Screening Assessment for the Challenge
This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Archived
Phenol, 4-[[2-methoxy-4-[(4-nitrophenyl)azo]phenyl]azo]-
Disperse Orange 29
Chemical Abstracts Service Registry Number
19800-42-1
Environment Canada
Health Canada
September 2011
Table of Contents
- Synopsis
- Introduction
- Substance Identity
- Physical and Chemical Properties
- Sources
- Uses
- Releases to the Environment
- Environmental Fate
- Persistence and Bioaccumulation Potential
- Potential for Bioaccumulation
- Potential to Cause Ecological Harm
- Potential to Cause Harm to Human Health
- Conclusion
- References
- Appendix 1: Robust Study Summaries for Key Studies
- Appendix 2: PBT Model Inputs Summary Table
- Appendix 3: Estimated range of upper-bound intake of Disperse Orange 29 from Textiles by various age groups (µg/kg-bw per day) (Lower bound: estimated peak exposure to colourfast textile. Upper bound: conservative maximal exposure)
- Appendix 4: Exposure estimate for migration of dye from clothing
- Appendix 5: Summary of QSAR Results for Disperse Orange 29 and Potential Azo Cleavage Products
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a screening assessment on Phenol, 4-[[2-methoxy-4-[(4-nitrophenyl)azo]phenyl]azo]-, (herein referred to as Disperse Orange 29), Chemical Abstracts Service Registry Number[1] 19800-42-1.
This substance was identified as a high priority for screening assessment and included in the Challenge initiative under the Chemicals Management Plan because it was found to meet the ecological categorization criteria for persistence, bioaccumulation potential and inherent toxicity to non-human organisms and is believed to be in commerce in Canada. The substance, Disperse Orange 29, was not considered to be a high priority for assessment of potential risks to human health, based upon application of the simple exposure and hazard tools developed for categorization of substances on the Domestic Substances List.
Disperse Orange 29 is an organic substance that is used in Canada primarily as a textile dye. It is not naturally produced in the environment. Based on information provided in response to Canada’s Section 71 survey under CEPA 1999, Disperse Orange 29 was not reported to be manufactured in Canada in 2006 or 2005. One company reported importing 2000 kg of the substance into the country in 2006. In 2005, two companies reported importing Disperse Orange 29 into Canada with one company importing between 100–1000 kg and another company imported between 1001–100 000 kg, either in products or for use in the manufacturing of various coloured products.
Based on reported use patterns in Canada and certain assumptions, it is expected that the majority of the quantity of Disperse Orange 29 which is used in Canada, ultimately is deposited in waste disposal sites. A significant amount would however, be estimated to be released to sewers (14.8%). Disperse Orange 29 is not expected to be soluble in water or to be volatile, but is expected to adsorb on particles because of its hydrophobic nature. For these reasons, Disperse Orange 29 will likely be found in sediments if released directly to water, and, possibly to a lesser extent, in agricultural soil that has been amended with biosolids. Disperse Orange 29 is not expected to be significantly present in other media and is not expected to be subject to long-range atmospheric transport.
Based on the physical and chemical properties of Disperse Orange 29, it is expected to be persistent in soil, sediment, and water. However, new experimental data relating to the bioaccumulation potential of two relatively close structural analogues suggest that this dye has a low potential to accumulate in the lipid tissues of organisms. This substance, therefore, meets the persistence criteria but does not meet the bioaccumulation criteria as set out in the Persistence and Bioaccumulation Regulations. In addition, experimental toxicity data for chemical analogues suggest that Disperse Orange 29 does not cause acute harm to aquatic organisms exposed at low concentrations.
For this screening assessment, a conservative ecological exposure scenario was selected in which a single wastewater treatment plant was assumed to discharge the maximum quantity of Disperse Orange 29 based on the most recent survey. Additionally, since Disperse Orange 29 may be used in consumer products, a conservative consumer release scenario was developed based on an estimate of the quantity of this dye in Canadian commerce. The predicted environmental concentration in water was below the predicted no-effect concentration calculated for sensitive aquatic species.
Based on the information available, it is concluded that Disperse Orange 29 is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Exposure of the general population to Disperse Orange 29 from environmental media is expected to be negligible. The general population may be exposed to Disperse Orange 29 from its use as a dye in textiles and fabrics; however, dermal and oral exposure is expected to be low. No empirical health effects data were available for Disperse Orange 29 or for suitable analogues. Although the potential hazard of Disperse Orange 29 due to possible formation of component aromatic amines from azo cleavage is recognized, taking into consideration the expected low exposure to the general population, the potential risk to human health is considered to be low at current levels of exposure. It is concluded that Disperse Orange 29 is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
Based on the information available, it is concluded that Disperse Orange 29 does not meet any of the criteria set out in section 64 of CEPA 1999.
This substance will be considered for inclusion in theDomestic Substances List inventory update initiative. In addition and where relevant, research and monitoring will support verification of assumptions used during the screening assessment.
The Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or to human health.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherent toxicity to aquatic organisms (iT), and were believed to be in commerce in Canada; and/or
- met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE) and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in theCanada Gazette, Part I, on December 9, 2006 (Canada 2006a), that challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment, and to develop and benchmark best practices for the risk management and product stewardship of those substances identified as high priorities.
The substance, phenol, 4-[[2-methoxy-4-[(4-nitrophenyl)azo]phenyl]azo]-, (which will be referred to as Disperse Orange 29 for the purposes of this document) was identified as a high priority for assessment of ecological risk as it met the ecological categorization criteria for persistence, bioaccumulation potential and inherent toxicity to aquatic organisms, and was believed to be in commerce in Canada. The Challenge for this substance was published in the Canada Gazette on May 31, 2008 (Canada 2008a, 2008b). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, information was submitted pertaining to the properties, persistence, hazards and uses of Disperse Orange 29 and some of its formulation products.
Although Disperse Orange 29 was determined to be a high priority for assessment with respect to the environment, it did not meet the criteria for GPE or IPE and high hazard to human health based on classifications of other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
Screening assessments focus on information critical to determining whether a substance meets the criteria as set out in section 64 of CEPA 1999. Screening assessments examine scientific information and develop conclusions by incorporating a weight-of-evidence approach and precaution[2].
This screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including any information submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents, stakeholder research reports and from recent literature searches, up to July 2010. Key studies were critically evaluated; modelling results may have been used to reach conclusions. When available and relevant, information presented in a hazard assessment from other jurisdictions was considered. This screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents the most critical studies and lines of evidence pertinent to the conclusion.
This final screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. The ecological portion of this assessment has undergone external written peer review/consultation. Comments on the technical portions relevant to human health were received from scientific experts selected and directed by Toxicology Excellence for Risk Assessment (TERA) and included comments by Dr. Larry Claxton, Dr. Bernard Gadagbui, Dr. Pertti Hakkinen, Dr. Glenn Talaska, and Dr. Pam Williams. Additionally, the draft of this screening assessment was subject to a 60-day public comment period. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment Canada. Approaches used in the screening assessments under the Challenge have been reviewed by an independent Challenge Advisory Panel.
The critical information and considerations upon which this assessment is based are summarized below.
Substance Name
For the purposes of this document, Phenol, 4-[[2-methoxy-4-[(4-nitrophenyl)azo]phenyl]azo]- will be referred to as Disperse Orange 29, its Colour Index name (Colour Index Constitution Number: 26077; CII 2002-). Information on this substance’s identity is shown in Table 1 below.
Table 1. Substance identity for Disperse Orange 29
| Chemical Abstracts Service Registry Number (CAS RN) | 19800-42-1 |
| DSL name | Phenol, 4-[[2-methoxy-4-[(4-nitrophenyl)azo]phenyl]azo]- |
| Inventory names[1] | Phenol, 4-[[2-methoxy-4-[(4-nitrophenyl)azo]phenyl]azo]- (TSCA, AICS, PICCS, ASIA-PAC, NZIoC) 4-[[2-Methoxy-4-[(4-nitrophenyl)azo]phenyl]azo]phenol(EINECS) Disperse Orange 29 (ENCS) C.I. disperse orange 029 (ECL) C.I. Disperse Orange 29, (4-[[2-methoxy-4-[(4-nitrophenyl)azo]phenyl]azo]phenol)(PICCS) |
| Other names | 4-[[4-[(p-Nitrophenyl)azo]-2-methoxyphenyl]azo]phenol; C.I. Disperse Orange 29; Dianix Yellow Brown SE-R; Foron Yellow Brown SE-RL; Hisperse Orange C-GS;Intrasil Orange L 2R; Intrasil Orange L 2R200; Palanil Orange GL; Phenol, p-[[2-methoxy-4-[(p-nitrophenyl)azo]phenyl]azo]-; Resolin Yellow Brown 3GL; Samaron Yellow Brown HRSL; Sumikaron Orange SE-RBL; Synten Orange P-GRL; 4-({2-Methoxy-4-[(4-nitrophenyl)azo]phenyl}azo)phenol |
| Chemical group | Azo compounds |
| Chemical sub-group | Disazo compounds |
| Chemical formula | C19H15N5O4 |
| Chemical structure | 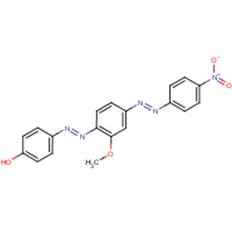 |
| SMILES[2] | N(=O)(=O)c(ccc(N=Nc(ccc(N=Nc(ccc(O)c1)c1)c2OC)c2)c3)c3 |
| Molecular mass | 377.36 g/mol |
[2] Simplified Molecular Line Input Entry System
Disperse Orange 29 is a disazo disperse dye. The two azo bonds (–N=N–) of this molecule are functional groups that produce colour (Danish EPA 1999). In addition to chemical structure, dyes may be classified according to their industrial applications and the methods by which they are applied to the substrate of interest (ETAD 1995). This classification system tends to reflect groupings based on physical and chemical behaviour. A brief discussion of the uses of this dye can be found later in this document under the Uses section.
Few experimental data on the physical and chemical properties of Disperse Orange 29 are available. At the Environment Canada-sponsored Quantitative Structure-Activity Relationship (QSAR) Workshop in 1999, invited modelling experts identified many structural classes of pigments and dyes as “difficult to model” using QSARs (Environment Canada 2000). The physical and chemical properties of many of the structural classes of pigments and dyes are not amenable to model prediction because they are considered “out of the model domain of applicability” (e.g., structural and/or property parameter domains). Therefore, to determine potential utility, the domains of applicability of QSAR models to pigments and dyes are reviewed on a case-by-case basis.
For this assessment it is considered that QSAR models used to predict physical and chemical properties that lack comparable substances to Disperse Orange 29 in their domain of applicability, may produce results with a high degree of uncertainty. Consequently, a "read-across" approach has been used to determine the approximate physical and chemical properties in Table 2. These properties were subsequently considered in evaluating various lines of evidence in this assessment. Table 2 shows some experimental and extrapolated physical and chemical properties of Disperse Orange 29.
An analogue is a chemical which is structurally similar to the substance under assessment and is therefore expected to have similar physical-chemical properties, behaviour in the environment and/or toxicity. Where there are experimental data for a given parameter for an analogue substance, these can be used directly or with adjustment as an estimate of that parameter value for the substance under assessment.
To find acceptable analogues, a review of data for several disperse azo dyes was performed (Anliker et al. 1981, Anliker and Moser 1987, Baughman and Perenich 1988, ETAD 1995, Brown 1992, Yen et al. 1989, Sijm et al. 1999). These compounds have structural similarities to Disperse Orange 29 but also share other important attributes that contribute to their suitability as analogues. This includes properties affecting their fate in the environment such as high molecular weights, generally >300 g/mol, similar cross sectional diameters (1.3-2.2 nm), solid particulate structures, decomposition at greater than 120° C, and “dispersibility” in water (i.e., not truly “soluble”). In addition, they have a negligible vapour pressure at room temperature and are stable under environmental conditions as they are designed to be so. Additional analogues have been chosen for the human health assessment, where data exist (see Potential to Cause Harm to Human Health section for further rationale and discussion).
Since some of the disazo dyes were studied under non-relevant environmental conditions (e.g., high temperature) or were not tested as pure compounds, and/or limited information on which to assess their reliability of certain studies, some supporting data for azo disperse dyes in general, are also presented in Table 2.
Table 2. Experimental physical and chemical properties for Disperse Orange 29 and its relevant analogues.
| Property | Type[1] | Value | Temperature (°C) | Reference |
|---|---|---|---|---|
| Physical state | Disperse Orange 29 | Powder | Study Submission 2008a | |
| Decomposition Point[2] (ºC) |
Disperse Orange 29 | 223 to 223.8 | ETAD 2005 | |
| Analogue Solvent Red 23 |
195 | PhysProp 2006 | ||
| Analogue Sudan IV (also known as Solvent Red 24) | 185 | MITI 1992 | ||
| Analogue Disperse Yellow 23 | 158 178 | Odabasoglu et al. 2003; Datyner 1978 | ||
| Analogue Disperse Orange 13 | 153 to 156.5 | Nishida et al. 1989 | ||
| Analogue Disperse Orange 30 | 126.9 to 128.5 | ETAD 2005 | ||
| Analogue Disperse Blue 79 | 157 | PhysProp 2006 | ||
| Analogue Disperse Blue 79:1 | 132 153 | Sijm et al. 1999; Yen et al. 1989 |
||
| Read-across for azo disperse dyes | 117 to 175 74 to 236 |
Anliker and Moser 1987; Baughman and Perenich 1988 |
||
| Boiling point[3] (ºC) |
Not applicable | |||
| Density (kg/m3) |
Not available | |||
| Vapour pressure (Pa) |
Analogue Disperse Blue 79 | 4.53 x 10-7 | Clariant 1996 | |
| Read-across for azo disperse dyes | 5.3 x 10-12 to 5.3 x 10-5 (4x10-14 to 4 x 10-7 mm Hg) |
25 | Baughman and Perenich 1988 | |
| Analogue Disperse Orange 13 | 0.18 to 0.42[4] | 191.5 to 211[4] | Nishida et al. 1989 | |
| Henry’s Law constant (Pa·m3/mol) |
Read-across for azo disperse dyes | 10-8 to 10-1 (10-13 to 10-6atm·m3/mol)[5] |
Baughman and Perenich 1988 | |
| Log Kow (Octanol-water partition coefficient) (dimensionless) |
Disperse Orange 29 | 4.6[6] | Study Submission 2008a | |
| Analogue Disperse Blue 79 | 4.1; 4.3[7] | Clariant 1996; Brown 1992 | ||
| Analogue Disperse Blue 79:1 | 4.4; 4.8 | Sijm et al. 1999; Yen et al. 1989 | ||
| Analogue Disperse Orange 30 | 4.2[8] | Brown 1992 | ||
| Read-across for azo disperse dyes | 1.8 to 5.1 | Baughman and Perenich 1988 | ||
| >2 to 5.1 | Anliker et al. 1981; Anliker and Moser 1987 | |||
| Log Koc (Organic carbon-water partition coefficient) (dimensionless) |
Read-across, calculated | 3.4 to 4.2[9] | Baughman and Perenich 1988 | |
| Water solubility (mg/L) |
Analogue Disperse Orange 29 | 42.9[6] | Study Submission 2008a | |
| Test substance poorly water soluble | Study Submission 2008a | |||
| 0.0037 | 25 | Baughman et al. 1996 (estimated) | ||
| Analogue Disperse Orange 13 | 0.345 | PhysProp 2006 | ||
| Analogue Disperse Yellow 23 | 0.00006 | 25 | Baughman and Perenich 1988 | |
| 0.00052 | Baughman et al. 1996 (estimated) | |||
| 15.7 to 34.8[4] | 130 | Braun 1991 | ||
| Analogue Disperse Yellow 68 | 16.6[4] | 125 | Prikryl et al. 1979 | |
| Analogue Disperse Blue 79 | 0.0054 | 25 | Clariant 1996 | |
| 0.027 | Brown 1992 | |||
| Analogue Disperse Blue 79:1 | 0.02 | Sijm et al. 1999 | ||
| 0.0052 | Yen et al. 1989 | |||
| 0.00063[4] | 100 to 125 | Baughman and Perenich 1988 | ||
| Analogue Disperse Orange 30 | 0.07[8] | Brown 1992 | ||
| Read-across for azo disperse dyes | <0.01 | 20 | Anliker and Moser 1987 | |
| Substantially water insoluble | ETAD 1995 | |||
| 1.2 x 10-5 to 35.5 (4x 10-11 to 1.8 x 10-4 mol/L) | Baughman and Perenich 1988 | |||
| n-octanol solubility (mg/L) | Analogue Disperse Orange 29 | 5086 | ETAD 2005 | |
| Analogue Disperse Orange 30 | 576 | ETAD 2005 | ||
| Analogue Disperse Blue 79:1 | 14 | Sijm et al. 1999 | ||
| Read-across for azo disperse dyes | 81 to 2100 | 20 | Anliker and Moser 1987 | |
| pKa (Acid dissociation constant) (dimensionless) |
Analogue Disperse Orange 29 | 9.03 | ACD/pKa DB 2005 | |
| Analogue Disperse Yellow 23 |
8.1 | Haag and Mill 1987 | ||
[2] The phrase “decomposition point” is used instead of melting point since disperse dyes are known to char at high temperatures (greater than 200°C) rather than melt (ETAD 1995).
[3] Boiling point is generally not applicable for disperse dyes. For powder dyes, charring or decomposition occurs at high temperatures instead of boiling. For liquids and pastes, boiling will only occur for the solvent component, while the unevaporated solid will decompose or char (ETAD 1995).
[4] Note that the water solubility tests in these studies were performed at very high temperatures and so are higher than expected at room temperature.
[5] Solubility values of five azo disperse dyes (Disperse Orange 3, Disperse Red 1, Solvent Yellow 2, Dis. A. 5, Dis. A. 7) at 25 and 80?C were used by Baughman and Perenich (1988) to calculate Henry’s Law constants for these dyes. These values are presented here as a range to illustrate the expected Henry’s Law constant for disazo dyes.
[6] The study indicates that the Disperse Orange 29 used in the test was a dispersion of 20% dye stuff that was tested (70% water and 10% Reax).
[7] The study indicates that the Disperse Blue 79 used in the test had a purity (as organic materials) of 76% and a dispersion of 20% dye stuff.
[8] The study indicates that the Disperse Orange 30 used in the test had a purity (as organic materials) of 73% and a dispersion of 20% dye stuff.
[9] Log Koc values are based on calculations by Baughman and Perenich (1988) using a range of measured solubility for commercial dyes and an assumed melting point of 200?C.
Because of the paucity of empirical data for Disperse Orange 29 and the error associated with model predictions for disperse dyes, selected empirical physical and chemical properties (Table 2), bioaccumulation data (Table 6a and 6b) and toxicity data from analogues (Table 7b) were used to support the weight of evidence and conclusions in this screening assessment. Specifically, data were obtained for 4 structurally similar disazo dyes (Disperse Yellow 23, Disperse Yellow 68, Disperse Orange 13, Solvent Red 23 and Sudan IV/Solvent Red 24) and 6 structurally similar monoazo dyes (Disperse Blue 79, Disperse Blue 79:1, Disperse Orange 30, Disperse Red 73, Disperse Orange 25 and Disperse Red 17). Substance identity information, as well as empirical data for analogues used in this report, are presented in Table 3a, while the molecular weights and cross-sectional diameters are presented in Table 3b.
Table 3a. Structural analogues for Disperse Orange 29 considered for ecological assessment
| Common name (CAS RN) |
DSL name | Structure | Major structural similarities and differences with Disperse Orange 29 | Available empirical data[1] |
|---|---|---|---|---|
| Disperse Orange 29 (19800-42-1) |
Phenol, 4-[[2-methoxy-4-[(4-nitrophenyl)azo]phenyl]azo]- |  |
Not applicable. Same substance. | Physical state, melting point, log Kow, water solubility, n-octanol solubility |
| Disperse Yellow 23 (6250-23-3) |
Phenol, 4-[[4-(phenylazo)phenyl]azo]- |  |
Similarity: Aromatic disazo compound with three rings and a terminal hydroxyl group Differences: Disperse Yellow 23 does not contain a terminal nitro group nor an ether group. |
Melting point, water solubility, pKa and aquatic toxicity |
| Disperse Yellow 68 (21811-64-3) |
Phenol, 4,4'-[1,4-phenylenebis(azo)]bis | 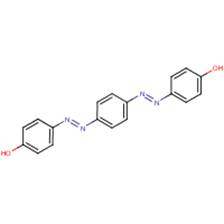 |
Similarity: Aromatic disazo compound with three rings and a terminal hydroxyl group Differences: Disperse Yellow 68 does not contain a terminal nitro group nor an ether group and has an additional hydroxyl group. |
Water solubility |
| Disperse Orange 13 (6253-10-7) |
Phenol, 4-[[4-(phenylazo)-1-naphthalenyl]azo]- | 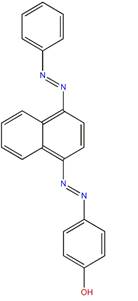 |
Similarity: Aromatic disazo compound with a terminal hydroxyl group Differences: Disperse Orange 13 contains a naphthalene ring and no terminal nitro group |
Melting point, water solubility, vapour pressure |
| Solvent Red 23 (85-86-9) |
2-Naphthalenol, 1-[[4-(phenylazo)phenyl]azo]- | 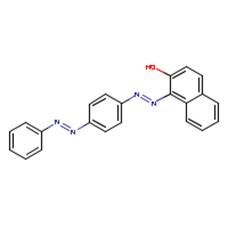 |
Similarity: Aromatic disazo compound with a hydroxyl group Differences: Solvent Red 23 contains a naphthalene ring and no terminal nitro group or ether group. |
Melting Point |
| Sudan IV (also known as Solvent Red 24) (85-83-6) |
2-Naphthalenol, 1-[[2-methyl-4-[(2-methylphenyl)azo]phenyl]azo]- | 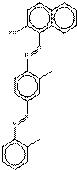 |
Similarity: Aromatic disazo compound with a naphthalene ring and a hydroxyl group attached. Same numbers of rings. Differences: Two additional methyl groups – one each attached to the single rings. |
Melting Point, toxicity, BCF. |
| Disperse Orange 25 (31482-56-1) |
3-(Ethyl(4-((4-nitro phenyl)azo)phenyl) amino)propanenitrile |  |
Similarity: Aromatic azo compound with a terminal nitro group Differences: No second azo group, Disperse Orange 25 contains a nitrile functional group and an amine group. |
Aquatic toxicity |
| Disperse Orange 30 (5261-31-4) |
Propanenitrile, 3-[[2-(acetyloxy)ethyl][4-[(2,6-dichloro-4-nitrophenyl) azo]phenyl]amino]- | 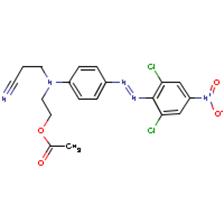 |
Similarity: Aromatic azo compound with a terminal nitro group Differences: No second azo group, Disperse Orange 30 contains nitrile and carboxylic functional groups as well as two chlorines. |
Bioaccumulation, aquatic toxicity, log Kow |
| Disperse Blue 79 (12239-34-8) |
Acetamide,N-[5-[bis[2-(acetyloxy)ethyl]amino]-2-[(2-bromo-4,6-dinitrophenyl)azo]-4-ethoxyphenyl]- |  |
Similarity: Aromatic azo compound with a terminal nitro group and an ether group. Differences: No second azo group, Disperse Blue 79 contains two carboxylic groups, an additional nitro functional group, an aniline with short two carbon chains and a bromine moiety. |
Melting point, vapour pressure, log Kow, water solubility, aquatic toxicity |
| Disperse Blue 79:1 (3618-72-2) |
Acetamide, N-[5-[bis[2-(acetyloxy)ethyl]amino]-2-[(2-bromo-4,6-dinitrophenyl)azo]-4-methoxyphenyl ]- |  |
Similarity: Aromatic azo compound with a terminal nitro group and an ether group. Differences: No second azo group, Disperse Blue 79:1 contains two carboxylic groups, an additional nitro functional group, and an aniline with two short carbon chains. |
Melting point, log Kow, water solubility, bioaccumulation, aquatic toxicity |
| Disperse Red 17 (3179-89-3) |
Ethanol, 2,2'-((3-methyl-4-(2-(4-nitrophenyl) diazenyl)phenyl) imino)bis- |  |
Similarity: Aromatic azo compound with a terminal nitro group. Differences: No second azo group, Disperse Red 17 contains an additional hydroxy group and aniline with two short carbon chains. |
Aquatic toxicity |
| Disperse Red 73 (16889-10-4) |
2-((4-((2-Cyanoethyl) ethylamino)phenyl)azo)-5-nitro benzonitrile |  |
Similarity: Aromatic azo compound with a terminal nitro group Differences: No second azo group, Disperse Red 73 contains two nitrile functional groups and no ether group as well as aniline with two short carbon chains. |
Aquatic toxicity |
Table 3b. Comparison of the molecular mass and cross-sectional diameter of the monoazo and disazo disperse dye structural analogues
| Substance | CAS RN | Common name | Molecular mass (g/mol) | Minimum–maximum Dmax(nm)[1] |
|---|---|---|---|---|
| Disazo dyes | 19800-42-1 | Disperse Orange 29 | 377 | 1.56–2.19 |
| 6250-23-3 | Disperse Yellow 23 | 302 | 1.50–2.07 | |
| 6253-10-7 | Disperse Orange 13 | 352 | 1.56–2.07 | |
| 85-86-9 | Solvent Red 23 | 352 | 1.50–2.03 | |
| 85-83-6 | Sudan IV | 380 | 1.50-2.05 | |
| 21811-64-3 | Disperse Yellow 68 | 318 | 2.09–2.14 | |
| Monoazo dye analogues | 12239-34-8 | Disperse Blue 79 | 639 | 1.69–2.05 |
| 3618-72-2 | Disperse Blue 79:1 | 625 | 1.43–2.03 | |
| 5261-31-4 | Disperse Orange 30 | 450 | 1.75–1.98 | |
| 16889-10-4 | Disperse Red 73 | 348 | 1.31–1.93 | |
| 31482-56-1 | Disperse Orange 25 | 323 | 1.37–1.95 | |
| 3179-89-3 | Disperse Red 17 | 344 | 1.41–1.86 |
It should be noted that there are several uncertainties associated with the use of physical and chemical, toxicological, and bioaccumulation data available for the substances. All these substances share the same chemical class--azo compounds (one subset has two azo bonds and another has one azo bond) and are used for similar industrial purposes (i.e., disperse dyes and 2 solvent dyes). However, there are differences between these substances associated with their unique functional groups (see Table 3a) and some of their molecular sizes. In spite of the fact that some of these monoazo dyes have larger molecular weights than the disazo dyes, their comparable physical state, melting points, water solubility, log Kow values and cross-sectional diameters (Tables 3b) provide a reasonable basis to conclude that the monoazo dyes will behave similarly to the disazo dyes in the environment and present an approximately equal bioavailability, and that their use as analogues for Disperse Orange 29 is therefore acceptable.
Disperse Orange 29 is not naturally produced in the environment.
Recent information was collected through industry surveys conducted for the years 2005 and 2006 under Canada Gazettenotices issued pursuant to section 71 of CEPA 1999 (Canada 2006b and 2008b). These notices required submission of data on the Canadian manufacture, import and use of Disperse Orange 29. In the notice for 2006, data were also required on use quantities of this dye. In association with the section 71 notices for 2005 and 2006, companies that did not meet the mandatory reporting requirements but had a business interest in Disperse Orange 29 were invited to identify themselves as stakeholders.
In 2006, one company reported importing 2000 kg of Disperse Orange 29 (Environment Canada 2008a). In 2005, a total of two companies reported importing Disperse Orange 29, with one company in the 100–1000 kg range and the other company in the 1001–100 000 kg range (Environment Canada 2006). No companies reported manufacturing Disperse Orange 29 above the 100 kg/year reporting threshold in either year.
In 2006, an importer of Disperse Orange 29 reported sales to thirteen companies, the highest amount sold being 261 kg in that year. In total, three companies reported a stakeholder interest in Disperse Orange 29 in 2005 and 2006 (Environment Canada 2006, 2008a).
During the development of the Domestic Substances List (DSL) the quantity of Disperse Orange 29 reported as being manufactured, imported or in commerce in 1986 was 42 000 kg from 8 companies (Environment Canada 1988).
Disperse Orange 29 has been identified as a low production volume chemical by the European Union (EU), indicating that production within the EU is estimated to be between 10 and 1000 tonnes per year (ESIS 2008). The national aggregate production volumes for Disperse Orange 29 in the United States were 10 000–500 000 pounds in each of the 1986, 1990, 1994, 1998 and 2002 reporting cycles under the US Environmental Protection Agency’s Inventory Update Reporting program (US EPA 1986–2002). Disperse Orange 29 was also used in Sweden from 1999 to 2006 and in Denmark from 2003 to 2006 (SPIN 2008).
Products containing Disperse Orange 29 may enter Canada even if they are not identified as such in the section 71 survey because they may be imported unknowingly in manufactured items, or in quantities below the 100 kg reporting threshold for the survey.
Information on uses for the 2005 and 2006 calendar years was gathered in response to the CEPA 1999 section 71 notices (Canada 2006b, 2008b). Companies importing Disperse Orange 29 in 2005 and 2006 reported that their business activities were textile and fabric finishing and chemical product preparation. The importer of Disperse Orange 29 in 2006 indicated that the substance was sold to 13 other companies (Environment Canada 2008a). According to additional information-gathering, these companies are in the textile industry and produce home fabrics, work wear, technical fabrics, children’s wear, webbing for seat belts and other uses, zippers, and other textile products. Textiles used by these companies include cotton, jersey, fleece, polyester and terry-cloth (Industry Canada 2008a).
During the DSL nomination (1984–1986), the DSL use codes for “Colourant - pigment/stain/dye/ink” “pigment, dye and printing ink”, “textile, primary manufacture” and “textile, product” were identified for Disperse Orange 29.
Review of the available scientific and technical information indicates that Disperse Orange 29 is used primarily in the textile industry (SPIN 2008) for dyeing polyester, acetate and nylon (CII 2002-). Application methods include thermosol dyeing and printing (QPC 2004). Textiles coloured with Disperse Orange 29 may be used in apparel goods, sports wear, work wear, and automotive and upholstery fabric (Farbchemie Braun KG 2008).
In Canada, Disperse Orange 29 is not listed as a permitted food additive under the Food and Drug Regulations nor has it been identified for use in food packaging applications (Health Canada 2007)
In Canada, Disperse Orange 29 is not listed in the Food and Drugs Regulations under section C.01.040.2 as a colouring agent permitted in drugs (Canada 1978). In addition, Disperse Orange 29 is not listed in the Drug Products Database, the Therapeutic Products Directorate's internal Non-Medicinal Ingredients Database, the Natural Health Products Ingredients Database nor the Licensed Natural Health Products Database as a medicinal or non-medicinal ingredient present in pharmaceutical drugs, natural health products or veterinary drugs (DPD 2010; 2008 email from Therapeutic Products Directorate, Health Canada, to Risk Management Bureau, Health Canada, unreferenced; NHPID 2010; LNHPD 2010).
Mass Flow
A method has been developed by Environment Canada to estimate a substance’s losses during different stages of its life cycle, including its fate within a finished product or article (Environment Canada 2008b). This method, referred to as Mass Flow, consists of a life cycle analysis and a spreadsheet tool (Mass Flow Tool or MFT) that integrates information on the manufacturing, importation and use data available for the substance. Starting with an identified mass of the substance, each life cycle stage is subsequently evaluated until no mass remains. Relevant factors are considered, uncertainties recognized and assumptions may be made during each stage, depending on information available. The estimated losses represent the complete mass balance of the substance over the life cycle of the substance and include releases to wastewater and other receiving compartments (land, air), chemical transformation, transfer to recycling activities and transfer to waste disposal sites (landfill, incineration). However, unless specific information on the rate or potential for release of the substance from landfills and incinerators is available, the method does not quantitatively account for releases to the environment from disposal. Ultimately, the estimated losses provide a first tier in the exposure analysis of a substance and help to estimate environmental releases and focus exposure characterization later in the assessment.
In general, releases of a substance to the environment depend upon various losses from its manufacture, industrial use, and/or consumer/commercial use. These losses can be grouped into seven types: (1) discharge to wastewater; (2) emission to air; (3) loss to land; (4) chemical transformation; (5) disposal to landfill; (6) loss to incineration; and (7) disposal through recycling (i.e., recycling is deemed a loss and not considered further). They are estimated using regulatory survey data, industry data and data published by different organizations. The discharge to wastewater refers to raw wastewater prior to any treatment, whether it be on-site industrial wastewater treatment or off-site municipal sewage treatment. In a similar manner, the loss via chemical transformation refers to changes in a substance's identity that may occur within the manufacture, industrial use, and consumer/commercial use stages, but excludes those during waste management operations such as incineration and wastewater treatment. The loss to land includes unintentional transfer or leakage to soil or paved/unpaved surfaces during the substance’s use and service life (e.g., from the use of agricultural machinery or automobiles). The loss to land, however, does not include transfers subsequent to a substance’s use and service life (e.g., land application of biosolids and atmospheric deposition).
The losses estimated for Disperse Orange 29 over its lifecycle (based on conservative assumptions) are presented in Table 4 (Environment Canada 2010a). Disperse Orange 29 is not manufactured in Canada above reporting thresholds, so estimated losses are based on import quantities reported in 2006.
Table 4. Estimated Losses of Disperse Orange 29 during Its Lifecycle
| Type of Loss | Proportion (%) | Pertinent Lifecycle Stages |
|---|---|---|
| Wastewater | 14.8 | Industrial use, consumer/commercial use |
| Air emission | 0 | |
| Land | 0 | |
| Chemical transformation | 0 | |
| Landfill | 82.6 | Industrial use, consumer/commercial use |
| Incineration | 2.6 | Industrial use, consumer/commercial use |
| Recycling | 0 | |
| Total | 100 |
Because Disperse Orange 29 is primarily used in the textile industry, the Mass Flow Tool used in this assessment was populated with inputs specific to textile dyes.
Disperse Orange 29 is estimated to be released to wastewater at 14.8% during the industrial use and consumer/commercial use stages. Assumptions made during this step include losses during container handling and dyeing operations. The majority of Disperse Orange 29 in textiles is estimated to be lost through waste disposal of manufactured items (incineration 2.6% and landfill 82.6%). It is assumed that losses to recycling of textiles are negligible.
The above loss estimates indicate that Disperse Orange 29 used in textiles has a potential for release to the environment. In general, wastewater is a common point-of-entry of a substance to water through wastewater treatment facilities and a point-of-entry to soil through the subsequent waste management of sludge. Emissions to air can result in atmospheric deposition to soil and water. When a substance is unintentionally transferred to land, it may be washed into the sewer or transferred by wind or rain to nearby soil. As a result of recycling activities, a substance could find its way to water or soil, depending upon the operational characteristics of facilities. Finally, landfills have the potential to leach substances into groundwater or there may be releases of substances to the atmosphere.
Based on Statistics Canada information and an analysis by Industry Canada (2008b), it is proposed that textile dyes, such as Disperse Orange 29, assessed in this report, may be imported in manufactured articles. Following this proposal, a ratio of the amount of textiles manufactured in Canada relative to the amount of imported textiles of 30:70 has been used to estimate the amount of dye imported in finished textiles (Industry Canada 2008b, Environment Canada 2008c). This import quantity was included in the Mass Flow Tool calculations for Disperse Orange 29 used in the textile sector.
The calculations assume that there is no release of the substance from landfill sites, although long-term releases may be possible. A small fraction of solid waste is incinerated, which is expected to result in chemical transformation of the substance. Based largely on information contained in OECD emission scenario documents for processing and uses associated with this type of substance (OECD 2004), it is estimated that 14.8% of Disperse Orange 29 used in textile dyes may be released to sewers (5.4% from industrial processing and 9.4% from consumer uses).
Based on the above, waste water effluent is the medium potentially receiving the greatest proportion of Disperse Orange 29 during product use. It is anticipated that the majority of the substance, whether it is bound in manufactured textiles or bound to sewage sludge from down-the-drain releases to sewage treatment plants (STPs), will be sent in solid form (textiles) or entrained in sludge to landfills. In addition to being sent to landfill, some of the biosolids from wastewater treatment facilities can be applied to land as a fertilizer or soil condition for uses in agriculture, forestry and reclamation and a small percentage may be incinerated.
As indicated by the results of the Mass Flow Tool (Table 4), Disperse Orange 29 is expected to be released to wastewater effluents from industrial processing and downthe-drain uses. The high log Kow value (4.6) and high read-across log Koc (3.4 to 4.2) values (see Table 2) indicate that this substance may have affinity for solids. However, the log Koc values are calculated, not strictly experimental (see footnote 8 below Table 2) and the adsorption potential of disperse particulate dye structures is generally not well understood; therefore the degree to which this particular behaviour applies is uncertain.
According to aerobic biodegradation models, Disperse Orange 29 is not expected to biodegrade quickly (see Table 5 below). This substance may inadvertently be applied to land in Canada as a component of biosolids, which is commonly used for soil enrichment. Moreover, it may also be released from coloured textiles deposited in landfills.
Given an experimental pKa of 8.1 for the analogue Disperse Yellow 23 and an estimated pKa value of 9.03 for Disperse Orange 29 (Table 2), this chemical is expected to behave as a weak acid and be partially ionized in water at the higher end of environmentally relevant pHs (8–9). However, given the expected low water solubility of Disperse Orange 29 (Table 2) and its particulate state, it is unlikely that ionization at elevated pH will have significant impact on the partitioning or water solubility of this substance. Instead, when released into water, this substance is expected to be mostly present as a particulate solid or adsorbed to suspended particles and eventually sink to surface bed sediments where Disperse Orange 29 is expected to remain in a relatively biologically unavailable form. It has been stated generally that, due to the recalcitrant nature of azo dyes in aerobic environments, they eventually end up in anaerobic sediments, shallow aquifers and in groundwater (Razo-Flores et al. 1997). However, since Disperse Orange 29 has low water solubility and a relatively high Koc it is not likely to leach from sediments and soils.
The rate of volatilization from the surface of water is proportional to the Henry’s law constant (Baughman and Perenich 1988). Baughman and Perenich (1988) also state that volatilization from aquatic systems will not be an important loss process for disperse dyes, which agrees with the low to negligible read-across Henry’s Law constant value (10-8 to 10-1Pa•m3/mol; Table 2). Transport in air due to the loss of this substance from moist and dry soil surfaces is not likely to be important for this substance as indicated by very low read-across vapour pressures (5.33 x 10-12 to 5.33 x 10-5 Pa; Table 2). These data are consistent with the physical state (solid particle) of the disazo dyes which makes them unlikely candidates for volatilization. The experimental vapour pressure for Disperse Orange 13 is not a useful indicator of volatilization for Disperse Orange 29 since it was measured at an elevated temperature.
Environmental Persistence
Dyes must have a high degree of chemical and photolytic stability in order to be useful, so most are generally considered non-degradable under environmentally relevant aerobic conditions (Danish EPA 1999; ETAD 1995). Studies applying commonly accepted screening tests (e.g., OECD guidelines) for ready and inherent biodegradability have confirmed this point (ETAD 1992; Pagga and Brown 1986). Abiotic degradation, including photolysis and hydrolysis, is not thought to play a significant role in the environmental fate of azo dyes (Danish EPA 1999), although one study showed strongly accelerated photo decomposition of azo dyes in the presence of natural humic materials (Brown and Anliker 1988).
Biotic degradation of azo dyes may take place relatively rapidly under anaerobic or reducing conditions (Baughman and Weber 1994; Danish EPA 1999; ETAD 1995; Isik and Sponza 2004; Yen et al. 1991). Permeability of the bacterial cell wall has been found to be the rate-limiting step in the reduction process (Danish EPA 1999). Azo dyes have a high tendency to cleave at the azo bond with the formation of aromatic amines (Danish EPA 1999; Hunger 2005). The carcinogenic potential of aromatic amines varies considerably with molecular structure, with carcinogenic breakdown products being associated with the moieties of benzidine, aniline, toluene or naphthalene. However, the formation of such metabolites in deep anoxic sediments would typically not result in exposure to aquatic organisms. Total mineralization or further degradation of these metabolites could take place if they are transferred (e.g., by sediment resuspension) to aerobic environments (Danish EPA 1999; Isik and Sponza 2004). Aromatic amines may also be present as impurities in commercially available azo dyes, although the metabolic cleavage of azo dyes is the main source of these compounds (Danish EPA 1999).
A bioelimination study was submitted for the analogue Disperse Yellow 23 indicating that it undergoes 51% degradation in 14 days (Study Submission 2008b). However, due to lack of experimental details, this study was considered to have low reliability and its experimental result could not be used to support the persistence assessment of Disperse Orange 29 (see Robust Study Summary in Appendix 1). Other than this study, no experimental or read-across degradation data for Disperse Orange 29 or analogues have been identified. No environmental monitoring data relating to the persistence of these dyes in the Canadian environment (air, water, soil, sediment) have been identified.
Given the expected release of Disperse Orange 29 as a dye into wastewater, persistence was primarily examined using predictive QSAR models for aerobic biodegradation in water. These models are considered acceptable for use in this situation as they are based on chemical structure and the disazo structure is represented in the training sets of all the BIOWIN models used, thereby increasing the reliability of the predictions (Environment Canada 2007). The following analysis applies primarily to the portion of this substance that is present in the environment in the dissolved form, recognizing that a significant proportion would also likely exist in dispersed form as solid particles. Disperse Orange 29 and its analogues do not contain functional groups expected to undergo hydrolysis in aerobic environments (dyes are designed to be stable in aqueous conditions). Table 5 summarizes the results of available QSAR models for aerobic biodegradation in water.
Table 5. Modelled data for degradation of Disperse Orange 29
| Fate Process | Model and model basis |
Model Result and Prediction | Extrapolated Half-life (days) |
|---|---|---|---|
| Primary biodegradation | |||
| Biodegradation (aerobic) | BIOWIN 2008[1] Sub-model 4: Expert Survey (qualitative results) |
3.2[2] “biodegrades slowly” |
≥182 |
| Ultimate biodegradation | |||
| Biodegradation (aerobic) | BIOWIN 2008[1] Sub-model 3: Expert Survey (qualitative results) |
1.6[2] “biodegrades very slowly” |
≥182 |
| Biodegradation (aerobic) | BIOWIN 2008[1] Sub-model 5: MITI linear probability |
-0.34[3] “biodegrades very slowly” |
≥182 |
| Biodegradation (aerobic) | BIOWIN 2008[2] Sub-model 6: MITI non-linear probability |
0[3] “biodegrades slowly” |
≥182 |
| Biodegradation (aerobic) | TOPKAT 2004 Probability |
n/a[4] “biodegrades slowly |
≥182 |
| Biodegradation (aerobic) | CATABOL c2004-2008 % BOD[5] (biological oxygen demand) |
% BOD = 0 “biodegrades very slowly” |
≥182 |
[2] Output is a numerical score from 0 to 5.
[3] Output is a probability score.
[4] n/a: not available (out of model domain)
[5] BOD: Biological oxygen demand
The results from Table 5 reveal that all ultimate biodegradation models (BIOWIN 3, 5, 6, CATABOL and TOPKAT) suggest that Disperse Orange 29 biodegrades slowly aerobically in water. However, output from the TOPKAT model was flagged as unreliable for this type of structure. Both BIOWIN 5 and 6 probability results are much less than 0.3, the cut-off suggested by Aronson et al. (2006) to identify substances as having a half-life >60 days (based on the MITI probability models). Furthermore, both of the other ultimate degradation models, BIOWIN 3 and CATABOL, predict that Disperse Orange 29 will be persistent in water.
When the results of the probability and the other ultimate degradation models are considered, there is model consensus suggesting that the ultimate biodegradation half-life in water is =182 days. This finding is consistent with what would be expected for these chemical structures (i.e., few degradable functional groups, sparingly soluble solid particle).
Using an extrapolation ratio of 1:1:4 for a water:soil:sediment biodegradation half-life (Boethling et al. 1995), the ultimate degradation half-life in aerobic soil is =182 days and the half-life in aerobic sediments is =365 days. This suggests that Disperse Orange 29 is expected to be persistent in soil and sediment.
Based on modelled ultimate degradation data (Table 5) and expert judgment (Danish EPA 1999, ETAD 1995), Disperse Orange 29 meets the persistence criteria in water, soil and sediment (half-lives in aerobic soil and water =182 days and half-life in aerobic sediment =365 days) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
No experimental bioaccumulation data are available for Disperse Orange 29. Since bioaccumulation models are known to predict poorly for pigments and dyes, predictions from such models are considered unreliable for disazo dyes. As a result, bioaccumulation modelling has not been used to evaluate the bioaccumulation status of these substances.
In the absence of experimental and modelled data, empirical bioconcentration (BCF) and bioaccumulation (BAF) factors for structural analogues were used to estimate the bioaccumulation potential of Disperse Orange 29. To that end, bioconcentration studies for relatively close structural analogues Sudan IV (MITI 1992) and Disperse Orange 30 (Shen and Hu 2008), suggest that Solvent Red 23 is unlikely to accumulate in fish.
The test on Solvent Red 23 (Table 6a) performed by the Japanese Ministry of International Trade and Industry (MITI) using common carp resulted in a series of low bioconcentration factors less than 11 L/kg.
Table 6a. Empirical data for bioaccumulation and bioconcentration of Sudan IV, an analogue of Solvent Red 23
| Test Organism | Experimental Concentration (mg/L) and/or Exposure Source | Endpoint (BCF, L/kg) | Reference |
|---|---|---|---|
| Common carp (Cyprinus carpio) |
0.35 | <0.29-2.9 | MITI 1992 |
| Common carp (Cyprinus carpio) |
0.035 | <2.9-11 | MITI 1992 |
The bioconcentration test by Shen and Hu (2008) was performed according to OECD Guidelines (OECD 1996). This test was performed according to OECD Guidelines (OECD 1996). The bioconcentration of Disperse Orange 30 in zebra fish (Brachydanio rerio) was determined in a 28-day semi-static test with test medium renewal every two days. An exposure test at a nominal concentration of 20 mg/L (mean measured concentration 0.028 ~ 0.28 mg/L) was performed in accordance with the result of the fish acute toxicity test to check the bioconcentration potential of the test substance. Samples from both test solutions and test organisms were taken daily from Day 26 to Day 28 during the 28-day exposure test period. Samples were prepared by extracting the lipid component from the test fish. The measured concentration of test substance, fish lipid content and BCF calculation are reported in Table 6b.
Table 6b. Measured concentrations, fish lipid content and BCF calculation for analogue Disperse Orange 30 from Shen and Hu (2008)
| Treatments (20 mg/L) | Sampling Time | ||
|---|---|---|---|
| Day 26 | Day 27 | Day 28 | |
| Measured concentration of the test substance in extracted solutions (mg/L) | <0.028 | <0.028 | <0.028 |
| Content of the test substance in the fish lipids (mg) | <1.68 | <1.68 | <1.68 |
| Fish total weight (g) | 2.07 | 2.13 | 2.53 |
| Concentration of the test substance in the fish Cf (mg/kg) | <0.81 | <0.79 | <0.66 |
| Measured concentration of the test substance in the water Cw (mg/L) | 0.028 ~ 0.28 | 0.028 ~ 0.28 | 0.028 ~ 0.28 |
| Fish lipid content (%) | 0.81 | 0.57 | 1.25 |
| BCF | <100 | <100 | <100 |
| Average BCF | <100 | ||
The Shen and Hu (2008) study has been reviewed and considered acceptable (see Appendix 1). The very low level of detection in fish extracts (<0.028 mg/L) suggests a limited solubility in lipids and/or limited potential to partition into fish tissue from aqueous systems. However, there is some uncertainty associated with limit-bounded values in any study because the actual value is not known. But given the structure and likely behavior of disperse dyes in aqueous systems, a low BCF result is expected. Most disperse dyes, as their name suggests, exist as fine dispersible particles with limited truly soluble fractions. Solubility, however, can be increased by adding polar functional groups to the molecule. While Disperse Orange 29 contains some of these solubilizing functional groups (phenol groups), relevant experimental solubility values (i.e., 0.00006–0.345 mg/L) are relatively low – being below or comparable to (less than one order of magnitude above) the water solubility for Disperse Orange 30 (i.e., 0.07 mg/L). As a result, it is expected that Disperse Orange 29 would have a bioavailability and bioconcentration potential that is similar to or lower than that of Disperse Orange 30.
While the above study serves as primary evidence to indicate the lack of bioaccumulation potential for Disperse Orange 29, other research corroborates this conclusion. Anliker et al. (1981) reported experimental fish bioaccumulation values for 18 monoazo disperse dyes, performed according to test methods specified by the Japanese Ministry of International Trade and Industry (MITI). Expressed on the basis of wet body weight of the fishes, these log bioaccumulation factors ranged from 0.00 to 1.76 (Anliker et al. 1981). A lack of reporting of chemical registry numbers and chemical structures limited the utility of this study for read-across purposes to Disperse Orange 29. However, follow-up studies, which provided the chemical structures for the disperse dyes tested, confirmed low bioaccumulation potential for ten nitro-substituted azo dyes, with reported log bioaccumulation factors ranging from 0.3 to 1.76 (Anliker and Moser 1987; Anliker et al. 1988). Studies available from MITI also support low bioaccumulation potential for disperse azo dyes. Reported BCFs for 3 disperse azo dyes (CAS Nos. 40690-89-9, 6196852-3 and 71767-67-4) tested at a concentration of 0.01 mg/L were in the range of <0.3 to 47 L/kg (MITI 1992). An accumulation study by Brown (1987) also showed that none of the twelve disperse dyes tested accumulated during an eight week study with carp.
A high log Kow value for Disperse Orange 29 and read-across values for other related azo analogues (Table 2) is the only line of evidence that suggests the substance may have a high potential for bioaccumulation. In spite of the high Kowvalues for Disperse Orange 29 and the other azo structural analogues, evidence for bioaccumulation of disperse azo dyes is lacking (Anliker et al. 1981; Anliker and Moser 1987; Anliker et al. 1988; MITI 1992). Authors who have measured high log Kows and concomitant low bioaccumulation factors for disperse azo dyes suggest that the low accumulation factors may be due to their low absolute fat solubility (Brown 1987) or relatively high molecular weight, which may make transport across fish membranes difficult (Anliker et al. 1981; Anliker and Moser 1987). It is also likely that the lack of bioavailability and limited capacity to partition under BCF test conditions limits accumulation in fish lipids.
Recent investigations relating fish BCF data and molecular size parameters (Dimitrov et al. 2002, 2005) suggest that the probability of a molecule crossing cell membranes as a result of passive diffusion declines significantly with increasing maximum diameter (Dmax). The probability of passive diffusion decreases appreciably when the maximum diameter is greater than ~1.5 nm and much more so for molecules having a maximum diameter of greater than 1.7 nm. Sakuratani et al. (2008) have also investigated the effect of cross-sectional diameter on passive diffusion in a BCF test set of about 1200 new and existing chemicals. They observed that substances that do not have a very high bioconcentration potential (BCF <5000) often have a Dmax of >2.0 nm and an effective diameter (Deff) >1.1 nm.
Disperse Orange 29 and its closest analogues (the disazo dyes) have molecular weights ranging between 302 and 377 g/mol (see Table 3b) and their molecular structures are relatively uncomplicated; both these characteristics indicate a bioaccumulation capability of these substances if molecular weight is used as the only indicator. In addition, Arnot et al. (2010) points out that there are no clear relationships for establishing strict molecular size cutoffs for assessing bioaccumulation potential. However, the report does not dispute the notion that a reduction in uptake rate can be associated with increasing cross-sectional diameter as demonstrated by Dimitrov et al. (2002, 2005). The maximum diameter of Disperse Orange 29, its closest analogues and their conformers ranges from 1.5 to 2.2 nm (BBM 2008), suggesting that a potential for a significantly reduced uptake rate from water and reduced in vivo bioavailability exists with these dyes.
However, as Arnot et al. (2010) have noted, there are uncertainties associated with the thresholds proposed by Dimitrov et al. (2002, 2005) and Sakuratani et al. (2008) since the BCF studies used to derive them were not critically evaluated. Arnot et al. (2010) point out that molecular size influences solubility and diffusivity in water and organic phases (membranes), and larger molecules may have slower uptake rates. However, these same kinetic constraints apply to diffusive routes of chemical elimination (i.e., slow in = slow out). Thus, significant bioaccumulation potential may remain for substances that are subject to slow absorption processes, if they are slowly biotransformed or slowly eliminated by other processes. Consequently, when evaluating bioaccumulation potential, molecular size information should be considered with care and used together with other relevant lines of evidence in a weight-of-evidence approach.
Based on a lack of accumulation observed in bioconcentration tests with Sudan IV, Disperse Orange 30 and other related disperse azo dyes that showed similar results, as well as data showing large cross-sectional diameters for Disperse Orange 29 and its analogues that likely limit their partitioning behavior, Disperse Orange 29 is expected to have a low potential for bioaccumulation. Therefore, considering the available evidence, Disperse Orange 29 does not meet the bioaccumulation criteria (BCF or BAF >5000) as set out in thePersistence and Bioaccumulation Regulations (Canada 2000).
Ecological Effects Assessment
A - In the Aquatic Compartment
Toxicity studies were submitted for Disperse Orange 29 and the analogue Disperse Yellow 23 (Study Submission 2008a,b) to support the hazard assessments for these substances. According to these studies, Disperse Orange 29 has a 72hour EC50 of 6 mg/L in algae (Scenedesmus subspicatus) and a 48-hour EC50 in Daphnia magna of 70 mg/L (Table 7a). In addition, Disperse Yellow 23 has a 48-hour LC50 of >1000 mg/L in rainbow trout (Oncorhynchus mykiss) and Disperse Orange 29 has a 96-hour LC50 of 480 mg/L in zebra fish (Brachydanio rerio) (Table 7b). Due to lack of details, these studies were deemed of uncertain reliability (See Appendix 1). However, these data were considered usable in this screening assessment in a weight-of-evidence context.
Table 7a. Empirical data aquatic toxicity for Disperse Orange 29
| Substance | Test organism | Type of test | Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|---|
| Disperse Orange 29 | Scenedesmus subspicatus | Chronic (72 hours) |
EC50[1] | 6 | Study Submission 2008a[3] |
| Daphnia magna | Acute (48 hours) |
EC50 | 70 | ||
| Brachydanio rerio | Acute (96 hours) |
LC50[2] | 480 |
[2] LC50 – The concentration of a substance that is estimated to be lethal to 50% of the test organisms.
[3] The study reports that a 20% dispersive dye stuff was used for these toxicity tests.
Additional ecotoxicological data have been located for several analogues of Disperse Orange 29. A study submitted on behalf of ETAD provides acute ecotoxicity data in fish, invertebrates, algae and bacteria for 5 nitro-substituted azo disperse dyes (Brown 1992). Acute zebra fish, Daphnia magna and Scenedesmus subspicatus toxicity for the 5 analogues ranged from 17 to 710 mg/L, from 4.5 to 110 mg/L and from 6.7 to 54 mg/L, respectively (Table 7b). In addition, all bacteria tests had an IC50exceeding 100 mg/L. The experimental details for the dyes tested were not provided, which greatly limited evaluation of these studies (Brown 1992). However, these data were considered usable and are included in this screening assessment as part of the weight of evidence.
Another acute fish toxicity study was submitted for the analogue Disperse Blue 79 (BASF 1990). According to the study, Disperse Blue 79 has a 96-hour LC50 in golden orfe between 100 and 220 mg/L (Table 7b). However, due to lack of details, this study was also considered of uncertain reliability (Appendix 1). A fish toxicity study on analogue Sudan IV of >100 mg/L (MITI 1992) was also included in Table 7b to contribute to the weight of evidence but was not preferred as a critical value since the endpoint is not bounded.
Ecotoxicological data for another disperse azo dye were received through the New Substances Notification Regulations(Environment Canada 1995). An acute fish toxicity study submitted to meet notification requirements revealed this substance has a 96-hour LC50 of 505 mg/L in rainbow trout (Table 7b). The test was conducted according to OECD guideline No. 203. The Material Safety Data Sheet provided as part of this notification also contained information on bacterial toxic effects. The results indicate an activated sludge respiration inhibition EC50of >1000 mg/L. Based on the available ecotoxicity information, the new substance was considered to be of low concern for toxic effects to aquatic organisms. Reliability of the study was assessed using a robust study summary and considered as satisfactory (Appendix 1).
Lastly, a chronic study submitted for the analogue Disperse Blue 79:1, revealed its 122 day noobserved-effect concentration (NOEC) in rainbow trout to be greater than 0.0048 mg/L (Table 7b). Reliability of this study was assessed as high (Appendix 1). However, this value was not used to calculate the predicted no-effect concentration because the value is an unbounded result (i.e., no certainty as to the threshold for effects).
When considering all structural analogue toxicity information in concert with the toxicity values for Disperse Yellow 23 and Disperse Orange 29, these data suggest that Disperse Orange 29 is not highly hazardous to aquatic organisms (i.e., acute LC50 values are >1 mg/L).
Table 7b. Empirical aquatic toxicity data for analogues of Disperse Orange 29
| Common name or (CAS RN) | Test organism | Severity (Duration) |
Endpoint | Value (mg/L) | Reference |
|---|---|---|---|---|---|
| Disperse Yellow 23 (6250-23-3) |
Oncorhynchus mykiss | Acute (48 hours) |
LC50[1] | >1000 | Study Submission 2008b |
| Sudan IV (85-83-6) |
Oryzias latipes | Acute (48 hours) |
LC50[1] | >100 | MITI 1992 |
| Disperse Blue 79[2] (12239-34-8) |
Golden orfe | Acute (96 hours) |
LC50[1] | 100< LC50 <220 | BASF 1990 |
| Zebra fish | Acute (96 hours) |
LC50 | 340 | Brown 1992 | |
| Daphnia magna | Acute (48 hours) |
EC50[3] | 4.5[*] | ||
| Scenedesmus subspicatus | Chronic – growth (72 hours) |
EC50 | 9.5 | ||
| Bacteria | Not available | IC50[4] | >100 | ||
| Disperse Red 73[5] (16889-10-4) |
Zebra fish | Acute (96 hours) |
LC50 | 17 | |
| Daphnia magna | Acute (48 hours) |
EC50 | 23 | ||
| Scenedesmus subspicatus | Chronic – growth (72 hours) |
EC50 | >10 | ||
| Bacteria | Not available | IC50 | >100 | ||
| Disperse Orange 30[6] (5261-31-4) |
Zebra fish | Acute (96 hours) |
LC50 | 710 | |
| Daphnia magna | Acute (48 hours) |
EC50 | 5.8 | ||
| Scenedesmus subspicatus | Chronic – growth (72 hours) |
EC50 | 6.7 | ||
| Bacteria | Not available | IC50 | >100 | ||
| Disperse Orange 25[7] (31482-56-1) |
Zebra fish | Acute (96 hours) |
IC50 | 268 | |
| Daphnia magna | Acute (48 hours) |
LC50 | 110 | ||
| Scenedesmus subspicatus | Chronic – growth (72 hours) |
EC50 | 54 | ||
| Bacteria | Not available | EC50 | >100 | ||
| Disperse Red 17[8] (3179-89-3) |
Zebra fish | Acute (96 hours) |
LC50 | 103 | |
| Daphnia magna | Acute (48 hours) |
EC50 | 98 | ||
| Scenedesmus subspicatus | Chronic – growth (72 hours) |
EC50 | 7 | ||
| Bacteria | Not available | IC50 | >100 | ||
| Analogue azo disperse dye (CAS# confidential) |
Rainbow trout | Acute (96 hours) |
LC50 | 505 | Environment Canada 1995 |
| Disperse Blue 79:1 (3618-72-2) |
Rainbow trout | Chronic (122 days) |
NOEC[9] | >0.0048 | Cohle and Mihalik 1991 |
[2] The study indicates that the Disperse Blue 79 used in the test had a purity (as organic materials) of 76% and a dispersion of 20% dye stuff.
[3] EC50 - The concentration of a substance that is estimated to cause some toxic sublethal effect on 50% of the test organisms.
[4] IC50 – The inhibiting concentration for a specified percent effect. A point estimate of the concentration of a test substance that causes a 50% reduction in a quantitative biological measurement such as growth rate.
[5] The study indicates that the Disperse Red 73 used in the test had a purity of 96.6%.
[6] The study indicates that the Disperse Orange 30 used in the test had a purity (as organic materials) of 73% and a dispersion of 20% dye stuff.
[7] The study indicates that the Disperse Orange 25 used in the test had a purity of 94%.
[8] The study indicates that the Disperse Red 17 used in the test had a purity of 98.8%.
[9] NOEC – The no-observed-effect concentration is the highest concentration in a toxicity test not causing a statistically significant effect in comparison to the controls.
[*] The critical toxicity value used to derive a predicted no-effect concentration.
In general, due to their poor solubility (i.e., <1 mg/L), disperse dyes are expected to have a low acute ecological impact (Hunger 2003). The results of empirical toxicity studies with Disperse Orange 29 and its analogues are consistent with this expectation, indicating fish LC50 values in the 17505 mg/L range, with Daphnia being the most sensitive organism tested (EC50/LC50s from 4.5 to 110 mg/L). The critical value chosen to derive a probable no effect concentration was deemed to be the Daphnia magna EC50 value of 4.5 mg/L (Brown 1992).
The interpretation of results from these tests is complicated by the fact that some of the reported effect values (i.e., EC50 and LC50s) are greater than the reported solubility of the substances tested. In effect, some of the concentrations reported in Tables 7a and 7b may represent the loading levels of the test substance. Thus, a subset of the actual LC50 or EC50 values may be lower than the level reported, as the actual concentration dissolved in water that may cause an effect is not known. In other cases (see footnotes of Table 7b), substances tested were in formulations and so were not 100% pure. Therefore, other chemicals in the formulation may have increased solubility and also contributed to the total toxicity. Despite uncertainties in regards to water solubility and the purity of some analogues, the experimental and analogue data available do indicate that the toxicity of Disperse Orange 29 is likely to be low.
A range of aquatic toxicity predictions for Disperse Orange 29 were also obtained from various QSAR models. However, as with bioaccumulation, the QSAR ecotoxicity predictions for these substances are not considered reliable because of the potential error associated with input parameters and the unique nature of disperse dyes--specifically physical state, structural and/or physical and chemical properties which fall outside of the models’ domain of applicability.
The available empirical ecotoxicity information for Disperse Orange 29 and its analogues suggests that Disperse Orange 29 is not likely to be highly hazardous to aquatic organisms.
B - In Other Environmental Compartments
No ecological effects studies were found for Disperse Orange 29 in media other than water. However, this substance could end up in soil or sediment as a result of release to the aquatic environment, landfill disposal of sludge from wastewater treatment plants, disposal of products containing these substances, or biosolids application to soils. Therefore, toxicity data for soil and sediment organisms would be desirable.
This being said, the toxicity potential is likely to be low in sediment- and soil-dwelling species, considering the low bioaccumulation potential and the physical and chemical properties of this substance. However, this cannot be confirmed due to the lack of suitable whole organism toxicity data.
Ecological Exposure Assessment
No data concerning concentrations of Disperse Orange 29 in water in Canada have been identified. Environmental concentrations are therefore estimated from available information, including substance quantities, estimated release rates, and characteristics of receiving water bodies.
The Mass Flow Tool was used to predict releases to water (sewers) from formulation use and from consumer use of products containing this substance.
A – Industrial Release
The aquatic exposure of Disperse Orange 29 is expected if the substance is released from industrial use to a wastewater treatment plant and the treatment plant discharges its effluent to a receiving water body. The concentration of the substance in the receiving water near the discharge point of the wastewater treatment plant is used as the predicted environmental concentration (PEC) in evaluating the aquatic risk of the substance. It can be calculated using the equation
Cwater-ind = [1000 × Q × L × (1 - R)] / [N × F × D]
where
Cwater-ind: aquatic concentration resulting from industrial releases, mg/L
Q: total substance quantity used annually at an industrial site, kg/yr
L: loss to wastewater, fraction
R: wastewater treatment plant removal rate, fraction
N: number of annual release days, d/yr
F: wastewater treatment plant effluent flow, m3/d
D: receiving water dilution factor, dimensionless
A conservative industrial release scenario is used to estimate the aquatic concentration of Disperse Orange 29 as a dye in the production of textile products with the help of Environment Canada's (2009) Industrial Generic Exposure Tool – Aquatic (IGETA). The scenario is made conservative by assuming that the total quantity of the substance used by Canadian industry is used by one single industrial facility at a small, hypothetical site. Such a small site is selected to have an STP effluent flow at the 10th percentile (3 456 m3/d) of the STP discharge rates across Canada. The scenario also assumes that the release occurs 150 days per year, as textiles dyes are consumed in very low quantity and applied on a specialty basis. Further, it is assumed that 16% (i.e., 14% loss from unfixed dye and 2% loss from transfer and cleaning of vessels) is being lost during industrial activities with a primary removal rate of 60% at the STP, into a relatively small receiving watercourse with a dilution capacity of 10.
Based on the above assumptions, the IGETA model yields an aquatic concentration of 0.0247 mg/L (Environment Canada 20010b). This PEC value represents the level of exposure in the receiving water near the point of the discharge from the wastewater treatment plant at the site.
B – Consumer Release
Environment Canada’s spreadsheet model to estimate down-the-drain releases from consumer uses (Mega Flush) was employed to estimate the potential substance concentration in multiple water bodies receiving STP effluents to which consumer products containing this substance may have been released (Environment Canada 2008c). The spreadsheet tool provides these estimates for approximately 1000 release sites across Canada based on conservative assumptions.
The conservative assumptions include:
- loss to sewer at 100%,
- STP removal rate estimated at 0.0%,
- number of annual release days at 365 days/year,
- receiving water dilution factor in the range of 1 to 10.
The consumer release scenario was based on the maximum import quantities of 2000 kg of Disperse Orange 29 from the most recent survey (i.e., 2000 kg). Only the quantity remaining after the manufacturing of articles in Canada was considered (i.e., after considering a 16% loss at the industrial stage). In addition, the potential quantities in textiles in Canada resulting from the 30/70 ratio of textiles manufactured in Canada /imported textiles were also taken into account (Industry Canada 2008b). Therefore, the total quantity considered for the consumer use scenario of Disperse Orange 29 in textile dyes was 5628 kg. A 10% loss of textile dyes was predicted to potentially be released annually to water, as a result of loss to sewers during the laundering of manufactured articles that contain these dyes (Danish EPA 1999). The resulting maximum PEC from Megaflush was 8.6 x 10-4 mg/L (Environment Canada 2010c), based on the 10% flow for all watercourses.
Characterization of Ecological Risk
The approach taken in this ecological screening assessment was to examine available scientific information and develop conclusions based on a weight-of-evidence approach and precaution as required under CEPA 1999.
Based on read-across physical and chemical properties, Disperse Orange 29 is predicted to degrade slowly in the aerobic environment and is expected to be persistent in water, soil and sediment. This substance is expected to have low bioaccumulation potential. Although the percentage of Disperse Orange 29 that is expected to be released into sewers is quite high (14.8%), the low importation quantities of this dye into Canada, along with information on physical and chemical properties and its uses, indicate a low potential for overall releases into the Canadian environment. If released into the environment, this substance is expected to be discharged mainly to surface waters, although it is expected to ultimately be transferred to sediment. Through use of experimental and analogue data, Disperse Orange 29 has also been demonstrated to have only a moderate potential for acute toxicity to aquatic organisms.
A predicted no-effect concentration (PNEC) was estimated based on the 48-hour EC50 of 4.5 mg/L in Daphnia magna for analogue Disperse Blue 79 (Table 7b). A factor of 100 was then applied to account for acute to chronic toxicity and lab to field extrapolations and use of a surrogate substance. The resulting PNEC is 0.045 mg/L.
A risk quotient analysis, integrating a conservative PEC with a conservative estimate of the potential to cause adverse effects, or PNEC, was conducted for the aquatic environment and the resulting risk quotient (PEC/PNEC) is an important line of evidence in evaluating the potential risk to the environment.
When compared to the PEC calculated above for industrial releases to water through an STP (0.0148 mg/L), the resulting risk quotient (PEC/PNEC) is 0.0247/0.045 = 0.55. Therefore, it is estimated that the concentration of Disperse Orange 29 in surface waters in Canada resulting from industrial releases through a primary STP is not expected to harm aquatic organisms.
For exposure resulting from down-the-drain releases through consumer uses (conservative scenario), Mega Flush results estimate that the PEC for Disperse Orange 29 will not exceed the PNEC at any sites (i.e., all risk quotients <1). The maximum risk quotient calculated from the highest PEC (8.6 x 10-4 mg/L) divided by the PNEC (0.045 mg/L) is 0.019. This indicates that down-the-drain consumer releases of Disperse Orange 29 are not expected to harm aquatic organisms.
Therefore, Disperse Orange 29 is unlikely to be causing harm to populations of aquatic organisms in Canada.
Uncertainties in Evaluation of Ecological Risk
The persistence assessment is limited by the absence of biodegradation data, which necessitated generation of model predictions. Although all model prediction has some degree of error, the aerobic biodegradation model outputs confirmed the expected persistence of Disperse Orange 29 given its uses and structural characteristics. In addition, the persistence assessment is limited by the uncertainty about the rate and extent to which degradation occurs in anaerobic sediments and whether the degradation products (e.g., aromatic amines) would be biologically available. Although the degradation products are expected to be of limited biological availability because they are expected to form only in relatively deep anoxic sediment, there is still potential for sediment perturbation. Therefore, this issue is a source of uncertainty in the toxicity assessment of Disperse Orange 29.
The bioaccumulation assessment for this substance was limited by the lack of empirical data for Disperse Orange 29 and the inability of available models to reliably estimate bioaccumulation for disazo dyes. Instead the assessment relied on the use of bioaccumulation data for a structural analogue (Disperse Orange 30).
Uncertainties are also present due to the lack of information on environmental concentrations in Canada for Disperse Orange 29. However the lack of reports of manufacturing in Canada, low import quantities, the relatively high fixation level of these dyes to textiles, and the anticipated removal from the effluent suggests low potential for releases of these chemicals into the Canadian aquatic environment.
Uncertainties are also associated with the fraction of the substance that is released, and with the fraction that is removed in STPs. Those uncertainties were addressed through the use of conservative assumptions in the exposure modelling.
The experimental concentrations associated with toxicity for aquatic organisms may have an additional source of uncertainty when these concentrations exceed the solubility of the chemical in water (either experimental or predicted). There is also some uncertainty related to the purity of the substances used in the solubility and toxicity tests. Due to the low solubility of dyes, often they are mixed with water and a solubilising agent in order to complete the test. This convention can produce solubility values that are artificially high and may affect the result of aquatic toxicity tests as well. However, efforts to make the colourants more soluble are likely to artificially increase bioavailability rather than decrease it. Therefore, despite these uncertainties, the available data indicate that Disperse Orange 29 and its analogues are not highly hazardous to aquatic organisms in the water column.
Also, regarding ecotoxicity, based on the predicted partitioning behaviour of Disperse Orange 29 and its analogues, the significance of soil and sediment as important media of exposure is not well addressed by the effects data available. Indeed, the only effects data identified apply primarily to pelagic aquatic exposures, although the water column may not be the medium of primary long-term concern based on partitioning estimates and release patterns.
Given the use of this substance in other countries, it is possible that the substance is entering the Canadian market as a component of manufactured items and/or consumer products. Information obtained from the section 71 survey and other information sources indicated that it may be present in a limited number of these types of products in Canada. Available information is currently not sufficient to derive a quantitative estimate to help determine the importance of this source in ecological assessment. However, it is anticipated that the proportions of Disperse Orange 29 released to the various environmental media would not be significantly different from those estimated here, although quantities transferred to recycling and/or waste disposal may be higher. It is also recognized that releases from waste disposal sites are possible, although difficult to quantify due to the lack of data, and would contribute to overall environmental concentrations.
Exposure Assessment
There were no data identified for Disperse Orange 29 in environmental media. Environmental concentrations estimated using ChemCAN version 6 software (ChemCAN 2003) were based on the loss percentages predicted by the Mass Flow tool as shown in Table 4 (Environment Canada 2010a). The percentages were applied to the total quantity of 2000 kg of Disperse Orange 29 in Canadian commerce in 2006 (Environment Canada 2008a). The loss quantities are estimated to be 296 kg to water from wastewater, 0 kg to air from air emissions and 0 kg to soil from loss to land. The resulting upper-bounding estimates of Disperse Orange 29 intake for each age group in the general population of Canada from environmental media were predicted to be negligible.
Disperse Orange 29 is mainly used in the textile/fabric mill sector to dye or print fabrics, textiles and apparel such as polyesters and polyamides. Disperse dyes derive their name from the dyeing process employed (Danish EPA 1999). Because of their low water solubility the dye compounds are typically milled to produce a fine powder and applied a dispersion in water.
Personal apparel made from fabric dyed with Disperse Orange 29 is a potential source of general population exposure. Infants and toddlers could also be exposed via the oral route by mouthing fabric containing this dye. Upper-bounding intake estimates via both dermal and oral exposure routes are summarized in Appendix 3. Details of the assumptions used in these exposure estimates are described in Appendix 4. Dermal exposure was estimated to range from 0.1 – 2 µg/kg-bw per day for adults to 0.2 – 4 µg/kg-bw per day for infants aged 0 – 6 months at an estimated leaching rate of 0.03% to 0.5% (ETAD 2004, Kraetke & Platzek 2005). The leaching rate range presented applies to a new unwashed garment possessing good to poor colourfastness properties (ETAD 1997). Exposure to Disperse Orange 29 for infants and toddlers (6 months to 4 years old) would expect to have additional contribution from oral route via mouthing where exposure is estimated to be approximately 0.1 µg/kg-bw per day. These estimates represent upper-bounding exposures as the dyes are expected to be primarily leached out of fabric during laundering. Furthermore, a recent study compared the migration of a disperse dye out of a garment onto the skin of human volunteers versus its migration out of a garment into a sweat stimulant. This study found that the amount of a disperse dye that migrated onto the skin of human volunteers was up to 600 times lower than that leached by sweat simulants (Meinke et al. 2009). Overall, the conservative nature of the upper-bound exposure estimates is supported.
Health Effects Assessment
No empirical health effects data were identified for Disperse Orange 29 and no structurally related disperse azo dye analogues were identified for which health effects data were available. Therefore, the toxicity profile for Disperse Orange 29 is based primarily on data for potential azo cleavage products and consideration of (quantitative) structural activity relationship [QSAR] model results.
Since Disperse Orange 29 is a member of the family of azo colourants, information for the azo class of substances has been considered in this assessment. It has been demonstrated that azo colourants can undergo reductive cleavage mediated by azoreductase enzymes present in mammalian tissues and in bacteria of the intestine and skin (e.g. Golka et al. 2004; Platzek 1999; Chen 2006; Stingley et al. 2010). While it is recognized that the degree of azo reduction is likely influenced by various factors (e.g. solubility of parent, presence/position of molecular substituents, etc.), health effects information on potential azo cleavage products are considered relevant to the health effects characterization of the parent compound, as exposure to azo colourants may result in exposure to its corresponding azo cleavage products, typically aromatic amines. Based on the potential of Disperse Orange 29 to undergo azo cleavage, relevant information on the potential cleavage products are also considered in this assessment: 4-aminophenol (CAS RN 123-30-8), 2,5-diaminoanisole (CAS RN 5307-02-8), and 4-nitroaniline (CAS RN 100-01-6) (see Table 8 for structures and types of available data). International assessments and toxicity profiles of the above-noted potential azo cleavage products were reviewed to inform the health effects database for Disperse Orange 29. A summary of this information is provided below.
Table 8. Chemical structure for Disperse Orange 29 and potential azo cleavage products considered in the human health effects assessment
| Substance Identification | Structure | Data considered/available |
|---|---|---|
| Disperse Orange 29 | ||
| Disperse Orange 29 CI 26077 CAS RN 19800-42-1 |
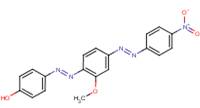 |
QSAR |
| Potential Azo Cleavage Products | ||
| 4-nitroaniline CAS RN 100-01-6 |
 |
QSAR 2-year bioassays in rat and mouse Reproductive/Development studies in rats and mice. Ames assay |
| 4-aminophenol CAS RN 123-30-8 |
|
QSAR IUCLID dataset (IUCLID 2000) Mouse lymphoma assay In vivo micronucleus assay Ames Assays 6-month oral study in rat Reproductive/Development study in rat |
| 2,5-diaminoanisole CAS RN 5307-02-8 |
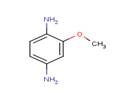 |
QSAR Ames assay Dominant lethal assay in rat Analogue data for isomer (2,5-diaminoanisole) |
4-Aminophenol (CAS RN 123-30-8) is classified by the European Commission under the CLP Directive as a mutagen[3] (European Commission 2008; ESIS 2010). Positive results were obtained in the mouse lymphoma forward mutation assay (Majeska and Holden 1995). 4-aminophenol induced an increase in the number of micronucleated hepatocytes when administered to mice intraperitoneally (IP) (Cliet et al 1989). In addition, administration to mice via the IP route caused micronucleus formation in the bone marrow (Wild et al 1981). However, 4-aminophenol did not cause increased micronucleus formation in the bone marrow when administered to mice orally (Wild et al 1981). In vitro tests for mutagenicity in bacteria were largely negative (Zeiger et al. 1988). The Scientific Committee on Consumer Products (SCCP) concluded 4-aminophenol to be genotoxic in vivo and in vitro (SCCP 2005). No empirical data is available to assess carcinogenicity of 4-aminophenol. 4-Aminophenol demonstrated effects on renal proximal tubules in an in vitro model (Lock et al. 1993) and induced kidney effects (nephrosis) at the high-dose in rats receiving 0.07, 0.2, or 0.7% in the diet for 6 months (Burnett et al. 1989). A recent study investigated the reproductive and developmental toxicity of 4-aminophenol in groups of 12 rats administered 0, 20, 100, or 500 mg/kg-bw/day by gavage. In females, exposure was from 14 days before mating until day 3 of lactation. Males were dosed for 49 days beginning 14 days before mating. Four males and two females died at the high dose and all survivors had brown urine at 100 mg/kg-bw/day and above. Body-weight gain and food consumption were decreased at 500 mg/kg bw/day. Effects on the male reproductive tract were evident at the high dose as were effects on the developing fetuses. The authors identified a reproductive/developmental no adverse effect level (NO(A)EL) of 100 mg/kg-bw/day to the study (Harada et al. 2008).
2,5-diaminoanisole (CAS RN 5307-02-8) tested positive for reverse mutagenicity in standard Ames’ assays usingSalmonella typhimurium strains TA98 and TA100 with and without S9 activation (Degawa et al. 1979; Koovi et al. 1987; Esancy et al. 1990), in TA1538 with S9 activation (Ames et al. 1975; White et al. 1977; Robertson et al. 1983), and in TA100 with a modified activation system using purified prostaglandin H synthase (Sarkar et al. 1992). This substance also caused single-strand DNA breaks in cultured human skin lymphocytes without metabolic activation suggesting direct interaction with DNA (Nordenskjold et al. 1984) and also induced unscheduled DNA synthesis in primary rat hepatocytes (Bradlaw et al. 1981). However, 2,5-diaminoanisole was negative for mutagenicity at the TK locus in mouse lymphoma L5781Y cells with and without S9 activation (Palmer et al. 1978) and was also negative in the dominant lethal test in rats (Sheu and Green 1979). No empirical data are available to assess carcinogenicity of 2,5-diaminoanisole. The structural isomer of 2,5-diaminoanisole, 2,4-diaminoanisole (CAS RN 615-05-4) was positive for carcinogenicity in both sexes of mice and rats in a chronic feeding study (NTP 1978) and is "reasonably considered as a human carcinogen" by the National Toxicology Program (NTP 2005). The 2,4- isomer is also classified as a carcinogen and mutagen by the European Commission under the CLP Regulation (European Commission 2008), as an IARC Group 2B carcinogen (IARC 2001), and is regulated in Europe under REACH as one of 22 aromatic amines liberated from azo dyes which may not be found in certain textiles or leather articles (European Commission 2006). Both 2,5- and 2,4-diaminoanisole and their salts have been prohibited for use in cosmetics under the Canadian Cosmetic Ingredient Hotlist (Health Canada 2009) and under Annex II of the European Cosmetics Directive (European Commission 2010).
4-nitroaniline (CAS RN 100-01-6) was positive in somein vitro bacterial tests for mutagenicity, but not in others (ACGIH 1991). Hamster liver S9 rendered 4-nitroaniline more mutagenic than rat liver S9 in tests for bacterial reverse mutation (ACGIH 1991). 4-Nitroaniline was negative for carcinogenicity and reproductive toxicity in an oral exposure study in rat (Nair et al 1990). In mice, liver hemangiosarcomas were observed, but were considered equivocal evidence of carcinogenicity (CCRIS 2009). 4-Nitroaniline induces methaemoglobin in humans, which may lead to anoxia and haemolysis if exposure is sufficiently high or prolonged. Adverse effects on the liver have also been reported in humans (ACGIH 1991).
In a recent study a mixture containing 4-aminophenol, 4-nitroaniline and another compound (4-nitrophenol) was administered to male and female rats intragastrically, at doses of 5, 25, or 50 mg/kg-bw/day for 4 weeks. Significant differences were observed between controls and animals administered 25 or 50 mg/kg-bw/day in body weight increases (p<0.01 for males; p<0.05 for females). Biochemical parameters (ALT, AST, GTP, urea, total cholesterol, total protein, albumin and cratinine) were significantly increased in animals administered 50 mg/kg-bw/day. Erythrocyte, leukocyte, and reticulocyte counts and haemoglobin concentrations were increased significantly in the high dose group compared to controls. Methaemoglobin concentrations were significantly increased after 3 weeks of treatment in animals exposed to 50 mg/kg-bw/day and after 4 weeks in both the 25 and 50 mg/kg-bw/day groups. Lesions affecting the liver, kidneys, spleen, cerebellum and haematopoietic system were observed (Zhang and Wang 2009).
Predictions from (quantitative) structural activity relationship [QSAR] models (CASETOX, DEREK, TOPKAT) on genotoxicity and carcinogenicity of Disperse Orange 29 were also considered in this assessment. Predictions for cancer from the (Q)SAR program DEREK were positive. However, CASETOX predictions were largely inconclusive. TOPKAT results were equivocal. For models of genotoxicity, DEREK predicted that Disperse Orange 29 would be positive in the Ames assay and was inconclusive in a model of chromosome aberration. CASETOX predicted a negative result in the Ames assay, a positive result in the chromosome aberration assay, was negative for micronuclei induction, and inconclusive in a model of the mouse lymphoma mutation assay. TOPKAT was inconclusive forin vitro mutagenicity. Results for the model predictions of Disperse Orange 29 and its potential azo cleavage products are presented in Appendix 5.
Characterization of Risk to Human Health
There are no empirical health effects data available for Disperse Orange 29. Disperse Orange 29 belongs to the group of azo substances that may potentially release aromatic amines by reductive cleavage of the azo linkage. While no chemical-specific information on azo reduction of Disperse Orange 29 was identified, a conservative assumption was to assume the potential release of aromatic amines following exposure to this substance. Because carcinogenicity and genotoxicity are often associated with aromatic amines, these are considered to be the critical effects that may be associated with exposure to Disperse Orange 29. Therefore, health effects data on aromatic amines potentially released from Disperse Orange 29 were considered. 4-Aminophenol has been classified as a mutagen by the European Union (ESIS 2010). Additionally, 2,5-diaminoanisole has tested positive in mutagenicity assays, and is structurally similar to 2,4’-diaminoanisole, which has demonstrated genotoxic and carcinogenic potential (IARC 2001; NTP 2005). 4-Nitroaniline demonstrated mixed results in mutagenicity assays. Based on information for these potential azo cleavage products, it is considered that there may be a potential hazard associated with Disperse Orange 29.
Exposure of the general population to Disperse Orange 29 from environmental media is expected to be negligible. The general population may be exposed to Disperse Orange 29 from its use as a dye in textiles and fabrics; however, the estimated dermal and oral exposure is expected to be low.
Although potential hazards associated with Disperse Orange 29, due to the possible formation of component aromatic amines from azo cleavage are recognized, taking into consideration the expected low exposure to the general population, the potential risk to human health is considered to be low at current levels of exposure.
Uncertainties in Evaluation of Risk to Human Health
The confidence in the health effects database for Disperse Orange 29 is considered to be low as no empirical data on health effects were identified for Disperse Orange 29 or structural azo dye analogues. Furthermore, as there are no empirical data indicating the potential of this substance to undergo azo reductive cleavage, data reviewed pertaining to potential metabolites may not necessarily be associated with exposure to Disperse Orange 29.
There are uncertainties associated with the exposure assessment. The sources of exposure for Disperse Orange 29 have been broadly characterized as synthetic fabrics and exposure estimates were derived for conservative scenarios to include personal apparel in the absence of more specific information regarding its use profile. However, confidence is high that the modelled exposure values presented in this assessment overestimate actual exposure due to conservative nature of assumptions used.
Based on the information available, it is concluded that Disperse Orange 29 is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends. Additionally, Disperse Orange 29 meets the criteria for persistence but does not meet the criteria for bioaccumulation potential as set out in thePersistence and Bioaccumulation Regulations (Canada 2000).
Although the potential hazard of Disperse Orange 29 is recognized, based on an overall consideration of available health effects information, together with expected low exposure to the general population, risk to human health is considered to be low. It is concluded that Disperse Orange 29 is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that Disperse Orange 29 does not meet any of the criteria under section 64 of CEPA 1999.
Considerations for Follow-up
Disperse Orange 29 belongs to a group of azo substances that may metabolize to aromatic amines, which as a chemical class are known to exhibit hazardous properties, including carcinogenicity. Therefore, additional activity (e.g. research, assessment, monitoring and surveillance) to characterize the risk to human health in Canada of this broader group of azo substances may be undertaken. A Notice of Intent outlining how the Government of Canada will address this group of substances is available on theChemical Substances website.
ACD/pKaDB [Prediction Module]. 2005. Version 9.04. Toronto (ON): Advanced Chemistry Development. [restricted access]
[ACGIH] American Conference of Governmental Industrial Hygienists, Inc. 1991. Documentation of the Threshold Limit Values and Biological Exposure Indices. 6th ed. Volumes I, II, III. Cincinnati, OH: ACGIH, p. 1094.
Ames BN, Kammen HO, Yamasaki E. 1975. Hair Dyes Are Mutagenic: Identification of a Variety of Mutagenic Ingredients. Proc. Nat. Acad. Sci. 72(6): 2423-2427.
Anliker R, Clarke EA, Moser P. 1981. Use of the partition coefficient as an indicator of bioaccumulation tendency of dyestuffs in fish. Chemosphere 10(3):263-274.
Anliker R, Moser P. 1987. The limits of bioaccumulation of organic pigments in fish: their relation to the partition coefficient and the solubility in water and octanol. Ecotoxicol and Environ Safety 13:43-52.
Anliker R, Moser P, Poppinger D. 1988. Bioaccumulation of dyestuffs and organic pigments in fish. Relationships to hydrophobicity and steric factors. Chemosphere 17(8):1631-1644.
Arnot JA, Arnot M, Mackay D, Couillard Y, MacDonald D, Bonnell M, Doyle P. 2010. Molecular size cut-off criteria for screening bioaccumulation potential: Fact or fiction? Integrated Environmental Assessment and Management 6(2):210–224.
Aronson D, Boethling B, Howard P, Stiteler W. 2006. Estimating biodegradation half-lives for use in chemical screening. Chemosphere 63: 1953-1960.
BASF. 1990. Bericht uber die Prufung der akuten Toxizitit an der Goldorfe (Leuciscus idus L.,. Goldvariante). Submitted by ETAD to Environment Canada on August 13, 2008 via e-mail.
Baughman GL, Perenich TA. 1988. Fate of dyes in aquatic systems: I. Solubility and partitioning of some hydrophobic dyes and related compounds. Environ Toxicol Chem 7(3):183-199.
Baughman GL, Weber EJ. 1994. Transformation of dyes and related compounds in anoxic sediment: kinetics and products. Environ Sci Technol 28(2): 267-276.
Baughman GL, Bannerjee S, Perenich TA. 1996. Dye Solubility. In: Physico-chemical Principles of Color Chemistry. A.T. Peters and H.S. Freeman (Eds.). Advances in Color Chemistry Series, Vol. 4. Blackie Academic and Professional Publishers, Glasgow. p. 145-195.
[BBM] Baseline Bioaccumulation Model. 2008. Gatineau (QC): Environment Canada, Existing Substances Division. [Model developed based on Dimitrov et al. 2005]. Available upon request.
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2008. Version 4.02. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4): 741-752.
Bradlaw JA, Hauswirth JW, Thomas CA. 1981. Induction of unscheduled DNA synthesis (UDS) in primary rat hepatocytes by some hair dye components. Environ Mut. 3:398 (meeting abstract).
Braun H. 1991. A new method for the determination of the solubility of disperse dyes. JSDC 107:77-83.
Brown D (ICI Group Environmental Laboratory, Brixham, UK). 1992. Environmental assessment of dyestuffs. Prepared for Ecological and Toxicological Association of the Dyes and Organic Pigments Manufacturers, Basel, Switzerland. ETAD ecological sub-committee project E3020. Submitted to Environment Canada May 9, 2008.
Brown D. 1987. Effects of colorants in the aquatic environment. Ecotox Environ Safe 13:139-47.
Burnett CM, Ta RE, Rodiguez S, Loehr RF, Dressler WE. 1989. The toxicity of p-aminophenol in the Sprague-Dawley rat: effects on growth, reproduction and foetal development. Fd. Chem. Toxic. 27(10):691-698.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33.
Canada. 2000. Canadian Environmental Protection Act, 1999: Persistence and Bioaccumulation Regulations, P.C. 2000-348, 23 March, 2000, SOR/2000-107. Canada Gazette, Part II, Vol. 134, No. 7.
Canada, Dept. of the Environment, Dept. of Health. 2006a.Canadian Environmental Protection Act, 1999: Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette, Part I, Vol. 140, No. 49.
Canada, Dept. of the Environment, Dept. of Health. 2006b.Canadian Environmental Protection Act, 1999: Notice with respect to selected substances identified as priority for action. Canada Gazette, Part I, Vol. 140, No. 9.
Canada, Dept. of the Environment, Dept. of Health. 2008a.Canadian Environmental Protection Act, 1999: Notice of sixth release of technical information relevant to substances identified in the Challenge. Canada Gazette, Part I, Vol. 142, No. 22.
Canada, Dept. of the Environment. 2008b. Canadian Environmental Protection Act, 1999: Notice with respect to Batch 6 Challenge substances. Canada Gazette, Part I, Vol. 142, No. 22.
CASETOX [Prediction module]. 2008. Version 2.0. Beachwood (OH): MultiCASE Inc. CASETOX [restricted access].
[CATABOL] Probabilistic assessment of biodegradability and metabolic pathways [Computer Model]. c2004-2008. Version 5.10.2. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. CATALOGIC.
[CCRIS] Chemical Carcinogenesis Research Information System. 2009. P-Nitroaniline: CASRN: 100-01-6. Accessed March, 2010.
ChemCAN [Level III fugacity model of 24 regions of Canada]. 2003. Version 6.00. Peterborough (ON): Trent University, The Canadian Centre for Environmental Modelling and Chemistry[cited 2010 Jan].
Chen H. 2006. Recent advances in azo dye degrading enzyme research. Curr Prot Pept Sci 7:101-111.
[CII] Colour Index International [database on the Internet]. 2002- . 4th ed. Research Triangle Park (NC): American Association of Textile Chemists and Colorists. [cited 2009 Feb 03].
Clariant. 1996. IUCLID dataset for C.I. Disperse Blue 79 (CAS No 12239-34-8). [Database on the Internet]. [cited 2008 Oct 21].
Cliet I, Fournier E, Melcion C, Cordier A. 1989. In vivo micronucleus test using mouse hepatocytes. Mut Res, 216:321-326.
Cohle P, Mihalik R. 1991. Early life stage toxicity of C.I. Disperse Blue 79:1 purified presscake to rainbow trout (Oncorhynchus mykiss) in a flow-through system. Final report. Columbia (MO): ABC Laboratories Inc.
[ConsExpo] Consumer Exposure Model [Internet]. 2006. Version 4.1. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu (National Institute for Public Health and the Environment).Product safety.
[CPOPs] Canadian POPs Model. 2008. Gatineau (QC): Environment Canada, Existing Substances Division; Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. [Model developed based on Mekenyan et al. 2005]. Available upon request.
[Danish EPA] Danish Environmental Protection Agency. 1999.Survey of azo-colorants in Denmark. Consumption, use, health and environmental aspects. MiljØprojekt No. 509. Henriette, Danish Technological Institute, Environment, Ministry of Environment and Energy, Denmark, Danish Environmental Protection Agency, 1999. Report prepared by Øllgaard H, Frost L, Galster J, Hansen OC.
Datyner A. 1978. The solubilisation of disperse dyes by dispersing agents at 127ºC. JSDC June: 256-260.
Degawa M, Shoji Y, Masuko K, Hashimoto Y. 1979. Mutagenicity of metabolites of carcinogenic aminoazo dyes. Cancer Lett. 8(1):71-6.
[DEREK] Deductive Estimation of Risk from Existing Knowledge [Prediction module on CD ROM]. 2008. Version 10.0.2. Cambridge (MA): Harvard University, LHASA Group. Toxicology Prediction [restricted access]
Dimitrov S, Dimitrova N, Walker J, Veith G, Mekenyan O. 2002. Predicting bioconcentration potential of highly hydrophobic chemicals. Effect of molecular size. Pure Appl Chem 74(10): 1823-1830.
Dimitrov S, Dimitrova N, Parkerton T, Comber M, Bonnell M, Mekenyan O. 2005. Base-line model for identifying the bioaccumulation potential of chemicals. SAR QSAR Environ Res 16(6): 531–554.
Environment Canada. 1988. Data relating to the Domestic Substance List (DSL) 1984-1986, collected under CEPA, 1988, s. 25(1). Based on Reporting for the Domestic Substances List [guide] 1988. Data prepared by: Environment Canada.
Environment Canada. 1995. NSN submission. Submitted to New Substances Branch, Environment Canada under New Substances Notification Program.
Environment Canada. 2000. Chemicals Evaluation Division. Environmental Categorization for Persistence, Bioaccumulation and Inherent Toxicity of Substances on the Domestic Substances List Using QSARs. Final Report. Environment Canada. July.
Environment Canada. 2006. Data for selected substances collected under the Canadian Environmental Protection Act, 1999, Section 71: Notice with respect to selected substances identified as priority for action. Prepared by: Environment Canada, Health Canada, Existing Substances Program.
Environment Canada. 2008a. Data for Batch 6 substances collected under the Canadian Environmental Protection Act, 1999, Section 71:Notice with respect to certain Batch 2 Challenge substances. Data prepared by: Environment Canada, Existing Substances Program.
Environment Canada. 2008b. Guidance for conducting ecological assessments under CEPA, 1999: science resource technical series, technical guidance module: Mass Flow Tool. Preliminary draft working document. Gatineau (QC): Environment Canada, Existing Substances Division.
Environment Canada. 2008c. Guidance for conducting ecological assessments under CEPA 1999: science resource technical series, technical guidance module: Mega Flush consumer release scenario. Preliminary draft working document. Gatineau (QC): Environment Canada, Existing Substances Division.
Environment Canada. 2009. Guidance for conducting ecological assessments under CEPA, 1999: science resource technical series, technical guidance module: the Industrial Generic Exposure Tool – Aquatic (IGETA). Working document. Gatineau (QC): Environment Canada, Existing Substances Division.
Environment Canada. 2010a. Assumptions, limitations and uncertainties of the Mass Flow Tool for Disperse Orange 29, CAS RN 19800-42-1. [cited 2010 Jul 27]. Internal draft document. Gatineau (QC): Environment Canada, Existing Substances Division. Available on request.
Environment Canada. 2010b. IGETA report: Disperse Orange 29, CAS RN 19800-42-1, version [cited 2010-07-27]. Unpublished report. Gatineau (QC): Environment Canada, Existing Substances Division.
Environment Canada. 2010c. MegaFlush report : Disperse Orange 29, CAS RN 19800-42-1,[cited 2010 Jul 27]. Unpublished report. Gatineau (QC): Environment Canada, Existing Substances Division.
[EPIsuite] Estimation Programs Interface Suite for Microsoft Windows [Estimation Model]. 2008. Version 4.0 Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Exposure Assessment Tools and Models.
Esancy JF, Freeman HS, Claxton LD. 1990. The effect of alkoxy substituents on the mutagenicity of some aminoazobenzene dyes and their reductive-cleavage products. Mut Res. 238:1-22.
[ESIS] European Chemical Substances Information System [database on the Internet]. 2008. Version 5. European Chemical Bureau (ECB). [cited 2009 Feb 13].
[ESIS] European Chemical Substances Information System [database on the Internet]. 2010. Database developed by the European Chemicals Bureau. CLP/GHS Search of Annex VI [cited 2010 June ].
[ETAD] Ecological and Toxicological Association of Dyes and Organic Pigments Canadian Affiliates, Dayan J, Trebitz H, consultants. 1995. Health and environmental information on dyes used in Canada. Unpublished report submitted to Environment Canada, New Substances Division. On the cover: An overview to assist in the implementation of the New Substances Notification Regulations under the Canadian Environmental Protection Act.
[ETAD] Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 1992. Draft Guidelines for the Assessment of Environmental Exposure to Dyestuffs.
[ETAD] Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 1997
Extractability of dyestuff from textiles over a normal lifetime of use. March 1997
[ETAD] Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 2005. Information received in support of DSL categorization for 12 disperse dyes. Email correspondence dated October 27, 2005.
European Commission. 2006. Regulation (EC) No 1907/2006 of the European Parliament and of the Council (REACH).
European Commission. 2008. Regulation (EC) No 1272/2008 on classification, labelling and packaging of substances and mixtures (CLP).
European Commission 2010. European Commission "Cosmetics Directive" 76/768/EEC (Cosmetics Directive) Database of Ingredients and Substances (CosIng).
Farbchemie Braun KG. 2008. Fantagen Disperse Dyes for Polyester. [Internet].
Golka K, Kopps S, Myslak ZW. 2004. Carcinogenicity of azo colorants: influence of solubility and bioavailabilty. Toxicol. Lett. 151(1):203-210.
Haag WR, Mill T. 1987. Direct and indirect photolysis of water-soluble azodyes: kinetic measurements and structure-activity relationships. Environ Toxicol Chem 6:359-369.
Harada T, Kimura E, Hirata-Koizumi M, Hirose A, Kamata E, Ema M. 2008. Reproductive and developmental toxicity screening study of 4-aminophenol in rats. Drug Chem. Toxicol. 31:473-486.
Health Canada. 1995. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada. Environmental Health Directorate.
Health Canada 2007. Food and Drug Regulations, Part B – Foods.
Health Canada. 2009. Cosmetic Ingredient Hotlist.
Hunger K, editor. 2003. Industrial dyes; chemistry, properties, applications. Weinheim (DE): WILEY-VCH Verlag GmbH & Co. KGaA.
[IARC] International Agency for Research on Cancer. 2001.IARC Monographs on the Evaluations of Carcinogenic Risks to Humans, Volume 79: Some Thyrotropic Agents. Summary of Data Reported and Evaluation.
India. 1997. Ministry of Environment and Forests Notification, Prohibition on the Handling of Azodyes. The Gazette of India Extraordinary. Part II- Sec. 3(ii). Annexure 10. REGD. NO. D.L.-33004/97.
Industry Canada. 2008a. Canadian Company Capabilities (CCC).
Industry Canada. 2008b. Textile and Fabric Finishing [NAICS 31331]: 2004-2007 and Fabrics Coating 2004-2007. [NAICS 31332]: 2004-2007. Prepared by:Apparel and Textiles Directorate, Service Industries and Consumer Products Branch, Industry Canada, Enquiries B John (Jazz) Szabo, 613-957-1242, szabo.john@ic.gc.ca.
[IUCLID] International Uniform Chemical Information Database. 2000. IUCLID Dataset: Substance ID: 123-30-8, 4-aminophenol.
Koovi DG, Mamane R, Callais F, Festy B, Lich NP. 1987. Study on the genotoxicity of 2-amino-5-nitroanisole (ANA) and its metabolites. Ann. Falsif. Expert. Chim. Toxicol., 80(854), 25-39.
Kraetke RM & Platzek T. 2005. Exposure to chemicals in clothing textiles: Methods and models. Occupational and Environmental Exposure of Skin to Chemicals.
Lock EA, Cross TJ, and Schnellmann RG. 1993. Studeis on the mechanisms of 4-aminophenol-induced toxicity to renal proximal tubules. Hum Exp Toxicol, 12(5):383-388.
Majeska JB, Holden HE. 1995. Genotoxic effects of p-aminophenol in Chinese hamster ovary and mouse lymphoma cells: Results of a multiple endpoint test. Environ. Mol. Mutagen. 26:163-170.
Meinke M, Abdollahnia M, Gähr F, Platzek T, Sterry W, Lademann J. 2009. Migration and penetration of a fluorescent textile dye into the skin--in vivo versus in vitro methods. Experimental Dermatology. 18(9): 789–792.
Mekenyan G, Dimitrov SD, Pavlov TS, Veith GD. 2005. POPs: A QSAR system for creating PBT profiles of chemicals and their metabolites. SAR QSAR Environ Res 16(1-2):103-133.
[MITI] Ministry of International Trade & Industry (Japan). 1992. Biodegradation and bioaccumulation data of existing chemicals based on the CSCL Japan, Basic Industries Bureau, Chemical Products Safety Division. Japan Chemical Industry Ecology-Toxicology & Information Centre,Tokyo (Jpn).
Nair RS, Auletta CS, Schroeder RE, Johannsen FR. 1990. Chronic toxicity, oncogenic potential, and reproductive toxicity of p-nitroaniline in rats. Fund Appl Toxicol 15:507-621.
[NCI] National Chemical Inventories [database on CD-ROM]. 2006. Columbus (OH): American Chemical Society. [cited 2006 Dec 11].
Nishida K, Ando Y, Ohwada K, Mori T, Koide M, Koukitsu A. 1989. Vapour pressures and heats of sublimation of some azo disperse dyes. JSDC 105:112-114.
Nordenskjold M, Andersson B, Rahimtula A, Moldeus P. 1984. Prostaglandin synthase-catalyzed metabolic activation of some aromatic amines to genotoxic products. Mut Res. 127:107-112.
Norris B, Smith S. 2002. Research into the mouthing behaviour of children up to 5 years old. Report commissioned by Consumer and Competition Policy Directorate, UK Department of Trade and Industry, London, UK.
[NTP] United States National Toxicology Program. 1978. Toxicology Report TR-84: Bioassay of 2,4-Diaminoanisole Sulfate for Possible Carcinogenicity (CASRN 615-05-4).
[NTP] United States National Toxicology Program. 2005. 11th Report on Carcinogens (RoC): 2,4-Diaminoanisole Sulfate (CASRN 615-05-4).
Odabasoglu M, Çakmak S, Turgut G, Içbudak H. 2003. Preparation and characterization of chromophor group containing cyclotriphosphazenes: III bis-azo chromophor carrying some cyclotriphosphazenes. Phosphorus, Sulfur and Silicon 178:549-558.
[OECD] Organisation for Economic Co-operation and Development. 1996. OECD Guidelines for Testing of Chemicals, No. 305B Bioconcentration: Semi-Static Fish Test. Paris: OECD, Adopted June 1996.
[OECD] Organisation for Economic Co-operation and Development. 2004. Draft emission scenario on textile manufacturing wool mills [Internet]. Paris (FR): OECD, Environment Directorate. Report No.: ENV/JM/EEA(2004)8/1/REV, JT00175156. [cited 2009 Feb 16].
Pagga U, Brown D. 1986. The degradation of dyestuffs: Part II Behaviour of dyestuffs in aerobic biodegradation tests. Chemosphere 15(4):479-491.
Palmer KA, Denunzio A, Green S. 1978. The mutagenic assay of some hair dye components, using the thymidine kinase locus of L5178Y mouse lymphoma cells. J Environ Pathol Toxicol. 1(1):87-91.
[PhysProp] Interactive PhysProp Database [database on the Internet]. 2006. Syracuse (NY): Syracuse Research Corporation. [cited 2009-02-16].
Prikryl J, Rusicka, Burgert L. 1979. A new method of determining the solubility of disperse dyes. JSDC October: 349-351.
Platzek T, Lang C, Grohmann G, Gi US, Baltes W. 1999. Formation of a carcinogenic aromatic amine from an azo dye by human skin bacteria in vitro. Hum. Exp. Toxicol. 18(9):552-559.
[QPC] Qianjiang Printing & Chemical Co., Ltd. 2004. Products: Disperse Orange 29. [Internet].
Razo-Flores E. Luijten M, Donlon B, Lettinga G, Field J. 1997. Biodegradation of selected azo dyes under methanogenic conditions. Wat. Sci Technol 36(6-7): 65-72.
Robertson ICG, Sivarajah K, Eling TE, Zeiger E. 1983. Activation of Some Aromatic Amines to Mutagenic Products by Prostaglandin Endoperoxide Synthetase. Cancer Res. 43: 476-480
Sakuratani Y, Noguchi Y, Kobayashi K, Yamada J, Nishihara T. 2008. Molecular size as a limiting characteristic for bioconcentration in fish. J Environ Biol 29(1):89-92.
Sarkar FH, Radcliff G, Callewaert DM. 1992. Purified prostaglandin synthase activates aromatic amines to derivatives that are mutagenic to Salmonella typhimurium. 282:273-281.
[SCCP] European Commission Scientific Committee on Consumer Products. 2005. SCCP Opinion on p-Aminophenol. COLIPA No. A16.
Shen G, Hu S. 2008. Bioconcentration Test of C.I. Disperse Orange 30 in Fish. Prepared by Environmental Testing Laboratory, Shanghai Academy of Environmental Sciences, Shanghai, China for Dystar in the name of Ecological and Toxicological Association of the Dyes and Organic Pigments Manufacturers (ETAD) Basel, Switzerland. Report No. S-070-2007. Submitted to Environment Canada in April 2008. Challenge Submission ID#8351.
Sheu CJW, Green S. 1979. Dominant lethal assay of some hair-dye components in random-bred male rats. Mutat. Res. 68:85-98.
Sijm DTHM, Schuurmann G, deVries PJ, Opperhuizen A. 1999. Aqueous solubility, octanol solubility, and octanol/water partition coefficient of nine hydrophobic dyes. Environ Toxicol Chem 18(6):1109-1117.
[SPIN] Substances in Preparations in Nordic Countries [database on the Internet]. 2008. Financed by the Nordic Council of Ministers, Chemical group. [cited 2008 Dec].
Stingley RL, Zou W, Heinze TM, Chen H, Cerniglia CE. 2010. Metabolism of azo dyes by human skin microbiota. J. Med. Microbiol. 59(Pt.1):108-114.
Study Submission. 2008a. Unpublished confidential study submitted to Environment Canada, Existing Substances Division under the Chemical Management Plan Challenge initiative. Available as Robust Study Summary, Identification No.: 11887Challenge006.
Study Submission. 2008b. Unpublished confidential study submitted to Environment Canada, Existing Substances Division under the Chemical Management Plan Challenge initiative. Available as Robust Study Summary, Identification No.: 12890Challenge006.
[TOPKAT] TOxicity Prediction by Komputer Assisted Technology [Internet]. 2004. Version 6.2. San Diego (CA): Accelrys Software Inc. [cited 2009 Jan 7].
[US EPA] United States Environmental Protection Agency. 1986-2002. Toxic Substances Control Act: Inventory Update Reporting (TSCA-IUR). Non-confidential Production Volume Information for 1986, 1990, 1994, 1998 and 2002 reporting cycles [CD-ROM]. Washington (DC): U.S. EPA.
White TJ, Goodman D, Sulgin AT, Castagnolli N, Lee R, Petrakis NL. 1977. Mutagenic activity of some centrally active aromatic amines in Salonella typhimurium. Mutation Research. 56:199-202.
Wild D, King MT, Eckhardt K, Gocke E. 1981 Mutagenic activity of aminophenols and diphenols, and relations with chemical structure. Mut Res 85:456.
Yen CC, Perenich TA, Baughman GL. 1989. Fate of dyes in aquatic systems II. Solubility and octanol/water partition coefficients of disperse dyes. Environ Toxicol Chem 8 (11):981-986.
Yen CC, Perenich TA, Baughman GL. 1991. Fate of commercial disperse dyes in sediments. Environ Toxicol Chem 10:1009-1017.
Zeiger E, Anderson B, Haworth S, lawlor T, Mortelmans K. 1988.Salmonella mutagenicity tests: IV. Results from the testing of 300 chemicals. Environ. Mol. Mutagen. 11(Suppl 12):1-157.
Zhang, X, Wang, ,G. 2009. Four-week oral toxicity study of p-nitrophenol, paminophenol
and p-nitroaniline in rats. Journal of Veterinary Pharmacology and Therapeutics, 32 (SUPPL. 1), pp. 122-123.
Robust Study Summaries Form and Instructions: Persistence in Water, Sediments and Soil
| No. | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 1 | Reference: Bio-elimination study for CAS# 6250-23-3 (Disperse Yellow 23) Clariant dyestuff product = Foron Yellow E RGFL (Study Submission 2008b) | |||
| 2 | Substance identity: CAS RN | n/a[1] | Y | 6250-23-3 |
| 3 | Substance identity: chemical name(s) | n/a | Y | Disperse Yellow 23 |
| 4 | Chemical composition of the substance | 2 | N | |
| 5 | Chemical purity | 1 | Y | 54% Disperse Yellow 23 |
| Method | ||||
| 6 | Reference | 1 | N | |
| 7 | OECD, EU, national or other standard method? | 3 | N | |
| 8 | Justification of the method/protocol if a nonstandard method was used | 2 | N | |
| 9 | GLP (good laboratory practice) | 3 | Not applicable (study conducted 1976) | |
| Test design / conditions | ||||
| 10 | Test type (i.e., hydrolysis, biodegradation) | n/a | Y | Biodegradation |
| 11 | Test conditions type (aerobic or anaerobic) | n/a | N | Not reported |
| 12 | Test medium (water, sediment or soil) | n/a | Y | Water (test medium is inferred as water since test concentration given in mg/L) |
| 13 | Test duration | n/a | Y | 14 days |
| 14 | Negative or positive controls? | 1 | N | |
| 15 | Number of replicates (including controls) | 1 | N | |
| 16 | Measured concentrations reported? | 3 | N | |
| 17 | Analytical method / instrument | 1 | N | |
| Details on biodegradation | ||||
| 18 | Type of biodegradation (ready or inherent) reported? | 2 | N | Not reported |
| 19 | When type of biodegradation (ready or inherent) is not reported, is there indirect information that allows for the identification of biodegradation type? | 1 | N | |
| 20 | Inoculum source | 1 | N | |
| 21 | Inoculum concentration or number of microorganisms | 1 | N | |
| 22 | Were inoculum pre-conditioning and pre-adaptation reported? | 1 | N | |
| 23 | Were inoculum pre-conditioning and pre-adaptation appropriate for the method used? | n/a | N | |
| 24 | Temperature | 1 | N | Not reported |
| 25 | Has percentage degradation of the reference compound reached the pass levels by Day 14? | n/a | N | No reference compound tested |
| 26 | Soil: soil moisture reported? | 1 | ||
| 27 | Soil and sediments: background SOM (soil organic matter) content reported? | 1 | ||
| 28 | Soil and sediments: clay content reported? | 1 | ||
| 29 | Soil and sediments: CEC (cation exchange capacity) reported? | 1 | ||
| Details on hydrolysis | ||||
| 30 | pH values reported? | 1 | ||
| 31 | Temperature | 1 | ||
| 32 | Were appropriate concentrations of the substance used? | |||
| 33 | If solvent was used, was it done appropriately? | |||
| Details on photodegradation | ||||
| 34 | Temperature | 1 | ||
| 35 | Light source | 1 | ||
| 36 | Light spectrum (nm) | 1 | ||
| 37 | Relative intensity based on sunlight intensity | 1 | ||
| 38 | Spectrum of a substance | 1 | ||
| 39 | Indirect photolysis: sensitizer (type) | 1 | ||
| 40 | Indirect photolysis: concentration of sensitizer | 1 | ||
| Results | ||||
| 41 | Endpoint and value | n/a | n/a | Avg. 14-day biodegradation = 51% |
| 42 | Breakdown products | n/a | N | |
| 43 | Score: .% | 4.5 | ||
| 44 | EC reliability code: | 4 | ||
| 45 | Reliability category (high, satisfactory, low): | Not Satisfactory | ||
| 46 | Comments | |||
Robust Study Summaries Form: Aquatic B
| No. | Item | Weight | Yes/No | Specify | |
|---|---|---|---|---|---|
| 1 | Reference: Shen G, Hu S. 2008. Bioconcentration Test of C.I. Disperse Orange 30 in Fish. Prepared by Environmental Testing Laboratory, Shanghai Academy of Environmental Sciences, Shanghai, China for Dystar in the name of Ecological and Toxicological Association of the Dyes and Organic Pigments Manufacturers (ETAD) Basel, Switzerland. Report No. S-070-2007. Submitted to Environment Canada in April 2008. Challenge Submission ID#8351 | ||||
| 2 | Substance identity: CAS RN | n/a[1] | Y | 5261-31-4 | |
| 3 | Substance identity: chemical name(s) | n/a | Y | Propanenitrile, 3-[[2-(acetyloxy)ethyl][4-[(2,6-dichloro-4-nitrophenyl)azo]phenyl]amino]- | |
| 4 | Chemical composition of the substance | 2 | N | ||
| 5 | Chemical purity | 1 | N | ||
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | N | ||
| 7 | If test material is radiolabelled, were precise position(s) of the labelled atom(s) and the percentage of radioactivity associated with impurities reported? | 2 | N | ||
| Method | |||||
| 8 | Reference | 1 | Y | OECD guidelines for the testing of chemicals No 305B-1996 | |
| 9 | OECD, EU, national or other standard method? | 3 | Y | OECD | |
| 10 | Justification of the method/protocol if a nonstandard method was used | 2 | Not applicable | ||
| 11 | GLP (good laboratory practice) | 3 | N | ||
| Test organism | |||||
| 12 | Organism identity: name | n/a | Y | Zebra fish, Brachydanio rerio | |
| 13 | Latin or both Latin and common names reported? | 1 | Y | Both | |
| 14 | Life cycle age / stage of test organism | 1 | N | ||
| 15 | Length and/or weight | 1 | Y | Mean body length 3.91+/-0.18 cm and mean body weight 0.32+/-0.06 g | |
| 16 | Sex | 1 | N | ||
| 17 | Number of organisms per replicate | 1 | Y | 7 | |
| 18 | Organism loading rate | 1 | Y | 20 mg/L | |
| 19 | Food type and feeding periods during the acclimation period | 1 | Y | Fed a commercial fish diet until one day before start of test | |
| Test design / conditions | |||||
| 20 | Experiment type (laboratory or field) | n/a | Y | Laboratory | |
| 21 | Exposure pathways (food, water, both) | n/a | Y | Water | |
| 22 | Exposure duration | n/a | Y | 28 days | |
| 23 | Number of replicates (including controls) | 1 | Y | ||
| 24 | Concentrations | 1 | Y | 20 mg/L | |
| 25 | Food type/composition and feeding periods during the test | 1 | Y | Fish were fed two hours before water renewal | |
| 26 | If BCF/BAF derived as a ratio of chemical concentration in the organism and in water, was experiment duration equal to or longer than the time required for the chemical concentrations to reach steady state? | 3 | Y | 28 days | |
| 27 | If BCF/BAF derived as a ratio of chemical concentration in the organism and in water, were measured concentrations in both water and organism reported? | 3 | Y | ||
| 28 | Were concentrations in the test water measured periodically? | 1 | Y | On three separate days | |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for metal toxicity - pH, DOC/TOC, water hardness, temperature) | 3 | Y | Yes, every second day | |
| 30 | Photoperiod and light intensity | 1 | Y | 12:12 | |
| 31 | Stock and test solution preparation | 1 | Y | ||
| 32 | Analytical monitoring intervals | 1 | Y | Every second day for dissolved oxygen, pH and temperature | |
| 33 | Statistical methods used | 1 | Y | ||
| 34 | Was solubilizer/emulsifier used if the chemical was unstable or poorly soluble? | n/a | N | ||
| Information relevant to the data quality | |||||
| 35 | Was the test organism relevant to the Canadian environment? | 3 | Y | ||
| 36 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Y | ||
| 37 | Does system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and organism's nature/habits? | 2 | Y | Semi-static | |
| 38 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | Y | 7.22–7.84 | |
| 39 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | 22–23 | |
| 40 | Was lipid content (or lipid-normalized BAF/BCF) reported? | 2 | Y | ||
| 41 | Were measured concentrations of a chemical in the test water below the chemical’s water solubility? | 3 | N | ||
| 42 | If radiolabelled test substance was used, was BCF determination based on the parent compound (i.e., not on total radiolabelled residues)? | 3 | N | ||
| Results | |||||
| 43 | Endpoints (BAF, BCF) and values | n/a | n/a | BCF | |
| 44 | BAF or BCF determined as: 1) the ratio of chemical concentration in the organism and in water, or 2) the ratio of the chemical uptake and elimination rate constants | n/a | n/a | 1 | |
| 45 | Was BAF/BCF was derived from a 1) tissue sample or 2) whole organism? | n/a | n/a | 2 | |
| 46 | Was 1) average or 2) maximum BAF/BCF used? | n/a | n/a | 1 | |
| 47 | Score: .% | 67.9 | |||
| 48 | EC reliability code: | 2 | |||
| 49 | Reliability category (high, satisfactory, low): | Satisfactory Confidence | |||
| 50 | Comments | The present procedure is based on semi-static conditions (renewal of test solutions every 2 days). Therefore, test chemicals with very low water solubility like the disazo dyes can also be characterized as to their bioconcentration potential without adding solvents or other auxiliary substances that may affect the results. | |||
Robust Study Summaries Form and Instructions: Aquatic iT
| No. | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 1 | Reference: Fish toxicity study for CAS# 6250-23-3 (Disperse Yellow 23) Clariant dyestuff product=Foron Yellow E RGFL (Study Submission 2008b) | |||
| 2 | Substance identity: CAS RN | n/a[1] | Y | 6250-23-3 |
| 3 | Substance identity: chemical name(s) | n/a | Y | Disperse Yellow 23 |
| 4 | Chemical composition of the substance | 2 | N | |
| 5 | Chemical purity | 1 | Y | 54% DY23 |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | N | |
| Method | ||||
| 7 | Reference | 1 | N | |
| 8 | OECD, EU, national or other standard method? | 3 | N | |
| 9 | Justification of the method/protocol if a nonstandard method was used | 2 | N | |
| 10 | GLP (good laboratory practice) | 3 | Not applicable (study conducted 1974) | |
| Test organism | ||||
| 11 | Organism identity: name | n/a | Y | Rainbow trout |
| 12 | Latin or both Latin and common names reported? | 1 | N | |
| 13 | Life cycle age / stage of test organism | 1 | Y | |
| 14 | Length and/or weight | 1 | Y | 11 cm length, 15 g weight |
| 15 | Sex | 1 | N | |
| 16 | Number of organisms per replicate | 1 | N | |
| 17 | Organism loading rate | 1 | N | |
| 18 | Food type and feeding periods during the acclimation period | 1 | N | |
| Test design / conditions | ||||
| 19 | Test type (acute or chronic) | n/a | Y | Acute |
| 20 | Experiment type (laboratory or field) | n/a | Y | Lab |
| 21 | Exposure pathways (food, water, both) | n/a | Y | Water |
| 22 | Exposure duration | n/a | Y | 48 hours |
| 23 | Negative or positive controls (specify) | 1 | N | |
| 24 | Number of replicates (including controls) | 1 | N | |
| 25 | Nominal concentrations reported? | 1 | N | |
| 26 | Measured concentrations reported? | 3 | N | |
| 27 | Food type and feeding periods during the long-term tests | 1 | Not applicable | |
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | N | |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for metal toxicity - pH, DOC/TOC, water hardness, temperature) | 3 | N | |
| 30 | Photoperiod and light intensity | 1 | N | |
| 31 | Stock and test solution preparation | 1 | N | |
| 32 | Was solubilizer/emulsifier used if the chemical was poorly soluble or unstable? | 1 | N | |
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | ||
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | ||
| 35 | Analytical monitoring intervals | 1 | N | |
| 36 | Statistical methods used | 1 | N | |
| Information relevant to the data quality | ||||
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by organism’s health (e.g., when mortality in the control >10%) or physical effects (e.g. “shading effect”)? | n/a | Y | |
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | |
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | N | Temperature only variable reported (temp is typical for test organism) |
| 40 | Does system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and organism's nature/habits? | 2 | Not able to assess | |
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | pH not specified | |
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | 20oC |
| 43 | Was toxicity value below the chemical’s water solubility? | 3 | N | The reported LC50 is 8 orders of magnitude higher than the WS measured in Baughmann and Perenich 1989. |
| Results | ||||
| 44 | Toxicity values (specify endpoint and value) | n/a | n/a | 48-hour LC50 1000 mg/L |
| 45 | Other endpoints reported - e.g., BCF/BAF, LOEC/NOEC (specify)? | n/a | N | |
| 46 | Other adverse effects (e.g., carcinogenicity, mutagenicity) reported? | n/a | N | |
| 47 | Score: % | 17.5 | ||
| 48 | EC reliability code: | 4 | ||
| 49 | Reliability category (high, satisfactory, low): | Not Satisfactory | ||
| 50 | Comments | |||
Robust Study Summaries Form and Instructions: Aquatic iT
| No. | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 1 | Reference: ETAD: Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 2005. Project E 3020: Information received in support of DSL categorization for 12 disperse dyes. Email correspondence dated October 27, 2005) | |||
| 2 | Substance identity: CAS RN | n/a[1] | Y | 19800-42-1 |
| 3 | Substance identity: chemical name(s) | n/a | Y | Disperse Orange 29 |
| 4 | Chemical composition of the substance | 2 | Y | 20% dyestuff, 10% Reax 85A, 70% water |
| 5 | Chemical purity | 1 | Y | Dispersion 20% dyestuff |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | N | |
| Method | ||||
| 7 | Reference | 1 | N | |
| 8 | OECD, EU, national or other standard method? | 3 | Y | OECD 203 |
| 9 | Justification of the method/protocol if a nonstandard method was used | 2 | Not applicable | |
| 10 | GLP (good laboratory practice) | 3 | Not applicable (study conducted 1990) | |
| Test organism | ||||
| 11 | Organism identity: name | n/a | Y | zebra fish, Brachydanio rerio |
| 12 | Latin or both Latin and common names reported? | 1 | Y | |
| 13 | Life cycle age / stage of test organism | 1 | N | |
| 14 | Length and/or weight | 1 | N | |
| 15 | Sex | 1 | Not applicable | |
| 16 | Number of organisms per replicate | 1 | N | |
| 17 | Organism loading rate | 1 | N | |
| 18 | Food type and feeding periods during the acclimation period | 1 | N | |
| Test design / conditions | ||||
| 19 | Test type (acute or chronic) | n/a | Y | Acute |
| 20 | Experiment type (laboratory or field) | n/a | Y | Lab |
| 21 | Exposure pathways (food, water, both) | n/a | Y | Water |
| 22 | Exposure duration | n/a | Y | 48 hours |
| 23 | Negative or positive controls (specify) | 1 | N | |
| 24 | Number of replicates (including controls) | 1 | N | |
| 25 | Nominal concentrations reported? | 1 | N | |
| 26 | Measured concentrations reported? | 3 | N | |
| 27 | Food type and feeding periods during the long-term tests | 1 | Not applicable | |
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | N | |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for metal toxicity - pH, DOC/TOC, water hardness, temperature) | 3 | N | |
| 30 | Photoperiod and light intensity | 1 | N | |
| 31 | Stock and test solution preparation | 1 | N | |
| 32 | Was solubilizer/emulsifier used if the chemical was poorly soluble or unstable? | 1 | N | |
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | ||
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | ||
| 35 | Analytical monitoring intervals | 1 | N | |
| 36 | Statistical methods used | 1 | N | |
| Information relevant to the data quality | ||||
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by the organism’s health (e.g., when mortality in the control >10%) or physical effects (e.g., “shading effect”)? | n/a | Y | |
| 38 | Was the test organism relevant to the Canadian environment? | 3 | N | |
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Not reported | |
| 40 | Does system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and organism's nature/habits? | 2 | Not able to assess | |
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | pH not specified | |
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Not specified | |
| 43 | Was toxicity value below the chemical’s water solubility? | 3 | Y | The reported LC50 and WS are within 1 order of magnitude of each other. |
| Results | ||||
| 44 | Toxicity values (specify endpoint and value) | n/a | n/a | 96-hour LC50 = 480 mg/L |
| 45 | Other endpoints reported - e.g., BCF/BAF, LOEC/NOEC (specify)? | n/a | N | |
| 46 | Other adverse effects (e.g., carcinogenicity, mutagenicity) reported? | n/a | N | |
| 47 | Score:. % | 28.6 | ||
| 48 | EC reliability code: | 4 | ||
| 49 | Reliability category (high, satisfactory, low): | Not Satisfactory | ||
| 50 | Comments | |||
Robust Study Summaries Form and Instructions: Aquatic iT
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 1 | Reference: ETAD: Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 2005. Project E 3020: Information received in support of DSL categorization for 12 disperse dyes. Email correspondence dated October 27, 2005) | |||
| 2 | Substance identity: CAS RN | n/a[1] | Y | 19800-42-1 |
| 3 | Substance identity: chemical name(s) | n/a | Y | Disperse Orange 29 |
| 4 | Chemical composition of the substance | 2 | Y | 20% dyestuff, 10% Reax 85A, 70% water |
| 5 | Chemical purity | 1 | Y | Dispersion 20% dyestuff |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | N | |
| Method | ||||
| 7 | Reference | 1 | N | |
| 8 | OECD, EU, national or other standard method? | 3 | Y | OECD 201 |
| 9 | Justification of the method/protocol if a nonstandard method was used | 2 | Not applicable | |
| 10 | GLP (good laboratory practice) | 3 | Not applicable (study conducted 1990) | |
| Test organism | ||||
| 11 | Organism identity: name | n/a | Y | Scenedesmus subspicatus |
| 12 | Latin or both Latin and common names reported? | 1 | Y | |
| 13 | Life cycle age / stage of test organism | 1 | N | |
| 14 | Length and/or weight | 1 | Not applicable | |
| 15 | Sex | 1 | Not applicable | |
| 16 | Number of organisms per replicate | 1 | N | |
| 17 | Organism loading rate | 1 | N | |
| 18 | Food type and feeding periods during the acclimation period | 1 | N | |
| Test design / conditions | ||||
| 19 | Test type (acute or chronic) | n/a | Y | Acute |
| 20 | Experiment type (laboratory or field) | n/a | Y | Lab |
| 21 | Exposure pathways (food, water, both) | n/a | Y | Water |
| 22 | Exposure duration | n/a | Y | 72 hours |
| 23 | Negative or positive controls (specify) | 1 | N | |
| 24 | Number of replicates (including controls) | 1 | N | |
| 25 | Nominal concentrations reported? | 1 | N | |
| 26 | Measured concentrations reported? | 3 | N | |
| 27 | Food type and feeding periods during the long-term tests | 1 | Not applicable | |
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | N | |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for metal toxicity - pH, DOC/TOC, water hardness, temperature) | 3 | N | |
| 30 | Photoperiod and light intensity | 1 | N | |
| 31 | Stock and test solution preparation | 1 | N | |
| 32 | Was solubilizer/emulsifier used if the chemical was poorly soluble or unstable? | 1 | N | |
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | ||
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | ||
| 35 | Analytical monitoring intervals | 1 | N | |
| 36 | Statistical methods used | 1 | N | |
| Information relevant to the data quality | ||||
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by the organism’s health (e.g., when mortality in the control >10%) or physical effects (e.g., “shading effect”)? | n/a | Y | |
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | |
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Not reported | |
| 40 | Does system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and organism's nature/habits? | 2 | Not able to assess | |
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | pH not specified | |
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Not specified | |
| 43 | Was toxicity value below the chemical’s water solubility? | 3 | Y | EC50s and WS are within 1 order of magnitude of each other. |
| Results | ||||
| 44 | Toxicity values (specify endpoint and value) | n/a | n/a | 72-hr biomass EC50 = 6 mg/L |
| 45 | Other endpoints reported - e.g., BCF/BAF, LOEC/NOEC (specify)? | n/a | Y | Growth EC50 = 86 mg/L; biomass/ growth EC10 = 1.7/5.4 mg/L |
| 46 | Other adverse effects (e.g., carcinogenicity, mutagenicity) reported? | n/a | N | |
| 47 | Score: % | 38.2 | ||
| 48 | EC reliability code: | 4 | ||
| 49 | Reliability category (high, satisfactory, low): | Not Satisfactory | ||
| 50 | Comments | |||
Robust Study Summaries Form and Instructions: Aquatic iT
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 1 | Reference: ETAD: Ecological and Toxicological Association of Dyes and Organic Pigments Manufacturers. 2005. Project E 3020: Information received in support of DSL categorization for 12 disperse dyes. Email correspondence dated October 27, 2005) | |||
| 2 | Substance identity: CAS RN | n/a[1] | Y | 19800-42-1 |
| 3 | Substance identity: chemical name(s) | n/a | Y | Disperse Orange 29 |
| 4 | Chemical composition of the substance | 2 | Y | 20% dyestuff, 10% Reax 85A, 70% water |
| 5 | Chemical purity | 1 | Y | Dispersion 20% dyestuff |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | N | |
| Method | ||||
| 7 | Reference | 1 | N | |
| 8 | OECD, EU, national or other standard method? | 3 | Y | OECD 202 |
| 9 | Justification of the method/protocol if a nonstandard method was used | 2 | Not applicable | |
| 10 | GLP (good laboratory practice) | 3 | Not applicable (study conducted 1990) | |
| Test organism | ||||
| 11 | Organism identity: name | n/a | Y | Daphnia magna |
| 12 | Latin or both Latin and common names reported? | 1 | Y | |
| 13 | Life cycle age / stage of test organism | 1 | N | |
| 14 | Length and/or weight | 1 | Not applicable | |
| 15 | Sex | 1 | Not applicable | |
| 16 | Number of organisms per replicate | 1 | N | |
| 17 | Organism loading rate | 1 | N | |
| 18 | Food type and feeding periods during the acclimation period | 1 | N | |
| Test design / conditions | ||||
| 19 | Test type (acute or chronic) | n/a | Y | Acute |
| 20 | Experiment type (laboratory or field) | n/a | Y | Lab |
| 21 | Exposure pathways (food, water, both) | n/a | Y | Water |
| 22 | Exposure duration | n/a | Y | 24 and 48 hours |
| 23 | Negative or positive controls (specify) | 1 | N | |
| 24 | Number of replicates (including controls) | 1 | N | |
| 25 | Nominal concentrations reported? | 1 | N | |
| 26 | Measured concentrations reported? | 3 | N | |
| 27 | Food type and feeding periods during the long-term tests | 1 | Not applicable | |
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | N | |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for metal toxicity - pH, DOC/TOC, water hardness, temperature) | 3 | N | |
| 30 | Photoperiod and light intensity | 1 | N | |
| 31 | Stock and test solution preparation | 1 | N | |
| 32 | Was solubilizer/emulsifier used if the chemical was poorly soluble or unstable? | 1 | N | |
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | ||
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | ||
| 35 | Analytical monitoring intervals | 1 | N | |
| 36 | Statistical methods used | 1 | N | |
| Information relevant to the data quality | ||||
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by organism’s health (e.g., when mortality in the control >10%) or physical effects (e.g., “shading effect”)? | n/a | Y | |
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | |
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Not reported | |
| 40 | Does system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and organism's nature/habits? | 2 | Not able to assess | |
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | pH not specified | |
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Not specified | |
| 43 | Was toxicity value below the chemical’s water solubility? | 3 | N | The reported LC50 is greater than the WS provided. |
| Results | ||||
| 44 | Toxicity values (specify endpoint and value) | n/a | n/a | 48-hr ELC50 = 70 mg/L |
| 45 | Other endpoints reported - e.g., BCF/BAF, LOEC/NOEC (specify)? | n/a | Y | 24-hr EC50 >100 mg/L |
| 46 | Other adverse effects (e.g., carcinogenicity, mutagenicity) reported? | n/a | N | |
| 47 | Score: % | 29.4 | ||
| 48 | EC reliability code: | 4 | ||
| 49 | Reliability category (high, satisfactory, low): | Not Satisfactory | ||
| 50 | Comments | |||
Robust Study Summaries Form: Aquatic iT
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 1 | Reference: BASF. 1990. Bericht uber die Prufung der akuten Toxizitit an der Goldorfe (Leuciscus idus L.,. Goldvariante). Submitted by ETAD to Environment Canada, August 2008. | |||
| 2 | Substance identity: CAS RN | n/a[1] | ||
| 3 | Substance identity: chemical name(s) | n/a | ||
| 4 | Chemical composition of the substance | 2 | N | |
| 5 | Chemical purity | 1 | N | |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | N | |
| Method | ||||
| 7 | Reference | 1 | N | |
| 8 | OECD, EU, national or other standard method? | 3 | N | |
| 9 | Justification of the method/protocol if a nonstandard method was used | 2 | N | |
| 10 | GLP (good laboratory practice) | 3 | ||
| Test organism | ||||
| 11 | Organism identity: name | n/a | Y | Golden orfe |
| 12 | Latin or both Latin and common names reported? | 1 | Y | |
| 13 | Life cycle age / stage of test organism | 1 | N | |
| 14 | Length and/or weight | 1 | N | |
| 15 | Sex | 1 | N | |
| 16 | Number of organisms per replicate | 1 | N | |
| 17 | Organism loading rate | 1 | N | |
| 18 | Food type and feeding periods during the acclimation period | 1 | N | |
| Test design / conditions | ||||
| 19 | Test type (acute or chronic) | n/a | Y | Acute |
| 20 | Experiment type (laboratory or field) | n/a | N | |
| 21 | Exposure pathways (food, water, both) | n/a | N | |
| 22 | Exposure duration | n/a | Y | 96 hr |
| 23 | Negative or positive controls (specify) | 1 | N | |
| 24 | Number of replicates (including controls) | 1 | N | |
| 25 | Nominal concentrations reported? | 1 | N | |
| 26 | Measured concentrations reported? | 3 | N | |
| 27 | Food type and feeding periods during the long-term tests | 1 | Not applicable | |
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | N | |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for metal toxicity - pH, DOC/TOC, water hardness, temperature) | 3 | N | |
| 30 | Photoperiod and light intensity | 1 | N | |
| 31 | Stock and test solution preparation | 1 | N | |
| 32 | Was solubilizer/emulsifier used if the chemical was poorly soluble or unstable? | 1 | N | |
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | N | |
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | N | |
| 35 | Analytical monitoring intervals | 1 | N | |
| 36 | Statistical methods used | 1 | N | |
| Information relevant to the data quality | ||||
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by the organism’s health (e.g., when mortality in the control >10%) or physical effects (e.g., “shading effect”)? | n/a | N | |
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | |
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | N | |
| 40 | Does system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and organism's nature/habits? | 2 | N | |
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | N | |
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | N | |
| 43 | Was toxicity value below the chemical’s water solubility? | 3 | ||
| Results | ||||
| 44 | Toxicity values (specify endpoint and value) | n/a | LC50 = >100 <220 mg/L | |
| 45 | Other endpoints reported - e.g., BCF/BAF, LOEC/NOEC (specify)? | n/a | NOEC = 100 mg/L | |
| 46 | Other adverse effects (e.g., carcinogenicity, mutagenicity) reported? | n/a | ||
| 47 | Score: % | 9.5 | ||
| 48 | EC reliability code: | 4 | ||
| 49 | Reliability category (high, satisfactory, low): | Not Satisfactory | ||
| 50 | Comments | Not enough data submitted to properly assess the reliability of this study. | ||
Robust Study Summaries Form: Aquatic iT
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 1 | Reference: Environment Canada. 1995. NS submission. Submitted to New Substances Branch, Environment Canada under New Substances Notification Program. | |||
| 2 | Substance identity: CAS RN | n/a[1] | N | |
| 3 | Substance identity: chemical name(s) | n/a | Y | |
| 4 | Chemical composition of the substance | 2 | N | |
| 5 | Chemical purity | 1 | N | |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | N | |
| Method | ||||
| 7 | Reference | 1 | Y | OECD 203 |
| 8 | OECD, EU, national or other standard method? | 3 | Y | |
| 9 | Justification of the method/protocol if a nonstandard method was used | 2 | Not applicable | |
| 10 | GLP (good laboratory practice) | 3 | Y | |
| Test organism | ||||
| 11 | Organism identity: name | n/a | Y | Rainbow trout |
| 12 | Latin or both Latin and common names reported? | 1 | Y | |
| 13 | Life cycle age / stage of test organism | 1 | Y | Mean length 51 mm and mean weight 1.54 g |
| 14 | Length and/or weight | 1 | Y | See above |
| 15 | Sex | 1 | Not applicable | |
| 16 | Number of organisms per replicate | 1 | Y | 10 |
| 17 | Organism loading rate | 1 | Y | |
| 18 | Food type and feeding periods during the acclimation period | 1 | Y | |
| Test design / conditions | ||||
| 19 | Test type (acute or chronic) | n/a | Y | Acute |
| 20 | Experiment type (laboratory or field) | n/a | y | Lab |
| 21 | Exposure pathways (food, water, both) | n/a | y | Water |
| 22 | Exposure duration | n/a | y | 96 hr |
| 23 | Negative or positive controls (specify) | 1 | Y | 3 |
| 24 | Number of replicates (including controls) | 1 | Y | 2 |
| 25 | Nominal concentrations reported? | 1 | Y | 320–3200 mg/L |
| 26 | Measured concentrations reported? | 3 | N | |
| 27 | Food type and feeding periods during the long-term tests | 1 | Not applicable | |
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | N | |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for metal toxicity - pH, DOC/TOC, water hardness, temperature) | 3 | Y | |
| 30 | Photoperiod and light intensity | 1 | Y | |
| 31 | Stock and test solution preparation | 1 | Y | |
| 32 | Was solubilizer/emulsifier used if the chemical was poorly soluble or unstable? | 1 | N | |
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | ||
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | ||
| 35 | Analytical monitoring intervals | 1 | Y | |
| 36 | Statistical methods used | 1 | Y | |
| Information relevant to the data quality | ||||
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by the organism’s health (e.g., when mortality in the control >10%) or physical effects (e.g., ”shading effect”)? | n/a | Y | |
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | |
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Y | |
| 40 | Does system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and organism's nature/habits? | 2 | Y | |
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | Y | |
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | |
| 43 | Was toxicity value below the chemical’s water solubility? | 3 | Unknown water solubility | |
| Results | ||||
| 44 | Toxicity values (specify endpoint and value) | n/a | n/a | 96-hr LC50 |
| 45 | Other endpoints reported - e.g., BCF/BAF, LOEC/NOEC (specify)? | n/a | N | |
| 46 | Other adverse effects (e.g., carcinogenicity, mutagenicity) reported? | n/a | N | |
| 47 | Score: % | 77.5 | ||
| 48 | EC reliability code: | 2 | ||
| 49 | Reliability category (high, satisfactory, low): | Satisfactory Confidence | ||
| 50 | Comments | |||
Robust Study Summaries Form and Instructions: Aquatic iT
| No | Item | Weight | Yes/No | Specify |
|---|---|---|---|---|
| 1 | Reference: Cohle P and R Mihalik. 1991. Early life stage toxicity of C.I. Disperse Blue 79:1 purified presscake to rainbow trout (Oncorhynchus mykiss) in a flow-through system. | |||
| 2 | Substance identity: CAS RN | n/a[1] | ||
| 3 | Substance identity: chemical name(s) | n/a | Disperse Blue 79:1 | |
| 4 | Chemical composition of the substance | 2 | Y | |
| 5 | Chemical purity | 1 | Y | 96.61 |
| 6 | Persistence/stability of test substance in aquatic solution reported? | 1 | N | |
| Method | ||||
| 7 | Reference | 1 | Y | |
| 8 | OECD, EU, national or other standard method? | 3 | Y | ASTM 1983 |
| 9 | Justification of the method/protocol if a nonstandard method was used | 2 | Not applicable | |
| 10 | GLP (good laboratory practice) | 3 | Y | |
| Test organism | ||||
| 11 | Organism identity: name | n/a | Rainbow trout | |
| 12 | Latin or both Latin and common names reported? | 1 | Y | |
| 13 | Life cycle age / stage of test organism | 1 | Y | |
| 14 | Length and/or weight | 1 | Y | |
| 15 | Sex | 1 | Not applicable | |
| 16 | Number of organisms per replicate | 1 | Y | 20 |
| 17 | Organism loading rate | 1 | Y | 0.36 to 4.8 µg/L |
| 18 | Food type and feeding periods during the acclimation period | 1 | Y | |
| Test design / conditions | ||||
| 19 | Test type (acute or chronic) | n/a | Y | Chronic |
| 20 | Experiment type (laboratory or field) | n/a | Y | Lab |
| 21 | Exposure pathways (food, water, both) | n/a | Y | Water |
| 22 | Exposure duration | n/a | Y | 122 days |
| 23 | Negative or positive controls (specify) | 1 | Y | Control and carrier blank |
| 24 | Number of replicates (including controls) | 1 | Y | 2 |
| 25 | Nominal concentrations reported? | 1 | Y | 5 |
| 26 | Measured concentrations reported? | 3 | Y | |
| 27 | Food type and feeding periods during the long-term tests | 1 | Y | |
| 28 | Were concentrations measured periodically (especially in the chronic test)? | 1 | Y | |
| 29 | Were the exposure media conditions relevant to the particular chemical reported? (e.g., for metal toxicity - pH, DOC/TOC, water hardness, temperature) | 3 | Y | |
| 30 | Photoperiod and light intensity | 1 | Y | |
| 31 | Stock and test solution preparation | 1 | Y | |
| 32 | Was solubilizer/emulsifier used if the chemical was poorly soluble or unstable? | 1 | Y | |
| 33 | If solubilizer/emulsifier was used, was its concentration reported? | 1 | Y | |
| 34 | If solubilizer/emulsifier was used, was its ecotoxicity reported? | 1 | Y | No tox value but it was used as a control |
| 35 | Analytical monitoring intervals | 1 | Y | |
| 36 | Statistical methods used | 1 | Y | |
| Information relevant to the data quality | ||||
| 37 | Was the endpoint directly caused by the chemical's toxicity, not by the organism’s health (e.g., when mortality in the control >10%) or physical effects (e.g., “shading effect”)? | n/a | Y | |
| 38 | Was the test organism relevant to the Canadian environment? | 3 | Y | |
| 39 | Were the test conditions (pH, temperature, DO, etc.) typical for the test organism? | 1 | Y | |
| 40 | Does system type and design (static, semi-static, flow-through; sealed or open; etc.) correspond to the substance's properties and organism's nature/habits? | 2 | Y | Flow-through |
| 41 | Was pH of the test water within the range typical for the Canadian environment (6 to 9)? | 1 | Y | |
| 42 | Was temperature of the test water within the range typical for the Canadian environment (5 to 27°C)? | 1 | Y | |
| 43 | Was toxicity value below the chemical’s water solubility? | 3 | Cannot assess. Highest dose tested believed to be at limit of solubility. | |
| Results | ||||
| 44 | Toxicity values (specify endpoint and value) | n/a | n/a | NOEC > 0.005 mg/L |
| 45 | Other endpoints reported - e.g., BCF/BAF, LOEC/NOEC (specify)? | n/a | ||
| 46 | Other adverse effects (e.g., carcinogenicity, mutagenicity) reported? | n/a | ||
| 47 | Score: % | 97.7 | ||
| 48 | EC reliability code: | 1 | ||
| 49 | Reliability category (high, satisfactory, low): | High Confidence | ||
| 50 | Comments | |||
Disperse Orange 29 (19800-42-1)
| Parameter | Phys-Chem/Fate | PBT Profiling | Ecotoxicity |
|---|---|---|---|
| Model Input Parameters | EPIWIN Suite (all models, including: AOPWIN, KOCWIN, BCFWIN BIOWIN and ECOSAR) |
Canadian-POPs (including: CATABOL, BCF Mitigating Factors Model, OASIS Toxicity Model) |
Artificial Intelligence Expert System (AIES)/ TOPKAT/ ASTER |
| SMILES Code[1] | x | x | x |
Appendix 3: Estimated range of upper-bound intake of Disperse Orange 29 from Textiles by various age groups (µg/kg-bw per day) (Lower bound: estimated peak exposure to colourfast textile. Upper bound: conservative maximal exposure)[1]
| Consumer product | 0–6 months[2] | 0.5–4 years[3] | 5–11 years[4] | 12–19 years[5] | 20+ years[6] |
|---|---|---|---|---|---|
| Dermal: wearing of textiles | 0.2 – 4 | 0.2 – 3 | 0.2 – 3 | 0.1 – 2 | 0.1 – 2 |
| Oral: mouthing | 0.1 | 0.1 | N/A[7] | N/A | N/A |
[2] Assumed to weigh 7.5 kg, have body surface area (excluding head and hands) of 0.28 m2(Health Canada 1995) and spend 23 min/d mouthing (Norris & Smith 2002).
[3] Assumed to weigh 15.5 kg, have body surface area (excluding head and hands) of 0.46 m2(Health Canada 1995) and spend 29 min/d mouthing (Norris & Smith 2002).
[4] Assumed to weigh 31.0 kg and have body surface area (excluding head and hands) of 0.80m2(Health Canada 1995).
[5] Assumed to weigh 59.4 kg and have body surface area (excluding head and hands) of 1.4 m2(Health Canada 1995).
[6] Assumed to weigh 70.9 kg and have body surface area (excluding head and hands) of 1.6 m2(Health Canada 1995).
[7] Not applicable
| Consumer product scenario | Assumptions | Estimated exposure |
|---|---|---|
| Wearing of dyed clothing made from synthetic fabrics | Exposure scenario for infants: ConsExpo 4.0, direct dermal contact with product: migration (RIVM 2005). Concentration: 1 wt % (Kraetke & Platzek 2005) Fabric Density: 100 g/m2 (Kraetke & Platzek 2005) General assumptions
Dermal route: Direct dermal contact with product: migration (RIVM 2005)
|
Dermal Chronic dose = 4 µg/kg-bw per day |
| Mouthing of dyed fabrics | Exposure is estimated below for infants aged 0–6 months. The estimated daily intake for ingestion from mouthing:
where:
= (42.8 mg/L × 0.22 mL/min × 0.001 L/mL × 0.005 × 1 × 23 min/day) / 7.5 kg-bw = 0.0001 mg/kg-bw per day |
Oral Chronic dose = 0.1 µg/kg-bw per day |
[2] Product amount = Fabric Density´Amount of fabric´Concentration=100g/m2´0.28m2´1wt%=0.28g.
Carcinogenicity
| CAS RN | DEREK (2008) | CASETOX (2008) | TOPKAT (2008) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer | m-rat | f-rat | m-mice | f-mice | NTP Rodent | NTP m-rat | NTP f-rat | NTP m-mouse | NTP f-mouse | |
| 19800-42-1 (parent) | P | IC | N | IC | IC | IC | P | ND | P | IC |
| 100-01-6 | P | IC | IC | IC | IC | N | P | IC | P | N |
| 123-30-8 | ND | IC | IC | N | IC | IC | P | N | N | N |
| 5307-02-8 | P | N | N | N | N | IC | P | IC | N | P |
Genotoxicity
| CAS RN | Ames | ChrAb | Micronuclei Induction | Mouse Lymphoma mutation | |||
|---|---|---|---|---|---|---|---|
| Derek | CT | TK | Derek | CT# | CT | CT | |
| 19800-42-1 (parent) | P | N | IC | IC | P | N | IC |
| 100-01-6 | P | IC | N | IC | P | N | IC |
| 123-30-8 | ND | N | N* | P | P | N | P |
| 5307-02-8 | P | P | P | ND | P | N | P |
Footnotes
[2] A determination of whether one or more of the criteria of section 64 are met is based upon an assessment of potential risks to the environment and/or to human health associated with exposures in the general environment. For humans, this includes, but is not limited to, exposures from ambient and indoor air, drinking water, foodstuffs, and the use of consumer products. A conclusion under CEPA 1999 on the substances in the Chemicals Management Plan (CMP) Challenge Batches 1-12 is not relevant to, nor does it preclude, an assessment against the hazard criteria specified in the Controlled Products Regulations, which is part of the regulatory framework for the Workplace Hazardous Materials Information System [WHMIS] for products intended for workplace use. Similarly, a conclusion based on the criteria contained in section 64 of CEPA 1999 does not preclude actions being taken under other sections of CEPA or other Acts.
[3] Mutagen 2 in CLP Annex VI Table 3.1, Mutagen category 3 in CLP Annex VI Table 3.2 (European Commission 2008; ESIS 2010).