Screening Assessment for the Challenge
Trisiloxane, octamethyl-
(MDM)
Chemical Abstracts Service Registry Number
107-51-7
Environment Canada
Health Canada
March 2015
Table of Contents
- Synopsis
- Introduction
- Substance Identity
- Physical and Chemical Properties
- Sources
- Uses
- Releases to the Environment
- Measured Environmental Concentrations
- Environmental Fate
- Persistence and Bioaccumulation Potential
- Potential to Cause Ecological Harm
- Potential to Cause Harm to Human Health
- Conclusion
- References
- Appendix I: Model Inputs Summary Table
- Appendix II: Fugacity and Critical Body Burden Analysis for MDM
- Appendix III: Upper-bound Estimates of Daily Intake of MDM by the General Population in Canada
- Appendix IV: Upper-bounding Estimates of Exposure to Octamethyltrisiloxane in Personal Care Products Using ConsExpo 4.1 (ConsExpo 2007)
- Appendix V: Structures and Property Data for MDM and Analogues Considered in the Screening Assessment
- Appendix VI: Summary of Health Effects Information for MDM
- Appendix VII: Summary of Health Effects Information for Analogues of MDM
- Appendix VIII: Summary of Health Effects Information for Analogues of MDM
Synopsis
Pursuant to section 74 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), the Ministers of the Environment and of Health have conducted a screening assessment on Trisiloxane, octamethyl-, Chemical Abstracts Service Registry NumberFootnote[1]107-51-7. This substance is referred to by its derived acronym, MDM, in the assessment. MDM was identified as a high priority for screening assessment and included in the Challenge initiative under the Chemicals Management Plan because it was found to meet the ecological categorization criteria for persistence, bioaccumulation potential and inherent toxicity to non-human organisms and was believed to be in commerce in Canada.
The substance, MDM, was not considered to be a high priority for assessment of potential risks to human health, based upon application of the simple exposure and hazard tools developed by Health Canada for categorization of substances on the Domestic Substances List.
MDM is an organic substance that is primarily used as an ingredient in the preparation of polydimethylsiloxane (PDMS) polymers or mixtures. PDMS formulations containing MDM are in turn used as ingredients in industrial products,,in consumer products such as cleaning and degreasing products, lubricants, diluents and solvents, and in cosmetics including personal care productsFootnote[2] . MDM may also be added in its pure form to cosmetics, drugs, and natural health products and may be present as an impurity in as a result of PDMS processing. MDM is not manufactured in Canada; however, imports for the calendar years 2005 and 2006 were in the range of 100 to 100 000 kg and 10 000 to 100 000 kg, respectively. The substance does not occur naturally in the environment.
Based on certain assumptions and reported use patterns, much of the MDM imported into Canada is expected to be exported out of the country in products, recycled during industrial use, or present in products that are eventually directed to landfills or incineration. Approximately half of the MDM used in Canada is expected to be released into the environment, with the majority of the emissions occurring to air and a small proportion (approximately 1%) occurring to pre-treatment wastewaters. The high vapour pressure of MDM indicates that, when released into environmental media other than air, it will tend to volatilize out of these media and into air.
MDM present in air will undergo abiotic degradation through reaction with photochemically-produced atmospheric hydroxyl radicals, with atmospheric half-lives of 6 to 9 days. Modelling predicts that MDM will have significant atmospheric transport potential but is unlikely to be deposited from air into water or soil in remote regions. Abiotic processes such as volatilization and hydrolysis are important removal processes for MDM in water and soil, with hydrolysis half-lives of 0.12 to 60.9 days and 1.5 to 120 days determined for water and soil, respectively. No degradation data was found for MDM in sediment and a calculated biodegradation half-life of 365 days was determined using analogue data. This half-life indicates that MDM may remain for long periods in sediment. However, MDM has demonstrated low potential for microbial biodegradation and, given the evidence for active abiotic degradation of the substance in both soil and water, it seems likely that an analysis of persistence in sediment based only on biodegradation data would underestimate the potential for removal in this medium.
MDM has demonstrated significant bioconcentration capacity in laboratory testing with fish and may also have significant potential to accumulate in organisms through dietary exposures. An empirical biomagnification factor (BMF) of less than 1 indicates that MDM is unlikely to transfer from one trophic level to the next highest level in the foodweb studied.
MDM has demonstrated low hazard potential in aquatic species, with no adverse effects observed following prolonged exposures at concentrations up to the limit of water solubility. Adverse effects were reported in one of two laboratory studies conducted with the sediment species, Lumbriculus variegatus. However, no adverse effects were seen in a second Lumbriculus study, nor were effects seen in laboratory testing with two other sediment species. The lowest effect level determined in testing with Lumbriculus is substantially higher than MDM levels measured or estimated to be present in the environment. No information was found on the potential for effects in terrestrial species; however, results obtained for a mechanistically-similar compound suggest that MDM is not likely to be hazardous to terrestrial invertebrates or plants.
Monitoring data indicate that exposure levels of MDM in the environment are very low. The substance was below detection limits in surface water, soil and sediment samples, including those collected near potential MDM sources of release. MDM has been detected at low levels in some air samples and was also measured in some wastewater treatment plant influents and effluents, as well as in some pre-treatment industrial process waters and landfill leachates. However, the concentrations and frequency of occurrence are lower in effluents relative to influents collected at the same time and from the same treatment plants, indicating that wastewater treatment is effective at reducing the amount of MDM available to enter receiving waters. The results of quantitative risk quotient analyses conducted for surface waters and sediment determined that the highest predicted concentrations of MDM in the Canadian environment are much less than the experimentally-determined no-effect levels.
Evidence for the active abiotic degradation of MDM, together with limited direct release of the substance to the environment and its effective removal at wastewater treatment plants, indicate that MDM will have low exposure potential in the environment. On the basis of limited environmental presence, MDM is expected to pose a low risk to organisms. This low exposure and hazard potential indicate that there is low risk of harm to organisms or to the broader integrity of the environment from MDM. It is therefore concluded that MDM does not meet the criteria under paragraphs 64(a) or (b) of CEPA 1999 as it is not entering the environment in a quantity or concentration or under conditions that have or may have immediate or long-term harmful effects on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
In terms of human health, the predominant source of exposure to MDM through environmental media is likely to be via indoor air. Exposure of the general population to MDM may occur primarily through the use of cosmetics, including some personal care products.
Limited empirical health effects data was available for MDM. Effects on the liver, kidney and lung, as well as reduced body weight gain were observed in rats following repeated-dose exposure to MDM and its analogues. The margins between the upper-bounding estimates of exposure from environmental media and use of products containing MDM and critical effect levels in experimental animals are considered adequate to address uncertainties in the health effects and exposure databases.
On the basis of the adequacy of the margins between upper-bounding estimates of exposure to MDM and critical effect levels in experimental animals, it is concluded that MDM does not meet the criteria under paragraph 64(c) of CEPA 1999 as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health. Based on available information for human health considerations, it is concluded that MDM does not constitute a danger in Canada to human life or health.
Therefore, based on the information available, it is concluded that MDM does not meet any of the criteria set out in section 64 of CEPA 1999.
Introduction
The Canadian Environmental Protection Act, 1999(CEPA 1999) (Canada 1999) requires the Minister of the Environment and the Minister of Health to conduct screening assessments of substances that have met the categorization criteria set out in the Act to determine whether these substances present or may present a risk to the environment or to human health.
Based on the information obtained through the categorization process, the Ministers identified a number of substances as high priorities for action. These include substances that
- met all of the ecological categorization criteria, including persistence (P), bioaccumulation potential (B) and inherent toxicity to aquatic organisms (iT), and were believed to be in commerce in Canada; and/or
- met the categorization criteria for greatest potential for exposure (GPE) or presented an intermediate potential for exposure (IPE) and had been identified as posing a high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
The Ministers therefore published a notice of intent in the Canada Gazette, Part I, on December 9, 2006 (Canada 2006), that challenged industry and other interested stakeholders to submit, within specified timelines, specific information that may be used to inform risk assessment, and to develop and benchmark best practices for the risk management and product stewardship of those substances identified as high priorities.
The substance Trisiloxane, octamethyl- was identified as a high priority for assessment of ecological risk as it was determined during categorization to meet criteria for persistence, bioaccumulation potential and inherent toxicity to aquatic organisms and is believed to be in commerce in Canada. The Challenge for this substance was published in the Canada Gazette on December 26, 2009 (Canada 2009a, 2009b). A substance profile was released at the same time. The substance profile presented the technical information available prior to December 2005 that formed the basis for categorization of this substance. As a result of the Challenge, submissions of information pertaining to the chemical properties, bioaccumulation potential, persistence, hazard, uses and exposure potential of the substance were received.
Although Trisiloxane, octamethyl- was determined to be a high priority for assessment with respect to the environment, it did not meet the criteria for GPE or IPE and high hazard to human health based on classifications by other national or international agencies for carcinogenicity, genotoxicity, developmental toxicity or reproductive toxicity.
Screening assessments focus on information critical to determining whether a substance meets the criteria as set out in section 64 of CEPA 1999. Screening assessments examine scientific information and develop conclusions by incorporating a weight-of-evidence approach and precaution Footnote[3]. The use of the term “conservative” throughout this assessment refers to the protective approach taken.
This screening assessment includes consideration of information on chemical properties, fate, hazards, uses and exposure, including additional information submitted by stakeholders. Relevant data were identified up to September 2014. Key studies were critically evaluated and used, along with modelled results, to reach conclusions. When available and relevant, information presented in risk and hazard assessments from other jurisdictions was considered. The screening assessment does not represent an exhaustive or critical review of all available data; rather, it presents the most critical studies and lines of evidence pertinent to the conclusion.
This screening assessment was prepared by staff in the Existing Substances Programs at Health Canada and Environment Canada and incorporates input from other programs within these departments. The ecological and human health portions of this assessment have undergone external written peer review and consultation. The original draft of this screening assessment was released in January 2011 and was subject to a 60-day public comment period. Following receipt of substantial new information of relevance to this evaluation, extensive revisions were made to the ecological portion of this screening assessment and an updated draft was published in March 2014 for a second 60-day public comment period. Further comments were received on the updated draft during the second 60-day public comment period and were taken into consideration during preparation of the final screening assessment report.
Approaches used in the screening assessments conducted under the Challenge have been reviewed by an independent Challenge Advisory Panel. While external comments were taken into consideration, the final content and outcome of the screening assessment remain the responsibility of Health Canada and Environment Canada.
The critical information and considerations upon which the assessment is based are summarized below.
Substance Identity
Substance Name
For the purposes of this document, Trisiloxane, octamethyl- will be referred to as MDM, derived following the nomenclature rules for polydimethylsiloxanes presented in Fendinger et al. (1997), where M represents a (CH3)3Si- unit and D represents a -Si(CH3)2O- unit.
| Chemical Abstracts Service Registry Number (CAS RN) | 107-51-7 |
|---|---|
| DSL name | Trisiloxane, octamethyl- |
| National Chemical Inventories (NCI) namesFootnote Table 1[a] | Trisiloxane, 1,1,1,3,3,5,5,5-octamethyl- (TSCA); Trisiloxane, octamethyl- (AICS, PICCS, ASIA-PAC, NZIoC); Octamethyltrisiloxane (EINECS, ECL) |
| Other names | Dimethylbis(trimethylsiloxy)silane; Pentamethyl(trimethylsiloxy)disiloxane; Pentamethyl(trimethylsilyloxy)disiloxane; L3 Trisiloxane (INCI)Footnote Table 1[b] |
| Chemical group (DSL Stream) |
Discrete organics |
| Major chemical class or use | Organosilicones |
| Major chemical sub-class | Linear volatile methyl siloxanes (linear VMS) |
| Chemical formula | C8H24O2Si3 |
| Chemical structure | 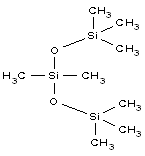 |
| SMILESFootnote Table 1[c] | C[Si](C)(C)O[Si](C)(C)O[Si](C)(C)C |
| Molecular mass | 236.5 g/mol |
Physical and Chemical Properties
Table 2 contains experimental and modelled physical and chemical properties of MDM that are relevant to its environmental fate.
| Property | Type | ValueaFootnote Table 2[a].1 | Temperature (°C) | Reference |
|---|---|---|---|---|
| Melting point (ºC) |
Experimental | -82Footnote Table 2 [*] | n/a | SEHSC 2006 |
| Melting point (ºC) |
Modelled | -53 | n/a | MPBPVPWIN 2008 |
| Boiling point (ºC) |
Experimental | 152.5[*] | n/a | SEHSC 2006 |
| Boiling point (ºC) |
Modelled | 142 | n/a | MPBPVPWIN 2008 |
| Density (kg/m3) |
Experimental | 820 (0.820 g/cm3) |
20 | Mazzoni et al. 1997 |
| Vapour pressure (Pa) |
Experimental | 520[*] (3.9 mm Hg) |
n/a | SEHSC 2006 |
| Vapour pressure (Pa) |
Modelled | 465 (3.5 mm Hg) |
25 | MPBPVPWIN 2008 |
| Henry’s Law constant (Pa·m3/mol) |
Experimental | 3.0 × 105 (121 dimensionless) |
25 | SEHSC 2006 |
| Henry’s Law constant (Pa·m3/mol) |
Experimental | 2.9 × 106[*] | 25 | Xu and Kropscott 2010 |
| Henry’s Law constant (Pa·m3/mol) |
Modelled | 4.23 × 104 (0.418 atm·m3/mole; Bond method) |
25 | HENRYWIN 2008 |
| Henry’s Law constant (Pa·m3/mol) |
Modelled | 4.07 × 106 (40.2 atm·m3/mole; VP/Wsol method)Footnote Table 2[b] |
25 | HENRYWIN 2008 |
| Henry’s Law constant (Pa·m3/mol) |
Modelled | 3.62 × 106 (35.7 atm·m3/mole; VP/Wsol method)Footnote Table 2[c] | 25 | HENRYWIN 2008 |
| Log Kow (Octanol-water partition coefficient) (dimensionless) |
Experimental | 4.80 | 25 | Bruggeman et al. 1984 |
| Log Kow (Octanol-water partition coefficient) (dimensionless) |
Experimental | 6.60[*] | 24.1 | SEHSC 2010 |
| Log Kow (Octanol-water partition coefficient) (dimensionless) |
Modelled | 6.70 | 25 | KOWWIN 2008 |
| Log Koa (Octanol-air partition coefficient) (dimensionless) |
Experimental | 3.72[*] | 25 | Xu and Kropscott 2010 |
| Log Koa (Octanol-air partition coefficient) (dimensionless) |
Modelled | 4.5 | 25 | KOAWIN 2008 |
| Log Koc (Organic carbon-water partition coefficient) (dimensionless) |
Experimental | 4.34[*] | 25 | Durham and Kozerski 2010 |
| Log Koc (Organic carbon-water partition coefficient) (dimensionless) |
Modelled | 3.4–5.7 | 25 | KOCWIN 2008 |
| Water solubility (mg/L) |
Experimental | 0.034[*] | 23 | Varaprath et al. 1996 |
| Water solubility (mg/L) |
Modelled | 0.027 | 25 | WSKOWWIN 2008 |
MDM is one of a group of organosilicone compounds (i.e., substances containing an alternating silicon-oxygen backbone) termed volatile methyl siloxanes (VMS) (Chandra 1997). VMS are oligomeric alkylsiloxanes with low molecular weight (less than 600 g/mol) and significant vapour pressure under ambient environmental conditions (Allen et al. 1997; Hobson et al. 1997). The group is also highly hydrophobic and has low aqueous solubility. MDM is a linear VMS, indicating that the structural components of the siloxane molecule are arranged in linear rather than cyclic fashion (Table 1).
Sources
There are no known natural sources of MDM.
Responses to a survey notice published under section 71 of CEPA 1999 indicated that, for the 2005 calendar year, MDM was not manufactured in Canada at or above the reporting threshold of 100 kg. However, seven Canadian companies reported importing MDM into Canada, with one company below the reporting limit of 100 kg, two companies importing in the 100 to 1000 kg/year range and four companies in the 1001 to 100 000 kg/year range (Environment Canada 2007a). In addition, two Canadian companies were identified as having a stakeholder interest in the substance.
A subsequent section 71 survey conducted for the 2006 calendar year indicated that MDM was not manufactured in Canada in a quantity at or above the 100 kg reporting threshold. Total reported imports for that year were in the range of 10 000 to 100 000 kg (Environment Canada 2010a). MDM was imported into the country either as a pure substance, a mixture, as a component of finished products, or as a residual in silicone polymers and oligomers (Environment Canada 2010a).
MDM was used in Denmark from 2001 to 2010 (the most recent reporting year), with a total use quantity of 400 kg reported for 2010 (SPIN 2013). Total use quantities in Norway remained relatively constant over the period 2001 to 2010, ranging from 300 to 600 kg/year. With the exception of a 300 kg use quantity reported by Finland in 2010, no other entries have been provided to the Substances in Preparations in Nordic Countries (SPIN) database by the three other participating Nordic countries of Sweden, Finland and Norway. MDM is not currently listed as a high production volume chemical (HPVC) or low production volume chemical (LPVC) in the European Union (ESIS c1995–2009) and is not included in the U.S. EPA High Production Volume (HPV) Challenge Program (HPVIS 2012).
Uses
Information provided in the section 71 surveys indicated that, for the 2005 and 2006 calendar years, business activities associated with the use of MDM in Canada included those in the wholesale trade and distribution of chemicals (except agricultural) and allied products and the retail trade of health and personal care stores (Environment Canada 2007a, 2010a). Other reported activities were the manufacture of basic chemicals; paints, coatings and adhesives; soaps, cleaning compounds and toiletries; plastics products; and semiconductors and other electronic components. Activities were also reported for the construction of foundations, structures and building exteriors.
MDM is primarily used as an ingredient in the preparation of polydimethylsiloxane (PDMS) polymers, oligomers and mixtures (Hobson et al. 1997; SEHSC 2010; Environment Canada 2010a). These PDMS formulations are applied as formulation components in a range of industrial, medical and consumer products (Fendinger et al. 1997). PDMS formulations containing MDM, also known as dimethicone, may also be found in a variety of cleaning and degreasing products, lubricants, diluents and solvents, as well as in cosmetics including personal care products and cosmetics (SEHSC 2005; DowCorning Corporation 2009d, 2009e, 2010a).
In cosmetics, PDMS formulations (polymers, oligomers, and mixtures) are generally called dimethicone. MDM may be present in dimethicone as a component of the PDMS mixture or as an impurity (left over from the processing of the PDMS polymers and oligomers) (Environment Canada 2010a). MDM may be added in its pure form to cosmetics as an antifoaming agent and/or skin conditioning agent (CosIng 1976–). The substance also acts as a carrier and emollient in skin and hair care products and in antiperspirants (DowCorning Corporation 2009d). MDM is not present on the List of Prohibited and Restricted Cosmetic Ingredients (more commonly referred to as the Cosmetic Ingredient Hotlist or simply the Hotlist - an administrative tool that Health Canada uses to communicate to manufacturers and others that certain substances, when present in a cosmetic, may contravene (a) the general prohibition found in section 16 of the Food and Drugs Act or (b) a provision of the Cosmetic Regulations) as a restricted or prohibited substance, and therefore may be used in the formulation of cosmetic products (Health Canada 2014). Based on notifications submitted under the Cosmetic Regulations to Health Canada, MDM is used in certain cosmetic products (see exposure section for product types) (April 2013 email from Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced) .
MDM is listed in the Natural Health Products Ingredients Database (NHPID) with a non-medicinal role as an antifoaming and skin-conditioning agent for use in topical natural health products only (NHPID 2014). It is listed in the Licensed Natural Health Products Database (LNHPD) as being present as a non-medicinal ingredient in currently licensed natural health products (LNHPD 2014).
MDM is not listed in the Drug Product Database (DPD) as a medicinal ingredient in pharmaceutical drugs or veterinary drugs (DPD 2014). However, it is listed in the Therapeutic Product Directorate's internal Non-Medicinal Ingredients Database as a non-medicinal ingredient present in marketed therapeutic products used in sunscreen applications but not in any veterinary drugs (2014 personal communication from Therapeutic Products Directorate, Health Canada; unreferenced).
MDM is not listed as an approved food additive in the Lists of Permitted Food Additives which have been incorporated by reference in Marketing Authorizations under the authority of the Food and Drugs Act (Canada 2014). MDM was not identified to be present in the formulation of incidental additives or in food packaging materials ( 2014 personal communication from Food Directorate, Health Canada; unreferenced). However, PDMS (CAS RN 9006-65-9; USP 2010; dimethylpolysiloxane) is a permitted food additive as per Item D.1 of List of Permitted Food Additives with Other Generally Accepted Uses. PDMS is commonly a component of food processing aids used to control foam in food applications. Finally, MDM has been identified as a formulant impurity in one herbicide pest control product (2013 personal communication from Pest Management Regulatory Agency, Health Canada; unreferenced).
Releases to the Environment
As a component of products with a range of industrial and consumer applications, releases of MDM to the Canadian environment could occur during processing operations, including the transportation and storage of materials, as well as during service life and disposal of products containing MDM. Based on this, both non-dispersive and dispersive releases of MDM to the environment are possible. Results from the section 71 notice conducted for the year 2006 (Environment Canada 2010a) were used to estimate potential releases of MDM to the Canadian environment.
A method has been developed by Environment Canada to estimate losses of a substance during different stages of its life cycle, including its fate within a finished product or article (Environment Canada 2008). This method, referred to as Mass Flow, consists of a life cycle analysis and a spreadsheet tool (Mass Flow Tool or MFT) that integrates information on the manufacturing, importation and use patterns available for the substance. Starting with an identified mass of the substance, each life cycle stage is subsequently evaluated until no mass remains. Relevant factors are considered, uncertainties recognized and assumptions may be made during each stage, depending on information available. The estimated losses represent the complete mass balance of the substance over the life cycle and include releases to wastewater and other receiving compartments (land, air), chemical transformation, transfer to recycling activities and transfer to waste disposal sites (landfill, incineration). However, unless specific information on the rate or potential for release of the substance from landfills and incinerators is available, the method does not quantitatively account for releases to the environment during or after disposal.
In general, releases of a substance to the environment depend upon various losses from its manufacture, industrial use, and/or consumer/commercial use. These losses can be grouped into seven types: (1) discharge to wastewater; (2) emission to air; (3) loss to land; (4) chemical transformation; (5) disposal to landfill; (6) loss to incineration; and (7) disposal through recycling (i.e., recycling is deemed a loss and not considered further). They are estimated using regulatory survey data, industry data and data published by different organizations. The discharge to wastewater refers to raw wastewater prior to any treatment, whether it is on-site industrial wastewater treatment or off-site wastewater treatment. In a similar manner, loss via chemical transformation refers to changes in a substance's identity that may occur during manufacture, industrial use, and consumer/commercial use, but excludes those during waste management operations such as incineration and wastewater treatment. The loss to land includes unintentional transfer or leakage to soil or paved/unpaved surfaces during the substance’s use and service life (e.g., from the use of agricultural machinery or automobiles). The loss to land, however, does not include transfers subsequent to a substance’s use and service life (e.g., land application of biosolids and atmospheric deposition).
The losses estimated for MDM over its life cycle (based on conservative assumptions) are presented in Table 3 (Environment Canada 2010b). As MDM was not manufactured in Canada above reporting thresholds, estimated losses are based on import quantities reported for 2006.
| Type of Loss | Proportion (%) | Pertinent Lifecycle Stages |
|---|---|---|
| Wastewater (prior to wastewater treatment) |
1.1 | Industrial use and consumer/commercial use |
| Air emission | 45.4 | Industrial use and consumer/commercial use |
| Land | 0.0 | - |
| Chemical transformation | 0.0 | - |
| Landfill | 5.1 | Industrial use and consumer/commercial use |
| Incineration | 1.3 | Industrial use and consumer/commercial use |
| Recycling | 16.1 | Industrial use |
| Export | 31.0 | Industrial use and consumer/commercial use |
Most MDM is expected to be emitted to air during consumer and commercial applications or industrial use (45.4%) or exported in end-use products (31.0%). Significant recycling (16.1) during industrial use is also expected, with smaller losses to wastewater (1.1%) or disposal to landfill (5.1%) and incineration sites (1.3%) during industrial or consumer and commercial applications.
Measured Environmental Concentrations
Data concerning the measured presence of MDM in the environment are presented in Appendix II. Recent Canadian monitoring data were obtained for air, sediment, process effluents and wastewaters (i.e., wastewater treatment plant influents and effluents, landfill leachate, and industrial waters), and biota.
Air
Cheng et al. (2011) measured concentrations of 0.97 to 2.00 ng/m3 (mean value 1.53 ng/m3; n = 8) in air samples collected at an Ontario wastewater treatment plant over the period July to September 2009 . Levels upwind and downwind of the plant were 0.66 to 0.77 ng/m3 (mean value 0.73 ng/m3; n = 3) and 1.41 ng/m3 (n = 1), respectively. Concentrations in two air samples collected in November 2009 above the aeration tank of a second Ontario wastewater treatment plant were 1.88 and 2.64 ng/m3. The same study measured air concentrations of 5.60 and 6.14 ng/m3 at two sites located downwind of two Ontario landfills. Concentrations upwind of the sites were 0.49 and 0.87 ng/m3. Sampling for the landfill sites occurred from June to August 2009.
Genualdi et al. (2011) examined the occurrence of linear and cyclic VMS at 20 locations worldwide, including sites in the Arctic, remote background locations and urban areas . MDM was detected in four of 12 background sites (Whistler, BC; Point Reyes, CA; Groton, CT; Hilo, HI) at concentrations ranging from 0.011 to 0.019 ng/m3, and in two of three urban sites (Downsview, ON and Paris, France but not Sydney, FL) at concentrations of 0.12 and 0.029 ng/m3. The substance was not detected in any of the four Arctic samples (detection limit 0.011 ng/m3) and was not found in the one agricultural sample (Bratt’s Lake, SK). Only one of the three linear VMS examined in the study (MDM, L4, L5) was present above detection limits in an Arctic sample, with L4 having a measured concentration of 0.013 ng/m3at the polar location of Little Fox Lake, Yukon. Concentrations of linear VMS were up to three orders of magnitude lower than those of the cyclic VMS (D3, D4, D5, D6), with higher average concentrations of the sum of linear VMS associated with urban areas (0.63 ng/m3) as compared with background sites (0.03 ng/m3). The low concentrations determined for background and Arctic sites was taken to indicate that MDM and other linear VMS do not undergo significant long-range atmospheric transport, while the higher concentrations observed in more populated areas were attributed to personal care product use and other indoor sources (Genualdi et al. 2011). However, it is also possible that the low concentrations observed in northern and remote areas resulted from dilution of the substance to levels below detection limits as it travelled further from sources.
Krogseth et al. (2013) reported concentrations of 0.39 to 6.43 ng/m3 (mean value 1.80 ng/m3; n = 41) in air samples collected on the campus of the University of Toronto Scarborough in a suburban area of Toronto, Ontario. The samples were collected over a four-month period from March to June, 2012.
MDM was not detected (detection limit 8 ng/m3) in 24 air samples collected in 2004 and 2005 from various locations in six Nordic countries (Kaj et al. 2005b).
Water
MDM was not detected (detection limits 0.5 to 0.8 ng/L) in surface grab samples of freshwater (n = 10) and seawater (n = 8) collected from 2004 to 2006 from various locations in Denmark, Norway, Iceland and Sweden (Kaj et al. 2005b; Schlabach et al. 2007 ). A grab sample is one in which all test material in the sample is collected at the same time; therefore, a grab sample represents conditions specific to the location and time at which the sample was taken.
Sediment
MDM was not detected (detection limits 0.2 to 7 ng/g dw) in 93 sediment grab samples collected in 2011 from various locations in the Great Lakes region (Backus et al. 2012), nor was it found in sediment grab samples collected in the same year from sites on Lake Ontario (n = 10) and Lake Pepin, Minnesota (n = 24; detection limits 0.18 to 0.76 ng/g dw) (CES 2012). MDM was also not detected (detection limit 20 ng/g dw) in 126 sediment grab samples collected in 2012 from locations in Newfoundland (n = 1), Nova Scotia (n = 3), New Brunswick (n = 2), Quebec (n = 78), Ontario (n = 39) and British Columbia (n = 3) (Pelletier et al. 2012). For all three studies, sampling locations were selected to include sites situated near to known or potential point sources of MDM, as well as sites away from potential point or non-point sources (i.e., reference sites).
MDM was not detected (detection limits 0.02 to 0.71 ng/g dw) in 36 sediment grab samples, including six from the Norwegian Arctic, collected from 2003 to 2008 at sites in Norway, Denmark, Finland and the Faroe Islands (Kaj et al. 2005b; Schlabach et al. 2007; Evenset et al. 2009).
Process Effluents and Wastewaters
Alaee (2012) analyzed influent and effluent grab samples collected in 2011 from 15 wastewater treatment plants (WWTPs) in Ontario, Quebec and British Columbia. MDM was present in 10 of 16 influent samples at concentrations of 6 to 96 ng/L, and in three of 15 effluent samples at concentrations of 3 to 10 ng/L ( detection limits 1 to 13 ng/L).
Preliminary data from Alaee (2014) determined concentrations of 1 to 531 ng/L in all 16 WWTP influent samples and 0.4 to 114 ng/L in nine of 16 WWTP effluent samples collected in 2012 from WWTPs in Ontario, Quebec and British Columbia (detection limit 1 ng/L).
Preliminary measurements conducted by Khera (2014) found MDM in all of 17 influent samples collected in 2012 from WWTPs across Canada. Concentrations in the influents ranged from 14 to 531 ng/L, while effluent samples collected from the same plants contained from 0.5 to 115 ng/L (in 12 of 18 samples, detection limit 0.40 ng/L). Influent samples collected in the first part of 2013 contained from 4.7 to 388 ng/L (in 12 of 16 samples), while effluent samples contained from 0.9 to 66 ng/L (in 13 of 16 samples, detection limit 0.40 ng/L). In July of 2013, the detection limit for both influent and effluent samples was increased to 53 ng/L. MDM was not detected in 24 influent and effluent samples collected over the balance of 2013 (detection limit 53 ng/L). MDM was present at concentrations of 60 to 128 ng/L in three of 21 WWTP influent samples collected in 2014 but was not found in 21 effluent samples (detection limit 53 ng/L).
Concentrations of 1 to 32 ng/L were measured in four of six WWTP influent grab samples collected from 2004 to 2006 in several Nordic countries (Kaj et al. 2005b; Schlabach et al. 2007). MDM was not detected (detection limits 0.3 to 1 ng/L) in 11 WWTP effluent grab samples collected over the same period at similar locations .
MDM was not detected (detection limit 13 ng/L) in nine water grab samples collected in 2011 from the on-site treatment plants of four industrial facilities in Ontario and Quebec (Alaee 2012 ). The substance was present at 828 ng/L in one of four intermediate process water grab samples collected at a fifth facility but was not detected (detection limit 13 ng/L) in the final effluent from the facility. Concentrations ranging from 17 to 15 300 ng/L were measured in all four water grab samples collected at a sixth industrial facility, with effluent from the facility containing 5000 ng/L. These concentrations occurred in process waters prior to the process waters being discharged to a publicly-owned wastewater treatment plant.
In preliminary data from Alaee (2014), MDM was not detected (detection limit 2 ng/L) in three process waters collected in 2012 from an industrial facility in Ontario.
A grab sample of leachate collected in 2011 from a landfill in Ontario contained MDM at the detection limit of 1 ng/L (Alaee 2012 ). MDM was not detected (detection limit 13 ng/L) in leachate grab samples collected in the same year from two other landfills in Ontario and Quebec.
Preliminary data from landfills in Ontario, Quebec and British Columbia measured MDM at concentrations of 0.7 to 6.2 ng/L in nine of 15 leachate samples collected in 2012 (Alaee 2014).
MDM was not detected (detection limits 0.5 to 4 ng/L) in 10 water grab samples collected from Nordic landfills in 2004 to 2005 (Kaj et al. 2005b ).
WWTP sludge
No Canadian data were found for MDM present in wastewater treatment plant sludge; however, concentrations of 7 to 31 ng/g dw were reported in 23 of 72 sludge grab samples collected from Nordic WWTP facilities over the period from 2004 to 2006 (Kaj et al. 2005a,b; Schlabach et al. 2007 ).
Soil
No Canadian or North American soil data were found for MDM.
MDM was not detected (detection limit 0.1 ng/g dw) in soil grab samples collected in 2004 from an abandoned landfill and an operating landfill in the Faroe Islands (Kaj et al. 2005b ).
Biota
MDM concentrations of 0.062 to 0.088 ng/g ww (mean value 0.071 ng/g ww) were measured in blood collected from three of five Northwest Atlantic harbour seal pups (Phoca vitulina) sampled in 2008 from a contaminated area of the St. Lawrence Estuary, but was not detected (detection limit 0.034 ng/g ww) in the blood of 10 harbour seal pups collected at a nearby reference site in the Gulf of St. Lawrence (Wang et al. 2012). MDM was also not detected in the blood of snapping turtles (Chelydra s. serpentina; n = 32) and cormorant (Phalacrocorax auritus; n = 22) collected from reference and contaminated sites in the Great Lakes region of Canada. Contamination was attributed to the proximity of urban and industrial centres, with reference sites located upstream and/or at a greater distance from potential sources of MDM.
MDM was not detected (detection limit 0.42 ng/g ww) in whole body homogenates of lake trout (Salvelinus namaycush; n = 60) and walleye (Sander vitreus; n = 17) collected from the Great Lakes, Kusawa Lake (Yukon), Lake Athabasca (Alberta) and Lake Winnipeg (Manitoba) (McGoldrick et al., 2014).
MDM was detected in one of 7 whole body samples of northern pike (Esox lucius) at a concentration of 0.30 ng/g ww, and was also present in all of four whole body samples of walleye (Sander vitreus) at concentrations of 0.19 to 1.77 ng/g ww (Pelletier 2013). It was also measured at 0.29 ng/g ww in one of 7 pooled samples of mussels (Elliptio complanata; pooled samples contained 5 mussels each). The substance was below detection limits in two whole body yellow perch (Perca flavescens) samples and four pooled samples of round goby (Neogobius melanostomus; pooled samples each contained 8 to 12 gobies). The detection limit for all species was 0.17 ng/g dw. The samples were collected in 2012 and 2013 from an area of the St. Lawrence River that was within the immediate dispersion plume of effluent originating from the dense urban centre of Montréal, and are therefore reflective of near-source exposure conditions to urban contamination.
MDM was not detected in pooled samples of mysid shrimp (Mysis relicta; n = 4), round goby (Neogobius melanostomus; n = 12; total of 337 fish), rainbow smelt (Osmerus mordax; n = 9; total of 54 fish) and alewife (Alosa pseudoharengus; n = 5; total of 13 fish) collected in 2011 from Lake Ontario (CES 2013). MDM was also not detected in 19 lake trout (Salvelinus namaycush) collected during the same sampling program. The detection limit for all samples in the study was 1.63 ng/g ww.
MDM was not detected in pooled samples of zooplankton (n = 4), mayfly larvae (Hexagenia sp.; n = 5; total of 496 larvae) and young gizzard shad (Dorosoma cepedianum; n = 11; total of 105 fish) collected in 2011 from Lake Pepin in Minnesota (CES 2012 ). The substance was also not detected in 20 sauger (Sander canadensis) collected concurrently with the pooled samples. Detection limits for the study ranged from 0.18 to 0.76 ng/g ww.
MDM was not detected (detection limit 0.3 ng/g ww) in 45 samples of fish, marine mammals and seabird eggs collected from various Nordic locations in 2004 to 2005 (Kaj et al. 2005b). The study examined pooled liver samples from 9 species of marine and freshwater fish (eelpout, flounder, cod, sculpin, dab, Arctic char, brown trout, pike and vendace), as well as pooled blubber samples from 4 types of marine mammal (seal, pilot whale, whiteside dolphin, and common porpoise) and eggs from 3 species of seabirds (fulmar, black guillemot and herring gull). MDM was also not detected (detection limit 0.04 ng/g ww) in samples of mussel (Mytilus edulis; n = 3), flounder liver (Platichthys flesus; n = 2) and Atlantic cod stomach contents (Gadus morhua; n = 3) collected from 2004 to 2006 in Norway (Schlabach et al. 2007 ).
However, MDM at a concentration of 0.1 ng/g ww was measured in 2 of 4 Atlantic cod (Gadus morhua) liver samples collected in Norwegian waters in 2004 to 2006 (Schlabach et al. 2007 ). The substance was also present at 0.33 ng/g ww in 1 of 5 Atlantic cod liver samples, and at 0.17 ng/g ww in 1 of 11 polar cod (Boreogadus saida) livers collected in the Norwegian Arctic in 2008 (Evenset et al. 2009). MDM was not detected in 14 seabird (kittiwake, Rissa tridactyla, and common eider, Somateria mollissima) and 6 sediment samples collected from the same region (detection limits 0.08 to 0.18 ng/g ww and 0.19 to 0.3 ng /g dw, respectively) (Evenset et al. 2009).
Environmental Fate
Level III fugacity modelling (EQC 2011) simulates the distribution of a substance in a hypothetical, evaluative environment known as the “unit world”. The updated 2011 EQC model simulates the environmental distribution of a chemical at a regional scale (i.e., 100,000 km2) and outputs the fraction of the total mass in each compartment from an emission into the unit world and the resulting concentration in each compartment.
The mass-fraction distribution of MDM determined using the EQC model is given in Table 4, using individual steady-state emissions to air, water and soil. The Level III EQC model assumes non-equilibrium conditions between environmental compartments, but equilibrium within compartments. The results in Table 4 represent the net effect of chemical partitioning, inter-media transport, and loss by both advection (out of the modelled region) and degradation/transformation processes.
The results of Level III fugacity modelling suggest that MDM can be expected to predominantly reside in air when the substance is released into this compartment or into soil. When released into water, MDM is predicted to distribute mainly within the water or into sediment with a small proportion distributing into air. Input values used in the modelling are provided in Appendix I.
| Substance released to: | Air | Water | Soil | Sediment |
|---|---|---|---|---|
| Air (100%) | 100 | 0 | 0 | 0 |
| Water (100%) | 7 | 40 | 0 | 53 |
| Soil (100%) | 91 | 0 | 9 | 0 |
The high vapour pressure (520 Pa) indicates that MDM is volatile. Therefore, if released into air, the substance is expected to remain within this compartment with little tendency to move into other environmental compartments. The EQC model predicts that approximately 67% of the amount emitted to air will be advected out of the unit world and undergo further atmospheric transport, while the remaining 33% will be reacted (degraded) in the atmosphere.
The low water solubility of 0.034 mg/L (25°C) and high log Koc values of 3.4 to 5.7 (Table 2) indicate that MDM released into water will tend to adsorb to suspended solids and sediment. The EQC model predicts that under steady-state conditions of continuous release into water, approximately 40% will remain in the water and the remaining amount will distribute to sediment (53%) or escape from water surfaces into air (7%). While the calculated Henry’s Law constant for MDM is high (2.9 × 106 Pa·m3/mol at 25°C), volatilization from water surfaces is not predicted to be a dominant fate process according to the Level III model. However, in the environment, evaporation from the water surface could be enhanced under some environmental conditions such as those of increased surface turbulence and temperature. As well, other factors will influence the relative importance of sorption and volatilization in the partitioning of MDM in water. These include the nature of the receiving water body, in particular the concentrations of suspended sediment and organic matter, as well as a longer predicted half-life in sediment as compared with water, resulting in a larger mass fraction being retained in the sediment compartment due to slower removal processes.
If released to soil, the high vapour pressure suggests there will be significant tendency (91%) for MDM to volatilize from the soil surface into air. About 9% of the amount released to soil is expected to remain within the soil compartment (Table 4), with approximately 30% of this amount expected to exist in soil pore air and 70% adsorbed to solids. This adsorptivity, along with low water solubility (0.034 mg/L; Table 2), suggests that MDM will be relatively immobile in soil.
Long-range Transport Potential
The Transport and Persistence Level III Model (TaPL3) (TaPL3 2000) was used to estimate the Characteristic Travel Distance (CTD), defined as the maximum distance traveled in air by 63% of the substance. Beyer et al. (2000) have proposed that CTDs of greater than 2000 km represent high long-range atmospheric transport potential (LRATP), 700to 2000 km represent moderate LRATP, and less than 700 km represent low LRATP. Based on a TaPL3 CTD estimate of 2882 km, the LRATP of MDM is considered to be high. This means that MDM is judged to be subject to atmospheric transport to remote regions such as the Arctic.
The OECD POPs Screening Model can also be used to help identify chemicals with high persistence and long-range transport potential (Scheringer et al. 2009). The OECD model is a global model that compartmentalizes the earth into air, water and soil. This model is “transport-oriented” rather than “target-oriented”, as it simply identifies the CTD without indicating specifically where a substance may be transported to (Fenner et al. 2005). Klasmeier et al. (2006) have suggested that a threshold of 5098 km, based on the model’s CTD estimate for PCB-180, can be used to identify substances with high long-range-transport potential. PCB-180 is empirically known to be found in remote regions. The CTD calculated for MDM using the OECD model is 2881 km, indicating that MDM has significant potential for transport in air, although this is below the boundary suggested for global pollutants by Klasmeier et al. (2006). The OECD POPs Screening Model also calculates the transfer efficiency (TE), which is the percentage of emission flux to air that is deposited to the surface (water and soil) in a remote region (TE % = D/E × 100, where E is the emission flux to air and D is the deposition flux to surface media in a target region). The TE for MDM was calculated to be 1.3 × 10−6 %, which is below the boundary of 2.248 % (PCB-28) established based on the model’s reference substances empirically known to be deposited from air to soil or water. The low TE means that although MDM has the potential for long-range travel in the atmosphere, it is unlikely to be deposited to Earth’s surface in any remote region, even cold environments.
Input values used to model the long-range transport potential of MDM are provided in Appendix I.
In addition, the log Koa of 3.72 and log Kaw of 3.06 (Xu and Kropscott 2010) suggest that MDM will have a low Arctic contamination potential (ACP) when examined using chemical partitioning space plots as described by Wania (2003, 2006). Chemicals such as these are often referred to as “fliers”, in that they have LRATP, but do not necessarily end up in other environmental media due to their high vapour pressures.
Model estimates indicate that MDM has significant atmospheric transport potential and may be capable of reaching areas far from its emission sources. While an analysis of TE and space plots based on log Koa and log Kaw suggest that MDM will have low potential to be deposited to water or soil in remote regions and low Arctic contamination potential (ACP), a recent study conducted in the Norwegian Arctic (Evenset et al. 2009) reported the presence of MDM in two of 16 cod liver samples. The substance was below detection limits in sediment and seabird samples collected from the same region. As there is no evidence for the natural production of MDM, detection of MDM in the fish samples is indicative of contamination from anthropogenic sources, although the nature of these sources is unclear. A recent study by Warner et al. (2010) found that levels of cyclic VMS measured in fish samples collected from sites in the European Arctic were influenced mostly by the presence of nearby human settlements. In addition, for migratory fish species such as cod, tissue VMS concentrations may result from exposure of the fish to sources in more populated southern regions (Warner et al. 2010).
Persistence and Bioaccumulation Potential
Environmental Persistence
Relevant Media
Based on the results of Level III fugacity modelling, air, water and sediment are considered to be media of relevance for MDM, depending upon the compartment of release. The soil compartment is not predicted to be a medium of relevance, although the substance may remain to a minor extent within soil when released directly into this compartment (see Table 4).
Data Sources
Both empirical and modelled data were considered in the analysis of potential for environmental persistence.
Empirical Data for Persistence
A summary of empirical degradation data for MDM is presented in Table 5a.
| Medium | Fate process | Degradation value | Degradation endpoint / units | Reference |
|---|---|---|---|---|
| Air | Photodegradation | 1.83 × 10−12 | Rate constant / cm3/molecule·sec Atmospheric lifetime / days |
Markgraf and Wells 1997 |
| Air | Photodegradation | 6.3 | Rate constant / cm3/molecule·sec Atmospheric lifetime / days |
Markgraf and Wells 1997 |
| Air | Photodegradation | 8.77 | Atmospheric reaction half-life/ days | Whelan et al. 2004 |
| Air | Photodegradation | 5.79 | Half-life / days | SEHSC 2011 |
| Water | Hydrolysis | 60.9 (pH 7; 10°C) |
Half-life / days | Mosey and Kozerski 2008 |
| Water | Hydrolysis | 13.7 (pH 7; 25°C) |
Half-life / days | Mosey and Kozerski 2008 |
| Water | Hydrolysis | 0.12 (pH 9; 35°C) |
Half-life / days | Mosey and Kozerski 2008 |
| Water | Biodegradation | -3.7 | Biodegradation / % | Schaefer and Matthews 2009 |
| Soil | Catalysis | 1.5 (32% RH; 21°C) |
Half-life / days | Xu and Doede 2010; Xu et al. 2012 |
| Soil | Catalysis | 3.6 (42% RH; 21°C) |
Half-life / days | Xu and Doede 2010; Xu et al. 2012 |
| Soil | Catalysis | 6.2 (92% RH; 21°C) |
Half-life / days | Xu and Doede 2010; Xu et al. 2012 |
| Soil | Catalysis | 120 (100% RH; 21°C) |
Half-life / days | Xu and Doede 2010; Xu et al. 2012 |
| Soil | Catalysis | 4.54Footnote Table 5a[a] | Half-life / days | SEHSC 2011 |
| Soil | Volatilization | No significant volatilization (32% RH; 21°C) |
Half-life / days | Xu and Doede 2010; Xu et al. 2012 |
| Soil | Volatilization | 0.52 (100% RH; 21°C) |
Half-life / days | Xu and Doede 2010; Xu et al. 2012 |
| Sediment | Biodegradation | 365Footnote Table 5a[b] | Half-life / days | SEHSC 2011 |
Biodegradation
MDM demonstrated low potential for microbial biodegradation in OECD Test Guideline 310 ready biodegradation testing (OECD 2006), with a mean percent biodegradation of -3.7% reported in test vessels at the end of the 28-day test (Schaefer and Matthews 2009).
A sediment biodegradation half-life of 365 days (8760 hours) was calculated for MDM based on data provided in Xu (2009) for the cyclic VMS octamethylcyclotetrasiloxane (D4; CAS RN 556-67-2) in anaerobic lake sediment (SEHSC 2011).
Abiotic degradation
A reaction rate constant of 1.83 × 10−12cm3/molecule·sec was determined for the gas-phase reaction of MDM with photochemically produced hydroxyl radicals at approximately 24°C (Markgraf and Wells 1997). Assuming an hydroxyl radical concentration of 1 × 106 molecules/cm, the authors calculated an atmospheric lifetime for MDM of 6.3 days. The major observed reaction products were two siloxane alcohols (silanols), both considered to be second-generation products of the reaction of atmospheric water with an intermediary siloxane ester product. Smaller amounts of two cyclic siloxanes were also observed. Using this rate constant and a steady-state hydroxyl radical concentration of 5 × 105 molecule/cm, Whelan et al. (2004) calculated an approximate atmospheric reaction half-life of 8.77 days for MDM.
Using the reaction rate constant of 1.83 × 10−12cm3/molecule·sec provided by Markgraf and Wells (1997) and a 12-hour average hydroxyl radical concentration of 1.5 × 106 molecules/cm as determined by Mount and Eisele (1992), SEHSC (2011) calculated a half-life value of 5.79 days (139 hours) for MDM in air.
Mosey and Kozerski (2008) reported hydrolysis half-lives of 60.9 and 13.7 days for MDM present in water at a pH of 7 and temperatures of 10 and 25°C, respectively. A half-life of 0.12 day was measured in pH 9 water at 35°C. The results indicate that hydrolysis rates for MDM are both pH and temperature dependent. Proposed hydrolysis final products included dimethylsilanediol (CAS RN 1066-42-8) and trimethylsilanol (CAS RN 1066-40-6), with pentamethyldisiloxanol (CAS RN 56428-93-4) formed as an intermediate species.
Xu and Doede (2010) investigated the relative significance of degradation and volatilization to the fate of MDM in soil. Michigan Londo soilFootnote[4]spiked with 14C-labelled MDM at an approximate initial concentration of 10 µg/g dry weight (dw) was incubated at 21°C in closed and open tube systems and four constant soil moisture levels (32%, 42%, 92% and 100% relative humidity or RH). Extracts from the test systems were analyzed for the presence of MDM and degradation products using high performance liquid chromatography equipped with a radiometric detector, and liquid scintillation counting. For estimation of mass balance of the spiked radioactivity, the non-extractable radioactive residual in the soil was also determined by combustion using a biological oxidizer. Results from the closed tube systems indicated that degradation appeared to be more rapid as the soil became drier, with half-lives of 1.5, 3.7, 6.2 and 120 days at 32%, 42%, 92% and 100% RH, respectively. In the open tube systems, volatilization was not significant at the lowest relative humidity of 32% but was the predominant removal mechanism at 100% RH (volatilization half-life 0.52 day). The results indicated that both transformation and volatilization can be significant removal mechanisms for MDM in soil, depending upon environmental conditions. Xu et al. (2012) confirmed the importance of hydrolysis as the predominant removal mechanism for MDM in drier soils, with volatilization becoming more important with increasing soil humidity.
Xu and Chandra (1999) proposed that drier soil conditions may limit biological degradation processes for cyclic VMS but promote abiotic reactions such as surface-acid-catalyzed hydrolysis. If this also applies to linear VMS such as MDM, then the more rapid degradation observed by Xu and Doede (2010) at lower relative humidity may reflect similar catalysis reactions occurring in the soil substrate. Both surface-acid-catalyzed hydrolysis and volatilization are severely restricted under the sealed-vessel and water-based conditions of the standard ready biodegradation test and this may account for the very slow rate of degradation observed by Schaefer and Matthews (2009).
Using results obtained by Xu and Doede (2010) for a soil at 92% RH and 20.9°C, SEHSC (2011) estimated a half-life for MDM in soil of approximately 4.54 days (109 hours)
Modelling of Persistence
Although experimental degradation data are available for MDM, Quantitative Structure-Activity Relationships (QSARs) were also considered in a weight-of-evidence approach as described in Environment Canada (2007b). The results are summarized in Table 5b below. Given the ecological importance of the water compartment and the fact that MDM can be expected to be released to this compartment, biodegradation in water was primarily examined. In the absence of suitable biodegradation models for soil and sediment, the results obtained for water were extrapolated to obtain estimates for the biodegradation potential of MDM in these media.
| Fate process | Model and model basis |
Model result and prediction | Extrapolated half-life (days) |
|---|---|---|---|
| Atmospheric oxidation | AOPWIN 2008Footnote Table 5b[a] | t 1/2 = 8.9 days | greater than 2 |
| Ozone reaction | AOPWIN 2008[a] | n/aFootnote Table 5b[b] | n/a |
| Hydrolysis | HYDROWIN 2008[a] | n/a[b] | n/a |
| Primary biodegradation (aerobic) | BIOWIN 2008[a] Sub-model 4: Expert Survey (qualitative results) |
3.5Footnote Table 5b[c] “biodegrades fast” |
less than 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2008[a] Sub-model 3: Expert Survey (qualitative results) |
2.7[c] “biodegrades fast” |
less than 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2008[a] Sub-model 5: MITI linear probability |
0.01Footnote Table 5b[d] “biodegrades very slowly” |
greater than 182 |
| Ultimate biodegradation (aerobic) | BIOWIN 2008[a] Sub-model 6: MITI non-linear probability |
0.02[d] “biodegrades very slowly” |
greater than 182 |
| Ultimate biodegradation (aerobic) | CATABOL c2004-2008 % BOD (biological oxygen demand) |
% BOD = 8 “biodegrades very slowly” |
greater than 182 |
The predicted atmospheric half-life of 8.9 days (AOPWIN 2008; Table 5b) agrees well with empirically derived half-life values of 5.79 days (SEHSC 2011) and 8.77 days (Whelan et al. 2004; Table 5a), providing further evidence that MDM is likely to be oxidized slowly in air. There is no estimate for the reaction half-life of this substance with other photo-oxidative species in the atmosphere, such as ozone. However, it is expected that reactions with hydroxyl radicals will be the most important degradation process in the atmosphere for gas-phase MDM.
HYDROWIN (2008) provides no estimate for the hydrolysis potential of MDM, as organosilicones such as MDM are not represented in the training set of the model and are therefore outside of the model domain.
BIOWIN Sub-model 4, a primary survey model, estimates that MDM will undergo primary biodegradation in water with a half-life of less than 182 days. This prediction differs from the empirically-derived value of -3.7% biodegradation over 28 days reported by Schaefer and Matthews (2009) in standard ready biodegradation testing. Results from three of the four ultimate biodegradation models (BIOWIN Sub-models 5 and 6, CATABOL c2004-2008; Table 5b) suggest that the half-life for ultimate biodegradation of MDM in water will likely exceed 182 days. While organosilicone substances have limited representation in the training sets of the selected models, the predicted slow ultimate biodegradation is in agreement with empirical data for both water and sediment, which report low potential for microbial biodegradation. For this reason, the modelling results were considered in the weight-of-evidence evaluation of potential for persistence.
Using an extrapolation ratio of 1:1:4 for water: soil: sediment biodegradation half-life (Boethling et al. 1995), the ultimate biodegradation half-life of MDM in soil based on model predictions is greater than 182 days and the half-life in sediment is estimated to be in the order of years.
Summary of Persistence Potential
Empirical half-lives of 5.79 and 8.77 days, and a modelled estimate of 8.9 days, have been determined for the degradation of MDM in air. The results indicate that MDM is not recalcitrant in air, although the longer residence time suggests that MDM may have significant atmospheric transport potential and may be capable of reaching areas far from its emission sources. However, it has low Arctic contamination potential (see Environmental Fate section ) as it is not likely to be deposited to water or soil in remote regions.
While empirical ready biodegradation results and modelled biodegradation estimates suggest that MDM will biodegrade only slowly in water, hydrolysis half-lives of 60.9 days or less have been reported and this suggests that abiotic processes may significantly affect the presence of this substance in the water column. Empirical soil degradation data derived for the abiotic process of surface-acid-catalyzed hydrolysis provide a maximum half-life of 120 days under the reported study conditions. These data are considered sufficient to establish that both biotic and abiotic processes may be relevant removal mechanisms for MDM in water and soil. Based on the available information, MDM is not expected to persist in these media.
The calculated biodegradation half-life of 365 days provided by SEHSC (2011) is the only value available upon which to analyze the potential for persistence of MDM in sediment, and this value was derived for the cyclic VMS D4. There is uncertainty in applying degradation rate data for a cyclic VMS to a linear VMS such as MDM, as the difference in geometries of the molecules (i.e., cyclic vs. linear) is likely to influence their availability to undergo biological degradation. However, both MDM and D4 are low molecular weight organosilicones and can therefore be expected to respond similarly to microbial action. For this reason, the sediment biodegradation half-life value derived for D4 is considered to provide an acceptable estimate for that of MDM. Based on this value of 365 days, as well as an extrapolated sediment half-life in the order of years, MDM has high potential to remain resident in sediment for an extended period of time.
Potential for Bioaccumulation
Data Gathering
In order to provide the best possible weight of evidence for bioaccumulation potential of MDM, empirical and modelled property data for MDM as well as property data for the structurally and mechanistically similar substances L4, L5 and M4Q were considered. Their structures and relevant physical-chemical property data are given in Appendix VI for comparison purposes.
The analogous structures described in Appendix VI have greater than 85% structural comparability using the CHEMID (2010) software. Based on data in Appendix VI, MDM is considered to be the most bioavailable of the substances in Appendix VI. MDM is slightly more water soluble than L4, has the same log Koc, but has smaller molecular dimensions, suggesting that uptake in fish during laboratory BCF testing will be less restricted than L4 (and therefore potentially a higher observed BCF). The bioavailability of L5 and M4Q for uptake via the water column under environmental conditions is expected to be much lower than MDM (i.e., measured water solubility, estimated log Kow, log Koc). MDM generally has smaller molecular dimensions (based on conformational analysis) compared to L5 and M4Q. Therefore, for reasons explained further on in this section, it is reasonable to consider L4 as the primary analogue for bioconcentration. It is reasonable, however, to consider all three analogues for dietary uptake (i.e., BMF) for real world exposures. Factors addressing aqueous bioavailability via the gills may not apply to dietary uptake. Also, molecular dimensions of these chemicals are generally larger than MDM and the gastrointestinal tract (GIT) is not subject to the same molecular resistance as gill surfaces (Arnot et al. 2010) (see below discussion).
Bioconcentration Factor (BCF)
Drottar (2006) performed a flow-through bioconcentration study using MDM and fathead minnow, Pimephales promelas. Fish were exposed for 42 days to nominal concentrations of 0.034 and 0.0034 mg/L, with the highest test concentration being near the reported water solubility of the substance (Table 2). Due to the low water solubility and high volatility of MDM, dimethylformamide was used as a solvent and the test concentrations were prepared using sealed mixing jars. Despite these precautions, some loss of the test substance occurred and mean measured test concentrations were 0.021 and 0.0017 mg/L for the high and low doses, respectively. Steady-state bioconcentration factor (BCF) values determined on the basis of fish tissue and water concentrations were 5030 for the 0.0017 mg/L test concentration and 7730 for the 0.021 mg/L concentration (Table 6). In addition, kinetic BCF values were calculated based on uptake and depuration rates and these values were 3610 and 5600 for the 0.0017 and 0.021 mg/L doses, respectively. Depuration of the test substance occurred at a moderate rate, with greater than 90% removal within 10 days following cessation of exposure. The kinetic rate constants from this study are reported in Table 8. However, as noted in the Drottar (2006) report, little metabolism of parent MDM was observed. Metabolite characterization was performed on fish collected at apparent steady-state. The percentage of radioactivity associated with MDM averaged 97.7 plus or minus 1.6%. The percentage of radioactivity associated with an unknown metabolite averaged 1.4 plus or minus 1.9%. The percentage of radioactivity not extracted averaged 0.9 plus or minus 0.3%. Consequently, the majority of the radioactivity found in the fish tissues was present as parent MDM (Drottar 2006).
A similar bioconcentration study was conducted for L4 (decamethyltetrasiloxane; CAS RN 141-62-8) (SEHSC 2006). Fathead minnows were exposed for 35 days to nominal concentrations of 0.0067 and 0.00067 mg/L in a flow-through test system, followed by a depuration period of 28 days. The water solubility of L4 is 0.0067 mg/L. As with MDM, the high volatility and low water solubility of L4 required application of special procedures to minimize loss of the substance from the test system. These included use of a solvent, sealed mixing jars, and maximizing of diluter system flow rates in order to reduce evaporative losses. Despite these precautions, some loss of the test substance occurred and measured doses were 0.0053 and 0.00043 mg/L in the high and low dose concentrations, respectively. Steady-state BCFs of 1610 and 3870 were determined for the 0.0053 and 0.00043 mg/L concentrations, respectively, while the respective kinetic BCF values calculated from uptake and depuration rates were 1760 and 3830 (Table 6). Greater than 90% of the test substance was removed from the fish tissue over the 28-day depuration period. The kinetic rate constants from this study are reported in Table 8.
| Substance | Test organism | Endpoint | Kinetic and Steady-State Values (L/kg)Footnote Table 6[a].4 | Reference |
|---|---|---|---|---|
| MDM | Fathead minnow, Pimephales promelas | BCF | 5600–7730 (0.021 mg/L) 3610–5030 (0.0017 mg/L) |
Drottar 2006Footnote Table 6[*] |
| L4 | Fathead minnow, Pimephales promelas | BCF | 1610-1760 (0.0053 mg/L) 3830-3870 (0.00043 mg/L) |
SEHSC 2006 |
The BCF of MDM and L4 were estimated using a kinetic mass-balance model based on Arnot and Gobas (2003a). The results of this modelling, which included normalized metabolic rate constants (as explained in the kinetic rate constants discussion below), predict that the BCFs for MDM and L4 for the fish used in the empirical studies are 7762 and 3890, respectively (99% accurate for the upper limit of observed BCFs in Table 6). The BCF predicted for a middle trophic level fish representative of Canadian waters using the same model is 7585. The predicted BCF using the Arnot-Gobas mass balance model (v1.11) using a kM of 0.02/day for a 10g fish with a 5% lipid content fish results in a BCF of 7943, which is also very comparable to the steady-state BCFs reported by Drottar (2006).
Arnot and Gobas (2006) critically evaluated available bioaccumulation data (BCF and BAF) for fish and other organisms and created an empirical database of quality BCF and BAF values (Arnot and Gobas 2003b). In Arnot and Gobas (2006), at a log Kow of 6.6 for MDM, the empirical distribution of “acceptable” fish BCF data shows values ranging up to 10 000 . This is largely a function of metabolic biotransformation.
The ranges of steady-state and kinetic BCFs for L4 in Table 6 are in the range 1610 to 3870, whereas the BCF for MDM are generally higher; in the range 3610 to 7730. This is likely due to the uptake rate of L4 from water being mitigated to some extent by steric hindrance, thus permitting other elimination process to mitigate the overall bioconcentration. Information regarding molecular size and cross-sectional diameters are useful to consider and are commonly used by international jurisdictions such as the European Union (ECHA 2012) as weight of evidence for bioaccumulation potential. Recent investigations relating fish BCF data and molecular size parameters (Dimitrov et al. 2002, 2005) suggest that the probability of a molecule crossing cell membranes as a result of passive diffusion declines significantly with increasing maximum diameter (Dmax). The probability of passive diffusion decreases appreciably when the maximum diameter is greater than approximately 1.5 nm and much more so for molecules having a maximum diameter of greater than 1.7 nm. Sakuratani et al. (2008) have also investigated the effect of cross-sectional diameter on passive diffusion in a BCF test set of about 1200 new and existing chemicals. They observed that substances that do not have a very high bioconcentration potential (BCF less than 5000) often have a Dmax of greater than 2.0 nm and an effective diameter (Deff) greater than 1.1 nm.
However, as Arnot et al. (2010) have noted, there are uncertainties associated with the thresholds proposed by Dimitrov et al. (2002, 2005) and Sakuratani et al. (2008) since the BCF studies used to derive them were not critically evaluated. Arnot et al. (2010) point out that molecular size influences solubility and diffusivity in water and organic phases (membranes), and larger molecules may have slower uptake rates. However, these same kinetic constraints apply to diffusive routes of chemical elimination (i.e., slow in = slow out). Thus, significant bioaccumulation potential may remain for substances that are subject to slow absorption processes, if they are slowly biotransformed or slowly eliminated by other processes. Therefore, when evaluating bioaccumulation potential, molecular size information is examined with care and considered together with other relevant lines of evidence.
Based on 3D analysis of 30 conformers calculated using the Baseline Bioaccumulation Model with Mitigating Factors (Dimitrov et al. 2005), the maximum and effective diameters of MDM are smaller than L4. This suggests that MDM is less likely to experience restricted uptake from steric effects at the gill surface and this may explain the higher empirical BCF.
Biomagnification Factor (BMF)
A growth-corrected steady-state kinetic biomagnification factor (BMF) of 0.26 and a lipid-adjusted steady-state kinetic BMFL/L of 0.94 were reported for juvenile rainbow trout, Oncorhynchus mykiss, exposed to 14C-radiolabelled MDM on fish food (approximately 500 µg/g) for a 35-day period, followed by a 28-day clearance period with clean food (SEHSC 2010; Table 7). The lipid-adjusted BMFL/L was subsequently refined to a value of 0.86 (SEHSC 2011). The steady-state BMF calculated based on MDM concentrations in fish tissue and feed was 0.11 and the corresponding lipid-normalized value was 0.38 (Drottar 2010). The lipid-normalized BMF is considered to be a more relevant endpoint for assessing biomagnification potential (Arnot and Gobas 2006). Dietary assimilation efficiency in the exposed fish was calculated to be 32% and the elimination or clearance half-life was 18 days, based on a clearance rate constant of 0.0378 d-1. Kinetic rate constants from this study are reported in Table 8.
BMF values describe the process in which the concentration of a chemical in an organism reaches a level that is higher than that in the organism’s diet, due to dietary absorption (Gobas and Morrison 2000). A BMF exceeding 1 indicates that biomagnification is occurring. BMF data are considered as indicators of the potential for uptake and accumulation in biota via the diet. For the foodweb examined in the SEHSC (2010, 2011) study, the BMF of MDM did not exceed 1; however, the lipid-normalized BMF was very close to this value, suggesting that dietary exposures may significantly contribute to body burdens in the environment.
Comparison of the parent MDM concentrations and total radioactivity in fish tissue and digestive tract samples showed that the radioactivity was associated with parent MDM; however, comparison of parent MDM concentrations and total radioactivity in liver extracts collected on Day 1 of depuration indicated the presence of one or more metabolites (Drottar 2010). While this provides evidence for some degree of metabolism of MDM by rainbow trout, the study results suggest that little biotransformation of MDM occurred. In addition, the presence of unknown metabolites does not establish that MDM was completely metabolized nor is there any information on the rate of metabolism.
DowCorning Corporation (2010b) reported an apparent steady-state BMF for the branched VMS, Trisiloxane, 1,1,1,5,5,5-hexamethyl-3,3-bis[(trimethylsilyl)oxy]- (M4Q; CAS RN 3555-47-3) of 0.045 and a lipid-normalized BMF of 0.16 for juvenile rainbow trout, Oncorhynchus mykiss, exposed to 14C-radio-labelled M4Q on fish food (approximately 400 µg/kg) for a 42-day period, followed by a 28-day clearance period with clean food (Table 7). Kinetic BMFs based on uptake and depuration rates of 0.00252 g/g/d and 0.0245 d-1, respectively, were 0.10 and 0.37 (for the lipid-adjusted value) and were estimated not including growth rate dilution of the fish over the study period. As with MDM, the DowCorning Corporation (2010b) report noted that comparison of the parent M4Q concentrations and total radioactivity in fish tissue demonstrated that they were essentially the same. This indicated that the radioactivity present in the fish tissue was generally parent M4Q. Comparison of the parent M4Q concentrations and total radioactivity in the digestive tract over time indicated that the radioactivity present in the digestive tract was unchanged M4Q. Comparison of the parent M4Q concentrations with the total radioactivity found in the liver indicated that the radioactivity present in the liver was also primarily parent M4Q. The kinetic rate constants from this study are also reported in Table 8.
| Substance | Test organism | Endpoint | Steady-State, Kinetic and Lipid Normalized Values (/kg) | Reference |
|---|---|---|---|---|
| MDM | Rainbow Trout Oncorhynchus mykiss |
BMF | 0.11-0.86 | SEHSC 2010, 2011; Drottar 2010Footnote Table 7[*] |
| M4Q | Rainbow Trout Oncorhynchus mykiss |
BMF | 0.045-0.37 | DowCorning Corporation 2010b[*] |
Dietary assimilation efficiency (ED) is also a key parameter for estimating the BAF using kinetic mass-balance models such as that of Arnot and Gobas (2003a, 2004) because it is used to calculate the dietary uptake rate constant (kD) and is related to log Kow of the substance in question (Kelly et al. 2004). As noted by Arnot (2010), some chemicals are subject to degradation in the gastrointestinal tract (GIT) and gut epithelial tissues and these processes can reduce the chemical transfer efficiency into the organism and thus the overall biomagnification. In theory, a substance that is highly metabolized in the GIT should have low dietary assimilation efficiency and slowly metabolized substances a potentially higher assimilation and thus higher biomagnification.
The dietary assimilation efficiency of MDM reported by SEHSC (2010) is 32% which is below, but close to that of the 40 to 60% range reported for some polyhalogenated biphenyl compounds known to have BMF greater than 1 (Kelly et al. 2004). This further suggests some limitation to the uptake of MDM from the GIT either from steric effects, bound residues in the food, or both. It should be noted that a BMF using the proposed equation in the OECD dietary portion of the draft revision to the 305 guideline (OECD 2011) cannot be calculated for MDM because the growth rate is higher that the depuration rate constant (by approximately 0.005 d-1) leading to a negative growth corrected depuration rate constant (i.e., k2g). This shows the effect of growth rate “swamping” the kinetics of the BMF test (see Table 8). Efforts are being made via the OECD to deal with growth rate influences in the 305 dietary test (OECD 2011).
Kinetic Rate Constants
The Arnot-Gobas model was employed using metabolic rate constants initially normalized to the weight, temperature and lipid content of the fish in the BCF and BMF studies. This was performed using the approach outlined in Arnot et al. (2008a) when BCF or the depuration rate constant is known. The purpose of this is to fit the kinetic model to agree with the observed BCF data, thus providing reasonable estimations of rate constants. The empirically observed and calculated kinetic rate constants are summarized in a mass-balance format in Table 8 below.
| Substance | Study endpoint | Uptake rate constant day-1 (k1) |
Depuration rate constant day-1 (kD)a |
Gill elimination rate constant day-1 (k2) | Metabolic rate constant day-1 (kM) |
|---|---|---|---|---|---|
| MDM | BCFss (5030 – 7730) |
n/a | n/a | n/a | n/a |
| MDM | BCFkinetic (3610 – 5600) |
1210 and 1040Footnote Table 8a[a] | 0.336 and 0.186[a] | n/a | n/a |
| MDM | BCFkinetic (7762) |
1427Footnote Table 8a[b] | n/a | 0.028[b] | 0.058[b] |
| MDM | BMFss (0.11 – 0.38) |
0.01[a] | 0.038[a] | 0.00Footnote Table 8a[c] | 0.028[c] |
| MDM | BMFkinetic (0.26 – 0.86) |
n/a | n/a | n/a | n/a |
| L4 | BCFss (1610 – 3870) |
n/a | n/a | n/a | n/a |
| L4 | BCFkinetic (1760 – 3830) |
n/a | n/a | n/a | n/a |
| L4Footnote Table 8a[*] | BCFkinetic (3890) |
1427[b] | n/a | 0.007[b] | 0.066[b] |
| M4Q | BMFss (0.045 – 0.16) |
n/a | n/a | n/a | n/a |
| M4Q | BMFkinetic (0.10 – 0.37) |
0.0025[a] | 0.025[a] | 0.0000[c] | approximately 0.020[c] |
| Substance | Study endpoint | Growth rate constant day-1 (kG) |
Fecal egestion rate constant day-1 (kE) | Total elimination rate constant day-1 (kT)Footnote Table 8b[d].1 | Reference |
|---|---|---|---|---|---|
| MDM | BCFss (5030 – 7730) |
n/a | n/a | n/a | Drottar 2006 |
| MDM | BCFkinetic (3610 – 5600) |
n/a | n/a | n/a | Drottar 2006 |
| MDM | BCFkinetic (7762) |
0.002Footnote Table 8b[b].5 | 0.011[b] | 0.099 | Environment Canada (see text) |
| MDM | BMFss (0.11 – 0.38) |
0.040Footnote Table 8b[a].6 | 0.010Footnote Table 8b[c].4 | 0.078[c] | Drottar 2010 |
| MDM | BMFkinetic (0.26 – 0.86) |
n/a | n/a | n/a | SEHSC 2010, 2011 |
| L4 | BCFss (1610 – 3870) |
n/a | n/a | n/a | SEHSC 2006 |
| L4 | BCFkinetic (1760 – 3830) |
n/a | n/a | n/a | SEHSC 2006 |
| L4Footnote Table 8b[*].4 | BCFkinetic (3890) |
0.002[b] | 0.009[b] | 0.084[b] | Environment Canada (see text) |
| M4Q | BMFss (0.045 – 0.16) |
n/a | n/a | n/a | DowCorning Corp 2010b |
| M4Q | BMFkinetic (0.10 – 0.37) |
0.037[a] | 0.005[c] | 0.062[c] | Environment Canada (see text) |
The kinetic mass-balance approach fitted to the analogue BCF data predicts BCF values of 7762 and 3890 for MDM and L4, respectively (see Table 8), and these values agree well with those of 3610 to 5600 and 1760 to 3830 derived empirically for MDM and L4 (Table 6). Thus, there is good confidence that the kinetic rate constants approximate those under laboratory conditions. The calculated total elimination rate constants are in very good agreement with each other (0.06 to 0.10 d-1) whether derived based on BCF or BMF data. The metabolic rate constants are also in very good agreement and range from 0.020 to 0.066 d-1 suggesting a slow rate of biotransformation and supporting observations reported in the empirical studies of little biotransformation of parent MDM or L4. For comparison, depuration rate constants of 0.035 and 0.040 d-1 were calculated for D4 and D5, respectively, in rainbow trout, Oncorhynchus mykiss (Woodburn et al. 2013).
The metabolic competency of an organism can be related to body weight and temperature (e.g., Hu and Layton 2001; Nichols et al. 2007). In order to provide a more representative metabolic rate constant, the geometric mean of the aqueous and dietary metabolic rate constants (i.e., 0.038 d-1) for all compounds in Table 8 was determined. This rate constant was further normalized to the conditions of the middle trophic level scenario in the Arnot-Gobas model (fish weight =184 g, lipid content = 6.8%, temperature = 10°C) according to the procedures outlined in Arnot et al. (2008b). The resulting kM when rounded is 0.010 d-1. To provide some context within the broader class of VMS substances, this kM value lies within the ranges of the values available for other low molecular weight VMS of 0.008 to 0.08 (median value 0.02) and 0.001 to 0.01 (median 0.004) for D4 and D5, respectively (Environment Canada, Health Canada 2008a, 2008b). The middle trophic level fish was used to represent overall model output as suggested by the model developer and is most representative of fish weight likely to be consumed by an avian or terrestrial piscivore; it also has a lipid content of 6.8%, which is considered representative of Canadian conditions. The calculated kM value is consistent with the analysis for metabolites conducted at steady-state conditions in the bioaccumulation study from Drottar (2006) in which 98% of the radio-labelled material was present as the parent MDM, thus supporting the notion of “slow metabolic breakdown” of this substance.
The kM based on the data from Table 8 for MDM for a 10 g fish at 15°C was calculated to be 0.03 d-1using the method of Arnot et al. (2008b). Examination of the kM database from Arnot et al. (2008b) for a 10 g fish at 15°C shows that chemicals with log Kow approximately 6.6 and a comparable kM to MDM, such as 2,3,7,8-tetrachlorodibenzodioxin (CAS RN 1746-01-6; log Kow 6.8, kM-normalized of 0.01 to 0.02 d-1); phenol, 2-(2H-benzotriazol-2-yl)-4,6-bis(1,1-dimethylethyl) (CAS RN 3846-71-7; log Kow 6.3, kM-normalized of 0.04 to 0.05 d-1) and DDE (CAS RN 72-55-9; log Kow 6.5, kM-normalized 0.02 d-1) have measured BCFs that can range above 5000 (Gobas et al. 1989; Gobas and Sharp 1990; NITE 2002).
Trophic Magnification Factor (TMF)
The TMF is a measure of the biomagnification potential of a substance within a studied foodweb under field conditions. It is estimated by correlating the normalized substance concentrations in biota at different trophic levels. A positive slope of the lipid-normalized concentration to trophic level regression line indicates that the substance concentration increases over several trophic levels and biomagnification is occurring (Weisbrod et al. 2009). Conversely, a TMF below 1 indicates trophic dilution, which is largely a function of metabolism.
No TMF values were available for MDM or its analogues at the time of this analysis. Based on an empirical BMF range of 0.11 to 0.86 (Table 7), MDM is expected to have low potential to biomagnify through foodwebs and is therefore likely to have a TMF of less than 1.
Bioaccumulation Factor (BAF)
Bioaccumulation factors are measured under field conditions as the ratio of the whole body burden of chemical taken up from all exposures to that of the ambient water concentrations. No such field data are available for any of the VMS considered in this bioaccumulation analysis. Measures of BAF are a preferred metric for assessing the bioaccumulation potential of substances because they incorporate all chemical exposures to an organism, including the diet which predominates for substances with log Kowgreater than approximately 4.0 (Arnot and Gobas 2003a).
At a log Kow of 6.6, the predicted bioavailable fraction of MDM in the water column (excluding loss from volatilization) according to mass-balance fish models is approximately 54%, which suggests that uptake from water via the gills is a very relevant exposure for MDM. However, if the log Koc of 4.3 is used, the majority fraction (approximately 99%) of MDM will be in the dissolved phase in water. This analysis suggests that gill uptake is very relevant for this chemical.
In the absence of empirical data, estimates of BAF were generated using a three trophic level version of the mass-balance kinetic model from Arnot and Gobas (2003a) by correcting the default dietary assimilation efficiency of 46% according to the alpha value of 32% reported in the MDM BMF study from SEHSC (2010). This has a direct impact on the default dietary uptake rate (kD) assumed by the model. The BAF prediction for the middle trophic level fish using the normalized metabolic rate constant of 0.010 d-1 and ED of 32% results in a BAF for the middle trophic level fish of approximately 288 400 which is within the domain of the modelFootnote[5].
Possible overestimation of the middle trophic level fish BAF was considered, given the potential for higher biomagnification potential calculated by the model. However, the predicted BMF used in the model for calculation of the BAF is in good agreement with the kinetic BMF results for MDM (model BMF = 0.8 vs. kinetic BMF = approximately 0.9). The BAF model calculates a “realized foodweb magnification factor”Footnote[6] of 1.0, which is not an extreme value for this parameter. If this value is set to less than 1 (using an upper bound TMF of 0.7), the resulting BAF is approximately 18 620. Although there is some uncertainty regarding the absolute value of BAF generated by the model, it is not likely that the BAF is substantially less than the value calculated, given the corrections made to the model and considering the kinetic information, particularly slow metabolic rate, relatively high aquatic bioavailability and efficient dietary uptake.
Fugacity and Critical Body Residue Analysis
Calculation of fugacity ratios and fugacity capacities based on Burkhard et al. (2012) and critical body residues (McCarty and Mackay 1993) for water, sediment and dietary exposures (i.e., using BCF, BSAF and BMF data) as well as critical body burden calculations are provided in Appendix III. BSAF data for D4 were used for this analysis, given the similar water solubility, Kow and Koc to MDM, as no data were available for MDM. Fugacity ratios (Fbiota-water,sediment,diet) in biota from exposure to MDM in water, sediment and the diet are all less than one ranging up to only 0.05. These values suggest little biomagnification potential and little contribution of the diet to overall bioaccumulation of MDM. This is consistent with observed BMF data. The fugacity and CBR analysis suggests that critical body residues, which range from much less than 1 mmol/kg to 1.4 mmol/kg can be reached in benthic and pelagic organisms given the overall bioaccumulation potential of MDM. The upper range of these values exceeds the internal chronic CBR of 0.2 to 0.8 mmol/kg suggested by McCarty and MacKay (1993) for chronic effects in fish and invertebrates and while adverse effects have been observed in one sediment worm test, none have been reported in other sediment tests or for other pelagic organisms. This lack of correlation with pelagic organisms suggests that: (1) MDM may bioaccumulate, but not act as other narcotic chemicals at target sites (less reactive than other narcotic chemicals), (2) there could be error associated with the bioconcentration factor in fish or the sediment toxicity test, (3) accumulation in organisms is not strictly a result of hydrophobic partitioning (e.g., hydrogen bonding) and that distribution in the organism does not result in toxicity at target sites as a result, or (4) octanol is not a good surrogate for target lipid resulting in error estimating internal concentrations. Burkhard et al. (2012) discuss the limitations of fugacity analysis for bioaccumulation as well.
Summary of Bioaccumulation Potential
There is consistency between lines of evidence, to infer that MDM will be highly bioaccumulated from both water and the diet. However, MDM is not likely to biomagnify in foodwebs. The intrinsic properties of MDM indicate that there is potentially a significant bioavailable fraction of MDM in natural waters and thus water remains a relevant exposure medium contributing to potential body burdens of MDM in biota, in addition to the diet. MDM has a log Kow and slow rate of metabolism comparable to other chemicals that have been empirically observed to highly bioconcentrate from water. There is also very good consistency between the kinetic parameters calculated for all the VMS analysed in this assessment which suggests that elimination of MDM for fish is likely not a result of significant metabolism. This has important implications for exposures at individual trophic levels of foodwebs, but perhaps not the entire foodweb. This is consistent with calculated fugacity ratios well below one, suggesting biomagnification is not likely for MDM.
A high bioaccumulation potential, regardless of route of exposure, provides information on the potential for adverse effects at a given exposure, particularly where a narcotic mode of action is known or predicted. Fugacity and CBR analysis suggest that chronic narcotic thresholds could be reached at sufficiently high environmental concentrations in sediment or water. However, there are some uncertainties associated with the fugacity and CBR analysis as well as the potency of MDM as a narcotic chemical. MDM has only been observed to cause adverse effects in one sediment test and not in other sediment or pelagic tests. Critical body residues are subject to exposure concentrations, which if well below maximum solubility, will limit the tissue residue concentration in exposed organisms further reducing the potential for adverse effects. MDM has been detected in a limited number of fish, marine mammals and invertebrates, but at levels well below those resulting in narcosis, and has not been detected in other species of fish, invertebrates, reptiles or birds.
Potential to Cause Ecological Harm
Ecological Effects Assessment
Data Sources
The empirical aquatic toxicity database for MDM was sufficient to evaluate the potential for hazard in this medium. However, no terrestrial toxicity data were found and empirical data for the cyclic VMS D5, which is a relatively data-rich low molecular weight VMS, were used for comparative purposes in the examination of potential for terrestrial effects. As MDM and D5 are expected to both act via non-polar narcosis and are within the same general class of VMS substances, examining terrestrial toxicity data for D5 in the context of potential MDM terrestrial toxicity was considered meaningful.
Mode of action
Drottar (2006) reported an average steady-state tissue concentration of 162.2 mg/kg, equivalent to a molar concentration of 0.69 mmol/kg, in fathead minnow, Pimephalespromelas, exposed for 42 days to 0.021 mg/L MDM. This value lies within the critical body residue range in fish of 0.2 to 0.8 mmol/kg proposed by McCarty et al. (1992) as being attributable to chronic effects due to a narcosis mode of action. Based on the absence of observed effects such as substance-related mortality and other clinical signs of toxicity at this tissue concentration, non-polar narcosis is considered to be the most likely mode of toxic action for MDM (SEHSC 2006). A non-specific, non-polar narcosis mechanism of toxicity has also been proposed for some cyclic VMS (Hobson and Silberhorn 1995; Redman et al. 2012).
Empirical Studies – Aquatic/Sediment Compartment
Experimental ecological effects data for MDM that were used to evaluate the potential for adverse effects in the Canadian aquatic environment are summarized in Table 9.
| Test organism | Type of test | Endpoint | Value Footnote Table 9[a].7 | Reference |
|---|---|---|---|---|
| Oncorhynchus mykiss, rainbow trout | Acute (96 hours) |
LC50Footnote Table 9[b] | greater than 0.019 | SEHSC 2010 |
| Oncorhynchus mykiss, rainbow trout | Acute (96 hours) |
NOECFootnote Table 9[c] | 0.019 | SEHSC 2010 |
| Oncorhynchus mykiss, rainbow trout | Acute (96 hours) |
LOECFootnote Table 9[d] | greater than 0.019 | SEHSC 2010 |
| Oncorhynchus mykiss, rainbow trout | Chronic (90 days) |
NOEC | 0.027 | Lee 2010Footnote Table 9[*] |
| Oncorhynchus mykiss, rainbow trout | Chronic (90 days) |
LOEC | greater than 0.027 | Lee 2010[*] |
| Daphnia magna, water flea | Acute (48 hours) |
EC50Footnote Table 9[e] | greater than 0.020 | SEHSC 2010 |
| Daphnia magna, water flea | Chronic (21 days) |
EC50 | greater than 0.015 | SEHSC 2010 |
| Daphnia magna, water flea | Chronic (21 days) |
NOEC | 0.015 | SEHSC 2010 |
| Daphnia magna, water flea | Chronic (21 days) |
LOEC | greater than 0.015 | SEHSC 2010 |
| Pseudokirchneriella subcapitata, green alga | Acute (72 hours) |
EC50 | greater than 0.0094 | SEHSC 2010 |
| Pseudokirchneriella subcapitata, green alga | Acute (72 hours) |
NOEC | 0.0094 | SEHSC 2010 |
| Lumbriculus variegates, oligochaete | Chronic (28 days) |
EC50 | greater than 17 | Thomas et al. 2009b[*] |
| Lumbriculus variegates, oligochaete | Chronic (28 days) |
NOEC (survival, reproduction) |
1.1 | Thomas et al. 2009b[*] |
| Lumbriculus variegates, oligochaete | Chronic (28 days) |
LOEC (survival, reproduction) |
1.6 | Thomas et al. 2009b[*] |
| Lumbriculus variegates, oligochaete | Chronic (28 days) |
NOEC (dry weight) |
17 | Thomas et al. 2009b[*] |
| Lumbriculus variegates, oligochaete | Chronic (28 days) |
LOEC (dry weight) |
greater than 17 | Thomas et al. 2009b[*] |
| Lumbriculus variegates, oligochaete | Chronic (28 days) |
EC50 | greater than 38 | Bradley 2013 |
| Lumbriculus variegates, oligochaete | Chronic (28 days) |
NOEC (survival, reproduction, biomass) |
38 | Bradley 2013 |
| Lumbriculus variegates, oligochaete | Chronic (28 days) |
LOEC | greater than 38 | Bradley 2013 |
| Chironomus riparius, midge | Chronic (28 days) |
LC50 | 166 | Thomas et al. 2009a |
| Chironomus riparius, midge | Chronic (28 days) |
NOEC (development time, emergence, emergence ratios) |
84 | Thomas et al. 2009a |
| Chironomus riparius, midge | Chronic (28 days) |
LOEC (development time, emergence, emergence ratios) |
29 | Thomas et al. 2009a |
| Chironomus riparius, midge | Chronic (28 days) |
NOEC (development rate) |
39 | Thomas et al. 2009a |
| Chironomus riparius, midge | Chronic (28 days) |
LOEC (development rate) |
84 | Thomas et al. 2009a |
| Hyalella azteca, amphipod | Chronic (28 days) |
LC50 | greater than 70 | Bradley 2012 |
| Hyalella azteca, amphipod | Chronic (28 days) |
NOEC (survival, growth) |
70 | Bradley 2012 |
| Hyalella azteca, amphipod | Chronic (28 days) |
LOEC (survival, growth) |
greater than 70 | Bradley 2012 |
No observable adverse effects were seen at test concentrations up to the reported water solubility of 0.034 mg/L in acute testing with rainbow trout, Oncorhynchus mykiss, the water flea, Daphnia magna and the green alga, Pseudokirchneriella subcapitata, as well as in chronic testing with O.mykiss and D. magna (SEHSC 2010; Lee 2010). Analytical determinations were performed in all studies, and the results presented in Table 9 are expressed in terms of mean measured concentrations.
No dose-related toxicity was observed in fathead minnow, Pimephales promelas, exposed for 42 days to test concentrations of 0.0017 mg/L (0.0034 nominal) and 0.021 mg/L (0.034 nominal) MDM (Drottar 2006; see Potential for Bioaccumulation section). Although occasional mortalities occurred throughout the study (i.e., 8 to 10 mortalities in each treatment of 120 fish), all surviving fish appeared normal and displayed no overt signs of toxicity.
A 28-day toxicity test with the freshwater oligochaete, Lumbriculus variegates, and MDM incorporated into a formulated sediment (sediment organic carbon (OC) content 1.9%), found significantly reduced survival and reproduction at a lowest measured test concentration (LOEC) of 1.6 mg/kg sediment dry weight (dw) (Thomas et al. 2009b). The lowest no-effect concentration (NOEC) for the study was 1.1 mg/kg dw, based also on endpoints of survival and reproduction. Mean dry weight was not significantly reduced at any test concentration relative to the controls. Survival and reproduction were reported as a single endpoint as specified in toxicity testing guidelines for Lumbriculus(e.g., OECD 2007) and were determined as the mean number of living worms present in the test containers at test termination.
The saturation concentration of MDM in sediment can be determined using the relationship:
Cs = Cw × Koc × foc
where:
- C s:
- saturation concentration (mg/kg dw)
- C w:
- water solubility of MDM (mg/L) = 0.034 mg/L (Table 2)
- K oc:
- organic carbon-water partition coefficient of MDM = 21,878 L/kg OC (log K oc is 4.34; Table 2)
- f oc:
- fraction of organic carbon (OC) in the sediment (unitless)
Saturation concentration reflects the theoretical thermodynamic saturation concentration of a compound in a given medium at equilibrium. It cannot be exceeded according to thermodynamic principles. In surface water, however, the presence of co-solvents or surfactants can create conditions that allow for an “apparent solubility” to be observed which is greater than the maximum solubility. In solid phases, such as sediments and soils, saturation concentration is a direct function of the amount of organic carbon present in the matrix if it is assumed that only hydrophobic interactions with organic matter occur. Sediment organic carbon content can vary from location to location and often average carbon contents are used for calculating saturation concentrations in sediments. The apparent solubility in water, and saturation concentrations in sediment or soil, can increase or decrease the bioavailability of a compound. The values calculated above therefore represent the theoretical saturation concentrations which, for the purposes of bioavailability, may be exceeded under some circumstances. For example, it is difficult to be certain that only hydrophobic interactions are responsible for defining the theoretical saturation concentration in solid phases. These circumstances cannot be easily predicted without specific information regarding the nature of release and the characteristics of the receiving environment.
For a sediment with 1.9% OC, the saturation concentration of MDM is 14 mg/kg dw. As test concentrations used in the study are below this value, the saturation concentration for MDM was not exceeded.
Subsequent Lumbriculus testing conducted using natural sediment found no statistically significant adverse effects on survival, reproduction or biomass (mean weight) up to and including the highest mean measured test concentration of 38 mg/kg dw of sediment (Bradley 2013). Therefore, the NOEC and LOEC determined from the study were 38 and greater than 38 mg/kg dw, respectively. As the test sediment contained 3.1% OC, the saturation concentration of MDM was 23 mg/kg dw. The saturation concentration was therefore exceeded under the conditions of the study, although no adverse effects were observed in the test organisms.
Survival, development time, emergence ratios and development rates were examined in 28-day sediment toxicity testing (formulated sediment, OC content 2.2%) with the freshwater midge, Chironomus riparius (Thomas et al. 2009a). Development rates were the most sensitive endpoints, with a LOEC of 84 mg/kg dw and a NOEC of 39 mg/kg dw. The saturation concentration of MDM in sediment having 2.2% OC is 16 mg/kg dw, therefore the endpoint values exceeded the saturation concentration of MDM in this sediment. This suggests that free MDM was present in the test system and may have contributed to the observed effects through factors such as the physical clogging of respiratory surfaces.
Bradley (2012) reported no adverse effects on survival and growth in freshwater amphipods, Hyalella azteca, exposed for 28 days to mean measured sediment MDM concentrations of 6.0, 10, 19, 31 and 70 mg/kg dw of sediment (natural sediment, OC content 3.7%). The NOEC for the study was therefore 70 mg/kg dw and the LOEC was greater than 70 mg/kg dw. Both values exceed the saturation concentration of 27.5 mg/kg dw for MDM in a sediment having this OC content. However, no adverse effects were seen in the test system.
In summary, no adverse effects were seen in laboratory testing with fish, Daphnia and algae exposed to MDM concentrations at or below the limit of water solubility for periods of 48 hours to 90 days. A range of toxicity endpoint values was observed in sediment testing with MDM, including two studies conducted with the same species (Lumbriculus variegatus) where effects were seen in one study but not in the other. The observed differences in response likely reflect variable sensitivities between the three test species (Lumbriculus sp., Chironomus sp., and Hyalella sp.), but may also relate to variations in study conditions. While all toxicity studies were conducted in accordance with internationally-accepted standard methodologies and followed Principles of Good Laboratory Practice (GLP), differences such as the use of formulated vs. natural sediment and slight variations in the organic carbon (OC) content of the sediment, may have influenced the test results. Lumbriculus variegatuswas the most sensitive species tested, with a lowest effect level of 1.6 mg/kg dw in a formulated sediment containing 1.9% OC. The experimental results suggest that adverse effects may occur in some sediment species exposed to MDM, based on laboratory tests.
Empirical studies – Terrestrial Compartment
No ecological effects data were found for MDM in terrestrial plants, soil-dwelling organisms (such as earthworms) or wildlife. Laboratory studies using rodents have been conducted with MDM in order to evaluate the potential for impacts to human health and relevant data from these studies are presented in the Human Health Effects section of this assessment.
Terrestrial effects information was also not found for other linear VMS, such as L4 and L5. However, soil toxicity studies with the cyclic VMS D5 are described in the literature and, based on a probable similarity in mode of action, these results are considered relevant to MDM. A median inhibition concentration (IC50; concentration causing a 50% reduction in a biological measurement) of 767 mg/kg dw of soil was reported for significantly reduced young production in the springtail, Folsomia candida, exposed to D5 for 28 days, while the 56-d IC50 for the same endpoint in earthworm, Eisenia fetida, was greater than the highest test concentration of 4074 mg/kg dw (Environment Canada 2010c). In 14-d testing with D5 and four terrestrial plant species, the most sensitive species was barley, Hordeum vulgare, with an IC50 of 209 mg/kg dw of soil based on significantly reduced dry mass of roots. The same endpoint was greater than the highest test concentration of 3533 to 4306 mg/kg dw for the other three species tested, red clover (Trifolium pretense), durum wheat (Triticum durum) and radish (Raphanus sativus) (Environment Canada 2010c). By comparison, D5 levels measured in samples of biosolids-amended soils collected from sites in southern Ontario and Quebec ranged from 0.006 to 0.221 mg/kg dw (Wang et al. 2010). Although soils amended with biosolids represent a maximum or worst-case scenario for D5 in this medium, the measured range is well below the lowest laboratory-derived effect level of 209 mg/kg dw. The proposed similarity in mode of action for D5 and MDM suggests that terrestrial toxicity endpoint values will be similar between the two substances. As well, MDM is expected to be present at lower levels in soils than D5, given the higher use of D5 in Canada relative to that of MDM (Environment Canada, Health Canada 2008a). Based on this, it is likely that MDM poses low hazard to terrestrial invertebrates and plants.
Derivation of the PNEC
Aquatic compartment
No adverse effects were observed in testing with water column species and a Critical Toxicity Value (CTV) for the aquatic compartment was derived using a no-effect rather than a lowest-effect level. The highest NOEC of 0.027 mg/L, reported by Lee (2010) for 90-day toxicity testing with rainbow trout, Oncorhynchus mykiss, was selected as the CTV. In addition to providing the highest measured test concentrations available, the 90 day study duration ensured that exposure to the test substance occurred over a sustained period of time. As this endpoint is already a no-effect value, no Assessment Factor (AF) was applied and the Predicted No-Effect Concentration (PNEC) is therefore 0.027 mg/L.
Sediment compartment
The lowest endpoint value obtained in testing with a sediment species is a 28-day LOEC of 1.6 mg/kg dw reported for reduced survival and reproduction in the oligochaete, Lumbriculus variegates (Thomas et al. 2009b). This value represents the toxicity threshold for sediment organisms based on the available data, and is therefore selected as the CTV for sediment. An AF of 10 was applied to the CTV of 1.6 mg/kg dw in order to account for inter- and intraspecies variability in sensitivity to MDM, resulting in a PNEC of 0.16 mg/kg dw. The LOEC of 1.6 mg/kg dw was obtained in sediment having an organic carbon (OC) content of 1.9%. This PNEC value was standardized to an OC of 3% in order to better facilitate comparison with a Predicted Environmental Concentration (PEC) on an equal OC basis (see Ecological Exposure Assessment section). The resulting PNEC is then 2.5 mg/kg dw.
Terrestrial compartment
No ecological effects data were found for MDM in terrestrial plants, soil-dwelling organisms or wildlife species; therefore, a PNEC could not be derived for the terrestrial compartment.
Ecological Exposure Assessment
The chemical properties and reported uses of MDM indicate that release into wastewaters and receiving waters could occur during both consumer and industrial applications. Once released into receiving waters, MDM is predicted to distribute within the water column and into sediment, although a proportion will also volatilize into the atmosphere. Release of MDM from consumer applications is expected to be diffuse and, for this reason, industrial sources are considered to provide the highest potential for more concentrated releases into the environment.
MDM was below detection limits in surface water and sediment grab samples collected in North America and Europe, including areas located near wastewater treatment plant (WWTP) outfalls (see Appendix II and Measured Environmental Concentrations section). Potential concentrations in surface waters near WWTP outfalls were estimated using a modelling approach which considered information relating to import, use and estimated release quantities of the substance, as well as characteristics of Canadian receiving environments.
The concentration of MDM calculated to be present in receiving waters situated near the discharge point of a wastewater treatment plant was used as the Predicted Environmental Concentration (PEC) in evaluating risk in Canadian surface waters. This surface water PEC was calculated using the equation:
Cwater-ind = 1000 × Q × L × (1 - R) / N × F × D
where:
- C water-ind:
- aquatic concentration resulting from industrial releases, mg/L
- Q:
- total substance quantity used annually at an industrial site, kg/yr
- L:
- loss to wastewater, fraction
- R:
- wastewater treatment plant removal rate, fraction
- N:
- number of annual release days, d/yr
- F:
- wastewater treatment plant effluent flow, m 3/d
- D:
- receiving water dilution factor, dimensionless
An exposure analysis was conducted for the aquatic compartment at five sites where MDM was used industrially in 2006 (Environment Canada 2010a). These sites were selected to represent realistic worst-case release scenarios across Canada based on the general assumption that the quantity of a substance released is proportional to the quantity consumed or produced. In the site-specific exposure analysis, each scenario included one facility, one wastewater treatment plant and one receiving water body. The PEC in the receiving water was estimated based on the concentration in the wastewater treatment plant effluent and a dilution factor in the receiving water body limited to a maximum value of 10. The concentration in the wastewater treatment effluent was determined based on reported data and a secondary wastewater treatment plant removal rate of from 87 to 90% derived using the computer model ASTreat (2006). The effluent flow of a local wastewater treatment plant is proportional to the population served and was in the range of 4000 to 200 000 m3 per day for the sites considered.
The assumed number of days of release for industrial users (small- or medium-sized facilities) used in the estimation was 300 days/year, for a continuous release scenario. The PEC values obtained are considered to represent a steady-state level of exposure under a realistic worst-case release scenario in receiving waters near the point of discharge from a WWTP at an industrial site in Canada. To better account for the difference in concentration due to volatilization between the industrial point of release to the sewer and the WWTP influent, the fraction released to wastewater was calibrated based on monitoring data available for two substances used in similar industrial processes (D5 and D6). The measured concentrations of D5 and D6 in the influent of a WWTP in the municipality known to receive the highest concentrations of these substances were used to calculate the total loading. The proportion released to wastewater was then calculated by dividing the total loading in the influent of the WWTP in the municipality by the known quantity of D5 and D6 used at industrial sites discharging their effluent to the WWTP in the municipality and was determined to be 0.25%.
Based on the above input values and assumptions, PECs for MDM are estimated to be in the range of 0.00001 to 0.00011 mg/L. The highest PEC value of 110 ng/L (0.00011 mg/L) is in the range of the highest measured Canadian WWTP effluent concentration of 115 ng/L (see Appendix II).
An equilibrium partitioning relationship (EqP) was applied to the highest surface water PEC value of 0.00011 mg/L in order to derive an estimated PEC for the sediment compartment. Based on the principles of hydrophobic interactions,
PECsediment = PECwater × Kocsediment × foc
where:
- PEC sediment:
- Predicted Exposure Concentration in sediment (mg/kg dw)
- PEC water:
- Predicted Exposure Concentration in water (mg/L) = 0.00011 mg/L
- K oc:
- organic carbon – water partitioning coefficient (L/kg OC) = 21878 (see Table 2)
- F oc sediment:
- fraction of organic carbon in sediment (unitless)
The fraction of organic carbon (OC) present in sediment (Foc sediment) is expected to vary substantially between locations and an average value of 3% OC was used to represent Canadian sediments.
The resulting PECsediment value is then 0.072 mg/kg dw of sediment.
Characterization of Ecological Risk
This screening assessment examines information critical to determining whether MDM meets criteria under section 64 of CEPA 1999, including whether the substance has the potential to cause ecological harm in Canada. Lines of evidence considered in reaching a conclusion include those pertaining to import and use patterns, environmental release and distribution, potential for environmental persistence, bioaccumulation potential, toxicity and hazard potential, environmental monitoring results, and the results of quantitative risk quotient analyses based on empirical and modelled exposure and effects data.
Information obtained for the year 2006 determined that MDM was not manufactured in Canada but was imported in a quantity range of 10 000 to 100 000 kg/y. The substance is primarily used as an ingredient in industrial, medical and consumer products such as cleaning and degreasing products, lubricants, diluents and solvents, and in cosmetics. Based on reported use patterns, much of the MDM imported into Canada is expected to be exported out of the country in products (31%), recycled during industrial use (16%), or present in products that are eventually directed to landfills (5%) or incineration (1%). Approximately half of the MDM used in Canada (47%) is expected to be released directly into the environment, with the majority of the emissions occurring to air (46%) and the remaining proportion (1%) occurring to pre-treatment wastewaters. The high vapour pressure of MDM indicates that, when released into environmental media other than air, it will tend to volatilize out of these media and into air. . Some distribution into water and sediment is also expected to occur when the substance enters the aquatic environment.
MDM present in air will primarily undergo abiotic degradation through reaction with photochemically-produced atmospheric hydroxyl radicals, with calculated half-lives of 6 to 9 days. These results indicate that MDM is not recalcitrant in air, although the longer residence time suggests that MDM may be capable of moving in atmospheric currents to areas some distance from the site of release. However, MDM is considered to have low Arctic contamination potential, as it is unlikely to deposit from air to water and soil in remote regions. MDM has not been detected in Arctic air monitoring programs conducted in North America and Europe, although it has been found at low concentrations in a small number of air samples collected from North American background sites. MDM is more likely to be detected in air samples collected in the vicinity of source activities, such as industrial sites, waste treatment facilities and areas of urbanization. A highest measured air concentration of 6. 43 ng/m3 was determined in an air sample collected from a suburban area of Toronto, Ontario, Canada and this value is much lower than the lowest adverse effect concentration (LOAEC) of 7740 mg/m3reported for inhalation effects in rats (see Human Health Effects Assessment). This suggests that environmental concentrations of MDM in air are well below those expected to elicit adverse effects in organisms.
A small proportion (approximately 1%) of the MDM used in Canada is expected to be released into pre-treatment wastewaters. Empirical data indicate that abiotic processes such as volatilization at the air-water interface and hydrolysis within the water column are important for removal of MDM in aqueous media, and both processes will contribute to reductions within wastewater treatment plants (WWTPs) and in surface waters. Levels of MDM measured in WWTP effluents are much lower than those in influents, indicating that treatment processes are effective at reducing the quantity of MDM leaving the plant to enter surface receiving waters. Empirical hydrolysis half-lives of 0.12 to 60.9 days confirm that MDM will be degraded in the water column.
Removal processes such as volatilization at the water surface and hydrolysis within the water column are expected to substantially reduce the quantity of MDM that ultimately reaches the sediment bed. No information was found on the abiotic degradation of MDM in sediment and an analogue-based calculated biodegradation half-life of 365 days was used in the analysis of the potential for persistence in this medium. This half-life indicates that MDM may remain for long periods in sediment. However, MDM has demonstrated low potential for microbial biodegradation and given the evidence for active abiotic degradation of the substance in both soil and water, it seems likely that an analysis of persistence in sediment based only on biodegradation data would underestimate the potential for removal in this medium.
Land treatment with biosolids (amended WWTP sludge) likely represents the highest potential source of MDM to soil. The high vapour pressure suggests that MDM present in sludge will be removed through volatilization during the processing of sludge to biosolids, and some hydrolysis may also occur prior to sludge de-watering. These processes are expected to reduce the total quantity of MDM present in biosolids that are applied to soils. In addition, acid-catalyzed hydrolysis and volatilization have been demonstrated to be important removal processes for MDM in soil. Based on the available information, MDM is not expected to persist for extended periods of time in soil.
MDM has demonstrated significant bioconcentration capacity in laboratory testing with fish. Bioaccumulation Factor (BAF) estimates calculated using empirical data for MDM and several suitable analogue substances indicate that MDM may also have significant potential to accumulate in organisms through dietary exposures. The measured presence of MDM in some aquatic biota samples confirms that the substance is indeed bioavailable. However, MDM is only occasionally detected in monitoring programs and no clear patterns are evident in terms of regional characteristics or foodwebs. An empirical biomagnification factor (BMF) of less than 1 indicates that MDM is unlikely to transfer from one trophic level to the next highest trophic level in the foodweb studied.
No link has been established between accumulation of MDM in organisms and the potential for effects. No adverse effects were observed in water column species exposed for prolonged periods in a laboratory setting to MDM concentrations up to the limit of water solubility. Adverse effects were reported in one of two laboratory studies conducted with the sediment species, Lumbriculus variegatus. However, no adverse effects were seen in a second Lumbriculus study nor were effects seen in laboratory testing with two other sediment species, the freshwater amphipod Hyalella azteca and larval midge Chironomus riparius. The lowest effect level of 1.6 mg MDM/kg dw of sediment determined in Lumbriculus testing is substantially higher than MDM levels that have been measured in the environment. MDM was below detection limits in all sediment and surface water samples collected in environmental monitoring studies. This included a total of 289 sediment samples, some of which were collected from regions of known industrial and urban contamination. No information was found on the potential for effects in terrestrial species; however, results obtained for a mechanistically-similar compound suggest that MDM is not likely to be hazardous to terrestrial invertebrates or plants.
The environmental occurrence of MDM is closely associated with urban and industrial activities and waste treatment processes. MDM has been measured in some WWTP influents and effluents, as well as in some pre-treatment industrial process waters and landfill leachates, and this confirms that the substance can be released during product use and at disposal. Canadian WWTP influent concentrations are in the range of 1 to 531 ng/L (in 58 of 110 grab samples) and effluent levels are 0.4 to 115 ng/L (in 37 of 110 grab samples). The noticeable reduction in concentration and detection frequency between WWTP effluents and influents collected at the same time and from the same treatment plant indicates that wastewater treatment processes are effective at reducing the quantity of MDM available to enter surface waters. Volatilization and hydrolysis are likely to be important removal processes within and even prior to entering the treatment plant. As well, the high log Koc of 3 to 7 (empirical value 4.34) suggests that MDM present in influents will tend to adsorb onto suspended particulates with subsequent removal from the aqueous phase through settling. MDM was detected in 32% of WWTP sludge samples (23 of 72) collected at Nordic facilities and it is likely that MDM is also present in some Canadian sludges, although no data on this are currently available.
A quantitative estimation of potential for ecological harm was conducted by comparing Predicted Exposure Concentrations (PECs) in the Canadian aquatic environment with Predicted No-Effect Concentrations (PNECs) in a risk quotient analysis. For the pelagic compartment, a surface water PEC of 0.00011 mg/L was determined as the highest value in a range of concentrations derived from five industrial exposure scenarios. Comparing the PEC value with a PNEC of 0.027 mg/L, derived from the 90-day chronic no-effect value for rainbow trout, Oncorhynchus mykiss, yielded a risk quotient (PEC / PNEC) of 0.004. This risk quotient indicates that MDM is unlikely to harm aquatic organisms.
For the sediment compartment, a PEC of 0.072 mg/kg dw based on a 3% OC sediment was determined by applying the principles of Equilibrium Partitioning (EqP) to the highest predicted surface water concentration (PEC) of 0.00011 mg/L. By comparison, MDM was not detected in any of 229 sediment samples collected from sites in six Canadian provinces, with detection limits of 0.0002 to 0. 02 mg/kg dw of sediment. Comparing the PEC with a PNEC of 2.5 mg/kg dw, derived from the lowest effect level for chronic testing with the oligochaete, Lumbriculus variegatus, yielded a risk quotient (PEC / PNEC) of 0.03. Therefore, based on the conservative assumptions used to derive both the surface water and sediment PEC values, it is unlikely that MDM will harm sediment organisms in Canada.
Evidence for the active abiotic degradation of MDM in water and soil, together with limited direct release of the substance to the environment and its effective removal at WWTPs, indicate that MDM will have low exposure potential in the environment. MDM may remain for longer periods in air, although it is not recalcitrant in this medium, and may also persist in sediment. However, given the compositional similarity between soil and sediment, and the demonstrated ability of the substance to degrade in soil, it seems likely that some degree of abiotic degradation will also occur in sediment. In addition, hydrolysis within the water column is expected to reduce the quantity of MDM reaching the sediment bed. While laboratory and modelling data indicate that MDM may have significant bioaccumulation potential, the substance is not expected to biomagnify within foodwebs. In addition, there is an absence of adverse effects in organisms exposed for prolonged periods to MDM concentrations up to the solubility limit of the substance. On the basis of limited environmental presence, MDM is expected to pose low risk to sediment and terrestrial species . This low exposure and hazard potential indicate that there is low risk of harm to organisms or to the broader integrity of the environment from MDM. It is therefore concluded that MDM does not meet the criteria under paragraphs 64(a) or (b) of CEPA 1999 as it is not entering the environment in a quantity or concentration or under conditions that have or may have immediate or long-term harmful effects on the environment or its biological diversity, or that constitute or may constitute a danger to the environment on which life depends.
Uncertainties in Evaluation of Ecological Risk
Risk quotients were developed for both the pelagic and sediment compartments; however, both quotients contain uncertainties. For the pelagic compartment, a toxicity threshold value was not available and the PNEC was based on a no-effect rather than a lowest effect level. This means that the PNEC is unbounded, with water solubility providing the only limiting parameter. This uncertainty was addressed by not applying an Assessment Factor to the Critical Toxicity Value (CTV), given that the CTV was already a no-effect value. For the sediment compartment, uncertainty is associated with deriving a PEC value for MDM based on conservative assumptions and modelled estimates when the substance has not in fact been detected in sediment samples. The derived PEC cannot therefore be compared with environmental levels in order to assess the realism of the value.
Model predictions have been used to provide information relating to the environmental distribution, persistence and bioaccumulation potential of MDM. However, there may be higher uncertainties associated with the use of modelling for this substance, as few siloxanes data have been included in the models. Programs such EPI Suite and EQC have recently begun to incorporate data for VMS, primarily cyclic VMS, into the training sets of the models and this should help to decrease uncertainties associated with applying these models to organosilicones such as MDM. The most recent EPI and EQC versions were used to provide model output in this screening assessment and both incorporate some consideration of siloxanes. Therefore, estimates derived from modelling were deemed sufficiently reliable for use in the evaluation of potential for ecological harm.
Potential to Cause Harm to Human Health
Exposure Assessment
Environmental Media and Food
Limited empirical data are available on levels of MDM in environmental media. In Canada, MDM has been detected in ambient air at wastewater treatment plants and landfill sites in Ontario and upwind and downwind of these facilities (0.66 to 6.14 ng/m3 ; see Appendix II) (Cheng et al. 2011). Since these ambient air measurements were obtained at point sources, they were not considered relevant to general population exposures and were not used to characterize exposure from ambient air. MDM has also been detected in the Nordic environment ( see Appendix II). Specifically, it was detected in sludge and water in and near the vicinity of point sources, but was not detected in soil (less than 0.1 µg/kg), surface water (less than 0.5 to 0.8 ng/L), or ambient air (less than 8 ng/m3) in samples taken from background sources (Kaj et al. 2005a, 2005b). In follow-up surveys, MDM was also detected in sludge and water but again was not detected in sediment (less than 0.19 to less than 0.4 ng/g) or surface water (less than 0.3 ng/L) (Schalbach et al. 2007; Evenset et al 2009). In a recent study, MDM was detected above detection limit in ambient air in 6 of 20 locations worldwide at a range of 0.011 to 0.12 ng/m3 (Genualdi et al 2011).
In the indoor environment, MDM was detected in 2 of 400 Swedish homes at a range of 2.5 to 12.3 µg/m3 (Kaj et al. 2005a). Preliminary data also show detectable levels of MDM (1.73 to 5.2 ng/m3, n=6) in a Toronto office building (2010 personal communication from Environment Canada; unreferenced). Additionally, linear (decamethyltetrasiloxane) and cyclic siloxanes were also detected in bedrooms of Vancouver homes (Harner et al. 2010). These observations indicate that MDM may be present in the indoor air environment.
MDM was also detected in 6 of 39 breast milk samples at a concentration range of 0.003 to 0.008 µg/L (Kaj et al. 2005a).
Finally, MDM is not listed as an approved food additive and was not identified to be present in the formulation of incidental additives or in food packaging materials. However, PDMS (CAS RN 9006-65-9) is a permitted food additive as an antifoaming or release agent in specified foods in Canada. Health Canada does not have current data on the amount of MDM, if any, in the PDMS used in food applications. It should be noted, though, that the maximum level of PDMS when used as a food additive is 10 ppm; any MDM present would therefore be considered negligible (2010 personal communication from Food Directorate, Health Canada; unreferenced).
Levels of MDM in fish (e.g., trout, walleye) and shellfish (e.g., mussel) tissue from freshwater and marine locations have been monitored in Canada, US and European Nordic countries (see Appendix II and the “Measured Environmental Concentrations” section). Most reported levels were either below the method detection limit or near urban point sources (and therefore not representative of fish likely to be consumed by the general population); one study reported detectable levels (0.17 and 0.33 ng/g) away from point sources, in Norwegian Arctic cod and Polar cod livers, however prevalence was low (1 in 5 and 1 in 11 samples) (Evenset et al., 2009). Based on this, exposure to MDM from consumption of fish is expected to be low for the Canadian general population.
Exposure from environmental media was estimated using the data from the Nordic surveys (Kaj et al. 2005a, 2005b) and the total intake (6.5 µg/kg/day) from all routes of exposure was highest in the 0.5 to 4 years age group (see details in Appendix IV).
Uncertainty in estimates of exposure is high, as Canadian empirical data were not available for a majority of environmental sources. Monitoring data showing MDM presence in the Nordic environment were used as a surrogate for Canadian data. Also, there is uncertainty related to the absence of current data on the amount of MDM, if any, in PDMS used in food applications. However, the maximum level of PDMS when used as a food additive is 10 ppm; any MDM present would therefore be considered negligible (2010 personal communication from Food Directorate, Health Canada; unreferenced).
Based on conservative assumptions (use of limits of detection in calculations, maximal concentrations), there is confidence that the estimates of exposure from environmental media and breast milk are upper bounding.
Consumer Products, and Cosmetics including Personal Care products
Estimates of exposure from use of cosmetics, including some personal care products, were derived using ConsExpo 4.1 software (ConsExpo 2007). Manufacturers of cosmetics are required to notify Health Canada of the concentrations of siloxanes including MDM and PDMS formulations (generally termed dimethicone)Footnote[7] in these types of products. Based on notifications submitted under the Cosmetic Regulations to Health Canada , MDM was reported to be present in 20 to 30 product types (see table 10), most of which are associated with “leave-on” applications ( April 2013 email from Consumer Product Safety Directorate, Health Canada, to the Existing Substances Risk Assessment Bureau, Health Canada; unreferenced).
MDM has high volatility (molecular weight 236.54 g/mol, vapour pressure 3.9 mmHg; Table 2 ); therefore, both inhalation and dermal routes were addressed in this assessment.
In the assessment of another siloxane, D4, conducted by Health Canada (Environment Canada, Health Canada 2008b), it was assumed that 90% of cosmetics and personal care product exposures would occur through the inhalation route, with 10% occurring through the dermal route. This was based on experimental data which showed that 88 to 95 % of D4 evaporated from the skin (Zareba et al. 2002; Jovanovic et al. 2008; Environment Canada, Health Canada 2008b). Based on the similarity in molecular weight and vapour pressure between MDM and D4 (D4 molecular weight: 296.62 g/mol, vapour pressure 1.05 mmHg), this assumption was also used in estimating exposure associated with the use of cosmetics containing MDM. Additionally, based on the type of products (i.e., the majority are “leave-on”), inhalation and dermal exposure were assumed to occur over a 12 -hour period.
Aggregate daily exposure was calculated for an individual using products containing MDM over a 12-hour period. Exposure was estimated to be 0.090 to 2.8 mg/kg-bw (kilograms of body weight) per day and 6.8 × 10−3 to 0.42mg/kg-bw per day for dermal and inhalation routes, respectively (see Table 10). Additionally, an aggregate mean event concentration of 0.050 to 4.2 mg/m3 was estimated for use of these products over the course of the 12-hour period.
Table 10. Estimates of adult exposure to MDM from use of cosmetics, including personal care products Footnote Table 10[a].8
| Product | Concentration range (%)Footnote Table 10[b].7 | Mean event concentration (mg/m3) | Inhalation (mg/kg-bw per day) | Dermal (mg/kg-bw per day) |
|---|---|---|---|---|
| Foundation | 1 – 3 | 4.6 × 10−3 – 0.014 | 6.4 × 10−4 – 1.9 × 10−3 | 9.1 × 10−3 – 0.027 |
| Make-up primer | 1 – 100 | 4.6 × 10−3 – 0.18 | 6.4 × 10−4 – 0.025 | 9.1 × 10−3 – 0.35 |
| ConcealerFootnote Table 10[d] | 1 – 30 | n/a | n/a | 2.1 × 10−3 – 0.063 |
| Blush[d] | 1 – 3 | n/a | n/a | 2.4 × 10−3 – 7.1 × 10−3 |
| Mascara[d] | 0.3 – 30 | n/a | n/a | 7.1 × 10−5 – 7.1 × 10−3 |
| Eye shadow[d] | 3 – 100 | n/a | n/a | 4.6 × 10−4 – 1.5 × 10−2 |
| Eye lotion[d] | 0.3 – 3 | n/a | n/a | 9.7 × 10−4 – 0.010 |
| Face cream | 0.3 – 30 | 3.1 × 10−3 – 0.31 | 6.4 × 10−4 – 0.064 | 9.1 × 10−3 – 0.91 |
| Body lotion | 0.3 – 10 | 0.011 – 0.38 | 1.4 × 10−3 – 0.047 | 0.021 – 0.68 |
| Leave-in conditioner | 0.1 – 30 | 0.011 – 3.2 | 8.7 × 10−4 – 0.26 | 1.2 × 10−3 – 0.37 |
| Shampoo[d] | 0.1 – 0.3 | n/a | n/a | 2.0 × 10−3 – 6.0 × 10−3 |
| Rinse-out conditioner[d] | 0.1 – 0.3 | n/a | n/a | 7.2 × 10−4 – 2.2 × 10−3 |
| Antiperspirant/ deodorantFootnote Table 10[c] |
1 – 10 | n/a | n/a | 0.011 – 0.11 |
| Perfume | 3 – 30 | 8.5 × 10−3 – 0.08 | 2.4 × 10−3– 0.024 | 0.024 – 0.24 |
| Total | n/a | 0.050 – 4.2 | 6.8 × 10−3– 0.42 | 0.090 – 2.8 |
| Product | Concentration range (%)[b] | Mean event concentration (mg/m3) | Inhalation (mg/kg-bw per day) | Dermal (mg/kg-bw per day) |
|---|---|---|---|---|
| Detangler[c] | 5 | 0.27 | 0.14 | 0.20 |
| Shampoo[d] | 0.3 | n/a | n/a | 2.0 × 10−3 |
| Total | n/a | 0.27 | 0.14 | 0.20 |
In addition to the inhalation and dermal routes, oral exposure from lipstick use was also calculated, assuming 100% absorption via the GI tract. Exposure was estimated to be 3.0 to 8.9 µg/kg-bw per day. These estimates were low compared to exposure from the other “leave-on” products and therefore were not presented as part of the aggregate daily exposure from cosmetics, including personal care products (Table 10).
The general population may be exposed to paints containing MDM as an impurity (0.01%); however, this exposure was estimated to be significantly lower than exposure from cosmetics including personal care products.
MDM is also present in a silicone cleaner (Environment Canada 2010a) applied to automobiles to remove streaks after application of silicone-based wax. However, current market trends indicate an increased prevalence of streak-free wax products. Since this product is not expected to be used frequently by the general population, it is not considered a major source of exposure.
There is uncertainty associated with assumptions used in estimating exposure from the inhalation route (90% availability for inhalation). However, given that MDM and other linear and cyclic siloxanes have been observed in indoor air (Kaj et al. 2005a; Harner et al. 2010), there is confidence that this route of exposure is relevant for this substance. There is also some uncertainty regarding the concentrations and presence of MDM in various products (not reported under section 71 or notifications submitted under the Cosmetic Regulations to Health Canada); however, given the use of conservative assumptions (maximum concentrations, 100% dermal and inhalation absorption, co-occurrence of product use) there is confidence that these estimates of exposure are upper bounding.
Health Effects Assessment
Structure and identities of relevant analogues of MDM are presented in Appendix VI. The available health effects information for MDM is summarized in Appendix VII. Data on analogues of MDM are included in Appendix VIII.
No health effects classification or assessments by national or international regulatory agencies were identified for MDM. MDM did not induce gene mutations in bacterial cells (Salmonella typhimurium and Escherichia coli) and did not induce chromosomal aberrations in Chinese hamster ovary cells, with and without metabolic activation (Seifried et al. 2006; BioReliance 2008, 2009). In a mammalian cell mutation assay, positive results were reported in mouse lymphoma in absence of metabolic activation but negative results were reported in presence of metabolic activation (Seifried et al. 2006).
In a combined repeated-dose/reproductive/developmental toxicity screening test, increases in serum cholesterol and increases in absolute and relative liver weights were observed in both male and female rats exposed by inhalation to MDM for up to 28 to 29 days at 7740 mg/m3 and above (DowCorning Corporation 2007a). Hyaline droplet nephropathy was also observed in males exposed to this concentration, but appeared to be characteristic of kidney lesions induced by male-rat-specific alpha-2-urinary globulin. Because alpha-2-urinary globulin is only synthesized in the liver of sexually mature male rats, this mechanism of toxicity is not considered relevant to human health risk assessment. No treatment-related effects were observed in any of the reproductive or developmental parameters evaluated. No adverse reproductive or developmental effects were observed at 31 000 mg/m3, the highest concentration tested.
In another inhalation study, protoporphyrin accumulations along with secondary effects of cholangitis/pericholangitis and bile duct proliferation were observed in male and female rats after exposure to 31 000 mg MDM/m3 for 90 days (SEHSC 2011).
Rats were exposed to MDM by gavage in an oral 28-day study. Significant increase in absolute and relative liver weight accompanied by hepatocellular hypertrophy and protoporphyrin accumulation with associated bile duct proliferation and chronic inflammation was observed in males at 250 mg/kg-bw per day and above (Harland Laboratories Ltd 2010).
In the rabbit, MDM was not irritating to the skin following single exposure but was minimally to moderately irritating when applied following a repeated exposure (10 applications over a 14-day period) (DowCorning Corporation 1994, 1999a). In a human patch test, none of the subjects exhibited signs of irritation or sensitization to MDM during any part of the study. It was concluded that MDM is not a skin sensitizer (DowCorning Corporation 1998).
Quantitative structure-activity relationship (QSAR) predictions were considered; however, three of the four models (DEREK 2008; CASETOX 2008; Model Applier 2008) typically used for this purpose were not applicable to this specific substance, and outputs of the fourth model (TOPKAT 2004) were inconclusive for all endpoints (predictions were unreliable, based on user-defined model specific criteria other than models’ applicability domain).
The available health effects database for MDM was considered limited and relevant information on analogue substances was taken into consideration to further inform the human health assessment. Three suitable analogues were identified based on chemical similarity and availability of empirical health effects data: CAS RN 107-46-0 (hexamethyldisiloxane [HMDS]), CAS RN 141-62-8 (decamethyltetrasiloxane [L4]) and CAS RN 141-63-9 (dodecamethylpentasiloxane [L5]). The degree of structural similarity was quantified using the Tanimoto association coefficient in SciFinder; this coefficient was 67%, 86% and 73% between MDM and HMDS, L4 and L5, respectively, and was considered adequate. Additionally, one physico-chemical property (i.e., water solubility) fell within comparable range for MDM and its analogues (see Appendix VI for structures, similarity scores and water solubility values).
A summary of the available health effects data for the three analogues is presented below.
In in vitro assays, L5 was not mutagenic in S. typhimurium reverse-mutation assays, with and without metabolic activation (DowCorning Corporation 1979). HMDShas shown negative results in Salmonella and Escherichia coli reverse-mutation assays, Saccharomyces mitotic recombination assays, a mouse lymphoma forward-mutation assay, a sister chromatid exchange (SCE) assay in mouse lymphoma cells and two DNA damage assays (pol assay in Escherichia coli and mouse lymphoma alkaline elution assay), all in presence and absence of metabolic activation (Isquith et al. 1988b; Shin-Etsu 1994a). Chromosome damage was evident in mouse lymphoma L5178Y cells but this effect was not related to dose and was only seen in the absence of metabolic activation (Isquith et al. 1988a). Chromosome damage was absent in Chinese hamster lung fibroblasts in presence and absence of metabolic activation (Shin-Etsu 1994b).
One in vivo assay was identified in the literature for the analogues. In a chromosome aberration assay using HMDS via intraperitoneal injection, no chromosomal damage was seen in the bone marrow of male rats (Isquith et al. 1988b).
Based on the collective evidence on genotoxicity, it is considered that the analogues of MDM, especially HMDS, are not genotoxic.
In a chronic study in rats exposed by inhalation to HMDS, there was a dose-related increase in incidence of Leydig cell tumours (LCTs) in males in all treated groups after one year of exposure (2/20, 10/20, 9/20, 12/20 and 15/20 at 0, 665, 2660, 10 640 and 33 300 mg/m3, respectively). After 24 months, LCTs were observed in almost all control and treated males. Additionally, several renal tubular adenomas and carcinomas occurred in males at 10 640 mg/m3 (in 3 of 65 animals) and at 33 000 mg/m3 (in 6 of 65 animals). No evidence of neoplastic lesions was observed in females after 12 or 24 months. Non-neoplastic effects observed in rats included Leydig cell hyperplasia and increased incidence of eosinophilic inclusions in the olfactory epithelium in males in all exposure groups, increased liver weight and increased incidence of intraluminal mineralization in the kidney in males at 10 640 mg/m3 and above and a slight reduction in females body weights at 10 640 mg/m3and above which was likely associated with reduction in food consumption (DowCorning Corporation 1999b; Jovanovic et al. 2005). The lowest lowest-observed-adverse-effect concentration (LOAEC) for non-neoplastic effects was 665 mg/m3 based on Leydig cell hyperplasia and increased incidence of eosinophilic inclusions in the olfactory epithelium in males.
It has been shown that LCTs are common in one-year-old F344 rats and that these tumours become increasingly more frequent with age (Boorman et al. 1990). There are several lines of evidence that suggest human Leydig cells are quantitatively less sensitive than rats cells to chemically induced LCTs (Shenker et al. 1993; Quigley et al. 1995; Clegg et al. 1997). Further epidemiology studies available on a number of compounds that induce LCTs in rats did not demonstrate an association between human exposure to these compounds and induction of Leydig cell hyperplasia or adenomas (Preston-Martin 1991; Wilmer et al. 1994; Longnecker 1995; Himmelstein et al. 1997). Based on these findings, induction of LCTs are considered unlikely to be relevant to human risk assessment. Concerning the renal tubular adenomas and carcinomas observed in males rats, recent demonstration of reversible binding of HMDS or metabolites to alpha-2μ-globulin in male rat kidney samples from HMDS treated rats, along with observations of increased hyaline droplet formation in male rats, have shown that alpha 2μ-nephropathy, a species-specific mechanism, is likely to be responsible for the rat kidney neoplasia observed (DowCorning Corporation 2007b). Therefore, occurrence of kidney tumours in male rats following administration of HMDS is considered species-specific and not relevant to human risk assessment.
In a 2-week inhalation study with HMDS in rats, the only adverse effect observed was a dose-related increased severity of hyaline droplets in the proximal convoluted tubule epithelium of male kidneys at 3319 mg/m3. However, these lesions, as described previously for MDM, were consistent with male-rat-specific alpha-2-urinary globulin nephropathy and were not considered relevant to human health risk assessment (DowCorning Corporation 1992b). In a 1-month inhalation study, an increase in the incidence and severity of renal tubule regeneration, which indicates increase in kidney damage, was observed in males exposed to HMDS at 12 700 mg/m3 and above (DowCorning Corporation 1997a). In a study in which rats were exposed by inhalation to HMDS for 3 months, inflammation associated with alveolar macrophage aggregation was observed in the lungs of females rats at the lowest dose tested (140 mg/m3) and above (DowCorning Corporation 1997b). The lowest LOAEC for repeated-dose inhalation exposure was 140 mg/m3 based on induction of inflammatory reaction in lungs of female rats.
When rats were dosed by oral gavage with HMDS for 28 days, histopathological changes in the kidney (increase in eosinophilic bodies) were observed in males at 40 mg/kg-bw per day (Shin-Etsu 1994c). In a study in which rats were dosed by oral gavage with L5 for 28 days, significant increase in absolute and relative liver weight accompanied with liver lesions such as periportal hepatocellular vacuolation was observed in females at 25 mg/kg-bw per day and higher (DowCorning Corporation 2009c). When L4 was administered by gavage to rats for the same dose term, the incidence and severity of perilobular fatty change was increased in female rats at 25 mg/kg-bw per day and higher (DowCorning Corporation 2009a). However, no treatment-related effects were observed in both male and female rats exposed in the diet to 500 mg L4/kg-bw per day, the only dose tested, in a 1-year study (DowCorning Corporation 1966). The lowest lowest-observed-adverse-effect-level (LOAEL) for repeated-dose oral exposure to these analogues was 25 mg/kg-bw per day, based on adverse effects in the liver of female rats exposed to L4 and L5.
Dermal repeated-dose studies were identified for two of the analogues of MDM. No treatment-related adverse effects were noted in male rats exposed to 1000 mg L4/kg-bw per day dermally, once daily for 28 days (Hobbs et al. 1972). A NOAEL for dermal repeated exposure to HMDS was identified at 500 mg/kg bw/d based on statistically significant reduction in body weight gain, food consumption, absolute liver weights and relative liver and relative kidney weights at the next dose tested (1000 mg/kg-bw per day) in male rats exposed via the dermal route for 28 days (DowCorning Corporation 1993).
In a one-generation reproduction study in rats exposed by inhalation, HMDS did not elicit reproductive effects in males and females at concentrations up to 33 700 mg/m3, but a parental lowest-observed-effect concentration (LOEC) was identified based on increased lung and liver weights in males, transient reduction in body weight gain in females and transient reduction in food consumption in both sexes (DowCorning Corporation 2000). This finding is consistent with the absence of reproductive effects observed following inhalation exposure to high concentrations of MDM.
In an acute inhalation study with exposure to HMDS for 4 hours, treatment-related mortality and congestion and/or hemorrhage of various lobes of the lungs in male and female rats were reported to occur at a dose of 93 500 mg/m3 (DowCorning 1996). Acute oral studies using HMDS were identified, but none reported a LOAEL.
HMDS, as MDM, did not induce skin sensitization in human patch tests (DowCorning Corporation 1980, 1992a). No incidence of skin irritation or sensitization was observed in a guinea pig maximization test (DowCorning Corporation 1992c). Evaluation of skin irritancy with HMDS gave similar results as those observed with MDM. Following a single application of HDMS to the skin of rabbits, no irritation was noted (DowCorning Corporation 1978, 1991). When applied following a repeated exposure, HMDS was not or slightly irritating to the skin (DowCorning Corporation 1976, 1978).
The confidence in the health effects database of MDM is considered to be low to moderate, as limited empirical data were identified but data on analogues were used (especially HMDS) to read across missing endpoints for MDM.
Characterization of Risk to Human Health
Since limited health effects information was available for MDM, relevant data on analogue substances was considered. No evidence of carcinogenicity that would be considered relevant to a human health risk assessment was identified in a chronic inhalation study in rats exposed to the analogue HMDS. Consideration of the available information on genotoxicity for MDM and its analogues indicates that MDM is not likely to be genotoxic. Therefore, characterization of the risk to human health is based on non-cancer effects.
Based on estimates derived from limited data on levels of MDM in environmental media, the predominant source of exposure to MDM was found to be indoor air. Exposure from ambient air, drinking water and soil were significantly lower in comparison. Exposure from food is not expected for the majority of the age groups of the general population. However, MDM was detected in breast milk samples, and thus breast-fed infants could be exposed to the substance.
The LOAEC from a short-term inhalation study conducted with MDM was considered adequate to characterize risk for the general population from exposure to MDM via indoor air. This LOAEC (7740 mg/m3, based on liver effects) is several orders of magnitude higher than the maximum air concentration measured in houses (12.3 μg/m3), and the margin of exposure is considered adequate to address uncertainties in the health effects and exposure database.
The LOAEL from a 28-day oral study conducted with MDM was considered adequate for use in the characterization of risk from oral exposure. Although a lower LOAEL was identified for the analogues L4 and L5 from 28-day oral studies, the short-term study conducted with MDM specifically was deemed more appropriate. A comparison of this LOAEL (250 mg/kg-bw per day, based on adverse effects in the liver of male and female rats) with the upper-bounding estimate of oral daily intake of MDM for breast-fed infants (7.9 × 10−4 μg/kg-bw per day; Appendix V) results in a margin of exposure of several orders of magnitude. This margin is considered adequate to address uncertainties in the health effects and exposure databases.
Exposure of the general population from use of cosmetics (including some personal care products) is expected to occur via inhalation, dermal and oral routes.
Comparison of the upper-bounding estimate of oral exposure from use of lipstick to the above-noted short-term oral LOAEL of 250 mg/kg-bw per day for MDM results in margins of exposure of 28 090 to 83 330. These margins are considered adequate to address uncertainties in the health effects and exposure databases.
With respect to dermal exposure from use of cosmetics, no effects were observed in experimental animals at a dose of 500 mg/kg-bw per day in a 28-day dermal study in rats using HMDS. A NOAEL of 500 mg/kg-bw per day and a LOAEL of 1000 mg/kg-bw per day could be derived from this study (DowCorning Corporation 1993). In a dermal study conducted with L4, no adverse effects were observed in male New Zealand albino rabbits at 1000 mg/kg-bw per day (Hobbs et al. 1972). Comparison of these critical effects levels with estimates of aggregate daily dermal exposure to MDM from use of cosmetics (0.090 to 2.8 mg/kg-bw per day) results in margins of exposure ranging from 180 to 5560 when using the NOAEL from the HMDS study, and 360 to 11 110 using the NOAEL from the L4 study. Taking into consideration that exposure estimates were based on conservative assumptions (i.e., maximum concentrations, use of all product types on the same day, all products contain MDM), and given that these margins are based on no-observed-adverse-effect levels, these margins are considered adequate to address uncertainties in the health effects and exposure database.
Due to the high volatility of MDM and its occurrence in products with “leave-on” applications, inhalation is also considered to be a relevant route of exposure. Comparison of the estimated range of mean-event concentration (0.050 to 4.2 mg/m3), from use of cosmetics including personal care products, with the lowest LOAEC following short-term inhalation exposure to MDM (7740 mg/m3), results in margins of exposure ranging from 1840 to 154 800. These margins are considered adequate to address uncertainties in the health effects and exposure databases.
Uncertainties in Evaluation of Risk to Human Health
Empirical health effects data for MDM are limited and there is uncertainty associated with the use of analogues to characterize human health effects. There is uncertainty regarding the carcinogenicity of MDM due to the lack of long-term studies, although the available information from genotoxicity tests and carcinogenicity studies that are available for the analogue HMDS do not indicate a concern. There is also uncertainty in the exposure estimates. Specifically, there is uncertainty in using environmental concentrations from the Nordic environment to derive exposure estimates (environmental media and breast milk) in the Canadian situation and uncertainty associated with the presence of MDM, if any, in PDMS (CAS RN 9006-65-9) used as a food additive.
Additionally, there is uncertainty associated with the assumptions used in estimating exposure from cosmetics, including personal care products. The assumption that an individual would wear all MDM-containing products on the same day is considered very conservative. There is also uncertainty related to assumptions pertaining to characterizing exposure via the inhalation route from use of cosmetics. However, given that MDM has been detected in indoor air, this is considered a relevant route of exposure for MDM. Finally, there is also some uncertainty regarding the concentrations and presence of MDM in various products.
Conclusion
Considering all available lines of evidence presented in this screening assessment, there is low risk of harm to organisms and the broader integrity of the environment from MDM. It is concluded that MDM does not meet the criteria under paragraphs 64(a) or (b) of CEPA 1999 as it is not entering the environment in a quantity or concentration or under conditions that have or may have an immediate or long-term harmful effect on the environment or its biological diversity or that constitute or may constitute a danger to the environment on which life depends.
Based on the information presented in this screening assessment, it is concluded that MDM does not meet the criteria under paragraph 64(c) of CEPA 1999 as it is not entering the environment in a quantity or concentration or under conditions that constitute or may constitute a danger in Canada to human life or health.
It is concluded that MDM does not meet any of the criteria set out in section 64 of CEPA 1999.
References
Alaee M. 2014. Unpublished CMP2 monitoring data submitted to Ecological Assessment Division of Environment Canada, Gatineau QC.
Alaee M. 2012. Linear and cyclic volatile methyl siloxanes in the leachate, influent, and effluent in various landfills, wastewater treatment plants, facilities from Canada. Unpublished report. Burlington (ON): Environment Canada, Water Science and Technology Directorate.
Allen RB, Kochs P, Chandra G. 1997. Industrial organosilicon materials, their environmental entry and predicted fate. In: Chandra G, editor. The handbook of environmental chemistry, Volume 3, Part H: Organosilicon materials. New York (NY): Springer-Verlag. p. 181-223.
[AOPWIN] Atmospheric Oxidation Program for Windows [Estimation Model]. 2008. Version 1.92a. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Arnot JA, Gobas FAPC. 2003a. A generic QSAR for assessing the bioaccumulation potential of organic chemicals in aquatic food webs. QSAR Comb Sci 22(3):337-345.
Arnot JA, Gobas FAPC. 2003b.Categorization of organic substances on the Domestic Substances List for bioaccumulation potential. Report to Environment Canada, Existing Substances Branch. June 2003. Gatineau (QC): Environment Canada.
Arnot JA, Gobas FAPC. 2004. A food web bioaccumulation model for organic chemicals in aquatic ecosystems. Environ Toxicol Chem 23:2343-2355.
Arnot JA, Gobas FAPC. 2006. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ Rev 14:257-297.
Arnot JA, Mackay D, Bonnell M. 2008a. Estimating metabolic biotransformation rates in fish from laboratory data. Environ Toxicol Chem 27(2):341-351.
Arnot JA, Mackay D, Parkerton TF, Bonnell M. 2008b. A database of fish biotransformation rates for organic chemicals. Environ Toxicol Chem 27(11):2263-2270.
Arnot, JA. 2010. Estimating chemical absorption efficiency from dietary exposures and degradation rate constants in the gastro-intestinal tract. Draft Report. Prepared for Ecological Assessment Division, Environment Canada. March 2010. Gatineau (QC): Environment Canada.
Arnot JA, Arnot MI, Mackay D, Couillard Y, MacDonald D, Bonnell M, Doyle P. 2010. Molecular size cutoff criteria for screening bioaccumulation potential: fact or fiction? Integr Environ Assess Manag 6(2):210–224.
ASTreat Model [sewage treatment plant removal model]. 2006. Version 1.0. Cincinnati (US): Procter & Gamble Company. Available from Procter & Gamble Company, P.O. Box 538707, Cincinnati, OH 45253-8707, U.S.
Backus S, Moore S, Pelletier M. 2012. Unpublished CMP2 sediment monitoring data submitted to Ecological Assessment Division of Environment Canada, Gatineau QC.
Beyer A, Mackay D, Matthies M, Wania F, Webster E. 2000. Assessing long-range transport potential of persistent organic pollutants. Environ Sci Technol 34(4):699-703
BioReliance. 2008. Bacterial reverse mutation assay with Octamethyltrisiloxane. Study No. AC09FV.503.BTL.
BioReliance. 2009. L3 (Octamethyltrisiloxane; CAS No. 107-51-7): In vitro mammalian chromosome aberration test. Study Number AC19VU.331.BTL.
[BIOWIN] Biodegradation Probability Program for Windows [Estimation Model]. 2008. Version 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Boethling RS, Howard PH, Beauman JA, Larosche ME. 1995. Factors for intermedia extrapolations in biodegradability assessment. Chemosphere 30(4):741-752.
Boorman GA, Chapin RE, Mitsumori K. 1990. Testis and epididymis in pathology of the Fisher rat. San Diego (CA): Academic Press. p. 405-418.
Bradley MJ. 2012. Octamethyltrisiloxane (L3): A 28-day toxicity test exposing freshwater amphipods (Hyalellaazteca) to a test substance applied to sediment under static-renewal conditions following OPPTS draft guideline 850.1735. HES Reference Number 12085-826. Revised draft III. 18 September 2012. Wareham (MA): Smithers Viscient.
Bradley MJ. 2013. Octamethyltrisiloxane (L3) – Sediment-water Lumbriculus toxicity test using spiked sediment, following OECD Guideline 225. Smithers Viscient Study No. 12023.6221. Wareham (MA): Smithers Viscient.
[BRRC] Bushy Run Research Center. 1982. Unpublished report to Union Carbide Corporation. Silicone Fluid Y-4081. Acute toxicity and primary irritancy studies. BRRC Project Report 44-108.
Bruggeman WA, Weber-Fung D, Opperhuizen A, VanDerSteen J, Wijbenga A, Hutzinger O. 1984. Absorption and retention of polydimethylsiloxanes (silicones) in fish: Preliminary experiments. Toxicol Environ Chem
7(4):287-296.
Burkhard LP, Arnot JA, Embry MR, Farley KJ, Hoke RA, Kitano M, Leslie HA, Lotufo GR, Parkerton TF, Sappington KG, Tomy GT, Woodburn KB. 2012. Comparing laboratory and field measured bioaccumulation endpoints. Integ Environ Assess Manag 8(1):17-31.
Canada. 1999. Canadian Environmental Protection Act, 1999. S.C., 1999, c. 33, Canada Gazette. Part III, vol. 22, no. 3. Available from: http://www.gazette.gc.ca/archives/p3/1999/g3-02203.pdf
Canada, Dept. of the Environment, Dept. of Health. 2006. Canadian Environmental Protection Act, 1999 : Notice of intent to develop and implement measures to assess and manage the risks posed by certain substances to the health of Canadians and their environment. Canada Gazette Part I, vol. 140, no. 49, p. 4109-4117. Available from: http://www.gazette.gc.ca/archives/p1/2006/2006-12-09/pdf/g1-14049.pdf
Canada, Dept. of the Environment, Dept. of Health. 2009a. Canadian Environmental Protection Act, 1999: Notice of twelfth release of technical information relevant to substances identified in the Challenge. Canada Gazette Part I, vol. 143, no. 52, p. 3839-3843. Available from: http://www.gazette.gc.ca/rp-pr/p1/2009/2009-12-26/pdf/g1-14352.pdf#page=33
Canada, Dept. of the Environment. 2009b. Canadian Environmental Protection Act, 1999: Notice with respect to Batch 12 Challenge substances. Canada Gazette Part I, vol. 143, no. 52, p. 3813-3836. Available from: http://www.gazette.gc.ca/rp-pr/p1/2009/2009-12-26/pdf/g1-14352.pdf#page=7
Canada.2014.. Food and Drug Regulations, C.R.C., c.870 as amended September 15, 2010. Available from: http://laws.justice.gc.ca/eng/C.R.C.-c.870/index.html
CASETOX [Prediction module]. 2008. Version 2.0. Beachwood (OH): MultiCASE [cited 2010 August 6]. Available from: http://www.multicase.com/products/prod03.htm [restricted access].
Cassidy SL, Dotti A, Kolesar GB, Dochterman W, Meeks RG, Chevalier HJ. 2001. Hexamethyldisiloxane: A 13-week subchronic whole-body vapor inhalation toxicity study in Fisher 344 rats. J Toxicol 20:391-399.
[CATABOL] Probabilistic assessment of biodegradability and metabolic pathways [Computer Model]. c2004−2008. Version 5.10.2. Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. Available from: http://oasis-lmc.org/?section=software&swid=1
[CES] Centre Européen des Silicones. 2012. Preliminary assessment of octamethyltrisiloxane (L3) in sediments and biota in aquatic ecosystems. Interim report. 06 August 2012. Submission to Environment Canada under the Chemicals Management Plan Challenge initiative.
[CES] Centre Européen des Silicones. 2013. Survey of surface sediment and aquatic biota from Lake Ontario for the presence of octamethyltrisiloxane (MDM) (2011). 14 October 2013. Submission to Environment Canada under the Chemicals Management Plan Challenge initiative.
Chandra G. 1997. Introduction. In: Chandra G, editor. The handbook of environmental chemistry, Volume 3, Part H: Organosilicon materials. New York (NY): Springer-Verlag. p. 181-223.
[ChemID] ChemIDplus Advanced [website on the internet]. 2010. Bethesda, MD: United States National Library of Medicine. National Institutes of Health. Available from: http://chem.sis.nlm.nih.gov/chemidplus/
Cheng Y, Shoeib M, Ahrens L, Harner T, Ma J. 2011. Wastewater treatment plants and landfills emit volatile methyl siloxanes (VMSs) to the atmosphere: Investigations using a new passive air sampler. Environ Pollut 159:2380-2386.
Clegg ED, Cook JC, Chapin RE, Foster PMD, Daston GP. 1997. Leydig Cell hyperplasia and adenoma formation: mechanisms and relevance to humans. Reprod Toxicol 11:107-121.
[ConsExpo] Consumer Exposure Model [Internet]. 2007. Version 4.1. Bilthoven (NL): Rijksinstituut voor Volksgezondheid en Milieu (National Institute for Public Health and the Environment). Available from: http://www.ConsExpo.nl/en/healthanddisease/productsafety/ConsExpo.jsp#tcm:13-42840
[CosIng] Cosmetic Ingredients and Substances [database on the Internet]. 1976– . Brussels (BE): European Commission, Enterprise and Industry. [revised 2009 Mar 12; cited 2010 Jun 7]. Available from: http://ec.europa.eu/enterprise/cosmetics/cosing/index.cfm?fuseaction=search.simple
[CPOPs] Canadian POPs Model. 2008. Gatineau (QC): Environment Canada, Ecological Assessment Division; Bourgas (BG): Bourgas Prof. Assen Zlatarov University, Laboratory of Mathematical Chemistry. [Model developed based on Mekenyan et al. 2005]. Available from: Environment Canada, Ecological Assessment Division.
[CTFA] Cosmetic, Toiletry and Fragrance Association. 1983. Summary for the results of surveys of the amount and frequency of use of cosmetic products by women. Report Prepared by Pitkin B, Rodericks JV, Turnbull D. Washington (DC): Environ Corporation.
[DEREK] Deducting Estimation from Existing Knowledge [Prediction module on CD ROM]. 2008. Version 10.0.2. Cambridge (MA): Harvard University, LHASA Group [cited 2010 August 6]. Available from: http://lhasa.harvard.edu/?page=toxicology.htm [restricted access].
Dimitrov S, Dimitrova N,Walker J, Veith G, Mekenyan O. 2002. Predicting bioconcentration potential of highly hydrophobic chemicals. Effect of molecular size. Pure and Appl Chem 74(10): 1823-1830.
Dimitrov S, Dimitrova N, Parkerton T, Comber M, Bonnell M, Mekenyan O. 2005. Base-line model for identifying the bioaccumulation potential of chemicals. SAR QSAR Environ Res 16(6):531–554.
DowCorning Corporation. 1965. Chronic (8-month) feeding studies with methyl siloxanes in rabbits with cover letter dated 04/20/94. TSCA OTS0556517, Document ID: 1965-I0065-1179-01.
DowCorning Corporation. 1966. Chronic (one-year) feeding studies with methyl siloxanes in rats with cover letter dated 04/20/94. TSCA OTS0572253, Document ID: 1976-I0065-1010-04.
DowCorning Corporation. 1976. Skin contact irritation tests with 200 Fluid/0.65 cs. with cover letter dated 04/20/94. TSCA OTS0590160, Document ID: 1976-I0065-1410Y03.
DowCorning Corporation. 1978. Comparison of primary skin irritation and effects of repeated prolonged skin exposure to various volatile experimental cosmetic fluids w/SD alcohol 40W with cover letter dated 04/20/94. TSCA OTS0572276, Document ID: 86940001270.
DowCorning Corporation. 1979. Mutagenicity evaluation of DowCorning 200 Fluid in the Ames Bacterial Assay, with cover letter dated 04/20/94. TSCA OTS0558128, Document ID: 86940001347.
DowCorning Corporation. 1980. Human repeated insult patch test with DowCorning Q2-1096 Fluid, with cover letter dated 04/20/94. TSCA OTS0558105, Document ID: 86940001324.
DowCorning Corporation. 1990. A 28-day subchronic oral gavage feasibility study of various low molecular weight silicone oligomers in rats with cover letter dated 04/20/94. TSCA OTS0590105, Document ID: 1990-I0000-35105.
DowCorning Corporation. 1991. An acute skin irritation study of hexamethyldisiloxane in rabbits with cover letter dated 04/20/94. TSCA OTS0572296, Document ID: 1991-I0000-36741.
DowCorning Corporation. 1992a. Human repeated insult patch test with hexamethylsiloxane with cover letter dated 04/20/94. TSCA OTS0572322, Document ID: 1992-I0000-37036.
DowCorning Corporation. 1992b. A two-week repeated dose inhalation toxicity study of hexamethyldisiloxane in albino rats with cover letter dated 04/20/94. TSCA OTS0572321, Document ID: 1992-I0000-37011.
DowCorning Corporation. 1992c. A skin sensitization study of hexamethyldisiloxane in guinea pigs with cover letter dated 04/20/94. TSCA OTS0572320, Document ID: 86940001808.
DowCorning Corporation. 1993. A 28-day repeated dose dermal toxicity study of hexamethyldisiloxane in rats with cover letter dated 04/20/94. TSCA OTS0572811, Document ID: 86940001823.
DowCorning Corporation. 1994. Skin contact irritation tests with Tx-1302 A, Tx-1302 B, Tx-1302 C, Tx-1302 D and Tx 1302 E in Albino rabbits with cover letter dated 04/20/94. TSCA OTS0590160, Document ID: 1976-I0065-1410Y03.
DowCorning Corporation. 1996. An acute whole body inhalation study of HMDS in Albino rats. Report no. 1996-I0000-41477.
DowCorning Corporation. 1997a. 1-month repeated dose inhalation toxicity study with hexamethyldisiloxane in rats, with cover letter dated 11/21/97. TSCA OTS0559379, Document ID: 86-980000041.
DowCorning Corporation. 1997b. 3-month repeated dose inhalation toxicity study with hexamethyldisiloxane in rats with a 1-month recovery period, with cover letter dated 11/25/97. TSCA OTS0559386, Document ID: 86-980000048.
DowCorning Corporation. 1998. Repeat insult patch test of six DowCorning materials in human subjects. Report no. 1998-I0000-45918.
DowCorning Corporation. 1999a. Skin irritation of DowCorning 200® Fluid, 1 cSt in the rabbit. Report no. 1999-I0000-47455 [cited in ECB 2010].
DowCorning Corporation. 1999b. Initial submission: LTR FR DowCorning Corp to USEPA Re 24-mo combined chronic toxicity and oncogenicity vapor inhalation study of hexamethyldisiloxane in Fisher rats, dated 110899. TSCA OTS0559838, Document ID: 88000000031.
DowCorning Corporation. 2000. A one-generation inhalation reproductive toxicity study of hexamethyldisiloxane in rats, with cover letter dated 04/18/00. TSCA OTS0574055, Document ID: 86000000019.
Dow Corning Corporation. 2004. An acute whole-body inhalation toxicity study of octamethyltrisiloxane in rats. Report no. 2004-I0000-54030.
Dow Corning Corporation. 2006. An acute oral toxicity study in rats with octamethyltrisiloxane. Report no. 2004-I0000-53879.
Dow Corning Corporation. 2007a. Combined repeated dose toxicity study with the reproductive/developmental toxicity screening test for octamethyltrisiloxane (L3) in Sprague-Dawley rats via inhalation exposure. HES Report number 2007-I0000-58159, 11 April 2008.
Dow Corning Corporation. 2007b. Non-regulated study: hexamethyldisiloxane (HMDS): Determination of the reverse binding of HMDS/metabolites to Alpha 2u-Globulin in male Fischer 244 rats following oral gavage Administration. Report number 2007-I0000-57893, 21 September 2007.
Dow Corning Corporation. 2009a. Decamethyltetrasiloxane (L4): 28-day oral (gavage) toxicity study in the Sprague-Dawley rat with decamethyltetrasiloxane. Information submitted to EPA under TSCA; TSCA Document no. 8EHQ-09-17631B, Dated 09/10/09.
Dow Corning Corporation. 2009b. A 28-day subchronic oral gavage feasibility study of various low molecular weight silicone oligomers in rats. Information submitted to EPA under TSCA; TSCA Document no. 8EHQ-09-17732A, Dated 11/23/09.
Dow Corning Corporation. 2009c. 28-day oral (gavage) toxicity study in the Sprague-Dawley rat with dodecamethylpentasiloxane. Information submitted to EPA under TSCA; TSCA Document no. 8EHQ-09-17683A, Dated 10/14/09.
Dow Corning Corporation. 2009d. Environmental information. An overview of volatile methylsiloxane (VMS) fluids in the environment. Midland (MI): Dow Corning Corporation. Available from: https://www.xiameter.com/en/ExploreSilicones/Documents/95-724-01%20Overview%20of%20Volatile%20Methylsiloxane.pdf.
Dow Corning Corporation. 2009e. Xiameter® PMX-200 Silicone Fluid 1CS. Material safety data sheet. Version 1.0. January 20, 2009. Midland (MI): Dow Corning Corporation. Available from https: www.xiameter.com/EN/Products/.
Dow Corning Corporation. 2010a. Dow Corning® Cleaner and Surface Prep Solvent. Material safety data sheet. Version 1.4. April 22, 2010. Midland (MI): Dow Corning Corporation. Available from: http://www2.dowcorning.com/DataFiles/090007b2814676c0.pdf
Dow Corning Corporation. 2010b. 14C-Tetrakis(trimethylsiloxy)silane (14C-M4Q): Dietary bioaccumulation in the rainbow trout (Oncorhynchus mykiss) under flow-through test conditions. Dow Corning Corporation Health and Environmental Sciences Technical Report. October 11, 2010.
[DPD] Drug Products Database [database on the internet]. 2014. Canada: Health Canada [cited August 2014]. Available from: http://webprod.hc-sc.gc.ca/dpd-bdpp/index-eng.jsp
Drottar KR. 2006. 14C-Octamethyltrisiloxane (14C-L3): Bioconcentration in the fathead minnow (Pimephales promelas) under flow-through conditions. Silicones Environmental, Health and Safety Council (SEHSC). DowCorning Internal Report - 2006-10000-56380.
Drottar KR. 2010. 14C-Octamethyltrisiloxane (14C-L3): Dietary bioaccumulation in the rainbow trout (Oncorhynchusmykiss) under flow-through test conditions. Silicones Environmental, Health and Safety Council (SEHSC). HES Study Number 11245-102.
Durham J, Kozerski GE. 2010. Soil-water distribution of 13C-Octamethyltrisiloxane (13C-L3) (107-51-7) and 13C-Decamethyltetrasiloxane (13C-L4) (141-62-8) using a batch equilibrium method. Final report to SEHSC. DCC HES Study No. 10959-102 [cited in SEHSC 2011].
[ECB] European Chemicals Bureau. 2010. IUCLID dataset: Octamethyltrisilixane, CAS No. 107-51-7, Unpublished.
[ECHA] European Chemicals Agency. 2012. Guidance on information requirements and chemical safety assessment. Chapter R.11: PBT assessment. November 2012. Version 1.1. Guidance for the implementation of REACH. Helsinki (FI): European Chemicals Agency.
Environment Canada. 2007a. Data for selected substances collected underthe Canadian Environmental Protection Act, 1999, Section71: Notice with respect to selected substances identified as priority for action. Data compiled by: Environment Canada, Program Development and Engagement Division.
Environment Canada. 2007b. Guidance for Conducting Ecological Assessments under CEPA 1999, Science Resource Technical Series, Technical Guidance Module: QSARs. Reviewed draft working document. Gatineau (QC): Environment Canada, Ecological Assessment Division.
Environment Canada. 2008. Guidance for Conducting Ecological Assessments under CEPA 1999, Science Resource Technical Series, Technical Guidance Module: Mass Flow Tool. Working document. Gatineau (QC): Environment Canada, Ecological Assessment Division.
Environment Canada. 2010a. Data for Batch 12 substances collected underthe Canadian Environmental Protection Act, 1999, Section71: Notice with respect to certain Batch 12 Challenge substances. Data compiled by: Environment Canada, Program Development and Engagement Division.
Environment Canada. 2010b. Mass Flow Tool report. Trisiloxane, octamethyl- (MDM). CAS No.: 107-51-7. Internal technical supporting document. Gatineau (QC): Environment Canada, Ecological Assessment Division.
Environment Canada. 2010c. Evaluation of the ecological effects of siloxane D5 in soil. Unpublished report. December 2010. Ottawa (ON): Environment Canada, Soil Toxicology Laboratory, Biological Assessment and Standardization Section.
Environment Canada, Health Canada. 2008a. Screening assessment for the Challenge. Decamethylcyclopentasiloxane (D5). Chemical Abstracts Service Registry Number 541-02-6. November 2008. Ottawa (ON): Environment Canada, Health Canada.
Environment Canada, Health Canada. 2008b. Screening assessment for the Challenge. Octamethylcyclotetrasiloxane (D4). Chemical Abstracts Service Registry Number 556-67-2. November 2008. Ottawa (ON): Environment Canada, Health Canada.
Environment Canada, Health Canada. 2014. Screening assessment for the Challenge. Trisiloxane, 1,1,1,5,5,5-hexamethyl-3,3-bis[(trimethylsilyl)oxy]- (M4Q). Chemical Abstracts Service Registry Number 3555-47-3. Ottawa (ON): Environment Canada, Health Canada.
[EPI Suite] Estimation Programs Interface Suite for Microsoft Windows [Estimation Model]. 2000-2008. Version 4.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: http://www.epa.gov/oppt/exposure/pubs/episuitedl.htm
[EQC] Equilibrium Criterion Model. 2011. Version 1.00. Released August 2011. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre. Available from: http://www.trentu.ca/academic/aminss/envmodel/models/NewEQCv100.html
[ESIS] European Chemical Substances Information System [database on the Internet]. c1995–2009. European Chemicals Bureau (ECB) [cited 2012 Sep 18]. Available from: http://esis.jrc.ec.europa.eu/
Evenset A, Leknes H, Christensen GN, Warner N, Remberger M, Gabrielsen GW. 2009. Screening of new contaminants in samples from the Norwegian Arctic. SFT Report 1049/2009 (TA-2510/2009). Oslo (NO): Norwegian Pollution Control Authority (SFT), Akvaplan-niva, Norwegian Polar Institute and Norwegian Institute for Air Research (NILU).
Fendinger NJ, Lehmann RG, and Mihaich EM. 1997. Polydimethylsiloxane. In: Chandra G. editor. The handbook of environmental chemistry, Volume 3, Part H: Organosilicon materials. New York (NY): Springer-Verlag. p. 181-223.
Fenner K, Scheringer M, MacLeod M, Matthies M, McKone TE, Stroebe M, Beyer A, Bonnell M, Le Gall A, Klasmeier J, et al. 2005. Comparing estimates of persistence and long-range transport potential among multimedia models. Environ Sci Technol 39:1932-1942.
Genualdi S, Harner T, Cheng Y, MacLeod M, Hansen KM, van Egmond R, Shoeib M, Lee SC. 2011. Global distribution of linear and cyclic volatile methyl siloxanes in air. Environ Sci Technol 45:3349-3354.
Gobas FACP, Morrison HA. 2000. Bioconcentration and biomagnification in the aquatic environment. In: Boethling RS, Mackay D, editors. Handbook of property estimation methods for chemicals, environmental and health sciences. Boca Raton (FL): CRC Press. p. 189-231.
Gobas FAPC, Sharp SM. 1990. Bioaccumulation of some polychlorinated dibenzo-p-dioxins and octachlorodibenzofuran in the guppy (Poecilia reticulata). Chemosphere 20(5):495-512.
Gobas FAPC, Clark KE, Shiu WY, Mackay D. 1989. Bioconcentration of polybrominated benzenes and biphenyls and related superhydrophobic chemicals in fish: Role of bioavailability and elimination into the feces. Environ Toxicol Chem 8:231-245.
Gobas FAPC, Hugget DB, Springer TA. 2011. D5 bioaccumulation assessment. Unpublished report submitted to Environment Canada, Gatineau, Québec.
Harland Laboratories Ltd. 2010. 28-Day oral toxicity (gavage) study in the Sprague-Dawley rat with octamethyltrisiloxane (L3). Study number C53655.
Harner T, Shoeib M, Ahrens L, Genualdi S, Cheng C, Lee SC, Lane D, Smyth SA. 2010. Polyfluoroalkyl compounds (PFCs) and siloxanes in air in support of the Chemicals Management Plan: new sampling and analysis methods, emissions from the waste sector and long-range transport – April 2008 to May 2010. Unpublished report. Downsview (ON): Atmospheric Science and Technology Directorate, Environment Canada.
Health Canada. 1994. Human health risk assessment for priority substances. Ottawa (ON): Health Canada, Environmental Health Directorate. Available from: http://www.hc-sc.gc.ca/ewh-semt/pubs/contaminants/approach/index_e.html
Health Canada. 1995. Investigating human exposure to contaminants in the environment: A handbook for exposure calculations. Ottawa (ON): Health Canada.
Health Canada. 1998. Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Unpublished report. Ottawa (ON): Health Canada, Environmental Health Directorate.
Health Canada. 2014. The cosmetic ingredient hotlist--June 2014 [Internet]. Ottawa (ON): Health Canada, Consumer Product Safety Directorate. [cited Aug 2014]. Available from: http://www.hc-sc.gc.ca/cps-spc/person/cosmet/info-ind-prof/_hot-list-critique/prohibited-eng.php
[HENRYWIN] Henry’s Law Constant Program for Microsoft Windows [Estimation Model]. 2008. Version 3.20. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Himmelstein MW, Acquavella JF, Reccio L, Medinsky MA, Bond JA. 1997. Toxicology and epidemiology of
1,3-Butadiene. Crit Rev Toxicol 27:1-108.
Hobbs EJ, Fancher OE, Calandra JC. 1972. Effect of selected organopolysiloxanes on male rats and rabbits reproductive organs. Toxicol Appl Pharmacol 21:45-54.
Hobson JF, Atkinson R, Carter WPL. 1997. Volatile methylsiloxanes. In: Chandra G. editor. The handbook of environmental chemistry, Volume 3, Part H: Organosilicon materials. New York (NY): Springer-Verlag. p. 137-180.
Hobson JF, Silberhorn EM. 1995. Octamethylcyclotetrasiloxane (OMCTS), a case study: Summary and aquatic risk assessment. Environ Toxicol Chem 14(10):1667-1673.
[HPVIS] High Production Volume Information System [database on the Internet]. 2012. United States Environmental Protection Agency [last updated 2012 Mar 22; cited 2012 Sep 18]. Available from: http://www.epa.gov/chemrtk/hpvis/index.html
Hu TM, Layton WL. 2001. Allometric scaling of xenobiotic clearance: uncertainty versus universality. AAPS PharmSci [Internet] [cited 2010 May 25]. Vol. 3(4): Article 29. Available from: http://www.aapsj.org/view.asp?art=ps030429
[HYDROWIN] Hydrolysis Rates Program for Microsoft Windows [Estimation Model]. 2008. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Industrial Bio-Test Laboratories, Inc. 1964. Report to Dow Corning Corporation irritation studies on seven samples (TX-46A, TX-46B, TX-46C, TX-46D, TX-46E, TX-47A, TX-47B) with cover letter dated 04/20/94. TSCA OTS0556505, Document ID: 89940001051.
Industrial Bio-Test Laboratories, Inc. 1966. Report to Dow Corning Corporation toxicity studies on TX-135A with cover letter dated 04/20/94. TSCA OTS0556522, Document ID: 86940001068.
Industrial Bio-Test Laboratories, Inc. 1967a. Range finding eye irritation test on TX-212A (Hexamethyldisiloxane). TSCA OTS0572647, Document ID: 86940001612.
Industrial Bio-Test Laboratories, Inc. 1967b. Report to Dow Corning Corporation range finding eye irritation test on TX-212B with cover letter dated 04/20/94. TSCA OTS0556570, Document ID: 1967-I0065-1156-01.
Isquith A, Matheson D, Slesinski R. 1988a. Genotoxicity studies on selected organosilicon compounds: in vitro assays. Food Chem Toxicol 26:255-261.
Isquith A, Slesinski R, Matheson D. 1988b. Genotoxicity studies on selected organosilicon compounds: in vivo assays. Food Chem Toxicol 26:263-266.
Jovanovic ML, Crofoot SD, Crissman JW, Smith PA, Plotzke KP, Meeks RG. 2005. Chronic toxicity and oncogenicity study of hexamethyldisiloxane (HMDS) in Fisher-344 rats. Toxicol Sci 84:308.
Jovanovic ML, McMahaon JM, McNett DA, Tobin JM, Plotzke KP. 2008. In vitro and in vivo percutaneous absorption of 14C-octmethylcyclotetrasiloxane (14C-D4) and 14C decamethylcyclopentasiloxane (14C-D5). Regul Toxicol Pharmacol 50:239-248.
Kaj L, Andersson J, Cousins AP, Remberger M, Ekheden Y, Dusan B, Brorström-Lundén E. 2005a. Results from the Swedish National Screening Programme 2004. Subreport 4: siloxanes. IVL Report B1643. Stockholm (SE): IVL Swedish Environmental Research Institute Ltd.
Kaj L, Schlabach M, Andersson J, Cousins AP, Schmidbauer N, Brorström-Lundén E. 2005b. Siloxanes in the Nordic environment. TemaNord 2005:593. Copenhagen (DK): Nordic Council of Ministers.
Kelly BC, Gobas FAPC, McLachlan MS. 2004. Intestinal absorption and biomagnification of organic contaminants in fish, wildlife, and humans. Environ Toxicol Chem 23(10):2324-2336.
Khera N. 2014. Unpublished CMP2 wastewater monitoring data submitted to Ecological Assessment Division of Environment Canada, Gatineau QC.
Klasmeier J, Matthies M, MacLeod M, Fenner K, Scheringer M, Stroebe M, Le Gall AC, McKone TE, van de Meent D, Wania F. 2006. Application of multimedia models for screening assessment of long-range transport potential and overall persistence. Environ Sci Technol 40:53-60.
[KOAWIN] Octanol Air Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2008.
Version 1.10. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
[KOCWIN] The Soil Adsorption Coefficient Program [Estimation Model]. 2008. Version 2.00. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
[KOWWIN] Octanol-Water Partition Coefficient Program for Microsoft Windows [Estimation Model]. 2008. Version 1.68. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Krogseth IS, Zhang X, Lei YD, Wania F, Breivik K. 2013. Calibration and application of a passive air sampler (XAD-PAS) for volatile methyl siloxanes. Environ Sci Technol 47:4463-4470.
Lee MR. 2010. Early life-stage toxicity test with rainbow trout (Oncorhynchus mykiss) following OECD Guideline #210 with octamethyltrisiloxane (CAS No. 107-51-7). Springborn Smithers Laboratories Study No.: 12023.6205 [cited in SEHSC 2011].
[LNHPD] Licensed Natural Health Products Database [database on the internet]. 2014. Canada: Health Canada [cited Aug 2014]. Available from: http://205.193.93.55/lnhpd-bdpsnh/start-debuter.do
Longnecker MP. 1995. Alcohol consumption and risk of cancer in humans: an overview. Alcohol 12:87-96.
Loretz LG, Api AM, Barraj LM, Burdick J, Dressler WE, Gettings SD, Han Hsu H, Pan YHL, Re TA, Renskers KJ, Rothenstein A, Scrafford CG, Sewall C. 2005. Exposure data for cosmetic products: lipstick, body lotion, and face cream. Food Chem Toxicol 43: 279-291.
Loretz L, Api AM, Barraj L, Burdick J, Davis DA, Dressler W, Gilberti E, Jarrett G, Mann S, Pan YHL, et al. 2006. Exposure data for personal care products: Hairspray, spray perfume, liquid foundation, shampoo, body wash, and solid antiperspirant. Food Chem Toxicol 44:2008-2018.
Loretz LG, Api AM, Babcock L, Barraj LM, Burdick J, Cater KC, Jarrett G, Mann S, Pan YHL, Re TA, Renskers KJ, Scrafford CG. 2008. Exposure data for cosmetic products: Facial cleanser, hair conditioner, and eye shadow. Food Chem Toxicol 46: 1516-1524.
Markgraf SJ, Wells JR. 1997. The hydroxyl radical reaction rate constants and atmospheric reaction products of three siloxanes. Int J Chem Kinet 29:445-451.
Mazzoni SM, Roy S, Grigoras S. 1997. Eco-relevant properties of selected organosilicon materials. In: Chandra G, editor. The handbook of environmental chemistry. Volume 3. Part H. New York (NY): Springer-Verlag. p. 53-81.
McCarty LS, Mackay D, Smith AD, Ozburn GW, Dixon DG. 1992. Residue-based interpretation of toxicity and bioconcentration QSARs from aquatic bioassays: neutral narcotic organics. Environ Toxicol Chem 11(7):917-930.
McCarty LS, Mackay D. 1993. Enhancing ecotoxicological modelling and assessment: Body residues and modes of toxic action. Environ Sci Technol 27:1718-1728.
McGoldrick DJ, Letcher RJ, Barresi E, Keir MJ, Small J, Clark MG, Sverko E, Backus SM. 2014. Organophosphate flame retardants and organosiloxanes in predatory freshwater fish from locations across Canada. Environ Pollut 193:254-261.
McKim JM, Wilga PC, Breslin WJ, Plotzke KP, Gallavan RH, Meeks RG. 2001. Potential estrogenic and antiestrogenic activity of the cyclic siloxane octamethylcyclotetrasiloxane (d4) and the linear siloxane hexamethyldisiloxane (HMDS) in immature rats using the uterotrophic assay. Toxicol Sci 63:37-46.
Mekenyan G, Dimitrov SD, Pavlov TS, Veith GD. 2005. POPs: A QSAR system for creating PBT profiles of chemicals and their metabolites. SAR QSAR Environ Res 16(1-2):103−133.
[Model Applier] Leadscope FDA Model Applier [Prediction module on CD ROM]. 2008. Version 1.2.0–3. Columbus (OH): Leadscope, Inc. [cited 2010 August 6]. Available from: http://www.leadscope.com/model_appliers/ [restricted access].
Mosey JL, Kozerski GE. 2008. Hydrolysis of octamethyltrisiloxane (MDM). Silicones Environmental, Health and Safety Council (SEHSC). Dow Corning Study No. 10390-102 [cited in SEHSC 2010].
Mount GH, Eisele FL. 1992. An intercomparison of tropospheric OH measurements at Fritz Peak Observatory, Colorado. Science 256:1187 [cited in SEHSC 2011].
[MPBPVPWIN] Melting Point Boiling Point Program for Microsoft Windows [Estimation Model]. 2008. Version 1.43. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
[NCI] National Chemical Inventories [database on CD-ROM]. 2009. Issue 1. Columbus (OH): American Chemical Society. [cited 2010 Mar 15]. Available from: http://www.cas.org/products/cd/nci/index.html
[NHIPD] Natural Health Ingredients Products Database [database on the internet]. 2014. Ottawa (ON): Health Canada [cited Aug 2014]. Available from: http://webprod.hc-sc.gc.ca/nhpid-bdipsn/search-rechercheReq.do
[NHW] Dept. of National Health and Welfare (CA). 1990. Present patterns and trends in infant feeding in Canada. Ottawa (ON): Department of National Health and Welfare. NHW Cat. No. H39-199/1990E [cited in Health Canada 1998].
Nichols JW, Fitzsimmons PN, Burkhard LP. 2007. In vitro – in vivo extrapolation of quantitative hepatic biotransformation data for fish. II. Modeled effects on chemical bioaccumulation. Environ Toxicol Chem 26:1304−1319.
[NITE] National Institute of Technology and Evaluation. 2002. Tokyo (JP): National Institute of Technology and Evaluation, Chemical Management Centre. Available from: http://www.safe.nite.go.jp/data/hazkizon/pk_e_kizon_input_second.home_object
[OECD] Organisation for Economic Co-operation and Development. 2006. Guidelines for the testing of chemicals. Test Guideline 310. Ready biodegradability – CO2 in sealed vessels (Headspace test). Adopted 23 March 2006. Paris (FR): OECD, Environment Directorate.
[OECD] Organisation for Economic Co-operation and Development. 2007. Guidelines for the testing of chemicals. Test Guideline 225. Sediment-water Lumbriculus toxicity test using spiked sediment. Adopted 16 October 2007. Paris (FR): OECD, Environment Directorate.
[OECD] Organisation for Economic Co-operation and Development. 2011. Guidelines for the testing of chemicals. Bioaccumulation in fish: Aqueous and dietary exposure. Draft document V.10. Paris (FR): OECD, Environment Directorate.
Pelletier M. 2013. Unpublished CMP2 monitoring data submitted to Ecological Assessment Division of Environment Canada, Gatineau QC.
Pelletier M, Moore S, Backus S. 2012. Unpublished CMP2 sediment monitoring data submitted to Ecological Assessment Division of Environment Canada, Gatineau QC.
Preston-Martin SP. 1991. Evaluation of the evidence that tobacco-specific nitrosamines (TSNA) cause cancer in humans. Crit Rev Toxicol 21:295-298.
Quigley CA, DE Bellis A, Marschke KB, El-Awady MK, Wilson EM, French FS. 1995. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev 16:19-34.
Redman AD, Mihaich E, Woodburn K, Paquin P, Powell D, McGrath JA, Di Toro DM. 2012. Tissue-based risk assessment of cyclic volatile methyl siloxanes. Environ Toxicol Chem 31(8):1911-1919.
Rowe VK, Spencer HC, Bass SL. 1948. Toxicological studies on certain commercial silicones and hydrolyzable silane intermediates. J Ind Hyg Toxicol 30:332-52.
[RTECS] Registry of Toxic Effects of Chemical Substances. 2009. Record for Disiloxane, hexamethyl- (107-46-0) [updated 2009 November]. Hamilton (ON): Canadian Centre for Occupational Health and Safety.
Sakuratani Y, Noguchi Y, Kobayashi K, Yamada J, Nishihara T. 2008. Molecular size as a limiting characteristic for bioconcentration in fish. J Environ Biol. 29(1):89-92.
Schaefer EC, Matthews ME. 2009. Octamethyltrisiloxane (L3; CAS No. 107-51-7): Ready biodegradability – CO2 in sealed vessels (headspace test). Silicones Environmental, Health and Safety Council (SEHSC). Wildlife International Ltd. Study No. – 570E-101 [cited in SEHSC 2010].
Scheringer M, MacLeod M, Wegmann F. 2009. The OECD POV and LRTP Screening Tool [Internet]. Version 2.2. Zurich (CH): Swiss Federal Institute of Technology Zurich. Available from: http://www.oecd.org/document/24/0,3746,en_2649_34373_45373336_1_1_1_1,00.html#download
Schlabach M, Strand Andersen M, Green N, Schøyen M, Kaj L. 2007. Siloxanes in the environment of the Inner Oslofjord. SFT Report 986/2007 (TA-2269/2007). Oslo (NO): Norwegian Pollution Control Authority (SFT), Norwegian Institute for Air Research (NILU) and Norwegian Institute for Water Research (NIVA).
SciFinder [database on CD-ROM]. 2007. Version 2007.3. Columbus (OH): American Chemical Society [cited 2010 Sep 9]. Available from: http://www.cas.org/products/scifindr/index.html
[SEHSC] Silicones Environmental, Health and Safety Council. 2005. Linear volatile methyl siloxanes group justification. CAS Nos. 107-51-7, 141-62-8, 141-63-9, and 1000-05-1. August 31, 2005. Submission to Environment Canada under the Chemicals Management Plan Challenge initiative.
[SEHSC] Silicones Environmental, Health and Safety Council. 2006. Linear volatile methyl siloxanes category. CAS Nos. 107-51-7, 141-62-8, 141-63-9, and 1000-05-1. June 9, 2006. Submission to Environment Canada under the Chemicals Management Plan Challenge initiative.
[SEHSC] Silicones Environmental, Health and Safety Council. 2010. Octamethyltrisiloxane (L3). Background information. CAS No. 107-51-7. June 23, 2010. Submission to Environment Canada under the Chemicals Management Plan Challenge initiative.
[SEHSC] Silicones Environmental, Health and Safety Council. 2011. Octamethyltrisiloxane (L3). SEHSC comments on Health and Environment Canada’s draft screening assessment of octamethyltrisiloxane (MDM). March 11, 2011. Submission to Environment Canada under the Chemicals Management Plan Challenge initiative.
Seifried HE, Seifried RM, Clarke JJ, Junghans TB, San RHC. 2006. A compilation of two decades of mutagenicity test results with the Ames Salmonella typhimurium and L5178Y mouse lymphoma cell mutation assays. Chem Res Toxicol 19:627-624.
Shenker A, Laue L, Kosugi S, Merendino JJ, Minegishi T, Cutler GB. 1993. A constitutively activating mutation of the luteinizing hormone receptor in familial male precocious puberty. Nature 265:652-654.
[Shin-Etsu] Shin-Etsu Silicones of America Inc. 1994a. Final Report, Bacterial reverse mutation test with LS-7130, Hexamethyldisiloxane, with cover letter dated 06/29/95. TSCA OTS0557700, Document ID: 86950000188.
[Shin-Etsu] Shin-Etsu Silicones of America Inc. 1994b. Final report, chromosomal aberration test with LS-7131, Hexamethyldisiloxane, using cultured mammalian cells, with cover letter dated 06/29/95. TSCA OTS0557699, Document ID: 86950000187.
[Shin-Etsu] Shin-Etsu Silicones of America Inc. 1994c. Final report, sub-acute oral toxicity study on LS-7131, Hexamethyldisiloxane, in rats, with cover letter dated 06/29/95. TSCA OTS0557701, Document ID: 86950000189.
[SPIN] Substances in Preparations in Nordic Countries [database on the Internet]. 2013. Copenhagen (DK): Nordic Council of Ministers [cited 2012 Sep 18] Available from: http://90.184.2.100/DotNetNuke/default.aspx
[TaPL3] Long Range Transport and Persistence Level III model [Internet]. 2000. Version 2.10. Peterborough (ON): Trent University, Canadian Environmental Modelling Centre. Available from: http://www.trentu.ca/academic/aminss/envmodel/models/TaPL3.html
Thomas ST, Krueger HO, Kendall TZ. 2009a. Octamethyltrisiloxane (L3; CAS No. 107-51-7): A prolonged sediment toxicity test with Chironomus riparius using spiked sediment. Wildlife International, Ltd. Project Number: 570A-114. Final report. Easton (MD): Wildlife International, Ltd.
Thomas ST, Krueger HO, Kendall TZ. 2009b. Octamethyltrisiloxane (L3; CAS No. 107-51-7): A prolonged sediment toxicity test with Lumbriculus variegatus using spiked sediment. Wildlife International, Ltd. Project Number: 570A-115. Final report. Easton (MD): Wildlife International, Ltd.
[TOPKAT] TOxicity Prediction by Komputer Assisted Technology [Internet]. 2004. Version 6.2. San Diego (CA): Accelrys Software Inc. [cited 2010 August 6]. Available from: http://www.accelrys.com/products/topkat/index.html
[USP] United States Pharmacopeia. 2010. Food Chemicals Codex, 7th Edition. Rockville (MD): The United States Pharmacopeial Convention
Varaprath S, Frye CL, Hamelink J. 1996. Aqueous solubility of permethylsiloxanes (silicones). Environ Sci Technol 15(8):1263-1265.
Wang D, Steer H, Young T, Tait T, Williams Z, Pacepavicius G, Ng T, Smyth SA, Kinsman L, Sverko E, Svoboda L, Fazal S, Alaee M. 2010. Concentrations of cyclic volatile methyl siloxanes (cVMS) in various environmental media from Southern Ontario and Southern Quebec. Unpublished report. Burlington (ON): Environment Canada, Water Science and Technology Directorate.
Wang D, de Solla SR, Lebeuf M, Bisbicos T, Barrett GC, Alaee M. 2012. Determination of linear and cyclic volatile methylsiloxanes in blood of turtle, cormorant, and seal from Canada. Unpublished report. Burlington (ON): Water Science and Technology Directorate, Environment Canada.
Wania F. 2003. Assessing the potential of persistent organic chemicals for long-range transport and accumulation in polar regions. Environ Sci Technol 37:1344-1351.
Wania F. 2006. Potential of degradable organic chemicals for absolute and relative enrichment in the Arctic. Environ Sci Technol 40:569-577.
Warner NA, Evenset A, Christensen G, Gabrielsen GW, Borgå K, Leknes H. 2010. Volatile siloxanes in the European Arctic: Assessment of sources and spatial distribution. Environ Sci Technol 44:7705-7710.
Weisbrod AV, Woodburn KB, Koelmans AA, Parkerton TF, McElroy AE, Borgå K. 2009. Evaluation of bioaccumulation using in vivo laboratory and field studies. Integr Environ Assess Manag 5(4): 598-623.
Whelan MJ, Estrada E, van Egmond R. 2004. A modelling assessment of the atmospheric fate of volatile methyl siloxanes and their reaction products. Chemosphere 57:1427-1437.
Wilmer J, Bloeman L, Farrar D, Farny V, Green T, Millischer RJ, Vrijhor H. 1994. Trichloroethylene: Assessment of human carcinogenic hazard. Technical Report 60. May 1994. Brussels (BE): European Centre for Ecotoxicology and Toxicology of Chemicals.
Woodburn K, Drottar K, Domoradzki J, Durham J, McNett D, Jezowski R. 2013. Determination of the dietary biomagnification of octamethylcyclotetrasiloxane and decamethylcyclopentasiloxane with the rainbow trout (Oncorhynchus mykiss). Chemosphere 93(5):779-788.
[WSKOWWIN] Water Solubility for Organic Compounds Program for Microsoft Windows [Estimation Model]. 2008. Version 1.42. Washington (DC): US Environmental Protection Agency, Office of Pollution Prevention and Toxics; Syracuse (NY): Syracuse Research Corporation. Available from: www.epa.gov/oppt/exposure/pubs/episuite.htm
Wu X, Bennett DH, Ritz B, Cassady DL, Lee K, Hertz-Picciotto I. 2010. Usage pattern of personal care products in California households. Food Chem Toxicol 48: 3109-3119.
Xu S. 2009. Anaerobic transformation of octamethylcyclotetrasiloxane (14C-D4) in aquatic sediment systems. Dow Corning Report No. 2009-10000-61734 [cited in SEHSC 2011].
Xu S, Chandra G. 1999. Fate of cyclic methylsiloxanes in soils. 2. Rates of degradation and volatilization. Environ Sci Technol 33: 4034-4039.
Xu S, Doede K. 2010. Degradation octamethyltrisiloxane (L3) (CAS No. 107-51-7) in soils. Final report to SEHSC. DCC HES Study No. 11297-102 [cited in SEHSC 2010, 2011].
Xu S, Kropscott BE. 2010. Temperature dependence of air/water, 1-octanol/air, and 1-octanol/water partitioning coefficients of octamethyltrisiloxane (L3) (CAS 107-51-7) Final report to SEHSC. DCC HES Study No. 10958-102 [cited in SEHSC 2011].
Xu S, Doede K, Staples C. 2012. Degradation and volatilization of octamethyltrisiloxane (L3) in soils. Poster presented at the 33rd Annual North American Meeting of The Society of Environmental Toxicology and Chemistry (SETAC), held November 11–15 in Long Beach, CA.
Zareba G, Gelein R, Morrow PE, Utell MJ. 2002. Percutaneous absorption studies of octamethylcyclotetrasiloxane using the human skin /nude mouse model. Skin Pharmacol Appl Skin Physiol 15:184-194.
Appendix I: Model Inputs Summary Table
| Parameter | Phys-Chem / Fate | Fate | Fate | Fate | PBT Profiling |
|---|---|---|---|---|---|
| Model | EPI suite (all models) |
EQC (Type II chemical) |
TaPL3 (Type II chemical) |
OECD Pov – LRTP Tool | Canadian POPs (includes CATABOL, BCF Mitigating Factors, OASIS) |
| SMILES code | C[Si](C)(C)O[Si](C)(C)O[Si](C)(C)C | n/a | n/a | n/a | C[Si](C)(C)O[Si](C)(C)O[Si](C)(C)C |
| Molecular weight (g/mol) | n/a | 236.5 | 236.5 | 236.5 | n/a |
| Melting point (ºC) | -82 | -82 | -82 | n/a | n/a |
| Boiling point (ºC) | 152.5 | n/a | n/a | n/a | n/a |
| Data temperature (ºC) | n/a | 25 | 20 | n/a | n/a |
| Vapour pressure (Pa) | 520 | 520 | 520 | n/a | n/a |
| Water solubility (mg/L) | 0.034 | 0.034 | 0.034 | n/a | n/a |
| Henry’s Law constant (Pa·m3/mol) | 2.9 × 106 | 2.9 × 106 | n/a | n/a | n/a |
| Log Kaw (Air-water partition coefficient; dimensionless) |
n/a | 3.06Footnote Appendix I Table 1[a] | n/a | 3.06[a] | n/a |
| Log Kow (Octanol-water partition coefficient; dimensionless) |
6.6 | 6.6 | 6.6 | 6.6 | n/a |
| Soil-water partition coefficient (L/kg) | n/a | 438 | n/a | n/a | n/a |
| Sediment-water partition coefficient (L/kg) | n/a | 876 | n/a | n/a | n/a |
| Suspended particles-water partition coefficient (L/kg) | n/a | 4380 | n/a | n/a | n/a |
| Fish-water partition coefficient (L/kg) | n/a | 5030 | n/a | n/a | n/a |
| Half-life in air (days) | n/a | 5.79Footnote Appendix I Table 1[b] | 5.79[b] | 5.79[b] | n/a |
| Half-life in water (days) | n/a | 13.7[b] | 13.7[b] | 13.7[b] | n/a |
| Half-life in sediment (days) | n/a | 365[b] | 365[b] | n/a | n/a |
| Half-life in soil (days) | n/a | 4.54[b] | 4.54[b] | 4.54[b] | n/a |
| Half-life in suspended sediment (days) | n/a | n/a | 13.7Footnote Appendix I Table 1[c] | n/a | n/a |
| Half-life in fish (days) | n/a | n/a | 13.7[c] | n/a | n/a |
| Half-life in aerosol (days) | n/a | n/a | 1 × 1011[c] | n/a | n/a |
Appendix II: Environmental concentrations
| Location; year | Concentration (ng/m3) |
No. of samples | Reference |
|---|---|---|---|
| Ontario, Canada; 2009 WWTP #1 Primary clarifier |
1.84, 2.00 | 2 | Cheng et al. 2011 |
| Ontario, Canada; 2009 WWTP #1 Aeration tank |
0.97, 1.17, 1.73, 1.84 | 4 | Cheng et al. 2011 |
| Ontario, Canada; 2009 WWTP #1 Secondary clarifier |
1.28, 1.43 | 2 | Cheng et al. 2011 |
| Ontario, Canada; 2009 WWTP #1 Background |
0.66, 0.76, 0.77, 1.41 | 4 | Cheng et al. 2011 |
| Ontario, Canada; 2009 WWTP #2 Aeration tank |
1.88, 2.64 | 2 | Cheng et al. 2011 |
| Ontario, Canada; 2009 Landfill #1 Upwind |
0.49 | 1 | Cheng et al. 2011 |
| Ontario, Canada; 2009 Landfill #1 Downwind |
6.14 | 1 | Cheng et al. 2011 |
| Ontario, Canada; 2009 Landfill #2 Upwind |
0.87 | 1 | Cheng et al. 2011 |
| Ontario, Canada; 2009 Landfill #2 Downwind |
5.60 | 1 | Cheng et al. 2011 |
| Polar/Arctic; 2009 Barrow, AK |
BDL MDL: 0.011 |
1 | Genualdi et al. 2011 |
| Polar/Arctic; 2009 Little Fox Lake, YK |
BDL MDL: 0.011 |
1 | Genualdi et al. 2011 |
| Polar/Arctic; 2009 Alert, NU |
BDL MDL: 0.011 |
1 | Genualdi et al. 2011 |
| Polar/Arctic; 2009 Ny-Alesund, Norway |
BDL MDL: 0.011 |
1 | Genualdi et al. 2011 |
| Canada; 2009 Ucluelet, BC |
BDL MDL: 0.011 |
1 | Genualdi et al. 2011 |
| Canada; 2009 Whistler, BC |
0.016 MDL: 0.011 |
1 | Genualdi et al. 2011 |
| Canada; 2009 Bratt’s Lake, SK |
BDL MDL: 0.011 |
1 | Genualdi et al. 2011 |
| Canada; 2009 Fraserdale, ON |
BDL MDL: 0.011 |
1 | Genualdi et al. 2011 |
| Canada; 2009 Downsview, ON |
0.12 MDL: 0.011 |
1 | Genualdi et al. 2011 |
| Canada; 2009 Sable Island, NS |
BDL MDL: 0.011 |
1 | Genualdi et al. 2011 |
| U.S.; 2009 Point Reyes, CA |
0.011 MDL: 0.011 |
1 | Genualdi et al. 2011 |
| U.S.; 2009 Sydney, FL |
BDL MDL: 0.011 |
1 | Genualdi et al. 2011 |
| U.S.; 2009 Groton, CT |
0.013 MDL: 0.011 |
1 | Genualdi et al. 2011 |
| U.S.; 2009 Hilo, HI |
0.019 MDL: 0.011 |
1 | Genualdi et al. 2011 |
| Europe; 2009 Storhofdi, Iceland |
BDL MDL: 0.011 |
1 | Genualdi et al. 2011 |
| Europe; 2009 Malin Head, Ireland |
BDL MDL: 0.011 |
1 | Genualdi et al. 2011 |
| Europe; 2009 Paris, France |
0.029 MDL: 0.011 |
1 | Genualdi et al. 2011 |
| Europe; 2009 Kosetice, Czech Rep. |
BDL MDL: 0.011 |
1 | Genualdi et al. 2011 |
| Cape Grim, AU; 2009 | BDL MDL: 0.011 |
1 | Genualdi et al. 2011 |
| Tudor Hill, BM; 2009 | BDL MDL: 0.011 |
1 | Genualdi et al. 2011 |
| Toronto, ON; 2012 | 0.39–6.43 | 41 IDL = 0.39 |
Krogseth et al. 2013 |
| Nordic countriesFootnote Appendix II Table 1[a]; 2004–2005 |
BDL MDL: 8 |
24 | Kaj et al. 2005b |
| Location; year | Concentration (ng/L) |
No. of samples | Reference |
|---|---|---|---|
| Nordic countriesFootnote Appendix II Table 2[a]; 2004–2005 Surface freshwater |
BDL MDL: 0.5–0.8 |
10 | Kaj et al. 2005b |
| Iceland; 2005 Seawater |
BDL MDL: 0.5 |
4 | Kaj et al. 2005b |
| Norway; 2006 Seawater |
BDL MDL: 0.3 |
4 | Schlabach et al. 2007 |
| Ontario, Quebec, British Columbia, Canada; 2011 WWTP influents |
6–96 DL: 13 |
in 10 of 16 | Alaee 2012 |
| Ontario, Quebec, British Columbia, Canada; 2011 WWTP effluents |
3–10 DLs: 1, 13 |
in 3 of 15 | Alaee 2012 |
| Ontario, Quebec, British Columbia, Canada; 2012 WWTP influents |
1–531 DL: 1 |
in 16 of 16 | Alaee 2014 |
| Ontario, Quebec, British Columbia, Canada; 2012 WWTP effluents |
0.4–114 DL: 1 |
in 9 of 16 | Alaee 2014 |
| Across Canada; 2012 WWTP influents |
14.15 – 530.99 DL: 0.40 |
17 | Khera 2014 |
| Across Canada; 2012 WWTP effluents |
0.49 – 114.94 DL: 0.40 |
in 12 of 18 | Khera 2014 |
| Across Canada; 2013 WWTP influents |
4.73 – 388.18 DL: 0.40 |
in 12 of 16 | Khera 2014 |
| Across Canada; 2013 WWTP effluents |
0.90 – 65.42 DL: 0.40 |
in 13 of 16 | Khera 2014 |
| Across Canada; 2013 WWTP influents |
BDL DL: 53 |
24 | Khera 2014 |
| Across Canada; 2013 WWTP effluents |
BDL DL: 53 |
24 | Khera 2014 |
| Across Canada; 2014 WWTP influents |
60.10 – 128.19 DL: 53 |
in 3 of 21 | Khera 2014 |
| Across Canada; 2014 WWTP effluents |
BDL DL: 53 |
21 | Khera 2014 |
| Ontario, Quebec, Canada; 2011 Industrial process waters |
17–15 300 DL: 13 |
in 4 of 13 | Alaee 2012 |
| Ontario, Quebec, Canada; 2011 Industrial effluents |
5000 DL: 13 |
in 1 of 5 | Alaee 2012 |
| Ontario, Canada; 2012 Industrial process waters |
BDL DL: 2 |
3 | Alaee 2014 |
| Ontario or Quebec, Canada; 2011 Landfill leachate |
1 DL: 1 |
in 1 of 3 | Alaee 2012 |
| Ontario, Quebec, British Columbia, Canada; 2012 Landfill leachate |
0.7–6.2 DL: 0.3–3 |
in 9 of 15 | Alaee 2014 |
| Nordic countriesFootnote Appendix II Table 2[b]; 2004–2005 WWTP influents |
3.4, 14 DL: 0.5–1 |
in 2 of 4 | Kaj et al. 2005b |
| Nordic countries[b]; 2004–2005 WWTP effluents |
BDL DL: 0.5–1 |
9 | Kaj et al. 2005b |
| Norway; 2006 WWTP influents |
1, 32 DL: 0.3 |
2 | Schlabach et al. 2007 |
| Norway; 2006 WWTP effluents |
BDL DL: 0.3 |
2 | Schlabach et al. 2007 |
| Nordic countries[b]; 2004–2005 Landfill leachate |
BDL DL: 0.5–4 |
10 | Kaj et al. 2005b |
| Location; year | Concentration (ng/g dw) | No. of samples | Reference |
|---|---|---|---|
| Great Lakes region, Canada; 2011 | BDL DL: 0.2–7 |
93 | Backus et al. 2012 |
| Lake Ontario, Canada; 2011 | BDL DL: 0.18–0.76 |
10 | CES 2012 |
| Newfoundland, Nova Scotia, New Brunswick, Quebec, Ontario, British Columbia; 2012 | BDL DL: 20 |
126 | Pelletier et al. 2012 |
| Lake Pepin MN, USA; 2011 | BDL DL: 0.18–0.76 ng/g ww |
24 | CES 2012 |
| Nordic countriesFootnote Appendix II Table 3[a]; 2003–2005 |
BDL DL: 0.02–0.71 |
24 | Kaj et al. 2005b |
| Norway; 2006 | BDL DL: 0.2–0.4 |
6 | Schlabach et al. 2007 |
| Norwegian Arctic; 2008 | BDL DL: 0.19–0.3 |
6 | Evenset et al. 2009 |
| Location; year | Concentration (ng/g dw) | No. of samples | Reference |
|---|---|---|---|
| Sweden; 2004–2005 WWTP sludge |
(max) 37 (mean) 7 |
in 12 of 54 | Kaj et al. 2005a |
| Nordic countriesFootnote Appendix II Table 4[a]; 2004–2005 WWTP sludge |
1–64 (mean) 12 |
in 8 of 14 | Kaj et al. 2005b |
| Norway; 2006 WWTP sludge |
11–31 | in 3 of 4 | Schlabach et al. 2007 |
| Faroe Islands; 2004 | BDL DL: 0.1 |
2 | Kaj et al. 2005b |
| Location; year | Organism | Concentration (ng/g ww) | No. of samples | Reference |
|---|---|---|---|---|
| Ontario, Canada; 2008 | Common snapping turtle (Chelydra s. serpentine) |
BDL DL: 0.034 |
32 | Wang 2012 |
| Ontario, Canada; 2008 | Double-crested cormorant (Phalacrocorax auritus) |
BDL DL: 0.034 |
22 | Wang 2012 |
| Ontario, Canada; 2008 | Northwest Atlantic harbour seal (Phoca vitulina) |
0.062–0.088 DL: 0.026 |
in 3 of 15 | Wang 2012 |
| Ontario, Manitoba, Alberta, Yukon, Canada; 2009-2010 |
Lake trout (Salvelinus namaycush) |
BDL DL: 0.42 |
60 | McGoldrick et al., 2014 |
| Ontario, Manitoba, Alberta, Yukon, Canada; 2009-2010 |
Walleye (Sander vitreus) |
BDL DL: 0.42 |
17 | McGoldrick et al., 2014 |
| Lake Ontario, Canada; 2011 | Mysid (Mysis relicta) |
BDL DL: 1.63 |
4Footnote Appendix II Table 5[a] | CES 2013 |
| Lake Ontario, Canada; 2011 | Round goby (Neogobius melanostomus) |
BDL DL: 1.63 |
12Footnote Appendix II Table 5[b] | CES 2013 |
| Lake Ontario, Canada; 2011 | Rainbow smelt (Osmerus mordax) |
BDL DL: 1.63 |
9Footnote Appendix II Table 5[c] | CES 2013 |
| Lake Ontario, Canada; 2011 | Alewife (Alosa pseudoharengus) |
BDL DL: 1.63 |
5Footnote Appendix II Table 5[d] | CES 2013 |
| Lake Ontario, Canada; 2011 | Lake trout (Salvelinus namaycush) |
BDL DL: 1.63 |
19 | CES 2013 |
| Québec, Canada; 2012-2013 |
Northern pike (Esox lucius) |
0.30 DL: 0.17 |
in 1 of 7 | Pelletier 2013 |
| Québec, Canada; 2012-2013 |
Walleye (Sander vitreus) |
0.19–1.77 DL: 0.17 |
4 | Pelletier 2013 |
| Québec, Canada; 2012-2013 |
Yellow perch (Perca flavescens) |
BDL DL: 0.17 |
2 | Pelletier 2013 |
| Québec, Canada; 2012-2013 |
Round goby (Neogobius melanostomus) |
BDL DL: 0.17 |
4Footnote Appendix II Table 5[e] | Pelletier 2013 |
| Québec, Canada; 2012-2013 |
Eastern elliptio mussel (Elliptio complanata) | 0.29 DL: 0.17 |
in 1 of 7Footnote Appendix II Table 5 [f] | Pelletier 2013 |
| Lake Pepin, USA; 2011 | Zooplankton | BDL DL: 0.18–0.76 |
4[a] | CES 2012 |
| Lake Pepin, USA; 2011 | Mayfly larvae (Hexagenia sp.) |
BDL DL: 0.18–0.76 |
5[a] | CES 2012 |
| Lake Pepin, USA; 2011 | Gizzard shad (YOY) (Dorosoma cepedianum) | BDL DL: 0.18–0.76 |
11[a] | CES 2012 |
| Lake Pepin, USA; 2011 | Sauger (Sander canadensis) |
BDL DL: 0.18–0.76 |
20 | CES 2012 |
| Nordic countriesFootnote Appendix II Table 5[g]; 2004–2005 |
Marine and freshwater fish, marine mammals, seabird eggs | BDL DL: 0.3 |
45 | Kaj et al. 2005b |
| Norway; 2004–2006 |
Mussel (Mytilus edulis) |
BDL DL: 0.04 |
3 | Schlabach et al. 2007 |
| Norway; 2004–2006 |
Flounder, liver (Platichthys flesus) |
BDL DL: 0.04 |
2 | Schlabach et al. 2007 |
| Norway; 2004–2006 |
Cod, stomach (Gadus morhua) |
BDL DL: 0.04 |
3 | Schlabach et al. 2007 |
| Norway; 2004–2006 |
Cod, liver (Gadus morhua) |
0.1 DL: 0.04 |
in 2 of 4 | Schlabach et al. 2007 |
| Norwegian Arctic; 2008 | Atlantic cod, liver (G. morhua) |
0.33 DL: 0.08–0.18 |
in 1 of 5 | Evenset et al. 2009 |
| Norwegian Arctic; 2008 | Polar cod, liver (Boreogadus saida) |
0.17 DL: 0.08–0.18 |
in 1 of 5 | Evenset et al. 2009 |
| Norwegian Arctic; 2008 | Kittiwake (Rissa tridactyla) |
BDL DL: 0.08–0.18 |
9 | Evenset et al. 2009 |
| Norwegian Arctic; 2008 | Eider (Somateria collissima) | BDL DL: 0.08–0.18 |
5 | Evenset et al. 2009 |
Appendix III: Fugacity and Critical Body Burden Analysis for MDM
The following analysis was based on the equations presented in Burkhard et al. (2012) and Gobas et al. (2011).
Fugacity Ratios:
Fugacity ratios were calculated using the equations:
Fbiota-water = BCF (L/kg) × dbiota(kg/L) × Zwater ÷ Zbiota
Fbiota-sediment = BSAF (kg/kg) × dbiota(kg/L) ÷ dsediment (kg/L) × Zsediment ÷ Zbiota
Fbiota-diet = BMF (kg/kg) × dbiota (kg/L) ÷ ddiet (kg/L) × Zdiet ÷ Zbiota
| Parameter | Value Calculated |
|---|---|
| Zwater = 1/H | 3.4E-07 |
| Zbiota = Kbiota-water Zwater = Φlipid × (dbiota/dlipid) × Kow × Zwater | 0.07, 0.05 (invert) |
| Zsediment = Ksediment-waterZwater = ΦOC × dsediment × Koc × Zwater | 0.0002 |
| Zdiet = Kdiet-water Zwater = Φlipid × (ddiet/dlipid) × Kow × Zwater | 1.9E-08 |
| BCF = bioconcentration factor (L/kg) | 7730 |
| BMF = biomagnification factor (kg/kg) | 0.86 |
| BSAF = biota-sediment accumulation factor (kg/kg) | 16 (max D4 value) |
| H = Henry’s Law constant (Pa·m3·mol-1) | 2900000 |
| Φlipid = lipid content of the biota (kg/kg) | 0.05, 0.02 (invert) |
| ΦOC = organic carbon content of the sediment (kg/kg) | 0.03 (D4 study) |
| dbiota = the density of biota (kg/L) | 1 |
| dlipid = the density of lipids (kg/L) | 0.9 |
| dsediment = density of the sediments (kg/L) | 1.5 |
| Koc = organic carbon water partition coefficient L/kg OC | 21878 |
| Kow = octanol-water partition coefficient (L/kg) | 3981072 |
| VP = vapour pressure (Pa) | 520 |
| Fbiota-water = | 0.035 |
| Fbiota-sediment = | 0.050 |
| Fbiota-diet = | 2.2E-07 |
Fugacity capacity was calculated in a 5% lipid fish as:
C = F × Z
where C is the internal maximum fugacity capacity of MDM (mmol/kg), F = fugacity (Pa) (as VP × Fratio) and Z is Zbiota. In fish, using the BCF data, a maximum capacity of 1.4 mmol/kg was calculated, which is equal to a fugacity of 18.2 Pa. Using read-across D4 BSAF data a value of 1.3 mmol/kg (25.9 Pa) was calculated as the maximum capacity in sediment invertebrates. Using the BMF data, a value of 8.6 E-06 mmol/kg was calculated.
Critical body residues (CBR) for a 5% lipid fish (MDM BCF already at 5% lipid) was also calculated using a steady state approach where the CBR in mmol/kg was calculated as
CBR = WS × BCF ÷ MW
where:
- WS:
- the maximum solubility in water (mg/L)
- BCF:
- bioconcentration factor (L/kg)
- MW:
- molecular weight (g/mol)
When the units are cancelled out in the above equation a value of 1.1 mmol/kg was calculated for MDM which is very comparable to the value of 1.4 calculated using the fugacity approach above for fish.
The median critical body burden threshold for chemicals with a narcotic mode of action cited in McCarty and MacKay (1993) is about 5 mmol/kg (but ranges from 2-8 mmol/kg). Median chronic narcotic internal body burden thresholds are about a factor of 10 lower than the acute values with the same range of values (i.e., 0.2 to 0.8 mmol/kg).
The above fugacity and CBR analysis suggests that critical body residues can be reached for chronic effects in fish and invertebrates and while some adverse effects have been observed in sediment invertebrates, none have been reported in fish or other pelagic organisms. This lack of correlation in fish suggests that: (1) MDM may bioaccumulate but not act as other narcotic chemicals at target sites (less reactive than other narcotic chemicals), (2) there could be error associated with the bioconcentration factor in fish (too high), (3) that accumulation in organisms is not strictly a result of hydrophobic partitioning (e.g., hydrogen bonding) and that distribution in the organism does not result in toxicity at target sites as a result, and, (4) that octanol is not a good surrogate for target lipid resulting in error estimating internal concentrations. Burkhard et al. (2012) discuss the limitations of this method.
Appendix IV: Upper-bound Estimates of Daily Intake of MDM by the General Population in Canada
| Estimated intake (µg/kg-bw per day) by age group | 0–6 monthsFootnote Appendix 4 Table 1[a].10 Breast fedFootnote Appendix 4 Table 1[b].9 |
0–6 months[a] Formula fedFootnote Appendix 4 Table 1[c].8 |
0–6 months[a] Not formula fed |
0.5–4 yearsFootnote Appendix 4 Table 1[d].4 | 5–11 yearsFootnote Appendix 4 Table 1[e].1 | 12–19 yearsFootnote Appendix 4 Table 1[f] | 20–59 yearsFootnote Appendix 4 Table 1[g] | 60+ yearsFootnote Appendix 4 Table 1[h] |
|---|---|---|---|---|---|---|---|---|
| Ambient airFootnote Appendix 4 Table 1[i] | 2.8 × 10−4 | 2.8 × 10−4 | 2.8 × 10−4 | 6.0 × 10−4 | 4.7 × 10−4 | 2.7 × 10−4 | 2.3 × 10−4 | 2.0 × 10−4 |
| Indoor airFootnote Appendix 4 Table 1[j] | 3.0 | 3.0 | 3.0 | 6.5 | 5.0 | 2.9 | 2.5 | 2.1 |
| Drinking waterFootnote Appendix 4 Table 1[k] | N/A | 8.5 × 10−5 | 3.2 × 10−5 | 3.6 × 10−5 | 2.8 × 10−5 | 1.6 × 10−5 | 1.7 × 10−5 | 1.8 × 10−5 |
| Food and beveragesFootnote Appendix 4 Table 1[l] | 7.9 × 10−4 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| SoilFootnote Appendix 4 Table 1[m] | 4.0 × 10−7 | 4.0 × 10−7 | 4.0 × 10−7 | 6.5 × 10−7 | 2.1 × 10−7 | 5.1 × 10−8 | 4.2 × 10−8 | 4.2 × 10−8 |
| Total intake | 3.0 | 3.0 | 3.0 | 6.5 | 5.0 | 2.9 | 2.5 | 2.1 |
Maximum total intake from all routes of exposure:6.5 µg/kg-bw per day
Appendix V: Upper-bounding Estimates of Exposure to Octamethyltrisiloxane in Personal Care Products Using ConsExpo 4.1 (ConsExpo 2007)
| Product | Scenario | AssumptionsFootnote Appendix 5a Table 1[a].11 | External applied doseFootnote Appendix 5a Table 1[b].10 (mg/kg-bw per day) |
|---|---|---|---|
| Foundation | Facial makeup | Concentration of MDM = 1–3% Exposure frequency: 438×/year (Loretz et al 2006) Exposed area: 637 cm2 (Health Canada 1995) Amount product applied: 0.54 g (Loretz et al 2006) (10% partitioning, model input 0.054 g) |
9.1 × 10−3 – 0.027 |
| Makeup primer | Facial makeup |
Concentration of MDM = 1–100% Exposure frequency: 438×/year (Loretz et al 2006) Exposed area: 637 cm2 (Health Canada 1995) Amount product applied: 0.54 g (Loretz et al 2006) (10% partitioning, model input 0.054 g) |
9.1 × 10−3 – 0.35 |
| Concealer (spot treatment) | Facial makeup | Concentration of MDM = 1–30% Exposure frequency: 365×/year Exposed area: 50 cm2 (estimated) Amount product applied: 0.15 g (10% partitioning, model input 0.015 g) |
2.1 × 10−3– 0.063 |
| Blush | Facial makeup | Concentration of MDM = 1–3% Exposure frequency: 438×/year (Loretz et al 2006) Exposed area: 160 cm2 (estimated ¼ face) Amount product applied: 0.14 g (10% partitioning, model input 0.014 g) (Loretz et al 2006) |
2.4 × 10−3 – 7.1 × 10−3 |
| Mascara | Mascara | Concentration of MDM = 0.3–30% Exposure frequency: 244×/year (Wu et al 2010) Exposed area: 1.6 cm2 (ConsExpo 2007) Amount product applied: 0.025 g (10% partitioning, model input 0.0025 g) |
7.1 × 10−5 – 7.1 × 10−3 |
| Eye shadow/ colour |
Eye shadow | Concentration of MDM = 3–100 % Exposure frequency: 438×/year (Loretz et al. 2008) Exposed area: 24 cm2 (ConsExpo 2007) Amount product applied: 0.009 g (10% partitioning, model input 0.0009 g) (Loretz et al 2008) |
4.6 × 10−4 – 1.5 × 10−2 |
| Eye lotion | Eye makeup remover | Concentration of MDM = 0.3–3% Exposure frequency: 166×/year (CTFA 1983) Exposed area: 50 cm2 (Health Canada 1995) Amount product applied: 0.5 g (10% partitioning, model input 0.05 g) |
9.7 × 10−4 – 0.010 |
| Face cream | Face cream | Concentration of MDM = 0.3–30% Exposure frequency: 657×/year (Loretz et al 2005) Exposed area: 637 cm2 (Health Canada 1995) Amount product applied: 1.2 g (Loretz et al 2005) (10% partitioning, model input 0.12 g) |
9.1 × 10−3 – 0.91 |
| Body lotion | Body lotion | Concentration of MDM = 0.3–10% Exposure frequency: 402×/year (Loretz et al 2005) Exposed area: 16 925 cm2 (Health Canada 1995) Amount product applied: 4.4 g (Loretz et al 2005) (10% partitioning, model input 0.44 g) |
0.021 – 0.68 |
| Leave-in conditioner | Conditioner (no retention factor) | Concentration of MDM = 0.1–30% Exposure frequency: 260×/year Exposed area: 1550 cm2 (Health Canada 1995) Deposition factor of 10% was appliedFootnote Appendix 5a Table 1[d] Amount product applied: 0.124 g (10% partitioning, model input 1.24 g) |
1.2 × 10−3– 0.37 |
| Shampoo | Shampoo | Concentration of MDM = 0.1–0.3% Exposure frequency: 260×/year Exposed area: 1550 cm2 (Health Canada 1995) Retention factor of 10% was appliedFootnote Appendix 5a Table 1[c] Amount product applied: 20 g (10% partitioning, model input 2 g) |
2.0 × 10−3 – 6.0 × 10−3 |
| Rinse-off conditioner | Conditioner | Concentration of MDM = 0.1–0.3% Exposure frequency: 104×/year Exposed area: 1550 cm2 (Health Canada 1995) Retention factor of 10% was applied[c] Amount product applied: 18 g (10% partitioning, model input 1.8 g) |
7.2 × 10−4 – 2.2 × 10−3 |
| Deodorant (stick) | Deodorant | Concentration of MDM = 1–10% Exposure frequency: 475×/year (Loretz et al 2006) Exposed area: 240 cm2 (estimated) Amount product applied: 0.6 g (Loretz et al 2006) (10% partitioning, model input 0.06 g) |
0.011 – 0.11 |
| Perfume (amount left on skin after spraying) | Fragrance | Concentration of MDM = 3–30% Exposure frequency: 621×/year (Loretz et al. 2006) Exposed area: 200 cm2 (ConsExpo 2007) Amount product applied: 0.33 g (Loretz et al. 2006) (10% partitioning, model input 0.033 g) |
0.024 – 0.24 |
| Detangler (Children) |
Leave-in Conditioner | Maximum Concentration of MDM = 5% (Environment Canada 2010a) Frequency: 365 (professional judgement) Exposed Area: 785 cm2 (Health Canada 1995) Deposition factor of 10% was applied[d] Amount of product applied: 6.3 g (10% partitioning, model input 0.63 g), obtained applied product amount by correcting adult leave-in conditioner for surface area differences |
0.20 |
| Shampoo (Children) | Shampoo | Concentration of MDM = 0.1–0.3% Exposure frequency: 164×/year (Wu et al 2010) Exposed area: 785 cm2 (Health Canada 1995) Retention factor of 10% was applied[c] Amount product applied: 10 g (10% partitioning, model input 1.0 g) obtained applied product amount by correcting adult shampoo for surface area differences |
2.0 × 10−3 |
| Product | AssumptionsFootnote Appendix 5b Table 1[a].12 | Estimated chronic external oral exposureFootnote Appendix 5b Table 1[b].11 (mg/kg-bw per day) |
|---|---|---|
| Lipstick | Concentration of MDM = 1–3% Exposure frequency: 767 times/year (Loretz et al 2005) Exposure type: direct intake (ConsExpo 2007) Amount product ingested: 0.01 g |
0030 – 0.0089 |
| Product | Scenario | AssumptionsFootnote Appendix 5c Table 1[a].13 | Estimated Chronic exposure (per application) |
|---|---|---|---|
| Foundation | Facial makeup | Concentration of MDM: 1–3% Frequency: 438×/year (Loretz et al 2006) Exposure to vapour, constant rate Exposure duration: 12 h Emission duration: 12 h Room volume: 80 m3 Ventilation rate: 1/h Applied amount: 0.54g (90% partitioning, model input 0.49 g) (Loretz et al 2006) Inhalation rate: 16.2 m3/day Uptake fraction: 1 |
Mean event concentration: Chronic internal dose: |
| Make-up Primer | Facial makeup | Concentration of MDM: 1–100% Frequency: 438×/year (Loretz et al 2006) Exposure to vapour, constant rate Exposure duration: 12 h Emission duration: 12 h Room volume: 80 m3 Ventilation rate: 1/h 0.54g (90% partitioning, model input 0.49 g) (Loretz et al 2006) Inhalation rate: 16.2 m3/day Uptake fraction: 1 |
Mean event concentration: Chronic internal dose: |
| Face cream | Face cream | Concentration of MDM: 0.3–30% Frequency: 657×/year (Loretz et al 2005) Exposure to vapour, constant rate Exposure duration: 12 h Emission duration: 12 h Room volume: 80 m3 Ventilation rate: 1/h Applied amount: 1.2 (90% partitioning, model input 1.1 g) (Loretz et al 2005) Inhalation rate: 16.2 m3/day Uptake fraction: 1 |
Mean event concentration: Chronic internal dose: |
| Body lotion | Body lotion | Concentration of MDM: 0.3–10% Frequency: 402×/year (Loretz et al 2005 Exposure to vapour, constant rate Exposure duration: 12 h Emission duration: 12 h Room volume: 80 m3 Ventilation rate: 1/h Applied amount: 4.4 g (90% partitioning, model input 4.0 g) (Loretz et al 2005) Inhalation rate: 16.2 m3/day Uptake fraction: 1 |
Mean event concentration: Chronic internal dose: |
| Leave-in conditioner | Conditioner | Concentration of MDM: 0.1–30% Frequency: 260×/year Exposure to vapour, constant rate Exposure duration: 12 h Emission duration: 12 h Room volume: 80 m3 Ventilation rate: 1/h Applied amount: 12.4 g (90% partitioning, model input 11.2 g) Inhalation rate: 16.2 m3/day Uptake fraction: 1 |
Mean event concentration: Chronic internal dose: |
| Perfume | Amount volatilizing from skin deposit | Concentration of MDM: 3–30% Frequency: 621×/year (Loretz et al 2006) Exposure duration: 12 h Room volume: 80 m3 Ventilation rate: 1/h Amount product applied: 0.33 g (Loretz et al. 2006) (90% partitioning, model input 0.30 g) Inhalation rate: 16.2 m3/day Uptake fraction: 1 |
Mean event concentration: Chronic external applied dose: |
| Children’s Detangler |
Leave-in conditioner | Concentration of MDM = 5 % (Environment Canada 2010a) Frequency: 365 (professional judgement) Limited air concentration to vapour pressure of pure substance Exposure duration: 12 h Room volume: 80 m3 Ventilation rate: 1/h Amount of product applied: 6.3 g (90% partitioning, model input 5.67 g), obtained applied product amount by correcting adult leave-in conditioner for surface area differences Inhalation rate: 9.3 m3/day Uptake fraction: 1 |
Mean event concentration: Chronic internal dose: |
Appendix VI: Structures and Property Data for MDM and Analogues Considered in the Screening Assessment
| Name / CAS RN / short name | Structure | Molecular formula / molecular weight (g/mol) / chemical properties | Analogue identification method (% similar) |
|---|---|---|---|
| Octamethyl- trisiloxane 107-51-7 MDM |
 |
C8H24O2Si3 MW: 236.5 Low water solubility (34.0 μg/L)Footnote Appendix 6 Table 1[a] Log Kow: 6.6[a] Log Koc: 4.3[a] Dmax, Deff (nm)Footnote Appendix 6 Table 1[b]: 1.2, 0.9 |
n/a |
| Hexamethyl-disiloxane 107-46-0 HMDS |
 |
C6H18OSi2 MW: 162.62 Low water solubility (964 μg /L) |
SciFinder: 67% |
| Decamethyl- tetrasiloxane 141-62-8 L4 |
 |
C10H30O3Si4 MW: 310.70 Low water solubility (6.7 μg/L)Footnote Appendix 6 Table 1[c] Log Kow: 7.2Footnote Appendix 6 Table 1[d] Log Koc: 4.3Footnote Appendix 6 Table 1[e] Dmax, Deff(nm)[b]: 1.5, 1.0 |
SciFinder: 86% ChemID: 89% |
| Dodecamethyl-pentasiloxane 141-63-9 L5 |
 |
C12H36O4Si5 MW: 384.9 Low water solubility (0.07 μg/L)[c] Log Kow: 7.8[d] Log Koc: 5.2[e] Dmax, Deff(nm)[b]: 1.7, 1.1 |
SciFinder: 73% ChemID: 87% |
| Trisiloxane, 1,1,1,5,5,5-hexamethyl-3,3-bis[(trimethylsilyl)oxy]- 3555-47-3 M4Q |
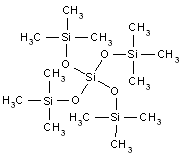 |
C12H36O4Si5 MW: 384.85 Low water solubility (0.15 μg/L)Footnote Appendix 6 Table 1[f] Log Kow: 9.6[f] Log Koc: 5.2[e],[f] Dmax, Deff(nm)[b]: 1.3, 1.2 |
SciFinder: 73% ChemID: 83% |
Appendix VII: Summary of Health Effects Information for MDM
| Endpoint | LD50/LC50 or lowest/no effect levelsFootnote Appendix 7 Table 1[a].15/results |
|---|---|
| Acute toxicity | Oral LD50 (rat) = greater than 2000 mg/kg-bw (DowCorning Corporation 2006) Inhalation LC50 (rat, 4 h) = greater than 2350 ppm (22 600 mg/m3) (DowCorning Corporation 2004) Dermal LD50 (rat) = greater than 2000 mg/kg-bw (DowCorning Corporation 1999a) |
| Short-term repeated-dose toxicity | Inhalation LOAEC = 7740 mg/m3, based on significant increases in serum cholesterol in males, significant increases in absolute and relative liver weights in females, and hyaline droplet nephropathy, which was consistent in appearance with alpha-2μ nephropathy, in male Sprague-Dawley rats (10/sex/group) exposed via whole-body inhalation to 0, 7.74, 15.5 or 31.0 mg/L (equal to 0, 7740, 15 500 or 31 000 mg/m3), 6 h/day, 7 days/week, for 28–29 days in a combined repeated-dose/reproductive/developmental toxicity study. Significant increases in serum cholesterol and increases in relative liver weight were also observed in females at 15 500 mg/m3 and in males at 31 000 mg/m3, respectively. Other observations included centrilobular hypertrophy, which was considered an adaptative change, in females exposed to 7740 mg/m3 and above and in males exposed at the highest dose and hepatic protoporphyrinosis in males at 15 500 mg/m3 and above. No treatment-related effects on body weight, food consumption and neurobehavioral responses were noted (DowCorning Corporation 2007a). Oral LOAEL = 250 mg/kg-bw per day, based on significant increase in liver weight in both sexes in Sprague-Dawley rats (5 per group) dosed by gavage at 0, 5, 25, 250 and 1000 mg/kg-bw per day for 28 days. This increase was accompanied with hepatocellular hypertrophy and protoporphyrin accumulation with associated bile duct proliferation and periportal chronic inflammation in males at 250 and 1000 mg/kg-bw per day and in females at the highest dose only. After a 14-day recovery period, hepatocellular hypertrophy showed complete regression while protoporphyrin accumulation and periportal chronic inflammation was still present in both sexes at 1000 mg/kg-bw per day. An increase incidence and severity of hyaline droplets and higher levels of alpha-2μ-globulin was observed in males at 25 mg/kg-bw per day and above and at all dose levels, respectively. However, hyaline deposits showed complete regression at the end of the recovery period. Thyroid gland follicular cell hypertrophy of minimal severity was observed in both sexes at 1000 mg/kg-bw per day. A reduction in body weight gain was also noted in males at the highest dose at the end of the treatment period (Harland Laboratories Ltd 2010). No dermal studies were identified. |
| Subchronic toxicity | Inhalation LOAEL = 31 000 mg/m3, based on protoporphyrin accumulations along with secondary effects of cholangitis/pericholangitis and bile duct proliferation in both sexes in Sprague-Dawley rats (number of animals per group unknown) exposed via whole-body inhalation to 0, 95, 400 or 3200 ppm (equal to 0, 919, 3870 or 31 000 mg/m3), 6 h/day, 7 days/week, for 90 days. By the end of the 28-day recovery period, partial recovery was observed. Centrilobular hepatocellular hypertrophy associated with a change in organ weight was also observed in males at 3870 mg/m3 and in both sexes at the highest concentration. This was reversible and considered to represent an adaptative process due to enzyme-induction rather than to be a toxic effect. In the kidney, hyaline droplets and higher levels of alpha-2μ-globulin was observed in males. There was evidence of incomplete recovery at the highest dose (SEHSC 2011). No oral or dermal studies were identified. |
| Chronic toxicity/ carcinogenicity | No studies were identified. |
| Reproductive toxicity | NOAEC for reproductive toxicity = 31 000 mg/m3 based on no treatment-related reproductive toxicity observed in a combined repeated-dose/reproductive/ developmental toxicity screening test in which rats 10/sex/concentration) were exposed via whole-body inhalation to 0, 7.74, 15.5 or 31.0 mg/L (0, 7740, 15 500 or 31 000 mg/m3), 6 h/day, 7 days/week for 28 or 42 days (males treated 14 days prior to mating and 14 days after mating; females treated from 14 days prior to mating until gestation day 19). Systemic toxicity in parents is reported in the short-term toxicity section (DowCorning Corporation 2007a). No other reproductive toxicity studies were identified. |
| Developmental toxicity | NOAEC for developmental toxicity = 31 000 mg/m3 based on no evidence of treatment-related developmental toxicity observed in fetuses in a combined repeated dose/reproductive/ developmental toxicity screening test in which rats (10/sex/concentration) were exposed via whole-body inhalation to 0, 7.74, 15.5 or 31.0 mg/L (equal to 0, 7740, 15 500 or 31 000 mg/m3), 6 h/day, 7 days/week for 28 or 42 days (males treated 14 days prior to mating and 14 days after mating; females treated from 14 days prior to mating until gestation day 19). LOEC for maternal toxicity = 7740 mg/m3, based on significant increases in liver weights accompanied with centrilobular hypertrophy (DowCorning Corporation 2007a). No other developmental toxicity studies were identified. |
| Genotoxicity and related endpoints: in vivo | No studies were identified. |
| Genotoxicity and related endpoints: in vitro | Mutagenicity in bacteria Chromosome aberration assay Mammalian cell mutation assay |
| Irritation | Skin irritation: Tx-1302 A (MDM) was applied to 3 albino rabbits (sex not specified) under a 1-inch by 1-inch cotton pad on the shaved abdomen and held by a cloth bandage. Ten applications were made over a 14-day period. Minimal to moderate irritation to the skin was noted (DowCorning Corporation 1994). Eye irritation: |
| Human studies Sensitization | In a human patch test, 103 subjects (male and female) were exposed to the test material (MDM) for two phases. The first phase (induction) consisted of nine consecutive patch applications of 0.2 mL of test material to the same site every 48 hours under semiocclusive wraps; the patches were removed after 24 hours of exposure. After a 12- to 14-day rest period, the same dose method was used on a previously unexposed site (challenge phase) and the volunteers removed the patches after 24 hours. None of the subjects exhibited signs of irritation or sensitization to MDM during any part of the study (DowCorning Corporation 1998). |
Appendix VIII: Summary of Health Effects Information for Analogues of MDM
| Endpoint | Lowest/no effect levelsFootnote Appendix 8a Table 1[a].16 / results |
|---|---|
| Acute toxicity | No studies were identified. |
| Short-term repeated-dose toxicity | Oral LOAEL = 25 mg/kg-bw per day, based on increased incidence and severity of perilobular fatty change in female Sprague-Dawley rats (5 per group) dosed by gavage with 0, 25, 250 or 1000 mg/kg-bw per day for 28 days. This increase in incidence and severity was still present after a 14-day recovery period for the females treated with the highest dose in comparison with the controls. Also, an increase in absolute and relative liver weights was observed in male. This increase was accompanied by liver effects such as brown pigment in intrahepatic bile ducts at the highest and intermediate dose and bile duct proliferation, chronic inflammation and hepatocellular hypertrophy at the highest dose, which were not present in the control group males. While increases in absolute and relative liver weights were also observed in females at 250 mg/kg-bw per day and above, bile duct pigment accumulation and associated bile duct proliferation/chronic inflammation were not present in treated females at any dose level. There were also statistically significant increases in group mean locomotor activity (early intervals and/or total session) at the highest dose in males and females (DowCorning Corporation 2009a). Oral LOEL = 1500 mg/kg-bw per day, based on increase in relative kidney weight in male Sprague-Dawley rats (6 per group) dosed by gavage with 0 or Dermal NOAEL = 1000 mg/kg-bw per day based on no significant adverse effects with respect to body weight, mortality and behavioural reactions and no evidence of testicular atrophy and reduced testicular function in male New Zealand albino rabbits (10 per group) exposed dermally to 0 or 1000 mg/kg-bw per day, once daily, for 28 consecutive days (Hobbs et al. 1972). No other dermal studies were identified. No inhalation studies were identified. |
| Subchronic toxicity | Oral NOAEL = 500 mg/kg-bw per day based on no treatment-related effects on growth, physiological status, organ weights or gross and microscopic appearance of tissues (all organ systems) in albino weanling rats (5/sex/dose) exposed via the diet to 0 or 1% 200 Fluid 1.5 cs (decamethyltetrasiloxane) (equivalent to 0 or Other oral NOAEL = 500 mg/kg-bw per day based on no treatment-related effects on hematological and urinalysis parameters, organ weights and gross or microscopic appearance of tissues from all organ systems in albino rabbits (3 males and females per dose) exposed via the diet to 0 or 1% 200 Fluid 1.5 cs (decamethyltetrasiloxane) (equivalent to 0 or 500 mg/kg-bw per day, using a dose conversion by Health Canada 1994), for 8 months (DowCorning Corporation 1965). No inhalation or dermal studies were identified. |
| Chronic toxicity/ carcinogenicity | No studies were identified. |
| Reproductive toxicity | No studies were identified. |
| Developmental toxicity | No studies were identified. |
| Genotoxicity and related endpoints: in vivo | No studies were identified. |
| Genotoxicity and related endpoints: in vitro | No studies were identified. |
| Sensitization | No studies were identified. |
| Irritation | Skin irritation: Eye irritation: |
| Human studies | No studies were identified. |
| Endpoint | Lowest/no effect levelsFootnote Appendix 8b Table 1[a].17/results |
|---|---|
| Acute toxicity | No studies were identified. |
| Short-term repeated-dose toxicity | Oral LOAEL = 25 mg/kg-bw per day, based on significant increase in absolute and relative liver weight accompanied with liver lesions such as periportal hepatocellular vacuolation in female Sprague-Dawley rats (5 per group) dosed by gavage with 0, 25, 250 or 1000 mg/kg-bw per day for 28 days. Increased absolute and relative liver weight and periportal hepatocellular vacuolation were observed in males only at 250 and 1000 mg/kg-bw per day. Bile duct proliferation was noted in males at 1000 mg/kg-bw per day and in females at 250 mg/kg-bw per day and above. Accentuated lobular pattern was also noted on the liver of the males treated at the highest dose (DowCorning Corporation 2009c). Oral NOAEL = 1500 mg/kg-bw per day, based on no treatment-related effects on survival, body weight, food consumption, organ weights (kidney and liver), gross pathological changes and behavioural changes in male and female Sprague-Dawley rats (6/sex/dose) dosed by gavage with 0 or 1500 mg/kg-bw per day, 5 days/week for 4 consecutive weeks (DowCorning Corporation 1990). No inhalation or dermal studies were identified. |
| Subchronic toxicity | No studies were identified. |
| Chronic toxicity/ carcinogenicity | No studies were identified. |
| Reproductive toxicity | No studies were identified. |
| Developmental toxicity | No studies were identified. |
| Genotoxicity and related endpoints: in vivo | No studies were identified. |
| Genotoxicity and related endpoints: in vitro | Mutagenicity in bacteria Negative: Salmonella typhimurium,strains TA98, TA100, TA1535, TA1537 and TA1538, with and without metabolic activation (DowCorning Corporation 1979). |
| Sensitization | No studies were identified. |
| Irritation | Skin irritation: Eye irritation: |
| Human studies | No studies were identified. |
| Endpoint | Lowest effect levelsFootnote Appendix 8c Table 1[a]/results |
|---|---|
| Acute toxicity | Lowest oral LD50 (rat) = greater than 16 000 mg/kg-bw (BRRC 1982) Lowest inhalation LC50 (rat, 4 h) = 15 956 ppm (1.06 × 105 mg/m3) (RTECS 2009). No dermal studies were identified. |
| Short-term repeated-dose toxicity | Lowest oral LOAEL = 40 mg/kg-bw per day based on histopathological changes in the kidney (increase in eosinophilic bodies) in male Crj:CD(SD) rats dosed by oral gavage at 0, 8, 40, 160 or 640 mg/kg-bw per day for 28 days. Other effects observed include apparent spotty pattern of surface in male kidneys at 160 mg/kg-bw per day and above and dark brownish change in the liver and enlargement of hepatic lymph node in males at 640 mg/kg-bw per day. Histopathological changes in the liver such as bile stasis, cell infiltration around bile stasis and swelling of hepatocytes were noted in males and females at the highest dose tested. Bile duct proliferation, single cell necrosis in hepatocytes and deposition of brown pigment and increase of histiocytic cells in the hepatic lymph node were observed as well at this dose level but in males only. A dose-related reduction in food consumption and body weight gain and an increase in spleen and liver weight were noted in male at the highest dose. In haematological examinations, an increase in the white blood cell count was also noted in males at that dose level (Shin-Etsu 1994c). LOAEL = 1500 mg/kg-bw per day, based on liver protoporphyrinosis (presence of dark brown pigment in bile duct accompanied by bile duct proliferation and chronic inflammation) in male Sprague-Dawley rats (6 per group) dosed by gavage with 0 or 1500 mg/kg-bw per day 5 days/week for 4 consecutive weeks. An increase in relative kidney weight was also observed in males. No mortality, change in general appearance, behavioural abnormalities and other signs of toxicity were reported in both males and females (DowCorning Corporation 1990, 2009b). NOAEL = 1200 mg/kg-bw per day based on no treatment-related clinical abnormalities, no dose-related changes in body weight and no effect on uterine weight in Sprague Dawley rat pups (12 per group) dosed by gavage with 0, 600 or 1200 mg/kg-bw per day once per day, for 4 consecutive days starting at postnatal day 18 (McKim et al. 2001). Lowest inhalation LOAEC = 3319 mg/m3, based on dose-related increased severity of hyaline droplets in the proximal convoluted tubule epithelium, which was consistent in appearance with alpha-2μ nephropathy, in male Sprague-Dawley rats (5 per group) exposed via whole-body inhalation to 0, 499 or 1004 ppm (approximately 0, 3319 or 6678 mg/m3), 6 h/day, 5 days/week, for 2 weeks. Significant increases in relative kidney weight were also observed in male at the highest dose. No treatment-related toxic effects were observed in female rats (DowCorning Corporation 1992b). Other inhalation LOAEC = 12 700 mg/m3, based on increase in the incidence and severity of renal tubule regeneration, which indicate increase in kidney damage, in male Fischer 344 albino rats (10 per group) exposed by inhalation (nose-only) at 0, 0.9, 3.4, 12.7 or 59.2 mg/l (0, 900, 3400, 12 700 or 59 200 mg/m3) for 6 h/day, 5 days/week, for 1 month. A slight to moderate dose-dependent increase in liver weights was observed in males at all doses tested and in females exposed to the intermediate and highest doses. This increase was accompanied by minimal hepatocellular hypertrophy in males at 12 700 and 59 200 mg/m3 and in one female at 59 200 mg/m3 and by a slight increase in pigment accumulation in bile ducts in males at the highest dose. Hyaline droplet accumulation protein casts and granular casts were also observed in kidneys of several males at the highest dose (DowCorning Corporation 1997a). Dermal LOAEL = 1000 mg/kg-bw per day based on statistically significant reduction in body weight gain, food consumption, absolute liver weight and relative liver and kidney weights (organ to brain ratio) in male Sprague-Dawley rats (10 per group) exposed to 0, 100, 500 or 1000 mg/kg-bw per day through dermal route, 6 h/day, 5 days/week for 28 days. Those effects were not observed in female rats. No treatment related changes in haematological, clinical chemistry or histopathological parameters were reported in treated males and females (DowCorning Corporation 1993). Dermal NOAEL = 200 mg/kg-bw per day based on no significant adverse effects with respect to body weight, mortality and behavioural reactions and no evidence of testicular atrophy and reduced testicular function in male New Zealand albino rabbits (10 per group) exposed to 0 or 200 mg/kg-bw per day through dermal route once daily, for 28 consecutive days (Hobbs et al. 1972). |
| Subchronic toxicity | Lowest inhalation LOAEC = 140 mg/m3, based on slight but significant increase in multifocal, subpleural, subacute to chronic interstitial inflammation, associated with alveolar macrophage aggregation, in the lungs of female Fischer 344 (F344) albino rats (20–30 per group) exposed by inhalation (nose-only) at 0, 0.14, 0.73, 3.42 or 13.64 mg/L (0, 140, 730, 3420 or 13 640 mg/m3) for 6 h/day, 5 days/week, for 3 months. The incidence of these findings was increased in males at the highest dose only. A higher incidence of occult blood in the urine and a minimal increase in severity of testicular tubular atrophy were also observed in males exposed to the highest dose. Minor hematological and clinical biochemical changes were seen but were not considered to be of toxicological relevance. No treatment-related signs of clinical toxicity or mortality, statistically significant effects upon body-weight gain or food consumption, ophthalmoscopic changes, gross macroscopic necropsy findings, or organ weight changes were noted (DowCorning Corporation 1997b). Other inhalation LOAEC = 3944 mg/m3, based on histological lesions in the kidney, which were consistent in appearance with male-rat-specific alpha-2-urinary globulin nephropathy, in male Fisher 344 (F344) rats (20 per group) exposed via whole-body inhalation to 0, 50, 194, 593, 1509 and 5012 ppm (approximately 0, 333, 1290, 3944, 10037 and 33 335 mg/m3), 6 h/day, 5 days/week, for 13 weeks. These lesions were accompanied by slightly increased plasma urea and creatinine concentrations. Minor hematological, clinical biochemical, and urinalysis changes were seen but were not considered to be of toxicological relevance. No No oral or dermal studies were identified. |
| Chronic toxicity/ carcinogenicity | Inhalation study in rats: Groups of 20 F344 rats of each sex were exposed to HMDS by vapour inhalation at 0, 100, 400, 1600 or 5000 ppm (approximately 0, 665, 2660, 10 640, 33 300 mg/m3), 6 h/day, 5 days/week, for 1 or 2 years. After Non-neoplastic LOAEC= 665 mg/m3based on Leydig cell hyperplasia in males. This effect was seen in all exposure groups including controls and tended to increase in severity as the exposure concentration increased. Increased incidence of eosinophilic inclusions in the olfactory epithelium in males at all doses was consistent with chronic inhalation of a mild irritant. Increased liver weight and increased incidence of intraluminal mineralzation in the kidney were also observed in males at 10640 mg/m3 and above. Slight reduction in female body weights at 10 640 mg/m3 and above were likely associated with reduction in food consumption. No oral or dermal studies were identified. |
| Reproductive toxicity | NOAEC for reproductive toxicity= 33 700 mg/m3 based on no treatment-related effects on reproductive parameters in male and female Crl:CD (Sprague-Dawley) rats (24/sex/group) exposed by inhalation to 0, 99, 1030 or 5067 ppm (0, 658, 6850 or 33 700 mg/m3) in a one-generation study (males treated 28 days prior to mating, throughout mating and through the day prior necropsy; females treated 28 days prior to mating, throughout mating and through gestation day 20. Exposure of the females was re-initiated on lactation day 5 and continued through the day prior to necropsy). LOEC for systemic toxicity = 33 700 based on increase in lung and liver weight in males, transient reduction in body weight gain in females and transient reduction in food consumption in both sexes (DowCorning Corporation 2000). No oral or dermal studies were identified. |
| Developmental toxicity | No studies were identified. |
| Genotoxicity and related endpoints: in vivo | Chromosome aberration assay Negative: Bone marrow; male Sprague-Dawley rats; intraperitoneal (0, 255, 515 or 1030 mg/kg-bw, single injection) (Isquith et al. 1988b). |
| Genotoxicity and related endpoints: in vitro | Mutagenicity in bacteria Mammalian cell mutation assay DNA damage (DNA alkaline elution assay) Chromosome aberration assay Sister chromatid exchanges (SCE) assay |
| Sensitization | In a guinea pig maximization test, no incidence of skin irritation or sensitization was observed in the animals (10) exposed to HMDS at 24 or 48 hours post-challenge (DowCorning Corporation 1992c) |
| Irritation | Skin irritation: HMDS was applied to 6 albino rabbits (sex not specified) under a 1-inch by 1-inch cotton pad on the shaved abdomen and held by a cloth bandage. Ten applications were made over a 14-day period. Slight irritation to the intact skin was noted (DowCorning Corporation 1976). Single or repeated dermal contact of HMDS with skin for several hours (up to 72h) or days (up to 16 days) was reported not to cause irritation in rabbit (DowCorning Corporation 1978). Eye irritation: |
| Human studies Sensitization | In a human patch test, 100 subjects (87 Caucasian females, 2 Hispanic females and 11 Caucasian males) were exposed to the test material (HMDS) for two phases. The first phase (induction) consisted of nine consecutive patch applications of 0.2 mL of test material to the same site every 48 hours under occlusive or semi-occlusive wraps; the patches were removed after 24 hours of exposure. After a 14-day rest period, the same dose method was used on a previously unexposed site (challenge phase) and the volunteers removed the patches after 24 hours. No evidence of sensitization was observed following this challenge application (DowCorning Corporation 1992a). In a human patch test, 64 subjects (males and females) were exposed to the test material (HMDS) for two phases. The first phase (induction) consisted of ten consecutive patch applications of the test material to the same site every 48 hours under non-occlusive dressing; the patches were removed after 48 hours of exposure. After a 14-day rest period, the same dose method was used on a previously unexposed site (challenge phase) and the volunteers removed the patches after |