Screening assessment 2H-1-Benzopyran-2-one, 7-(diethylamino)-4-methyl-(Coumarin 1)
Officia title: Screening assessment 2H-1-Benzopyran-2-one, 7-(diethylamino)-4-methyl-(Coumarin 1)
Chemical Abstracts Service Registry Number
91-44-1
Environment and Climate Change Canada
Health Canada
May 2023
Cat. No.: En84-332/2023E-PDF
ISBN: 978-0-660-47973-6
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of the Environment and the Minister of Health have conducted a screening assessment of 2H-1-benzopyran-2-one, 7-(diethylamino)-4-methyl-, hereinafter referred to as coumarin 1. The Chemical Abstracts Service Registry Number (CAS RNFootnote 1 ) for coumarin 1 is 91-44-1. This substance was identified as a priority for assessment as it met categorization criteria under subsection 73(1) of CEPA.
According to information submitted in response to a CEPA section 71 survey, coumarin 1 was not reported to be manufactured in Canada above the reporting threshold of 100 kg in 2011, while a quantity in the range of 1000 kg to 10 000 kg was imported into Canada in the same calendar year. Reported uses in Canada included commercial applications in fabric, textile and leather articles. Coumarin 1 is also used in certain cosmetic products in Canada, such as temporary hair dyes, nail polishes, and body, lip and facial makeup (including eye makeup). Coumarin 1 is also used as a stabilizer in a carpet cleaner and as a fragrance ingredient in other cleaning products (for example, multi-purpose cleaners).
The ecological risk of coumarin 1 was characterized using the ecological risk classification of organic substances (ERC), which is a risk-based approach that employs multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances based on their hazard and exposure profiles. Based on the outcome of the ERC analysis, coumarin 1 is considered unlikely to be causing ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from coumarin 1. It is concluded that coumarin 1 does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
The health effects dataset for coumarin 1 was considered to be limited. To address this limitation, a read-across approach was applied to inform the health effects assessment. Based on the available data on the analogues, developmental toxicity was considered to be the critical effect. Exposure of the general population in Canada to coumarin 1 occurs predominantly through the use of certain cosmetic products, such as temporary hair dyes, nail polishes, and body, lip and facial makeup (including eye makeup), as well as cleaning products. A comparison of levels of coumarin 1 that Canadians may be exposed to in drinking water, nail polish, temporary powder hair dye, facial makeup, lipstick/lip gloss, multi-purpose spray cleaner and carpet cleaner with levels associated with adverse effects in laboratory studies results in margins that are considered adequate to address uncertainties in the health effects and exposure datasets. However, the margins between exposure to coumarin 1 from occasional-use specialty body makeup (for ages 4 years and older) and temporary gel hair dye (for ages 2 to 13 years) and the critical effect levels for coumarin 1 are considered inadequate to address uncertainties in the health effects and exposure datasets.
Considering all the information presented in this screening assessment, it is concluded that coumarin 1 meets the criteria under paragraph 64(c) of CEPA as it is entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that coumarin 1 meets one or more of the criteria set out in section 64 of CEPA.
It is also concluded that coumarin 1 meets the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
1. Introduction
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health have conducted a screening assessment of 2H-1-benzopyran-2-one, 7-(diethylamino)-4-methyl-, hereinafter referred to as coumarin 1. This substance was identified as a priority for assessment as it met categorization criteria under subsection 73(1) of CEPA (ECCC, HC [modified 2017]).
The ecological risk of coumarin 1 was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC describes the hazard of a substance using key metrics, including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity, and considers the possible exposure of organisms in the aquatic and terrestrial environments on the basis of such factors as potential emission rates, overall persistence, and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposures, including additional information submitted by stakeholders. Relevant data were identified up to April 2019, with additional targeted literature searches up to July 2021. Empirical data from key studies as well as results from models were used to reach conclusions. When available and relevant, information presented in assessments from other jurisdictions (that is, Australia’s National Industrial Chemicals Notification and Assessment Scheme (NICNAS), the Joint FAO/WHO Expert Committee on Food Additives (JECFA) and the European Food Safety Authority (EFSA)) was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Program at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The human health portions of this assessment have undergone external review and/or consultation. Comments on the technical portions relevant to human health were received from Tetra Tech Inc. (Theresa Lopez, Jennifer Flippin and Joan Garey). The ecological portion of this assessment is based on the ERC document (published July 30, 2016), which was subject to an external review as well as a 60-day public comment period. Additionally, the draft of this screening assessment (published October 31, 2020) was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of this screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA by examining scientific information and incorporating a weight-of-evidence approach and precaution.Footnote 2 This screening assessment presents the critical information and considerations on which the conclusion is based.
2. Substance identity
The Chemical Abstracts Service Registry Number (CAS RN), Domestic Substances List (DSL) name, common name and molecular structure for coumarin 1 are presented in Table 2‑1.
| CAS RN | DSL name (common name) |
Chemical structure and molecular formula | Molecular weight (g/mol) | Reference |
|---|---|---|---|---|
| 91-44-1 | 2H-1-Benzopyran-2-one, 7-(diethylamino)-4-methyl- (coumarin 1) |
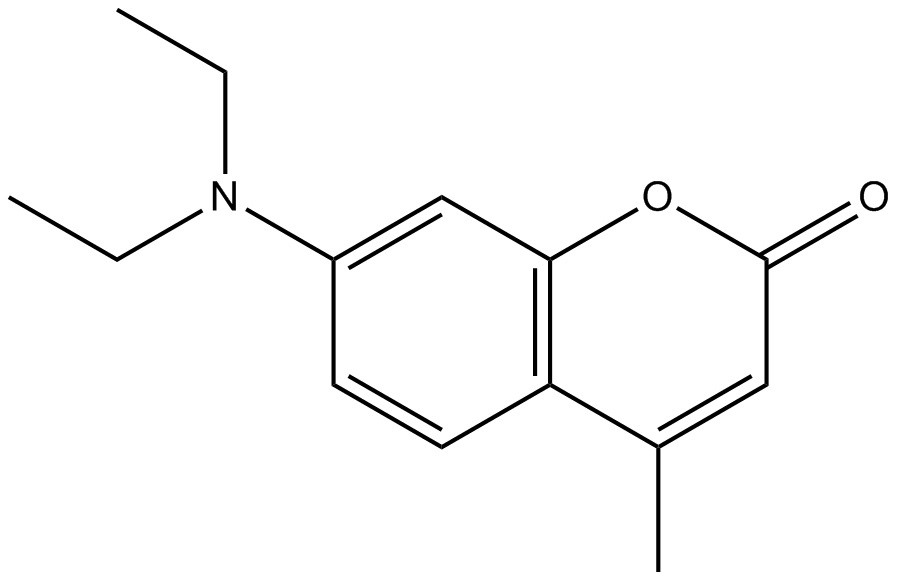 C14H17NO2
C14H17NO2
|
231.30 | ChemIDplus 1993- |
2.1 Selection of analogues
A read-across approach using data from analogues was used to inform the human health assessment. Analogues were selected that were structurally similar to coumarin 1 (similar physical-chemical properties, metabolism) and that had relevant empirical data that could be used to read-across to endpoints with limited empirical data for coumarin 1. Information on the identities and chemical structure of the analogues used to inform this assessment are presented in Table 2‑2. Appendix A provides further details on the factors considered in the identification of analogues. For further information on the physical-chemical properties and health effects data available on the analogues, refer to Appendix B.
| CAS RN | DSL name (common name) |
Chemical structure and molecular formula | Molecular weight (g/mol) | Reference |
|---|---|---|---|---|
| 92-48-8 | 2H-1-Benzopyran-2-one, 6-methyl- (6-methylcoumarin) |
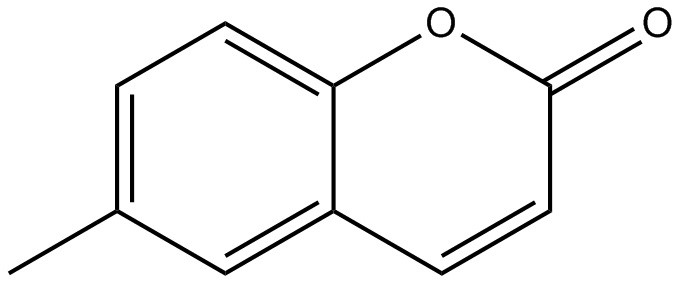 C10H8O2
C10H8O2 |
160.172 | ChemIDplus 1993- |
| 91-64-5 | 2H-1-Benzopyran-2-one (coumarin) |
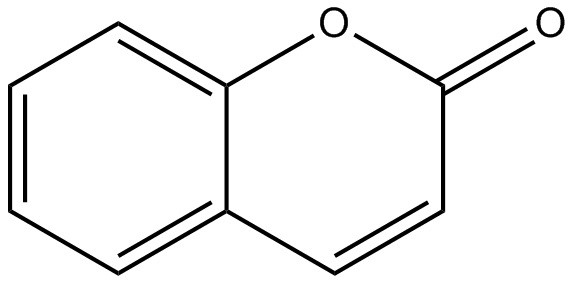 C9H6O2
C9H6O2 |
146.133 | ChemIDplus 1993- |
6-Methylcoumarin was found to be the closest analogue to coumarin 1 for which data on chronic toxicity were identified. However, no data on reproductive/developmental toxicity was identified for 6-methylcoumarin and as such, coumarin was used to inform this endpoint.
3. Physical and chemical properties
A summary of physical and chemical property data for coumarin 1 is presented in Table 3‑1. Additional physical and chemical properties are reported in ECCC (2016b).
| Property | Value | Key reference(s) |
|---|---|---|
| Physical state | light yellow powder solid | SDS 2019 |
| Melting point (°C) | 72 – 75 | Epi Suite c2000-2012 |
| Vapour pressure (Pa) | 0.00257 at 25 °C | Epi Suite c2000-2012 |
| Henry’s law constant (Pa·m3/mol) | 0.0309 | Epi Suite c2000-2012 |
| Water solubility (mg/L) | 53.28 at 25 °C | Epi Suite c2000-2012 |
| log Kow (dimensionless) | 3.22 | Epi Suite c2000-2012 |
Abbreviation: Kow, octanol-water partition coefficient
4. Sources and uses
In a survey issued pursuant to section 71 of CEPA (Canada 2012), coumarin 1 was not reported to be manufactured in Canada above the reporting threshold of 100 kg in 2011. For the same calendar year, it was reported to be imported into Canada in a quantity of between 1000 and 10 000 kg (Environment Canada 2013). Coumarin 1 was also reported to be used as a dye in commercial fabric, textile and leather articles in Canada (Environment Canada 2013). Information received as part of the public comment period on the draft screening assessment indicates that coumarin 1 is used in the manufacture of engine components and as a fragrance ingredient in cleaning products.
Based on notifications submitted under the Cosmetic Regulations, coumarin 1 is present in cosmetics in Canada (personal communication, emails from Consumer and Hazardous Products Safety Directorate (CHPSD), Health Canada (HC), to Existing Substances Risk Assessment Bureau (ESRAB), HC, dated October 2018; unreferenced). Coumarin 1 may be used as a marker ingredient in an adhesive for meat packaging with no potential for direct food contact (personal communication, email from the Food Directorate, HC, to the ESRAB, HC, and July 2021; unreferenced). Coumarin 1 was also identified as a stabilizer in a carpet cleaner available in Canada (SDS 2015).
Internationally, coumarin 1 has been identified in tattoo ink (Piccinini et al. 2015; Landeg et al. 2016), cleaning products (HCPA 2019; CPID c2001-2019; RB c2012-2019), and leather and textile treatment products, paper chemicals and dyes (ECHA c2007-2021).
5. Environmental fate and behaviour
5.1 Environmental persistence
According to models used in ERC (ECCC 2016b), coumarin 1 is expected to persist in water, sediment and soil, but not in air.
5.2 Potential for bioaccumulation
Given its low Kow and low bioconcentration factors (ECCC 2016b), coumarin 1 is not expected to significantly bioaccumulate in organisms.
6. Potential to cause ecological harm
6.1 Characterization of ecological risk
The ecological risk of coumarin 1 was characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure, with weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (for example, median lethal concentration) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, and fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were collected from the scientific literature, from available empirical databases (for example, OECD QSAR Toolbox 2014), from responses to surveys issued pursuant to section 71 of CEPA, or they were generated using selected (quantitative) structure-activity relationship ([Q]SAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles were based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Exposure profiles were also based on multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate, or high. Additional rules were applied (for example, classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance on the basis of its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (that is, in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under- classification of hazard and exposure, and of subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modelled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (that is, mode of toxic action), many of which are predicted values from (Q)SAR models (OECD QSAR Toolbox 2014). However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue value used for critical body residue analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada on the basis of what is estimated to be the current use quantity, and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profile for coumarin 1, and the hazard, exposure and risk classification results, are presented in ECCC (2016b).
On the basis of low hazard and low exposure classifications according to information considered under ERC, coumarin 1 was classified as having a low potential for ecological risk. It is unlikely that this substance is resulting in concerns for the environment in Canada.
7. Potential to cause harm to human health
7.1 Exposure assessment
Environmental media and food
No empirical monitoring data were identified for coumarin 1 in air, water or soil in Canada or elsewhere. Given the physical and chemical properties of coumarin 1 (that is, it is solid at room temperature and has a low vapour pressure), as well as its identified uses in Canada, disperse releases of coumarin 1 to air are not expected. No occurrence data on coumarin 1 in food has been identified. Coumarin 1 may be used as a marker ingredient in an adhesive for meat packaging with no potential for direct food contact (personal communication, email from the Food Directorate, HC, to the ESRAB, HC, dated October 2018 and July 2021; unreferenced). Therefore, exposure to this substance from food is not expected.
Given the absence of surface water and drinking water monitoring data for coumarin 1 in Canada, an industrial release scenario based on Environmental Assessment Unit Drinking Water Spreadsheets (Health Canada 2015) was used to estimate the concentration of coumarin 1 in surface water as a surrogate for drinking water. Total annual usage corresponding to the maximum import quantity identified through information submitted in response to a CEPA section 71 survey (that is, 10 000 kg), removal percentage by wastewater treatment plants of 16% (ECCC 2016b), and a maximum loss percent release to wastewater of 1% (Health Canada 2015) were used as inputs. The resulting conservatively estimated surface water concentration was 0.18 µg/L. This concentration was used to estimate exposure to coumarin 1 from drinking water for the general population of Canada.
The estimated potential daily intakes for coumarin 1 for the general population of Canada from drinking water ranged from 0.003 µg/kg bw/day for persons aged 14 to 18 years to 0.02 µg/kg bw/day for formula-fed infants (see Appendix C, Table C-1).
Products available to consumers
According to notifications submitted to Health Canada under the Cosmetic Regulations, coumarin 1 is used in certain cosmetic products in Canada, such as temporary hair dyes, nail polishes, body, lip and facial makeup (including eye makeup) at concentrations ranging from less than 0.1% to 10% (personal communications, emails from CHPSD, HC, to ESRAB, HC, dated October 2018 to May 2019; unreferenced). According to information received through the public comment period, coumarin 1 may be used as a fragrance ingredient in various cleaning products (for example, multi-purpose cleaners) at concentrations less than 0.5%. In addition, coumarin 1 may be found in carpet cleaning products (CPID c2001-2019; RB c2012-2019; SDS 2015), with potential exposures occurring during both application and post-application. Exposures to coumarin 1 from its use as a dye in commercial fabric, textile and leather articles (Environment Canada 2013) are expected to be less than those found in cleaning products.
No dermal absorption data were identified for coumarin 1. Dermal absorption studies, both in vitro and in vivo, for the analogue coumarin, were identified. They indicated dermal absorption ranging from approximately 45% to 98%, depending on the vehicle used (values at the lower range were measured in studies with ethanol solvents, while those at the higher end were in oil-water emulsions) (Beckley-Kartey et al. 1997; Yourick et al. 1997; Minghetti et al. 2000; Ford et al. 2001). On the basis of the dermal absorption values measured in oil-water emulsions (which are considered to be more relevant for estimating exposures to coumarin 1 from cosmetic products presented in Table 7-1), the dermal absorption for coumarin 1 was assumed to be 100%. Inhalation exposure to coumarin 1 is not expected from use of nail polish, gel hair dyes, lip, body and facial makeup due to its low vapour pressure. For powder and spray products, estimated inhalation exposures were insignificant in comparison to dermal exposures. Estimated dermal and oral exposures were derived for all relevant age groups. For most products, only the age groups with the highest and lowest exposures are presented in Table 7‑1, representing the range of exposures. Refer to Appendix D for details on parameters used.
| Exposure scenario | Maximum concentrationa | Estimated exposure |
|---|---|---|
| Nail polish – 2 coats (dermal) – 2 to 3 years | 2% | 0.08 mg/kg bw/event |
| Nail polish – 2 coats (dermal) – ≥ 19 years | 2% | 0.043 mg/kg bw/event |
| Temporary powder hair dye (dermal) – 2 to 3 years | 0.5% | 0.06 mg/kg bw/event |
| Temporary powder hair dye (dermal) – ≥ 19 years | 0.5% | 0.012 mg/kg bw/event |
| Temporary gel hair dye (dermal)b – 2 to 3 years | 1% | 0.19 mg/kg bw/event |
| Temporary gel hair dye (dermal)b – 4 to 8 years | 1% | 0.14 mg/kg bw/event |
| Temporary gel hair dye (dermal)b – 9 to 13 years | 1% | 0.083 mg/kg bw/event |
| Temporary gel hair dye (dermal)b – 14 to 18 years | 1% | 0.06 mg/kg bw/event |
| Temporary gel hair dye (dermal)b – ≥ 19 years | 1% | 0.05 mg/kg bw/event |
| Facial makeup (dermal) – 4 to 8 years | 0.3% | 0.044 mg/kg bw/event |
| Facial makeup (dermal) – ≥ 19 years | 0.3% | 0.026 mg/kg bw/day |
| Body makeup (dermal)c – 4 to 8 years | 0.3% | 0.27 mg/kg bw/event |
| Body makeup (dermal)c – ≥ 19 years | 0.3% | 0.17 mg/kg bw/event |
| Lipstick/lip gloss (oral)c,f – 2 to 3 years | 1% | 0.015 mg/kg bw/day |
| Lipstick/lip gloss (oral)d – ≥ 19 years | 1% | 0.0059 mg/kg bw/day |
| Carpet cleaner application (dermal) – ≥ 19 years | 0.01%e | 0.002 mg/kg bw/event |
| Carpet cleaner post-application (dermal from crawling on floor) – 6 to 11 months | 0.01%e | 0.0036 mg/kg bw/day |
| Carpet cleaner post-application (oral hand-to-mouth) – 6 to 11 months | 0.01%e | 0.00078 mg/kg bw/day |
| Carpet cleaner post-application (oral and dermal combined) – 6 to 11 months | 0.01%e | 0.0044 mg/kg bw/day |
| Multi-purpose spray cleaner - spraying (dermal) – ≥ 19 years | 0.5%f | 0.0083 mg/kg bw/day |
| Multi-purpose spray cleaner – wiping (dermal) – ≥ 19 years | 0.5%f | 0.042 mg/kg bw/day |
| Multi-purpose spray cleaner – spraying + wiping (dermal) – ≥ 19 years | 0.5%f | 0.0503 mg/kg bw/day |
a Personal communication, emails from CHPSD, HC, to ESRAB, HC, October 2018; unreferenced, unless specified otherwise.
b All age groups are presented for temporary gel hair dyes given potential health concerns for this product (refer to section 7.3).
c Specialty product that would be used on occasion on face and/or body.
d Specialty product that would be used on occasion.
e SDS 2015.
f According to information received through the public comment period; unreferenced.
7.2 Health effects assessment
Limited chemical-specific hazard data were identified for coumarin 1. Health effects studies pertaining to carcinogenicity and to reproductive and developmental toxicity were not identified. Health effects data from analogues were therefore used to inform the assessment for coumarin 1. Analogues were considered based on similarities in their physical and chemical properties, metabolism, and structure. The chemical-specific data will be presented first, followed by the analogue data used to inform the health effects characterization of coumarin 1.
Coumarin 1 has been reviewed as part of the “Coumarins” group by Australia’s National Industrial Chemicals Notification and Assessment Scheme (NICNAS 2016). A registration dossier submitted to ECHA (ECHA c2007-2021) is also available for coumarin 1.
In a limited study conducted according to the Draize method, rabbits were exposed to coumarin 1 for a period of 8-14 days. No signs of systemic toxicity following topical application of coumarin 1 in rabbits were observed (Thomann and Krüger 1975). However, the study does not quantify the amount of coumarin 1 applied to the rabbits.
A limited 14-week oral repeated-dose study in rats is presented in the ECHA dossier for coumarin 1. However, it is stated that the results from the study could not be interpreted due to limited reporting (ECHA c2007-2021).
With respect to genotoxicity, coumarin 1 was found to be negative in an Ames test with and without metabolic activation (NICNAS 2016). It was also negative in an in vitro mammalian cell hypoxanthine-guanine phosphoribosyltransferase (HPRT) gene mutation test in Chinese lung (V79) fibroblasts (ECHA c2007-2021).
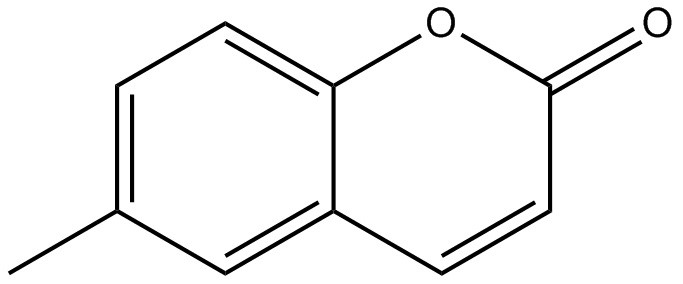
No studies on the effects of coumarin 1 from chronic exposure were identified. 6-Methylcoumarin (CAS RN 92-48-8) was found to be the closest analogue to coumarin 1 for which data on chronic toxicity were identified.
6-Methylcoumarin and coumarin 1 both contain a coumarin skeleton, which consists of two 6-membered rings fused together: a benzene ring and an α,β-unsaturated lactone ring. Coumarin 1 also has a C-4 methyl group and a C-7 diethylamino group. With respect to physical-chemical properties, both coumarin 1 and 6-methylcoumarin have relatively similar molecular weights (231 g/mol vs. 160 g/mol, respectively), melting points (72 ºC to 75 ºC vs. 76.5 ºC, respectively), boiling points (240 ºC vs. 304 ºC, respectively) and log Kow values (3.22 vs. 2.06). However, coumarin 1 and 6-methylcoumarin differ in water solubility (53.28 mg/L vs. 1189 mg/L, respectively) and vapour pressure (0.00257 Pa vs. 0.068 Pa, respectively) by orders of magnitude (Appendix B).
Based on their chemical structure, 6-methylcoumarin and coumarin 1 are likely to be metabolized via a 7-hydroxylation pathway. In contrast, coumarin is primarily metabolized by a 3,4-epoxidation pathway (Lake 1999). 6-Methylcoumarin was found to be the closest analogue to coumarin 1 for which data on chronic toxicity were identified. However, no data on reproductive/developmental toxicity was identified for 6-methylcoumain and as such coumarin was used to inform this endpoint.
6-Methylcoumarin has been reviewed internationally by the Joint FAO/WHO Expert Committee on Food Additives (JECFA 2004) and the European Food Safety Authority (EFSA 2019). These reviews were used to inform the health effects assessment section.
In a 13-week repeated-dose study, B6C3F1 mice (10/sex/dose) were administered 6-methylcoumarin via gavage at doses of 0, 50, 100, 200, 400 or 800 mg/kg bw/day. During the course of the study, one mouse died in the 400 mg/kg bw/day group. At the highest dose tested, 3 mice died and there were reports of prostration, bradycardia, bradypnea, hypoactivity, hypothermia, and loss of the grasping reflex. There were no significant changes in body or organ weights and no significant findings in the clinical, macroscopic and microscopic examinations at any of the other doses tested (NTP 2002, as cited in JECFA 2004).
In another 13-week repeated-dose study, F344/N rats (10/sex/dose) were administered 6-methylcoumarin via gavage at doses of 0, 75, 150, 300, 600 or 1200 mg/kg bw/day. In the first week of the study, 1 rat at the 600 mg/kg bw/day dose and all the rats at the highest dose tested died. At necropsy, there were microscopic hepatic lesions with varying degrees of congestion, degeneration, necrosis and hepatitis in rats receiving 1200 mg/kg-bw/day. In males and females at 600 mg/kg bw/day and 1200 mg/kg bw/day, clinical effects including hypoactivity, lacrimation, ataxia, impaired righting reflex and decreased limb tone were observed. In males and females at 600 mg/kg bw/day, there was a statistically significant decrease in body weight at week 13. There were increased mean absolute and relative liver weights in both males and females at 300 mg/kg bw/day and 600 mg/kg bw/day. There was a significant decrease in serum cholinesterase activity in females at 300 mg/kg bw/day and 600 mg/kg bw/day. There were no other changes reported in hematological, serum biochemical or urinary parameters at any dose. There were no treatment-related effects at 150 mg/kg bw/day (NTP 2002, as cited in JECFA 2004).
In a 13-week repeated-dose study, male Sprague-Dawley rats (6 to 8 rats/group) that were exposed to 6-methylcoumarin in diet at 0.82% (695 mg/kg bw/day) were reported to have vacuolation of hepatocytes and increased relative liver weights. There were no increases in plasma aminotransferase activity and no bile duct hyperplasia or cholangiofibrosis reported (Lake et al. 1994, as cited in JECFA 2004 and NICNAS 2016).
In a 14-week repeated-dose study, Osborne-Mendel rats (25/sex/group) were exposed to 6-methylcoumarin in diet at a concentration of 0, 1000 or 10 000 ppm (calculated to be equivalent to 0, 100 and 1000 mg/kg bw/day). There were no treatment-related effects on general health and behaviour, body weight, food consumption, organ weights, macroscopic or microscopic changes in the tissues or in hematological examinations at any of the doses tested (Hagan et al. 1967, as cited in JECFA 2004).
In a 2-year carcinogenicity study, Osborne-Mendel rats (25/sex/group) were exposed to 6-methylcoumarin in the diet at 0, 25, 50, 175, 250, 375 or 750 mg/kg bw/day. In males, there was moderate growth depression along with decreased food intake at the 375 mg/kg bw/day dose and severe growth depression at the 750 mg/kg bw/day dose. At the highest dose tested, there were observations of fatty metamorphosis, focal telangiectasis and bile duct proliferation in the liver. Testicular atrophy was observed at the 750 mg/kg bw/day dose. There were no other treatment-related effects, including carcinogenicity, at any of the doses tested (Hagan et al. 1967, as cited in EFSA 2019).
With respect to genotoxicity, 6-methylcoumarin was found to be negative in two Ames tests (with and without metabolic activation), equivocal in an Ames test with Salmonella typhimurium TA100 (with metabolic activation), and negative (with metabolic activation) in a mouse lymphoma assay (ESFA 2019). In in vivo studies of limited validity, 6-methylcoumarin was found to be negative in a mouse bone marrow micronucleus assay via gavage and a mouse peripheral blood micronucleus 90-day assay via intraperitoneal injection (EFSA 2019). The available data indicates that 6-methylcoumarin is not expected to be genotoxic (EFSA 2019).
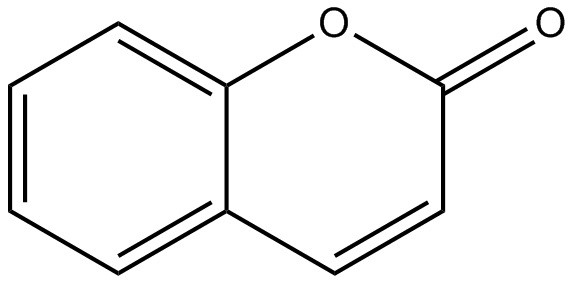
There were no reproductive/developmental studies identified for coumarin 1 or 6-methylcoumarin. As such, using a conservative approach, coumarin is being used to inform characterization of reproductive/developmental toxicity of coumarin 1. In a developmental study with pregnant NMRI mice (31 – 39 mice/group), mice were fed 0%, 0.05%, 0.10% or 0.25% (equivalent to 0, 75, 150 and 375 mg/kg bw/day, respectively) coumarin in the diet on days 6 to 17 of pregnancy. The study did not investigate maternal effects. There was a significant increase in total mortality at all dose levels (that is, the number of still births and the number of dead young up to 3 weeks of age) (Roll and Bär 1967, NICNAS 2016). At 375 mg/kg bw/day, there was also an increase in late resorptions, and delayed ossification.. The lowest observed adverse effect level (LOAEL) is considered to be 75 mg/kg bw/day based on increased mortality up to 3 weeks of age.
There were no carcinogenicity studies identified for coumarin 1. As discussed earlier, a 2-year repeated-dose study in Osborne-Mendel rats exposed to 6-methylcoumarin did not report any carcinogenic effects up to 750 mg/kg bw/day (Hagan et al. 1967, as cited in EFSA 2019). There is evidence that coumarin is carcinogenic in animal tests (NTP 1993, as cited in NICNAS 2016). However, the carcinogenic effects of coumarin have been linked to its metabolism by the 3,4-epoxidation pathway (EFSA 2008). In contrast, based on their chemical structure, 6-methylcoumarin and coumarin 1 are likely to be metabolised via a 7-hydroxylation pathway (Lake 1999). In light of the available data, coumarin 1 is likely not carcinogenic.
7.3 Characterization of risk to human health
Exposure of the general population in Canada to coumarin 1 may occur from drinking water as a result of point source releases to water. Canadians may be exposed to coumarin 1 through the use of cosmetic products such as temporary hair dyes, nail polishes, body makeup and facial makeup (including eye and lip makeup). Coumarin 1 has also been identified in various cleaning products including multi-purpose cleaners and a carpet cleaner.
Based on the available data for coumarin 1 and from the analogue 6-methylcoumarin, coumarin 1 is likely not genotoxic or carcinogenic (NICNAS 2016; ECHA c2007-2021; EFSA 2019).
In light of the severity of effects observed and the absence of reproductive/ developmental studies for coumarin 1 and 6-methylcoumarin, a LOAEL of 75 mg/kg bw/day based on increased mortality up to 3 weeks of age from a reproductive/developmental toxicity study conducted with coumarin, an analogue for coumarin 1, was identified as the critical effect level for characterization of risk.
Table 7‑2 provides all relevant exposure and hazard values for coumarin 1, as well as resultant margins of exposure (MOEs), for determination of risk.
| Exposure scenario | Estimated exposure | Critical effect level | MOE |
|---|---|---|---|
| Drinking water | 2.4E-5 mg/kg bw/day | LOAEL = 75 mg/kg bw/day | 3 125 000 |
| Nail polish – 2 coats (dermal) – 2 to ≥ 19 years | 0.043 – 0.08 mg/kg bw/event | LOAEL = 75 mg/kg bw/day | 937 – 1 744 |
| Temporary powder hair dye (dermal) – 2 to ≥ 19 years | 0.012 – 0.06 mg/kg bw/event | LOAEL = 75 mg/kg bw/day | 1250 – 6250 |
| Temporary gel hair dye (dermal)b – 2 to 3 years | 0.19 mg/kg bw/event | LOAEL = 75 mg/kg bw/day | 395 |
| Temporary gel hair dye (dermal)b – 4 to 8 years | 0.13 mg/kg bw/event | LOAEL = 75 mg/kg bw/day | 577 |
| Temporary gel hair dye (dermal)b – 9 to 13 years | 0.083 mg/kg bw/event | LOAEL = 75 mg/kg bw/day | 904 |
| Temporary gel hair dye (dermal)b – 14 to 18 years | 0.06 mg/kg bw/event | LOAEL = 75 mg/kg bw/day | 1250 |
| Temporary gel hair dye (dermal)b – ≥ 19 years | 0.05 mg/kg bw/event | LOAEL = 75 mg/kg bw/day | 1500 |
| Facial makeup (dermal) – 4 to ≥ 19 years | 0.026 – 0.044 mg/kg bw/day | LOAEL = 75 mg/kg bw/day | 1704 – 2884 |
| Body makeup (dermal)c – 4 to ≥ 19 years | 0.17 – 0.27 mg/kg bw/event | LOAEL = 75 mg/kg bw/day | 280 – 447 |
| Lipstick/lip gloss (oral) – 2 to ≥ 19 years | 0.0089 – 0.015 mg/kg bw/event | LOAEL = 75 mg/kg bw/day | 5000 – 8427 |
| Carpet cleaner application (dermal) – ≥ 19 years | 0.002 mg/kg bw/event | LOAEL = 75 mg/kg bw/day | 37 500 |
| Carpet cleaner post-application (oral and dermal combined) – 6 to 11 months | 0.00437 mg/kg bw/day | LOAEL = 75 mg/kg bw/day | 17 162 |
| Multi-purpose spray cleaner – spraying + wiping (dermal) – ≥ 19 years | 0.0503 mg/kg bw/day | LOAEL = 75 mg/kg bw/day | 1 491 |
Abbreviations: MOE, margin of exposure; LOAEL, lowest observed adverse effect level
a For most cosmetics, only the age groups with the highest and lowest exposures are presented, representing the full range of exposures.
b For temporary gel hair dye, all age groups are presented given the health concerns for this product.
c Specialty product that would be used on occasion on face or body
The MOEs for environmental media, nail polish, temporary powder hair dye, facial makeup, lipstick/lip gloss, carpet cleaner and multi-purpose spray cleaner are considered adequate to address uncertainties in the health effects and exposure datasets. However, the MOEs for body makeup (4 years and older) and temporary gel hair dye (2 to 13 years) are considered inadequate to address uncertainties in the health effects and exposure datasets.
7.4 Uncertainties in evaluation of risk to human health
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| The dermal absorption data for coumarin 1 is unavailable. Therefore, dermal absorption data from the analogue coumarin was considered and assumed equivalent to oral absorption. | +/- |
| No environmental monitoring data for coumarin 1. | +/- |
| There are no data on chronic toxicity or reproductive/developmental toxicity of coumarin 1 and limited information on dermal toxicity. | +/- |
8. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from coumarin 1. It is concluded that coumarin 1 does not meet the criteria under paragraphs 64(a) or (b) of CEPA as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Considering all the information presented in this screening assessment, it is concluded that coumarin 1 meets the criteria under paragraph 64(c) of CEPA as it is entering or may enter the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that coumarin 1 meets one or more of the criteria set out in section 64 of CEPA.
It is also concluded that coumarin 1 meets the persistence criteria but not the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations of CEPA.
References
Beckley-Kartey SAJ, Hotchkiss SAM, Capel M. 1997. Comparative in vitro skin absorption and metabolism of coumarin (1,2-benzopyrone) in human, rat and mouse. Toxicol Appl Pharmacol. 145:34-42.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c.33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2012. Canadian Environmental Protection Act, 1999 [PDF]: Notice with respect to certain substances on the Domestic Substances List. Canada Gazette, Part I, vol. 146, no. 48, Supplement.
ChemIDplus [database]. 1993- . Bethesda (MD): US National Library of Medicine. [accessed 2018 August 28].
ChemSpider [database]. 2015. Search results for CAS RN 91-44-1. London (UK): Royal Society of Chemistry. [accessed 2019 May 3].
[ConsExpo Web] Consumer Exposure Web Model. 2016. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment].
[CPID] Consumer Product Information Database USA and Canada. c2001-2019. Resolve Professional Carpet Extraction Cleaner, Professional Use. [accessed May 2019].
[ECCC] Environment and Climate Change Canada. 2016a. Science approach document: ecological risk classification of organic substances. Ottawa (ON): Government of Canada.
[ECCC] Environment and Climate Change Canada. 2016b. Supporting documentation: data used to create substance-specific hazard and exposure profiles and assign risk classifications. Gatineau (QC): ECCC. Information in support of the science approach document: ecological risk classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada. [accessed 2019 April 30].
[ECHA] European Chemicals Agency. c2007-2021. Registered substances database; search results for CAS RN 91-44-1. Helsinki (FI): ECHA. [accessed 2021 July 30].
[EFSA] European Food Safety Authority. 2004. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Coumarin Question Number: EFSA-Q-2003-118.
[EFSA] European Food Safety Authority. 2008. Coumarin in flavourings and other food ingredients with flavouring properties - Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC).
[EFSA] European Food Safety Authority. 2019. Scientific Opinion on Flavouring Group Evaluation 217 Revision 2 (FGE.217Rev2), consideration of genotoxic potential for α,β‐unsaturated ketones and precursors from chemical subgroup 4.1 of FGE.19: lactones. EFSA Journal. Volume 17: Issue 1.
Environment Canada. 2013. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain substances on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
[EPI Suite] Estimation Program Interface Suite for Microsoft Windows [estimation model]. c2000-2012. Ver. 4.11. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Ficheux AS, Chevillotte G, Wesolek N, Morisset T, Dornic N, Bernard A, Bertho A, Romanet A, Leroy L, Mercat AC, er al. 2016. Consumption of cosmetic products by the French population second part: Amount data. Food Chem Toxicol. 90:130-141.
Ficheux AS, Morriset T, Chevillotte G, Postic C, Roudot AC. 2014. Probabilistic assessment of exposure to nail cosmetics in French consumers. Food Chem Toxicol. 66:36-43.
Ford RA, Hawkins DR, Mayo BC, Api AM. 2001. The in vivo dermal absorption and metabolism of [4-14C] coumarin by rats and by human volunteers under simulated conditions of use in fragrances. Food Chem Toxicol. 39(2):153-62.
Garcia-Hidalgo E, von Goetz N, Siegrist M, Hungerbühle K. 2017. Use-patterns of personal care and household cleaning products in Switzerland. Food Chem Toxicol 99: 24-39.
Hagan EC, Hansen WH, Fitzhugh OG, Jenner PM, Jones WI, Taylor JM, Long EL, Nelson AA, Brouwer JB. 1967. Food flavourings and compounds of related structure. II.Subacute and chronic toxicity. Food Cosmet Toxicol. 5(2):141-57.
[HCPA] Household & Commercial Products Association. 2019. Consumer Product Ingredients Database: Diethylaminomethylcoumarin [Internet]. Washington (DC): HCPA.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Government of Canada.
Health Canada. 2015. Environmental Assessment Unit Drinking Water Spreadsheets. [Excel format]. Ottawa (ON): Health Canada.
Health Canada. [modified 2021 Jun 25]. Canadian exposure factors used in human health risk assessments. Ottawa (ON): Government of Canada. [accessed 2021 Aug 20].
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 2004. Safety evaluation of certain food additives and contaminants prepared by the sixty-first meeting of the Joint FAO/WHO Expert Committee on Food Additives. WHO Food Additives Series No. 52.
Lake BG. 1999. Coumarin metabolism, toxicity and carcinogenicity: relevance for human risk assessment. Food Chem Toxicol. 37(4):423-53.
Landeg S, Kirby A, Lee S, Bartlett F, Titmarsh K, Donovan E, Griffin C, Gothard L, Locke I, McNair H. 2016. A randomized control trial evaluating fluorescent ink versus dark ink tattoos for breast radiotherapy. Br J Radiol. 89(1068):20160288. [accessed 2019 May].
Loretz L, Api AM, Barraj L, Burdick J, Davis DA, Dressler W, Gilberti E, Jarrett G, Mann S, Pan YHL, et al. 2006. Exposure data for personal care products: Hairspray, spray perfume, liquid foundation, shampoo, body wash, and solid antiperspirant. Food Chem Toxicol. 44:2008-2018.
Minghetti P, Casiraghi A, Cilurzo F, Montanari L. 2000. Development of local patches containing melilot extract and ex vivo–in vivo evaluation of skin permeation. Eur J Pharm Sci. 10(2):111-117.
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme. 2016. Coumarins: Human health tier II assessment. Australian Government Department of Health. [accessed 2019 April].
OECD QSAR Toolbox. [read-across tool]. 2014. Version 3.3. Paris (FR): Organisation for Economic Co-operation and Development, Laboratory of Mathematical Chemistry.
Piccinini P, Contor L, Pakalin S, Raemaekers T, Senaldi C. 2015. Safety of tattoos and permanent make-up. State of play and trends in tattoo practices. Report on Work Package 2. Administrative Arrangement N. 2014-33617. Analysis conducted on behalf of DG JUST. Piccinini P, Contor L, Pakalin S, Raemaekers T, Senaldi C. JRC Technical Reports. Report EUR 27528. European Commission, 2015.
RB. c2012-2019. Professional Resolve Carpet Extraction Cleaner. [Internet]. Parsippany (NJ): Reckitt Benckiser LLC. [accessed 2019 May].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2006. Cosmetics fact sheet: to assess the risks for the consumer: updated version for ConsExpo 4 [PDF]. Bilthoven (NL): RIVM. Report No.: 320104001/2006. [accessed 2019 May].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2018. Cleaning products fact sheet: default parameters for estimating consumer exposure – updated version 2018. Bilthoven (NL): RIVM. Report No.: 2016-0179. [accessed 2019 May].
Roll R, Bär F. 1967. [Effect of coumarin (o-hydroxyeinnamic acid-lactone) on pregnant female mice]. Arzneimittelforschung. 1:97-100. German.
[SDS] Safety Data Sheet. 2015. Electrolux Pet Stain & Odor W/BS2X with Baking Soda [PDF]. Michigan City (IN): Fas-Pak. [accessed 2021 August].
[SDS] Safety Data Sheet. 2019. 7-Diethylamino-4-methylcoumarin [PDF]. Lancashire (UK): Thermo Fisher Scientific. [accessed 2019 May 3].
Thomann P, Krüger L. 1975. Acute oral, dermal, and inhalation studies. Environ Qual Saf Suppl. 4:193-8.
[US EPA] United States Environmental Protection Agency. 2012. Standard Operating Procedures for Residential Pesticide Exposure Assessment. [PDF] Washington (DC): Health Effects Division, Office of Pesticide Programs, Office of Chemical Safety and Pollution Prevention.
Vocanson M, Valeyrie M, Rozières A, Hennino A, Floc'h F, Gard A, Nicolas JF. 2007. Lack of evidence for allergenic properties of coumarin in a fragrance allergy mouse model. Contact Dermatitis. 6:361-4.
Yourick JJ, Bronaugh RL. 1997. Percutaneous absorption and metabolism of coumarin in human and rat skin. J Appl Toxicol. 13(3):153-158.
Appendix A. Read-across approach
| Consideration | Rationale |
|---|---|
| 1) Chemical structure. Emphasis was placed on analogues with a coumarin skeleton, which consists of two 6-membered rings fused together: a benzene ring and an α,β-unsaturated lactone ring. | Analogues that have similar chemical structure are expected to have similar toxicity profiles. |
| 2) Similar metabolites (predicted or observed). | Analogues that are metabolized through similar pathways to similar degradation products are expected to have similar toxicity profiles. |
| 3) Common structural alerts. | Analogues with similar structural alerts are expected to share greater similarity in terms of toxicity. |
| 4) Similar physical-chemical properties. Emphasis was placed on chemical structures with similar molecular weight, water solubility, vapour pressure, and log Kow. | Analogues with similar physical-chemical properties may potentially share similar toxicological profiles. |
Appendix B. Hazard summary for coumarin 1, 6-methylcoumarin and coumarin
| Chemical name | Coumarin 1 | 6-Methylcoumarin | Coumarin |
|---|---|---|---|
| Role | Target substance | Analogue | Analogue |
| CAS RN | 91-44-1 | 92-48-8 | 91-64-5 |
| Chemical structure | 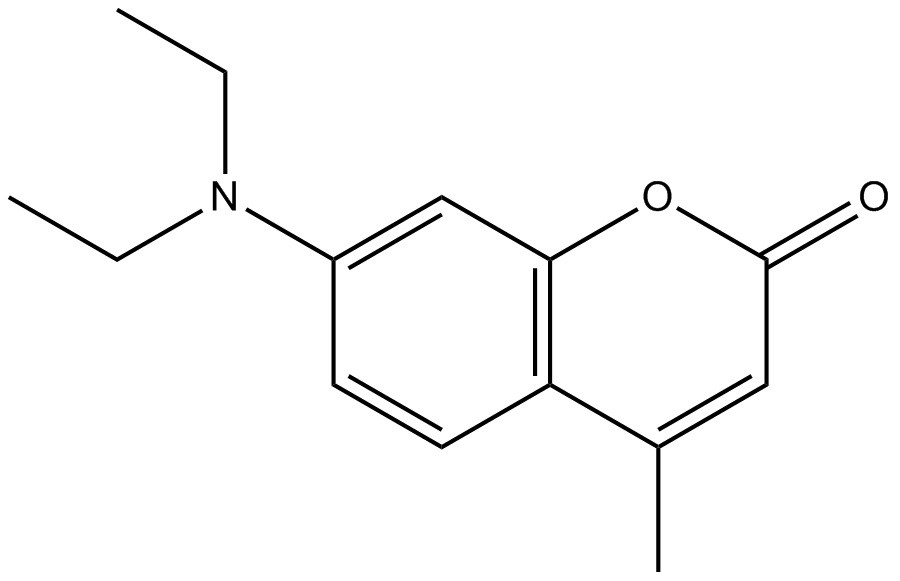
|
 |
 |
| Molecular weight (g/mol) | 231.290 (ChemIDplus 1993-) | 160.172 (ChemIDplus 1993-) | 146.144 (ChemIDplus 1993-) |
| Melting point (ºC) | 72–75 (Epi Suite c2000-2012) | 76.5 (ChemIDplus 1993-) | 71 (ChemIDplus 1993-) |
| Boiling point (ºC) | 240 (ChemSpider 2015) | 304 (ChemIDplus 1993-) | 301.7 (ChemIDplus 1993-) |
| Vapour pressure (Pa) | 0.00257 (Epi Suite c2000-2012) | 0.068 (Epi Suite c2000-2012) | 0.087 (Epi Suite c2000-2012) |
| Water solubility (mg/L) | 53.28 at 25 °C (Epi Suite c2000-2012) | 1189 at 25 °C (Epi Suite c2000-2012) | 5126 at 25 °C (Epi Suite c2000-2012) |
| log Kow (dimensionless) | 3.22 at 25 °C (Epi Suite c2000-2012) | 2.060 at 25 °C (Epi Suite c2000-2012) | 1.51 at 25 °C (Epi Suite c2000-2012) |
| Acute toxicity (oral) | LD50: 5000 mg/kg bw in rats and 1780 mg/kg bw in mice (NICNAS 2016) | LD50: 1680 mg/kg bw in rats (NICNAS 2016) | LD50: 290–680 mg/kg bw in various rat strains; 196–780 mg/kg bw in various mouse strains (NICNAS 2016). |
| Skin sensitization | In a maximization test in female guinea pigs, coumarin 1 was observed to be non-sensitizing (ECHA c2007-2021). In a limited patch test in humans, there were no positive reactions (ECHA c2007-2021). |
No evidence of photoallergenic potential in guinea pigs and humans (NICNAS 2016). In a maximization test in humans, there were no skin reactions when volunteers were exposed to 6-methylcoumarin at 4% in petrolatum (NICNAS 2006). |
No skin-sensitizing potential (SCCP 2005, as cited in NICNAS 2016). Pure coumarin has very weak sensitizing capacities (Vocanson et al. 2007). |
| Sub-chronic repeat dose toxicity (oral) | Oral study via diet in rats (14 weeks): Limited study as described in the Health effects assessment section. (ECHA c2007-20121) |
Gavage study in rats (13 weeks): Vehicle: not indicated NOAEL = 150 mg/kg bw/day based on a significant decrease in serum cholinesterase activity in females and increased mean absolute and relative liver weights in both sexes at the next dose of 300 mg/kg-bw/day (NTP 2002, as cited in JECFA 2004). Oral via diet study in rats (13 weeks): 695 mg/kg bw = slight vacuolation of hepatocytes (Lake et al. 1994, as cited in JECFA 2004). Oral study via diet in rats (14 weeks): NOAEL = 1 000 mg/kg bw/day (highest dose tested) (Hagan et al. 1967, as cited in JECFA 2004). |
Gavage study in rats (13 weeks): Vehicle: corn oil NOAEL = 75 mg/kg bw/day based on centrilobular hepatocellular degeneration and necrosis along with chronic active inflammation and bile duct hyperplasia at 150 mg/kg bw/day (NTP 1993, as cited in NICNAS 2016). |
| Long-term repeat dose toxicity (oral) | N/A | Oral study via diet in rats (2 years): NOAEL = 250 mg/kg bw/day based on moderate growth depression at the next dose of 375 mg/kg-bw/day (Hagan et al. 1967). |
Gavage study in rats (2 years): Vehicle: corn oil LOAEL = 25 mg/kg bw/day based on lesions in the liver, kidney, and forestomach (NTP 1993). |
| Reproductive/developmental Toxicity (oral) | N/A | N/A | Oral study via diet in mice (days 6 to 17): LOAEL = 75 mg/kg bw/day based on increased mortality up to 3 weeks of age (Roll and Bär 1967). |
| Genetic toxicity | Negative (NICNAS 2016) | Negative (EFSA 2019) | Negative (EFSA 2004) |
| Carcinogenicity (oral) | N/A | Oral study via diet in rats (2 years): No carcinogenicity was observed up to 750 mg/kg bw/day (EFSA 2019). |
Gavage study in rats and mice (2 years): Vehicle: corn oil LOAEL = 50 mg/kg bw/day based on increased incidences of renal tubule adenomas in male rats; increased incidences of alveolar/bronchiolar adenomas, alveolar/bronchiolar carcinomas, and hepatocellular adenomas in female mice (NTP 1993, as cited in NICNAS 2016). Oral study via diet in dogs (2 years): NOAEL = 10 mg/kg-bw/day based on histological lesions in the liver at the next dose of 25 mg/kg bw/day (NICNAS 2016). |
Abbreviations: N/A, not available; LD50, median lethal dose; NOAEL, no observed adverse effect level; LOAEL, lowest observed adverse effect level.
Appendix C. Estimates of daily intake by various age groups within the general population of Canada
| Route of exposure | 0 to 5 monthsa (breast -fed)b |
0 to 5 monthsa (formula fed)c |
6 to 11 monthsd | 1 yeare | 2 to 3 yearsf | 4 to 8 yearsg | 9 to 13 yearsh | 14 to 18 yearsi | ≥ 19 yearsj |
|---|---|---|---|---|---|---|---|---|---|
| Drinking waterk | N/A | 0.024 | 0.015 | 5.9E-3 | 5.2E-3 | 4.2E-3 | 3.2E-3 | 3.2E-3 | 3.7E-3 |
Abbreviations: N/A, not applicable.
a Assumed to weigh 6.3 kg, to breathe 3.7 m3 of air per day , and to ingest 21.6 mg of dust per day . It is assumed that no soil ingestion occurs due to typical caregiver practices (Health Canada 2021).
b Exclusively for breast milk-fed infants, assumed to consume 0.744 L of breast milk per day (Health Canada 2021), and breast milk is assumed to be the only dietary source.
c Exclusively for formula-fed infants, assumed to drink 0.826 L of water per day (Health Canada 2021), where water is used to reconstitute formula. See footnote on drinking water for details.
d Assumed to weigh 9.1 kg , to breathe 5.4 m3 of air per day , to drink 0 L of water per day ), to ingest 7.3 mg of soil per day, and to ingest 27.0 mg of dust per day . For breast milk-fed infants, assumed to consume 0.632 L of breast milk per day). For formula-fed infants, assumed to drink 0.764 L of water per day (Health Canada 2021), where water is used to reconstitute formula. See footnote on drinking water for details.
e Assumed to weigh 11.0 kg , to breathe 8.0 m3 of air per day , to drink 0.36 L of water per day , to ingest 8.8 mg of soil per day, and to ingest 35.0 mg of dust per day (Health Canada 2021).
f Assumed to weigh 15 kg , to breathe 9.2 m3 of air per day , to drink 0.43 L of water per day , to ingest 6.2 mg of soil per day, and to ingest 21.4 mg of dust per day (Health Canada 2021).
g Assumed to weigh 23 kg , to breathe 11.1 m3 of air per day modified]), to drink 0.53 L of water per day , to ingest 8.7 mg of soil per day, and to ingest 24.4 mg of dust per day (Health Canada 2021).
h Assumed to weigh 42 kg (), to breathe 13.9 m3 of air per day , to drink 0.74 L of water per day , to ingest 6.9 mg of soil per day, and to ingest 23.8 mg of dust per day (Health Canada 2021).
i Assumed to weigh 62 kg , to breathe 15.9 m3 of air per day , to drink 1.09 L of water per day, to ingest 1.4 mg of soil per day, and to ingest 2.1 mg of dust per day (Health Canada 2021).
j Assumed to weigh 74 kg , to breathe 15.1 m3 of air per day , to drink 1.53 L of water per day , to ingest 1.6 mg of soil per day, and to ingest 2.6 mg of dust per day (Health Canada 2021).
k Estimated to be 0.18 µg/L using the NSACB EAU Drinking Water Spreadsheet (2003) and the upper-end volume data (that is, 10 000 kg).
Appendix D. Parameters used to estimate human exposures from use of products available to consumers
Exposure estimates were calculated on the basis of default body weights of 6.3 kg (0 to 5 months), 9.1 kg (6 to 11 months), 11 kg (1 year), 15 kg (2 to 3 years), 23 kg (4 to 8 years), 42 kg (9 to 13 years), 62 kg (14 to 18 years) and 74 kg (≥ 19 years) (Health Canada 2021). Estimated dermal and oral exposures to cosmetics as well as the use of carpet cleaner were derived using ConsExpo Web (2016). Post-application exposures to coumarin 1 from carpet cleaners were derived using US EPA (2012) for young children. The estimated inhalation exposures for all scenarios were insignificant in comparison to the dermal exposures, and therefore are not presented. The estimated exposure parameters are described in Table D-1.
| Exposure scenario | Assumptionsa,b |
|---|---|
| Nail polish (dermal) Assumes 2 coats of nail polish applied to fingernails and toenails |
Maximum reported concentration: 2% (personal communication, emails from CHPSD, HC, to ESRAB, HC, dated October 2018; unreferenced) Product amount on skin: 0.06 g/use (2 to 3 years), 0.16 g/use (14 to 18 years, ≥ 19 years) (modified from Ficheux et al. 2014, adjusted by a factor of 0.206 for adults and 0.205 for children to account for how much nail polish ends up on skin) |
| Temporary powder hair dye (dermal)c |
Maximum reported concentration: 0.5% (personal communication, emails from CHPSD, HC, to ESRAB, HC, dated October 2018; unreferenced) Loading: Instant application Product amount: 1.75 g (personal communication, emails from CHPSD, HC, to ESRAB, HC, dated May 2019; unreferenced) Retention Factor: 0.1 |
| Temporary gel hair dye (dermal)c | Maximum reported concentration: 1% (personal communication, emails from CHPSD, HC, to ESRAB, HC, dated October 2018; unreferenced) Scenario: assumed to be similar to applying hair gel Loading: Instant application Product amount: 3.7 g (14 to 18 years, ≥ 19 years) (Ficheux et al. 2016), 2.8 g (2 to 3 years), 3.1 g (4 to 8 years), 3.5 g (9 to 13 years) (based on value for 14 – 18 years and adjusting for differences in surface area of half the head) Transfer Factor: 0.1d |
| Lipstick/lip glosse (oral) |
Maximum reported concentration: 1% (personal communication, emails from CHPSD, HC, to ESRAB, HC, dated October 2018; unreferenced) Frequency: 1/day (2 to 3 years), 2.5/day (14 to 18 years), 2/day (≥ 19 years) Amount ingested: 0.022 g (Ficheux et al. 2016) |
| Facial makeup (dermal) |
Maximum reported concentration: 0.3% (personal communication, emails from CHPSD, HC, to ESRAB, HC, dated October 2018; unreferenced) Facial makeup scenario from Cosmetic Fact Sheet (RIVM 2006), with additional information on amount applied Frequency: 1.2/day (≥ 19 years ) (Loretz et al. 2006); per event exposures were derived for 4 to 8 years given frequency of use is <1/day (Garcia-Hidalgo et al. (2017) and professional judgement) Loading: Instant application Product amount: 0.34 (4 to 8 years), 0.54 g (≥ 19 years) (Loretz et al. 2006) |
| Body makeupf (dermal) |
Maximum reported concentration: 0.3% (personal communication, emails from CHPSD, HC, to ESRAB, HC, October 2018; unreferenced) Body moisturizer scenario from Cosmetics Fact Sheet (RIVM 2006), with additional information on amount applied Surface area: 3393 cm2 (4 to 8 years – assume face, arms and 3/4 of legs are exposed), 7263 cm2 (≥ 19 years – assume face, arms, 3/4 of legs and half trunk are exposed) Product amount: 2.05 g (Ficheux et al. 2016, with surface area adjustment) (4 to 8 years), 4.14 g (Ficheux et al. 2016, with surface area adjustment) (≥ 19 years) |
| Carpet cleaner application (dermal) |
Concentration: 0.01% (SDS 2015) Age group: ≥ 19 years Exposed area: 2200 cm2 Loading: Instant application Weight fraction substance: 0.01% Product amount 1.5 g |
| Carpet cleaner post-application (dermal) |
Concentration: 0.01% (SDS 2015) Scenario based on US EPA (2012) Residential SOPs Dermal intake = surface residue x concentration x floor-to-skin transfer efficiency x transfer coefficient x exposure duration x (1 m2 / 10 000 cm2) x 1 000 000 µg/g / bw Age group: 6 to 11 months Surface residue: 9 g/m2 (based on ConsExpo default for dislodgeable amount of carpet cleaning liquid post-application; RIVM 2018) Floor-to-skin transfer efficiency: 0.06 (US EPA 2012 default for carpets) Transfer coefficient: 1528 cm2/hr (US EPA 2012 default of 1800 cm2/hr for hard surfaces and carpets adjusted for surface area of a 6- to 11-month-old child)g Exposure duration: 4 hr (US EPA 2012 default for carpets) Conversion factors: 1 m2 / 10 000 cm2, 1 000 000 µg/g |
| Carpet cleaner post-application (oral) |
Concentration: 0.01% (SDS 2015) Scenario based on US EPA (2012) for oral hand-to-mouth intake Age group: 6 to 11 months Oral hand-to-mouth intake = hand residue loading x surface area mouthed x [exposure time x number of replenishment intervals per hr] x [1- (1-saliva extraction factor, 0.48)frequency of hand-to-mouth, 20 / number of replenishments, 4] / bw Hand residue loading (mg/cm2): fraction of substance on hands compared to total surface residue from jazzercise study x total dermal deposition calculated from dermal scenario (mg/day) / surface areas of both hands. There may be slight differences due to rounding. Dermal deposition: dermal exposure (mg/kg bw/day) x bw Fraction of substance on hands compared to total surface residue from jazzercise study: 0.15 Surface area of both hands: 240 cm2 Surface area mouthed: 22 cm2/event Exposure time: 4 hr/day Number of replenishment intervals per hr: 4 intervals/hr Saliva extraction factor: 0.48 Frequency of hand-to-mouth: 20 events/hr Number of replenishments: 4 |
| Multi-purpose spray cleaner – spraying (dermal) | Concentration: 0.5% (according to information received through the public comment period) Scenario: Bathroom cleaner spray - spraying in Cleaning Products Fact Sheet (RIVM 2018). Age group: ≥ 19 Exposed area: 2200 cm2 Loading: Constant rate Weight fraction substance: 0.5% Contact rate: 46 mg/min Release duration: 2.67 min |
| Multi-purpose spray cleaner – wiping (dermal) |
Concentration: 0.5% (according to information received through the public comment period) Scenario: Bathroom cleaner spray – rinsing in Cleaning Products Fact Sheet (RIVM 2018). Age group: ≥ 19 years Exposed area: 225 cm2 Loading: Instant application Weight fraction substance: 0.5% Product amount 0.62 g |
a Cosmetic exposures were estimated using ConsExpo Web (2016).
b Unless specified, the defaults come from the relevant ConsExpo Fact Sheet for the scenario presented.
c Applied using a sponge. Scenario accounts for product that ends up on hands during product application and is washed off. Assumes half the container is used.
d Transfer factor assumes only 10% of product applied to hair is in contact with scalp and available for dermal absorption.
e Specialty product that would be used on occasion, likely reapplied throughout the day.
f Specialty product that would be used on occasion.
g Transfer coefficient = (1800 cm2 x 4500 cm2)/ 5300 cm2 = 1528 cm2/hr.
