Screening Assessment for the Challenge
This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Archived
Methyloxirane
(propylene oxide)
Chemical Abstracts Service Registry Number
75-56-9
Environment Canada
Health Canada
July 2008
Table of Contents
- Synopsis
- Introduction
- Substance Identity
- Physical and Chemical Properties
- Sources
- Uses
- Releases to the Environment
- Environmental Fate
- Persistence and Bioaccumulation Potential
- Potential to Cause Ecological Harm
- Potential to Cause Harm to Human Health
- Conclusions
- References
- Appendix 1: Upper-bounding estimates of daily intake of Methyloxirane (CAS No. 75-56-9) for the general population in Canada
- Appendix 2: Predicted concentrations1 of methyloxirane in environmental media
- Appendix 3: Estimates of exposure to methyloxirane from consumer products by adults
- Appendix 4: Summary of health effects information for methyloxirane
The Ministers of the Environment and of Health have conducted a screening assessment of Oxirane, methyl- (methyloxirane), Chemical Abstracts Service Registry Number (CAS RN) 75-56-9, a substance identified in the categorization of the Domestic Substances List as a high priority for action under the Ministerial Challenge. Methyloxirane was identified as a high priority as it was considered to pose greatest potential for exposure (GPE) to individuals in Canada and had been classified by other agencies on the basis of carcinogenicity and genotoxicity. While the substance did meet the ecological categorization criterion for persistence, it did not meet the criteria for bioaccumulation or inherently toxic to aquatic organisms. Therefore, the focus of this assessment of methyloxirane relates to human health aspects.
Methyloxirane was reported to be imported into Canada in 2006 in a quantity greater than 10 000 000 kg. It is used mainly as a monomer in polymer production of polyether polyols, and to a lesser degree in the production of propylene glycol.
Population exposure to methyloxirane in Canada is expected to be predominantly in air, based on its potential releases to this medium and its high vapour pressure. Based on very limited information on levels in environmental media and the results of fugacity modelling, exposure in the general environment is expected to be low. However, exposure to methyloxirane may be elevated during use of consumer products containing the substance.
Based principally on weight of evidence based assessments by several international and national agencies, a critical effect for the characterization of risk to human health is carcinogenicity, based on the observation of nasal cavity tumours in rats and mice. Methyloxirane was also genotoxic in several in vitro and in vivo assays. Therefore, although the mode of induction of tumours has not been fully elucidated, it cannot be precluded that the tumours observed in experimental animals resulted from direct interaction with genetic material. In addition, the upper-bounding estimate of exposure via inhalation during use of consumer products containing methyloxirane may approach or exceed the critical effect level for non-cancer effects in the nasal cavity.
On the basis of the carcinogencity of methyloxirane, for which there may be a probability of harm at any level of exposure, as well as the potential inadequacy of the margin between estimated exposure from products and the critical effect level for non-cancer effects, it is concluded that methyloxirane is a substance that may be entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
On the basis of ecological hazard and reported releases of methyloxirane, it is concluded that this substance is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity, or that constitute or may constitute a danger to the environment on which life depends. Methyloxirane meets the criterion for persistence but it does not meet the criterion for bioaccumulation potential as set out in the Persistence and Bioaccumulation Regulations.
This substance will be included in the Domestic Substances List inventory update initiative, to be launched in 2009. In addition and where relevant, research and monitoring will support verification of assumptions used during the screening assessment and, where appropriate, the performance of potential control measures identified during the risk management phase.
Based on the information available, methyloxirane meets one or more of the criteria set out in Section 64 of the Canadian Environmental Protection Act, 1999.
The Canadian Environmental Protection Act, 1999 (CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or human health. Based on the results of a screening assessment, the Ministers can propose to take no further action with respect to the substance, to add the substance to the Priority Substances List (PSL) for further assessment, or to recommend that the substance be added to the List of Toxic Substances in Schedule 1 of the Act and, where applicable, the implementation of virtual elimination.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherently toxic to aquatic organisms (iT), and were believed to be in commerce; and/or
- met the categorization criteria for greatest potential for human exposure (GPE) or presented an intermediate potential for exposure (IPE), and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in theCanada Gazette, Part I, on December 9, 2006 (Canada 2006), that challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment and to develop and benchmark best practices for the risk management and product stewardship of those substances identified as the highest priorities.
The substance methyloxirane was identified as a high priority for assessment of human health risk because it was considered to present GPE and had been classified by other agencies on the basis of carcinogenicity and genotoxicity. The Challenge for methyloxirane was published in the Canada Gazette on February 3, 2007 (Canada 2007a). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, submissions of information were received.
Although methyloxirane was determined to be a high priority for assessment with respect to human health and it also met the ecological categorization criterion for persistence, it did not meet the criteria for potential for bioaccumulation and inherent toxicity for aquatic organisms. Therefore, this assessment focuses principally on information relevant to the evaluation of risks to human health.
Under CEPA 1999, screening assessments focus on information critical to determining whether a substance meets the criteria for defining a chemical as toxic as set out in section 64 of the Act, where
"64. [...] a substance is toxic if it is entering or may enter the environment in a quantity or concentration or under conditions that
- have or may have an immediate or long-term harmful effect on the environment or its biological diversity;
- constitute or may constitute a danger to the environment on which life depends; or
- constitute or may constitute a danger in Canada to human life or health."
Screening assessments examine scientific information and develop conclusions by incorporating a weight of evidence approach and precaution.
This screening assessment includes consideration of information on chemical properties, hazards, uses and exposure, including the additional information submitted under the Challenge. Data relevant to the screening assessment of this substance were identified in original literature, review and assessment documents, stakeholder research reports and from recent literature searches, up to July 2007. Key studies were critically evaluated; modelling results may have been used to reach conclusions. Evaluation of risk to human health involves consideration of data relevant to estimation of exposure (non-occupational) of the general population, as well as information on health hazards (based principally on the weight of evidence assessments of other agencies that were used for prioritization the substance). Decisions for human health are based on the nature of the critical effect and/or margins between conservative effect levels and estimates of exposure, taking into account confidence in the completeness of the identified databases on both exposure and effects, within a screening context. The screening assessment does not represent an exhaustive or critical review of all available data. Rather, it presents a summary of the critical information upon which the conclusion is based.
This screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. This assessment has undergone external written peer review/consultation. Comments on the technical portions relevant to human health were received from scientific experts selected and directed by Toxicology Excellence for Risk Assessment (TERA), including John Christopher (California Department of Toxic Substances Control), Michael Jayjock (The Lifeline Group), Donna Vorhees (The Science Collaborative) and Joan Strawson (TERA). Comments on these section were also received from Gradient Corporation. While external comments were taken into consideration, the final content and outcome of the screening risk assessment remain the responsibility of Health Canada and Environment Canada. Additionally, the draft of this screening assessment was subject to a 60-day public comment period. The critical information and considerations upon which the assessment is based are summarized below.
Common synonyms: propylene oxide, 1,2-epoxypropane, methyl ethylene oxide.
A summary of key physical and chemical properties for methyloxirane is presented in Table 1. At room temperature, methyloxirane is a colourless liquid, is highly soluble in water and very volatile.
| Property | Value/Units | Reference |
|---|---|---|
| [*] Temperature 20–25°C [**] Original values reported in non-SI units |
||
| Chemical structure | 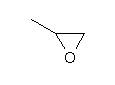 |
|
| Molecular weight | 58.08 g/mol | (PhysProp 2003) |
| Physical state (normal temperature and pressure) | Colourless liquid with a sweet, ether-like odour | (EURAR 2000) |
| Density | 831 kg/m3 (at 20°C) | BASF 1977a |
| Boiling point (BP) | 33.9–35°C | (American Chemical Society 1952; Jefferson Chemical Company 1960; Plunkett 1987; BASF 1995; PhysProp 2003) |
| Melting point (MP) | -112.16 to -111°C | (McDonald et al. 1959; Oetting 1964; Howard 1989; BASF 1995; PhysProp 2003) |
| Log Kow | 0.03; 0.08 ± 0.05 | (Deneer et al. 1988; Howard 1989; Hansch et al. 1995) |
| Log Koc | 0.37 (est.) | (PCKOCWIN 2000) |
| Henry's Law constant (HLC) | 1.226 × 10-4 atm·m3/mol (est.) | (HENRYWIN 2000) |
| Vapour pressure (VP)[*] | 60.7–71.55 kPa; 59.2 kPa (8.59 PSI[**]); 70.9–71.7 kPa (532.1–538 mm Hg[**]) |
(American Chemical Society 1952; BASF 1967; Dow 1977; Boubliket al. 1984; Howard 1989; Aldrich Chemical Co. 2003) |
| Water solubility (WS)[*] | 400 - 590 g/L | (Bogyo et al. 1980; BASF 1995) |
Methyloxirane is not known to occur as a natural product. Peroxidation and chlorohydrination are the two major processes used to manufacture methyloxirane from propylene in large quantities.
Under information reported pursuant to the CEPA 1999 section 71 Notice with respect to methyloxirane, no Canadian companies reported manufacturing this substance in a quantity greater than or equal to 100 kg in 2006. Canadian companies reported importing methyloxirane in a quantity greater than 10 000 000 kg for the 2006 calendar year (Canada 2007b).
According to submissions made under section 71 of CEPA 1999, from the Challenge questionnaire submission and other data voluntarily submitted (Canada 2007b), as well as from other available scientific and technical literature, methyloxirane is used mainly as a monomer in polymer production of polyether polyols. Polyether polyols are used in the production of polyurethane foams for the furniture and automotive industries. Methyloxirane may also be used in the manufacture of propylene glycol, as a starch modifying agent in food, in potential food contact applications, in cosmetics and personal care products, resins, ink, synthetic lubricants and in the automotive industry as a detergent additive and corrosion inhibitor in motor fuels, gasket removers, cleaners, petroleum defoamers, fuel additives and adhesives (Dow 1981; Sack et al. 1992; Trent 2001; European Union 2002; Dow 2007). Propylene glycol manufactured from methyloxirane can also be used in the production of unsaturated polyester resin especially in the textile and plastic industries, in pharmaceuticals, and aircraft de-icers (Dow 1981; Trent 2001; European Union 2002; Dow 2007). Propylene glycol is also used in the manufacture of glycol ethers, for use as solvents in paints and varnish (Trent 2001). Methyloxirane itself is also found in paint stripper (Henkel 2008).
Methyloxirane is used for fumigation of dried fruit products and as a fumigant for bulk quantities of several food products such as cocoa, spices, processed nutmeats, starch and gum in the United States (European Union 2002; US EPA 2005). However, the substance is not a registered pesticide in Canada, and food treated in the United States (or elsewhere) and imported into Canada cannot contain more than 0.1 ppm methyloxirane (PMRA 2006).
Methyloxirane is an approved food additive under Health Canada’s Food and Drugs Regulations (Justice Canada 1985) and is subject to conditions which ensure limited use, precluding human exposure through food consumption. It is included as a preservative substance (antimicrobial) on Health Canada’s Natural Health Products non-medicinal ingredient list (HPFB 2007).
Methyloxirane is not manufactured for commercial purposes in quantities greater than 100 kg in Canada (Canada 2007b). Domestic supply is met by imports from the United States. In 1999, the total Canadian supply and consumption of methyloxirane was 51 500 tonnes, but in 2002, only 38 000 tonnes were imported (CPI 2003). The Canadian National Pollutant Release Inventory reported only one emitter in Ontario in 2005. The reported releases were exclusively to air and the volume released decreased steadily from 10.4 tonnes in 1999 to 5.16 tonnes in 2002 and to 0.059 tonnes in 2005 (NPRI 2006). In recent information gathered under a CEPA 1999 section 71 Notice with respect to methyloxirane, companies reported the release of this substance in 2006 in a quantity less than 50 kg, the cut-off quantity for reporting (Canada 2007b).
The major source of release to the environment is through evaporation and vented gases during production of substances where methyloxirane is used as a chemical intermediate, storage, handling, transport and use. Process vents appear to be the most important sources of atmospheric pollution. Methyloxirane releases may also originate from automobile exhaust and combustion exhaust from stationary sources that burn hydrocarbons. Emissions from waste gas are often removed by air scrubbing, with liquid waste being controlled by incineration (IPCS 1985; Ontario MOE 2001).
Methyloxirane, with a vapour pressure of 71.5 kPa, is expected to exist solely as a vapour in the ambient atmosphere. Methyloxirane may be reduced from the atmosphere by rain washout, considering the very high water solubility of this chemical (IPCS 1985). In water, methyloxirane hydrolyzes rapidly within 11-22 days (table 3), forming propylene glycol. Degradation is accelerated by the presence of chloride ions, indicating that chemical removal in seawater will be more rapid than in freshwater (European Union 2002). Volatilization from water surfaces, based upon an estimated Henry's Law constant of 1.23 ×10-4atm-m3/mole, is expected to be moderate. Methyloxirane is not expected to adsorb to suspended solid and sediment, based on an estimated log Koc value of 0.37, and will have high mobility in this environmental compartment. Evaporation from soil surfaces is therefore likely to be high (Meylan et al. 1986).
The results of a level III fugacity model, which predicts the distribution of methyloxirane in the environment, are summarized in Table 2. It should be noted that the high percentages predicted to partition to soil could be overestimates since the model did not consider fate processes such as volatilization from dry soil and vertical migration in soil. These fate processes are expected to be important for methyloxirane based on its physical and chemical properties.
| Substance released to | Fraction of substance partitioning into each compartment (%) | |||
|---|---|---|---|---|
| Air | Water | Soil | Sediment | |
| Air (100%) | 88.0 | 10.3 | 1.6 | 0.02 |
| Water (100%) | 4.5 | 95.3 | 0.1 | 0.18 |
| Soil (100%) | 7.6 | 18.0 | 74.4 | 0.03 |
| Air, water, soil (33.3% each) | 15.4 | 44.0 | 40.6 | 0.08 |
Persistence
The half-life from the reaction of methyloxirane with photochemically produced hydroxyl radicals in air varies from approximately 7 to 32 days (Table 3), which indicates that this chemical meets the persistence criterion in air (half-life of ≥ 2 days) set out in the Persistence and Bioaccumulation Regulations (Canada 2000)
Methyloxirane is hydrolysable with a half-life of 10.7–21.7 days. Methyloxirane is up to 100% biodegradable and is not persistent in water (Table 3). A further model-predicted biodegradation half-life of 15 days in water (BIOWIN 2000) was obtained and used to predict the half-life of this chemical in soil and sediment by applying Boethling's extrapolation factors (t1/2 water : t1/2 soil : t1/2 sediment = 1:1:4) (Boethling 1995). According to these values, it can be concluded that this chemical does not meet the persistence criteria in water and soil (half-lives = 182 days) and sediments (half-life = 365 days) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
| Fate process | Degradation value | Degradation endpoint/units |
Reference |
|---|---|---|---|
| · OH radical reaction in air | Rate constant (10-12 cm3/molecule · sec) |
Pitts 1979; Winer et al. 1979 ; Zetsch and Stahl 1981; Edney et al. 1986; Wallington et al. 1988; Atkinson 1989 | |
| 6.6–32.3 | Half-life (days) | ||
| Hydrolysis (at 20–25 °C) | Rate constant (10-6 · s-1) |
Nichols and Ingham 1955; Koskikallio and Whalley 1959; Mabey and Mill 1978; Sato et al. 1985 | |
| 10.7 - 21.7 | Half-life (days) | ||
| Biodegradation in water | 12–14; 67; 74; 93–98; 90–100 |
Biodegradation (%) | Waggy and Payne 1974; BASF 1977b; Dow Deutschland 1978; Miller and Watkinson 1985; Chemicals Inspection and Testing Institute 1992 |
Bioaccumulation
Experimental and modelled log Kow values of 0.03 and 0.37, respectively, indicate that the potential for bioaccumulation is likely to be low. Modelled bioaccumulation (BAF) and bioconcentration (BCF) factors of 1 to 13 L/kg (Table 4) indicate that methyloxirane does not meet the bioaccumulation criteria (BCF /BAF = 5000) as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
| Test organism | Endpoint/Units | Value | Reference |
|---|---|---|---|
| Fish | BAF (wet weight, L/kg) | 1 | Modified Gobas BAF T2MTL (Arnot and Gobas 2003) |
| Fish | BCF (wet weight, L/kg) | 1–13 | OASIS Forecast 2005; Modified Gobas BCF 5% T2LTL (Arnot and Gobas 2003) BCFWIN 2000 |
As indicated earlier, methyloxirane meets the criterion for persistence but it does not meet the criterion for bioaccumulation potential as set out in the Persistence and Bioaccumulation Regulations (Canada 2000).
Experimental and modelled ecotoxicological data for methyloxirane indicate low to moderate toxicity to aquatic organisms. Acute experimental LC50 values for fish vary from 89 to 219 mg/L (Bridie et al. 1979; Crews, 1974). No toxicity data for non-aquatic non-mammalian organisms were identified.
The National Pollutant Release Inventory reports releases of 0.059 tonnes of methyloxirane to air by one emitter in Ontario in 2005 (NPRI 2006). In recent information gathered under section 71 of CEPA 1999, companies reported the release of this substance in 2006 in a quantity less than 50 kg, the cut off quantity for reporting (Canada 2007b). Methyloxirane release may also originate from automobile exhaust and combustion exhaust from stationary sources that burn hydrocarbons (IPCS 1985; Ontario MOE 2001). Given the quantity and nature of these releases, they are deemed unlikely to result in significant exposure of organisms in the environment.
Exposure Assessment
Very limited information is available on concentrations of methyloxirane in environmental media, on which to base a quantitative estimate of population exposure. In limited studies, methyloxirane was not detected in different media; however, detection limits are available from these studies, i.e., a US pilot study for indoor and outdoor air, as well as surface water and sediment data from a Japanese monitoring study (Sheldon and Jenkins 1990; Japan MOE 1995). Use of detection limits from the Japanese study as surrogates for drinking water and soil in Canada is considered to be inappropriate for estimating intake. Based on the Canadian uses and release patterns and physical and chemical properties of methyloxirane, its presence in water and soil is expected to be minimal in relation to levels in air, the expected predominant source of exposure. Using the detection limits for indoor and outdoor air in the US study, the upper bounding estimates of exposure to methyloxirane for the general population range from 0.06 µg/kg-bw per day in the 60 + years age group to 0.18 µg/kg-bw per day in the 0.5 to 4 years age group (Appendix 1). In addition, the maximum residue limit of 0.1 ppm methyloxirane in imported fumigated nutmeat was used to calculate the intake estimate from the nuts and seeds category, as no other quantitative data for methyloxirane in food were identified. The calculated intake estimates from food were negligible compared to those from air. In light of the uncertainty associated with the use of detection limits as surrogate concentrations for air and the lack of quantitative information on levels in water and soil in Canada, estimated concentrations for these media were modelled using ChemCan Ver.6.0 (CEMC 2003) based on the release quantity of 50 kg/year, the cutoff quantity for reporting under the recent section 71 notice of CEPA 1999 (Canada 2007b). When modelled as a release to air, the expected predominant medium of release, the predicted concentrations for air, water and soil (Appendix 2) are much lower than those used to estimate the intakes presented in Appendix 1. This further illustrates the conservative nature of these estimates. Although the release quantity of 50 kg/year does not take into account potential releases from products containing methyloxirane, potential releases from these products are expected to be predominantly to air based on the uses of this substance in Canada. Contribution of releases from products to the general environment would likely already be accounted for when using the detection limits in the upper-bounding estimate of exposure outlined above. Confidence in the database on environmental exposure is considered very low, since method detection limits and modelling are used to estimate exposures and because the initial inputs to the exposure models are based solely on theoretical release amounts.
Based on the available information on uses of methyloxirane in Canada, consumer products could represent a source of direct exposure for users. To assess the potential increase in exposure to methyloxirane from use of consumer products containing this substance, estimates of resulting airborne concentrations and daily intake for the Canadian adult population (20–59 years old) were made for paint stripper and acrylic aerosol spray paint (Appendix 3). These products were selected because they represent important product uses of methyloxirane or contain substances in which methyloxirane may be present as a residual. The Canadian adult population is expected to be the principal user of these products. Based on these screening estimates, inhalation from paint stripper could contribute substantially to exposure, while estimated inhalation exposure from acrylic aerosol spray paint is lower. Dermal intake from the use of these consumer products is considered to be negligible based on the high vapour pressure and low log KOW of methyloxirane. Confidence in these estimates of exposure from consumer products is low, however, as they were predicted in the absence of data on actual emissions of methyloxirane from the products.
Health Effects Assessment
On the basis of investigations in experimental animals, methyloxirane has been classified by the International Agency for Research on Cancer (IARC) as Group 2B – “possibly carcinogenic to humans” (IARC 1994), by the European Community as Category 2 Carcinogen – “regarded as if carcinogenic to humans” (European Commission 1999; European Commission 2001; European Union 2002; ESIS 2006), by the US EPA as Group B2 – “probable human carcinogen” (US EPA 1994) and by the National Toxicology Program (NTP) as “reasonably anticipated to be a human carcinogen” (NTP 2005). These classifications were based on observed nasal cavity epithelial tumours in male and female mice and a low incidence of papillary adenomas in the nasal cavity of both sexes of rats exposed via inhalation to 400 ppm (964 mg/m3) methyloxirane for 103 weeks (NTP 1985; Renne et al. 1986) (see Appendix 4 for an overview of the toxicological database). In an additional inhalation study, male rats exposed to 300 ppm (723 mg/m3) methyloxirane for 123–124 weeks had an increased incidence of carcinomas of the respiratory tract and three males exposed to 30 or 300 ppm (72 or 723 mg/m3) displayed malignant nasal cavity tumours (Kuper et al. 1988). As well, mice exposed via inhalation to 400 ppm (964 mg/m3) had significantly increased combined incidences of hemangiomas and hemangiosarcomas in the nasal cavity (NTP 1985; Renne et al. 1986). When female rats were exposed orally to 15 or 60 mg/kg-bw methyloxirane twice weekly for 150 weeks, an exposure-dependent increase in the incidence of stomach tumours was observed (no statistical analysis) (Dunkelberg 1982). Studies by Dunkelberg (1979; 1981) and Walpole (1958) demonstrated that methyloxirane can induce local sarcomas at the site of subcutaneous injection in mice and rats, respectively. While in some of the aforementioned studies the incidences of nasal cavity tumour did not reach statistical significance, they were considered to be exposure related in view of the rarity of this tumour in concurrent and historical controls (NTP 1985; Kuper et al. 1988; EU 2002). The only data available from human studies are inadequate to evaluate the carcinogenicity of methyloxirane in humans (IARC 1994; EU 2002).
The European Community has classified methyloxirane as a Category 2 Mutagen – “Regarded as if mutagenic to humans” (European Commission 1999; European Commission 2001; EU 2002; ESIS 2006). This classification was based on the consensus opinion of a panel of specialized experts that there was “clear evidence for mutagenicity of methyloxirane in somatic cells in vitroand in vivo” (European Commission 1999). Methyloxirane was genotoxic in a number of in vitro assays, including tests for gene mutation in mammalian and non-mammalian cells, and chromosomal aberrations and micronucleus induction in mammalian cells (Appendix 4). It also induced DNA strand breaks and formed DNA adducts in vitro. Additionally, positive results were observed in vivo in rodents and in Drosophila,including micronuclei induction, chromosomal aberrations, DNA lesions or the formation of DNA adducts; however some in vivo assays for these endpoints were also negative. The results of dominant lethal assays in rodents were negative. A review (EU 2002) of six studies involving occupational exposure noted that no conclusions could be drawn from these studies regarding the mutagenicity of methyloxirane in humans (Theiss et al. 1981; Pero et al. 1982 and 1985; Van Sittert and de Jong, 1985; de Jong et al. 1988; Hogstedt et al. 1990; Viktorova et al. 1994). A recent small pilot study has shown significantly increased blood levels of DNA adducts and sister chromatid exchanges in workers exposed to methyloxirane although confounding factors contributing to cytogenetic effects could not be excluded (Czene et al. 2002).
Although a thorough analysis of the mode of action is beyond the scope of this screening assessment, it is recognized that non-neoplastic cellular proliferation may contribute to tumorgenesis above a certain level of exposure. However, a potential role of genetic damage in the development of tumours cannot be discounted in light of the considerable evidence of genotoxicity. A number of studies have been recently conducted to examine the role of glutathione depletion in methyloxirane related tumourgenesis in both rats and mice (Morris et al. 2004; Lee et al. 2005; Morris and Pottenger 2006). The potential mode of action for induction of tumours by methyloxirane is currently being evaluated by the United States Environmental Protection Agency (US EPA 2005) After an initial review of data submitted by industry, the US EPA has indicated that a threshold mode of action for the carcinogenic effects is “highly plausible”; however, the information is being reviewed further (US EPA 2006). In addition, Albertini and Sweeney (2007) examined the genotoxicity profile for methyloxirane and concluded that its DNA-reactive genotoxicity may be necessary but not sufficient for carcinogenicity and that a complex mode of action is likely.
In an assessment by the European Union (EU 2002) it was concluded that the carcinogenicity of methyloxirane is primarily confined to the sites of initial contact but that the relative contribution to the carcinogenic process made by irritation, consequential proliferative response, and genotoxicity is unclear based on current scientific knowledge. The EU concluded that due to methyloxirane’s direct acting nature and mutagenic activity, the carcinogenic hazard of methyloxirane expressed in animals is considered relevant to humans.
The critical non-neoplastic effects induced by methyloxirane occur in the nasal cavity. The lowest-observed-(adverse)-effect concentration (LO(A)EC) identified was 71 mg/m3 in rats exposed via inhalation for 123–124 weeks, based on a slight increase in the incidence of “nest-like infolds” in the nasal epithelium of exposed animals (Kuper et al. 1988). The EU (2002) report identifies this value as the ‘minimal’ LOAEC for nasal epithelial effects. As well, the US EPA (US EPA 1990) considers this effect level a LOAEC for deriving their Reference Concentration (RfC) for chronic inhalation exposure for methyloxirane. Likewise, in terms of a recent reregistration eligibility decision for the use of methyoxirane as a pesticide, the US EPA considered this effect level to be a no-observed-adverse-effects concentration, or NOAEC (US EPA 2005). Similar effects were observed in chronic studies in mice at a higher exposure level of 474 mg/m3 (NTP 1985; Renne et al. 1986) as well as in both rats and mice exposed for shorter durations at 5- to 15-fold higher concentrations, e.g., 362 mg/m3 (Eldridge et al. 1995). In chronic studies in both species, these effects were observed at the lowest exposure levels tested.
The confidence in the toxicity database is high, as data for acute toxicity, carcinogenicity, repeated dose toxicity, genetic toxicity and reproductive and developmental toxicity are available, although there is uncertainty in the mode of induction of tumours.
Characterization of Risk to Human Health
Based principally on the weight of evidence based assessments of several international and national agencies (IARC, EU, US EPA and US NTP), a critical effect for characterization of risk to human health for methyloxirane is carcinogenicity, for which a mode of induction involving direct interaction with genetic material cannot be precluded.
With respect to non-cancer effects, comparison of the critical non-neoplastic effect level in chronically exposed experimental animals (i.e., 71 mg/m3) with the upper bounding estimate of general population exposure via inhalation--the expected principal route of exposure, the estimation of which was based on detection limits in a U.S. study (i.e., 0.31 µg/m3)--results in a margin of exposure of approximately 229 000. However, if the conservative upper bounding estimate of air-borne concentration during use of consumer products containing methyloxirane is considered (i.e., 200 mg/m3in paint stripper), the resulting margin of exposure would be less than 1. If estimated exposure from products is compared to the lowest effect level for short-term repeated exposure (i.e., 362 mg/m3), which may be more appropriate in light of the infrequent use patterns for such products, the resulting margin of exposure would be approximately 2. Thus, while the margin of exposure for non-neoplastic effects is adequate for exposure in the general environment, the margin for consumer product exposure scenarios (although conservative in nature), may not be adequate to account for uncertainties in the databases on exposure and effects.
Uncertainties in Evaluation of Risk to Human Health
The scope of this screening assessment does not take into account possible differences between humans and experimental species in sensitivity to effects induced by this substance, particularly in light of the limited data available for humans. However, similar effects are observed in both rodent species tested and physiological toxicokinetic modelling (Csandy and Filser 2007) suggests that the toxicokinetics of methyloxriane are similar in humans and rats. In addition, the mechanism of tumour induction has not been fully elucidated; however, the data suggest that both genotoxic and non-genotoxic mechanisms may play a role. As well, there are significant uncertainties with respect to the extent of the exposure of the general population to methyloxirane. However, since the estimates of exposure presented here are conservative, confidence is high that actual exposure levels do not exceed these estimates.
Based on the available information, it is concluded that methyloxirane is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity, or that constitute or may constitute a danger to the environment on which life depends.
On the basis of the carcinogencity of methyloxirane, for which there may be a probability of harm at any level of exposure, as well as the potential inadequacy of the margin between estimated exposure from products and the critical effect level for non-cancer effects, it is concluded that methyloxirane is a substance that may be entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is therefore concluded that methyloxirane does not meet the criteria in paragraph 64a and 64b of CEPA 1999, but it does meet the criteria in paragraph 64c of CEPA 1999. Additionally, methyloxirane meets the criterion for persistence but it does not meet the criterion for bioaccumulation potential as set out in thePersistence and Bioaccumulation Regulations.
Agurell E, Cederberg H, Ehrenberg L, Lindahl-Kiessling K, Rannug U, Tornqvist M. 1991. Genotoxic effects of ethylene oxide and propylene oxide: a comparative study. Mutat Res 250(1-2):229-37 [cited in IARC 1994; EU 2002].
Aldrich Chemical Co. 2003. Material safety data sheet: propylene oxide. Catalog No. 240397.
[ACC] American Chemistry Council. 2005. Methyl oxirane: results of a 20-day repeated dose inhalation toxicity study in male mice and male rats. Report No.: TSCATS 8EHQ--05-16044B.
Albertini RJ, Sweeney LM. 2007. Propylene Oxide: Genotoxicity Profile of a Rodent Nasal Carcinogen. Crit Rev Toxicol 37:489-520.
American Chemical Society. 1952. Monograph series: Glycols. Curme GO Jr., Johnston F, editors. New York (NY): Reinhold. [cited in European Union Risk Assessment Report 2000].
Arnot JA, Gobas FA. 2003. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci 22(3):337-345.
Atkinson R. 1989. Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds. J Phys Chem Ref Data Monograph 1. 246 p.
Antonova VI, Zommer EA, Kuznetsova AD, Petrova NA. 1981. Toxicology of propylene oxide and regulation of its level in water. Gig Sanit 7:76-9 [cited in EU 2002; US EPA 2005].
BASF AG. 1967. Analytisches Labor, unveroeffentlichte Untersuchung, TA/PV-Vg L 540 [cited in European Union Risk Assessment Report, 2000].
BASF AG. 1977a. Analytisches Labor, unveroeffentlichte Untersuchung, BRU 77.119 [cited in European Union Risk Assessment Report, 2000].
BASF AG. 1977b. Oekologielabor, unpublished report of BASF AG, May 6 1977 [cited in European Union Risk Assessment Report, 2000].
BASF AG. 1995. Sicherheitsdatenblatt Propylenoxid [cited in European Union Risk Assessment Report, 2000].
[BCFWIN] BioConcentration Factor Program for Windows. 2000. Version 2.15. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[BIOWIN] Biodegradation Probability Program for Windows. 2000. Version 4.01. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Blair D, Osborne GP. 1977. Acute toxicity studies on propylene oxide: acute inhalation toxicity and 10 day repeated exposure study. London : Shell Research. Report No.: TLGR.0032.77 [cited in EU 2002].
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4):741-752.
Bogyo DA, Lande SS, Meylan WM, Howard PH, Santodonato J. 1980. Investigation of selected potential environmental contaminants: Epoxides. Report to U. S. Environmental Protection Agency, Office of Toxic Substances, Washington, DC. New Jersey (US): Syracuse Research Corporation.
Bridie, A.L., C.J.M. Wolff, and M. Winter, 1979. The Acute Toxicity of Some Petrochemicals to Goldfish. Water Res. 13(7):623-626.
Boogaard PJ, Rocchi PS, Van Sittert NJ. 1999. Biomonitoring of exposure to ethylene oxide and propylene oxide by determination of hemoglobin adducts: correlations between airborne exposure and adduct levels. Int Arch Occup Environ Health 72(3):142-50.
Bootman J, Lodge DC, Whalley HE. 1979. Mutagenic activity of propylene oxide in bacterial and mammalian systems. Mutat Res 67(2):101-12 [cited in IARC 1994; EU 2002].
Boublik T, Fried V, Hala E. 1984. The vapor pressure of pure substances: selected values of the temperature dependence of the vapor pressures of some pure substances in the normal and low-pressure region. (Vol 17). 2nd rev. ed. Amsterdam (NL): Elsevier. 241 p.
Canada. 1999. Canadian Environmental Protection Act, 1999 = Loi canadienne sur la protection de l’environnement, 1999. Statutes of Canada = Statuts du Canada, Chapter 33. Act assented to September 14, 1999. Ottawa: Queen’s Printer. Available at Canada Gazette Part III, Vol. 22, No. 3 (accessed August 3, 2007).
Canada. 2000. Persistence and Bioaccumulation Regulations. Ottawa : Public Works and Government Services Canada. Canada Gazette, Part II. Vol. 134. [cited 2006 August].
Canada. 2006. Department of the Environment, Department of Health. Canadian Environmental Protection Act, 1999: Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Ottawa: Public Works and Government Services Canada. Canada Gazette Part I, Vol. 140, No. 49.
Canada, 2007a, Department of the Environment, Department of Health. Substance profile for the challenge methyl oxirane (propylene oxide) CAS No. 75-56-9.
Canada. 2007b. Canadian Environmental Protection Act, 1999. Notice with respect to certain substances on the Domestic Substances List (DSL) . Ottawa : Public Works and Government Services. Canada Gazette Part I, Vol. 141, No.5.
Canter DA, Zeiger E, Haworth S, Lawlor T, Mortelmans K, Speck W. 1986. Comparative mutagenicity of aliphatic epoxides in salmonella. Mutat Res 172(2):105-38 [cited in IARC 1994; EU 2002].
Castelain P, Criado B, Cornet M, Laib R, Rogiers V, Kirsch-Volders M. 1993. Comparative mutagenicity of structurally related aliphatic epoxides in a modified Salmonella/microsome assay. Mutagenesis 8(5):387-93 [cited in IARC 1994].
Center for Chemical Hazards Assessment. Report No.: (EPA-560/11-80-005). Available from: NTIS No. PB80-183197.
ChemCAN. 2003. ChemCAN: lvel III. fugacity model of regional fate of chemicals. Version 6.00. Peterborough (ON): Canadian Environmental Modelling Centre, Trent University.
Chemicals Inspection and Testing Institute. 1992. Biodegradation and bioaccumulation data of existing chemicals based on the CSCL Japan. Japan : Chemical Industry Ecology, Toxicology and Information Center.
Chovanec M, Naslund M, Spivak I, Dusinska M, Cedervall B, Kolman A. 1998. Rejoining of DNA strand breaks induced by propylene oxide and epichlorohydrin in human diploid fibroblasts. Environ Mol Mutagen 32(3):223-8.
[CPI] Consumer Price Index. 2003. CPI Product Profiles. [Internet]. Toronto (ON): Camford Information Service.
Cookson MJ, Sims P, Grover PL. 1971. Mutagenicity of expoxides of polycyclic hydrocarbons correlated with carcinogenicity of parent hydrocharbons. Nat New Biol 234:86-187.
Crews RC. 1974. Effects of Propylene Oxide on Selected Species of Fishes. Tech. Rep. AFATL-TR-74-183, Environics Office, Air Force Armament Lab., Eglin Air Force Base, F L:13.
Csanady GA, Filser JG. 2007. A physiological toxicokinetic model for inhaled propylene oxide in rat and human with special emphasis on the nose. Toxicol Sci 95(1):37-62.
Czene K, Osterman-Golkar S, Yun x , Li G, Zhao F, Perez HL, Li M, Natarajan AT, Segerback D. 2002. Analysis of DNA and hemoglobin adducts and sister chromatid exchanges in a human population occupationally exposed to propylene oxide: a pilot study. Cancer Epidemiol Biomarkers Prev 11(3):315-8.
de Jong G, Van Sittert NJ, Natarajan AT. 1988. Cytogenetic monitoring of industrial populations potentially exposed to genotoxic chemicals and of control populations. Mutat Res 204(3):451-64. [cited in EU 2002; IARC 1994]
Dean BJ, Brooks TM, Hodson-Walker G, Hutson DH. 1985. Genetic toxicology testing of 41 industrial chemicals. Mutat Res 153(1-2):57-77. [cited in IARC 1994; EU 2002].
Dean BJ, Hodson-Walker G. 1979. An in vitro chromosome assay using cultured rat-liver cells. Mutat Res 64(5):329-37. [cited in IARC 1994; EU 2002].
Deneer JW, Sinnige TL, Seinen W, and Hermens JLM. 1988. Quantitative Structure-Activity Relationship for the Acute Toxicity of Some Epoxy Compounds to the Guppy. Aquatic Toxicology, 13, 195-204 [cited in EU 2002].
Djuric Z, Hooberman BH, Rosman L, Sinsheimer JE. 1986. Reactivity of mutagenic propylene oxides with deoxynucleosides and DNA. Environ Mutagen 8(3):369-83. [cited in IARC 1994; EU 2002].
[Dow] Dow Chemical Company. 2007. Technical data sheet [Internet]. Dow Chemical Company. [cited 2007 Sept].
[Dow] Dow Chemical Company. 1981. A guide to glycols. Midland (MI): Dow Chemical Company. Form No. 117-00991-89.
[Dow] Dow Chemical Company. 1977. Among the most versatile of intermediates...Alkylene oxides from Dow. Michigan (US): Dow Chemical Company [cited in EURAR 2000].
Dow Deutschland. 1978. NTIS/OTS 0509917 # 407875003 [cited in IUCLID dataset 2000].
Dunkelberg H. 1981. Carcinogenic activity of ethylene oxide and its reaction products 2-chloroethanol, 2-bromoethanol, ethylene glycol and diethylene glycol: 1. Carcinogenicity of ethylene oxide in comparison with 1,2-propylene oxide after subcutaneous administration in mice. Zentralbl Bakteriol Mikrobiol Hyg [B] 174(5):383-404 [cited in IARC 1994; EU 2002]
Dunkelberg H. 1982. Carcinogenicity of ethylene oxide and 1,2-propylene oxide upon intragastric administration to rats. Br J Cancer 46(6):924-33.
Dunkelberg H. 1979. On the oncogenic activity of ethylene oxide and propylene oxide in mice. Br J Cancer 39(5):588-9.
[EHD] Environmental Health Directorate.1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report December 1998. Ottawa (ON): Health Canada, Environmental Health Directorate, Priority Substances Section.
Edney EO, Kleindienst TE, Corse EW. 1986. Room temperature rate constants for the reactions of OH with selected chlorinated and oxygenated hydrocarbons. Int J Chem Kinet 18:1355-1371 [cited in European Union Risk Assessment Report 2000].
Eldridge SR, Bogdanffy MS, Jokinen MP, Andrews LS. 1995. Effects of propylene oxide on nasal epithelial cell proliferation in F344 rats. Fundam Appl Toxicol 27(1):25-32 [cited in IARC 1994]
Emmert B, Bunger J, Keuch K, Muller M, Emmert S, Hallier E, Westphal GA. 2006. Mutagenicity of cytochrome P450 2E1 substrates in the Ames test with the metabolic competent S. typhimurium strain YG7108pin3ERb5. Toxicology 228(1):66-76.
[EPIWIN] Estimation Programs Interface for Microsoft Windows. 2004. Version 3.12. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
[ESIS] European Chemical Substances Information System [database on the Internet]. 2006. Methyloxirane. CAS No. 75-56-9. Version 4.50. European Chemical Bureau (ECB).
[EURAR] European Union Risk Assessment Report. 2000. CAS No. 75-56-9.
Methyloxirane (propylene oxide). European Chemicals Bureau, Existing Substances. 2nd Priority List, Volume 23. Luxembourg: Office for Official Publications of the European Communities.
European Commission. 1999. Methyloxirane. Summary Record Meeting of the Commission Working Group on the Classification and Labelling of Dangerous Substances. ECB Ispra 13-15 October, 1999.ECBI/61/99 - Rev. 3. 17.04.
European Commission. 2001. Methyloxirane. Commission Directive 2001/59/EC of 6 August 2001. Annex 1B. Official Journal of the European Communities 21.8.2001. L 225/26.
European Union. 2002. Methyloxirane (Propylene Oxide). European Commission. Joint Research Centre. Institute for Health and Consumer Protection. European Chemicals Bureau (ECB). European Union Risk Assessment Report. 2 nd Priority List. Report No. EUR 20512 EN 23:1-149.
Farooqi Z, Tornqvist M, Ehrenberg L, Natarajan AT. 1993. Genotoxic effects of ethylene oxide and propylene oxide in mouse bone marrow cells. Mutat Res 288(2):223-8 [cited in IARC 1994; EU 2002].
Foureman P, Mason JM, Valencia R, Zimmering S. 1994. Chemical mutagenesis testing in Drosophila. × : results of 70 coded chemicals tested for the National Toxicology Program. Environ Mol Mutagen 23(3):208-27.
Gardner MJ, Coggon D, Pannett B, Harris EC. 1989. Workers exposed to ethylene oxide: a follow up study. Br J Ind Med 46(12):860-5 [cited in IARC 1994].
Garro AJ, Phillips RA. 1980. Detection of mutagen-induced lesions in isolated DNA by marker rescue of Bacillus subtilis phage PHI105. Mutat Res 73 (1):1-14 [cited in EU 2002].
Gosselin RE, Smith RP, Hodge HC, Braddock JE, editors. 1984. Clinical toxicology of commercial products. 5th ed. Baltimore (MD): Williams & Wilkins; Section II Ingredients Index, Propylene oxide; p. 97-98 [cited in EU 2002].
Gulati DK, Witt K, Anderson B, Zeiger E, Shelby MD. 1989. Chromosome aberration and sister chromatid exchange tests in Chinese hamster ovary cells in- vitro. III. Results with 27 chemicals. Environ Mol Mutagen 13 (2):133-93 [cited in IARC 1994; EU 2002].
Hackett PL, Brown MG, Buschbom RL, Clark ML, Miller RA, Music RL, Rowe SE, Schirmer RE, Sikov MR. 1982. Teratogenic study of ethylene and propylene oxide and n-Butyl Acetate. Cincinnati (OH): Department of Health and Human Services (US), Centers for Disease Control & Prevention; NIOSH (National Institute for Occupational Safety and Health). Contract No. 210-80-0013, PB83-258-038.127 p. [cited in IARC 1994].
Hansch C, Leo A, Hoekman D. 1995. Exploring QSAR: hydrophobic, electronic, and steric constants. Washington (DC): American Chemical Society.
Hardin BD, Niemeier RW, Sikov MR, Hackett PL. 1983a. Reproductive-toxicologic assessment of the epoxides ethylene oxide, propylene oxide, butylene oxide, and styrene oxide. Scand J Work Environ Health 9(2 Spec No):94-102.
Hardin BD, Schuler RL, Mcginnis PM, Niemeier RW, Smith RJ. 1983b. Evaluation of propylene oxide for mutagenic activity in 3 in vivo test systems. Mutat Res 117 (3-4):337-44 [cited in IARC 1994; EU 2002].
Harris SB, Schardein JL, Ulrich CE, Ridlon SA. 1989. Inhalation developmental toxicity study of propylene oxide in Fischer 344 rats. Fundam Appl Toxicol 13(2):323-31 [cited in IARC 1994].
Hayes WC, Kirk HD, Gushow TS, Young JT. 1988. Effect of inhaled propylene oxide on reproductive parameters in Fischer 344 rats. Fundam Appl Toxicol 10(1):82-8 [cited in IARC 1994].
Hayes WC, Gushow TS, Kirk HD, Young JT. 1985. Propylene oxide: two-generation inhalation reproduction study in Fischer 344 rats. Midland (MI): Dow Chemical, Health and Environmental Sciences, Mammalian and Environmental Toxicology Research Laboratory. Report No.: D-1734 [cited in EU 2002].
Hemminki A, Vayrynen T, Hemminki K. 1994. Reaction kinetics of alkyl epoxides with DNA and other nucleophiles. Chem Biol Interact 93(1):51-8.
Hemminki K, Falck K. 1979. Correlation of Mutagenicity and 4-p Nitrobenzyl pyridine alkylation by epoxides. Toxicol Lett 4 (2):103-6 [cited in IARC 1994; EU 2002].
Hemminki K, Paasivirta J, Kurkirinne T, Virkki L. 1980. Alkylation products of DNA bases by simple epoxides. Chem Biol Interact 30(3):259-70 [cited in EU 2002].
Henkel Technologies 2008. Material Safety Data Sheet for Loctite Paint stripper.
[HENRYWIN] Henry's Law Constant Program for Microsoft Windows. 2000. Version 3.10. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation.
Hogstedt C, Aringer L, Gustavsson A. 1986. Epidemiologic support for ethylene oxide as a cancer-causing agent. JAMA 255(12):1575-8 [cited in IARC 1994].
Hogstedt B, Bergmark E, Tornqvist M, Osterman-Golkar S. 1990. Chromosomal aberrations and micronuclei in lymphocytes in relation to alkylation of hemoglobin in workers exposed to ethylene oxide and propylene oxide. Hereditas 113(2):133-8 [cited in EU 2002; IARC 1994].
Hogstedt C, Rohlen O, Berndtsson BS, Axelson O, Ehrenberg L. 1979. A cohort study of mortality and cancer incidence in ethylene oxide production workers. Br J Ind Med 36(4):276-80 [cited in IARC 1994].
Hogstedt LC. 1988. Epidemiological studies on ethylene oxide and cancer: an updating. IARC Sci Publ (89):265-70 [cited in IARC 1994].
Howard PH. 1989. Handbook of environmental fate and exposure data for organic chemicals: Vol. I, Large production and priority pollutants. Chelsea (MI): Lewis Publishers.
HPFB (Health Products and Food Branch) 2007. Personal communication from Shahriar Khadem, Natural Health Products Directorate, Health Products and Food Branch
Hughes TJ, Simmons DM, Monteith LG, Claxton LD. 1987. Vaporization technique to measure mutagenic activity of volatile organic chemicals in the Amesalmonella assay. Environ Mol Mutagen 9(4):421-41 [cited in IARC 1994; EU 2002].
IARC (International Agency for Research on Cancer). 1994. Propylene oxide.
IARC Monogr Eval Carcinog Risks Hum 60:181-213.
[IPCS] International Program on Chemical Safety. 1985.Environmental Health Criteria 56, Propylene oxide . Geneva (CH): World Health Organization, United Nations Environmental Programme, International Labour Organization, International Programme on Chemical Safety.
Jacobson KH, Hackley WB, Feinsilver L. 1956. The toxicity of inhaled ethylene oxide and propylene oxide vapors; acute and chronic toxicity of ethylene oxide and acute toxicity of propylene oxide. AMA Arch Ind Health 13(3):237-44 [cited in EU 2002].
Japan, Government of Japan, Ministry of Environment. 1995. Summary of the results of monitoring
of water and bottom sediment: Report on environment survey and wildlife monitoring of chemicals in the environment. [cited 2007 Aug]. Japan Ministry of the Environment
Jefferson Chemical Company. 1960. Technische Broschuere Propylene Oxide [cited in EU 2002].
Jensen O. 1981. Contact allergy to propylene oxide and isopropyl alcohol in a skin disinfectant swab. Contact Dermatitis 7(3):148-50 [cited in IARC 1994]
Jorritsma U, Cornet M, Van Hummelen P, Bolt HM, Vercruysse A, Kirsch-Volders M, Rogiers V. 1995. Comparative mutagenicity of 2-methylpropene (isobutene), its epoxide 2-methyl-1,2-epoxypropane and propylene oxide in the in vitro micronucleus test using human lymphocytes. Mutagenesis 10(2):101-4 [cited in EU 2002].
Justice Canada 1985. Food and Drugs Act (R.S., 1985, c. F-27) [cited 2007 Sept.]
Kautiainen A, Tornqvist M. 1991. Monitoring exposure to simple epoxides and alkenes through gas chromatographic determination of hemoglobin adducts. Int Arch Occup Environ Health 63(1):27-31 [cited in IARC 1994; EU 2002].
Kohda KH, Ninomiya S, Washizu K, Shiraki K, Ebie M, Kawazoe Y. 1987. Mutagenicity of a series of N-alkyl-, N-hydroxyalkyl-, N-haloalkyl- and N-carboxyalkyl-N-nitrosoureas in Escherichia coli tester strains: dependence on the uvrA DNA-repair system. Mutat Res 177(2):219-28 [cited in IARC 1994].
Kolman A, Dusinska M. 1995. Comparison of propylene oxide and epichlorohydrin effects in two transformation tests (C3H/10T1/2 and SHE cells). Toxicol Lett 81(2-3):213-21 [cited in EU 2002].
Kolman A, Spivak I, Naslund M, Dusinska M, Cedervall B. 1997. Propylene oxide and epichlorohydrin induce DNA strand breaks in human diploid fibroblasts. Environ Mol Mutagen 30 (1):40-6.
Kolmark G, Giles NH. 1955. Comparative Studies of Monoepoxides as Inducers of Reverse Mutations in Neurospora. Genetics 40:890-902. [cited in IARC 1994; EU 2002].
Kumar R, Staffas J, Forsti A, Hemminki K. 1995. 32P-postlabelling method for the detection of 7-alkylguanine adducts formed by the reaction of different 1,2-alkyl epoxides with DNA. Carcinogenesis 16(9):483-9.
Kuper CF, Reuzel PG, Feron VJ, Verschuuren H. 1988. Chronic inhalation toxicity and carcinogenicity study of propylene oxide in Wistar rats. Food Chem Toxicol 26(2):159-67.
Koskikallio J, Whalley E. 1959. Effect of pressure on the spontaneous and the base-catalysed hydrolysis of epoxides. Can J Chem 37:783-787.
Lawley PD, Jarman M. 1972. Alkylation by propylene oxide of deoxyribonucleic acid, adenine, guanosine and deoxyguanylic acid. Biochem J 126:893-900
Lee MS, Faller TH, Kreuzer PE, Kessler W, Csanady GA, Putz C, Rios-Blanco MN, Pottenger LH, Segerback D, Osterman-Golkar S et al. 2005. Propylene oxide in blood and soluble nonprotein thiols in nasal mucosa and other tissues of male Fischer 344/N rats exposed to propylene oxide vapors--relevance of glutathione depletion for propylene oxide-induced rat nasal tumors. Toxicol Sci 83(1):177-89.
Lynch DW, Lewis TR, Moorman WJ, Burg JR, Groth DH, Khan A, Ackerman LJ, Cockrell BY. 1984a. Carcinogenic and toxicologic effects of inhaled ethylene oxide and propylene oxide in F344 rats. Toxicol Appl Pharmacol 76(1):69-84 [cited in IARC 1994].
Lynch DW, Lewis TR, Moorman WJ, Burg JR, Gulati DK, Kaur P, Sabharwal PS. 1984b. Sister-chromatid exchanges and chromosome aberrations in lymphocytes from monkeys exposed to ethylene oxide and propylene oxide by inhalation. Toxicol Appl Pharmacol 76(1):85-95 [cited in IARC 1994; EU 2002].
Mabey W, Mill T. 1978. Critical review of hydrolysis of organic compounds in water under environmental conditions. J Phys Chem Ref Data. 7:383-415.
Matthews EJ, Spalding JW, Tennant RW. 1993. Transformation of BALB/c-3T3 cells: V. Transformation responses of 168 chemicals compared with mutagenicity in Salmonella and carcinogenicity in rodent bioassays. Environ Health Perspect 101(Suppl 2):347-482.
McDonald RA, Shrader SA Stull DR. 1959. Vapour pressure and freezing points of 30 organics. J Chem Eng Data 4 (4):311-313 [cited in European Union Risk Assessment Report, 2000].
McGregor D, Brown AG, Cattanach P, Edwards I, Mcbride D, Riach C, Shepherd W, Caspary WJ. 1991. Responses of the L5178Y mouse lymphoma forward mutation assay: V. Gases and vapors. Environ Mol Mutagen 17(2):122-9 [cited in IARC 1994; EU 2002].
McMahon RE, Cline JC, Thompson CZ. 1979. Assay of 855 test chemicals in ten tester strains using a new modification of the Ames test for bacterial mutagens. Cancer Res 39 (3):682-93 [cited in EU 2002].
Meylan W, Papa L, De Rosa CT, Stara JF. 1986. Chemicals of current interest - propylene oxide : health and environmental effects profile. Toxicol Ind Health 2(3): 219-60
Migliore L, Rossi AM, Loprieno N. 1982. Mutagenic action of structurally related alkene oxides on Schizosaccharomyces pombe: the influence, 'in vitro', of mouse-liver metabolizing system. Mutat Res 102(4):425-37 [cited in IARC 1994; EU 2002].
Miller RC, Watkinson RJ. 1985. Propylene oxide: an assessment of ready biodegradability. Sittingbourne (GB): Shell Research, Sittingbourne Research Centre. Group Research Report SBGR.85.064. [cited in UK IUCLID dataset 2000]
Morris AD, Ratcliffe J, Dalziel KL, English JSC. 1998. Allergic contact dermatitis from epoxy propane [abstract]. Contact Dermatitis 38(1):57.
Morris JB, Banton MI, Pottenger LH. 2004. Uptake of inspired propylene oxide in the upper respiratory tract of the F344 rat. Toxicol Sci 81(1):216-24.
Morris JB, Pottenger LH. 2006. Nasal NPSH depletion and propylene oxide uptake in the upper respiratory tract of the mouse. Toxicol Sci 92(1):228-34.
Nichols PL Jr, Ingham JD. 1955. The inductive effect in ethylene oxide ring openings. Amer Chem Soc 77:6547-6551 [cited in European Union Risk Assessment Report, 2000].
Nivard MJ, Czene K, Segerback D, Vogel EW. 2003. Mutagenic activity of ethylene oxide and propylene oxide under XPG proficient and deficient conditions in relation to N-7-(2-hydroxyalkyl)guanine levels in Drosophila. Mutat Res 529(1-2):95-107.
[NHW] Department of National Health and Welfare (CA). 1990. Present patterns and trends in infant feeding in Canada. Ottawa : Canada, Department of National Health and Welfare. NHW Cat No H39-199/1990E. [cited in EHD 1998].
[NPRI] National Pollutant Release Inventory [database on the Internet]. 2006. Gatineau (QC): Environment Canada. [cited 2006 Mar].
[NTP] National Toxicology Program. 2005. 11 th Report on carcinogens. Substance profile: propylene oxide. Research Triangle Park (NC): National Toxicology Program.
[NTP] National Toxicology Program. 1985. Toxicology and carcinogenesis studies of propylene oxide in F344/N rats and B6C3F1 mice (inhalation studies). Research Triangle Park (NC): National Toxicology Program. Technical Report Series 267.
[NTP] National Toxicology Program. 1989-1993. [database on the Internet]. Research Triangle Park (NC): National Toxicology Program. Study number 669217.
[OASIS Forecast] Optimized Approach Based on Structural Indices Set [Estimation Model]. 2005. Version 1.20. Bourgas, Bulgaria: Laboratory of Mathematical Chemistry.
Oetting FL.1964. Low-temperature heat capacity and related thermodynamic functions of propylene oxide. J Chem Physics 41(1):149-153 [cited in European Union Risk Assessment Report, 2000].
Ohnishi A, Hori H, Tanaka I, Yamamoto T, Murai Y. 1988. [Determination of the exposure concentration of propylene oxide to produce neuropathy in rats]. J UOEH 10(3):227-81.
Okuda H, Takeuchi T, Senoh H, Arito H, Nagano K, Yamamoto S, Matsushima T. 2006. Effects of inhalation exposure to propylene oxide on respiratory tract, reproduction and development in rats. J Occup Health 48(6):462-73.
Omura M, Tanaka A, Mori K, Hirata M, Zao M, Inoue N. 1994. Dose-dependent testicular toxicity of propylene oxide in rats induced by repeated intraperitoneal injections. Fukuoka Igaku Zasshi 85(7):204-10.
Ong T, Stewart J, Wen Y, Whong WZ. 1987. Application of SOS umu-test for the detection of genotoxic volatile chemicals and air pollutants. Environ Mutagen 9(2):171-6 [cited in IARC 1994; EU 2002].
[Ontario MOE], Ministry of Environment, Standard Development Branch. 2001 Mar. Ontario Air Standards for propylene oxide . Toronto (ON). Standard Development Branch.
Osterman-Golkar S, Bailey E, Farmer PB, Gorf SM, Lamb JH. 1984. Monitoring exposure to propylene oxide through the determination of hemoglobin alkylation. Scand J Work Environ Health 10(2):99-102 [cited in IARC 1994].
Osterman-Golkar S, Czene K, Lee MS, Faller TH, Csanady GA, Kessler W, Perez HL, Filser JG, Segerback, D. 2003. Dosimetry by means of DNA and hemoglobin adducts in propylene oxide-exposed rats. Toxicol Appl Pharmacol 191(3):245-54.
Ott MG, Teta J, Greenberg HL. 1989. Lymphatic and hematopoietic tissue cancer in a chemical manufacturing environment. Am J Ind Med 16 (6):631-44 [cited in IARC 1994].
[PCKOCWIN] Organic Carbon Partition Coefficient Program for Windows. 2000. Version 1.66. Washington (DC): U.S. Environmental Protection Agency, Office of Pollution Prevention and Toxics. Syracuse (NY): Syracuse Research Corporation.
Pero RW, Bryngelsson T, Widegren B, Hogstedt B, Welinder H. 1982. A reduced capacity for unscheduled DNA synthesis in lymphocytes from individuals exposed to propylene oxide and ethylene oxide. Mutat Res 104(1-3):193-200 [cited in EU 2002].
Pero RW, Osterman-Golkar S, Hogstedt B. 1985. Unscheduled DNA synthesis correlated to alkylation of hemoglobin in individuals occupationally exposed to propylene oxide. Cell Biol Toxicol 1(4):309-14 [cited in EU 2002].
Pfeiffer EH, Dunkelberg H. 1980. Mutagenicity of ethylene oxide and propylene oxide and of the glycols and halohydrins formed from them during the fumigation of foodstuffs. Food Cosmet Toxicol 18(2):115-8 [cited in IARC 1994; EU 2002].
[PhysProp] Interactive PhysProp Database [Internet]. 2003. Syracuse (NY): Syracuse Research Corporation [cited 2003 Mar].
Pitts JN. 1979. Chemical consequences of air quality standards and of control implementation programmes: roles of hydrocarbons, oxides of nitrogen, oxides of sulphur and aged smog in the production of photochemical oxidant and aerosol. California: CA State-wide Air Pollution Center. [cited in IUCLID dataset 2000].
Plna K, Nilsson R, Koskinen M, Segerback D. 1999. 32P-postlabelling of propylene oxide 1- and N(6)-substituted adenine and 3-substituted cytosine/uracil: formation and persistence in vitro and in vivo. Carcinogenesis 20(10):2025-32.
Plunkett ER. 1987. Handbook of Industrial Toxicology. 3rd ed. New York (NY): Chemical Pub. Co.
[PMRA] Pest Management Regulatory Agency. 2006. Pest Management Regulatory Agency Discussion Document June 2006 : Revocation of 0.1 ppm as a General Maximum Residue Limit for Food Pesticide Residues [Regulation B.15.002(1)]
Randerath K, Reddy MV, Gupta RC. 1981. 32P-labeling test for DNA damage. Proc Natl Acad Sci U S A 78(10):6126-9 [cited in IARC 1994].
Renne RA, Giddens WE, Boorman GA, Kovatch R, Haseman JE, Clarke WJ. 1986. Nasal cavity neoplasia in F344/N rats and (C57BL/6 × C3H)F1 mice inhaling propylene oxide for up to two years. J Natl Cancer Inst 77(2):573-82 [cited in IARC 1994].
Reuzel PGJ, Kuper CF. 1981. Subchronic (13 week) inhalation toxicity study of propylene oxide in rats. Zeist, NL: CIVO Institutes TNO. [cited in EU 2002].
Rios-Blanco MN, Ranasinghe A, Lee MS, Faller T, Filser JG, Swenberg JA. 2003a. Molecular dosimetry of N7-(2-hydroxypropyl)guanine in tissues of F344 rats after inhalation exposure to propylene oxide. Carcinogenesis 24(7):1233-8
Rios-Blanco MN, Yamaguchi S, Dhawan-Robl M, Kessler W, Schoonhoven R, Filser JG, Swenberg JA. 2003b. Effects of propylene oxide exposure on rat nasal respiratory cell proliferation. Toxicol Sci 75(2):279-88.
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu. 2007a. Consumer Exposure (ConsExpo) Model [Internet]. Version 4.1. The Netherlands: The National Institute for Public Health and the Environment (Rijksinstituut voor Volksgezondheid en Milieu).
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu. 2007b. Do-it-yourself Products Fact Sheet: To assess the risks for the consumer [Internet]. Report 320104007/2007. RIVM. [cited 2008 April].
[RIVM] Rijksinstituut voor Volksgezondheid en Milieu. 2007c. Paint Products fact Sheet : To assess the risks for the consumer [Internet]. Report 320104008/2007. RIVM. [cited 2008 April].
Rowe VK, Hollingsworth RL, Oyen F, Mccollister DD, Spencer HC. 1956. Toxicity of propylene oxide determined on experimental animals. AMA Arch Ind Health 13(3):228-36 [cited in IARC 1994].
Sack TM, Steele DH, Hammerstrom K, Remmers J. 1992. A survey of household products for volatile organic compounds. Atmos Environ 26A(6):1063-1070.
Sato H, Kidata T, Hori M. 1985. Sterilization of therapeutic immunoadsorbents with aqueous propylene oxide solutions. Int J Artif Organs 8:109-114.
Segerback D, Osterman-Golkar S, Molholt B, Nilsson R. 1994. In vivo tissue dosimetry as a basis for cross-species extrapolation in cancer risk assessment of propylene oxide. Regul Toxicol Pharmacol 20(1 Pt 1):1-14 [cited in IARC 1994].
Segerback D, Plna K, Faller T, Kreuzer PE, Hakansson K, Filser JG, Nilsson R. 1998. Tissue distribution of DNA adducts in male Fischer rats exposed to 500 ppm of propylene oxide: Quantitative analysis of 7-(2-hydroxypropyl)guanine by 32P-postlabelling. Chem Biol Interact 115 (3):229-46.
Shelby MD, Erexson GL, Hook GJ, Tice RR. 1993. Evaluation of a three-exposure mouse bone marrow micronucleus protocol: Results with 49 chemicals. Environ Mol Mutagen 21(2):160-79.
Sheldon LS. Jenkins, P. 1990. Indoor pollutant concentrations and exposures for air toxics: a pilot study.
Proceeding of the 5 th International Conference on Indoor air Quality and Climate; Toronto, Canada; p.759-764.
Simmon VF. 1981. Applications of the Salmonella/Microsome Assay. New York (NY): Springer-Verlag. p 120-6 [cited in IARC 1994].
Sina JF, Bean CL, Dysart GR, Taylor VI, Bradley MO. 1983. Evaluation of the alkaline elution/rat hepatocyte assay as a predictor of carcinogenic/mutagenic potential. Mutat Res 113:357-91 [cited in IARC 1994; EU 2002].
Smyth HF, Carpenter CP, Weil CS, Pozzani US, Striegel JA, Nycum JS. 1969. Range finding toxicity data. (List VII). AIHA J 30:470-6 [cited in EU 2002].
Smyth HF, Seaton J, Fischer L. 1941. The single dose toxicity of some glycols and derivatives. J Ind Hyg Toxicol 23:259-68 [cited in EU 2002]
Snyder CA, Solomon JJ. 1993. The extent and persistence of binding to respiratory mucosal DNA by inhaled tritiated propylene oxide. Cancer Lett 72(3):157-61 [cited in IARC 1994].
Solomon JJ, Mukai F, Fedyk J, Segal A. 1988. Reactions of propylene oxide with 2'-deoxynucleosides and in vitro with calf thymus DNA. Chem Biol Interact 67(3):275-94 [cited in IARC 1994; EU 2002].
Sprinz H, Matzke H, Carter J.(1982) Neuropathological evaluation of monkeys exposed to ethylene and propylene oxide. Kansas City (MI):Midwest Research Institute. (Prepared for NIOSH) (PB 83-134817).
Steinkraus V, Hausen BM. 1994. Contact allergy to propylene oxide. Contact Dermatitis 31(2):120.
Stocker WG, Thiess AM. 1979. Morbidity study on workers exposed to ethylene oxide/propylene oxide. In: The 7th Medichem Congress; 15 September; Gera ;1979. p. 534, 29.3.7.3. [cited in EU 2002].
Svensson K, Olofsson K, Osterman-Golkar S. 1991. Alkylation of DNA and hemoglobin in the mouse following exposure to propene and propylene oxide. Chem Biol Interact 78(1):55-66 [cited in IARC 1994].
Thiess AM, Frentzel-Beyme R, Link R, Stocker WG. 1982. Mortality study on employees exposed to alkylene oxides (ethylene oxide/propylene oxide) and their derivatives. In: Prevention of Occupational Cancer International Symposium; April 1981; Helsinki, Finland. International Labour Office, Geneva, Switzerland : Occupational Safety and Health Series, No. 46. p. 249-259 [cited in IARC 1994; EU 2002].
Thiess AM, Schwegler H, Fleig I, Stocker WG. 1981. Mutagenicity study of workers exposed to alkylene oxides (ethylene oxide/propylene oxide) and derivatives. J Occup Med 23(5):343-7 [cited in EU 2002; IARC 1994].
Trent DL. 2001. Propylene oxide [Internet]. Kirk-Othmer Encyclopedia of Chemical Technology, online version.
Tsuda S, Matsusaka N, Madarame H, Miyamae Y, Ishida K, Satoh M, Sekihashi K, Sasaki YF. 2000. The alkaline single cell electrophoresis assay with eight mouse organs: results with 22 mono-functional alkylating agents (including 9 dialkyl N-nitrosoamines) and 10 DNA crosslinkers. Mutat Res 467(1):83-98.
Tucker JD, Xu J, Stewart J, Baciu PC, Ong TM. 1986. Detection of sister chromatid exchanges induced by volatile genotoxicants. Teratog Carcinog Mutagen 6(1):15-21 [cited in IARC 1994; EU 2002].
[US EPA] United States Environmental Protection Agency. 1986 Sept. Standard scenario for estimating exposure to chemical substances during use of consumer products Vol.1. (EPA contract No. 68-02-3968)
[US EPA] States Environmental Protection Agency. 1997 Aug. Exposure Factors Handbook Vol.3 Activity factors.(EPA/600/P-95/002Fc)
[US EPA] United States Environmental Protection Agency. 1994. Integrated Risk Information System (IRIS): Propylene oxide (CASRN 75-56-9).
[US EPA] United States Environmental Protection Agency. 2006 Aug. Reregistration Eligibility Decision (RED) for Propylene Oxide (EPA 738-R-06-029).
[US EPA] United States Environmental Protection Agency. 1990. Integrated Risk Information System (IRIS): Propylene oxide (CASRN 75-56-9).
[US EPA] United States Environmental Protection Agency. 2005 Sept 26. Propylene Oxide Revised HED Risk Assessment for Reregistration Eligibility Decision (RED) Document (PC Code: 042501, DP Barcode: D321759).
Van Ketel WG. 1979. Contact dermatitis from propylene oxide. Contact Dermatitis 5(3):191-2 [cited in IARC 1994].
Van Sittert NJ, De Jong G. 1985. Biomonitoring of exposure to potential mutagens and carcinogens in industrial populations. Food Chem Toxicol 23(1):23-31. [cited in EU 2002].
Viktorova TV, Karimova LK, Khusnutdinova EK, Rafikov KH S. 1994. Assessment of chromosomal aberrations in workers of propylene and ethylene oxides production. Med Tr Prom Ekol 4:20-3 [cited in EU 2002].
Vogel EW, Nivard MJ. 1998. Genotoxic effects of inhaled ethylene oxide, propylene oxide and butylene oxide on germ cells: sensitivity of genetic endpoints in relation to dose and repair status. Mutat Res 405(2):259-71.
Vogel EW, Nivard MJ. 1997. The response of germ cells to ethylene oxide, propylene oxide, propylene imine and methyl methanesulfonate is a matter of cell stage-related DNA repair. Environ Mol Mutagen 29(2):124-35.
von der Hude W, Carstensen S, Gurtler R, Obe G. 1992. Structure-activity relationships of epoxides: induction of sister-chromatid exchanges in V79 Cells by enantiomeric epoxides. Mutat Res 278(4):289-97 [cited in IARC 1994].
Von der Hude W, Carstensen S, Obe G. 1991. Structure-activity relationships of epoxides: Induction of sister-chromatid exchanges in Chinese hamster V79 cells. Mutat Res 249 (1):55-70 [cited in EU 2002].
von der Hude W, Seelbach A, Basler A. 1990. Epoxides: Comparison of the induction of SOS repair in Escherichia coli PQ37 and the bacterial mutagenicity in the Ames test. Mutat Res 231 (2):205-18 [cited in EU 2002].
Voogd CE, Van Der Stel JJ, Jacobs JJ. 1981. The mutagenic action of aliphatic epoxides. Mutat Res 89(4):269-82. [cited in IARC 1994; EU 2002].
Wade DR, Airy SC, Sinsheimer JE. 1978. Mutagenicity of aliphatic epoxides. Mutat Res 58 (2-3):217-24. [cited in IARC 1994; EU 2002].
Waggy GT, Payne JR. 1974. Environmental impact analysis, product biodegradability testing. Progress report. August 12, 1974. File No. 19751, Research and Development Department, Union Carbide Corporation [cited in European Union Risk Assessment Report 2000].
Wakata A, Miyamae Y, Sato SI, Suzuki T, Morita T, Asano N, Awogi T, Kondo K, Hayashi M. 1998. Evaluation of the rat micronucleus test with bone marrow and peripheral blood: Summary of the 9th collaborative study by CSGMT MMS. Environ Mol Mutagen 32 (1):84-100.
Walles SA. 1974. The influence of some alkylating agents on the structure of DNA in vitro. Chem Biol Interact 9(2):97-103 [cited in EU 2002].
Wallington TJ, Liu R., Dagaut P, and Kurylo M.J. 1988. The gas phase reactions of hydroxyl radicals with a series of aliphatic ethers over the temperature range 240-440 K. Int J Chem Kinet 20:41-49 [cited in European Union Risk Assessment Report, 2000].
Walpole AL. 1958. Carcinogenic action of alkylating agents. Ann NY Acad Sci 68:750-61 [cited in IARC 1994].
Weil CS, Condra N, Haun C, Streigel JA. 1963. Experimental carcinogenicity and acute toxicity of representative epoxides. Am Ind Hyg Assoc J 24:305-25 [cited in EU 2002].
Winer AM et al. 1979. Cited in Atkinson R, Darnall KR, Lloyd AC, Winer AMand Pitts JN Jr. Kinetics and mechanisms of the reactions of the hydroxyl radical with organic compounds in the gas phase. Advances in Photochemistry, 11: 3741.
Yamaguchi T. 1982. Mutagenicity of trioses and methyl glyoxal on Salmonella typhimurium. Agric Biol Chem 46:849-51 [cited in IARC 1994].
Yasunaga K, Kiyonari A, Oikawa T, Abe N, Yoshikawa K. 2004. Evaluation of the Salmonella umu test with 83 NTP chemicals. Environ Mol Mutagen 44(4):329-45.
Young JT, Mattsson JL, Albee RR, Schuetz DJ. 1985. Propylene oxide: Assessment of neurotoxic potential in male rats. Report (D-1831) of the Mammalian and Environmental Toxicology Research Laboratory. Midland (MI): Dow Chemical, Health and Environmental Sciences. [cited in EU 2002].
Zamora PO, Benson JM, Li AP, Brooks AL. 1983. Evaluation of an exposure system using cells grown on collagen gels for detecting highly volatile mutagens in the CHO mutation assay Environ Mutagen 5(6):795-801 [cited in IARC 1994; EU 2002].
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K. 1988. Salmonella mutagenicity tests: IV. Results from the testing of 300 chemicals. Environ Mol Mutagen 11 Suppl 12:1-157 [cited in IARC 1994].
Zetsch C, Stahl F. 1981. Rate constants for reactions of OH with partly oxidised hydrocarbons: carbonic acids and epoxides. Paper presented during "Discussion meeting of working party 2" in Leuven, Belgium, February 11/12, 1981, in the framework of COST 61A bis "Physicochemical behaviour of atmospheric pollutants" (Report prepared by RA Cox, AERE, Harwell) [cited in European Union Risk Assessment Report, 2000).
| Route of exposure | Estimated intake (µg/kg-bw per day) of methyloxirane by various age groups | ||||||
|---|---|---|---|---|---|---|---|
| 0-6 months [1] | 0.5-4 years[3] | 5-11 years[4] | 12-19 years[5] | 20-59 years[6] | 60+ years[7] | ||
| formula fed[2] | not formula fed | ||||||
| NA = not applicable ID = no data [1] No data were identified for methyloxirane in breast milk [2] Assumed to weigh 7.5 kg, to breathe 2.1 m3 of air per day, to drink 0.8 L of water per day (formula fed) or 0.3 L/day (not formula fed) and to ingest 30 mg of soil per day (EHD 1998). [3] For formula-fed infants, intake from water is synonymous with intake from food. No data on concentrations of methyoxirane in formulae were identified. No data for methyloxirane in water in Canada were identified and a detection limit from a Japanese surface water monitoring study (Japan MOE) was considered inappropriate for use as based on the release pattern, physical chemical properties and uses of methyloxirane its presence in water is expected to be minimal in comparison to levels in air. [4] Assumed to weigh 15.5 kg, to breathe 9.3 m3 of air per day, to drink 0.7 L of water per day and to ingest 100 mg of soil and 0.1 g of nutmeat per day (EHD 1998). [5] Assumed to weigh 31.0 kg, to breathe 14.5 m3 of air per day, to drink 1.1 L of water per day and to ingest 65 mg of soil and 0.4 g of nutmeat per day (EHD 1998). [6] Assumed to weigh 59.4 kg, to breathe 15.8 m3 of air per day, to drink 1.2 L of water per day and to ingest 30 mg of soil and 0.5 g of nutmeat per day (EHD 1998). [7] Assumed to weigh 70.9 kg, to breathe 16.2 m3 of air per day, to drink 1.5 L of water per day and to ingest 30 mg of soil and 0.8 g of nutmeat per day (EHD 1998). [8] Assumed to weigh 72.0 kg, to breathe 14.3 m3 of air per day, to drink 1.6 L of water per day and to ingest 30 mg of soil and 0.4 g of nutmeat per day (EHD 1998). [9] No data for methyloxirane in ambient air in Canada were identified. The detection limit of 0.31 µg/m3 for ambient air from a pilot study of 4 homes in Woodland, California was used to calculate the upper bounding limit of exposure estimate (Sheldon et al. 1990). Canadians are assumed to spend three hours outdoor each day (EHD 1998). [10] No data for methyloxirane in indoor air in Canada were identified. The detection limit of 0.31µg/m3 for indoor air from a pilot study of 2 homes in Woodland, California, in which methyloxirane was not deteted, was used to calculate the upper-bounding limit of exposure estimate (Sheldon et al. 1990). Canadians are assumed to spend 21 hours indoors each day (EHD 1998). [11] No data for methyloxirane in water in Canada were identified. The maximum detection limit of 5 µg/L from a 1980 survey of 36 Japanese surface water sources (Japan MOE searched July 2007) was considered inappropriate for calculating the upper bounding limit of exposure estimate or concentrations in reconstituted formula for infants, as based on the release pattern, physical chemical properties and uses of methyloxirane its presence in water is expected to be minimal in comparison to levels in air. [12] The general maximum residue limit of 0.1 ppm for residues of methyloxirane in/on imported fumigated almonds, walnuts and pecans was used to calculate the upper-bounding limits of exposure estimate. These are the only nutmeats known to be fumigated with this pesticide; no data on methyloxirane in other food was identified. (PMRA 2006). In a study submitted to the US EPA on treated nutmeats, the highest residue level of methyloxirane, i.e., of 273 ppm, was found in almonds 28 days after fumigation (for treated nutmeats, the current US label requires the fumigators to hold (i.e., off-gas) the treated nutmeat for 28 days before shipment) (US EPA 2005). Since Canada has not established import MRLs for this pesticide this means that food treated in the US (or elsewhere) coming into Canada cannot contain more than 0.1 ppm of methyl oxirane, as this would be a violation of the Food and Drugs Act (PMRA 2006). Consumption of nut and seeds from this food group--but excluding peanut butter--was used, as almonds, pecans and walnuts are the only known nutmeats being fumigated. No data of methyloxirane in other food was identified. [13] No data for methyloxirane in soil in Canada were identified. The detection limit of 2 µg/kg found in 12 sediment samples from a Japanese monitoring study in 1980 (Japan MOE) was considered inappropriate for calculating the upper-bounding limit of exposure estimate (similar to water above) as, based on the release pattern, physical chemical properties and uses of methyloxirane, its presence in soil is expected to be minimal in comparison to levels in air. |
|||||||
| Ambient air[9] | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | |
| Indoor air[10] | 0.08 | 0.16 | 0.13 | 0.07 | 0.06 | 0.05 | |
| Drinking water[11] | -- | -- | -- | -- | -- | -- | -- |
| Food and beverages[12] | NA | 0.0007 | 0.001 | 0.0008 | 0.001 | 0.0006 | |
| Soil[13] | ID | ID | ID | ID | ID | ID | |
| Total intake | 0.09 | 0.09 | 0.18 | 0.15 | 0.08 | 0.07 | 0.06 |
| Media | Air | Water | Soil |
|---|---|---|---|
| [1] Concentrations prediction using Chemcan (version 6.0) (CEMC 2003) [2] Model predictions were based on 100% release to air in Southern Ontario of a quantity of 50 kg/year, the cutoff reporting quantity from the recent section 71 notice under CEPA 1999 (Canada 2007). |
|||
| Concentration[2] | 5.4 × 10-7 µg/m3 | 1.8 × 10-5 µg/L | 1.2 × 10-9 ng/g |
| Consumer Product Type | Assumptions | Estimated exposure (µg/kg bw/day) |
|---|---|---|
| [a] Since these products are used primarily by adults (20–59 years old), estimated exposure have been derived for this age group only. [b] ConsExpo model version 4.1, exposure to vapour, constant rate as mode of release (for which chemical is assumed release with a constant rate in a certain time) was estimated for comparison, the exposed mean event concentration is identical at 200 mg/m3. [c] Molecular weight matrix is calculated based on the molecular weight of the main solvent, methylene chloride in the paint stripper, [d] Molecular weight matrix is calculated based on the molecular weight of the main solvent , propylene carbonate in spray paint. |
||
| Paint stripper | Inhalation
|
Mean event concentration = 200 mg/m3 |
| Acrylic aerosol spray paint | Inhalation
|
Mean event concentration = 1.3 mg/m3 |
| Endpoint | Results and reference | |||
|---|---|---|---|---|
| Species, strain | Result | Metabolic activation | Reference | |
| Genotoxicity and related endpoints: in vitro | ||||
| Gene mutation | Salmonella typhimurium TA 97 | Positive | +/- | Canter et al. 1986 |
| Negative | +/- | Zeiger et al. 1988 | ||
| Salmonella typhimurium TA 98 | Negative | + | Bootman et al. 1979 Canter et al. 1986 Zeiger et al. 1988 |
|
| - | Bootman et al. 1979 McMahon et al. 1979 Pfeiffer and Dunkelberg 1980 Canter et al. 1986 Zeiger et al. 1988 |
|||
| Salmonel la typhimurium TA100 | Positive | + | Bootman et al. 1979 Canter et al. 1986 Hughes 1987 Castelain et al. 1993 |
|
| - | Wade et al. 1978 Bootman et al. 1979 McMahon et al. 1979 Pfeiffer and Dunkelberg 1980 Simmon 1981 Yamaguchi 1982 Canter et al. 1986 Djuric et al. 1986 Agurell et al. 1991 Castelain et al. 1993 |
|||
| Negative | + | Zeiger et al. 1988 | ||
| - | Hemminki and Falck 1979 Zeiger et al. 1988 |
|||
| Salmonella typhimurium TA1535 | Positive | + | Bootman et al. 1979 Canter et al. 1986 Zeiger et al. 1988 Castelain et al. 1993 |
|
| - | Wade et al. 1978 Bootman et al. 1979 McMahon et al. 1979 Pfeiffer and Dunkelberg 1980 Simmon 1981 Canter et al. 1986 Djuric et al. 1986 Agurell et al. 1991 Castelain et al. 1993 |
|||
| Negative | - | Zeiger et al. 1988 | ||
| Salmonella typhimurium TA1537 | Negative | + | Bootman et al. 1979 Zeiger et al. 1988 |
|
| - | Bootman et al. 1979 McMahon et al. 1979 Pfeiffer and Dunkelberg 1980 Zeiger et al. 1988 |
|||
| Inconclusive | +/- | Canter et al. 1986 | ||
| Salmonella typhimurium TA1538 | Negative | + | Dean et al. 1985 | |
| - | Dean et al. 1985 McMahon et al. 1979 |
|||
| Salmonella typhimurium YG7108 | Positive | Not specified | Emmert et al. 2006 | |
| Salmonella typhimurium G46 | Negative | - | McMahon et al. 1979 | |
| Escherichia coli WP2 uvr A | Positive | + | Dean et al. 1985 | |
| - | Dean et al. 1985 McMahon et al. 1979 |
|||
| Negative | - | Hemminki and Falck 1979 | ||
| Escherichia coli WP2 | Positive | + | Bootman et al. 1979 | |
| - | Bootman et al. 1979 McMahon et al. 1979 Dean et al. 1985 |
|||
| Negative | + | Dean et al. 1985 | ||
| Escherichia coli CM891 | Positive | - | Bootman et al. 1979 | |
| Escherichia coli CM871 | Positive | - | Bootman et al. 1979 | |
| Escherichia coli B (Arg-) Hs30R | Positive | - | Kohda et al. 1987 | |
| Escherichia coli PQ37 | Negative | +/- | Von der Hude et al. 1990 | |
| Klebsiella pneumoniae | Positive | - | Voogd et al. 1981 | |
| Saccharamyces cerevisiae | Positive | - | Agurell et al. 1991 | |
| Schizosaccharomyces pombe p1 | Positive | +/- | Migliore et al. 1982 | |
| Neurospora crassa | Positive | - | Kolmark and Giles 1955 | |
| Bacteriophage | Positive | Not specified | Garro and Phillips 1980 | |
| Negative | - | Cookson et al. 1971 | ||
| Mouse L5178Y cells | Positive | - | McGregor et al. 1991 | |
| Chinese hamster ovary cells, hprt locus | Positive | - | Zamora et al. 1983 | |
| Endpoint | Results and reference | ||||
|---|---|---|---|---|---|
| Genotoxicity and related endpoints: in vitro | |||||
| Sister chromatid exchange | Positive: Chinese hamster CHO (+/-S9) (Gulati et al. 1989) Chinese hamster V79 cells (-S9) (Von der Hude et al. 1991; Von der Hude et al. 1992) Rat liver cells in vitro (-S9) (Dean and Hodson-Walker, 1979) Human lymphocytes in vitro (Tucker et al. 1986; Agurell et al. 1991) |
||||
| Chromosomal aberrations | Positive: Chinese hamster CHO (+/-S9) (Gulati et al. 1989) Rat liver cells in vitro (-S9) (Dean and Hodson-Walker, 1979; Dean et al. 1985) Human lymphocytes in vitro (Bootman et al. 1979) |
||||
| Micronucleus induction | Positive: Human lymphocytes in vitro (Jorritsma et al. 1995) |
||||
| DNA strand breaks | Positive: Rat hepatocytes in vitro (-S9) (Sina et al. 1983) Human diploid fibroblasts (Kolman et al. 1997; Chovanec et al. 1998) Calf thymus DNA (Walles 1974) |
||||
| DNA adducts | Positive: (Lawley and Jarman 1972; Walles, 1974; Hemminki et al. 1980; Randerath et al. 1981; Djuric et al. 1986; Solomon et al. 1988; Hemminki et al. 1994; Kumar et al. 1995; Plna et al. 1999) |
||||
| Differential toxicity | Positive: Bacteria (other) (-S9) (Bootman et al. 1979) |
||||
| SOS induction | Positive: Salmonella typhimurium TA1535/pSK1002 (+/-S9) (Ong et al. 1987) Salmonella typhimurium TA1535/pSK1002 (-S9) (Yasunaga et al. 2004) |
||||
| Gene conversion | Positive: Saccharomyces cerevisiae (- S9) (Agurell et al. 1991) |
||||
| Cell transformation | Positive: Mouse embryo fibroblasts (Kolman and Duniska 1995) Syrian hamster embryo cells (Kolman and Duniska 1995) BALB/c-3T3 cells (-S9) (Matthews et al. 1993) |
||||
| Genotoxicity and related endpoints: in vivo | |||||
| Micronucleus induction (bone marrow) | Positive: Negative: |
||||
| Micronucleus induction (peripheral blood) | Positive: Negative: |
||||
| Chromosomal aberrations (bone marrow) | Positive: Mouse (i.p.) (Farooqi et al. 1993; NTP database 1989–1993) |
||||
| Sister chromatid exchange (bone marrow) | Positive: Mouse (i.p.) (Farooqi et al. 1993; NTP database 1989) |
||||
| Sister chromatid exchange (peripheral blood) |
Negative: cynomolgus monkeys (inhalation) (Lynch et al. 1984b) |
||||
| DNA lesions in bone marrow, stomach and colon | Positive: Mouse (i.p.) (Tsuda et al. 2000) |
||||
| Dominant lethal assay | Negative: Rat (inhalation) (Hardin et al. 1983b) Mouse (oral) (Bootman et al. 1979) |
||||
| Sex-linked recessive lethal mutation | Positive: Drosophila (vapour) (Hardin et al. 1983b; Vogel and Nivard 1997; Vogel and Nivard 1998; Nivard et al. 2003) Drosophila (oral) (Foureman et al. 1994) |
||||
| Reciprocal translocations | Positive: Drosophila (oral) (Foureman et al. 1994) |
||||
| DNA Adducts | Positive: Drosophila (vapour) (Nivard et al. 2003) Mouse, rat or dog (inhalation, i.p. or i.v.) (Segerback et al. 1994) Mouse (i.p.) (Svensson et al. 1991) Rats (inhalation) (Snyder and Solomon 1993; Segerback et al. 1998; Plna et al. 1999; Osterman-Golkar et al. 2003; Rios-Blanco et al. 2003a) |
||||
| Chromosome loss | Positive : Drosophila (vapour) (Vogel and Nivard, 1998) |
||||
| Humans | |||||
| Chronic toxicity/carcinogenicity | IARC, 1994 and EU, 2002 examined a number of occupational cohort studies and an occupational case-control study and concluded that no conclusions with respect to carcinogenicity of methyloxirane could be drawn (Stocker and Thiess, 1979; Thiess et al. 1982; Hogstedt et al. 1979, 1986; Hogstedt, 1988; Gardner et al. 1989; Ott et al. 1989) | ||||
| Genotoxicity and related endpoints |
EU 2002 reviewed six studies involving occupational exposure and noted that no conclusions could be drawn from these studies regarding the mutagenicity of methyloxirane in humans (Theiss et al. 1981; Pero et al. 1982 and 1985; Van Sittert and de Jong 1985; de Jong et al. 1988; Hogstedt et al. 1990; Viktorova et al. 1994) A pilot study of a group of eight workers exposed to methyloxirane for 2 years or more (levels historically < 10 ppm and measured to be up to 7 ppm the day before blood sampling), had significantly increased blood levels of DNA adducts and sister chromatid exchanges when compared to 8 control subjects although confounding factors contributing to cytogenetic effects could not be excluded (Czene et al. 2002) |
||||
| Sensitization, irritation and other | Case reports of allergic contact dermatitis (Van Ketel 1979; Jensen 1981; Steinkraus and Hausen 1994; Morris et al. 1998) Case report of corneal burns (McLaughlin 1946) Case report of symptoms, including respiratory tract and eye irritation (Gosselin et al. 1984) |
||||
| Haemoglobin adducts | Increased levels observed in small sample populations; (Osterman-Golkar et al. 1984; Kautiainen and Tornqvist 1991; Boogaard et al. 1999; Czene et al. 2002) | ||||