Screening assessment - corn, steep liquor
Official title: Screening assessment - corn, steep liquor
Chemical Abstracts Service Registry Number
66071-94-1
Environment and Climate Change Canada
Health Canada
Cat. No.: En84-275/2021E-PDF
ISBN 978-0-660-39168-7
July 2021
Synopsis
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of corn, steep liquor, hereinafter referred to as CSL. The Chemical Abstracts Service Registry Number (CAS RNFootnote 1 ) for CSL is 66071-94-1.
CSL is a chemical substance of Unknown or Variable composition, Complex reaction products or Biological materials (UVCB). CSL does not occur naturally in the environment; it is a by-product of the corn wet milling process, consisting of the water soluble extracts of corn soaked (steeped) in water. In Canada, CSL is primarily used as a corrosion inhibitor and anti-scaling agent in anti-freeze and de-icing products, as a formulant in registered pest control products, and as an animal feed ingredient. It is also used as an attractant in carp bait. According to information submitted in response to a CEPA section 71 survey for the 2011 reporting year, CSL was manufactured in Canada in quantities over 10 000 000 kg and was imported into Canada in quantities between 100 000 kg and 1 000 000 kg.
The ecological risk of CSL was characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances on the basis of their hazard and exposure profiles. Based on the outcome of the ERC analysis, CSL is considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from CSL. It is concluded that CSL does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
With respect to human health, the United States Environmental Protection Agency found CSL to be of low concern for hazard for human health. Furthermore, the Organisation for Economic Co-operation and Development (OECD) determined that lactic acid, which is the major individual component of CSL on a dry weight basis, does not present a hazard for human health based on its low hazard profile. The Joint Food and Agriculture Organization/World Health Organization (FAO/WHO) Expert Committee on Food Additives (JECFA) concluded that no limit needs to be set for the acceptable daily intake for lactic acid from food and that there is no concern for human health at current levels of intake when used as a food flavouring agent since lactic acid is endogenous in humans. In consideration of the available information from international assessments, CSL is considered to be a substance of low hazard potential and therefore risk to human health is considered to be low.
On the basis of the information presented in this screening assessment, it is concluded that CSL does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that CSL does not meet any of the criteria set out in section 64 of CEPA.
1. Introduction
Pursuant to section 68 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of corn, steep liquor, hereinafter referred to as CSL, to determine whether this substance presents or may present a risk to the environment or to human health. This substance was considered a priority on the basis of other human health concerns (ECCC, HC [modified 2017]).
The ecological risk of CSL was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
The substance currently being evaluated and its major individual component on a dry weight basis, lactic acid, have been reviewed internationally through the United States Environmental Protection Agency (US EPA), the Organisation for Economic Co-operation and Development (OECD) Cooperative Chemicals Assessment Programme, and the Joint Food and Agriculture Organization/World Health Organization (FAO/WHO) Expert Committee on Food Additives (JECFA), and there are existing assessments available. These international assessments were used to inform the health effects characterization in this screening assessment.
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to August 2018. Targeted literature searches were conducted up to November 2018. Empirical data from key studies as well as results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. Additionally, the draft of this screening assessment (published December 7, 2019) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight of evidence approach and precaution.Footnote 2 This screening assessment presents the critical information and considerations on which the conclusions are based.
2. Substance identity
CSL (CAS RNFootnote 3 66071-94-1) is a complex mixture consisting of the water soluble extracts of corn soaked (steeped) in water, and is a by-product from the initial stages of the corn wet milling process. It contains water, water-soluble proteins, carbohydrates (such as reducing sugars), organic acids, minerals, and vitamins. Its composition can vary depending on the type and condition of the corn, and the variables involved in the milling process (Hofer et al. 2018; US EPA 2015; White and Johnson 2003). Additional information on the identity of the components in CSL is presented in Appendix A.
CSL is a UVCB, which is an acronym for “Unknown or Variable composition, Complex reaction products, or Biological materials”. These materials are derived from natural sources or complex reactions and cannot practicably be synthesized by simply combining individual constituents. . A UVCB is not an intentional mixture of discrete substances and is considered a single substance.
2.1 Selection of analogues
CSL is associated with limited empirical data. The major individual component of CSL on a dry weight basis, lactic acid(28.8%, see Appendix A), is an undefined mixture of the L(+) and D(‒) isomers of lactic acid in variable proportions. The ratio of the two isomers depends on the type of Lactobacillus bacteria present in the corn steep, and this ratio changes as the steeping process progresses (Hull et al. 1996).
DL-Lactic acid (a 1:1 mixture of the L(+) and D(‒) isomers of lactic acid), L(+)-lactic acid and calcium lactate (the calcium salt of lactic acid) were used as analogues as they were considered appropriate on the basis of structural similarities to the undefined lactic acid components present in CSL. L(+)-lactic acid also occurs naturally in mammals and is a normal intermediary of mammalian metabolism (WHO 1974).
Information on the identities and chemical structures of analogues used to inform this assessment is presented in Table 2-1. Details of the read-across data chosen to inform the human health assessment of CSL are further discussed in the relevant sections of this report.
| CAS RN | DSL name (common name) | Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 50-21-5 | Propanoic acid, 2-hydroxy- (DL-Lactic acid) | 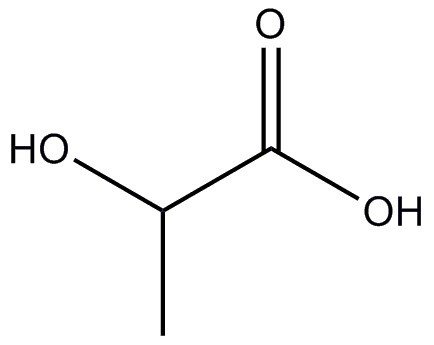 C3H6O3 C3H6O3 | 90.08 |
| 79-33-4 | Propanoic acid, 2-hydroxy-, (S)- (L(+)-Lactic acid) | =O)O](/content/dam/eccc/images/pded/corn-steep-liquor/20210604-t21b.jpg) C3H6O3 C3H6O3 | 90.08 |
| 814-80-2 | Propanoic acid, 2-hydroxy-, calcium salt (2:1) (Calcium lactate) | ![CC(C([O-])=O)O.CC(C([O-])=O)O.[Ca+2]](/content/dam/eccc/images/pded/corn-steep-liquor/20210604-t21c.jpg) C6H12O6Ca C6H12O6Ca | 218.22 |
3. Physical and chemical properties
A summary of physical and chemical property data of CSL is presented in Table 3‑1, with the range in values indicated for each property as applicable. Additional physical and chemical properties are reported in ECCC (2016b).
| Property | Value | Key reference |
|---|---|---|
| Physical state | Tan to brown liquid, viscous | CRA 2006 |
| Vapour pressure (Pa) | NAa | US EPA 2015 |
| Water solubility | Soluble | CRA 2006 |
| Density (g/cm3) | 1.2 – 1.4 | CRA 2006 |
Abbreviations: NA, Not Available
a The vapour pressure reported in CRA (2006) reflects the properties of impure water and is not representative of the organic constituents of the substance. The organic constituents have negligible to moderate vapour pressure (US EPA 2015).
4. Sources and uses
CSL does not occur naturally in the environment and is a by-product of the corn wet milling process. This is a refinement process that transforms corn kernels into higher value products including starches, sweeteners, oils and alcohols. This substance has been included in a survey notice issued pursuant to section 71 of CEPA (Canada 2012). According to quantities reported in response to the CEPA section 71 survey for the 2011 reporting year (Environment Canada 2013),4 CSL was manufactured in Canada in a quantity over 10 000 000 kg and was imported into Canada in the range of 100 000 to 1 000 000 kg.
According to non-confidential use information reported in response to a CEPA section 71 surveyFootnote 4 (Environment Canada 2013), CSL is used in anti-freeze and de-icing products as a corrosion inhibitor and anti-scaling agent in Canada. Other uses were also reported, but are not disclosed herein due to business confidentiality claims. Publically available information indicated that CSL may be present in de-icing products used for road maintenance (SDS 2013).
Although “corn, steep liquor” is not listed as an approved feed ingredient name in Schedule IV or V of the Feeds Regulations of the Feeds Act (Canada 1985), it is considered to fall under the definition of “maize extractives fermented condensed” (IFN 4-02-890). As such, CSL is a permitted ingredient in animal feed in Canada (personal communication, emails from the Canadian Food Inspection Agency (CFIA), to the Existing Substances Risk Assessment Bureau, Health Canada, dated May to July 2018; unreferenced).
CSL is listed on the Pest Management Regulatory Agency (PMRA) Pesticide Formulants List, and was identified as being present in two registered pest control products (personal communication, email from the Pest Management Regulatory Agency, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated July 2018; unreferenced).
Other uses have been identified for CSL. In Canada, this substance is used as an attractant in carp bait in sport fishing feeds (Canadian Carp Club Shop 2018). Internationally, CSL is also used in fertilisers and pH regulators, water treatment products, fine chemical and food product production (ECHA c2007-2018; ECHA 2017), and industrial fermentation processes (e.g., growth medium for large-scale production of penicillin) (ECHA c2007-2018; Hofer et al. 2018).
5. Potential to cause ecological harm
5.1 Characterization of ecological risk
The ecological risk of CSL was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., median lethal concentration) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox 2014), and from responses to survey notices issued pursuant to section 71 of CEPA, or they were generated using selected (Q)SAR or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under- classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for CSL and the hazard, exposure and risk classification results, are presented in ECCC (2016b).
On the basis of low hazard and low exposure classifications according to information considered under ERC, CSL was classified as having a low potential for ecological risk. It is therefore unlikely that this substance is resulting in concerns for the environment in Canada.
6. Potential to cause harm to human health
6.1 Exposure assessment
No Canadian or recent international data on levels of CSL in environmental media or food were identified. It is expected that the components of CSL would partition into water and soil (US EPA 2015; CRA 2006).
The Canadian general population may be exposed to CSL from the commercial use of anti-freeze and de-icing products on Canadian roads and subsequent use of treated roads by the public, and during the use of carp bait products while fishing for sport or recreation. Both of these potential exposures are expected to occur primarily through the dermal route.
As CSL is considered to be of low hazard potential (see Section 6.2), quantitative estimates of exposure for the general population were not derived.
6.2 Health effects assessment
No health effects studies were found for CSL. In 2015, the US EPA published a screening level hazard characterization of CSL and determined that there is low concern for hazard for human health endpoints because the substance is comprised of approximately 50% water, with the majority of the remaining 50% comprised of lactic acid, protein, and other nutritive substances (US EPA 2015).
Lactic acid
The major individual component of CSL on a dry weight basis, lactic acid, was reviewed by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) (WHO 1974, 2000) and the Organisation for Economic Co-operation and Development (OECD 2011). These reports were used to inform the health effects characterization in this screening assessment. A literature search was conducted for the period of January 2011 to November 2018. No new health effects studies which could impact the risk characterization were identified.
As discussed in Section 2.1, various forms of lactic acid were selected to inform the human health assessment of CSL. L(+)-lactic acid is a natural, functional metabolite in mammals and represents a major means of distributing carbohydrate potential energy for oxidation and gluconeogenesis. The production rate of endogenous L(+)-lactic acid in a resting human is about 1.3 mole per day for a 70 kg person, which corresponds to 1670 mg/kg bw/day (OECD 2011). JECFA indicated that humans have consumed foods containing DL-lactic acid for centuries without any adverse effects (WHO 1974). As such, JECFA stated that no limit needs to be set for the acceptable daily intake (ADI) of lactic acid; its subsequent evaluation of lactic acid in 2002 confirmed that the ADI is not specified, and that there is no concern for human health at current levels of intake when used as a flavouring agent since lactic acid is endogenous in humans (WHO 2002).
Repeated dose toxicity
In a repeated dose study (OECD 2011), male and female F344 rats (5/sex/dose) were administered calcium lactate via drinking water at 0%, 0.3%, 0.6%, 1.25%, 2.5%, or 5% (corresponding to 0, approximately 30, 60, 125, 250, or 500 mg/kg bw/day) for 13 weeks, and a basic diet was given ad libitum to all groups. No mortalities were observed. A slight decrease in body weight gain (less than 10%) compared to controls was observed at all concentrations. No adverse treatment effects were observed up to the highest dose tested. The OECD set a no observed adverse effect level (NOAEL) at 500 mg/kg bw/day, the highest dose tested.
Genotoxicity/Mutagenicity
On the basis of the overall results of in vitro Ames and chromosomal aberration tests, the OECD concluded that L(+)-lactic acid is not genotoxic.
Carcinogenicity
A 2-year carcinogencity study of calcium lactate in F344 rats (50/sex/dose, calcium lactate in drinking water at 0%, 2.5%, or 5%) showed no evidence of carcinogenicity or organ-specific toxicity, though a significant reduction in mean body weight gain was observed at the high dose (OECD 2011). Limited details were available on this study.
Reproductive/Developmental toxicity
In a developmental toxicity study, DL-lactic acid was not toxic to either dams or offspring when administered orally to pregnant CD-1 mice via gavage at either 0 or 570 mg/kg bw/day during days 6 to 15 of gestation. The NOAEL for maternal and developmental toxicity was deemed to be 570 mg/kg bw/day (the highest dose tested) by the OECD. No reproductive toxicity studies were identified (OECD 2011).
6.3 Characterization of risk to human health
On the basis of the collective information included in several assessments from international agencies on CSL and its main component lactic acid, CSL is considered to be of low hazard potential; this is consistent with the hazard-based approach outlined in the Science approach document for substances with low human health hazard potential (Health Canada 2017). As such, quantitative exposure estimates were not derived and the risk of CSL to human health is considered to be low.
6.4 Uncertainties in evaluation of risk to human health
Although there may be some limitations in the health effects database, no significant uncertainties were identified and additional data is considered unlikely to impact the determination of low hazard potential for CSL.
7. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from CSL. It is concluded that CSL does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the information presented in this screening assessment, it is concluded that CSL does not meet the criteria under paragraph 64(c) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that CSL does not meet any of the criteria set out in section 64 of CEPA.
References
Canada. 1985. Feeds Act. R.S.C., 1985, c. F-9.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List [PDF]. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
Canadian Carp Club Shop. 2018. CC Moore Corn steep liquor powder.
[CRA] Corn Refiners Association. 2006. Assessment plan for corn, steep liquor (CAS #66071-94-1) in accordance with the US EPA High Production Volume Chemical Challenge Program [PDF]. Washington (DC): Keller and Heckman LLP.
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications in the Ecological Risk Classification of organic substances. Gatineau (QC). ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2018 Nov 14].
[ECHA] European Chemicals Agency. c2007-2018a. Registered substances database. Search results for CAS RN [66071-94-1]. Helsinki (FI): ECHA. [updated 2018 Nov 20; accessed 2018 Nov 27].
[ECHA] European Chemicals Agency. 2017. Brief profile: Corn, steep liquor; CAS RN 66071-94-1. Helsinki (FI): ECHA. [updated 2017 Apr 4; accessed 2018 Mar 13].
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Health Canada. 2017. Science approach document: Science approach document for substances with low human health hazard potential. Ottawa (ON): Government of Canada.
Hofer A, Kamravamanesh D, Bona-Lovaz J, Limbeck A, Lendl B, Herwig C, Fricke J. 2018. Prediction of filamentous process performance attributes by CSL quality assessment using mid-infared specroscopy and chemometrics. J Biotechnol. 265:93-100.
Hull SR, Yang BY, Venzke D, Kulhavy K, Montgomery R. 1996. Composition of corn steep water during steeping. J Agric Food Chem. 44:1857-1863.
[OECD] Organisation for Economic Co-operation and Development. 2011. SIDS Initial Assessment Profile [PDF]. Lactic acid. Paris (FR): OECD HPV Chemicals Program. [accessed 2018 Jul 25].
OECD QSAR Toolbox [Read-across tool]. 2014. Version 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
[SDS] Safety Data Sheet. 2013. Ice Ban 200M [PDF]. Saskatoon (SK): Pounder Emulsions, A division of Husky Oil Limited. [accessed 2019 Feb 05].
[US EPA] US Environmental Protection Agency. 2015. Screening-level hazard characterization: Corn steep liquor [PDF]. Washington (DC): US EPA, HPV Challenge Program.
White PJ, Johnson LA. 2003. Corn: Chemistry and Technology. St. Paul (MN): American Association of Cereal Chemists, Inc.
[WHO] World Health Organization. 1974. Toxicological evaluation of some food additives including anti-caking agents, antimicrobials, antioxidants, emulsifiers and thickening agents: seventeenth meeting of the Joint FAO/WHO Expert Committee on Food Additives. WHO Food Additives Series, No. 5. Geneva (CH): World Health Organization. [accessed 2018 Jul 25].
[WHO] World Health Organization. 2002. Safety evaluation of certain food additives and contaminants: fifty-seventh meeting of the Joint FAO/WHO Expert Committee on Food Additives. WHO Food Additives Series, No. 48. Geneva (CH): World Health Organization. [accessed 2018 Jul 25].
Appendix A. Substance identity information
| Chemical classa | Percentage of mixture (on a dry weight basis)b | Major individual chemical component common name | Major individual chemical component percentage of mixture (on a dry weight basis) |
|---|---|---|---|
| Amino acids and polypeptides | 45.3c | Arginine | 7.2 |
| Organic acids | 36.8 | Lactic acidd | 28.8 |
| Reducing sugars | 4.7 | Glucose | NA |
| Vitamins | 2.8 | NA | NA |
| Minerals/trace elements | 7.0 | NA | NA |
| Other (unidentified) | 3.5 | NA | NA |
Abbreviations: NA, Not Available; UVCB, Unknown or Variable composition, Complex reaction products, and Biological material
a CSL is a mixture consisting of the water soluble extracts of corn soaked in water, and consists of approximately 50% water (US EPA 2015; White and Johnson 2003). The substance also contains lactic acid-producing bacteria (Lactobacillus bacteria) and residual amounts of SO2 from the manufacturing process (<0.01%) (White and Johnson 2003). The US EPA reports water, crude protein, lactic acid, ash, phytic acid, reducing sugars, and fat as the components of CSL (US EPA 2015).
b The analysis by Hofer et al. (2018) is presented as the representative composition of CSL primarily due to its level of granularity. The data are generally consistent with other published analyses (US EPA 2015; White and Johnson 2003).
c Although the chemical class that is the most highly represented in CSL is amino acids and polypeptides, the predominant individual component within this class (arginine) is only present in CSL at 7.2% (on a dry weight basis). The individual chemical component with the highest concentration in CSL on a dry weight basis is lactic acid (28.8%).
d The ratio of D to L isomer found in CSL depends on the type of Lactobacillus bacteria present in the corn steep, and this ratio changes as the steeping process progresses (Hull et al. 1996).
