Screening Assessment for the Challenge 2-Naphthalenol, 1-[(2,4-dinitrophenyl)azo]- (Pigment Orange 5)
Chemical Abstracts Service Registry Number 3468-63-1
Environment Canada
Health Canada
February 2009
Table of Contents
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a screening assessment on 2-Naphthalenol, 1-[(2,4-dinitrophenyl)azo]- (Pigment Orange 5), Chemical Abstracts Service Registry Number 3468-63-1. This substance was identified as a high priority for screening assessment and included in the Challenge because it was originally found to meet the ecological categorization criteria for persistence, bioaccumulation potential and inherent toxicity to non-human organisms and is believed to be in commerce in Canada.
The substance Pigment Orange 5 was not considered to be a high priority for assessment of potential risks to human health, based upon application of the simple exposure and hazard tools developed by Health Canada for categorization of substances on the Domestic Substances List. Therefore, this assessment focuses on information relevant to the evaluation of ecological risks.
Pigment Orange 5 is an organic substance that is used in Canada and elsewhere as an orange pigment in paints, printing inks and textiles. The substance is not naturally produced in the environment. In 2006, twelve companies reported import of this substance, in a total quantity of 10 000 to 100 000 kg. Eleven companies reported using this substance in 2006, in a total quantity of 10 000 to 100 000 kg. The quantity of Pigment Orange 5 in commerce in Canada, along with the potentially dispersive uses of this substance, indicate that it could be released into the Canadian environment.
Based on reported use patterns and certain assumptions, most of the substance ends up in waste disposal sites. Small proportions are estimated to be released to water (20%), and soil (3%). Pigment Orange 5 exists in the environment as a solid particle, which is not soluble in water or volatile. For these reasons, Pigment Orange 5 is likely to settle by gravity to sediments if released to water, and will tend to remain in soils if released to terrestrial environments. It is not expected to be significantly present in other media. It is also not expected to be subject to long-range atmospheric transport.
Based on its physical and chemical properties, Pigment Orange 5 is expected to be persistent in water, soil and sediment. New experimental data relating to its solubility in water and octanol suggest that this pigment has a low potential to accumulate in the lipid tissues of organisms. The substance therefore meets the persistence criteria but does not meet the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations. In addition, new experimental toxicity data, as well as new toxicity predictions that take into account revised estimates of bioaccumulation potential, suggest that the substance has negligible to low potential for acute toxicity to aquatic organisms.
For this screening assessment, a very conservative exposure scenario was designed in which it is assumed that all industrial operations (users of the pigment) discharge Pigment Orange 5 into the aquatic environment. The predicted environmental concentration in water was below the predicted no-effect concentration calculated for sensitive aquatic organisms. Additionally, since Pigment Orange 5 may be used in consumer products, a conservative consumer release scenario was also developed based on the quantity of Pigment Orange 5 in commerce. This scenario predicted that all of the Canadian watercourses modelled would have predicted environmental concentrations below the predicted no-effect concentration.
This substance will be included in the upcoming Domestic Substances List inventory update initiative. In addition and where relevant, research and monitoring will support verification of assumptions used during the screening assessment.
Based on the information available, it is concluded that Pigment Orange 5 does not meet any of the criteria set out in section 64 of CEPA 1999.
Introduction
The Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or human health. Based on the results of a screening assessment, the Ministers can propose to take no further action with respect to the substance, to add the substance to the Priority Substances List (PSL) for further assessment, or to recommend that the substance be added to the List of Toxic Substances in Schedule 1 of the Act and, where applicable, the implementation of virtual elimination.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherent toxicity to aquatic organisms (iT), and were believed to be in commerce in Canada; and/or
- met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE), and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in the Canada Gazette, Part I, on December 9, 2006 (Canada 2006a), that challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment, and to develop and benchmark best practices for the risk management and product stewardship of those substances identified as high priorities.
The substance 2-Naphthalenol, 1-[(2,4-dinitrophenyl)azo]- was identified as a high priority for assessment of ecological risk as it had been found to be persistent, bioaccumulative and inherently toxic to aquatic organisms and is believed to be in commerce in Canada. The Challenge for this substance was published in the Canada Gazette on August 18, 2007 (Canada 2007a). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, submissions of information pertaining to the properties, bioaccumulation potential, hazard and uses of the substance were received.
Although 2-Naphthalenol, 1-[(2,4-dinitrophenyl)azo]- was determined to be a high priority for assessment with respect to the environment, it did not meet the criteria for GPE or IPE and high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity. Therefore, this assessment focuses principally on information relevant to the evaluation of ecological risks.
Under CEPA 1999, screening assessments focus on information critical to determining whether a substance meets the criteria for defining a chemical as toxic as set out in section 64 of the Act, where
"64. [...] a substance is toxic if it is entering or may enter the environment in a quantity or concentration or under conditions that
- have or may have an immediate or long-term harmful effect on the environment or its biological diversity;
- constitute or may constitute a danger to the environment on which life depends; or
- constitute or may constitute a danger in Canada to human life or health.”
Screening assessments examine scientific information and develop conclusions by incorporating a weight of evidence approach and precaution as required under CEPA 1999.
This screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including the additional information submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents, stakeholder research reports and from recent literature searches, up to July 2007 ecological sections of the document. Key studies were critically evaluated; modelling results may have been used to reach conclusions. When available and relevant, information presented in hazard assessment from other jurisdictions was considered. The screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents the most critical studies and lines of evidence pertinent to the conclusion.
This screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. Additionally, the draft of this screening assessment was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening risk assessment remain the responsibility of Health Canada and Environment Canada. The critical information and considerations upon which the assessment is based are summarized below.
Substance Identity
For the purposes of this screening assessment, this substance will be referred to as Pigment Orange 5. Other names and identifying characteristics of this substance are presented in Table 1 below.
| Chemical Abstracts Service Registry Number (CAS RN) | 3468-63-1 |
|---|---|
| DSL name | 2-Naphthalenol, 1-[(2,4-dinitrophenyl)azo]- |
| National Chemical Inventories (NCI) namesTable note a | 2-Naphthalenol, 1-[2-(2,4-dinitrophenyl)diazenyl]-(TSCA) 2-Naphthalenol, 1-[(2,4-dinitrophenyl)azo]- (AICS, PICCS, ASIA-PAC, NZIoC) 1-[(2,4-dinitrophenyl)azo]-2-naphthol (EINECS) Pigment Orange 5 (ENCS) 1-[(2,4-Dinitrophenyl)azo]-2-naphthalenol (ECL) C.I. PIGMENT ORANGE 5, 2-NAPHTHOL, 1-[(2,4-DINITROPHENYL)AZO]- (PICCS) BASIS PIGMENT ORANGE 5 (PICCS) |
| Other names | 1-(2,4-Dinitrophenylazo)-2-naphthol 2-Naphthol, 1-(2,4-dinitrophenylazo)- C.I. 12075 C.I. Pigment Orange 5 |
| Chemical group (DSL Stream) |
Discrete organics |
| Major chemical class or use | Azo compounds; naphthalenes |
| Major chemical sub-class | Beta-naphthol pigment |
| Chemical formula | C16H10N4O5 |
| Chemical structure |  |
| Simplified Molecular Input Line Entry Specification (SMILES) | N(=O)(=O)c(c(N=Nc(c(c(ccc1)cc2)c1)c2O)ccc3N(=O)(=O))c3 |
| Molecular mass | 338.28 g/mol |
Physical and Chemical Properties
The pigment industry synthesizes organic pigments that have low to very low solubilities in nearly all solvents (i.e., less than 1 mg L[1] to less than 0.01 mg L[1]). This arises from the desire of the industry to produce chemicals that will retain their colour for a long time and in any type of material. Low solubility is enhanced by designing chemicals that have strong interactive forces within and between molecules. For Beta Naphthol compounds, this is achieved by the intramolecular, bifurcated hydrogen bonds. Although the structure of Pigment Orange 5 is often depicted as in Table 1, based on the measured bond lengths, the keto-hydrazone tautomer was found to be favoured. The keto-hydrazone tautomer is different with respect to certain bonds. Namely, there is a ketone oxygen on the naphthalene ring instead of the hydroxyl group, a double bond exists between the nitrogen and the naphthalene ring and the azo bond is a single bond (Figure 1). This structure creates bifurcated hydrogen bonds between the ortho substituents on the phenyl ring (like -Cl, or -NO2), the azo group and the ketone oxygen on the naphthalene group. The molecules may be linked by weak van der Waals forces and charge-transfer forces causing the molecules to stack in columns within a crystal (Herbst and Hunger 2004; Whitaker 1978; Lincke 2003).

Figure 1. Beta-Naphthol pigment structure (Whitaker 1978)
As is the case with the majority of organic pigments, Beta Naphthol pigments generally do not exist as individual molecules but are principally particles in the submicron range. The pigment powder is typically composed of primary particles (i.e., the crystal lattice of a pigment), aggregates and agglomerates. Manufacturers usually provide the physical specifications of their pigments, which include the average particle size of the pigment powder (see Table 2a). In doing so, users can determine which pigment is the most appropriate to colour their product(s), since performance is chiefly controlled by the particle size distribution (Herbst and Hunger 2004).
Table 2a contains modelled and experimental physical and chemical properties of Pigment Orange 5 that are relevant to its environmental fate. Modelled estimates for these properties are typically generated using quantitative structure-activity relationship (QSAR) models. These models, in turn, base their predictions on the characteristics of the individual molecules. Pigment Orange 5 is expected to exist in the crystalline form in the environment; therefore there is uncertainty associated with the modelled physical and chemical data. The modelled log Kow of 5.72 (KOWWIN 2000) used for categorization implies that the solubility of Pigment Orange 5 is much higher in octanol than in water. Experimental solubility data, however, reveal that the difference in the solubility in the two solvents is not that sizeable, indicating that the modelled partition coefficient is likely overestimated. The ratio log (CO/CW) has been estimated from the experimental solubilities of Pigment Orange 5 in octanol (CO) and water (CW) determined individually, and this experimentally derived ratio has been preferred over the model-derived log Kow for this pigment. The modelled estimate of log Kow has therefore been disregarded for this assessment, and the ratio log (CO/CW) of 2.4 has been used instead (Table 2a).
The experimental solubilities in Table 2a have been determined using an aggressive approach with long contact times between pigment particles and the solvent, and a filtration step removing as much of the particulate matter in the suspension as possible. These studies have been critically reviewed and, although none reported using reference chemicals of known solubilities, they were determined to have a satisfactory degree of reliability for the present risk assessment (Appendix I). Additional solubility studies were submitted by industry. However due to a lack of detail in the description of the procedures, they were not considered reliable for this risk assessment. The values were higher than the values shown in Table 2a, with water solubility ranging from 0.093 to 0.410 mg/L and octanol solubility ranging from 2.6 to 4.8 mg/L, resulting in calculated log (CO/CW) values of approximately 1.2.
| Type | Value | Temperature (°C) | Reference | |
|---|---|---|---|---|
| Physical state | Experimental | Orange powder | MSDS 2003 | |
| Physical state | Experimental | Orange paste | MSDS 2005 | |
| Average particle size (nm) | Experimental | 285 | Clariant 2007 | |
| Average particle size (nm) | Experimental | 320-370 | NPIRI 2000 | |
| Melting point (°C) | Experimental | 302 | Danish EPA 1998 NPIRI 2000 |
|
| Melting point (°C) | Modelled | 220.89 | MPBPWIN 2000 | |
| Boiling point (°C) | Experimental | Not available | ||
| Boiling point (°C) | Modelled | 517.75 | MPBPWIN 2000 | |
| Density (kg/m3) | Experimental | 1100 (1.1 g/cm3) |
20 | MSDS 2005 |
| Density (kg/m3) | Experimental | 1700 (1.7 g/cm3) |
20 | MSDS 2003 |
| Density (kg/m3) | Modelled | Not available | ||
| Vapour pressure (Pa) | Experimental | Not available | ||
| Vapour pressure (Pa) | Modelled | 1.243 × 10-9 (9.32 × 10-12 mm Hg) |
25 | MPBPWIN 2000 |
| Henry's Law constant (Pa·m3/mol) |
Experimental | Not available | ||
| Henry's Law constant (Pa·m3/mol) |
Modelled (Bond estimation method) | 2.357 × 10-10 (2.326 × 10-15 atm·m3/mol) |
25 | HENRYWIN 2000 |
| Henry's Law constant (Pa·m3/mol) |
Modelled (Group estimation method) | 9.208 × 10-10 (9.088 × 10-15 atm·m3/mol) |
25 | HENRYWIN 2000 |
| Log Kow (Octanol-water partition coefficient) (dimensionless) |
Experimental | Not available | ||
| Log Kow (dimensionless) |
Modelled | Not applicable | KOWWIN 2000 | |
| Log (Co/Cw) (dimensionless) |
Experimental | 2.4 | Calculated based on data in Table 2a | |
| Log Koc (Organic carbon-water partition coefficient - L/kg) (dimensionless) |
Experimental | Not available | ||
| Log Koc (L/kg) (dimensionless) |
Modelled | Not available | ||
| Water solubility (mg/L) | Experimental | 0.0068 | 26-27 | Study Submission 2007a |
| Water solubility (mg/L) | Modelled | 0.1336 | 25 | WSKOWWIN 2000 |
| Other solubilities (mg/L) | Experimental (octanol) |
1.76 | 26-27 | Study Submission 2007b |
| pKa (Acid dissociation constant) (dimensionless) | Modelled | 13.5 for acid form |
ACD 2005 | |
| pKa (dimensionless) | Experimental | NA |
Octanol and water solubility studies were performed on two structurally similar substances, Pigment Red 4 and Pigment Red 3 (CAS RN 2814-77-9 and CAS 2425-85-6 respectively). These results support the low water solubility and octanol solubility reported for Pigment Orange 5. The ratio log (Co/Cw) has been presented as a surrogate for log Kow (Table 2b).
| Chemical substance | Structure | Notes |
|---|---|---|
| Analog Pigment Red 4 (CAS RN 2814-77-9) |
 |
Pigment Red 4 differs from Pigment Orange 5 in one chemical feature: a Cl is substituted for a NO2 on the terminal benzene ring. |
| Analog Pigment Red 3 (CAS RN 2425-85-6) |
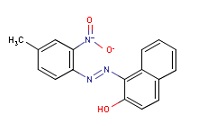 |
Pigment Red 3 differs from Pigment Orange 5 in one chemical feature: a methyl group is substituted for a NO2 group on the terminal benzene ring. |
| Property | Value | Temperature (°C) | Reference |
|---|---|---|---|
| Melting Point (ºC) | 276 | NPIRI 2000 | |
| Water solubility (mg/L) | 0.0033 | 22-23 | Study Submission 2007c |
| Octanol solubility (mg/L) | 9.4 | 22-23 | Study Submission 2007d |
| Log (Co/Cw) (dimensionless) | 3.5 | Calculated |
| Property | Value | Temperature (°C) | Reference |
|---|---|---|---|
| Melting Point (ºC) | 276 | Danish EPA 1998 | |
| Water solubility (mg/L) | 0.0033 | 23-24 | Study Submission 2007e |
| Octanol solubility (mg/L) | 1.79 | 23-24 | Study Submission 2007f |
| Log (Co/Cw) (dimensionless) | 3.7 | Calculated |
Sources
Pigment Orange 5 is not naturally produced in the environment.
Recent information was collected through industry surveys conducted for the years 2005 and 2006 under Canada Gazette notices issued pursuant to section 71 of CEPA 1999 (Canada 2006b, 2007b). These notices requested data on the Canadian manufacture and import of the substance.
Under the CEPA section 71 notice with respect to certain Batch 3 Challenge substances (Canada 2007b), Canadian companies who manufactured or imported in 2006 greater than 100 kg of a substance listed in the notice were required to provide specific data regarding the substance to Environment Canada. Information gathered from this survey notice indicates that Pigment Orange 5 was not manufactured in Canada in 2006 in a quantity greater than the 100 kg reporting threshold. In total, twelve companies reported import of this substance. The total import was reported to be in the range of 10,000 to 100,000 kg in 2006. In total, eleven companies reported using this substance in 2006, in a total quantity range of 10,000 to 100,000 kg. In addition, twelve Canadian companies, one American industrial association, and one American company, identified themselves as having a stakeholder interest in the substance. The American company voluntarily reported exporting this substance to Canada in 2006.
Under the CEPA section 71 notice with respect to selected substances identified as priority for action (Canada 2006b), Canadian companies who manufactured or imported in 2005 greater than 100 kg of a substance listed in the notice were required to provide specific data regarding the substance to Environment Canada. Information gathered from this survey notice indicates that Pigment Orange 5 was not manufactured in Canada in 2005 in a quantity greater than the 100-kg reporting threshold. In total, nine companies reported import of this substance, with four companies in the 100-1000 kg/yr range, four companies in the 1001-100 000 kg/yr range and one company reporting in the greater than 100 000 kg/yr range. In addition, nine Canadian companies, one American company and one American industrial association identified themselves as having a stakeholder interest in the substance. The American company voluntarily reported export to Canada of this substance in the 1,001-100 000 kg/yr range.
Under the Inventory Update Rule in the United States, the reported use of Pigment Orange 5 was 4.5 to 225 tonnes in 2002 and 225 to 455 tonnes in 1998. It is a European Union (EU) low production volume chemical, indicating that production within the EU has been estimated to be between 10 and 1000 tonnes per year. The database for Substances in Preparations in Nordic Countries indicates that in 2004, approximately 33.1 tonnes were used in Norway, 32.6 tonnes were used in Denmark and 20 tonnes were used in Sweden (SPIN 2006).
Uses
Information on uses was gathered in response to CEPA section 71 notices (Canada 2006b, 2007b). Importing companies in 2005 and 2006 indicated the substance is used in Paint, Coating, and Adhesive Manufacturing; Plastic Coloration; Other Chemical Product and Preparation Manufacturing (including printing inks and cartridges); and Chemical Wholesale and Distribution. One company reported importing the substance for use in Other Miscellaneous Chemical Product Manufacturing as a colourant in the textile industry.
One of the companies that identified itself as having a stakeholder interest in Pigment Orange 5 in 2005 identified its business activity as Basic Chemical Manufacturing. The American company that voluntarily reported export to Canada identified its business activity as Paint, Coating and Adhesive Manufacturing.
Pigment Orange 5 is included on Health Canada's List of Prohibited and Restricted Cosmetic Ingredients (Health Canada 2007).
Reported use categories in Sweden, Denmark and Norway include colouring agents, reprographic agents, paints, lacquers and varnishes (SPIN 2006). Other possible uses elsewhere include textile manufacturing and in the rubber industry (Colour Index International 2002). Azo colourants that degrade to certain carcinogenic aromatic amines have been restricted in Europe (European Commission 2002), however Pigment Orange 5 has not been restricted.
Releases to the Environment
Pigment Orange 5 was identified to be primarily used in paints, printing inks and textiles according to the results of the CEPA section 71 notice (Canada 2007b). Releases were estimated based on the use of 50% of Pigment Orange 5 in paints, 40% in printing inks and 10% in textiles.
Mass Flow Tool
To estimate potential release of the substance to the environment at different stages of its life cycle, a Mass Flow Tool was developed (Environment Canada 2008a). Empirical data concerning releases of specific substances to the environment are seldom available. Therefore, for each identified type of use of the substance, the proportion and quantity of release to the different environmental media are estimated, as is the proportion of the substance chemically transformed or sent for waste disposal. Unless specific information on the rate or potential for release of the substance from landfills and incinerators is available, the Mass Flow Tool does not quantitatively account for releases to the environment from disposal.
Assumptions and input parameters used in making these estimates are based on information obtained from a variety of sources including responses to regulatory surveys, Statistics Canada, manufacturers' websites and technical databases. Of particular relevance are emission factors, which are generally expressed as the fraction of a substance released to the environment, particularly during its manufacture, processing, and use associated with industrial processes. Sources of such information include emission scenario documents, often developed under the auspices of the Organisation for Economic Co-operation and Development (OECD), and default assumptions used by different international chemical regulatory agencies. It is noted that the level of uncertainty in the mass of substance and quantity released to the environment generally increase towards the end of the life cycle.
| Fate | Proportion of the mass (%)Table note b | Major life cycle stage involvedTable note c |
|---|---|---|
| Released to soil | 2.5 | Industrial use, consumer use |
| Released to air | 0.0 | - |
| Released to sewerTable note d | 18.5 | Formulation, industrial use, consumer use |
| Chemically transformed | 0 | - |
| Transferred to waste disposal sites (e.g., landfill, incineration) |
79 | Formulation, industrial use, consumer use, waste disposal |
Results indicate that the majority of Pigment Orange 5 (79%) is expected to be found in waste management sites, due mostly to the eventual disposal of manufactured items containing it. A small fraction of solid waste is incinerated, which is expected to result in transformation of the substance. Based largely on information contained in OECD emission scenario documents for processing and uses associated with this substance, it is estimated that approximately 3% and 20% of Pigment Orange 5 may be released to soil and wastewater, respectively. Releases of Pigment Orange 5 to soil are expected to occur from flaking and chipping of paints during industrial and consumer use. Releases of Pigment Orange 5 to wastewater are predicted to be mostly due to releases from industrial recycling of printing ink. Releases to wastewater are also predicted to occur from transport, handling and transfer line clean-out of paints and printing inks during formulation (e.g., paint manufacturing), from the consumer use of textiles containing it (mostly as a result of laundering) as well as from cleaning paint brush residues during industrial and consumer use of paints.
Although no information is available on the quantity of importation of consumer products containing Pigment Orange 5, it is anticipated that because losses from use of consumer products during their lifetime are expected to be relatively small, the quantities of releases to the various environmental media would not be significantly different from those estimated here. However, the quantities sent for waste management would be higher if importation of these products were taken into consideration.
Environmental Fate
The very low modelled vapour pressure and a negligible Henry's Law constant of ~ 10-9 Pa·m3/mol for Pigment Orange 5 are consistent with the fact that it is a large and complex molecule (Baughman and Perenich 1988; Danish EPA 1998). This pigment is not expected to volatilize at environmentally realistic temperatures, and will thus not be subject to long range atmospheric transport.
The particulate character of Pigment Orange 5 should have a key influence on its fate in the environment. Its particle size and density, together with its chemical stability and low aqueous solubility, indicate that it will partition by gravity to sediments if released to surface waters, and will tend to remain in soil if released to terrestrial environments.
Persistence and Bioaccumulation Potential
Environmental Persistence
Because of its very low solubility in water, this pigment may be considered to be not available for aerobic biodegradation if released to water during product manufacturing. Jaffe (1996) has stated that once a pigment is incorporated into a matrix (e.g., paint), it is expected to be durable and withstand the combined chemical and physical stresses of weather, solar radiation, heat, water and industrial pollutants. Therefore, direct contact with biota probably does not occur when the pigment is incorporated in paint, printing inks or textiles and it is not expected that the pigment would be susceptible to abiotic degradation.
Industries manufacturing pigments recognize that their substances are persistent. For example, the Color Pigments Manufacturers Association, Inc. has indicated that pigments are designed to be durable or persistent in the environment in order to provide colour to finished coatings, inks and paints (CPMA 2003).
The environmental persistence of Beta Naphthol pigments such as Pigment Orange 5 in anoxic environments is an important area of uncertainty. More generally for the broader chemical class, azo dyes are reported to be degraded in anoxic waters and sediment via anaerobic reduction of the azo bond (-N=N-) (Weber and Wolfe 1987). A mutagenic potential is attributed to their breakdown products, which include aromatic amines (Van der Zee 2002). More specifically, Beta Naphthol pigments have azo chromophores in their structure as well; however, no documentation has been found regarding a possible degradation potential of these pigments in the absence of oxygen. In principle, the crystal would have to dissolve first, releasing its constituent molecules. Then, the azo bonds in these molecules would be available for reduction. However given its limited solubility, it is expected that only a very small proportion of the pigment would be reduced in this manner.
Some disperse azo dyes have been shown to undergo anaerobic degradation in sediment at depth where anoxic conditions persist (Yen et al 1991, Baughman and Weber 1994, Weber and Adams 1995). Disperse dyes and pigments are expected to eventually settle to the aerobic layers of surface sediment where they will persist until sediment burial creates conditions suitable for reducing conditions. The rate of sediment deposition varies from site to site and thus is very difficult to ascertain the residence time of dyes in anaerobic sediment layers as a function of sediment burial. It is likely however, that this is much greater than 365 days. Once under anaerobic or reducing conditions, azo dyes may undergo degradation to substituted aromatic constituents. At depth, these biodegradation transformation products are not expected to present a high degree of exposure potential to most aquatic organisms and therefore not likely to present an ecological concern. It is also expected that if the azo pigment is reduced, it would not likely present an ecological harm.
Based on the weight of evidence provided by the above-described literature, Pigment Orange 5 is considered to meet the persistence criteria defined in the Persistence and Bioaccumulation Regulations (Canada 2000).
Potential for Bioaccumulation
For most organic compounds there is a predictable relationship between Kow and the bioconcentration factor in lipids (Mackay 1982). However this relationship is not considered to be applicable for Pigment Orange 5.
The ratio log (CO/CW) has been estimated from the experimental solubilities of Pigment Orange 5 determined independently in octanol (CO) and water (CW) (Table 2a), and this experimentally derived ratio is preferred over the model-derived log Kow for this pigment. This approach is supported by the observation that partitioning into octanol is a good indicator of a substance's potential to partition into the lipid phase of aquatic biota (Bertelsen et al. 1998) and, for pigments, the observation that a reduced solubility in octanol translates into a similarly reduced bioconcentration factor (BCF) and bioaccumulation factor (BAF) in an aquatic organism (Banerjee and Baughman 1991).
A revised set of BCF and BAF estimates for Pigment Orange 5, different from those used during categorization, have been obtained from quantitative structure-activity relationship (QSAR)-based bioaccumulation models, using the experimentally based value log (CO/CW) in place of the QSAR-estimated log Kow. Similar log (CO/CW) values have been derived from experimental solubilities for reasonably close analogues, Pigment Red 4 and Pigment Red 3 (Table 2b). Table 4 shows that all of the revised BCF and BAF estimates for Pigment Orange 5 are all well below 1000.
| Test Organism | Endpoint | Value wet weight (L/kg) |
Reference |
|---|---|---|---|
| Fish | BAF | 18 | Gobas BAF T2MTL (Arnot and Gobas 2003) |
| Fish | BCF | 16 | Gobas BCF T2LTL (Arnot and Gobas 2003) |
| Fish | BCF | 87 | OASIS Forecast 2005 |
| Fish | BCF | 10Table note e | BCFWIN 2000 |
Pigment Orange 5 is therefore expected to present a low bioaccumulation potential, because of its very limited affinity for the lipid phase of living organisms. This is in agreement with the conclusion of a Danish assessment report (Danish EPA 1998) that based on published information, organic pigments are generally not bioaccumulative.
The results of the QSAR models indicate that Pigment Orange 5 does not meet the bioaccumulation criterion (BCF, BAF greater than or equal to 5000) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Potential to Cause Ecological Harm
Ecological Effects Assessment
A - In the Aquatic Compartment
There is experimental evidence that Pigment Orange 5 does not cause acute harm to aquatic organisms at the level of saturation. Furthermore, predicted ecotoxicity values were obtained using the experimental log (Co/Cw) of Pigment Orange 5.
The effect of a saturated solution of Pigment Orange 5 on the immobilisation of Daphnia magna was determined under static conditions over 48 hours (Table 5a). Twenty test organisms were exposed to the saturated solution and a control. Water quality parameters were measured at the start and end of the test. The pH was maintained between 7.58 and 7.88 and oxygen between 8.46 and 8.64 mg/L. The temperature ranged from 18 to 22ºC. Saturation was achieved by shaking the stock solution for 24 hours and removing un-dissolved particles by membrane filtration (0.45 microns). The concentration of the pigment in solution was measured by dissolved organic carbon (DOC) analysis at the start and end of the test. It was observed that 0.7 mg/L DOC was present at the beginning and 1.2 mg/L was present at the end of the test, indicating that the concentration of the pigment was maintained throughout the test. Based on the measured DOC, the concentration of pigment at saturation is estimated to be approximately 1.6 mg/L. No biologically significant effects (immobilization) were observed at saturation. This study is considered to be of high reliability for the present assessment as good laboratory practices (GLP) were followed, control and reference toxicants were used and the dissolved organic carbon concentration was measured at the beginning and end of the experiment. However, according to the guidance provided by the OECD for sparingly soluble substances, when a substance is found to have no effects at saturation, this saturation concentration is typically below the water solubility value obtained in a water solubility test (OECD 2000). The water solubility of Pigment Orange 5 was measured to be 0.0068 mg/L. Therefore the measured DOC in this test may not be representative of the dissolved concentration, but rather a measure of the pigment particles and perhaps a small fraction of dissolved pigment. Although the stock solution was shaken for 24 hours to increase solubility, the maximum solubility may not have been achieved. During the solubility test, the pigment was shaken in water for 2 hours at 30ºC and then for 70 hours at 26-27ºC. Even during the solubility test, after this thorough shaking, a residue of undissolved colorant was still observed on the 0.05-µm filter. Also, the average particle size for Pigment Orange 5 is 0.285 microns, indicating that the filter would not have removed most particles. Therefore, it is expected that undissolved pigment was also present in the toxicity test.
In a similar toxicity test on the analogue, Pigment Red 3 (2425-85-6), it was also found that no biologically significant effects were observed in Daphnia magna at saturation. This study was also considered to be of high reliability. The DOC was also measured in this test and was found to correspond to a concentration of 0.9 mg/L of pigment. This concentration is also expected to represent the concentration of pigment particles and a small fraction of dissolved pigment. Therefore, the dissolved and particulate forms of Pigment Orange 5 are expected to have a negligible to low acute toxicity to Daphnia magna.
Aquatic toxicity predictions, recalculated using log (CO/CW) instead of the modelled log Kow, were obtained from the ECOSAR program (ECOSAR 2004). It is assumed that Pigment Orange 5 has a narcotic mode of action (MOA) similar to that of phenols. However, the ASTER (1999) model predicted the MOA "uncoupling of oxidative phosphorylation" for this pigment, in addition to narcosis. An application factor of 100 (Environment Canada 2003) was therefore applied to the ECOSAR estimate to extrapolate from baseline toxicity to this more toxic MOA. It should be noted that the above MOAs are predicted for the solubilized molecule, which is likely released in very low amounts in solution as suggested by the solubility test in water. Furthermore, the training sets for ECOSAR phenols and ASTER do not contain pigments, introducing more uncertainty into these estimates. Table 5b presents these modelled ecotoxicity results, which are consistent with the empirical studies indicating that there would be no acute effect at saturation.
| Substance | Organism | Test type | Endpoint | Duration | Value | Reference |
|---|---|---|---|---|---|---|
| Pigment Orange 5 | Daphnid | Acute | EC50Table note f | 48 hours | No effect at saturation (1.6mg/L) |
Study Submission 2007g |
| Pigment Red 3 | Daphnid | Acute | EC50Table note f | 48 hours | No effect at saturation (0.9mg/L) |
Study Submission 2007h |
| Organism | Endpoint | Duration | Value (mg/L) |
Chemical class/ mode of action |
Reference |
|---|---|---|---|---|---|
| Fish | LC50 | 14 days | 203.647Table note g | Neutral Organic SAR (baseline toxicity) |
ECOSAR 2004 |
| Fish | LC50 | 14 days | 2.036 | Uncoupling of oxidative phosphorylation | CalculatedTable note h |
| Fish | LC50 | 96 hours | 28.176Table note g | Phenols | ECOSAR 2004 |
| Daphnid | LC50 | 48 hours | 12.493Table note g | Phenols | ECOSAR 2004 |
| Green algae | EC50 | 96 hours | 74.349Table note g | Phenols | ECOSAR 2004 |
| Fish | LC50 | 96 hours | 28.176Table note g | Dinitrobenzenes | ECOSAR 2004 |
| Daphnid | LC50 | 48 hours | 12.493Table note g | Dinitrobenzenes | ECOSAR 2004 |
| Green algae | EC50 | 96 hours | 74.349Table note g | Dinitrobenzenes | ECOSAR 2004 |
Chronic exposure to Pigment Orange 5 is likely to be low in water due to its low water solubility, relatively low potential for bioaccumulation, high molecular weight and particulate nature.
Overall, experimental and modelled toxicity data indicate that Pigment Orange 5 is predicted to have a negligible to low potential for acute toxicity to aquatic organisms (LC50/EC50s are above saturation).
B - In Other Environmental Compartments
No empirical or predicted effects data for non-aquatic organisms were identified for this compound. Pigment Orange 5 is expected to reside in sediment or soil; however, effect levels in these media have not been identified.
Ecological Exposure Assessment
No data have been found regarding concentrations of Pigment Orange 5 in the Canadian environment. The Mass Flow Tool estimated that more than 75% of the mass of this pigment ends up in waste disposal facilities. Off-site chemical migration from these facilities is unlikely, or can be predicted to be minor, because of the negligible geochemical mobility of the pigment indicated by its very low solubility in water and in organic solvents. Consequently, it is anticipated that there are negligible releases associated with the waste management stage of this substance.
The Mass Flow Tool estimated that up to about 18.5% of the total mass of Pigment Orange 5 in use could be released to wastewater. Available industrial information suggested that these releases would be generated during industrial processes using the pigment to manufacture other coloured products and during the recycling of printing inks (Environment Canada 2007a). A conservative, site-specific industrial releases scenario was developed to obtain predicted environmental concentrations (PEC). The scenario conservatively considered four sites in Canada that had the highest aquatic exposure based on the quantity of substance used, the loss to wastewater, STP flow and dilution factors at each site. These four sites represent sectors that use or process the pigment. Based on the site-specific scenario, PECs ranged from 0.00043 to 0.0077 mg/L (Environment Canada 2008b).
Because Pigment Orange 5 is used in paints, coatings and printing inks it is likely that releases occur from its use in these products. Environment Canada's tool to estimate down-the-drain releases from consumer uses (Mega Flush) was employed to estimate the potential substance concentration in multiple water bodies receiving sewage treatment plant effluents to which consumer products containing the substance may have been released (Environment Canada 2008c). The spreadsheet model is designed to provide these estimates based on conservative assumptions regarding the amount of chemical used and released by consumers. It was assumed that primary and secondary sewage treatment plant (STP) removal rates were 52.2% and 70.9% respectively, losses from use to be 1.5%, consumer use of the substance to be over 365 days/year, and the flow rate used at all sites to be the 10th percentile value. These estimates were made for approximately 1000 release sites across Canada, which account for all of the major STPs in Canada. The equation and inputs used to calculate the predicted environmental concentration (PEC) of Pigment Orange 5 in the receiving water bodies are described in Environment Canada (2008d). A scenario was run assuming a total consumer use quantity of 100,000 kg/yr, based on the upper limit of the range reported under the section 71 notice (Environment Canada 2007b).
Using this scenario, the tool estimates that the PEC in the receiving water bodies ranges from 4.5 × 10-6 to 9.5 × 10-3 mg/L (Table 6).
Characterization of Ecological Risk
The approach taken in this ecological screening assessment was to examine the available scientific information and develop conclusions based on a weight-of-evidence approach and using precaution as required under CEPA 1999. Particular consideration has been given to risk quotient analysis, persistence, bioaccumulation, inherent toxicity, sources and fate in the environment.
Pigment Orange 5 is determined to be persistent, based on published evidence and comments submitted by industry. However, it has been determined not to be bioaccumulative in accordance with the Persistence and Bioaccumulation Regulations of CEPA 1999 (Canada 2000), based on observations of its very low solubility in octanol and low modelled BCFs.
Newly acquired empirical data and modelled aquatic toxicity results also suggest that this pigment is not very harmful, showing a negligible to low potential for toxicity to aquatic organisms (LC50/EC50 above saturation).
Since Pigment Orange 5 is considered persistent but not bioaccumulative, a quantitative evaluation of exposure and of ecological effects was conducted as part of the weight-of-evidence evaluation of this pigment's potential to cause harm.
An experimental toxicity study performed on Daphnia magna revealed that no effects were observed at 1.6 mg/L. The lowest modelled LC50 predicted by the toxicity models was 2.036 mg/L. The predicted toxicity will be used as a critical toxicity value (CTV) as the substance was in the particulate form in the experiment and no effects were observed. Furthermore, the modelled result is felt to address an endpoint that is not captured by existing experimental results With the application factor of 100 to extrapolate from a model prediction to an experiment and an acute to a chronic endpoint, the resulting Predicted No Effect Concentration (PNEC) is 0.02 mg/L and is deemed very conservative due to the multiple application factors applied to the original prediction.
When PEC to PNEC are compared to determine the risk quotient, the resulting risk quotients in all cases are less than 1 (Table 6), suggesting that there is negligible risk to the environment. For exposure resulting from down-the-drain releases through consumer uses (conservative scenario), MegaFlush results estimate that the PNEC will not be exceeded at any of the approximately 1000 sites considered (i.e. all risk quotients less than 1). This indicates that down-the-drain consumer releases of Pigment Orange 5 are not expected to harm aquatic organisms. The conservative risk quotients from the site-specific industrial scenario is also less than 1 (Table 6) indicating that there is little risk.
| Organism | CTV (mg/L) | PNEC (mg/L) | PEC (mg/L) | Scenario | Risk quotient (PEC/PNEC) |
|---|---|---|---|---|---|
| Fish | 2.036 | 0.02 | 0-0.00095 | Mega Flush Consumer Release Scenario: discharge to 960 watercourses in Canada | 0-0.05 |
| Fish | 2.036 | 0.02 | 0.00043-0.0077 | Site-specific industrial releases | 0.022-0.38 |
Considering these findings and the high degree of conservatism in the underlying assumptions, it is concluded that Pigment Orange 5 is unlikely to be causing ecological harm in Canada.
Uncertainties in Evaluation of Ecological Risk
This section summarizes the key uncertainties associated with the risk assessment of Pigment Orange 5.
Pigment Orange 5 is expected to partition primarily to sediment; however, experimental data on its fate and toxicity in sediments are lacking. Specifically, the long-term stability of Pigment Orange 5 in anoxic sediments, as well as in anoxic layers in the soil column of waste disposal sites, has not been studied. It is however considered likely that the crystalline structure of Pigment Orange 5 would be maintained in these compartments, and that the substance would remain unavailable to sediment-dwelling organisms and unavailable for reduction of the azo bond, which could release bioavailable aromatic amines. Although acute and chronic toxicity data are not available for sediment or soil-dwelling organisms, toxicity is expected to be low based on information for aquatic organisms.
Nanoscale materials are informally defined as substances having at least one dimension less than 100 nm. Evidence is accumulating to the effect that nanoparticles can be absorbed by non-specific biouptake pathways such as pinocytosis (Leroueil et al. 2007). Organic pigments, such as Pigment Orange 5, may have a certain proportion of their particle size spectra in the nanoparticle range (e.g., Table 2). Presently, the bioaccumulation mechanisms and potential of these particles is poorly understood, as is the nature of the relationship between their bioaccumulation and their toxicity. Furthermore, certain less commonly considered environmental fate processes may have an important influence on the propensity of the pigment nanoparticles to be taken up by biota (e.g., importance of aggregation in nature: Wiesner et al. (2006)).
Conclusion
Based on the information presented in this screening assessment, it is concluded that Pigment Orange 5 is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
It is therefore concluded that Pigment Orange 5 does not meet the definition of toxic as set out in section 64 of CEPA 1999. Additionally, Pigment Orange 5 meets the criteria for persistence but does not meet the criteria for bioaccumulation as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
References
ACD/pKa DB [Prediction Module]. 2005. Version 9.04. Toronto (ON): Advanced Chemistry Development. http://www.acdlabs.com/products/phys_chem_lab/pka/
Arnot JA, Gobas FAPC. 2003. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci 22(3): 337-345.
[ASTER] Assessment Tools for the Evaluation of Risk [Internet]. 1999. Duluth (MN): US Environmental Protection Agency, Mid-Continent Ecology Division. Restricted access. http:www.epa.gov/med/Prods_Pubs/aster.htm
Banerjee S, Baughan GL. 1991. Bioconcentration factors and lipid solubility. Environ Sci Technol. 26: 536-539.
Baughman GL, Perenich TA. 1988. Fate of dyes in aquatic systems: I. Solubility and partitioning of some hydrophobic dyes and related compounds. Environ Toxicol Chem. 7: 183-199.
[BCFWIN] BioConcentration Factor Program for Windows [Estimation Model]. 2000. Version 2.15. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Bertelsen SL, Hoffman AD, Gallinat CA, Elonen CM, Nichols JW. 1998. Evaluation of Log Kow and tissue lipid content as predictors of chemical partitioning in fish tissues. Environ Toxicol Chem. 17: 1447-1455.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33, Canada Gazette. Part III. vol. 22, no. 3. http://canadagazette.gc.ca/partIII/1999/g3-02203.pdf
Canada. 2000. Canadian Environmental Protection Act: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March, 2000, SOR/2000-107, Canada Gazette. Part II, vol. 134, no. 7, p. 607-612. http://canadagazette.gc.ca/partII/2000/20000329/pdf/g2-13407.pdf
Canada, Dept. of the Environment, Dept. of Health. 2006a.Canadian Environmental Protection Act, 1999 : Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette, Part I, vol. 140, no. 49, p. 4109-4117. http://canadagazette.gc.ca/partI/2006/20061209/pdf/g1-14049.pdf
Canada, Dept. of the Environment. 2006b. Canadian Environmental Protection Act, 1999: Notice with respect to selected substances identified as priority for action. Canada Gazette, Part I, vol. 140, no. 9, p. 435-459. http://canadagazette.gc.ca/partI/2006/20060304/pdf/g1-14009.pdf
Canada, Dept. of the Environment, Dept. of Health. 2007a.Canadian Environmental Protection Act, 1999: Notice of third release of technical information relevant to substances identified in the Challenge. Canada Gazette, Part I, vol. 141. no. 33, p. 2375-2379. http://canadagazette.gc.ca/partI/2007/20070818/pdf/g1-14133.pdf#page=7
Canada, Dept. of the Environment. 2007b. Canadian Environmental Protection Act, 1999: Notice with respect to Batch 3 substances. Canada Gazette, Part I, vol. 141. no. 33, p. 2379-2394. http://canadagazette.gc.ca/partI/2007/20070818/pdf/g1-14133.pdf#page=7
Clariant. 2007. Colorants for the Paint Industry. [cited 2007 June 3]. http://www.clariant.com/C1256C70004EEA54/vwLookupDownloads/DP8523ED.pdf/$File/DP8523ED.pdf
Colour Index International. 2002. Fourth Edition Online. Society of Dyers and Colourists and American Association of Textile Chemists and Colorists. [cited 2008 Feb 26]. http://www.colour-index.org/
CPMA (Color Pigments Manufacturers Association, Inc.). 2003. Comments of the Color Pigments Manufacturers Association, Inc. on the Draft Guidance Manual for the Categorization of Organic and Inorganic Substances on Canada 's Domestic Substances List ('DSL') and Environment Canada's Computer Generated Estimates and Empirical Data on Approximately 12,000 Discrete Organic Chemicals on the DSL. Letter to Danie Dubé, Existing Substances Branch, September 30, 2003.
Danish EPA (Environmental Protection Agency). 1998. Survey of azo-colorants in Denmark : Consumption, use, health and environmental aspects. Ministry of Environment and Energy, Denmark.
[ECOSAR] Ecological Structural Activity Relationships [Internet]. 2004. Version 0.99h. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Environment Canada. 2003. Guidance manual for the categorization of organic and inorganic substances on Canada's DSL. Gatineau (QC): Environment Canada, Existing Substances Division. Available on request.
Environment Canada. 2007a. Assumptions, limitations and uncertainties of the mass flow tool for Pigment Orange 5 CAS RN 3468-63-1. Internal draft document. Gatineau (QC): Environment Canada, Existing Substances Division. Available on request.
Environment Canada. 2007b. Data for Batch 3 substances collected under the Canadian Environmental Protection Act, 1999, Section 71: Notice with respect to Batch 3 substances. Prepared by : Environment Canada, Health Canada, Existing Substances Program.
Environment Canada. 2008a. Guidance for conducting ecological assessments under CEPA, 1999: science resource technical s eries, technical guidance module: Mass Flow Tool. Preliminary draft working document. Gatineau (QC): Environment Canada, Existing Substances Division.
Environment Canada. 2008b. Guidance for conducting ecological assessments under CEPA, 1999: science resource technical s eries, technical guidance module: the Industrial Generic Exposure Tool - Aquatic (IGETA). Working document. Gatineau (QC): Environment Canada, Existing Substances Division.
Environment Canada. 2008c. IGETA report: CAS RN 3468-63-1. 2008-05-20. Unpublished report. Gatineau (QC): Environment Canada, Existing Substances Division.
Environment Canada. 2008d. Guidance for conducting ecological assessments under CEPA 1999, Science Resource Technical Series, Technical Guidance Module: Mega Flush Consumer Release Scenario. Preliminary draft working document. Gatineau (QC): Environment Canada, Existing Substances Division.
European Commission. 2002. Council Directive of 19 July, 2002 relating to restrictions on the marketing and use of certain dangerous substances and preparations (azocolourants) (76/769/EEC) (OJL 243. 2002. p. 15. [accessed 2008 December 8]. http://eur-lex.europa.eu/pri/en/oj/dat/2002/l_243/l_24320020911en00150018.pdf
Health Canada. 2007. List of prohibited and restricted cosmetic ingredients (The cosmetic ingredient "hotlist"). Ottawa, Ontario: Cosmetic Division, Consumer Product Safety Bureau, Healthy Environments and Consumer Safety Branch. http://www.hc-sc.gc.ca/cps-spc/person/cosmet/info-ind-prof/_hot-list-critique/hotlist-liste_e.html
[HENRYWIN] Henry's Law Constant Program for Microsoft Windows [Estimation Model]. 2000. Version 3.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Jaffe EE. 1996. Pigments (Organic). In: Kroschwitz JI and Howe-Grant M (eds.). Kirk-Othmer encyclopedia of chemical technology, 4 th ed. John Wiley and Sons, New York, NY. Vol. 19: 41-78.
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2000. Version 1.67. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Leroueil PR, Hong S, Mecke A, Baker JR Jr, Orr BG, Banaszak Holl MM. 2007. Nanoparticle interaction with biological membranes: does nanotechnology present a Janus face? Acc Chem Res. 40: 335-342.
Lincke G. 2003. Molecular stacks as a common characteristic in the crystal lattice of organic pigment dyes. A contribution to the "soluble-insoluble" dichotomy of dyes and pigments from the technological point of view. Dyes and Pigments. 59: 1-24.
Mackay D. 1982. Correlation of bioconcentration factors. Environ Sci Technol. 16: 274-278.
[MPBPWIN] Melting Point Boiling Point Program for Microsoft Windows [Estimation Model]. 2000. Version 1.41. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
[MSDS] Material Safety Data Sheet. 2003. Suco Or L 3052 HD [Internet]. Toronto (ON). BASF Canada. [cited 2006 Dec 11]. http://www.msdsonline.com/
[MSDS] Material Safety Data Sheet. 2005. Pigmatex L Crange RC [Internet]. Kobenhavnsvej (DK). Sun Chemical A/S. [cited 2006 Dec 11]. http://www.msdsonline.com/
[NCI] National Chemical Inventories [database on CD-ROM]. 2006. Columbus (OH): American Chemical Society, Chemical Abstracts Service. [cited 2006 Dec 11]. http://www.cas.org/products/cd/nci/index.html
NPIRI Raw Materials Data Handbook. 2000. A reference guide to regulatory data and technical performance properties. Volume 4 Pigments, Second Edition. National Printing Research Institute. Woodbridge, NJ. Page 62.
[OASIS Forecast] Optimized Approach based on Structural Indices Set [Internet]. 2005. Version 1.20. Bourgas, Bulgaria: Laboratory of Mathematical Chemistry. http://oasis-lmc.org/?section=software
[OECD] Organisation for Economic Co-operation and Development. 2000. Guidance document on aquatic toxicity testing of difficult substances and mixtures. OECD Environmental Health and Safety Publications Series on Testing and Assessment. Paris (FR): Environment Directorate, OECD. Number 23. [cited 2008 Feb 28]. http://www.epa.gov/oscpmont/oscpendo/pubs/ref-2_oecd_gd23_difficult_substances.pdf
[OECD] Organisation for Economic Co-operation and Development. 2004. Emission Scenario Document on textile finishing industry [Internet]. Paris (FR): OECD Environmental Directorate, Environmental Health and Safety Division. ENV/JM/MONO(2004)12, JT00166691 [cited 2008 Dec]. http://www.olis.oecd.org/olis/2004doc.nsf/LinkTo/NT0000452A/$FILE/JT00166691.PDF
[OECD] Organisation for Economic Co-operation and Development. 2006. Draft emission scenario document on coating industry (paints, lacquers and varnishes). Draft document. Paris (FR): OECD.
[OECD] Organisation for Economic Co-operation and Development. 2007. Emission scenario document on adhesive formulation [Internet]. Final report. Paris (FR): OECD, Environment Directorate. (Series on Emission Scenario Documents). [cited 2008 Feb 26]. http://ascouncil.org/news/adhesives/docs/EPAFormulation.pdf
[SPIN] Substances in Products in Nordic Countries [database on the Internet]. 2006. Copenhagen (DK): Nordic Council of Ministers. [cited 2006 Dec 11]. http://195.215.251.229/Dotnetnuke/Home/tabid/58/Default.aspx
Study Submission. 2007a. Unpublished confidential study submitted to Environment Canada, Existing Substances Division under the Chemical Management Plan Challenge initiative. Available as Robust Study Summary, Identification No.: 13365Submission016. (See Appendix I).
Study Submission. 2007b. Unpublished confidential study submitted to Environment Canada, Existing Substances Division under the Chemical Management Plan Challenge initiative. Available as Robust Study Summary, Identification No.: 13365Submission017. (See Appendix I).
Study Submission. 2007c. Unpublished confidential study submitted to Environment Canada, Existing Substances Division under the Chemical Management Plan Challenge initiative. Available as Robust Study Summary, Identification No.: 13365Submission013. (See Appendix I).
Study Submission. 2007d. Unpublished confidential study submitted to Environment Canada, Existing Substances Division under the Chemical Management Plan Challenge initiative. Available as Robust Study Summary, Identification No.: 13365Submission014. (See Appendix I).
Study Submission. 2007e Unpublished confidential study submitted to Environment Canada, Existing Substances Division under the Chemical Management Plan Challenge initiative. Available as Robust Study Summary, Identification No.: 13365Submission011. (See Appendix I).
Study Submission. 2007f. Unpublished confidential study submitted to Environment Canada, Existing Substances Division under the Chemical Management Plan Challenge initiative. Available as Robust Study Summary, Identification No.: 13365Submission012. (See Appendix I).
Study Submission. 2007g. Unpublished confidential study submitted to Environment Canada, Existing Substances Division under the Chemical Management Plan Challenge initiative. Available as Robust Study Summary, Identification No.: 13365Submission015. (See Appendix I).
Study Submission. 2007h. Unpublished confidential study submitted to Environment Canada, Existing Substances Division under the Chemical Management Plan Challenge initiative. Available as Robust Study Summary, Identification No.: 13365Challenge020. (See Appendix I).
Van der Zee FP. 2002. Anaerobic azo dye reduction. Doctoral Thesis, Wageningen University. Wageningen, The Netherlands. pp 142.
Weber EJ, Adams RL. 1995. Chemical- and sediment-mediated reduction of the azo dye Disperse Blue 79. Environ Sci Technol 29: 1163-1170.
Weber EJ, Wolfe NL. 1987. Kinetic studies of the reduction of aromatic azo compounds in anaerobic sediment/water systems. Environ Toxicol Chem. 6: 911-919.
Whitaker A. 1978. Crystal structure analysis of azo pigments involving -Naphthol: A review. Journal of the Society of Dyers and Colourists (Communications). 431-435.
Wiesner MR, Lowry GV, Alvarez P, Dionysiou D, Biswas P. 2006. Assessing the risks of manufactured nanomaterials. Environ Sci Technol. 40: 4336-4345.
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [Estimation Model]. 2000. Version 1.41 Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Yen CC, Perenich TA, Baughman GL. 1991. Fate of commercial disperse dyes in sediments. Environ Toxicol Chem 10:1009-1017.
Appendix I - Robust study summaries
Evaluation of experimental data using Kollig's approach
Kollig, H.P. 1988. Criteria for evaluating the reliability of literature data on environmental process constants. Toxicol. Environ. Chem. 17: 287-311.
Table A-1. Evaluation of experimental data using Kollig's approach for 13365Submission016
| Reference : | Study Submission 2007e. 13365Submission016. Water Solubility Following ETAD Method |
|---|---|
| Test substance : | CAS RN 3468-63-1, Pigment Orange 5 |
| Item | Weight | Response | Mark |
|---|---|---|---|
| Could you repeat the experiment with available information? | 5 | Yes | 5 |
| Is a clear objective stated? | 1 | Yes | 1 |
| Is water quality characterized or identified (distilled or deionized)? | 2 | No | 2 |
| Are the results presented in detail, clearly and understandably? | 3 | Yes | 3 |
| Are the data from a primary source and not from a referenced article? | 3 | Yes | 3 |
| Was the chemical tested at concentrations below its water solubility? | 5 | N/A | |
| Were particulates absent? | 2 | Yes | 2 |
| Was a reference chemical of known constant tested? | 3 | No | 0 |
| Were other fate processes considered? | 5 | N/A | |
| Was a control (blank) run? | 3 | Yes | 1.5 |
| Was temperature kept constant? | 5 | Assumed | 5 |
| Was the experiment done near room temperature (15 - 30°C)? | 3 | Yes | 3 |
| Is the purity of the test chemical reported (greater than 98%)? | 3 | Yes | 3 |
| Was the chemical's identity proven? | 3 | Yes | 3 |
| Is the source of the chemical reported? | 1 | No | 0 |
| Results: | (X±SE) |
|---|---|
| Solubility: | 6.8 µg/L |
| Score: | 31.5/37=85% |
| Degree of reliability (the reliability code for ecotoxicological studies of DSL categorization is used): |
High |
| Comments: |
Table A-2. Evaluation of experimental data using Kollig's approach for 13365Submission017
| Reference : | Study Submission 2007f. 13365Submission017. Octanol Solubility Following ETAD Method |
|---|---|
| Test substance : | CAS RN 3468-63-1, Pigment Orange 5 |
| Item | Weight | Response | Mark |
|---|---|---|---|
| Could you repeat the experiment with available information? | 5 | Yes | 5 |
| Is a clear objective stated? | 1 | Yes | 1 |
| Is water quality characterized or identified (distilled or deionized)? | 2 | No | 2 |
| Are the results presented in detail, clearly and understandably? | 3 | Yes | 3 |
| Are the data from a primary source and not from a referenced article? | 3 | Yes | 3 |
| Was the chemical tested at concentrations below its water solubility? | 5 | N/A | |
| Were particulates absent? | 2 | Yes | 2 |
| Was a reference chemical of known constant tested? | 3 | No | 0 |
| Were other fate processes considered? | 5 | N/A | |
| Was a control (blank) run? | 3 | Yes | 1.5 |
| Was temperature kept constant? | 5 | Assumed | 5 |
| Was the experiment done near room temperature (15 - 30°C)? | 3 | Yes | 3 |
| Is the purity of the test chemical reported (greater than 98%)? | 3 | Yes | 3 |
| Was the chemical's identity proven? | 3 | Yes | 3 |
| Is the source of the chemical reported? | 1 | No | 0 |
| Results: | (X±SE) |
|---|---|
| Solubility: | 1.76 mg/L |
| Score: | 31.5/37=85% |
| Degree of reliability (the reliability code for ecotoxicological studies of DSL categorization is used): |
High |
| Comments: |
Table A-3. Evaluation of experimental data using Kollig's approach for 13365Submission013
| Reference : | Study Submission 2007c. 13365Submission013. Water Solubility Following ETAD Method |
|---|---|
| Test substance : | CAS RN 2814-77-9, Pigment Red 4 |
| Item | Weight | Response | Mark |
|---|---|---|---|
| Could you repeat the experiment with available information? | 5 | Yes | 5 |
| Is a clear objective stated? | 1 | Yes | 1 |
| Is water quality characterized or identified (distilled or deionized)? | 2 | No | 2 |
| Are the results presented in detail, clearly and understandably? | 3 | Yes | 3 |
| Are the data from a primary source and not from a referenced article? | 3 | Yes | 3 |
| Was the chemical tested at concentrations below its water solubility? | 5 | N/A | |
| Were particulates absent? | 2 | Yes | 2 |
| Was a reference chemical of known constant tested? | 3 | No | 0 |
| Were other fate processes considered? | 5 | N/A | |
| Was a control (blank) run? | 3 | Yes | 1.5 |
| Was temperature kept constant? | 5 | Assumed | 5 |
| Was the experiment done near room temperature (15 - 30°C)? | 3 | Yes | 3 |
| Is the purity of the test chemical reported (greater than 98%)? | 3 | Yes | 3 |
| Was the chemical's identity proven? | 3 | Yes | 3 |
| Is the source of the chemical reported? | 1 | No | 0 |
| Results: | (X±SE) |
|---|---|
| Solubility: | 3.3 µg/L |
| Score: | 31.5/37=85% |
| Degree of reliability (the reliability code for ecotoxicological studies of DSL categorization is used): |
High |
| Comments: |
Table A-4. Evaluation of experimental data using Kollig's approach for 13365Submission014
| Reference : | Study Submission 2007d. 13365Submission014. Octanol Solubility Following ETAD Method |
|---|---|
| Test substance : | CAS RN 2814-77-9, Pigment Red 4 |
| Item | Weight | Response | Mark |
|---|---|---|---|
| Could you repeat the experiment with available information? | 5 | Yes | 5 |
| Is a clear objective stated? | 1 | Yes | 1 |
| Is water quality characterized or identified (distilled or deionized)? | 2 | No | 2 |
| Are the results presented in detail, clearly and understandably? | 3 | Yes | 3 |
| Are the data from a primary source and not from a referenced article? | 3 | Yes | 3 |
| Was the chemical tested at concentrations below its water solubility? | 5 | N/A | |
| Were particulates absent? | 2 | Yes | 2 |
| Was a reference chemical of known constant tested? | 3 | No | 0 |
| Were other fate processes considered? | 5 | N/A | |
| Was a control (blank) run? | 3 | Yes | 1.5 |
| Was temperature kept constant? | 5 | Assumed | 5 |
| Was the experiment done near room temperature (15-30°C)? | 3 | Yes | 3 |
| Is the purity of the test chemical reported (greater than 98%)? | 3 | Yes | 3 |
| Was the chemical's identity proven? | 3 | Yes | 3 |
| Is the source of the chemical reported? | 1 | No | 0 |
| Results: | (X±SE) |
|---|---|
| Solubility: | 9.4 mg/L |
| Score: | 31.5/37=85% |
| Degree of reliability (the reliability code for ecotoxicological studies of DSL categorization is used): |
High |
| Comments: |
Table A-5. Evaluation of experimental data using Kollig's approach for 13365Submission011
| Reference : | Study Submission 2007a.13365Submission011. Water Solubility Following ETAD Method |
|---|---|
| Test substance : | CAS RN 2425-85-6, Pigment Red 3 |
| Item | Weight | Response | Mark |
|---|---|---|---|
| Could you repeat the experiment with available information? | 5 | Yes | 5 |
| Is a clear objective stated? | 1 | Yes | 1 |
| Is water quality characterized or identified (distilled or deionized)? | 2 | No | 2 |
| Are the results presented in detail, clearly and understandably? | 3 | Yes | 3 |
| Are the data from a primary source and not from a referenced article? | 3 | Yes | 3 |
| Was the chemical tested at concentrations below its water solubility? | 5 | N/A | |
| Were particulates absent? | 2 | Yes | 2 |
| Was a reference chemical of known constant tested? | 3 | No | 0 |
| Were other fate processes considered? | 5 | N/A | |
| Was a control (blank) run? | 3 | Yes | 1.5 |
| Was temperature kept constant? | 5 | Assumed | 5 |
| Was the experiment done near room temperature (15 - 30°C)? | 3 | Yes | 3 |
| Is the purity of the test chemical reported (greater than 98%)? | 3 | Yes | 3 |
| Was the chemical's identity proven? | 3 | Yes | 3 |
| Is the source of the chemical reported? | 1 | No | 0 |
| Results: | (X±SE) |
|---|---|
| Solubility: | 3.3 µg/L |
| Score: | 31.5/37=85% |
| Degree of reliability (the reliability code for ecotoxicological studies of DSL categorization is used): |
High |
| Comments: |
Table A-6. Evaluation of experimental data using Kollig's approach for 13365Submission012
| Reference : | Study Submission 2007b. 13365Submission012. Octanol Solubility Following ETAD Method |
|---|---|
| Test substance : | CAS RN 2425-85-6, Pigment Red 3 |
| Item | Weight | Response | Mark |
|---|---|---|---|
| Could you repeat the experiment with available information? | 5 | Yes | 5 |
| Is a clear objective stated? | 1 | Yes | 1 |
| Is water quality characterized or identified (distilled or deionized)? | 2 | No | 2 |
| Are the results presented in detail, clearly and understandably? | 3 | Yes | 3 |
| Are the data from a primary source and not from a referenced article? | 3 | Yes | 3 |
| Was the chemical tested at concentrations below its water solubility? | 5 | N/A | |
| Were particulates absent? | 2 | Yes | 2 |
| Was a reference chemical of known constant tested? | 3 | No | 0 |
| Were other fate processes considered? | 5 | N/A | |
| Was a control (blank) run? | 3 | Yes | 1.5 |
| Was temperature kept constant? | 5 | Assumed | 5 |
| Was the experiment done near room temperature (15 - 30°C)? | 3 | Yes | 3 |
| Is the purity of the test chemical reported (greater than 98%)? | 3 | Yes | 3 |
| Was the chemical's identity proven? | 3 | Yes | 3 |
| Is the source of the chemical reported? | 1 | No | 0 |
| Results: | (X±SE) |
|---|---|
| Solubility: | 17.9 mg/L |
| Score: | 31.5/37=85% |
| Degree of reliability (the reliability code for ecotoxicological studies of DSL categorization is used): |
High |
| Comments: |
Robust Study Summaries Form: Aquatic iT
Table A-7. Robust Study Summaries Form and Instructions for 13365Submission015: Aquatic iT
Reference: Study submission 2007g. 13365Submission015. Acute Immobilisation Test (Static, 48h) to Daphnia magna, Limit Test.
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 2 | Substance identity: CAS RN | n/a | Y | 3468-63-1 |
| 3 | Substance identity: chemical name(s) | n/a | Y | Pigment Orange 5 |
| 4 | Chemical composition of the substance | 2 | n/a | |
| 5 | Chemical purity | 1 | Y | 98.78% |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | Y |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 7 | Reference | 1 | Y | |
| 8 | OECD, EU, national, or other standard method? | 3 | Y | OECD 202 |
| 9 | Justification of the method/protocol if a standard method was not used | 2 | n/a | |
| 10 | GLP (Good Laboratory Practice) | 3 | Y |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 11 | Organism identity: name | n/a | Y | Daphnia magna STRAUS |
| 12 | Latin or both Latin & common names reported? | 1 | Y | |
| 13 | Life cycle age / stage of test organism | 1 | Y | |
| 14 | Length and/or weight | 1 | Y | |
| 15 | Sex | 1 | n/a | |
| 16 | Number of organisms per replicate | 1 | Y | 5 |
| 17 | Organism loading rate | 1 | N | |
| 18 | Food type and feeding periods during the acclimation period | 1 | Y |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 19 | Test type (acute or chronic | n/a | Y | Acute |
| 20 | Experiment type (laboratory or field | n/a | Y | Laboratory |
| 21 | Exposure pathways (food, water, both) | n/a | Y | Water |
| 22 | Exposure duration | n/a | Y | 48h |
| 23 | Negative or positive controls (specify) | 1 | Y | Positive & Negative |
| 24 | Number of replicates (including controls) | 1 | Y | 4 |
| 25 | Nominal concentrations reported? | 1 | N | |
| 26 | Measured concentrations reported? | 3 | Y | Reported as DOC |
| 27 | Food type and feeding periods during the long-term tests | 1 | n/a | |
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | Y | 2 measurements |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for the metal toxicity - pH, DOC/TOC, water hardness, temperature) | 3 | Y | |
| 30 | Photoperiod and light intensity | 1 | Y | |
| 31 | Stock and test solution preparation | 1 | Y | |
| 32 | Was solubilizer/emulsifier used, if the chemical was poorly soluble or unstable? | 1 | N | |
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | n/a | |
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | n/a | |
| 35 | Analytical monitoring intervals | 1 | Y | |
| 36 | Statistical methods used | 1 | Y |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by organism's health (e.g. when mortality in the control is greater than 10%) or physical effects (e.g. "shading effect")? | n/a | Y | |
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | |
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Y | |
| 40 | Does system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and organism's nature/habits? | 2 | Y | |
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | Y | |
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | |
| 43 | Was toxicity value below the chemical's water solubility? | 3 | n/a. Was tested at saturation and no effect was observed. |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 44 | Toxicity values (specify endpoint and value) | n/a | n/a | No effect at DOC=2.2mg/L |
| 45 | Other endpoints reported - e.g. BCF/BAF, LOEC/NOEC (specify)? | n/a | N | |
| 46 | Other adverse effects (e.g. carcinogenicity, mutagenicity) reported? | n/a | N |
| No | Item | Specify |
|---|---|---|
| 47 | Score: ... % | 92.1 |
| 48 | EC Reliability code: | 1 |
| 49 | Reliability category (high, satisfactory, low): | High Confidence |
| 50 | Comments | The average DOC of the pigment is 0.9 mg/L. The TOC of the pigment is 56.81%. The average concentration of the pigment in water can be calculated based on [DOC]= [DOC of pigment] × purity of pigment/fraction OC of pigment=0.9 mg DOC/L × 0.988 /0.568 = 1.6 mg/L pigment. The pigment's water solubility is only 6.8 µg/L (experimental). i.e., the "saturation" value dramatically exceeds the water solubility value; therefore it is assumed that 0.9 mg/L corresponds to a mixture of pigment particles and a small dissolved fraction. The stock solution was shaken for 24 hours and filtered with a 0.45-um filter, however the average particle size of Pigment Orange 5 is only 285 nm. This indicates that the filter wouldn't remove the particles, which confirms the above conclusion that the pigment in the test solution is represented by the pigment particles and the water-soluble fraction of the substance. There is uncertainty that the maximum solubility was achieved, as the solution was only shaken for 24 hours and the temperature was not elevated. The OECD Guidance for aquatic toxicity testing of difficult substances also indicates that the concentration measured will typically be less than the water solubility if it is saturated. The study demonstrates that the pigment particles and dissolved pigment caused no effects on Daphnia magna. |
Table A-8. Robust Study Summaries Form and Instructions for 13365Submission020: Aquatic iT
Reference: Study Submission 2007g. 13365Submission020. Acute Immobilisation Test (Static, 48h) to Daphnia magna, Limit Test
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 2 | Substance identity: CAS RN | n/a | Y | 2425-85-6 |
| 3 | Substance identity: chemical name(s) | n/a | Y | Pigment Red 3 |
| 4 | Chemical composition of the substance | 2 | n/a | |
| 5 | Chemical purity | 1 | Y | 98.20% |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | Y |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 7 | Reference | 1 | Y | |
| 8 | OECD, EU, national, or other standard method? | 3 | Y | OECD 202 |
| 9 | Justification of the method/protocol if a standard method was not used | 2 | n/a | |
| 10 | GLP (Good Laboratory Practice) | 3 | Y |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 11 | Organism identity: name | n/a | Y | Daphnia magna STRAUS |
| 12 | Latin or both Latin & common names reported? | 1 | Y | |
| 13 | Life cycle age / stage of test organis | 1 | Y | |
| 14 | Length and/or weight | 1 | Y | |
| 15 | Sex | 1 | n/a | |
| 16 | Number of organisms per replicate | 1 | Y | 5 |
| 17 | Organism loading rate | 1 | N | |
| 18 | Food type and feeding periods during the acclimation period | 1 | Y |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 19 | Test type (acute or chronic | n/a | Y | Acute |
| 20 | Experiment type (laboratory or field | n/a | Y | Laboratory |
| 21 | Exposure pathways (food, water, both) | n/a | Y | Water |
| 22 | Exposure duration | n/a | Y | 48h |
| 23 | Negative or positive controls (specify) | 1 | Y | Positive & Negative |
| 24 | Number of replicates (including controls) | 1 | Y | 4 |
| 25 | Nominal concentrations reported? | 1 | N | |
| 26 | Measured concentrations reported? | 3 | Y | Reported as DOC |
| 27 | Food type and feeding periods during the long-term tests | 1 | n/a | |
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | Y | 2 measurements |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for the metal toxicity - pH, DOC/TOC, water hardness, temperature) | 3 | Y | |
| 30 | Photoperiod and light intensity | 1 | Y | |
| 31 | Stock and test solution preparation | 1 | Y | |
| 32 | Was solubilizer/emulsifier used, if the chemical was poorly soluble or unstable? | 1 | N | |
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | n/a | |
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | n/a | |
| 35 | Analytical monitoring intervals | 1 | Y | |
| 36 | Statistical methods used | 1 | Y |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by organism's health (e.g. when mortality in the control is greater than 10%) or physical effects (e.g. "shading effect")? | n/a | Y | |
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | |
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Y | |
| 40 | Does system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and organism's nature/habits? | 2 | Y | |
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | Y | |
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | |
| 43 | Was toxicity value below the chemical's water solubility? | 3 | n/a. Was tested at saturation and no effect was observed. |
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 44 | Toxicity values (specify endpoint and value) | n/a | n/a | No effect at DOC=2.6mg/L |
| 45 | Other endpoints (e.g., BCF/BAF, LOEC/NOEC) reported? (specify) | n/a | N | |
| 46 | Other adverse effects (e.g., carcinogenicity, mutagenicity) reported? | n/a | N |
| No | Item | Specify |
|---|---|---|
| 47 | Score: ... % | 92.1 |
| 48 | EC Reliability code: | 1 |
| 49 | Reliability category (high, satisfactory, low): | High Confidence |
| 50 | Comments | The DOC of the pigment is 0.6 mg/L at t=0 hr and t=48 hr. The TOC of the pigment is 66.4%. The average concentration of the pigment in water can be calculated based on [DOC]= [DOC of pigment] × purity of pigment/fraction OC of pigment=0.6 mg DOC/L × 0.982 /0.644 = 0.9 mg/L pigment. The pigment's water solubility is 3.3 µg/L (experimental). i.e., the 'saturation' value dramatically exceeds the water solubility value; therefore it is assumed that 0.9 mg/L corresponds to a mixture of pigment particles and a small dissolved fraction. The stock solution was shaken for 24 hours, followed by centrifugation. There is uncertainty that the maximum solubility was achieved, as the solution was only shaken for 24 hours and the temperature was not elevated. The OECD Guidance for aquatic toxicity testing of difficult substances also indicates that the concentration measured is typically less than the water solubility if it is saturated. The study demonstrates that the pigment particles and dissolved pigment caused no effects on Daphnia magna. |