Screening Assessment for the Challenge Ethanol, 2-ethoxy-, acetate
Chemical Abstracts Service Registry Number 111-15-9
Environment Canada
Health Canada
February 2009
Table of Contents
- Synopsis
- Introduction
- Substance Identity
- Physical and Chemical Properties
- Sources
- Uses
- Releases to the Environment
- Environmental Fate
- Persistence and Bioaccumulation Potential
- Potential to Cause Ecological Harm
- Potential to Cause Harm to Human Health
- Conclusion
- References
- Appendix 1. Summary of health effects information for 2-EEA
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a screening assessment of ethanol, 2-ethoxy-, acetate (2-ethoxyethanol acetate; 2-EEA), Chemical Abstracts Service Registry Number 111-15-9. The substance 2-EEA was identified in the categorization of the Domestic Substances List as a high priority for action under the Challenge. 2-EEA was identified as a high priority because it was considered to pose greatest potential for exposure of individuals in Canada and had been classified by the European Commission on the basis of reproductive and developmental toxicity. The substance did not meet the ecological categorization criteria for persistence, bioaccumulation potential and inherent toxicity to aquatic organisms. Therefore, the focus of this assessment of 2-EEA relates principally to human health risks.
According to data submitted in CEPA 1999 section 71 responses, 2-EEA was not manufactured in Canada in 2006 above the reporting threshold of 100 kg. The total quantity imported into Canada in the same calendar year was reported to be in the range of 10 000-100 000 kg. Its principal uses include solvents, paints, coatings and cleaning solution for industrial applications.
Population exposure to 2-EEA is expected to be predominantly via the air. Based on very limited information on concentrations in environmental media and the results of fugacity modelling, exposure in the general environment is expected to be low. 2-EEA is primarily used in industrial settings, and consumer exposure to 2-EEA is not expected to be significant. The health effects associated with exposure to 2-EEA are primarily developmental and reproductive toxicity and hematological effects, based on observations in experimental animals and exposed workers. The margins between an upper bounding estimate of concentrations in indoor air and levels associated with effects in occupationally exposed humans and experimental animals are considered to be adequately protective.
On the basis of the adequacy of the margins between conservative estimates of exposure to 2-EEA from environmental media and critical effect levels in exposed human workers and experimental animals, it is concluded that 2-EEA is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
On the basis of low ecological hazard, expected releases and low environmental exposure of 2-EEA, it is concluded that this substance is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends. 2-EEA does not meet the criteria for persistence or bioaccumulation potential as set out in the Persistence and Bioaccumulation Regulations.
This substance will be included in the upcoming Domestic Substances List (DSL) inventory update initiative. In addition and where relevant, research and monitoring will support verification of assumptions used during the screening assessment and, where appropriate, the performance of potential control measures identified during the risk management phase.
Based on the information available, it is concluded that 2-EEA does not meet the criteria set out in section 64 of CEPA 1999.
Introduction
The Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or to human health. Based on the results of a screening assessment, the Ministers can propose to take no further action with respect to the substance, to add the substance to the Priority Substances List (PSL) for further assessment or to recommend that the substance be added to the List of Toxic Substances in Schedule 1 of the Act and, where applicable, the implementation of virtual elimination.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherent toxicity to aquatic organisms (iT), and were believed to be in commerce; and/or
- met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE) and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in theCanada Gazette, Part I, on December 9, 2006 (Canada 2006), which challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment and to develop and benchmark best practices for the risk management and product stewardship of those substances identified as high priorities.
The substance ethanol, 2-ethoxy-, acetate (2-ethoxyethanol acetate, referred to as 2-EEA) was identified as a high priority for assessment of human health risk because it was considered to present GPE and had been classified by another agency on the basis of reproductive and developmental toxicity.
The Challenge for 2-EEA was published in the Canada Gazette on August 18, 2007 (Canada 2007). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, submissions of information were received (Environment Canada 2008).
Although 2-EEA was determined to be a high priority for assessment with respect to human health, it did not meet the criteria for potential for persistence, bioaccumulation or inherent toxicity to aquatic organisms. Therefore, this assessment focuses principally on information relevant to the evaluation of risks to human health.
Under CEPA 1999, screening assessments focus on information critical to determining whether a substance meets the criteria for defining a chemical as "toxic" as set out in section 64 of the Act, where
"64. [...] a substance is toxic if it is entering or may enter the environment in a quantity or concentration or under conditions that
- have or may have an immediate or long-term harmful effect on the environment or its biological diversity;
- constitute or may constitute a danger to the environment on which life depends; or
- constitute or may constitute a danger in Canada to human life or health.”
Screening assessments examine scientific information and develop conclusions by incorporating a weight of evidence approach and precaution as required under CEPA 1999.
This screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including the additional information submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents and stakeholder research reports and from recent literature searches, up to December 2007 for the exposure section of the document and up to September 2007 for the health effects section. Key studies were critically evaluated; modelling results may have been used to reach conclusions. Evaluation of risk to human health involves consideration of data relevant to estimation of exposure (non-occupational) of the general population, as well as information on health hazards (based principally on the weight of evidence assessments of other agencies that were used for prioritization of the substance). Decisions for human health are based on the nature of the critical effect and/or margins between conservative effect levels and estimates of exposure, taking into account confidence in the completeness of the identified databases on both exposure and effects, within a screening context. The screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents a summary of the critical information upon which the conclusion is based.
This screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. This assessment has undergone external written peer review/consultation. Comments on the technical portions relevant to human health were received from scientific experts selected and directed by Toxicology Excellence for Risk Assessment (TERA), including John Christopher (California Department of Toxic Substances Control), Michael Jayjock (The Lifeline Group) and Joan Strawson (TERA). Comments on these sections were also received from ToxEcology Environmental Consulting Ltd. Additionally, the draft of this screening assessment was subject to a 60-day public comment period. Although external comments were taken into consideration, the final content and outcome of the screening risk assessment remain the responsibility of Health Canada and Environment Canada.
The critical information and considerations upon which the assessment is based are summarized below.
Substance Identity
2-Ethoxyethanol acetate (2-EEA) is the ester of 2-ethoxyethanol (2-EE) (Wenninger and McEwen 1997). Ethylene glycol monoalkyl ether acetates including 2-EEA are generally produced via esterification of the particular glycol ether with acetic acid, acetic acid anhydride or chloride (NIOSH 1991). 2-EEA is described as a colourless liquid with a mild characteristic fruity odour at room temperature and normal pressure (European Commission 2005) and is soluble in water (Nikitakis and McEwen 1990). Additional information on the identity of 2-EEA is summarized in Table 1.
| CAS RN | 111-15-9 |
|---|---|
| DSL Name | Ethanol, 2-ethoxy-, acetate |
| National Chemical Inventories (NCI) namesTable note a | Ethanol, 2-ethoxy-, 1-acetate (TSCA) 2-Ethoxyethyl acetate (EINECS) |
| Other names | Acetate, ethoxyethyl; Acetate, 2-ethoxyethyl; Acetic acid, 2-ethoxyethyl ester; 1-Acetoxy-2-ethoxyethane; Cellosolve acetate; 2-Ethoxy acetate; 2-Ethoxyethanol acetate; 2-Ethoxyethanol acetate ester; 2-Ethoxyethyl acetone; 2-Ethoxyethylethanoate; Ethyl cellosolve acetate; Ethyl glycol acetate; O-Ethylglycol acetate; Ethylene glycol acetate monoethyl ether; Ethylene glycol ethyl ether acetate; Ethyleneglycol monoethyl ether acetate; Ethylene glycol monoethyl ether monoacetate; Glycol monoethyl ether acetate; Oxitol acetate; Poly-Solv EE acetate |
| Chemical group (DSL stream) | Organics |
| Chemical subgroup | Esters |
| Chemical formula | C6H12O3 |
| Chemical structure | 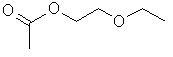 |
| Simplified Molecular Input Line Entry Specification (SMILES) | O=C(OCCOCC)C |
| Molecular mass | 132.16 g/mol |
Physical and Chemical Properties
Table 2 summarizes experimental and modelled physical and chemical properties of 2-EEA.
| Type | Value | Temperature (°C)Table note b | ReferenceTable note c | |
|---|---|---|---|---|
| Melting point (ºC) | Experimental | -61.7 | PhysProp 2006; Lewis 2007 | |
| Boiling point (ºC) | Experimental | 156; 156.4 |
PhysProp 2006; Budavari 1996 | |
| Relative density | 0.9730; 0.975 | 20; 25 | Kirk-Othmer 1980; Budavari 1996 | |
| Vapour pressure (Pa) | Experimental | 270; 326.64 | 20; 25 | Kirk-Othmer 1980; Daubert and Danner 1989 |
| Henry's Law constant (Pa·m3/mol) |
Experimental | 3.24 × 10-4 (3.2 × 10-6 atm·m3/mol)Table note d |
25 | PhysProp 2006 |
| Log Kow (Octanol-water partition coefficient) (dimensionless) |
Experimental | 0.24 | Hüls 1989a | |
| Log Koc (Organic carbon partition coefficient) (dimensionless) |
Modelled | 0.32 | 25 | PCKOCWIN 2000 |
| Log Koc (dimensionless) |
Modelled | 1.62 | 25 | ACD 2007 |
| Water solubility (mg/L) | Experimental | 2.47 × 105 | 15 - 25 | Yalkowsky and Dannenfelser 1992 |
| Water solubility (mg/L) | Experimental | 2.29 × 105 | 20 | Kirk-Othmer 1980 |
Sources
The majority of reports noted that 2-EEA is not expected to occur naturally (IPCS 1990; European Commission 2005; NPI 2006), whereas one study reported biogenic emissions from wood species in the Mediterranean region (Peñuelas and Llusià 2001).
Anthropogenic sources of 2-EEA include manufacturing processes where the acetates are produced by standard esterification of 2-EE with acid anhydride or acid chloride and acid catalyst (Kirk-Othmer 1980).
According to data submitted in response to a survey conducted under CEPA 1999 section 71, 2-EEA was not manufactured in Canada in 2006 above the reporting threshold of 100 kg. The total quantity imported into Canada in the same calendar year was reported to be in the range of 10 000-100 000 kg (Environment Canada 2008).
All producers in the European Union ceased production of 2-EEA in 2002, and it is not expected that any production or import of this substance will start again in the future (European Commission 2006).
Uses
Glycol ethers in general have a wide range of applications, such as coupling agents for a variety of chemical specialties and as intermediates in the production of plasticizers and solvents. 2-EEA has been widely used as an industrial solvent for lacquers and varnish removers, as a blush retardant, as a solvent for nitrocellulose and in oils, resins, dyes, paints and stains. It has also been used in photographic and photolithographic processes and as a general solvent in a wide variety of cleaners (IPCS 1990). It is used in automobile lacquers to retard evaporation and impart high gloss; its primary function is to dissolve components of mixtures in both aqueous and non-aqueous systems and to keep the mixtures in solution until the final stages of evaporation (Montgomery 2000; NPI 2006). It may also be used in the production of adhesives and in the semiconductor industry (ACGIH 1991; Kim et al. 1999; NPI 2006). As a chemical intermediate, 2-EEA may be used in the synthesis of 2-ethoxyethylcyanoacrylate, which is used in low-odour cyanoacrylate adhesives (HSDB 2005).
Based on the information obtained from section 71 submissions, uses of 2-EEA in Canada include solvents, paints, coatings and cleaning solution for industrial applications (Environment Canada 2008).
2-EEA is listed among the ingredients of adhesives that may be used as components of articles intended for use in packaging, transporting or holding food in the United States (US FDA 2007). While it was also used as a solvent in the manufacture of coatings and adhesives in food packaging in Canada in the past (i.e., 15 years ago), no current known uses of this substance in food packaging are recognized in Canada (personal communication with Food Packaging Materials and Incidental Additives Section, Health Products and Food Branch, Health Canada, 2008-04-18; unreferenced).
2-EEA is listed as a solvent and viscosity-decreasing agent in the International Cosmetic Ingredient Dictionary and Handbook (Wenninger and McEwen 1997). Historically, 2-EEA was present in an eye makeup product (30-100%) and skin moisturizer (1-3%). However, both 2-EEA and 2-EE are currently prohibited for use in cosmetic products in Canada (CNS 2008), as well as in the European Union (European Commission 1999).
Use of short straight-chain glycol ethers including 2-EEA and 2-EE has progressively decreased over the last 20 years worldwide (Johanson and Rick 1996; De Kettenis 2005; US EPA 2005). The United States Environmental Protection Agency (EPA) has indicated that there was no ongoing use of 2-EEA and 2-EE in consumer products in the United States and that notification would be required for any significant new use of these substances (US EPA 2005).
Releases to the Environment
The greatest environmental exposure from 2-EEA is a result of direct release to the atmosphere from its use as an evaporative solvent (IPCS 1990). Table 3 summarizes the environmental releases reported under the National Pollutant Release Inventory (NPRI) during the 1994-2006 period (NPRI 2007). The total on-site releases reported mainly refer to atmospheric releases. The increase in releases of 2-EEA in recent years is primarily from one company in Ontario.
| Reporting year |
Number of reporting companies |
Total on-site releases |
Reporting year |
Number of reporting companies |
Total on-site releases |
|---|---|---|---|---|---|
| 2006 | 2 | 1.4 | 1999 | 6 | 4.4 |
| 2005 | 1 | 1.6 | 1998 | 6 | 4.4 |
| 2004 | 2 | 1.8 | 1997 | 8 | 33 |
| 2003 | 3 | 1.5 | 1996 | 6 | 0.31 |
| 2002 | 3 | 0.13 | 1995 | 7 | 4.4 |
| 2001 | 4 | 0.12 | 1994 | 3 | 0.00 |
| 2000 | 4 | 0.15 |
Environmental Fate
Level III fugacity modelling was performed for 2-EEA based on its physical and chemical properties (Table 2), and the results are summarized in Table 4. The results indicate that 2-EEA is expected to reside predominantly in the compartment of release.
| Fraction of substance partitioning to air (%) | Fraction of substance partitioning to water (%) | Fraction of substance partitioning to soil (%) | Fraction of substance partitioning to sediment (%) | |
|---|---|---|---|---|
| Substance released to air (100%) | 50.8 | 19.5 | 29.7 | 0.03 |
| Substance released to waster (100%) | 0.02 | 99.8 | 0.01 | 0.17 |
| Substance released to soil (100%) | 0.15 | 21.1 | 78.7 | 0.04 |
Persistence and Bioaccumulation Potential
Environmental Persistence
When released into the environment, 2-EEA is not expected to be persistent in air, water, soil or sediment. Table 5 summarizes the persistence prediction based on the modelled and empirical data.
| Fate process | Data type | Degradation value | Endpoint / Units | Reference |
|---|---|---|---|---|
| Atmospheric oxidation | Modelled | 0.823 | t½ (days) | AOPWIN 2000 |
| OH reaction in air | Empirical | 0.45 | t½ (days) | Atkinson 1989 |
| Biodegradation in water | Modelled | 15 | t½ (days) | BIOWIN 2000 (Ultimate Survey Model) |
| Biodegradation in water | Modelled | 0.915 | Probability | BIOWIN 2000 (Ministry of International Trade & Industry, Japan (MITI) Nonlinear Model) |
2-EEA reacts with sunlight and other chemicals in the atmosphere and is typically broken down to hydroesters, hydroxyacids, hydroxycarbonyls, peroxyacyl nitrates and formaldehyde (NPI 2006). High photochemical reactivity of 2-EEA has also been reported in the presence of nitrogen oxides in smog chamber tests (Yanagihara et al. 1977).
Much of the 2-EEA is expected to be deposited onto land or water by rainfall, as the gaseous 2-EEA dissolves in water upon contact (NPI 2006). It is expected to hydrolyse readily and subsequently biodegrade to carbon dioxide and water under aerobic conditions. Under anaerobic conditions, methane and carbon dioxide are the major end products (IPCS 1990). In soil, 2-EEA is broken down by bacteria (NPI 2006).
Using an extrapolation ratio of 1:1:4 for a water:soil:sediment biodegradation half-life (Boethling et al. 1995), the half-life in soil is less than 182 days, and the half-life in sediments is less than 365 days. This indicates that 2-EEA is not expected to be persistent in soil or sediment.
The weight of evidence, based on the data described above, indicates that 2-EEA does not meet the persistence criteria for air (half-life in air greater than or equal to 2 days), water or soil (half-life in soil or water greater than or equal to 182 days) or sediment (half-life in sediment greater than or equal to 365 days) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Potential for Bioaccumulation
Modelled data for the bioaccumulation potential of 2-EEA, presented in Table 6, indicate that this substance is not expected to bioaccumulate in the environment.
| Endpoint | Value wet wt (L/kg) | Reference |
|---|---|---|
| Bioaccumulation factor (BAF) | 1.18 | Arnot and Gobas 2003 (Gobas BAF T2MTL) |
| Bioconcentration factor (BCF) | 1.15 | Arnot and Gobas 2003 (Gobas BCF 5% T2LTL) |
| BCF | 1.29 | ACD 2007 |
| BCF | 14.37 | OASIS Forecast 2005 |
| BCF | 3.16 | BCFWIN 2000 |
The Modified Gobas BAF middle trophic level model for fish produced a BAF value of 1.18 L/kg wet weight, indicating that 2-EEA is not likely to bioconcentrate or biomagnify in the environment. The BCF models also provide a weight of evidence to support the low bioconcentration potential of the substance.
The weight of evidence indicates that 2-EEA does not meet the bioaccumulation criteria (BCF, BAF greater than or equal to 5000) as set out in thePersistence and Bioaccumulation Regulations (Canada 2000).
Potential to Cause Ecological Harm
As noted below in the exposure assessment section for human health, concentrations of 2-EEA in air, water and soil are expected to be low.
The experimental results for aquatic toxicity summarized in Table 7 show that acute median lethal concentrations (LC50 values) were much greater than 1.0 mg/L. The experimental data therefore provide a weight of evidence indicating that 2-EEA is not highly hazardous to aquatic organisms. The range of values predicted in Table 7 indicate that 2-EEA has low to moderate acute toxicity to aquatic organisms and that 2-EEA is unlikely to cause harm to aquatic organisms at relatively low concentrations.
| Test organism | Type of test | Endpoint | Value (mg/L) |
|---|---|---|---|
| Daphnia | Acute | LC50 | 560 |
| Shrimp | Acute | LC50 | 4000 |
| Fish | Acute | LC50 | 40-197 |
Reported releases of 2-EEA are predominantly to air. When released into the atmosphere 2-EEA is dispersed quickly and the resulting concentrations in air are low. Atmospheric releases from the substance from finished products containing the substance would be diffuse, from a large number of small sources rather than from a few large point sources, so the resulting concentrations of 2-EEA in air would be low. Releases to water are reported to be low, so exposure of aquatic organisms to 2-EEA would be minimal.
As indicated in the previous sections, 2-EEA does not meet the persistence or bioaccumulation criteria as set out in thePersistence and Bioaccumulation Regulations (Canada 2000). Based on the information available, 2-EEA is unlikely to be causing ecological harm in Canada.
Uncertainties in Evaluation of Ecological Risk
Quantitative structure-activity relationship (QSAR) models were used to estimate persistence and bioaccumulation. There are uncertainties associated with the use of these models to estimate the ecological risk. In addition, the value for Kow, which is used as input to the QSAR models, was also estimated.
There is uncertainty about the environmental exposure to 2-EEA, but it is believed that concentrations in the various environmental media are low.
Potential to Cause Harm to Human Health
Exposure Assessment
Multimedia intake estimates were not derived in this assessment due to insufficient available data. No data were identified on concentrations of 2-EEA in environmental media in Canada except for one study in Windsor, Ontario, where the concentration of 2-EEA in ambient air was below the detection limit of 0.55-2.9 µg/m3 (OMEE 1994). Modelled estimates based on industrial releases to the atmosphere reported under NPRI in 2006 (NPRI 2007) indicate that concentrations of 2-EEA in air, water and soil are not expected to be significant (i.e., in the range of ng/m3, ng/L and ng/g, respectively) (ChemCAN 2003). Likewise, concentrations of 2-EEA in food and beverages are not expected to be significant based on available information on uses and physical and chemical properties.
The maximum concentration of 130 µg/m3 was measured in six samples of indoor air in an Italian study (De Bortoli et al. 1986), whereas in other larger studies conducted in Finland (n = 50 samples) and Germany (n = 89 samples), reported indoor air concentrations of 2-EEA ranged up to a maximum of 5 µg/m3 only (Jungers and Sheldon 1987; Kostiainen 1995; Schleibinger et al. 2001). Thus, it is considered likely that the general population in Canada is not exposed to concentrations in indoor air greater than 5 µg/m3, the highest concentration detected in the more comprehensive surveys (Schleibinger et al. 2001). In addition, since the use of 2-EEA has declined in the United States and Europe (US EPA 2005; European Commission 2006), the higher concentrations detected in the earlier samples are likely not representative of current exposure. Danish studies have reported emission of 2-EEA from a finished product (e.g., polyvinyl chloride flooring) or during use of a product (e.g., hair dryer), which may contribute to levels of 2-EEA observed in the indoor environment (Lundgren et al. 1999; Danish Ministry of the Environment 2005).
Although only limited information is available on the presence of 2-EEA in products, available information indicates that it is used in paints (Akzo Nobel 2005), spray paints (Borden Inc. 1985, 1987), spray lubricant (Dow Corning Corporation 2007), photoresist (Injectorall Electronics Corporation 1992) and rear window defogger repair kits (Permatex, Inc. 2001). The United States Household Products Database (HPD 2007) also includes two varnishes containing 2-EEA on its ingredient listings; however, the concentration is listed as 0%. Although data were identified on the concentration of 2-EEA in yacht paint (Akzo Nobel 2005), use of this product was limited to professional use only and was not considered to be widespread enough to be appropriate for extrapolation to the general population. Likewise, all the other products were determined not to be in the Canadian marketplace or were considered to be used in limited or specialized activities. Therefore, consumer product exposure scenarios were not developed.
There is a high degree of uncertainty in the estimates of exposure, as only very limited data on concentrations of 2-EEA were identified for environmental media in Canada.
Health Effects Assessment
The available health effects information for 2-EEA is summarized in Appendix 1.
The European Commission has classified 2-EEA as a Category 2 substance with risk phrases R60 ("May impair fertility") and R61 ("May cause harm to the unborn child") (ESIS 2007). In addition, the International Programme on Chemical Safety has assessed the toxicity of 2-EEA, together with 2-methoxyethanol (2-ME) and its acetate (2-MEA) and 2-ethoxyethanol (2-EE), and concluded that "the major effects of concern [of these chemicals] for humans are developmental, testicular, and haematological toxicity. These are demonstrated by extensive and consistent data in animals and some human data" (IPCS 1990). Since 2-EEA is rapidly hydrolysed to 2-EE via esterases present in various tissues in the body, information on the toxicity of 2-EE is considered relevant to assessment of the acetate. In the Priority Substances List assessment prepared on 2-EE under CEPA 1999, the critical health effects were also considered to be reproductive and developmental toxicity, as well as effects on the hematological system (Environment Canada and Health Canada 2002).Footnote 1
Reproductive effects were observed in mice orally exposed to 2-EEA for 5 weeks or longer. Dose-dependent testicular atrophy was observed in mice administered 2-EEA by gavage for 5 weeks, with a lowest-observed-(adverse-)effect level (LO(A)EL) of 1000 mg/kg body weight (kg-bw) per day (Nagano et al. 1979, 1984). In a continuous breeding study, there were reductions in number of litters per pair, number of pups per litter, pup viability and fetal body weight at 1860 mg/kg-bw per day and above in drinking water, whereas at a higher dose, significantly reduced fertility index, testis and epididymis weights, and sperm density were noted, as well as increased incidence of sperm abnormality. Histopathological changes in testes and epididymis were also present (Gulati et al. 1985; Morrissey et al. 1989; Chapin and Sloane 1997).
Developmental effects were observed in rodents and rabbits exposed to 2-EEA via oral, dermal and inhalation administration. Following oral exposure, significantly decreased number of litters per fertile pair, reduced fetal body weights and reduced pup viability were observed in mice exposed to 1% or 2% 2-EEA in drinking water (1860 or 3000 mg/kg-bw per day, respectively) in the absence of maternal toxicity. No effects were observed in mice administered 0.5% 2-EEA (930 mg/kg-bw per day) (Gulati et al. 1985; Morrissey et al. 1989; Chapin and Sloane 1997). In inhalation studies in various strains of pregnant rats and rabbits exposed to 2-EEA, there were significantly increased incidences of visceral and skeletal variations at multiple sites, increased fetal resorption, reduced fetal viability and reduced fetal body weights in the presence of maternal toxicity at 100 parts per million (ppm) (550 mg/m3) or more, for both species. No effects were observed at 50 ppm (275 mg/m3) (Tinston et al. 1983; Doe 1984; Nelson et al. 1984; Tyl et al. 1988). Rabbits were more sensitive than rats, as there was a significant increase in the number of totally resorbed litters at 200-300 ppm (1099-1649 mg/m3), whereas such effects were observed in rats only at 600 ppm (3298 mg/m3) (Nelson et al. 1984; Tyl et al. 1988). In the only available dermal developmental toxicity study, significantly increased incidence of cardiovascular and skeletal variations, increased fetal resorption and decreased fetal body weights and fetal viability were observed in rats exposed to 2-EEA at 5826 mg/kg-bw per day, the only dose tested. Maternal toxicity was also observed at this dose level (Hardin et al. 1984).
The hematological system is also a primary target of 2-EEA. Hematological effects were consistently observed in experimental animals following acute or repeated inhalation exposure. The lowest-observed-(adverse-)effect concentration (LO(A)EC) of 2-EEA in acute inhalation studies was 62 ppm (341 mg/m3), which was based on increased erythrocyte osmotic fragility in female rats (Carpenter et al. 1956). In the developmental toxicity studies described above, a concentration related decrease in the number of platelets and an elevated mean corpuscular volume were observed in rabbit dams and significantly decreased red blood cell counts, hemoglobin, hematocrit and red blood cell size as well as increased white blood cell counts were observed in rat dams at 100 ppm (550 mg/m3) or more for both species, with a no-observed-(adverse-)effect concentration (NO(A)EC) of 50 ppm (275 mg/m3) (Tyl et al. 1988). Additionally, leucopenia was observed in orally exposed mice at 1000 mg/kg-bw per day or more, but not at 500 mg/kg-bw per day (Nagano et al. 1979, 1984).
No long-term animal bioassay conducted with 2-EEA was identified. Since 2-EEA is rapidly hydrolysed to 2-EE via esterases present in various tissues in the body (Environment Canada and Health Canada 2002), information on carcinogenicity and chronic toxicity of 2-EE is considered relevant to its acetate. One report of a chronic study was identified in which rats and mice were orally exposed to 2-EE. Although no increases in tumour incidences were noted, reporting on histopathological data was limited. However, the testes were reported to be the principal target organ in both species (Melnick 1984). Based on the analysis of available data on genotoxicity of 2-EE and 2-EEA (for the latter, the database is more limited), it was concluded in the Priority Substances assessment (Environment Canada and Health Canada 2002) that 2-EE "may have some weak potential, at most, to induce cytogenetic damage, but there is no evidence that it induces mutation." 2-EEA did not induce gene mutation in various strains ofSalmonella typhimurium (Slesinski et al. 1988; JCIETIC 2000) or in Escherichia coli (JCIETIC 2000), with or without metabolic activation. Although the clastogenicity of 2-EEA in Chinese hamster ovary cells in vitro was enhanced by metabolic activation, 2-EEA did not induce micronuclei formation in mouse bone marrow cells in vivo (Slesinski et al. 1988).
Reproductive and developmental effects were also noted in humans exposed to glycol ethers, including 2-EEA, in occupational environments. Although exposure to 2-EEA at a time-weighted average (TWA) concentration of 0.51 ppm (2.8 mg/m3) did not affect the menstrual patterns of occupationally exposed women in a liquid crystal display manufacturing facility (Chia et al. 1997), several epidemiological investigations showed that significantly increased risks of spontaneous abortion and/or conception delays (subfertility) and congenital malformations were associated with maternal occupational exposure to glycol ether mixtures, including 2-EEA as well as 2-EE, 2-ME and 2-MEA (Gray et al. 1993, 1996; Beaumont et al. 1995; Schenker et al. 1995; Swan et al. 1995; Correa et al. 1996; Ha et al. 1996; Schenker 1996; Swan and Forest 1996; Cordier et al. 1997, 2001). However, the relative contribution of 2-EEA to these observed effects could not be elucidated, as available data indicate that 2-ME or its acetate could induce similar effects at lower exposure levels in experimental animals (Environment Canada and Health Canada 2002). In a review of these studies (Maldonado et al. 2003), it was stated that the evidence available at that time was insufficient to determine whether occupational exposure to glycol ethers causes human congenital malformations.
With respect to hematological effects, it was reported in a cross-sectional study that exposure to a geometric mean concentration of 2-EEA of 9.34 ppm (51.2 mg/m3) was associated with significant decreases in hemoglobin and hematocrit in exposed women in a silk screening shop, but not in exposed men who, on average, were exposed to lower concentrations (Loh et al. 2003). In the follow-up surveys, the hematological effects observed in the female workers were no longer significant or present after the implementation of protective measures at the workplace for 1 year or longer (Chen et al. 2007). In another cross-sectional study, hematological effects, such as increased leucopenia, were also observed in occupationally exposed men in a shipyard. The TWA level of 2-EEA at the workplace ranged from 1.76 to 3.03 ppm (9.67 to 16.65 mg/m3), with peak exposures of up to 8.12-18.27 ppm (44.6-100.4 mg/m3). The workers in this study were also exposed to other solvents, but not to other glycol ethers (Kim et al. 1999). In the only epidemiological investigation of the potential genotoxicity of 2-EEA, no cytogenic effects (sister chromatid exchange or micronuclei induction) were observed in varnish production workers who were exposed to glycol ethers, including 2-EEA, at levels up to 3.07 ppm (20.34 mg/m3) (Söhnlein et al. 1993).
Although a thorough analysis of mode of action is beyond the scope of this screening assessment, it is recognized that the systemic toxicity of 2-EEA is mediated by its principal major metabolite, 2-ethoxyacetic acid (ECETOC 2005).
The confidence in the database for reproductive and developmental toxicities and for hematological effects of 2-EEA is high, as consistent data are available from different experimental animals and by various routes of administration. These data are also supported by some epidemiological observations as well as by the toxicity information for 2-EE and 2-ethoxyacetic acid (Environment Canada and Health Canada 2002). However, with respect to chronic effects and carcinogenicity of 2-EEA, information is limited.
Characterization of Risk to Human Health
Based on consideration of the weight of evidence-based classification of 2-EEA by the European Commission as a Category 2 substance for reproductive and developmental effects (ESIS 2007), assessments prepared by another international body (IPCS 1990) and within Health Canada (Environment Canada and Health Canada 2002), and consideration of the available relevant data, the critical effects for characterization of risk to human health for 2-EEA are reproductive, developmental and hematological toxicities. Therefore, where sufficient data are available, margins of exposure are derived between lowest exposure levels associated with induction of critical effects and estimates of population exposure to 2-EEA.
The principal source of exposure to 2-EEA for the general population is expected to be indoor air. A comparison between the concentration of 2-EEA (9.67 mg/m3) at which adverse hematological effects have been reported in workers (Kim et al. 1999) (although it is recognized that these workers were also exposed to other potentially confounding substances) and the highest concentration identified in surveys of indoor air considered most relevant to potential current exposures in Canada (5 µg/m3 [Schleibinger et al. 2001]) results in a margin of exposure of approximately 1930. Comparison of the lowest LO(A)EC (550 mg/m3) at which developmental toxicity and hematological effects were observed in experimental animals (Tyl et al. 1988) with this indoor air concentration yields a larger margin (110 000). These margins are considered adequate to account for uncertainties in the database, in light of the conservative nature of the estimates of exposure and critical effect levels in studies in humans and experimental animals.
Potential consumer products containing 2-EEA were determined not to be in the Canadian marketplace or were considered to be used in limited/specialized activities. Therefore, exposure to 2-EEA via consumer products is not expected to be significant.
Uncertainties in Evaluation of Risk to Human Health
There is some uncertainty regarding the precise magnitude of exposure to 2-EEA in the general environment, although exposures are expected to be very low. There is also uncertaintyassociated with the limited data on the environment and the presence of 2-EEA in consumer products used in Canada. Available sources of information indicate that 2-EEA is not currently used in products that would result in significant exposure of the general population.
Although quantification of differences in sensitivity to 2-EEA induced effects between laboratory animals and humans and across the population is beyond the scope of this screening assessment, it is noteworthy that hematological effects were reported in workers at concentrations lower than effect levels observed in experimental animals. There is some uncertainty with respect to the carcinogenicity potential of 2-EEA due to lack of sufficient information, although the results of genotoxicity studies as well as the primary chronic toxicity data for 2-EE do not suggest that 2-EEA is carcinogenic. Additionally, there is some uncertainty associated with the lack of a reproductive toxicity study in experimental animals exposed by inhalation (the route most relevant to population exposure); however, the available database for other endpoints observed in animals exposed by oral, dermal or inhalation routes does not suggest that effect levels for reproductive toxicity for inhaled 2-EEA would be lower than those for other effects.
Conclusion
Based upon consideration of the margins of exposure between conservative estimates of exposure to 2-EEA from environmental media and concentrations associated with developmental and hematological effects in experimental animals or exposed workers, it is concluded that 2-EEA should not be considered to be "toxic" as defined in paragraph 64(c) of CEPA 1999: i.e., 2-EEA is not a substance entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Based on the information presented in this screening assessment, it is concluded that 2-EEA is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
It is therefore concluded that 2-EEA does not meet the definition of "toxic" as set out in section 64 of CEPA 1999. Additionally, 2-EEA does not meet the criteria for persistence or bioaccumulation potential as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
References
[ACD] Advanced Chemistry Development. 2007. Calculated values using Advanced Chemistry Development (ACD/Labs) Software V9.04 for Solaris (© 1994-2007, presented in SciFinder database, searched July 25, 2007.
[ACGIH] American Conference on Governmental Industrial Hygienists. 1991. American Conference of Governmental Industrial Hygienists. Documentation of the threshold limit values and biological exposure indices. 6 th Edition. Cincinnati. 567 - 568.
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2000. Version 1.91. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Arnot JA, Gobas FAPC. 2003. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci [Internet]; 22(3): 337-345. [restricted access]. http://www3.interscience.wiley.com/journal/104557877/home_
Atkinson R. 1989. Kinetics and Mechanisms of the Gas-Phase Reactions of the Hydroxyl Radical With Organic Compounds. Journal of Physical and Chemical Reference Data. Monograph No. 1.
Akzo Nobel. 2005. Material safety data sheets (various). http://www.awlgrip.com/support/msds/Pages/MSDS.aspx
[BCFWIN] BioConcentration Factor Program for Windows [Estimation Model]. 2000. Version 2.15. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Beaumont JJ, Swan SH, Hammond SK, Samuels SJ, Green RS, Hallock MF, Dominguez C, Boyd P, Schenker MB. 1995. Historical cohort investigation of spontaneous abortion in the semiconductor health study: epidemiologic methods and analysis of risk in fabrication overall and in fabrication work groups. Am J Ind Med 28: 735-750.
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2000. Version 4.02. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Boethling RS, Howard PH, Beauman JA, and Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4):741-752.
Borden Inc. 1985. Material Safety Data Sheets for Krylon Interio/Exter Enamel Aerosol 1809. http://www.biosci.ohio-state.edu/safety/MSDS/KRYLON%20ENAMEL%20AEROSOL.htm
Borden Inc. 1987. Material Safety Data Sheets for Product for SPARVAR S-100 GLOSSY WHITE (OLD FORM). MSDS Date: 01/01/1987. http://hazard.com/msds/f2/bdw/bdwyf.html
Budavari S (ed.). 1996. The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 12 th Edition. Whitehouse Station, NJ : Merck and Co., Inc. p. 640.
Canada. 1999. Canadian Environmental Protection Act, 1999: Loi canadienne sur la protection de l'environnement, 1999. Statutes of Canada = Statuts du Canada, Chapter 33. Act assented to September 14, 1999. Ottawa : Queen's Printer. Available at Canada Gazette (Part III) 22(3): chapter 33. http://canadagazette.gc.ca/partIII/1999/g3-02203.pdf
Canada. 2000. Canadian Environmental Protection Act: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March, 2000, SOR/2000-107, Canada Gazette. Part II, vol. 134, no. 7, p. 607-612. Ottawa : Queen's Printer. http://canadagazette.gc.ca/partII/2000/20000329/pdf/g2-13407.pdf
Canada, Dept. of the Environment, Dept. of Health. 2006.Canadian Environmental Protection Act, 1999: Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette, Part I, vol. 140, no. 49, p. 4109-4117. http://canadagazette.gc.ca/partI/2006/20061209/pdf/g1-14049.pdf
Canada, Dept. of the Environment, Dept. of Health. 2007.Canadian Environmental Protection Act, 1999: Notice with respect to Batch 3 Challenge substances. Canada Gazette, Part I, vol. 141, no. 33, p. 2379-2394. http://canadagazette.gc.ca/partI/2007/20070818/pdf/g1-14133.pdf
Carpenter CP. 1947. Cellosolve (Queries and minor notes). J Am Med Ass 135:880.
Carpenter CP, Smyth HF. 1946. Chemical burns of the rabbit cornea. Am J Ophthalmol 29: 1363-1372. [cited in ECETOC 2005].
Carpenter CP, Pozzani UC, Weil CS, Nair JH, Keck GA, Smyth HF. 1956. The toxicity of butyl cellosolve solvent. AMA Arch Ind Health 14: 114-31.
[CCRIS] Chemical Carcinogenesis Research Information System. [database on the Internet]. 2003. Record for Ethylene glycol monoethyl ether acetate. CAS registry number: 111-15-9. Bethesda (MD): National Library of Medicine (US). (2004 update). http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?CCRIS
Chapin RE, Sloane RA. 1997. Reproductive assessment by continuous breeding: evolving study design and summaries of ninety studies; Ethylene glycol monoethyl ether acetate. Environ Health Perspect 105 (Suppl 1):199-395; 221-222.
ChemCAN [Level III fugacity model of 24 regions of Canada]. 2003. Version 6.00. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry. http://www.trentu.ca/academic/aminss/envmodel/models/CC600.html
Chen HI, Liou SH, Hsieh MH, Shih TS, Sun CW, Wu TN, Chang HY, Loh CH. 2007. Hematological follow-up of an intervention program adding rubber glove-wearing to local ventilation for 2-ethoxyethanol acetate-exposed workers. J Occup Health 49:285-93.
Chia SE, Foo SC, Khoo NY, Jeyaratnam J. 1997. Menstrual patterns of workers exposed to low levels of 2-ethoxyethylacetate (EGEEA). Am J Ind Med 31:148-52.
[CNS] Cosmetic Notification System. [Proprietary Database]. March 2008. Ottawa (ON): Health Canada. [cited April 2008].
Cordier S, Bergeret A, Goujard J, Ha MC, Aymé S, Bianchi F, Calzolari E, De Walle HEK, Knill-Jones R, Candela S, Dale I, Dananché B, de Vigan C, Fevotte J, Kiel G, Mandereau L. 1997. Congenital malformations and maternal occupational exposure to glycol ethers. Epidemiology 8:355-63.
Cordier S, Szabova E, Fevotte J, Bergeret A, Plackova S, Mandereau L. 2001. Congenital malformations and maternal exposure to glycol ethers in the Slovak Republic. Epidemiology 12:592-3.
Correa A, Gray RH, Cohen R, Rothman N, Shah F, Seacat H, Corn M. 1996. Ethylene glycol ethers and risks of spontaneous abortion and subfertility. Am J Epidemiol 143:707-17.
Danish Ministry of the Environment. 2005. Emission and evaluation of chemical substances from selected electrical and electronic products - part 2. Survey of Chemical Substances in Consumer Products, No. 66. Environmental Protection Agency.
Daubert TE, Danner RP. 1989. Physical and Thermodynamic Properties of Pure Chemicals Data Compilation. Washington, D.C. : Taylor and Francis.
De Bortoli N, Knöppel H, Pecchio E, Peil A, Rogora L, Schauenburg H, Schlitt H, Vissers H. 1986. Concentrations of selected organic pollutants in indoor and outdoor air in Northern Italy. Environment International 12: 343-350.
De Kettenis P. 2005. The historic and current use of glycol ethers: a picture of change. Toxicol Lett 156: 5-11.
Doe JE. 1984. Ethylene glycol monoethyl ether and ethylene glycol monoethyl ether acetate teratology studies. Envir Health Perspect 57: 33-41.
Dow Corning Corporation. 2007. Material Safety Data Sheets for MolyKote® 3402C Spray. http://www3.dowcorning.com/DataFiles/090007b280e49bba.pdf
Eastman Kodak Company. 1958. Unpublished data. Corporate Health, Safety and Environment. [cited ECB 2000].
Eastman Kodak Company. 1968. Unpublished data. Corporate Health, Safety and Environment. [cited ECB 2000].
[ECB] European Chemical Bureau. 2000. IUCLID Dataset for 2-ethoxyethyl acetate (CAS Number 111-15-9). http://ecb.jrc.it/esis/
[ECETOC] European Centre for Ecotoxicology and Toxicology of Chemicals. 2005. The toxicology of glycol ethers and its relevance to man. Technical report No. 95, vol II. 4.10. Substance profile: EGEEA (CAS number: 111-15-9). Brussels, Belgium.
[ECOTOX] ECOTOXicology database [database on the Internet]. 2006. Washington (DC): US Environmental Protection Agency, Office of Research and Development; US Environmental Protection Agency, National Health and Environmental Effects Research Laboratory's, Mid-Continent Ecology Division. http://cfpub.epa.gov/ecotox
Environment Canada. 2008. Data for Batch 3 substances collected underCanadian Environmental Protection Act, 1999, Section 71: Notice with respect to Batch 3 Challenge substances. Data prepared by: Environment Canada, Existing Substances Program.
Environment Canada and Health Canada. 2002. Priority Substance List Assessment Report for 2-Ethoxyethanol [Internet]. Ottawa (ON): Environment Canada; Health Canada. http://www.hc-sc.gc.ca/ewh-semt/pubs/contaminants/psl2-lsp2
[EQC] Equilibrium Criterion Model. 2003. Version 2.02. Peterborough (ON): Trent University, Canadian Centre for Environmental Modelling and Chemistry. http://www.trentu.ca/academic/aminss/envmodel/models/EQC2.html
[ESIS] European Chemical Substances Information System. 2007. Version 5. 2-Ethoxyethanol acetate, CAS No 111-15-9. http://ecb.jrc.it/esis/
European Commission. 1999. The rules governing cosmetic products in the European Union, Volume 1, Cosmetics legislation, Cosmetic products. Council Directive 76/768. Annex II. Euromean Commission, Enterprise Directorate-General, Pharmaceuticals and cosmetics. http://www.leffingwell.com/cosmetics/vol_1en.pdf
European Commission. 2005. Risk Assessment: Draft of 24.02.2005. 2-Ethoxyethyl Acetate. Federal Institute for Occupational Safety and Health Notification Unit, Dortmund, Germany.
European Commission. 2006. Risk Assessment: Draft of 24.02.2006 Ethoxyethyl Acetate (CAS -No: 111-15-9, EINECS-No: 203-839-2). Note - Human Health Section. Federal Institute for Occupational Safety and Health Notification Unit, Dortmund, Germany.
Gig Sanit. 1988. 53 (10) 78, cited in RTECS 1993 [cited in ECB 2000].
Gray RH, Corn M, Cohen R, Correa A, Hakim R, Hou W, Shah F, Zauer H. 1993. Final report. The Johns Hopkins University retrospective and prospective studies of reproductive health among IBM employees in semiconductor manufacturing. The Johns Hopkins University. Baltimore, MD, USA. [cited in Swan and Forest 1996; Maldonado et al. 2003].
Gray RH, Correa A, Hakim R, Cohen R, Corn M, Shah F, Rothman N, Hou W, Secat H. 1996. Ethylene glycol ethers and reproductive health in semiconductor workers. Occupational Hygiene 2:331-8.
Gulati DK, Barnes LH, Russell S, Poonacha KB, Lamb JC. 1985. Ethylene glycol monoethyl ether acetate. Reproduction and fertility assessment in CD-1 mice when administered in drinking water. Final report, NTP-85-320. PB 86-139565. National Toxicology Program, National Institute of Environmental Health Sciences.
Ha MC, Cordier S, Dananche B, Bergeret A, Mandereau L, Bruno F.1996. Congenital malformations and occupational exposure to glycol ethers: a European collaborative case-control study. Occup Hyg 2:417-421.
Hardin BD, Goad PT, Burg JR. 1984. Developmental toxicity of four glycol ethers applied cutaneously to rats. Environ Health Perspect 57:69-74.
[HPD] Household Products Database [database on the Internet]. 2007. 2-Ethoxyethanol acetate: U.S Department of Health and Human Services, National Institutes of Health, National Library of Medicine, Toxicology Data Network. [cited September 2007]. http://household products.nlm.nih.gov/
[HSDB] Hazardous Substances Data Bank [database on the Internet]. 2005. Ethylene glycol monoethyl ether acetate. Bethesda (MD): National Library of Medicine (US). [updated 2005 Jun 26; cited 2007 Jun]. http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB
Hüls AG. 1989a. Verteilungskoeffizient n-Octanol - Wasser für "Altstoffe". Test report from 10.01.1989. [cited in European Commission 2005].
Hüls AG. 1989b. Bestimmung der Mutagenität von Ethyglykolacetat in Salmonella/Säuger-Mikrosomen-Mutagenitätstest nach Ames, gemäß Eg-Richtlinie 84/449/EGW B14. Unpulished report AM-89/22. Schöberl, Ps-Biologie/Toxikologie. Hüls, Marl, Germany. [cited in ECETOC 2005
Injectorall Electronics Corp. 1992. Material Safety Data Sheet for Positive Photo Resist PC197. http://www.injectorall.com/MSDS197.htm
[IPCS] International Programme on Chemical Safety. 1990. 2-methoxyethanol, 2-ethoxyethanol, and their acetates. Geneva (CH): World health Organization. (Environmental Health Criteria 115). Jointly sponsored by the United Nations Environment Programme, the International Labour Organization, and the World Health Organization.
[JCIETIC] Japan Chemical Industry Ecology-Toxicology and Information Center. 2000. Mutagenicity test data of exostng xhemixal substances based on the toxicity investigation of the industrial safety and health law. Suppl 2 [cited in CCRIS 2004].
Johanson G, Rick U. 1996. Use and use patterns of glycol ethers in Sweden. Occup Hyg 2: 105-110.
Johnson W. Jr. 2002. CIR (Cosmetic Ingredient Review). Final Report on the Safety Assessment of Ethoxyethanol and Ethoxyethanol Acetate. Int. J. Toxicol. 21 (Suppl. 1): 9-62. http://lib.bioinfo.pl/auth:Johnson%20Jr,W
Jungers RH, Sheldon LS. 1987. Characterization of Volatile Organic Chemicals in Public Access Buildings. In: Proceedings of the Fourth International Conference on Indoor Air Quality and Climate. Vol.1. Institute for Water, Soil, and Air Hygiene. Berlin, West Germany. 144 - 148.
Kennah HF, Hignet S, Laux PE, Dorko JD, Barrow CS. 1989. An objective procedure for quantitating eye irritation based upon changes of corneal thickness. Fundam Appl Toxicol 12:258-68. [cited in ECETOX 2005]
Kim Y, Lee N, Sakai T, Kim JS, Yang JS, Park S, Lee CR, Cheong HK, Moon Y. 1999. Evaluation of exposure to ethylene glycol monoethyl ether acetates and their possible haematological effects on shipyard painters. Occup Environ Med 56:378-82.
Kirk-Othmer. 1980. Encyclopaedia of Chemical Technology Vol. 11. Glycols (Ethylene and Propylene). 3 rd Edition. New York (NY). [cited in European Commission 2005].
Kostiainen R. 1995. Volatile organic compounds in the indoor air of normal and sick houses. Atmospheric Environment 29 (6): 693-702.
Lewis RJ, Sr. 2007. Hawley's Condensed Chemical Dictionary. 15 th Edition. Wiley-Interscience. A John Wiley & Sons, Inc. Publication. 529.
Loh CH, Shih TS, Liou SH, Lin YC, Hsieh AT, Chen CY, Liao GD. 2003. Haematological effets among silk screening workers exposed to 2-ethoxy ethyl acetate. Occup Environ Med 60:e7.
Lundgren B, Jonsson B, and Ek-Olausson, B. 1999. Material emission of chemicals - PVC flooring materials. Indoor Air 9:202-208.
Maldonado G, Delzell E, Tyl RW, Sever LE. 2003. Occupational exposure to glycol ethers and human congenital malformations. Int Arch Occup Environ Health 76:405-23.
Melnick RL. 1984. Toxicities of ethylene glycol and ethylene glycol monoethyl ether in Fisher 344/N rats and B6C3F1 mice. Environ Health Perspect 57:147-55.
[MITI] Ministry of International Trade & Industry (Jpn), Basic Industries Bureau, Chemical Products Safety Division. 1992. Biodegradation and bioaccumulation data of existing chemicals based on the CSCL Japan. Tokyo (Jpn): Japan Chemical Industry Ecology-Toxicology & Information Centre.
Montgomery JH. 2000. Groundwater Chemicals Desk Reference. CRC Press, Boca Raton.
Morrissey RE, Lamb JC, Morris RW, Chapin RE, Gulati DK, Heindel JJ. 1989. Results and evaluations of 48 continuous breeding reproduction studies conducted in mice. Fundam Appl. Toxicol 13:747-77.
Nagano K, Nakayama E, Koyano M, Oobayashi H, Adachi H, Yamada T. 1979. Testicular atrophy of mice induced by ethylene glycol mono alkyl ethers. Sanyo Igaku (Japanese Journal of Industrial Health) 21:29-35 (in Japanese, with English abstract and tables).
Nagano K, Nakayama E, Oobayashi H, Nishizawa T, Okuda H, Yamazaki K. 1984. Experimental studies on toxicity of ethylene glycol alkyl ethers in Japan. Environ Health Perspect 57:75-84.
[NCI] National Chemical Inventories [database on CD-ROM]. 2007. Columbus (OH): American Chemical Society. [cited 2007 Nov]. http://www.cas.org/products/cd/nci/index.html
Nelson BK, Setzer JV, Brightwell WS, MAthinos PR, Kuczuk MH, Weaver TE, Goad PT. 1984. Comparative inhalation teratogenicity of four glycol ether solvents and an amino derivative in rats. Environ Health Perspect 57:261-71.
Nikitakis JM, McEwen GN, Jr. eds. 1990. CTFA compendium of cosmetic ingredient composition - descriptions. I. Washington, DC: CTFA. [cited in Johnson 2002]
NIOSH. 1991. Criteria for a recommended standard. Occupational exposure to ethylene glycol monomethyl ether, ethylene glycol monoethyl ether, and their acetates. (DHHS [NIOSH] Publication No. 91 - 119.) Cincinnnati: NIOSH. [cited in Johnson 2002]
[NPI] National Pollutant Inventory. 2006. Substance fact sheet. 2-Ethoxyethanol acetate. Parkes (AU): Australian Government, Department of the Environment, Water, Heritage and the Arts. [cited 2008 Jan 21]. http://www.npi.gov.au/cgi-bin/npireport.pl?proc=substance;substance=37#Details
[NPRI] National Pollutant Release Inventory [database on the Internet]. 2007. Gatineau (QC): Environment Canada. [cited 2007 Jul 5]. http://www.ec.gc.ca/pdb/querysite/query_e.cfm
[OASIS Forecast] Optimized Approach based on Structural Indices Set [Internet]. 2005. Version 1.20. Bourgas, Bulgaria : Laboratory of Mathematical Chemistry. http://oasis-lmc.org/?section=software
[OMEE] Ontario Ministry of Environment and Energy. 1994. Windsor Air Quality Study: TAGA 6000 survey results. Windsor Air Quality Committee; Queen's Printer for Ontario (PIBS 3152E; ISBN 0-7778-2831-6, Fall 1994).
[PCKOCWIN] Organic Carbon Partition Coefficient Program for Windows [Estimation Model]. 2000. Version 1.66. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. http://www.epa.gov/oppt/exposure/pubs/episuite.htm
Peñuelas J, Llusià J. 2001. Seasonal patterns of non-terpenoid C 6 -C 10 VOC emission from seven Mediterranean woody species. Chemosphere 45: 237-244.
Permatex, Inc. 2001. Material Safety Data Sheet: Quick Grid TM Repair Kit. 05FO CG. https://corporateportal.ppg.com/NR/rdonlyres/9B08C0E3-63DD-47CE-8BB7-3DEAC80D4BE0/0/permatex_15067_msds.pdf
[PhysProp] Interactive PhysProp Database [database on the Internet]. 2006. Syracuse (NY): Syracuse Research Corporation. http://www.syrres.com/esc/physdemo.htm
Pozzani UC, Weil CS, Carpenter CP. 1959. The toxicological basis of threshold limit values. 5. The experimental inhalation of vapor mixtures by rats, with notes upon the relationship between single dose inhalation and single dose oral data. Am Ind Hyg Ass J 20:364-9.
Schenker MB. 1996. Reproductive health effects of glycol ether exposure in the semiconductor industry. Occup Hy. 2:367-72.
Schenker MB, Gold EB, Beaumont JJ, Eskenazi B, Hammond SK, Lasley BL, McCurdy SA, Samuels JJ, Saiki CL, Swan SH. 1995. The association of spontaneous abortion and other reproductive effects with work in the semiconductor industry. Am J Ind Med 28:639-59.
Schleibinger H, Hott U, Marchl D, Braun P, Plieninger P, Ruden H. 2001. VOC-concentrations in Berlin indoor environments between 1988 and 1999. Gefahrstoffe Reinhaltung der Luft. 61(1-2). 26-38.
Shaw GM, Velie EM, Katz EA, Morland KB, Schaff DM, Nelson V. 1999. Materanl Occupational and hobby chemical exposures as risk factors for neural tube defects. Epidemiology 10:124-9.
Shell Chemical Co. Undated. Unpublished report. [cited in Tyl et al. 1988].
Slesinski RS, Guzzie PJ, Tyler TR. 1988. Cytotoxicity and genotoxic potential of ethylene glycol monethyl ether acetate (EGEE.Ac) in a battery of short term test systems. Environ Molec Mutagen 11, Suppl 11:97 (Abstract).
Smyth HF, Seaton J, Fisher L. 1941. The single dose toxicity of some glycols and derivatives. J Ind Hyg Toxicol 23:259-68.
Söhnlein B, Letzel S, Weltle D, Rüdiger HW, Angerer J. 1993. Occupational chronic exposure to organic solvents. XIV. Examinations concerning the evaluation of a limit value for 2-ethoxyethanol and 2-ethoxyethyl acetate and genotoxic effects of these glycol ethers. Int Arch Occup Environ Health 64:479-84.
Swan SH, Beaumont JJ, Hammond SK, VonBehren J, Green RS, Hallock MF, Woskie SR, Hines CJ, Schenker MB. 1995. Historical cohort study of spontaneous abortion among fabrication workers in the semiconductor health study: agent-level analysis. Am J Ind Med 28:751-769.
Swan SH, Forest W. 1996. Reproductive risks of glycol ethers and other agents used in semiconductor manufacturing. Occup Hyg 2: 373-85.
Tinston DJ, Doe JE, Killick M, Thomas M. 1983. Ethylene glycol monoethyl ether acetate (EEAc). Inhalation teratogenicity study in rabbits. Imperial Chemical Industries PLC. Report No. CTL /P/840 to Chemical Manufacturer's Association.
Truhaut R, Dutertre-Catella H, Phu-Lich N, Huyen VN. 1979. Comparative toxicological study of ethylglycol acetate and butylglycol acetate. Toxicol Appl Pharmac 51:117-127.
Tyl RW, Pritts IM, France KA, Fisher LC, Tyler TR. 1988. Developmental toxicity evaluation of inhaled 2-ethoxyethanol acetate in Fischer 344 rats and New Zealand white rabbits. Fund Appl Toxicol 10:20-39.
[US EPA] US Environmental Protection Agency. 2005. Federal Register Environmental Documents Volume 70 Number 228. November 29, 2005. Rules and Regulations. 71401-71406. http://www.epa.gov/EPA- TOX /2005/November/Day-29/t23421.htm
[US FDA] US Food and Drug Administration. Code of Federal Regulations. Title 21. Volume 3. Revised as of April 1 2007. Cite: 21 CFR 175.105. Department of Health and Human Services. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm
Von Oettingen WF, Jirouch EA. 1931. The pharmacology of ethylene glycol and some of its derivatives in relation to their chemical constitution and physical properties. J Pharmacol Exp Ther 42:355-72. [cited in ECETOX 2005]
Wenninger and McEwen. 1997. International Cosmetic Ingredient Dictionary and Handbook. 7 th ed. 505. Washington, DC. CTFA. [cited in Johnson 2002]
Yalkowsky SH, Dannenfelser RM. 1992. The Aquasol Database of Aqueous Solubility. Fifth Ed, Tucson, AZ : Univ Az, College of Pharmacy.
Yanagihara S, Shimada I, Shinoyama E, Chisaka P, Saito K. 1977. Photochemical Reactivities of Hydrocarbons. Proceeding of the Fourth International Clean Air Congress. 472-477.
Zissu D. 1995. Experimental study of cutaneous tolerance to glycol ethers. Contact
Appendix 1. Summary of health effects information for 2-EEA (CAS RN 111-15-9)
| Endpoint | Lowest effect levels/Results |
|---|---|
| Acute toxicity | Lowest oral median lethal dose (LD50) (guinea pig) = 1910 mg/kg-bw (Smyth et al. 1941; Carpenter 1947) Lowest dermal LD50 (mouse) = 10 300 mg/kg-bw (Carpenter 1947) Lowest inhalation LC50 (rat)= 8250 mg/m3 (Shell Chemical Co. [undated]) Lowest inhalation LO(A)EC (rat, female) = 62 ppm, equivalent to 341 mg/m3, based on hemolytic effect on erythrocyte osmotic fragility (Carpenter et al. 1956) |
Short-term repeated-dose toxicity
|
Lowest oral LO(A)EL (mouse) = 1000 mg/kg-bw per day (male mice, 5/group, gavage for 5 weeks), based on dose-dependent testicular atrophy and leucopenia; no-observed-(adverse-)effect level (NO(A)EL) = 500 mg/kg-bw per day (Nagano et al. 1979, 1984) Lowest dermal LO(A)EL (rat) = 5826 mg/kg-bw per day (the only dose tested -- pregnant rats, 18/group, 4 times daily on gestation days 7-16), based on maternal toxicity, including significantly reduced body weight gain and gravid uterus weight in dams (Hardin et al. 1984) Lowest inhalation LO(A)EC (rat and rabbit) = 100 ppm, equivalent to 550 mg/m3 (pregnant rabbits, 24/group, 6 h/day, on gestation days 6-18; pregnant rats, 30/group, 6 h/day, on gestation days 6-15), based on maternal toxicity, including significantly reduced body weight gain, altered hematological parameters, increased absolute liver weights and clinical signs; NO(A)EC = 50 ppm, equivalent to 275 mg/m3 (Tyl et al. 1988) |
Subchronic toxicity
|
Lowest inhalation LO(A)EC (rat and rabbit) = 200 ppm, equivalent to 1099 mg/m3 (2 rabbits/sex per group; 10 rats/sex per group; 4 h/day, 5 days/week for 10 months). This was the only concentration used in the study. Discrete lesions of tubular nephritis with clear degeneration of the epithelium with hyaline and granular tubular casts were observed in the kidneys of male and female rabbits and male rats but not in female rats (Truhaut et al. 1979). Lowest oral LO(A)EL (mouse) = 2% in drinking water, equivalent to 3000 mg/kg-bw per day (Swiss CD mice, 20/sex per group, administered in drinking water for 119 days), based on significantly reduced body weights in the F0 male mice in a continuous breeding study (Gulati et al. 1985; Morrissey et al. 1989; Chapin and Sloane 1997) No dermal subchronic toxicity data identified |
| Chronic toxicity/ carcinogenicity | No chronic toxicity/carcinogenicity data identified |
| Reproductive toxicity | Lowest oral LO(A)EL (mouse) = 1000 mg/kg-bw per day (male mice, 5/group, gavage for 5 weeks), based on dose-dependent testicular atrophy; NO(A)EL = 500 mg/kg-bw per day (Nagano et al. 1979, 1984) In a continuous breeding study (male/female mice, administered in drinking water for two generations), a LO(A)EL = 1.0%, equivalent to 1860 mg/kg-bw per day, was identified, based on reduced number of litters per pair, reduced number of pups per litter, reduced pup viability and reduced fetal body weight. At a higher dose level (3000 mg/kg-bw per day), significantly reduced fertility index and testis weights and significantly increased incidence of abnormal sperm were observed in the F0 generation, and significantly reduced epididymis weight and sperm density and prominent histopathological changes in the testes and epididymis were observed in the F1 animals. Significant liver weight changes were also observed in the F1 generation. NO(A)EL = 0.5%, equivalent to 930 mg/kg-bw per day (Gulati et al. 1985; Morrissey et al. 1989; Chapin and Sloane 1997). |
| Developmental toxicity | Lowest oral LO(A)EL (mouse) =1% in drinking water, equivalent to 1860 mg/kg-bw per day (male/female mice, continuous breeding study), based on significantly decreased number of litters per fertile pair and reduced fetal body weights and pup viability in the absence of maternal toxicity; NO(A)EL = 0.5% in drinking water (930 mg/kg-bw per day) (Gulati et al. 1985; Morrissey et al. 1989; Chapin and Sloane 1997) Lowest dermal LO(A)EL (rat) = 5826 mg/kg-bw per day (the only dose tested, 18 rats/group, 4 times daily on gestation days 7-16). Significantly increased incidence of cardiovascular variation and skeletal variations in ribs and vertebrae, retarded ossification, increased fetal resorption and decreased fetal body weights and fetal viability were observed. Maternal toxicity was also observed (see above) (Hardin et al. 1984). Lowest inhalation LO(A)EC (rat and rabbit) = 100 ppm, equivalent to 550 mg/m3 (F344 rats, 30/group, gestation days 6-15; New Zealand White rabbits, 24/group, gestation days 6-18), based on significantly increased incidence of visceral and skeletal variations in fetuses. At higher concentrations (200-300 ppm), significantly increased incidence of external variations, visceral variations (predominantly in cardiovascular and renal systems in both species and in pulmonary system in rabbits) and skeletal variations/malformation (multiple sites) in fetuses, increased fetal resorption and reduced fetal body weights and viability were observed. NO(A)EC = 50 ppm, equivalent to 275 mg/m3. Maternal toxicity was observed at 100-300 ppm (see above) (Tyl et al. 1988). |
| Genotoxicity and related endpoints: in vivo | Micronuclei induction Negative results: |
| Genotoxicity and related endpoints: in vitro | Mutagenicity Mutation in Escherichia coli WP2uvrA/pKM101, with and without activation (JCIETIC 2000) HGPRT locus mutation in Chinese hamster ovary (CHO) cells, with and without activation (Slesinski et al. 1988) Sister chromatid exchange (SCE) Clastogenicity test in CHO cells (no further details), positive results with activation; weak positive results without activation (Slesinski et al. 1988) |
| Sensitization | Negative in guinea pig (Zissu 1995) |
| Irritation | Skin irritation Eye irritation |
| Endpoint | Lowest effect levels/Results |
|---|---|
| Genotoxicity | No increased incidence of SCE and micronuclei induction was detected in 19 varnish production workers exposed to a glycol ether mixture, including 2-EEA, compared with 15 controls (age and smoking habits were largely matched). The 2-EEA level in the varnish workplace was in the range of 0.1-3.7 ppm, equivalent to 0.55-20.34 mg/m3 (Söhnlein et al. 1993). |
| Reproductive and developmental toxicity | Menstrual patterns Spontaneous abortion Similar conclusions were derived from cohort studies, including a historical cohort (561 pregnancies to exposed women and 589 pregnancies to the wives of exposed men) and a prospective cohort (148 women), conducted at two IBM semiconductor manufacturing companies. Glycol ethers used at the workplace included diethylene glycol dimethyl ether and/or 2-EEA. In the historical cohort study, significantly increased RR of spontaneous abortion was observed in women who might have experienced high-level exposure (RR = 2.8, 95% CI = 1.4-5.6), and not in the wives of exposed men. In the prospective study, the RR of fetal loss was 2.5 (95% CI = 0.8-8.5) in the exposed women. The result was not significant. In addition, in the retrospective cohort study, the odds ratio (OR) of subfertility (conception delay for more than a year) was also significantly increased in those women who might have experienced high-level exposure (440 pregnancies, OR = 3.9, 95% CI = 1.4-11.4), and there was a non-significantly increased risk of subfertility in the wives of men who might have experienced high-level exposure (495 pregnancies, OR = 1.6, 95% CI = 0.6-3.8); no exposure estimates were provided (Gray et al. 1993, 1996; Correa et al. 1996). Congenital malformation In two case-control studies, increased risk of congenital malformation was associated with maternal occupational exposure to a glycol ether mixture. One was a study (984 cases versus 1134 controls) of western European workers in a wide diversity of occupational settings (Ha et al. 1996; Cordier et al. 1997); the second was a study (196 cases versus 196 controls) in the Slovak Republic (Cordier et al. 2001). No information regarding the risk specifically attributed to 2-EEA exposure was provided in these studies. |
| Hematological effects | A cross-sectional study was conducted in workers with high-exposure experience (n = 29: 17 males, 12 females) versus those with low/no-exposure experience (n = 56: 29 males, 27 females) at a silk screening shop. The geometric mean (GM) concentration of 2-EEA in the high-exposure group was 7.4 ppm (range 1.35-16.5 ppm), equivalent to 40.66 mg/m3 (range 7.42-90.68 mg/m3); the GM concentration for female workers was 9.34 ppm, equivalent to 51.2 mg/m3; the GM concentration for male workers was 4.87 ppm, equivalent to 26.8 mg/m3. The GM concentration in the low/no-exposure group (n = 26) was 0.07 ppm (range from undetectable to 3.62 ppm), equivalent to 0.39 mg/m3 (range from undetectable to 19.9 mg/m3). The mean values of hemoglobin and hematocrit in the female workers in the high-exposure group were significantly decreased (12 exposed versus 27 controls). No such effects were observed in exposed male workers (Loh et al. 2003). In the follow-up surveys done 1 year (11 exposed versus 24 controls) or 3 years (11 exposed versus 19 controls) after implementation of a rubber glove-wearing policy and the improvement of the ventilation system at the workplace, there were no longer any significant differences in hematological parameters between the female workers in the high-exposure group and controls. The authors stated that 2-EEA was the main component of the screen cleaning solvent; these workers may be exposed to small amounts of methyl isobutyl ketone and toluene as well (Chen et al. 2007). Hematological effects associated with 2-EEA exposure were reported in another cross-sectional investigation among male shipyard painters with high-exposure experience (n = 30, mean years of work = 8.0 ± 5.4 [standard deviation (SD)]) or low-exposure experience (n = 27, mean years of work = 11.0 ± 0.7 [SD]), versus a control group (mainly office staff, n = 41, mean years of work = 11.0 ± 6.6 [SD]). The TWA concentration of 2-EEA in the high-exposure scenario was 3.03 ppm, equivalent to 16.65 mg/m3, with an exposure peak up to 18.27 ppm (100.4 mg/m3); the TWA concentration of 2-EEA in the low-exposure areas was 1.76 ppm (9.67 mg/m3), with an exposure peak up to 8.12 ppm (44.6 mg/m3). The occurrence of leucopenia was significantly increased in the exposure groups (6/57 = 11%). In the high exposure group, the mean white cell and granulocyte counts were significantly decreased, and the mean corpuscular volume was significantly increased, compared with the controls. The workers in the exposure groups were also exposed to other chemicals (toluene, xylene, butanol, ethyl benzene, isopropanol, ethanol, ethyl acetate, butyl acetate, methyl isobutyl ketone and nonane), but not to other glycol ethers (Kim et al. 1999). |