Screening Assessment Ethylene Glycol Ethers Group
Chemical abstracts service registry numbers
110-71-4, 111-46-6, 111-90-0, 112-07-2, 112-27-6, 112-34-5, 112-60-7
Environment and Climate Change Canada
Health Canada
August 2018
Cat. No.: En14-329/2018E-PDF
ISBN 978-0-660-27544-4
Table of contents
- Synopsis
- 1. Introduction
- 2. Identity of substances
- 3. Physical and chemical properties
- 4. Sources
- 5. Uses
- 6. Potential to cause ecological harm
- 7. Potential to cause harm to human health
- 8. Conclusion
- References
- Appendicies
List of tables
- Table 2-1. Substance identities for ethylene glycol ethers
- Table 2-2. Subgroups of ethylene glycol ethers substances
- Table 2-3. Analogue identity and availability of read-across data used in this assessment
- Table 3-1. Range of chemical and physical properties for ethylene glycols
- Table 3-2. Range of chemical and physical properties for ethylene glycol ethers
- Table 3-3. Physical and chemical property values for monoglyme
- Table 4-1. Summary of canadian manufacture and import of substances in the ethylene glycol ethers group
- Table 5-1. Summary of canadian non-confidential business information uses of substances in the EGs and EGEs subgroups
- Table 6-1. Ecological risk classification results for substances in the ethylene glycol ethers group
- Table 7-1. Summary of dermal absorption values for substances in the EGEs group
- Table 7-2. Summary of estimates of dermal exposure of an adult to ethylene glycols and ethylene glycol ethers from use of cosmetics, natural health products, and over the counter drugs
- Table 7-3. Summary of estimates of acute dermal exposures of an adult to ethylene glycols and ethylene glycol ethers from use of cosmetics
- Table 7-4. Summary of estimates of dermal and air exposures of an adult to ethylene glycols and ethylene glycol ethers from use of paints and coatings
- Table 7-5. Estimated exposures to DEGEE and DEGBE from mouthing of a painted toy by children (Hansen and Pederson 2005)
- Table 7-6. 24-hour and 6-hour mean event air concentrations from air freshener use
- Table 7-7. Estimated dermal exposure and air concentrations of ethylene glycol ethers from use of household cleaning products
- Table 7-8. Updated critical endpoint values for DEGBE
- Table 7-9. Relevant exposure and hazard values for DEG, and resulting MOEs
- Table 7-10. Relevant exposure and hazard values for TEG, and resulting MOEs
- Table 7-11. Relevant exposure and hazard values for TTEG, and resulting MOEs
- Table 7-12. Relevant exposure and hazard values for DEGEE, and resulting MOEs
- Table 7-13. Relevant exposure and hazard values for EGBEA, and resulting MOEs
- Table 7-14. Relevant exposure and hazard values for DEGBE, and resulting MOEs
- Table 7-15. Relevant exposure and hazard values for monoglyme and its metabolite 2-ME, as well as resulting MOEs
- Table 7-16. Sources of uncertainty in the risk characterization
List of figures
Synopsis
Pursuant to sections 68 or 74 of the Canadian Environmental Protection Act, 1999 (CEPA), the Minister of Environment and the Minister of Health have conducted a screening assessment of seven of the nine substances referred to collectively under the Chemicals Management Plan as the Ethylene Glycol Ethers Group. These seven substances were identified as priorities for assessment as they met the categorization criteria under subsection 73(1) of CEPA or considered a priority based on other human health concerns. Two of the nine substances were subsequently determined to be of low concern through other approaches, and proposed decisions for these substances are provided in a separate report.Footnote 1 Accordingly, this screening assessment addresses the seven substances listed in the table below.
| CAS RNa | Domestic Substances List (DSL) name | Common name | Acronyms |
|---|---|---|---|
| 110-71-4b | Ethane, 1,2-Dimethoxy- | Monoglyme or Ethylene glycol dimethyl ether | EGDME |
| 111-46-6 | Ethanol, 2,2'-oxybis- | Diethylene glycol | DEG |
| 112-27-6 | Ethanol, 2,2'-[1,2-ethanediylbis(oxy)]bis- | Triethylene glycol | TEG |
| 112-60-7 | Ethanol, 2,2'-[oxybis(2,1-ethanediyloxy)]bis- | Tetraethylene glycol | TTEG |
| 111-90-0 | Ethanol, 2-(2-ethoxyethoxy)- | Diethylene glycol monoethyl ether | DEGEE |
| 112-07-2 | Ethanol, 2-butoxy-, acetate | Ethylene glycol monobutyl ether acetate | EGBEA |
| 112-34-5 | Ethanol, 2-(2-butoxyethoxy)- | Diethylene glycol monobutyl ether | DEGBE |
a The CAS RN is the property of the American Chemical Society and any use or redistribution, except as required in supporting regulatory requirements and/or for reports to the Government of Canada when the information and the reports are required by law or administrative policy, is not permitted without the prior, written permission of the American Chemical Society.
b This substance was not identified under subsection 73(1) of CEPA but was included in this screening assessment as it was considered a priority based on other human health concerns.
In Canada, substances in the Ethylene Glycol Ethers Group are used in a variety of products including cosmetics and non-prescription drugs, paint and coating products and air fresheners, as well as in adhesives, batteries, and textiles.
All of the substances in this group are imported into Canada at reported quantities ranging from 100 to 10 000 000 kg/year. Four of the seven substances (TTEG, DEGEE, EGBEA, and DEGBE) in this group are manufactured in Canada at reported quantities ranging from 1000 to 10 000 000 kg/year. In the United States, production volumes range from 10 000 000 to 450 000 000 kg/year for these substances.
The ecological risks of the seven substances in the Ethylene Glycol Ethers Group were characterized using the Ecological Risk Classification of organic substances (ERC). The ERC is a risk-based approach that employs multiple metrics for both hazard and exposure based on weighted consideration of multiple lines of evidence for determining risk classification. Hazard profiles are established based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity. Metrics considered in the exposure profiles include potential emission rate, overall persistence, and long-range transport potential. A risk matrix is used to assign a low, moderate or high level of potential concern for substances based on their hazard and exposure profiles. The ERC identified the seven substances in the Ethylene Glycol Ethers Group as having low potential to cause ecological harm.
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from the seven substances in the Ethylene Glycol Ethers Group. It is concluded that the seven substances in the Ethylene Glycol Ethers Group do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
For the human health risk assessment, the seven substances in this group were separated into 3 subgroups: Ethylene Glycols (EGs), Ethylene Glycol Ethers (EGEs), and glymes. Environmental media and food were not identified as significant sources of exposure to Canadians. For the EGs and EGEs, estimates of exposure were derived based on levels of substances in products available to consumers, such as cosmetics and non-prescription drugs, paint and coating products and household cleaning products. For monoglyme, estimates of exposure were based on levels in indoor air and from use of air fresheners.
For these substances, adverse health effects are observed at high dose levels in laboratory studies, with target organs being the liver and kidney. For some of the substances (DEGEE, EGBEA, and DEGBE), hemolytic effects observed in laboratory studies are not relevant to human health as humans are much less sensitive to these effects. For monoglyme, developmental effects are observed in laboratory studies at doses lower than those for the other ethylene glycol ethers substances in this group, along with effects on testes, blood, thymus and adrenal glands.
For all subgroups, based on a comparison of the estimates of exposure from use of products available to consumers and levels at which critical effects are observed, the margins are considered to be adequate to address uncertainties in the health effects and exposure databases.
Based on the information presented in this screening assessment, it is concluded that the seven substances in the Ethylene Glycol Ethers Group do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that the seven substances in the Ethylene Glycol Ethers group in this screening assessment do not meet any of the criteria under section 64 of CEPA.
1. Introduction
Pursuant to sections 68 and 74 of the Canadian Environmental Protection Act, 1999 (CEPA) (Canada 1999), the Minister of the Environment and the Minister of Health conduct screening assessments of substances to determine whether they present or may present a risk to the environment or to human health. The seven substances addressed in this screening assessment are referred to collectively as the Ethylene Glycol Ethers Group. They were identified as priorities for assessment as they met categorization criteria under subsection 73(1) of CEPA (Environment and Climate Change Canada, Health Canada [modified 2007]), or were considered a priority based on other human health concerns.
Two other substances, 1, 1'-oxybis[2-methoxy] Ethane (CAS RNootnote 2 111-96-6) and 2,5,8,11-Tetraoxadodecane (CAS RN 112-49-2) were considered in the Ecological Risk Classification of Organic Substances (ERC) and the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances science approach documents (ECCC 2016a; Health Canada 2016b), and were identified as being of low concern to both human health and the environment. As such, these substances are not further addressed in this report. Proposed conclusions for these substances are provided in the Substances Identified as Being of Low Concern based on the Ecological Risk Classification of Organic Substances and the Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances Screening Assessment (ECCC,HC 2017). The seven substances addressed in this screening assessment will hereinafter be referred to as the Ethylene Glycol Ethers Group.
The ecological risks of the seven substances in the Ethylene Glycol Ethers Group were characterized using the ecological risk classification of organic substances (ERC) (ECCC 2016a). The ERC describes the hazard of a substance using key metrics including mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity and considers the possible exposure of organisms in the aquatic and terrestrial environments based on factors including potential emission rates, overall persistence and long-range transport potential in air. The various lines of evidence are combined to identify substances as warranting further evaluation of their potential to cause harm to the environment or as having a low likelihood of causing harm to the environment.
Substances in the Ethylene Glycol Ethers Group were reviewed internationally through different programs, such as the Organisation for Economic Cooperation and Development’s Cooperative Chemicals Assessment Programme, and by the European Commission (a number of assessments are available). These assessments undergo rigorous review and endorsement by international governmental authorities. Environment and Climate Change Canada and Health Canada are active participants in this process and consider these assessments to be reliable. The data in these assessments will be used to inform the health effects characterization for the Ethylene Glycol Ethers Group.
This screening assessment includes consideration of information on chemical properties, environmental fate, hazards, uses and exposure, including additional information submitted by stakeholders. Relevant data were identified up to April 2016. However, more recent studies or information provided via internal and external peer consultation or via the public comment period for the draft version (see below) may also be cited. Empirical data from key studies as well as results from models were used. When available and relevant, information presented in assessments from other jurisdictions was considered.
This screening assessment was prepared by staff in the CEPA Risk Assessment Programs at Health Canada and Environment and Climate Change Canada and incorporates input from other programs within these departments. The ecological portion of this assessment is based on the ERC document which was subject to an external peer-review. Additionally, the ERC document was published on July 30, 2016 and subject to a 60-day public comment period. The human health portions of this screening assessment have undergone external peer review and/or consultation. Comments on the technical portions relevant to human health were received from Lisa Sweeney (Henry M. Jackson Foundation for the Advancement of Military Medicine), Chris Bevan (CJB Consulting LLC.), Ray York (RG York and Associates LLC), and John Reichard (Toxicology Excellence for Risk Assessment Center, University of Cincinnati). While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Environment and Climate Change Canada and Health Canada. Additionally, the draft of this screening assessment was subject to a 60 day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment and Climate Change Canada.
This screening assessment focuses on information critical to determining whether substances meet the criteria as set out in section 64 of CEPA. It examines scientific information and develops a conclusion by incorporating a weight-of-evidence approach and precaution.ootnote 3 The screening assessment presents the critical information and considerations that form the basis of the conclusion.
2. Identity of substances
The seven substances assessed in this screening assessment belong to a larger chemical group of substances known as glycol ethers. The general formula for glycol ethers is shown in Figure 2-1. Glycol ethers may be subgrouped based on the nature of the functional groups (R1 or R2), the number of glycol units, the number of carbon atoms in the ether side chain (R1 or R2) or the degree of branching in R3 and R4 (Mangelsdorf et al. 2016). Glycol esters with acetic acid, also known as glycol ether acetates, are also included in the chemical group (Mangelsdorf et al. 2016).
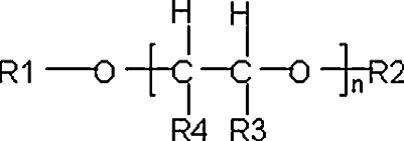
Long description for figure 2-1
General structural formula of glycol ethers, glycol esters and glycol diethers (from Mangelsdorf et al. 2016)
The Chemical Abstracts Service Registry Numbers (CAS RNFootnote 4), Domestic Substances List (DSL) names, common names, and acronyms for the individual substances in the Ethylene Glycol Ethers are presented in Table 2-1. A list of additional chemical names (e.g., trade names) is available from the National Chemical Inventories (NCI 2014).
The seven substances discussed in this screening assessment are representative substances from three different subgroups: ethylene glycols (EGs), ethylene glycol ethers (EGEs) and glymes (see Tables 2-1 and 2-2).
| CAS RN (acronym) |
DSL name (common name)a |
Chemical structure and molecular formula | Molecular weight (g/mol) |
|---|---|---|---|
| 111-46-6 (DEG) |
Diethylene Glycol | 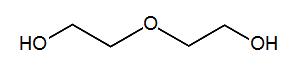 |
106.12 |
| 112-27-6 (TEG) |
Triethylene Glycol | 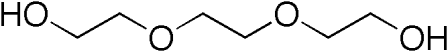 |
150.17 |
| 112-60-7 (TTEG) |
Tetraethylene Glycol | 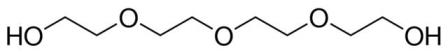 |
194.23 |
| 111-90-0 (DEGEE) |
Diethylene Glycol Monoethyl Ether | 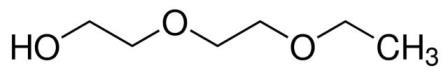 |
134.17 |
| 112-07-2 (EGBEA) |
2-Butoxyethyl Acetate | 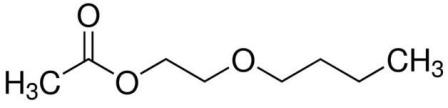 |
160.21 |
| 112-34-5 (DEGBE) |
2-(2-Butoxyethoxy) ethanol | 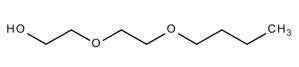 |
162.23 |
| 110-71-4 (EGDME) |
Monoglyme | 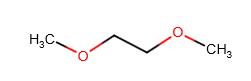 |
90.12 |
a ChemIDplus 1993- a,b,c,d,e,f,g
| Subgroup | Chemicals | Critical functional groups | Molecular weight range (g/mol) |
|---|---|---|---|
| Ethylene Glycols (n=3) | DEG, TEG, TTEG | Ethylene glycol ethers that consist of 1, 2, or 3 glycol ether groups with 2 terminal alcohol groups | 106.12-194.23 |
| Ethylene Glycol Ethers (n=3) | DEGEE, EGBEA, DEGBE | Ethylene glycol ethers that consist of 1 or 2 glycol ether groups with 1 terminal alkyl group of 2 or 4 carbons and 1 terminal alcohol or ester group | 134.17-162.23 |
| Glymes (n=1) | Monoglyme | Ethylene glycol ether consisting of two glycol ether units and two terminal methyl groups. | 90.12 |
2.1 Selection of analogues
A read-across approach using data from analogues has been used to inform the human health assessments. The analogues selected were structurally similar and/or functionally similar to substances within this group (e.g., based on physical-chemical properties and toxicokinetics) and had relevant empirical data that could be used to read-across to substances that were data poor. In addition to the analogues, read-across from one substance to another within the EGs subgroup are discussed in the relevant sections of this report (see Health effects assessment of the Ethylene Glycols subgroup). A list of the analogues used to inform this assessment is presented in Table 2-3 along with an indication of the potential read-across data.
| CAS RN for analogue | DSL/other name (common name) |
Chemical structure and molecular formula | Mole-cular weight (g/mol) | Human health data | Target substance for analogue |
|---|---|---|---|---|---|
| 111-76-2a | 2-Butoxy-ethanol (EGBE: Ethylene glycol monobutyl ether) |
 C6H14O2 |
118.18 | Y | EGBEA (CAS RN 112-07-2)a |
| 109-86-4b | 2-Methoxy-ethanol (2-ME) |
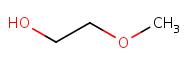 C3H8O2 |
76.09 | Yc | Monoglyme (CAS RN 110-71-4)b |
a EGBE chemical structure, molecular formula and molecular weight from ChemIDplus 1993- h.
b 2-ME chemical structure, molecular formula and molecular weight from ChemIDplus 1993- i.
c As shown in the "Health effects assessment of Monoglyme" section, 2-ME is actually a metabolite of monoglyme in experimental animals.
3. Physical and chemical properties
Generally, glycol ethers are high boiling point, semi-volatile liquids that behave as solvents for water and many organic liquids (Mangelsdorf et al. 2016). The EG, EGE, and monoglyme subgroups have different melting and boiling point, specific gravity, density, and vapour pressure ranges. Furthermore, monoglyme shows a higher vapour pressure, higher log octanol-water partition coefficient (Kow) and lower boiling point than substances in the other two subgroups and this may be due in part to its low molecular weight.
A summary of physical and chemical properties of the substances in the Ethylene Glycol Ethers Group are presented in Tables 3-1, 3-2 and 3-3. Additional physical and chemical properties are presented in ECCC (2016b).
| Property | Type | Value | Temperature (°C) | Reference |
|---|---|---|---|---|
| Physical form | - | Liquid | 25 | - |
| Melting point (°C) |
Experimental | -10 to -6.2 | NA | Lide 2007 HSDB 2007b |
| Boiling point (°C) |
Experimental | 246-327.3 | NA | Lide 2007 HSDB 2007b |
| Specific gravity (dimensionless) |
Experimental | 1.1197-1.1285 | 25; 15 | Lide 2007 HSDB 2007b |
| Density (kg/m3) |
Experimental | 1119.7-1128.5 | 25; 15 | Lide 2007 HSDB 2007b |
| Vapour pressure (mmHg) |
Experimental | 4.65 x 10-5-5.7 x 10-3 | 26; 25 | HSDB 2007a,b |
| Henry's Law constant (atm·m3/mol) |
Estimated; Experimental |
2.61 x 10-10-2.0 x 10-9 | 25; NS | HSDB 2007a HSDB 2009a |
| Log Kow (dimensionless) |
Modelled; Estimated |
-2.02 to -1.47 | NS | HSDB 2007b HSDB 2009a |
| Water solubility (mg/L) |
Experimental | 1 x 106 | 20 | HSDB 2007a,b HSDB 2009a |
| Property | Type | Value | Temperature (°C) | Reference |
|---|---|---|---|---|
| Physical form | - | Liquid | 25 | - |
| Melting point (°C) |
Experimental | -68.1 to -54 | NA | HSDB 2007c,d |
| Boiling point (°C) |
Experimental | 192-230.4 | NA | HSDB 2007b HSDB 2009b |
| Specific gravity (dimensionless) |
Experimental | 0.9422-0.9885 | 20 | HSDB 2007c HSDB 2009b |
| Density (kg/m3) |
Experimental | 942.2-988.5 | 20 | HSDB 2007c HSDB 2009b |
| Vapour pressure (mmHg) |
Experimental | 0.0219-0.375 | 25; 20 | HSDB 2007d HSDB 2009b |
| Henry's Law constant (atm·m3/mol) |
Experimental | 7.29 x 10-9-5.46 x 10-6 | 25 | HSDB 2007d HSDB 2009b |
| Log Kow (dimensionless) |
Experimental; Estimated |
-0.54 to 1.57 | NS | HSDB 2007c HSDB 2009b |
| Water solubility (mg/L) |
Experimental | 9 x 103-1 x 106 | 20 | HSDB 2007c,d HSDB 2009b |
| Property | Type | Value | Temperature (°C) | Reference |
|---|---|---|---|---|
| Physical Form | - | Liquid | 25 | - |
| Melting point (°C) |
Experimental | -71 to -58 | NA | HSDB 2002 |
| Boiling point (°C) |
Experimental | 82-83 | NA | HSDB 2002 |
| Specific gravity (dimensionless) |
Experimental | 0.86285 | 20; 4 | HSDB 2002 |
| Density (kg/m3) |
Experimental | 862.85 | 25; 15 | HSDB 2002 |
| Vapour pressure (mmHg) |
Experimental | 48 | 20 | HSDB 2002 |
| Henry's Law constant (atm-m3/mol) |
Estimated | 1.1 x 10-6 | 25 | HSDD 2002 |
| Log Kow (dimensionless) |
Modelled; Estimated | -0.21 | NA | HSDS 2002 |
| Water solubility (mg/L) |
Experimental | 1 x 106 | 20 | HSDB 2002 |
NA = Not available.
4. Sources
All seven substances in this group are commercially produced and do not occur naturally.
Three separate surveys, issued pursuant to section 71 of CEPA, were conducted in 2001, 2008 and 2012 to obtain information about quantities in commerce for various substances in Canada (Environment Canada 2001, 2009, 2014).
The section 71 surveys indicated that one substance in the EGs subgroup, TTEG, and all three substances in the EGEs subgroup were manufactured in Canada above the reporting threshold. All seven substances in the Ethylene Glycol Ethers Group were imported into Canada above the reporting threshold (Environment Canada 2001, 2009. 2014; see Table 4-1).
| Subgroup | Common name | Total manufacture (kg)* | Total imports (kg)* |
|---|---|---|---|
| Ethylene Glycols | DEG | - | 100 000-1 000 000 |
| Ethylene Glycols | TEG | - | 1 000 000-10 000 000 |
| Ethylene Glycols | TTEG | 1 000 000-10 000 000 | 100 000-1 000 000 |
| Ethylene Glycol Ethers | DEGEE | 10 000-100 000 | 100 000-1 000 000 |
| Ethylene Glycol Ethers | EGBEA | 1000-10 000 | 100 000-1 000 000 |
| Ethylene Glycol Ethers | DEGBE | 10 000-100 000 | 1 000 000-10 000 000 |
| Glymes | Monoglyme | - | 100-100 000 |
a Values reflect quantities reported in response to surveys conducted under section 71 of CEPA (Environment Canada 2001, 2009, 2014). See survey for specific inclusions and exclusions (schedules 2 and 3).
In the United States (US), production volumes ranged from 10 000 000 to 450 000 000 kg for the EG and EGE substances in 2012 (US EPA 2012) and from 453 600 to 4 536 000 for monoglyme in 2005 (ECHA 2012b).Footnote 5 In the European Union, manufacture and import of all seven substances ranged from 100 000 to 1 000 000 000 kg in 2015 (ECHA 2015).
5. Uses
Two of the section 71 surveys collected information about the uses in Canada of all six substances in the EGs and EGEs subgroups; they are presented in Table 5-1 (Environment Canada 2001, 2014).
| Major usesa | Ethylene Glycols | Ethylene Glycol Ethers |
|---|---|---|
| Adhesives | TEG, TTEG | EGBEA, DEGBE |
| Automotive, aircraft and transportation | DEG, TEG, TTEG | DEGEE, EGBEA, DEGBE |
| Cleaning productsb | DEG, TEG | DEGEE, EGBEA, DEGBE |
| Personal care productsc, including cosmetics | - | DEGEE |
| Dyes and pigments | - | DEGEE, DEGBE |
| Medical, health products and veterinary | - | DEGEE |
| Paints and coatingsb | DEG, TEG, TTEG | DEGEE, EGBEA, DEGBE |
| Plastics and plasticizers | TEG, TTEG | DEGEE, DEGBE |
| Printing and writing products and printing inks | TEG, TTEG | DEGEE, DEGBE |
| Rubber | TEG | DEGBE |
| Textile, leather and tanning | - | DEGEE |
| Toys, sporting equipment and playground equipment | - | EGBEA |
a All information obtained from section 71 surveys under CEPA (Environment Canada 2001, 2014).
b Submitted section 71 data along with other sources of concentration data (e.g. MSDS concentration data, personal communication from other Branches in Health Canada) were used to characterize exposure in Section 7.
c For the purpose of this document, a personal care product is defined as a product that is generally recognized by the public for use in personal cleansing or grooming. Depending on how the product is represented for sale and its composition, personal care products may fall into one of three regulatory categories in Canada: cosmetics, drugs or natural health products.
DEG is not permitted in oral or leave-on cosmetic productsFootnote 6 (Health Canada [modified 2015]). Based on notifications submitted under the Cosmetic Regulations to Health Canada, TEG, DEGEE and DEGBE were notified to be present in cosmetic productsFootnote 7 (personal communication, emails from the Consumer Product Safety Directorate (CPSD), Health Canada, to the Existing Substances Risk Assessment Bureau (ESRAB), Health Canada, dated September 2015; unreferenced).
TEG and DEGBE are listed in the Drug Product Database as being present as active ingredients in hard-surface disinfectant drugs (DPD 2016). DEG, TEG, DEGEE and DEGBE are listed in the Therapeutic Product Directorate’s internal Non-Medical Ingredients Database as being present in human and disinfectant drugs.ootnote 8 DEG is listed in the Natural Health Products Ingredients Database (NHPID) with a non-medicinal role for use as fragrance ingredient, solvent, or viscosity decreasing agent in topical rinse-off products only, DEG is not permitted in oral or leave-on products (NHPID 2018, Personal communication Natural and Non-prescription Health Products Directorate (NNHPD), Health Canada to the Existing Substances Risk Assessment Bureau (ESRAB), Health Canada, dated August 3, 2017). DEGBE is listed in the NHPID with a non-medicinal role for topical use only as fragrance ingredient, solvent, or viscosity decreasing agent (NHPID 2018). DEG and DEGBE are not listed in the Licensed Natural Health Products Database (LNHPD) as being present in currently licensed natural health products (LNHPD 2018). DEGEE is listed in the NHPID with a non-NHP role as not a naturally occurring substance falling under Schedule 1 to the Natural Health Products Regulations, whereas DEGEE and TEG are listed in the NHPID with a non-medicinal role for topical use only as fragrance ingredient, solvent, or viscosity decreasing agent (NHPID 2018). DEGEE and TEG are listed in the LNHPD as being present in currently licensed natural health products (LNHPD 2018).Footnote 9
In Canada, the six substances in the EGs and EGEs subgroups are used in food packaging; however, only DEG and EGBEA could be in direct contact with food (personal communication, emails from the Food Directorate, Health Canada, to the Risk Management Bureau, Health Canada, dated October 2015; unreferenced). None of the substances are listed in the Lists of Permitted Food Additives as approved food additives under the Food and Drugs Act and associated Marketing Authorizations (personal communication, emails from the Food Directorate, Health Canada, to the Risk Management Bureau, Health Canada, dated October 2015; unreferenced).
Finally, five of the six substances in the EGs and EGEs subgroups (DEG, TEG, DEGEE, EGBEA and DEGBE) are identified as formulants in pest control products. Only TEG is a registered active pesticidal ingredient, but the registered product is only formulated in Canada and exported (personal communication, emails from the Pest Management Regulatory Agency, Health Canada to the ESRAB, Health Canada, dated September 2015 and September 2016; unreferenced).
Monoglyme is used as an additive at concentrations ranging from 1 to 10% w/w in lithium for the manufacture of all types of batteries based on material safety data sheets (MSDSs) for products in Canada. Monoglyme is also found as an impurity in air fresheners and laundry products at concentrations less than 0.01% (Environment Canada 2009). Monoglyme is identified in the Therapeutic Products Directorate’s guidance document Q3C(R5): Impurities: Guideline for Residual Solvents as a Class 2 solvent and should therefore not exceed 100 ppm (equal to 1.0 mg/day permissible daily exposure) in drugs (Health Canada 2016a).
Major uses identified internationally for the EGs and EGEs subgroup substances, in addition to those listed in Table 5-1, include antifreeze, do-it-yourself materials, pest control products, indirect additives in food contact substances, additives to polyurethanes and polyester resins, dehydrating agents for natural gas activities, and additives for industrial or commercial use (JECFA 1995; Wagner 2006; Tønning et al. 2008; OECD 2007, 2009a; Scorecard 2011; US FDA 2011; GoodGuide 2014; CCOHS 2015; DOW 2015; ECHA 2015; EWG 2015; NICNAS 2015; NYSDEC 2015; U.S. Government Publishing Office 2015). Major uses identified internationally for monoglyme include cleaning products, medical, health and veterinary products, paints and coatings, printing, writing products and printing inks, and industrial or commercial use (ECHA 2012b; NICNAS 2016a).
Identified uses in Canada, carried forward for human exposure assessment are outlined in Section 7.1.2. Briefly, exposures to the substances in this group were characterized for waterborne paints, wood sealant/stains, cosmetics, non-prescription drugs air fresheners, household cleaning products, and painted toys. There are also potential infrequent exposures from other products, such as adhesives, batteries and textiles (Environment Canada 2001, 2009, 2014; see Table 5-1).
6. Potential to cause ecological harm
6.1 Characterization of ecological risk
The ecological risks of the seven substances in the Ethylene Glycol Ethers Group were characterized using the ecological risk classification of organic substances (ERC) approach (ECCC 2016a). The ERC is a risk-based approach that considers multiple metrics for both hazard and exposure based on weighted consideration of multiple lines of evidence for determining risk classification. The various lines of evidence are combined to discriminate between substances of lower or higher potency and lower or higher potential for exposure in various media. This approach reduces the overall uncertainty with risk characterization compared to an approach that relies on a single metric in a single medium (e.g., LC50) for characterization. The following summarizes the approach, which is described in detail in ECCC (2016a).
Data on physical-chemical properties, fate (chemical half-lives in various media and biota, partition coefficients, fish bioconcentration), acute fish ecotoxicity, and chemical import or manufacture volume in Canada were either collected from scientific literature, from available empirical databases (e.g., OECD QSAR Toolbox) and from responses to surveys under section 71 of CEPA or were generated using selected quantitative structure-activity relationship (QSAR) or mass-balance fate and bioaccumulation models. These data were used as inputs to other mass-balance models or to complete the substance hazard and exposure profiles.
Hazard profiles based principally on metrics regarding mode of toxic action, chemical reactivity, food web-derived internal toxicity thresholds, bioavailability, and chemical and biological activity were established. Exposure profiles were also composed of multiple metrics, including potential emission rate, overall persistence, and long-range transport potential. Hazard and exposure profiles were compared to decision criteria in order to classify the hazard and exposure potentials for each organic substance as low, moderate or high. Additional rules were applied (e.g., classification consistency, margin of exposure) to refine the preliminary classifications of hazard or exposure.
A risk matrix was used to assign a low, moderate or high classification of potential risk for each substance based on its hazard and exposure classifications. ERC classifications of potential risk were verified using a two-step approach. The first step adjusted the risk classification outcomes from moderate or high to low for substances that had a low estimated rate of emission to water after wastewater treatment, representing a low potential for exposure. The second step reviewed low risk potential classification outcomes using relatively conservative, local-scale (i.e., in the area immediately surrounding a point-source of discharge) risk scenarios, designed to be protective of the environment, to determine whether the classification of potential risk should be increased.
ERC uses a weighted approach to minimize the potential for both over- and under-classification of hazard and exposure and subsequent risk. The balanced approaches for dealing with uncertainties are described in greater detail in ECCC (2016a). The following describes two of the more substantial areas of uncertainty. Error with empirical or modeled acute toxicity values could result in changes in classification of hazard, particularly metrics relying on tissue residue values (i.e., mode of toxic action), many of which are predicted values from QSAR models. However, the impact of this error is mitigated by the fact that overestimation of median lethality will result in a conservative (protective) tissue residue used for critical body residue analysis. Error with underestimation of acute toxicity will be mitigated through the use of other hazard metrics, such as structural profiling of mode of action, reactivity and/or estrogen binding affinity. Changes or errors in chemical quantity could result in differences in classification of exposure as the exposure and risk classifications are highly sensitive to emission rate and use quantity. The ERC classifications thus reflect exposure and risk in Canada based on what is believed to be the current use quantity and may not reflect future trends.
Critical data and considerations used to develop the substance-specific profiles for the seven substances in the Ethylene Glycol Ethers Group and the hazard, exposure and risk classification results are presented in ECCC (2016b).
The hazard and exposure classifications of the seven substances in the Ethylene Glycol Ethers Group are summarized in Table 6-1.
| Substance (CAS RN) |
ERC hazard classification | ERC exposure classification | ERC risk classification |
|---|---|---|---|
| DEG (111-46-6) | low | low | low |
| TEG (112-27-6) | low | low | low |
| TTEG (112-60-7) | low | low | low |
| DEGEE (111-90-0) | low | low | low |
| EGBEA (112-07-2) | low | low | low |
| DEGBE (112-34-5) | low | low | low |
| [EGDME] (110-71-4) | low | low | low |
Based on low hazard and low exposure classifications according to ERC for the seven substances in the Ethylene Glycol Ethers Group, these substances were classified as having a low potential for ecological risk. It is therefore unlikely that these substances result in concerns for the environment in Canada.
7. Potential to cause harm to human health
7.1 Exposure assessment
7.1.1 Environment media and food
Measured concentrations of the seven substances in this group in ambient air, water, and food were not identified in Canada.ootnote 10
Two substances, DEGBE and EGBEA, were reported as released to air in Canada at a rate of 20 389 and 10 876 tons/year, respectively, in 2013 (NPRI 1993-2013). Based on this information and using commercial quantities reported in Canada of all six substances in the EG and EGE subgroups and monoglyme, concentrations were modelled (using ChemCAN version 6.0) in environmental matrices after emission into air. Using these modelled estimates for each substance, drinking water intakes were derived, which resulted in nanogram-level or minimal exposures (less than 1 to 45 ng/kg bw/day, depending on the substance) (Health Canada 2016a).
In Canada, all seven substances in this group are used in food packaging. However, only DEG and EGBEA could potentially be in contact with food (personal communication, emails from the HPFB, Health Canada, to the RMB, Health Canada, dated October 2015; unreferenced). Probable exposure was estimated to be 0.3 and 0.03 μg/kg bw/day for DEG and EGBEA, respectively.
DEGEE was surveyed in a comprehensive indoor air study conducted in 18 cities and towns across Canada (2009 to 2011). It was detected in 3% of the 3857 residences at a geometric mean of 0.54 μg/m3 with a range of 0.4 to 6.93 μg/m3 (Zhu et al. 2013 a, b). Monoglyme was reported in indoor air in Germany at a maximum concentration of 13 μg/m3 (95th percentile: 0.5 μg/m3) based on 12 of 500 measurements (ECHA 2012b).Footnote 11
DEG has been reported to be present in soil from hazardous waste sites or facilities in the US; however, the value reported was not relevant to this assessment (ASTDR 2013).
7.1.2 Products available to consumers
Dermal absorption
DEG dermally applied to rat skin in vivo was rapidly absorbed, and 15 minutes after application, approximately 50% of the dose, based on radioactivity, was located in the skin.Footnote 12 At each timepoint (15 min, 8 and 24 h), the skin site of each animal was wiped with Q-tips, the animals were sacrificed and then the skin site was removed and stored frozen before analysis. Eight and 24 hours after dermal application, radioactivity of 12 and 32%, respectively, was detected in rat urine, thus indicating dermal absorption and systemic exposure (DOW 1981). Based on these data, dermal absorption for TEG and TTEG was considered to be 50% (DOW 2016a).
For DEGEE, three well-conducted in vitro studies on dermal absorption through human skin tested a shampoo formulation containing 5 or 10% DEGEE, a hydro-alcoholic (leave-on) formulation containing 15% DEGEE, and emulsified (leave-on) formulations containing 2, 5 or 10% DEGEE. Total absorption was 21.6 and 17.5%, 51.5%, and 43.2 to 56%, respectively, for the three studies (SCCP 2006a). Based on these studies, dermal absorption was considered to be 50% for DEGEE.
For DEGBE, none of the available studies on dermal absorption in human or rat skin were suitable for derivation of a dermal absorption value. However, based on these same experiments and well-conducted dermal absorption studies with DEGEE and EGBE, SCCP (2006b) stated that "… it is unlikely that the dermal absorption is larger than 50%".
As shown in Section 7.2.2, once absorbed, EGBEA is rapidly hydrolysed in blood to EGBE and acetate, and all systemic effects observed with EGBEA are typically also observed with EGBE. Based on studies in rats, pigs and humans, a range of absorption values were observed, but in general, dermal absorption of liquid EGBE varied between 20 and 30% in rats, and EC (2006-2008a) stated that, "for the dermal route, extrapolation is likely to be equal or less than EGBE. It can be anticipated that dermal penetration of liquid EGBEA would be of about 30% and vapour EGBEA of about 39%". Thus, based on the available information for EGBE, a dermal absorption value of 30% was selected for EGBEA.
A summary of the dermal absorption values is presented in Table 7-1.
| Substance (CAS RN) |
Dermal absorption | Reference |
|---|---|---|
| DEG (111-46-6) | 50% | DOW 1981, 2016a |
| TEG (112-27-6) | 50%a | DOW 2016a |
| TTEG (112-60-7) | 50%a | DOW 2016a |
| DEGEE (111-90-0) | 50% | SCCP 2006a |
| EGBEA (112-07-2) | 30% | EC 2006-2008a |
| DEGBE(112-34-5) | 50% | SCCP 2006b |
a Using dermal absorption reported for DEG as a surrogate.
Cosmetics, natural health products, personal care products and over the counter drugsFootnote 13
Based on notifications to Health Canada, three substances in this group (TEG DEGEE, DEGBE) are in cosmetic products in CanadaFootnote 14 (personal communication, emails from the CPSD, Health Canada, to the ESRAB, Health Canada, dated September 2015; unreferenced). TEG and DEGEE are also present as non-medicinal ingredients in natural health products in Canada (LNHPD 2018).ootnote 15 Health Canada internal sources of information indicated that DEG, TEG and DEGEE are also used as non-medicinal ingredients in non-prescription drugs (personal communication, emails from the Health Products and Food Branch, Health Canada, to the Risk Management Bureau, Health Canada, dated October 2015; unreferenced).
Based on the information above, exposures to DEG, TEG, DEGEE and DEGBE from use of selected product types was characterized and scenarios presented in this section are sentinel exposure scenarios covering other product types such as Natural Health Products and personal care products. Dermal absorption values are presented in Table 7-1 and the estimates of dermal exposure for each substance are presented in Tables 7-2 and 7-3, respectively (see also Appendix A for the parameters used in the model developed for these scenarios).
| Substance (CAS RN) |
Product scenario | Concentration (% w/w) |
Estimated daily exposure (mg/kg bw/day) |
|---|---|---|---|
| DEG (111-46-6) |
Antibacterial shampoo | 3a | 0.027 (internal)b 0.054 (external)b |
| TEG (112-27-6) |
Body cream | less than or equal to 0.3c | less than or equal to 0.10 (internal) less than or equal to 0.20 (external) |
| DEGEE (111-90-0) |
Deodorant/ antiperspirant |
10-30c | 0.55-1.7 (internal) 1.1-3.4 (external) |
| DEGEE (111-90-0) |
Body cream | 1-3c | 0.34-1.0 (internal) 0.68-2.0 (external) |
| DEGBE (112-34-5) |
Facial makeup | 10-30c | 0.47-1.4 (internal) 0.94-2.8 (external) |
a Non-medicinal ingredient in antibacterial shampoo (personal communication, emails from the Health Products and Food Branch, Health Canada, to the Risk Management Bureau, Health Canada, dated October 2015; unreferenced).
b Estimates of external exposure are estimates of dermal deposition. Estimates of internal exposure are estimates of systemic exposure based on the dermal absorption values for each substance.
c Concentrations are based on notifications submitted under the Cosmetic Regulations to Health Canada (personal communication, emails from the CPSD, Health Canada, to the ESRAB, Health Canada, dated September 2015; unreferenced).
| Substance (CAS RN) |
Product scenario | Concentration (% w/w)a |
Estimated per application exposure (mg/kg bw) |
|---|---|---|---|
| DEGEE (111-90-0) |
Hair dye | 3-10b | 4.2-14.0 (external)c 2.1-7.0 (internal) |
| DEGBE (112-34-5) |
Hair dye | 1-3 | 1.4-4.2 (external) 0.71-2.1 (internal) |
a Concentrations are based on notifications submitted under the Cosmetic Regulations to Health Canada (personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated September 2015; unreferenced).
b 97.5% of products contain 3-10% DEGEE; only 2.5% contain greater than 10% (personal communication, emails from the CPSD, Health Canada, to the ESRAB, Health Canada, dated September 2015; unreferenced).
c Estimates of external exposure are estimates of dermal deposition. Estimates of internal exposure are estimates of systemic exposure based on the dermal absorption values for each substance.
Paint and coating products
In Canada, some of these substances are also used in paints and coatings (Environment Canada 2001; Environment Canada 2014). Globally, substances in this group may be present in a variety of paints and coatings, building products and do-it-yourself materials (Household Products Database 1993-2015; DOW 2007a, b; DOW 2014a).
Dermal and inhalation exposures to substances in this group from use of waterborne roller wall paints and floor finish/sealers were estimated using ConsExpo v4.1 (ConsExpo 2006) and are presented in Table 7-4.Footnote 16 Scenarios were modelled to simulate the painting of either a wall or floor and include inhalation and incidental dermal routes (internal dermal estimates were derived using dermal absorption values from Table 7-1). No data were available for TTEG concentrations in paints; therefore, the concentration of TEG in paint was used as a surrogate for TTEG, and exposures are therefore expected to be similar.
| Substance (CAS RN) |
Product scenarioa | Concentration (% w/w) |
Estimated dermal exposure per application (mg/kg bw)b |
Instantaneous mean event air concentration during use (mg/m3) |
|---|---|---|---|---|
| DEG (111-46-6) |
Water-borne roller wall paints | 1c | 0.254 internal; 0.51 external |
0.27 |
| TEG (112-27-6) |
Water-borne roller wall paints | 0.1-4%d | 1.02 Internal; 2.04 external |
0.25 |
| TTEG (112-60-7) |
Water-borne roller wall paints | 0.1-4%d | 1.02 Internal; 2.04 external |
0.25 |
| DEGEE (111-90-0) |
Floor sealant | 1-10e | 0.176 internal; 0.353 external |
20.1 |
| EGBEA (112-07-2) |
Wood sealant | 1-5f | 0.053 internal; 0.176 external |
58.4 |
| DEGBE (112-34-5) |
Water-borne roller wall paints | 2g | 0.51 internal; 1.02 external |
0.5 |
a All scenarios conducted using ConsExpo (Health Canada 2016a; see also Appendix A).
b Estimates of external exposure are estimates of dermal deposition. Estimates of internal exposure are estimates of systemic exposure based on the dermal absorption values for each substance.
c MSDS 2012c
d Industry data submitted under section 71 of CEPA indicated that TEG is present in concentrations between the range of 0.1 to 4%. No data was available for TTEG concentrations in paints;-however, industry submissions indicated a potential presence in paints and coatings. Therefore, the concentration of TEG in paint was used as a surrogate for TTEG and exposure is expected to be similar.
e MSDS 2012d.
f MSDS 2010d.
g MSDS 2015u.
The instantaneous mean event concentrations presented above may overestimate exposure as these substances have low to moderate vapour pressures (see Physical and Chemical Properties section).
Painted toys mouthed by children
In Canada, some of these substances may be present in children's toys and articles (Environment Canada 2001; Environment Canada 2014); internationally, substances in this group have also been reported in painted toys (Hansen and Pederson 2005).
The Danish Ministry of the Environment reported migration of certain substances in this group from painted wooden toys into simulated saliva (Hansen and Pederson 2005). They analyzed all six of the substances in the EGs and EGEs subgroups, and four of the six substances (TEG, EGBEA, DEG, TTEG) were detected at low concentrations in simulated saliva from fewer than five products. Therefore, exposure to these four substances via this route is not expected to be significant.
Two of the substances (DEGEE and DEGBE) were detected in simulated saliva from four or more products (n=15) at moderate to high concentrations, and potential exposures to these substances via mouthing were estimated. The Danish Ministry of the Environment assessment of oral exposure from mouthing of toys was adopted for this assessment and the highest reported oral uptakes for both substances are reported in Table 7-5.Footnote 17
| Substance (CAS RN) |
Migration rate (μg/10 cm2/h |
Highest oral uptake reported (μg/kg bw/day)a |
Reference |
|---|---|---|---|
| DEGEE (111-90-0) |
12.6 | 18.9 | Hansen and Pederson 2005 |
| DEGBE (112-34-5) |
55.7 | 16.7 | Hansen and Pederson 2005 |
a Absorption rate was assumed to be 100%, exposure for 3 hours, 10 kg body weight.
Air fresheners
Spray-operated air fresheners containing concentrations of up to 1% DEG, up to 10% TEG and up to 30% DEGEE are sold in Canada (MSDS 2009b, 2014d, 2014e, Environment Canada 2009). Additionally, monoglyme may be found as a potential impurity (at concentrations < 0.01%, Environment Canada 2009) of EGEs used in air fresheners. However, it is not thought to be present in a widespread portion of products available to consumers.
Twenty-four hour mean event air concentrations from use of air fresheners containing DEGEE, TEG and monoglyme were estimated using ConsExpo v4.1 (ConsExpo 2006)Footnote 18 and are presented in Table 7-6 below. In addition, for monoglyme, a shorter duration mean event concentration (6 hours) matching the daily exposure duration of the critical study for risk characterization was also estimated.
| Substance (CAS RN) |
Concentration (% w/w) |
24-h (6-h) air concentrations |
|---|---|---|
| DEG (111-46-6) |
1 | 0.187 mg/m3 |
| TEG (112-27-6) |
10 | 1.87 mg/m3 |
| DEGEE (111-90-0) |
30 | 5.62 mg/m3 |
| Monoglyme (110-71-4) |
0.01 | 0.0019 mg/m3 (0.0073 mg/m3) |
Household cleaning products
In Canada, a majority of the EGEs have also been reported to be used in cleaning and disinfection products in the home and in professional settings such as hospitals and kitchens (Environment Canada 2001; Environment Canada 2014; personal communication, emails from the Health Products and Food Branch, Health Canada, to the Risk Management Bureau, Health Canada, dated October 2015; unreferenced). Globally, substances in this group may also be present in cleaning products including dish soaps, all purpose cleaners, and oven cleaners (Household Products Database 1993-2015).
Bathroom or all-purpose spray window cleaners containing concentrations of DEG, DEGEE, EGBEA, and DEGBE may be available in Canada (personal communication, emails from the Health Products and Food Branch, Health Canada, to the Risk Management Bureau, Health Canada, dated October 2015; unreferenced, MSDS 2013f; MSDS 2015v; OECD 2007). Twenty-four hour mean event concentrations and dermal exposure estimates (internal dermal estimates were derived using dermal absorption values from Table 7-1) were estimated using ConsExpo v4.1 (ConsExpo 2006) and are presented in Table 7-7 (see also Appendix A for the parameters used in ConsExpo v4.1).
| Substance (CAS RN) |
Product | Concentration (% w/w) |
Estimated dermal exposure (mg/kg bw/event; mg/kg bw/day) |
24-h mean event concentrations (mg/m3) |
|---|---|---|---|---|
| DEG (111-46-6) |
Bathroom cleaner | 4a | 0.10 internal; 0.20 external |
2.92 |
| DEGEE (111-90-0) |
Window Cleaner | 8b | 0.19 internal; 0.38 external |
3.14 |
| EGBEA (112-07-2) |
Window Cleaner | 5c | 0.070 internal; 0.23 external |
1.96 |
| DEGBE (112-34-5) |
Bathroom Cleaner | 10d | 0.26 internal; 0.52 external |
7.31 |
a Personal communication, emails from the Health Products and Food Branch, Health Canada, to the Risk Management Bureau, Health Canada, dated October 2015; unreferenced.
b OECD (2007: p. 61).
c MSDS (2015v).
d MSDS (2013f).
The 24-h mean event concentrations are comparable to reported peak air (10.8 mg/m3) and breathing zone (5.4 mg/m3) concentrations from the use of a bathroom spray cleaner containing DEGBE, measured by HERA (2005). However, the modelled concentrations outlined above are estimated over 24 hours (during and post use) and highlight the conservatism of this model for these specific set of substances.
Other products
Exposures from products such as adhesives, batteries, and textiles were not assessed, as the sentinel products presented above (cosmetics and non-perscription drugs, paints and coatings, air fresheners, household cleaning products) are expected to account for inhalation and dermal exposures from other less frequently used products.
7.2 Health effects assessment
7.2.1 Health effects assessment of the ethylene glycols subgroup
The majority of the health effects information for this subgroup is based on the Organisation for Economic Co-operation and Development (OECD) Screening Information Data Set (SIDS) Initial Assessment Report for the ethylene glycol category and the specific glycols: diethylene glycol (DEG), triethylene glycol (TEG) and tetraethylene glycol (TTEG) (OECD 2009a, 2009b, 2009c, 2009d). The scientific literature for these documents was updated by the OECD sponsor country (Canada). More recent information, based on a search of published literature conducted from January 2003 to September 2015, identified new health effects studies and resulted in the use in this screening assessment of different critical endpoints than those stated in OECD (2009a).
The EGs subgroup includes di-, tri-, and tetra-ethylene glycols, and this section provides critical endpoints and corresponding effect levels for DEG, TEG and TTEG to be used for risk characterization, as cited directly from the OECD (2009a, 2009b, 2009c, 2009d) and more recent publications, if applicable.
Diethylene glycol (DEG)
The toxicokinetics of DEG was summarized in OECD (2009b) as follows: "Based on studies in rats and/or dogs, the main metabolic pathway is oxidation via alcohol dehydrogenases and aldehyde dehydrogenases (ADH/ALD). DEG and its metabolites do not significantly accumulate in tissues. DEG is primarily eliminated in urine as DEG and 2-(hydroxyethoxy)acetic acid [HEAA] and is also eliminated as exhaled CO2. DEG is readily absorbed by the oral route, but dermal absorption is limited (9% based on a dose of 200 mg/kg bw in rats)".Footnote 19 Although not stated in OECD (2009a, 2009b), in another study conducted by DOW (1981), dermal absorption in rats was higher than 9% at a lower applied dose within 24-h post-dosing. These data are described in the "Exposure Assessment" section.
For DEG, a short-term oral study was conducted in rats via the oral route. Test animals were exposed to 0, 11, 46, 180 or 850 mg/kg bw/day in feed for 32 days. The no- observed adverse effect level (NOAEL) was 850 mg/kg bw/day. Although this value is listed as a low observed effect level (LOEL) in OECD (2009b), new information confirms that the NOAEL should be established at 850 mg/kg bw/day.Footnote 20 A short-term inhalation study was also conducted in rats exposed to 0, 530, 3000 or 5000 mg/m3 DEG, for 6 h/day for 9 days. The no-observed effect concentration (NOEC) and lowest-observed effect concentration (LOEC) were 3000 and 5000 mg/m3, respectively, based on minor changes in hematological and clinical chemistry parameters (OECD 2009a, 2009b). No short-term dermal studies were identified for DEG.
Two chronic repeated-dose oral studies were conducted in rats. In one study, animals were exposed to 0, 1200, or 2300 mg/kg bw/day DEG in feed for 3 to 24 months. An oral LOAEL of 2300 mg/kg bw/day was determined based on observation of bladder stones in males at 24 months; the NOAEL was 1200 mg/kg bw/day. In the other study, in contrast, no effects were observed in rats exposed to 25 000 ppm (equivalent to 3500 mg/kg bw/day) in drinking water for 2 years (OECD 2009b).
Short-term toxicity has also been observed in humans based on a case-control study of 63 children aged one month to 13 years who had been exposed to acetaminophen syrup contaminated with DEG. These children showed idiopathic anuria and severe oliguria for 24 h or more, with other effects sometimes noted (hepatitis, pancreatitis, and severe neurological manifestations) with a median of 6 days from first dose to onset. The mean number of medications (doses) was 4 to 5 in renal failure patients and 2 in controls. Based on 32 affected children and 17 control children with no renal failure, for which maximum ingested doses of DEG could be estimated, a median dose of 1500 mg/kg bw (range 246-4942 mg/kg bw) was determined as the probable effect level for renal failure in children aged one month to 13 years. The estimated ingestion of DEG in 17 control children (no renal failure) was 56 to 2773 mg/kg bw. Note that there is a large overlap in estimated toxic and non-toxic doses of DEG in children (O'Brien et al. 1998; OECD 2009b). Due to this large overlap, this study is not considered to be of utility in derivation of points of departure for characterization of risk for DEG.
Further details of the studies described above and other study summaries (oral 13- and 32-week studies in rats, oral continuous breeding study conducted in mice and oral developmental toxicity studies in rats, mice and rabbits, other human studies) are provided in the supporting document (Health Canada 2017).
Triethylene glycol (TEG)
The toxicokinetics of TEG was summarized in OECD (2009a, 2009c). Based on studies in rats and/or rabbits, the main TEG metabolic pathway is oxidation via ADH/ALD. TEG was primarily eliminated in urine as TEG and ethylenedioxydiacetic acid, and only 1% was degraded and eliminated as exhaled CO2 (OECD 2009c; DOW 2016a). OECD (2009a, 2009c) did not provide a dermal absorption value for TEG. However, based on unpublished data for DEG submitted to Health Canada, TEG dermal absorption was considered to be 50% (see Section 7.1.2).
In a short-term oral study conducted in rats, animals were exposed to 0, 1132–1177, 2311–2411, or 5916–6209 mg/kg bw/day of TEG in feed for 14 days. The NOAEL was 5916 mg/kg bw/day. Although changes in urinary parameters (increased urine volume, decreased urinary pH and phosphite crystals) were observed at the high dose, they were not considered adverse (Ballantyne and Snellings 2007). No short-term dermal studies were identified for TEG. Two 9-day inhalation studies were conducted in rats, and an adverse effect level was determined in one of them. In this study, animals were exposed nose-only to 0, 102, 517 or 1036 mg/m3 for 6 h/day for 9 days. The no-observed adverse effect concentration (NOAEC) was 1036 mg/m3, based on a lack of significant effects at all concentrations (Ballantyne et al. 2006; OECD 2009a, 2009c).
A subchronic repeated-dose oral study was conducted in rats exposed to 0, 748–848, 1522–1699 or 3849–4360 mg/kg bw/day TEG in diet for 13 weeks. An oral NOAEL and LOAEL of 1699 and 3489 mg/kg bw/day, respectively, were determined based on decreased body weights in both sexes and decreased body-weight gain in females (Ballantyne and Snellings 2007; OECD 2009c). In two inhalation studies conducted in monkeys for periods up to 13 months, no adverse effects were observed at concentrations up to 6.14 mg/m3 (exposure duration/day not stated) (Ballantyne and Snellings 2007; US EPA 2005).
Summaries of chronic repeated-dose oral studies conducted in rats and monkeys, an oral continuous breeding study conducted in mice, and oral developmental toxicity studies using TEG in rats and mice are provided in the supporting document (Health Canada 2017).
In humans, no literature documenting cases of oral ingestion of pure TEG were identified. For the few reported cases of ingestion, any interpretation of effects was complicated by the presence of other substances (Ballantyne and Snellings 2007).
Tetraethylene glycol (TTEG)
In vivo toxicokinetic studies using TTEG were not identified. As stated in OECD (2009a, 2009d), TTEG may be assumed to be metabolized predominantly by ADH/ALD in vivo. Although OECD (2009a, 2009c) did not provide a dermal absorption value for TTEG, based on unpublished dermal data for DEG submitted to Health Canada, dermal absorption for TTEG was considered to be 50% (See Section 7.1.2).
For TTEG, three short-term oral (one 14-day and two 4-week) studies were conducted in rats. Treatment-related effects were observed in only one of the three studies; increased volume and specific gravity of the urine in both sexes and decreased urinary pH in females at a dose of 4500 mg/kg bw/day in one of the two 4-week studies. The NOAEL in this 28-32 day gavage study was 3380 mg/kg bw/day (Healing et al. 2016).
A subchronic repeated-dose dermal study was conducted in rats, in which animals were exposed to 0 or approximately 3360 mg/kg bw/day, for 6 h/day, 5 days/week for 13 weeks. This study was conducted according to OECD and U.S. Environmental Protection Agency (US EPA) guidelines and a wide range of parameters were examined. The NOAEL was 3360 mg/kg bw/day (OECD 2009d).
Though the available genotoxicity studies for DEG and TEG showed negative results, similar tests (in vitro chromosome aberration and sister chromatid exchange assays, in vivo assays for chromosome levels) conducted with TTEG have shown positive or equivocal results. However, based on the overall genotoxicity database and quantitative structure-activity relationship (QSAR) investigation of genotoxicity for TTEG, as well as two-year rat and/or mouse studies using DEG or ethylene glycol (EG), OECD (2009a) concluded that the members of the EG category [EG, DEG, TEG, TTEG, and pentaEG] were not regarded as carcinogens.
Although no standard reproductive toxicity studies or any developmental toxicity studies were identified for TTEG, OECD (2009a) concluded that TTEG showed a low potential for reproductive toxicity based on lack of histopathological changes in the testes and epididymides of rats administered TTEG for either 14 days or 4 weeks.
Read-across/analogue hazard data for risk characterization
As noted above, because there was a lack of dermal absorption data for TEG and TTEG, DEG dermal absorption data (50%) was used to estimate dermal absorption for TEG and TTEG. There was also no inhalation toxicity data for TTEG. However, since these substances were evaluated as a group by the OECD (2009a), the inhalation data for TEG was considered as an appropriate analogue for TTEG. As stated in OECD (2009a), the data for five EG substances show that as the molecular weight increases above that of DEG in the homologous series, the potential for systemic, reproductive, and developmental toxicity decreases. This pattern is consistent with a likely decrease in absorption with increasing molecular weight, although available data for direct comparison were limited and inconclusive.
Although the only repeated-dose dermal study identified was a 13-week TTEG dermal study in rats, TTEG was not considered validfor read-acrossfor dermal repeated dosing for DEG and TEG, as the rabbit dermal LD50 increases from 12 500 mg/kg bw for DEG to 22 600 mg/kg bw for TTEG, and the oral toxicity database shows that the severity of toxicological effects decreases from DEG to TEG to TTEG at similar dose levels.Footnote 21 Thus, substance-specific oral studies with critical effect levels (LOAEL or NOAEL) for the substance in question (DEG or TEG) will be used as surrogates for dermal toxicity in risk characterization of these substances.
In contrast, although acute and repeated-dose toxicity shows decreasing severity of toxicological effects from DEG to TEG to TTEG, this trend is not apparent for developmental toxicity, as shown in the supporting document (Health Canada 2017).
7.2.2 Health effects assessment of the ethylene glycol ethers subgroup
Different international organizations (e.g., OECD and the European Chemicals Agency [ECHA]) analyzed and summarized the health effects information and characterized hazard for the Ethylene Glycol Ethers subgroup. Therefore, these documents are used to inform the health effects assessment for the respective substances, including selection of effect levels for critical endpoints and NOAELs/NOAECs.
Substance-specific health effects data for risk characterization
This subgroup includes one monoethylene glycol acetate and two diethylene glycol ethers, and this section provides critical endpoints and corresponding effect levels for DEGEE, EGBEA and DEGBE, to be used for risk characterization.
Diethylene glycol monoethyl ether (DEGEE)
OECD (2007) and SCCP (2006a) summarize the health effects literature related to DEGEE. A literature search was conducted from the year prior to the OECD SIAMFootnote 22 (i.e., October 2004 to April 2016), and no health effects studies that would result in critical effect levels lower than those identified by OECD (2007) were identified.
Available animal metabolism studies for members of the diethylene glycol ether substances indicate that the principal route of elimination is via urine. Only low or trace amounts of metabolites are found in expired air or faeces. The two major urinary metabolites for DEGEE, ethoxyethoxyacetic acid and DEG, are excreted within the first 24 hours. Based on limited data, similar metabolism of DEGEE is expected in humans (OECD 2007).
Available in vitro and in vivo data suggest that DEGEE is not considered to be genotoxic. Two carcinogenicity studies for DEGEE reported no increases in tumoral responses; however, these studies had limitations (see Health Canada 2017). The oral bioavailability of DEGEE in experimental animals is reported to be 79–95% (DOW 2016b).
DEGEE is of low inhalation acute toxicity given that at the maximum attainable vapour concentration (560 mg/m3), no rats died when exposed for 7 hours (OECD 2007).
In an oral short-term study, rats were exposed to 0, 1340, 2680 or 5360 mg/kg bw/day of DEGEE by gavage for 5 days/week for 6 weeks. Mortality was observed at the highest dose along with presence of bloody urine. At 2680 mg/kg bw/day, no significant effects were observed; however, relative liver, heart, and kidney weights (but not absolute weights of these organs) were also increased with respect to controls. Pathological changes included hyperkeratosis of the stomach and splenic congestion. The NOAEL for this study is 1340 mg/kg bw/day (Krasavage and Vlaovic 1982 as cited in OECD 2007).
In a 90-day oral study, pigs were administered 0, 167, 500 or 1500 mg/kg bw/day in the dietFootnote23. A wide variety of parameters, including body and organ weights, hematological and urinary parameters, and tissue histopathology, were examined. Hydropic degeneration of liver and kidney proximal tubules were observed in one of two females treated with 500 mg/kg bw/day and all animals treated with 1000-1500 mg/kg bw/day. The relative weight of kidneys was increased and red blood cell counts were also reduced in males treated with 1500 mg/kg bw/day. The NOAEL was determined to be 167 mg/kg bw/day (Gaunt et al. 1968 as cited in OECD 2007).
In a 28-day dermal study, rabbits were administered 0, 100, 300 or 1000 mg/kg bw/day of DEGEE. There were no treatment-related signs of gross toxicity, adverse health effects or abnormal behaviour. The NOEL for systemic toxicity is considered to be 1000 mg/kg bw/day (Gattefosse 1994 as cited in SCCP 2006a).
With respect to sub-chronic exposure, rabbits were dermally administered DEGEE (0, 99, 296, 986 or 2957 mg/kg bw/day) for 5 days/week for 12 weeks. Increased blood urea nitrogen levels and severe kidney injury were reported at 2957 mg/kg bw/day in 1 of 4 surviving animals. Moderate kidney changes in 3 of 4 surviving animals were observed at the 986 mg/kg bw/day dose level. Based on these effects, the NOAEL was determined to be 296 mg/kg bw/day. However, this study had low numbers of animals and limited information on specific effects and may therefore be of low reliability (Dow Chemical Company 1950, as cited in OECD 2007).
For DEGEE, a chronic oral drinking water study was conducted in rats over three generations (two groups each of 8/sex/dose: DEGEE with 29.5% ethylene glycol or DEGEE with less than 0.2% ethylene glycol - high purity). Groups were administered the equivalent of 0, 10, 40, 200 or 950 mg/kg bw/day of high or low purity DEGEE for up to 718 days. Only livers and kidneys were examined microscopically in all animals. Kidney damage and bladder stones were observed in 2 of 28 animals administered 950 mg/kg bw/day of high purity DEGEE. The NOAEL for high purity DEGEE was 200 mg/kg bw/day. The NOAEL for the low purity DEGEE was 10 mg/kg bw/day based on 4, 11, and 39% of rats with pathological changes at 40, 200 and 950 mg/kg bw/day, respectively. Although effects were not specified at 40 mg/kg bw/day, in secondary sources, kidney damage, bladder stones and cloudy swelling in the livers were observed at 200 and 950 mg/kg bw/day (Smyth et al. 1944 as cited in SCCP 2006a; Smyth et al. 1944 as cited OECD 2007). No chronic or sub-chronic inhalation studies were identified for DEGEE.
Summaries of an oral mouse two-generation reproduction study and limited developmental toxicity studies in rats and mice, as well as summaries of a 28-day inhalation study conducted in rats and daily exposure of mice, rabbits, cats and guinea pigs subjected to a saturated air concentration of DEGEE, are provided in the supporting document (Health Canada 2017). Repeated-dose studies and reproductive/developmental toxicity studies conducted using DEGEE (administered up to 2 years) show that the target organs (when effects were observed) were kidney and liver.Footnote 24 Additionally, these effects were also observed in multiple species through different routes of exposure and at similar doses (between 1000–2700 mg/kg bw/day), indicating that systemic effects occur predominantly at doses greater than 1000 mg/kg bw/day.
A recent epidemiological study was conducted on 204 mother-child pairs in France to examine the association between levels of glycol ether metabolites in mother's urine collected before 19 weeks of gestation and the neurocognitive abilities of their children 6 years after birth. Five different metabolites were measured in urine and for two potential metabolites of DEGEE, median values were 0.016 mg/L for ethoxyacetic acid [EAA] and 0.028 mg/L for ethoxyethoxyacetic acid [EEAA]. An association between mother's urinary levels and the scores for 6-year old children was identified for only one of the two potential metabolites of DEGEE (EAA) and this association was noted in only one of four different neurocognitive tests in 6 year old children (Béranger et al. 2017). Thus, the results are equivocal with respect to suggesting that DEGEE may affect neurocognitive abilities and have limited utility for risk characterization.
Ethylene glycol monobutyl ether acetate (EGBEA)
OECD (2006) and EC (2006-2008a) summarize the available health effects literature related to EGBEA, and a literature search conducted from the year prior to OECD (2006) (i.e., August 2005 to April 2016) did not identify new health effects information. Only limited data was available on inhalation toxicity and repeated-dose toxicity by the oral and dermal routes.
The inhalation LC50 for EGBEA was greater than 2660 mg/m3 in rats and rabbits (EC 2006-2008a). Repeated-dose inhalation studies showed signs of hematotoxicity and associated lesions in all species except guinea pigs at a concentration in air of 400 ppm = 2621 mg/m3 (only concentration tested). However, these studies have significant limitations and were not considered by the European Commission to be reliable for risk assessment (EC 2006-2008a).
Available evidence demonstrates that EGBEA is rapidly hydrolyzed to EGBE (CAS RN 111-76-2) by esterases present in mammalian blood and other tissues (Environment Canada and Health Canada 2002a; OECD 2006a).Footnote 25 Therefore, due to the limitations of the EGBEA studies and since EGBEA is rapidly hydrolyzed to EGBE, toxicity data for EGBE were used to characterize risk for EGBEA.
Read-across/analogue health effects data for risk characterization
EC (2006-2008b) summarized the available health effects literature related to EGBE. A literature search was conducted from the year prior to EC (2006-2008b) (i.e., August 2007 to April 2016), and no additional health effects studies that would result in lower critical effect levels were identified.
EGBE is classified as an International Agency for Research on Cancer (IARC) Group 3 substance (inadequate evidence of carcinogenicity in humans and limited evidence of carcinogenicity in animals) (IARC 2006). However, "As humans are less sensitive than rodents to this toxicity, EGBE is not likely to represent a carcinogenic hazard under conditions of normal handling and use." (OECD 2006b). In vivo and most in vitro genotoxicity assays suggest that EGBE is not considered to be genotoxic.
Two studies were available to assess the short-term dermal toxicity of EGBE. In a 9-day study, signs of toxicity were recorded and were limited to transient signs of hemolysis in rabbits after a 9-day exposure to EGBE at 900 mg/kg bw/day (doses of 0, 45, 225, 450 or 900 mg/kg bw/day). This study NOAEL of 450 mg/kg bw/day was based on hematological effects (Bushy Run Research Center 1980 as cited in EC 2006-2008b). To assess immune system effects, EGBE was administered dermally to mice at 0, 100, 500, 1000 or 1500 mg/kg bw/day for 4 days. At the 1500 mg/kg bw/day dose, an increase in relative spleen weight and in splenic cellularity were observed. No decreases were observed in the proliferation of B cells whereas T lymphocytes exhibited a statistically significant decrease of proliferative responses at doses of 500 and 1000 mg/kg bw/day. In the mixed lymphocyte response assay, natural killer cells, cytotoxic T lymphocyte activity and T-dependent plaque-forming cell responses were unchanged after treatment. A NOAEL of 1000 mg/kg bw/day based on relative spleen weight increases at 1500 mg/kg bw/day was determined (Singh et al. 2001 as cited in EC 2006-2008b).
In a 13-week study, rabbits were treated dermally with 0, 10, 50 or 150 mg/kg bw/day, 5 days/week for 13 weeks. No effects were observed up to the highest dose tested of 150 mg/kg bw/day (Wil Research laboratories Inc. 1983 as cited in EC 2006-2008b).
In EGBE repeated-dose inhalation studies (1–3 week, 14-week, and 2-year studies in rats and a 2-year study in mice), the principal effect consistently observed was hemolysis, which was occasionally associated with secondary hepatic effects (Kupffer cells pigmentation and absolute and relative liver weight increases) (see Health Canada 2017).
In an oral gavage developmental toxicity study, pregnant female rats were dosed during gestation days (GD) 9 to 11 with 0, 30, 100 or 200 mg/kg bw/day of EGBE or during GD 11 to 13 with 0, 30, 100 or 300 mg/kg bw/day of EGBE. The maternal NOAEL and LOAEL were 30 and 100 mg/kg bw/day, respectively, based on decreased body weight and/or weight gain, increased kidney and spleen weights, and severe hematotoxicity typical of hemolytic anaemia. As stated in EC (2006-2008b), a developmental NOAEL and LOAEL of 100 and 200 mg/kg bw/day, respectively, were determined based on increased fetal lethality without malformations. However, these effects were observed only in the presence of maternal toxicity.
Another oral developmental study reported reproductive effects (decreased number of fertile females, decrease in litters/fertile pair) and maternal effects (increased female deaths, decreased body weights, etc.) at doses higher than reported above (EC 2006-2008b).
Although the lowest effect levels for EGBE administration induce hemolytic toxicity in rodents, mechanistic and mode of action studies have shown that EGBE's metabolite, 2-butoxyacetic acid (BAA), is responsible for the hematoxicity in rodent species and that humans are 30 times less sensitive than rats to this hemotoxic effect (EC 2006-2008b). Complex mode of action analyses have also shown that human red blood cells are 40 to 150 less sensitive to hematoxicity than rat blood cells and that under different susceptibility conditions (physiological or blood diseases), humans are still resistant to the hemolytic toxicity of BAA (US EPA 2010). This is evident, as following high oral doses of EGBE (up to 4500 mg/kg bw/day) in humans, the main toxic effect was metabolic acidosis and central nervous system depression, accompanied with hematotoxicity (hematuria) in some cases (based on case reports in adult humans ingesting products containing 10-35% EGBE). The lowest acute dose leading to metabolic acidosis in humans was 400 mg/kg bw (LOAEL) (EC 2006-2008b).
Further details of the studies described above and other study summaries (inhalation developmental toxicity studies in rats and rabbits) are provided in the supporting document (Health Canada 2017).
Diethylene glycol monobutyl ether (DEGBE)
EC (2000) summarizes the health effects literature related to DEGBE. A literature search was conducted from the year prior to finalization of the EC (2000) (i.e., July 1998 to April 2016). One study potentially relevant to the risk characterization was identified, and information on this study is provided below.
DEGBE is of low inhalation acute toxicity given that at the maximum attainable vapour concentration (120 mg/m3DEGBE), no rats died when exposed for 7 hours.
Available in vitro and in vivo data suggest that DEGBE is not considered to be mutagenic or genotoxic (see Health Canada 2017).
The literature search identified a 2005 subchronic study in which DEGBE was administered to rats for 13 weeks at doses of 0, 50, 250 or 1000 mg/kg bw/day in drinking water (see Table 7-8 for details).
| Endpoint | Study details | Critical effect level and health effect endpoint | Citation |
|---|---|---|---|
| Repeated-exposure; subchronic toxicity (13 weeks) |
Species: Rat (10/sex/dose) Route: Oral (drinking water) Concentration and Duration: 50, 250 or 1000 mg/kg bw/day for 13 weeks |
NOAEL = 250 mg/kg bw/day LOAEL = 1000 mg/kg bw/day Changes in hematology and clinical chemistry parameters. Changes in organ weights of liver and kidney. Histopathological lesions in liver of females. |
Johnson et al. 2005 |
EC (2000) reported one 6-week oral gavage study and one 13-week oral gavage study in rats, but a NOAEL was not determined in either study. The 6-week study utilized high doses (891 to 3564 mg/kg bw/day), and although the 13-week study utilized lower doses (51 to 1630 mg/kg bw/day), it was considered of limited quality because the effects observed were not consistent with the known toxicity profile of glycol ethers (e.g., hyperkeratosis of the stomach, high unclarified mortality at the doses of 254 to 1630 mg/kg bw/day). Thus, the study by Johnson et al. (2005) described above (Table 7-8) is used in the risk characterization of oral exposures.
In a 13-week dermal study, rats were exposed to 0, 200, 600 or 2000 mg/kg bw/day of DEGBE for 5 days/week, 6 h/day (occluded). No mortality or effects on body weight, organ weights, pathology or clinical chemistry were observed at any of the doses tested, including no effects observed based on analysis of neurological parameters DEGBE caused local effects, such as erythema, in all groups, and severity and time of onset were concentration dependent. The NOAEL for systemic effects was determined to be 2000 mg/kg bw/day (Auletta et al. 1993 and Beyrouty et al. 1993 as cited in EC 2000).
DEGBE is not considered harmful by inhalation, possibly due in part to its low vapour pressure and volatility (Patty 1994 as cited in EC 2000). This is confirmed by 2-week, 5-week, and 90-day inhalation studies conducted in rats, in which effects were observed at vapour concentrations ≥ 100 mg/m3 (effects on the lungs, spleen and liver observed in the 2- and 5-week studies; in the 90-day study, the NOAEC was established at 94 mg/m3, the highest concentration tested) (see Health Canada 2017).
In a dermal developmental study, pregnant female rabbits were administered 0, 100, 300 or 1000 mg/kg bw/day DEGBE, 4 hours a day, from gestation days (GD) 7 to 18 (12 days, non-occluded), and were sacrificed on GD 29. Maternal body weight gain was slightly decreased at the two higher dose levels but not statistically significant. The NOAEL for systemic toxicity is 1000 mg/kg bw/day (Nolen et al. 1985; EC 2000).
In a one-generation dermal reproduction study conducted in rats, no reproductive or systemic toxicity was observed at the only dose tested, 2000 mg/kg bw/day (EC 2000).
A 30-day oral study and an oral one-generation reproduction study in rats, as well as an oral developmental toxicity study in mice are summarized in the supporting document (Health Canada 2017).
In the oral rat repeated-dose studies mentioned above, DEGBE showed hemolytic toxicity at doses greater than or equal to 250 mg/kg bw/day. In vitro studies of putative metabolites of DEGBE have shown that human red blood cells are much less sensitive to hematoxicity than rat blood cells (SCCP 2006b; OECD 2007). Humans are considered to be more resistant than rats to the hemolytic toxicity of DEGBE's metabolites (2-butoxyacetic acid, butoxyethoxyacetic acid) based on interpretation of the proposed metabolic pathway described in SCCP (2006b).
7.2.3 Health effects assessment of monoglyme
Monoglyme is classified under Europe's harmonised classification and labelling system as a substance that may damage fertility and may damage the unborn child (Repr. 1B: H360FD). Under the European REACH program, it is classified as a substance that may damage fertility or the unborn child and is suspected of causing cancer (European Union 2016).
ECHA (2012b) assessed monoglyme and selected the developmental and reproductive toxicity studies as the most robust and sensitive studies for dose-response analysis. NICNAS (2016a) was also used as supplementary information for monoglyme. Monoglyme metabolizes to 2-methoxyethanol (2-ME) and then to 2-methyoxyacetic acid, and the toxicity of monoglyme is attributed to these two metabolites (ECHA 2012b; NICNAS 2016a). No toxicokinetic studies were identified for monoglyme, but based on NICNAS (2016a) and ECHA (2012b), monoglyme and diglyme (CAS No. 111-96-6), of which the toxicokinetics have been studied, are considered to be metabolized by the same enzymatic metabolic pathway and demonstrated similarity of metabolic products such as 2-ME and 2-methoxyacetic acid, with only 2% of the administered dose remaining in the carcass of mice. 2-ME was previously assessed by Environment Canada and Health Canada (2002b), and relevant data are discussed under "Read-across/analogue hazard data for risk characterization".
The potential health effects associated with monoglyme are based on the results of repeated-dose (effects on blood, thymus, adrenal gland, and testicular degeneration) and developmental (increased fetal death and skeletal effects and decreased fetal body weight) toxicity studies at relatively low doses. Available in vitro data also suggest that monoglyme is not genotoxic; however, no in vivo genotoxicity data were available (NICNAS 2016a).
Monoglyme has low acute oral and dermal toxicity (LD50 greater than 2000 mg/kg bw in rabbits) and has low to moderate acute toxicity via the inhalation route (LC50 between 2000 and 6300 mg/m3). No repeated-dose toxicity data were identified via the oral or dermal route for monoglyme.
In a repeated-dose inhalation study, rats were exposed by whole-body inhalation to monoglyme vapours for 6 h/day, 5 days/week for 2 weeks. The NOAEC and LOAEC for this study were 50 and 250 ppm (187 and 935 mg/m3), respectively, based on reversible reduction of cell layers of seminiferous epithelium in male rats. In contrast, in a similar study using rabbits exposed by whole-body inhalation, irreversible changes to the seminiferous epithelium of the testes occurred at 935 mg/m3 (ECHA 1986a, 1986b, 2012a, 2012b).
In a developmental study, pregnant female rats were exposed by oral gavage to monoglyme during GD 8 to 18. At doses of greater than 120 mg/kg bw/day, 100% fetal death was observed. At doses of 30 or 60 mg/kg bw/day, fetotoxic effects were also observed; therefore, a LOAEL of 30 mg/kg bw/day based on increased frequency of stillborn pups, fetal edema, and increased gestation length was derived. A maternal NOAEL of 60 mg/kg bw/day was determined based on decreased body weight gains (partly due to early deaths) at doses of 120 to 1000 mg/kg bw/day (Leonhardt et al.1991 as cited in ECHA 2012b).
In an earlier developmental toxicity study, pregnant female rats were exposed to monoglyme via inhalation (whole body) for 6 h/day during GD 7 to 16 (10 days). Fetal resorptions along with delayed ossification and other fetal effects (fragmented thoracic and lumbar vertebrae, malformations of ribs, enlarged ureter, blood in the pericardium, etc.) were observed at 32 and 100 ppm (120 and 374 mg/m3). Fetal death and other fetal effects (malformations of extremities, body weight decreases, etc.) were observed at the highest dose of 100 ppm (374 mg/m3). The NOAEC and LOAEC for developmental toxicity were determined to be 10 and 32 ppm (37 and 120 mg/m3), respectively, based on retarded development and increased incidence of malformations. A maternal NOAEC of 100 ppm (374 mg/m3) was also determined (ECHA 1988a, 2012b). In a similar study conducted at the same time, pregnant rabbits were exposed to monoglyme via inhalation (whole body) for 6 h/day during GD 6 to 18 (13 days), followed by a recovery period of 10 days. At the highest dose of 50 ppm (187 mg/m3), vitality of the litters within the first 24 hours after Caesarean section was considerably decreased and fetal anomalies were noted in several fetuses (abnormal orientation of fore-paws, skull malformations and ossification irregularities, red-bordered spots on skin). A developmental NOAEC and LOAEC of 16 and 50 ppm (60 and 187 mg/m3), respectively, were determined based on the decreased vitality in pups and the fetal anomalies. The maternal NOAEC and LOAEC was also 16 and 50 ppm (60 and 187 mg/m3), respectively, based on slightly decreased food consumption at both of these concentrations (ECHA 1988a, 1988b; ECHA 2012b). Although this inhalation study in rabbits suggests that fetal effects were seen at the same doses as maternal effects; however, in the rat inhalation study, fetal effects (increased incidence of malformations and delayed ossification) were observed at 120 mg/m3 in the absence of maternal toxicity.
Read-across/analogue health effects data for risk characterization
As noted above, monoglyme metabolizes to 2-ME and then to 2-methyoxyacetic acid, and its toxicity is attributed to these two metabolites (ECHA 2012b; NICNAS 2016a). This supports the use of 2-ME as an analogue for assessing the toxicity of monoglyme. Based on similar arguments, a submission to the US EPA by Ferro Corporation (2002) also supported the use of 2-ME as an analogue.Footnote 26 2-ME was previously assessed by Environment Canada and Health Canada (2002b) and NICNAS (2016b).
As cited in NICNAS (2016b), 2-ME and other chemicals in the alkoxyethanols and acetates groupingFootnote 27 are well absorbed by all routes of exposure. Uptake through the exposed skin is considered to be a significant source of exposure in humans exposed to vapours. Once absorbed, these chemicals are distributed throughout the body, including the developing fetus. This may potentially result in levels of metabolites that are greater in the fetus than those observed in pregnant women.Footnote 28 The alkoxyethanols and acetates are extensively metabolized, with the majority excreted in the urine.
2-ME is not mutagenic in vitro but there is evidence that it may be clastogenic, and there is consistent evidence that the initial metabolite, 2-methoxyacetaldehyde, is genotoxic in several cell lines. Likewise, the main metabolite, 2-methoxyacetic acid, was not mutagenic in vitro, but gave mostly positive results for clastogenicity in different assays. In vivo studies suggest that 2-ME is not genotoxic in somatic cells, but there is evidence that it causes genetic damage to male germ cells in rats at doses higher than 500 mg/kg bw/day (Environment Canada and Health Canada 2002b).
A number of repeated-dose toxicity studies on the metabolite, 2-ME, are available. 2-ME has been shown to cause adverse effects to the male reproductive system in a number of species by all routes of exposure. Effects on the female reproductive system have also been reported (changes in the oestrus cycle and hormone levels along with histopathological changes in the ovaries) (Environment Canada and Health Canada 2002b). In two 13-week inhalation studies conducted in rats and rabbits, a LOAEC of 93 mg/m3 was determined in the rabbit study based on testicular effects (dose-related degeneration of the testes and testicular weight), whereas in the rat study, a LOAEC for degenerative effects on the testes was 933 mg/m3, but the overall NOAEC and LOAEC were 93 and 311 mg/m3, respectively, based on decreased body weights in females (Miller et al. 1983; Environment Canada and Health Canada 2002b). These studies show that the rabbit was more sensitive than rats to the testicular toxicity of 2-ME.
As shown below, 2-ME causes adverse developmental effects in the absence of maternal toxicity in a number of species by all routes of exposure. Developmental effects were generally observed at doses lower than those observed for both reproductive and hematological effects. Also, the rabbit was more sensitive than rodent species to the developmental toxicity of 2-ME (Environment Canada and Health Canada 2002b).
In a developmental toxicity study, pregnant female rabbits were exposed daily via inhalation (whole body) during GD 6 to 18 (13 days) and sacrificed on GD 29. Significant teratologic effects (external, visceral and skeletal malformations) and decreased fetal body weight were observed at 156 mg/m3. Skeletal variations (delayed ossification of the sternebrae) were significantly increased in fetuses of animals exposed at 31 and 156 mg/m3. The NOEC and LOEC for delayed ossification were 9 and 31 mg/m³ respectively, in rabbits. The LOAEC, based on fetal malformations, was 156 mg/m3 (Hanley et al. 1984a, 1984b; Environment Canada and Health Canada 2002b).
Hanley et al. (1984a, 1984b) also conducted inhalation developmental toxicity studies in rats and mice using the same concentrations of 2-ME as those used in the rabbit study. In the rat study (pregnant females exposed whole body), an increase in skeletal variations (lumbar spurs and delayed ossification of the vertebrae) was observed in fetuses of animals exposed to 156 mg/m3. In the mouse study (pregnant females exposed whole body), an increase in testicular hypoplasia and skeletal variations (extra lumbar ribs) were observed in fetuses of animals exposed to 156 mg/m3. Transient decreases in maternal body weight gain among rats, mice and rabbits exposed at the highest dose (156 mg/m3) were the only consistent signs of maternal effects (Hanley et al. 1984a, 1984b; Environment Canada and Health Canada 2002b).
In a study conducted by Nelson et al. (1984), male rats were exposed to 0 or 78 mg/m3 of 2-ME, 7 h/day, 7 days/week for 6 weeks, while pregnant female rats were exposed on GD 7 to 13 or 14 to 20. Exposed males were then mated with unexposed females and all groups of females were allowed to deliver normally. Selected groups of pups/litter were subjected to neuromotor behavioural tests and neurochemical studies of their brains. No signs of toxicity were observed in exposed male or female parents. In the pups of rats exposed to 78 mg/m3, neurochemical changes (significant differences in levels of acetylcholine, dopamine, norepinephrine and 5-hydroxytryptamine in the brains of 21-day old pups) and behavioural effects were observed (Environment Canada and Health Canada 2002b).
The inhalation developmental toxicity studies above indicate a LOEC of 31 mg/m3 (and corresponding NOEC of 9 mg/m3) for increased incidence of skeletal variations in fetuses of rabbits, which is also stated in Environment Canada and Health Canada (2002b). This increased incidence of skeletal variations occurred in the absence of maternal toxicity. Increased incidences of skeletal variations were observed at 156 mg/m3 in the rat and mouse inhalation developmental toxicity studies, not at 31 mg/m3. In terms of fetal malformations, increased incidences of this effect were observed at 156 mg/m3 in both rabbits and mice. Although 2-ME did not appear to increase the incidence of fetal malformations at 156 mg/m3 in rats; however, in an independent monoglyme inhalation developmental toxicity study, an increased incidence of malformations (enlarged ureter and skeletal malformations) and delayed ossification was observed at 120 mg/m3 in rat fetuses. These effects occurred in the absence of maternal toxicity.
The inhalation developmental toxicity studies were based on protocols of exposing the pregnant females to the substance for 6 h/day for 10 or 13 days during gestation. However, as shown above, effects were also observed in pups of female rats exposed to a single concentration of 2-ME over a shorter dosing period, i.e., for 7 days for 7 h/day, at a concentration of 78 mg/m3. Although gross necropsy or histopathological parameters were not examined in this study, the limited information suggests that short in utero fetal exposure during gestation (7 days) may result in neurochemical brain effects.
Further details of the studies described above and other study summaries (13-week rat drinking water study, a 28-day rat dermal study, a 13-week guinea pig dermal study) are provided in the supporting document (Health Canada 2017).
Human studies - 2-ME
Effects on blood, bone marrow and sperm have been observed in workers exposed to 2-ME via inhalation or dermal contact. These effects include increased prevalence of anaemia, increased incidence of leukopenia, and hypoplasia of the bone marrow. Data were also available that indicate an association between exposures to the chemicals and adverse reproductive or developmental effects, including decreased sperm production, changes in sperm morphology, spontaneous abortion and congenital malformations. While confounding factors such as exposure to other chemicals cannot be ruled out (all of the populations studied were also exposed to other glycol ether solvents), these observations are consistent with those in experimental animals (Environment Canada and Health Canada 2002b).
A recent epidemiological study was conducted on 204 mother-child pairs in France to try to determine the association between levels of glycol ether metabolites in mother's urine collected before 19 weeks of gestation and the neurocognitive abilities of their children 6 years after birth. Five different metabolites were measured in urine and for the potential metabolite of 2-ME, the median value was 0.062 mg/L for methoxyacetic acid [MAA]. No association was identified between mother's urinary levels of this metabolite and the scores for 6-year old children in four different neurocognitive tests (Béranger et al. 2017).
7.3 Characterization of risk to human health
7.3.1 Characterization of risk to human health for the ethylene glycols subgroup
As noted in section 7.1, environmental media and food are not expected to be significant sources of exposure for this subgroup. Exposure is expected to occur mainly from paints and coatings, cosmetics and non-prescription products, air fresheners, and household cleaning products. Available dermal or inhalation studies, though limited, were used to characterize risk where possible. However, oral short-term and subchronic critical effect levels were also used to characterize dermal risk.
The tables below provide all relevant exposure (sentinel product scenarios) from different routes and durations) and critical effect values for the EGs subgroup, as well as resulting margins of exposure (MOEs).
| Exposure scenario | Exposure concentration | Critical effect level | Critical hazard endpoint | Margin of exposure (MOE) |
|---|---|---|---|---|
| Acute dermal exposure via waterborne roller paint | Acute dermal exposure (internal) = 0.25 mg/kg bw | NOAEL = 850 mg/kg bw/day based on 32-day feeding study in rats | No significant effects (highest dose) | 3400 |
| Daily dermal exposure via antibacterial shampoo | Daily dermal internal exposure = 0.027 mg/kg bw/day | NOAEL = 1200 mg/kg bw/day based on 90 day to 2 year feeding study in rats | Bladder stones in male rats at 2300 mg/kg bw/day | 44 450 |
| Inhalation acute exposure via waterborne roller paint | Acute inhalation exposure = 0.269 mg/m3 | NOEC = 3000 mg/m3 (9-day rat inhalation study) | Changes in hematology and clinical chemistry parameters in rats at 5000 mg/m3 | 11 150 |
| 24 hour mean event concentrations via DEG in bathroom cleaner | 24 hour mean event concentration = 2.92 mg/m3 | NOEC = 3000 mg/m3 based on 9-day rat inhalation study | Changes in hematology and clinical chemistry parameters in rats at 5000 mg/m3 | 1000 |
| Exposure scenario | Exposure concentration | Critical effect level | Critical hazard endpoint | Margin of exposure (MOE) |
|---|---|---|---|---|
| Acute dermal exposure via waterborne roller painta | Acute dermal exposure (internal) = 1.02 mg/kg bw | NOAEL = 5916 mg/kg bw/day TEG based on 14-day feeding study in rats | Highest dose; no significant effects. | 5800 |
| Acute inhalation exposure via waterborne roller paint | Acute inhalation exposure = 0.269 mg/m3 | NOAEC = 1036 mg/m3 TEG based on 9-day inhalation study in rats | Highest concentration; no significant effects. | 3850 |
| Daily dermal exposure via body cream | Daily dermal internal exposure = 0.1 mg/kg bw/day | NOAEL = 1699 mg/kg bw/day based on 13-wk feeding study in rats | Decreased body-weights decreased urine pH and increased kidney weights in rats at 3489 mg/kg bw/day. | 16 990 |
| 24 hour mean event concentrations via air freshener (spray) | 1.87 mg/m3 | NOAEC = 1036 mg/m3 based on 9-day inhalation study in rats | Highest concentration; no significant effects. | 550 |
| Exposure scenario | Exposure concentration | Critical effect level | Critical hazard endpoint | Margin of exposure (MOE) |
|---|---|---|---|---|
| Dermal acute exposure via waterborne roller painta | Acute dermal exposure (internal) = 1.02 mg/kg bw | NOAEL = 3380 mg/kg bw/day TTEG based on 28-32 day gavage study in rats | Increased urine volume and specific gravity and decreased urine pH in rats at 4500 mg/kg bw/day | 3310 |
| Inhalation acute exposure via waterborne roller painta | Acute inhalation exposure = 0.269 mg/m3 | NOAEC = 1036 mg/m3 TEGb based on 9-day inhalation study in rats | Highest concentration; no significant effects. Read-across from TEG NOAEC in rats | 3850 |
a Using TEG max concentration (4 %) as a surrogate.
b The 9-day inhalation study in rats and the 13-week oral study in rats, using TEG, were also used as a surrogate to help determine risk to TTEG from inhalation exposures to paints and coatings.
The MOEs listed above are considered adequate to account for uncertainties in the health effects and exposure databases.
For the paint scenario (see Tables 7-9 to 7-11), a comparison between relevant NOAELs and NO(A)ECs and the estimates of dermal and inhalation exposure results in MOEs of approximately 3400 to 11 150 for this subgroup.
For cosmetics and non-prescription products (i.e., body cream and antibacterial shampoo; see Tables 7-9 and 7-10), a comparison between relevant NOAELs and estimates of dermal exposure results in MOEs of 16 990 to 44 450 for the subgroup. For household cleaning products (i.e., air freshener and bathroom cleaner; see Tables 7-9 and 7-10), comparison between relevant NOAECs and the estimates of inhalation exposure results in MOEs of 550 to 1000 for the subgroup. A MOE of 550 was determined for the acute air freshener scenario using TEG when the 24-h mean event concentration was compared to the NOAEC of 1036 mg/m3 in a 9-day nose-only exposure inhalation study in rats. This inhalation NOAEC from exposures over 9 days when compared to the mean event concentration of 1.87 mg/m3 (based on the maximum concentration of TEG in air fresheners), results in a conservative and protective MOE.
For all other products and exposure scenarios investigated, the MOEs determined were greater than those presented in the risk characterization tables above and thus are considered adequate to account for uncertainties in the health effects and exposure databases.
7.3.2 Characterization of risk to human health for the ethylene glycol ethers subgroup
As mentioned in section 7.1, environmental media and food are not expected to be significant sources of exposure for this subgroup. Exposure is expected to occur mainly from cosmetics, do it yourself products (i.e. floor sealant, waterborne roller paint) and household cleaning products. Exposure from the mouthing of toys was also considered in the risk characterization. Oral, dermal or inhalation studies, where available, were used to characterize risk. For dermal exposure, oral studies were also used to characterize risk in certain instances.
The tables below provide all relevant exposures (highest exposure products from different routes and durations) and hazard values for the EGEs subgroup, as well as resulting MOEs, for determination of risk.
| Exposure scenario | Exposure concentration | Critical effect level | Critical hazard endpoint | Margin of exposure (MOE) |
|---|---|---|---|---|
| Daily oral exposure via mouthing of painted toy | 0.0189 mg/kg bw/day | NOAEL = 167 mg/kg bw/day (90-day oral study in pigs) |
Liver and kidney effects in female pigs at 500 mg/kg bw/day | 8840 |
| Acute dermal exposure via hair dye | 4.2-14.0 mg/kg bw (external exposure) 2.1-7.0 mg/kg bw/day (internal exposure) |
NOEL = 1000 mg/kg bw/day (28-day dermal study in rabbits) NOAEL = 1340 mg/kg bw/day (6-week oral study in rats) |
No effects Limited pathological changes and organ weights changes at 2680 mg/kg bw/day |
No MOE was determined. 191-638 |
| Daily dermal exposure via deodorant/anti-perspirant | 1.1-3.4 mg/kg bw/day (external exposure) 0.55-1.7 mg/kg bw/day (internal exposure) |
NOAEL = 296 mg/kg bw/day (12-week dermal study in rabbits) NOAEL = 200 mg/kg bw/day (2-year oral study in rats) |
Liver and kidney effects at 986 mg/kg bw/day. Kidney and bladder effects at 950 mg/kg bw/day |
87-269 118-364 |
| Exposure scenario | Exposure concentration | Critical effect level | Critical hazard endpoint | Margin of exposure (MOE) |
|---|---|---|---|---|
| Acute dermal exposure via window cleaner | 0.23 mg/kg bw (external dermal exposure of EGBEA) 0.07 mg/kg bw (internal exposure of EGBEA) |
NOAEL = 1000 mg/kg bw/day (4-day dermal study in mice with EGBE) Maternal NOAEL = 30 mg/kg bw/day (Oral developmental toxicity study; rat dams dosed during three GD with EGBE) |
Relative spleen weight increase at 1500 mg/kg bw/day. Decreased body weight and/or weight gain, increased kidney and spleen weights, and severe hematotoxicity in dams at 100 mg/kg bw/day |
4350 429 |
| Exposure scenario | Exposure concentration | Critical effect level | Critical hazard endpoint | Margin of exposure (MOE) |
|---|---|---|---|---|
| Daily oral exposure via mouthing of toy | 0.0167 mg/kg bw/day | NOAEL = 250 mg/kg bw/day (13-week oral study in rats) |
Liver and kidney effects at 1000 mg/kg bw/day | 14 970 |
| Acute dermal exposure via hair dye | 4.2 mg/kg bw/day (external exposure) |
NOAEL = 1000 mg/kg bw/day (12-day developmental dermal study in rabbits) |
No effects | 238 |
| Daily dermal exposure via facial makeup | 2.8 mg/kg bw/day (external exposure) |
NOAEL = 2000 mg/kg bw/day (13-week dermal study in rats) |
No effects | 714 |
For the toy scenario (see Tables 7-12 and 7-14), a comparison between relevant NOAELs and the highest oral exposure estimates results in MOEs of approximately 8840 to 14 970 for the subgroup.
For the cosmetic scenarios (i.e., hair dye, antiperspirant/deodorant and facial makeup; see Tables 7-12 and 7-14), a comparison between relevant NOAELs and the estimates of dermal exposure results in MOEs of 87 to 714 for this subgroup. MOEs below 100 applied to products containing DEGEE only. However, these MOEs are considered acceptable for three reasons: 1) the dermal and oral studies used for the daily deodorant/antiperspirant scenario both showed effects at doses close to 1000 mg/kg bw/day, indicating that the MOEs based on much lower NOAELs identified for both studies (200 and 296 mg/kg bw/day) are conservative; 2) as stated in the "Health Effects" section, systemic effects observed in multiple species through different routes of exposure occur predominantly at doses greater than the limit dose of 1000 mg/kg bw/day of DEGEE; and 3) for the acute dermal hair dye scenario, the NOEL of 1000 mg/kg bw/day in the short-term dermal rabbit study was the highest dose tested, i.e. the limit dose, and thus, a NOAEL was not determined for acute dermal exposure. When this scenario considered using data from a short-term oral rat study and internal exposures that accounted for dermal absorption, the MOE was greater than or equal to 191. Therefore, the evidence indicates that acute and daily dermal risk is low for DEGEE.
For household cleaning products (see Table 7-13), a comparison between relevant NOAELs and the estimates of dermal exposure results in MOEs of 429 to 4350 for EGBEA.
All other products and scenarios resulted in acceptable MOEs that were larger than MOEs (comparable by exposure duration and route) presented in the risk characterization tables above.
With respect to the modelled air concentrations and inhalation exposures, bathroom cleaners, window cleaners and floor sealants are used infrequently and have clear guidelines regarding labelling (especially floor sealants).
Additionally, as mentioned in the "Exposure Assessment" section, measured peak air concentrations (right after bathroom cleaner use) are comparable to modelled 24-hour mean event concentrations, indicating the conservativeness of the ConsExpo inhalation model for this specific set of substances. However, EGEs are not considered harmful by inhalation, due in part to their low volatility and vapour pressure (Patty 1994 as cited in EC 2000). In addition, no adverse effects were observed in acute studies, and limited effects observed in longer duration studies also support low toxicity for DEGEE and DEGBE at maximal attainable vapour concentrations. For EGBE (the metabolite of EGBEA), developmental and maternal effects were observed at 483 mg/m3 (NOAEC: 242 mg/m3). There is a low likelihood that the general population would be exposed to the maximal attainable vapour concentration over an extended period of time. Therefore, given the above considerations, risk from inhalation is expected to be low and MOEs were not quantified for these products.
Therefore, all exposures presented above are considered to have MOEs that are adequate to address the uncertainties in the health effects and exposure databases.
7.3.3 Characterization of risk to human health for monoglyme
As cited in ECHA (2012b), the reproductive toxicity of monoglyme is considered to occur through its sequential metabolism to 2-ME and then to 2-methoxyacetic acid.
As noted in section 7.1, indoor air and the use of air fresheners are the major sources of exposure. The tables below provide all relevant exposures (highest exposure products from different routes and durations) and hazard values for monoglyme and 2-ME, as well as resulting MOEs, for risk characterization.
As noted in section 7.2.3, delayed ossification was observed after administration of 2-ME in fetuses of rabbits at a dose of 31 mg/m3. In contrast, fetal malformations were observed after administration of monoglyme and 2-ME in rats, mice and rabbits at a higher dose range of 120-156 mg/m3. Given that delayed ossification (at the lower dose) was only observed in rabbits and that malformations were observed in three species (at higher doses), MOEs were determined for both effects.
| Exposure scenario | Exposure concentration | Critical effect level | Critical hazard endpoint | MOE |
|---|---|---|---|---|
| Indoor air | 0.013 mg/m3 (monoglyme) |
NOEC = 9 mg/m3 (2-ME) ≈ 10.7 mg/m3 (monoglyme) NOAEC = 37 mg/m3 (monoglyme)a |
Skeletal variations (delayed ossification of the sternebrae) in fetuses at 31 mg/m3 in a rabbit developmental toxicity study Skeletal and visceral fetal malformations at 120 mg/m3in a rat developmental toxicity study |
823 2850 |
a Note: Fetal malformations were observed at 156 mg/m3 after 2-ME administration. On a molar basis, 31 mg/m3 2-ME is the same as 37 mg/m3 monoglyme:
0.037 g/90.12 g/mol ≈ 0.00041 mol = 0.41 mmol ≈ 10 ppm.
0.031 g/76.09 g/mol ≈ 0.00041 mol = 0.41 mmol ≈ 10 ppm.
To evaluate the risk of monoglyme and its metabolite 2-ME, the lowest NO(A)ECs observed from developmental toxicity studies using 2-ME (9 mg/m3) and monoglyme (37 mg/m3) were considered. This approach is considered to be protective for susceptible subpopulations (pregnant women, fetuses and newborns) and to account for male reproductive system effects observed in different animal studies (LOAEC = 93 mg/m3 for 2-ME; LOAEC = 935 mg/m3 for monoglyme). Based on concentrations of monoglyme in indoor air, a comparison to the relevant NO(A)ECs results in MOEs of 823 to 2850. However, note that the rabbit inhalation developmental toxicity study showed fetal effects at 156 mg/m3, whereas the skeletal variations observed at 31 mg/m3 of 2-ME are of unknown biological significance. The use of a NOEC (9 mg/m3) for this study is considered to be protective of susceptible populations, as stated above. The rat inhalation developmental toxicity study using monoglyme itself showed fetal skeletal and visceral effects at 120 mg/m3, and the resultant MOE for the indoor air scenario based on the NOAEC from this study was greater than 2800. Thus, there is a high level of confidence in the conservativeness of the MOEs developed.
Modelled product concentrations (see "Exposure Assessment" section) were lower than reported indoor air concentrations; therefore, the MOEs for these modelled scenarios were not derived. Based on both the lowest NO(A)ECs for monoglyme and 2-ME, all derived MOEs are considered adequate to account for uncertainties in the health effects and exposure databases.
One of the metabolites of monoglyme, 2-ME, was previously assessed by Environment Canada and Health Canada (2002b) and was determined to be of high hazard potential due to a range of adverse effects in experimental animals, including the developmental toxicity described above. As a result, it is currently listed on Schedule 1 (Toxic Substances List) of CEPA. While exposure of the general population to monoglyme is not of concern at current levels, it might have a potential health effect of concern should exposures increase and should levels of its metabolite 2-ME increase.
7.4 Uncertainties in evaluation of risk to human health for the ethylene glycol ethers
The key sources of uncertainty are presented in the table below.
| Key source of uncertainty | Impact |
|---|---|
| Lack of chronic studies for majority of the seven ethylene glycol ether substances. | - |
| Route-to-route extrapolation of oral dose toxicity studies for dermal exposures | + |
| Use of sub-chronic toxicity studies for long-term exposures (due to lack of chronic toxicity studies) | - |
| Significance of skeletal variations as an adverse developmental endpoint for monoglyme | + |
+ = uncertainty with potential to cause over-estimation of exposure/risk,
- = uncertainty with potential to cause under-estimation of exposure/risk,
+/- = unknown potential to cause over or under estimation of risk.
In the available health effects database, there were no chronic studies for most of the EGEs. Extrapolation from subchronic studies or from one exposure route to another exposure route due to lack of an appropriate dermal and/or inhalation study may also contribute some additional uncertainties. However, for the available dermal toxicity studies in the EGs and EGEs subgroups (TTEG, DEGEE, DEGBE, EGBEA), there was no evidence of health effects below the limit dose of 1000 mg/kg bw/day.
For monoglyme, developmental effects based on inhalation exposure to either monoglyme or 2-ME occurred at concentrations of 120 to 156 mg/m3 in three different species. An increased incidence of skeletal variations was observed in only one of three species (rabbit) at lower concentrations. The biological significance of skeletal variations at these lower concentrations is uncertain.
Limited data were available on consumer use exposure patterns (duration of exposure) associated with air fresheners, window cleaners and tanning products. Also, uncertainties are associated with the market share and prevalence of higher concentrations of EGE-containing cosmetic products in Canada. Also, although some of the EGEs may occur in several products (e.g., DEGEE), aggregate exposures for individual substances were not estimated because they are not expected to account for a significant market share in Canada. Additionally, there may be uncertainty related to proportion of products used by consumers with the presence of monoglyme as an impurity.
8. Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to the environment from the seven substances in the Ethylene Glycol Ethers Group. It is concluded that the seven substances in the Ethylene Glycol Ethers Group do not meet the criteria under paragraphs 64(a) or (b) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Based on the information presented in this screening assessment, it is concluded that the seven substances in the Ethylene Glycol Ethers Group do not meet the criteria under paragraph 64(c) of CEPA as they are not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that the seven substances in the Ethylene Glycol Ethers Group do not meet any of the criteria set out in section 64 of CEPA.
ReferencesFootnote 29
Akuse RM, Eke FU, Ademola AD, Fajolu IB, Gbelee HO, Ihejiahi U, Bugaje MA, Anochie IC, Asinobi AO, Okafor HU, et al. 2012. Diagnosing renal failure due to diethylene glycol in children in a resource-constrained setting. Pediatr Nephrol. 27(6):1021–1028.
[ATSDR] Agency for Toxic Substances & Disease Registry [Internet]. 2013. SUMMARY DATA FOR 2013 PRIORITY LIST OF HAZARDOUS SUBSTANCES. Atlanta (GA): Agency for Toxic Substances & Disease Registry. 19 pages.
Auletta CS, Schroeder RE, Krasavage WJ, Stack CR. 1993. Toxicology of diethylene glycol butyl ether, 4. Dermal subchronic/reproduction study in rats. J Am Coll Toxicol. 12(2):161-168.
Ballantyne B, Snellings WM. 2005a. Developmental toxicity study with diethylene glycol dosed by gavage to CD rats and CD-1 mice. Food Chem Toxicol. 43:1637–46.
Ballantyne B, Snellings WM. 2005b. Developmental toxicity study with triethylene glycol given by gavage to CD rats and CD-1 mice. J. Appl. Toxicol. 25:418–426.
Ballantyne B, Snellings WM. 2007. Triethylene glycol HO(CH2CH2O)3H; Toxicology update. J. Appl. Toxicol. 27:291–299.
Ballantyne B, Snellings WM, Norris JC. 2006. Respiratory peripheral chemosensory irritation, acute and repeated exposure toxicity studies with aerosols of triethylene glycol. J. Appl. Toxicol. 26:387–396.
Belgaqua. 23 March 2010. Annex to the HYDROCHECK procedure for acceptance of Materials in contact with Drinking Water. Brussels. 13 pages.
Béranger R, Garlantézec R. Le Maner-Idrissi G, Lacroix A, Rouget F, Trowbridge J, Warembourg C, Monfort C, Le Gléau F, Jourdin M, et al. 2017. Prenatal exposure to glycol ethers and neurocognitive abilities in 6-year-old children: The PELAGIE cohort study. Environmental Health Perspectives 125(4): 684-690.
Besenhofer LM, McLaren MC, Latimer B, Bartels M, Filary MJ, Perala AW, McMartin KE. 2011. Role of Tissue Metabolite Accumulation in the Renal Toxicity of Diethylene Glycol. Toxicological Sciences 123(2):374–383.
Beyrouty P, Broxop B, Losos G, Robinson K, Maurissen JPJ, Gill MW, Stack CR. 1993. Toxicology of diethylene glycol butyl ether, 5. Dermal subchronic neurotoxicity study in rats. J. Am. Coll. Toxicol. 12:169–174.
[Cal. EPA] California Environmental Protection Agency. 2016. Ethylene glycol mono‐n‐butyl ether reference exposure levels. Technical support document for the derivation of noncancer reference exposure levels. Appendix D1: Scientific Review Panel Review Draft, January 2016. Air Toxics Hot Spots Program. Air, Community, and Environmental Research Branch. 43 pp.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C. 1999, c. 33. Canada Gazette Part III, vol. 22, no. 3.
Canada, Dept. of the Environment. 2001. Canadian Environmental Protection Act, 1999: Notice with respect to certain substances on the Domestic Substances List (DSL). Canada Gazette, Part I, vol. 135, no. 46, p. 4194-4210.
Canada, Dept. of the Environment. 2009. Canadian Environmental Protection Act, 1999: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List. Canada Gazette, Part I, vol. 143, no. 40, p. 2945–2956.
ChemCAN 2003. Level III fugacity model of 24 regions of Canada. Ver. 6.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry.
[CCOHS] Canadian Centre for Occupational Health and Safety.2006. CHEMINFO Search [database on the Internet].. Hamilton (ON). [cited 2015 Dec 3].
ChemIDplus [database]. 1993- a. CAS RN 111-46-6. Bethesda (MD): US National Library of Medicine. [updated NS; accessed 2016 Mar 15].
ChemIDplus [database]. 1993- b. CAS RN 112-27-6. Bethesda (MD): US National Library of Medicine. [updated NS; accessed 2016 Mar 15].
ChemIDplus [database]. 1993- c. CAS RN 112-60-7. Bethesda (MD): US National Library of Medicine. [updated NS; accessed 2016 Mar 15].
ChemIDplus [database]. 1993-d. CAS RN 111-90-0. Bethesda (MD): US National Library of Medicine. [updated NS; accessed 2016 Mar 15].
ChemIDplus [database]. 1993- e. CAS RN 112-07-2. Bethesda (MD): US National Library of Medicine. [updated NS; accessed 2016 Mar 15].
ChemIDplus [database]. 1993- f. CAS RN 112-34-5. Bethesda (MD): US National Library of Medicine. [updated NS; accessed 2016 Mar 15].
ChemIDplus [database]. 1993- g. CAS RN 110-71-4. Bethesda (MD): US National Library of Medicine. [updated NS; accessed 2016 Mar 15].
ChemIDplus [database]. 1993- h. CAS RN 111-76-2. Bethesda (MD): US National Library of Medicine. [updated NS; accessed 2016 Mar 15].
ChemIDplus [database]. 1993- i. CAS RN 109-86-4. Bethesda (MD): US National Library of Medicine. [updated NS; accessed 2016 Mar 15].
Conrad T, Landry GM, Yee Aw T, Nichols R, McMartin KE. 2016. Diglycolic acid, the toxic metabolite of diethylene glycol, chelates calcium and produces renal mitochondrial dysfunction in vitro. Clinical Toxicology 54(6):501–511.
[ConsExpo] Consumer Exposure Model [Internet]. 2006. Version 4.1. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu (National Institute for Public Health and the Environment).
Devoti E, Marta E, Belotti E, Bregoli L, Liut F, Maiorca P, Mazzucotelli V, Cancarini G. 2015. Diethylene glycol poisoning from transcutaneous absorption. Am J Kidney Dis. 65(4):603–606.
[DOW] The DOW Chemical Company. 1981. DEG: Method Development for Skin Penetration Study in Rats (using diethylene glycol). Unpublished report of The Dow Chemical Company, Midland, MI 48674, USA. Submitted to Health Canada, March 2016. [See supporting document (Health Canada 2017) for details].
[DOW] The DOW Chemical Company. 2007a. Product Safety Assessment, Diethylene Glycol Butyl Ether Acetate. Form No. 233-00340-MM-1207. [created 15 Dec. 2007].
[DOW] The DOW Chemical Company. 2007b. Product Safety Assessment, Diethylene Glycol Monoethyl Ether. Form No. 233-00344-MM-0907. [created 24 Sep 2007].
[DOW] The DOW Chemical Company. 2014a. Product Safety Assessment, Diethylene Glycol Butyl Ether. Form No. 233-00262-MM-1214X. [revised 23 Dec 2014]..
[DOW] The DOW Chemical Company. 2014b. Triethylene glycol mouse developmental toxicity study: Developmental toxicity is secondary to maternal toxicity. Unpublished update submitted by The Dow Chemical Company to Health Canada, March 2016. 3 pp. [See supporting document (Health Canada 2017) for details].
[DOW] The DOW Chemical Company. 2015. Product Safety Assessment Finder [database on the Internet]. [cited 2015 Dec 3
[DOW] The DOW Chemical Company. 2016a. Response to data gaps request on ethylene glycols to Health Canada. Received 8 March 2016. [See supporting document (Health Canada 2017) for details].
[DOW] The DOW Chemical Company. 2016b. Updated response to data gaps request on ethylene glycol ethers to Health Canada. Received 7 April 2016. [See supporting document (Health Canada 2017) for details].
[DPD] Drug Product Database [database]. [modified 2016 Sep 27]. Ottawa (ON): Health Canada. [accessed 2016 Oct.]
[EC] European Commission. 2000. European Union Risk Assessment Report. CAS RN 112-34-5: 2-(2-Butoxyethoxy)ethanol. Luxembourg: Office for Official Publications of the European Communities. Report No.: EUR 18998 EN 114 pp.
[EC] European Commission. 2006-2008a. European Union Risk Assessment Report. CAS RN 112-07-2: 2-Butoxyethyl acetate (EGBEA) Part I – Environment; EUR 22475 EN, 2006; and Part II Human Health, final approved version, August 2008. 204 pp.
[EC] European Commission. 2006-2008b. European Union Risk Assessment Report. CAS RN 111-76-2: 2-Butoxyethanol (EGBE). Luxembourg: Office for Official Publications of the European Communities. Volume 68 Part I – Environment; EUR 22501 EN, 2006; and Part II Human Health, final approved version, August 2008. 418 pp.
[ECCC] Environment and Climate Change Canada. 2016a. Ecological Science Approach: Ecological Risk Classification of Organic Substances.
[ECCC] Environment and Climate Change Canada. 2016b. Gatineau (QC): Data used to create substance-specific hazard and exposure profiles and assign risk classifications in the Ecological Risk Classification of organic substances. Available from: substances@ec.gc.ca.
[ECCC, HC] Environment and Climate Change Canada, Health Canada. 2017. Screening assessment: Draft screening assessment substances identified as being of low concern using the Ecological Risk Classification of organic substances and the Threshold of Toxicological Concern (TTC)-based approach for certain substances. Ottawa (ON): ECCC, HC.
[ECETOC] European Centre for Ecotoxicology and Toxicology of Chemicals. 2005. The toxicology of glycol ethers and its relevance to man (Fourth edition). Volume 1. ECETOC Technical Report No. 95. Brussels, Belgium. 202 pp.
[ECHA] European Chemicals Agency. 1986a. 1,2-Dimethoxyethane, CAS RN 110-71-4. Exp Key Repeated dose toxicity: inhalation. 002. Industry Submission to ECHA, Updated 6 January 2016. [cited 2016 March].
[ECHA] European Chemicals Agency. 1986b. 1,2-Dimethoxyethane, CAS RN 110-71-4. Exp Supporting Repeated dose toxicity: inhalation. 004. Industry Submission to ECHA, Updated 6 January 2016. [cited 2016 Mar].
[ECHA] European Chemicals Agency. 1988a. 1,2-Dimethoxyethane, CAS RN 110-71-4. Exp Supporting Developmental toxicity / teratogenicity.001. Industry Submission to ECHA, Updated 6 January 2016. [cited 2016 Mar].
[ECHA] European Chemicals Agency. 1988b. 1,2-Dimethoxyethane, CAS RN 110-71-4. Exp Key Developmental toxicity / teratogenicity.002. Industry Submission to ECHA, Updated 6 January 2016. [cited 2016 Mar].
[ECHA] European Chemical Agency. 2012a. Support document for identification of 1,2-Dimethyoxyethane (EGDME) as substance of very high concern because of its CMR properties. 21 pp.
[ECHA] European Chemicals Agency. 2012b. Annex XV dossier. Proposal for identification of a Substance as a Category 1A OR 1B CMR, PBT, vPvB or a substance of an equivalent level of concern. Public version. 1,2-dimethoxyethane (EGDME). CAS RN 110-71-4. EC number 203-794-9.
[ECHA] European Chemicals Agency. 2015. Dossier for 2-(2-ethoxyethoxy)ethanol, CAS RN 111-90-0. [accessed 2015 Sep].
Environment Canada. 2001. Data for certain substances collected under the Canadian Environmental Protection Act, 1999, Section71: Notice with respect to certain substances on the Domestic Substances List (DSL). Data prepared by: Environment Canada, Health Canada, Existing Substances Program.
Environment Canada. 2009. DSL Inventory Update data collected under the Canadian Environmental Protection Act, 1999, section 71: Notice with respect to certain inanimate substances (chemicals) on the Domestic Substances List. Data prepared by: Environment Canada, Health Canada; Existing Substances Program.
Environment Canada. 2014. Results from Phase Two of the Domestic Substances List Inventory Update Rapid Screening Assessment of Substances of Lower Ecological Concern – Detailed Spreadsheet. Ecological Assessment Division, Environment Canada, Gatineau, QC, Canada.
Environment Canada, Health Canada. 2002a. Canadian Environmental Protection Act: Priority Substances List assessment report: 2-butoxyethanol. Ottawa (ON).
Environment Canada, Health Canada. 2002b. Canadian Environmental Protection Act: Priority Substances List assessment report: 2-methoxyethanol. Ottawa (ON).
Environment Canada, Health Canada. [modified 2017 Mar 12]. Categorization. Ottawa (ON): Government of Canada.
Environment Canada, Health Canada. 2015. State of the science report: phthalate substance grouping: 1,2-benzenedicarboxylic acid, diisononyl ester; 1,2-benzenedicarboxylic acid, di-C8-10-branched alkyl esters, C9-rich; (diisononyl phthalate; DINP). Ottawa (ON): Environment Canada, Health Canada. On the cover: Chemical Abstracts Service Registry Numbers: 28553-12-0 and 68515-48-0.
European Union. 2016. 1,2-dimethoxyethane - Brief Profile - ECHA. [cited 2016 Apr].
[EWG] ] Environmental Working Group; 2015; EWG’s Skin Deep Cosmetics Database [database on the Internet]. 2015. Washington (DC): EWG. [cited 2015 Dec 3].
Ferrari LA, Giannuzzi L. 2005. Clinical parameters, postmortem analysis and estimation of lethal dose in victims of a massive intoxication with diethylene glycol. Forensic Sci Int. 153(1):45–51.
Ferro Corporation. 2002. 1,2-dimethoxyethane: CAS Number 110-71-4. US EPA HPV Challenge Program Submission. December 2001, Revised August 2002. Pages 1–21 of 93.
GoodGuide [database on the Internet]. 2014. [cited 2015 Dec 3].
Hanley TR Jr, Young JT, John JA, Rao KS. 1984a. Ethylene glycol monomethyl ether (EGME) and propylene glycol monomethyl ether (PGME): Inhalation fertility and teratogenicity studies in rats, mice and rabbits. Environ. Health Perspect. 57:7–12.
Hanley TR Jr, Yano BL, Nitschke KD, John JA. 1984b. Comparison of the teratogenic potential of inhaled ethylene glycol monomethyl ether in rats, mice, and rabbits. Toxicol. Appl. Pharmacol. 75:409–422.
Hansen O, Pedersen E. 2005. Migration and health assessment of chemical substances in surface treated wooden toys. Danish Ministry of the Environment.
Healing G, Sulemann T, Cotton P, Harris J, Hargreaves A, Finney R, Kirk S, Schramm C, Garner C, Pivette P, Burdett L. 2016. Safety data on 19 vehicles for use in 1 month oral rodent pre-clinical studies: administration of hydroxypropyl-ß-cyclodextrin causes renal toxicity. J. Appl. Toxicol. 36:140–150.
Health Canada. 1994. Human health risk assessment for priority substances. Ottawa (ON): Minister of Supply and Services Canada. Cat. No.: En40-215/41E. Available from substances@ec.gc.ca.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Health Canada. 1999. Canadian Environmental Protection Act: Priority Substances List Supporting documentation: Health-related sections - 2-methoxyethanol. 157 pp. [See supporting document (Health Canada 2017) for details].
Health Canada. [modified 2015 Dec 14]. Cosmetic ingredient hotlist: list of ingredients that are prohibited for use in cosmetic products. Ottawa (ON): Health Canada, Consumer Product Safety Directorate. [accessed 2016 January].
Health Canada. 2016a. Impurities: Guideline for Residual Solvents. ICH Topic Q3C(R5). Adoption of International Conference on Harmonisation of Technical Requirements for the Registration of Pharmaceuticals for Human Use (ICH) Guidance Document: Q3C(R5): Guideline for Residual Solvents. 25 pp.
Health Canada. 2016b. Science Approach Document: Threshold of Toxicological Concern (TTC)-based Approach for Certain Substances. September 2016. 54 pp.
Health Canada. 2017. Supporting documentation: Physical and chemical properties, exposure estimate methods, and human health effects. Gatineau (QC): HC. Information in support of the Screening Assessment for Ethylene Glycol Ethers. Available from: substances@ec.gc.ca.
Heilmair R, Lenk W, Löhr D. 1993. Toxicokinetics of diethylene glycol (DEG) in the rat. Arch. Toxicol. 67:655–666.
[HERA] Human and Environmental Risk Assessment on ingredients of household cleaning products. 2005. 2-(2-Butoxyethoxy)ethanol; CAS No: 112-34-5. Edition 1.0. August 2005. 12 pp.
Household Products Database [database on the Internet]. 1993—2015. Bethesda (MD): US National Library of Medicine. [updated 2015 Aug; cited 2015 Dec 7].
[HSDB] Hazardous Substances Data Bank [database]. 2002. CAS RN 110-71-4. Bethesda (MD): National Library of Medicine (US). [updated fields; 2007 Feb. and 2014 Dec.; accessed 2016 Mar 9].
[HSDB] Hazardous Substances Data Bank [database]. 2007a. CAS RN 112-27-6. Bethesda (MD): National Library of Medicine (US). [updated 2007 October; accessed 2016 Mar 8].
[HSDB] Hazardous Substances Data Bank [database]. 2007b. CAS RN 112-60-7. Bethesda (MD): National Library of Medicine (US). [updated 2007 October; accessed 2016 Mar 8]..
[HSDB] Hazardous Substances Data Bank [database]. 2007c. CAS RN 111-90-0. Bethesda (MD): National Library of Medicine (US). [updated 2007 October; accessed 2016 Mar 9].
[HSDB ] Hazardous Substances Data Bank [database]. 2007d. CAS RN 112-34-5. Bethesda (MD): National Library of Medicine (US). [updated 2007 October; accessed 2016 Mar 9].
[HSDB] Hazardous Substances Data Bank [database]. 2009a. CAS RN 111-46-6. Bethesda (MD): National Library of Medicine (US). [updated 2009 December; accessed 2016 Mar 7].
[HSDB ] Hazardous Substances Data Bank [database]. 2009b. CAS RN 112-07-2. Bethesda (MD): National Library of Medicine (US). [updated field 2012 April; accessed 2016 Mar 9].
[IARC] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 2006. IARC Monographs on the evaluation of carcinogenic Volume 88: Formaldehyde, 2-butoxyethanol and 1-tert-butoxypropan-2-ol. Summary of data reported and evaluation. Technical Publication no. 39. Lyon, France.
[IFRA] International Fragrance Association. 2012. REACH exposure scenarios for fragrance substances. IFRA Operations, Brussels, Belgium. 126 pp.
[JECFA] Joint FAO/WHO Expert Committee on Food Additives. 1995. Evaluation of Certain Food Additives and Contaminants. Geneva: World Health Organization. 54 pages. Forty-fourth report.
Johnson KA, Baker PC, Kan HL, Maurissen JP, Spencer PJ, Marty MS. 2005. Diethylene glycol monobutyl ether (DGBE): two- and thirteen-week oral toxicity studies in Fischer 344 rats. Food and Chemical Toxicology 43:467–481.
Kassotis CD, Tillitt DE, Wade Davis J, Hormann AM, Nagel SC. 2014. Estrogen and androgen receptor activities of hydraulic fracturing chemicals and surface and ground water in a drilling-dense region. Endocrinology 155(3):897-907.
Kassotis CD, Klemp KC, Vu DC, Lin C-H, Meng C-X, Besch-Williford CL, Pinatti, Thomas Zoeller R, Drobnis EZ, Balise VD, et al. 2015. Endocrine-disrupting activity of hydraulic fracturing chemicals and adverse health outcomes after prenatal exposure in male mice. Endocrinology 156(12):4458-4473.
Kenyon MO, Coffing SL, Ackerman JI, Gunther WC, Dertinger SD, Criswell K, Dobo KL. 2015. Compensatory erythropoiesis has no impact on the outcome of the in vivo Pig-a mutation assay in rats following treatment with the haemolytic agent 2-butoxyethanol. Mutagenesis 30(3):325–334.
Kraut, J.A. 2015. Diagnosis of toxic alcohols: limitations of present methods. Clinical Toxicology 53:589–595.
Landry GM, Dunning CL, Abreo F, Latimer B, Orchard E, McMartin KE. 2015. Diethylene glycol-induced toxicities show marked threshold dose response in rats. Tox Appl Pharm. 282:244–251.
Lide DR, editor. 2007. CRC handbook of chemistry and physics. 88th ed. Boca Raton (FL): CRC Press.
[LNHPD] Licensed Natural Health Products Database [database]. [modified 2018 Feb 6]. Ottawa (ON): Health Canada.
Loretz LG, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, Rothenstein A, Scrafford CG, Sewall C. 2005. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol. 43: 279–291.
Loretz L, Api AM, Barraj L, Burdick J, Davis DA, Dressler W, Gilberti E, Jarrett G, Mann S, Pan YHL et al. 2006. Exposure data for personal care products: Hairspray, spray perfume, liquid foundation, shampoo, body wash, and solid antiperspirant. Food Chem Toxicol. 44:2008-2018.
Loretz LG, Api AM, Babcock L, Barraj LM, Burdick J, Cater KC, Jarrett G, Mann S, Pan YHL, Re TA, Renskers KJ, Scrafford CG. 2008. Exposure data for cosmetic products: Facial cleanser, hair conditioner, and eye shadow. Food Chem Toxicol. 46:1516–1524.
Mangelsdorf I, Kleppe SN, Heinzow B, Sagunski H. 2016. Indoor air guide values for glycol ethers and glycol esters—A category approach. Int J Hyg Environ. Health 219(4-5):419–436.
Miller RR, Ayres JA, Young JT, McKenna MJ. 1983. Ethylene glycol monomethyl ether. I. Subchronic vapor inhalation study with rats and rabbits. Fundam Appl Toxicol. 3:49–54.
[MSDS] Material Safety Data Sheet. 2007. FUTURE PREMIUM FLOOR FINISH. Racine, WI: S.C. Johnson & Son, Inc. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2009a. Shell Flushing Oil D 22. Bangkok, THA: The Shell Company of Thailand Ltd. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2009b. GLADE® TOUGH ODOR SOLUTIONS SURFACE DISINFECTANT & AIR SANITIZER - CLEAR SPRINGS. S.C. Johnson & Son, Inc., Racine W.I [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2010a. FROTH-PAK(TM) 620BF HFC CLASS A POLYOL Spray Polyurethane Foam. Midland, MI: The Dow Chemical Company. [accessed 2015 Dec 14].
[MSDS] Material Safety Data Sheet. 2010b. RT201504 - RT-2015-2.0-W. El Segundo, CA: Henry Company. [accessed 2015 Dec 2].
[MSDS] Material Safety Data Sheet. 2010c. PROFIN 1GL 4PK WB 275VOC SANDING SEALER. Vernon Hills, IL: Rust-Oleum Corporation. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2010d. 27X37, CLEAR WOOD FINISH GLOSS. Irvine, CA: Deft, Inc. [accessed 2016 Feb 22].
[MSDS] Material Safety Data Sheet. 2010e. Tetra-ThinTM. Tetra-Etch Products Ltd, Whitburn West Lothian, Scotland [accessed 2016 Mar 13].
[MSDS] Material Safety Data Sheet. 2011a. Driveway, Concrete & Masonry Cleaner Concentrate. Atlanta, GA: Zep. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2011b. Artisan Anti-Graffiti Protectant. Moorestown, NJ: Chemique, Inc. [accessed 2015 Dec 2].
[MSDS] Material Safety Data Sheet. 2011c. CLASSIC WHITE FINISH LINE IND. MENOMONEE FALLS, WI: RAABE COMPANY. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2012a. NanoSet Color – All Colors. Salt Lake City, UT: NewLook International, Inc. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2012b. Shell Irus Fluid C-NA. Houston, TX: SOPUS Products. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2012c. Dynamic PTC Pro Tint. Mississauga, ON: Dynamic Paint Products Inc. [accessed 2016 Feb 22].
[MSDS] Material Safety Data Sheet. 2012d. Wet-Look Floor Finish. Atlanta, GA: Zep Commercial Sales and Service. [accessed 2016 Feb 22].
[MSDS] Material Safety Data Sheet. 2012e. GLADE® SENSE & SPRAY® AUTOMATIC ROOM SPRAY - CLEAR SPRINGS®. S.C. Johnson & Son, Inc., Racine W.I [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2013a. SN214 - PREMIUM ALL SEASON CONCRETE ACCELERATOR. El Segundo, CA: Henry Company. [accessed 2015 Dec 2].
[MSDS] Material Safety Data Sheet. 2013b. GRANITE GOLD DAILY CLEANER. San Diego, CA: Granite Gold, Inc. [accessed 2015 Dec 2].
[MSDS] Material Safety Data Sheet. 2013c. ULTRACOAT POLISH REMOVER. Laval, QC: Mapei. [accessed 2015 Dec 2].
[MSDS] Material Safety Data Sheet. 2013d. SimpleGrout Premixed Grout. Seal Beach, CA: Custom Building Products. [accessed 2015 Dec 2].
[MSDS] Material Safety Data Sheet. 2013e. Energel X. Tokyo, Jpn: Pentel Co., LTD. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2013f. DISINFECTANT SCRUBBING BUBBLES® BATHROOM CLEANER - FRESH CITRUS SCENT. [accessed 2015 Sep 30].
[MSDS] Material Safety Data Sheet. 2013g. AIR CARE air conditioner hygienizer; Electrolux code 9029794261. Mariel refrigeration and air conditioning. Busto Arsizio, Italy (translated from German). [accessed 2016 Feb.].
[MSDS] Material Safety Data Sheet. 2014a. EPOXY 2-GL BLACKTOP COATING. Vernon Hills, IL: Rust-Oleum Corporation. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2014b. TB 670 LVT Flooring Adhesive. Columbus, OH: Franklin International. [accessed 2015 Dec 11].
[MSDS] Material Safety Data Sheet. 2014c. TractionCote Anti-Slip Coating. Maplewood, NJ: Surface Solutions, LLC. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2014d. Mulberry Air Freshener/Air Delights Air Neutralizer 9000. K-G Spray-Pak Inc., Concord, Ontario, Canada. http://msds.kgpackaging.com/ [accessed 2016 Mar 18].
[MSDS] Material Safety Data Sheet. 2014e. Air Freshener 70%. Air Guard Control (Canada) Ltd. Concord, Ontario, Canada. [accessed 2016 Mar 18].
[MSDS] Material Safety Data Sheet. 2014f. Vectair V-Air Air Freshener. K-G Spray-Pak Inc., Concord, Ontario, Canada. [accessed 2016 Mar 18].
[MSDS] Material Safety Data Sheet. 2015a. Glance HC 1 Glass & Multi-Surface Cleaner. Sturtevant, WI: Diversey, Inc. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2015b. Citrus II Hospital Germicidal Deodorizing Cleaner. Kennesaw, GA: Beaumont Products, Inc. [accessed 2015 Dec 2].
[MSDS] Material Safety Data Sheet. 2015c. ARMOR ALL Extreme Wheel and Tire Cleaner. Danbury, CT: The Armor All/STP Products Company. [accessed 2015 Dec 2].
[MSDS] Material Safety Data Sheet. 2015d. Liquid Wrench White Lithium Grease. Indian Trail, NC: RSC Chemical Solutions. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2015e. MAPEI-ACTIVE DP02-S. Milan, IT: Mapei. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2015f. MAPEFLOOR WAX REMOVER. Castanheira do Ribatejo, Prt: Mapei. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2015g. DIF 22-OZ 12 WALLPAPER STRIPPER CONC. Vernon Hills, IL: Rust-Oleum Corporation. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2015h. MAPEGROUT SVT DF GRAVIER. Milan, IT: Mapei. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2015i. WDCARE 1-GL 2PK VARA UNIVSL SAND SEALER. Vernon Hills, IL: Rust-Oleum Corporation. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2015j. SILANCOLOR CLEANER PLUS. Milan, IT: Mapei. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2015k. StoneSpecific Polished Granite Sealer. Atlanta, GA: Custom Building Products. [accessed 2015 Dec 2].
[MSDS] Material Safety Data Sheet. 2015l. ELIMINATOR III ENGINE DEGREASER 15 OZ. Hartford, CT, USA: Permatex, Inc. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2015m. ROCK SOLID 1GL 4PK WOODSTRIPPER128. Vernon Hills, IL: Rust-Oleum Corporation. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2015n. ULTRACOAT TOP DECK OIL. Milan, IT: Mapei. [accessed 2015 Dec 2].
[MSDS] Material Safety Data Sheet. 2015o. ACCENT SSPR 6PK MICA STONE TRAVERTINE. Vernon Hills, IL: Rust-Oleum Corporation. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2015p. ADHESION PROMOTER. Hickory, NC: RPM Wood Finishes group. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2015q. CSL850 Heat Sink Compound. Guelph, ON: CSL Silicones Inc. [accessed 2015 Dec 2].
[MSDS] Material Safety Data Sheet. 2015r. VIBROMIX E. Budaörs, Hun: Mapei. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2015s. ULTRA PENETRATING STAIN MEDIUM MAHOGANY GAL. Hickory, NC: MOHAWK Finishing Products. [accessed 2015 Dec 17].
[MSDS] Material Safety Data Sheet. 2015t. MINWAX Water-Based HELMSMAN Urethane. Upper Saddle River, NJ: Minwax. [accessed 2015 Dec 3].
[MSDS] Material Safety Data Sheet. 2015u. COLOR ACCENTS™ Interior Latex Satin Paint, Ultradeep Base. Cleveland, OH: THE SHERWIN-WILLIAMS COMPANY. [accessed 2016 Feb 22].
[MSDS] Material Safety Data Sheet. 2015v. KLEAN STRIP MULTI-SURFACE CLEANER AEROSOL. [accessed 2016 Apr 29].
[MSDS] Material Safety Data Sheet. 2015w. Tetra-Etch® Etchant. Tetra-Etch Products Ltd, Whitburn West Lothian, Scotland [accessed 2016 Mar 13].
Nagano K, Nakayama E, Oobayashi H, Nishizawa T, Okudat H, Yamazaki K. 1984. Experimental studies of ethylene glycol ethers in Japan. Environ. Health Perspectives 57:75–84.
Neeper-Bradley TL, Tyl RW, Fisher LC, Kubena MF, Vrbanic MA, Losco PE. 1995. Determination of a no-observed-effect level for developmental toxicity of ethylene glycol administered by gavage to CD rats and CD-1 mice. Fundam Appl Toxicol. 27:121–130. [cited in OECD 2009a].
Nelson BK, Brightwell WS, Burg JR, Massari VJ. 1984. Behavioral and neurochemical alterations in the offspring of rats after maternal or paternal inhalation exposure to the industrial solvent 2-methoxyethanol. Pharmacol Biochem Behav. 20:269–279.
[NCI] National Chemical Inventories [database on a CD-ROM]. 2014. Columbus (OH): American Chemical Society, Chemical Abstracts Service. [cited 2014].
[NHPID] Natural Health Products Ingredients Database [database]. [modified 2018 Feb 21]. Ottawa (ON): Health Canada.
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme. 2015. HUMAN HEALTH TIER II ASSESSMENT FOR Ethanol, 2,2'-oxybis-. Australian Government Department of Health and Ageing. [updated 2015 Jul 31; cited 2015 Dec 14].
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme. 2016a. Human Health Tier II Assessment for Ethane, 1,2-dimethoxy-. CAS RN 110-71-4. Inventory multi-tiered assessment and prioritisation (IMAP). Australian Government Department of Health and Ageing. [accessed 2016 March].
[NICNAS] National Industrial Chemicals Notification and Assessment Scheme. 2016b. Human Health Tier II Assessment for Alkoxyethanols (C1-C2) and their acetates. Inventory multi-tiered assessment and prioritisation (IMAP). Australian Government Department of Health and Ageing. [accessed 2016 April].
Nolen GA, Gibson WB, Benedict JH, Briggs DW, Schardein JL. 1985. Fertility and teratogenic studies of diethylene glycol monobutyl ether in rats and rabbits. Fundam Appl Toxicol. 5:1137–1143.
[NPRI] National Pollutant Release Inventory [database]. 1993-2013. Gatineau (QC): Environment Canada. [updated 2016 January 4; accessed 2015 November].
[NYSDEC] New York State Department of Environmental Conservation. 2015. Final Supplemental Generic Environmental Impact Statement On The Oil, Gas and Solution Mining Regulatory Program [Internet]. 2011 Dec 12. Revised Draft. Albany, NY: NYSDEC. 1537. [cited 2015 Dec 3].
O'Brien KL, Selanikio JD, Hecdivert C, Placide M-F, Louis M, Barr JR, Hospendales CJ, Lewis MJ, Schawartz B, Philen RM, et al. 1998. Epidemic of pediatric deaths from acute renal failure caused by diethylene glycol poisoning. JAMA 279:1175–1180.
[OECD] Organisation for Economic Co-operation and Development. 1981. OECD Guideline for testing of chemicals: Test guideline 411, "Subchronic Dermal Toxicity: 90-day Study". Adopted 12 May 1981. 9 pp.
[OECD] Organisation for Economic Co-operation and Development. 2006a. SIDS Initial Assessment Report for: Monoethylene glycol ethers category (Mono EGEs); CAS RN 2807-30-9, 111-76-2(surrogate only), 112-07-2, 112-25-4. SIDS Initial Assessment Meeting 19, 19-22 October 2004 [NOTE: This is a summary of EC 2006-2008a].
[OECD] Organisation for Economic Co-operation and Development. 2006b. SIDS Initial Assessment Profile for: 2-butoxyethanol (Ethylene Glycol Butyl Ether = EGBE); CAS RN 111-76-2. SIDS Initial Assessment Meeting 23; 17-20 October 2006 [NOTE: This is a summary of EC 2006-2008b].
[OECD] Organisation for Economic Co-operation and Development. 2007. SIDS Initial Assessment Report for: Diethylene glycol ethers category (Di EGEs); CAS RN 111-90-0, 112-15-2, 6881-94-3, 124-17-4, 112-59-4. SIDS Initial Assessment Meeting, 21 October 2005.
[OECD] Organisation for Economic Co-operation and Development. 2009a. SIDS Initial Assessment Report for: Ethylene Glycol Category. SIDS Initial Assessment Meeting 18; April, 2004. Search "all records", then "ethylene glycol" at http://webnet.oecd.org/hpv/ui/Publications.aspx
[OECD] Organisation for Economic Cooperation and Development. 2009b. SIDS Dossier on the HPV chemical Diethylene Glycol; CAS No.: 111-46-6. Sponsor Country: Canada. 89 pp.
[OECD] Organisation for Economic Cooperation and Development. 2009c. SIDS Dossier on the HPV chemical Triethylene Glycol; CAS No.: 112-27-6. Sponsor Country: Canada. 65 pp.
[OECD] Organisation for Economic Cooperation and Development. 2009d. SIDS Dossier on the HPV chemical Tetraethylene Glycol; CAS No.: 112-60-7. Sponsor Country: Canada. 72 pp.
Pomierny B, Krzyzanowska W, Niedzielska E, Broniowska Z, Budziszewska B. 2016. Ethylene glycol ethers induce apoptosis and disturb glucose metabolism in the rat brain. Pharmacological Reports. 68:162–171.
Rentz ED, Lewis L, Mujica OJ, Barr DB, Schier JG, Weerasekera G, Kuklenyik P, McGeehin M, Osterloh J, Wamsley J. et al. 2008. Outbreak of acute renal failure in Panama in 2006: a case-control study. Bull World Health Organ. 86:749–756.
Renwick JH, Cameron KM. 1992. Fetal detriment used as an index of effects of diethylene glycol on Syrian hamster fetuses. J Toxicol Environ Health. 36:377–400.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2005. Consumer Exposure and Uptake Models Program Manual. Bilthoven (NL): RIVM. Report No.: 320104004/2005. [accessed 2016 Mar 2].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2006a. Cleaning products fact sheet: to assess the risks for the consumer. Bilthoven (NL): RIVM. Report No.: 320104003/2006. [accessed 2016 Feb 22].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2006b. Pest control products fact sheet: to assess the risks for the consumer. Bilthoven (NL): RIVM. Report No.: 320005002/2006. [accessed 2016 Mar 11].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2007a. Paint products fact sheet: to assess the risks for the consumer: updated version for ConsExpo 4. Bilthoven (NL): RIVM. Report No.: 320104008/2007. [accessed 2016 Feb 22].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment]. 2007b. Do-it-yourself products fact sheet: to assess the risks for the consumer. Bilthoven (NL): RIVM. Report No.: 320104007/2007. [accessed 2016 Feb 22].
[SCCP] Scientific Committee on Consumer Products. 2006a. Opinion on diethylene glycol monoethyl ether (DEGEE) [published in 2007]. 27 pp.
[SCCP] Scientific Committee on Consumer Products. 2006b. Opinion on diethylene glycol monobutyl ether (DEGBE) [published in 2007]. 22 pp.
[SCCP] Scientific Committee on Consumer Products. 2008. Opinion on diethylene glycol (DEG). 25 pp.
[SCCS] Scientific Committee on Consumer Safety. 2012. The SCCS’s Notes of Guidance for the Testing of Cosmetic Ingredients and their Safety Evaluation, 8th Revision.
Schep LJ, Slaughter RJ. 2005. Comments on diethylene glycol concentrations (Letter to the editor). Forensic Sci Int. 155:233.
Schep, LJ, Slaughter RJ, Temple WA, Beasley DMG. 2009. Diethylene glycol poisoning. Clinical Toxicology 47: 525-535.
Schier JG, Hunt DR, Peralas A, McMartin KE, Bartels MJ, Lewis LS, McGeehin MA, Flanders WD. 2013. Characterizing concentrations of diethylene glycol and suspected metabolites in human serum, urine, and cerebrospinal fluid samples from the Panama DEG mass poisoning. Clin. Toxicol. 51(10):923–929.
Scorecard [database on the internet]. 2011. [cited 2015 Dec 17
Sitarek K, Gromadzinska J, Lutz P, Stetkiewicz J, Śweircz J, Wasowicz W. 2012. Fertility and developmental toxicity studies of diethylene glycol monobutyl ether (DGBE) in rats. Int J Occup Environ Med. 25(4):404-417.
Snellings WM, McMartin KE, Banton MI, Reitman F, Klapacz J. 2017. Human health assessment for long-term oral ingestion of diethylene glycol. Reg Tox Pharm. 87:Si-S20.
Starek-Świechowicz B, Miranowicz-Dzierżawska K, Budziszewska B, Starek A. 2015. The effects of 2-methoxyethanol and 2-ethoxyethanol on hematological changes induced by 2-butoxyethanol. Medycyna Pracy. 66(3):303–315.
Statistics Canada. 2012. Canadian Health Measure Survey. (CHMS). Cycle 2. 2009-2011.
Tønning K, Pedersen E, Drøjdahl Lomholt A, Malmgren-Hansen B, Woin P, Møller L, Bernth N. 2008. Survey, emission and health assessment of chemical substances in baby products [Internet]. Copenhagen: Danish Environmental Protection Agency. 98. No. 90. [accessed 2015 Dec 4].
Uemura K. 1980. The teratogenic effects of ethylene glycol dimethyl ether on mouse. Acta Obst Gynaec Jpn. 32:113–121.
Union Carbide [BRRC] Bushy Run Research Center. 1983. 1,2-Dimethoxyethane (Ethylene Glycol Dimethylether) In Vitro Mutagenesis Studies: 3-Test Battery. Final report with cover letter. U.S. NTIS Record OTS0534903. 2/10/1983. Part 1.
[US FDA] U.S. Food and Drug Administration. 2011. List of Indirect Additives Used in Food Contact Substances [database on the Internet]. Silver Spring (Maryland) [updated 2011 Nov 14; cited 2015 Dec 7].
US Federal Register. 2011. Glymes; Proposed Significant New Use Rule. United States Environmental Protection Agency. 76 FR 40850.
US Federal Register. 2014. Ethylene Glycol Ethers: Significant New Use Rule. United States Environmental Protection Agency. 79 FR 74639.
[US EPA] United States Environmental Protection Agency. 1996. Health Effects Test Guidelines: OPPTS 870.3250, Subchronic Dermal Toxicity—90 days. "Public draft". Report number EPA 712–C–96–202, June 1996. 13 pp.
[US EPA] United States Environmental Protection Agency. 2005 Triethylene glycol - Revised report of the Antimicrobials Division Toxicology Endpoint Selection Committee. 13 pp. [cited in HSDB. 2007a. Triethylene glycol; CASRN: 112-27-6].
[US EPA] United States Environmental Protection Agency. 2008. Supporting Documents for Initial Risk-Based Prioritization of High Production Volume Chemicals. Chemical/Category: CAS No. 110-71-4, Ethane, 1,2-dimethoxy- (also known as 1,2-Dimethyoxyethane, or Monoglyme). March 18, 2008. 24 pp. [cited in ECHA ca 2012]. Note: U.S. website cited in ECHA (2012b) no longer accessible.
[US EPA] United States Environmental Protection Agency. 2010. Toxicological review of ethylene glycol monobutyl ether (EGBE) (CAS No. 111-76-2): In support of summary information on the Integrated Risk Information System (IRIS). Report No. EPA/635/R-08/006F. Washington, D.C. 206 pp.
[US EPA] United States Environmental Protection Agency. 2012. Chemical Data Access Tool (CDAT) [database on the Internet]. [updated 2016 Apr 5; cited 2016 Apr 15].
[US NTP] United States National Toxicology Program. 1993. NTP technical report on toxicity studies of ethylene glycol ethers 2-methoxyethanol, 2-ethoxyethanol, 2-butoxyethanol (CAS Nos. 109-86-4, 110-80-5, 111-76-2) administered in drinking water to F344/N rats and B6C3F1 mice. U.S. Department of Health and Human Services. 122 pp. + appendices. (NTP Toxicity Report Series No. 26; NIH Publication No. 93-3349).
[US NTP] United States National Toxicology Program. 2000. Toxicology and Carcinogenesis Studies of 2-Butoxyethanol (CAS No. 111-76-2) in F344/N rats and B6C3F1 mice (Inhalation studies). NTP Technical Report Series No.484. NIH Publication No. 00-3974.
Vale A. 2007. Ethylene and diethylene glycol. Medicine 35(11):617–618.
Wagner P. 2006. Reassessment of 3 Tolerance Exemptions for Ethylene Glycol, Diethylene Glycol, and the Combination of Diethylene Glycol Monomethyl Ether, Diethylene Glycol Monoethyl Ether, and Diethylene Glycol Monobutyl Ether [Internet]. 2006. Washington: United States Environmental Protection Agency. 17 pages. [cited 2015 Dec 7].
Zhu J, Wong SL, Cakmak S. 2013a. Nationally representative levels of selected volatile organic compounds in Canadian residential indoor air: Population-based survey. Env Sci Tech. 47:13276–13283.
Zhu J, Wong SL, Cakmak S. 2013b. Supporting information: Nationally representative levels of selected volatile organic compounds in Canadian residential indoor air: Population-based survey. 8 pp. [cited in Env Sci Tech. 47:13276-13283].
Appendices
Appendix A. Exposure estimate parameters for ethylene glycol ethers from use of products
Exposures were estimated based on the assumed weight (70.9 kg) of an adult (Health Canada 1998), inhalation rate of an adult (16.2 m3/day) and use behaviours of an adult, unless noted otherwise. Exposures were estimated using ConsExpo version 4.1 or algorithms from the model (ConsExpo 2006).
| Product type and scenario | Sub-stances | Model parameter |
|---|---|---|
| Personal care products: Permanent hair dye (wash-in) |
DEGEE; DEGBE |
Concentration of DEGEE = 3-10%f DEGBE = 1-3% (personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated September 2015; unreferenced) Product Amount = 100 g per application (RIVM 2006a) Frequency = 3.57 times per year (Statistics Canada 2012) Retention Factor = 0.10 (SCCS 2012) |
| Personal care products: Tanning product |
DEGEE | Concentration of DEGEE = 3-10% (personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated September 2015; unreferenced) Product Amount = 4.4 g per day (application) (Loretz et al. 2005) Frequency = 1 application per day (expert judgement) |
| Personal care products: Body cream |
TEG; DEGEE |
Concentration of TEG = ≤0.3%; DEGEE = 1-3% (personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated September 2015; unreferenced) Product Amount = 4.4 g per application (Loretz et al. 2005) Frequency = 1.1 times per day (Loretz et al. 2005) |
| Personal care products: Facial makeup |
DEGBE | Concentration of DEGBE = 10-30% (personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated September 2015; unreferenced) Product Amount = 1.2 g per application (Loretz et al. 2006) Frequency = 1.8 times per day (Loretz et al. 2006) |
| Personal care products: Antibacterial shampoo |
DEG | Concentration of DEG = 3% personal communication, emails from the Health Products and Food Branch, Health Canada, to the Risk Management Bureau, Health Canada, dated October 2015; unreferenced) Product Amount = 11.8 g per application (Loretz et al. 2006) Frequency = 1.1 times per day (Loretz et al. 2006) |
| Personal care products: Deodorant/anti-perspirant |
DEGEE | Concentration of DEGEE = 10-30% (personal communication, emails from the Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada, dated September 2015; unreferenced) Product Amount = 0.6 g per application (Loretz et al. 2006) Frequency = 1.3 times per day (Loretz et al. 2006) |
| Paints and coatings: Waterborne roller wall painta |
DEG | Concentration of DEG in paint colourant = 13% (MSDS 2012c) Pigment in wall paint = 8% (RIVM 2007a) Therefore, maximum expected concentration of DEG in whole paint = 1% Dermal absorption rate = 50% (DOW 2016a) |
| Paints and coatings: Waterborne roller wall painta |
TEG; TTEG |
Industry data submitted under section 71 of CEPA indicated that TEG is present in concentrations between the range of 0.1 to 4 %. No data was available for TTEG concentrations in paints. However, industry submissions indicated a potential presence in paints and coatings. Therefore, the concentration of TEG in paint was used as a surrogate for TTEG and exposure is expected to be similar. |
| Paints and coatings: Waterborne roller wall painta |
DEGBE | Maximum concentration of DEGBE = 2% (MSDS 2015u) Dermal absorption rate = 50% (SCCP 2006a) Mass transfer rate = 353 m/s (Thibodeaux; calculated from RIVM 2005, p. 41) MW matrix = 20.5 m/s (calculated from RIVM 2005, p. 41) |
| Paints and coatings: Floor/concrete/stone sealant/finishb |
DEGEE | Concentration of DEGEE = 1-10% (MSDS 2012d) Dermal absorption rate = 50% (SCCP 2006a) Mass transfer rate = 22 m/s (Thibodeaux; calculated from RIVM 2005, p. 41) MW matrix = 27.6 m/s (calculated from RIVM 2005, p. 41) |
| Paints and coatings: Wood/deck stainb |
EGBEA | Concentration of EGBEA = 1-5% (MSDS 2010d) Dermal absorption rate = 30% (EC 2006-2008a) Mass transfer rate = 22 m/s (Thibodeaux; calculated from RIVM 2005, p. 41) MW matrix = 27.6 m/s (calculated from RIVM 2005, p. 41) |
| Cleaning products and air fresheners: Window cleanerc |
DEGEE; EGBEA |
Concentration of DEGEE = 8% (OECD 2007: p. 61) Concentration of EGBEA = 5% (MSDS 2015v) Exposure duration = 24 h (expert judgement) Dermal estimate = instant application and constant rate values added (expert judgement) |
| Cleaning products and air fresheners: Bathroom cleanerd |
DEG; DEGBE |
Concentration of DEGBE = 10% (MSDS 2013f) Concentration of DEG = 4% (personal communication, emails from the Health Products and Food Branch, Health Canada, to the Risk Management Bureau, Health Canada, dated October 2015; unreferenced) Exposure duration = 24 h (expert judgement) Dermal estimate = instant application and constant rate values summed (expert judgement) |
| Cleaning products and air fresheners: Air freshenere |
TEG; DEGEE; Monoglyme |
Concentration of DEGEE = 10-30% (MSDS 2014d,e) Concentration of TEG = 5-10% (MSDS 2009b) Concentration of monoglyme (as impurity of other EGEs used in air fresheners) = 0.01% (Environment Canada 2009). Aerosol air freshener (non-aqueous): Discharge rate = 0.9 g/sec; Use frequency = 1/day; Spray duration = 6 sec (IFRA 2012) Chronic exposure duration (for air fresheners containing DEGEE, TEG, or monoglyme) = 24 h (expert judgement) Acute exposure duration (for air freshener containing monoglyme) = 6 h (based on single day exposures in 2-ME rabbit and monoglyme rat developmental toxicity studies) Room volume = 20 m3 (IFRA 2012; RIVM 2006b) Ventilation rate = 0.6/h (RIVM 2006b) |
a Exposures were estimated using the ConsExpo scenario for ‘Brush/roller painting, waterborne wall paint’ (RIVM 2007a). All recommended default values from this scenario were used, except for parameters listed below.
b General coating on a floor (RIVM 2007b) best fit the product scenarios for a floor/concrete/stone finish/sealant and wood/deck stain. All recommended default ConsExpo values from this scenario were used, except for parameters listed here.
c Exposures were estimated using the ConsExpo scenario for ‘Glass cleaners’ (RIVM 2006a). All recommended default values from this scenario were used, except for parameters listed here.
d Exposures were estimated using the ConsExpo scenario for ‘Bathroom cleaners’ (RIVM 2006a). All recommended default values from this scenario were used, except for parameters listed here.
e ConsExpo does not currently have a scenario for air fresheners. However, based on IFRA (2012) and internal consultations, the parameters used in ConsExpo v4.1 are listed under "Model parameter".
f 97.5% of hair dyes in Canada contain 3-10% DEGEE; only 2.5% contain greater than 10%.
