared for Environment Canada.
Conestoga-Rovers and Associates. 2013. Landfill Monitoring Data - Correlation, Trends, and Perspectives. Report Number 9. Unpublished report prepared for Environment Canada. 435 p.
Corazza M, Baldo, F, Pagnoni A, Virgili A. 2009. Measurement of nickel, cobalt and chromium in toy make-up by atomic absorption spectroscopy. Acta Derm Venerol 89:130-133.
[CoRC] The Cobalt REACH Consortium Ltd. 2004. A Survey of Consumer Exposure to Cobalt. July 2004. 44p. Unpublished report submitted to Environment Canada under the Chemicals Management Plan.
[CoRC] The Cobalt REACH Consortium Ltd. 2010a. Cobalt di(acetate), CAS RN 71-48-7, EC Number 200-755-8. September 2010. 308 p. Unpublished report submitted to Environment Canada under the Chemical Management Plan.
[CoRC] The Cobalt REACH Consortium Ltd. 2010b. Cobalt carbonate, CAS RN 513-79-1, EC Number 208-169-4. September 2010. 309 p. Unpublished report submitted to Environment Canada under the Chemical Management Plan.
[CoRC] The Cobalt REACH Consortium Ltd. 2010c. Cobalt sulphide, CAS RN 1317-42-6, EC Number 215-273-3. September 2010. 309 p. Unpublished report submitted to Environment Canada under the Chemical Management Plan.
[CoRC] The Cobalt REACH Consortium Ltd. 2010d. Cobalt oxide, CAS RN 1307-96-6, EC Number 215-154-6. September 2010. 309 p. Unpublished report submitted to Environment Canada under the Chemical Management Plan.
[CoRC] The Cobalt REACH Consortium Ltd. 2010e. Cobalt dihydroxide, CAS RN 21041-93-0, EC Number 244-166-4. September 2010. 322 p. Unpublished report submitted to Environment Canada under the Chemical Management Plan.
[CoRC] The Cobalt REACH Consortium Ltd. 2012. Chemical Safety Report for Cobalt, CAS RN 7440-48-4, EC Number 231-158-0. November 2012. 241 p. Unpublished report submitted to Environment Canada under the Chemicals Management Plan.
[CoRC] The Cobalt REACH Consortium Ltd. 2013a. Stearic acid, cobalt salt (Octadecanoic acid, cobalt salt), CAS RN 13586-84-0, EC Number 237-016-4. April 2013. 307 p. Unpublished report submitted to Environment Canada under the Chemical Management Plan.
[CoRC] The Cobalt REACH Consortium Ltd. 2013b. Chemical Safety Report for Naphthenic acid, cobalt salt, CAS RN 61789-51-3, EC Number 263-064-0. April 2013. 316 p. Unpublished report submitted to Environment Canada under the Chemical Management Plan.
Corrier DE, Mollenhauer HH, Clark DE, Hare MF, Elissalde MH. 1985. Testicular degeneration and necrosis induced by dietary cobalt. Veterinary Pathology, 22:610-616.
Cotton FA, Wilkinson G. 1988. Advanced inorganic chemistry, 5th edition. Toronto (ON): John Wiley and Sons. 1455 p.
Couillard Y, Grapentine LC, Borgmann U, Doyle P, Masson S. 2008. The amphipod Hyalella azteca as a biomonitor in field deployment studies for metal mining. Environ. Pollut. 156: 1314-1324.
[CRD] Capital Regional District. 2008. 2008 Treated Water Quality Below Japan Gulch Plant.
[CRD] Capital Regional District. 2009. 2009 Treated Water Quality Below Japan Gulch Plant.
[CRD] Capital Regional District. 2010. 2010 Treated Water Quality Below Japan Gulch Plant.
[CRD] Capital Regional District. 2011. 2011 Treated Water Quality Below Japan Gulch Plant.
CSIRO 2007. Toxicity and Bioavailability of Cobalt in Terrestrial Environments. Wendling, L., Kirby, J., McLaughlin, M. and Ma, Y. CSIRO Centre for Environmental Contaminants IN Redeker E, F Degryse, E Smolders. 2008. Toxicity of Cobalt in soils: Summary of conclusion (i) Toxicity data and modeling bioavailability for effects assessment. Final report to Cobalt Development Institute. September 2008.
Dabeka RW, McKenzie AD. 1995. Survey of lead, cadmium, fluoride, nickel, and cobalt in food composites and estimation of dietary intakes of these elements by Canadians in 1986 - 1988. J AOAC Int 78(4): 897-909.
Dabeka RW. 1989. Survey of lead, cadmium, cobalt and nickel in infant formulas and evaporated milks and estimation of dietary intakes of the elements by infants 0-12 months old. Sci Total Environ 89: 279-289.
Dave G, Xiu R. 1991. Toxicity of mercury, copper, nickel, lead, and cobalt to embryos and larvae of zebrafish, Brachydanio rerio. Arch Environ Contam Toxicol. 21: 126-134.
Davis JE, Fields JP. 1958. Experimental production of polycythemia in humans by administration of cobalt chloride. Proc Soc Exp Biol Med 99:493-495. [cited in IPCS 2006]
De Schamphelaere KAC, Lien TH Nguyen and Colin R Janssen. 2008. Bioavailability and Aging of Cobalt in Soils: Invertebrate Toxicity Testing. Testing laboratory: Laboratory of Environmental Toxicology and Aquatic Ecology Ghent University. A report to the Cobalt Development Institute. Guildford, Surrey, United Kingdom.
DeForest, DK, Brix KV, Adams WJ. 2007. Assessing metal accumulation in aquatic environments: the inverse relationship between bioaccumulation factors, trophic transfer factors and exposure concentration. Aquat Toxicol. 84: 236-246.
Degryse F, Smolders E, Parker DR. 2009. Partitioning of metals (Cd, Co, Cu, Ni, Pb, Zn) in soils: concepts, methodologies, prediction and applications - a review. Eur J Soil Sci. 60: 590-612.
Demont M, Boutakhrit K, Fekete V, Bolle F, Van Loco J. 2012. Migration of 18 trace elements from ceramic food contact material: influence of pigment, pH, nature of acid and temperature. Food and Chem Toxicol. 50: 734-743.
Di Toro DM, Mahony JD, Hansen DJ, Scott KJ, Carlson A, Ankley GT. 1992. Acid volatile sulphide predicts the acute toxicityof cadmium and nickel in sediments. Environ Sci Technol. 26, 96-101.
Di Toro DM, Kavvadas CD, Mathew R, Paquin PR, Winfield RP. 2001. The Persistence and Availability of Metals in Aquatic Environments. ICME, International Council on Metals in the Environment, 67p.
Diamond J, Winchester D, Mackler W, Rasnake J, Fanelli J, Gruber D.. 1992. Toxicity of cobalt to freshwater indicator species as a function of water hardness. Aquatic Toxicol. 22, 163-180.
Diamond ML, Mackay D, Cornett RJ, Chant LA. 1990. A model of the exchange of inorganic chemicals between water and sediments. Environ Sci Technol. 24: 713-722.
Domingo JL, Paternain JL, Llobet JM, Corbella J. 1985. Effects of cobalt on postnatal development and late gestation in rats upon oral administration. Rev Esp Fisiol 41:293-298.
[DPD] Drug Product Database [database on the Internet]. 2013. Ottawa (ON): Health Canada. [cited 2013 Aug].
Duckham JM, Lee HA. 1976. The treatment of refractory anaemia of chronic renal failure with cobalt chloride. Q J Med 178:277-294.
[ECHA] European Chemicals Agency. 2012. Guidance on information requirements and chemical safety assessment. Chapter R.16: Environmental exposure estimation, Version 2.1. Helsinki (FI): European Chemicals Agency.
Elbetieha A., Al-Thani AS, Al-Thani, RK, Darmani H, Owais W. 2008. Effects of Chronic Exposure to Cobalt Chloride on the Fertility and Testes in Mice. J. Appl Biol Sci. 2(1):01-06.
Eleftheriadis K, Colbeck I. 2001. Coarse atmospheric aerosol: size distributions of trace elements. Atmos. Environ. 35: 5321-5330
Elinder CG, Friberg L. 1986. Cobalt. Chapter 9 in Handbook on the Toxicology of Metals, 2nd edition. Eds. Friberg, Nordberg, Vouk editors.
Environment Canada. 1988. Data relating to the Domestic Substance List (DSL) 1984-1986, collected under CEPA, 1988, s. 25(1). Based on Reporting for the Domestic Substances List [guide] 1988. Data prepared by: Environment Canada.
Environment Canada. 2006. Guidance for conducting ecological assessments under CEPA, 1999: science resource technical series, technical guidance module: Sludge amendment. Working document. Gatineau (QC): Environment Canada, Ecological Assessment Division.
Environment Canada. 2007a. Ecological categorization of substances on the Domestic Substances List (DSL) [Internet]. [updated and reviewed 2007 May]. Environment Canada.
Environment Canada. 2007b. MSW Thermal Treatment in Canada 2006. Report submitted to Environment Canada by GENIVAR Ontario Inc., in Association with Ramboll Danmark A/S. March 2007.
Environment Canada. 2009a. Data for certain inanimate substances collected under Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain inanimate chemicals (substances) on the Domestic Substances List. Data prepared by: Environment Canada, Existing Substances Program.
Environment Canada. 2010a. Data for Batch 10 Substances collected under Canadian Environmental Protection Act, 1999, section 71 and voluntary data submission: Notices with respect to Batch 10 Challenge substances. Data prepared by: Environment Canada, Existing Substances Program.
Environment Canada. 2010b. Battery recycling in Canada 2009 update -executive summary. Pollution and Waste Department.
Environment Canada. 2010c. Evaluation of the Ecological Effects of Cobalt in Soil. Technical Report Ottawa (ON) Environment Canada, Soil Toxicology Laboratory, Biological Assessment and Standardization Section.
Environment Canada. 2011. Waste to Energy: A Technical Review of Municipal Solid Waste Thermal Treatment Practices - Final Report. Unpublished report prepared for Environment Canada by Stantec, Burnaby, B.C. Project No. 1231-10166. March 2011. 339 p.
Environment Canada. 2012a. Data for certain cobalt-containing substances collected under Canadian Environmental Protection Act, 1999, section 71 and voluntary data submission: Notices with respect to certain cobalt-containing substances. Data prepared by: Environment Canada, Existing Substances Program.
Environment Canada. 2012b. Great Lakes Surveillance Program. Data collected under the National Fish Contaminants Program. Database shared from the Great Lakes Water Quality Monitoring Division to the Ecological Assessment Division.
Environment Canada. 2013a. Federal Environmental Quality Guidelines: Cobalt. Gatineau (QC): Environment Canada, National Guidelines and Standards Office.
Environment Canada. 2013b. Biomonitoring Information System for the Yukon (BISY) [CD-Rom on the Internet] Environment Canada. [cited 2013 August].
Environment Canada. 2013c. Data collected under the Chemicals Management Plan Environmental Monitoring and Surveillance Program. Unpublished data.
Environment Canada. 2013d. Unpublished confidential reports submitted to Environment Canada under the Environmental Effects Monitoring (EEM) Program of the Metal Mining Effluent Regulations (MMER) and Pulp and Paper Effluent Regulations (PPER). Gatineau (QC): Environment Canada, Forestry Products and Fisheries Act Division.
[ECCC] Environment and Climate Change Canada 2016a. Supporting documentation: Physical/Chemical Properties, Environmental Fate and Potential for Bioaccumulation. Gatineau (QC): ECCC. Information in support of the Screening Assessment for Cobalt and Cobalt-containing Substances.
[ECCC] Environment and Climate Change Canada 2016b. Supporting documentation: Analysis and potential for release of cobalt-containing inorganic UVCBs substances generated by metal smelting and refining. Gatineau (QC): ECCC. Information in support of the Screening Assessment for Cobalt and Cobalt-containing Substances.
[ECCC] Environment and Climate Change Canada 2016c. Supporting documentation: Ecological Effects. Gatineau (QC): ECCC. Information in support of the Screening Assessment for Cobalt and Cobalt-containing Substances.
[ECCC] Environment and Climate Change Canada 2016d. Supporting documentation: Ecological Exposure Assessment and Characterization of Ecological Risk Gatineau (QC): ECCC. Information in support of the Screening Assessment for Cobalt and Cobalt-containing Substances.
Environment Canada, Health Canada. 1994. Priority Substances List (PSL1) Assessment: Chromium and its Compounds: [Internet]. Ottawa (ON): Environment Canada, Health Canada. [July 19 2013].
Environment Canada, Health Canada. 2011a. Screening assessment for the Challenge: Cobalt (elemental cobalt): Chemical Abstracts Service Registry Number 7440-48-4; Cobalt chloride: Chemical Abstracts Service Registry Number 7646-79-9; Sulfuric acid, cobalt(2+) salt (1:1): Chemical Abstracts Service Registry Number 10124-43-3; Sulfuric acid, cobalt salt (cobalt sulfate): Chemical Abstracts Service Registry Number 10393-49-2 [Internet]. Ottawa (ON): Environment Canada, Health Canada.
Environment Canada, Health Canada. 2011b. Screening assessment for the Challenge: 2-ethylhexanoic acid or 2-EHA: Chemical Abstracts Service Registry Number (CAS) 149-57-5 [Internet]. Ottawa (ON): Environment Canada, Health Canada. [July 19 2013].
Ephraim JH, Marinsky JA, Cramer SJ. 1989. Complex-forming properties of natural organic acids - fulving acid complexes with cobalt, zinc and europium. Talanta 36(4): 437-443.
[ESIS] European Chemical Substances Information System [database on the Internet]. 2006. Version 4.5. European Chemical Bureau (ECB).
[ETRMA] 2010. Tyre and General Rubber Goods Generic Exposure Scenario Emission Factor Guidance for Formulation and Industrial Version 2.0. Prepared for: ETRMA, Brussels, Belgium Prepared by: ChemRisk LLC Pittsburgh, PA.
[EURAR] European Union risk assessment report [final?]. Nickel CAS No.: 7440-02-0 [Internet]. 2008. Luxembourg: Office for Official Publications of the European Communities. [cited 2013 Jul 22].
European Commission. 2004a. Summary Record Meeting of the Technical Committee C&L on the Classification and Labelling of Dangerous Substances. Meeting at Riga, 12-14 May 2004. European Commission Directorate General JRC. Joint Research Centre. Institute for Health and Consumer Protection. European Chemicals Bureau. ECBI/147/04 - Rev. 3.
European Commission. c2000a. IUCLID Dataset, Octanoic acid, CAS No. 124-07-2 [Internet]. Year 2000 CD-ROM edition. [place unknown]: European Chemicals Agency, European Commission. [created 2000 Feb 18; cited 2013 July 18].
European Commission. c2000b. IUCLID Dataset, Sodium phosphate acid, CAS No. 7558-80-7 [Internet]. Year 2000 CD-ROM edition. [place unknown]: European Chemicals Agency, European Commission. [created 2000 Feb 19; cited 2013 July 18].
European Commission. 2004b. Cobalt sulphate. Commission Directive 2004/73/EC of 29 April 2004. Annex IA. Official Journal of the European Union. 16.6.2004. L216/20. European Commission. 29th ATP.
Evans JC, Abel KH, Olsen KB, Lepel EA, Sanders RW, Wilkerson CL, Hayes DJ, Mangelson NF. 1985. Characterization of trace constituents at Canadian coal-fired power plants. Report for the Canadian Electricity Association, vol. 1, Phase I, 172 p. as cited in Goodarzi, F. 2013.
Evans LJ. 2000. Fractionation and Aqueous Speciation of Zinc in a Lake Polluted by Mining Activities, Flin Flon, Canada. Water Air Soil Poll. 122: 299-316.
Expert Group on Vitamins and Minerals (EGVM). 2003. Safe Upper Levels for Vitamins and Minerals. Food Standards Agency, London.
[FAO] Food and Agriculture Organization of the United Nations. 1990. Energy conservation in the mechanical forest industries. FAO Forestry Paper 93. Rome (Italy).
FDRL. 1984. Acute oral LD50 study of cobalt sulphate lot no. S88336/A in Sprague-Dawley rats. FDRL study no. 8005D. Food and Drug Research Laboratories, Inc., Waverly, NY. April 11, 1984. [cited in ATSDR 2004].
Filon FL, D'Agostin F, Crosera M, Adami G, Bovenzi M, Maina G. 2009. In vitro absorption of metal powders through intact and damaged human skin. Toxicol in Vitro. 23: 574-579.
Finley BL, Monnot AD, Gaffney SH, Paustenbach DJ. 2012. Dose-response relationships for blood cobalt concentrations and health effects: a review of the literature and application of a biokinetic model. J Toxicol Environ Health- Part B. 15, 493-523.
Finley BL, Unice KM, Kerger BD, Otani JM, Paustenbach DJ, Galbraith DA, Tvermoes BE. 31-day study of cobalt(II) chloride ingestion in humans: pharmacokinetics and clinical effects. J Toxicol Environ Health A 2013;76:1210-24.
Firriolo JM, Ayala-Fierro F, Sipes IG, Carter DE. 1999. Absorption and disposition of cobalt naphthenate in rats after a single oral dose. J Toxicol EnvironHealth A, 58:383-395.
Fischer 2008. "Material Safety Data Sheet: Cobalt Cyclohexanebutyrate" Fischer Scientific Web 20 Nov. 2008.
Förstner U, Wittmann GTW. 1981. Metal pollution in the aquatic environment, 2nd ed. Berlin: Springer. 532 pp.
Fréry N, Saoudi A, Garnier R, Zeghnoun A, Falq G, Guldner L. 2010. Exposure of the French population to environmental pollutants -Environmental components of the French National Survey on Nutrition and Health - Initial results. Saint-Maurice (Fra): French Institute for Public Health Surveillance, September 2010, 12 p.
Friel JK, Andrews WL, Jackson SE, Longerich HP, Mercer C, McDonald A, Dawson B, Sutradhar B. 1999. Elemental composition of human milk from mothers of premature and full-term infants during the first 3 months of lactation. Biol Trace Elem Res 67: 225-247.
Gál J, Hursthouse A, Tatner P, Stewart F, Welton R. 2008. Cobalt and secondary poisoning in the terrestrial food chain: Data review and research gaps to support risk assessment. Environ. Int. 34: 821-838.
Garrett RG. 2005. Natural distribution and abundance of elements. In: Selinus O (ed.). The essentials of medical geology. Amsterdam (The Netherlands): Elsevier Academic Press. p. 17-41.
Gaur JP, Noraho N, Chauhan YS. 1994. Relationship between heavy metal accumulation and toxicity in Spirodela polyrhiza (L.) Schleid and Azolla pinnata (R.Br.) Aquatic Botany 49: 183-192.
Gentsia Consulting Inc. 2009. Status report and technologies for management of liquid effluents in petroleum refining and bitumen upgrading sectors. Unpublished report submitted to Environment Canada. 420 p.
Geological Survey of Canada. 2001. National Geochemical Reconnaissance (NGR) - Geoscience Data Repository. Searched for Lake, Sediments and Streams data. Open file No. 2952.
Geological Survey of Canada and Teck Cominco Metals Ltd. 2001. Preliminary Assessment of Background Concentrations of Elements in Soil from the Trail Area. December 2001.
Geological Survey of Canada. 2002. Open file 4171: The deposition of trace elements on the land/surface soil in the Wabamun Lake area, Alberta, Canada. Ottawa (ON). 66 p.
Gobas FACP, Morrison HA. 2000. Bioconcentration and biomagnification in the aquatic environment. In Boethling RS, Mackay D, editors. Handbook of property estimation methods for chemicals, environmental and health sciences. Boca Raton (FL): CRC Press. p. 189-231.
Goodarzi, F. 2013. Characteristics and elemental composition of milled coals, bottom and fly ashes from power plants in Alberta and Nova Scotia. Unpublished reported prepared by FG and Partners Ltd., Calgary (AB) and submitted to Environment Canada, January 2013. 108 p.
Government of Alberta. 2010. Alberta Biomonitoring Program. Chemicals in Serum of Children in Southern Alberta 2004-2006. Influence of Age Comparison to Pregnant Women. [cited 2013 May 2].
Gross RT, Kriss JP, Spaet TH. 1955. The hematopoietic and goitrogenic effects of cobaltous chloride in patients with sickle cell anaemia. Pediatrics 15:284-290.
Haga Y, Clyne N, Hatori N, Hoffman-Bang C. Pehrsson, SK, Ryden L. 1996. Impaired myocardial function following chronic cobalt exposure in an isolated rat heart model. Trace Elem Electrolytes 13(2):69-74.
Hamilton-Taylor J, Postill AS, Tipping E, Harper MP. 2002. Laboratory measurements and modeling of metal-humic interactions under estuarine conditions. Geochim. Cosmochim. Acta 66(3): 403-415.
Hamilton-Taylor JM, Willis, CS. 1984. Reynolds Depositional fluxes of metals and phytoplankton in Windemere as measured by sediment traps. Limnol Oceanogr. 29(4): 695-710.
Hansen S, Nieboer E, Sandanger T, Wilsgaard T, Thomassen Y, Veyhe A, Oyvind, Odland J. 2011. Changes in maternal blood concentrations of selected essential and toxic elements during and after pregnancy. J. Environ. Monit. 13:2143-2152.
Harp MJ, Scoular FI. 1952. Cobalt metabolism of young college women on self-selected diets. Journal of Nutrition, 47:67-72.
Hatch. 2000. Review of environmental releases for the base metals smelting sector. Appendix A. Prepared for Environment Canada.
Hays SM, Aylward LL, LaKind JS, Bartels MJ, Barton HA, Boogaard PJ, Brunk C, DiZio S, Dourson M, Goldstein DA et al. 2008. Biomonitoring Equivalents Expert Workshop. Guidelines for the derivation of Biomonitoring Equivalents: report from the Biomonitoring Equivalents Expert Workshop. Regul Toxicol Pharmacol.51(3 Suppl):S4-15.
Health Canada 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Health Canada. 2009a. Categorization of substances on the Domestic Substances List[Internet]. [modified 2009 Sep]. Ottawa (ON): Health Canada.
Health Canada. 2009b. Canadian Total Diet Study [Internet]. Ottawa (ON): Health Canada. [cited: 2013 May].
Health Canada. 2009c. Guidance on Controlling Corrosion in Drinking Water Distribution Systems. Ottawa (ON): Health Canada. [cited: 2013 July].
Health Canada. 2010. Canadian Dietary Reference Intake [Internet]. Ottawa (ON): Health Canada. [cited: 2013 October].
Health Canada. 2012. Supporting hazard data for the screening assessment report of cobalt-containing substances. Internal document. Available upon request.
Health Canada. 2013. Second Report on Human Biomonitoring of Environmental Chemicals in Canada. Results of the Canadian Health Measures Survey Cycle 2 (2009-2011). April 2013.
[HC] Health Canada. 2016. Supporting Documentation: Health Effects of Cobalt-Containing Substances. Ottawa (ON): HC. Information in support of the Screening Assessment for Cobalt and Cobalt-containing Substances.
Heijerick D, Ghekiere A, Van Sprang P, De Schamphelaere K, Deleebeeck N, Janssen C. 2007. Effect of cobalt (CoCl2.6H2O) on freshwater organisms. Testing laboratory: EURAS & Laboratory of Environmental Toxicology, Ghent University. A report to the Cobalt Development Institute. Guildford, Surrey, United Kingdom. CDI Study Number 20.
Heitland P, Koster H. 2006. Biomonitoring of 30 trace elements in urine of children and adults by ICP-MS. Clinica Chimica Acta. 365:310-318.
Holly RG. 1955. Studies on iron and cobalt metabolism. JAMA. 158:1349-1352.
Holloway PC, Etsell TH, Bunnell CF. 2005. Atmospheric leaching of Oil Sands fly ash from Syncrude and Suncor. Miner Metall Proc 22(3):145-152.
Horev-Azaria L, Kirkpatrick CJ, Korenstein R, Marche PN, Maimon O, Ponti J, Romano R, Rossi F, Golla-Schindler U, Sommer D, et al. 2011. Predictive toxicology of cobalt nanaoparticles and ions: Comparative in vitro study of different cellular models using methods of knowledge discovery from data. Toxicol. Sci. 122(2): 489-501.
[HSDB] Hazardous Substances Data Bank [database on the Internet]. 1983a. Octanoic acid (CAS 124-7-2): National Library of Medicine (US). [revised 2007 Oct 07; cited 2013 July 18].
[HSDB] Hazardous Substances Data Bank [database on the Internet]. 1983b. Sodium hydrogen phosphate (CAS 7558-79-4): National Library of Medicine (US). [revised 2009 Aug 17; cited 2013 July 18].
[HSDB] Hazardous Substances Data Bank [database on the Internet]. 1983c. Potassium nitrite (CAS 7758-09-0): National Library of Medicine (US). [revised 2007 Jun 4; cited 2013 July 18].
[HSDB] Hazardous Substances Data Bank [database on the Internet]. 1985. Sodium octanoate (CAS 1984-6-1): National Library of Medicine (US). [revised 2003 Feb 14; cited 2013 July 18].
[HSDB] Hazardous Substances Data Bank [database on the Internet]. 1986. Naphthenic acid (CAS 1338-24-5): National Library of Medicine (US). [revised 2002 Nov 8; cited 2013 July 18].
[HSDB] Hazardous Substances Data Bank [database on the Internet]. 1992. Chromic acid (CAS 7738-94-5): National Library of Medicine (US). [revised 2005 Jun 23; cited 2013 July 18].
Hydromantis Inc., University of Waterloo, Trent University. 2010. Emerging substances of concern in biosolids: concentrations and effects of treatment processes. Final report - Field sampling program; CCME Project No. 447- 2009. Report preprared for the Canadian Council of Ministers of the Environment. Winnipeg (MB): Canadian Council of Ministers of the Environment. 255 p.
IARC (International Agency for Research on Cancer). 2006. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 86. Cobalt in hard metals and cobalt sulphate, gallium arsenide, indium phosphide and vanadium pentoxide.
ICRP (International Commission on Radiological Protection), 2006. Human alimentary tract model for radiological protection. ICRP Publication 100: a report of the International Commission on Radiological Protection. Ann. ICRP 36, 25-327.
Ikarashi Y, Ohno K, Tsuchiya T, Nakamura A. 1992a. Differences of draining lymph node cell proliferation among mice, rats and guinea pigs following exposure to metal allergens. Toxicology. 76:283-292.
Ikarashi Y, Tsuchiya T, Nakamura A. 1992b. Detection of contact sensitivity of metal salts using the murine local lymph node assay. Toxicol Lett. 62:53-61.
Ikemoto T, Tu NPC, Okuda N, Iwata A, Omori K, Tanabe S, Tuyen BC and Takeuchi I. 2008. Biomagnification of trace elements in the aqautic food web in the Mekong delta, South Vietnam using stable carbon and nitrogen isotope analysis. Arch. Environ. Contam. Toxicol. 54: 504-515.
[INSPQ] Institut national de santé publique du Québec. 2004. Étude sur l'établissement de valeurs de référence d'éléments traces et de métaux dans le sang, le sérum et l'urine de la population de la grande région de Québec. Institut national de santé publique du Québec, Québec, INSPQ-2004-030.
[ICMM] International Council on Mining and Metals. 2007a. MERAG: Metals Environmental Risk Assessment Guidance, Factsheet no. 6 Incorporation of Bioavailability for Soils. London, UK, 16 p.
[ICMM] International Council on Mining and Metals. 2007b, MERAG: Metals Environmental Risk Assessment Guidance, Factsheet no. 2. London, UK, 50 p.
Intrinsik Environmental Sciences Inc. 2010. Human Health Risk Assessment of Flin Flon, Manitoba, and Creighton, Saskatchewan - Final Report. June 2010.
Intrinsik Environmental Sciences Inc., Swansin Environmental Strategies Ltd., Delphinium Holdings Inc., Teck Metals Ltd. 2011. Terrestrial ecological risk assessment for the Teck Metals Ltd. Smelter at Trail, BC. Main report, revised May 2011. 162 p.
[IOM] Institute of Medicine. 1998. Cobalt, in: Institute of Medicine, Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. National Academy Press, Washington, D.C., pp. 306-356.
[IPCS] International Programme on Chemical Safety. 2006. Cobalt and inorganic cobalt compounds. Geneva (CH): World Health Organization. (Concise International Chemical Assessment Document 69). Jointly sponsored by the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals.
Jaimet CH, Thode HG. 1955. Thyroid function studies on children receiving cobalt therapy. J Am Med Assoc. 158: 1353-1355.
Johansson A, Camner P, Jarstrand C, Wiernik A. 1983. Rabbit alveolar macrophages after inhalation of soluble cadmium, cobalt, and copper: A comparison with the effects of soluble nickel. Environ. Res. 31: 340-354.
Johansson A, Curstedt T, Robertson B, Camner P. 1984. Lung morphology and phospholipids after experimental inhalation of soluble cadmium, copper, and cobalt. Environ Res. 34:295-309.
Johansson A, Robertson B, Camner P. 1987. Nodular accumulation of type II cells and inflammatory lesions caused by inhalation of low cobalt concentrations. Environ Res. 43:227-243.
Johnke B. 2000. Emissions from waste incineration. In "Good Practice Guidance and Uncertainty Management in National Greenhouse Gas Inventories" by Intergovernmental Panel on Climate Change (IPCC). pp.455-468. Available from:
Kapustka LA, Eskew D, Yogum JM. 2006. Plant toxicity testing to derive ecological soil screening levels for cobalt and nickel. Environmental Toxicology and Chemistry 25(3):865-874.
Kelleher Environmental. 2009. Battery Recycling in Canada - 2009 Update. Unpublished report prepared for Environment Canada and Natural Resources Canada. January 2009. 147 pages.
Keller W, Heneberry J, Gunn JM, Snucins E, Morgan G, Leduc J. 2004. Recovery of acid and metal damaged lakes near Sudbury Ontario: trends and status. Cooperative Freshwater Ecology Unit. Department of Biology, Laurentian University, Sudbury, Ontario. 55 p.
[KEMI] Hazardous chemicals in textiles - report of a government assignment [Internet] April 2013 Stockholm: Swedish Chemical Agency. 12 pages. [cited: 2013 May 16].
Kerfoot EJ. 1975. Semi-chronic inhalation study on cobalt. Diss Abstr Int B 35:6054-6055.
Kesteloot H, Roelandt J, Willems J, Claes JH, Joosens JV. 1968. An enquiry into the role of cobalt in the heart disease of chronic beer drinkers. Circulation 37: 854-864. [cited in IPCS 2006]
Khangarot BS, Rathore RS, Singh BB. 2003. pH-Dependent toxicity of heavy metals to a freshwater sludgeworm Tubifex tubifex Muller. Bulletin of Environmental Contamination and Toxicology 71(2): 283-289.
Kimball G. 1978. The effects of lesser known metals and one organic to fathead minnows (Pimephales promelas) and Daphnia magna. Department of Entomology, Fisheries and Wildlife, University of Minnesota.
Klaassen CD, editor. 2001. Casarett & Doull's Toxicology: basic science of poisons. 6th ed. NY, McGraw-Hill, absorption, distribution and excretion of toxicants. p 107-132.
Kniga M. 1980. HYSAAV (113095 Moscow, USSR). Vol 45(10): 72.
Krachler M, Rossipal E, Micetic-Turk D. 1999. Concentrations of Trace Elements in Sera of Newborns, Young Infants and Adults. Biol Trace Elem Res.68:121-135.
Krachler M, Prohaska T, Koellensperger G, Rossipal E, Stingeder G. 2000. Concentrations of selected trace elements in human milk and in infant formulas determined by magnetic sector field inductively coupled plasma-mass spectrometry. Biol Trace Elem Res.76: 97-112.
Krasovskii GN, Fridlyand SA. 1971. Experimental data for the validation of the maximum permissible concentration of cobalt in water bodies. Hyg Sanit 26:277-279.
Kreider ML, Panko JM, McAtee BL, Sweet LI, Finley BL. 2010. Physical and chemical characterization of tire-related particles: Comparison of particles generated using different methodologies. Sci Total Environ, 408, pp.652-659.
Kriss JP, Carnes WH, Ross RT. 1955. Hypothyroidism and thyroid hyperplasia in patients treated with cobalt. JAMA 157(2): 117-121.
Kurk DN, Choppin GR. 2000. Determination of Co(II) and Ni(II)-humate stability constants at high ionic strength NaCl solutions. Radiochim. Acta 88: 583-586.
Lantin AC,Vermeulen J, Mallants A, Vanoverschelde JL, Speybroeck N, Swennen B, Hoet P Lison D. 2013. Occupational exposure to cobalt is not associated with incipient signs of dilated cardiomyopathy in a Belgian refinery. Occup Environ Med 70(6): 386-92.
Lauwerys R, Lison D. 1994. Health risks associated with cobalt exposure-an overview. Sci Total Environ.150:1-6.
Lee J, Joansson JL. 1983. Contribution of organic complexation to Ni, Co and Cu speciation in surface waters: implications for hydrogeochemical surveys. J Geochem Explor. 18: 25-48.
Lee Y, Tebo BM. 1994. Cobalt oxidation by the marine manganese (II) oxidizing Bacillus sp. Strain SG-1. Applied and Environmental Microbiology, 60(8): 2949-2957.
Leggett RW. 2008. The biokinetics of inorganic cobalt in the human body. Sci. Total Environ. 389: 259-269.
Letourneau EG, Jack GC, McCullough RS, Hollins JG. 1972. The metabolism of cobalt by the normal human male: whole body retention and radiation dosimetry. Health Phys;22:451-9.
Lewis RJ. 1996. Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold, p. 901.
Li HFC, Gray C,. Micó,Zhao FJ, McGrath SP.2009. Phytotoxicity and bioavailability of cobalt to plants in a range of soils. Chemosphere 75:979-986.
Liden C, Wahlberg JE. 1994. Cross-reactivity to metal compounds studied in guinea pigs induced with chromate or cobalt. Acta Derm. Venereol. 74: 341-343.
Linna A, Oksa P, Groundstroem K, Halkosaari M, Palmroos P, Huikko S, Uitti J. 2004. Exposure to cobalt in the production of cobalt and cobalt compounds and its effect on the heart. Occup. Envir. Med. 61(11): 877-85.
Linna A, Oksa P, Palmroos P, Roto P, Laippala P, Uitti J. 2003. Respiratory health of cobalt production workers. American Journal of Industrial Medicine. 44: 124-132.
Llobet JM, Domingo JL. 1983. Toxicidad aguda y alteraciones hematológicas y séricas por algunas sales de cobalto en ratas. Revista Española de Fisiologia, 39: 291 - 298.
[LNHPD] Licensed Natural Health Products Database [database on the Internet]. 2013. Version 1.0. Ottawa (ON): Health Canada. [cited 2013 April 10]. Available from: webprod3.hc-sc.gc.ca/lnhpd-bdpsnh/index-eng.jsp
Lock K., Schamphelaere KAC, Because S, Criel P, van Eeckhout H, Janssen CR. 2006. Development and validation of an acute biotic ligand model (BLM) predicting cobalt toxicity in soil to potworm Enchtraeus albidus. Soil Biology and Biochemistry 38(2006):1924-1932.
Lock K., Schamphelaere KAC, Because S, Criel P, van Eeckhout H, Janssen CR. 2007. Development and validation of a terrestrial biotic ligand model predicting the effect of cobalt on root growth of barley (Hordeum vulgare). Environmental Pollution 147:626-633.
Lock K., Because S, Criel P, van Eeckhout H, Janssen CR. 2004. Ecotoxicity of cobalt to the springtail Folsomia candida. Comparative Biochemistry and Physiology. Part C 139(2004):195-199.
Luckey TD. 1975. Introduction to heavy metal toxicity, safety and hormology. Environmental Quality and Safety; 1:1-4: 55-56.
[MAC] Mining Association of Canada. 2015. Comments from MAC dated February 15, 2015, received during the public comment period following the publication of the draft screening assessment on cobalt. Gatineau (QC): Environment Canada, Ecological Assessment Division. 23 p. Table 23.
MacFarlane A, Greene-Finestone LS, Yipu S. 2011. Vitamin B-12 and homocysteine status in a folate-replete population: results from the Canadian Health Measures Survey. Am J Clin Nutr 94(4):1079-87.
Macfie SM, Tarmohamed Y, Welbourn PM. 1994. Effects of cadmium, cobalt, copper, and nickel on growth of the green alga Chlamydomonas reinhardtii: The influences of the cell wall and pH. Archives of Environ Contam Toxicol. 27: 454-458.
MacLean RS, Borgmann U, Dixon DG. 1996. Bioaccumulation kinetics and toxicity of lead in Hyalella azteca (Crustacea, Amphipoda). Can J Fish Aquat Sci. 53: 2212-2220.
Manitoba Conservation. 2003. Metal concentrations in surface soils of Thompson, Manitoba, September 2001. Report No. 03-01. 28 p.
Manitoba Conservation. 2007. Concentrations of Metals and Other Elements in Surface Soils of Flin Flon, Manitoba, and Creighton, Saskatchewan, 2006. Report No. 2007-01. 77 p.
Mathews T, Fisher NS, Jeffree RA, Teyssié JL. 2008. Assimilation and retention of metals in teleost and elasmobranch fishes following dietary exposure. Mar. Ecol. Prog. Ser. 360: 1-12.
[MCC] Metal Carboxylates Coalition. 2007. Cobalt borate neodecanoate complex: In vitro mammalian chromosome aberration test in Chinese Hamster Ovary Cells. Testing laboratory: E.I. du Pont de Neumors & Company, Haskell Laboratory for Health & Environmental Sciences, USA. Project ID: DuPont-20901. Work request number: 16637, Service code number 531. Contact company: Synthetic Organic Manufactures Association (SOCMA), 1850, M Street, Suite 700, Washington DC, 20036, USA.
McGeer JC, Brix KV, Skeaff JM, DeForest DK, Brigham SI, Adams WJ, and Green A. 2003. Inverse relationship between bioconcentration factor and exposure concentration for metals: implications for hazard assessment of metals in the aquatic environment. Environ Toxicol Chem. 22(5): 1017-1037.
McLaughlin M.J. 2001 Ageing of metals in soils changes bioavailability. Fact Sheet 4 on Environmental Risk Assessment, ICME (International Council on Metals and the Environment), 6.
[MDDEP] Ministère du Développement Durable, de l’Environnement et des Parcs. 2010. Évaluation des rejets d’eaux usées des usines de pâtes et papiers du Québec en fonction du milieu récepteur. Gouvernement du Québec, 193 p.
Méranger JC, Subramanian KS, Chalifoux C. 1981. Survey for cadmium, cobalt, chromium, copper, nickel, lead, zinc, calcium, and magnesium in Canadian drinking water supplies. J Assoc Off Anal Chem. 64(1): 44-53.
Metian M, Warnau M, Hédouin L, Busamante P. 2009. Bioaccumulation of essential metals (Co, Mn and Zn) in the king scallop Pecten maximus: seawater, food and sediment exposures. Mar Biol. 156: 2063-2075.
Micó C, Li HF, Zhao FJ, McGrath SP. 2008. Use of Co speciation and soil properties to explain variation in Co toxicity to root growth of barley (Hordeum vulgare L.) in different soils. Environmental Pollution 156 (2008):883-890.
Mohiuddin SM, Taskar PK, Rheault M, Roy P-E, Chenard J, Morin Y. 1970. Experimental cobaltcardiomyopathy. Am Heart J. 80(4):532-543. [cited in ATSDR 2004].
Mollenhauer HH, Corrier DE, Clark DE, Hare MF, Elissalde MH. 1985. Effects of dietary cobalt on testicular structure. Virchows Archiv B: Cell Pathology Including Molecular Pathology, 49:241-248.
Morin Y, Daniel P. 1967. Quebec beer-drinkers' cardiomyopathy: etiological considerations. Can Med Assoc J 97: 926-928. [cited in IPCS 2006].
Morin Y, Tetu A, Mercier G. 1971. Cobalt cardiomyopathy: Clinical Aspects. Br Heart J 33: 175-178. [cited in IPCS 2006].
Morsy SM, El-Assaly FM. 1970. Body elimination rates of 134Cs, 60Co and 203Hg. Health Phys 19:769-773.
Mosconi G, Bacis M, Vitali MT, Leghissa P, Sabbioni E. 1994. Cobalt excretion in urine: Results of a study on workers producing diamond grinding tools and on a control group. Sci Total Environ 150:133-139.
Moulin JJ. Wild P, Mur JM, Fournier-Betz M, Mercier-Gallay M. 1993. A mortalitiy study of cobalt production workers: An extension of the follow-up. Am J Ind Med. 23: 281 - 288. [cited in IARC 2006]
Mur JM Moulin JJ, Charruyer-Seinerra MP, Lafitte J. 1987. A cohort mortality study among cobalt and sodium workers in an electrochemical plant. Am J Ind Med., 11: 75 - 81. [cited in IARC 2006]
[NAPS] National Air Pollutants Surveillance Network. Forincoming 2003 - 2008. [Prepublication NAPS data on Excel spreadsheet] Environment Canada. Air Monitoring Data.
Nation JR, Bourgeois AE, Clark DE, Hare MF. 1983. The effects of chronic cobalt exposure on behavior and metallothionein levels in the adult rat. Neurobehav Toxicol Teratol. 5: 9-15.
Natural Resources Canada. 2007. Benchmarking energy intensity in the Canadian steel industry. ISBN 0-662-43410-2. Cat. No. M144-125/2006E. 100 p.
Natural Resources Canada. 2009a. Statistics on Annual Mineral Production. [Modified on 30 August 2013, cited on 17 September 2013].
Natural Resources Canada. 2009b. Canadian Minerals Yearbook. [Modified on 30 June 2011, cited on 6 December 2012].
Nautilus Environmental. 2009. Environmental. Summary of ecotoxicity data for the purpose of developing a species sensitivity distribution (SSD) for cobalt and its compounds Final Report, Available upon request.
[NCASI] National Council for Air and Stream Improvement. 2006. Unpublished draft confidential report submitted to Environment Canada. Gatineau (QC): Environment Canada, Forestry Products and Fisheries Act Division.
[NCI] National Chemical Inventories [database on CD-ROM]. 2007. Issue 1. Columbus (OH): American Chemical Society. [cited 2010 Jan 11].
Nemery B, Casier P, Roosels D, Lahaye D, Demedts M. 1992. Survey of cobalt exposure and respiratory health in diamond polishers. Am Rev Respir Dis 145: 610-616.
Nfon E, Cousins IT, Jarvinen O, Mukherjee AB, Verta M, Broman D. 2009. Trophodynamics of mercury and other trace elements in a pelagic food chain from the Baltic Sea. Sci Total Environ. 407: 6267-6274.
Nguyen LTH, Vandegehuchte MB,Roman YE, Janssen CR. 2009a. Ecotoxicity of cobalt to Tubifex tubifex, Chironomus riparius and Hyallela azteca tested in natural freshwater sediment. A report to Cobalt Development Institute, Guildford, Surrey, United Kingdom.
Nguyen LTH, Vandegehuchte MB,Roman YE, Janssen CR. 2009b. Ecotoxicity of cobalt to Ephoron virgo, Gammarus pulex, and Lumbriculus variegatus tested in natural freshwater sediment. A report to Cobalt Development Institute, Guildford, Surrey, United Kingdom.
[NHPID] Natural Health Products Ingredients Database [database on the Internet]. 2013. Version 2.1. Ottawa (ON): Health Canada. [cited 2013 Apr].
Nielsen NH, Kristiansen J, Borg L, Christensen JM, Poulsen LK, Menne T. 2000. Repeated exposures to cobalt or chromate on the hands of patients with hand eczema and contact allergy to that metal. Contact Dermatitis, 43(4):212-215. [cited in IPCS 2006].
Nong A, Ashrafi M, Macey K and Poddalgoda D. 2013. Derivation of biomonitoring equivalent for cobalt. Health Canada (unpublished).
Norwood WP, Borgmann U, Dixon DG. 2006. Saturation models of arsenic, cobalt, chromium and manganese bioacccumulation by Hyalella azteca. Environ Pollut. 143:519-528.
Norwood WP, Borgmann U, Dixon DG. 2007. Chronic toxicity of arsenic, cobalt, chromium and manganese to Hyalella azteca in relation to exposure and bioaccumulation. Environ. Poll. 147: 262-272.
Nota B, Verweij RA, Molenaar D, Ylstra B, van Straalen NM, Roelofs D. 2010. Gene expression analysis reveals a gene set discriminatory to different metals in soil. Toxicol. Sci. 115 (1): 34-40.
[NPRI] National Pollutant Release Inventory [database on the Internet]. 1995. NPRI data search for "Cobalt (and its compounds)". Gatineau (QC): Environment Canada. [cited 2015 May]. Available from:
[NPRI] National Pollutant Release Inventory. 2013. Glossary of terms and expressions used by the NPRI. Gatineau (QC): Environment Canada. [cited 2015 Nov].
Nriagu, JO. 1989. A global assessment of natural sources of atmospheric trace metals. Nature 338: 47-49.
[NTE] National Agri-Environmental Health Analysis and Reporting Program (NAHARP) Trace Element Model. 2011. Pinawa (MB): Ecomatters Inc, Unpublished model developed for Environment Canada. Latest version dated March 2011.
NTP (National Toxicology Program). 1991. Toxicity studies of cobalt sulfate heptahydrate (CAS No. 10026-24-1) in F344/N rats and B6C3F1 mice (inhalation studies). Research Triangle Park, NC, United States Department of Health and Human Services, National Institutes of Health, (NIH Publication No. 91-3124) [cited in IARC 2006, IPCS 2006].
NTP (National Toxicology Program). 1998. Report on the toxicology and carcinogenesis studies of cobalt sulfate heptahydrate (CAS No. 10026-24-1) in F344/N rats and B6C3F1 mice (inhalation studies). Research Triangle Park, NC, United States Department of Health and Human Services, National Institutes of Health, National Toxicology Program (NIH Publication No. 471). [cited in IARC 2006, IPCS 2006].
NTP [National Toxicology Program]. 2005. 11th Report on carcinogens. Substance Profile: Cobalt Sulfate CAS No. 10124-43-3.
NTP [National Toxicology Program]. 2014. NTP technical report on the toxicology studies of cobalt metal(CAS No. 7440-48-4) in F344/N rats and B6C3F1 mice and toxicology and carcinogenesis studies of cobalt metal in F344/NTac rats and B6C3F1/N mice (inhalation studies). Research Triangle Park, NC, United States Department of Health and Human Services, National Institutes of Health, National Toxicology Program (NTP TR 581). [Accessed 2015 April]
NTP [National Toxicology Program]. 2016. Cobalt-related exposures. In 14th Report on Carcinogens. Research Triangle Park, NC: National Toxicology Program. [Access 2016 November].
[NWRI] National Water Research Institute. Received in 2009. EC data collection program for Prairies provinces and North-West territories.
[OECD] Organisation for Economic Co-operation and Development. 1993. Bioaccumulation: sequential static fish test. OECD Guidelines for the Testing of Chemicals No. 305A. Organisation for Economic Co-operation and Development, Paris, France. 13 p.
[OECD] Organisation for Economic Co-operation and Development. 1996. Bioconcentration: flow-through fish test. OECD guidelines for the testing chemicals No. 305E. Organization for Economic Co-operation and Development, Paris, France. 23 p.
[OECD] Organisation for Economic Co-operation and Development. 2001. Guidance Document on Transformation/Dissolution of Metals and Metal Compounds in Aqueous Media [Internet]. Paris (FR): OECD, Environment Directorate. Report No.: ENV/JM/MONO(2001)9.
[OECD] Organisation for Economic Co-operation and Development. 2008a. Series on Testing and Assessment No. 87. Report of the Ring Test and Statistical Analysis of Performance of the Guidance on Transformation/Dissolution of Metals and Metal Compounds in Aqueous Media (Transformation/Dissolution Protocol) [Internet]. Paris (FR): OECD, Environment Directorate. Report No.: ENV/JM/MONO(2008)8.
[OECD] Organisation for Economic Co-operation and Development. 2008b. Series on Testing and Assessment No. 98. Considerations Regarding Applicability of the Guidance on Transformation/Dissolution of Metals and Metal Compounds in Aqueous Media(Transformation/Dissolution Protocol) [Internet]. Paris (FR): OECD, Environment Directorate. Report No.: ENV/JM/MONO(2008)25.
[OECD] Organisation for Economic Co-operation and Development. 2009. Emission Scenario Documents on Coating Industry (Paints, Laquers and Varnishes). OECD Series on Emission Scenario Documents. Paris (FR). Jul 8, 2009.
[OECD] Organisation for Economic Co-operation and Development. 2014. SIDS Initial Assessment Profile (SIAP) for soluble cobalt salts. CoCAM [Cooperative Chemicals Assessment Meeting] 6, 30-3 October 2014. [Acessed April 2015].
[OMOE] Ontario Ministry of the Environment. 1989. Preliminary Report for the First Six Months of Monitoring in the Petroleum Refining Sector (December 1, 1988 to May 31, 1989). ISBN 0-7729-6375-4. 42 p.
[OMOE] Ontario Ministry of the Environment. 1991. Status Report on the Effluent Monitoring Data for the Iron and Steel Sector for the Period from November 1, 1989 to October 31, 1990. 256 p. ISBN 0-7729-8819-6.
[OMOE] Ontario Ministry of the Environment. 2000. Phytotoxicology soil investigation: INCO - Port Colborne(1998). Report No. SDB-031-3511-1999. 100 p.
[OMOE] Ontario Ministry of the Environment. 2002. Soil Investigation and Human Health Risk Assessment for the Rodney Street Comminity, Port Colborne, Part B - Human Health Risk Assessment: Appendix 1 - Environmental Monitoring of Metals in the Rodney Street Community and Port Colborne [Internet]. Toronto (ON): Ontario Ministry of the Environment. [Accessed 2009 Sept 9].
[OMOE] Ontario Ministry of the Environment. 2004. Sudbury Soils Study, Volume I: Background, Study Organization and 2001 Soils Survey, Appendix C: 2001 Soil Survey Reports, City of Greater Sudbury 2001 Urban Soil Survey. Report No. SDB-008-3511-2003. Toronto, ON. 1351 p. [Cited 2013 Mar].
[OMOE] Ontario Ministry of the Environment. 2011. Final Report: Cobalt Coleman Mining Camp Soil Assessment[Internet]. Toronto (ON): Ontario Ministry of the Environment. [Accessed May 2013].
[OMOE] Ontario Ministry of the Environment. 2013. Data downloads. Provincial Water Quality Monitoring Network[database on the Internet]. 2013 Ontario. [cited 2013 May].
Pacific Ecorisk. 2005. An Evaluation of the Acute Toxicity of Cobalt in Panther Creek Water to Three Resident Invertebrate Species (Brachycentrus americanus, Centroptilum conturbatum, and Serratella tibialis) and the Acute and Chronic Toxicity of Cobalt in Panther Creek Water to Chironomus tentans and Oncorhynchus mykiss. Testing laboratory: Pacific Ecorisk, Martinez, CA. A report to the Blackbird Mine Site Group.
Paley KR, Sobel, ES, Yalow, RS. 1958. Effect of oral and intravenous cobaltous chloride on thyroid function. J. Clin. Endocrinol. Metab. 18: 850-859.
Pandey AK, Pandey SD, Misra V. 2000. Stability constants of metal-humic acid complexes and its role in environmental detoxification. Ecotoxicol Environ Safety. 47: 195-200.
Paquin PR, Gorsuch JW, Apte S, Batley GE, Bowles KC, Campbell PGC, Delos CG, Di Toro DM, Dwyer RL, Galvez F, Gensemer RW, Goss GG, Hogstrand C, Janssen CR, McGeer JC, Naddy RB, Playle RC, Santore RC, Schneider U, Stubblefield WA, Wood CM, Wu KB. 2002. The biotic ligand model: a historical overview. Comp. Biochem. Physiol. Part C 133, 3-35.
Parametrix. 2010a. Acute and chronic toxicity of cobalt to freshwater organisms: Cobalt AWQC/PNEC development - Study 20. Testing laboratory: Parametrix Environmental Research Laboratory. A report to the Cobalt Development Institute. Guildford, Surrey, United Kingdom. CDI Study Number 20.
Parametrix. 2010b. Chronic toxicity of cobalt to the cladoceran, Ceriodaphnia dubia: Framework for the Development of a Biotic Ligand Model (BLM). Testing laboratory: Parametrix Environmental Research Laboratory. A report to the Cobalt Development Institute. Guildford, Surrey, United Kingdom. CDI Study Number 44.
Parametrix. 2010c. Chronic toxicity of cobalt to the fathead minnow (Pimephales promelas): Framework for the Development of a Biotic Ligand Model (BLM). Testing laboratory: Parametrix Environmental Research Laboratory. A report to the Cobalt Development Institute. Guildford, Surrey, United Kingdom. CDI Study Number 44.
Paternain JL, Domingo JL, Corbella J. 1988. Developmental toxicity of cobalt in the rat. J Toxicol Environ Health. 24:193-200.
Paustenbach DJ, Tvermoes BE, Unice KM, Finley BL, Kerger BD. 2013. A review of the health hazards posed by cobalt. Crit Rev Toxicol. 43(4): 316-362.
Pedigo NG, George WJ, Anderson MB. 1988. Effects of acute and chronic exposure to cobalt in male reproduction in mice. Reprod Toxicol 2: 45-53.
Pedigo NG, Vernon MW. 1993. Embryonic losses after 10-week administration of cobalt to male mice. Reproductive Toxicology, 7:111-116.
Pennington JAT, Church HN. 1985. Food Values of Portions Commonly Used. 14th edition. Harper and Row, New York.
Pham M, Garnier J-M. 1998. Distribution of trace elements associated with dissolved compounds (less than 0.45 μm - 1 nm) in freshwater using coupled (frontal cascade) ultrafiltration and chromatographic separations. Environ Sci Technol. 32(4): 440-449.
Pirila V. 1953. Sensitization to cobalt in pottery workers. Acta Dermato-Venereologica, 33(3): 193-198.
Prado AGS, Airoldi C. 2003. Humic acid-divalent cation interactions. Thermochim. Acta 405: 287-292.
Pratt, M.D., D.V. Belsito, V.A. DeLeo, J.F. Fowler Jr, A.F. Fransway, H.I. Maibach, J.G. Marks, C.G. Mathias, R.L. Rietschel, D. Sasseville, E.F. Sherertz, F.J. Storrs, J.S. Taylor and K. Zug. 2004. North American contact dermatitis group patch-test results, 2001-2002 study period. Dermatitis 15: 176-183.
Qian J, Xue HB, Sigg L, Albrecht A. 1998. Complexation of cobalt by natural ligands in freshwater. Environ Sci Technol. 32: 2043-2050.
[RAMP] Regional Aquatics Monitoring Program. 2012. Monitoring Database. Searched for water and sediment quality data. [cited 2013 Apr].8
Rasmussen JB, Gunn JM, Sherwood GD, Iles A, Gagnon A, Campbell PGC, Hontela A. 2008. Direct and indirect (foodweb mediated) effects of metal exposure on the growth of yellow perch (Perca flavescens): implications for ecological risk assessment. Human Ecol Risk Assess. 14. 317-350.
Rasmussen PE.2013. Preliminary data from the Windsor Exposure Assessment Study and the Canadian House Dust Study. Exposure and Biomonitoring Division, Health Canada. Unpublished Data.
Rasmussen PE, Subramanian KS, Jessiman BJ. 2001. A multi-element profile of housedust in relation to exterior dust and soils in the city of Ottawa, Canada. The Science of the Total Environment. 267: 125-140.
Rathore RS, Khangarot BS. 2003. Effects of Water Hardness and metal concentration on a Freshwater Tubifex tubifex Muller. Water Air Soil Poll. 142: 341-356.
Reagan EL. 1992. Acute oral toxicity study in rats with cobalt (II) sulfide. Int J of Toxicol 11(6): 693.
Reddy MS, Basha S, Joshi HV, Jha B. 2005. Evaluation of the emission characteristics of trace metals from coal and fuel oil fired power plants and their fate during combustion. J Hazard Mater. B123: 242-249.
Redeker E, F Degryse, E Smolders. 2008. Toxicity of Cobalt in soils: Summary of conclusion (i) Toxicity data and modeling bioavailability for effects assessment. Final report to Cobalt Development Institute. September 2008.
Rehfisch P, Anderson M, Berg P, Lampa E, Nordling Y, Svartengren M, Westberg H, Gunnarsson L. 2012. Lung function and respiratory symptoms in hard metal workers exposed to cobalt. J Occup Environ Med. 54 (4): 409-413.
Reimann C, de Caritat P. 1998. Chemical elements in the environment. Berlin (Germany): Springer-Verlag. 398 p.
Rencz A, Adcock G, Bonham-Carter G. 2006. Geochemical Background in Soil and Till. Geological Survey of Canada Open File.
Reuber S, Krcuzer M, Kirchgessner M. 1994. Interactions of cobalt and iron in absorption and retention. J Trace Elem Electrolytes Health Dis 8:151-158.
Roche, M., and Layrisse, M. 1956. Effect of cobalt on thyroidal uptake of I131. J Clin Endocrinol Metab 16:831-833.
Roto P. 1980. Asthma, symptoms of chronic bronchitis and ventilatory capacity among cobalt and zinc production workers. Scan J Work Environ Health. 6 (1): 1-49.
Salpeteur L, Van Laer L, Oorts K, Smolders E. 2007. Development of a predictive model of bioavailability and toxicity of cobalt in soils: Microbial toxicity. Testing laboratory: Laboratory of Soil and Water Management, Katholieke Universiteit Leuven. A report to the Cobalt Development Institute. Guildford, Surrey, United Kingdom. CDI Study Number 28.
Santschi PH. 1984. Particle flux and trace metal residence time in natural waters. Limnol. Oceanogr. 29: 1100-1108.
[SARA] Sudbury Area Risk Assessment. Volume II Appendix M: Indoor Dust Survey - Data Report. 2005. The SARA Group. Sudbury, ON. Aug 2005. 45p. [Cited 2013 Aug].
[SARA] Sudbury Area Risk Assessment. 2008. Summary of Volume II: Human Health Risk Assessment. The SARA Group. Sudbury, ON. May 2008. 48p. [Cited 2013 Aug].
[SARA] Sudbury Area Risk Assessment. 2009. Sudbury Soils Study, Volume III: Ecological Risk Assessment, Chapter 5: Aquatic Problem Formulation. The SARA Group. Sudbury, ON. March 2009. 158p. [Cited 2013 Mar].
Sarantis, H. A. Report on Heavy Metals in Face Paints [Internet] October 2009, Campaign for Safe Cosmetics: Breast Cancer Fund and Commonwealth. 36 pages [cited: 2013 May 16].
Sauni R. Linna A, Oksa P, Nordman H, Tuppurainen, Uitti J. 2010. Cobalt asthma - a case series from a cobalt plant. Occupational Medicine (Lond), 60 (4): 301-306.
Scansetti G, Botta GC, Spinelli P, Reviglione L, Ponzetti C. 1994. Absorption and excretion of cobalt in the hard metal industry. Sci Total Environ 150:141-144.
Schlekat CE, McGeer JC, Blust R, Borgmann U, Brix KV, Bury N, Couillard Y, Dwyer RL, Luoma SN, Robertson S, Sappington KG, Schoeters I, Sijm DTHM. 2007. Bioaccumulation; hazard identification of metals and inorganic metal substances. In: Adams WJ, Chapman PM, editors. Assessing the hazard of metals and inorganic metal substances in aquatic and terrestrial systems. Pensacola (FL): SETAC Publications, CRC Press. Chap 4: 55-87.
Sheppard SC, CA Grant, MI Sheppard, R de Jong, J Long. 2009. Risk indicator for agricultural inputs of trace elements to Canadian soils. J Env Quality, 38(3): 919-932.
Sherwood GD, Pazzia I, Moeser A, Hontela A Rasmussen JB 2002. Shifting gears:enzymatic evidence for the energetic advantage of switching diet in wild-living fish. Can J Fish Aqua. Sci. 59(2): 229-241
Shirakawa T, Kusaka Y, Fujimura N, Goto S, Kato M, Heki S, Morimoto K. 1989 Occupational asthma from cobalt sensitivity in workers exposed to hard metal dust. Chest. 95(1):29-37.
Shirakawa T, Kusaka Y, Fujimura N, Goto S, Morimoto K. 1988. The existence of specific antibodies to cobalt in hard metal asthma. Clinical Allergy, 18:451-460.
Shrivastava V, David C, Khare N. 1996. Cobalt chloride induced histopathological changes in thyroid gland of female mice, Mus musculus (P). Pollution Research. 15:307-309.
Siddique R. 2010. Utilization of coal combustion by-products in sustainable construction materials. Resources, Conservation and Recycling. 54: 1060-1066.
Simonsen LO, Harbak H, Bennekou P. 2012. Cobalt metabolism and toxicology- a brief update. Sci Total Environ, 432C, 210-15.
Singh PP, Junnarkar AY. 1991. Behavioral and toxic profile of some essential trace metal salts in mice and rats. Indian J Pharmacol 23:153-159.
Smith RM, Martell AE. 2004. Critical constants for metal complexes [database on a CD-ROM]. NIST Standard Reference database 46 Version 8. Gaithersburgh (MD): U.S. Department of Commerce, National Institute of Standards and Technology. [updated 2004].
Smith T, Edmonds CJ, Barnaby CF. 1972. Absorption and retention of cobalt in man by whole-body counting. Health Phys. 22: 359-367.
Smolders E, McGrath S, Fairbrother A, Hale BA, Lombi E, McLaughlin M, Rutgers M, Van der Vliet L. 2007. In: Adams WJ, Chapman PM (editors.). Hazard identification approaches for metals and inorganic metal substances. Pensacola (FL): SETAC Press. p. 113-133.
Smolders E, Oorts K, Van Sprang P, Schoeters I, Janssen CR, McGrath SP, McLaughlin MJ. 2009. Toxicity of trace metals in soil as affected by soil type and aging after contamination: using calibrated bioavailability models to set ecological soil standards. Environ Toxicol Chem. 28(8): 1633-1642.
Sorbie J, Olatunbosun D, Corbett WEN, Valberg LS. 1971. Cobalt excretion test for the assessment of body iron stores. Can Med Assoc J 104: 777-782.
Speijers GJA, Krajnc EI, Berkvens JM, van Logten MJ. 1982. Acute oral toxicity of inorganic cobalt compounds in rats. Food Chem Toxicol 20:311-314.
[SSD Master] Determination of Hazardous Concentrations with Species Sensitivity Distributions [Computer Model]. 2010. Version 3. Ottawa (ON): Intrinsikscience.
Stanley AJ, Hopps HC, Shideler AM. 1947. Cobalt polycythemia. II. Relative effects of oral and subcutaneous administration of cobaltous chloride. Proc Soc Exp Biol Med 66:19-20.
Statistics Canada. 2007. Analysis in brief: heavy fuel oil consumption in Canada. Ottawa (ON): Statistics Canada, Manufacturing, Construction and Energy Division. Catalogue No. 11-621-MIE-No. 062. Available from:
Statistics Canada. 2013a. Canadian International Merchandise Trade Database. [accessed 14-06-2013].
Statistics Canada. 2013b. Waste management industry survey: business and government sectors - 2010. Catalogue no. 16F0023X. Statistics Canada, Environment Accounts and Statistics Division. Ottawa. 38 pages.
Statistics Canada. 2013c. Canadian Health Measures Survey: Cycle 2 Data Tables. 2009 to 2011. Catalogue no. 82-626-X. Statistics Canada, Health Statistics Division. Ottawa [cited 13 May 2013].
Stephan CE, Mount DI, HansenDJ, Gentile JH, Chapman, GA, Brungs, WA. 1985. Guidelines for deriving numerical national water quality criteria for the protection of aquatic organisms and their uses. U.S. Environmental Protection Agency. PB85-227049.
Stopford W, Turner J, Cappellini D, Brock T. 2003. Bioaccessability Testing of Cobalt Compounds. Journal of Environmental Monitorinq, 5:675-680.
Sullivan JF, Egan JD, George RP. 1969. A distinctive myocardiopathy occurring in Omaha, Nebraska: Clinical aspects. Ann N Y Acad Sci 156(1): 526-543 [cited in IPCS 2006].
Swennen B, Buchet J-P, Stanescu D, Lison D, Lauwerys R. 1993. Epidemiological survey of workers exposed to cobalt oxides, cobalt salts, and cobalt metal. Br J Ind Med 50: 835-842.
[SYSTAT] Statistics software [Computer Software]. 2013. Version 13. Chicago (USA): Systat Software, inc.
Szakmary E, Ungvary G, Hudak A, Tatrai E, Naray M, Morvai V. 2001. Effects of cobalt sulfate on prenatal development of mice, rats, and rabbits, and on early postnatal development of rats. J Toxicol Environ Health A 62: 367-386.
Szkokan-Emilson EJ, Watmough S, Gunn JM. 2014. Wetlands as long-term sources of metals to receiving waters in mining-impacted landscapes. Environ Pollut 192: 91-103.
Taylor A, Marks V, Shabaan AA, Mahmood HA, Duckham JM, Lee HA. 1977. Cobalt induced lipaemia and erthropoiesis. Dev Toxicol Environ Sci 1:105-108 [cited in ATSDR 2004].
Takeno N. 2005. Atlas of Eh-pH diagrams. Geological Survey of Japan Open File Report No. 419. National Institute of Advanced Industrial Science and Technology. 285 p.
Tessier A, Campbell PGC, Bisson M. 1979. Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem. 51(7): 844-850.
Tipping E. 2002. Cation binding by humic substances. Cambridge (UK): Cambridge University Press. 434 p.
Tugulea AM. 2013. National survey of disinfection by-products and selected drinking water contaminants in Canadian drinking water (2009-2010) [personal communication, unpublished data].
Tvermoes B E, Otani J M, Unice K M, Finley B L, Paustenbach D J, and Galbraith DA. 2013. A 14 day cobalt supplement study to investigate cobalt steadystate levels in five healthy adult volunteers. Food Chem Toxicol. 53: 432-439
Tvermoes B E, Unice K M, Paustenbach D J, Finley B L, Otani J M, and Galbraith DA. 2014. Effects and blood concentrations of cobalt after ingestion of 1 mg/d by human volunteers for 90 d. Am J Clin Nutr. 99(4): 632-646
Uding N, Schreder E. 2013. Chemicals Revealed: Over 5,000 Kids Products Contain Toxic Chemicals. Washington Toxics Coalition,
Unice K, Monnot A, Gaffney S H, Tvermoes B, Thuett K, Paustenbach D, Finley B. 2012. Inorganic cobalt supplementation: Prediction of cobalt levels in whole blood and urine using a biokinetic model. Food Chem Toxicol.50: 2456-2461.
[UNIDO] United Nations Industrial Development Organization and [IFDC] International Fertilizer Development Center. 1998. Fertilizer Manual. Dordrecht (The Netherlands): Kluwer Academic Publishers. p. 166.
Union Carbide Corp. 1992. Initial submission: Industrial fellowship Special Report on the Toxicity of Cobalt oxide, cover letter dated 09/08/91. TSCATS, EPA/OTS; Doc #88-920009381.
United Nations Environment Programme (UNEP) and International Solid Waste Association (ISWA). 2002. Training Resource Pack for Hazardous Waste Management in Developing Economies, Chapter 6.5 - Thermal treatment. United Nations Publication, ISBN 92-807-2235-2. CD-ROM. [accessed Feb 2013]
[US CDC] United States Centres for Disease Control and Prevention. 2009. Fourth National Report on Human Exposure to Environmental Chemicals. US Centre for Disease Control and Prevention. [cited 2013 April 2].
[US CDC] United States Centres for Disease Control and Prevention. 2013. Fourth National Report on Human Exposure to Environmental Chemicals, updated Tables, March, 2013. US Centre for Disease Control and Prevention [cited 2013 April 2].
[US EPA] US Environmental Protection Agency. 2005. Initial risk-based prioritization of high production volume (HPV) chemicals. Cobalt salts of C8 to C13 Carboxylic acids including neodecanoic acid, cobalt salt, (CAS 27253-31-2), Fatty acids, C9 to C13 Neo, cobalt salts (CAS 68955-83-9) and hexanoic acid, 2-ethyl, cobalt salt (CAS 136-52-7). Washington (DC): US EPA, HPV Challenge Program. [cited 2013-10-01].
[US EPA] US Environmental Protection Agency. 2009. Initial risk-based prioritization of high production volume (HPV) chemicals. Octanoic acid (CAS 124-07-2). Washington (DC): US EPA, HPV Challenge Program. [cited 2013-07-18].
[US EPA] US Environmental Protection Agency. 2013. Coal Combustion Products, Industrial Materials Recycling. [Internet] Last updated 2013-05-21.
[US EPA] US Environmental Protection Agency. 1987. Ambient Water Quality Criteria for Zinc - 1987. Office of Water, U.S. Environmental Protection Agency, Washington, D.C, EPA-440/5-87-003.
[US EPA] US Environmental Protection Agency. 2009. Initial risk-based prioritization of high production volume (HPV) chemicals. Octanoic acid (CAS 124-07-2). Washington (DC): US EPA, HPV Challenge Program [cited 2013 Jul 8].
Uter W, Hegewald J, Aberer W, Ayala F, Bircher AJ, Brasch J, Coenraads PJ, Schuttelaar ML, Elsner P, Fartasch M, Mahler V, Belloni Fortina A, Frosch PJ, Fuchs T, Johansen JD, Menne T, Jolanki R, Krecisz B, Kiec-Swierczynska M, Larese F, Orton D, Peserico A, Rantanen T and Schnuch A 2005. The European standard series in 9 European countries, 2002/2003 - first results of the European Surveillance System on Contact Allergies. Contact Dermatitis 53: 136-145.
Valberg LS, Ludwig J, Olatunbosun D. 1969. Alteration in cobalt absorption in patients with disorders of iron metabolism. Gastroenterology 56: 241-251.
Valberg LS, Ludwig J, Olatunbosun D. 1969. Alteration in cobalt absorption in patients with disorders of iron metabolism. Gastroenterology 56: 241-251.
Vangheluwe M, Van Sprang P, Verdonck F, Heijerick D, Versonnen B, Vandenbroele M, Van Hyfte A. 2007. Metals Environmental Risk Assessment Guidance. [Internet]. London (UK): International Council of Mining and Metals [ICMN]. 32 p.
WAQB 2013a. ICPMS data from Edmonton Indoor Air Quality Study (2010) [unpublished data].
WAQB 2013b. ICPMS data from Calgary spatial study (2010, 2011) [unpublished data].
WAQB 2013c. ICPMS data from Halifax Spatial Study (2010, 2011) [unpublished data].
Warshaw EM, Ahmed R, Belsito DV, Deleo VA, Fowler JF Jr., Maibach HI, Marks, JGJr., Toby Mathias CG, Pratt MD, Rietschel RL, Sasseville D, Storrs FJ, Taylor JS, Zug KA. 2007. Contact dermatitis of the hands: cross-sectional analyses of North American Contact Dermatitis Group Data, 1994-2004. J Am Acad Dermatol. 57(2): 301-314.
Wehner AP, Busch RH, Olson RJ, Craig DK. 1977. Chronic inhalation of cobalt oxide and cigarette smoke by hamsters. Am Ind Hyg Assoc J 38: 338-346.
[WHO] World Health Organization. 1989. Minor and trace elements in breast milk. Geneva (CH): World Health Organization / International Atomic Energy Association.
Williams JH. 1999. Regulations on additions of sludge-borne metals to soil and their adaptation to local conditions. In: L'Hermite P, editor. Treatment and use of sewage sludge and liquid agricultural wastes. London (GB): Elsevier Applied Science, p. 243-250.
Wilson R, Jones-Otazo H, Petrovic S, Bitchell I, Bonvalot Y, Williams D, Richardson MG. 2013. Revisiting dust and soil ingestion rates based on hand-to-mouth transfer. Human and Ecological Risk Assessment. 19(1): 158-188.
Wiseman C, Zereini F, Wilhelm P. 2013. Traffic-realted trace elemnt fate and uptake by plants cultivated in roadside soils in Toronto, Canada. Sci Total Environ. 442: 86-95.
Yamano T, Shimizu M, Noda T. 2006. Allergenicity and cross-reactivity of naphthenic acid and its metallic salts in experimental animals. Contact Dermatitis. 54: 25-28.
Yanagi M. Masatoshi H, Masaaki M, Yoshio K. 2001. Modified short-term guinea pig sensitization tests for detecting contact allergens as an alternative to the conventional test. Contact Dermatitis. 44: 140-145.
Zajdlik and Associates Inc. 2009. Evaluation of potential standardization models for Canadian Water Quality Guidelines. Prepared for the Water Quality Task Group, Canadian Council of Ministers of the Environment. Project # 387-2006.
Ziaee H, Daniel J, Datta AK, Blunt S and McMinn DJ. 2007. Transplacental transfer of cobalt and chromium in patients with metal-on-metal hip arthroplasty: a controlled study. J Bone Joint Surg Br. 89: 301-305.
Appendices
- Appendix A: identities of cobalt-containing substances that met categorization criteria
- Appendix B: quantities, activities and uses of cobalt-containing substance for which information was received pursuant to section 71 surveys
- Appendix C: releases reported for 2011 to the NPRI for "Cobalt and its compounds"
- Appendix D: summary of reliable data for chronic toxicity of cobalt to aquatic, benthic and soil organisms
- Appendix E: summary of human health Effects information
- Appendix F: summary of human biomonitoring data
- Appendix G: summary of human exposure data