Screening Assessment for the Challenge Amines, tallow alkyl, ethoxylated, phosphates
Chemical Abstracts Service Registry Number 68308-48-5
Environment Canada
Health Canada
August 2009
Table of Contents
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a screening assessment of Amines, tallow alkyl, ethoxylated, phosphates (ATAEP), Chemical Abstracts Service Registry Number 68308-48-5. This substance was identified as a high priority for screening assessment and included in the Challenge because it was originally found to meet the ecological categorization criteria for persistence, bioaccumulation potential and inherent toxicity to non-human organisms and is believed to be in commerce in Canada.
The substance ATAEP was not considered to be a high priority for assessment of potential risks to human health, based upon application of the simple exposure and hazard tools developed by Health Canada for categorization of substances on the Domestic Substances List. Therefore, this assessment focuses on information relevant to the evaluation of ecological risks.
ATAEP is an organic substance that is in commerce in Canada. A new use of this substance (the use and activity are regarded as a confidential business activity) has been identified since 2005 when it was reported to be used mainly in down-the-drain consumer products such as soaps and lotions. The amount manufactured in 2006 in Canada was reported to range from 100 to 1000 kg. This information on manufacture and use indicates that ATAEP could potentially be released into the Canadian environment.
Based on certain assumptions and reported use patterns, most of the substance that is manufactured in Canada ends up in soil. Small proportions are estimated to be transferred to waste disposal sites (2%) or released to water (1.4%), with the majority being emitted to soil (96.6%).
ATAEP is an alkyl phosphate ester, that is expected to have a net charge of zero at ambient pHs. It is therefore considered to behave as a neutral molecule, having a low solubility in water, a low volatility and a high Koc. It will thus have a tendency to partition to particles or to lipids (fat) of organisms. For these reasons, ATAEP will likely be found mostly in soil or sediments. It is not expected to be subject to long-range atmospheric transport.
Based on its physical and chemical properties, ATAEP does degrade quickly in the environment. It is therefore not expected to be persistent in air, water, soil or sediments. ATAEP has a low potential to accumulate in organisms. The substance has been determined to not meet the persistence and bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations. Modelled acute aquatic toxicity values suggest however that the substance has a relatively high potential for toxicity to aquatic organisms.
In this screening assessment, conservative exposure scenarios were developed to estimate releases into the aquatic environment from industrial operations as well as consumer uses, and resulting aquatic concentrations. The obtained predicted environmental concentrations in water are lower than the predicted no-effect concentration for fish, daphnids and algae. This indicates that the substance is not anticipated to cause ecological harm in the aquatic environment. In addition, because of the relatively small quantity in commerce and the nature of its use, little exposure of soil-dwelling biota is anticipated.. Therefore, ATAEP is unlikely to pose significant risk to soil organisms.
Based on the information presented in this screening assessment, it is concluded that ATAEP is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
This substance will be included in the upcoming Domestic Substances List (DSL) inventory update initiative. In addition and where relevant, research and monitoring will support verification of assumptions used during the screening assessment.
Based on the information available, it is concluded that ATAEP does not meet any of the criteria set out in section 64 of CEPA 1999.
Introduction
The Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or human health. Based on the results of a screening assessment, the Ministers can propose to take no further action with respect to the substance, to add the substance to the Priority Substances List (PSL) for further assessment, or to recommend that the substance be added to the List of Toxic Substances in Schedule 1 of the Act and, where applicable, the implementation of virtual elimination.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that:
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherent toxicity to aquatic organisms (iT), and were believed to be in commerce in Canada; and/or
- met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE), and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in the Canada Gazette, Part I, on December 9, 2006 (Canada 2006), that challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment, and to develop and benchmark best practices for the risk management and product stewardship of those substances identified as high priorities.
The substance Amines, tallow alkyl, ethoxylated, phosphates was identified as a high priority for assessment of ecological risk, as it had been found to be persistent, bioaccumulative and inherently toxic to aquatic organisms and is believed to be in commerce in Canada. The Challenge for this substance was published in the Canada Gazette on November 17, 2007 (Canada 2007). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, submissions of information pertaining to the uses of the substance were received.
Although Amines, tallow alkyl, ethoxylated, phosphates was determined to be a high priority for assessment with respect to the environment, it did not meet the criteria for GPE or IPE and was not identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity. Therefore, this assessment focuses principally on information relevant to the evaluation of ecological risks.
Under CEPA 1999, screening assessments focus on information critical to determining whether a substance meets the criteria for defining a chemical as toxic as set out in section 64 of the Act, where
"64. [...] a substance is toxic if it is entering or may enter the environment in a quantity or concentration or under conditions that
- have or may have an immediate or long-term harmful effect on the environment or its biological diversity;
- constitute or may constitute a danger to the environment on which life depends; or
- constitute or may constitute a danger in Canada to human life or health."
Screening assessments examine scientific information and develop conclusions by incorporating a weight-of-evidence approach and precaution.
This screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including the additional information submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents, stakeholder research reports and from recent literature searches, up to March 2008. Key studies were critically evaluated; modelling results may have been used to reach conclusions. When available and relevant, information presented in hazard assessments from other jurisdictions was considered. The screening assessmentdoes not represent an exhaustive or critical review of all available data. Rather, it presents the most critical studies and lines of evidence pertinent to the conclusion.
This screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. This assessment has undergone external written peer review/consultation. Additionally, a draft of this screening assessment was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening risk assessment remain the responsibility of Environment Canada and Health Canada. The critical information and considerations upon which the assessment is based are summarized below.
Substance Identity
For the purposes of this document, this substance will be referred to as ATAEP, derived from the name on the Domestic Substances List (DSL). This substance is a UVCB (Unknown or Variable Composition,Complex Reaction Products, orBiological Materials); that is, it is not a discrete chemical and thus may be characterized by a variety of structures. To assist with modelling and further assessments, the structure and corresponding SMILES presented here were chosen to represent the substance.
| Chemical Abstracts Service Registry Number (CAS RN) | 68308-48-5 |
|---|---|
| Domestic Substances List (DSL) Name | Amines, tallow alkyl, ethoxylated, phosphates |
| National Chemical Inventories (NCI) nameTable note a | Amines, tallow alkyl, ethoxylate, phosphates (TSCA, AICS, ECL, PICCS, ASIA-PAC, NZIoC) |
| Other names | Tallow amine, ethoxylated, phosphated; Tallowamine, ethoxylated, phosphate salt; Phosphates (chemical category); Polyoxyalkylenes (chemical category); Tallow (chemical category) |
| Chemical group (DSL stream) | Unknown or Variable Composition, Complex Reaction Product, or Biological Material (UVCB) |
| Major chemical class or use | Surfactant |
| Major chemical sub-class | Alkyl phosphate ester |
| Chemical formula | C28H60N1O8P |
| Representative chemical structure used to run the estimation modelTable note b | 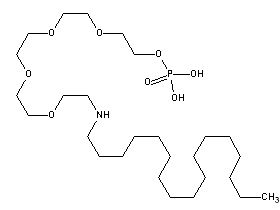 |
| Representative Simplified Molecular Input Line Entry System (SMILES) used to run the estimation modelTable note b | |
| Molecular mass | 569.77 g/mol |
Physical and Chemical Properties
No experimental data are available for ATAEP.
Table 2 contains modelled physical and chemical properties of ATAEP that are relevant to its environmental fate.
| Type | ValueTable note c | Temperature (°C) | Reference | |
|---|---|---|---|---|
| Melting point (°C) | Modelled | 90.27 | MPBPWIN 2000 | |
| Boiling point (°C) | Modelled | 480 | MPBPWIN 2000 | |
| Density (kg/m3) | Not applicable | Not applicable | Not applicable | Not applicable |
| Vapour pressure (Pa) | Modelled | 8.07 × 10-9 (6.06 × 10-11 mm Hg) |
25 | MPBPWIN 2000 |
| Henry's Law constant (Pa·m3/mol) | Modelled | 5.32 × 10-016 (5.25 × 10-21 atm m3/mol) |
25 | HENRYWIN 2000 |
| Log Kow (Octanol-water partition coefficient) (dimensionless) | Modelled | 6.02 | KOWWIN 2000 ADME Boxes 2008 | |
| Log Koc (Organic carbon-water partition coefficient - L/kg) (dimensionless) | Modelled | 3.95 | PCKOCWIN 2000 | |
| Water solubility (mg/L) | Modelled | 0.018 (neutral form) | 25 | WSKOWWIN 2000 ADME Boxes 2008 |
| pKa (Acid dissociation constant) (dimensionless) | Modelled | 1.2 (acid form) 9.6 (base form) | ADME Boxes 2008 |
Sources
The substance ATAEP is not naturally produced in the environment.
Information was submitted for this substance as part of the Challenge for the 2006 calendar year (Environment Canada 2008b). The substance was manufactured in a quantity in the range of 100-1000 kg. Five companies indicated stakeholder interest in this substance.
In response to the section 71 survey notice for the 2005 calendar year, fewer than five companies reported importing ATAEP (either alone, or contained in a mixture, a product or manufactured item) in a quantity greater than or equal to 100 kg. In total, between 100 and 1000 kg of this substance were reported to be imported into Canada in 2005 (Environment Canada 2007).
The quantity reported to be manufactured, imported or in commerce in Canada during the calendar year 1986 was between 10 000 and 100 000 kg (Environment Canada 1986). The number of notifiers was fewer than five.
Uses
As a result of a survey conducted under section 71 for the calendar year 2006 (Environment Canada 2008), the substance ATAEP is known to have been manufactured in Canada. However, the use of ATAEP is considered confidential business information.
Information submitted in response to the section 71 survey notice for the year 2005 (Environment Canada 2007), and information voluntarily submitted in response to the Challenge (Environment Canada 2007) indicated that ATAEP was imported into Canada for business activity related to soap, cleaning compound and toiletries preparation manufacturing, which includes the manufacture of perfumes, shaving and hair preparations, face creams and lotions including sunscreens in the 2005 calendar year. The use pattern codes and corresponding applications noted by companies included: 60 - cosmetics; and 93 - soap and cleaning products. Given the uses, this substance may be released to the environment in a widely dispersive manner.
The DSL use codes for the year 2005 are consistent with the use codes that were identified for ATAEP in 1986.
In Sweden and Denmark, ATAEP was reported to be used as a formulation component and in the cosmetic industry (SPIN 2006). The total amount used in Sweden and Denmark was 1.6 tonnes between 1999 and 2003 and 0.1 tonnes in 2005.
Releases to the Environment
Mass Flow Tool
To estimate potential releases of substances to the environment at different stages of their life cycle, a Mass Flow Tool was developed (Environment Canada 2008c). Empirical data concerning releases of specific substances to the environment are seldom available. Therefore, for each identified type of use of the substance, the proportion and quantity of releases to the different environmental media are estimated, as is the proportion of the substance chemically transformed or sent for waste disposal. Unless specific information on the rate of, or potential for, release of the substance from landfills and incinerators is available, the Mass Flow Tool does not quantitatively account for releases to the environment from disposal.
Assumptions and input parameters used in making the release estimates are based on information obtained from a variety of sources including responses to regulatory surveys, Statistics Canada, manufacturers' websites, technical databases and documents. Of particular relevance are emission factors, which are generally expressed as the fraction of a substance released to the environment, particularly during its manufacture, processing, and use associated with industrial processes. Sources of such information include emission scenario documents, often developed under the auspices of the Organisation for Economic Co-operation and Development (OECD), and default assumptions used by different international chemical regulatory agencies. The level of uncertainty in the mass of substance and quantity released to the environment generally increases towards the end of the life cycle.
Given the use identified for 2006, confidential results of Mass Flow Tool modelling indicate that the ATAEP that is manufactured in Canada can be expected to be released largely to soil (96.6%) (Table 3). Based largely on information in OECD emission scenario documents on processing and uses associated with this type of substance, it is estimated that 1.4%, 0.0% and 96.6% of ATAEP may be released to water, air and soil, respectively.
| Fate | Proportion of the mass (%)Table note d | Major life cycle stage involvedTable note e |
|---|---|---|
| Releases to soil | 96.6 | Consumer use |
| Releases to air | 0.0 | Production |
| Releases to sewerTable note f | 1.4 | Production, formulation, consumer use |
| Chemically transformed | 0.0 | |
| Transferred to waste disposal sites (e.g., landfill, incineration) | 2.0 | Waste disposal |
Environmental Fate
Based on ATAEP's physical and chemical properties (Table 2) and the results of Level III fugacity modelling (Table 4), ATAEP is expected to predominantly reside in soil or sediment, depending on the compartment of release, with a lesser amount partitioning to water.
| Fraction of substance partitioning to air (%) | Fraction of substance partitioning to water (%) | Fraction of substance partitioning to soil (%) | Fraction of substance partitioning to sediment (%) | |
|---|---|---|---|---|
| Substance released to air (100%) | 1.4 | 1.4 | 80.9 | 16.3 |
| Substance released to water (100%) | 1E-7 | 7.81 | 5.7E-6 | 92.2 |
| Substance released to soil (100%) | 2E-8 | 4.0E-3 | 99.9 | 0.05 |
With an acid dissociation constant (pKa) of 1.2 and a base dissociation constant of 9.6 (Table 2), ATAEP is expected to have a net charge of zero at ambient pHs (6-8). Although it is predicted to have "zwitterion" characteristics (ADME Boxes, 2008), for the purposes of this assessment ATAEP will be assumed to behave as a neutral hydrophobic (log Kow = 6.02) molecule.
If released to soil, ATAEP is expected to have a high adsorptivity to soil (i.e., expected to be relatively immobile) based upon an estimated log Koc of 3.95. Volatilization from moist soil surfaces seems to be an unimportant fate process based upon an estimated Henry's Law constant. This chemical is not expected to volatilize from dry soil surfaces based upon its vapour pressure. Therefore, if released to soil, ATAEP will mainly remain in this environmental compartment, which can be illustrated by the results of the Level III fugacity modelling (Table 4).
If released into water, ATAEP is expected to exhibit high adsorption to suspended solids and sediment based upon a high value of estimated log Koc of 3.95. Volatilization from water surfaces is expected to be an unimportant fate process based upon this compound's estimated Henry's Law constant of 5.32 ×10-16 Pa m3/mol. Thus, if water is a receiving medium, ATAEP is expected to partition mainly to sediments and to a much lesser extent to remain in water (Table 4).
If ATAEP is released to air, the Level III fugacity model indicates a negligible amount of the substance will remain in air (Table 4). A modelled vapour pressure of 8.07 × 10-9 Pa and Henry's Law constant of 5.32 × 10-16 Pa m3/mol indicate that ATAEP is non-volatile. Therefore, if released solely to air, it is not expected to remain in this compartment. The major two media to which this substance will partition will be soil and sediment (Table 4).
Persistence and Bioaccumulation Potential
Environmental Persistence
No experimental degradation data for ATAEP have been identified. Table 5 summarizes the results of available quantitative structure-activity relationship (QSAR) models for degradation in various environmental media.
| Fate Process | Model and model basis | Model Result and Prediction | Extrapolated Half-life (days) |
|---|---|---|---|
| Atmospheric oxidation | AOPWIN 2000 | t½ = 0.052 day | less than 2 |
| Ozone reaction | AOPWIN 2000 | n/aTable note g | n/a |
| Fate Process | Model and model basis | Model Result and Prediction | Extrapolated Half-life (days) |
|---|---|---|---|
| Hydrolysis | HYDROWIN 2000 | n/aTable note h | n/a |
| Biodegradation (aerobic) | BIOWIN 2000 Sub-model 3: Expert Survey (ultimate biodegradation) |
2.23Table note i "months" | less than 182Table note k |
| Biodegradation (aerobic) | BIOWIN 2000 Sub-model 4: Expert Survey (primary biodegradation) |
3.29Table note i "weeks" | less than 182Table note k |
| Biodegradation (aerobic) | BIOWIN 2000 Sub-model 5: MITI linear probability |
0.39Table note j | less than 182Table note k |
| Biodegradation (aerobic) | BIOWIN 2000 Sub-model 6: MITI non-linear probability |
0.04Table note j "does not biodegrade fast" | greater than 182Table note k |
| Biodegradation (aerobic) | CATABOL 2004-2008 % BOD (biological oxygen demand) |
% BOD = 80 "biodegrades rapidly" | less than 182Table note k |
In air, a predicted atmospheric oxidation half-life value of 0.052 days (see Table 5 above) demonstrates that this chemical is likely to be rapidly oxidized. The compound is not expected to react with other photo-oxidative species in the atmosphere, such as O3. Therefore, it is expected that reactions with hydroxyl radicals will be the most important fate process in the atmosphere for ATAEP. With a half-life of 0.052 days via reactions with hydroxyl radicals, ATAEP is considered not persistent in air.
For ATAEP, the results from BIOWIN sub-models 3 and 4 (ultimate and primary degradation) indicate that the biodegradation half life is less than 182 days. For BIOWIN sub-model 5, the result of 0.39 has been suggested to equate to a half-life of less than 60 days (Aronson et al. 2006). Catabol results similarly suggest that the half-life in water would be much less than 182 days (about 12 days assuming first order degradation kinetics). Only the BIOWIN sub-model 6 result suggests that the half-life of ATAEP could be more than 182 days. Therefore, in light of the BIOWIN 3, 4 and 5 predictions and the Catabol result, the weight of evidence indicates that the ultimate degradation half life of ATAEP in water is likely much less than 182 days.
To extrapolate a half-life in water to half-lives in soil and sediment, Boethling's factors t½ water: t½ soil : t½ sediment = 1: 1: 4 (Boethling et al. 1995) can be used. Therefore, since the half-life in water is likely much less than 182 days (and probably less than 90 days; see discussion above), the half-life for ATAEP is expected to be less than 182 days in soil and the half-life in sediment is expected to be less than 365 days.
Thus the modelled data (see Table 5 above) demonstrate that ATAEP does not meet the persistence criteria in air, water, soil or sediment] (half-life in air greater than or equal to 2 days, half-lives in soil and water greater than 182 days and half-life in sediment greater than or equal to 365 days as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).]
The Transport and Persistence Level III Model (TaPL3) (TaPL3 2000) was used to estimate the characteristic travel distance (CTD), defined as the maximum distance travelled in air by 63% of the substance. Beyer et al. (2000) have proposed CTDs of greater than 2000 km as representing high long-range atmospheric transport potential (LRATP), 700-2000 km as moderate LRATP, and less than 700 km as low LRATP. Based on the CTD estimate of 469 km, the long-range atmospheric transport potential of ATAEP is considered to be low. This means that ATAEP is not expected to be transported through the atmosphere a significant distance from its emission sources.
Potential for Bioaccumulation
A modelled log Kow value for ATAEP of approximately 6.02 suggests that this chemical has the potential to bioaccumulate.
Since no experimental bioaccumulation factor (BAF) and/or bioconcentration factor (BCF) data for ATAEP were available, a predictive approach was applied using available BAF and BCF models as shown in Table 6. According to the Persistence and Bioaccumulation Regulations (Canada 2000), measures of BAF are the preferred metric for the assessment of the bioaccumulation potential of substances. This is because BCF does not adequately account for the bioaccumulation potential of substances via the diet, which predominates for substances with log Kow greater than ~4.0 (Arnot and Gobas 2003). Kinetic mass-balance modelling is in principle considered to provide the most reliable prediction method to determine bioaccumulation potential, because it allows for metabolism correction when such data are available. Although there are no substances like ATAEP in the training set of the Gobas' kinetic mass balance model (it has mostly halogenated compounds and PAHs), ATAEP is within the mechanistic domain (passive diffusion) and global parameter domain (log Kow range) of the model. Log Kows of 6.02 were used for these estimations.
| Test organism | Endpoint | Value wet weight (L/kg) | Reference |
|---|---|---|---|
| Fish | BAF | 1278 | Gobas BAF middle trophic level (Arnot and Gobas 2003) |
| Fish | BCF | 654 | Gobas BCF middle trophic level (Arnot and Gobas 2003) |
| Fish | BCF | 5 | Bioaccumulation model with mitigating factors (Dimitrov et al. 2005) |
| Fish | BCF | 185 | BCFWIN 2000 |
The results of the bioaccumulation modelling using structural fragments indicate that ATAEP does not have the potential to bioconcentrate and biomagnify in the environment at significant levels.. Also, based on the chemical structure of the substance, metabolism is likely via aliphatic carbon oxidation, so results for the Gobas model would likely have been lower if metabolism had been taken into account.
Based on the available modelled values, ATAEP does not meet the bioaccumulation criteria (BCF or BAF greater than or equal to 5000) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Potential to Cause Ecological Harm
Ecological Effects Assessment
A - In the Aquatic Compartment
No experimental ecotoxicity data were identified for ATAEP. Therefore, QSAR modelling and a weight-of-evidence approach were used to estimate the potential for aquatic toxicity for ATAEP.
Table 7 contains the modelled ecotoxicological values obtained using ECOSAR (non-ionic surfactant class) for ATAEP. The acute LC50 value for ATAEP of 0.192 mg/L is chosen as the critical toxicity value (CTV). Although the predicted water solubility is below this LC50 value, the difference is acceptable, taking into account the uncertainty in QSAR predictions of solubility and toxicity, since the LC50 value is within about 1 order of magnitude of the water solubility An application factor of 100I s applied to the CTV to account for extrapolating from acute to chronic (long-term) toxicity, and from laboratory results to other potentially sensitive species in the field resulting in a predicted no-effect concentration (PNEC) in water of 0.00192 mg/L.
| Organism | Test type | Endpoint | Value | Reference |
|---|---|---|---|---|
| Daphnid | 48-hour | The concentration of a substance that is estimated to be lethal to 50% of the test organisms (LC50) | 0.192 | ECOSAR 2004 |
| Fish | 96-hours | LC50 | 0.192 | ECOSAR 2004 |
Based on this information, ATAEP is potentially highly hazardous to aquatic organisms (i.e., acute LC50/EC50s are less than 1 mg/L).
B - In Other Environmental Compartments
No experimental or predicted-effects data for non-aquatic non-mammalian organisms in any other media were identified for ATAEP.
Ecological Exposure Assessment and Risk Quotient Estimates
No monitoring data on the presence of ATAEP in environmental media (air, soil or sediment) have been found.
Water: Industrial Effluents
No data concerning concentrations of this substance in water in Canada have been identified. Environmental concentrations are, therefore, estimated from available information, including estimated substance quantities, release rates and receiving water bodies.
To address the possibility of releases to water from manufacturing facilities, a water exposure scenario was developed. Environment Canada's Industrial Generic Exposure Tool - Aquatic (IGETA) was employed to estimate the substance concentration in a generic watercourse receiving industrial effluents (Environment Canada 2008d). The generic scenario is designed to provide these estimates based on conservative assumptions regarding the amount of chemical processed and released, the number of processing days, the sewage treatment plant removal rate, and the size of the receiving watercourse. The tool models an industrial-release scenario based on loading data from sources such as industrial surveys and knowledge of the distribution of industrial discharges in the country, and calculates a predicted environmental concentration (PEC). The equation and inputs used to calculate the PEC in the receiving watercourse are described in the IGETA report for ATAEP (Environment Canada 2008e). Assuming a manufactured quantity equal to that reported in the 2006 S. 71 survey (note: exact quantity and uses are confidential business information), the PEC in water for a conservative scenario (i.e.,a manufacturing facility with 5% loss to sewers 0% removal during waste-water treatment, discharging to a small generic surface water body) does not exceed the predicted no-effect concentration (PNEC = 0.0019 mg/L), the resulting risk quotient (PEC/PNEC) being less than 1. This indicates that industrial releases of ATAEP to water are not expected to harm aquatic organisms.
Water: Consumer Use
Recent use pattern data (Environment Canada 2006) indicate that ATAEP was used in consumer products such as perfumes, shaving and hair preparations, and face creams and lotions including sunscreens in the 2005 calendar year. To estimate releases to water from these "down-the-drain" consumer uses, Environment Canada's MegaFlush tool was used (Environment Canada 2008d). As with the IGETA model run, conservative assumptions were used (e.g., total quantity of ATAEP is 1000 kg - equal to the high end of the S. 71 reporting range for 2006 - all of which is lost down the drain with no STP removal). The MegaFlush results for ATAEP (Environment Canada 2008f) show that the risk quotients for all scenarios are below one, indicating that harm to aquatic organisms is unlikely to occur because of consumer of ATAEP use in Canada.
Soil
Although most ATAEP manufactured in Canada is expected to be released to soil, given the low quantity involved and the type of use (confidential information) little exposure of soil-dwelling organisms is anticipated. While on-land disposal of wastes containing small amounts of ATAEP is possible such practices are unlikely to cause environmental harm when provincial guidelines for such disposal are followed. Therefore, ATAEP is not expected to pose a significant risk to soil-dwelling organisms in Canada.
Characterization of Ecological Risk
The quantities of ATAEP imported into or manufactured in Canada are not particularly large, and the amounts used appear to have decreased since the mid-1980s. However, because of ATEAP's dispersive consumer uses there is potential for releases into the Canadian environment over a wide area. ATAEP does not however persist in the environment, and it is unlikely to bioaccumulate in large amounts. It has also demonstrated potential for relatively high toxicity to aquatic organisms.
This information taken together with the exposure scenario information presented above, suggests that ATAEP is unlikely to cause ecological harm in Canada.
Uncertainties in Evaluation of Ecological Risk
Given that the substance ATAEP is of variable composition, a representative structure was identified and used to estimate physical-chemical properties as well as persistence, bioaccumulation and toxicity and in subsequent modelling in the assessment. There are uncertainties associated with the structure chosen, and the properties of the substance estimated using QSAR models, which were whenever possible addressed by making conservative assumptions.
There are also uncertainties associated with the exposure scenarios developed, which as a consequence incorporate a moderate to a relatively high degree of conservatism,
Regarding ecotoxicity, based on the predicted partitioning behaviour of this chemical, the significance of soil and sediment as important media of exposure is not well addressed by the effects data available. Indeed, the only effects data identified apply to pelagic aquatic exposures, although the water column may not be the medium of primary concern based on partitioning and release estimates.
Conclusion
Based on the information presented in this screening assessment, it is proposed that ATAEP is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
It is therefore proposed that ATAEP does not meet the definition of toxic as set out in section 64 of CEPA 1999. Additionally, ATAEP does not meet the criteria for persistence and bioaccumulation potential as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
References
[ADME Boxes] [desktop software module]. 2008. Version 3.5. Toronto (ON): Pharma Algorithms. http://pharma-algorithms.com/adme_boxes.htm
BCE Place - TD Canada Trust Tower161 Bay Street, 27th Floor Toronto, Ontario
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2000. Version 1.91. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Arnot JA, Gobas FAPC. 2003. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci 22(3): 337-345.
Aronson D, Howard PH. 1999. Evaluating potential POP/PBT compounds for environmental persistence. North Syracuse (NY): Syracuse Research Corp., Environmental Science Centre. Report No.: SRC-TR-99-020.
Aronson D, Boethling B, Howard P, Stiteler W. 2006. Estimating biodegradation half-lives for use in chemical screening. Chemosphere 63: 1953-1960.
[BCFWIN] BioConcentration Factor Program for Windows [Estimation Model]. 2000. Version 2.15. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Beyer A, Mackay D, Matthies M, Wania F, Webster E. 2000. Assessing long-range transport potential of persistent organic pollutants. Environmental Science and Technology 34(4): 699-703.
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2000. Version 4.02. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4): 741-752.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33, Canada Gazette. Part III. vol. 22, no. 3. http://canadagazette.gc.ca/partIII/1999/g3-02203.pdf
Canada. 2000. Canadian Environmental Protection Act: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March, 2000, SOR/2000-107, Canada Gazette. Part II, vol. 134, no. 7, p. 607-612. http://canadagazette.gc.ca/partII/2000/20000329/pdf/g2-13407.pdf
Canada, Dept. of the Environment, Dept. of Health. 2007.Canadian Environmental Protection Act, 1999: Notice of fourth release of technical information relevant to substances identified in the Challenge. Canada Gazette, Part I, vol. 141. no. 46, p. 3192-3197. http://canadagazette.gc.ca/partI/2007/20071117/pdf/g1-14146.pdf
[CATABOL] Probabilistic assessment of biodegradability and metabolic pathways [Computer Model]. c2004-2008. Version 5.10.2. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. http://oasis-lmc.org/?section=software&swid=1
Dimitrov S, Dimitrova N, Parkerton T, Comber M, Bonnell M, Mekenyan O. 2005. Base-line model for identifying the bioaccumulation potential of chemicals. SAR QSAR Environ Res 16(6):531-554.
Di Toro DM, Zarba CS, Hansen DJ, Berry WJ, Swartz RC, Cowan CE, Pavlou SP, Allen HE, Thomas NA, Paquin PR. Technical basis for establishing sediment quality criteria for nonionic organic chemicals using equilibrium partitioning. 1991. Environ. Toxicol. Chem 10(2):1541-1583.
[ECOSAR] Ecological Structural Activity Relationships [Internet]. 2004. Version 0.99h. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Environment Canada. 1986. Domestic Substance list Report. Based on data collected for the Domestic Substance List Survey during 1984-1986. Gatineau, Quebec: Environment Canada. New Substances Division. Available upon request from: New Substances Division, Environment Canada. Ottawa. K1A 0H3.
Environment Canada. 2001. Screening Level Ecological Risk Assessments for Existing Substances Under the Canadian Environmental Protection Act, 1999: Guidance Manual.
Environment Canada. 2007. Data for selected substances collected under the Canadian Environmental Protection Act, 1999, Section 71: Notice with respect to selected substances identified as priority for action. Data prepared by: Environment Canada, Health Canada, Existing Substances Program.
Environment Canada. 2008a. Ionization data for batch 4 substances. Internal draft document. Gatineau (QC): Environment Canada, Existing Substances Division. Available on request.
Environment Canada. 2008b. Data for Batch 4 substances collected under the Canadian Environmental Protection Act, 1999, Section 71Notice with respect to certain substances identified in the Challenge published in the December 9, 2006 Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Prepared by: Environment Canada, Health Canada, Existing Substances Program.
Environment Canada. 2008c. Assumptions, limitations and uncertainties of the mass flow tool for ATAEP, CAS RN 68308-48-5. Internal draft document. Gatineau (QC): Environment Canada, Existing Substances Division. Available on request.
Environment Canada. 2008d. Guidance for conducting ecological assessments under CEPA, 1999: science resource technical series, technical guidance module: Mega Flush consumer release scenario. Preliminary draft working document. Gatineau (QC): Environment Canada, Existing Substances Division.
Environment Canada. 2008e. IGETA report: CAS RN 68308-48-5, 2008-07-31. Unpublished report. Gatineau (QC): Environment Canada, Existing Substances Division.
Environment Canada. 2008f. MegaFlush report: CAS RN 68308-48-5, 2008-09-05. Unpublished report. Gatineau (QC): Environment Canada, Existing Substances Division.
[EPIWIN] Estimation Programs Interface for Microsoft Windows [Estimation Model]. 2004. Version 3.12. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
[HENRYWIN] Henry's Law Constant Program for Microsoft Windows [Estimation Model]. 2000. Version 3.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
[HYDROWIN] Hydrolysis Rates Program for Microsoft Windows [Estimation Model]. 2000. Version 1.67. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2000. Version 1.67. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
[MPBPWIN] Melting Point Boiling Point Program for Microsoft Windows [Estimation Model]. 2000. Version 1.41. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Mekenyan G, Dimitrov SD, Pavlov TS, Veith, GD. 2005. POPs: A QSAR system for creating PBT profiles of chemicals and their chemicals andtheir metabolites. SAR QSAR Envir Res, 16(1-2): 103-133.
[NCI] National Chemical Inventories [database on CD-ROM]. 2006. Columbus (OH): American Chemical Society. [cited 2006 Dec 11]. http://www.cas.org/products/cd/nci/index.html
[PCKOCWIN] Organic Carbon Partition Coefficient Program for Windows [Estimation Model]. 2000. Version 1.66. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
[SPIN] Substances in Products in Nordic Countries [database on the Internet]. 2006. Financed by the Nordic Council of Ministers, Chemical group. [cited 2006 Dec]. http://195.215.251.229/Dotnetnuke/Home/tabid/58/Default.aspx
[TaPL3] Long Range Transport and Persistence Level III model [Internet]. 2000. Version 2.10. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre. http://www.trentu.ca/academic/aminss/envmodel/models/TaPL3.html
[TOPKAT] Toxicity Prediction by Komputer Assisted Technology [Internet]. 2004. Version 6.2. San Diego (CA): Accelrys Software Inc. [cited 2006 August 11]. http://www.accelrys.com/products/topkat/index.html
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [Estimation Model]. 2000. Version 1.41 Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
[US EPA] U.S. Environmental Protection Agency. 2002. PBT Profiler Methodology [Internet]. Washington (DC): US EPA, Office of Pollution Prevention and Toxics. [cited 2008 July]. http://www.pbtprofiler.net/methodology.asp
Appendix I - PBT Model Inputs Summary Table
| Phys-Chem/Fate | Fate | Fate | Fate | Fate | PBT Profiling | Ecotoxicity | |
|---|---|---|---|---|---|---|---|
| Model Input Parameters | EPIWIN Suite (all models, including: AOPWIN, KOCWIN, BCFWIN BIOWIN and ECOSAR) | STP (1) ASTreat (2) SimpleTreat (3) (required inputs are different depending on model) | EQC (required inputs are different if Type I vs. Type II chemical) | TaPL3 (required inputs are different if Type 1 vs. Type 2 chemical) | Arnot- Gobas BCF/BAF Model | Canadian-POPs (including: Catabol, BCF Mitigating Factors Model, OASIS Toxicity Model) | Artificial Intelligence Expert System (AIES)/ TOPKAT/ ASTER |
| SMILES Code | CCCCCC CCCCCC CCCCCC NCCOCC OCC OC COCCOP (=O)(O)O |
CCCCC CCCCC CCCCC CCCNC COCCO CCOCC OCCOP (=O)(O)O |
CCCCCC CCCCCC CCCCCC NCCOCC OCCOCC OCCOP (=O)(O)O |
CCCCCC CCCCCC CCCCCC NCCOCC OCCOCC OCCOP (=O)(O)O |
|||
| Molecular weight (g/mol) | 569.77 g/mol | 569.77 g/mol | 569.77 g/mol | ||||
| Melting point (ºC) | 90.27 | 90.27 | 90.27 | ||||
| Boiling point (ºC) | 480 | ||||||
| Data temperature (ºC) | 20 | 20 | 20 | ||||
| Density (kg/m3) | Not available | ||||||
| Vapour pressure (Pa) | 8.07 × 10-9 | 8.07 × 10-9 | 8.07 × 10-9 | 8.07 × 10-9 | |||
| Henry's Law constant (Pa·m3/mol) | 5.32 × 10-16 | ||||||
| Log Kaw (Air-water partition coefficient; dimensionless) | |||||||
| Log Kow (Octanol-water partition coefficient; dimensionless) | 6.02 | 6.02 | 6.02 | 6.02 | 6.02 | ||
| Kow (Octanol-water partition coefficient; dimensionless) | |||||||
| Log Koc (Organic carbon-water partition coefficient - L/kg) | 3.95 | ||||||
| Water solubility (mg/L) | 0.0185 | 0.0185 | 0.0185 | 0.0185 | |||
| Log Koa (Octanol-air partition coefficient; dimensionless) | |||||||
| Soil-water partition coefficient (L/kg)Table note l | |||||||
| Sediment-water partition coefficient (L/kg)Table note l | |||||||
| Suspended particles-water partition coefficient (L/kg)Table note l | |||||||
| Fish-water partition coefficient (L/kg)Table note m | |||||||
| Aerosol-water partition coefficient; dimensionlessTable note n | |||||||
| Vegetation-water partition coefficient; dimensionlessTable note l | |||||||
| Enthalpy (Kow) | |||||||
| Enthalpy (Kaw) | |||||||
| Half-life in air (days) | |||||||
| Half-life in water (days) | |||||||
| Half-life in sediment (days) | |||||||
| Half-life in soil (days) | |||||||
| Half-life in vegetation (days)Table note o | |||||||
| Metabolic rate constant (1/days) | 0.0256 | ||||||
| Biodegradation rate constant (1/days) or (1/hr) -specify | |||||||
| Biodegradation half-life in primary clarifier (t½-p) (hr) | 6930 | ||||||
| Biodegradation half-life in aeration vessel (t½-s) (hr) | 693 | ||||||
| Biodegradation half-life in settling tank (t½-s) (hr) | 693 |