Screening Assessment for the Challenge
This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Archived
Siloxanes and Silicones, di-Me, hydrogen-terminated
Chemical Abstracts Service Registry Number
70900-21-9
Environment Canada
Health Canada
September 2011
Table of Contents
- Synopsis
- Introduction
- Substance Identity
- Physical and Chemical Properties
- Sources
- Uses
- Releases to the Environment
- Environmental Fate
- Persistence and Bioaccumulation Potential
- Potential for Bioaccumulation
- Potential to Cause Ecological Harm
- Potential to Cause Harm to Human Health
- Conclusion
- References
- Appendix I: Relationship between viscosity, degree of polymerisation and molecular weight (Wacker 1992 and Fendinger et al. 1997)
- Appendix II: PBT Model Inputs Summary Table
- Appendix III: Estimated concentrations of MHDnMH in environmental media using ChemCAN version 6.00 (ChemCAN 2003)
- Appendix IV: Estimates of dermal exposure to MHDnMH from use of disposable bras, skin whitening cream, eye makeup and lipstick
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a screening assessment on siloxanes and silicones, di-Me, hydrogen-terminated (MHDnMH), Chemical Abstracts Service Registry Number[1] 70900-21-9. This substance was identified as a high priority for screening assessment and included in the Challenge initiative under the Chemicals Management Plan because, based on model predictions, it was found to meet the ecological categorization criteria for persistence, bioaccumulation potential and inherent toxicity to non-human organisms and is believed to be in commerce in Canada.
The substance, MHDnMH, was not considered to be a high priority for assessment of potential risks to human health, based upon application of the simple exposure and hazard tools developed for categorization of substances on the Domestic Substances List (DSL).
MHDnMH was originally classified as an organic UVCB (Unknown or Variable Composition, Complex Reaction Products or Biological Material) during the DSL Categorization. Based on new information received, the substance is considered to be an organic siloxane polymer.
MHDnMH is not naturally produced in the environment. In 2006, between 10 000 and 100 000 kg of the substance were manufactured in Canada, and less than 100 kg were imported into the country.
Based on reported use, MHDnMH is manufactured as an intermediate polymer, and then exported in bulk form out of the country for producing plastics. During the industrial process, a small amount of the polymer is released to wastewater, and to a lesser extent air and land; however, the total environmental release is not significant.
Based on the available information, it is determined that the form of MHDnMH in commerce in Canada meets the Reduced Regulatory Requirement polymer criteria as specified in the New Substances Notification Regulations (Chemicals and Polymers).Given that polymers are often complex mixtures and the molecular weight of a polymer varies as a function of the number of repeating units, two forms of the polymer of different molecular weights were considered in this assessment. This was done in order to address concerns relating to both number-average (e.g., MHDnMH where n = 34) and low (e.g., MHDnMH where n = 5) molecular weight forms of the polymer.
Information for an analogous polymer, polydimethylsiloxanes (PDMS), has been used for assessing MHDnMH. Based on the read-across data of the analogue, the substance is expected to be persistent in the environment. Based on the information for the analogue on the potential for bioaccumulation, and taking into account its relatively large molecular size, the substance is not likely to be bioavailable and is expected to have a low potential to accumulate in the lipid tissues of organisms. The substance therefore meets the persistence criteria but does not meet the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations. In addition, new modelled toxicity data for the substance and experimental data for an analogous polymer indicate that MHDnMH has a low potential to cause harm to organisms in water, soil, and sediment.
For this screening assessment, a conservative exposure scenario was developed in which an industrial operation discharges MHDnMH into the aquatic environment. The predicted environmental concentration in water was well below the predicted no-effect concentration calculated for the aquatic organisms. Therefore, based on the information presented in this screening assessment, it is expected that MHDnMH is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Empirical health effects data were not identified for MHDnMH. Based on health effects data for PDMS, and on the weight-of-evidence based risk assessments for PDMS conducted by international agencies, it is considered that MHDnMH demonstrates low hazard potential.
Based on the estimated concentrations of MHDnMH in environmental media (air, drinking water and soil), exposure of the general population is expected to be negligible. General population exposure can occur through use of consumer products containing MHDnMH. Margins between conservative upper-bounding estimates of exposure for MHDnMH and effect levels from health effects studies with the analogue (PDMS) are considered adequate to address uncertainties in the health effects and exposure databases. It is therefore concluded that MHDnMH is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Based on the information available, it is concluded that the Reduced Regulatory Requirement form of MHDnMH does not meet any of the criteria set out in section 64 of CEPA 1999.
Given the complexity associated with the polymer formulation and the potentially hazardous properties associated with low molecular weight polymers, there is concern that new activities for MHDnMH which have not been identified or assessed under CEPA 1999 could lead to the substances meeting the criteria as set out in section 64 of the Act. Therefore, it is recommended that the DSL be amended to indicate that MHDnMH meets the Reduced Regulatory Requirement (RRR) Polymer criteria. Should other forms of MHDnMH, not meeting the Reduced Regulatory Requirement polymer criteria, be introduced on the Canadian market, those forms would be subject to the requirements of the New Substances Notification Regulations.
The Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or to human health.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherent toxicity to aquatic organisms (iT), and were believed to be in commerce in Canada; and/or
- met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE) and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in theCanada Gazette, Part I, on December 9, 2006 (Canada 2006), that challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment, and to develop and benchmark best practices for the risk management and product stewardship of those substances identified as high priorities.
The substance, siloxanes and silicones, di-Me, hydrogen-terminated (MHDnMH), was initially identified as a high priority for assessment of ecological risk during the DSL Categorization as it was found to be persistent, bioaccumulative and inherently toxic to aquatic organisms and is believed to be in commerce in Canada. The Challenge for this substance was published in the Canada Gazette on September 26, 2009 (Canada 2009). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, submissions of information pertaining to the properties and uses of the substance were received.
Although MHDnMH was determined to be a high priority for assessment with respect to the environment, it did not meet the criteria for GPE or IPE and high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
Screening assessments focus on information critical to determining whether a substance meets the criteria set out in section 64 of CEPA 1999. Screening assessments examine scientific information and develop conclusions by incorporating a weight-of-evidence approach and precaution[2].
This screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including the additional information submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents, stakeholder research reports and from recent literature searches, up to May 2010 for the human health sections and December 2010 for ecological sections of the document. Key studies were critically evaluated; modelling results may have been used to reach conclusions.
When available and relevant, information presented in hazard assessments from other jurisdictions was considered. The screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents the most critical studies and lines of evidence pertinent to the conclusion.
This screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. The ecological portion of this assessment has undergone external written peer review/consultation. Additionally, the draft of this screening assessment was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment Canada. Approaches used in the screening assessments under the Challenge have been reviewed by an independent Challenge Advisory Panel.
The critical information and considerations upon which the assessment is based are summarized below.
For the purposes of this document, the substance, siloxanes and silicones, di-Me, hydrogen-terminated, will be referred to as MHDnMH, derived from the chemical structure. MH refers to the silicon atom in end groups, having one shared bond to oxygen, two shared bonds to methyl substituents and one shared bond to hydrogen. D symbolizes the silicon atoms in the backbone, having two shared bonds to oxygen and two shared bonds to methyl substituents, while n is the number of D in the polymer molecule.
MHDnMH was originally classified as an organic UVCB in the DSL Categorization. Based on new information received, MHDnMH can be characterized by 1) the monomer unit of dimethyl-siloxy and 2) hydrogen and dimethyl groups attached to the silicon atom functioning as the polymer terminating groups (Environment Canada 2010a).
According to results from gel permeation chromatography (GPC), MHDnMH has been reported to have a number-average molecular weight (Mn) of approximately 2 700 g/mol (Environment Canada 2010a), equivalent to an average of 34 monomer units of dimethyl-siloxy (n=34, based on the molecular weight of the monomer unit of dimethyl-siloxy as 74.15 g/mol). Any single molecule of MHDnMH contains more than three monomer units. Differences in molecular weights (MW) are primarily attributable to differences in the number of units. Therefore, MHDnMH meets the definition of a polymer (OECD 1994), as summarized below:
- molecules characterized by the sequence of one or more types of monomer units;
- greater than 50% by weight of molecules having three of more monomer units that are covalently bound to one or more other monomer units or reactants;
- less than 50% by weight of molecules of the same molecular weight; and
- molecules distributed over a range of molecular weights whose differences in molecular weights are primarily attributable to differences in the number of monomer units.
There have been considerably smaller oligomers (the lowest molecular weight of ~500 g/mol) detected in the polymer product but at a very low concentration. Furthermore, looking at the global market, there are other products manufactured and sold under the same CAS RN (70900-21-9), including the polymer with an average molecular weight around 500 g/mol (Gelest 2010). A molecular weight of ~500 g/mol corresponds to a polymer with five monomer units of dimethyl-siloxy (n=5). Given the above, both MHD34MH and MHD5MH are considered in the assessment, to represent both the number-average molecular weight and low molecular weight polymers.
The assessment considers critical data on key parameters for assessing persistence, bioaccumulation, and ecotoxicity for both the number-average molecular weight and the low molecular weight polymers. In some cases when experimental data are not available for MHDnMH, experimental data for analogous chemicals and Quantitative Structure-Activity Relationship (QSAR) models will be used to fill the data gaps.
The identity of MHDnMH is summarized in Table 1 below.
Table 1. Substance identity for MHDnMH
| Chemical Abstracts Service Registry Number (CAS RN) | 70900-21-9 |
| DSL name | Siloxanes and silicones, di-Me, hydrogen-terminated |
| National Chemical Inventories(NCI) names[1] | Siloxanes and Silicones, di-Me, hydrogen-terminated (TSCA, REACH, ENCS, ECL, PICCS, ASIA-PAC, NZIoC) Siloxanes and silicones, dimethyl hydrogen terminated (AICS) SILOXANE, DI-ME, HYDROGEN TERMINATED (PICCS) |
| Other names | Di-Me, dimethylsilyl-terminated siloxanes FH 0023 Hydrogen-terminated di-Me silicones Hydrogen-terminated di-Me siloxanes MCR-H 07 MHD6MH Polysiloxanes, di-Me, hydrogen-terminated PS 542 SE 1886A SE 1887A Silicones, di-Me, dimethylsilyl-terminated Siloxanes and Silicones, di-Me, (dimethylsilyl)-terminated XF 40A6100 Dimethylsiloxanes et silicones, termines par un atome d'hydrogene |
| Chemical group (DSL Stream) | Polymer |
| Major chemical class or use | Organosilicon |
| Major chemical sub-class | Siloxane |
| Chemical formula | C(4+2n)H(14+6n)O(1+n)Si(2+n) |
| Representative chemical structure used to run the estimation model[2] | 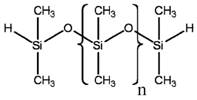 |
| SMILES[3] | MHDnMH (n=34) or MHD34MH: not available MHDnMH (n=5) or MHD5MH: Si(C)(C)OSi(C)(C)OSi(C)(C)OSi(C)(C)OSi(C)(C)OSi(C)(C)OSi(C)(C) |
| Molecular mass | MHD34MH: 2655.45 g/mol MHD5MH: 505.10 g/mol |
[2] This substance is an organic polymer; i.e., it is not a discrete chemical and thus can be characterized by a representative structure. For this assessment, two representative structures were used, one with n=34 and one with n=5.
[3] Simplified Molecular Input Line Entry System
It is noted that the polymer may contain a small fraction of impurities, i.e., residual monomers or by-products which have much lower molecular weights. The quantities and the types of impurities vary, based on a number of factors, including different manufacturing processes and operations, reaction conditions, and use of catalysts and promoters. Based on a data submission received (Environment Canada 2010a),
octamethylcyclotetrasiloxane (D4, CAS RN 556-67-2), decamethylcyclopentasiloxane (D5, CAS RN 541-02-6), dodecamethylcyclohexasiloxane (D6, CAS RN 540-97-6), and 1,3-dihydrotetramethyldisiloxane (CAS RN 3277-26-7) have been reported as residual monomers or byproducts in the polymer products under the same CAS RN 70900-21-9, however the concentrations of these residual monomers and byproducts vary in the polymers manufactured by different companies. D4, D5 and D6 were previously assessed under the Challenge; and according to the GPC results, 1,3-dihydrotetramethyldisiloxane was not detected in MHDnMH in commerce in Canada. Therefore risk associated with impurities in the polymer will not be addressed in this assessment on MHDnMH.
Unlike any discrete substance which has a defined molecular weight, the molecular weight of a polymer varies as a function of the number of repeating units, which can range from three to many thousands. Therefore, a polymer is usually characterized by the average number of repeating units it contains or its number-average molecular weight.
As mentioned in the previous section of Substance Identity, MHD34MH represents the polymer which is manufactured in Canada; while MHD5MH refers to the polymer product produced internationally that has a molecular weight of 505.10 g/mol. There is a difference in assessment approaches for these two polymer products based on the molecular weights.
For the purpose of the assessment, the number of repeating units (n) may be referred to in a certain case if information is only applicable to either one of the above two forms. If there is no indication of the number of repeating units, the assessment applies to the polymer MHDnMH (n=5) in general.
There are no experimental physical and chemical properties for MHDnMH, thus information on analogous substance, polymethylsixolanes (PDMS), is considered in the assessment.
An analogue is a chemical that is structurally similar to the substance under assessment and is therefore expected to have similar physical and chemical properties, similar fate in the environment, and/or similar toxicity. Where there are experimental data for a given property of an analogue substance, they can be used to characterize the substance under assessment, though differences in structure which may affect bioavailability should be noted.
PDMS is another siloxane polymer, having the same repeating unit of di-methyl groups attached to the silicon of the –Si-O-Si- backbone. The difference between MHDnMH and PDMS lies in the terminating units. While there is a hydrogen atom and two methyl groups at both end units in MHDnMH, PDMS has three methyl groups (see Table 2a below). The chemical formula of PDMS can be referred to as MDnM. Such differences are not anticipated to significantly affect the substances’ physical and chemical properties between MHDnMH and PDMS (when numbers of repeating units are similar). Given the similarities of these chemicals, the experimental data for PDMS were used to evaluate environmental fate, potential for persistence and bioaccumulation, and ecological toxicity for both forms of MHDnMH (n=5 and n=34) with no need for qualitative adjustment.
Table 2a. Chemical structures of PDMS and MHDnMH
| Chemical | PDMS | MHDnMH |
|---|---|---|
| Representative Structure | 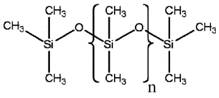 |
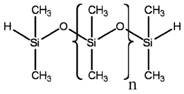 |
MHD5MH
For the low molecular weight form of MHDnMH (n=5), several linear methyl siloxanes (MDnM) have been used as analogues (see Table 2b below). These linear methyl siloxanes (n=3) are low molecular weight PDMS.
Table 2b. Linear methyl siloxanes used as analogues of MHD5MH
| Chemical Name and CAS RN | Formula | Molecular Weight (g/mol) |
|---|---|---|
| Dodecamethylpentasiloxane (141-63-9) | MD3M | 384.84 |
| Tetradecamethylhexasiloxane (107-52-8) | MD4M | 459.00 |
| Hexadecamethylheptasiloxane (541-01-5) | MD5M | 533.15 |
| Siloxanes and silicones, di-Me, hydrogen-terminated (70900-21-9) | MHD5MH | 505.10 |
- M refers to the silicon atoms in end groups, having one shared bond to oxygen and three shared bonds to methyl substituents.
- MH refers to the silicon atoms in end groups, having one shared bond to oxygen, two shared bonds to methyl substituents and one shared bond to hydrogen.
- D symbolizes the silicon atoms in the backbone, having two shared bonds to oxygen and two shared bonds to methyl substituents.
Selected experimental physical and chemical property information for MDnM was read-across to MHD5MH with some adjustments for structural differences (i.e., the number of repeating units). QSAR models were also used to fill data gaps where there was no experimental information for certain physical and chemical properties (see Appendix II). These models (except WSKOWWIN 2008) are mainly based on fragment addition methods, i.e., they rely on the structure of a chemical. It is noted that most models do not have siloxanes in their training sets, and therefore MHD5MH is technically outside the domain of the models. However, given that low molecular weight material such as MHD5MH remains in a neutral form under environmental conditions, estimations based on its fragments and molecular weight are expected to provide approximate values.
The modelled physical and chemical properties for MHD5MH are summarized in Table 2c below. Experimental data for the analogous linear methyl siloxanes (see Table 2b) are also presented in the table. For enhancing the reliability of model predictions, an experimental value adjustment (EVA) method was applied using sub-models in EPISUITE to derive an estimate from an experimental measure, with consideration of the structural differences between the analogue and MHD5MH.
Table 2c. Estimated physical and chemical properties for MHD5MH (MW=505.10 g/mol)
| Property | Value[1] | Temperature (°C) |
Reference |
|---|---|---|---|
| Melting point (ºC) |
12.02 | MPBPWIN 2008 | |
| -59 (for MD4M) | Gelest 2008a[5] | ||
| Boiling point (ºC) |
317.45 | MPBPWIN 2008 | |
| 245 (for MD4M) | Gelest 2008a | ||
| Density (kg/m3) |
890 (for MD4M) | Gelest 2008a | |
| Viscosity[2] (centistoke, cs) |
2.6 (for MD4M) | Gelest 2008a | |
| Vapour pressure (Pa) |
0.10 (7.69 × 10-4 mmHg) |
25 | MPBPWIN 2008 |
| <1 mm Hg (for MD4M) | 25 | Gelest 2008a | |
| Henry’s Law constant (Pa·m3/mol) |
2.0 × 107 (2.0 × 102atm·m3/mole) | HENRYWIN 2008 | |
| Log Kow (Octanol-water partition coefficient) (dimensionless) |
6. 6 | KOWWIN 2008 | |
| 6.7 (EVA extrapolation from MD4M and MD5M)[3] |
KOWWIN2008 | ||
| 6.0 (for MD3M) 6.6 (for MD4M) 7.2 (for MD5M) 7.7 (for MD6M) |
Bruggeman et al. 1984 | ||
| Log Koc (Organic carbon-water partition coefficient) (dimensionless) |
5.7 | PCKOCWIN 2008 | |
| Water solubility (mg/L) |
2.2 × 10-6 – 2.7 × 10-6 (based on log Kow = 6.7 – 6.6) |
25 | WSKOWWIN 2008 |
| 5.7 × 10-9 – 2.2 × 10-6 (EVA extrapolation from MD4M and MD5M)[3] |
WATERNT 2008 | ||
| 3.4 × 10-2 (for MDM) 7.0 × 10-5 (for MD3M) 1.3 × 10-5 (estimated for MD4M) 5.4 × 10-7 (estimated for MD5M) |
25 | Varaprath et al. 1996 WSKOWWIN 2008 |
|
| Cross-sectional diameter (nm)[4] | 1.46 – 2.18 | CPOPs 2008 |
[2] The viscosity of a polymer is used to characterize the molecular size and molecular weight of polymers. The unit for kinematic viscosity of a polymer is centistoke (cs).
[3] An experimental value adjustment (EVA) method was applied using EPISUITE to derive an estimate from an experimental measure, with consideration of the structural differences
[4] Values represent the range of possible maximum molecular diameters (i.e., Dmax values) estimated by CPOPs
[5] Information cited from Gelest 2008a is for a product of MD4M (>95%), with fraction of MD3M and higher linear siloxanes (<5%)
According to the experimental data on log Kow and water solubility for MD3-6M (see Table 2c), the low molecular weight linear methyl siloxanes are extremely hydrophobic. They possess low water solubilities (at the level of 10-4 mg/L or lower) and high octanol-water partition coefficients (log Kow=6 or higher).
Having a hydride in the terminating unit, MHD5MH is expected to demonstrate similar water solubility and octanol-water partition coefficient to those parameters of the analogous PDMS with the similar molecular weight. The modelled predictions of log Kow for MHD5MH are 6.7 with the experimental value adjustment and 6.6 without, close to the octanol-water partition coefficients for MD4M (log Kow = 6.6) and MD5M (log Kow = 7.2). The estimated water solubilities for MHD5MH (derived based on the log Kow values) are 2.2 × 10-6 – 2.7 × 10-6 mg/L, between the water solubilities of MD4M (1.3 × 10-5 mg/L) and MD5M (5.4 × 10-7 mg/L).
Based on the model predictions, MHD5MH is not expected to be particularly volatile and not soluble in water. The estimated vapour pressure is moderate (0.10 Pa) and the water solubility is very low (~2.5 × 10-6 mg/L), which consequently make the Henry’s Law constant very high, estimated as 2.0 × 107 Pa·m3/mol.
MHD34MH
The polymer in commerce in Canada (MHD34MH) has a number-average molecular weight of ~2 700 g/mol. There is no component in the polymer having a molecular weight less than 500 g/mol and less than 5% of the components have molecular weights less than 1000 g/mol. There are no cationic or reactive functional groups in either the terminating unit or the repeating unit in the polymer, thus the functional group equivalent weight[3] (FGEW) for MHD34MH is more than 1000 g/mol. Given this, the polymer meets Reduced Regulatory Requirement polymer criteria as specified the New Substances Notification Regulations (Chemicals and Polymers).
Having a number-average molecular weight of ~ 2 700 g/mol, which is higher than the limit acceptable for the QSAR models, MHD34MH is outside the domains of applicability and there are no QSAR models that can be used for assessing the substance. Consequently, read-across data from PDMS were considered when characterizing the physical and chemical properties for MHD34MH.
In a toxicity test, a hydroxyl terminated PDMS (approximately equivalent to n=45 and MW=3 500 g/mol, derived from the viscosity of PDMS as 55 cs) was reported with a maximum water solubility of 0.26 mg/L and an equilibrium concentration of 0.04 to 0.05 mg/L (Gettings and Lane 1982). Given that the hydroxyl in the terminating unit would make the polymer somewhat more soluble, the water solubility MHD34MH is expected to be inferior to 0.26 mg/L.
For large molecular weight polymers, there is a relationship between viscosity and the average molecular weight (see Appendix I). Viscosity is a measure of how much a fluid resists flow when a force is applied to it. It is also a measure for ability to flow. The larger the polymer molecule is (higher number of the monomer units and average molecular weight), the more “thickness” the polymer will display (indicating a higher viscosity value) (see Appendix I). In many studies on PDMS which have been referred in the assessment, viscosity has been reported and used to characterize the molecular weight of a polymer.
High molecular weight siloxane polymers (including PDMS) are not volatile and not expected to partition into air and degrade (Graiver et al. 2003). For a polymer with an average molecular weight >1 000 g/mol, the vapour pressure is expected to be less than 1.33 × 10-6 Pa (10-8 mm Hg) and the Henry’s Law constant less than 1.01 × 10-3Pa•m3/mol (10-8 atm•m3/mol) (USEPA 2008). Given the large molecular weight of MHD34MH (2655.45 g/mol), the n=34 form of the polymer is expected to possess a low vapour pressure and low Henry’s Law constant.
Siloxane polymers are relatively free-flowing liquids, even at high molecular weight (viscosity up to 500 000 cs, which is equivalent to an average molecular weight around 100 000 g/mol) (Fendinger et al. 1997). This may be attributed to the high degree of flexibility of the siloxane backbone and to the resulting low inter-molecular attractive forces. The polymer is characterized by a low melting point and a high boiling point, and remains as a liquid at standard environmental temperatures (Fendinger et al. 1997; Graiver et al. 2003).
Based on the data for PDMS, MHD34MH is anticipated to be insoluble in water, while the octanol-water partition coefficient (Kow) is anticipated to be high. The polymer contains no functional groups which could undergo hydrolysis in water. If released to water, the polymer may form a thin layer on the surface of the water, due to a specific gravity slightly less than water (approximately 960 kg/m3) (Fendinger et al. 1997; Environment Canada 2010a; ECETOC 1994).
Given the above information, the n=5 form of MHDnMH is expected to have a moderate vapour pressure; however the n=34 form of the polymer is anticipated to be much less volatile with the increase of the chain length.
The polymer (both n=5 and n=34 forms) is expected to be insoluble in water, with a specific gravity slightly less than water, and remains in the liquid state at environmental temperatures.
MHDnMH is not reported to be naturally produced in the environment.
Recent information was collected through industry surveys conducted for the 2005 and 2006 calendar years by means ofCanada Gazette notices issued pursuant to section 71 of CEPA 1999 (Canada 2006). These notices required submission of data on the Canadian manufacture and import of MHDnMH. In the notice for 2006, data were also required on the use quantity of the substance.
In 2005, fewer than 4 companies identified themselves as manufacturing 100-1000 kg or importing 1 001 to 100 000 kg of MHDnMH in Canada. There were also fewer than 4 companies reporting a stakeholder interest in this substance.
In 2006, fewer than 4 companies identified themselves as manufacturing between 10 000 to 100 000 kg of MHDnMH in Canada and fewer than 4 companies identified themselves as importing less than 100 kg of the substance into the country. There were also fewer than 4 companies reporting a stakeholder interest in this substance.
The quantity reported to the Domestic Substances List (DSL) as being manufactured, imported or in commerce in Canada during the 1986 calendar year was between 100 – 1000 kg.
According to information from the United States Environmental Protection Agency (USEPA), the import/production of MHDnMH was in the range of 4 500 to 225 000 kg (originally reported as 10 000 to 500 000 lb) in 1994; no import or production data were reported in 1998 and 2002 (USEPA 2005).
MHDnMH was also used in Nordic Countries in the range of 6 000 to 60 000 kg from 1999 to 2008 in Sweden, and in the range of 400 to 13 000 kg from 2000 to 2008 in Denmark (SPIN 2006).
Information on uses for the 2005 and 2006 calendar years was also obtained as part of the response to the CEPA 1999 section 71 notices (Canada 2006 and 2009).
In 2005, MHDnMH was imported mainly for basic chemical manufacturing.
In 2006, the polymer was manufactured as a chemical intermediate for use in further plastic synthesis outside Canada. The substance was also imported in 2006 in a silicone elastomer mixture that contains between 1% and 59% of MHDnMH. It is used as a curing agent for silicone rubber, adhesive or protective coating resins (Environment Canada 2010a).
MHDnMH was notified as an ingredient in four types of cosmetic products in Canada: in bra adhesive (30-100% by weight); skin whitening cream (0.1-0.3% by weight); lipstick (< 0.1 % by weight); and eye makeup (< 0.1 % by weight) (CNS 2010). However, MHDnMH does not appear on the Cosmetic Ingredient Hotlist, Health Canada’s administrative list of ingredients that are intended to be prohibited or restricted for use in cosmetics in Canada (Health Canada 2009). MHDnMH is not currently used in any pest control products registered for use in Canada as either an active ingredient or a formulant (PMRA 2007). MHDnMH is not listed as an approved food additive under Division 16 of the Food and Drug Regulations (Canada 1978). MHDnMH was not identified in food packaging applications or in incidental additives (April 2010 email from Food Directorate, Health Canada, to Risk Management Bureau, Health Canada; unreferenced). MHDnMH is not listed in the Drug Product Database (DPD), the Therapeutic Products Directorate's internal Non-Medicinal Ingredient Database, the Natural Health Products Ingredients Database or the Licensed Natural Health Products Database as a medicinal or a non-medicinal ingredient present in final pharmaceutical products, natural health products or veterinary drugs (DPD 2010; NHPID 2010; LNHPD 2010; April 2010 email from Therapeutic Products Directorate, Health Canada to Risk Management Bureau, Health Canada; unreferenced).
The following DSL use codes have been identified for MHDnMH:
04- Adhesive / binder / sealant / filler
25- Humectant/dewatering aid/dehumidifier/dehydrating agent
89- Printing and Publishing
Other uses of MHDnMH have not been identified.
A method has been developed by Environment Canada to estimate a substance’s losses during different stages of its life cycle, including its fate after being incorporated into a finished product or article (Environment Canada 2008). This method consists of a life cycle analysis and a spreadsheet tool (Mass Flow Tool or MFT) that integrates information on the manufacturing, importation and use data available for the substance. Starting with an identified mass of the substance, each life cycle stage is subsequently evaluated until all of the mass is accounted for. Relevant factors are considered, uncertainties recognized and assumptions may be made during each stage, depending on information available. The estimated losses represent a complete mass balance over the life cycle of the substance and include releases to wastewater and other receiving compartments (land, air), chemical transformation, transfer to recycling activities and transfer to waste disposal sites (landfill, incineration). However, unless specific information on the rate or potential for release of the substance from landfills and incinerators is available, the method does not quantitatively account for releases to the environment from disposal. Ultimately, the estimated losses provide a first tier in the exposure analysis of a substance and help to estimate environmental releases and focus exposure characterization in the assessment.
In general, releases of a substance to the environment depend upon various losses from its manufacture, industrial use, and/or consumer/commercial use. These losses can be grouped into seven types: (1) discharge to wastewater; (2) emission to air; (3) loss to land; (4) chemical transformation; (5) disposal to landfill; (6) loss to incineration; and (7) disposal through recycling (i.e., recycling is deemed a loss and not considered further). They are estimated using regulatory survey data, industry data and data published by different organizations. The discharge to wastewater refers to raw wastewater prior to any treatment, whether it is on-site industrial wastewater treatment or off-site wastewater treatment. In a similar manner, the loss via chemical transformation refers to changes in a substance's identity that may occur within the manufacture, industrial use, and consumer/commercial use stages, but excludes those during waste management operations such as incineration and wastewater treatment. The loss to land includes unintentional transfer or leakage to soil or paved/unpaved surfaces during the substance’s use and service life (e.g., from the use of agricultural machinery or automobiles). The loss to land, however, does not include transfers subsequent to a substance’s use and service life (e.g., land application of biosolids and atmospheric deposition).
The losses estimated for MHDnMH over its lifecycle (based on conservative assumptions) are presented in Table 3 (Environment Canada 2010b). The estimated losses are calculated based on the identified uses in 2006.
Table 3. Estimated Losses of MHDnMH during Its Lifecycle
| Type of Loss | Proportion (%) | Pertinent Lifecycle Stages |
|---|---|---|
| Wastewater | 1.0 | Manufacture and industrial use |
| Air emission | <0.1 | Manufacture and industrial use |
| Land | 0.3 | Manufacture and industrial use |
| Chemical transformation | - | - |
| Landfill | 1.0 | Manufacture and industrial use |
| Incineration | - | - |
| Recycling | - | - |
| Export | 97.7 | Manufacture and industrial use |
According to the section 71 survey, the majority of the polymer was exported out of the country as a chemical intermediate in bulk to manufacture plastics (Environment Canada 2010a).
Although there is the possibility that other consumer/commercial products containing MHDnMH may be imported into Canada in addition to those reported as a result of industry surveys conducted pursuant to Section 71 of CEPA 1999, no information is available on the quantity of such imports. However, the actual mass of the substance lost from each of the life cycle stages may be higher than the estimates provided above, if such information was available for consideration.
Environmental fate of MHD5MH and MHD34MH may be inferred from their physical and chemical properties as indicated previously.
MHD5MH
Based on estimated physical and chemical properties (Table 2c), MHD5MH is characterized by very low water solubility (~2.5 × 10-6 mg/L), moderate vapour pressure (0.1 Pa), high log Koc value (5.7), and very high Henry’s Law Constant (2.0 × 107
Pa·m3/mol). Thus, partitioning to each of the air, soil, sediment and water compartments is potentially significant, depending on the compartment of release and the rates of partitioning relative to other fate processes such as advection and degradation.
The Level III fugacity model has been used to predict the environmental partitioning of the low molecular weight polymer, with consideration of the half-lives in air (estimated as 122.64 hours, equivalent to 5.11 days), water (4386 hours, equivalent to 182 days), soil (670 hours, equivalent to 4 weeks), and sediments (8760 hours, equivalent to 365 days). Half-lives used for Level III fugacity modelling are estimated later in this report (see Environmental Persistence section).
Table 4. Results of the Level III fugacity modelling for MHD5MH (EQC 2003)
| Substance released to: | Percentage of substance partitioning into each compartment | |||
|---|---|---|---|---|
| Air | Water | Soil | Sediment | |
| Air (100%) | 97.6 | 0.1 | 0.2 | 2.1 |
| Water (100%) | 0.1 | 3.4 | <0.1 | 96.4 |
| Soil (100%) | 55.1 | <0.1 | 43.6 | 1.2 |
If released to air, a high amount of the substance is expected to reside in this environmental compartment. Based on the moderate vapour pressure (modelled as 0.10 Pa) and very high Henry’s Law constant (calculated as 2.0 × 107Pa•m3/mole), the polymer is semi-volatile. Therefore, if released solely to air, MHD5MH will tend to reside in this compartment, with little partitioning to other environmental media.
If released into water, MHD5MH is expected to strongly adsorb to suspended solids and sediment based upon its high estimated log Koc value of ~6. Volatilization from water surfaces is expected to be an unimportant fate process. Thus, if water is a receiving medium, MHD5MH is expected to mainly partition to sediment with a small amount residing in water.
If released to soil, MHD5MH is expected to have moderate adsorptivity to soil (i.e., expected to be relatively immobile based upon its estimated log Koc). Volatilization from soil surfaces seems to be a significant fate process based upon the expectation that this compound’s Henry's Law constant is very high. This chemical may volatilize from soil surfaces based upon its vapour pressure (estimated at 0.1 Pa). Therefore, if released to soil, it is expected that less than half of the total quantity of MHD5MH will remain in the soil.
MHD34MH
Due to the high molecular weight, MHD34MH is not suitable for use with any model to predict its environmental fate. Based on the read-across of physical and chemical properties of PDMS, MHD34MH remains in a liquid state at environmental temperatures and is expected to be non-volatile and insoluble in water.
If released to air, a negligible amount of the substance is expected to reside in this environmental compartment, based on the assumptions that MHD34MH has a low vapour pressure, and the polymer is non-volatile. Therefore, if released solely to air, the polymer will tend to be deposited to soil and sediment.
If released into water, MHD34MH in emulsions is expected to strongly adsorb to suspended solids and sediment. In a study by Gettings and Lane (1982), a hydroxyl-endblocked PDMS (the physical and chemical properties equivalent to PDMS 55 cs) was adsorbed from water phase to suspended particulates and settled in the sediment without further remobilisation over a 6 week period. If mixed with sediment prior to the test, the polymer remained immobile during the test period. Eales and Taylor (1983) also studied the movement of PDMS (50cs) in sea sediment. The polymer was concluded to be bound firmly to marine sediment and unlikely to be remobilised by seawater. Volatilization from water surfaces is expected to be an unimportant fate process. Thus, if water is a receiving medium, MHD34MH is expected to mainly partition to sediment.
If released to soil, MHD34MH is expected to behave similarly to PDMS and demonstrate high adsorptivity to soil (i.e., expected to be relatively immobile based upon its large molecular size and assumption of high log Koc). In a study on the distribution in soil (Battelle 1992), there was no movement of a14C-PDMS (200 cs) after it was released into this environmental compartment, and it was not shown to partition into the aqueous or atmospheric phases. Therefore, if released to soil, MHD34MH is expected to mainly reside in this environmental compartment.
Environmental Persistence
No experimental degradation data for MHDnMH have been identified.
MHD5MH
QSAR models have been applied to the low molecular weight polymer MHD5MH to assess the potential for degradation. Such predictions are considered acceptable for use in this situation, as the molecular weight of MHD5MH is in the domain of all the models.
Table 5 summarizes the results of available QSAR models for the degradation of MHD5MH in water and air.
Table 5. Modelled data for degradation of MHD5MH
| Fate Process | Model and model basis | Model Result and Prediction | Extrapolated Half-life (days OR hours ) |
|---|---|---|---|
| Air | |||
| Atmospheric oxidation | AOPWIN 2008[1] | t 1/2 = 5.11 days | ≥ 2 |
| Water | |||
| Hydrolysis | HYDROWIN 2008[1] | n/a[2] | n/a |
| Primary biodegradation | |||
| Biodegradation (aerobic) | BIOWIN 2008[1] Sub-model 4: Expert Survey (qualitative results) |
3.11[3] “biodegrades fast” |
< 182 |
| Ultimate biodegradation | |||
| Biodegradation (aerobic) | BIOWIN 2008[1] Sub-model 3: Expert Survey (qualitative results) |
2.08[3] “does not biodegrade fast” |
≥ 182 |
| Biodegradation (aerobic) | BIOWIN 2008[1] Sub-model 5: MITI linear probability |
-0.78[4] “does not biodegrade fast” |
≥ 182 |
| Biodegradation (aerobic) | BIOWIN 2008[1] Sub-model 6: MITI non-linear probability |
0.00[4] “does not biodegrade fast” |
≥ 182 |
| Biodegradation (aerobic) | CATABOL c2004–2008 % BOD (biological oxygen demand) |
1.68 “biodegrades very slowly” |
≥ 182 |
[2] Model does not provide an estimate for this type of structure.
[3] Output is a numerical score from 0 to 5.
[4] Output is a probability score.
In air, a predicted atmospheric oxidation half-life of 5.11 days (see Table 5) indicates that MHD5MH is not likely to be rapidly oxidized. Reaction with ozone is not anticipated to be significant either. Therefore, MHD5MH is considered to be persistent in air. Depending on the media of release, and because of the substance’s anticipated moderate volatility and very high Henry’s Law constant, it is expected to be found in air in a significant portion, compared to its distribution to the other environmental media.
In water, MHD5MH is not expected to undergo significant hydrolysis in this environmental compartment. Although the BIOWIN (2008) aerobic biodegradation model (BIOWIN submodel 4) suggests fast primary degradation for the polymer, the four ultimate biodegradation models (BIOWIN submodels 3, 5, 6 and CATABOL) suggest that the substance does not biodegrade rapidly in water. When these results are considered, there is model consensus that the ultimate biodegradation half-life for MHD5MH in water is > 182 days.
MHD34MH
Due to its large molecular weight (2655.45 g/mol), the n=34 form of this polymer is considered to be out of the domain of QSAR models for predicting degradation in the aquatic environment. For the purpose of this assessment, experimental data for PDMS (presented below) are used to evaluate the potential for persistence of MHD34MH in the environment.
Air and Water
PDMS is not volatile and not expected to partition into the atmospheric compartment, and is thus not considered available for further degradation in this compartment (Graiver et al. 2003; ETETOC 1994). There is also no evidence indicating that PDMS would degrade fast in the aquatic environment. From the studies of wastewater treatment, PDMS was found not to degrade or decompose in the wastewater treatment process but end up in the solid sludge (Graiver et al. 2003). Having a hydrogen-dimethyl terminating group, MHD34MH is slightly more reactive than PDMS, but no significant reaction is expected in environmental conditions. Based on this information for PDMS, MHD34MH is thus not expected to degrade rapidly in air or water.
Soil
For degradation in soil, there have been a number of studies reporting the depolymerisation and rapid clay-catalyzed hydrolysis of PDMS such that the polymer is broken down to low molecule weight oligomers.
Buch and Ingebrigtson (1979) reported a half-life of approximately 30 days of PDMS (1 000 cs, equivalent to a molecular weight of ~15 000 g/mol) at a 6 000 mg/kg concentration in a dry Iowa topsoil. Such reaction was inhibited even with only 1% moisture content. In the same study, they also reported an extensive loss of the polymer (50 cs) due to degradation over the 53-day duration of the test.
There are several studies on the environmental behaviour of PDMS in soil (summarized in Graiver et al. 2003). Results from these studies indicate that PDMS quickly depolymerises by soil hydrolysis, thereby breaking the siloxane bonds and producing lower molecular weight oligomeric PDMS. Such soil-catalyzed hydrolytic degradation is random, not specific to certain bonds, and does not necessarily start from the chain end. Furthermore, the hydrolysis catalyzed by clay minerals in soil does not occur in any of the other media (air, water or sediment) to a significant degree.
Lehmann et al. (1994a) and Carpenter et al. (1995) also studied degradation under more realistic soil conditions, by analyzing the degradation product extracted with either 0.01 M CaCl2or tetrahydrofuran. The results indicate that the degradation of PDMS (204 cs) took place in the soil, which was gradually air drying from 12% to 3% moisture faster than the degradation of the substance maintained at constant moisture (12-13% moisture) in incubation chambers.
In another experiment, Lehmann et al. (1995) further studied 7 soils with widely differing properties. PDMS (350 cs) was spiked into moist soil and allowed to gradually dry for 2 weeks. PDMS degraded extensively after 14 days to lower molecular weight materials. Although degradation took place when the soil was still moist, the degradation rates were greatest in the most weathered soil. However, there is no correlation observed between PDMS degradation rate and the soil properties measured (pH, % organic matter, and % clay).
The rate of depolymerization by hydrolytic degradation in soil is fast but dependent on several factors such as the moisture level and the temperature. Lehmann et al. (1995) reported that the overall degradation rates were slower in moist soil than in the soil which was allowed to gradually dry for two weeks, indicating that continuous moisture could be an important factor, but moisture content is inversely related to the degradation rate (Lehmann et al. 1998b). In addition, the study results also demonstrated that warm temperatures favour the degradation rate during a yearly weather cycle.
In another study (Xu 1998), no PDMS was detected after 30 days of incubation in goethite and even faster degradation was observed when the polymer was incubated in Al-montmorillonite. For the impact of water content, the study reported a faster depolymerisation rate at the relative humidity (RH) of 32% compared to a RH of 100%. Considering the field data from different types of soil in the study, the PDMS depolymerization half-life is expected to be no longer than 3 weeks.
Based on results from two experimental studies, PDMS is anticipated to undergo clay-catalyzed hydrolysis rapidly in soil with a half-life of a few weeks. Given this evidence for PDMS, MHD34MH is anticipated to hydrolyze rapidly in soil with a half-life of a few weeks, which is much less than 182 days; therefore the polymer is considered to be not persistent in this environmental medium.
Dimethylsilanediol (DMSD) is the major product from soil-catalyzed hydrolysis of PDMS (Stevens 1998; Craig 2003). Following the polymer decomposition phase of the experiment, radio-labelled 14C was used to detect the source of CO2, which was eventually coming from the biodegradation of the 14C-PDMS degradation products. DMSD biodegradation was illustrated by Sabourin et al. (1996), who reported that biodegradation of DMSD began with the oxidation of a methyl group producing methylsilanetriol. Methylsilanetriol then further degrades to CO2 and silica.
Volatilization may also account for DMSD loss from the soil and compete with losses that result from biodegradation. After its release in the air, the methyl groups of DMSD will undergo reaction with OH radicals that are generated in the presence of sunlight (Atkinson 1991). Neither ozone nor the NO3 radicals were found by these authors to react with methylsiloxanes, but the reaction with OH radicals yielded atmospheric lifetimes of 10-30 days for several oligomeric methylsiloxanes. In another similar study (Sommerlade et al. 1993), lifetimes of 2-9 days in air were found for various oligomeric methylsiloxanes and trimethylsilanol, and the identification of several reaction products showed that methyl groups were being replaced with hydroxy groups.
Based on the experimental evidence from PDMS and the structural similarity between the analogue and the polymer, MHD34MH is expected to degrade rapidly in soil and does not persist in this environmental medium.
Sediment
In sediment, degradation of PDMS has also been observed; however, the rate is apparently much slower than in soil, as the half-life is more than a year (Environment Canada 2010a).
An experiment (Christensen 1994) has examined the potential for PDMS degradation in freshwater sediment. A concentration of 500 ppm of 14C-labelled PDMS (350 cs) was added into sediment/water columns. Water soluble materials and the off-gas were monitored as the evidence of degradation. After 56 days, no evidence of 14CO2 or water soluble14C was observed, indicating no degradation in sediment occurred during the test period.
In other longer incubation studies, degradation of PDMS in sediment was, however, evident (Fendinger et al. 1997). Following a year of incubation under aerobic conditions, 5-10% of the PDMS (350 cs) was hydrolyzed to DMSD and approximately 0.25% of the total14C had been oxidized to carbon dioxide (Carpenter et al. 1995). The rate and extent of DMSD formation and carbon dioxide production from sediment agree closely with the rates observed by Lehmann et al. (1994a, 1994b) in moist soil (3% DMSD and 0.13% carbon dioxide formed in 6 months). Given its high adsorption and immobility in sediment in combination with the water content of the substrate, it is anticipated that PDSM degrades very slowly in sediment and the half-life of the polymer in this environmental medium is expected to be longer than 365 days. The prediction can be extrapolated to the polymer under assessment such that MHD34MH is not expected to degrade rapidly in sediment either, and the half-life in this environmental medium is expected to be more than 365 days.
Based on the modelled data for the low molecular weight polymer (MHD5MH) (see Table 5) and experimental studies on the analogue polymer (PDMS) as read-across for the high molecular weight polymer (MHD34MH), MHDnMH is expected to degrade rapidly in soil (half-life in soil = 182 days). However the substance meets the persistence criteria in air, water and sediment (half-life in air = 2 days, half-life in water = 182 days, and half-life in sediment = 365 days) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000), and therefore is concluded to be persistent in the environment.
There are no experimental data on the potential for bioaccumulation of MHDnMH.
Experimental data for the analogous linear methyl siloxane have been used to predict the bioaccumulation potential for MHD5MH. Additionally, QSAR models have also been applied for estimating a bioaccumulation factor (BAF) and bioconcentration factor (BCF) for this low molecular weight compound.
For the large molecular weight polymer, MHD34MH, experimental data for PDMS have been used to assess potential for bioaccumulation.
MHD5MH
Modelled data for bioaccumulation for MHD5MH are summarized in Table 6 below.
Table 6a. Modelled data for bioaccumulation for MHD5MH
| Test organism | Endpoint | Value wet weight (L/kg) | Reference |
|---|---|---|---|
| Fish | BCF | 1.04 × 104 | BCFBAF 2008 (mid trophic level) |
| Fish | BCF | 7.41 × 104 | Arnot and Gobas 2003 (mid trophic level) |
| Fish | BCF | 3.24 × 104 | BBM with Mitigating Factors 2008 |
| Fish | BAF | 5.48 × 106 | BCFBAF 2008 (mid trophic level) |
| Fish | BAF | 3.39 × 106 | Arnot and Gobas 2003 (mid trophic level) |
According to the model predictions (based on the modelled log Kow= 6.60), MHD5MH may have a high BAF and BCF in fish. However, experimental results indicate different bioaccumulation potentials of the low molecular weight linear methyl siloxanes.
Annelin and Frye (1989) reported no intake of the linear PDMS oligomers, having molecular weights greater than 384 g/mol (MD3M, which is the smallest form of PDMS), by fish after an exposure period of greater than 5 weeks. The experimental results indicated that none of the MD3M, MD4M, or MD7M (MW=607 g/mol) was bioconcentrated in rainbow trout after 35 to 77 days exposure.
In another study on absorption and retention of PDMS in fish (Bruggeman et al. 1984), a number of linear methyl siloxanes were present in food at the average daily intake of 1 × 10-3mg/g of fish for 6-8 weeks. At the end of feeding period, MD3M and MD4M were found below detection limits (approximately 3 × 10-4 mg /g) in all fish samples, while MD5M and other longer linear methyl siloxanes did not exceed 2 × 10-3 mg/g. Comparing to the average daily intake (1 × 10-3 mg/g), these results indicated that absorption and retention and accumulation of MD3-6M via food is not significant. It therefore can be expected that MHD5MH (with a similar linear structure and a molecular weight higher than MD3M and MD4M) possesses a similar bioaccumulation potential and is also not likely to bioaccumulate in fish.
Investigations by Dimitrov et al. (2002), Dimitrov et al. (2005) and the Baseline Bioaccumulation Model (BBM 2008) suggests that the probability of a molecule crossing cell membranes as a result of passive diffusion declines significantly with increasing maximum cross-sectional diameter (Dmax). The probability of passive diffusion lowers appreciably when cross-sectional diameter is > ~1.5 nm and more significantly for molecules having a cross-sectional diameter of >1.7 nm. Sakuratani et al. (2008) have also investigated the effect of cross-sectional diameter on passive diffusion from a test set of about 1200 new and existing chemicals. They also observed that substances not having a very high bioconcentration potential often have a Dmax>2.0 nm and an effective diameter (Deff) >1.1 nm.
According to the estimation of physical and chemical properties for MHD5MH, the substance has a cross-sectional diameter in the range 1.46 to 2.18 nm. Based on the observation mentioned above (Sakuratani et al. 2008), the n=5 form of the polymer is associated with a potential for reduced uptake rate from water and reduced in vivo bioavailability of MHD5MH.
Based on the experimental data for analogous linear methyl siloxanes, with consideration of the molecular size, MHD5MH is not expected to bioaccumulate in aquatic organisms.
MHD34MH
Compared to the low molecular weight form of MHDnMH (n=5), MHD34MH would have a correspondingly much larger molecular size. Such character would limit the partitioning behaviour (i.e., crossing biological membranes), and therefore make the polymer even less bioavailable and less likely to bioaccumulate in aquatic organisms. Data from studies on PDMS are used to characterize the bioaccumulation potential for MHDnMH (see data summarized in Table 6b below).
Table 6b. Empirical data for bioaccumulation and bioconcentration of PDMS
| Polymer Character (Molecular Weight, Viscosity or Emulsion) |
Test Organism | Experimental Concentration (mg/L) and/or Exposure Source | Endpoint of BAF/BCF (L/kg) and/or BMF[1](dimensionless) | Reference |
|---|---|---|---|---|
| 300 cs 15% emulsion | Bluegill sunfish (Lepomis macrochirus) |
1.5 | BCF = 0.16 – 0.5 (after 2 weeks) |
Hobbs et al. 1975 |
| MD15-17M | Guppies (Poecilia reticulate) |
Saturation | BCF < 10 | Opperhuizen et al. 1987 |
| Approximately 32 mg/kg in dry food | BAF < 0.01 | |||
| Commercial PDMS MW (g/mol): 1200 6000 25000 56000 |
Carp sp. | Saturation solutions measured concentrations 1.330 0.486 0.135 0.060 |
BCF = 2.9 BCF = 7.1 BCF = 386 BCF = 1250 |
Watanabe et al. 1984a |
| 50 cs 35% emulsion |
Level 1: Phytoplankton (Tetraselmis sp.) Level 2: Mollusc (Mytilus edulis) |
70 Direct exposure from water Exposure from water and food |
BCF = 2.08 BMF = 0.12 |
Aubert et al. 1985 Guillemaut et al. 1987 |
| Level 1: Plankton (Tatreselmis sp.Artemia salina) Level 2: Fish (Carassius auratus) |
70 Direct exposure from water Exposure from water and food |
BCF = 1.9 BMF = 0.05 |
||
| Level 1: Annelid (Nereis diversicolor) Level 2: Fish (Scorpaena porcus) |
70 Direct exposure from water Exposure from water and food |
BCF = 0.036 BMF = 1.4 |
||
| Level 1: Annelid (Nereis diversicolor) Level 2: crab (Carcinus maenas) |
70 Direct exposure from water Exposure from water and food |
BCF = 0.036 BMF = 1.09 |
||
| 50 cs | Bullhead catfish (Ictalurus melas) |
Exposure from food | < detection level | Annelin 1979 |
Generally, PDMS with a variety of molecular weights (MW=1200 g/mol and above) demonstrates low bioaccumulation and bioconcentration in aquatic organisms and terrestrial species.
Opperhuizen et al. (1987) conducted a bioaccumulation study on guppies (Poecilla reticulate) using PDMS (5 cs, MD15-17M). After 12 weeks of exposure, siloxane polymers were not detected in the whole fish.
In another study, bluegill sunfish (Lepomis macrochirus) were exposed to a PDMS (molecular weight > 5 000 g/mol) for 30 days (Hobbs et al. 1975). There was no relationship found between the polymer tissue levels and duration or level of exposure. It was observed, however, that the polymer was adsorbed to surfaces of the fish, and not taken up into the cells.
In addition, there were studies of PDMS uptake by other species in other environmental media. In a 28-day study by Garvey et al. (1996), earthworms (Eisenia foetida) were exposed to 100 and 1 000 mg/kg PDMS in an artificial soil matrix. At termination of the exposure phase, the earthworms from each replicate were transferred to exposure media that contained no PDMS for the 14-day depuration phase of the study. Samples were collected through the 28-day exposure phase; however there was no statistical difference in the tissue residue concentrations between any the two groups. Furthermore, PDMS ingested by the earthworms and present in the gut was rapidly depurated during a following 14-day period.
Kukkonen and Landrum (1995) examined aquatic worms living in the sediments and concluded that there was no bioaccumulation of PDMS observed in these worms.
Results from studies on other benthic and terrestrial organisms (Putt 1994; Putt and Mihaich 1996) also indicated very limited bioaccumulation potential for PDMS.
There is one study that reports a contradictory finding, in which the higher molecular weight PDMS materials bioaccumulated more than lower molecular weight compounds – based on tests using silver carp (Watanabe et al. 1984a). The fact that the relationship between bioaccumulation and the molecular weight is the reverse of what would be expected is probably the result of surface contamination, and no evidence was available to indicate whether whole fish or fillet were analysed. Thus, these results are of uncertain quality.
Biomagnification or food-chain transfer of a PDMS (50 cs) emulsion in the marine environment has been studied (Aubert et al. 1985; Guillemaut et al. 1987). Organisms in the first level of the food chain were exposed to emulsified PDMS (50 cs) at a concentration of 70 mg/L in water. PDMS was either not bioconcentrated in water or the BCF was <2 in the first level of the food chain. The bioaccumulation between the two trophic levels was low, ranging between 0.05 and 1.4, indicating that the biomagnification of PDMS between two trophic levels is not significant in the aquatic food chain tested.
Based on the above information on PDMS, it is expect that MHD34MH is not bioavailable in either the aquatic or soil dwelling organisms. The polymer does not bioaccumulate in those organisms either.
Given the assessment on the bioaccumulation potentials for both the low and number-average molecular weight polymers, it is concluded that MHDnMH does not meet the bioaccumulation criteria (BAF or BCF = 5000) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Ecological Effects Assessment
A - In the Aquatic Compartment
There were no experimental toxicity data found for MHDnMH.
MHD5MH
For MHD5MH, QSAR models have been used to estimate the aquatic toxicity and the modelled data are summarized in Table 7a below.
Table 7a. Modelled data for aquatic toxicity of MHD5MH
| Test organism | Type of test | Endpoint | Value (mg/L) |
Model |
|---|---|---|---|---|
| Fish | Acute (96 hours) | LC50[1] | 0.035[*] | ECOSAR 2008 |
| Acute (14 days) | 0.038[*], [!] | |||
| Fish (sea water) | Acute (96 hours) | LC50 | 0.035[*] | |
| Daphnid | Acute (48 hours) | LC50 | 0.039[*] | |
| Green Algae | Acute (96 hours) | EC50[2] | 0.137[*] | |
| Mysid Shrimp | Acute (96 hours) | LC50 | 0.001[*] | |
| Fish | Chronic (30 days) | ChV[3] | 0.005[*] | |
| Daphnid | Chronic | ChV | 0.009[*] | |
| Green Algae | Chronic | ChV | 0.112[*] | |
| Mysid Shrimp | Chronic | ChV | 0.00003[*] | |
| Fish (sea water) | Chronic | ChV | 0.072[*] | |
| Earthworm | Chronic (14 days) | LC50 | 293.13[*] | |
| Fathead Minnow | Acute (96 hours) | LC50 | 146[*] | AIEPS 2003-2007 |
| Daphnia magna | Acute (48 hours) | LC50 | 13.19[*] | |
| Pseudokirchneriella subcapitata | Acute (72 hours) | EC50 | 14.09[*] | |
| Daphnia magna | Acute (48 hours) | EC50 | 0.18[*] | OASIS Forecast 2005 |
| Pimephales promelas | Acute (96 hours) | EC50 | 0.43[*] |
[2] EC50- The concentration of a substance that is estimated to cause some effect on 50% of the test organisms.
[3] ChV – Chronic toxicity value
[*] The exposure concentration is at least 10 times higher than the estimated water solubility, indicating that no effects at saturation are predicted for the subject chemical.
[!] Critical toxicity value (CTV). It is further used to derive the predicted no effect concentration (PNEC).
The model estimates of aquatic toxicity for MHD5MH have been summarized in Table 7a. It is noted that the modelled values of all endpoints are far higher than the modelled water solubility of MHD5MH (2.7 × 10-6 mg/L). These results therefore suggest that, at the maximum soluble level, MHD5MH does not have any observable effect on aquatic organisms in short term exposures. Nonetheless for the purposes of a conservative toxicity assessment for MHD5MH, a modelled 14-day LC50 = 0.038 mg/L on fish has been used as the critical toxicity value (CTV) to characterize the risk of MHD5MH on aquatic organisms.
MHD34MH
There are no QSAR models applicable for modelling the ecotoxicity for the polymer with a large molecular weight. Information on PDMS has thus been used to assess the toxicity of MHD34MH.
There are a number of toxicity studies reporting effects of PDMS on aquatic organisms and their results are summarized in Table 7b. As mentioned earlier in this report (see Potential for Bioaccumulation section), PDMS is not taken up by aquatic organisms (Annelin and Frye 1989; Bruggeman et al. 1984); therefore, effects are not expected. Because PDMS is extremely hydrophobic and tends to adsorb strongly to solid particles, the polymer in emulsion form has been used in many studies to maintain constant and nominal exposure concentrations. These concentrations are higher than the water solubility of PDMS.
In general, PDMS has demonstrated low toxicity to fish (see Table 7b). A chronic no observed effect concentration (NOEC) of 91 mg/L of PDMS (50 cs emulsion) to the sheepshead minnow was reported by Hill et al. (1984). In another study, Hill et al. (1980) reported an LC50 at the exposure level of 350 mg/L of PDMS (50 cs emulsion) to plaice. The toxic effects of PDMS emulsions at high concentrations were shown to have been caused by the emulsifier used in the formulation (Hobbs et al. 1975; Aubert et al. 1985).
PDMS has also shown low toxicity to aquatic invertebrates (see Table 7b). An LC50 of 1980 mg/L of PDMS (50 cs emulsion) has been reported from a study on the mussel Mytilus sp. (Hill et al. 1984). However the adverse effects are somewhat difficult to interpret. PDMS fluids are insoluble in water and have a specific gravity less than 1000 kg/m3. Therefore the polymer forms a surface film in which the aquatic invertebrate tends to be caught. Such entrapment may occur and therefore interfere with the assessment of the chemical toxicity of the polymer.
Toxicity to aquatic microorganisms has also been studied and PDMS has been found not to be very hazardous to such organisms (see Table 7b).
Since the exposure concentrations of PDMS are well above the maximum solubility expected for the polymer in the available aquatic toxicity studies, the polymer likely poses low hazard to aquatic organisms under environmental conditions.
The Critical Toxicity Value (CTV) is selected from a study by Hill et al. (1977) conducted with PDMS (50 cs, 35% emulsion), since it has a molecular weight closest to the average molecular weight of MHD34MH. For the purpose of characterizing the risk of MHD34MH on aquatic organisms, the NOEC of 91 mg/L reported in this study has been used as the CTV to derive the predicted no effect concentration (PNEC).
Table 7b. Toxicity data of PDMS on aquatic organisms
| Polymer Character -- (viscosity and/or emulsion) |
Test Organism | Type of Test | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|---|
| Fish | |||||
| Not specified | Fish | Acute | LC50[1] | >1000 | Annelin and Humble 1978; Hobbs et al. 1975 |
| Not specified | Minnows (Phoxinus phoxinus) |
Semi-chronic | LT40[2] | 3000 (8 days) | Cabridenc 1978 |
| 100, 350, 12500 cs | Pomatoschistus minutus Gasterosteus aculeatus |
Acute (96 hours) |
LC0[3] | At saturation | Maggi and Alzieu 1977 |
| 50 cs 20% emulsion |
Plaice (Pleuronetes platessa) |
Acute (96 hours) |
EC[4] | 88 | Firmin 1984 |
| 50 cs 35% emulsion |
Scorpion fish Scorpaena porcus Carassius auratus |
Acute | LT50[5] | 1000 (12 hrs) 2000 (50 hrs) 10 000 (hrs) 700 (50 hours) 3500 (24 hours) |
Aubert et al. 1985 |
| 50 cs | Plaice (Pleuronectes platessa) |
Acute (96 hours) |
LC50 | 350 > 10 000 at the surface of the water (5 mg/L in water) |
Hill et al. 1980 Hill 1980 |
| 50 cs 35% emulsion |
Sheepshead minnow (Cyprinodon variegatus) |
Chronic (33 days) |
NOEC[6] EC |
91[!] 235 (minor effects observed) |
Hill et al. 1984 |
| 350 cs | Rainbow trout Oncorhynchus mykiss) almo gairdneri) |
Sub-chronic spiked food (28 days) | NOEL[7] | 10 000 mg/kg bw/day | Mann et al. 1977 |
| 350 cs 30% emulsion |
Bluegill sunfish (Lepomis macrochirus) Rainbow trout (Salmo gairdneri) |
Acute (96 hours) |
LC50 Hypoactivity |
> 10 000 At both 1000 and 10000 |
Hobbs et al. 1975 |
| Aquatic invertebrate and molluscs | |||||
| 10 – 60000 cs 66% emulsion |
Daphnia | Acute | NOEC | >200 | Annelin et al. 1994 |
| 350 cs emulsion |
Daphnia magna | Acute (48 hrs) |
LC50 | 1000 | Spacie 1972 |
| 100, 350, and 12,500 cs | European oyster (Ostrea edulis) Mussel sp. (Mytilus edulis) Periwinkle (Littorina littorea) |
Acute (96 hours) |
LC0 | Saturation in sea water | Maggi and Alzieu 1977 |
| 50 cs 35% emulsion |
Mussel (Mytilus edulis) Crab (Artemia salina Carcinus maenas) |
Acute Semi-chronic (10 days) Semi-chronic |
LT50 LT50 LC0 LC50 Mortality |
3500 (96 hrs) 10000 (80 hrs) (emulsifier only) 700 7000 (9 days) 3500 (10 days) |
Aubert et al. 1985 |
| 50 cs 20% emulsion |
Mytilus sp. | Acute (96 hours) |
LC50 | 1980 | Hill et al. 1984 |
| 50 cs | Mytilus sp. | Acute (96 hours) |
LC50 | 1020 | Hill et al. 1984 |
| 100 cs 100 cs 30% emulsion 350 cs 30% emulsion |
Daphnia magna Daphnia magna Cockles (Prothaca spaminea) Mummichog (Fundulus heteroclitus) Shore crabs (Pachygrapsus crassipes) Brown shrimp (Penaeus oxtecus) |
Acute (48 hrs) Acute (48 hrs) Acute (96 hrs) |
LC50 LC50 LC50 |
44.5 (polymer formed a layer on the surface) 73.4 >1000 |
Hobbs et al. 1975 |
| Aquatic microorganisms (bacteria, fungi, and phytoplankton) | |||||
| 55 cs hydroxyl-endblock | Aerobic and anaerobic bacteria, algae, and protozoa | Chronic (24 weeks) |
NOEC | > 0.26 | Gettings and Lane 1982 |
| 100, 350, and 12,500 cs | Marine algae (diatoms and flagellates) |
Semi-chronic (9 days) |
NOEC | Saturation in sea water | Maggi and Alzieu 1977 |
| 50 cs 35% emulsion |
Marine phytoplankton Tetraselmis sp.) |
Not specified | LOEC[8] | 350 | Maggi and Alzieu 1977 |
[2] LT40 – The concentration of a substance that is used in time-until-death to determine 40% lethality of the test organisms in hours.
[3] LC0- The concentration of a substance that is estimated to have no lethal effect on the test organisms.
[4] EC - The concentration of a substance that is estimated to demonstrate toxic effect on the test. organisms, however the percentage of affected test organisms can not be determined in the study.
[5] LT50 – The concentration of a substance that is used in time-until-death to determine 50% lethality of the test organisms in hours.
[6] NOEC – The No Observed Effect Concentration is the highest concentration in a toxicity test not causing a statistically significant effect in comparison to the controls.
[7] NOEL – The No Observed Effect Level is the highest level of exposure in a toxicity test not causing a statistically significant effect in comparison to the controls.
[8] LOEC – The Lowest Observed Effect Concentration is the lowest concentration in a toxicity test causing a statistically significant effect in comparison to the controls.
[!] CTV – Critical Toxicity Value. It is further used to derive the predicted no effect concentration (PNEC).
Based on the model predictions, MHD5MH is not expected to demonstrate observable effects on aquatic organisms at its maximum water solubility level. For MHD34MH, considering the structural similarity to PDMS, it is also not anticipated to have any significant effects on aquatic organisms also within the range of its water solubility. Therefore, it is concluded that MHDnMH has low toxicity to aquatic organisms.
B - In Other Environmental Compartments
The primary route of exposure in an aquatic environment would be through contact with the polymer absorbed to sediments, due to its low water solubility; and therefore, the potential effects of the polymer on sediment-dwelling organisms is also considered in this screening assessment. Furthermore, since biosolids from treated wastewater system sludge is often used to augment agricultural soils and reclaim land, this pathway of exposure to the polymer to terrestrial organisms is also considered.
Since there are no experimental sediment or soil toxicity data for MHDnMH, information on PDMS is used to assess the toxicity of MHDnMH (see Table 7c below). In summary, PDMS demonstrates low toxicity to sediment and soil-dwelling organisms.
Table 7c. Toxicity data for PDMS on sediment and soil organisms
| Polymer Character (viscosity and/or emulsion) |
Test Organism | Test Medium and Type |
Endpoint | Concentration Value | Reference |
|---|---|---|---|---|---|
| Sediment organisms | |||||
| 350 cs | Marine amphipod (Ampelisca abdita) | Sediment Semi-chronic (10 days) |
LC0[1] | >2300 | Putt and Mihaich 1996 |
| 350 cs | Freshwater amphipod (Hyallela azteca) | Sediment Chronic (28 days) |
EC0[2] | >2200 | Putt and Mihaich 1996 |
| PDMS (viscosity/ emulsion characteristics not noted) | Polychaete worm (Nereis diversicolor) | Sediment Chronic (28 days) |
EC0 | 1000 | Craig et al. 1984 |
| 50 cs | Annelids | Sediment Acute (96 hours) Chronic (28 days) |
EC0 | >10 000 | Craig and Caunter 1990 |
| 50 cs 35 % emulsion (sea water) |
Polychaete worm (Nereis diversicolor) | Sediment Semi-chronic (9 days) |
LC0 EC0 | > 350 > 700 | Aubert et al. 1985 |
| 350 cs | Benthic invertebrates (H. azteca and C. tentans) |
Sediment Semi-chronic (10 days) Life-cycle (28 days for H. azteca and 50-65 days forC. tentans) |
LC0 EC0 | > 1000 | Henry et al. 2001 |
| Soil organisms | |||||
| 350 cs fluid |
Earthworm (Eisenia foetida) | Soil Chronic (21 days) |
EC0 | 1100 | Garvey et al. 1996 |
| 350 cs fluid |
Springtail (Folsomia candida) | Soil Chronic (21 days) |
NOEC[3] | 250 | Garvey et al. 1996 |
| Plant | |||||
| 10 cs 10% emulsion |
Conifer plantation | Soil Acute (right after the treatment) Chronic (1 year) |
NOEL[4] | 30 ml/m3 | Belt et al. 1977 |
| Birds | |||||
| 100 cs | Mallard duck (Anas platyrhynchos) Bobwhite quail (Colinus virginatus) |
Semi-chronic (5 days) |
LC0 | >5000 | Hobbs et al. 1975 |
| 100 cs | White Leghorn chickens | Chronic (24 weeks) |
EC0 | >5000 | Hobbs et al. 1975 |
[2] EC0- The concentration of a substance that is estimated to observed adverse effect on the test organisms.
[3] NOEC – The No Observed Effect Concentration is the highest concentration in a toxicity test not causing a statistically significant effect in comparison to the controls.
[4] NOEL – The No Observed Effect level is the highest level of exposure in a toxicity test not causing a statistically significant effect in comparison to the controls.
The effect of PDMS (5 to 1 000 cs) was also evaluated on adultAcheta domesticus (cricket) by direct application of the polymer (5 × 10-3 mg) to the ventral thorax (Levier 1988). The time of loss of righting reflex (the ability of a cricket to flip back over when placed on its back) increased with the viscosity of the PDMS and the mortality at 48 hours decreased 2 fold when the viscosity of PDMS increased 200 fold. A similar effect of PDMS (10 cs) was observed in another study (Nielson et al. 1975).
Based on study results for PDMS, it is expected that MHDnMH does not have a significant potential for toxicity to either soil or sediment-dwelling organisms.
Ecological Exposure Assessment
A – Industrial Release
No data concerning concentrations of MHDnMH in the environmental media in Canada have been identified. Environmental concentrations are therefore estimated based on the exposure analysis for the polymer with consideration of the quantities of MHD34MH manufactured in Canada, as well as MHD5MH contained in the polymer.
The Mass Flow Tool applied realistic worst-case assumptions for release of MHDnMH to water (via wastewater effluent) from industrial use (Table 3). To address releases from industrial activities, a site-specific scenario was employed to estimate a conservative substance concentration in a watercourse receiving industrial effluents (Environment Canada 2010c). The scenario is designed to provide these estimates based on the amount of chemical processed and released, the number of processing days, wastewater treatment plant (WWTP) removal rate, and the size of the receiving watercourse.
The industrial-release in the site-specific scenario was based on loading data from sources such as industrial surveys and knowledge of the distribution of industrial discharges in the country, and calculates a predicted environmental concentration (PEC).
The aquatic exposure of siloxane polymer is expected if the substance is released from industrial use to a wastewater treatment plant that subsequently discharges its effluent to a receiving water body. The concentration of the substance in the receiving water near the discharge point of the wastewater treatment plant is used as the predicted environmental concentration (PEC) in evaluating the aquatic risk of the substance. It can be calculated using the equation:
Cwater-ind = [1000 × Q × L × (1 - R)] / [N × F × D]
where
Cwater-ind: aquatic concentration resulting from industrial releases, mg/L
Q: total substance quantity used annually at an industrial site, kg/yr
L: loss to wastewater, fraction
R: wastewater treatment plant removal rate, fraction
N: number of annual release days, d/yr
F: wastewater treatment plant effluent flow, m3/d
D: receiving water dilution factor, dimensionless
The site-specific scenario is made conservative by assuming that the total quantity of the substance used by Canadian industry is used by one single industrial facility at a small, hypothetical site and the loss to a local WWTP is high at 1% of the total quantity resulting from the cleaning of chemical containers and process equipment. At the selected site, the WWTP effluent flow is estimated at the 10th percentile (3.3 × 104 m3/d) of its discharge rate. The scenario also assumes that the release occurs 250 days per year, typical for small and medium-sized facilities.
Removal of a polymer in wastewater treatment is dependent on its solubility and potential to adsorb to sludge. In addition, the molecular weight is considered since large molecule weight polymers are associated with a higher rates of removal from effluent (i.e., to biosolids) in comparison to those with lower molecular weights (USEPA 2008). Based on these considerations, the WWTP removal rate is estimated as 75% for the MHD5MH and 95% for MHD34MH. The receiving water dilution factor is 3.8 for this site-specific scenario.
For the n=5 form, the relevant upper bound of a reporting range (100 – 1000 kg/year), is used to calculate the environmental concentration.
For the n=34 form, the upper bound of the reported use quantity range of 10 000 to 100 000 kg/year in 2006 was applied.
Based on the above assumptions, the predicted environmental concentration (PEC) is 8 × 10-5 mg/L for MHD5MH, and 1.6 × 10-3 mg/L for MHD34MH (Environment Canada 2010c).
It is noted that the predominant pathway of MHDnMH release to in the environment is through soil amendment with biosolids. Based on the substance’s high molecular weight and very low water solubility, and with consideration given to the toxicity data for the analogue (PDMS), it can be assumed that landfilled or incinerated sludge associated MHDnMH is not expected to be dispersed in the environment. In soil, the substance is expected to be immobile and undergo rapid degradation, and consequently will result in no significant exposure to aquatic or terrestrial organisms.
B – Consumer/Commercial Releases
When used as a curing agent for silicone rubber, adhesive or protective coating resins, most of MHDnMH is incorporated into the polymers via cross-linking. The majority of the adhesive matrix is expected to be sent to waste disposal sites with the cured objects. The overall release of the substance to environmental media (water and soil) due to consumer use is expected to be very low. Therefore, wildlife exposure to the substance is anticipated to be negligible.
Characterization of Ecological Risk
The approach taken in this ecological screening assessment was to examine various supporting information and develop conclusions based on a weight-of-evidence approach and using precaution as required under CEPA 1999. Lines of evidence considered include results from a conservative risk quotient calculation, as well as information on persistence, bioaccumulation, toxicity, sources and fate of the substance.
MHDnMH is expected to be persistent in air, water, and sediment. The polymer is expected to have a low bioaccumulation potential. The moderate volumes of MHDnMH that are manufactured or used in Canada, along with information on its uses, indicate a low potential for release into the Canadian environment. When released to water, it may initially float like PDMS and will eventually partition into sediment. For the effects in water, the polymer is expected to have low potential for toxicity to aquatic organisms.
PNECs were calculated for both low and number-average molecular weight polymers separately.
For MHD5MH, a modelled 14-day LC50 = 0.038 mg/L on fish has been used as the CTV value to characterize the risk on aquatic organisms. It is noted that this modelled value is lower than those reported from laboratory studies. An assessment factor of 100 (to account for interspecies and intraspecies variability in sensitivity, extrapolation from acute toxicity in a laboratory to chronic toxicity in the field, and uncertainty associated with the model prediction) is applied to this CTV resulting in a PNEC for MHD5MH of 3.8 × 10-4mg/L.
For MHD34MH, the PNEC was derived from the chronic no effect toxicity value of 91 mg/L (as the most sensitive valid experimental value for a PDMS, which has the closest molecular weight to MHD34MH), by dividing this value with an assessment factor of 100 (to account for interspecies and intraspecies variability in sensitivity and uncertainty associated with the data from an analogue polymer) to give a value of 0.91 mg/L.
The risk quotient analysis was conducted for both MHD5MH and MHD34MH. This analysis was performed for the aquatic medium to determine whether there is potential for ecological harm in Canada, by integrating conservative estimates of exposure with toxicity information.
The site-specific industrial scenario (considering the actual receiving water body) presented above yielded the PECs of 8 × 10-5 and 1.6 × 10-3 mg/L for MHD5MH and MHD34MH respectively (Environment Canada 2010c). The risk quotients (RQ=PEC/PNEC) are approximately 0.21 for MHD5MH and 2 × 10-3 for MHD34MH. Therefore harm to aquatic organisms is unlikely.
This information suggests that MHDnMH (n=5) does not have the potential to cause ecological harm to aquatic organisms in Canada.
When MHDnMH is released into a water body, it partitions into suspended particulate matter and to bottom sediments, where sediment-dwelling organisms would be exposed to the substance. However, no environmental monitoring data specific to sediment-dwelling organisms are available for this substance in Canada. Based on the experimental data for the analogue (PDMS), it is expected that MHDnMH would demonstrate low toxicity to benthic species. Considering the possible exposure to the polymer, harm to sediment-dwelling organisms from MHDnMH in Canada is unlikely.
Uncertainties in Evaluation of Ecological Risk
One major uncertainty in the assessment is due to the complexity associated with the polymer. Unlike any discrete chemical, the polymer can only be described by a distribution of homologous structures with varying molecular weight. The average molecular weight, the content of any molecular weight species, and impurities vary depending on different polymer formulations. It is noted that the assessment has addressed concerns on the polymer in commerce in Canada (represented as MHD34MH), meanwhile also included the molecular weight component as 500 g/mol (represented as MHD5MH).
Another major uncertainty is due to a lack of data on the physical and chemical properties specific to MHDnMH. The polymer can only be characterized by structures of the monomer. Given the molecular weight >1 000 g/mol, the polymer has generally been considered out of the domains of available QSAR models. Therefore, model predictions are only applied for the polymer MHD5MH, while experimental data for analogous siloxane chemicals have been used to assess both MHD5MH and MHD34MH. Comparison of the chemical compositions in MHDnMH and the analogues, suggests that the difference in the repeating units and the terminating units will not significantly influence their physical and chemical properties.
Given the QSAR models do not contain many siloxane compounds in the training set, the model predictions on MHD5MH are uncertain and interpreted with scientific judgment. Key parameters of physical and chemical properties are estimated by QSAR models and compared to experimental measures of the analogous linear methyl siloxanes for further modelling. When there is conflict in predicting the bioaccumulation potential, more weight is given to the observation in the experimental studies on the suitable analogues.
The uncertainty is also associated with the lack of information on environmental concentrations in Canada of MHDnMH. However, the majority of manufactured polymer is expected to be exported out of the country, and releases into the Canadian environment are expected to be low.
Metabolism of the polymer in environmental organisms is not fully understood. Although the siloxane polymer (PDMS) has demonstrated a rapid clearance from fish; however it is not clear whether this is definitively related to metabolism.
Given the use of this substance in other countries, it is possible that MHDnMH is entering the Canadian market as a component of manufactured items or consumer products. However, information obtained from the S71 survey and other information sources did not indicate that it was present in these types of products in Canada. Available information is currently not sufficient to derive a quantitative estimate that would help determine the importance of this source. However, it is anticipated that the proportions of MHDnMH released to the various environmental media would not be significantly different from those estimated here. It is also recognised that releases from waste disposal sites are possible and could contribute to overall environmental concentrations.
Exposure Assessment
Environmental Media
Empirical data on concentrations of MHDnMH in environmental media in Canada were not identified. Based on its use pattern, MHDnMH is not expected to be found in food or beverages in Canada. As the majority of the manufactured polymer is expected to be exported out of the country, releases into the Canadian environment are expected to be low. Environmental concentrations were estimated using the loss percentages predicted by the Mass Flow tool as shown in Table 3 of the “Release to the Environment” section (Environment Canada 2010b). The percentages were applied to the total quantity of MHDnMH in Canadian commerce in 2006. The total quantity in commerce was conservatively assumed to be up to 100 000 kg (Environment Canada 2010a). The loss quantities are estimated to be 1 000 kg to water from wastewater, 100 kg to air from air emissions, 1 300 kg to soil from landfill and loss to land.
The estimated losses were used in ChemCAN, a Canada-specific regional far-field level III fugacity model (ChemCAN 2003). For the purposes of modelling, an n=5 form of the oligomeric structure was used with molecular weight of approximately 500 g/mol. This n=5 form is known to be marketed under CAS RN 70900-21-9 in some commercial versions of the polymer and may be present in very low concentrations in other higher molecular weight commercial versions of the polymer (eg. number-averaged n=34 form) and is considered suitable for ChemCAN modelling. The estimated environmental concentrations are presented in Appendix III. Conservative upper-bound estimates of daily intake of MHDnMH for the general population in Canada were derived based on estimated environmental concentrations, and were found to be in the order of nanograms (10-9 g) per kg-bw (kilogram of body weight) per day. Additionally, given the very low vapour pressure of MHDnMH, levels in air are expected to be low.
Consumer Products
MHDnMH is mainly used as an intermediate in the production of silicone eugenol polymers not intended for use by the general population (Environment Canada 2010a).
MHDnMH was, however, notified as an ingredient in four types of cosmetic products in Canada: bra adhesive (30-100% by weight); skin whitening cream (0.1-0.3% by weight); lipstick (< 0.1 % by weight); and eye makeup (< 0.1 % by weight) (CNS 2010). Estimates of exposure from use of these consumer products are presented in Appendix IV. The bra adhesive is present in the inside liner of disposable bras used with strapless and backless personal apparel. Based on this, exposure from use of disposable bras is expected to be of short duration, and the potential acute dermal exposure associated with this scenario was estimated to be 4.0 × 10-3 mg/kg-bw per event. Skin whitening cream is typically applied on a daily basis over a period of approximately three months, and dermal exposure was estimated to be 0.2 mg/kg-bw per day. Lipstick is assumed to be applied four times daily and the daily oral exposure from this use is estimated to be 5.6 × 10-4 mg/kg-bw per day. Eye colour is assumed to be applied twice daily and the daily dermal exposure from this use is estimated to be 2.8 × 10-4 mg/kg-bw per day.
Confidence in estimates of exposure from environmental media is low. Data on concentrations of this substance in environmental media were not identified in the literature. The estimates of consumer product exposure were based on modelling and confidence in the estimates is moderate.
Health Effects Assessment
No empirical health effects data were identified for MHDnMH. Currently available quantitative or qualitative structure – activity relationship ((Q)SAR) models are not considered suitable for predicting the health effects of polymers.
MHDnMH commercial products consist of molecules with various molecular masses, depending on the number of repeating units in these molecules. MHDnMH and linear polydimethysiloxanes (PDMS, CAS Registry Number: 63148-62-9) are considered to be structural analogues (shown in Table 1 of the “Substance Identity” section). Therefore, health effects data on PDMS were considered suitable to inform the assessment of potential health effects associated with MHDnMH exposure.
The European Centre for Ecotoxicology and Toxicology of Chemicals has conducted a Joint Assessment of Commodity Chemicals for PDMS with viscosity of 10 – 100 000 cs (ECETOC 1994). This assessment noted that results of in vitro genotoxicity studies conducted with bacteria and mammalian cells provided no indications of mutagenic or clastogenic potential of PDMS (ECETOC 1994).
PDMS represents a low potential for acute toxicity, with an absence of mortality, when tested at concentrations exceeding limit doses by oral, dermal or inhalation routes of exposure. Low viscosity PDMS has been shown to be mildly irritating to the eyes but is not a skin irritant or sensitizer (ECETOC 1994).
Effects of PDMS on rats, mice and dogs via oral administration have been investigated in studies with exposure periods varying from four weeks to two years. The oral Lowest-Observed-Effect-Level (LOEL) was determined to be 375 mg/kg bw per day, based on increased superficial stomach ulcers observed in male mice (but not in female mice), exposed to PDMS in the diet for 76 weeks. Effects from dermal administration of PDMS for periods of 4 weeks to 1 year have been investigated in rabbits and monkeys. The dermal No-Observed-Adverse-Effect-Level (NOAEL) was determined to be 2000 mg/kg bw per day, the only dose tested, in monkeys (4 male Stumptailed macaques per group) after dermal application of PDMS (350 cs), 5 times per week for 26 months. Inhalation studies with PDMS have been conducted in the cat, rabbit, guinea pig (one animal each), rats and mice. However, due to its low vapour pressure, effect concentrations of PDMS could not be determined (ECETOC 1994).
Developmental toxicity of PDMS was investigated in rabbits administered PDMS during gestation via dermal administration and subcutaneous injection and in rats via subcutaneous injection. In the two rabbit dermal studies, PDMS was administered at 200 mg/kg bw/day; one study using 10 cs and the other 350 cs PDMS. Although the dermal studies reported low incidences of umbilical hernia, clubbed feet and frontal skull deformities, the results were not statistically significant. The four subcutaneous injection studies also demonstrated an absence of significant adverse developmental effects at all doses tested. In addition, PDMS (10 to 1 000 cs) did not induce cytotoxicity in human embryonic cells (ECETOC 1994; Siddiqui 1994; Siddiqui et al. 2002).
Reproductive toxicity of PDMS (350 cs) was investigated in male and female rats administered the substance by subcutaneous injection for 2 weeks prior to mating and in male monkeys by dermal administration of 2000 mg/kg bw per day for 26 months before mating with receptive females. No effects on fertility, male reproductive performance or development were observed (ECETOC 1994).
A two-year dietary study was conducted with PDMS (10 cs) in rats administered 0, 100, 300 or 1000 mg/kg bw per day PDMS (Meeks et al. 2005). Evidence of local irritation was noted in the eyes of male and female rats from all treatment groups at 24 months and in the nasolacrimal duct (microscopically) of 1000 mg/kg bw per day group males. A statistically significant increase in incidence of pancreatic islet cell adenomas was identified in the 1000 mg/kg bw per day group males. This was not considered to be treatment-related by the study investigators due to the absence of an increase in relevant preneoplastic changes, lack of an effect in treated females, high incidence of islet cell adenomas in the recovery group of control animals and the published incidence range of this type of tumour for the control animals. No other treatment related effects were observed. The study investigators identified a NOEL for oncogenicity of 1000 mg/kg-bw per day (highest dose tested). In addition, eye irritation was also observed in a 13-wk dietary study in rats exposed to 5000 ppm (700 mg/kg bw per day) of 10 or 350 cs PDMS (Siddiqui et al. 2002).
The oral absorption of PDMS (35 to 1200 cs) has been studied in rats via single or repeated (13 days) administration and the overall absorption rates were less than 0.2% at dose levels up to 2500 mg/kg bw. The dermal absorption of PDMS (100 cs) has been investigated in human volunteers at a dose level of 50 mg/kg bw per day for 10 days and no dermal absorption was observed (ECETOC 1994). In addition, PDMS did not interfere with gastrointestinal absorption of glucose in humans (ECETOC 1994).
Overall, the available data indicate that PDMS does not present a health hazard for humans (ECETOC 1994). IPCS (2009) has established a temporary Acceptable Daily Intake (ADI) of 0.8 mg/kg bw/day for PDMS based on the determination of a NOEL of 150 mg/kg bw/day from a 3-month rat (diet) study (150 mg/kg bw/day was the only dose tested in this study), and based on a number of considerations, including but not exclusively related to ocular irritation that was observed in other subchronic and chronic oral studies. In this screening assessment, the lowest adverse effect level of 375 mg/kg bw/day derived from a 76-week mouse study conducted with PDMS as shown above, is used for the characterization of risk associated with MHDnMH.
Confidence in the assessment of toxicological effects of MHDnMH is moderate. Although substance specific toxicological information was not identified, it was considered appropriate to use information on the toxicity of PDMS to inform the assessment of potential health effects of MHDnMH, based on structural, physical and chemical similarities between the two substances.
Characterization of Risk to Human Health
No empirical health effects data for MHDnMH were identified. Based on health effects data for the analogue PDMS, and on the weight-of-evidence based risk assessments for PDMS conducted by international agencies, it is considered that MHDnMH demonstrates low hazard potential.
Environmental exposure (via air, drinking water and soil) to MHDnMH was estimated to be in the order of nanograms (10-9 mg) per kg-bw per day, and therefore was considered to be negligible. Exposure is not expected from food and beverages. General population exposure can occur through use of consumer products containing MHDnMH. Conservative upper-bounding estimates of exposure to MHDnMH by the dermal and oral routes from use of consumer products (2.8 x 10-4 to 0.2 mg/kg-bw per day for daily dermal exposures; 5.6 x 10-4 mg/kg-bw per day for daily oral exposures; and 4.0 x 10-3 mg/kg-bw per event for acute dermal exposures) are several orders of magnitude below the lowest effect level for oral exposure (375 mg/kg bw/day) and several orders of magnitude below the no effect level for dermal exposure (2000 mg/kg bw/day; an effect level was not determined) established for the analogue PDMS (IPCS 2009). These margins are considered adequate to address uncertainties in the health effects and exposure databases.
Based on its low vapour pressure, inhalation exposure to MHDnMH from use of consumer products containing MHDnMH is considered to be negligible.
Uncertainty in Evaluation of Risk to Human Health
There is uncertainty associated with the estimate of exposure of the general population to environmental levels of MHDnMH, based on the use of modelling and the lack of Canadian data. However, quantities in commerce for the 2006 calendar year are known and were combined with estimated loss percentages from Environment Canada’s Mass Flow tool to model environmental concentrations. As the maximum value of the quantity in commerce range was used in the modeling, it is likely that the modeled results are conservative estimates of environmental exposure.
Regarding consumer product exposure, use of the maximum concentrations for cosmetic products (identified in the Cosmetic Notification System database) for modelling dermal and oral exposure was considered to result in conservative estimates. There is uncertainty around the likelihood of use of bra adhesives and whitening cream since the only use identified in a response to a notice issued under section 71 of CEPA 1999 was in the manufacture of an item not intended for use by the general population in Canada.
Due to the absence of data for MHDnMH, a structural analogue, PDMS, was used to inform possible health effects for MHDnMH. Although the human health assessment is based mainly on the analogue, margins between exposure levels from environmental media or from use of consumer products and levels associated with health effects in experimental studies were considered adequate to address uncertainties in the health effects and exposure databases.
Based on the information presented in this screening assessment, it is determined that the form of MHDnMH in commerce in Canada meets the Reduced Regulatory Requirement polymer criteria as specified the New Substances Notification Regulations (Chemicals and Polymers).
Based on the information available, it is concluded that MHDnMH is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends. Additionally, MHDnMH meets the criteria for persistence but does not meet the criteria for bioaccumulation as set out in thePersistence and Bioaccumulation Regulations (Canada 2000).
Based on the information available, it is concluded that MHDnMH is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Therefore, it is concluded that the Reduced Regulatory Requirement form of MHDnMH does not meet any of the criteria under section 64 of CEPA 1999.
Given the complexity associated with the polymer formulation and the potentially hazardous properties associated with low molecular weight polymers, there is concern that new activities for MHDnMH which have not been identified or assessed under CEPA 1999 could lead to the substances meeting the criteria as set out in section 64 of the Act. Therefore, it is recommended that the DSL be amended to indicate that MHDnMH meets the Reduced Regulatory Requirement polymer criteria. Should other forms of MHDnMH not meeting the reduced regulatory requirements, be introduced on the Canadian market, those forms would be subject to the requirements of the New Substances Notification Regulations.
Annelin RB. 1979. Dow Corning internal report no. I-0005-0669. Cited in Fendinger et al. 1997.
[AIEPS] Artificial Intelligence Expert Predictive System. 2003–2007. Version 2.05. Ottawa (ON): Environment Canada. Model developed by Stephen Niculescu. Available from: Environment Canada, Ecological Assessment Division, New Chemicals Evaluation Section.
Annelin RB, Frye CL. 1989. The piscine bioconcentration characteristics of cyclic and linear oligomeric permethylsiloxane. The Science of the Total Environment. 83:1
Annelin RB, Humble DA. 1978. Dow Corning report I-0005-0596. Cited in Fendinger et al. 1997.
Annelin RB, Klein R, and Varaprath S. 1994. Dow Corning Corp Internal Report no. 1994-I-0000. Cited in Fendinger et al. 1997.
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2008. Version 1.92a. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Arnot JA, Gobas FAPC. 2003. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci 22(3):337–345.
Atkinson R. 1991. Kinetics of the Gas-Phase Reactions of a Series of Organosilicon
Compounds with OH and NO3 Radicals and Ozone at 297 ± 2K. Environmental Science and Technology. 25: 863.
Aubert M, Aubert J, Augier H, Guillemaut C. 1985. Study of the toxicity of some silicone compounds in relation to marine biological chains. Chemosphere. 14: 127-138.
Battelle. 1992. Fate and effects of PDMS: Soil amendment sludge disposal. Report to Dow Corning Corporation, Battelle Study No SC 900090. May 8, 1992. Battelle, Columbus Lab., Ohio, USA. Cited in ECETOC 1994.
[BBM] Baseline Bioaccumulation Model. 2008. Gatineau (QC): Environment Canada, Existing Substances Division. [Model based on Dimitrov et al 2005]. Available upon request.
[BCFBAF] Bioaccumulation Program for Windows [Estimation Model]. 2008. Version 3.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Belt GH, King JG, and Haupt HF. 1977. Augmenting summer stream flow by use of silicone antitranspirant. Water Resource Research. 13(2): 267-272.
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2008. Version 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Bruggeman WA, Weber-Fung D, Opperhuizen A, Van der Steen J, Wijbenga A, Hutzinger O. 1984. Absorption and retention of polydimethylsiloxanes (silicones) in fish: preliminary experiments. Toxicological and experimental chemistry. 7: 287-296.
Buch RR, Ingebrigtson DN. 1979. Rearrangement of Polydimethylsiloxane Fluids on Soil. Environmental Science and Technol. 13: 676.
Cabridenc R. 1978. Polydimethylsiloxanes, IRNS. Cited in TNO Report to EEC No U/83/208(605). Evaluation of the impact of Organo Silicone Compounds on the Aquatic Environment. TNO, The Hague, The Netherlands. Cited in ECETOC 1994.
Canada. 1978. Food and Drug Regulations, C.R.C., c. 870.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33, Canada Gazette, Part III, Vol. 22, No. 3.
Canada. 2000. Canadian Environmental Protection Act, 1999: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March, 2000, SOR/2000-107, Canada Gazette, Part II, Vol. 134, No. 7.
Canada, Dept. of the Environment, Dept. of Health. 2006. Canadian Environmental Protection Act, 1999: Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette, Part I, Vol. 140, No. 49.
Canada, Dept. of the Environment. 2009. Canadian Environmental Protection Act, 1999: Notice with respect to Batch 11 Challenge substances. Canada Gazette, Part I, Vol. 143, No.39.
Carpenter JC, Cella JA, Dorn SB. 1995. Study of the degradation of polydimethylsiloxanes on soil. Environmental Science and Technology. 29: 864-868.
Carpenter JC, Leib TK, Sabourin CL, Spivack JL. 1996. Abstract no. PO 578, SETAC 17th Annual Meeting, Washington, DC. Cited in Fendinger et al. 1997.
[CATABOL] Probabilistic assessment of biodegradability and metabolic pathways [Computer Model]. c2004-2008. Version 5.10.2. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. CATALOGIC.
ChemCAN [Level III fugacity model of 24 regions of Canada]. 2003. Version 6.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry. [cited 2010 May]. The Canadian Centre for Environmental Modelling and Chemistry.
Christensen K. 1994. Silicone Environmental Health and Safety Council Study Report, Document ID no. 47. Cited in Fendinger et al. 1997.
[CNS] Cosmetic Notification System [Proprietary Database]. 2010. Available from Health Canada, Cosmetics Division.
[ConsExpo] Consumer Exposure Model [Internet]. 2006. Version 4.1. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu (National Institute for Public Health and the Environment). RIVM Product safety.
[CPOPs] Canadian POPs Model. 2008. Gatineau (QC): Environment Canada, Ecological Assessment Division; Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. [Model developed based on Mekenyan et al. 2005]. Available from: Environment Canada, Ecological Assessment Division. Also see Mekenyan et al. 2005.
Craig PJ. 2003. Organometallic compounds in the environment. John Wiley & Sons Ltd, England.
Craig NCD, Caunter JE, and Eales GJ. 1984. ICI Let, Brixham, UK, prepared for Centre Europeen des Cilicones, Dow Corning report no. I-9039-0004.
Craig NCD and Caunter JE. 1990. The effects of polydimethylsiloxane (PDMS) in sediment on the polychaete worm Nereis diversicolor. Chemosphere. 21(6):751-759.
Dimitrov SD, Dimitrova NC, Walker JD, Veith GD, Mekenyan OG. 2002. Predicting bioconcentration factors of highly hydrophobic chemicals. Effects of molecular size. Pure Appl. Chem. 74(10):1823-1830.
Dimitrov S, Dimitrova N, Parkerton T, Comber M, Bonnell M, Mekenyan O. 2005. Base-line model for identifying the bioaccumulation potential of chemicals. SAR QSAR Environ Res 16(6):531–554.
[DPD] Drug Products Database [database on the Internet]. 2010. Ottawa (ON): Therapeutic Products Directorate, Health Canada. [cited 2010 Apr]. Drug Product Database.
Eales GJ and Taylor D. 1983. A study of the movement of a polydimethylsiloxane fluid in sediment columns – Phase 2. Dow Corning Report I-9039-002. Cited in ECETOC 1994.
[ECETOC] European Centre for Ecotoxicology and Toxicology of Chemicals. 1994. Joint Assessment of Commodity Chemicals No. 26: Linear Polymethylsiloxanes (viscosity 10 – 100 000 centistokes) CAS No. 63148-62-9. European Centre for Ecotoxicology and Toxicology of Chemicals, Brussels, Belgium. JACC Reports.
[ECOSAR] Ecological Structural Activity Relationships [Internet]. 2008. Version 1.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Environment Canada. 2008. Guidance for Conducting Ecological Assessments under CEPA, 1999, Science Resource Technical Series, Technical Guidance Module: Mass Flow Tool. Working Document. Gatineau (QC): Environment Canada, Ecological Assessment Division. Available upon request.
Environment Canada. 2010a. Data for Batch 11 substances collected under the Canadian Environmental Protection Act, 1999, Section 71: Notice with respect to certain Batch 11 Challenge substances. Data compiled by: Environment Canada, Program Development and Engagement Division.
Environment Canada. 2010b. Assumptions, limitations and uncertainties of the Mass Flow Tool for MHDnMH, CAS RN 70900-21-9. Internal draft document. Gatineau (QC): Environment Canada, Ecological Assessment Division. Available upon request.
Environment Canada. 2010c. Industrial release report: CAS RN 70900-21-9, 2010-05-19. Unpublished report. Gatineau (QC): Environment Canada, Ecological Assessment Division. Available upon request.
Environment Canada, Health Canada. 2008a. Screening Assessment for the Challenge: Octamethylcyclotetrasiloxane(D4): Chemical Abstracts Service Registry Number 556-67-2 [Internet]. Ottawa(ON): Environment Canada, Health Canada. [cited 2010 May].
Environment Canada, Health Canada. 2008b. Screening Assessment for the Challenge: Decamethylcyclopentasiloxane (D5): Chemical Abstracts Service Registry Number 541-02-6 [Internet]. Ottawa(ON): Environment Canada, Health Canada. [cited 2010 May].
Environment Canada, Health Canada. 2008c. Screening Assessment for the Challenge: Dodecamethylcyclohexasiloxane (D6): Chemical Abstracts Service Registry Number 540-97-6 [Internet]. Ottawa(ON): Environment Canada, Health Canada. [cited 2010 May].
[EPIsuite] Estimation Programs Interface Suite for Microsoft Windows [Estimation Model]. 2008. Version 4.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
[EQC] Equilibrium Criterion Model. 2003. Version 2.02. Peterborough (ON): Trent University, The Canadian Environmental Modelling Centre.
Fendinger NJ, Lehmann RG, and Mihaich EM. 1997. Polydimethylsiloxane. Organosilicon Materials. The Handbook of Environmental Chemistry. Springer-Verlag Berlin Heidelberg.
Firmin R. 1984. Les composes organosiliciés et l’environnement aquatique. Mémoire, Sciences Environnement, Université Libre Bruxelles. Cited in TNO Report to EEC No U/83/208(605). Evaluation of the Impact of Organo Silicon Compounds on the Aquatic Environment. TNO, The Hague, The Netherlands. Cited in ECETOC 1994.
Garvey N, Collins MK, Mihaich EM. 1996. Abs no. 256, SETAC 17th Annual Meeting, Washington, DC (cited in Graiver et al. 2003).
Gelest. 2008a. Material Safety Data Sheet: Tetradecamethylhexasiloxane - SIT7089.0. Gelest, Inc., Morrisville, PA 19067. [cited 2010 December].
Gelest. 2008b. Material Safety Data Sheet: Poly(dimethylsiloxane), hydride terminated – DMS-H03. Morrisville (PA): Gelest, Inc. [cited 2010 May 17].
Gelest. 2010. Reactive silicone: forging new polymer links. Gelest, Inc., Morrisville, PA 19067.
Gettings RL and Lane TH. 1982. Dow Corning Corp, Internal Report no. I0005-10957. Cited in ECETOC 1994.
Graiver D, Farminer KW, Narayan R. 2003. A review of the fate and effects of silicones in the environment. Journal of Polymers and the Environment. 11 (4): 129-136.
Guillenaut G, Aubert J, Augier H. 1987. Study on organosilicon contamination and toxicity in relation to the marine biomass. Revue Internationale d’Océanographie Médicale. 85/86: 88.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Health Canada. 2009. The cosmetic ingredient hotlist – September 2009 [Internet]. Ottawa (ON): Health Canada, Consumer Product Safety. [cited 2010 May 14]. Cosmetics and Personal Care.
Henry KS, Wieland WH, Powell DE, Giesy JP. 2001. Laboratory analyses of the potential toxicity of sediment-associated polydimethylsiloxne to benthic macroinvertebrates. Environmental Toxicology and Chemistry. 20(11): 2611-2616.
[HENRYWIN] Henry’s Law Constant Program for Microsoft Windows [Estimation Model]. 2008. Version 3.20. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Hill RW. 1980. Determination of acute toxicity of DC 561 and DC X2-3168 to mussel (Mytilus edulis), report DC 19039 (6 and 8). Cite in TNO Report to EEC No U/83/208(605). Evaluation of the Impact of Organo Silicone Compounds on the Aquatic Environment. TNO, The Hague, The Netherlands. Cited in ECETOC 1994.
Hill RW, Young BE, Eales GJ. 1980. Imperial Chemical Industries Ltd., Brixham, TQ58BA, England. Determination of acute toxicity of Dow Corning DC 561 to the plaice (Pleuronectes platessa). Report No. BL/B/2052, prepared for Dow Corning, (DC Report no. I-9030-5).
Hill RW, Young BE, Eales GJ. 1984. Imperial Chemical Industries Ltd., Brixham, TQ58BA, England. Effect of an organosilicon formulation on embryos and larvae of the sheepshead minnow (Cyprinodon variegatus). Report No. BL/B/2392A, prepared for Centre European des Silicones, 34 pp (DC Report no. I-9039-0003). Cited in Fendinger et al. 1997 and ECETOC 1994.
Hobbs EJ, Keplinger ML, and Calandra JC. 1975. Toxicity of polydimethylsiloxanes in certain environmental systems. Environmental Research. 10: 397-406.
[HYDROWIN] Hydrolysis Rates Program for Microsoft Windows [Estimation Model]. 2008. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
[IARC] International Agency for Research on Cancer Working Group on the Evaluation of Carcinogenic Risks to Humans. 1999. IARC Monographs on the evaluation of carcinogenic risks to humans. Vol.74: Surgical implants and other foreign bodies.
[IPCS] International Programme on Chemical Safety. 2009.Polydimethylsiloxane. Geneva (CH): World Health Organization. (WHO Food Additives Series 60, 3.1.7). Jointly sponsored by the Food and Agriculture Organization of the Unites Nations and the World Health Organization.
Kukkonen J and Landrum PF. 1995. Effects of sediment-bound polydimethylsiloxane on the bioavailability and distribution of benzo[a]pyrene in lake sediment to Lumbriculus variegates. Environmental Toxicology and Chemistry. 14: 523-531.
[KOCWIN] The Soil Adsorption Coefficient Program [Estimation Model]. 2008. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2008. Version 1.67. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Lehmann RG, Varaprath S, Frye CL. 1994a. Degradation of silicone polymers in soil. Environmental Toxicology and Chemistry. 13: 1061-1064.
Lehmann RG, Varaprath S, Frye CL. 1994b. Fate of silicone degradation products (silanols) in soil. Environmental Toxicology and Chemistry. 13: 1753-1759.
Lehmann RG, Varaprath S, Annelin RB, Arndt J. 1995. Degradation of silicone polymer in a variety of soils. Environmental Toxicology and Chemistry. 14: 1299-1305.
Lehmann RG, Miller JR, Xu S, Singh UB, Reece CF. 1998. Degradation of silicone polymer at different soil moistures. Environmental Science and Technology. 32: 1260-1264.
Levier RR. 1988. Permethylated siloxane insect toxicants. Silicon chemistry. COREY-GASPAR – Ellis Howood Publishers – Chap. 15 : 153-160. Cited in ECETOC 1994.
[LNHPD] Licensed Natural Health Products Database [database on the Internet]. 2010. Natural Health Products Directorate, Health Canada. [cited 2010 Apr]. Licensed Natural Health Products Database.
Maggi P and Alzieu C. 1977. Etude de la nocivité d'huiles polydimethyl -siloxane à l'égard d'organismes marins. Science et Péche, Bulletin de l’Institut des Péches Maritimes. 269: 1-3. Cited in Fendinger et al. 1997, Stevens et al. 2001, and ECETOC 1994.
Mann H, Ollenschlager B, Reichenback-Klinke HH. 1977. Reichenbach-Klinke, Untersuchunger zur Wirkung von Siliconölen auf Regenbogenforellen. Fisch Umwelt. 3:19. Cited in Fendinger et al. 1997 and ECETOC 1994.
Meeks R.G., Jean P.A., Mertens J.W., Regan K.S., Crissman J.W., Plotzke K.P. 2005. Chronic toxicity and oncogenicity study of polydimethylsiloxane (PDMS) 10 Cst fluid in Fisher 344 rats. Toxicol Sci 84(1-S):308.
Mekenyan G, Dimitrov SD, Pavlov TS, Veith GD. 2005. POPs: A QSAR system for creating PBT profiles of chemicals and their metabolites. SAR and QSAR Environmental Research. 16(1-2):103-133.
[MPBPWIN] Melting Point Boiling Point Program for Microsoft Windows [Estimation Model]. 2008. Version 1.43. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
[NCI] National Chemical Inventories [database on CD-ROM]. 2009. Issue 1. Columbus (OH): American Chemical Society. [cited 2010 May]. National Chemical Inventories.
Nielson WR, Heinlein JP and Haigh WG. 1975. Acaridical and insectisidal activity of various silicone fluids. TSCA 8(c) Submission 87-8215084. Cited in ECETOC 1994.
[NHPID] Natural Health Products Ingredients Database [database on the Internet]. 2010. Natural Health Products Directorate, Health Canada. [cited 2010 Apr].
[NHPID]. Natural Health Products Ingredients Database [database on the Internet]. 2010. Natural Health Products Directorate, Health Canada. [cited 2010 Apr]. Natural Health Products Ingredients Database.
Noll, W. 1968. Chemistry and Technology of Silicones. Academic Press, New York. Cited in USEPA 2008.
OECD. 1994. Organization for Economic Co-operation and Development. Chemicals Group and Management Committee. Chairman’s Report, Third Meeting of OECD Experts on Polymers, Tokyo, 14-16; April 1993.
[OASIS Forecast] Optimized Approach based on Structural Indices Set [Internet]. 2005. Version 1.20. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. OASIS Software.
Opperhuizen A, Van Der Velde EW, Gobas FAPC, Liem DAK, Van Der Steen JMD, Hutzinger O. 1985. Relationship between bioconcentration in fish and steric factors of hydrophobic chemicals. Chemosphere. 14: 1871-1896.
[PCKOCWIN] Organic Carbon Partition Coefficient Program for Windows [Estimation Model]. 2008. Version 2.00. Washington (DC): United States Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
[PMRA] Pest Management Regulatory Agency. 2007. Regulatory Note REG 2007-04: PMRA list of formulants.. Ottawa (ON): Health Canada, Pest Management Regulatory Agency. [cited 2010-05-14].
Putt AE. 1994. Polydimethylsiloxane (PDMS) – the subchronic toxicity to midge larvae (chironomus tentans) under flow-through conditions. Springborn Laboratories, Inc. report #94-4-5235, submitted to Silicones Environmental Health and Safety Council (cited in Fendinger et al. 1997).
Putt AE, Mihaich EM. 1996. Effect of sediment-bound polydimethyl-siloxane (PDMS) to aquatic invertebrates. Abs no. 257, SETAC 17h Annual Meeting, Washington, DC (cited in Fendinger et al. 1997).
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu. 2006a. General fact sheet: Limiting conditions and reliability, ventilation, room size, body surface area. Updated version for ConsExpo 4. [Internet]. [cited 2010 May]. Bilthoven (NL): RIVM (National Institute for Public Health and the Environment).RIVM Report 320104002/2006.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu. 2006b. Cosmetics fact sheet: To assess the risks for the consumer. [Internet]. [cited 2010 May]. Bilthoven (NL): RIVM (National Institute for Public Health and the Environment). RIVM Report No.: 320104001/2006.
Sabourin CL, Carpenter JC, Leib TK, Spivack JL. 1996. Biodegradation of dimethylsilanediol in soils. Appl Environ Microbiol. 62(12): 4352-60.
Sakuratani Y, Noguchi Y, Kobayashi K, Yamada J, Nishihara T. 2008. Molecular size as a limiting characteristic for bioconcentration in fish. J. Environ. Biol. 29(1): 89-92.
Siddiqui W.H. 1994. Developmental toxicity evaluation of Dow Corning & Ntilde; antifoam A compound, food grad in rabbits. Teratology 49:397.
Siddiqui W.H., Schardein J.L., Cassidy S.L., Meeks R.G. 1994. Reproductive and developmental toxicity studies of silicone elastomer Q7-2423/Qw7-2551 in rats and rabbits.
Siddiqui W.H., Blee M.B., Tompkins E.C., Meeks R.G. 2002. 13-weeks dietary and developmental toxicity studies of polydimethylsiloxanes (PDMS) in rats and rabbits. Toxicologist 22(1-S):232.
Sommerlade R, Parlar H, Wrobel D, Kochs P. 1993. Product analysis and kinetics of the gas-phase reactions of selected organosilicon compounds with OH radicals using a smog chamber-mass spectrometer system. Environmental Science and Technology. 27 (12): 2435–2440.
Spacie A. 1972. Acute toxicity of SAG 10 and SAG 350 silicone antifoam to aquatic organisms. Internal Report, Union Carbide Chemicals Corp,. Danbury, USA. Cited in ECETOC 1994.
[SPIN] Substances in Preparations in Nordic Countries [database on the Internet]. 2006. Copenhagen (DK): Nordic Council of Ministers. [cited 2010 May]. SPIN - Substances in Preparation in Nordic Countries.
Stevens C. 1998. Environmental degradation pathways for the breakdown of polydimethylsiloxanes. Journal of Inorganic Biochemistry. 69(3): 203-207.
Stevens C, Powell DE, Mäkelä P, Karman C. 2001. Fate and effects of polydimethylsiloxane (PDMS) in marine environments. Marine Pollution Bulletin. 42 (7): 536-543.
[TPD NMID] Therapeutic Products Directorate’s Non-Medicinal Ingredients Database [Proprietary Database]. 2010. [cited 2010 Apr]. Available from: Therapeutic Products Directorate, Health Canada.
[USEPA] United States Environmental Protection Agency. 2008. Interpretive assistance document for assessment of polymers. United States Environmental Protect Agency.
[USEPA] United States Environmental Protection Agency. 2005. Toxic Substances Control Act-Inventory Update Rule (TSCA-IUR) - Information on High Production Volume (HPV) Chemicals. United States Environmental Protection Agency. [cited December 2005 – January 2006].
Varaprath S, Frye CL, Hamelink J. 1996. Aqueous solubility of permethylsiloxanes (silicones). Environmental Toxicology and Chemistry. 15 (8): 1263-1265.
Wacker. 1992. Wacker-Broschüre: Silicone Fluids AK, Wacker Chemie AG, München, Germany. Cited in ECETOC 1994.
Watanabe N, Nakamura T, Watanabe E, Sato E, Ose Y. 1984a. Bioconcentration potential of polydimethylsiloxane (PDMS) fluids by fish. Science of the Total Environment. 38: 167.
Watanabe N, Yasuda Y, Kato K, Nakanura T, Funasaka R, Shimokawa K, Sato E, Ose Y. 1984b. Determination of trace amounts of siloxanes in water, sediments and fish tissues by inductively coupled plasma emission spectrometry. Science of the Total Environment. 34 (1-2): 169-176.
[WATERNT] Water Solubility Program [Estimation Model]. 2008. Version 1.01. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [Estimation Model]. 2008. Version 1.41. Washington (DC): United States Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Xu S. 1998. Hydrolysis of poly(dimethylsiloxanes) on clay minerals as influenced by exchangeable cations and moisture. Environmental Science and Technology. 32(20): 3162–3168.
Appendix I: Relationship between viscosity, degree of polymerisation and molecular weight (Wacker 1992 and Fendinger et al. 1997)
Appendix Ia. PDMS data (Wacker 1992 and Fendinger et al. 1997)
| Viscosity (cs) | Average Number of D-Units[*] | Number-Average Molecular Weight (g/mol) |
|---|---|---|
| 10 | 15 | 1 300 |
| 50 | 40 | 3 000 |
| 100 | 70 | 5 000 |
| 1 000 | 200 | 15 000 |
| 10 000 | 500 | 37 000 |
| 100 000 | 1 000 | 74 000 |
Appendix Ib. PDMS data from Noll (1968, cited in USEPA 2008)
| Viscosity (cs) | Average Number of D-Units | Number-Average Molecular Weight (g/mol) |
|---|---|---|
| 60 | 50 | 3 600 |
| 140 | 110 | 8 000 |
| 440 | 230 | 17 000 |
| 680 | 280 | 21 000 |
| 1 440 | 400 | 30 000 |
| 10 000 | 800 | 60 000 |
| 50 000 | 1 200 | 88 000 |
| 100 000 | 1 400 | 103 000 |
| 300 000 | 1 900 | 143 000 |
The model prediction has only been applied on MHD5MH and its analogues (low molecular weight linear methyl siloxanes), with input of SMILES, molecular weights and the experimental data available only.
| Model Input Parameters | Phys-Chem/Fate EPI Suite 2008 (all models, including: |
|||
|---|---|---|---|---|
| MHD5MH (70900-21-9) |
MD3M (141-63-9) |
MD4M (107-52-8) |
MD5M (541-01-5) |
|
| SMILES Code | Si(C)(C)OSi(C) (C)OSi(C)(C) OSi(C)(C)OSi (C)(C)OSi(C) (C)OSi(C)(C) |
O([Si](O[Si](O [Si](C)(C)C)(C) C)(C)C)[Si](O [Si](C)(C)C)(C) C |
O([Si](O[Si](O [Si](C)(C)C)(C) C)(C)C)[Si](O [Si](O[Si](C)(C) C)(C)C)(C)C |
O([Si](O[Si](O [Si](O[Si](C)(C) C)(C)C)(C)C) (C)C)[Si](O[Si] (O[Si](C)(C)C) (C)C)(C)C |
| Molecular weight (g/mol) | 505.10 | 384.84 | 459.00 | 533.15 |
| Melting point (ºC) | ||||
| Boiling point (ºC) | ||||
| Data temperature (ºC) | ||||
| Density (kg/m3) | ||||
| Vapour pressure (Pa) | ||||
| Henry’s Law constant (Pa·m3/mol) | ||||
| Kow (Octanol-water partition coefficient; dimensionless) |
6.0 | 6.6 | 7.2 | |
| Water solubility (mg/L) | 7.0 × 10-5 | |||
Appendix III: Estimated concentrations of MHDnMH in environmental media using ChemCAN version 6.00 (ChemCAN 2003)[1]
| Medium[2] | Estimated concentration |
|---|---|
| Ambient air[3] | 1.74 ng/m3 |
| Surface water | 0.128 ng/L |
| Soil | 1.47 × 10-3 ng/g solids |
| Sediment | 9.09 ng/g solids |
[2] Default inflow concentrations of 2 ng/m3 in air and 3 ng/L in water were specified by ChemCAN v6.00.
[3] The oxidative degradation half-life in air was assumed to be 5.125 days (AOPWIN 2008) and in soil 3 weeks (based upon PDMS soil degradation data).
Appendix IV: Estimates of dermal exposure to MHDnMH from use of disposable bras, skin whitening cream, eye makeup and lipstick
| Product | Assumptions | Exposure estimate |
|---|---|---|
| Bra adhesive[1] | Applied dose = (thickness of product on skin) (exposed surface area)(100%) (density of product) / BW
|
Acute applied dose = 4.0 × 10-3 mg/kg-bw per event |
| Skin whitening cream[1] | Used ConsExpo v4.1 for modelling (ConsExpo 2006).
|
Daily applied dermal dose: 0.2 mg/kg-bw per day |
| Eye makeup[1] | Used ConsExpo v4.1 for modelling (ConsExpo 2006).
|
Dermal chronic dose: 2.8× 10-4 mg/kg-bw per day |
| Lipstick[1] | Used ConsExpo v4.1 for modelling (ConsExpo 2006). - Maximum weight percent: 0.1% (CNS 2010) - Exposure frequency: 1460/yr (RIVM 2006b) - Amount ingested: 0.01 g (RIVM 2006b) - Uptake fraction: 1 |
Oral chronic dose: 5.6 × 10-4 mg/kg-bw per day |
Footnotes
[2] A determination of whether one or more of the criteria of section 64 are met is based upon an assessment of potential risks to the environment and/or to human health associated with exposures in the general environment. For humans, this includes, but is not limited to, exposures from ambient and indoor air, drinking water, foodstuffs, and the use of consumer products. A conclusion under CEPA 1999 on the substances in the Chemicals Management Plan (CMP) Challenge Batches 1-12 is not relevant to, nor does it preclude, an assessment against the hazard criteria specified in the Controlled Products Regulations, which is part of regulatory framework for the Workplace Hazardous Materials Information System [WHMIS] for products intended for workplace use. Similarly, a conclusion based on the criteria contained in section 64 of CEPA 1999 does not preclude actions being taken under other sections of CEPA or other Acts.
[3] Functional group equivalent weight (FGEW) for the cationic groups is the ratio of the mass of the polymer to the number of moles of the cationic group. Consequently, larger FGEW values represent polymers that have relatively few cationic species.